Other Conditions of the Hip
neck-shaft angle below the normal. Coxa vara was initially classified
by Elmslie (1). This classification has
subsequently been modified by Fairbank and condensed into three broad
categories: congenital coxa vara, acquired coxa vara, and developmental
coxa vara (2) (Table 27.1).
cartilaginous defect in the femoral neck. This can be a congenital
short femur, a congenital bowed femur, or part of a proximal femoral
focal deficiency (PFFD) (Fig. 27.1). The deformity is not always evident clinically at birth; however, it is usually evident on radiographs (3,4). Significant leg length inequality is often present.
slipped capital femoral epiphysis, and Legg-Calvé-Perthes disease
(LCPD). The resulting coxa vara can be secondary to necrosis of the
femoral head or altered physeal growth. Coxa vara can also be
associated with skeletal dysplasias and, although these conditions
probably should be classified separately, they are included under
“acquired causes” for the sake of simplicity (5–8) (Fig. 27.4).
term reserved for coxa vara of the proximal femur in early childhood
with classical radiographic changes and no other skeletal
manifestations
or obvious underlying causes (9,10) (Fig. 27.5).
There is associated mild limb-length inequality that develops secondary
to the progressive varus deformity, but not because of a significant
decrease in the length of the femoral shaft. The growth behavior and
radiographic changes help differentiate this condition from the others
mentioned previously.
|
TABLE 27.1 CLASSIFICATION OF COXA VARA
|
|
|---|---|
|
 |
|
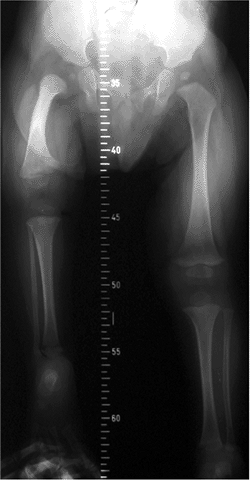
Figure 27.1
The radiographic appearance of congenital coxa vara in an 18-month-old child with a congenital short femur. (Courtesy of Perry L. Schoenecker.) |
 |
|
Figure 27.2 The radiographic appearance of acquired coxa vara in a 7-year-old girl who had an intertrochanteric left hip fracture.
|
 |
|
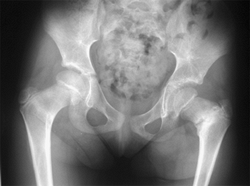
Figure 27.3
The radiographic appearance of acquired coxa vara in an 8-year-old child who had fibrous dysplasia and a shepherd-crook deformity of the proximal femur. (Courtesy of Perry L. Schoeneker.) |
 |
|
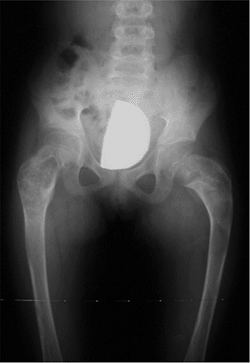
Figure 27.4
The radiographic appearance of coxa vara associated with spondylometaphyseal dysplasia in a 4-year-old child. (Courtesy of Perry L. Schoenecker.) |
 |
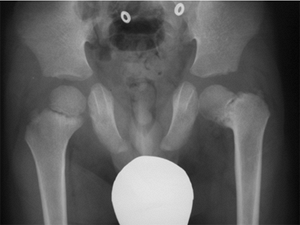
|
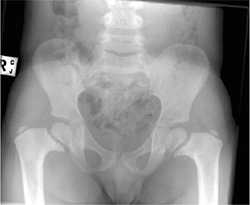
Figure 27.5 Radiographic appearance of developmental coxa vara in a 3-year-old child.
|
Bilateral cases may more likely be associated with a skeletal
dysplasia, so the examiner should investigate for this possibility
during the physical and radiographic examination. There is presumed to
be a genetic cause of developmental coxa vara, with several reports
suggesting an autosomal dominant pattern of inheritance with incomplete
penetrance (10,13,171819,20).
unknown. The most widely accepted theory is that the deformity in the
proximal femur results from a primary defect in endochondral
ossification of the medial part of the femoral neck (21).
This results in dystrophic bone along the medial inferior aspect of the
femoral neck, which fatigues with weight bearing, resulting in the
progressive varus deformity that is seen clinically. In this regard,
the condition has been likened to infantile Blount disease of the
proximal tibia; however, the two conditions have not been shown to
coexist (22,23).
is caused by excessive intrauterine pressure on the developing fetal
hip, resulting in a depression in the neck of the femur (9).
A vascular insult causing a growth arrest to the developing femoral
head and neck has also been proposed as a cause of coxa vara (24).
Yet another theory is that the varus deformity results from faulty
maturation of the cartilage and metaphyseal bone of the femoral neck (10).
Clinically, the child presents with a painless limp that is caused by
both the functional abductor muscle weakness and a relatively minor
limb-length inequality in unilateral cases. When the disease is
bilateral, the child presents with a waddling gait and increased lumbar
lordosis as seen in bilateral developmental hip dislocation (2,10,25,27,28,29).
Although pain is seldom reported as a symptom, older children may
report a deep ache in the buttock muscles after prolonged exercise.
more prominent and proximal than the contralateral normal side, thereby
altering normal hip joint mechanics. With increasing coxa vara
deformity, the origin and insertion of the hip abductors approach each
other, resulting in functional hip abductor weakness and a positive
Trendelenburg test. An associated limb-length inequality is present in
unilateral cases but is rarely greater than 3 cm at skeletal maturity,
even in untreated patients (13,30).
of motion, with limitations of abduction and internal rotation being
the greatest (12,25).
The limitation in abduction is due to impingement of the greater
trochanter on the side of the pelvis. The loss of internal rotation is
due to the loss of the femoral neck anteversion that is a feature of
developmental coxa vara. As part of the general clinical examination
other causes of coxa vara should be ruled out, for example, skeletal
dysplasias (15,31).
with a plain anteroposterior radiograph of the affected hip. The
typical radiographic findings are listed in Table 27.2. Mild acetabular dysplasia is sometimes present as well (4,10,15,16,21,26,31,32). The inverted Y pattern seen in the inferior femoral neck remains the sine qua non
of this condition. The inverted Y pattern is formed by a triangular
piece of bone in the medial femoral neck, abutting the physis and
bounded by two radiolucent bands. Although these bands were once
postulated to be two physeal plates, biopsy specimens and magnetic
resonance studies have shown this to be an area of widening of the
physeal plate with associated abnormal ossification (22).
Kim et al. used computed tomography (CT) scanning in three patients and
suggested that the triangular metaphyseal fragment is a Salter-Harris
type 2 “separation” through the defective femoral neck (32).
|
TABLE 27.2 RADIOGRAPHIC FEATURES OF DEVELOPMENTAL COXA VARA
|
|
|---|---|
|
quantified on anteroposterior radiographs by measuring the neck-shaft
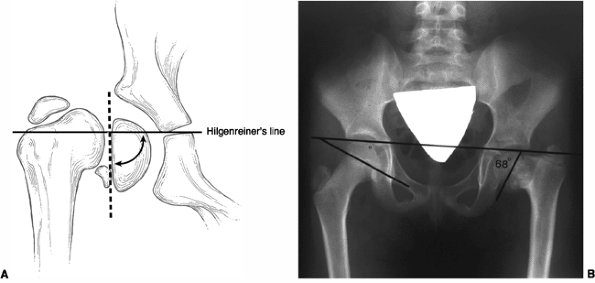
angle, the head-shaft angle, or the Hilgenreiner-epiphyseal angle (H-E)
(33). Neither the neck-shaft angle nor the
head-shaft angle provides an accurate reflection of the severity of the
deformity and its likely progression or correction (24,29). On the other hand, the H-E angle, described by Weinstein, has been shown to have good prognostic value (33). The H-E angle is the angle between the physeal plate and Hilgenreiner’s line (33) (Fig. 27.6).
In 100 healthy patients, this angle averaged 16 degrees. In
developmental coxa vara, the angle is between 40 and 70 degrees. Using
this measurement in 22 patients with coxa vara, Weinstein was able to
make recommendations concerning the natural history and treatment
options for this group of children. These are discussed in the
subsequent text.
extends across the entire proximal femur. The cartilage columns that
make this physis then differentiate into cervical epiphyseal and
trochanteric apophyseal portions. The medial cervical portion matures
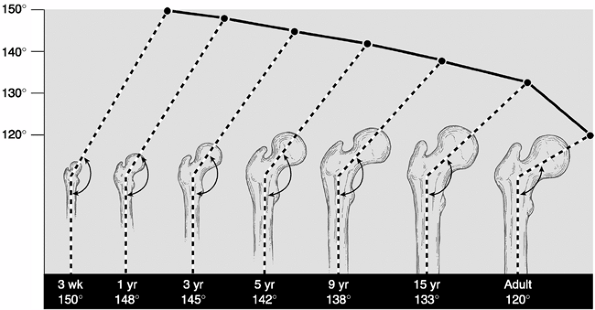
first, elongating the femoral neck. The neck-shaft angle is determined
by the relative amount of growth at these two sites (34,35,36,37,38). The mean angle of the femoral neck-shaft angle is 150 degrees at 3 weeks of age, decreasing to 120 degrees in adulthood (39) (Fig. 27.7).
taken from both the proximal femoral physis and femoral neck in
patients with developmental coxa vara (12,34,40).
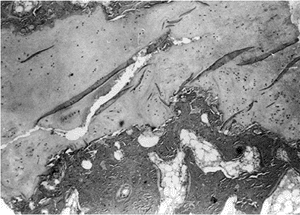
These have shown defects in cartilage production and secondary
metaphyseal bone formation in the inferior portion of the proximal
femoral physeal plate and adjacent femoral neck. The cartilage cell
numbers are decreased and the remaining cells are not well organized in
regular columns as seen in a healthy physis. The adjacent metaphyseal
bone is osteoporotic and infiltrated with nests of cartilage cells (34,40) (Fig. 27.8).
Chung and Riser reported on the postmortem findings in a 5-year-old boy
with unilateral coxa vara. They noted that the acetabular volume and
femoral head were smaller, the femoral neck was shorter, and the physis
was wider on the affected side than on the normal contralateral side.
They found that endochondral ossification was altered in the affected
hip as well as in the “normal” contralateral side. They also
observed
that there was a “reduction in the number and caliber of intraosseous
arteries supplying the metaphyseal sides of the growth plates in the
proximal femur and those supplying the subchondral region and
extraosseous medial ascending cervical arteries on the surface of the
femoral neck” (34).
 |
|
Figure 27.6 Hilgenreiner-epiphyseal (H-E) angle. A: The H-E angle is the angle between Hilgenreiner’s line and a line drawn parallel to the capital femoral physis. B: H-E angle of 68 degrees in a patient with developmental coxa vara.
|
underlying pathology and the altered mechanical forces across the hip.
With progressive varus deformity of the femoral neck, the force across
the proximal femoral physis changes from compression to shear as it
assumes a more vertical orientation. The shortened lever arm and
relative proximal migration of the greater trochanter also leads to
altered muscular forces in the abductor group.
 |
|
Figure 27.7 Evolution of the neck-shaft angle in the normal hip.
|
condition in which increased tensile forces on the superior femoral
neck led to progressive varus deformity of the proximal femur,
ultimately resulting in the development of a stress-fracture-related
nonunion of the femoral neck and
premature degenerative arthritic changes within the hip joint in almost all the affected patients (41). Weinstein et al. (33),
however, showed that not all patients with developmental coxa vara
follow such a progressive course. Their study demonstrated that the
determining factor for progression of the varus deformity is the H-E
angle. If the H-E angle is less than 45 degrees, the condition is
stable and progressive deformity is unlikely. If the H-E angle is
greater than 60 degrees, surgical intervention is recommended because
the deformity invariably progresses. Between 45 and 60 degrees the
natural history is not as clear, and these patients must have serial
radiographs to reevaluate their varus deformity (33). Serafin et al. (16), Carroll et al. (22), Cordes et al. (23), and Desai et al. (15)
have all confirmed these parameters in their own patient populations.
What is not clear from natural history studies is the time of onset of
developmental coxa vara and the speed of progression of the deformity.
 |
|
Figure 27.8
Photomicrograph of a biopsy specimen of the proximal femoral physeal plate of a patient with developmental coxa vara demonstrates irregularly distributed germinal cells in the resting zone; an absence of normal, orderly progression of the cartilage columns; and a poorly defined zone of provisional calcification. Nests of cartilage cells reside at the margin of the metaphyseal bone. |
based on the natural history studies mentioned in the preceding text.
Because the cause of the abnormal pathology is not known, a biologic
cure is not possible. Treatment, therefore, is aimed at preventing the
secondary deformities of the proximal femur created by the condition’s
natural history. Borden et al. (42) identified
the main objectives of current treatment approaches: correction of the
varus angulation into a more normal physiologic range; changing the
loading characteristics seen by the abnormal femoral neck from shear to
compression; correction of limb-length inequality; and reestablishment
of a proper abductor muscle length-tension relation.
and are asymptomatic need to be assessed for limb-length inequality (in
unilateral cases) and for evidence of skeletal dysplasia. These
patients should also have periodic radiographic assessments to assess
for progressive deformity until skeletal maturity. In patients with an
H-E angle between 45 and 59 degrees, serial radiographs are essential
so as to assess for progression. In those who develop a symptomatic
limp, Trendelenburg gait, or progressive deformity, surgical treatment
is warranted. In general, nonsurgical treatments including bed rest,
traction, and hip immobilization in a spica cast have not altered the
natural course of the disease (24,29,43). Zadek (21),
in a review of conservative treatment of developmental coxa vara,
concluded that the previously attempted nonoperative methods had
universally little or no value.
H-E angle of 60 degrees or greater, a progressive decrease in the
femoral neck-shaft angle to 90 to 100 degrees or less, or for patients
who develop a symptomatic limp or Trendelenburg gait (25,33,44).
for developmental coxa vara over the years, many of which are of
historical interest only. One such procedure is epiphysiodesis of the
greater trochanter, which has been shown to be unreliable as the sole
surgical treatment of this condition (12,27,45).
Other historical surgical procedures included pin fixation and bone
grafting of the femoral neck defect, which did not correct the varus
deformity, did not prevent progression, and sometimes resulted in
growth arrest of the capital femoral physis (27).
restore more normal hip joint mechanics is with a derotational
valgus-producing proximal femoral osteotomy. A valgus osteotomy
converts the shear forces across the physes into compressive forces,
and this appears to improve ossification in the femoral neck.
Correction of the neck-shaft angle to normal also restores the muscle
function to the hip abductors. Restoration of a normal neck-shaft angle
allows proximal femoral remodeling and normal ossification to occur.
The proximal femoral osteotomy has been performed at the level of the
neck, the intertrochanteric region, and the subtrochanteric region, all
with the goal of restoring the normal anatomy of the hip joint (2,12,29,42,44,46,47).
Femoral neck osteotomies have had a higher morbidity rate and poorer
clinical results than either the intertrochanteric or subtrochanteric
osteotomies, which are the treatments of choice (14,15,22,23,31,33,48,49,50).
Many intertrochanteric and subtrochanteric osteotomies have been
described for correcting coxa vara, thereby indicating that no one
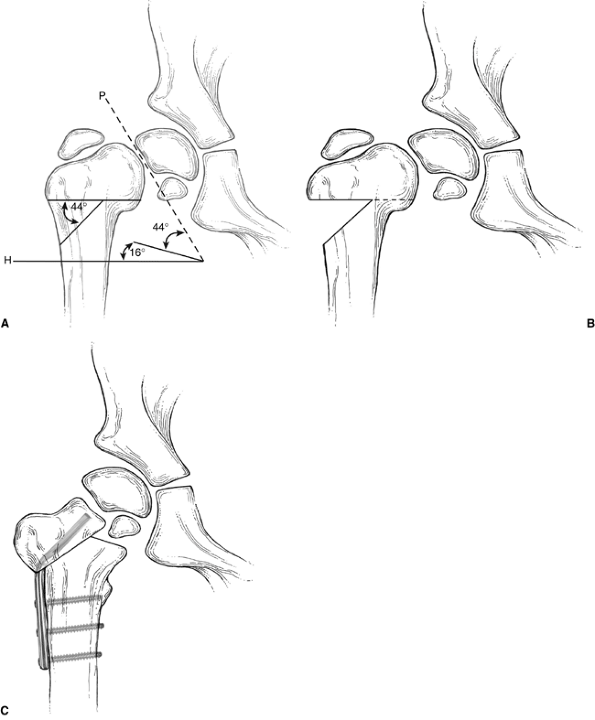
method has proved to be totally satisfactory. Langenskiöld’s
valgus-producing osteotomy (12) (Fig. 27.9) and Pauwel Y-shaped
osteotomy (23,51) (Fig. 27.10)
are examples of intertrochanteric corrective osteotomies that have
produced good results. Pauwel osteotomy is technically demanding and
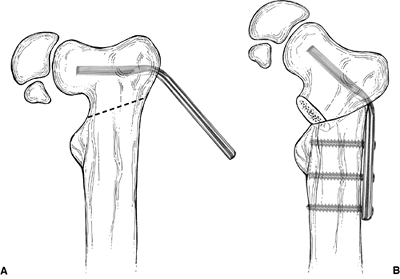
does not allow rotational correction of the upper femur. Borden et al.
describe a subtrochanteric valgus-producing osteotomy that has been
used successfully in achieving and maintaining the goals of surgical
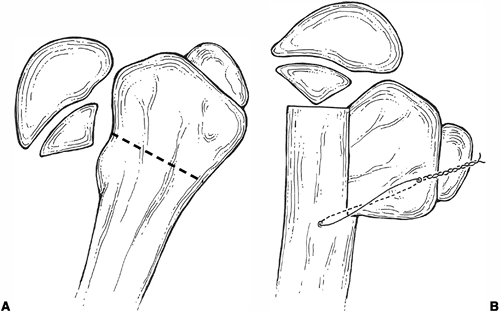
treatment (42) (Fig. 27.11).
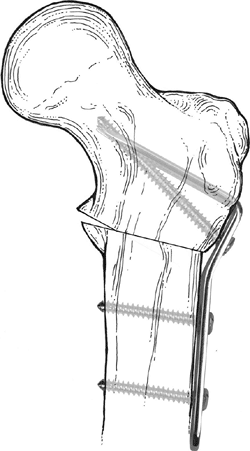
An alternative form of internal fixation suggested by Wagner is
performed with a bifurcated plate driven through the intramedullary
surface of the proximal fragment and secured to the distal fragment
with screws (Fig. 27.12).
 |
|
Figure 27.9 Langenskiöld intertrochanteric osteotomy. A: Site of osteotomy in proximal femur. B: After osteotomy with fixation in place and resulting coxa valga.
|
osteotomy. Some orthopaedists advocate performing the osteotomy as soon
as it is clinically indicated, whereas others prefer to wait until the
child is older. Pylkkanen (12), Weighill (47), and Serafin (52) recommend that the osteotomy be performed at an early age, even as young as 18 months. Weinstein et al. (33), and Duncan (10),
on the other hand, recommend delaying surgery until the patient is 5 to
6 years of age. In very young children, it is difficult to obtain
adequate fixation because of the mostly cartilaginous proximal femur,
and this may accentuate the propensity for recurrence of the deformity
in this age group. On the other hand, the amount of acetabular
dysplasia associated with developmental coxa vara most likely increases
with increasing age, and the capacity for acetabular remodeling
decreases with increasing age. Therefore, the appropriate time for
surgical intervention in indicated patients is as soon as there is
adequate bone development to allow secure internal fixation.
with either a blade plate or sliding hip screw. A number of other
devices have been used, including cerclage wire (53), hook plates (54), and external fixators (55),
all of which have a higher incidence of fixation failure. The advantage
of the blade plate over the sliding hip screw is that it does not cross
the proximal femoral physis, and so it can be used in very young
patients. Good results have also been reported with the use of a spike
osteotomy and no internal fixation (56).
Stability is obtained in these cases by careful fashioning of the spike
so that there is a tight fit into the cancellous bone of the metaphysis.
overcorrection of the neck-shaft angle of the proximal femur,
regardless of the patient’s age. A number of authors have reported
recurrence rates of between 30% and 70% because of insufficient
correction at the time of surgery, or loss of correction in the
postoperative period because of inadequate fixation of the osteotomy (15,22,23). Carroll et al. (22) found that if the H-E angle is reduced to less than 38 degrees, 95% of the patients showed no evidence of recurrence (Fig. 27.13).
In contrast, 93% of the osteotomies that retained a physeal angle
greater than 40 degrees required revision for recurrent varus deformity.
valgus osteotomy, the femur must be internally rotated to recreate the
normal femoral neck anteversion. The amount of derotation required is a
clinical decision made during surgery. An adductor tenotomy performed
at the time of the valgus osteotomy allows easier positioning of the
proximal femur (47). In cases where it is
difficult to correct the varus deformity, a proximal femoral shortening
procedure at the level of the osteotomy can be used (25).
Care should be taken to avoid crossing the physeal plate with the
fixation device, if possible. A spica cast may be applied, depending on
the stability of the internal fixation.
valgus correction and restoration of more normal growth of the proximal
physis. By converting the shear stresses to compression, the osteotomy
allows this more normal development.
The
triangular metaphyseal defect in the femoral neck spontaneously closes
within the first months postoperatively in most cases, if adequate
valgus has been created (25) (Fig. 27.14).
The results of most studies show that a correction of the H-E angle to
less than 40 degrees will result in a good clinical outcome. The
published results of valgus osteotomies for coxa vara invariably
include multiple etiologies for this deformity; hence, some conclusions
are not necessarily specific for developmental coxa vara. In a review
of 14 patients who had had a Pauwel Y-shaped intertrochanteric
osteotomy for coxa vara, Cordes et al. (23)
reported good maintenance of correction at 11 years average follow-up
in patients in whom the H-E angle had been corrected to less than 40
degrees. Desai and Johnson (15) reviewed 20
hips in 12 patients for an average of 20 years and found that
satisfactory results were achieved if the H-E angle was 35 degrees or
less. Twelve hips had trochanteric overgrowth; however, only 5 of these
patients
had weakness of the abductor. Yang and Huang (50)
showed that the acetabular depth improves significantly in patients
with developmental coxa vara who are treated with a valgus
intertrochanteric osteotomy, especially if it is performed before the
child reaches 6 years of age. Carroll et al. (22)
reviewed 37 affected hips in 26 children following a valgus osteotomy
for congenital or acquired coxa vara. They reported a 50% recurrence
rate that was unrelated to age at the time of surgery, the type of
internal fixation, or the etiology. Of the children in whom the H-E
angle was corrected to less than 38 degrees, 95% had no recurrence of
the deformity. If the femoral osteotomy is performed before 10 years of
age, 83% of the patients will have excellent acetabular development.
 |
|
Figure 27.10 Pauwel Y-shaped osteotomy. A: Lines are drawn corresponding to the axes of the physis (P) and parallel to Hilgenreiner’s line several centimeters below the lesser trochanter (H). The angle between lines P and H,
less 16 degrees (the normal Hilgenreiner-epiphyseal angle), describes the amount of deformity, and therefore the angle of wedge to be resected (in this case, 44 degrees). B: Proximal femur after the wedge of bone has been removed. C: Proximal femur with osteotomy completed and hardware in place. |
 |
|
Figure 27.11 Borden subtrochanteric osteotomy. A: Line of osteotomy and insertion of 140-degree angle blade plate parallel to the superior border of the femoral neck. B:
Varus deformity corrected. Note that the lateral cortex of the proximal fragment is approximated to the upper end of the distal fragment. |
 |
|
Figure 27.12
Internal fixation of valgus osteotomy with the Wagner bifurcated plate. The bifurcated end of the plate is driven into the proximal fragment through its intramedullary surface. |
Once diagnosed, the child should be followed up every 4 to 6 months
with anteroposterior radiographs of the pelvis. Surgical intervention
is recommended for hips with an H-E angle of 60 degrees or greater, a
progressive decrease in the femoral neck-shaft angle of 90 to 100
degrees or less, or in patients with developmental coxa vara who
develop a symptomatic limp or Trendelenburg gait. The authors prefer a
subtrochanteric valgus-producing derotational osteotomy of the proximal
femur. The preferred fixation device is either a blade plate or a
sliding hip screw (Fig. 27.15). An adductor
tenotomy is frequently used so as to facilitate correction of the
deformity. Spica cast immobilization is used, in addition, for 6 to 8
weeks in most patients.
that 89% of hips that have had an osteotomy have premature closure of
the proximal femoral physeal plate. This usually occurs in the first 12
to 24 months after surgery (Fig. 27.14). This phenomenon is probably due to an inherently abnormal
physis that has a compressive force applied across it rather than any
physeal injury at the time of surgery. The surgery itself may also
accelerate physeal closure. This premature closure may lead to both
limb-length inequality and trochanteric overgrowth with resultant
recurrent coxa vara. To prevent this recurrent deformity, it is
recommended that after premature closure of the proximal femoral
epiphyseal plate has been documented, an apophyseodesis of the greater
trochanter or a trochanteric advancement be performed before the
development of a recurrent deformity (25).
If the varus deformity does recur, a repeat valgus-producing femoral
osteotomy can be performed. The residual limb-length inequality is
usually mild and can be addressed in most cases with a shoe lift.
Contralateral epiphysiodesis around the knee can be used in more severe
cases to achieve equal limb lengths.
 |
|
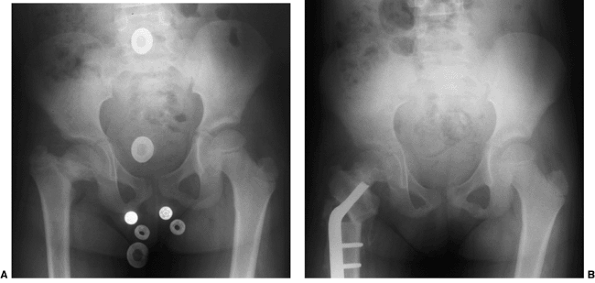
Figure 27.13 Anteroposterior pelvic radiographs of a 4-year-old child with developmental coxa vara. A: Preoperative radiograph. B:
Postoperative radiograph. A subtrochanteric derotational proximal femoral osteotomy successfully achieved the objectives of surgical correction, including correction of the varus angulation, restoring the Hilgenreiner-epiphyseal (H-E) angle to less than 30 degrees, and lateralizing the distal fragment to help reestablish the proper abductor muscle length-tension relation. (Courtesy of Perry L. Schoenecker.) |
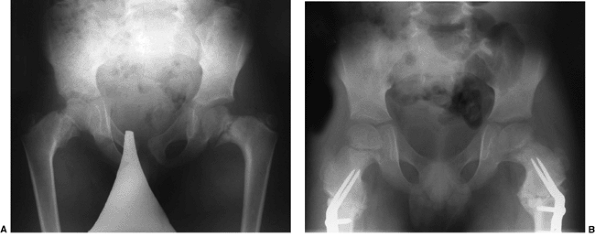 |
|
Figure 27.14 Anteroposterior pelvic radiographs of a child with developmental coxa vara. A: The preoperative radiograph demonstrates a classic inferior femoral neck triangular fragment. B:
Two months postoperatively, the radiograph demonstrates correction of the physeal angle, with spontaneous closure of the femoral neck triangular metaphyseal fragment. (Courtesy of Perry L. Schoenecker.) |
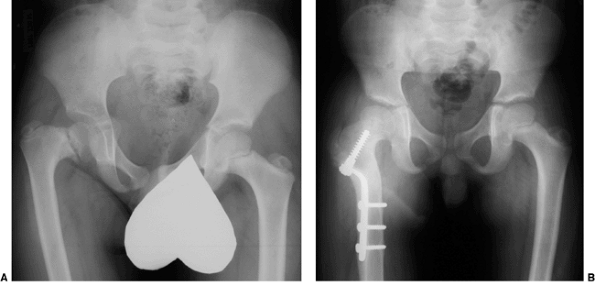 |
|
Figure 27.15 The anteroposterior pelvic radiographs of an 8-year-old child with developmental coxa vara. A: Preoperative radiograph. B:
The postoperative radiograph 11 months after the subtrochanteric proximal femoral derotational osteotomy and fixation with a sliding hip screw demonstrates spontaneous closure of the proximal femoral epiphyseal plate. The greater trochanteric apophyses remain open. (Courtesy of Perry L. Schoenecker.) |
which may involve, to varying degrees, the bladder, pelvis, intestinal
tract, and external genitalia. The prototypical and most common form is
“classic” exstrophy, involving a widened pelvis with an anterior
diastasis, an open bladder, and a complete epispadias (57).
The most minor form of this spectrum is epispadias, which may have a
closed bladder but widened pelvic symphysis. The most pronounced
expression of this spectrum is cloacal exstrophy, which usually
involves all of the findings discussed in the preceding text, as well
as omphalocele and often, a lumbosacral neural tube defect. Although
classic exstrophy is a relatively uniform anomaly, cloacal exstrophy is
extremely variable from patient to patient and often includes anomalies
of the spine and extremities. The orthopaedic surgeon may be consulted
with questions about prognosis of the pelvic defect, for assistance
during closure of the bladder, and for treatment of associated
anomalies of the spine and extremities.
Boys are much more commonly affected, with a gender ratio of at least
2.5:1 between boys and girls. “Classic” exstrophy is the most common
type seen, with cloacal exstrophy being about one fifth as common. When
parents have a child with exstrophy, the risk of their having a
subsequent child with the same defect is approximately 1:100.
pathogenesis is thought to be a failure of the cloacal membrane to get
reinforced by the ingrowth of mesoderm (57).
The cloacal membrane is the caudal end of the embryonic abdominal wall.
It initially forms the anterior boundary of the bladder and hindgut.
Mesenchymal ingrowth allows formation of the anterior part of the
pelvis and the abdominal wall muscles. This defective structure leads
to the development of a large open bladder and urethra. In cloacal
exstrophy, the hindgut is exposed as well.
abdominal wall at the level of the pubic symphysis, exposing an open
bladder and urethra. This usually measures at least 3 to 4 cm at birth
in classic exstrophy (Fig. 27.16). The bladder
itself is a flat plate instead of a closed sac. In classic exstrophy,
the innervation is normal, the hips are usually stable, and the
extremities are functional. In cloacal exstrophy, the abdominal wall
defect is much larger and the lower intestinal tract is variably
exposed (Fig. 27.17). A spinal exam and a neurologic exam of the lower extremities should be performed. Often, in patients with cloacal
exstrophy, there is a lower lumbar neurologic deficit caused by
lipomeningocele or myelomeningocele. Hip dislocation, foot deformity,
and hemisacral agenesis are not uncommon. Other anomalies that may
coexist with cloacal exstrophy include partial failures of formation of
the lower extremity or a distal duplication of the spine.
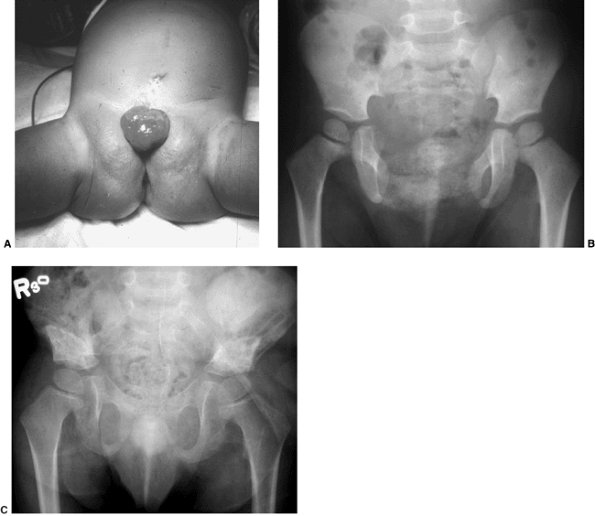 |
|
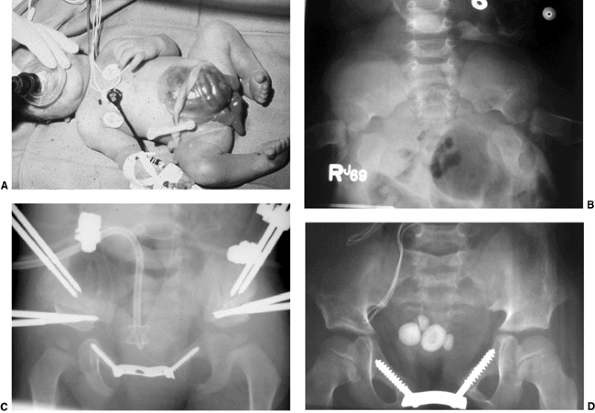
Figure 27.16 Patient with classic exstrophy before closure. A: Clinical photo. B: Radiograph prior to closure. C: Radiograph after closure. (Courtesy of Paul Sponseller.)
|
usually symmetrically formed in patients with classic exstrophy. This
separation is typically approximately 4 to 5 cm at birth and increases
steadily with age (58) (Fig. 27.16B).
In normal persons, this separation is constant at approximately 1 cm
throughout life. The iliac wings are externally rotated by
approximately 15 degrees each, and assume a “flattened” or “flared”
shape. The anterior (ischiopubic) portions of the pelvis are slightly
underdeveloped, having a decreased transverse diameter. The hips
themselves, apart from being in a retroverted position, rarely show
dysplasia. In cloacal exstrophy, the diastasis is much larger (Fig. 27.17B)
and many other spinopelvic anomalies exist: posterior laminar defects,
vertebral body anomalies, sacroiliac asymmetry, and hip dysplasia (Fig. 27.18).
cloacal exstrophy, CT scan may show the sacroiliac and pelvic
malformations more completely. Magnetic resonance imaging (MRI) of the
spine may be valuable in patients with cloacal exstrophy for the
purpose of assessing anomalies.
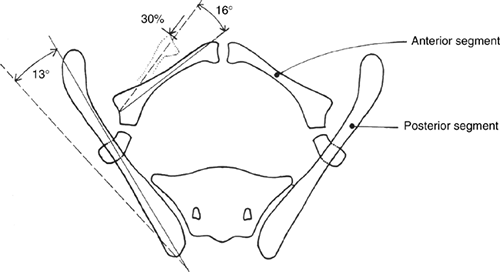
the following (59,60,61) (Fig. 27.19):
-
The pubic bones are foreshortened by about one third in transverse length.
-
The ilia are normal in size but externally rotated approximately 13 degrees.
-
The acetabulae are retroverted but
femoral version is normal. Biomechanical modeling has shown that the
total stress on the hip joint is increased by approximately 30% above
normal, mainly because of the increased transverse distance of the
center of hip rotation from the center of body mass, as well as the
change in orientation of the trochanter and acetabulum. -
The sacroiliac joints are also externally rotated and the pelvis is angled caudally.
-
The muscles of the pelvic floor are divergent, causing a risk of prolapse.
-
The bladder itself is opened into the shape of a flat plate, small and fibrotic. The external genitalia are hypoplastic.
-
In cloacal exstrophy, there may be
absence, hypoplasia, or asymmetry of the sacroiliac joint, as well as a
dislocation of the hip(s). In these patients, the bone density is
usually diminished. The genitalia may be severely anomalous.
 |
|
Figure 27.17 Patient with cloacal exstrophy.A: Clinical photo. B: Radiograph before closure. C: Radiograph 2 months after closure. D: Radiograph 6 years after closure. (Courtesy of Paul Sponseller.)
|
good in the untreated patient with classic bladder exstrophy. Children
learn to walk at a normal age, although they
have an increased external foot-progression angle. This becomes less pronounced over time (58).
Athletic ability is not impaired. Adults with exstrophy seem to have an
increased incidence of pain in the region of the sacroiliac joints. One
natural history study suggested that there is an increased incidence of
degenerative disease of the hip in patients with uncorrected exstrophy (61).
However, the number of patients in the study was small, and the
affected patients appeared to have a degree of acetabular dysplasia
that is not commonly seen in most patients with classic exstrophy. It
is not currently established that osteotomy of the pelvis for exstrophy
is necessary to protect against premature osteoarthritis of the hip in
a patient with no associated acetabular dysplasia. There are some
reports of uterine prolapse in adult women with exstrophy, but the
frequency of this is not known. Both men and women patients with
exstrophy are usually fertile, and a number of women have successfully
given birth, usually by cesarean section.
 |
|
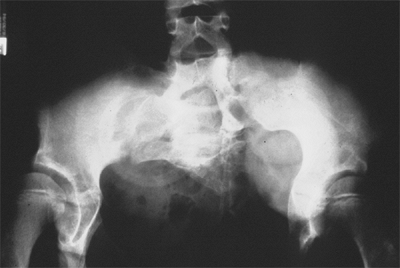
Figure 27.18 Patient with severe multiple neurologic and pelvic anomalies associated with cloacal exstrophy. (Courtesy of Paul Sponseller.)
|
 |
|
Figure 27.19
Schematic representation of pelvic differences in classic exstrophy versus normal in the transverse plane. (Courtesy of Paul Sponseller.) |
reconstruction in several surgical procedures, including closure of the
bladder and lower abdominal wall soon after birth, followed by
epispadias closure at a later date. Surgery for achieving continence is
commonly performed after the age at which children normally become
continent and may consist of bladder neck suspension and/or collagen
injections. Patients who are unable to become continent are offered a
bladder augmentation and a catheterizable umbilical stoma. Finally, in
the older child, occasionally plastic surgery is an option to optimize
the appearance of the perineum. In general, orthopaedic surgery of the
pelvic deformity is indicated as part of one of these procedures only
if it is needed for achieving urologic goals. These goals include
achieving a closed bladder, urinary continence, and acceptable
appearance of the perineum. In the past, it was most common for boys
with cloacal exstrophy to be reconstructed as girls with appropriate
endocrine supplementation because of the extreme abnormalities of the
external genitalia. However, long-term studies have shown that
psychological distress at maturity is not uncommon after this
procedure, so families are given both gender options.
managed without osteotomy may not need any procedures to be done on the
pelvic bones. These are patients who may have been managed without
bladder closure or had closure done in the newborn period without
osteotomy. They may have had closures with soft-tissue rotational
procedures to manage a mild-to-moderate sized exstrophy defect.
have their pubic symphysis closed down by manually approximating the
two halves of the pelvis and placing a strong suture between the pubic
bones. The lower extremities are immobilized in traction or a cast or
splint. The mobility to allow the approximation of the pubis probably
occurs by plastic deformation of the sacral ala and by the laxity of
the sacroiliac joints. Although the pelvis gradually assumes its
original diastasis over time, the tissue relaxation achieved by this
sequence of events lasts long enough for the midline closure to become
mature in most patients.
the pelvis is a bladder and lower urinary tract that cannot be closed
without approximation of the pubic bones. This is usually the case in a
patient who presents for closure after about the first month of age, or
in whom a prior closure without osteotomy has failed. Another
indication is in an older patient with a closed bladder who requires
osteotomy and pubic reapproximation to achieve continence. The final,
and least common, indication is in an older child in whom perineal
reconstruction is aided by bringing the pelvis closer together. This
decreases the width
of
the perineum and restores a more normal appearance to the external
genital structures. Patients who have acetabular dysplasia with classic
exstrophy should undergo osteotomy.
structures with the aid of pelvic osteotomy was first described by
Shultz and O’Phelan in Minneapolis nearly half a century ago (62).
They employed bilateral vertical iliac osteotomies through a posterior
approach, with a midline wire to hold the pubic bones together (63).
This necessitated turning the patient from prone to supine in the
middle of the procedure. Although the original procedure remains
popular, other approaches have been developed. Transverse
supra-acetabular iliac osteotomies from an anterior approach were
described in the 1980s by several surgeons. A group in Toronto has also
described an oblique osteotomy of the ilium, midway between the two
approaches discussed earlier, which can help bring the wings of the
ilium together (64). Anterior pubic ramotomy is
a simple procedure that can aid in the approximation of the two pubic
bones, although the effect is not as pronounced as iliac osteotomy.
rest and a cast, traction, external fixation, or internal fixation. A
combination of external and internal fixation provides the most
consistent results.
closing all classic exstrophy within a few days after birth, when the
relaxin is maximal and the pelvic bones are soft. In most babies, the
exstrophy can be successfully closed without osteotomy. We prefer to
immobilize these patients in modified Bryant traction with the legs
suspended vertically so that the pelvis is slightly off the bed (Fig. 23.29 in Chapter 23
of Lovell and Winter’s Pediatric Orthopaedics 5th Edition). This
maintains an anterior closing force to the anterior pelvis while the
closure heals. Other surgeons employ casts or splints to adduct or
internally rotate the hips, cutting out the surgical site for urologic
access.
whom simpler procedures to achieve continence fail, the authors prefer
anterior supraacetabular iliac osteotomies. In children older than 1
year, an additional closing-wedge greenstick osteotomy is added lateral
to the sacroiliac joints to rotate the iliac wings together. Pins are
inserted for external fixation, and the urologist closes the bladder,
perineum, and abdomen. The external fixator is then assembled.
much larger and the bone is softer. An anterior plate is especially
helpful in maintaining the closure. For this reason, we prefer to defer
cloacal closure until the child is at least 6 to 12 months of age and
plating is feasible.
they grow after an osteotomy, although it is less than it would have
been without closure. The patients who have osteotomy at the youngest
ages have greatest recurrence. The goal of maintaining pelvic
approximation while the urologic reconstruction heals appears to be
successful in most cases.
These were classified as bony or neurologic complications at the
osteotomy site, complications of traction, and infection. Bony
complications included vertical migration or nonunion after posterior
iliac osteotomies, as well as inadvertent osteotomy through the
sacroiliac joints because the procedure does allow visualization of
these joints. The most frequent neurologic complication was femoral
nerve palsy after anterior osteotomy. This appears to be caused by
medial pressure and tension on the nerve, and resolves spontaneously
within 3 months. There were two reports of sciatic palsy, by mechanisms
unknown. Complications of immobilization include skin breakdown from
wrapping the two legs tightly adducted together. Deep infection at the
osteotomy site does not occur with more frequency than in other
elective surgeries, despite the proximity to the incontinent bladder.
audible snapping that usually occurs with flexion and extension of the
hip. This snapping can be accompanied by pain and often occurs during
physical activity. It can be divided into three types: external,
intraarticular, and internal, with the external type being by far the
most common (66). The external type is caused
by snapping of either the posterior border of the iliotibial band or
the anterior border of the gluteus maximus over the greater trochanter
when the hip is flexed from an extended position (66,67,68,69).
The internal type, which is still the most poorly understood, has a
variety of presumed etiologies, with snapping of the iliopsoas tendon
over the iliopectineal eminence (70) or over the femoral head (69)
being the most common. The intraarticular type is caused by a loose
body in the joint, such as a fracture fragment or a torn piece of
labrum. It usually has a distinctive presentation and, unlike the other
types of snapping, almost always requires surgery for symptomatic
relief (71,72).
often unrecognized or misdiagnosed. In addition, internal snapping can
be asymptomatic and therefore not reported, making it difficult to
assess the true incidence (73,74). One study demonstrated that only 14 of 26 (54%) sonographically diagnosed snapping hips were clinically painful (75). From the few reports in the literature, patients of both sexes are affected equally (66,67,74,76).
In the adolescent population, symptomatic internal snapping is most
common among teenagers (aged 14 to 17 years) who are engaged in sports
activities that involve running (74,76). There is no evidence in support of a genetic basis for this condition.
The cause of this condition was believed to be snapping of the
iliopsoas tendon over the iliopectineal eminence. Two of the three
patients in the initial report had good relief after iliopsoas
lengthening. Other reported causes of internal snapping include
snapping of the iliopsoas over an exostosis of the lesser trochanter (69), snapping attributed to the iliopsoas bursa (77), and snapping caused by habitual dislocation of the hip (78).
Slipping of the iliofemoral ligaments over the femoral head and
slipping of the long head of the biceps femoris tendon over the ischial
tuberosity have also been proposed as causes of snapping, but no
pathologic or surgical basis has yet been identified (79).
The pain associated with iliopsoas snapping hip is generally believed
to be caused by the sudden movement of the iliopsoas tendon over the
pelvic pectineal eminence, femoral head, or lesser trochanter.
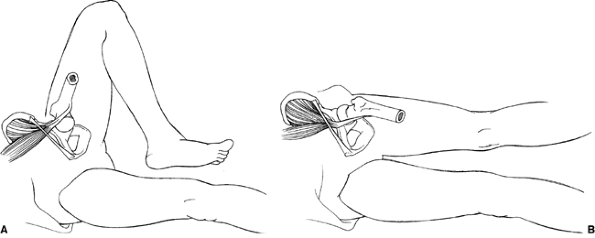 |
|
Figure 27.20 A schematic demonstrating the snapping iliopsoas tendon. A: The iliopsoas tendon is lateral to the pelvic brim with the hip in flexion and abduction. B: Snapping is produced as the tendon moves to a more medial position on the pelvic brim with extension and adduction of the hip.
|
diagnostic of the internal type of coxa saltans, with the patient
describing a painful snapping sensation localized to the anterior part
of the groin. However, because of its rarity, this type of snapping hip
can present a formidable diagnostic challenge. The snapping can often
be reproduced at will by the patient in either the supine or standing
position. In addition, the examiner can frequently reproduce the
snapping by having the patient lie supine and bringing the hip from a
flexed and abducted position to an extended and adducted position (Fig. 27.20). This is due to the iliopsoas tendon shifting from lateral to medial over the iliopectineal eminence (70,74) and/or the femoral head (69)
when the hip is brought from flexion into extension. If the snapping
occurs with these motions, blocking the snapping by applying finger
pressure over the iliopsoas tendon at the level of the femoral head
and/or iliopectineal eminence will corroborate the diagnosis.
A specific event typically precipitates the symptoms; the most common
precipitating activities are sprinting and long-distance running (74).
The snapping gradually increases in frequency and intensity so that it
occurs with daily activities and inhibits participation in sports
activities.
arthrogram may be useful for ruling out labral tears and intraarticular
loose bodies. Bursography, tenography, and ultrasonography have been
recommended for evaluating snapping of the iliopsoas tendon, but each
modality has its limitations, and they are generally unnecessary for
the diagnosis (69,75,80).
Bursography involves injecting contrast material into the iliopsoas
bursa whereas tenography involves injecting into the iliopsoas tendon
itself. Both are performed under fluoroscopic guidance with the use of
a local anesthetic. One limitation of bursography is that the tendon
itself is not injected and is therefore only visualized indirectly as a
negative defect impressing upon the bursa. Tenography allows direct
observation of the iliopsoas tendon’s movement under fluoroscopic
examination. Unlike bursography, this technique allows direct
visualization of the tendon. Although tenography has been useful in
furthering our understanding of the etiology of the snapping, it is not
necessary for clinical diagnosis. Recently, dynamic sonography has
emerged as a noninvasive technique for examining a snapping iliopsoas
tendon (75). This has the advantage of being noninvasive and can be used in cases in which the clinical diagnosis is not obvious.
understood. Although iliopsoas tendon snapping can occur without pain,
symptomatic iliopsoas tendon snapping in adolescence follows a somewhat
predictable course (74). A specific event
typically precipitates the symptoms; the most common precipitating
activities are sports that involve running. The snapping initially
occurs only rarely and does not inhibit participation in sports. Over a
period of months to a few years, the snapping increases in severity to
a point that participation in sports is no longer possible. The
snapping often begins to occur even with daily activities. It is often
at this point that the individual seeks medical attention.
Initial treatment includes rest, avoidance of activities that produced
the snapping, nonsteroidal antiinflammatory medications, and a 3-month
physical therapy program emphasizing stretching of the iliopsoas tendon
(74).
tendon is continued snapping of the iliopsoas tendon with resulting
pain despite an intensive 3-month supervised physical therapy program.
The snapping and pain at this stage often occur with daily activities
but, occasionally, are present only during physical exertion.
evolved for the treatment of refractory cases of the iliopsoas snapping
tendon. In 1984, Schaberg et al. (69) reviewed
six patients treated with lengthening of the iliopsoas tendon,
performed through a modified anterior approach to the hip, with the
tendon being partially divided near its insertion on the lesser
trochanter. Two of the patients had an exostosis removed from the
anteromedial aspect of the lesser trochanter; these exostoses were
believed to have been contributing to the symptomatic snapping. In
1990, Jacobson et al. (67) reviewed these 6
patients and also reported on an additional 14 patients who had
undergone lengthening of the iliopsoas tendon as a treatment for
internal snapping hip. The authors noted that their skin incision
changed from an anterior vertical incision to a more cosmetically
appealing incision running just distal to the inguinal crease in the
last 14 patients. In all the patients, the tendon was partially divided
below the pelvic brim near its insertion onto the lesser trochanter. Of
the 20 patients in the series, 6 (30%) had recurrent snapping, 3 (15%)
reported weakness in hip flexion, and 2 (10%) required reoperation. In
addition, 10 of 20 patients (50%) reported periincisional loss of
sensation.
reported on 14 patients with internal snapping treated by partial
iliopsoas tendon release. They described a medial approach through a
horizontal incision several centimeters below the inguinal skin crease.
As with previously reported approaches, the tendon lengthening was
performed below the pelvic brim, close to the insertion of the tendon
on the lesser trochanter. Of the 14 patients, 2 reported postoperative
hip-flexion weakness. Six patients continued to have snapping after
surgery.
described 11 patients with internal snapping hip treated with iliopsoas
tendon lengthening above the pelvic brim through an ilioinguinal
approach. Of the 11 patients, 5 reported postoperative hip-flexion
weakness. No patients experienced continued snapping postoperatively,
but two patients reported continued hip pain.
describe 11 hips in 9 patients. These internal snapping hips were
unresponsive to conservative measures. All the hips were treated with a
fractional iliopsoas tendon lengthening above the pelvic brim through a
modified iliofemoral approach. This approach allows excellent
visualization of the iliopsoas
musculotendinous
junction and facilitates complete transection of all tendon fibers at
this level. One patient in this study described recurrent snapping in
one operatively treated hip, but stated that the symptoms were much
less frequent and severe than they had been preoperatively. All
patients returned to their preoperative level of activity. No patient
had a detectable loss of hip-flexion strength. Two patients had a
transient decrease in sensation that was localized to the anterolateral
aspect of the thigh.
and symptomatic iliopsoas snapping hip. A supervised physical therapy
program emphasizing iliopsoas stretching for a minimum of 3 months is
the first line of treatment. For patients with continued pain and
popping that limits activities, a fractional iliopsoas tendon
lengthening through a modified iliofemoral approach is recommended (74).
This approach has been effective in relieving symptoms and allowing
patients to return to their preoperative level of functioning while
preserving hip-flexion strength.
the surgeon to partially divide the iliopsoas tendon just above its
insertion on the lesser trochanter. The advantages of these two
approaches include good visualization of the tendon insertion and
direct access to any contributing exostoses on the lesser trochanter
and femoral head. The major problem with these two surgical approaches
that attempt to lengthen the tendon below the pelvic brim lies in
judging the amount of tendon to release. Insufficient lengthening
results in recurrent snapping, whereas overlengthening results in
hip-flexion weakness (67,69,81). Other problems include the potential for a cosmetically unappealing scar resulting from an anterior vertical incision (67),
and the frequent periincisional loss of sensation with the medial
approach through a horizontal incision below the inguinal crease (81).
brim have better reported maintenance of hip-flexion strength and less
recurrence of snapping than those that lengthen the tendon below the
pelvic brim (74,76). A
disadvantage of the ilioinguinal approach for this condition is the
relative unfamiliarity of this approach to many pediatric orthopaedic
surgeons. The modified iliofemoral approach (74),
on the other hand, is used frequently by pediatric orthopaedists in
performing pelvic osteotomies. Extreme care should be taken to
correctly identify the tendon before transection because the femoral
nerve lies nearby. To minimize risk of injury of the femoral nerve, the
authors recommend that the patient have no paralyzing agents
administered during the procedure and the surgeon use very low-level
electrocautery to stimulate the tendon before cutting its fibers so as
to ensure that nerve fibers are not included. Care should also be taken
to avoid injury to the lateral femoral cutaneous nerve. The nerve
should be identified on the sartorius side of the sartorius-tensor
muscle interval and retracted medially.
of pain in the young child. Transient synovitis is characterized by an
acute onset of hip pain associated with a limp in a child that has no
other musculoskeletal or constitutional symptoms. This clinical problem
has been often referred to as irritable hip, observation hip, toxic synovitis, transitory coxitis,coxitis serosa, coxalgia fugax, and phantom hip. Transient synovitis is the term most commonly used because it aptly describes the short duration of this benign condition.
the average patient being between 5 and 6 years of age. However, the
condition has been reported in children as young as 3 months. The
annual hospital admissions for the diagnosis of transient synovitis is
0.4% to 0.9%, but the actual incidence of the condition may be higher
because many patients never seek medical attention and only a few
patients are hospitalized when the diagnosis is made. Landin et al. (82)
reported that the risk that a child will be affected by at least one
episode of acute transient synovitis of the hip is 3%. They also
reported a seasonal variation, with more cases in the fall than in the
winter. Boys are twice as often affected than girls, and there is a
much lower incidence among African Americans (83).
Right and left hips are affected equally. Ninety-five percent of the
cases are unilateral. After a child has had an episode of transient
synovitis of the hip, the annual risk of recurrence for that child is
4% (82,84).
and differentiated it from tuberculosis. Since then, many investigators
have described a similar painful condition that is self-limiting and
rapidly resolving, and that is now considered as transient synovitis of
the hip. However, its cause still remains unknown.
synovitis present with a recent history of an upper respiratory tract
infection, a viral etiology has been suggested. Leibowitz et al. (86)
found that blood interferon levels were significantly raised in 40% of
patients with acute transient synovitis of the hip. In healthy
subjects, these levels
are
usually not measurable, but various viral diseases cause significantly
raised concentrations. They concluded that a viral infection was the
cause. Tolat et al. (87)
evaluated 80 children who were admitted with acute transient synovitis
of the hip. Their management protocol included a clinical examination,
venous samples, synovial fluid samples taken when ultrasonography had
detected an effusion, and recall after 3 or 4 weeks for clinical
examination and procurement of samples for convalescent viral titers.
Twenty-eight of 65 patients (43%) had raised blood interferon levels.
Fifteen of the 16 patients with an effusion that was successfully
aspirated showed raised levels of interferon in the synovial fluid.
Bacterial and viral cultures of all synovial fluid samples were
negative. Viral serology in 67 patients showed raised antibody titers
to viruses including rubella, enterovirus, and Epstein-Barr. Other
investigators have evaluated different viruses including parvovirus
B-19 and herpes virus-6, and were unable to confirm any correlation
between transient synovitis and infection with these specific viruses (88,89,90).
otitis media, and gastrointestinal problems have also been associated
with transient synovitis of the hip in up to 70% of the patients. Spock
(91) reported a higher incidence of nose and throat β-hemolytic streptococci in patients with transient synovitis when compared to asymptomatic patients. However, Hardinge (92) did not find any correlation between infection sources and transient synovitis.
transient synovitis of the hip in up to 25% of patients. In 1952,
Edwards reported that patients with transient synovitis recovered in a
few days with the use of antihistamines, and Rothschild et al. found a
rapid clinical improvement when steroid injections were given
intramuscularly. However, Nachemson and Scheller (93)
did not find any association between allergic hypersensitivity and
transient synovitis in a group of 73 patients, compared to the general
population.
investigators proposed trauma as a cause of transient synovitis, with
local trauma to the involved hip being present in up to 30% of the
cases (91,94,95,96,97).
However, as with most childhood musculoskeletal conditions, a
significant number of patients may relate an episode of trauma to the
onset of symptoms, but this may not be the actual cause of this
condition. No other studies have reported on this association.
found a three times greater incidence of transient synovitis in obese,
stocky children compared with a randomly selected, age-matched control
group. Vila-Verde and da Silva (98) evaluated
children with LCPD and transient synovitis, and found that in the
active stage of both diseases there was a bone age delay that persisted
after healing, but became normal by puberty.
of limping, unilateral hip pain, and subsequent refusal to bear weight
on the involved extremity in an otherwise healthy child. The pain is
usually unilateral, with fewer than 5% of cases being bilateral. The
pain is usually located in the groin and hip area, with referred pain
to the anteromedial aspect of the thigh and knee. The pain is acute in
about half of the patients, with symptoms being present for 1 to 3 days
before presentation. In other patients, the symptoms may be more
chronic, with symptoms being present for several weeks in some cases.
The pain is usually mild, but in some children, mostly very young ones,
it can be severe enough to awaken them at night. Because the symptoms
sometimes follow a recent upper respiratory tract infection, the
patient may have a low-grade fever at presentation (rarely greater than
38°C).
distress who will not bear weight or walk, or who does so reluctantly
and with an antalgic limp. The extremity is held in flexion and
external rotation, and there is decreased range of motion, especially
for hip abduction and internal rotation. The irritability of the hip is
usually mild or moderate. If it is severe, a diagnosis of septic
arthritis should be considered. Ipsilateral muscle atrophy may be
present, and this implies a longstanding duration of the symptoms,
although this is not very common. In this situation, a diagnosis other
than transient synovitis should be considered.
normal or may demonstrate slightly widened joint space medially. The
main purpose of the radiograph is to exclude other conditions that may
involve the hip joint such as LCPD, eosinophilic granuloma,
osteomyelitis, osteoid osteoma, and so on. Bone density is normal in
all cases. Loss of the hip capsular shadow has been reported in cases
of transient synovitis; however, this sign is not specific and it is
related to holding the hip in abduction and external rotation.
evaluated the diagnostic significance of some radiographic signs
(abnormal hip “joint space” and periarticular fat layers) as indicators
of hip joint effusion or hip complaints without effusion. These
indicators were studied with ultrasonography and radiography in 47
children (58 examinations), of whom 40 had acute unilateral transient
synovitis. It was found that “joint depth” was not influenced by
presence of intra-articular fluid collections; blurring and/or
displacement of the periarticular fat pads medial and lateral to the
hip joint occurred more frequently when joint effusion was present than
in symptom-free hips or in painful hips without effusion. However, the
radiographic signs provided too low a diagnostic accuracy to be of
practical value (100). It is suggested that ultrasonography is a valuable means of
obtaining a better definition of the hip joint, thereby aiding in the diagnosis of transient synovitis.
presence of an effusion in the hip joint, and it is usually performed
prior to hip aspiration to be certain that an effusion accompanies the
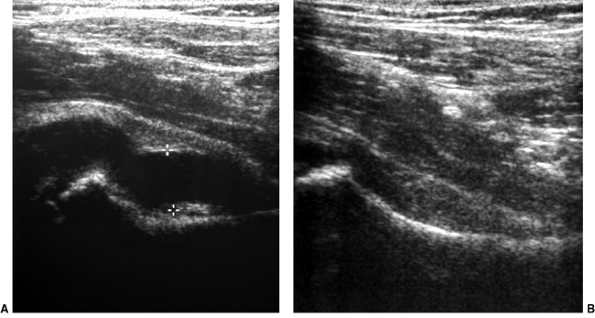
clinical findings (101–106) (Fig. 27.21).
Although ultrasonography cannot identify the cause of an effusion in
the joint, a negative result directs attention to other causes of hip
pain.
prospective study of 111 children with acute hip pain to assess whether
ultrasonography can replace traditional radiography. An effusion was
diagnosed in 71% of the cases by ultrasonography but in only 15% by
radiography. This effusion persisted for a mean of 9 days; symptoms
lasted for 5 days. Interestingly, in patients without an effusion there
was no obvious factor that could be causing their pain, so the pressure
of an effusion from a transient synovitis does not account for all
patients with irritable hips. Patients with an effusion persisting for
over 24 days had more symptoms, a significantly larger effusion, and
greater limitation of movement. The investigators proposed a protocol
of management for irritable hip, using ultrasonography at the first
presentation of certain categories of patients, thereby reducing the
number of early radiographs by 75%.
protocol for the management of irritable hip with the aim of avoiding
hospital admissions while identifying all other serious causes of hip
pain, in particular, septic arthritis. Fifty children with painful hips
were studied prospectively with immediate ultrasonographically guided
aspiration and Gram stain of all hip effusions. Bone scintigraphy at an
early stage was reserved for patients with unremitting symptoms.
Thirty-six hips were aspirated. Only two patients were admitted to
hospital. The final diagnoses were transient synovitis (45 patients),
Perthes disease (3 patients), fracture (1 patient), and septic
arthritis (1 patient). The single case of hip sepsis was diagnosed on
presentation.
 |
|
Figure 27.21 Longitudinal linear ultrasonographic view of the hips in a girl 6 years and 6 months of age. A:
Ultrasonographic scan of the symptomatic right hip demonstrates a large effusion in the joint, as indicated between the cursor markings. B: Ultrasonographic scan of asymptomatic left hip, for comparison, demonstrates no effusion. |
prospectively evaluated 500 children with painful hips or limps by
using plain films and ultrasonography. The clinical, radiographic, and
ultrasonographic findings were correlated with the final diagnoses.
Ultrasonography disclosed hip effusion in 235 patients, and plain films
were abnormal in 58 of these 235 patients and in 4 others. Both
ultrasonography and plain films were normal in 261 patients. There were
no ultrasonographic signs that were useful in differentiating sterile,
purulent, or hemorrhagic effusion. Ultrasonography showed that 73% of
patients with presumed transient synovitis had no effusion 2 weeks
after diagnosis. Patients with hip disorders other than transient
synovitis had persistent effusion for more than 2 weeks; however, this
was also observed in 27% of the patients with presumed transient
synovitis. Ultrasonography was more sensitive than plain films in
detecting hip effusion, but ultrasonographic detection of effusion
changed the therapeutic approach in only six patients. Therefore,
although ultrasonography can be useful in documenting and following a
hip effusion, it is not
in and of itself diagnostic of this condition, and is not routinely required in making the diagnosis.
investigation of young children who present with limping as their only
or predominant symptom. In cases of transient synovitis, it usually
demonstrates a variety of possible patterns of isotope uptake,
including those showing normal, increased, or decreased activity of the
femoral epiphysis (110,111,112,113,114,115).
scintigraphy and pinhole collimator technique. A decrease in isotope
uptake in the proximal femoral epiphysis was observed in 13 children.
This was correlated with a reduced uptake in the growth plate,
indicating a disturbance of blood supply to these regions. A
characteristic pattern of isotope uptake relating to the duration of
symptoms was observed, with a decrease in uptake during the first week
followed by rebound hyperemia within 1 month. The significance of this
finding is uncertain, but there has been a report of coxa magna
following transient synovitis of the hip, which may be caused by this
increase in blood supply (117).
on a 4-year study during which 192 patients with a typical transient
synovitis syndrome underwent radionuclide scintigraphy shortly after
presentation. Three different patterns were found, suggesting that not
all the cases may have shared the same etiology. Fifteen patients had
evidence of ischemia of the femoral head, but only four patients went
on to develop the typical radiographic features of Perthes disease. The
other 11 patients are thought to represent a minor, radiographically
silent form of Perthes disease.
be an early, transient decrease in vascular perfusion of the proximal
femoral epiphysis, but it will resolve spontaneously. The role of bone
scintigraphy in the diagnosis of transient synovitis and in the
decision-making about the management of the condition remains
undetermined, and its routine use is not recommended.
resonance arthrography, the imaging algorithm for hip pain is evolving.
Toby et al. (119) reported the use of MRI in
the assessment of pediatric hip disease (24 children, 8 of them with
transient synovitis). MRI accurately showed articular cartilage
thickening and effusion in the joint in two of the patients with
transient synovitis. The images in the other six patients were
unremarkable.
conventional radiography, radioisotope bone scan, and MRI for
evaluating 45 children who presented with acute hip pain. The final
diagnoses were transient synovitis (n=17) septic arthritis (n=2) LCPD
(n=13) epiphyseal dysplasia (n=2) other conditions (n=4) and normal
findings (n=7) In the workup, MRI provided more morphologic information
than other techniques and enlarged the diagnostic possibilities. MRI is
extremely sensitive to alterations in the bone marrow that may
represent pathology that remains occult to plain radiography and bone
scintigraphy of the hips. For diagnosis and treatment planning, MRI of
the hips should be performed early in patients with persistent pain and
negative radiography findings.
differences in MRI findings between septic arthritis (7 patients) and
transient synovitis (14 patients). The diagnoses were made by means of
aspiration of the joint, bacteriologic study, arthrotomy, and clinical
evaluation. MRI findings were analyzed with emphasis on the grade of
effusion and alterations in signal intensity in the soft tissue and
bone marrow of the affected hip joint. Alterations in the signal
intensity in bone marrow (i.e., low signal intensity on fat-suppressed
gadolinium-enhanced T1-weighted spin-echo images and high signal
intensity on fat-suppressed T2-weighted fast spin-echo images) were
seen in 8 of 9 patients with septic arthritis. Such alterations in
signal intensity were not seen in the 14 patients with transient
synovitis. The investigators concluded that signal intensity
alterations in the bone marrow of the affected hip joint are useful in
differentiating septic arthritis from transient synovitis.
erythrocyte sedimentation rate (ESR) (range 1 to 63 mm/h) and
C-reactive protein (CRP) level (less than 0.5). Urinalysis, serum
electrophoresis, rheumatoid factor, blood culture, and tuberculin skin
test results are usually within normal limits.
septic arthritis is suspected. Gram stain of the aspirated fluid will
confirm the diagnosis of septic arthritis in 30% to 50% of the
patients. The cell count of the fluid in the joint can vary, but it is
usually less than 25,000 cells per mm3 The glucose concentration of the aspirate is normal in transient synovitis. Zawin et al. (122)
found that ESR and the WBC count of the synovial fluid were
significantly higher in patients with septic arthritis than in those
with transient synovitis.
arthritis, which requires emergent treatment to prevent proximal
femoral destruction and subsequent permanent deformity leading to early
degenerative arthritis of the hip. Because of these disabling
possibilities, many institutions have a policy of hospital admissions
and workup investigations for all patients who present with an acutely
painful hip.
diseases at the early stages, the differential diagnosis should remain
one of exclusion. Classically, septic arthritis presents with more
severe pain and marked limitation of motion of the hip because of the
pain. However, low-grade septic arthritis in an older child or in a
child who has received antibiotics for another problem (such as upper
respiratory infection) may have a less acute presentation. Many
investigators agree that if the diagnosis is not clear from the
history, physical examination, and radiography, hip aspiration should
be performed, preferably with fluoroscopy or ultrasonography guidance.
If the initial attempt is “dry” during fluoroscopy, dye should be
injected to confirm that the needle has entered the joint.
algorithms that are designed to help differentiate septic arthritis
from transient synovitis (122–128). However, there are different
opinions about the parameters to be used for this indication. It has
been suggested that aspiration should be performed in patients with a
temperature higher that 37.5°C (99.5°F) or an ESR greater than 20 mm/h.
With the use of these two criteria, 97% of the patients with septic
arthritis would have been identified as requiring a hip aspiration. On
the other hand, 50% of the patients with transient synovitis would have
undergone an unnecessary aspiration (123).
evaluated 97 patients with transient synovitis and 27 patients with
septic arthritis. Plain radiographs showed a displacement or blurring
of periarticular fat pads in all patients with acute septic arthritis,
and multivariate regression analysis showed that body temperature
greater than 37°C, ESR higher than 20 mm/h, CRP greater than 1 mg/dL,
WBC count greater than 11,000 per mL, and an increased hip joint space
of more than 2 mm were independent multivariate predictors of acute
septic arthritis. Eich et al. (129) found that
all children with septic arthritis had hip effusion detectable by
ultrasonography, and at least two of the following criteria: fever,
elevation of ESR and elevation of CRP.
identified four independent multivariate clinical predictors to
differentiate between septic arthritis and transient synovitis: history
of fever, non–weight bearing, ESR greater than 40 mm per hour, and
serum WBC greater than 12,000 cells per mL. However, Luhmann et al. (127)
found that this algorithm was not as useful in their institution. Given
the devastating effects of a missed septic arthritis, the surgeons
should rule out this possibility by setting a very low threshold to
indicate the need for aspiration of the joint.
femoral or pelvic bacterial osteomyelitis and tuberculosis. These
conditions may present with very similar manifestations including hip
pain, limited range of motion and effusion in the joint. Some patients
may demonstrate minimal elevation of body temperature and of laboratory
values (WBC, ESR, and CRP). MRI and bone scintigraphy are very useful
in differentiating between these conditions and can demonstrate
characteristic bone marrow changes. Skin testing will be diagnostic for
tuberculous arthritis.
group A streptococcal infections usually occurs 2 to 4 weeks
postinfection. The joint is usually warm, erythematous, and exquisitely
painful to any range of motion, and there may be an associated skin
rash. Several joints can become affected over time (migratory
arthritis). In addition, juvenile rheumatoid arthritis or one of the
seronegative spondyloarthropathies should also be considered in the
differential diagnosis. In these cases, the arthritis is more insidious
in onset and will persist beyond the 2 weeks that are typical for
transient synovitis. A careful examination of other joints and serology
analysis will help clarify the diagnosis.
the same age range, but it has a slightly greater male predominance.
Pain is usually more insidious in onset, and more protracted in
duration. Hip motion at the onset of symptoms tends to be limited to a
lesser degree than in transient synovitis. Radiographs may show joint
space widening and a smaller femoral ossified nucleus on the affected
side. Bone scintigraphy and MRI in the early stages of LCPD may show a
decreased uptake of the femoral head, and bone marrow abnormalities,
respectively.
proximal femur, must also be included in the differential diagnosis.
Osteoid osteoma is usually associated with night pain that is relieved
by aspirin.
pathoanatomy of this condition. Biopsy material demonstrated
nonspecific inflammatory changes and synovial hypertrophy without
pyogenic-related abnormalities. Aspiration of the hip joint has shown
culture-negative synovial effusion, usually measuring between 1 and 5
mL (83,117,131,132,133).
could cause ischemia of the proximal femoral epiphysis by a tamponade
effect from increased intraarticular pressure. However, many other
studies have shown little or no evidence of such a causative
association. One of the reasons for this confusion is the fact that
many cases of LCPD may initially present as synovitis of the hip before
any changes can be seen in radiograms; therefore, subsequent proximal
femoral epiphyseal changes may be misdiagnosed as transient synovitis.
self-limiting condition that resolves spontaneously. Most short-term
studies of patients with transient synovitis usually demonstrate a
limited duration of the symptoms
with no evidence of residual clinical or radiographic abnormalities (134).
However, longer follow-up studies have demonstrated some abnormalities
in the proximal femur. Sequelae or conditions associated with transient
synovitis of the hip include coxa magna, LCPD, and mild degenerative
cystic changes of the femoral neck.
the proximal femoral epiphysis, has been noted in up to 32% of patients
(93,117,135).
The reason for this increase in size is not clear, but it has been
suggested that a reactive increase in the blood supply to the femur or
an increased growth of the articular cartilage secondary to the
transient inflammation may be associated with this finding (102). De Valderrama (135)
reported a 21-year follow-up of patients who had transient synovitis of
the hip. He found a 50% incidence of radiographic changes including
coxa magna, widening of the femoral neck, and changes consistent with
degenerative arthritis of the hip. However, Nachemson and Scheller (93)
did not find any abnormalities of the hip joint. The full importance of
these radiographic changes remains unknown, and whether these patients
will develop degenerative arthritis over the long term remains
uncertain.
synovitis of the hip ranges from 1% to 3%. A direct correlation between
transient synovitis of the hip and the development of LCPD has,
however, never been documented. Therefore, it is reasonable to conclude
that there is no association between these two conditions and many of
the reported instances of correlation undoubtedly represent an initial
misdiagnosis of early LCPD.
frequently at the emergency department. The main aim of the treatment
is to resolve the underlying synovitis with its associated
symptomatology. Bed rest and non–weight bearing on the affected side is
the primary method of treatment of this condition. Light skin traction
can be applied for comfort in patients with recalcitrant or recurrent
symptoms. On the basis of ultrasonographic studies and intraarticular
pressure recording it was found that the best position is with the hip
in 30 to 45 degrees of flexion and some abduction (133,136,137).
For patients in whom the diagnosis is uncertain, hospital admission is
often necessary. Close observation is essential in these cases, and
worsening of the symptoms suggests septic arthritis.
period of time, and this often results in rapid improvement. Kermond et
al. (138) performed a randomized, double-blind,
placebo-controlled trial using ibuprofen syrup (10 mg per kg three
times a day for 5 days). They found that ibuprofen shortened the
duration of the symptoms to 2 days compared to 4.5 days in the placebo
group. Because many children may have an associated upper respiratory
tract viral infection, the use of aspirin should be avoided so as to
prevent Reye syndrome. There is no indication for the use of
antibiotics if the diagnosis is certain.
full range of motion of the hip is reestablished and the pain has
improved. Most patients will have resolution of their symptoms in 5 to
7 days. Recurrences are uncommon unless the child returns to full
activities prematurely (96). Patients should be
reevaluated if significant residual symptoms persist 7 to 10 days after
the initial presentation, so as to rule out other pathologies. In some
cases, however, low-grade symptoms can last up to several weeks.
disorder that occurs during adolescence. It is characterized by pain
and a limp, with a rapid loss of the articular cartilage of the hip
joint resulting in narrowing of the joint space and consequent
stiffness in the joint. This condition should be differentiated from
chondrolysis secondary to prolonged immobilization, trauma, severe
burns, infection, juvenile idiopathic arthritis, Marfan syndrome, or
slipped capital femoral epiphysis (139,140,141,142).
in 1971. He reported a series of nine adolescent girls who
spontaneously developed symptoms and signs similar to the description
of chondrolysis secondary to slipped capital femoral epiphysis. Since
then several investigators have documented the pathology, clinical
presentation, natural history, prognosis, and treatment of idiopathic
chondrolysis of the hip (139,144,145,146,147,148,149).
remains unclear. Although there are fewer than 100 patients recorded in
the literature to date with idiopathic chondrolysis of the hip, it may
be more common than was once thought. In fact, Kozlowski and Scougall (150) believe that it is probably one of the most common causes of degenerative arthritis of the hip in women.
There is an approximately sixfold predominance among girls. Onset is
most frequently around the age of 11 to 12 years of age, but it may
occur until the age of 20 years. When first described it appeared to be
more common among individuals of African descent, but it has since been
documented as occurring ubiquitously in African, Asian, Indian,
Australian, Hispanic, American, and European populations (143,145,147,148,150,151,152,153,154,155,156,157).
origin of this process. These include nutritionally based abnormalities
in the joint because of abnormal synovium (140,142); mechanical insult to the articular cartilage, resulting in a release of chondrolytic enzymes (158); abnormal intracapsular pressure (159); and intrinsic abnormal chondrocyte metabolism that can be triggered by an unknown environmental event (145,150,160).
cartilage resorption seen in idiopathic chondrolysis of the hip is
caused by an autoimmune response within the hip joint in genetically
susceptible individuals (159,161).
This theory is supported by the fact that microscopic evaluation of the
synovial tissue from affected joints shows an increase in chronic
inflammatory cells, and these patients have serologic abnormalities as
well (143,147,162,163).
edematous and demonstrates villous formation, nodular lymphoid
hyperplasia of the subsynovium, and perivascular infiltrates of
lymphocytes, plasma cells, and monocytes (160).
No fibrinoid necrosis or granuloma formation is seen. There is minimal
synovial fluid in the joint. The changes to the articular cartilage
include loss and thinning of the superficial areas on both sides of the
joint, with more significant destruction on the femoral side. There may
be complete loss of articular cartilage in the weight-bearing areas of
the femoral head. Thickening of the joint capsule is common. The
adjacent bone is osteopenic without evidence of necrosis, but there may
be cysts filled with synovium.
inflammation. The articular cartilage is fragmented, with the
superficial zone I missing and with necrotic chondrocytes. However, the
basal zone II shows abnormalities in the organization and size of the
collagen fibrils, with viable chondrocytes interspersed with necrosis
and debris (Fig. 27.22). These chondrocytes are important for the subsequent regeneration of the articular cartilage in some cases (162,164).
demonstrated deposition of IgM and the C3 component of the complement
in the synovium in patients with idiopathic chondrolysis. However,
other investigators reported normal levels of serum immunoglobulins and
normal immunofluorescence studies of the synovium and cartilage (144,156).
the hip is that of an adolescent girl (mean age 11 years, range 6 to 15
years) with a 2- to 3-month history of unexplained hip pain, stiffness,
and a limp. Pain is usually insidious in onset, and is located in the
hip, anterior thigh, or knee area. There is a distinct absence of
systemic symptoms. Physical examination demonstrates significant
restriction of the range of motion in all planes with associated muscle
spasm. If presentation for orthopaedic treatment is delayed, many
patients will demonstrate fixed contractures about the hip, the most
common being in the flexed, abducted, and externally rotated position (143,144,145,149,152).
Contractures about the hip can result in secondary leg-length
inequality, pelvic obliquity caused by an adduction contracture, and
increased lumbar lordosis.
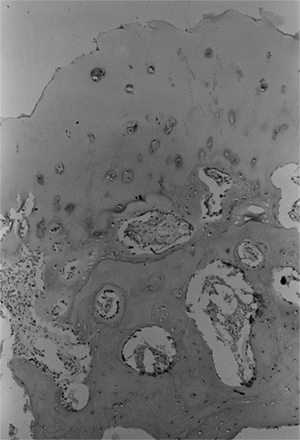 |
|
Figure 27.22
The photomicrograph of a biopsy specimen of the femoral head from a patient with idiopathic chondrolysis demonstrates a frayed and fragmented superficial layer of articular cartilage, with viable chondrocytes remaining in the more basal layers. The subchondral bone appears histologically normal. |
urinalysis, rheumatoid factor, human leucocyte antigen HLA-B27,
antinuclear antibody, blood cultures, and tuberculin skin test are
usually within normal limits. The ESR is usually normal although in
some cases it can be slightly elevated (less than 30 mm per hour) (146,150,152,165).
of the hip includes trauma, slipped capital femoral epiphysis, LCPD,
juvenile rheumatoid arthritis, septic arthritis, tuberculosis,
migratory or transitory osteoporosis, idiopathic protrusio acetabuli,
reflex sympathetic dystrophy, pigmented villonodular synovitis, and
synovioma. Septic arthritis of the hip is one of the most important
entities that should be examined for and excluded on presentation
because this condition requires urgent surgical treatment. Although the
findings of the physical examination can be similar in the acute stages
of both clinical entities, the history, and laboratory results are
usually quite different. Septic arthritis usually has a more acute
onset and the patient may by systemically ill with high fever.
Laboratory studies in septic
arthritis typically show a significant elevation in the WBC count and ESR, which is not the norm for idiopathic chondrolysis (166).
 |
|
Figure 27.23
Anteroposterior hip radiograph of a girl 13 years and 6 months of age. Radiographic findings in this case of bilateral idiopathic chondrolysis of the hip include a narrowing of the joint space to less than 3 mm and diffuse osteopenia. |
in the course of the disease are often normal, but are important to
exclude other causes of hip pain. Early features of cartilage
destruction include periarticular osteopenia, narrowing of the joint
space, and small subchondral bone erosions. Concentric diminution of
the articular space to less than 3 mm is considered to be a diagnostic
criterion of chondrolysis (normal space measures between 3.5 and 7 mm) (144,145) (Fig. 27.23).
There may be slight overgrowth of the proximal femur, seen as widening
and altered angulation of the epiphysis and femoral neck.
 |
|
Figure 27.24 A:
Anteroposterior radiograph of the pelvis of a girl, 12 years and 4 months of age, with idiopathic chondrolysis of the right hip. Late radiographic signs shown here include obliteration of the joint space, subchondral bone cysts, narrowing or early closing of the growth plate, and pelvic obliquity. B: Anteroposterior radiograph of the same patient 1 year after a hip arthrodesis. |
space, subchondral bone cysts, protrusio acetabuli, and narrowing or
early closing of the growth plate (Fig. 27.24A).
This early closing of the growth plate rarely results in any major
growth abnormality or significant change in the proximal femur.
Protrusio acetabuli has been reported in as many as 50% of the patients
and is thought to be caused by a softening of the acetabular floor,
paralleling the loss of joint space seen radiographically (167) (Fig. 27.25).
Widening of the symphysis pubis has also been noted. With resolution of
the disease, there will be up to 2 mm restoration of the joint space in
as many as 50% of the patients. Long-term radiographic results
demonstrate osteopenia with degenerative changes, osteophytes, and
cavities in the two articular surfaces, leading to complete ankylosis
of the joint (144,149).
pool, but delayed scans demonstrate periarticular increase in uptake
within the femoral head and the acetabulum
(Fig. 27.26) (168).
MRI is used in children with severe symptoms or unusual presentation,
or where there is doubt about the diagnosis. In idiopathic
chondrolysis, there may be a small effusion in the joint, without
synovial enhancement, and with cartilage loss being confined initially
to areas of focal irregularity with deeper erosions. Interestingly, on
serial examinations the cartilage loss was seen to extend peripherally
from the center. Some degree of bone marrow edema and bone remodeling
can be observed, with progressive protrusio acetabuli. Profound muscle
wasting around the affected hip can be observed in most cases (169).
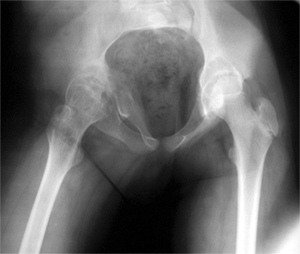 |
|
Figure 27.25
Anteroposterior radiograph of the pelvis of a girl 13 years and 2 months of age, with idiopathic chondrolysis of the right hip. This radiograph reveals complete loss of the joint space with resultant acetabuli protrusio. |
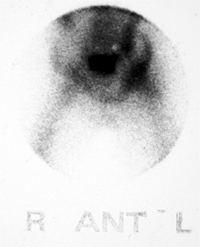 |
|
Figure 27.26
The technetium bone scan of the pelvis of a patient with idiopathic chondrolysis demonstrating a diffuse uptake of the isotope by both sides of the affected left hip. |
without treatment is unpredictable. Outcomes vary from spontaneous
resolution to ankylosis of the hip joint, avascular necrosis, and
long-term hip deformity. It was originally thought that idiopathic
chondrolysis of the hip always followed the same progressive downhill
course resulting in pain and a dramatic loss of range of motion and
function in the affected hip. In 1971, Jones (143)
in his original description of idiopathic chondrolysis of the hip,
described nine cases, all of which had poor outcomes. All nine patients
developed progressive loss of motion of the involved hip, leading to
complete ankylosis in eight of them. Sparks and Dall (155)
in 1982 reported a follow-up of Jones’s original nine patients and
included nine additional patients. Of Jones’s original nine patients,
two had an arthrodesis, one had a cup arthroplasty, one had a femoral
head resection, two had complete spontaneous ankylosis, and two had
what was described as severe limitation in hip motion. The additional
nine patients also displayed the same natural history, which consisted
of progressive stiffening and malposition of the affected hips. The
authors concluded that idiopathic chondrolysis inevitably results in
the development of a malpositioned fibrous ankylosis of the involved
hip joint.
more favorable outcome for patients affected with idiopathic
chondrolysis of the hip. At a mean follow-up of 6.2 years, six of the
nine patients had either no symptoms or only minor intermittent
discomfort in the hip, with an improved range of hip motion and a
partial restoration of joint space width. Roy et al. (170)
in 1988 published the results for three patients with idiopathic
chondrolysis followed for a mean of 3 years after onset of symptoms.
All three patients had improved range of motion and reconstitution of
the joint space at latest follow-up. Similarly, Daluga and Millar (145)
in 1989 reported 14 patients (16 hips) with idiopathic chondrolysis of
the hip, after a mean follow-up of 84 months. Partial restoration of
the joint space occurred in eight hips. Nine hips demonstrated improved
range of motion, and five of these hips had a full restoration of
motion.
idiopathic chondrolysis of the hip vary, the disease process appears to
have two distinct stages in most patients. The acute stage commences
with the onset of the condition and typically lasts for 6 to 16 months.
The patient presents with an insidious onset of pain and decreased
motion of the hip caused by an inflammatory response. Radiographically,
there
is concentric narrowing of the articular space, caused by loss of
articular cartilage in the femoral head and acetabulum. As the synovial
inflammation decreases toward the end of the acute stage, there is an
increase in fibrous tissue deposition. The acute stage is followed by
the chronic stage, which lasts from 3 to 5 years and follows a less
predictable course than the acute stage. At the end of the chronic
stage, the affected hip will manifest one of three possible outcomes:
(a) the involved hip may continue to deteriorate to a painful and
malpositioned ankylosis; (b) the involved hip may become stiff and
ankylosed in a position that limits function but causes minimal pain;
and (c) the involved hip may show resolution of pain, improved motion,
and partial or complete return of joint space, as evidenced by
radiographs (Fig. 27.27).
the etiology of idiopathic chondrolysis of the hip and improve the
understanding of its natural history.
and are still in evolution as more information is collected concerning
the natural history of idiopathic chondrolysis of the hip.
Historically, the prognosis of idiopathic chondrolysis was viewed as
universally poor. Therefore, early definitive surgical intervention was
routinely practiced, including hip fusion, joint arthroplasty, and
corrective osteotomies. This form of early definitive treatment has
been largely abandoned because of reports that as many as 50% to 60% of
affected hips in some series achieve satisfactory function, motion, and
improved joint space, as seen radiographically, because of various
management strategies (144,145,152,170).
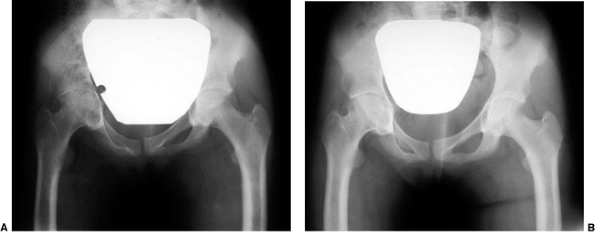 |
|
Figure 27.27 Anteroposterior pelvic radiographs of a girl 13 years and 3 months of age, with idiopathic chondrolysis of the right hip. A: A radiograph made at the time of diagnosis demonstrates significant loss of joint space and osteopenia at the involved hip. B:
A radiograph of the same patient 18 months after diagnosis demonstrates partial regeneration of the joint space width in the affected hip. (Courtesy of Perry L. Schoenecker.) |
Mild cases, defined as those without severe pain or contractures, were
treated with physical therapy, non–weight bearing, and analgesics.
Joint space improved radiographically in four hips, and range of motion
was reported as approximately two thirds of normal. More severely
involved hips were treated with prolonged spica casting until fibrous
ankylosis was achieved. These patients were reported to be functioning
well at latest evaluation with an ankylosed pain-free hip and
satisfactory gait.
antiinflammatory medications, aggressive physical therapy, periodic
traction with bed rest, and prolonged protected weight bearing for the
involved hip was reported on by two different groups in the 1980s for
the treatment of idiopathic chondrolysis of the hip (144,145).
In both groups, 50% to 60% of the patients showed clinical improvement
in the range of motion of the involved joint, and improved radiographic
appearance of the joint space. In 1985, Hughes (152)
reported the use of continuous passive motion in the acute stage of
idiopathic chondrolysis of the hip in one patient, in whom a good range
of hip motion was maintained even during the acute stage.
reported one patient who underwent surgical release of tendon
contractures as well as an anterior capsulotomy. This patient at final
follow-up was pain-free, with functional range of motion of the hip and
improved joint space radiographically. In 1988, Roy and Crawford (170) reported on
three patients with idiopathic chondrolysis of the hip, who were
treated with a subtotal circumferential capsulectomy of the involved
hip. Concomitant muscle releases were performed as necessary to relieve
joint contractures. Surgery was followed by a period of traction as
well as aggressive physical therapy emphasizing non–weight bearing
range-of-motion exercises. Continuous passive motion was also tried in
some patients. At an average follow-up of 3 years, all the patients
were reported to be symptom-free, with full return of hip motion and an
improved joint space radiographically. Del Couz Garcia et al. (149)
reported on eight hips in seven patients with idiopathic chondrolysis
of the hip treated by capsulectomy and tenotomy of the iliopsoas and
adductor muscles with an average 13.2-year follow-up. Postoperative
skin traction was used for 3 weeks, followed by an aggressive
range-of-motion rehabilitation program. Although there was some initial
improvement in the range of motion of the affected hips
postoperatively, long-term follow-up showed progressive degeneration of
the joint with resultant stiffness and pain.
chondrolysis of the hip include controlling inflammation and
maintaining hip motion during the acute stage. This is accomplished in
most patients with the use of antiinflammatory medications, periodic
skin traction and bed rest during the time of acute exacerbation of
pain and loss of motion, surgical release of unresolved contractures,
and an aggressive physical therapy program that emphasizes both passive
and active range of motion. During the acute stage the patient is also
maintained on non–weight bearing or limited–weight bearing status for
the involved hip, until all pain has resolved and progressive joint
space loss has ceased as shown radiographically. Although the initial
results of aggressive subtotal capsulectomy and tendon release were
favorable, more recent long-term follow-up indicates that the
improvements achieved may deteriorate with time. For this reason, the
routine use of this procedure for the treatment of idiopathic
chondrolysis of the hip cannot yet be recommended. In patients with
hips that are painful, despite nonoperative measures, or are
malaligned, a hip arthrodesis is the preferred operative intervention (Fig. 27.24B).
HC. Developmental (infantile) coxa vara—a distinct entity. Report of
two patients with previously normal roentgenograms. Clin Orthop 1970;72:242–247.
HT, Chambers HG, Mubarak SJ, et al. Congenital coxa vara: computed
tomographic analysis of femoral retroversion and the triangular
metaphyseal fragment. J Pediatr Orthop 2000;20: 551–556.
FJ. The treatment of developmental coxa vara by abduction
subtrochanteric and intertrochanteric femoral osteotomy with special
reference to the role of adductor tenotomy. Clin Orthop 1976;116:116–124.
AA, Pannu HK, Tadros YE, et al. Pelvic floor anatomy in classic bladder
exstrophy using 3-dimensional computerized tomography: initial
insights. J Urol 2001;166:1444–1449.
RJ, O’Phelan EH, Chisholm TC, et al. Exstrophy of the bladder:
long-term results of bilateral posterior iliac osteotomies and
two-stage anatomic repair. Clin Orthop 1980;151:193–200.
PH, Khoury AE, McLorie GA, et al. Iliac osteotomy: a model to compare
the options in bladder and cloacal exstrophy reconstruction. J Urol 1994;151:182–186; discussion 186–187.
DL, Partridge E, Logan PM, et al. The snapping hip: clinical and
imaging findings in transient subluxation of the iliopsoas tendon. Can Assoc Radiol J 1996;47:202–208.
LA, Danielsson LG, Wattsgard C. Transient synovitis of the hip. Its
incidence, epidemiology and relation to Perthes’ disease. J Bone Joint Surg Br 1987;69:238–242.
A, Reif S, Ashkenazi S. Lack of association of transient synovitis of
the hip joint with human parvovirus B19 infection in children. Pediatr Infect Dis J 1998;17:843–844.
GR, Longobardi YL, Ehrlich M. Transient synovitis: lack of serologic
evidence for acute parvovirus B-19 or human herpesvirus-6 infection. J Pediatr Orthop 1999;19:185–187.
TT, Su CT, Chiu LC, et al. Evaluation of hip disorders by radiography,
radionuclide scanning and magnetic resonance imaging. J Formos Med Assoc 1993;92:737–744.
AM, Berman L, Edwards D, et al. The irritable hip: immediate ultrasound
guided aspiration and prevention of hospital admission. Arch Dis Child 1995;72:110–113; discussion 113–114.
H, Bauer GC, Brismar J, et al. Transient ischaemia of the proximal
femoral epiphysis in the child. Interpretation of bone scintimetry for
diagnosis in hip pain. Acta Orthop Scand 1985;56:197–203.
SK, Suh KJ, Kim YW, et al. Septic arthritis versus transient synovitis
at MR imaging: preliminary assessment with signal intensity alterations
in bone marrow. Radiology 1999;211: 459–465.
Beccaro MA, Champoux AN, Bockers T, et al. Septic arthritis versus
transient synovitis of the hip: the value of screening laboratory
tests. Ann Emerg Med 1992;21:1418–1422.
MS, Zurakowski D, Kasser JR. Differentiating between septic arthritis
and transient synovitis of the hip in children: an evidence-based
clinical prediction algorithm. J Bone Joint Surg Am 1999;81:1662–1670.
ST, Rowe SM, Moon ES, et al. Significance of laboratory and radiologic
findings for differentiating between septic arthritis and transient
synovitis of the hip. J Pediatr Orthop 2003; 23:368–372.
SJ, Jones A, Schootman M, et al. Differentiation between septic
arthritis and transient synovitis of the hip in children with clinical
prediction algorithms. J Bone Joint Surg Am 2004;86-A:956–962.
MS, Mandiga R, Zurakowski D, et al. Validation of a clinical prediction
rule for the differentiation between septic arthritis and transient
synovitis of the hip in children. J Bone Joint Surg Am 2004;86-A:1629–1635.
U, Wingstrand H, Forsberg L, et al. The effect of arthrocentesis in
transient synovitis of the hip in the child: a longitudinal sonographic
study. J Pediatr Orthop 1996;16:24–29.
A, Turner A, Ferguson J, et al. Seven year follow up of children
presenting to the accident and emergency department with irritable hip.
J Accid Emerg Med 1999;16:345–347.
S, Fink M, Graham K, et al. A randomized clinical trial: should the
child with transient synovitis of the hip be treated with nonsteroidal
anti-inflammatory drugs? Ann Emerg Med 2002;40:294–299.
RL. The pathology of acute necrosis of cartilage in slipping of the
capital femoral epiphysis. A report of two cases with pathological
sections. J Bone Joint Surg Am 1963;45:1013–1024.
E, Bellocci M, Santori FS, et al. Idiopathic chondrolysis of the hip:
an ultrastructural study of the articular cartilage of the femoral
head. Orthopedics 1986;9:1383–1387.
