The Lower Extremity
Differences in appearance related to foot position during walking or
running are most often just that, differences, not pathologic
conditions. Foot position during walking is described by the direction
of the foot relative to the body’s line of progression during the gait
cycle (internal, external, or neutral). This is torsion. It results from the summation of several factors that include version of the bones, capsular pliability, and muscle control (1,3,4,5).Version
is tilt or inclination within a bone, such as the relation of the
femoral head/neck to the shaft of the femur. Contracture of a joint
capsule may restrict rotation. Similarly, capsular laxity may allow a
greater than normal arc of motion. Arthrosis or incongruity may also
restrict motion. The balance between opposing muscle groups is also a
determinant of foot position and may introduce a significant, dynamic
component to the rotational profile. Age is another important variable
because version, soft-tissue pliability, and muscle coordination change
as the child matures (1,2,3,4,5,6,7,8).
dynamic components. The static examination describes the available
range of rotational motion. The dynamic examination displays the effect
of various torsional forces at play during the walking cycle (Fig. 28.1).
The static examination is best performed on a firm examining table,
with the child in comfortable, loose clothing such as shorts or a
diaper. Rotation is best assessed with the child in the prone position,
keeping the pelvis flat and level on the examination table (3,4,5).
Flexion of the knee to 90 degrees allows the leg to be used like a
goniometer relative to the thigh. Some children will not allow
examination other than on a parent’s lap. This is usually adequate
although not as controlled as in the prone position. The arc of
rotation may be more generous if measured with the hip flexed as in a
sitting position (5).
assess the degree of available hip rotation. When there is a greater
degree of internal or medial rotation (outward movement of the foot in
the prone position) than external or lateral rotation, a toed-in gait
is more likely to be observed. Similarly, if there is greater external
rotation than internal rotation, the gait pattern is usually toed-out.
A greater ability to rotate the thigh internally than externally is
frequently referred to as anteversion. It should correctly be called antetorsion because hip rotation is the combined effect of version of the femur, joint mobility, and muscle function (1,3,5).
Variability is greater in younger children. Static medial hip rotation
averages 40 degrees in infants, but can range from 10 to 60 degrees. It
increases slightly by the age of 10 years and then decreases gradually
in adulthood. Lateral hip rotation is greater than medial rotation in
infants; it averages 65 degrees (range, 45 to 90 degrees), compared to
children over the age of 10 years who average 40 degrees (range, 20 to
55 degrees) (1,2,3,5,9,10). It increases slightly in adults.
relative to the thigh, which is held in neutral rotation, determines
the thigh-foot angle (TFA), as shown in Figure 28.1B.
This relation also describes the contribution of the leg segment or the
degree of tibial torsion. Foot deformity, which may contribute to
rotational abnormalities, can easily be assessed in this position.
Forefoot adduction or abduction and hindfoot varus or valgus is noted.
Many young children have significant rotational laxity through the
knee. Internal and external rotation of the tibia with the knee flexed
demonstrates the degree of knee joint laxity. This may contribute to
variation in foot position. Average TFA is 5 degrees internal in
infants (range, -30 to +20 degrees) and 10 degrees external by 8 years
of age (range, -5 to +30 degrees) (1,2,3,5). TFA changes very little after 12 years of age.
dynamic examination is important. The child’s walking pattern should be
observed in an area large enough to allow comfortable and safe walking
and running. The child should be able to walk alone (i.e., not holding
someone’s hand). It is often helpful to observe the child in shoes as
well as barefoot. Often the in-toeing is more apparent with shoes on,
presumably because the weight of the shoe may be more taxing on the
muscle control of foot position. An oversized shoe may also compound
the appearance of in-toeing. Variations are common with change in speed
or direction of walking. Foot and knee position should be observed over
several cycles of gait. The relation of foot position to an imaginary
line along the path being walked describes the foot-progression angle
(FPA). Variability is considerable in children up to 5 years of age.
Out-toed position predominates in older children. Once a mature gait
pattern is established, usually by 5 years of age, FPA changes very
little (1,2,3,4,5,6,9,10).
of each of these components to the gait pattern. The FPA, TFA, and
position of the knee relative to the body (femoral torsion), all
contribute to the sum of rotational factors.
Each
area needs to be assessed along with its contribution, either positive
(internal rotation) or negative (external rotation), to the overall
gait pattern. Those children whose rotational profiles are beyond two
standard deviations of the mean are considered abnormal according to
Staheli’s criteria (3,4) (Fig. 28.2).
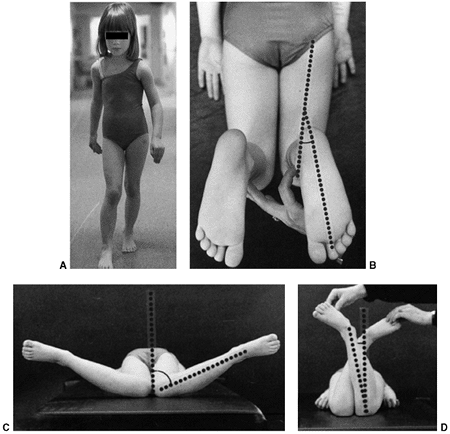 |
|
Figure 28.1 How the rotational profile measurements are made. A:
The foot-progression angle (FPA) is estimated by observing the child’s gait. It is defined as the angular difference between the axis of the foot and the line of progression. This child’s FPA is 0 degrees. B: The thigh-foot angle (TFA) is the angular difference between the axis of the foot and thigh as viewed from above. The TFA in this child is 18 degrees. From this view the shape of the foot is apparent. C: Medial hip rotation is the maximum angular difference between the vertical and the axis of the tibia. In these hips, this measurement is 70 degrees. D: Lateral hip rotation is the corresponding measurement. On this child, the angle is 10 degrees. |
rotational abnormality. Residual foot deformities, disorders of the
hip, and neuromuscular diseases are the most common causes of
pathologic in-toeing or out-toeing. In-toeing may be caused by residual
foot deformity from metatarsus adductus, clubfoot, or skewfoot (1,4,5). These conditions are discussed in detail elsewhere in this text (see Chapter 29).
In-toeing, which is only apparent during swing phase, may be the result
of overpull of the posterior tibial tendon, often seen in spastic
hemiplegia (11,12).
quadriplegia. This may be a combination of excess femoral anteversion
with contracture of the adductor and medial hamstring muscles (4,5,13). Excess valgus and pronation of the foot, which contribute to an out-toed foot progression, may also be seen (13).
For some children with spasticity, extremes in rotational posture are a
compensatory mechanism for limited hip, knee, or ankle motion. The
combination of excess internal femoral rotation and external tibial
rotation (malicious malalignment) is often observed in children with
spasticity. Children with diplegia
and
quadriplegia often have heel cord tightness. To maintain foot contact
with the floor, the calcaneus tends to rotate laterally beneath the
talus, which allows the talar head to drop plantarward, producing a
valgus deformity (14).
If the peroneal tendons are also spastic, the forefoot will also be
pulled into an abducted position creating a planovalgus deformity. If
the child is unable to clear the foot during swing phase, the foot is
repeatedly dragged along the floor, adding to the external torsional
force applied to the foot. This produces a malalignment, with the foot
externally rotated from the planovalgus deformity and the knee
internally rotated from femoral antetorsion.
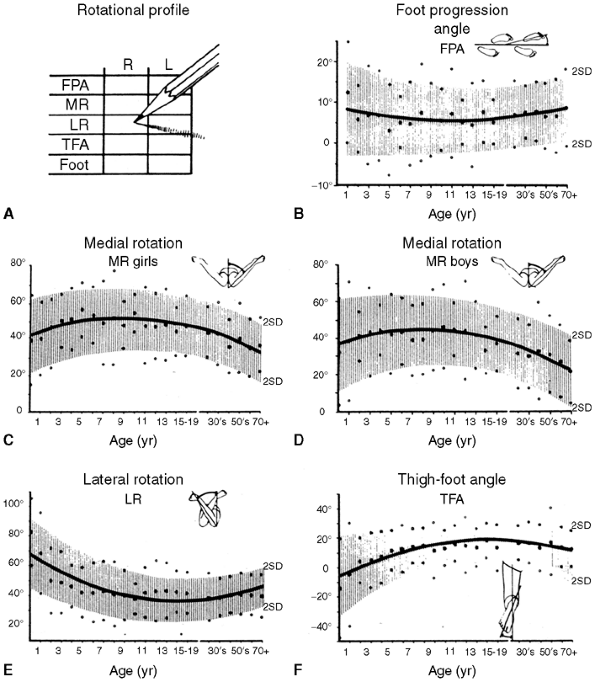 |
|
Figure 28.2 The rotational profile. A:
The method of recording the degree measurements for each element of the profile is depicted. This simple chart includes the vital information necessary to establish the diagnosis and to document severity. B–F: Normative values for the profile based on 1000 healthy limbs are shown. In each figure the age is listed on the abscissa on a logarithmic scale and the degrees are shown on the ordinate scale. Mean values are shown by the solid line with standard deviations; reference range is shown in shaded areas. A sex difference was found to affect the values for medial rotation, so the values are shown independently. |
This may be secondary to tarsal coalition, but may also be seen in
adolescents with rigid flat feet without a coalition. It is unclear
whether the out-toed position is an adaptation to a rigid flat foot, or
whether the lack of foot flexibility promotes the development of
external rotation. The combination of femoral retrotorsion, external
tibial torsion, and pes planovalgus can also be seen, particularly in
large adolescents, which produces a striking out-toed gait. Slipped
capital femoral epiphysis (SCFE) should be considered in a differential
diagnosis of out-toeing, particularly when the deviation is asymmetric
or of recent onset (4,16). Hip dysplasia can alter rotation, but its effect is highly variable (7).
An asymmetric hip may be apparent upon examination, but is not reliable
in detecting hip dysplasia. In such instances, further evaluation with
radiographic hip examination is warranted (3,4). Coxa vara may also present as an out-toed gait.
orthopaedic surgeon with concerns of a gait abnormality have an
anteroposterior pelvis radiograph. Children older than 8 years with a
recent change in gait or with complaints of pain should also have a
lateral radiograph of the hips (3,4,16).
A cross-table lateral film is optimal to detect a minimally displaced
SCFE. Although the incidence of otherwise occult pathology is low, the
consequence of a missed diagnosis, such as hip dysplasia or an SCFE, is
significant for the child.
hips or lower extremities to follow the course of normal rotational
development (4,5). Special views to quantify version of the femur or tibia are not indicated in the routine evaluation of rotation (3,4,5).
Three-dimensional imaging studies [computed tomography (CT) and
magnetic resonance imaging (MRI)] are rarely indicated in the
assessment or follow-up of rotational variations, but are more accurate
in quantifying rotation than clinical or biplane radiography (17).
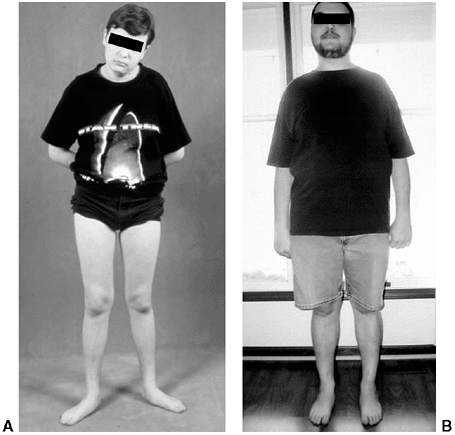 |
|
Figure 28.3 A:
Excess external rotational deformity may not resolve. It may be associated with symptomatic flatfoot, with or without tarsal coalition. The use of medial support orthotics may reduce symptoms, but will not alter rotation. B: Alignment at 18 years of age following tibial internal rotation osteotomy to correct excessive external tibial rotation. |
out-toeing are normal. Rotational profiles are highly variable,
particularly in toddlers who have not mastered the basic skills needed
for normal walking, which includes just about every child younger than
2 years and many of those who are 2 to 5 years of age (1,2,6,10).
Internal and external rotational variations should be considered just
that, variations of normal, not a pathologic condition. The natural
history of rotational variations is gradual normalization, usually
accomplished by 5 to 6 years of age. There are no conclusive studies to
show that any nonsurgical intervention speeds up or assures the
normalization of gait (4,5,18).
It is often associated with physiologic bowlegs and decreases 1 to 2
years after resolution of the bowing. Occasionally, it will persist
into preadolescence. External tibial torsion is less common, but is
more likely to persist through adolescence (4,5).
Most healthy infants have an external rotation contracture about the
hip, which is likely to be a result of intrauterine positioning. This
may not fully resolve until they become established walkers at 18 to 24
months of age. An in-toed gait may then be more apparent. Lateral hip
rotation gradually decreases as medial rotation increases.
Paradoxically, anteversion in the femoral neck typically decreases from
30 degrees (range, 15 to 50 degrees) at birth to 20 degrees (range, 10
to 35 degrees) by 10 years of age (1,2,3,4,5,7,8).
A decrease in anteversion of the femoral neck would be expected to
produce greater external rotation of the hip; however, changes in
muscle balance and hip capsule pliability appear to have greater
influence on gait. By 8 years of age, a child who toes in while
walking, but has at least 20 degrees of hip external rotation, is
within normal limits as defined by the mean plus or minus two standard
deviations. Similarly, one who toes out while walking, but has at least
20 degrees of hip internal rotation, has motion within a normal range (4,5,10).
Several authors have tried to correlate the degree of femoral
anteversion with the presence of osteoarthritis of the hip using
postmortem studies or by preoperative CT scan. Most studies have shown
a similar measure of anteversion in hips with and without arthritic
changes (20,21,22,23,24).
One study measured anteversion in hips of patients undergoing proximal
femoral osteotomy or total hip replacement. Patients with bilateral
disease had an average 9 degrees greater anteversion than patients with
healthy hips. Patients with unilateral arthritis averaged 4 degrees
more anteversion in the arthritic hip than in the healthy hip (24).
Some authors have demonstrated a relation of anteversion with
degenerative changes in the knee, presumably from increased shear loads
(25). Hip pain is rarely a complaint in children with increased femoral anteversion alone (19,21,26). Anterior knee pain may be associated with increased medial rotation
of the femur, but not generally with patellofemoral changes (21,22,26).
Although some recent publications have suggested that torsional
variations may increase the risk of osteoarthritis, anatomic studies
have not conclusively shown a causal relation between femoral
anteversion and osteoarthritis. A subset of patients exists in whom the
limits of tolerance in hip or knee range of motion is exceeded and for
whom the risk for early degenerative changes may be increased (25,27,28).
In these patients, the alteration in rotation places increased stress
across the hip or knee, which can promote the development of
osteoarthritic changes. The precise limit of tolerance remains
undefined. However, the presence of hip or knee pain should be
considered as indications for osteotomy.
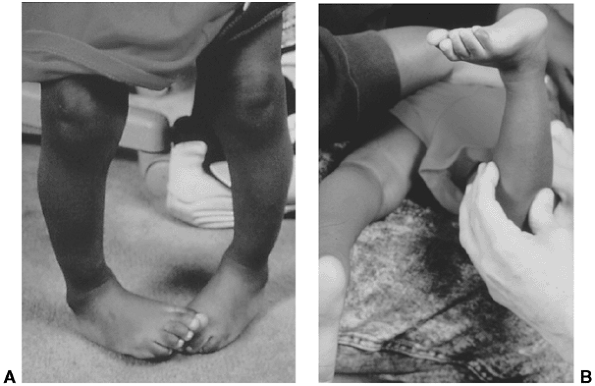 |
|
Figure 28.4 A: Internal tibial torsion is often seen in toddlers in association with physiologic bowlegs and results in an in-toed gait. B: The thigh-foot angle is best assessed in the prone position.
|
augment posterior shear loads, an increased incidence of slipped
epiphysis has not been found in patients with femoral retroversion
without other contributory factors being present (16).
Athletic ability does not correlate with the position assumed during
normal walking, although some activities may be hindered by rotational
variations, particularly hip or tibial external rotation (29,30,31). High-performance sprinters, however, tend to adopt a toed-in position during running regardless of their walking style (31).
Functional impairments such as tripping, clumsiness, or lack of running
ability, although often attributed to rotational differences, have not
been shown to correlate with rotational profile (29).
rotation that their appearance is unacceptable to them or their
parents. The natural history of rotational variation, the lack of
evidence for musculoskeletal sequelae, and the absence of objective
functional disability must be kept in mind and the family educated in
this regard (1,3,4,5).
The natural history of rotational variation should be clearly
communicated to the parents and understood by them prior to any
recommendation for active treatment.
normalization. No treatment is necessary in most children who present
with concerns of in-toeing or out-toeing. The use of shoe
modifications, orthotics, or positioning devices is ineffective (5,16).
Although these measures are not harmful, there are few data to show any
positive effect. Their use reinforces the errant notion that in-toeing
or out-toeing is an abnormal condition or disorder that requires
treatment. The cost of orthopaedic shoes and orthotics can be
considerable. Muscle-strengthening exercises or activities may diminish
the dynamic component that produces variation in gait; however, no
studies have addressed this specific topic. Treatment options for foot
and ankle abnormalities are covered in Chapter 29.
otherwise healthy children who have persistent rotational abnormality
into later childhood and adolescence and find the appearance of their
gait or their function unacceptable (4,5,25,32,33).
An awkward gait may appear less following osteotomy. Patients and their
parents often express satisfaction with the change in appearance
following osteotomy. Improvement in function is variable and less
predictable. Those with in-toeing typically note less tripping. Those
with out-toeing are more likely to notice an improvement in running
ability.
Most often, this occurs in patients with malicious malalignment or the
combination of femoral internal rotation with tibial external rotation (25,34).
These patients may experience knee symptoms preoperatively, likely
related to the increased shear forces through the knee. This
combination of rotational abnormalities has been associated with some
risk of patellofemoral arthritis (35,36).
fibula has less potential for serious neurovascular complication than
does proximal tibial osteotomy (32,37). The
authors’ preferred technique uses fixation with crossed Kirschner wires
(K-wires), supplemented with a long-leg cast. Alternatively, a small
fragment T-plate supplemented with a short-leg cast can be used. The
tibia should be cut 2 to 3 cm above the physis and parallel to the
ankle. Rotation through an oblique cut will tilt the articular surface
of the tibia. Sectioning of the fibula also allows easier rotation of
the tibia without displacement. The degree of correction is determined
by aligning the foot and ankle, such that the second toe is in line
with the tibial tubercle and the center of the patella. As the foot is
dorsiflexed and knee bent, the foot should remain in line with the
lateral thigh. Two or three stainless steel wires of appropriate size
are inserted, one from proximal medial to distal lateral and another
from distal medial to proximal lateral, avoiding the growth plate of
the distal tibia. A third wire is used if rotational stability does not
seem adequate with two wires. A long-leg, bent-knee cast is used for 6
weeks. Healing is usually sufficient at that time for pin removal and
application of a short-leg weight-bearing cast for an additional 4 to 6
weeks.
along with a short-leg non–weight-bearing cast for 6 to 8 weeks. The
T-plate may be preferable in older patients. Plate removal may be
necessitated by the subcutaneous position of the plate.
rotation is obtained by equalizing the degree of internal versus
external rotation. The amount to be rotated can be estimated during the
prone examination, comparing internal and external hip rotation,
preoperatively (5). Femoral osteotomy may be
performed proximally or distally depending on the surgeon’s preference
and the type of fixation to be used (33,38,39).
Proximal femoral osteotomy is preferred if there is also varus, valgus,
flexion, or extension deformity of the hip. Similarly, angular
deformities about the knee are better addressed by a distal femoral
osteotomy. A standard lateral approach to the femur is used, whether
proximal or distal. In proximal osteotomy, a pediatric or adolescent
blade plate is used for fixation. If a distal femoral osteotomy is
performed in a skeletally immature patient, a small straight
compression or 95-degree condylar (adolescent) blade plate fixation may
be used. Use of cast or orthotic immobilization is at the discretion of
the surgeon. Alternatively, a medial approach can be used along with
K-wire fixation (33). This latter technique
should be reserved for smaller children, and must be supplemented with
a spica cast. The desired amount of rotational correction is typically
that which achieves an equal amount of internal and external rotation.
Locking screws or similar mechanisms are needed proximal and distal to
the osteotomy because the osteotomy has no intrinsic stability. In the
skeletally immature and in those with a relatively narrow medullary
canal, the osteotomy is generally performed by a limited open
technique. The proximal femur entry site is made lateral and distal to
the tip of the greater trochanter. Great care is taken to avoid any
dissection (including penetration) on the medial side of the
trochanter. A recent modification in femoral nail design (proximally
angulated 15 degrees) facilitates correct nail placement. This lateral
trochanter entry site is necessary to avoid injury to the medial
portion of the trochanteric growth plate and injury to the terminal
branches of the medial circumflex artery in the trochanteric fossa.
Neither coxa valga nor avascular necrosis has occurred in the early
experiences with this approach (40). An
intramedullary saw can be used to section the femur in skeletally
mature patients who have a larger medullary canal. Fixation with an
intramedullary rod does not allow for any concomitant correction of
varus, valgus, flexion, or extension. However, intramedullary fixation
does allow for early weight bearing, a particular advantage if
bilateral procedures are performed.
Manipulation may be hindered by the pull of the soft tissues,
particularly in large adolescents (41,42).
Pins should be inserted, taking into account the rotation to be
accomplished. The amount of change in rotation can be reassessed in the
early postoperative period and adjusted if needed.
Knee pain, which is usually diffuse, may be present. Patellar
mal-tracking usually is not present. Because the deformities are
complementary, foot progression may be normal, but the static
examination will demonstrate the abnormality. Combined osteotomy of the
femur and tibia may be necessary to correct symptomatic malrotation (4,25,34).
Femoral rotation is corrected as the first step of the procedure. The
foot is then aligned with a tibial osteotomy completing the correction.
Correction of bilateral deformities can be performed as staged
unilateral procedures 6 to 12 months apart.
Because most patients who have surgical alteration of their rotation do
so to effect a change in the appearance of their gait, the surgical
procedure must accomplish the desired change in rotation. Functional
change is noted in some children, but not consistently.
for other osteotomies. Problems related to nonunion, infection, blood
loss, joint stiffness, scarring, and anesthesia are the most serious (25,32,33,37).
Distal tibial osteotomy has less risk of compartment syndrome and
peroneal nerve injury than proximal osteotomy. Whether performed
proximally or distally, the use of a blade plate for fixation of a
femoral osteotomy may inadvertently produce undesirable frontal or
sagittal plane deformity.
Similarly,
inadvertent deformity can be produced in the distal tibia if an oblique
osteotomy is made, rather than a transverse cut, in performing a distal
tibial osteotomy. The distal tibial physis should not be violated when
using K-wires to fix a supramalleolar tibial osteotomy. The authors
have observed distal tibial physeal growth arrest and deformity
following K-wire penetration of the medial tibial physis. Asymmetric
growth arrest can occur from periosteal stripping and injury to the
lateral distal femoral physis. Injury to the greater trochanteric
apophysis can produce valgus deformity in the proximal femur. Scars may
be less visible with proximal osteotomy, a consideration for a
procedure that is largely cosmetic.
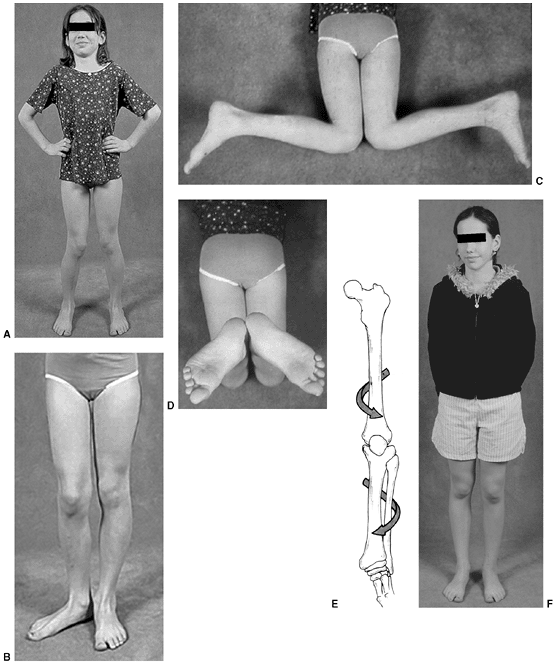 |
|
Figure 28.5 A:
This 10-year-old girl is attempting to stand with her feet directly forward. The patellae face medially, and with effort she can direct the feet straight ahead. B: When the limb is positioned with the patella facing forward, the outward rotation of the foot becomes apparent. C: Examination in the prone position demonstrates approximately 80 degrees of internal rotation of the thigh, or medial femoral torsion. D: With knees flexed, the outward direction of the foot can also be appreciated. If the rotational deformities are complementary, foot progression may be deceptively normal. E: The combination of increased medial thigh rotation and external rotation of the lower leg segment may result in symptomatic torsional malalignment. This deformity may be a cause of nonspecific knee pain in adolescents because of the increased shear forces through the knee. F: Combined femoral external rotation and tibial internal rotation osteotomies correct the malicious malalignment. Knee symptoms are predictably improved. |
intramedullary fixation device is used; however, adjustments in
position can be difficult once the rod is locked. Use of a lateral
entry point helps minimize the risk of avascular necrosis to the
femoral head and potential disruption of normal growth of the proximal
femur (39,40,42,43,44).
Recent changes in pediatric intramedullary nail design permit safer
entry through the lateral aspect of the greater trochanter, minimizing
both the potential for avascular necrosis and the disruption of normal
growth of the proximal femur (40). In the skeletally immature patient, use of this lateral entry site is imperative.
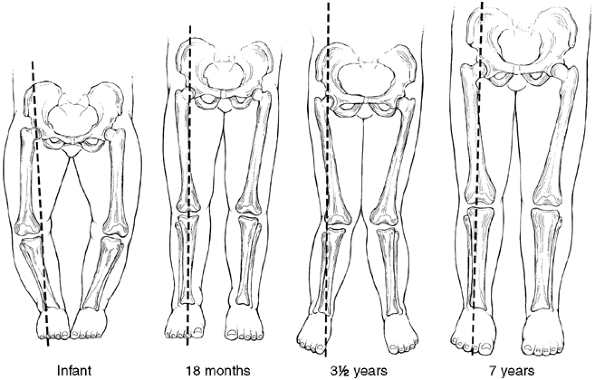
The normal knee alignment at birth is 10 to 15 degrees of varus, which
remodels to a neutral femoral-tibial alignment at approximately 14
months of age (1,49,50) (Fig. 28.6). Levine and Drennan (51)
have defined physiologic bowing radiographically as more than 10
degrees of bilateral femoral-tibial varus noted after the age of 18
months.
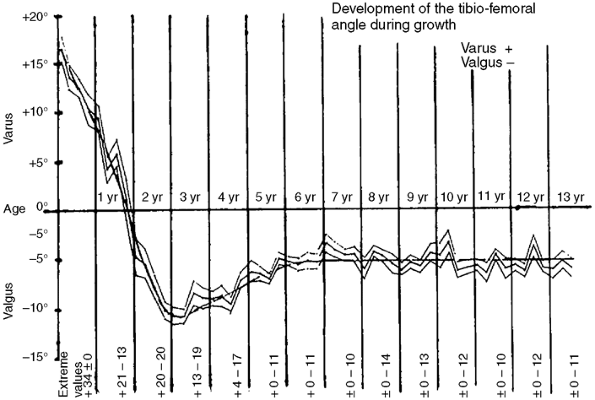 |
|
Figure 28.6
The graph depicts the expected change in genu varum and genu valgum with age. Children with bowlegs after 2 years of age are outside the normal range and should be thoroughly evaluated. (From Salenius P, Vankka E. The development of the tibiofemoral angle in children.J Bone Joint Surg Am 1975;57: 259, with permission.) |
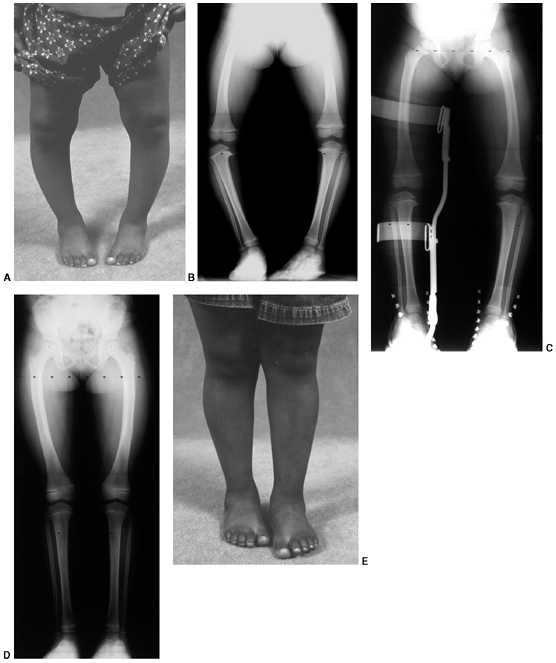
of the bowing deformity and the associated problems of excessive
tripping. A family history of bowleg deformity is common (45,52). Examination typically reveals bowing of both the lower extremities with in-toeing (Fig. 28.7).
Although the bowing deformity is bilateral, its severity in the left
versus right extremities can vary. Despite their bowlegged, toed-in
gait, these young children are characteristically very agile walkers.
The child should be observed walking, both toward and away from the
examiner, to assess the severity of the dynamic varus and associated
internal rotation deformity. Internal rotation of the extremity permits
contact of the foot with the floor as the child stands. This also
maintains the body center over the midline as the child walks. The
presence of a lateral thrust should be noted. This is a brief, dynamic,
lateral, knee joint protrusion that occurs in stance. It represents a
lateral subluxation or shifting of the femur on the depressed medial
tibia and is accentuated by ligamentous laxity. It is characteristic of
pathologic bowlegs.
measured with a goniometer with the knees extended and patella facing
forward. Photographs are helpful in documenting the deformity. Angular
and rotational alignment and joint range of motion of the entire lower
extremity are assessed. Knee joint laxity typically is not present in
physiologic bowing.
 |
|
Figure 28.7
Examination of the child with physiologic bowing shows symmetric bowing throughout the tibia and internal tibial torsion which is often more noticeable with walking. |
radiographs can document the degree and location of the varus
deformity, but usually will not distinguish between physiologic bowing
and early Blount disease. Radiographs are an essential part of the
evaluation in the older child (over 18 months of age), for those with
more pronounced deformities (greater than 20 degrees), when a lateral
thrust is observed, if the child is of short stature (below fifth
percentile), or if a metabolic bone disease is suspected (Fig. 28.8).
The radiograph is taken with the patient standing, if possible, and the
patella pointing straight ahead. The relative degree of varus deformity
is noted by observing the shaft-to-shaft angle of the femur and tibia (51,52). More importantly, the distribution of bowing deformity is noted (53). When physiologic, the bowing occurs throughout the distal femur, proximal tibia, and distal tibia (54). In contrast, early Blount disease has more focal deformity limited to the proximal tibia.
B–D). The clinical and radiographic features associated with metabolic
bone disease or skeletal dysplasia readily differentiate these
pathologic conditions from physiologic bowing. Most often, the
differentiation is between physiologic bowing and infantile Blount
disease. If the bowing is physiologic, the entire lower extremity will
appear to be bowed. If the varus results from a relatively greater
deformity of the proximal tibia, infantile tibia vara or Blount disease
may be present (54,55,56).
Children with either physiologic bowing or Blount disease usually are
early walkers and typically present for evaluation at 15 to 18 months
of age. Often, there is a positive family history of bowing deformity
(in siblings, parents, uncles, and aunts) (45,46,52,53).
Physiologic bowing and Blount disease ought to be perceived as two
points within the same spectrum, with Blount disease being the
pathologic result of unresolved infantile bowing (46,51,55). Frequently, one extremity will have physiologic bowing with Blount disease affecting the contralateral tibia.
|
TABLE 28.1 DIFFERENTIAL DIAGNOSIS OF BOWED LEGS
|
|
|---|---|
|
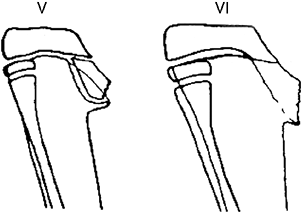
Blount disease is not obvious in very young children. The Langenskiöld
changes, diagnostic of Blount disease, are not always distinct before 2
to 3 years of age (47,50,51,53,56).
Measuring the metaphyseal-diaphyseal (MD) angle of both the proximal
tibia and distal femur further helps to identify the specific location
and relative severity of varus deformity (51,56,57).
Although an absolute MD angle is not diagnostic, it does serve as a
guide in differentiating Langenskiöld stage I infantile Blount disease
from physiologic bowing (50,53) (Fig. 28.9). Measurement can be affected by limb position (58).
A study of the proximal tibial MD angle in patients with bowing
(physiologic bowing or Blount disease) identified two distinct
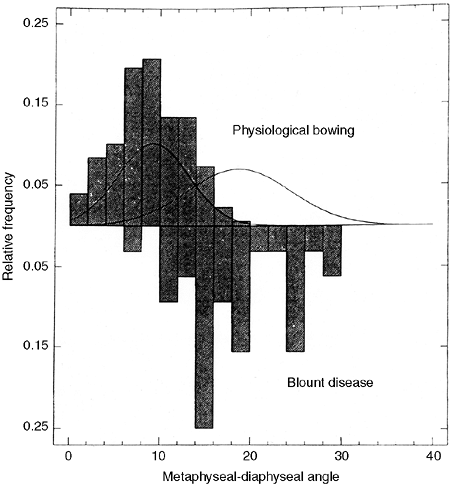
populations with considerable overlap (56) (Fig. 28.10).
On the basis of this study by Feldman and Schoenecker, when the MD
angle is less than 10 degrees, there is a 95% probability that the
diagnosis is physiologic bowing. Conversely, if the MD angle is greater
than 16 degrees, then there is a 95% probability that the diagnosis is
Blount disease. For those patients with an MD angle between 10 and 16
degrees, follow-up for at least 1 to 2 years is necessary to determine
whether the metaphyseal changes resolve (physiologic bowing) or
progress (Blount disease). In a recent report, Bowen et al. (54) noted that all children with a tibial MD angle greater than 16 degrees showed progression of the varus deformity.
physiologic bowing from early Blount disease. This is measured on the
anteroposterior lower extremity x-ray film by constructing a distal
femoral MD angle similar to that measured in the proximal tibia. This
angle represents the contribution of varus in the distal femur to the
overall measure of femoral-tibial varus in the limb. The distal femoral
MD angle is divided by the proximal tibial MD angle. This quotient
represents the proportion of varus found in the femur relative to the
tibia. A ratio of greater than one suggests the bowing is physiologic,
that is the femur contributes as much as the proximal tibia to the
varus, and resolution is expected to occur (57).
A ratio of less than one indicates that the bowing is predominantly
within the tibia and is more likely to evolve into Blount disease.
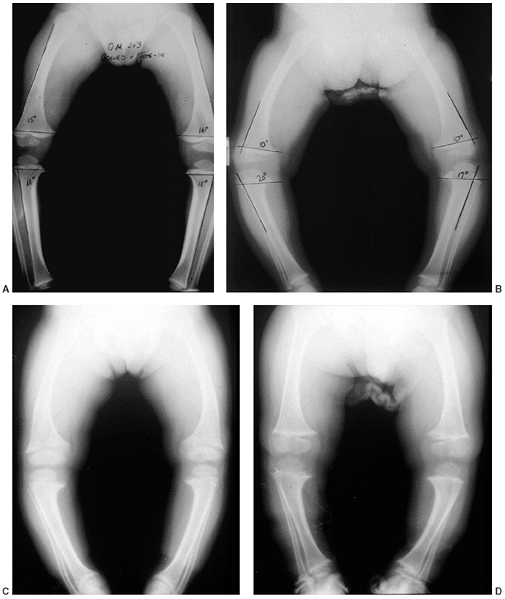 |
|
Figure 28.8 Standing anteroposterior films of both lower extremities help distinguish physiologic bowing from pathologic causes. A:
Tibial metaphyseal-diaphyseal (MD) angles are typically 11 degrees or less. A similar angle constructed in the distal femur is the same or greater, indicating that the femur and tibia contribute similarly to the bowing. The ratio of femoral to tibial MD angle is greater than 1. B: Early Blount disease may be difficult to distinguish from severe physiologic bowing. The MD angle in Blount disease is usually greater than 16 degrees and the ratio of femur to tibia is less than 1. Fragmentation of the medial tibial metaphysis may not be evident. C: This patient with X-linked hypophosphatemic rickets (XLH) has multiple widened physes. Osteopenia is evident in the adjacent metaphysis, which is also flared. Bowing tends to be more diffuse throughout the bone rather than focal in the proximal tibia. D: Skeletal dysplasia, such as chondrometaphyseal dysplasia, may cause genu varus. These children are usually of short stature. Skeletal abnormalities are multifocal as in this example of Schmid metaphyseal chondrodysplasia. The proximal and distal metaphyses of the femur and tibia are all abnormal. The epiphyses, physes, and bone density are normal. |
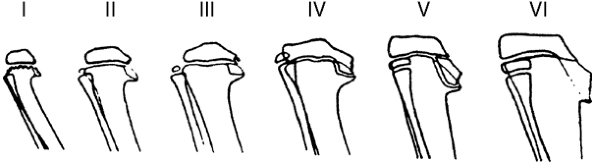 |
|
Figure 28.9
Depiction of the six stages of radiographic changes seen in Langenskiöld classification of tibia vara. (From Langenskiöld A. Tibia vara, A critical review. Clin Orthop Relat Res 1989;246:195, with permission.) |
 |
|
Figure 28.10
The relative frequency of metaphyseal-diaphyseal (MD) angle measured in children with physiologic and Blount disease is presented. MD angle in physiologic bowing is graphed above the horizontal line; similar measurements in Blount disease are graphed below. The bell curve to the left shows the distribution in physiologic bowing. The bell curve to the right is the distribution in Blount disease. Although peak distributions are clearly separate, there is significant overlap between MD angles of 10 and 16 degrees. Below 10 degrees, there is a 95% probability that the bowing is physiologic. Above 16 degrees, there is a 95% probability that the bowing observed is in fact Blount disease. Angles in between are indeterminate. Additional risk factors such as obesity, instability (lateral thrust), and family history must be considered. |
differentiated from the pathologic bowing associated with metabolic
bone disease or skeletal dysplasia (Fig. 28.8).
Rickets [usually X-linked hypophosphatemic rickets (XLH)] is the most
likely metabolic bone disease to present as a bowed leg deformity in a
toddler (59,60).
Nutritional rickets should be suspected if the child was breast-fed and
did not receive supplemental vitamin D. Infants presenting with rickets
are short; the measured height is often below the tenth percentile.
Radiographs of infants with bowing deformity secondary to rickets
should not be mistaken for physiologic bowing (53).
In patients with XLH, the physis appears abnormally wide, and the
metaphysis is flared and curves like a trumpet around the physis.
Similar changes are found within all growth plates. Bone density will
be diminished overall, including thinned diaphyseal and metaphyseal
cortices. The severity of changes in bone morphology and the degree of
osteomalacia is variable. The diagnosis of XLH is made by analyzing
calcium and phosphate in serum and in urine (59,60). Patients suspected of having rickets should be referred to an endocrinologist for a thorough metabolic workup.
the lower extremity. The child who presents with bowing deformity in
association with a skeletal dysplasia will be short, typically below
the fifth percentile. The radiographic changes for each skeletal
dysplasia vary with the site of involvement, which may be principally
epiphyseal, physeal, metaphyseal, or diaphyseal or may involve multiple
sites and may include the spine. Achondroplasia often presents with
bowing. Distinctive physical findings, including a knee-centered varus
deformity with an elongated fibula, are characteristic of
achondroplasia. Patients with pseudoachondroplasia may present with a
varus deformity (although valgus is sometimes present) in association
with ligamentous laxity. Metaphyseal chondrodysplasia (Schmid or
McKusick type) typically presents with persistent bowing and short
stature in an otherwise normal-appearing child (Fig. 28.8D).
The radiographic changes about the physis and metaphysis are not those
associated with osteomalacia, and bone density will appear normal.
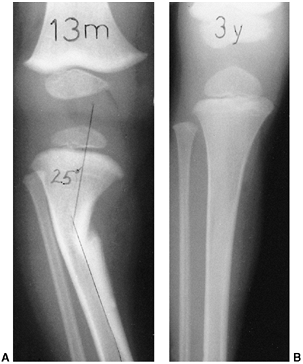
but progressive, unilateral, focal deformity that can occur either in
the proximal tibial metaphysis or distal femoral metaphysis (61,62,63,64,65,66) (Fig. 28.11).
The clinical presentation is similar to unilateral infantile Blount
disease. The lesion consists of a focus of fibroblastic and
cartilaginous tissue, usually below the insertion of the pes anserinus
on the tibia. This produces an acute angulation below the metaphysis.
The diagnosis is made radiographically. A characteristic indentation is
noted in the medial cortex at the junction of the metaphysis and
diaphysis, and a focal varus
deformity
is associated with it. The lesion is radiolucent and well
circumscribed, often with a rim of reactive bone. The physis and
epiphysis are normal. An identical process has also been observed in a
corresponding location in the distal medial femur (66). Some authors have reported spontaneous improvement in angulation.
 |
|
Figure 28.11 Focal fibrocartilaginous dysplasia. A:
Initial radiograph of a patient at 13 months of age shows anterior and lateral bowing of the tibia, but bowing is more proximal than in congenital pseudarthrosis. B: The deformity resolved spontaneously, as seen in a radiograph made at 3 years of age. |
 |
|
Figure 28.12
Lower-limb alignment follows a predictable pattern. Infants typically have a gentle varus bow throughout the femur and tibia. By 18 to 24 months, the lower leg is nearly straight with a neutral mechanical axis. Valgus gradually develops and is most apparent between 3 and 4 years of age. By 7 years of age, the lower limb is in slight valgus and changes very little thereafter. Varus should not recur nor should valgus increase. |
of physiologic bowing is anticipated as predicted by the data of
Salenius and Vankka (1,49) (Fig. 28.6).
Salenius and Vankka followed the changes in frontal plane alignment of
the lower extremities over time. Bowing, although present in infants,
may not be noticed until the child begins weight bearing. The bowing
resolves, typically by 2 years of age, and physiologic valgus develops
between 3 to 4 years of age (Fig. 28.12).
Nonoperative treatment (orthotic insert, shoe modification, or
nighttime splinting) is ineffective. Follow-up visits may not be
necessary in these physiologic, mild deformities because the bowing and
its associated internal tibial torsion predictably resolve. For those
with more pronounced or persistent deformities, follow-up visits are
scheduled at 4- to 6-month intervals. Correction or progression of the
varus that occurs with growth can be documented by subsequent physical
examination. Serial photos can be compared with the initial image to
determine the degree of resolution or progression of the bowing
deformity. Serial radiographs should be obtained if the initial tibial
MD angle was greater than 10 degrees, or if the varus does not appear
improved on clinical appearance or by serial goniometric measurement.
Uncommonly, physiologic varus may persist into late childhood,
warranting longer follow-up and the possible need for treatment with
hemiepiphyseal stapling.
published his classic review of tibia vara or osteochondrosis deformans
of the proximal tibia, noting the progressive course of both the
clinical deformity and the correlative radiographic pathology. The
distal femur is typically normal, but occasionally it will develop a
valgus deformity (46,49,68). These changes
described by Blount and histopathologic changes described by Langenskiöld (47,69,70,71)
were felt to be secondary to a disruption of normal growth of cartilage
and bone caused by excessive pressure on the proximal medial tibial
growth plate and adjacent bone from abnormal weight bearing. Avascular
necrosis of bone in Blount disease has not been observed (71). Progression of this developmental, pathologic tibia vara can be corrected with treatment (67,70,72,73,74).
Physiologic bowing is a continuum with Blount disease. When unilateral
infantile Blount disease is noted, often the contralateral extremity is
bowed physiologically, and this can be indistinguishable from early
infantile Blount disease (Fig. 28.13 A,B).
These children typically are early walkers and often are overweight. As
with physiologic bowing, there may be a family history of bowing
deformity, (46,47,48,50,51,53,55,67,69,79,80,81).
element analysis, calculated that the compressive force resultant from
weight-bearing stresses on a bowed leg were sufficient to cause growth
disturbance. Obesity increases the potential for growth-plate injury.
In some extremities, the bowing resolves through remodelling (46,50),
but in others, the focal pathologic changes in the proximal medial
tibia growth plate cause clinical varus to progress (Blount disease).
The chronic growth disturbance results in the osteochondrosis of the
medial proximal tibial physis and adjacent epiphysis and metaphysis as
described by Blount (46,52,55,67,77,80,81).
Inevitably, the proximal medial tibia fails to grow normally, and tibia
vara of variable severity develops. This results in extremity
shortening and intraarticular depression of the medial tibial condyle.
radiographic stages of development of the proximal tibia depicting the
natural progression of untreated infantile Blount disease (69) (Fig. 28.9). The stage and the age at which it occurs has prognostic significance (50,52,53,69,71).
In stages I and II, the irregular metaphyseal ossification changes are
often indistinguishable from physiologic bowing. These changes may be
reversible. Clear-cut stage I or stage II changes of Blount disease are
typically not apparent until the patient is 2 to 2½ years of age. Stage
III shows definite deformity in the proximal tibial physis, often with
some fragmentation. Stage IV lesions can be associated with early bar
formation across the deformed physis, as it assumes a vertical
orientation (50,53).
These physeal bars are often difficult to detect. There is profound
disruption of the physeal cartilage and abnormal growth in the adjacent
bone as stage V develops, usually in children older than 8 years.
Eventually this process results in severe depression of the articular
surface and stage VI disease (47).
younger age than Langenskiöld reported. All of Langenskiöld’s patients
were whites from Finland, whereas in the United States, a high
proportion of patients with infantile Blount disease are African
American. The greater severity of disease may also be attributed to a
higher proportion of overweight children in North America. These
children tend to show more rapid progression with more severe,
irreversible changes at an earlier age than their European
counterparts. Stage IV changes are seen in 4- to 5-year-old children in
the United States compared to 7- to 8-year-old children in Finland.
normal. Although distal femoral varus does not occur in infantile
Blount disease, valgus does occur in some children with more advanced
tibial changes. It may be a response to the asymmetric load across the
knee, allowing relative overgrowth of the distal medial femur (46,68,77,83).
less than 2½years of age with early Blount disease changes
(Langenskiöld stages I, II) and in patients older than 2 years who have
persistent bowing and risk factors for Blount disease. A tibial MD
angle of greater than 16 degrees is a radiographic sign of significant
risk for Blount disease (50,53,56).
A sign of relative risk is an MD angle between 10 and 16 degrees along
with the clinical appearance of a varus deformity or progression of
varus. Additional risk factors include obesity, ligamentous
instability, or the presence of a lateral thrust, any of which may
potentiate a varus deformity (53,56). Improvement in the tibial MD angle should be apparent within 12 months of brace treatment.
can correct both the varus deformity and the pathologic proximal-medial
tibial growth disturbance (72,73,74).
In three studies that were reported, 79 extremities were treated using
a brace. The best results were obtained with unilateral deformity,
where brace treatment was successful in 22 of 23 patients. In contrast,
brace treatment was less successful for treating bilateral deformities,
with only 18 of 28 patients noted to be successfully treated.
Compliance was much more difficult to achieve for the child with
bilateral deformity, as is understandable. It is also less effective in
obese patients. Bracing should not be initiated after 3 years of age,
nor should brace treatment be continued if Langenskiöld stage III
changes develop (72,73,74).
 |
|
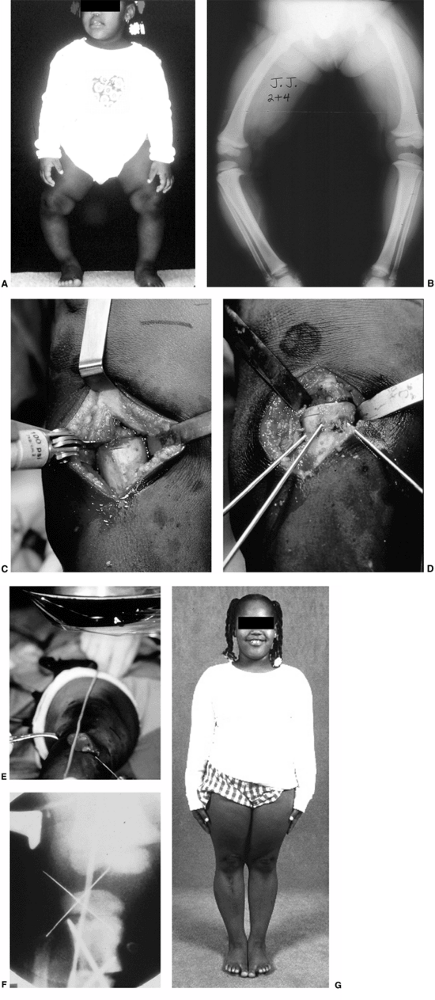
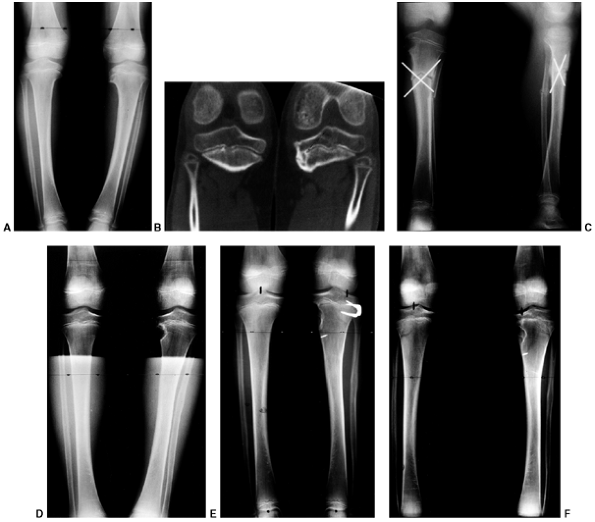
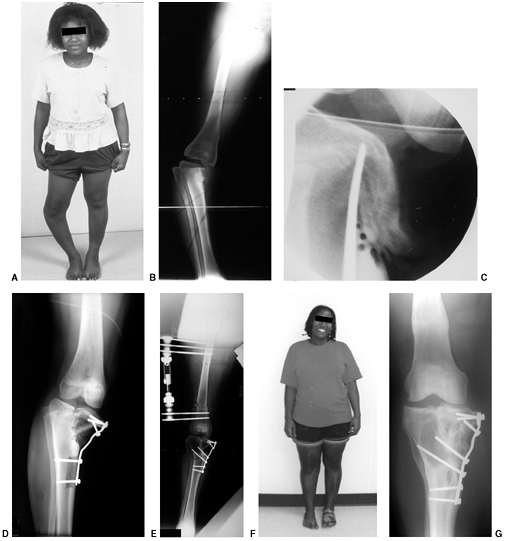
Figure 28.13 A: This 30-month-old girl shows clinically asymmetric bowing. She is large for her age (>95% weight). B:
The metaphyseal-diaphyseal (MD) angle on the right is 20 degrees, compared to 10 degrees on the left. This is consistent with stage II changes of Blount disease on the right and physiologic bowing on the left. C: A transverse osteotomy is performed distal to the tibial apophysis. An appropriately sized wedge is removed to allow slight overcorrection. D: Smooth Kirschner (K) wires are used for fixation, supplemented with cast immobilization. E: Clinical alignment can be assessed using a bovie cord, which is visualized radiographically. The leg should be allowed to rest in its neutral position. F: Intraoperative films of a right proximal tibial osteotomy show slight overcorrection to valgus. A bovie cord centered over the hip and ankle is an easy method to assess mechanical axis intraoperatively. G: A clinical photo taken 2 years later shows maintenance of correction on the right. Spontaneous correction of physiologic bowing has occurred on the left. |
The locked KAFO counteracts the pathologic medial compressive forces,
allowing resumption of more normal growth and correction of the genu
varum. The bowleg deformity typically improves over the ensuing months.
The pathologic radiographic changes at the proximal medial tibial
metaphysis, physis, and epiphysis are slow to remodel. Brace treatment
is continued until the bony changes in the proximal medial tibia
resolve; typically, this takes 1½ to 2 years of brace treatment (72,73,74).
Sustained, successful correction with nonoperative treatment requires
that correction be achieved before 4 years of age. Appropriate
alignment means that the mechanical axis of the lower extremity passes
through the center of the knee (28,48,49,50,53).
either noncompliant or not good candidates for brace treatment because
of obesity or bilateral involvement, should be treated with a
varus-correcting osteotomy (50,72).
The proximal tibial varus should decrease within 12 months in those
children who are compliant with bracing. The radiographic appearance of
the medial epiphysis and metaphysis should normalize by 5 years of age.
If such improvement does not occur, varus-correcting osteotomy should
be recommended. Early surgery to realign the leg (that is, osteotomy
performed by 4 years of age) is necessary to prevent progression to
stage IV disease, which is the formation of a physeal bar. The
osteotomy unloads the medial compartment of the knee and facilitates
growth of the proximal medial physis. Restoration of normal growth in
the medial tibial physis is less likely to occur if surgery is delayed (50,52,53,71,76) (Fig. 28.13 A,B).
Simple osteotomy after 5 years of age does not assure permanent
correction and carries a higher risk of recurrent deformity because of
the greater pathologic change and potential for physeal bar formation.
to the risks related to osteotomy in the upper tibia and the need for
obtaining adequate correction of the
deformity (50,53,85,86).
The fibula is osteotomized through a separate lateral incision, taking
care to avoid injury to the deep motor branches of the peroneal nerve (37). The tibial osteotomy can be accomplished in a variety of ways (50,53,71,87,88,89,90).
A straight transverse osteotomy optimally allows for necessary
adjustment in correction of frontal, sagittal, and rotational
deformity. The fragments are stabilized with smooth K-wires (Fig. 28.13 C,D). Alternatively, Price et al. (91)
have effectively utilized monolateral external fixation to stabilize
the tibial osteotomy. A slight “overcorrection” into valgus with or
without translation of the distal fragment laterally is desirable (50,52,53,92).
This places the mechanical axis of the leg within the lateral
compartment of the knee, optimally unloading the medial proximal tibia.
The mechanical axis of the leg can be assessed intraoperatively using
the bovie cord. The cord is stretched from the center of the hip and
across the center of the ankle with the leg resting on a radiolucent
table. The leg should not be held, but simply allowed to rest on the
table. The wire within the bovie cord, which is visible on the C-arm,
is used to verify the position of the mechanical axis by taking an
anteroposterior spot radiograph of the knee (Fig. 28.13 E,F).
This simple technique provides a reproducible method to verify that the
mechanical axis has actually been transferred lateral to the center of
the knee joint (91). Alternatively, an
intraoperative anteroposterior x-ray film of the entire lower extremity
can be taken to assess the correction obtained. If a unilateral
deformity is present, clinical comparison to the normal extremity is
helpful in determining whether adequate correction has been obtained. A
subcutaneous fasciotomy of the anterior compartment is performed prior
to wound closure. Suction drains are also routinely used.
 |
|
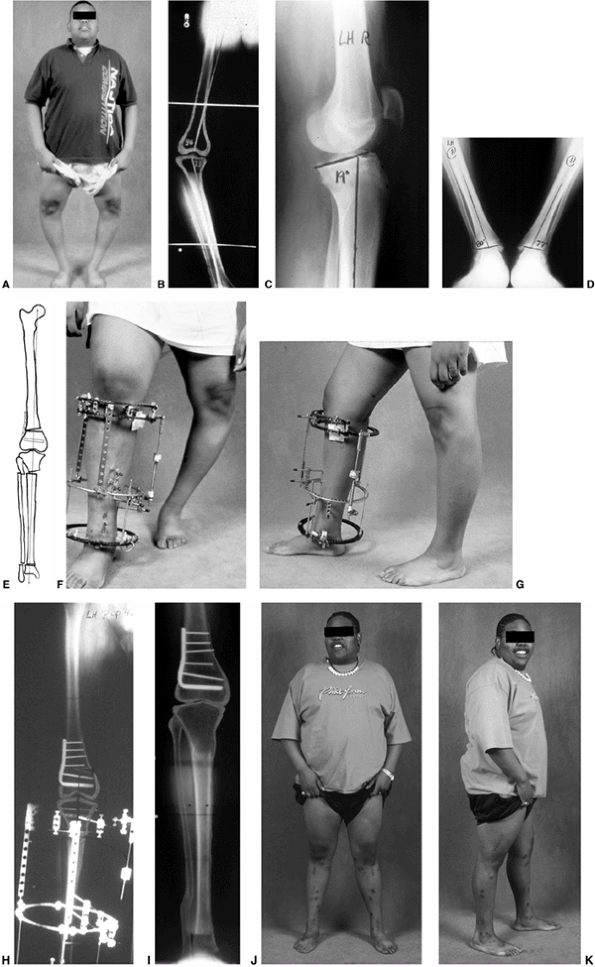
Figure 28.14 Orthotic treatment of Blount disease. A: This 2-year-old girl presented with asymmetric bowing. B: Lower limb radiographs show an increased metaphyseal-diaphyseal (MD) angle with changes of stage II Blount disease. C: A locked knee-ankle-foot orthosis (KAFO) was worn full time. D: Radiographic appearance improved following 18 months of orthotic use. E: Clinical appearance at 4 years of age.
|
non–weight-bearing long-leg, bent-knee cast. Alternatively, a spica
cast is used for the child with relatively short, fat extremities. On
occasion, a KAFO is used following cast removal if the preoperative
deformity was severe and associated with ligamentous laxity. Following
corrective osteotomy, the pathologic changes in the proximal medial
tibia must be carefully monitored. These bony changes are not always
reversible, and further progression in their development may be
associated with a recurrence of deformity (47,50,53).
It is essential to document that valgus alignment has been obtained and
maintained by using long cassette radiographs and comparing serial
examinations until skeletal maturity (Fig. 28.13G).
A careful subperiosteal exposure minimizes direct nerve and vessel
trauma. Prophylactic limited fasciotomy and the use of drains help
prevent increased compartment pressure (37,52,53).
If a compartment syndrome is suspected postoperatively, immediate
fasciotomy should be performed. On the basis of our recent clinical
experience, an acute traction or impingement injury to the peroneal
nerve may also occur (37). Prompt surgical release of any peroneal nerve compression has typically resulted in a satisfactory outcome.
females, children with Langenskiöld stage III or stage IV disease,
marked obesity (>95th percentile weight), or ligamentous laxity (50,52,53,76) (Fig. 28.15).
Worsening varus deformity or persistent pathologic radiographic changes
can occur despite restorative osteotomy performed prior to 4 or 5 years
of age (52,53,76).
Advanced changes in Langenskiöld stage IV or V can occur even in these
young patients, independent of race or body habitus. If patients
present with advanced pathologic changes or with recurrent deformity
following brace or surgical treatment, MRI, CT scan, or conventional
tomography should be utilized to search for the presence of a physeal
bar within the distorted proximal medial tibia anatomy. Identification
of a bar may be difficult because of the serpentine course of this
abnormal physis, which takes on a vertical slope as the deformity
worsens. Early bridging across the physis typically occurs at the
inferior aspect of the vertical limb of the distorted medial physis (Fig. 28.15B).
This occurs rather subtly because of a relatively decreased growth rate
of the proximal medial tibial physis and can occur any time prior to
skeletal maturity. Careful observation is needed to detect this change
early in the course of treatment. Children need regular follow-up until
skeletal maturity. Premature closure of the medial tibial physis
frequently occurs because of persistent abnormal growth in the medial
physis. If this occurs, the varus deformity can be controlled with
temporary hemiepiphyseal stapling (50,97,98). The staples are removed after a slight overcorrection is obtained.
surgery is indicated. Left untreated, progression of bar formation will
result in complete arrest of the medial physis (Langenskiöld stage VI).
Physeal bar resection in conjunction with a varus-correcting osteotomy
will be most effective if the patient is younger than 10 years, the bar
is relatively small, and the patient is not excessively overweight (50,53,71,76,99,100,101).
longitudinal incision and the physeal bar is resected first. Often the
bar is not discreet, but is a collection of several small punctate foci
of bone that coalesce and function as a tether. The medial edge of the
normal physis is often difficult to locate. Excision of the bony bridge
is done cautiously, preserving as much normal physeal tissue as
possible. However, resection must be complete so that an intact physeal
line coursing 180 degrees from the posteromedial
to
the anteromedial cortical edge of the tibia is defined. The C-arm can
be helpful in monitoring the procedure. Methylmethacrylate
(Cranioplast) is used to fill the void to inhibit formation of another
osseous tether. Varus-correcting osteotomies of the tibia and fibula
are then performed (Fig. 28.15C).
Alternatively, a temporary lateral hemiepiphyseal staple can be used in
conjunction with the medial epiphysiolysis. This is most applicable if
the varus deformity is mild, the patient is not obese, and there is no
lateral thrust of the knee.
 |
|
Figure 28.15 A:
These anteroposterior radiographs show focal changes of unilateral stage IV Blount disease. The medial tibial physis is indistinct. These changes are suggestive of physeal bar formation. B: A computed tomography (CT) scan shows the deformity in the medial physis. The growth plate has a vertical rather than horizontal orientation. A bridge of bone is clearly evident here. Varus will rapidly recur following osteotomy if the physeal bar is not recognized and treated. C: These postoperative films show correction of the varus and resection of the bar. The defect created by excision is filled with radiolucent methylmethacrylate (Cranioplast). D: Subsequent films show recurrent bar formation with gradual loss of correction over 2 years. E: A second excision of the physeal bar along with lateral physeal stapling has resulted in improved alignment. F: Following removal of the staples, varus has gradually recurred. The abnormal medial physis tends to close prematurely. |
Continued normal growth in the medial physis generally does not equal
growth of the lateral physis. There is a risk of retethering or
premature medial physeal closure. A second bar resection and varus
corrective osteotomy can be performed for small
recurrent bars in younger patients (Fig. 28.15D).
If lateral hemiepiphyseal stapling has been done, the patient requires
close follow-up for the appropriate timing of staple removal (Fig. 28.15 E and F).
in obese or older patients, a permanent lateral epiphysiodesis of the
tibia and fibula is indicated, and is performed in conjunction with
osteotomies to correct depression of the medial plateau and any
residual varus, as described in the next section (50,53,71,76).
A potential for leg-length inequality exists with any of the above
approaches. If anticipated, it often can be corrected with an
appropriately timed contralateral epiphysiodesis or with lengthening of
the tibia.
irreversible changes in the medial tibial physis. The architecture of
the proximal tibia is distorted, including the medial tibial condylar
surface (Fig. 28.16). Typically, they are seen
in children older than 10 years, yet these changes may be seen as in
patients as young as 6 years. The proximal tibial varus deformity is
characterized by severe depression of the medial tibial plateau, often
with ligamentous laxity. Compensatory distal femoral valgus deformity
may develop (68,83,90).
In severe cases, the tibia will be subluxed medially on the femur. Left
untreated, degenerative arthritis is likely to occur early in life (103,104).
For a lasting satisfactory outcome, the surgical goal is to correct
both the abnormal limb alignment and the pathologic depression of the
medial tibial plateau (Fig. 28.17). This
deformity occurs because the medial physis is closed or so extensively
involved that epiphysiolysis, as in stage IV disease, will not restore
proximal tibial growth (71).
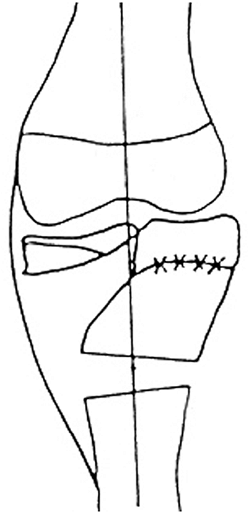
is a combination of medial tibial plateau elevation and realignment
osteotomy of the proximal tibia (Fig. 28.18 A,B). If significant distal femoral valgus is present, osteotomy of the distal femur is performed as well (47,68,71,92,105,106,107).
The proximal medial tibia should not be approached subperiosteally.
Rather, soft-tissue attachments to the proximal medial tibia need to be
preserved to minimize devascularization of the medial tibial condyle
following the plateau-elevating osteotomy. A medial parapatellar
arthrotomy is usually performed to visualize the articular surface of
the tibia. The posterior neurovascular structures are at risk and are
protected by placing a curved retractor between the neurovascular
structures and the tibia. A drill bit or osteotomes are used to
complete the curved osteotomy (Fig. 28.18C).
The osteotomy is begun distally, starting at the apex of angulation in
the proximal medial metaphysis, and curves proximally toward the tibial
eminence. Intraoperative radiography or fluoroscopy is used to assure
that the plane of the osteotomy is directed superolaterally in order to
bisect the tibia intercondylar eminence. The depressed tibial condyle
must be sufficiently elevated to restore the articular surface. A
smooth laminar spreader is helpful in maintaining elevation of the
medial tibial plateau while the bone grafting and internal fixation is
completed. Autogenous graft is preferred. A segment of fibula obtained
at the time of fibular osteotomy or adjacent tibial cortex can be used.
Alternatively, a cortical iliac crest graft may be used. Fixation may
be difficult. The elevated fragment is small, and fixation must not
disturb the articular cartilage (Fig. 28.18D). The correction obtained following plateau elevation typically restores the tibial condylar angle to a near normal value (105).
A concomitant epiphysiodesis of the lateral proximal tibia and proximal
fibula is always done because there is no growth potential remaining in
the medial physis.
 |
|
Figure 28.16
Stage V and stage VI Blount disease are complicated by depression of the medial tibial condyle. The physis has a vertical orientation. This marked growth disturbance is the result of physeal bar formation seen at the junction of the normal horizontal physis and the depressed medial plateau. Insufficient normal physis remains for growth of the medial physis to be restored by resection of these large physeal bars. |
 |
|
Figure 28.17
Restoration of normal limb alignment requires complex reconstruction. An arcuate osteotomy is performed to restore the tibial condyle to its normal position. A proximal lateral hemiepiphysiodesis is performed to prevent recurrent deformity because the medial physis is no longer functional. A valgus osteotomy of the proximal tibia is also usually needed to restore a normal mechanical axis and orientation of the proximal tibia. |
 |
|
Figure 28.18 Treatment of stage V Blount disease. A: Severe deformity and a lateral thrust are noted clinically. B:
Blount disease that progresses to physeal bar formation results in severe depression of the medial metaphysis. Valgus may develop in the distal femur because of overgrowth of the medial femoral condyle. Restoration of medial physeal growth is not possible. C: Image intensification is useful to control the direction of the medial elevation osteotomy. The cut begins at the apex of deformity in the medial cortex and is completed between the tibial spines. D: A cortical strut is used to support the elevated plateau. E: Osteotomy of the tibia or femur, or both, is performed to correct residual tibial varus or femoral valgus. F: Normal anatomic and mechanical alignment can be achieved with this approach. Residual limb-length inequality can be managed by contralateral epiphysiodesis if needed. G: Radiographic appearance after healing of the osteotomies shows restoration of joint orientation and mechanical alignment. |
correction of the preoperative varus deformity. However, correction may
be incomplete, and a proximal tibial varus-correcting osteotomy is
often needed to restore normal alignment to the tibia. Occasionally a
distal femoral osteotomy may also be required to correct valgus
deformity. Alternatively, a hemiepiphyseal stapling of the distal
lateral femur can be used for gradual correction. The surgical goal of
this comprehensive approach is correction of all components of the
deformity, including medial tibial plateau depression, asymmetric
proximal tibial growth, varus of the tibia, and valgus of the distal
femur (Fig. 28.18E). By addressing all aspects
of the deformity, both joint orientation (relation of the knee and the
ankle) and alignment of the extremity (mechanical axis of the limb
bisects the center of the knee joint) can be normalized (Fig. 28.18 F,G).
limb-length inequality may remain. This may be managed by
epiphysiodesis of the contralateral limb, or in the case of a more
severe discrepancy, a combination of lengthening and shortening may be
required to equalize limb length. Davidson (92)
has utilized a circular external fixator to stabilize the elevated
plateau fragment and perform a gradual correcting osteotomy of the
proximal tibia. Use of the external fixator provides the option of
lengthening, in addition to deformity correction of the proximal tibia.
to concomitantly elevate the medial tibial plateau and perform a
varus-correcting proximal tibial osteotomy. The medial proximal tibia
is more prominent and elongated following the plateau elevation. Wound
closure may be compromised and needs to be performed as a delayed
closure. In a series by Schoenecker et al. (105),
3 of 22 patients treated by this comprehensive approach experienced
wound healing complications. In two patients, eventual wound healing
occurred with local care, and one required operative repair with
subsequent satisfactory secondary wound healing.
 |
|
Figure 28.19
A 13-year-old boy with adolescent Blount disease. As is often seen in this group of patients, he is morbidly obese. The large thigh circumference in such patients contributes to the deformity and increased load across the medial distal femur and proximal tibia. |
to perform a tibial plateau elevation also increases the risk of
avascular necrosis of the medial tibial condyle. This occurred in 1 of
22 tibial plateau elevations. Satisfactory revascularization and
reossification occurred in this morbidly obese 8-year-old child
following a 1-year period of non–weight bearing.
group of patients who developed varus deformity of the tibia in later
childhood or early adolescence. He described this deformity as
adolescent tibia vara (Fig. 28.19). Typically,
these children present for evaluation of bowlegged deformity that
develops later in childhood. They are usually overweight, sometimes
morbidly so (108,109,110,111).
The varus deformity in this group of patients with late-onset or
adolescent Blount disease typically involves both the proximal medial
tibia and the distal femur. In contrast, children with deformity from
persistent early–onset or
infantile
Blount disease have varus of the proximal tibia only. There should be
no confusion in differentiating patients who present as adolescents
with Blount-like changes from those who have had varus deformity since
infancy. Patients in this latter group will typically have advanced
pathologic changes involving the proximal medial tibia by later
childhood. Children with infantile Blount disease develop advanced
pathologic changes in the proximal medial tibia, as described by
Langenskiöld, whereas those with juvenile or adolescent Blount disease
develop varus without medial joint depression. Distal femoral deformity
is also more common in these older children (68).
patients with characteristically wide thighs; however, there are
exceptions (77,78,108,109,110,112).
Obesity potentiates the occurrence of adolescent Blount disease because
of the increased load across the medial compartment of the knee (108,109,112,113).
The varus develops after 9 to 10 years of age and often involves both
the proximal tibia and distal femur. Increased thigh circumference
makes it difficult for these children to keep their center of mass over
the weight-bearing foot during the single stance phase of gait. Their
extreme weight adds to an already increased load on the medial knee
joint and promotes development of the varus deformity.
examined the gait deviations that compensate for the increased thigh
girth associated with obesity. During ambulation, individuals tend to
minimize the horizontal displacement of their center of mass by
positioning the foot during single leg stance as centrally as possible
along the line of progression. This optimizes energy expenditure during
normal gait. An obese individual, with large thighs, has difficulty
adducting the hip adequately in order to allow placement of the foot
along the line of progression. Davids speculated that this “fat thigh
syndrome” produces a varus moment on the knee that leads to increased
pressure on the medial proximal tibial physis and inhibits growth in
accordance with the Hueter-Volkmann principle (77).
This work supports the observation that preexisting varus of the knee
is not necessary to initiate the pathologic mechanical changes that
result in adolescent tibia vara. The incidence of adolescent Blount
disease has markedly increased in the past 20 years corresponding to
the development of earlier and more severe adolescent obesity (109,116).
varus. They are older than those with infantile Blount disease and
generally are not morbidly obese. In retrospect, these are children
with mild bowing that never resolved. They do not develop the
pathologic changes seen in infantile Blount disease. The varus
deformity in these children becomes more apparent during the rapid
growth of early adolescence (108,109,110,111).
aberrations throughout the entire physis; however, the medial physis is
more affected than the lateral physis (110). The histologic changes are very similar to those found in infantile Blount disease and SCFE (110,111).
In adolescent Blount disease, the radiographic changes in the epiphysis
and metaphysis are less apparent compared to infantile Blount disease,
because the secondary ossification center is larger and better
established in these older patients (92,93).
affects the posteromedial physis and initially produces varus, followed
by progressive procurvation of the proximal tibia (50,83,110).
Although the name adolescent tibia vara would suggest that varus of the
proximal tibia is the only deformity present, distal femoral varus
deformity is common, because that physis can also undergo excessive
loading (83,110,116) (Fig. 28.20 A,B,C).
This is in contradistinction to infantile tibia vara in which the
distal femur is either normal or in valgus. The in-toeing noted in
adolescent Blount disease is generally less severe than it is in
infantile tibia vara. The combination of varus, procurvatum, and
internal rotation results in a complex three-dimensional deformity of
the proximal tibia. As the proximal tibial and distal femoral varus
deformities increase, there is a significant strain placed on the
lateral collateral ligament of the knee, which leads to laxity and
varus deformity within the knee joint. In some very severe cases,
compensatory distal tibial valgus develops to allow the patient to
place the foot flat on the floor (Fig. 28.20D).
Although the natural progression of advanced infantile Blount disease
is the formation of a medial proximal tibial physeal bar, discreet
physeal bar formation is not noted in adolescent Blount disease.
However, early closure of all lower extremity physes can occur, perhaps
because of overload related to obesity.
obese male who presents with complaints of bowing, often with knee pain
and/or instability (109,110,112,117,118).
The patient should be assessed while walking both toward and away from
the examiner, who should note gait mechanics and, specifically, the
presence of a limp or lateral thrust to one or both knees. Although
unilateral complaints are more common, attention should be paid to both
limbs because the patient’s obesity can mask mild bowing on the
contralateral limb (108,112,117).
Knee stability is assessed because lateral collateral ligament laxity
can occur. Proximal tibia procurvatum deformity may occur, producing a
relative knee flexion deformity. The patient may have anterior knee
pain secondary to holding the knee in a flexed position during gait.
Patients complain of medial knee pain secondary to medial knee joint
stress. The hips should also be examined for evidence of SCFE (110,114,115). Morbidly obese patients presenting with adolescent Blount disease may have varying amounts of respiratory distress. The walk
from the waiting room to the examination area can be quite taxing to
these patients. Sleep disorders related to apnea are also common.
 |
|
Figure 28.20 A: Adolescent Blount disease frequently occurs in very large teenagers. The deformity is often bilateral. B:
Long cassette films are used to assess mechanical alignment as well as the anatomic axes of the femur and tibia. Distal femoral deformity is often present as well as proximal tibial varus. C: Procurvatum of the proximal tibia develops, with increased posterior slope of the proximal tibia. D: Distal tibial valgus develops to allow the foot to have flat contact with the floor. E: Restoration of normal alignment may include multilevel osteotomies. Preoperative templates are useful for planning operative strategies. F: In this example, the plan included immediate correction of distal femoral varus using a blade plate and gradual correction of proximal tibial varus and distal tibial valgus using a circular small wire frame. G: Multiplane correction is facilitated with this technique. Adjustments can also be made to correct the procurvatum that may be present. H: The circular frame provides flexibility. It also allows lengthening as needed in cases of unilateral or asymmetric deformity. I: Radiographs confirm the restoration of alignment. Correction is generally well maintained. J: Clinical photo after bilateral treatment shows satisfactory clinical correction compared to the preoperative photo. K: Correction of procurvatum restores normal orientation of the knee. |
extremities is essential for evaluating the patient with adolescent
tibia vara (Fig. 28.20B). Most adolescent
patients require a 51-in (129.5 cm) cassette. Simultaneous satisfactory
visualization of the hips through an abundance of soft tissue as well
as the ankles through a relative paucity of soft tissue can be
difficult. Care should be taken when positioning the patient to ensure
that the knees are straight ahead with the patella centered. This is
particularly difficult in large patients in whom the palpation of bony
landmarks is uncertain. Radiology technicians who are not experienced
in obtaining these radiographs frequently compensate for the inability
to identify the patella by simply turning the feet straight ahead.
Because of the internal tibial torsion, this produces external rotation
of the knees and an inadequate radiograph for accurate assessment of
bony deformity. Occasionally, because of the width of the patient or
the patient’s inability to sufficiently rotate the hip internally, it
may be necessary to obtain separate standing radiographs of each lower
extremity. A true lateral supine radiograph of the proximal tibia is
obtained to evaluate the magnitude of the procurvatum deformity. The
radiograph should be centered on the knee and the film positioned so
that a significant portion of the tibial diaphysis can be visualized.
Finally, with the feet positioned straight ahead, a standing
anteroposterior radiograph of both ankles is obtained.
Initially, the mechanical axis deviation should be measured using a
line drawn from the center of the femoral head to the center of the
ankle (Fig. 28.21). The surgeon should also
look for a limb-length discrepancy at this point. Both the lateral
distal femoral angle and the medial proximal tibial angle should be
measured to evaluate the frontal plane deformity of both the distal
femur and the proximal tibia. It is incorrect to assume that the distal
femur is normal because the knee joint appears to be parallel to the
floor (28,68). The genu
varum produces relative abduction at the hip and can mask a significant
femoral deformity. The radiograph of the knee in the weight-bearing
position must be examined to assess the presence of significant lateral
collateral laxity and an increased joint line congruency angle (the
angle formed by an intersect of two lines, one drawn parallel to the
distal articular surface of the femur and one parallel to the proximal
articular surface of the tibia). Likewise, the lateral view of the knee
should be evaluated to assess the size and location of the procurvatum
deformity (Fig. 28.20C). The presence of compensatory distal tibial valgus is assessed on the anteroposterior ankle film (28,68,121)
of the proximal tibia and distal femur and, occasionally, a secondary
valgus deformity of the distal tibia. The goal of surgery is to restore
normal anatomical orientation of the knee and ankle joint and a normal
mechanical axis of the
leg (91,95,112,116,117,119,120,121).
For a unilateral deformity, leg-length inequality also needs to be
addressed. Osteotomy is the definitive treatment of adolescent Blount
disease. However, depending on the patient’s age, limb realignment can
be attained on occasion by staple hemiepiphysiodesis or, more likely,
by a combination of hemiepiphysiodesis and osteotomy.
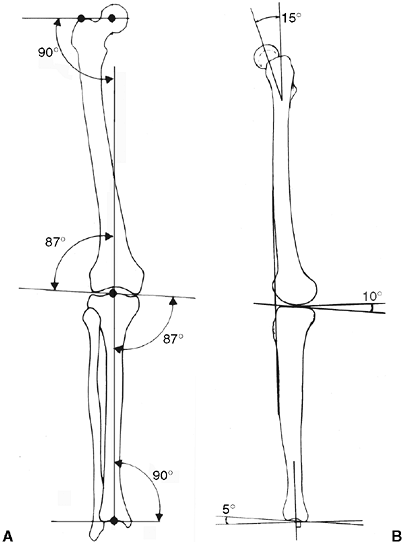 |
|
Figure 28.21 A:
Frontal plane mechanical axis of the lower extremity consists of two components: colinear centers of the femoral head, knee joint, and ankle joint; and an almost perpendicular relation of the hip, knee, and ankle joints’ orientation lines to the mechanical axis. B: Normal sagittal plane mechanical axis and joint orientation lines. |
appropriate, detailed, preoperative plan is essential to the hope of
obtaining a mechanically well-aligned limb (Fig. 28.20E).
The magnitude and location of the various bony deformities, the
presence of soft-tissue laxity at the lateral collateral ligament, and
the presence of joint contractures and leg-length discrepancy should
all be assessed and incorporated into an overall plan for addressing
the deformity.
obese, a thorough evaluation of their cardiopulmonary system is
essential prior to any consideration of operative treatment. The
extreme size of these patients can lead to nocturnal hypoxia with
significant decreases in sleeping O2 levels and accompanying hypercarbia (122). If these changes are prolonged, significant pulmonary hypertension can result, leading to right-heart hypertrophy (122).
If symptoms such as marked snoring or irregular breathing patterns at
night are present, sleep studies with pulmonary and cardiology
evaluations may be indicated prior to a general anesthesia.
indicated if the growth plates are still open and the varus deformity
is not too severe (69,117,123) (Fig. 28.22 A,B).
Staple hemiepiphysiodesis is preferred. Two to three “Blount staples”
(Zimmer, Inc., Warsaw, IN, USA) with reinforced corners are placed
extraperiosteally at the lateral distal femoral physis, the lateral
proximal tibial physis, or the medial distal tibial physis depending on
the site of deformity (50,97).
Once hemiepiphyseal stapling is performed, it is critical to follow up
the patients closely with clinical and radiographic examinations to
monitor the correction obtained and possible staple displacement and/or
breakage. If complete deformity correction is obtained, staple removal
may be necessary to prevent overcorrection. Relative rebound varus
growth is unpredictable in this clinical setting (97).
Complete epiphyseodesis may be preferred to avoid loss of correction by
rebound. Hemiepiphyseal stapling has resulted in reduction of deformity
or arrest of progression of the proximal tibial or distal femoral
deformities in some of the patients who had at least 15 to 18 months of
growth remaining. This simple technique may obviate the need for
subsequent femoral and sometimes tibial varus-correcting osteotomy (Fig. 28.22 C,D).
The adolescent who may not be a good candidate for staple
hemiepiphysiodesis is one with a history of progressive varus deformity
and knee joint pain. A dynamic lateral thrust is usually present in the
patients who have knee joint laxity. Hemiepiphyseal stapling does not
allow rapid enough resolution of the bony deformity or correction of
the ligamentous laxity that is present.
disease will require an osteotomy of the proximal tibia, and sometimes
the distal femur as well, to achieve restoration of a normal mechanical
axis and joint orientation. In skeletally mature or nearly mature
patients, osteotomy is indicated if the mechanical axis of the lower
extremity is deviated medially to the center third of the knee joint.
The extent of deformity in both the proximal tibia medial angle (PTMA)
and distal femur lateral angle (DFLA) is determined (28,124).
In addition, the sagittal plane alignment is assessed and procurvatum
deformity noted. All patients will require a proximal tibial
varus-correcting osteotomy, ideally restoring a normal anatomical
alignment of the proximal tibia. Regardless of the method chosen, the
proximal tibial deformity should be completely corrected by the
redirectional osteotomy including varus, procurvatum, and internal
rotation.
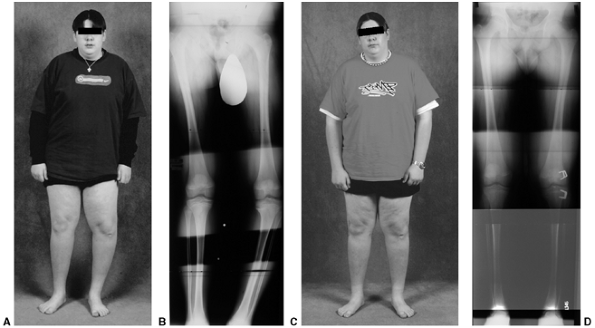 |
|
Figure 28.22 A: Clinical photo of a teenager with unilateral adolescent Blount disease. B:
Long cassette radiograph demonstrates varus deformity in the distal femur and proximal tibia in a skeletally immature individual. C: Clinical appearance following correction. D: Hemiepiphyseal stapling was used. Correction is noted 1 year after staple insertion in the lateral distal femur and proximal tibia. This technique is optimal in mild to moderate deformities where 1 to 2 years of growth remain. |
with a concomitant femoral varus-correcting osteotomy is indicated if
the DFLA measures more than 5 degrees varus (normal DFLA is 87 degrees)
(68,121). If there is
sufficient growth remaining, the distal femoral deformity may be
amenable to hemiepiphyseal stapling. If a distal femoral osteotomy is
performed, the transverse cut can be made through the physeal scar in
skeletally mature patients or proximal to the physis as in the
skeletally immature. This allows correction closest to the anatomic
site of the deformity. A blade plate is used for fixation.
and is assessed on the anteroposterior ankle radiograph. Hemiepiphyseal
stapling is used if there are 5 degrees or more of distal tibial valgus
and the physis is open. If the physis is closed and more than 8 degrees
of valgus is present, an osteotomy is performed.
of treatment of infantile Blount disease, is generally not recommended
in adolescent Blount disease. A supplemental long-leg cast often adds
minimal additional protection for these extraordinarily large patients.
The difficulties associated with non–weight bearing on the affected
extremity make ambulation difficult in these patients. Outcome of
treatment with this approach has far too often been unsatisfactory (112).
internal fixation, can be effectively utilized in patients with
mild-to-moderate varus and procurvatum deformity (50,125,126).
Because the deformity occurs at the level of the physis, a transverse
osteotomy of the tibia in the metaphysis distal to the tubercle must be
translated laterally, sometimes as much as the entire diameter of the
tibia in order to prevent creating an offsetting deformity. To
circumvent this deformity, Millis (125) has
utilized an oblique, laterally based, closing wedge osteotomy in
skeletally mature patients which hinges at the intact cortex just
distal to the proximal tibial physeal scar (127) (Fig. 28.23).
Fixation is achieved using a laterally placed compression plate that
serves as a tension band and permits weight bearing without external
immobilization. As the hinge point is near the physeal scar, the
correction is achieved at the level of the deformity and a
translational deformity is not created.
correction of severe deformities. The dynamic axial monolateral fixator
has the advantages of ease of achieving an acute correction,
adjustability after the initial surgical correction, and relative
patient acceptance (127). Price et al. obtained satisfactory stability of the osteotomy fragments even in heavier patients (91). In applying a monolateral fixator of any type, pin placement
parallel to the knee and ankle joint aids in obtaining a satisfactory acute correction of deformity (127).
Similarly, Gaudinez et al. and Stanitski et al. have effectively
incorporated the Garche clamp when utilizing a monolateral fixation in
the treatment of Blount disease in adolescents (128,129).
The Garche external fixator optimizes fixation and allows for
postoperative frontal plain adjustment and lengthening. It is, however,
not conducive to postoperative adjustments in the sagittal plane or in
rotation (129).
gradual correction of the proximal tibia; it allows for the maximal
adjustability of the alignment in all planes and is ideally applicable
in the most severe deformities and more obese patients (95,121,130).
Advantages include stable fixation with improved patient mobility, the
ability to evaluate alignment in a functional, standing position, and
the ability to correct accurately all of the tibial deformities
including proximal tibial varus and procurvatum, internal tibial
torsion, and distal tibial valgus (121,129,130).
A hybrid circular fixator such as an Ilizarov or Taylor spatial frame
can be used. The latter device is our preferred method of fixation (Fig. 28.20 F,G).
single surgical procedure. If indicated, the distal femoral varus is
acutely corrected first. The distal femoral deformity may be addressed
by supracondylar osteotomy and stable fixation of the distal femur
using a blade plate to maintain alignment. This may be performed
through a medial or lateral approach, with an opening or closing wedge
osteotomy technique. A lateral approach is preferred for performing an
opening wedge osteotomy of the distal femur; the fragments are fixed
with an adult 95-degree condylar blade plate.
posterolateral incision over the midshaft of the fibula at least 10 cm
distal to the fibular head. Great care should be taken in obtaining
subperiosteal exposure of the fibula to avoid injury to the branches of
the deep peroneal nerve, the extensor hallucis longus, or the peroneal
vessels, which lie just medial to the fibula. A 1-cm section of the
fibula is removed and used as bone graft for the distal femoral
osteotomy if needed.
leg and applied with a combination of transfixing wires and half-pins.
Ring strategy and placement of transfixing wire vary depending on the
presence of open growth plates, the need for fibular transfer to
correct ligamentous laxity, and the need for distal tibial
valgus-correcting osteotomy (121,129).
The proximal tibial osteotomy is performed through a limited incision,
using drill bits and osteotomes. If indicated, a distal tibial
osteotomy is created in an identical fashion.
three, and serial radiographs are obtained to monitor the correction
and bone formation (Fig. 28.20H). The patient
is encouraged to undertake full weight bearing as early as possible,
and vigorous physical therapy is instituted to maintain mobility and
joint range of motion. Adjustments in the circular frame are made as
necessary to correct all planes of the deformity. The fixator is left
in place and progressively dynamized until consolidation and
cortication of the osteotomy site is complete. Bilateral deformities
are corrected one side at a time. Correction of the second extremity is
usually planned within 6 months of completion of the first side. This
comprehensive approach has provided very satisfactory results in this
most difficult group of patients and has been a marked improvement over
traditional methods of treatment (112,129,131) (Fig. 28.20 I,J,K). There have been very few surgical complications in these patients despite their extremely large size (121,130).
All osteotomies, including those that included lengthening, have healed
without delay. The knee and ankle joints have been realigned and the
normal mechanical axis of the leg restored. Knee pain has resolved.
Patients are satisfied with their more normal lower-extremity alignment.
Physiologic knock-knee generally becomes a concern when the child is
between 3 and 5 years of age, and the normal femoral-tibial angle is at
its maximum valgus angle (5,132,133) (Fig. 28.24).
Parents often notice the flat appearance of the foot before the valgus
knee position is noted. There may be occasional complaints of medial
foot or medial knee pain.
years of age, reaching a maximum femoral-tibial angle of 8 to 10
degrees at approximately 3 to 4 years of age, a time when it is most
noticeable (2,5,49).
As demonstrated by Salenius and Vankka, valgus gradually decreases to a
stable “adult” level of 5 degrees to 7 degrees of femoral-tibial valgus
angulation by 6 to 7 years of age (Fig. 28.6).
There is, however, wide variation among normal children, that is, those
who fall within two standard deviations of the mean. This range
includes measurements of ±8 to 10 degrees, which means that normal
femoral-tibial angles may range from 2 degrees of varus to 20 degrees
of valgus at 3 to 4 years of age and neutral to 12 degrees of valgus
after 7 years of age (49,132,133).
The severity of the deformity can easily be assessed and documented by
standing photographs. Radiographic evaluation is indicated in those
children with clinically excessive femoral-tibial angles, those who
present outside of typical age range for physiologic valgus, those with
asymmetric deformity, or those who fall below the tenth percentile of
height.
from persistent knock-knee in a young child, but more often develops
during early adolescence (Fig. 28.25).
Typically, the deformity arises from asymmetric growth in the distal
femur and does not spontaneously resolve. In some cases, the proximal
tibia is also abnormal.
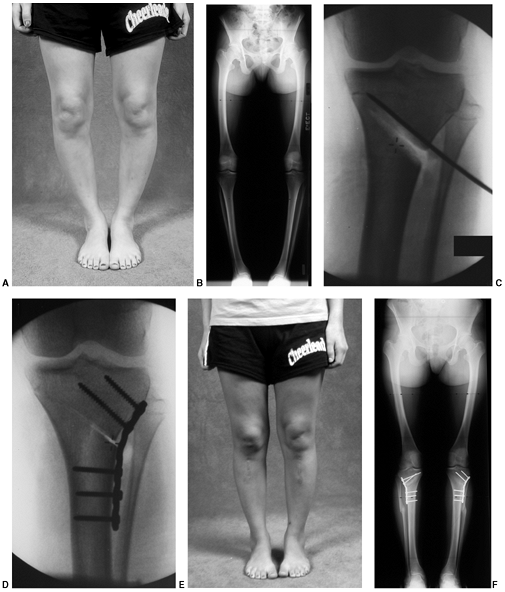 |
|
Figure 28.23 A:
There is a subgroup of patients with adolescent Blount disease who are not obese. Deformity is typically bilateral and mild to moderate. B: Radiographs in this nearly skeletally mature girl demonstrate deformity only in the proximal tibia. C: The oblique osteotomy directed toward the physeal scar. D: A laterally based wedge is removed, and the osteotomy fixed with a “tension band” plate. E: Clinical photo after bilateral correction. F: Bilateral lower extremity radiographs following osteotomy. |
 |
|
Figure 28.24
Physiologic genu valgus peaks between 3 and 4 years of age. It may be associated with asymptomatic flat feet. It is typically symmetric and shows gradual resolution by 8 years of age. |
If the onset of rickets (osteomalacia) occurs when physiologic valgus
is present, a knock-knee deformity is more likely to develop. Valgus
may result from overgrowth of the proximal medial tibia following a
proximal tibia fracture (Cozen fracture) or from an injury to the
distal lateral femoral physis (137,138,139).
Skeletal dysplasias most typically associated with genu valgum are
chondroectodermal dysplasia (Ellis–van Creveld), mucopolysaccharidosis
type IV, and spondyloepiphyseal dysplasia tarda (136).
Benign neoplastic processes such as multiple hereditary exostoses and
focal fibrocartilaginous dysplasia may also produce a valgus deformity (140).
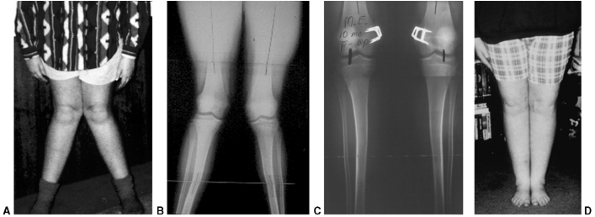 |
|
Figure 28.25 A:
Lower limb valgus that persists past 8 years of age is not physiologic. It may cause an awkward gait, in addition to concerns about appearance. B: These long cassette films of a 12-year-old girl confirm the presence of valgus in both the femur and tibia. She is not skeletally mature, making hemiepiphyseal stapling a treatment option. C: Stapling of the medial physis of both distal femurs and tibiae in a growing adolescent results in rapid correction. Correction of the mechanical axis is achieved in this patient within a year. D: There is marked clinical improvement in alignment. Correction is generally well maintained. |
early adolescence. Knee pain is a common feature. The clinical
deformity is often more striking than the radiographic appearance. It
may be associated with an out-toed gait. Lateral patellar subluxation
may develop, but is uncommon. Many of these children are above the 90th
percentile in height and weight. Walking may become awkward because of
the knees rubbing or hitting together as the child tries to narrow the
base of support. This degree of deformity is not physiologic and
typically does not resolve on its own. Valgus of the knee which
increases after 7 years of age is not physiologic. This genu valgum is
a pathologic state and often requires surgical treatment (140,141).
|
TABLE 28.2 DIFFERENTIAL DIAGNOSIS OF KNOCK-KNEES
|
||
|---|---|---|
|
of both lower extremities, which includes the hips, knees, and ankles,
is best for assessing the mechanical axis and any deviation in joint
alignment. The lower extremities should be placed so that the kneecaps
are facing directly forward. This must be done without regard to foot
position. This technique produces the truest anteroposterior x-ray film
of the knees and allows for reliable serial comparisons if needed. The
mechanical axis of the lower extremity is drawn from the center of the
hip through the center of the ankle. A normal mechanical axis passes
through the central third of the knee, roughly defined by the tibial
spines, or through zones +1 to -1 where positive values represent
valgus and negative values are varus (28,98,141,142).
portion of the lateral tibial plateau (zone +2 or +3), can result in
relative overload of the lateral compartment (28,142) (Figs. 28.26 and 28.27).
The severity of deformity sufficient to produce premature degenerative
changes in the lateral compartment of the knee, is not known. Genu
valgum that results in mechanical axis deviation beyond the lateral
margin of the tibia is pathologic and warrants correction. In addition
to improving the appearance of lower limb alignment, correction can
restore a normal mechanical axis (138,141,143). Gait analysis has demonstrated abnormal moments about the hip and knee in proportion to the deviation from normal.
parents regarding the natural history of this physiologic condition,
which is anticipation of spontaneous correction by 7 years of age (133). Lower-extremity bracing is not indicated (133).
Long-leg bracing is poorly tolerated in these children, who are
typically older than 4 years. Unlike Blount disease, the deformity may
be present above and below the knee, making bracing less effective.
cal management of the osteomalacia. Genu valgum associated with bone
diseases such as XLH and skeletal dysplasias may worsen with time,
necessitating surgical treatment (see Chapters 7 and 8).
Postfracture valgus deformity also may require treatment, as
spontaneous correction does not always occur. Observation is the
appropriate initial management; however, if the valgus deformity does
not resolve, hemiepiphyseal stapling or proximal tibial osteotomy is
recommended to restore normal limb alignment.
mechanical axis passes through zone 3, that is to say, at or beyond the
lateral cortex of the tibial plateau, or if it passes through zone 2
and knee pain is present. In most cases, surgery can be deferred until
the child is 10 to 11 years of age.
hemiepiphysiodesis, preferably by hemiepiphyseal stapling (97,98,123,141,143,144,145,146,147) (Fig. 28.25C).
Whether medial physeal stapling is performed in the distal femur,
proximal tibia, or both will depend on the location of the deformity
and the amount of growth remaining (123,138,147).
Most often valgus deformity arises primarily from the femur. The
mechanical axis of the femur and tibia and the relative abnormality in
the distal lateral femoral and proximal medial tibial angles should be
utilized to determine the appropriate site for hemiepiphysiodesis (28,125,141).
The technique of stapling, although simple, requires attention to a few
important details to maximize its effectiveness and minimize the
potential for growth-plate injury (97,98,138,144,145).
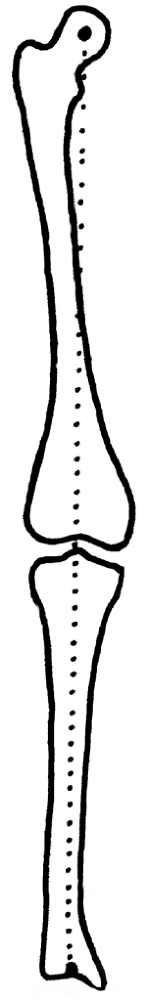 |
|
Figure 28.26
The mechanical axis is assessed on a standing, anteroposterior, long cassette radiograph that includes the hips and ankles. A line is constructed from the center of the femoral head to the center of the ankle. For consistent serial measurements, the knees are positioned with the patellae facing forward. |
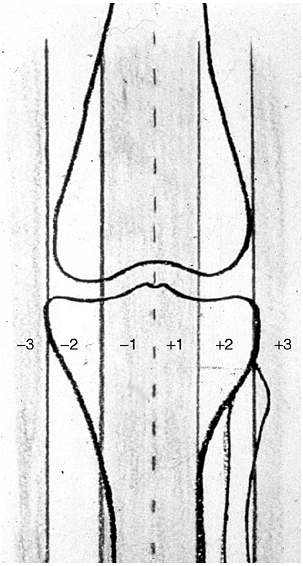 |
|
Figure 28.27
To determine the mechanical axis of the tibia, the proximal tibia is longitudinally divided into four parts. Positive values are lateral to the midline or valgus. Negative values are medial to the midline or varus. Zone 1 is centered over the tibial spines, zone 2 is within the tibial condyle, and zone 3 is beyond the cortex. A normal mechanical axis falls within zone 1. |
of C-arm image intensification is critical in assuring optimal staple
placement. On the anteroposterior or frontal view, the staple prongs
should both span and be parallel to the physis. This may require a
slightly oblique orientation to the cortex. On the lateral view the
staples should be placed centrally (equidistant from the anterior and
posterior edges of the physis) to avoid inadvertent creation of a
sagittal plane deformity. Two staples per location are generally
sufficient. Three may be used if the bone is unusually large. We use
staples made of cobalt-chrome alloy (Zimmer, Inc., Warsaw, IN, USA)
that have reenforced corners and are less likely to break or be
ineffective (143,144).
particularly those with more than 2 years of growth remaining, must
have reliable follow-up. Overcorrection to a varus position can occur
and is not desirable. Long cassette radiographs of the lower
extremities should be obtained at 3-month intervals. Some improvement
in the lower extremity mechanical axis should be apparent 3 to 6 months
after stapling. Once the mechanical axis passes through the central
third of the knee joint, the staples should be removed to avoid
overcorrection into varus unless the lateral physis is closed (Fig. 28.25D).
Following staple removal, rebound medial overgrowth can occur resulting
in some loss of correction. This is more common in children who are
younger than 10 years when stapling is performed (97,143,144,145).
It is unclear how long extraperiosteal staples can safely span a growth
plate without affecting future growth. It has been our practice to
remove staples within 18 to 24 months if resumption of growth is
desired. Stevens has reported resumption of growth following removal of
staples that were across the physis for more than 2 years in patients
with a variety of deformities (98,141).
It has been our experience that in children with XLH or skeletal
dysplasia, staples can safely remain for more than 2 years, and growth
will resume following removal. If rebound overgrowth is a concern and
the possibility of another surgical procedure is not acceptable, a
complete epiphysiodesis can be performed; however, this will result in
limb shortening in proportion to the amount of growth remaining. As the
procedure is usually bilateral and performed close to skeletal
maturity, the absolute amount of shortening is usually not significant.
It may be preferred in very young children with severe deformity such
as valgus associated with a skeletal dysplasia or in those who are
skeletally mature. The site of deformity correction is dependent on the
anatomic deviations present in the tibia and/or femur, just as in the
determination for hemiepiphyseal stapling (125,150,151,152).
The femur is more often the primary site of valgus deformity. In young
children, valgus is corrected using a transverse osteotomy in the
distal femur. Appropriately sized K-wires or a small fragment plate can
be used for fixation and supplemented with a long-leg cast. Immediate
correction of femoral valgus using internal fixation with a 95 degree
condylar blade plate is preferred for older children and adolescents. A
lateral approach to the distal femur is preferred. It also allows
exploration and release of the peroneal nerve, which is sometimes
necessary in severe deformities.
in whom it reduces the risk of peroneal nerve neurapraxia, and for
those with limb-length inequality when lengthening is also needed (42,125,149). Circular frame fixation may facilitate angulatory correction in combination with lengthening (42,148,149). A unilateral frame may be considered when external fixation is used with immediate correction.
the tibia. In young children, correction can be accomplished by simple,
closing wedge technique in the proximal tibia, using two or three
crossed stainless steel wires as described regarding rotational
variation earlier in this chapter (148). In
adolescents and young adults, tibial valgus deformity can be corrected
by a proximal tibial osteotomy that uses a medially based oblique wedge
osteotomy and hinges proximally and laterally near the physeal scar,
analogous to that described in Figure 28.23.
The wedge is carefully removed and the distal medial cortex is
compressed together utilizing a short compression plate to produce a
controlled fracture of the lateral cortex.
potential for injury to the involved growth plate. Errors in technique
can lead to failure with hemiepiphyseal stapling. Poor fixation and
inadequate design or number of staples will compromise results (97,98,144). Timing of placement, follow-up, and removal are sources of error (123,143).
If the stapling is performed too late, there will not be adequate
growth remaining to correct the deformity. Lack of appropriate and
timely evaluation poststapling, resulting in overcorrection, is the
most common serious complication of hemiepiphyseal stapling. The
resultant varus alignment produces greater mechanical loads across the
medial compartment of the knee than the same degree of valgus would
produce over the lateral compartment (50). Increased medial compartment load can accelerate degenerative changes.
premature physeal closure beneath the staple. The length of time
extraperiosteal staples can be left across a growth plate without
permanently affecting growth is unclear. We have used 18 to 24 months
as the upper limit if resumption of growth is desired. The actual limit
may be dependent on the patient’s age at the time of stapling and the
number of staples and their design. In a recent report by Stevens (141), none of his patients had premature growth arrest or rebound when treated with temporary physeal stapling for genu valgum.
union or fixation, infection, blood loss, knee stiffness, and scar
formation. None of these is unique to distal femoral or tibial
osteotomy for valgus correction. Peroneal nerve injury is a serious
concern in the process of valgus correction. Mobility of the peroneal
nerve is limited above the knee as it passes around the distal femur
and across the lateral edge of the biceps femoris tendon and below the
knee as it curves around the proximal fibula and through the crural
fascia (37). More severe deformities may
require release of one or more of these sites to reduce the risk of
permanent injury. Closing wedge technique for immediate correction is
less likely to stretch the peroneal nerve than opening wedge. Gradual
correction of severe deformities may allow the nerve to accommodate to
these changes more safely than does acute correction. Injury to the
nerve must be avoided during pin placement, however.
either anterior, anterolateral, or posterior medial. Anterior tibial
bowing that occurs in association with a deficient or absent fibula is
diagnostic of fibular hemimelia. Posterior medial bowing occurs in
association with calcaneovalgus foot deformity and has a relatively
excellent prognosis. In contrast, anterolateral bowing, which usually
presents soon after birth, is typically a progressive deformity which
often results in a pseudarthrosis. Anterolateral bowing associated with
congenital pseudarthrosis of the tibia is rare (1:140,000), yet it is
the most common type of congenital pseudarthrosis (153,154).
Neurofibromatosis occurs in more than 50% of patients with
anterolateral bowing, with or without pseudarthrosis of the tibia (153,154,155,156,157). This bowing may be the first clinical manifestation of neurofibromatosis (155,156).
Rather, the tibia with an anterolateral bow appears dysplastic, with
failure of tubulation, cystic prefracture, or frank pseudarthrosis, or
a combination of these features, with narrowing of the fragments (153,155). Fracture with resultant pseudarthrosis typically occurs in the first 4 to 5 years of life (Fig. 28.28).
Once established, the natural history of a pseudarthrosis is that of
persistent instability and progressive deformity. Numerous
classification systems have been proposed in an attempt to
prognosticate the natural history and outcome of treatment. In reality,
there has been very little correlation with initial radiograph
classification and eventual outcome of treatment (155,159,160,161,162). The radiographic appearance of congenital pseudarthrosis of the tibia has been classified by Boyd and by Crawford (153,155). Both noted the variable natural history and prognosis of each of the types they described (Table 28.3). Consolidation of the pseudarthrosis was most difficult to obtain in the Boyd type II or Crawford dysplastic type II-C (Fig. 28.29).
full-time brace treatment is indicated. An ankle-foot orthosis (AFO) is
appropriate protection prior to walking, and a KAFO is fit as the
infant begins walking. Orthotic support is continued indefinitely
during the growing years with or without surgical reconstruction of a
pseudarthrosis. Although very uncommon, some forms of anterolateral
bowing, such as Boyd type IV, occasionally do not progress (158).
These unusual patients present with a bowed tibia, with or without a
previous fracture that consolidates with immobilization (158). Supplemental bone grafting may be beneficial (163). These unique patients seem to carry less chance of progression and fracture following the bone graft procedure.
treatment in the past 100 years, congenital pseudarthrosis of the tibia
remains one of the most challenging problems in pediatric orthopaedics.
Even with consolidation, the tenuous status of the atrophic bone
markedly limits functional potential (156,157,158).
A major improvement in outcome can be traced to Boyd’s use of dual
onlay grafts, Moore’s use of staged bone graft and external fixator,
McFarland’s bypass graft, and Sofield and Millar’s innovative use of
intramedullary rod stabilization (164,165,166,167,168,169,170). Less invasive technical innovations were developed by Brighton and Bassett (171,172)
utilizing implanted cathode leads or pulsed electromagnetic current,
either alone or in combination with surgical stabilization.
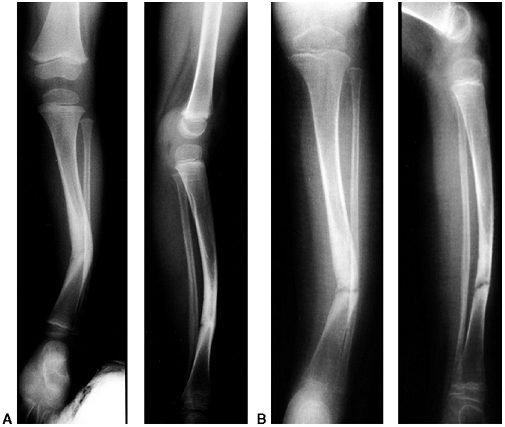 |
|
Figure 28.28 A:
Anterolateral bowing of the tibia may be apparent at birth or may progress with weight bearing. Bowing usually occurs between the middle and distal thirds of the tibia. The adjacent bone may appear sclerotic, with increased density, or it may be atrophic and spindle shaped. Once the deformity is recognized, the leg should be protected with a total-contact orthosis. Although fracture is not likely to be avoided, it may be delayed until the child is bigger. B: Fracture occurs at the apex of the bow, usually without prodromal symptoms and with minimal or no trauma. Once fracture occurs, surgical management begins. |
All techniques include excision of the pseudarthrosis site. Techniques
used to achieve union of the pseudarthrosis include intramedullary
fixation with autogenous iliac crest bone graft, circular ring fixation
with bone transport, and vascularized fibular graft. However, even
after obtaining consolidation with any of these techniques, long-term
follow-up is critical to address associated deformities that frequently
occur later in the course of the disease. These include refracture,
persistent or increasing valgus deformity, and limb-length inequality.
use of an intramedullary rod that both stabilized the pseudarthrosis
site and transfixed the ankle joint (173). In a similar technique, Umber et al. (174) popularized the use of the two-part Peter Williams intramedullary (IM) solid rod in North America (175).
This IM rod technique, in conjunction with pseudarthrosis excision and
iliac crest bone grafting, has been further refined in dealing with all
aspects of the deformity (176,177,178,179).
It is our approach of choice in treating congenital pseudarthrosis of
the tibia. A posterior iliac bone graft is obtained consisting of
adequate corticocancellous strips and cancellous bone graft. The
pseudarthrosis is excised and the bone fragments stabilized with a
Williams rod (177,179).
The entire rod assembly is inserted at the pseudarthrosis site into the
medullary canal of the distal fragment and is advanced in an antegrade
direction through the distal tibia and across the
talus and calcaneus as it exits through the heel pad (Fig. 28.31 A,B).
During the passage of the rod across the ankle joint, it is imperative
that the foot be positioned to correct the calcaneus position of the
foot and valgus deformity of the distal tibia. The desired foot
position is neutral dorsiflexion/plantar flexion verified clinically
and with the C-arm. The tibial fragments are anatomically reduced at
the pseudarthrosis site, and the rod is driven retrograde into the
proximal fragment (Fig. 28.31C). In selecting
an appropriately sized rod, consideration should be given to the
desired length of rod based on the need to transfix the ankle joint and
the amount of growth remaining in the distal tibia (Fig. 28.32 A,B,C,D,E).
In smaller children and with distal defects, ankle-joint fixation for 1
to 2 years may be desirable to minimize stress on the pseudarthrosis
site, which facilitates consolidation. Distally, the rod should extend
into the talus, calcaneus, or both. Proximally, the rod should remain
within the proximal tibia metaphysis and not cross the physis. The rod
may be advanced across the ankle joint once the defect has united (at
least 1 to 2 years following placement) to allow ankle motion. Ideally,
there will have been sufficient growth in the proximal tibia so that
the rod can be advanced into the tibia and not cross the proximal
tibial physis. Occasionally, the presence of deformity in the proximal
tibia necessitates an additional osteotomy in the proximal tibia to
assure intramedullary passage of the rod and anatomic alignment of the
tibia. An intact fibula is not osteotomized unless it distracts the
tibial fragments. If the fibula is not intact, it may be possible to
stabilize it with an intramedullary rod. A small-diameter K-wire is
used for fibular fixation and is placed into the distal fragment
through a separate incision. The wire is directed antegrade and out the
of distal tip of the fibula, then in a retrograde direction into the
proximal fragment. Fibular fixation adds stability to the construct (178,179).
|
TABLE 28.3 CLASSIFICATION OF CONGENITAL PSEUDARTHROSIS OF THE TIBIA
|
||||
|---|---|---|---|---|
|
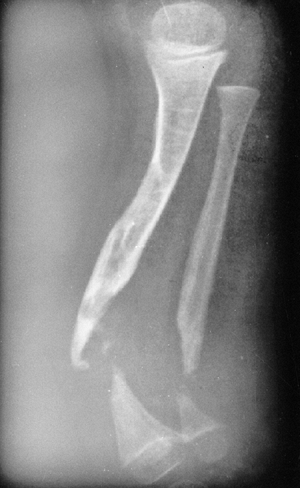 |
|
Figure 28.29 Early (Boyd type II, Crawford II-C) congenital pseudarthrosis of the tibia and fibula in a patient with neurofibromatosis.
|
end of the rod “migrates” proximally. Therefore, the anticipated
remaining growth determines the appropriate placement of the distal end
of the rod at the time of surgery. The previously harvested iliac bone
graft strips are then placed circumferentially around the
pseudarthrosis site and secured with absorbable suture as a
barrel-stave construction. By protocol for children 6 years and
younger, a one and one-half spica cast is applied to assure minimal
rotational stress at the pseudarthrosis site. The spica cast is
replaced with a long-leg cast after 6 to 8 weeks, and cast
immobilization is discontinued after approximately 4 months. Older
children are treated with long-leg casts for the entire 4 months. Once
cast protection is discontinued, the involved extremity is protected
with a custom-fabricated KAFO with a locked ankle joint and free
knee joint (Fig. 28.32F).
The presence of the rod across the ankle joint and in the hindfoot has
a considerable advantage in providing optimal immobilization and
protection of the consolidating pseudarthrosis. Refracture may occur;
although spontaneous healing with plaster immobilization may occur it
is not always assured, and repeat grafting, with or without rerodding,
may be required. With longitudinal growth of the tibia, the distal end
of the rod “migrates” more proximally and eventually will be positioned
in the distal tibia. As the tip of the rod crosses the ankle joint, the
potential
for
disruption of articular cartilage exists. To minimize such an
occurrence, the rod can be surgically pushed across the ankle joint as
the tip approaches the articular surface of the talus. This adjustment
is easily accomplished with a slightly larger diameter, concave-tipped,
IM pusher rod inserted through the calcaneus and guided with C-arm
assistance (Fig. 28.32 G,H,I).
On occasion, despite consolidation of the pseudarthrosis and growth of
the tibia, the rod does not migrate. If this occurs, the rod should be
replaced, with or without repeat grafting. In the case of failure of
rod migration, the replacement rod should be secured proximally with
methylmethacrylate (176,177,179).
 |
|
Figure 28.30 Operative options for pseudarthrosis of the tibia. A: Solid intramedullary rod fixation. B: Circular fixation with bone transport and compression. C: Vascularized fibula graft.
|
 |
|
Figure 28.31 Technique of solid intramedullary rod fixation. A:
After excision of the pseudarthrosis, the Williams rod is inserted, antegrade, through the distal tibia, talus, and calcaneus and exits through the heel pad. Care is taken to maintain the foot in a neutral, plantigrade position as the rod passes across the ankle and subtalar joints. B: The tibial surfaces are opposed. C: The rod is then passed into the proximal fragment, just below the proximal tibial physis. If the tibia is distracted, a fibular osteotomy is performed to allow contact across the tibia. |
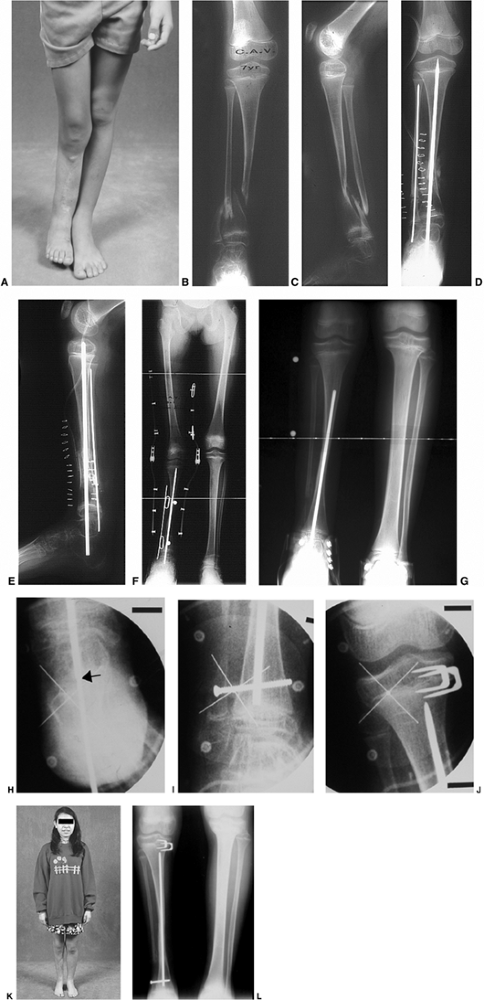 |
|
Figure 28.32 A:
This is another example of a tibial pseudarthrosis. Treatment includes stabilization of the fracture, establishment of a source of healthy bone, and maintenance of alignment. B: Atrophic pseudarthrosis of the tibia and fibula. C: Boyd type II or Crawford II-C dysplasia. D: The pathologic bone was resected; a Williams rod was used in the tibia and a Steinman pin in the fibula. Iliac crest graft was packed around the pseudarthrosis. E: The ankle and foot are held in a neutral position during placement of the rod. Transfixing the ankle and subtalar joint increases stabilization of the pseudarthrosis. F: A spica cast is applied postoperatively and a total contact orthosis used once union is established. Ankle motion is prohibited as long as the rod remains across the subtalar and ankle joints. A KAFO is used in smaller children. A patellar-tendon-bearing (PTB) orthosis can be used in most children over 7 years of age. G: With growth, the rod typically is drawn proximally and comes to lie within the tibia. In this case, the rod has remained with the distal fragment and continues to cross the ankle. Note the increased distance from the rod tip to the proximal tibial physis. H: A second rod can be used to push the Williams rod across the ankle. Image intensification is helpful in guiding placement. I: A syndesmosis screw was added to stabilize the persistent fibular pseudarthrosis. J: Hemiepiphyseal stapling of the proximal and/or distal medial tibia is used to correct valgus. K: This comprehensive treatment has resulted in a stable, healed pseudarthrosis. L: Satisfactory tibial alignment and stabilization of the pseudarthrosis have been achieved. Limb-length inequality can be managed with contralateral epiphysiodesis. Lengthening of the affected leg may be complicated by delayed bone formation and healing. |
can compromise the functional result. This valgus deformity is part of
the natural history of the pseudarthrosis that has a deficient fibula
and therefore lacks lateral support. It is not a result of traversing
the physis with the rod. Long-term bracing protects the distal tibia
and ankle and is mandatory during growth to minimize the risk of valgus
deformity. This is particularly true once the rod no longer crosses the
distal tibial physis. Valgus has been satisfactorily treated by
placement of staples across the medial tibial physis, either
proximally, distally, or across both (Fig. 28.32J).
The valgus deformity usually improves over a 1- to 2-year period. Once
correction is achieved, the staples are removed. A transverse
tibiofibular syndesmosis screw is inserted at the time of staple
insertion or removal if the distal fibula remains nonunited or has
migrated proximally, compromising ankle stability. With this approach,
symmetric growth of the distal tibia has generally continued and the
valgus deformity has not recurred.
In a recently reported review of our congenital pseudarthrosis of the
tibia (CPT) treatment experience, 11 of 21 patients had a significant
limb-length inequality (179). The average
discrepancy in these 11 patients was 5 cm and ranged from 2 to 9 cm.
Six of these patients were treated by epiphysiodesis
for
an average predicted leg-length discrepancy at maturity of 4 cm. Two
patients underwent a proximal tibial leg lengthening. Two patients with
leg-length discrepancies of 6 cm and 9 cm underwent amputations at
parental request, one at 8 years of age and the other at 15 years of
age.
The remaining five patients have had an amputation because of
limb-length inequality (two patients), refracture with persistent
nonunion (two patients), or significant residual angulatory deformity
(one patient). The quality of ankle motion and of gait has varied
considerably. The best results have been noted in those patients in
whom the tibia is anatomically aligned and the foot is plantigrade.
results were generally satisfactory. More recently treated patients
fared better than those patients treated earlier in the series. This is
likely because of modifications made in techniques based on earlier
experience. One recommendation that has improved outcome is
stabilization of the fibula. This enhances stability of the
pseudarthrosis and reduces the risk of valgus deformity in the distal
tibia and ankle. Second, advancement of the rod across the ankle
minimizes the effect on ankle range of motion and function. A third
lesson learned is that secondary deformities such as refracture,
valgus, and leg-length discrepancy must be more aggressively managed.
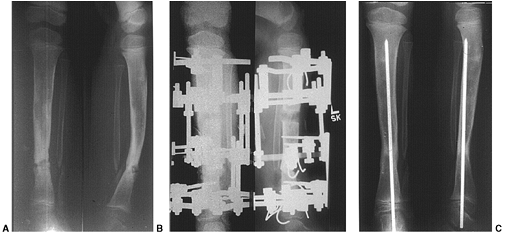 |
|
Figure 28.33 A:
Tibial pseudarthrosis recurred following multiple bone graft procedures and intramedullary pinning in this 6-year-old with neurofibromatosis. B: The pseudarthrosis was resected. The circular frame was used to apply compression across the distal site and to lengthen proximally. C: Subsequently, an intramedullary rod was placed across both osteotomy sites in the tibia and the ankle. A solid AFO has also been used. Alignment and bone union have been maintained as shown in these radiographs 2 years later. |
treatments of CPT cited relatively poor push-off following IM rod
treatment (180). Gait analysis of our more
recently treated patients has shown a better outcome concerning
push-off ability. We now utilize an active ankle-strengthening program
once the rod is positioned within the tibia.
fixator that used a combination of compression, distraction, resection
of the pseudarthrosis, and bone transfer (Fig. 28.33). Subsequently, numerous authors have refined this technique (181,182,183,184). The report by Paley et al. (183)
on their initial experience with 16 patients cited a primary union rate
of 94% after one procedure and 100% after two. The mean age at
treatment was 8 years. Five refractures occurred, and all were
successfully treated with additional procedures. Paley now incorporates
the use of an intramedullary rod along with the frame assembly in the
treatment of pseudarthrosis. The rod is left in place at the time of
fixator removal, providing additional protection against refracture and
recurrent deformity.
reported failures in 33% of patients with CPT managed by Ilizarov
technique. In their study, all 21 patients had neurofibromatosis. The
best results were obtained with sclerotic and normotrophic bone where
all six tibias had early consolidation (10 to 12 months) and did not
refracture. In contrast, only 5 of 11 tibias with an hourglass
appearance
eventually
healed. In the same series, consolidation was obtained in 86% of those
patients who were older than 5 years at the time of operation and only
14% of those younger than 5 years.
surgical patients, followed up to maturity. To date he has observed a
satisfactory outcome of treatment, that is, sustained consolidation and
independent ambulation in 87% of 20 of these patients. One patient had
an amputation. The average age at the time of treatment was 11 years
(range, 8 months to 29 years), and 18 of 21 patients had multiple prior
surgical procedures. Dahl (186) noted
refractures in 33% of the first 12 patients he treated. Refinements in
his techniques led to fewer fractures. To achieve prompt and lasting
union, he made the following recommendations: (a) resection of the
pseudarthrosis and pathologic periosteum, (b) creation of a stable
intrinsic fit of the bone end, (c) bone graft of the pseudarthrosis to
maximize the cross-sectional area, (d) ideal axial alignment to
eliminate bending forces, (e) judicious correction of length and
angulatory deformity through the proximal tibia, and (f) prophylactic
intramedullary pinning after fixator removal (186). The intramedullary rod is placed 8 weeks after fixator removal to reduce the risk of contamination.
utilized for the past 100 years in the surgical treatment of congenital
pseudarthrosis of the tibia (187,188,189).
In the last 25 years, refinements in microsurgical techniques have been
developed that allow for successful transfer of vascularized bone from
a variety of donor sites to remote bony defects (190,191) (Fig. 28.34).
Early reports demonstrated that a free vascularized fibular graft could
successfully be used as a biologic bridge across a congenital
pseudarthrosis of the tibia (192,193,194,195,196,197). Tolo’s (192)
current indications for the use of a vascularized graft in the
treatment of pseudarthrosis of the tibia are lesions with marked bony
atrophy or measurable gap at the pseudarthrosis site. His recommended
technique for implanting a free vascularized fibular graft for a
congenital pseudarthrosis of the tibia includes the following essential
steps. Preoperative arteriography of both legs is obtained. Two
surgical teams are used to facilitate treatment by allowing
simultaneous harvest of an autologous bone graft from the contralateral
fibula and preparation of the pseudarthrosis site. The distal fibula on
the involved side is stabilized with a screw into the tibia. The
pseudarthrosis is resected extraperiosteally to normal bone. Next, the
harvested fibula is dowel fitted into the host tibia and fixed with a
plate or an external fixator. Vessels are anastomosed, and a skin
paddle is used to monitor blood flow. A spica cast is used for 2 to 3
months and a clamshell orthosis for several years.
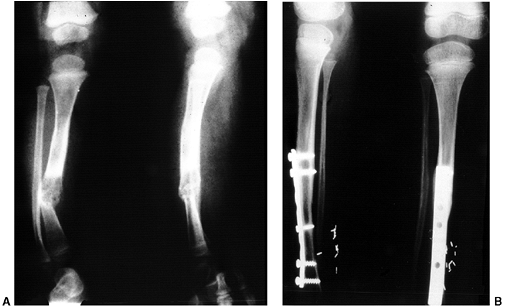 |
|
Figure 28.34 A: This film shows another fracture resulting from anterolateral bowing. Note the quality of the bone at the pseudarthrosis site. B:
A contralateral vascularized fibular graft has been used to bridge the pseudarthrosis. Healing proximally and distally has occurred. |
have reported on treatment of CPT by a vascularized fibular graft.
Combination of this data allowed review of 65 cases with sufficient
follow-up to assess outcome. Fifty-six cases eventually healed. Often a
second bone graft was necessary to achieve union (195). As with
intramedullary rod and external fixation techniques, sequelae may have
to be addressed including refracture, nonunion at one end of the graft
site, and recurrent pseudarthrosis with failure of the fibular graft (195,196,197).
Subsequent additional surgical treatment was often necessary, with
variable success in achieving a functional consolidation. Anterior
bowing and valgus deformity were also problems (193). The angulatory deformity did not remodel and was often progressive (190,193).
Fourteen of Weiland’s patients were noted to have residual angular
deformity. In a recent report of six patients with Crawford type IV
atrophic pseudarthrosis, Dimeglio et al. (198)
added an intramedullary rod to his technique of fibular transfer to
maintain length and improve stability of the reconstruction.
with proximal migration of the distal fibula on the normal side can
occur following harvest of the fibula (191). The occurrence of this most concerning deformity has not consistently been addressed (190,191,193,194,195,196,197).
Prophylactic stabilization of the donor site distal tibiafibular
syndesmosis was done by Weiland and Kanaya and was not done by Dormans
or Simonis (190,193,194,196). In follow-up, Dormans and Simonis both noted minimal donor site morbidity such as progressive valgus deformity.
continue to be a very challenging problem. A stable consolidation is
essential for a long-term satisfactory functional outcome. Each case is
unique, and as such, the treatment plan must often be modified. The
newer techniques of intramedullary stabilization, external fixation
with bone transport, and vascularized fibular graft have been refined
and successfully used by a few. All of these authors have had
considerable experience in learning how to apply and adapt their
particular surgical technique to the variables of each individual case.
Early results demonstrate satisfactory outcomes with these refined
surgical techniques and are encouraging. However, as Boyd and Sage (199)
suggested years ago, the true success of treatment of CPT in the
growing child can only be known by following up these patients until
maturity.
Amputation should be considered when there is failure to obtain
consolidation despite application of these described surgical
techniques, or if the outcome is otherwise unsatisfactory for the
patient as in those with poor limb function or severe limb-length
inequality. Traditionally, a below-knee amputation is performed.
Crawford and Jacobsen et al. (155,200)
recommended preserving the hindfoot with a Boyd or Syme procedure,
which preserves length of the residual limb and uses a prosthesis to
stabilize and protect the pseudarthrosis. These authors suggest that a
longer lever may enhance prosthetic fitting and minimize increased
energy expenditure, compared to a below-knee amputee. We have not had
the same success with a Syme procedure performed in two patients and
have found the need to revise both of them to a below knee level
because of residual limb instability and problems related to prosthetic
fit.
in association with congenital pseudarthrosis of the tibia. It rarely
occurs as an isolated entity. Only 21 cases of isolated fibular
pseudarthrosis have been reported in the literature (201,202,203,204,205,206,207). Congenital pseudarthrosis of the fibula presents later in life than congenital pseudarthrosis of the tibia (Fig. 28.35).
This entity typically presents because of an abnormal gait or because a
valgus deformity of the leg and a prominent fibula are noted (201,202,205,206).
The condition is frequently linked to neurofibromatosis. The tibia is
not normal. The deformity is variable and represents a
prepseudarthrosis (201).
through pathologic bone or through an area of mesodermal maldevelopment (202). Dooley et al. (202)
outlined a gradation in the severity of the condition: (a) fibular
bowing without pseudarthrosis, (b) fibular pseudarthrosis without ankle
deformity, (c) fibular pseudarthrosis with ankle deformity, and (d)
fibular pseudarthrosis with late development of tibial pseudarthrosis.
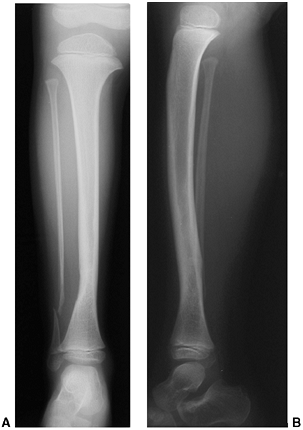 |
|
Figure 28.35 A: Rare presentation of a fibular pseudarthrosis. B: The tibia is also abnormal. Most are associated with neurofibromatosis.
|
felt that the potential for ankle valgus was significant without an
intact fibula and suggested interpositional bone graft to reconstitute
the fibula. If pseudarthrosis of the fibula is associated with ankle
valgus, it should be treated. Langenskiöld recommended synostosis of
the fibula to the tibia to prevent progression of valgus deformity in
the growing child (201,202,204).
If there is associated significant distal tibial valgus deformity, it
should be surgically addressed. In less severe deformities and in
skeletally immature patients, this can be accomplished with medial
tibial staple hemiepiphysiodesis, either proximal or distal. In severe
deformities or in skeletally mature patients, this is best done with a
supramalleolar valgus-correcting osteotomy.
Unlike its counterpart, anterior lateral bowing, posterior medial
bowing is not associated with pathologic fracture or pseudarthrosis of
the tibia, and has generally been considered a relatively benign
condition. Although the severity of the bow does diminish, considerable
leg-length discrepancy develops (208,209). This residual deformity presents the greatest need for orthopaedic management.
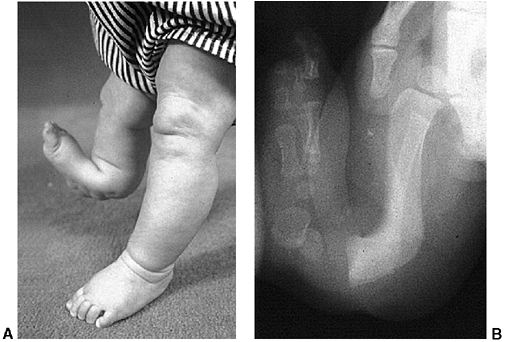 |
|
Figure 28.36 A:
Infants with posteromedial bowing usually present because the foot is in an abnormal, severely dorsiflexed position. The bow can be palpated along the subcutaneous border of the tibia. A skin dimple is usually present over the apex of the bow. Shortening of the leg may not be as obvious as the bowing deformity. B: This infant’s lateral radiograph shows how the foot seems to nestle against the curve of the tibia. The bone appears normal or may show signs of remodeling with thickening of the anterior cortex and smoothing posteriorly. |
Mechanical forces (the dorsiflexed foot against the tibia) and
embryologic vagaries of tibial development (circulatory or limb bud
anomaly) have been suggested as causes but remain unproven. The rapid
decrease of bow in the tibia in the first 6 to 12 months supports
mechanical factors as a cause of bowing (208,210). However, it cannot account for the growth inhibition.
Plantar flexion of the foot may be limited. The bow is most easily felt
by palpation of the anterior border of the tibia. A normal infant has
an anterior bow, which is distinct from the posterior defect palpated
in infants with posterior medial bow. A skin dimple is often present
over the posterior apex of the bow. Shortening of the affected tibia
may not be readily apparent in newborns, but is expected to increase as
the child grows.
radiographs of both tibias. The severity of the posterior and medial
deformity can be measured and a percent inhibition of tibial growth
calculated. Serial measurement of tibial length in infants is more
accurate when the lateral tibial radiograph is used. Orthoradiographs
or similar radiographic techniques can be used for serial assessments
of limb-length inequality. The posterior angulation usually remodels,
often producing a mildly “S-shaped” tibia (Fig. 28.37). The medial angulation is less likely to resolve completely, and significant residual valgus may remain.
Growth inhibition is constant as the absolute leg-length difference increases with growth (209).
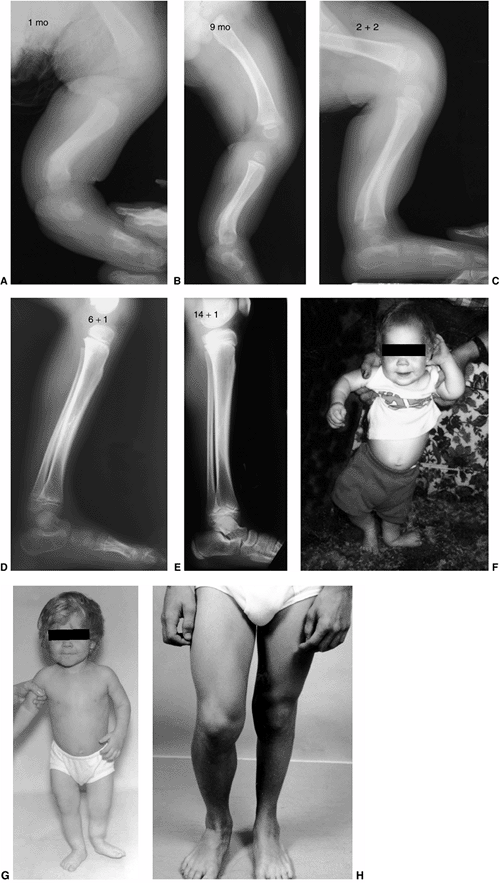 |
|
Figure 28.37 A–E:
Composite serial lateral radiographs show the degree of spontaneous resolution of the posterior bow. Improvement is often very dramatic within the first year. Similarly, there is improvement in the medial component; however, this bow may not fully resolve, resulting in residual valgus. Posteromedial bow is not associated with risk of pathologic fracture. F–H: Shortening of the involved tibia is expected and increases with growth as seen in these serial photographs. The percent inhibition remains fairly constant after 3 years of age. |
diagnosis of posterior medial bowing. Its direction is clearly
different from the more serious pathology of anterolateral bowing.
Posterior medial bowing may initially be overlooked in the presence of
severe calcaneovalgus foot deformity. Occasionally, adolescents will
present with limb inequality and mild valgus, the result of previously
unrecognized posterior medial bowing. Metabolic bone disease such as
osteogenesis imperfecta rarely results in this type of bowing deformity.
stretching of the foot. Serial cast application may be used for severe
deformities. The bowing generally corrects rapidly in the first few
years (208,209,210).
Rarely, extreme dorsiflexion and valgus persist such that plantigrade
weight bearing cannot be accomplished. In such cases, a solid AFO may
facilitate weight bearing for walking-aged children (211,212).
As tibial length inequality increases, a shoe lift may be needed to
balance the pelvis. Gradual contracture of the plantarflexors may occur
as a compensation for leg-length discrepancy. Passive stretching is
usually adequate treatment in young children.
tibia were reviewed at the Shriners Hospital for Children in St. Louis.
All had some degree of limb-length inequality. The minimum discrepancy
was 1.4 cm at 1 month of age, and the projected difference at maturity
ranged from 3 to 8 cm. Pes planovalgus that was seen in some children
resulted in decreased foot height as well. This degree of inequality is
best managed by surgical equalization, either by shortening the long
tibia by epiphyseodesis or lengthening the short tibia. Residual valgus
deformity can also be corrected. In the past 10 years, most patients
have chosen lengthening and about half have had bi-level osteotomies to
allow lengthening proximally and deformity correction distally. The
others have been treated with epiphyseodesis, often preceded by
proximal medial tibia hemiepiphyseal stapling to correct residual
valgus deformity (209).
differences as great as 4 to 5 cm. Shortening is usually confined to
the tibia. Epiphyseodesis of the proximal tibia is usually sufficient.
There are several techniques that can be employed. A staged
hemiepiphysiodesis may be used to correct residual angulation prior to
completion of the epiphyseodesis.
with projected discrepancies greater than 4 cm and for those with
residual valgus angulation (Fig. 28.38).
Lengthening alone in this group can exaggerate a valgus deformity,
particularly when unilateral frames are used. Circular frame fixation
allows better control of valgus (42,124).
Bi-level osteotomies in the tibia (lengthening proximally, deformity
correction distally) provide optimum management of both length and
angulation. The foot may assume a varus position to compensate for
residual tibial valgus and require modification of the frame if the
foot deformity is rigid. Lengthening and deformity correction in our
patients with posterior medial bowing has been successful, with few
complications compared to procedures in children with other forms of
congenital shortening.
rare deformity that varies from simple hyperextension to anterior
dislocation of the tibia on the femur. The spectrum of deformity in CDK
has been classified as recurvatum, subluxation, and dislocation (213,214) (Fig. 28.39). The incidence of CDK is estimated at 1 per 100,000 live births,
that is, 1% of the incidence of developmental hip dysplasia (215,216,217). Although there are some reports of occurrence within families, most cases are sporadic (218,219,220). The deformity may be unilateral or bilateral.
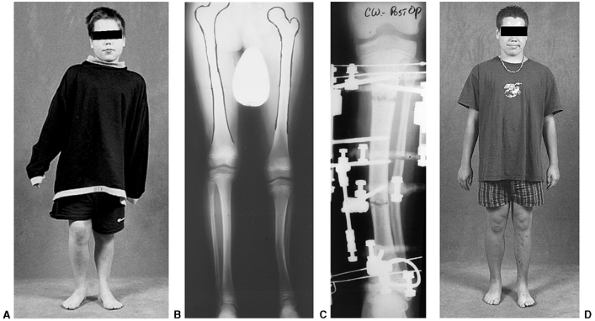 |
|
Figure 28.38 A:
This 10-year-old boy had posteromedial bowing of the left tibia. He had 4.3 cm shortening and a 13% growth inhibition in the tibia. B: A long cassette radiograph demonstrates the limb-length inequality as well as the residual valgus in the distal tibia. C: A bi-level tibial osteotomy was performed. Residual valgus was corrected through the distal tibia, and limb-length equalization was accomplished with proximal lengthening. D: Valgus has been corrected and the pelvis balanced as shown in this follow-up photograph at 16 years of age. |
 |
|
Figure 28.39 Congenital knee dislocation can range from simple hyperextension (recurvatum) (A) to subluxation to (B) complete anterior dislocation of the tibia on the femur (C).
|
The familial occurrence suggests a possible genetic basis. CDK has also
been seen in association with developmental hip dysplasia, idiopathic
clubfoot, and congenital vertical talus (213,214,215,221). All of these have a genetic basis or polygenic mode of inheritance, which suggests a genetic link for CDK as well (213,215,222).
In one study, 41% of otherwise healthy newborns with CDK were of breech
presentation. These are generally believed to be positional, not
pathologic deformities (215). Severe CDK often
occurs in the presence of muscle imbalance and/or ligamentous laxity,
such as that occurring in myelodysplasia, arthrogryposis, Larsen
syndrome, and oligohydramnios (213,215,221,223).
or contracted quadriceps muscle can, along with deficient hamstrings,
lead to anterior dislocation of the knee. These infants typically have
severe hyperextension in utero and,
presumably, decreased fetal mobility. Chronic knee hyperextension
results in anterior subluxation of the hamstrings, allowing them to
function as knee extensors (224). The quadriceps muscle is short and contracted. Uhthoff and Ogata (225)
were able to study a 19½-week-old fetus with such a deformity. They
found fibrosis of the quadriceps, absence of the suprapatellar pouch,
and incomplete patellofemoral cavitation. The authors suggest that knee
subluxation resulted from these abnormalities, intrinsic to the
extensor mechanism, rather than from some secondary, extrinsic cause.
The pathologic findings in this fetus are the same as those found in
patients treated surgically.
In cases of subluxation, passive flexion is limited, but improves with
splinting and gentle stretching. Milder forms, such as hyperextension
or recurvatum, are usually isolated abnormalities. In the case of
dislocation, there is inability to flex the knee actively or passively.
The quadriceps tendon is often severely contracted. A dimple or deep
crease may be present over the anterior aspect of the knee. The patella
is difficult to palpate and often laterally displaced. Those with a
more severe variant of CDK are more likely to have hip dysplasia and
congenital foot deformities. When it occurs in association with a
neuromuscular or genetic syndrome, CDK is typically very severe and
difficult to manage (213,214,226,227).
Radiographs help to differentiate the mild hyperextension deformity
from the more severe type with fixed anterior dislocation of the tibia
on the distal femur (Fig. 28.41).
 |
|
Figure 28.40
This infant has bilateral anterior knee subluxation and clubfoot deformities. These deformities are often associated with intrauterine breech position. Deep skin creases are often found across the front of the knee. The skin may be dimpled posteriorly. |
easily manipulated over the femoral condyle as flexion increases.
Serial casts are used to hold the knee in flexion. They are changed
every few days in neonates and then weekly as the knee position
improves. This is followed by the use of removable splints to maintain
flexion. Mild deformities may be treated with splinting alone. These
knees often show rapid improvement in flexion. A Pavlik harness is
useful for maintaining knee flexion achieved by stretching, splinting,
or casting (228,229,230).
The femoral condyles should be easily palpable once flexion beyond 90
degrees is achieved. Once this degree of flexion is obtained, it is
unlikely that additional treatment will be needed. A lateral radiograph
or ultrasound image of the knee can be obtained to document anatomic
restoration of the femoral-tibial articulation.
dislocation do not respond to passive stretching or splinting. Traction
has been suggested as a means to achieve gradual reduction (213,227,231).
During attempted closed treatment, it is particularly important to
document restoration of a normal relation of the tibia on the femur
with a lateral knee radiograph. Ultrasound can also be used. It is
possible to create an iatrogenic physeal separation of the distal femur
or to deform the proximal tibia plastically. Rather,
the
correction obtained must occur through the knee joint, allowing the
tibia to translate on the femur. Closed treatment should be abandoned
if appropriate reduction of the tibia cannot be confirmed.
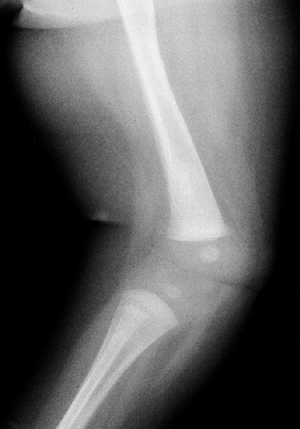 |
|
Figure 28.41
A lateral radiograph differentiates among simple hyperextension, subluxation, and anterior dislocation. The anterior aspect of the knee is to the left. Note the deep skin crease anteriorly. The ossification center of the proximal tibial epiphysis is anterior to that of the distal femur. Serial lateral radiographs should be used to document anatomic reduction of the knee. Failure to achieve anatomic reduction by closed manipulation or inability to flex the knee more than 45 degrees are indications for surgical treatment. |
anteriorly dislocated tibia on the end of the femur and therefore lack
flexion, early surgical treatment at 1 to 2 months of age should be
considered (217). As suggested by Roy and Crawford (221)
this release is done through a variable limited incision in the distal
thigh. The quadriceps tendon and adjacent medial and lateral
retinaculum are transected as necessary to obtain both reduction and
flexion of the tibia on the femur, followed by serial casting.
Range-of-motion exercises, along with intermittent splinting in flexion
or extension as needed, are used to maintain the reduction and knee
mobility. These authors have reported favorable outcome from surgical
treatment in these infants; however, our experience with this limited
approach has been less satisfactory.
becomes more severe and requires a more extensive release as the infant
grows. Some authors have had success correcting moderate contractures
using a simple V-Y advancement of the quadriceps tendon (213,214).
This is insufficient for more severe dislocations that require greater
lengthening of the quadriceps mechanism and release of contractures.
quadriceps mechanism to the patella is recommended to allow correction
of this complex deformity. A serpentine incision extends from the
proximal thigh to slightly past the tibial tubercle. This incision,
rather than a straight incision, facilitates wound closure and results
in fewer problems related to wound healing. Subcutaneous flaps are
raised to expose the quadriceps mechanism. In these cases of severe
contracture, fibrosis and scarring of the muscle is more extensive. It
may be adherent to the periosteum of the femur. The patella is usually
very small and often laterally displaced. The extensor mechanism is
usually malrotated and pulls the tibia into valgus. The quadriceps
mechanism requires considerable lengthening, yet it must remain
attached both proximally and distally. The release requires extensive
dissection through fibrotic tissue. The quadriceps tendon is lengthened
using a long Z-plasty or V-Y advancement, depending on the degree of
quadriceps contracture present (213,214).
The capsule of the anterior knee joint is released transversely to the
collateral ligaments, which often must be reflected and/or partially
released. The knee can usually be flexed to 90 degrees following this
release. Occasionally the medial hamstrings, iliotibial band, and the
lateral intermuscular septum must be released to correct valgus and
external rotational deformity (217). The cruciate ligaments are typically present but may be attenuated (213,214,222,224).
The authors have not found it necessary to release the cruciates to
gain flexion. The elongated quadriceps mechanism, which may be tenuous
in these infants, is repaired with the knee in approximately 30 degrees
of flexion (213,217,224).
A spica cast is used for immobilization, with the knee placed in enough
flexion (approximately 45 degrees) to prevent recurrent tibial
subluxation (215,217). Too much flexion jeopardizes the quadriceps mechanism repair and potentiates necrosis of the skin and subcutaneous tissue (213).
Long-term orthotic splinting and range-of-motion exercises are
essential to maintain maximal flexion and minimize loss of extension. A
knee flexion contracture can be more debilitating than a lack of full
flexion.
either at the time of reduction of the knee dislocation or later as a
staged procedure. Following release of the contracted quadriceps
mechanism, the knee can be flexed, facilitating treatment of the hip (213,214,215,217).
Mild hip dysplasia, which is amenable to simple closed reduction or
limited open reduction using a medial approach, can be treated
concurrently with the spica cast utilized to immobilize a surgically
reduced CDK. More severe hip dysplasia, which requires open reduction
by an anterior approach, should be done later. If a coexisting foot
deformity requires operative treatment, this can be staged or done in
conjunction with the knee reconstruction. Knee flexion facilitates cast
application necessary for the treatment of associated foot deformities.
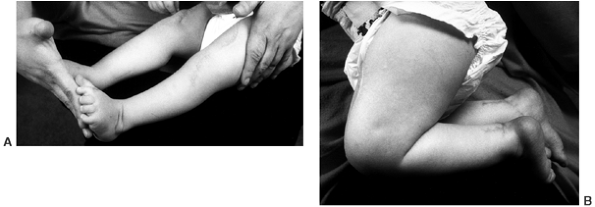 |
|
Figure 28.42 A:
The upper photo shows knee extension following open reduction of these bilateral knee deformities. Hyperextension is no longer evident. B: Knee flexion greater than 90 degrees has been accomplished. Range-of-motion exercises and intermittent use of orthotics may be needed to maintain this correction. |
Those who have had hyperextension or mild dislocation and required only
stretching and minimal intervention as infants have the best results.
They do not report problems later on. Function is excellent and
radiographs appear nearly normal (216,226,229,230).
Children with more severe dislocation who required an open reduction,
but do not have any other musculoskeletal problems, generally do well (Fig. 28.42).
In these patients knee range of motion includes full to nearly full
extension and flexion that averages 80 to 120 degrees. This allows very
functional, independent ambulation, and participation in most normal
play activities; however, activities such as running or bicycling,
which requires flexion beyond 90 degrees, may be limited. Radiographic
abnormalities are found in some, but not all knees. This usually
consists of flattening of the femoral and tibial articular contours (213,215).
those with unilateral deformity. These also tend to be children with
associated neuromuscular disorders. Early repair generally has a more
satisfactory functional result than late repair (213,215,221).
Recurrent hyperextension deformity is not likely. However, loss of
flexion, particularly that gained at surgery, can be a late
complication. Similarly, the development of a flexion contracture can
compromise long-term function.
toe-walking. The habit of doing so is not that uncommon or abnormal in
2- to 3-year-old children. Typically, the habitual toe-walkers, with
coaxing, can walk plantar grade. By the age of three, children should
walk with a heel strike (6,232,233). Persistent toe-walking beyond this age is abnormal.
first begin weight bearing. If the toe-walking persists, a contracture
develops and worsens over time. In the child who is otherwise
neurologically normal, toe-walking may be associated with a shortened
heel cord; however, this is generally not recognized at birth or within
the first year (234,235,236).
retrospectively reviewed 80 patients who were evaluated and treated for
ITW. Forty-eight of the 80 patients, generally those with the mildest
deformity, were observed without treatment from ages 3 years to 6
years. The degree of heel cord contracture was mild and remained
essentially unchanged in these patients. Only 25% of parents noted a
spontaneous improvement; that is, appreciably less toe-walking. For the
children with more than 5 degrees of passive dorsiflexion, persistent
toe-walking is not a functional problem and does not result in any foot
deformity or pain. For many patients, toe-walking either diminishes or
ceases with time. This may occur as the body mass becomes too large to
be supported by the triceps surae or as a result of secondary
development of external tibial torsion (235).
The development of a mid-foot break to permit foot contact with the
floor is frequently seen in neuromuscular conditions, but is not
typically seen in ITW (14).
The condition predominates in the male sex, and the family history
(siblings and generation to generation) is often positive for similar
ITW persisting into adulthood (234,236,237).
The suggested inheritance pattern is autosomal dominant with variable penetrance (238,239). Although Stricker et al. (236)
noted a frequent history of both prematurity and developmental delay,
walking was not delayed. On examination, ITW occurs bilaterally and is
best seen when the child is walking barefoot (Fig. 28.43).
When the child stands still, the feet may be flat on the ground. With
time, toeing-out and splaying of the forefoot frequently occur.
Idiopathic toe-walkers, in contrast to children with spastic diplegia,
do not have a tendency to walk with a back-knee thrust in order to
achieve foot flat position.
flexion will be normal and dorsiflexion will be limited, secondary to a
variable degree of true shortening of the heel cord (234).
The posterior calf muscles are typically very well developed; in fact,
they may appear to be enlarged. However, the muscle texture will feel
normal on examination and there will be no evidence of any weakness,
proximal or distal, in the upper or lower extremities. Importantly, no
spasticity is present and clonus will not be elicited by a rapid
stretch of the triceps surae. The diagnosis of ITW is a diagnosis of
exclusion, and typically can be made from the child’s history and
physical examination (234,237,240,241,242).
ITW that develops in a child with a previously normal gait must be
differentiated from primary muscle diseases such as muscular dystrophy,
myotonic dystrophy, dystonia, and tethered cord syndrome, and from
central nervous system (CNS) neoplastic processes (236,237). Further evaluation by a neurologist may be indicated to exclude such consequential neuromuscular diagnoses.
computerized gait analysis or dynamic electromyograph (EMG); however,
an EMG may be helpful if it is essential to differentiate between ITW
and spastic diplegia (235,240,241,242,243).
Children with mild cerebral palsy who walk on their toes often have
out-of-phase gastrocnemius soleus muscle activity, whereas idiopathic
toe-walkers will be in phase (242,243).
Both groups show lack of heel strike. Children with mild diplegia show
greater sustained knee flexion at terminal swing. Maximal knee
extension occurs at ground contact for idiopathic toe-walkers and at
mid-to-late stance for diplegics (240). In the
ITW gait, ankle dorsiflexion occurs early in maximal swing, followed by
plantar flexion. Ankle dorsiflexion occurs throughout swing in
diplegics.
 |
|
Figure 28.43 A:
Children with idiopathic toe-walking assume an externally rotated posture to facilitate a foot flat position. They typically do not hyperextend the knee. B: While up on their toes, the heel moves to a varus position. Chronic toe-walking can cause the forefoot to splay because of the overload of the intermetatarsal ligaments. Forefoot splay and tibial external rotation typically do not resolve after heel cord lengthening. |
the parents regarding the importance of a long-term commitment to
assisting the child with both heel cord stretching and dorsiflexion
strengthening exercises. This is particularly true in early childhood,
when stretching exercises are more likely to make a difference. It is
important to maintain inversion of the hindfoot to optimize stretch of
the heel cord. If the calcaneus is allowed to evert, the forefoot will
be allowed to dorsiflex independent of the hindfoot and create a
mid-foot break without effectively stretching the heel cord. A 3- to
4-month course of twice daily dorsiflexion manipulations may be
effective in obtaining increased dorsiflexion. The response to
treatment varies with the duration and severity of the equinus
deformity and compliance with the exercise regimen. Clinical
improvement will be sustained with continued daily heel cord stretching
exercises and active dorsiflexion strengthening exercises supplemented
with orthotics. An articulated AFO with a plantar-flexion stop and free
dorsiflexion is used to maintain position.
casts should be considered. Predictably, stretching casts will be more
effective when there is a “springy” rather than a firm
feel
of maximal heel cord stretch during foot dorsiflexion. The casts are
applied with the foot maximally dorsiflexed and the heel in a neutral
position or slightly inverted. Flexing the knee or prone positioning
facilitates placement of the cast. A series of two or three sets of
short-leg casts will often elongate the heel cord and result in greater
passive dorsiflexion. This immobilization also produces short-term
weakness. The combination of stretch and slight weakness usually
produces a marked decrease in the tendency to toe-walk. After casting,
articulated AFOs with plantar-flexion stops are used full time, and the
heel cord stretching and dorsiflexion-strengthening regimen is resumed.
If dorsiflexion can be maintained for 3 to 6 months, the children are
weaned from daytime use. Nighttime splinting can be discontinued if
there is no recurrence of the toe-walking. Although serial casting and
subsequent orthotic usage is widely recommended, very little is known
of its long-term effectiveness (241).
This approach will often be successful in younger patients (younger
than 4 years), and even in some of the older patients. However, relapse
is common due to lack of compliance, often requiring repeat serial
casting, AFO use, or surgical treatment. Compliance with a
comprehensive program of heel cord stretching, dorsiflexor
strengthening, and bracing is essential for long-term success.
satisfactory clinical improvement in the tendency to toe-walk, then
heel cord lengthening procedures will be necessary to effect a change
in gait (234,236). Age
also becomes an important determinant in treatment recommendations. The
authors feel that by 6 to 7 years of age, children should have improved
sufficiently to consistently demonstrate a normal heel-toe, rather than
toe-heel or toe-toe gait. Far too often, parents are inappropriately
advised that ITW will resolve on its own. In fact, toe-walking after 6
years of age often does not improve, and the heel cord contracture
slowly worsens.
contracture can potentiate both forefoot splay and a disproportionately
wide forefoot compared to the heel. Standard footwear may not
accommodate the wide forefoot and narrow heel. External tibial torsion
frequently develops to compensate for the lack of foot flat contact.
This external tibial torsion deformity becomes more obvious once the
heel cord has been lengthened. It may be severe enough to warrant
corrective osteotomy.
techniques. The authors prefer performing a fractional lengthening at
the junction of the middle and distal thirds of the triceps surae
complex if the foot can be brought to neutral with the knee flexed.
Lengthening is performed sufficient to obtain 10 degrees of
dorsiflexion with the knee extended. In some, release of the posterior
ankle capsule may be necessary. Short-leg walking casts are used for 5
to 6 weeks, then the child is fitted with AFOs, which are used 22 of 24
hours for 4 to 6 weeks, then gradually weaned during daytime but used
at night for up to 6 months postoperatively. Heel cord stretching and
ankle strengthening exercises are performed twice daily.
outcome. Generally, parents are very satisfied with the improved gait
following heel cord lengthening; however, the parents need to be well
informed as to the anticipated postoperative course. The child’s
postoperative gait (relatively weak plantar flexor power) will be
considerably different from the preoperative gait (relatively strong
plantar flexion power). The predominately equinus gait is replaced by a
gait with relatively weak push off. Quick cadence is replaced by a
slower cadence gait. It takes time for the push-off power to recover to
near normal levels (234). Younger children
regain a relatively normal gait pattern soon after weaning from
orthotics, whereas those older than 8 years at the time of surgical
treatment may take a year or more to normalize their gait. Hall et al. (234),
at an average length of follow-up of 3 years following heel cord
lengthening, reported a satisfactory outcome for all 20 patients. In a
review of all treatment methods, Stricker and Angulo (236)
noted that surgical lengthening of the heel cord was the only treatment
that permanently improved ankle dorsiflexion. Although 33 of 56
patients still exhibited some degree of toe-walking, most parents were
satisfied with the outcome of heel cord lengthening. A study by Stott
et al. (244) of toe-walkers evaluated at
skeletal maturity concluded that, although kinematic studies continued
to demonstrate some gait abnormalities, no abnormalities were apparent
clinically.
JM. Comparison of normal and abnormal human fetal hip joints: a
quantitative study with significance to congenital hip disease. J Pediatr Orthop 1983;3:173.
RM, deSwart RJ, Dodgin DA, et al. Kinematic and kinetic analysis of
distal derotational osteotomy of the leg in children with cerebral
palsy. J Pediatr Orthop 1998;18:81.
DA, deSwart RJ, Stefko RM, et al. Distal tibial/fibular derotation
osteotomy for correction of tibial torsion: review of technique and
results in 63 cases. J Pediatr Orthop 1998; 18:95.
DP, Schoenecker PL, Rich MM. Peroneal nerve injury as a complication of
pediatric tibial osteotomies: a review of 345 osteotomies. J Pediatr Orthop 1994;14:166.
MA. Current trends in the treatment of simple and complex bone
deformities using the Ilizarov method. In: Eilert RE, ed. AAOS Instructional Course Lecture Volume 41. Rosemont, IL: American Academy of Orthopaedic Surgeons, 1992:423.
JH, Austin SM, Warner WC, et al. Interlocking intramedullary nailing of
femoral-shaft fractures in adolescents: preliminary results and
complications. J Pediatr Orthop 1994; 14:178.
K. Etiology of Blount’s disease. The Pediatric Orthopaedic Society of
North America 28th Annual Meeting, Orlando, FL, May 16, 1999.
AM, Drennan JC. Physiological bowing and tibia vara. The
metaphyseal-diaphyseal angle in the measurement of bowleg deformities. J Bone Joint Surg Am 1982;64:1158.
RE, Dorey FJ, Moseley CF. Relative tibial and femoral varus as a
predictor of progression of varus deformities of the lower limbs in
young children. J Pediatr Orthop 2002;22:105.
BC, Israelite CL, Betz RR, et al. Reliable early radiographic
differentiation of infantile Blount’s disease and physiologic bowing:
utilizing the ratio of the femoral and tibial metaphyseal-diaphyseal
angles. Ortho Trans 1991;15:27.
JD, Radomisli TE, Simoncini J, et al. Variability of the
metaphyseal-diaphyseal angle in tibia vara. A comparison of two
methods. J Pediatr Orthop 2004;24:75.
DJ, Boniface AM, Schranck FW, et al. X-linked hypophosphatemic rickets:
a study (with literature review) of linear growth response to
calcitriol and phosphate therapy. J Bone Miner Res 1992;7:583.
JH, Barrett IR. Unilateral angular deformity of the distal end of the
femur secondary to a focal fibrous tether: a report of four cases. J Bone Joint Surg Am 1989;71:440.
JE, Schoenecker PL, Luhmann SE, Rich MM. Distal femoral deformity in
children with tibia vara. American Association for Orthopaedic
Surgeons’ Annual Meeting, San Francisco, CA, March, 2004.
RS. Epiphyseal deformity of infantile Blount’s disease. The Pediatric
Orthopaedic Society of North America 28th Annual Meeting, Orlando, FL,
May 16, 1999.
DF, Dahl MT, Louie K, et al. Management of late-onset tibia vara in the
obese patient by using circular external fixation. J Pediatr Orthop 1997;17:691.
JG, Lapinsky AS. Results of partial physeal bar resection in patients
with Langenskiold stage VI infantile Blount’s disease, POSNA, Memphis,
Tennessee 1994.
A, Wosko I, Kandzierski G, et al. Double elevating osteotomy of tibia
in the treatment of severe cases of Blount’s disease. J Pediatr Orthop 1989;9:178.
JR, Leeson MC, Thompson GH, et al. Late-onset tibia vara: a
histopathologic analysis. A comparative evaluation with infantile tibia
vara and slipped capital femoral epiphysis. J Pediatr Orthop 1988;8:187.
D, Tetsworth K. Mechanical axis deviation of the lower limbs.
Preoperative planning of multiapical frontal plane angular and bowing
deformities of the tibia or femur. Clin Orthop Relat Res 1992;280:65.
D, Tetsworth K. Mechanical axis deviation of the lower limbs.
Preoperative planning of uniapical angular deformities of the tibia or
femur. Clin Orthop Relat Res 1992;280:65.
MB. Juvenile/adolescent Blount’s disease: treatment with osteotomy and
internal fixation. The Pediatric Orthopaedic Society North America 28th
Annual Meeting, Orlando, FL May 16, 1999.
DF, Srivastava P, Stanitski CL. Correction of proximal tibial
deformities in adolescents with the T-garche external fixator. J Pediatr Orthop 1998;18:512.
JE. Treatment of Blount’s disease using the Taylor spatial frame. The
Pediatric Orthopaedic Society of North America 28th Annual Meeting,
Orlando, FL May 16, 1999.
PG, Fox JA, Fitch RD. Treatment of adolescent Blount disease with the
circular external fixation device and distraction osteogenesis. J Pediatr Orthop 1996;16:450.
CA, Maranji K, Frederick N, et al. Comparison of crossed pins and
external fixation for correction of angular deformities about the knee
in children. J Pediatr Orthop 1998;18:502.
MB, Rich MM, Gordon JE, et al. Use of an intramedullary rod for
treatment of congenital pseudarthrosis of the tibia. A long-term
follow-up study. J Bone Joint Surg Am 2004;86:1186.
LA, Haideri NF, Halliday SE, Johnson CE, et al. Gait analysis and
muscle strength in children with congenital pseudarthrosis of the
tibia: The effect of treatment. J Pediatr Orthop 1998; 18:381.
S, Catagni M, Donzelli O, et al. Congenital pseudarthrosis of the tibia
associated with neurofibromatosis-1: treatment with Ilizarov’s device. J Pediatr Orthop 1997;17:675.
MT. Congenital pseudarthrosis of the tibia treated with circular
external fixation. POSNA One Day Course, Orlando, FL, May 16, 1999.
VT. The role of free vascularized fibular grafts in congenital tibial
pseudoarthrosis treatment. POSNA One Day Course, Orlando, FL, May 16,
1999.
JP, Krajbich JI, Zucker R, et al. Congenital pseudarthrosis of the
tibia: treatment with free vascularized fibular grafts. J Pediatr Orthop 1990;10:623.
SS, Coleman DA. Congenital pseudarthrosis of the tibia—treatment by
transfer of the ipsilateral fibular with vascular pedicle. J Pediatr Orthop 1994;14:156.
A, Moukoko D, Chammas M. Treatment of severe congenital pseudarthrosis
of the tibia: A combined method associating intramedullary nailing and
free folded vascularized fibular transfer. The Pediatric Orthopaedic
Society of North America Annual Meeting, Amelia Island, FL 2003.
A. Pseudarthrosis of the fibula and progressive valgus deformity of the
ankle in children: treatment by fusion of the distal tibial and fibular
metaphyses. J Bone Joint Surg Am 1967;49:463.
MM. Posterior medial bowing of the tibia. Shriners Hospitals for
Children experience. POSNA One Day Course, Orlando, FL, May 16, 1999.
CH, Herndon CH, Heiple KG. Congenital posterior angulation of the tibia
with talipes calcaneus. A long-term report of eleven patients. J Bone Joint Surg Am 1959;41:476.
DR, Crawford AH. Percutaneous quadriceps recession: a technique for
management of congenital hyperextension deformities of the knee in the
neonate. J Pediatr Orthop 1989;9:717.
S, Klemms A, Muller K. Gait analysis by measuring ground reaction
forces in children: changes to an adaptive gait pattern between the
ages of one and five years. Dev Med Child Neurol 1997;39:228.
JF, Torburn L, Rinsky LA, et al. Electromyographic test to
differentiate mild diplegic cerebral palsy and idiopathic toe-walking. J Pediatr Orthop 2001;21:784.
