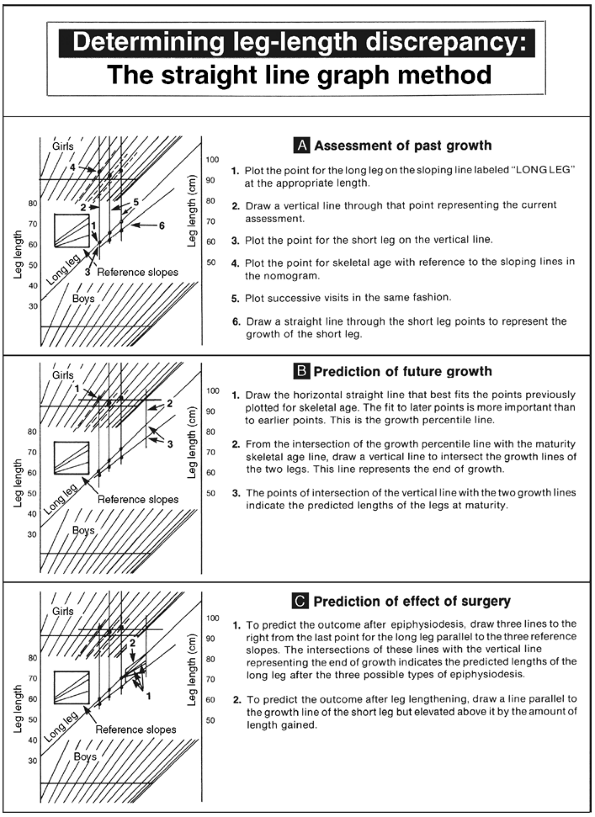
Leg-Length Discrepancy
challenges to the orthopaedic surgeon, who must understand the
mechanisms and concepts of growth, including the relations among age,
maturity, and leg length. Because the patient usually presents during
the growing years, the orthopaedic surgeon must understand the need to
correct the discrepancy as it will exist at maturity and not the
discrepancy that is present in the growing child. The surgeon must be
familiar with the methods used to analyze growth and to predict future
growth and the effects of surgery. The techniques of leg lengthening
evolve rapidly, and the orthopaedic surgeon must consider these new
techniques in choosing the most appropriate management. As surgeons
become more confident in their ability to utilize leg-lengthening
procedures, they accept discrepancies of greater magnitude for
correction, and they must be confident that the improvements in their
abilities outweigh the increased risks. The final challenge facing the
orthopaedic surgeon is to maintain a holistic perspective and to resist
the temptation to direct attention solely toward the lengths of the
legs, thereby neglecting the other factors that are important in the
patient’s overall function and appearance.
by careful and sometimes difficult education of the patient and
parents. In the case of epiphysiodesis, parents frequently find it
difficult to understand why a problem in one leg requires an operation
on the normal leg, and they are not pleased at the thought that it will
make their child shorter. In the case of leg lengthening, the parents
and the patients must understand that a fairly high morbidity is
associated with this procedure even if things go well because the child
must wear an external device for many months and must restrict his or
her recreation and athletics. They also must understand that the risk
of complications is high and that these complications can compromise
the final result. The orthopaedic surgeon knows that surgery is
necessary to correct leg-length discrepancy, but parents are often
anxious to find some nonsurgical method of stimulating the growth of
the short leg. If risks were equal, it would certainly be better to
correct the leg-length discrepancy by lengthening the abnormally short
leg than to compensate for the discrepancy by shortening the normal
long leg.
discrepancy are immediately apparent. The long-term effects, however,
are less understood. Despite a consensus in the orthopaedic and lay
communities that leg-length discrepancy does have deleterious effects
on the spine and the hips, good documentation to support that consensus
is lacking.
compensates better than the adult, probably because of a higher
strength-to-weight ratio. The child can compensate for minor degrees of
leg-length discrepancy by walking on the toes of the short leg with the
heel never touching the ground. This can result in a smooth,
symmetrical gait that shows no abnormality except for the lack of heel
strike on the short side.
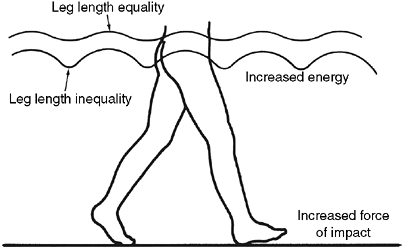
manner but tends to walk with a heel-to-toe gait even on the short side
and to vault over the long leg. This action produces excessive
up-and-down motion of the pelvis and trunk (Fig. 29.1).
Although it is theoretically possible to compensate for the leg-length
discrepancy by flexing the knee on the long side, this is almost never
done by either adults or children, probably because it would require
too much of the quadriceps mechanism.
Liu et al. proposed the “symmetry index” (SI) as a measure of the
quality of gait and found that correction of discrepancy by a heel lift
considerably improves the SI (3).
for their discrepancies. The movements about the lower limb joints with
simulated and real leg-length discrepancies have been found to be
essentially unchanged with small discrepancies (4).
Song found that discrepancies greater than 5.5% of the long extremity
increased the mechanical work performed by the long limb and increased
the vertical displacement of the center of body mass, with consequent
energy penalty. Children with lesser discrepancies were able to normalize the work performed by the two extremities.
 |
|
Figure 29.1
Motion of pelvis during gait. The amplitude of vertical pelvic motion is increased by leg-length discrepancy. The patient vaults over the long leg and descends to plant the heel of the short leg. |
in the elderly patient may actually be the result of some previously
unrecognized minor problem, such as mild dysplasia, slipping of the
capital femoral epiphysis, or, possibly, leg-length discrepancy. In
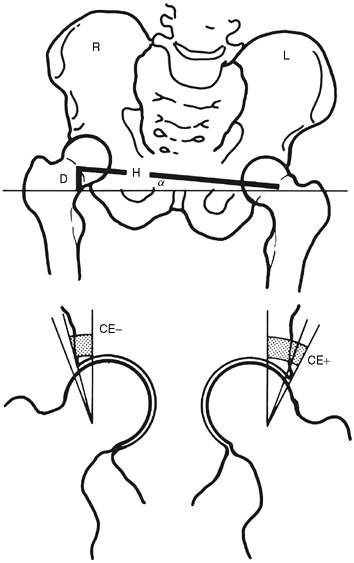
two-legged stance with the legs straight, the patient with leg-length
discrepancy has a pelvic obliquity, with respect to the floor, that
relatively uncovers the hip of the long leg and that increases the
coverage of the hip of the short leg (Fig. 29.2).
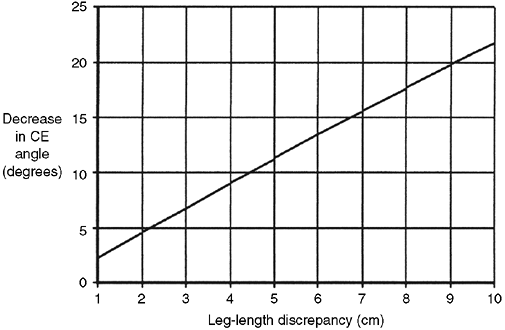
on the high side also increases, with a decrease in the center-edge
(CE) angle. This relation is illustrated in Figure 29.3.
It is reasonable to suspect that the patient with a leg-length
discrepancy throughout his or her life may be subject to an increased
risk of developing degenerative arthritis in the hip of the long leg (5),
however, there is no documentation to prove this hypothesis. The effect
of the leg-length discrepancy in decreasing coverage is present only
during two-legged stance and perhaps during gait if the pelvis is
lowered on the short side during swing phase as a compensatory
mechanism. When the patient is in one-legged stance, sitting, or lying
down, as is the case most of the time, this effect disappears.
 |
|
Figure 29.2
Decrease in center-edge (CE) angle with pelvic obliquity. The CE angle is decreased on the side of the long leg. Coverage is decreased and the resulting decrease in the load-bearing area causes an increase in pressure. Such a hip may be susceptible to late degenerative arthritis (L, left; R, right; H, Hilgenrine’s line; D, leg-length discrepancy). (From Morscher E. Etiology and pathophysiology of leg length discrepancies. Prog Orthop Surg 1977;1:9.) |
of knee pain in athletes, although the nature of the relation has not
been elucidated (6).
also not clearly established. The parents of young children with
leg-length discrepancy worry about the children developing degenerative
arthritis of the spine, low back pain, and scoliosis. Contradictory
evidence exists about the possibility that leg-length discrepancy
causes low back pain in the long term (7,8,9).
Low back pain is unusual in the younger child and is more common in the
adolescent, but there is no evidence that low back pain and leg-length
discrepancy are related in this age-group. Froh et al. (10)
studied whether leg-length discrepancy had any effect on the
orientation of the facet joints in adults and found none, whereas Giles
and Taylor (11) found changes in the facet
joints of cadavers with leg-length discrepancy. It is not clear whether
the incidence of back pain is higher in patients with leg-length
discrepancy than it is in the general population. Radiographs of the
spine should be examined carefully to rule out anomalous development of
the vertebrae.
leg-length discrepancy leads to scoliosis. Gibson et al. assessed 15
patients with leg-length discrepancy following femoral fractures and
found that after 10 years none had structural scoliosis (12), but minor structural changes have been reported in such patients (13).
Studies have demonstrated an increased incidence of structural
scoliosis in patients with leg-length discrepancy when compared with
the general population (14), but the leg-length
discrepancy has not been established as being the cause of scoliosis.
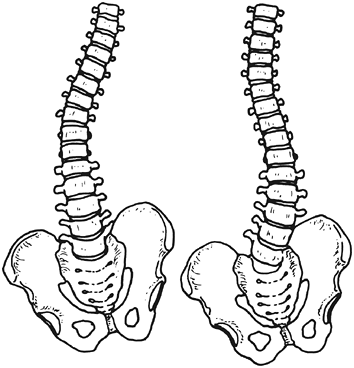
If leg-length discrepancy were the cause, the scoliosis would have been
expected to be in the direction that would compensate for the
leg-length discrepancy, but in up to one third of the cases in these
studies, the scoliosis was in the opposite direction (Fig. 29.4).
Because the leg-length discrepancy affects the spine only during
two-legged stance, and to some extent while walking, some of the
skepticism toward the cause-and-effect
hypothesis
seems justified. It has been suggested, however, that scoliosis
develops more as the result of the dynamic forces of walking and not
the static forces of standing (15).
 |
|
Figure 29.3
Relation between leg-length discrepancy and center-edge (CE) angle. The CE angle and coverage decrease with increasing leg-length discrepancy. For every centimeter of leg-length discrepancy, there is a decrease of approximately 2.6 degrees in the CE angle. (From Morscher E. Etiology and pathophysiology of leg length discrepancies. Prog Orthop Surg 1977;1:9.) |
spine or hips, it is reasonable to suspect that the severity of the
problem is related to the severity of the discrepancy, the degree to
which it remains uncompensated or uncorrected, and the age of the
patient at onset.
 |
|
Figure 29.4
Oblique pelvis with scoliosis in compensatory and noncompensatory directions. If leg-length discrepancy causes scoliosis, the direction of the spinal curvature is expected to be in the direction that is compensatory for the scoliosis. When scoliosis occurs in the other direction, it must be concluded that the leg-length discrepancy is not responsible. |
to the treatment of patients with leg-length discrepancy. The
mechanisms of growth are discussed in Chapter 1.
In the study of leg-length discrepancy, we are concerned with the rates
and patterns of growth. Growth of the leg is the result of both growth
at the four physeal plates at the proximal and distal ends of the tibia
and femur and an increase in size of the four adjacent epiphyses. The
growth of the epiphyses contributes only 5% to the total growth of the
legs, and this is usually ignored in treating patients with leg-length
discrepancy.
Their second study was longitudinal in that a single group of children
was followed up until maturity. They published their data in two forms.
The first form related the lengths of the femur and the tibia of boys
and girls to their ages (from 1 to 18 years) (17).
These data can be combined to show the total leg lengths rather than
the lengths of the individual bones. The total leg-length data are
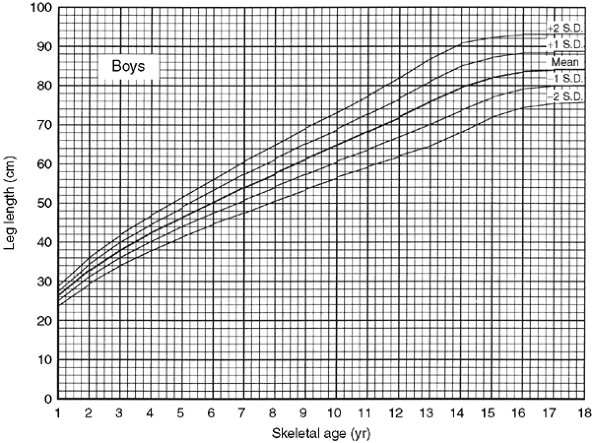
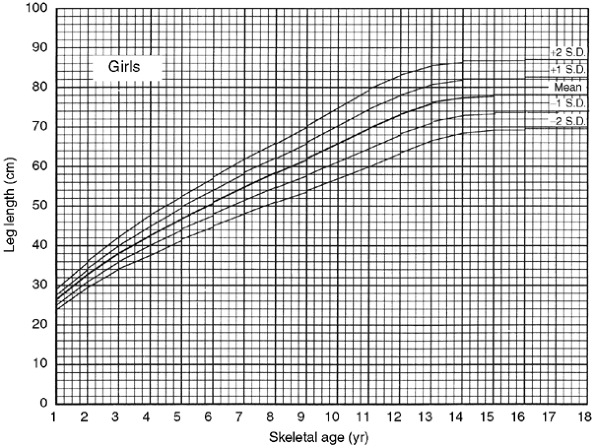
shown here in tabular (Table 29.1) and in graphic forms (Figs. 29.5 and 29.6).
The graph showing leg lengths related to age is a useful tool in the
analysis of leg-length data. They later published their data in the
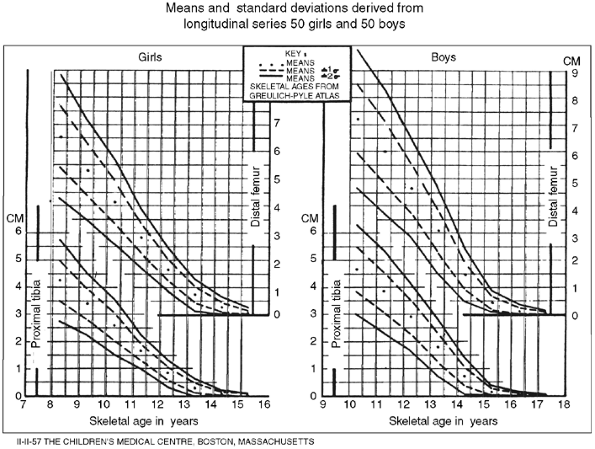
form of a graph showing the growth remaining at the distal femoral and
proximal tibial physes of boys and girls as a function of skeletal age (Fig. 29.7) (18).
physes near the knee, as opposed to the upper extremity where most of
the growth is contributed by the physes farthest from the elbow. The
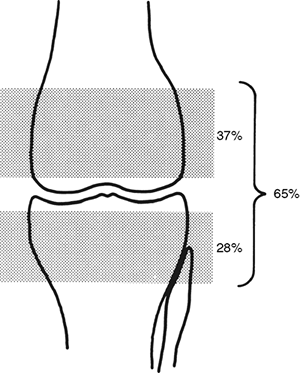
four growth plates of the lower limb contribute consistent proportions
of growth to their individual bones and to the entire lower extremity (Fig. 29.8) (19). These percentages are worth remembering
because they can be useful in clinical situations. For example, a child
with avascular necrosis of the femoral head in infancy cannot lose more
than 15% of future growth of the affected leg, and a child whose distal
femoral growth plate was destroyed because of infection will loose at
the most 38% of future growth. Also noteworthy is that the femur is
longer than the tibia, comprising 54% of the total length of the leg.
|
TABLE 29.1 LENGTH AS A FUNCTION OF SKELETAL AGE
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
maturity, and chronologic age (Fig. 29.9). Although these relations are familiar, some aspects deserve elaboration.
 |
|
Figure 29.5
Graph showing total leg length versus skeletal age for boys allows a specific boy to be related to the population by plotting his leg length as a function of his skeletal age. It is useful in the analysis of leg-length data because it allows a projection into the future on the basis of the present situation. (From Anderson M, Green WT. Lengths of the femur and tibia; norms derived from orthoroent-genograms of children from five years of age until epiphyseal closure. Am J Dis Child 1948;75:279–290.) |
 |
|
Figure 29.6 Graph showing total leg length versus skeletal age for girls serves the same purpose for girls that Figure 29.5
serves for boys. (From Anderson M, Green WT. Lengths of the femur and tibia; norms derived from orthoroent-genograms of children from five years of age until epiphyseal closure. Am J Dis Child 1948;75:279–290.) |
 |
|
Figure 29.7
Green and Anderson growth-remaining graph. This graph shows the amount of growth potential remaining in the growth plates of the distal femur and the proximal tibia of boys and girls as functions of skeletal age. The graph is useful in determining the amount of shortening that will result from epiphysiodesis. (From Anderson M, Messner M, Green W. Distribution of lengths of the normal femur and tibia in children from one to eighteen years of age. J Bone Joint Surg 1964;46-A(6):1197–1202.) |
 |
|
Figure 29.8
The growth plates of the lower limb contribute definite and constant proportions to the growth of the long bones of the leg and the total growth of the limb. These contributions determine the slopes of the reference lines of the straight-line graph method and are therefore automatically taken into account by it. |
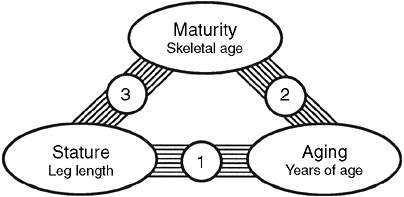
that maturation and aging are only weakly related. Some children mature
rapidly and go through their growth spurts early. These children appear
tall during the growing years not because they are of a taller growth
percentile but because they are of advanced maturity. Many of these
children who are tall for their chronologic age during early
adolescence who mature early stop growing at a younger age than usual,
and are shorter than the mean height at maturity. Pediatricians, in
their studies of stature, and orthopaedic surgeons, in their studies of
leg lengths, need a measure of maturity rather than a measure of age.
Although leg lengths and chronologic age can be measured accurately and
easily, maturity cannot be.
 |
|
Figure 29.9
Leg length, maturity, and age change with time, but the way they do so is not identical in every child. The three relations among these factors can be examined individually, and it is important to do so to understand parents’ perceptions and to predict the patient’s future growth. Relation 2 is about maturation, whereas relations 1 and 3 are both about growth but from different perspectives. |
development of the bones of the skeleton as seen on radiographs. By
comparing the radiographs of a patient with standard radiographs in an
atlas (20), it is possible to derive a number known as the skeletal age.
The skeletal age is actually the most likely age of a person with the
given radiograph, but orthopaedic surgeons use it as a measure of
maturity. It correlates well with menarche and other signs of
maturation such as the appearance of secondary sexual characteristics
and also correlates more closely with the growth of the legs than
chronologic age does. Because the skeletal age standards represent an
average of the general population, it should be apparent that for a
random group of children, the mean skeletal age should be equal to the
mean chronologic age.
can be examined individually, and it is instructive to do so. First,
consider the relation between growth and chronologic age. This relation
is obvious to parents. The parent who is concerned about his or her
child being too short or too tall repeatedly compares the child to
classmates and other children of the same age. In this relation, there
is steady growth throughout life, with a growth spurt in early
adolescence and cessation of growth at the age of 16 to 17 years in
boys and 14 to 15 years in girls (Fig. 29.10) (21).
Although this relation is the most obvious, it is virtually meaningless
without a consideration of maturity, and its variability from child to
child presents problems for the treating doctor. A more consistent
relation must be used.
skeletal age is extremely variable within the population, children tend
to pass the various landmarks of maturity in the same orderly and
consistent manner. The child who develops secondary sexual
characteristics early also goes through the growth spurt early, reaches
menarche early, and has advanced skeletal age. The implication is that
children who mature more quickly or slowly than the average rate do so
throughout their growing years, but this is not necessarily so. Some
children appear to be going through a maturation spurt, during which
they mature faster than they age. This maturity spurt tends to coincide
with the growth spurt, and the orthopaedic surgeon who is waiting for
just the right time to do an epiphysiodesis can be caught unawares. It
is as if these children deal with their rapid growth rate by turning
the pages of the skeletal age atlas at a faster rate.
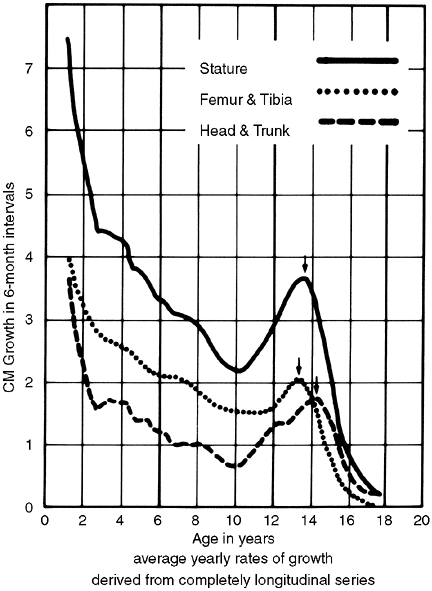
correlates more closely than the relation between chronologic age and
skeletal age and is of most interest to the orthopaedic surgeon. This
is the relation shown in the data of Green and Anderson (Figs. 29.5 and 29.6),
and it has interesting properties. The first property is that growth is
never faster as the child becomes older, in either absolute
or
relative terms, than it was when he or she was a newborn. The growth
rate continually slows down until it stops completely at maturity.
There is no inflection point in the curve, which means that the growth
spurt that is obvious when growth is correlated with chronologic age
disappears when growth is correlated to skeletal age. The absence of
the growth spurt has two possible explanations. The first is that these
curves are derived from averages of a number of patients and that
averaging data tends to minimize individual variations that occur at
different times. The second, more interesting, explanation is that the
growth spurt may actually represent a maturation spurt in which growth
maintains its customary relation to skeletal age.
 |
|
Figure 29.10
Green and Anderson growth curve. The examination of growth rate as a function of chronologic age shows a major growth spurt during adolescence. Interestingly, no such spurt appears in the growth curve of Figures 29.6 and 29.7. (From Green W, Anderson M. Skeletal age and the control of bone growth. Instr Lect Am Acad Orthop Surg 1960;17:199–217.) |
of the relation between skeletal age and leg length for the population
studied, but there is no guarantee that these data hold for children of
other races or of other genetic stocks from within the same race.
Indeed, there is one report that shows that Dutch children are taller
than those described by Green and Anderson (22).
It is not clear, however, that these children actually follow a pattern
of growth that is different from that described by Green and Anderson.
They may just behave like children taller than Green and Anderson’s
mean. Indeed, Paley supported this concept by reporting an extensive
review of published growth patterns of children of varied races and by
concluding that they all share the same pattern of growth (23).
etiology, but the concepts involved in understanding the disorder and
in treating patients suggest a more logical approach. Leg-length
discrepancy can result from two types of processes: those that change
the length of the leg directly and those that alter its growth. A
fracture that heals with overriding is an obvious example of an
alteration in length with no effect on growth, and injury to the growth
plate from osteomyelitis is an example of an alteration in growth rate
with no immediate effect on length. These two effects determine whether
a discrepancy is static or dynamic and, therefore, greatly influence
the choice of treatment. The causes of leg-length discrepancy can be
classified according to their effect on length or growth, but the
classification system breaks down because certain causes can affect
different patients differently, and some causes affect both length and
growth. A fracture, for example, can cause leg shortening in one
patient and overgrowth in another, and a congenitally short femur can
be thought of as being both short and retarded in its growth. Such a
classification is shown in Table 29.2.
present as having leg-length discrepancy and may be treated in the same
manner as for leg-length discrepancy, although their legs may be of
equal length. Examples include high-riding congenital dislocation of
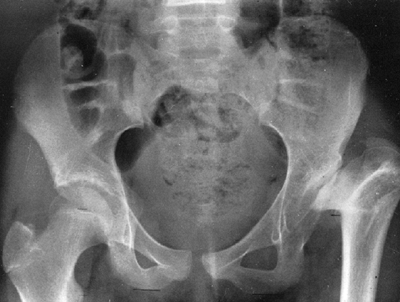
the hip (Fig. 29.11) and fixed pelvic obliquity
secondary to scoliosis, both of which illustrate the difference between
functional and anatomic leg-length discrepancy.
affect the length of the leg are fractures and dislocations. Whether a
congenitally short bone has suffered direct interference with its
length is a moot point, because this interference occurred before
birth, and it is the inhibition growth that is the important factor.
The terminal deletions and proximal focal femoral deficiency and its
variants can be thought of as growth inhibition superimposed on a short
limb.
or by angular deformity. In the latter case, the shortening often
disappears when the angulation is corrected (24).
Sugi and Cole have shown that shortening of up to 10% of the femoral
length can be accepted in the treatment of femoral fractures by early
spica without causing considerable discrepancy (25). Overgrowth frequently accompanies healing of fractures and can spontaneously correct the shortening (26). Excessive length can result when excessive force is applied in traction.
|
TABLE 29.2 CLASSIFICATION OF CAUSES OF LEG-LENGTH DISCREPANCY
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||
mechanisms. First, congenital short bones grow more slowly than normal
bones because of abnormal programming of the genetic mechanism that
determines growth rate. Second, the growth plate can be injured in such
a way that a part or all of it is no longer able to grow, and it
eventually gets converted to solid bone in the form of a physeal bridge
or a prematurely closed plate. Any part of the plate that has retained
its ability to grow cannot do so effectively because of tethering by
the fused part. Third, a change in the environment of the plate can
influence its growth rate. Unusual vascular malformations can stimulate
or inhibit growth (27,28).
Children with paralysis usually have shortening of the more severely
affected leg, presumably because the growth rate of the plate responds
to the decreased compressive forces across it. The concept that
pressure might change the direction of the growth of the plate is
commonly known as the Heuter-Volkmann law (29,30), but this concept was first proposed by Delpech (31,32).
Delpech treated an angular deformity of the ankle with casting that
caused the distal tibial plate to change its direction of growth.
 |
|
Figure 29.11
High-riding dislocation of hip. This patient has a functional leg-length discrepancy that is greater than the actual discrepancy in the lengths of the legs because of the adduction of the short leg. |
are otherwise normal, it is often impossible to know which leg is the
abnormal one. Because the more severe cases clearly involve shortening,
it is appropriate to think of these cases as hemiatrophy rather than
hemihypertrophy. Beals has stated that hemiatrophy and hemihypertrophy
are distinct clinical syndromes, partly because of their associated
anomalies (33). The dysplasia usually involves
the entire limb, with some shortening of all components, and usually is
accompanied by a diminution in girth. Each leg appears to be
genetically programmed to be of a different size (34).
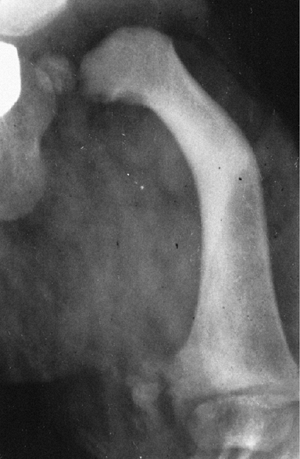
and absence of the lateral rays of the foot. Indeed, the congenital
short femur is thought by some to be a variant of proximal focal
femoral deficiency (43).
 |
|
Figure 29.12
In proximal focal femoral deficiency, the leg-length discrepancy is accompanied by qualitative changes, including coxa vara and bowing. |
 |
|
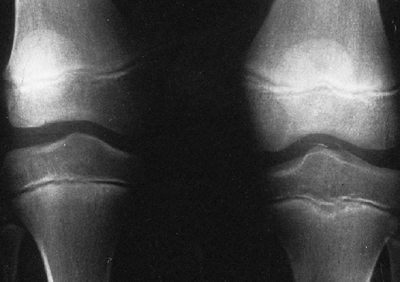
Figure 29.13 Hypoplasia of femoral condyle is frequently found in association with congenital shortening of the femur.
|
rate of growth either by direct injury to the cells responsible for
growth or by formation of a bony bridge that tethers the epiphysis to
the metaphysis. Salter and Harris provided a classification of
fractures of the physeal plate that is useful in anticipating the
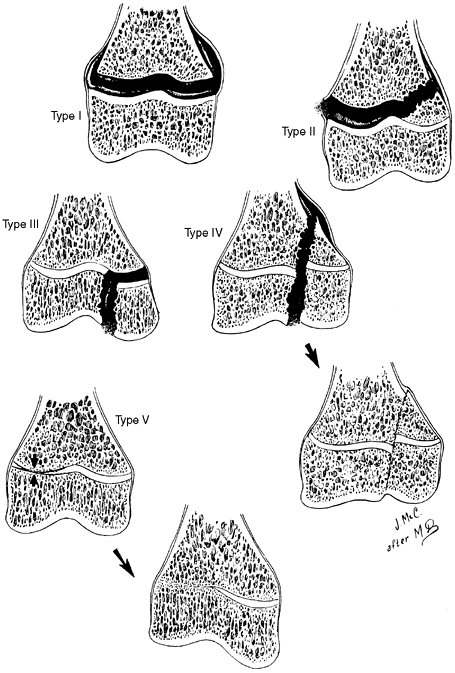
effect of fractures on future growth (49). This classification is shown diagrammatically in Figure 29.14.
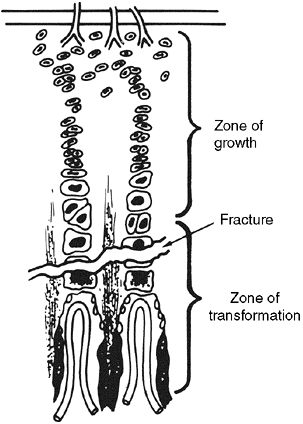
Fractures can wander through all zones of the plate, but tend to pass
through the zone of cell hypertrophy where the material is weakest and
the amount of material is least. The material in that zone is
cartilage, which is weaker than bone, and because the cells there are
large, the ratio of matrix volume to cell volume is low (Fig. 29.15).
It is important to note that this part of the plate is the site of
conversion of cartilage to bone, but it is not primarily responsible
for growth that occurs by virtue of cell multiplication and matrix
production in the zones nearer the epiphysis.
the growth zone, they are less likely to interfere with growth than
other fractures are. Both types of fractures, however, may be
associated with a crush injury, which injures the cells by compression.
This mechanism may account for the higher than expected incidence of
growth disturbance in type II fractures of the weight-bearing bones,
such as the distal femur, where growth arrest is found in more than one
third of patients (50). Type III and IV
fractures do, however, cross the growth zone and are therefore more
likely to result in growth arrest. The type IV fracture, in particular,
can result in a bony bridge when the fracture fragment displaces in the
diaphyseal direction (Fig. 29.14). This is one
reason why these fractures must be anatomically reduced. Type V
fractures can occur in isolation or can accompany any of the other
types of fractures. Type V fractures are insidious because they are not
initially recognizable on radiographs and they always demonstrate their
presence by a disturbance of growth, either a shortening or
a
combination of shortening and angulation, usually in the first year
after the occurrence of the fracture. Although the fracture
classifications provide guidelines about the likelihood of growth
arrest, the orthopaedic surgeon must be wary of giving a definite
prognosis for a given epiphyseal fracture until enough time has elapsed
to rule out a type V injury.
 |
|
Figure 29.14
Salter-Harris classification of epiphyseal fractures. Fractures of types I and II do not cross the part of the growth plate that is responsible for growth, whereas those of types III and IV do. In the type IV fracture, approximation of epiphyseal bone to metaphyseal bone can result in formation of a bony bridge. (From Salter R, Harris W. Injuries involving the epiphyseal plate. J Bone Joint Surg 1963;45A: 587–622.) |
following physeal fractures usually is discrete and well defined and
lends itself to excision if the fractures are small and peripheral.
Bridge resections usually are limited to those that involve less than
50% of the plate in patients who have at least 2 years of growth
remaining. Even more extensive resections can be considered in very
young children because, if successful, difficult treatment of severe
leg-length discrepancy might be avoided. Resection of a bony bridge
always should be considered if there is significant growth remaining,
even if leg-length discrepancy is already present. The angular
deformity that may also be present because of the bridge can influence
treatment of the discrepancy because both deformities can be corrected
at once.
destruction of physeal cells and disturbance of growth if not treated
early (51). The infection is usually
hematogenous osteomyelitis of the metaphysis, but it can be epiphyseal
in infants and can follow or precede septic arthritis of the joint. The
bony bridge that results from infection is more difficult to treat than
that following trauma because it is not so amenable to resection. The
bridge tends to be larger,
more
central, and less discrete than that following trauma and can even
consist of multiple small bridges. It is difficult to define by
radiograph, is usually more extensive than it appears, and is more
difficult to define during resection. There is the danger that minor
components of the bridge can be left behind because the usual end point
of resection, a continuous line of physis around the resection tunnel,
can be achieved despite incomplete resection of all components of the
bridge.
 |
|
Figure 29.15
Location of fractures in the growth plate. Fractures tend to occur through the part of the growth plate where the matrix is least, although individual fractures may wander from zone to zone. |
discrepancy problems than trauma does because it occurs commonly in
younger children with much growth ahead of them. As with trauma,
angular deformity and leg-length discrepancy can coexist.
paralysis of the leg, but the mechanism of this inhibition is not
clear. It may be true that blood flow to the limb is reduced because of
the reduced muscle mass, but this does not necessarily mean that flow
to the plate is also reduced. Venous return results partly from muscle
activity and, therefore, it is conceivable that reduced muscle activity
and decreased pumping effect could reduce blood flow to the limb and
perhaps to the plate. Alternatively, abnormal vasomotor control from
the neurologic abnormality could affect blood flow.
suggests that the growth rate of the physis responds to the compression
forces across it, and this law is frequently used to explain how a
spontaneous reorientation of the physis occurs while contributing to
the remodeling of angular deformities in the immature child. This
mechanism also can explain the decrease in the overall growth rate that
occurs in children with muscle weakness.
frequently concerned about leg-length discrepancy, and minor degrees of
discrepancy can be seen in this condition. More often, however, the
discrepancy is more apparent than real and occurs because of pelvic
obliquity due to hip contractures or asymmetric posturing due to
asymmetric spasticity. It is likely that serious discrepancies do not
occur more often because even dysfunctional spasticity can be
effective, through the Heuter-Volkmann law, in stimulating the physis
to grow.
several ways. The first way involves destruction of the plate by direct
tumor invasion, the mechanism of invasion in this instance being
similar to that of infection.
Irradiation has a particularly harmful effect because the osteocytes of
the neighboring bone also are killed, and the bone can take many years
to become revascularized and repopulated with healthy osteocytes. The
absence of healthy osteoblasts and precursors can complicate the
treatment of the ensuing leg-length discrepancy by precluding
lengthening procedures through the affected bone. Radiation damage to
regional soft tissues also complicates lengthening procedures (53,54).
involves the diversion of cartilage cells of the physis into the tumor,
thereby stealing growth potential from the plate. Examples of this are
enchondromatosis, Ollier disease (55), and
osteochondromatosis, all of which are derived from chondrocytes of the
growth plate. Although unicameral cysts usually do not result in
significant leg-length discrepancy, some of the growth disturbances can
result from aggressive attempts to remove the cyst wall when the cyst
is active and immediately adjacent to the plate. Unicameral cysts and
fibrous dysplasia cause leg-length discrepancy because of both growth
inhibition and repeated fractures with minimal displacement that
produce progressive varus.
the epiphyseal circulation, avascular necrosis of the epiphysis
frequently involves the growth plate as well, resulting in leg-length
discrepancy. This may be seen in Legg-Perthes disease and in avascular
necrosis following treatment of developmental dislocation of the hip.
Peterson has reported a case in which a discrepancy resulted from a
temporary but substantial episode of vascular insufficiency during
surgery in an infant (56).
In the case of congenital dislocation of the hip, the effect is
maximized by onset at an early age and by the years of future growth
that are affected but is moderated by the fact that the growth plate of
the proximal femur contributes only approximately 15% of the growth of
the limb. The likelihood of substantial discrepancy has been correlated
with the pattern of ischemic damage to the head and increases with
increasing involvement (57). The fact that the
vascular damage to the epiphysis does not always significantly affect
the physis is indicated by the observation that patients with
Legg-Perthes disease do not usually develop substantial deformity (58). Leg-length discrepancy also has been reported as a complication of catheterization of the umbilical or femoral artery (59), presumably because of impairment of the arterial supply to the physis.
Although increased circulation is thought to be the mechanism of growth
stimulation, there is only circumstantial evidence to support this
theory. Attempts to stimulate growth in the treatment of leg-length
discrepancy have been made by numerous means, including sympathectomy
to increase blood flow, insertion of foreign materials next to the
physis, stripping and elevation of the periosteum (60,61,62), surgical establishment of an arteriovenous fistula (63), shortwave diathermy (64), and electrical stimulation (65). None of these methods has consistently produced sufficient growth stimulation to be clinically useful (66,67),
but the fact that the arteriovenous fistula does produce stimulation at
all supports the hypothesis that increased circulation can be a final
common pathway for the conditions that stimulate growth.
large portions of the limb, produce growth stimulation that often
involves all growth plates of the limb and not just the ones in
proximity to the tumor or the plates of the involved bone. This
stimulation is seen with hemangiomatosis and Klippel-Trenaunay-Weber
syndrome (68). Stimulation is also seen with
certain nonvascular tumors, such as neurofibromatosis, fibrous
dysplasia, and Wilms tumor, although an increase in circulation could
be the final common pathway to growth stimulation.
chronic osteomyelitis, presumably because of the increased blood flow
to the limb as part of the inflammation. Infection, therefore, can both
inhibit and stimulate growth. Overgrowth of the affected limb can be
seen in pauciarticular juvenile rheumatoid arthritis (69), particularly in those cases with onset before the age of 3 years (70), and has also been reported in a patient with hemophilia and with chronic knee synovitis (71).
bones in children and also is believed to result from the increased
blood flow to the limb that is part of the healing process (72).
One particularly pernicious example of this effect is the overgrowth of
the tibia and valgus deformity that can follow minimally displaced
proximal metaphyseal fractures (73). The mechanism involves overgrowth of the medial side of the tibial growth plate (74), possibly because of tethering by the fibula or by release of the torn medial periosteum (75,76,77,78).
Some studies have reported that the stimulatory effect can last for
years, but it is believed to occur principally during the healing and
remodeling periods in the first 2 years after the fracture (81).
The stimulation has been reported variously to be the greatest in
fractures in the proximal third of the femur, the middle third of the
femur (82), and in those fractures with greater degrees of overriding (83); it can be accompanied by overgrowth of the fractured tibia on the same side. Conversely, Meals (84)
found that the patient’s age and the type and location of the fracture
did not influence the extent of overgrowth, although, inexplicably,
handedness did.
leg-length discrepancy to concentrate attention on the lengths of the
legs and the discrepancy and to ignore other factors that are important
in the patient’s function and the ultimate outcome of treatment. When
the process of choosing treatment goals is discussed later in this
chapter, the importance of a complete and thorough assessment of the
patient is emphasized.
its previous treatment should be obtained. The cause of the leg-length
discrepancy is important because knowledge of whether length, growth,
or both is affected is essential to understanding the growth pattern.
Also important is knowledge of the affected physeal plates, because
this permits an estimation of the future increase in the discrepancy.
Parents of patients expect this kind of information on the first visit,
and it is important in establishing a good parent-patient-surgeon
relationship to be as knowledgeable as possible about the condition and
its future. The history of surgery, including surgery to correct
angular deformity, is needed because the numeric data about leg lengths
can be misinterpreted if the examiner is
unaware of previous surgery that might have affected the leg lengths.
deformity, and neuromuscular deficits, is referred to during selection
of the treatment goal. The fact that a patient’s discrepancy is of
congenital origin suggests increased risk and indicates that certain
precautionary steps to avoid complications must be taken in conjunction
with lengthening. Instability of an adjacent joint can preclude
lengthening. Weakness of the leg suggests that the weak leg should be
left a little short to facilitate floor clearance during swing phase.
measurements of the patient should not blind the physician to the
necessity of conducting a careful and complete physical examination.
There are two reasons for this. First, the radiographic measurements
can be wrong because of artifacts caused by angular deformity,
positioning, or patient movement. Second, there are important factors
that are not measured by routine radiographs but that can be crucial to
the outcome of the treatment. The second point is discussed in the
section on goal selection. After assessment of the patient is complete,
the clinical assessment must be consistent with the radiologic
assessment, or else an explanation must be sought. Additional
radiographs or reexamination can be necessary to determine the reason
for the inconsistency. In the final analysis, it is the functional
leg-length discrepancy that must be treated, and that cannot be
determined by radiographs.
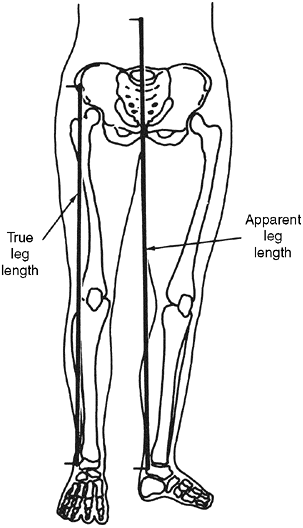
each leg from the anterior superior iliac spine to the tip of the
medial malleolus. The apparent length is measured from the umbilicus to
the tip of the medial malleolus. These two measurements of the
leg-length discrepancy may yield different results, because the
apparent length is affected by pelvic obliquity and hip position. The
leg on the side of the adducted hip appears shorter, but the real
length is minimally affected (Fig. 29.16). The
patient should be undressed completely for this measurement to avoid
tenting of the tape over the clothes. In any case, the tape often tents
over or around the knee, impairing the accuracy of the measurement.
Because different landmarks are used, the lengths of the legs measured
in this way are not the same as those measured by radiograph, but the
discrepancies should correspond closely.
used as a landmark for measuring the segment lengths of the tibia and
femur. Although this method is less accurate than the radiologic
measurement, it is useful for comparison to prevent errors. If the
relative heights of the knees will be a strategy factor, then the
lengths of the individual bones can be determined as well.
 |
|
Figure 29.16
The measurement of real length is relatively immune from error because of pelvic obliquity. Measurement of apparent length is susceptible to error. |
The block height required to produce a level pelvis should correspond
to the measured discrepancy. Blocks also can be used to estimate the
extent of correction that feels best to the patient and provides him or
her with the best correction. This amount can be different from the
radiographic measurement, and it may indicate that the goal of
treatment should not be exact correction of leg length. This assessment
is most useful in the mature patient whose discrepancy will not be
changing with growth. Although it only measures the present length and
does not indicate the desired amount of correction, it can be helpful
in immature patients to indicate that exact correction is not the best
goal. Carrying this idea further, the patient can be provided with a
temporary shoe lift and can be assessed after a period of ambulation.
These techniques are especially useful for patients with complex
deformities, because they take into account the combined effects of
asymmetric feet, angular deformities, contractures, pelvic obliquity,
and spinal balance.
because they affect the measurement of leg length or influence the
final outcome of the patient’s treatment.
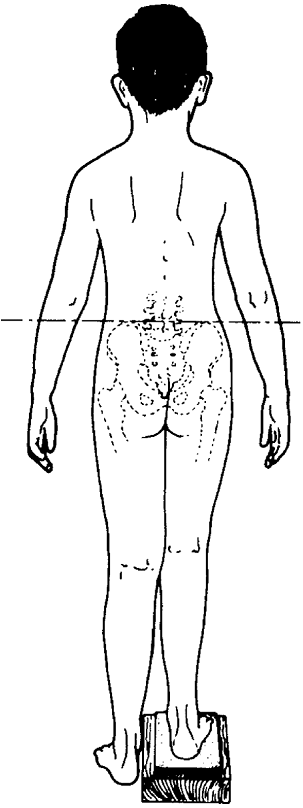 |
|
Figure 29.17
Placing blocks beneath the heel of the short leg allows assessment of the combined effect of all factors that produce functional leg-length discrepancy. |
contractures tend to shorten the leg, an equinus contracture of the
ankle tends to lengthen it, and pelvic obliquity also tends to affect
apparent length. The term pelvic obliquity has a broader meaning here
than when used by spinal surgeons, who use it to refer to the relation
of the position of the pelvis to that of the spine (85).
In the context of leg-length discrepancy, it refers to the relation of
the position of the pelvis to that of the legs, and it is affected both
by adduction and abduction contractures of the hips and by spinal
deformity. An adduction contracture of the hip causes the leg on the
affected side to appear short and to be functionally short, whereas an
abduction contracture has the opposite effect (86).
This situation is common in patients with cerebral palsy and those with
the residuals of poliomyelitis. A difference between the measured real
and apparent discrepancies indicates that pelvic obliquity is present.
adduction contractures produce a functionally short leg and abduction
contractures produce a functionally long leg (87).
These effects occur even in the absence of an actual discrepancy as
measured by a scanogram. Conversely, in a patient with true shortening,
a limitation of ipsilateral hip abduction or other side hip abduction
will prevent him or her from compensating easily and will exaggerate
the effect of the discrepancy.
the measurements of leg length and influences the final outcome if it
is to be corrected later. Joint stability must be assessed because it
pertains particularly to the risks of leg lengthening. Femoral
lengthening is contraindicated in the presence of hip instability.
Congenitally short femurs always are associated with laxity of the
anterior cruciate ligament (37) and with hypoplasia of the lateral femoral condyle (Fig. 29.13)
that predisposes to posterolateral subluxation of the tibial plateau.
Lengthening of the tibia can be contraindicated in the presence of an
unstable ankle or a useless foot which might be better handled by
amputation and prosthetic fitting.
there is stiff suprapelvic obliquity such that the axis of the trunk
cannot be made perpendicular to the transverse axis of the pelvis, an
equalization of leg lengths will cause an imbalance of the trunk, and
some modification of that goal will be necessary. Adduction and
abduction contractures of the hips produce infrapelvic obliquity, with
apparent and functional leg-length discrepancy (88).
because leg-length discrepancy in patients with paralysis or weakness
is usually best handled by undercorrection, leaving the weak leg short
to facilitate swing-through, particularly if the leg is braced with the
knee locked in extension. Patients who require bracing of the short leg
to walk can have their leg-length discrepancy corrected in the brace
and may not require surgical correction at all.
of the parents and patient must be taken into account. This aspect is
particularly important when lengthening is being considered, because
this is a long and difficult process requiring understanding and
cooperation by all involved. Despite excellent education and
preparation, parents and patients always underestimate the challenge of
dealing with the duration of lengthening and the later restriction of
activities. If there is a lack of understanding or a suggestion of poor
compliance, then another approach may be more appropriate. The surgeon
constantly must be aware that patients frequently express concerns
about function when they are actually concerned about cosmetic effect,
which may be less important when compared with the risks of surgery.
leg length. These methods are more accurate than clinical methods, but
each has its advantages and disadvantages. The nonexistent, ideal
method would allow the hip and ankle to be viewed, minimize radiation,
use only one exposure, use a film of convenient size, demonstrate
angular deformity, have no magnification, give true readings from a
scale on the film, and be inexpensive.
mortise is slightly saddle shaped, and the midpoint of the saddle can
be easily identified. Although these techniques allow the measurement
of the femur and tibia individually, these values are not required in
analyzing and predicting growth, and the length of the entire leg
suffices.
patient’s record but must review all radiographs before performing
surgery to check their accuracy and reliability. There is the
possibility that radiographs taken over the years have been read by
different individuals using different techniques and landmarks. It is
also possible that the scales have been misread or that arithmetic
errors have been made in determining lengths and discrepancies.
lengths directly and others that provide useful information. The
terminology is confusing because names used for these techniques are
inconsistent in the literature and in use. The term “scanogram,” for
example, was derived from the technique of split scanography, in which
the x-ray beam was tightly collimated to a thin transverse slit that
exposed the film as the x-ray tube was moved from one end of the leg to
the other. The principles are more important than the terminology.
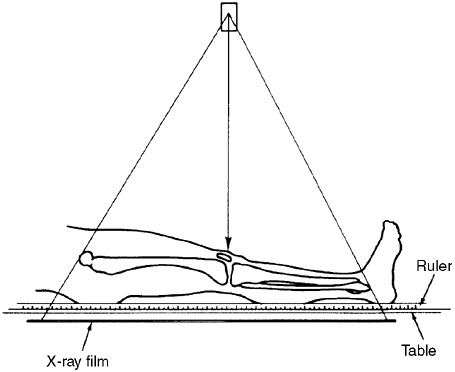
is a single exposure of both legs on a long, 35 cm × 90 cm (14 in × 36
in) film. It is taken from a 2-m (6-ft) distance, usually with the
patient standing and with a radiopaque ruler placed on the cassette. It
has the advantage of showing angular deformities and of using a single
exposure, but it produces a radiographic film that is inconvenient to
handle and measurements that are subject to magnification because of
parallax of the x-ray beam. There is no significant difference between
measurements of the leg taken supine and those taken standing (89).
 |
|
Figure 29.18
The teleoroentgenogram reveals angular deformity but is subject to errors of magnification. It is probably the best technique for children who cannot reliably comply with instructions to remain still for multiple exposures. |
 |
|
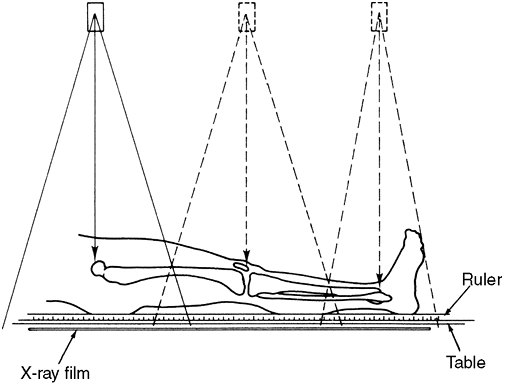
Figure 29.19
The orthoradiograph technique exposes each joint individually, thereby ensuring that the x-ray beam through each joint is perpendicular to the x-ray film, thereby avoiding errors of magnification. |
avoids the magnification factor by taking separate exposures of the
hip, knee, and ankle so that the central x-ray beam passes through the
joints, giving true readings from the scale (90).
However, the orthoradiographic film is still cumbersome, and the need
for multiple exposures introduces the risk of error if the patient
moves.
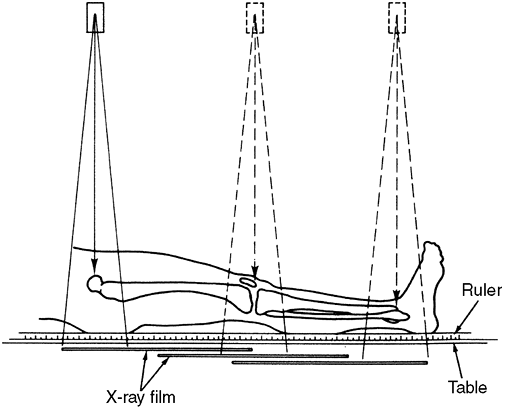
avoids magnification in the same way but reduces the size of the
resulting film by moving the film cassette beneath the patient between
exposures (91). This technique is preferred in
children older than 5 or 6 years who can be compliant with instructions
not to move, because it gives true measurements without
magnification, but younger children are better assessed using the teleoradiograph.
 |
|
Figure 29.20
The scanogram technique avoids magnification error in the same manner as the orthoroentgenogram does and is the preferred technique for children who can remain still for three exposures. |
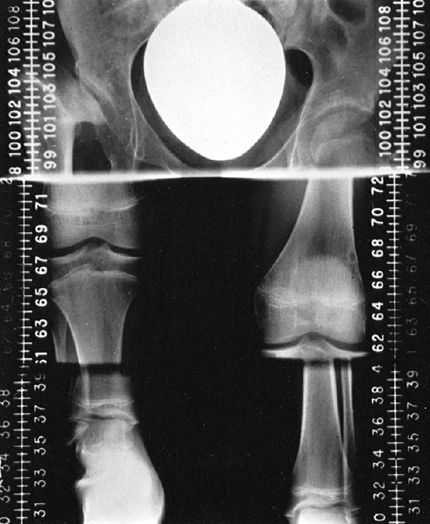 |
|
Figure 29.21
Scanogram technique allows the images of the three joints to be captured on a film of convenient size by moving the film beneath the patient between exposures. |
patients with contractures of the hip or knee. Patients with hip
flexion contractures can have accurate measurements taken in the
reclining position. In those with only knee contractures, the femur can
be measured in either the lateral or the prone position, and the tibia
can be measured in the lateral position. Assessment of both bones can
be made on one x-ray film in the lateral position if two rulers are
used, one parallel to each bone. If a hip contracture is also present,
the femur must be assessed in the lateral position. The scanogram of
both femur and tibia can be done in the lateral position.
Computed tomography scan can be used to measure the distances between
points on the radiographic film, reducing errors from angular deformity
(94). If the examination is done specifically for this purpose, the cost is comparable with more traditional techniques (95,96,97), multiple sections are unnecessary, and the radiation exposure is less, especially with microdose techniques (98).
consistent and to not mix true and magnified measurements when
analyzing data to determine the timing of surgery. Because errors are
possible with all of these techniques, the resulting measurements
should be compared with, and should correspond with, the clinical
measurements.
with the patient standing and the legs straight (on blocks if
necessary), is occasionally useful in assessing the combined effect of
leg-length discrepancy, angular deformity, and asymmetry of the foot
and pelvis because it more closely approximates the functional
discrepancy. The leg-length discrepancy is calculated from the heights
of the femoral heads from the floor, by taking into account the height
of the blocks. This assessment can supplement the clinical examination
done with blocks.
information from the various methods of measuring leg length while
preparing a treatment strategy.
radiographs of the patient with standards in an atlas. Methods have
been described using the bones of the pelvis and hip, the knee, and the
hand and wrist (99,100).
The estimation of skeletal age is only moderately accurate and is the
weak link in the techniques of analysis and prediction of growth.
consists of reproductions of radiographs of the left hand and wrists of
boys and girls that the authors considered typical for the stated
skeletal age. To estimate the skeletal age of the patient, a radiograph
of the left hand and wrist is taken according to the technique
described in the atlas, and this film is then compared with the
standard radiographs of the appropriate gender according to qualitative
(e.g., the visibility of the hook of the hamate) and quantitative
(e.g., the degree of conformity of an epiphysis to its metaphysis)
criteria. The standard that most closely matches the patient’s
radiograph is taken as the skeletal age.
that in some parts of the atlas the standards represent skeletal ages
that are far apart, with a gap as great as 14 months. There is
therefore a large standard error built into the technique. With
practice, it is possible to interpolate between standards, but this
practice is difficult, and studies have shown significant interobserver
and intraobserver errors (101). A second
problem is that some children do not follow the same orderly succession
of maturity indicators shown in the atlas, and an arbitrary choice must
be made in assigning a skeletal age. Third, some children with
leg-length discrepancy of congenital origin also have congenital
anomalies of the hand and wrist, making it impossible to reliably
compare their radiographs with the standards. Finally, one of the
features of almost all atlases of skeletal ages is that the mean
skeletal age of a sample of similarly aged children is equal to their
chronologic age so that the skeletal age is, in fact, the best possible
predictor of chronologic age. That is, of a random sampling of boys or
girls of the stated chronologic age, half would be more developed and
half would be less developed than the standard radiograph. Greulich and
Pyle, however, in selecting standards
for
their atlas, did not follow this principle exactly, and in some cases
they selected radiographs that they believed were more representative.
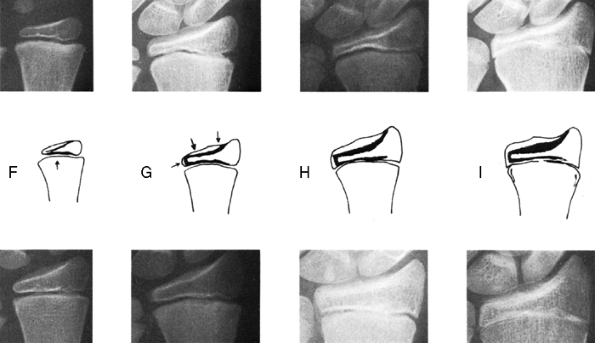 |
|
Figure 29.22
The Tanner-Whitehouse atlas provides standards such as this for 20 different landmarks in the hand and wrist. This technique allows the determination of skeletal age to an accuracy of months but is not consistent with the Greulich-Pyle atlas. (From Tanner J, Whitehouse R, Marshall W, et al. Assessment of skeletal maturity and prediction of adult height (TW2 method), London: Academic Press, 1975.) |
is similar in that it uses radiographs of the hand and wrist but was
developed using modern computerized mathematical procedures. It adds a
level of refinement and accuracy to the Greulich-Pyle technique by
defining and showing examples of successive stages of development of 20
specific bony landmarks in the hand and wrist (Fig. 29.22).
The same standards are used for boys and girls. The patient’s
radiograph is compared with the standards, and a letter score is
assigned to each of the 20 landmarks. The letter score then is
converted to a numeric score by consulting a table for the appropriate
gender. The sum of these 20 scores represents the level of skeletal
maturity attained by the patient, and it can be converted to years and
months with a much smaller standard error than with the Greulich-Pyle
technique. Of special interest in dealing with leg-length discrepancy
is that the bony landmarks can be divided into two groups. Tanner and
Whitehouse provide tables for including either the 12 long bone
standards of the hand or the eight cuboid bone standards of the wrist
in the assessment. When these two approaches give different results, it
is reasonable to assume that the long bone standards give a skeletal
age that is more pertinent to the growth of the long bones of the leg.
The concept that the long bones are more important than the cuboid
bones in the context of leg-length discrepancy can be useful, even with
the Greulich-Pyle atlas, in allowing the resolution of difficulty in
selecting the appropriate standard for a given radiograph.
the Greulich-Pyle method. Its use in the context of leg-length
discrepancy is problematic because this method and the Greulich-Pyle
method can produce different skeletal ages from the same x-ray. This is
surprising and probably is related in part to the fact that different
populations and different techniques were used to develop the standards.
as reliable as we would like it to be, and we must accept the relative
inaccuracy of skeletal age estimation. It is still possible to make
acceptably accurate predictions.
growth and no possibility of a changing discrepancy, presents with no
need of analyzing data. The growing child, on the other hand, whose
legs may be growing at different rates and whose discrepancy may be
changing, presents another level of difficulty. The treatment goal must
be chosen by considering the discrepancy that would be present at
maturity and not the present discrepancy, and therefore, before
performing surgery, the orthopaedic surgeon must be able to predict the
situation at maturity. The importance of proper data analysis cannot be
overemphasized. Blair et al. (103) reviewed 67
epiphysiodeses and found that correction to within 1 cm had been
achieved in only 22 cases, and 35 failures had occurred because of
incorrect use of the Green and Anderson data.
the arithmetic method, the growth-remaining method, the multiplier
method, and the straight-line graph method. These methods differ
significantly in their convenience, complexity, and accuracy, but the
analysis moves through the same stages in all four. The first stage is
the analysis of past growth, including the determination of the present
discrepancy, and, depending on the method, the growth percentile and
the growth inhibition. The second stage involves the prediction of
future growth, including the lengths of the legs and discrepancy at
maturity. The third stage is the prediction of the effects of
corrective surgery.
of the orthopaedic surgeon. To be used properly and without error, all
require a good understanding of the methodologies and the principles of
growth. The four methods are discussed here in general terms, and
step-by-step instructions for their use are shown in Figures 29.23, 29.24, and 29.25. The charts depicted in these figures are examples
of the use of these methods in a specific case. It should be noted that
the arithmetic method, the growth-remaining method, and the multiplier
method are designed solely to arrive at the correct timing of
epiphysiodesis. The straight-line graph method, on the other hand,
provides a visualization of the child’s entire pattern of growth and
also provides for the prediction of future growth.
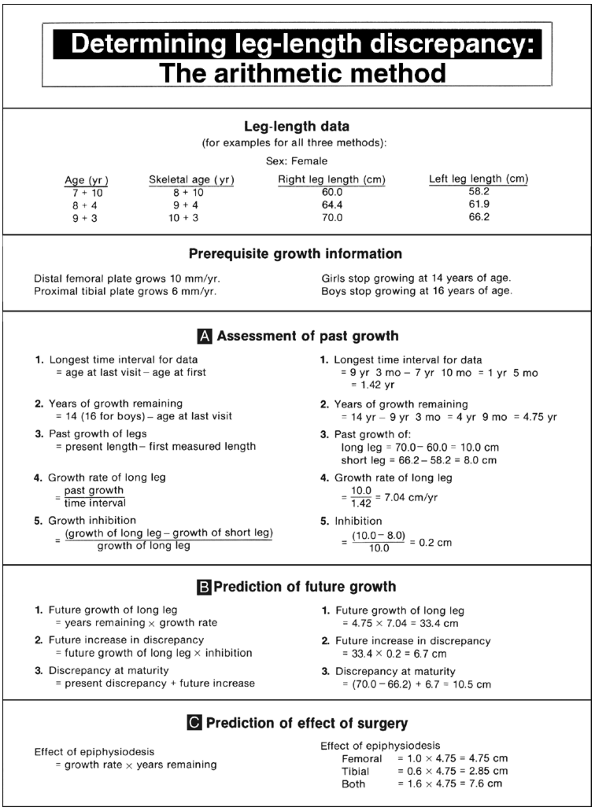 |
|
Figure 29.23
Step-by-step instructions for use of the arithmetic method. The method presented here is modified from that presented by Menelaus and Westh, in that the future increase in discrepancy is calculated from growth acquired in the past instead of being assumed to be 0.3175 cm per year of growth remaining. An example is shown in the panels in the right column. |
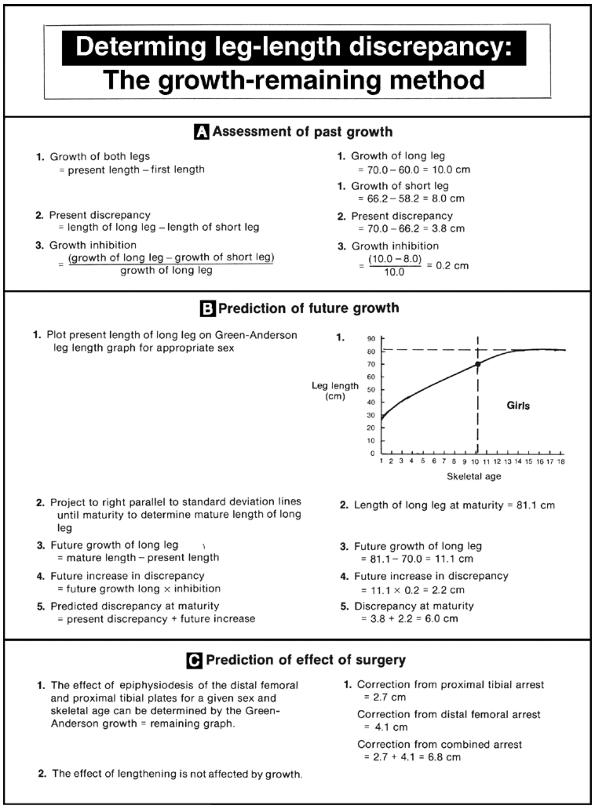 |
|
Figure 29.24
Step-by-step instructions for use of the growth-remaining method. An example, shown in the right column, uses the same data as in the example in Figure 29.23. |
 |
|
Figure 29.25 Step-by-step instructions for use of the straight-line graph method. The data used are the same as in the example in Figure 29.24.
|
nature of available data. The calculation of growth inhibition, for
example, is more accurate with data over longer intervals. It is the
interval over which data is collected and not the number of visits that
is important, and data should be gathered for at least 1 or,
preferably, 2 years. Minor errors in measurement over a short time can
lead to major errors in estimating the growth inhibition and,
consequently, to major errors in predicting the discrepancy at
maturity. In the straight-line graph method, a greater number of visits
can be useful in recognizing values that are in error, because they do
not fit the pattern established by other visits. For example, one
erroneous radiographic reading can be noticed in a group of other valid
points, but can be missed if the patient makes only one or two visits.
The straight-line graph is the only one of the three methods that uses
all available skeletal ages, and the accumulation of more skeletal age
estimates diminishes the errors in single estimates.
skeletal age estimation, have suggested that the use of chronologic age
is just as accurate and have reported on series in which that appears
to be true (104). The validity of that
suggestion is difficult to accept, however, when one considers that it
would lead to treating a 14-year-old boy with a skeletal age of 16
years in the same manner as a 14-year-old boy with a skeletal age of 12
years. Indeed, although Menelaus is frequently reported as using
chronologic and not skeletal age, he said that his method should not be
used if the difference was more than 1 year.
It is a method designed solely to manage discrepancy and to determine
the timing of epiphysiodesis and is not a general method to describe
growth. Menelaus intended the method to be based on measurements of
discrepancy by blocks and not on measurements of leg length (Menelaus
M, Personal communication, 1997). Although
the method uses calendar age and not skeletal age, Menelaus suggests
that it be used only in children whose skeletal and chronologic ages
are less than a year apart, rendering moot the question of calendar
versus skeletal age (Menelaus M, Personal communication, 1997).
statements, all of which are first approximations of the true growth
pattern described by Green and Anderson.
-
Girls stop growing at the age of 14 years.
-
Boys stop growing at the age of 16 years.
-
The distal femoral plate grows 3/8 in. (10 mm) per year.
-
The proximal tibial plate grows 1/4 in. (6 mm) per year.
-
The discrepancy increases by 1/8 in. (3 mm) per year.
years of growth but are inaccurate in young children. The statement
that the discrepancy increases by 1/8 in. per year is obviously not
true in all cases. It is, however, fairly accurate in those children
who are in the last few years of growth, whose discrepancies began at
birth, whose maturation is not considerably advanced or delayed
relative to their chronologic ages, and whose discrepancies are within
the clinical range for epiphysiodesis.
its convenience, because no special tools are needed for its use.
Menelaus recommended using English measure rather than metric measure
because the arithmetic required to deal with one eighths of inches is
elementary. There are several disadvantages of this method. It uses
chronologic age rather than skeletal age and is therefore subject to
error in children who grow and mature very early or very late. It uses
an approximation of the growth curve rather than the growth curve
itself and is only accurate in children approaching skeletal maturity.
If, however, its use is restricted to determining the timing of
epiphysiodesis and is applied only to the patients described earlier,
then good results can be anticipated as have been reported by Menelaus
et al. (106). Step-by-step instructions for the use of this method are shown in Figure 29.23.
the growth-remaining graph, in which the remaining growth in the
epiphyses of boys and girls is determined by first-order equations
using skeletal age, but this approach lacks the simplicity of the
arithmetic method (107).
These studies are to my knowledge the only good studies published that
relate leg lengths to chronologic and skeletal age and that serve as
the foundations of the more accurate methods of analyzing growth. Their
graphs describing the lengths of the legs of boys and girls as a
function of age can be used to determine the growth percentile of the
child and the future growth of the long leg. Their graph showing the
growth remaining in the distal femur and proximal tibia can be used to
predict the effects of epiphysiodesis (108) (Fig. 29.7).
age, is based on an accurate description of the growth pattern, takes
into account the child’s growth percentile in predicting future growth,
and has been demonstrated to be accurate over decades of use. The
disadvantages are that it
requires
the availability of the two sets of graphs, does not take into account
the growth percentile in predicting the effect of epiphysiodesis, and,
because it uses only the most recent skeletal age estimation, it will
be in error to the extent that the skeletal age estimate is in error.
An inherent hazard of this method is that the growth-remaining graph is
so familiar and easy to use that unwary physicians are tempted to
correct the present discrepancy in a growing child, neglecting the
steps that involve the prediction of future change. Step-by-step
instructions for the use of this method are shown in Figure 29.24.
show the leg lengths achieved at various ages. By simple division, this
data can be used to determine the proportion of adult leg length
achieved by any specific age. Assuming that congenital discrepancies
increase in proportion to leg length, it follows that the proportion of
ultimate discrepancy achieved is the same as the proportion of adult
length achieved. Paley et al. have calculated these proportions and, by
inverting them, have provided a table of multipliers that can be used
to predict the discrepancy at maturity given the present discrepancy
and age of the child (23). These multipliers are shown in Table 29.3.
The Green and Anderson data, for example, show that a boy has achieved
50% of his adult leg length by the age of 4 years. The multiplier for
4-year-old boys is 2, that is, a 4-year-old boy with a congenital
discrepancy of 3 cm can be expected to have a 2 × 3 = 6 cm discrepancy
at maturity.
|
TABLE 29.3 MULTIPLIERS
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
epiphysiodesis becomes more complex and requires calculations according
to formulas provided by Paley et al. (23). This
is particularly the case in developmental discrepancies where the
discrepancy does not increase in proportion to leg length. Paley et al.
have shown that the accuracy of this method is similar to that of other
methods (23).
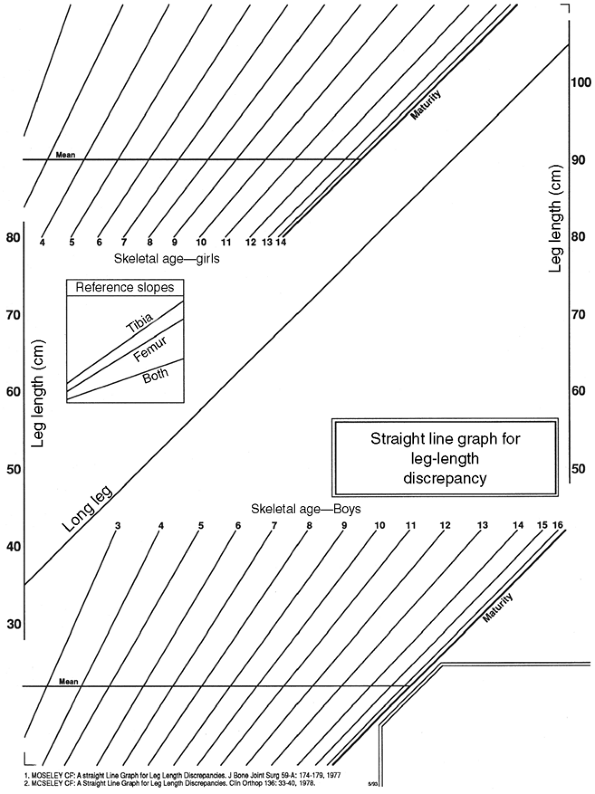
a method of presenting the relative growth of the legs in a clear,
graphic manner (Fig. 29.26). By incorporating the data of Green and Anderson (17), it evolved into a method of recording, analyzing, and predicting growth (109,110).
The method is based on two principles: the growth of the legs can be
represented graphically by straight lines, and a nomogram can be used
to determine the growth percentile from the skeletal age and leg length.
appears to contradict the Green and Anderson description of growth, in
which the growth lines of the legs are clearly curved. It is
accomplished by manipulating the scale of the abscissa (x-axis) in
strict accordance with the Green and Anderson data so that the curve
that disappears from the growth lines reappears as an irregularity of
that scale. This curve actually appears on the straight-line graph as a
variable distance between the skeletal age lines. The important fact is
that the straight line is not an approximation of the Green and
Anderson data but represents the data just as accurately as their
original graphs did. In the absence of active disease or treatment, the
relative rates of growth of the two legs stay constant, with the result
that the growth line of the short leg also follows a straight line on
the graph. This means that the length of the leg is represented on the
graph by the vertical position of its growth line, and its growth rate
by the slope of the growth line. The discrepancy, therefore, is
represented by the vertical distance between the two growth lines and
the inhibition by the difference in slope.
points in a way that relates the length of the patient’s long leg to
the population and, in a sense, depicts the growth percentile. The
nomogram is constructed so that all points for the child whose growth
pattern exactly follows the pattern described by Green and Anderson lie
on a horizontal straight line. In practice, this is rarely the case,
partly because of the inaccuracy of estimation of skeletal age, and
also in part because of the possibility that children of other races or
other genetic stock have different patterns of growth. It is likely
that the plotting of every skeletal age point on the nomogram before
drawing the horizontal line representing the growth percentile
“averages out” the inaccuracies of individual determinations of
skeletal age. Similarly, the risk of error due to single estimates of
skeletal age is likely to decrease with an increasing number of
estimates.
 |
|
Figure 29.26
The straight-line graph comprises three parts: the leg-length area with the predefined line for the growth of the long leg, the areas of sloping lines for plotting skeletal ages, and reference slopes to predict growth following epiphysiodesis. |
the effect of surgery. Lengthening or shortening will be represented on
the graph by a vertical shift of its growth line either upward or
downward to the appropriate extent without any change in its rate of
growth. Conversely, the effect of an epiphysiodesis will be to decrease
the slope of the growth line of the long leg. This is a strict
mathematical relation, and because the contributions of the individual
growth plates to the overall growth of the leg are known, the future
slope of the growth line can be predicted accurately. Reference slopes
on the graph depict the slopes to be followed after each of the three
possible types of epiphysiodeses: distal femoral, proximal tibial, or
both. For example, the slope of the growth line of the leg following a
proximal tibial epiphysiodesis will be reduced to 73%, having lost the
27% normally contributed by that epiphysis. Because the unoperated long
leg is defined on the graph as having a slope of 1.0, the slope
following that surgery would be 0.73.
skeletal age and the actual growth pattern described by Green and
Anderson; (ii) takes into account the growth percentile in predicting
future growth and the effect of surgery; (iii) minimizes errors due to
the inaccuracy of skeletal age estimation; (iv) facilitates the
flagging of erroneous values; and (v) avoids arithmetic errors (111).
It is a general tool for analysis, illustration, and prediction in
leg-length discrepancy and can be used in children with large
discrepancies and inhibitions, extreme growth percentiles, and marked
delay or advancement of maturation. Step-by-step instructions for the
use of this method are shown in Figure 29.25.1
inhibition is determined on the basis of past growth and is then used
to predict future growth. Evidence supports the
assumption that the growth inhibition will remain constant throughout the growing years (112).
Indeed, in my study of patients who went on to have epiphysiodeses, the
linear correlation coefficient between the lengths of the two legs was
greater than 0.955 in every case (109,110).
This is an extremely close fit and suggests that growth inhibition does
indeed remain constant. It should be noted, however, that none of the
children in this group had active disease or were under treatment that
could affect growth.
reported that the growth rate of the femur tends to increase, and that
of the tibia to decrease, following a lengthening procedure (113).
This effect is relatively short-lived and is not of sufficient
magnitude to affect clinical decisions. Koman et al. have demonstrated
constant inhibition in unilateral and bilateral proximal focal femoral
deficiency (114), and Hootnick et al. have shown constant inhibition in congenitally short tibias (115).
concerns the possibility of errors in correction due to changing
inhibition. Does changing inhibition cause clinically significant
errors in clinical judgment? A partial answer can be derived from my
study mentioned earlier, in which the inhibition did not appear to
change, and the straight-line graph predicted the final outcome within
1 cm in all cases. This suggests that whatever effect the changing
inhibition has, it is not sufficient to prevent reaching predictions of
future growth that are sufficiently accurate for good clinical results.
late, it is suspected that the time for epiphysiodesis is imminent, and
there is insufficient time to accumulate data required for accurate
assessment and prediction. In some cases, it is still possible to make
reliable assumptions about the growth pattern that allow accurate
prediction and confident treatment planning.
any prior data, and the only information available is from that
particular visit. The difficulty here is that it is impossible to
calculate the growth inhibition and predict the discrepancy at
maturity. If, however, the onset of the growth inhibition can be
determined, it can be assumed that the legs were of equal length at
that time. The length of the long (and therefore the short) leg at that
time can be estimated using either the Green and Anderson growth graph
or the straight-line graph, and the growth inhibition can then be
calculated in the usual way. Likewise, in the case of a congenital
discrepancy with inhibition beginning before birth, it can be assumed
that there was a time before birth when the length of both legs was
zero or, in other words, that the growth inhibition is equal to the
percentage difference in the length of the legs. This assumption is
consistent with the conclusion of Herron et al. (116).
leg-length measurements without skeletal age when the patient is not
immediately available to obtain them. In this case, the child’s
chronologic age can be used in place of the skeletal age, and this
approach is validated if the development of secondary sexual
characteristics and menarche are consistent with the chronologic age.
Little et al. have suggested, in fact, that chronologic age is as
accurate as skeletal age in determining the timing of epiphysiodesis,
but his study eliminated several patients on the basis of skeletal age
and dealt with a select population (104). In my
view, a surgeon should not base his timing for epiphysiodesis on
chronologic age and should take this approach only as a rough guideline.
longer active, there exists no continuing growth inhibition or
accentuation. Patients with leg-length discrepancy caused by
stimulation from fracture healing or osteomyelitis, or with shortening
caused by fracture malunion that occurred more than 2 years earlier,
can confidently be assumed to have a static discrepancy. The
discrepancy at maturity therefore will be the same as that at present,
and attention can be directed at correcting the present discrepancy.
of trauma or infection can present with an early minimal discrepancy,
but significant inhibition that, with growth, will certainly lead to a
greater discrepancy requiring treatment. In select cases, these
children can be treated with an epiphysiodesis of the corresponding
plate of the other limb. This plan does not correct the discrepancy but
ensures equal inhibition in both legs and an unchanging discrepancy
throughout the growth period.
decision reliable enough to undertake surgery cannot be made. In these
cases, it is best to abandon the possibility of epiphysiodesis and wait
until maturity, when the discrepancy can be corrected without error by
shortening or lengthening.
treatment method are two different and independent steps in the
treatment of patients with leg-length discrepancy and are discussed
separately here. The selection of the treatment goal depends on the
careful and thorough assessment of the patient, both clinically and
radiologically, and the reader is referred back to the earlier sections
on patient assessment.
Undercorrection of 1 or 2 cm is best for patients with paralysis of the
short leg. The residual discrepancy facilitates clearing of the floor
by the weak short leg during the swing phase of gait, and this is even
more important in patients who wear braces and have the knee locked in
extension to ambulate. In patients who cannot walk without braces, the
leg-length discrepancy usually can be made up in the brace, and
corrective surgery may not be indicated.
asymmetry that extends beyond the legs. Patients with congenital
shortening, for example, may have a small foot or hemipelvis, and in
these cases perfectly equal leg-lengths result in residual pelvic
obliquity and tilting of the lumbosacral joint. A similar situation can
arise in patients who have leg-length discrepancy from avascular
necrosis of the femoral head following treatment of congenital
dislocation of the hip and who have also had an innominate osteotomy.
These patients should be examined in the standing position, with blocks
beneath the short leg to relate the desired correction to the
leg-length discrepancy. The treatment goal can be modified as required.
junction cannot achieve a level pelvis and a vertical lumbar spine at
the same time. Usually, a vertical lumbar spine and good balance of the
spine are more important than a level pelvis, and treatment of the
leg-length discrepancy should be consistent with these more important
goals. It is interesting to consider the possibility that such a
patient could benefit from lengthening of the already long leg if the
pelvic obliquity were greater than the leg-length discrepancy.
correction. The traditional guideline has been that a femur that is
less than half of the length of the femur of the other side and legs
that eventually grow to be shorter than 15 cm are candidates for
prosthetic fitting. This is often done in conjunction with other
procedures, including knee fusion, Syme amputation, and Van Nes
rotationplasty. Modern advances in lengthening have led us to hope that
we are more proficient at correcting very large discrepancies with
acceptable morbidity (117,118,119).
It is my opinion that some discrepancies up to 20 cm are reasonable
candidates for correction by a variety of combined simultaneous or
staged shortening and lengthening procedures, but there is no reason
why the traditional guideline should be abandoned.
that are prerequisites to surgical treatment of the leg-length
discrepancy itself. These intermediate goals can include stabilization
of unstable joints, release of contractures, correction of angular
deformity, correction of spinal deformity, completion of partial growth
arrests causing angular deformity, and excision of bony bridges in an
attempt to restore growth.
deformities before correcting leg-length discrepancy, because the
correction of some deformities changes the treatment goal. For example,
the correction of angular deformity of the limb usually increases the
length of the leg, and the correction of spinal imbalance often changes
pelvic obliquity and the desired amount of correction of leg length.
magnitude of the predicted discrepancy at maturity than on the
etiology. Fairly straightforward guidelines expressed in terms of the
magnitude of the predicted discrepancy can be used to choose from among
the major treatment categories:
-
0 to 2 cm: No treatment
-
2 to 6 cm: Shoe lift, epiphysiodesis, shortening
-
6 to 20 cm: Lengthening, which may or may not be combined with other procedures
-
>20cm: Prosthetic fitting
for factors such as environment, motivation, intelligence, compliance,
emotional stability, patient’s and parents’ wishes, and associated
pathology in the limbs.
thresholds. It has been shown that discrepancies of less than 2 cm are
of no functional or clinical consequence in adults and that these
discrepancies do not require treatment (120). Indeed, Rush and Steiner found leg-length discrepancy in 71% of new recruits into the United States Armed Forces (121). Because there is some advantage to being tall (122,123,124,125),
lengthening procedures are often preferred by parents and patients, but
lengthening is not generally done for discrepancies less than 6 cm
because there are other alternatives. Because of the high morbidity and
complication rate of lengthening, this procedure should be avoided in
favor of epiphysiodesis or shortening whenever possible.
Because
these alternatives are reasonable for corrections of up to 6 cm, they
are the procedures of choice. Shortening procedures are usually not
appropriate for correction of greater than 6 cm, because a
disproportionate appearance results that is not pleasing to the
patient. Epiphysiodesis can be performed to correct a discrepancy of
any magnitude when the long leg is clearly the abnormal one, because
this procedure corrects the abnormally long leg and does not result in
abnormal proportions in these cases. Patient preference for lengthening
over shortening is considered only in the 5- to 6-cm range of
correction because there are overriding considerations outside that
range.
of making the patient’s body as symmetrical as possible, with knees
being as level as possible. This involves lengthening the shortest bone
or shortening the bone corresponding to the shortest bone on the other
side leg. This principle can be ignored in some cases of correction by
epiphysiodesis in which a single plate is arrested to reduce the
magnitude of surgery and risk of problems, although a combined femoral
and tibial epiphysiodesis might produce the most symmetrical result. It
also can be ignored if more time for data gathering allows a more
confident prediction of future growth and a more dependable treatment
plan. Symmetry of knee height is a secondary consideration to equality
of leg length. Knee height is not a factor in function or comfort and
is not very important in cosmesis.
up to 6 cm. Though believed to be less desirable than surgical
correction of the discrepancy, it is a satisfactory answer for those
patients who do not wish or are not appropriate for surgery. The shoe
lift is only effective when the patient is walking or is in two-legged
stance and is only prescribed for its benefit on gait.
For larger discrepancies, the height of the lift should be less than
the discrepancy. For reasons of cosmesis, up to 2 cm of the lift can be
put inside the shoe with the remainder, if necessary, on the outside.
Lifts higher than 5 cm are poorly tolerated because the muscles
controlling the subtalar joint are not strong enough to resist
inversion stress and frequent ankle strains result. If a higher lift is
required, an orthotic extension up the posterior calf can be added for
stability. The optimum height of the lift can be determined by clinical
trials in which the lift height is temporarily modified to suit the
patient. Liu found that the benefit provided by a heel lift, although
it affects the SI, is unpredictable (3).
amputation, is a treatment of last resort but is useful for those
patients with very large discrepancies and for those with deformed and
functionally useless feet (126,127).
Discrepancies anticipated to become greater than 15 to 20 cm and those
involving a femoral length less than 50% of the other side femur should
be treated in this way (38). This approach has
the advantage of involving only one hospitalization and one definitive
operation. Patients with fibular hemimelia and an unstable ankle do
better with this approach than with multiple hospitalizations and
surgical procedures in a futile attempt to conserve the foot; the
latter situation usually results in late amputation, which is more
difficult to accept.
amputated for fibular hemimelia, do very well functionally. They have
an almost normal walking gait and can participate in recreational and
sporting activities. Children who are treated for proximal focal
femoral deficiency require above-knee prostheses and they function
well, although not as well as the children who have undergone
below-knee amputations. Some of the above-the-knee prostheses can
function as below-the-knee prostheses following a Van Nes
rotationplasty, in which the reversed ankle functions as a knee, which
provides active control and motor power to the prosthetic knee (128).
amputation on young children, the children who undergo surgery and
prosthetic fitting early in life show the best results. The optimal
time for performing the Syme amputation is toward the end of the first
year of life and for performing the rotationplasty is at approximately
3 years of age. It is helpful for parents of children who are
candidates for these procedures to meet older children who have
undergone the same procedure and to talk with their parents.
complication rate, and is the treatment of choice for the surgical
correction of leg-length discrepancy (19,105,129,130).
The operation is effective by slowing the growth rate of the long leg
and by allowing the short leg to catch up. In planning for this
procedure, therefore, it is necessary to take into account the ability
of the short leg to catch up; this is done using the growth inhibition
to predict the discrepancy at maturity. For all surgical treatments of
leg-length discrepancy, it is the discrepancy at maturity that should
be corrected and not the present discrepancy in a growing child.
Epiphysiodesis is a highly acceptable procedure because it is
straightforward, does not require postoperative immobilization, and
disables the child minimally and for a short duration. It is only
suitable for those children who have sufficient leg-length data to
enable a confident prediction of the discrepancy at maturity and for
those whose discrepancies require correction of 2 to 6 cm (131).
the leg grows at a slower rate, having lost the contribution of the
operated physis toward the process of growth. The loss is 27% for the
proximal tibial, 38% for the distal femoral, and 65% for combined
epiphysiodesis of both plates. The surgeon therefore induces a known
degree of growth inhibition and has before him not a continuous
spectrum of shortenings but only three discrete choices. The exact
amount of desired shortening can be achieved only by performing the
surgery at exactly the correct time. Performing the operation too late
results in undercorrection, and performing it too early results in
overcorrection. This is in contrast to shortening and lengthening
procedures that can be performed at any time.
it is better to err on the side of undercorrection than overcorrection.
Because slight discrepancies are well tolerated, it is best to aim for
0.5 to 1.0 cm of undercorrection by doing the epiphysiodesis slightly
later than the time for perfect correction. To improve symmetry, the
procedure is best done in the bone that is opposite the shortest one on
the other-side leg, although this principle may have to be compromised
if future growth is insufficient for such an epiphysiodesis to be
effective.
bony bridge that tethers the physis and prevents future growth. The
traditional open techniques involve removing a block of bone from the
medial and lateral aspects of the plate, extirpating the plate with a
curette, and replacing the block of bone in such a manner as to produce
a bony bridge. Phemister described removal of a rectangular block,
two-thirds on the metaphyseal side and one-third on the epiphyseal side
of the plate, and its replacement in the reversed position (132) (Fig. 29.27). White and Stubbins used a special chisel to remove a square block that was later rotated 90 degrees before replacement (130),
and Blount used a circular trephine to remove a cylindrical block that
was rotated in the same way. Macnicol and Gupta have reported a
percutaneous version of the Blount technique (133). All these procedures serve to bridge the physis medially and laterally with solid bone.
across the physis, both medially and laterally, thereby producing a
tethering effect resulting in arrest of growth (134,135,136) (Fig. 29.28). The rationale was that the arrest was temporary and that growth would resume after removal of the staples (137,138).
This concept was attractive because it alleviated the need to make
accurate predictions of future growth. In certain patients, however,
the physes fused while the staples were in place; on the removal of the
staples, growth did not resume, resulting in overcorrection of the
discrepancies (139,140).
Stapling therefore lost proponents and was considered to be a permanent
form of growth arrest. Staples caused problems by extruding, entering
the adjacent joint, or causing overlying bursitis (141). Growth arrest was occasionally asymmetrical, and a second operation was sometimes necessary to remove the staples.
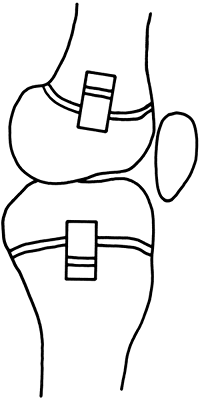 |
|
Figure 29.27
Epiphysiodesis by the Phemister technique. A rectangular bone block is replaced in reverse position to produce a bar across the growth plate. |
stapling in such a way that normal growth resumes when the staples are
removed (142,143,144). Technical details are important, among them, remaining extraperiosteal and not directly exposing the growth plate.
the medial and lateral aspects of the knee, a total of four incisions
if both tibial and femoral epiphysiodeses are performed. Several
authors have reported percutaneous
techniques developed to avoid unsightly scarring (133,145,146).
They are done with a drill or burr through small medial and lateral
incisions under image intensifier control. This technique has gained
wide acceptance and is considered by most to be the technique of
choice. Great care must be taken to line up the image intensifier beam
perfectly to ensure that the tool is in the plate.
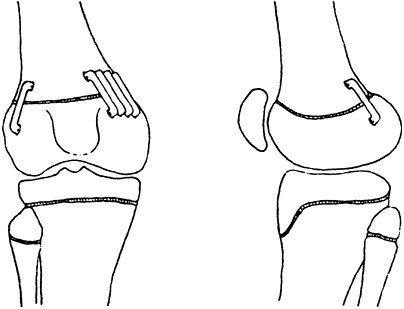 |
|
Figure 29.28
Epiphysiodesis by stapling. Growth arrest can be accomplished by careful placement of three extraperiosteal staples over the medial and lateral aspects of the plate. |
two incisions, one medial and one lateral. Because the growth plates,
particularly the distal femoral plate, are not perfectly flat, there is
a significant technical challenge in making sure that the tip of the
tool is in the plate and that it stays there. Scott, for example,
reported a rate of continued growth of the physis of 12% (147).
In the distal femur, the intercondylar notch intrudes into the
posterior aspect of the plate, and the surgeon must be careful not to
inadvertently enter the notch by being sensitive to the feel of the
tool touching cortical bone. The operator might not notice the tool
entering the notch because it is not obvious on the anteroposterior
view of the image intensifier.
This extent of removal is sufficient to ensure arrest of the physis and
maintains enough bone strength through residual plate and surrounding
periosteum and perichondral ring to make postoperative immobilization
unnecessary. Tibial epiphysiodesis should be accompanied by arrest of
the proximal fibular physis if the tibial shortening is greater than
2.5 cm (148). The fibular epiphysiodesis can be
performed through the same skin incision as the lateral aspect of the
tibia, but the surgeon must approach the fibula from the anterior
aspect to avoid the peroneal nerve.
approaches because of its low morbidity and low complication rate, but
there are minor disadvantages (149,150).
It is a compensatory and not a corrective operation, in that it makes
the normal leg abnormal. Compared to lengthening, it results in a
decrease in the patient’s stature that may be undesirable.
 |
|
Figure 29.29
Area of plate to be removed in epiphysiodesis. Obliteration of medial and lateral circular segments of the plate, leaving the central part and the strong periphery, successfully stops growth, yet the bone retains sufficient strength to forego immobilization. |
epiphysiodesis but is offered to patients who do not meet the
prerequisites for epiphysiodesis either because they are too old or
because their conditions are such that the extent of the discrepancy at
maturity cannot be confidently predicted. The advantage of this
procedure over epiphysiodesis is that it can be done in the mature
patient, when the discrepancy is known and unchanging and the desired
degree of correction can be obtained precisely.
very rarely done except in cases in which the femur does not lend
itself to shortening. Although shortening of 7.5 cm in the femur and 5
cm in the tibia have been reported with no loss of function (151),
it is believed that no more than 3 cm of shortening can safely be
achieved in the tibia, and it is unusual to perform shortenings of more
than 5 cm in the femur. The risk of neurovascular complications is
higher in the tibia because of the proximity and the tethering of
neurovascular structures, as is the risk of delayed union and nonunion.
Fasciotomy is advisable to reduce the risk of compartment syndrome.
Internal fixation is more difficult in the tibia; closed techniques
cannot be used because the bone is subcutaneous, and the muscles of the
leg are slower to recover strength than those of the thigh.
muscles of the leg recovering strength more slowly than those of the
thigh. The ability of a muscle to adjust to shortening of the
underlying bone rests on its ability to remove sarcomeres from the ends
of its fibers until the average length of the remaining sarcomeres
returns to normal, which is relatively constant throughout the body.
The lengths of the hamstring and quadriceps muscles, crossing two
joints, are approximately twice as long as the soleus, but more
importantly, there is an even greater difference in the lengths of the
fibers within those muscles. The soleus muscle, requiring high strength
and short excursion, has a high number of short fibers oriented
obliquely, whereas the hamstrings, requiring excursion more than
strength, have a smaller number of longer fibers oriented
longitudinally. The fiber lengths in any one muscle are relatively
equal, and the fiber length in the hamstrings is much longer than the
4-cm length of fibers in the soleus muscle (152).
It is not difficult to imagine that a shortening of the tibia by more
than 3 cm might completely overwhelm the ability of the fibers of the
soleus muscle to accommodate.
cuts or other complex cuts in the diaphysis of the bone, using
interfragmentary screws or intramedullary rods for fixation (153,154). These techniques are only of historical interest, because better techniques with more secure fixation are now available (155). The two principal techniques in use
today are proximal shortening with blade plate fixation and
middiaphyseal shortening, open or closed, with intramedullary rod
fixation. Both approaches provide secure fixation and neither requires
postoperative immobilization.
the lesser trochanter with blade plate fixation has the advantage of
being proximal to most of the quadriceps origin and therefore not as
disadvantageous to the knee as shortening in the midshaft is. Patients
recover strength and the ability to climb stairs more quickly. This
approach leaves a large scar on the lateral thigh and requires a second
later operation of moderate magnitude to remove the plate.
pioneered a technique that involves the use of a special set of
instruments designed to allow the procedure to be performed entirely
from within the medullary cavity and without any direct approach to the
shaft of the femur. The bone is cut by a special eccentric cam saw that
is passed down the shaft and cuts through the cortex from within. The
size of the saw required is determined by the outside diameter of the
bone, and the femoral shaft is first reamed to an internal diameter
that is sufficiently large to accept it. The distal cut is made first,
and a second cut is then made more proximally at the precise location
to give the desired amount of correction. The cuts should be placed so
that the cylindrical fragment is removed from the isthmus of the femur
where the internal diameter is least, because this site provides the
best fixation to the rod both proximally and distally. The cylindrical
piece of bone can be cut into two sections using a special hook-shaped
reverse-cutting osteotome, and the pieces are pushed aside. The gap can
then be closed over an intramedullary rod to provide rigid internal
fixation. Locking at both ends maintains shortening and rotation. A
second operation is later required to remove the rod.
result from less than rigid fixation of the fragments, usually due to
inadequate reaming without locking. It is apparent, in using an
unlocked nail, that if a 5-cm segment of bone is to be removed and 2.5
cm of fixation is desired both proximally and distally, then the bone
has to be reamed until 10 cm of the shaft is reamed to the minimum
diameter. This in itself can be a problem because the cortex can become
very thin at the level of the osteotomies, especially because the
reaming is usually eccentric, and can lead to fracture of the shaft.
Less than rigid fixation can lead to loss of rotational control and
opening of the shortening gap, two problems that are difficult to
control without locking. Less reaming is necessary for a locked nail,
but in this case, the reduced internal diameter of the canal may not
allow passage of a cam saw large enough to cut completely through the
cortex, and a percutaneous osteotomy may be required to complete the
cut. Nevertheless, the benefits of control of position with the locked
nail far outweigh this disadvantage, and locking is desirable in such
situations.
during or following closed intramedullary shortening and has been
believed to be the result of fat embolization caused by reaming (158).
Possible preventative measures include venting of the distal metaphysis
and use of reamers with flutes sufficiently deep to allow the unimpeded
egress of reamed material, but the effectiveness of these measures has
not been demonstrated. It is not advisable to use this technique until
this serious complication can be avoided with certainty.
familiarity with the instruments, is technically demanding, and has
best results in experienced hands. The major disadvantages are the
technical complications and risk of respiratory distress syndrome, as
noted in the preceding text, and the significant quadriceps weakness
that results. Patients with greater shortening require 6 to 12 months
to regain normal knee control and function (159).
It leaves a small, cosmetically acceptable scar, and the later
procedure to remove the rod is of lesser magnitude than that required
to remove a blade plate.
growth inhibition would be to stimulate his growth to achieve a normal
level. Many techniques have been used in attempts to accomplish this,
but none has been successful enough to be clinically useful. On the
basis of the concept that the periosteum acts as a tether inhibiting
growth, circumferential release of the periosteum has been assessed
both clinically and experimentally and has been found to stimulate
growth (160,161). A variety of foreign materials have been implanted next to the growth plate (162), but the stimulation, if it occurred, was too little and too short-lived to be of use. Sympathectomy (163,164) and surgically constructed arteriovenous fistulae (165,166)
temporarily stimulate growth, presumably by altering the circulation to
the physis, as does stripping and lifting the periosteum by packing
bone beneath it (60,61,167).
However, these techniques have little or no clinical significance.
Electrical stimulation inconsistently stimulates physeal growth, but
even the maximum effect is insufficient to correct clinical
discrepancies (65).
method of growth stimulation available at this time that is useful in
the treatment of leg-length discrepancy.
discrepancy because it is a corrective procedure. It involves operating
on the abnormal leg to correct its abnormality, as opposed to
epiphysiodesis and shortening, which make the normal leg abnormal and
only compensate for the discrepancy.
an account of injuries sustained in battle by Ignatius of Loyola. It
was next reported by Codivilla at the beginning of the 20th century (168).
A number of advances have been made in the surgical technique, or the
lengthening apparatus, or both, and each change has been greeted with
hopes that it would solve the problems associated with lengthening (169).
reserved for those situations in which other methods of correction are
inappropriate. Lengthening is usually not appropriate for patients
requiring correction of less than 6 cm because in these cases other
procedures of lesser risk and morbidity can be used. A reasonable goal
of lengthening for most patients is less than 10 cm for the femur and 7
cm for the tibia. Patients requiring large corrections may require
simultaneous lengthenings of femur and tibia, repeated staged
lengthenings of the same bone (170), or
supplementary shortening procedures on the long leg. There is a
threshold, at approximately 15 to 20 cm, where the risks outweigh the
benefits, and lengthening is abandoned in favor of amputation and
prosthetic fitting.
and may be indicated in cases with both infrapelvic asymmetry and
decompensated scoliosis cases requiring concurrent hip stabilization (181,182). Techniques of instantaneous lengthening of the femur (116,155,183,184) and tibia (185)
have been reported but have not gained widespread support because the
amount of length to be gained is limited. Simultaneous shortening of
one femur and lengthening of the other with the excised bone segment
from the other-side leg has been recommended (186,187).
The Anderson device, using large pins and an external fixator with
threaded rods for lengthening, became widely used but confined the
patient to bed (Fig. 29.30). Some of the older
methods persist in nonindustrialized nations (e.g., double oblique
osteotomy followed by elongation by balanced skeletal traction) (188).
Many of these methods became obsolete with the introduction of the
Wagner device and, later, the Ilizarov and Orthofix devices.
adopted enthusiastically by the orthopaedic community and has been used
extensively with great optimism. However, the complication rate has
remained high, and the patient’s course difficult, leading to the
realization that the human leg has not made similar advances and that
lengthening is still a difficult matter for both the surgeon and the
patient.
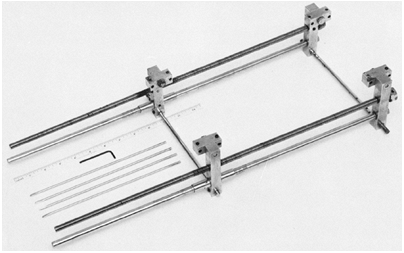 |
|
Figure 29.30
The Anderson lengthening device was commonly used until it was superseded by the Wagner device and later by the Ilizarov and Orthofix devices. It accomplished stable fixation and gradual lengthening, but it was not appropriate for application to the femur, and it confined the patient to bed. |
lengthening device the patient will require intensive education and
observation. This allows time for the initial delay and for the patient
and parents to understand and become comfortable with the lengthening
mechanism, pin site care, an exercise program to maintain mobility and
to attain ambulation with weight bearing. Inpatients undergoing
lengthening should be examined daily for blood pressure, neurologic
status, and range of motion. The reading from the scale of the
lengthener should be recorded daily and should be checked to be sure
that the lengthening process is going according to plan. These
assessments also should be made at weekly or biweekly visits after
discharge. Radiographs are taken at intervals of 2 to 4 weeks to
evaluate alignment and the quality of bone in the lengthening gap
(i.e., the regenerate). Ultrasonography can be used instead of
radiographs to measure the lengthening gap (189,190). The rate of distraction can be modified according to clinical progress or radiologic appearance.
lengthening procedure. Patients and parents should be instructed in a
home exercise program, and the patient’s range of motion should be
monitored regularly. Stopping the lengthening should be considered if
limitation of motion that is resistant to a more intensive motion
program develops. Wagner recommended that distraction should not be
performed on any given day if the patient cannot achieve 60 degrees of
flexion (191). My personal guidelines are to
discontinue lengthening of the femur when a knee flexion contracture is
greater than 10 degrees or knee flexion is less than 30 degrees.
Lengthening can be started again if those guidelines are later met
before consolidation of the regenerate prevents it. All patients regain
flexion in the first year after lengthening (192),
and it appears that maintaining extension is more important. Patients
are allowed to ambulate with full weight bearing, with aids if
necessary. The pin sites are cleaned twice daily and, if necessary, the
stab wounds are elongated weekly under local anesthesia to prevent
tenting and ischemia of the skin, which could lead to pin tract
infection. Cutting pins and small wires circumvent the need to release
pin sites.
been achieved or when an unresolvable complication, usually loss of
motion, supervenes. The device is retained until radiographs show
consolidation and suggest adequate strength of the regenerate bone. In
the consolidation period, dynamization of the device is considered to
be important to subject the bone to cyclic longitudinal loading to
stimulate bone formation. If the bone in the lengthening gap is slow to
consolidate, the device can be shortened to put the bone under
longitudinal compression, either leaving it somewhat shortened or
lengthening it once again when the regenerate responds. Valid objective
guidelines for what constitutes adequate consolidation for removal of
the lengthening device have not been established. Findings such as
corticalization with three cortices visible on two radiographs and the
appearance of a medullary cavity are considered to be signs of adequate
strength, but the decision to remove the device is still empiric.
cast or brace after device removal, allowing removal from the tibia
earlier than from the femur. In addition, the mechanical and anatomic
axes of the tibia are collinear, and the bone is subject mainly to
compressive forces. This is not the case for the femur, in which the
regenerate bone, especially for proximal lengthenings, is eccentric and
is subject to bending loads. Patients should be restricted from violent
body-contact sports for a long time, and perhaps until the bone is
radiologically normal, because fractures through the lengthening gap
have been reported years later.
It is unilateral and uses half-pins instead of through-and-through
pins, thereby facilitating application to the femur. It is small and
light, and it was the first device to allow the patient to be
ambulatory, whereas older devices required confinement to bed. The
patient can perform the lengthening procedure simply by turning a knob
at one end. It is adjustable in varus-valgus and anteroposterior
angulation but not in rotation (Fig. 29.31), so that minor angulation
that occurs during lengthening can be corrected without removing the pins.
 |
|
Figure 29.31
The Wagner device. The device can be adjusted in two planes, but not in rotation. It allows correction of angulation that develops during lengthening but does not allow simultaneous lengthening and gradual correction of angular deformity. |
procedures. The first involves performing an osteotomy, releasing soft
tissues if necessary, and applying the device. At the end of the
lengthening phase, a second procedure involves bone grafting the
lengthening gap, plating the bone, and removal of the lengthener.
Months or years later, when the bone has achieved sufficient strength,
a third procedure is done to remove the plate. There is a prolonged
period of restriction of activities to protect the bone and to avoid
late fracture.
utility, it has become clear that it is ill-advised to plate the bone
in the presence of contaminated pin tracts. Therefore, the technique
has largely been replaced by newer methods that involve neither plating
nor grafting and appear to reduce the complication rate. The device,
however, remains simple and satisfactory and can be used with the newer
biologic principles.
This arrangement allows the pin sets to start close together and
provides long excursion. It allows the use of up to three screws in
each set, which is an advantage, especially in the proximal femur. It
is technically similar in operation to the Wagner device but, although
it offers more stable fixation to bone, it has a more cumbersome method
of elongation and is not easily adjustable once in place. Encouraging
results have been reported with this method, with a complication rate
similar to that of other techniques (198).
 |
|
Figure 29.32
The Orthofix track lengthening device. This device places the lengthening mechanism beside the pins instead of between them, thereby increasing the excursion and obviating device exchange during lengthening. Like the standard device, it is not adjustable once the pins are in place. |
It is achieved by applying a distraction force across the physis until
it fractures. Lengthening can then be obtained by gradual distraction.
This method has the disadvantages that the lysis is sudden, painful,
and not well tolerated and that the physis can be injured, thereby
compounding the leg length inequality (206,207). The complication rate is high (208)
and, if it is to be used at all, it should be reserved for children who
are very near the end of growth to minimize the consequences of physeal
damage.
Wagner and Orthofix devices but are also more versatile in that they
lend themselves to the correction of complex deformities. They can
control more than two segments (213),
can extend across joints, and can be used to translate segments of bone
in the treatment of congenital pseudarthrosis and acquired absences (209).
Fixation is accomplished by tensioned through-and-through wires
attached to complete or partial rings. Unwillingness to use
through-and-through wires in the proximal femur has led to the
development of half-pins, which are now gaining favor at all levels.
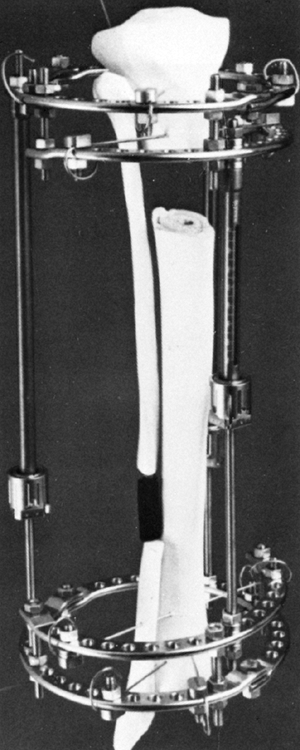 |
|
Figure 29.33
Ilizarov lengthening device. External fixation is accomplished by tensioned wires fixed to circumferential rings. (From Ilizarov G, Deviatov A. Surgical lengthening of the shin with simultaneous correction of deformities. Ortop Travmatol Protez 1969;30:32–37.) |
lengthening devices, the device is responsible for both maintaining
alignment and achieving distraction. Numerous unsightly scars result
because of the multiple percutaneous pins or wires used to achieve
sufficient stability and because they must be left in place for a
prolonged period until the bone is strong. In 1956, Bost and Larsen
introduced the concept of lengthening over an intramedullary rod (175).
The rod serves to maintain alignment during both the distraction and
the consolidation phases, and the external device serves only to
achieve length (214). In this way, the number
of percutaneous tracts can be reduced, the external device can be
removed at the conclusion of lengthening, and the complication rate,
particularly the rate of regenerate fracture, may be reduced (215). Lin described two cases of 15 cases of lengthening over a rod that required bone grafting (216), so the effect of rodding on osteogenesis remains unclear.
with other lengthening techniques, that alignment of the anatomic axis
of the femur cannot be changed and that the mechanical axis of the leg
cannot be controlled. Because the anatomic axes of the femur and tibia
are not collinear, elongation in this manner increases valgus of the
mechanical axis of the leg. Whether or not this is a problem depends on
the initial configuration of the leg. The correction, if necessary, of
preexisting angular deformity or
the valgus produced by the lengthening must be performed at another time, when the rod is no longer in place.
 |
|
Figure 29.34
Metaphyseal leng-thening. Elongation through the metaphysis promotes osteogenesis in the lengthening gap because metaphyseal bone is so active, and it promotes strength by the large cross-sectional area. |
approach because of the fear of producing a serious intra-medullary
infection with the intramedullary foreign material in continuity with
the exterior through the pin tracts. Early experience with this
technique is varied. Some studies report a low infection rate and
recommend the technique (217,218), but others are less enthusiastic, mainly because of a significant rate of infection (219,220,221).
Paley studied a series of 29 cases with case-matched controls and
reported a reduction of time in the external lengthener by 50% and a
2.2 times faster recovery of knee motion. There were no infections and
no fractures, compared to six regenerate fractures in the control group
(218).
that are straight enough to accept the rod and to children who are old
enough not to be at risk for avascular necrosis of the femoral head.
have to do with infection resulting from pins that traverse the skin
and fractures through the regenerate, there has been much interest in
the development of an elongating intramedullary rod that would avoid
both of these types of complication. Lengthening by motorization (223) and by electronically induced shape memory actuation (224)
have been used experimentally, but reports of mechanical ratcheting
devices are starting to appear in the clinical literature (225,226,227,228).
Rotational movement of the distal femoral segment in relation to the
proximal segment operates an internal ratcheting mechanism and, in the
case of the Albizzia nail, 15 such movements achieve 1 mm of
lengthening. Significant complications have been reported, especially
in the form of mechanical problems with the lengthening (226,227), but the concept is promising.
last 3 decades to develop not only improved devices but also improved
concepts and methods of lengthening. In particular, Ilizarov and De
Bastiani have contributed new concepts and a new understanding of the
biology of lengthening that are more important contributions than the
devices themselves (196,197,209,210,211).
Because the biologic principles are not device-specific, they are
considered here in principle. Then, with that foundation, technologic
issues are considered.
the cortex is cut but care is taken that the medullary contents are not
disturbed (Fig. 29.35). He felt it was
important to preserve the intramedullary blood supply of the bone. It
is difficult, however, even with careful technique, to avoid disturbing
intramedullary contents, and it has been demonstrated that the
intramedullary circulation reconstitutes very quickly, even if
interrupted (229). The current consensus is
that it is not necessary to perform a corticotomy, but care should be
taken to avoid burning the bone with a saw so as to preserve the
periosteum.
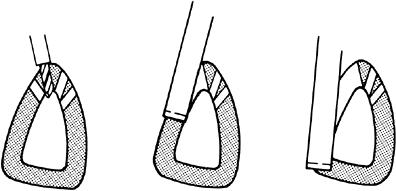 |
|
Figure 29.35
The corticotomy technique. In this technique, care is taken to preserve the contents of the medullary cavity of the bone so that they may make their greatest contribution to osteogenesis during lengthening. Drill holes of controlled depth are made through the anterior cortex; the lateral cortices are cut with a narrow osteotome to avoid entering the medullary cavity; and the posterior cortex is cracked by bending. |
disturbance, massive bone production in the lengthening gap following
peripheral decortication near the osteotomy has been reported (230).
metaphysis, where the bone is more active and there are greater numbers
of active osteoblasts to participate in the process of regeneration (Fig. 29.34) (231).
A second benefit of this location is purely mechanical and is based on
the principle that the strength of a structure in bending varies with
the fourth power of its diameter. Because the diameter of the
metaphysis is so much greater than that of the diaphysis, the bone is
stronger at any stage of healing. This allows earlier removal of the
fixator and decreased risk of fracture when the bone is subjected to
bending loads.
two segments of the same bone make it possible to lengthen a single
bone both proximally and distally at the same time. Although this
theoretically doubles the rate of bone elongation, the soft tissues do
not easily double their elongation rate, and articular cartilage has
been shown experimentally in animals to suffer with rapid elongation (232); this concept has therefore been abandoned clinically.
allow the osteogenic process to become established. Osteogenesis can
then keep up with the elongating gap. Delays of several days for young
children, 1 week for adolescents, and 10 days for adults appear
appropriate without risking premature consolidation, which would
prevent distraction.
distraction of 1 mm per day, a rate that exceeds the ability of the
regenerating bone in the gap to effect union but is not so fast that it
inhibits bone formation. The rate may have to be slowed if radiographs
show inadequate regeneration and a widening lucency in the regenerating
bone. Faster rates often induce ischemia and considerably slow the rate
of osteogenesis, but some patients who show excellent regeneration
radiologically can have their distraction rate increased. The rate of 1
mm per day also appears to be appropriate for the soft tissues that
must grow in length in tandem with the bone (233).
changing the rate promotes faster consolidation experimentally and
reduces the tension stress on the regenerating bone. Lengthening by
0.25 mm four times per day is better than lengthening by 1 mm one time
per day, and it appears that gradual, continuous elongation, perhaps by
a motorized device as suggested by Ilizarov, is ideal.
expands to fill the gap. Multipotential cells become osteoblasts and
form bone without a cartilage foundation in a manner somewhat like
membranous bone formation. Microscopic examination of the regenerating
bone, elongated according to modern concepts, shows that it has a
longitudinal orientation, probably because its formation is guided
carefully by the architecture of the osteotomy surface (234).
The architecture of the regenerate resembles haversian bone and is said
to be stronger than woven bone and to remodel and calcify faster.
wires instead of large screws. The wires cause less reaction of
surrounding skin and bone, move through the skin more easily, and allow
some axial dynamization of the bone fragments.
lengthening. Reports on the new techniques suggest that greater
lengthening may be possible than was formerly thought. Carroll et al.
have shown that permanent changes occur in muscle and joint cartilage
with tibial lengthening greater than 11% (235), and Bell has shown effects on the adjacent joints in animal experiments (232).
These effects may be related more to the rate than the magnitude of
lengthening, and the degree to which they occur in human patients is
uncertain.
full weight bearing from the start, and they should participate in
vigorous calisthenics and physical therapy to maintain normal joint
motion and muscle strength. Weight bearing takes advantage of the
flexibility of the devices to apply dynamic compression, which is
believed to stimulate bone formation.
osteogenesis. The ring fixators have the advantage of allowing dynamic
loading of the lengthening gap throughout the period of fixation while
they simultaneously control length. Their construct of thin wires and
circumferential rings provides rigidity against bending in the sagittal
and coronal planes but is not so rigid in the axial direction, allowing
slight axial movement in response to applied loads. The Orthofix device
can be dynamized by the application of an elastic buttress; when the
regenerate bone appears strong enough to resist shortening, the
buttress can be removed. The device then maintains alignment but not
length, thereby allowing dynamization. All of the devices allow some
dynamization by virtue of their elasticity and can be made less rigid
by the removal of wires or pins in a staged manner.
fixation required per centimeter of lengthening. It is generally
between 1 and 1.5 months per cm and tends to be greater for lesser
lengthenings because one component of this time, the interval from the
arrest of elongation to the removal, tends to be constant. This number
is a very rough guideline to predict the duration of fixation prior to
undergo lengthening but is of little clinical importance (236).
are, for the most part, independent of the device used. The choice of
lengthening device is not, however, completely arbitrary. The devices
have certain characteristics that affect their ease of use in specific
situations. Because deformity correction often goes hand in hand with
lengthening, this discussion includes some aspects of device selection
and use in the correction of angular deformity.
There is a long and successful history of acute correction of deformity
by osteotomy with internal or external fixation, or no fixation at all.
Although there can be advantages in the use of external fixation, in
some cases Ilizarov’s technique of gradual correction appears to offer
little benefit (237,238).
or disadvantages of gradual correction if an external fixator is
already required to accomplish lengthening. There is good evidence to
suggest that, if an external device is already in place for
lengthening, either gradual or acute correction of coexisting deformity
can achieve good results (238). Acute
correction has the effect of simplifying the lengthening and widens the
selection of devices, whereas gradual correction with the Ilizarov or
another ring fixator allows the physician to monitor and modify the
correction on an ongoing basis.
correction, then the Ilizarov device or another ring fixator, or the
Orthofix device with the Garches clamp, can be used. Using the Ilizarov
apparatus to perform gradual angular correction, with or without
lengthening, requires attention to the geometric principles of hinge
placement as described by Herzenberg and Waanders (239).
Careful hinge placement can control both translation and angular
correction. Placing hinges so that the axis of rotation is outside the
bone can accomplish lengthening and angular correction simultaneously.
whereas the Ilizarov device can be used with either thin wires or
half-pins. Thin wires appear to pass through the skin more easily, but
they leave twice as many unsightly scars in the skin and tether the
muscles in twice as many locations, thereby interfering with joint
motion (231).
of the proximal femur, and most orthopaedic surgeons prefer half-pins
and partial rings at that level. There is a growing trend of using
half-pins at other levels as well. Wires must be tensioned to close to
their elastic limit, and it does not take much additional force to
stretch the wires plastically, thereby compromising the rigidity of the
frame.
toward the tip. If the pins loosen, they can be tightened by advancing
them slightly. Conversely, if the pins are inserted too far, they
cannot be retracted without loosening. Hydroxyapatite coatings of the
pins provide better adherence to bone and correspondingly greater
resistance to their removal.
placed at almost any level and angle, there is no problem in removing
troublesome wires and in inserting new ones. The monolateral fixators,
on the other hand, are more limited in that the pin clamps have
predesignated locations and that all the pins in one cluster are in the
same plane. Replacement of one pin may require replacement of all pins
of that cluster.
Ilizarov device sets no limits on the number of pins or wires. There
can be an advantage to using more than two pins to fix the proximal
femur, especially if the lengthening is through the proximal part of
the shaft, and the pins must be placed proximally. In this
configuration, the loading is eccentric and three pins provide better
fixation with less risk of loosening.
than two segments, although with some difficulty, and with special
clamps and hinges it can traverse joints. The Ilizarov device can
easily be extended to provide fixation of as many segments as are
required and can be extended across joints with or without hinges.
of deformities in more than one plane. Not only can multiple segments
be controlled and multiple hinges incorporated, but the system can also
be modified as correction progresses to meet emerging demands.
of the pins, and the procedure requires general anesthesia and special
pin clamps. The Ilizarov method is well suited for continual
modification of its configuration by differential lengthening of the
individual rods. In addition, its components can be changed and the
hinges realigned repeatedly if required. This can be done without the
patient returning to the operating room for anesthesia.
while patients are hospitalized under the care of trained personnel,
but it becomes an issue when they are discharged home. A simple
mechanism results in less anxiety and fewer errors. The Wagner
mechanism is the simplest requiring only the turn of a single knob. The
Orthofix devices require use of a special extender that is advanced
with a wrench. The Ilizarov device requires elongation of up to three
or four rods individually, perhaps by different amounts.
devices, none of which has a clear advantage over another, except that
it might be argued that the simplest device capable of solving the
problem is preferable. Gradual correction of complex deformities can
present demands that can only be met by a more versatile device such as
the Ilizarov device.
Most studies report more complications than there are patients, and
many patients do not reach their anticipated lengthening goals with
uncompromised function. The complication rate, however, is related to
the amount of lengthening (245), and if the goal is modest the rate is reduced and the proportion of patients reaching their preoperative goals is increased (117).
Ghoneem et al. found that all of the studied children who had undergone
lengthening had a normal psychological score; 92% had no limitations in
daily activities, and 81% were satisfied with the overall result (118).
lengthening, and angular deformity of the tibia has been related to the
degree of elongation (252). The Ilizarov
technique has been proposed to supplant amputation and prosthetic
fitting in fibular hemimelia, but Cheng et al. report that
uncontrollable deformity of the tibia and ankle joint was encountered (253).
Ultimate alignment may be improved by using arthrography to delineate
the joint surfaces when applying the lengthening device (254).
The mechanism is not clear, but it resolves dependably with shortening
of the lengthening gap and may not recur when lengthening is resumed.
consequence in itself. True pin tract infection usually responds well
to local care and systemic antibiotics. It can, however, be elevated to
a serious complication if the pin tract infection contaminates a
surgical procedure.
break or loosen and require replacement. Fractures through the
lengthening gap (or deformation of the bone in the lengthening gap) can
occur early or late in the procedure. It can occur soon after removal
of the device, indicating that it was removed too soon, or can occur
late, while the bone is still remodeling and has not yet regained its
normal strength (258). In my series of Wagner
lengthenings, there was one fracture 8 years after the lengthening,
which suggests that the bone is extremely slow to remodel and regain
full strength and is probably weak even after it looks normal
radiologically. Long-term follow-up is important in assessing the
results of leg lengthening because complications can occur late.
in the lengthening gap and concluded that axial loading while the
device is in place may not be enough to increase the diameter but that
the diameter increases dependably after removal of the device (259).
This finding has profound implications for the strength of the bone
because strength in bending varies as the fourth power of the diameter.
Indeed, there is a report substantiating this conclusion (260).
The subluxation appears to be a posterior subluxation of the lateral
tibial plateau and is always preceded by a loss of extension of the
knee. Dysplasia or absence of the anterior cruciate ligament and
hypoplasia of the lateral femoral condyle are usually found in
association with congenitally short femurs (263,264)
and contribute to this complication. Routine lengthening of the lateral
structures (the biceps tendon and iliotibial band), determined
maintenance of knee extension, and avoidance of continued distraction
in the face of a knee flexion contracture will prevent knee subluxation.
even in the early stages of distraction and tends to be associated with
previous hip surgery and residual instability (265).
lengthening, but there is no doubt that certain cases take
significantly longer to consolidate than others. True nonunion occurs
and is similar in appearance and treatment to posttraumatic nonunion,
except that it can occur in narrowed and spindle-shaped regenerate so
that it is fragile even when united.
quality of the underlying bone, but inconsistently so. For example
Naudie et al. showed that the complication rate is higher in patients
with an underlying bone disorder (266), but
patients with achondroplasia seem to have a lower complication rate.
Reasonable lengthening expectations in femoral hypoplasia are unclear (267).
from stretching, entrapment by tense tissues, movement of wires or pins
through the tissues, or direct trauma (268). Nerve conduction is affected in one third or more of patients undergoing lengthening (269,270).
prone to complications than were tibias and that the complication rate
increased with lengthenings exceeding 25% of the initial bone length (271).
We found that the complication rate is significantly higher in children
younger than 8 years than in older children, and this may reflect the
inability of younger children to understand, or remain motivated to
comply with, the instructions therapists and surgeons (262).
Conversely, Noonan et al. found that the complication rate in both
femoral and tibial lengthening increased in patients older than 14
years (236). To some extent, the amount of
possible lengthening depends on the length of the bone to start with,
and this is another reason to perform lengthening only in older
children whose bones are nearing mature length.
Price and Carantzas have reported a case of severe growth retardation
in the lengthened limb (272). Viehweger has
found that lengthening the tibia by more than 14% slows down its growth
but that this has a negligible effect on the ultimate clinical result
because it is compensated for by femoral overgrowth (273).
problems, and complications that surround the care of patients
undergoing lengthening, it facilitates their management to form a
lengthening team in a program with shared responsibilities. A nurse,
physical therapist, social worker, and a skilled technician are
important members of the
team
and should join the surgeon in preparing patients and families for the
lengthening procedure. The team can respond to ongoing needs and offer
support during the lengthening procedure. Families and patients never
fully appreciate the depth and breadth of the hardship they will face,
and they will require more support than is needed for most orthopaedic
cases.
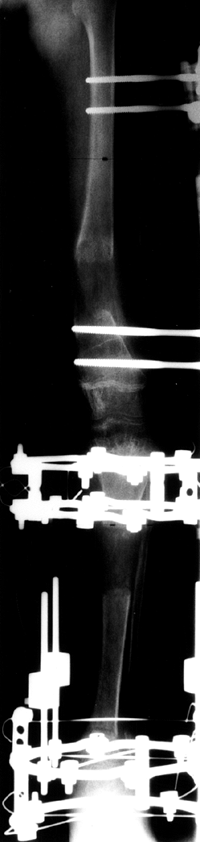 |
|
Figure 29.36
The course of Ilizarov and Orthofix lengthening. This patient had complex deformities resulting from meningococcemia. The femur was managed by an acute correction of the distal femoral varus deformity and subsequent lengthening, using the Orthofix rail lengthener. The distal pins were placed carefully, before the osteotomy was performed. The tibial deformity required valgus osteotomies both distally and proximally. Because fixation of three fragments was required, the more versatile Ilizarov device was used. |
with leg-length discrepancy requires comfortable familiarity with the
techniques for patient assessment, the methods for prediction of future
growth and discrepancy, the factors important in the selection of
treatment goals, and the approaches to treatment. Maintaining
familiarity with up-to-date techniques and philosophies of surgical
treatment is challenging, but the improvement in our capabilities
should be an adequate reward.
 |
|
Figure 29.37 Patients with the Wagner, Orthofix, and Ilizarov devices can be mobile and undertake partial to full weight bearing.
|
R, Kirby R, MacLeod D. Simulated leg-length discrepancy: its effect on
mean center-of-pressure position and postural sway. Arch Phys Med Rehabil 1985;66:822–824.
U, Friberg O, Aalto T, et al. Lower limb asymmetry and patellofemoral
joint incongruence in the etiology of knee exertion injuries in
athletes. Int J Sports Med 1987;8:214–220.
A, Alaranta H, Tallroth K, et al. Leg-length inequality in people of
working age. The association between mild inequality and low-back pain
is questionable. Spine 1991;16:429–431.
M, Green WT. Lengths of the femur and tibia; norms derived from
orthoroentgenograms of children from five years of age until epiphyseal
closure. Am J Dis Child 1948;75:279–290.
M, Messner M, Green W. Distribution of lengths of the normal femur and
tibia in children from one to eighteen years of age. J Bone Joint Surg 1964;46-A(6):1197–1202.
W, Anderson M. Experiences with epiphyseal arrest in correcting
discrepancies in length of the lower extremities in infantile
paralysis. J Bone Joint Surg 1947;29:659–675.
A, Lampe H, Swierstra B, et al. The straight line graph in limb length
inequality. A new design based on 182 Dutch children. Acta Orthop Scand 1997;68(4):355–360.
J, Clancy M, Harcke H, et al. Congenital diastasis of the inferior
tibiofibular joint: a review of the literature and report of two cases.
J Pediatr Orthop 1985;5:225–228.
G. The effect of division of the periosteum on the rate of longitudinal
bone growth: an experimental study in the rabbit. Orthop Trans 1980;4(3):400–401.
R, Best T. Pathogenesis and prevention of valgus deformity following
fractures of the proximal metaphyseal region of the tibia in children. J Bone Joint Surg 1972;54B:767.
R, Kushner D, Ogden M, et al. Determination of leg length discrepancy.
A comparison of weight-bearing and supine imaging. Invest Radiol 1988;23:301–304.
A, Weinstein D, Thickman D, et al. Comparison of orthoroentgenography
and computed tomography in the measurement of limb-length discrepancy. J Bone Joint Surg 1992;74A: 897–902.
D, Boyd N, Fixsen J, et al. The natural history and management of
congenital short tibia with dysplasia or absence of the fibula. J Bone Joint Surg 1977;59B:267–271.
H, Wright J, Cole W, et al. The Ilizarov method for correction of
complex deformities. Psychological and functional outcomes. J Bone Joint Surg Am 1996;78(10):1480–1485.
Y. Comparison of gait patterns and energy efficiency of unilateral PFFD
in patients treated by symes amputation and by knee fusion and
rotational osteotomy. In: ACPOC Annual Meeting. Minneapolis, MN, 1994.
B, Hansson LI, Selvik G. Pattern of growth retardation after blount
stapling: a roentgen stereophotogrammetric analysis. J Pediatr Orthop 1983;3:63–72.
R, Bowen J, Guille J, et al. Prospective evaluation of fifty-three
consecutive percutaneous epiphysiodeses of the distal femur and
proximal tibia and fibula. J Pediatr Orthop 1991; 11:350–357.
D’Aubigne R, Dubousset J. Surgical correction of large length
discrepancies in the lower extremities of children and adults. J Bone Joint Surg 1971;53A:411–430.
I, Grosse A, Abalo C. Locked intramedullary nailing. Its application to
femoral and tibial axial, rotational, lengthening and shortening
osteotomies. Clin Orthop 1986;212:165–173.
HR. Experiments with foreign materials in the region of the epiphyseal
cartilage plate of growing bones to increase their longitudinal growth.
J Bone Joint Surg 1929;11:365–384.
P. Lengthening of shortened bones of the leg by operation. Ivory screws
with removable heads as a means of holding the two bone fragments. Surg Gynecol Obstet 1913;16:63–71.
M, Hall J. Transiliac lengthening of the lower extremity. A modified
innominate osteotomy for the treatment of postural imbalance. J Bone Joint Surg 1979;61A:1182–1194.
D, Choi I, Ahn J, et al. Triple innominate osteotomy for hip
stabilisation and transiliac leg lengthening after poliomyelitis. J Bone Joint Surg 1993;75B:858–864.
Bastiani G, Aldegheri R, Renzi-Brivio L, et al.
Chondrodiatasis—controlled symmetrical distraction of the epiphyseal
plate. Limb lengthening in children. J Bone Joint Surg 1986;68B: 550–556.
I, Correll J, Niethard F. Closed distraction epiphyseolysis for leg
lengthening and axis correction of the leg in children. Z Orthop Ihre Grenzgeb 1986;124:743–750.
J, Huurman W, Lippiello L, et al. Epiphyseal traction to correct
acquired growth deformities. An animal and clinical investigation. Clin Orthop 1986;202:258–268.
M, Mann J, Oedekoven G, et al. Segmental transport after unreamed
intramedullary nailing. Preliminary report of a monorail system. Clin Orthop 1992;282:233–240.
D, Herzenberg JE, Paremain G, et al. Femoral lengthening over an
intramedullary nail. A matched-case comparison with Ilizarov femoral
lengthening. J Bone Joint Surg Am 1997;79(10): 1464–1480.
LP, Steen H. Lengthening of the tibia over an intramedullary nail,
using the Ilizarov external fixator. Major complications and slow
consolidation in 9 lengthenings. Acta Orthop Scand 1999;70(3):271–274.
C, Shih C, Chen W. Nonunion and shortening after femoral fracture
treated with one-stage lengthening using locked nailing teechnique.
Good results in 48/51 patients. Acta Orthop Scand 1999;70(1):33–36.
JD, Justin D, Kasparis T, et al. The intramedullary skeletal kinetic
distractor (ISKD): first clinical results of a new intramedullary nail
for lengthening of the femur and tibia. Injury 2001;32(Suppl.
4):SD129–SD139.
S, Pape HC, Gosling T, et al. Improved comfort in lower limb
lengthening with the intramedullary skeletal kinetic distractor.
Principles and preliminary clinical experiences. Arch Orthop Trauma Surg 2004;124(2):129–133.
J, Levy E, Donohue M. Comparison of knee motion and callous formation
in femoral lengthening with the wagner or monolateral-ring device. J Pediatr Orthop 1993;13:467–472.
G, Irianov I, Migalkin N, et al. Ultrastructural characteristics of
elastogenesis in the major arteries of the canine hindlimb during
lengthening. Arkh Anat Gistol Embriol 1987;93: 94–98.
G, Palienko L, Shreiner A. Bone marrow hematopoietic function and its
relationship to osteogenesis activity during reparative regeneration in
leg lengthening in the dog. Ontogenez 1984;15:146–152.
K, Leyes M, Forriol F, et al. Distraction osteogenesis of the lower
extremity with use of monolateral external fixation. A study of two
hundred and sixty-one femora and tibiae. J Bone Joint Surg Am 1998;80-A(6):793–806.
K, Price C, Sproul J, et al. Acute correction and distraction
osteogensis for the malaligned and shortened lower extremity. J Pediatr Orthop 1998;18(2):178–186.
C, Pouliquen J, Langlais J, et al. Femoral lengthening using the
callotasis method: study of the complications in a series of 70 cases
in children and adolescents. J Pediatr Orthop 1996;16(2):161–167.
F, Harper G, Hill R. Introperative arthrography and the Ilizarov
technique. Role in the correction of paediatric deformity and leg
lengthening. J Bone Joint Surg Br 1997;79(5): 731–733.
K, Matsushita T, Mamada K, et al. Changes of callus diameter during
axial loading and after fixator removal in leg lengthening. Arch Orthop Trauma Surg 1998;117(8): 464–467.
K, Nakamura K, Matsushita T, et al. The diameter of callus in leg
lengtheining: 28 tibial lengthenings in 14 patients with
achondroplasia. Acta Orthop Scand 1998;9(3):306–310.
A, Aldegheri R, Zambito A, et al. Lower-limb lengthening in short
stature. An electrophysiological and clinical assessment of peripheral
nerve function. J Bone Joint Surg Br 1997;79(6): 1014–1018.
N, Davis R, Bell D, et al. Electromyographic and nerve conduction
changes after tibial lengthening by the Ilizarov method. J Pediatr Orthop 1993;13:473–477.
N, Lombari C, Matarazzo L, et al. A review of 240 patients undergoing
distraction osteogenesis for congenital, post-traumatic or
postinfective lower limb length discrepancy. J Am Coll Surg 1996;182(5):394–402.
