Musculoskeletal Tissue Healing
operative treatment of many musculoskeletal diseases and deformities
depends on healing the musculoskeletal tissues: that is, restoration of
tissue structure and function following injury. Healing occurs by
repair, replacement of damaged or lost tissue by fibrous or
fibrocartilaginous tissue that fails to duplicate the normal tissue
structure and function, or by regeneration, replacement of damaged or
lost tissue by tissue that duplicates the normal tissue structure and
function. Most isolated muscle lacerations heal by repair with scar;
bone fractures heal by regeneration of bone. Over the last two decades
orthopaedic surgeons have dramatically advanced their ability to
promote healing of damaged bones and joints. Using new methods of
internal fixation, external fixation, and rehabilitation, they now
successfully treat even the most severe fractures and many severe joint
injuries.
Patients
with injuries of dense fibrous tissue structures (tendon, ligament,
joint capsule, and meniscus) or skeletal muscle present problems as
difficult as patients with bone or articular cartilage injuries.
Furthermore, injuries to these latter tissues may leave patients with
more significant disability than fractures. For these reasons optimal
treatment of musculoskeletal injuries and diseases requires
understanding of the responses of the musculoskeletal tissues to injury
and the healing of these tissues.
applications of forces to the skeleton that exceed the strength of the
tissue. The intensity of the applied force determines the severity of
the injury as measured by the extent of bone and soft tissue damage. A
fracture initiates a sequence of inflammation, repair, and remodeling
that can restore the injured bone to its original state. Inflammation
begins immediately after injury and is followed rapidly by repair.
After repair has replaced the lost and damaged cells and matrix, a
prolonged remodeling phase begins that commonly restores normal bone
structure and function.
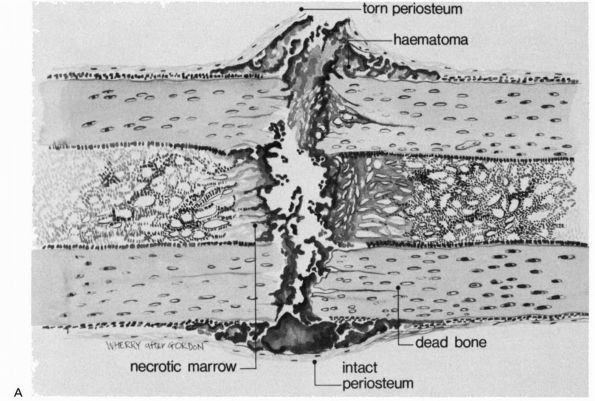 |
|
FIGURE 2-1. Initial events following fracture of a long bone diaphysis. (A)
Drawing showing that the periosteum is torn opposite the point of impact, and may remain intact on the other side. A hematoma accumulates beneath the periosteum and between the fracture ends. There is necrotic marrow and cortical bone close to the fracture line. (B) A photomicrograph of a fractured rat femur 3 days after injury showing the proliferation of the periosteal repair tissue. |
but also the surrounding soft tissues, including the periosteum and
muscle. A hematoma accumulates within the medullary canal, between the
fracture ends and beneath elevated periosteum. The damage to the bone
blood vessels deprives osteocytes of their nutrition, and they die as
far back as the junction of collateral channels, leaving the immediate
ends of the fracture without living cells (see Figure 2-2).
Severely damaged periosteum and marrow, as well as other surrounding
muscle, may also contribute necrotic material to the fracture site.
dilate and exude plasma leading to the acute edema seen in the region
of a fresh fracture. Inflammatory cells migrate to the region,
including polymorphonuclear leukocytes followed by macrophages and
lymphocytes. These cells also release cytokines that stimulate
angiogenesis. As the inflammatory response subsides, necrotic tissue
and exudate are resorbed, and fibroblasts and chondrocytes appear
and start producing a new matrix, the fracture callus (Figures 2-2 and 2-3).
 |
|
FIGURE 2-1. (Continued)
|
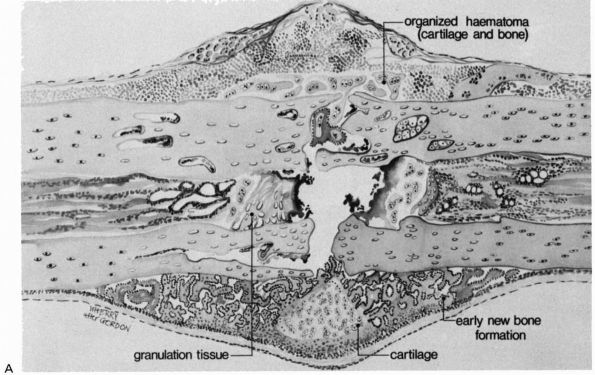 |
|
FIGURE 2-2. Early repair of a diaphyseal fracture of a long bone. (A)
Drawing showing organization of the hematoma, early woven bone formation in the subperiosteal regions, and cartilage formation in other areas. Periosteal cells contribute to healing this type of injury. If the fracture is rigidly immobilized or if it occurs primarily through cancellous bone and the cancellous surfaces lie in close apposition, there will be little evidence of fracture callus. (B) Photomicrograph of a fractured rat femur 9 days after injury showing cartilage and bone formation in the subperiosteal regions. (Reprinted from Clin Ortho Rel Res with permission [5]) |
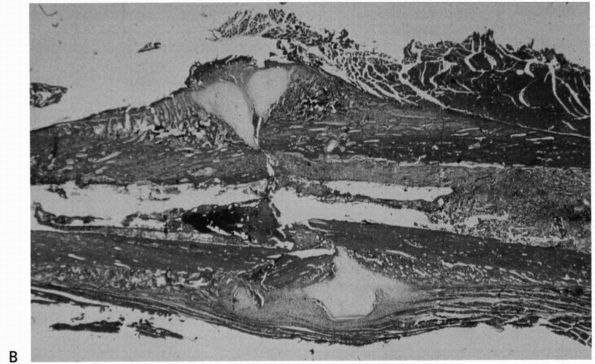 |
|
FIGURE 2-2. (Continued)
|
the repair process. The summaries of fracture repair and remodeling
that follow immediately below first describe healing of closed
fractures that are not rigidly stabilized; that is, fractures where
repair proceeds in the presence of motion at the fracture site (Figure 2-4).
A closed clavicle fracture that is not treated by internal fixation
provides an example of repair and remodeling of an unstable fracture.
The second summary describes healing of stable fractures; that is,
fractures where repair proceeds at a rigidly stable fracture site with
the fracture surfaces held in contact. Transverse diaphyseal fractures
of the radius and ulna treated by open anatomic reduction and rigid
internal fixation provide examples of the repair and remodeling of
stabile fractures.
periosteum, and surrounding tissue at the time of injury results in the
extravasation of blood at the fracture site and the formation of a
hematoma. Organization of this hematoma is usually recognized as the
first step in fracture repair (Figure 2-2).
Loss of the hematoma impairs or slows fracture healing suggesting that
the hematoma and an intact surrounding periosteal soft tissue envelope
that contains the hematoma may facilitate the initial stages of repair.
increases shortly after fracture, presumably because of vasodilation,
vascular proliferation also occurs in the region of the fracture. It
appears that, under ordinary circumstances, the periosteal vessels
contribute the majority of capillary buds early in normal bone healing,
with the nutrient medullary artery becoming more important later in the
process. Growth factors may be important mediators of the angiogenesis
in fracture healing, but the exact stimuli responsible for vascular
invasion and endothelial cell proliferation have not been defined.
blood supply, become necrotic and are resorbed. In some fractures this
may create a radiographically apparent gap at the fracture site several
weeks or more after the fracture. The cells responsible for this
function, the osteoclasts, come from a different cell line than the
cells responsible for bone formation. They are derived from circulating
monocytes in the blood and monocytic precursor cells from the bone
marrow, whereas the osteoblasts develop from the undifferentiated
mesenchymal cells that migrate into the fracture site.
origin, form fibrous tissue, cartilage, and eventually bone at the
fracture site. Some of these cells originate in the injured tissues,
while others migrate to the injury site with the blood vessels. Cells
from the cambium layer of the periosteum form the earliest bone (Figure 2-1A).
Osteoblasts from the endosteal surface also participate in bone
formation, but surviving osteocytes do not appear to form repair
tissue. The majority of cells responsible for osteogenesis during
fracture healing appear in the fracture site with the granulation
tissue that replaces the hematoma.
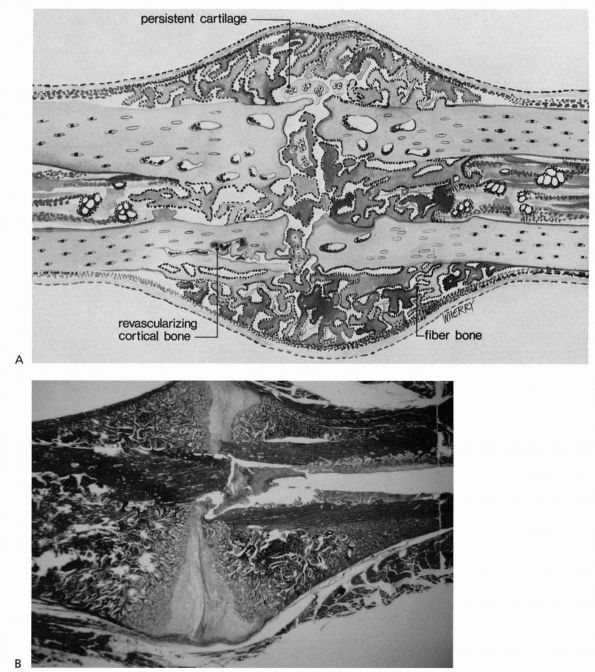 |
|
FIGURE 2-3. Progressive fracture healing by fracture callus. (A)
Drawing showing woven or fiber bone bridging the fracture gap and uniting the fracture fragments. Cartilage remains in the regions most distant from ingrowing capillary buds. In many instances, the capillaries are surrounded by new bone. Vessels revascularize the cortical bone at the fracture site. (B) Photomicrograph of a fractured rat femur 21 days after injury showing fracture callus united the fracture fragments. (Reprinted from Clin Ortho Rel Res with permission [5]) |
The fracture callus fills and surrounds the fracture site, and in the
early stages of healing can be divided into the hard or bony callus and
the softer fibrous and cartilaginous callus. The bone formed initially
at the periphery of the callus by intramembranous bone formation is the
hard callus. The soft callus
forms in the central regions and consists primarily of cartilage and
fibrous tissue. Bone gradually replaces the cartilage through the
process of endochondral ossification, enlarging the hard callus and
increasing the stability of the fracture
fragments (see Figure 2-4). This process continues until new bone bridges the fracture site, reestablishing continuity between the cortical bone ends.
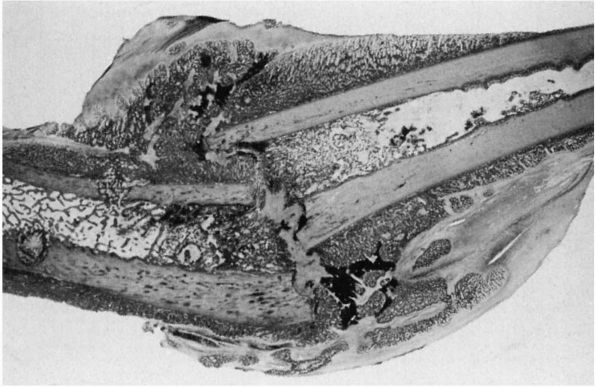 |
|
FIGURE 2-4.
Light micrograph showing healing of a diaphyseal fracture under conditions of loading and motion. This femur fracture occurred in a pig that continued to use the limb for 3 weeks. Even though the fracture was not stabilized, it is healing. A large fracture callus consisting primarily of woven bone surrounds and unites the two fracture fragments. As the callus matures, it progressively stabilizes the fracture. Notice that the fracture callus contains areas of mineralized and unmineralized cartilage. |
ends gradually become enveloped in a fusiform mass of callus containing
increasing amounts of woven bone. The increasing mineral content is
closely associated with increasing stiffness of the fracture callus.
Stability of the fracture fragments progressively increases because of
the internal and external callus formation, and eventually clinical union occurs—that is, the fracture site becomes stable and pain-free. Radiographic union
occurs when plain radiographs show bone trabeculae or cortical bone
crossing the fracture site and often occurs later than clinical union.
However, even at this stage healing is not complete. The immature
fracture callus is weaker than normal bone, and it only gains full
strength during remodeling.
repair tissue begins with replacement of woven bone by lamellar bone
and resorption of unneeded callus. Although fracture callus remodeling
results from an elaborate sequence of cellular and matrix changes, the
important functional result for the patient is an increase in
mechanical stability.
limits at a fracture site, fracture callus progressively stabilizes the
bone fragments, and remodeling of the fracture callus eventually
produces lamellar bone. However, when the fracture surfaces are rigidly
held in contact, fracture healing can occur without grossly visible
callus in either cancellous or cortical bone. Some surgeons refer to
this type of fracture healing as primary bone healing, indicating that it occurs without the formation and replacement of visible fracture callus.
bone ends directly apposed, the bone ends are in contact in some
regions of the fracture line and other areas where there are small
gaps. Where between bone ends contact, lamellar bone can form directly
across the fracture line by extension of osteons. A cluster of
osteoclasts cuts across the fracture line, osteoblasts following the
osteoclasts deposit new bone, and blood vessels follow the osteoblasts.
The new bone matrix, enclosed osteocytes, and blood vessels form new
haversian systems. Where gaps exist that prevent direct extension of
osteons across the fracture site, osteoblasts fill the defects with
woven bone. After the gap fills with woven bone, haversian remodeling
begins, reestablishing normal cortical bone structure. Cutting cones
consisting of osteoclasts followed by osteoblasts and blood vessels
traverse the woven bone in the fracture gap, depositing lamellar bone
and reestablishing the cortical bone blood supply across the fracture
site without grossly visible fracture callus. If a segment of cortical
bone is necrotic, gap healing by direct extension of osteons still can
occur, but at a slower rate, and areas of necrotic cortical bone remain
unremodeled for a prolonged period.
body fractures where cancellous and in some regions cortical bone
surfaces interlock have
sufficient
stability to permit primary bone healing at sites where bone surfaces
make direct contact. The same type of cancellous bone healing can occur
at osteotomies through metaphyseal bone, rigidly stabilized
intra-articular fractures, and surgical arthrodesis treated with rigid
stabilization. Most diaphyseal osteotomies, acute diaphyseal fractures
of long bones, and unstable metaphyseal fractures require use of
devices that compress and rigidly stabilize the fracture site to allow
primary healing.
fail to heal. It is difficult to set the time when a given fracture
should be united, but when healing progresses more slowly than average,
the slow progress is referred to as delayed union.
This indolent fracture healing may be related to the severity of the
injury, poor blood supply, the age and nutritional status of the
patient, or other factors. Failure of bone healing, or nonunion,
results from an arrest of the healing process. A nonunion that occurs
despite the formation of a large volume of callus around the fracture
site is referred to as a hypertrophic nonunion (Figure 2-5), in contrast to an atrophic nonunion (Figure 2-6)
where little or no callus forms and bone resorption occurs at the
fracture site. In some nonunions, cartilaginous tissue forms over the
fracture surfaces and the cavity between the surfaces fills with a
clear fluid resembling normal joint or bursal fluid creating a pseudoarthrosis,
or false joint. Pseudoarthroses may or may not be painful, but they
almost uniformly remain unstable indefinitely. In other nonunions the
gap between the bone ends fills with fibrous or fibrocartilaginous
tissue. Occasionally dense fibrous and cartilaginous tissue firmly
stabilizes a fracture creating a fibrous union. Although fibrous unions may be painless and unite the fracture fragments, they fail to restore the normal strength of the bone.
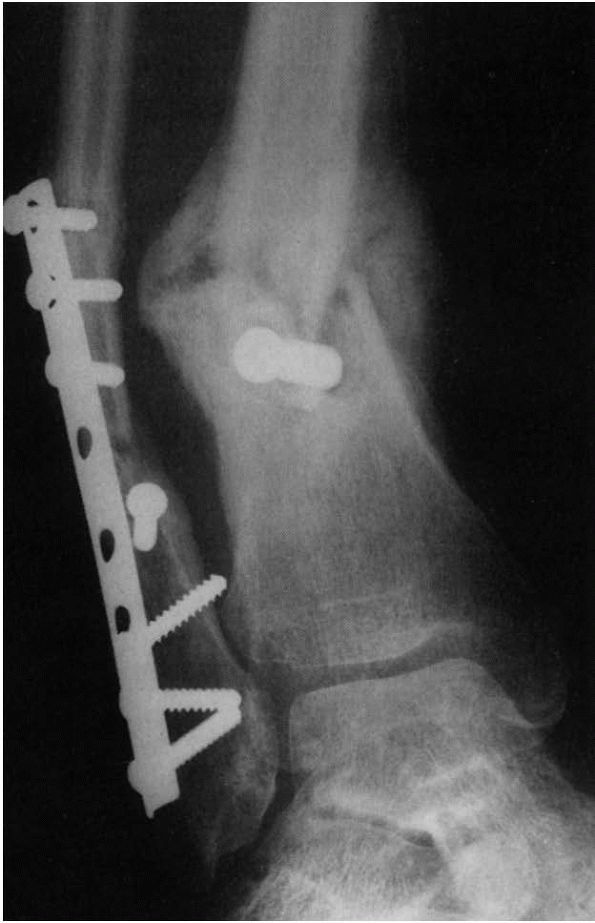 |
|
FIGURE 2-5.
Hypertrophic delayed union of a distal tibial fracture 5 months after injury. Note the abundant callus but incomplete bridging of the fracture gap. |
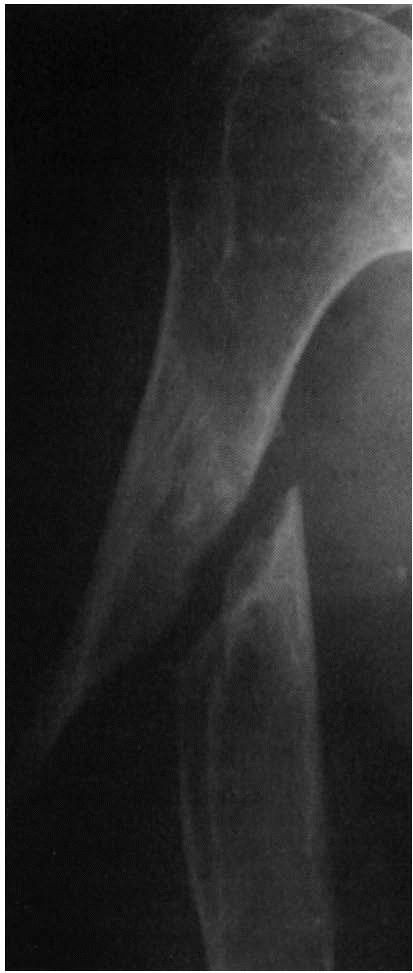 |
|
FIGURE 2-6. Atrophic nonunion of a humeral shaft fracture 18 months after fracture. Note the absence of callus.
|
synovial joints. It may be injured by mechanical forces that disrupt
the articular cartilage alone or the articular cartilage and the
underlying bone. Visible disruptions of articular cartilage are
referred to as osteochondral or intra-articular fractures
when they involve both the articular cartilage and subchondral bone;
when they involve only the cartilage, they are referred to as chondral fractures. In addition to direct mechanical injury, articular cartilage can sustain damage by disruption of the synovial membrane
leading to exposure of the articular cartilage to air. Because of these
special features, acute traumatic injuries to synovial joints can be
separated into the following categories: disruption of the soft tissues
of the synovial joint without direct mechanical cartilage injury and
mechanical injury of articular cartilage. Because cartilage lacks blood
vessels, it cannot respond to cell damage with inflammation. However,
injuries that disrupt subchondral bone as well as the overlying
cartilage initiate the fracture healing process, and the repair tissue
from bone will fill an articular cartilage defect. Cartilage healing
then follows the sequence of inflammation, repair, and remodeling like
that seen in bone or dense fibrous tissue. Unlike these tissues, the
repair tissue that fills cartilage defects from subchondral bone
initially differentiates toward articular cartilage rather than toward
dense fibrous tissue or bone.
capsule and synovial membrane can alter cartilage matrix composition by
stimulating degradation of proteoglycans or suppressing synthesis of
proteoglycans. A decrease in matrix proteoglycan concentration
decreases cartilage stiffness and may make the tissue more vulnerable
to damage from impact loading. Prompt restoration of the synovial
environment by closure of the synovial membrane allows chondrocytes to
repair the damage to the macromolecular framework of the matrix, and
the tissue may regain its normal composition and function. However,
prolonged exposure of the articular surface to air can desiccate the
tissue and kill chondrocytes.
and bone tissue at the fracture site, but in addition, osteochondral
fractures may be associated with blunt trauma limited to cartilage,
abrasions of the articular surface, or chondral fractures.
Alternatively, blunt trauma to a synovial joint may occur without an
associated bone or cartilage fracture. Therefore, acute articular
cartilage injuries can be separated into those caused by blunt trauma
that does not disrupt or fracture tissue and those caused by blunt
trauma that mechanically disrupts the tissue. Injuries that fracture or
disrupt cartilage can be further divided into those limited to
articular cartilage and those affecting both cartilage and subchondral
bone.
when there is no grossly apparent tissue disruption; and these injuries
may lead to later degeneration of the articular surface. Physiologic
levels of impact loading have not been demonstrated to produce
cartilage injury, and clinical experience suggests that acute impact
loading considerably greater than physiologic loading, but less than
that necessary to produce detectable fractures, rarely causes
significant articular cartilage injury. However, acute impact loading
less than that necessary to produce visible tissue disruption may cause
cartilage swelling and alter the relationships between collagen fibrils
and proteoglycans. This observation suggests that blunt trauma may
disrupt the macromolecular framework of the cartilage matrix and
possibly injure cells without producing detectable fracture of the
cartilage or bone. Presumably this tissue damage makes cartilage more
vulnerable to subsequent injury and progressive deterioration if the
cells do not rapidly restore the matrix. This type of injury may help
explain the development of articular cartilage degeneration following
joint dislocations or other types of acute joint trauma that do not
cause visible damage to the articular surface.
cartilage perpendicular to the surface, or chondral fractures kill
chondrocytes at the site of the injury and disrupt the matrix. Viable
chondrocytes near the injury may proliferate, form clusters of new
cells, and synthesize new matrix. They do not migrate to the site of
the injury, and the matrix they synthesize does not fill the defect. A
hematoma does not form, and inflammatory cells and fibroblasts do not
migrate to the site of injury. This minimal response may be due to the
inability of
chondrocytes
to respond effectively to injury, the inability of undifferentiated
mesenchymal cells to invade the tissue defect, and the lack of a clot
that attracts cells and gives them a temporary matrix to adhere to and
replace with more permanent tissue. Although the response of
chondrocytes to injury will not heal a clinically significant cartilage
defect, most traumatic defects limited to small areas of articular
cartilage do not progress.
subchondral bone stimulates bone fracture healing including
inflammation, repair, and remodeling. Blood from ruptured bone blood
vessels fills the injury site with a hematoma that extends from the
bony injury into the chondral defect. The clot may fill a small
chondral defect, generally one less than several millimeters wide, but
it usually does not completely fill larger defects. Inflammatory cells
migrate through the clot followed by fibroblasts that begin to
synthesize a collagenous matrix. In the bone defect and the chondral
defect some of the mesenchymal cells assume a rounded shape and begin
to synthesize a matrix that closely resembles the matrix of articular
cartilage.
chondral portion of the defect and the tissue forming in the bony
portion of the defect begin to differ. Tissue in the chondral defect
has a higher proportion of repair cells and matrix which resemble
hyaline cartilage (Figure 2-7), while the
repair tissue in the bone defect has started to form new bone. Within 6
weeks of injury repair tissue in the two locations is distinguished by
the new bone formed in the bone defect, the absence of bone in the
chondral defect, and the higher proportion of hyaline cartilage repair
tissue in the chondral defect.
usually follows a predictable course, subsequent changes in the
cartilage repair tissue vary considerably among similar defects. In
some chondral defects the production of a cartilaginous matrix
continues, and the cells may retain the appearance and some of the
functions of chondrocytes, including production of type II collagen and
proteoglycans. They rarely, if ever, restore the matrix to the original
state, but they may succeed in producing fibrocartilaginous tissue that
maintains the integrity of the articular surface and provides
clinically satisfactory joint function for years. Unfortunately, in
many other injuries the cartilage repair tissue deteriorates rather
than remodels. It becomes progressively more fibrillar, and the cells
lose the appearance of chondrocytes and appear to become more
fibroblastic. The fibrous matrix may begin to fibrillate and fragment,
eventually leaving exposed bone (Figure 2-7).
The reasons why healing of some osteochondral injuries results in
formation of fibrocartilage that may provide at least temporary joint
function, while others fail to repair, have not been well defined.
articular surface, or the cartilaginous repair tissue deteriorates, the
joint loses its ability to provide pain-free motion. It becomes stiff
and frequently becomes painful, a condition called posttraumatic
osteoarthritis. The risk of posttraumatic osteoarthritis increases with
the severity of the joint injury as measured by the degree of
disruption of the articular surface and with the age of the patient.
Residual joint articular surface incongruity and joint instability
increase the risk of degeneration of remaining normal articular
cartilage, and thereby increase the risk of posttraumatic
osteoarthritis.
as blunt, tearing, or penetrating injuries or combinations of these
types of injury. Blunt injuries compress and crush tissue and range from mild contusion to severe crushing. Tearing injuries can range from minimal elongation or stretching to rupture, avulsion, or tearing away of tissue. Penetrating injuries
vary in depth and the extent to which they cleanly lacerate tissue or
cause combinations of blunt and tearing injuries. Generally the extent
of tissue damage from penetrating injuries can be relatively easily
determined. It is more difficult to define the extent of cell and
matrix injury from blunt or tearing trauma.
tissue to acute injury includes inflammation, repair, and remodeling,
and the repair tissue matrix consists primarily of type I collagen.
Although the repair tissue formed following injury to dense fibrous
tissue can replace damaged or lost tissue, it rarely duplicates the
structure and properties of the uninjured tissue. The specialized forms
of dense fibrous tissue follow the same general pattern of healing, but
because of the differences in their structure and function, tendon,
ligament, and joint capsule and meniscus healing present different
clinical problems.
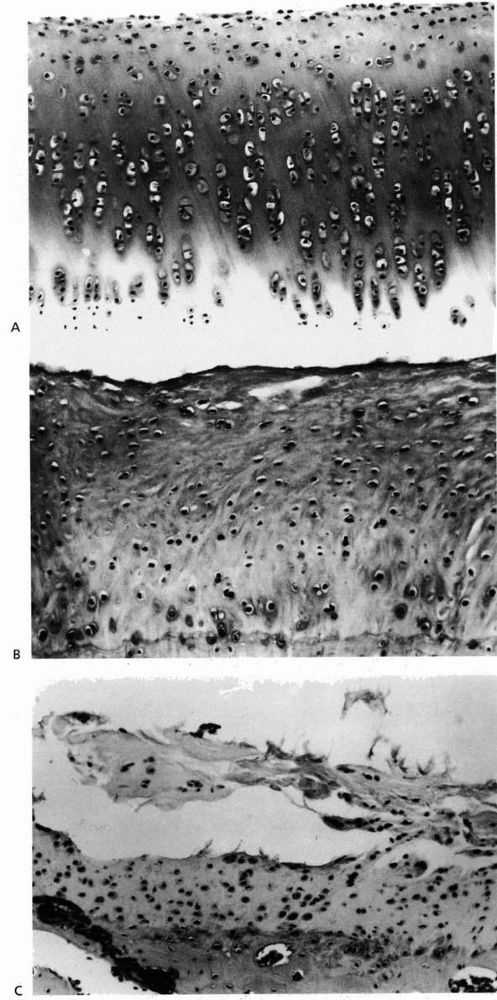 |
|
FIGURE 2-7. (A)
Normal rabbit articular cartilage showing the homogenous extracellular matrix. The chondrocytes near the articular surface are relatively small and flattened, whereas those in the middle and deeper zones of the articular cartilage have a more spherical shape. (B) Well-formed fibrocartilaginous repair cartilage. Notice that the extracellular matrix is more fibrillar and the chondrocytes do not show the same organization as normal articular cartilage. Nonetheless, this repair cartilage does fill the defect in the articular surface. In most instances after osteochondral injury, this type of tissue forms within 6 to 8 weeks. (C) Photomicrograph showing fibrillation and fragmentation of fibrocartilaginous repair tissue. Because fibrocartilaginous repair tissue lacks the mechanical properties of normal articular cartilage, it often degenerates over time. (Reprinted from Buckwalter JA, Mow VC. Cartilage repair and osteoarthritis. In: Moskowitz RW, Howell DS, Goldberg VM, et al., eds. Osteoarthritis Diagnosis and Medical/Surgical Management, 2nd Ed. Philadelphia: WB Saunders, 1992:86-87, with permission) |
insertions, and muscle-tendon junctions, may suffer acute traumatic
injuries. Complete disruption of any part of the muscle-tendon unit
allows the muscle to retract, increasing the gap at the injury site. If
the injury is left untreated, scar tissue may eventually fill the gap
between the tendon ends, but it will leave the muscle-tendon unit
longer than before injury and may bind the tendon to the surrounding
tissues. Without restoration of normal tendon length and gliding, the
function of the muscle-tendon unit will be compromised. Furthermore,
even when tendons are repaired, gaps at the repair site may prevent
healing tendons from gaining strength and stiffness at the same rate as
repaired tendons with gaps. The decreased strength increases the risk
of tendon rupture. For these reasons restoration of muscle-tendon unit
function following a complete disruption usually requires a surgical
repair that reestablishes normal muscle-tendon unit length and has
sufficient strength to allow immediate motion of the tendon relative to
the surrounding tissues.
for them to transmit the force of muscle contraction to bone, thereby
producing joint motion. Some tendons pass through well-defined
synovial-lined sheaths and dense fibrous tissue pulleys. Achieving
healing of lacerated digital flexor tendons within these tendon sheaths
while preserving the pulleys and the tendon motion presents a unique
problem in the treatment of musculoskeletal injuries. The cut tendon
ends can be sutured and will heal, but if the repair tissue scars the
tendon to the sheath or the pulleys, tendon motion will be restricted
and may cause joint contracture. Tendons without sheaths do not usually
present this problem because scarring of their repair tissue to
surrounding loose areolar tissue often will not severely restrict
motion.
fibroblast migration into the site of injury. Granulation tissue
proliferates around the injury site and between the ends of the sutured
tendons and deposits randomly-oriented collagen fibrils (Figure 2-8).
The density of fibroblasts increases up to 3 weeks after injury when
granulation tissue fills and surrounds the repaired area. If the tendon
has been sutured, the suture material holds the tendon ends together
until the fibroblasts have produced sufficient collagen to form a
“tendon callus.” The tensile strength of the repaired tendon depends on
the collagen concentration and the orientation of the collagen fibrils.
The collagen fibrils become longitudinally oriented by about 4 weeks,
and during the next 2 to 3 months the repair tissue remodels until it
resembles normal tendon (Figure 2-8). The
amount and density of the scar tissue adhesions between the tendon
injury site and surrounding tissues depend on the intensity, extent,
and duration of the inflammatory and repair phases of healing and the
mobility of the tendon during repair.
reduce scar adhesions between the tendon injury site and the
surrounding tissue and facilitate healing, but excessive loading may
disrupt the repair tissue and create gaps at tendon repair sites. Thus,
optimal tendon healing depends on surgical apposition and mechanical
stabilization of the tendon ends without excessive soft tissue damage
and on creating the optimal mechanical environment for healing. This
mechanical environment includes sufficient tendon mobility to prevent
adhesions and sufficient loading to stimulate remodeling of the repair
tissue matrix along the lines of stress, but loads applied to the
tendon must not exceed the strength of the surgical repair.
a fracture or avulsion of a bone fragment at the site of injury. These
injuries usually can be treated by surgically reducing and stabilizing
the fracture or reinserting the tendon into the bone and stabilizing
the insertion. Healing occurs either by bony union or by union of the
bone to the tendon substance.
heal successfully if further injury can be prevented, but complete or
nearly complete avulsions or tears
can
present difficult problems because attempts to suture muscle tissue
consisting primarily of muscle cells to tendon is unlikely to restore
the structure and function of the muscle-tendon junction. Optimal
healing of these injuries depends on approximation of the avulsed
tendon and any remnants of the tendon remaining attached to the muscle
or, when available, muscle fascia. Although it may appear that muscles
attach to tendons over a small area, in many muscles thin extensions of
their tendons penetrate long distances within the muscle bellies.
Identification of these thin bands of tendon within muscle may make it
possible to suture them to an avulsed or partially avulsed tendon in
the proximal and distal thirds of many muscles and as far as the middle
third of some muscles.
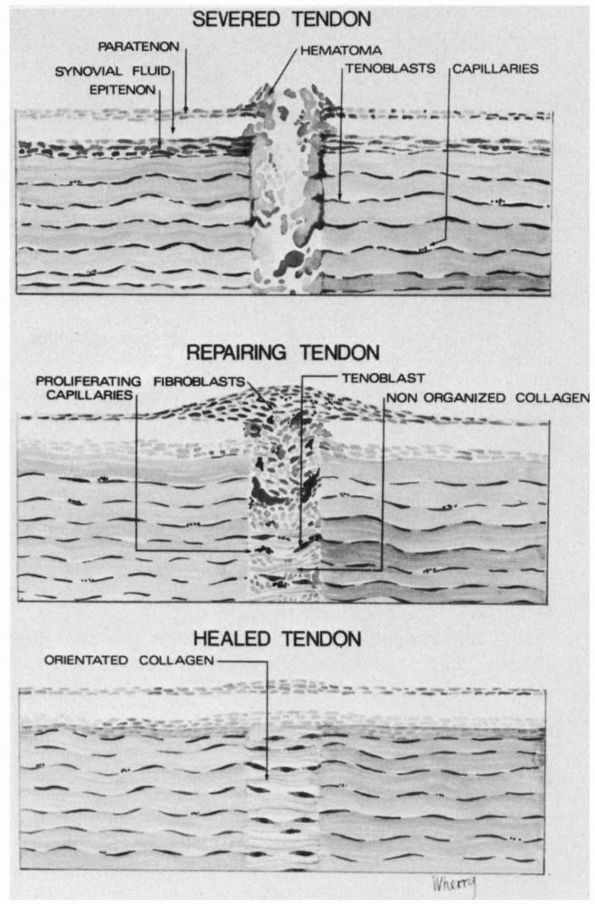 |
|
FIGURE 2-8.
The sequence of events following a tendon laceration. A hematoma forms between the tendon ends. Inflammatory cells, undifferentiated mesenchymal cells, fibroblasts and blood vessels grow into the gap between the tendon ends. The fibroblasts repair the tendon defect by proliferating and synthesizing a new matrix. This repair tissue remodels until it closely resembles the uninjured tendon tissue. |
Also, as in tendon healing, early motion and loading of injured
ligaments can stimulate healing. Because controlled normal motion of a
joint does not necessarily cause large forces in the ligaments and
joint capsule, limited joint motion will not necessarily disrupt the
repair of the tissue.
gap or fail to heal, the resultant joint instability may increase the
probability of subsequent joint injury and degenerative joint disease.
For this reason, restoration or maintenance of near-normal ligament and
capsule length and maintenance of normal joint
motion
should be the objectives of treatment. The most favorable condition for
healing divided ligaments and joint capsules is direct apposition of
the divided surfaces. Apposition and stabilization of the injury site
decreases the volume of repair tissue required to heal the injury,
minimizes scarring, and may help provide near-normal tissue length. A
sutured ligament can heal with a minimal gap. When tested under
tension, sutured ligaments are stronger than those that heal with a
significant length of scar tissue, and ligaments that heal with a gap
between the cut ends may have a decreased ability to stabilize the
adjacent joint. However, many ligament and joint capsule tears heal
without surgical repair and function as well or better than surgically
repaired ligaments and capsules if the torn ends do not retract and the
tear occurs through tissue with an adequate blood supply.
whether the tear occurs through a vascular or an avascular portion of
the meniscus. The vascular regions respond to injury like other
vascularized dense fibrous tissues. This response can heal a meniscal
injury and restore the tissue structure and function if the torn edges
remain apposed and if the repair tissue is not disrupted in the early
stages of healing. Providing these conditions frequently requires
surgical repair of meniscal tears or tears of meniscal attachments. The
avascular regions of meniscal tissue, like articular cartilage, do not
repair significant tissue defects. Cells in the region of the injury,
like chondrocytes in the region of the injury limited to articular
cartilage, may proliferate and synthesize new matrix but there is no
evidence that the cells migrate into the defect site or produce new
matrix that can fill the defect site.
menisci may fail to heal despite treatment. Instead of a firm scar
aligned along the lines of stress, the injury site contains filmy loose
connective tissue, myxoid tissue, or granulation tissue. The reasons
for failure of healing are unclear in some instances, but identifiable
causes include a large gap at the injury site, extensive damage to the
surrounding tissue including loss of vascular supply, excessive early
loading and motion of the repair tissue, and injury-related necrosis of
the tissue. Surgical treatment may also contribute to poor healing.
Extensive dissection can devascularize traumatized tissue, and
inappropriate suture technique may also damage the blood supply to the
injury site or place excessive tension on a sutured tissue.
fibrous tissue structures (i.e., blunt trauma, lacerations, and tearing
injuries) also injure muscle.
that differ in their potential for healing based on the components of
the muscle left intact (Table 2-1).
damages muscle fibers but leaves the extracellular matrix, blood
vessels, and nerve supply intact. Blunt trauma, including surgical
trauma, mild stretching injuries, and temporary ischemia can cause a
type I injury. The muscle fibers will be damaged but the basal lamina
and other components of the extracellular matrix, the blood supply, and
the nerve supply remain intact. These injuries occur frequently and can
heal through spontaneous muscle fiber regeneration that restores the
original structure, composition, and function of the muscle.
damages the nerve supply and may include damage to the myofibers, but
leaves the extracellular matrix and blood supply intact. Type II
injuries may result from isolated peripheral nerve damage, blunt
trauma, or stretching of nerve and muscle. Because the matrix maintains
the muscle structure, if regenerating nerve fibers reach intact
neuromuscular junctions, the potential for restoration of function
exists.
causes loss or necrosis of all muscle tissue components, including
myofibers and extracellular matrix and/or prolonged loss of blood and
nerve supply. Type III injuries result from severe blunt trauma,
tearing, or penetrating trauma. If the vascular supply remains intact,
the inflammatory response can remove the necrotic tissue, but some type
III injuries compromise the blood supply, and the necrotic muscle is
not removed and must be surgically debrided. If the necrotic tissue is
removed, repair can begin. Cells capable of differentiating into
myoblasts
survive even severe injuries or migrate into the injury site. However,
the lack of an extracellular matrix to guide regeneration of myofibers
usually prevents formation of organized muscle tissue. Even if such
tissue forms, lack of guidance for reinnervation prevents regenerated
myofibers from regaining function. For these reasons, the usual result
of a type III muscle injury is healing by scar formation with scattered
myoblasts attempting to form myofibers.
|
TABLE 2-1. Acute Skeletal Muscle Injuries
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||
isolated injury or in association with fractures. The results vary from
type I to type III muscle injuries. Mild blunt trauma to skeletal
muscle damages myofibers without disruption of extracellular matrix,
nerves, or vessels—a type I muscle tissue injury. A slightly more
severe injury ruptures blood vessels as well as myofibers, causing
hemorrhage and inflammation. Healing of these injuries generally
results in restoration of normal function. At the other extreme, blunt
trauma can crush all components of skeletal muscle resulting in a type
III muscle tissue injury that heals with scar tissue or that may not
heal. If the area of the crushing injury is relatively small, muscle
function may not be noticeably altered. However, following an extensive
crushing injury, the cells replace large areas of the muscle with
noncontractile regenerating myofibers and scar, permanently decreasing
muscle strength.
lacerations or combinations of blunt trauma and lacerations. Because
lacerations necessarily damage myofibrils, extracellular matrix,
nerves, and blood vessels, they are type III tissue injuries. Following
complete laceration and suture repair the separated muscle fragments
heal primarily by scar, with a small number of regenerated myotubes
within the scar. True regeneration of functional muscle tissue and
nerves across complete lacerations has not been demonstrated, and
muscle fragments separated from their nerve supply show the changes of
denervation. Transected myofibers may form buds, but these buds fail to
restore normal tissue across the laceration.
includes migration of inflammatory cells into the injured muscle and,
in most injuries, hemorrhage and formation of a hematoma. In addition
to hematoma
formation,
and the other events seen following injury to vascularized tissues, an
important part of the inflammatory process in skeletal muscle is the
removal of damaged muscle fibers by phagocytic inflammatory cells that
penetrate and fragment necrotic myofibers. After they enter damaged
muscle fibers, these cells phagocytize bundles of contractile filaments
and other cytoplasmic debris. This macrophage activity not only removes
damaged cell organelles, it may have an important role in stimulating
regeneration of myofibers.
spindle-shaped myogenic cells appear and begin to proliferate and fuse
with one another to form long syncytial myotubes with chains of central
nuclei. Frequently, several of these early regenerating myotubes form
within the basement membrane tube of a single necrotic muscle fiber. As
they enlarge, the myotubes construct their sarcoplasmic reticulum and
begin to assemble organized bundles of contractile filaments. The
central chains of nuclei break up and migrate to the periphery of the
myotube, completing the transition of the myotube into a muscle fiber.
Contractile proteins continue to accumulate and form myofibrils. To
become functional, a regenerating muscle fiber must be innervated,
including formation of a neuromuscular junction.
are producing granulation tissue necessary to repair the matrix of the
muscle. However, this granulation tissue can interfere with the orderly
regeneration of the myofibers producing a disorganized mass of scar and
partially regenerated myofibers. This type of tissue may restore the
continuity of the muscle, but not its contractile function. Therefore,
the optimal results of muscle healing require a balance between
myofiber regeneration and synthesis of new matrix and appropriate
organization and orientation of these two components of the healing
muscle.
matrix continues to remodel. If excessive scar formation can be avoided
and the muscle cells are innervated, controlled muscle contraction and
loading increases the strength of the injured muscle.
extracellular matrix heals with scar. In some instances, the scar joins
remaining intact muscle to either tendon or adjacent intact muscle. If
the remaining muscle hypertrophies it can restore at least some normal
function. Blunt trauma to muscle may also stimulate bone formation,
myositis ossificans. The new bone can be contiguous with periosteum or
lie entirely within muscle, free of any connection with underlying
bone. Extensive myositis ossificans may weaken the muscle and restrict
joint motion.
and articular cartilage differ in their composition, structure, and
capacity for healing. Bone fractures initiate a response that begins
with inflammation (the cellular and vascular response to injury),
proceeds through repair (the replacement of damaged or lost cells and
matrices with new cells and matrices), and ends with remodeling
(removal, replacement, and reorganization of the repair tissue, usually
along the lines of mechanical stress). Injury to articular cartilage
does not trigger an inflammatory response, but the cells respond to
injury with an effort at cell proliferation and synthesis of new
matrix. This effort rarely, if ever, restores a normal articular
surface. When injuries extend through articular cartilage into bone,
the repair tissue that forms in the bone extends into the region of the
chondral injury and produces a fibrocartilaginous tissue that in some
instances restores a functional articular surface. The principles of
treating acute bone and joint injuries include preventing further
tissue damage, avoiding treatments that compromise the natural healing
process, and creating the optimal mechanical and biological conditions
for healing. This treatment may include removing necrotic tissue,
preventing infection, rapidly restoring blood and nerve supply when
necessary, and in some circumstances providing apposition, alignment,
and stabilization of injured tissue. The ideal result of healing—
restoration of the original structure, function, and composition of the
tissue—may occur following certain dense fibrous tissue injuries and
some skeletal muscle injuries. Achieving ideal results is most likely
to occur following fibrous tissue injuries
in
tissues with an excellent blood supply and injuries that do not cause
segmental tissue loss. Following type I and type II injuries, skeletal
muscle can regain normal structure and function. Dense fibrous tissue
injuries that lead to segmental tissue loss and type III muscle
injuries heal by formation of scar consisting primarily of a dense
collagenous matrix containing primarily type I collagen and
fibroblasts. Scar tissue may restore clinically acceptable function of
injured tissue, especially in some tendon and ligament injuries and
lacerations of skeletal muscle. As with bone and joint injuries, the
principles of treating acute musculoskeletal soft tissue injuries
include preventing further tissue damage, avoiding treatments that
compromise the natural healing process, and creating the optimal
mechanical and biological conditions for healing. Treatment includes
removing necrotic tissue, preventing infection, rapidly restoring blood
and nerve supply when necessary, and in some circumstances providing
apposition, alignment, and stabilization of injured tissue. Early
controlled loading and motion of the repair and remodeling tissues
improves healing of many injuries, but uncontrolled or excessive
loading can adversely affect or even prevent healing.
JA. Tendon, Ligament, Meniscus and Skeletal Muscle Healing. In: Green
DP, Bucholz RW, Heckman JD, eds. Fractures. 5th Ed. Philadelphia: JB
Lippincott, 2001:273-284. This book chapter reviews in detail the healing of tendon, ligament, meniscus, and skeletal muscle.
JA, Einhorn TA, Marsh JL. Bone and Joint Healing. In: Green DP, Bucholz
RW, Heckman JD, eds. Fractures. 5th Ed. Philadelphia: JB Lippincott,
2001:245-271. This book chapter reviews bone healing in detail.
SM, Ostrum RF, Chao EYS, Rubin CT, Aro HT, Einhorn TA. Bone Injury,
Regeneration and Repair. In: Buckwalter JA, Einhorn T, Simon S, eds.
Orthopaedic Basic Science—The Biology and Biomechanics of the
Musculoskeletal System. Rosemont, IL: American Academy of Orthopaedic
Surgeons, 2000:371-400. This book chapter discusses bone regeneration and repair.
SL-Y, Buckwalter JA, eds. Injury and Repair of the Musculoskeletal Soft
Tissues. Park Ridge, IL: American Academy of Orthopaedic Surgeons,
1988. This book reviews the structure,
composition, function, and response to injury of the musculoskeletal
soft tissues: tendon, ligament, tendon and ligament insertions into
bone, muscle-tendon junctions, skeletal muscle, peripheral nerve,
peripheral blood vessel, articular cartilage, and meniscus.
