Complications of Musculoskeletal Trauma
distressingly common. Some complications are a consequence of the
injury itself and may be unavoidable, while others are iatrogenic and
potentially preventable. Regardless of the etiology of the
complication, prompt recognition and appropriate treatment lessen the
impact of the complication and improve the outcome.
-
SIRS has many manifestations ranging from occult hypoxemia to multiorgan dysfunction (MOD) (1).
Fat embolism syndrome (FES) and the adult respiratory distress syndrome
(ARDS) are other clinical manifestations of similar phenomenon that are
related to SIRS. FES may be one of the etiologic factors contributing
to SIRS, while ARDS is now recognized as the “final common pathway” of
the pulmonary consequences of SIRS. The fat embolism syndrome is
generally a self-limited pulmonary disease that usually occurs within 3
days of a fracture. The diagnosis is suspected if the following symptoms and signs are present in a patient with a fracture (2,3,4):-
Disturbances of consciousness (i.e., confusion, delirium, coma)
-
Tachycardia and dyspnea
-
History of hypovolemic shock
-
Petechial hemorrhages
Any combination of the above symptoms may be present in
patients with isolated or multiple fractures. Patients with major long
bone fractures should be monitored for occult hypoxemia with
continuous, noninvasive pulse oximetry (5).
When hypoxia is documented, supplemental oxygen is provided. Patients
with hypoxia should be evaluated for coagulopathy and monitored for
pulmonary, renal, and hepatic dysfunction that may develop in
full-blown SIRS. -
-
Pertinent laboratory findings
-
Of all the laboratory values, a platelet count of less than 150,000 and an arterial oxygen tension (Pao2) of less than 60 mm Hg are the most useful diagnostic tests. Hypoxemia itself is very common in trauma patients and may or may not suggest pulmonary compromise (5).
Recent data suggests that patients with elevated interleukin 6 (IL-6)
levels are at increased risk of SIRS, and this is a useful marker to
follow in patients with multiple injuries. Patients with multiple
injuries and elevated IL-6 levels seem to be at increased risk for
complications following surgery, and when possible, nonemergent
orthopedic procedures should be deferred until the abnormal systemic
inflammatory response has resolved (1). -
Electrocardiographic changes
may be present and include tachycardia, a prominent S wave on lead I, a
prominent Q wave on lead II, a shift in the transition zone to the
left, arrhythmias, inverted T waves, depressed RST segments, and a
right bundle branch block. Serial electrocardiograms are useful. -
Increased serum lipase is indicative of FES, but is of little practical value.
-
Chest roentgenographic changes,
when present, are patchy pulmonary infiltrates. The clinical
manifestations of fat embolism usually precede these changes. The
pulmonary findings become more severe in those patients that meet
criteria for ARDS.
-
-
Recommended treatment
-
Respiratory support is the cornerstone of prevention and treatment of FES, ARDS, and MOD. Respiratory support is provided to keep the Pao2
between 50 and 100 mm Hg. Patients with ARDS and MOD usually need
prolonged ventilatory support with continuous positive airway pressure
(CPAP). In patients with isolated fractures, early (within 24 hours)
fixation of femur fractures helps limit the incidence of this
complication (4,6). -
Shock is treated as outlined in Chap. 1, I.A.3.
-
Coagulopathy is monitored and treated
with fresh frozen plasma and/or cryoprecipitate. Platelet counts should
ideally be maintained above 50,000.
-
-
Carpal tunnel syndrome (CTS, median nerve entrapment at the wrist)
-
The diagnosis
is suspected with a history of pain, tingling, and numbness in the
first three digits; the symptoms are usually worse at night. CTS is
only rarely associated with acute trauma and is generally a chronic
condition typically associated with repetitive microtrauma. When
occurring as a complication of trauma, the condition may develop and
progress rapidly. Acute CTS is most commonly associated with distal
radius fractures but can also occur with perilunate dislocation and
other more subtle injuries. CTS must be recognized and treated
emergently, first with fracture reduction and then with carpal tunnel
release if symptoms do not immediately resolve (see Chap. 20).
-
-
Ulnar nerve compression at the elbow
(“tardy” ulnar nerve palsy, acute ulnar palsy) is commonly associated
with fractures and dislocations about the elbow in children as well as
adults. Acute ulnar neuropathy following injury is most often the
result of iatrogenic damage such as injury occurring during pinning of
a supracondylar fracture in a child, or retraction during internal
fixation of a distal humerus fracture in an adult.-
An early diagnostic sign
is the inability to separate the fingers (interosseous weakness). There
is usually decreased sensation in the fourth and fifth fingers. Light
pressure on the cubital tunnel may reproduce the pain. Nerve conduction
studies show a slowing of the ulnar nerve conduction velocity as it
crosses the elbow (see App. F); this test is not useful diagnostically until 3 weeks after injury. -
If symptoms are minimal, ulnar nerve compression is managed with observation and passive range of motion of the fingers. Surgical therapy
consists of exploration and transposition of the ulnar nerve beneath
the flexor muscle mass anterior to the medial epicondyle when the
pattern of injury or fracture permits. This treatment usually stops any
progressive neuropathy but does not guarantee complete regression of
the neurologic symptoms or signs.
-
-
Peroneal nerve palsy may be due to compression of the common peroneal nerve
in the area of the fibular head or as the nerve enters the anterior
compartment. Apparent peroneal palsy may also be a manifestation of
more proximal injury to the peroneal division of the sciatic nerve. Thus peroneal palsy may be a complication of hip or pelvic fracture/dislocation.-
Diagnosis
often is based on the motor loss, which includes weakness of
dorsiflexion of the ankle and toes as well as eversion of the foot.
History of a hip, tibia, ankle, or foot injury is likely. Pain is
usually on the lateral aspect of the leg and dorsal aspect of the foot.
Pressure over the nerve trunk may cause local pain as well as radiation
into the sensory distribution of the nerve. Pressure over the nerve as
it courses around the proximal fibula results from patient positioning
in the operating room or intensive care unit or from poorly applied
splints. -
Treatment.
Associated hip, knee, or ankle dislocations are emergently reduced. If
there is an operable cause, then neurolysis is indicated. During the
recovery stage, a lateral shoe wedge or plastic ankle-foot orthosis
maintains eversion of the foot. Tendon transfer may be appropriate for
some patients with a permanent foot drop.
-
-
Sciatic nerve
neuropraxia can accompany hip dislocation or fracture dislocation
(acetabular fracture). Note that some sciatic palsies may present as an
isolated peroneal palsy, as discussed above.-
The main differentiating factor in the diagnosis
of a sciatic neuropathy is an L5 or S1 root injury resulting from
pelvic or spine fracture. A sciatic neuropathy must be suspected when
multiple neurologic (L5–S3) segments are involved. A helpful
differentiating test is straight-leg raising just short of discomfort;
pain caused by a sciatic neuropathy is increased by internal rotation
and relieved by external rotation of the hips. This reaction is not
seen with lumbar radiculopathies. -
Treatment is
aimed at the cause of the sciatic neuropathy, and the neuropathy itself
is treated with observation. If the sciatic nerve is known to be
damaged and is not improving, neurolysis may be indicated. In general,
the tibial portion of the nerve recovers well, but the peroneal portion
does not (7). This may relate to the fact that
it is the peroneal portion that lies against the pelvis as it exits
through the greater sciatic foramen.
-
which increased pressure within the space compromises the circulation
to the contents of that space” (8). Although
most commonly applied to the osteomyofascial compartments of the
extremities, compartment syndrome can occur in the abdomen and in major
muscle groups about the spine and pelvis. Other terms that have been
used to describe compartment syndrome are Volkmann ischemia, local
ischemia, traumatic tension in muscles, impending ischemic contracture,
exercise ischemia, exercise myopathy, anterior tibial syndrome, medial
tibial syndrome, rhabdomyolysis, and calf hypertension.
-
Locations
-
In the upper extremity, typical locations include the volar and dorsal compartments of the forearm (Fig. 2-1). There are also several intrinsic compartments of the hand.
-
In the lower extremity,
typical locations include the anterior, lateral, superficial posterior
(gastrocnemius, soleus), and deep posterior compartments of the leg (Figs. 2-2, 2-3). Compartmental syndromes are also seen in the thigh, arm, buttocks (gluteal), and foot compartments (9).
-
-
Etiologies
-
Decreased compartment volume,
such as occurs following closure of fascial defects, application of
tight circumferential dressings, and localized external pressure, can
precipitate a compartmental syndrome (10).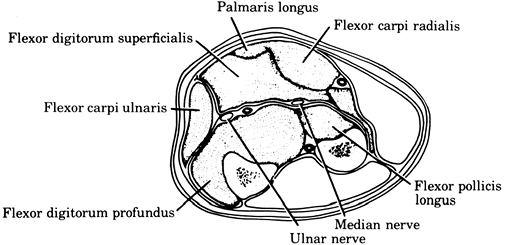 Figure 2-1.
Figure 2-1.
Volar compartmental syndrome of the forearm. Symptoms and signs of
weakness of finger and wrist flexion, pain on finger and wrist
extension, hypesthesia of the volar aspect of the fingers, and
tenseness of the volar forearm fascia.![]() Figure 2-2.
Figure 2-2.
Anterior compartmental syndrome of the leg. Symptoms and signs are
weakness of toe extension and foot dorsiflexion, pain on passive toe
flexion and foot plantar flexion, hypesthesia in the dorsal first web
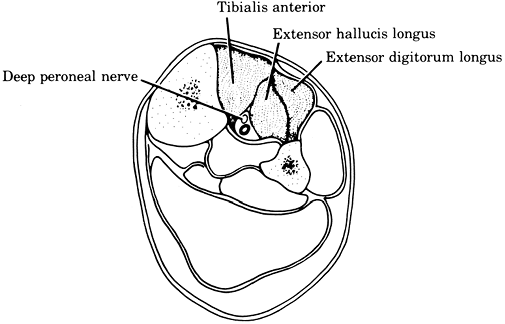
space, and tenseness of the anterior compartmental fascia. -
Increased compartment content arises from:
-
Bleeding caused by a major vascular injury, edema from massive tissue crushing, or a bleeding disorder
-
Increased capillary permeability due to shock, postischemic swelling, exercise, direct trauma, burns, intraarterial drugs, or orthopaedic surgery
-
Increased capillary pressure from exercise or venous obstruction
-
Muscle hypertrophy
-
Direct infusion (infiltrated intravenous line, injection gun)
-
Application of excessive traction (Fig. 2-4)
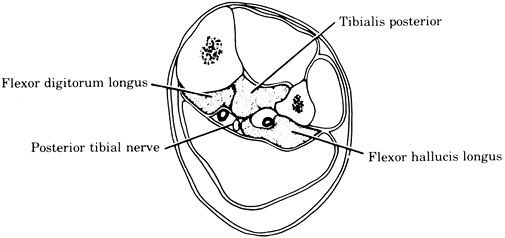 Figure 2-3.
Figure 2-3.
Deep posterior compartmental syndrome of the leg. Symptoms and signs
are weakness of toe flexion and foot inversion, pain on passive toe
extension and foot eversion, hypesthesia of the plantar aspect of the
foot and toes, and tenseness of the deep posterior compartmental fascia
(between the tibia and Achilles tendon).![]() Figure 2-4.
Figure 2-4.
Distraction of fracture fragments (excessive traction) can increase
compartmental tissue pressure and be a cause of a compartmental
syndrome.
-
P.30 -
-
Increased tissue pressure
is the key feature of compartmental syndromes. Once the pressure is
elevated, it can compromise the local circulation by at least three
mechanisms: decreased perfusion pressure, arteriolar closure, and
reflex vasospasm. Muscle cell death and nerve dysfunction begin at
approximately 6 hours after the pressure begins to approach 20 mm Hg
lower than the patients diastolic pressure. -
The clinical approach.
Note that compartment syndrome may be divided into “chronic” and
“acute.” Chronic compartment syndromes are related to exertion and tend
to occur when a given activity level causes transiently increased
tissue pressures that resolve with rest. The topic of this chapter
relates to acute compartment syndromes, which are limb-threatening and
must be treated as potential emergencies.-
Identify the patients at risk as early as possible and examine them frequently.
Continuous real-time monitoring of intramuscular pressure should be
done if the patient’s mental status and/or the ability to examine the
patient is compromised in any way. If the risk is high and the patient
is under anesthesia, consider prophylactic decompression. Patients who have been hypotensive for any reason are at particular risk. -
Carefully document the time and findings of each examination.
-
The appearance of excess pain, sensory deficits, or muscle weakness demands a thorough examination to rule out a compartmental syndrome (Table 2-1). Because the compartmental syndrome is usually progressive, frequent examination
is indicated in questionable cases. Of the 5 “Ps” traditionally taught
to be associated with compartmental syndrome (pain, pulselessness,
pallor, paresthesias, paralysis), only pain and paresthesias are useful
for the early diagnosis of compartment syndrome. Classically, pain with
gentle passive motion is the first sign, and pulselessness is the last.
Patients who are at risk for developing compartment syndrome of an
injured extremity should not have regional anesthesia.
Patient-controlled anesthesia techniques are also capable of masking
the pain associated with compartment syndrome and should be used with
caution in “at-risk” patients.-
Check each potentially involved nerve using two-point discrimination and light touch because both are more sensitive than the commonly used pin.
-
Grade the strengths of all potentially involved muscles (see App. B).
-
The passive muscle stretch test causes severe pain if the muscle is ischemic.TABLE 2-1 Diagnostic Factors in Compartmental Syndromes of the Lower Extremity
Compartment Distribution of sensory changes Muscles weakened Painful passive movement Location of tenseness Anterior Deep peroneal (first web space) Toe extensors and tibialis anterior Toe flexion Anteriorly between tibia and fibula Lateral Superficial and deep peroneal (dorsum of foot) Peronei Inversion of foot Laterally over fibula Deep posterior Posterior tibial (sole of foot) Toe flexors and tibialis posterior Toe extension in distal half Posteromedially of leg between Achilles tendon and tibia Superficial posterior None Gastrocnemius and soleus Foot dorsiflexion Over the bulk of the calf From Matsen III, FA. Compartmental syndrome. Clin Orthop 1976;113:8, with permission. -
Palpation of
each compartment is important because tenseness is a specific sign of a
compartmental syndrome. This sign is obscured unless the dressing and
plaster are adequately opened. Warm and red skin overlying the affected
compartment suggests a cellulitis or thrombophlebitis. -
The peripheral pulse
frequently is normal in the presence of a compartmental syndrome. If it
is abnormal, the diagnosis of a major arterial occlusion or
compartmental syndrome must be entertained. -
Laboratory findings are nonspecific.
P.32 -
-
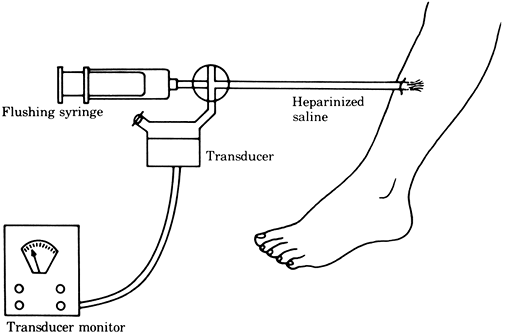
The tissue pressure can be accurately measured by the infusion or wick techniques (Figs. 2-5, 2-6), which give similar pressure readings (11). A simpler but less reliable measurement can be obtained by the injection technique (Fig. 2-7).
Tissue pressure readings within 30 mm Hg of the patient’s diastolic
blood pressure (perfusion pressure <30 mm Hg) are strongly
suggestive of a compartmental syndrome (12). The various techniques for pressure measurement are described by Whitesides (10). The anterior compartment is nearly
P.33P.34
always involved in patients with compartment syndrome and has been
called the “sentinel” compartment. Continuous monitoring of the
anterior compartment is simply performed by connecting a saline-filled
intravenous (IV) tube to an angiocath inserted into the muscle, which
is connected to a standard pressure transducer. A three-way valve
allows the line to be flushed periodically. Although these measuring
techniques are useful, the clinician should rely largely on the
patient’s history and ongoing (or repeat) examinations to establish the
proper diagnosis and treatment program. In the presence of head injury,
intoxication, or an unreliable or unconscious patient, monitoring
techniques become indispensable. The evaluation should include
measurement of the tissue pressure at multiple levels in the
compartment (13).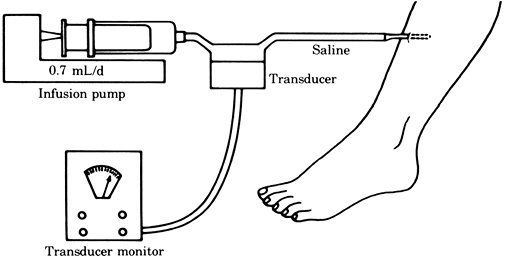 Figure 2-5. Tissue pressure measured by the infusion technique.
Figure 2-5. Tissue pressure measured by the infusion technique.![]() Figure 2-6. Tissue pressure measured by the wick technique.
Figure 2-6. Tissue pressure measured by the wick technique.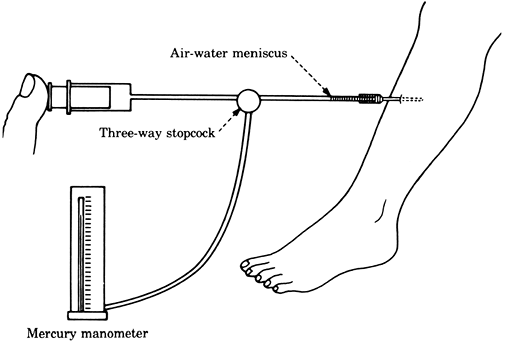 Figure 2-7. Tissue pressure measured by the injection technique.
Figure 2-7. Tissue pressure measured by the injection technique. -
If the examination suggests a compartmental syndrome, decompression of the involved compartments by fasciotomy
should be performed emergently, ideally within 8 hours of the onset of
symptoms. It is very important to perform a longitudinal skin incision
that spans the majority of the length of the involved compartment;
inadequate skin release does not provide adequate decompression (14) and is a common reason for continued tissue ischemia and poor outcome. -
If decompression does not produce the
expected improvement, one should consider the possibilities of
inadequate decompression, another compartmental syndrome, incorrect
diagnosis, or secondary arterial occlusion. Careful reexploration and possibly arteriography are indicated. -
Because myoglobinuria and renal failure
can complicate compartmental syndromes, adequate hydration and urinary
output, with alkalinization of the urine using IV sodium bicarbonate,
should be ensured. Dark urine may usually be attributed to
myoglobinuria if the benzidine test is positive and in the absence of
hematuria.If the compartment syndrome is recognized more than 24
hours after the onset of symptoms, fasciotomy should not be performed.
The risk of deep infection is high and often results in limb loss. When
the time of onset is known to be 24 hours or longer, observation with
urine alkalinization should be the recommendation (15).
-
Sympathetically Maintained Pain Syndrome; Sudeck Atrophy; Reflex
Sympathetic Dystrophy (RSD)]
complaints of pain, especially when associated with hyperesthesia of
the skin and/or abnormal pseudomotor response. For example, an
excessively sweaty extremity that has severe pain with light touch
(such as with bedding sheets or clothing) should be suspected as having
CRPS. For successful treatment, the diagnosis must be made before the classic signs
of thin shiny skin, excessive hair growth, attrition of nails, and
diffuse osteoporosis occur. Whenever the diagnosis is suspected,
institute treatment immediately. Treatment consists of regional
sympathetic nerve blocks plus vigorous active physical therapy to
mobilize any edema as well as to increase the muscle activity and the
range of joint motion. The condition can occur in the upper extremity
as well as in the lower extremity from the knee distally.
-
Deep vein thrombosis (DVT) is extremely common in the trauma patients (16).
The risk is greatest in patients with spine, pelvic, and hip trauma but
is sufficiently high to warrant prophylactic treatment in all injured
patients. The risk of DVT is further increased in patients with
hereditary (often occult) thrombophilia, women who receive hormone
replacement therapy, pregnant patients, and those who are obese, have
cancer, or a previous history of DVT.The diagnosis of DVT should
be entertained in any patient with lymphedema and or pain in an injured
extremity. Pulmonary embolism (PE) is rarely the first manifestation of
DVT. When the diagnosis of DVT is considered, screening the extremities
with duplex Doppler venous ultrasound is usually done first. Contrast
venography is not usually done except in the research setting because
of its invasiveness and potential complications. Magnetic resonance
venography is an
P.35
emerging
technique that is especially promising for diagnosing DVT in the
pelvis, whereas ultrasound has been shown to be less reliable. -
When DVT is identified, patients are usually anticoagulated.
Although intravenous therapeutic heparin infusion followed by oral
warfarin is the traditional regimen, new data supports the use of
intermittent, therapeutic doses (1 mg/ kg/day) of low-molecular weight
heparin. Treatment is usually provided for 3 to 6 months. When
anticoagulation is not possible, often because of associated injuries,
a vena cava filter should be inserted.Prophylactic treatment to prevent DVT is initiated in
every trauma patient upon admission. Mechanical devices such as
sequential compression stockings or intermittent plantar compression
pumps should be applied to both legs of injured persons when possible.
Intermittent fixed-dose heparin (5,000 units heparin every 8 hours) or
low-molecular weight heparin should be started when possible.Screening of patients with venous ultrasound should be
done in patients who have had a delay in the initiation of prophylaxis
for any reason. Screening can also be considered at discharge to assist
with decisions about continuing prophylaxis following discharge.
-
Heterotopic bone formation often occurs
after injury or surgery and can occur in any collagenous supportive
tissue of skeletal muscles, tendons, ligaments, and fascia. There are four clinical types; three may be seen in injured patients:-
Myositis ossificans progressiva
is rare and can be genetic. It usually occurs between the ages of 5 and
10 years (younger than age 20) and proceeds relentlessly to progressive
ossification of skeletal muscles. It is often present in the shoulders
and neck as firm subcutaneous masses, which can be hot and tender and
can undergo ossification. Often associated are microdactyly of the
great toes and thumbs, ankylosis of the interphalangeal and
metatarsophalangeal joints, and bilateral hallux valgus. Minor trauma
often causes exacerbations. Treatment may include diphosphonate
combined with surgery for severe joint malpositioning and functional
impairment. -
Myositis ossificans paralytica
occurs in proximal paralyzed muscles. The ossification occurs 1 to 10
months after a spinal cord injury. This process causes decreased
passive range of motion. The three classic sites are in the vastus
medialis, the quadratus femoris, and the hip abductors. Surgical
treatment is indicated only if the position and function of the
extremity are unacceptable and when the ossification has matured. After
excision, the dead space created must be drained by closed suction and
the wound carefully observed for a hematoma. -
Myositis ossificans circumscripta
can be idiopathic but is more commonly caused by focal trauma and is
common as a sports injury in the contact setting. It is more common in
teenage or young adult males. It presents as an uncomfortable,
indistinct mass that shows local induration and a local increase of
temperature. The lesion occurs 80% of the time in the arm (biceps
brachialis) but also occurs in the thigh (abductors and quadratus
femoris). Roentgenograms show fluffy calcification 2 to 4 weeks after
injury. In 14 weeks, the calcification has matured, and in 5 months,
ossification has occurred. The differential diagnosis includes
osteosarcoma and periosteal osteogenic sarcoma. Treatment is by
excision, only if the lesion is unusually large or painful and after
ossification is mature. -
Myositis ossificans traumatica,
the most common type of hetertopic ossification presents the same way
as the circumscripta type except for a clear history of trauma, with
ossification of a single muscle group in the traumatized area (17).
Treatment is controversial but generally is aimed at the prevention of
ossification by immediate application of cold and compression to the
area of muscle injury. Later, heat is applied. An operation is
indicated only when the ossification causes permanent impairment and
only after the process has stabilized, often as soon as 6 to 8 months
after injury.
-
-
The precise pathophysiology of myositis ossificans is not known. Preventive treatment should be designed to stop the sequence of osteogenesis.P.36
-
Pharmacologic treatment
is generally prophylactic and has historically included bisphosphonates
to inhibit hydroxyapatite crystallization, mithramycin to interfere
with mobilization of calcium, and cortisone to decrease bone formation
at the site of injury. None of these drugs, however, has proved to be
an extremely beneficial therapeutic agent. Indomethacin and Naprosyn
have been shown to help minimize posttraumatic heterotopic ossification
associated with acetabular fractures and arthroplasty (18,19,20).
Similarly, low-dose irradiation with 800 to 1,000 rad has been shown to
be very effective at preventing heterotopic ossification (21). -
When surgical treatment
is indicated, traditional teaching has been to wait until the
ossification is mature that is, when the bone scan is negative and the
alkaline phosphatase level is decreasing. Many authors have recently
advocated earlier resection before these tests have returned to normal (22).
-
PV, Pape HC, Cohen AP, et al. Review: systemic effects of femoral
nailing: from Kuntscher to the immune reactivity era. Clin Orthop 2002;404:378–386.
MM, Whitesides TE, Greve SR, et al. Compartment pressure in association
with closed tibia fractures the relationship between tissue pressure,
compartment and the distance from the site of fracture. J Bone Joint Surg (Am) 1994; 76:1285–1292.
G, Buckley RE, Pineo GE, et al. Incidence of deep-vein thrombosis in
patients with fractures of the lower extremity distal to the hip. J Orthop Trauma 1996;10:230–235.
N, Shaw DL, Bouen SR. Myositis ossification: calcification of the
entire tibialis anterior after ischaemic injury (compartment syndrome).
J Bone Joint Surg (Br) 1995;78:318–319.
JM, Siebenrock KA. Does indomethacin reduce heterotopic bone formation
after operations for acetabular fractures? A prospective randomized
study. J Bone Joint Surg (Br) 1997;79:959–963.
BR, Karzes DE. Prophylactic indomethacin for the prevention of
heterotopic ossification after acetabular fracture in high risk
patients. J Orthop Trauma 1993;7:33–38.
SA, Kjaersgaard-Andersen P, Pedersen NW, et al. The use of indomethacin
to prevent the formation of heterotopic bone after total hip
replacement: a randomized, double-blind clinical trial. J Bone Joint Surg (Am) 1988;70:834–838.
MJ, Poka A, Reinert CM, et al. Heterotopic ossification as a
complication of acetabular fracture: prophylaxis with low-dose
irradiation. J Bone Joint Surg (Am) 1988;70:1231–1237.
EB, von Bonsdorff H, Hakkinen S, et.al. Prevention of fat embolism by
early internal fixation of fractures in patients with multiple
injuries. Injury 1976;8:110–116.