Physeal Injuries and Growth Disturbances
the presence of the physis (or growth plate), which provides
longitudinal growth of children’s long bones. Physeal injuries are a
common and unique feature of children’s bony injuries, in part because
the physis is structurally more susceptible to loads that would produce
metaphyseal or juxta-articular fractures in adults.13,26,59,76,102,106,122,124,129 Physeal injury may occur in a variety of ways in addition to trauma.12,13,17,21,24,30,36,49,61,73,81,83,111,115,123,135,138,140
Although physes, similar to the children with them, are resilient to
permanent injury, uneventful outcomes are by no means assured.1,8,15,18,23,28,57,71,86,94,96,100,103,110,121,130 In this discussion of management of physeal injuries and associated growth disturbances the term epiphysis
is used to refer to the bulbous end of a long bone incorporating the
“growth plate” or “physis” and the secondary ossification center, and
the term physis is used rather than “growth plate.”
articular cartilage-covered ends (epiphyses) tapering to the
funnel-shaped metaphyses, with the central diaphysis interposed between
the metaphyses. During growth, the epiphyseal and metaphyseal regions
are separated by the organized cartilaginous physis, which is the major
contributor to longitudinal growth of the bone. The larger long bones
(clavicle, humerus, radius, ulna, femur, tibia, and fibula) have physes
at both ends, whereas the smaller tubular bones (metacarpals,
metatarsals, and phalanges) usually have a physis at one end only.
occasionally the proximal tibia, all of the above-mentioned epiphyses
are purely cartilaginous. At various stages of postnatal growth and
development, a secondary ossification center forms within
the
epiphysis. This development helps define the radiolucent zone of the
physis, which persists until the physis closes at skeletal maturation.
Typical ages for appearance of the major secondary ossification centers
and physeal closure are summarized in Figures 5-1 and 5-2.
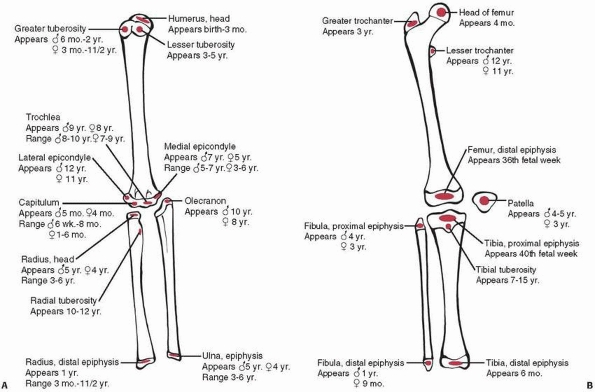 |
|
Figure 5-1 Typical age (and range) of development of the secondary ossification centers of the epiphyses in the (A) upper extremity and (B) lower extremity.
|
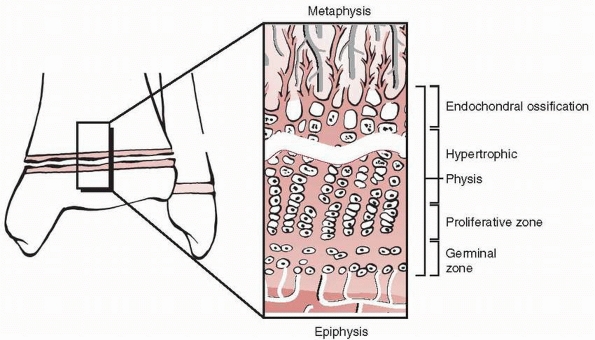
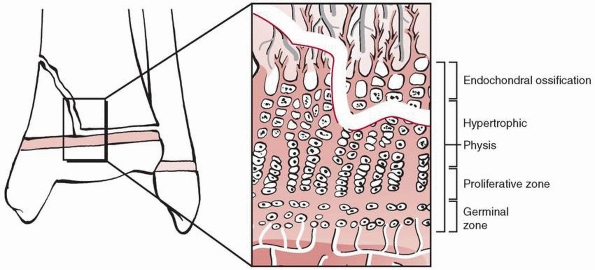
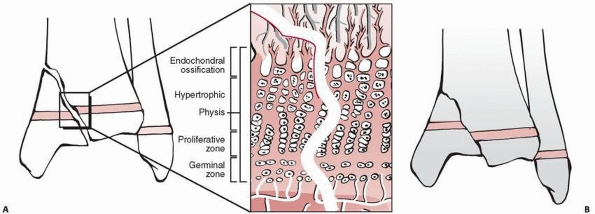
Traditionally, the physis is divided into four zones from the center of
the epiphysis to the metaphysis: germinal, proliferative, hypertrophic,
and provisional calcification (or enchondral ossification) (Fig. 5-3).
The germinal and proliferative zones are the location of cellular
proliferation, whereas the hypertrophic and provisional calcification
zones are characterized by matrix production, cellular hypertrophy,
apoptosis, and matrix calcification. Normal longitudinal growth is
dependent on the interaction of many factors, both hormonal and
mechanical.
specialized areas important to the mechanical integrity and peripheral
growth of the physis (see Fig. 5-3). The zone
(or groove) of Ranvier is a triangular microscopic structure at the
periphery of the physis, containing fibroblasts, chondroblasts, and
osteoblasts. It is responsible for peripheral growth of the physis. The
perichondral ring of LaCroix is a fibrous structure overlying the zone
of Ranvier, connecting the metaphyseal periosteum and cartilaginous
epiphysis, and has the important mechanical function of stabilizing the
epiphysis to the metaphysis.
Type A epiphyses (such as the proximal humeral and proximal femoral
epiphyses) are nearly completely covered with articular cartilage;
therefore, most of the blood supply must enter from the perichondrium.
The blood supply to these epiphyses may be easily compromised by
epiphyseal separation. Type B epiphyses (such as the proximal and
distal tibia and the distal radius) have only a portion of their
surface covered with articular cartilage and are theoretically less
susceptible to devascularization from epiphyseal separation.
the physeal zones permits an understanding of the theoretical line of
least resistance (and hence fracture) within the physis. The germinal
and proliferative zones are characterized by an abundance of
extracellular matrix, whereas the hypertrophic and enchondral
ossification zones are primarily apoptotic cells and vascular channels.
As a consequence, fracture lines can be predicted to pass through the
hypertrophic and enchondral ossification zones, a finding that Salter
and Harris reported in their experimental investigation in rats.125 Theoretically, Salter-Harris types I and II fractures should involve these zones only, not affecting
the germinal and proliferative zones, and thus should be at lower risk
for subsequent growth disturbance. However, types III and IV physeal
fractures traverse the entire physis, including the germinal and
proliferative zones. In addition, displacement between bone fragments
containing portions of the physis may occur. Consequently, growth
disturbance is more likely from type III or IV injuries.
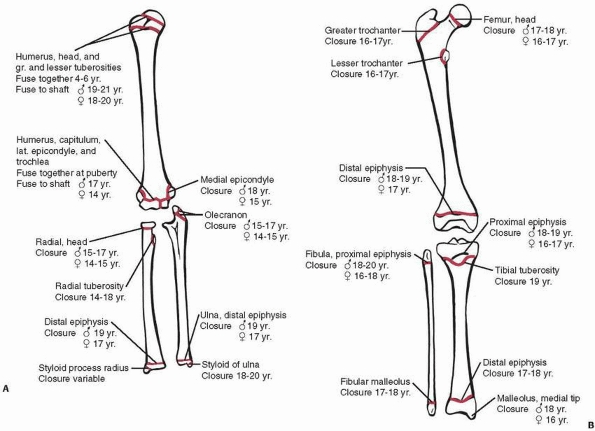 |
|
Figure 5-2 Typical age (and range) of closure of physes in the (A) upper extremity and (B) lower extremity.
|
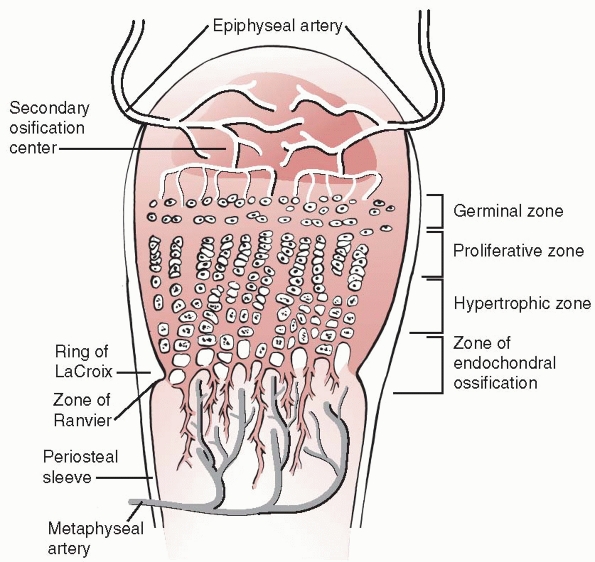 |
|
Figure 5-3
Schematic diagram of the organization of the physis. Four zones are illustrated: the germinal, proliferative, hypertrophic, and provisional calcification (or enchondral ossification) layers. Note also the groove of Ranvier and the perichondral ring of LaCroix. |
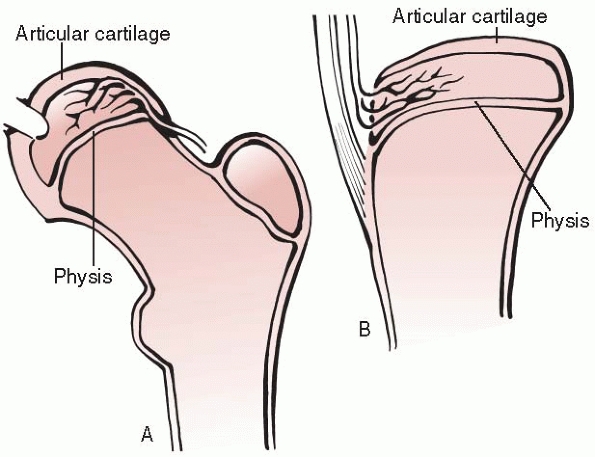 |
|
Figure 5-4 Classification of epiphyseal blood supply according to Dale and Harris. A.
Type A epiphyses are nearly completely covered by articular cartilage. Blood supply must enter via the perichondrium. This blood supply is susceptible to disruption by epiphyseal separation. The proximal femur and proximal humerus are examples of type A epiphyses. B. Type B epiphyses are only partially covered by articular cartilage. Such epiphyses are more resistant to blood supply impairment by epiphyseal separation. The distal femur, proximal and distal tibia, and distal radius are clinical examples of type B epiphyses. |
microscopic fracture patterns have demonstrated that fracture lines
through the physeal layers are more complex than this simplistic view,
and often undulate through the various zones.26,59,75,102,134,142 Smith et al.134
reported a Salter-Harris type I fracture of the distal tibia examined
microscopically after associated traumatic lower leg amputation. In
this high-energy injury, they found that the fracture line involved all
four layers of the physis, in part because of the relatively straight
plane of fracture and the undulations of the physis. Bright et al.,26
in a study of experimentally induced physeal fractures in immature
rats, found that not only was the fracture line usually complex,
involving all four layers of the physis, but also that the physis
contained a number of horizontal “cracks” separate from the fracture
itself. They also observed a statistically significant lower force
required to produce a physeal fracture in male and prepubescent
animals, which might have clinical relevance to the epidemiologic
aspects of physeal fractures (see “Epidemiology”). The rate, direction,
and magnitude of force are also factors that contribute to the
histologic pattern of physeal fractures. Moen and Pelker,102
in an experimental study in calves, found that compression forces
produced fractures in the zone of provisional calcification and
metaphysis, shear caused fractures in the proliferative and
hypertrophic zones, and torque produced fracture lines involving all
four layers of the physis. Finally, the energy of injury is a factor in
the extent of physeal injury. Distal femoral physeal fractures are a
good example of the overriding significance of the energy of injury in
potential for subsequent growth disturbance. High-energy mechanisms of
injury are frequent in this region, and the risk of subsequent growth
disturbance is high.121,91
elongation occurring at their ends. However, as a generality, the
physes at the end of long bones contribute known average lengths in
percentage of total bone growth and percentage contributions to the
total length between two physes at either end of a long bone. This
information has come from observations of longitudinal growth by a
number of authors.9,10,11,60,67,93 Knowledge of this information is paramount for the surgeon managing physeal injuries to long bones. Figure 5-5
outlines the generally accepted percentage of longitudinal growth
contribution of pairs of physes for each long bone in the upper and
lower extremities. Table 5-1 outlines the
average amount of growth in millimeters per year of skeletal growth
contributed by these same physes. These are estimations only, and
growth tables should be consulted when more specific information is
required.10,11,60,67,93
subtle. Obviously, the most frequent mechanism of injury is fracture.
Most commonly, fracture injury is direct, with the fracture pattern
involving the physis itself. Occasionally, physeal injury from trauma
is indirect and associated with a fracture elsewhere in the limb
segment, either as a result of ischemia115 or perhaps compression1,8,23,71,97,103,141 (see discussion of Salter-Harris type V physeal fractures below). Other mechanisms of injuries to the physes include infection,17,21,83,111 disruption by tumor, cysts,135 and tumor-like disorders, vascular insult,115 repetitive stress,7,24,37,38,90,146 irradiation,32,123 and other rare etiologies.16,30,36,126
(particularly of the shoulder, hip, and knee) can cause physeal damage
resulting in either physeal growth disturbance or frank growth arrest.12,17,21,49,61,73,81,83,111
These septic injuries may be further complicated by joint disruption
resulting from associated epiphyseal destruction, articular cartilage
damage, and capsular adhesions, particularly in the hip and shoulder.
deformity requiring multiple surgical procedures. The most common
causes are fulminant neonatal sepsis, particularly in premature infants
or those with neonatal sepsis associated with maternal diabetes, and
multiple septic arrests associated with meningococcemia (Fig. 5-6).
In the latter case, physeal damage may also result from the
cardiovascular collapse and disseminated intravascular coagulation
known as purpura fulminans.12,61,73,81
disorders can disrupt normal physeal architecture, resulting in direct
physeal destruction. In the case of malignant tumors, the extent of
growth lost as the result of local irradiation or limb salvage
surgery must be taken into consideration in planning and recommending the therapeutic reconstruction to be undertaken.
 |
|
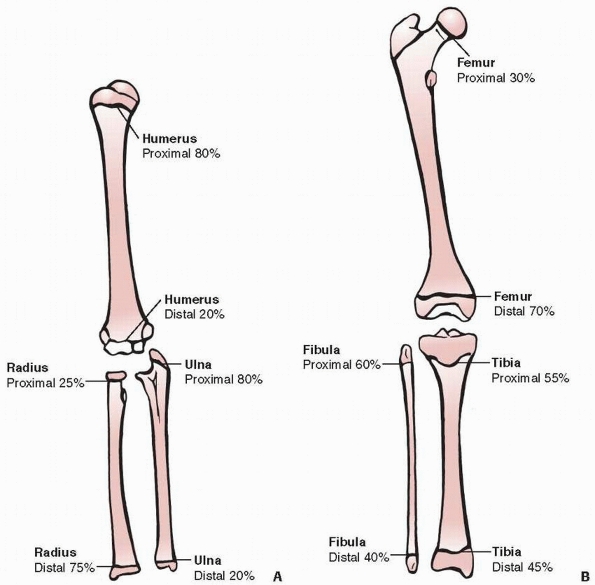
Figure 5-5 Approximate percentage of longitudinal growth provided by the proximal and distal physes for each long bone in the upper (A) and lower (B) extremities.
|
|
TABLE 5-1 Average Growth Per Year (in millimeters) of Specific Physes of the Upper and Lower Extremities*
|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
destruction of all or part of a physis. Examples include enchondromata,
either isolated or multiple (Ollier disease) (Fig. 5-7), and unicameral bone cysts.135
Growth disturbance as a consequence of physeal damage from these
disorders generally cannot be corrected by surgical physeal arrest
resection (see “Physeal Arrests”), and other treatment strategies must
be adopted as clinically indicated.
Unrecognized vascular insult may represent the mechanism of subsequent
growth disturbance after an injury in an adjacent part of a limb and
may represent Salter-Harris type V injuries; the most common location
for this is the tibial tubercle after femoral shaft or distal femoral
physeal fractures. In addition, ischemia may be the cause of physeal
damage associated with purpura fulminans.12,61,73,81
); and the proximal humerus, as in baseball pitchers.37
These injuries should be managed by rest, judicious resumption of
activities, and longitudinal observation to monitor for potential
physeal growth disturbance.
 |
|
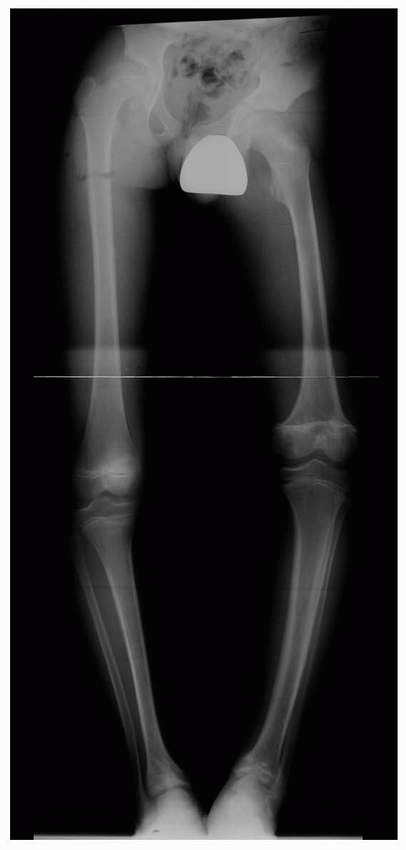
Figure 5-6
Standing anteroposterior lower extremity radiograph of a 12-year-old boy with multifocal physeal disturbance from purpura fulminans associated with meningococcemia. Radiograph abnormalities are present in the left proximal femur; both distal femoral epiphyses, including partial arrest of the left distal femoral physis; and both distal tibial epiphyses. The patient also has digital amputations and extensive soft-tissue scarring resulting from this septic event. |
 |
|
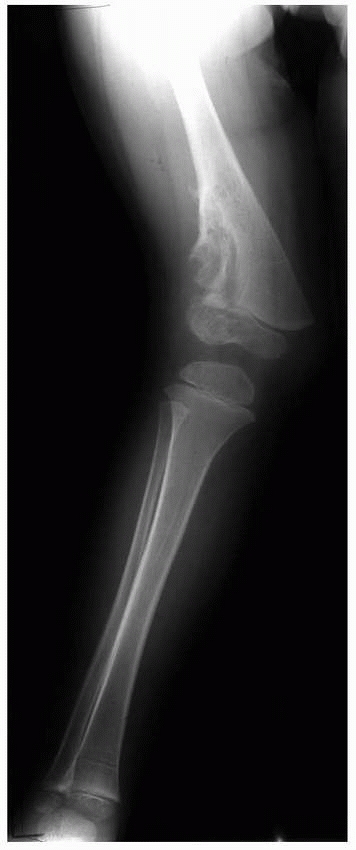
Figure 5-7
Valgus deformity of the distal femur associated with the presence of an enchondroma of the distal lateral femur involving the lateral physis. |
 |
|
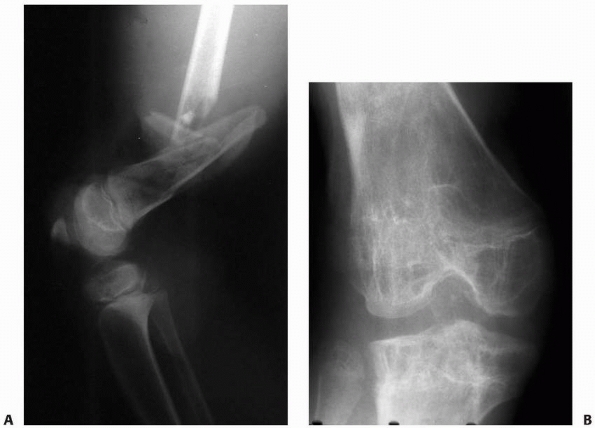
Figure 5-8 Physeal injury from presumed vascular insult. A.
The patient’s leg was caught under heavy pipes rolling off a rack, resulting in stripping of the soft tissues from the distal thigh, open comminuted fracture of the distal femur, and popliteal artery injury. B. In follow-up, after arterial and soft tissue reconstruction, the patient has physeal growth arrests of the distal femur and proximal tibia. The mechanism of injury to the proximal tibial physis was presumed to be vascular because of the associated femoral artery injury. |
 |
|
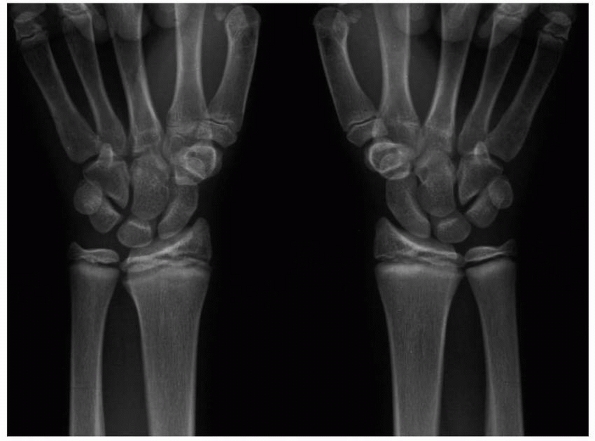
Figure 5-9
Stress injury of the distal radius and ulna in both wrists of a competitive gymnast. There was no history of specific injury. The wrists were tender to touch. Note distal radial and ulnar physeal widening and irregularity. |
burns; and electrical injuries. On rare occasions, physeal growth
disturbance noted on clinical findings and radiographs has no
identifiable cause. Presumably, such events represent unrecognized
trauma or infection involving the physis.
 |
|
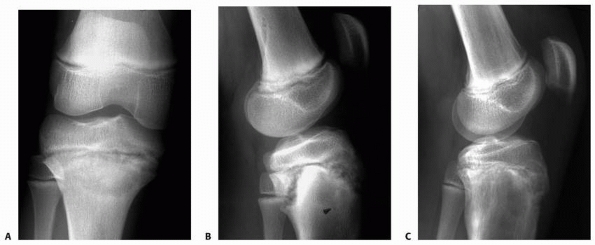
Figure 5-10 Stress injury of the proximal tibia in an elite soccer player. A. Anteroposterior radiograph film demonstrates subtle proximal tibial physeal widening. B.
Lateral radiograph shows widening, a metaphyseal Thurston-Holland fragment, and some posterior displacement of the proximal epiphysis. C. Significant radiograph improvement noted after discontinuing athletic activities for 3 months. |
 |
|
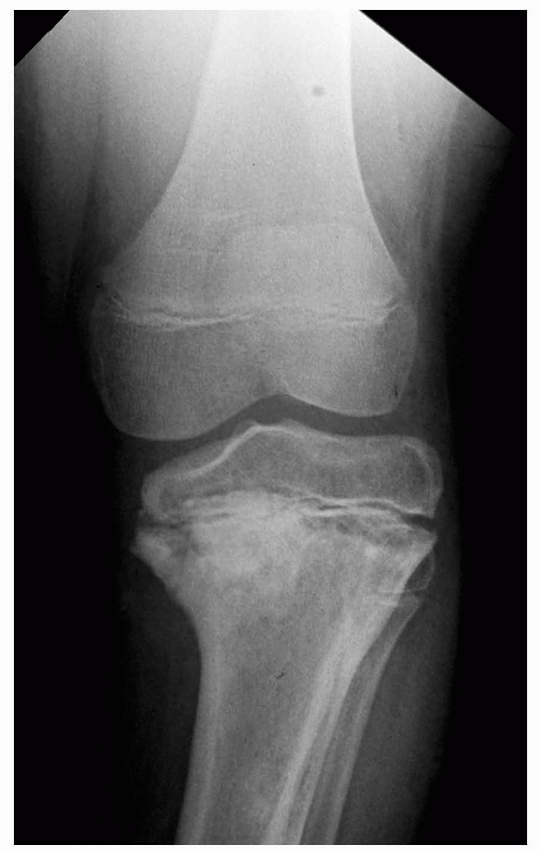
Figure 5-11 Proximal tibial physeal growth disturbance with angular deformity after irradiation for Ewing sarcoma.
|
ancient times. Hippocrates is credited with the first written account
of this injury. Poland (see “Classification of Physeal Fractures”)
reviewed accounts of physeal injuries in his 1898 book, Traumatic Separation of the Epiphysis.119
Poland is also credited with the first classification of the patterns
of physeal fracture, and the publication of his text closely followed
Roentgen’s discovery of radiographs in 1895.
by a number of authors,2,3,4,5,43,46,92,106,107,113,114,118,125 including Aitken,4 Salter and Harris,125 Ogden et al.,107 and Peterson.113,114
Classifications of physeal fractures are important because they alert
the practitioner to potentially subtle radiographic fracture patterns,
can be of prognostic significance with respect to growth disturbance
potential, and guide general treatment principles based on that risk
and associated joint disruption. To some extent, fracture pattern
provides some insight into mechanism of injury and the extent of
potential physeal microscopic injury (“Normal Physeal Anatomy” and
“Mechanical Features of the Physis and Patterns of Injury”).
 |
|
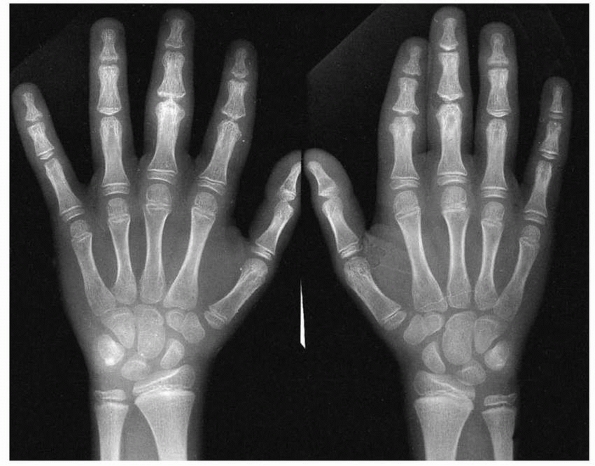
Figure 5-12 Premature closure of the distal phalangeal physes after a frostbite injury to the digits.
|
is firmly entrenched in the literature and most orthopaedists’ minds.
Therefore, evolution and specifics of the nature of physeal fractures
of the various classification schemes are discussed relative to the
Salter-Harris classification. The reader also should be aware of some
deficiencies in that classification, as pointed out by Peterson.113,114,116
Types I, II, and III were the foundation of the Salter-Harris
classification, as described below. Poland’s type IV fracture was
effectively a T-condylar fracture of the epiphysis and physis.
His type I corresponded to Poland and Salter-Harris type II fractures,
his type II to Poland and Salter-Harris type III fractures, and his
type III was an intra-articular transphyseal metaphyseal-epiphyseal
fracture equivalent to a Salter-Harris type IV fracture.
 |
|
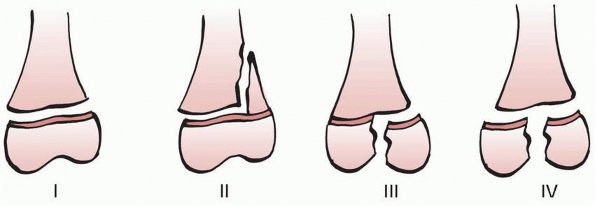
Figure 5-13
Poland classification of physeal fractures. Compare to the Salter-Harris classification. Poland type I: epiphyseal separation without metaphyseal fragment, or extension into the epiphysis. Poland type II: physeal fracture line extends into the metaphysis. Poland type III: fracture extends from the articular surface to the physis and continues peripherally through the physis. Poland type IV: T-condylar fracture of the epiphysis and physis. |
The first four types were adopted from Poland (types I, II, and III)
and Aitken (Aitken type III became Salter-Harris type IV) (Fig. 5-15).
Salter and Harris added a fifth type, which they postulated was an
unrecognized compression injury characterized by normal radiographs and
late physeal closure. Peterson challenged the existence of true type V
injuries,116 but other authors have subsequently documented its existence in some form.1,8,15,23,70,71,80,141,116
Because we believe that delayed physeal closure can occur after some
occult injuries, we have chosen to retain this type of injury in our
preferred classification scheme.
by a transphyseal plane of injury, with no bony fracture line through
either the metaphysis or the epiphysis. Radiographs of undisplaced type
I physeal fractures, therefore, are normal except for associated soft
tissue swelling, making careful patient examination particularly
important in this injury. In the Olmstead County Survey of physeal
fractures,117 type I fractures
occurred most frequently in the phalanges, metacarpals, distal tibia,
and distal ulna. Epiphyseal separations in infants occur most commonly
in the proximal humerus, distal humerus, and proximal femur. If an
urgency to make the diagnosis is deemed necessary for patients
suspected of having a type I injury, further imaging by ultrasound,
magnetic resonance imaging (MRI),34,41,74,118,133 or arthrography may be helpful.6,63,95,145
Stress radiographs to document displacement are generally unnecessary
and probably unwise. Ultrasound is particularly helpful for assessing
epiphyseal separations in infants (especially in the proximal femur and
elbow regions) without the need for sedation, anesthetic, or invasive
procedure.29,45,47,69,128
zone of hypertrophy of the physis, as the path of least resistance
during the propagation of the injury (see “Normal Physeal Anatomy”) (Fig. 5-16).
As a consequence, in theory, the essential resting and proliferative
zones are relatively spared, and, assuming that there is no vascular
insult to these zones as a consequence of the injury, subsequent growth
disturbance is relatively uncommon. As discussed above, however,
studies have shown this to be a simplistic view of the fracture line
through a physis, and that, due to uneven loading and macroscopic
undulations in
the physis, any zone of the physis can be affected by the fracture line.26,75,102,124,129,134
 |
|
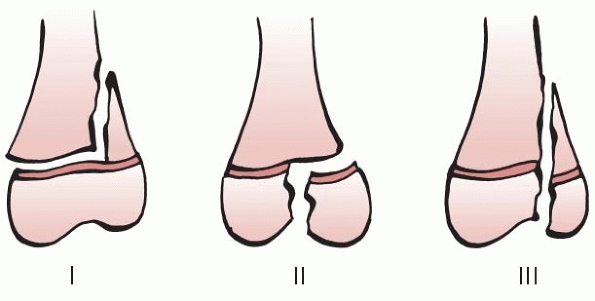
Figure 5-14 Aitken classification of physeal fractures: types I, II, and III. Type III is equivalent of Salter-Harris type IV.
|
 |
|
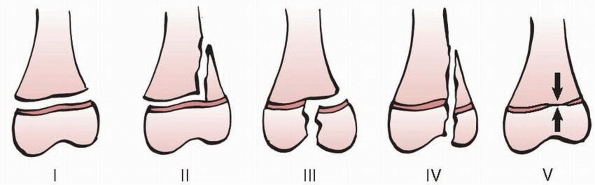
Figure 5-15
Salter-Harris classification of physeal fractures. In Salter-Harris type I fractures, the fracture line is entirely within the physis, referred to by Poland as type I. In Salter-Harris type II fractures, the fracture line extends from the physis into the metaphysis; described by Poland as type II and Aitken as type I. In Salter-Harris type III fractures, the fracture enters the epiphysis from the physis and almost always exits the articular surface. Poland described this injury as type III and Aitken as type II. In Salter-Harris type IV, the fracture extends across the physis from the articular surface and epiphysis, to exit in the margin of the metaphysis. Aitken described this as a type III injury in his classification. Salter-Harris type V fractures were described by Salter and Harris as a crush injury to the physis with initially normal radiographs with late identification of premature physeal closure. |
 |
|
Figure 5-16
Scheme of theoretic fracture plane of Salter-Harris type I fractures. Because the hypertrophic zone is the weakest zone structurally, separation should occur at this level. Experimental and clinical studies have confirmed that the fracture plane is more complex than this concept and frequently involves other physeal zones as well. |
 |
|
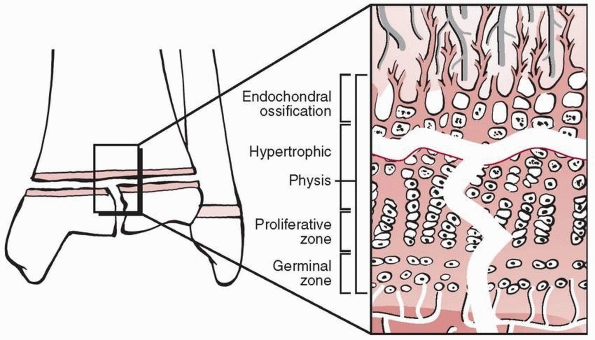
Figure 5-17 Fracture plane of Salter-Harris type II fractures. The fracture extends from the physis into the periphery of the metaphysis.
|
the germinal and proliferative layers of the physis are not displaced,
the general principles of fracture management are to secure a gentle
and adequate reduction of the epiphysis on the metaphysis and stabilize
the fragments as needed.
components; the fracture line extends from the physeal margin
peripherally across a variable portion of the physis and exits into the
metaphysis at the opposite end of the fracture (Fig. 5-17).
The epiphyseal fragment thus comprises all of the epiphysis and some
portion of the peripheral metaphysis (the Thurston-Holland fragment or
sign). The physeal portion of this fracture has microscopic
characteristics similar to those of type I injuries, but the fracture
line exits the physis to enter the metaphysis (i.e., away from the
germinal and proliferative layers) at one margin. Similar to type I
injuries, these fractures should have a limited propensity to
subsequent growth disturbance as a consequence of direct physeal
injury. However, the metaphyseal “spike” of the diaphyseal/metaphyseal
fragment may be driven into the physis of the epiphyseal fragment,
which can damage the physis (Fig. 5-18).
Similar to type I injuries, the articular surface is not affected and
the general principles of fracture management are effectively the same.
 |
|
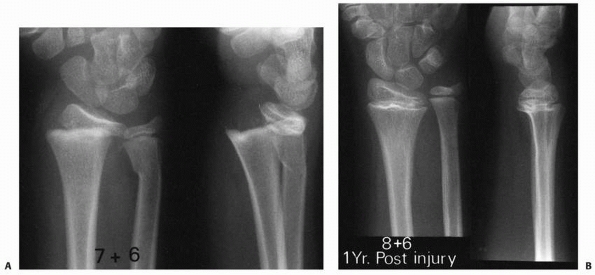
Figure 5-18 Potential mechanism of physeal arrest development after Salter-Harris type II fracture of the distal radius. A.
Dorsally displaced type II fracture of the distal radius. Note the evidence of impaction of the epiphyseal fragment (with the physis) by the dorsal margin of the proximal fragment metaphysis. B. One year later, there is radiographic evidence of physeal arrest formation in the distal radial physis. |
epiphysis (with only rare exception) as a fracture through the
articular surface and extend vertically toward the physis. The fracture
then courses peripherally through the physis (Fig. 5-19).
There are two fracture fragments: a small fragment consisting of a
portion of the epiphysis and physis, and a large fragment consisting
the remaining epiphysis and long bone. This fracture pattern is
important for two main reasons: the articular surface is involved (Fig. 5-20)
and the fracture line involves the germinal and proliferative layers of
the physis. In addition, type III injuries are often associated with
high-energy or compression mechanisms of injury, which imply greater
potential disruption of the physis and higher risk of subsequent growth
disturbance. Anatomic reduction (usually open) and stabilization are
required to restore the articular surface and to minimize the potential
for growth disturbance.
 |
|
Figure 5-19
Scheme of fracture plane in Salter-Harris type III fractures. The fracture plane extends from the physis into the epiphysis and articular surface. “Extra-articular” type III fractures in which the articular surface is intact have been reported but are quite rare. |
distal humerus, high-energy injuries produce either a T-condylar or
other complex pattern of injury, with at least three fragments,
resulting in a combination of physeal and epiphyseal injuries (Fig. 5-21).
These fractures are important because they disrupt the articular
surface, violate all the physeal layers in crossing from the epiphysis
to the metaphysis, and, with displacement, may result in
metaphyseal-epiphyseal cross-union (see Fig. 5-22B).39,58
The latter occurrence almost invariably results in subsequent growth
disturbance. This fracture pattern is frequent around the medial
malleolus, but may occur in other epiphyses. Lateral condylar fractures
of the distal humerus and intra-articular two-part triplane fractures
of the distal tibia may be thought of as complex Salter-Harris type IV
fractures. Rang120 described an
extra-articular variant (as if a peripheral extra-articular
epiphyseal-physeal-metaphyseal fragment were “scooped out” from the
rest of the bone) sometimes referred to as a Salter-Harris type VI
fracture pattern.
 |
|
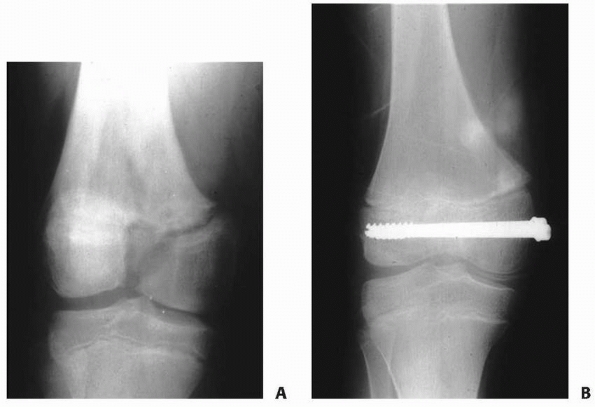
Figure 5-20 A. Salter-Harris type III fracture of the distal femur. B. Fixation with cannulated screws.
|
reduction and adequate stabilization to restore the articular surface
and prevent metaphyseal-epiphyseal cross union.
Harris was not described by Poland or Aitken. Salter and Harris
postulated that type V fractures represented unrecognized compression
injuries with normal initial radiographs that later produced premature
physeal closure. The existence of true type V injuries was questioned
by Peterson116 and subsequently became a subject of debate.1,8,15,23,70,71,80,141
We believe that delayed physeal closure clearly occurs. The most common
example of such an injury is closure of the tibial tubercle, often with
the development
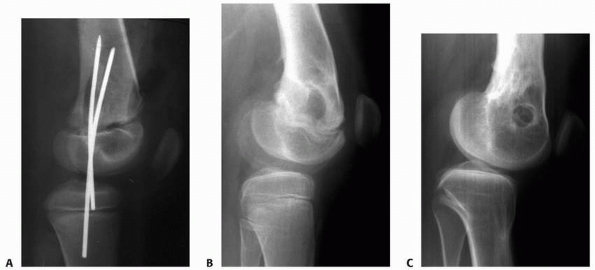
of recurvatum deformity of the proximal tibia, after fractures of the femur or distal femoral epiphysis (Fig. 5-23).23,70,80
While the mechanism of such injuries may be unclear (perhaps vascular
rather than compression trauma), the traditionally held view that such
injuries occurred as a result of inadvertent direct injury during the
insertion of proximal tibial skeletal traction pins has been
unequivocally discounted in some cases.23,70,80
Other locations and case reports of late physeal closure after
extremity injury and apparently normal initial radiographs exist in the
literature.1,8,15,71,101,141
By definition, this pattern of injury is unrecognized on initial
radiographs. Undoubtedly, more sophisticated imaging of injured
extremities (such as with MRI) will identify physeal injuries in the
presence of normal plain radiographs (Fig. 5-24). Although the mechanism of injury in type V injuries may be in dispute, in our opinion, the existence of such injuries is not.
 |
|
Figure 5-21
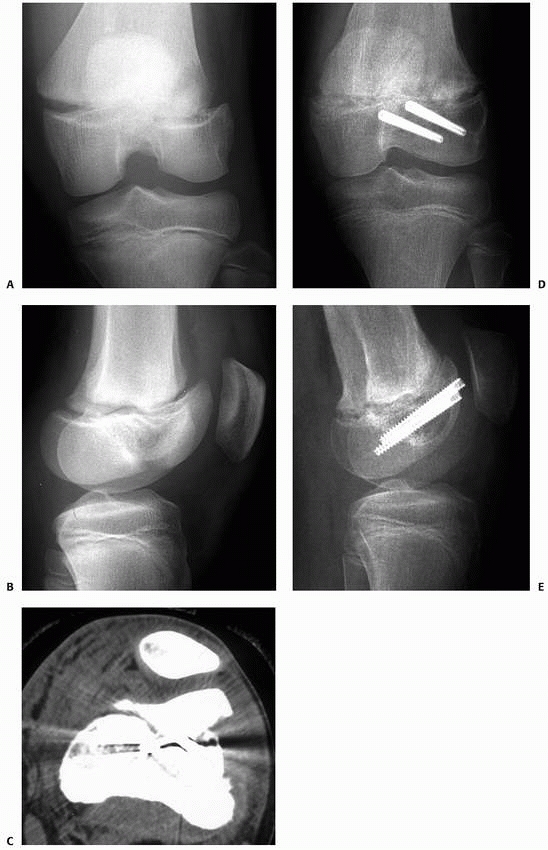
Complex fracture of the distal femur. There is a Salter-Harris type II fracture of the distal femoral physis. In addition, there is an additional coronal plane epiphyseal fracture of the major portion of the lateral femoral condyle, not involving the physis, which was not recognized at the time of initial treatment. The type II component was treated by closed reduction and cross-pinning. The epiphyseal fracture was treated separately and subsequently by open reduction and headless screw fixation. A. Initial anteroposterior radiograph showing what appears to be simple Salter-Harris type II fracture of the distal femur. B. Lateral radiograph after reduction appears acceptable; however, careful review demonstrates the coronal plane, intra-articular fracture of the lateral condyle. C. CT scan demonstrates the epiphyseal fracture of the lateral femoral condyle. D,E. Radiograph appearance after healing of the fractures. Patient was asymptomatic and recovered full knee motion. In follow-up, the patient developed symmetric distal physeal closure not requiring further treatment. |
 |
|
Figure 5-22 Scheme of the Salter-Harris type IV fracture. A. The fracture line extends across the physis from the epiphysis and articular surface into the peripheral metaphysis. B. Displacement of the fragments can lead to horizontal apposition (and cross union) of the epiphyseal and metaphyseal bone.
|
 |
|
Figure 5-23
Posttraumatic closure of the anterior proximal tibial physis after displaced Salter-Harris type II fracture of the distal femoral physis. A. Lateral radiographs after reduction. No injury to the proximal tibia was noted at the time of treatment of the distal femoral injury. B. At follow-up, distal femoral physeal growth disturbance with flexion deformity is apparent. C. At skeletal maturity, proximal tibial extension deformity with sclerosis of the tibial tubercle area is evident, suggestive of arrest in this area. The patient has undergone a distal femoral extension osteotomy. |
identified several deficiencies of the Salter-Harris classification and
subsequently developed a new classification of physeal fractures (Fig. 5-25).
They were not able to identify any Salter-Harris type V injuries caused
by compression in this epidemiologic study, challenged their existence,
and excluded that type from the classification. This classification
retained Salter-Harris types I through IV as Peterson types II, III,
IV, and V and added two new types.113,114 It is important to be cognizant of the two new patterns that Peterson et al described, because they are clinically relevant.
 |
|
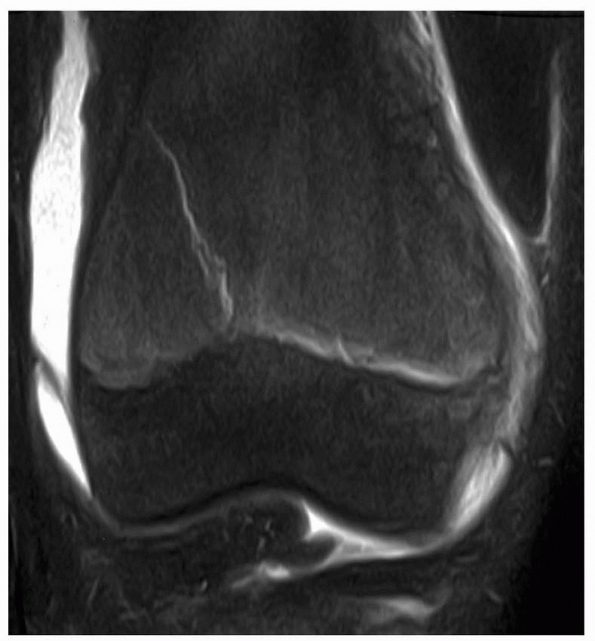
Figure 5-24
MRI of patient after injury with normal radiographs. MRI clearly documents the presence of a Salter-Harris type II fracture of the distal femur. |
Unfortunately, this pattern of injury currently is common, largely as a
consequence of lawnmower injuries. Soft tissue loss, neurovascular
injury, and partial physeal loss (usually including the epiphysis so
that articular impairment also results) further complicate this
often-devastating injury.
an easily recognized and recalled classification scheme embracing most
physeal injuries and continue to use it to describe most physeal
fracture patterns. It provides generally useful prognostic and
treatment guidelines. We encourage the continued recognition of the
Salter-Harris type V physeal injury as a delayed, indirect, or occult
injury-induced physeal closure, whose mechanism may be compression,
other unrecognized direct injury, or vascular insult. We also believe
that Peterson types I and VI physeal fractures are not classifiable by
the Salter-Harris scheme and refer to them as Peterson type I and VI
fractures, respectively.
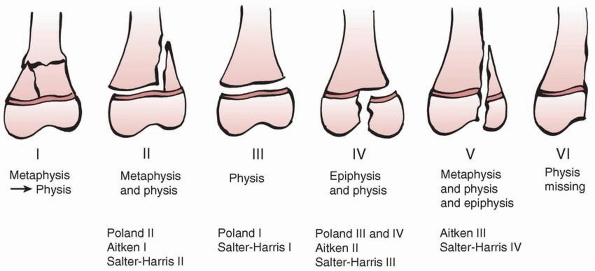 |
|
Figure 5-25
Peterson classification of physeal fractures. Type I is a fracture of the metaphysis extending to the physis. Types II to V are the equivalents of Salter-Harris types I, II, III, and IV, respectively. Peterson type VI is epiphyseal (and usually articular surface) loss. Lawnmower injuries are a frequent mechanism for type VI injuries (see text for further discussion). |
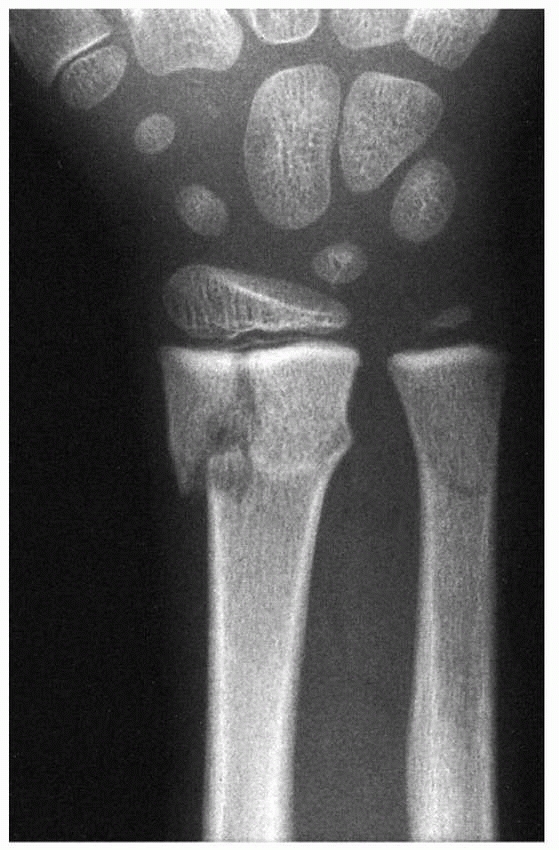 |
|
Figure 5-26
Peterson type I injury of the distal radius. These injuries typically have a benign course with respect to subsequent growth disturbance. |
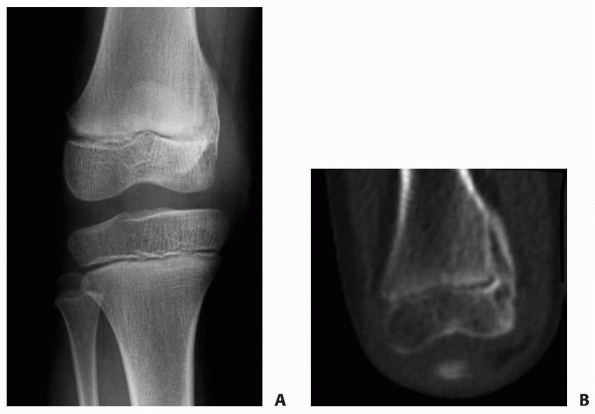 |
|
Figure 5-27 Sequelae of a Peterson type VI physeal injury. A.
Anteroposterior radiograph of distal femur of a young girl who suffered a Peterson type VI injury. This particular injury was the result of direct abrasion of the distal femur when the unrestrained child was ejected from a car. B. CT scan 1 year after injury demonstrates the development of a peripheral physeal arrest with valgus deformity. |
20% to 30% of all childhood fractures were physeal injuries. The
phalanges represent the most common location of physeal injuries.
This study of the frequency of physeal fractures in a stable population
base was performed between 1979 and 1988, in Olmstead County,
Minnesota. The most relevant components are summarized in Tables 5-2 and 5-3.
During the study period, 951 physeal fractures were identified: 37% of
fractures occurred in the finger phalanges, with the next most common
site the distal radius; 71% fractures occurred in the upper extremity,
28% in the lower, and 1% in the axial skeleton. Other salient findings
of the Olmstead County survey included a 2:1 male to female ratio and
age-related incidence by gender (peak incidence at age 14 in boys and
11 to 12 in girls) (Fig. 5-28). The Adelaide, Australia, survey by Mizuta et al.101
had similar findings: 30% of physeal fractures were phalangeal, males
outnumbered females approximately 2:1, and the prepubertal age groups
had the highest relative frequency of physeal fracture.
|
TABLE 5-2 Frequency of Physeal Fracture by Location
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
injuries include plain radiographs, computed tomography scans (CT), and
MRI scans,34,41,56,74,75,118,133 arthrography,6,44,63,95,145 and ultrasound.29,45,47,69,128
Plain radiographs remain the preferred initial modality for the
assessment of most physeal injuries. Radiographs should be taken in
true orthogonal views and include the joint both above and below the
fracture. If a physeal injury is suspected, dedicated views centered
over the suspected physis
should
be obtained to decrease parallax and increase detail. Oblique views may
be of value in assessing minimally displaced injuries.
|
TABLE 5-3 Distribution of Physeal Fracture Patterns by Salter-Harris and Peterson Types I and VI Classification*
|
|||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||
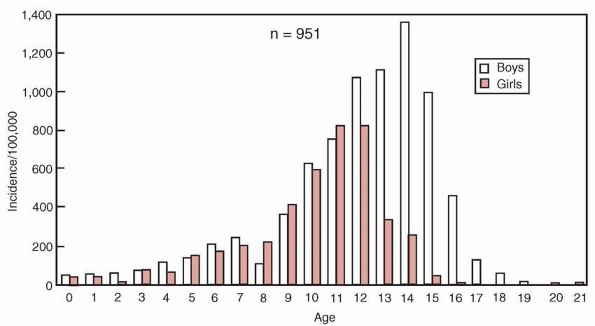 |
|
Figure 5-28 Relative frequency of physeal fractures by age and sex according to the Olmstead County survey by Peterson et al.81 Peak incidence age 14 in boys, and 11 to 12 in girls.
|
the assessment and treatment of most physeal injuries, occasionally
greater anatomic detail is necessary. CT scans provide excellent
definition of bony anatomy, particularly using reconstructed images.
They may be helpful in assessing complex or highly comminuted
fractures, as well as the articular congruency of minimally displaced
fractures (Fig. 5-29). MRI scans are excellent
for demonstrating soft tissue lesions and “minor osseous injuries,”
which may not be seen using standard radiation techniques.
assess the congruency of articular surfaces. Arthrography may help
define the anatomy in young patients with small or no secondary
ossification centers in the epiphyses.6,44,63,95,145 Ultrasonography is occasionally useful for diagnostic purposes to identify epiphyseal separation in infants (Fig. 5-30).29,44,45,47,69
essentially the same as those for injuries not involving the physis,
including radiographs of all areas with abnormal physical findings.
Once the patient has been stabilized and the initial assessment
completed, further studies may be obtained as indicated. Open physeal
injuries and those involving neurovascular compromise or impending
compartment syndrome should be managed emergently. In most cases,
stabilization of the physeal fractures will help facilitate management
of the soft tissue injury. All trauma patients should be reassessed as
their condition stabilizes to identify occult injuries not identified
in the initial assessment.
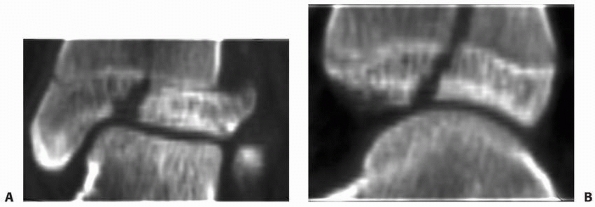 |
|
Figure 5-29 CT scans with or without reconstructed images can be helpful in the assessment of physeal fractures. Coronal (A) and sagittal (B) plane reconstructions of a triplane fracture of the distal tibia.
|
injuries, heal more rapidly than in adults, and they are less likely to
experience morbidity or mortality from prolonged immobilization.
Additionally, children are also often less compliant with postoperative
activity restrictions, making cast immobilization a frequently
necessary adjunct to therapy.
in a consistent methodical manner that includes a general assessment
and stabilization of the polytraumatized patient, evaluation of the
neurovascular and soft tissue status of the traumatized limb, and
reduction and stabilization of the fracture. What
constitutes
an “acceptable” reduction is dictated in part by the fracture pattern
and remodeling potential of the fracture. Intra-articular fractures
(such as Salter-Harris types III and IV) require anatomic reduction to
restore the articular surface and prevent epiphyseal-metaphyseal cross
union. Salter-Harris types I and II fractures, particularly those that
are the result of low-energy injuries, have minimal risk of growth
disturbance (excepting injuries of the distal femur and proximal tibia)
and excellent remodeling potential in most patients; in such patients,
the surgeon must be cautious not to create physeal injury by excessively forceful or invasive reductions.
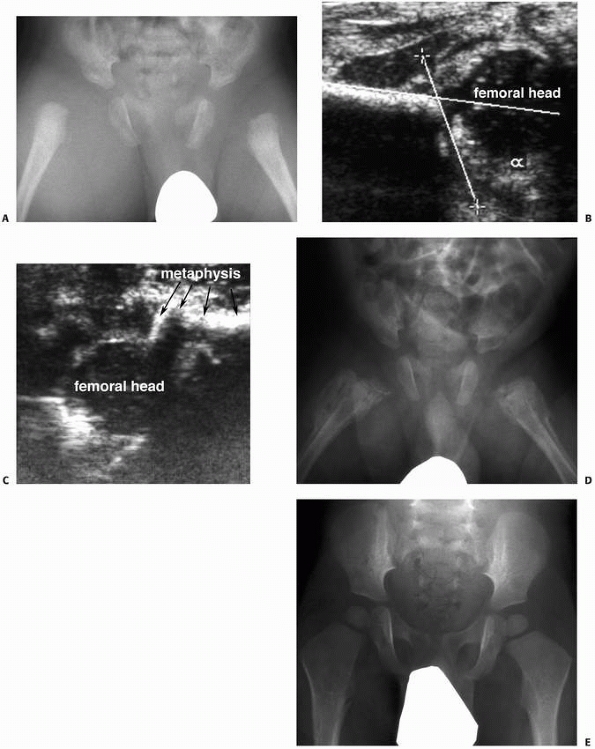 |
|
Figure 5-30
Ultrasonography can be useful as a noninvasive investigation confirming intra-articular effusion or epiphyseal separation, particularly in infants. A. Anteroposterior radiograph of a 2-month-old infant with bilateral hip pain and generalized irritability. Septic arthritis is included in the differential diagnosis. B. Ultrasonographic image of the right hip demonstrates a femoral head contained in the acetabulum, without significant hip effusion. C. This ultrasonographic image demonstrates separation of the proximal epiphysis from the femoral metaphysis. The diagnosis is nonaccidental trauma. D. One month later, radiograph demonstrates extensive periosteal reaction bilaterally. E. At 18 months of age, radiograph demonstrates remarkable remodeling, without evidence of physeal growth disturbance or epiphyseal abnormality. |
disturbance, the potential complications of physeal injuries are no
different than other traumatic musculoskeletal injuries. Neurovascular
compromise and compartment syndrome represent the most serious potential complications.28,110
It is important to remember that, although a high degree of suspicion
and diligence may avoid some of these potentially devastating
complications, they can occur even with “ideal” management. Infection
and soft tissue loss can complicate physeal fracture management, just
as they can in other fractures. The one complication unique to physeal
injuries is growth disturbance. Most commonly, this “disturbance” is
the result of a tethering (physeal bar or arrest) that may produce
angular deformity or shortening. However, growth disturbance may occur
without an obvious tether or bar and growth acceleration also occurs (Fig. 5-31). Finally, growth disturbance may occur without injury to the physis.
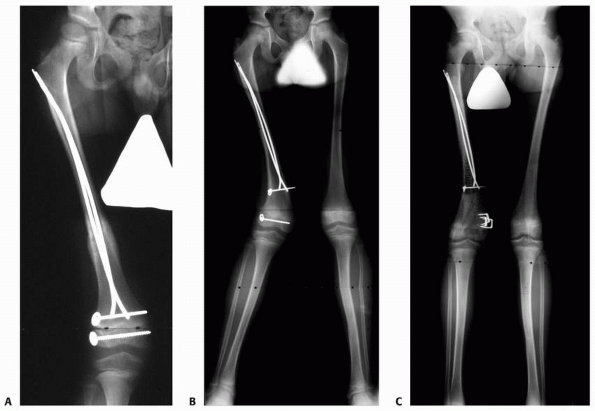 |
|
Figure 5-31
Growth deceleration in the absence of a true physeal arrest. This patient sustained concurrent ipsilateral femoral shaft and Salter-Harris type IV distal femoral epiphyseal fractures. A. Anteroposterior radiograph of the healed femur. Both fractures were treated with internal fixation. B. The patient developed valgus deformity of the distal femur due to asymmetric growth of the distal femoral physis. Note that the distance between the screws on either side of the physis has increased asymmetrically, confirming asymmetric growth rather than cessation of growth laterally. C. The angular deformity was treated with medial distal femoral epiphyseal stapling. |
The potential consequences of physeal growth disturbance include the
development of angular deformity, limb length inequality, epiphyseal
distortion, or various combinations of these. Development of these
abnormalities, if any, depends on the physis affected, location within
the affected physis, the duration of time present, and the skeletal
maturity of the patient. Frequently, further surgery, often repeated
and extensive, is required to correct or prevent deformity caused by an
established growth disturbance.25,27,64,78,82,84,127,143
physical loss of the physis (such as after Peterson type VI injuries),
from disruption of normal physeal architecture and function without
actual radiograph loss of the physis, or by the formation of a physeal
arrest, also called bony bridges or physeal bars.142
Careful identification of the nature of physeal growth disruption is
important, because treatment strategies may differ based on the
etiology of growth disturbance and the presence or absence of a true
growth arrest.
abnormality noted on serial radiographs in a patient known to be at
risk after fracture or infection, clinically with established limb
deformity (angular deformity, shortening, or both), or occasionally
incidentally on radiographs obtained for other reasons. The hallmark of
plain radiographic features of physeal growth disturbance
is
the loss of normal physeal contour and the sharply defined radiolucency
between epiphyseal and metaphyseal bone. Frank physeal arrests
typically are characterized by sclerosis in the region of the arrest.
If asymmetric growth has occurred, there may be tapering of a growth
arrest line to the area of arrest,65,105 angular deformity, epiphyseal distortion, or shortening (Fig. 5-32).
Physeal growth disturbance without frank arrest typically appears on
plain radiographs as a thinner or thicker physeal area with an
indistinct metaphyseal border because of alteration in normal
enchondral ossification. There may be an asymmetric growth arrest line
indicating angular deformity, but the arrest line will not taper to the
physis itself (Fig. 5-33).105
This indicates altered physeal growth (either asymmetric acceleration
or deceleration) but not a complete cessation of growth. This
distinction is important, because the consequences and treatment are
different from those caused by complete growth arrest.
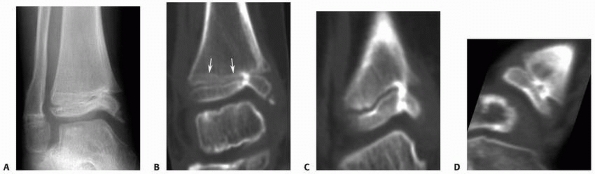 |
|
Figure 5-32
Harris growth arrest line tapering to the physis at the level of the growth arrest can serve as an excellent radiograph confirmation of the presence of the true growth arrest. Although most commonly noted on plain radiographs, these arrest lines can be seen on CT scans and MRIs as well. A. Anteroposterior radiograph of the distal tibia after Salter-Harris type IV fracture demonstrates a Harris growth arrest line tapering to the medial distal tibial physis, where a partial physeal arrest has formed. B. Harris growth arrest line as noted on CT. CT scans with coronal (C) and sagittal (D) reconstructions corrected for bone distortion provide excellent images of the location and size of arrest. |
a skeletally immature child, further evaluation often is warranted. CT
scanning with sagittal and coronal reconstructions (orthogonal to the
area of interest) may demonstrate clearly an area of bone bridging the
physis between the epiphysis and metaphysis (see Fig. 5-32C,D). MRI is also a sensitive method of assessing normal physeal architecture (Fig. 5-34).34,48,56
Revealing images of the physis and the region of physeal growth
disturbance can be obtained using three-dimensional spoiled recalled
gradient echo images with fat saturation or fast spin echo proton
density images with fat saturation (Fig. 5-35).
MRI has the additional advantage of the opportunity to assess the
organization of the residual physis that may indicate its relative
“health.” This assessment may be helpful in cases of infection,
irradiation, or tumor to determine if arrest resection is feasible
based on the integrity of the remaining physis. With either CT or MRI,
physeal arrests are characterized by an identifiable bridge of bone
between the epiphysis and metaphysis, whereas growth disruption without
arrest demonstrates some degree of loss of normal physeal contour and
architecture without the bony bridge or physeal bar.
 |
|
Figure 5-33
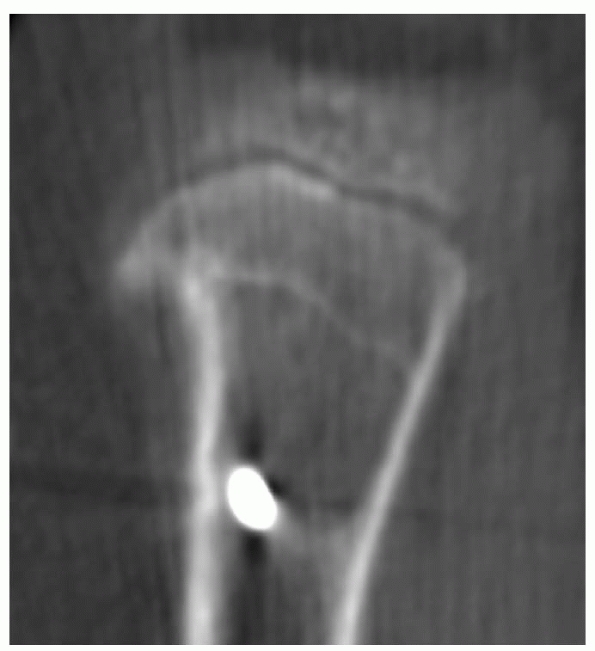
Asymmetric growth arrest line that does not taper to the physis is a strong indication of the presence of physeal growth disturbance without frank physeal arrest. In this case, the asymmetric growth arrest line is noted in the proximal tibial metaphysis on CT scan. |
by plain radiographs is also beneficial. Radiographs of the entire
affected limb should be obtained to document the magnitude of angular
deformity. Existing limb length inequality should be assessed by
scanogram. An estimation of predicted growth remaining in the
contralateral unaffected physis should be made based on a determination
of the child’s skeletal age and reference to an appropriate growth
table.9,10,11,60,67,68,93
 |
|
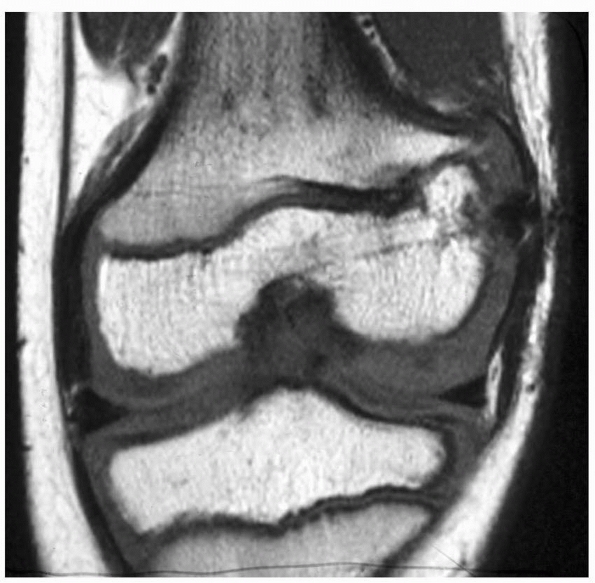
Figure 5-34
MRI scan of a patient with traumatic lateral distal femoral partial growth arrest. Note Harris arrest line tapering to the site of the arrest. |
 |
|
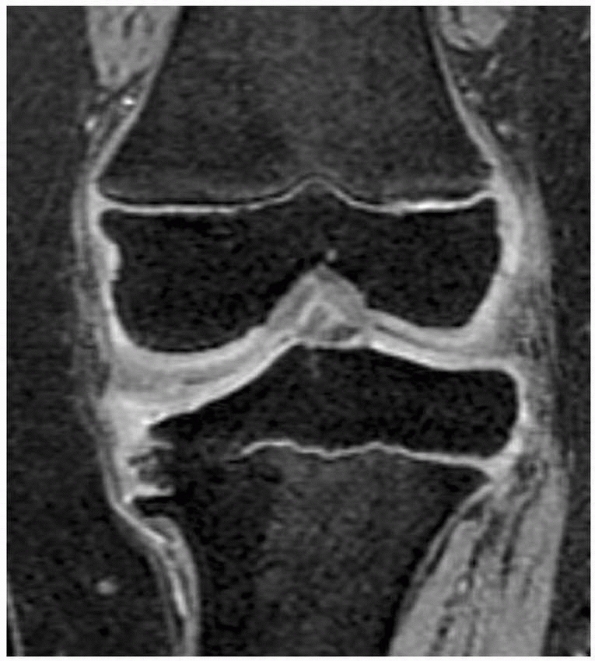
Figure 5-35
MRI scan (three-dimensional spoiled recalled gradient echo images with fat saturation) provides excellent visualization of the affected area and some sense of the integrity of the residual physis. This patient has infantile Blount disease. |
physis, tethering of the metaphyseal and epiphyseal bone together may
result (Table 5-4). These partial physeal
arrests can result in angular deformity, joint distortion, limb length
inequality, or combinations of these, depending on the location of the
arrest, the rate and extent of growth remaining in the physis involved,
and the health of the residual affected physis. Although these partial
arrests are not common, their presence usually requires preventive or
corrective treatment to minimize the long-term sequelae of the
disturbance of normal growth they can create (Fig. 5-36).
|
TABLE 5-4 Potential Causes of Physeal Arrest Formation
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
|
 |
|
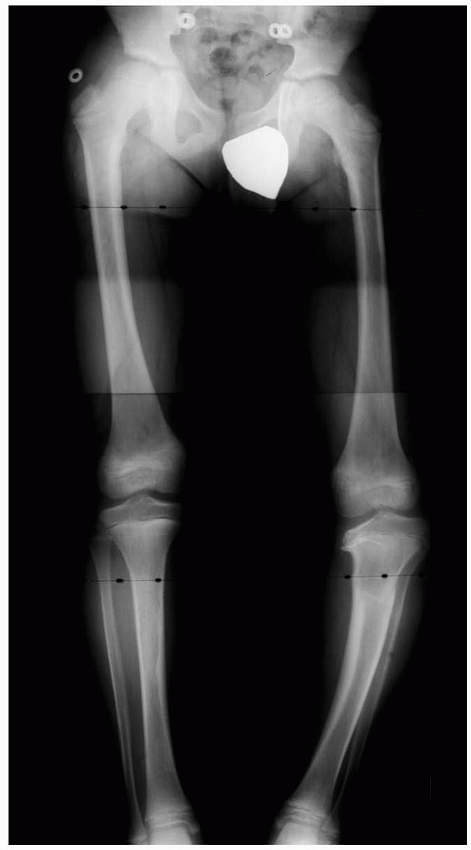
Figure 5-36
Physeal arrests create variable amounts of limb shortening, angular deformity, and epiphyseal distortion, depending on the duration of the arrest, the physis affected, and the size of the arrest. A long, standing film of the lower extremities with the hip, knee, and ankle joints included provides an overall assessment of angular deformity and shortening. |
and by anatomic pattern. Potential etiologies of physeal arrest are
summarized in Table 5-4 and include physeal
fracture, Langenskiöld stage VI infantile Blount disease, infection,
tumor, and irradiation. Physeal arrests also can be classified based on
the anatomic relationship of the arrest to the residual “healthy”
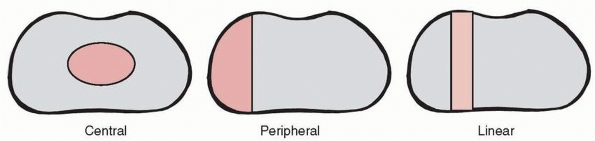
physis. Three basic patterns are recognized (Fig. 5-37): central, peripheral, and linear. A central
arrest is surrounded by a perimeter of normal physis, like an island
within the remaining physis. Central arrests are most likely to cause
tenting of the articular surface, but also may result in angular
deformity if eccentrically located and limb length inequality (Fig. 5-38). A peripheral
arrest is located at the perimeter of the affected physis. This type of
arrest primarily causes progressive angular deformity and variable
shortening. A linear arrest is a
“through-and-through” lesion with anatomic characteristics of both a
central and peripheral arrest; specifically, the affected area includes
the perimeter of the physis, but there is normal physis on either side
of the affected area. Linear arrests most commonly develop after
Salter-Harris type III or IV physeal fractures of the medial malleolus.
important to be aware of these and to weigh carefully the
appropriateness of each for the individual situation.
 |
|
Figure 5-37
Anatomic classification of physeal arrests. Central arrests are surrounded by a perimeter of normal physis. Peripheral arrests are located at the perimeter of the physis. Linear arrests are “through-and-through” lesions with normal physis on either side of the arrest area. |
should be proactive in the prevention of physeal arrest formation. Most
commonly, this can be accomplished by adhering to the general treatment
principles of physeal fractures: gentle, anatomic, and secure reduction
of the fracture, especially Salter-Harris types III and IV injuries.
Damaged, exposed physes can be protected by immediate fat grafting,53
similar to the principle of interposition material insertion for the
resection of established arrests (see following discussion). The most
common situation in which this can be considered appropriately is
during open reduction of medial malleolar fractures, where comminution
or partial physeal damage is identified during reduction.
indicates that nonsteroidal anti-inflammatory medications (specifically
indomethacin) given for a period of time after physeal injury may
prevent formation of physeal arrest. There is, however, no clinical
study supporting this experimental study, so the use of nonsteroidal
anti-inflammatory medications is empiric and not common clinical
practice.
The principle is to remove the bony tether between the metaphysis and
physis and fill the physeal defect with a bone reformation retardant,
anticipating that the residual healthy physis will resume normal
longitudinal growth.25,55,82,84,85,109
However, this procedure can be technically demanding, and results in
our practice are modest. To determine if this procedure is indicated,
careful consideration must be given to the location and extent of the
arrest and the amount of longitudinal growth to be potentially salvaged.
 |
|
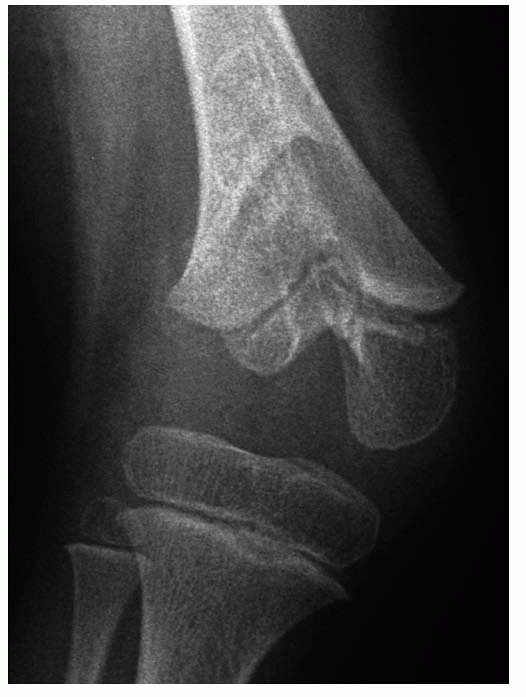
Figure 5-38
Central arrests are characterized by tenting of the articular surface. Variable shortening and angular deformity will develop, depending on the size and location of the arrest. |
with the application of an external fixator spanning the arrest and
gradual distraction until the arrest “separates.”33,42
Angular deformity correction and lengthening can be accomplished after
separation as well. However, distraction injury usually results in
complete cessation of subsequent normal physeal growth at the
distracted level.51 Furthermore, the
fixation wires or halfpins may have tenuous fixation in the epiphysis
or violate the articular space, risking septic arthritis. Thus, this
modality is rarely used in patients near the end of growth.
to correct angular deformity associated with physeal arrests is
corrective osteotomy in the adjacent metaphysis. Of course, neither
significant limb length inequality nor epiphyseal distortion that may
result from the arrest is corrected by this strategy. However, in young
patients with a great deal of growth remaining in whom previous physeal
arrest resection has been unsuccessful or is technically not possible,
this treatment may be a reasonable interim alternative until more
definitive completion of arrest and management of limb length
inequality is feasible.
Limb Length Discrepancy. An alternative strategy for the management of
physeal arrests is to complete the epiphysiodesis to prevent recurrent
angular deformity or epiphyseal distortion and manage the existing or
potential limb length discrepancy appropriately. Management of the
latter may be by simultaneous or subsequent lengthening of the affected
limb segment or contralateral epiphysiodesis if the existing
discrepancy is tolerable and lengthening is not desired. We believe
that this course of management is specifically indicated if arrest
resection has failed to result in restoration of longitudinal growth
and in patients in whom the amount of growth remaining does not warrant
an attempt at arrest resection. In our opinion, this treatment should
be considered carefully in all patients with a physeal arrest.
be considered before determining if physeal arrest resection is indicated.
have a relatively good prognosis for resumption of normal growth,
whereas those secondary to infection, tumor or tumorlike conditions, or
irradiation are less likely to demonstrate growth after resection.
are difficult to expose, a technically adequate resection is less
likely in these areas. Distal femoral bars have a poorer prognosis for
growth after resection, whereas those of the distal tibia have a more
favorable prognosis for the resumption of growth.
after arrest resection is influenced by the amount of physeal surface
area affected.25,27,78
Arrests affecting more than 25% of the total surface area are unlikely
to grow, and, except in patients in whom significant growth potential
remains, alternative treatment strategies should be used.
 |
|
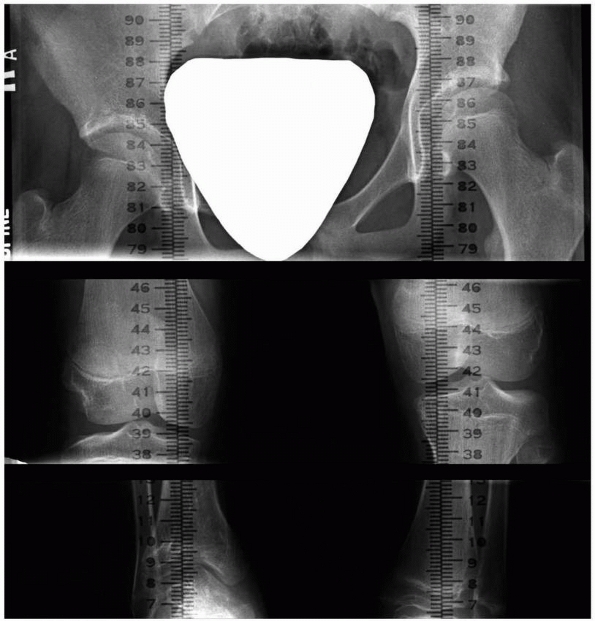
Figure 5-39
Scanogram indicates the existing limb length inequality. Bone age determination and growth contribution per year of the affected physis will allow calculation of the extent of limb segment shortening to be expected. |
have stated that 2 years of growth remaining based on skeletal age
determination is a prerequisite for arrest resection to be considered.
Based on our results with this procedure, we find that 2 years of
growth remaining is an inadequate indication for physeal arrest
resection. We believe that the decision to perform arrest resection
should be made on a combination of the calculated amount of growth
remaining in the affected physis and the likelihood of resumption of
growth. Scanogram and determination of skeletal age (Fig. 5-39) will document the existing discrepancy, and consultation with the growth remaining tables for the affected physis9,10,11,57,70,93 will allow calculation of growth remaining in the affected physis.
some planning is required to maximize the opportunity for resumption of
longitudinal growth.
the rest of the physis must be carefully documented. The most
cost-effective method to accurately evaluate an arrest is with
reconstructed sagittal and coronal CT images to provide views
orthogonal to the affected physis. MRI may also be used and, with
recent
advancements in the capability to identify and quantify physeal
arrests, may soon become the imaging study of choice. We currently
prefer three-dimensional spoiled recalled gradient echo images with fat
saturation or fast spin echo proton density images with fat saturation
to visualize the physis. CT images allow precise delineation of bony
margins and, at the current time, is cheaper than MRI. An estimation of
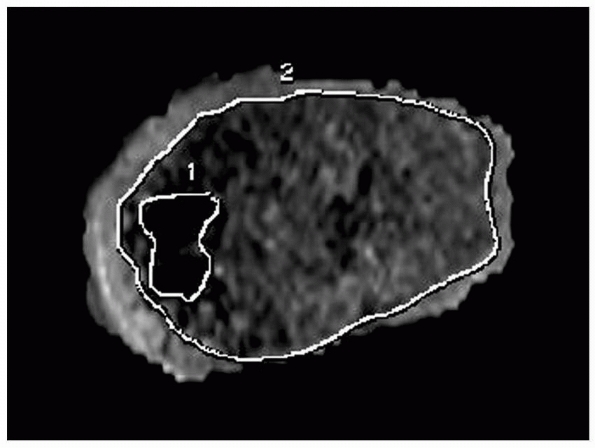
the affected surface area can be computed with the assistance of the
radiologist using a modification of the method of Carlson and Wenger (Fig. 5-40).35 The procedure should be planned with consideration of the principles discussed in the following section.
 |
|
Figure 5-40
Reconstructed MRIs allow estimation of the percentage of surface area of the physis affected by a growth arrest. This workstation reconstruction delineates the perimeter of normal physis (border 2) and that of the physeal arrest (border 1). Surface area affected can be calculated from these reconstructions. |
that minimizes trauma to the residual physis. Central lesions should be
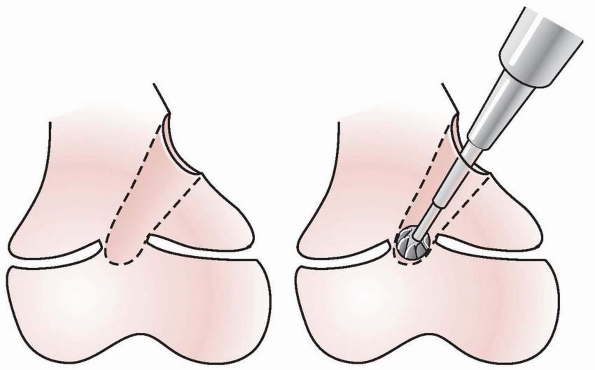
approached through either a metaphyseal window (Fig. 5-41)
or through the intramedullary canal after a metaphyseal osteotomy.
Peripheral lesions are approached directly, resecting the overlying
periosteum to help prevent reformation of the arrest. Intraoperative
imaging (fluoroscopy) is needed to keep the surgeon oriented properly
to the arrest and the residual healthy physis. Care to provide adequate
visualization of the surgical cavity is essential, because
visualization is usually difficult even under “ideal” circumstances. A
brilliant light source, magnification, and a dry surgical field are
very helpful. An arthroscope can be inserted into a metaphyseal cavity
to permit a circumferential view of the resection area. A high-speed
burr worked in a gentle to-and-fro movement perpendicular to the physis
is usually the most effective way to gradually remove the bone
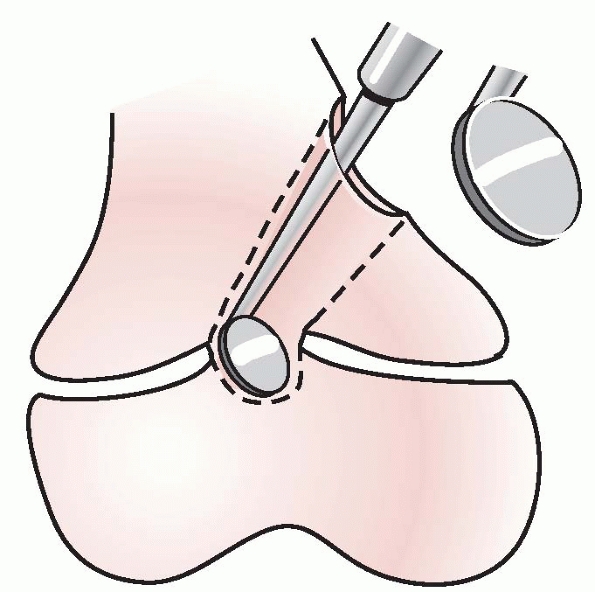
composing the arrest and expose the residual healthy physis (Fig. 5-42).
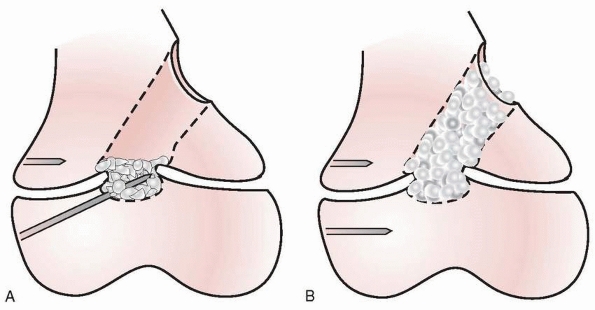
By the end of the resection, all of the bridging bone between the
metaphysis and epiphysis should be removed, leaving a void in the
physis where the arrest had been, and the perimeter of the healthy
residual physis should be visible circumferentially at the margins of
the surgically created cavity (Fig. 5-43).
 |
|
Figure 5-41 Central arrests are approached through a metaphyseal “window” or the medullary canal after metaphyseal osteotomy.
|
 |
|
Figure 5-42
The arrest is removed, leaving in its place a metaphyseal-epiphyseal cavity with intact physis surrounding the area of resection. |
Epiphysis. A bone-growth retardant or “spacer” material should be
placed in the cavity created by the arrest resection to prevent
re-forming of the bony bridge between the metaphysis and epiphysis.
Four compounds have been used for this purpose either clinically or
experimentally: autogenous fat,27,78,82,84,85,87,88,143
methylmethacrylate,20,78,112 silicone rubber,25 and autogenous cartilage.14,16,52,62,79,89
Silicone rubber is no longer available and, to our knowledge,
autogenous cartilage has been used only experimentally as a press-fit
plug or cultured chondroblasts. Currently, only autogenous fat graft,
harvested either locally or from the buttock, and methylmethacrylate
are used clinically. Autogenous fat has at least a theoretic advantage
of the ability to hypertrophy and migrate with longitudinal and
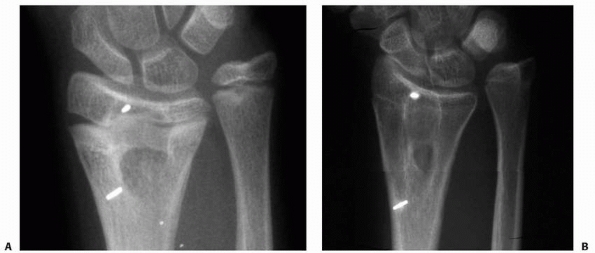
interstitial growth (Fig. 5-44).87,88 Methylmethacrylate is inert, but provides some immediate structural stability.31
This feature may be important with large arrest resections in
weight-bearing areas, as in the proximal tibia in association with
infantile Blount disease (Fig. 5-45). However,
embedded methylmethacrylate, especially products without barium to
clearly delineate its location on radiograph, can be extremely
difficult to remove and can jeopardize bone fixation if subsequent
surgery is required.
 |
|
Figure 5-43
After complete resection, the healthy physis should be evident circumferentially within the cavity produced by the arrest resection. |
in the epiphysis and metaphysis at the time of arrest resection to
allow reasonably accurate estimation of the amount of longitudinal
growth that occurs across the operated physis, as well as to identify
the deceleration or cessation of that growth (Fig. 5-46).
We believe that precise monitoring of subsequent longitudinal growth is
an important aspect of the management of patients after arrest
resection. First, resumption of longitudinal growth may not occur
despite technically adequate arrest resection in patients with good
clinical indications. Perhaps more importantly, resumption of normal or
even accelerated longitudinal growth may be followed by late
deceleration or cessation of that growth.64
It is imperative that the treating surgeon be alert to those
developments, so that proper intervention can be instituted promptly.
Embedded metallic markers serve those purposes admirably.
 |
|
Figure 5-44
Fat used as an interposition material in partial physeal arrest resection can persist and hypertrophy during longitudinal growth. A. Radiograph appearance after traumatic distal radial physeal arrest resection. B. Appearance 5 years later. Longitudinal growth between the metallic markers is obvious. The fat-filled cavity created at physeal arrest resection has persisted and elongated with distal radial growth. |
observation that even patients who have significant resumption of
growth following arrest resection will experience premature cessation
of longitudinal growth of the affected physis relative to the
contralateral uninvolved physis. We believe that even if growth resumes
after bar resection, the previously injured physis will cease growing
before the contralateral physis. Thus, the percent of predicted growth
might be expected to decrease over the length of follow-up.
 |
|
Figure 5-45
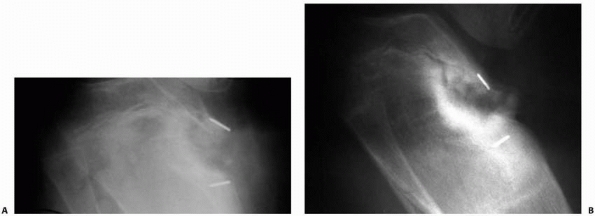
Resection of substantial physeal arrests in weight-bearing areas may allow subsidence of the articular surface. This is of particular concern in the proximal tibia of patients with infantile Blount disease. A. Early postoperative radiograph after partial physeal arrest resection in an obese patient with infantile Blount disease. B. One year later, the metallic markers are actually closer together, in addition to demonstrating increased varus. Subsidence of the medial proximal tibial articular surface is the likely explanation of this radiograph finding. Protected weight-bearing or methylmethacrylate as the interposition material may be indicated in such cases. |
-
On average, approximately 60% of physeal
arrests demonstrate clear radiograph evidence of resumption of
longitudinal growth of the affected physis after physeal arrest
resection. -
There is a correlation between the amount
of surface area of the physis affected and the prognosis for subsequent
longitudinal growth after arrest resection. Physeal arrests affecting
less than 10% of the surface area of the physis have a better prognosis
than larger arrests. -
Langenskiöld stage VI infantile Blount disease has results comparable to posttraumatic physeal arrests.
-
Etiologies other than posttraumatic and infantile Blount disease have poor prognoses for subsequent growth.
-
Central and peripheral arrests have equivalent prognoses with respect to resumption of growth.
-
Early growth resumption may be followed
by cessation of longitudinal growth before skeletal maturity. As a
consequence, patients must be evaluated regularly until skeletal
maturity with some reliable method (such as metaphyseal and epiphyseal
radiograph markers) to detect such development as promptly as possible.
in patients with significant longitudinal growth remaining. However,
the benefits of such surgery must be weighed against the actual amount
of growth remaining, and the etiology, location, and extent of the
physeal arrest must be considered. The appropriate time to add a
corrective osteotomy to bony bar resection is controversial. Generally,
when the angular deformity is more than 10 to 15 degrees from normal,
corrective osteotomy should be considered.
arrest. Both growth deceleration and, less frequently, acceleration
have been reported. Growth deceleration without arrest is characterized
radiographically by the appearance of an injured physis (usually
relative widening of the physis with indistinct metaphyseal
boundaries). There may be associated clinical or radiographic deformity
if the disturbance is severe and long standing. It is important to make
a distinction between growth deceleration without complete cessation
and true physeal arrest, because management and outcome are typically
different in these two disorders. The concept of growth deceleration
without arrest is most readily appreciated in patients with adolescent
Blount disease and the milder stages of infantile Blount disease.
Recently, growth deceleration without physeal arrest has also been
reported to produce distal femoral valgus deformity in obese
adolescents.146 Growth deceleration
may also occur after infection and physeal fracture. In contrast to
physeal arrests, there is no sclerotic area of arrest on plain
radiographs (see Fig. 5-33). A growth arrest
line, if present, may be asymmetric but will not taper to the physis,
thereby suggesting growth asymmetry but not complete arrest.
Furthermore, in some cases, deformity will not be relentlessly
progressive and can actually improve over time.
proximal tibial fracture in young patients resulting in valgus
deformity which usually spontaneously resolves.72,77,108,131,132,147
Interestingly, it has also recently been reported to occur in patients
younger than 10 years who have had curettage of benign lesions of the
proximal tibial metaphysis.66
made incidentally by noting physeal abnormality on radiographs during
physeal fracture follow-up or after a diagnosis of frank physeal arrest
has been excluded during the evaluation of a patient with angular
deformity and physeal abnormality on plain radiographs. Once a growth
disturbance has been identified in a patient, its full impact should be
assessed by determining the
presence and extent of limb length inequality and the calculated amount of potential growth remaining for the affected physis.
 |
|
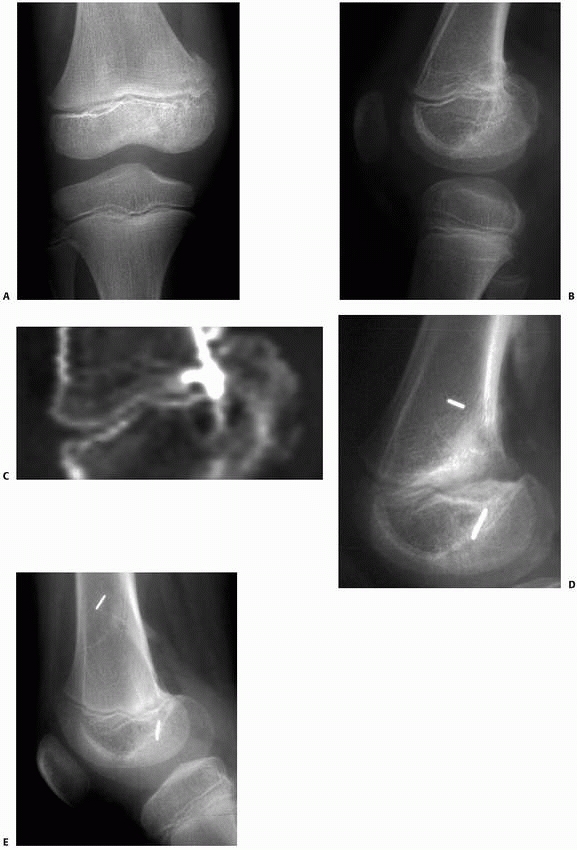
Figure 5-46
Intraosseous metallic markers in the epiphysis and metaphysis spanning the area of arrest resection allow sensitive radiographic documentation of the presence and extent of growth after arrest resection and permit early detection of the cessation of restored longitudinal growth. This patient had a small central arrest of the lateral portion of the distal femoral physis after a Salter-Harris type IV fracture. A. Injury films show a mildly displaced Salter-Harris type IV fracture of the lateral distal femur. B. Several years later, a small central arrest has developed involving a portion of the lateral distal femoral physis. A tapering growth arrest line is faintly visible. C. The posterior location of the partial arrest can be seen on the sagittal CT reconstructions. D. After arrest resection through a metaphyseal window, a cavity is evident in the region of the original bar. Metallic markers have been placed in the metaphysis and epiphysis. E. Three years after arrest resection, substantial growth has occurred, as documented by the increased distance between the markers. However, on radiographs taken at 4 years postoperatively, no further growth was documented. This event was treated by completion of the epiphysiodesis and contralateral distal femoral epiphysiodesis to prevent the development of limb length discrepancy from developing. |
and only longitudinal observation is required. This observation must be
regular and careful, because progressive deformity will require
treatment. If angular deformity is present or progressive, treatment
options include hemiepiphysiodesis or physeal “tethering” with staples,
screws, or tension plates22,40,50,54,98,99,104,136 and corrective osteotomy, with or without completion of the epiphysiodesis.
hemiepiphysiodesis or “tethering” the affected physis with staples,
screws, or tension plates on the convex side may result in gradual
correction of the deformity. If correction occurs, options include
completion of the epiphysiodesis (with contralateral epiphysiodesis if
necessary to prevent the development of significant leg length
deformity) and removal of the tethering device with careful
longitudinal observation for recurrence or overcorrection of deformity.
management of growth disturbance with established angular deformity.
Angular deformity correction in the early stages of infantile and
adolescent Blount disease is known to result in resolution of the
physeal growth disturbance in some patients, both on radiographs and
clinically. We are unaware of confirmation of similar outcome when the
etiology of growth disturbance is infection or trauma, although it may
occur. Thus, the treating surgeon must decide whether to perform
epiphysiodesis of the affected physis (with contralateral
epiphysiodesis, if appropriate) to prevent recurrence or to ensure
careful longitudinal observation of the growth performance of the
affected physis until skeletal maturity.
pediatric orthopaedics. These injuries are common and usually have a
favorable outcome without long-term sequelae. Physeal fractures must be
treated gently and expertly to maximize restoration of normal limb
function and longitudinal growth. Depending on the severity and nature
of physeal injury, longitudinal followup to identify the development of
physeal growth disturbance is important.
LJ, Thompson GH. Deformity after premature closure of the distal radial
physis following a torus fracture with a physeal compression injury.
Report of a case. J Bone Joint Surg Am 1987;69(9):1450-1453.
BA, Silvrestein MJ, Rende RJ, et al. Arthrography in the diagnosis of
fractures of the distal end of the humerus in infants. J Bone Joint
Surg Am 1986;68(4):599-602.
SA, Palmer AK, Kerr DR, et al. Wrist pain and distal growth plate
closure of the radius in gymnasts. J Pediatr Orthop 1989;9(1):23-28.
A, Schoenecker PL. Premature closure of the distal radial physis after
fracture of the distal radial metaphysis. J Pediatr Orthop
1995;15(4):495-498.
M, Green WT. Length of femur and tibia; norms derived from
orthoroentgenogram of children from 5 years of age until epiphyseal
closure. Am J Dis Child 1948;75(3):279-290.
M, Messner MB, Green WT. Distribution of lengths of the normal femur
and tibia in children from 1 to 18 years of age. J Bone Joint Surg Am
1964;46:1197-1202.
M, Pauleto AC, Cunha LAM. Osteochondral sequelae of meningococcemia:
radiographic aspects. J Pediatr Orthop 2002;22(4):511-516.
F, Forriol F, Cañadell J. Histomorphometric study of growth plate
subjected to different mechanical conditions (compression, tension, and
neutralization): an experimental study in lambs. Mechanical growth
plate behavior. J Pediatr Orthop B 2001; 10(4):334-338.
SJ, Zaleske DJ. Physeal reconstruction with blocks of cartilage of
varying developmental time. J Pediatr Orthop 1992;12(6):766-773.
S, Ekengren K, Eriksson M. Neonatal hematogenous osteomyelitis: risk
factors for long-term sequelae. J Pediatr Orthop 1985;5(5):564-568.
G, Tallet JM, Jacquemier M, et al. New procedure to remove a centrally
located bone bar. J Pediatr Orthop 1990;10(5):662-666.
CF, Mol LJ, Obermann WR, et al. Late sequelae of neonatal septic
arthritis of the shoulder. J Bone Joint Surg Br 1998;80(4):645-650.
JR, Mubarak SJ, Wenger DR. Tibial physeal closure and genu recurvatum
after femoral fracture: occurrence without a tibial traction pin. J
Pediatr Orthop 1990;10(5): 653-657.
KT, Batt ME. Stress fracture of the proximal humeral epiphysis in an
elite junior badminton player. Br J Sports Med 1997;31(3):252-253.
RW. Operative correction of partial epiphyseal plate closure by
osseous-bridge resection and silicone-rubber implant. An experimental
study in dogs. J Bone Joint Surg Am 1974;56(4):655-664.
RW, Burstein AH, Elmore SM. Epiphyseal-plate cartilage. A biomechanical
and histological analysis of failure modes. J Bone Joint Surg Am
1974;56(4):688-703.
NS, Dickens DR, Cole WG, et al. Epiphysiolysis for partial growth plate
arrest. Results after 4 years or at maturity. J Bone Joint Surg Br
1989;71(1):13-16.
PJ, Gaffney JT, Denton JR. Acute compartment syndrome complicating a
distal tibial physeal fracture in a neonate. Am J Orthop
1999;28(10):587-589.
FH, Burbach T. Ultrasonic diagnosis of separation of the proximal
humeral epiphysis in the newborn. J Bone Joint Surg Am
1990;72(2):187-191.
MJ, Phillips WA, Gordon J, et al. Effect of interposition material on
mechanical behavior in partial physeal resection: a canine model. J
Pediatr Orthop 1990;10(4): 459-462.
MS, Robertson WW Jr, Rate W, et al. Skeletal sequelae of radiation
therapy for malignant childhood tumors. Clin Orthop Relat Res
1990;251:235-240.
J, Spence L, Blickman H, et al. MRI of pediatric growth plate injury:
correlation with plain film radiographs and clinical outcome. Skeletal
Radiol 1998;27(5):250-255.
WO, Wenger DR. A mapping method to prepare for surgical excision of a
partial physeal arrest. J Pediatr Orthop 1984;4(2):232-238.
GF, Kozin F, Flaherty L et al. Radiographic changes in the hands
following childhood frostbite injury. Skeletal Radiol 1981;6(1):33-37.
JR, Peterson HA. Salter-Harris type-IV injuries of the distal tibial
epiphyseal growth plate, with emphasis on those involving the medial
malleolus. J Bone Joint Surg Am 1983;65(8):1059-1070.
P, Urquhart B, Sullivan E, et al. Hemiepiphysiodesis for the correction
of angular deformity about the knee. J Pediatr Orthop
2008;28(2):188-191.
JF, Huurman WW, Ray S. Physeal distraction treatment of fracture
deformities. Orthopaedic Transactions 1991;3(2):231-232.
RS, Markowitz RI, Dormans J, et al. Ultrasonographic evaluation of the
elbow in infants and young children after suspected trauma. J Bone
Joint Surg Am 1994; 76(12):1804-1813.
JJ, Lamont AC, Jones JM. Ultrasonic diagnosis of neonatal separation of
the distal humeral epiphysis. J Bone Joint Surg Br 1988;70(5):825-828.
MJ, Hedlund GL. Sonographic diagnosis of traumatic separation of the
proximal femoral epiphysis in the neonate. Pediatr Radiol
1991;21(3):238-240.
K, Jaramillo D. Patterns of premature physeal arrest: MR imaging of 111
children. AJR Am J Roentgenol 2002;178(4):967-72.
BK, Frierson MA, Raney EM, et al. Humerus varus: a complication of
neonatal, infantile, and childhood injury and infection. J Pediatr
Orthop 1994;14(4):479-486.
MR, Birch JG, Albright M. Correction of non-Blount’s angular knee
deformity by permanent hemiepiphysiodesis. J Pediatr Orthop
2004;24(4):397-402.
TO, Steen H. Growth retardation after experimental limb lengthening by
epiphyseal distraction. J Pediatr Orthop 1990;10(4):463-466.
BK, Hansen AL, Gibson GJ, et al. Reimplantation of growth plate
chondrocytes into growth plate defects in sheep. J Orthop Res
1990;8(4):555-564.
BK, John B, Hasler C. Free fat interpositional graft in acute physeal
injuries: the anticipatory Langenskiöld procedure. J Pediatr Orthop
2000;20(3):282-285.
RK, Dickens DR, Cole WG. Medial physeal stapling for primary and
secondary genu valgum in late childhood. J Bone Joint Surg Br
1995;77(5):733-735.
GT, Peterson HA, Berquist TH. Premature partial physeal arrest.
Diagnosis by magnetic resonance imaging in two cases. Clin Orthop Relat
Res 1991;272:242-247.
CA, Bassett GS, Sullivan S, et al. Retrosternal displacement after
physeal fracture of the medial clavicle in children treatment by open
reduction and internal fixation. J Bone Joint Surg
Br2001;83(8):1168-1172.
LS, Volpon JB. Experimental physeal fracture-separations treated with
rigid internal fixation. J Bone Joint Surg Am 1993;75(12):1756-1764.
LS, Volpon JB, Goncalves RP. Traumatic separation of epiphyses. An
experimental study in rats. Clin Orthop Relat Res 1988;236:286-295.
DP, Love SM, Ogden JA, et al. Chondro-osseous growth abnormalities
after meningococcemia. A clinical and histopathological study. J Bone
Joint Surg Am 1989; 71(6):920-928.
AL, Foster BK, Gibson GJ, et al. Growth-plate chondrocyte cultures for
reimplantation into growth-plate defects in sheep: characterization of
cultures. Clin Orthop Relat Res 1990;256:286-298.
PE, Barnes DA, Tullos HS. Arthrographic diagnosis of an injury pattern
in the distal humerus of an infant. J Pediatr Orthop 1982;2(5):569-572.
CC, Foster BK. Secondary tethers after physeal bar resection: a common
source of failure. Clin Orthop Relat Res 2002;405:242-249.
H. Lines of arrested growth in the long bones in childhood: the
correlation of histological and radiographic appearance in clinical and
experimental conditions. Br J Radiol 1931;4:561-588.
RK Jr, Sawyer JR, Warner WC, et al. Progressive valgus deformity after
curettage of benign lesions of the proximal. J Pediatr Orthop
2008;28(7):757-760.
R. Linear growth of long bones of the lower extremity form infancy to
adolescence. In: Hensinger R, Raven P, eds. Standards in Pediatric
Orthopaedics: Tables, Charts, and Graphs Illustrating Growth. New York:
Raven Press Books, 1986, 232-233.
R. Standards in Pediatric Orthopaedics: Tables, Charts, and Graphs
Illustrating Growth. New York: Raven Press Books, 1986.
CB, Shinwell E, Nyska M, et al. Ultrasound diagnosis of neonatal
fracture separation of the upper humeral epiphysis. J Bone Joint Surg
Br 1992;74(3):471-472.
MT, Kasser JR. Physeal arrest about the knee associated with nonphyseal
fractures in the lower extremity. J Bone Joint Surg Am
1989;71(5):698-703.
LY, Hensinger RN. Premature monomelic growth arrest following fracture
of the femoral shaft: a case report. J Bone Joint Surg Am
1978;60(6):850-852.
E, Pentimalli G. Posttraumatic valgus deformity of the knee in proximal
tibial metaphyseal fractures in children. Ital J Orthop Traumatol
1984;10(1):103-108.
ST, Crawford AH. Amputation following meningococcemia. A sequela to
purpura fulminans. Clin Orthop Relat Res 1984;185:214-219.
R, Bielski RJ. Fracture of lower femoral epiphysis in an infant at
birth: a rare obstetrical injury. J Perinatol 2001;21(8):550-552.
D, Kammen BF, Shapiro F. Cartilaginous path of physeal
fracture-separations: evaluation with MR imaging—an experimental study
with histologic correlation in rabbits. Radiology 2000;215(2):504-511.
SE, Alonso JE, Cook FF. The etiology of valgus angulation after
metaphyseal fractures of the tibia in children. J Pediatr Orthop
1987;7(4):450-457.
N, Ehrlich MG, Mankin HJ. Growth plate reconstruction using chondrocyte
allograft transplants. J Pediatr Orthop 1987;7(4):381-388.
A. An operation for partial closure of an epiphysial plate in children,
and its experimental basis. J Bone Joint Surg Br 1975;57(3):325-330.
A. The possibilities of eliminating premature partial closure of an
epiphyseal plate caused by trauma or disease. Acta Orthop Scand
1967;38:267-279.
A, Osterman K, Valle M. Growth of fat grafts after operation for
partial bone growth arrest: demonstration by computed tomography
scanning. J Pedaitr Orthop 1987;7(4):389-394.
A, Videman T, Nevalainen T. The fate of fat transplants in operations
for partial closure of the growth plate. Clinical examples and an
experimental study. J Bone Joint Surg Br 1986;68(2):234-238.
DW, Goldner RD, Sussman MD. Cartilage as an interposition material to
prevent transphyseal bone bridge formation: an experimental model. J
Pediatr Orthop 1983;3(2):207-210.
MS, Berdon WE, Ruzal-Shapiro C, et al. Gymnast’s wrist (pseudorickets
growth plate abnormality) in adolescent athletes: findings on plain
films and MR imaging. AJR Am J Roentgenol 1995;164(1):157-159.
S, Harvey J. Fractures of the distal femoral epiphysis. Factors
influencing prognosis: a review of 34 cases. J Bone Joint Surg Am
1977;59(6):742-751.
DC, Rajmaira S. Distribution of physeal and nonphyseal fractures in
2650 long-bone fractures in children aged 0 to 16 years. J Pediatr
Orthop 1990;10(6):713-716.
MM. Linear growth of long bones of the extremities from infancy through
adolescence. AMA Am J Dis Child 1955;89(6):725-742.
RP, Parsons DL. Avascular necrosis of the proximal humeral epiphysis
after physeal fracture. A case report. J Bone Joint Surg Am
1997;79(5):760-762.
JM, d’Amato C, Strong M, et al. Usefulness and accuracy of arthrography
in management of lateral humeral condyle fractures in children. J
Pediatr Orthop 1990; 10(3):317-321.
AA, Bartal E, Grillot MB, et al. Compression (Salter-Harris type V)
physeal fracture: an experimental model in the rat. J Pediatr Orthop
1992;12(1):29-37.
JP, Wong-Chung J, Bertrand H, et al. Percutaneous epiphysiodesis using
transphyseal screws (PETS). J Pediatr Orthop 1998;18(3):363-369.
CH, Stevens PM. Hemiepiphyseal stapling for knee deformities in
children younger than 10 years: a preliminary report. J Pediatr Orthop
1996;16(4):423-429.
A, Sugawara M. Humeral trochlear hypoplasia secondary to epiphyseal
injury as a cause of ulnar nerve palsy. Clin Orthop Relat Res
1988;228:227-232.
T, Benson WM, Foster BK, et al. Statistical analysis of the incidence
of physeal injuries. J Pedaitr Orthop 1987;7(5):518-523.
JA, González-López JL, López-Valverde S, et al. Premature physeal
closure after tibial diaphyseal fractures in adolescents. J Pediatr
Orthop 2000;20(2):193-196.
F, Kuo LA. Percutaneous epiphysiodesis using transphyseal screws
(PETS): prospective case study and review. J Pediatr Orthop
2004;24(6):721-725.
JA, Ganey T, Light TR, et al. The pathology of acute chondro-osseous
injury in the child. Yale J Biol Med 1993;66(3):219-233.
JA, Ogden DA, Pugh L, et al. Tibia valga after proximal metaphyseal
fractures in childhood: a normal biologic response. J Pediatr Orthop
1995;15(4):489-494.
K. Operative elimination of partial premature epiphyseal closure: an
experimental study. Acta Orthop Scand Suppl 1972;3-79.
JM, Goulet JA, Hensinger RN. Compartment syndrome complicating tibial
tubercle avulsion. Clin Orthop Relat Res 1993;295:201-204.
W, Irving J, Letts M. Long-term effects of neonatal bone and joint
infection on adjacent growth plates. J Pediatr Orthop
1992;12(6):806-810.
HA. Premature physeal arrest of the distal tibia associated with
temporary arterial insufficiency. J Pediatr Orthop 1993;13(5):672-675.
HA, Burkhart SS. Compression injury of the epiphyseal growth plate:
fact or fiction? J Pediatr Orthop 1981;1(4):377-384.
HA, Madhok R, Benson JT, et al. Physeal fractures: part 1. Epidemiology
in Olmsted County, Minnesota, 1979-1988. J Pediatr Orthop
1994;14(4):423-430.
P, Panuel M, Faure F, et al. Acute fracture of the distal tibial
physis: role of gradient-echo MR imaging versus plain film examination.
AJR Am J Roentgenol 1996; 166(5):1203-1206.
M, ed. Injuries of the epiphyses, the growth plate, and the
perichondral ring. Children’s Fractures. Philadelphia: JB Lippincott,
1983, 10-25.
EJ, Barrett IR, Shapiro F. Growth disturbances following distal femoral
physeal fracture-separations. J Bone Joint Surg Am 1983;65(7):885-893.
R, Shapiro F. Structural stages in the development of the long bones
and epiphyses: a study in the New Zealand white rabbit. J Bone Joint
Surg Am 2002;84A-1: 85-100.
WW Jr, Butler MS, D’Angio GJ, et al. Leg length discrepancy following
irradiation for childhood tumors. J Pediatr Orthop 1991;11(3):284-287.
S, Pelker RR, Lee KE, et al. Shear fractures through the capital
femoral physis of the skeletally immature rabbit. J Pediatr Orthop
1985;5(1):27-31.
MM, Peterson HA. Opening-wedge osteotomy for angular deformities of
long bones in children. J Bone Joint Surg Am 1994;76(3):325-334.
NK. Fracture separation of the medial clavicular epiphysis:
ultrasonography findings. Arch Orthop Trauma Surg 2003;123(7):367-369.
WR, Canale ST. Fractures of the tibia through the proximal tibial
epiphyseal cartilage. J Bone Joint Surg Am 1979;61(2):167-173.
BG, Rand F, Jaramillo D, et al. Early MR imaging of lower-extremity
physeal fracture-separations: a preliminary report. J Pediatr Orthop
1994;14(4):526-533.
DG, Geist RW, Cooperman DR. Microscopic examination of a naturally
occurring epiphyseal plate fracture. J Pediatr Orthop 1985;5(3):306-308.
E, Husby OS, Bang G. Inhibition of partial closure of epiphyseal plate
in rabbits by indomethacin. Acta Orthop Scand 1982;53(4):507-511.
J, Amato VP. The vascular contribution to osteogenesis. III. Changes in
the growth cartilage caused by experimentally induced ischaemia. J Bone
Joint Surg Br 1960;42-B:571-587.
J, Morgan J. The vascular contribution to osteogenesis. I. Studies by
the injection method. J Bone Joint Surg Br 1960;42-B:97-109.
J, Trias A. The vascular contribution to osteogenesis. IV. The effect
of pressure upon the epiphyseal cartilage of the rabbit. J Bone Joint
Surg Br 1961;43-B:800-813.
JA, Albiñana J, Certucha JA. Early posttraumatic physeal arrest in
distal radius after a compression injury. J Pediatr Orthop B
1996;5(1):57-60.
JM, Gruber HE, Phieffer LS. Physeal fractures, part I: histologic
features of bone, cartilage, and bar formation in a small animal model.
J Pediatr Orthop 2002; 22(6):703-709.
RV, Staheli LT. Partial physeal growth arrest: treatment by bridge
resection and fat interposition. J Pediatr Orthop 1990;10(6):769-776.
AL, Exner GU, Wenger DR. Progressive genu valgum resulting from
idiopathic lateral distal femoral physeal. J Pediatr Orthop
2008;28(7):752-756.
LE, Harcke HT, Brooks KM, et al. Posttraumatic tibia valga: a case
demonstrating asymmetric activity at the proximal growth plate on
technetium bone scan. J Pediatr Orthop 1987;7(4):458-462.
