TUMORS OF THE SPINE
survival and improve the quality of life for many patients with spinal
column tumors. The goals of treatment are pain relief, improved
function, and the best possible chance of local control and cure of the
disease. Aggressive surgical approaches, combined with improved
adjuvant therapy, now offer good short-term and long-term outcomes for
many lesions previously thought untreatable or unresectable.
age groups and at all levels of the spine. Primary tumors can arise
from any of the hard or soft tissues of the spinal column, or they can
extend directly to the spinal column from contiguous paraspinal
lesions. Metastatic tumors, which migrate from distant sites by either
lymphatic or hematogenous routes, account for 97% of all spinal column
tumors.
adenocarcinoma. Between 50% and 70% of all patients with carcinoma
develop skeletal metastases during the course of their disease, as do
85% of women with breast cancer (9,25,34).
The spine is the most common site for these metastases. The most common
metastatic lesions are adenocarcinomas from the following:
-
Lung
-
Breast
-
Prostate
-
Kidney
-
Gastrointestinal tract
-
Thyroid
and plasmacytoma, show a preference for the spinal column, but they
represent a small proportion of all spinal lesions.
spinal lesions in all age groups are more likely to be malignant than
benign. This is particularly true in adult patients,
where
an increasing incidence of metastatic disease, an increased risk of
systemic diseases such as myeloma and lymphoma, and a greater
likelihood of having a malignant primary tumor combine to present a
particularly grim prognosis. Seventy percent of primary spine tumors in
patients over 25 are malignant, compared with only 30% in patients
under 21 years of age (73).
originate in the vertebral body, involving one or both pedicles. The
vertebral body contains most of the bone, hematogenous marrow, and
cartilage from which primary lesions arise, and the notochordal rests
that give rise to chordoma. Most of the hematogenous
marrow is also contained in the bodies. Finally, retrograde flow
through the venous drainage of the spinal column (Batson’s plexus)
permits tumor cells from the abdominal cavity to seed the vertebral
bodies directly (29). Lesions of the posterior elements are more commonly benign.
ubiquitous in the age group most at risk for spinal tumors. Whereas
idiopathic back pain is typically mechanical, activity related, and
self-limiting, neoplastic pain is more often
-
progressive and unrelenting,
-
unrelated to activity and unresponsive to rest,
-
well localized to the spinal segment involved,
-
reproduced by palpation or percussion over the involved area,
-
and more severe or disturbing at night.
-
Pathologic fracture
-
Expansion of the vertebral cortex and surrounding tissues by tumor
-
Compression or invasion of nerve roots
-
Segmental instability
-
Spinal cord compression
with more aggressive, malignant tumors, whereas symptoms that progress
over years are typical of slow-growing and often benign processes.
Spine tumor patients most often present with pain. Common presenting
symptoms occur as follows:
-
Eighty-five percent of spine tumor patients present with pain.
-
Back pain is the only symptom in 30%.
-
Leg pain is the only symptom in 10%.
-
Twenty-eight percent present with a combination of pain and neurologic deficit.
-
Forty-two percent of all patients have a neurologic deficit at presentation.
-
Sixteen percent have a mass or deformity.
-
Spinal tumor is an incidental finding in 2% of patients.
nucleus pulposis, symptoms from lumbar and sacral neoplasms do not
respond to rest and recumbency, and they tend to progress relentlessly (62).
neoplasia, except when vertebral collapse results in severe kyphosis.
Osteoid osteoma and osteoblastoma may produce a scoliosis that is
typically painful, with localized pain, muscle spasm, and limited
motion. Deformities associated with tumors may come on suddenly and
progress rapidly. Unless addressed early on, these curves will become
structural and difficult to manage (35). If the
primary lesion is addressed in a timely manner, the curve will often
resolve with observation or bracing. However, if the deformity is
allowed to persist, surgical correction may be necessary (50).
the physician must be alert to any patient with persistent,
nonmechanical back pain, age- or activity-related risk factors, or,
particularly, a previous history of malignancy (23).
Although neurologic injury is rarely the first sign of a spinal
neoplasm, it may be present in as many as half of patients by the time
they seek medical attention, and they may be recognized in more than
70% by the time a diagnosis is made.
-
Complete blood cell count (CBC), differential, sedimentation rate, urinalysis, electrolytes, calcium, and basic chemistry panel
-
Serum and urine protein electrophoresis; if positive, bone survey and bone marrow aspirate
-
Renal ultrasound or abdominal computed tomography (CT)
-
Chest CT
-
Bone scan
-
Physical exam: breasts, prostate, rectal, stool guaiac, thyroid
patients with suspected spinal tumor. Good-quality studies of the
symptomatic spinal segment may be sufficient to define the
characteristic changes of bone destruction and tumor expansion, and
they may establish a specific diagnosis in some tumor types. Plain
roentgenograms will demonstrate abnormalities in 80% to 90% of patients
with a spinal neoplasm (73,74).
Even when the precise tumor type cannot be identified, the benign or
malignant nature of the lesion can often be implied from the pattern of
bone destruction (Fig. 152.1A, Fig. 152.1B).
 |
|
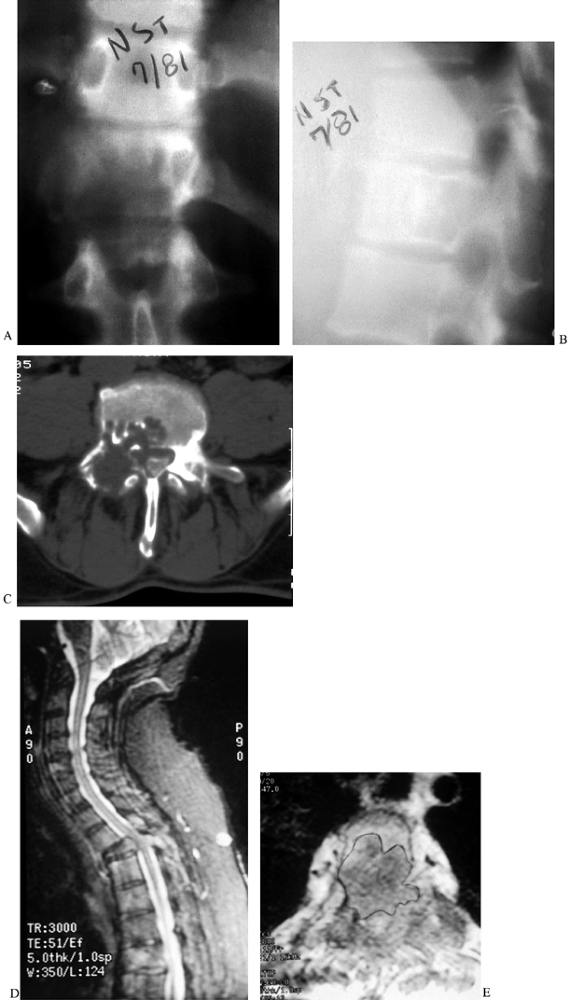
Figure 152.1. The 35-year-old patient in (A) and (B) has giant cell tumor of the T-12 vertebral body. AP (A) radiograph shows a classic “winking owl” sign, suggesting destruction of the left pedicle by tumor. The lateral view (B)
shows a rarified vertebral body with a geographic pattern of bony replacement. In a patient with metastatic renal carcinoma, CT demonstrates bony destruction and expansion of the anterior vertebral cortex (C). Sagittal (D) and transverse (E) MRIs showing vertebral destruction, collapse, and cord compression caused by a breast metastasis to the T-3 vertebral body. |
on plain films. Because roentgenographic evidence of bony destruction
is not apparent until after 30% to 50% of the trabecular bone has been
demineralized or destroyed, bone scans are far more sensitive for
picking up early involvement (17).
on small tumor foci early in their development, before extensive bony
destruction or intramedullary extension has occurred, and before
cortical erosion has advanced to the point of impending fracture. CT is
time consuming and not suitable for screening large segments of the
spine. Once the suspected lesion is identified on plain films or bone
scan, however, CT provides unsurpassed imaging of the bony architecture
(Fig. 152.1C). It may also demonstrate
characteristic features of soft-tissue calcification or trabecular
remodeling, which may be pathognomonic for some types of tumors.
and readily available to most patients. It provides multiplanar images
of large segments of the spine and surrounding tissues and can be used
to screen for disseminated disease. MRI delineates soft-tissue
extension from other processes, and newer techniques can accurately
differentiate tumor from hematoma, edema, and inflammation (Fig. 152.1D, Fig. 152.1E). MRI directly images the spinal cord, cauda equina, and nerve roots without the aid of intrathecal contrast (24).
It can reveal invasion of paravertebral structures better than either
CT or myelography, and with gadolinium enhancement it can differentiate
osteoporotic compression fractures from metastatic disease (13).
Characteristics of tumors on MRI are (a) convex posterior cortex, (b)
epidural mass, (c) low-intensity T1 signal, (d) high or inhomogenous T2
signal intensity, (e) high-intensity signal after gadolinium injection (13,69).
test has been largely replaced by MRI. When MRI cannot be done,
myelography with postmyelogram CT may provide the same information.
-
Percutaneous needle or trocar biopsy
allows aspiration and removal of fine tissue fragments; the advantages
are that needle biopsy is minimally invasive and uses local anesthetic.
It is most suitable for lesions that are easily differentiated. Because
samples are small, they are difficult to read, and they are frequently
not diagnostic. When the differential diagnosis is limited to lesions
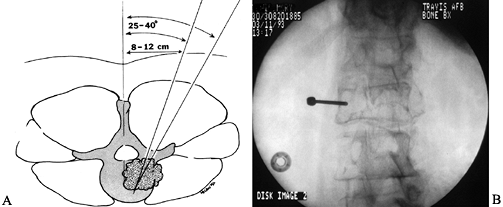
that are easily distinguished histologically, needle biopsy is ideal (Fig. 152.2).![]() Figure 152.2. A:
Figure 152.2. A:
Percutaneous needle biopsy of the thoracolumbar spine is performed
through a posterior, percutaneous route. The needle is positioned 8–12
cm lateral of the midline at the level of the documented lesion. By
advancing the needle at a 30° to 45° angle, under fluoroscopic control,
the posterolateral aspect of the vertebral body is targeted. B:
Alternatively, a small posterior exposure is made over the documented
lesion, and a burr is used to take down the cortex overlying the
pedicle. A Craig needle or a small curet is then passed down the
pedicle to harvest bone and tumor from the vertebral body. -
Open, incisional biopsy provides
moderate-size specimens showing cellular architecture and marginal
tissue. It provides diagnostic tissue and may be done just prior to
formal excision when used with frozen section. Place the incision so it
can be excised with the definitive procedure. Carry out the incisional
biopsy as the last step in tumor staging, just before or at the time of
definitive resection. The incision must be longitudinal, never
transverse. Handle the tissues gently, and provide meticulous
hemostasis to prevent tumor spread. Take a section of tissue large
enough to allow histologic and ultrastructural analysis as well as
immunologic staining from the margin of the lesion (central sections
may be necrotic). Take the specimen with a sharp scalpel and be careful
not to crush or distort the tissue during harvest. Avoid using
electrocautery on the biopsy specimen itself. -
Open, excisional biopsy includes removal
of all tumor tissue at the time of biopsy. It provides complete
treatment of local disease. Few tumors are suitable, however; the
surgeon must already have a good idea of the diagnosis to plan
appropriate excision.
renal cell metastases must be approached more cautiously than most
other tumors, although metastatic thyroid, melanoma, and some breast
lesions may also be highly vascular (67). An
abdominal ultrasound or CT will reveal the primary renal lesion before
the surgeon performs a biopsy that may lead to uncontrollable bleeding.
Preoperative angiography will reveal the extensive neovasculature often
associated with these tumors, allowing embolization of abnormal vessels
and nonessential segmental arteries.
lesions. Survival rates are most directly, but not entirely, related to
tumor type; some low-grade lesions may permit a long
survival
despite their malignant nature, whereas some histologically benign
lesions may prove lethal because of their location in the spine.
osteochondromas, but symptomatic lesions are rare. Eighty percent of
symptomatic osteochondromas occur in the cervicothoracic spine, above
T-6 (41). MRI demonstrates the radiolucent cartilage cap, which usually causes spinal cord compression. Excision of the tumor, en bloc
or piecemeal, provides reliable neurologic recovery with little risk of
recurrence. Enchondromas rarely produce any symptoms but may prove a
diagnostic dilemma when encountered incidentally. Enchondromas develop
a well-defined, benign-appearing cortical margin, but calcific
stippling of the lesion may suggest chondrosarcoma, prompting an
excisional biopsy (46).
lesions frequently found in the spine, originating in or from the
posterior vertebral elements (35,43). Symptoms in the spine are similar to extremity lesions (see Chapter 127), except for the occasional development of a painful scoliosis.
overlying shadows of the vertebral body, it is most readily localized
by bone scan. Excision of the lesion reliably and immediately relieves
the patient’s pain. The key to successful treatment is accurate
localization of the tumor nidus, confirmed by directed CT of the area.
Osteoblastoma is considerably larger than the 2 cm osteoid osteoma; it
is characterized by the expansion of the overlying cortical bone and a
thin rim of reactive bone among the trabeculae. When complete excision
of the osteoblastoma is not feasible, curettage and bone grafting may
provide an acceptable long-term result (27,43).
Scoliosis related to these lesions is often flexible and will usually
improve or resolve after the lesion is removed. Instrumentation and
fusion of the curve may be required if the scoliosis has been present
for a long period and has become structural. Corrective surgery may be
planned after the patient has recovered from the tumor surgery and has
had a chance to improve spontaneously.
hemangiomas are common, occurring in approximately 10% of all adults.
Fortunately, only a small proportion are ever symptomatic. Reports of
deformity or pain associated with hemangioma are rare, and surgeons
should hesitate before attributing mechanical or chronic pain symptoms
to these lesions. Asymptomatic hemangiomas rarely develop into
symptomatic ones, and follow-up is unnecessary (19).
Plain radiographs typically show vertical striations indicative of
thickened trabeculae within the involved vertebral body, and CT usually
demonstrates these same trabeculae dotting the region of the lesion.
These lesions often respond to radiotherapy or vascular embolization
alone. If vertebral collapse or neural compression occurs, surgical
decompression and reconstruction through an anterior approach are
indicated.
these histologically benign lesions may behave in a far more malignant
fashion in the spinal column, resulting in significant mortality as
well as morbidity (45,70).
Usually seen in the third or fourth decade of life, the tumors appear
lucent on plain radiographs, with marginal sclerosis and a geographic
pattern of bone destruction. These slow-growing tumors are usually
anterior and may expand the surrounding cortical bone as they grow.
Some authors have suggested that spinal tumors are less aggressive than
extremity lesions (15,56),
whereas others have acknowledged the aggressive nature of the tumor in
this vulnerable region and have recommended adjuvant irradiation (55) or cryotherapy (42) for local control.
locally, CT and MR imaging are particularly important in planning an
operation that will provide as wide a margin as possible. Complete
excision is the key to eradicating these tumors (Fig. 152.3). Anterior/posterior vertebrectomy with an en bloc
excision, followed by a combined reconstruction, limits the likelihood
of recurrence and allows the most rapid return to function.
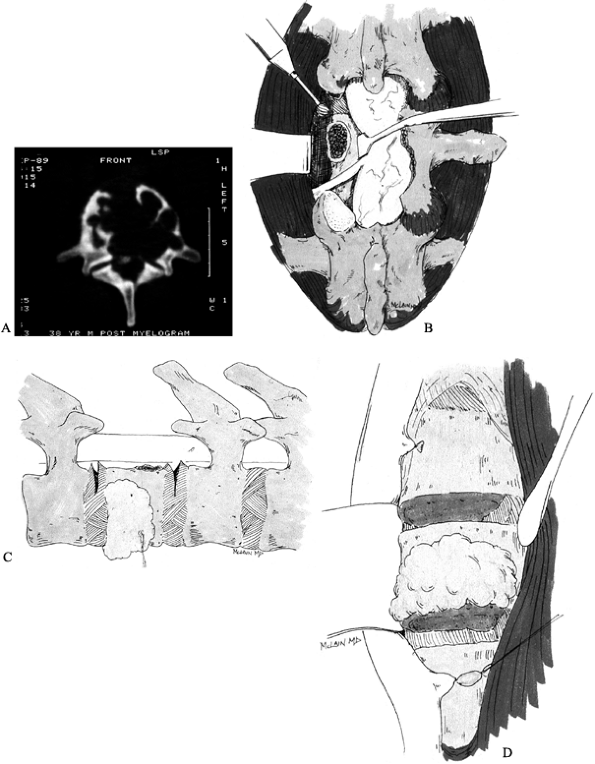 |
|
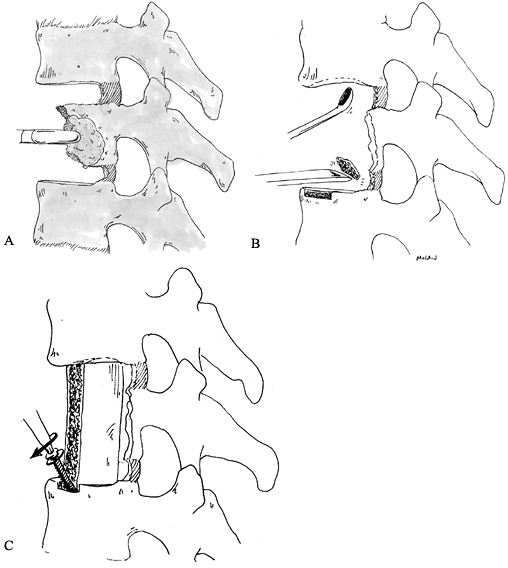
Figure 152.3. Treatment of giant cell tumor. A: CT scan showing extensive destruction of L-1 vertebral body. B:
An aggressive approach is taken to limit the chance of local recurrence. Wide laminectomy over the tumor level allows pedicle resection and release of the posterior annulus. C: After the posterior annulus is sectioned, the dorsal instrumentation is applied to stabilize the spine. D: An anterior approach allows en bloc vertebrectomy by completing the discectomies and removing the tumor with the overlying soft tissues adherent. |
commonly seen in children under the age of 10 years. Vertebral
involvement occurs in approximately 15% of all cases and can be
associated with any of three syndromes: isolated eosinophilic
granuloma, Hand-Schüller-Christian disease, and Letterer-Siwe disease.
The classic radiographic presentation is caused by near-complete
collapse of the vertebral body, resulting in a vertebra plana, or
“coin” lesion (57). Although classic, this
appearance is not pathognomonic, and a similar picture can result from
either infection or Ewing’s sarcoma (51). Once
a definitive diagnosis is established, usually by trocar biopsy, the
patient may be effectively treated by bracing and observation. Although
radiotherapy has been advocated in the past, it can be avoided in most
patients.
present, either with or without vertebral collapse, the established
course of biopsy followed by irradiation and immobilization remains the
most widely accepted (26).
Recovery of neurologic function is usually excellent, and some
reconstitution of vertebral height is seen in most young patients.
column. When they do, they usually involve the posterior elements and
are most commonly seen in the lumbar spine. Radiographs demonstrate an
expansile lesion with an osteolytic cavity that may extend across
segmental levels to involve two or even three adjacent vertebrae. The
cortex is often eggshell thin and blown out, and the cyst contains
numerous strands of bone which give the “bubbly” appearance typical of
ABCs. Curettage usually eradicates the lesion, and recurrences, which
do not tend to invade vital structures, may be successfully treated by
repeated curettage or excision (32).
in the axial skeleton, most often in the spine and sacrum. The tumor is
derived from rests of notochordal tissue residing in the skull base,
sacrococcygeal region, and the vertebral segments in between (49).
The tumor is characterized by slow but relentless local progression. It
metastasizes late, but it has an aggressive tendency to recur at the
surgical site, which makes it highly lethal. Although uncommon in
children, chordomas are more histologically variable and more
clinically aggressive in this age group than in adults (11).
Because of their insidious development, chordomas can reach remarkable
size before they are recognized. Patients may present after months or
even years of progressive pain, sitting intolerance, urinary
obstruction, and constipation. Sacrococcygeal tumors are easily
detected on rectal examination as firm, fixed lesions displacing the
posterior rectal wall.
chordomas. A wide margin is crucial to local control because these
lesions are generally unresponsive to radiotherapy and chemotherapy.
Whereas only 5% of patients with spinal chordoma develop metastases,
nearly 70% will die of their disease, reflecting the seriousness of
local tumor extension (3). Intralesional resection is associated with a high rate of local recurrence (82%) and a high mortality (71%) (7).
Carry out biopsy of a suspected chordoma through a posterior approach,
after all other staging studies are done. Never biopsy a sacral lesion
through the rectal vault; violation of the rectal wall necessitates
colectomy.
median survival following diagnosis has ranged from 6 to 18 months,
irrespective of surgical approach (1,58,65).
When effective local control can be obtained surgically, survival is
comparable to that of extremity lesions; fewer than half of all spine
patients achieve complete local excision, however (2).
body. Radiographs reveal cortical destruction, soft-tissue
calcification, and periosteal reaction. The paraspinal soft-tissue mass
may be extensive and may encase or invest the great vessels or other
contiguous structures. Intraspinal extension of the soft-tissue mass
may result in either cord or cauda equina compression.
aggressive treatment protocols have improved overall survival. By
combining current adjuvant therapy with extensive anterior/posterior
resections, surgeons have provided patients with improved local
control, neurologic function, and improved survivals (65,73).
column or sacrum. Resistant to both radiotherapy and chemotherapy,
these tumors are slow growing, locally invasive, and difficult to
eradicate from the spinal column. Although survival may be prolonged in
spite of residual disease, the final prognosis for patients with spinal
chondrosarcoma is poor.
CT and MR imaging are crucial to determining soft-tissue extensions and
the potential for surgical resection. Although long-term survivals are
occasionally associated with intralesional resection, a wide margin is
the most reliable means of local control and cure (59,63).
metastatic lesion. Approximately 3.5% of Ewing’s lesions are thought to
arise in the spinal column primarily. These tumors produce a permeative
destructive pattern that can be difficult to discern on plain
radiographs, so that the first radiographic finding may be vertebral
collapse and vertebra planum (51). Intraspinal
extension may produce neurologic symptoms before bony involvement
becomes apparent on plain radiographs. MRI will demonstrate the lesion
and its extension, as well as showing occasional epidural metastasis
that do not involve bone.
program of multiagent chemotherapy and high-dose radiotherapy. Surgical
treatment is indicated to decompress neurologic structures and
stabilize the spinal column. Thoracic and thoracolumbar laminectomies
should be instrumented to prevent kyphosis (28). Although the prognosis is generally worse than for extremity lesions, encouraging
disease-free survival rates have been obtained using current multimodality regimens (28,36).
ends to the continuum of B-cell lymphoproliferative diseases. Multiple
myeloma is rapidly progressive and highly lethal, requiring little more
than supportive care for spinal involvement. Solitary plasmacytoma may
remain localized for years before eventually disseminating. Prolonged
survival is possible if local control can be obtained (44).
cell neoplasms. Whereas spinal involvement in multiple myeloma is
associated with a poor 1-year survival rate, patients with solitary
plasmacytoma of the spine have a 60% 5-year survival rate (44,71).
Although most, if not all, of these lesions will eventually degenerate
into disseminated multiple myeloma with a rapidly lethal course,
survivals of 20 years or more have been reported.
Surgical treatment is indicated to stabilize the spine and reduce
mechanical pain and to decompress neurologic elements in patients with
rapidly progressive symptoms. Surgery is also warranted for those
patients with recurrent disease or tumors that have not responded to
radiotherapy (Fig. 152.4). Follow-up with MRI and serum protein electrophoresis provides the earliest indication of recurrence or dissemination.
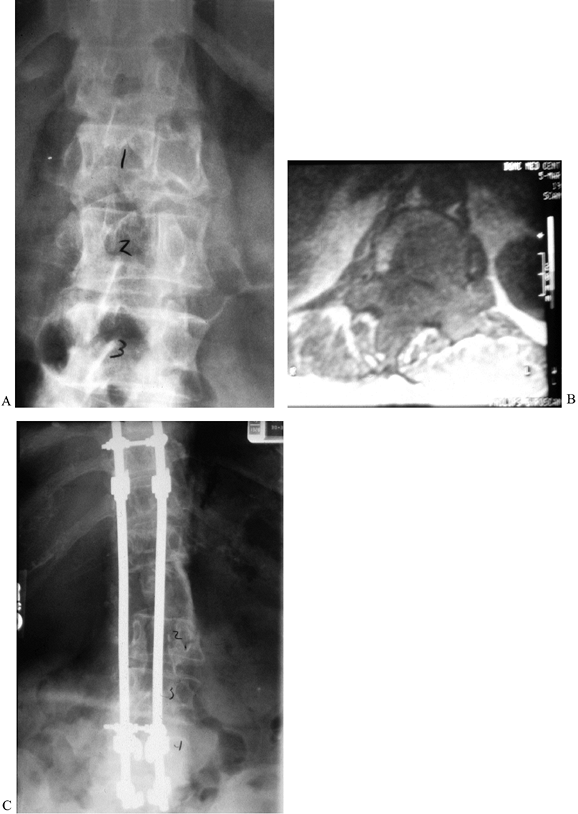 |
|
Figure 152.4. A: AP radiograph of a 68-year-old woman with solitary plasmacytoma of the T-12 vertebra, refractory to radiotherapy. B: MRI shows the extent of the tumor. C:
Complete anterior/posterior excision was followed by anterior tricortical graft and long segmental instrumentation posteriorly. She was disease free for 5 years before recurrence and dissemination occurred. |
manifestation of a disseminated disease. As with plasmacytoma, surgical
treatment is an adjuvant to systemic therapy and radiotherapy. Surgical
decompression is indicated to decompress cord, cauda equina, or nerve
root injured by tumor extension or pathologic fracture, and to
stabilize damaged spinal segments.
disease by its vascular anatomy, its architecture, and its proximity to
common sources of disease. The venous drainage of the spine is
contiguous with that of the thoracic and abdominal viscera. Retrograde
venous flow provides a variety of tumors access to the vertebral body.
There, metastatic emboli settle and implant in the capillary end-loops
adjacent to the vertebral endplates. The red marrow of the vertebral
body provides a physiologically favorable environment for tumor cell
proliferation. The vertebral trabecular bone has a rich blood supply,
with few barriers to tumor extension; once established, the tumor can
grow for some time before it becomes clinically apparent.
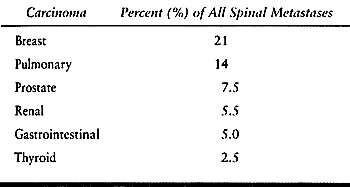
metastases; however, certain tumors are particularly adept at reaching
and surviving in the trabecular environment (Table 152.1).
Breast, lung, prostate, and lymphoreticular disease account for
approximately 60% of all spinal column metastases requiring treatment.
Whether a tumor requires surgical treatment is determined by the
behavior of the primary lesion and the metastasis. Patients with
breast, prostate, renal, thyroid, or gastrointestinal carcinoma may
experience extended survivals with current adjuvant protocols, despite
established metastases. Patients with multiple myeloma or pulmonary
carcinoma typically deteriorate and die soon after metastasis. Breast,
prostate, and renal carcinomas tend to establish spinal metastases
early in the disease process, whereas gastrointestinal carcinomas
typically seed the liver and lungs first. Hence, patients with breast,
prostate, and renal carcinoma tend to live long enough for their spinal
metastases to become a specific threat to function and quality of life,
while patients with lung carcinoma and myeloma will often die before
the spinal metastasis needs more than palliation, and patients with
gastrointestinal carcinoma are often more directly affected by their
visceral metastases than their skeletal disease.
 |
|
Table 152.1. Trabecular Carcinomas
|
spine in children; neuroblastoma accounts for nearly one third of all
pediatric spinal tumors (20,39). Ewing’s sarcoma is the most common primary malignancy, but it is still more often a metastasis than a primary lesion (73). However, 70% of primary pediatric tumors are benign.
spread to the spine either by vascular dissemination or by contiguous
spread from the primary lesion. Treated with a combination of
chemotherapy, radiotherapy, and surgical excision, patients with these
tumors have a poor prognosis overall. Those patients that do survive
are at high risk of developing a progressive spinal deformity as a
result of either rib resection or hemibody irradiation (21,52).
initial finding in a patient with systemic disease. Most of these
patients will have back pain, and some may have vertebral collapse at
the time of presentation (53). Nonspecific
complaints of muscular aches and pains, lethargy, fatigue, and fever,
as well as findings of anemia, should prompt a search for the
underlying disease. Radiographs are not characteristic: They may show
vertebral collapse, focal lytic changes, or sclerotic, geographic
lesions, or they may
be entirely normal. Bone scan may also be equivocal, but MRI will reliably demonstrate the infiltrate (10).
-
Is the tumor benign or malignant? Primary or metastatic?
-
Is the patient systemically ill, or fit?
-
Is the tumor slow growing, locally aggressive, or widely disseminated?
-
Is there any neurologic compromise?
-
Is there a fracture or instability?
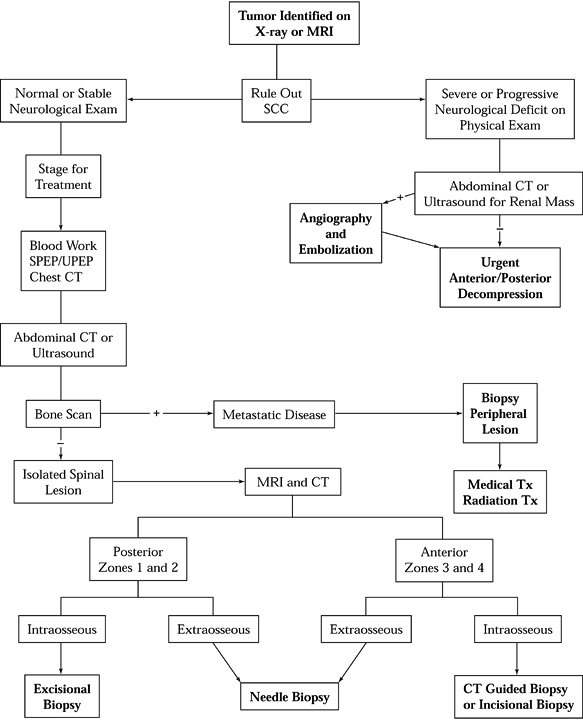
treatment until these questions have been answered. See the algorithms
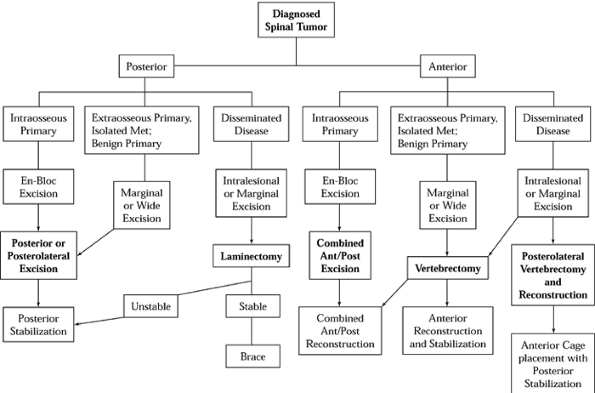
for diagnostic workup (Fig. 152.5) and treatment and reconstruction (Fig. 152.6).
 |
|
Figure 152.5. Diagnostic workup algorithm for spinal tumor. SCC, spinal cord compression; SPEP, serum protein electrophoresis; UPEP, urine protein electrophoresis; TX, treatment; Zones, see Figure 152.7.
|
 |
|
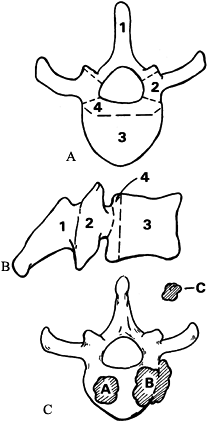
Figure 152.7. Tumor staging. Axial (A) and lateral (B) views of vertebral body showing four zones of tumor involvement. C: Grade A represents intraosseous spread; grade B, extraosseous extension; and grade C, distant metastasis.
|
 |
|
Figure 152.6. Treatment and reconstruction algorithm for spinal tumor. Met, metastasis.
|
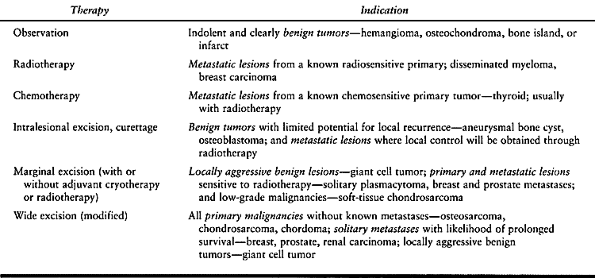
to treat spinal tumors, ranging from observation to total spondylectomy
(Table 152.2). Both undertreatment and overtreatment can lead to trouble.
 |
|
Table 152.2. Medical Therapies to Treat Spinal Tumors
|
because any break in the vertebral ring violates the osseous
“compartment.” The necessary cuts through the bony ring could expose
normal tissues to contamination even in well-circumscribed tumors.
Hemorrhage from the cut bone can carry tumor cells throughout the
field, reducing the chance for local control. Once the tumor has
extended beyond the vertebral cortex, even a marginal excision may be
difficult to obtain. A tumor that adheres to or invades the dura or
aorta may prove difficult or impossible to resect, and a tumor that
involves the vena cava is usually unresectable. In these cases, the
risks of attempting a wide resection with vascular or dural grafting
must be weighed against those of following up a marginal excision with
adjuvant radiation.
be easily biopsied, may not require any spinal surgery. Unless there is
neurologic impingement or mechanical instability, radiation or
chemotherapy can retard tumor progression and control the spinal lesion.
-
Inability to obtain a tissue diagnosis by other methods
-
Neurologic compression due to pathologic fracture or bony impingement
-
Mechanical instability, with severe pain or impending neurologic injury
-
Tumor progression despite, or following, radiotherapy
-
Known radioresistant tumor
-
Primary malignant tumor
-
Resectable solitary metastasis in patient with potential long-term survival
the patient suffers from neurologic compromise, spinal in-stability, or
collapse, surgical treatment will be needed following biopsy. Three
issues must be considered in developing a surgical plan—first, the
proper margin of resection (of primary concern in locally aggressive
and malignant primary tumors); second, the need for neurologic
decompression; and third, the means of reconstruction.
In locally aggressive tumors, resect the anterior and posterior
longitudinal ligaments, vertebral body, adjacent discs, and the
overlying dura, if necessary, to avoid leaving residual tumor behind.
It is sometimes necessary to sacrifice one or more nerve roots to
provide a suitable margin of excision.
-
Zone 4 lesions involve the posterior
portion of the vertebral body and that portion of the cortex just
anterior to the spinal cord or neural elements. -
To address any lesion involving zone 4,
the surgeon must cross zone 3 and must release the vertebra from the
pedicles, resecting zones 1 and 2 as well. -
Zone 4 lesions frequently require a
subtotal or total vertebrectomy to obtain a clean margin. This assumes
that the tumor is still intraosseous (grade A), without extraosseous
spread (grade B), or distant metastases (grade C).
information needed for staging, with bone scan and serologies added to
determine metastatic status. Pay particular attention to the
possibility of extraosseous extension—Grade B lesions may prove
unresectable if vital structures are directly invested by tumor. The
decision to attempt a wide resection in these lesions must be weighed
against the risks of vascular or neurologic injury. In some cases, the
most prudent approach may be to accept a marginal or intralesional
margin, supplementing local treatment with adjuvant radiotherapy or
cryotherapy.
-
Zone 1 lesions are best approached
through a standard posterior incision, with the extent of the incision
based on the extent of the soft-tissue mass, if any. -
Zone 2 lesions require a posterolateral approach (Fig. 152.8).
The laminectomy and bone resection necessary for tumor excision
generally results in some degree of segmental instability, and
posterior instrumentation and fusion is usually necessary.![]() Figure 152.8.
Figure 152.8.
Resection of zone 2 lesion. Posterolateral approach allows access to
uninvolved lamina on contralateral side along with uninvolved
ipsilateral pedicle. A marginal margin is obtainable if the pedicle is
free of tumor. -
Zone 3 lesions can often be addressed through an anterior approach alone.
-
3A lesions can be adequately resected at
any level of the spine, but 3B lesions may present different challenges
at different levels. Depending on the extent of resection, a formal
reconstruction may or may not be necessary. -
Zone 4 lesions require a combined
surgical approach if a marginal or wide margin is to be obtained. Zones
1, 2, and/or 3 must be crossed to gain access to the zone 4 lesion, and
more than one zone is usually involved with the tumor. -
Complete resection of the vertebral body
requires separating the posterior structures (zones 1 and 2) from the
anterior structures (zones 3 and 4), at the junction between the
pedicles and the vertebral body (Fig. 152.9).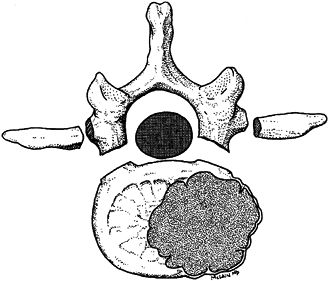 Figure 152.9. A zone 4 lesion en bloc excision of the involved thoracolumbar vertebra.
Figure 152.9. A zone 4 lesion en bloc excision of the involved thoracolumbar vertebra.
midline posterior incision with either a retroperitoneal, a
thoracoabdominal, or a transthoracic approach to the anterior vertebral
body. An alternative approach is to extend the posterior dissection
around the side of the vertebral body, completing the vertebrectomy
through a posterolateral
resection (18). If at least one pedicle is uninvolved, a wide margin is possible (6).
Complete vertebrectomy requires both anterior and posterior
stabilization, but experience has shown that this aggressive surgical
approach does improve patient survival and neurologic function even
when cure cannot be obtained (66).
This combined anterior/posterior sacral approach provides improved
outcome with surprisingly little long-term morbidity; as long as the
S-2 nerve roots are spared bilaterally, or S-2 and S-3 are retained
unilaterally, bowel and bladder function are usually unharmed (22,54). In more proximal tumors, these roots must be sacrificed to obtain local control and a reasonable likelihood of survival.
To prevent permanent neurologic injury, the surgeon must recognize and
treat spinal cord compression early in its development. Compression may
result when an enlarging soft-tissue mass encroaches on cord or nerve
roots, or when a pathologic fracture results in retropulsion of bone
fragments into the canal, vertebral collapse, or kyphosis. Soft-tissue
metastasis to the meninges or epidural space may directly compress
neural elements (5,30).
back pain, radicular symptoms or “girdle” pain, lower extremity
weakness, sensory loss, and bowel or bladder dysfunction. Acute spinal
cord compression typically results from rapid tumor growth or
pathologic fracture caused by extensive bony destruction. Early
treatment is crucial:
-
Patients with rapidly progressive
paralysis have a poor prognosis for recovery compared to those who
develop symptoms over a prolonged period. -
In ambulatory patients, 60% to 95% will retain that function after treatment.
-
Only 35% to 65% of paraparetic patients will walk independently after treatment.
-
Less than 30% of paraplegic patients will regain ambulation after either surgical or medical treatment (31,38).
most patients with spinal column metastases. Different tumor types
exhibit different levels of radiosensitivity, however, and different
clones of the same primary tumor may behave differently as well:
-
Prostatic and lymphoreticular neoplasms
are typically radiosensitive, and satisfactory local control can be
gained through postoperative radiotherapy, even after an intralesional
resection (68). -
Gastrointestinal and renal neoplasms, on the other hand, are often unresponsive to irradiation.
-
A number of primary tumors (e.g.,
chondrosarcoma, chordoma) are not radiosensitive, and, consequently,
neurologic compromise resulting from these lesions is best treated by
operative methods.
is properly matched to the compressive lesion: anterior decompression
for anterior tumors and posterior decompression for posterior lesions.
Using the wrong approach (e.g., laminectomy for anterior compression)
provides little benefit and increases complications. For example,
laminectomy has shown no added benefit relative to radiation alone in
treating anterior spinal lesions, and it can compound problems by
introducing or increasing segmental instability in the compromised
segment (23,30).
Overall, decompressive laminectomy provides neurologic improvement in
only 33% of cases, and an overall satisfactory outcome (maintenance of
ambulation and sphincter control) in 37% (45). By comparison, anterior decompression results in 79% improvement and 80% satisfactory outcome in similar patients (Fig. 152.10).
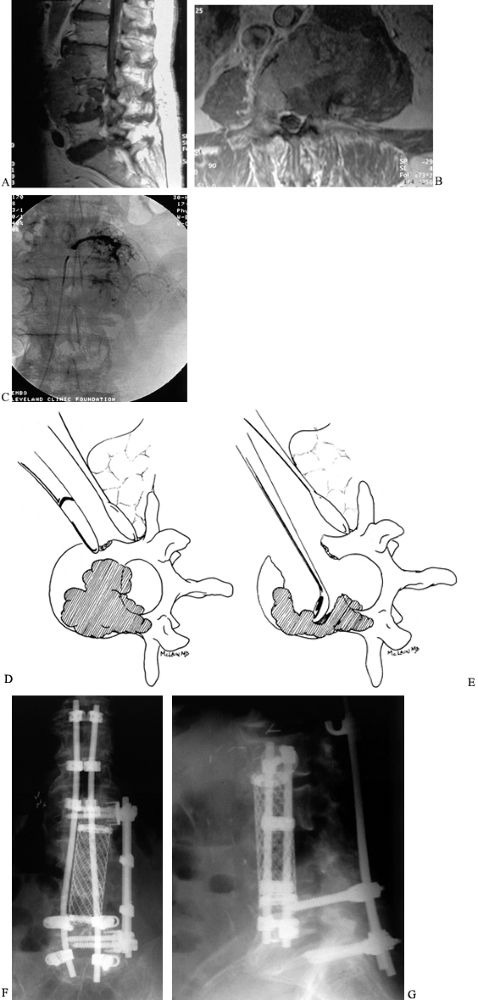 |
|
Figure 152.10. Anterior vertebrectomy for metastatic disease. Sagittal (A) and axial (B)
MRIs demonstrating extent of an isolated renal cell metastasis involving both L-3 and L-4 vertebral bodies. The lesion probably seeded in one vertebral body, then spread contiguously to the adjacent level. C: Angiography prior to surgery shows blush of neovasculature in the tumor mass just prior to embolization. D: Anterior decompression of radiosensitive tumors begins by resecting the normal bone exposed during the anterior approach, then excising the tumor tissue in as few pieces as can be managed, moving quickly to limit blood loss. E: After removal of the bulk of involved vertebra, meticulous dissection is carried out to remove retropulsed fragments and extruded tumor from in front of the thecal sac, and to curet away all gross tumor from the resection margins. AP (F) and lateral (G) radiographic views, 2 years postoperative. After resection, the anterior column is reconstructed with a strut or cage. The titanium cage selected here is packed with autograft bone because successful treatment may provide this patient with several years of life. An anterior construct stabilizes the spine until the posterior reconstruction, using segmental instrumentation, can be performed. |
tumor resection to restore stability, prevent progressive deformity,
and facilitate graft incorporation and fusion. The surgeon must choose
an instrumentation construct that (a) can meet the mechanical demands
it will face following tumor resection, (b) can compensate for loss of
bony elements due to resection or laminectomy, and (c) will permit
postoperative imaging with CT and MRI. Key principles to reconstruction
are the following:
-
Restore or augment the anterior weight-bearing column to prevent vertebral collapse and kyphosis.
-
Use posterior instrumentation to provide
a tension-band effect after laminectomy, to compensate for lost
muscular attachments, and to prevent progressive kyphosis. -
Combine anterior and posterior constructs to restore axial, sagittal, and torsional stability after vertebrectomy.
-
Anticipate disease progression—extend
fixation over longer segments, maximize fixation points, and combine
anterior and posterior constructs to ensure construct survival. -
Anticipate patient survival—strive for spinal fusion in patients likely to live more than 3 to 6 months.
combined with sublaminar or Drummond wires to provide segmental
fixation in the thoracic spine, but they do not contour well to the
lumbar spine and they tend to flatten the normal lumbar lordosis,
resulting in a painful lumbar deformity. These systems are inexpensive
and are adequate to stabilize thoracic compression fractures or
laminectomies. They are not the best choice for cases with extensive
bone destruction, however. Rod breakage and hook pullout are common,
particularly when applied to patients with combined anterior and middle
column insufficiency. These systems are vulnerable to fatigue failure,
particularly in tumor patients where perioperative irradiation and
systemic disease increase the risk of delayed union, and nonunion.
The Luque rods–sublaminar wire system has been used successfully in
treating degenerative and neoplastic disease of the cervical, thoracic,
and lumbar spine. The system has good stability in torsion and flexion
but cannot resist pure axial loads—the sublaminar wires are free to
slide down the rod, allowing the instrumented segment to collapse
considerably along the axis of the rods.
and resilient. They allow the surgeon to neutralize the overall length
of the spine while either compressing or distracting the intercalary
segments involved in the reconstruction. Hook and screw fixation at
multiple levels improves fixation strength, and pedicle screws allow
fixation to levels where posterior elements have been removed. These
systems have superior torsional and sagittal strength and are widely
available in titanium, improving postoperative imaging capabilities.
These versatile systems also allow the surgeon to address multiple
levels of vertebral involvement, restoring normal thoracic kyphosis and
lumbar lordosis in the same construct.
patients who have undergone previous laminectomy. They allow the
surgeon to minimize the number of segments instrumented, limiting the
need to extend fusions to additional levels for support. Combined with
an anterior strut, screw-and-rod and screw-and-plate constructs provide
sufficient axial, torsional, and sagittal rigidity to allow the surgeon
to instrument only two motion segments when treating primary and
metastatic lesions of the thoracolumbar spine (47). Screw failure can be expected, however, if the anterior weight-bearing column is incompetent and is not reconstructed (48).
lumbar regions, where pedicles are relatively large and the spinal cord
is not at risk. They may prove useful for lower thoracic lesions, as
well, by securely anchoring the caudal end of a longer thoracolumbar
construct. Use in the thoracic spine is more limited, although some
authors have found that screw fixation is an important alternative in
patients with extensive laminectomies. Screw-and-plate constructs can
be used in the upper thoracic spine to stabilize the cervicothoracic
junction, to treat laminectomized segments, and to limit the bulk of
instrumentation placed under thin, irradiated soft tissues (Fig. 152.11).
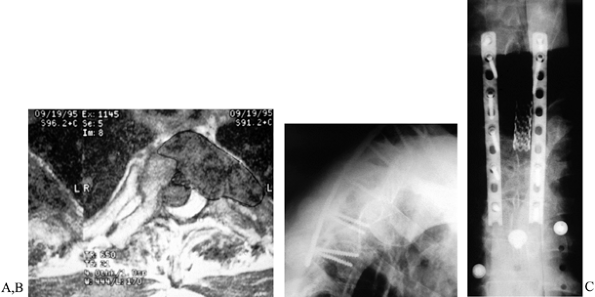 |
|
Figure 152.11. A: MRI of T2 metastasis. B,C:
Lateral and AP views of screw and plate construct for upper thoracic spine. An alternative to rod-and-hook constructs, low-profile plates may be useful in patients with absent or incompetent posterior elements, or those with tenuous skin following irradiation. |
addition to posterior procedures, or as the primary treatment in some
patients. Posterior instrumentation alone cannot provide adequate
stability in all cases. When posterior decompression is superimposed on
anterior and middle column vertebral collapse, the resulting
instability can be severe (16), and untreated anterior column deficiency leads to pedicle screw fatigue and breakage (47,48).
Moreover, there is a significant incidence of wound complications
associated with posterior surgery. These patients are often
systemically ill. Many have undergone regional radiation therapy, have
lost muscle mass and subcutaneous fat, and have impaired healing
potential. Wound dehiscence, infection, and skin problems are common
enough to prompt many surgeons to consider anterior reconstruction as
their primary avenue of treatment.
column must be reconstructed. Depending on the situation, the surgeon
can chose from bone, methylmethacrylate, or a variety of prosthetic
struts (Table 152.3).
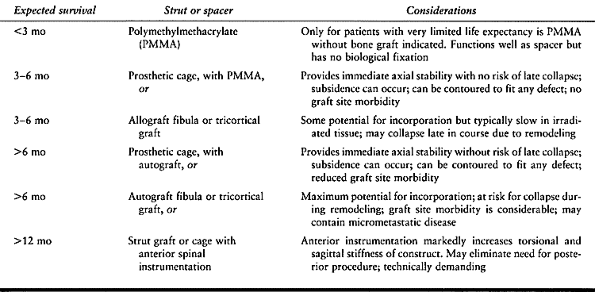 |
|
Table 152.3. Choice of Struts or Spacers for Reconstruction of Anterior Spinal Tumors
|
reconstruct the vertebral column in metastatic disease. It is resilient
in compression, but because it has no potential for biological
incorporation it has a tendency to loosen and extrude over time.
-
Incorporate longitudinal Steinmann pins
into the PMMA mass and drive them proximally and distally into the
adjacent vertebrae to anchor the spacer and improve its bending
resistance (Fig. 152.12). Alternatively, insert
Harrington distraction rods or Knodt rods into the vertebrectomy site
to distract the defect and anchor the PMMA mass (61).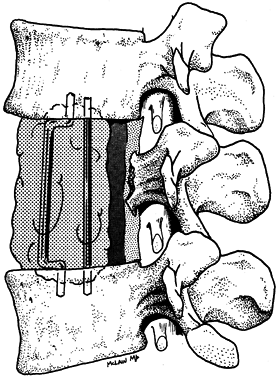 Figure 152.12. Reconstruction of the anterior column with polymethylmethacrylate.
Figure 152.12. Reconstruction of the anterior column with polymethylmethacrylate. -
Countersink the rod ends into the opposing endplates and distract to restore alignment.
-
Apply PMMA in its dough phase to fill the defect.
-
Place a Silastic or Gelfoam dam in front
of the dura to protect the spinal cord from compression or thermal
injury, and wash the PMMA mass constantly with cool saline during
polymerization.
morcelized autograft is favored in the treatment of benign or
slow-growing tumors in patients whose survival is likely to be measured
in years, and similarly in malignant primaries, where successful
treatment will result in prolonged survival.
-
Cut tricortical struts from the anterior
superior iliac crest, measuring 5–10 mm longer than the defect to be
filled. Cut graft with a saw, not an osteotome. -
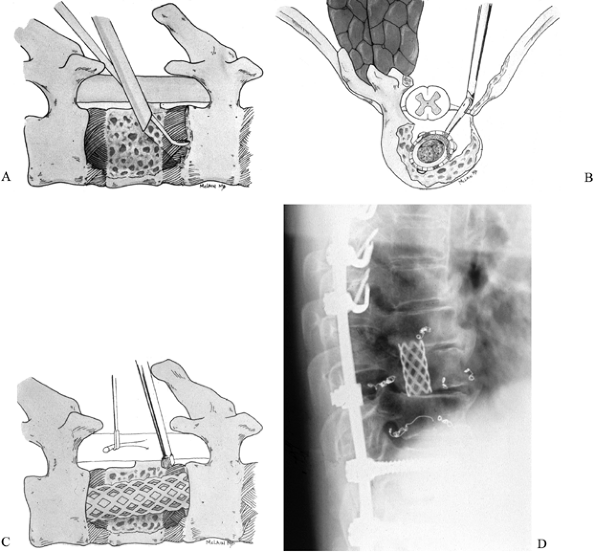
Key the graft into the vertebral endplates to prevent displacement when the patient is mobilized (Fig. 152.13).
![]() Figure 152.13. Anterior reconstruction with tricortical graft. A:
Figure 152.13. Anterior reconstruction with tricortical graft. A:
Anterior vertebrectomy provides a wide defect. Anterior superior iliac
crest is harvested and contoured to fill and distract the vertebrectomy
defect. B: Graft ends are contoured to articulate with a groove and pit fashioned in the vertebral endplates. C: Graft is impacted into place and locked with a single 6.5 mm cancellous screw. -
To prevent displacement, drive a single
6.5 mm cancellous interference screw into the vertebral endplate just
lateral to the graft. This will keep the graft from slipping back out
through the keyhole defect.
-
Impact the cage into the vertebrectomy defect.
-
Sagittal compressive forces will tend to hold the cage in place until anterior or posterior instrumentation can be added.
-
Do not penetrate the endplates during cage placement. Do not “key” the cage into place.
-
If fusion is intended, pack the cage full
of morcelized autograft bone before placing it into the defect. After
inserting the cage, place more graft anteriorly to augment the fusion. -
Because the cage provides little
torsional rigidity on its own, apply an anterior fixation system to
stabilize the spinal construct.
disease, the physician must determine whether surgery is likely to
improve the patient’s function, quality of life, or longevity. In cases
of severe pain, segmental instability, or neurologic compromise,
operative intervention may be indicated.
spinal metastasis often do not require surgery. Patients who may
require surgery include those with known radioresistant tumors,
solitary metastasis with potential for wide resection, unknown tumor
type despite systemic workup and needle biopsy, bony compression of
neural elements, and mechanical instability and bone destruction.
-
Treat patients with mechanical
instability and neck or back pain with bracing and irradiation, unless
other factors dictate surgery. -
Consider surgical reconstruction once bony destruction is advanced.
-
If the tumor is radiosensitive, stabilize
the spine with posterior instrumentation and control tumor growth with
adjuvant radiotherapy. -
If the tumor is not radiosensitive, or if
bony destruction is advanced, perform an anterior/posterior or
posterolateral decompression and stabilization, and mobilize the
patient early. -
If the patient presents with a solitary
metastasis from a tumor with potential long-term survival (breast,
colon, prostate, kidney), consider a combined procedure to obtain a
wide excision of the lesion. Treat the tumor in the same way as a
primary malignancy.
-
If the tumor is radiosensitive and neural
progression is gradual, radiotherapy is the initial treatment of
choice. If progression is rapid, unresponsive to radiotherapy, or
secondary to bony as opposed to soft-tissue encroachment, decompress
the cord or roots through the most direct approach. -
Use the posterior surgical approach in the cervical spine, above the level of C-3.
-
Below C-3, the posterior approach should
be limited to lesions of the dorsal elements. Address lesions of the
vertebral body successfully through the appropriate anterior approach. -
As an alternative in lesions of the
thoracic spine, use a costotransversectomy or transpedicular technique
to access the vertebral body and decompress the anterior aspect of the
spinal cord (37,38), (Fig. 152.14).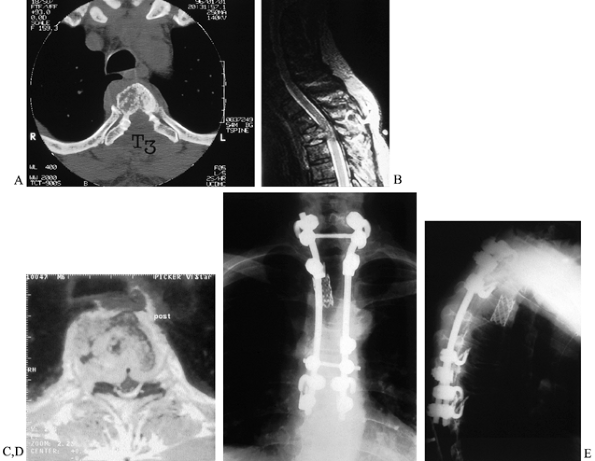 Figure 152.14. Posterolateral decompression and reconstruction. CT (A) and MRI (B,C) images of a 65-year-old man with T-3 solitary plasmacytoma, cord compression, and incomplete paraplegia. D,E:
Figure 152.14. Posterolateral decompression and reconstruction. CT (A) and MRI (B,C) images of a 65-year-old man with T-3 solitary plasmacytoma, cord compression, and incomplete paraplegia. D,E:
Endoscopically assisted posterolateral approach allowed complete
vertebrectomy and spinal cord decompression. Titanium cage
reconstruction and posterior instrumentation through the single dorsal
incision provided immediate stability as seen on AP(D) and lateral (E) radiographs. Complete neurologic recovery was facilitated by rapid mobilization and rehabilitation.
 |
|
Figure 152.15. Endoscopically assisted posterolateral vertebrectomy. A:
After laminectomy and pedicle resection, the posterolateral aspect of the vertebral body and tumor mass are cavitated with pituitaries and curets. The endoscope is then used to visualize the undersurface of the cord, the endplates, and the far pedicle. Complete decompression can be confirmed without manipulating the neural tissues. B,C: Once the endplates are prepared, an appropriately sized cage, packed with autograft bone or PMMA, is introduced into the defect between the ipsilateral nerve roots and impacted into place. The endoscope confirms a safe interval between the cage and thecal sack before posterior instrumentation is applied. D: Lateral radiograph of a patient with metastatic adenocarcinoma treated with posterolateral decompression and reconstruction. |
to treat pain and to prevent local tumor expansion. Intralesional
excisions are adequate in many tumor types (e.g., aneurysmal bone cyst,
osteoblastoma) and should be carried out through the most direct
approach with the least disruption of normal vertebral elements.
-
Treat locally aggressive (aggressive
benign and low-grade malignant) tumors rigorously, ensuring a clear
margin wherever possible. Because recurrent tumors are more difficult
to eradicate than primary lesions, these locally aggressive lesions may
become unresectable if not adequately addressed in the first place. -
Resect giant cell tumors en bloc when possible, and check the bone margins for residual tumor.
-
Repeat curetments if necessary to obtain
a clean margin, and consider adjuvant cryotherapy or postoperative
radiotherapy to ensure a clean tumor bed (42,55). -
As an alternative to the traditional
anterior/posterior approach, consider a posterolateral dissection and
vertebrectomy through a T posterior incision (18).
treatment is local control of the disease. Plan the approach and
resection to give the best chance of an adequate resection margin with
the least disruption of vertebral stability.
-
Approach dorsal tumors through a longitudinal posterior approach, incorporating any previous biopsy wound in the incision.
-
Take care not to enter the soft-tissue mass of the tumor, either surgically or with retractors or rakes.
-
Excise a cuff of normal muscle tissue with the tumor.
-
Perform a laminectomy above and below the
involved level and cut through uninvolved lamina or pedicle to isolate
and resect the involved elements. -
Remove the tumor en bloc and stabilize the spine with a posterior instrumentation construct.
-
Embolize the lesion preoperatively to limit blood loss during the procedure.
-
Resect the posterior elements and posterior disc first to allow the vertebral body to be removed en bloc through an anterior approach.
-
Use a transthoracic or retroperitoneal approach to reach the tumor from the front.
-
Separate adherent tumor from the dura
using a Freer elevator as the body is excised. Excise any involved dura
and patch the defect with a fascial graft to improve local control. -
Stabilize the spine posteriorly with a segmental system at the time of posterior release.
-
Restore the anterior weight-bearing
column, filling the vertebrectomy defect with tricortical or fibular
graft, or with a prosthetic cage. -
Use anterior plate fixation to augment overall stability.
plana, a clear surgical margin is not possible, and local control is
dependent on adjuvant therapy.
-
Perform an intralesional resection of the
vertebral body to debulk the tumor and prepare the vertebrectomy site
for anterior reconstruction (40,44). -
In radioresistant tumors such as chondrosarcoma or chordoma, make every effort to obtain a clear surgical margin.
and reconstructive dilemma. A chordoma involving the distal sacrum
requires a partial sacral amputation through a combined anterior and
posterior approach, sacrificing whatever nerve roots exit the involved
segment. For higher sacral lesions, more roots will need to be
sacrificed. If the S-2 roots can be spared bilaterally, or if S-2 and
S-3 roots are spared on one side, bowel and bladder function should be
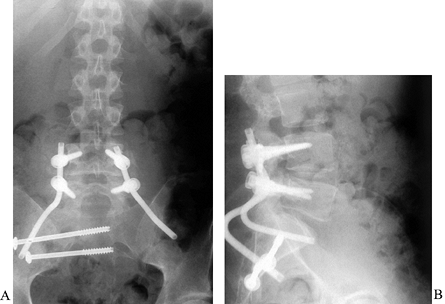
retained (22). Reconstruction following sacral amputation is most challenging when one or both of the sacroiliac joints is involved (Fig. 152.16).
 |
|
Figure 152.16. AP (A) and lateral (B)
radiographs after resection and reconstruction of sacral metastasis. Patient with destructive lesion of the sacral ala, presenting with pain and weakness. Posterior intralesional resection was followed by reconstruction of the ala with a bicortical iliac autograft contoured to fill the defect and transfixed with transiliac screws. Lumbosacral stability was provided with a short pedicle construct using Galveston-type fixation into the pelvis. This construct is suitable for limited resections such as this, or for more extensive tumors requiring sacrectomy and prosthetic reconstruction. |
neurologic deficit generally have one of three problems: a pathologic
fracture, which may either retropulse bone fragments into the spinal
canal or produce kyphosis; extraosseous extension through the posterior
vertebral cortex, producing direct compression of the neural elements;
or direct extension of the tumor involving one or more nerve roots.
-
In every case, the resection margins are likely to be contaminated, and adjuvant therapy is the key to eventual outcome.
-
Resect any nerve roots directly involved by tumor along with the primary mass.
-
Adherence to or investment of the dura or
great vessels is an ominous finding. Consider nonoperative or
palliative modalities in these cases.
becomes dependent on systemic therapy. Local control is still important
to prevent neurologic compromise and pain, but the impetus toward more
aggressive and potentially dangerous procedures is reduced. Reconstruct
the involved segments carefully, however, addressing both anterior and
posterior columns to ensure that late collapse, kyphosis, and pain will
not occur.
enhance cancer survival in patients with both primary and metastatic
disease. As patients live longer, metastatic lesions will pose a
greater threat to independence and survival, and musculoskeletal
lesions will require treatment that provides pain relief and protects
function for years rather than months. It is no longer acceptable to
assume that the patient with spinal metastasis is near death
and
beyond help; benign neglect is not benign, and it is neither fiscally
or ethically conscionable. Vertebrectomy, considered a radical
procedure in the past, is coming to be seen as the conservative
approach to tumor management in many situations. Advanced technologies
have made aggressive surgery less invasive and dangerous, and improved
instrumentation has all but eliminated the prolonged immobilization
associated with spinal reconstruction. Appropriate surgical management
can have an immediate and dramatic impact on patient function and
survival, and it should never be dismissed without consideration.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
SS, Wulff B, Delling G, et al. Osteosarcoma of the Trunk Treated by
Multimodality Therapy: Experience of the Cooperative Osteosarcoma Study
Group. Med Pediatr Oncol 1995;24:6.
S, Biagini R, De Lure F, et al. En-bloc Resections of Bone Tumors of
the Thoracolumbar Spine. A Preliminary Report on 29 Patients. Spine 1996;21:1927.
KH, Jenny AB, Saul T, et al. Posterior Segmental Spinal Instrumentation
(PSSI) with Posterior Decompression and Debulking for Metastatic
Thoracic and Lumbar Spinal Disease. Spine 1988;13:1383.
N, Gotze H, Pedersen A, et al. Skeletal Scintigraphy and Radiography at
Onset of Acute Lymphocytic Leukemia in Children. Med Pediatr Oncol 1983;11:291.
CA, Laredo JD, Chevret S, et al. Acute Vertebral Fracture due to
Osteoporosis vs. Malignancy: Appearance on Unenhanced and
Gadolinium-enhanced MR Images. Radiology 1996;199:541.
RL, Bridwell KH, Prodromas C, Rodts MF. Reconstructive Spinal Surgery
as Palliation for Metastatic Malignancies of the Spine. Spine 1985;10(1):21.
M, Shimizu A, Nakamura Y, Nemoto R. Role of the Vertebral Venous System
in Metastatic Spread of Cancer Cells to the Bone. Adv Exp Med Biol 1992;324:83.
KD. Anterior Decompression and Stabilization of the Spine as a
Treatment for Vertebral Collapse and Spinal Cord Compression from
Metastatic Malignancy. Clin Orthop 1988;233:177.
JP. Anterior Spinal Cord Decompression for Lesions of the Thoracic and
Lumbar Spine, Techniques, New Methods of Internal Fixation, Results. Spine 1983;8:512.
RC, Sheth DS, Brien EW, et al. Conservative Surgery for Giant Cell
Tumors of the Sacrum: The Role of Cryosurgery as a Supplement to
Curettage and Partial Resection. Cancer 1994;74:1253.
RF, Sparling E, Benson DR. Failure of Short Segment Pedicle
Instrumentation in Thoracolumbar Fractures: Complications of
Cotrel-Dubousset Instrumentation. J Bone Joint Surg Am 1993;75:162.
WR, Butler MS, Robertson WW Jr, D’Angio GJ. Late Orthopaedic Effects in
Children with Wilm’s Tumor Treated with Abdominal Irradiation. Med Pediatr Oncol 1991;19:265.