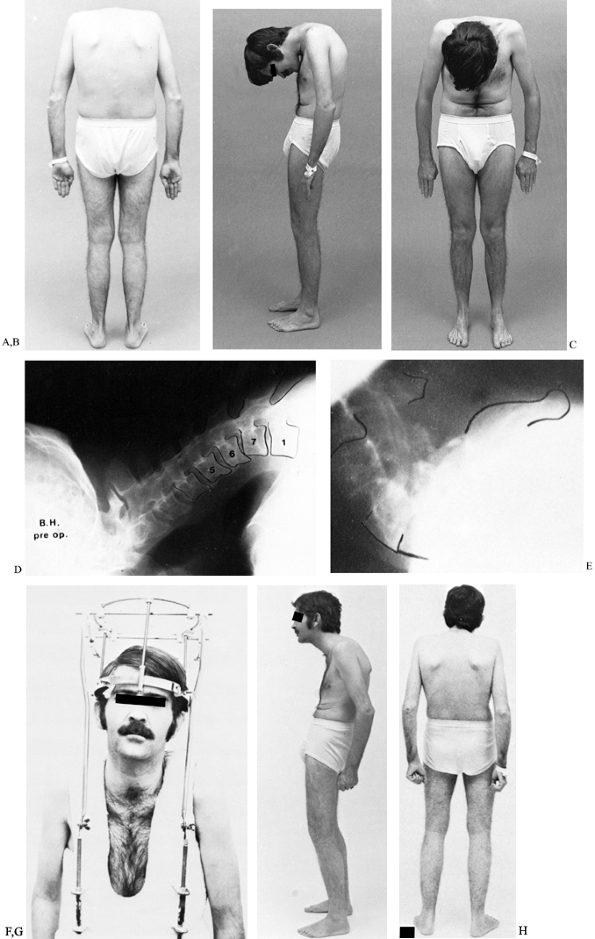
SURGERY OF THE SPINE IN ANKYLOSING SPONDYLITIS
VIII – THE SPINE > Rheumatoid Disease > CHAPTER 153 – SURGERY OF
THE SPINE IN ANKYLOSING SPONDYLITIS
although commonly considered together, are two distinct diseases.
Ankylosing spondylitis has often been described as “rheumatoid”
spondylitis, but it is a different disease with a different serology.
It is more common in men than in women and has a predilection for the
spine and major joints; rheumatoid arthritis is more common in women
and tends to affect smaller joints and the joints of the appendicular
skeleton (10,15,16). Clinical manifestations of ankylosing spondylitis occur in 0.2% to 0.3% of the general population.
of the spine are gross fixed deformities. In rheumatoid arthritis the
spine is subject to local destruction and instability. Atlantoaxial
subluxation and dislocation may occur in both diseases, however.
rheumatoid arthritis and ankylosing spondylitis requires careful
clinical monitoring. Rheumatoid disease is discussed in greater detail
in Chapter 154. In ankylosing spondylitis, a
solid column of bone below may place excessive stress at the
craniocervical junction. With the attritional effects of inflammation
of the transverse ligament and associated hyperemia on its bony
attachments, atlantoaxial subluxation and dislocation may occur. With
subluxation, the joint may subsequently stabilize in the subluxated
position without significant symptoms.
planning surgery on any patient who might undergo neck manipulation,
either by the anesthesiologist or during the surgery itself. Obtain
routine flexion and extension lateral radiographs of the cervical spine
before performing any such procedures.
symptomatic atlantoaxial instability and the patient is at risk.
Stabilization can be effectively achieved by a Gallie-type posterior
atlantoaxial arthrodesis (12,19,22,23,25).
-
Shape a modified H graft from the iliac crest and contour it to fit over the posterior arches of C-1 and C-2, astride the spinous process of C-2.
-
Pass a single piece of 22-gauge
stainless-steel wire inferior to the spinous process of C-2 and through
the interspinous ligament of C2–C3. Carry the ends of the wire upward
posteriorly to the graft around a notch in its upper border and under
the arch of C-1 on each side of the graft. -
Tie the wire ends posteriorly over the
graft, fixing it into position and pulling back the arch of C-1 into
alignment with the arch of C-2 (Fig. 153.1). Some form of wire tightener is needed, such as that developed by Harris (8). The wire tightener grasps the wire, allowing tension to be applied, so that a knot
P.3961
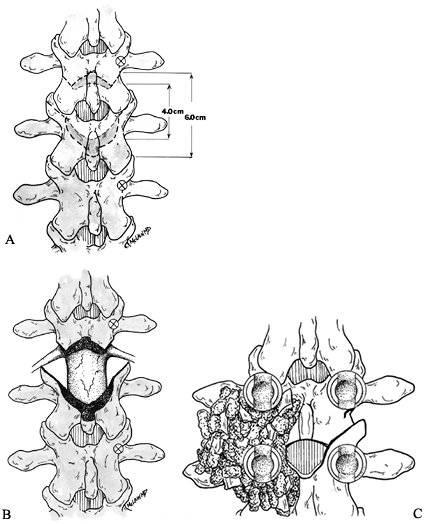
can be tied firmly in the narrow confines of the wound with only gentle manual pressure (discussed in more detail in Chapter 154).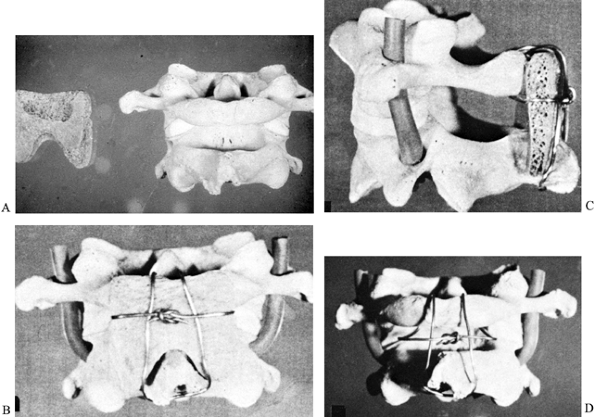 Figure 153.1. A: Skeletal model showing deep cancellous surface of Gallie modified H-graft,
Figure 153.1. A: Skeletal model showing deep cancellous surface of Gallie modified H-graft,
which is contoured from the posterior iliac crest to fit over the
posterior arches of C-1 and C-2, sitting astride the spinous process of
C-2. B: Posterior view showing the graft
in position with cortical surface superficially. Pass the Gallie wire
through the interspinous ligament of C2–C3 below the spinous process of
C-2, upward over the graft on each side. Bring it out below the arch of
C-1 laterally and tie it firmly posteriorly. C:
Lateral view showing the position of the modified H-graft, with the
wire pulling back the C-1 vertebra into a normal relationship with the
odontoid. The inferior portion of the wire passes below the spinous
process of C-2 through the interspinous ligament of C2–C3, which should
be preserved during exposure and wiring because it assists in
maintaining the wire in position until it is tightened. D: Posterior view of model showing the configuration of the wire without the graft in position.
anterior pressure that cannot be reduced, transoral resection of the
odontoid may be required. This procedure is more hazardous than
posterior decompression and stabilization; if the instability is
recognized earlier, the procedure will be less frequently required.
Transoral surgery is reserved for patients with significant anterior
compression.
differentiated: In the first, basilar invagination is present without
gross instability, and in the second there is additional atlantoaxial
instability and luxation. Posterior column involvement also may be
evident in the second type, caused by posterior compression of the
spinal cord by the posterior arch of C-1, which slides anteriorly.
cerebellomedullary cistern is the primary indication for transoral
decompression. Decompression may involve the removal of not only the
odontoid process, but also the body of C-2 and the space-occupying
portion of the clivus. In cases of pure atlantoaxial instability, a
stable posterior atlantoaxial arthrodesis is preferred. Results of
primary anterior decompression combined with simultaneous anterior
fusion are not encouraging, and it would appear preferable to perform a
posterior fusion as a secondary procedure following a primary anterior
decompression.
infection or dental sepsis. Obtain nasal, oral, and pharyngeal swabs
for bacterial culture and sensitivity, and administer the most
appropriate antibiotic combination before surgery, intravenously during
surgery, and in the immediate postoperative period.
-
Position the patient supine for surgery,
with the neck extended. Lateral radiographic control is necessary. Some
recommend routine tracheostomy and endotracheal anesthesia (6). However, the endotracheal tube is not an obstacle at surgery, and in some ways it is best to leave the airway intact. -
In patients who do not have a
tracheostomy, place a gastric tube for postoperative nutrition, with
normal oral nutrition beginning 7–8 days after surgery. If tracheostomy
is indicated, perform it at the beginning of the operation with
ventilation continued through a cuffed tracheal tube. -
After preparation of the oral cavity,
introduce a Whitehead retractor and depress the tongue. Pack off the
nasopharynx and hypopharynx. -
The procedure can usually be done without
division of the soft palate. Fold the soft palate back on itself and
suture it to the junction of the hard and soft palates to expose the
lower portion of the nasopharynx. Release these sutures at the end of
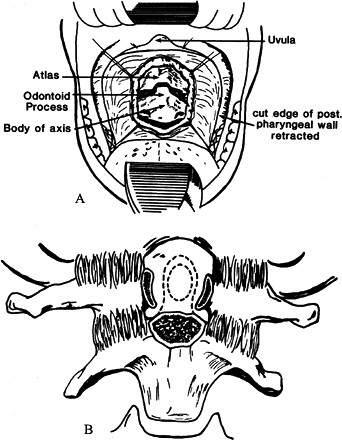
the procedure (Fig. 153.2).![]() Figure 153.2.
Figure 153.2.
Diagram of transoral exposure. Use two stay sutures to fold the soft
palate on itself and keep it in position. Incise the posterior
pharyngeal wall vertically. -
If division of the soft palate is
considered necessary, incise it on one side of the midline to avoid the
uvula, and retract the flaps laterally (Fig. 153.3). Figure 153.3. Exposure showing incision of the soft palate on one side, avoiding the uvula.
Figure 153.3. Exposure showing incision of the soft palate on one side, avoiding the uvula. -
Palpate the posterior pharyngeal wall to
locate the prominence of the anterior tubercle of the atlas. In
atlantoaxial dislocation, it is quite evident. -
Make a 2-inch midline incision from the lower portion of the clivus to the lower portion of C-2.
-
Center the incision one finger breadth below the anterior tubercle of the atlas.
-
Carry the incision down to bone.
-
Strip the soft tissues laterally to the
outer margin of the lateral masses of the atlas and axis. The soft
tissues may be anchored with retraction stay sutures. -
Expose the anterior arch of the atlas and the body of the axis.
-
Remove the anterior arch of the atlas with sharp rongeurs and expose the odontoid (Fig. 153.4).
![]() Figure 153.4. A: Anterior exposure of the atlas and axis with the soft tissues stripped laterally. B: Excision of the anterior arch of the atlas allowing exposure of the odontoid.
Figure 153.4. A: Anterior exposure of the atlas and axis with the soft tissues stripped laterally. B: Excision of the anterior arch of the atlas allowing exposure of the odontoid. -
Remove the odontoid process with a high-speed drill and a diamond burr.
-
Carefully and gently free the odontoid of its soft-tissue attachments, using sharp dissection as necessary.
-
If resection of the odontoid is difficult
because of its elevation and the angle of approach, commence the
resection in the central part of the body of the axis. -
Remove the lower portion of the clivus
(anterior margin of the foramen magnum) only when necessary; when it is
required, I recommend a diamond burr and upward-cutting bone forceps. -
If lateral fusion of the C1–C2 joint is
to be attempted, clear the articular cartilage from the joints and
wedge iliac grafts between the lateral masses. -
Close the posterior pharyngeal wall.
the anterior ligament, the buccopharyngeal fascia and constrictor
muscles, and the pharyngeal mucosa. Many, however, recommend a loose,
single-layer closure because it allows wound secretions to drain more
easily and it has technical advantages (6,7,14). Defer oral feedings 6–7 days to allow adequate healing; introduce liquids before solids. See Chapter 138 and Chapter 154.
disability may arise from destructive changes at the atlanto-occipital
joint. Destructive changes may produce intractable pain associated with
minimal persisting motion, justifying surgical stabilization. On
occasion, subluxation or even rotatory deformity can occur, which may
warrant gradual reduction with halo traction followed by stabilization
with occipitocervical fusion.
iliac grafts fixed to the base of the skull and the upper cervical
spine, reinforced by multiple cancellous bone grafts. The onlay grafts
are contoured to fit over the posterior arches of the upper cervical
vertebrae (usually down to C-4), the cancellous surface of the graft
being contoured to fit over the posterior arches.
-
Apply a cranial halo preoperatively to support the head during surgery.
-
Position the patient supine and prepare and drape the neck well up onto the head and to the ears.
-
Hold the grafts firmly against the skull
and fix them directly to the posterior arches of the cervical vertebrae
using transfixing threaded Compere wires. Drive these wires
percutaneously from the side of the neck, through the graft on one side
of the spine, then through the base of the spinous process, and then
through the graft on the opposite side. Cut the wire free on the side
of its entrance in the wound lateral to the graft. -
Before the grafts are fixed to the upper
cervical spine, pass a wire through drill holes in the skull to fix the
grafts against the skull, which has been denuded; then pass it around
the ends of the threaded Compere wires for fixation to the skull and to
fix the grafts to the posterior arches of the cervical spine. -
Reinforce the main grafts with cancellous strips laterally on both sides and superiorly against the skull (Fig. 153.5).
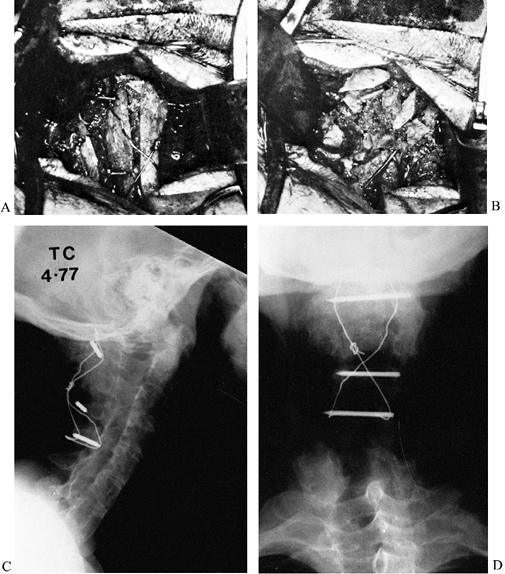 Figure 153.5. Occipitocervical Fusion: A: Posterior operative view showing double onlay cortical and cancellous iliac grafts. B:
Figure 153.5. Occipitocervical Fusion: A: Posterior operative view showing double onlay cortical and cancellous iliac grafts. B:
Operative view showing additional onlay cancellous bone grafts, which
reinforce the main grafts and extend from the cervical spine to the
skull. C: Postoperative lateral radiograph
showing a solid fusion extending from the occiput to the cervical
spine. The wiring passes through the skull. Compere wires fix onlay
grafts to the posterior arches of the cervical spine. D: Postoperative anteroposterior radiograph demonstrating the configuration of the wire loop, Compere wires, and grafts. -
Postoperative immobilization in a well-fitted halo cast is essential to protect the area of fusion from abnormal stress (5).
joints is a typical radiographic feature of ankylosing spondylitis.
This erosive, sclerotic process may occasionally extend into the
intervertebral disc and adjacent bone, and
it is then called spondylodiscitis. Such lesions were first reported by Andersson in 1937 (2).
lesions. The first view is that spondylodiscitis is an inflammatory
process affecting the intervertebral disc and surrounding bone.
Detailed assessment and biopsy specimens support this view. The second,
less commonly held view is that spondylodiscitis is secondary to trauma
with excessive forces localized at one intervertebral segment,
resulting in mechanical destruction and a functional pseudarthrosis.
The erosive process widens the disc space, breaking down the
subchondral bony plates. The surrounding bone becomes sclerotic and
radiodense. The prominence of either erosion or sclerosis varies.
Spondylodiscitis has a reported incidence of 5% to 6% in ankylosing
spondylitis (9,17,18).
Most of the lesions develop in the lower thoracic spine. A little more
than half the lesions are discovered on routine radiographic studies
and are asymptomatic (9). About half the patients with spondylodiscitis present with back pain.
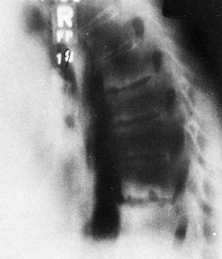 |
|
Figure 153.6.
Lateral radiograph showing the typical lesion of spondylodiscitis in the lower thoracic spine (the most frequent location). Erosive sclerotic changes involve the vertebral endplates adjacent to the disc space. |
responds to conservative management. Surgical stabilization may
occasionally be required for intractable pain, particularly when there
is an associated fracture or disruption of the posterior fused spine
resulting from loss of bony substance anteriorly. If the lesion
presents with intractable pain and no deformity, as in the mid-thoracic
spine, then anterior resection and strut grafting may be indicated. The
involved disc space and adjacent eroded bone are resected, and fibular
or iliac strut grafts are fixed into the defect in a keystone fashion (24). Supplemental rib and iliac grafts are added as necessary.
lower thoracic spine—an area subject to shear stress—with increasing
deformity shifting the weight-bearing line anterior to the lesion,
causing further erosion and increasing pain. In my experience,
successful handling of this lesion requires correction of the overall
spinal deformity. In my experience with more than 20 patients with this
problem, the lesion has healed following resection–extension osteotomy
of the mid-lumbar spine, shifting the weight-bearing line posteriorly,
and changing the direction of the area of spondylodiscitis to
horizontal, thus converting the shear force to a compression force (Fig. 153.7).
This outcome is preferable to multiple anterior and posterior
procedures, which have a higher failure rate when the overall deformity
is not corrected, and which do not alleviate the shear stress.
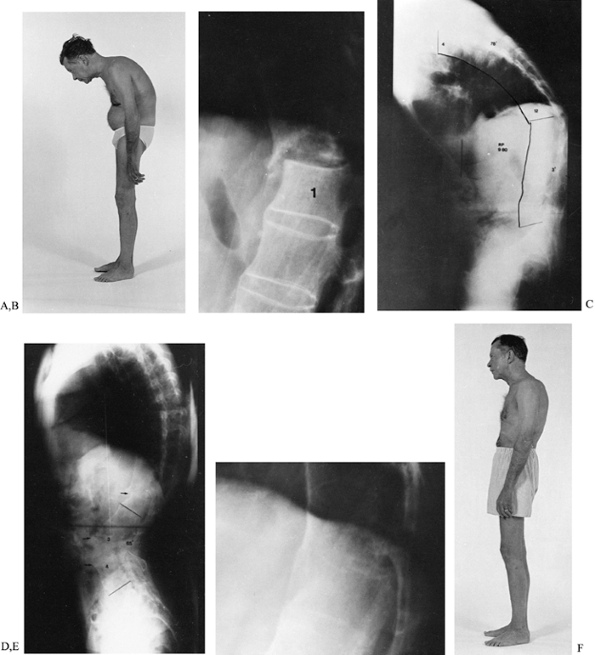 |
|
Figure 153.7. A:
A 58-year-old man had severely painful spondylodiscitis at T12–L1, which resulted in an increasing flexion deformity of the thoracolumbar spine. B: A lateral radiograph of T12–L1 shows gross destructive spondylodiscitis at the apex of the deformity. C: A lateral standing 3-foot radiograph shows the weight-bearing line to be anterior to the area of spondylodiscitis, producing shear stress on the lesion. D: A standing lateral 3-foot radiograph after an extension osteotomy of the mid-lumbar spine shows that the weight-bearing line has been shifted posteriorly, converting shear force to compression force at the site of the spondylodiscitis. E: A lateral radiograph at T12–L1, 4 months after surgery, shows spontaneous healing of the area of spondylodiscitis as a result of the conversion of shear stress to compression. F: The patient following healing of the osteotomy and area of spondylodiscitis. The extension osteotomy of his lumbar spine corrected his deformity and allowed spontaneous healing of the spondylodiscitis. |
previously suggested. In my review of 124 patients referred for
surgical correction of spinal deformity for
whom
radiographs of the entire spine were available for study, 28 (23%) were
found to have spondylodiscitis. When the lesion was in the area of the
deformity and contributed to it, correction of the deformity resulted
in fusion at the site of the spondylodiscitis (20,21).
patients suffering from Marie-Strümpell spondylitis or ankylosing
spondylitis associated with psoriasis. Prevention of deformity by early
recognition and appropriate medical care should be the aim. Despite
improvements in medical care, however, patients still present with
gross disability from advanced kyphotic deformity of the trunk.
extent of deformity, the degree of functional impairment, the general
condition and age of the patient, the feasibility of correction, and,
perhaps most important, the willingness of the patient to accept the
risks and undergo the reconstructive and rehabilitative measures
required for correction.
address the area of involvement. For example, a patient with apparent
spinal deformity actually may have a major deformity in the hip joints.
If a hip flexion deformity is the cause of the patient’s malalignment,
then it should be corrected through surgery on the hip joints. Also,
accurate assessment and measurement of any deformity are required to
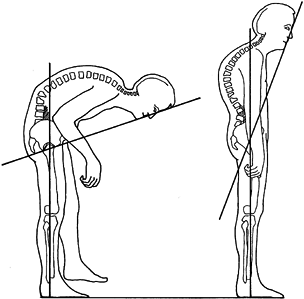
gauge the results of treatment. The most effective and reliable measure
of a spine flexion deformity is the chin-brow-to-vertical angle. It is
a measure of the angle formed by a line from the brow to the chin
through the vertical, when the patient stands with the hips and knees
fully extended, and the neck in its neutral or fixed position (Fig. 153.8).
 |
|
Figure 153.8.
Technique for measuring the degree of flexion deformity of the spine in ankylosing spondylitis. The chin-brow-to-vertical angle is measured from the brow to the chin to the vertical, with the patient standing with hips and knees extended and the neck in its fixed or neutral position. |
type of deformity corrected surgically in arthritic disease, as
reported by Smith-Peterson et al. in 1945 (26).
The initial procedure was done under general anesthesia with the
patient lying prone. Difficulties with the prone position were later
avoided by performing the surgery with the patient on the side, as
recommended by Adams (1).
procedure with surgical division of the longitudinal ligament
anteriorly. I do not find it necessary: Correction can be achieved
through the posterior approach alone.
dilatation and abdominal ileus. As the spine is extended, the superior
mesenteric artery is stretched over the third part of the duodenum,
predisposing to gastric dilatation. A nasogastric tube must be in
position after surgery until intestinal motility is established.
mortality rate of 8% to 10% with this procedure; some degree of
neurologic deficit, including paraplegia, occurred in up to 30% of
patients. Two thirds of the deaths were related to the use of general
anesthesia. As a result, I used my experience in cervical osteotomy, as
well as the recommendations of others, to perform surgical correction
of lumbar flexion deformity with a resection–extension osteotomy under
local anesthesia. From the 1970s to the early 1990s, I used this method
in a series now totaling 100 patients. Generally, correction under
local anesthesia has proved to be a safe, reliable, and practical
procedure (19,21,23).
The main advantage of local anesthesia is that it allows a critical
assessment of the patient’s neurologic and vital functions throughout
the operation.
improvements in anesthesia, including fiberoptic intubation and spinal
cord monitoring. The risks of surgery under general anesthesia have
decreased; therefore, today I use general anesthesia following the
strict guidelines that will be discussed later.
lordosis. There may be some associated increase in thoracic kyphosis,
which can be balanced by overcorrecting the lumbar deformity so that
the chin-brow-to-vertical angle will be normal. This angle is measured
and transposed to a lateral radiograph of the lumbar spine, placing the
apex of the angle at the posterior longitudinal ligament over the L3-4
disc space. The L3–L4 level is selected for correction because it is
the center of the lumbar lordosis and is below the spinal cord. The
L4–L5 level is used only on rare occasions.
assessment, pulmonary function tests, and electrocardiography. Instruct
the patient in deep breathing and extremity exercises, which will be
used postoperatively. Psychological preparation includes explaining to
the patient the procedure and the importance of awake intubation and
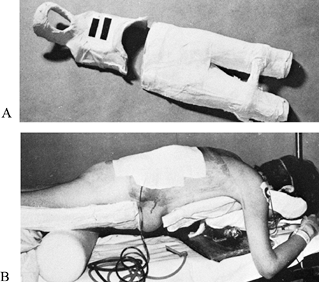
positioning, prior to inducing general anesthesia. Make plaster molds
of the upper and lower halves of the body before surgery. The upper
half extends from the waist up, supporting the chest and head; the
lower half extends from the waist to the knees. These shells are used
for support when the patient is turned prone after the correction is
done. The molds protect the face and allow the anesthesiologist to
administer oxygen by face mask through the opening in the shell (Fig. 153.9).
 |
|
Figure 153.9. A: Cephalad and caudad anterior molds. The cephalad mold supports the head and has an opening for the face. B:
Postoperative view. After correction of deformity, the patient is supported in the prone position by the anterior molds with a jack below the upper mold. Note the restoration of lordosis with correction of the flexion deformity. |
whether the patient has normal spinal canal dimensions. If the patient
has a canal of normal or above-average size, then less bone may be
removed posteriorly with closer approximation of the laminae. However,
if the canal dimensions are fairly small (less than 20 mm on the
anteroposterior diameter) or in the stenotic range, then considerably
more caution must be taken to ensure that the posterior decompression
is generous, particularly superiorly. In this situation, it is best to
leave the central laminectomy area open significantly above and below
to avoid any compression from postoperative edema, buckling of the
dura, or minimal translation. Some indication of adequate canal size
may be noted on a lateral view of the spine; patients with large
foramina and long pedicles have a greater canal size than those in whom
the foramina appear small and the pedicles short. If a narrow canal is
suspected, make accurate computed tomography (CT) canal measurements.
They described a posterior wedge resection of the mid-lumbar spine in a
V fashion. This resection was carried superiorly and laterally through
the superior facet of the vertebra above and the inferior facet of the
vertebra below in an oblique fashion. More bone was resected superiorly
than inferiorly. The oblique plane of the osteotomy was designed to
produce overlap of the posterior elements following correction, in an
effort to prevent displacement. Since the deformity was corrected by
manipulation of the spine after the posterior resection was completed,
the anteriorly longitudinal ligament was fractured. This technique is
the basis for the procedure described next.
-
Have your neuromonitoring technician
apply the spinal cord monitoring electrodes and confirm that the spinal
cord monitoring is satisfactory. -
Have the anesthesiologist intubate the
patient while he is awake and lying on the gurney on which he was
brought into the operating room. When the endotracheal tube is in
position, ask the patient to stand (with assistance) and transfer to a
suitable spinal OR table with his knees and hips flexed. Adjust the
supports to provide comfortable positing for his head, chest, and
pelvis, avoiding any strain on his neck. Ask him to give an OK signal
with his hand when he is comfortable, and then induce general
anesthesia (28). -
Compared to our previous use of local
anesthesia only, general anesthesia makes the posterior bony resection
easier to perform. It is much easier to undercut the pedicles above and
below the area of resection, and a more complete decompression of the
L-3 nerve roots is possible. -
Prepare and drape the back.
-
Make a midline exposure and confirm the
proposed L3–L4 osteotomy site radiographically with towel clips placed
on the base of the spinous processes at the level of the laminae of L-3
and L-4. -
I now routinely use pedicle screw
fixation; therefore the next step is the insertion of the pedicle
screws prior to the performance of the osteotomy. The number of levels
of fixation required depends on bony quality, but usually I place
screws in the pedicles of L-1, L-2, L-3, as well as L5 and S-1 (Fig. 153.10A).![]() Figure 153.10. Resection-Extension Osteotomy, L3–4: A:
Figure 153.10. Resection-Extension Osteotomy, L3–4: A:
The osteotomy is planned to completely decompress the thecal sack in
the midline, with symmetrical lateral resections extending cranially
and laterally. Pedicle screw insertion sites are identified, and screws
may be placed prior to osteotomy. B:
Following decompression, the Smith-Peterson osteotomy provides two
wedges of posterior bone that will lock together when the spine is
placed in extension. Relieve the undersurface of the lamina to prevent
impingement as the spine is extended into its reduced position. C:
Closure of the osteotomy by extension of the spine. Pack resected bone
and autograft posterolaterally over the transverse processes and dorsal
cortex of the osteotomy. Place the fixation rods into the screws and
compress the osteotomy site, locking the wedges together to ensure
stability and reliable arthrodesis. -
Next, perform a V-shaped wedge resection osteotomy at the interlaminar space, as recommended by Smith-Petersen et al. (26), resecting the ossified ligamentum flavum and adjacent laminae.
-
Extend the resection upward and laterally
on each side through the fused posterior joints at L3–L4. The amount of
bone to be removed posteriorly is measured from the radiograph at the
level of the tips of the spinous processes, the laminae, the posterior
aspect of the posterior joints, and inside the canal at the level of
the pedicles. The obliquity of this resection allows locking of the
vertebrae following correction. -
Remove the spinous processes in small strips to be used for grafting. Remove the bone with bone cutters and rongeurs.
-
A power burr may be a useful tool for
cutting slowly through the fused posterior elements toward the spinal
canal, which is opened. The ossified ligamentum flavum is harder and
denser than the vertebral elements. -
Protect the dura with cotton patties. In
many instances, the dura is atrophic and occasionally it may adhere to
the laminae, making separation difficult. -
Completely expose the dural sac laterally on each side to the level of the pedicles.
-
Undercut the laminae above and below to
avoid impingement on the dura following extension. Undercut the
pedicles to avoid any impingement on the third lumbar nerve roots
following extension. -
Remove bone from each side symmetrically, to allow symmetrical closure of the defect (Fig. 153.10B).
-
When the osteotomy is completed, correct
the kyphotic deformity by extending the hips with the adjustments of
the spine table. This will produce an anterior osteoclasis and an
opening wedge anteriorly with closure of the posterior osteotomy.
Perform this maneuver gently and carefully, constantly watching the
neuromonitoring for any evidence of compromise of neurologic function,
and close the osteotomy down to obtain good posterior bone apposition (Fig. 153.10C). -
Full correction must be obtained with the
weight-bearing line shifted posterior to the osteotomy site, so that
gravity will maintain and increase the correction as it heals; it will
also stimulate bone formation across the osteotomy sites of the
resected posterior fusion masses (Fig. 153.11). -
Complete the stabilization by fixing the rods to the pedicle
P.3970
screws using TSRH instrumentation (Sofamor-Danek, Memphis, TN).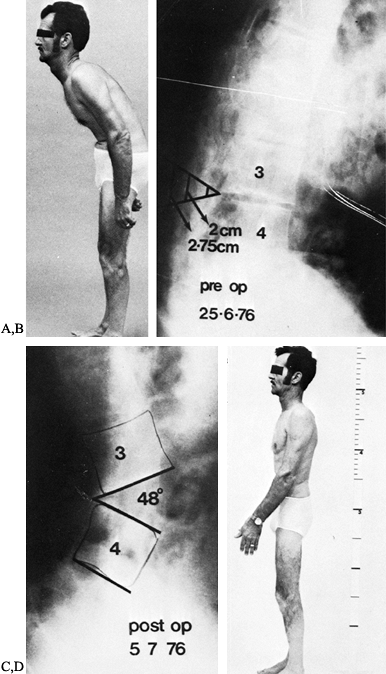 Figure 153.11. A:
Figure 153.11. A:
Lateral view of a patient standing with hips and knees extended. This
patient still had mobility of his cervical spine and was compensating
with the neck hyperextended. When the neck was in the neutral or
comfortable position, he had a chin-brow-to-vertical angle of 45°. B:
Lateral radiograph of lumbar spine showing the chin-brow-to-vertical
angle superimposed with the apex at the L3-4 disc space. The amount of
bone to be resected is indicated at each depth posteriorly. C:
Postoperative lateral radiograph following correction under local
anesthesia showing the angle of correction obtained after closure of
the resected defect posteriorly with an opening osteoclasis at L3-4 of
48°. The weight-bearing line has been shifted posterior to the
osteotomy site. D: Postoperative standing
lateral radiograph showing complete correction of the deformity after
removal of a calculated wedge of bone based on preoperative assessment.
configuration of the osteotomy to provide locking and reasonable
stability. The patients were kept recumbent for 6 weeks, followed by
ambulation in a Minerva plaster cast (19,21,23).
Subsequently, I used internal wire loop fixation for additional
stability and comfort in the early postoperative interval. Before the
advent of pedicle screw fixation, I used a Luque rectangle with
posterior segmental instrumentation and Drummond buttons and wires. The
advantage of internal fixation with segmental instrumentation was that
it decreased the risk of translation and allowed earlier postoperative
mobilization (Fig. 153.12). I now prefer
pedicle screw and rod fixation with the decompression technique just
described, as it allows easier and more liberal decompression; the
rigid internal fixation decreases the risk of displacement and allows
easier and quicker mobilization of the patients, who seem to have less
postoperative pain. The disadvantages of pedicle screw and rod fixation
are increased operating time, and increased risk of neurologic injury
from improper insertion of bone screws because of the altered anatomy
of the pedicles. Overall, however, I feel that the advantages outweigh
the disadvantages, as illustrated by the patient in Figure 153.13.
 |
|
Figure 153.12.
Postoperative lateral radiograph showing Luque instrumentation in position using Drummond buttons and wires, which add stability to the osteotomy. A rectangle is usually used and is bent to the desired angle of correction at the time of insertion. The use of Drummond buttons avoids the necessity to invade the spinal canal, the insertion being done under local anesthesia. |
 |
|
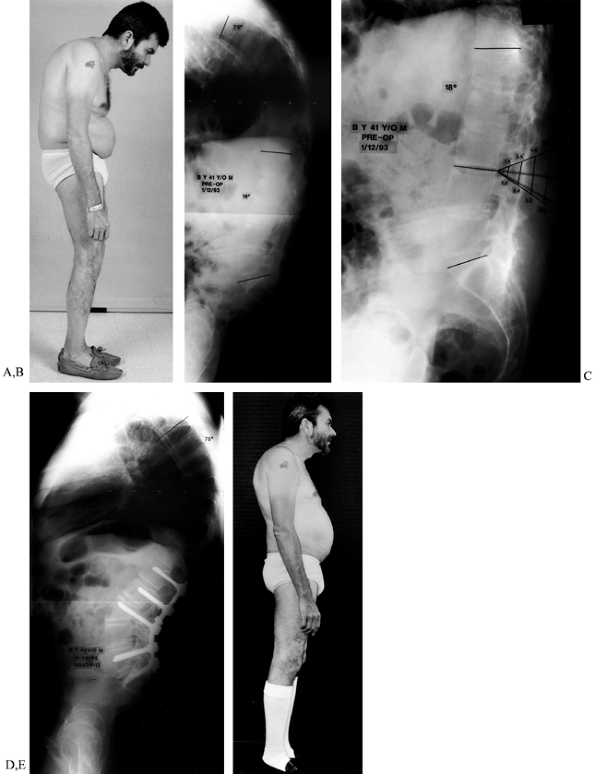
Figure 153.13. A:
This 41-year-old man with a 21-year history of ankylosing spondylitis has had a major kyphotic deformity for 10 years, with a chin-brow-to-vertical angle measuring 55°. He has been unable to work for about 6 years. B: Lateral standing 3-foot radiograph of the spine shows a thoracic kyphosis of 75° and a decreased lumbar lordosis of 18°. The weight-bearing line is well anterior to the mid-lumbar spine. C: Preoperative lateral radiograph of the lumbar spine shows the planned resection/osteotomy of 50° to 55° located at L3–L4. D: This lateral standing 3-foot radiograph, taken 16 months postoperatively, shows the healed lumbar osteotomy with pedicle screw fixation and rods in place. The lumbar lordosis now measures 74°; the thoracic kyphosis is approximately 70° and the spine is in balance. The weight-bearing line is posterior to the osteotomy site. E: The patient 16 months after surgery. He returned to a normal lifestyle. (From White AH, Schofferman JA. Spine Care: Operative Treatment, vol.2. Philadelphia: Mosby, 1995:1678, with permission.) |
-
Close the wound with suction drainage.
-
When the patient is still prone after
wound closure, apply a well-molded posterior plastic shell, before
transfer to a Roto-rest bed. The shell must be rigid and the contour of
the spine must be maintained with adequate support under the pelvis, so
that the rounded hump of the thoracic spine is not pushed forward in
relation to the lumbar spine as the patient rests in the supine
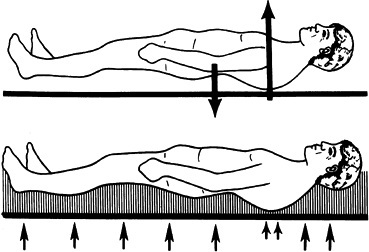
position (Fig. 153.14). The posterior shell is
necessary to support the patient adequately when the Roto-rest trap
door is removed for use of a bedpan.![]() Figure 153.14. A:
Figure 153.14. A:
Lateral diagrammatic view showing the contour of the corrected spine
when lying supine unsupported. The posteriorly projecting thoracic hump
bears most of the weight, with less support on the lumbar spine and the
spine below the osteotomy. As a result, the rigid thoracic hump tends
to be displaced forward and the lumbar spine posteriorly. B:
With the patient supine in a well-molded, rigid shell, equal support is
created throughout the spine, with elimination of any uneven contact
forces that would tend toward displacement.
expelling flatus. About 7–10 days after surgery, when the patient is
comfortable, he is lifted to an orthopaedic table in the posterior
shell. Partly suspend the patient by the lower portion of the shell
while the upper portion and sides are trimmed away, and then immobilize
him in a well-molded plaster jacket. Take care in transferring and
supporting him during application of the jacket, to avoid any loss of
position.
under local anesthesia, was used in 100 patients between 1969 and 1993
without major intraoperative difficulty. Satisfactory correction was
achieved, with restoration of normal functional alignment. There were
no major respiratory complications and there was no pneumonitis in the
series. Three nonunions occurred, one in a patient without internal
fixation who subsequently responded to posterior instrumentation and
fusion. In one patient, the fusion site appeared to be united, but a
fracture of the osteotomy site was sustained in an automobile accident,
requiring anterior strut-grafting. In a third patient who had severe
osteopenia, union was not achieved and subsequent anterior
strut-grafting with allograft and autogenous bone was performed.
suffered a sudden fatal pulmonary embolism 15 days after surgery, at
which time she had been ambulatory in a plaster jacket. Eight patients
developed L-3 root or cauda equina compression within 2 to 14 days
after surgery; all except two compressions occurred in patients in whom
internal fixation with segmental instrumentation was not used. In one
of these, the compression was thought to be vascular in nature—he was
an obese patient (about 300 pounds) who developed vena caval
compression from thrombosis with neural venous stasis.
compression included patients slipping during the course of turning on
a Circoelectric frame, the removal of the trap door on a Roto-rest bed
with the patient not supported in a posterior shell, and in one
patient, manipulation on a table for cast application. In most of
these, estimates of canal dimension had not been done and in retrospect
were probably on the low side. In most of these patients, the
osteoclasis occurred through the body of L-2 rather than through the
disc space, and this would appear to be a risk factor.
is considerably greater than at the vertebral body, selected
preliminary anterior L3–L4 disc division might be considered, but I
have not done this. Patients who presented with these problems were
promptly reexplored and further decompression was carried out (usually
superiorly), with the spine stabilized by internal fixation in the form
of Luque instrumentation and Drummond buttons. On the whole, this
prompt treatment allowed satisfactory recovery.
weight-bearing line is shifted posterior to the osteotomy site, fusion
will occur with a 97% success rate and the correction will be
maintained. Our results using pedicle screw fixation under general
anesthesia are equally good.
reasonably safe, practical, and reliable procedure in this high-risk
type of patient. This technique allows a greater degree of correction
of purely lumbar spinal deformity, in that it produces more
hyperextension at the osteotomy site than can be obtained with multiple
anterior and posterior osteotomies using compression instrumentation
(either
Harrington
or Zielke) of the thoracolumbar spine. The range of osteotomy
correction in our patients was from 40° to 104° (average, 56°). The
preoperative chin-brow-to-vertical angle varied from 35° to 134° (the
latter was in a patient with associated hip deformity), with an average
of 60°. The postoperative chin-brow-to-vertical angle ranged from – 5°
to +15°, with an average of +15° (Fig. 153.11).
to the opening wedge technique just described. In this approach, carry
out the dorsal osteotomy and decompression as described, but complete
the anterior correction through the posterior approach by removing the
vertebral pedicles, and by decancellating and collapsing the vertebral
body to correct the sagittal alignment. Then stabilize corrected
segments with a pedicle screw fixation construct.
-
Position the patient prone as described,
and use the routine midline longitudinal dorsal approach to the
thoracic and thoracolumbar spine. -
Begin by removing the spinous processes and laminae over the L2–L3 interspace, cutting a wedge-shaped defect posteriorly.
-
Isolate and remove the pedicles and
transverse processes of L-2, carefully resecting the pedicles until
they are flush with the back of the vertebral body. -
Working through the pedicles, use curets
and pituitary rongeurs to break up and remove the cancellous bone from
within the vertebral body. -
Use curved curets or a burr to remove cancellous bone from between the pedicles and along the dorsal cortex of the body.
-
Take care not to breach the anterior cortical wall.
-
Place pedicle screws at adjacent levels before attempting the final correction.
-
Use a blunt impactor to fracture the
posterolateral cortex of the body, then score the dorsal cortex with an
osteotome and implode it into the vertebral body cavity, away from the
thecal sack. -
Slowly hyperextend the operating table,
or bring the patient’s legs into extension to collapse the dorsal
aspect of the vertebral body and close the dorsal osteotomy. -
Instrument the spine from L-4 to the thoracic spine and graft for fusion (Fig. 153.15).
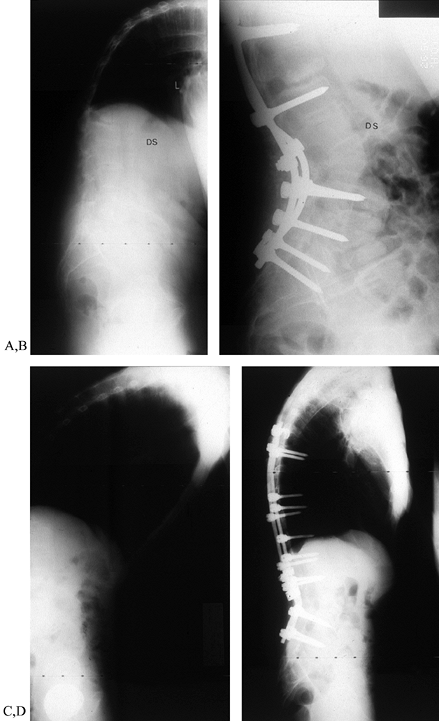 Figure 153.15. A:
Figure 153.15. A:
Lateral view of a 50-year-old man with multilevel deformity. Severe
cervical deformity was corrected prior to treating the thoracolumbar
kyphosis. He also suffered from a persistent thoracolumbar
pseudarthrosis. An L-2 decancellation and pedicle reduction osteotomy
was carried out, fully correcting sagittal balance. B: Posterior segmental instrumentation and grafting provided immediate stability and a solid fusion. C: A 58-year-old man with severe, fixed deformity, localized primarily to the thoracolumbar junction. D:
After “eggshell” osteotomy of L-2, posterior instrumentation was
extended from the upper thoracic segments to L-5. Sagittal correction
was excellent and well maintained. The patient’s visual horizon was
restored to normal. -
Place the patient in a molded thoracolumbosacral orthosis for 4–6 months after surgery.
deformity associated with ankylosing spondylitis. It is much less
common for the only deformity or primary deformity to be in the
thoracic region, requiring the correction to be confined to that area.
In the first group, the main or primary deformity is in the thoracic
spine, but in addition there is a loss of lumbar lordosis. If the
thoracic kyphosis is mild or moderate and the lumbar spine rigid and
flattened, the overall deformity can be satisfactorily corrected by a
compensatory osteotomy in the midlumbar spine. With sufficient
extension of the lumbar spine, the thoracic kyphosis can be compensated
for with restoration of spinal balance and a normal
chin-brow-to-vertical angle.
normal or exaggerated cervical and lumbar lordosis. These patients
require correction of the primary thoracic deformity. It is impossible
to do this safely with a single major angular correction. Correction of
purely thoracic deformity requires multiple anterior and posterior
intervertebral osteotomies, instrumentation, and grafting.
and lumbar lordosis can be further subdivided into two subgroups,
depending on the rigidity of the thoracic spine. In the first subgroup,
with incomplete ossification of the thoracic spine or extensive areas
of destructive spondylodiscitis, preliminary correction can be obtained
by halo-dependent traction; this is followed by multiple posterior
resection osteotomies and compression instrumentation with second-stage
anterior resection of the areas of spondylodiscitis and the disc
spaces, with supportive strut-grafting (Fig. 153.16).
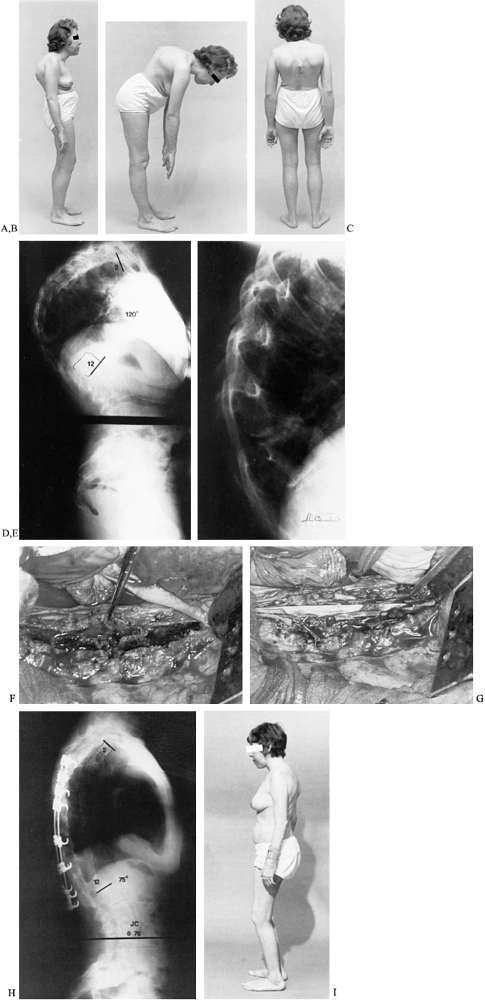 |
|
Figure 153.16. A:
Lateral view of a 32-year-old woman with severe kyphosis confined to the thoracic region, associated with ankylosing spondylitis and steroid therapy. Patient had lost 6 inches in height, and her ribs impinged against the pelvis. B: Lateral bending view showing angular kyphotic deformity. C: Posterior view showing impending skin breakdown. D: Standing lateral radiograph showing thoracic kyphosis of 120° with increased lumbar lordosis. E: Lateral radiograph showing destructive spondylodiscitis at the apex of the thoracic deformity, indicating that it is likely to be flexible. The patient was treated with halo-dependent traction to reduce the main deformity, followed by posterior joint resections with bilateral Harrington compression instrumentation and fusion. F: Operative view at second-stage anterior procedure (following previous posterior instrumentation) showing resection of areas of spondylodiscitis with a trough prepared from T-6 to T-11 for a fibular strut graft. G: Operative view showing the fibular strut graft locked into the spine from T-6 to T-11 with onlay rib grafts. H: Postoperative standing lateral radiograph showing posterior Harrington compression instrumentation with fibular strut grafts supporting the spine anteriorly. I: Postoperative standing lateral view of the patient showing correction of the deformity. She had regained 5 inches in height. |
with rigid thoracic kyphosis and relatively complete ossification.
These patients require a first-stage anterior transthoracic procedure.
-
Approach the thoracic spine through a
right-sided thoracotomy with removal of the rib at the level of the
apex of the deformity in the midaxillary line. -
Reflect the pleura and resect the
ossified disc spaces completely from right to left, anterior to the
posterior longitudinal ligament. Thoroughly curet the disc spaces and
pack them with autogenous bone, using portions of the removed rib
supplemented by iliac crest bone where necessary. -
Apply halo-dependent traction after surgery.
-
Perform the second-stage procedure about
7–10 days later using a posterior approach. Perform multiple V-shaped
resection osteotomies at each level, resecting the
P.3974P.3975P.3976P.3977
ossified
ligamentum flavum and adjacent portions of the laminae upward and
outward on each side through the intervertebral foramen, removing
enough bone to allow adequate correction following closure. -
Apply bilateral segmental compression instrumentation, gradually closing the osteotomy sites and correcting the deformity (Fig. 153.17).
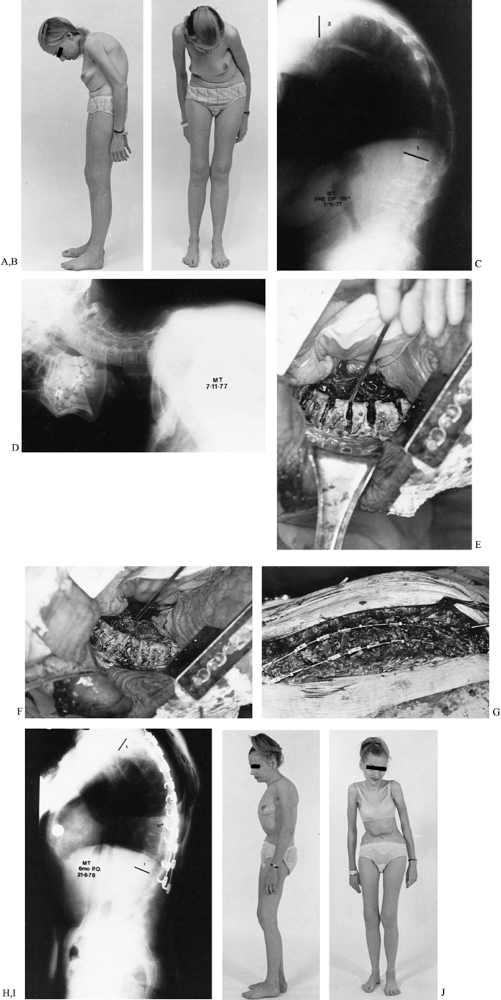 Figure 153.17. A:
Figure 153.17. A:
Lateral view of a 34-year-old woman with rigid thoracic kyphosis and
normal lordosis of the cervical and lumbar spines. The patient had lost
5 inches in height. B: Anterior view of
the patient showing restricted field of vision with rib impingement on
the pelvis. Pulmonary function was reduced to 54% of predicted normal. C: Preoperative standing lateral 3-foot radiograph of the spine showing thoracic kyphosis of 110° with normal lumbar lordosis. D: Lateral radiograph of the cervical spine showing normal or increased cervical lordosis. E:
Operative view showing anterior transthoracic resection of the ossified
disc spaces completely through, from one side to the other. F: Operative view showing rib grafting of resected ossified disc spaces. G:
Operative view showing multiple posterior V-shaped osteotomies, which
were followed by bilateral posterior Harrington compression
instrumentation with fusion. The ossified ligamentum flavum has been
resected at each level, passing upward and laterally through the fused
posterior joints. The margins are undercut before the compression
instrumentation was performed. H:
Postoperative lateral standing 3-foot radiograph showing correction of
major deformity with restoration of normal spinal alignment. I:
Postoperative lateral view of patient showing correction of the
thoracic kyphosis with the ribs being lifted out of the pelvis. J: Postoperative anterior view of the patient showing restoration of normal field of vision.
-
Perform multiple osteotomies in the
thoracic region so that correction at any one level is minimal, yet the
cumulative effect allows substantial improvement. -
Anterior and posterior osteotomies with
interval traction produce some of the correction while the patient is
awake, reducing neurologic risk. -
Place the posterior instrumentation using
spinal cord monitoring to allow reasonable correction in a critical
area with greater safety. -
The technique of multiple, two-stage
anterior and posterior osteotomies with posterior instrumentation is
the obvious choice for primary thoracic deformity with a normal
cervical and lumbar lordosis. -
Resection–extension osteotomy of the
mid-lumbar spine is ideal for purely lumbar kyphosis with a normal or
reduced thoracic kyphosis.
osteotomies under general anesthesia in two procedures are more
hazardous than a single-stage extension correction of the lumbar spine
under local anesthesia. Tracheostomy, with its attendant problems, may
be required in the management of patients with kyphotic deformity.
However, where the primary deformity is thoracic with a normal lordosis
above and below, these risks must be accepted if the deformity is to be
corrected.
instrumentation systems have been used in the past, current segmental
instrumentation systems offer more reliable correction and fixation. I
favor the TSRH system, which provides a variety of pedicle-screw
fixation options that allow the surgeon to tailor the instrumentation
construct to the individual. Other systems are available that provide
similar features. The surgeon should be well versed in the technical
features of any selected instrumentation before undertaking this sort
of reconstruction.
-
In kyphosis procedures, place pedicle screws above and below the selected level before performing the osteotomy.
-
Use fluoroscopy or plain radiography to
confirm position and alignment during placement. I use spinal cord
monitoring in all patients done under general anesthesia, and I
stimulate the screws to confirm safe placement during instrumentation. -
After the osteotomy is completed, extend
the patient’s hips until the osteoclasis occurs, then place contoured
fixation rods into the screws and fix them in place. -
Pack milled bone graft along the lateral
gutters and across the osteotomy site. Place a well-molded plaster
shell over the patient’s back before rolling him off the operating
table. This will prevent excessive pressure over the dorsal kyphosis in
the immediate postoperative period, and it will be converted to a
plaster cast when abdominal distension resolves.
deformity of the spine occurs primarily in the cervical region. This
deformity can be severely disabling, with restriction of the field of
vision, and it may progress to the point where it interferes with
opening of the mouth. Surgical correction is fraught with hazard.
Anecdotal reports of isolated attempts to correct this deformity under
general anesthesia indicate a high rate of disastrous complications
including death.
of deformity, it is important that the surgeon clearly understand the
principles related to its possible correction and its indications. Over
the past 30 years, I have routinely used a technique of correction
under local anesthesia that has allowed a consistent, satisfactory, and
somewhat dramatic correction with relative safety (3,4 and 5,7).
ankylosing spondylitis require cervical osteotomy, as the underlying
problem is fracture. Those who present with a recent onset of a painful
flexion deformity often do not require osteotomy, as, again, an
underlying problem is fracture. In fact, any patient with ankylosing
spondylitis whose spinal alignment has been relatively unchanged over
time and who has had little pain, who then experiences painful,
progressive flexion deformity after minor trauma, has a fracture of the
cervical spine until proven otherwise. The fracture is usually at the
base of the neck at the cervicothoracic junction. Unfortunately, the
pain is often attributed to the patient’s disease.
resembles a fracture of an osteoporotic tubular bone, with a transverse
shear pattern. The fracture is difficult to recognize
radiographically,
being obscured by the shoulders, and it may vary in its location from
C-6 to T-2, although most commonly it is in the area of C-7 or T-1.
compression and collapse anteriorly, with the chin approaching the
chest. The patient is aware that the position of the head varies during
the day, being more elevated on waking in the morning and dropping more
toward the chest after being ambulatory during the day. The patient may
hold the head with the hands to ease the distress.
tomography at the cervicothoracic junction. Patients with this fracture
do not require cervical osteotomy. Apply a cranial halo and initiate
traction along the line of the deformed neck. Then slowly restore
normal alignment under careful observation until the head is restored
to its normal functional position. It is usually possible to obtain a
fairly normal chin-brow-to-vertical angle, after which place the
patient in a well-molded halo cast for 4 months. The halo cast is
essential: A halo vest does not provide adequate immobilization.
cervical spine where lesions may occur that cause painful flexion
deformities. Destructive arthritis at the atlanto-occipital joint may
cause the patient to flex the neck at this joint with the chin held
downward, while the lateral radiograph of the cervical spine shows a
relatively normal lordosis. Correct this by graduated halo traction to
restore a normal chin-brow-to-vertical angle, followed by posterior
stabilization, usually with occipitocervical fusion and, where
necessary, excision of the posterior fragmented arch of C-1.
cervical spine ultimately heal, at which time the pain disappears,
leaving the patient with a painless, fixed flexion deformity. At this
stage, osteotomy is required for correction.
osteotomy, 36% have shown evidence of previous cervical fracture. In
31%, the fracture contributed significantly to the spinal deformity. In
only 15% of those who presented with evidence of fracture had the
fracture been diagnosed previously. This experience underscores the
fact that early recognition of the fracture and adequate immobilization
are essential if the risk of further deformity is to be avoided.
preoperative radiographs. Measure the chin-brow-to-vertical angle and
transpose it to a lateral radiograph of the cervical spine with the
apex of the angle at the posterior margin of the C7–T1 disc space. A
lateral tomogram probably will be necessary to show this clearly.
Center the angle over the posterior arch of C-7. The amount of bone to
be resected is determined from the radiograph with the angle
superimposed over the spinous processes, the laminae, the posterior
margin of the facet joints, and the posterior margin of the spinal
canal at the level of the pedicles. The lines of resection are beveled
upward at the superior margin and downward at the inferior margin.
Following correction, the two surfaces will be parallel and in
apposition.
rigid plaster or fiberglass body jacket incorporating the supports for
the halo unit. The jacket must be rigid and made of a material that
will not soften with body heat. It must be very skillfully contoured to
the patient’s trunk so that it cannot slide up or down. It must be
molded under the rib cage and the sides of the chest to prevent it from
going upward, and over the pelvis and iliac crests to prevent it from
moving distally.
postoperative immobilization required for these patients. In most
instances, because the spine is completely solid above and below the
area of osteotomy, a flexible jacket creates excessive forces that tend
to cause movement at the osteotomy site with almost any activity. If
the patient is not rigidly immobilized, he will have extreme distress
when trying to get in or out of bed or to move about. Excessive
mobility can even result in some neurologic compromise with irritation
of the C-8 nerve root. Apply the jacket and test it carefully with the
patient up and about to make certain that it is well contoured and
secure; do this far enough before the surgery that there is time to
make any necessary adjustments. Fit a halo to the skull preoperatively
under local anesthesia in a stable position, below the maximum
circumference of the skull.
perform the operation under local anesthesia with the patient in the
sitting position. Use a dental-type chair, so that the patient can be
placed in a recumbent position if necessary. Having the patient awake
avoids any major anesthetic hazards, and allows accurate monitoring of
neurologic and other vital functions. The patient can assist with
anatomic localization of the level during the decompression by
indicating at any time any paresthesias or discomfort along the
distribution of a cervical nerve root: This ability is of real value in
confirming the location of the C-8 nerve root canal and the level of
the root.
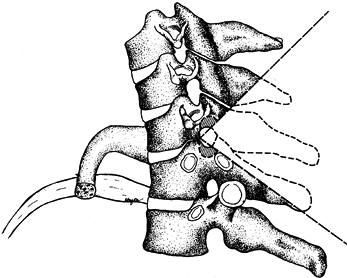
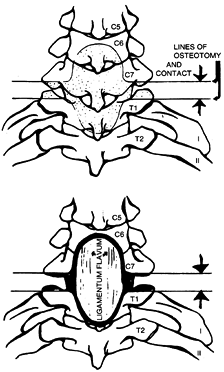
junction. The preferred level for correction is between C-7 and T-1 (Fig. 153.18), because, as Mason et al. (11) and Urist (27)
have indicated, this interspace is more receptive to surgical treatment
than any other level of the cervical region. The advantages are many:
 |
|
Figure 153.18.
Diagrammatic posterior view of the area of resection for cervical osteotomy. Bevel the margins of the resection of the lateral fused joints slightly away from each other extending posteriorly, so that after correction the two surfaces are parallel and in apposition. Undercut the pedicles significantly to avoid impingement on the C-8 nerve roots. Bevel the midline resection on its deep surface above and below to avoid impingement against the dura after extension correction. (From Simmons EH. Surgery of the Spine in Rheumatoid Arthritis and Ankylosing Spondylitis. In: Cruess RL, Mitchell NS, eds.Surgery of Rheumatoid Arthritis. Philadelphia: Lippincott, 1971, with permission.) |
-
The spinal canal is relatively wide.
-
The cervical cord and C-8 nerve root have reasonable mobility in this area.
-
Any weakness caused by compromise of the C-8 nerve root results in less disability than with other roots.
-
The vertebral artery and veins usually
pass in front of the transverse process of C-7 and enter the transverse
foramen at the sixth vertebra. -
The position of these vessels above the level of T-1 protects them from injury during osteotomy at the C7–T1 level (Fig. 153.19).
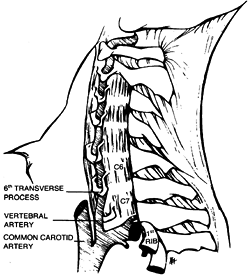 Figure 153.19.
Figure 153.19.
Lateral anatomic diagram showing the normal passage of the vertebral
arteries and veins in front of the transverse process of the seventh
vertebra, entering the transverse foramen at the sixth vertebra.
(Simmons EH. Surgery of the Spine in Rheumatoid Arthritis and
Ankylosing Spondylitis. In: Cruess RL, Mitchell NS, eds.Surgery of Rheumatoid Arthritis. Philadelphia: Lippincott, 1971, with permission.)
exposure carried out posteriorly under local infiltration (1% lidocaine
and epinephrine 1:200,000). Use fentanyl for supplementary analgesia
during the procedure.
-
Identify the last bifid spinous process,
which is usually C-6. Compare the architecture of the lower cervical
spinous processes using a lateral radiograph of the cervical spine. If
you encounter any difficulty in anatomic localization, obtain
radiographic confirmation of the level. -
Remove the C-7 spinous process, the
inferior spine of C-6, and the superior spine of T-1 in strips and
preserve them for grafting. Remove the entire posterior arch of C-7
along with the inferior half of the arch of C-6 and the superior half
of the arch of T-1 (Fig. 153.20).![]() Figure 153.20. Lateral diagrammatic outline of the area of posterior resection at the C7–T1 level. The shaded areas represent where the pedicles are undercut to avoid nerve root impingement after extension correction.
Figure 153.20. Lateral diagrammatic outline of the area of posterior resection at the C7–T1 level. The shaded areas represent where the pedicles are undercut to avoid nerve root impingement after extension correction. -
Open the spinal canal. Protect the dura and spinal cord with cotton patties.
-
Extend the decompression laterally on
each side beyond the lateral margin of the spinal cord to the level of
the pedicles. Undercut the laminae above and below the decompression to
avoid impingement following extension correction. -
Identify the C-8 nerve root and pass a
curved probe into its canal. The patient may be able to assist in
confirmation of the level by indicating the distribution of any
paresthesias associated with displacement of the root. Extend the
resection through the fused area of the posterior joints of C7–T1,
decompressing the C-8 nerve root completely. -
Expose the inferior aspect of the pedicles of C-7 and the superior aspect of the pedicles of T-1.
-
Cut the pedicles through in a curved
fashion away from the C-8 root, leaving a shell to protect the nerve
root while the main bone is removed; the shell is removed
P.3982
at
the completion of the nerve root decompression. This is done to
undercut the pedicles above and below adequately, so that after the
extension correction, there will be a bony recess for the eighth nerve
root to avoid impingement or a pincer effect. -
Follow the nerve root laterally, and remove all bone that could impinge on it.
-
Resect the lateral masses completely
through from medial to lateral so that there is no remaining bridge of
bone laterally that could interfere with extension correction (Fig. 153.21A).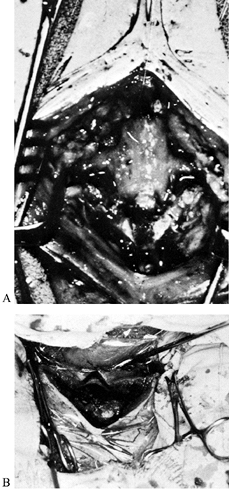 Figure 153.21. A:
Figure 153.21. A:
Posterior operative view showing midline decompression with resection
of lateral masses, decompression of C-8 nerve roots, and undercutting
of pedicles. B: Posterior operative view
of wound after anterior osteoclasis and extension correction. The
lateral masses have come together posteriorly on each side. The wound
has been transformed from a vertical configuration to a transverse one. -
Insert the deep closing sutures and a suction drain before the osteoclasis is done.
-
Have the anesthesiologist give
supplemental oxygen during the procedure, either by nasal catheter or
face mask. The patient is allowed to listen to music from a radio or
tape recorder. This is an important part of the anesthetic management,
along with a continuing, cheerful conversation between the
anesthesiologist or an attendant and the patient. When this is done
well, the amount of discomfort or concern expressed by the patient can
be minimized. -
Complete the decompression. The patient is given a small dose of a short-acting barbiturate, such as brevitol.
-
When the anesthesiologist indicates the
sedation is effective, extend the neck by grasping the halo firmly and
tilting the neck backward. An audible snap may be heard and a physical
sense of fracture will be appreciated. -
Extend the neck until resistance occurs. The lateral masses can be palpated as they come together posteriorly on each side (Fig. 153.21B).
-
The patient is allowed to awaken almost instantly and can confirm normal neurologic function of the extremities.
-
Hold the head firmly in the corrected
position while an assistant stabilizes it by connecting the anterior
supports for the halo unit to the cast. -
Avoid overcorrection, particularly in
patients with a rigid cervical spine and no compensatory movement at
the occipitocervical junction. The final position should effect a
compromise for the patient between looking ahead for walking and being
able to work at a desk (Fig. 153.22).![]() Figure 153.22. A:
Figure 153.22. A:
Posterior view of a male patient with a severe rigid flexion deformity
of the cervical spine. His head is not visible from the posterior
aspect. B: Lateral view of the patient
showing marked restriction of the field of vision. His chin is rigidly
fixed against his chest, interfering with his ability to open his
mouth. C: Anterior view showing complete restriction of his field of vision. D: Lateral view of the cervical spine showing ossification of posterior joints with previous subluxation of C6–C7. E: Postoperative lateral radiograph of the cervical spine showing extension–resection osteotomy correction. F: Postoperative anterior view of the patient in a halo cast, showing return of his normal field of vision. G:
Postoperative lateral view of the patient after union of the osteotomy,
demonstrating return of normal chin-brow-to-vertical angle. H: Postoperative posterior view of the patient demonstrating return of normal head–trunk configuration. -
Place the bone that has been removed
during the course of the decompression posterolaterally on both sides
over the apposed lateral masses; do not place it in the midline over
the exposed dura. -
Tie the deep sutures after extension correction, and complete the wound closure.
if there has been an adequate and complete decompression posteriorly.
If the spine does not fracture readily, check to be sure there is not a
bridge of bone remaining laterally and that the correct level has been
operated on.
initial procedure, correction of a severe deformity is sometimes
limited by tightness of the anterior musculature, or by the patient’s
apprehension that the deformity is going to be overcorrected. In this
case, most of the correction can be established at the time of surgery,
with further correction added 7–10 days later by adjustment of the
halo-jacket. By this time, the soft tissues will have had an
opportunity to stretch and the patient will have had an opportunity to
assess the amount of correction that has been achieved. With the
patient supine under diazepam and fentanyl sedation, the head may be
supported by the surgeon, and the attachments for the halo released,
and the neck allowed to extend to obtain full correction.
immediate postoperative period. It allows the patient to be brought to
a vertical position fairly easily, to stand and walk about and then
return to the recumbent position without difficulty. When sufficiently
mobile to get in and out of a regular bed, the patient is transferred
to a hospital bed with or without a trapeze attachment.
Immobilize
the patient in a halo cast for 4 months, then remove the cast and
perform careful radiographic studies, including lateral tomography
centered at C7–T1. When there is radiographic evidence of union and
clinical evidence of stability and no pain, remove the halo. Have the
patient wear a skull-occiput-mandibular immobilizer (SOMI) brace for at
least 2 more months or until it is certain that there is solid union,
clinically and radiographically.
major deviations over the past 20 years in a total of 130 patients. The
results have been exceedingly satisfactory, with a minimum of
complications, considering the nature of the deformity and the
associated disease process. Nonunion occurred in four patients (3%).
Three of these responded to anterior cervical fusion at the C7–T1 level
with an iliac crest graft. The fourth patient required not only
anterior cervical fusion, but also posterior segmental instrumentation
and fusion to obtain solid union. Transient neurologic complaints
occurred in 16 patients, including 13 patients with transient C-8
paresthesias, one with Horner’s syndrome, one with mild central cord
syndrome 2 weeks after surgery, and one with transient paresis of the
ninth and tenth cranial nerves thought to be secondary to traction. All
of these cleared spontaneously without treatment. Five other patients
had persistent C-8 nerve root deficits; one of these underwent further
decompression, with subsequent improvement. All improved with minimal
residual signs and no gross functional handicap.
cord. One dramatic intraoperative experience demonstrates the necessity
for surgery performed under local anesthesia. A 70-year-old man had a
severe flexion deformity accentuated by an unrecognized fracture that
had healed in such a way that his chin rested on his chest. On
posterior decompression, dense scarring was noted about the dura
related to the previous fracture. As the spine was decompressed in the
midline, the patient suffered increasing weakness of his lower limbs
and had difficulty with speech.
it. In view of this tension, it was split longitudinally down to the
arachnoid. As this was done, there was an immediate, dramatic return of
neurologic function in the lower extremities, and the patient’s speech
returned to normal. The operation was continued and as the
decompression was being completed on both sides, he again developed
weakness of his lower extremities. The remaining exposed dura was split
further distally, again with immediate recovery of neurologic function.
The operation was completed without any further difficulty and the
patient went on to a satisfactory result without neurologic deficit.
describing the results of laminectomy for cord compression associated
with kyphoscoliosis. They related the compressive effect of the dura to
the kyphosis and recommended that the dura be split both longitudinally
and transversely. Surgeons undertaking this type of surgery should be
familiar with this recommendation. If this problem should occur during
surgery, adequate splitting of the dura should be done longitudinally
and transversely. When incising the dura, take care not to violate the
arachnoid; cerebrospinal fluid leakage is thus avoided. If leakage
should occur, it can be stopped with a sheet of Gelfoam or a free fat
graft.
following the osteoclasis. However, a sudden cardiac arrest occurred in
one patient toward the end of the decompression. The chair was
flattened and the patient responded to resuscitation without late
sequelae. The cause of this arrest remains unknown. Air embolism was
considered, but aspiration of the heart showed negative for it. Air
embolism is a possibility with surgery in the sitting position, and a
doplar is now routinely used over the chest and care is taken to
control venous bleeding.
embolism 21 days after surgery; her lungs showed multiple previous
areas of subclinical embolism. Another patient suffered a fatal
pulmonary embolism before the osteotomy was done. Autopsy revealed
multiple thrombi in his leg veins, with evidence of previous pulmonary
infarctions. Other complications have been related to the age of the
patient and associated disease processes, including one nonfatal
pulmonary embolism, a perforated peptic ulcer, and myocardial
infarction.
patients, the results and complication rates compare favorably with any
other type of major reconstructive procedure in a similar group of
patients with the same disease process. These patients have an
increased tendency for peptic ulceration. Considering the lethal nature
of any intra-abdominal catastrophe in these patients, who breathe
entirely with the diaphragm, I now place all patients routinely on
cimetidine.
correction was 60°. In 15 patients, cervical osteotomy was combined
with lumbar osteotomy for major deformity in both areas; the procedures
were performed either on separate admissions or during the same
hospitalization. When you plan to repair severe deformity in both areas
during the same admission, do the cervical osteotomy first, followed by
lumbar osteotomy 1–2 weeks later. Both procedures are done under local
anesthesia (Fig. 153.23).
 |
|
Figure 153.23. A:
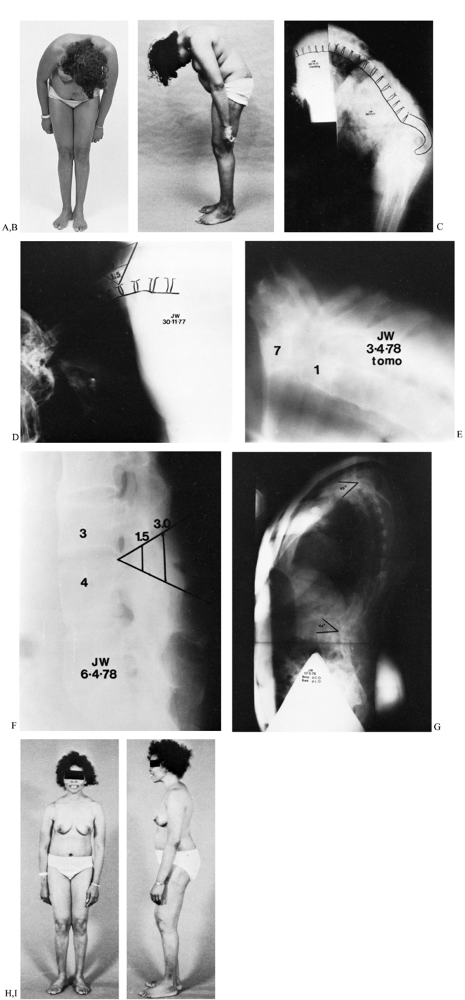
Anterior view of a 43-year-old woman suffering combined neck and lumbar flexion deformities with severe restriction of field of vision. She had suffered arthritic disease since her teens. B: Lateral view demonstrating severe flexion deformity of the cervical spine combined with flexion deformity of the lumbar spine. Patient had undergone total hip replacement arthroplasties with some residual hip flexion deformity. C: Composite lateral radiograph showing combined neck and lumbar flexion deformities. D: Lateral radiograph of the cervicothoracic spine showing the plan of the extension osteotomy. E: Postoperative lateral tomogram showing resection–extension osteotomy at C7–T1. F: Planned resection–extension osteotomy of the flattened lumbar spine, which was performed 6 weeks after cervical osteotomy. Both procedures were performed under local anesthesia. G: Standing lateral 3-foot radiograph of the spine showing cervical and lumbar extension osteotomies. The weight-bearing line is shifted posterior to the lumbar osteotomy site. H: Postoperative anterior view demonstrating normal field of vision. I: Postoperative lateral view showing complete correction of spinal deformities with restoration of normal chin-brow-to-vertical angle. |
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
W, Duff IF, Preston RE, et al. The Cervical Spine and Rheumatoid
Arthritis: Correlation of Radiographic Clinical Manifestations
(abstr.). Arthritis Rheum 1964;7:326.