The Cervical Spine
the pediatric cervical spine are simply a reflection of aberrant growth
and developmental processes. This chapter discusses these diseases and
anomalies in this framework. A basic knowledge of the normal
embryology, growth, and development of the pediatric cervical spine is
necessary in order to understand these conditions. Most of the
anomalies and diseases involving the pediatric cervical spine are
easily divided into those of the upper (occiput, C1, C2) and lower
(C3-C7) segments.
somites. All definitive vertebrae develop from the caudal sclerotome
half of one segment and the cranial sclerotome half of the succeeding
segment (1). These areas of primitive
mesenchyme separate from each other during fetal growth, then undergo
chondrification and subsequent ossification. Chondrification and
ossification are passive processes that follow the blueprint laid down
by the mesenchymal anlage. Because of this sequencing, the cranial half
of the first cervical sclerotome remains as a half segment between the
occipital and the atlantal rudiments and is known as the proatlas.
The primitive centrum of this proatlas becomes the tip of the odontoid
process, and its arch rudiments assist in the formation of the
occipital condyles (2). The vertebral arch of
the atlas separates from its centrum, becoming the ring of C1; the
separated centrum fuses with the proatlas above and the centrum of C2
below to become the odontoid process and body of C2. The axis forms
from the second definitive cervical vertebral mesenchymal segment. The
odontoid process is the fusion of the primitive centra of the atlas and
the proatlas half-segment. The posterior arches of C2 form only from
the second definitive cervical segment.
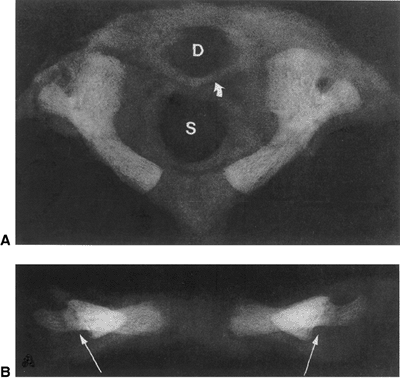 |
|
Figure 22.1 A:
Cross-sectional radiograph of C1 in a full-term neonate. The posterior ossification centers are present. No ossification is present in the anterior cartilage. The transverse ligament (arrow) separates the dens (D) from the spinal canal (S). B: Anteroposterior radiograph of C1 in a full-term neonate. (From Ogden JA. Radiology of postnatal skeletal development. XI. The first cervical vertebrae. Skeletal Radiol 1984;12:12–20.) |
and the two neural arches. The axis comprises four main components: the
body, two neural arches, and the odontoid (or five components, if the
proatlas rudiment is also considered) (Figs. 22.1 and 22.2).
A portion of the mesenchyme from the sclerotomal centrum creates two
neural arches that migrate posteriorly and around the neural tube. This
eventually forms the pedicles, the laminae, the spinous processes, and
a very small portion of the body. Most of the body is formed by the
centrum. An ossification center develops in each of the two neural
arches and in the vertebral center, with a synchondrosis formed by the
cartilage between the ossification centers.
knowledge regarding the human genome, and how it relates to normal
developmental processes and pathologic conditions. Vertebral
segmentation begins with clustering segments of the paraxial mesoderm,
the somites. Segmentation of the mesoderm into somites is an important
and fundamental
process that allows for spatial specialization in the organism and is under genetic control.
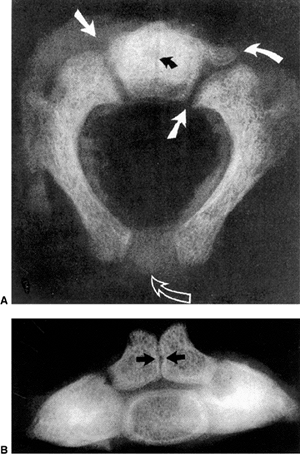 |
|
Figure 22.2 A: Cross-sectional radiograph of C2 in a neonate. The neurocentral (solid white arrows) and posterior (open arrow)
synchondroses are evident. A small area of accessory ossification is present in the right neurocentral synchondrosis anteriorly (curved arrow at top right). Also note the central linear radiolucency (black arrow) indicating the synchondrosis between the dens ossification centers. The posterior ossification centers extend into the eventual vertebral body. B: Anteroposterior radiograph of C2 in a neonate. In this specimen, the dens ossification centers have not fused, leaving a midline synchondrosis (arrows) that extends from the chondrum terminale to the dentocentral synchondrosis. The superior margin of the eventual vertebral body is above the lower level of the dens. The neurocentral synchondroses are continuous with the “ring apophyseal” cartilage inferiorly, the facet cartilage inferiorly, and the dentocentral synchondrosis superiorly. (Ogden JA. Radiology of postnatal skeletal development. XI. The first cervical vertebrae. Skeletal Radiol 1984;12:12–20.) |
genes encode a highly conserved family of transcription factors that
play fundamental roles in morphogenesis during embryonic development.
Vertebrate Hox genes help control
developmental patterning in the embryo along the primary (head to tail)
and secondary (genital and limb bud) axes. There are 39 Hox genes in vertebrates, organized into four clusters located on different chromosomes. In humans these clusters are named HOXA, HOXB, HOXC, and HOXD, located on chromosomes 7p14, 17q21, 12q13, and 2q31, respectively (4). When referring to animals, the names of the genes are written in title case (e.g., Hoxc); when referring to humans they are written in all caps (e.g., HOXC).
Each cluster contains 9 to 11 genes, all oriented in the same 5′ to 3′
direction of transcription. There are 13 possible subsets of genes; no
single cluster contains a representative from all 13 known numbered
subsets (paralogous groups). The numbering of the genes in each cluster
is based on their sequence similarity and relative positions, starting
from the end of the complex that is expressed most anteriorly
(cranially). The equivalent genes in each complex are called a paralogous group.
gene mutation intentionally produced by the investigator, or more
random hits by teratogens (methanol, boric acid, retinoic acid, and
maternal hyperthermia) (5,6,7,8).
|
TABLE 22.1 AXIAL SKELETAL MALFORMATIONS CAUSED BY GENETIC ABNORMALITIES
|
||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
genes are a highly conserved family of developmental control genes that
encode transcription factors containing a 128-amino acid DNA-binding
domain (17,18) called the paired box (19). To date, there are nine known Pax genes (16,20). The Pax gene family is broken down into four subgroups (Pax1 and Pax9; Pax2, Pax5, and Pax8; Pax3 and Pax7; Pax4 and Pax6). Pax1 and Pax9 induce chondrogenic differentiation in the paraxial mesenchymal mesoderm of the sclerotome (18,19,21). They are therefore critically involved in vertebral formation. Abnormalities in the PAX1 sequence in humans have been associated with Klippel-Feil syndrome in some patients (16).
increasingly recognized as being crucial in axial skeletal development.
The best known of these proteins is the sonic hedgehog (shh), which is expressed in the notochord (22,23); shh is believed to be the signal for induction of the ventral somite to differentiate into the sclerotome (23). In shh knockout mice, most sclerotomal derivatives are absent, in conjunction with reduced expression of Pax1 (23). Therefore, absence of shh leads to absence of Pax1 expression and subsequent failure of the mesenchymal cells to chondrify. Defective shh signaling during embryogenesis in mice results in anomalies similar to those seen in association with the human (VACTERL) (24).
These ossification centers extend posteriorly toward the rudimentary
spinous process to form the posterior synchondrosis and anteriorly into
the articular facet region to form all of the bone present in the
facets. Anteromedial to each facet the neurocentral synchondroses form,
joining the neural arches and the body; this occurs on each side of the
expanding anterior ossification center. The body starts to ossify
between 6 months and 2 years of age, usually in a single center. By 4
to 6 years of age the posterior synchondrosis fuses, followed by the
anterior ones slightly thereafter. The final internal diameter of the
pediatric C1 spinal canal is determined by 6 to 7 years of age. Further
growth is obtained only by periosteal appositional growth on the
external surface, which leads to thickening and an increased height,
but without changing the size of the spinal canal. Therefore, a spinal
fusion after the age of 6 or 7 years has minimal impact on the internal
canal diameter; when possible, surgical fusion should not be performed
before this age because of the potential for later cervical stenosis.
that usually coalesce within the first 3 months of life; these centers
are separated from the C2 centrum by the dentocentral synchondrosis (26,27).
This synchondrosis is below the level of the C1 and C2 facets and
contributes to the overall height of the odontoid, as well as to the
body of C2. It is continuous with the vertebral body and facets, and it
coalesces with the anterior neurocentral synchondroses and finally at
the dentocentral synchondrosis. This closure occurs between 3 and 6
years of age. The tip of the dens is composed of a cartilaginous region
similar to an epiphysis, known as the chondrum terminale. In patients between 5 and 8 years of age, this develops an ossification center, becoming the ossiculum terminale. The ossiculum terminale fuses to the remainder of the odontoid between 10 and 13 years of age.
birth, and are joined by the posterior synchondrosis. By 3 months of
age these arches, growing more posteriorly,
form
the rudimentary spinous process. By 1 year of age, ossification fills
the spinous process, and by 3 years of age, the posterior synchondrosis
has fused. Therefore, both the posterior and the anterior synchondroses
are closed by 6 years of age, and there is no further increase in
spinal canal size after this age.
The anterior synchondrosis (i.e., neurocentral synchondrosis) is
slightly anterior to the base of the pedicles; it usually closes
between 3 and 6 years of age. The posterior synchondrosis is at the
junction of the two neural arches; it usually closes by 2 to 4 years of
age. In the neonate and young child the articular facets are horizontal
but become more vertically oriented as the child grows older and
reaches the normal adult configuration. They are also more horizontal
in the upper cervical spine than in the lower cervical spine. The
vertebral bodies enlarge circumferentially by periosteal appositional
growth, whereas their vertical growth is by endochondral ossification.
Secondary ossification centers develop at the tips of the spinous
processes and the cartilaginous ring apophyses of the bodies around the
time of puberty. These ring apophyses are involved in the vertical
growth of the body. These secondary ossification centers fuse with the
vertebral body by 25 years of age.
than the mature adult vertebrae, there are significant differences
between normal vertebral measurements in the child compared to the
adult (28). As the child grows older, the
vertebral body height increases relative to the vertebral body depth.
This is because the activity of the apophyseal end plates contributes
proportionately more growth to the height of the vertebral body than
the appositional growth contributes to the depth of the vertebral body.
The height-to-depth ratio of the vertebral body increases from
approximately 0.5 in children less than 1 year of age to 0.8 to 0.9 in
adults. This ratio remains relatively constant for all vertebral bodies
from C3 to C7. With these changes in the vertebral body height relative
to depth, there are also changes in the sagittal diameter of the canal
relative to vertebral body depth. The ratio of the sagittal diameter of
the canal to vertebral body depth is stable at 1.4 in children from
birth to 7 to 8 years of age, and then gradually decreases to the
normal 1.0 adult value (28). Knowledge of these
normal growth parameters is important when determining the possibility
of occurrence of platyspondyly or spinal stenosis.
of the cervical spine in adults represent normal developmental
processes in children. These parameters are the atlantooccipital motion
and atlantodens interval (ADI), pseudosubluxation and
pseudoinstability, variations in the curvature of the cervical spine
that may resemble spasm and ligamentous injury, variations in the
presence of skeletal growth and growth centers that may resemble
fractures, and anterior soft tissue widening. Normal cervical spine
motion in children is also discussed.
extension radiographic views, with the movements performed voluntarily
by the patient while awake. The ADI is the space between the anterior
aspect of the dens and the posterior aspect of the anterior ring of the
atlas (Fig. 22.3). An ADI of more than 5 mm on flexion and extension lateral radiographs indicates instability (29,30).
This is more than the 3-mm adult value because there is increased
cartilage in the odontoid and ring of the atlas in children, as well as
increased ligamentous laxity. In extension, overriding of the anterior
arch of the atlas on top of the odontoid can be seen in up to 20% of
children (31).
disruption of the transverse atlantal ligament. In adults an ADI
greater than 5 mm indicates ligament rupture (32).
In chronic atlantoaxial conditions (e.g., rheumatoid arthritis, Down
syndrome, congenital anomalies) the ADI is less useful. In children
with these disorders who are frequently hypermobile but do not have
ruptured transverse atlantal ligaments, the ADI is increased beyond the
3 to 5 mm
range.
The complement of the ADI, the SAC, is a more useful measure in this
situation. This space is the distance between the posterior aspect of
the dens and the anterior aspect of the posterior ring of the atlas or
the foramen magnum. A SAC of less than 13 mm may be associated with
neurologic problems (33).
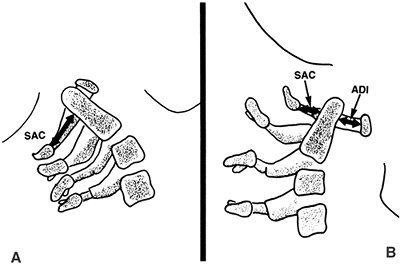 |
|
Figure 22.3
Lateral view of the atlantoaxial joint. The atlantodens interval (ADI) is the distance between the anterior aspect of the dens and the posterior aspect of the anterior portion of the ring of the atlas. The space available for the cord (SAC) is the distance between the posterior aspect of the dens and the anterior aspect of the posterior portion of the ring of the atlas. In children, an ADI of 5 mm or larger is abnormal. In teenagers and adults, a SAC of 13 mm or smaller can be associated with canal compromise. In younger children, spinal cord impingement is imminent if the SAC is equal to or less than the transverse diameter of the odontoid. A: The relations in extension. B: The relations in flexion. |
transverse atlantal ligament without rupture, the alar ligament
provides some stability. It acts like a checkrein (34),
first tightening up in rotation, then becoming completely taut as the
odontoid process continues to move posteriorly for a distance
equivalent to its full transverse diameter. This safety zone between
the anterior wall of the spinal canal of the atlas, the axis, and the
neural structures is an anatomic constant equal to the transverse
diameter of the odontoid. This constant defines Steel’s rule of thirds:
one third cord, one third odontoid, and one third space. This rule
remains constant throughout the growth of the cervical spine (35).
The cord can move into this space (safe zone) when the odontoid moves
posteriorly because of an attenuated transverse atlantal ligament. It
is here that the alar ligament becomes taut, acting as a checkrein and
secondary restraint, preventing further movement of the odontoid into
the cord. In the chronic situation, it is important to recognize when
this safe zone has been exceeded and the child is entering the stage of
impending spinal cord compression. The alar ligament will be
insufficient to prevent a fatal cord injury in the event of another
neck injury similar to the one that caused the initial interruption of
the transverse atlantal ligament.
are not well defined. In a series of 40 healthy college freshmen, the
tip of the odontoid remained directly below the basion of the skull in
both flexion and extension (36). That is, the joint should not normally allow any horizontal translation during flexion and extension. Tredwell et al. (37)
believe that a posterior subluxation of the atlantooccipital relation
of more than 4 mm in extension position indicates instability (Fig. 22.4).
This subluxation can be measured as the distance between the anterior
margin of the condyles at the base of the skull and the sharp contour
of the anterior aspect of the concave joint of the atlas
anteriorly,
or as the distance between the occipital protuberance and the superior
arch of the atlas posteriorly. Another method of measuring posterior
subluxation of the atlantooccipital joint is that of Wiesel and Rothman
(38) (Fig. 22.5).
With this technique, occiput-C1 translation from maximum flexion to
maximum extension should measure no more than 1 mm in normal adults.
The corresponding norms in children have not yet been established.
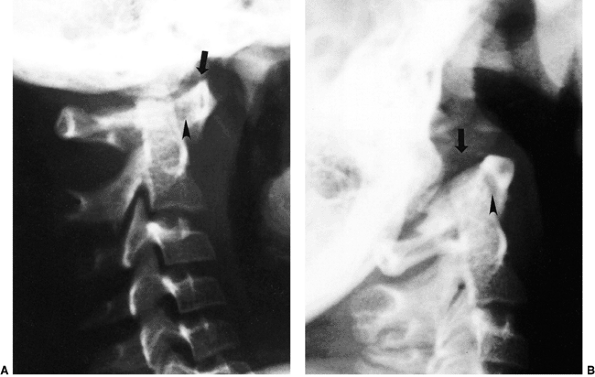 |
|
Figure 22.4 Lateral flexion (A and extension) B
radiographs of an 11-year-old boy with Down syndrome. The child presented with loss of hand control when flexing his neck. Using the method of Tredwell et al. (37), the atlantooccipital distance is measured as the distance between the anterior margin of the condyles at the base of the skull and the sharp contour of the anterior aspect of the concave joint of the atlas. More than 4 mm of posterior translation is abnormal. The atlantooccipital distance (arrows) measures 10 mm in extension and 1 mm in flexion. The atlantodens interval is 1 mm in extension and 6 mm in flexion, for a total of 5 mm of motion (arrowheads). The space available for the cord is 17 mm in flexion and 20 mm in extension. Both occipitoatlantal instability (more than 4 mm posterior translation) and atlantodens hypermobility (5 mm atlantodens interval in flexion) are present. |
 |
|
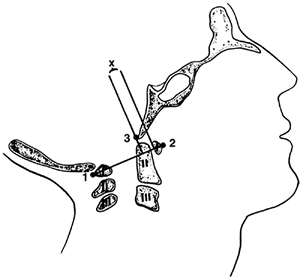
Figure 22.5
The method of measuring atlantooccipital instability according to Weisel and Rothman (38). The atlantal line joins points 1 and 2. A perpendicular to the atlantal line is drawn at the posterior margin of the anterior arch of the atlas. The distance (x) from the basion (3) to the perpendicular line is measured in flexion and extension. The difference between flexion and extension represents the anteroposterior translation at the occipitoatlantal joint; in normal adults, this translation should be no more than 1 mm. [From Gabriel KR, Mason DE, Carango P. Occipito-atlantal translation in Down’s syndrome. Spine 1990;15:996–1002, with permission (39).] |
in children have a normal physiologic displacement. In a study of 161
children (31), marked anterior displacement of
C2 on C3 was observed in 9% of the subjects between 1 and 7 years of
age. In a more recent study, 22% of 108 polytrauma children
demonstrated pseudosubluxation that had no association with intubation
status or severity of the injury (40). In some
children, the anterior physiologic displacement of C2 on C3 is so
pronounced that it appears pathologic (pseudosubluxation). In order to
differentiate physiologic from pathologic subluxation, Swischuk (41)
has proposed using, as a reference line, the posterior cervical line
drawn from the anterior cortex of the posterior arch of C1 to the
anterior cortex of the posterior arch of C3 (Fig. 22.6).
In physiologic displacement of C2 on C3, the posterior cervical line
may pass through the cortex of the posterior arch of C2, touch the
anterior aspect of the cortex of the posterior arch of C2, or come
within 1 mm of the anterior cortex of the posterior arch of C2. In
pathologic dislocation of C2 on C3, the posterior cervical line misses
the posterior arch of C2 by 2 mm or more.
The facets of the lower cervical spine change from 55 to 70 degrees,
whereas the upper facets (i.e., C2-C4) may have initial angles as low
as 30 degrees, gradually increasing to 60 to 70 degrees. This variation
in facet angulation, together with normal looseness of the soft tissues
and the relative increase in size and weight of the skull compared with
the trunk, are the major factors responsible for this
pseudosubluxation. No treatment is needed for this normal physiologic
subluxation.
16% of normal children showed a marked angulation at a single
interspace, suggestive of injury to the interspinous or posterior
longitudinal ligament; 14% showed an absence of the normal lordosis in
the neutral position; and 16% showed an absence of the flexion
curvature between the 2nd and 7th cervical vertebrae, which could be
erroneously interpreted as splinting secondary to injury. These
findings may occur in children up to 16 years of age.
ossification centers of the ring of C1, may mimic fractures. They can
be distinguished from fractures by their smooth cortical margins. In
some children the posterior ring of C1 remains cartilaginous, and this
is usually of no clinical significance (42).
Spina bifida may also occur at other cervical levels and, on
anteroposterior radiographs, the overlapping lucent areas crossing a
vertebral body may mimic a vertical fracture of the body.
and may be erroneously interpreted as an undisplaced fracture.
Similarly, the apical odontoid epiphysis (i.e., ossiculum terminale)
may appear by 5 years of age, although it most typically appears at
approximately 8 years of age. This may be misinterpreted as an odontoid
tip fracture.
If there is a history of trauma, and if it is unclear whether the
wedging is a normal variation or a true compression fracture, a
computerized tomography (CT) scan will demonstrate fracture lines
through the body if a fracture is present. In the lower cervical
levels, secondary centers of ossification of the spinous processes may
resemble avulsion fractures (31).
until 15 years of age, at which stage this distance is largest at C5-C6 (30).
The anteroposterior displacement, from hyperflexion to hyperextension,
decreases from C2-C3 to C6-C7. The angular displacement is greatest (15
degrees) at C3-C4 and C4-C5 in children 3 to 8 years of age, is
greatest (17 degrees) at C4-C5 in children 9 to 11 years of age, and is
greatest (15 degrees) at C5-C6 in children 12 to 15 years of age.
 |
|
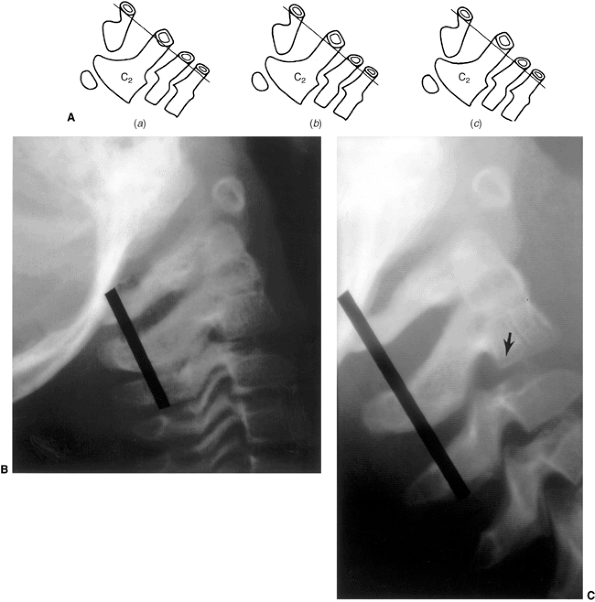
Figure 22.6 A: The posterior cervical line referred to by Swischuk. In C2-C3 pseudosubluxation, the posterior cervical line may pass through (a), touch (b), or lie 1 mm in front of (c) the cortex of the posterior arch of C2. B and C:
Lateral cervical radiographs of a child 2 years and 6 months of age with pseudosubluxation at C2-C3. The radiograph in extension (B) demonstrates no step-off at C2-C3, whereas the radiograph in flexion (C) demonstrates a step-off at C2-C3 (arrow), but with a normal posterior cervical line (solid line). Also note the anterior wedging of the C3 vertebral body, and the overriding of the anterior arch of the atlas on the tip of the odontoid in extension. (A from Shaw M, Burnett H, Wilson A, et al. Pseudosubluxation of C2 on C3 in polytraumatized children—prevalence and significance. Clin Radiol 1999;54:377–380, with permision.) |
deformity. Torticollis indicates a problem at C1-C2, because 50% of the
cervical spine rotation occurs at this joint. A head tilt
alone
indicates a more generalized problem in the cervical spine. The
differential diagnosis of torticollis is large and can be divided into
osseous and nonosseous types. In a recent large series from a tertiary
care pediatric orthopaedic center (44),
a nonmuscular etiology of torticollis was found in 18% of the patients,
most frequently Klippel-Feil syndrome or a neurologic disorder (ocular
pathology, or central nervous system lesion).
odontoid anomalies are the most common congenital and developmental
malformations of the occipitovertebral junction, with an incidence of
1.4 to 2.5 per 100 children (45). These lesions arise from a malformation of the mesenchymal anlages at the occipitovertebral junction.
by the upper cervical spine. The tip of the dens is more cephalad and
sometimes protrudes into the opening of the foramen magnum. This may
encroach on the brain stem, risking neurologic damage from direct
injury, vascular compromise, or alterations in cerebrospinal fluid flow
(46).
basilar impression, the most common type, is a congenital abnormality
often associated with other vertebral defects (e.g., Klippel-Feil
syndrome, odontoid abnormalities, atlantooccipital fusion, and atlas
hypoplasia). The incidence of primary basilar impression in the general
population is 1% (47).
condition attributed to softening of the osseous structures at the base
of the skull. Any disorder of osseous softening can lead to secondary
basilar impression. These include: metabolic bone diseases [e.g., Paget
disease (48), renal osteodystrophy, rickets, and osteomalacia (49)], bone dysplasias and mesenchymal syndromes [e.g., osteogenesis imperfecta (50,51,52,53,54), achondroplasia (55), hypochondroplasia (56), and neurofibromatosis (57)],
and rheumatologic disorders (e.g., rheumatoid arthritis and ankylosing
spondylitis). The softening allows the odontoid to migrate cephalad and
into the foramen magnum.
This shortening is only an apparent deformity because of the basilar
impression. Asymmetry of the skull and face (68%), painful cervical
motion (53%), and torticollis (15%) can also occur. Neurologic signs
and symptoms are often present (59). Many children will have acute onset of symptoms precipitated by minor trauma (60).
In cases of isolated basilar impression, the neurologic involvement is
primarily a pyramidal syndrome associated with proprioceptive sensory
disturbances (motor weakness, 85%; limb paresthesias, 85%). In cases of
basilar impression associated with Arnold-Chiari malformations, the
neurologic involvement is usually cerebellar, and symptoms include
motor incoordination with ataxia, dizziness, and nystagmus. In both
types, the patients may complain of neck pain and headache from the
distribution of the greater occipital nerve and of cranial nerves,
particularly those that emerge from the medulla oblongata [trigeminal
(V), glossopharyngeal (IX), vagus (X), and hypoglossal (XII)]. Ataxia
is a very common finding in children with basilar impression (60).
Hydrocephalus may develop because of obstruction of the cerebrospinal
fluid flow caused by obstruction of the foramen magnum from the
odontoid.
McGregor’s line is the best line for screening because the landmarks
can be clearly defined at all ages on a routine lateral radiograph.
McRae’s line is helpful in assessing the clinical significance of
basilar impression because it defines the opening of the foramen
magnum; in patients who are symptomatic, the odontoid projects above
this line. Nowadays, CT scans with sagittal plane reconstructions can
show the osseous relations at the occipitocervical junction more
clearly, and magnetic resonance imaging (MRI) clearly delineates the
neural anatomy. Occasionally, vertebral angiography is needed (64).
requires a multidisciplinary approach (orthopaedic, neurosurgical, and
neuroradiologic) (53,54,65,66). The symptoms can rarely be relieved with customized orthoses (67);
the primary treatment is surgical. If the symptoms are caused by a
hypermobile odontoid, surgical stabilization in extension at the
occipitocervical junction is needed. Anterior excision of the odontoid
is needed if it cannot be reduced (68), but this should be preceded by
posterior stabilization and fusion. If the symptoms result from
posterior impingement, suboccipital decompression and, often, upper
cervical laminectomy are needed. The dura often needs to be opened so
that the surgeon can look for a tight posterior band (58,69).
Posterior stabilization should also be performed. In a recent series of
190 cases, decompression of the foramen magnum was found to be
appropriate for those without an Arnold-Chiari malformation; transoral
anterior decompression was reserved for those with an associated
Arnold-Chiari malformation (70). These are
general statements, and each case must be considered individually.
Secondary basilar impression tends to progress despite arthrodesis (54).
 |
|
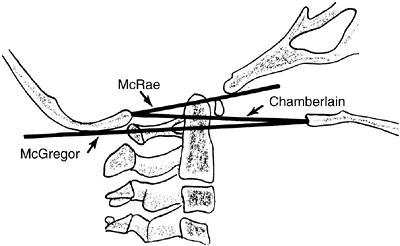
Figure 22.7
The landmarks used on a lateral radiograph of the skull and upper cervical spine used to assess basilar impression. McRae’s line defines the opening of the foramen magnum. Chamberlain’s line is drawn from the posterior lip of the foramen magnum to the dorsal margin of the hard palate. McGregor’s line is drawn from the upper surface of the posterior edge of the hard palate to the most caudal point of the occipital curve of the skull. McGregor’s line is the best for screening because of the clarity of the radiographic landmarks in children of all ages. |
atlantooccipital junction present with a wide spectrum of deformities.
In these patients, the anterior arch of C1 is commonly assimilated to
the occiput, usually in association with a hypoplastic ring posteriorly
(Fig. 22.8) as well as condylar hypoplasia. The
height of C1 is variably decreased, allowing the odontoid to project
upward into the foramen magnum (i.e., primary basilar impression). More
distal cervical anomalies can also occur in association with the
atlantooccipital anomaly. The odontoid may be misshapen, or directed
more posteriorly than normal. Up to 70% of children with this condition
have a congenital fusion of C2 and C3 (Fig. 22.8).
(Posterior congenital fusion of C2 and C3 is a clue that occiput–C1
anomalies, or other more distal cervical fusions, may be present. These
may be cartilaginous initially, and may not appear on plain radiographs
until the child becomes more mature.)
Klippel-Feil syndrome: short, broad necks; restricted neck motion; low
hairline; high scapula; and torticollis (69,71). Recently hemifacial microsomia has been noted to have associated atlantooccipital anomalies (72).
The skull may demonstrate a positional deformational plagiocephaly.
These patients may also have other associated anomalies, including
dwarfism, funnel chest, jaw anomalies, cleft palate, congenital ear
deformities, hypospadias, genitourinary tract defects, and syndactyly.
They can present with neurologic symptoms during childhood, but more
often present at between 40 and 50 years of age. These symptoms can be
initiated by traumatic or inflammatory processes, and they progress
slowly and relentlessly. Rarely do they present suddenly or
dramatically, although they have been reported as a cause of sudden
death. The most common signs and symptoms, in decreasing order of
frequency, are neck and occipital pain, vertigo, ataxia, limb paresis,
paresthesias, speech disturbances, hoarseness, diplopia, syncope,
auditory malfunction, and dysphagia (73,74).
fixed bony deformities and overlapping shadows from the mandible,
occiput, and foramen magnum. An x-ray beam directed 90 degrees
perpendicular to the skull (rather than to the cervical spine) usually
gives a satisfactory view of the occipitocervical junction. The anomaly
is usually studied further with a CT scan. In young children, the
head-wag autotomography technique can be quite useful (75).
This technique involves side-to-side rotation of the child’s head while
a slow anteroposterior radiographic exposure of the upper cervical
spine is performed. This rotation blurs the overlying head and
mandibular structures, allowing for improved visualization of the
occiput–C1-C2 complex.
the foramen magnum has been described as the distance measured from the
posterior aspect of the odontoid to the posterior ring of C1 or the
posterior lip of the foramen magnum, whichever is closer (71,76).
This should be determined in flexion, because this position maximizes
the reduction in the SAC. If this distance is less than 19 mm, a
neurologic deficit is usually present. Lateral flexion and extension
views of the upper cervical spine often show up to 12 mm of space
between the odontoid and the C1 ring anteriorly (71); associated C1-C2 instability has been reported to develop eventually in 50% of these patients.
Compression of the brain stem or upper cervical cord anteriorly occurs
because of the backward-projecting odontoid. This produces a range of
findings and symptoms, depending on the location and degree of
compression. Pyramidal tract signs and symptoms (e.g., spasticity,
hyperreflexia, muscle weakness, and gait disturbances) are most common,
although signs of cranial nerve involvement (e.g., diplopia, tinnitus,
dysphagia, and auditory disturbances) can also be seen. Compression
from the posterior lip of the foramen magnum or dural constricting band
can disturb the posterior columns, leading to a loss of proprioception
as well as vibration and tactile sensation. Nystagmus also occurs
frequently because of posterior cerebellar compression. Vascular
disturbances from vertebral artery involvement can result in brain stem
ischemia, manifested by syncope, seizures, vertigo, and unsteady gait.
Cerebellar tonsil herniation can occur. The altered mechanics of the
cervical spine may result in a dull, aching pain in the posterior
occiput and neck with intermittent stiffness and torticollis.
Irritation of the greater occipital nerve may cause tenderness in the
posterior scalp.
unknown. The neurologic symptoms may develop so late and progress so
slowly because the frequently associated C1-C2 instability progresses
slowly with age, and the increased demands placed on the C1-C2 interval
only gradually produce spinal cord or vertebral artery compromise.
For this reason nonoperative methods should be initially attempted.
Cervical collars, braces, and traction often help patients with
persistent head and neck pain, especially after minor trauma or
infection.
Immobilization
may achieve only temporary relief if neurologic deficits are present.
Patients with evidence of a compromised upper cervical area should take
precautions not to expose themselves to undue trauma.
 |
|
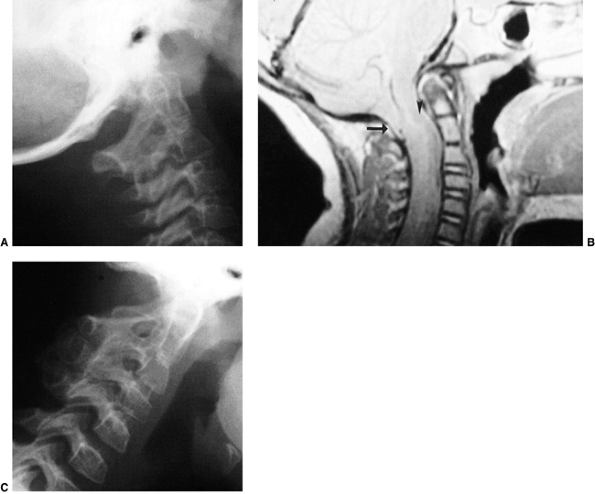
Figure 22.8
This girl, 3 years and 9 months of age, had a history of vertex headaches for 1 year. One month prior to presentation, she developed a painful, left-sided torticollis. A: Plain lateral radiograph shows fusion of C2 and C3 and absence of the ring of C1 with occipitalization. B: Magnetic resonance image (MRI) shows an Arnold-Chiari malformation, with herniation of the cerebellar tonsils into the foramen magnum (arrow). Also note the cordal edema (arrowhead). C: The child underwent an occipital decompression and laminectomy to C3, posterior cervical fusion from the occiput to C4, and halo cast immobilization for 4 months. Flexion and extension lateral radiographs 1 year after treatment show solid incorporation of the fusion from C2 to C4, with dissolution of the graft from the occiput to C2. However, there is no atlantooccipital instability. The child’s symptoms resolved. |
present, a posterior C1-C2 fusion is indicated. Preliminary traction to
attempt reduction is used if necessary. If a reduction is possible and
there are no neurologic signs, surgery has an improved prognosis (69,73,74).
Posterior signs and symptoms may be an indication for posterior
decompression depending on the evidence of dural or osseous
compression. Results vary from complete resolution to increased
deficits or death (69,78).
In situations where there is no instability but only compressive
pathology, the role of concomitant posterior fusion has not yet been
determined. However, if decompression (whether anterior or posterior)
could lead to a destabilized spine, then concomitant posterior fusion
should be considered.
vertebra is, in essence, a hemiatlas or a congenital scoliosis of C1.
Doubousset (79) described 17 patients with this
condition. No definite population incidence is known. The problem is
often associated with other anomalies common to children with
congenital spine deformities (e.g., tracheoesophageal fistula).
develops. A lateral translation of the head on the trunk, with variable
degrees of lateral tilt and rotation (best appreciated from the back),
is the typical finding. There also may be severe tilting of the eye
line. The sternocleidomastoid muscle is not tight, although there is
regional aplasia of the muscles in the nuchal concavity of the tilted
side. Neck flexibility is variable and decreases with age. The
condition is not painful. Plagiocephaly can occur, and increases as the
deformity increases. Neurologic signs (e.g., headache, vertigo,
myelopathy) are present in about one-fourth of the patients. The
natural history is unknown.
give the diagnosis, although the open-mouth odontoid view may suggest
it. Tomograms or CT scans usually are needed in order to see the
anomaly (Fig. 22.9). The defect can range from
a hypoplasia of the lateral mass to a complete hemiatlas with
rotational instability and basilar impression. Occasionally the atlas
is occipitalized. Doubousset classifies this disorder as one of the
three types (71). Type I is an isolated
hemiatlas. Type II is a partial or complete aplasia of one hemiatlas,
with other associated anomalies of the cervical spine (e.g., fusion of
C3-C4 and congenital bars in the lower cervical vertebrae). Type III is
a partial or complete atlantooccipital fusion and symmetric or
asymmetric hemiatlas aplasia, with or without anomalies of the odontoid
and the lower cervical vertebrae.
entire spine should be taken in order to rule out other congenital
vertebral anomalies. Other imaging studies that may be needed are
vertebral angiography and MRI. Angiography should be performed if
operative intervention is to be undertaken, because arterial anomalies
(e.g., multiple loops, vessels smaller than normal, and abnormal routes
between C1 and C2) are often found on the aplastic side. MRI also
should be performed if operative intervention is undertaken, because
many of these children will have stenosis of the foramen magnum, and a
few may have an Arnold-Chiari malformation.
the presence or absence of progression. This observation is primarily
clinical (e.g., photographs) because radiographic measurements are
difficult if not impossible to obtain. Bracing does not halt
progression of the deformity. Surgical intervention is recommended in
patients with severe deformities. A preoperative halo is used for
gradual traction correction over 6 to 8 days. An ambulatory method of
gradual correction of cervical spine deformity has been described using
the halo-Ilizarov technique (80). A posterior
fusion from the occiput to C2 or C3 is then performed, depending on the
extent of the anomaly. Decompression of the spinal canal is necessary
if the canal size is not ample or if projections show that it will not
be able to fully accommodate the
developed
spinal cord. The ideal age for posterior fusion is between 5 and 8
years, corresponding to the age at which the canal size reaches adult
proportions.
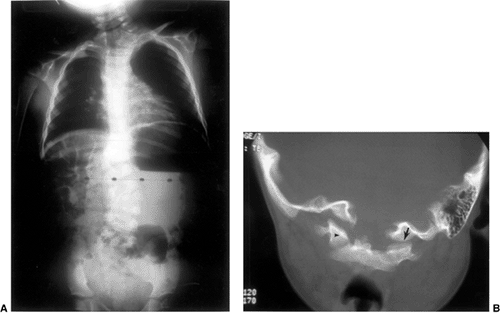 |
|
Figure 22.9 This boy, 4 years and 10 months of age, presented with a torticollis. A:
The anteroposterior radiograph of his entire spine, taken with him in the standing position, documents the head tilt to the left, along with left-sided hemivertebrae in the left lower cervical spine. Also note the multiple hemivertebrae in the thoracic and lumbar spine. B: A computed tomography scan with frontal reconstruction clearly demonstrates an absent lateral mass of C1 (arrow) with a normal right lateral mass (arrowhead). This represents a Doubousset type II, C1 unilateral absence. |
is not known. Clinical presentation varies from an incidental finding
to a passively correctable head tilt, suboccipital pain, decreased
cervical motion, or a clunking of the upper cervical spine.
anomalies of C1, most commonly a partial absence of the posterior ring
of C1, are typically seen. Various anomalies of C2 also commonly exist,
for example, a shallow hypoplastic left facet. Other dysplasias of the
lateral masses, facets, and posterior elements are seen, as are
occasional spondylolistheses. Occiput–C1 instability is seen
frequently, whereas C1-C2 instability rarely occurs. The delineation of
this complex anatomy is often seen best with a CT scan and
three-dimensional reconstruction (Fig. 22.10).
When symptoms of instability are present, MRI in flexion and extension
is recommended in order to assess the presence and magnitude of neural
compression. Instability of the occipitocervical junction caused by the
malformation may lead to neural compromise.
12 months to ensure that instability does not develop, either
clinically (e.g., progressive weakness and fatigue or objective signs
of myelopathy) or radiographically on lateral flexion and extension
radiographs. Surgical intervention is recommended for persistent pain,
torticollis, and neurologic symptoms. A posterior fusion from the
occiput to C2 is usually required, after gradual preoperative reduction
using an adjustable halo cast (80).
common causes of childhood torticollis. Rotary displacements are
characteristically a pediatric problem, but they may occur in adults.
There are several causes. Because the resultant radiographic findings
and treatment regimens are the same for all pediatric causes, they are
discussed as a unit and individual exceptions are noted where necessary.
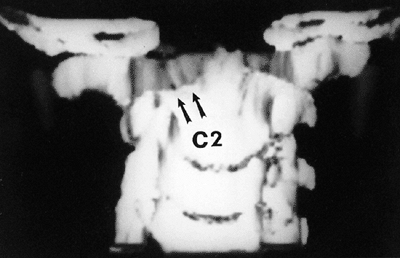 |
|
Figure 22.10
A three-dimensional computerized tomographic scan of the upper cervical cord of a child with familial cervical dysplasia. The left superior facet of C2 is shallow and hypoplastic (arrows). (From Saltzman CL, Hensinger RN, Blane CE, et al. Familial cervical dysplasia. J Bone Joint Surg Am 1991;73-A:163–171, with permission.) |
is misleading, however, because cases of “subluxation” usually present
within the normal range of motion of the atlantoaxial joint. Rotary displacement
is a more appropriate and descriptive term because it includes the
entire range of pathology, from mild subluxation to complete
dislocation. If the deformity persists, the children present with a
resistant and unresolving torticollis that is best termed atlantoaxial rotary fixation or fixed atlantoaxial displacement.
Gradations exist between very mild, easily correctable rotary
displacement to rigid fixation. Complete atlantoaxial rotary
dislocation has rarely been reported in surviving patients.
In rotary torticollis, the lateral mass of C1 that has rotated to the
anterior appears wider and closer to the midline (medial offset),
whereas the opposite lateral mass is narrower and away from the midline
(lateral offset). The facet joints may be obscured because of apparent
overlapping. The lateral view shows the wedge-shaped lateral mass of
the atlas lying anteriorly (where the oval arch of the atlas normally
lies) and the posterior arches failing to superimpose because of the
head tilt (Fig. 22.11). These findings may
suggest occipitalization of C1 because the neck tilt may cause the
skull to obscure C1 in the radiographic image. It is believed that the
normal relation between the occiput and C1 is maintained in children
with atlantoaxial rotary displacement. A lateral radiograph of the
skull may demonstrate the relative positions of C1 and C2 more clearly
than a lateral radiograph of the cervical spine. This is because
tilting of the head also tilts C1, which creates overlapping shadows
and makes interpretation of a lateral spinal radiograph difficult.
child with subluxation appears to be the same as that in a normal child
whose head is rotated. Open-mouth views are difficult to obtain and
interpret, and the lack of cooperation and diminished motion on the
part of the child often make it impossible to obtain these special
views. Cineradiography has been recommended, but the radiation dose is
high, and it still may be difficult to obtain the patient’s cooperation
because of muscle spasms (84,85). CT scans are helpful in this situation if they are done properly (86).
A CT scan, when taken with the head in the torticollic position, may be
interpreted by the casual observer as showing rotation of C1 on C2. If
the rotation of C1 on C2 is within the normal range, as it usually is
early in this
condition,
the observer may attribute this rotation to the positioning of the
patient. A dynamic-rotation CT scan is helpful here. Views with the
head maximally rotated to the right, then to the left, will demonstrate
atlantoaxial rotary fixation when there is a loss of normal rotation (Fig. 22.11).
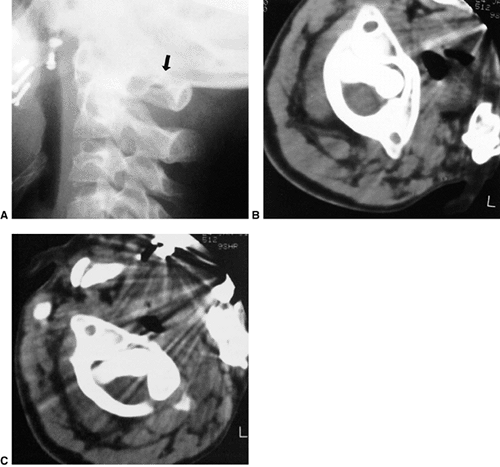 |
|
Figure 22.11 Radiographic findings in atlantoaxial rotary subluxation. A: The lateral cervical spinal radiograph. The posterior arches fail to superimpose because of the head tilt (arrow). B:
Dynamic computerized tomography (CT) scans in a 9-year-old girl with a fixed atlantoaxial rotary displacement, with the head maximally rotated to the left. C: Her head maximally rotated to the right, in this case, does not reach the midline. The ring of C1 is still in the exact relation to the odontoid as in B, indicating a fixed displacement. |
type I is a simple rotary displacement without an anterior shift, type
II is rotary displacement with an anterior shift of 5 mm or less, type
III is rotary displacement with an anterior shift greater than 5 mm,
and type IV is rotary displacement with a posterior shift. The amount
of anterior displacement considered to be pathologic is greater than 3
mm in older children and adults and greater than 4 mm in younger
children (29). Flexion and extension lateral-stress radiographs are suggested to rule out the possibility of anterior displacement.
benign and frequently resolves by itself. Type II deformity is
potentially more dangerous. Types III and IV are very rare, but because
of the potential for neurologic involvement and even instant death,
their management must be approached with great caution.
Several causative mechanisms are possible. Cervical spine fracture is
rarely a cause. More commonly, atlantoaxial rotary displacement occurs
following minor trauma [e.g., clavicle fractures (88)], after head and neck surgery including simple central line insertion (89),
or after an upper respiratory tract infection. The children present
with a “cock-robin” torticollis, and resist any attempt to move the
head because of pain. The associated muscle spasm is noted on the side
of the long sternocleidomastoid muscle. This is because the muscle is
attempting to correct the deformity, unlike in congenital muscular torticollis in which the muscle causes
the torticollis. If the deformity becomes fixed, the pain subsides but
the torticollis persists, along with decreased neck motion. In
long-standing cases, plagiocephaly and facial flattening may develop on
the side of the tilt.
pharyngovertebral veins and the periodontal venous plexus and suboccipital epidural sinuses (91).
This may provide a route for hematogenous transport of peripharyngeal
septic exudates to the upper cervical spine, a possible anatomic
explanation for the atlantoaxial hyperemia of Grisel syndrome. Regional
lymphadenitis is known to cause spastic contracture of the cervical
muscles. This muscular spasm, in the presence of abnormally loose
ligaments (hypothetically caused by the hyperemia of the
pharyngovertebral vein drainage), could produce locking of the
overlapping lateral joint edges of the articular facets. This situation
prevents easy repositioning, resulting in atlantoaxial rotary
displacement. The hyperemia after surgery of the oral pharynx, most
frequently tonsillectomy and adenoidectomy, enhances the passage of the
inflammatory products into the pharyngovertebral veins. It is known
that patients may develop Grisel syndrome after otolaryngological
procedures (92), especially with monopolar electrocautery (93). Kawabe et al. (94)
have demonstrated meniscuslike synovial folds in the atlantooccipital
and lateral atlantoaxial joints of children, but not in those of
adults, and have found that the dens-facet angle of the axis is steeper
in children than in adults. They postulate that excessive C1-C2
rotation, caused by the steeper angle and compounded by ligament laxity
from an underlying hyperemia, allows the meniscuslike synovial folds to
become impinged in the lateral atlantoaxial joint, leading to rotary
fixation. The predominance of this syndrome in childhood correlates
with the predilection for the adenoids to be maximally hypertrophied
and inflamed at this same time, and located in the area drained by the
pharyngovertebral veins.
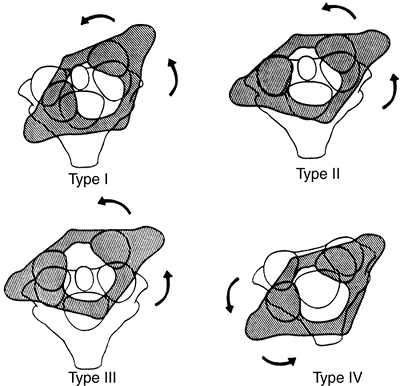 |
|
Figure 22.12 The four types of atlantoaxial rotary displacement. (From Fielding JW, Hawkins RJ. Atlanto-axial rotatory fixation. J Bone Joint Surg Am 1977;59-A:37–44, with permission.)
|
 |
|
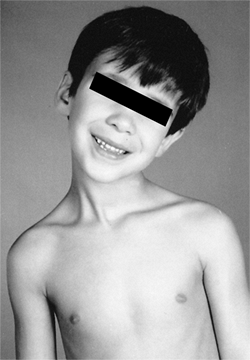
Figure 22.13
A 5-year-old boy developed an atlantoaxial rotary subluxation after an upper respiratory viral infection (Grisel syndrome). It rapidly resolved after treatment with a soft collar and mild doses of diazepam. |
spontaneously. Rarely, however, the pain subsides and the torticollis
becomes fixed. The duration of symptoms and deformity dictates the
treatment recommended (95).
duration can be treated with immobilization in a soft cervical collar
and rest for approximately 1 week. Close follow-up is mandatory. If
spontaneous reduction does not occur with this initial treatment,
hospitalization and the use of halter traction, muscle relaxants (e.g.,
diazepam), and analgesics are recommended next. Patients with rotary
subluxation of more than 1 week but less than 1 month should be
hospitalized immediately for cervical traction, relaxants, and
analgesics. Gentle halo traction is occasionally needed in order to
achieve reduction. The reduction is noted clinically and confirmed with
a dynamic CT scan. If no anterior displacement is noted after
reduction, cervical support should be continued only so long as
symptoms persist. If there is anterior displacement, immobilization
should be continued for 6 weeks to allow ligamentous healing to occur.
In patients with rotary subluxation for more than 1 month, cervical
traction (usually halo skeletal) can be tried for up to 3 weeks, but
the prognosis is guarded. These children usually fall into two groups:
those whose rotary subluxation can be reduced with halo traction but,
despite a prolonged period of immobilization, resubluxate when the
immobilization is stopped; and those whose subluxation cannot be
reduced, and is fixed. It has been recently shown that patients with
recurrence of deformity have a larger difference in the lateral
mass–dens interval on the initial anteroposterior radiograph compared
to those who do not have recurrence (96).
displacement is present, the transverse atlantal ligament is
compromised, presenting a potential for catastrophe. In this situation,
posterior C1-C2 fusion should be performed. The indications for fusion
are neurologic involvement, anterior displacement, failure to achieve
and maintain correction, a deformity that has been present for more
than 3 months, and recurrence of deformity following an adequate trial
of conservative management (at least 6 weeks of immobilization after
reduction). Before surgical fusion, halo traction is used for several
days in order to obtain as much straightening of the head and neck as
possible; a forceful or manipulative reduction should not be performed.
Postoperatively, the child is simply positioned in a halo cast or vest
in the straightened position obtained preoperatively; this usually
achieves satisfactory alignment. A Gallie-type fusion with sublaminar
wiring at the ring of C1 and through the spinous process of C2 is
preferred to a Brooks-type fusion in which the wire is sublaminar at
both C1 and C2. This is because of the decreased SAC at C2 and the
consequent higher risk of neurologic injury. This wiring does not
reduce the displacement but simply provides some internal stability for
the arthrodesis. The overall results for a Gallie fusion are very good (Fig. 22.14) (97). Long-term results do not indicate any significant abnormalities of the sagittal profile (98).
 |
|
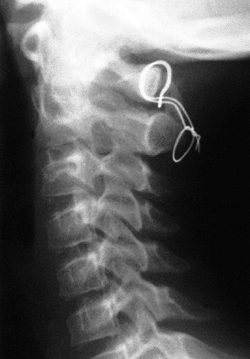
Figure 22.14 The child in Figure 22.11
had a fixed deformity that occurred 6 months earlier, immediately after reconstructive maxillofacial surgery for Goldenhar syndrome. It did not respond to traction, including halo traction. She underwent a posterior C1-C2 (Gallie-type) fusion. A solid fusion was present 9 months later; clinically, the patient achieved 80 degrees of rotation to the left and 45 degrees of rotation to the right. |
duration are treated with immobilization in a soft cervical collar and
rest for approximately 1 week. If spontaneous reduction does not occur,
halter traction, muscle relaxants (e.g., diazepam), and analgesics are
prescribed. Patients with rotary subluxation of more than 1 week but
less than 1 month should be hospitalized immediately for cervical
traction, relaxants, and analgesics. Gentle halo traction is
occasionally needed to achieve reduction. All reductions are confirmed
with a dynamic CT scan. In patients with rotary subluxation for more
than 1 month, cervical traction (usually halo skeletal) can be tried
for up to 3 weeks, but the prognosis is guarded. If reduction cannot be
achieved or maintained, then posterior C1-C2 arthrodesis is recommended.
is the most common cause of torticollis in the infant and young child,
presenting at a median age of 2 months (99).
The deformity is caused by contracture of the sternocleidomastoid
muscle, with the head tilted toward the involved side and the chin
rotated toward the opposite shoulder. A disproportionate number of
these children have a history of a primiparous birth or a breech birth
or other kind of difficult delivery. However, it has also been reported
in children who were born normally and in those born by cesarean
section (99,100,101).
theories. Because of the birth history, one theory is that a
compartment syndrome occurs due to soft tissue compression of the neck
at the time of delivery (102). Surgical histopathologic sections suggest venous occlusion of the sternocleidomastoid muscle (103).
This occlusion may result in a compartment syndrome, as manifested by
edema, degeneration of muscle fibers, and muscle fibrosis. This
fibrosis is variable, ranging from small amounts to the entire muscle.
It has been suggested that the clinical deformity is related to the
ratio of fibrosis to remaining functional muscle. If ample muscle
remains, the sternocleidomastoid will probably stretch with growth, and
the child will not develop torticollis; if fibrosis predominates, there
is little elastic potential, and torticollis will develop.
The fact that this condition can occur in children with normal birth
histories or in children born by cesarean section challenges the
perinatal compartment syndrome theory and supports the in utero crowding theory. The fact that it can occur in families (106,107,108) (supporting a genetic predisposition) also calls the compartment syndrome theory into question.
The primary myopathy initially may result from trauma, ischemia, or
both, and unequally involves the two heads of the sternocleidomastoid
muscle. With continuing fibrosis of the sternal head, the branch of the
spinal accessory nerve to the clavicular head of the muscle can be
entrapped, leading to a later progressive deformity (109).
the sternocleidomastoid from fetal embyrogenesis. Recent
histopathologic studies have demonstrated the presence of both
myoblasts and fibroblasts in sternocleidomastoid tumors in varying
stages of differentiation and degeneration (110).
The source of these myoblasts and fibroblasts is unknown. After birth,
environmental changes stimulate these cells to differentiate, and the
sternocleidomastoid tumor develops. Hemorrhagic and inflammatory
reactions would be expected if the tumor were a result of perinatal
birth trauma or intrauterine positioning, yet these cells were not seen
in sternocleidomastoid histopathologic studies. The occurrence of
torticollis depends on the fate of the myoblasts in the mass. If the
myoblasts undergo normal development and differentiation, no persistent
torticollis will occur and conservative treatment will likely succeed.
If the myoblasts mainly undergo degeneration, then the remaining
fibroblasts produce large amounts of collagen, with a scarlike
contraction of the sternocleidomastoid muscle and the typical
torticollis.
sternocleidomastoid tumor (43% of cases), those with muscular
torticollis (31%), and those with postural torticollis (22%) (111).
The clinical features of congenital muscular torticollis depend on the
age of the child. The condition is often discovered in the first 6 to 8
weeks of life. If the child is examined during the first 4 weeks of
life, a mass or “tumor” may be palpable in the neck (100). Although it may be palpable, it is unrecognized up to 80% of the time (112).
Characteristically, it is a nontender, soft enlargement beneath the
skin, and is located within the sternocleidomastoid muscle belly. This
so-called tumor reaches its maximum size within the first 4 weeks of
life then gradually regresses. After 4 to 6 months of life the
contracture and the torticollis are the only clinical findings. In some
children the deformity is not noticed until after 1 year of age, which
raises questions about both the congenital nature of this entity and
the perinatal compartment syndrome theory. Recent studies (113)
indicate that the rate of associated hip dysplasia in children with
congenital muscular torticollis is 8%, lower than the previously cited
20% (105). The sternocleidomastoid tumor
subgroup, the most severe group, presents at an earlier age and is
associated with a higher incidence of breech presentation (19%),
difficult labor (56%), and hip dysplasia (6.8%) (111).
deformities can develop (plagiocephaly), often within the first year of
life. The facial flattening occurs on the side of the contracted
muscle, and is probably caused by the sleeping position of the child (114).
In the United States children usually sleep prone, and in this position
it is more comfortable for them to lie with the affected side down. The
face thus remodels to conform to the bed. If the child sleeps supine,
reverse modeling of the contralateral skull occurs. In the child who is
untreated for many years, the level of the eyes and ears becomes
unequal and can result in considerable cosmetic deformity.
order to rule out associated congenital anomalies. Plain radiographs of
the cervical spine in children with muscular torticollis are always
normal, aside from the head tilt and rotation. If any suspicion exists
about the status of the hips, appropriate imaging (e.g.,
ultrasonography or radiography) should be done, depending on the age of
the child and expertise of the ultrasonographer.
The muscle diameter is two to four times greater than that of the
contralateral muscle. In older patients the signals are consistent with
atrophy and fibrosis, similar to those encountered in compartment
syndromes of the leg and forearm.
the incidence of a previous stenocleidomastoid tumor, hip dysplasia,
and the likelihood of needing surgery increase (115,116). Treatment initially consists of conservative measures (100,101,112,117,118). Good results can be expected with stretching exercises alone, with one series reporting 90% success (117) and another 95% (116).
Children with a sternocleidomastoid tumor respond less favorably to
conservative stretching exercises than do those with a simple muscle
torticollis; none of the children with postural torticollis need
surgery (116). The extent of sternocleidomastoid fibrosis on ultrasound examination is also predictive of the need for surgery (119,120).
In one series, conservative therapy was effective for all the patients
in whom only the lower one-third of the muscle was involved with
fibrosis; surgery was needed in 35% of the children in whom the entire
length of the muscle was involved (121).
guided by the physiotherapist. The ear opposite the contracted muscle
should be positioned to the shoulder, and the chin should be positioned
to touch the shoulder on the same side as the contracted muscle. When
adequate stretching has occurred in the neutral position, the exercises
should be graduated up to the extended position, which achieves maximum
stretching and prevents residual contractures. Treatment measures to be
used along with stretching include room modifications; the child’s toys
and crib should be modified so that the neck is stretched when the
infant is reaching for or looking at objects of interest. The exact
extent of the efficacy of these stretching measures, compared against a
natural history of spontaneous
resolution, is not known (122);
there are many anecdotal cases of spontaneous resolution. Occasionally,
muscle stretching itself will result in partial or complete rupture of
the sternocleidomastoid muscle (123).
surgery is recommended. The child’s neck and anatomic structures are
larger by this age, making surgery easier. Established facial deformity
or a limitation of more than 30 degrees of motion usually precludes a
good result, and surgery is required to prevent further facial
flattening and further cosmetic deterioration (118).
Asymmetry of the face and skull can improve so long as adequate growth
potential remains after the deforming pull of the sternocleidomastoid
is removed; good but not perfect results can be obtained with surgery
performed on children as old as 12 years (112,125).
at the sternoclavicular or mastoid pole, bipolar release, middle third
transection, and even complete resection. Although these surgical
procedures are usually done open, endoscopic (127) and percutaneous (distal) approaches have been recently described (128). Bipolar release combined with a Z-plasty of the sternal attachment (Fig. 22.15) yielded 92% satisfactory results in one series, whereas only 15% satisfactory results were obtained with other procedures (124). Similar results, although not perfect, can be achieved even in older children by using a bipolar release technique (129).
In a more recent series of surgical cases, excellent results were
obtained with a unipolar release and aggressive postoperative
stretching (125). Middle-third transection has also been reported to give 90% satisfactory results (130).
Z-plasty lengthening maintains the V-contour of the neck and cosmesis,
which the middle-third transection does not. Structures that can be
injured by surgery are the spinal accessory nerve, the anterior and
external jugular veins, the carotid vessels and sheath, and the facial
nerve. Skin incisions should never be located directly over the
clavicle because of cosmetically unacceptable scar spreading; rather,
they should be made one finger’s breadth proximal to the medial end of
the clavicle and sternal notch, and in line with the cervical skin
creases. The postoperative protocol can vary from simple stretching
exercises to cast immobilization. Some type of a bracing device to
maintain alignment of the head and neck is probably a desirable part of
the postoperative protocol.
 |
|
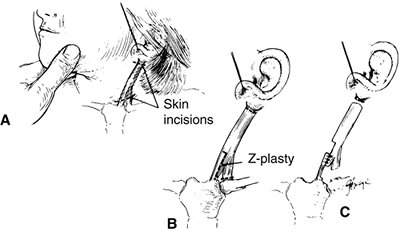
Figure 22.15 The Z-plasty procedure for torticollis. A: The location of the skin incisions. B:
The clavicular and mastoid attachments of the sternocleidomastoid muscle are cut, and a Z-plasty is performed. Note that the medial aspect of the sternal attachment is preserved. C: The completed procedure after release of the proximal muscle insertion. (From Ferkel RD, Westin GW, Dawson EG, et al. Muscular torticollis. A modified surgical approach. J Bone Joint Surg Am 1983;65-A:894–900, with permission.) |
stretching exercises and room modifications is tried first. If this
approach fails, or if the child presents after 1 year of age, a bipolar
sternocleidomastoid release is performed. Postoperative orthotic
immobilization is used along with frequent physiotherapy for at least 3
months after surgery.
differential diagnosis of any atypical torticollis, especially when the
condition is unresponsive or progressive in the face of therapy that is
believed to be appropriate. The major neurogenic etiologies are central
nervous system tumors (i.e., of the posterior fossa or spinal cord),
syringomyelia with or without cord tumor, Arnold-Chiari malformation,
ocular dysfunction, and paroxysmal torticollis of infancy.
has described three children with torticollis, photophobia, and
epiphora (tearing). In all three children, the diagnosis was delayed by
an initial diagnosis of a local ocular inflammatory condition. The age
at presentation ranged from 1 to 23 months. The delay in diagnosis
ranged from 5 months to 4 years. The neoplastic diagnosis was not
considered initially by the ophthalmologists because the primary signs
of posterior fossa tumors are extraocular muscle paresis, nystagmus,
and papilledema.
The peculiar, often overlooked signs of the tumor are spinal rigidity,
early spinal deformity, and spontaneous or induced vertebral pain. In
young children, pain may be expressed as irritability and restlessness (136).
system tumor should consist of plain radiographs of the skull and
cervical spine followed by CT scan and MRI. Vertebral angiography may
also be needed, both diagnostically and in neurosurgical planning.
deformities of the brain stem and cerebellum (137,138).
It may be associated with myelomeningocele (i.e., Chiari type II
malformation). The Chiari type I malformation is a downward
displacement of the medulla oblongata with extrusion of the cerebellar
tonsils through the foramen magnum; it is encountered in older
children. Dure et al. (137) described 11
children with Chiari type I malformations; torticollis was the
presenting complaint of 1 of the 11 children, who was 5 years of age.
It was associated with headaches and paracervical muscle spasm; the
torticollis was left sided. As with tumors, the workup in a child with
the potential diagnosis of Chiari malformation consists of plain
radiographs of the skull and cervical spine followed by an MRI (137). The treatment is neurosurgical.
These children typically present at approximately 1 year of age. The
face can be turned about a vertical axis, the head can be tilted to one
shoulder with the frontal plane of the face remaining coronal, the chin
can be elevated or depressed, or a combination of any of these
positions can occur. These abnormal head positions optimize visual
acuity and maintain binocularity. An ocular cause is likely if the head
is tilted but not rotated or if the tilt changes when the child is
lying versus sitting or standing up. Children with ocular torticollis
have a full range of cervical motion without the fibrotic
sternocleidomastoid muscle seen in congenital muscular torticollis.
Ophthalmologic evaluation is usually positive for paralytic squint or
nystagmus. Detailed tests conducted by an experienced ophthalmologist
are diagnostic. Treatment for ocular torticollis is usually by
ophthalmic surgery.
episodic torticollis lasting for minutes to days, with spontaneous
recovery (141,142,143).
The attacks usually occur in the morning and last from minutes to days,
with a frequency ranging from less than one episode per month to three
to four episodes per month. The attacks may be associated with lateral
trunk curvature, eye movements or deviations, and alternating sides of
torticollis. The children are usually girls (71%), the average age at
onset is 3 months (range, 1 week to 30 months), and the average
recovery period is 24 months (range, 6 months to 5 years). It has been
suggested that paroxysmal torticollis of infancy is equivalent to a
migraine headache (144,145)
because family histories of migraines were reported for 29% of the
patients in one study, or that it could be a forerunner of benign
paroxysmal vertigo of childhood (142). Whatever the cause, it is usually self-limiting and does not require therapy. It may be linked to a mutation in the CACNA1A gene (145), which is associated with familial hemiplegic migraine.
from a hiatal hernia, and abnormal posturing of the neck and trunk,
usually torticollis (146,147).
The torticollis is likely an attempt of the child to decrease
esophageal discomfort resulting from the reflux. The abnormal posturing
may also present as opisthotonos or neural tics, and often mimics
central nervous system disorders. Most patients present in infancy. The
incidence of gastroesophageal reflux is high (up to 40% of infants) (148),
with the principal symptoms being vomiting, failure to thrive,
recurrent respiratory disease, dysphagia, various neural signs,
torticollis, and respiratory arrest. The diagnosis of symptom-causing
gastroesophageal reflux is frequently overlooked. On careful
examination of these infants, it is found that the sternocleidomastoid
muscle is not tight or short, and there is no tumor; this eliminates
the possibility of congenital muscular torticollis. Further workup
excludes dysplasias and congenital anomalies of the cervical spine as
well as central nervous system disorders. In these situations the
physician should consider Sandifer syndrome in the differential
diagnosis.
congenital anomalies or dysplasias; contrast studies of the upper
gastrointestinal tract usually demonstrate the hiatal hernia and
gastroesophageal reflux (149). Esophageal pH
studies may be necessary; many children, both asymptomatic and
symptomatic, show evidence of gastroesophageal reflux (150). Treatment begins with medical therapy. If this fails, fundoplication can be considered, which is usually curative (151).
the cervical vertebrae, clinically exhibited by the triad of a low
posterior hairline, a short neck, and variably limited neck motion (Fig. 22.16 A and B) (152). Its incidence is approximately 0.7% (153).
Other associated anomalies are often present both in the
musculoskeletal and other organ systems. The congenital fusions result
from abnormal embryologic formation of the cervical vertebral
mesenchymal anlages. This unknown embryologic insult is not limited to
the cervical vertebrae and explains the other anomalies associated with
the Klippel-Feil syndrome. In some instances the Klippel-Feil syndrome
is familial, indicating a genetic transmission (154,155,156).
associated Sprengel deformity. Other anomalies associated with the
syndrome are scoliosis (both congenital and idiopathic) (152), congenital limb deficiency (157), renal anomalies (158), deafness (159), synkinesis (mirror movements) (160), pulmonary dysfunction (161), and congenital heart disease (162).
Radiographs demonstrate a wide range of deformities, ranging from
simple block vertebrae to multiple and bizarre anomalies. Klippel-Feil
syndrome can be divided into three types, depending upon the extent of
vertebral involvement: type I involves the cervical and upper thoracic
vertebrae, type II involves the cervical vertebrae alone, and type III
involves the cervical vertebrae as
well as lower thoracic or upper lumbar vertebrae (163).
Associated scoliosis makes interpretation of the radiographs even more
difficult. Flexion and extension lateral radiographs are used to assess
for instability, and this should always be done prior to administering
any general anesthetic. If instability is noted on the flexion and
extension radiographs, the anesthesiologist should be informed
accordingly. The anesthesiologist may elect to undertake intubation
differently (e.g., awake nasotracheal, fiberoptic guided). Any segment
adjacent to unfused segments may develop hypermobility and neurologic
symptoms (164). A common pattern is fusion of C1 to C2 and of C3 to C4, leading to a high risk of instability at the unfused C2-C3 level (165).
If the flexion and extension radiographs are difficult to interpret, a
flexion and extension CT or MRI scan can be useful. A CT scan is
especially helpful at the C1-C2 level in assessing the SAC; sagittal
MRI is more helpful at other levels.
 |
|
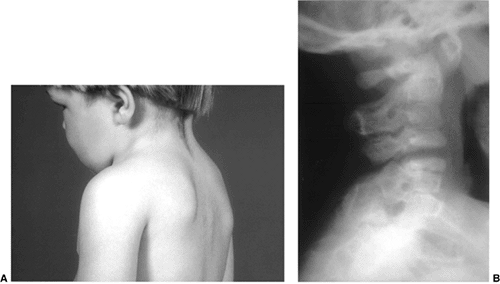
Figure 22.16 This boy, 3 years and 6 months of age, presented with a short neck and reduced motion. A: Note the short neck and low posterior hair line. B:
The lateral cervical spine radiograph demonstrates complete fusion of the posterior elements of C2 and C3, with reduced disc height anteriorly at C2-C3. Note the reduced space between C3 and C4, which most likely represents a cartilage fusion between C3 and C4 that will likely become an osseous fusion later. |
further evaluated for other organ system problems. A general pediatric
evaluation should be undertaken by a qualified pediatrician to ensure
that no congenital cardiac or other neurologic abnormalities exist.
Renal imaging should be done in all cases; simple renal ultrasonography
is usually adequate for the initial evaluation (166).
MRI should be performed whenever there is a clinical basis for any
concern about neurologic involvement, in order to define the site and
cause of neurologic pathology. Also, an MRI should be performed before
any orthopaedic spinal procedure; this is to rule out any other
intraspinal pathology that might not be seen clinically or
radiographically (e.g., Arnold-Chiari malformation, tethered cord,
nonosseous diastematomyelia) (167).
problems are present, since they have the potential to lead to organ
system failure and death. Cervical spine instability (168) can develop with neurologic involvement, especially in the upper segments, or in patients with iniencephaly (168,169). The more numerous the occipitoatlantal anomalies, the higher the neurologic risk (170).
Degenerative joint and disc disease develops in patients with lower
segment instabilities. In adulthood, many patients with Klippel-Feil
syndrome will complain of headaches, upper-extremity weakness, or
numbness and tingling. On neurologic examination, subtle findings can
be seen in up to half of these adults. Those with mirror-movement
disorders are likely to have cervicomedullary neuroschisis (171). Degenerative disc disease, as seen on MRI scans, occurs in nearly 100% of these patients (172).
are at high risk for developing instabilities, strenuous activities
should be avoided, especially contact sports. Other nonsurgical methods
of treatment are cervical traction, collars, and analgesics when
mechanical symptoms appear, usually in the adolescent or adult patient.
Arthrodesis is needed for the management of neurologic symptoms caused
by instability. Asymptomatic hypermobile segments pose a dilemma
regarding stabilization.
Unfortunately,
no guidelines exist for this problem. The need for decompression at the
time of stabilization depends on the exact anatomic circumstance, as
will the choice of combined anterior and posterior rather than simple
posterior fusions. Surgery for cosmesis alone is usually unwarranted
and risky.
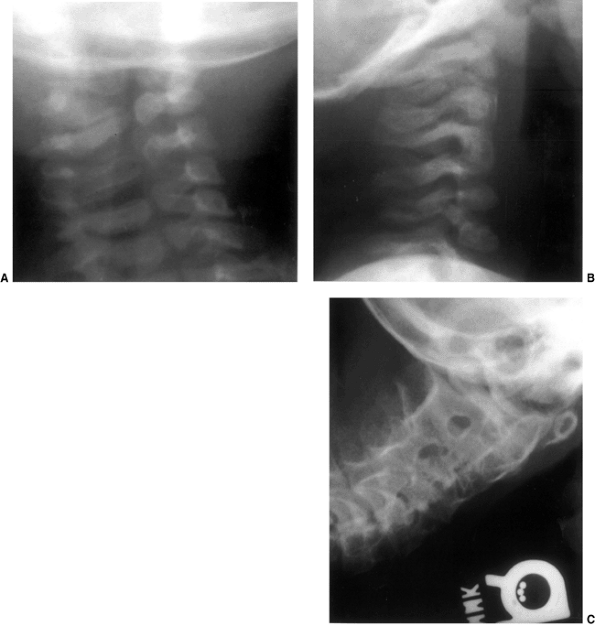 |
|
Figure 22.17 This 2-month-old girl presented with a left-sided torticollis. The anteroposterior (A) and lateral (B) cervical spine radiographs demonstrate congenital anomalies of the cervical spine at C1-C2. C:
At 7 years and 3 months of age these anomalies have further ossified and matured, demonstrating massive congenital fusions of the cervical spine. |
that imaging of the genitourinary system be performed if it has not
already been done. The patient is counseled against contact sports,
especially collision sports (football, wrestling, ice hockey) and
sports that may place the cervical spine under stress (e.g.,
gymnastics, diving, basketball, soccer, volleyball). We typically ask
the child what sports he/she likes to participate in, and then
determine whether that sport is likely to place the cervical spine
under stress. If so, that activity should be avoided. Arthrodesis is
reserved for the very rare instances of symptomatic hypermobility.
odontoid process is divided by a wide transverse gap, leaving the
apical segment without its basilar support (173).
The exact rate of incidence is not known. It most likely represents an
unrecognized fracture at the base of the odontoid or damage to the
epiphyseal plate during the first few
years of life (173,174).
Either of these conditions can compromise the blood supply to the
developing odontoid, resulting in the os odontoideum. MRI scans have
further documented the presence of nuchal cord changes consistent with
trauma (175). A congenital etiology has also been proposed (176),
and may represent an embryological anomaly characterized by
segmentation at the junction of the proximal 1½ somites of the 2½
somites from which the odontoid forms (177).
presentation; transitory episodes of paresis, myelopathy, and cerebral
brain stem ischemia due to vertebral artery compression from the upper
cervical instability are less common. Sudden death rarely occurs.
smooth sclerotic border of variable size located in the position of the
normal odontoid tip. It is occasionally located near the basioccipital
bone in the foramen magnum area. There are three radiographic types of
os odontoideum; round, cone, and blunt-tooth (178).
The base of the dens is usually hypoplastic. The gap between the os and
the hypoplastic dens is wider than in a fracture, usually well above
the level of the facets. However, it may be difficult to differentiate
an os odontoideum from nonunion following a fracture. Tomograms and CT
scans are useful in further delineating the bony anatomy, and flexion
and extension lateral radiographs help in assessing instability. The
instability index and sagittal plane rotation angle can be measured (Fig. 22.18) (179).
The presence of myelopathy is highly correlated with a sagittal plane
rotation angle of 20 degrees or more and an instability index of 40% or
higher; it is also most common in the round type of os odontoideum (178).
Myelopathy is also associated with cystic or fibrocartilaginous masses
behind the odontoid, within the transverse ligament, or at the level of
the articulation between the os odontoideum and the remainder of the
odontoid (176,180,181,182); these typically regress after successful stabilization and arthrodesis (183).
from posterior translation of the os into the cord in extension or from
the odontoid into the cord in flexion. Hypermobility at the C1 and C2
level may cause vertebral artery occlusion with ischemia of the brain
stem and posterior fossa structures; this will result in seizures,
syncope, vertigo, and visual disturbances.
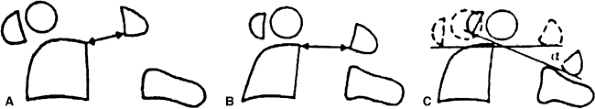 |
|
Figure 22.18 Radiographic parameters used for determining the instability index and sagittal plane rotation in os odontoideum. The minimum (A) and maximum (B)
distance from the posterior border of the body of C2 to the posterior atlantal arch. The instability index = [(maximum distance- minimum distance)/maximum distance) × 100% C: The change in the atlantoaxial angle between flexion and extension is the sagittal plane rotation. (From Watanabe M, Toyama Y, Fujimura Y. Atlantoaxial instability in os odontoideum with myelopathy. Spine 1996;21:1435–1439, with permission.) |
recover with immobilization. Subsequently, only nonstrenuous activities
should be allowed, but curtailment of activities in the pediatric age
group can be difficult. The risk of a small insult leading to
catastrophic quadriplegia and death must be weighed. The long-term
natural history is unknown.
neurologic involvement, progressive instability, or persistent neck
pain. Surgery should also be strongly considered in patients who are
asymptomatic but have an instability index greater than 40% and/or a
sagittal plane rotation angle greater than 20 degrees. A Gallie fusion
is recommended. The surgeon must be careful when tightening the wire so
that the os is not pulled back posteriorly into the canal and cord,
because the consequences would be disastrous. In small children, the
wire may be eliminated. In all children, a Minerva or halo cast or vest
is also used for at least 6 weeks, and often for 12 weeks. Recently,
C1-C2 screw fixation has been reported to be helpful in treating
pediatric atlantoaxial instability in children older than 4 years of
age (184). In all children undergoing C1-C2
posterior arthrodesis, care should be taken to avoid fixation of the
C1-C2 segment in hyperlordosis, as that will lead to subaxial cervical
kyphosis postoperatively (185).
this syndrome, cervical instabilities can develop at both the
occiput-C1 and C1-C2 levels. The instability may occur at more than one
level and in more than one plane (e.g., sagittal and rotary planes).
With the advent of the Special Olympics, there has been much concern
regarding the participation of children with Down syndrome, and much
confusion regarding the appropriate approach to the
problem
of upper cervical instability in these children. Outlined in following
text are the most recent recommendations regarding this problem.
No guidelines exist regarding the frequency of periodic screening or
indications for surgery, with the exception of those for
atlantooccipital fusion in the symptomatic child. Tredwell et al. (37)
believe that treatment plans for these children should depend on the
amount of room available for the cord rather than absolute values of
displacement for both atlantoaxial and atlantooccipital instability.
in 1961. Subsequently there have been many reports on this instability.
However, there are none that document the true incidence of
atlantoaxial dislocation (in contrast to instability), and there are no
long-term studies of the natural history of this problem.
in a series of 236 patients with Down syndrome. Progressive instability
and neurologic deficits are more likely to develop in boys older than
10 years (193). Children with Down syndrome
have a significantly greater incidence of cervical skeletal anomalies,
especially persistent synchondrosis and spina bifida occulta of C1,
than do normal children (194). Also, children
with both Down syndrome and atlantoaxial instability have an increased
frequency of cervical spine anomalies compared with Down syndrome
children without atlantoaxial instability (194). These spinal anomalies may be a contributing factor in the cause of atlantoaxial instability in these children.
hypermobility are asymptomatic. When symptoms occur, they are usually
pyramidal tract symptoms, such as gait abnormalities, hyperreflexia,
easy fatigability, and quadriparesis. Occasionally, local symptoms
exist such as head tilt, torticollis, neck pain, and limited neck
mobility. The neurologic deficits are not necessarily attributable to
hypermobility of the atlantoaxial or occipitoatlantal joints.
Neurologic symptoms in one series of adult patients with Down syndrome
were equally common in those with an increased ADI as in those with a
normal ADI (195). In this situation, further evaluation with flexion-extension CT or MRI scans is needed to assess for cord compression.
all the patients, catastrophic injury to the spinal cord has been
preceded by weeks to years of less severe neurologic abnormalities. In
a review by the American Academy of Pediatrics, 41 cases of symptomatic
atlantoaxial instability were compiled. In only 3 of these 41 children
did the initiation or worsening of symptoms of atlantoaxial instability
occur after trauma during organized sports activities (192).
However, symptomatic atlantoaxial instability is very rare, and the
chances of a sports-related catastrophic injury even rarer. The
reproducibility of radiographic screening for atlantoaxial and
occipitoatlantal mobility is poor (188,189,197). Furthermore, the radiologic picture can change over time, most frequently from abnormal to normal (193).
Because of all these factors, and in the absence of any evidence that a
screening program is effective in preventing symptomatic atlantoaxial
and occipitoatlantal mobility, lateral cervical radiographs are
believed to be of unproven value, and the previous recommendations for
screening radiographs by the American Academy of Pediatrics have been
retired (192).
consistent with symptomatic spinal cord injury is thus more important
than radiographs. Neurologic examination is often difficult to perform
and interpret in these children (37). Parental
education about the early signs of myelopathy is extremely important
(e.g., increasing clumsiness, more episodes of falling, and worsening
of upper-extremity function). A thorough history and neurologic
examination of the patient are more important than screening
radiographs before a decision is made about participation in sports.
However, further research is needed in this confusing matter, and
because of persistent concerns, the Special Olympics does not plan to
remove its requirement that all Down syndrome athletes have radiographs
of the cervical spine before participating in athletic events.
often obtained in the absence of neurologic symptoms. When such
radiographs are available, they should be reviewed to determine whether
there are any other associated anomalies, such as persistent
synchondrosis of C2, spina bifida occulta of C1, ossiculum terminale,
os odontoideum, and other less common anomalies. When the plain
radiographs indicate atlantoaxial or atlantooccipital instability of 6
mm or more in an asymptomatic patient, CT and MRI scans in flexion and
extension can determine the extent of neural encroachment and cord
compression.
radiographic instability, what treatment should be instituted? Those
with asymptomatic atlantoaxial or occipitoatlantal hypermobility should
probably be followed up with repeat neurologic examinations; the role
of repeat radiographs, however, is unclear, as noted in the previous
discussion. Because the risk of a catastrophic spinal cord injury is
extremely low with organized sports in Down syndrome children in the
absence of any neurologic findings, the avoidance of high risk
activities must be individualized. For those children with sudden onset
or recent progression
of
neurologic symptoms, immediate fusion should be undertaken if
appropriate imaging confirms cord compromise. The most difficult
question concerns the patient with upper cervical hypermobility with
minimal or nonprogressive chronic symptoms. Before embarking upon
arthrodesis, imaging with flexion-extension MRI (77)
or CT scan should be undertaken to confirm cord compression from the
hypermobility, and to eliminate other central nervous system causes of
neurologic symptoms. A CT scan is faster, reducing the need for
sedation, which can be potentially dangerous in these children. A CT
scan also visualizes the C1-C2 relations that are necessary to measure
the SAC. MRI is more useful for evaluating other central nervous system
lesions. Even if successfully stabilized, patients with chronic
symptoms often show little symptomatic improvement after arthrodesis (198).
recommended surgical treatment. The classic technique for posterior
C1-C2 fusion uses autogenous iliac crest bone graft with wiring and
postoperative halo cast immobilization. Internal fixation with wiring
and/or transarticular screws (199) provides
protection against displacement, shortens the time of postoperative
immobilization, permits the possible use of less rigid forms of
external immobilization, and is reported to aid in obtaining fusion (200).
However, internal fixation with sublaminar wiring poses added risk. If
the instability does not reduce as observed on routine films in the
extension position, the patient would be at high risk for developing
iatrogenic quadriplegia if sublaminar wiring and acute manipulative
reduction were tried (201,202).
For this reason it has been recommended that preoperative traction be
used to effect the reduction. If reduction does not occur with
traction, then only an onlay bone grafting should be performed, without
sublaminar wiring (201). Sublaminar wiring at
C2 is not recommended regardless of the success of reduction;
sublaminar wiring at C2 was associated with the only death of a patient
in one series (203). If wiring is to be
performed, pliable, smaller caliber wires should be used. Satisfactory
results can be obtained with onlay bone grafts and rigid external
immobilization without internal fixation (204).
A posterior translation of the ring of C1 and the os fragment into the
SAC can result from this overreduction. In a study of the results of
surgical fusion in 35 symptomatic Down syndrome children, 8 made a
complete recovery, 14 showed improvement, 7 did not improve, 4 died,
and the outcome for 2 is unknown (191).
Patients with long-standing symptoms and marked neural damage showed
little or no postoperative improvement, whereas patients with a more
recent onset of symptoms usually made an excellent recovery. Other
complications are loss of reduction despite halo cast immobilization
and resorption of the bone graft, with a stable fibrous union or an
unstable nonunion (Fig. 22.19) (204,205).
known. Individuals with Down syndrome who undergo short cervical
fusions are at risk for developing instability above the level of
fusion, such as occiput-C1 after a C1-C2 fusion or C1-C2 after lower
level fusions (208). This later instability occurred in four of five children between 6 months and 7 years after surgery.
flexion-extension lateral radiographs, should avoid collision sports
(boxing, football, wrestling). This seems prudent in view of the known
underlying ligamentous laxity and potential for development of cervical
instability. Also, all children with Down syndrome should avoid any
sports or activities that do or potentially may stress the cervical
spine (e.g., boxing, football, wrestling, ice hockey, basketball,
diving, and gymnastics). Certainly any child with progressive
instability, although he or she is neurologically intact, should also
not participate in any activities that have the potential to stress the
cervical spine. These children should also be followed up closely from
a clinical perspective to watch for the development of any neurologic
signs or symptoms. Children with neurologic signs or symptoms and
cervical instability should undergo arthrodesis, usually posterior.
Most instabilities are corrected with simple positioning. Internal
fixation is advised except for sublaminar wires at C2. If instability
is present and does not reduce as seen on routine films taken in the
extension position, the patient is at high risk for developing
iatrogenic quadriplegia if sublaminar wiring and acute manipulative
reduction were tried. Preoperative traction should be used in such a
situation to effect reduction. If reduction does not occur, then only
an onlay bone grafting should be performed without internal fixation.
The high complication rate associated with these procedures should be
remembered and parents should be counseled accordingly.
morphology. It is caused by a mutation in the glycoprotein fibrillin,
which has been mapped to the long arm of chromosome 15. Abnormalities
regarding the cervical spine in this syndrome have only recently been
described (209,210).
These are primarily abnormalities that are noted on radiographs. Focal
cervical kyphosis involving at least three consecutive vertebrae occurs
in 16% of patients with Marfan syndrome, with an average kyphosis of 22
degrees. The normal cervical lordosis is absent in 35% of patients.
Atlantoaxial hypermobility is common, occurring in approximately 54% of
patients. There is also an increased incidence of basilar impression
(36%) seen on radiographs.
Unlike
in Down syndrome, there is no increased incidence of cervical skeletal
anomalies such as persistent synchondrosis and spina bifida occulta of
C1. In spite of the abnormalities seen in patients with Marfan
syndrome, symptoms and neurologic compromise are rare. The incidence of
neck pain is not greater than that in the general population. Patients
with Marfan syndrome should be advised to avoid sports with high-impact
loading on the cervical spine; it does not appear necessary to
routinely perform cervical spine radiographs for those undergoing
general anesthesia. Atlantoaxial rotatory subluxation may be increased
in those with Marfan syndrome, and this should be specially noted
during surgical positioning.
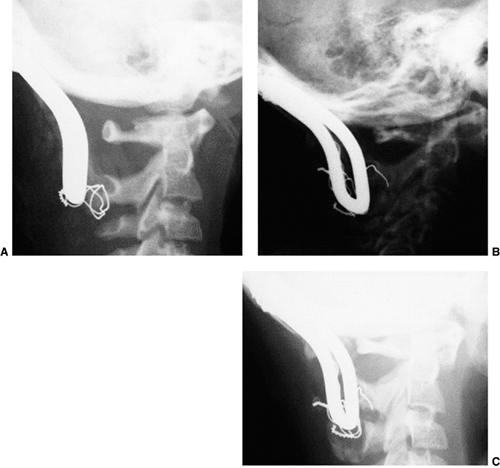 |
|
Figure 22.19 A: The child in Figure 22.3 underwent posterior cervical fusion from the occiput to C2 with internal fixation and autogenous iliac crest bone graft. B, C:
Halo-vest immobilization was maintained for 4 months postoperatively and was followed by a Philadelphia collar. Despite this postoperative treatment, the boy progressed to a nonunion, evidenced by graft resorption, wire breakage, and subsidence of the Luque rectangle, although flexion and extension radiographs 1.5 years postoperatively showed a marked decrease in hypermobility. The atlantooccipital distance was 4 mm, with only a 1-mm change in the atlanto-dens interval; the space available for the cord measured 18 mm in flexion and 19 mm in extension. The child’s neurologic symptoms also disappeared. |
absence of any underlying syndrome (e.g., Down syndrome). Georgopoulos
et al. (211) have described pediatric
nontraumatic atlantooccipital instability. Congenital enlargement of
the occipital condyles may have caused this instability by increasing
motion at this joint. The presenting symptoms were severe vertigo in
one 14-year-old boy and nausea with projectile vomiting in one
6-year-old girl. These symptoms are postulated to be a result of
vertebrobasilar arterial insufficiency resulting from the hypermobility
at the occiput-C1 junction. The diagnosis of instability was suggested
by plain radiographs initially and confirmed by cineradiography. Both
children were treated with a posterior occiput-C1 fusion, and the
symptoms resolved.
were first described in the athetoid types and subsequently in the
spastic types. Patients with athetoid cerebral palsy develop cervical
disc degeneration at a younger age than the general population. This
degeneration progresses more rapidly and involves more levels than
would normally be expected. Angular and listhetic instabilities also
are more frequent and appear at a younger age (216).
The combination of disc degeneration and listhetic instability
predisposes these patients to a relatively rapid, progressive
neurologic deficit.
extremity with decreased functional use or increased paraparesis or
tetraparesis (213,214,215). In ambulatory patients, a
loss of ambulatory ability is often seen on presentation. Occasional
loss of bowel and bladder control also occurs. Atlantoaxial instability
has been recently described in patients with severe spastic
quadriplegia; the symptoms are usually apnea, opisthotonos,
torticollis, respiratory problems, muscle tone abnormalities and
hyperreflexia, and bradycardia (217).
include narrowing of the spinal canal and premature development of
cervical spondylosis; malalignment of the cervical spine with localized
kyphosis, increased lordosis, or both; and instability of the cervical
spine manifested as spondylolisthesis. Flattening of the anterosuperior
margins of the vertebral bodies and beaklike projections of the
anteroinferior margins are radiographic findings relating to the
spondylosis. Myelography demonstrates stenosis, disc protrusion,
osteophyte projection, and blocks in dye flow, most commonly at the
C3-C4 and C4-C5 levels.
nerve root and cord compression. It is believed that the exaggerated
flexion and extension of the neck in these young adults with cerebral
palsy causes accelerated cervical degeneration and cervical stenosis
earlier than in people who do not have cerebral palsy, who develop
stenosis in the late fourth and fifth decades of life. Exaggerated
flexion and extension occur in patients with athetosis and writhing
movements. Difficulty with head control also can cause exaggerated
flexion and extension in patients with spastic cerebral palsy.
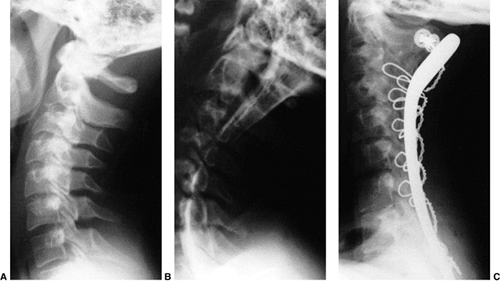 |
|
Figure 22.20
A 14-year-old girl with spastic quadriparesis showed progressive loss of upper-extremity function with loss of ability to control her wheelchair and feed herself. She also complained of some mild neck pain. A: The lateral radiograph shows marked stenosis from C3 to C6, as evidenced by a spinal canal-to-vertebral body ratio (Torg ratio) of less than 0.8. B: The myelogram shows near complete block of the dye column from C3 to C5. This stenosis was treated with posterior laminectomy from C3 to C7 and posterior cervical fusion from C2 to T1 using Luque rectangle fixation with spinous process and facet wiring. C: Eight months postoperatively, there is stable fixation and solid facet joint fusion. The girl’s upper-extremity strength is improved, and she is able to feed herself. [From Loder RT, Hensinger RN. Developmental abnormalities of the cervical spine. In: Weinstein SL, ed. The pediatric spine: principles and practice New York: Raven Press, 1994, with permission (218).] |
resection of osteophytes, and interbody fusion have been the most
effective methods. A halo cast is best and is well tolerated in some
patients with athetosis (214). However,
postoperative immobilization can be a problem for some other patients,
thus some surgeons recommend a posterior wiring of the facets as well
in order to minimize the duration of postoperative immobilization (213). Posterior laminectomy when performed alone (214)
is contraindicated in patients with cerebral palsy with developmental
cervical stenosis because this will increase the instability. Long-term
follow-up of surgically treated patients demonstrates late disc
degeneration and increased range of motion at adjacent segments in
those who underwent anterior arthrodesis (219).
immature, growing children. It has been duplicated in animal models; a
C3-C6 laminectomy in growing cats uniformly resulted in kyphosis,
whereas normal cervical curves were maintained in adult cats (227).
The natural history of postlaminectomy kyphosis is unknown; however,
the incidence of kyphosis when extensive cervical laminectomies are
performed in childhood varies from 33% to 100%, with an overall average
of 70% (225). Postlaminectomy kyphosis is
weakly correlated with age (mean age at laminectomy, 10.5 years) and is
not dependent upon the total number of levels decompressed or the
location of these levels (225). Postlaminectomy lordosis is less common and is strongly correlated with a peak age at decompression of 4 years (225).
In one study, 12 of 15 children who had undergone a cervical or
cervicothoracic laminectomy prior to 15 years of age developed kyphosis
(224). The normal posterior muscular
attachments to the spinous processes and laminae, as well as facet
capsules, the ligamentum nuchae, and the ligamentum flavum, are
violated by the laminectomy. This loss of posterior supporting
structures allows for a progressive deformity, which, if kyphotic in
nature, can eventually result in neurologic symptoms and deficits.
Early radiographic features show a simple kyphosis; later, vertebral
body wedging and anterior translations of one vertebral body on another
can develop. A late, severe deformity is the swan neck deformity (223).
Neurologic problems result from cord stretch and compression from the
anterior kyphotic vertebral bodies. MRI is useful in delineating the
extent of cord attenuation and compression.
frequent radiographic follow-up studies; the role of prophylactic
bracing is not yet known. When kyphotic deformities develop, anterior
vertebral body fusion, followed by immobilization with a Minerva cast
or halo cast or vest, is recommended (222) (Figs. 22.21 and 22.22). The role of a prophylactic posterior fusion at the time of laminectomy is not yet known (220), nor is the role of osteoplastic laminotomy instead of laminectomy (228), although this approach might not always be suitable in the context of the primary pathology.
deficiencies, facial anomalies, and variable major and minor
malformations are the characteristics of fetal alcohol syndrome. The
children present with developmental delay, especially in motor
milestones, failure to thrive, mild to moderate retardation, mild
microcephaly, distinct facies (hypoplasia of the facial bones and
circumoral tissues), and congenital cardiovascular anomalies. The
cervical findings are similar to those in Klippel-Feil syndrome.
Radiography reveals congenital fusion of two or more cervical
vertebrae, resembling Klippel-Feil syndrome, in approximately half of
the children (229). The major visceral anomaly
in fetal alcohol syndrome occurs in the cardiovascular system, whereas
in Klippel-Feil syndrome the major anomaly is in the genitourinary
system (229).
and treatment recommendations regarding the cervical spine are the same
as those for Klippel-Feil syndrome.
anomaly. It can be a solitary finding, but more often it is associated
with other syndromes and anomalies. Children with cleft palate
anomalies have a 13% to 18% incidence of cervical spinal anomalies
compared with the 0.8% incidence in children undergoing orthodontia
care for other reasons (230,231).
This incidence is highest in patients with soft palate and submucous
clefts (45%). These anomalies, usually spina bifida and vertebral body
hypoplasia, are predominantly in the upper cervical spine. The
potential for instability is unknown, as is the natural history. No
documented information regarding treatment is available; however, the
clinician should be aware of this association and make sound clinical
judgments as needed. These patients also demonstrate a reduced cervical
lordosis compared to those without cleft lip and/or palate (231).
Goldenhar, and Saethre-Chotzen—exhibit cervical spine fusions,
atlantooccipital fusions, and butterfly vertebrae (232,233,234,235,236,237). Fusions are more common in Apert syndrome (71%) than in Crouzon syndrome (38%) (232). Upper cervical fusions are most common in Crouzon and Pfeiffer syndromes (234), whereas in Apert syndrome the fusions are more likely to be complex and involve C5 and C6 (232).
However, this syndrome variation is not accurate enough for syndromic
differentiation. Congenital cervicothoracic scoliosis with rib fusions
is seen in Goldenhar syndrome, usually from hemivertebrae (234,238).
C1-C2 instability in Goldenhar syndrome may be as high as 33%, and
these children should be monitored carefully for this potential problem
(239).
younger children the vertebrae appear to be separated by intervertebral
discs, but as the children grow older the vertebrae fuse. There are no
specific, standard recommendations for treatment. The author recommends
following the same principles as for Klippel-Feil syndrome. The main
concern is the potential difficulty with intubation in these children.
Odontoid anomalies are rare; however, if any question exists regarding
the stability of the cervical spine, lateral flexion and extension
radiographs should be obtained. Children with Goldenhar syndrome have a
high incidence of C1-C2 instability (240).
There a high incidence of diabetes among the mothers of children with
Goldenhar syndrome; it has recently been suggested that children with
Goldenhar
syndrome should be assessed for maternal diabetes exposure, which
should aid in counseling concerning cause and risk of recurrence (239).
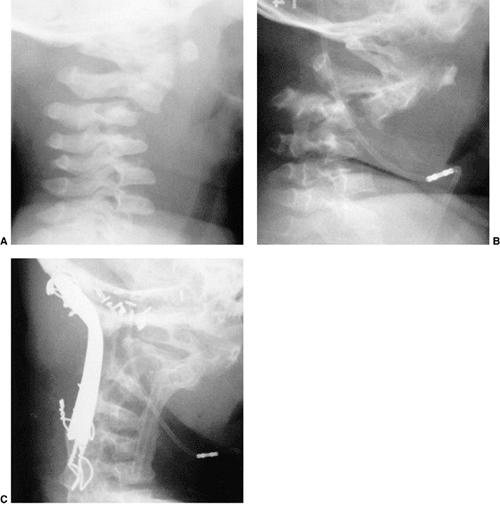 |
|
Figure 22.21 A:
Lateral cervical spine radiograph of a 9-month-old boy with neurofibromatosis. Note the preexisting cervical kyphosis at C2-3. At 3 years of age, he underwent a suboccipital craniotomy and cervical laminectomy from C1 to C4 for resection of neurofibromata. B: By 3 years and 10 months of age he had developed a 90-degree kyphosis. C: He underwent combined anterior cervical fusion from C2 to C6 with a fibular strut graft and posterior cervical fusion from the occiput to C6 with internal fixation consisting of a Luque U-rod. Three years postoperatively, solid fusion with a residual 70-degree kyphosis is present. |
disorder in humans. The proportion of patients with neurofibromatosis
and cervical spine involvement is difficult to assess: 30% of
neurofibromatosis patients in the series of Yong-Hing et al. (241)
and 44% with neurofibromatosis and scoliosis or kyphosis had cervical
spine lesions. The cervical lesions are often asymptomatic (241).
Symptoms, when they do occur, include diminished or painful neck
motion, torticollis, dysphagia, deformity, and neurologic signs ranging
from mild pain and weakness to paraparesis and quadriparesis (57,242). Neck masses constituted 20% of presenting symptoms in one study of patients with neurofibromatosis (243).
Lateral flexion and extension radiographs are recommended for all
patients with neurofibromatosis before general anesthesia or surgery (241).
MRI is helpful in assessing the involvement of neural structures and
dural ectasia. CT scan is useful in evaluating the upper cervical spine
complex and the bony definition of the neural foramen. The natural
history regarding the cervical spine in patients with this condition is
unknown, but those with severe kyphosis often develop neurologic
deterioration.
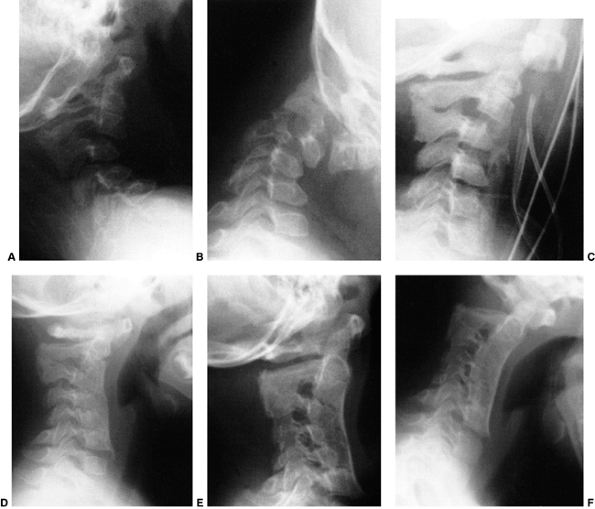 |
|
Figure 22.22
This girl underwent a cervical laminectomy from C2 to C6 for a low-grade astrocytoma of the cervical cord. At 1 year and 7 months of age, she had a postlaminectomy kyphosis that was 45 degrees in extension (A) and 82 degrees in flexion (B). C: An anterior cervical discectomy and fusion from C2 to C6 was performed with autogenous iliac crest strut graft. Immediately after surgery, the kyphosis was corrected to 20 degrees. Halo-vest immobilization was used for 3 months. D: Solid incorporation of the fusion occurred by 6 months postoperatively. At 4 years and 7 months of age, flexion (E) and extension (F) lateral radiographs show maintenance of the correction, solid fusion, and no instability at the remaining levels. |
Fusion, with or without internal fixation, is usually achieved with
simple interspinous wiring; a halo cast or vest is usually needed after
surgery. Kyphosis requires both anterior and posterior fusion (Fig. 22.21).
Pseudarthroses are frequent with isolated posterior fusions.
Vascularized fibular grafts may be necessary to effect fusion in
difficult cases (244,246). If there are no indications for surgical treatment, the patient should be followed up closely.
of connective tissue with progressive soft tissue ossification. The
disorder itself is rare; most cases represent new spontaneous
mutations. Eventually all patients with this disorder develop cervical
spine changes (248), often starting in childhood. These patients usually present with neck stiffness (249)
within the first 5 years of life. No cases of neurologic compromise
have been reported. Other general clinical features are big toe
malformations, reduction defects of all digits, deafness, baldness, and
mental retardation. Early in the course of the disease
small
vertebral bodies and large pedicles are seen radiographically.
Occasionally nuchal musculature ossification is also seen. Later,
neural arch fusions are seen. This factor reflects the progressive
ossification of the cervical spinal musculature, ligament ossification,
and spontaneous fusion of the cervical discs and apophyseal joints. No
effective medical treatment is known. Surgical treatment of the
cervical spine is not necessary.
occur more in boys than in girls. In one study, the age- and
gender-adjusted incidence in the general population was 7.41 per
100,000 per year (250); this incidence was much
less in children younger than 11 years, 1.19 per 100,000, than in
adolescents (older than 11 years, 13.24 per 100,000). The cause of the
injury in children is frequently a fall, whereas in adolescents it is
frequently related to sports, recreational activities, or motor vehicle
crashes. Children involved in side impact crashes are more likely to
have cervical spine injuries than those involved in frontal crashes (251). Unrestrained children are more likely to sustain cervical spine injuries in motor vehicle crashes than restrained children (252,253).
In general, children (younger than 11 years of age) are more likely to
sustain ligamentous injuries and injuries to the upper cervical spine,
whereas adolescents are more likely to sustain fractures and injuries
to the lower cervical spine (250). In a large
series of 1098 children with cervical spine injury, upper spine
injuries occurred in 52%, lower cervical spine injuries in 28%, and
both upper and lower injuries in 7% (254). Upper cervical spine injuries carry a significantly higher mortality than do lower cervical spine injuries (254).
By the age of 10 years the bony cervical spine has reached adult
configurations, and the injuries sustained are essentially those of the
adult. Therefore, this chapter will concentrate on injuries sustained
in the first decade of life.
have sustained polytrauma and frequently arrive immobilized on
backboards and wearing cervical collars. If the child is comatose or
semiconscious, if there are external signs of head injury, or if the
child complains of neck pain, cervical spine radiographs are needed.
All children involved in motor vehicle crashes who have head trauma and
neck pain, or who have neurologic signs or symptoms, should have
cervical spine radiographs (255,256).
The views recommended for this initial screening are the cross-table
lateral and anteroposterior views. The need for an open-mouth odontoid
is controversial, especially in children less than 5 years of age (257,258).
If the child is too critically ill to be positioned for all views, then
the cross-table lateral view is adequate until a complete evaluation
can be performed. Cervical spine precautions must be maintained until a
complete evaluation has demonstrated no injury. Once a cervical injury
has been identified, close scrutiny must be undertaken to ensure that
there are no other injuries in the remainder of the axial skeleton.
standard backboard. Young children have a disproportionately large
head, and positioning them on a standard backboard leads to a flexed
posture of the neck (Fig. 22.23A) (259).
This flexion can lead to further anterior angulation or translation of
an unstable cervical spine injury and can also cause pseudosubluxation,
which in itself in an injured child can be difficult to interpret. To
prevent this undesirable cervical flexion in young children during
emergency transport and radiography, modifications must be made by
either creating a recess for the occiput of the larger head or using a
double mattress to raise the chest (Fig. 22.23B). A simple clinical guideline is to align the external auditory meatus with the shoulder.
necessary in order to determine the stability of the cervical spine;
hyperflexion ligamentous injuries may not be seen immediately, and
flexion and extension views a few weeks later, after the spasm has
subsided, may document instability. In one series of children with
ligamentous injuries of the cervical spine, 8 of 11 children with lower
cervical instability were diagnosed between 2 weeks and 4 months after
the trauma (260).
seen before the actual injury or fracture itself. Malalignment of the
spinous processes on the anteroposterior radiograph should be regarded
as highly indicative of a jumped facet joint. Widening of the posterior
interspinous distances should be regarded as highly indicative of a
posterior ligamentous injury. In adults, an increase in the
retropharyngeal soft tissue space can indicate a hematoma in the
setting of trauma, and raise the suspicion that an upper cervical
fracture exists. In children, however, the pharyngeal wall is close to
the spine in inspiration, whereas there may be a large increase in this
space with forced expiration, as when the child cries (261). This should be remembered
when considering the significance of prevertebral pharyngeal soft
tissue in the cervical spine radiographs of a frightened, crying child.
 |
|
Figure 22.23 A:
Positioning a young child on a standard backboard forces the neck into a kyphotic position because of the relatively large head. B: Positioning a young child on a double mattress, which raises the chest and torso and allows the head to translate posteriorly, creates a normal alignment of the cervical spine. (From Herzenberg JE, Hensinger RN, Dedrick DK, et al. Emergency transport and positioning of young children who have an injury of the cervical spine. J Bone Joint Surg Am 1989;71-A:15–22, with permission.) |
cervical spine, especially the ring of the atlas and, occasionally, the
odontoid. As a rule, CT scan is not recommended for screening but only
in order to further study suspicious areas on plain radiographs or in
planning treatment. It should be used for studying all fractures of C1.
MRIs are useful in assessing the spinal cord and discs. In an injured
child, an MRI is the method of choice for assessing the cervical spine
when (a) the child is obtunded and/or nonverbal, and a cervical spine
injury is suspected; (b) the plain radiograph findings are equivocal;
(c) neurologic symptoms are present without radiographic findings; or
(d) there is inability to clear the cervical spine in a timely manner (262).
With the present rapid response to trauma victims and aggressive field
care, more of these children now survive. These children are usually
polytrauma victims with severe head injuries, and they present with a
range of clinical neurologic pictures (263,264).
In the past, those who survived had incomplete lesions, often
demonstrating cranial nerve dysfunctions and varying degrees of
quadriplegia. Many of the children who presently survive have complete
loss of neurologic function below the brain stem and live only because
of outpatient ventilatory support. Other presentations may range from a
responsive child with hypotension or tachycardia to a patient in
complete cardiac arrest. Occasionally, some patients present with
normal findings on neurologic examination. As of 2001, there were 29
children with atlantooccipital dislocation who survived (269).
of the cases do not demonstrate marked radiographic displacement. In
the past, a Powers ratio of greater than 1.0 (Fig. 22.24A) was used as an indication of the presence of atlantooccipital dislocation (270).
This criterion can cause the practitioner to miss isolated distraction
injuries, anterior atlantooccipital dislocations that have
spontaneously reduced after injury, and posterior atlantooccipital
injuries (263). For this reason, the distance between the tip of the dens and the basion (Fig. 22.24B)
has been used as a sign, in which a distance of more than 12.5 mm
indicates the potential for atlantooccipital dislocation. Recent
studies have described the stabilizing nature of the tectorial
membrane. When this membrane is disrupted, there is a high likelihood
of atlantooccipital instability. If the C1-C2 to C2-C3 posterior
interspinous ratio is greater than 2.5, there is a high chance of
tectorial membrane disruption, and MRI evaluation is warranted (271).
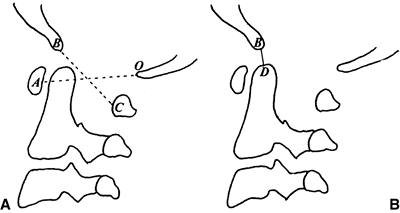 |
|
Figure 22.24 The BC:OA ratio (Powers ratio) (A) and the DB distance (B)
are used for assessing traumatic atlantooccipital dislocation. A ratio greater than 1.0 and a DB distance greater than 12.5 mm indicates the potential for atlantooccipital dislocation. (From Bulas DI, Fitz CR, Johnson DL. Traumatic atlanto-occipital dislocation in children. Radiology 1993;188:155–158, with permission.) |
its diagnosis. If the suspicion of craniocervical trauma persists after
inconclusive plain radiography, CT or MRI scans can be quite useful (Fig. 22.25). Subarachnoid hemorrhage at the craniocervical junction will be seen after atlantooccipital dislocation (272); a CT scan can also assist in assessing osseous alignment (273).
Once diagnosed, standard respiratory and other supporting measures are
given. Early definitive immobilization of the dislocation should be
undertaken. The immobilization can be with a halo cast alone or with
supplemental internal fixation and posterior fusion (272,274). Traction should be avoided because it can distract the joint and cause further neurologic injury (275).
These children must be moved rapidly into an upright position in order
to maximize pulmonary care. Late neurologic deterioration may indicate
progressive hydrocephalus or retropharyngeal pseudomeningocele (276).
It is caused by an axial load from the head into the lateral masses.
Unlike in adults, a single fracture through the ring in children may be
isolated, hinging on the synchondrosis (278,280)
instead of a double break in the ring. Alternatively, a bifocal
posterior arch fracture can occur—a Jefferson fracture variant (281). A transverse atlantal ligament rupture may occur as the lateral masses separate, resulting in C1-C2 instability.
and the assessment of healing. This injury in children is not commonly
seen on plain radiographs, which usually show only an asymmetry between
the odontoid and the lateral masses. Even if it is clearly seen on
plain radiographs, a CT scan should also be performed to confirm the
diagnosis and to rule out a transverse alar ligament rupture. Treatment
is usually simple immobilization with
a
Minerva or halo cast. Rarely is surgery necessary unless rupture of the
transverse alar ligament occurs, rendering the spine unstable. The
timing of surgery needs to be individualized.
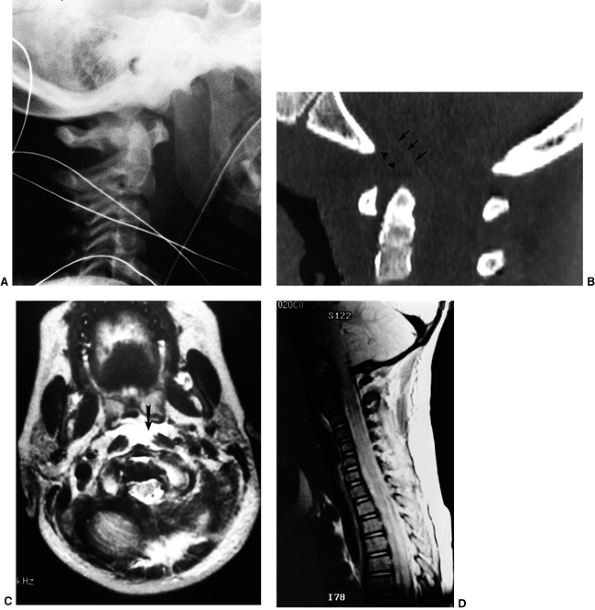 |
|
Figure 22.25
This girl, 5 years and 6 months of age, was hit by a van from behind, and presented with bilateral palsies of the cranial nerve VI. A: The lateral radiograph of the upper cervical spine demonstrates a rotational malalignment: the basion hemi-shadows fail to overlap while the C1 arches nearly superimpose upon each other, raising the possibility of atlantooccipital dislocation. B: A computed tomography (CT) scan with sagittal reconstruction demonstrates elevation of the periosteum at the caudal level of the clivus (arrows) and hemorrhage (arrowheads). C: An axial image from the magnetic resonance imaging (MRI) scan demonstrates abnormal fluid accumulation immediately anterior to the atlantooccipital junction (arrow). D: The MRI scan sagittal view demonstrates subarachnoid space narrowing at the level of the foramen magnum and atlantooccipital joint. |
As seen on radiographs, the ADI is increased, usually well beyond the
normal 5 mm. Adequate ligamentous healing and stability are not
achieved by simple immobilization. The recommended treatment is
reduction in extension, posterior cervical C1-C2 fusion with autogenous
bone graft, and immobilization with a halo or Minerva cast. A solid
arthrodesis is documented on flexion and extension lateral radiographs
after 2 to 3 months of immobilization. If the ligament is
avulsed
from the lateral masses of C1 and the bony avulsion attached to the
ligament is close to the lateral mass, simple immobilization may be
adequate (282).
They are usually physeal fractures of the dentocentral synchondrosis,
usually Salter-Harris type I fractures. These may occur after major or
minor trauma. Neurologic deficits are rare. These fractures usually
displace anteriorly with the dens posteriorly angulated (Fig. 22.26).
This fracture is usually seen only on the lateral view. If it is
difficult to tell from the radiographs whether there is a fracture or
merely a mild, normal, posterior angulation of the dens [which occurs
in up to 4% of normal children (284)], dynamic
flexion and extension CT scans with sagittal reconstructions can be
performed to evaluate for any motion or instability.
with mild extension and posterior translation. In most circumstances
the simple double mattress technique is all that is needed to obtain a
reduction. After a few days of recumbence and early healing, the
fracture can be immobilized easily with the use of a Minerva or halo
cast. As with all physeal fractures, healing is rapid, and
immobilization can usually be discontinued in 6 to 10 weeks. Flexion
and extension lateral radiographs should be taken to confirm union with
stability. These fractures, unlike those in adults, do not have a
significant nonunion rate requiring subsequent C1-C2 fusion. The intact
hinge of anterior periosteum most likely aids in the ease of reduction
and accounts for the stability of reduction and rapid healing.
reported on a series of five cases in children. Care must be taken to
not confuse this fracture with congenital anomalies that may mimic a
hangman fracture and lead to overtreatment (286,287,288,289). Similarly, it may be caused by child abuse (290).
These fractures readily heal with immobilization in either a Minerva or
halo cast after gentle positioning to obtain a reduction. Traction by
itself, as with most cervical injuries in children, should be avoided
because it overdistracts the spine and is associated with an increased
potential for nonunion and more serious neurologic injury. Posterior
cervical fusion of C1-C3 is indicated for the rare case of nonunion or
instability.
The typical patterns of fracture are usually compression fractures of
the vertebral body, or facet fractures and dislocations caused by
hyperflexion. These injuries are adult in pattern, and standard adult
treatment should be used. Physeal fractures, usually of the inferior
end plates, also can occur (292) because of
hyperextension. In older children they are usually ring apophyseal
fractures with minimal instability or neurologic damage. In younger
children they usually involve the entire end plate. Physeal fractures
are frequently not recognized in severely injured children, and they
may be noted for the first time at autopsy (292). These fractures are associated with a high incidence of neurologic injury (Fig. 22.27).
In these children, simple positioning (e.g., double mattresses or,
rarely, traction) followed by immobilization is all that is needed for
treatment. Because these are physeal injuries, healing is rapid.
The pivot point for younger children is in the upper cervical spine
because of the large size of the head, weak cervical musculature,
incompletely ossified wedge-shaped vertebrae, physiologic ligamentous
laxity, and horizontal facet joints in this region. The upper cervical
spine offers little resistance to traumatic shear forces, which often
result in ligamentous instability. The goal is to differentiate this
traumatic ligamentous instability from pseudosubluxation using the
posterior cervical line.
described true ligamentous instability at C2-C3, and not
pseudosubluxation. Most of the children with this condition sustained
an injury with the head and neck in flexion, usually caused by falls or
sports injuries (260,293).
The patients complain of severe neck pain. Immediately after injury the
instability may not be radiographically apparent, and becomes
noticeable only after progressive kyphosis is noted. In younger
children, avulsion of the cartilaginous tips of the C2 spinous process
is not visible on plain radiographs or CT scan. Later on, the avulsion
fragments become ossified. This late ossification, along with the
development of C2-C3 kyphosis, leads to the diagnosis. In one study of
ligamentous injuries of the cervical spine in children, 7 of 11
injuries occurred at the C2-C3 level. Treatment must be individualized;
both simple immobilization and posterior cervical arthrodesis have been
used with good results. When instability exists, treatment should
consist of posterior cervical fusion with Minerva or halo
immobilization (Fig. 22.28). However, children
who have undergone arthrodesis for cervical spine injury do demonstrate
decreased mobility and increased osteoarthritis at long-term follow-up (294).
Treatment for a mild sprain is immobilization for comfort followed by
flexion and extension radiographs several weeks to a few months later
to ensure that late instability does not occur.
cord with transient quadriplegia. It is seen most often in collegiate
and professional athletes (295,296), although there are several instances among younger athletes also (297).
The incidence in the National Collegiate Athletic Association is 1.3 per 10,000 athletes per season (296).
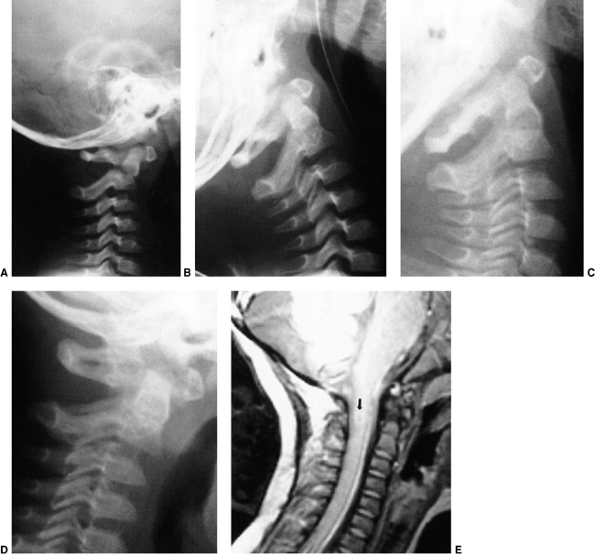 |
|
Figure 22.26
This boy, 2 years and 9 months of age, was brought to the emergency department unable to move his upper extremities, and withdrew his lower extremities only in response to noxious stimuli. He had a reported history of falling off couches. On investigation, it became clear that the child had been battered. A: A lateral radiograph demonstrates the odontoid fracture through the dentocentral synchondrosis with anterior angulation and translation. A magnetic resonance imaging (MRI) scan did not reveal any abnormalities in the cord. B: Simple positioning with a double mattress allowed for reduction of the fracture; the child was maintained on a double mattress for several days to allow for subsidence of cord edema and early healing. He was then placed into a Minerva cast 10 days after the injury. The cast was removed 6 weeks after the injury, followed by immobilization with a soft collar. Flexion (C) and extension (D) radiographs demonstrated no instability with the healed fracture. E: The child improved remarkably. Two months after the injury he was running and walking without difficulty. There were some subtle upper-extremity changes, indicated by a change in hand dominance from right to left. MRIs showed signal changes in the cord (arrow), which were interpreted as development of an early posttraumatic syrinx. |
decreased in the athletes with transient quadriparesis. The spinal cord
is compressed on forced hyperextension or hyperflexion, causing the
transient quadriparesis. Sensory changes such as burning pain,
numbness, tingling, and loss of sensation and motor changes ranging
from weakness to complete paralysis are seen. These episodes are
transient, and recovery occurs in 10 to 15 minutes; neck pain is
not
present at the time of injury. Transient quadriparesis needs to be
differentiated from a brachial plexus stretch, or “burner.” Patients
with the latter condition present with a monoparesis of the upper
extremity and often with neck pain.
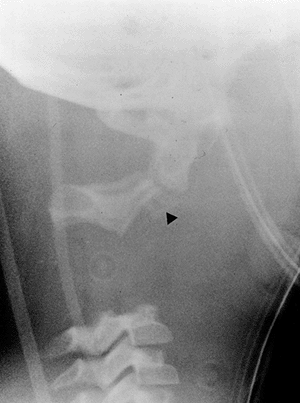 |
|
Figure 22.27
This girl, 7 years and 2 months of age, sustained polytrauma and presented in an agonal state. A lateral radiograph demonstrates complete separation at the C2-C3 level along with an associated C2 hangman fracture. Also note the small fleck of bone (arrowhead) attached to the base of the C2 body; this likely represents an avulsion of the superior aspect of the body of C3 with the C2-C3 disc. |
the spinal canal to the vertebral body is decreased; a value of 0.8 for
this ratio indicates significant developmental cervical stenosis.
Congenital fusions, cervical instability, and intervertebral disc
disease may also exist. In children, this spinal canal-to-vertebral
body ratio is not as accurate, and is inconsistent in predicting spinal
cord concussion (297). An MRI may be necessary in order to assess the presence or absence of a herniated nucleus pulposus.
nonsurgical treatments that are needed are collars, analgesics, and
antispasmodics. The efficacy of fusions for coexisting instability,
discectomy for herniated nucleus pulposus, and decompression for
congenital cervical stenosis is not known.
to participate in athletic activities, and if he or she were to do so,
what would be the risk of a permanent quadriplegia developing with a
later episode. Torg et al. (298) believe that
athletes with pure developmental spinal stenosis are not predisposed to
more severe injuries if they return to sports and that only those with
instability or degenerative changes should be precluded from
participation in contact sports. Odor et al. (299)
found that one third of professional and rookie football players have a
spinal canal ratio of less than 0.8 and that it is difficult to make
decisions about continuing with the sport on the basis of this ratio
alone. Eismont et al. (300), however, have
shown that smaller cervical canals are correlated with significant
neurologic injury in routine trauma. Considering this finding, and the
fact that narrowing of the spinal canal correlates even more closely
with spinal cord concussion in children (297),
it is prudent to keep any child who has had a cervical cord concussion
from playing contact sports until further epidemiologic data have been
established.
(SCIWORA) occurs in 5% to 55% of all pediatric spinal cord injuries
according to the neurosurgical literature (301); however, multicenter databases in a recent spinal cord injury study suggest that the incidence is much less (302).
By definition, no disruption, malalignment, or other abnormalities are
seen on plain radiographs. The immature and elastic pediatric spine is
more easily deformed than that of an adult. Momentary displacement
caused by external forces endangers the spinal cord without disrupting
bone or ligaments. The four major factors involved in such injuries are
hyperextension, flexion, distraction, and spinal cord ischemia.
Ischemia may arise from cord contusion or direct vascular insult (303). Spinal stenosis is not a factor; in a recent study of 145 children with cervical SCIWORA (304), the average Torg ratio was greater than 1.0.
spinal cord function to partial cord deficits. The physiologic
disruption of the spinal cord is not necessarily associated with
anatomic disruption. Most deficits (78%) are cervical; patients with
upper cervical SCIWORA are more likely to have severe neurologic
lesions than patients with lower cervical SCIWORA. An MRI is most
useful for studying the cord and disc-ligament complexes, and it
correlates well with clinical outcome (305). Positive MRI findings are typically seen only in children with very severe neurologic involvement at presentation (306).
The outcome usually is determined by the presenting neurologic status.
Approximately one fourth of these children have a secondary
deterioration in neurologic function.
approach. Pang and Pollack recommend immobilization in a Guilford brace
for 3 months and complete avoidance of all sports (301).
However, in the same series, no instability was noted in any of the
children at initial evaluation, and only one child later developed
instability as seen on flexion and extension radiographs. Without
documented
radiographic instability, the biomechanical usefulness of brace
immobilization is questionable. Pang and Pollack, however, describe
this as treatment for “incipient instability” (301).
More recent studies question both the reality of the recurrence of a
SCIWORA and the efficacy of bracing in the treatment of SCIWORA (304).
Most ligamentous spine injuries, when allowed to heal with simple
immobilization, do not return to the stability seen in the preinjury
state; fusion is usually needed. It is atypical for SCIWORA to behave
differently regarding instability, incipient or otherwise. Regardless
of whether the child is braced, close follow-up of neurologic function
is needed. Flexion and extension radiographs should be taken after 3
months of bracing; any late development of instability requires
surgical stabilization.
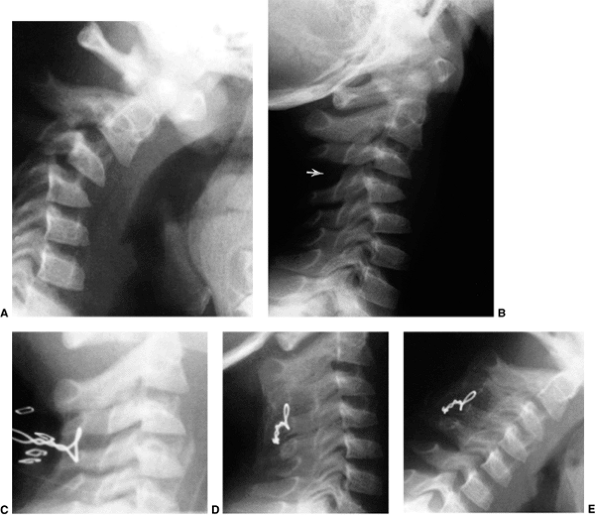 |
|
Figure 22.28
This boy, 4 years and 9 months of age, was run over by a snowmobile trailer 2 weeks before these radiographs were taken. He had complained of some neck pain and had been treated by chiropractic manipulation during these 2 weeks. The flexion (A) and extension (B) radiographs demonstrate marked instability at the C3-C4 interspace, which does not completely reduce, even with extension (arrow). C: He was treated by posterior fusion with iliac crest bone graft and interspinous wiring at C3-C4, as shown in this intraoperative radiograph. Halo-vest immobilization was used for 3 months. D and E: One year postoperatively, there was no instability at the C3-C4 level, but there was solid fusion, which had extended to C2 and C5, despite meticulous care not to expose the laminae of C2 and C5 or the interspinous ligaments of C2-C3 and C4-C5. |
cord, and during delivery with prolonged distraction it may be tethered
by nerve ends and blood vessels, injuring the cord but not the
chondroosseous structures. Damage to the vertebral artery, with
resultant ischemia of the cord, can also occur (310).
In battered children, the large head, poorly supported by the cervical
musculature, makes the upper cervical spine vulnerable to repeated
shaking, leading to either SCIWORA or a fracture. In a recent Canadian
study, the incidence of cervical spine injury is 4% of victims of
shaken baby syndrome (311).
Temperature regulation dysfunction can cause fevers, reflex movements
may be mistaken for voluntary movements, and respiratory distress can
occur from paralyzed intercostal muscles. These patients may also
present with symptoms that resemble a cerebral palsy–like picture (313) or sudden infant death syndrome (314). In one study, the diagnosis was delayed in three of four children, and the delay averaged 4.4 years from birth (315).
Typically, no fractures are seen radiographically; MRI is often helpful
in assessing cord damage. Anterior rupture of the lower cervical
intervertebral discs is seen on pathologic examination in children who
are victims of shaking (316).
neurologically. Treatment is usually nonsurgical because these are very
young infants. Bed rest, respiratory support, and physical therapy to
prevent paralytic contractures should be instituted. Older children
with bony injuries may need Minerva casts or halo immobilization.
Surgical fusion with stabilization is rarely needed.
When these devices, which clearly make automobile travel safer for
children, are not adequately tightened, serious and potentially fatal
injuries can occur. The harness must be adjusted periodically to
account for normal growth and seasonal changes in clothing thickness.
Car seat styles that allow the main lock to be attached to the crotch
strap (Fig. 22.29) prevent infants from sliding
forward, which can apply hyperextension forces to the head, the neck,
and the upper chest. Neurocentral synchondrosis separation between the
body and the neural arches is a common pattern (318,320).
One third of these injuries involve the cervical spine. Various degrees
of neurologic loss are noted, with complete lesions in 75% of the
patients in one series (321). Nonetheless, additional injuries to other body areas may cause more morbidity than the trauma to the neck.
 |
|
Figure 22.29 A child in a car seat without locking the crotch strap (arrow)
is in a dangerous situation that can allow serious injury in a collision. (From Conry BG, Hall CM. Cervical spine fractures and rear car seat restraints. Arch Dis Child 1987;62:1267–1268, with permission.) |
fracture and intracanal bullets may be seen radiographically. Other
imaging studies, such as arteriography and esophagography, are often
needed in order to look for other injuries. Panendoscopy is useful for
assessing injury to the trachea and esophagus. Spinal decompression is
not indicated in either complete or incomplete lesions. For patients
with complete injuries, removal of retained bullet fragments from the
canal does not improve neurologic outcome. Patients who undergo
decompression have a higher risk of meningitis and spinal instability,
without any added benefit. Spinal instability is rare unless
laminectomy is performed. Indications for neck exploration surgery are
a positive arteriogram, impending airway obstruction, tracheal
deviation, widened mediastinum, expanding hematoma, and appropriate
pathology on panendoscopy. Routine exploration of the neck and wound is
not advised.
that can affect the joints of the cervical spine as well. The subtypes
that usually involve the cervical spine are the polyarticular and
systemic onset types; only rarely does the pauciarticular type affect
the cervical spine (323).
to 2 years from disease onset and presents with stiffness. Pain and
torticollis are rare, and when they occur in a patient with juvenile
rheumatoid arthritis other causes should be explored, such as fracture,
infection, and tumor.
Torticollis was present in only 4 of 92 children in the series of Fried et al. (324) and in 1 of 121 children in the series of Hensinger et al. (323). Abnormal neurologic findings are also infrequent in these children.
-
Anterior erosion of the odontoid process
-
Anteroposterior erosion of the odontoid process (apple-core odontoid)
-
Subluxation of C1 on C2
-
Focal soft tissue calcification appearing adjacent to the ring of C1 anteriorly
-
Ankylosis of the apophyseal joints
-
Growth abnormalities
-
Subluxations between C2 and C7
neck stiffness are soft tissue calcification at the leading edge of C1,
anterior erosion of the odontoid process, and apophyseal joint
ankylosis (Fig. 22.30). Although there may be mild hypermobility at C1-C2 with flexion
and extension, true instability and myelopathy are rare. Basilar
invagination, which often occurs in adult rheumatoid arthritis, also is
rare in juvenile rheumatoid arthritis (324).
The radiographic findings in juvenile rheumatoid arthritis that differ
most from those in adult rheumatoid arthritis are late destruction of
articular cartilage and bone, growth disturbances, spondylitis with
associated vertebral subluxation and apophyseal joint ankylosis, and
micrognathia (325,326). In five patients who had long-standing disease (average age, 19 years), Hallah et al. (327) described a nonreducible head tilt caused by collapse of an atlantoaxial lateral mass.
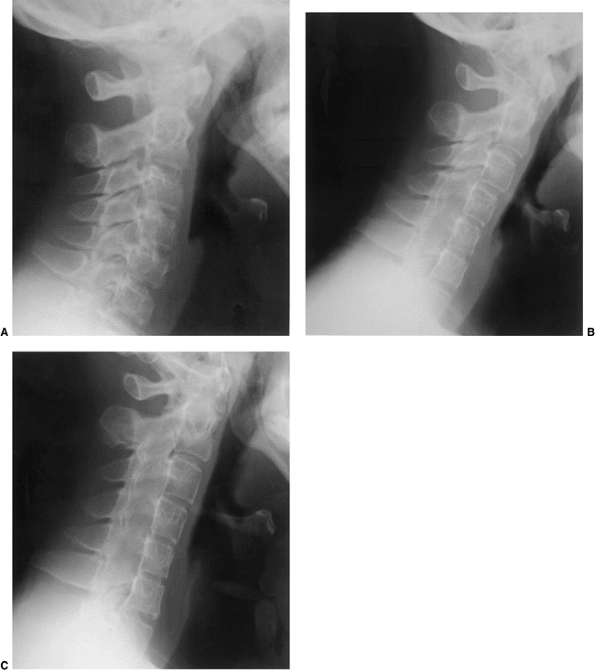 |
|
Figure 22.30 Cervical spine radiographs of a boy with systemic onset juvenile rheumatoid arthritis. A: At 10 years of age, note the facet joint narrowing posteriorly from C2 to C6. B:
At 17 years of age the facet joints from C3 to C6 have totally fused, with complete bony ankylosis. Also note the apple-core odontoid. C: By 21 years of age, there has been complete bony ankylosis between C2 and C3. The facet joint at C6-C7 is narrowed but not completely fused. Also note that the C4, C5, and C6 vertebral bodies are smaller in both height and depth. |
juvenile rheumatoid arthritis and neck pain. A bone scan is used for
pinpointing the exact anatomic location of activity, and the anatomy is
further studied with a CT scan. These studies can be helpful in looking
for occult fractures, infections, and bony tumors.
and the pannus of the synovial ring surrounding the odontoid process.
The pannus erodes the odontoid anteriorly and posteriorly, but leaves
the apical and alar ligament attachments free, creating the apple-core
lesion (Fig. 22.30B). This lesion is more
susceptible to fracture, both from erosions and from vascular
compromise to the odontoid, because the blood supply to the odontoid,
which courses along its side (328), may be
disturbed by the invading pannus. Ankylosis of the apophyseal joints is
most common in the systemic onset subtype. In these young children,
posterior ankylosis of the immature spine creates a tether, preventing
further anterior growth. Decreased disc-space height and smaller
vertebral bodies, both longitudinally and circumferentially, are the
result (Fig. 22.30C) (325).
with good rheumatologic care. Patients rarely develop flexion
deformities; early in the course of the disease, a cervical collar may
prevent this deformity (324). A cervical collar
is recommended for patients who have involvement of the odontoid
process or subaxial subluxation whenever they are in an automobile or
other mode of travel. If these patients need surgery for any reason,
intubation can be difficult because of the micrognathia, flexion
deformity, and neck stiffness. Cervical fusion is rarely needed and
should be reserved for children with documented instability or
progressive neurologic deterioration.
was in 1924, and there are now more than 100 cases reported in the
literature (329). It is slightly more common in
boys than in girls (ratio of 7.5), with an average age at presentation
of 8 years (range, 8 days to 13 years). It occurs most often in the
cervical spine and is especially symptomatic when located there. The
etiology is unclear. Theories proposed are antecedent trauma (present
in 30% of patients) and recent upper respiratory tract infections
(present in 15% of patients, which may only reflect the normally high
incidence of pediatric upper respiratory tract infections). There is no
evidence to suggest metabolic disorders.
The onset of symptoms is abrupt, between 12 and 48 hours. Twenty-three
percent of the children are febrile on presentation. Torticollis occurs
in one fourth of the children. Decreased cervical motion and spinal
tenderness also can occur. Radicular signs and symptoms are rarely
seen, and they are never without local symptoms. Myelopathy is rare (3
of 127 cases).
No protrusions have been seen in the asymptomatic group; 38% of the
symptomatic children have detectable protrusions. Recent reports have
also shown signal changes in the vertebrae on MRI scan (330).
weeks and 95% are free of symptoms by 6 months. The radiographs show
regression or disappearance of the calcific deposits in 90% of
patients; approximately one half of the radiographic improvement occurs
within 6 months. Children who are asymptomatic may not show regression
on radiographic images, even when followed up for long periods.
Children with multiple lesions show different rates of regression at
the different disc levels. In some cases, persistent flattening of the
vertebral bodies is noted into adulthood and may result in early
degenerative changes (331).
symptomatic unless there is spinal cord compression. Analgesics,
sedation, and cervical traction can all be used depending on the
severity of symptoms. A short trial of a soft cervical collar also may
be helpful. Contact sports should probably be avoided. Surgical
intervention is rarely needed. Two cases have been reported in which
anterior discectomy was performed (332,333).
disease defined as a symptomatic narrowing of the disc space, often
associated with fever and infection-like symptoms and signs. It affects
all pediatric age ranges, and is more common in boys than in girls. The
etiology is most likely infectious in nature; in about one third of the
children an organism can be isolated, usually Staphylococcus aureus (334,335).
and standing, fever, and malaise. It usually involves the lumbar spine,
with cervical involvement being rare. Early on there is a loss of
disc-space height; later, end-plate irregularities
on
both sides of the disc appear. Bone scans are very useful in
identifying the presence of discitis and osteomyelitis in a child with
systemic symptoms when the anatomic location cannot be localized on
clinical examination. The MRI findings are consistent with vertebral
osteomyelitis (334,336).
Other helpful diagnostic studies are the erythrocyte sedimentation rate
and blood cultures. Disc and bone cultures are necessary only if the
child does not respond to an initial course of rest and antibiotic
treatment.
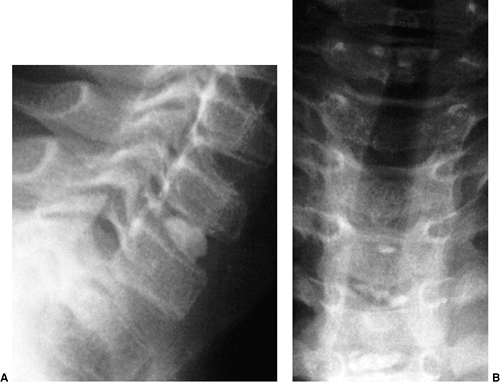 |
|
Figure 22.31 A: A 7-year-old boy with symptomatic intervertebral disc calcification at the C6-C7 level, as seen on a lateral radiograph. B: He also showed asymptomatic involvement at the T3-T4, T4-T5, and T5-T6 levels, as seen on an anteroposterior radiograph.
|
treatment. The intervertebral disc space reconstitutes to varying
degrees, but never to the normal height prior to illness. Sometimes
spontaneous vertebral body fusion occurs. Initially, nonsurgical
treatment is given. This includes rest, immobilization, and intravenous
antistaphylococcal antibiotics. Surgery is necessary only if there is
no response to nonsurgical management; usually, biopsy and culture to
isolate the infectious agent is all that is needed.
infection in the cervical spine is rare compared with other levels of
the spine. There will likely be an increase in North America because of
the increasing number of immigrants from Third World countries, the
rise of human immunodeficiency virus infection, and the emergence of
drug-resistant tuberculosis strains. There have been two very thorough
reviews of this subject (337,338).
Four of the 6 patients with upper cervical spine involvement and 24 of
40 with lower cervical spine involvement were children. Involvement at
the cervicodorsal junction is also frequent in children (339).
children present with neck pain and stiffness; torticollis, headaches,
and constitutional symptoms may also be present. Neurologic symptoms
vary from none to severe quadriparesis. In cases of lower cervical
spine involvement the children present with the same symptoms, and in
addition may have dysphagia, asphyxia, inspiratory stridor, and
kyphosis. In children younger than 10 years, more diffuse and extensive
involvement is seen, with large abscesses but with a decreased
incidence of paraplegia and quadriplegia. The neurologic symptoms have
a gradual onset over a period of 4 to 8 weeks. Sinus formation is not a
prominent feature because of the thick cervical prevertebral fascia
that contains the abscess. Cord compression occurs from the abscess and
the kyphosis. Cultures and biopsies are not always positive. Because
the infection is anterior, most cases will progress to spinal cord
compression and paralysis if left untreated. Patients with involvement
at the cervicodorsal junction have a very high incidence of neurologic
loss (339).
is seen radiographically, as are osteolytic erosions. Instability at
the C1-C2 level can be seen in some children; rarely is there a fixed
C1-C2 rotatory subluxation. A kyphosis is present in one fourth of
patients with lower cervical spine involvement. Other useful imaging
studies are chest radiography and renal studies.
children. Surgery also is recommended for the cervical spine, because
it gives rapid resolution of the pain, upper respiratory obstruction,
and spinal cord compression. This is in contrast to the thoracic and
lumbar spine, in which chemotherapy alone is an established method of
treating tuberculosis (340). Debridement is
performed with or without grafting. For children younger than 2 years,
grafting is usually not needed. For children with upper cervical spine
involvement, consideration should be given to anterior transoral
drainage and fusion across the lateral facet joints. Most children need
halo traction with reduction prior to drainage, if possible.
Cervicodorsal involvement typically needs anterior decompression
through an extended lower cervical approach (339).
The involvement is usually asymptomatic, although mild neck discomfort
may occur. Diminished lateral rotation may be noted on physical
examination. Radiographic findings, which begin to occur in adolescence
and early adulthood, consist of cystic changes in the vertebral bodies
or end-plate irregularities. Rarely is C1-C2 instability present. These
changes seen on radiographic examination can occur in patients with all
degrees of severity of hemophilia. The pathoanatomy of these changes in
the cervical spine is not known.
than in the normal population; the natural history of these premature
changes in the hemophiliac population is not known. There are no
treatment recommendations at present, other than the standard
precautions for patients with hemophilia.
are Langerhans cell histiocytosis, osteoid osteoma and osteoblastoma,
osteochondroma, and aneurysmal bone cyst. All can be defined as
neoplastic disorders without the propensity to metastasize. Although
pathologically and physiologically benign, they can be clinically
malignant if their surgical accessibility or risk of recurrence places
the neural structures at high risk.
gross motor or sensory deficits are much less common. Neoplasms can
cause torticollis. Probably the most common neoplasm causing childhood
torticollis is osteoid osteoma. In one series, all four children with
cervical osteoid osteomas presented with painful torticollis and
decreased neck motion (345). In the literature,
the incidence of torticollis is reported as ranging from 10% to 100% in
children with cervical osteoid osteomas (346,347,348).
The pain of an osteoid osteoma classically responds to aspirin or other
nonsteroidal antiinflammatory medications. When basilar invagination is
noted, Langerhans cell histiocytosis should be suspected (349).
although it is not always evident. Bone scans are very helpful in
locating the lesion; CT scan is then used to further delineate the
anatomy. The osteoid osteoma causes a sclerotic reaction in the
surrounding bone but usually does not invade the epidural space. It is
usually located in the laminae but can also be found in the pedicle and
in the vertebral body. An osteoblastoma is usually a mixture of lytic
and blastic elements (350). Bone scans are also
positive but are usually not needed for determining the presence or
absence of disease because most tumors are seen on plain radiographs. A
CT scan is very helpful in further assessing the anatomy, especially
the presence or absence of epidural invasion, which is common in
osteoblastoma. Typically, osteoid osteomas and osteoblastomas are
located in the posterior elements or pedicles (351) and, less commonly, in the vertebral body (352). Osteochondromas of the cervical spine (353,354,355)
demonstrate the same typical radiographic appearance that they do in
any other part of the body: expansile lesions with intact cortices and
normal trabecular patterns, absence of calcification, and absence of
soft tissue masses. One half of the patients have multiple
osteochondromatosis. Most of these are in the laminae or spinous
processes and can be mistaken for osteoblastomas. Aneurysmal bone cysts
are typically expansile lytic lesions with a thin rim of cortical bone
and may involve contiguous vertebral elements (e.g., the posterior
elements, pedicle, and body). A CT scan is useful for determining the
exact extent and the potential involvement and proximity of the
vertebral artery and neural elements. Angiography may be needed.
Aneurysmal bone cysts usually arise in the posterior elements (356). Eosinophilic granuloma usually exhibits vertebra plana as seen on radiographic images (357).
The CT or MRI scan is useful in determining the potential encroachment
on the neural structures. Eosinophilic granuloma usually arises in the
vertebral body, with varying degrees of involvement and collapse.
involvement of the vertebral artery and neural element because this can
lead to neurologic dysfunction. The intense
inflammatory
nature of the osteoid osteoma or osteoblastoma, which is so close to
the neural elements, causes nerve root irritation. This irritation,
pain, and muscle spasm may result in torticollis. Compressive
myelopathy may also occur, especially in patients with epidural
compression such as that seen with aneurysmal bone cysts (356,358,359).
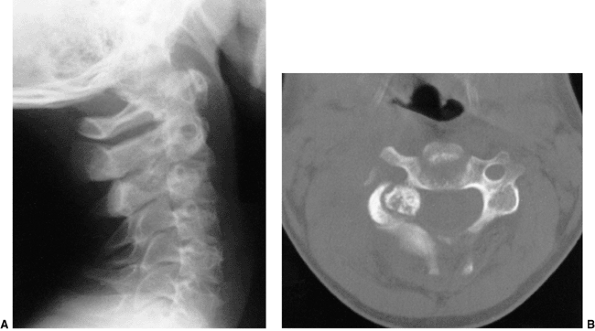 |
|
Figure 22.32 A 13-year-old boy had a 2-year history of neck pain that did not resolve with long-term chiropractic treatment. A: Plain radiographs show a sclerotic nidus with a surrounding lucency at the level of the C3 pedicle and C2-C3 foramen. B:
Computerized tomography (CT) scan confirms the typical appearance of an osteoid osteoma; note the proximity of the lesion to both the foramen and the nerve root as well as the vertebral artery. |
cervical spine has traditionally consisted of immobilization (e.g.,
collars, Minerva casts) and low-dose irradiation. Immobilization is
continued until early healing is seen radiographically. Low-dose
irradiation should be reserved for lesions that are associated with
neurologic deficits and are not surgically accessible. Multiple
laminectomies should be avoided. Rarely has immobilization alone been
used, and one of the children who underwent this treatment presented
with total collapse of the vertebral body (357).
Osteoid osteomas do not undergo malignant transformation. However,
continued torticollis and pain may lead to fixed spinal deformities.
For this reason the author advocates surgical resection. Pain relief
with complete resection is dramatic. Significant complaints of
postoperative pain resembling the preoperative pain indicate either
incomplete resection or recurrence. For osteoblastoma and aneurysmal
bone cysts, the primary treatment is surgical; nonsurgical treatment is
used only as adjunctive therapy. The surgical goal is complete primary
excision; however, this is often impossible because of the particular
anatomic location of the cysts. In these situations, adjunctive therapy
is useful (e.g., radiotherapy for eosinophilic granuloma, or
embolization for aneurysmal bone cysts if the nondominant vertebral
artery is involved) (360). Intralesional injection using both steroids and calcitonin may be useful in the difficult case of an aneurysmal bone cyst (361).
renders the spine unstable. The amount of resection necessary to render
the spine unstable is not known in children; however, in adults
resection of more than 50% of one facet likely leads to segmental
instability (362). Because the development of
postlaminectomy cervical instability is even more likely in children
than in adults, the author recommends an arthrodesis along with any
degree of facetectomy in children. Strong consideration should also be
given to an arthrodesis after any degree of laminectomy. Multiple
laminectomies should be avoided if at all possible; if it is necessary
to perform laminectomies, then fusion and stabilization also should be
performed. Anterior fusion is often necessary because of insufficient
posterior elements after surgical excision; supplemental halo cast/vest
or Minerva cast immobilization is usually needed if fusion is done. The
overall surgical management is individualized and multidisciplinary
(e.g., orthopaedics, neurosurgery, radiotherapy, interventional
radiology). Surgical complications include recurrence, pseudarthrosis
of the fusion, neurologic deterioration, and vertebral artery injury (363).
the cervical spine occur in adults; rarely, the cervical spine in
children can be involved by chordoma (364), leukemia, Ewing sarcoma (365), or metastatic neuroblastoma.
R, Meyer DB. The timing and sequence of events in the development of
the human vertebral column during the embryonic period proper. Anat Embryol 1979;157:167–176.
R, Muller F, Meyer DB. The human vertebral column at the end of the
embryonic period proper. 1. The column as a whole. J Anat 1980;131:565–575.
N, Narotsky MG, Pacico N, et al. Defects in cervical vertebrae in boric
acid-exposed rat embryos are associated with anterior shifts of hox gene expressed domains. Birth Def Res (Part A) 2003;67:59–67.
M, Gruss P. Homeotic transformations of murine vertebrae and
concomitant alteration of Hox codes induced by retinoic acid. Cell 1991;67:89–104.
Z-L, Shiota K. Stage-specific homeotic vertebral transformations in
mouse fetuses induced by maternal hyperthermia during somatogenesis. Dev Dyn 1999;216:336–348.
R, Zheng H, Whiting J, et al. Hoxb-4 mutant mice show homeotic
transformation of a cervical vertebra and defects in the closure of the
sternal rudiments. Cell 1993;73:279–295.
M, Neidhardt L, Haening B, et al. The paired homeobox gene Uncx4.1
specifies pedicles, transverse processes and proximal ribs of the
vertebral column. Development 2000;127:2259–2267.
den Akker E, Forlani S, Chawengsaksophak K, et al. Cdx1 and Cdx2 have
overlapping functions in anteroposterior patterning and posterior axis
elongation. Development 2002;129: 2181–2193.
VM, Heinänen MT, Kinnunen JS, et al. Reference values for radiological
evaluation of cervical vertebral body shape and spinal canal. Pediatr Radiol 2000;30:190–195.
JW, Cochran GVB, Lawsing JF III, et al. Tears of the transverse
ligament of the atlas. A clinical and biomechanical study. J Bone Joint Surg Am 1974;56-A:1683–1691.
M, Parker G, Ell J, et al. Basilar impression complicating osteogenesis
imperfecta type IV: the clinical and neuroradiological findings in four
cases. J Neurol Neurosurg Psychiatry 1999;66: 357–364.
PD, Menezes AH. Basilar invagination in osteogenesis imperfecta and
related osteochondrodysplasias: medical and surgical management. J Neurosurg 1997;86:950–960.
WE. Basilar impression (platybasia): bizarre developmental anomaly of
occipital bone and upper cervical spine with striking and misleading
neurologic manifestations. Yale J Biol Med 1939;11:487–496.
DE, Good TL, Hahn J, et al. Decompression of the brain stem and
superior cervical spine for congenital/acquired craniovertebral
invagination: an interdisciplinary approach. Laryngoscope 1990;100:926–931.
AH, Shaffery CI, Gruss JS, et al. Atypical hemifacial microsomia
associated with Chiari I malformation and syrinx: further evidence
indicating that Chiari I malformation is a disorder of the paraxial
mesoderm. J Neurosurg 2001;95:1034–1039.
JW. Normal and selected abnormal motion of the cervical spine from the
second cervical vertebra to the seventh cervical vertebra based on
cineroentgenography. J Bone Joint Surg Am 1964;46-A:1779–1781.
RE, Mah JY, Otsuka NY. Midshaft clavicle fractures associated with
atlantoaxial rotatory displacement: a report of two cases. J Orthop Trauma 2003;17:444–447.
P, Patel H, Scorpio R, et al. Rotatory atlanto-axial subluxation with
torticollis following central-venous catheter insertion. Pediatr Surg Int 2000;16:421–423.
KK, White DR, Weissler MC, et al. Nontraumatic atlantoaxial subluxation
(Grisel syndrome): a rare complication of otolaryngological procedures.
Laryngoscope 2003;113:1047–1049.
C, Yavuz SS, Sahin FI. Congenital muscular torticollis: is heredity a
possible factor in a family with five torticollis patients in three
generations? Plast Reconstr Surg 1997;99:1147–1150.
S, Liu Z, Quan X, et al. Sternocleidomastoid pseudotumor of infants and
congenital muscular torticollis: fine structure research. J Pediatr Orthop 1998;18:214–218.
JCY, Tang SP, Chen TMK, et al. The clinical presentation and outcome of
treatment of congenital muscular torticollis in infants—a study of 1086
cases. J Pediatr Surg 2000;35:1091–1096.
JCY, Wong MWN, Tang SP, et al. Clinical determinants of the outcome of
manual stretching in the treatment of congenital muscular torticollis
in infants. J Bone Joint Surg Am 2001;83-A:679–687.
H, Eng GD, Gaiser JF, et al. Congenital muscular torticollis: results
of conservative management with long-term follow-up in 85 cases. Arch Phys Med Rehabil 1987;68:222–225.
JC-Y, Metrewell C, Chen TM-K, et al. Correlation of ultrasonographic
imaging of congenital muscular torticollis with clinical assessment in
infants. Ultrasound Med Biol 2000;26: 1237–1241.
J-N, Chou M-L. Ultrasonographic study of the sternocleidomastoid muscle
in the management of congenital muscular torticollis. J Pediatr Surg 1997;32:1648–1651.
R, Jungert J, Rupprecht T, et al. Torticollis revealing as a symptom of
acute lymphoblastic leukaemia in a fourteen-month-old girl. Acta Paeditar 2001;90:587–588.
DB, Fisher JH, Gellis SS. Hiatal hernia and gastroesophageal reflux in
infants and children: analysis of the incidence in North American
children. Pediatrics 1974;54:450–455.
SG, Johnson DG, Herbst JJ, et al. An assessment of gastroesophageal
reflux in children by extended pH monitoring of the distal esophagus. Surgery 1978;84:16–24.
M, Krober M, Schneider U, et al. Congenital limb deficiencies
associated with Klippel-Feil syndrome. A survey of 57 subjects. Acta Orthop Scand 2000;71:461–464.
JE, Simmons ED, Danylchuk K, et al. Instability of the cervical spine
and neurological involvement in Klippel-Feil syndrome. J Bone Joint Surg Am 1990;72-A:460–462.
NE, Epstein JA, Zilkha A. Traumatic myelopathy in a seventeen-year-old
child with cervical spinal stenosis (without fracture or dislocation)
and a C2-3 Klippel-Feil fusion. Spine 1984;9:344–347.
DM, Ruderman RJ, Conrad RW, et al. Congenital scoliosis and urinary
tract abnormalities: are intravenous pyelograms necessary? J Pediatr Orthop 1987;7:441–443.
HH, Shut L, Chung S. Iniencephalic deformity of the cervical spine with
Klippel-Feil anomalies and congenital evaluation of the scapula. J Bone Joint Surg Am 1974;56-A:1254–1259.
P, Glorion C, Langlais J, et al. Assessment and neurologic involvement
of patients with cervical spine congenital synostosis as in
Klippel-Feil syndrome: study of 19 cases. J Pediatr Orthop B 1998;7:179–185.
SA, Tubbs S, D’Antonio MG, et al. Investigations into the association
between cervicomedullary neuroschisis and mirror movements in patients
with Klippel-Feil syndrome. Am J Neuroradiol 2002;23:724–729.
JT, Miller A, Bowen JR, et al. The natural history of Klippel-Feil
syndrome: clinical, roentgenographic, and magnetic resonance imaging
findings at adulthood. J Pediatr Orthop 1995;15:617–626.
G. Segmentation defect in the midontoid process and its possible
relationship to the congenital type of os odontoideum. Pediatr Radiol 2002;32:34–40.
FG, Gomori JM. Symptomatic cervical synovial cyst associated with an os
odontoideum diagnosed by magnetic resonance imaging. Spine 2000;25:1300–1302.
H, Park J-B, Kim K-W. Synovial cyst of the transverse ligament of the
atlas in a patient with os odontoideum and atlantoaxial instability. Spine 2000;25:741–744.
H, Park J-B, Kim K-W, et al. Retro-dental reactive lesions related to
development of myelopathy in patients with atlantoaxial instability
secondary to os odontoideum. Spine 2000;25:2777–2783.
B-Y. Complete reduction of retro-odontoid soft tissue mass in os
odontoideum following the posterior C1-C2 transarticular screw
fixation. Spine 1999;24:1961–1964.
H, Ito M, Abumi K, et al. A retrospective radiographic analysis of
subaxial sagittal alignment after posterior C1-C2 fusion. Spine 2004;29:175–181.
KA, Newton RW, Gupta S, et al. Clinical predictors and radiological
reliability in atlantoaxial subluxation in Down’s syndrome. Arch Dis Child 1991;66:876–878.
LA, Sheffield EG, Crawford K, et al. Reproducibility in the measurement
of atlanto-occipital instability in children with Down syndrome. Spine 1996;21:2463–2468.
TA, Bertrand SL, Powers MJ, et al. Posterior occipitoatlantal
hypermobility in Down syndrome: an analysis of 199 patients. J Pediatr Orthop 1994;14:304–308.
on Sports Medicine and Fitness of the American Academy of Pediatrics.
Atlantoaxial instability in Down syndrome: subject review. Pediatrics 1995;96:151–154.
RL, Putney ME, Allen BL Jr. Comparison of neurologic deficits with
atlanto-dens intervals in patients with Down syndrome. J Spinal Disord 1997;10:246–252.
CC, Sturm PF, Hatch RS, et al. Intraobserver reproducibility and
interobserver reliability of cervical spine measurements. J Pediatr Orthop 2000;20:66–70.
SM, Findley TW, Furia J, et al. Atlantoaxial instability in Down
syndrome: roentgenographic, neurologic, and somatosensory evoked
potential studies. J Pediatr 1987;110:515–521.
MD, Phillips WA, Hensinger RN. Fusion of the upper cervical spine in
children and adolescents. An analysis of 17 patients. Spine 1990;16:695–701.
LS, Drummond DS, Zanotti RM, et al. Complications of posterior
arthrodesis of the cervical spine in patients with have Down syndrome. J Bone Joint Surg Am 1991;73-A:1547–1554.
JS, Lauerman WC, Wood KB, et al. Complications and long-term outcome of
upper cervical spine arthrodesis in patients with Down syndrome. Spine 1996;21:1223–1231.
N, Tnabe G, Nakahara S, et al. Surgical treatment of cervical
spondylotic myelopathy complicating athetoid cerebral palsy. J Bone Joint Surg Br 1984;66-B:504–508.
H, Komori H, Okawa A, et al. Surgical treatment of cervical spondylotic
myelopathy associated with athetoid cerebral palsy. J Orthop Sci 2002;7:629–636.
DD, Kahn RH, Canady A, et al. Instability of the cervical spine after
decompression in patients who have Arnold-Chiari malformation. J Bone Joint Surg Am 1991;73-A:898–906.
S, Peterson H, Laws ER Jr, et al. Pathogenesis and prophylaxis of
postlaminectomy deformity of the spine after multiple level
laminectomy: difference between children and adults. Neurosurgery 1981;9:145–152.
JC, Ravitch MM. The operative management of von Recklinghausen’s
neurofibromatosis in children, with special reference to lesions of the
head and neck. Surgery 1977;82:342–348.
EA, van den Berg MP, Wuisman PIJM, et al. Correction of a dystrophic
cervicothoracic spine deformity in Recklinghausen’s disease. Clin Orthop 1998;349:149–155.
T, Yamagishi M, Nemoto K, et al. Spinal fusion using a vascularized
fibular bone graft for a patient with cervical kyphosis due to
neurofibromatosis. J Spinal Disord 1997;10:537–540.
KM, Edgerton EA, Bulas DI, et al. Patterns of injury to restrained
children in side impact motor vehicle crashes: the side impact
syndrome. J Trauma Inj Infect Crit Care 2003;54: 1094–1101.
RL, Brunn MA, Garcia VF. Cervical spine injuries in children: a review
of 103 patients treated consecutively at a level 1 pediatric trauma
center. J Pediatr Surg 2001;36:1107–1114.
JE, Hensinger RN, Dedrick DK, et al. Emergency transport and
positioning of young children who have an injury of the cervical spine.
J Bone Joint Surg Am 1989;71-A:15–22.
JM, Closkey RF, Mahboubi S, et al. Role of magnetic resonance imaging
in the assessment of pediatric cervical spine injuries. J Pediatr Orthop 2002;22:573–577.
GJ, Clyde BL, Fitz CR. Craniocervical junction subarachnoid hemorrhage
associated with atlanto-occipital dislocation. Spine 1996;21:1761–1768.
RV, Palma AMS, Abgussen CMB, et al. Traumatic vertical atlantoaxial
instability: the risk associated with skull traction. Eur Spine J 2000;9:430–433.
DB, Liem LK, Petermann G. Pediatric atlas fracture: a case of fracture
through a synchondrosis and review of the literature. Neurosurgery 2000;46:991–995.
PA, Drake JM, Hedden D, et al. Avulsion transverse ligament injuries in
children: successful treatment with nonoperative management. J Neurosurg (Spine 3) 2002;96:338–342.
PD, Rocha EF, D’Astous J, et al. Bilateral fracture of the pedicle of
the second cervical vertebra in the young child. J Bone Joint Surg Am 1986;68-A:892–896.
DM, Cross JLL, Antoun NM, et al. Helical computed tomography and
three-dimensional reconstruction of a bipedicular developmental anomaly
of the C2 vertebra. Spine 1999; 24:984–986.
J, Kaptain G, Sheehan J, et al. Congenital absence of a cervical
pedicle: report of two cases and review of the literature. Neurosurgery 2000;47:1439–1442.
M, Toyama Y, Chiba K, et al. Traumatic subluxation of the axis after
hyperflexion injury of the cervical spine in children. J Spinal Disord 2001;14:172–179.
JH, Naranja RJ, Pavlov H, et al. The relationship of developmental
narrowing of the cervical spinal canal to reversible and irreversible
injury of the cervical spinal cord in football players. An
epidemiological study. J Bone Joint Surg Am 1996;78-A:1308–1314.
GW, Wolfson AB, Mower WR, et al. Spinal cord injury without
radiographic abnormality: results of the National Emergency
X-radiography Utilization Study in Blunt Cervical Trauma. J Trauma Inj Infect Crit Care 2002;53:1–4.
PP, Vogt MT, Ward WT. Pediatric spinal cord injury without radiographic
abnormality (SCIWORA). The absence of occult instability and lack of
indication for bracing. Spine 2002;27:2788–2800.
PA, Pang D. Magnetic resonance imaging in the evaluation of spinal cord
injury without radiographic abnormality in children. Neurosurgery 1994;35:406–414.
AO, Dias MS, Li V. Magnetic resonance imaging correlation in pediatric
spinal cord injury without radiographic abnormality. J Neurosurg (Spine 1) 2002;93:33–39.
IF, Bresnan MJ, Zuckerman JE, et al. Cervical cord injuries secondary
to hyperextension of the head in breech presentations. Obstet Gynecol 1973;41:369–378.
JT, Fallahi S, Hardin JG. Nonreducible rotational head tilt and
atlantoaxial lateral mass collapse. Clinical and roentgenographic
features in patients with juvenile rheumatoid arthritis and ankylosing
spondylitis. Arch Intern Med 1983;143:471–474.
M, Terada K, Kikuchi N, et al. Herniation of calcified cervical
intervertebral disc causes dissociated motor loss in a child. Spine 1993;18:2347–2350.
D, Johnston CE II, Wenger DR. Pyogenic infectious spondylitis in
children: the convergence of discitis and vertebral osteomyelitis. J Pediatr Orthop 1995;15:652–660.
Report of the Medical Research Council Working Party on Tuberculosis of
the Spine. A 10-year assessment of a controlled trial comparing
debridement and anterior spinal fusion in the management of
tuberculosis of the spine in patients on standard chemotherapy in Hong
Kong. J Bone Joint Surg Br 1982;64-B:393–398.
EM, Kozlowski K, Marton D, et al. Osteoid osteoma and osteoblastoma of
the spine in children: report of 22 cases with brief literature review.
Pediatr Radiol 1986;16:25–31.
EOG, Hutton PAN, Pozo JL, et al. Osteoid osteoma and benign
osteoblastoma of the spine: clinical presentation and treatment. J Bone Joint Surg Br 1984;66-B:159–167.
NJ, Chandy KJ, Kellerman AJ. Osteoid osteomas of the body of the
cervical spine. Case report and review of the literature. Br J Neurosurg 2002;16:69–71.
M, Nakatani F, Ikuta K, et al. Treatment of cervical cord compression,
caused by hereditary multiple exostosis, with laminoplasty. Spine 2000;25:1290–1292.
SP, Grubb RL Jr, Gado MH, et al. Aneurysmal bone cyst of the
cervicothoracic spine: computed tomographic evaluation of the value of
preoperative embolization. Neurosurgery 1986;19: 290–293.
ML Jr, Gillingham BL, Hennrikus W, et al. Aneursymal bone cyst of the
first cervical vertebrae in a child with percutaneous intralesional
injection of calcitonin and methylprednisolone. Spine 2000;25:527–530.
RK, Day DL, Dehner LP, et al. Metastasizing chordoma in early
childhood: a pathological and immunohistochemical study with review of
the literature. Pediatr Pathol 1987;7:287–301.
