SPINAL SURGERY IN CONGENITAL SYNDROMES
VIII – THE SPINE > Spinal Deformity > CHAPTER 158 – SPINAL
SURGERY IN CONGENITAL SYNDROMES
abnormalities of connective tissue, muscle balance, or ossification.
Although a congenital syndrome is by definition present at birth, an
associated spinal deformity in most cases is not. It may develop with
growth as a result of bone dysplasia, a connective tissue disorder, or
miscellaneous chromosomal abnormalities. The orthopaedic surgeon must
understand the natural history of these growth disturbances to
determine when, as well as how, to intervene. Three factors should
always be kept in mind when evaluating the patient with a congenital
syndrome: (a) coexisting medical problems, (b) characteristics of bone
shape and quality, and (c) the effect of the syndrome on the neural
elements.
vulnerable to deformity, stenosis, and, most important, instability.
The surgical team must rule out or characterize the potentially
unstable cervical spine before general anesthesia is administered or
any skeletal surgery is performed. Plain film radiography with or
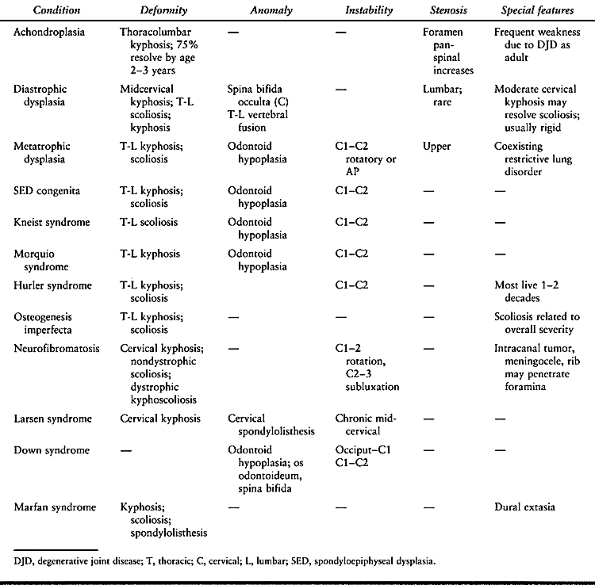
without flexion–extension may be helpful. Table 158.1 lists characteristic cervical spine problems in most common congenital syndromes.
 |
|
Table 158.1. Characteristic Cervical Spine Problems in Common Congential Syndroms
|
generally refers to an odontoid process that does not extend to the
midportion of the ring of the atlas. It may be seen in numerous
conditions;
the most common are skeletal dysplasia and Down syndrome (21,31,35). Odontoid hypoplasia may also be an idiopathic occurrence. In extreme cases, the dens may be essentially absent (aplasia).
The majority of these cases of odontoid hypoplasia are the result of
skeletal dysplasias. Another condition that exhibits similar clinical
symptoms is transverse ligament insufficiency, which may be caused by ligamentous laxity or damage to the ligament. Finally, a number of patients have an os odontoideum,
a chronic condition in which the odontoid is present only as a small
ossicle, not united to the body of the axis. Although os odontoideum
was long presumed to be a congenital lesion, more recent evidence
suggests that most cases may be the result of unrecognized fracture of
the odontoid.
of symptoms: (a) They may show signs and symptoms of neck instability,
manifested by the muscles’ response to guard it: neck pain and spasm,
torticollis, or headache. These symptoms appear most often after
activity or a fall. (b) Neurologic symptoms involving the long cervical
tracts may be present, such as developmental delay, hyporeflexia or
hyperreflexia, and weakness. (c) Finally, cerebrovascular symptoms may
prevail, from ischemia to stroke involving the posterior circulation.
Plain radiographs are helpful in establishing the diagnosis, and
computed tomography (CT) can usually demonstrate the pathology clearly,
if needed.
extension will help to quantify the instability. The normal space
available for the cord at this level should be at least 13 mm, and the
translation of the ring of the atlas should be less than 5 mm. Magnetic
resonance imaging (MRI) may be helpful to demonstrate cord impingement,
but this can often be deduced from plain films and clinical exam alone.
critical limit (more than 5–8 mm of translation on flexion/extension
films), stenosis, or neurologic signs, surgical fusion of C-1 to C-2 is
indicated (24). Reduction to a neutral position is the goal; if this cannot be accomplished, decompression may also be required.
or idiopathic congenital cervical stenosis. Signs and symptoms include
those of acute compression (numbness and tingling in the extremities,
acute weakness) or chronic myelopathy with developmental delay,
spasticity, weakness, and muscle atrophy. In the teenage athlete with
idiopathic cervical stenosis, transient quadriparesis is a common
presenting phenomenon, with forced hyperextension in the presence of a
narrowed spinal canal (41). Fortunately, this
symptom tends to resolve rapidly if there is no vertebral subluxation
or dislocation. In the child with achondroplasia, the greatest degree
of stenosis occurs at the foramen magnum, causing failure to meet
developmental milestones and a tendency to develop sleep apnea.
Clinically significant stenosis of the remainder of the cervical spine
in the person with achondroplasia generally develops only in adulthood,
if at all.
spondyloepiphyseal dysplasia and mucopolysaccharidoses) may produce
localized stenosis of the ring of the axis as well as atlantoaxial
instability; these can cause additive damage to the cord. On lateral
radiographs, cervical stenosis should be suspected if the distance from
the posterior laminar line to the posterior vertebral body line is less
than 80% of the width of the vertebral bodies (Pavlov’s ratio) (41). Also, the distance from the posterior laminar line to the line of the facets is diminished (Fig. 158.1).
In patients with Klippel-Feil syndrome, this finding may be missed
because attention is drawn to the vertebral fusions. The stenosis is
made more problematic if there are large blocks of fusion with just a
few motion segments.
 |
|
Figure 158.1.
Torticollis in a young child, in this case due to three consecutive hemivertebrae in the upper cervical spine. In infants, computed tomograms provide superior visualization compared with plain films, because of the baby’s large head, difficulty positioning, and the complexity of the case. Treatment was by realignment with distraction of the concave side and derotation in a halo-vest, followed by fusion. |
counseled to avoid contact sports, especially those that produce
forcible flexion or extension of the cervical spine, such as wrestling
and playing lineman in American football. Surgical decompression of the
lower cervical spine is generally best avoided, as it could produce a
region of decreased stability adjacent to further stenosis. Localized
decompression and fusion may be carried out if indicated. Patients with
congenital stenosis of the upper cervical spine may require
decompression. If this is so, fusion should be considered if there is
associated instability or if the decompression involves more than two
segments, in order to prevent development of localized kyphosis.
two congenital syndromes: Larsen syndrome and diastrophic dysplasia.
Larsen syndrome is characterized by multiple joint dislocations, foot
deformities, and an accessory calcaneal apophysis. In one series, more
than half the patients
had cervical spina bifida and resultant kyphosis (22).
In diastrophic dysplasia, diastrophic patients are often born with
significantly short stature, rigid clubfeet, joint contractures, and a
closed cervical spina bifida, although the incidence of kyphosis is not
as high.
indicates a deficiency of posterior ligamentous support (interspinous
ligament, ligamentum flavum) as well as of posterior muscle control.
This may be a reason for the development and progression of kyphosis.
In addition, the vertebral bodies in the region are hypoplastic and may
be rounded or wedge shaped. The kyphosis may progress as the child
becomes upright. Initially, the physical features of kyphosis are not
externally evident, except for a slight loss of the normal cervical
lordosis. There are no external clues to the presence of bifid cervical
laminae. Therefore, a high index of suspicion must be maintained for
these conditions.
to detect in children with severe skeletal deformities. Signs such as
muscle weakness, failure to achieve normal milestones, and
hyperreflexia or clonus may be seen. Endotracheal intubation for other
surgical procedures in the presence of this kyphosis may worsen the
neurologic condition if not done by knowledgeable persons. In some
patients with diastrophic dysplasia, a mild cervical kyphosis may
improve spontaneously with time (18).
Observation may be indicated if there are no established signs of
neurologic compromise. Bracing, however, does not seem to be feasible
or warranted. In Larsen syndrome, progression is more likely (22).
which may function as a tether and allow spontaneous correction of the
deformity.
-
Perform fusion early in the patient with
Larsen syndrome, before the kyphosis exceeds 50° and becomes rigid.
Consider fusion in patients with diastrophic dysplasia who do not
improve over the first several years of life, or whose deformity or
neurologic condition worsens. -
Perform posterior fusion over the levels
involved in the kyphosis, using autogenous bone graft from the iliac
crest or the tibial metaphysis. -
Use a halo-vest or halo-cast to control
the head and prevent the kyphosis from worsening during incorporation
of the fusion. For mild deformities, a Minerva-type cast or orthosis is
also an option. Order them in advance of the procedure. -
Take care in exposing the spine, because of the open laminae.
-
Dissect the muscles off only the extent
of the spine intended for fusion, since extension of the fusion to
adjacent exposed levels is a risk. Confirm levels radiographically. -
Decorticate the spine gently and perform a bone graft.
patients of a very young age. Some degree of correction in the
halo-vest may be possible by a combination of three maneuvers: (a)
positioning the head in slight extension and posterior translation, (b)
securing the shoulder straps of the vest so that they are snug (but not
too tight), to maintain the length of the cervical spine rather than
allowing it to settle, and (c) placing a padded sling behind the apex
of the kyphosis, which is attached to the bars of the halo-vest, to
prevent the kyphosis from settling posteriorly. When this is done, the
tension of the strap must be checked periodically to be certain that
there is not too much pressure on the skin.
neurologic compromise, an anterior decompression and strut graft may be
needed in addition to the posterior fusion. The spine should be
immobilized for a minimum of 3 months, and continuity of the fusion
mass should be demonstrated radiographically at the end of this period
to prevent loss of position due to a pseudarthrosis.
syndrome, may occur with congenital upper or lower thoracic or lumbar
fusion, or it may be present as an isolated finding. It has been
classified into three types: Type I involves fusion of cervical and upper thoracic vertebrae, type II involves isolated fusions of the cervical spine, and type III refers to cervical fusions associated with lower thoracic or upper lumbar fusion (36). Surgery is almost never required for the cervical anomaly itself.
search for other anomalies both within and outside the spine, such as
Sprengel deformity, hearing impairment, spina bifida, and associated
scoliosis. Scoliosis is most common in types I and III. Progressive
congenital cervical scoliosis is rare and usually involves the
cervicothoracic junction. Monitor young children with this finding
closely, since the shoulder tilt it produces may be highly deforming.
Perform surgery if progression of more than 10° is seen. A posterior
fusion in situ is the gold standard for this region.
usually presents as torticollis, which must be differentiated from
muscular torticollis, Grisel syndrome, ocular disturbance, and
abnormalities of the brainstem and cord. Other causes of fusion in a
young child include juvenile rheumatoid arthritis, as well as residua
of infection in the region. The upper cervical spine may be very hard
to image in children under age 5; it is frequently necessary to obtain
a multiplanar CT or an MRI under sedation. If a vertebral anomaly is
seen that is deforming, surgery may be indicated (Fig. 158.1).
-
Achieve a degree of correction of the
head tilt by applying a halo-vest and gradually adjusting the head
position using the uprights. -
Any malrotation may be improved by using bilateral rotation with Ilizarov turnbuckles attached to the halo (15).
-
Once an acceptable head position is achieved, carry out fusion.
-
If more than two levels are fused, consider an anterior epiphyseodesis as well, to prevent progressive lordosis with growth.
quality affect halo application and upper cervical fusion, these
techniques deserve special consideration (10).
Also, if there is risk of positional neurologic damage in children too
young to cooperate with examination, evoked potentials are useful.
-
General anesthesia is preferred for halo application, although local anesthesia and sedation are possible.
-
Because of the danger of hyperflexion
caused in part by children’s relatively large head size, elevate the
torso or have an assistant hold the head off the end of the table. -
Do not place pins in the thin temporal regions (14).
-
In children under 2 years of age, Mubarak et al. (28)
recommend placement of six to ten pins at low torque (finger tightness
through 2 inch-pounds) in the four traditional regions (Fig. 158.2).) Kopits and Steingass (25)
found four pins to be sufficient in most cases, and loads up to 5
inch-pounds to be safe in older children. My experience confirms these
findings. At our institution, we use 4 inch-pounds of torque for
children up to age 4 years, 6 inch-pounds for those aged 5 to 10, and 8
inch-pounds for those over 10.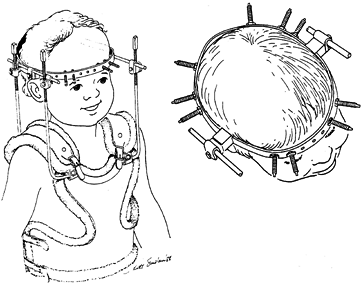 Figure 158.2.
Figure 158.2.
A technique of halo application in the infant or child under age 2. The
increased number of pins allows decreased torque on each. (Reproduced
with permission from Mubarak SJ, Camp JF, Vuletich W, et al. Halo
Application in the Infant. J Pediatr Orthop 1989;9:612.) -
The vest may be custom ordered from a
prior cast or from tape measurements, or it may be made of plaster
using a special frame. The pins may be retightened on the first or
second day after halo placement using intravenous analgesia, but do not
retighten after this time. If a pin becomes painful later, it is
usually that it has become loose or infected. Try oral antibiotics
first if there is no obvious loosening; if there is no relief, replace
the pin in an alternative site.
cervical spine for deformity or instability. Techniques of fusion are
well described elsewhere (in Chapter 139, Chapter 140, and Chapter 154).
Two special aspects require further comment. First, anomalies of the
posterior arches are common in several syndromes. Second, in very young
children, the posterior elements and occipital cortex may not allow
wire fixation of any substantial strength, or the lamina may be
resected in cases of stenosis. Study good plain radiographs and, in
most cases, CT scans in the areas of planned fusion to rule out spina
bifida or anomalies of the arches. If anomalies are present, start
dissection from a “normal” area, where depth can safely be established,
and proceed up and down over the facets in the deficient areas.
90% for upper cervical fusion in children with halo immobilization,
even when grafts are not wired in place (24).
This finding is relevant for infants or certain patients with skeletal
dysplasia who do not have adequate bone size or quality for wire
fixation.
-
Apply the halo as described previously for young children.
-
Turn the patient prone and affix the halo
to a halo-holder. Use spinal cord monitoring during turning and
throughout the entire procedure. -
Check a lateral radiograph to confirm proper alignment of the neck.
-
Gently expose the spine, taking care to remain medial to the vertebral arteries at C1–C2.
-
Avoid unnecessary exposure of caudal
levels, which often leads to unwanted extension of the fusion distally.
If a distal level is exposed unintentionally, covering it with bone wax
may prevent it from incorporating into the fusion mass. -
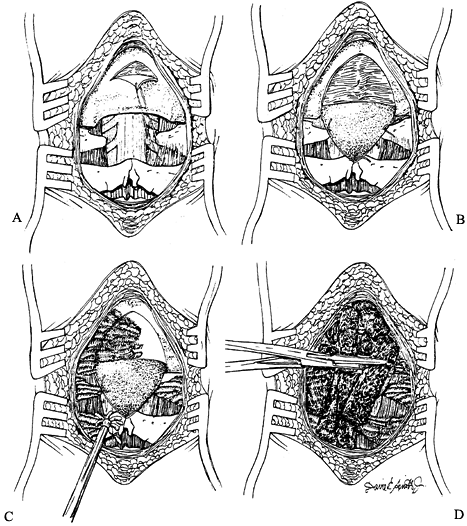
After exposure and wide decortication, place autologous bone in the desired areas (Fig. 158.3).
![]() Figure 158.3. Technique of occipitocervical fusion in infants. A,B: An occipital periosteal flap is raised and sutured distally. C,D:
Figure 158.3. Technique of occipitocervical fusion in infants. A,B: An occipital periosteal flap is raised and sutured distally. C,D:
An air drill is used for decortication, and the graft is inserted.
(Reproduced with permission from Koop SE, Winter RB, Lonstein JE. The
Surgical Treatment of Instability of the Upper Part of the Cervical
Spine in Children and Adolescents. J Bone Joint Surg Am 1984;66:403.) -
When fusion to the occiput is desired,
use a triangular periosteal flap equal to the distance to C-2. Using a
stay suture, dissect the flap, leaving it attached at its base, and
suture it to C-1 and C-2. Place the bone graft on top, abutting the
decorticated occiput.
and Drummond for children whose bone is adequate to permit wire
fixation (11). Use of autogenous bicortical
iliac crest in combination with occipitocervical wires forms a
construct that is stable in flexion and extension.
-
Perform the halo placement, with positioning and exposure as previously described.
-
Fashion a trough in the outer table of
the base of the occiput below the inion, at a level so that a graft can
be inserted on top of the laminae (Fig. 158.4).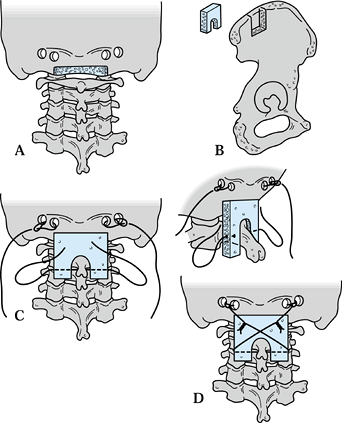 Figure 158.4.
Figure 158.4.
Technique of occipitocervical fusion (Drummond), used for children with
slightly better bone density that can support wire fixation. A: A trough is made in the base of the occiput, and two burr-holes are made on either side. B:
A corticocancellous graft is taken from the ilium and shaped to fit the
space between the occiput and the second or third cervical vertebra. C:
Four strands of wire are passed to be ready to twist together: one from
each side of the occiput, and one Drummond button-wire from each side
of the C-2 or C-3 spinous process. D: The
wires are twisted together to lock the graft into place. (Redrawn from
Dormans JP, Drummond DS, Sutton LN. Occipitocervical Arthrodesis in
Children: A New Technique and Analysis of Results. J Bone Joint Surg Am 1995;77:1234.) -
Make two burr holes through both cortices of the occiput just superior and lateral to the trough.
-
Loop a 16- to 18-gauge wire through these holes.
-
Make a hole at the base of the most
caudal lamina to be fused, and pass a pair of Wisconsin (Drummond)
wires through the hole in opposite directions. -
Obtain a corticocancellous autogenous
iliac crest graft the width of the laminae and the height of the
combined levels to be fused. -
Fashion a notch in the inferior edge to
fit around the lowest spinous process, and lay it in place after the
Wisconsin wires have been placed. -
Start each Wisconsin wire distally under
the graft, then pass it around the graft and over it, across to the
spinal wire in the opposite side of the occiput. -
Control the extension of the spine by the head position and the size and shape of the graft.
-
Tighten the wires and take a lateral radiograph to confirm alignment.
-
Place extra bone graft at the upper and lower edges of the main graft.
-
Take flexion–extension radiographs about every 4 weeks.
-
The halo may be removed after a mean of
only 8 weeks with excellent results; have the patient wear a hard
collar for 4–6 weeks after the halo is removed.
described a modified Gallie method of wiring around the base of the
spinous process to preserve strength yet avoid the risks of wire
passage under C-2 (Fig. 158.5). It also avoids
the risks of cutout or dorsal displacement of the wires, which could
otherwise occur in children with standard Gallie technique.
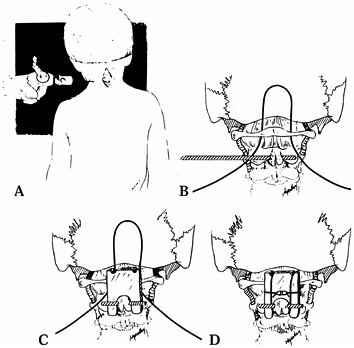 |
|
Figure 158.5. Atlantoaxial fusion by the modified Dewar technique. A: After C-1 and C-2 are exposed, a threaded Steinmann pin is inserted percutaneously through the base of C-2. B: A sublaminar wire is passed under the C-1 arch. C,D:
The wire is brought over the contoured graft and held under the Steinmann pin. (Reproduced with permission from Mah JY, Thometz J, Emans J, et al. Threaded K-Wire Spinous Process Fixation of the Axis for Modified Gallie Fusion in Children and Adolescents. J Pediatr Orthop 1989;9:675.) |
-
Position the patient in the standard
fashion, with the cervical spine reduced to an optimal position, and
the neck draped widely, allowing access to the lateral portion of the
neck from both sides. -
Contour a rectangular unicortical iliac crest graft to fit over the C-1 arch and straddle the C-2 spinous process.
-
Drill a threaded Steinmann pin
percutaneously through the widest portion of the base of the spinous
process of C-2 and cut it to leave 1 cm on each side. -
Pass an 18-gauge sublaminar wire under the C-1 arch.
-
Place the loop of the wire deep to the
Steinmann pin and draw it tightly over the graft, keeping it apposed to
the lamina. This placement allows the wire to obtain good cortical
purchase around C-2 and prevents dorsal migration. -
Add extra cancellous graft. Tie the free ends of the wire transversely over the graft.
translatory instability of the atlantoaxial segment is not too great. A
halo-vest may be used at the surgeon’s discretion.
posterior ileum in children. If the amount is inadequate, such as in an
infant with a small pelvis in whom long cortical and cancellous grafts
are needed, the grafts may be obtained from one or both tibiae (see Chapter 9).
seen mainly in achondroplasia; to a lesser degree, it is seen in other
skeletal dysplasias such as hypochondroplasia, diastrophic dysplasia,
and spondyloepiphyseal dysplasia. Patients with isolated congenital
spinal malformations such as scoliosis and kyphosis also commonly have
associated narrowing of the spinal canal in the region. Take this
narrowing into account when planning deformity correction and
instrumentation.
It is usually present at the thoracolumbar junction and may be
associated with segmental instability, scoliosis, or kyphosis. In
patients with complete neurologic deficit, return has not been seen
after decompression and stabilization, but these are indicated in those
with preservation of at least some distal neurologic function who have
progression or instability.
congenital malformation, who exhibit spinal stenosis that has been made
symptomatic by disc protrusion or mild degenerative change. Symptoms
include pain and tingling or numbness in the lower extremities more
than pain in the back. Standing or walking worsens these symptoms, and
rest usually relieves them. Plain films suggest stenosis by virtue of
the narrow distance between the posterior laminar line and the
posterior vertebral line, but they are less accurate in identification
of stenosis in the thoracic and lumbar spine than they are in the
cervical spine. MRI more clearly delineates the degree of neurologic
compression.
a flexion back brace may alleviate symptoms. Hip flexion contractures
may need to be addressed, as they increase the obligatory lumbar
lordosis. Decompressive laminectomy may be necessary if these measures
fail; careful examination and judgment are necessary to determine the
extent of unroofing required. Techniques of decompression may involve
traditional laminectomy, laminoplasty (enlarging the canal by hinging
open the lamina), or fenestration (removal of the stenotic inferior
portion of each involved lamina and the medial facets). Further details
are discussed in the later section on achondroplasia.
more serious neurologic consequences than congenital scoliosis. The
basic types include failure of vertebral formation (type I) and failure
of segmentation (type II) (see Chapter 161). If progression is seen, treatment is required. Bracing has no value in halting the increase of the curve.
posterior fusion of the level above and the one below the abnormal
vertebra in a type I kyphosis, unless it exceeds about 55°. If it
exceeds this value, the fusion mass will be under tension and will not
effectively halt growth; an anterior epiphyseodesis may be needed as
well. A type II kyphosis may be fused posteriorly between the two
involved vertebrae, to match the anterior bar; this may be extended one
level above and one below in young children if it is desired to achieve
some correction with cast and growth.
rule; follow-up should be performed to rule out pseudarthrosis and
progression. Undertake osteotomy or vertebrectomy in treatment of
congenital kyphosis only if the deformity is severe and is causing
neurologic compromise or an unacceptable appearance.
laminectomy, especially of the cervicothoracic or thoracolumbar
junctions. In congenital syndromes, this situation may occur after
decompression of spinal stenosis (in achondroplasia) or of intradural
tumors (in neurofibromatosis) (17,19).
More detail is given later in the sections on these conditions. It is
important to realize that the risk of this phenomenon is greater in
children than it is in adults. When there is preexisting kyphosis or
vertebral wedging, it becomes even more likely.
treatment of an established deformity, if it can be anticipated.
Limited posterior fusion in situ over the
region of the junction is usually effective. Another alternative is
laminoplasty, which allows many of the interlaminar ligaments to remain
intact and may prevent kyphosis from developing.
association is with the VATER syndrome (vertebral anomalies, anal
atresia, tracheoesophageal fistula, renal and radial abnormalities).
Some physicians include a C
for cardiac abnormalities. The vertebral anomalies are the most common
component of the VATER syndrome, so orthopaedic surgeons will see most
of these children. Other syndromes that include congenital vertebral
anomalies are Goldenhar (oculoauriculovertebral) syndrome,
myelomeningocele, Klippel-Feil syndrome, and Jarcho-Levin syndrome
(spondylothoracic dysplasia).
patch of hair over the spine in the occasional case of congenital
scoliosis, often patients have no external physical findings except for
the deformity, which may be mild in early childhood. Early diagnosis
usually comes about because of an incidental event such as a radiograph
for trauma or a chest film. Vertebral anomalies are frequently seen on
ultrasound of fetuses, and concerned parents as well as sonographers
often consult the orthopaedist for a prognosis. Isolated hemivertebrae
without neural tube defects or other sonographic anomalies typically
have a good outcome. The presence of other abnormalities reduces the
rate of survival.
include hemivertebra, wedged vertebra, or fusion of vertebrae (bar).
Many times there are elements of both in a given curve. The best
opportunity to understand the underlying growth abnormality is to study
the films of the patient at the youngest possible age; they will show
the asymmetries of ossification and allow diagnosis of hemivertebrae
and fusion.
surfaces, or if it is “carved into” the adjacent vertebra
(incarcerated), it is less likely to produce an increasing curve. Upon
diagnosis of congenital scoliosis, do a thorough exam, searching for
limb atrophy or other deformities. Chest auscultation should be done,
but cardiac imaging is not routinely indicated. However, the
genitourinary tract should be visualized at least once by ultrasound or
intravenous pyelogram. Some experts recommend a routine MRI on all
children with this diagnosis, since at least 25% will show some
abnormality such as a Chiari malformation, syrinx, or tether. This is
not a well-accepted recommendation, however, as the indications for
treating these conditions in the asymptomatic stage are highly
debatable. Most surgeons instead prefer to order an MRI only when
corrective surgery is planned, or if unexplained progression occurs.
There is no documented efficacy of brace treatment. Some curves such as
those with a segmented hemivertebra and a contralateral bar have a
virtual certainty of progression and should be fused when first seen.
All others should be followed during growth with serial radiographs,
always comparing them to the first film, rather than to the last prior
film.
recommend surgery. There are several surgical options, whose
indications depend on the characteristics of the curve, the
acceptability of the current deformity, and the likelihood of future
increase in the curve. Options include the following:
-
Posterior fusion in situ
-
Anterior and posterior fusion
-
Hemiepiphyseal fusion
-
Hemivertebral excision
-
Spinal osteotomy for correction
the most widely accepted procedure. It is indicated for progressive
curves if the deformity is acceptable and the likelihood of anterior
crankshaft progression is not high.
-
Take care in exposing the spine, since midline laminar defects are sometimes seen in congenital curves.
-
Fuse all vertebrae within the curve.
-
Some correction may be obtained through bracing if there is flexibility in the curve.
-
Postoperatively, immobilize the patient
in a cast or brace for 3 to 4 months, when consolidation of the fusion
should be demonstrated.
exists anteriorly that could cause a deformity due to the crankshaft
phenomenon, perform anterior and posterior fusion.
-
Perform the anterior procedure in the
traditional open fashion, through a thoracoscopic approach, or by a
transpedicular or costotransversectomy approach. See Chapter 155. -
Consider a hemiepiphyseodesis, as a
variation on this theme, for young patients’ curves with some growth
potential on the concave side. -
Fuse the curve anteriorly and posteriorly
only on the convexity, to allow for some corrective growth on the
concavity. Measurable correction is seen only in children under age 6
at surgery, and the amount of correction rarely exceeds 10° to 20°. -
Hemivertebra excision is now accepted as a safe alternative for curve correction in experienced hands (8) (see the Surgical Techniques
section later). It is mostly, although not solely, applicable to
anomalies at or below the thoracolumbar junction. Use this technique
for curves too large to be fused in situ. -
Both anterior and posterior procedures may be performed in the same operative session.
-
In all cases where corrective surgery is
planned for congenital deformities, a preoperative MRI of the spinal
canal is indicated.
chromosome 21) is commonly associated with cervical abnormalities.
Anterior subluxation of C-1 on C-2 of more than 5 mm in flexion is seen
in 15% to 20% of patients. Over 4 mm posterior translation of the
occiput on C-1 is seen in 60% (29,31). Also seen is increased frequency of os odontoideum, ossiculum terminale, and spina bifida of any upper cervical vertebra (31). Management of the instability is controversial.
high-risk sports those with more than 5 mm C1–C2 subluxation. Perform
fusion for those with more than 1 cm subluxation, neurologic deficit,
or persistent neck pain. In cases to be fused, it may be necessary to
extend the fusion to the occiput (42) if there
is significant posterior atlanto-occipital translation in extension.
Increasing quadriparesis during surgery has been reported in cases of
preoperative myelopathy or longstanding displacement. It appears that
in such cases there may be chronic degeneration within the cord,
rendering it extremely susceptible to insult. In addition, the space
available for passing wires is decreased. Reduction, if necessary,
should be achieved before surgery with evoked potential monitoring or
preoperative awake traction. If significant reduction cannot be
achieved but the patient’s neurologic condition is acceptable, only a
fusion without wires is recommended. Use a CT scan to rule out spina
bifida.
dysplasias. Odontoid hypoplasia and ligamentous laxity are common in
spondyloepiphyseal dysplasia (congenita more than tarda), Morquio
syndrome, Kniest syndrome, and metatrophic dysplasia (2,38).
It may also be seen in the occasional patient with
pseudoachondroplasia. Symptomatic instability frequently results. In
addition, cervical stenosis may be seen with metatrophic dysplasia,
Maroteaux-Lamy syndrome, or achondroplasia. Obtain neutral, flexion,
and extension cervical spine films in all patients with these
conditions. Diagnosis of cervical myelopathy is difficult in infants
and may be aided by checking motor milestones, and spinal cord
monitoring, flexion–extension MRI, and sleep studies. Metatrophic
patients may also have painful torticollis due to rotatory C1–C2
instability (Fig. 158.6).
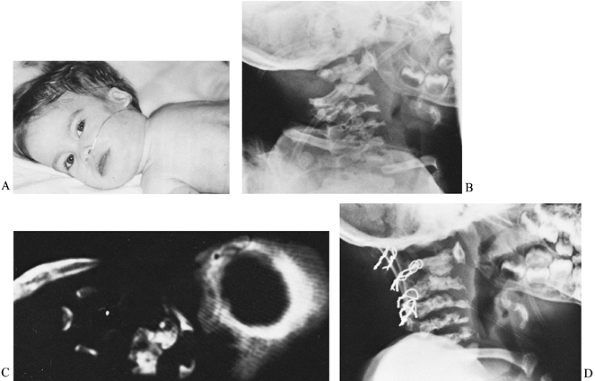 |
|
Figure 158.6. A: A 15-month-old child with metatrophic dysplasia and painful torticollis. The head is kept in marked hyperextension. B: Lateral roentgenogram shows some anterior C-1 displacement with rotation and stenosis. C: CT-myelogram confirms rotational malalignment and stenosis. D:
Posterior C1–C2 decompression and occiput to C-3 fusion done by the method of Koop. Wires seen are through facets (Southwick type). Tibial graft is used. Unfortunately, the patient died 3 months postoperatively due to the restrictive lung disease associated with metatrophic dysplasia. |
other dysplasias, diastrophic dysplasia frequently causes mild cervical
kyphosis and spina bifida (19). Surprisingly,
many of these kyphoses, especially those that are less than 80°,
resolve over time with or without bracing. Quadriplegia has been
reported with some larger kyphoses, however, so surgical treatment is
indicated for those with progression or neurologic deficit.
by postural reduction and posterior fusion. Place the patient in a halo
body jacket, and gradually extend the head over several days with
serial neurologic examinations. A posterior sling may be added at the
apex of the curve. If satisfactory improvement is obtained, identify
bifid areas on CT and perform a posterior fusion with a tibial cortical
graft. If the kyphosis is rigid, anterior release and strut graft
fusion, followed by posterior fusion, are indicated. Apply the halo
before the fusion, to protect the strut graft in young patients. The
anterior bar of the frame should be removable on the side of the
anterior approach.
flattened facies is occasionally associated with cervical spondylolysis
and kyphosis, causing neurologic deficit (22).
Screening of the cervical spine is recommended for all patients with
this diagnosis. Treatment follows the guidelines given for diastrophic
dwarfism. Note, however, that spontaneous resolution has not been
documented in this condition, and the posterior arches may also be
deficient.
sagittal deformity, and arthrosis combine to produce compressive
neurologic lesions in 30% to 80% of patients. Infantile kyphosis at the
thoracolumbar junction, resulting from muscular hypotonia, ligamentous
laxity, and a relatively large head, resolves in 75% to 85% of cases
but persists or progresses in the remainder, leading to wedging of
thoracolumbar vertebrae (19,26,37,40).
Wedging may be focal, involving a single vertebra, or gradual,
involving multiple levels. Some geneticists feel that it is important
to prevent children with achondroplasia from sitting unsupported, and
to use hard-backed sitting devices (30). I feel
that it is impossible to prevent a child from sitting who is
developmentally ready, and that the only effective support is a
thoracolumbosacral orthosis. Therefore, it seems prudent to brace all
achondroplastic children with significant kyphosis after 2 to 3 years
of age.
-
For any curve more than 50° to 60° with focal wedging, in patients over age 5 to 6 years
-
In any patient undergoing laminectomy with a curve over 30° in the thoracolumbar region or 50° in the thoracic region
-
For any curve that progresses on its own (37)
because of deformity and small posterior elements, especially after
laminectomy. If the kyphosis is sharp and angular, and if neurologic
deficit is present, perform a corpectomy with strut graft fusion.
Follow with posterior fusion. Both procedures can be done on 1 day if
the patient is young, or 1–2 weeks apart in older patients or those
requiring extensive laminectomy. Correction of deformity may be either
by cast or instrumentation. There is a 25% or greater chance of
somatosensory evoked potential or clinical neurologic deterioration
when instrumentation is used, although recovery is common (37).
This effect is probably caused by instrumentation impinging on a
narrowed canal, downward pressure on apical laminae, or stretch of
nerve roots in lordotic segments. This risk can be minimized by using
cast correction only, with 4–6 months of recumbency. If instrumentation
is used, it should include only pedicle screws in the lower thoracic or
lumbar region (Fig. 158.7). Stabilization and
fusion, rather than significant correction, should be the goals. It is
best not to fuse below L-4 in most cases, because mobility is always a
problem in patients with achondroplasia. Laminectomies should be done
in marginally stenotic levels. Spinal stenosis in achondroplasia is
caused by deficient endochondral growth in the neurocentral
synchondroses, with decreased sagittal and coronal canal dimensions,
increasing in severity caudally. Foramen magnum and cervical
stenosis may occur in addition to the more common thoracolumbar stenosis.
 |
|
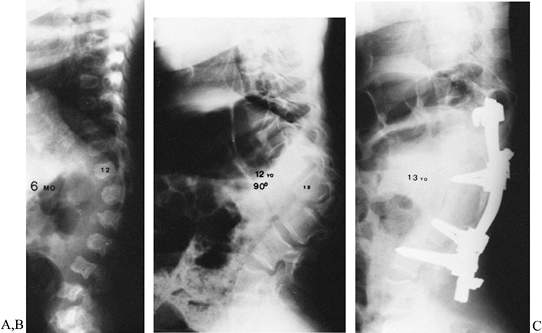
Figure 158.7. A: A 6-month-old girl with achondroplasia, never braced. B: The same patient at age 12 with severe 90° wedging of L-2 with early weakness. C:
One year after anterior decompression and posterior fusion. The patient had an initial postoperative increase in weakness, but recovered fully within 3 months. |
narrowing symptomatic. True disc herniations are a distinct minority,
however. Symptoms in older teenagers or adults include leg pain while
standing or walking, decreased endurance, numbness, and urgency or
incontinence. On examination, an upper or lower motor neuron picture
may be seen, depending on the level of compression. Evaluation should
include CT-myelography, cystometrogram, and postvoid residual. MRI is
less helpful because it does not show the bony compressive structures
as well. If stenosis symptoms or any neurologic deficit is present,
decompressive laminectomy should be done, after ruling out disc
herniation (43). Laminectomy should include all
involved levels, most commonly T8–S1. The most frequently reported
surgical error is insufficient length of laminectomy. Because of the
limited canal space, dural tear or cord contusion during decompression
is not infrequent.
having been seen in over 70% of patients in the largest reported
series. Only 30% of the curves, however, were over 30°. Two curve types
are seen: benign and idiopathic-like, and severe, rigid types with
kyphosis. The latter are considered by Tolo to be the result of wedged
or unsegmented vertebrae like those seen in congenital scoliosis (39). These curves are apparent before age 4, often in infancy.
successful for the gradual idiopathic-like curves. If the curve
progresses past 45° despite bracing, consider instrumentation without
fusion in young children if there is not too much kyphosis. Tolo and
Kopits (39) state that significant growth
ceases at age 9–10 in these patients, so fusion at this age, if it is
necessary, would have little effect on height. At any point where the
curve progresses significantly despite subcutaneous instrumentation,
perform fusion, for little of what is lost in progression can be
regained.
and fusion if the kyphosis or scoliosis is large or if there is much
growth remaining before skeletal maturity. Although the canal is
relatively stenotic in the lower lumbar region, hook placement can be
done safely (38). The incidence of postoperative neurologic deficit in hook placement was more than 50% in one series (5). The deficit
seems to be due to zealous attempts to correct these rigid curves, rather than to the instrumentation itself.
thoracolumbar kyphosis and hip flexion contractures. Treat the kyphosis
by anterior and posterior fusion if severe. Neurologic injury from
surgery is less common than in achondroplasia because the canal is
larger. Sublaminar instrumentation may be used. If excessive lumbar
lordosis is present and flexible, correction of any hip flexion
contracture by femoral extension osteotomies should be the first step.
that appear early and are difficult to control. The most common pattern
is a double major scoliotic curve with a severe junctional kyphosis,
which may equal or exceed the scoliosis in magnitude. The curves are
rigid, and bracing is poorly tolerated. The kyphosis and poor bone
quality contraindicate subcutaneous instrumentation. Definitive spine
fusion is frequently necessary at an early age. Restrictive lung
disease is common because of short ribs. Consult a pulmonary specialist
if you are contemplating anterior fusion or to determine if even
posterior fusion will be tolerated. Fusion with cast correction is the
most common method used.
to metatrophic dysplasia but less severe. Rib length is normal, and
restrictive lung disease is not as frequent as in metatrophic dysplasia
(2).
scoliosis is common in Hurler syndrome. It most often has an apex at
the thoracolumbar junction, where wedging of vertebrae and translation
may occur. Bracing is warranted, but its efficacy remains unproven. The
limited life expectancy of these patients historically has made fusion
untenable. With the increasing success of bone marrow transplantation,
patients who are longer-term survivors may require treatment by a
limited posterior fusion over the kyphotic segment if it is progressive.
by marked platyspondyly and, frequently, thoracic kyphosis and
scoliosis (2). Bracing is advised for scoliosis
less than 45° or for any increased kyphosis in growing children. In
some cases, the kyphosis has been permanently improved by bracing.
Scoliosis should be fused if the curve is over 45°. Pseudarthrosis is
common after posterior fusion of either kyphosis or scoliosis,
resulting in significant loss of correction. Therefore, patients with
severe curves may need an anterior as well as a posterior fusion if the
curve is rigid, if the patient is adult, or if he has had prior
laminectomy.
Although many classification systems have been proposed, the
radiographic system of Hanscom has been best correlated with spinal
involvement (16). Type A patients, those with
only bowing of the long bones, have the best bone quality and generally
maintain some correction if scoliosis surgery and instrumentation are
required. Type B patients, who also have biconcave vertebrae, and type
C, who have a trefoil pelvis, have a greater tendency to kyphosis. Type
D patients are more severely involved, having also cystic changes in
the metaphyses. With these latter three types, less correction is
obtainable, and there is more postoperative loss. Type E patients, with
absent long-bone cortices, should not be subjected to instrumentation
at all.
imperfecta curves except for postoperative protection because of the
potential for rib deformation. Posterior fusion should be done for
curves of more than 45° in type A, or 35° to 40° in types B through E.
Even at a young age, delaying fusion to preserve trunk height should
not be a consideration, because the trunk is so short in nonscoliotic
adults of these types, let alone those with curves.
carefully applied halo–gravity traction after anterior release may be
used to decrease the amount of force that must be applied through the
rods. All patients should be evaluated preoperatively for basilar
invagination and for pulmonary compromise. Segmental fixation using
hooks at as many levels as possible, augmented when necessary by
doubled Luque wires, is the preferred technique. The following points
should be noted:
-
Hooks placed on fragile laminae may be supplemented with methylmethacrylate.
-
Pack the methylmethacrylate after the hook is inserted; it should extend to the lamina above and below.
-
Preserve the spinous processes at these levels.
-
Supplement the fusion with banked bone.
-
Bone from other spinous processes is also helpful; these may be relatively large in osteogenesis imperfecta.
-
Blood loss is usually greater than in other conditions.
-
Use postoperative recumbency and orthoses as the quality of fixation dictates.
the curve is a long, uncompensated, “paralytic” type. Increased lumbar
lordosis may occur, especially with hip flexion contractures. In a
sizable minority, congenital anomalies may occur; take care to
distinguish these patients from those with multiple pterygium syndrome.
usual rules. Noncongenital curves can be braced if less than 50°, but
fusion should be performed for larger curves. The spine, like the
joints, is stiff, and correction is not often great unless the spine is
mobilized extensively anteriorly and posteriorly. Bone is osteoporotic
and hypervascular. Low lumbar curves with pelvic obliquity should have
fixation extended to the pelvis. Where excessive lumbar lordosis is the
main problem, patients respond poorly to posterior distraction, and
anterior column shortening by multiple partial vertebrectomies is most
successful.
scoliosis clinic population, so its signs should be looked for on all
initial examinations (9,44).
The diagnosis can be made with the presence of two or more of the
criteria from the 1987 Consensus Development Conference of the National
Institutes of Health (Table 158.2). In a patient with neurofibromatosis, it is important to make the distinction between dystrophic and nondystrophic curves (44).
Nondystrophic curves can be treated with brace or surgery according to
guidelines for idiopathic scoliosis. They are in the minority, however,
making up 25% to 35% of most series.
 |
|
Table 158.2. Relevant Aspects of Neurofibromatosis
|
Bracing is unsuccessful. Obtain cervical spine films and MRI or a
myelogram with CT before surgery. To rule out cervical deformity, which
is frequently associated with thoracolumbar deformity, obtain
radiographs of the neck before general anesthesia is done or halo
traction is applied (46). Abnormalities
identified within the canal by MRI or myelogram, such as thinning of
laminae, neurofibromas, meningoceles, and rib penetration, have obvious
implications for both the technique of the dissection and the choice of
fixation levels (6,12,23).
regardless of age because progression may be rapid, and loss of height
will be greater if the curve is allowed to progress than if early
fusion is accomplished. If the curve is less than 50°, kyphosis less
than 60°, and no obvious anterior scalloping or bony involvement is
present, posterior fusion alone is indicated (9).
Six months postoperatively, obtain oblique films or tomograms, or
perform routine reexploration to detect and treat early pseudarthrosis.
deficiency, or scoliosis more than 50° should have anterior and
posterior fusion. Because of potential vertebral body destruction by
tumor, anterior surgery has a more important mechanical role in
neurofibromatosis than in other conditions. Note the following points:
-
Fuse all involved levels.
-
If there is significant anterior tumor,
use strut grafts of fibula or vascularized rib, and establish good bone
continuity with vascularized tissue on the concavity of the curve. -
Halo traction may be used to optimize correction at the time strut grafts are inserted.
-
Posteriorly, segmental hook fixation is desirable; increasing rigidity of fixation will increase success of surgery (20).
-
Use postoperative bracing if the
vertebrae are weakened or the severity or location of the kyphosis is
causing excessive strain on end hooks, or if there are not optimum
numbers of fixation points above and below the apex (three on each
side).
it is due to intracanal tumor or rib penetration, decompress it
posteriorly and do subsequent fusion according to previous guidelines.
If it is due to kyphosis, correct it anteriorly and posteriorly with
decompression if focal.
patients ranks as a major threat to patient welfare. Take all possible
care in preoperative planning, surgery, and postoperative follow-up.
the life expectancy of patients with Marfan syndrome to nearly that of
the general population, thereby increasing the importance of
appropriate treatment of spinal disorders. Scoliosis of greater than
10° is present in approximately half of these patients. Less than 10%,
however, will require a brace or surgery (33).
There is no typical curve: In Marfan syndrome, the patient may have any
of the curve types seen in idiopathic scoliosis. Sagittal plane
deformities are equally common and vary from hyperkyphosis to
hypokyphosis. There is a fairly common finding of thoracolumbar
kyphosis. Use bracing for the same standard indications as in
idiopathic scoliosis; although the success rate is lower, there are
cases where the brace has been associated with curve stabilization.
cases treated with subcutaneous distraction instrumentation if they are
greater than 50° (34). This technique is
contraindicated, however, in cases where significant kyphosis exists.
The rod should be contoured to match the patient’s sagittal
profile—that is, not too straight. Dorsal displacement of hooks is a
frequent cause of failure of this technique, and it is due in part to
inadequate contouring. Postoperative bracing is mandatory. Despite all
of these precautions, the rate of hook cutout or continued progression
is significant. If cutout occurs, undertake posterior fusion with or
without anterior fusion, depending on curve size and the patient’s
overall condition.
One difference is the tendency to develop moderate thoracolumbar
kyphosis and the marked rotational listhesis that sometimes occurs in
lumbar curves. Evidence suggests an increased risk of pseudarthrosis in
patients with Marfan syndrome, especially in regions of kyphosis at the
thoracolumbar junction (7). Anterior release and fusion should be added in such cases (Fig. 158.8)
or when curves are large and rigid. Spondy lolisthesis of severe degree
occurs in approximately 2% of Marfan patients. Check for it on lateral
radiographs.
 |
|
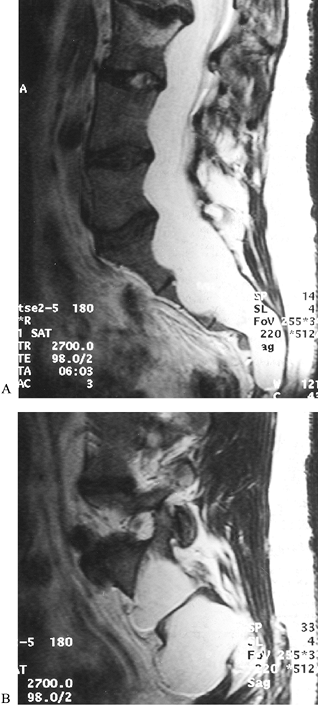
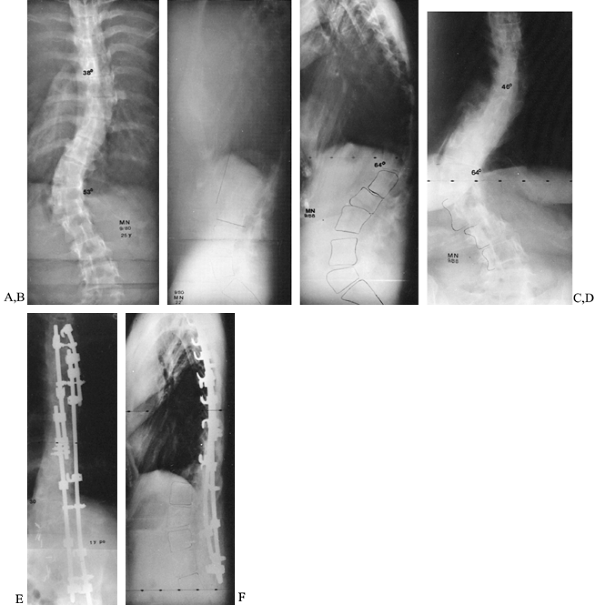
Figure 158.8. Progressive kyphoscoliosis in Marfan syndrome. A,B: Posteroanterior and lateral films at age 25, with 53° thoracolumbar scoliosis and 22° kyphosis. C,D: Repeat films 8 years later (after two pregnancies) show increase of scoliosis to 64° and, especially, of kyphosis to 64°. E,F:
One year after anterior release and fusion and posterior fusion with Cotrel-Dubousset instrumentation. Note that standard rods were not long enough in this patient; longer rods may be specially ordered. |
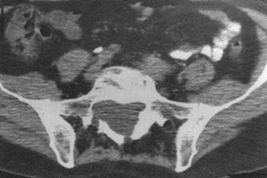
mind include the following: (a) The rate of dural ectasia is high (63%)
in the lower lumbar or sacral canal (32) (Fig. 158.9).
The dural ectasia is probably another manifestation of the effect of
gravity on abnormal connective tissues. The enlarged sac has thin dural
walls and may leak or erode laminae; take care with decortication and
instrumentation in these areas. (b) Instrumentation of
double curves in these already tall patients may require special ordering of long rods (Fig. 158.8).
(c) The patients have implanted cardiovascular devices that must be
considered when ordering prophylactic antibiotics or treating a
postoperative infection.
 |
|
Figure 158.9.
CT scan without contrast shows dural ectasia with foramenal meningocele. This is common in Marfan syndrome, in the lower lumbar spine and sacrum. Exercise care if working inside the canal. Marked thinning of laminae may compromise fixation strength. |
young patients with considerable growth remaining. I try to avoid
subcutaneous instrumentation in almost all cases, because the gains
over time are minimal and not worth the time and morbidity.
only to prevent progression of a congenital curve but also to allow
some correction of the curve with growth (45).
It is indicated for patients under about age 6 years who have some
growth potential on the concavity of the curve. The advantage of the
procedure is that it does not destabilize the spine and does not
require internal fixation, even though it is a corrective procedure.
The disadvantage is that it does not work for very large curves and is
not recommended for curves over 70°. There should be no significant
kyphosis or lordosis in the area to be fused. Although the anterior
portion of the procedure may be performed endoscopically, the patient
must be a satisfactory candidate for a thoracotomy. Take bending films
to assess the flexibility of the spine preoperatively. If some
correction of the curve is possible, accomplish it in the cast after
surgery.
-
Place the patient in the lateral position so that both anterior and posterior exposures may be performed without repositioning (Fig. 158.10B).
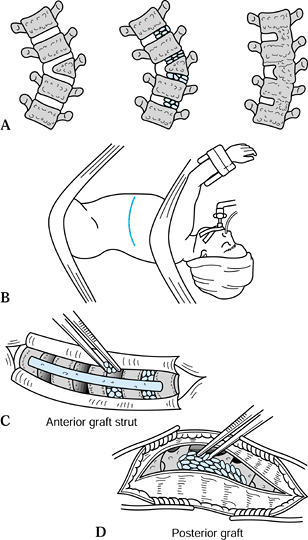 Figure 158.10. Technique of hemiepiphyseodesis for congenital scoliosis. A: Concept: The spine is fused anteriorly and posteriorly over the convexity, allowing some correction to occur with growth. B: The patient is placed in the mid-lateral position so that anterior and posterior approaches may be made simultaneously. C:
Figure 158.10. Technique of hemiepiphyseodesis for congenital scoliosis. A: Concept: The spine is fused anteriorly and posteriorly over the convexity, allowing some correction to occur with growth. B: The patient is placed in the mid-lateral position so that anterior and posterior approaches may be made simultaneously. C:
Anteriorly, 30% to 50% of the disc and endplates are removed and
replaced with bone graft; a strut graft is added if available. D:
Posteriorly, only the convex side of the curve is exposed and grafted
over the involved levels. (Redrawn with permission from Winter RB,
Lonstein JE, Denis F, de la Rosa HS. Convex Growth Arrest for
Progressive Congenital Scoliosis due to Hemivertebrae. J Pediatr Orthop 1988;8:633.) -
In the open technique, expose the spine anteriorly through the rib that is one level above the most cranial to be fused.
-
Confine dissection primarily to the
convexity of the curve, and confirm the levels either by the
characteristic shapes of the vertebrae, or by an intraoperative
radiograph with markers both anteriorly and posteriorly over the levels
to be fused. -
Remove the lateral one third to one half of the disc along with the corresponding portion of the endplates of the vertebrae.
-
Obtain bone graft from the morcelized rib or from another source and pack into the disc spaces to promote fusion (Fig. 158.10C).
-
Make a trough across consecutive vertebrae to allow a bone graft (such as rib) to be placed longitudinally, bridging them.
-
Perform posterior exposure at the same time, to be sure that the levels fused in the front and in the back correspond exactly (Fig. 158.10D).
-
Expose only the convexity of the posterior curve.
-
Avoid elevating the muscles from the concavity of the curve, to prevent fusion from occurring on this side as well.
-
Verify which levels are the end vertebrae to be fused by palpating the vertebrae from the front and the back simultaneously.
-
If in doubt, pass small Kirschner wires from front to back at the tip of a transverse process to help confirm levels.
-
Excise the convex facets and decorticate the spine.
-
If additional correction is desired, a
level above and below the curve itself may be partially fused as well,
to allow further correction with growth. -
Postoperatively, place the patient in a
cast to correct as much of the flexible portion of the deformity as
possible. Apply the cast either in the operating room, or a few days
after surgery, if there is significant edema or need to have access to
the patient. The patient wears the cast, or a cast followed by a brace,
for at least 6 months postoperatively. In the series of 13 patients
reported by Winter et al. (45), prevention of
curve progression was achieved in all but one, and in five of these,
curve correction occurred with growth. The mean correction for these
five patients was 10° (Fig. 158.11).![]() Figure 158.11. Result of convex hemiepiphyseodesis at 6-year follow-up. A:
Figure 158.11. Result of convex hemiepiphyseodesis at 6-year follow-up. A:
Curve measures 27° at age 4, due to unincarcerated hemivertebra with a
bar just distal to it on the opposite side. The hemiepiphyseodesis
extended two levels above and one level below the hemivertebra. B: At age 10½, the curve has corrected itself to 10°.
spine due to a hemivertebra. It entails somewhat more risk than a
hemiepiphyseodesis because the spinal canal is entered both anteriorly
and posteriorly, and the spine is partially destabilized to achieve the
correction. A significant degree of correction is possible, however,
and the risks are generally acceptable with current techniques in
experienced hands (8).
determine whether the desired degree of correction can be obtained
without vertebral resection. In addition, MRI should be performed in
all patients preoperatively because there is an increased frequency of
abnormalities within the spinal canal (Chiari malformation, syrinx,
diastematomyelia, and fibrous tether), which may predispose the patient
to neurologic complications. Hemivertebra excision in the thoracic
spine generally entails more neurologic risk as well as less
correction, but it is not contraindicated.
the procedure are performed in the same surgical session, if possible.
Use sensory and motor spinal cord monitoring.
-
Place the patient in the straight lateral position (Fig. 158.12).
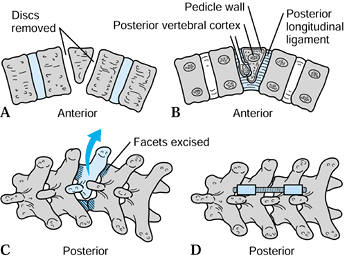 Figure 158.12. Hemivertebra excision. A: Remove discs and endplates above and below the hemivertebra. B: Curet and remove the hemivertebra. C: Resect the corresponding posterior elements. D: Complete the correction with posterior compression rod or wire fixation.
Figure 158.12. Hemivertebra excision. A: Remove discs and endplates above and below the hemivertebra. B: Curet and remove the hemivertebra. C: Resect the corresponding posterior elements. D: Complete the correction with posterior compression rod or wire fixation. -
Make a transpleural, transdiaphragmatic, or retroperitoneal anterior approach as dictated by the level of the curve.
-
Identification may be possible by local
landmarks as well as by the shape of the vertebrae, but it should be
confirmed by a radiograph if there is any question. -
If segmental arteries are to be ligated in the thoracic spine of a patient with congenital anomalies, some surgeons
P.4096
recommend placing a “bulldog” vascular clamp on the vessels to occlude
flow for 10 minutes, using spinal cord monitoring to be sure that the
intended vessels do not provide critical perfusion to the cord (1).
-
Resect the discs above and below the vertebrae first, followed by the body.
-
Leave the posterior portion of the
vertebra and the medial cortex of the pedicle intact until last, as
their resection may cause epidural bleeding. -
Place bone graft into the defect, but not so much as to limit the correction.
-
Resect the posterior elements over the corresponding level.
excessive difficulty, a pantaloon cast may be all that is necessary for
correction. However, if the patient’s size and bone density are
adequate, use internal fixation, which may include a wire for a simple
resection, or more rigid and complex fixation. It is the surgeon’s
judgment whether to perform these procedures in the same position, or
whether to turn the patient prone for the posterior fixation. It
depends on the complexity of the fixation intended.
fused. Bone from the resected vertebra and rib usually provides
adequate graft. The need for a postoperative brace depends on the
security of fixation and the presence of other, noncongenital curves in
the spine (Fig. 158.12, Fig. 158.13). In the largest recently reported series (21), the mean final correction was 35%, and there were 16% neurologic complications, but only 3% were permanent.
 |
|
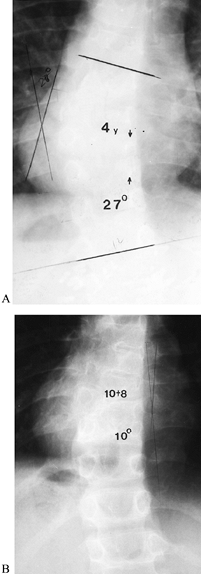
Figure 158.13. Patient with congenital scoliosis due to thoracic hemivertebra, treated with anterior and posterior convex hemiepiphyseodesis. A: At age 4, immediately before surgery, the curve had progressed to 27°. B: At 6 years postoperatively, the curve has improved to 10°.
|
indicated for tumor or for stenosis, as in achondroplasia. In both
cases, the presence of mild preexisting kyphosis when there is
remaining growth increases the risk of progression postoperatively.
This is greatest at the cervicothoracic and thoracolumbar junctions.
Progressive kyphosis may be prevented by performing a fusion at the
time of decompression, or in some cases by performing a laminoplasty.
achondroplasia, Uematsu et al. recommend a technique that involves
minimal use of instruments in the canal (Fig. 158.14) (43). Spinal motor and sensory monitoring is helpful.
 |
|
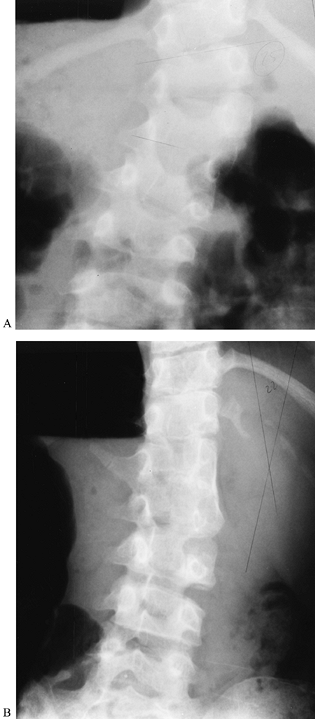
Figure 158.14.
A 5-year-old girl with congenital scoliosis due to hemivertebra at <2. She has the VATER association. Her curve has progressed to 45°. Treatment by hemivertebra excision was selected because the hemivertebra is easily accessible and the patient is significantly off-balance. A: Preoperatively, the hemivertebra may be easily seen. B: Two years after excision and fusion, the patient is in much better balance. |
-
Position the patient prone, taking care to reverse as much of the increased lumbar lordosis as possible.
-
Make bilateral laminar grooves just medial to the facets, using a high-speed burr.
-
Carry these down to the deep cortex, and gently lift off the laminae.
-
Preserve the facets if possible.
-
Perform the amount of length and width of decompression necessary.
-
A small (#10) rubber catheter should be able to pass centrally into the opening in the canal when the decompression is adequate.
-
Suture paraspinous muscles over the defect.
decompressed, posterior (with possible anterior) fusion should be done
as described previously. Even if no significant kyphosis is present
preoperatively, it should be watched for postoperatively and fused if
it develops. If laminoplasty is to be performed, the laminae with
interspinous ligaments are elevated in one continuous strip, and
reattached at the end with sutures into the adjacent facets, using bony
“shims” if needed to elevate the laminae.
scheme: 01, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
DM, Marrero G, King J, et al. Avoiding Paraplegia during Anterior Spine
Surgery: The Role of Somatosensory Evoked Potential Monitoring with
Temporary Occlusion of Segmental Spinal Arteries. Spine 1991;16(suppl):365.
DS, Boachie-Adjei O. One-Stage Anterior and Posterior Hemivertebral
Resection and Arthrodesis for Congenital Scoliosis. J Bone Joint Surg Am 1990;72:536.
DC, Winter RB, Lonstein JE, Denis F. Excision of Hemivertebrae and
Wedge Resection in the Treatment of Congenital Scoliosis. J Bone Joint Surg Am 1995;77:159.
SE, Winter RB, Lonstein JE. The Surgical Treatment of Instability of
the Upper Part of the Cervical Spine in Children and Adolescents. J Bone Joint Surg Am 1984;66:403.
JE. Treatment of Kyphosis and Lumbar Stenosis in Achondroplasia. In:
Nicoletti B, Kopits SE, Ascani E, McKusick VA, eds. Human Achondroplasia. Basic Life Science, 48:283. New York: Plenum Press, 1988.
JY, Thometz J, Emans J, et al. Threaded K-Wire Spinous Process Fixation
of the Axis for Modified Gallie Fusion in Children and Adolescents. J Pediatr Orthop 1989;9:675.
S, Wang H, Hurko O, Kopits SE. The Subarachnoid Space in
Achondroplastic Spinal Stenosis: The Surgical Implications. In:
Nicoletti B, Kopits SE, Ascani E, McKusick VA, eds. Human Achondroplasia. Basic Life Science, 48:275. New York: Plenum Press, 1988.