SPINAL SURGERY FOR MYELO MENINGOCELE
VIII – THE SPINE > Spinal Deformity > CHAPTER 157 – SPINAL
SURGERY FOR MYELO MENINGOCELE
abnormality associated with a host of problems that cross several
disciplines of medicine and rehabilitation. Of the problems presenting
to pediatric orthopaedic surgeons, none is as daunting, complex, and
fraught with pitfalls as the management of the associated spinal
deformities. In addition to the kyphotic, lordotic, or scoliotic
deformities typically faced by spinal-deformity surgeons, patients with
myelomeningocele bring the added challenge of a markedly abnormal
anatomy marked by the absence of a significant portion of the posterior
spinal elements. Additionally, these structures are frequently smaller,
the bone is more osteopenic, the skin, muscle, and fascia available for
coverage of hardware are frequently poor, and significant additional
medical problems frequently coexist (13). A
solid grounding in the treatment of idiopathic spinal deformities is
therefore a prerequisite to embarking on the management of patients
with myelomeningocele (6,34).
not exist in a vacuum. More than perhaps any other condition with which
orthopaedists are concerned, myelomeningocele should be managed by a
team of pediatric specialists. In addition to an orthopaedic surgeon, a
pediatric neurosurgeon, a urologist, a neurologist, and a physiatrist
must be allied with a health care team consisting of occupational and
physical therapy, social workers, and nutritionists. As patient demands
on each of these individuals is fairly high, most major pediatric
centers employ specialty clinics, where the specialists convene on
individual patients, rather than each specialist seeing patients
individually. Once thought to be a chronic and stable condition,
myelomeningocele is now known to be condition that changes throughout
life (8). It is therefore necessary for patients to be followed well into their adult years.
embryologically is unknown. Two major theories exist, and both have
their proponents. Von Recklinghausen, who initially described the
condition, felt that the abnormality was due to a failure of closure of
the neural canal. The opposing theory is that a closed neural tube
ruptures, exposing the neural elements (16). This theory was initially advanced by Morgagni (33).
A long discussion of the points that make both theories at least
histologically plausible is beyond the scope of this chapter; suffice
it to say that a certain modicum of evidence exists for both
viewpoints. The abnormality resulting in the formation of a
myelomeningocele occurs very early in gestation—most likely at the
third to fourth week after conception. In many cases, the defect is
present before a woman recognizes that she is pregnant.
that causes the formation of a myelomeningocele, many risk factors have
been identified that markedly increase the incidence of this birth
defect. Use of the the antiseizure medication valproic acid (Depakene)
during pregnancy has been demonstrated to markedly increase the risk of
myelomeningocele (9). As valproic acid can alter serum folate levels, this adds credence to the work of Yates et al. (45),
who showed an association between the incidence of neural tube defects
and depressed red cell folate levels. In their study, the diminished
folate levels could not be entirely attributed to decreased dietary
intake, suggesting that an inborn error of folate metabolism existed.
This study was subsequently supported by Seller and Nevin (40),
who noted a decreased incidence of myelomeningocele and other neural
tube defects when adequate vitamin and folate supplementation began
before conception. These studies led to the general dietary
recommendation that all women of childbearing age consume a minimum of
1.0 µg of folic acid daily (9). Many foods are being supplemented with folic acid in direct response to this work.
myelomeningocele remains the amniotic fluid level of alpha-fetoprotein
(AFP) obtained through amniocentesis. A high concentration of AFP
within the amniotic fluid, which is usually sequestered within the
cerebrospinal fluid (CSF) of the fetus, is pathognomonic for an open or
otherwise compromised neural canal. Most centers continue to use serum
AFP levels to screen expectant mothers. While high AFP concentrations
have a relatively high correlation with neural tube defects, the
sensitivity and specificity of this test is only about 80% (1).
Prenatal diagnosis is most useful because it is important to plan for
delivery at a tertiary care facility, where neurosurgical and
neurosurgical intensive care unit support is available; it is also
important because it is believed that elective cesarean section may
result in neural function at one to two levels higher than otherwise.
begins with closure of the defect, typically by a neurosurgeon, within
the first 12–24 hours of life. Without treatment, such children will
typically die. Several series of untreated children reported in the
1970s demonstrated mortality rates in the range of 90% to 100% (20,23).
The cause of death in untreated infants is bacterial
meningoventriculitis. Those who survive meningoventriculitis have
higher levels of paralysis, more profound mental retardation, and
frequently uncontrolled hydrocephalus, leading to additional problems.
Therapeutic nihilism was frequently practiced in the United States 40
years ago because of the difficulty in treating many of the sequelae of
myelomeningocele. However, with advances in orthopaedic, neurologic,
and urologic care, myelomeningocele is very treatable. It has therefore
become the standard of care in the United States to perform early sac
closure and ventriculoperitoneal shunting in infants with
myelomeningocele.
important to protect the fragile soft tissues overlying the repair.
Additionally, this period of prone recumbency is advantageous in
positioning the hips to avoid subluxation or dislocation.
require the services of an orthopaedist when performing the initial
shunt closure. Occasionally, the child will be born with a severe
gibbous deformity that will necessitate a two- to three-level
kyphectomy to facilitate closure.
careful history and perform a thorough physical examination. Do not
immediately focus on the spinal deformity. It is important relative to
surgical indications, as well as other prognostic factors, to know
about the general health of the child. Inquire about the patient’s
mental functioning, school attendance, and level of socialization, as
well as the activities of daily living that the patient is able to
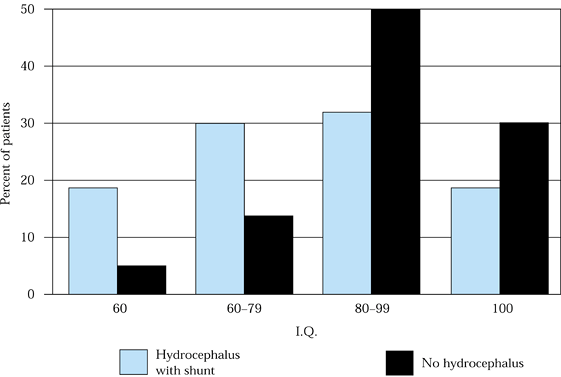
perform. The presence or absence of hydrocephalus or a shunt is
prognostic relative to overall intellectual function (Fig. 157.1) (18). Inquire about the patient’s urologic status and bowel function. Knowledge of the status of these
matters is yet another reason why a multidisciplinary clinic is so
valuable. Finally, it is important to have a good understanding of
parents’ perceptions and patients’ needs, desires, and expectations
regarding eventual ambulation and the use of ambulatory aids.
 |
|
Figure 157.1. Average I.Q. as a function of shunt presence in patients with myelomeningocele.
|
neurologic function, the perception of patients and caregivers
regarding spinal curvature progression, and any symptoms or problems
directly related to the curve, including pain, skin breakdown, loss of
balance, difficulty with breathing, or changes in gastrointestinal (GI)
or genitourinary (GU) function. It is also important to inquire about
previous trials of bracing and spinal surgery.
myelomeningocele is sensitivity to latex. It is mandatory to ask a
dermatologist to test patients for latex allergy in the preoperative
period. Use a latex-free protocol in surgery and in the postoperative
period, even with nonallergic patients. Allergic reactions to latex
have become a significant health problem for patients and health care
professionals over the last 5 years. It is believed that 50% of spina
bifida patients in the United States have evidence of severe allergy to
latex. As many as 5% to 10% of myelomeningocele patients may have a
potentially life-threatening form of this allergy (3,11).
The allergen causing the hypersensitivity response is a component of
the latex substance itself, and therefore only strict avoidance of
latex contact is effective (42). Immediate
hypersensitivity reactions to latex have been reported through several
different routes of exposure. Deaths have been reported after mucous
membrane exposure and infusion through latex-containing intravenous
lines (3,11).
Intraoperative anaphylactic reactions have been reported simply after
the opening of latex gloves in the operating suite. It is believed that
patients with myelodysplasia are at increased risk for latex allergy
due to the multiple contacts that they have had with the substance (11), although some feel that there is a yet unknown reason for their proclivity to an allergic response.
care materials become available, inadvertent exposure to latex will
become less and less common. The Joint Commission for the Accreditation
of Hospital and Healthcare Organizations currently requires that all
patients be asked specifically about a history of latex sensitivity at
their initial outpatient or inpatient visit. All hospitals should have
policies and procedures in place regarding bed assignment and room
preparation and strict protocols for direct patient care activities in
a nonlatex environment. It behooves the surgeon and operative team,
however, to doubly reinforce this concept in the operating room and
with the ancillary staff.
the extremities, paying special attention to the neurologic
examination. Whereas the lower extremities are typically the sites of
major neurologic compromise, conditions
such
as hydromyelia or syringomyelia may also compromise strength and
sensation in the upper extremities. Careful neurologic examination to
identify the specific level of neurologic involvement is important as a
predictor of both ambulatory ability and the likelihood of curve
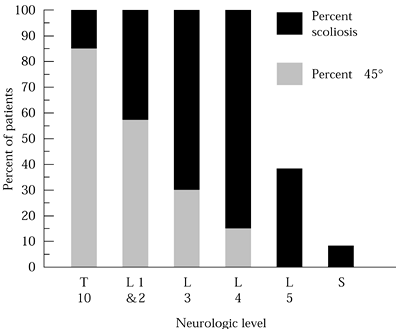
progression (Fig. 157.2).
It must be kept in mind that the neurologic pattern may differ from one
extremity to the other, even (in rare cases) nearly to the point of
having one normal extremity and near-total paralysis of the other
extremity, a condition known as hemimyelodysplasia (29).
 |
|
Figure 157.2. The incidence and the severity of scoliosis depend on the level of neurologic compromise.
|
spine (lordosis, kyphosis, scoliosis), examine the spine to assess
flexibility and balance. Additionally, assess the quality and the
condition of the skin throughout the torso. This is important not only
for wound coverage and closure but also for any postoperative casting
or bracing that may be anticipated. While radiographic studies will
convey a good deal of information regarding the underlying bony
structure, careful and thorough palpation of the spine for the presence
or absence of bony elements can immeasurably add to one’s understanding
of the deformity.
posteroanterior (PA) and lateral projections and supine bending films,
are essential for preoperative planning. Magnetic resonance imaging
(MRI) of the spine, as well as spiral computed tomography (CT) with
three-dimensional (3D) reconstruction, adds to one’s ability to
understand the nuances of the curve, note congenital elements and
dysplastic features, and undertake rigorous preoperative planning.
There is no aspect of spinal surgery where the adage “failure to plan
is a plan for failure” better applies than in myelosurgery.
ossified spine and in areas where there is no bone overlying the neural
elements. For the most part, myelography has been replaced by MRI.
ambulation with or without braces and orthotics, depending on the level
of neurologic involvement
which neurologic change can be expected. The observation of diminished
extremity function frequently leads to the diagnosis of subtle
neurologic deterioration.
and balanced seating pressure diminishes the likelihood of skin breakdown in insensate areas.
 |
|
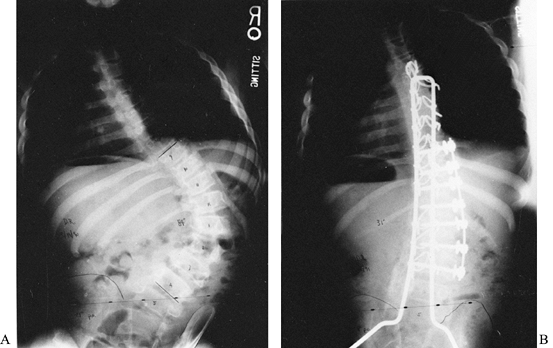
Figure 157.3. A:
A 13-year-old boy with a thoracic-level myelomeningocele. He had a severe, progressive right thoracolumbar paralytic curve of 84° and significant pelvic obliquity of 21°. Staged anterior and posterior procedures 1 week apart were necessary to correct the rigid spinal deformity and pelvic obliquity. The combination of an anterior Zielke procedure followed by posterior instrumentation and fusion with a Luque rod with Galveston pelvic fixation provided adequate correction of his deformities (B). |
management include observation, bracing, and surgery. However, unlike
idiopathic adolescent spinal deformities, there is no “routine”
management for the myelodysplastic spine. Patient size and age, level
of curve involvement, coexisting hemivertebrae, and wildly variant
anatomy make a highly individualized treatment plan mandatory. However,
certain rules do apply and are useful to bear in mind.
patients with spina bifida tends to progress faster and more
relentlessly than in idiopathic scoliosis. When scoliosis is simply
being observed, this option is generally chosen not so much because of
the low-level magnitude of the curve but because the patient either may
not be of appropriate size for definitive surgery or may have a curve
that is not amenable to orthotic management.
temporary measure, intended to allow further spinal growth and patient
maturation before definitive operative management. Many potential
problems exist with bracing the paralytic spine. First, the brace
exerts pressure on insensate or sensory-impaired skin, which can result
in decubitus ulcers, particularly over the bony prominences of the
spine and the edges of the rib cage and pelvis. Second, the degree of
compression necessary to maintain control of the curve will frequently
compromise an already restricted pulmonary capacity and definitely
impair an already compromised ability to be mobile. Any orthotic must
be custom-made and requires very careful fitting and supervised wear.
Insensate skin can break down very quickly, and children must be
introduced to the orthotic slowly, beginning with 1 hour at a time.
Inspect the skin carefully after removal of the orthosis. Increase the
wearing time slowly over 3–4 weeks until the patient is wearing it
throughout the waking hours. Remember that night- and nap-time brace
wear is not as beneficial in slowing the progression in the paralytic
spine as it is in the idiopathic spine.
are fully developed by 10 years of age (25,37).
Congenital deformities (unsegmented bars and hemivertebrae, and
combinations thereof) can coexist. These will be noted at birth and
tend to require surgery at an earlier age than the paralytic deformity (2).
Despite the high percentage of spinal deformities, one cannot be
complacent regarding the exact cause of any given curve. As in any
spinal deformity, investigation is necessary to determine whether there
is an underlying correctable cause for the disorder, and therefore MRI
and CT are useful in evaluating the spine for hydromyelia, a tethered
spinal cord, or ventricular porencephaly, which can contribute to the
deformity (8).
balanced spine with the head centered over a nonoblique pelvis,
allowing good balance and affording patients the use of both hands.
Those with collapsing deformities, who must push up with their hands to
support themselves or to maintain good pulmonary or GI function, are
severely hindered in their ability to perform activities of daily
living (27).
require surgery. It is assumed that most children with
myelomeningocele, who will eventually require spinal surgery, will have
curves greater than 50° well before skeletal maturity. Whereas it is
preferable to wait until age 10–12 years for instrumented fusion, one
will occasionally need to intervene at an earlier age to prevent
irretrievable progression. As with surgery for other medically
“delicate” patients, such as those with muscular dystrophy,
optimization of general medical health at the time of surgery is
important. Before surgery, any hydrocephalus should be well controlled,
and shunts, if in place, should function normally. Ideally, the urinary
tract should be free of any obstruction, ureteral reflux managed, and
the urine sterile. In many cases, of course, obtaining sterile urine is
temporary at best, and in these instances, the known colonizing
bacteria should be suppressed with appropriate antibiotic prophylaxis.
Failure to control bacteria in the urinary tract can lead to an
inordinately high infection rate (2). Finally,
the condition of the skin generally and in the operative area, in
particular, must be at its best. Whenever the potential for the
introduction of infection into the fusion through skin contaminants is
high, it is advisable to do appropriate plastic surgical reconstruction
first. This will often require tissue expansion, midline scar excision,
and closure. After the new area is well healed, definitive spinal
surgery can then proceed with less risk of infection.
greatly enhanced the ability to image the deformed spine, immeasurably
improving preoperative planning. Despite these advances, however, plain
radiographs (standing or sitting PA and lateral views and supine
bending films) are critical for initial planning. These images will
demonstrate the existing spinal alignment, head and shoulder balance,
and pelvic obliquity. Bending films assess the degree of correction
that may be possible. Keep in mind that the bone in the paralytic spine
is likely not to be as strong as in the idiopathic spine and may not
tolerate attempts to restore true anatomic alignment.
useful in diagnosing a preexistent underlying osteomyelitis, which
would be a short-term contraindication for instrumented fusion.
Patients with chronic skin breakdown over the deformity or a history of
prolonged drainage may have chronic osteomyelitis. Bone infection must
be eradicated before correction of the deformity.
when 3D reconstructions are performed. Spiral CT data can be
manipulated into excellent 3D images, and this minimizes the amount of
radiation to which patients are exposed. 3D reconstructions allow
virtual spinal visualization and can be useful when placement angles of
hooks or pedicular screws are being planned.
who underwent a surgical closure after birth have by definition a
degree of cord tethering (21). In some
patients, the tethering is a major factor in scoliosis progression. In
addition to demonstrating significant tethering, MRI will show any
other cord abnormalities that may be present, including syringomyelia
and hydromyelia, which may need correction before a definitive spinal
fusion. MRI will also demonstrate the degree of Arnold–Chiari
malformation and any change from baseline.
planning more important than in the paralytic spinal deformity. Be
prepared for the following:
anterior and posterior spinal instrumentation combined with fusion that
includes the sacrum.
-
Start the fusion typically two levels above the end of the curve, and extend to the sacrum or pelvis (25,34).
-
The most common technical error is to perform too short a fusion that necessitates later revision for progressive imbalance.
-
Wait until age 10–12 years if the curve magnitude is moderate.
-
It is better to accept some permanent truncal shortening than to let a curve progress beyond 70° to 80°.
-
The majority of patients with
myelodysplasia have a small, underdeveloped, osteopenic pelvis. Plan to
use allograft bone and other bone-graft extenders for the primary
grafting material.
-
Use subcutaneous epinephrine
(1:400,000–500,000) and a Bovie electrocautery, a bipolar coagulator,
and in some cases an argon laser to maintain a dry operative field.
Employ hypotensive anesthesia judiciously to minimize uncontrolled
blood loss. -
Remember that one of the most important factors related to complications and blood loss is the prevention of hypothermia (24).
-
Use blood and intravenous fluid warmers,
as well as warming blankets, to maintain normothermia. Simply turning
the room temperature up to 85° will not have the desired effect. -
Intraoperative salvage of blood with a
cell saver is useful because the volume of blood in these patients as a
result of their size is frequently not enough for salvage by
conventional means. Use of state-of-the-art processing units and
intraoperative plasmapheresis is an exciting new concept for
intraoperative cell salvage that requires markedly less volume of
salvaged cells to be useful.
myelomeningocele must deal with the issue of deficient bone stock on
two levels. First, is a congenital deficiency of the posterior elements
that provides limited sites for traditional sublaminar wires or hooks.
Secure the rods to the spine at these levels with a cable or a wire
encircling the pedicle through the neural foramen; otherwise do not
instrument these levels. Where soft-tissue coverage is adequate, the
newer, smaller pedicle screw systems appear to be an excellent means of
securing these levels of the spine to the rod. Remember that the
majority of pull-out strength with a pedicle screw is within the
pedicle itself. The screw should be optimally sized to the pedicle and
need not extend farther than 50% of the way through the vertebral body.
Be fully familiar with the available instrumentation, and arrange to
have a wide array of screw sizes and lengths available on the day of
surgery.
availability of autogenous graft. Portions of both iliac crests, the
ribs, and portions of the spinous processes were once the only bone
available. With modern bone banking technology and the availability of
graft extenders, the use of autogenous graft is now typically not
advised unless a patient is unusually robust; commercially available
graft extenders include Grafton (Grafton Corporation, Edison, NJ), a
demineralized bone matrix; Coralene (Interpore International, Irvine,
CA), and Osteoset pellets (Wright Medical Products, Arlington, TN).
Additional products on the horizon will make the issue of deficient
autogenous graft a moot point.
devastating complications that may follow instrumented spinal fusion in
this patient population. At our institution, dual-coverage antibiotics
(a first-generation cephalosporin and an aminoglycoside) are typically
given for 48–72 hours postoperatively.
-
Prolong antibiotic coverage if drains remain in place or significant drainage persists.
-
A potential for later infection is
present if systemic urosepsis occurs or if the soft tissue overlying
the instrumentation breaks down. -
Use all available means of obtaining
thick, well vascularized flaps to cover the spinal instrumentation,
particularly in the lumbar region, where the overlying tissue is
typically most atrophic and poorly vascularized. -
Stage procedures in instances where the
scarred area has had repeated episodes of breakdown or the scar is in
particularly poor repair. -
Excise nonviable scar initially, and mobilize flaps to bring new, healthy skin to the midline.
-
As simple flap mobilization may not be
possible, consider placing tissue-expansion bladders on either side of
the midline to create new skin. -
Then resect the midline scar, bring the
new expanded skin together in the midline, and allow the wound to heal
for 4–6 weeks before the definitive procedure.
for whom the best results for spinal fusion in these patients involved
combined anterior and posterior instrumentation. They noted that the
anterior–posterior approach gave the best degree of correction, best
maintenance of correction, and best correction of pelvic obliquity. The
instrumentation in this series was with Harrington rods and either the
Dwyer or Zielke device anteriorly. These three systems have long since
passed out of favor, but the concept of a 360° fusion still makes sense
from the standpoint of maximal rigidity
and stability. However, with improved posterior segmental instrumentation, a separate anterior approach is not always necessary.
pelvis. One problem with fusion to the pelvis is the historically high
rate of pseudarthrosis (10,14,15,34). The development by Allen and Ferguson (2)
of the Galveston technique, in which Luque rods are inserted into the
iliac wing, was a significant advance in pelvic fixation. Their first
reported series in 1979 demonstrated that the technique, although not
foolproof, was a major improvement over previous attempts to fix the
rod to the sacrum.
pelvis, particularly in nonambulators, tends to be osteopenic and
hypoplastic. Rods can migrate within the bone and can break out through
the thin cortex anteriorly or posteriorly. The other frequent problem
is the difficulty in securing adequate fixation in the lower lumbar
spine, where the posterior elements are absent (10).
No study has yet been published in which pedicular hooks were used at
the lower lumbar levels along with the Galveston technique; however,
one would expect that problems with iliac wing breakage would be less
common.
In this technique, the rod is contoured to fit just anterior to the
sacral ala or passed through a window in the superior aspect of the ala
down into the sacrum itself, paralleling the sacroiliac joint rather
than going into the ilium. Our experience with the latter intraosseous
modification suggests that rod pull-out by fracture of the posterior
sacral cortex is a major drawback.
to the pelvis employs an intrailiac bolt or a long pedicle screw
inserted in the same manner as the end of the rod in the
Luque-Galveston technique. The rod is then appropriately contoured and
attached to the screw or bolt in the same fashion as the screw would
otherwise be attached to a rod. The advantage of this technique is that
the rod–screw construct may be inserted in a “modular” fashion. The
screw has a greater initial pull-out strength than the smooth end of a
contoured rod. Studies have not shown a long-term benefit from this
construct relative to improved lumbosacral fusion. It is, however,
easier for surgeons to place. Be certain that the screw–rod interface
is sufficiently tight, as any modular system can disengage over time.
With improved segmental fixation, a patient who is beginning to show
signs of skeletal maturity or in whom the triradiate cartilage of the
acetabulum has closed (39), may not require
additional anterior fixation. However, when the anterior procedure is
performed, it is done first and consists of anterior release or
discectomy and fusion, anterior release with strut grafting, or, most
commonly, anterior fusion and correction with CD Horizon, CD Hopf
(Sofamor-Danek, Medtronic-Sofamor-Danek, Memphis, TN), or Kaneda
(Johnson & Johnson, Depuy-Acromed, Raytham, MA) style
instrumentation.
-
After induction of general anesthesia,
place the patient in the lateral decubitus position with the convexity
of the lumbar curve placed upward. Place the apex of the curve at the
break in the operating table so that the table can be gently flexed,
with the head and foot portion lower than the middle. A deflatable bean
bag, with or without kidney rests, maintains excellent positioning of
the patient. Carefully pad all bony prominences, and ensure that the
Bovie pad is not over a minimally protected bony prominence. Perform a
wide field preparation of the skin. If an adhesive surgical drape is
not utilized over the entire surgical field, seal the edges of the
drapes. -
Enter the thorax one or two levels above
the superiormost vertebrae to be instrumented. Utilizing two levels
above will facilitate placing the uppermost screw in its best position,
parallel to the end plates.-
If not experienced in using the Bovie
cautery for all cutting beyond the dermal level, use subcutaneous
epinephrine to secure hemostasis. -
A needle-tip Bovie unit is particularly useful for the subcutaneous dissection.
-
Start the incision proximally,
approximately a hand’s-breadth from the midline over the rib in
question, and extend along the rib to the costochondral junction. Then
curve the incision gently distally, following the lateral edge of the
rectus sheath. -
The distal extent of the incision will
depend on whether the instrumentation is to be carried down to the L-3
or L-4 level. It is exceedingly difficult and perhaps dangerous to
attempt to get to the L-5 level through this approach. -
Carry the incision down proximally to the periosteum of the rib, and incise it longitudinally.
-
Expose the rib subperiosteally without
violating the chest cavity. When the rib is freed, disarticulate it
from its chondral end, and with blunt finger dissection remove it
entirely from its vertebral articulation. -
Place a ringed lap sponge into the proximalmost portion of the wound to maintain hemostasis.
-
Incise the costal cartilage longitudinally with a knife, and find the extraperitoneal plane immediately beneath
P.4065
with blunt finger dissection. The key to this exposure is developing
the correct retroperitoneal plane. Open the rib bed longitudinally to
enter the chest, and use a self-retaining chest retractor to spread the
ribs. -
Carry the incision distally under direct
vision, taking care to not transect the external oblique, internal
oblique, and transverse abdominal muscles other than through their
tendinous condensations at the lateral aspect of the rectus sheath. -
Take down the diaphragm from the chest
wall from the site of the chondral split, working posteriorly toward
the diaphragmatic crus. The diaphragm may be taken off the chest wall
nearly flush, as some surgeons prefer, or, more typically, with a 1–1.5
cm cuff left, which is useful for reapproximation at closure. -
Place marking sutures of alternating colors every 3–4 cm to facilitate a more anatomic closure.
-
After the diaphragm has been taken down
to the vertebral body or the crus and the distal extent of the
abdominal portion is complete, use a self-retaining retractor system,
such as a laparotomy retractor (Omnitract, Omnitract Surgical,
Minneapolis, MN), to maintain broad exposure. -
Incise the pleura over the thoracic
levels to be instrumented, and using a Kidner sponge, elevate the
pleura generously toward the opposite side of the vertebral body and
equally generously back over the heads of the ribs. Taking time at this
point to mobilize the good pleura will facilitate covering the upper
end of the hardware construct at the end of the procedure. -
If instrumentation is placed, then gently
and carefully isolate, ligate, and tease the vertebral segmental
vessels from the vertebral body with a Kidner-type sponge. -
Vessels may be ligated with suture or
with vascular clips. Clips should be made of titanium, and use of an
automatic clip loader will speed the process. The vessels are readily
and easily exposed to the level of L-1, beyond which they are obscured
by the psoas musculature. -
Be careful of the nearby genitofemoral and ilioinguinal nerves.
-
Pay close attention to the tributaries of
the thoracic duct, as well as the sympathetic chain. If a portion of
the lymphatic chain is inadvertently cut, chylous spillage will be
evident; the two duct ends may be ligated without complication. -
After ligating the segmental arteries, remove the intervertebral discs.
-
Use a long handled #15 blade to incise the annulus as near the endplate as possible.
-
Resect the annulus as fully as possible,
except posteriorly, where a portion is frequently left adherent to the
posterior longitudinal ligament. -
The endplates are generally thick and are
easily removed with a large curet within the intervertebral space. Pay
particular attention not to stray through the fairly soft subchondral
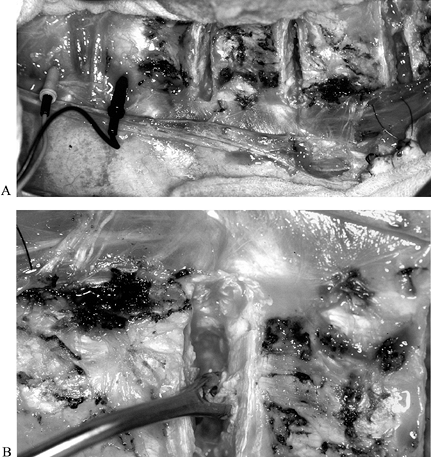
bone into the vertebral body itself (Fig. 157.4).![]() Figure 157.4. Anterior discectomy technique. A:
Figure 157.4. Anterior discectomy technique. A:
Intraoperative photograph demonstrates the anterior lumbar
discectomies. Note the motor-potential monitoring electrodes to the
left. This patient had some distal motor sparing. B:
A uterine curet may be useful to remove the cartilaginous endplate.
Care needs to be exercised in endplate removal, as the underlying bone
is frequently very soft. -
As each discectomy is performed, pack the intervertebral space with thrombin-soaked Surgicel to provide hemostasis.
-
-
When the desired degree of release has
been achieved, place the vertebral screws across the vertebral bodies.
The dual-screw systems available (CD-Horizon) and the Kaneda anterior
scoliosis system (KASS) demonstrate improved pull-out strength compared
with single-screw systems (Fig. 157.5) (32).
However, the small myelodysplastic spine may not always accommodate two
screws. The choice of whether to use a one- or two-screw vertebral
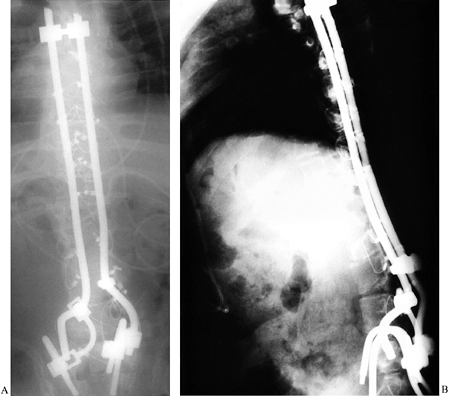
construct is an essential part of the preoperative planning.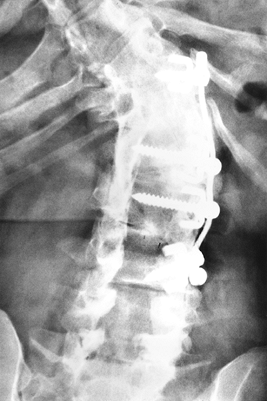 Figure 157.5.
Figure 157.5.
PA radiograph of the lumbar spine after Dwyer instrumentation. The
lumbar component is well fused despite hardware pull-out. However, note
the decompensation at the thoracolumbar junction secondary to “fusing
too short.”-
If possible, place screws with bicortical fixation to maximize pull-out strength.
-
After placement of the screws in each vertebral body, contour a flexible template rod to the spinal deformity.
-
Cut and carefully contour an appropriate-length implantable rod to match the curvature of the template rod.
-
After fixing the rod to the vertebral screws, reinflate the vacuum bean bag to soften it and make the table surface flat.
-
Now, slowly and gently rotate the rod
anteriorly to convert the scoliotic curve into a gentle lumbar curve.
When 90° of rotation has been achieved, lock the rod into position. -
Sequentially, beginning from the center,
gently distract the intervertebral disc spaces, remove the
thrombin-soaked Surgicel, and pack the disc space with bone graft. Use
the excised rib and additional allograft cancellous bone as needed.
Pack the disc space completely and tightly, and then gently compress
the disc.
P.4066 -
-
Fill each vertebral level with bone graft
so that at completion of the grafting, the spine appears as though
arthrodesis has already occurred. Leaving large segments of unfilled
disc space will increase the likelihood of fibrous union or nonunion,
which can lead to hardware failure (6,15,31). -
Allow the reflected psoas musculature to
overlie the distal construct, and close the pleura superiorly with a
running 2-0 absorbable suture attached to a tapered needle; the use of
a cutting needle will result in excessive tearing of the pleura.
Reattach the diaphragm either in layers or in a single layer, depending
on your preference. Alternate #1 Vicryl and Ethibond sutures for a very
strong and secure diaphragmatic closure. Place an appropriate-size
chest tube in the anterior axillary line, and tubularize the rib bed to
facilitate regrowth of the rib. Close the remainder of the wound in
layers. Typically,
P.4067
no
drain other than the chest tube is needed. If the anterior–posterior
fusion is to be staged, do the posterior procedure in 7 days. However,
there is no reason why the procedure must be staged, provided it is
safe to proceed with several hours of additional anesthesia. Blood loss
from the anterior approach can be kept to a reasonable amount—usually
less than 150 ml.
that the nutritional status of any patient is usually at its best at
the time of the first surgery (4,35). It is therefore advisable, whenever possible, to do anterior and posterior surgery under the same anesthetic.
the surgeon’s preference, and associated contractures and deformities,
any of several positioning devices is appropriate. I believe that the
four-post frame is the most useful for smaller adolescents. Once again,
it is extremely important to carefully pad all bony prominences, as
skin slough at the chest and anterior iliac wings is a significant
potential pitfall from prolonged prone positioning.
is mandatory. If the original scar remains in place, take meticulous
care to keep the flap edges as thick as possible. Do not use
subcutaneous epinephrine in this scarred area because of the increased
risk of postoperative skin slough.
-
After positioning the patient in the prone position, make a straight midline incision as for any posterior spinal approach.
-
As the distal anatomy is often quite
difficult, approach the abnormal anatomy from areas of normal anatomy
demonstrated cephalad. -
As the paraspinal musculature is stripped
from the posterior elements, maintain good hemostasis and use Bovie
cautery for the majority of soft-tissue cutting. -
To avoid the midline scarred area, use an inverted-Y
incision. With this approach, one entirely avoids the midline sac area,
and the midline lumbar area is not undermined at all. This allows good
exposure of the transverse processes and pedicles but avoids entering
the dural sac, which increases the likelihood of wound breakdown or
infection. Be prepared for a small skin slough at the junction of the Y, and be careful that the distal limbs of the Y portion are far enough apart to allow adequate collateral blood flow to this “island” flap. -
When the spine has been subperiosteally
exposed to the transverse processes throughout the length of the
incision, facilitate further lateral exposure at the caudal end by
transecting the paraspinal muscles in an L-type fashion.-
Contour the appropriate-length rods to maintain the sagittal curves, especially the lumbar lordosis.
-
The degree of contouring toward the
scoliosis will depend on the degree of correction that can be obtained
once the rod is in place. Gentle pressure on the spine from two persons
will allow fairly accurately prediction of what curvature correction
will be possible. -
Affix the upper end of the rod to the
spine with a sublaminar hook or sublaminar wires or cables. Sublaminar
cables have the advantage of being more flexible; however, they are
clearly more expensive, and retightening a cable once crimped is
generally not possible unless a second crimp device has been added
first. If one desires to maintain an all-titanium construct, however,
cables may be the only option (Fig. 157.6).![]() Figure 157.6. PA (A)
Figure 157.6. PA (A)
view of a patient who initially underwent pelvic fixation with
insertion of rod ends into the sacral ala. Approximately 2 weeks
postoperatively, the rod ends displaced dorsally through the bone.
Revision surgery utilized a construct with a separate rod placed
anterior to the ala and attached posteriorly with Texas Scottish Rite
Hospital links (TSRH, Medtronic-Sofamor-Danek, Inc., Memphis, TN). The
patient did well after the revision. (See Figure 157.11 for clinical photos.) Lateral (B)
view of a patient who initially underwent pelvic fixation with
insertion of rod ends into the sacral ala. Approximately 2 weeks
postoperatively, the rod ends displaced dorsally through the bone.
Revision surgery utilized a construct with a separate rod placed
anterior to the ala and attached posteriorly with Texas Scottish Rite
Hospital links (TSRH, Medtronic-Sofamor-Danek, Inc., Memphis, TN). The
patient did well after the revision. (See Figure 157.11 for clinical photos.) -
Pass the sublaminar wires or cables in
the usual fashion in the normal part of the spine and around the
remaining pedicle through the neural foramen in the bifid portion of
the spine. -
Pedicle screws can be used where the posterior elements are deficient, as noted previously.
P.4068 -
-
Fixation to the pelvis, also previously
discussed, is based on surgeon preference and bony anatomy. The
traditional one-piece Luque “unit” rod is technically demanding, but
fortunately alternatives exist. The one-piece bilateral rod may be cut
and then reconnected with any of several rod cross-links available on
the market. The overall stability will not be compromised by this
technique (Fig. 157.7). Additionally, there may
even be cases in which the surgeon may wish to use two rods on each
side connected by rod-to-rod connectors.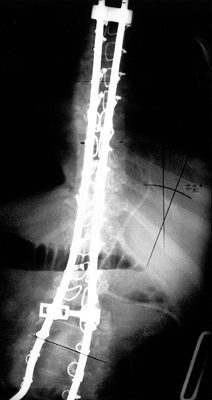 Figure 157.7.
Figure 157.7.
PA radiograph of the thoracolumbar spine demonstrates a segmental
sublaminar fixation utilizing flexible stainless steel cables.
Cross-links are required to maintain rod–rod stability. Fixation to the
pelvis (not seen here) is by the Galveston technique.![]() Figure 157.11. Pre- (A) and postoperative (B)
Figure 157.11. Pre- (A) and postoperative (B)
photographs show a 12-year-old girl who underwent kyphectomy and
thoracolumbar instrumented fusion. Note the improved sitting balance
and the better contour for seating. (The same patient is the subject as
Figure 157.6.) -
After completion of the instrumentation,
carefully decorticate the spine before applying the bone graft. If the
soft tissue has been satisfactorily removed from the bone, this may be
sufficient for the myelodysplastic spine. Meticulous decortication, as
done in idiopathic scoliosis, runs the risk of further weakening
already weak bone, which results in lamina fracture and wire
pull-through. The experienced surgeon will need to straddle this fine
line between risk of hardware failure and potential for nonunion.
surgery is to allow patients to be free of bracing or an orthosis
postoperatively, such is not the case in the myelodysplastic spine.
There is much greater risk for hardware failure, and most surgeons
place patients into a custom-made bivalved underarm body jacket 5–7
days after surgery. It should be worn for 9–15 months after surgery (34). Whereas bracing has significant pitfalls in attempting to improve a curve, the likelihood of skin problems with a postoperative brace that is simply holding
position is much less. However, do advise the family that skin problems
can still occur. It is important that an experienced orthotist make
these braces.
to their curve. Congenital curves are best addressed early and are most frequently treated by in situ
fusion of the congenital curve. Resection of hemivertebrae is rarely
indicated, except in the lower-lumbar regions, most typically at L-5. A
hemiepiphysiodesis will occasionally allow the contralateral portion of
the spine to grow and actually provide some correction. However, in the
majority of patients, epiphysiodesis serves to stop all growth, and
significant improvement is never realized.
curves that have progressed relentlessly to this point through the
desire of the family to refrain from surgery or by fear that early
surgery will result in too much truncal shortening. Surgeons must be
aggressive about treating the progressive and significant curve, even
in a patient with 6–8 years of growth remaining. It is better to have a
short trunk than to end up with a severe curve and loss of function.
The subcutaneous “growth” rod remains an excellent short-term way of
slowing some curve progression. The growth rod is similar to the
concept developed for juvenile idiopathic curves, but in the
myelodysplastic spine, it frequently requires additional points of
skeletal fixation. The Luque trolley, utilizing loose sublaminar wires
and no bone graft, was introduced in an attempt to maintain position
until formal fusion (36). The chief problem
with it is the difficulty in getting the spine to truly “slide.” This
may still be worth a try if it is the only alternative to fusing a
spine prematurely, and has been shown of greater utility when combined
with short-segment epiphysiodesis (36).
Operative management of collapsing kyphosis routinely requires an
anterior and posterior approach, and unlike the typical anterior
procedure, these frequently require some additional posterior-element
resection so that the kyphotic component can be corrected toward a more
anatomic configuration.
a mid- to upper-level lumbar meningocele. The absence of the posterior
elements and overlying musculature, combined with tight hamstrings
rocking the pelvis posteriorly, creates a hypolordotic, if not
kyphotic, lumbar spine. In severe cases, the kyphosis becomes profound,
and in some the lumbar spine will virtually fold over on itself,
creating a near 180° gibbous deformity (Fig. 157.8).
Lumbar kyphosis is more disabling than scoliosis in many respects
because the forward-flexed lumbar spine creates a relatively
hypokyphotic thoracic spine. Such spines are not mechanically stable,
and patients must prop themselves up on their hands. This creates
problems with pulmonary function, as well as with GI and GU tract
motility in the long-term. In addition, by constantly having to prop
themselves up with their hands, patients are unable to function well in
activities of daily living.
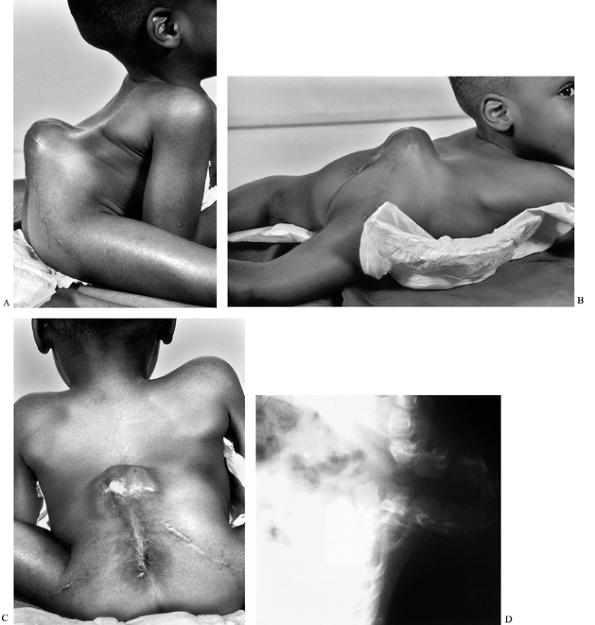 |
|
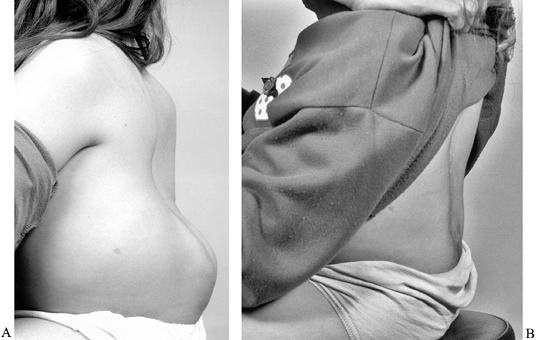
Figure 157.8. A 4-year-old boy with a 180° gibbus. A:
Lateral sitting view demonstrates the problems with a significant gibbus. There is limited upper-extremity mobility, as the elbows support much of his weight. B: A recumbent view demonstrates no appreciable mobility of the gibbous segment. C: Note the extensive scarring in the midline and the fragility of the skin overlying the gibbus. D: Lateral radiograph shows the hairpin bony deformity. |
significant very early on. Bracing is very difficult because of the
very prominent bone underlying poor skin (5).
more common type is a rigid kyphotic deformity, usually with the apex
at the L1-2 level. This is associated with a rigid compensatory
lower-thoracic lordosis. The other, less common, less severe kyphosis
is typically less rigid and has no proximal lordosis (19).
Indications for correcting lumbar kyphosis include recurrent skin
ulcerations over the prominence, difficulty with wheelchair fitting,
progressive pulmonary, GI, or GU problems, and difficulties with
activities of daily living secondary to the forward-flexed posture (5). Surgical reconstruction (26,43), a major undertaking, should accomplish three goals, according to Lindseth and Slezer (22):
It should (a) straighten and stabilize the spine for better sitting
posture, (b) markedly diminish the prominence of the kyphos, and (c)
increase the overall height of the abdominal cavity.
describe resection of vertebral bodies in the management of this
condition. His 1968 study demonstrated good correction, but follow-up
studies have demonstrated that simple excision of the apex leads to an
unacceptable recurrence rate of the initial kyphosis (22). Lindseth and Slezer (22)
showed that excision of one or two vertebrae proximal to the apex of
the kyphosis both realigned the lumbar spine to the proximal thoracic
portion and improved long-term results.
or most commonly in young adolescents. A one- to two-vertebral-level
kyphectomy may be required at birth for the very rare situation in
which skin closure is not possible. For the 2–5-year-old group, in whom
the gibbus is a problem, a procedure has been described in which the
proximal segment of the gibbus is resected and the remaining lumbar
spine is brought into apposition with the thoracic spine and held with
a single-level wiring of one pedicle to the other (Fig. 157.9) (22).
No fusion or other instrumentation is necessary in these young, usually
quite lightweight patients who then wear a pantaloon cast or brace for
6 months followed by an additional 6 months of underarm bracing. The
advantage of doing this procedure earlier rather than later stems from
the fact that only one level is fused and the remainder of the lumbar
and thoracic vertebral bodies continue to grow. The majority of these
patients will likely require additional surgery in the long term, but
the benefits tend to outweigh this minor drawback. The Luque trolley,
as noted previously, has also been utilized for kyphotic deformities (36). I prefer to wait until a child is 10–12 years of age before proceeding with a multisegmented instrumentation.
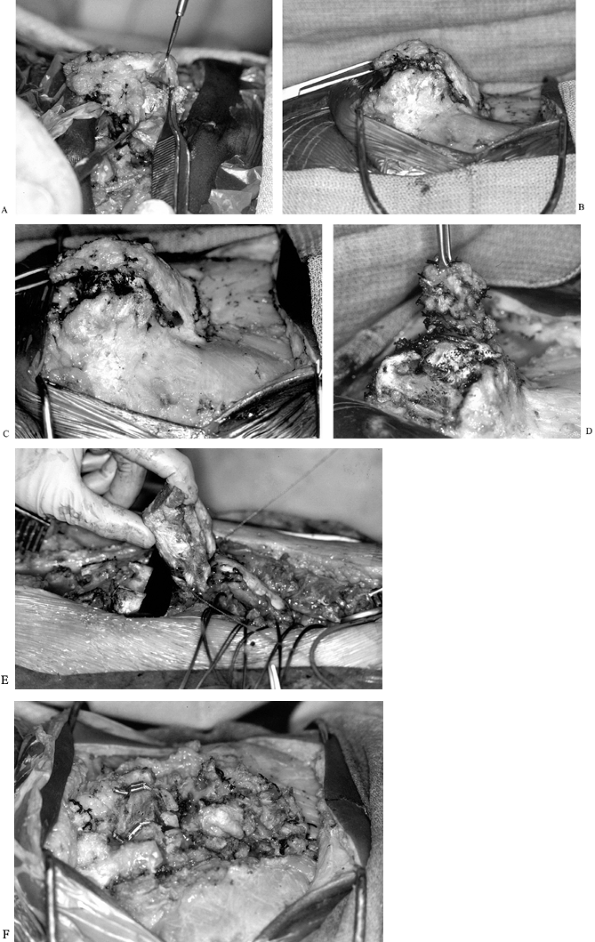 |
|
Figure 157.9. Intraoperative photographs of the patient in Figure 157.8. A:
In a patient undergoing a gibbus resection in whom significant growth remains, the spine is opposed after resection with single-level wiring of one pedicle to the other. Note the transverse incision. Here, the dural sac is being freed from the posterior aspect of the bony canal. B,C: Nonfunctional nerve roots are ligated. D: The dural sac is carefully elevated in a subperiosteal manner. E: While the sac is retracted cephalad, a Gigli saw is used to resect the superior portion of the gibbus. F: The vertebral bodies have been opposed and cabled together. |
involves the same technique in which the superior portion of the apex
is resected and the lumbar and thoracic segments are joined back
together with an accompanying segmental instrumentation construct
extending from the pelvis to the upper-lumbar spine (Fig. 157.10 and Fig. 157.11).
In contrast to the approach in 2–5-year-old patients, in whom a
transverse incision works very nicely, use a traditional longitudinal
incision for this procedure. Start in the midline from the more normal
upper-thoracic spine and extend downward to the sacrum. The dura is
typically just beneath the skin, and care must be exercised as the
dissection proceeds from the midline laterally to leave the dura intact
and the overlying skin as thick as possible. It is very easy to violate
the dural sac. Promptly close any durotomy.
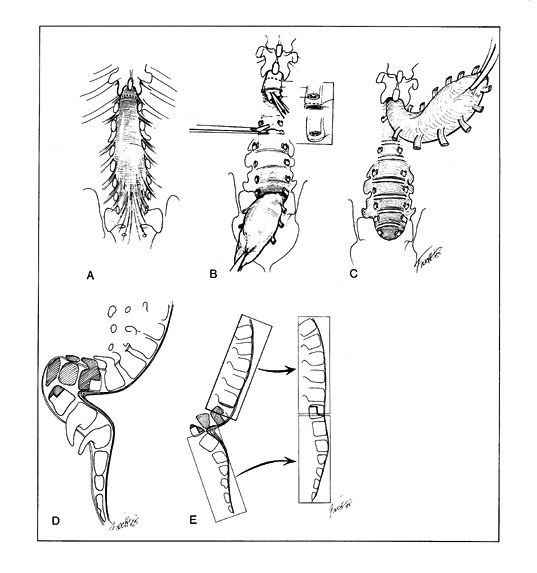 |
|
Figure 157.10. A: A typical total spina bifida of the lumbar spine as seen in congenital lumbar kyphosis. The dotted line shows the level of dural transection. B:
The nonfunctional cord is transected, and the dura is sutured distal to the cord transection. The central canal of the cord must be left open to communicate with the subdural space. The nerve roots are transected at each foramen. The discs are removed from behind. C: An alternative technique is distal detachment of the neural sac and progressive upward dissection with division of the nerve roots at the level of the foramina. The sac is usually not entered, and the cord does not have to be transected. The risk of acute hydrocephalus is reduced, and the sac is laid down over the instrumentation to provide better coverage. D: A typical rigid myelomeningocele kyphosis. The hatched area represents the vertebrae to be resected, usually those between the apex of the kyphosis and the apex of the lordosis. E: The general principle is to relate the distal segment (sacrum and lower-lumbar vertebrae) to the proximal segment (thorax) so that the two are in a stable vertical relationship. The anterior longitudinal ligament is preserved as the hinge on which the segments are aligned. (From Bradford DS, Lonstein JE, Moe JH, et al. Moe’s Textbook of Scoliosis and Other Spinal Deformities. Philadelphia: WB Saunders, 1987:322, with permission.) |
-
While dissecting laterally after exposing
the posterior elements, elevate the dural sac from the spinal canal to
expose the posterior aspects of the vertebral bodies. This is done
after distal transection of the dura. (These
P.4073P.4074
patients typically have a functioning level at L-1 or higher.)-
Typically, when the dura is transected at
the sacral level, the spinal cord itself, even in cases of significant
tethering, is well proximal. Take extreme care when closing this dural
transection not to ligate any element of the cord itself, as this can
result in sudden increased CSF pressure that will cause profound
intraoperative hypotension and possibly death. The central canal often
functions as a major CSF conduit when there are poorly functioning
ventricles (18,44). -
Cut the nerve roots at each level, and
ligate them to prevent CSF leakage. (The roots may be ligated, as there
is no CSF circulation within the nerve roots themselves.) -
After these have been ligated, elevate the sac, from distal to proximal, off the posterior aspect of the vertebral bodies (Fig. 157.9D).
-
Avoid significant blood loss by taking
care to stop the bleeding from the numerous venous sinuses lying within
the canal. Bovie electrocautery is useful for extraosseous bleeding,
and bone wax packed into the interstices is useful for bone bleeding.
-
-
After the sac has been mobilized
anteriorly and the stump is closed without evidence of leakage, turn
your attention to the kyphotic segment. With the nerve roots gone,
gently elevate the structures on either side of the transverse
processes subperiosteally, and proceed anteriorly back toward the
midline. This dissection generally proceeds quite easily if one takes
time and care to stay, if not subperiosteal, immediately
supraperiosteal. Remember that the aorta and vena cava are in this
soft-tissue mass that you are dissecting off the anterior portion of
the vertebral body and these vessels could be injured by any sharp
transgression into the mass.-
After the spine is circumferentially freed (Fig. 157.9E),
determine the segments for excision. It is always better to start with
a fewer number than your preoperative template led you to believe. -
Typically, two to three vertebral bodies must be removed to realign the spine in a reasonable configuration.
-
-
After the appropriate vertebral segments are resected en bloc (Fig. 157.9E),
the proximal and distal portions will still be prominent. Use gentle
finger pressure to approximate the two ends, which can then be
approximated with pedicle-to-pedicle wire or cable, following with an
overlying construct of hooks, wires, cables, or pedicle screws (Fig. 157.9F). Pelvic fixation may be of
P.4075
the Galveston type in the manner described by Dunn (14) or the variation described by McCarthy et al. (28). -
After instrumentation is completed, allow
the dural sac to fall back into its original position, and close the
wound in routine fashion. Some authors advise against using a suction
drain because it may increase the potential for CSF leak in their view.
down over the lumbar spine, several intravertebral discs will be
exposed. This represents an excellent opportunity to perform a
posterior lumbar interbody fusion (PLIF) and obviate a second stage of
fusion to be performed later from the front. Obviously, in certain
situations, a formal anterior fusion may be needed, but with several
levels of PLIF in a patient who is unlikely to crankshaft, a
second-stage anterior procedure is not always necessary.
fabrication of a custom bivalved underarm body jacket, which is worn
for 12–18 months after surgery.
operations in children with multiple medical problems. Blood loss can
be high, anesthesia times are long, and a postoperative pediatric
intensive care unit is therefore mandatory. Many patients will remain
intubated for 24 hours, and some will require short-term ventilatory
support. Aggressive blood replacement therapy is indicated, and good
urinary output must be maintained. As these patients are at higher risk
for postoperative infection, maintain a longer period of prophylactic
antibiotic therapy (often 5–7 days after surgery), and at the very
least prescribe one dose beyond the point when all drains and central
lines are removed. Use an aminoglycoside together with a cephalosporin
to adequately cover the gram-negative organisms associated with chronic
GU tract colonization and infection.
nutrition for patients undergoing staged procedures whose nutritional
status is poor. This is certainly an option if the anterior–posterior
fusion cannot be accomplished under one anesthetic. While monitoring of
somatosensory-evoked potentials is typically not used intraoperatively,
postoperative clinical neurologic monitoring is extremely important,
especially in patients who have a history of hydrocephalus and a
working shunt.
imperative to mobilize them as quickly as possible. However, do not
place a patient in a sitting or standing position until the custom
orthosis is available. A good relationship with the orthotist will
facilitate obtaining a brace with 24° to 48° postoperatively. Early
mobilization helps prevent muscle atrophy and acute postoperative
osteopenia. There is typically no reason or indication for prolonged
bed rest after surgery.
gained, are rapidly lost after surgery, and physical therapy is
exceedingly important to regaining their preoperative level of
function. Whereas the surgery should improve sitting and standing
balance, there is definitely a risk for marginal ambulators to become
wheelchair-bound, particularly after fusion to the pelvis. This
functional loss frequently occurs in patients with myelomeningocele in
the early teenage years, but this possibility or eventuality should be
discussed and well understood by patients and families before the
surgical procedure. As socialization of these patients is of high
importance as well, they should be allowed to return to their school
setting as soon as they are ready—usually at about 4 weeks after
surgery.
myelodysplasia is difficult because of the severity of the deformity,
the abnormal anatomy, and the multiple medical problems with which
patients present. The first caveat therefore regarding this sort of
spine surgery is that it is not for the casual spinal surgeon. Even in
the best of hands, the postoperative complication rate is high. Without
an experienced surgical team and an experienced surgeon in charge, the
results can be disastrous.
approached on an individual case-by-case basis, patients with
myelomeningocele, perhaps even more so, cannot be treated in
assembly-line or cookbook fashion. While the newer instrumentation may
make it somewhat less necessary to combine anterior with posterior
fusion, one must be careful not to neglect to perform both when both
are necessary. It can be difficult to achieve a good, solid fusion if
the posterior elements do not lend themselves to good bone-rod fixation
or the exposed areas are too sparse to achieve a good fusion mass.
the surgeon must be willing and able to compromise, devise, create, and
improvise intraoperatively. Routine surgery always needs a flexible
plan. Surgery of this nature requires not only plans A and B but plans
C, D, and E, as well.
desirable in virtually any other spinal surgery, it is a significant
mistake to leave the L5-S1 junction mobile in the paralytic spine. Not
only will this area degenerate and cause pain over time in some
patients, but it will almost certainly be unstable and can result in a
junctional kyphosis or hyperlordosis in the paralytic spine. Similarly,
stopping the fusion too short proximally can allow the upper portion of
the thoracic spine to develop forward kyphosis, which will cause
postural difficulties.
stock present is rarely sufficient to provide significant stability by
fusion of only one side of the spine.
As previously noted, prophylaxis with two antibiotics is considered the
standard of care. It is very difficult to achieve true urine sterility
in most patients, but if the organisms can be suppressed to the
colonization level, postoperative sepsis secondary to GU contamination
is less likely. Infection secondary to hematoma and dead-space and
wound breakdown secondary to poor skin is best addressed
preoperatively, as previously noted. The two best options for
preventing deep infection because of skin problems include using an
inverted-Y incision or doing a two-stage tissue expansion, as previously outlined.
With the availability of bank bone graft and bone-graft extenders, the
pseudarthrosis rate should decline in future studies. However, it is
important to realize that bone must fuse to bone and therefore
meticulous preparation of the bone graft bed is a requirement.
intraoperatively and in the early postoperative period. Its cause is
rarely metal breakage, but rather rod, hook, or wire pull-out from
osteopenic bone. Addressing calcium balance and osteopenia
preoperatively can be of use. One must be careful about being overly
aggressive in tightening wires and distracting hooks. Despite adequate
segmental fixation, the postoperative brace is still key in the
postoperative management. Late instrumentation failure (wire or rod
breakage) must be considered a pseudarthrosis until proven otherwise.
Again, meticulous attention to bone grafting technique will minimize
the pseudarthrosis rate.
exercised in providing postoperative hardware coverage, a sore will
form over a prominent portion of the hardware. Once this covering is
violated to the point where the metal is observable from the outside,
securing a sterile environment is virtually impossible until all metal
has been removed. If the wound becomes colonized and infected,
significant long-term complications can ensue, resulting in
difficult-to-treat chronic deep infections. If spinal osteomyelitis
occurs, I have used debridement, long-term parenteral antibiotics,
free-flap coverage to provide improved blood supply, and even
hyperbaric oxygen to manage this most difficult problem.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
J, Park SM. Improvement in Pulmonary Function in Patients Having
Combined Anterior and Posterior Spine Fusion for Myelomeningocoele
Scoliosis. Spine 1983;8:766.
for Disease Control and Prevention. Recommendations for the Use of
Folic Acid to Reduce the Number of Cases of Spina Bifida and Other
Neural Tube Defects. MMWR 1992;41(RR-14):1.
DS, Moreau M, Cruess RL. The Results and Complications of Surgery for
the Paralytic Hip and Spine in Myelomeningocoele. J Bone Joint Surg Br 1980;62:49.
PV, Lindseth RE, Campbell RL, et al. Scoliosis and Hydrocephalus in
Myelocele Patients: The Effects of Ventricular Shunting. J Neurosurg 1979;50:174.
JS, Gillespie R. Management of Myelomeningocele Kyphosis in the Older
Child by Kyphectomy and Segmental Spinal Instrumentation. Spine 1987;12:37.
DW, Williams HP, Ellis HL. The Outlook for the Child with a
Myelomeningocele for Whom Early Surgery was Considered Inadvisable. Dev Med Child Neruol 1972;14:304.
AJ, Smith JT, Dunn HK. Risk Factors Associated with Anterior Surgery of
the Thoracic Spine. Presented at the Pediatric Orthopaedic Society of
North America Annual Meeting, Cleveland, Ohio, May 1998.
J, Menelaus MB, Dickens DR, et al. Efficacy of Surgical Management for
Scoliosis in Myelomeningocele: Correction of Deformity and Alteration
of Functional Status. J Pediatr Orthop 1986;6:568.
ET, Krengel WF, King HA. Comparison of Same-day Sequential Anterior and
Posterior Spinal Fusion with Delayed Two-stage Anterior and Posterior
Spinal Fusion. Spine 1994;19:1256.
RK, Webb JK, Burwell RB, Cummings SL. Luque Trolley and Convex
Epiphysiodesis in the Management of Infantile and Juvenile Idiopathic
Scoliosis. Spine 1999;24:1538.
JO, Nerring JA, Browne RH. Posterior Arthrodesis and Instrumentation in
the Immature (Risser Grade-0) Spine in Idiopathic Scoliosis. J Bone Joint Surg Am 1995;77:39.
MD, Nevin NC. Periconceptional Vitamin Supplementation and the
Prevention of Neural Tube Defects in South-east England and North
Ireland. J Med Genet 1984;21:325.
K, Hall J, Johnson D, Micheli L. Acute Elevation of Intracranial
Pressure Following Transection of Non-functional Spinal Cord. Clin Orthop 1977;128:41.
JR, Ferguson-Smith MA, Shenkin A, et al. Is Disordered Folate
Metabolism the Basis for the Genetic Predisposition to Neural Tube
Defects? Clin Genet 1987;31:279.