Resurfacing Elbow Replacement Arthroplasty
destroyed elbow, depending on diagnosis, age, and activity level of the
patient, as well as the condition of the bone and ligaments. This
chapter reports the indications, planning, surgical technique, and
results of resurfacing elbow arthroplasty. Although many resurfacing
elbow prostheses are available, this chapter describes only two designs
particularly popular in Europe and Asia (4,5,6,7), specifically the Souter and the Kudo.
arthroplasty prostheses are those with rheumatoid arthritis who have
failed nonoperative measures having intractable pain, limited elbow
joint motion, and radiographic evidence of elbow joint destruction.
There must be sufficient bone stock to seat and anchor the humeral
component on the capitellum and trochlea, and the ulnar component in
the trochlear notch of the ulna with bone cement. Low-demand older
patients with osteoarthritis or posttraumatic degeneration of the elbow
joint are also appropriate indications if the bone stock is adequate,
the ligaments and capsule are competent, and the joint is not grossly
malaligned.
insufficient bone stock to seat the prosthesis, history of sepsis,
high-demand young patients, and an absent or attenuated olecranon.
These implants are also not usually effective for revision.
criteria. First, the patient must have sufficient bone stock to support
the implant; that is, both epicondyles and epicondylar ridges of the
humerus must be present. Second, erosion of the olecranon should not
preclude adequate
support
and fixation of the olecranon component. Finally, the elbow must be
stable before surgery. As would be expected, this type of unlinked
arthroplasty relies completely on the soft tissues, particularly the
collateral ligaments for postoperative stability. It is therefore
essential that these be intact and in a satisfactory state before
insertion, although the degree of integrity that is required varies
according to individual surgeon judgment.
 |
|
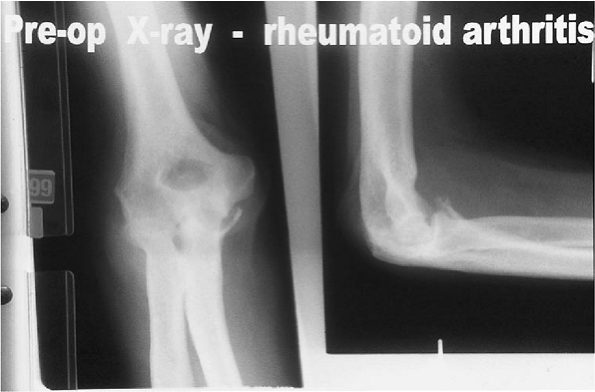
Figure 17-1.
Rheumatoid involvement of a 58-year-old female with considerable destruction and alteration of the architecture. There is adequate bone, and the ligaments appear to be intact, allowing consideration of resurfacing implantation. |
knowledge of the restrictions imposed and the care required after
arthroplasty has been undertaken. More specifically, the elbow should
be treated with respect, and repeated heavy lifting, or indeed periods
on crutches, or walking sticks, should be avoided. Hence if hip or knee
replacement is contemplated, these procedures should precede elbow
replacement. Although the Souter is typically not considered in
unstable conditions, the Kudo total elbow replacement has been offered
for some patients who have either painful stiffness of the elbow or
painful instability. Painful stiffness is defined as elbow pain with
less than 100 degrees of flexion. This invariably makes it difficult
for patients to bring their hand to their face.
range of movement, the elbow cannot be actively moved while carrying
any weight.
replacement is appropriate usually lie between grades III and V of the
Larsen et al. classification (8) (Fig. 17-1). Professor Kudo has also on occasion used the implant for mutilans deformity in rheumatoid arthritis (7). In this group of patients, bone grafting is necessary at the time of insertion of the implant.
appropriate sizing of the humeral and other components can be assessed.
The final determination is of course made at the time of surgery.
medial and lateral supracondylar columns for stable fixation.
Currently, both humeral and ulnar components employ stems (Fig. 17-2).
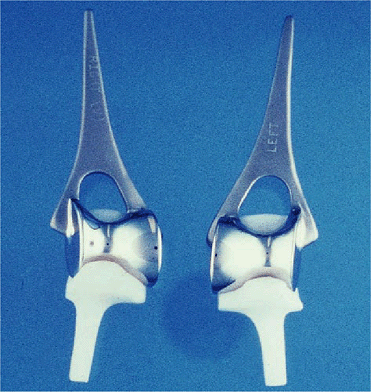 |
|
Figure 17-2. The current Souter implant involves an intramedullary stem on the humeral component and a smaller stem on the ulnar component.
|
anesthetic. The patient is brought into the operating room in the
lateral decubitus with the arm suspended over a support, which allows
free flexion and extension as well as pro-supination of the forearm (Fig. 17-3). A high tourniquet is applied and the operation proceeds under strict asepsis using body exhaust suits and antibiotic cover.
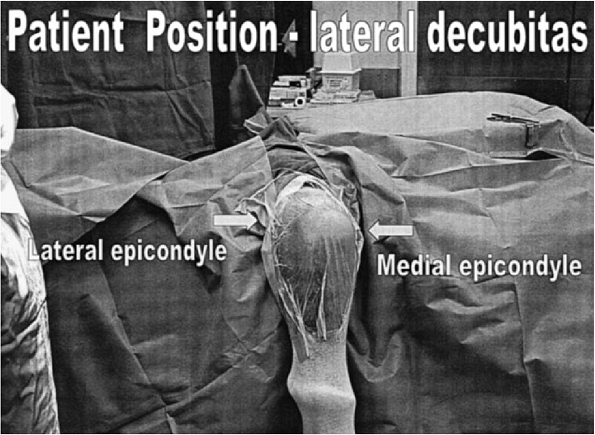 |
|
Figure 17-3. The patient prepped and draped in the prone or lateral decubitus position.
|
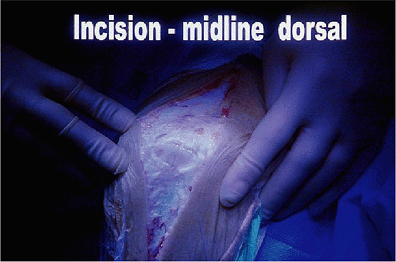 |
|
Figure 17-4. A straight posterior incision is preferred coursing just medial or lateral to the tip of the olecranon.
|
 |
|
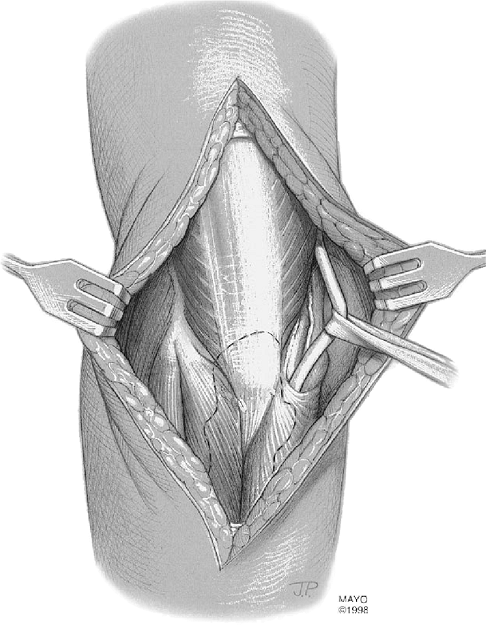
Figure 17-5. The ulnar nerve is identified and tagged but is not routinely translocated.
|
The incision is taken down to the plane of the triceps and deep fascia.
The skin flaps are then reflected out to both epicondyles and a
self-retaining retractor is inserted. The ulnar nerve is then
identified proximally and tagged (Fig. 17-5).
cubital tunnel into the forearm, releasing Osborne’s band. Note that
the nerve is not transposed, as our results have shown increased
postoperative morbidity of the ulnar nerve following transposition.
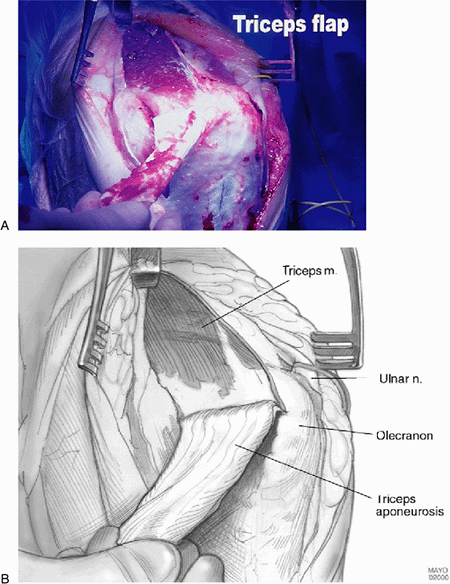
It is important to leave 5-mm strips of triceps tendon laterally and
medially to allow resuture at the end of the procedure. The triceps
muscle is then split in the midline and reflected again medially and
laterally out to the epicondylar ridges and epicondyles. The dorsal fat
pad is identified and preserved.
distally on the radial side down along the lateral border of the
olecranon at a distance of approximately half a centimeter from that
bone. Again this remaining flap allows resuture at the end of the
procedure. The incision is taken down to the level of the neck of the
radial head, which is isolated by sharp dissection. The head is removed
proximal to the annular ligament by an oscillating saw.
distally, this time, however, over a much shorter distance to the
dorsal edge of the medial collateral ligament. It is the author’s view
with this particular prosthesis that the anterior part of the medial
collateral ligament
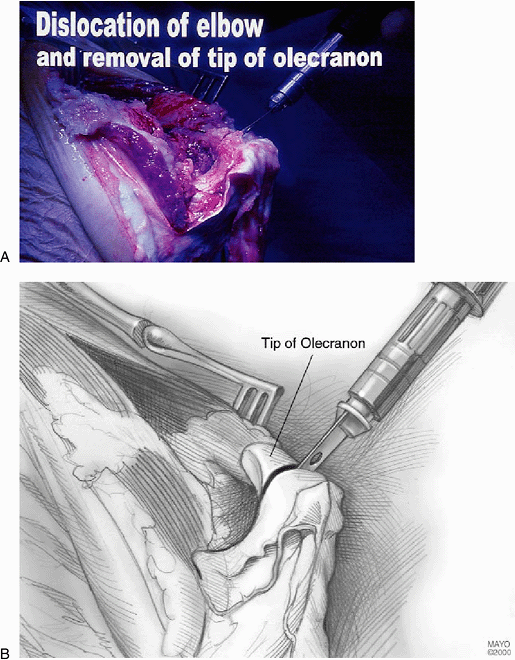
should never
be compromised. Once this dissection has been undertaken and the tip of
the olecranon has been removed with an oscillating saw, it should be
possible to dislocate the elbow (Fig. 17-7). If
this is not the case, then sometimes a coronoid osteotomy and possibly
an anterior capsular release can facilitate the dislocation.
 |
|
Figure 17-6. A,B:
The authors prefer the Van Gorder “turndown procedure” of a sleeve of fascia. The triceps is then split to expose the distal humerus and ulna. |
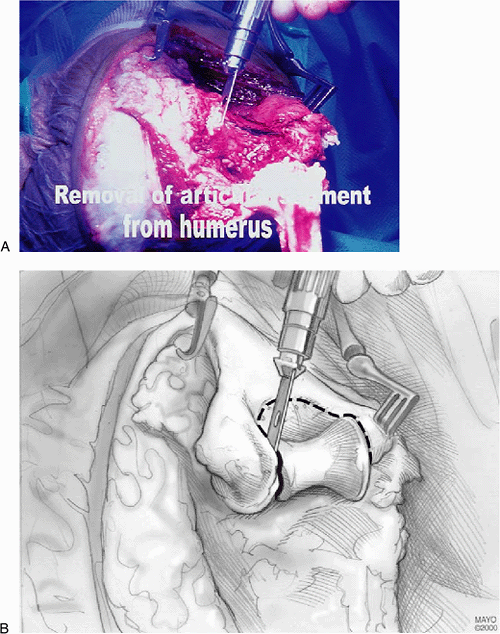
the patient’s head, the humerus is prepared for insertion of the
humeral component. First, part of the articular surface of the trochlea
is removed, together with the trochlear notch, and a small reamer is
passed up the intramedullary canal (Fig. 17-8).
Using a power bur, the cancellous bone contained within both
epicondylar ridges and epicondyles is removed. This is the most tedious
portion of the procedure (Fig. 17-9). An
alignment jig and a cutting block allow an appropriate amount of
humerus to be removed to accept the body of the humeral component. A
trial component is then inserted (Fig. 17-10).
At this stage it is extremely important not only to have the implant
aligned correctly in both the anterior-posterior (AP) and lateral
planes, but also to insert the implant to the correct depth and
rotation. The former is achieved by the use of cutting jigs and the
latter by using the patient’s normal anatomy as a guide. More
specifically, the articulating surface of the implant matches and is at
the same level as the capitellum.
of soft tissue. A hole is made in the base of the coronoid to gain
access to the ulnar intramedullary canal, and a trough from the
coronoid through the olecranon is developed (Fig. 17-11). An intramedullary rod and cutting
jig are applied and the articulated surface is removed using an
oscillating saw. A power bur is used for final modifications. It is
important that the ulnar component be inserted to the correct depth
within the olecranon and that it be totally supported by bone. In
addition, alignment in both the AP and lateral planes should be
checked. At this time a trial reduction is undertaken (Fig. 17-12)
to assess the stability of the reduction in all positions and ensure
unrestricted flexion and extension. The intramedullary canals are then
prepared first by the use of hydrogen peroxide, then by saline; then
they are dried. Bone blocks are prepared and inserted up the humeral
canal for use as cement restrictors. Then, using a gun, the cement is
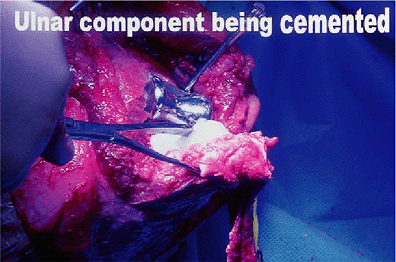
inserted under pressure and the components are inserted (Fig. 17-13).
Clamps are available, particularly for the ulnar component, to hold the
component in place while the cement sets. Once this has occurred the
arthroplasty is reduced.
 |
|
Figure 17-7. A,B: The tip of the olecranon is removed and a complete synovectomy is carried out as necessary.
|
 |
|
Figure 17-8. A,B:
The trochlear portion of the distal humerus is removed with an oscillating saw and the medullary canal of the humerus is identified. |
 |
|
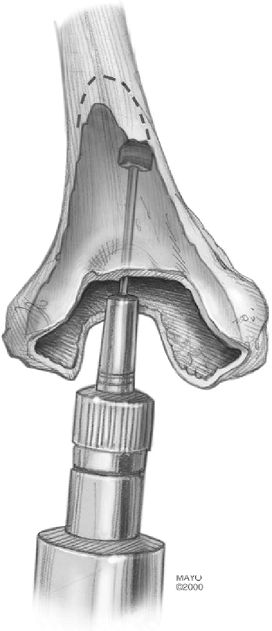
Figure 17-9. A bur is used to excavate bone from both the medial and the lateral supracondylar ridge to accommodate the wings of the device.
|
 |
|
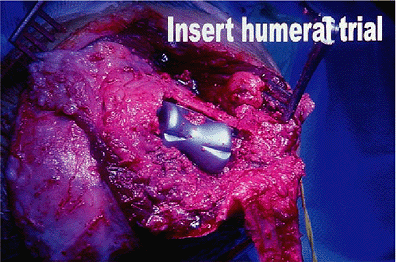
Figure 17-10. A trial reduction of the humeral component. Particular care should be taken to ensure proper rotational orientation.
|
 |
|
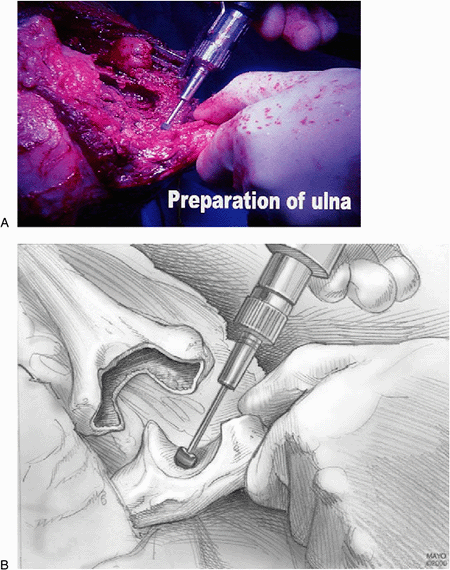
Figure 17-11. A,B:
The triceps fascial sleeve is grasped to secure the ulna, and the medullary canal is identified with a bur at the base of the olecranon. |
 |
|
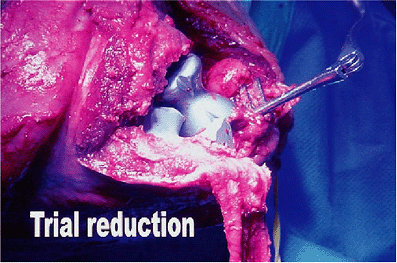
Figure 17-12.
A trial reduction of both the humeral and ulnar components. Axial rotation as well as varus/valgus alignment are extremely important to ensure proper stability and function. |
 |
|
Figure 17-13. The components may be cemented simultaneously with special devices used to hold the device securely while the cement hardens.
|
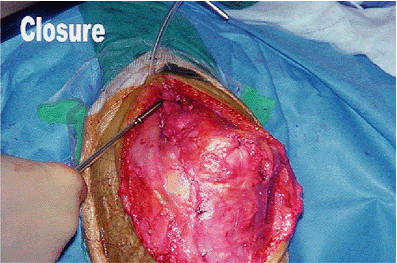
The incisions on the lateral and medial borders of the olecranon and
the triceps muscle are closed to the tip of the olecranon. A deep drain
is then inserted and the triceps fascial tongue is reattached, again
using a strong suture. It is at this point that any preoperative
flexion contracture can be addressed by allowing the tongue to slide
distally. A second,
more
superficial drain is then inserted and the skin is closed. A plaster
back-slap is applied with the elbow at 90 degrees and the forearm in
neutral rotation.
 |
|
Figure 17-14.
A careful closure of the triceps sleeve and fascial tongue is important, and a drain is routinely placed deep to the triceps reflection. |
inspected. A radiograph is taken to confirm alignment of the components
and the integrity of the implant. If they are satisfactory, early
active mobilization is begun. Almost invariably in the first instance,
this is done in conjunction with a therapist. At the same time the
patient is given a resting splint at 90 degrees. After 6 weeks this
splint is used only at night. Full active movement, including light
weights, is allowed at 3 months. Finally, if there is concern about the
integrity of the soft-tissue repair, then the author often immobilizes
the elbow at 90 degrees flexion and in neutral forearm rotation for 3
weeks.
1972. This consisted of a humeral component made of stainless steel,
which in appearance was basically a cylinder and went over the distal
humeral articular surface. The implant was a surface fit and had no
intramedullary system. The ulnar component was made of high-density
polyethylene with a short intramedullary stem. Its articulating surface
was reciprocal to that of the humeral component.
surface became saddle-shaped, rather than cylindrical. The ulna
articulation was also modified to allow for the redesign of the humeral
component.
component and the humeral articular surface was designed with 5 degrees
of valgus angulation and 20 degrees of anterior flexion. The current
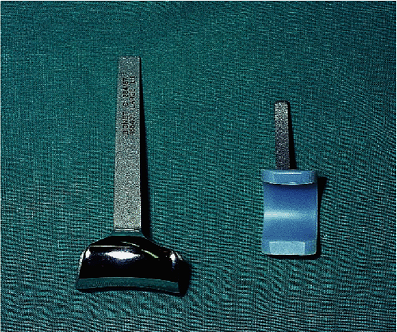
implant is made from a cobalt-chromium alloy (Fig. 17-15).
 |
|
Figure 17-15. The current Kudo design involves stemmed humeral and ulnar components and a nonconstrained articulation.
|
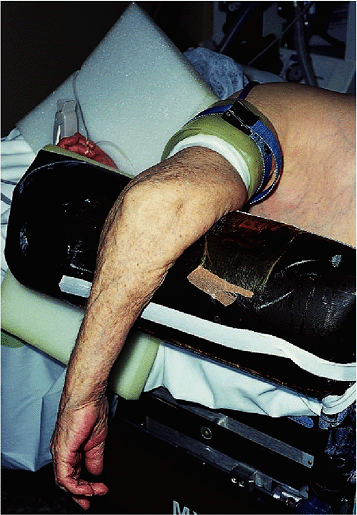
position with the arm across the chest and with the posterior aspect of
the elbow facing superiorly or the patient can be put in the full
lateral position with the upper arm horizontal and the forearm hanging
vertically (Fig. 17-16). This is the operative
position of the author. A nonsterile tourniquet is applied and a
straight midline skin incision is made centered at the tip of the
olecranon.
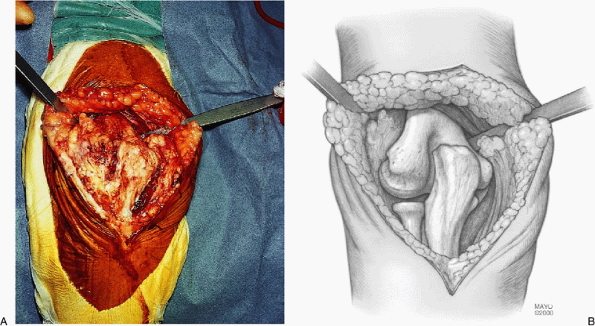
The exact surgical exposure used depends on the surgeon’s preference,
but triceps splitting or reflecting techniques are effective (1,2,14) (Fig. 17-17).
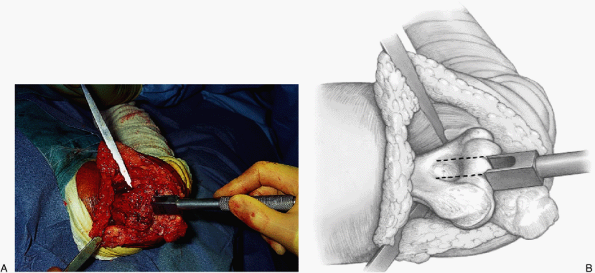
collateral ligament, including the tight anterior band, is released
from the humerus, enabling dislocation of the elbow joint. This gives
adequate access to both the distal humerus and the proximal ulna.
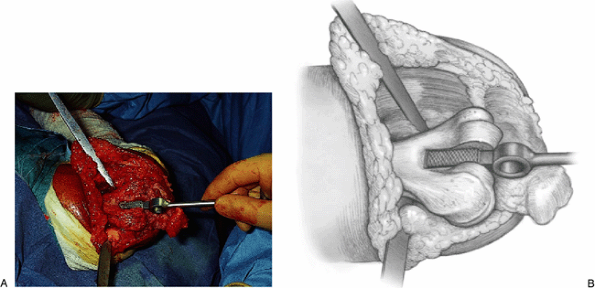
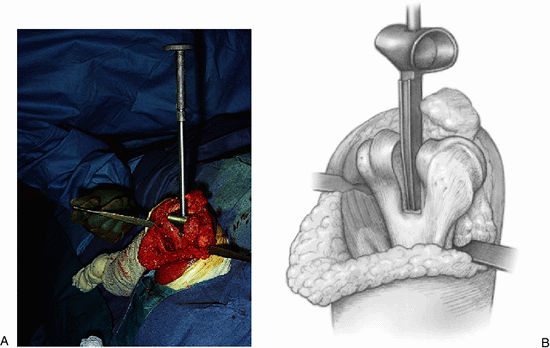
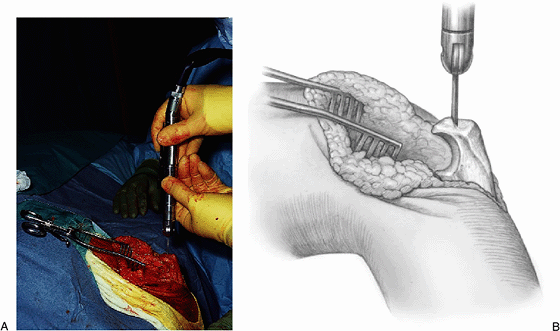
A special rasp is used to prepare the humeral canal, with care being
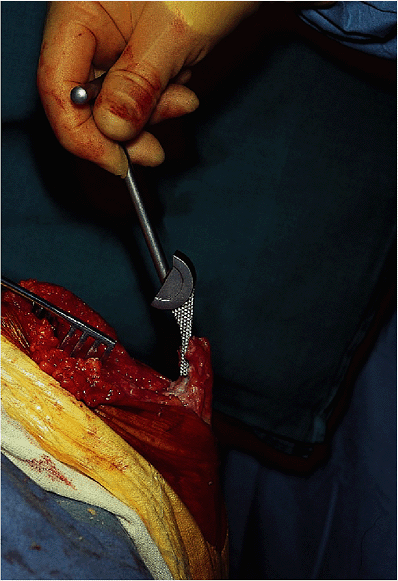
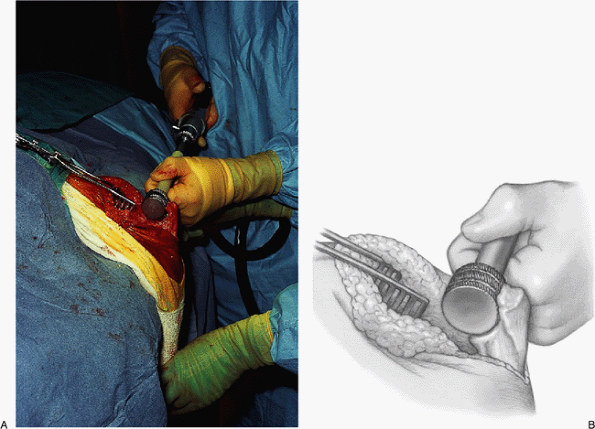
taken to ensure proper alignment in varus/valgus and axial rotation (Fig. 17-20). The ulna is prepared by isolating the olecranon, removing the tip of the olecranon (Fig. 17-21), and identifying the canal (Fig. 17-22). A special circular rasp is used to create a circular bed for the olecranon component (Fig. 17-23).
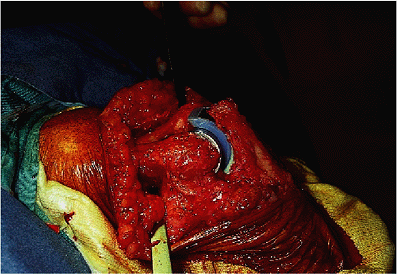
the appropriate implants are secured. Although Kudo has recently
advocated an uncemented technique, the author always cements both
devices (Fig. 17-24).
by the initial surgical approach. The wound is closed over suction
drainage to reduce the risk of postoperative hematoma formation (Fig. 17-25). A plaster slab is applied, maintaining the arm in as much extension
as possible. The plaster slab is removed after 48 hours, and the patient is permitted to begin mobilization of the elbow.
 |
|
Figure 17-16. The position of choice is in the lateral decubitus position with the arm brought across a padded bolster.
|
 |
|
Figure 17-17. A,B:
A triceps splitting incision reflecting the triceps medially and laterally and from the subcutaneous border of the ulna is the most commonly used exposure. |
 |
|
Figure 17-18. A,B:
After the distal humerus has been exposed the lateral collateral ligament is released and the ulna is rotated and dislocated, the midportion of the trochlea is removed with a box osteotome in line with the olecranon fossa. |
 |
|
Figure 17-19. A,B: A bone rasp is used to enlarge the humeral canal.
|
 |
|
Figure 17-20. A,B:
A specially designed spacer is used to further demonstrate the amount of bone to be removed and to orient and align the humeral component in a varus/valgus and from a rotatory perspective. |
 |
|
Figure 17-21. A,B: The tip of the olecranon is removed with an oscillating saw.
|
 |
|
Figure 17-22. The medullary canal is identified at the base of the coronoid and entered with a rasp.
|
 |
|
Figure 17-23. A,B:
A specially designed circular reamer is used to contour the bone of the olecranon to conform to the ulnar component and appropriately fit the ulna. |
 |
|
Figure 17-24. Both humeral and ulnar components are secured with methylmethacrylate.
|
 |
|
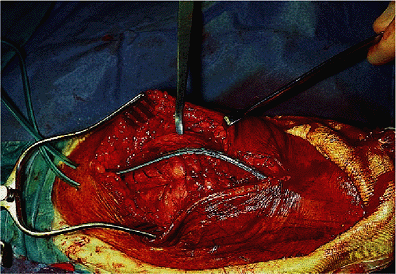
Figure 17-25.
After closure of the triceps a drain is placed in the subcutaneous tissue to minimize the likelihood of hematoma formation and problems of wound healing. |
excellent results in up to 90% of patients, provided that the
indications for surgery were appropriate. Pain relief is frequently
significantly improved (6), as is the patient’s
ability to undertake activities of daily living. An average increase in
flexion postoperatively of 25 to 130 degrees has been reported (4,6,12,17).
No improvement in extension might be expected. However, with the Souter
device the flexion contracture is felt to lessen the likelihood of
dislocation (11,17).
Improvement in rotation movements has also been noted, with a mean of
about 100 degrees, greater pronation and supination arc. In 1989,
Souter published his experience with 250 cases, reporting pain relief
in 92% and an improvement in flexion from 127 to 135 degrees but no
gain in extension.
replacement implants from linked devices is the incidence of
dislocation. The rate varies from 3% to 15% (3,4,11,13,17).
In our experience of 86 Kudo elbow arthroplasties inserted since 1992,
three have dislocated (Stanley, unpublished). The first dislocation
occurred early in Stanley’s clinical experience. In hindsight it was
not an appropriate joint to insert into the patient. The gross
instability was present preoperatively. Of the other two dislocations,
one required soft-tissue tensioning and the other required revision,
owing to a poorly positioned ulna component.
 |
|
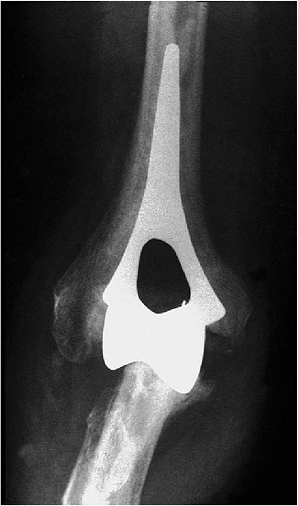
Figure 17-26. The Souter-Strathclyde resurfacing device.
|
Five (15%) were revised—three for dislocation, one for loosening, and
one for infection. The mean arc of flexion was 31 to 138 degrees. A
similar experience was reported by Chiu and co-workers. Of 20
replacements followed a mean of 43 months, only one had instability and
one required revision (3). The complication
rate was 45%, and the loss of extension averaged 7 degrees, but the
flexion arc increased a mean of 14 degrees. More recent reports have
revealed radiographic loosening rates of up to 30% and instability in
15% (10,15).
revision was recorded after 186 Souter procedures. The causes of
failure, which accounted for 75% of all revisions, included infection
and instability, and aseptic loosening of the humeral component (17).
to 115 degrees as a result of rheumatoid arthritis, but acceptable bone
stock was present (Fig. 17-1). She continues to do well 5 years after replacement with the Souter implant (Fig. 17-26).
 |
|
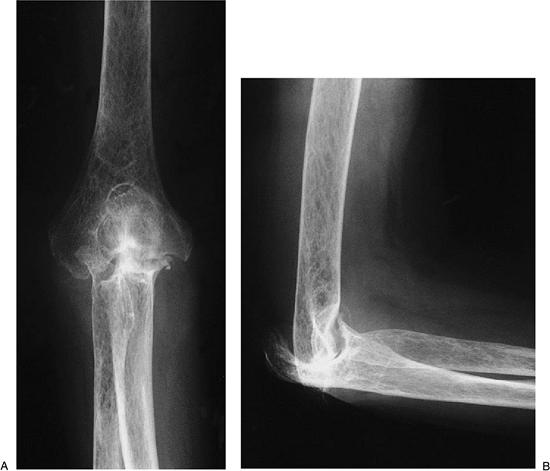
Figure 17-27. A,B:
Patient with significant bone deterioration but adequate stock and collateral ligaments for the minimally constrained Kudo implant. |
 |
|
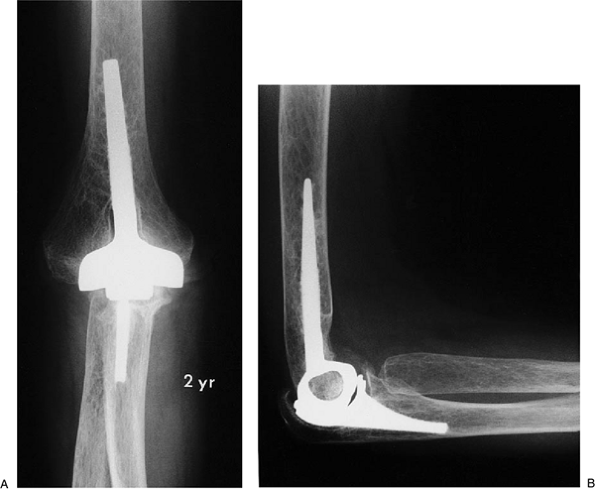
Figure 17-28. A,B:
Two years after implantation with methylmethacrylate, the patient has functional motion and no pain, and there is no evidence of implant loosening. |
H, Iwano K. Total elbow arthroplasty with a nonconstrained surface
replacement prosthesis in patients who have rheumatoid arthritis. J Bone Joint Surg 1990;72A:355.
H, Iwano K, Nishino J. Cementless or hybrid total elbow arthroplasty
with titanium-alloy implants: a study of interim clinical results and
specific complications. J Arthroplasty 1994;9:269.
HA, Cohen B, Clatworthy M, et al. Results of the Souter-Strathclyde
total elbow arthroplasty in patients with rheumatoid arthritis: a
preliminary report. J Arthroplasty 1994;9:279–284.
GO, Lundberg A, Blomgren GA. Late results of the Souter-Strathclyde
total elbow prosthesis in rheumatoid arthritis: 6/19 implants loose
after 5 years. Acta Orthop Scand 1995;66:391–394.
D, Nuttall D, Stanley JK. Survivorship and radiological analysis of the
standard Souter-Strathclyde total elbow arthroplasty. J Bone Joint Surg 1999;81B:80–83.
