RECONSTRUCTION OF THE UPPER EXTREMITY IN TETRAPLEGIA
III – THE HAND > Reconstructive Procedures > CHAPTER 68 –
RECONSTRUCTION OF THE UPPER EXTREMITY IN TETRAPLEGIA
Associate Clinical Professor, Department of Orthopaedic Surgery,
University of California, Davis; Shriners Hospital for Children,
Northern California, Sacramento, California, 95817.
devastating impairment. The abilities of the person with tetraplegia
are drastically diminished. This loss of independence is especially
catastrophic for young men with poor impulse control, the most common
victims of SCI. People with complete tetraplegia have loss of normal
use of their upper extremities and may require assistance with basic
activities of daily living (ADLs), such as eating, dressing, and
bladder and bowel care. They are unable to stand or walk, and because
they lack sensation below the level of their SCI they must learn to
protect their skin from pressure sores.
survived for only a brief time, succumbing to pneumonia or renal
failure. In the 1940s the prevention and care of the medical
complications of tetraplegia began to improve, allowing people with
this condition to survive longer. At this same time, the field of hand
surgery was developing, and by the late 1940s hand surgeons began
devising ways
to improve upper extremity (UE) function in patients with tetraplegia. Pioneers in this field include Bunnell (2), Lipscomb et al. (37), Nickel et al. (47), and later Freehafer et al. (11,12), Lamb and Landry (34,35), Moberg (41,42), Zancolli (60,61), House et al. (21,22), McDowell et al. (39,40), Hentz et al. (17,18), Waters et al. (3,14,57), Allieu et al. (1),
and others. Although current surgical techniques fall far short of
returning normal function, the challenges of functional restoration in
the tetraplegic hand have inspired some of the most innovative
developments in hand surgery today.
by bony or ligamentous injury to the cervical spine. The cervical spine
injury may be stable (for instance, gunshot wounds typically cause
stable injuries) or unstable. The highest orthopaedic priority in the
treatment of acute tetraplegia is to recognize spinal instability, and
to prevent the unstable spine from further damaging the spinal cord
(see Chapter 139 and Chapter 140).
After internal fixation of the cervical spine provides stability, the
patient can sit and upper extremity function can be fully assessed.
thereof) following SCI reflects the level and type of injury to the
spinal cord. The level of cord damage often does not correlate with the
level of bony injury. The cord is frequently damaged above or below the
level of spine injury, and cord damage of varying severity may occur
over several levels. The cord may be transected, or more commonly
crushed or contused, and the body’s response to the injury may cause
further damage. Other neurologic injury—including injury to the brain,
cervical nerve roots, and brachial plexus—may accompany SCI. The
presence of lower motor neuron injury compounding upper motor neuron
injury reduces treatment options, because muscles without an intact
lower motor neuron cannot contract in response to functional electrical
stimulation (see the section on Indications, Assessment, and Relative Results, below).
From a functional standpoint, the designation “incomplete” can mean
anything from total upper and lower extremity paralysis with retained
perianal sensation, to minimal upper or lower extremity weakness, or
both. In the first months after injury, an incompletely injured spinal
cord may partially recover (55). A completely
injured spinal cord will not recover, but muscles that are weak but not
paralyzed immediately after injury (due to less severe SCI cephalad to
the complete injury) will usually regain normal strength in the year
after injury (7,30). Surgical reconstruction of the upper extremity should wait until further improvement is unlikely (33,39,40,56).
make surgical reconstruction less effective or even contraindicated.
Some changes, such as severe spasticity and pain, are difficult to
prevent or treat. Other changes, such as contractures, may be
preventable with passive range of motion (ROM) exercises.
Steroids or other medications given within hours of injury may reduce
further damage caused by the body’s response to SCI. The major focus of
treatment is rehabilitation, however, an on-going process in which
people with SCI learn how to take care of themselves. As part of
rehabilitation, upper extremity function may be improved or partially
replaced by orthotic devices; modifications to cars, wheelchairs, and
computers; environmental control units; service dogs; and upper
extremity surgery. People with higher level tetraplegia require the
help of another person with activities such as lower extremity
dressing, food preparation, bathing, toileting, and transferring, and
they must learn to instruct others in these activities (59).
team approach. The team is usually headed by a specialist in physical
medicine and rehabilitation. Other physician team members include an
orthopaedic spine surgeon, a urologist, a psychiatrist, and an upper
extremity surgeon. Other professional team members include clinical
nurse specialists in rehabilitation, physical and occupational
therapists, therapeutic recreation specialists, and social workers. The
patient and his family are important members of the team, because
patient attitude and family support are the most important factors in
successful rehabilitation. Although the team may have standardized
goals determined by the level of SCI, they must also help the patient
develop realistic goals and focus on these. Successful rehabilitation
helps the patient reconnect with mainstream life, including returning
to school or work. As a result of effective rehabilitation, many people
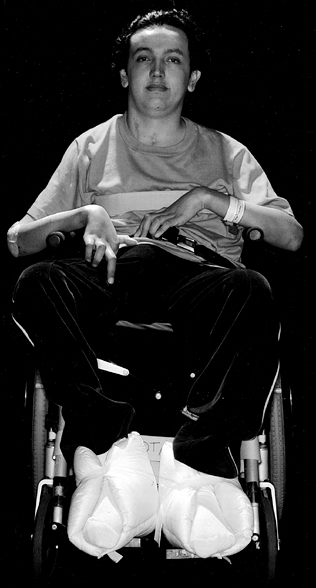
with tetraplegia lead happy, fulfilling lives (Fig. 68.1).
 |
|
Figure 68.1.
This young man sustained a spinal cord injury in a diving accident at age 16. He joined his father’s used car business as a salesman at age 18, following rehabilitation and bilateral deltoid to triceps transfers. |
patient early on, but it is not usually part of initial rehabilitation.
Surgery cannot be planned until upper extremity function is stable,
which usually occurs 1 year after injury. In addition, a person with
tetraplegia who has spent time at home following her initial
rehabilitation has more realistic goals and expectations of surgery.
The upper extremity
surgeon
can help the patient develop these goals, and provide encouragement and
contact with other people with tetraplegia who have undergone upper
extremity reconstruction. Contraindications to upper extremity
reconstruction are listed in Table 68.1.
 |
|
Table 68.1. Contraindications to Upper Extremity (UE) Reconstruction in Tetraplegia
|
although the tetraplegic patient may have so few donor muscles
available that the surgeon must be creative or even bend the rules.
Ideally, muscles selected for transfer have normal strength and
appropriate excursion for their new function, and they can be spared
without detriment to function. In tetraplegia, partially paralyzed
muscles may be transferred if this will augment function (for instance,
transfer of a portion of the deltoid muscle to the triceps improves
elbow extension even if the deltoid is partially paralyzed [23]),
and weakening one motion may be acceptable if the transfer supplies an
entirely absent function (such as biceps-to-triceps or extensor carpi
radialis longus [ECRL]–to–flexor digitorum profundus [FDP] transfers).
can restore because it enables people with tetraplegia to extend their
reach for eating, pushing elevator buttons, opening doors, transferring
in and out of a wheelchair, and propelling a manual wheelchair (33,36,41).
Following reconstruction of elbow extension, depending on which motors
are available for transfer, the surgeon may reconstruct key (lateral)
pinch, which is very useful for everyday activities such as eating with
a fork and writing with a pen (Fig. 68.2). If
additional motors are available for transfer, the surgeon may also
reconstruct hook grasp and release, and thumb opposition.
 |
|
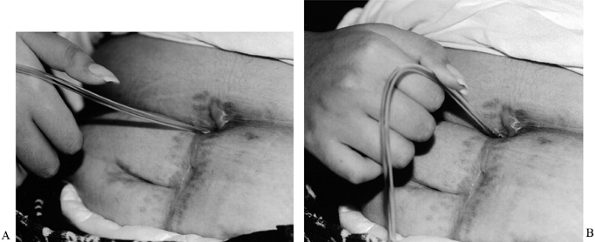
Figure 68.2.
This patient is using surgically reconstructed key pinch to catheterize a stoma between the bladder and umbilicus. This enables her to catheterize herself in her wheelchair. A: Thumb extended. B: Key pinch. |
All medical supplies that come into contact with people with
tetraplegia should be latex free. They are also at risk of autonomic
dysreflexia in response to noxious stimuli below their injury level,
such as postoperative pain or bladder distention. In addition, they are
at increased risk of postoperative pulmonary problems such as
atelectasis or pneumonia because of paralysis of accessory muscles of
respiration.
absent sensation, the surgeon must liberally pad splints or casts
applied postoperatively, to avoid causing pressure sores.
retaining passive range of motion (PROM) in the upper extremities. PROM
exercises should be performed regularly, especially before the patient
is able to sit. If these exercises are neglected, shoulder stiffness
and pain will develop and delay rehabilitation.
active ROM and strength are carefully evaluated and reevaluated at
frequent intervals for the first year after injury. Formal planning of
upper extremity reconstruction begins when function plateaus. Elbow
flexion contractures are frequently seen in patients with absent elbow
extension. These contractures diminish the patient’s ability to assist
with transfers; unless these can be overcome with stretching or serial
casting, reconstruction of elbow extension is contraindicated (15).
Supination contractures are less common; these contractures may require
osteotomy of the radius before pinch reconstruction. Other contractures
are uncommon. See Table 68.1 for other factors that contraindicate upper extremity surgery.
if the triceps muscle is paralyzed, elbow extension should be
reconstructed first. This is because pinch reconstruction uses the
brachioradialis or pronator teres as a donor, and these muscles cross
the elbow joint and are placed on optimal tension when the elbow is
extended (3). Because the elbow must be
immobilized in extension following tendon transfer to the triceps, this
procedure is not readily combined with other upper extremity surgery.
 |
|
Figure 68.3. Algorithm for upper extremity reconstruction in tetraplegia. Please see text for a description of the use of this algorithm.
|
 |
|
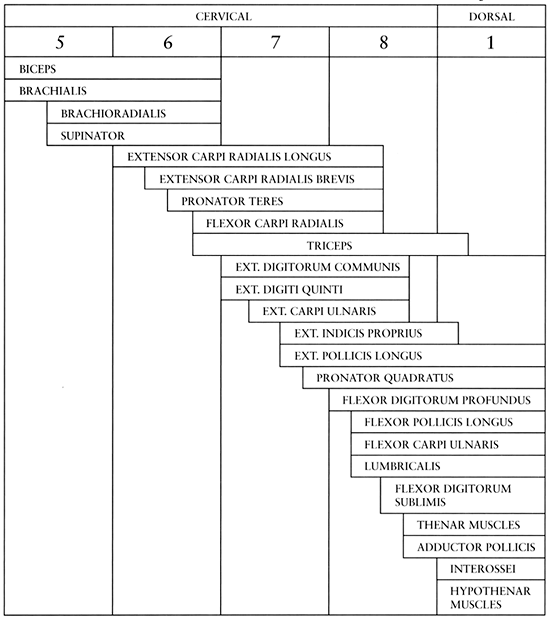
Figure 68.4. Segmental innervation of muscles of the elbow, forearm, and hand (61). (From Zancolli EA. Functional Restoration of the Upper Limbs in Tetraplegia. In: Zancolli EA, ed. Structural Dynamic Bases of Hand Function. Philadelphia: JB Lippincott, 1978:231.)
|
commonly used to reconstruct elbow extension. In this operation, the
posterior one third to one half of the deltoid muscle is detached at
its origin and transferred to the triceps tendon, usually using an
interposition graft. Fascia lata is easy to harvest and use as a graft (17). Good results have also been reported using toe extensor tendons and tibialis anterior (6,8,10,32,41,48), and using a turned-up portion of triceps to bridge the gap between the deltoid and the triceps (4,8).
Graft interposition material tends to stretch out over time,
diminishing active elbow extension; this deterioration may be reduced
when the interposition graft is reinforced with nonabsorbable suture
tape. Shoulder abduction strength is not diminished with this transfer,
because the transferred deltoid is able to continue to function as a
shoulder abductor (personal communication, Vincent R. Hentz, M.D).
Transfer of a weak, partially paralyzed deltoid muscle provides useful
elbow extension, also without diminishing shoulder abduction strength (23).
People with tetraplegia are usually very pleased with the results of
this procedure, which increases their reach and thereby their
independence; it may allow them to assist with transferring in and out
of their wheelchair, and
propelling
a manual wheelchair. In spite of the major drawbacks of this operation,
such as postoperative immobilization with the elbow in extension, and
prolonged rehabilitation to prevent the interposition graft from
stretching out, patients consistently request the same procedure on the
contralateral side (23,33).
is paralyzed. Although this transfer weakens elbow flexion, the
brachialis is usually strong enough to continue to flex the elbow
against gravity (13,31,39,50). This operation may not be as consistently reliable as a deltoid-to-triceps transfer.
the patient should be evaluated for a neuroprosthesis. Also called
implanted functional electrical stimulation, and commercially known as
the Freehand system, this complex system may provide grasp and key
pinch when tendon transfers are not possible (5,25,26,28,46).
A control unit implanted under the pectoralis muscle is programmed to
stimulate muscles via implanted electrodes. The patient controls this
unit by using a transducer attached to his chest wall and mechanically
activated by contralateral shoulder motion (Fig. 68.5).
In order to be stimulated by a neuroprosthesis, muscles must be
paralyzed but not denervated (their lower motor neuron must be intact).
The status of the peripheral nerves can be tested with transcutaneous
stimulation to determine if the patient is a candidate for this
operation. The surgeon and occupational therapist work together to
determine which muscles are available for stimulation and transfer. If
critically important muscles, such as wrist extensors, are denervated,
their function may be replaced by transferring and stimulating a
paralyzed but not denervated muscle. Planning, surgical implantation,
and postoperative training are quite complex and require an experienced
surgical and therapy team, and a highly motivated patient (53).
 |
|
Table 68.2. International Classification for the Upper Extremity in Tetraplegia (39)
|
 |
|
Figure 68.5. A: Diagram of the Freehand system. B:
Young man with IC 1 SCI, who has undergone left upper extremity implantation of the Freehand neuroprosthesis. He is using lateral pinch generated by the neuroprosthesis to hold his fork. C: The same young man, using grasp generated by the neuroprosthesis to hold a water bottle. |
for a neuroprosthesis (see above), or for a combination of tendon transfers designed to restore key pinch (10,11,20,41,44).
Key pinch is reconstructed by transferring the brachioradialis to
provide wrist extension, and tenodesing the extrinsic thumb flexor and
extensor tendons to the radius. After this surgery, wrist extension
activates key pinch, and wrist flexion (by gravity) activates release.
This provides approximately 1 kg of pinch strength; neuroprosthesis
pinch strength is at least twice as strong, but the patient requires
assistance donning the transducer to activate the neuroprosthesis (5,25). The thumb may require carpometacarpal arthrodesis (20) or interphalangeal joint stabilization with a transarticular Kirschner wire (41)
or arthrodesis to stabilize pinch. Alternatively, pinch can be
stabilized by transferring half of the flexor pollicis longus (FPL)
tendon to the extensor pollicis longus (EPL) tendon, just proximal to
the interphalangeal joint (split-FPL transfer) (45).
Because people with tetraplegia usually do not like to have stiff
hands, the split-FPL transfer, which provides stability without
stiffness, is preferred to arthrodesis. Restoration of tenodesis pinch
allows the patient to hold a fork, toothbrush, pen, catheter, or other
small object.
key pinch in the same manner as for IC-2 (see above) and one of the
wrist extensors (usually ECRL) can also be transferred to the finger
flexors to restore hook grasp (22,44).
No motor is available to provide active finger extension, so the
extrinsic finger extensors should be tenodesed to the radius. Because
the hand lacks intrinsic function, an intrinsic tenodesis, such as the
Zancolli lasso procedure in which the paralyzed flexor digitorum
superficialis (FDS) is tenodesed around the A1 pulley] (17,60), improves grasp strength by preventing metacarpophalangeal joint hyperextension, or clawing, with grasp (38).
These procedures are performed in two stages, an extensor phase and a
flexor phase, with different positions of immobilization. In the
extensor phase, EDC, EPL and
abductor pollicis longus are tenodesed to the radius, and an intrinsic tenodesis is performed.2
In the flexor phase, approximately 3 months later, the brachioradialis
is transferred to the FPL, and the the ECRL is transferred to the FDP (21,22).
hold larger objects, such as a cup, and to pull up pants. However, if
the extensor carpi radialis brevis (ECRB) is not full strength, the
weakening of wrist extension caused by transfer of ECRL may be
detrimental to key pinch, which is a more useful function. There is no
consistently reliable way to determine the strength of ECRB. Some
surgeons solve this dilemma by transferring ECRB instead of ECRL; this
leaves wrist extension strong but causes radial deviation with
extension. Another alternative is to omit grasp reconstruction and
simply reconstruct key pinch as for IC-2.
have an additional motor available for transfer. FCR is not usually
transferred because no other motor is available to flex the wrist, and
wrist flexion enhances release and improves the function of EDC
tenodesis (see the section on IC-3 earlier).
The PT can be transferred without detriment to function. In addition to
the two-stage reconstruction described earlier for IC-3, as part of the
flexor phase, the PT can be transferred to the ring FDS, which can then
be transferred to the abductor pollicis brevis. The insertion and
routing of this opponensplasty can be adjusted to restore adduction and
opposition, in order to improve key pinch contact by counteracting the
supination force of the FPL acting in the absence of thenar intrinsics (19,27).
Many variations of these procedures have been described, including
transferring the brachioradialis to the finger and thumb extensors
instead of to the FPL, and using the PT or ECRL to provide grasp by
transferring it to the FDP (14). Thumb carpometacarpal joint fusion may help improve grasp (22).
and EPLs) lack only active finger and thumb flexion and intrinsic
function (IC-6 and IC-7, Table 68.2). If the
EDC is present but the EPL is absent (IC-6), the extensor digiti quinti
(EDQ) can be transferred to EPL. Otherwise, key pinch, hook grasp,
intrinsic balance (Zancolli lasso), and adduction-opposition are
restored as described earlier for IC-4 and IC-5; the Zancolli lasso can
be performed at the same operation as pinch and grasp reconstruction
and opponensplasty. Reconstructed grasp averages 5.5 kg, and key pinch
averages 3.0 kg (22).
can benefit from opponensplasty using ring FDS and intrinsic tenodesis
(Zancolli lasso); the Zancolli lasso works as an active, rather than
passive, tether when the FDS is functional.
 |
|
Table 68.3. Classification of Spinal Cord Injury (52)
|
At his suggestion, O (for “oculo,” or visual afferent) and Cu (for
“cutaneous,” or sensory afferent) are added to the IC scheme to denote
the sensibility of the individual extremity (Table 68.3).
Although lack of sensibility may adversely affect the outcome of the
procedure, even Moberg, who considers sensibility of prime importance,
notes that in the absence of sensation, “restored grip was useful
nonetheless” (41).
orthopaedic surgeons and rehabilitation medicine specialists is based
on level of nerve root injury (Table 68.3) (52).
This system is not specific enough to help with planning upper
extremity tendon transfers. Furthermore, individual muscles are
innervated by multiple nerve roots (Fig. 68.4)
and different individuals may have different innervation patterns.
Triceps innervation is especially variable. For these reasons, the
International Classification for the Upper Extremity in Tetraplegia
(IC) was developed (Table 68.3). This is the classification system used to plan upper extremity reconstruction in tetraplegia.
-
Perform the operation under general
anesthesia. Place the patient on a large beanbag or other positioning
device in the lateral decubitus position with the operated side up. Pad
the contralateral arm and both legs carefully. Inject the planned
incision sites (Fig. 68.6), including the
fascia lata graft donor site on the ipsilateral leg, with bupivacaine
(Marcaine) with epinephrine (the patient probably has no sensation in
the leg and the majority of the arm, but painful stimulus below the
level of injury can cause autonomic dysreflexia).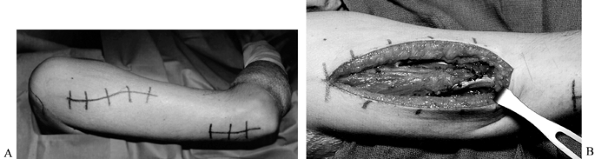 Figure 68.6. A: Incisions for deltoid to triceps transfer. B: Proximal surgical wound showing fascia lata graft attached to posterior deltoid.
Figure 68.6. A: Incisions for deltoid to triceps transfer. B: Proximal surgical wound showing fascia lata graft attached to posterior deltoid. -
Through a curvilinear incision over the
posterior deltoid, extending to the deltoid insertion, expose the
deltoid muscle. The overlying fascia is not well developed. Visualize
the anterior and posterior borders, and select one third to one half of
the posterior deltoid to dissect free for transfer. Harvest periosteum
and brachialis fascia distally to supplement the deltoid insertion as a
sturdy attachment point for the transfer. A leash of vessels is
consistently located just posterior to the deltoid insertion. Leave the
anterior portion of the deltoid insertion attached to the humerus. -
Dissect the posterior deltoid proximally,
separating the fibers. Stop at least 5 cm distal to the acromion, or no
more than 7 cm proximal to the insertion, to avoid damaging the
axillary nerve (44). Test the passive excursion of the posterior deltoid; it should be at least 2 cm, preferably 3 to 4 cm. -
Through a separate posterior longitudinal
incision, expose the triceps aponeurosis. Make two 3 cm transverse
incisions about 2 cm apart in the triceps tendon, reinforcing the
corners with #0 nonabsorbable suture. Create a subfascial tunnel
between the two triceps tendon incisions, and a subcutaneous tunnel
between the two skin incisions. Measure the distance between the end of
the deltoid and the triceps tendon with the elbow in 10° to 20° flexion
and add 5 cm; this is the length of fascia lata interposition graft
needed. -
Through a straight lateral incision
extending from just below the greater trochanter to 5 to 7 cm proximal
to the knee joint, expose the fascia lata. Harvest a piece of fascia
lata about 5 cm wide and as long as needed. Detach the fascia lata at
one end and weave #5 nonabsorbable suture tape through the fascia lata,
then detach the other end. -
Pass the fascia lata graft through the
subcutaneous tunnel. Wrap the proximal end around the posterior deltoid
tendon, attaching it with several #0 non-absorbable sutures. While
holding the elbow in 10° to 20° flexion, pass the graft into the
proximal incision in the triceps and out the distal incision, firmly
attaching it at both contact points with several #0 nonabsorbable
sutures. Fold the excess back on itself and suture in place. Do not
allow the elbow to flex more than 20°. -
Close all wounds with deep interrupted absorbable sutures, and skin with running subcuticular sutures.
-
Apply a well-padded long arm cast with the elbow in 10° to 20° flexion.
-
Immobilize the arm, with the elbow in 10°
to 20° flexion for 4 weeks. During immobilization, avoid active or
passive adduction across the midline of the body, or forward flexion or
abduction above 45°. When the cast is removed, fit the patient with a
long arm splint with elbow hinges with hinge stops (Fig. 68.7).
The purpose of this brace is to allow strengthening of the transfer
without stretching out the interposition graft. Under the close
supervision of a therapist, the patient actively strengthens the
transfer by elbow extension exercises, starting with an arc of 20° to
0°, and increasing by 10° increments as soon as she can extend against
gravity through the allowed arc. At night the brace is set at no more
than 20° flexion. When the patient is able to extend against gravity
from 90° to 0°, the brace is removed during the day. Nighttime bracing
should be continued for at least 6 to 12 months.![]() Figure 68.7. Elbow hinge brace used after deltoid to triceps transfer.
Figure 68.7. Elbow hinge brace used after deltoid to triceps transfer.
-
The indications for this operation are
controversial and are still being developed. At present, it is
indicated when the deltoid is paralyzed, which occurs rarely in
patients eligible for tendon transfer. Please refer to Revol et al. (50) for details.
-
The implantation of this system is
complex and highly individualized, and the details are beyond the scope
of this text. Please refer to work by Keith and others for details (25,28,53).
-
Perform the operation under general
anesthesia and tourniquet. Inject subcutaneous bupivacaine
preoperatively in the planned incision sites. -
Through a curvilinear incision on the
dorsoradial border of the forearm, identify the brachioradialis muscle
and detach its insertion (just proximal to the floor of the first
dorsal compartment). Dissect it proximally, to approximately the distal
three quarters and proximal one quarter junction of the muscle-tendon
unit, or until it has 3 cm excursion. Do not damage the dorsal radial
sensory nerve, which travels immediately deep to the brachioradialis. -
Split FPL transfer:
Through a zig-zag palmar incision centered over the interphalangeal
joint of the thumb, identify the FPL tendon just proximal to its
insertion (Fig. 68.8). Transect the radial half
of the tendon and dissect proximally, placing a nonabsorbable suture at
the base of the split of the Y. Expose the EPL through a zigzag dorsal
incision just proximal to the interphalangeal joint flexion crease.
Reroute the radial split FPL around the radial side of the thumb and
weave it through the EPL, adjusting the tension so that the thumb
interphalangeal joint flexes less than 20° when the wrist is passively
extended; attach it to the EPL with nonabsorbable sutures, and splint
the transfer internally with a transarticular Kirschner wire.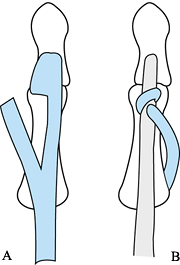 Figure 68.8. Split FPL transfer (45). A: The radial half of the FPL tendon is detached. B: The detached portion of the FPL is inserted into the EPL. From Mohammed K, Walsh W, Peljovich AE, et al. Anatomy and Neurological Relations to the Distal Deltoid. May 1998; VI International Conference: Surgical Re-habilitation of the Upper Limb in Tetraplegia, Cleveland, OH: 874.
Figure 68.8. Split FPL transfer (45). A: The radial half of the FPL tendon is detached. B: The detached portion of the FPL is inserted into the EPL. From Mohammed K, Walsh W, Peljovich AE, et al. Anatomy and Neurological Relations to the Distal Deltoid. May 1998; VI International Conference: Surgical Re-habilitation of the Upper Limb in Tetraplegia, Cleveland, OH: 874. -
EPL tenodesis:
Identify the EPL tendon proximal to the dorsal retinaculum. Transect it
and allow the proximal end to retract. Loop the distal end over the
dorsal retinaculum, adjust the tension so that the thumb pad does not
contact the index finger when the wrist is in flexion, and attach it to
the retinaculum and to itself with nonabsorbable sutures.
Alternatively, perform EPL tenodesis
P.1866
by
passing it through two small holes in the distal radius (one on either
side of Lister’s tubercle) and suturing it back on itself. In addition,
perform abductor pollicis longus (APL) tenodesis if this will help
balance the thumb. -
FPL tenodesis:
Through a longitudinal palmar incision over the distal forearm,
identify the FPL tendon and transect it distal to its musculotendinous
junction. Pass the tendon through two small elliptical windows in the
cortex of the distal radius and adjust tension so the thumb pad firmly
contacts the radial side of the index finger when the wrist is
passively extended. -
Determine which radial wrist extensor
tendon (ECRB, ECRL, or both) provides centralized wrist extension under
passive tension, and weave the brachioradialis tendon through this
wrist extensor (usually the ECRB alone). Set the tension with the elbow
flexed at 90°, so that the transfer passively holds the wrist in slight
extension, but still allows passive wrist flexion. -
Close all wounds with absorbable suture
and apply a well-padded long arm cast with the elbow in 90° flexion,
the forearm in neutral rotation, the wrist in 30° to 40° extension and
the thumb in midrange radial and palmar abduction, with the
interphalangeal joint in neutral.
-
Immobilize the arm for 6 weeks, then
begin active pinch training. No further protection is needed.
Alternatively, remove the cast after 4 weeks and begin active ROM and
pinch exercises supervised by an occupational therapist. Splint the
wrist in 30° extension and the thumb in midrange abduction between
exercises for 2 more weeks.
-
Perform the operation under general
anesthesia and tourniquet. Inject subcutaneous bupivcaine
preoperatively in the planned incision sites. -
Perform the brachioradialis dissection and split FPL transfer and EPL tenodesis as outlined earlier (see the section on Restoration of Key Pinch for IC-1).
-
Attach the brachioradialis tendon to the
FPL tendon in the distal forearm with nonabsorbable suture. The FPL may
be too small to accommodate weaving the brachioradialis; if this is the
case, use an end-to-side attachment. Tension should be set with the
elbow in 90° of flexion, so that the thumb contacts the long finger
with the wrist in neutral. -
Close all wounds with absorbable suture
and apply a bulky compressive dressing covered by a well-padded long
arm cast, with the elbow in 90° flexion, the forearm in neutral
rotation, the wrist in 30° to 40° extension, the thumb in midrange
radial and palmar abduction, and the interphalangeal joint in neutral.
-
Immobilize the arm for 6 weeks, then
begin active pinch training. No further protection is needed.
Alternatively, remove the cast after 4 weeks and begin active ROM and
pinch exercises supervised by an occupational therapist. Splint the
wrist in 30° extension and the thumb in midrange abduction between
exercises for 2 more weeks.
RELEASE, AND INTRINSIC BALANCE, AND (IC-4 AND IC-5 ONLY) RESTORATION OF
THUMB ABDUCTION/OPPOSITION (8,9,10,12,14,21,22,27,38,44,60,61)
-
Perform the operations under general
anesthesia and tourniquet. Inject subcutaneous bupivacaine
preoperatively in the planned incision sites. -
IC-3 Extensor phase: intrinsic tenodesis (Zancolli lasso) (38,61).
Through zigzag incisions centered over the distal palmar flexion crease
of the finger, carefully pull on the FDS tendon distal to the A1 pulley. Transect it at the chiasm. Avoid handling the FDP tendon. Attach the transected tendon to the palmar surface of the A1
P.1867
pulley with nonabsorbable suture, weaving it, if possible, after
adjusting tension to create dynamic metacarpophalangeal joint flexion
when the wrist is extended. Perform the same operation for the index,
long, ring, and small fingers. -
IC-4 and IC-5 extensor phase: intrinsic tenodesis (Zancolli lasso).
If the PT is present, it may be transferred during the flexor phase to
abductor pollicis brevis (APB) to provide thumb abduction. Do not
perform the Zancolli lasso procedure on the ring and small fingers if
the PT is to be transferred to provide thumb abduction, because the
ring FDS will be used to extend the PT. -
IC-3, IC-4, and IC-5 extensor phase: extensor tenodesis.
Through a dorsal curvilinear forearm incision, identify the EDC, EPL,
and APL tendons. Transect them distal to their musculotendinous
junctions. Perform tenodeses by passing the EDC tendons through two
small elliptical holes in the dorsal radius and by looping the EPL and
APL around their dorsal retinacula. Adjust tension so that the fingers
and thumb start to open (release) when the wrist is in neutral
position, and fully release when the wrist is in flexion. Attach the
EDC to itself, and the EPL and APL to the retinaculum, with
nonabsorbable suture. Close all incisions with absorbable suture. Apply
a bulky compressive dressing covered with a short arm cast,
incorporating the thumb and metacarpophalangeal joints of the fingers.
The wrist should be in extension, the metacarpophalangeal joints in 45°
of flexion, and the thumb in radial and palmar abduction. -
IC-3 flexor phase (at least 3 months after extensor phase; see General Rehabilitation and Postoperative Principles below): Perform a split FPL transfer as described earlier (see the section on Restoration of Key Pinch for IC-1). Transfer the brachioradialis to the FPL as described above (see the section on Restoration of Key Pinch for IC-2).
Transfer the ECRL to the index, long, ring, and small FDP as follows.
First, through a palmar curvilinear forearm incision, suture the FDP
tendons together with nonabsorbable suture, synchronizing them by
adjusting their tension so that they flex in unison, with slightly more
flexion in the ulnar digits; do not transect them (60).
Make a generous window in the interosseous membrane proximal to the
pronator quadratus. Transect the ECRL as far distal as possible, and
pass it through the window to the palmar side of the forearm. Split it
longitudinally and wrap it around the FDP tendons distal to their
interattachment. Adjust the tension so that the fingertips touch the
palm when the wrist is extended, and attach the ECRL to the FDP with
nonabsorbable suture. -
IC-4 and IC-5 flexor phase (at least 3 months after extensor phase; seeGeneral Rehabilitation and Postoperative Principles below): Perform a split FPL transfer as described above (see the section on Restoration of Key Pinch for IC-1). Transfer the brachioradialis to the FPL as described earlier (see the section on Restoration of Key Pinch for IC-2).
Transfer the ECRL to the index, long, ring, and small FDP as described
earlier. Through the incision used to harvest the brachioradialis,
identify and detach the PT. Transfer the PT to the ring FDS tendon in
the distal forearm; because the PT tendon is broad, use an end-to-side
or split and wrap around attachment, with nonabsorbable suture. Then
detach the ring FDS at the chiasm, through a palmar incision at the
level of the distal wrist flexion crease. Through a radial thumb
incision at the level of the metacarpophalangeal flexion crease, expose
the abductor pollicis brevis (APB) insertion. Split the ring FDS into
two tails; attach one tail to the APB tendon and the other to the
extensor hood, using nonabsorbable suture under enough tension to hold
the thumb in radial abduction and to prevent it from supinating during
pinch (9). Alternatively, the PT can be transferred to the FPL and the brachioradialis to the ring FDS (22). -
Close all incisions with absorbable
suture. Apply a bulky compressive dressing covered with a long arm
fiberglass cast with the elbow in 90° of flexion, the forearm in
neutral rotation, the wrist in neutral, the fingers with
metacarpophalangeal joints flexed and proximal interphalangeal joints
extended, and the thumb in radial and palmar abduction.
-
After the first stage, immobilize the arm for 6 weeks, then begin active transfer training and passive ROM exercises.
-
Once the patient can activate the
transfer well and ROM has returned to preoperative status (usually at
least 3 months after the first operation), perform the second stage.
After the second stage, immobilize the arm for 6 weeks, then resume
active transfer training. No further protection is needed.
Alternatively, remove the cast after 4 weeks and begin active ROM and
pinch exercises supervised by an occupational therapist. Splint between
exercises for 2 more weeks, with the wrist in neutral, the thumb in
midrange abduction, and the fingers in flexion at the
metacarpophalangeal joints and in extension at the proximal
interphalangeal joints.
-
Perform the operations under general
anesthesia and tourniquet. Inject subcutaneous bupivacaine
preoperatively in the planned incision sites. -
Perform the flexor phase as described
earlier for IC-4 and IC-5. At the same anesthetic, add the Zancolli
lasso as described earlier for IC-4 and IC-5. -
IC-6:
Transfer EDQ to EPL, weaving the EDQ through the EPL and attaching it
with nonabsorbable suture, after adjusting the tension as for EPL
tenodesis. -
Close all incisions with absorbable
suture. Apply a bulky compressive dressing covered with a long arm
fiberglass cast with the elbow in 90° of flexion, the forearm in
neutral rotation, the wrist in neutral, the fingers with
metacarpophalangeal joints flexed and proximal interphalangeal joints
extended, and the thumb in radial and palmar abduction.
-
Immobilize the arm for 6 weeks, then
begin active pinch, grasp, and (IC-6 only) thumb extension training. No
further protection is needed. Alternatively, remove the cast after 4
weeks and begin active ROM and pinch exercises supervised by an
occupational therapist. Splint between exercises for 2 more weeks, with
the wrist in neutral, the thumb in midrange abduction, and the fingers
in flexion at the metacarpophalangeal joints and in extension at the
proximal interphalangeal joints.
-
Perform the operations under general
anesthesia and tourniquet. Inject subcutaneous bupivacaine
preoperatively in the planned incision sites. -
Perform the intrinsic tenodesis as
described earlier. Transfer the ring FDS to the APB as described
earlier, except the proximal attachment to PT is unnecessary if the
ring FDS is sufficiently strong enough to provide thumb
abduction/opposition. -
Close all incisions with absorbable
suture. Apply a bulky compressive dressing covered with a short arm
fiberglass cast with the wrist in neutral, the fingers with
metacarpophalangeal joints flexed and proximal interphalangeal joints
extended, and the thumb in radial and palmar abduction.
-
Immobilize the arm for 6 weeks, then
begin active pinch and grasp training. No further protection is needed.
Alternatively, remove the cast after 4 weeks and begin active ROM and
pinch exercises supervised by an occupational therapist. Splint between
exercises for 2 more weeks, with the wrist in neutral, the thumb in
midrange abduction, and the fingers in flexion at the
metacarpophalangeal joints and in extension at the proximal
interphalangeal joints.
specialized preoperative, intraoperative, and postoperative care to
prevent complications such as latex allergy, hypotension, pressure
sores, pneumonia, wound infections, urinary tract infections, and
autonomic dysreflexia.
-
Upper extremity reconstruction in
tetraplegia is best performed by an experienced hand surgeon, because
proper tensioning of tendon transfers remains a skill honed by
practice. The surgeon’s job is easier and the results of surgery are
probably better if a rehabilitation team is available to help care for
the patient in the perioperative period. -
Many patients benefit from intensive
in-patient occupational and physical therapy after cast immobilization
is discontinued. Intensive (twice-per-day) therapy sessions before and
after large muscle transfer, such as deltoid-to-triceps transfer, allow
the patient to strengthen the transferred muscle, and thereby improve
the outcome of this operation. Travel to and from the therapist can be
difficult for people with tetraplegia, so performing the therapy in an
in-patient setting also enhances compliance. -
People with tetraplegia who are
contemplating surgery can benefit enormously from meeting with others
who have undergone the same operation or operations. If possible, these
meetings should take place away from the hospital or physician’s
office, so the patients can talk freely. Tetraplegics who have worked
hard to gain independence may be especially reluctant to undergo
surgery because of fear of decreased independence during immobilization
or after surgery. -
Although neuroprostheses are an exciting
development, from a practical standpoint, they are not widely useful.
Implantation and postoperative therapy are very complicated and require
a highly motivated patient, surgeon, and therapist. The cost of the
system (excluding the cost of the implantation surgery and preoperative
and postoperative therapy) is more than $25,000. The biggest drawback,
however, is that the patient with a neuroprosthesis in place requires
assistance to don and doff the apparatus that makes the neuroprosthesis
work. -
A weak deltoid muscle is not a
contraindication to deltoid-to-triceps transfer. If the muscle is weak,
a larger portion—or even the entire deltoid—can be transferred to
increase elbow extension power.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
SE, Mulcahey MJ, Betz RR, et al. Outcomes of Upper Extremity Tendon
Transfers and Functional Electrical Stimulation in an Adolescent with
C5 Tetraplegia. Am J Occup Ther 1997;51:307.
JFJr, Stover SL, Freed MM, Ahn JH. Motor Recovery of the Upper
Extremities in Traumatic Quadriplegia: A Multicenter Study. Arch Phys Med Rehabil 1992;73:436.
AA, Kelly CM, Peckham PH. Tendon Transfer for the Restoration of Upper
Limb Function after a Cervical Spinal Cord Injury. J Hand Surg 1984;9A:887.
J, Waters R, Gellman H. Transfer of the Pronator Teres Tendon to the
Tendons of the Flexor Digitorum Profundus in Tetraplegia. J Bone Joint Surg 1990;72A:427.
J, Gellman H, Waters RL. The Effect of a Flexion Contracture of the
Elbow on the Ability to Transfer in Patients Who Have Quadriplegia at
the Sixth Cervical Level. J Bone Joint Surg 1996;78A:1397.
AW, Yarkony GM, Roth EJ, et al. Functional Outcome Following Spinal
Cord Injury. A Comparison of Specialized Spinal Cord Injury Center vs.
General Hospital Short-term Care. Arch Neurol 1989;46:1098.
VR, Brown M, Keoshian L. Upper Limb Reconstruction in Quadriplegia:
Functional Assessment and Proposed Treatment Modifications. J Hand Surg 1983;8:119.
JH, Gwathmey FW, Lundsgaard DK. Restoration of Strong Grasp and Lateral
Pinch in Tetraplegia due to Cervical Spinal Cord Injury. J Hand Surg 1976;1:152.
JH, Shannon MA. Restoration of Strong Grasp and Lateral Pinch in
Tetraplegia: A Comparison of Two Methods of Thumb Control in Each
Patient. J Hand Surg 1985;10A:22.
DL, Gellman H, Waters RL, Tognella M. Brachioradialis Transfer for
Wrist Extension in Tetraplegic Patients Who Have Fifth-cervical-level
Neurological Function. J Bone Joint Surg 1996;78A:1063.
MW, Kilgore KL, Peckham PH, et al. Tendon Transfers and Functional
Electrical Stimulation for Restoration of Hand Function in Spinal Cord
Injury. J Hand Surg 1996;21A:89.
CM, Freehafer AA, Peckham PH, Stroh K. Postoperative Results of
Oppenensplasty and Flexor Tendon Transfer in Patients with Spinal Cord
Injuries. J Hand Surg 1985;10A:890.
RF, Acosta AM, Perreault EJ, Keith MW. Measurement of Isometric Elbow
and Shoulder Moments: Postition-dependent Strength of Posterior
Deltoid-to-triceps Muscle Tendon Transfer in Tetraplegia. IEEE Trans Rehabil Eng 1996;4:403.
LM, Herbison GJ, Decena BF, Ditunno JFJ. Biceps vs. Extensor Carpi
Radialis Recovery in Frankel Grades A and B in Spinal Cord Injury
Patients. Paraplegia 1994;32:340.
J, Van Heest A, House J. Biceps-to-triceps Transfer in Tetraplegic
Patients: Report of the Medial Routing Techique and Follow-up of Three
Cases. J Hand Surg 1999;24A:161.
SH, Wilber G, Peckham PH, Freehafer AA. The Posterior Deltoid to
Triceps Transfer: A Clinical and Biomechanical Assessment. J Hand Surg 1986;11A:542.
PR, Elkins EC, Henderson ED. Tendon Transfers to Restore Function of
Hands in Tetraplegia, Especially Fracture-dislocation of the Sixth
Cervical Vertebra on the Seventh. J Bone Joint Surg 1958;40A:1071.
CL, Moberg EA, House JH. The Second International Conference on
Surgical Rehabilitation of the Upper Limb in Tetraplegia
(Quadriplegia). J Hand Surg 1986;11A:604.
E. Reconstructive Hand Surgery in Tetraplegia, Stroke, and Cerebral
Palsy: Some Basic Concepts in Physiology and Neurology. J Hand Surg 1976;1:29.
MJ, Betz RR, Smith BT, et al. Implanted Functional Electrical
Stimulation Hand System in Adolescents with Spinal Injuries: An
Evaluation. Arch Phys Med Rehabil 1997;78:597.
SD, Gellman H, Waters R, et al. Single Stage Reconstruction of Key
Pinch and Extension of the Elbow in Tetraplegic Patients [See
comments]. J Bone Joint Surg 1994;76A:1451.
R, Moore KR, Graboff SR, Paris K. Brachioradialis to Flexor Pollicis
Longus Tendon Transfer for Active Lateral Pinch in the Tetraplegic. J Hand Surg 1985;10A:385.
RL, Stark LZ, Gubernick I, et al. Electromyographic Analysis of
Brachioradialis to Flexor Pollicis Longus Tendon Transfer in
Quadriplegia. J Hand Surg 1990;15A:335.
Some experts believe that IC-2 cannot be distinguished from IC-3 on
examination alone, and surgical exposure under local anesthesia is
required to assess ECRB strength (39). In many
cases, however, patients with IC-2 SCI will show radial deviation with
active wrist extension. In patients with IC-3 SCI, a groove may be
visible between ECRB and ECRL muscle bellies in the proximal forearm
with resisted wrist extension (44).
In theory, this may reduce the likelihood of FDP adhesions, because
active FDP motion will begin sooner after the flexor stage than after
the extensor stage. In addition, the optimal position of immobilization
following intrinsic tenodesis is more compatible with the flexor stage.
However, immobilization of the metacarpophalangeal joints in neutral or
extension, however desirable to optimize finger extension following
extensor tenodesis, is likely to lead to extension contractures of
these joints, rendering the intrinsic tenodesis less effective.

