MANAGEMENT OF UPPER EXTREMITY DYSFUNCTION FOLLOWING STROKE OR BRAIN INJURY
III – THE HAND > Reconstructive Procedures > CHAPTER 67 –
MANAGEMENT OF UPPER EXTREMITY DYSFUNCTION FOLLOWING STROKE OR BRAIN
INJURY
Director, Neuro-Orthopaedics Program, Department of Orthopaedic
Surgery, Albert Einstein Medical Center, Philadelphia, PA, 19141;
Professor of Orthopaedic Surgery, Professor of Rehabilitation Medicine,
Temple University School of Medicine, Philadelphia, PA, 19132.
injury (TBI) are distinct syndromes. However, the stroke and
brain-injured patient share many features (see, e.g.,3,42,46,52,55,56,84,85,89,92,109,128,
Both patient groups exhibit upper motoneuron (UMN) syndromes with
impairment of motor control, spasticity, and stereotypical patterns of
movement (synergy). Cognitive, memory, and sensory deficits are also
commonly seen in these patients. Because of the similarities between
stroke and TBI, there is a great deal of overlap in terms of specific
surgical and nonsurgical techniques for treating the upper extremity
problems caused by these conditions.
peripheral nervous system, and the musculoskeletal system, and lesions
causing pain may lead directly or indirectly to syndromes of restricted
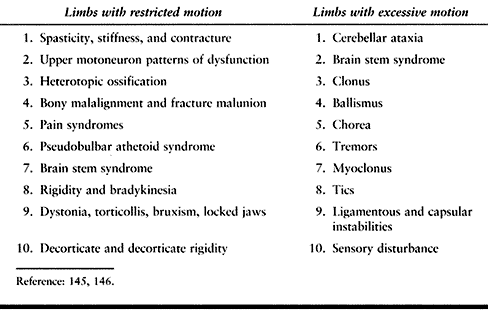
or excessive motion of the limbs. Syndromes of restricted limb motion
are the most common type of movement impairment. Syndromes of excessive
motion are less common. A distinction between restricted versus
excessive motion is made. This is because the functional implications
of each and the treatment of the problems they generate are very
different. Syndromes of restricted limb motion are manifested by
impaired access of the limb to targets in the environment during
voluntary movement. Limbs are unable or are poorly able to move toward
objects or places because motion across joints is restricted. An
example is a patient with spastic finger flexors who attempts to open
the hand to grasp an object. Another example seen after head injury is
heterotopic bone formation about the elbow, which restricts joint
motion and impairs use of the upper extremity even in the presence of
voluntary muscle action. Limbs with restricted motion lose their
operating range and are unable to be positioned adequately for
function. The general treatment strategy for limbs with restricted
motion is to identify and reduce sources of limb restriction.
impaired tolerances in the production of voluntary movement parameters
such as movement amplitude, accuracy, timing, and force. Clinical
conditions associated with excessive motion seen after head injury
include such movement disorders such as hemiballismus, athetosis,
tremors, and cerebellar ataxia. Biomechanical laxity in the
musculoskeletal system may also be associated with excessive motion.
For example, inferior subluxation of the shoulder may lead to excessive
motion.
confines of the skull and the subsequent cognitive function of the
brain. The musculoskeletal system is profoundly effected by brain
dysfunction. Hypertonicity, the unmasking of primitive reflexes, and
impaired motor control contribute to the abnormal limb positions,
contractures, and impaired mobility so frequently encountered in
persons with brain injury.
affected by dysfunction of the musculoskeletal system. Just as the
shoulder and elbow position the hand for grasping and manipulating
objects, the musculoskeletal system gives mobility to the brain and
positions it to interact with the world. Mobility of the individual is
a key element of human life and of fundamental importance to our
well-being.
stroke rehabilitation are knowledgeable about the cognitive and
behavioral deficits that accompany brain injury. It has been our
experience that less importance has been given to the musculoskeletal
impairment that results from brain trauma or stroke. The penalties of
musculoskeletal limitations for the individual can be devastating.
Improving an individual’s physical mobility is often therapeutic,
leading to increases in their cognitive, behavioral, and emotional
capacities.
care. Which in the physically disabled population, means maximizing
function and mobility in order to avoid the complications of chronic
incapacity. Potential complications of physical immobility include
decubiti, infection, pain, social isolation, and physical and emotional
dependence. For society, this results in a costly loss of productivity
for the patient and often family members as well.
commonly arise regarding the indications for surgery, the cost, what
outcome to expect, and the practicality of this approach. Consider
these issues on an individual basis for each patient. General
principles have been delineated that can serve as guidelines for
decision making:
-
Operate early—before deformities are
severe and fixed. Orthopaedic surgery is a powerful rehabilitation
tool. It is often the only treatment that will correct a limb deformity
or improve function. Surgery should not be considered a treatment of
last resort when conservative measures have failed. Physical and
occupational therapy cannot affect a permanent change in motor control.
Drug therapy for increased muscle tone
P.1811
has
generalized effects and cannot be targeted to specific offending
muscles. Phenol blocks and botulinum toxin injections provide only
temporary modulation of muscle tone. When a permanent treatment is
needed to decrease muscle tone or redirect muscle force, consider
surgery. The results of surgical intervention are improved when
deformities are corrected early. Less muscle lengthening is needed when
deformities are mild and there is little or no fixed contracture to
overcome. Early surgery preserves maximum muscle strength, joint
capsule and ligament flexibility, and articular cartilage integrity. In
general, the patient will also be in better physiologic condition to
undergo surgery if there has not been a period of several years of
immobility. -
Better underlying motor control means
better function for the extremity. Orthopaedic surgery cannot impart
control to a muscle. Lengthening a spastic muscle can improve its
function by diminishing the overactive stretch response and uncovering
any control that was present. Successful surgery depends on a careful
evaluation preoperatively to determine the amount of volitional control
present in each individual muscle that is affecting limb posture and
movement.Motor performance occurs on a continuous scale, with the
disabled at the lower end and the elite athlete at the upper end.
Infinitesimal improvements in the performance of elite athletes
distinguish between the winner and loser. Incremental changes in limb
function also result in performance improvements for the disabled
individual. Surgery should not be reserved for patients with severe
impairment and deformity. Individuals with milder degrees of impairment
can benefit greatly from relatively simple procedures such as
lengthening of the extrinsic finger flexors to regain sufficient fine
motor control to perform more intricate hand functions. The amount of
improvement correlates best with the degree of underlying motor control
and not with the severity of the deformity -
Distinguish between the function of the
extremity and the function of the individual. We commonly speak of
“functional” and “nonfunctional” surgical procedures. These terms refer
to the expected outcomes for a limb but do not indicate the outcome for
the person as a whole. Surgical releases of an arm contracted in a
flexed and internally rotated position in a hemiplegic patient often
allows the person to become independent in dressing, even though the
arm itself remains nonfunctional. -
Consider the cost of not correcting limb
deformities. The cost of motor control evaluation using dynamic
electromyography (EMG) is relatively modest for the benefits it
provides. Dynamic EMG is a one time expense. The cost of performing an
incorrect surgical procedure that fails to correct or worsens a limb
deformity is much greater. The cost of performing a surgical procedure
is likewise more cost effective when compared with a lifetime of
attendant care, spasticity medications, repeated blocks, orthotics to
control limb position, complications such as skin ulceration and
infection, and lost productivity for the patient and caretakers.
It is also the third leading cause of death. Roughly 600,000 people
suffer a new or recurrent stroke each year. Management of the stroke
patient has become a major priority for physicians treating the
elderly. There are 4,000,000 stroke survivors alive today. The average
patient who survives beyond the first few months has a life expectancy
of greater than 6 years (10,79,80). Stroke victims survive long enough and achieve adequate function to justify aggressive rehabilitation.
oxygen. Any significant interruption of oxygenation by thrombosis,
emboli, or hemorrhage results in neuron death and subsequent deficits
in cognitive, sensory, and motor functions. Thrombosis is the most
common cause of infarction and accounts for nearly three fourths of all
CVAs (79,80). Arteriosclerosis is the most significant predisposing factor.
of all CVAs, and includes spontaneous intracerebral hemorrhage and
subarachnoid hemorrhage. Hypertension is commonly present in these
patients. Isolated cerebral emboli account for less than 10% of CVAs.
increasing age, genetic predisposition, hypertension, hyperlipidemia,
hypercholesterolemia, obesity, cardiac anomalies (arrhythmias,
myocardial infarction, hypotension, mural thrombosis), diabetes
mellitus, collagen vascular disease (vasculitis, polyarteritis),
hyperviscosity states (polycythemia, sickle cell anemia), oral
contraceptive use, tobacco smoking, severe cerebrovascular spasm
secondary to migraine headaches, and septic vasculitis (tuberculosis,
syphilis, and mucormycosis).
specific areas of the cerebral cortex. CVAs involving the middle
cerebral artery are the most common, and produce the typical hemiplegic
picture of greater impairment in the upper extremity, face, and speech
compared with lower extremity involvement. The middle cerebral artery
supplies the largest area of cerebral cortex. This area controls
sensory and motor function of the trunk, upper extremity, and face, as
well as the functions of speech.
the sagittal plane. This area of cerebral cortex controls sensory and
motor function predominantly in the lower extremity. CVAs involving the
anterior cerebral artery result in a hemiplegic picture of sensory and
motor deficits chiefly involving the lower extremity.
in the occipital region. Involvement of this artery typically results
in visual impairment. Bilateral cortical involvement may lead to severe
mental impairment, frontal release signs, loss of short-term memory,
and inability to learn.
in balance and coordination arise from interruption of afferent and
efferent pathways between the brain and spinal cord. Balance reactions
are also dependent on limb control and proprioception (95,119,141,201).
mentation, decreased learning ability, and loss of short-term memory
may occur (10,14,17,20,24,27,33,41,51,77,79,80,93,104,
The patient’s ability to cooperate with treatment affects
rehabilitation potential. In patients with extensive frontal lobe
deficits, these deficiencies may be severe. Patients with extensive
frontal lobe pathology exhibit clinical features similar to senility,
with lack of attention span and little motivation for recovery. Their
prognosis for rehabilitation is poor.
receptive or expressive in nature. It usually involves both components.
Aphasia occurs with lesions of the left hemisphere, usually without
regard to hand dominance. A receptive aphasia hinders rehabilitation
most strongly because the patient cannot understand instructions.
Persistent receptive loss has a poor prognosis.
rehabilitation, allowing a patient to comprehend and follow
instructions. Expressive aphasia may resolve significantly.
characterized by a loss of ability to perform a previously learned
action, such as tying shoe laces or waving good-bye. Apraxia is not the
result of motor or sensory loss. It occurs more commonly with right
hemispheric involvement (left hemiparesis). The apraxia, however,
occurs on both sides of the body. The prognosis with severe apraxia is
generally poor. Some improvement with practice and repetition may
occur. If impairment persists after 3 months, further improvement is
unlikely (10).
Sensory perception occurs in the cerebral cortex and is most often
affected by lesions of the middle cerebral artery. Sensory loss may be
manifest by impairment of touch, pinprick, two-point discrimination,
proprioception, discrimination of size, shape, texture, or point
localization, or the presence of astereognosis. Impairment of sensory
function in the upper extremity is a poor prognostic sign, even though
motor function may be intact or only minimally impaired (14,20,25,26,27,33,34,41,53,77,93,97,104,121,126,137,138,141,154,164,165,167,168,
hemisphere may result in a lack of awareness of the involved side of
the body (neglect). A failure to recognize and use the involved side
may occur despite minimal motor involvement.
hemianopia (blindness in one eye), disturbance of perception, poor
perceptual organization, loss of geometric sense, inability to copy
figures, and failure of tasks involving spatial analysis. Hemianopia is
likely to be permanent but it usually has little impact on
rehabilitation potential. Disturbances in visual perception are more
significant and may result in failures in activities of daily living (216).
stroke. Recovery follows a fairly typical pattern. A period of flaccid
paralysis occurs, lasting from 24 hours to several weeks. This is
followed by a period of increasing muscle tone. In general, the longer
the period of flaccidity, the poorer the prognosis for functional
recovery. In the arm, the shoulder adductor and internal rotator
muscles become tight. The elbow, wrist, and finger flexors also develop
marked tone. These changes are usually evident within 48 hours after
the stroke. Any paralysis remaining after 3 months usually persists,
although some slight improvement may occur over 6 months (10,14,17,20,24,25,26,27,29,31,33,34,41,51,53,67,77,79,
Functional improvement may continue as a result of further sensorimotor
reeducation. Increasing muscle tone usually leads to muscle spasticity.
Hyperactive deep tendon reflexes and clonus may appear.
muscle groups of the limbs and follows in a proximal-to-distal
direction or pattern of recovery. Voluntary movement should be sought
and examined during the early recovery phase, when flaccidity is
present.
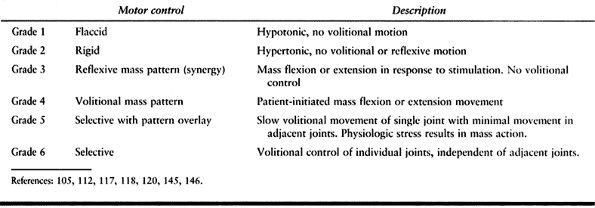
The extremity may be hypotonic or flaccid and without any volitional
movement. A spastic extremity may be held rigidly without any
volitional or reflexive movement. Patterned or synergistic motor
control is defined as a mass flexion or extension response involving
the entire extremity. Mass flexion in the upper extremity consists of
shoulder abduction, forearm pronation, and flexion of the elbow, wrist,
and fingers. Mass extension in the lower extremity consists of
extension
of the hip and knee with equinovarus of the foot and ankle. Synergistic
movement may be reflexive, in response to a stimulus, but without
volitional control. Some patients can also volitionally initiate the
synergistic movement. Selective motor control with pattern overlay is
defined as the ability to move a single joint or digit with minimal
movement in the adjacent joints when performing an activity slowly.
Rapid movements or physiologic stress make the mass pattern more
pronounced. Selective motor control is the ability to volitionally move
a single joint or digit independently of the adjacent joints.
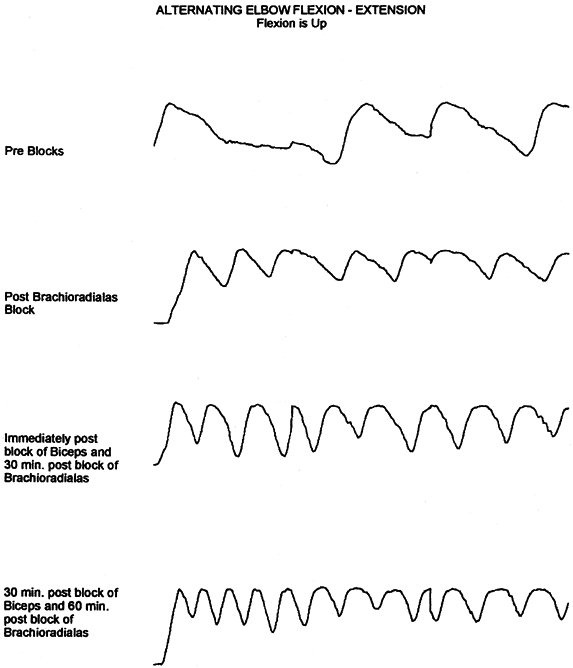
Spasticity can mask underlying motor control (Fig. 67.1).
 |
|
Figure 67.1.
This series of tracings records by electrogoniometer the maximum arc and frequency of elbow flexion—extension movement in a patient with traumatic brain injury. The first tracing is maximal effort before placement of a block. The second tracing shows improvement after a bupivacaine motor point block of the brachioradialis. Further improvement is noted immediately after bupivacaine block of the biceps and 30 minutes after block of the brachioradialis. After the biceps block has been allowed to set up, further improvement is noted. The blocks were used to demonstrate that there was volitional motor control in the elbow flexors that was previously masked by the spastic response of the muscles to movement. The blocks also preview the improvement to be gained by fractional lengthening of the elbow flexors. (With permission from Hisey MS, Keenan MAE. Orthopaedic Management of Upper Extremity Dysfunction Following Stroke or Brain Injury. In Green DP, Hotchkiss RN, Pederson WC, eds. Operative Hand Surgery, 4th ed. New York: Churchill Livingstone, 1998:287.) |
a primitive form of motor control and of no functional use in the upper
extremity. The hand requires some selective control for functional use.
The lower extremity can more successfully use synergistic motions for
functional activities, such as transfers or walking. For example, the
patient can be taught to use the flexion movement to advance the limb
and the extension pattern to provide limb stability during stance.
cerebral cortex, where basic sensory information is integrated to
complex sensory phenomena such as proprioception, spatial
relationships, shape, sight, and texture. Patients with severe parietal
dysfunction and sensory loss may lack sufficient perception of space
and awareness of the involved segment of their body to ambulate.
Patients with severe perceptual loss may lack balance to sit, stand, or
walk.
stabilization of the patient. The orthopaedic surgeon is rarely
involved in the acute care of the stroke patient. In some situations,
the orthopaedic surgeon may be asked to assist with splinting
extremities to prevent limb deformities.
the first 6 months following a stroke. This is particularly true for
recovery of muscle function. During the subacute phase, limb flaccidity
changes to spasticity. The patient is commonly in a rehabilitation
facility for a portion of this time. Muscle weakness can result in
joint subluxation or ligamentous laxity if the limb is not protected
using a sling to support the shoulder or splints to support the wrist.
When spasticity becomes pronounced, temporary measures are used to
prevent contracture formation until spontaneous neurologic recovery has
ceased.
months. Decisions can then be made regarding surgery to correct limb
deformities and rebalance the muscle forces. This is the time of
greatest contribution by the orthopaedic surgeon.
An epidemiologic study of physician-documented cases of TBIs occurring
in San Diego County, California, in 1981 determined an annual incidence
of 180 per 100,000 population (139).
provides an estimate of 410,000 new cases of TBI cases each year.
Eleven percent of these patients die shortly after the
injury.
Approximately 80% of the survivors have a good or moderate neurologic
recovery. Most traumatic injuries to the brain are in individuals who
are younger than 45 years old, and those who survive have a normal life
span despite the injury.
Glasgow Outcome Scale is frequently used to determine outcome following brain injury (Table 67.3).
Using the GCS score obtained within 24 hours of the patient’s admission
to the hospital, a coma score of 11 or greater is associated with an
82% probability of moderate or good neurologic recovery. Lower scores
have a significantly higher incidence of severe sequelae.
 |
|
Table 67.2. Glasgow Coma Scale*
|
 |
|
Table 67.3. Glasgow Outcome Scale*
|
outcome following brain injury regardless of the severity of injury.
Patients younger than the age of 20 years at the time of brain injury
experience as a group a 62% moderate or good neurologic recovery.
Patients between the ages of 20 and 30 can expect a 46% chance of
moderate or good neurologic recovery. In a series of pediatric patients
with brain injury, overall 90% achieved a moderate or good neurologic
recovery and only 8% expired or remained in a persistent vegetative
state (94,95). Young
children with a GCS score of 5 or better have a good prognosis for
recovery. In addition to having a poorer prognosis for recovery, the
cost and time required for rehabilitation of older patients is higher
than that for younger patients (39).
accurately, recent studies have suggested the GCS as a single variable
may have limited value as a predictor of functional outcome and many
trauma centers are now using the Revised Trauma Score (RTS) to assist
with triage of multitrauma patients. The RTS combines the GCS as well
as the systolic blood pressure and respiratory rate, and is used to
predict both mortality and disability (246,247).
emersion from coma occurs within the first 2 weeks of brain injury, 70%
of patients can be expected to achieve a good recovery. If the coma
persists beyond 4 weeks, the chance of good recovery is much
diminished. Brain stem involvement, as indicated by the presence of
decerebrate or decorticate posturing, has a poor prognosis for outcome.
If decerebrate posturing occurs and resolves within the first week
after injury, 40% of patients will have a good neurologic recovery. If
decerebrate posturing persist beyond the first week, only 9% of
patients will achieve a good neurologic recovery. In a similar manner,
the duration of posttraumatic confusion can also be an indicator of
prognosis. If the period of posttraumatic confusion persists for more
than 4 weeks, then one third of these patients will have a poor
neurologic outcome. It should be remembered, however, that prognosis is
a probability statement, and although various factors can be used as
guidelines, none is an absolute indicator in the individual patient.
The initial phase of management occurs immediately following the injury
in the acute care hospital. The majority of TBIs are the result of a
motor vehicle accident. Multiple trauma is common. The orthopaedic
surgeon is a consultant with a critical roll. Aggressive treatment of
orthopaedic injuries at an early stage is important to functional
outcome.
It is common for injuries such as fractures or major peripheral nerve
injuries to go undetected. Garland reported an 11% incidence of delayed
diagnosis of fractures, with an average time to diagnosis of 57 days (65,214). In the comatose patient, obtain radiographs of all major joints and any
other areas of suspect for injury. It is important not to assume that
all neurologic deficits present are from the CNS injury. Stone and
Keenan reported that 34% of brain-injured patients have missed
peripheral nerve injuries (214). Especially in the presence of a limb fracture, look for a peripheral nerve injury (65,214).
sympathetic dystrophy (RSD), deep vein thrombophlebitis, spasticity,
occult fracture, and the formation of heterotopic ossification (HO) (36,61,62,81,82,170,209,214,215,228).
If pain is treated promptly (and this depends on an accurate and early
diagnosis), prolonged restriction of motion may be avoided. HO,
fracture and fracture malunion restrict motion on the basis of lost
structural integrity. Many brain-injured patients who recover
cognitively have residual spasticity and impaired balance and
consequently, are less able to compensate for such structural
impediments (61,158). Peripheral nerve injury produces weakness and pain, both potential causes of restricted motion.
They are particularly prevalent in blunt trauma, especially motorcycle
accidents and ejections from a motor vehicle. They are often associated
with fracture or dislocation, particularly clavicle fractures. If the
plexopathy results in a flail arm in a patient with ipsilateral humerus
fracture, there is a predisposition for delayed union.
the patient will make a good neurologic recovery. All orthopaedic
injuries should be treated promptly and appropriately. When possible,
internal fixation is best. Spasticity develops, and casting a spastic
joint in a flexed position may result in a joint contracture or an
unsatisfactory reduction. Fracture healing is accelerated, presumably
by the same humoral factors that contribute to heterotopic bone
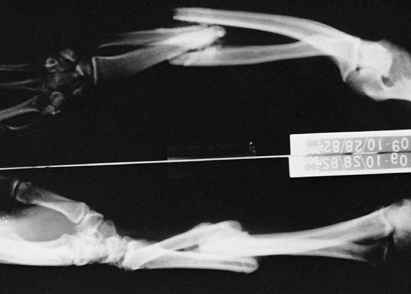
formation (8,111,194,226). Fracture malunion is a common and potentially avoidable complication (Fig. 67.2).
 |
|
Figure 67.2.
Malunited fractures of the radius and ulna in a brain-injured patient. Because the patient was not expected to survive, the fractures were not treated initially. |
cooperation. As the patient emerges from coma, they may be anticipated
to go through a period of agitation and confusion. Fracture care should
be made as foolproof as possible because patient cooperation cannot be
expected. Anticipating a possible period of agitation, traction and
external fixators for treatment of extremity fractures should be
avoided when possible (61,68,70,73,76,78,158,228,237,245).
prolonged period of time. The majority of improvement in motor control
occurs within the first 6 months following injury (42).
Cognitive changes are made most rapidly in the early phases following
brain injury but can continue for a very prolonged period of time,
often years (Table 67.4) (88).
Because the period of potential neurologic recovery following head
injury is prolonged, definitive surgical procedures are avoided during
the transitional stage. There is not an exact time that must elapse
before considering surgery to improve musculoskeletal function (109).
The orthopaedic surgeon must consider the rate of continued improvement
in motor control when deciding at what point to intervene surgically.
If the additional improvement in motor control will be overridden by
the complications of contracture formation, osteopenia, peripheral
nerve compression, and
muscle atrophy, then early surgical intervention is appropriate.
 |
|
Table 67.4. Rancho Levels of Cognitive Functioning
|
is commonly in a rehabilitation facility. Serious head injury is
usually complicated by UMN syndrome (7,19,71,104,105,112,120,136,145).
Spasticity is commonly severe and prevents adequate joint range of
motion. Spasticity also interferes with the maintenance of limb
position despite the most conscientious and aggressive treatment. Even
in those situations in which joint motion can be maintained by a
knowledgeable therapist, it commonly requires much force, which is
painful for the patient, potentially harmful, and very time consuming.
Lesser degrees of spasticity can also impede a patient’s function or
require the use of positioning devices that interfere with the use of
an extremity.
Contractures are common. Limited positioning and myostatic contractures
combined with the patient’s diminished nutritional status can result in
pressure sores or hygiene problems. When fractures are present,
malunions can occur in the face of uncontrolled muscle tone and
accelerated fracture healing. Joint subluxation can also occur from
prolonged spasticity or the attempts to range a joint in the face of
severe spasticity. If a ligamentous injury occurred at the time of
injury, frank dislocation of a joint can be caused by hypertonicity.
Spasticity also appears to be one of several etiologic factors in the
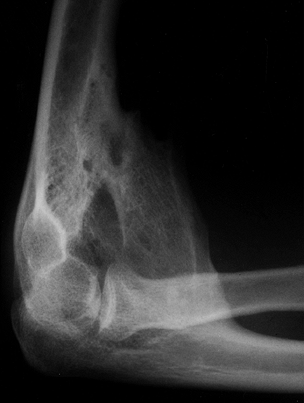
formation of HO in a periarticular location (Fig. 67.3). (12,18,40,43,60,64,66,69,70,83,86,98,111,113,127,132,147,153,157,163,171,192,194,226,227,238). Another common complication of spasticity is acquired peripheral neuropathy (38,45,49,59,65,170,214).
The most common peripheral neuropathies acquired with severe spasticity
and contracture formation are ulnar neuropathy at the elbow, resulting
from severe flexion and continuous pressure on the ulnar nerve, and
carpal tunnel syndrome, secondary to severe wrist flexion and pressure
of the median
nerve against the leading edge of the transverse carpal ligament (Fig. 67.4) (170,214).
During the period of physiologic recovery, the temporary control of
spasticity is the major focus of treatment. Prevention of additional
complications such as disuse muscle atrophy, joint contractures, HO,
and peripheral neuropathies is critical to a good functional outcome (6,8,11,17,18,22,23,27,32,35,36,38,40,57,59,60,61,65,66,68,70,73,76,
Early joint contractures are also best corrected during this phase of
treatment. This is accomplished by first reducing spasticity and then
correcting contractures by splinting, casting, and range-of-motion
therapy.
 |
|
Table 67.5. Complications of Spasticity
|
 |
|
Figure 67.3.
Lateral radiograph of the elbow in a patient with traumatic brain injury showing heterotopic ossification anteriorly causing ankylosis in 90° of flexion. There was no concomitant injury to the elbow and the radio-capitellar joint is not involved. |
 |
|
Figure 67.4. Clinical photograph (A) and radiograph (B)
of a patient with severe wrist flexion deformity and subsequent carpal tunnel syndrome from compression of the median nerve against the proximal edge of the transverse carpal ligament. |
brain-injured patient is commonly left with residual limb deformities
from spasticity, contractures, and muscle imbalance. It is at this time
that definitive orthopaedic surgical procedures are performed to
rebalance the muscle forces and correct the residual deformities (206).
reaction about the joint seen as redness, warmth, severe pain, and
rapidly decreasing range of motion (4,12,18,35,36,38,40,43,47,54,59,60,61,62,
Although the time of initial occurrence is variable, HO is usually
detected 2 months following the onset of TBI. Generally, radiographs
show evidence of the heterotopic bone in the form of spotty
periarticular calcification.
Although the exact etiology of HO is not known, and likely
multifactorial, it clearly has a predilection for joints surrounded by
spastic or paretic muscle (66,226,238).
A dramatic increase in incidence to 85% is seen in patients who have
concomitant musculoskeletal injuries. Because of this increased
incidence, consideration should be given to prophylaxis. Several
modalities have been used with varying success. High-dose
diphosphonates (Didronel) and nonsteroidal anti-inflammatory drugs,
particularly indomethacin have been used in the early postinjury period
(4,63,65,134,
Joint manipulation is also used, but the benefit of this procedure is
unclear if the heterotopic bone has already begun to form (74,218).
Formation of HO can be followed radiographically and by following
serial alkaline phosphatase measurements. The HO is mature when the
alkaline phosphatase has returned to a normal value, and the
radiographs show a well-defined, bone mass with a cortex. Technetium
bone
scans
show increased uptake for many years and are of little value in this
situation. Serum osteocalcin levels to diagnose HO or determine its
maturation are unreliable (163).
Surgical excision is thought to carry a higher risk of recurrence if it
is attempted before maturation. This comes from work with HO in spinal
cord injury patients and has not been shown to be applicable to TBI
patients (35,36,62,63,
Early excision should be considered in cases in which the HO is causing
progressive nerve or vascular compromise, or is threatening joint
ankylosis.
burning pain and is usually associated with hypo- and hyperesthesia,
hyperpathia, and allodynia, along with vasomotor and sudomotor
disturbances that, if persistent, result in trophic changes (82).
It commonly develops following CVA (posthemiplegic dystrophy), TBI, and
surgery. It may be associated with trauma, which occurred concurrently
with TBI, although the severity of the initial injury is unrelated to
the severity of the ensuing symptoms.
several weeks; however, with stroke and brain injury, the onset may be
delayed and atypical. Because of this, RSD may remain undiagnosed in
the stroke or brain-injured population until it becomes irreversible.
involved epiphyses even during the first phase aid diagnosis.
Subperiosteal resorption, tunneling of the cortex, and striation may be
evident on good quality films. Unfortunately, none of these changes are
specific for RSD. Triple phase bone scan may help with diagnosis. The
pattern seen varies with the phase of the disease. In the acute phase,
the flow (immediate), blood pool (early), and delayed (static) scan
patterns all show increased uptake, usually in a periarticular
distribution. False-negative results are frequent in the dystrophic
phase, in which the flow and blood pool scans are normal, and the
delayed scan shows a somewhat less prominent periarticular increase in
uptake. In the atrophic phase, both the flow and early scans show
decreased uptake, whereas the delayed phase is normal.
particularly active and active assisted range of motion, gentle muscle
strengthening and conditioning, massage, and heat therapy have been
effective. Tricyclic antidepressants (amitriptyline) may have their
effect because of their inhibition of serotonin uptake at
pain-suppressing neurons, prolonging the serotonin effect at the
receptor. Narcotics have a role in low-dose epidural infusions in
combination with local anesthetics. Systemic corticosteroids and
adrenergic blocking agents have both been advocated. Nerve blocks,
including stellate ganglion blocks, Bier blocks, surgical
sympathectomy, and chemical sympathectomy have been reported with high
percentages of patients improved. At present, we use a regimen of
amitriptyline, physical therapy, and percutaneous sympathetic blockade.
flaccidity before the gradual onset of increasing muscle tone or
spasticity (resistance to quick stretch). Because of the mean older age
of these patients compared with brain-injured patients, their muscles
are also weaker. This, combined with the shorter period of spontaneous
neurologic recovery (6 months), makes the temporary control of
spasticity an easier task in stroke patients. The use of phenol nerve
blocks or intramuscular botulinum toxin injection is therefore less
common than in the TBI patient.
intense level of spasticity (response to fast stretch), the presence of
rigidity (resistance to slow stretch), and the strong muscles found in
young TBI patients make the temporary control of spasticity much more
difficult. Phenol nerve blocks, botulinum toxin injections, and casting
are used more commonly and aggressively.
infarcts) have hemiplegic involvement with a nonfunctional upper
extremity and a lower extremity with greater potential for function.
The surgical procedures used to correct residual deformities in the
upper extremity are more likely to be for the correction of contractual
deformities than to improve function. Even when functional procedures
are employed, the gains from these procedures are more moderate than in
the brain-injured patient.
likely to have quadriplegic involvement, concomitant peripheral nerve
injuries, residual deformities from fractures, and joint limitation
from HO but better return of motor control. Functional surgical
procedures are more common in these patients.
injury and the prognosis for further recovery. In the period of
physiologic recovery, temporizing interventions are used because
permanent changes may result in chronic imbalance of forces across
joints (11,13,16,28,44,50,70,71,72,102,104,107,122,123,129,130,131,156,159,200,205,229,240,242).
Prevention of additional complications such as disuse muscle atrophy,
joint contractures, HO, and peripheral neuropathies is critical to a
good functional outcome. Several choices are available for treatment.
splinting techniques are commonly used to give temporary relief of
spasticity. Casting maintains muscles fiber length and diminishes
muscle tone by decreasing sensory input (11,70).
Local anesthetic nerve blocks are very helpful when administered before
cast application, because relieving the spasticity allows for easier
limb positioning. Casts are used primarily for the correction of
contractual deformities by applying a cast on a weekly basis. Serial
casting is most successful when a contracture has been present for less
than 6 months.
Antispastic agents that have sedating properties such as baclofen,
diazepam, and clonidine may compromise patients with attention deficits
or memory disorders, or both. Even a drug such as dantrolene sodium,
which has a peripheral mechanism of action, may also cause drowsiness.
Other serious side effects such as hepatotoxicity can occur. Continuous
infusion of intrathecal baclofen has been reported to be useful in
managing spasticity secondary to spinal cord injury. Such delivery
avoids the cognitive side effects seen with oral delivery. Early
studies have shown that intrathecal bolus infusions of baclofen via a
catheter placed in the lumbar space may also be capable of reducing
spastic hypertonia associated with brain injury (1,2,152).
agents is the most suitable approach for treating restricted motion
secondary to spasticity. Neurolytic agents such as phenol and
chemodenervation agents such as botulinum toxin A are used in this
period because their effects are temporary, lasting only 3 to 5 months (13,16,28,44,70,71,72,101,102,104,107,122,123,129,130,131,156,159,229,242).
These agents are used when restricted motion occurs as a result of
focal spasticity. When these agents wear off, reevaluation is done to
determine whether additional recovery has taken place and whether there
is further indication for re-blocking. It is critical that the
functional problems of the patient can be accurately ascribed to
specific muscles. This is done by evaluation using multi-channel
dynamic EMG (58,105,110,112,117,120,134,136,145,146,177,184).
Even if many muscles in a limb are involved, a number of focal
injections are possible and by doing so CNS side affects can be
avoided. For patients with pathologies causing excessive motion,
environmental modification weights, bracing, and oral medications may
be considered during the period of physiologic recovery.
membrane of peripheral nerves in aqueous concentrations of 5% or more.
When phenol is injected in or near a nerve bundle, phenol’s neurolytic
action on the myelin sheath or the cell membrane of axons with which it
makes contact serves to reduce neural traffic along the nerve. The
onset of the destructive process with higher concentrations of phenol
may begin to show effects several days after injection. The denaturing
process induced by phenol extends biologically on the order of weeks,
but eventually regeneration occurs. A phenol block is used as a
temporizing measure rather than a permanent intervention. In our
clinical experience and the experience of others, the effect of a
phenol block typically lasts 3 to 5 months.
axons of all sizes in a patchy distribution but more so on the outer
aspect of the nerve bundle, onto which phenol is dripped. When phenol
is percutaneously injected, it is likely that the nerve block will be
incomplete. This is especially useful in situations in which a spastic
muscle also has volitional capacity because under these circumstances,
it is desirable to reduce spasticity while still preserving volitional
capacity of a given muscle or muscle group.
stimulation. Motor branches are injected close to the offending muscle
or muscle group. These are referred to as motor points. A surface
stimulator is briefly used to approximate the percutaneous stimulation
site in advance. A 25 gauge Teflon-coated hypodermic needle is advanced
toward the motor nerve. Electrical stimulation is adjusted by noting
whether muscle contraction of the index muscle takes place. As the
electrode gets closer to the motor nerve, less current intensity is
required to produce a contractile response. The motor nerve is injected
when minimal current produces a visible or palpable contraction of the
muscle. Generally, 4 to 7 ml of 5% to 7% aqueous phenol is injected at
each site. As with any injection, care needs to be taken not to inject
into a blood vessel and this is done by aspirating before the injection
(13,16,28,44,70,71,72,101,102,104,107,122,123,129,130,131,145,146,156,159,229).
treatment of spasticity. Ordinarily, an action potential propagating
along a motor nerve to the neuromuscular junction triggers the release
of acetylcholine (ACh) into
the
synaptic space. The released ACh causes depolarization of the muscle
membrane, activating a biochemical sequence that leads to muscle
contraction. Botulinum toxin type A is a protein produced by Clostridium botulinum
that inhibits this calcium-mediated release of ACh at the neuromuscular
junction. Botulinum toxin A attaches to the presynaptic nerve terminal
and divides into a light and a heavy component. The light component
invades the nerve cell and interferes with fusion proteins affiliated
with vesicles of ACh, thereby preventing the release of ACh from their
storage vesicles.
variety of dystonias and is currently approved by the Food and Drug
Administration (FDA) for the treatment of blepharospasm, facial spasm,
and strabismus. A number of studies have reported its use in treating
spasticity in individuals with cerebral palsy, stroke, head trauma, and
multiple sclerosis (37,50,58,145,146,200,205,240).
Clinical benefit lasts 3 to 5 months but may be more variable.
Botulinum toxin is injected directly into an offending muscle, and
depending on the size of the muscle being injected, dosing has ranged
between 10 and 200 units (U). Current practice is to wait at least 12
weeks before re-injection and not to administer a total of more than
400 U in a single treatment session. Because this upper limit of 400 U
may be reached rather quickly, a different strategy is needed for the
limb requiring many proximal and distal injections. Botulinum toxin A
and phenol may be combined, the former being injected into smaller
distal muscles and the latter aimed at larger proximal ones. A 3- to
7-day delay between injection of botulinum toxin A and the onset of
clinical effect is typical. Effects will not be seen by the patient
immediately and usually a follow-up visit is arranged to check the
result. The amount of toxin given for a particular muscle is variable.
physicians inject through a syringe attached to a hypodermic needle
that doubles as a monopolar EMG recording electrode. Patients may be
asked to make an effort to contract the targeted muscle or the muscle
may be contracting involuntarily as in dystonia. After inserting the
needle electrode, injection is made when EMG activity is recorded. For
deep or small spastic muscles (e.g., finger flexors), electrical
stimulation is preferred as a means of localizing the muscle before
injection.
in the past several years. The advantages of botulinum toxin are (a)
ease of injection and (b) the lack of residual scaring after injection.
The disadvantages of botulinum toxin are (a) high cost and (b)
stimulation of antibody formation that requires higher doses for
repeated injections. Phenol, in contrast, requires more technical
expertise to localize the nerve or motor points for injection. Phenol
is caustic and causes localized scarring of the nerve and muscle.
Phenol, however, is inexpensive and readily available.
common in the upper extremity of both stroke and brain-injured
patients. This deformity interferes with hygiene and upper body
dressing. Phenol motor point blocks or botulinum toxin injections of
the pectoralis major muscle are effective in reducing tone and
improving shoulder adduction during the physiologic recovery phase (16,37,49,104,145,146,200,205,240).
-
Inject botulinum toxin directly into the muscle.
-
The effects of the botulinum toxin
injection appear slowly over the ensuing 48 hours. Start physical
therapy as the muscle relaxes. -
For phenol injections, localize the motor
points using a surface stimulator over the pectoralis major muscle.
Then use an insulated, Teflon-coated needle in conjunction with a nerve
stimulator to localize more accurately the points where the motor
nerves enter the muscle. -
Inject approximately 1 ml of a 5% aqueous
solution of phenol at each point. A decrease in tone can be expected to
last approximately 2 months, during which time an active therapy
program can continue. -
There is an initial reduction in muscle
tone immediately after the block. The muscle will continue to relax
gradually over the next 24 hours. -
Begin physical therapy immediately using
both active and passive techniques to increase shoulder range of
motion. The duration of the block is approximately 2 months. -
Repeat blocks as needed during the period
of time in which neurologic recovery can be expected to continue. In
addition, blocks of the thoracodorsal nerve can also be performed.
flexor spasticity requires the elimination or decrease of excessive
tone in each of the three flexor muscles. The brachioradialis muscle
has been shown by dynamic EMG studies to be the most spastic of the
elbow flexor muscles (112). Because the radial
nerve innervates this muscle, it is necessary to perform a motor point
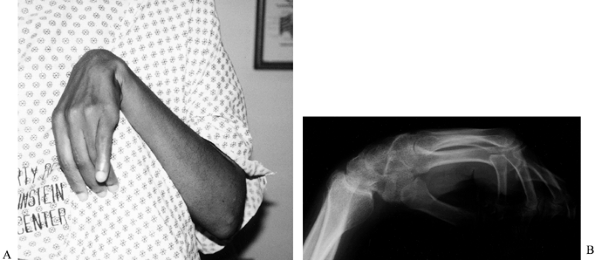
block of the brachioradialis muscle (Fig. 67.5).
 |
|
Figure 67.5. A phenol injection of the motor branches of the radial nerve to the brachioradialis is used to reduce spasticity temporarily.
|
-
Localize the motor points on the surface
of the brachioradialis muscle using a surface stimulator. Then use an
insulated Teflon-coated needle in conjunction with a nerve stimulator
to localize more accurately to the motor points.
interfere with elbow extension. Botulinum toxin can be injected into
the individual elbow flexor muscles. Alternately, a phenol block of the
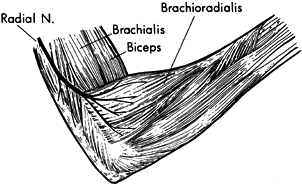
musculocutaneous nerve will provide temporary relief (Fig. 67.6) (112).
The block is commonly performed percutaneously. The musculocutaneous
nerve has minimal corticosensory representation. Percutaneous phenol
injection of the musculocutaneous nerve therefore does not interfere
with sensation in the upper extremity. The advantages of performing the
musculocutaneous nerve block percutaneously are (a) it does not require
general anesthesia; (b) because no surgery is required, it is more
readily done; and (c) it provides only a partial blockade of the action
of the biceps and brachialis muscle. The partial block preserves the
potential for upper extremity functional training.
 |
|
Figure 67.6.
A percutaneous injection of the musculocutaneous nerve can be used to diminish temporarily spasticity of the biceps and brachialis muscles. |
-
Perform phenol injection of the musculocutaneous nerve using a Teflon-coated needle and a nerve stimulator.
-
Introduce the needle from the medial
aspect of the arm and pass between the lower edge of the short head of
the biceps and the brachialis. In a spastic patient, this interval is
easily identified. -
Move the needle while applying stimulation until the point of maximal response is noted.
-
Inject 3 ml of an aqueous solution of 5%
phenol at this location. Use an aqueous solution for percutaneous
injections to provide better diffusion of the phenol.
-
Treat spastic forearm flexor muscles
causing wrist and finger flexion deformities during the physiologic
recovery phase with botulinum toxin injection of the muscles or with
phenol motor point blocks (37,70,71,72,200,205,240).
Because of the large sensory components of both the median and ulnar
nerves, direct injection of the nerves with phenol is undesirable. -
To localize the point of entry of the
motor branches into the muscles, use surface electrical stimulation.
Mark the points of maximal response on the skin. -
For insulation, insert at these points a
needle coated with Teflon. Use additional stimulation to define the
motor points of the muscles further. -
When the motor point has been localized,
inject an aqueous solution of phenol at each site. Do not inject more
than 5 points in a forearm in 1 day to avoid excessive swelling and
inflammation. -
Residual spasticity and mild contracture
are commonly present despite motor point blocks or botulinum toxin. The
blocks can be supplemented with functional electrical stimulation of
the wrist and finger extensor muscles and by casting or splinting
techniques. -
Perform gentle passive range of motion of
the wrist and fingers. When motor control is present, include a program
of active exercise and functional training.
is common but is usually masked by spasticity in the extrinsic finger
flexors. An adducted thumb, limited extension of the
metacarpophalangeal joints, or swan-neck positioning of the fingers
should alert the physician to the possibility of underlying intrinsic
spasticity. Botulinum toxin can be injected into the offending muscles.
Alternately, a phenol block of the motor branches of the ulnar nerve in
Guyon’s canal can be administered after surgical exposure (Fig. 67.7) (103,104,115,122,131).
The close proximity of the sensory branch of the nerve makes
percutaneous injection undesirable because loss of sensation in the
hand or painful dysesthesia could develop with phenol injection of the
sensory nerve.
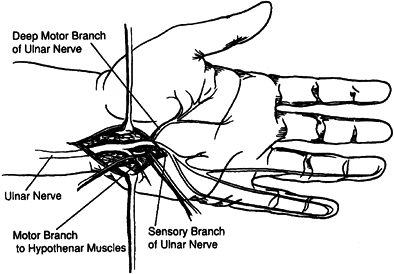 |
|
Figure 67.7.
Isolation of the motor branches of the ulnar nerve distal to Guyon’s Canal. (Reprinted with permission from Keenan MA, Kozin SH, Berlet AC. Manual of Orthopaedic Surgery for Spasticity. New York: Raven Press, 1993.) |
-
Make an incision on the palmar surface of
the hand radial to the pisiform bone and extend distally for 1 inch.
Take care to prevent harm to the ulnar artery. -
Expose the ulnar nerve and identify the
motor branches using a nerve stimulator. Generally, two motor branches
are seen; the main branch lies beneath the sensory branch and a smaller
motor branch can be seen entering the hypothenar muscles. -
Place a moistened gauze sponge under the nerves to be injected to protect the surrounding soft tissues.
-
Inject the motor branch with 5% phenol in
glycerin. Use phenol for surgical blocks when the nerve is being
injected under direct vision. The glycerin allows the phenol to be
released more slowly into the nerve, thereby prolonging its effect. The
duration of the block lasts approximately 6 months. -
Unless combined with other surgery no
splinting or casting is used postoperatively. Apply a soft dressing to
the hand and begin active and passive exercises on the first
postoperative day.
If flexion of the interphalangeal joint of the thumb is present, then
perform a botulinum toxin injection or a motor point block of the
flexor pollicis longus (71). When the thumb is in a severely adducted position, use phenol for a surgical block of the motor branches of the ulnar nerve (122).
-
When the adduction deformity of the thumb
is also secondary to spasticity of the median innervated muscles of the
thenar eminence, a phenol block of the recurrent motor branch of the
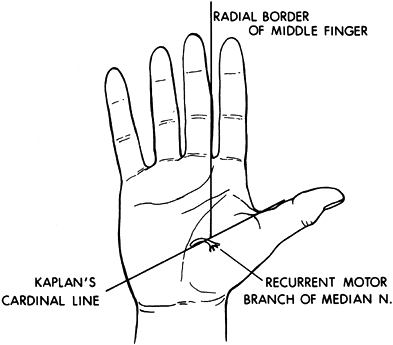
median nerve can be performed (107). Perform the block percutaneously using a Teflon-coated needle and nerve stimulator. -
The recurrent motor branch of the median
nerve enters the thenar mass at the junction of a line drawn along the
radial border of the long finger and Kaplan’s cardinal line. Kaplan’s
cardinal line is drawn parallel to the proximal palmar crease beginning
at the apex of the first web space (Fig. 67.8).![]() Figure 67.8.
Figure 67.8.
The recurrent branch of the median nerve enters the thenar mass at the
junction of a line drawn along the radial border of the long finger and
Kaplan’s cardinal line. -
Use a nerve stimulator and insulated
needle to localize the nerve during the percutaneous injection. Then
inject approximately 2 ml of aqueous phenol at the point of maximum
response to stimulation. -
Casting or splinting may be needed after
the block if a contracture is present. Employ active and passive range
of motion exercises. Perform functional training when any motor control
is present in the upper extremity.
upper extremity function requires careful systematic evaluation before
surgery. The goals of surgery must be practical and clearly understood
by the patient and the family. Assessment includes an evaluation of
cognition and communication skills (15,104,117,127,133,136,231).
The patient must be capable of following simple commands and should
also be able to cooperate with a postoperative therapy program.
The basic modalities of pain, light touch, and temperature must be
present. Two-point discrimination is a valuable predictive test. A
patient rarely uses the hand for functional activities if the
discrimination is greater than 10 mm. Proprioception and kinesthetic
awareness of the limb in space are also important. Kinesthetic
awareness is tested in a hemiplegic individual by placing the spastic
limb in a position and asking the patient to duplicate this position
with the sound limb while keeping the eyes closed. Stereognosis is not
a practical test in spastic patients. They lack the fine motor control
necessary to manipulate an object in the hand. It is helpful to observe
the patient’s spontaneous use of the hand. Visual perceptual deficits
add increased problems involving motion of the limb and even awareness
of the limb itself.
and diffuse hypoxic encephalopathy lead to a large variety of
posttraumatic motor phenomena, many of which are functionally
significant (Table 67.6). Lesions affecting the
corticospinal system, the cerebellum and its pathways, and the
extrapyramidal system are common. Hemiparesis is the most common
long-term residual problem of head injury. Many patients, however, have
a brain stem syndrome consisting of ipsilateral ataxia and
contralateral spastic hemiparesis. A small percentage have a
pseudobulbar athetoid type of picture. The literature also identifies
patients with residuals of bilateral hemiparesis, ataxia involving both
sides of the body, and severe dystonic decerebrate posturing or
rigidity. Many patients, especially during the early recovery stage
from head injury reveal mixed signs such as spasticity combined with
tremor and ataxia. Peripheral neuropathy is common after head injury,
and focal dystonia, although unusual, is also seen. Because so many
different aspects of the motor control system may be affected by a head
injury, we present a way of organizing the unwieldy array of symptoms
that emerge from a damaged nervous system. Our perspective is a
practical one, namely taking into account the impact of movement
disorders on the patient’s ability to function in real life.
 |
|
Table
67.6. Clinical Phenomena Associated with Impaired Movement That Functionally Lead to Restricted or Excessive Motion After Traumatic Brain Injury or Cerebrovascular Accident |
problems are formulated and described in focal rather than diffuse
terms. Treatment of focal problems lends itself well to surgical
intervention, which can target particular muscles. Surgical
lengthening, transfer, or release of targeted muscles can provide very
effective solutions to problems of function that are clearly identified
from the outset. The localizing approach is useful because it forces
the clinician to indicate the desired outcome in advance. The outcome
is based on an analysis that identifies the specific spastic muscles
responsible for the problem. For example, if the clinical problem is an
adducted shoulder that hinders access to the axilla, surgically
releasing the pectoralis major will not solve the problem if the teres
major and latissimus dorsi are really the culprits responsible for the
problem. Identifying the specific offending muscles is critically
important to localized strategies of intervention.
difficult to distinguish between the many potential causes of limited
joint motion. The possibilities include increase muscle tone, a
myostatic contracture, the presence of periarticular HO, an undetected
fracture or dislocation, joint subluxation, pain, or the lack of
patient cooperation secondary to diminished cognition. Bony deformities
may not exhibit an obvious clinical deformity but can be detected by
radiography.
three factors: (a) the clinical pattern of motor dysfunction, (b) the
patient’s ability to control muscles involved in the clinical pattern,
and (c) the role of muscle stiffness and contracture in relation to the
functional problem. For purposes of convenience, we identify six
clinical patterns of upper extremity motor dysfunction that are most
commonly seen (Table 67.7). Other variations of
motor dysfunction occur but are less common. Various muscles may
contribute to motor dysfunction across joints and limb segments in
these clinical patterns. During evaluation, focus on the following
characteristics of the involved part: (a) voluntary or selective
control, (b) spastic reactivity, (c) rheologic stiffness, and (d)
contracture. Ask five specific questions:
 |
|
Table 67.7. Common Clinical Patterns of Motor Dysfunction in the Upper Extremity*
|
-
Does the patient have voluntary control over a given muscle?
-
Is the muscle spastic to passive stretch?
-
Is the muscle, as an antagonist, activated during active movement generated by an agonist?
-
Does the muscle have increased stiffness when stretched?
-
Does the muscle have fixed shortening (contracture)?
each muscle may vary. Because each muscle may contribute to motion and
movement of the joint, information about each muscle’s contribution is
useful to the assessment as a whole. Treatment depends on such
information (58,145,146).
upper extremity, the most common pattern of spasticity is one of
flexion. Passive range of motion of each joint should be established
first. This is tested by slow extension of the joint to avoid the
velocity sensitive response of the muscle spindle. When spasticity is
significant and passive joint motion is incomplete, perform an
anesthetic nerve block to assess whether a myostatic contracture is
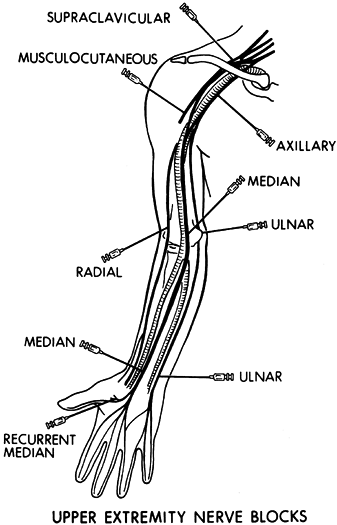
present (104,105,117,118,126).
In order to evaluate passive joint motion in the entire upper
extremity, perform a brachial plexus block using a local anesthetic.
contributes to the motor impairment. Spasticity (hyperactive response
to quick stretch), rigidity (resistance to slow movement), or movement
dystonias may be present. The degree of spasticity within selected
muscles can be graded clinically in response to a quick stretch as
mild, moderate, or severe. There is surprising consistency between
observers using this simple grading system. Another method of
quantifying muscle tone, which is readily accessible and easily
performed at the bedside, is to measure the amount of intramuscular
pressure generated by a passive quick stretch or during functional use
of the limb. Intramuscular pressure can be measured using a wick or
slit catheter technique. The pressure generated within the muscle is
proportional to the force of contraction (5,182).
 |
|
Table 67.1. Clinical Scale of Motor Control
|
pain, increased muscle tone, and contracture to a limb deformity can be
difficult. Anesthetic nerve blocks are extremely useful in assessing
joint range of motion. The
blocks
can be easily performed without the use of special devices. By
temporarily eliminating pain and muscle tone, patient cooperation is
gained and the amount of myostatic contracture can be determined. By
using local anesthetic blocks, the strength and motor control of the
antagonistic muscle group can also be evaluated (Fig. 67.9).
 |
|
Figure 67.9. Commonly performed diagnostic nerve blocks in the upper extremity using a local anesthestic.
|
the mainstay of evaluation. The clinical questions of interest
regarding a given problem include the following:
-
Does the patient have selective voluntary control over the given muscle?
-
Is the muscle activated dyssynergically (i.e., in antagonism to movement) when the patient attempts to move the relevant joint?
-
Is the muscle resistive to passive stretch (i.e., spastic)?
-
Does the given muscle have fixed shortening (i.e., contracture)?
and procedural costs involved in treating complicated movement
dysfunction in patients with CVA and TBI, clinical examination alone
may not be sufficient to answer these questions with a high degree of
confidence. Technology-driven laboratory assessments that may include
formal gait and motion analysis, dynamic EMG studies, and nerve blocks
may be helpful (105,112,117,118,120).
Dynamic multichannel EMG is acquired with simultaneous measurements of
joint motion (kinematics) in the upper extremity. Kinetic, kinematic,
and dynamic EMG data assist the clinician in interpreting whether
voluntary function (effort-related initiation, modulation, and
termination of activity) is present in a given muscle and whether that
muscle’s behavior is also dyssynergic (sometimes referred to as “out of
phase” behavior). In addition, responses to different rates of passive
stretch of muscle before and after local anesthetic nerve block can
help the clinician distinguish between the dynamic, velocity-sensitive
reflex resistance of spasticity versus passive muscle tissue stiffness
and contracture. Somatosensory evoked potentials (SEPs) and motor
evoked potentials (MEPs) provide information on the integrity of the
sensory and motor pathways and may be helpful in predicting recovery of
motor function after stroke (93). Combined with
clinical information, laboratory measurements of muscle function often
provide the degree of detail and confidence necessary for making
conservative and surgical treatment decisions.
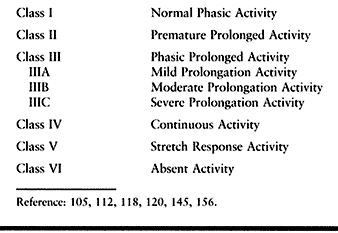
spastic patients from our institution the following classification of
EMG activity was devised to standardize terminology and may be used for
either the upper or lower extremity (Table 67.8) (105,112,117,118,120).
 |
|
Table 67.8. Classification of Electromyographic Activity
|
-
Class I constitutes a normal phasic pattern with appropriate on and off EMG activity.
-
Class II consists of EMG activity that,
although phasic, begins prematurely and continues for a short period
beyond the normal duration of activity for that muscle. This is more
commonly seen in the lower extremity. -
Class III consists of phasic activity
with prolongation beyond the normal timing of the muscle. Class III
activity can be further subdivided into three patterns, depending on
the degree of prolongation. -
Class IIIA consists of phasic activity
with a short period of low-intensity EMG activity extending into the
next phase of the flexion–extension cycle secondary to mild spasticity. -
Class IIIB consists of phasic activity
with prolongation extending for at least half of the next phase of
motion. This is indicative of a moderate amount of spasticity. -
Class IIIC represents a severely spastic
muscle and consists of phasic activity with severe prolongation in
which EMG activity is continued throughout the next phase of motion at
a high intensity but the underlying phasic nature of the muscle
activity is still distinguishable. -
Class IV consists of continuous EMG activity without phasic variations.
-
Class V consists of EMG activity seen
only in response to a quick stretch by the antagonist muscles. There is
no volitional activation of the muscle. This pattern is common in the
finger extensors. -
Class VI consists of absent EMG activity.
and TBI are very similar. The same orthopaedic procedures can be used
in both patient populations. The orthopaedic treatment interventions
will be described together. These procedures, however, are not applied
equally to both patient groups. The degree of spasticity, the timing of
neurologic recovery, and the pattern of spontaneous neurologic recovery
are different between stroke and brain-injured patients. These
differences account for the variation in the need for specific
treatments between the two groups.
challenges to the surgical team. The patients may have behavioral
deficits or cognitive limitations that would make them difficult to
manage with regional anesthesia and sedation. Therefore, general
anesthesia is preferred (67,215).
The patients often have previously had a tracheostomy performed;
therefore, an anesthesia team familiar with airway difficulties caused
by this procedure is important. Great care must be taken when
positioning these patients for long procedures because contractures of
other portions of their bodies may increase the risk of pressure ulcer
formation.
is due to a combination of factors. Trauma concurrent with the brain
injury may cause complex fractures, which are predisposed to malunion.
Injuries may be missed in the initial resuscitation. Fracture healing
may be acelerated by the same mechanisms that cause HO. The prognosis
of the brain injury may be sufficiently poor that optimal internal
fixation of fractures is not sought or obtained. Hemodynamic or
pulmonary instability may cause optimal fracture fixation to be
delayed. Poor patient compliance, agitation, and spasticity may alter
the initial reduction. If this is not anticipated malunion may result.
acromioclavicular [AC] joint) are the most common upper extremity
injuries in TBI (61,65).
Most commonly, these injuries can be treated nonoperatively. Patient
agitation and muscle spasticity may necessitate open reduction and
internal fixation. Malunion of the proximal humerus may result from
inadequate closed reduction or failed internal fixation. These problems
are difficult to treat because of excessive scar formation, HO, and
retraction of the tuberosities. Treatment of malunited tuberosity
fractures is similar to acute injuries, with open reduction of the
tuberosity to prevent subacromial impingement (9).
In three-part and four-part fracture malunions, prosthetic replacement
is generally indicated. If there is no evidence of avascular necrosis
of the head and a congruent joint in a three-part fracture, osteotomy
and internal fixation may be considered.
perform stable internal fixation so that early motion may be initiated.
Prophylaxis for HO should also be considered in the acute injury phase
when performing internal fixation in brain-injured patients. The drug
therapy we employ is diphosphonate 20 mg/kg/day combined with
indomethacin 50 mg tid. Low-dose radiation can also be employed (132).
predictably gives unsatisfactory results, particularly loss of
supination and pronation. Open reduction results in a 31% incidence of
interosseus membrane ossification. For this reason, when performing
open reduction and internal fixation of these fractures, use a
two-incision technique. Minimize dissection, and use bone grafting
cautiously (68). Consider prophylaxis for HO.
Distal radius fractures are common. Treat malunions with osteotomy and
internal fixation with bone grafting.
malunions are more symptomatic. This is especially true with fractures
that are more proximal and with the radial metacarpal bones. This
causes a pseudoclaw and painful grip. Treat with dorsal closing wedge
osteotomy with percutaneous pin fixation or internal fixation. The
rotationally malaligned fractures cause a gap between fingers and makes
manipulation of small objects more difficult. This is particularly
important to TBI patients, who may have motor control deficits that
aggravate the situation. Correct these deformities with rotational
osteotomy and fixation (87).
function and when volitional movement of the involved joint is present (69).
In addition, surgical excision of functionally significant
periarticular HO often results in significant improvements in range of
motion, independence, and quality of life (62,63,69,132). Radiographically immature lesions also have a higher rate of recurrence (18,47,60,62,63,69,111,127,132,147,157,195).
It may not be feasible or desirable to allow the HO to reach maturity
before resection, because although the risk of recurrence is higher,
ankylosis of the joint and subsequent contracture worsen the over all
functional result.
Shoulder HO radiographically appears to form inferomedial to the joint.
This can be deceiving and computed tomography may be needed to localize
the abnormal bone. When the bone is located anterior to the joint, use
a deltopectoral incision. When it is necessary to resect a shoulder HO,
it may also be necessary to release or lengthen internal rotators
simultaneously without entering the joint capsule (63,127,132).
Releasing the pectoralis major may produce a cosmetic defect, but its
release is necessary when there is a severe internal rotation and
adduction deformity. Close the wound in layers over a drain. When the
HO is located posterior to the shoulder, it commonly follows the teres
muscles from the scapula to the posterior humerus; therefore, use a
posterior approach. Great care must be taken to identify the axillary
nerve in normal tissue because it is commonly encased within the mass
of heterotopic bone and is easily transected. Following HO resection
begin range-of-motion exercises immediately.
these cases, the HO does not involve the collateral ligaments or the
radio-capitellar joint (Fig. 67.3). Even when joint ankylosis occurs, forearm rotation is maintained (63,127,132). HO forms in 90% of fractured or dislocated elbows in head injured adults (73).
When HO follows trauma, it commonly forms in the collateral ligaments
and may involve the radio-capitellar joint. Consider prophylactic
treatment with either anti-inflammatory agents, diphosphonates, or
radiation in patients with concurrent head injury and elbow trauma.
When elbow HO does form, tardy ulnar nerve palsy should be suspected,
particularly when the bone is located medial or posteromedial (113). Elbow HO occurs in 20% of patients with TBI and forearm fractures (68). Anterior HO occurs roughly one third as often as posterior HO, with a posterolateral location the most frequent (61,63,69).
Surgical approaches for elbow HO are posterolateral, medial, and
anterolateral. Base the decision on which approach to use on plain
radiographs, computed tomography with or without three-dimensional
reconstructions, and the need to decompress any nerves. Unfortunately,
delaying surgical excision for the suggested 12 to 18 months often
results in severe elbow impairment. Even with this delay, however,
recurrence is common (69,132).
between the lateral humeral condyle and the lateral olecranon. The
elbow is commonly ankylosed in mild flexion. When excising the HO, it
is helpful to resect the central bridge first, allowing some motion of
the elbow and exposure of the posterior fat pad. Once motion is
obtained, the landmarks can be identified more easily and the remainder
of the HO can be resected from the humerus and olecranon. Preserve the
fat pad to prevent recurrent contracture.
transposition is necessary, when posterior HO extends medially, or when
HO of the medial collateral ligament limits motion. In this approach,
the ulnar nerve is identified proximally and protected. Occasionally,
as the nerve is followed distally, it will dive through a gap between
posterior and medial HO. If it is thus encircled, it must be freed and
transposed anteriorly before HO resection (69,113,127,132).
the brachialis to the coronoid. If the biceps is involved, proximal
forearm synostosis may occur. With anterior HO the elbow is most often
fixed in 90° of flexion. The anterolateral approach allows exposure of
the HO, identification of the radial and median nerves, and the
brachial artery. Should lengthening of the brachialis and biceps be
necessary, this is also facilitated by this approach. First, resect the
central bridge, establishing motion, then extend the elbow and resect
the remaining HO. Anterior capsular release may be performed if
necessary (63,127,132).
Close wounds over drains in layers. Following resection of elbow HO,
arc of motion averages 65°. Complications of resections include wound
breakdown, posterior interosseous nerve palsy, and re-ankylosis (69,126,132,157).
trauma, radioulnar HO can be particularly troublesome. When it is
present, the posterolateral incision can be extended along the
posterolateral border of the proximal ulna to gain access for removal.
Remember that less extensive dissection minimizes the chances of a
recurrence (68).
it is a common source of pain. A variety of different factors
contribute to the painful, immobile shoulder: RSD; brachial plexitis;
inferior subluxation; spasticity with adduction, internal rotation
contracture; adhesive capsulitis; spastic abduction; HO; and traumatic
lesions, such as rotator cuff tears or fractures and dislocations.
following stroke and in those with RSD. They have a characteristically
painful shoulder with limited glenohumeral motion. Three clinical and
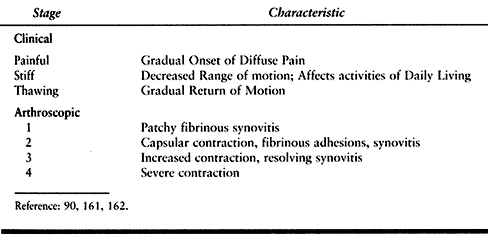
four arthroscopic stages have been identified (90,161,162,203) (Table 67.9).
The treatment in this group of patients is similar to that for the
general population. Nonsteroidal anti-inflammatory drugs, physical
therapy, and intra-articular injections are all useful. Selected cases
may benefit from manipulation under anesthesia.
 |
|
Table 67.9. Stages of Adhesive Capsulitis
|
-
Arthroscopic capsular release may be
performed for adhesive capsulitis. Perform capsular release via a
three-portal technique, with a standard anterior port and two posterior
ports, one just above and one just below the posterior soft spot. -
Release the posterior superior capsule
using the posterior superior port for a straight punch, with the
arthroscope in the anterior port. -
Establish the posterior inferior port and
with the arthroscope in the superior port, use a straight punch through
the inferior port to release the inferior capsule. Take care to dissect
the inferior capsule away bluntly from the underlying tissue to avoid
injuring the axillary nerve. -
Begin range-of-motion exercises
immediately. These exercises may be facilitated by a long-acting
interscalene block. Botulinum toxin injections or phenol blocks of
spastic shoulder muscles also fascilitate the postoperative
rehabilitation.
This is most commonly seen in stroke patients or in brain-injured
patients with concomitant brachial plexus trauma. The subluxation is
usually self-limiting, but occasionally, the shoulder will be
chronically subluxated, causing pain. The patients typically have no
functional use of the extremity. Patients complain of increased pain
when upright. The pain may be due to chronic stretch on the shoulder
capsule or from traction on the brachial plexus. Physical examination
shows a positive sulcus sign, with little to no active motion of the
involved shoulder. There is a prominence of the acromion and atrophy of
the deltoid. There may be contracture of the shoulder in adduction and
internal rotation. Subacromial or intra-articular injections of local
anesthetics do not relieve the symptoms. Radiographs show inferior
subluxation of the humerus on the glenoid (Fig. 67.10). Brachial
plexopathy must be ruled out by using diagnostic EMG.
 |
|
Figure 67.10.
AP radiographs of the shoulder in a patient with painful inferior subluxation before and after biceps suspension. Note inferior subluxation of the humerus preoperatively (A) with reduction after surgery (B) (With permission from Hisey MS, Keenan MAE. Orthopaedic Management of Upper Extremity Dysfunction Following Stroke or Brain Injury. In Green DP, Hotchkiss RN, Pederson WC, eds. Operative Hand Surgery, 4th ed. New York: Churchill Livingstone, 1998:287.) |
stimulation to the deltoid and supraspinatus muscles use of a sling.
This relieves the symptoms by elevating the humeral head in the
glenoid. Although this technique is usually successful in the short
run, this is frequently unacceptable to the patient as a permanent
solution. A surgical solution to this problem of excessive laxity is
the biceps suspension procedure (Fig. 67.11).
 |
|
Figure 67.11. The biceps suspension procedure.
|
paralytic inferior subluxation of the shoulder, but none has gained
widespread acceptance. Braun (31) has advocated
using the coracoacromial ligament to suspend the humeral head. The
lateral portion of the coracoid process together with the
coracoacromial ligament is detached and transferred to the humeral
head, where it is fixed using a cancellous lag screw. Garland has
advocated detaching the proximal end of the long head biceps tendon,
looping the tendon over the clavicle and securing it back on itself.
With time, the paretic biceps muscle tends to stretch and the humerus
once again subluxates inferiorly. Shoulder arthrodesis has also been
performed but is not well accepted by the patient because it produces a
rigid joint, which interferes with passive positioning, hygiene, and
nursing care.
-
The procedure we prefer is to convert the
long head of the biceps tendon to a proximally based suspensory
ligament. This preserves passive shoulder motion while correcting the
subluxation. Because only the tendon is being used, there is no
opportunity for paretic muscle to develop laxity and for the deformity
to recur. -
Place the patient in the beach chair position with a small bolster under the scapula.
-
Use a standard deltopectoral approach. If
the pectoralis major is causing an adduction or internal rotation
deformity, release it at its insertion. -
Identify the musculotendinous region,
where the tendon of the long head of the biceps joins the muscle belly.
Take care to preserve the musculocutaneous nerve, which enters the
medial aspect of the muscle. -
Detach the long head of the biceps. To
obtain the greatest length of tendon, attach the distal, muscular
portion of the long head of the biceps to the conjoined tendon medially
to preserve elbow flexion and supination strength. Then dissect the
proximal portion of the long head of biceps tendon in the biceps groove
and rotator interval. It is important to direct the biceps tendon in
the groove in order to pull the humeral head proximally and medially
during final tensioning of the graft and reduction of the glenohumeral
joint (Fig. 67.11). -
Use a burr to drill a hole in the humeral
neck in an anterior to posterior direction, just distal to the biceps
groove. Burr a second hole in the proximal portion of the biceps
groove, between the greater and lesser tuberosities. Use an angled
curet to connect the two holes, forming a bony tunnel in the proximal
humerus. Place a braided, nonabsorbable traction suture in the distal
portion of the biceps tendon. Use a wire loop to pass the tendon from
proximal to distal through the tunnel. -
Position the arm with the humeral head
reduced in the glenoid and the arm in 30° of internal rotation. Using
the suture, attach the distal tendon back to the proximal tendon, thus
creating a suspensory loop of biceps tendon. Protect the repair in a
sling for three months to allow bone-to-tendon healing.
spastic abduction posturing. The deformity is usually dynamic, becoming
more prominent with ambulation, transfers, or other attempted
activities (Fig. 67.12). The affected arm is
held in an abducted posture, making balance while ambulating difficult.
Patients complain that their balance is thrown off because of bumping
into furniture, doorways, and people in crowds. Diagnosis requires
examination of the patient at rest and during a variety of activities.
It is also helpful to elicit from caretakers or family members any
history of activities that trigger this posture. Use dynamic EMG to
confirm that spasticity of the supraspinatus muscle is causing the
deformity.
 |
|
Figure 67.12.
Dynamic EMG of shoulder muscles in a patient with spastic abduction of the shoulder. Although standing causes a mild increase in activity of the deltoid muscle, the supraspinatus exhibits severe spasticity and is the cause of the dynamic abduction deformity. |
by means of a slide procedure. This procedure has been used
successfully to correct spastic abduction deformity.
-
Place the patient in the lateral
decubitus position, with the affected extremity uppermost. To protect
the brachial plexus, place an axillary roll in the unaffected axilla
and ensure that all bony prominences are well padded. -
Make a 10 to 15 cm incision parallel to the scapular spine.
-
Detach the trapezius insertion from the spine of the scapula, leaving a cuff of fascia for later reattachment (Fig. 67.13).
![]() Figure 67.13. The supraspinatus slide procedure for spastic abduction of the shoulder.
Figure 67.13. The supraspinatus slide procedure for spastic abduction of the shoulder. -
Retract the deltoid laterally. Using a
small periosteal elevator, elevate the origin of the supraspinatus
subperiosteally from the medial border of the scapula. Continue the
dissection laterally, taking care to avoid injury to the neurovascular
pedicle at the suprascapular notch. Allow the muscle to slide laterally. -
Reattach the trapezius to the scapular
spine. The remainder of the closure is performed in routine fashion.
Allow the patient full, unrestricted postoperative motion.
wall, and shoulder internal rotation causes the forearm to lie against
the middle of the chest. The tendon of pectoralis major is often
prominent when the examiner attempts to abduct and externally rotate
the shoulder but other muscles contribute to the deformity. The
glenohumeral joint normally functions as a universal joint, enabling
the hand to reach an almost spherical volume of locations in
three-dimensional space. When patients attempt to reach forward,
spastic adductors and internal rotators can severely restrict
acquisition of targets in the environment and on the body. The
patient’s ability to stabilize, push, or apply force to an object is
also compromised. From the perspective of passive function goals such
as skin care and axillary hygiene, spastic adductors and internal
rotators hinder efforts of caregivers to gain access to the axilla to
provide needed care. Restricted motion may impair dressing, washing,
and bathing, and promote skin irritation and maceration. Passive
manipulation of the shoulder during personal care may cause pain and
trigger spastic resistance in reactive muscles.
internal rotation dysfunction of the shoulder include latissimus dorsi,
teres major, the clavicular and sternal heads of pectoralis major, and
subscapularis. Involvement of latissimus dorsi and teres major should
be considered when hyperextension posturing of the shoulder is
observed. Antagonistic activity in these muscles may be masking a
patient’s potential for active flexion. Diagnostic lidocaine block to
the thoracodorsal nerve or the lower subscapular nerve may unmask that
voluntary potential. When the pectoralis major is chronically spastic,
the musculotendinous insertion of pectoralis major is prominent and
tight. However, the two heads of this muscle may be differentially
spastic, and EMG recordings from each or diagnostic
lidocaine
blocks to medial and lateral pectoral nerves may help to distinguish
whether one or both heads are pathophysiologically active. Release of
all four muscles may be required to relieve the deformity in a
nonfunctional extremity (31,104,126).
In patients who have evidence of underlying control of muscle function
despite the presence of dyssynergy, the pectoralis major, latissimus
dorsi, and teres major muscles can be fractionally lengthened at their
muscle tendon junctions. Alternately, the teres major muscle can be
partially released from its origin on the scapula and allowed to slide
distally in the same manor as the supraspinatus slide.
-
Place the patient in a slight beach chair
position with a small bolster under the scapula. Prep and drape the arm
so that the entire arm and shoulder are accessible. -
Make an incision on the anterior shoulder beginning at the coracoid process and extending distally for approximately 7 cm.
-
Identify the tendinous insertion of the pectoralis major tendon and use electrocautery to release it near its insertion (Fig. 67.14).
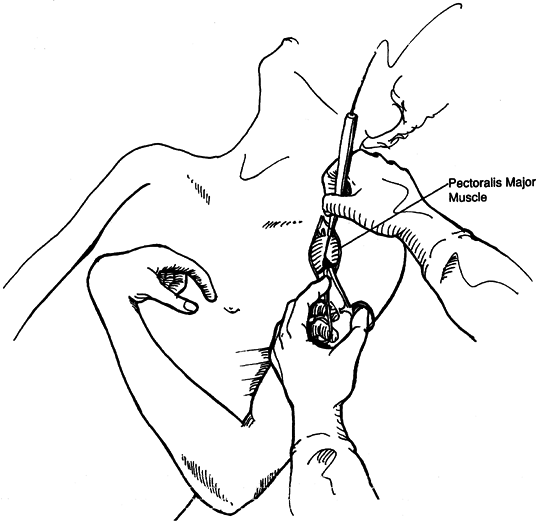 Figure 67.14.
Figure 67.14.
Anterior shoulder release for internal rotation and adduction
deformity. Release of pectoralis major muscle with electrocautery.
(Reprinted with permission from Keenan MA, Kozin SH, Berlet AC. Manual of Orthopaedic Surgery for Spasticity. New York: Raven Press, 1993.) -
Expose the subscapularis and isolate it
from the shoulder capsule near its insertion on the humerus. Release
the subscapularis muscle without violating the glenohumeral joint
capsule (Fig. 67.15).![]() Figure 67.15.
Figure 67.15.
Anterior shoulder release for internal rotation and adduction
deformity. Isolation and release of subscapularis muscle. (Reprinted
with permission from Keenan MA, Kozin SH, Berlet AC. Manual of Orthopaedic Surgery for Spasticity. New York: Raven Press, 1993.) -
Do not open the joint capsule or instability, or intra-articular adhesions may result.
-
After identifying the latissimus dorsi
and teres major muscles through the interval between the short head of
the biceps and deltoid musculature, release these deforming muscles (Fig. 67.15). -
Place a drain deep into the wound prior to closure.
-
Postoperatively, institute an aggressive
mobilization program following skin healing and employ gentle
range-of-motion exercises to correct any remaining contracture. Careful
positioning of the limb in abduction and external rotation is necessary
for several months to prevent recurrence.
-
Place the patient in a 45° lateral position. Prep and drape the arm so that the entire arm and shoulder are accessible.
-
Make an incision on the anterior shoulder
directly over the palpable insertion of the pectoralis on the humerus.
Retract the pectoralis major muscle upwards to visualize the muscle
tendon junction on its undersurface near its insertion on the humerus.
Sharply transect the tendinous fibers as they overlie the muscle belly.
This allows the tendon to slide distally on the muscle belly while
maintaining the integrity of the muscle tendon unit. -
Make a second incision over the posterior
axillary fold and identify the lattissimus dorsi and teres major
muscles at their insertion’s on the humerus. Again, sharply transect
the fibers of the tendons overlying the muscle bellies. -
Close the wounds in a routine fashion.
-
Postoperatively, employ a sling for
comfort for 2 to 3 weeks, and begin gentle active assisted
range-of-motion exercises on the first postoperative day. Further
lengthening of the muscles will occur with functional use of the arm
during the next several weeks.
elbow flexors of the upper limb. In the patient without motor control,
severe flexion posturing can lead to skin maceration in the antecubital
fossa, malodor, and skin breakdown. In reality, a continuum of
volitional control is seen. Many patients complain that their elbows
persistently “ride up” when they stand up and walk. They also complain
that their flexed elbow hooks door frames and other people, and that
putting on a shirt or jacket is a struggle. Kinesiologically, the elbow
lengthens and shortens the upper extremity. Consequently, active
dysfunction is characterized by impaired reaching for objects in the
environment, placing them elsewhere or bringing them to the body.
elbow control. Smooth control of elbow flexion and extension is
frequently impaired. The usual clinical picture is one of cogwheel
motion on attempted extension of the elbow. Elbow extension range is
often limited with a very prolonged period of extension. Elbow flexion
is relatively normal. Laboratory examination using dynamic EMG helps to
confirm the presence of volitional capacity as well as dyssynergy
during movement for each of the elbow flexors. Obtain dynamic
recordings from biceps, brachialis, brachioradialis, lateral, medial
and long head of the triceps. Dynamic EMG combined with
electrogoniometric measurement of elbow motion of stroke and TBI
patients has revealed a consistent pattern of muscle activity
responsible for this clinical picture (112,116).
The pattern most commonly seen is that all three heads of the triceps
muscle are operating in a normal phasic pattern. The brachioradialis
muscle most frequently shows continuous spastic activity. One or both
heads of the biceps muscle are also spastic. Less spasticity is
observed in the brachialis muscle. This pattern of muscle activity is
also common in patients with cerebral palsy. Armed with this
information, a rational surgical plan can be devised to improve elbow
control.
whenever possible in spastic muscles. This allows the underlying tone
and strength of the muscle to determine the amount of lengthening
rather than having the surgeon estimate this elusive quantity.
Lengthening over the muscle belly eliminates the need for suturing, and
this diminishes the amount of scarring that occurs. A new tendon
reforms and fills in the gap within several months. The fractional
lengthening technique allows the patient to begin gentle active motion
immediately after surgery because the muscle tendon unit remains
intact. In contrast, a Z-lengthening technique requires immobilization
for a minimum of 4 weeks to allow healing of the relatively avascular
tendon and prevent inadvertent rupture.
spastic brachioradialis. Surgical lengthening of the brachioradialis
can be used if volitional control is demonstrated on EMG. If little or
no control is demonstrated, release of the severely spastic
brachioradialis muscle at the level of the elbow may be performed.
Lengthening of the spastic biceps and brachialis muscles as indicated
by dynamic EMG is also performed to improve elbow motion and hand
placement. Meals has advocated neurectomy of the radial nerve branches
to the brachioradialis (151). This technique would not correct an established contracture of the brachioradialis.
-
Beginning with the biceps, make a
longitudinal incision over the anterior arm starting just distal to the
lower edge of the pectoralis major tendon and extending distally 5 cm. -
Close the incision in a routine fashion.
-
Apply a sterile tourniquet to the upper arm and inflate after exsanguination of the limb.
-
Make a curved incision on the volar
aspect of the elbow in the antecubital space. Begin the incision over
the lateral border of the biceps muscle, pass medially across the
antecubital crease and gently curve distally onto the anteromedial
aspect of the forearm. -
Dissection develops the internervous
interval between the brachioradialis and the biceps musculature.
Identify and protect the radial nerve. If the brachioradialis has been
demonstrated by dynamic EMG to be spastic and without any functional
capacity, use electrocautery to transect it through its muscle belly
proximally. -
Divide the lacertus fibrosis and expose
the biceps tendon. Retract the biceps tendon to expose the underlying
brachialis muscle. Sharply transect the broad band of tendinous fibers
on the anterior surface of the brachialis muscle, leaving the
underlying muscle tissue intact. Then extend the elbow, fractionally
lengthening the brachialis by approximately 1 cm (Fig. 67.16). Figure 67.16. Fractional lengthening of the long and short heads of the biceps at their proximal musculotendinous junction.
Figure 67.16. Fractional lengthening of the long and short heads of the biceps at their proximal musculotendinous junction. -
In the presence of a severe rigid
contracture, it may be necessary to perform a Z-lengthening of the
biceps tendon. Make a Z-step cut for the entire length of the biceps
tendon. Then suture the ends of the biceps tendon in a lengthened
position using a nonabsorbable suture. -
Close the subcutaneous tissues and skin edges over a drain.
-
In patients who have volitional function in the brachioradialis, fractionally lengthen the muscle at the myotendinous junction.
-
Make a separate 3 cm longitudinal
incision on the radial aspect of the forearm to identify the
myotendinous junction of the brachioradialis. Roll the muscle, exposing
its deep surface. Here the tendon extends more proximally. Sharply
transect the tendinous fibers, allowing the brachioradialis to lengthen. -
If preoperative nerve conduction studies
have shown an ulnar neuropathy at the level of the elbow, perform
anterior transposition of the ulnar nerve. -
Close the skin incision over a drain in routine manner.
-
When the biceps has been lengthened
proximally, no immobilization is needed. Remove the drain within the
first 24 hours following surgery and begin active range-of-motion
exercises. Allow no resistive exercises for 3 weeks to prevent
overlengthening of the muscles with resulting weakness. If a
Z-lengthening of the biceps tendon was done distally, place the patient
in a posterior splint with the elbow in 90° of flexion for 4 weeks to
protect the biceps tendon repair. Functional elbow flexor lengthening
has significantly enhanced the fluid control of elbow motion and
improved hand placement in properly selected patients.
myostatic contracture and flexion deformity of the elbow. This results
in skin maceration and breakdown of the antecubital space (115,116,126).
This position of severe elbow flexion also predisposes the ulnar nerve
to an acquired compression neuropathy by increasing the vulnerability
to direct pressure and decreasing the cross sectional area of the
cubital tunnel (113,214).
Perform surgical release of the biceps tendon and brachioradialis
muscle combined with lengthening or release of the brachialis,
depending on the severity of the contracture. The joint capsule is
generally not released. Gradual extension of the elbow with serial
casting or physical therapy corrects the preoperative deformity and
decreases the ulnar nerve compression. Anterior transposition of the
ulnar nerve may be necessary to improve ulnar nerve function further.
-
Make a curved incision on the volar
aspect of the elbow in the antecubital space. Begin the incision over
the lateral border of the biceps muscle, pass medially across the
antecubital crease, and gently curve distally onto the anteromedial
aspect of the forearm. When the contracture is severe and long
standing, use a straight longitudinal incision to facilitate wound
closure. -
Dissection develops the interval between the brachioradialis and the biceps musculature. Identify and protect the radial nerve.
-
Using electrocautery, transect the brachioradialis through its muscle belly proximally (Fig. 67.17). Transect the biceps tendon and lacertus fibrosis at the level of the elbow.
![]() Figure 67.17.
Figure 67.17.
Release of a nonfunctional elbow flexion contracture. The biceps tendon
is transected. The brachioradialis muscle belly is divided proximally
using electrocautery. The brachialis muscle is fractionally lengthened.
(With persmission from from Keenan MA, Kozin SH, Berlet, AC. Manual of Orthopaedic Surgery for Spasticity. New York: Raven Press, 1993.) -
When the elbow flexion contracture is
severe and has been present for many years, it may be necessary to
completely transect the brachialis muscle. -
Avoid an anterior capsulectomy because of the associated increased stiffness and intra-articular adhesions postoperatively.
-
If preoperative nerve conduction studies
have shown an ulnar neuropathy at the level of the elbow, perform an
anterior transposition of the ulnar nerve. -
Close the skin incision over a drain in
routine manner. Approximately 50% correction of the deformity can be
expected at surgery without causing excessive tension on the contracted
neurovascular structures. Serial casting or drop out casts can be used
to obtain further correction over the ensuing weeks (11,70,103,108,133).
spastic flexion. These patients have frequently had a brain stem
infarct or injury. They complain of difficulty reaching their face for
activities of daily living.
limited because it is an uncommon problem. When needed, however, good
results have been reported with surgical lengthening (187).
A V-Y triceps plasty allows improved flexion range of motion, at the
cost of decreased extension power and extensor lag. Use this procedure
with caution in patients who rely on their arms to assist with
ambulation or transfers because strength is lost with any lengthening
procedure.
patients for a number of reasons. Prolonged elbow flexion with traction
on the nerve can lead to decreased volume of the cubital tunnel,
resulting in nerve compression. Support of the torso by leaning on a
chronically flexed elbow may result in direct compression of the nerve.
HO, particularly when it is in a posterior location, may involve the
ulnar nerve causing a neuropathy. The patients are often limited in
their ability to complain about ulnar nerve symptoms because of limited
cognitive and communicative abilities. The diagnosis is usually
suspected because of intrinsic atrophy, and it is confirmed using nerve
conduction studies. A 2.5% incidence has been shown in patients with
TBI at a large brain injury referral center (113,214).
same time as elbow flexor lengthenings, flexor releases, or resection
of HO. Subcutaneous transposition is preferred to avoid stimulation of
HO formation.
associated with elbow spasticity, wrist spasticity, or both. Pronation
deformities are much more common. These deformities are most often
treated together with the associated deformities. They seldom require
treatment individually.
makes it difficult for a person to reach for a target underhand,
whereas supination deformity impairs reaching for targets that require
overhand reach. Many activities of daily living depend on active
supination. The use of feeding and grooming utensils and clothes
fasteners becomes problematic when spastic or contracted pronators
restrict supination. Physical examination reveals a fully pronated
resting position of the forearm. When passive supination range of
motion exceeds active supination range, the possibility of pronator
muscle dyssynergy during active supination should be suspected. Muscles
that potentially contribute include pronator teres and pronator
quadratus. Dynamic EMG studies of pronator teres, pronator quadratus,
and biceps greatly augment clinical examination. Clinical examination
does not easily predict which of the pronators might be retaining
volitional capacity and which might be spastic. Both pronator muscles
may show varying degrees of volition and spasticity. Interestingly,
flexor spasticity of the powerful biceps often coexists with a pronated
forearm deformity.
botulinum toxin may be injected into either or both pronators,
depending on clinical and laboratory analyses. In the period of
residual deficits, surgical lengthening of pronator teres and pronator
quadratus may be performed depending on their individual voluntary
capacities and the clinical goal is to improve active supination
function by reducing pronator dyssynergy. The possibility of
lengthening the pronator teres has long been recognized, but fractional
lengthening of the pronator quadratus is of recent vintage. Release of
the flexor-pronator origin was previously a common operation. We do not
advocate this procedure because it does not target the individual
muscles responsible for deformities. When an excessive amount of the
pronator teres origin was released or when there was no function in the
pronator quadratus muscle, an iatrogenic supination deformity occurred.
The supination deformity was much less functional than a pronated
forearm. When the pronators are contracted and not active volitionally,
muscle releases may be considered, although passive functional
advantages, other than cosmesis, may be difficult to come by.
-
Approach the pronator teres in the
interval between the mobile wad and the flexor carpi radialis in the
midforearm. Take care to preserve the superficial radial nerve and
radial artery that occupy this interval. -
Identify the pronator teres as it inserts
on the radius. Perform myotendinous lengthening by cutting the
tendinous fibers of the musculotendinous junction and allowing the
tendon fibers to slide on the muscle belly, thereby lengthening the
muscle tendon unit. -
When dynamic EMG has demonstrated that
the pronator is spastic but does not have any volitional activity,
release the pronator from its insertion on the radius. In either case,
take care not to extend the dissection proximally into the supinator to
avoid injury to the posterior interosseus nerve. -
Approach the pronator quadratus via an incision over the volar aspect of the forearm just proximal to the wrist crease.
-
Retract the finger flexor tendons
radially to expose the broad myotendinous junction of the pronator
quadratus. Transect the tendon fibers, leaving the underlying muscle
fibers intact. Then supinate the arm, separating the ends of the tendon
fibers and lengthening the pronator quadratus (Fig. 67.18).
When the pronator quadratus is contracted and does not have any
functional capacity, transect to eliminate it as a deforming force.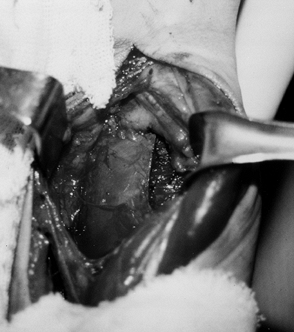 Figure 67.18.
Figure 67.18.
Intraoperative photograph showing pronator quadratus myotendinous
lengthening. The arrow indicates the cut edge of the pronator quadratus
tendon. (With permission from Hisey MS, Keenan MAE. Orthopaedic
Management of Upper Extremity Dysfunction Following Stroke or Brain
Injury. In Green DP, Hotchkiss RN, Pederson WC, eds. Operative Hand Surgery, 4th ed. New York: Churchill Livingstone, 1998:287.)
also associated with elbow flexion deformities. Most often, this
deformity is seen as a complication of the surgical flexor-pronator
origin release. The biceps, supinator, or both may cause supination
deformity. Physical examination supplemented by dynamic EMG may be used
to determine the relative contribution of each.
conjunction with correction of the elbow flexion deformity. As
described previously, in the functional extremity, perform a biceps
Z-lengthening. In a nonfunctional extremity, perform a distal biceps
release. Often, at the conclusion of this procedure, the arm is able to
achieve a functional range of pronation. If not, attention must be
turned to the supinator.
-
Lengthen the supinator by elevating the
insertion from its radial insertion. Use a standard anterior approach
to the elbow and proximal radius. An extension of the approach, as
noted for the elbow release, is generally used. -
Approach the supinator in the plane between the brachialis and the brachioradialis.
-
Ligate the radial recurrent artery to allow exposure of the supinator.
-
The existing supination deformity
facilitates exposure of the insertion of the supinator by pulling the
posterior interosseus nerve laterally. Nevertheless, take extreme care
must to visualize and preserve the nerve as it enters the arcade of
Frohse. -
Incise the broad insertion of the
supinator through the periosteum, exposing the shaft of the radius.
Then elevate the supinator in a subperiosteal plane. Be careful to
avoid excessive traction on the posterior interosseus nerve when
elevating the supinator. Also, keep the dissection in the subperiosteal
plane to avoid injury to the posterior interosseus nerve that comes in
contact with the radius in 25% of patients. After elevating, the
supinator, allow it slide. It will subsequently scar down in a
lengthened position. -
Close the wound in routine fashion.
deformity may also be seen. Patients complain of difficulty inserting
their hand into shirts, jackets, and other narrow openings and they
frequently have pain on passive motion. They may also have symptoms of
carpal tunnel syndrome secondary to compression of the median nerve
against the transverse carpal ligament by taut flexor tendons (170,214). In severe cases, wrist subluxation may be present. Radial or ulnar deviation and a clenched fist are often present as well.
include the flexor carpi radialis (FCR), flexor carpi ulnaris (FCU),
palmaris longus (PL), flexor digitorum sublimis (FDS), and flexor
digitorum profundus (FDP). Singly or in combination, these muscles may
have variable features of spasticity, contracture, and voluntary
control. Because they have a larger cross-sectional area, wrist flexor
muscles are generally stronger than their extensor counterparts.
Despite a net balance of forces favoring flexion, the extent to which a
patient may have voluntary control over wrist extensors should be
investigated. Dynamic EMG studies and temporary diagnostic motor point
blocks are helpful. When wrist hyperextension deformity is present,
wrist extensors are typically volitional and spastic, wrist flexors are
often poorly volitional and mildly spastic, and the fingers are tightly
clenched into the palm.
posture of the wrist. FCR, FCU, or both may bowstring across the wrist,
and radial or ulnar deviation suggests their respective involvement. A
clenched fist points to extrinsic finger flexors as having a role. If
fingernails dig into the palm, FDP is likely to be involved. If the
proximal interphalangeal (PIP) joint is markedly flexed but the distal
interphalangeal (DIP) joint is not, involvement of FDS is likely.
includes recordings from FCR, FCU, ECR, ECU, FDS, and FDP. Kinesiologic
study includes “isolated” muscle group testing along with whole limb
movements such as reaching, grasping, and releasing objects. In
addition, passive stretch of the wrist flexors and finger flexors
provides an indication of spastic reactivity as seen
electromyographically. Dynamic EMG findings are not easily predictable
from clinical examination. Some patients show extensive activation of
wrist extensors during extension and reaching efforts. Nevertheless,
the wrist remains flexed because tension in the wrist flexors and
finger flexors overbalances extensor forces. EMG activity in FCR is
often present during attempts at wrist extension, and activity in FDS
or FDP may be present. In patients with ulnar deviation, EMG activity
is typically seen in FCU on reaching effort, but “isolated” testing
also suggests that patients can often activate FCU voluntarily. Because
EMG activity is not correlated with force production, diagnostic nerve
blocks are often helpful in unmasking movement. Temporary chemical
“weakening” of a dyssynergic wrist flexor may unmask strength in the
wrist extensors sufficient to improve active wrist motion. A similar
hypothesis can be used for the extrinsic finger flexors after dynamic
EMG reveals whether the FDS or FDP is generating antagonistic activity
acting to restrain wrist extension. Motor point block of the target
muscle group or median or ulnar nerve blocks at the elbow may be
performed to examine for active wrist extension during reach. Combined
median and ulnar nerve blocks at or above the elbow also reveal the
presence of muscle contracture.
surgical treatment for flexed and hyperextended wrists are myotendinous
lengthenings. Selective muscle releases, wrist fusion, and proximal row
carpectomy can be considered in the presence of severe deformities.
Subtotal carpectomy
combined
with radio-carpal or radio-metacarpal fusion has been suggested for the
treatment of severe flexion contracture either not amenable to, or
refractory to, soft-tissue releases alone (185,189).
single-stage procedure consisting of superficialis-to-profundus
transfer, wrist flexor release, FPL lengthening, wrist arthrodesis,
carpal tunnel release, and ulnar motor branch neurectomy or intrinsic
release can provide comprehensive correction. Such a definitive
approach to severe deformities is associated with acceptable morbidity
and eliminates the possibility of recurrence or undercorrection from
untreated intrinsic pathology (189).
flexion deformities are treated during the physiologic recovery phase
by phenol motor point blocks (70,71 and 72).
Because of the large sensory components of both the median ulnar
nerves, perform motor point injections of the wrist and finger flexors
using surface stimulation to localize the motor points. The blocks can
be supplemented with functional electrical stimulation of the wrist and
finger extensor muscles, and by casting or splinting techniques.
Perform gentle passive range of motion of the wrist and fingers. When
motor control is present, a program of active exercise and functional
training is also employed. Electrical orthoses, which rely on
functional electrical stimulation (FES), are available and have been
shown to provide improvement in hand function in people with spinal
cord injury or stroke. These devices are still in their early stages of
development; however, technical advances in the field of FES provide
hope for more widespread applicability in the future.
perform fractional lengthening of the appropriate wrist flexors. This
is done in conjunction with lengthening of the extrinsic finger
flexors, when indicated.
-
Make a longitudinal incision on the volar
surface of the forearm. Extend this incision distally if a carpal
tunnel release is necessary. Divide the palmaris longus tendon if it is
tight. -
Perform myotendinous lengthening by transecting the tendinous portion overlying the myotendinous junction (Fig. 67.19).
![]() Figure 67.19. Intraoperative photograph showing myotendinous lengthening of the flexor carpi radialis (A).
Figure 67.19. Intraoperative photograph showing myotendinous lengthening of the flexor carpi radialis (A).
At reoperation, intraoperative photograph of a different patient shows
excellent healing of the myotendinous junction that had been lengthened
2 cm (B). (With permission from Hisey MS,
Keenan MAE. Orthopaedic Management of Upper Extremity Dysfunction
Following Stroke or Brain Injury. In Green DP, Hotchkiss RN, Pederson
WC, eds. Operative Hand Surgery, 4th ed. New York: Churchill Livingstone, 1998:287.) -
Do not use immobilization after surgery.
Begin a program of active exercise immediately. Don’t allow passive
stretching of the wrist for 3 weeks. Resistive exercises can be started
6 weeks after surgery.
little or no function seen in the hand, perform a release of the wrist
flexors. A proximal row carpectomy may be useful in patients with
severe, long-standing contracture to correct the deformity. Then
stabilize the wrist with a wrist fusion to eliminate the need for a
wrist orthosis after surgery. Splints tend to be lost by these patients
and their caretakers. Gravity alone can cause a recurrence of the
flexion deformity. Unopposed
wrist
or finger extensor tone can result in a hyperextension deformity.
Because the median nerve is compressed against the proximal transverse
carpal ligament causing a painful neuropathy, perform a carpal tunnel
release as well.
-
Position the patient supine and apply an arm tourniquet.
-
Make a volar forearm incision extending to the transverse carpal ligament.
-
Identify and transect the tendons of the
palmaris longus, flexor carpi radialis, and flexor carpi ulnaris. Take
care to protect the radial and ulnar artery as well as the median,
radial, and ulnar nerves. -
Perform carpal tunnel release.
-
Close the volar incision in routine fashion.
-
If wrist fusion is to be performed,
supinate the forearm and make a longitudinal dorsal incision exposing
the dorsal wrist joint. Remove Lister’s tubercle. -
Perform capsulotomy of the radiocarpal, midcarpal, and third carpal-metacarpal joints.
-
Using a high-speed burr, remove the
articular cartilage and hard subchondral bone of the radiolunate,
radioscaphoid, scaphocapitate, lunocapitate, and capitate-third
metacarpal joints. Hyperflex the wrist to facilitate exposure. -
Position the wrist in 15° of extension,
with the third metacarpal in line with the shaft of the radius. If this
position is achieved only with excessive tension on the volar tissues,
perform proximal row carpectomy. -
Graft the denuded surfaces with local autologous bone with or without allograft.
-
Stabilize the wrist using a prebent
titanium wrist fusion plate fixed distally to the third metacarpal with
2.7 mm screws and proximally to the radius with 3.5 mm screws (Fig. 67.20) (115,185,189).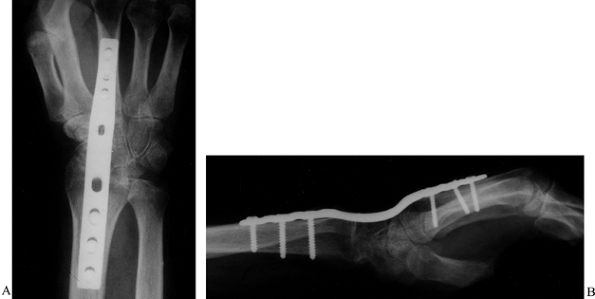 Figure 67.20.
Figure 67.20.
Postoperative AP and lateral radiographs showing a dorsally placed
wrist fusion plate. (With permission from Hisey MS, Keenan MAE.
Orthopaedic Management of Upper Extremity Dysfunction Following Stroke
or Brain Injury. In Green DP, Hotchkiss RN, Pederson WC, eds. Operative Hand Surgery, 4th ed. New York: Churchill Livingstone, 1998:287.) -
Close in a routine manner. Place the
wrist in a well-padded splint or cast for 3 weeks. Remove the cast and
sutures, and apply a splint for the following 3 weeks.
and may prevent release in patients with poor digital extension. Median
nerve compression may also be caused by prolonged extension. If median
nerve compression is diagnosed, perform carpal tunnel release.
-
When volitional control has been
demonstrated in the dyssynergic wrist extensors, perform myotendinous
lengthening of the extensor carpi ulnaris through a short longitudinal
incision on the ulnar border of the forearm. Identify the myotendinous
junction and transect the tendinous portion, allowing the muscle to
stretch. -
Close in routine fashion. Begin active motion immediately after surgery.
-
When no volitional activity is seen in
the wrist extensor muscle by clinical examination and dynamic EMG,
perform tendon release with wrist fusion with or without proximal row
carpectomy. -
Make a midline dorsal incision.
-
Dissect medially, and laterally expose
the distal extensor carpi radialis longus and brevis and the distal
extensor carpi ulnaris. Transect these tendons proximal to their
insertions. -
Perform wrist fusion with or without proximal row carpectomy, as described above.
extremities have sufficient volitional control of the muscles to allow
surgical procedures aimed at restoring function to the hand. Often,
severe deformities are present, but there is insufficient or no
volitional activity in the muscles. In these cases, perform contracture
releases to decrease pain, improve position and cosmesis of the hand,
and to ease basic skin care and hygiene. The criterion for determining
which procedures are most appropriate are summarized in Table 67.10.
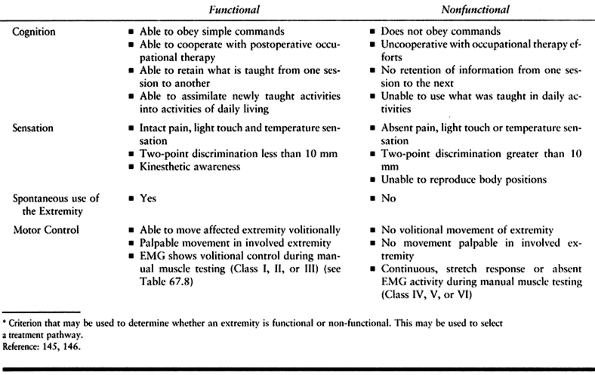 |
|
Table 67.10. Criterion For Functional vs. Nonfunctional Procedures*
|
injury or stroke involving the upper extremity. This pattern results
from unmasking of the primitive grasp reflex. The fingers are typically
clasped into the palm. Fingernails may dig into palmar skin, and access
to the palm for washing may be compromised. When access is chronically
restricted, skin maceration, breakdown, and malodor occurs. Patients
may complain of pain when they or their caregivers attempt to pry the
fingers open in order to gain palmar access. Some relaxation of finger
tightness may occur if the wrist is positioned in extreme flexion. The
deformity, however, is often accompanied by wrist flexion as well.
amount of tone present in the finger flexors. First, establish passive
range of motion. Following this, ask the patient to open and close the
fingers and to flex and extend the wrist. If no active wrist or finger
extension is seen, it is still important to assess whether there
appears to be active control of finger flexion. In the continuum of
neurologic impairment and recovery, control of wrist and finger flexion
is seen before active control of extension. Place a finger in the
patient’s palm and ask the patient to grasp. Often, an increase in the
pressure of grasp can be felt indicating underlying muscle control.
the antecubital space to eliminate flexor tone temporarily. A block of
the ulnar nerve in the cubital canal can supplement relaxation. With
the flexor muscles relaxed, the activity of the extensor muscles can be
more accurately evaluated. When extensor control returns, it is
generally seen in the extensor indicis proprius muscle first (105,117,118,120).
include the FDS and FDP. If the PIP joints flex while the DIP joints
remain extended, spasticity of FDS rather than FDP may be suspected.
Dynamic EMG studies have shown that the flexor digitorum superficialis
muscles exhibit a marked degree of spasticity, whereas the flexor
digitorum profundus muscles are often normal or minimally spastic (105,117,118,120).
Despite the marked increase in tone, it often has some underlying
volitional control. The flexor profundus has less spasticity and better
volitional control. Volitional control of the finger extensors is
present in 50% of patients with spastic flexion deformities.
extrinsics, but an intrinsic plus posture (i.e. combined MCP flexion
and PIP extension) is not seen because spastic extrinsic flexors
dominate by flexing the PIP joints. Some degree of contracture of the
extrinsics is typical of the chronically clenched fist. From the
perspective of active functional potential, some degree of volitional
control may also be present in either or both sets of extrinsic finger
flexors. Spastic finger flexors may override and mask the patient’s
potential to extend the fingers. Sometimes, a patient presents with
spasticity in just one or two muscle slips of either FDP or FDS. For
example, we have seen a number of cases of index finger flexion traced
to a spastic FDP muscle slip for that finger alone.
function, a variety of orthopaedic options are available. When
volitional control is demonstrated in the extrinsic flexor muscles by
dynamic EMG, the fractional lengthening is indicated. In a hand with
skin maceration and malodor from a clenched fist deformity in which no
volitional movement is detected, more significant lengthening of the
flexor tendons is required. In this situation, perform a
superficialis-to-profundus (STP) tendon transfer.
-
Make a longitudinal incision on the volar surface of the forearm, commonly at the same sitting with wrist flexor lengthenings (Fig. 67.19).
-
Divide the palmaris longus tendon if it
is tight. Perform the lengthening of the individual FDS and FDP tendons
by sharply incising the tendon fibers as they overlie the muscle belly
at the musculotendinous junction, allowing the tendon to slide distally. -
Lengthen the flexor pollicis longus
tendon in an identical manner. This technique allows the tendons to
lengthen with minimal scarring. By transecting the tendon over the
muscle belly, no sutures are needed. This eliminates scarring from
foreign body reaction to suture material. The underlying support and
vascularity of the muscle provides an optimal environment for the
tendons to heal and reconstitute themselves. -
Do not immobilize postoperatively. Begin
the patient on a program of active and active assisted exercises on the
first postoperative day. If the patient has significant pain or
spasticity, a short volar splint can be used for comfort. Remove the
splint for exercise. In patients with limited motor control, it is
often useful to use the splint to position the wrist while the patient
exercises the fingers. This immediate active motion allows the flexor
tendons to continue to lengthen in the postoperative period as
necessary. -
Ultimately, the amount of flexor
lengthening is determined for each individual muscle by its underlying
tone and control rather than by the surgeon’s “educated guess” of tone
while the patient is under anesthesia. By using this technique, we have
had marked improvement in functional results when compared with our
previous regimen of postoperative immobilization. In cases in which the
motor control is very limited, extrinsic finger flexor lengthening can
be combined with wrist fusion.
clenched fist deformity in which no volitional movement is detected,
more significant lengthening of the flexor tendons is required. In this
situation, an STP tendon transfer is performed (21,30,114,115,189) (Fig. 21).
This provides a more cosmetically pleasing hand position, aids in
hygiene by getting the fingers out of the palms, and provides, at best,
a mass action grasp pattern and at least a passive restraint to
extension.
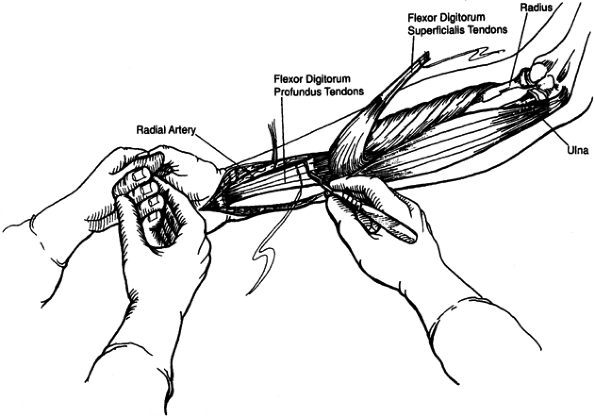 |
|
Figure 67.21.
Superficialis-to-profundus tendon transfer: The four superficialis tendons are sutured together distally and then transected for transfer. The profundus tendons are sutured together proximally and then cut. The fingers are extended, and the distal end of the superficialis tendons are then sutured en masse to the proximal end of the profundus tendons. (Reprinted with permission from Keenan MA, Kozin SH, Berlet, AC. Manual of Orthopaedic Surgery for Spasticity. New York: Raven Press, 1993.) |
-
Perform the STP tendon transfer through a
longitudinal palmar incision that may be extended distally to allow
release of the carpal tunnel and access to Guyon’s canal. -
Identify and transect the palmaris longus
tendon. Suture together the four superficialis tendons distally and
then transect for the en masse transfer (21,114,115,189). -
Suture together the profundus tendons
proximally and then cut. Extend the fingers and suture the distal end
of the superficialis tendons en masse to the proximal end of the
profundus tendons.
performed in combination with the STP tendon transfer to treat the
concurrent deformities. A neurectomy of the motor branch of the ulnar
nerve is needed to prevent an intrinsic plus deformity from developing (21,114,115,122,189).
If an intrinsic contracture is seen at the time of surgery following
the STP lengthening, perform a release of the intrinsics.
-
Use a carpal tunnel release to decompress the median nerve.
-
To prevent a recurrent wrist flexion
deformity from occurring secondary to passive wrist flexion, stabilize
the wrist. Wrist extensor tenodesis has been attempted but often will
stretch out with time. -
A cock-up wrist splint can be worn, but patient compliance is poor.
-
A fusion of the wrist in 15° of extension
provides the most reliable means of maintaining hand position and is
now routinely performed. The surgical technique has been described
earlier. A proximal release of the thenar muscles is often needed to
correct a thumb-in-palm deformity (19,48,104,143,144,189,193,199,204,220). -
Because the STP transfer and wrist fusion
are extensive surgery, we prefer to perform the thenar slide procedure
at a later time, if it is indicated. -
Postoperatively, immobilize the wrist for
three weeks in a short arm splint that includes the fingers and thumb.
Hold the wrist in 15° of extension and immobilize the fingers with the
metacarpophalangeal joints in 60° of flexion and the interphalangeal
joints extended. Begin gentle range of motion of the
metacarpophalangeal joints after cast removal. Use a volar wrist splint
until the wrist fusion is healed.
including the flexor pollicis longus muscle and the median and ulnar innervated thenar muscles (19,48,96,104,143,144,189,193,199,204,220).
The thumb is held within the palm, the DIP joint of the thumb is
commonly flexed, and the thumb is unable to function during key grasp
or in three-jaw chuck grasp (i.e., in opposition to the pads of the
index and third fingers). In addition, skin maceration and breakdown
can occur if proper hygiene is prevented.
indicated by flexion of the interphalangeal joint. Some patients may be
able to extend the thumb if the wrist is flexed, suggesting that a
spastic flexor pollicis longus (FPL) may be impeding active thumb
extension when the wrist is more extended and FPL is tighter. The
thumb-in-palm deformity may result from spastic activity in FPL,
adductor pollicis (AP), or the thenar muscles, particularly flexor
pollicis brevis. Adduction of the thumb metacarpal indicates spasticity
of the AP muscle and possibly the first dorsal interosseous muscle. A
quick stretch of the thumb into abduction often elicits a clonic
response. An anesthetic block of the ulnar nerve in Guyon’s canal at
the wrist temporarily eliminates intrinsic tone. This will demonstrate
the presence of any myostatic contractures and will also confirm that
the AP was an offending muscle in the deformity. Contracture of the
skin of the web space and interphalangeal joint contracture of the
thumb may also develop over time. If some volitional potential in thumb
extensors or thumb abductors is present, lengthening of the spastic FPL
and AP will facilitate key grasp. Dynamic EMG and lidocaine blocks are
helpful to elucidate the specifics of motor control.
function, orthopaedic treatment consists of fractional lengthening of
the FPL at the myotendinous junction combined with a thenar muscle
slide, in which the origins of the thenar muscles are detached from the
transverse palmar ligament while preserving the neurovascular pedicle.
Fractional lengthening of the FPL at the myotendinous junction will
improve thumb extension. This is generally performed in conjunction
with wrist or digital flexor lengthening. In order to provide a
functional lateral pinch, it is desirable to stabilize the
interphalangeal joint of the thumb. This may be done using a Moberg
screw or a Herbert screw (Fig. 67.22).
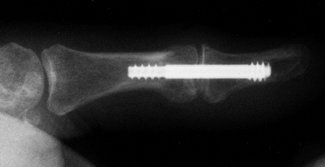 |
|
Figure 67.22.
Radiograph showing a Herbert screw that has been placed across the thumb interphalangeal (IP) joint in a thumb IP fusion. (With permission from Hisey MS, Keenan MAE. Orthopaedic Management of Upper Extremity Dysfunction Following Stroke or Brain Injury. In Green DP, Hotchkiss RN, Pederson WC, eds. Operative Hand Surgery, 4th ed. New York: Churchill Livingstone, 1998:287.) |
-
Make a stab incision on the tip of the
thumb pulp, and under fluoroscopic guidance, pass the pilot hole for
the through the tuft and shaft of the distal phalanx, across the joint,
and into the proximal phalanx. -
Advance the screw through this hole,
compressing the articular surfaces. In this patient group, we have not
found it necessary to denude the interphalangeal joint surfaces. -
Protect the thumb in a thumb spica splint or cast for 3 weeks.
Generally, all of the thenar muscles are spastic or contracted, and a
proximal myotomy is required to reposition the thumb and decrease the
underlying tone in order to improve pinch function. Avoid distal
releases because these often result in a hyperextension deformity of
the metacarpophalangeal joint of the thumb (19).
rerouting procedure in combination with intrinsic muscle releases has
been suggested for the treatment of severe deformities. In this method,
the EPL tendon is transected proximal to Lister’s tubercle. The distal
stump is passed through the first compartment tunnel and is sutured to
the proximal EPL stump. For mild deformities the EPL, rerouting may be
performed alone (193).
-
Under tourniquet control, make an incision along the thenar crease on the palm.
-
Protect the neurovascular structures and flexor tendons ulnarly (Fig. 67.23).
 Figure 67.23. Proximal myotomy of the thenar muscles used to correct a spastic thumb-in-palm deformity.
Figure 67.23. Proximal myotomy of the thenar muscles used to correct a spastic thumb-in-palm deformity. -
Detach the origins of the flexor pollicis
brevis, opponens pollicis, and AP muscles from their origins while
protecting the recurrent branch of the median nerve. -
Preserve the transverse carpal ligament; do not enter the carpal canal.
-
Extend the thumb, allowing the released
muscles to slide radially and reattach in an improved position, thereby
preserving function and preventing a hyperextension deformity. -
Release the origin of the AP muscle from
the third metacarpal. Carefully dissect to retract the digital
neurovascular bundles and flexor tendons to the index and long digits. -
Identify the deep palmar vascular arch
and deep branch of the ulnar nerve as they penetrate the AP muscle
between its oblique and transverse heads before adductor muscle
release. Preserve the neurovascular supply of the adductor pollicis. -
If the first dorsal interosseous muscle
is contracted, perform a release through a dorsal incision along the
ulnar margin of the thumb metacarpal while protecting the radial
sensory nerve. Release the origin of the first dorsal interosseus from
its origin on the base of the first metacarpal. -
In persistent web space contractures despite appropriate muscle releases, perform a Z-plasty of the thumb web space.
-
Postoperatively, immobilize the patient
in a thumb spica splint for 3 weeks. Initiate active therapy a few days
after surgery. The splint is removed for therapy but used at other
times to position the thumb.
However, intrinsic spasticity and contracture are frequently masked by
the presence of extrinsic flexor spasticity or contracture. Extension
of the fingers at the metacarpophalangeal joints may be blocked by
spasticity of the interossei and lumbrical muscles of the hand. Another
manifestation of intrinsic spasticity is the tendency to swan-neck or
boutonnière positioning of the fingers. When a release or tendon
lengthening of the spastic extrinsic flexor muscles has already been
done, an intrinsic positive deformity of the hand is unmasked. These
hand deformities can be painful and disfiguring. Such contractures
often lead to maceration of the palmar skin and recurrent nail bed
infections from poor hygiene.
can be demonstrated by comparing the amount of proximal interphalangeal
joint flexion obtained with the metacarpophalangeal joints, both flexed
and extended. If there is less proximal interphalangeal joint flexion
with metacarpophalangeal joint extension, then the intrinsic tendons
are tight. Perform this test both before and after a lidocaine block of
the ulnar nerve at the wrist in order to distinguish between intrinsic
tone and contracture.
intrinsic spasticity. They result from a combination of intrinsic
spasticity combined with FDS tone. Swan-neck deformities may also
result from increased intrinsic tone (Fig. 67.24).
The central extensor band is relatively shortened relative to the
lateral bands because of tension exerted by the intrinsics and long
extensor. In both of these cases, take care to distinguish between
deformities caused by the intrinsic spasticity that should improve with
treatment and deformities resulting from the more usual mechanisms such
as traumatic central slip injury with lateral band subluxation or
traumatic mallet finger, because if not caused by spasticity the
deformity will not improve with treatment for spasticity.
 |
|
Figure 67.24.
Clinical photograph showing a patient with multiple swan-neck deformities following traumatic brain injury. (With permission from Hisey MS, Keenan MAE. Orthopaedic Management of Upper Extremity Dysfunction Following Stroke or Brain Injury. In Green DP, Hotchkiss RN, Pederson WC, eds. Operative Hand Surgery, 4th ed. New York: Churchill Livingstone, 1998:287.) |
the intrinsic and extrinsic muscles by clinical assessment alone, we
routinely obtain dynamic EMG studies of the intrinsic muscles before
embarking on treatment of hand deformities. This is especially
important before considering any surgical intervention.
function, three treatment options are available. The procedure chosen
is based on considerations of contracture and the presence or absence
of volitional activity in the intrinsic muscles. When no significant
intrinsic contracture is present and dynamic EMG indicates that there
is no volitional control in the intrinsic muscles, perform a neurectomy
of the motor branches of the ulnar nerve in the palm (Fig. 67.7).
Leave the sensory branches intact to preserve protective sensation in
the hand. When a contracture of the intrinsic muscles is present and
the dynamic EMG study shows no volitional activity, perform a release
of the lateral bands of the extensor hood mechanism at the level of the
proximal phalanx (Fig. 67.25). In these cases,
neurectomy of the motor branches of the ulnar nerve is performed
simultaneously to prevent recurrence of the intrinsic plus deformity
from spasticity of the interosseous muscles. When there is either a
dynamic or static intrinsic plus deformity but the EMG demonstrates
volitional control, release the interossei from their proximal origins
on the metacarpals and allow to slide distally (207,208).
A static deformity is one in which a myostatic contracture is present.
A dynamic deformity is one in which the deformity results mostly from
increased tone with little or no fixed contracture.
 |
|
Figure 67.25. Isolation and release of the intrinsic interossei and lumbricales muscles.
|
-
Be careful to prevent harm to the ulnar
artery. Expose the ulnar nerve and identify the motor branches using a
nerve stimulator. Generally, two motor branches are seen. The main
motor branch lies beneath the sensory branch and a smaller motor branch
can be seen entering the hypothenar muscles (Fig. 67.7) (115,122). -
After identifying these features,
transect the nerves, taking care to preserve the ulnar artery and the
sensory branch of the ulnar nerve (Fig. 67.7). -
Unless the procedure is combined with
other surgery, no splinting or casting is necessary postoperatively.
Apply a soft dressing to the hand and begin active and passive
exercises on the first postoperative day.
muscles is present and the dynamic EMG study shows no volitional
activity, perform lateral band releases (Fig. 67.25).
-
Make a midline longitudinal incision over
the dorsum of the metacarpophalangeal joint and proximal phalanx of
each finger. Dissect on both the ulnar and radial sides of the extensor
mechanism. -
Identify the palmar edge of the lateral
bands. Transect the lateral band and oblique fibers of the extensor
hood on each side. Take care to preserve the transverse fibers of the
sagittal extensor hood. -
Recurrent intrinsic plus deformities are
common. This is thought to be secondary to residual attachment of the
interossei muscles to the base of the proximal phalanges. To prevent
recurrent deformities, perform a concomitant neurectomy of the motor
branches of the ulnar nerve in Guyon’s canal.
-
In a hand with volitional control of the intrinsic musculature, perform intrinsic slides (207,208).
-
Make two dorsal longitudinal incisions, one between the second and third and one between the fourth and fifth metacarpals.
-
Preserve the extensor tendons while
exposing the origins of the interossei on the shafts of the
metacarpals. Elevate these origins from the metacarpals and extend the
metacarpophalangeal joints while the interphalangeal joints are flexed,
causing the intrinsics to be lengthened. -
Postoperatively, immobilize the hand in a bulky dressing for one week.
-
The abductor digiti quinti muscle may
contribute to a flexion deformity of the metacarpophalangeal joint of
the fifth finger. The muscle can be fractionally lengthened by
transecting the tendon within the muscle belly. This allows lengthening
while preserving the function of the muscle.
hand. In these patients, the intrinsic muscles have normal or weakened
tone, but there is spasticity of the extrinsic finger flexors. There
may be increased tone in the extrinsic extensors as well. This pattern
results in a claw hand posture,
with
hyperextension of the metacarpophalangeal joints and flexion of the
proximal and distal interphalangeal joints. Ulnar neuropathy must be
considered as a possible diagnosis. Hyperextension contracture of the
metacarpophalangeal joint capsule is common. When it is present, the
contractures require surgical release. Treatment of this deformity may
also require lengthening of the extrinsic digital flexors or STP
transfers, as described earlier.
joints from the metacarpal is often needed. Zancoli capsulodesis may
also be required to restore metacarpophalangeal flexion and place the
hand in a more functional and cosmetic position (220,248). Postoperatively, splint the hand with the metacarpalphalgeal joints flexed 70° for 3 weeks.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
JU, Sutherland DH, Hanggi A. Intramuscular Pressure During Walking: An
Experimental Study Using the Wick Catheter Technique. Clin Orthop 1979;145:292.
MJ, Keenan MA, Korchek JI, Waters RL. Modified Technique for the
Superficialis-to-profundus Transfer in the Treatment of Adults with
Spastic Clenched Fist Deformity. J Hand Surg [Am] 1987;12:639.
MJ, Waters RL, Keenan MAE, et al. Orthopaedic Management of the Stroke
Patient: Part I: Pathophysiology, Limb Deformity, and Patient
Evaluation. Orthop Rev 1988;27:637.
MJ, Waters RL, Keenan MAE, et al. Orthopaedic Mangement of the Stroke
Patient: Part II: Treating Deformities of the Upper and Lower
Extremities. Orthop Rev 1988;27:891.
BR, Baker LL, Waters RL. Positional Feedback and Electrical
Stimulation: An Automated Treatment for the Hemiplegic Wrist. Arch Phys Med Rehabil 1979;60:497.
RM, Mooney V, Nickel VL. Flexor-Origin Release for Pronation-Flexion
Deformity of the Forearm and Hand in the Stroke Patient. J Bone Joint Surg [Am] 1970;52:907.
JR. Manual Stretch: Effect on Finger Movement Control and Force Control
in Stroke Subjects with Spastic Extrinsic Finger Flexor Muscles. Arch Phys Med Rehabil 1990;71:888.
G, Judet T, de Loubresse CG, Piriou P. Articulated Radial Head
Replacement and Elbow Release for Post Head-injury Heterotopic
Ossification. J Orthop Trauma 1996;10:68.
G, Judet T, Garreau de Loubresse TC, Mollaret O. Excision of
Heterotopic Ossification Around the Knee Following Brain Injury. Injury 1996;27:125.
MK, Stacy M, Cooke DL, Stonnington HH. Comaparison of Two Injection
Techniques Using Botulinum Toxin in Spastic Hemiplegia. Am J Phys Med Rehabil 1996;75:462.
HC, Tan CB, Tjia H. A Case of Bilateral Ulnar Nerve Palsy in a Patient
with Traumatic Brain Injury and Heterotopic Ossification. Singapore Med J 1997;38:447.
DX, Kreutzer JS, Marwitz JH, et al. Functional Outcomes of Older Adults
with Traumatic Brain Injury: A Prospective, Multicenter Analysis. Arch Phys Med Rehabil 1996;77:883.
TJ, Alexander MA, Steg NL. Early Detection of Heterotopic Ossification
in Young Patients with Traumatic Brain Injury. Arch Phys Med Rehabil 1992;73:258.
RB, Hahn GV, Tabas JA, et al. The Natural History of Heterotopic
Ossification in Patients Who Have Fibrodysplasia Ossificans
Progressiva. A Study of Forty-four Patients. J Bone Joint Surg Am
1993;75:215.
JL, Vargo M, Reidy ME. A Prospective Study of Peripheral Nerve Lesions
Occurring in Traumatic Brain-injured Patients [see comments]. Am J Phys Med Rehabil 1989;68:15.
JP, Pellegrini VD Jr, Miller RJ, Jones JA. Treatment of Traumatic
Radioulnar Synostosis by Excision and Postoperative Low-dose
Irradiation. J Hand Surg [Am] 1994;19:394.
LB, Komoto-Tufvesson Y, Salgeback S. Surgery of the Spastic Hand in
Cerebral Palsy. Improvement in Stereognosis and Hand Function after
Surgery. J Hand Surg [Br] 1998;23:334.
P, Filipetti P, Feve A, et al. Peripheral Selective Neurotomy of the
Brachial Plexus Collateral Branches for Treatment of the Spastic
Shoulder: Anatomical Study and Clinical Results in Five Patients. J Neurosurg 1997;86:648.
R, Heffes Y, Abulaffio N. Electromyographic and Positional Changes in
the Elbows of Spastic Hemiparetic Patients During Walking. Electroencephalogr Clin Neurophysiol 1996;101:491.
MM, Stokic DS, Wawro AW, Wun CC. Modification of Motor Control of Wrist
Extension by Mesh-glove Electrical Afferent Stimulation in Stroke
Patients. Arch Phys Med Rehabil 1996;77:252.
S, Meek RN, Snelling CF, et al. Range of Motion and Complications After
Postburn Heterotopic Bone Excision about the Elbow. J Trauma 1996;41:825.
P, Cotterill G, Kneale TA, et al. Outcome of Intensive Rehabilitation
after Severe Brain Injury: A Long-term Follow-up Study. Brain Inj 1996;10:631.
AS, O’Dell MW, Guiffra LJ, Sandel ME. Radial Nerve Injury Associated
with Traumatic Myositis Ossificans in a Brain Injured Patient. Arch Phys Med Rehabil 1993;74:770.
DE. Clinical Observations on Fractures and Heterotopic Ossification in
the Spinal Cord and Traumatic Brain Injured Populations. Clin Orthop 1988;233:86.
C, Malkmus D, Durham P, Bowman. Levels of Cognitive Functioning.
Rehabilitation of the Brain Injured Adult: Comprehensive Physical
Management. Downey, CA: Professional Staff Association of Rancho Los
Amigos Hospital, 1979:87.
JK, Mueller BA, Rivara FA. Characteristics of Drivers and Driving
Record after Traumatic and Nontraumatic Brain Injury. Arch Phys Med Rehabil 1998;79:738.
HT, Hageman G, van Limbeek J. Prediction of Recovery from Upper
Extremity Paralysis after Stroke by Measuring Evoked Potentials. Scand J Rehabil Med 1997;29:155.
SL, Sharpe MH, Metzer J. Outcomes 5 Years Post-traumatic Brain Injury
(with Further Reference to Neurophysical Impairment and Disability). Brain Inj 1997;11:661.
H, Maier-Loth ML, Eickhof C. The Functional Value of Electrical Muscle
Stimulation for the Rehabilitation of the Hand in Stroke Patients. Scand J Rehabil Med 1997;29:3.
EA, Mandac BR, Davidoff G, et al. Risk Factors for Heterotopic
Ossification in Children and Adolescents with Severe Traumatic Brain
Injury. Arch Phys Med Rehabil 1992;73:459.
MA, Abrams RA, Garland DE, Waters RL. Results of Fractional Lengthening
of the Finger Flexors in Adults with Upper Extremity Spasticity. J Hand Surg [Am] 1987;12:575.
MA, Creighton J, Garland DE, Moore T. Surgical Correction of Spastic
Equinovarus Deformity in the Adult Head Trauma Patient. Foot Ankle 1984;5:35.
MA, Haider T. The Formation of Heterotopic Ossification after Traumatic
Brain Injury: A Biopsy Study with Ultrastructural Analysis. J Head Trauma Rehabil 1996;11:8.
MA, Korchek JI, Botte MJ, et al. Results of Transfer of the Flexor
Digitorum Superficialis Tendons to the Flexor Digitorum Profundus
Tendons in Adults with Acquired Spasticity of the Hand. J Bone Joint Surg [Am] 1987;69:1127.
MAE, Perry J. Evaluation of Upper Extremity Motor Control in Spastic
Brain-injured Patients Using Dynamic Electromyography. J Head Trauma Rehabil 1990;5:13.
MAE, Perry J. Motion Analysis: Upper Extremity. In: Nickel VL, Botte
MJ, eds. Orthopaedic Rehabilitation: Churchill Livingstone Publishers,
1992;243:2556.
MA, Romanelli RR, Lunsford BR. The Use of Dynamic Electromyography to
Evaluate Motor Control in the Hands of Adults Who Have Spasticity
Caused by Brain Injury. J Bone Joint Surg [Am] 1989;71:120.
MA, Todderud EP, Henderson R, Botte M. Management of Intrinsic
Spasticity in the Hand with Phenol Injection or Neurectomy of the Motor
Branch of the Ulnar Nerve. J Hand Surg [Am] 1987;12:734.
MA, Tomas ES, Stone L, Gersten LM. Percutaneous Phenol Block of the
Musculocutaneous Nerve to Control Elbow Flexor Spasticity. J Hand Surg [Am] 1990;15:340.
MA, Waters RL. Heterotopic Ossification. In: Skinner HB, ed. Diagnosis
and Treatment in Orthopaedics. New Jersey: Prentice Hall, 1995:604.
AA, Harmel MH, Forster S, Benton JG. Management of Spasticity by
Selective Peripheral Nerve Block with Dilute Phenol Solutions in
Clinical Rehabilitation. Arch Phys Med Rehabil 1964;45:513.
DJ, Katz SD, Keenan ME. Functional Outcome Following Surgical Resection
of Heterotopic Ossification in Patients with Brain Injury. J Head Trauma Rehabil 1996;44:4.
SH, Keenan MA. Using Dynamic Electromyography to Guide Surgical
Treatment of the Spastic Upper Extremity in the Brain-injured Patient. Clin Orthop 1993;288:109.
NH, Esquenazi A, Keenan ME. Analysis and Management of Spasticity,
Contracture, and Impaired Motor Control. In: Horn LJ, Zasler ND, eds. Medical Rehabilitation of Traumatic Brain Injury. Philadelphia: Hanley & Belfus, 1996:411.
JM, DeVivo MJ, Hadley M. Prospective Study on the Use of Bolus
Intrathecal Baclofen for Spastic Hypertonia due to Acquired Brain
Injury. Arch Phys Med Rehabil 1996;77:461.
JM, Tuel SM, Cross LL, Mathew MM. Heterotopic Ossification of the
Extensor Tendons in the Hand Associated with Traumatic Spinal Cord
Injury. J Am Paraplegia Soc 1992;15:229.
CA, Gelberman RH, Rhoades CE. Upper Extremity Tendon Transfers in
Cerebral Palsy: Electromyographic and Functional Analysis. J Pediatr Orthop 1985;5:69.
H, Jorgensen HS, Raaschou HO, Olsen TS. Compensation in Recovery of
Upper Extremity Function after Stroke: The Copenhagen Stroke Study. Arch Phys Med Rehabil 1994;75:852.
H, Jorgensen HS, Raaschou HO, Olsen TS. Recovery of Upper Extremity
Function in Stroke Patients: The Copenhagen Stroke Study. Arch Phys Med Rehabil 1994;75:394.
M, Balbi P, Russo G, Nilsson J. H-reflex Recovery Curve and Reciprocal
Inhibition of H-reflex of the Upper Limbs in Patients with Spasticity
Secondary to Stroke. Am J Phys Med Rehabil 1995;74:357.
Evaluation and Treatment of the Stroke Patient. Part III. Upper
Extremity Management and Surgery. American Academy of Orthopaedic
Surgeons Instructional Course Lectures, Vol. 24. St. Louis: C. V. Mosby, 1975:51.
MS, Wehner J, Kett N, Trilla M. Brachioradialis to Finger Extensor
Tendon Transfer to Achieve Hand Opening in Acquired Spasticity. J Hand Surg [Am] 1988;13:5492.
A, Gauthier M, Wieler M, Kenwell Z. The Bionic Glove: An Electrical
Stimulator Garment that Provides Controlled Grasp and Hand Opening in
Quadriplegia. Arch Phys Med Rehabil 1997;78:608.
DJ Jr, Kellerman WC, Bonner FJ Jr. Heterotopic Ossification
Masquerading as Deep Venous Thrombosis in Head-injured Adult:
Complications of Anticoagulation. Arch Phys Med Rehabil 1986;67:.
KJ, Banovac K, Hornicek FJ, et al. Evaluation of Serum Osteoblast
Mitogenic Activity in Spinal Cord and Head Injury Patients with Acute
Heterotopic Ossification. Spine 1994;19:740.
MA. Indomethacin: An Adjunct to Surgical Excision of Immature
Heterotopic Bone Formation in a Patient with a Severe Head Injury. A
Case Report. Orthopedics 1987;10:1379.
DM, Zasloff M, Peeper J, et al. Age- and Joint-specific Risk of Initial
Heterotopic Ossification in Patients Who Have Fibrodysplasia Ossificans
Progressiva. Clin Orthop 1994;301:243.
HT, Mital MA, Matza RA, Dimakopoulos P. Classification and Surgical
Treatment of the Thumb-in-palm Deformity in Cerebral Palsy and Spastic
Paralysis. J Hand Surg [Am] 1995;20:428.
C, Ferreira JJ, Pinto AA, et al. Botulinum Toxin Type A for the
Treatment of Arm and Hand Spasticity in Stroke Patients. Clin Rehabil 1997;11:3.
DM, Alexander DN, O’Brien CF, et al. Botulinum Toxin Type A in the
Treatment of Upper Extremity Spasticity: A Randomized, Double-blind,
Placebo-controlled Trial. Neurology 1996;46:1306.
KM, Alexander MA, Harcke HT. Undetected Musculoskeletal Trauma in
Children with Traumatic Brain Injury or Spinal Cord Injury. Arch Phys Med Rehabil 1993;74:902.
G, Gennarelli TA, Rogers CR. Disodium Etidronate: Its Role in
Preventing Heterotopic Ossification in Severe Head Injury. Arch Phys Med Rehabil 1983;64:539.
A, Sazbon L, Lotem M. Relationship Between Muscular Tone, Movement and
Periarticular New Bone Formation in Postcoma-unaware (PC-U) Patients. Brain Inj 1996;10:259.
LX, Bosse MJ, Mayo KA, et al. Results in Patients with Craniocerebral
Trauma and an Operatively Managed Acetabular Fracture. J Orthop Trauma 1990;4:376-82.
S, Grosswasser Z, Ohri A, et al. Histocompatibility (HLA) Antigens in
Heterotopic Ossification Associated with Neurological Injury. J Rheumatol 1979;6:88.
SL, Binder-MacLeod SA. Electromyographic Biofeedback Applications to
the Hemiplegic Patient. Changes in Upper Extremity Neuromuscular and
Functional Status. Phys Ther 1983;63:1393.
RD, Hammond FM, Mann NR, et al. Revised Trauma Score: An Additive
Predictor of Disability Following Traumatic Brain Injury? Am J Phys Med Rehabil 1996;75:456.
JP, Dugan SA, Young JL, et al. A Retrospective Review of the Incidence
and Rehabilitation Outcome of Concomitant Traumatic Brain Injury and
Ligamentous Knee Injury. Arch Phys Med Rehabil 1998;79:805.