Principles of Spine Trauma Care
result in a neurologic deficit and permanently impair a patient’s
function and quality of life, and in some cases may lead to death.
Additionally, the legal context of working in emergency rooms makes the
difficult work of evaluating trauma patients for a potential spine
injury even more stressful for training and practicing physicians.
Making this work a little less stressful is the goal of this chapter.
and this remains true today, but that does not that nothing can be done
for patients who sustain severe neurologic deficits. Patients with
spinal cord injury today regain mobility, improve their quality of
life, and achieve prolonged survival.19 Research at the cellular and genetic
level continues to improve our understanding of the fundamental processes,222
and clinical research methods to study spinal cord injury in real
patient populations have improved, renewing optimism for novel spinal
cord injury treatments.278 This
chapter focuses on general principles of spinal injury care. Subsequent
chapters in this section discuss specific injury patterns in greater
detail.
Although precise definitions for many of these terms are lacking, broad
interpretations are nevertheless useful for conveying a general meaning
when discussing related processes.
etiologybased categories: primary injury refers to physical tissue
disruption caused by mechanical forces, and secondary injury refers to
additional neural tissue damage resulting from the biologic response
initiated by the physical tissue disruption. The extent of structural
damage to neural tissue is indicated by other descriptive terms.
Concussion refers to physiologic disruption without anatomic injury.
Contusion refers to physical neural tissue disruption leading to
hemorrhage and swelling (the most common type of spinal cord injury).
Or laceration, which describes loss of structural continuity of the
neural tissue (rare in blunt trauma). The clinical response to injury
is typically described in temporal terms: acute refers to the first few
hours after injury; subacute typically refers to several hours to days
following injury, and chronic refers to intervals of weeks to months
after the injury. The functional consequences of spinal cord injury are
usually described by terms that refer to the severity and pattern of
neurologic dysfunction. Complete spinal cord injury, incomplete injury,
or transient spinal cord dysfunction describe different grades of
severity of neurologic injury. Names for different types of spinal cord
injury syndromes, such as anterior cord syndromes, central cord
syndrome, and Brown-Séquard syndrome, refer to patterns of neurologic
dysfunction observed during clinical evaluation.295
 |
|
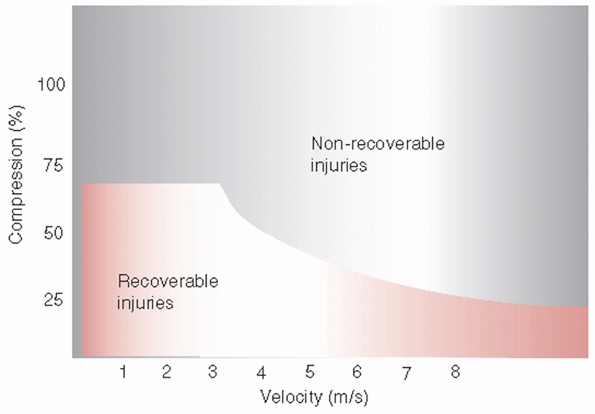
FIGURE 41-1
Effect of rate and severity of cord compression on potential for neurologic recovery. The threshold varies inversely with the magnitude of compression and the velocity of compression of the spinal cord. |
and ligament structures into the neural space, the spinal canal, and
neural frame. These displaced and disrupted structures apply forces on
the neural tissue that result in either functional or anatomic
disruption.25 Most spinal cord
injuries are crushing injuries resulting in acute neural tissue
contusion from applied physical forces. Laceration or transection of
the spinal cord is rare, even in markedly displaced fracture
dislocations.151
identified several characteristics of the injury force that determine
the extent of neural tissue damage. These include the rate of force
application, the degree of neural tissue compression, and the duration
of neural tissue compression.150 The severity of neural tissue disruption is proportional to the energy absorbed from the injury mechanism.10
For direct impact on neural tissue, contact velocity and maximum cord
compression are better predictors of injury severity than either force
or acceleration.
principal resistance to deformation in the early stages of impact
during compression injures.25,290 Spinal cord tolerance for compression decreases as the velocity of compression increases (Fig. 41-1).150 Minimum compression of the cord at high-contact velocity may produce severe anatomic injury and limited functional recovery.150 At about 50% cord compression, functional recovery is minimal regardless of the contact velocity.150
Although this threshold effect denotes an upper limit of compression in
the acute injury model, it is not apparent in extremely slow onset of
cord compression seen in chronic degenerative conditions, such as
cervical spondylotic myelopathy. Cord compression that develops over
years of progressive arthritic changes can be quite severe and yet
manifest minimal clinical symptoms.
displacement without sustaining structural or neurologic deficit.
Contrary to the relationship between nerve roots and neural foramina
during physiologic movement, the spinal cord does not slide up and down
in the spinal canal during spinal flexion and extension. Rather, the
cord appears to deform like an accordion.228
Physiologic movements can stretch the cord an average of 10%, and
maximum change can be as much as 18% of the longitudinal length of the
spinal cord.228 Maximum stretching occurs between C2 and T1. Cord deformations may be more severe
in patients with spondylosis, and in these patients, may contribute to
cord injury even in the absence of vertebral column disruption.
determinant of the presence and severity of neurologic damage and
cervical spine trauma.82,281
The preinjury spinal canal diameter, as measured on lateral radiographs
or sagittal magnetic resonance images (MRI), and the cross sectional
spinal canal area are important in determining the severity of cord
injury.183,281
A narrow spinal canal is associated with a greater likelihood of
neurologic injury and a higher probability of complete cord injury.
Cervical spondylosis can severely decrease the size of the spinal
canal, further predisposing the patient to neurologic injury.
immediate depolarization of axonal membranes in the neural tissue. This
results in a functional neurologic deficit that exceeds the actual
tissue disruption. This condition is referred to as “spinal shock.”148 Although the mechanism of spinal shock is not established, it may relate to immediate depolarization of the entire cord.75
The clinical examination more accurately reflects the neural injury
when spinal shock resolves and uninjured neural tissues neural
structures repolarize.
As physical displacement of the spinal cord damages neural tissue, the
inner most regions of the spinal cord sustain the most severe injury.42,226
In less severe injuries, this compressive force may lead to
demyelination and an acute central cord syndrome. The primary neural
injury also causes Wallerian degeneration in ascending dorsal columns
and descending motor tracks, independent of any secondary injury or
vascular insult. These changes may result in a gap at the injury site,
which is largely devoid of neural parenchymal matrix.
Hematomyelia, hemorrhage within the cord parenchyma, further displaces
cells and axons away from the primary injury point. Within the first
few hours following injury, tissue breakdown leads to expansion of the
zone of injury. The size of the neural injury zone is fairly well
defined by 1 week after injury.
macrophages remove damaged tissue and form a fluid-filled cavity. This
cavity can expand to fill the entire area of neural tissue disruption,
and form cysts at the injury site. This process reaches a steady state
if the surface of the cord remains intact and the spinal cord
membranes, the pia-arachnoid and dura, do not form adhesions. This can
result in stable, nonexpanding cysts that show cavitation with loose
borders and no astrocyte boundary at the periphery. If the layers of
the pia scar to the dura and the cysts develop a border of astrocytes,
the process can lead to expansile cysts or syrinx formation. Scarring
between the pia and dura can also lead to an apparent expansion of the
cord or progressive noncystic myelomalacia. These mechanisms may
contribute delayed neurologic worsening following spinal cord injury,
and they also form the basis for treatments for late neural
deterioration, such as duraplasty and untethering of the scarred spinal
cord.
in various in vivo and in vitro models that attempt to mimic the
processes in human spinal cord injury. The variability in the
experimental designs and differences across species of tested animals
has led to varied characterizations of the injury response. An optimal
experimental model of spinal cord injury has not been established.
Experimental constraints in the injury models somewhat limit the
generalizability of the findings to clinical conditions.
Local tissue elements undergo structural and chemical changes. These
changes in turn elicit systemic responses. Hemorrhage at the injury
site occurs within minutes of the injury in the gray matter and
radially expands to involve the white matter in lateral columns.
Endothelial cell disruption increases fluid extravasation and local
swelling in the neural tissue. Extensive neural cell death occurs
within the first few hours of injury.249
within the first hour of injury. White matter necrosis begins within 4
hours of injury. Neural tissue loss in spinal cord injury is not purely
a result of physical forces and cytotoxic processes, but also includes
programmed cell death (apoptosis).171
Apoptosis depends on active protein synthesis and begins to occur as
early as 4 hours after injury. Programmed cell death peaks initially at
24 hours following injury and then reoccurs in a second peak at
approximately 7 days following injury.
Cytoskeletal protein disruption in the axon membranes causes separation
of axons and necrosis ascending from the injury site (Wallerian
degeneration).223 Axons die back at approximately 1 mm per month.223
Above the lesion, sterile end bulbs form at failed regeneration
attempts in descending tracks. Below the lesion, abortive sprouting of
dorsal root ganglion cells results in proliferation of Schwann cells
(schwannosis).223
concentrations, and concentrations of chemical mediators lead to
propagation of interdependent reactions. This pathophysiologic
response, referred to as secondary injury, can propagate tissue
destruction and functional loss. Ischemia and inflammation are
prominent mechanisms in the secondary responses.48,49 Ischemia also contributes to delayed secondary injury.92,276 Severity of neurologic injury is also proportional to the duration of spinal cord deformation.209
In the injury zone, a reversible injury may become irreversible from
local ischemia and inflammation. Irreversible axonal injury can also
lead to cell death extending beyond the primary injury site.203
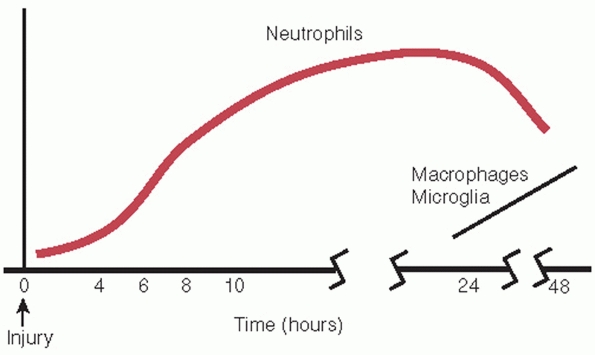
Inflammatory response consists of polymorphonuclear cell infiltration
within 6 hours of injury and macrophage infiltration beginning at 24
hours following injury (Fig. 41-2).50
Incomplete conversion of oxygen to carbon dioxide in water results in
free radical formation, lipid poor oxidation, and membrane breakdown.122,314
injury research. Sodium-calcium exchange mechanisms have been noted to operate in reverse following an acute injury.268
Agents that block sodium-calcium exchange, such as choline, ketamine,
and mk-801, decrease neural swelling. Other agents that increase
sodium-calcium exchange, such as glutamic, induce neuronal swelling.
Disruption of the cell membranes contributes to neuron death, and
agents that interfere with membrane peroxidation, such as
methylprednisolone and 21-amino steroid tirilazad mesylate, may be
useful in spinal cord injury by protecting cell membranes.
|
TABLE 41-1 Physiologic Response to Spinal Cord Injury
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Reduction of extracellular sodium is a neuroprotective, and increased
intracellular sodium exacerbates traumatic axonal injury. Agents that
inhibit sodium exchange, such as amiloride and harmaline, are
neuroprotective.3 In contrast to
neurons, reverse operation of the sodium-calcium exchange does not
explain the effects of sodium in white matter injury. Axons in spinal
cord white matter lack receptor-coupled and voltagesensitive calcium
channels.3
physiology. At this time, this basic science research has not led to any clinical diagnostic or therapeutic interventions.
 |
|
FIGURE 41-2 The inflammatory response begins within 4 hours of injury. Neutrophil infiltration precedes macrophage accumulation.
|
It is not clear whether this recovery is related to resolution to the
acute physiologic responses to injury or to active repair mechanisms in
the nerve tissue. In animal experiments, ability to regain ambulation
correlates with the amount of white matter remaining after the injury.21 The primary injury frequently spares a peripheral rim of white matter.315
Even a small number of intact axons traversing the injury zone, as few
as 5% to 10% in small animal experiments, may be sufficient to support
significant functional recovery.315
Fish and amphibians show successful regeneration of axons in the
central nervous system. Higher vertebrates only show this capacity in
the embryonic and perinatal periods.142 Adult mammals do retain some capacity for regeneration, and this process may be activated under controlled circumstances.
pluripotential cells to recreate the complex microenvironment necessary
for neural regeneration.6,248,311
Although results in smaller animals and in primates are promising, this
technology has not yet advanced to large scale human trials.141,229
Monosialotetrahexosylganglioside GM-1 (Sygen; Sygen International PLC,
Berkeley, CA) was given to 697 subjects, divided equally among one of
two different drug dosages and a placebo group.103,104 Despite an earlier phase 1 trial of 37 patients that suggested encouraging findings,105 this larger investigation failed to demonstrate significance in the predetermined primary efficacy analysis.103,104
All of those subjects received methylprednisolone according to National
Acute Spinal Cord Injury Study II protocol (NASCIS). In a
placebo-controlled study examining effectiveness of nimodipine, the
latter was used in combination to methylprednisolone and compared with
steroid alone and with placebo.218
No significant differences in sensory or motor recovery were observed 1
year postintervention. Finally, the autologous incubated macrophage
trial (Proneuron Biotechnologies Inc., Los Angeles, CA) surgically
implanted the patient’s own cells at the level of the spinal cord
lesion, necessitating two surgeries in a 10- to 14-day window
postinjury to accomplish the transplantation. All participants eligible
for NASCIS protocol received steroid treatment. Although halted for
financial reasons in 2006, preliminary analysis of the 50 subjects who
completed the study has not suggested a significant benefit to the
procedure.7,27,146,160,161 Cellular therapies remain unproven.27,88,162,263,283
Injury mechanisms do not have direct or exclusive relationships with
injury patterns. Similar injury mechanisms can result in different
clinical patterns of spine injury. In addition to the magnitudes and
directions of injury forces, the orientation of the spine at the moment
of injury and structural predispositions of the vertebral column
influence the resulting injury.
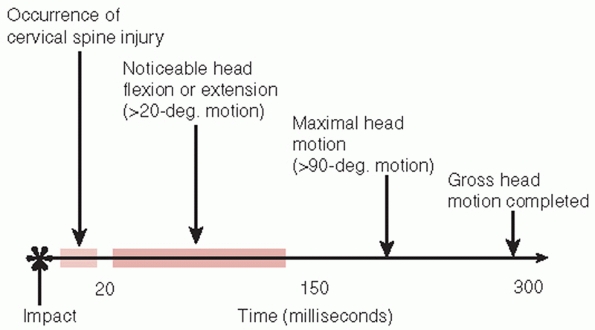
the failure of the vertebral column precedes the occurrence of any
measurable head motion (Fig. 41-3).202
For a given magnitude, direction, and locale of head impact, variations
in the local vertebral alignment at the level of injury at the moment
of impact change observed pattern of injury, with facet joint
disruptions occurring when the injured vertebrae are in relative
flexion and burst-type vertebral body fractures when the vertebrae are
in relatively neutral or extended position. Similar injury patterns of
cervical spine fractures and fracture-dislocations can also occur in
patients who do not sustain any direct head impact, such as restrained
front-seat occupants of motor vehicle crashes.136
These observations explain a long-standing and fundamental problem of
spinal injury classification systems based on presumed injury
mechanisms: the same injury pattern may result in morphologically
different injuries; similar morphologic patterns can be the result of
different injury mechanisms, and the patterns of head deflection do not
predict the spinal injury patterns.
between injury mechanisms and clinical manifestations is the role
played by time during the injury process. Injuries are a series of
dynamic events that take place over time, whereas clinical assessment
takes
place with the patient in a relatively static state with respect to
forces and movements. Dynamic events occurring at the time of injury
are not reflected in subsequent static anatomic assessments of injured
tissues.304,305
For example, the degree of spinal occlusion that occurs during a
burst-type fracture of the vertebral body greatly exceeds the amount of
spinal canal occlusion seen on computed tomography (CT) scans performed
after the injury event.51,282
Given this consideration, it is not surprising that radiographic
measurements of spinal canal size in patients with cervical spine
injuries show an increased likelihood of cord injury for patients with
a narrow spinal canal. Poor correlation exists between the severity of
spinal canal occlusion and the severity of neurologic deficit.82,85,286
 |
|
FIGURE 41-3
Timing of head and neck movement in impact injuries of the cervical spine. Spinal column failure precedes head deflection in impact injuries. |
by way of rate of force application. With faster rates of load
application, the transient displacement of failed structural elements
is greater, and associated neural space occlusion and spinal cord
compression are greater.51 At high
loading rates, bone fails first, as opposed to the ligaments or the
intervertebral disc. At low loading rates and with rotational forces,
the vertebral column fails through the soft tissues.238
junctional areas: the craniocervical junction (occiput-C2), the
cervicothoracic junction (C7-T1), and the thoracolumbar junction
(T11-L2). These areas represent regions of stress concentration, where
a rigid segment of the spine meets a more flexible segment. Also
contributing to stress concentration in these regions are changes at
these levels in the movement constraints of vertebrae. These junctional
areas are transition zones where the predominant pattern of movement
between vertebrae changes from facet joint orientation allowing
side-bending, flexion-extension, and rotation in the cervical spine, to
allowing predominantly rotation in the thoracic spine, to permitting no
rotation in the lumbar region. Cervical rotational facet injuries are
accompanied by facet fractures and bilateral damage of the rotated
vertebra.251
associated with motor vehicle crashes, and most thoracic-lumbar
injuries occur between T11 and L2.8,114
fractures are less likely to have associated neurologic injury. In
part, this is because of a high incidence of low-energy thoracic
fractures related to osteoporosis, which rarely cause cord injury.
Since the spinal cord ends typically at the L1 level, the thecal sac
caudal to this level contains only thin nerve roots and has much
greater cerebrospinal fluid space than the thoracic or cervical
regions. Only 3% of thoracic and lumbar vertebral body fractures are
associated with a neurologic deficit.230
In general, fracture-dislocations are associated with a neurologic
injury in majority of the cases. Also, burst-type fracture patterns
that manifest a neurologic injury demonstrate more severe vertebral
column disruption with greater vertebral body collapse, more severe
deformity, and greater spinal canal occlusion, than burst-type injuries
without neurologic involvement. In the lower lumbar vertebrae (L4 and
L5), however, severe canal occlusions often have no associated
neurologic injury.
displacement of the injured structures may have severe associated
neurologic deficit. This clinical association is consistent
observations from biomechanic studies of injury mechanisms. A
high-impact loading rate produces fractures with significant canal
encroachment and a high potential for neurologic injury.282 A time interval of 400 msec from zero to peak load results in 7% spinal canal encroachment with fracture fragments.282 Decreasing this interval to 20 msec leads to 48% spinal canal encroachment.282
In these injuries, the subject’s torso hinges across a lap belt,
causing extreme flexion and distraction at a focal vertebral level. Lap
belt injuries can cause bowel rupture or major vessel, liver, spleen,
and urologic injury, noted in approximately 65% of patients with these
injuries.107 In the absence of
osteoporosis or neoplastic disease, spinal fracture requires high
energy and external trauma. Although rare, forces generated during a
tonic-clonic seizure can also result in axial skeletal trauma,
including thoracic and lumbar burst fractures.316 Total fracture incidence is 2.4% in patients with seizure disorders.316
of the caudal spine. They are usually associated with pelvic
disruptions or falls. A fall from a height usually results in a
transverse sacral fracture.243 The
particular sacral fracture pattern, such as vertical, transverse, or
combinations such as “H-shaped” or “U-shaped” fracture patterns, is
dependent on the sagittal plane position of the lumbar spine at the
time of impact.243
associated with injury patterns can guide prioritization and sequencing
interventions in the evaluation and management of individual trauma
victims. This section presents the general estimates for numbers
associated with vertebral column and spinal cord injuries.
been involved in trauma are skeletal injuries and head injuries. The
prevalence of skeletal injury is roughly equal to the prevalence of
head injury and has been reported to be as high as 78% in multiply
injured patients.232 Skeletal injury occurs four times as frequently as abdominal injury and twice as frequently as thoracic injury.
frequently than injuries to the appendicular skeleton. Vertebral column
injuries are reported to occur in approximately 6% of trauma patients,
with half of these patients (2.6%) sustaining spinal cord or nerve root
level neurologic injury.43 The vertebral injury can occur at multiple noncontiguous levels in 15% to 20% of the patents sustaining a spinal injury.285 Often, patients with multiple spinal injuries have an injury to the cranial-cervical
junction in addition to an injury in the lower cervical, thoracic, or
lumbar spine. Chest and abdominal injuries are common with fractures in
the thoracic and lumbar region. The incidence of concurrent abdominal
injury in association with cervical fractures is low, approximately
2.6%.257
patients sustaining tetraplegia or paraplegia, 80% have concurrent
multiple system injuries and 40%-74% have associated head injury.65,66,279
The presence of a spinal cord injury dramatically affects a patient’s
chances of surviving the initial hospitalization and in achieving the
permanent function and quality of life level subsequently. For patients
with a spinal cord injury, the overall mortality during the initial
hospitalization was 17% based on a study in the early 1980s.43
As of 2008, the National Spinal Cord Injury Statistical Center
estimates mortality of traumatic spinal cord injury patients among 15
model systems of care to be 2.6%.284
However, this figure is a substantial underestimate since many centers
are unable to attribute acute care deaths to spinal cord injury alone.
With complete ascertainment, the number is closer to 5%-10%.294 Improvements in acute care have been partially attributed to this modest improvement in survival following immediate injury.267
more by the preservation of neurologic status than by other
intermediate results of treatment, such as the quality of long bone and
joint reduction in healing, the length of time in the intensive care
unit or acute care facility, or by the social and demographic
characteristics of the patient. Patients with a permanent residual
neurologic deficit require life-long social adjustments and supportive
care. Incremental loss of neurologic function disproportionately
increases disability.63,187
Since spinal cord injury primarily affects young individuals, the
functional, medical, and social burden of illness of spinal cord injury
is amplified in terms of loss of productive life years.
victims depends on the demographics of the population served by a
trauma center. In general, approximately 2% to 6% of trauma patients
sustain a cervical spine fracture. Of those trauma patients sustaining
a spinal injury, more than half of the spinal injuries involve the
cervical region. Various characteristics of the patient and the injury
mechanism influence the likelihood of an individual patient having a
cervical spine fracture (Table 41-2).26 The highest risk occurs in patients who manifest a focal neurologic deficit (20%).26
Other characteristics associated with an increased risk are age greater
than 50 years, an injury mechanism involving high energy, and the
presence of a head injury. The same injury mechanism can impart widely
different risk of injury to different patients. For example, the risk
of a cervical spine fracture from a low-energy mechanism, such as fall
from a standing height, in a person aged less than 50 years is 0.04%.26
The same mechanism in a person aged greater than 50 years carries a
risk of cervical spine fracture of 0.5%, a risk estimate more than 10
times greater than the younger person.26,40
related to osteoporosis and involve minimal or no trauma. In fact,
osteoporosis-related fractures far outnumber trauma-related thoracic
and lumbar fractures. Osteoporosis leads to approximately 750,000
vertebral fractures each year in the United States.231 The annual rate of trauma-related thoracic and lumbar fractures is approximately 15,000.114 Thoracic and lumbar fractures account for 30% to 50% of all spinal injuries in trauma victims.114
In trauma patients, thoracic and lumbar fractures are concentrated at
the thoracolumbar junction, with 60% of thoracic and lumbar fractures
occurring between T11 and L2 vertebral levels.229
Although spinal cord injury is exceptionally rare with osteoporotic
fractures, neurologic injury occurs in one fourth of thoracic and
lumbar fractures associated with trauma.230 Many of these patients (37%) also sustain concomitant injuries to other regions of the body.
|
TABLE 41-2 Risk of Cervical Spine Fracture in Trauma Patients
|
||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||
The gender ratio is 4:1 (males:females), but data in recent years
indicate a growing percentage of females with spinal cord injury.143 The mean age is 39.5
years and the median age 34. Within National Institute on Disability
and Rehabilitation Research funded Model Spinal Cord Injury Care
Systems, the percentage of persons over age 60 with spinal cord injury
has increased from 4.7% in 1980 to 11.5% since 2000.284
Prevalence of a condition is determined by the size of the affected
population and both the incidence rates and the survival rates
associated with the condition. Despite changes in automotive
technology, such as introduction of seatbelts and air bags, and despite
changes in laws regulating the use of these safety devices and other
safety measures, such as highway speeds, the incidence of
trauma-induced spinal cord injury has not changed much over the past 30
years. It is estimated to be between 29 and 50 cases per million
persons per year, excluding spinal cord injuries which are fatal at the
scene.43 Spinal cord injuries fatal
at the scene are estimated to occur at a rate of approximately 20 cases
per million persons per year. Each year, 12,000 patients are admitted
to trauma centers for acute spinal cord injury.43
Approximately 200,000 persons with trauma-related tetraplegia or
paraplegia currently live in the United States, and this population is
expected to increase due to an growing number of older patients with
spinal cord injury.163
a motor vehicle crash, which accounts for 42% of all trauma-related
cord injuries. Other common causes are falls (27.1%), gunshot injuries
(15.3%), and sports injuries (7.4%).119,284,309
Older individuals are more likely to sustain a cord injury from a minor
fall, such as a fall from standing height. Falls in persons of age
greater than 65 years are 2.8 times more likely to be associated with a
spinal cord injury than similar falls in patients younger than 65 years.2
The increasing rates of falls are largely responsible for the shift
toward incomplete tetraplegia as the most common neurologic category.
Neurologic injury is localized to the lumbar region (conus medullaris
or cauda equina) in 20% to 24% of patients and to the thoracic cord in
17% to 19%.274 Approximately 41% of
the patients with an acute spinal cord injury have a complete injury on
initial evaluation, with no preservation of motor or sensory function
in the sacral cord segments. Cervical injuries (tetraplegia) are more
often incomplete neurologic deficits than complete, whereas thoracic
injuries are more often complete.
cord injury admissions, while 15%-20% of spinal cord injuries occur in
persons under age.18,72,143,210,211
Patients with an immature skeleton can sustain a spinal cord injury
without overtly disrupting the structural components of the vertebral
column. This type of a cord injury, labeled as spinal cord injury
without radiographic abnormality (SCIWORA) in the publications
preceding the use of MRI, may occur in nearly half of very young spinal
cord injury patients (42% of patients aged less than 9 years).73 It is present much less frequently in patients with mature skeletons (8% of patients aged 15 to 17 years).73 A majority of the younger patients (70%) with SCIWORA had an incomplete spinal cord injury.73
vertebral column can also occur in older patients who have a narrow
spinal canal, either a congenitally narrow spinal canal (spinal canal
diameter less than 80% of the midbody vertebral body diameter) or a
pathologically narrow canal from osteophytes and degenerative changes.241 These patients also typically have an incomplete cord injury, usually of a central cord injury pattern.
aspect of cost measurement. Although it is the most difficult cost to
measure quantitatively, the greatest cost to society for spinal cord
injury is the loss of many years of quality of life in the young
population of patients who sustain these injuries, especially since
improvements in rehabilitation have resulted in nearly normal life
expectancy for many young individuals with a spinal cord injury. The
lifetime direct medical cost of spinal cord injury is estimated to be
from $680,000-$3 million for persons injured at age 25 and $500,000 for
persons experiencing a spinal cord injury at age 50.284 In the United States, the aggregate annual direct medical cost of traumatic spinal cord injury is estimated at $7.74 billion.71
Although high-level tetraplegia (upper cervical segments) only
represents 10% of spinal cord injury patients, it accounts for 80% of
the direct medical cost of spinal cord injury.71 Paraplegia accounts for 4% of the overall aggregate cost, and incomplete injuries account for approximately 15% of the costs.71
spinal injury can be complex, but priorities of Advanced Trauma Life
Support still apply.14 Critical
decisions are necessary at each step as more information about the
patient becomes available. Each subsequent event in the patient’s
triage is influenced by the findings of the initial evaluation, both
for management of the spinal injury and for management of other
potential injuries.
investigated for spinal injury. Even mild complaints of pain or
posterior midline tenderness in trauma patients should not be dismissed
without full evaluation, including imaging studies. Unclear findings on
imaging studies should be assumed to reflect acute injury until further
evaluation clarifies their significance. Persistent symptoms despite
normal initial imaging studies may require further evaluation with
dynamic imaging studies such as upright radiographs or
flexion-extension radiographs. Unresolved findings from the patient’s
history, physical examination, and imaging studies should be clearly
and efficiently communicated to all providers involved in the patient’s
care.
management of trauma victims requires keeping the possibility of an
unstable spine injury in the forefront of active concerns until spinal
injury is definitively excluded or treated. Treatment priorities are
preserving life, limb, and function. The spine must be protected as
these priorities are addressed sequentially.
the cervical spine at the accident scene are critical to avoid further
neurologic injury.217 The head and neck need to be aligned with the long axis of the trunk and immobilized in this position.17 Emergency medical technicians are challenged by rescue attempts that entail removal of patients from tight spaces or vertical
drops. In such situations, the Kendrick Extrication Device has proven to be an effective means of spinal immobilization.306
It can also be used in pediatric patients needing accommodation for a
large occiput while maintaining neutral spine positioning.180 Immobilization with cervical collar, sandbags, tape, and spine board is superior to immobilization with a collar alone.115
For field transportation of injured patients, log-rolling still allows
motion at the spinal injury site, and a scoop-stretcher is useful
adjunct to the spine board.186 Cervical extension should be avoided since it narrows the spinal canal more than flexion.56 Neutral flexion-extension head and neck alignment is optimal during prehospital transport of cervical spine injury patients.217
To maintain neutral head-neck alignment, the relatively larger head of
pediatric patients should be accommodated with elevating the trunk on
padding or using a special pediatric spine board containing a cutout
for the occiput.12 For injured
athletes, motorcycle riders, and cyclists, helmet and shoulder gear
should be left in position until personnel trained in safe removal
techniques are available.13,76,102,190
helps prioritize subsequent treatment interventions in the emergency
room. Observations of the patient’s spontaneous physical movements and
function should be recorded in field records and conveyed to subsequent
caregivers. These observations are extremely valuable in determining
the possible presence and sometimes the general extent of neurologic
injury. Eliciting subjective symptoms in alert and communicative
patients and specifically asking about neck pain, back pain, numbness,
and weakness helps identify and localize spinal injury.
be started in the field. The steroid dosage and administration schedule
was established by three publications of results from three phases of
the NASCIS I, II, and III (Table 41-3).30,31,33
following spinal cord injury makes it difficult to determine the
optimal interruption point for preserving neurologic function.249
The secondary responses to spinal injury are both reparative and
contributory to additional injury. Interrupting the cascade of events
has the potential to change either aspect of the physiologic response.
Experimental treatments have investigated agents that block specific
pathophysiologic events occurring after the injury. Only a few of these
treatment interventions have shown sufficient promise in laboratory
studies to prompt clinical trials (Table 41-4).31,32,33,105,215,216,221
|
TABLE 41-3 Summary of National Acute Spinal Cord Injury Study I, II, and III Protocols
|
|||||||
|---|---|---|---|---|---|---|---|
|
The results showed no difference in outcome and increased infection
rate in the high-dose group. The second trial compared
methylprednisolone (30 mg/kg loading dose + 5.4 mg/kg/h for 23h) to
naloxone and placebo.32
Statistically significant improvement in motor and sensory scores in
both complete and incomplete injuries occurred in the group given
methylprednisolone.32 The magnitude
of effect was small: neurologic change score (improvement in motor
score) was 16.0 in the treatment group and 11.2 in the control group,
with a p-value of 0.03 for the difference.32 Pinprick score change was 11.4 in the treatment group and 6.6 in the control group (p = 0.02).32
These differences reached statistical significance because of the large
sample size for the study; they may have little or no clinical
significance. Statistically significant advantage of 3 to 7 points in
motor score may have limited functional benefit (see section on
controversies later in this chapter). A crucial assessment missing from
clinical trials of spinal cord injury is measurement of clinically
meaningful functional changes. NASCIS trials demonstrate that
large-scale, high-quality randomized clinical trials are
methodologically feasible, even when addressing difficult problems such
as the emergent management of spinal cord injury. This achievement is
as significant for future work in this area.
promise in the clinical trial stage to become established interventions.105,215,216 Lazaroids are one category of candidate drugs. Lazaroids are 21-amino-steroid free radical scavengers.9
One such agent, 21-aminosteroid U7-4006F, inhibits membrane
peroxidation. Another category of potential drugs for spinal cord
injury treatment is gangliosides. Gangliosides are large glycolipid
molecules found on the outer surface of most cell membranes.105
They are highly concentrated in neural tissues and are involved in
immunologic processes, binding, transport, and nerve cytogenesis.
Gangliosides have a trophic effect on nerve cells. They can stimulate
dendritic outgrowth and neuronal recovery. Further research is needed
to understand their application to spinal cord injury treatment.
monitoring to diagnose conditions not readily apparent early in their
clinical course. Spinal evaluation is concurrent with resuscitative
measures. Spine evaluation within the first few minutes of arrival in
the emergency room includes:
|
TABLE 41-4 Randomized Clinical Trials of Pharmacologic Treatment of Spinal Cord Injury
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
-
Assessment of gross neurologic function
by report from field personnel, direct observation, or initial gross
examination during primary survey, -
Diagnosis of severely unstable injuries
such as fracture-dislocations or distractive injuries on trauma
radiographs that include lateral cervical spine radiograph and
anteroposterior (AP) chest radiograph, and -
Assessment of hemodynamic parameters for potential neurogenic shock.
Loss of vasoconstrictive sympathetic control of the peripheral
vasculature can accentuate hemodynamic effects of hemorrhage.
Nonetheless, recognizing neurogenic shock as distinct from hemorrhagic
shock is critical for safe initial resuscitation of a trauma patient (Table 41-5).14,167
Treatment of neurogenic shock is pharmacologic intervention (typically
dopamine) to augment peripheral vascular tone. It may be essential for
effective resuscitation. Fluid overload from excessive fluid volume
administration, typical in treatment of hemorrhagic shock, can result
in pulmonary edema in the setting of neurogenic shock. For this reason,
clinical practice guidelines have advised the use of vasopressors,
rather than large volumes of intravenous fluid, to maintain mean
arterial pressure.60 Because of these complicated
fluid dynamics, these patients often merit invasive monitors such as
central lines or Swan Ganz catheters to accurately assess fluid status.
In a prospective trial that examined aggressive blood pressure
management in postinjury days 3-7, findings suggest favorable
neurologic recovery in patients whose main arterial pressure is
maintained above 85 mm Hg.288
|
TABLE 41-5 Comparison of Neurogenic and Hypovolemic Shock
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||
|
TABLE 41-6 Spinal Cord and Conus Medullaris Reflex Pathways
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Presence of severe hemodynamic parameter abnormalities in the initial
phases of resuscitation is associated with a poor prognosis for
neurologic recovery.167 Normal hemodynamics, on the other hand, do not predict neurologic recovery.
activity during resuscitation, complete spine examination and
neurologic assessment follows resuscitation. The spine assessment
begins with a review of the reports from the field. The sequence of
evaluation and intervention steps differs in unresponsive patients from
awake and cooperative patients. Assessment of acute symptoms is
critical in evaluation of awake patients. Neurologic examination should
be performed concurrently with resuscitation and hemodynamic
stabilization of the patient. Perineal reflex assessment and rectal
examination are essential components of the physical examination in
every trauma patient (Tables 41-6 and 41-7).
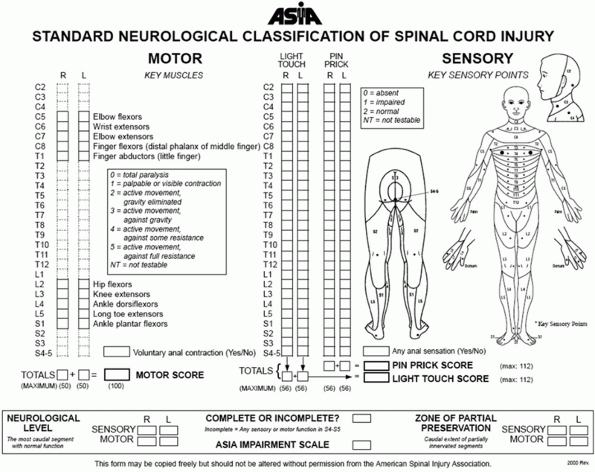
neurologic examination. The American Spinal Injury Association (ASIA)
has identified essential minimal elements of neurologic assessment
recommended for all patients with a spinal injury (Fig. 41-4).15,147,184,197
The essential elements of neurologic function are strength assessment
of five specific muscles in each limb and pinprick discrimination
assessment at 28 specific sensory locations on each side of the body.
On each side of the body, five muscles representing the segments of the
cervical cord and five muscles representing segments of the lumbar cord
are scored on a 5-point muscle grading scale (Table 41-8).
The sum of all 20 muscles yields a total motor score for each patient,
with a maximum possible score of 100 points for patients with no
weakness. For the 28 sensory dermatomes on each side of the body,
sensory levels are scored on a 0 to 2 point scale. A patient with
normal sensation would be assigned a maximum possible light touch score
of 112 points and a similar pinprick score of 112 points. The findings
of sensory testing of the sacral segments distinguish complete from
incomplete spinal cord injury. The sensory examination and motor
strength testing allow designation of sensory and motor levels for each
side of the body and of the overall neurologic level.147,184,197
|
TABLE 41-7 Assessments during Rectal Examination in a Trauma Patient
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
and palpation of the spine. The patient must be rolled on his or her
side using a log-rolling maneuver. The patient’s head and neck are
supported by one assistant and the patient’s trunk is supported by two
to three other assistants. The head and trunk are then rolled by the
assistants in unison while the examiner inspects and palpates the spine
to check for hemorrhage, abrasion, laceration, malalignment, or
palpable gap in the spinous processes. The chest and abdomen are also
examined for contusions or abrasions suggestive of a seatbelt or
steering-wheel induced injury.
minimum necessary assessment by ASIA were chosen because of their
reproducibility.15,147,184,197
They constitute a minimal data set desirable in all spinal injury
patients for accurate communication, particularly for clinical research
study populations. Clinical management, however, requires neurologic
assessment extending beyond essential examination elements recommended
by ASIA. In fact, the examination elements considered optional by ASIA
are often necessary components for actual
patient
care. Assessment of lower extremity and perineal reflexes is important
in determining the severity of neurologic involvement. These elements
are considered optional in the ASIA standards since they do not meet
sufficient reproducibility standards to allow categorization of spinal
cord injury patients for objective comparisons. Although categorization
of injury severity is essential to allow comparisons, guide treatment,
and determine prognosis, neurologic deficits span a continuous spectrum
of severity and may not always fit into clean, distinct categories (Table 41-9).
 |
|
FIGURE 41-4 Neurologic examination recommended by ASIA.
|
division of a classification requires some arbitration and judgment.
The variability in these judgments sometimes makes comparisons across
different research studies difficult. Older spinal cord injury
literature contains variable interpretations of many commonly used
terms. The ASIA definitions have provided guidance for use of these
terms with greater clarity (Table 41-10). These
specific definitions will hopefully improve categorization of spinal
cord injury patients in scientific communication and allow more
meaningful analyses of collective experience.
is the most caudal segment of the spinal cord with normal motor and
sensory function on both sides: right and left sensation, right and
left motor function (see Tables 41-8 and 41-9).128,184,197 Complete injury is defined by the absence of sensory and motor function in the lowest sacral segment.15,18,128,147,295
Sacral sensation refers to sensation at the anal mucocutaneous junction
and deep anal sensation. Sacral motor function is voluntary anal
sphincter contraction on digital examination. Incomplete injuries have
partial preservation of sensory or motor function in the lowest sacral
segment. For a patient to be classified as sensory incomplete, he or
she must demonstrate either sensory preservation (light touch,
pinprick, or both) in the S4-S5 dermatome or
deep anal sensation. To be considered motor incomplete, a patient must
demonstrate either voluntary anal sphincter contraction or a
combination of sacral sensory sparing and presence of lower extremity
motor function more than 3 levels below the designated motor level of
injury (Table 41-11). Details of the examination grading are described in Table 41-8.
carries an improved prognosis of regaining the ability to walk even in patients who are initially motor complete.62,206
In addition, pinprick preservation in more than 50% of the lower
extremity dermatomes L2-S1 in the first 72 hours of injury is
associated with improved prognosis for ambulation (Table 41-12).206
|
TABLE 41-8 Neurologic Assessment Recommended by the American Spinal Injury Association
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
TABLE 41-9 Definitions of American Spinal Injury Association Impairment Scale Categories
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||
a review of observations recorded in the field and during transport by
emergency medical system personnel. The neurologic assessment must be
systematically re-evaluated and updated over time in unresponsive
patients until a complete examination is possible.
identifying a spine injury in unresponsive patients. Spine injury
precautions are maintained in effect until a spine injury is identified
and treated or until assessment is complete and injury excluded. If
imaging studies identify a spinal column injury, the treating spine
surgeon makes a decision regarding the urgency of assessing the
integrity of neurologic structures. In an unresponsive patient, the
options for this assessment are serial neurologic examinations, MRI to
identify structural neural tissue disruption, and sensory or motor
evoked potentials to assess function in neural pathways.95,96,97
Several reports suggest that presence of intramedullary hemorrhage at
the time of initial spinal cord injury is indicative of a poor
prognosis,246,247,250 with many subjects remaining complete96 or at least motor complete.179
Although the degree of bone or soft tissue injury did not correlate
with injury severity, rostral extent of edema and total length of cord
edema did have prognostic value.28,96
One report found that a hemorrhage longer than 4 mm suggested poor
neurologic recovery, but smaller ones were not as ominous for prognosis.28 Miyanji et al.194 recently demonstrated that degree of spinal cord compression had greater predictive value than the amount of canal compromise.
|
TABLE 41-10 Definitions of Terms Describing Spinal Cord Injury
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||
First, spinal shock should be distinguished from neurogenic shock,
which refers to hypotension associated with loss of peripheral vascular
resistance in spinal cord injury. Second, the etiology and significance
of spinal shock are unclear.75 The
confusion surrounding the concept of spinal shock is responsible for
some complacency during the management of spinal cord injury patients
during the initial few hours following injury, the time interval where
intervention may have the most beneficial results. Spinal shock may
involve immediate depolarization of the axonal membranes from kinetic
energy of the injury.148 Spinal
shock disrupts all cord function distal to injury, including reflexes.
Although many effects of spinal shock, such as return of deep tendon
reflexes, may take weeks or even months, early effects of spinal shock
typically resolve within 24 hours of injury.148
The difficulty for practitioners is the varying definitions of spinal shock and different interpretations of its resolution.75
In addition, the delayed plantar reflex, the first sign of emergence
from spinal shock, is present only transiently and can easily be missed
during the focus of immediate life-saving measures. Moreover, it may be
several days before the next series of reflexes (bulbocavernosus,
cremasteric, or anal wink) are observed.
|
TABLE 41-11 Descriptions of Incomplete Spinal Cord Injury Patterns
|
|||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||
substance abuse withdrawal.45 Patients have also been known to worsen in the first 72 hours as maximal cord swelling is reached.38,44,45
Examinations conducted between 72 hours and 1 week after injury more
accurately predict functional muscle recovery than examinations
conducted within the first 24 hours.38
For this reason, distinction of spinal cord injury as complete or
incomplete on the basis of clinical examination is problematic.295
Suspending treatment interventions until resolution of this depressed
reflex state may waste a potentially timesensitive opportunity to
arrest or diminish the secondary injury process in patients with spinal
cord injury.193
|
TABLE 41-12 Ambulation According To ASIA Grade
|
|||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||
evaluation is complete and the patient does not have a spinal injury
requiring treatment. All trauma patients are at risk for spinal injury.
Two to 4.6% of patients presenting with blunt trauma have cervical
spine injuries.53 Systematic
evaluation is necessary to achieve the goal of no missed injuries.
Based on mechanism of injury and physical examination, physician
judgment alone is not accurate in predicting cervical spine fractures.144
Clinical prediction rules are based on measurement of patient
characteristics, injury circumstances, and findings on initial
evaluation associated with spinal injuries. These measures, in
combination with physician clinical judgment, allow efficient
utilization of radiologic imaging studies (Table 41-13).26
-
History to assess for high-risk events and high-risk factors.
-
Physical examination to check for physical signs of spinal injury or neurologic deficit.
-
Imaging studies based on an initial evaluation.
not intoxicated, provided they have an isolated blunt trauma without
neck tenderness on physical examination.133 However, this estimate of “no false negatives” is based on small numbers of patients with fractures.133
Each patient with a diagnosed fracture had at least one of the
following four characteristics: midline neck tenderness, evidence of
intoxication, abnormal level of alertness, or several painful injuries
elsewhere.133 Lack of these clinical
findings suggests absence of a spine fracture, but does not
definitively exclude injury. To reduce routine cervical spine imaging
in trauma patient, two competing prediction rules have been developed
and validated: the National Emergency X-ray Utilization Study (NEXUS)
criteria132 and the Canadian C-spine Rule265 (Fig. 41-5).
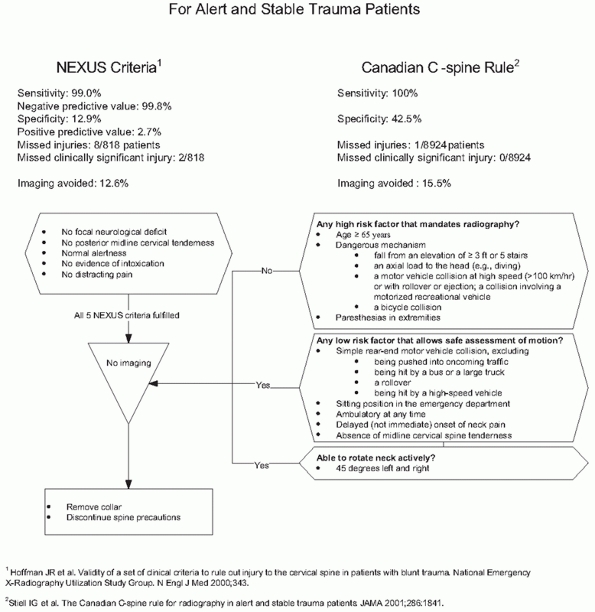
The Canadian C-spine injury prediction rules have better sensitivity
and specificity and reduce unnecessary imaging to a greater extent,264,265 but they are more complex to apply routinely.129,130,131,132,133 Applying Canadian C-spine rules in the field may prevent 38% of out-of-hospital spine immobilizations.26
The clinical presentation of an unrecognized spinal cord injury, such
as a neurologic deficit from a cervical injury, may be misinterpreted
as a stroke.214 In obtunded patients
found unresponsive at the scene, spinal cord injury may present as a
medical emergency, such as bradycardia with hypotension,
hydronephrosis, renal failure, or pyelonephritis associated with
urinary retention. Focused emergency room evaluation of medical
symptoms may overlook the underlying spinal cord injury.20
Spinal cord injury without an associated cervical fracture or
dislocation, advanced age, unusual or changing neurologic deficits,
intoxication, and psychiatric problems all contribute to clinical
confusion and missed diagnoses of cervical fracture.24 Neurologic problems in these patients may be attributed to hysteria, intoxication, or other disease.
similar to that for clearing the cervical spine. Only AP and lateral
view radiographs are necessary. Patients with a clear mental status, no
back pain, and no other major injuries do not need radiographs of the
spine to exclude a spinal fracture.189
The spine in these patients can be safely cleared by a physical
examination alone. Any physician with adequate training and experience
can clear the spine following a directed spine and neurologic
examination of the patient and careful review of appropriate and
adequate quality imaging.237
deteriorate from a missed spine injury. To meet this goal, immediate
recognition of a potential cervical spine injury is essential.83 Patients with missed fractures can develop neurologic deterioration.83,294 Assessment of cervical spine is an essential component of the advanced trauma life support system of trauma care.14
Every trauma patient requires a definitive decision regarding the
presence or absence of spine injury. At minimum, the assessment
requires a careful review of history and a thorough examination.
Usually, spine evaluation involves serial clinical examinations and
review of cervical spine radiographs.
Standard imaging (three views of the cervical spine and CT as
necessary) has a false negative rate of 0.1%. The radiographic
evaluation should be correlated to clinical considerations. The aim of
careful decision-making is to have a zero missed injury rate.
visualization of the cervical spine and include an open-mouth view, are
fairly sensitive in identifying cervical spine fractures. The risk of
missing significant fractures is less than 1% of patients.83 The sensitivity of the lateral radiograph alone is 83% and specificity is 97%.173 The addition of open-mouth and AP view increases the sensitivity to approximately 100%.173
with the patient supine and secure on a backboard. The patient is not
moved to position for the various views; rather, the x-ray beam and
film position is adjusted to provide the desired image perspective.
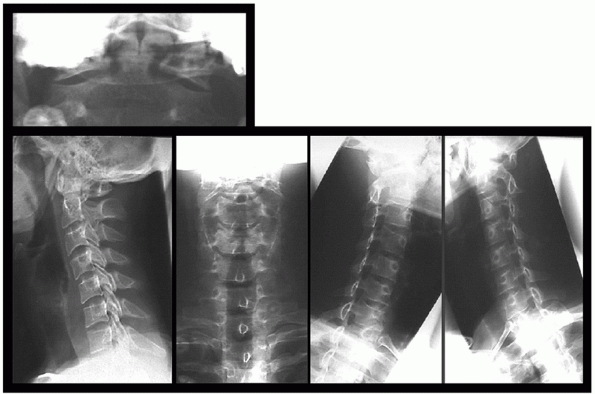
Opinions for minimum number of plain radiographs necessary in trauma
patients range from 0 to 7 (AP, lateral, open-mouth, oblique,
flexion-extension) (Figs. 41-6 and 41-7).125
radiograph is essential, yet this interpretation may frequently be
erroneous because of the stressed circumstances in an emergency setting
and inexperience of the individuals responsible for initial care. The
first step in interpreting radiographs is to make sure they are of
adequate quality for the intended purpose. Lower
quality films significantly increase error rates.213
Adequate lateral cervical spine radiographs require clear visualization
of the spine from the occiput to the first thoracic vertebra. If the
lower cervical spine is not visualized on a lateral radiograph, a
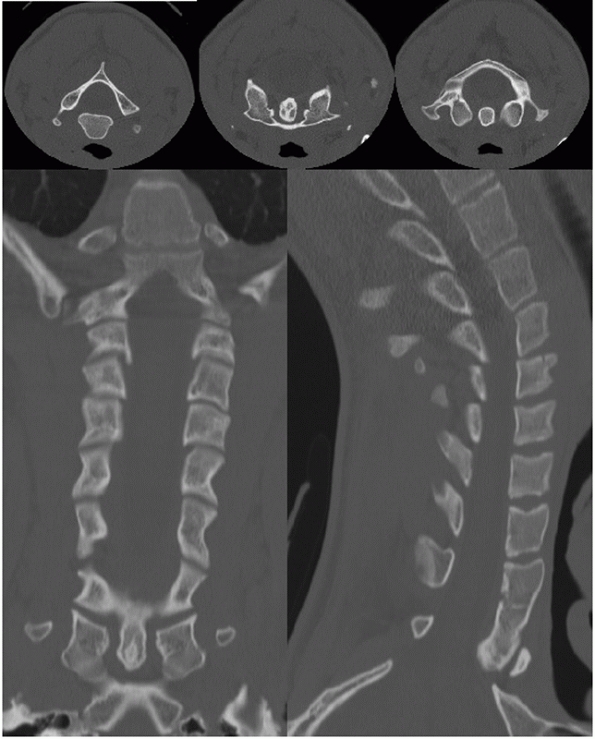
swimmer’s lateral view or a CT scan can visualize this region (Fig. 41-8).
|
TABLE 41-13 Concepts Underlying the White and Panjabi Instability Checklist for the Lower Cervical Spine (C3-C7)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
acute injuries. A change in alignment of the uncovertebral joints and
spinous processes can indicate an acute injury. The open-mouth view is
essential for excluding a C1 arch or C2 odontoid process fractures.
Tomograms or a CT scan may be necessary to substitute for the
open-mouth view in unresponsive patients. Oblique views can identify
injuries of the facet joints, pedicles, and lateral masses,
particularly at the cervicothoracic junction. For this reason, oblique
views in the trauma setting can increase the diagnostic sensitivity of
cervical radiographs.
 |
|
FIGURE 41-5 Comparison of NEXUS Criteria and Canadian C-spine Rule for avoiding imaging in alert, examinable trauma patients.
|
craniocervical, cervicothoracic, and thoracolumbar. They are often the
most difficult to see on standard radiographs. Among these injuries,
the most serious and most frequently missed is craniocervical
dissociation. Harris measurements based on the distance between the
dens and the basion are probably the simplest and most reliable
measurements for identifying craniocervical dissociation.126 Suspicion of injury and careful scrutiny of radiographs minimizes errors of missed injury.224
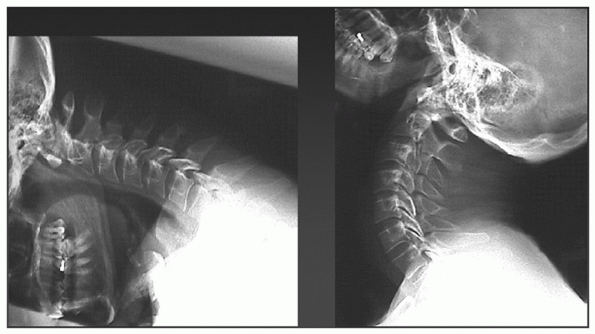
radiographs, flexion-extension views can identify occult cervical
ligamentous injury (see Fig. 41-7).
Flexion-extension views in the acute setting of an emergency room,
however, can be nondiagnostic or even dangerous. Patients in acute pain
may have limited mobility related to muscle spasm, limiting cervical
spine motion on dynamic views. Unsupervised or forceful flexion in a
patient with an occult ligamentous injury may precipitate a neurologic
injury. When necessary, flexion-extension radiographs should be
performed in alert patients, under supervision, and with voluntary
unassisted positioning by the patient.
 |
|
FIGURE 41-6 Standard radiographs of the cervical spine.
|
of anatomy and clinical experience are important for accurate
interpretation of radiographs.199
Landmarks for measurements can be difficult to identify. A systematic
approach to reading cervical radiographs can help reduce the chances of
missing an important injury. Alignment of the cervical vertebrae is
assessed by examining longitudinal lines along vertebral bodies,
lamina, and spinous processes. Examining alignment of the lamina in the
upper cervical vertebrae is particularly helpful in excluding injuries
of the craniocervical junction in both children and adults.
indicator of swelling from acute hemorrhage. Increased thickness and
altered contour of the pharyngeal tissue anterior to C2 (i.e.,
convexity instead of concavity caudal to the C1 anterior arch) suggest
acute craniocervical injury. The prevertebral soft tissue shadow
thickness, however, becomes unreliable in the presence of oropharyngeal
tubes. Also, soft tissue swelling can occur without bony injury, and
conversely, bony injuries can occur without significant soft tissue
swelling.192 Prevertebral soft tissue widening resolves to normal after 2 weeks in 50% of patients, and in 3 weeks in 90%.213
 |
|
FIGURE 41-7 Flexion-extension radiographs.
|
MRI is superior in demonstrating spinal cord pathology and
intervertebral disc herniation. CT is superior to MRI in demonstrating
osseous injury. However, injuries purely localized to the transverse
plane, such as odontoid fracture, can be missed on axial CT images. For
these types of injuries, direct coronal CT can provide superior demonstration of skeletal features in the upper cervical spine.212
 |
|
FIGURE 41-8 Images from a screening cervical spine CT.
|
expose edema and hemorrhage associated with acute spinal cord injury.
Increased cord signal and parenchymal cord hemorrhage indicate poor
prognosis for neurologic recovery.28,96,97,179,246,247
MRI is particularly useful for assessing the craniocervical junction.
Edema in the occipitocervical facet capsules and basicervical ligaments
or acute cervicomedullary angulation suggests craniocervical injury.41
While MRI can offer exceptional imaging of soft tissues and may prove
valuable in the management of spinal injuries, its use for primary
spine clearance is limited. MRI (usually with sagittal short tau
inversion recovery images) is very sensitive to muscular and soft
tissue injury and often does not correlate with clinical instability.278
MRI has high sensitivity for identifying injuries to the disc space,
posterior longitudinal ligament, facet joint, and posterior
interspinous tissues; it is less sensitive for anterior longitudinal
ligament and ligamentum flavum.111
In unstable injuries, MRI within 24 hours of injury may show edema
across the entire vertebral column: prevertebral space, disc space,
facet joints, and interspinous ligaments.111 If increased MRI signal is not present in all four regions, correlation with unstable injury is lower.111
vertebral artery blood flow in cervical trauma, which can be frequently
disrupted in cervical spine injuries.100 MR angiograms are abnormal in 24% of patients.100 However, MR diagnosis of arterial artery may not be functionally significant.255
Patients often need emergent care and transport prior to the spine
being cleared. Emergent life-saving interventions, such as intubation,
anesthesia, and abdominal or chest surgery, can be performed relatively
safely using appropriate precautions in a patient with incompletely
evaluated cervical spine.
Obtaining an AP chest radiograph, AP pelvis radiograph, and a lateral
cervical spine radiograph does not interfere the urgent management of
multiply injured patients during resuscitation. These three images
provide crucial information that facilitates resuscitation and comprise
the standard “trauma series” in trauma centers. Additional emergent
spinal imaging is only necessary if these initial views demonstrate a
spine injury or if the primary survey examination suggests a neurologic
deficit. Otherwise, further spinal imaging can follow resuscitation and
hemodynamic stabilization. Plain radiographic studies are increasingly
being replaced by CT for initial cervical spine imaging in trauma
patients since radiographs. Cervical spine CT is frequently performed
in conjunction with head CT. CT imaging adds diagnostic information in
approximately half of the injuries identified on plain radiographs.58
initial trauma lateral radiograph are completed once the patient is
medically stable (see Fig. 41-5). These
additional views include open-mouth, AP, and in some institutions,
right and left oblique radiographs. Alternatively, trauma patients at
high risk for cervical spine injury can be screened for a cervical
injury with a rapid-sequence helical CT scan (see Fig. 41-7).124
Patients with an incomplete spinal cord injury may require an emergent
MRI to identify the source of cord injury, if radiographs and CT do not
show vertebral column injury consistent with the neurologic exam.236
progressive neurologic deficit. In an important publication that
stimulated debate about acute MRI, 6 patients were described as having
an unrecognized disc herniation that potentially contributed to
neurologic worsening after reduction of a cervical spine facet
dislocation.81 Many surgeons cite
this report as justification for obtaining prereduction MRI in patients
with spinal cord injury associated with cervical facet dislocation.
However, this report acknowledged other treatment-related complications
that may have also contributed to deterioration in three of the six
reported cases.
In patients with spinal cord injury associated with cervical spine
fracture-dislocations, the most urgent priority after life-saving
measures is mechanical decompression of the spinal cord. This is most
expeditiously and efficiently achieved through closed reduction. Rapid
closed reduction is successful and safe.113
Waiting for MRI in this setting should not delay closed reduction.
Other interventions or other diagnostic tests for nonlife-threatening
conditions also should not supersede the priority for closed reduction.
Postreduction MRI, however, is useful to look for disc extrusion, if
neurologic deterioration occurs or if planning approach for definitive
surgical treatment is required.239
dislocation do not have the time-urgency of spinal cord injury. In
these patients, MRI may be obtained prior to reduction without
adversely influencing outcome. The disc at the level of dislocation is
usually abnormal on these images.78
Such disc abnormalities have been advanced as an argument for
decompressing the damaged disc prior to reduction. However, induction
of anesthesia and positioning for surgery remain challenging in a
patient with a dislocated cervical spine. Furthermore, performing an
open reduction through an anterior approach is a difficult task. Neck
swelling occurs more commonly after an anterior approach, leading to
potential swallowing deficits or prolonged intubation postoperatively.
The improved stability gained from reduction, safer induction of
anesthetic, and the option of posterior surgery are arguments for
attempted closed reduction even in neurologically intact patients.
may have an occult fracture or ligamentous injury. Also, fractures may
be difficult to see if patients have severe degenerative disease,
osteoporosis, or ankylosis of the spine. In these settings, MRI or a
technetium bone scan can facilitate diagnosis of occult injuries.22,94
patients with high-energy injury mechanisms. In these patients, the
spine is sometimes deferred until the patient is examinable. However,
that may be weeks to months in some patients. Meanwhile, these patients
may develop complications associated with external bracing and mobility
restrictions imposed by spine precautions. If a CT scan is completely
normal, the spine may be cleared without further imaging or examination.127
When the CT scan is difficult to interpret due to severe degeneration,
osteoporosis, DISH, or ankylosing spondylitis, then additional imaging
such as MRI, clinical examination, or dynamic imaging such as a
traction test, may be needed to rule out injury of the cervical spine.
Thoracic and lumbar injuries can be reliably excluded by radiographs
alone.
of cervical spine injuries suggested neurologic deterioration occurred
after admission in 5% of spine injury patients.181 An identifiable specific management event is associated with 86% of these deteriorations.181 Unstable spinal injury should be assumed and the patient protected.116
Log-rolling does not keep the spine immobile, and unstable injuries
should be stabilized immediately when the patient’s medical condition
permits doing so safely.186
Extremely combative patients with closed head injury may require
intubation and chemical paralysis to protect against neurologic injury
from an associated spine fracture. Fiberoptic intubation or laryngeal
mask airway should be considered in the management of patients with
cervical spine instability.37
inadequate radiographs (44%) and misinterpretation of adequate-quality
radiographs (47%). More frequently than cervical injuries, the
diagnosis of thoracolumbar fractures may be delayed in 11% of trauma
patients and missed in 5.5%.189 A
thoracolumbar fracture may not be recognized despite complaints of back
pain by 66% of these patients during their initial evaluation.189
Patients with a missed thoracolumbar fracture that do not complain of
back pain have either altered mental status or other major associated
injuries.189 Back pain in trauma
patients should be taken seriously and evaluated thoroughly, and the
evaluation should include radiographic imaging.
In trauma care, this goal implies protecting all patients until a
spinal injury is definitively excluded or identified and treated. Also,
caring for a trauma patient requires that associated injuries be
expeditiously identified and appropriately addressed. For patients
sustaining a spinal column injury, the treatment focus is protecting
uninjured neural tissues, maximizing recovery of injured neural
tissues, and optimizing conditions for the musculoskeletal portions of
the spinal column to heal in a satisfactory position.
patients requires concerted activity of a trauma team. Experienced
field personnel, emergency room physicians, general surgeons,
orthopaedic surgeons, neurosurgeons, radiologists, anesthesiologists,
physiatrists, and nursing personnel are integral members of this team.
The overriding general principle in efficient care for trauma patients
is early involvement by appropriate members of this team. Physicians
ultimately assuming the long-term management of trauma patients,
frequently orthopaedic surgeons
and physiatrists, are particularly critical in directing optimal initial care.112
|
TABLE 41-14 Goals of Spine Trauma Care
|
|||||||
|---|---|---|---|---|---|---|---|
|
the spine until spinal injury is definitively excluded or treated. This
general principle has specific patient-care implications commonly
referred to as “spine precautions.” All trauma patients should be
maintained in the supine position at strict bedrest with the bed flat,
transfers with a spine board, and frequent log-rolling for decubitus
ulcer prophylaxis. Alternatively, patients may be placed in a rotating
frame for improved pulmonary mechanics and skin care.
skull traction, except injuries with complete ligamentous disruption,
usually indicated by distraction between vertebrae on imaging studies.
Distraction injuries are the most unstable spine injuries. Skull
traction in these patients will lead to catastrophic neurologic
deterioration or even fatal vascular injury. Patients with distraction
injuries are best immobilized with sandbags and tape or a halo
apparatus. Even when immobilized in the halo apparatus, these patients
should be maintained in strict bedrest with full spine precautions
until definitive surgical stabilization.
the external auditory meatus, 1 cm above the pinna. Carbon fiber tongs
with titanium pins should be used initially to permit subsequent MRI
evaluation if necessary. Carbon fiber tongs, however, grip the skull
less strongly than steel tongs. Pins in carbon fiber tongs may pull out
of the skull with traction weights above 80 pounds. Pins in steel tongs
can withstand traction up to 140 pounds.157
On occasion, if closed reduction in a cervical injury requires weights
greater than 80 pounds, carbon fiber tongs may be exchanged for steel
tongs prior to applying heavier weights.
In young patients with no osteoporosis, traction weight up to 70% of
body weight is generally safe, provided steel tongs are used and pins
are properly placed.244 Cervical
traction at weights larger than 80 pounds should not be applied to most
carbon fiber MRI compatible tongs; traditional steel Gardner-Wells
tongs are less like to slip with larger weights.157 Traction should not be applied in injuries showing distraction of the spinal column.
The window of opportunity for maximal neurologic improvement through
decompression may be as short as the first few hours following spinal
cord injury.48,49,50,68 Spinal cord injury patients may have an excellent capacity for spinal cord recovery regardless of initial presentation.39
Reduction within the first few hours of injury may lead to dramatic
improvement in neurologic status. Reduction within 2 hours of injury
has been reported to reverse tetraplegia.270
Emerging evidence from the Surgical Treatment of Acute Spinal Cord
Injury Study indicates that decompression within 24 hours is associated
with improved neurologic recovery. Although results of this
observational study are preliminary, investigators found a 2-3 grade
improvement in the ASIA Impairment Score in those decompressed within
24 hours. Moreover, higher rates of surgical complications were found
in subjects who underwent surgery after the first 24 hours.89,91a,161,162a
decompression. Emergent attempted closed reduction is the treatment of
choice for alert cooperative patients with acute spinal cord injury
from a cervical spine dislocation.259 In these patients, MRI is not necessary prior to reduction and should not delay reduction.165
Reduction in an unconscious or unexaminable patient should be preceded
by an MRI scan. In this situation, the presence of a herniated disc may
be treated with surgical decompression before reduction.81,236
craniocervical dissociation or a cervical injury that shows distraction
at the injured segment, require compression for reduction, not further
traction. Compression across the cervical spine can be applied by a
halo vest.196
In fact, a case in one of these series was of patient with a large disc
herniation seen on a prereduction MRI scan resolved with closed
reduction.113 Closed reduction also decreases the need for more complicated surgical procedures later.269
spinal injuries. Clinical observation reports, biomechanical
investigations of stability, and radiographic measurements of stability
have not produced definitive recommendations applicable to specific
cases in deciding closed or operative treatment. The only consistent
indication for surgical treatment may be skeletal disruption in the
presence of a neurologic deficit. A consistent contraindication to
closed treatment is an unstable purely ligamentous spinal column injury
in a skeletally mature patient. Although these injuries may heal
sufficiently in pediatric patients with significant growth remaining,
in adult patients the healing response does not restore sufficient
strength to provide spinal column stability, regardless of the length
of bedrest or external immobilization. Unstable ligamentous injuries
require fusion. Osseous injuries heal adequately but require treatment
to control deformity.
Bedrest as definitive treatment may be indicated in rare cases of
patients unable or unwilling to undergo bracing or surgery: severe
pre-existing deformity, morbid obesity, medical comorbidity, or
personal preference. Bedrest for the initial few weeks preceding
bracing is an option for severely unstable injuries. If bedrest is
determined as a treatment option, measures must be taken to provide
pressure relief to areas at risk for skin breakdown. Sacral, calcaneal,
and occipital pressure ulcers are particularly problematic in persons
with spinal cord injury remaining on bedrest for undefined periods of
time.60,159
category are equivalent.23
Custom molded trunk orthosis provide added rotational control. Casts
can be applied in hyperextension to improve kyphosis. Bracing is
continued until bone healing is sufficient for load bearing: 8 weeks in
cervical injuries and 12 weeks in thoracolumbar injuries.
based on reports of experience and observation, not rigorous clinical
trials. Surgical stabilization of the spinal column can prevent further
mechanical injury to the damaged cord tissue. Removing residual
compressive mass effect may additionally allow better neurologic
recovery. Closed treatment of unreduced injuries may lead to chronic
pain requiring later surgical treatment.23
recognized as potentially pivotal in affecting neurologic recovery.
Early intervention in this setting is not defined in days after the
injury, but rather in minutes and hours. Animal studies have suggested
a potential window of opportunity in the first 3 to 6 hours after
injury in which significant neurologic recovery may be possible (Fig. 41-9).48,49,68
exceptions: odontoid fractures and C2 arch fractures. These two
injuries in specific circumstances may be treated with internal
fixation (osteosynthesis). Open reduction and instrumentation may be
just as effective fusion for spinal fractures.208 Early surgery reduces hospital stay.207
Injured vertebrae are associated with injury to nearby soft tissues.
Anterior interbody grafts without fixation may work for anterior
cervical fusion following discectomy. However, grafts of this nature
are prone to displacement if there is posterior instability or gross
deformity of the vertebral body, unless supplemented by fixation.289 Anterior plating and posterior plating are equally successful in cervical trauma.91,110 Anterior plating provides immediate stabilization even with posterior ligamentous injury.52
Although the strength of the fixated spine is relatively unchanged by
corpectomy and anterior grafting, anterior grafting improves alignment.177 Fixation maintains alignment.195 Anterior fusion allows early mobilization, shorter stay, and less cost.271 Spinal cord blood flow may, however, be adversely affected by an anterior surgical approaches.
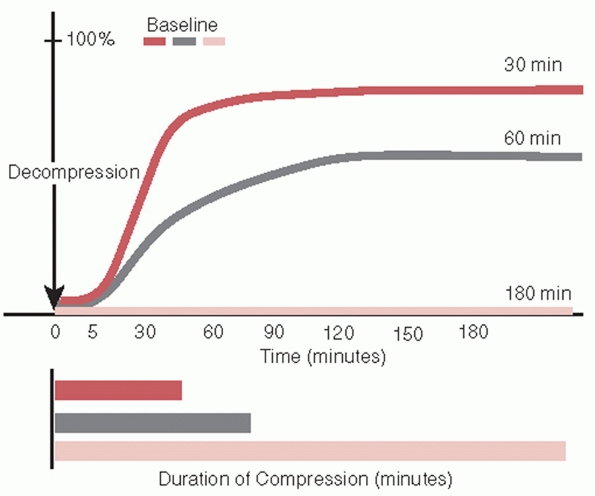 |
|
FIGURE 41-9 Electrophysiologic recovery diminishes with longer duration of cord compression.
|
treated with anterior instrumentation should be either augmented with
posterior instrumentation or postoperatively immobilized in a rigid
external brace.178 In burst
fractures, anterior reconstruction with fixation is more stable than
the posterior instrumentation systems in all loading conditions.253
fractures should not be based on any hypothetical differences in
reductive power.86,291
Canal clearance is most effective when carried out in the first 4 days
after injury and in patients with an initial canal compromise of 34% to
66%.108 Percentage of encroachment decrease in posterior systems is small.55 Laminectomy increases deformity and neurologic deficit unless combined with internal stabilization.46,261 Traumatic dural tear should be repaired before any anterior or posterior spinal reduction maneuver.70
Primary anterior decompression and fusion is preferred in an axial
loading or flexion compression injury with a large midline retropulsed
fragment that produces a significant neurologic deficit.185,232
As injury progresses to involve all three structural columns, the
ability of the transpedicular constructs to restore preinjury stiffness
decreases. Several reports have identified failure of fixation when
thoracolumbar vertebral body fractures are treated with short-segment
posterior fixation.80,188
One option to reduce risk of hardware failure prior to solid fusion is
to augment transpedicular constructs with anterior bone grafting.47,252,256 However, external bracing to protect posterior fixation during healing remains an alternative to anterior surgery.
steroids in acute spinal cord injury. The magnitude of observed
neurologic improvement is minimal and controversial compared to risk of
infection and gastrointestinal bleeding complications. We recommend a
screening CT to image the cervical spine in obtunded patients, and
discontinuing cervical spine immobilization and mobility precautions if
the study is normal (Fig. 41-10). Decompression
should be achieved expediently in patient with spinal cord injury,
whether complete or incomplete. For medically stable, examinable
patients with cervical spine dislocation and severe neurologic injury,
such as complete tetraplegia, we recommend emergent closed reduction
prior to MRI or other time-consuming interventions.
serious adverse consequences for subsequent care. For optimal spine
evaluation of trauma patients and reducing risk of complications, we
recommend observing the following principles.
-
Personally review outside imaging studies; do not assume studies were adequate or interpretation was accurate.
-
Convey your level of concern for
potential spine injury clearly to all members of the team caring for
the patient. Trauma care is multidisciplinary; coordination of care is
critical for successful outcomes, both when spinal injury is identified
and also when spinal injury is excluded. -
Do not accept inadequate quality
radiographs or other imaging for definitive decision-making. An initial
lateral radiograph “clear to C5” does not mean “no cervical spine
injury.” -
Look for thoracic fracture-dislocation on
the trauma AP chest radiograph: lateral translation between vertebrae
or widened disc space. -
Perform a neurologic examination
unobtrusively during resuscitation, if possible, prior to intubation or
pharmacologic paralysis. Identifying a neurologic deficit may change
treatment priorities. -
Record personal neurologic examination
accurately in the medical record: moving all extremities (MAE),
neurovascular intact (NVI), and “wiggle your toes” are not adequate
assessment or documentation of neurologic function. -
Check sacral pinprick sensation. Sacral
sparing assessment is essential for distinguishing a complete spinal
cord injury from an incomplete injury, and this distinction is
important for prognosis of neurologic recovery. -
Do not attribute neurologic deficit to
intoxication, effect drugs, or pain from extremity injuries. This
dangerous assumption that may limit proper management of neurologic
injury. -
Do not dismiss unusual or complex
neurologic deficits as nonphysiologic. Incomplete spinal cord injury
can present with complex neurologic deficits, and not recording or
investigating them urgently can delay appropriate acute treatment of
spinal cord injury. -
Pay careful attention to any discrepancy
between neurologic level on examination and radiographic level of
injury. The difference may be due to another vertebral injury level,
hemorrhage, ligamentous disruption, or other process causing cord
compression. -
Differentiate between neurogenic shock
and hypovolemic shock. Treating neurogenic shock with fluid
resuscitation instead of vasopressors may result in volume overload,
pulmonary edema, or adult respiratory distress syndrome. -
Do not delay closed reduction of a
dislocated cervical spine in a patient with complete tetraplegia. Delay
to obtain CT, MRI, or other diagnostic tests not essential to reduction
may miss an opportunity to decompress the spinal cord during an early
postinjury period when it has the best potential for recovery. -
Do not apply cranial traction pins in a
patient with a skull fracture without consulting a neurosurgeon.
Placing pins in fracture fragments may exacerbate cranial injury. -
Use traction tongs, weights, and pins
compatible with MR. Some pins and tongs may develop heat, displace, or
cause interference with the image. -
Do not apply cervical traction in an
injury that shows widened disc space or other distraction.
Overdistraction and vertebral artery injury is a potentially fatal
complication. -
Look for associated injury patterns. A
patient with a flexion-distraction injury will frequently have lapbelt
marks and abdominal injury, bilateral calcaneus fractures may be
associated with a lumbar burst fracture, a thoracic
fracture-dislocation may be associated with a vascular injury, and
cervical fracture in ankylosing spondylitis may be associated with
esophageal injury. -
Look for additional levels of spinal injury. Approximately 15% of patients have multiple discontiguous levels of spinal injury.
-
Remove the backboard within 2 hours of
application; pressure sores can develop in patients laying on a
backboard for more than 2 hours. -
Consider age and mechanism of injury before allowing a patient to be discharged home without additional evaluation.
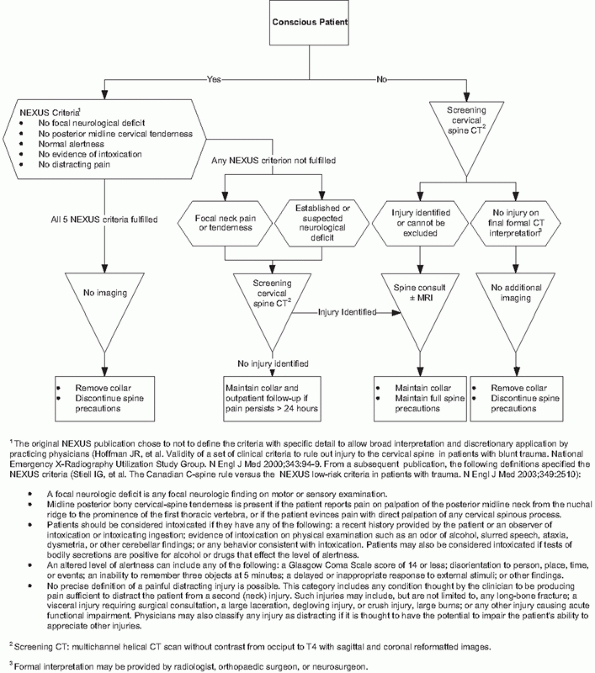 |
|
FIGURE 41-10 Dartmouth Spine Clearance Protocol.
|
Harm to a patient with spine injury takes the form of missed diagnosis,
missed associated injuries such as an aortic tear with a thoracic
fracture-dislocation, a missed abdominal injury with a lumbar
flexion-distraction injury, or marked neurologic deterioration in a
neurologically intact patient. The frequency of complications during
acute hospitalization is increased in the presence of a neurologic
deficit (Table 41-15).98
Even when they are not life-threatening, complications can prolong
hospitalization and compromise outcome. Complications during initial
hospitalization add $1.5 billion annually to the cost of caring for
patients with vertebral fractures in the United States.98
|
TABLE 41-15 Complications in Spine Injury Patients
|
|||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||
Function after spinal injury seems dependent primarily on the injury
itself and less on the method of treatment, residual spinal mobility,
or the radiographic results.36,166,219,220 Nonsurgical treatment of neurologically intact patients is associated results equivalent to surgical treatment.57,198,307 Anterior and posterior surgery yield equivalent function in neurologically intact patients.308 In the absence of neurologic injury, pain and function approach population norms at 3 to 8 years following the injury.166
patients with spinal cord injury, clinical studies have not shown an
association between neurologic and skeletal outcome.77 Clinical outcome in general is not related to deformity.11,200,272
Kyphosis of more than 30 degrees may increase pain, but this threshold
is based on a impressions of surgeons, not rigorous clinical research.106 Residual canal occlusion does not result in late symptoms of spinal stenosis.
A recent report identified several important secondary health
predictors that significantly improved prediction models of mortality
when compared with
injury severity models alone.158
Secondary health conditions thought to influence mortality include
surgery to repair pressure ulcers (which strongly correlates with
severity of pressure ulcer); infection symptoms, notably urinary tract
infections; amputations; additional bone fractures remote from acute
injury; and probable major depression. Older patients with complete
tetraplegia have very high acute mortality (60%-100%).4,301
In contrast, over 90% of patients with central cord injury survive the
initial hospitalization. Life expectancy following spinal cord injury
is related to the severity of neurologic deficit, with decreased
survival in patients with more severe deficits (Table 41-16).266 Older age at time of spinal cord injury is also associated decreased survival (Table 41-17).168
|
TABLE 41-16 Life Expectancy Following Spinal Cord Injury, for Those Surviving the Initial 24 Hours
|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||
nature and magnitude of initial injury. The pattern of spinal cord
injury does not correlate with the pattern of skeletal injury on plain
radiographs.266 Cord hemorrhage is
associated with less neurologic recovery. When controlling for
neurologic level (paraplegia versus tetraplegia) and completeness of
spinal cord injury, motor recovery does not differ for type of injury
(penetrating versus nonpenetrating) or type of fracture.298
Complete cord injuries are more likely in flexion-rotation patterns of
injury, bilateral facet dislocation, and gunshot injury with bullet
traversing the canal. Incomplete injuries are associated with
pre-existing spondylosis and gunshot injury with bullet not traversing
the canal.298 The initial motor
index score correlates with overall function at the time of discharge
from rehabilitation in tetraplegia and complete injuries but not in
paraplegia and incomplete injuries.164
Levels that have some voluntary motor function at 1 week after injury
are likely to achieve three fifths of original strength by 1 year
postinjury.75 Pediatric patients with incomplete injuries have a good prognosis; neurologic deficit improves in 74% and resolves in 59%.123 Pediatric patients with complete injuries show improvement in 10% and resolution in none.123
|
TABLE 41-17 Life Expectancy for Persons with SCI Surviving at least 1 Year Postinjury
|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||
found conversion rates of complete to incomplete injuries as high as
20% within the first month, the study included patients before and
after the shift in the ASIA definitions which were changed in the year
2000. Burns and colleagues45 found
that up to 9.3% of subjects initially considered complete at 72 hours
were reclassified as motor complete, sensory incomplete (ASIA
Impairment Score B) in the first week of injury, due to challenges
affecting the reliability of the early examination. In contrast, only
2.6% of subjects without factors impeding accuracy of early exam
converted to sensory incomplete and no subjects were upgraded to motor
incomplete.45
can occur. Early studies suggest approximately 4% of injuries complete
at 21 days convert to incomplete. Three of 6 patients with late
conversion regained bladder and bowel control and 2 regained the
ability to ambulate.299
In the largest series to date, 987 subjects were evaluated for changes
in ASIA Impairment Score, Motor Index Score, motor level, and
neurologic level of injury. Of the 539 subjects who were neurologically
complete at 1 year, 94.4% remained complete at 5 years postinjury, with
3.5% converting to motor complete sensory incomplete and only 2.1%
improving to motor incomplete.152
Complete paraplegia at 1 month following injury is associated with
essentially no motor recovery in the lower extremities if the injury
level is cephalad to T9.299 With a more caudal level, 38% regain some lower extremity motor function.299
Of patients with neurologic levels at or below T12, 20% gained
sufficient lower extremity motor function to reciprocally ambulate with
conventional orthoses.
Incomplete injuries carry a much better prognosis for recovery than
incomplete injuries. For example, most patients with Brown-Séquard type
of incomplete tetraplegia ambulate independently (75%) and nearly all
regain bladder and bowel control.242
Patients with ASIA Impairment Score B incomplete paraplegia, motor
complete but preserved bilateral sacral pin sensation, gained an
average of 12 motor score points at 1 year.297 Nearly half (46%) of these patients are able to ambulate with a reciprocal gait.297
Mechanism of injury is likely physeal failure through a fracture in
hypertrophic zone of endplate, leading to distraction of cord and
ischemic injury. Children have a greater capacity for neurologic
recovery following spinal cord injury than adults, and the recovery can
continue over prolonged periods.293
Studies on the use of methylprednisolone in spinal cord injury did not
include children and, therefore, no data exist to comment on the use of
such agents in the pediatric population.
Diagnosis is often missed. The patients are sent home as normal, called
hysterical, or undiagnosed during stupor or coma. Cervical spine injury
commonly occurs with relatively minor trauma in patients more than 65
years of age.172 C2 injuries, especially odontoid fractures, must be ruled out in older patients with neck pain after even a minor injury.172,258 Overall mortality rate these patients is 26%.4 Patients older than 50 years of age with complete spinal cord injury have a 60% mortality rate.4,5,258 Injury severity decreases survival, particularly in older patients.117 Bedrest, traction, and halo immobilization are poorly tolerated by older people.170
Surgery may be necessary for dural repair in patients with a
cerebrospinal fluid leak or fistula. Débridement and bullet removal is
an option if laparotomy for abdominal injury exposes the spinal injury
area without added surgical morbidity. If the projectile traverses the
orophrynx or colon, intravenous antibiotics should be administered for
7 to 14 days for infection prophylaxis.29 NASCIS trials excluded penetrating spinal injury and no data exists as to the efficacy in such patients.
of spine surgery. Spinal fusion and fixation surgery, in fact, is
performed primarily to restore stability of the spinal column following
instability from injury, degeneration, or decompression to address
neural tissue. It is natural to expect that a concept so integral to
the daily work of spine surgeons would be well understood and well
defined. Unfortunately, the contrary is true: spinal instability is
variably defined, widely interpreted, and inconsistently measured. The
components included in the list of “spine instability” are so
wide-ranging that the concept has little meaning without specific
definition in each context of its use. Stable and unstable lesions can
have an associated neurologic injury. Stable and unstable lesions are
reported to be equivalently managed with surgery and nonsurgical
treatments and are reported to have similar results of treatment.
Attempts to assign universal meaning to the term “instability” have
rendered it useless. It should be explicitly defined wherever it is
used. Since treatment and outcome of spine injuries is integrally
related to the neurologic status, definition of spinal stability in
trauma should be centered on preservation of neural function.
focused on the vertebral column and not on neural structures or neural
function.69,93,134,300
For the cervical spine, a commonly advanced principle is that the
functional spinal unit, composed of two adjacent vertebrae and their
intervening ligaments and intervertebral disc, is stable if all
anterior structures plus one posterior structure are intact or,
alternatively, if all posterior structures and one anterior structure
is intact.302 In this enticingly
elegant rule, anterior structures are the vertebral body and the
intervertebral disc and posterior structures are facet joints, laminae,
spinous processes, and the posterior intervertebral ligaments. The rule
is based on biomechanical laboratory studies where investigators
applied opposing anteriorly and posteriorly directed force to adjacent
cadaveric vertebrae and sequentially sectioned each structure.302 This work was later summarized into a checklist to help clinicians evaluate cervical spine stability (see Table 41-13).303
checklist for instability assessment is helpful in providing a
framework for evaluating a spinal injury or other vertebral column
destructive lesions. Although the elements scored in the assessment
were compiled for the cervical region of the spine, similar anatomic,
functional, and physiologic considerations also apply to the evaluation
of thoracic and lumbar lesions. The checklist organizes the various
considerations
necessary
in thoroughly analyzing a spinal injury and formulating the treatment
plan for an individual patient. Calculation of specific numerical
instability score, however, does not provide a reliable cookbook
formula for treatment determination. The scores are difficult to
confidently calculate in actual patients with real injuries, and no
clinical studies have confirmed the validity of the numerical score
method for determining which injuries should be surgically stabilized.
Also, in our (the authors’) opinion, neurologic considerations are
likely undervalued in the checklist scoring method. If a patient has
vertebral column disruption associated with a spinal cord injury, the
stabilizing role of the vertebral column has failed, and in this
setting, we most often will prefer surgical stabilization over
nonsurgical treatments.
The first trial (NASCIS I) compared low-dose (100 mg bolus and 25 mg
every 6 hours for 10 days) to high-dose (1000 mg bolus and 250 mg every
6 hours for 10 days) methylprednisolone administered within 48 hours of
injury.31 The results showed no
difference in motor or sensory outcome at 6 weeks, 6 months, and 1 year
following injury. An increased infection rate was seen in the high dose
group.
(30 mg/kg loading dose and 5.4 mg/kg/h every 23 hours) to naloxone and
placebo.32 The authors claimed
statistically significant improvements in motor and sensory scores in
both complete and incomplete injuries in the group receiving steroids.32
The magnitude of effect was small: neurologic change score (improvement
in motor score) was 16.0 in the treatment group and 11.2 in the control
group, with a p value of 0.03 for the difference.32 Pinprick score change was 11.4 in the treatment group and 6.6 in the control group (p = 0.02).32 These differences reached statistical significance because of the large sample size for the study.
compared the motor and sensory outcomes from spinal cord injuries when
treated with the dose regimen shown effective in NASCIS II (30 mg/kg
bolus followed by 5.4 mg/kg/hr for 23 hours) versus an extended dose of
methylprednisolone (same bolus dose with the infusion lasting 48 hours)
versus an initial bolus of methylprednisolone with a 48 hour
administration of tirilazad (a lazaroid with free radical scavenging
benefit). The authors concluded that all three arms achieved equal
benefit if the treatment was started within 3 hours of the injury.
After 3 hours, but within 8 hours of injury, the extended dose regimen
of 48 hours of methylprednisolone gave superior results.
generally accepted by medical practitioners and the dosing regimens of
either NASCIS II or III were widely adopted. However, several
limitations were brought forth by critics and an ongoing conflict has
yet to be resolved regarding the utility of steroids in spinal cord
injuries.
In brief, the papers were criticized on a number of issues: statistical
analyses, lack of standardizations in treatment, failure to show
significant functional recovery, lack of minimum injury inclusion
criteria, and an inability for independent reviewers to access the raw
data for further investigation.
the development of the 8 hour cutoff in NASCIS II and the 0-3-hour and
3-8-hour window in NASCIS III. The original NASCIS II hypothesis
included a plan for analysis of early versus late treatments. While the
critics are correct in stating the exact times were selected from post
hoc analyses, the 8-hour window was created since the median time to
treatment was 8.5 hours. A similar argument concerns the development of
the 0-3-hour and 3-8-hour window in NASCIS III. Researchers have
requested the data to see if the treatment is a time limited effect
(i.e., the earlier steroids are received, the more likely a better
outcome regardless of a hard 3-hour or 8-hour window). Unfortunately,
to date, no independent assessment of the data has been published.
motor score of 7 muscle groups (using a 0-5 point scale) on both sides
of the body. Only the scores from the right side were used for
analyses. Improvement was graded as a percentage and not evaluated as a
functional gain in NASCIS II. While function was evaluated in NASCIS
III, the improvements are modest and need to be weighed against the
potential complications.
created in any of the trials. While this may reflect the
implementations of the treatments in real world settings, it opens the
data to potential bias with unaccounted interventions.
risk. NASCIS III data showed the 48-hour regimen to result in a
statistically significant increase in pneumonia and a trend toward
increased severe sepsis. Further reports have additionally shown higher
rates of complications (infection, pulmonary, gastrointestinal
bleeding) in patients receiving high dose steroids.182,218
have undergone continued re-evaluation with continued critiques and
rebuttals from the lead author. As the criticisms mounted against the
papers and the request for third party review of the data has yet to
appear, the acceptance of steroid use has declined. Hurlbert, one of
the most vocal critics of the trials, and Hamilton140
document a dramatic turn in the use of steroids in spinal cord injury
in Canada. Their survey suggests that in 2001, 76% of surgeons
prescribed steroids for spinal cord injury (concerningly, many for
reasons of “being sued or from peer pressure”).140
Five years later, the number had dropped to 24%. Similar trends are
occurring in the United States, perhaps the most notable being the
American Association of Neurological Surgeons/Congress of Neurological
Surgeons joint statement on the Guidelines for the Management of Acute
Cervical Spine and Spinal Cord Injuries in which they concluded there
is insufficient evidence to support treatment standards or guidelines
and the use of “methylprednisolone for either 24 or 48 hours is
recommended as an option in the treatment of patients with acute spinal
cord injuries that should be undertaken only with the knowledge that
the evidence suggesting harmful side effects is more consistent than
any suggestion of clinical benefit.”118
steroids in the setting of acute spinal cord injury is far from
accepted. In patients evaluated after 8 hours from injury, the
consensus is clear that there is no indication for steroid use.
However, the
use
within 8 hours is still hotly debated and will likely require further
randomized, controlled studies for clarification of the issue.
much of orthopaedics, including spinal injuries. These studies usually
yield isolated observations related to injury mechanisms and their
consequences, or the effects of various fixation constructs used to
reconstruct the vertebral column. It is difficult to assemble these
isolated observations into a cohesive or simple conceptualization of
the mechanics of the spine. In fact, sometimes the results of
experiments lead to seemingly contrary conclusions. For example,
fractures of the C2 arch may result from hyperextension, hyperflexion,
or both. The cervical spine facet joint capsules provide resistance to
disruption in both flexion and extension. Partly, these differing
results are due to the artificial conditions selected in the
experimental design of biomechanical studies. Experiments are designed
to answer individual questions, and experimental models simplify
conditions in order to clarify interpretation of the results. Another
limitation on the generalizability of experimental findings is the aim
of homogeneity in experimental protocols. For practical considerations
of limited time and resources, biomechanical experiments seek to
minimize variation among individual specimens. Reallife injuries, on
the other hand, may occur under conditions that are much more complex
than experimental setups, and they may be highly influenced by
individual variations.
general principles are clear from biomechanical studies. Injuries with
distraction between adjacent vertebrae require near complete disruption
of essentially all intervening structural elements. As such,
distraction injuries represent the most unstable injury pattern.
Examples of these injuries are craniocervical dissociation, distracted
fracture-dislocations, and displaced extension fractures in patients
with ankylosing spondylitis or DISH. These injuries are often
associated with severe neurologic deficits, vascular disruption,
stroke, and death. In these types injuries, the static views in imaging
studies are useful for identifying distraction as a crucial component
of injury categorization, but the particular direction of displacement
is not indicative of any mechanistic anterior of posterior or lateral
displacement; it is simply a reflection of the particular position of
the patient at the moment the image was taken. Patients with these
injuries are susceptible to further deterioration during physical
transfers for diagnostic studies. Furthermore, application of cervical
traction, for obvious reasons, would exacerbate the injury.
Strategies to prevent or minimize the functional loss include changing
modifiable risk factors, altering the mechanics of the injury event,
mechanisms of initial injury, or interrupting the ensuing deleterious
biologic responses. Implementing injury prevention measures requires a
high initial investment of resources. Success in these efforts is
difficult to achieve and difficult to measure.310
PA, Farley T, Freni LW, et al. Traumatic spinal cord injury in
Arkansas, 1980 to 1989. Arch Phys Med Rehabil 1993;74(10):1035-1040.
SK, Fehlings MG. Mechanisms of secondary injury to spinal cord axons in
vitro: role of Na+, Na(+)-K(+)-ATPase, the Na(+)-H+ exchanger, and the
Na(+)-Ca2+ exchanger. J Neurosci 1996;16(2):545-552.
DH, Andreychik DA, Stauffer ES. Early outcome in cervical spinal cord
injured patients older than 50 years of age. Spine
1994;19(20):2299-2301.
DH, Parker J, Stauffer ES. Intermediate-term outcome of cervical spinal
cord-injured patients older than 50 years of age. Spine
1997;22(11):1189-1192.
MS, Anderson KD, Biering-Sorensen F, et al. Outcome measures in spinal
cord injury: recent assessments and recommendations for future
directions. Spinal Cord 2009;47(8):582-591.
GJ Jr, Oh YS, Leslie EV. High cervical spine and craniocervical
junction injuries in fatal traffic accidents: a radiological study.
Orthop Clin North Am 1978;9(4): 1003-1010.
DK, Braughler JM, Hall ED, et al. Effects of treatment with U-74006F on
neurological outcome following experimental spinal cord injury. J
Neurosurg 1988; 69(4):562-567.
TE. Spinal cord contusion injury: experimental dissociation of
hemorrhagic necrosis and subacute loss of axonal conduction. J
Neurosurg 1985;62(1):115-119.
DA, Alander DH, Senica KM, et al. Burst fractures of the second through
fifth lumbar vertebrae. Clinical and radiographic results. J Bone Joint
Surg Am 1996; 78(8):1156-1166.
C, Thompson BM, Darin JC. Recommended helmet removal techniques in a
cervical spine injured patient. J Trauma 1984;24(9):841-842.
Spinal Injury Association. International standards for neurological
classification of spinal injury. Atlanta, GA: Author, 2008.
SP, O’Neill B, Haddon W Jr, et al. The injury severity score: a method
for describing patients with multiple injuries and evaluating emergency
care. J Trauma 1974;14(3):187-196.
G, ed. Emergency Care and Transportation of the Sick and Injured.
Menasha, WI: American Academy of Orthopaedic Surgeons, 1987.
P. Application of the International Standards for the Neurological and
Functional Classification of Spinal Cord Injuries (the ASIA scale).
Ortop Traumatol Rehabil 2000;2(1):31-34.
RN, Kendall MD, Amsters DI, et al. The relationship between quality of
life and disability across the lifespan for people with spinal cord
injury. Spinal Cord 2009; 47(2):149-155.
DM, Beattie MS, Bresnahan JC, et al. MASCIS evaluation of open field
locomotor scores: effects of experience and teamwork on reliability.
Multicenter Animal Spinal Cord Injury Study. J Neurotrauma
1996;13(7):343-359.
CA, Cabanela ME, Berquist TH. Unilateral facet dislocations and
fracture-dislocations of the cervical spine. J Bone Joint Surg Br
1991;73(6):977-981.
JM, Fielder K. Unrecognized incomplete cervical spinal cord injury:
review of nine new and 28 previously reported cases. Am J Emerg Med
1992;10(4):336-343.
CC, Emerson SS, Mann FA, et al. Cervical spine imaging in patients with
trauma: determination of fracture risk to optimize use. Radiology
1999;211(3): 759-765.
A, Curt A, Ditunno JF, et al. Position statement on the sale of
unproven cellular therapies for spinal cord injury. The International
Campaign for Cures of Spinal Cord Injury Paralysis. Spinal Cord 2009
May 5 (Epub ahead of print).
C, Raith J, Fankhauser F, et al. Predicting neurologic recovery in
cervical spinal cord injury with postoperative MR imaging. Spine
2006;31(5):554-559.
MB. Treatment of acute spinal cord injury with methylprednisolone:
results of a multicenter, randomized clinical trial. J Neurotrauma
1991;8(Suppl 1):S47-50; discussion S1-2.
MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of
methylprednisolone or naloxone in the treatment of acute spinal-cord
injury. Results of the Second National Acute Spinal Cord Injury Study.
N Engl J Med 1990;322(20): 1405-1411.
MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone
for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment
of acute spinal cord injury. Results of the Third National Acute Spinal
Cord Injury Randomized Controlled Trial. National Acute Spinal Cord
Injury Study. JAMA 1997;277(20):1597-1604.
JM, Hall ED. Pharmacokinetics of methylprednisolone in cat plasma and
spinal cord following a single intravenous dose of the sodium succinate
ester. Drug Metab Dispos 1982;10(5):551-552.
BS, Kunkel-Bagden E, Schnell L, et al. Recovery from spinal cord injury
mediated by antibodies to neurite growth inhibitors. Nature
1995;378(6556):498-501.
D, Lehmann W, Ruecker AH, et al. Factors influencing the quality of
life after burst fractures of the thoracolumbar transition. Arch Orthop
Trauma Surg 2004; 124(7):461-468.
J, Berry A. Laryngeal mask airway insertion. A comparison of the
standard versus neutral position in normal patients with a view to its
use in cervical spine instability. Anaesthesia 1993;48(8):670-671.
PJ, Marino RJ, Herbison GJ, et al. The 72-hour examination as a
predictor of recovery in motor complete quadriplegia. Arch Phys Med
Rehabil 1991;72(8): 546-548.
DD, Rockswold GL. Neurologic recovery following rapid spinal
realignment for complete cervical spinal cord injury. J Trauma
1987;27(4):445-447.
LD, Blackmore CC, Mann FA, et al. Cervical spine fractures in patients
65 years and older: a clinical prediction rule for blunt trauma.
Radiology 2005;234(1):143-149.
CV, Alley JB, Ross M, et al. Magnetic resonance imaging of suspected
atlanto-occipital dislocation. Two case reports. Spine
1992;17(2):245-248.
RP, Puckett WR, Becerra JL, et al. Observations on the pathology of
human spinal cord injury. A review and classification of 22 new cases
with details from a case of chronic cord compression with extensive
focal demyelination. Adv Neurol 1993; 59:75-89.
RE, Maio RF, Maynard F, et al. Incidence, characteristics, and outcome
of spinal cord injury at trauma centers in North America. Arch Surg
1993;128(5):596-599.
AS, Ditunno JF. Establishing prognosis and maximizing functional
outcomes after spinal cord injury: a review of current and future
directions in rehabilitation management. Spine 2001;26(Suppl
24):S137-145.
AS, Lee BS, Ditunno JF Jr, et al. Patient selection for clinical
trials: the reliability of the early spinal cord injury examination. J
Neurotrauma 2003;20(5):477-482.
SP, Golding DG, Rolle WA, et al. Recovery of ambulation in motor
incomplete tetraplegia. Arch Phys Med Rehab 1997;78(11):1169-1172.
RA, Johnson RM, Margolis RN, et al. Cervical facet fusion for control
of instability following laminectomy. J Bone Joint Surg Am
1977;59(8):991-1002.
AL, Tromanhauser SG, Roger DJ. Pedicle screw instrumentation for
thoracolumbar burst fractures and fracture-dislocations. Spine
1992;17(Suppl 8):S317-324.
GD, Minato Y, Okada A, et al. Early time-dependent decompression for
spinal cord injury: vascular mechanisms of recovery. J Neurotrauma
1997;14(12):951-962.
GD, Warden KE, Barbeau JM, et al. Viscoelastic relaxation and regional
blood flow response to spinal cord compression and decompression. Spine
1997;22(12): 1285-1291.
JW, Mirza SK, Tencer AF, et al. Canal geometry changes associated with
axial compressive cervical spine fracture. Spine 2000;25(1):46-54.
W, Barbier DD, Klara PM. Anterior cervical fusion and Caspar plate
stabilization for cervical trauma. Neurosurgery 1989;25(4):491-502.
RP, Watson NA, Carter JW, et al. The effect of postinjury spinal
position on canal occlusion in a cervical spine burst fracture model.
Spine 1997;22(15):1710-1715.
GH, Nelson BJ, Gebhard JS, et al. Functional outcome of thoracolumbar
burst fractures managed with hyperextension casting or bracing and
early mobilization. Spine 1996;21(18):2170-2175.
for Spinal Cord Medicine. Early acute management in adults with spinal
cord injury: a clinical practice guideline for health-care
professionals. J Spinal Cord Med 2008;31(4):403-479.
HB, Miller LS, DeLucia FA, et al. Closed reduction of cervical spine
dislocations. Clin Orthop Relat Res 1987;214:185-199.
KS, Graziani V, Ditunno JF Jr, et al. Spinal cord injury: prognosis for
ambulation based on sensory examination in patients who are initially
motor complete. Arch Phys Med Rehabil 1991;72(2):119-121.
A, Keck ME, Dietz V. Functional outcome following spinal cord injury:
significance of motor-evoked potentials and ASIA scores. Arch Phys Med
Rehabil 1998;79(1): 81-86.
S, Aguayo AJ. Axonal elongation into peripheral nervous system
“bridges” after central nervous system injury in adult rats. Science
1981;214(4523):931-933.
G, Roth E, Morris J, et al. Assessment of closed head injury in
trauma-related spinal cord injury. Paraplegia 1986;24(2):97-104.
GN, Roth EJ, Richards JS. Cognitive deficits in spinal cord injury:
epidemiology and outcome. Arch Phys Med Rehabil 1992;73(3):275-284.
RB, Sherman J, Carr JB. Pathophysiology of spinal cord injury. Recovery
after immediate and delayed decompression. J Bone Joint Surg Am
1995;77(7): 1042-1049.
F. The three column spine and its significance in the classification of
acute thoracolumbar spinal injuries. Spine 1983;8(8):817-831.
F, Burkus JK. Diagnosis and treatment of cauda equina entrapment in the
vertical lamina fracture of lumbar burst fractures. Spine1991;16(8
Suppl):S433-439.
CA, Zabramski JM, Hadley MN, et al. Pediatric spinal cord injury
without radiographic abnormalities: report of 26 cases and review of
the literature. J Spinal Disord 1991;4(3):296-305.
JF Jr, Stover SL, Freed MM, et al. Motor recovery of the upper
extremities in traumatic quadriplegia: a multicenter study. Arch Phys
Med Rehabil 1992;73(5): 431-436.
WF 3rd, Lauerman WC, Heil B, et al. Helmet and shoulder pad removal
from a player with suspected cervical spine injury. A cadaveric model.
Spine 1998; 23(16):1729-1733.
WH, Kopaniky D, Stolzmann E, et al. The neurological and skeletal
outcome in patients with closed cervical spinal cord injury. J
Neurosurg 1987;66(5):690-694.
SE, Papadopoulos SM, Ducker TB, et al. Magnetic resonance imaging
documentation of coexistent traumatic locked facets of the cervical
spine and disc herniation. J Neurosurg 1993;79(3):341-345.
TB, Salcman M, Perot PL Jr, et al. Experimental spinal cord trauma, I:
correlation of blood flow, tissue oxygen and neurologic status in the
dog. Surg Neurol 1978;10(1): 60-63.
DK, Asher MA, Neff JR, et al. Survivorship analysis of VSP spine
instrumentation in the treatment of thoracolumbar and lumbar burst
fractures. Spine 1991;16(8 Suppl): S428-432.
FJ, Arena MJ, Green BA. Extrusion of an intervertebral disc associated
with traumatic subluxation or dislocation of cervical facets. Case
report. J Bone Joint Surg Am 1991;73(10):1555-1560.
BL, Reath DB, Meadors J, et al. The tertiary trauma survey: a
prospective study of missed injury. J Trauma 1990;30(6):666-670.
A, Hasman A, Kristensen K, et al. Do doctors know the content of the
Hippocratic Oath and other medical oaths and declarations? Bull Med
Ethics 2000;154: 13-16.
JW, Curt A, Steeves JD, et al. Guidelines for the conduct of clinical
trials for spinal cord injury as developed by the ICCP panel:
spontaneous recovery after spinal cord injury and statistical power
needed for therapeutic clinical trials. Spinal Cord 2007;45(3):190-205.
MG. Update on “Hot” Pharmacological, Bioengineer, and Cellular
Therapies for Spinal Cord Injuries. Presented at 3rd National Spinal
Cord Injury Conference. Toronto, Canada; November 7, 2008.
MG, Cooper PR, Errico TJ. Posterior plates in the management of
cervical instability: long-term results in 44 patients. J Neurosurg
1994;81(3):341-349.
MG, Tator CH, Linden RD. The relationships among the severity of spinal
cord injury, motor and somatosensory evoked potentials and spinal cord
blood flow. Electroencephalogr Clin Neurophysiol 1989;74(4):241-259.
AE, Schaefer DM, Doan HT, et al. Acute cervical spine trauma:
correlation of MR imaging findings with degree of neurologic deficit.
Radiology 1990;177(1): 25-33.
AE, Spettell CM, Friedman DP, et al. The relationship between the
functional abilities of patients with cervical spinal cord injury and
the severity of damage revealed by MR imaging. AJNR Am J Neuroradiol
1999;20(5):926-934.
AE, Spettell CM, Tartaglino LM, et al. Forecasting motor recovery after
cervical spinal cord injury: value of MR imaging. Radiology
1996;201(3):649-655.
DJ, Taddonio RF, Byrne DW, et al. Incidence of acute care complications
in vertebral column fracture patients with and without spinal cord
injury. Spine 1995; 20(10):1136-1146.
HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the
initial management of closed injuries of the spine with paraplegia and
tetraplegia. I. Paraplegia. 1969;7(3):179-192.
D, Flanders A, Thomas C, et al. Vertebral artery injury after acute
cervical spine trauma: rate of occurrence as detected by MR angiography
and assessment of clinical consequences. AJR Am J Roentgenol
1995;164(2):443-447; discussion 448-449.
JE, Haddad GG. Removal of extracellular sodium prevents anoxia-induced
injury in freshly dissociated rat CA1 hippocampal neurons. Brain Res
199428;641(1): 57-64.
JA, Palumbo MA, Hulstyn MJ, et al. Emergency removal of football
equipment: a cadaveric cervical spine injury model. Ann Emerg Med
1998;32(4):411-417.
FH, Coleman WP, Grieco G, et al. Measurements and recovery patterns in
a multicenter study of acute spinal cord injury. Spine 2001;26(Suppl
24):S68-86.
FH, Dorsey FC, Coleman WP. Recovery of motor function after spinal-cord
injury—a randomized, placebo-controlled trial with GM-1 ganglioside. N
Engl J Med 1991;324(26):1829-1838.
SD, Court-Brown CM. Flexion-distraction injuries of the lumbar spine.
Mechanisms of injury and classification. Clin Orthop Relat Res
1988;227:52-60.
SD, Crowe PJ, Fazl M, et al. Canal clearance in burst fractures using
the AO internal fixator. Spine 1992;17(5):558-560.
J, Plets C, Van den Bergh R. Anterior cervical fusion and
osteosynthetic stabilization according to Caspar: a prospective study
of 41 patients with fractures and/or dislocations of the cervical
spine. Neurosurgery 1989;25(6):865-871.
D, Linnau KF, Cohen WA, et al. Correlation of MR imaging findings with
intraoperative findings after cervical spine trauma. AJNR Am J
Neuroradiol 2007;28(2): 209-215.
GA, Mirza SK, Chapman JR, et al. Risk of early closed reduction in
cervical spine subluxation injuries. J Neurosurg 1999;90(Suppl 1):13-18.
H, Praemer, A, eds. Musculoskeletal conditions in the United States.
Rosemont, IL: American Academy of Orthopaedic Surgeons, 1999.
BA, Eismont FJ, O’Heir JT. Spinal cord injury—a systems approach:
prevention, emergency medical services, and emergency room management.
Crit Care Clin 1987; 3(3):471-493.
L, McLellan BA, Greig H. Abbreviated Injury Scale and Injury Severity
Score: a scoring chart. J Trauma 1985;25(1):60-64.
MN, Walters BC, Grabb PA, et al. Guidelines for the management of acute
cervical spine and spinal cord injuries. Clin Neurosurg 2002;49:407-498.
ED. Pharmacological treatment of acute spinal cord injury: how do we
build on past success? J Spinal Cord Med 2001;24(3):142-146.
ED, Braughler JM. Effects of intravenous methylprednisolone on spinal
cord lipid peroxidation and Na+ + K+)-ATPase activity. Dose-response
analysis during 1st hour after contusion injury in the cat. J Neurosurg
1982;57(2):247-253.
ED, Braughler JM. Role of lipid peroxidation in post-traumatic spinal
cord degeneration: a review. Cent Nerv Syst Trauma 1986;3(4):281-294.
JA, Blackmore CC, Mann FA, et al. Cervical spine injury: a clinical
decision rule to identify high-risk patients for helical CT screening.
AJR Am J Roentgenol 2000; 174(3):713-717.
JH Jr. What is the minimum number of plain radiographs necessary to
evaluate the cervical spine in patients who have had trauma? AJR Am J
Roentgenol 1994;163(1): 217-218.
JH Jr, Carson GC, Wagner LK, et al. Radiologic diagnosis of traumatic
occipitovertebral dissociation: 2. Comparison of three methods of
detecting occipitovertebral relationships on lateral radiographs of
supine subjects. AJR Am J Roentgenol 1994; 162(4):887-892.
KC, Hsieh JT, Wolfe DL, et al. Classifying incomplete spinal cord
injury syndromes: algorithms based on the International Standards for
Neurological and Functional Classification of Spinal Cord Injury
Patients. Arch Phys Med Rehabil 2000;81(5): 644-652.
JR, Mower WR. National Emergency X-radiography Utilization Study: doing
what’s right for your patients. Emerg Med Australas 2005;17(4):406-407.
JR, Mower WR, Wolfson AB, et al. Picking a winner among decision aids.
Ann Emerg Med 2004;43(6):789-790; author reply 790-791.
JR, Mower WR, Wolfson AB, et al. Validity of a set of clinical criteria
to rule out injury to the cervical spine in patients with blunt trauma.
National Emergency X-Radiography Utilization Study Group. N Engl J Med
2000;343(2):94-99.
JR, Schriger DL, Mower W, et al. Low-risk criteria for cervical-spine
radiography in blunt trauma: a prospective study. Ann Emerg Med
1992;21(12):1454-1460.
DA, Schwartz ML, Klettke KA. Neurophysiologic diagnosis in
uncooperative trauma patients: confounding factors. J Trauma
1992;33(2):244-251.
DF, Mackay GM, Morris A, et al. A review of cervical fractures and
fracture-dislocations without head impacts sustained by restrained
occupants. Accid Anal Prev 1993;25(6):731-743.
JT. The Edwin Smith Surgical Papyrus: an analysis of the first case
reports of spinal cord injuries. Paraplegia 1988;26(2):71-82.
RJ. Methylprednisolone for acute spinal cord injury: an inappropriate
standard of care. J Neurosurg 2000;93(Suppl 1):1-7.
RJ, Hamilton MG. Methylprednisolone for acute spinal cord injury:
5-year practice reversal. Can J Neurol Sci 2008;35(1):41-45.
A, Kaneko S, Nakamura M, et al. Transplantation of human neural stem
cells for spinal cord injury in primates. J Neurosci Res
2005;80(2):182-190.
Y, Kawaguchi S, Murata M. Restoration of function by replacement of
spinal cord segments in the rat. Nature 1994;367(6459):167-170.
AB, Dijkers M, Devivo MJ, et al. A demographic profile of new traumatic
spinal cord injuries: change and stability over 30 years. Arch Phys Med
Rehabil 2004;85(11): 1740-1748.
LM, Schwartz R. Prospective analysis of acute cervical spine injury: a
methodology to predict injury. Ann Emerg Med 1986;15(1):44-49.
A, Lammertse DP, Coll JR, et al. Apolipoprotein E epsilon4 allele and
outcomes of traumatic spinal cord injury. J Spinal Cord Med
2008;31(2):171-176.
M, Tollback A, Gonzales H, et al. Inter-rater reliability of the 1992
international standards for neurological and functional classification
of incomplete spinal cord injury. Spinal Cord 2000;38(11):675-679.
K, Taneichi H, Abumi K, et al. Anterior decompression and stabilization
with the Kaneda device for thoracolumbar burst fractures associated
with neurological deficits. J Bone Joint Surg Am 1997;79(1):69-83.
PA, Ridella SA, Viano DC, et al. Interaction of contact velocity and
cord compression in determining the severity of spinal cord injury. J
Neurotrauma 1988; 5(3):187-208.
S, Millis S, McKinley W, et al. Late neurologic recovery after
traumatic spinal cord injury. Arch Phys Med Rehabil
2004;85(11):1811-1817.
J. The results of early conservative and surgical treatment of cervical
spinal cord injured patients. Int J Rehabil Res 1986;9(2):149-154.
N, Auerbach G, Fulga V, et al. Clinical experience using incubated
autologous macrophages as a treatment for complete spinal cord injury:
phase I study results. J Neurosurg Spine 2005;3(3):173-181.
JS, Carter RE, Pickelsimer EE, et al. A prospective study of health and
risk of mortality after spinal cord injury. Arch Phys Med Rehabil
2008;89(8):1482-1491.
JS, Vines CL, Farley TL, et al. An exploratory study of pressure ulcers
after spinal cord injury: relationship to protective behaviors and risk
factors. Arch Phys Med Rehabil 2001;82(1):107-113.
D. Autologous Cellular Therapy in SCI: Lessons Learned from the
Multicenter Macrophage Trial. Presented at Association of Academic
Physiatrists Annual Meeting. Colorado Springs, CO, February 26, 2009.
D, Tuszynski MH, Steeves JD, et al. Guidelines for the conduct of
clinical trials for spinal cord injury as developed by the ICCP panel:
clinical trial design. Spinal Cord 2007;45(3):232-242.
G, Conti A, Cardali S, et al. Does early decompression improve
neurological outcomes of spinal cord injured patients? Spinal Cord
2004;42:503-512.
JE, Custis D, Morrone F, et al. A model for estimating spinal cord
injury prevalence in the United States. Paraplegia 1995;33(2):62-68.
RB, Yarkony GM, Ortolano D, et al. Prediction of functional outcome by
motor capability after spinal cord injury. Arch Phys Med Rehabil
1989;70(12):819-822.
AS, MacLean JC, Newton DA. Rapid traction for reduction of cervical
spine dislocations. J Bone Joint Surg Br 1994;76(3):352-356.
VJ, Keizer HJ, Oosterhuis JK, et al. Functional outcome in patients
with thoracolumbar burst fractures treated with dorsal instrumentation
and transpedicular cancellous bone grafting. Eur Spine J
2003;12(3):261-267.
L, Wolf A, Belzberg H. Hemodynamic parameters in patients with acute
cervical cord trauma: description, intervention, and prediction of
outcome. Neurosurgery 1993; 33(6):1007-1016; discussion 1016-1017.
AM, Waters RL, Yoshida GM. Prognosis of spinal cord injuries. In:
Levine AM, ed. Orthopaedic Knowledge Update: Trauma. Rosemont, IL:
American Academy of Orthopaedic Surgeons, 1996:303-310.
MA, Flanders AE. Diagnostic capabilities of magnetic resonance imaging
and computed tomography in acute cervical spinal column injury. Am J
Emerg Med 1991; 9(2):131-135.
FM, Blackmore CC, Mirza SK, et al. Cervical spine injuries in patients
65 years old and older: epidemiologic analysis regarding the effects of
age and injury mechanism on distribution, type, and stability of
injuries. AJR Am J Roentgenol 2002; 178(3):573-577.
RL, Schwartz ML, Mirich D, et al. Diagnosis of cervical spine injury in
motor vehicle crash victims: how many x-rays are enough? J Trauma
1990;30(4): 392-397.
YJ, Silver JR. Progressive paralysis after bilateral facet dislocation
of the cervical spine. J Bone Joint Surg Br 1992;74(2):219-223.
DJ, Pintar F, Yoganandan N, et al. Effects of anterior vertebral
grafting on the traumatized lumbar spine after pedicle screw-plate
fixation. Spine 1993;18(16): 2423-2430.
KA, McGowan DP, Fredrickson BE, et al. A biomechanical investigation of
short segment spinal fixation for burst fractures with varying degrees
of posterior disruption. Spine 1990;15(6):470-478.
MA, Flanders AE, Herbison GJ, et al. Magnetic resonance imaging related
to neurologic outcome in cervical spinal cord injury. Arch Phys Med
Rehabil 1993;74(9): 940-946.
D, Foltin G, Tunik M, et al. The Kendrick extrication device used for
pediatric spinal immobilization. Prehosp Emerg Care 1999;3(1):66-69.
LF, Knowlton S, Garfin SR, et al. Deterioration following spinal cord
injury. A multicenter study. J Neurosurg 1987;66(3):400-404.
T, Tamaki T, Kawakami M, et al. Early complications of high-dose
methylprednisolone sodium succinate treatment in the follow-up of acute
cervical spinal cord injury. Spine 2001;26(4):426-430.
P, Waters RL, Adkins RH, et al. Comparison of computerized tomography
parameters of the cervical spine in normal control subjects and spinal
cord-injured patients. J Bone Joint Surg Am 1989;71(2):183-188.
FM Jr, Bracken MB, Creasey G, et al. International Standards for
Neurological and Functional Classification of Spinal Cord Injury.
American Spinal Injury Association. Spinal Cord 1997;35(5):266-274.
PC, Bohlman HH, Yuan HA. Anterior decompression of traumatic
thoracolumbar fractures with incomplete neurological deficit using a
retroperitoneal approach. J Bone Joint Surg Am 1985;67(1):89-104.
RF. Functional outcomes after surgery for spinal fractures: return to
work and activity. Spine 2004;29(4):470-477; discussion Z6.
RF, Sparling E, Benson DR. Early failure of short-segment pedicle
instrumentation for thoracolumbar fractures. A preliminary report. J
Bone Joint Surg Am 1993; 75(2):162-167.
SW, Moettus LN. Thoracolumbar spine fractures: clinical presentation
and the effect of altered sensorium and major injury. J Trauma
1995;39(6):1110-1114.
CM, Kuhn JE, Greenfield ML. Cervical spine alignment in immobilized
hockey players: radiographic analysis with and without helmets and
shoulder pads. Clin J Sport Med 1998;8(2):92-95.
KA, Finlay D. Is prevertebral soft tissue swelling a useful sign in
injury of the cervical spine? Injury 1988;19(3):177-179.
SK, Krengel WF 3rd, Chapman JR, et al. Early versus delayed surgery for
acute cervical spinal cord injury. Clin Orthop Relat Res
1999;359:104-114.
F, Furlan JC, Aarabi B, et al. Acute cervical traumatic spinal cord
injury: MR imaging findings correlated with neurologic
outcome—prospective study with 100 consecutive patients. Radiology
2007;243(3):820-827.
J, Harth A, Van Trimpont I, et al. Treatment of unstable fractures,
dislocations and fracture-dislocations of the cervical spine with
Senegas plate fixation. Acta Orthop Belg 1994;60(1):30-35.
MJ, Gaughan J, Betz RR, et al. The International Standards for
Neurological Classification of Spinal Cord Injury: reliability of data
when applied to children and youths. Spinal Cord 2007;45(6):452-459.
J, Weinstein JN, Spratt KF, et al. Thoracolumbar burst fractures. The
clinical efficacy and outcome of nonoperative management. Spine
1993;18(8):955-970.
RW, McElhaney JH, Richardson WJ, et al. Dynamic responses of the head
and cervical spine to axial impact loading. J Biomech
1996;29(3):307-318.
RW, McElhaney JH, Richardson WJ, et al. Experimental impact injury to
the cervical spine: relating motion of the head and the mechanism of
injury. J Bone Joint Surg Am 1996;78(3):412-421.
R, Young W. Pharmacologic strategies in the treatment of experimental
spinal cord injury. J Neurotrauma 1992;9(Suppl 1):S211-217.
DD, Wilmot CB, Hall KM, et al. Benefits of early admission to a
comprehensive trauma center for patients with spinal cord injury. Arch
Phys Med Rehabil 1990;71(9): 637-643.
C, Jonsson H Jr. Compression of the cervical spine cord after reduction
of fracture dislocations. Report of 2 cases. Acta Orthop Scand
1991;62(6):599-601.
CV, Burns AS, Ditunno JF, et al. Prognostic value of pinprick
preservation in motor complete, sensory incomplete spinal cord injury.
Arch Phys Med Rehabil 2005; 86(5):988-992.
OL, Fraser RD, Cornish BL. Fractures and fractures-dislocations of the
lumbar spine. A retrospective study of 70 patients. Int Orthop
1987;11(4):323-329.
JH, Naito M, Bridwell KH, et al. Relationship between duration of
spinal cord ischemia and postoperative neurologic deficits in animals.
Spine 1990;15(7):618-622.
KC, Lammertse DP. Rehabilitation in spinal cord disorders. 1.
Epidemiology, prevention, and system of care of spinal cord disorders.
Arch Phys Med Rehabil 1991; 72(4-S):S293-294.
L. Prevertebral hematoma in cervical spine injury: incidence and
etiologic significance. AJR Am J Roentgenol 1981;136(3):553-561.
JW, Bourne RB, Hawkins RJ. Odontoid fractures, with special reference
to the elderly patient. Clin Orthop Relat Res 1985;193:178-183.
ME, Pointillart V, Dixmerias F, et al. [Medical treatment of spinal
cord injury in the acute stage]. Ann Fr Anesth Reanim
1998;17(2):114-122.
LH, Ross A, Chase GA, et al. Treatment with thyrotropin-releasing
hormone (TRH) in patients with traumatic spinal cord injuries. J
Neurotrauma 1995;12(3):235-243.
V, Petitjean ME, Wiart L, et al. Pharmacological therapy of spinal cord
injury during the acute phase. Spinal Cord 2000;38(2):71-76.
RB, Keizer HJ, Leferink VJ, et al. Functional outcome 5 years after
non-operative treatment of type A spinal fractures. Eur Spine J
2005;15(4):472-478.
RB, Leferink VJ. Sagittal range of motion after a spinal fracture: does
ROM correlate with functional outcome? Eur Spine J 2004;13(6):489-494.
PJ, Hayes KC, Segal JL, et al. Randomized double-blind crossover trial
of fampridine-SR (sustained release 4-aminopyridine) in patients with
incomplete spinal cord injury. J Neurotrauma 1998;15(10):837-849.
RM, Bunge RP. The injured spinal cord: imaging, histopathologic
clinical correlates, and basic science approaches to enhancing neural
function after spinal cord injury. Spine 1996;21(18):2064-2066.
RM, Bunge RP, Egnor M, et al. Acute traumatic central cord syndrome:
MRIpathological correlations. Neuroradiology 1992;34(2):85-94.
JD. Effects of flexion-extension movements of the head and spine upon
the spinal cord and nerve roots. J Neurol Neurosurg Psychiatry
1960;23:214-221.
EB, Myllynen P, Bostman O. Anterolateral decompression for neural
involvement in thoracolumbar fractures. A review of 78 cases. J Bone
Joint Surg Br 1987;69(5): 704-708.
EB, von Bonsdorff H, Hakkinen S, et al. Primary operative fixation of
long bone fractures in patients with multiple injuries. J Trauma
1977;17(2):111-121.
SJ, Piazza MR, Cotler JM, et al. Intervertebral disc injury
complicating cervical spine trauma. Spine 1991;16(Suppl 6):S187-189.
PA, Ryan MD. Neurological deterioration after reduction of cervical
subluxation. Mechanical compression by disc tissue. J Bone Joint Surg
Br 1992;74(2):224-227.
EJ, Park T, Pang T, et al. Traumatic cervical Brown-Séquard and
Brown-Séquardplus syndromes: the spectrum of presentations and
outcomes. Paraplegia 1991;29(9): 582-589.
R, Saillant G, Gagna G, et al. Transverse fracture of the upper sacrum.
Suicidal jumper’s fracture. Spine 1985;10(9):838-845.
CP, Wing PC, Schweigel JF, et al. Closed reduction of dislocations of
the lower cervical spine. J Trauma 1988;28(6):832-835.
FT, Kronvall E, Nilsson OG. Methylprednisolone treatment in acute
spinal cord injury: the myth challenged through a structured analysis
of published literature. Spine J 2006;6(3):335-343.
DM, Flanders A, Northrup BE, et al. Magnetic resonance imaging of acute
cervical spine trauma. Correlation with severity of neurologic injury.
Spine 1989; 14(10):1090-1095.
DM, Flanders AE, Osterholm JL, et al. Prognostic significance of
magnetic resonance imaging in the acute phase of cervical spine injury.
J Neurosurg 1992;76(2): 218-223.
NR, Quint DJ, Patel N, et al. Emergency magnetic resonance imaging of
cervical spinal cord injuries: clinical correlation and prognosis.
Neurosurgery 1999;44(4): 785-792; discussion 792-793.
K, Mirvis SE, Levine AM. Rotational injury of cervical facets: CT
analysis of fracture patterns with implications for management and
neurologic outcome. AJR Am J Roentgenol 1994;163(5):1165-1169.
K, Katsuki M, Ueta T, et al. Transpedicular fixation with Zielke
instrumentation in the treatment of thoracolumbar and lumbar injuries.
Spine 1994;19(17):1940-1949.
Y, McAfee PC, Cunningham BW. Experimental study of thoracolumbar burst
fractures. A radiographic and biomechanical analysis of anterior and
posterior instrumentation systems. Spine 1994;19(15):1711-1722.
E, Schwarz N, Biowski-Fasching I, et al. Color-coded Duplex sonography
of vertebral arteries. 11 cases of blunt cervical spine injury. Acta
Orthop Scand 1993;64(2): 133-137.
PJ Jr, Patwardhan AG, Lorenz M, et al. Instability of the lumbar burst
fracture and limitations of transpedicular instrumentation. Spine
1995;20(13):1452-1461.
CA, McArdle DQ, Ducker TB, et al. The diagnosis of intra-abdominal
injury in patients with cervical cord trauma. J Trauma
1983;23(12):1061-1065.
AM, Jones AA, Cotler JM, et al. Immediate closed reduction of cervical
spine dislocations using traction. Spine 1990;15(10):1068-1072.
ES, Kelly EG. Fracture-dislocations of the cervical spine. Instability
and recurrent deformity following treatment by anterior interbody
fusion. J Bone Joint Surg Am 1977;59(1):45-48.
ES, Wood RW, Kelly EG. Gunshot wounds of the spine: the effects of
laminectomy. J Bone Joint Surg Am 1979;61(3):389-392.
JD, Lammertse D, Curt A, et al. Guidelines for the conduct of clinical
trials for spinal cord injury (SCI) as developed by the ICCP panel:
clinical trial outcome measures. Spinal Cord 2007;45(3):206-221.
IG, Clement CM, McKnight RD, et al. The Canadian C-spine rule versus
the NEXUS low-risk criteria in patients with trauma. N Engl J Med
2003;349(26): 2510-2518.
IG, Wells GA, Vandemheen KL, et al. The Canadian C-spine rule for
radiography in alert and stable trauma patients. JAMA
2001;286(15):1841-1848.
DJ, Devivo MJ, Paculdo DR, et al. Trends in life expectancy after
spinal cord injury. Arch Phys Med Rehabil 2006;87(8):1079-1085.
PK, Waxman SG, Ransom BR. Ionic mechanisms of anoxic injury in
mammalian CNS white matter: role of Na+ channels and Na(+)-Ca2+
exchanger. J Neurosci 1992;12(2):430-439.
B, Liu JF, Marshall GJ, et al. Transoral anterior decompression and
fusion of chronic irreducible atlantoaxial dislocation with spinal cord
compression. Spine 1995; 20(11):1233-1240.
NA, Cronqvist S, Delgado T, et al. Treatment of severe cervical spine
injuries by anterior interbody fusion with early mobilization. Acta
Neurochir (Wien) 1982;60(1-2):91-105.
E, Tibbs PA. Long-term follow-up results of thoracolumbar fractures
after posterior instrumentation. Spine 1995;20(15):1704-1708.
CH. Review of treatment trials in human spinal cord injury: issues,
difficulties, and recommendations. Neurosurgery 2006;59(5):957-982;
discussion 982-987.
CH, Duncan EG, Edmonds VE, et al. Neurological recovery, mortality and
length of stay after acute spinal cord injury associated with changes
in management. Paraplegia 1995;33(5):254-262.
CH, Fehlings MG. Review of the secondary injury theory of acute spinal
cord trauma with emphasis on vascular mechanisms. J Neurosurg
1991;75(1):15-26.
AR. The mechanism of injury to the spinal cord in the neck without
damage to vertebral column. J Bone Joint Surg Br 1951;33-B(4):543-547.
A, Turkka J, Salonen O, et al. Traumatic brain injury is
under-diagnosed in patients with spinal cord injury. J Rehabil Med
2007;39(8):622-626.
ND, Chew BG, Chang YF, et al. MRI is unnecessary to clear the cervical
spine in obtunded/comatose trauma patients: the four-year experience of
a level I trauma center. J Trauma 2008;64(5):1258-1263.
JS, Corcoran TA, Thibault LE, et al. Cervical cord neurapraxia:
classification, pathomechanics, morbidity, and management guidelines. J
Neurosurg 1997;87(6): 843-850.
NT, Watson NA, Tencer AF, et al. Mechanism of the burst fracture in the
thoracolumbar spine. The effect of loading rate. Spine
1995;20(18):1984-1988.
MH, Steeves JD, Fawcett JW, et al. Guidelines for the conduct of
clinical trials for spinal cord injury as developed by the ICCP Panel:
clinical trial inclusion/exclusion criteria and ethics. Spinal Cord
2007;45(3):222-231.
of Alabama. Spinal Cord Injury: Facts and Figures at a Glance.
Birmingham: University of Alabama at Birmingham National SCI Center;
2008. Available at: http://www.spinalcord.uab.edu/show.asp?durki=24480.
Accessed: March 14, 2009.
AR, Nachwalter RS, Klein GR, et al. The significance of thoracolumbar
spinal canal size in spinal cord injury patients. Spine
2001;26(4):371-376.
C, Stiell IG, Beaudoin T, et al. The out-of-hospital validation of the
Canadian C-Spine rule by paramedics. Ann Emerg Med 2009 Apr 23 (Epub
ahead of print).
FL, Burns J, Jackson AB, et al. Combined medical and surgical treatment
after acute spinal cord injury: results of a prospective pilot study to
assess the merits of aggressive medical resuscitation and blood
pressure management. J Neurosurg 1997; 87(2):239-246.
Peteghem PK, Schweigel JF. The fractured cervical spine rendered
unstable by anterior cervical fusion. J Trauma 1979;19(2):110-114.
MJ, Bostman OM, Myllynen PJ. Reduction of bone retropulsed into the
spinal canal in thoracolumbar vertebral body compression burst
fractures. A prospective randomized comparative study between
Harrington rods and two transpedicular devices. Spine
1995;20(15):1699-1703.
AW, Wamil BD, Hellerqvist CG. CM101-mediated recovery of walking
ability in adult mice paralyzed by spinal cord injury. Proc Natl Acad
Sci U S A 1998;95(22): 13188-13193.
MY, Hoh DJ, Leary SP, et al. High rates of neurological improvement
following severe traumatic pediatric spinal cord injury. Spine
2004;29(13):1493-1497; discussion E266.
RL, Adkins RH, Yakura JS, et al. Motor and sensory recovery following
complete tetraplegia. Arch Phys Med Rehabil 1993;74(3):242-247.
RL, Adkins RH, Yakura JS, et al. Motor and sensory recovery following
incomplete tetraplegia. Arch Phys Med Rehabil 1994;75(3):306-311.
RL, Sie I, Adkins RH, et al. Injury pattern effect on motor recovery
after traumatic spinal cord injury. Arch Phys Med Rehabil
1995;76(5):440-443.
SI, Graham PM. Falls resulting in spinal cord injury: patterns and
outcomes in an older population. Paraplegia 1989;27(6):423-427.
AA III, Johnson RM, Panjabi MM, et al. Biomechanical analysis of
clinical stability in the cervical spine. Clin Orthop Relat Res
1975;109:85-96.
RK, Boerger TO, Allen DJ, et al. A dynamic study of thoracolumbar burst
fractures. J Bone Joint Surg Am 2003;85-A(11):2184-2189.
RK, Boerger TO, Hall RM, et al. Measurement of canal occlusion during
the thoracolumbar burst fracture process. J Biomech 2002;35(3):381-384.
E, Jacomet H, Zafren K, et al. The use of extrication devices in
crevasse accidents: official statement of the International Commission
for Mountain Emergency Medicine and the Terrestrial Rescue Commission
of the International Commission for Alpine Rescue intended for
physicians, paramedics, and mountain rescuers. Wilderness Environ Med
2008;19(2):108-110.
K, Buttermann G, Mehbod A, et al. Operative compared with nonoperative
treatment of a thoracolumbar burst fracture without neurological
deficit. A prospective, randomized study. J Bone Joint Surg Am
2003;85-A(5):773-781.
KB, Bohn D, Mehbod A. Anterior versus posterior treatment of stable
thoracolumbar burst fractures without neurologic deficit: a
prospective, randomized study. J Spinal Disord Tech 2005;18
Suppl:S15-23.
BA, Baron RC. A description of nonfatal spinal cord injury using a
hospital-based registry. Am J Prev Med 1994;10(1):10-14.
M, Rivara FP, Ferse D. Evaluation of the Think First head and spinal
cord injury prevention program. Inj Prev 1995;1(2):81-85.
S, Pan W, Kastin AJ. Strategies to create a regenerating environment
for the injured spinal cord. Curr Pharm Des 2005;11(10):1267-1277.
IG, Palumbo M, Spatz E, et al. Nerve root recovery in complete injuries
of the cervical spine. Spine 1991;16(Suppl 10):S518-521.
D, Jane JA, White RJ. Prognosis and management of spinal cord and cauda
equina bullet injuries in sixty-five civilians. J Neurosurg
1970;32(2):163-170.
RI, Scalea TM, Trooskin SZ, et al. Hemodynamic responses to penetrating
spinal cord injuries. J Trauma 1993;35(4):578-582; discussion 582-583.
