METABOLIC BONE DISEASE
VII – NEOPLASTIC, INFECTIOUS, NEUROLOGIC AND OTHER SKELETAL DISORDERS
> Tumors and Tumor-Like Conditions > CHAPTER 131 – METABOLIC BONE
DISEASE
primarily in its mechanical role as the body’s framework, often
overlooking its metabolic aspects. Bone is dynamic tissue, and
disturbances in its metabolic activity may have significant
consequences for the skeleton and the body as a whole. In this chapter,
we will explore the implications for orthopaedic surgery of bone
metabolism.
when the body’s serum ionized calcium concentration is low; the net
effect of PTH is an increase in serum calcium. PTH causes bone
resorption by indirect activation of the osteoclasts (increased number
and activity) through osteoblast coupling factors. At high
concentrations of PTH, osteoblast activity is inhibited, and at low
concentrations, bone formation is stimulated. In the kidney, PTH causes
decreased resorption of phosphate in the proximal tubule and increased
distal resorption of calcium. PTH also increases renal conversion of
25-hydroxy vitamin D to active 1,25-dihydroxy vitamin D, resulting in
increased calcium absorption from the gut (see later).
in calcium metabolism. It is synthesized from 7-dihydrocholesterol in
the skin under the influence of ultraviolet light. As little as 15
minutes a day of direct sunlight exposure on a Caucasian person will
produce the daily requirement of vitamin D, but many people,
particularly in northern cities, do not meet this requirement during
the winter (28). The cytochrome P450 system
forms 25-hydroxy vitamin D, which has a 3-day half-life, from vitamin D
in the liver. The kidney then converts this into the active form,
1,25-dihydroxy vitamin D, which has a half-life of 8 hours. This step
is under the influence of PTH.
primarily to increase the concentration of calcium. Vitamin D enhances
PTH-stimulated bone resorption and promotes increased intestinal
absorption of calcium. In the gut, 1,25-dihydroxy vitamin D acts to
mature the intestinal villi and to induce the formation of
calcium-binding
protein
by the villi. This binding protein is responsible for active transport
of calcium across the gut. In high dietary calcium situations, it is
absorbed across the gut by passive diffusion. Vitamin D also promotes
the differentiation of cells in the monocyte–macrophage line, which
should increase the number of osteoclasts. The possible effect of
vitamin D on osteoid mineralization may be mediated through alterations
in osteoblasts, enhanced osteocalcin production, or modification of
phospholipid metabolism. Vitamin D, however, does not directly
stimulate bone formation.
metabolism. It produces potent and rapid hypocalcemic activity through
inhibition of the activity and number of osteoclasts. Calcitonin binds
directly to receptors on osteoclasts, resulting in detachment of
osteoclasts from the bone and disappearance of the ruffled border.
Continued administration of calcitonin produces a gradually smaller
response in the osteoclasts. It stimulates bone formation, but this
effect wanes as the number of osteoclasts decreases. Calcitonin also
acts in the kidney to decrease resorption of calcium from the proximal
tubules. It also appears to inhibit production of the active
1,25-dihydroxy vitamin D.
effect on calcium metabolism through estrogen receptors on the surface
of osteoblasts (17). Although the mechanisms of
estrogen’s effect on bone metabolism have not been clearly identified,
accelerated loss of bone following natural, chemical, or surgical
oophorectomy is well established (57,72). Administration of estrogen with calcium has been shown to slow the accelerated loss of skeletal calcium following menopause (14,18,32,33,39,57).
and upper gut; about 15% to 40% of the dietary dose is absorbed,
depending on the patient’s age and hormonal status. The body’s
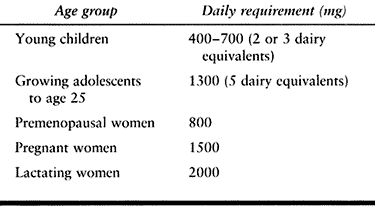
requirement for calcium changes with age (Table 131.1).
Children need 400–700 mg/day ingested calcium (two or three dairy
equivalents daily). During the peak acquisition of skeletal bone mass,
between adolescence and age 25, daily intake of about 1,300 mg of
calcium (five dairy equivalents) is required. Healthy adults generally
require 500 mg/day, pregnant women 1,500 mg/day, and lactating women
2,000 mg/day. Postmenopausal women generally require 1,500 mg/day, as
do patients who have had a major fracture.
 |
|
Table 131.1. Daily Calcium Requirements
|
gastric pH, adequate 1,25-dihydroxy vitamin D, and a
calcium-to-phosphate ratio of 1:1 or 1:2. Achlorhydria; decreased
vitamin D; increased phosphates; increased fat, phytates, or oxylates
in the diet; sprue; blind-loop disorders; and renal disorders can
decrease the absorption of calcium.
excreted in the urine. The daily requirement is about 1,000–1,500 mg
and is rarely a limiting factor in American diets. Aluminum and
beryllium can decrease the absorption of phosphate; PTH increases its
excretion.
disturbance in the rate of bone turnover, initiated by an early
increase in bone resorption by osteoclasts and followed by excessive
and pathologic bone formation by osteoblasts. The result is distorted
bones with thickened cortices, coarse trabeculations, and an immature
woven appearance. Paget’s disease can affect any bone but most commonly
afflicts the femur, spine, pelvis, and cranium. Paget’s disease may be
monostotic or polyostotic, and it may or may not be symptomatic.
appears to be a familial clustering, with a positive family history in
14% to 30% of patients with Paget’s disease. A correlation of Paget’s
disease and certain human leukocyte antigen (HLA) patterns has been
noted (83). In addition, a viral etiology for Paget’s disease has been proposed (64,65,78).
Inclusions resembling viral nuclear capsules have been described in
osteoclasts in pagetic bone; these are not seen in osteoclasts or
osteoblasts in normal sites in the same patient. These viruslike
particles resemble paramyxovirus or measles virus (65).
Viral etiology has not been definitively proved, but it is possible
that a susceptible host may contract a slow type of virus, predisposing
the host to Paget’s disease later in life.
a 3:2 ratio, and it is seen most often in patients over age 50. It is
estimated that 3% to 4% of people over
age
45 and as many as 8% of those over age 80 in the United States may have
Paget’s disease, a total of more than 3 million people (84).
This disease is most common in Europe, North America, Australia, and
New Zealand, and it appears more common in people of northern European
ancestry than of southern European ancestry, although Scandinavians
have a particularly low rate. The disease is uncommon in Asians and
blacks, although most clinical series in North America have black
patients.
asymptomatic when diagnosed incidentally through radiology or
laboratory work (e.g., by an elevated alkaline phosphatase). In
symptomatic Paget’s disease, patients experience bone pain either with
rest or with motion. Pagetic bone also has increased vascularity,
leading to a warmth of the bone or of the skin over the bone. Back pain
is common in patients with Paget’s disease, secondary to spinal
stenosis or vertebral compression fractures. Paraplegia can occur
acutely, often in association with a giant-cell tumor secondary to
Paget’s. Paget’s disease may cause an increase in the size of the
skull, with or without frontal bossing. Hearing loss may also occur.
lucencies or pseudofractures, typically on the convex surfaces of the
weight-bearing long bones. Bowing of the femur or tibia may also occur,
either with or without pseudofracture. Typical radiologic appearance
includes areas of focal bone resorption and formation and a disordered
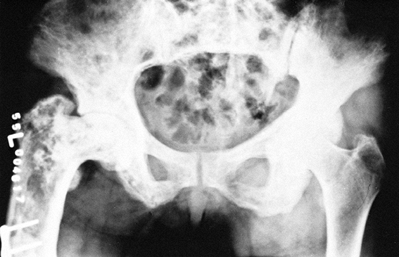
trabecular pattern (Fig. 131.1, Fig. 131.2).
Bone size is expanded and the cortices are thickened. A flame-shaped
osteolytic wedge may be seen, as may bony deformities, incomplete
pseudofractures, and frank pathologic fractures.
 |
|
Figure 131.1. Paget’s disease. Notice coarse trabeculation.
|
 |
|
Figure 131.2. Paget’s disease. Notice enlargement of bone and coarse trabeculation.
|
of the bone progresses toward the other end. A bone scan is more useful
than a skeletal survey to discover the extent of the disease. A gallium
scan will differentiate tumor from Paget’s disease (only tumor is
positive). Elevated serum alkaline phosphatase and bone collagen
products (N-telopeptide, pyridinoline) are
typical findings. The alkaline phosphatase level is more useful as a
screening tool. Either alkaline phosphatase or collagen breakdown
products
may also be used to monitor response to treatment, with a 50% reduction considered to be a good clinical response.
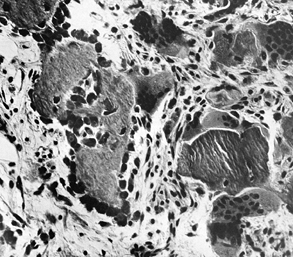
abnormalities. There is an increase in the cellular activity of both
osteoblasts and osteoclasts, with fibrosis and fibrovascular invasion
of the marrow. The osteoclasts seen in Paget’s disease have extremely
numerous nuclei with very large nucleoli (Fig. 131.3).
Active Paget’s disease as just described presents a classic histologic
appearance. “Burned-out” sclerotic pagetic bone, however, is more
difficult to diagnose definitively because the bone reverts to near
normal, although a mosaic-type bone pattern can be seen microscopically
with polarized light.
 |
|
Figure 131.3.
Photomicrograph shows resorption by large osteoclasts with numerous nuclei, as well as bone formation by osteoblasts (hematoxylin and eosin, ×80). |
those who do not have the disease in the weight-bearing bones in the
lower extremity, do not require intervention other than periodic
monitoring. In patients with symptomatic Paget’s disease, the primary
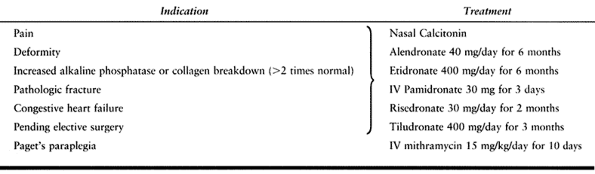
indication for treatment is pain (Table 131.2).
Bone pain, excessive warmth over bones, headaches, and some low back
pain secondary to vertebral changes are most likely to be relieved with
drug treatment. Secondary arthritic pain is less likely to be relieved
by antipagetic agents. Treatment is also indicated when it is felt that
suppression of the disease would be beneficial to the patient (e.g., in
the presence of neurologic deficits, fracture, proposed elective
skeletal surgery, congestive heart failure due to polyostotic disease,
and severe polyostotic disease itself). A third indication is an
alkaline phosphatase or bone biomarker level twice normal, with the
disease in weight-bearing bones.
 |
|
Table 131.2. Management of Paget’s Disease
|
disease in the United States are calcitonin, bisphosphonates such as
etidronate, and occasionally mithramycin. Calcitonin is available as
salmon or human calcitonin; the salmon calcitonin is more potent by
weight. Calcitonin is a peptide and is currently given by an intranasal
spray. In patients with painful pseudofracture or frank fractures,
calcitonin is given initially at 200 units/day until there is evidence
of decreased bone pain or fracture healing, and the alkaline
phosphatase level falls. The dose can then be decreased to three times
a week to maintain the alkaline phosphatase at a lower level. Side
effects of nasal calcitonin include dry nares (2%).
bisphosphonate. Several compounds that have shown excellent efficacy
are oral alendronate, residronate, and tulidronate, as well as
intravenous pamidronate. These agents essentially inhibit bone
resorption with no significant
inhibition of bone formation. Etidronate affects both aspects of bone turnover.
strong inhibitor of both osteoclasts and osteoblasts. It can rapidly
relieve bone pain in Paget’s disease, but because of its significant
side effects it is recommended only in Paget’s paraplegia (62,79). In this case, it is given intravenously in doses of 15 mg/kg/day for 10 days.
most common complication in Paget’s disease. An estimated 10% of
patients with extensive Paget’s disease will suffer pathologic
fracture, usually of the femur, tibia, or forearm (62). Nonunions are common, particularly with femoral neck and subtrochanteric fractures (5,15,24,69).
Pseudofractures in Paget’s disease occur most commonly in the tibia and
the proximal femur, typically on the convex side of the bone, although
they may be seen on the medial concavity of the femur as well.
Treatment includes bracing and protected weight bearing in addition to
pharmacologic treatment until the patient becomes pain free. Radiologic
healing often lags behind clinical healing.
oblique, or transverse. If they fail closed management, they are best
treated by an intramedullary device. Passage of an intramedullary
device may be technically difficult because of the disordered
architecture of the pagetic bone.
often do not heal in patients with Paget’s disease; the reported rate
of nonunion is 75% to 90% (62). If these
fractures are displaced, perform prosthetic replacement.
Intertrochanteric fractures, on the other hand, usually unite after
fixation with a sliding screw-plate device.
handle in patients with Paget’s disease. Intramedullary fixation is
preferable from a biomechanical standpoint but may be difficult because
of excessively soft or sclerotic bone and because of bony deformity of
the femur. Sometimes a corrective osteotomy in addition to
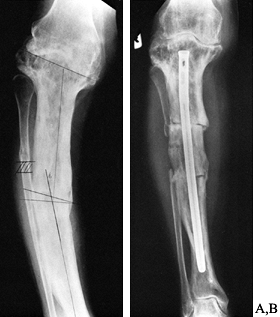
intramedullary fixation may be required (Fig. 131.4).
Femoral shaft fractures are typically managed in a similar way, but
tibial fractures can often be managed by closed means because of the
transverse configuration of the fracture. Surgical treatment of a
fracture, however, may provide an opportunity to correct a deformity.
 |
|
Figure 131.4. Corrective osteotomy with intramedullary fixation. A: Preoperative. B: Postoperative.
|
to heal with abundant callus. Delayed union or nonunion is more common
in the sclerotic burned-out phase of the disease. Even after successful
union, protective bracing may be needed for 3–6 months to allow for
remodeling, which is slow.
incidence of tumors is about 2% to 4%; however, when all patients who
have Paget’s disease of any variety are included, the incidence is
usually about 0.1% (62). The most commonly seen
benign tumors are giant-cell tumors, which are common in patients with
an ancestry from Arolina, Italy. They contain viral particles and are
exquisitely sensitive to corticosteroid therapy (84). The most common malignant tumors are osteogenic sarcomas, although fibrosarcoma and chondrosarcoma may be seen as well.
At Memorial Sloan-Kettering Cancer Center, there have been no survivors
for axial tumors, but 23% of appendicular tumors have a 5-year
survival. Any patient with Paget’s disease who develops new pain in a
previously pain-free area of Paget’s disease, or a lytic area in
sclerotic Paget’s bone, must be evaluated for a possible tumor.
factors or pagetic involvement of the joints. For patients with severe
disabling arthritis of the hip, total joint replacement may be
considered, but several technical considerations should be noted. There
is often significant varus deformity of the proximal femur, and the
medullary canal may be distorted. The bone may also be extremely
sclerotic, making reaming difficult.
loss may be expected, and postoperatively the patients have a higher
incidence of heterotopic ossification. On the acetabular side, about
25% of patients may have a protrusio acetabuli (27).
Because many patients with Paget’s disease also have involvement of the
lumbar spine, it is extremely important to carefully separate possible
lumbar spine pain from hip pain before undertaking arthroplasty of the
hip. Local instillation of anesthesia into the hip may help determine
the primary cause of pain.
patients with Paget’s disease shows a slightly increased incidence of
revision for aseptic loosening, but otherwise good or excellent results
(55,58,61).
These studies reviewed cemented total hip arthroplasty; there have been
no long-term studies of porous ingrowth implants in patients with
Paget’s disease. Because of the modeling and remodeling disorders in
Paget’s, uncemented total hip replacement is not recommended. Treat
patients undergoing total joint arthroplasty with calcitonin, 50 units
three times a week, beginning 1 month preoperatively and continuing for
about 5–6 months postoperatively, so as to decrease perioperative
bleeding and postoperative hypercalcemia.
patients with Paget’s disease, with up to a 12-year follow-up. No
unusual technical difficulties were encountered (7,9).
required in Paget’s disease is osteotomy to correct malalignment in the
leg. Osteotomy, particularly of the femur and tibial shaft, is
indicated for symptomatic and recurrent or recalcitrant
pseudofractures. Surgery is usually performed with an interlocking
intramedullary rod after one or more closing-wedge osteotomies. If the
intramedullary canal is obstructed, double plating with bone grafting
can be used.
and adult consequences of a failure to mineralize either physeal
cartilage (children) or osteoid (adults) due to disorders in vitamin D
metabolism.
is of an apathetic, irritable child who is shorter than usual and has a
positive Gower’s sign, frontal bossing, and an enlarged epiphysis. He
also may have a rachitic rosary or Harrison’s groove. The radiologic
findings in rickets include a wide epiphyseal plate with cupping, and
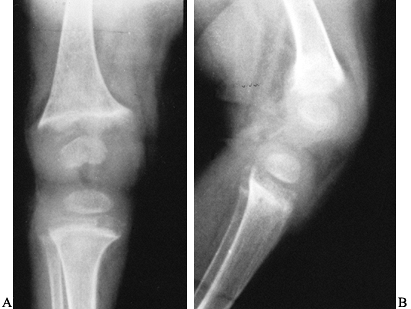
an indistinct zone of provisional calcification (Fig. 131.5).
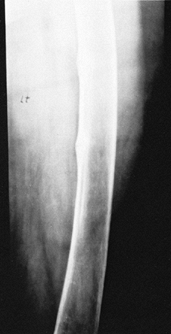
Long-bone bowing, genu valgum or genu varum, and stress fractures (Looser’s lines) are also seen (Fig. 131.6).
 |
|
Figure 131.5. Rickets: Notice the widened, cupped physes on the AP (A) and the lateral (B) radiographs.
|
 |
|
Figure 131.6. Looser’s lines in osteomalacia.
|
bone pain and muscle weakness. Radiologic findings may be identical to
those seen in osteoporosis, except that Looser’s lines appear in
patients with osteomalacia. Other radiologic findings, such as “rugger
jersey spine,” have also been described in patients with osteomalacia.
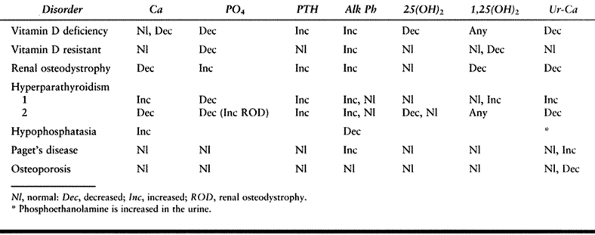
vary, depending on the etiology of the problem. Phosphorus is
decreased, alkaline phosphatase is increased, and 25-hydroxy vitamin D
is decreased in patients who have a vitamin D deficiency syndrome (Table 131.3).
Vitamin D deficiency may be secondary to decreased exposure to
ultraviolet light or to a vitamin D deficiency in the diet. Although
milk and dairy products in the United States are typically fortified
with vitamin D, certain people are at risk because of their dietary
habits. A significant number of American blacks are lactose intolerant
and therefore avoid dairy products. Strict vegetarians who do not eat
any dairy products are at risk for rickets or osteomalacia.
 |
|
Table 131.3. Disorders of Mineral Metabolism
|
is typically treated with 50,000 units of vitamin D plus calcium.
Vitamin D–resistant rickets (a proximal tubular defect in excretion of
phosphorus) requires increased phosphate in the diet plus
1,25-dihydroxy vitamin D in low doses.
to treatment once the diagnosis is made. Patients may consult an
orthopaedic surgeon because of bone pain or bony deformity. Specific
orthopaedic intervention is rarely necessary, but bracing of the
extremities or spine may be required,
particularly
in children. In addition, a patient with significant bony deformity may
need an osteotomy. With appropriate pharmacologic treatment, such a
patient can produce appropriate amounts of osteoid, and the osteotomy
should heal well.
diseases, ranging from a low-turnover bone disease resembling
osteomalacia to a high-turnover disease resembling secondary
hyperparathyroidism. In renal disease, the physiologic PTH–vitamin D
axis is altered. Phosphate retention and hyperphosphatemia occur as a
result of impaired renal phosphorus clearance. Hypocalcemia occurs,
partly as a result of increased calcium excretion. In addition,
hydroxylation to form 1,25-dihydroxy vitamin D is diminished, which in
turn impairs calcium absorption across the gut. The effect of PTH on
the kidney is also diminished.
hyperphosphatemia is an important factor in the development of the
secondary hyperparathyroidism. There is abundant histologic evidence of
active bone resorption, including increased number and size of
osteoclasts, increased percentage of bone surface covered with
osteoclasts, and increased number of Howship’s lacunae. Patients may
develop osteitis fibrosa, in which fibrous tissue accumulates in the
bone. Osteoblast activity also increases in patients with osteitis
fibrosa (80,82).
Moderate increases in the amount of osteoid may be seen, and the
osteoid often appears woven because of the increased bone turnover.
Clinically, bone pain, brown tumors, and ulcers may be seen. On
radiographs, the classic salt-and-pepper skull, soft-tissue
calcifications, osteopenia next to osteosclerosis, and erosions of the
phalangeal tuft and distal clavicle may be seen. These changes parallel
those seen in patients with primary hyperparathyroidism.
changes are common and have been described as rickets-like. Unlike
patients with congenital rickets, patients with renal osteodystrophy
prematurely develop genu valgum deformities. In addition, slipped
capital femoral epiphysis (SCFE) is a common complication of the
disease in children (59). Epiphyseal slippage may also occur in the lower femoral epiphysis or the distal tibial epiphysis.
as a low-turnover bone disease similar to osteomalacia and aplastic
bone disease. Initially thought to be the result of vitamin D
deficiency, it is now clear that aluminum toxicity can account for
osteomalacia seen with renal osteodystrophy (Fig. 131.7).
Aluminum contamination of water used in dialysis solutions has been
identified as a source; a decrease in the aluminum in the filtrate
reduces the prevalence of the osteomalacia (53,71,76).
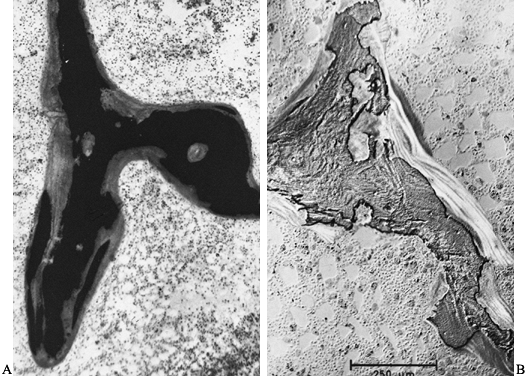 |
|
Figure 131.7. A: Photomicrograph shows extensive hyperosteoidosis with smudged mineralization front (Von Kossa, ×80). B:
Photomicrograph shows aluminum deposition at the mineralization front. Patient has renal osteodystrophy (aurin tricarboxylic acid stain, ×80). |
aluminum-containing phosphate binders to prevent hyperphosphatemia and
secondary hyperparathyroidism. These buffers can substantially increase
the aluminum load in the body. Aluminum is known to deposit along the
mineralization front, which may impair the calcification of the osteoid
(22,56). Aluminum is
also associated with a substantial decrease in the number of
osteoblasts. Histologically, patients who have a low-turnover type of
bone disease have bone characterized by excessive osteoid or
unmineralized collagen. The osteoid seams are widened and often
multilamellar in appearance, with more of the bone surface covered by
osteoid. Osteoblastic activity is markedly reduced. Aluminum can be
demonstrated in these areas by staining. The use of calcium carbonate
to buffer high phosphate has largely prevented this disorder.
renal osteodystrophy. Typically they have some type of mixed pattern
characterized by secondary hyperparathyroidism as well as evidence of
osteomalacia. The most common clinical manifestations of renal
osteodystrophy are bone pain, muscle weakness, skeletal deformities,
ectopic calcification, and growth retardation in children.
usually aggravated by weight bearing. It is most common in the lower
back, hips, and legs, although heel and ankle pain may also be noted.
Bone pain is more common in patients with aluminum-related bone disease
than in those with osteitis fibrosa (54).
Muscle weakness, often of a proximal myopathy type, may also be noted
with severe renal disease. In some cases, a favorable clinical response
has been seen after treatment with 1,25-dihydroxy vitamin D3 and with deferoxamine chelators for treatment of aluminum-related bone disease (12,68).
renal osteodystrophy. They are more common in children and may affect
either the axial or the appendicular skeleton. In adults, particularly
those with aluminum toxicity, deformities are predominantly confined to
the axial skeleton.
disease resemble those of vitamin D–deficiency rickets. In children
between ages 4 and 10, deformities of the long bones, particularly
bowing of the tibia and femur, are common; genu valgum is particularly
common. SCFE is common in pediatric patients, particularly those with
secondary hyperparathyroidism. Despite vitamin D treatment, 20% to 25%
of pediatric patients treated with long-term dialysis require
orthopaedic intervention to correct bony deformities (23).
often in a periarticular location. These calcifications are most common when the calcium–phosphorous ion products exceed 75 (23). Ectopic calcifications are more common in adults but can also occur in children with severe end-stage disease.
amyloidosis. The most common presenting syndrome is carpal tunnel
syndrome, but pathologic fracture or scapulohumeral periarthritis may
also be present (23,44).
Arthritis may involve the metacarpophalangeal and interphalangeal
joints, hand, shoulder, wrist, and knees. Bone cysts may be found in
the femoral head, acetabulum, humerus, radius, carpal bones, tibial
plateau, and pubis; these may resemble brown tumors (44).
Distinctive changes may occur in the bone with sites of amyloid
deposition, and fractures can occur through such cysts. There is no
clear treatment for this problem.
medical and dietary. Restrict phosphorus intake in the diet to 400–800
mg/day. Phosphate-binding antacids may be required to decrease the
absorption of phosphorus. Calcium supplements are useful; calcium
carbonate not only increases the amount of calcium available for
absorption but also acts as a phosphate-binding agent. Do not, however,
give calcium supplements until the serum phosphorus levels are reduced
to about 6.5 mg/dl or less or until the calcium–phosphorous ion product
is below 75, to minimize the risk of extraskeletal calcification (23).
If calcium carbonate is to be used to bind phosphate, it should be
taken with a meal to increase its phosphate-binding efficiency.
also been used in the treatment of renal osteodystrophy. Oral
calcitriol (0.25 to 1.5 µg/day) in patients with renal osteodystrophy
has improved muscle strength, decreased bone pain, and increased growth
in uremic children (12). The major side effect of this treatment is hypercalcemia. Hypercalcemia developing within the first few
weeks of treatment may indicate either a limited amount of bone disease or autonomous hyperparathyroidism.
manifestation of the renal disease, parathyroid surgery can be
considered, especially if patients have persistent hypercalcemia,
intractable pruritus, progressive ectopic calcifications, severe
skeletal pain, or fractures.
difficult. The chelator deferoxamine substantially increases the
removal of aluminum with both hemodialysis and peritoneal dialysis,
which may improve symptoms and bone histology (26,29,63).
Currently, it is recommended that deferoxamine be given only to
patients with symptomatic aluminum toxification. The dose should not
exceed 1–2 g/week; measure aluminum levels and plasma regularly.
have already been mentioned. In children, SCFE and deformities of the
long bones are most prominent. SCFE requires pinning and appropriate
medical treatment. Bowing of the extremities can sometimes be treated
with bracing but often require osteotomy. In both adults and children,
bone pain may be due to pseudofractures seen as Looser’s lines, and
these may progress to frank fractures. The treatment of bone pain
associated with pseudofractures should be medical management combined
with decreased weight bearing until the pain has resolved. An actual
fracture should be treated as any other fracture, with the expectation
that healing will be delayed.
is unlikely to be successfully managed by conservative treatment alone,
and surgery may be required. There are no good long-term studies
available for total joint replacement in patients with renal
osteodystrophy, but because of the severe derangement of bone
metabolism, uncemented arthroplasty cannot be recommended. Dialysis
patients have a high rate of prosthesis loosening, but patients who
have undergone transplantation do well.
osteodystrophy, do an extensive medical workup first. Patients with
severe renal disease may have clotting abnormalities and often have
severe anemia. They also have arteriovenous fistulae from dialysis,
which require careful management in the hospital. Infections can be
particularly severe in patients with renal osteodystrophy; make every
effort to minimize any chance of contamination. Overall, the patient’s
medical condition must guide any decisions about elective orthopaedic
surgery, and in emergency situations optimize the patient’s medical
status before doing any surgery.
of Health (NIH) Consensus Conference as an age-related disorder
characterized by decreased bone mass and increased fracture risk in the
absence of other recognizable causes of bone loss (69).
The fractures most commonly seen in osteoporosis involve the vertebral
body, proximal femur, wrist, humerus, pelvis, and ankle and foot.
United States, where it is estimated that more than 20 million people
have it. As the population ages, this number will increase
dramatically. More than 1.2 million fractures a year are attributed to
osteoporosis, including about 535,000 vertebral fractures, 172,000
wrist fractures, and 200,000 hip fractures (39). The financial cost of osteoporosis in 1982 was over $6 billion annually (60).
weight, and being postmenopausal, white, and of northwestern European
descent (77). Other factors include poor
calcium intake, a sedentary lifestyle, cigarette and alcohol use, and,
most prominently, any history of premature menopause or amenorrhea.
Factors that increase risk for hip fracture besides osteoporosis
include falls, lack of weight gain since age 25, greater height, poorer
self-rated health status, history of hyperparathyroidism, use of
long-acting benzodiazepines, inability or unwillingness to stand each
day (4 min), inability to rise from a chair, impaired vision, heart
rate over 80, and a history of a fragility fracture (15,64).
In addition, family history may be important. At the Hospital for
Special Surgery Osteoporosis Center, one third of patients with
osteoporosis have been found to have a strong family history of the
disease.
men and women. Men have a peak bone mass about 30% higher than women,
and blacks 10% higher than whites, although nutrition, exercise, and
general health can affect the peak bone mass (13,19,69). Declining bone mass after the age of 30 may represent an inescapable part of the aging process.
generally identified, slow and accelerated. The slow phase is loss of
bone that occurs at a nearly constant rate of 0.3% to 0.5% per year (85).
It affects men and women equally and is thought to be due to continuous
negative calcium balance. Rapid or accelerated bone loss begins after
natural or surgical menopause and can result in the loss of 2% to 3% of
bone mass per year (8% per year trabecular and 0.5% per year cortical).
From 6 to 10 years after menopause, the rate returns to the slow phase (51,52,57,72,74,75).
by Riggs and Melton: Type I is the rapid postmenopausal loss, and type
II is the slow loss (Table 131.4) (79).
The type I pattern is most common in recently postmenopausal woman,
predominantly age 51 to 65. It involves rapid bone loss primarily from
areas of trabecular bone and is characterized by vertebral and wrist
fractures. Men make up about 16% of patients manifesting this pattern
of osteoporosis. In about half of these men, hypergonadism may be a contributing factor to osteoporosis.
 |
|
Table 131.4. Types I and II Osteoporosis
|
cortical and trabecular bone, typically seen in men and women over age
75. Typical fractures resulting from this type of osteoporosis include
those of the hip, pelvis, humerus, and tibia. Type II bone loss is
related to aging and chronic calcium deficiency as well as decreased
vitamin D activity, increased PTH, and decreased bone formation. Women
age 65 to 75 may manifest elements of both types of bone loss. Several
laboratory tests can differentiate between high- and low-turnover
osteoporosis. These tests measure the level of bone resorption by
elevating bone collagen breakdown products. The most common tests are N-telopeptide, pyridinoline, and dehydroxypyridinoline tests (22).
and patterns of the various fracture syndromes seen in osteoporosis.
The incidence of vertebral fractures and Colles’ fractures rises soon
after menopause, as these areas derive much of their mechanical
strength from trabecular bone (73). The
incidence of hip fractures, in which cortical bone loss is more
important, increases slowly with age until late in life. One third of
women who live to age 90 suffer a hip fracture (69).
osteopenia, seen when more than 30% of mineral is absent; loss of
horizontal trabeculae of the vertebral bodies; wedge fractures to the
thoracic spine; end-plate fractures or crush fractures of the lumbar
spine; stress fractures of the pelvis; and fractures of the humerus,
wrist, hip, supracondylar femur, and tibial plateau. In pure
osteoporosis, laboratory studies are normal except for a slight
increase in alkaline phosphatase after new fractures. Although
osteoporosis is the number one cause for fractures in these locations,
other possible metabolic abnormalities must be ruled out before making
the diagnosis of osteoporosis.
Hyperparathyroidism is a relatively common cause of osteopenia, and
patients with the condition typically have an increased PTH level,
hypercalcemia, and hypophosphatemia. Most of the patients are
identified by incidental laboratory findings.
values may be elevated or may be within the high-normal limits.
Iatrogenic hyperthyroidism is common in women who are slightly obese
and have been placed on thyroid medication. The key diagnostic tests
for patients with suspected hyperthyroidism is the thyroid stimulating
hormone–radioimmunometric assay (TSH-IRMA). Other endocrinopathies
associated with osteoporosis are unstable diabetes and Cushing’s
disease. Over 99% of cases of Cushing’s disease leading to osteoporosis
are iatrogenic, with patients on doses of prednisone exceeding 7.5
mg/day. Diabetes can cause osteoporosis by hypercalciuria and negative
nitrogen balance.
should be sought in patients with localized vertebral fractures. Two
percent of osteopenic patients have bone-marrow abnormalities; 50% of
these are multiple myeloma. Typical laboratory findings for these
patients are decreased hemoglobin, increased sedimentation rate, and
abnormal serum or urinary protein electrophoresis. In about 15% of
patients with multiple myeloma, the serum electrophoresis may be
normal. Osteopenic patients with abnormal blood parameters may require
a bone-marrow biopsy. Once endocrinopathy and bone-marrow pathologies
have been eliminated as causes for osteopenia, the differential
diagnosis is between osteomalacia and osteoporosis.
degrees of osteomalacia. About half of these patients have abnormal
blood studies—most notably decreased urinary calcium, decreased to
low-normal serum calcium, decreased 25-hydroxy vitamin D, low
phosphorus, and elevated alkaline phosphatase. The remaining patients
with osteomalacia can be differentiated from those with true osteoporosis only by evaluating a transilial bone biopsy.
maximum information. Patients whose biopsies demonstrate more than 5%
osteoid volume are considered to have osteomalacia, and the source
should be sought. The bone biopsy can also help distinguish between
high-turnover and low-turnover forms of osteoporosis. With the biopsy
demonstrating less than 5% osteoid volume and less than 0.5% of the
trabecular surface with active resorption, the patient is classified as
having quiescent or low-turnover osteoporosis; this accounts for about
two thirds of the patients with osteoporosis. Patients with more than
0.5% active resorption on the trabecular surface and less than 5%
osteoid volume are classified as having high-turnover osteoporosis.
Treatments for the other forms—osteopenia and osteomalacia—should be
individualized to the underlying etiology (Table 131.5).
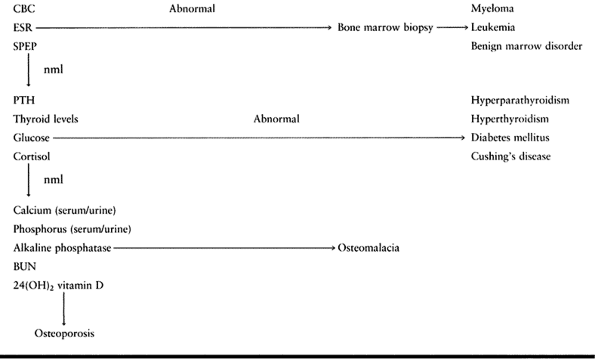 |
|
Table 131.5. Workup of Osteoporosis
|
quantification of bone mass is necessary for confirmation of the
diagnosis and longitudinal follow-up. Plain radiographs are of little
use in measuring a patient’s bone mass. A decrease or loss of the
horizontal trabecular pattern on spinal radiograph suggests osteopenia;
such a finding is, however, somewhat technique dependent, and up to 40%
of spinal bone mass can be lost before this finding is noted.
Dual-energy x-ray absorption uses an x-ray source that emits two
different energies, allowing the soft-tissue component to be
subtracted. This technique permits assessment of fracture risk of the
vertebral bodies and the femoral neck. The radiation dose is low (about
5–15 mrad), and the precision is 1% to 2% for the vertebral body and 2%
to 4% for the femoral neck (43). Compression fractures, scoliosis, and soft-tissue calcifications may distort the data.
can also be made. New studies using this method in a lateral position
appear to improve its sensitivity by 50% (85) (Table 131.6).
 |
|
Table 131.6. World Health Organization Diagnostic Criteria for Osteoporosis
|
the measurement of spinal trabecular bone density and is very sensitive
to change; its precision is 3% to 15% (43).
There is about 20 times as much radiation involved as in dual-energy
x-ray absorptiometry. The CT scan may be affected by artifacts caused
by marrow fat or vertebral veins.
CT scanning are used to identify patients with low initial bone mass,
or to monitor patients longitudinally to evaluate decreasing bone mass.
Other methods to identify osteopenic patients include radiographic
wedge, single-beam absorptiometry, and ultrasound. Although these
measures can be used to identify a population at risk for fracture
(those with spinal density below 1 g/cm3), they have proved
to be of little value in predicting whether a particular patient will
suffer a fracture during a specific period of time.
but uniform preventative measures have not been widely adopted.
Clearly, patients who develop a high peak bone mass by age 25, maintain
adequate calcium intake, and perform weight-bearing exercises will have
a decreased risk of developing symptomatic osteoporosis. During the
period of maximum bone accretion (age 13 to 25), we recommend a dietary
regimen of about 1,200 mg/day elemental calcium with 400 units vitamin
D, as well as an impact-type exercise program.
protein, carbohydrates, and calcium. Girls who develop amenorrhea or
oligomenorrhea at an early age are at particular risk for developing
osteoporosis later, because they never attain sufficient peak bone
mass. These patients can actually develop a negative calcium balance
and lose bone mass while young. Although some have suggested that
exercise causes both amenorrhea and bone loss, it has been shown that
girls who maintain normal menstrual cycles while exercising are at no
increased risk for osteoporosis. Young athletes who do develop
amenorrhea or oligomenorrhea should be considered for cyclic
estrogen/progesterone or birth-control pills in an effort to
reestablish normal menstrual cycles.
age) should be maintained on a calcium intake of about 800 to 1,000
mg/day with 400 units of vitamin D, as well as a reasonable exercise
program.
possible treatments once the diagnosis of osteoporosis is established.
Recommend behavior modification, such as the cessation of smoking and
decreasing alcohol intake, for all patients, but do not expect a high
level of compliance. Another nonpharmacologic intervention is to
increase the level of exercise. It is well known that immobilization
leads to increasing bone resorption (67).
Prospective studies have shown that 1 hour of exercise two or three
times a week is effective in increasing lumbar spine density and total
body calcium in postmenopausal women compared with sedentary control
groups (1,47). Spinal
extension, abdominal muscle strengthening, and impact-loading exercises
such as walking, square dancing, or weight lifting may be very
beneficial for patients with osteoporosis.
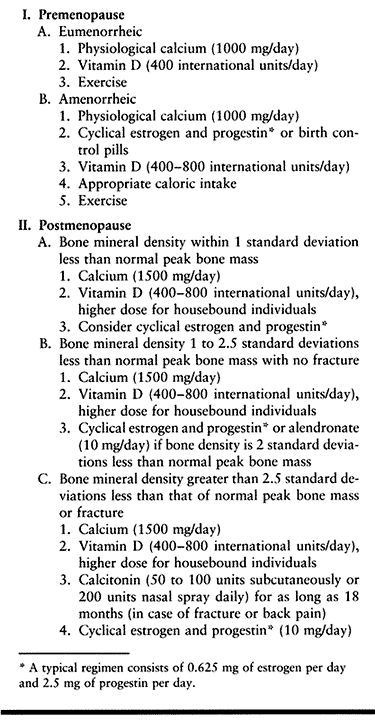 |
|
Table 131.7. Updated Protocol for Treatment of Osteoporosis at the Hospital for Special Surgery
|
adversely affect the skeleton, the role of calcium supplementation in
preserving or augmenting skeletal mass is unclear. Menopausal status
may determine whether calcium therapy will be effective in osteoporotic
patients.
osteoporotic women were put on 1.5 g/day calcium, 800 units/day vitamin
D, and an exercise program. The patients were divided into three age
groups: premenopausal, early postmenopausal (within 10 years of
menopause), and late postmenopausal (65 years or older). Serial
dual-photon absorptiometry measurements were obtained from the lumbar
spine and femoral neck. Calcium therapy (with exercise and vitamin D)
resulted in a significant 3% to 5% increase in bone mass of the femoral
neck among all three groups tested in the first and second year of
therapy.
per year. Late postmenopausal patients showed a slight increase in bone
density. Early postmenopausal patients, however, lost vertebral bone
density at the rate of 2.2% per year, the rate of loss reported
elsewhere (18,75).
supplementation was beneficial in the younger and oldest patients,
calcium supplementation alone will not reverse or significantly slow
the rate of acute postmenopausal bone loss. Women with calcium intake
below 400 mg/day, however, can be significantly aided by calcium. We
currently recommend that all women over age 35 take 1,200 mg/day of
calcium; postmenopausal women should take 1,500 mg/day. Give vitamin D,
400–800 units/day, to all patients.
Many studies demonstrate that low-dose estrogen therapy, particularly
when begun shortly after menopause, can arrest or decrease spinal bone
loss, and decrease by 50% the incidence of radial, vertebral, and
proximal femoral fractures (18,32,33,42,51,52).
Postmenopausal patients with a strong family history of osteoporosis,
those with a bone mass more than 1.5 standard deviations below the
mean, or those who demonstrate a loss of 4% or more of bone per year on
serial bone-density measurements are strong candidates for estrogen
therapy or other antiresorptive programs.
cancer in women taking only exogenous estrogens, low-dose estrogens
combined with progesterone actually lower the incidence of endometrial
cancer (10). Of somewhat greater concern is the
possibility of an increased risk of breast cancer in patients on
estrogen. Several studies of combined treatment with low-dose estrogen
and progesterone indicate that the risk of breast cancer may not be
appreciably increased (19,38,91).
The general consensus, however, is that estrogen, even in combination,
may marginally increase breast-cancer risk. Unopposed estrogen therapy
appears to have a beneficial effect on atherosclerotic heart disease
and mental function (20).
contraindicated include those with preexisting breast cancer,
regardless of receptor status, and patients with a history of
thromboembolic disease on hormonal therapy (95). Excess uterine bleeding, hypertriglyceridemia, liver disease, and cholelithiasis may also be contraindications to therapy.
about 90% of women eligible for estrogen therapy were reluctant to
undertake it because of the perceived risk of cancer. Clearly, it is
the orthopaedist’s responsibility to educate the patient on relative
risks and benefits of estrogen therapy. If hormonal therapy is chosen,
we recommend a minimum dose of 0.625 mg of conjugated estrogen cycled
with appropriate medroxyprogesterone acetate. Mammograms and
gynecologic evaluations must be done at least yearly.
(SERMs) such as relaxofen provide ERT-like bone benefits without the
associated breast and endometrial cancer risk. Preliminary data suggest
that spinal fractures decrease by 50% and the risk of breast cancer
decreases similarly. There are no data regarding hip fracture.
chronic administration may decrease the number of osteoclasts.
Calcitonin is a strong antiresorptive agent particularly useful for
patients with high-turnover osteoporosis who will not take estrogen or
progesterone. Currently, calcitonin must be given subcutaneously by
injection; in some patients, flushing accompanies injection. Although
40% of patients develop an antibody to the calcitonin, this
circumstance does not preclude its use. Patients on calcitonin have
shown increases in total body calcium, iliac crest bone volumes, and
bone content of the lumbar spine and femoral diaphysis (21,24,25). Most of the increase occurs in the first 6 months.
Calcitonin may also be particularly useful in patients with acute
recent spinal fractures, as there is evidence that it functions as a
neuropeptide and has an analgesic effect. It is also helpful in steroid
osteoporosis, in which it counteracts the secondary
hyperparathyroidism. The usual dose is 200 units daily nasally for 18
months, with concomitant calcium and vitamin D.
used for decades in the treatment of Paget’s disease; recently, they
have been used successfully to treat osteoporosis (92,94).
The cyclic use of etidronate, 400 mg/day for the first 14 days of each
quarter, significantly increased bone mass by 1% to 2% per year and
decreased the spinal fracture rate when compared with calcium alone (87,89).
Appendicular bone mass increased less, and there was no clear effect on
prevention of hip fractures. Some of the newer bisphosphonates, the
so-called third-generation bisphosphonates, including pamidronate and
alendronate, lead to a greater increase in spinal mass and may enhance
formation and decrease resorption more effectively than the
first-generation bisphosphonates such as etidronate (15). Alendronate use is supported by a large number of clinical trials using fracture as an end point (42).
Like ERT, alendronate increases bone mass and decreases fracture risk
for all bones by 50%. The major side effect is esophagitis. Alendronate
appears to have similar efficacy on osteoporosis in men.
inhibits bone formation and resorption and can lead to osteomalacia,
which is the reason for using short cycles. The newer bisphosphonates
do not affect formation and are quite safe.
increased morbidity and mortality. Economic consequences of these
fractures can also be quite severe. Cornell has developed the following
principles guiding the management of fractures in patients with
osteoporosis (14):
-
Elderly patients are best served by rapid
definitive fracture care aimed at early restoration of mobility and
function. Often patients are in their best health on the day of the
injury; if so, perform surgery at that time. Judicious medical
management of patients with coexisting medical problems can minimize
postoperative morbidity and mortality. Choose surgical procedures that
require minimal operative time and result in minimal blood loss while
providing definitive care. -
The operative procedure should allow
stable fracture fixation with early weight bearing or early return of
function. Intra-articular fractures should be anatomically restored,
but metaphyseal and diaphyseal fractures are best managed by achieving
primary stability rather than anatomic reduction. -
In osteoporotic patients, the primary
mode of failure of fracture repair is failure of the bone rather than
failure of the implant. Choose fracture fixation to allow stable
compaction of fracture fragments and to minimize stress shielding of
regional bone. -
Osteoporotic patients go through the
early stages of fracture healing by endochondral repair normally.
However, the last stage of remodeling is slowed in these patients. Hip
fractures can remain positive on bone scan as long as 3 years. In
addition, elderly patients often are malnourished at the time of
injury, and their diets are deficient in calcium, calories, and
protein. Administer 1,500 mg/day of calcium and physiologic vitamin D
to patients in the hospital, as well as a diet sufficient in calories
and protein to overcome malnutrition. -
Evaluate patients with fractures fully
for the type of osteoporosis they have, and initiate treatment on the
basis of the pathology.
fractures are the major cause of morbidity and mortality in patients
with osteoporosis (45). About 32% of women and
17% of men suffer a hip fracture before age 90, and 40% of
noninstitutionalized patients die within 2 years of the injury (4,30,31,35,36,40,90).
A return to preinjury level of function is seen in only 25% of patients
who suffer a hip fracture, and 50% of these patients have a significant
social deterioration (36,86).
noted. It has been suggested that the common use of tranquilizing
medication in this population results in poorer reaction time and an
increased incidence of falls (88,93).
Hayes has suggested that only falls that result in a direct blow to the
region of the greater trochanter result in hip fracture (31). Recent incidence studies by Cummings and Hayes now place falls as the primary etiology in 65% to
95% of cases (15). Osteopenia is almost universally found in patients with hip fractures.
patients with osteoporosis because of the high incidence of nonunion
and avascular necrosis. Closed reduction and internal fixation have
been associated with an incidence of about 14% nonunion and 15%
avascular necrosis (11). While these
complications can be minimized by accurate reduction, patients with
severe osteopenia have a higher incidence of failure secondary to loss
of bone fixation (3).
are closed reduction with internal fixation, and hemiarthroplasty.
Hemiarthroplasty would seem an ideal procedure, as nonunion and
avascular necrosis would not occur, but it is associated with its own
complications (dislocation, loosening and breakage of implants, late
infection). In addition, hemiarthroplasty does not provide hip function
equal to that of an intact hip joint. We recommend primary
hemiarthroplasty using a cemented bipolar prosthesis for patients who
are physiologically 70 years of age and who are active household or
community ambulators. Patients with severe osteopenia and those with
neurologic disorders who cannot comply with partial weight-bearing are
also selected for hemiarthroplasty.
closed reduction with internal fixation using cannulated screws,
Knowles pins, or compression screws. A study from New York
Hospital–Cornell Medical Center and the Hospital for Special Surgery
showed that among 251 patients undergoing this procedure between 1979
and 1981, 80% of those with grades III and IV fractures had good to
excellent results at 6 months when good reduction was obtained in the
operating room, compared with 95% good-to-excellent results for
hemiarthroplasty. The survival rate at 6 months was 96% for patients
with Knowles pins and 95% with hemiarthroplasty (Fig. 131.8) (2,6).
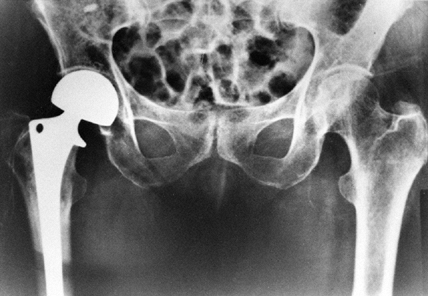 |
|
Figure 131.8. Hemiarthroplasty (bipolar) for treatment of displaced femoral neck fracture.
|
osteoporosis. These fractures do not have the same rate of nonunion and
avascular necrosis as do femoral neck fractures, but malunion with
varus and shortening in external rotation is a risk. Nail side-plate
devices, such as the Jewett nail, have been associated with loss of
fixation. This device is a load-bearing, not a load-sharing, device.
Use of these fixators in patients with poor bone quality often results
in loss of fixation or pin penetration into the joint.
controlled impaction. The sliding hip screw has been our primary means
of treatment of intertrochanteric fractures. Several technical points
should be noted. Place the screw in the center of the femoral head to
engage subchondral bone. A screw with a side-plate angle more than 140°
is optimal for biomechanical reasons. New screws with extra-large
threads have been introduced to improve fixation in poor-quality bone.
In patients with grossly unstable fractures in which a large amount of
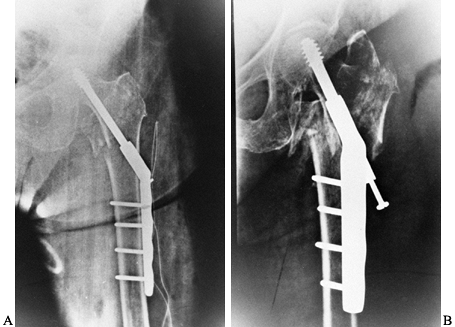
impaction is anticipated, use short-barrel side plates (Fig. 131.9).
Some authors have recommended lag-screw fixation for large posterior
medial fragments to improve the stability for three- and four-part
intertrochanteric fractures (48).
 |
|
Figure 131.9. A: Intertrochanteric hip fracture fixed with sliding hip screw. B: Further impaction. Patient went on to union in this position.
|
of the tibial plateau are also seen in patients with osteoporosis,
because they occur in areas of thin cortex and abundant trabecular
bone. In general, gear treatment toward producing early range of motion
in the knee and quadriceps rehabilitation. In debilitated patients with
limited ambulatory capacity preoperatively, or limited life expectancy,
either a long-leg cast or cast-brace may be the
best
treatment. If surgery is necessary, the standard principles of anatomic
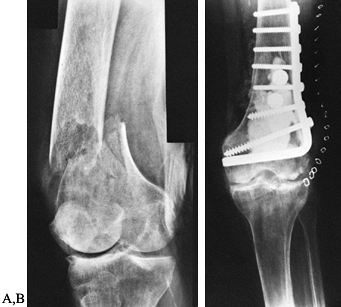
restoration and rigid fixation apply as in any fracture.
in extremely comminuted fractures, a condylar buttress plate can be
substituted. Autogenous bone graft can be applied to areas of
comminution. Rigid fixation will allow early knee rehabilitation,
including continuous passive motion (CPM). Ideally, in a stable
situation ambulation can be started, although a cast-brace may be
required.
 |
|
Figure 131.10. A: Supracondylar femur fracture. B: Fixation of supracondylar fracture.
|
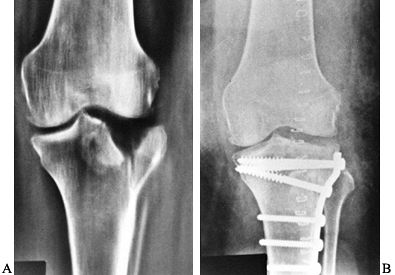
less than 5 mm of depression and are stable on examination can be
treated by a short period of knee immobilization, followed by active
knee motion. No weight should be put on the site until fracture healing
has occurred. CPM may be used after the injury; apply a hinged knee
brace for protection. Patients with displaced fractures of more than 8
mm or with an associated varus or valgus instability require open
reduction and internal fixation (66) (Fig. 131.11).
 |
|
Figure 131.11. A: Tibial plateau fracture. B: Fixation of tibial plateau fracture.
|
exposure; it is the incision of choice in elderly patients who may
later need a total knee arthroplasty. Use CPM and a cast-brace
postoperatively, and delay weight bearing for 8–10 weeks.
osteoporosis by the same principles as for ankle fractures in younger
patients. In osteoporotic bone, which often provides poor screw
purchase, Rush rods or tension band techniques may be superior.
Antiglide plate fixation of the lateral malleolus also offers
advantages in the elderly (8,81).
Pay close attention in the postoperative period to the wound.
Generally, use splints for 10–14 days; keep the fracture site elevated
and permit no weight bearing. Then apply a short-leg cast for 4–6
weeks, and begin joint range-of-motion and strengthening exercises.
fracture of a vertebral body. In 90% of the population, radiographic
evidence of a vertebral injury is discernible by age 75 (37).
The classic “dowager’s hump” is the result of compression and wedge
fractures primarily of the thoracic and lumbar spine, resulting in
kyphosis and diminished height. To maintain upper body posture, a
hyperlordotic curve can develop in the lumbar spine, resulting in
mechanical low-back pain.
loss of height of the vertebral body or anterior wedging of the
vertebral body, in addition to clinical symptoms of pain and
tenderness. Often, though, the fractures may be relatively asymptomatic
and discovered only on radiograph. As previously mentioned, a patient
with one isolated vertebral fracture must be fully evaluated to exclude
the diagnosis of malignancy. In addition, as with all osteoporotic
fractures, do a full evaluation of the underlying metabolic bone
disease.
discussed, and additional orthopaedic intervention may be required.
Pain may be a prominent finding in patients with vertebral body
fractures, and analgesics may be necessary. Narcotic analgesics are
unsuitable because of the potential for respiratory depression and the
effect of sedation on the patient’s ability to ambulate without falling.
and others believe that bracing should be avoided, because further
osteoporosis will result if the brace functions as a load-bearing
device. Studies by Krag et al. suggest that corset spinal braces do not
significantly reduce the load on the spine but do restrict motion (49,50). These braces may allow the patient to ambulate while restricting motion enough to minimize pain.
the spine and is undesirable in patients with severe osteoporosis. A
light hyperextension type of orthosis may be helpful in relieving pain
in some patients; however, patient compliance can be a problem. In
these cases, a very short period of bed rest followed by ambulation and
mobilization as tolerated may be sufficient.
operative means, except for the rare patient in whom significant
neurologic compromise develops. Patients who suffer a high-energy type
of burst fracture with a resultant unstable spine require posterior
fixation.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
and Steroid Hormone Study of the Centers For Disease Control and the
National Institutes of Child Health and Human Development. Combination
Oral Contraceptive Use and the Risk Of Endometrial Carcinoma. JAMA 1987;257:796.
SR, Nevett MC, Browner WS, et al. Risk Factors for Hip Fractures in
White Women. Study of Osteoporotic Fractures Research Group. N Engl J Med 1995;332:767.
PD, Bjarnason NH, Mitlak BH, et al. Effects of Raloxifene on Bone
Mineral Density, Serum Cholesterol Concentrations, and Uterine
Endometrium in Postmenopausal Women. N Engl J Med 1997;337:1641.
JP, Nagent de Deuxchaisnes C. The Treatment of Involutional
Osteoporosis with the Bisphosphonate APD (Disodium Pamidronate):
Nonlinear Increases in Lumbar Bone Mineral Density. J Bone Miner Res 1990;5(suppl 2):5251.
C, Chierichetti SM, Bigazzi S, et al. Comparative Effects on Bone
Mineral Content of Calcium and Calcium plus Salmon Calcitonin Given in
Two Different Regimens in Postmenopausal Osteoporosis. Clin Ther Res 1985;38:455.
WG, Henry DA, Horst R, et al. Parenteral Aluminum Administration in the
Dog. II. Induction of Osteomalacia and Effect on Vitamin D Metabolism. Kidney Int 1984;25:370.
W. Fall Biomechanics as Determinants of Osteoporotic Hip Fracture Risk.
Presented at the Steenbock Symposium on Osteoporosis, June 1989.
TA, Polansky SM, Feinstein AR. Postmenopausal Oestrogens Protect
against Fractures of the Hip and Distal Radius: A Case Control Study. Lancet 1979;2:705.
WJ. Spinal Osteoporosis, Spinal Deformity: What’s the Latest, What’s
the Best? Presented at meeting of American Academy of Orthopaedic
Surgeons, Baltimore, 1988.
MH, Byrne KB, Gilbertson LG, et al. Intra-Abdominal Pressurization:
Failure to Reduce Erector Spinae Loads During Lifting Tasks. Presented
to the International Society for Study of the Lumbar Spine, Dallas,
1986.
MH, Gilbertson LG, Pope MH. Intra-Abdominal and Intrathoracic Pressure
Effects upon Load-Bearing of the Spine. Presented at meeting of
Orthopaedic Research Society, Las Vegas, 1985.
B, Toft B, Pors Neilsen S, et al. Physical Exercise as Prophylaxis
against Involutional Vertebral Bone Loss: A Controlled Trial. Clin Sci 1983;64:541.
P, Wilson-MacDonald J. Total Arthroplasty in Paget’s Disease of the
Hip: A Clinical Review and Review of the Literature. Clin Orthop 1990;255:160.
BG, Weiner LP, Sutton SC, et al. Evidence for Both Respiratory
Syncytial Virus and Measles Virus Antigens in Osteoclasts of Patients
with Paget’s Disease of Bone. Clin Orthop 1984;183:303.
MM, Goods GC, Hislop JS. Composition of Domestic Water Supply and
Incidence of Fractures and Encephalopathy in Patients on Home Dialysis.
Br Med J 1977;2:657.
LS, Wahner HW, Melton LJ III, et al. The Relative Contributions of
Aging and Estrogen Deficiency to Postmenopausal Bone Loss. N Engl J Med 1984;311:1273.
BL, Wahner HW, Melton LJ III, et al. Rates of Bone Loss in Appendicular
and Axial Skeletons of Women: Evidence of Substantial Vertebral Bone
Loss before Menopause. J Clin Invest 1981;67:328.
E. Paget’s Disease of Bone. In: Favus ML, ed. Primer on Metabolic Bone
Disease and Disorders of Mineral Metabolism. Kelseyville: ASBMR, 1990.
T, Thamsborg G, et al. Effect of Intermittent Cyclical Etidronate
Therapy on Bone Mass and Fracture Rate in Women with Postmenopausal
Osteoporosis. N Engl J Med 1990;322:1265.
PA, Layde PM, Lee NC, et al. The Risk of Breast Cancer in
Postmenopausal Women Who Have Used Estrogen Replacement Therapy. JAMA 1987;257:209.
