METASTATIC BONE DISEASE
VII – NEOPLASTIC, INFECTIOUS, NEUROLOGIC AND OTHER SKELETAL DISORDERS
> Tumors and Tumor-Like Conditions > CHAPTER 130 – METASTATIC
BONE DISEASE
Professor of Radiation Oncology, Johns Hopkins University, Johns
Hopkins Radiation Oncology at Greenspring Station, Lutherville, MD.
destruction in the adult skeleton. Each year in the United States there
are approximately 1.3 million new cancer cases (16).
The most common carcinomas, those of the breast, lung, prostate gland,
and kidney, are also the most common cancers that metastasize to bone (9,10,11). The common distribution of bone metastases is to the spine, ribs, pelvis, and proximal limb girdles (1).
Lung carcinomas and melanomas have the ability not only to occur in the
axial skeleton but also to spread peripherally to the hands and feet (13,14).
It is important for the clinician to remember that virtually every
known cancer may metastasize to bone and that any bone in the body can
be involved.
both achieve local control of the tumor and reconstruct a limb that
will be functional. In contrast, the goals of treatment of patients
with metastatic bone disease are to stop progression of the tumor,
control pain, and maintain the quality of the remaining life of the
patient. Virtually all patients with metastatic bone disease will die
of their cancer. Patients and their families greatly appreciate the
clinician’s expertise and commitment to maintain the patient’s
independence in ambulation and the activities of daily living.
Uncontrolled pain, confinement to bed, pathologic fractures, and
neurologic compromise lead to despair and must be avoided.
with a working knowledge of the evaluation, decision making, operative
treatment, and postoperative care of the patient with metastatic bone
disease.
evaluate the patient to determine the source of the pain and to plan
treatment. The essential steps in evaluation are history, physical
examination, and plain radiographs (11).
patient’s pain. Pain at rest; severe pain; pain not responsive to
anti-inflammatory medications and narcotics such as Tylenol with
codeine, Tylox, and Darvocet; and pain at night are common complaints
of patients with bone metastases. The pain often begins as a dull ache
and then becomes constant. Activity-related pain is common with lower
extremity metastases. Patients with lower extremity metastases will
often relate a decrease in their ability to ambulate accompanied by
fatigue, pain, and weakness. Patients with vertebral metastases often
have pain and weakness, and they may have bladder and bowel dysfunction.
prominent physical findings. Atrophy is common with lower extremity
metastases, and the clinician can often elicit tenderness with deep
palpation. Perform a careful neurologic examination of the upper and
lower extremities. If the patient has back pain or reports bowel or
bladder dysfunction, perform a rectal examination to exclude cauda
equina syndrome.
areas. Many times, it is difficult to localize pain exactly in the
upper extremity, back, or pelvis. In these areas, it is often necessary
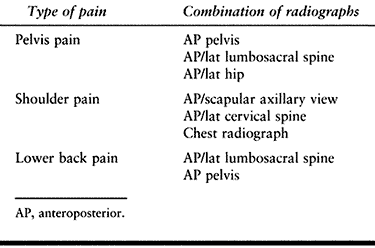
to obtain a combination of radiographs (Table 130.1). Carefully review the radiographs because the initial findings may be very subtle. The key features to look for are:
 |
|
Table 130.1. Combinations of Radiographs for Bone Pain in Cancer Patients
|
-
Cortical bone destruction: In metastatic bone disease, one may see the bone destruction on the endosteal or periosteal surface.
-
Periosteal reaction: Small areas of
periosteal reaction are often the only evidence that the tumor has left
the medullary cavity. -
Permeative pattern of bone destruction:
In contrast to bone sarcomas, which cause large areas of cortical bone
destruction, in metastatic bone disease there are often very subtle
areas of permeative bone destruction in the cortex. -
Destruction of the vertebral pedicle: In
the spine, the cortical destruction in the pedicle is an early
radiographic sign of metastatic bone disease. This finding is often
subtle and must be looked for carefully.
examination, and plain radiographs, there are generally four different
clinical scenarios:
-
Patients with advanced metastatic
disease, including diffuse visceral and bone metastases. In this
scenario, the orthopaedic surgeon is not called on to make a diagnosis
but rather to evaluate and manage either a fracture or an impending
fracture. -
Patients with a history of cancer and a
single destructive bone lesion. In this scenario, perform a very
careful evaluation because you cannot simply assume that an undiagnosed
bone lesion is necessarily related to the patient’s prior cancer. Plan
the evaluation as if you were evaluating the patient without the
history of the prior cancer. The staging evaluation includes a
technetium bone scan, chest radiograph, computerized tomographic (CT)
scan of the chest and abdomen, and serum laboratory studies (complete
blood count, erythrocyte sedimentation rate, chemistry group, and serum
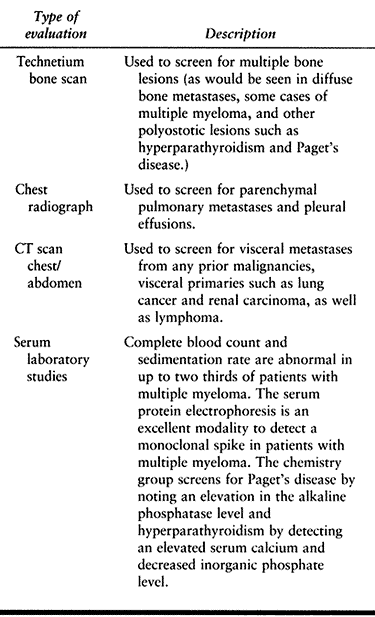
protein electrophoresis) (Table 130.2).![]() Table 130.2. Staging Evaluation for Patients with a Single Destructive Bone Lesion with and without a History of Cancer (22)
Table 130.2. Staging Evaluation for Patients with a Single Destructive Bone Lesion with and without a History of Cancer (22) -
Patients without a history of cancer and
a single destructive lesion. Many patients with metastatic bone disease
present with a destructive lesion without a history of cancer. Skeletal
pain or fracture heralds the presence of an occult primary malignancy
(sentinel metastasis). A sentinel metastasis is common in carcinomas of
the kidney, lung, and melanoma. The evaluation of the patient with a
single destructive lesion is exactly the same as the scheme for the
patient with a known cancer with a single destructive lesion. -
Patients with a history of cancer, bone
pain, and normal radiographs. Primary care physicians, medical and
radiation oncologists, and orthopaedic surgeons often evaluate cancer
patients with bone pain and normal
P.3471
radiographs.
This scenario is tricky. Do a careful history and physical examination
to avoid both undertreatment and overtreatment. If the plain
radiographs do not show a destructive lesion, try to establish the
cause of the pain. For example, if the patient has shoulder pain, look
for a degenerative, traumatic, or other anatomic problems such as
rotator cuff tendinitis, cervical spondylolysis, acromioclavicular
joint arthritis, and glenohumeral arthritis. If a reasonable working
diagnosis cannot be established, then search for bone metastases with
other imaging techniques. A technetium bone scan is very sensitive; it
will detect an occult metastatic focus in about 95% of patients (25).
Magnetic resonance imaging (MRI) is also highly sensitive and can
pinpoint the site of the pain. If the patient’s shoulder pain is
secondary to a cervical vertebral metastasis, however, an MRI of the
shoulder will not detect the lesion.
to evaluate patients with an unknown primary tumor that has
metastasized to bone (22). This plan is based
on the knowledge that the two most common occult primary carcinomas
that metastasize to bone are lung and kidney cancers. When one wishes
to search for the occult primary malignancy, a CT scan of the chest is
used to look for either a primary lung carcinoma and a CT scan of the
abdomen, or abdominal ultrasound is used to detect a renal cell
carcinoma. Serum studies are used to detect multiple myeloma. Patients
with multiple myeloma have a decreased hemoglobin level or an increased
erythrocyte sedimentation rate, or both, in approximately 60% to 70%.
This simple staging evaluation identifies the primary carcinoma in up
to 85% of patients. In contrast, biopsy of the bone lesion alone
identifies the primary in less than 5% of patients.
pursue further tests such as gastrointestinal contrast studies or
endoscopy. We designate the patient as having an unknown primary
carcinoma.
decide whether the patient should be treated with both surgery and
radiation or radiation alone. Virtually all patients with complete
fractures need surgery unless their medical condition is too precarious
or their life expectancy is very short (less than 2 to 4 weeks).
Fracture risk is multifactorial, and there are no absolute criteria,
although there have been a number of criteria described in the
literature (2,5,15,19). For most patients, the decision must be individualized, and there are several criteria that should be considered.
the amount of cortical bone destruction. Lesions that fill the
medullary cavity but have not destroyed any of the cortical bone are at
low risk for fracture. Many destructive lesions destroy bone
eccentrically, with damage to a single cortex visible on
anteroposterior and lateral radiographs. We try to quantify the amount
of bone destruction by studying the two sets of radiographs. If three
of the cortices are normal and a single cortex is 50% destroyed, then
the amount of bone destruction would be 12.5%. When the amount of
cortical bone destruction is assessed at less than 20% to 30%, the risk
of fracture is small. When the cortical bone destruction is between 40%
and 80%, the risk is much higher. Spontaneous fractures (without
trauma) commonly occur in the lower extremity if there is greater than
75% cortical bone destruction (or the equivalent of three missing
cortices).
amount of bone destruction. Lesions that are purely blastic seldom
fracture. In contrast, lesions that are purely lytic are prone to
fracture, especially when they are large or in high-stress regions.
Lung and renal carcinoma often produce purely lytic metastases, which
can progress rapidly. Many patients with prostate carcinoma have purely
blastic metastases, which tend to progress slowly. Patients with breast
cancer usually have one of three patterns: (a) purely lytic lesions,
which are aggressive; (b) purely blastic lesions, which grow very
slowly; and (c) a mixed pattern of bone destruction and bone formation.
must be considered. Very proximal lesions in the metaphysis and
epiphysis are less prone to fracture. In contrast, lesions that destroy
greater than 50% of the cortical bone in the subtrochanteric, midshaft,
or distal third regions of the femur are prone to fracture. In the
upper extremity, similar sized lesions in the surgical neck, humeral
shaft, and distal third of the humerus may fracture. Fortunately, most
pelvic lesions do not fracture unless they become very large or invade
the hip joint.
clue to impending fracture. Many patients describe marked pain with
ambulation and none when they rest. Place patients on crutches or a
walker if they have weight-bearing pain as they await treatment.
system based on the degree of bone destruction, presence of lytic or
blastic metastases, location of the lesion, and type of bone pain. For
each of these four variables, there is a score of 0 to 3 for a 12-point
grading system. Patients with a score of 9 or greater are prone to
fracture, whereas those with a score of 7 or less are less likely to
fracture. This system has not been widely accepted because of the
overlap between the fracture and nonfracture groups and the
subjectivity of the criteria.
explaining the risks and benefits of prophylactic fixation. The
benefits include (a) an elective procedure that can be optimally timed
between courses of chemotherapy, if necessary; (b) usually less blood
loss; (c) avoidance of the pain of a pathologic fracture; (d) often a
technically easier operation that can usually be performed using closed
techniques; and (e) a quicker recovery. The risks are the usual ones
for surgery with the potential for an anesthetic complication,
bleeding, infection, and a delay in chemotherapy.
-
Cortical bone destruction greater than 40% to 50%
-
Pure lytic metastases
-
Permeative pattern of bone destruction over a length of bone that exceeds two to three shaft diameters
-
Lesions in high-stress areas:Subtrochanteric region of the femurShaft of the femurSupracondylar region of the femurSurgical neck of the humerusShaft of the humerus
-
Weight-bearing pain
factors. Take into consideration the patient’s emotional needs, the
anticipated loading of the bone, the need for chemotherapy, and the
medical condition of the patient.
Most patients are treated with a course of either 2,000 cGy in 5
fractions or 3,000 cGy in 10 fractions. In patients who are terminally
ill, a single dose of 800 cGy often diminishes their pain. Patients
with a solitary lesion and a relatively good prognosis can be treated
with a longer course (4,000 to 5,000 cGy in 20 to 25 fractions).
Patients with renal carcinoma are frequently treated with a higher dose
(4,500 to 5,000 cGy) than those with other carcinomas because of the
tendency for disease progression with lower doses.
or crutches during treatment and for 2 to 3 months following treatment.
Take serial radiographs at 1-month intervals to judge healing of the
lesion. Caution patients with upper-extremity disorders or injuries to
avoid heavy loads such as changing a car tire, chopping wood, lifting
items greater than 5 to 10 pounds, twisting the cap off a tight jar,
carrying heavy suitcases, and so forth.
rigid fixation so that the patient can bear full weight following the
operation. In contrast to the treatment of nonpathologic fracture, in
which the surgeon depends on the bone to heal, there may or may not be
healing of the fracture in metastatic disease because of the tumor
itself or the postoperative radiation or chemotherapy. In patients who
live for 5 years following treatment, the failure rates due to disease
progression or inadequate fixation can be as high as 40% (26).
plates) and prosthetic devices are the most commonly used surgical
tools. Methyl methacrylate can be used to supplement the fixation and
to fill in defects caused by the tumor (12). If rigid fixation cannot be achieved with an internal
fixation device or if the joint surfaces have been destroyed, we prefer
to use an endoprosthesis. When we use a prosthetic device we always add
an aminoglycoside antibiotic (Tobramycin) to the cement. The risk of
infection is higher in patients with metastatic bone disease, so we
take the extra precaution.
of treatment is to remove the tumor with a wide margin, in metastatic
disease, the cancer is controlled with external beam irradiation,
hormonal treatment, or chemotherapy. When open surgery is performed,
the metastatic tissue is curetted down to the cortex, and methyl
methcrylate is usually used to fill the spaces that have been destroyed
by the tumor.
-
A lesion in which there is disease
progression despite one or two adequate courses of irradiation. If the
patient has a good prognosis, we perform a wide resection to achieve
local control and then reconstruct the limb as necessary. -
In a patient who has renal cell carcinoma
and an excellent prognosis, we sometimes perform a wide resection for
local control. Many renal cell carcinomas are difficult to control with
external beam irradiation alone, especially if the patient survives 3
to 5 years.
defects and to supplement the fixation of internal fixation devices.
When large defects are filled, the cement becomes load sharing in
compression and helps reduce the deformation of the bone–implant
construct. If large voids are left, there will be excessive bending
forces on the implant with weight bearing. Metal fatigue failure was
common before the use of the cement. The cement is an excellent
adjuvant to enhance the fixation of intramedullary rods and screws.
When applying the cement, one must be careful to confine the cement to
the intramedullary cavity. If the cement leaks into the soft tissue, it
can damage neurovascular structures; when large amounts are in the
fracture site, the two bones ends will not unite. Most of the healing
of pathologic fractures comes from the periosteal tissues.
of cortical and cancellous screws. The screw can be initially placed
and then removed. The cement is injected into the screw hole with a
5-ml syringe, and then the screw is placed and the cement is allowed to
harden. An alternative method is to place the cement and then drill and
tap through it. Both methods work well.
Make an assessment of the entire skeleton. This can be done by either
reviewing a recent technetium bone scan or asking the patient if there
is more than one site of bone pain. If you suspect cervical vertebral
involvement, study an anteroposterior or lateral view of the cervical
spine to make sure that the spine is stable before surgery. When lower
extremity surgery is planned, make sure that there are no large lesions
in the humeri. Humeral lesions may fracture in the postoperative period
while the patient is using a walker or crutches.
factors: marrow replacement by tumor cells, prior irradiation and
chemotherapy, and anemia of chronic disease. We generally transfuse
patients preoperatively if their hemoglobin levels are below 10 mg/dl.
For complex procedures or procedures in which major blood loss is
anticipated, we transfuse to a hemoglobin level of 13 mg/dl or higher.
Leukopenia is also common. Ascertain if the patient’s white blood cell
count is rising or falling if he has received recent chemotherapy. We
avoid operating if the absolute white blood cell count is below 500
cells/mm3 at the time of surgery or below that within 2
weeks of surgery. Thrombocytopenia is also common. If the platelet
count is below 50,000/mm3, transfuse the patients with 8
units of platelets on the morning of surgery. Depending on the blood
loss, platelets may also be needed intraoperatively.
Lung and breast carcinomas, lymphoma, and myeloma are the most common
malignancies with associated hypercalcemia. Symptoms from hypercalcemia
are relative to the serum calcium level and the acuteness of the rise.
A rapid small rise may precipitate symptoms, whereas a slow rise to a
higher value may not. Early symptoms of hypercalcemia include anorexia,
fatigue, nausea, and vomiting. These symptoms may mimic the common
cold, so keep a high index of suspicion in the cancer patient. Late
symptoms include coma and other neurologic symptoms. Correct
hypercalcemia before surgery. Hydration and saline diuresis are the
first steps in treatment. Intravenous pamidronate is very effective in
lowering the serum calcium level (usually within 24 hours).
metastatic lesions. Destructive lesions are more common in the proximal
limb girdle—in the scapula, clavicle, and proximal one-half of the
humerus. Most lesions of the clavicle, scapula, forearm, wrist, and
hand can be treated nonoperatively. Occasionally, a pathologic fracture
occurs in the radius and ulna that requires open reduction with a
dynamic compression plate with methyl methacrylate augmentation as
necessary.
-
The humeral articular joint surface has been destroyed.
-
A pathologic fracture of the anatomic neck is present.
-
A large destructive lesion is present so that rigid internal fixation is not feasible.
-
There is progressive disease despite external beam irradiation.
-
Perform prosthetic arthroplasty through a
deltopectoral approach in a manner similar to arthroplasty for
nonpathologic conditions. -
Take the subscapularis off the humerus and address the tumor according to the particular problem.
-
If a fracture has occurred through the
anatomic neck, resect the proximal fragment with the articular surface
and curet the tumor from the distal fragment with straight and angled
curets. -
Then prepare the humerus with reamers and broaches and perform a trial reduction.
-
Then cement the humeral component into place and test the appropriate head with the humeral endoprosthesis.
-
Close the wound in a routine fashion.
for prophylactic fixation of impending fractures. Usually, at least one
cortex (on the anteroposterior and lateral views) is intact. The intact
cortices and the intramedullary nail result in a rigid construct, so
the patient has use of the upper extremity immediately postoperatively
without restriction. Closed rodding can also be used once a fracture
has occurred if the amount of bone destruction is small. If there is a
large destructive lesion, then it is unlikely that rod fixation alone
will result in a rigid construct. In this scenario, we prefer open
rodding with methyl methacrylate augmentation.
-
Place the patient supine on a radiolucent
table with a bump under the affected shoulder. Before prepping the
patient, use the image intensifier to ensure visualization of the
humeral articular surface and the entire humerus. Some fluoroscopy
tables must be turned around 180° to image the upper extremity because
they are designed for imaging the lower extremity. -
Prep the limb from the fingers to the
angle of the jaw proximally. Include the anterior chest wall to the
midline and the posterior shoulder to the vertebral border of the
scapula. -
Cover the image intensifier with a
sterile drape and maintain sterility throughout the procedure. If the
sterile drape is contaminated at some point, change it. -
For closed nailing, make a 5 to 8 cm
longitudinal incision from the acromion distally just posterior to the
greater tuberosity. Incise the subcutaneous tissue and fascia over the
deltoid muscle. -
Divide the deltoid muscle longitudinally with electrocautery down to the rotator cuff.
-
Make a 3 cm incision into the rotator cuff about one finger breadth posterior to the greater tuberosity.
-
Bring the image intensifier into the
field and use a sharp pointed awl to enter the proximal humerus just
medial and posterior to the greater tuberosity. -
Use a pointed T-handled reamer to both
enlarge the entry point and develop a track in the proximal humerus for
the guide wire. Bend the tip of the guide wire at 30° over a 3 cm
length to facilitate passage of the guide across the fracture site. -
Then pass the guide wire into the proximal fragment to the level of the fracture site under image guidance.
-
Reduce the fracture under the image
intensifier and pass the guide wire across the fracture site. Then
advance the guide wire as far distally as possible. -
Measure the length of the intramedullary
rod by placing a second guide wire of the same length along the
intramedullary guide wire and placing the tip at the entry site with
the image intensifier. -
Place a Kocher clamp on the measuring
guide at the proximal end of the intramedullary guide wire. Use a nail
gauge to measure the difference, which represents the length of the
guide that is in the intramedullary cavity. An alternate and equally
effective method is to use the nail itself as a reference. -
Then sequentially ream the humerus in 0.5
mm increments. The diameter of the nail chosen is the largest nail that
will reasonably fit in the intramedullary cavity. We generally choose a
nail diameter 1.0 mm less than the last intramedullary reamer. -
Exchange the ball-tipped guide wire for a
smooth guide wire, and place the nail over the guide wire into the
proximal fragment under image intensification. With the help of the
image intensifier, pass the rod across the fracture site. By
alternately checking the entry site and the elbow, advance the rod
until the proximal end is buried in the humeral head. Then place a
proximal interlocking screw. -
If the destructive lesion is small and
rigid fixation has been achieved with the rod and proximal locking
screw, a distal interlocking is not necessary. When in doubt, add the
distal locking screw to the fixation.
destructive lesions and in most pathologic fractures in which we do not
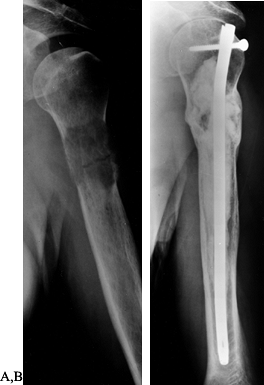
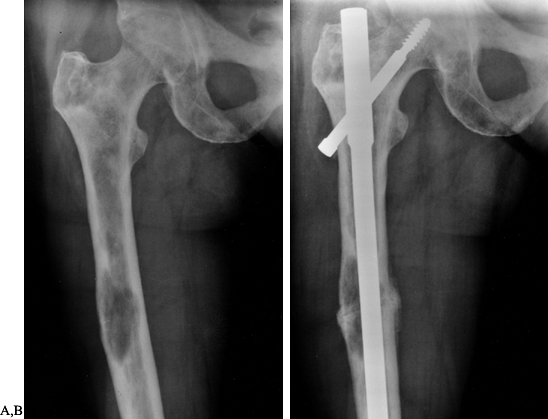
believe that we can obtain rigid fixation with closed rodding (Fig. 130.1). The rodding procedure is exactly the same as for closed rodding with the following modifications.
 |
|
Figure 130.1. Anteroposterior radiograph of the proximal humerus showing a purely lytic lesion with a pathologic fracture. B:
Anteroposterior radiograph following open reduction internal fixation with an intramedullary rod augmented with methyl methacrylate. Note the mature periosteal healing around the cement augmentation. |
-
Use a deltopectoral approach to expose
the proximal humerus and an anterolateral approach for lesions of the
diaphysis. For large lesions that involve both the proximal and mid
humerus, we combine the two approaches. -
Curet the tumor from the medullary canal
through a large cortical window in the patients who do not have a
fracture and through the fracture site in patients with fractures. -
Then prepare the humerus to accept the
intramedullary rod. We rehearse placement of the nail across the
fracture site and verify satisfactory reduction and position of the
nail before placing the cement in the intramedullary cavity. Be sure
that the exposure is adequate and the instruments and nail are ready. -
Withdraw the nail into the proximal
fragment proximal to the destructive lesion. We generally remove the
guide wire at this point. If the field is very bloody or the exposure
is less than adequate, you can leave the guide wire in place; however,
that makes it more difficult to inject the cement. -
Mix the cement and inject it with a
cement gun using a small nozzle into the distal fragment first and then
into the proximal fragment. Hand pressurize the cement to fill all the
defects. -
Drive the nail into place and then image
the humerus from the shoulder to the elbow to make sure the nail
placement is adequate and that there is not excessive cement in the
soft tissues. -
Place the proximal locking screws and close the wound in layers with a suction drain.
Most destructive lesions in the pelvis can be managed with external
beam irradiation alone. The most common lesions that require surgery
are lesions in the hip and femoral shaft.
The pattern of disease can be very diffuse with destructive lesions
involving the femoral head, neck, or intertrochanteric and
subtrochanteric regions alone or in various combinations. In addition,
there may be destructive lesions in the shaft of the femur that are
separated from the hip lesions by many centimeters. Preoperative
planning requires a high-quality plain radiograph of the entire femur.
Base the treatment plan on the location of the lesions.
The advantage of arthroplasty is that the patient can bear full weight
within a month of surgery and pain relief is excellent and
reproducible. Occasionally, if the patient has a small femoral neck
lesion (less than 40% cortical bone destruction), we perform cancellous
screw fixation with three screws followed by external beam irradiation.
Most metastatic lesions in the proximal femur are very diffuse,
however, and the screw fixation protects only the discrete femoral neck
lesions. We often treat patients with femoral neck lesions with
external beam irradiation alone and with crutches. Many patients wish
to avoid surgery but if the femoral neck fractures, the surgical
procedure remains the same—bipolar hemiarthroplasty.
 |
|
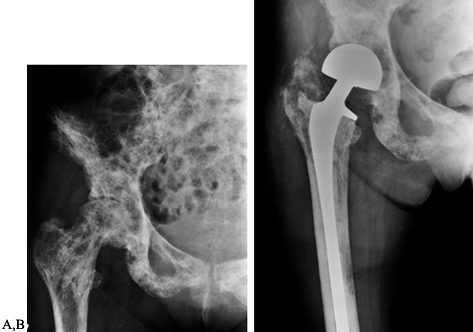
Figure 130.2. A:
Anteroposterior radiograph of the hip with extensive lytic and blastic areas in a woman with breast cancer. There is a minimally displaced femoral neck fracture. B: Anteroposterior radiograph of the hip following reconstruction with a long-stem bipolar endoprosthesis. Note the lytic lesion in the medial cortex of the midshaft of the femur. This lytic area is well bypassed by the femoral stem. |
-
Bipolar arthroplasty can be performed equally well through anterior or posterior approaches. We prefer
P.3476
the Hardinge approach because it provides excellent access to the
entire femoral shaft if curettage, wiring, or other procedures are also
necessary. It is important to choose a femoral stem that will
adequately bypass any lesions in the femoral shaft. -
Have available both head and neck
replacement prostheses and long-stem prostheses. The head and neck
prostheses are very useful if the proximal femoral disease is found to
be greater at surgery than what the radiographs showed. Many lesions
will progress while the patient waits for surgery. The long-stem
prostheses are very useful if an intraoperative fracture occurs. When a
long-stem prosthesis is used, it is very important not to pressurize
the cement because fat embolization may occur and result in
intraoperative cardiac arrest. To prevent this problem, vent the
intramedullary canal with a Silastic tube or drill one or two holes in
the proximal anterior cortex. -
Place the patient in the lateral decubitus position and pad all pressure points.
-
Using the Hardinge approach, incise the
skin and subcutaneous tissues directly down to the fascia and place a
Charnley self-retaining retractor to retract the deep fascia. -
Excise the trochanteric bursa and release
the anterior one third to one half of the gluteus medius and minimus
from the proximal femur. -
For bipolar arthroplasty, incise the hip
capsule with a T-type incision with the horizontal limb parallel to the
intertrochanteric line. Place no. 2 Ethibond sutures
P.3477
(Ethicon,
Inc., Somerville, NJ) in the capsule to facilitate retraction. Take the
vertical limb of the T to the acetabular labrum without cutting the
labrum. -
Be careful when dislocating the femoral
head. If the proximal femur is severely compromised by several
destructive lesions and you apply a large amount of force to dislocate
the head, the femur may fracture and significantly increase the
magnitude of the surgical problems. Three techniques can be used to
minimize the risk of fracture (we always employ the first two):-
Release the capsule anteriorly from the
proximal femur around the medial side by flexing and externally
rotating the hip while releasing the capsule. This can be accomplished
around the medial side and for a short distance posteriorly. -
Place a bone hook around the femoral neck to facilitate dislocation.
-
Osteotomize the femoral neck in situ
and remove in a manner similar to that for a patient who has had
femoral neck fracture. Be careful not to injure the acetabular
cartilage while removing the femoral head.
-
-
Once the femoral head has been removed,
inspect the articular cartilage visually and by palpation. If there are
no defects, then trial the acetabulum for the appropriate diameter
femoral head. We also measure the femoral head diameter with a caliper. -
Then ream and broach the proximal femur
to accept the femoral component. When cementing the component, do not
pressurize the cement as for nonpathologic cases. -
Close the wound in layers over suction drain.
sure to advise the anesthesiologist so that proper precautions can be
taken. These precautions include (a) decreasing the amount of
inhalation agent, (b) ensuring that the patient is not hypovolemic, and
(c) maximizing the oxygenation of the patient. The femoral canal is not
pressurized as is done in nonneoplastic cases.
-
Treat intertrochanteric lesions in a fashion similar to femoral neck lesions.
-
Always use the anterior Hardinge exposure.
-
Remove the compromised cortex and shape the intertrochanteric region to accept the head and neck prostheses.
-
Use straight and angled curets to remove
the gross disease. Do not be overly aggressive and thus destroy the
remaining cortical shell or there will not be sufficient bone stock to
perform the arthroplasty. -
Perform a trial reduction and cement the prosthesis as previously described.
proximal femur. In this scenario, we resect the proximal femur and
reconstruct the defect with a modular custom prosthesis. There are
three common situations in which this procedure is necessary:
-
There is extensive proximal femoral
disease and the bone stock is so poor that a standard prosthesis, head
and neck prosthesis, or an internal fixation device is not suitable. -
The patient has undergone one or two
courses of external beam irradiation to the hip and has progressive
disease. Here, the only way to halt progression is to resect the lesion. -
The patient has a failed internal
fixation device (the metal has fractured, the device has cut out of the
bone, or there is disease progression around the implant with loss of
fixation).
-
The positioning of the patient and the surgical approach is exactly the same as for bipolar hemiarthroplasty.
-
Divide the abductor mechanism
longitudinally into two equal anterior and posterior halves. When the
anterior half is dissected, release the gluteus medius and minimus from
the femur in longitudinal continuity with the vastus lateralis (it is
important for postoperative function not to separate them transversely). -
Remove the posterior flap from the
proximal femur in a similar fashion, although it is much more difficult
to maintain the continuity with the vastus lateralis. Also, release the
short external rotators. -
Release the hip capsule in a fashion
similar to that for bipolar arthroplasty, except now release the entire
capsule from the femur. Be very careful not to sever the acetabular
labrum because it is important for the stability of the prosthesis. -
Then osteotomize the femur at the desired
level and place a Verbrugge clamp on the end of the proximal fragment.
The Verbrugge is useful to position the proximal fragment as the soft
tissues are released. -
By placing the proximal fragment in
external rotation, abduction, and internal rotation, release the
adductors muscles, psoas tendon and all other soft tissues from the
femur. Having released all the soft tissues, dislocate the femoral head
from the acetabulum and section the ligament of the head. A hip skid is
often helpful to dislocate the head; however, be careful not to injure
the acetabular cartilage. Inspect the acetabular cartilage and
determine the bipolar head size. -
Then assemble the modular trial
prosthesis based on the amount of proximal femur removed. If the trial
modular components are too long, use a smaller body segment (decrease
by 20 mm increments). If only the stem and proximal trunnion are used
and the reduction is too tight, then resect 1 or 2 cm of femoral shaft
from
P.3478
the
remaining femur. Tag the hip capsule at four to six points along its
360° circumference to prevent soft-tissue entrapment during the trial
reductions of the bipolar component. -
Once the proper size is found, prepare
the femoral shaft with a flexible reamer so that a 1.0 to 1.5 mm cement
mantle can be achieved (the stem diameters are 9, 11, and 13 mm;
reaming would then be 11 mm for the 9 mm stem, 13 mm for the 11 mm stem
and 15 mm for the 13 mm stem). -
Then bring the actual prosthesis to be
used onto the field and assemble it. Cement the stem into the femur
with modern cement technique. -
Close the hip capsule carefully with no. 5 Ethibond sutures.
-
Close the anterior and posterior sleeves
of the abductor and vastus lateralis muscles carefully with a
combination of no. 5 Ethibond and no. 1 Vicryl sutures (Ethicon, Inc.,
Sommerville, NJ). -
Close the remainder of the wound in layers over a suction drain.
brace for 3 to 6 months, allowing full weight bearing immediately
following surgery.
has evolved as an excellent modality to internally fix both impending
and complete fractures of the femur (20) (Fig. 130.3). In contrast to the procedure with the humerus, we usually
do not open femoral shaft fractures to supplement the fixation with
methyl methacrylate. Rigid fixation can usually be achieved with
proximal and distal interlocking. We study anteroposterior and lateral
radiographs of the entire femur to identify sites of bone destruction.
If there are substantial lesions in the subtrochanteric region (within
10 cm of the lesser trochanter), we prefer to fix the femur with a
reconstruction nail. We use the standard femoral nail for lesions in
the femoral shaft to the supracondylar region. We do not routinely use
the reconstruction nail for all femoral lesions because the nail is
more difficult to insert than the standard nail. See Chapters 11 and, in particular, Chapter 20 for a description of closed intramedullary nailing. Specific details of the technique for metastatic lesions follows:
 |
|
Figure 130.3. A:
Anteroposterior radiograph of the hip showing a large subtrochanteric lytic lesion and diffuse disease in the intertrochanteric region. B: Anteroposterior radiograph following stabilization with a reconstruction nail. The femoral shaft fractured while placing the nail. Note the prominent periosteal healing. |
-
We prefer the supine position on the
fracture table. For reconstruction nailing, place the entry point a
little anterior to the exact midline. If the entry point is too
posterior with reconstruction nailing, you will not be able to place
the proximal interlocking screws into the femoral head. -
When advancing the guide pin in femoral
shafts with large destructive lesions, be careful because the guide can
penetrate the remaining cortex and enter the soft tissues. Avoid this
problem by turning the angle of the distal guide wire away from the
destructive lesion as the guide wire is advanced under image control.
In patients with complete fractures, advance the guide wire in the
proximal fragment until the fracture site is reached. With image
guidance, reduce the fracture and pass the guide wire across the
fracture site and advance it to the physeal scar. -
Plan to ream about 1 to 2 mm larger than the first reamer that obtains good cortical contact.
intramedullary cavity to the lungs during reaming and insertion of the
femoral nail. We do not routinely vent the femur by placing more holes
in the already compromised
bone.
Instead, we choose an intramedullary nail that is 1.5 to 2.0 mm smaller
than the last reamer. With this technique, the nail can be advanced by
hand or with gentle hammer taps. The proximal and distal interlocking
screws provide the rotational stability. If one has to drive the nail
with sharp hammer blows, then the femur should be reamed an additional
0.5 to 1.00 mm or a smaller diameter nail chosen.
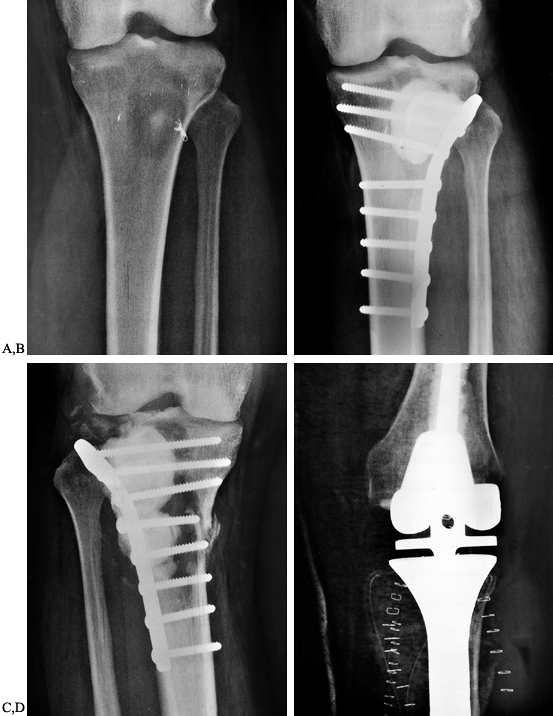
femur and proximal tibia are usually managed by curettage of the
lesion, cement augmentation, and plate fixation (Fig. 130.4).
It is more difficult to achieve rigid fixation with intramedullary rods
when the lesion approaches the articular surface. For the distal femur
we use a dynamic compression screw, whereas in the tibia we use lateral
tibia plateau plates or medial buttress plates, depending on the
location of the bone destruction. We avoid removing intact cortical
bone and generally do the exposure and plate fixation on the side of
the greatest bone destruction.
 |
|
Figure 130.4. A: Anteroposterior radiograph of the tibia showing a destructive lesion in a patient with renal cell carcinoma. B: Anteroposterior radiograph of the tibia following curettage and methyl methacrylate augmentation and plate fixation. C:
Anteroposterior radiograph of the tibia showing progressive bone destruction following two courses of external beam irradiation and plate fixation with cement augmentation. D: Anteroposterior radiograph demonstrating resection of the proximal tibia and reconstruction with a custom modular knee replacement. |
progression of the bone destruction, (b) avoid postoperative
complications, and (c) control pain.
Without postoperative irradiation, 20% to 30% of patients will have
disease progression with failure of the surgical procedure. In
addition, pain control can be achieved only when the tumor cells stop
destroying the bone. Begin radiation therapy
at
2 to 3 weeks following surgery, when the wound has healed. In
noncomplicated wounds, we often close the wound with staples. Nylon
sutures are used if we anticipate leaving the sutures in place for a
prolonged period because most radiation oncologists hesitate to radiate
over metal staples because of possible skin reaction. The dose of
radiation depends on the clinical situation and the general health of
the patient, according to the following guidelines:
-
Patients who are terminally ill and
cannot tolerate 2 weeks of daily treatments can be treated with a
single treatment 800 cGy in a single fraction. -
The most common prescription is 2,000 to
3,000 cGy in 5 to 10 fractions; this regimen is excellent for achieving
pain control and decreasing the risk of disease progression. -
For a solitary metastasis in a patient
with a good prognosis (survival greater than 12 to 24 months), 4,000 to
4,500 cGy in 20 to 25 fractions is often recommended. Patients with
renal cell carcinoma are also treated with higher doses because it is
often difficult to control metastatic renal cell carcinoma with lower
doses.
stabilizing bone disease in patients with metastatic bone disease.
Hormonal therapy plays a key role in appropriate patients with
metastatic breast and prostate carcinoma. Chemotherapy has less of an
effect on the healing of bone disease.
treating widespread bone metastases in patients with metastatic breast
cancer and multiple myeloma. The diphosphonates stop the osteoclasts
from resorbing bone, and this action helps to halt the relentless cycle
of bone destruction and further activation. Intravenous pamidronate is
the most commonly used agent. It is given over a 3-hour period, usually
on a monthly basis.
surgery because many of the patients are immunocompromised, poorly
nourished, and prone to deep vein thrombosis and hypercalcemia. The
following are some general guidelines to employ:
-
In patients who are immunocompromised,
administer intravenous cephalosporin and aminoglycoside for 3 days
following surgery. Continue with oral cephalosporin if the wound
continues to drain. -
Provide nutritional supplements to patients who have low albumin levels or lymphocyte counts.
-
Give low-dose warfarin (Coumadin) for 4
to 6 weeks following surgery to patients who have lower extremity
surgery. Encourage them to wear their thromboembolic disease (TED) hose
for 6 weeks when they are out of bed and ambulating. -
Check serum calcium levels
postoperatively in patients who exhibit any early symptoms of
hypercalcemia. Check the serum calcium routinely in patients who have a
history of hypercalcemia.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
KD, Sim FH, Enis JE, et al. Methylmethacrylate as an Adjunct in
Internal Fixation of Pathologic Fractures: Experience with Three
Hundred and Seventy Five Cases. J Bone Joint Surg [Am] 1976;58:1047.
JH, Twinbull AD, Miedema B, Lane JM. Acrometastases: A Study of Twenty
Nine Patients with Osseous Involvement of the Hands and Feet. J Bone Joint Surg [Am] 1986;68:743.
IH, Wiggers T, Bouma WH, et al. Treatment of Manifest and Impending
Pathologic Fractures of the Femoral Neck by Cemented Hemiarthroplasty. Clin Orthop 1990;260:220.
Y, Frassica FJ, Chao EYS, et al. Metastatic Bone Disease: A Study of
the Surgical Treatment of 166 Pathologic Humeral and Femoral Fractures.
Clin Orthop 1990;251:213.