Malignant Bone Lesions
Editors: Tornetta, Paul; Einhorn, Thomas A.; Damron, Timothy A.
Title: Oncology and Basic Science, 7th Edition
Copyright ©2008 Lippincott Williams & Wilkins
> Table of Contents > Section I
– Evaluation and Management of Musculoskeletal Oncology Problems > 4
– Treatment Principles > 4.3 – Malignant Bone Lesions
– Evaluation and Management of Musculoskeletal Oncology Problems > 4
– Treatment Principles > 4.3 – Malignant Bone Lesions
4.3
Malignant Bone Lesions
Francis R. Patterson
Timothy A. Damron
Carol D. Morris
Malignancies involving bone include metastatic disease,
myeloma, lymphoma, and bone sarcoma. Treatment options include surgery,
radiotherapy, and chemotherapy. This chapter will focus on the
treatment principles involved in choosing the appropriate surgical
treatment along with appropriate adjuvant radiotherapy and/or
chemotherapy according to the diagnosis.
myeloma, lymphoma, and bone sarcoma. Treatment options include surgery,
radiotherapy, and chemotherapy. This chapter will focus on the
treatment principles involved in choosing the appropriate surgical
treatment along with appropriate adjuvant radiotherapy and/or
chemotherapy according to the diagnosis.
Surgical Treatment
Surgical Indications/Contraindications
Indications for surgical intervention of bone malignancies vary according to the underlying disease process (Table 4.3-1).
-
Biopsy for diagnosis
-
Indications: plays a role in diagnosing
nearly all bone malignancies, although multiple myeloma may often be
diagnosed by serum or urine protein electrophoresis (SPEP or UPEP).
-
-
Prophylactic stabilization
-
Indications: Impending pathologic
fractures merit prophylactic fixation in many cases of metastatic
disease, myeloma, and lymphoma. -
Contraindications: Except in unusual
circumstances, bone sarcomas should be treated by wide excision, as
instrumentation will potentially disseminate tumor locally and possibly
systemically.
-
-
Open reduction and internal fixation (ORIF) pathologic fractures
-
Same as for prophylactic stabilization
-
-
Extended intralesional curettage with adjuncts
-
Indication: low-grade chondrosarcomas without soft tissue extension (as an alternative to wide resection)
-
Contraindication: more
aggressive-appearing chondrosarcomas (soft tissue extension and/or
intermediate or high grade), all other bone sarcomas
-
-
Resection for extensive destruction
-
Indications: Extensive symptomatic bony
destruction that is not amenable to stabilization may warrant resection
and reconstruction in the setting of metastatic disease, myeloma, and
lymphoma.-
Especially proximal femur and proximal humerus
-
Endoprosthetic reconstructions favored
-
Appropriate surgical margins range from intralesional to marginal or wide in this situation, as resection is not for cure.
-
-
Contraindications: When internal fixation will suffice in metastatic disease, myeloma, and lymphoma, ORIF is preferred.
-
-
Resection for cure
-
Indications
-
Solitary bone metastases (Box 4.3-1)
-
Most bone sarcomas (except intraosseous low-grade chondrosarcomas)
-
-
Resection of major segments of bone should not be
undertaken without considering (1) whether limb-sparing surgery or
amputation should be done, (2) whether reconstruction will be needed
for the defect left after limb-sparing surgery, and (3) what
reconstructive options should be considered.
undertaken without considering (1) whether limb-sparing surgery or
amputation should be done, (2) whether reconstruction will be needed
for the defect left after limb-sparing surgery, and (3) what
reconstructive options should be considered.
P.61
|
Table 4.3-1 Roles for Surgical Treatment According to Disease Process
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Limb Salvage Versus Amputation
The most important goal of the surgical treatment of
bone sarcoma is complete resection of the tumor with a wide margin.
Maximizing function and salvaging the limb are secondary but important
considerations in surgical planning. The decision to perform limb
salvage versus amputation is dependent on several factors.
bone sarcoma is complete resection of the tumor with a wide margin.
Maximizing function and salvaging the limb are secondary but important
considerations in surgical planning. The decision to perform limb
salvage versus amputation is dependent on several factors.
Indications for Limb-Sparing Surgery
-
Wide resection (complete resection of the tumor) must be attainable.
-
Function of the salvaged limb must be at
least as good as the function of the limb after amputation at the
appropriate level required for complete tumor resection (Fig. 4.3-1). -
Reconstructed limb must be stable and durable.
-
There must be adequate skin and soft tissue after resection of the tumor to allow for coverage of the limb/reconstruction.
-
Local rotation of tissues and the use of free tissue transfer have broadened the indications for limb salvage.
-
-
Usually major neurovascular bundles must not be involved or surrounded by tumor.
Box 4.3-1 Diagnoses for Which Resection of a Solitary Bone Metastasis may be Considered for Oncologic Purposes
-
Renal carcinoma
-
Thyroid carcinoma
-
Bone sarcoma
-
Soft tissue sarcoma
Evaluation and Management of Possible Neurovascular Involvement
-
Magnetic resonance imaging (MRI) is the
gold standard for imaging to determine the relationship of the tumor to
the surrounding structures. -
Major vessel resection en bloc with the sarcoma and reconstruction with vein graft or artificial vessel graft is possible.
-
Resection of major nerves is allowable as
long as the predicted function of the limb is at least as good as an
amputation with prosthesis.-
Upper extremity: As a general rule, any function saved is better than an amputation
-
Lower extremity
-
Patients without a sciatic nerve can walk; may require ankle–foot orthosis (AFO) and/or assistive device.
-
Femoral nerve resection/loss of extensor
mechanism is not an indication for amputation, as patients can walk
without active knee extension.
-
-
Relative Contraindications to Limb Salvage
-
Displaced pathologic fracture, due to tumor contamination throughout extent of fracture hematoma
-
Not absolute, as there is literature to
support limb salvage after pathologic fracture if there is a good
response to chemotherapy and the fracture heals
-
-
Misplaced biopsy site or prior “nononcologic” procedure performed with contamination of surrounding tissues
-
Reconstruction of limb not possible to allow function equivalent to an amputationP.62
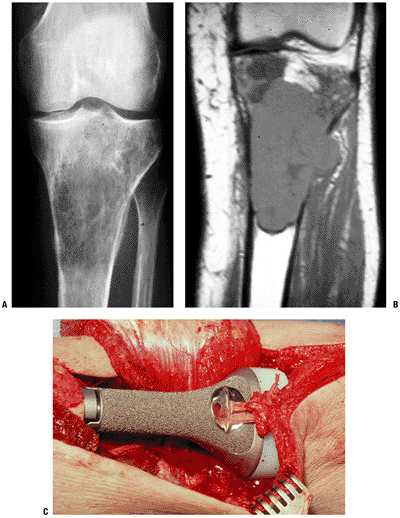
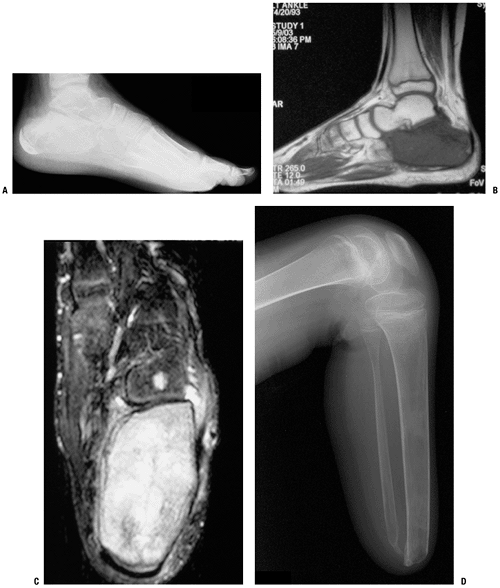 Figure 4.3-1
Figure 4.3-1
When the expected function following limb preservation is worse than
that following amputation, the latter is preferred, as in this patient
with a Ewing sarcoma of the calcaneus. Preoperative lateral foot
radiograph (A) and sagittal T1-weighted (B) and coronal T2-weighted magnetic resonance images (C) are shown. This patient underwent below-knee amputation (D). -
Poor response to chemotherapy
-
Vital neurovascular structures encased by tumor and not reconstructible or amenable to bypass
P.63
Amputation
-
Primary goal of amputation, like limb salvage surgery, is to resect the entire tumor with adequate “wide” margins.
-
Secondary goals of amputation are functional:
-
Must result in a stump that will allow for fitting of a prosthesis
-
Prosthetic fitting and rehabilitation is
an important part of recovery and can be difficult while patient is
still undergoing postoperative chemotherapy.
-
-
Risk of surgical complications is lower than for limb-sparing surgery.
-
Sometimes required after failed limb salvage attempts (e.g., infection, prosthesis failure, fracture)
-
Expendable Bone
After determining that limb-sparing surgery is
indicated, the decision of whether to reconstruct or not must be
weighed next. The specific sites of bone that are generally considered
expendable, and therefore not in need of reconstruction, are those
listed.
indicated, the decision of whether to reconstruct or not must be
weighed next. The specific sites of bone that are generally considered
expendable, and therefore not in need of reconstruction, are those
listed.
-
Fibula: usually no bony reconstruction
required (lateral collateral ligament [LCL] stabilization proximally or
augmentation distally is sometimes required) -
Iliac wing: when acetabulum not involved (some surgeons advocate reconstruction to restore pelvic continuity)
-
Pubis: if hip joint maintained, no bone reconstruction of inferior pelvis usually necessary
-
Rib: no bone reconstruction necessary
-
Distal ulna: no bone reconstruction is necessary, but soft tissue repair of the triangular fibrocartilage is recommended (Fig. 4.3-2)
Limb Reconstruction
Generalities of Available Options
There are several options for reconstructing skeletal
defects after resection of malignant or aggressive benign bone tumors.
Each has inherent advantages and disadvantages, and these should be
considered when planning limb salvage surgery. The seven “A’s” of limb
reconstruction are:
defects after resection of malignant or aggressive benign bone tumors.
Each has inherent advantages and disadvantages, and these should be
considered when planning limb salvage surgery. The seven “A’s” of limb
reconstruction are:
-
Amputation
-
Autograft
-
Arthrodesis
-
Allograft
-
Arthroplasty
-
Allograft-prosthetic composites (APC)
-
Alternative reconstructions
Amputation (see Fig. 4.3-1)
-
Advantages
-
Lowest complication rate
-
Least chance of requiring reoperation for failure of reconstruction
-
-
Disadvantages
-
Body image issues
-
Function of upper extremity or proximal lower extremity may be fair to poor.
-
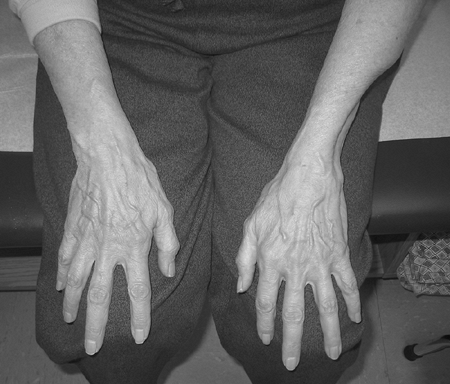 |
|
Figure 4.3-2
This patient underwent resection of the left distal ulna for a malignant fibrous histiocytoma adjacent to the bone. The distal ulna is an example of an expendable bone, since it does not require reconstruction. |
Autograft (Fig. 4.3-3)
-
Vascularized (e.g., free fibula) or nonvascularized (iliac crest, rib, fibula)
-
Advantages
-
“Normal” bone
-
Durable reconstruction
-
No risk of disease transmission
-
-
Disadvantages
-
Limited by size of defect and amount of bone available
-
Additional morbidity from donor site
-
Risk of cross-contamination (small)
-
Arthrodesis: Fusion of Bone With Elimination of Joint (Fig. 4.3-4)
-
Advantages
-
Stable reconstruction after union
-
No need for revision/repeat surgery after union
-
-
Disadvantages: does not allow immediate
function/weight bearing, may require additional bone graft (auto- or
allograft), delayed union/nonunion rates can be high depending on site
Allograft
-
Supplied by “bone bank”: sterilization with or without radiation (weakens), processing required for storage and transplantation
-
“Rejection” of transplanted bone does not occur, but role of “histocompatibility” is currently being evaluated.P.64
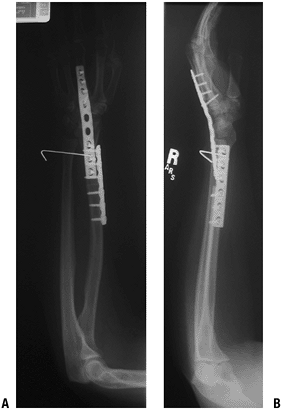 Figure 4.3-3
Figure 4.3-3
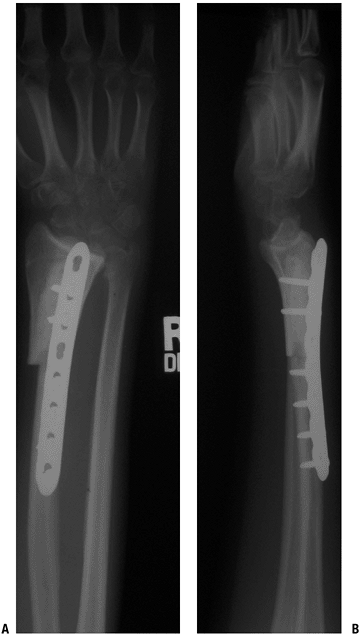
These early postoperative radiographs show an autograft nonvascularized
proximal fibula that has been used to reconstruct the defect following
resection of a distal radius for a giant cell tumor of bone. In this
case, the fibula was used to achieve an intercalary arthrodesis. A
proximal fibula may also be used with ligament reconstruction to
replace the distal radius and allow some wrist motion.![]() Figure 4.3-4 Radiographs of the proximal femur (A) and knee (B).
Figure 4.3-4 Radiographs of the proximal femur (A) and knee (B).
An extra-articular resection of the distal femur was performed
secondary to extensive tumor extension into the knee joint. An
intercalary allograft fusion of the knee with a long intramedullary
fusion nail was performed. -
Intercalary: segment of bone between joints maintained; host joint surfaces maintained; cylinder versus hemi-cylinder (Fig. 4.3-5)
-
Osteoarticular: articular surface of allograft used to reconstruct at least part of the joint surface (Fig. 4.3-6)
-
Advantages
-
Stable reconstruction after union
-
Not limited by size of reconstruction required
-
No donor morbidity
-
-
Disadvantages
-
Infection up to 20%
-
Nonunion/delayed union of host–graft junctions up to 20%
-
Fracture of allograft up to 15% to 20%
-
Disease transmission possible
-
Size of allograft needs to be matched to host.
-
Arthroplasty
-
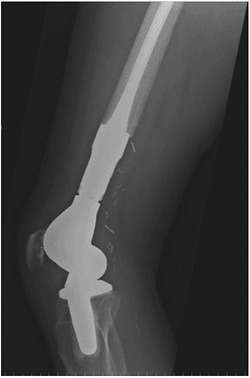
Usually by “megaprostheses”; modular endoprosthesis that can replace segments of bone and adjacent joint(s) (Fig. 4.3-7)
-
Advantages
-
Stable reconstruction that usually allows early weight bearing
-
Implant failure short term is low (less than fracture, nonunion of allograft)
-
No disease transmission
-
Size of reconstruction less of a problem (e.g., “total femur” and variable sizing of implant possible)
-
-
Disadvantages
-
Will likely require (several) revisions over lifetime
-
P.65
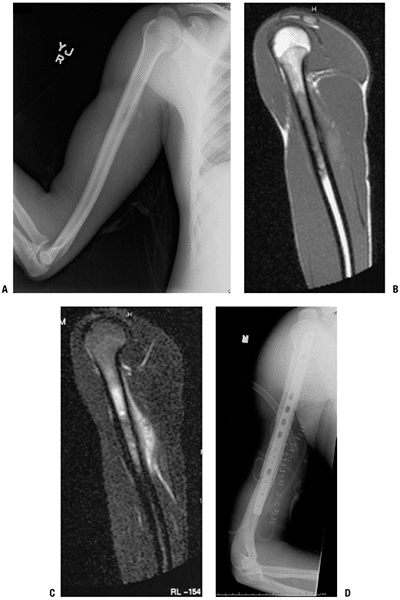 |
|
Figure 4.3-5
Intercalary allograft reconstruction of a right proximal humeral diaphyseal osteosarcoma. Preoperative studies include radiograph (A) and T1-weighted sagittal (B) and T2-weighted sagittal (C) magnetic resonance images. (D) Postoperative radiograph shows dual 90:90 plate fixation spanning the intercalary allograft, which is filled with antibiotic-loaded cement. |
P.66
 |
|
Figure 4.3-6
Following resection of a giant cell tumor of the distal radius, this patient underwent reconstruction using a distal radius osteoarticular allograft. |
 |
|
Figure 4.3-7
Lateral radiograph of the knee shows a rotating-hinge distal femoral replacement endoprosthesis. Both the femoral stem and tibial stem are cemented. The surgical clips are seen that were used to ligate the multiple branches off the popliteal artery at the time of resection of this distal femur osteosarcoma. |
Allograft-Prosthetic Composite (APC)
-
Combines segmental reconstruction of bone
with allograft and joint surface reconstruction with more standard
arthroplasty (cemented) components (Fig. 4.3-8) -
Advantages
-
May allow for
better soft tissue (tendon) attachment about the joint and therefore
more stability (e.g., host rotator cuff to proximal humeral APC or host
patellar tendon to proximal tibial APC or host gluteus medius tendon to
proximal femoral APC) -
Some surgeons believe this allows better function of that joint (controversial).
-
-
Disadvantages
-
Technically more difficult
-
All the risks of allograft and
arthroplasty combined, though stems of prosthesis should cross host
allograft junction to make nonunion and fracture less of a clinical
problem -
Disease transmission
-
Alternative Reconstructions
-
Site-specific options may have advantages over other types of reconstructions.
-
Rotationplasty: for resections about the knee (Fig. 4.3-9)
-
Single-bone forearm after radial/ulnar tumor resection (Fig. 4.3-10)
-
Bone transport/limb lengthening
-
Physeal distraction in children
-
Reconstruction in the Growing Child
Since bone sarcomas can occur at ages where there is
more bone growth left, resection of bone can result in significant leg
length discrepancies. This needs to be considered in the treatment of
these young patients. One indication for amputation is significant limb
length inequality after treatment. However, options do exist for limb
salvage surgery in growing children:
more bone growth left, resection of bone can result in significant leg
length discrepancies. This needs to be considered in the treatment of
these young patients. One indication for amputation is significant limb
length inequality after treatment. However, options do exist for limb
salvage surgery in growing children:
-
Expandable prostheses can be “lengthened” as the child grows (Fig. 4.3-11).
-
Rotationplasty requires preoperative planning to achieve both knees being at same level at skeletal maturity (see Fig. 4.3-9).P.67
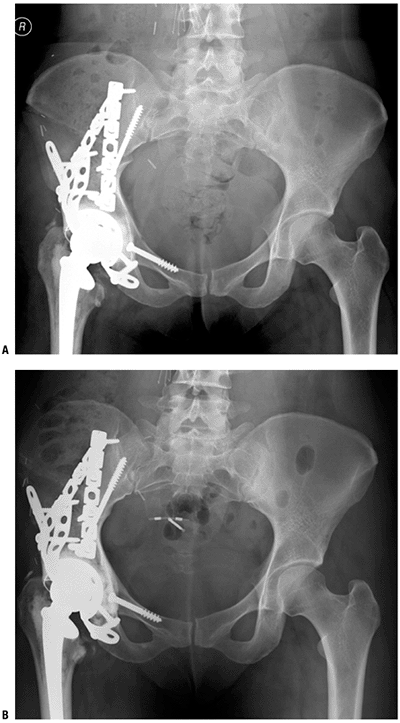
![]() Figure 4.3-8 After resection of an acetabular sarcoma, an allograft–prosthetic composite of the acetabulum was used for reconstruction. (A)
Figure 4.3-8 After resection of an acetabular sarcoma, an allograft–prosthetic composite of the acetabulum was used for reconstruction. (A)
An anteroposterior (AP) radiograph soon after surgery shows the
acetabular cage and constrained cup cemented into the acetabular
allograft and the cemented femoral stem. The allograft is secured with
interfragmentary lag screws and pelvic reconstruction plates. (B) The allograft–host junctions are healed at 9 months postoperatively.P.68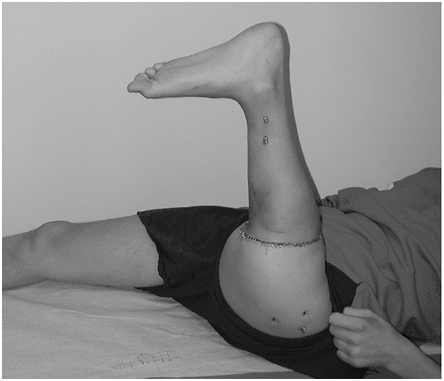 Figure 4.3-9
Figure 4.3-9
A postoperative picture of a skeletally immature patient after
rotationplasty was performed following extra-articular resection of a
distal femur osteosarcoma. The tibia has been turned 180 degrees and
osteosynthesis between the proximal femur and proximal tibia was
performed. The sciatic nerve and femoral arteries and veins were
preserved, as was active motion at the ankle. This allowed what would
have been an above-knee amputation to potentially function as a
below-knee amputation. -
Limb lengthening after treatment (e.g., bone transport or Ilizarov technique)
-
Claviculo-pro-humero (autograft clavicle used to replace proximal humerus)
-
Proximal fibular autograft
Location-Specific Reconstructive Options
There are unique anatomic considerations to each site of
bone tumor resection. Those, along with the reconstructive options, are
discussed below.
bone tumor resection. Those, along with the reconstructive options, are
discussed below.
Scapula (Fig. 4.3-12)
Many patients with scapular bone sarcomas undergo
resection without reconstruction, but scapular endoprostheses are
available; proponents cite improved functional outcome through
lateralization of the shoulder joint.
resection without reconstruction, but scapular endoprostheses are
available; proponents cite improved functional outcome through
lateralization of the shoulder joint.
-
Unique anatomic considerations
-
Soft tissue extent determines feasibility of scapular endoprosthetic reconstruction.
-
-
Reconstructive options
-
Flail shoulder reconstruction
-
Scapular endoprostheses (see Fig. 4.3-12)
-
Need rhomboids, trapezius, latissimus dorsi, serratus anterior, some of rotator cuff
-
-
Proximal Humerus (Figs. 4.3-13 and 4.3-14)
-
Unique anatomic considerations
-
Intra-articular involvement
-
Some surgeons feel the shoulder joint should be routinely resected en bloc with the proximal humerus due to a purported high incidence of intra-articular tumor extension.
-
Many surgeons feel the resection should be based upon individual radiographic assessment.
-
-
Rotator cuff tendon insertion at tuberosities
-
Any resection of the proximal humerus
entails sacrifice of the native rotator cuff attachments, and only
osteoarticular allograft and allograft prosthetic composite
reconstructions allow the potential for suturing of the host tendons to
the reconstruction.
-
-
Axillary nerve and deltoid muscle insertion at deltoid tuberosity
-
Axillary nerve and/or deltoid muscle resection is sometimes needed for tumor resection based upon soft tissue extension.
-
Without the axillary nerve and/or deltoid function, the best reconstructive options are arthrodesis or proximal humeral spacer.
-
If axillary nerve and deltoid function
can be preserved, mobile reconstructive options (osteoarticular
allograft, allograft prosthetic composite, and megaprosthesis) are
viable alternatives.
-
-
Glenohumeral joint stability
-
-
Reconstructive options (Table 4.3-2)
-
Osteoarticular allograft
-
Allograft prosthetic composite (see Fig. 4.3-13)
-
Proximal humeral megaprosthesis (see Fig. 4.3-14)
-
Proximal humeral spacer prosthesis (Fig. 4.3-15)
-
Humeral Shaft (Figs. 4.3-5 and 4.3-16)
-
Unique anatomic considerations
-
Deltoid muscle insertion at deltoid tuberosity
-
Remaining deltoid may be sewn to allograft soft tissue or to a sleeve of synthetic material around prosthesis.
-
P.69![]() Figure 4.3-10
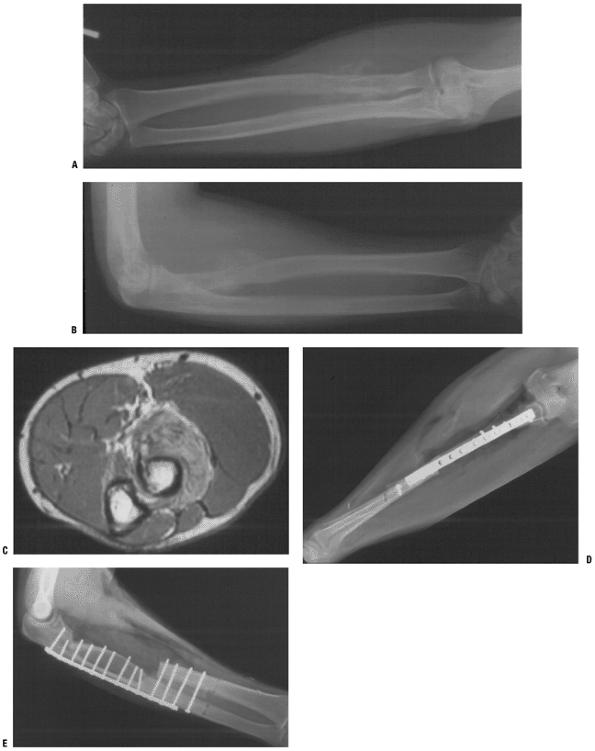
Figure 4.3-10
Single-bone forearm reconstruction following resection of a proximal
radial osteosarcoma. Preoperative studies include AP and lateral
radiographs (A,B) and an axial T1-weighted magnetic resonance image (C). (D,E) Postoperative AP and lateral radiographs.P.70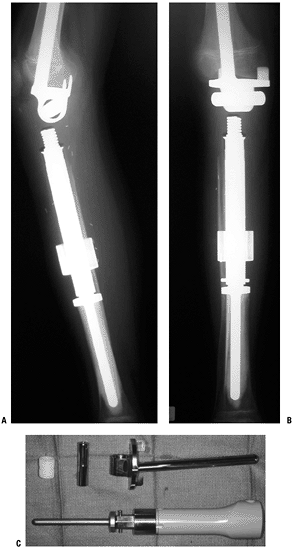 Figure 4.3-11 (A,B)
Figure 4.3-11 (A,B)
In this skeletally immature patient with a proximal tibial Ewing
sarcoma, the tibia was reconstructed using an expandable prosthesis,
maintaining the distal femoral physis. (C)
This specific expandable prosthesis has a deformable resin, which, when
placed in a magnetic coil, allows expansion of the encased preloaded
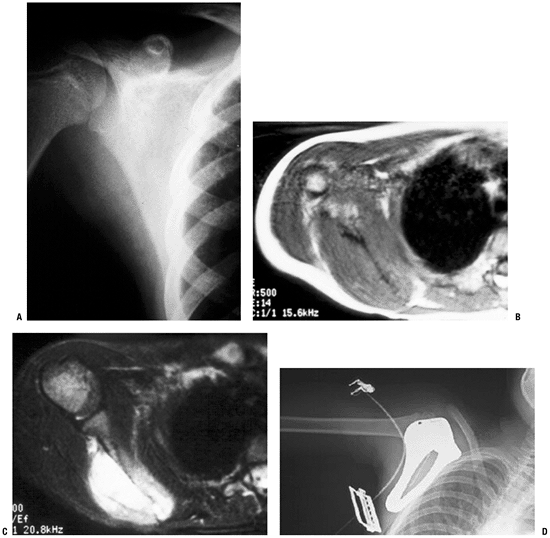
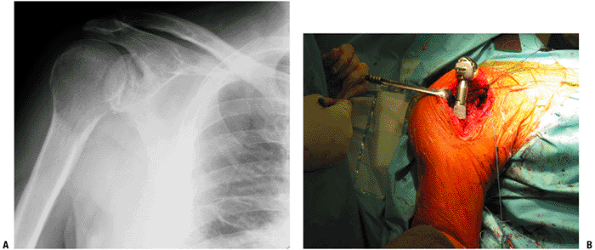
spring device. The expansion procedure does not require a skin incision.P.71![]() Figure 4.3-12 This patient with Ewing sarcoma of the right scapula shown on plain radiograph (A) and T1-weighted (B) and T2-weighted (C) axial magnetic resonance images underwent scapulectomy and prosthetic scapular reconstruction (D), maintaining the integrity of the proximal humeral metaphysis following neoadjuvant chemotherapy.P.72
Figure 4.3-12 This patient with Ewing sarcoma of the right scapula shown on plain radiograph (A) and T1-weighted (B) and T2-weighted (C) axial magnetic resonance images underwent scapulectomy and prosthetic scapular reconstruction (D), maintaining the integrity of the proximal humeral metaphysis following neoadjuvant chemotherapy.P.72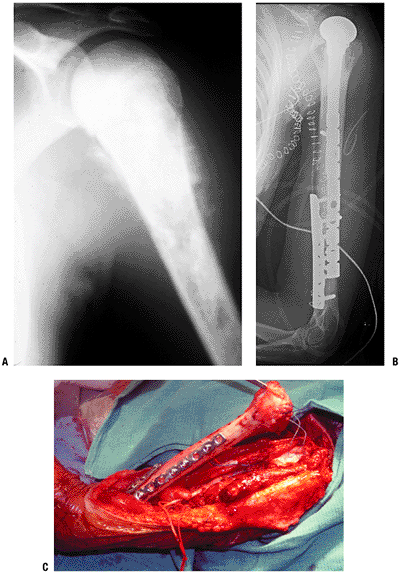 Figure 4.3-13 A proximal humeral osteosarcoma (A) has been reconstructed using an allograft–prosthetic composite reconstruction (B,C).
Figure 4.3-13 A proximal humeral osteosarcoma (A) has been reconstructed using an allograft–prosthetic composite reconstruction (B,C).
The intraoperative photo shows dual 90:90 plate fixation of the
allograft and sutures being used to repair the host rotator cuff to the
allograft rotator cuff (C).P.73![]() Figure 4.3-14 This patient with metastatic renal carcinoma to the right proximal humerus (A)
Figure 4.3-14 This patient with metastatic renal carcinoma to the right proximal humerus (A)
underwent resection and reconstruction using a proximal humeral
replacement endoprosthesis, shown here intraoperatively prior to
reduction and closure (B). -
-
Reconstructive options (Table 4.3-3)
-
Intercalary allograft (see Fig. 4.3-5)
-
Intercalary metallic spacer (see Fig. 4.3-16)
-
Distal Humerus
-
Unique anatomic considerations
-
Elbow joint
-
Use of osteoarticular allografts in this
site is usually limited to partial distal humeral resections and
requires soft tissue repair.
-
-
-
Reconstructive options (Table 4.3-4)
-
Distal humeral osteoarticular allograft
-
Custom distal humeral megaprosthesis total elbow replacement
-
-
Rehabilitative considerations
-
Early range of motion is crucial to maximize function.
-
|
Table 4.3-2 Reconstructive Options for the Proximal Humerus
|
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||
Distal Radius (see Figs. 4.3-3 and 4.3-6)
-
Unique anatomic considerations
-
Wrist joint instability
-
-
Reconstructive options
-
Mobile wrist reconstruction (see Fig. 4.3-6)
-
Wrist fusion (see Fig. 4.3-3)
-
P.74
 |
|
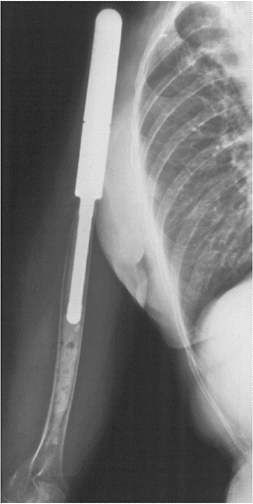
Figure 4.3-15
A proximal humeral spacer prosthesis has been cemented into the remaining humerus and used to suspend the limb to the chest wall following an extensive resection of the shoulder girdle, including the deltoid musculature. In this case, the prosthesis helps to provide a stable post for distal upper extremity function, but essentially no shoulder function is achieved. |
For each of the reconstructions, either an allograft or
the proximal fibula may be used. Mobile wrist reconstructions require
meticulous soft tissue repair to avoid instability. Proximal fibular
grafts rarely need to be vascularized, as the total length of the graft
needed is rarely long enough to encompass the region where the vascular
supply enters the proximal fibula. The lateral collateral ligament must
be repaired at the donor site.
the proximal fibula may be used. Mobile wrist reconstructions require
meticulous soft tissue repair to avoid instability. Proximal fibular
grafts rarely need to be vascularized, as the total length of the graft
needed is rarely long enough to encompass the region where the vascular
supply enters the proximal fibula. The lateral collateral ligament must
be repaired at the donor site.
Periacetabular Region
-
Unique anatomic considerations
-
Hip joint instability
-
-
Reconstructive options (Fig. 4.3-8 and Table 4.3-5)
-
Resection arthroplasty (flail hip reconstruction)
-
Hip arthrodesis (ischiofemoral or
iliofemoral, depending upon resection and remaining bone; with or
without intercalary allograft) -
Allograft prosthetic composite total hip replacement (see Fig. 4.3-8)
-
Saddle prosthesis
-
-
Rehabilitative considerations
-
Period of bracing (hip abduction orthosis) to allow for soft tissue healing used by some surgeons routinely
-
Weight bearing may be delayed to allow for healing of the allograft–host junction in a composite reconstruction.
-
Proximal Femur (Fig. 4.3-17)
-
Unique anatomic considerations
-
Gluteus medius tendon insertion
-
Level of tendon resection dictated by soft tissue extent of tumor
-
Even for completely intraosseous sarcoma,
approximately 2-cm cuff of tendon generally left with resected femur to
achieve wide margin
-
-
Hip joint instability
-
Degree of instability dependent on extent
of bone and soft tissue resection, including capsule, hip abductor,
iliopsoas, and adductors -
Standard soft tissue closure should attempt to restore stability.
-
Bipolar components generally considered more stable than total hip arthroplasty
-
Cerclage closure of remaining capsule when remains
-
Synthetic substitution when no remaining capsule (synthetic aortic grafts commonly used)
-
Gluteus medius tendon reefed into
allograft tendon, abductor attachment device on prosthesis, and/or
vastus lateralis muscle when feasible
-
-
-
-
Reconstructive options (Table 4.3-6)
-
Allograft–prosthetic composite
-
Proximal femoral megaprosthesis (see Fig. 4.3-17)
-
-
Rehabilitative considerations same as for periacetabular region
Femoral Shaft
-
Reconstructive options (Table 4.3-7)
-
Intercalary femoral allograft
-
Custom intercalary femoral metallic spacer
-
-
Rehabilitative considerations
-
Intercalary femoral allograft
reconstruction requires prolonged period of limited weight bearing
until radiographic signs of healing evident. -
Cemented custom intercalary femoral spacer allows immediate full weight bearing.
-
Distal Femur (Fig. 4.3-18)
-
Unique anatomic considerations
-
Ligamentous stability of knee joint
-
Most easily substituted for by use of
rotating hinge knee components (fully constrained), but this increases
stresses at bone–cement or bone–prosthesis (for cementless) stemmed
components -
Use of allograft–prosthetic composite
reconstruction with repair of allograft–host collateral ligament(s) or
capsule may allow use of a less constrained device than a hinge
(usually a constrained condylar type).
-
P.75![]() Figure 4.3-16
Figure 4.3-16
Intercalary humeral metallic spacers have been used predominately for
reconstruction of segmental diaphyseal defects in patients with
metastatic carcinoma and myeloma. (A) The radiograph shows the early male–female taper device with both components cemented and reduced. (B) This intraoperative photograph shows the current lap joint with Morse taper and compression set screw.Table 4.3-3 Reconstructive Options for the Humeral ShaftReconstructive Option Advantages Disadvantages Unique Issues Intercalary allograft Biologic reconstruction
Allows deltoid repairAllograft fracture
Allograft-host nonunion
Higher infection riskHigher fracture risk with plate/screws
Higher nonunion risk with intramedullary fixationIntercalary metallic spacer Immediate stability
Avoids allograft risksLess durable Usually reserved for patients with limited life expectancy Table 4.3-4 Reconstructive Options for the Distal HumerusReconstructive Option Advantages Disadvantages Unique Issues Distal humeral osteoarticular allograft Biological reconstruction Allograft fracture
Allograft-host nonunion
Infection riskExtremely difficult to achieve size matching Custom distal humeral megaprosthesis Avoids allograft risks Potential for aseptic loosening Usually custom component required P.76Table 4.3-5 Common Reconstructive Options for the Periacetabular RegionReconstructive Option Advantages Disadvantages Unique Issues Resection arthroplasty Minimizes complications Least potential functional outcome Hip arthrodesis Stable hip Technically demanding
Difficult to achieve unionIntercalary allograft more common for iliofemoral fusion Allograft-prosthetic composite Best potential functional outcome Extremely high risk: instability, allograft fracture, allograft-host nonunion, infection Cement cup into allograft acetabulum Saddle prosthesis Stable hip but preservation of some motion May dislocate from iliac notch Mersilene tape through drill holes in ilium often used to increase initial stability 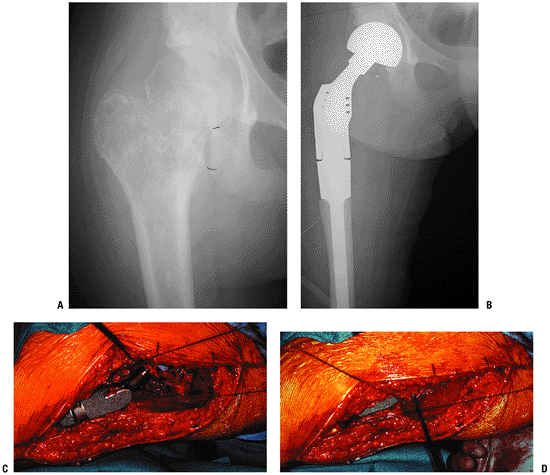 Figure 4.3-17 A proximal femoral Ewing sarcoma (A) was treated with neoadjuvant chemotherapy followed by resection and reconstruction with a proximal femoral prosthesis (B). (C) Intraoperative photos show the prosthesis in place with the bipolar component reduced in the acetabulum. (D)
Figure 4.3-17 A proximal femoral Ewing sarcoma (A) was treated with neoadjuvant chemotherapy followed by resection and reconstruction with a proximal femoral prosthesis (B). (C) Intraoperative photos show the prosthesis in place with the bipolar component reduced in the acetabulum. (D)
Sutures attached to the remaining hip abductors proximally and to the
vastus lateralis distally have been pulled in the direction of closure.
When possible, these structures are reefed together and may be attached
to the prosthesis with tape or nonabsorbable suture.P.77Table 4.3-6 Common Reconstructive Options for the Proximal FemurReconstructive Option Advantages Disadvantages Unique Issues Allograft-prosthetic composite Allows reattachment of hip abductors
Potential improvement in hip abductor function
Potential improvement in hip stabilityAllograft fracture
Allograft-host nonunion
Infection risk
PLUS all of prosthetic risksBipolar components favored for stability
Capsular reconstruction beneficialProximal femoral megaprosthesis Avoids allograft risks Hip instability
Aseptic loosening -
-
Reconstructive options (Table 4.3-8)
-
Allograft–prosthetic composite
-
Distal femoral megaprosthesis (see Fig. 4.3-18)
-
-
Rehabilitative considerations
-
Many surgeons rehabilitate these patients
similar to a standard total knee replacement, with early weight bearing
for cemented components and early aggressive range of motion. -
Weight bearing may be delayed to allow for healing of the allograft–host junction in a composite reconstruction.
-
Proximal Tibia (Figs. 4.3-19 and 4.3-20)
-
Unique anatomic considerations
-
Patellar tendon insertion/extensor mechanism
-
Level of reconstruction is dictated by
level of resection and may be through the patellar tendon (most
common), transpatellar, or through the quadriceps tendon. -
Level of resection is dictated by tumor extent.
-
-
Limited native soft tissue coverage
-
Medial gastrocnemius muscle flap is used by many surgeons for routine coverage of reconstruction.
-
-
-
Reconstructive options (Table 4.3-9)
-
Allograft–prosthetic composite (see Fig. 4.3-19)
-
Proximal tibial megaprosthesis (see Fig. 4.3-20)
-
-
Rehabilitative considerations are same as for distal femoral reconstruction.
Tibial Shaft (Fig. 4.3-21)
-
Unique anatomic considerations
-
Limited native soft tissue coverage
-
-
Reconstructive options (Table 4.3-10)
-
Intercalary allograft (see Fig. 4.3-21)
-
Fibular interposition graft (single or double barrel)
-
Custom tibial metallic prosthesis
-
|
Table 4.3-7 Common Reconstructive Options for the Femoral Shaft
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
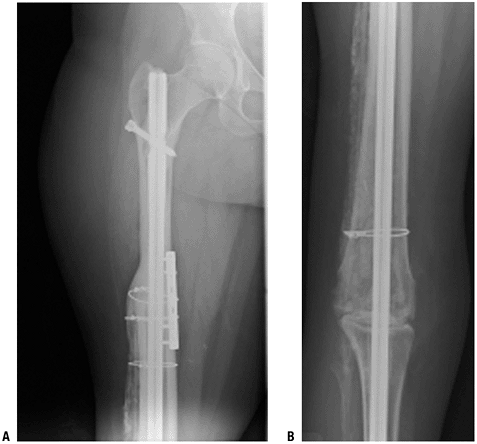
Distal Tibia (see Fig. 4.3-1)
Most patients with high-grade distal tibial bone
sarcomas warrant below-knee amputation for the prime reason that
function is better with the use of a prosthesis than after
reconstruction.
sarcomas warrant below-knee amputation for the prime reason that
function is better with the use of a prosthesis than after
reconstruction.
-
Unique anatomic considerations
-
Ankle joint
-
Soft tissue coverage of distal third of leg
-
Free-flap coverage should be considered.
-
Too distal for soleus flap
-
-
-
Reconstructive options
-
Distal tibial allograft–arthrodesis
-
May be accomplished with retrograde
intramedullary nail fixation through calcaneus, talus, and across
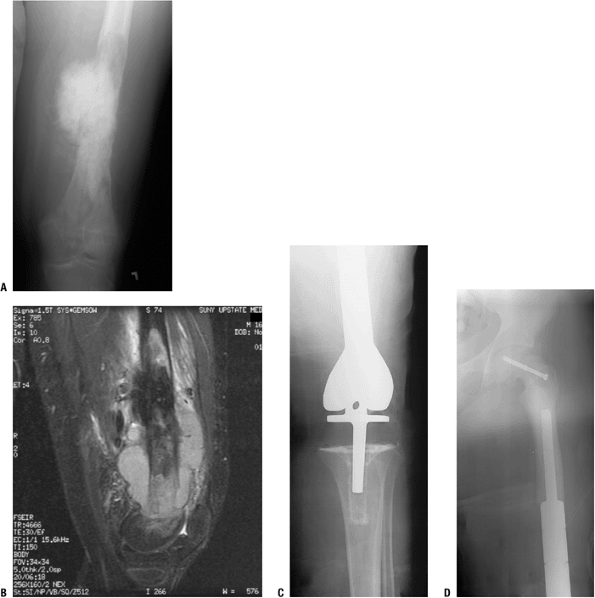
intercalary segmental allograft into proximal host boneP.78![]() Figure 4.3-18 A distal femoral osteosarcoma is shown on an AP plain radiograph (A) and sagittal fat-suppressed inversion recovery magnetic resonance image (B). (C,D)
Figure 4.3-18 A distal femoral osteosarcoma is shown on an AP plain radiograph (A) and sagittal fat-suppressed inversion recovery magnetic resonance image (B). (C,D)
After preoperative chemotherapy and resection, the distal femur was
reconstructed using a distal femoral megaprosthesis rotating hinge knee
replacement. Postoperative radiographs also show screw fixation of a
slipped capital femoral epiphysis, which occurred during postoperative
chemotherapy.Table 4.3-8 Common Reconstructive Options for the Distal FemurReconstructive Option Advantages Disadvantages Unique Issues Allograft-prosthetic composite Partial biological reconstruction
May allow less constrained total knee to be usedAllograft fracture
Allograft-host nonunion
Infection risk
Technically more difficultLong-stem femoral component should bypass allograft-host junction Distal femoral megaprosthesis Avoids allograft risks
Technically easierAseptic loosening P.79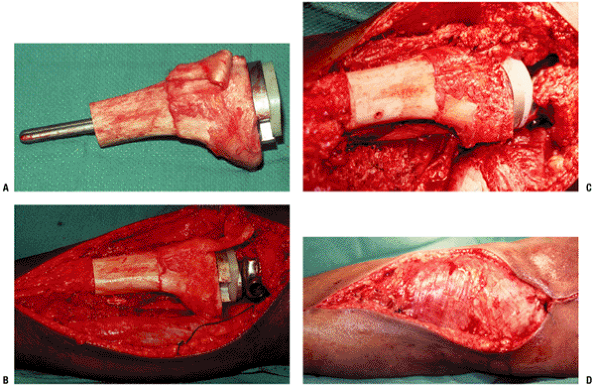 Figure 4.3-19 Intraoperative photographs during a proximal tibial allograft–prosthetic composite reconstruction. (A) The allograft has been prepared on the back table, and the trial tibial component has been placed through the bone. (B) The rotating hinge tibial and femoral components have been cemented into place. (C) The host and allograft patellar tendons have been sutured together. (D)
Figure 4.3-19 Intraoperative photographs during a proximal tibial allograft–prosthetic composite reconstruction. (A) The allograft has been prepared on the back table, and the trial tibial component has been placed through the bone. (B) The rotating hinge tibial and femoral components have been cemented into place. (C) The host and allograft patellar tendons have been sutured together. (D)
Finally, the medial gastrocnemius muscle flap has been closed over the
construct in preparation for split-thickness skin grafting. -
Prolonged limited weight bearing needed until signs of allograft–host healing
-
Normal allograft risks apply: fracture, delayed union, nonunion, and infection
-
-
Chemotherapy
Prior to 1970, the 5-year survival of patients with
nonmetastatic osteogenic sarcoma treated with surgical ablation of the
tumor was less than 20%. The primary mechanism of failure for these
patients was the development of fatal pulmonary metastases. In the
1970s, the benefit of adjuvant chemotherapy started to emerge, with
individual institutional protocols reporting increased survival in
patients treated with both surgery and chemotherapeutic agents.
Unfortunately, early trials provided conflicting results and confusion.
As a result, two prospective randomized trials were designed to define
the role of adjuvant chemotherapy in the treatment of osteosarcoma.
Both the Multi-Institutional Osteosarcoma Study Group and the UCLA
group unequivocally demonstrated that the addition of chemotherapy to
surgical excision of the tumor significantly improved survival. In the
modern era, standard treatment for nonmetastatic osteogenic sarcoma
consists of a combination of chemotherapy and surgery resulting in
5-year survival rates of 60% to 65%. Ewing sarcoma enjoys similar
survival statistics when chemotherapy is used along with local control.
nonmetastatic osteogenic sarcoma treated with surgical ablation of the
tumor was less than 20%. The primary mechanism of failure for these
patients was the development of fatal pulmonary metastases. In the
1970s, the benefit of adjuvant chemotherapy started to emerge, with
individual institutional protocols reporting increased survival in
patients treated with both surgery and chemotherapeutic agents.
Unfortunately, early trials provided conflicting results and confusion.
As a result, two prospective randomized trials were designed to define
the role of adjuvant chemotherapy in the treatment of osteosarcoma.
Both the Multi-Institutional Osteosarcoma Study Group and the UCLA
group unequivocally demonstrated that the addition of chemotherapy to
surgical excision of the tumor significantly improved survival. In the
modern era, standard treatment for nonmetastatic osteogenic sarcoma
consists of a combination of chemotherapy and surgery resulting in
5-year survival rates of 60% to 65%. Ewing sarcoma enjoys similar
survival statistics when chemotherapy is used along with local control.
Indications/Contraindications
Indications
Chemotherapy has been shown to be effective in improving survival for the majority of malignant bone tumors:
-
Osteogenic sarcoma (high-grade conventional)
-
Ewing sarcoma
-
Malignant fibrous histiocytoma of bone
-
Fibrosarcoma of bone
-
Leiomyosarcoma of bone
-
Lymphoma of bone
Relative Contraindications
-
High-grade chondrosarcoma
 |
|
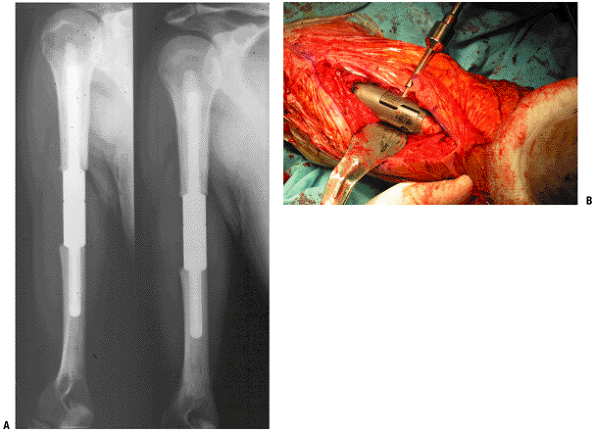
Figure 4.3-20
A proximal tibial endoprosthetic reconstruction following resection of an osteosarcoma. Preoperative anteroposterior radiograph (A) and coronal T1-weighted magnetic resonance image (B). (C) Intraoperative photograph shows the cemented proximal tibial megaprosthetic rotating hinge total knee replacement in place. The remaining host patellar tendon has been sutured with Mersilene tape to the polished loop prior to coverage with the medial gastrocnemius flap, which has been mobilized and is laid back medially (top) in this figure. |
|
Table 4.3-9 Common Reconstructive Options for the Proximal Tibia
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Absolute Contraindications
-
Nonmetastatic low-grade sarcomas (no role for chemotherapy)
-
Low-grade parosteal osteogenic sarcoma
-
Low-grade central osteosarcoma
-
Low-grade chondrosarcomas
-
Chemotherapeutic Drugs
In general, chemotherapy works by damaging DNA or
halting the cell cycle. It preferentially targets rapidly dividing
cells, those of both high-grade tumors and normal cells with high
division rates. Affected normal cells manifest some of the commonly
seen side effects from chemotherapy: alopecia from hair follicles,
mucositis from gastrointestinal mucosa, and myelosuppression from the
hematopoietic system. Fortunately, great strides have been made in
supportive measures to decrease the intensity of adverse side effects,
thereby allowing for clinically effective chemotherapeutic dosing (Table 4.3-11).
halting the cell cycle. It preferentially targets rapidly dividing
cells, those of both high-grade tumors and normal cells with high
division rates. Affected normal cells manifest some of the commonly
seen side effects from chemotherapy: alopecia from hair follicles,
mucositis from gastrointestinal mucosa, and myelosuppression from the
hematopoietic system. Fortunately, great strides have been made in
supportive measures to decrease the intensity of adverse side effects,
thereby allowing for clinically effective chemotherapeutic dosing (Table 4.3-11).
 |
|
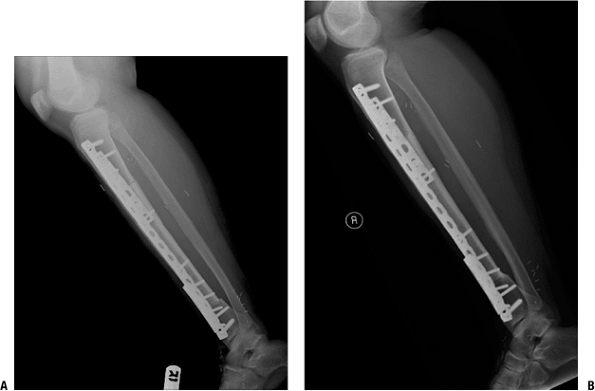
Figure 4.3-21 (A)
A lateral postoperative radiograph of the tibia shows an intercalary allograft fixed with multiple plates and screws. Graft–host junctions are still visible. (B) A radiograph obtained 9 months after surgery reveals complete healing of the proximal and distal graft junctions. The patient obtained near-normal postoperative function of the ipsilateral ankle and knee joints. |
The best outcomes in bone sarcoma treatment are
associated with multiagent or combination chemotherapy as opposed to
single-agent regimens. Most patients with malignant bone tumors are
treated in the setting of a clinical trial or on established protocols.
Table 4.3-12 outlines the most commonly used
chemotherapy agents for specific malignant bone tumors. While there is
some institutional variability
associated with multiagent or combination chemotherapy as opposed to
single-agent regimens. Most patients with malignant bone tumors are
treated in the setting of a clinical trial or on established protocols.
Table 4.3-12 outlines the most commonly used
chemotherapy agents for specific malignant bone tumors. While there is
some institutional variability
P.82
for
a given tumor, the tumor cytotoxicity of the drugs listed below has
been well established. The exact drugs, duration, and dosing used
remain controversial.
|
Table 4.3-10 Common Reconstructive Options for the Tibial Shaft
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Important Chemotherapy Concepts
Induction Chemotherapy
-
Definition: chemotherapy administered before gross total resection of the tumor
-
Synonyms: neoadjuvant or preoperative chemotherapy
-
-
Historical perspective: Originally
administered during the advent of limb salvage surgery in order to
treat patients while custom prostheses were being manufactured -
Advantages
-
Immediate treatment of micrometastases and potential metastatic sites
-
Reduces surrounding tumor edema and in some instances shrinks the tumor, facilitating surgical resection
-
Causes tumor necrosis, providing important prognostic information (see Assessment of Chemotherapy Response below)
-
-
While induction chemotherapy has become
the standard of care for osteogenic sarcoma and Ewing sarcoma, the
administration of chemotherapy prior to surgical removal of the tumor
has never been shown to improve patient survival.
|
Table 4.3-11 Biologic Response Modifiers Used to Treat Chemotherapy Toxicities
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Pathologic Assessment of Chemotherapy Response
-
Aside from the presence of metastatic
disease at presentation, histologic necrosis following induction
chemotherapy is the most powerful predictor of disease-free survival
available.-
Synonyms: Huvos grading system
-
-
Prognostic value has been established for both osteogenic sarcoma and Ewing sarcoma.
-
Calculated by quantifying tumor viability on grid constructed from cut sections of the tumor (Fig. 4.3-22)
-
Theoretically, the amount of necrosis reflects the effectiveness of the therapy.
-
Consists of a four-tiered grading system
-
Grade I (0% to 50% necrosis)
-
Grade II (51% to 90% necrosis)
-
Grade III (91% to 99% necrosis)
-
Grade IV (100% necrosis)
-
-
Clinical significance
-
“Good response” (grade III and IV) is associated with superior survival outcomes (as high as 89% at 5 years).
-
Grade I and II responders are at increased risk of relapse.
-
A grade I response is superior to no chemotherapy (5-year survival of 50% versus 17%, respectively).
-
-
Increasing necrosis by prolonging
chemotherapy induction time or dose intensification does not correlate
with increased survival (i.e., the grading system loses its prognostic
power).
-
Clinical and Radiographic Assessment of Chemotherapy Response
-
Decreased pain and swelling
-
Decreased alkaline phosphataseP.83Table 4.3-12 Chemotherapy Agents Used to Treat Malignant Bone Tumors
Sarcoma Type Commonly Used Chemotherapy Agents Mechanism of Action Associated Major Toxicity Osteogenic sarcoma Doxorubicin - Binds DNA via intercalation on the DNA helix, blocking DNA and RNA synthesis
- Inhibits topoisomerase II
- Produces free radicals, cleaving DNA and the cell membrane
Cardiomyopathy, myelosuppression Cisplatin Covalently binds DNA, disrupting DNA function Renal failure, neuropathy, ototoxicity, myelosuppression High-dose methotrexate Inhibits dehydrofolate reductase, thereby blocking thymidine synthesis and hence DNA synthesis Mucositis, renal toxicity Ifosfamide Alkylates DNA, leading to cross-linking Cystitis, renal failure, encephalopathy Ewing sarcoma Vincristine Prevents the polymerization of tubulin to form microtubules, thereby blocking mitosis Peripheral neuropathy Doxorubicin - Binds DNA via intercalation on the DNA helix, blocking DNA and RNA synthesis
- Inhibits topoisomerase II
- Produces free radicals, cleaving DNA and the cell membrane
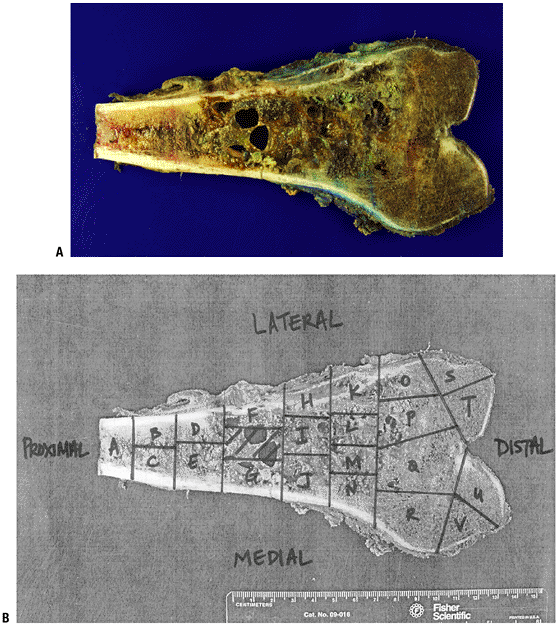
Cardiomyopathy, myelosuppression Cyclophosphamide Alkylates DNA, leading to cross-linking Cystitis, renal failure, encephalopathy Ifosfamide Structural analogue of cyclophosphamide with same mechanism of action Same as cyclophosphamide Etoposide Inhibits DNA topoisomerase II, thereby inhibiting DNA synthesis Neutropenia Malignant fibrous histiocytoma, fibrosarcoma, and leiomyosarcoma of bone Same as for osteogenic sarcoma Lymphoma (also see Chapter CHOP (cyclophosphamide, hydroxydoxorubicin, Oncovin [vincristine], prednisone) See above P.84![]() Figure 4.3-22 Pathologic assessment of chemotherapy response. (A) Gross photograph of a distal femoral osteogenic sarcoma. (B) Mapping of the gross specimen for histologic analysis.
Figure 4.3-22 Pathologic assessment of chemotherapy response. (A) Gross photograph of a distal femoral osteogenic sarcoma. (B) Mapping of the gross specimen for histologic analysis. -
“Normalization” of the tumor on x-ray (Fig. 4.3-23)
-
Decreased uptake on Tc-99 bone scan and thallium scan
-
Decreased edema on magnetic resonance imaging (Fig. 4.3-24)
-
Decreased size of the tumor
-
Most commonly seen in Ewing sarcoma
-
Seldom seen in osteogenic sarcoma secondary to osteoid matrix
-
Chemotherapy Tailoring
-
Definition: Refers to modifying the postoperative chemotherapy regimen for an inferior histologic response to chemotherapy
-
Most studies have failed to increase survival by changing or intensifying chemotherapeutics.
Radiation Therapy
Radiation therapy is a local treatment modality. The
most commonly used form of radiotherapy is a high-energy photon beam
delivered by a linear accelerator. When the beam collides with its
target, it “ionizes” its target by removing an orbiting electron from
an atom or group of atoms. In tumors, the target is water in the tumor
cells, which creates highly reactive free radicals capable of causing
DNA stand breaks and eventually cell death. For bone tumors, radiation
is usually delivered as fractionated (small doses) external beam
radiation administered on consecutive days for a specified period of
time. Fractionation allows for a large total dose to be delivered
without exceeding the threshold of the normal surrounding tissues.
most commonly used form of radiotherapy is a high-energy photon beam
delivered by a linear accelerator. When the beam collides with its
target, it “ionizes” its target by removing an orbiting electron from
an atom or group of atoms. In tumors, the target is water in the tumor
cells, which creates highly reactive free radicals capable of causing
DNA stand breaks and eventually cell death. For bone tumors, radiation
is usually delivered as fractionated (small doses) external beam
radiation administered on consecutive days for a specified period of
time. Fractionation allows for a large total dose to be delivered
without exceeding the threshold of the normal surrounding tissues.
P.85
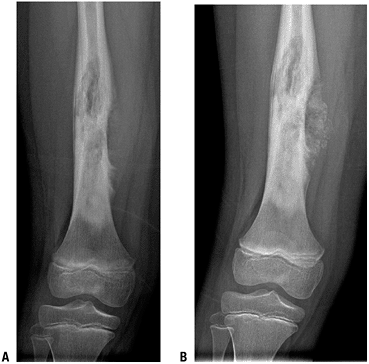 |
|
Figure 4.3-23 Normalization of the tumor following induction chemotherapy. (A) Preoperative x-ray of a diaphyseal femoral osteogenic sarcoma demonstrating periosteal elevation with soft tissue extension. (B) Following induction chemotherapy, there is increased sclerosis in the femur and thickening of the periosteum.
|
Dosage
Radiation dose is typically reported as the absorbed dose, which is measured in grays (Gy).
-
1 Gy = 1 J/kg
-
1 centigray (cGy) = 1/100 of a Gy
-
1 rad = 1 cGy
For bone tumors, a total dose of 4,500 to 6,000 cGy is
delivered in fractionated doses of 180 to 200 cGy per day, 5 days per
week.
delivered in fractionated doses of 180 to 200 cGy per day, 5 days per
week.
While the role of radiation is well established in the
management of soft tissue sarcomas, it has a less defined role in the
management of bone malignancies.
management of soft tissue sarcomas, it has a less defined role in the
management of bone malignancies.
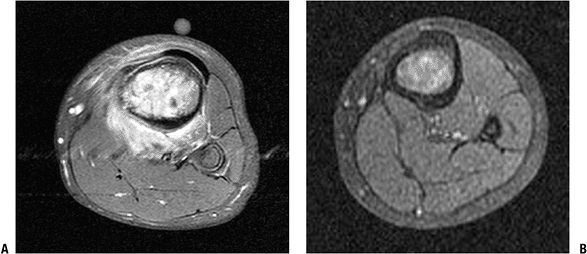 |
|
Figure 4.3-24 MRI assessment of chemotherapy response. T1-weighted fat-suppressed MR images of an osteogenic sarcoma of the proximal tibia. (A) Considerable edema surrounds the tumor at presentation. (B) The same tumor following 9 weeks of induction chemotherapy.
|
Use in Specific Tumors
Osteogenic Sarcoma
-
Very limited role for radiation
-
Primary indications
-
Anatomic locations where complete surgical resection is not feasible
-
Palliate symptomatic metastases
-
Ewing Sarcoma
-
Very radiosensitive
-
Radiation is a standard local control option, achieving local control rates of greater than 70%.
-
Overall survival is similar for patients treated with radiation compared to surgery.
-
Local recurrence rate is likely greater in irradiated patients compared to surgically treated patients.
-
Primary indications
-
When surgical treatment would cause unacceptable disfigurement, functional deficit, morbidity, or mortality
-
When surgical margins are positive or close
-
Metastases
-
P.86
Chondrosarcoma
-
Radioresistant
-
May have a role to palliate unresectable tumors
Chordoma
-
Definitive local control has been
reported with highly conformal therapy such as high-dose
proton/photon-beam radiation and intensity-modulated radiation therapy
(IMRT). -
Requires very high doses (>7,000 cGy) to maximize local control
-
For large tumors, preoperative radiation may facilitate surgical resection.
-
Postoperative radiation has been associated with improved local control.
Lymphoma of Bone
-
Used as the primary local control measure
Side Effects
-
Dermatologic
-
Acutely: erythema, desquamation, wound dehiscence
-
Long-term: hyperpigmentation
-
-
Myelosuppression (acutely)
-
Muscle fibrosis
-
Extremity edema
-
Joint contractures
-
Growth arrest
-
Fracture
-
Avascular necrosis
-
Secondary malignancy
-
Requires a latency period of at least 3 years, though typically occurs 10 to 20 years after radiation
-
Risk is ~1% following treatment for all childhood cancers.
-
Risk is ~5% following treatment for Ewing sarcoma.
-
Suggested Reading
Bacci
G, Ferrari S, Bertoni F, et al. Neoadjuvant chemotherapy for osseous
malignant fibrous histiocytoma of the extremity: results in 18 cases
and comparison with 112 contemporary osteosarcoma patients treated with
the same chemotherapy regimen. J Chemother 1997;9(4):293–299.
G, Ferrari S, Bertoni F, et al. Neoadjuvant chemotherapy for osseous
malignant fibrous histiocytoma of the extremity: results in 18 cases
and comparison with 112 contemporary osteosarcoma patients treated with
the same chemotherapy regimen. J Chemother 1997;9(4):293–299.
Bramwell
VH, Steward WP, Nooij M, et al. Neoadjuvant chemotherapy with
doxorubicin and cisplatin in malignant fibrous histiocytoma of bone: A
European Osteosarcoma Intergroup study. J Clin Oncol 1999;17(10):3260–3269.
VH, Steward WP, Nooij M, et al. Neoadjuvant chemotherapy with
doxorubicin and cisplatin in malignant fibrous histiocytoma of bone: A
European Osteosarcoma Intergroup study. J Clin Oncol 1999;17(10):3260–3269.
DeLaney TF, Park L, Goldberg SI, et al. Radiotherapy for local control of osteosarcoma. Int J Radiat Oncol Biol Phys 2005;61(2):492–498.
Donaldson
SS, Torrey M, Link MP, et al. A multidisciplinary study investigating
radiotherapy in Ewing’s sarcoma: end results of POG #8346. Pediatric
Oncology Group. Int J Radiat Oncol Biol Phys 1998;42(1):125–135.
SS, Torrey M, Link MP, et al. A multidisciplinary study investigating
radiotherapy in Ewing’s sarcoma: end results of POG #8346. Pediatric
Oncology Group. Int J Radiat Oncol Biol Phys 1998;42(1):125–135.
Eilber F, Giuliano A, Eckardt J, et al. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Oncol 1987;5(1):21–26.
Ferrari
S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose
ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for
patients with localized osteosarcoma of the extremity: a joint study by
the Italian and Scandinavian Sarcoma Groups. J Clin Oncol 2005;23(34):8845–8852.
S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose
ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for
patients with localized osteosarcoma of the extremity: a joint study by
the Italian and Scandinavian Sarcoma Groups. J Clin Oncol 2005;23(34):8845–8852.
Goorin
AM, Schwartzentruber DJ, Devidas M, et al. Presurgical chemotherapy
compared with immediate surgery and adjuvant chemotherapy for
nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol 2003;21(8):1574–1580.
AM, Schwartzentruber DJ, Devidas M, et al. Presurgical chemotherapy
compared with immediate surgery and adjuvant chemotherapy for
nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol 2003;21(8):1574–1580.
Gorlick
R, Anderson P, Andrulis I, et al. Biology of childhood osteogenic
sarcoma and potential targets for therapeutic development: meeting
summary. Clin Cancer Res 2003;9(15):5442–5453.
R, Anderson P, Andrulis I, et al. Biology of childhood osteogenic
sarcoma and potential targets for therapeutic development: meeting
summary. Clin Cancer Res 2003;9(15):5442–5453.
Kolb
EA, Kushner BH, Gorlick R, et al. Long-term event-free survival after
intensive chemotherapy for Ewing’s family of tumors in children and
young adults. J Clin Oncol 2003;21(18):3423–3430.
EA, Kushner BH, Gorlick R, et al. Long-term event-free survival after
intensive chemotherapy for Ewing’s family of tumors in children and
young adults. J Clin Oncol 2003;21(18):3423–3430.
Krasin
MJ, Rodriguez-Galindo C, Davidoff AM, et al. Efficacy of combined
surgery and irradiation for localized Ewing’s sarcoma family of tumors.
Pediatr Blood Cancer 2004;43(3):229–236.
MJ, Rodriguez-Galindo C, Davidoff AM, et al. Efficacy of combined
surgery and irradiation for localized Ewing’s sarcoma family of tumors.
Pediatr Blood Cancer 2004;43(3):229–236.
Link
MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on
relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med 1986;314(25):1600–1606.
MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on
relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med 1986;314(25):1600–1606.
Meyers
PA, Gorlick R, Heller G, et al. Intensification of preoperative
chemotherapy for osteogenic sarcoma: results of the Memorial
Sloan-Kettering (T12) protocol. J Clin Oncol 1998;16(7):2452–2458.
PA, Gorlick R, Heller G, et al. Intensification of preoperative
chemotherapy for osteogenic sarcoma: results of the Memorial
Sloan-Kettering (T12) protocol. J Clin Oncol 1998;16(7):2452–2458.
Meyers PA, Heller G, Healey J. Retrospective review of neoadjuvant chemotherapy for osteogenic sarcoma. J Natl Cancer Inst 1992;84(3):202–204.
Meyers
PA, Schwartz CL, Krailo M, et al. Osteosarcoma: a randomized,
prospective trial of the addition of ifosfamide and/or muramyl
tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol 2005;23(9):2004–2011.
PA, Schwartz CL, Krailo M, et al. Osteosarcoma: a randomized,
prospective trial of the addition of ifosfamide and/or muramyl
tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol 2005;23(9):2004–2011.
Paulino AC. Late effects of radiotherapy for pediatric extremity sarcomas. Int J Radiat Oncol Biol Phys 2004;60(1):265–274.
Provisor
AJ, Ettinger LJ, Nachman JB, et al. Treatment of nonmetastatic
osteosarcoma of the extremity with preoperative and postoperative
chemotherapy: a report from the Children’s Cancer Group. J Clin Oncol 1997;15(1):76–84.
AJ, Ettinger LJ, Nachman JB, et al. Treatment of nonmetastatic
osteosarcoma of the extremity with preoperative and postoperative
chemotherapy: a report from the Children’s Cancer Group. J Clin Oncol 1997;15(1):76–84.
Samson IR, Springfield DS, Suit HD, et al. Operative treatment of sacrococcygeal chordoma. A review of twenty-one cases. J Bone Joint Surg [Am] 1993;75(10):1476–1484.
Wexler
LH, DeLaney TF, Tsokos M, et al. Ifosfamide and etoposide plus
vincristine, doxorubicin, and cyclophosphamide for newly diagnosed
Ewing’s sarcoma family of tumors. Cancer 1996;78(4):901–911.
LH, DeLaney TF, Tsokos M, et al. Ifosfamide and etoposide plus
vincristine, doxorubicin, and cyclophosphamide for newly diagnosed
Ewing’s sarcoma family of tumors. Cancer 1996;78(4):901–911.
Wunder
JS, Paulian G, Huvos AG, et al. The histological response to
chemotherapy as a predictor of the oncological outcome of operative
treatment of Ewing sarcoma. J Bone Joint Surg [Am] 1998;80(7):1020–1033.
JS, Paulian G, Huvos AG, et al. The histological response to
chemotherapy as a predictor of the oncological outcome of operative
treatment of Ewing sarcoma. J Bone Joint Surg [Am] 1998;80(7):1020–1033.