Acute Compartment Syndrome
within a confined space in the body, resulting in a critical reduction
of the blood flow to the tissues contained within the space. Without
urgent decompression, tissue ischemia, necrosis, and functional
impairment occur. The acute compartment syndrome should be
differentiated from other related conditions.
compartment syndrome is important. Acute compartment syndrome is
defined as an elevation of intracompartmental pressure to a level and
for a duration that without decompression will cause tissue ischemia
and necrosis.
intercompartmental pressure during exercise, causing ischemia, pain,
and rarely neurological symptoms and signs. It is characterized by
resolution of symptoms with rest but may proceed to acute compartment
syndrome if exercise continues.
neglected acute compartment syndrome with irreversible muscle necrosis
leading to ischemic contractures.
necrosis commonly caused by prolonged external compression of an
extremity. In crush syndrome muscle necrosis is established by the time
of presentation, but intracompartmental pressure may rise as a result
of intracompartmental edema, causing a superimposed acute compartment
syndrome.
description of ischemic muscle contractures was published in the
medical literature.
The first report of the condition was attributed to Hamilton in 1850 by Hildebrand61
but Hamilton’s original description has never been found. The credit
for the first full description belongs to Richard Von Volkmann148
who published a summary of his views in 1881. He stated that paralysis
and contractures appeared after too tight bandaging of the forearm and
hand, were ischemic in nature, and were caused by prolonged blocking of
arterial blood. He recognized that muscle cannot survive longer than 6
hours with complete interruption of its blood flow and that 12 hours or
less of too tight bandaging were enough to result in “dismal permanent
crippling.” In 1888 Peterson recognized that ischemic contracture could
occur in the absence of bandaging but did not postulate a cause.115
literature in the early twentieth century. At this time it was
suggested that swelling after removal of tight bandaging might
contribute to the contracture and that the contracture was caused by
“fibroustissue forming elements” or a myositic process.28,125,150
By the early part of the twentieth century published accounts of the
sequence of events in acute compartment syndrome were remarkably
similar to what is known today, with differentiation between acute
ischemia caused by major vessel rupture, acute ischemia caused by
“subfascial tension,” the late stage of ischemic contracture, and the
separate concept of nerve involvement.9
This paper was the first description of fasciotomy to relieve the
pressure. The importance of early fasciotomy was suggested at this time9,107 and confirmed by prevention of the development of contractures in animal experiments.68
from these sound conclusions. A belief arose that ischemic contracture
was caused by arterial injury and spasm with reflex collateral spasm.
Successful results from excision of the damaged artery34,44
were undoubtedly owing to the fasciotomy carried out as part of the
exposure for the surgery. An unfortunate legacy of this belief persists
today in the dangerously mistaken view that an acute compartment
syndrome cannot exist in the presence of normal peripheral pulses.
He noted that in all cases of ischemic contracture there was early and
gross swelling requiring prompt fasciotomy, and that 50% of his cases
had palpable peripheral pulses. He was unable to explain muscle
infarcts at the same level as the injury on the basis of arterial
damage. He recommended early fasciotomy.
They described the vicious circle of increasing tension in an enclosed
compartment causing venous obstruction and subsequent reduction in
arterial inflow. Their most important conclusion was that delay in
performing a fasciotomy was the single cause of failure of treatment.
syndrome is important in defining the patient at risk of developing
acute compartment syndrome. The epidemiology of acute compartment
syndrome has been described in a cohort of 164 patients drawn from a
defined population in the United Kingdom.84 From adolescence, younger patients are at more risk of compartment syndrome.
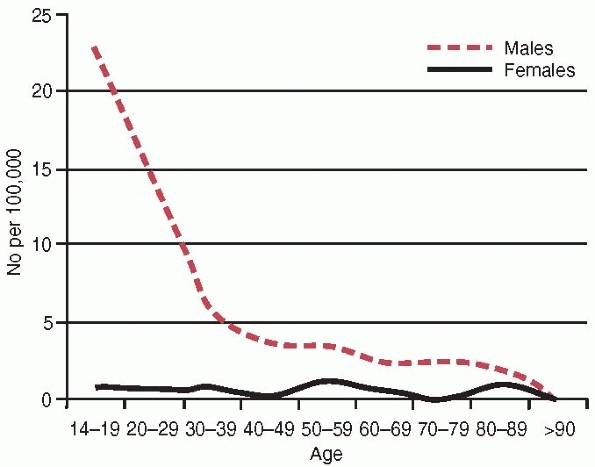
The annual incidence for males is 7.3 per 100,000 compared with 0.7 per
100,000 for females, a tenfold increase in risk for males. The age and
gender-specific incidences are illustrated in Fig. 27-1, showing a Type B pattern (see Chapter 3) or the L-shaped pattern described by Buhr and Cooke.18 The mean age for the whole group was 32 years; the median age for males was 30 years and for females 44 years.
 |
|
FIGURE 27-1 The annual age and gender specific incidence of acute compartment syndrome.
|
Similar figures have been reported in children, with 76% of cases
caused by fracture, predominantly tibial diaphyseal, distal radius, and
forearm.8 The most common fracture
associated with acute compartment syndrome in adults is tibial
diaphyseal fracture. The prevalence of acute compartment syndrome
in tibial diaphyseal fractures is reported as 2.7% to 15%,* with the differences in prevalences likely to be because of different diagnostic techniques and selection of patients.
|
TABLE 27-1 Conditions Associated with Injury Causing Acute Compartment Syndrome Presenting to an Orthopaedic Trauma Unit
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
TABLE 27-2 Less Common Causes of Acute Compartment Syndrome
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
syndrome is soft tissue injury, which added to tibial diaphyseal
fracture makes up almost two thirds of the cases. The second most
common fracture to be complicated by acute compartment syndrome is the
distal radius fracture, which occurs in approximately 0.25% of cases.
Forearm diaphyseal fractures are complicated by acute compartment
syndrome in 3% of cases. The prevalence of acute compartment syndrome
in other anatomic locations is rarely reported. Other less common
causes of acute compartment syndrome are listed in Table 27-2.
more risk of compartment syndrome. In tibial diaphyseal fracture the
prevalence of acute compartment syndrome was reported as being three
times greater in the under 35-year-old age group, and in distal radial
fractures the prevalence is 35 times less in the older age group.84 Adolescents have been recognized as having a high rate (8.3%) of compartment syndrome after tibial fracture.23
Analysis of 1403 tibial diaphyseal fractures presenting to the
Edinburgh Orthopaedic Trauma Unit over the period from 1995 to 2007
shows that there were 160 cases of acute compartment syndrome (11.4%).
Using univariate analysis, significant risk factors for the development
of acute compartment syndrome were youth (P
<0.001), and male gender. Males were almost six times more likely to
develop acute compartment syndrome if aged between 20 and 29 years
compared with those aged over 40 years. Youth, regardless of gender, is
therefore a significant risk factor for the development of acute
compartment syndrome after tibial fracture. The only exception to youth
being a risk factor in acute compartment syndrome is in cases with soft
tissue injury only. These patients have an average age of 36 years and
are significantly older than those with a fracture.63
risks of developing an acute compartment syndrome. Nevertheless, in
tibial diaphyseal fracture complicated by acute compartment syndrome
the proportion of high and low energy injury shows a slight
preponderance of low energy injury (59%).84
In the same population there is an equal number of high energy and low
energy injury in tibial diaphyseal fractures uncomplicated by acute
compartment syndrome.24 In the
larger Edinburgh series there was an increased risk of acute
compartment syndrome in closed compared with open fractures (P
<0.05). This suggests acute compartment syndrome may be more
prevalent after low energy injury, possibly because in low energy
injury the compartment boundaries are less likely to be disrupted and
an “autodecompression” effect is avoided. The concept of patients with
lower energy injury being at higher risk is supported by the
distribution of severe open fractures in each group. In those
complicated by acute compartment syndrome, 20% are Gustilo Type III84; in the whole population of tibial fractures, 60% were Type III.24
It is important to note that open tibial diaphyseal fractures remain at
risk of acute compartment syndrome, which occurs in approximately 3%,84
but it appears that the lower Gustilo types are at more risk, again
possibly because of the lack of disruption of the compartment
boundaries.
associated with high energy injury are more likely to be complicated by
acute compartment syndrome, probably because of the high preponderance
of young males who sustained these types of injury. This is illustrated
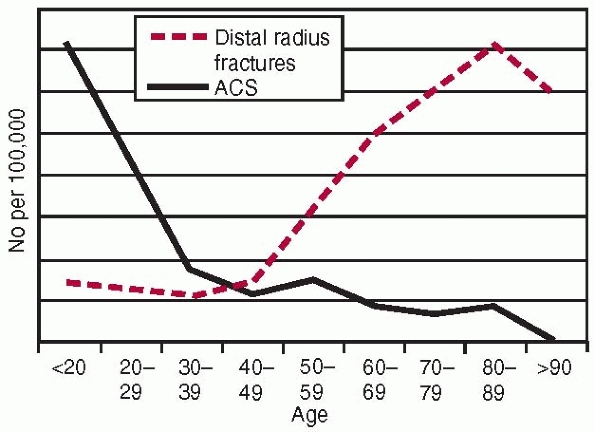
by a comparison of the age and gender related incidence of distal
radius fractures complicated by acute compartment syndrome (Fig. 27-2). The likely explanation for the
preponderance of young males with acute compartment syndrome is that
young men have relatively large muscle volumes, whereas their
compartment size does note change after growth is complete. Thus young
men may have less space for swelling of the muscle after injury.
Presumably the older person has smaller hypotrophic muscles allowing
more space for swelling. There may also be a protective effect of
hypertension in the older patient.
 |
|
FIGURE 27-2
The annual age specific incidence of all distal radius fractures compared with the annual age specific incidence of acute compartment syndrome in distal radial fractures. |
|
TABLE 27-3 Risk Factors for Development or Late Diagnosis of Acute Compartment Syndrome
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
syndrome is that arising in the absence of fractures. The majority of
these arise subsequent to soft tissue injury, particularly a crushing
type injury, but some arise with no preceding history of trauma.63
In children 61% of cases of acute compartment syndrome in the absence
of fracture are reported as being iatrogenic. 117 Patients with acute
compartment syndrome without fracture tend to be older and have more
medical comorbidities than those with a fracture. They are more evenly
distributed between the genders with a male to female ratio of five to
one. The use of anticoagulants seems also to be a risk factor for the
development of acute compartment syndrome.
in the diagnosis of their acute compartment syndrome so identification
of at risk factors in this group is of particular importance.35
Kosir and his coauthors examined risk factors for lower limb acute
compartment syndrome in 45 critically ill trauma patients with the
institution of an aggressive screening protocol.73
The prevalence of acute compartment syndrome was 20%. High base
deficits, lactate levels, and transfusion requirements were significant
risk factors in this group.
As well as demographic risk factors, altered pain perception can delay
diagnosis. This can occur if the patient has an altered conscious state
or with certain types of anesthesia or analgesia.52,87,105
mechanism of the reduction in blood flow in the acute compartment
syndrome, although it is generally accepted that the effect is at small
vessel level, either arteriolar, capillary, or venous levels.
is a critical closing pressure in the small vessels when the transmural
pressure (the difference between intravascular pressure and tissue
pressure) drops.19 Transmural
pressure (TM) is balanced by a constricting force (TC) consisting of
active and elastic tension derived from smooth muscle action in the
vessel walls. The equilibrium between expanding and contracting forces
is expressed in a derivation of Laplace’s law:
transmural pressure drops to a level such that elastic fibers in the
vessel wall are no longer stretched and therefore cannot contribute any
elastic tension, then there will be no further automatic decrease in
the radius. TC ÷ r then becomes greater than TM and active closure of
the vessel will occur. This concept has been verified in both animal
and human local vascular beds.6,112,121,160
Ashton was the first to relate these findings to acute compartment
syndrome and concluded that whatever the cause of the raised tissue
pressure, blood flow will be decreased and may temporarily cease
altogether as a result of a combination of active arteriolar closure
and passive capillary compression, depending on vasomotor tone and the
height of the total tissue pressure.7
Critics of this theory doubt the possibility of maintaining arteriolar
closure in the presence of ischemia, which is a strong local stimulus
for vasodilatation.91 Ashton noted
that flow resumes after 30 to 60 seconds of maintained tissue pressure
and attributes this to vessel re-opening possibly because of an
accumulation of vasodilator metabolites.6
According to this theory the increases in local tissue pressure reduce
the local arteriovenous pressure gradient and thus reduce blood flow.
When flow diminishes to less than the metabolic demands of the tissues
(not necessarily to zero), then functional abnormalities result. The
relationship between arteriovenous (AV) gradient and the local blood
flow (LBF) is summarized in the equation:
venous pressure, and R is the local vascular resistance. Veins are
collapsible tubes and the pressure within them can never be less than
the local tissue pressure. If tissue pressure rises as in the acute
compartment syndrome, then the Pv must rise also, thus reducing the AV
gradient (Pa – Pv) and therefore the local blood flow. At low AV
gradients compensation from local vascular resistance (R) is relatively
ineffective59 and local blood flow
is primarily determined by the AV gradient. Matsen and his colleagues
presented results on human subjects demonstrating reduction of the AV
gradient with elevation of the limb in the presence of raised tissue
pressure.98 This theory has been
supported by recent work that demonstrated that with external pressure
applied, simulating acute compartment syndrome, venous and capillary
flow ceased but arterioles were still capable of carrying flow.147
This disproves the critical closing theory but supports the hypothesis
of reduced arteriovenous gradient as the mechanism of reducing blood
flow.
postulates that capillary occlusion is the main mechanism reducing
blood flow in acute compartment syndrome.49 Measurement of capillary
pressure in dogs with normal tissue pressures revealed a mean level of
25 mm Hg. Hargens and his colleagues suggest that a tissue pressure of
similar value is sufficient to reduce capillary blood flow.49
Resultant muscle ischemia leads to increased capillary membrane
permeability to plasma proteins, increasing edema and obstruction of
lymphatic by the raised tissue pressure. Nonetheless, the authors admit
that reactive hyperemia and vasodilatation both tend to raise the
critical pressure level for microvascular occlusion. Note, however,
that this work was done in the presence of normal tissue pressures, and
it has also been pointed out that capillaries are collapsible tubes91 and their intravascular pressure ought to rise in the presence of raised tissue pressure. Hargens’ theory49
is supported by more recent work demonstrating reduction of the number
of perfused capillaries per unit area with raised tissue pressures.54
in blood flow in the acute compartment syndrome has a profound effect
on muscle tissue. Skeletal muscle is the tissue in the extremities most
vulnerable to ischemia and is therefore the most important tissue to be
considered in acute compartment syndrome. Both the magnitude and
duration of pressure elevation have been shown experimentally to be
important influences in the extent of muscle damage.
pressure leads to a reduction in muscle blood flow. A number of
experimental studies have highlighted the importance of perfusion
pressure as well as tissue pressure in the reduction of muscle blood
flow. MR measurements of cellular metabolic derangement (PH, tissue
oxygenation, and energy stores) and histological studies, including
electron microscopy and videomicroscopy studies of capillary blood
flow, have shown that critical tissue pressure thresholds are 10 to 20
mm Hg below diastolic blood pressure or 25 to 30 mm Hg below mean
arterial pressure.54,57,60,89
Increased vulnerability in previously traumatized or ischemic muscle
has been demonstrated when the critical threshold may occur at tissue
pressures more than 30 mm Hg below mean arterial pressure.11
muscle is ischemia followed by necrosis, with general agreement that
increasing periods of complete ischemia produce increasing irreversible
changes.56,75,116
Evidence indicates that muscle necrosis is present in its greatest
extent in the central position of the muscle, and that external
evaluation of the degree of muscle necrosis is unreliable. The duration
of muscle ischemia dictates the amount of necrosis, although some
muscle fibers are more vulnerable than others to ischemia. For example,
the muscles of the anterior compartment of the leg contain Type 1
fibers or red slow twitch fibers, whereas the gastrocnemius contains
mainly Type 2 or white fast twitch fibers. Type 1 fibers depend on
oxidative metabolism of triglycerides for their energy source and are
more vulnerable to oxygen depletion than Type 2 fibers whose metabolism
is primarily anaerobic.77 This may explain the particular vulnerability of the anterior compartment to raised intracompartmental pressure.
tissue pressure on neurological function. All investigators note a loss
of neuromuscular function with raised tissue pressures but at varying
pressure thresholds and duration.40,51,93,134
In a study on human neurological function, Matsen et al. found
considerable variation of pressure tolerance that could not be
attributed to differences in systemic pressure.95
could result from ischemia, ischemia plus compression, toxic effects,
or the effects of acidosis.
It was first suggested by Nario in 1938 that “Volkmann’s disease”
caused obliteration of the “musculodiaphyseal” vessels and caused
frequent pseudarthrosis.110 McQueen
observed a reduction in bone blood flow and bone union in rabbit tibiae
after an experimentally induced acute compartment syndrome.80
It is likely that muscle ischemia reduces the capacity for development
of the extraosseous blood supply on which long bones depend for healing.
following reestablishment of blood flow to the ischemic tissues and can
occur after fasciotomy and restoration of muscle blood flow in the
acute compartment syndrome. Reperfusion is followed by an inflammatory
response in the ischemic tissue that can cause further tissue damage.
The trigger for the inflammatory response is probably the breakdown
products of muscle.15 Some breakdown
products are procoagulants that activate the intrinsic clotting system.
This results in increasing microvascular thrombosis, which in turn
increases the extent of muscle damage.
ischemic process, the inflammatory response may become systemic. In
acute compartment syndrome this is most likely to occur in the crush
syndrome. Procoagulants escape into the systemic circulation and
produce systemic coagulopathy with parallel activation of inflammatory
mediators. These then damage vascular endothelium, leading to increased
permeability and subsequent multiple organ failure. Systemic clotting
and the breakdown products of dead and dying cells also lead to
activation of white blood cells, with the release of additional
inflammatory mediators such as histamine, interleukin, oxygen free
radicals, thromboxane, and many others.15
This is the basis for the use of agents such as antioxidants,
antithromboxanes, antileukotrienes, and anti-platelet-activating
factors that modify the inflammatory process. Some of these agents have
been shown in the laboratory to be capable of reducing muscle injury.1,70,71,149
key to a successful outcome. Delay in diagnosis has long been
recognized as the single cause of failure of the treatment of acute
compartment syndrome.85,92,122,124
Delay in diagnosis may be because of inexperience and lack of awareness
of the possibility of acute compartment syndrome, an indefinite and
confusing clinical presentation,99,143 or to anesthetic or analgesic techniques that mask the clinical signs.52,87,105
be catastrophic, leading to serious complications such as permanent
sensory and motor deficits, contractures, infection and at times,
amputation of the limb.106,117,124
In serious cases there may be systemic injury from the reperfusion
syndrome. A clear understanding of the clinical techniques necessary to
make an early diagnosis is therefore essential to any physician
treating acute compartment syndrome in order to avoid such
complications. As well as improving outcome, early recognition and
treatment of acute compartment syndrome is associated with decreased
indemnity risk in potential malpractice claims.14
compartment syndrome. The pain experienced by the patient is by nature
ischemic, and usually severe and out of proportion to the clinical
situation. Pain may, however, be an unreliable indication of the
presence of acute compartment syndrome because it can be variable in
its intensity.30,94,153 Pain may be absent in acute compartment syndrome associated with nerve injury62,159 or minimal in the deep posterior compartment syndrome.92,94
Pain is present in most cases because of the index injury but cannot be
elicited in the unconscious patient. Kosir and his coauthors abandoned
clinical examination as part of their screening protocol for critically
ill trauma patients because of the difficulty in eliciting reliable
symptoms and signs in this group.73
Children may not be able to express the severity of their pain, so
restlessness, agitation, and anxiety with increasing analgesic
requirements should raise the suspicion of the presence of an acute
compartment syndrome.8 Both Shereff135 and Myerson108 state that clinical diagnosis of acute compartment syndrome in the foot is so unreliable that other methods should be used.
and a specificity of 97% in the diagnosis of acute compartment syndrome
(i.e., a high proportion of false negative or missed cases but a low
proportion of false positive cases).143
There is general agreement, however, that pain if present is a
relatively early symptom of acute compartment syndrome in the awake
alert patient.143
recognized as a symptom of acute compartment syndrome. Thus pain is
increased for example in an anterior compartment syndrome when the toes
or foot are plantarflexed. This symptom is no more reliable than rest
pain, because the reasons for unreliability quoted above apply equally
to pain on passive stretch. The sensitivity and specificity of pain on
passive stretch are similar to those for rest pain.143
of the nerves traversing the affected compartment and are usually the
first signs of nerve ischemia, although sensory abnormality may be the
result of concomitant nerve injury.155,159
Ulmer reports a sensitivity of 13% and specificity of 98% for the
clinical finding of paraesthesia in acute compartment syndrome, a false
negative rate that precludes this symptom from being a useful
diagnostic tool.143
This sign has equally low sensitivity as others in predicting the
presence of acute compartment syndrome, probably because of the
difficulty of interpreting the underlying cause of the weakness, which
could be inhibition by pain, direct injury to muscle, or associated
nerve injury. If a motor deficit develops, full recovery is rare.17,26,122,127,157 Bradley17 reported full recovery in only 13% of patients with paralysis as a sign of their acute compartment syndrome.143
further sign of compartment syndrome; although the degree of swelling
is difficult to assess accurately, making this sign very subjective.
Casts or dressings often obscure compartments at risk and prevent
assessment of swelling.73 Some
compartments such as the deep posterior compartment of the leg are
completely buried under the muscle compartments, obscuring any swelling.
in acute compartment syndrome unless there is major arterial injury or
disease or in the very late stages of acute compartment syndrome when
amputation is inevitable. If acute compartment syndrome is suspected
and pulses are absent, then arteriography is indicated. Conversely, it
is dangerous to exclude the diagnosis of acute compartment syndrome
because distal pulses are present.
To achieve a probability of over 90% of acute compartment syndrome
being present, however, three clinical findings must be noted. The
third clinical finding is paresis; thus to achieve an accurate clinical
diagnosis of acute compartment syndrome the condition must be allowed
to progress until a late stage. This is clearly unacceptable and has
led to a search for earlier, more reliable methods of diagnosis.
intracompartmental pressure (ICP) once it was appreciated that acute
compartment syndrome was caused by increased tissue pressure within the
affected compartment. Because raised tissue pressure is the primary
event in acute compartment syndrome, changes in ICP will precede the
clinical symptoms and signs.83
One of the first to be used was the needle manometer method, using a
needle introduced into the compartment and connected to a column filled
partly with saline and partly with air.153
A syringe filled with air is attached to this column, as is a pressure
manometer or transducer. The ICP is the pressure that is required to
inject air into the tubing and flatten the meniscus between the saline
and the air. This method was modified by Matsen and his colleagues to
allow infusion of saline into the compartment.96,97
The ICP is the pressure resistance to infusion of saline. These
methods, although simple and inexpensive have some drawbacks. A danger
exists of too large a volume being infused, possibly inducing acute
compartment syndrome. It is probably the least accurate of the
measurement techniques available, with falsely high values having been
recorded in comparison with other techniques101 and falsely low values in cases of very high ICP.138 A needle with only one perforation at its tip also can become easily blocked.
This is a modification of the needle technique, in which fibrils
protrude from the bore of the catheter assembly. This allows a large
surface area for measurement and prevents obstruction of the needle; it
is ideal for continuous measurement. A disadvantage of this technique
is the possibility of a blood clot blocking the tip or air in the
column of fluid between the catheter and the transducer, which will
dampen the response and give falsely low readings. There is a
theoretical risk of retention of wick material in the tissues.
This operates on the same principal as the wick catheter in that it is
designed to increase the surface area at the tip of the catheter by
means of being cut axially at the end of the catheter (Fig. 27-3).
The interstitial pressure is measured through a column of saline
attached to a transducer. Patency can be confirmed by gentle pressure
over the catheter tip; an immediate rise in the pressure should be
seen. Care must be taken to avoid the presence of air bubbles in the
system as this can, like the wick catheter, result in falsely low
readings. The slit catheter is more accurate than the continuous
infusion method101 and is as accurate as the wick catheter.133
led to the placement of the pressure transducer directly into the
compartment by siting it within the lumen of a catheter. The solid
state transducer intracompartmental catheter (STIC) was described in
1984 and measurements were correlated with conventional pressure
monitoring systems.78 This device is
now commercially available and widely used, although to retain patency
of the catheter for continuous monitoring. an infusion must be used
with its attendant problems. The alternative is intermittent pressure
measurements, which is likely to cause significant discomfort to
patients and is more labor intensive. Newer systems with the transducer
placed at the tip of the catheter do not depend on a column of fluid
and therefore avoid the problems of patency.158 These systems are more expensive, however, and are a potential problem for resterilization.
way of measuring muscle blood flow and oxygenation. Near infrared
spectroscopy measures tissue oxygen saturation noninvasively by means
of a probe placed on the skin. This has proved to correlate to tissue
pressures experimentally5 and in human volunteers.41 It has also been used to demonstrate the hyperemic response to injury in tibial fracture.136 The technique has promise but requires further validation in humans subjected to injury.64
 |
|
FIGURE 27-3 The tip of a slit catheter, which can be made easily from standard equipment by cutting two slits in the tip of the catheter.
|
|
TABLE 27-4 Recommended Catheter Placements for Compartmental Pressure Monitoring
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
because this is most commonly involved in acute compartment syndrome
and is easily accessible.82 There is
a risk of missing an acute compartment syndrome in the deep posterior
compartments and some authors recommend measurement of both,58
but measuring two compartments is much more cumbersome. If the anterior
compartment alone is monitored, the surgeon must be aware of the small
chance of deep posterior acute compartment syndrome and measure the
deep posterior compartment pressures if there are unexplained symptoms
in the presence of anterior compartment pressures with a safe
difference between the perfusion pressure and the tissue pressure (ΔP).
It is important to measure the peak pressure within the limb, which
usually occurs within 5 cm of the level of the fracture.58 Recommended catheter placement for each of the anatomic areas is summarized in Table 27-4.
threshold, beyond which decompression of acute compartment syndrome is
required. After appreciation of the nature of acute compartment
syndrome being raised pressure, debate centered around the use of
tissue pressure alone as indication of the need for decompression. One
level believed to be critical was 30 mm Hg of ICP because this is a
value close to capillary blood pressure.50,104 Some authors felt that 40 mm Hg of tissue pressure should be the threshold for decompression,47,78,96,127 although some recognized a significant individual variation between individuals in their tolerance of raised ICP.47,98
In a series of patients with tibial fractures, a tissue pressure of 50
mm Hg was recommended as a pressure threshold for decompression in
normotensive patients.48
individuals in their tolerance of raised ICP is because of variations
in systemic blood pressure. Whitesides and his coauthors were the first
to suggest the importance of the difference between the diastolic blood
pressure and tissue pressure or ΔP.153
They stated that there is inadequate perfusion and relative ischemia
when the tissue pressure rises to within 10 to 30 mm Hg of the
diastolic pressure. There is now good evidence from experimental
work to support this concept57,89
or the similar concept that the difference between mean arterial
pressure and tissue pressure should not be less than 30 mm Hg in normal
muscle or 40 mm Hg in muscle subject to trauma60 or antecedent ischemia.11
test the hypothesis of the differential pressure as a threshold for
decompression.83 One hundred and
sixteen patients with tibial diaphyseal fractures underwent continuous
intracompartmental pressure monitoring both perioperatively and for at
least 24 hours postoperatively. The differential pressure between the
diastolic blood pressure and the ICP was recorded. Mean pressures over
a 12-hour period were calculated to include the duration of elevated
pressure in the analysis. Three patients had ΔP of less than 30 mm Hg
and underwent fasciotomy. Of the remaining patients, all maintained a
ΔP greater than 30 mm Hg despite a number with ICP greater than 40 mm
Hg. None of these patients underwent fasciotomy and none had any
sequelae of acute compartment syndrome at final review. The authors
concluded that a ΔP of 30 mm Hg is a safe threshold for decompression
in acute compartment syndrome. This has recently been validated by the
same group who examined the outcome in terms of muscle power and return
to function in two groups of patients with tibial fractures.154
The first group of patients all had an ICP of greater than 30 mm Hg and
the second all had an ICP less than 30 mm Hg. Both groups had
maintained a ΔP of greater than 30 mm Hg. There were no differences in
the outcomes between the two groups. The concept of the use of ΔP is
also supported by Ovre et al., who found an unacceptably high rate of
fasciotomies (29%) using an ICP of 30 mm Hg as a threshold for
decompression.114
with reference to leg compartment syndrome. The threshold may differ
for children who have a low diastolic pressure and are therefore more
likely to have a ΔP less than 30 mm Hg. Mars and Hadley recommend the
use of the mean arterial pressure rather than the diastolic pressure to
obviate this problem.88 It has been
assumed that these pressure thresholds apply equally to anatomic areas
other than the leg, although this has not been formally examined.
to proceed to fasciotomy. It is well established experimentally and
clinically that both the duration and severity of the pressure
elevation influence the development of muscle necrosis.*
Continuous pressure monitoring allows a clear record of the trend of
the tissue pressure measurements. In situations where the ΔP drops
below 30 mm Hg if the ICP is dropping and the ΔP is rising, then it is
safe to observe the patient in anticipation of the ΔP returning within
a short time to safe levels. If the ICP is rising, the ΔP is dropping
and less than 30 mm Hg, and this trend has been consistent for a period
of 1 to 2 hours, then fasciotomy should be performed. Fasciotomy should
not be performed based on a single pressure reading except in extreme
cases. Using this protocol, delay to fasciotomy and the sequelae of
acute compartment syndrome are reduced without unnecessary fasciotomies
being performed.82
but this study did not consider the importance of the duration of
raised ICP in the diagnosis of acute compartment syndrome. Some authors
have found compartment pressure monitoring less useful but used
clinical symptoms and signs as their indication for fasciotomy with
pressure monitoring only as an adjunct.3,53 For ICP monitoring to be most effective in reducing delay, it must be used as the primary indication for fasciotomy.
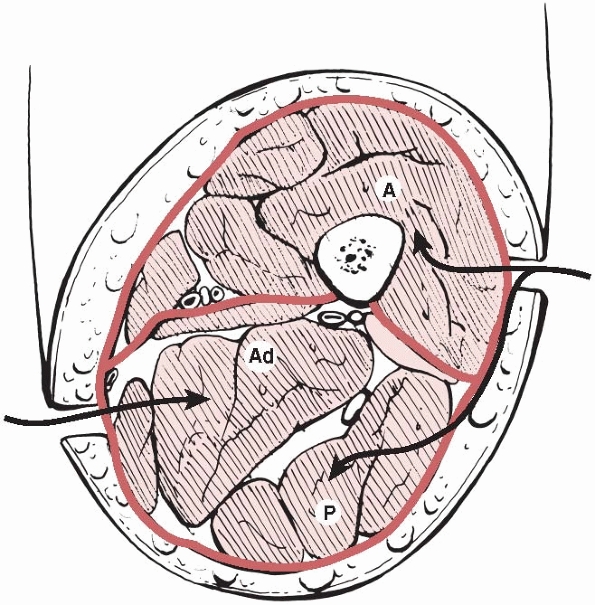
of which are bounded by the fascia lata and separated by the medial and
lateral intermuscular septa (Fig. 27-4). Their contents and the clinical signs of compartment syndrome in each compartment are summarized in Table 27-5. Involvement of the adductor compartment is rare.
and fascia, laterally by the intermuscular septum, posteriorly by the
fibula and interosseous membrane, and medially by the tibia. Its
contents and the clinical signs of acute compartment syndrome are
listed in Table 27-6.
by the fibula, and anteriorly by the anterior intermuscular septum. Its
contents and the clinical signs of involvement in acute compartment
syndrome are detailed in Table 27-6.
The deep peroneal nerve may rarely be affected as it passes through the
lateral compartment en route to the anterior compartment.
 |
|
FIGURE 27-4
A cross section of the thigh demonstrating the three compartments and the access to them. A, anterior; Ad, adductor; P, posterior. |
|
TABLE 27-5 Compartments of the Thigh, Their Contents, and Signs of Acute Compartment Syndrome
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
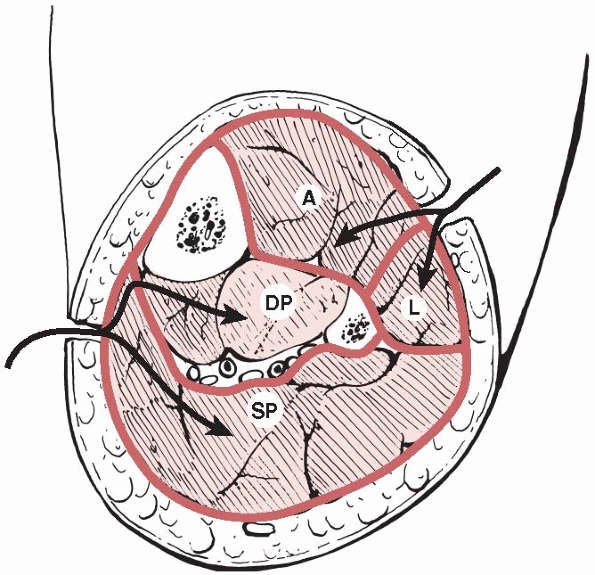 |
|
FIGURE 27-5 A cross section of the leg showing the four compartments. The arrows
show the routes for double incision four compartment fasciotomy. A, anterior compartment; DP, deep posterior compartment; L, lateral compartment; SP, superficial posterior compartment. |
|
TABLE 27-6 Compartments of the Leg with Their Contents and Clinical Signs of Acute Compartment Syndrome in Each
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
anteriorly by the intermuscular septum between the superficial and deep
compartments and posteriorly by skin and fascia. Its contents and the
clinical signs of acute compartment syndrome are summarized in Table 27-6.
the tibia and interosseous membrane, laterally by the fibula,
posteriorly by the intermuscular septum separating it from the
superficial posterior compartment, and medially by skin and fascia in
the distal part of the leg. Table 27-6 lists the contents of the deep posterior compartment and the likely clinical signs in acute compartment syndrome.
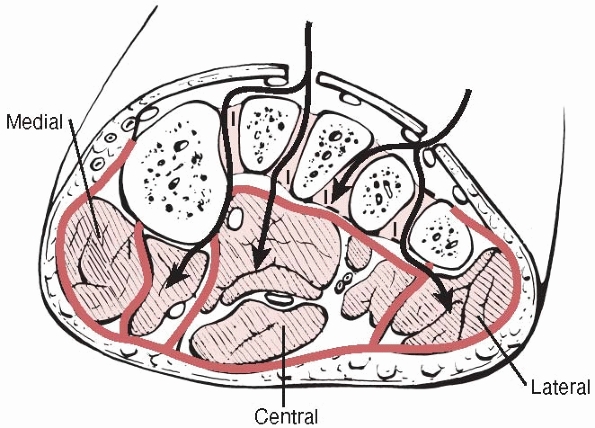
four compartments in the foot—medial, lateral, central, and
interosseous (Fig. 27-6). The medial
compartment lies on the plantar surface of the hallux, the lateral
compartment is on the plantar surface of the fifth metatarsal, and the
central compartment lies on the plantar surface of the foot. The
interosseous compartment lies dorsal to the others between the
metatarsals. Their contents are shown in Table 27-7.
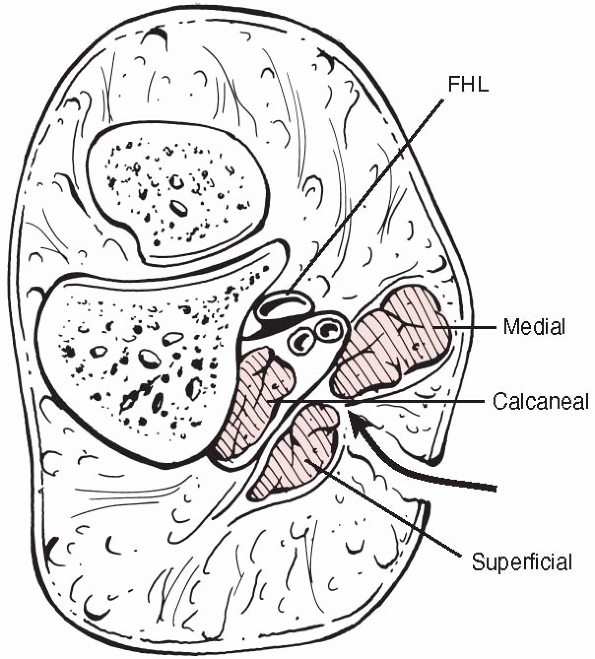
They believe that there are nine compartments in the foot, with two
central compartments, one superficial containing flexor digitorum
brevis, and one deep (the calcaneal compartment) (Fig. 27-7)
containing quadratus plantae, which communicates with the deep
posterior compartment of the leg. They demonstrated that each of the
four interosseous muscles and adductor hallucis lie in separate
compartments, thus increasing the number of compartments to nine. The
clinical importance of these anatomic findings has been challenged
after the finding that the barrier between the superficial and
calcaneal compartments becomes incompetent at a pressure of 10 mm Hg,
much lower than that required to produce an acute compartment syndrome.46
The clinical diagnosis of acute compartment syndrome should be
suspected in the presence of severe swelling, but differentiating the
affected compartments is extremely difficult.
 |
|
FIGURE 27-6 A cross section of the foot showing access from the dorsum of the foot to the compartments. I, interosseous.
|
|
TABLE 27-7 Compartments of the Foot and Their Contents
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
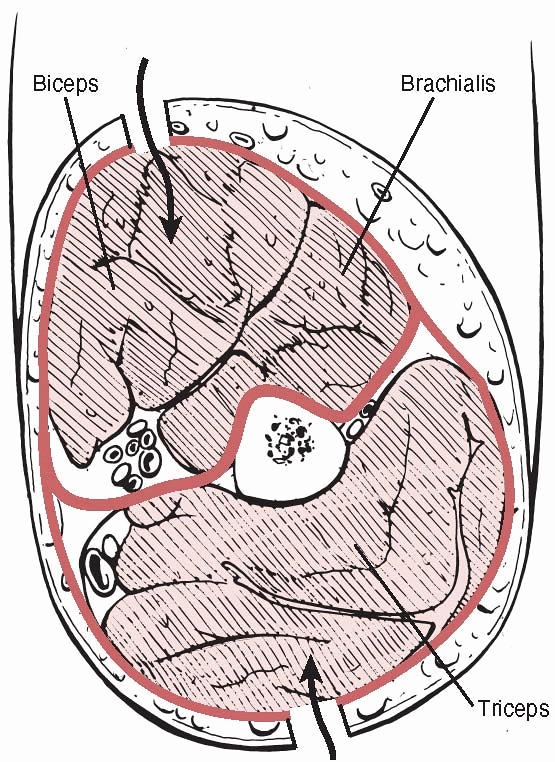
The anterior compartment is bounded by the humerus posteriorly, the
lateral and medial intermuscular septa, and the brachial fascia
anteriorly. Its contents and the clinical signs of acute compartment
syndrome are detailed in Table 27-8. In late cases paralysis of the muscles innervated by the median, ulna, and radial nerves is seen.
clinical signs of acute compartment syndrome are listed in Table 27-8.
 |
|
FIGURE 27-7
A section through the hindfoot showing the medial, superficial, and deep central (calcaneal) compartments. The medial approach for release of the calcaneal compartment is shown. FHL, flexor hallucis longus. |
 |
|
FIGURE 27-8
A cross section of the arm showing the anterior compartment containing biceps(B) and brachialis(Br), and the posterior compartment containing triceps(T). |
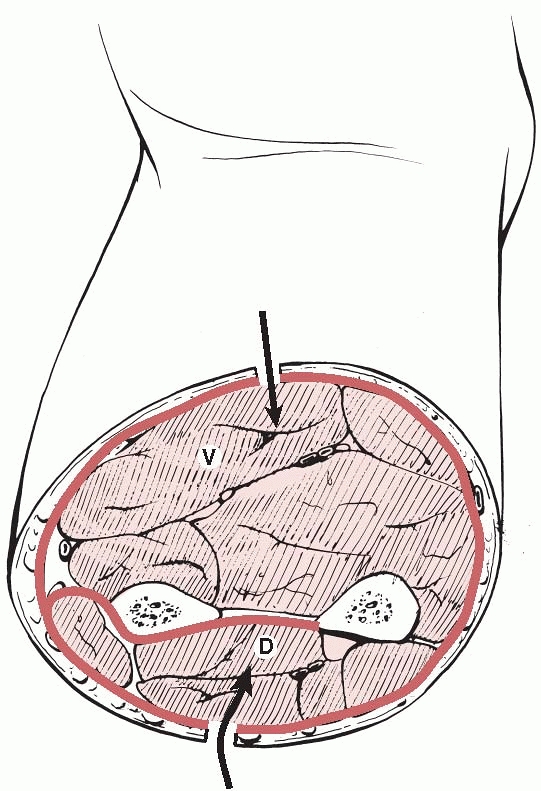
The volar compartment has the ulna, radius, and interosseous membrane
as its posterior limit and the antebrachial fascia as its anterior
limit. Table 27-9 lists the contents and
clinical signs of acute compartment syndrome in the volar compartment
of the forearm. A suggestion has been made that the volar compartment
of the forearm contains three spaces, the superficial volar, deep
volar, and pronator quadratus spaces,38 but in practice it is not usually necessary to distinguish between these at fasciotomy.21
|
TABLE 27-8 Compartments of the Arm, Their Contents, and Clinical Signs of Acute Compartment Syndrome
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
|
 |
|
FIGURE 27-9
A cross section of the midforearm. The pronator quadratus compartment is not shown as it lies in the distal forearm. D, dorsal; V, volar. |
radius, ulna, and interosseous membrane and contains the finger and
thumb extensors, abductor pollicis longus, and extensor carpi ulnaris.
Its contents and the clinical signs of acute compartment syndrome are
summarized in Table 27-9.
dorsal interosseous, and three volar interosseous compartments (Fig. 27-10).
The thenar compartment is surrounded by the thenar fascia, the thenar
septum, and the first metacarpal. The hypothenar compartment is
contained by the hypothenar fascia and septum and the fifth metacarpal.
The dorsal interosseous compartments lie between the metacarpals and
are bounded by them laterally and the interosseous fascia anteriorly
and posteriorly. The volar interosseous compartments lie on the volar
aspect of the metacarpals, but it is unlikely that these are
functionally separate from the dorsal interosseous compartments because
the tissue barrier between the two cannot withstand pressures of more
than 15 mm Hg.45 The contents of the hand compartments are detailed in Table 27-10.
|
TABLE 27-9 Compartments of The Forearm, Their Contents, and Signs of Acute Compartment Syndrome
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
compartment syndrome is fasciotomy, which if delayed can cause
devastating complications. Nevertheless, other preliminary measures
should be taken in cases of impending acute compartment syndrome. The
process may on occasion be aborted by release of external limiting
envelopes such as dressings or plaster casts, including the padding
under the cast. Splitting and spreading a cast has been shown to reduce
ICP as has release of dressings.36 The split and spread cast is the only method that can accommodate increasing limb swelling.161 The limb should not be elevated above the height of the heart as this reduces the arteriovenous gradient.98
Hypotension should be corrected, because this will reduce perfusion
pressure. Oxygen therapy should be instituted to ensure maximum oxygen
saturation.
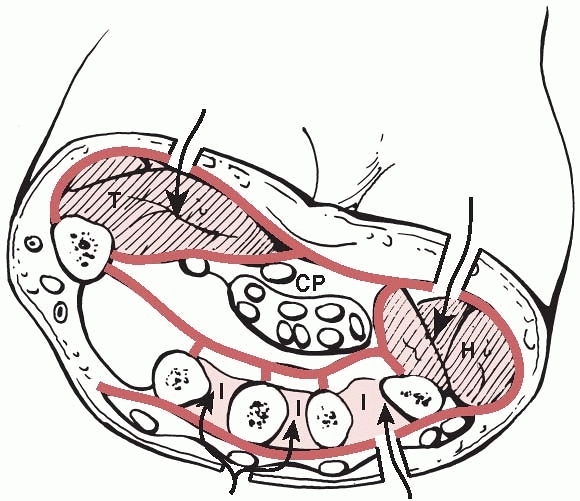 |
|
FIGURE 27-10
A cross section of the hand showing the muscle compartments. The adductor pollicis lies more distally. CP, central palmar; H, hypothenar; I, interosseous; T, thenar. |
full and adequate decompression. Skin incisions must be made along the
full length of the affected compartment. There is no place for limited
or subcutaneous fasciotomy in acute compartment syndrome. It is
essential to visualize all contained muscles in their entirety (Fig. 27-11) in order to assess their viability and any muscle necrosis must be thoroughly débrided to avoid infection.
Subcutaneous fasciotomy is contraindicated for these reasons and also because the skin may act as a limiting boundary.37
|
TABLE 27-10 The Compartments of The Hand and Their Contents
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
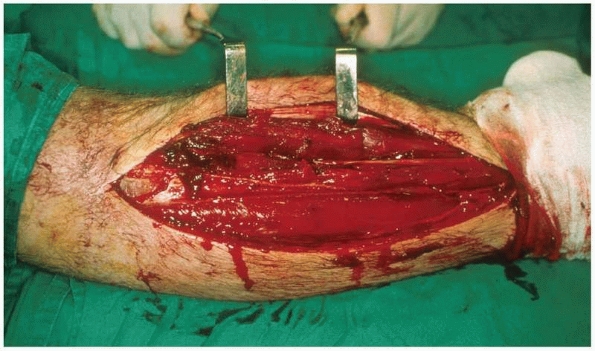 |
|
FIGURE 27-11
Fasciotomy of the anterior and lateral compartments of the leg. Note that the incision extends the whole length of the muscle compartment allowing inspection of all muscle groups. |
One of the most commonly used techniques is the double incision
four-compartment fasciotomy.103 The
anterior and lateral compartments are released through a lateral skin
incision over the intermuscular septum between the compartments (see Figure 27-5).
The skin may then be retracted to allow fascial incisions over both
compartments. Care must be taken not to injure the superficial peroneal
nerve that pierces the fascia and lies superficial to it in the distal
third of the leg (see Figure 27-11). There is
considerable variation in its course, with approximately three quarters
of the nerve remaining in the lateral compartment before its exit
through the deep fascia and one quarter passing into the anterior
compartment.2 The two posterior compartments are accessed through a skin incision 2 cm from the medial edge of the tibia (see Figure 27-5).
This allows a generous skin bridge to the lateral incision but is
anterior to the posterior tibial artery, especially in open fractures,
to protect perforating vessels that supply local fasciocutaneous flaps.120
The superficial posterior compartment is easily exposed by skin
retraction. The deep posterior compartment is exposed by posterior
retraction of the superficial compartment and is most easily identified
in the distal third of the leg (Fig. 27-12). It
is sometimes necessary to elevate the superficial compartment muscles
from the tibia for a short distance to allow release of the deep
posterior compartment along its length. Care must be taken to protect
the saphenous vein and nerve in this area and to protect the posterior
tibial vessels and nerves.118
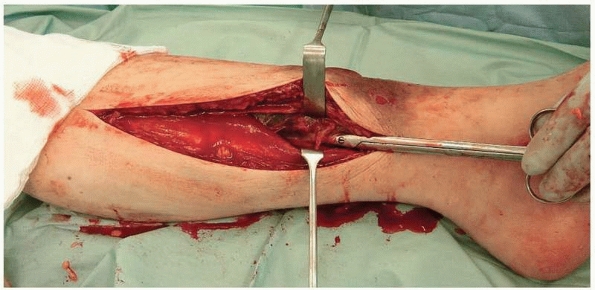 |
|
FIGURE 27-12
Decompression of the medial side of the leg. The superficial posterior compartment is being retracted to display the deep compartment. The scissors are deep to the fascia overlying the deep posterior compartment. |
but this is unnecessarily destructive and risks damage to the common
peroneal nerve. Single incision four-compartment fasciotomy without
fibulectomy can be performed through a lateral incision that affords
easy access to the anterior and lateral compartments.22
Anterior retraction of the peroneal muscles allows exposure of the
posterior intermuscular septum overlying the superficial posterior
compartment. The deep posterior compartment is entered by an incision
immediately posterior to the posterolateral border of the fibula.
than single incision methods because the fascial incisions are all
superficial. Using the single incision method, it can be difficult to
visualize the full extent of the deep posterior compartment. Both
methods seem to be equally effective at reducing ICP.103,146
and the compartments easily visualized. Both thigh compartments can be
approached through a single lateral skin incision (Fig. 27-13),140 although a medial incision can be used over the adductors if considered necessary (see Figure 27-4).
decompress, and a sound knowledge of the anatomy is essential. Dorsal
incisions overlying the second and fourth metacarpals allow sufficient
access to the interosseous compartments and the central compartment
that lies deep to the interosseous compartments (see Figure 27-6).
The medial and lateral compartments can be accessed around the deep
surfaces of the first and fifth metatarsal, respectively. Such a
decompression is usually sufficient in cases of forefoot injury, but
when a hindfoot injury, especially a calcaneal fracture, is present a
separate medial incision may be required to decompress the calcaneal
compartment (see Figure 27-7).109,126
performed. In most cases the volar compartment is approached first
through an incision extending from the biceps tendon at the elbow to
the palm of the hand, to allow carpal tunnel decompression
that is usually necessary (Fig. 27-14). Fascial incision then allows direct access to the compartment (see Figure 27-9).
The deep flexors must be carefully inspected after fascial incision.
Separate exposure and decompression of pronator quadratus may be
necessary.21 Often volar fasciotomy
is sufficient to decompress the forearm, but if ICP remains elevated in
the dorsal compartment perioperatively, then dorsal compression is
easily performed through a straight dorsal incision (see Figure 27-9).
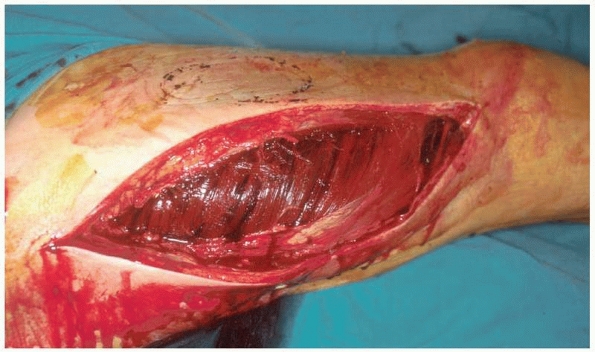 |
|
FIGURE 27-13 Fasciotomy of the thigh through a single lateral incision.
|
 |
|
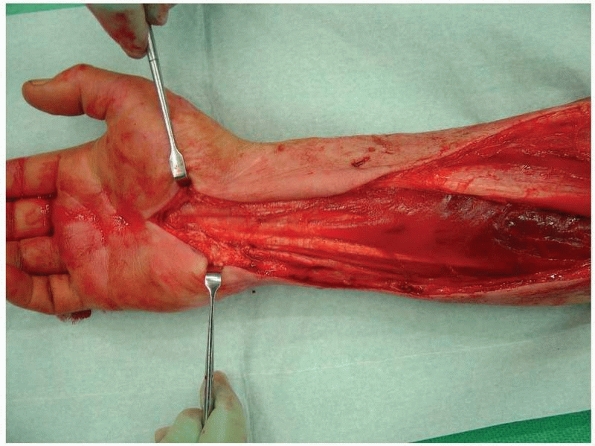
FIGURE 27-14
Fasciotomy of the forearm in a case of crush syndrome. There is necrosis of the forearm flexors proximally. The carpal tunnel has been decompressed. |
achieved using two dorsal incisions that allow access to the
interosseous compartments (see Figure 27-10).
This may often be sufficient, but if there is clinical suspicion or
raised ICP on measurement then incisions may be made over the thenar
and hypothenar eminences, allowing fasciotomy of these compartments.
The wounds should be left open and dressed, and approximately 48 hours
after fasciotomy a “second look” procedure should be undertaken to
ensure viability of all muscle groups. Skin closure or cover should not
be attempted unless all muscle groups are viable.
if possible, although this must be without tension on the skin edges.
Commonly in the leg this technique is possible in the medial but not
the lateral wound. If delayed primary closure cannot be achieved, then
the wound may be closed using either dermatotraction techniques or
split skin grafting. Dermatotraction or gradual closure techniques have
the advantage of avoiding the cosmetic problems of split skin grafting
but may cause skin edge necrosis.66 A further disadvantage is the prolonged time required to achieve closure, which may be up to 10 days.12,66
The recent introduction of vacuum assisted closure (VAC) systems is
likely to be a significant advantage in this area and may reduce the
need for split skin grafting with a low complication rate.151
the long bones, should be stabilized in the presence of acute
compartment syndrome treated by fasciotomy.39,42,122,142
In reality the treatment of the fracture should not be altered by the
presence of an acute compartment syndrome, although cast management of
a tibial fracture is contraindicated in the presence of acute
compartment syndrome. Fasciotomy should be performed prior to fracture
stabilization in order to eliminate any unnecessary delay in
decompression. Stabilization of the fracture allows easy access to the
soft tissues and protects the soft tissues, allowing them to heal.
excellent stabilization of a diaphyseal fracture and is now probably
the treatment of choice in most centers for tibial diaphyseal fracture.
Some authors, however, have implicated reaming as a possible cause of
acute compartment syndrome.74,100
This was refuted by other studies examining intercompartmental
pressures during and after tibial nailing. McQueen and coauthors
studying reamed intramedullary nailing,81 and Tornetta and French studying unreamed intramedullary nailing141
agreed that the ICP increased perioperatively and dissipated
postoperatively, and that nailing did not increase the likelihood of
acute compartment syndrome. Nassif and his coauthors found no
differences in ICP between reamed and unreamed nailing.111
tibial fractures. These include traction, which raises pressure in the
deep posterior compartment by approximately 6% per kilogram of weight
applied.132 Counter-traction using a
thigh bar can cause external calf compression if the bar is wrongly
positioned and can also decrease arterial flow and venous return,
making the leg more vulnerable to ischemia. Elevation of the leg as in
the 90-90 position decreases the tolerance of the limb to ischemia.95
Thus excessive traction, poor positioning of the thigh bar and high
elevation of the leg should be avoided in patients at risk of acute
compartment syndrome.
if the condition has been treated expeditiously. Delay in diagnosis has
been cited as the single reason for failure in the management of acute
compartment syndrome.85 Delay to fasciotomy of more than 6 hours is likely to cause significant sequelae,124 including muscle contractures, muscle weakness, sensory loss, infection, and nonunion of fractures.* In severe cases amputation may be necessary because of infection or lack of function.31
when the diagnosis is made late and muscle necrosis is inevitable,
whether because of a missed acute compartment syndrome or the crush
syndrome. Little can be gained in exploring a closed
crush
syndrome when complete muscle necrosis is inevitable, except in
circumstances where there are severe or potentially severe systemic
effects wherein amputation may be necessary. Increased sepsis rates
with potentially serious consequences have been reported when these
cases have been explored.119
Nonetheless, if partial muscle necrosis is suspected and compartment
monitoring reveals pressures above the threshold for decompression,
there may be an indication for fasciotomy to salvage remaining viable
muscle. In these circumstances debridement of necrotic muscle must be
thorough to reduce the chances of infection. In rare cases the ICP may
be high enough to occlude major vessels. This is a further indication
for fasciotomy to salvage the distal part of the limb.119
surviving muscle and compartment pressures are low, then fasciotomy
should be withheld. If there is any possibility of any remaining viable
muscle or if compartment pressures are above critical levels,
fasciotomy should be performed to preserve any viable muscle. In any
circumstances a thorough débridement of necrotic muscle is mandatory.
essential, and it is important to be aware of the patients at risk of
developing acute compartment syndrome. Good clinical examination
techniques in the alert patient will help to identify the compartments
at risk. Compartment monitoring should be used in all “at risk”
patients as defined in Table 27-3. In practice
this means that all tibial fractures should be monitored, but if
resources to do so are limited, then younger patients should be
selected for monitoring. The anterior compartment should be monitored,
but in rare cases where symptoms are present that cannot be explained
by the tissue pressures in the anterior compartment, the posterior
compartment should also be monitored.
differential pressure of less than 30 mm Hg. If the ΔP is less than 30
mm Hg but the tissue pressure is dropping, as can happen for instance
for a short time after tibial nailing, then the pressure may be
observed for a short period in anticipation of the ΔP rising. On the
other hand, if the ΔP remains less than 30 mm Hg or is reducing, then
immediate fasciotomy is indicated. Delay and complications are
minimized by making the decision to perform a fasciotomy primarily on
the level of ΔP, with clinical symptoms and signs being used as an
adjunct to diagnosis.
it is simpler and gives an excellent view of all compartments. At this
stage if a fracture is present, it should be stabilized if this has not
been done previously. Closure is with a suction type of dressing,
followed at 48 hours with either direct closure or split skin grafting.
There is no indication to prolong closure beyond this unless there is
residual muscle necrosis.
devastating complication of fracture that continues to be a significant
cause of disability and successful litigation.14
Delay to diagnosis was cited as the single cause of a poor outcome more
than 40 years ago, yet there remains a remarkable lack of consistency
in the methods used to diagnose the condition.152,156
Universally acceptable, clear, clinical guidelines are required to
improve speed of diagnosis in all units managing trauma and would
likely be the single biggest advance in the management of the condition.
methods of measuring blood flow directly rather than indirectly by ICP
measurement. Noninvasive methods of diagnosing acute compartment
syndrome are being examined.131 One
such example is near infrared spectroscopy, which measures the amount
of oxygenated hemoglobin in muscle tissues transcutaneously.5,52,64,136
syndrome are also likely to play a part in the future. Some basic
science research has already been published on the effects of
antioxidants on the outcome of acute compartment syndrome with
promising results.70 This work
should be extended to human studies in an attempt to reduce the effects
of acute compartment syndrome in the clinical situation.
goal in its management. Attempts have been made to reduce ICP with the
administration of hypertonic fluids intravenously,13
but these have never been successful clinically. Nevertheless, an
experiment on human subjects using tissue ultrafiltration to remove
fluid from the compartment has been shown to reduce ICP.113 Whether this technique can be useful clinically remains to be seen.
JG, Dhar A, Shukla SD, Silver D. Effect of pentoxifylline on tissue
injury and platelet-activating factor production during
ischemia-reperfusion injury. J Vasc Surg 1995;21:742-748.
DP, Bosse MJ, Gaccione DR, Gabriel KR. Anatomical variations in the
course of the superficial peroneal nerve. J Bone Joint Surg
(Am);73A:112-114.
OQ, Darrah C, Cooper A, et al. Continuous compartment pressure
monitoring vs clinical monitoring in tibial diaphyseal fractures.
Injury 2008;39:1204-1209.
SM, Due J, Samdal FA. Compartment syndrome with muscle necrosis
following repair of hernia of tibialis anterior. Case report. Acta Chir
Scand 1987;153: 695-697.
S, Brundage SI, Gentilello LM. Near-infrared spectroscopy: a potential
method for continuous transcutaneous monitoring for compartmental
syndrome in critically injured patients. J Trauma 1999;47:829-833.
DS, Kadiyala RK, Waters PM. Acute compartment syndrome in children:
contemporary diagnosis, treatment and outcome. J Paed Orthop
2001;21:680-688.
M, Fadale P. Compartment syndrome of the leg after arthroscopic
examination of a tibial plateau fracture. Case report and review of the
literature. Arthroscopy 1997; 13:646-651.
M, Gupta R, Dobrasz J, et al. The effect of antecedent ischemia on the
tolerance of skeletal muscle to increased interstitial pressure. J
Orthop Trauma 1996;10:555-559.
SS, Schilling JD, McIntyre KE, et al. Shoelace technique for delayed
primary closure of fasciotomies. Am J Surg 1994;169:435-436.
OS, Zinman C, Reis DN, et al. Hypertonic mannitol ameliorates
intracompartmental tamponade in a model compartment syndrome in the
dog. Nephron 1991;58: 344-346.
FW. The pathophysiology of skeletal muscle ischemia and the reperfusion
syndrome: a review. Cardiovasc Surg 2002;10:620-630.
RC, Irmay F, Magnin M, et al. Spontaneous anterior and lateral tibial
compartment syndrome in a Type 1 diabetic patient: Case report. J
Trauma 1997;43: 140-141.
PS, Steinberg DR, Pepe MD, Beredjiklian PK. The significance of the
three volar spaces in forearm compartment syndrome: a clinical and
cadaveric correlation. J Hand Surg (Am) 1998;23:1077-1081.
CM, Byrnes T, McLaughlin G. Intramedullary nailing of tibial diaphyseal
fractures in children with open physes. Injury 2003;34:781-785.
CG, Schmidt AH, Kyle RF, et al. A prospective randomized study of
intramedullary nails inserted with and without reaming for the
treatment of open and closed fractures of the tibial shaft. J Orthop
Trauma 2000;14:187-193.
M, Kalus AK, Kuther G, et al. Long-term results of compartment syndrome
of the lower limb in polytraumatised patients. Injury 2007;38:607-613.
SR, Mubarak SJ, Evans KL, et al. Quantification of intracompartmental
pressure and volume under plaster casts. J Bone Joint Surg (Am)
1981;63A:449-453.
A, Masquelet AC. Anatomy and intracompartmental pressure measurement
technique of the pronator quadratus compartment. J Hand Surg Am
2001;26: 1129-1134
RH. Upper extremity compartment syndromes. In: Mubarak SJ, Hargens AR,
eds. Compartment Syndromes and Volkmann’s Contracture. Philadelphia: WB
Saunders, 1981.
RH, Szabo RM, Williamson RV, et al. Tissue pressure threshold for
peripheral nerve viability. Clin Orthop 1983;178:285-291.
LM, Sanzone A, Wang L, et al. Near-infrared spectroscopy versus
compartment pressure for the diagnosis of lower extremity compartmental
syndrome using electromyelography-determined measurements of
neuromuscular function. J Trauma 2001;51:1-8.
DH, Mubarak SJ, Yani NC, et al. Fracture of the tibia complicated by
acute compartment syndrome. Clin Orthop 1987;217:221-227.
MJ, Barnes MR, Allen MJ, et al. Weakness of foot dorsiflexion and
changes in compartment pressures after tibial osteotomy. J Bone Joint
Surg Br 1986;68:471-475.
GP, Shearman CM, Saltzman CL. Compartmental divisions of the hand
revisited. Rethinking the validity of cadaver infusion experiments. J
Bone Joint Surg Br 2001;83:241-244.
GP, Shearman CM, Saltzman CL. The compartments of the foot revisited.
Rethinking the validity of cadaver infusion experiments. J Bone Joint
Surg Br 2001; 83:245-249.
AA, Nagel DA. Compartment syndrome of the forearm: early recognition
using tissue pressure measurement. J Hand Surg Am 1979;4:258-263.
AR, Akeson WH, Mubarak SJ, et al. Fluid balance within the canine
anterolateral compartment and its relationship to compartment
syndromes. J Bone Joint Surg Am 1978;60:499-505.
AR, Akeson WH, Mubarak SJ, et al. Kappa Delta Award paper: tissue fluid
pressures: from basic research tools to clinical applications. J Orthop
Res 1989;7: 902-909.
AR, Romine JS, Sipe JC, et al. Peripheral nerve conduction block by
high muscle compartment pressure. J Bone Joint Surg Am 1979;61:192-200.
P, Bunola J, Jennings AJ, et al. Acute compartment syndrome masked by
intravenous morphine from a patient-controlled analgesia pump. Injury
2000;31; 387-389.
IA, Kadir A, Donald G. Continuous compartment pressure monitoring for
tibia fractures: does it influence outcome? J Trauma 2006;60:1330-1335.
LA, O’Farrell D, Seaber AV, et al. Effect of increased compartment
pressure on the microcirculation of skeletal muscle. Microsurgery
1998;18:67-71.
MT, Leversedge FJ, Seiler JG. Forearm compartment pressures: an in
vitro analysis of open and endoscopic fasciotomy. J Hand Surg Am
1999;24:1289-1297.
G, Liauw S, Romaschin AD, et al. Separation of reperfusion injury from
ischemia induced necrosis. Surg Forum 1988;39:306-308.
MM, Whitesides TE, Greive SR, et al. Compartment pressure in
association with closed tibial fractures. The relationship between
tissue pressure, compartment and the distance from the site of the
fracture. J Bone Joint Surg Am 1994;76:1285-1292.
MM, Whitesides TE, Greve SR, et al. Histologic determination of the
ischemic threshold of muscle in the canine compartment syndrome model.
J Orth Trauma 1993; 7:199-210.
O. Orthostatic changes of blood flow in subcutaneous tissue in patients
with arterial insufficiency of the legs. Scand J Clin Lab Invest
1974;34:103-109.
RB, Sapega AA, Scott R, et al. The compartment syndrome. An
experimental and clinical study of muscular energy metabolism using
phosphorus nuclear magnetic resonance spectroscopy. Clin Orthop
1988;226:138-155.
MJ, Simpson H, McQueen MM. Noninvasive acute compartment syndrome
monitoring. The influence of haematoma on near-infrared spectroscopy.
Proc AAOS 2003; 4:514.
H, Broos P. Dermotraction: an effective technique for the closure of
fasciotomy: a preliminary report of 15 patients. J Orthop Trauma
2001;15:438-441.
HMJ, Broos PLO. Routine monitoring of compartment pressure in patients
with tibial fractures: beware of overtreatment! Injury 2001;32:415-421.
G, Lonnerholm T, Olerud S. Cavus deformity of the foot after fracture
of the tibial shaft. J Bone Joint Surg Am 1975;57:893-900.
SR, Daly AF, Sheehan K, et al. Oral vitamin C reduces the injury to
skeletal muscle caused by compartment syndrome. J Bone Joint Surg Br
2004;86:906-911.
SR, Moneley D, Murray P, et al. Oral vitamin C attenuates acute
ischemia-reperfusion injury in skeletal muscle. J Bone Joint Surg Br
2001;83:1202-1206.
R, Moore FA, Selby JH, et al. Acute lower extremity compartment
syndrome (ALECS) screening protocol in critically ill trauma patients.
J Trauma 2007;63: 268-275.
KJ, Clapper MF, Brumback RJ, et al. Complications of reamed
intramedullary nailing of the tibia. J Orthop Trauma 1991;5:184-189.
R, Lindsay T, Walker PM. The extent and distribution of skeletal muscle
necrosis after graded periods of complete ischemia. J Vasc Surg
1987;6:152-157.
R, Lin PH, Alankar S, et al. Acute limb ischemia secondary to
myositis-induced compartment syndrome in a patient with human
immunodeficiency virus infection. J Vasc Surg 2003;37:1103-1105.
TF, Liauw S, Rouraschin AD, et al. The effect of ischemia/reperfusion
on adenosine nucleotide metabolism and xanthine oxidase production in
skeletal muscle. J Vasc Surg 1990;12:8-15.
AGP, Marble AE, Yabsley RH. Monitoring acute compartment pressures with
the STIC catheter. Clin Orthop 1984;190:192-198.
MD, Jupiter JB. Acute exercise-induced bilateral anterolateral leg
compartment syndrome in a healthy young man. Am J Orthop 1995;24:862-864
MM. The effect of acute compartment syndrome on bone blood flow and
bone union. MD Thesis, University of Edinburgh, 1995.
MM, Christie J, Court-Brown CM. Acute compartment syndrome in tibial
diaphyseal fractures. J Bone Joint Surg Br 1996;78:95-98.
MM, Court-Brown CM. Compartment monitoring in tibial fractures. The
pressure threshold for decompression. J Bone Joint Surg Br
1996;78:99-104.
MM, Christie J, Court-Brown CM. Compartment pressures after
intramedullary nailing of the tibia. J Bone Joint Surg Br 1990;72:95-98.
A, Weber TG. Fasciotomy of the foot: an anatomical study with special
reference to release of the calcaneal compartment. Foot Ankle
1990;10:267-275.
GJ, Barrington MJ, McGuirk BR. Acute compartment syndrome of the lower
limb and the effect of postoperative analgesia on diagnosis. Br J
Anaesth 2009;102:3-11.
MS, Whitesides TE, Seiler JG, et al. Determination of the compartment
pressure threshold of muscle ischemia in a canine model. J Trauma
1994;37;50-58.
PV, Perry JJ, Murray PC. Compartment syndrome of the well leg as a
result of the hemilithotomy position: a report of two cases and review
of literature. J Orthop Trauma 2001;15:580-583.
FA, Mayo KA, Krugmire RB, et al. A model compartment syndrome in man
with particular reference to the quantification of nerve function. J
Bone Joint Surg Am 1978;59:648-653.
FA, Wyss CR, Krugmire RB, et al. The effect of limb elevation and
dependency on local arteriovenous gradients in normal human limbs with
particular reference to limbs with increased tissue pressure. Clin
Orthop 1980;150:187-195.
K, Llhowe DW, Vrahas MS, et al. Functional outcome after acute
compartment syndrome of the thigh. J Bone Joint Surg Am 2006;88:729-737.
BR, Strom DE. Compartment syndrome after closed intramedullary nailing
of the tibia: a canine model and report of two cases. J Orthop Trauma
1991;5:71-77.
BR, Thorderson PK. Measurement of ICP: a comparison of the slit
catheter, sideported needle, and simple needle. J Bone Joint Surg Am
1993;75:231-235.
SJ, Hargens AR, Owen CA, et al. The wick catheter technique for
measurement of intramuscular pressure. J Bone Joint Surg Am
1976;58:1016-1020.
SJ, Owen CA. Double incision fasciotomy of the leg for decompression in
compartment syndromes. J Bone Joint Surg Am 1977;59:184-187.
SJ, Owen CA, Hargens AR, et al. Acute compartment syndromes: diagnosis
and treatment with the aid of the wick catheter. J Bone Joint Surg Am
1978;60: 1091-1095.
H, Al-Abed K, Prasad C, et al. Outcome of compartment syndrome
following intramedullary nailing of tibial diaphyseal fractures. Injury
2001;32:411-413.
JM, Gorczyca JT, Cole JK, et al. Effect of acute reamed versus unreamed
intramedullary nailing on compartment pressure when treating closed
tibial shaft fractures: a randomized prospective study. J Orthop Trauma
2000;14:554-558.
J, Girling F, Jerrard W, et al. Fundamental instability of the small
blood vessels and critical closing pressures in vascular beds. Am J
Physiol 1951;164:330-344.
R, Schmidt AH, Hunter B, et al. Use of tissue ultrafiltration for
treatment of compartment syndrome: a pilot study using porcine
hindlimbs. J Orthop Trauma 2005; 19:267-275.
S, Hvaal K, Holm I, et al. Compartment pressure in nailed tibial
fractures. A threshold of 30 mm Hg for decompression gives 29%
fasciotomies. Arch Orthop Trauma Surg 1998;118:29-31.
ML, Ouellette EA, Livingstone A, et al. Acute pediatric upper extremity
compartment syndrome in the absence of fracture. J Pediatr Orthop
2009;29:263-268.
by the British Orthopaedic Association/British Association of Plastic
Surgeons working party on the management of open tibial fractures. Br J
Plast Surg 1997;50: 570-583.
IC, Shepherd JT. Evidence for critical closure of digital resistance
vessels with reduced transmural pressure and passive vasodilation with
increased venous pressure. J Physiol 1957;136:498-506.
CH, Castle GSP, Hardie R, et al. Compartmental pressure measurements:
an experimental investigation using the slit catheter. J Trauma
1981;21:446-449.
CH, Macnab L. Anterior tibial compartment syndrome complicating
fractures of the shaft of the tibia. J Bone Joint Surg Am
1976;58:549-550.
JT, Brumback RJ, Lakatos R, et al. Acute compartment syndrome of the
thigh: a spectrum of injury. J Bone Joint Surg Am 1989;71:392-400.
JG, Valadie AL, Frederick RW, et al. Perioperative compartment
syndrome. A report of four cases. J Bone Joint Surg Am 1996;78:600-602.
S, Carmel A, Cohen Y, et al. Iatrogenic forearm compartment syndrome in
a cardiac intensive care unit induced by brachial artery puncture and
acute anticoagulation. J Interv Cardiol 2002;15:107-109.
B, Menon M, O’Brien P, et al. Diagnostic techniques in acute
compartment syndrome of the leg. J Orthop Trauma 2008;22:581-587.
DT, Henderson NJ, Clough G. The slit catheter: a comparison with the
wick catheter in the measurement of compartment pressure. Injury
1982;13:404-408.
GW, Matsen FA, Krugmire RB. Further investigations on the
pathophysiology of the compartmental syndrome. Clin Orthop
1977;123:266-270.
MS, Reisman WM, Whitesides TE Jr, et al. Near infrared spectroscopy in
lower extremity trauma. J Bone Joint Surg Am 2009;91:1360-1368.
NS, Zionts LE, Holtom PD, et al. Acute hematogenous osteomyelitis. An
unusual case of compartment syndrome in a child. Clin Orthop
1995;317:210-222.
JR, Crenshaw A, Hargens AR. Intramuscular pressures during exercise.
Comparison of measurements with and without infusion. Acta Orthop Scand
1989;60:593-596.
HE, O’Brien F. Bilateral anterior tibial compartment syndrome in
association with the nephritic syndrome; report of a case. Arch Intern
Med 1965;116:487-488.
SD, Achterman CA, Hayhurst J, et al. Acute compartment syndrome in the
thigh complicating fracture of the femur. A report of three cases. J
Bone Joint Surg Am 1986;68:1439-1443.
CH, Burgess AR, Vanco B. Skeletal stabilization for tibial fractures
associated with acute compartment syndrome. Clin Orthop
1995;315:163-168.
T. The clinical diagnosis of compartment syndrome of the lower leg: are
clinical findings predictive of the disorder? J Orthop Trauma
2002;16:572-577.
RS, Paz IB, Nadeemanee A. Compartment syndrome of the leg secondary to
leukemic infiltration: a case report and review of the literature. J
Surg Oncol 1994;55:198-200.
GC, Richardson JD, George SM, et al. Fasciotomy for severe blunt and
penetrating trauma of the extremity. Surg Gynaecol Obstet
1988;166:397-401.
B, Westermann S, Menzer M. Microvascular response to compartment
syndrome-like external pressure elevation: an in vivo fluorescence
microscopic study in the hamster striated muscle. J Trauma
1999;46:91-96.
FC. Treatment of paralysis and muscular atrophy after the prolonged use
of splints or of an Esmarch’s cord. The Practitioner 1907;67:429-436.
CJ, Richardson MD, Lowe AJ, et al. Survey of management of acute
traumatic compartment syndrome of the leg in Australia. ANZ J Surg
2007;77:733-737.
TE, Haney TC, Morimoto K, et al. Tissue pressure measurements as a
determinant for the need of fasciotomy. Clin Orthop 1975;113:43-51.
TO, Howell GED, Will EM, et al. Elevated intramuscular compartment
pressures do not influence outcome after tibial fracture. J Trauma
2003;55:1133-1138.
PR, Russell ID, Mintowt-Czyz WJ. Compartment pressure
monitoring—current UK orthopaedic practice. Injury 1998;29:229-232.
C, Gerngross H, Sterk J. Measurement of intracompartmental pressure
with use of a new electronic transducer-tipped catheter system. J Bone
Joint Surg Am 1999;81: 158-168.
ASE, Curran P, McQueen MM. Backslabs and plaster casts: which will best
accommodate increasing intracompartmental pressures? Injury
1990;21:178-181.
