Skeletal Scintigraphy
Editors: Frassica, Frank J.; Sponseller, Paul D.; Wilckens, John H.
Title: 5-Minute Orthopaedic Consult, 2nd Edition
Copyright ©2007 Lippincott Williams & Wilkins
> Table of Contents > Skeletal Scintigraphy
Skeletal Scintigraphy
Heather A. Jacene MD
Description
-
Skeletal scintigraphy is a nuclear medicine imaging method for bone disease.
-
Advantages:
-
High sensitivity for early detection of disease
-
Whole body survey
-
-
Disadvantage:
-
Limited specificity
-
Signs and Symptoms
-
Indications
-
Whole body skeletal scintigraphy:
-
Primary bone tumors
-
Osteomyelitis
-
Joint prosthesis pain
-
Occult fractures
-
Stress fractures and shin splints
-
Spondylosis
-
Reflex sympathetic dystrophy
-
Fracture nonunion
-
AVN
-
Musculoskeletal pain, etiology uncertain
-
Heterotopic bone formation
-
Metastases to bone
-
Paget disease and fibrous dysplasia
-
-
TPSS:
-
Osteomyelitis
-
Stress fracture
-
Reflex sympathetic dystrophy
-
Osteoid osteoma
-
-
-
Radiopharmaceutical:
-
Technetium-99m-labeled phosphate and phosphonate compounds specifically localize in bone.
-
Regions of increased bone turnover (blastic response) have increased uptake.
-
Tests
-
Technique
-
Whole body skeletal scintigraphy:
-
20–30 mCi of technetium-99m-diphosphonate
-
Intravenous injection of radiopharmaceutical
-
Whole body imaging, 2–4 hours after radiopharmaceutical injection
-
-
TPSS (20–30 mCi of technetium-99m-diphosphonate):
-
Flow phase (phase 1): Bolus intravenous
injection; 1–3-second images for 60 seconds, beginning at time of
injection; spot imaging of area of interest for 1 minute to evaluate
for the presence of altered regional blood flow -
Extracellular (blood pool) phase (phase 2): High-count images immediately after the flow phase in various views
-
Delayed phase (phase 3): Whole body
imaging 2–4 hours after radiopharmaceutical injection and special views
of the region of interest
-
-
Optional imaging (phase 4):
-
24-hour delayed imaging: More time for
tracer localization in bone; for patients with poor blood flow (e.g.,
diabetes, peripheral vascular disease) and renal failure -
SPECT: Improved contrast resolution and 3D cross-sectional images
-
-
Pathological Findings
-
Primary malignant bone tumors:
-
Hyperemia on flow phase
-
Intense technetium-99m-diphosphonate in tumor on delayed images
-
-
Primary benign bone tumors:
-
Uptake varies widely
-
Osteoid osteoma has high uptake.
-
Hyperemia on flow phase
-
Intense accumulation in the lesion on extracellular and delayed images
-
High sensitivity for lesion detection
-
Radionuclide-guided surgery can be performed.
-
-
-
Osteomyelitis:
-
Findings (“3-phase positive”):
-
Focal arterial hyperemia
-
Extracellular phase: Increased focal bone accumulation
-
Delayed phase: Increased focal bone accumulation
-
-
Cellulitis without osteomyelitis: Increased regional flow and blood pool, but no delayed uptake
-
Cellulitis and osteomyelitis: Flow and extracellular phase may show diffuse uptake, but delayed images show focal bone uptake.
-
90% accuracy in absence of complicating factors (e.g., hardware, recent fracture or surgery) (1), which may result in false-positives
-
Other conditions also result in similar findings that decrease the specificity of TPSS.
-
Clinical history and comparison with radiographs is essential.
-
Differential diagnosis of a 3-phase positive bone scan:
-
Fractures
-
Gout
-
Osteoarthritis
-
Charcot joint
-
Reflex sympathetic dystrophy
-
Healing phase osteonecrosis
-
Primary malignant bone tumors
-
Osteotomy
-
-
False-negatives:
-
Neonates
-
Elderly, particularly in setting of conditions that cause decreased blood flow (e.g., diabetes, peripheral vascular disease)
-
Antibiotics (Fig. 1)
-
-
-
Septic joint:
-
3-phase positive
-
Periarticular bone and joint space accumulation of radiopharmaceutical
-
-
Complimentary nuclear medicine imaging
-
Radiolabeled leukocyte scintigraphy:
-
Leukocytes migrate to sites of infection.
-
Pitfall: Leukocytes also accumulate at sites of normal bone marrow.
-
Good accuracy, combined with bone scanning
-
Leukocyte/bone marrow scintigraphy: Accuracy of 89–98% (2); based on fact that only leukocytes but not bone marrow agents (sulfur colloid) will accumulate at a site of infection.
-
-
Sequential TPSS/gallium-67 scan:
-
Interpretative criteria are based on comparison of tracer localization and intensity of tracer uptake on the 2 scans (3).
-
Superior to bone scanning alone in the presence of incongruent image findings (4)
-
Large number of equivocal cases decreases the sensitivity and limits the utility of this combined study (3).
-
-
Special Considerations
-
Vertebral osteomyelitis:
-
Intense uptake in adjacent vertebral bodies
-
Sensitivity of delayed bone imaging in 86–100% (5)
-
Combined bone/gallium scan increases specificity.
-
Radiolabeled leukocyte imaging of limited value: 40–50% false-negative rate (5)
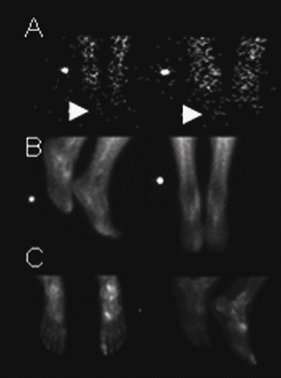 Fig. 1. 3-phase positive bone scan consistent with osteomyelitis of the right great toe. A: Flow phase with mildly increased flow to the right great toe. B and C: Increased tracer uptake in the right great toe on the extracellular and delayed phase images (C: Left image is plantar view).
Fig. 1. 3-phase positive bone scan consistent with osteomyelitis of the right great toe. A: Flow phase with mildly increased flow to the right great toe. B and C: Increased tracer uptake in the right great toe on the extracellular and delayed phase images (C: Left image is plantar view).
-
-
Diabetic foot:
-
TPSS: High negative predictive value for osteomyelitis
-
Forefoot:
-
Technetium-99m–exametazime-labeled leukocytes are the study of choice if the TPSS is positive or equivocal.
-
-
Mid- or hindfoot:
-
Neuropathic/Charcot joint can accumulate leukocytes in the absence of infection.
-
Study of choice: Combined indium-111-labeled leukocytes/technetium-99m-sulfur colloid
-
-
-
Arthritis:
-
Diffuse periarticular increase of radiotracer
-
Focal osteoblastic activity in subchondral bone
-
-
Occult fractures:
-
3-phase positive
-
Visualization on skeletal scintigraphy:
-
80% by 24 hours
-
95% by 72 hours in those <65 years old
-
Maximum sensitivity to detect fractures is at 7 days in patients >65 years old
-
-
Skeletal scintigraphy returns to normal (6).
-
Nondisplaced fractures: 60–80% at 1 year; 95% at 3 years
-
Displaced fractures: Positive indefinitely
-
-
-
Athletic injuries:
-
Stress fractures:
-
Positive 1–2 weeks before radiographic changes are visualized.
-
Intense, oval or fusiform uptake at fracture site
-
-
Shin splints:
-
Normal flow and extracellular phase
-
Mild to moderate, linear uptake on delayed imaging only
-
Most commonly, posteromedial aspects of bilateral tibias
-
-
-
Painful prosthesis evaluation:
-
Skeletal scintigraphy is nonspecific for differentiating prosthesis loosening and infection.
-
Typical appearance shows loosening on bone scan:
-
Increased uptake in region of greater and lesser trochanters and at tip of the prosthesis
-
-
Increased uptake can be seen up to 1 year
after cemented prosthesis placement and up to 2–3 years after
noncemented prosthesis placement. -
Study of choice is combined indium-111-labeled leukocytes/technetium-99m-sulfur colloid scan:
-
Sensitivity and specificities >90% (7)
-
False-positives (7): Displaced bone marrow (technetium-99m-sulfur colloid scan helps avoid this pitfall.)
-
-
-
Bone grafts:
-
Technetium-99m-diphosphonate scan can detect early changes in vascular patency if used within 1 week after surgery.
-
Vascularized graft: Normal or diffusely increased tracer uptake and focally increased uptake at osteotomy site
-
Failed graft: Photopenic defect
-
-
Pitfalls:
-
New bone formation on a nonviable graft
-
Osteoradionecrosis
-
Postoperative changes
-
-
-
Metastatic bone disease:
-
Metastatic bone disease from nonosseous tumors can present as several patterns on skeletal scintigraphy.
-
Multiple, randomly scattered lesions
-
Solitary lesion
-
Diffuse disease
-
Photopenic lesions
-
Reduced sensitivity and false-negatives with multiple myeloma, renal cell carcinoma, thyroid carcinoma, lymphoma
-
-
-
Spinal surgery:
-
Normal: Diffuse uptake at surgical sites secondary to new bone formation
-
Nonunion/pseudarthroses:
-
Focal areas of intense uptake
-
SPECT improves sensitivity
-
-
P.403
Pediatric Considerations
Young children may require sedation to remain still for the entire examination.
Pregnancy Considerations
-
Radiation exposure to a fetus from most studies using technetium-99m-labeled agent is <0.500 rad (8).
-
Fetal risk is considered negligible at radiation exposures of <5 rad (8).
-
However, the benefit of the single study
should outweigh the risk of the exposure to the fetus and be given
serious consideration.
-
Follow-up skeletal scintigraphy can assess:
-
Stability of metastatic bone disease
-
Residual or recurrent primary bone tumors
-
Assessment of response to therapy
-
Malignancy
-
Infections
-
Persistent nonunion at fracture sites
-
References
1. Maurer
AH, Chen DCP, Camargo EE, et al. Utility of three-phase skeletal
scintigraphy in suspected osteomyelitis: Concise communication. J Nucl Med 1981;22:941–949.
AH, Chen DCP, Camargo EE, et al. Utility of three-phase skeletal
scintigraphy in suspected osteomyelitis: Concise communication. J Nucl Med 1981;22:941–949.
2. Palestro
CJ, Roumanas P, Swyer AJ, et al. Diagnosis of musculoskeletal infection
using combined In-111 labeled leukocyte and Tc-99m SC marrow imaging. Clin Nucl Med 1992;17:269–273.
CJ, Roumanas P, Swyer AJ, et al. Diagnosis of musculoskeletal infection
using combined In-111 labeled leukocyte and Tc-99m SC marrow imaging. Clin Nucl Med 1992;17:269–273.
3. Palestro CJ, Torres MA. Radionuclide imaging in orthopaedic infections. Semin Nucl Med 1997;27:334–345.
4. Rosenthall L, Lisbona R, Hernandez M, et al. 99mTc-PP and 67Ga imaging following insertion of orthopaedic devices. Radiology 1979;133: 717–721.
5. Palestro
CJ, Kim CK, Swyer AJ, et al. Radionuclide diagnosis of vertebral
osteomyelitis: Indium-111-leukocyte and technetium-99m-methylene
diphosphonate bone scintigraphy. J Nucl Med 1991;32:1861–1865.
CJ, Kim CK, Swyer AJ, et al. Radionuclide diagnosis of vertebral
osteomyelitis: Indium-111-leukocyte and technetium-99m-methylene
diphosphonate bone scintigraphy. J Nucl Med 1991;32:1861–1865.
6. Matin P. The appearance of bone scans following fractures, including immediate and long-term studies. J Nucl Med 1979;20:1227–1231.
7. Palestro
CJ, Kim CK, Swyer AJ, et al. Total-hip arthroplasty: Periprosthetic
indium-111-labeled leukocyte activity and complementary
technetium-99m-sulfur colloid imaging in suspected infection. J Nucl Med 1990;31: 1950–1955.
CJ, Kim CK, Swyer AJ, et al. Total-hip arthroplasty: Periprosthetic
indium-111-labeled leukocyte activity and complementary
technetium-99m-sulfur colloid imaging in suspected infection. J Nucl Med 1990;31: 1950–1955.
8. Toppenberg KS, Hill DA, Miller DP. Safety of radiographic imaging during pregnancy. Am Fam Physician 1999;59:1813–1820.
Additional Reading
Holder L, ed. Orthopaedic nuclear medicine (part I). Semin Nucl Med 1997;27:309–400.
Holder L, ed. Orthopaedic nuclear medicine (part II). Semin Nucl Med 1998;28:3–131.
Palestro CJ. Radionuclide imaging after skeletal interventional procedures. Semin Nucl Med 1995;25:3–14.
Patient Teaching
-
Minimal preparation is required for patients undergoing skeletal scintigraphy.
-
Patients should be advised of the following:
-
The total time of the test from the injection of radiotracer until completion of imaging is ~4 hours.
-
Patients will be required to lie flat and still for image acquisition.
-
If patients have severe pain that will not permit them to lie still, then pain medication should be considered.
-
After radiotracer injection, patients should drink plenty of fluids to reduce the radiation dose to the bladder and kidneys.
-
FAQ
Q: How long after a fracture occurs will the bone scan return to normal?
A:
Most (60–90%) nondisplaced and uncomplicated fractures return to normal
in 1 year; 95% return to normal in 3 years. Displaced and comminuted
fractures and fractures around joints can have prolonged or indefinite
positivity.
Most (60–90%) nondisplaced and uncomplicated fractures return to normal
in 1 year; 95% return to normal in 3 years. Displaced and comminuted
fractures and fractures around joints can have prolonged or indefinite
positivity.
Q: Which test should be ordered to distinguish between loosening and infection of a joint prosthesis?
A:
In this setting, an indium-111-labeled- or technetium-99m-exametazime
white blood cell study is the most accurate test. White blood cells
accumulate in areas of infection but not in areas of bone remodeling
(loosening). Because bone marrow may be displaced during surgery and
normally accumulates labeled white cells, a technetium-99m-sulfur
colloid study often is performed in conjunction with a white cell
study. Infection is diagnosed in areas that accumulate white cells but
not sulfur colloid.
In this setting, an indium-111-labeled- or technetium-99m-exametazime
white blood cell study is the most accurate test. White blood cells
accumulate in areas of infection but not in areas of bone remodeling
(loosening). Because bone marrow may be displaced during surgery and
normally accumulates labeled white cells, a technetium-99m-sulfur
colloid study often is performed in conjunction with a white cell
study. Infection is diagnosed in areas that accumulate white cells but
not sulfur colloid.
