HETEROTOPIC OSSIFICATION AND CHARCOT NEUROARTHROPATHY
VII – NEOPLASTIC, INFECTIOUS, NEUROLOGIC AND OTHER SKELETAL DISORDERS
> Other Disorders > CHAPTER 124 – HETEROTOPIC OSSIFICATION AND
CHARCOT NEUROARTHROPATHY
is termed heterotopic ossification (HO) or heterotopic bone.
Histologically, it differs from soft-tissue calcification in terms of
its osteoblastic activity. Myositis ossificans is histologically
identical to HO but, by definition, forms in inflammatory muscle.
Periarticular calcification does not manifest the radiologic features
of organized bone and is usually limited to distinct structures, such
as the collateral ligaments. HO is metabolically active and
histologically similar to native bone, with increased numbers of
osteoblasts and osteoclasts.
it is clearly associated with certain clinical situations. HO can
result in severe limitation of motion and ankylosis. Chalmers et al. (19)
proposed three conditions necessary for HO formation: an osteogenic
precursor cell, an inducing agent, and an environment conducive to
osteogenesis. Mesenchymal cells, which have the ability to
differentiate to osteogenic stem cells, are found in the soft tissues
surrounding joints. Osteoinductive substances are probably released as
a result of the insult, and they may cause a proliferation of
mesenchymal cells. Growth factors, such as bone morphogenetic proteins
(BMPs), are potential inducers of undifferentiated mesenchymal cells to
proliferate and differentiate into cartilage and bone and may play a
role in the formation of HO (125,126).
risk has been investigated with bone scans and with laboratory tests,
such as erythrocyte sedimentation rates (ESRs) and alkaline phosphatase
levels. The results of these tests do not always correlate well with
the development of the condition Theoretically, an immediate
postoperative change
in
the serum level of substances related to osteoblast activity, such as
osteocalcin, circulating growth factors, or the bone isoenzyme of
alkaline phosphatase, might aid in the selection of high-risk patients
needing prophylaxis. Most investigators have observed an increase in
the serum alkaline phosphatase up to 3.5 times normal, beginning in the
first month of HO and peaking at about 3 months (84).
However, by the time these levels are noted to be elevated, the process
is well established and prophylaxis is unlikely to be useful.
literature with regard to which test is the most accurate predictor of
HO formation and maturation. Despite this, most surgeons usually allow
a triple-phase bone scan and alkaline phosphatase to return to baseline
before resecting heterotopic bone. Computerized tomography is often
useful for operative planning.
the Brooker classification, which is based on the extent of HO seen on
the AP radiograph of the hip (Table 124.1). Wright et al. (131)
tested the reliability and validity of this grading system. The
intraobserver reliability between two surgeons was 86% and 77%, and the
interobserver reliability was 68%. Although it is useful in determining
the radiographic appearance of the HO, the Brooker classification does
not address its functional effect.
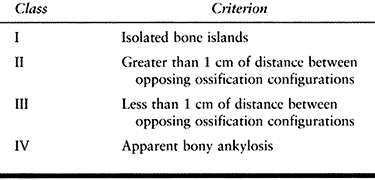 |
|
Table 124.1. Brooker Classification of Heterotopic Ossification (15)
|
mainstays of this prevention are nonsteroidal anti-inflammatory agents
(NSAIDs), ionizing radiation, and meticulous handling of surgical
tissue.
related to their inhibition of the activity of prostaglandin
synthetase. The resulting decrease in local concentrations of
osteoactive prostaglandins may be key to the inhibition of formation of
heterotopic bone. The change in environment tends to inhibit the
proliferation and maturation of pluripotential osteogenic precursors
and limits the development of heterotopic bone. Indomethacin has been
shown in an animal model to be effective only if administered in the
initial phase of bone induction (81). In
studies of animals, NSAIDs have been shown to delay the healing of bone
after local trauma. Rats that had been fed an aspirin-rich diet
revealed a dose-dependent delay in fracture healing (4). Dahl (24)
first reported on the use of indomethacin after total hip arthroplasty.
Its use as an analgesic showed reduction of HO compared with the use of
a placebo. Since that time, numerous studies have been conducted to
compare the different anti-inflammatory agents with varying dosing and
length of treatment. Because of the potential side effects of
dyspepsia, bleeding, and inhibited bone healing, much emphasis has
focused on finding the lowest dose and shortest length of treatment,
without compromising the results of suppression of HO formation.
Coumadin has been proven to be ineffective (103).
therapy. Most of their work centers around the use of such therapy
after total hip replacement (23,25,43,47,48,51,59,71,74,78,86,106,107,127).
procedure, especially around the hip and elbow, is also of importance
to minimize any trauma and subsequent inflammation. The role of proper
surgical technique in the formation of heterotopic bone is difficult to
quantitate.
had encouraging results with the transplantation of fat after excision
of HO, theorizing that filling the gap created in the soft tissues
would prevent recurrence. Biphosphonates have been employed because
they inhibit the transformation of amorphous calcium phosphate into
hydroxyapatite and thereby the mineralization of osteoid matrix.
Unfortunately, mineralization occurs when the medicine is discontinued.
Thomas and Amstutz (120) found no difference
when they compared biphosphonates to a placebo 2 years after the
operation. Furthermore, the medication is expensive and can adversely
affect calcium and phosphate metabolism, leading to conditions that
include osteomalacia.
Certain patients are at a higher risk for developing clinically
significant HO: those with ankylosing spondylitis, osteoarthritis with
large acetabular osteophytes,
Forestier’s
disease, Paget’s disease, bilateral disease, extensive operative
trauma, formation of a hematoma, and those who have developed HO after
a previous total hip arthroplasty. Men with osteoarthritis are at
higher risk for HO than women (93). Ahrengart and Lindgren (3)
concluded that men with osteoarthritis, men with fractures, women older
than 65 with osteoarthritis, and women with hypertrophic osteoarthritis
should be considered for prophylactic treatment.
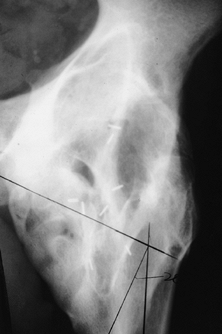 |
|
Figure 124.1. AP hip demonstrating Brooker IV heterotopic bone formation.
|
total hip arthroplasty without prophylactic treatment approximates 30%,
with a reported range from 5% (21) to 90% (99) (Table 124.2).
Of those who develop HO, 3% to 10% have pain and decreased range of
motion as a direct result of the HO. Maloney and Krushell (72)
found a significant increase in the severity of HO with uncemented
total hip arthroplasty as compared with hybrid arthroplasty. The rate
of clinically significant HO in cementless total hip arthroplasty is
approximately 30% (62).
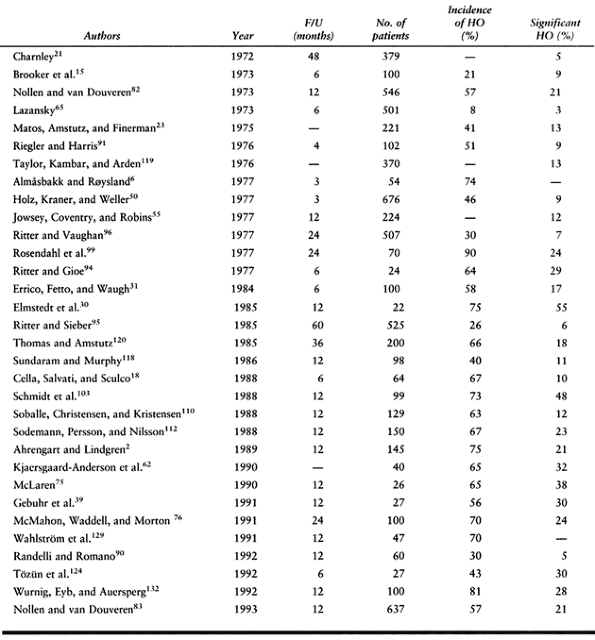 |
|
Table 124.2. Incidence of Heterotopic Ossification After Total Hip Arthroplasty
|
after operation and matures by 3 to 6 months. Histologically, HO is of
the membranous type and has a woven structure that is later transformed
into a more organized trabecular bone. Six months to 1 year after the
operation, the rate of remodeling decreases, and the tissue tends to
stabilize (Fig. 124.2).
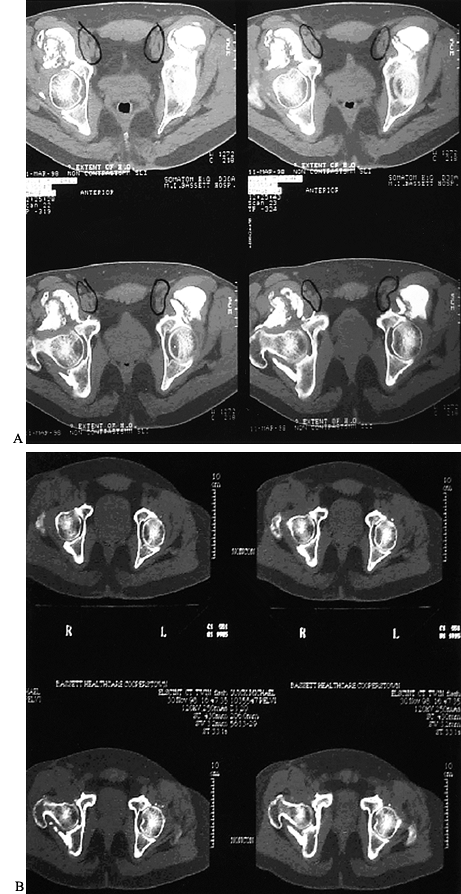 |
|
Figure 124.2. CT scan of a patient with Brooker IV heterotopic bone formation before and after resection.
|
found that 30% of their patients could not be treated with indomethacin
because of side effects. Indomethacin has been the drug of choice by
many physicians, although a recent study found no difference between
enteric-coated aspirin and indomethacin (60).
Because of animal studies showing decreased bone healing after NSAID
use, several authors have studied cementless total hip arthroplasty
results. Kjaersgaard-Anderson et al. (62) reported that NSAIDs had no effect on the clinical results of cementless total hip arthroplasty.
 |
|
Table 124.3. Previous Studies of Indomethacin to Prevent Heterotopic Ossification After Total Hip Arthroplasty
|
Radiation therapy is delivered locally and not systemically. Ionizing
radiation exerts its greatest influence on rapidly dividing cells by
altering nuclear DNA. It is believed that radiation therapy prevents
the differentiation of the pluripotential mesenchymal cells into
osteoblastic stem cells (32). Radiation therapy
may effectively block one of the earliest steps in a series of events
leading to H.O. It is imperative to begin the therapy immediately to
block the differentiation of stem cells, which peaks at 48 hours. It is
delivered through anteroposterior portals centered on the prosthesis,
which exposes the periarticular tissue but not the surrounding
radiosensitive organs.
 |
|
Table 124.4. Incidence of Heterotopic Ossification After Prophylactic Irradiation
|
wound breakdown. Single-dose protocols improve accuracy of radiation
delivery to the specific field, and minimizes patient discomfort,
transport, and scheduling issues. When irradiating a cementless total
hip arthroplasty, exclude the areas of porous coating. The radiation
portal is rectangular in shape, and the exposed field includes the area
surrounding the prosthetic femoral neck, the region between the lesser
trochanter and the ischium, and the lateral region between the greater
trochanter and the ilium.
wound healing or wound complications, providing that the incision is
excluded from the field of irradiation. Trochanteric nonunion after
radiation therapy in several studies of revision total hip arthroplasty
ranged from 25% to 43%. This compares with the incidence of
trochanteric nonunion in the absence of radiation of 2% to 15%. A
comparison is difficult to make because the bone quality in revision
total hip arthroplasty is usually poorer. Longo et al. (70)
studied bone ingrowth in rabbits after porous prostheses with radiation
therapy. The greatest delay of bony ingrowth was at 2 weeks, but by 6
weeks, the control group and irradiated groups were similar. No
radiation therapy protocol has been discontinued because of patient
intolerance, as has been reported with anti-inflammatory agents.
investigated by many authors. No soft-tissue sarcomas have been
reported with less than 3000 cGy (12). Radiation in low doses has been associated with the development of leukemia (109).
Although no definitive statement can be made regarding the risk of
development of sarcoma after prophylaxis with radiation therapy, this
possible side effect must be considered. The resistance of peripheral
nerves to radiation, combined with low-dose techniques, has led to no
documented cases of radiation-induced neuritis (74).
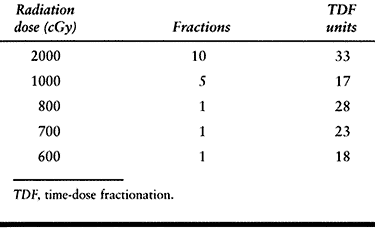
effect on tissue than fractionating doses. This greater tissue effect is referred to as the time-dose relationship (79). Table 124.5 shows a comparison of the time-dose fractionation (TDF) of various protocols.
 |
|
Table 124.5. Tissue Effect from Radiation Therapy
|
of acetabular fractures. It is seen in only about 5% of patients
treated conservatively (87) but in as many as 90% after extensile approaches to acetabular fractures (66). Severe HO has been reported in approximately 24% of patients (56).
Certain types of exposures, including the Kocher-Langenbeck, extended
iliofemoral, and triradiate, have resulted in a high occurrence of HO,
whereas the ilioinguinal exposure has not. The extended iliofemoral
approach has the highest prevalence of HO (66).
Postoperative HO prophylaxis is not required for fractures treated
through the ilioinguinal exposure. Prophylaxis with either NSAIDs,
single-dose local field radiation, or a combination should be used
routinely early after open reduction and internal fixation with
approaches other than the ilioinguinal approach. Moed and Letournel (78)
reviewed 53 patients who underwent the posterior or extended
iliofemoral approach and were treated with indomethacin and
irradiation. Indomethacin was given for 3 weeks, and radiation therapy
was given, either in divided or a single dose. Forty-four fractures
showed no HO, and 10 had grade I fractures. There was no difference
between radiation doses.
information about the incidence, pathophysiology, and treatment has
been applied to the upper extremity. HO around the elbow is associated
with local injury, electrical and thermal burns, and neurologic injury
to the brain and spinal cord. The most frequent cause of heterotopic
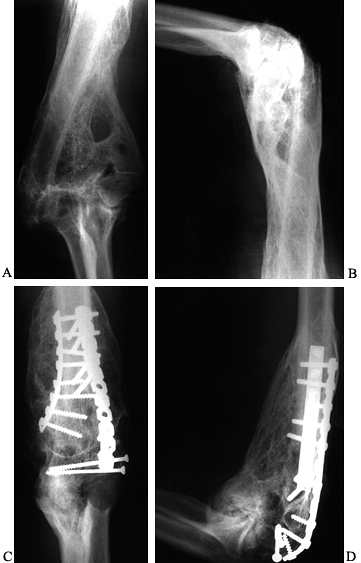
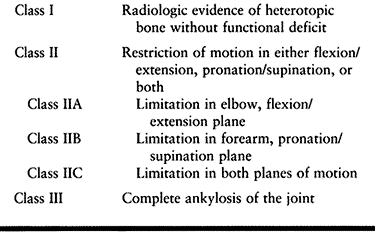
bone about the elbow is direct trauma (Fig. 124.3) (46).
There appears to be a direct correlation between the frequency of HO
and the magnitude of the injury. HO is usually seen in the collateral
ligaments when it is associated with trauma.
 |
|
Figure 124.3. A, B:
Twenty-one-year-old patient with head injury resulting from attempted suicide, with signs of heterotopic bone formation bilaterally. C,D: HO following fracture and fixation of a distal humerous fracture. |
sustained burns, 3% of patients with a local injury of the elbow, and
11% of patients with a head injury (36,88,121).
The elbow is the most frequent site after a burn. When a fracture
combined with a dislocation occurs, the incidence rises to 15% to 20%.
The incidence of HO about the forearm is approximately 2% to 4%, with
high-energy and open fractures most at risk. HO around the elbow,
associated with head injury, has been investigated by Garland and
O’Hollaren (38). The incidence of HO increases fifteenfold when the neurotraumatized patient has an associated elbow injury.
 |
|
Table 124.6. HO of Elbow and Forearm
|
 |
|
Table 124.7. Treatment Algorithm For Elbow Heterotopic Ossification
|
Early after injury, there is no completely effective prophylaxis. The
difficulty with the elbow is its smaller size, compared with the hip;
therefore, a small amount of bone results in more functional
limitations. Bridging bone in one plane is amenable to excision with
good results, whereas synostosis in two or more planes has poorer
results. Most surgeons wait 12 to 18 months before excision, with
recurrence demonstrated frequently in patients (Table 124.7) (37,88,97).
There is an advantage to early excision, particularly in those with
neurologic injury. This allows for early rehabilitation, enhancing
function and independence. In a recent review of eight patients who had
excision of HO at an average of 7 months followed by radiation therapy,
there was no recurrence and significant improvement in arc of motion (74). This compares with previous reports of recurrence rates up to 30% (37).
Because the preoperative testing for maturation of HO is not reliable
for recurrence, individualize the decision for excision.
posterolateral aspect of the elbow, from the olecranon to the lateral
humeral condyle. The elbow can be approached by various incisions
dictated by the location of HO (see Chapter 1).
Postoperatively, early motion is imperative, with the continuous
passive motion device used commonly. Initiate irradiation and
anti-inflammatory medications within 72 hours of surgery.
late 1800s, whereas its association with traumatic brain injury was first reported by Roberts in 1968 (98). The incidence of HO in adults who have had an injury to the brain ranges from 11% to 76% (100). Garland et al. (34,35,36 and 37)
have studied the traumatic brain injury patient in depth. The frequency
of HO in the upper extremity parallels that in the lower extremity. The
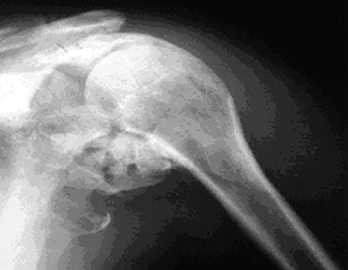
incidence of HO in the shoulder is similar to that in the elbow (Fig. 124.4).
Involvement of the upper extremities is more frequent in patients who
have had injuries to the brain than in those with spinal cord injuries (34).
 |
|
Figure 124.4. Brain-injured patient with ankylosed shoulder.
|
Patients with spinal cord injuries usually present with HO anteromedial
to the hip in the vicinity of the lesser trochanter, whereas in
traumatic brain injury patients, the location is more variable. New
bone formation in the head-injured patient is usually periarticular,
whereas patients with spinal cord injury develop HO at a distance from
the joint.
neurologically injured patients, the elbow most often becomes
ankylosed. New bone formation in the hip and elbow correlate with the
position of the extremity. It is thought that diffuse axonal injury
often produces spasticity, which is known to be associated with HO (1).
Patients with spastic quadriplegia and those with low levels of
recovery are particularly prone to the formation of HO and recurrence
after resection.
the lesion occurs after a spinal cord injury. Resection is usually
performed when serial alkaline phosphatase and bone scan have returned
to baseline. Brain-injured patients have a high variability of
neurologic involvement and severity, as well as variability in anatomic
location of HO. In a review of 23 patients with resection of HO in 37
joints, a recurrence of HO was found in 56%. The recurrence was closely
linked to the severity of cognitive deficit and physical disability (37).
NSAIDs. Biphosphonates are usually selected for prophylaxis if there is
a contraindication to NSAIDs. The size of the initial HO mass may be
the most important factor in predicting postoperative recurrence (116).
approach for resection. The shoulder is usually amenable to
manipulation and only uncommonly requires resection. Most resections
are carried out through a deltopectoral approach. The hip has three
areas of possible development of new bone: anterior, inferomedial to
the hip joint and distal to the lesser trochanter (associated with
adductor spasticity), and posterior to the femoral head (associated
with flexion contractures). The hip may be approached anteriorly or
posteriorly.
chronic form of a degenerative arthropathy that is associated with
decreased sensory innervation. It results in fragmentation,
destruction, and dislocation of the joints. Its most common locations
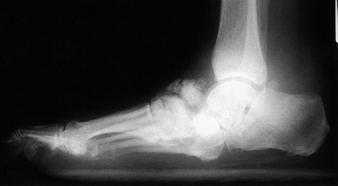
are the knee, ankle, and foot joints (Fig. 124.5 and Fig. 124.6).
 |
|
Figure 124.5. Lateral radiograph of a diabetic patient with Charcot arthropathy.
|
 |
|
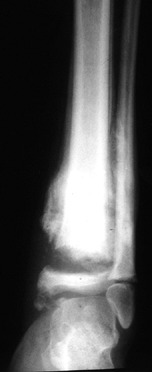
Figure 124.6. AP radiograph of a Charcot ankle in a skeletally immature girl with spina bifida.
|
described neuropathic arthropathy in 1868 as a “trophic effect” in a
patient with tabes dorsalis (20). He described
a process of severe osteoarthritic changes, including swelling and
instability of the knees. Prior to Charcot’s description, Mitchell (77a)
identified a destructive arthropathy associated with diseases involving
peripheral nerves. Volkmann and then Virchow proposed that the
underlying cause of the destruction was repeated traumatic events of
varying degrees unperceived by the insensitive joint. Steindler (44)
was one of the first to link together the destructive atrophic form and
the hypertrophic proliferative form, and to suggest that there were
many types and degrees of the condition. Before the advent of
antibiotics, syphilis was the most common cause involving primarily the
knee. The first report of arthropathy associated with diabetes was by
Jordan in 1936 (54), and even in 1966, the knee was still reported as the most common site (Table 124.8) (29).
 |
|
Table 124.8. Etiology of Charcot’s Neuroarthropathy
|
the three most common (in order) are diabetes, syphilis, and
syringomyelia. There are approximately 10 million diabetics in the
United States, with an incidence of Charcot arthropathy of 0.1% to 2.5%
(77) usually occurring after 10 years of the
disease. The most common diabetic complication requiring
hospitalization is foot disease. There is equal prevalence in men and
women and in type I and type II diabetes, and no correlation with
glucose
control.
Those with concomitant renal disease have a higher risk of
complications. Approximately 30% develop contralateral involvement (14).
The incidence of HO associated with tabes dorsalis is 6% to 10%. The
diabetic patient tends to have involvement of the tarsometatarsal
joints, tarsal, and ankle joints, whereas in syphilitic patients,
larger joints such as the knee are involved. The shoulder and elbow are
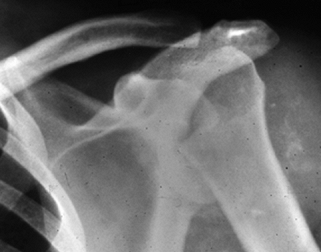
afflicted most commonly in syringomyelia (Fig. 124.7).
 |
|
Figure 124.7. Patient with syringomyelia causing Charcot’s arthropathy of the shoulder.
|
and neurovascular. The neurotraumatic theory proposed by Johnson in
1967 (53) involves repetitive trauma sustained
by a joint unable to sense pain. In cats, a denervated immobilized limb
does not produce a destructive arthropathy, but it can occur when the
cats are allowed to freely walk (16). This does
not account for the sensation of pain and neuroarthropathic changes
that can occur after acute fractures. It also does not explain the
gross destruction of joints that do not bear weight.
refers to a sympathetic dysfunction with persistent hyperemia and
active bone resorption by osteoclasts. As early as 1927, Leriche noted
that lesions of the sympathetic nerve led to an increase in blood flow (5). Blood flow may actually increase up to five times the normal rate in diabetic neuropathy.
brought on by a combination of factors that include damage to the
nociceptors of the joint and the periarticular tissues. This injury may
initially be caused by mechanical trauma, chronic infections such as
syphilis, or metabolic diseases such as diabetes associated with loss
of pain and proprioception. This leads to stretched ligaments, and lax
and subluxed joints. The activity of peptides such as substance P,
calcium gene–related peptide, and vasoactive intestinal peptide (VIP)
could result in increased vascularity and inflammation, contributing to
further joint destruction. Substance P can enhance the cellular
synthesis of collagenase and prostaglandin-E; activate T lymphocytes,
monocytes, and neutrophils; and take an active part in inflammation.
Glycolization of the supporting ligaments in the foot due to high
glucose levels in the blood can increase the stiffness and
cross-linking of the collagen fibers, making them brittle.
bone and cartilage. Recurrent effusions occur due to hyperplasia of the
synovium. The articular cartilage is slowly destroyed by a pannus,
which helps distinguish Charcot’s joints from other forms of
osteoarthritis. Histology of the fragments of cartilage and bone in
synovium shows deeply embedded tissue with normal metachromatic
staining. In contrast, in osteoarthritis the fragments are deposited
superficially and have staining characteristics suggestive of
degeneration (68).
for the early bone changes associated with Charcot’s neuroarthropathy
in diabetes mellitus. In a recent study by Gough et al. (41)
the serum carboxyterminal telopeptide of type 1 collagen, a marker of
osteoclastic bone resorption, had significantly increased levels in the
acute Charcot foot. The lack of an associated increase in osteoblastic
activity supports the idea that excess osteoclast activity is a feature
of the early stages of Charcot’s neuroarthropathy.
risk factor for ulceration in diabetics. In a study of 164 diabetic
patients, higher peak plantar pressures occurred in those with
Charcot’s arthropathy and those with neuropathic ulcers than in those
without. Measuring plantar pressures might be an effective means of
screening large numbers of patients (8).
diabetic patient is a single, painless, swollen, deformed foot and no
specific history of trauma. The joint has a large effusion, ligamentous
laxity, and increased warmth, but the peripheral white blood count
(WBC) and the ESR are normal. The swelling and warmth may suggest an
infection or pseudoseptic arthritis. The synovial fluid is clear, straw
colored, and viscous, although it can be bloody. Usually, there is
evidence of peripheral neuropathy in the presence of normal or bounding
pulses. Joint changes frequently precede the neurologic deficit, and as
many as one third of patients actually have pain at presentation (57).
The deep tendon reflexes at the knee are absent in a majority of
patients. Some patients present with nontraumatic dislocations or rapid
joint destruction without any known trauma. The spine is involved in 6%
to 21% of cases (40). Anand et al. (7)
reported on a patient with neuropathic spinal arthropathy in
Charcot-Marie-Tooth disease. Nonspiral fractures of the long bones
should heighten concern, because considerable force would normally be
necessary to cause such an injury. Rule out other disease processes
such as avascular necrosis (AVN), infection, crystal-induced arthritis,
hemophilia, and rheumatoid arthritis (RA).
to those observed in early osteoarthritis. Nontraumatic dislocations
may be an early sign. With time, there is radiographic evidence of
joint distention caused by fluid, hypertrophic synovitis, osteophytes,
and subluxation. The normal architecture of the joint is lost, with
dislocation, fragmentation, attempted repair by osteophytes, and
sclerosis. Various degrees of bone absorption have been observed, with
margins resembling surgical amputation coupled with periarticular
speckled calcification in the soft tissue. Infection usually produces
indistinct margins of bone as opposed to the sharp ones seen in
neuroarthropathy.
or cellulitis, with direct infectious spread to and through the cortex
of the adjacent bone. Establishing the diagnosis of osteomyelitis in
the foot may be difficult (see Chapter 116). Use imaging studies to confirm the clinical suspicion of osteomyelitis. Lipman et al. (67)
studied the diagnostic efficacy of combined three-phase bone
scintigraphy and indium-111–labeled WBC scintigraphy, magnetic
resonance imaging (MRI), and conventional radiography in detecting
osteomyelitis of the neuropathic foot. MRI was found to be comparable
to conventional radiography. In the midfoot and hindfoot, three-phase
or WBC scans were more specific than either MRI or conventional
radiography. MRI was most accurate in the forefoot.
uses both clinical and radiologic criteria. Stage I consists of an
acute, warm, swollen, erythematous foot. Radiographic changes of bony
fragmentation may occur and are often confused with osteomyelitis.
Stage II involves the beginning of the subacute process, in which
swelling subsides and new bone formation occurs. Stage III is chronic;
in it, the foot no longer is warm and swollen but is severely deformed.
Schon and Marks (105) have added a stage 0: the diabetic patient at risk for neuroarthropathy after an injury.
classification of neuroarthropathic joints that allows for recognition
of potential problems as well as helping to establish the prognosis
after treatment.
-
Type I neuropathy shows midfoot
manifestations in 60% to 70% of cases. It is characterized by
symptomatic medial and plantar bony prominences that may lead to
ulceration. A substantial indirect bending force is applied to the
ankle during the midstance and terminal-stance phases of gait. -
Type II involves the hindfoot,
representing 20% of Charcot’s joints; it is characterized by
instability and requires long periods of immobilization. -
Patients with type IIIA neuropathy have
an incidence of less than 10%. This type includes ankle injuries, and
are the most unstable and take the longest time to heal. -
Type IIIB involves the os calcis, which
presents clinically as a pathologic fracture of the tuberosity of the
calcaneus. These patients risk progressive pes planus and
tendo-Achilles incompetence. The Wagner classification (128) focuses on the progression of involvement of the soft tissue, which leads to eventual extension into the bone midjoints.
sound, plantigrade foot. The treatment of the injury should depend on
the timing, the stage of the injury, and the objective findings in the
physical exam. A choice must be made between conservative and operative
treatment based on the overall clinical scenario (Table 124.9). Education of the patient on the importance of foot care is of
paramount importance. Successful treatment results in a braceable ankle
without ulceration. The midfoot is afflicted most severely, followed by
the hindfoot and the ankle. Midfoot Charcot’s joints tend to become
more stable over time, in contrast to the hindfoot and ankle joint,
which become painful and prone to ulceration. Total-contact casts are
helpful in the management of the ulcers, with 86% of them healing
within 2 years (80).
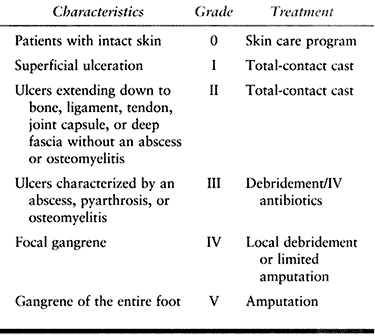 |
|
Table 124.9. Wagner Classification of Neuropathic Ulcerations (128)
|
mainstay in managing this condition in the early stages of the disease
process. Immobilization has been proven to slow down and sometimes
arrest destruction (28). Early stability helps
prevent bony prominences from causing ulceration. The length of
immobilization varies with the clinical exam. Saltzman et al. (100)
found that a properly fitted patellar tendon–bearing (PTB) brace can
reduce load transmission to the Charcot hindfoot. Selby et al. (108)
showed that biphosphonates can produce prompt reduction in the Charcot
activity. Ultrasound has been successful in the treatment of 28 of 29
patients with Charcot’s neuropathy of the foot and ankle (64). There are many who recommend protected weight bearing of the contralateral uninvolved foot and ankle (49).
Some patients develop deformity despite proper treatment or present
with significant deformity. This is especially true of patients who
have a varus or valgus deformity associated with involvement of the
ankle or subtalar joint. The foot is at risk for recurrent ulceration
and infection.
with serial-contact casting and avoidance of weight bearing until the
acute edema has resolved. Surgery can be considered for severe
dislocation that is unstable and manually reducible. Bone fragmentation
is a contraindication for surgery.
healing (stage II), allow progressive weight bearing. Replace the cast
with a brace once healing occurs. Johnson (53)
has shown that bracing is frequently effective in the treatment of the
deformed neuropathic foot. Bracing for ankle, hindfoot, and midfoot may
include a Charcot restraint orthotic walker, a double upright metal
foot orthosis, or a modified ankle-foot orthosis. The forefoot can be
managed with well-padded total-contact inserts.
Eichenholtz III, he or she can be managed in a long-term brace or with
continued use of the shoe insert. If the patient regresses back to the
acute stage, he or she must be placed into a total-contact cast and
weight bearing must be restricted. Bracing is successful in arresting
the deformity and providing a stable ankle-foot complex, and it is the
treatment of choice. It is imperative to evaluate the skin and soft
tissues at regular intervals for evidence of breakdown. Once the
deformity continues to progress with instability, the soft tissues
overlying the bone may begin to break down. Although the ulceration and
infection can be treated with local wound care and antibiotics, the
underlying cause is the deformity. With continued use of the extremity,
a cycle of breakdown and infection occurs. Bracing and foot and ankle
appliances are frequently not effective at this point. The deformed
limb ceases to be functional and requires surgery.
noted that nondisplaced neuropathic ankle fractures typically healed
uneventfully with casting and bracing. Closed reduction and casting for
displaced fractures has often resulted in loss of reduction and
progressive deterioration. Open reduction and internal fixation
provides better results (122). Fracture stability may be enhanced by multiple syndesmotic screws.
reported on six ankle fractures in five patients with longstanding
diabetes. One patient needed an amputation 10 days after a closed
injury, stressing that significant complications can occur.
disease because of the poor quality of the bone and the difficulty in
achieving a stable biomechanical construct with screws, plates, or
external fixation. The goal is a plantigrade foot with the ankle at
90°, the hindfoot in 5° to 10° of valgus angulation, and external
rotation of the foot with respect to the tibia equivalent to that of
the contralateral foot (85). Results of surgery have varied between series. Bono et al. (11a) performed 11 arthrodesis procedures for Charcot’s joint and achieved clinical union with good stability in 91%. Papa et al. (85) reported a 93% clinical success rate despite a 31% pseudarthrosis rate. Stuart and Morrey (117) reported on ankle arthrodesis procedures on 13 patients, with clinical union in seven patients. Pinzur and Kelikian (89)
reported on the stabilization of patients with a retrograde
intramedullary locked nail that had failed rigorous nonoperative
measures. Nineteen of 21 ankles progressed to fusion at an average of
20 months.
talectomy and a tibiocalcaneal arthrodesis. Consider ankle
disarticulation (Symes amputation) before ankle fusion in the most
severe cases to avoid progression to a more proximal amputation in the
future. Postoperatively, do not allow weight bearing. Observe the
patient closely for postoperative complications such as ulceration,
infection, and pseudarthrosis.
ulceration and deformity despite casting, bracing, and shoe
modifications. Exostectomy is most effective in the chronic, midfoot
deformity that is stable. If the foot is unstable, realignment and
arthrodesis is the procedure of choice. Early and Hansen (27)
reported on 21 patients with fusions who had midfoot collapse. Despite
50% postoperative complications and two amputations, 86% of patients
had successful results.
advocate immobilization in plaster. Consider arthrodesis for hindfoot
valgus with subluxation of the subtalar joint or midtarsals to prevent
ulceration and infection. Tisdel et al. (122a)
reported on eight triple arthrodeses in seven patients with 100% union
for peritalar neuroarthropathy. They stressed the principles outlined
by Papa et al. (85):
-
Careful removal of cartilage and debris.
-
Thorough removal of sclerotic bone.
-
Adequate fashioning of congruent bone surfaces for apposition.
-
Rigid fixation of the arthrodesis site.
-
Complete resection of fibrotic capsular tissue and synovium.
patients develop limitation of function that necessitates a
modification of their lifestyle (58). There is
an increased energy requirement to walk after amputation, which can be
a problem in diabetics with other comorbid conditions. There is also a
risk of an amputation on the contralateral limb, so limb salvage should
be attempted whenever possible.
joint, the critical element in treating a patient with a neuropathic
hip joint is to establish the diagnosis (Fig. 124.8) (9).
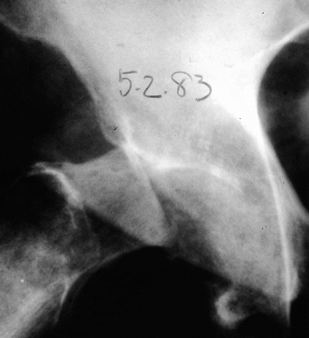 |
|
Figure 124.8. AP hip demonstrating Charcot’s arthropathy.
|
intervention is required. If pain is present and function is impaired,
pursue conservative treatment with protected weight bearing as long as
possible before considering any type of surgical procedure. Treatment
with hip arthrodesis results in a high rate of nonunion; historically,
it has been as high as 100% (53). Total hip
arthroplasty also has a high failure rate, especially in patients with
significant neurologic findings or ataxia. The overwhelming evidence in
the literature is that these patients do poorly with any type of
prosthetic replacement. The number of patients treated with total hip
arthroplasty reported in the literature is small, reflecting the
relatively low incidence of neuropathic hips. They have universally
done poorly (93,114).
This limited evidence strongly indicates that prosthetic replacement of
the Charcot hip joint is contraindicated. Of the dozen or so cases
reported in the literature, only one patient was stated to have done
well with a total hip joint arthroplasty (114).
The remainder did poorly even when treated with a broad range of total
hip designs. Resorting to a resection arthroplasty may be the only
viable solution in the treatment of the painful hip in these patients.
found that over 50% of fractures of the femoral neck in diabetics
developed Charcot’s joints. Many times, the neuropathic nature of this
fracture is not recognized, and hip nailing or a prosthesis is
inserted, only to result in failure. Internal fixation has been
documented to do as poorly as prosthetic replacements. Treatment with
restricted weight bearing may be the most prudent option with a delayed
resection arthroplasty if the joint subsequently becomes painful.
reported on three patients who developed Charcot’s changes after open
reduction internal fixation for fractures of the acetabulum. None had
any previous evidence of a neuropathy.
been reported as the most common neuropathic joint problem secondary to
syphilis and one of the most difficult to treat (53).
There is tremendous leverage, predisposing to instability, and little
soft-tissue envelope to cushion the joint. When the knee is painless,
bracing is the treatment of choice. Johnson (53)
recommends a corrective osteotomy to prevent shearing stress, followed
by bracing for severely damaged knees, particularly with bilateral
involvement. If only one knee is involved and destruction is severe,
fusion is indicated. Bracing is imperative to promote a solid union.
evidence of repair. The potential for complications with immobilization
is high. Arthrodesis, usually the treatment of choice in advanced
disease of the knee, may fail because of nonunion and breakage of
fixation devices. Drennan (26)
found a 55% clinically successful arthrodesis in his series of patients
with knee fusion. Arthroplasty is generally considered contraindicated
in Charcot’s joints. Loosening and breakage of the prosthesis is more
likely to occur secondary to lack of proprioception, although the
results of nine total knee arthroplasties were considered excellent in
eight knees after a 3-year follow-up (113).
Correct ligamentous balancing, use of long-stemmed components, and
meticulous surgical detail are imperative for good long-term results.
Despite these good reported early results, total knee arthroplasty
should be approached in these patients with great caution because the
long-term results undoubtedly will not be as favorable.
distinct, potentially devastating pathologic conditions affecting
joints. Although the two problems are different, the key to the
successful treatment and outcome of each is early recognition of
patients at risk and taking the appropriate preventive measures. Once
the conditions have been established, their outcomes, regardless of
treatment, are less favorable.
scheme: *, classic article; #, review article; ! basic research
article; and +, clinical results/outcome study.
JH, Graham DI, Murray LS, Scott G. Diffuse Axonal Injury due to
Nonmissile Head Injury in Humans: An Analysis of 45 Cases. Ann Neurol 1982;12:557.
HL, Wase A, Bear WT. Indomethacin and Aspirin: Effect of Nonsteroidal
Anti-inflammatory Agents on the Rate of Fracture Repair in the Rat. Acta Orthop Scand 1980;51:595.
A, Bowerman J, Robinson R, Riley L. Ectopic Ossification following
Total Hip Replacement. Incidence and a Method of Classification. J Bone Joint Surg 1973;55-A:1629.
TJ, Fetto JF, Waugh TR. Heterotopic Ossification: Incidence and
Relation to Trochanteric Osteotomy in 108 Total Hip Replacement. Clin Orthop 1984;190:138.
D. Clinical Observations on Fractures and Heterotopic Ossification in
the Spinal Cord and Traumatic Brain Injured Populations. Clin Orthop 1988;233:86.
A, Abraha H, Li F, et al. Measurement of Markers of Osteoclast and
Osteoblast Activity in Patients with Acute and Chronic Diabetic Charcot
Neuroarthropathy. Diabet Med 1997;14:527.
D, Colmar M, Penot P. Heterotopic Ossification of the Hip: The
Influence of the Duration of Postoperative Treatment with Indomethacin
on the Prevention of Ossification and the Effect of Uncemented
Acetabular Implants on the Development of Ossification. J Orthop Surg 1993;7:144.
S, Chadha M, Pellegrini V, et al. Randomized Trial Comparing
Pre-operative Versus Post-operative Irradiation for Prevention of
Heterotopic Ossification following Prosthetic Total Hip Replacement:
Preliminary Results. Int J Radiat Oncol Biol Phys 1994;30:55.
WL, Lo TCM, Covall DJ, et al. Single-dose Radiation Therapy for
Prevention of Heterotopic Ossification after Total Hip Arthroplasty. J Arthroplasty 1990;5:369.
AK, Mead LP, Hendren DH. The Prevention of Heterotopic Bone Formation
following Total Hip Arthroplasty Using 600 Rad in a Single Dose. J Arthroplasty 1989;4:319.
M, Schutzer S, Tepper J, et al. Radiation-blocking Shields to Localize
Periarticular Radiation Precisely for Prevention of Heterotopic Bone
Formation around Uncemented Total Hip Arthroplasties. Clin Orthop 1990;257:138.
Ossification: Theoretical Consideration, Possible Etiologic Factors,
and a Clinical Review of Total Hip Arthroplasty Patients Exhibiting
This Phenomenon. The Hip: Proceedings of the Fifth Open Scientific Meeting of the Hip Society. St Louis: CV Mosby, 1977.
F, Bone L, Border J. Open Reduction and Internal Fixation of Acetabular
Fractures: Heterotopic Ossification and Other Complications of
Treatment. J Orthop Trauma 1991;5:439.
W, Gruen T, Chessin H, et al. Radiation Therapy to Prevent Heterotopic
Ossification after Cementless Total Hip Arthroplasty. Clin Orthop 1991;262:185.
P, Ritter MA. Short-term Treatment with Nonsteroidal Antiinflammatory
Medications to Prevent Heterotopic Bone Formation after Total Hip
Arthroplasty: A Preliminary Report. Clin Orthop 1992;279:157.
P, Sletgard J, Gjerloff C, Lund F. Heterotopic Bone Formation after
Noncemented Total Hip Arthroplasty: Location of Ectopic Bone and the
Influence of Postoperative Antiinflammatory Treatment. Clin Orthop 1990;252:156.
K, Salzer M, Eyb R, et al. Heterotopic Ossification with Total Hip
Endoprostheses in Various Models of Thrombosis Prophylaxis. J Arthroplasty 1988;3:1.
D, Barthel T, Karrer A, et al. Prevention of Heterotropic Ossification
after Total Hip Replacement. A Prospective, Randomized Study Using
Acetylsalicylic Acid, Indomethacin, and Fractional or Single-Dose
Irradiation. J Bone Joint Surg Br 1997;79:596.
TK. The Effect of Low Power Specifically Programmed Ultrasound on the
Healing Time of Fresh Fractures Using a Colles’ Model. J Orthop Trauma 1990;4:227.
B, Collier D, Carrera G, et al. Detection of Osteomyelitis in the
Neuropathic Foot: Nuclear Medicine, MRI, and Conventional Radiography. Clin Nucl Med 1998;23:77.
TC, Healy WL, Covall DJ, et al. Heterotopic Bone Formation after Hip
Surgery: Prevention with Single-dose Postoperative Hip Irradiation. Radiology 1988;168:851.
JA, Hedley AK, Weinstein AM. Comparative Effects of EHDP, Indomethacin
and Irradiation on Bone Ingrowth in a Porous Polyethylene Rabbit Model.
Eur Soc Biomat 1985; 5:195.
I, Keys H, Evarts C, Rubin P. Usefulness of Postoperative Hip
Irradiation in the Prevention of Heterotopic Bone Formation in a
High-risk Group of Patients. Int J Radiat Oncol Biol Phys 1984;10:49.
W, Krushell R. Incidence of Heterotopic Ossification after Total Hip
Replacement: Effect of the Type of Fixation of the Femoral Component. J Bone Joint Surg 1991;73-A:191.
J, Waddell J, Morton J. Effect of Short-course Indomethacin on
Heterotopic Bone Formation after Uncemented Total Hip Arthroplasty. J Arthroplasty 1991;6:259.
OS, Bauer HC, Brosjo O, Tornkvist H. Influence of Indomethacin on
Induced Heterotopic Bone Formation in Rats: Importance of Length of
Treatment and of Age. Clin Orthop 1986;207:239.
AJG, van Douveren FQMP. Ectopic Ossification in Hip Arthroplasty: A
Retrospective Study of Predisposing Factors in 637 Cases. Acta Orthop Scand 1993;64:185.
VV, Konski AA, Gastel JA, et al. Prevention of Heterotopic Ossification
with Irradiation after Total Hip Arthroplasty. J Bone Joint Surg 1992;74-A:186.
E, Michelsson J. Treatment of Para-articular Ossification after Total
Hip Replacement by Excision and Use of Free Fat Transplants. Acta Orthop Scand 1979;50:751.
MA, Vaughan RB. Ectopic Ossification after Total Hip Arthroplasty:
Predisposing Factors, Frequency, and Effect on Results. J Bone Joint Surg 1977;59-A:345.
CL, Johnson KA, Goldstein RH, Donnelly RE. The Patellar Tendon-bearing
Brace as Treatment for Neurotrophic Arthropathy: A Dynamic Force
Monitoring Study. Foot Ankle Int 1992;13:14.
SA, Kjaersgaard-Anderson P, Pedersen NW, et al. The Use of Indomethacin
to Prevent the Formation of Heterotopic Bone after Total Hip
Replacement. A Randomized, Double-blind Clinical Trial. J Bone Joint Surg 1988;70-A:834.
MH, Goldmann AR, Martus P, et al. Prophylactic Radiation Therapy for
Prevention of Heterotopic Ossification after Hip Arthroplasty. Radiology 1993;188:257.
MH, Martus P, Goldmann AR, et al. Preoperative Versus Postoperative
Radiotherapy for Prevention of Heterotopic Ossification. Int J Radiat Oncol Biol Phys 1994;30:63.
B, Persson P, Nilsson O. Prevention of Heterotopic Ossification by
Nonsteroid Antiinflammatory Drugs after Total Hip Arthroplasty. Clin Orthop 1988;237:158.
B, Persson PE, Nilsson OS. Periarticular Heterotopic Ossification after
Total Hip Arthroplasty for Primary Coxarthrosis. Clin Orthop 1988;237:150.
BJ, Amstutz HC. Results of the Administration of Diphosphonate for the
Prevention of Heterotopic Ossification After Total Hip Arthroplasty. J Bone Joint Surg 1985;67-A:400.
E, Cronkite E. Autoradiographic Studies of Cell Proliferation in the
Periosteum of Intact and Fractured Femora of Mice Utilizing DNA
Labeling with H3-thymidine. Proc Cos Exper Biol Med 1961;107:719.
R, Pinar H, Yesiller E, Hamzaoglu A. Indomethacin for Prevention of
Heterotopic Ossification after Total Hip Arthroplasty. J Arthroplasty 1992;7:57.
der Werf GJ, van Hasselt NG, Tonino AJ. Radiotherapy in the Prevention
of Recurrence of Paraarticular Ossification in Total Hip Prostheses. Arch Orthop Trauma Surg 1985;104:85.
C, Eyb R, Auersperg V. Indomethacin for Prevention of Ectopic
Ossification in Cementless Hip Arthroplasties: A Prospective 1-year
Study of 100 Cases. Acta Orthop Scand 1992;63:628.
