Nonprosthetic Treatment of Elbow Arthritis
IV – Elbow Reconstruction > Part C – Operative Treatment Methods
> 62 – Nonprosthetic Treatment of Elbow Arthritis
inflammatory disease that affects synovial joints. The prevalence is
approximately 1.0% of adults, and the disease is associated with
significant morbidity and mortality. The treatment of RA has changed
significantly over the past decade. In addition to new medications that
have improved the treatment of inflammatory arthritis, advancements in
elbow arthroscopy have resulted in the introduction of new surgical
techniques.
mechanism involves the interaction between an unknown exogenous antigen
and the host immune system that precipitates a response that recruits
and activates monocytes and macrophages. The activated monocytes and
macrophages release proinflammatory cytokines such as tumor necrosis
factor-α (TNF-α) and interleukin-1 (IL-1)
into the joint. The cytokines mediate joint destruction by activating
chondrocytes and fibroblasts that release metalloproteinases and
collagenases capable of cartilage and bone destruction. A second
proposed pathway for joint destruction is through deregulation of B
lymphocytes that subsequently produce rheumatoid factor and
autoantibodies and promote the formation of destructive immune
complexes.
disease process, and ≤60% of patients will have radiographic evidence
of erosions by 2 years. Intra-articular symptoms associated with RA can
be clustered into three groups: (a) pain, (b) restricted motion, and
(c) instability. Extra-articular symptoms secondary to joint synovitis
include local nerve compression, tenosynovitis, and tendon rupture.
common feature of RA. A secondary fibrotic reaction can occur in
response to the inflammation and results in decreased elbow flexion and
extension. If the radiocapitellar articulation is affected, forearm
rotation can be restricted. As the disease progresses, cartilage
destruction, bone loss, and ligament incompetence can result in joint
instability.
includes nonsteroidal anti-inflammatory medications and
disease-modifying antirheumatic drugs (DMARDs) such as methotrexate,
prednisone, sulfasalazine, and gold. Combination therapy with these
agents has been shown to decrease disease activity and reduce
radiographic progression of bone erosions. In most patients, this form
of treatment is adequate.
understanding of the alteration in the immune system that contributes
to the development of RA.3 For example, leflunomide (Arava) inhibits the synthesis of pyrimidine by activated T lymphocytes, thereby hindering the
T lymphocytes’ ability to initiate an inflammatory response. Etanercept
(Enbrel), infliximab (Remicade), and adalimumab (Humira) all exploit
strategies to bind the inflammatory cytokine TNF-α.
Anakinra (Kineret) is an IL-1 receptor antagonist that competes with
IL-1 and blocks the production of metalloproteinases that have been
shown to destroy cartilage and create bone erosions. Each of these
medications has demonstrated efficacy according to the definition of
improvement in rheumatoid arthritis guidelines described by the
American College of Rheumatology.4
|
TABLE 62-1 Classification
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||
failure of an appropriately supervised course of pharmacotherapy,
symptoms of sufficient severity to justify the risks of surgery, and
satisfactory general health to permit the safe performance of a
surgical procedure. Ensuring satisfactory general health may require
the assistance of rheumatologists, anesthesiologists, and general
internists. In general, synovectomy is considered in patients with
stage I or II disease and in select cases of stage III disease in young
individuals. More advanced disease, manifested by joint destruction and
mechanical instability, is likely to benefit most from total elbow
arthroplasty (Fig. 62-1).
involves radial head excision and synovectomy. This procedure is
usually performed through
a
lateral exposure using the Kocher interval between anconeus and
extensor carpi ulnaris. Radial head excision is performed to improve
forearm rotation and to increase joint exposure, thereby facilitating
synovectomy.
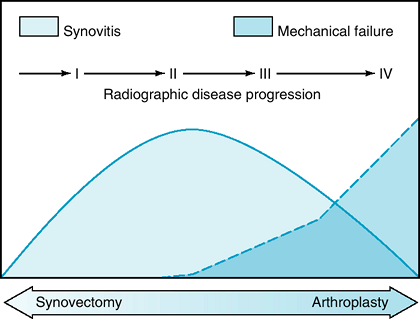 |
|
Figure 62-1 Inflammatory arthritis disease progression.
|
patients reporting diminished pain and improved range of motion. Ferlic
et al.5 reported that 77% of
patients described symptom relief following synovectomy and radial head
excision. A subgroup of patients treated with silicone radial head
arthroplasty was indistinguishable from the patients treated with
synovectomy and excision alone. The best results were observed in
patients with early disease. In a survivorship analysis, Gendi et al.6
identified factors associated with a poor outcome following open elbow
synovectomy including advanced disease, long duration of symptoms, a
significant reduction in the elbow flexion/extension arc, and poor
general health. Using severe pain or the need for revision surgery as
the end point, 80% of patients had a satisfactory response during the
first year following the intervention, but additional failures
accumulated at a rate of 2.6% per year.
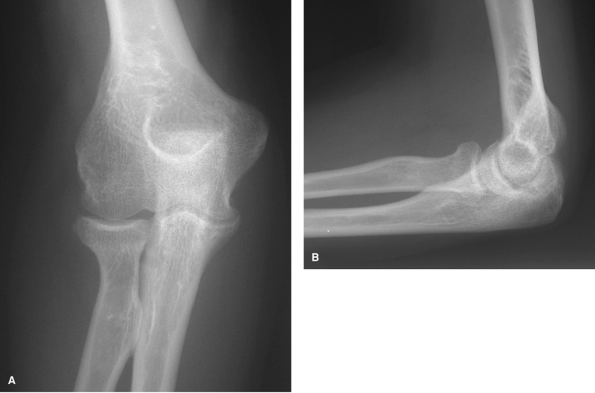 |
|
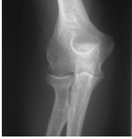
Figure 62-2 A: Stage I rheumatoid arthritis. B: Stage I rheumatoid arthritis.
|
found that 77% of elbow synovectomies did not require additional
surgery at a mean of 5 years following the primary synovectomy.
Patients undergoing synovectomy for late-stage arthritis were most
likely to be revised to an elbow arthroplasty. Elbow pain and patient
satisfaction both improved following synovectomy, but range of motion
was unchanged.
synovectomy are summarized in Table 61-2. Outcomes are difficult to
interpret because of methodologic shortcomings including a lack of
comparator groups, the use of nonstandardized outcome measures, and
deficient statistical measurements. The best indications for elbow
synovectomy, based on the papers cited, include pain, early
radiographic changes (I and II), and restricted elbow motion. Although
early results are rewarding, the durability of the results remains
questionable.
over open synovectomy. The improved joint visualization achieved with
arthroscopy allows for a more thorough synovectomy without sacrificing
the
radial head. The radiocapitellar articulation normally transmits 40% of
the forearm axial load across the elbow joint. Radial head excision may
result in accelerated wear of the ulnohumeral joint as a consequence of
altered joint reaction forces. Radial head excision can still be
performed if required using standard arthroscopic techniques. The
morbidity of synovectomy is reduced using portals that minimize muscle
injury and protect ligamentous stabilizers of the joint.
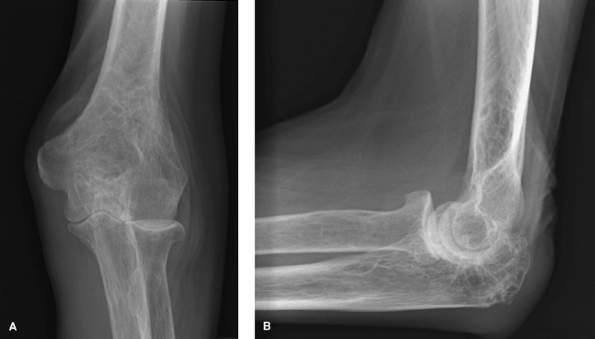 |
|
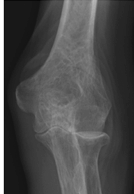
Figure 62-3 A: Stage II rheumatoid arthritis. B: Stage II rheumatoid arthritis.
|
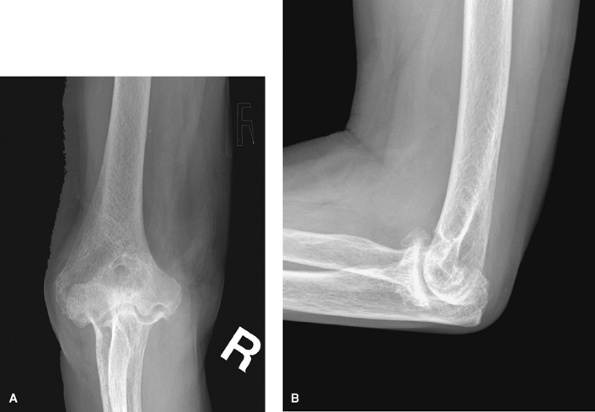 |
|
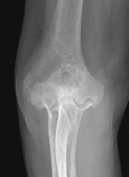
Figure 62-4 A: Stage III rheumatoid arthritis. B: Stage III rheumatoid arthritis.
|
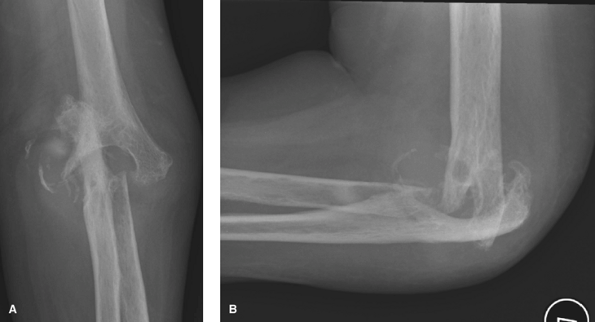 |
|
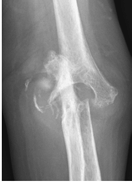
Figure 62-5 A: Stage IV rheumatoid arthritis. B: Stage IV rheumatoid arthritis.
|
a technically difficult procedure that requires advanced arthroscopy
skills. Furthermore, an assistant is required for limb positioning and
for intra-articular retraction of nerves and vessels to avoid
neurovascular injury. At the present
time, the superiority of arthroscopic synovectomy over open synovectomy has not been proven.
|
TABLE 62-2 Outcomes Reported Following Elbow Synovectomy
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
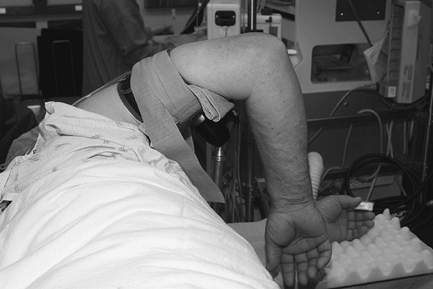 |
|
Figure 62-6
Patient positioned for elbow arthroscopy. The elbow is placed high to avoid contact with the arthroscope or other instruments against the patient. |
arthroscopic synovectomy. First, the surgeon must possess a thorough
knowledge of the three-dimensional anatomic relationships among nerves,
vessels, synovium, capsule, and articular structures. This allows for
safe portal placement and efficient insertion and removal of surgical
instruments. Second, arthroscopic irrigation fluid should not be
considered as a means to maintain joint distention but rather as the
medium to clear debris from the joint. Ideally, inflow pressure should
be minimized to prevent harmful fluid extravasations that obscure
surface anatomy landmarks and interfere with the use of arthroscopic
instruments. Third, a surgical assistant providing intra-articular
retraction helps to maintain joint visualization and to prevent
iatrogenic nerve injury. Finally, the procedure must proceed in a
sequential manner that minimizes the risk of complication. For example,
synovectomy precedes any planned bone resection since the synovium will
likely obscure the visualization of osseous structures within the
joint. Similarly, bone resection precedes planned capsulectomy since
satisfactory irrigation fluid management is difficult to maintain once
the capsule has been removed. By strictly adhering to the above
principles and by exercising patience during the procedure,
arthroscopic synovectomy can be safely performed.
 |
|
Figure 62-7 View of patient positioned for elbow arthroscopy.
|
reported the outcome of arthroscopic synovectomy in a series of 11
patients at 42 months following surgery and found that only 6 of the
patients continued to report a satisfactory result. Four of the
patients required revision surgery and were treated with total elbow
arthroplasty. Their recommendation was to cautiously advise patients
regarding arthroscopic synovectomy since the initial early satisfactory
response did not seem durable.
described the results of arthroscopic synovectomy in a series of 27
patients. Durable pain relief was observed at a mean follow-up of 97
months. The best results were obtained in patients with early disease
whereas the outcome in patients with more advanced disease was
unsatisfactory. Their conclusion was that arthroscopic synovectomy
could reliably relieve pain in patients with stage I or stage II
disease.
treatment for posttraumatic arthritis, there are several reports of the
outcome of interposition arthroplasty for the treatment of RA. A number
of interposition materials have been used including autogenous fascia,
dermis, and allograft tissue. Distraction with an articulated external
fixator is recommended to protect the interposition material during the
early postoperative period.
replace damaged articular surfaces with an interposition material that
eventually undergoes transformation into a new fibrocartilage articular
surface. This transformation requires biologic robustness that can
promote healing and soft tissue integration into the humerus and ulna.
In an immunosuppressed host with impaired tissue healing, it is unknown
whether the interposed tissue actually undergoes the expected changes.
reported their outcomes following interposition arthroplasty and found
that patients reported diminished pain, but that there were no
significant improvements in joint motion. Of concern, they observed
progressive bone loss in two thirds of humeri and in one third of
ulnae. In some instances, the bone loss interfered with their ability
to perform revision surgery. Total elbow arthroplasty was favored over
interposition arthroplasty based on their comparison of outcomes
following both procedures.
Primary osteoarthritis of the elbow tends to occur predominantly in
manual laborers and those who rely on wheelchairs or crutches for
ambulatory assistance.13,15,16,17
Three main pathologic processes are involved in osteoarthritis of the
elbow. Reactive bone and cartilage formation give rise to osteophytes.
Loss and fragmentation of cartilage can lead to loose body formation.
These two processes cause impingement and contribute to the third process of joint contracture.17,18 Symptoms include pain at the end points of motion, loss of extension, and mechanical symptoms such as catching or locking.12,15
Other commonly associated conditions include cubital tunnel syndrome
with paresthesias and weakness in the ulnar distribution and decreased
grip strength.15,19
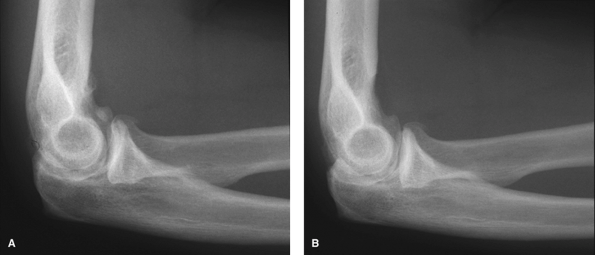 |
|
Figure 62-8 A:
Preoperative radiograph of a typical patient, a 47-year-old right hand–dominant man with right elbow osteoarthritis, demonstrates osteophyte formation and joint space narrowing. B: Postoperatively, the osteophytes and bony spurs have been removed. |
Total elbow arthroplasty, although it reliably provides pain relief and
improved range of motion, may be associated with early aseptic
loosening in young active patients and should rarely be done primarily
in this group.20 Elbow arthrodesis
is a potential procedure in this population; however, many patients
find the restricted motion postoperatively undesirable.20 Multiple open debridement procedures have been used with good success.12,15,17,21,22,23,24
Arthroscopic debridement and resection of osteophytes and capsule is a
technique that addresses the underlying pathologic processes and
provides outcomes similar to open procedures, and is associated with
minimal perioperative morbidity.13,25,26,27,28
The arm is cradled in a padded arm holder that attaches to the side to
the table. A nonsterile tourniquet is then placed high on the arm at
the level of the arm holder. The arm should be secured to the arm
holder. This is helpful during instrumentation since the arm remains
stable, similar to how a knee holder maintains stability during knee
arthroscopy. The elbow should be positioned slightly higher than the
shoulder. This will allow for 360-degree exposure of the elbow joint,
eliminating potential impingement of the arthroscope or shaver against
the side of the body.
surgery when the elbow is not distended or edematous and palpation of
bony landmarks is more precise. Surface landmarks that should be marked
with a pen in all patients include the ulnar nerve, the lateral
epicondyle, medial epicondyle, the radial head, capitellum, and
olecranon.
starting portal is an important step in contrast to techniques in the
shoulder or the knee. The elbow can be injected with 20 to 30 mL of
fluid at the location of the anterolateral portal just anterior to the
radiocapitellar articulation. With the elbow joint distended, the major
neurovascular structures are positioned farther from the starting
portal site and entry into the joint is easier.
preference. No starting portal has been shown to be better than
another, and ultimately the experience of the surgeon and his or her
knowledge of anatomy is the best guide to elbow arthroscopy.
Superficial cutaneous sensory nerves are common about the elbow and can
be injured during portal placement.
visualization can be maintained by pressure distention of the capsule
or by mechanical retraction. Retractors for the elbow are simple lever
retractors such as a Howarth or a large blunt Steinmann pin. Retractors
are placed into the elbow joint via an accessory portal, which is
typically 2 to 3 cm proximal to the arthroscopic viewing portal. By
holding the capsule and overlying soft tissue away from the bone with
retractors, adequate visualization can be achieved with a high-flow,
low-pressure system.
in which a triceps splitting approach is used to access and débride
osteophytes and loose bodies from the posterior aspect of the elbow
joint. Fenestration of the olecranon fossa allowed access to the
anterior aspect of the joint, and loose bodies and osteophyte removal
was facilitated by use of an osteotome and irrigation.22
Others have subsequently described arthroscopic modifications of this
procedure and have demonstrated satisfactory clinical outcomes
following use for treatment of osteoarthritis.13,16,28,29 Cohen et al.28
compared outcomes following arthroscopic debridement versus open
debridement of the elbow for osteoarthritis using the
Outerbridge-Kashiwagi procedure and the arthroscopic modification. Both
groups demonstrated improved range of elbow flexion, decrease in pain,
and a high level of patient satisfaction. Increases in elbow extension,
although improved in both groups, were more modest. However, neither
procedure included capsular release. Comparison between the open and
arthroscopic procedures demonstrated that the open procedure might be
more effective in improving flexion whereas the arthroscopic procedure
seemed to provide more pain relief. No differences between overall
effectiveness of the two procedures were noted.28
Heterotopic ossification prophylaxis should be considered in these
patients, as this complication has been demonstrated to occur in the
postoperative period following elbow procedures.30,31
anterior and posterior aspects of the elbow joint can be visualized and
pathology addressed (Fig. 62-8). To obtain a
similar view and access using open techniques would require large
surgical exposures and incisions with presumably attendant increased
morbidity. Despite the many advantages of arthroscopy in addressing
elbow pathology, it remains a technically demanding procedure that
requires a high level of arthroscopic experience and training to
perform safely.
range of motion postoperatively and leaves less opportunity for
recurrent scar or contracture formation. By removing the capsule, the
pliability of the joint and overlying soft tissues is improved, leading
to better possible range of motion. The surgeon should exercise caution
in performing this procedure owing to the potential for neurovascular
injury. In particular, care should be exercised when working about the
radial head. The fat pad near the posterior interosseous nerve can be
observed and avoided. In addition, the shaver should not be put to
suction, which may cause important structures to inadvertently be
pulled into the shaver and thus injured. Rather, the outflow of the
shaver should be put to gravity only.
be placed in immediate postoperative range of motion therapy. The two
common forms of treatment are static splinting and continuous passive
motion. There are currently no studies that demonstrate that one
technique is better than another.
DT, Anderson JJ, Boers M, et al. American College of Rheumatology.
Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38:727–735.
NS, Axon JM, Carr AJ, et al. Synovectomy of the elbow and radial head
excision in rheumatoid arthritis. Predictive factors and long-term
outcome. J Bone Joint Surg Br. 1997;79:918–923.
SA, Morrey BF, Adams RA, et al. Ulnohumeral arthroplasty for primary
degenerative arthritis of the elbow: long-term outcome and
complications. J Bone Joint Surg Am. 2002;84A:2168–2173.
B, Degreef I, De Smet L. Debridement arthroplasty for osteoarthritis of
the elbow (Outerbridge-Kashiwagi procedure). Acta Orthop Belg. 2004;70(4):306–310.
K, Mizuseki T. Debridement arthroplasty for advanced primary
osteoarthritis of the elbow. Results of a new technique used for 29
elbows. J Bone Joint Surg Br. 1994;76:641–646.
NJ, Ali A, Stanley D. Treatment of primary degenerative arthritis of
the elbow by ulnohumeral arthroplasty. A long-term follow-up. J Bone Joint Surg Br. 2003;85:347–350.