PERIPHERAL NERVE LESIONS OF THE FOOT AND ANKLE
a misnomer. Most recent literature supports the theory that the
condition we call interdigital neuroma is an entrapment neuropathy of
the common digital nerve beneath the transverse metatarsal ligament (26,38,39,44).
These studies demonstrate that almost all the histologic changes in the
nerve occur distal to the transverse metatarsal ligament, and that the
nerve proximal to the ligament appears normal. The histologic changes
are characterized by perineural fibrosis, degeneration of the nerve
fibers, deposition of an amorphous eosinophilic material, and a
decrease in both the number and
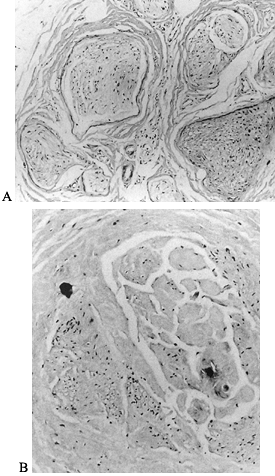
the diameter of thick myelinated fibers (Fig. 114.1).
Although these studies seem to support the theory that the main cause
of an interdigital neuroma is an entrapment neuropathy, there are
anatomic factors that need to be considered.
 |
|
Figure 114.1. Photomicrographs of an interdigital neuroma (trichrome stain). A: Low-power photomicrograph of a normal nerve. B: High-power photomicrograph of neuroma. Axonal degeneration and abundant vasculature are present.
|
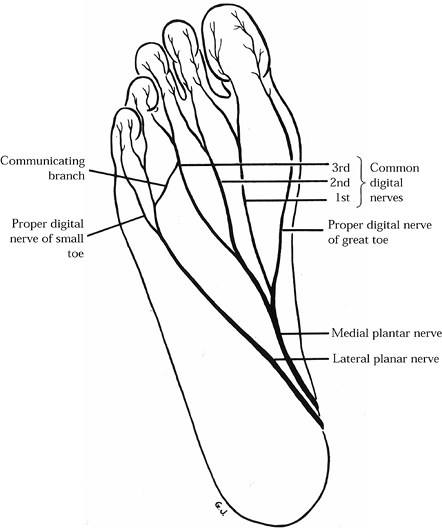
Subsequent studies of the anatomy of the plantar nerves and their
branches have demonstrated a communicating branch between the medial
and lateral plantar nerves (9,32) (Fig. 114.2).
This branch was noted to course from the common digital nerve in the
fourth interspace to the third digital branch of the medial plantar
nerve in the third interspace. Jones and Klenerman (32)
found this communicating branch to be present in all 20 of the cadaver
specimens dissected. In two of the female specimens, they found the
communicating branch was enlarged, and they hypothesized that this
enlargement may be a factor for the frequent involvement of the nerve
to the third interspace. Mann and Reynolds (44)
reported an equal distribution of interdigital neuromas in the second
and third interspaces. They believed their data supported the theory
that neuromas are caused by repetitive local trauma related to footwear
(e.g., tight shoes, high heels, and thin soles) and shed doubt on the
role of the communicating branch as the main cause of interdigital
neuroma formation. Levitsky et al. (39) found
the communicating branch was present in only 19 (26.8%) of 71 specimens
dissected, and the incidence of third-interspace neuromas was no
greater in those feet with the communicating branch as those without
it. The information also questions the role of the communicating branch
in the etiology of interdigital neuromas. Both Mann and Levitsky failed
to identify a neuroma in the first and fourth interspaces.
 |
|
Figure 114.2. Plantar nerves of the right foot.
|
branch in their study, Levitsky et al. (39)
also measured the distance between the metatarsal heads and the
diameter of the common digital nerve to each interspace. They found the
intermetatarsal head distances in the second and third interspaces were
significantly smaller than in the first and fourth. They then
calculated a mean ratio of the intermetatarsal head distance to the
diameter of the common digital nerve and found that ratio was
significantly lower in the second and third interspaces compared to the
first and fourth interspaces. They believed their data lent support to
the theories that explain the formation of a neuroma in both the second
and third interspaces on a mechanical basis.
more common in women than men. It is well known that with
hyperextension of the metatarsophalangeal joints, along with the action
of the plantar aponeurosis, the metatarsal heads are depressed. This
exposes the interdigital nerve to increased pressure. In a woman’s
fashionable high heel shoe, the metatarsophalangeal joints are
constantly hyperextended. This can cause the interdigital nerve to be
tethered across the transverse metatarsal ligament, leading to an
entrapment neuropathy. It is therefore realistic to consider a
“neuroma” as an entrapment neuropathy caused by a combination of
anatomic factors, such as a decreased ratio of the diameter of the
intermetatarsal head to that of the digital nerve, combined with
extrinsic factors, such as fashionable shoes and activity level.
-
Make the correct diagnosis.
-
Nonoperative treatment (shoewear
alterations, metatarsal pad, corticosteroid injection, nonsteroidal
anti-inflammatory medication) -
Operative treatment (dorsal approach, plantar approach)
-
1876—Morton (48) coined the term metatarsalgia to describe a painful affliction of the forefoot suspected to involve the interdigital nerves.
-
1893. Hoadley (29)
explored the digital nerves under the painful area, “found a small
neuroma,” excised it, and claimed the patient obtained “prompt and
perfect cure.” -
1940. Betts (9)
stated that “Morton’s metatarsalgia is a neuritis of the fourth digital
nerve.” He also noted branches of both the medial and lateral plantar
nerves formed the nerve in the third interspace. -
1979. Gauthier (22) speculated that the condition represented a nerve entrapment, a theory supported by Lassman (38) and later by Graham and Graham (26).
-
1983. Mann and Reynolds (44)
found an equal number of interdigital neuromas in the second and third
interspaces. They theorized that repetitive local trauma related to
footwear was the main etiologic factor rather than the communicating
branch. -
1984. Jones and Klenerman (32)
identified the communicating branch in all 20 cadaver feet studied.
They suggested enlargement of this communicating branch may be
responsible for the frequent involvement of the third interspace. -
1993. Levitsky et al. (39)
found the communicating branch in only 26.8% of specimens studied. They
developed a ratio of the distance between the metatarsal heads to the
diameter of the nerve and found it to be significantly smaller in the
second and third interspaces, suggesting neuromas are caused by an
entrapment neuropathy based on mechanical force. -
1993. Thompson and Deland (66)
coined the term “second neuroma, same foot,” or SNSF, to describe the
occurrence of a second primary neuroma in the same foot. They placed
the incidence at under 4%. They found it even more rare for the
neuromas to occur simultaneously and suggested that both interspaces
not be explored at the same time. -
1995. Bennett et al. (7) presented a comprehensive staged treatment protocol for interdigital neuromas, along with their results.
-
1996. Benedetti et al. (6)
reported on 19 feet that underwent simultaneous excision of two primary
interdigital neuromas in adjacent interspaces. They found a 3%
incidence. Patients had dense sensory loss. The authors recommended one
single dorsal longitudinal incision in the second webspace to resect
both neuromas.
interdigital neuroma is first making the correct diagnosis. The
examiner needs to determine not only if the patient’s symptoms are
consistent with the diagnosis of a neuroma, but also at which
interspace the neuroma is located. This can be very challenging. The
diagnosis is made on the basis of a patient’s symptoms and the physical
examination. Certainly there are patients that present with classic
symptoms and physical findings. Although magnetic resonance imaging
(MRI) and ultrasound have been reported to be sensitive in diagnosing
interdigital neuromas, they have not gained wide acceptance and should
not take the place of a directed history and physical examination.
Other studies present data without significant differences. A vast
majority of the patients are women in their fifties. Most of the time,
symptoms are unilateral, although bilateral neuromas occur about 15% of
the time. Either the second or third interspace can be involved, with
studies showing anywhere from equal involvement to predominance in the
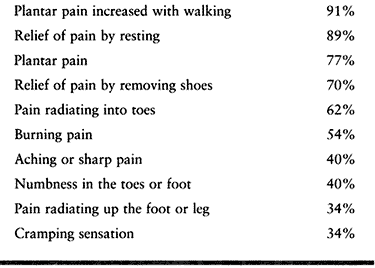
third interspace. The classic symptoms are burning plantar pain between
the metatarsal heads with radiation into the toes, exacerbated by
wearing tight toed, high heeled shoes and relieved by removing the
offending footwear. Patients may feel a “lump” or “mass,” as if their
sock is rolled up (but it is not). Some patients feel the need to “pop”
their foot to obtain relief. Runners and athletes are a group being
seen more often. They note the onset of their symptoms after a certain
distance or period of activity. Their pain crescendos to a peak and
does not subside until they stop and remove their athletic shoes.
 |
|
Table 114.1. Preoperative Symptoms
|
necessary to make the diagnosis of a neuroma. Look for positive
findings consistent with a neuroma, but also rule out other causes of
forefoot pain. First examine the foot in the weight-bearing position
and look for deformity and swelling. In particular, note any hindfoot
deformity, either varus or valgus, which affects the forefoot. Also
note hammer toe, claw toe, or other angular deformities of the digits.
Swelling or fullness in the interspaces or around the
metatarsophalangeal joints is not a typical finding in a patient with
an interdigital neuroma. Observe the patient’s gait and look for any
dynamic deformities or signs of pain. Then examine the
non-weight-bearing foot. Document range of motion of all the joints of
the foot and ankle, especially the metatarsophalangeal joints.
Neurovascular assessment, skin condition, temperature, and color of the
foot should be part of any foot examination and can give important
information to direct the diagnosis. Check for Tinel’s sign over the
tarsal tunnel, because diffuse burning plantar foot pain can be caused
by neuritis of the tibial nerve or a tarsal tunnel syndrome.
specifically looking for signs of a neuroma. Palpitate carefully each
metatarsophalangeal joint, under each metatarsal head, and in each
interspace. Tenderness directly under the metatarsal heads or in the
metatarsophalangeal joints is not a finding consistent with neuroma.
When palpating in the interspace, start proximal to the metatarsal
heads and work distally into the web space. This will often reproduce
the patient’s symptoms if a neuroma is present. Then reexamine the web
spaces by applying dorsal–plantar pressure with the thumb and
forefinger while squeezing the foot in a medial–lateral direction. This
maneuver may produce a click or crunching feeling (called Mulder’s
sign) and may reproduce the patient’s symptoms (49).
If so, this is considered diagnostic for an interdigital neuroma. One
can often feel a crunching or click without reproducing a patient’s
symptoms. In that case, the finding is not diagnostic for a neuroma. Table 114.2, also adapted from Mann and Reynolds (44), outlines the more common physical findings.
 |
|
Table 114.2. Physical Findings in Interdigital Neuromas
|
out any osseous abnormalities. Assess the metatarsophalangeal joints
for arthritis, subluxation, or widening, or any other abnormalities
such as a foreign body.
The authors note no false positives in the patients who eventually
required surgery, but they cannot comment on false negatives without
histologic verification of the diagnosis. Overall, the usefulness and
cost-effectiveness of MRI have yet to be
determined in the diagnosis of interdigital neuromas and directing treatment. Therefore, I do not recommend routine use of MRI.
as a useful study in diagnosing neuromas. The authors accurately
predicted the presence, location, and size of the neuroma in 98% of
cases. They feel it is particularly useful in diagnosing adjacent
neuromas in the same foot; this is the situation for which ultrasound
may have its greatest usefulness. The authors also admit that the
diagnosis is examiner dependent, and an experienced ultrasonographer
interested in foot and ankle problems is essential.
symptoms and physical findings, and the diagnosis seems
straightforward. Many, however, are difficult to diagnose with enough
confidence to suggest surgery. The best way to sort through the
findings for the difficult patient is to perform a series of
examinations over a period of time. Symptoms change, as do physical
findings. You will either feel more confident about the presence of a
neuroma or more sure that you are dealing with an entity different from
a neuroma. During the time you are following the patient, different
nonoperative treatments can be tried, and you may actually be able to
come to a diagnosis based on the patient’s response. A reasonable time
period between assessments is 6–8 weeks.
symptomatic neuroma difficult is the presence (in 28% of feet) of
adjacent neuromas (39), although this problem is becoming better understood. Thompson and Deland (66)
suggest the use of 1–2 ml of Xylocaine into one of the suspected
interspaces, with follow-up to determine its effect. If no or partial
relief is obtained, the other interspace is similarly injected, with
later follow-up. The authors suggest that if you feel strongly that the
patient indeed has simultaneous adjacent neuromas, they should not be
excised at the same time and the symptomatic one should be excised
first. Benedetti et al. (6) reported on the
surgical outcome of excision of adjacent neuromas, but they did not
have any specific suggestions on how to diagnose them. They did stress
the importance of ruling out any other condition that could cause more
diffuse forefoot pain, such as peripheral neuropathy, double crush
syndrome, and synovitis of the metatarsophalangeal joints.
nonoperatively, perform repeated examinations at appropriate intervals,
and do not operate.
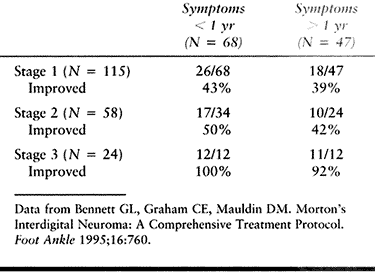
developed a three-stage treatment protocol, the first two stages of
which were nonoperative. The first stage included patient education
about the condition. Footwear modifications were made to include a
wider shoe, and a metatarsal pad was applied proximal to the involved
interspace. A letter was sent to the patient to reinforce the program.
If little or no improvement was seen within 3 months, the patient
returned, and 2 ml of 1% Xylocaine and 1 ml of Aristocort
(triamcinolone, 40 mg/ml) was injected into the interspace (stage 2).
Immediate short-term pain relief was seen in all patients. They were
encouraged to continue with the shoewear modifications. If satisfactory
relief of the patient’s symptoms was not seen 3 months later, surgical
excision was carried out (stage 3). The authors’ results are summarized
in Table 114.3. They conclude that although
the nonsurgical treatment was not successful in all patients, the
protocol helped select those patients who might benefit most from
surgery. They also felt that the injection was diagnostically helpful
and that a positive response to the injection is a prerequisite to
recommending surgical treatment.
 |
|
Table 114.3. Patient Satisfaction with Treatment Program
|
was similar to stage 1 of the preceding protocol. These authors found
that although the patients responded well to nonoperative management,
70% to 80% were sufficiently symptomatic and dissatisfied with the
change in shoewear that they opted for surgery. Anti-inflammatory
medications or injections, with or without a steroid, did not provide
long-term relief and was not part of their routine management.
Rasmussen et al. (55) reported on 51 feet that
received a single corticosteriod injection for treatment of third
interspace neuromas. They concluded that injections generally did not
provide a cure for interdigital neuromas, but they did provide
temporary relief and did not preclude a good
surgical outcome. After 4 years, only 11% of the feet had lasting improvement. The authors felt, as did Bennett et al. (7), that injection can be useful in confirming the diagnosis and as a predictor of operative success.
little subcutaneous tissue, such as the interspace in the foot, can
cause subcutaneous atrophy, altered cutaneous pigmentation, alopecia,
and telangectasia (56). The effects are more
pronounced with the use of relatively insoluble preparations, such as
triamcinolone. The more soluble preparations should be used in low
volume if a corticosteroid is to be injected into the foot for
treatment of an interdigital neuroma.
are from an interdigital neuroma, and the patient has failed
nonsurgical management, surgery is indicated. There are essentially
three options for surgical treatment: (a) dorsal approach, (b) plantar
approach, or (c) dividing the transverse metatarsal ligament and
leaving the nerve intact.
surgical treatment for interdigital neuroma. Overall, one can expect
80% to 85% good and excellent results with this surgical approach
combined with the staged treatment protocol outlined previously (7,19,44). It is my preferred technique.
-
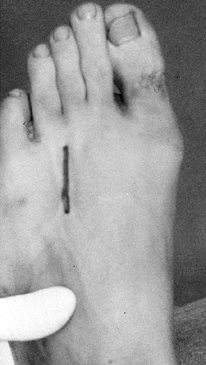
Under tourniquet control, make a 3 cm incision starting in the involved webspace (Fig. 114.3).
Place the incision between the metatarsals to avoid injury to a small
dorsal sensory nerve. Carry out blunt dissection with a thumb or small
scissors down through the subcutaneous tissue, avoiding injury to
superficial nerves.![]() Figure 114.3. Dorsal incision placed between the metatarsals.
Figure 114.3. Dorsal incision placed between the metatarsals. -
Place a Weitlander retractor or a small
lamina spreader into the wound between the metatarsal heads, and open
it, placing the transverse metatarsal ligament under tension (Fig. 114.4).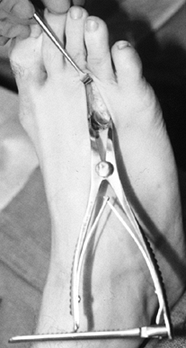 Figure 114.4. Weitlander retractor placed between the metatarsals for exposure.
Figure 114.4. Weitlander retractor placed between the metatarsals for exposure. -
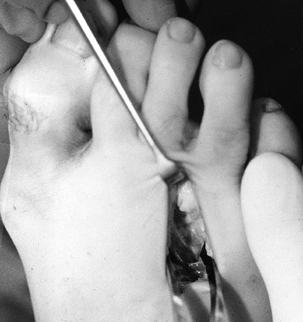
Use a Freer elevator to bluntly dissect
in line with the incision to identify the transverse metatarsal
ligament and the normal interdigital nerve proximally. The nerve
occasionally courses close to the metatarsal and is not always in the
center of the interspace. Plantar pressure under the web space should
reveal the enlarged neuroma protruding distal to the transverse
metatarsal ligament (Fig. 114.5). Then sharply divide the metatarsal ligament.![]() Figure 114.5. Neuroma protruding distal to the transverse metatarsal ligament.
Figure 114.5. Neuroma protruding distal to the transverse metatarsal ligament. -
Identify the nerve in the proximal part
of the wound and trace it distally into the irregular mass. Look for
any accessory branches coming from either metatarsal to join the common
digital nerve. If one is identified, dissect it and clearly define it.
I recommend leaving a divided accessory branch long and suturing the
end into the interosseous muscle or capsule of the metatarsal head with
a small suture. This prevents the nerve end from retracting and forming
a potential painful neuroma stump directly under the metatarsal head. -
Trace the common digital nerve and transect it in the most proximal aspect of the wound so the cut end does
P.3041
not end up between metatarsal heads. Then dissect the neuroma out distally and excise it (Fig. 114.6). Preserve as much plantar fat as possible.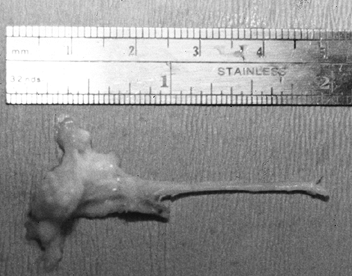 Figure 114.6. Typical surgical specimen showing normal interdigital nerve (right) leading into irregular neuroma mass.
Figure 114.6. Typical surgical specimen showing normal interdigital nerve (right) leading into irregular neuroma mass. -
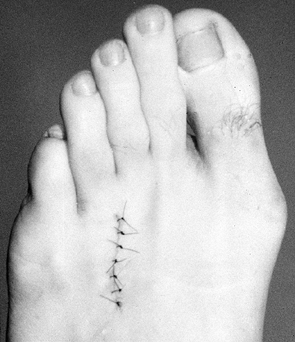
Close the skin with an interrupted 4-0 nylon suture and apply a compressive dressing (Fig. 114.7).
![]() Figure 114.7. Wound is closed with interrupted nylon suture to prevent hematoma formation.
Figure 114.7. Wound is closed with interrupted nylon suture to prevent hematoma formation.
performed 57 primary interdigital neuroma excisions through a plantar
approach. He felt that the improved exposure gave better results. He
reported no wound problems. There are other reports, however, of
painful plantar incisions from this approach. Because there is little
recourse to remedy a painful plantar scar, and because the dorsal
approach provides good exposure with less morbidity, the dorsal
approach is preferred for excision of a primary interdigital neuroma (42,44).
Some have advocated the plantar approach for treatment of recurrent
neuromas. The following is a brief description of the technique.
-
Palpate the metatarsal heads on each side of the involved interspace and draw them out with a skin marker.
-
Place a small-gauge needle (25- or
27-gauge) from a dorsal to plantar direction in the interspace,
piercing the plantar aspect of the foot. This identifies the interspace
plantarward so the incision can be made accurately. Draw the incision
in line with the interspace, but proximal to the metatarsal heads. -
Carry the incision through the
subcutaneous tissue. Place a Weitlander retractor. Carry out dissection
with a blunt scissors in line with the skin incision. Retract slips of
the plantar fascia, exposing the interdigital nerve and neuroma.
Transect the nerve proximally and continue dissection distal to the
neuroma. Excise the neuroma as in the dorsal approach. -
Close the skin with interrupted 5-0 nylon and apply a compressive dressing.
When surgery has been performed for a recurrent neuroma, it appears
that the transverse metatarsal ligament has completely reconstituted.
This casts some doubt on the long-term outcome of an interdigital
neuroma treated by this method, and it cannot be recommended at this
time (42).
is similar. Send the patient home the same day in a postoperative shoe.
Instruct the patient to elevate the foot as much as possible to
decrease swelling. Allow weight bearing, but the dressing should be
reapplied and kept in place for up to 3 weeks after surgery. The
patient may ambulate in the postoperative shoe during this time. After
3–4 weeks, the swelling and tenderness should subside enough for him to
begin ambulating in a wide, sensible shoe. Start passive and active
range-of-motion exercises of the foot and ankle at that time. The
patient can then slowly increase activities as tolerated, but he may
experience swelling and soreness for some months with vigorous
activities.
diagnosis and treatment of an interdigital neuroma. Making the correct
diagnosis of a neuroma and its location can be challenging. Suggestions
to avoid pitfalls in diagnosis have been given.
second neuroma in the same foot. This condition is rare, but it could
be the reason for unsatisfactory surgical outcomes. In addition, an
incorrect diagnosis about the interspace in which the neuroma is
located will lead to a poor surgical outcome. These two pitfalls have
some overlap, which is difficult to sort through at times.
interspace can lead to atrophy of the fat pad and pigment changes of
the skin. I recommend only one injection, if any, at this time, based
on the previously presented data.
dorsal approach. If done incorrectly, or if the patient forms a keloid,
it can lead to a painful incision that can be very difficult to cure.
Dissection outside the line of the incision or overvigorous activities
can lead to injury to the small dorsal cutaneous nerves, resulting in
uncomfortable paresthesias or dysesthesias in the toes.
neuromas in adjacent web spaces, the patient will experience dense
sensory loss under the third toe that can be
uncomfortable. Devascularization of the third toe due to injury to the digital arteries has been reported as well (Fig. 114.8).
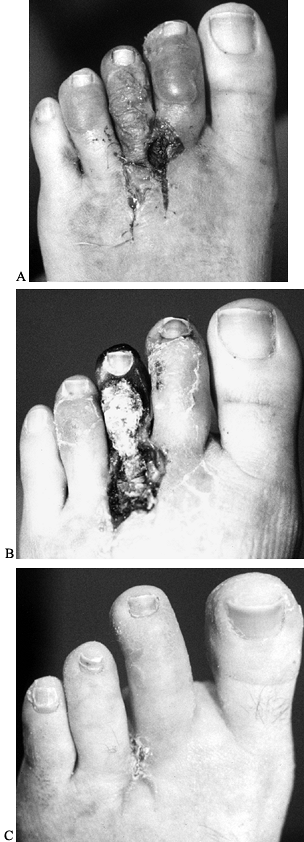 |
|
Figure 114.8. A: Patient 8 days after excision of presumed adjacent neuromas. B: Same patient 20 days postoperatively with gangrene secondary to devascularization of third toe. C: Same patient 3 weeks after amputation of third toe.
|
noted that two thirds of the 39 patients they treated for recurrent
neuroma had symptoms within 12 months of the original procedure. The
other one third noted symptoms 1–4 years later. Mann and Baxter (42)
hypothesized that the group with recurrent symptoms within 12 months
probably had surgery for the wrong diagnosis, and that the other third
had the interdigital nerve transected too far distal, and the neuroma
stump from the excision ended up between the metatarsal heads and took
1–4 years to become painful. In their own experience of 11 recurrent
neuromas, Mann and Reynolds (44) noted the
neuroma stump to be adherent to the plantar aspect of the metatarsal
head in all cases. They reported that 81% of these patients were
asymptomatic after surgery through a dorsal approach. The diagnosis,
nonoperative management, and surgical approach are almost identical to
those for the primary neuroma. At the time of surgery, you may need to
extend the incision a little more proximally to get down to virgin
tissue and nerve, then follow it distally into the neuroma stump. Also,
postoperatively, patients should be allowed a little longer healing
time before returning to vigorous activities.
diagnose and treat. By following the guidelines for diagnosis,
nonoperative management, and surgical treatment outlined in this
section, you may be able to avoid the many pitfalls and complications.
Nothing can take the place of experience. I cannot overstate the
importance of patient history and physical examination. All the
information needed to make the diagnosis is in the room with you and
the patient—you just need to find it. Those of us who treat foot and
ankle problems on a daily basis all too often see distressed patients
who have had neuromas excised from multiple interspaces on both feet.
Unfortunately, there is very little to offer them at that point.
Remember that adjacent neuromas in the same foot are very rare.
Neuromas occur bilaterally in about 15% of the patients, and even then
rarely at the same time. If you think a patient has multiple neuromas,
think again and again. If unsure as to the diagnosis or location, do
not operate. That is the best advice I can give.
understand tarsal tunnel syndrome. The tarsal tunnel is a fibro-osseous
canal bounded by the tibia and medial malleolus anteriorly, the talus
laterally, and the flexor retinaculum medially. The medial surface of
the talus with the sustenaculum tali and the medial surface of the os
calcis form the osseous floor. The tunnel is completed by the laciniate
ligament (flexor retinaculum). The contents of the canal are, from
medial to lateral, the tibialis posterior tendon and its sheath, the
flexor digitorum longus tendon and its sheath, the posterior tibial
neurovascular bundle, and the flexor hallucis longus muscle–tendon unit
(Fig. 114.9).
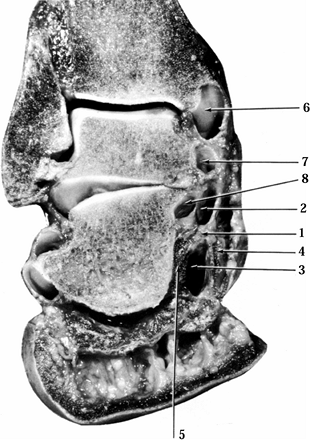 |
|
Figure 114.9. The compartments of the tarsal tunnel as seen in a frontal cross section of the ankle and hindfoot. 1, Interfascicular septum; 2, upper chamber containing medial plantar neurovascular bundle; 3, lower chamber containing lateral plantar neurovascular bundle; 4, abductor hallucis muscle; 5, quadratus plantae muscle; 6, tibialis posterior tunnel; 7, flexor digitorum longus tunnel; 8, flexor hallucis longus tunnel.
|
malleolar apex and an inferior base along the superior border of the
abductor hallucis muscle. The anterior and posterior borders are
difficult to define because they blend with the dorsal and superficial
aponeuroses of the foot and leg, respectively. The tibial nerve divides
into the medial and lateral plantar nerves and gives off its calcaneal
branch within the tarsal tunnel in most cases. There is, however, great
variation in this branching pattern (Fig. 114.10).
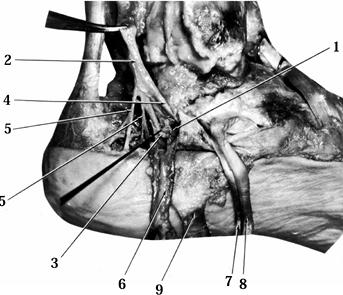 |
|
Figure 114.10. Medial aspect of tarsal tunnel. 1, Interfascicular ligament; 2, posterior tibial nerve; 3, lateral plantar nerve entering lower chamber; 4, medial plantar nerve entering upper chamber; 5, medial calcaneal nerves; 6, posterior tibial vessels reflected downward; 7, flexor digitorum longus reflected downward; 8, tibialis posterior tendon reflected downward; 9, reflected flexor retinaculum.
|
the posterior tibial nerve within the tarsal canal, or of one of its
terminal branches distal to the canal. Along its course, the nerve is
susceptible to compression from numerous sources, both intrinsic and
extrinsic. In about 60% of the patients with a tarsal tunnel syndrome,
a specific cause can be identified (41). Table 114.4 lists the differential diagnosis of tarsal tunnel syndrome (16).
 |
|
Table 114.4. Differential Diagnosis of Tarsal Tunnel Syndrome
|
syndrome is “burning” dysesthesias or paresthesias on the plantar
aspect of the foot. The symptoms are aggravated by prolonged standing
or activity and partially relieved at rest. Many patients describe
worsening of their symptoms while in bed at night, making it difficult
to fall asleep. Some patients describe the Valleix phenomenon, which is
proximal radiation of the pain along the medial aspect of the leg to
the midcalf (16). Often the symptoms are
diffuse and vague and not very well localized. Take a carefully
directed history to identify any of the alternative diagnoses listed in
Table 114.4. Look for a history of previous
trauma such as an ankle sprain or fractures about the ankle or
hindfoot. It is very important to elicit a history of diabetes or
peripheral vascular disease, which can cause a peripheral neuropathy
and mimic some of the symptoms of a tarsal tunnel syndrome.
looking for any deformity such as varus or valgus of the hindfoot.
Perform gross examination for swelling, color,
and
temperature. Carry out a detailed neurovascular exam. Check pulses,
warmth, and capillary refill. Gross sensory examination is usually not
very localizing or helpful. Patients may note some subjective decrease
in sensation on the plantar aspect of the foot, but it is difficult to
quantify. Bailie and Kelikian (2)
reported a significant increase in two-point discrimination (2-PD) of 7
mm in affected feet compared to normal controls. Postoperatively, they
noted an improvement of 4 mm in 2-PD. They suggest using the patient’s
uninvolved foot as the control in unilateral disease because of the
wide range of normal values (8–15 mm). Check the range of motion of the
ankle, subtalar, and transverse tarsal joints for possible
arthrofibrosis from an old injury, or the presence of a tarsal
coalition.
nerve, starting proximal to the tarsal tunnel and proceeding distally
along the medial and lateral plantar nerves. In a patient with a tarsal
tunnel syndrome, one can often demonstrate radiation of neuritic
symptoms into the plantar aspect of the foot in the distribution of the
medial or lateral plantar nerves or both (Tinel’s sign). Carefully
palpate the tarsal tunnel to identify the presence of any
space-occupying lesion such as a ganglion cyst or tumor. Motor function
of the intrinsic and extrinsic muscles of the foot rarely reveals
weakness. Look for any subtle atrophy of the abductor hallucis or
abductor digiti quinti muscles as compared to the opposite foot.
support the diagnosis of tarsal tunnel syndrome. Only when these
studies are abnormal, and combined with a history and physical
examination consistent with a tarsal tunnel syndrome, can the diagnosis
be made. Since Goodgold et al. (25) have
established the electrodiagnostic criteria for tarsal tunnel syndrome,
numerous authors have reported on the use of electrodiagnosis for
tarsal tunnel syndrome (2,11,16,20,21,25,31,33,41,50,52). As work continues in this area, recommendations for the best method are constantly changing.
categories: (a) nerve conduction studies of the medial and lateral
plantar nerve, (b) measurement of motor-evoked potentials, and (c)
sensory conduction velocity (42). Recent data suggest that the sensory nerve conduction velocity is the most accurate. Galardi et al. (21)
found sensory action potential (SAP) to be abnormal in all affected
limbs, thereby making it a very sensitive test. This coincides with the
findings of Oh et al. (50), who found abnormal SAPs in 90.5% of their patients. Galardi et al. (21)
were concerned about the poor specificity of pure sensory potentials.
They studied mixed nerve action potentials (NAPs) and found them
abnormal in 85.7% of the limbs with tarsal tunnel syndrome, with no
abnormal studies in asymptomatic limbs or normal controls, making it
very specific. They concluded that both SAPs and mixed NAPs are useful
in the diagnosis of tarsal tunnel syndrome. They recommend that the
coexistence of abnormal sensory and mixed nerve action potentials is
highly sensitive and specific for diagnosis of tarsal tunnel syndrome.
contents of the tarsal tunnel and the courses of the terminal branches
of the posterior tibial nerve (17). Most studies report that surgical outcome for tarsal tunnel syndrome is best when a definite lesion is identified (11,17,18,51,64). Frey and Kerr (18)
noted positive MRI findings in 88% of symptomatic feet studied. They
also noted positive MRI findings in 85% of the feet with abnormal
electrodiagnostic studies. They concluded that the information provided
by MRI could enhance surgical planning by indicating the extent of
decompression required. Pfeiffer and Cracchiolo (51)
suggest that, unless there is an identifiable lesion on MRI near or
within the tarsal tunnel preoperatively, surgical decompression of the
posterior tibial nerve should be considered with caution. Again, the
diagnosis of tarsal tunnel syndrome is based on clinical and
electrodiagnostic data. MRI is a useful adjunct when a space-occupying
lesion is suspected.
and identification of a cause. Nonsteroidal anti-inflammatory medicines
or an injection of a corticosteroid preparation can be tried if
tenosynovitis is suspected. If a flexible foot deformity is found, an
orthotic device can be helpful. If a lesion is found either on
examination or on MRI, surgical decompression is indicated. If a
patient’s symptoms persist after nonsurgical treatment, and you
strongly suspect compression or constriction of the posterior tibial
nerve within the tarsal tunnel, surgery is also indicated.
decompression of the posterior tibial nerve within the tarsal tunnel.
Identify and decompress the distal branches (i.e., the medial and
lateral plantar nerves) at the same time.
-
Approach the tarsal tunnel through a
curved incision starting 10 cm proximal to the tip of the medial
malleolus and 2 cm posterior to the medial tibial border. Carry the
incision distal to the tip of the medial malleolus, then gently curve
it distally and plantarward. Deepen
P.3046
the incision through the subcutaneous tissue to the level of the flexor retinaculum (laciniate ligament). -
Identify the retinaculum proximally. Note
the tibialis posterior tendon in its sheath. The flexor digitorum
longus tendon and posterior tibial nerve lie directly posterior to it.
Open the retinaculum from proximal to distal. As you dissect distally,
the retinaculum becomes more thick and taut. Place a curved clamp under
the retinaculum, and release it completely. -
Bluntly dissect the posterior tibial
nerve from proximal to distal. Note the condition of the nerve as well
as any compression along its course. If the normal amount of perineural
fat is present, it should be preserved. -
Trace the medial plantar nerve distally
to where it enters into a fibrous tunnel. If the nerve is felt to be
compressed at this point, open the tunnel and release the nerve. -
Then trace the lateral plantar nerve
distally to where it courses deep to the abductor hallucis muscle. The
dorsal portion of the muscle often requires release to decompress the
nerve branch. Identify the first branch off the lateral plantar nerve,
the nerve to the abductor digiti quinti, as it courses deep to the deep
investing fascia encompassing the abductor hallucis muscle. This is
discussed in more detail later in this chapter. -
Release the tourniquet to obtain
hemostasis. Observe whether the nerve’s normal color returns or if
there is a pale area from chronic constriction. Note in the operative
report if the nerve appears abnormal, for prognostic purposes. Then
close the wound in layers, without repairing the retinaculum. Apply a
sterile soft dressing and place a well-padded posterior splint.
removable walking cast for an additional 3 weeks. During that time, the
patient may remove the cast and start early range-of-motion exercises.
Physical therapy is rarely indicated. Patients may increase activities
as tolerated after 6 weeks.
lesion is identified and treated. If no specific etiology can be
identified, approximately 75% of patients obtain relief but 25% obtain
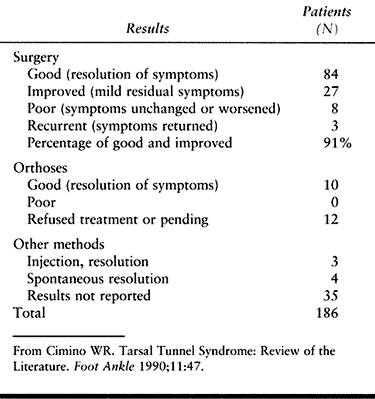
little or no relief (42). It is rare for a patient to be more symptomatic following uncomplicated tarsal tunnel release. In 1990, Cimino (11) reviewed 24 articles and compiled the overall treatment results from these reports. Those data are summarized in Table 114.5.
 |
|
Table 114.5. Results of Tarsal Tunnel Treatment: Summary of 24 Reports
|
reported 72% patient satisfaction following surgery. They also noted an
overall less-satisfied patient group in the presence of a worker’s
compensation claim. Pfeiffer and Cracchiolo (51) reported only 44% patient satisfaction after surgery and cautioned against surgery unless an obvious lesion is identified.
syndrome are related to misdiagnosis. Tarsal tunnel syndrome is a rare
entity. It implies compression of the posterior tibial nerve or its
branches within the tarsal tunnel. Pain or neuritis along the course of
the posterior tibial nerve is not a tarsal tunnel syndrome. Peripheral
neuropathy, often in conjunction with diabetes, can mimic tarsal tunnel
syndrome, but surgical decompression will rarely be of benefit.
Additional nerve lesions in the same lower extremity can affect the
treatment outcome of tarsal tunnel syndrome. These additional lesions,
which can occur anywhere along the course of the sciatic, common
peroneal, posterior tibial, or plantar nerves, can be detected with
electrodiagnostic tests as well as with a thorough history and physical
examination (58). As noted, the best results
occur when a specific mass or lesion is identified, either clinically
or on an MRI scan. When a lesion is not identified,
surgical
decompression can still be of benefit, but the patient needs to
understand that satisfactory outcomes occur only 75% of the time on
average, or even less.
diagnosing tarsal tunnel syndrome are ever changing. Patients are often
referred with an electromyogram and/or nerve conduction velocity study
with an interpretation by the examiner of a tarsal tunnel syndrome. The
diagnosis is not made on electrodiagnostic studies alone. These studies
serve only to support the clinical impression.
in the literature. A single case report of posterior tibial tendon
dislocation following decompression has been published (37).
One series reported an overall 13% complication rate, consisting of a
hematoma, suture abscesses, superficial wound infections, and
hypertrophic sensitive scars (2).
are believed to be secondary to adhesions or fibrosis around the nerve,
rather than the formation of another lesion. An MRI may be quite useful
to identify a possible surgical lesion. Revision surgery for tarsal
tunnel syndrome is rarely beneficial and should be avoided if possible.
This is especially true for the patient who did not obtain any relief
from the first surgical decompression.
overdiagnosed condition. If, after a thorough history and physical
examination, you feel strongly that the patient’s symptoms are caused
by compression of the posterior tibial nerve within the tarsal tunnel,
obtain an electrodiagnostic study. Most recent reports indicate that a
combination of SAPs and mixed NAPs is a highly sensitive and specific
electrophysiologic indication for tarsal tunnel syndrome. Work closely
with the person performing these tests and be familiar with their
techniques and expertise. If the electrodiagnostic tests are consistent
with the diagnosis, and a repeat history and physical examination are
unchanged from the first ones, treat the patient for tarsal tunnel
syndrome. If an extrinsic cause, such as varus or valgus of the
hindfoot, is identified, carry out nonsurgical treatment accordingly.
If after 3–6 months the treatment has not provided adequate relief,
consider surgical decompression. If a lesion or mass is appreciated on
physical examination, or is suspected, obtain an MRI to define the
extent of the lesion. If a fracture or tarsal coalition is suspected,
obtain a computed tomography scan to delineate the abnormality. Surgery
is indicated if an expected lesion is identified. Revision tarsal
tunnel surgery has poor results and I try to avoid it.
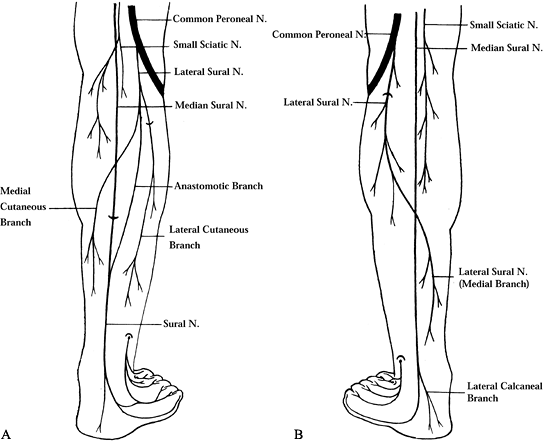
nerve, a branch of the tibial nerve. It may receive branches from the
lateral sural nerve or the common peroneal nerve (Fig. 114.11).
The sural nerve is accompanied by the short saphenous vein as they
course lateral to the Achilles tendon. It passes 1.0–1.5 cm distal to
the tip of the lateral malleolus. Readers interested in a detailed
description of the anatomy and the many variations of the sural nerve
are referred to Sarrafian’s textbook (59).
 |
|
Figure 114.11. A: Sural nerve type A (53%) formed by the medial sural nerve only. B: Sural nerve type B (40%) formed by two roots, the medial and lateral sural nerves, connected by an anastomotic branch.
|
being surrounded by subcutaneous tissue, and is not subjected to sharp
fascial edges or tight constricting bands. Coert and Dellon (12)
identified a potential site of entrapment of the lateral sural nerve as
it pierces through the deep vein fascia near the knee. As the knee is
extended, the fascia can exert a compressive force on the nerve. Most
sural neuropathies, however, are either related to trauma (e.g.,
lacerations, fractures, ankle sprains) or external pressure (e.g.,
ganglia, Baker’s cyst). There are few reports in the literature
regarding this entity. In 1974, Pringle et al. (53)
reported four cases of sural nerve entrapment. Their report found a
fracture at the base of the fifth metatarsal, a recurrent ankle sprain,
and ganglion cysts to be underlying causes. Also, external compression
of the nerve at different levels has been reported (57).
sural nerve entrapment is a history of neurogenic pain in the
distribution of the sural nerve. Often the symptoms occur only with
athletic activities. Physical findings are often absent, so the history
becomes paramount in making the diagnosis. On occasion one can palpate
a mass or cyst, or identify an associated fracture, but more often the
history is all there is to go on. Electrodiagnostic testing can be
performed to help localize an area of compression if the physical
examination is not helpful. Sensory nerve action potentials are reduced
in the affected extremity and normal in the contralateral limb. If a
mass is suspected, an MRI scan is useful to identify the extent and
location of the mass.
a history of external compression is identified, the symptoms resolve
1–2 months after removal of the offending
cause (57).
If the symptoms are secondary to trauma, a combination of casting or
rest, nonsteroidal anti-inflammatory medicines, and observation is
indicated. If nonsurgical methods fail to resolve the symptoms,
consider surgery. A trial injection of a local anesthetic agent around
the involved area with temporary resolution of symptoms preoperatively
will increase the chance that surgery may help. If a mass or ganglion
cyst is noted, surgical excision is indicated.
-
Place the patient either supine or in the lateral position on the operating room table. A tourniquet may be used.
-
Make a small incision over the involved
area identified preoperatively. Carry out blunt dissection carefully
down to the nerve. Excise the mass or release any compression. -
Transpose the nerve if external compression is the problem.
-
At closure, cover the nerve with
surrounding soft tissue and close the skin with interrupted suture.
Apply a compressive dressing.
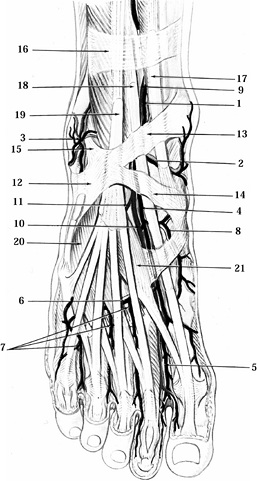
courses beneath the extensor hallucis longus muscle. As it approaches
the ankle, it is located between the extensor hallucis longus tendon
and the tendon of the extensor digitorum longus. It passes beneath the
superior and inferior extensor retinacula, then divides into a medial
and lateral branch. The medial branch accompanies the dorsalis pedis
artery, and it divides into two dorsal branches that supply the first
web space (Fig. 114.12). Just distal to the
inferior retinaculum, the lateral branch penetrates and innervates the
extensor digitorum brevis (EDB) muscle. These terminal branches of the
peroneal nerve may become compressed beneath the inferior extensor
retinaculum. Marinacci (45) called this entrapment the anterior tarsal tunnel
syndrome. Since that time, there have been scattered case reports in the literature (1,10,23,35,54), with the largest series being reported by Dellon (14).
 |
|
Figure 114.12. Second layer of the dorsum of the foot and ankle. 1, Anterior tibial artery; 2, anterior medial malleolar artery; 3, anterior lateral malleolar artery; 4, dorsalis pedis artery; 5, first dorsal metatarsal artery; 6, arcuate artery; 7, dorsal metatarsal arteries 2, 3, 4; 8, medial tarsal artery; 9,10, deep peroneal nerve; 11, motor nerve branch to extensor digitorum brevis (EDB); 12, inferior extensor retinaculum; 13, superomedial band of inferior extensor retinaculum; 14, inferomedial of inferior extensor retinaculum; 15, superolateral band of inferior extensor retinaculum; 16, superior extensor retinaculum; 17, tibialis anterior tendon; 18, extensor hallucis longus tendon; 19, extensor digitorum longus tendon; 20, EDB muscle to toes 2, 3, 4; 21, extensor hallucis brevis muscle.
|
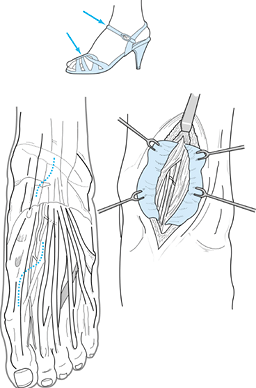
women. A history of trauma can often be elicited. Shoe straps or
tight-fitting boots, such as ski boots, that cross the dorsum of the
foot may irritate the nerve and lead to compression of the deep
peroneal nerve (Fig. 114.13). Classically,
patients complain of a deep ache over the dorsal and medial aspects of
the foot, with paresthesias into the first web space. Symptoms can
range from exacerbation with activities and relief with rest, to night
pain that awakens the patient. Physical findings include decreased
sensation in the first web space, a positive nerve percussion sign
(Tinel’s) over the course of the deep peroneal nerve, and atrophy of
the EDB muscle. Often, however, only a few—if any—of these signs are
present. There are a couple of reasons that atrophy of the EDB may be
lacking despite the presence of a true anterior tarsal tunnel syndrome.
If the entrapment occurs distal to the take-off of the motor branch, a
pure sensory neuropathy occurs. If the motor branch is involved,
atrophy and weakness of the EDB may be seen. Another reason is that in
22% of patients the accessory deep peroneal nerve, a branch of the
superficial peroneal nerve, innervates the EDB (27,36,43). In these cases, entrapment of the deep peroneal nerve would not manifest as denervation of the EDB muscle.
 |
|
Figure 114.13.
Entrapment sites over dorsum of ankle and foot. Shoe straps may have a direct relationship to nerve compressions in this region. Notice the more proximal site for release of the anterior tarsal tunnel syndrome at the inferior retinaculum. The incision for the release of the deep peroneal nerve over the mid dorsum of the foot is outlined (arrow). Deeper view demonstrates extensor hallucis brevis tendon crossing the neurovascular bundle. |
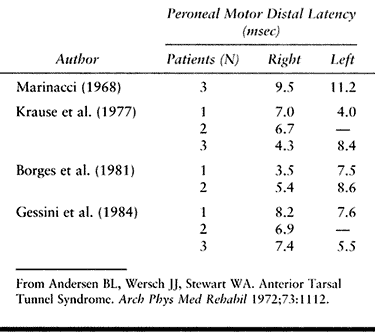
latencies of the peroneal nerve (Table 114.6) and needle findings described as representing “denervation” changes confined to the EDB muscle. Gessini et al. (23)
have suggested that a distal latency of greater than 5 msec confined to
the deep peroneal nerve is important in making the diagnosis. Because
abnormal needle findings in the EDB muscle have been reported in a high
number of normal subjects (1), one must be cautious about using this as the sole criterion for making a diagnosis.
 |
|
Table 114.6. Anterior Tarsal Tunnel Syndrome
|
Xylocaine without epinephrine into the dorsal aspect of the foot
proximal to the cuneiform bones. This should provide complete, but
temporary, relief of symptoms. This nerve block is recommended prior to
surgery in any patient for whom the diagnosis is not clear based on
history and physical examination.
underlying cause. Whatever the treatment, it should be tried for at
least 3 months before considering surgery. If shoewear is identified as
the problem, alterations can help. Adjusting tight ski boots is
possible and would be optimal compared to surgery. Providing relief
from straps (e.g., over a bone prominence) should also be tried before
surgery. Alteration in athletic or daily activities as well as
nonsteroidal anti-inflammatory medications can provide relief of
symptoms. If these treatment modalities fail, operative intervention is
indicated.
-
Place the patient supine on the operating
room table. Administer regional or general anesthesia. Operate with or
without a tourniquet. -
Make a dorsal incision over the proximal
first metatarsal, then curve it laterally between the junction of the
first and second metatarsals with the cuneiforms. Extend the incision
proximally to the level of the inferior extensor retinaculum. -
Identify the extensor hallucis brevis
tendon crossing the nerve at this point. Release this tendon, identify
the deep peroneal nerve, and dissect both proximally and distally until
the medial and lateral branches are exposed (Fig. 114.14). Release only a portion of the inferior retinaculum to make sure the nerve is not compressed.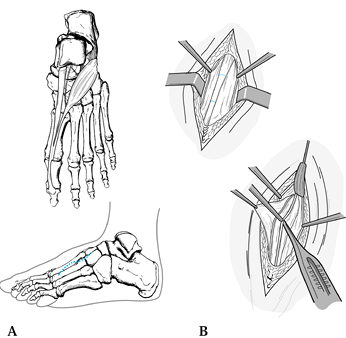 Figure 114.14. Anatomy of staged surgical release of distal deep peroneal nerve entrapment. A: Notice extensor hallucis brevis tendon crossing deep peroneal nerve at junction of metatarsals and cuneiform bones. B:
Figure 114.14. Anatomy of staged surgical release of distal deep peroneal nerve entrapment. A: Notice extensor hallucis brevis tendon crossing deep peroneal nerve at junction of metatarsals and cuneiform bones. B:
The initial operative management preserves the superficial peroneal
nerves (vessel loops) and resects a segment of the extensor hallucis
brevis tendon (between dotted lines). Subsequent release of the deep fascia permits neurolysis; take care to preserve the dorsalis pedis vascular bundle. -
Examine the nerve. If salvageable,
perform a neurolysis. If crushed or not deemed salvageable, resect it.
If the nerve is resected, dissect it proximally and bury the nerve end
into the muscles of the anterolateral compartment and secure it with
5-0 nylon suture (15). This prevents traction on the nerve end with ankle motion. -
If there is an underlying exostosis or bone fragment, excise it.
-
Close the skin with interrupted sutures and apply a compressive dressing.
peroneal nerve. It innervates the peroneus longus and brevis muscles in
the lateral compartment of the leg. The nerve is most predictably found
about 10.5 cm proximal to the tip of the lateral malleolus as it
pierces the fascia in the distal anterolateral leg. Because of its
subcutaneous position as it courses between the peroneal muscles and
the extensor digitorum longus, it is especially prone to injury at this
site. The superficial peroneal nerve then divides into two terminal
sensory branches to form the medial dorsal cutaneous and intermediate
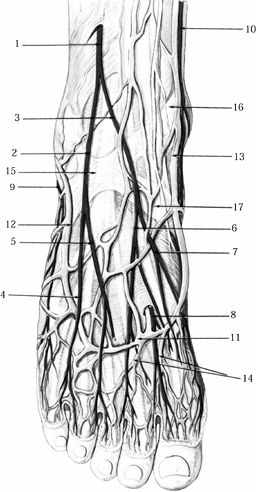
dorsal cutaneous nerves (Fig. 114.15).
 |
|
Figure 114.15. Superficial layer of the dorsum of the foot and ankle. 1, Superficial peroneal nerve; 2, intermediate dorsal cutaneous nerve; 3, medial dorsal cutaneous nerve; 4, dorsal cutaneous nerve to fourth web space; 5, dorsal cutaneous nerve to third web space; 6, dorsal cutaneous nerve to second web space with branch to dorsum of big toe; 7, dorsomedial cutaneous nerve to big toe; 8, nerve branch from deep peroneal nerve to first interspace; 9, sural nerve; 10, saphenous nerve; 11, dorsal venous arcade; 12, lesser saphenous vein; 13, greater saphenous vein; 14, dorsal metatarsal veins; 15, stem of inferior extensor retinaculum; 16, superomedial band of inferior extensor retinaculum; 17, inferomedial band of inferior extensor retinaculum.
|
the foot, sparing the first web space. The symptoms have typically been
present for years and are worse with activities such as walking,
jogging, or running. Patients also complain of pain over the lateral
distal leg and lateral ankle. About 25% of the patients report a prior
history of trauma, mostly ankle sprain. Injury to the nerve has also
been reported following fibular shaft fractures, or compression by a
lipoma (3,40,47,62).
start the physical examination with examination of the back, and look
for any sciatic tension signs. Examine the common peroneal nerve around
the fibula head for any swelling or a positive Tinel’s sign that
reproduces the location and quality of the patient’s complaints. The
most common area of entrapment is where the superficial peroneal nerve
pierces the deep fascia approximately 10.5 cm proximal to the tip of
the fibula. Point tenderness and a positive Tinel’s sign is often
elicited at this site. Styf and Morberg (63)
described three provocative tests for detection of nerve compression,
which are undertaken at rest and then again after exercise. In the
first, pressure is applied over the anterior intermuscular septum while
the patient actively dorsiflexes the ankle. In the second, the foot is
passively plantar flexed and inverted at the ankle. The third test is
performed by applying passive stretch with inversion, and percussing
over the course of the nerve. Pain or paresthesia caused by any of
these tests suggests entrapment of the superficial peroneal nerve.
According to the authors’ diagnostic criteria, entrapment of the
superficial peroneal nerve is diagnosed when sensation over the dorsum
of the foot is decreased and there is pain at rest or during exercise,
and when at least two of the three provocative tests are positive.
neuropathies, a trial of activity modification, anti-inflammatories,
and an ankle strap or orthotic device may prove useful. If this fails,
operative management is indicated.
-
Place the patient in the supine position under general or regional anesthesia. Use a thigh tourniquet.
-
Make a 6 cm incision just dorsal to the
previously marked painful sites. Carry the incision bluntly through the
subcutaneous tissue and identify the superficial peroneal nerve exiting
the deep fascia. -
Incise the roof of the peroneal tunnel
longitudinally until the nerve enters the muscle belly of the peroneal
longus. Keep the incision close to the anterior intermuscular septum (Fig. 114.16).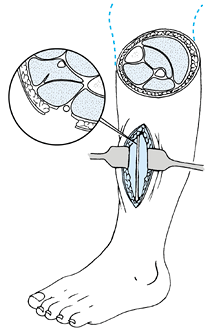 Figure 114.16. Diagram of the anatomy of the superficial peroneal tunnel.
Figure 114.16. Diagram of the anatomy of the superficial peroneal tunnel. -
Thoroughly decompress the nerve.
-
After the nerve is freed from any surrounding pressure, close the skin with interrupted sutures.
differential diagnosis is extensive. One cause of a painful heel
syndrome is an entrapment neuropathy of one of the branches of the
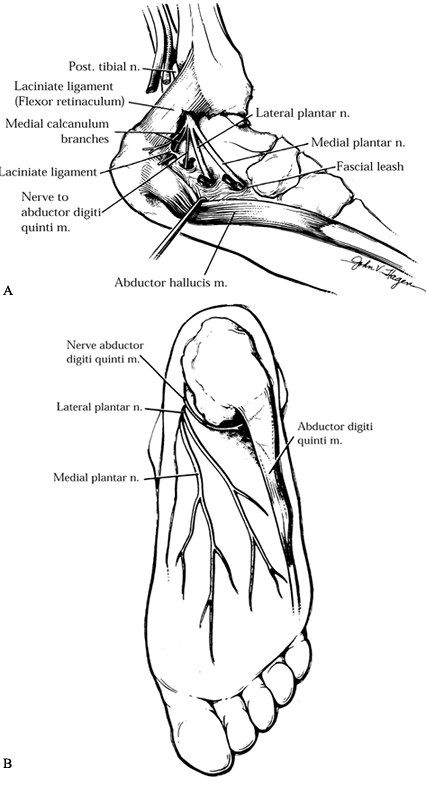
tibial nerve (4,5,28).
The first branch of the lateral plantar nerve has been implicated as a
source of heel pain. It supplies the abductor digiti quinti muscle on
the lateral side of the foot. Distal to the laciniate ligament (flexor
retinaculum), the tibial nerve divides into four or five branches.
There are one or two superficial branches, called the medial calcaneal
nerves. The medial and lateral plantar nerves, as well as the nerve to
the abductor digiti quinti, then course deep to the abductor hallucis
muscle. The nerve to the abductor digiti quinti may be a separate
branch of the tibial nerve or arise as a branch of the lateral plantar
nerve (Fig. 114.17). It passes beneath
the thick, inelastic, deep fascial edge of the abductor hallucis
muscle, where it can become compressed. The nerve continues laterally,
deep to the origin of the plantar fascia and flexor digitum brevis
muscle, and terminates in the proximal portion of the abductor digiti
quinti.
 |
|
Figure 114.17. A:
Nerve to abductor digiti quinti muscle as it migrates under the deep fascia of the abductor hallucis muscle. This nerve is deeper than the more superficial calcaneal nerve. B: Plantar view of nerve coursing to the abductor digiti quinti muscle. |
of heel pain exacerbated by weight bearing. Pain is worse with the
first step in the morning or on arising after a period of rest. The
onset is often gradual, and a history of competitive sports is present
in 50% of the cases. In contrast to other patients with heel pain who
are generally older, heavier, and less active, those with nerve
compression as the etiology are younger and more athletic. This patient
often has had symptoms for many months or years before seeking medical
attention. The pain normally starts medially but can radiate laterally.
Rarely does the patient complain of numbness or paresthesias into the
foot.
of the abductor hallucis muscle. Tenderness over the medial tubercle of
the calcaneus is a common finding as well. A Tinel’s sign is often
lacking, but on occasion one can elicit radiation of pain to the
lateral side of the foot. Electrodiagnostic studies can be useful as an
adjunct to the history and physical examination for entrapment of the
nerve to the abductor digiti quinti (60). However, the exact criteria and best method are yet to be determined.
quinti is suspected as a cause of a patient’s symptoms, treat
nonoperatively for 6 to 12 months. The treatment is much the same as
for heel pain syndrome caused by plantar fasciitis. This includes
education, stretching, heel cups, over-the-counter or custom-molded
orthoses, nonsteroidal anti-inflammatory medications, and alteration in
athletic shoes and/or activity.
operative results of 69 heels in 53 patients with recalcitrant heel
pain who had failed a minimum of 6 months of conservative therapy.
Their surgical technique is outlined below (4,5,42).
-
Place the patient in the supine position
on the operating table. Administer anesthesia, typically an ankle
block. A tourniquet is not required but can be used. -
Make a 4 cm oblique incision on the
medial aspect of the heel over the proximal abductor hallucis muscle.
Center the incision over the course of the first branch of the lateral
plantar nerve. The incision is oblique and placed distal to the medial
calcaneal sensory nerves. -
Divide the superficial fascia of the
abductor hallucis muscle with a #15 blade and retract the muscle
superiorly with a small retractor. -
Remove a section of the deep fascia along
the inferior border of the abductor hallucis directly over the area
where the nerve is compressed between the taut fascia and the medial
border of the quadratus muscle. A small portion of the medial plantar
fascia may be removed to facilitate exposure and clearly define the
plane between the deep abductor fascia and the plantar fascia (Fig. 114.18).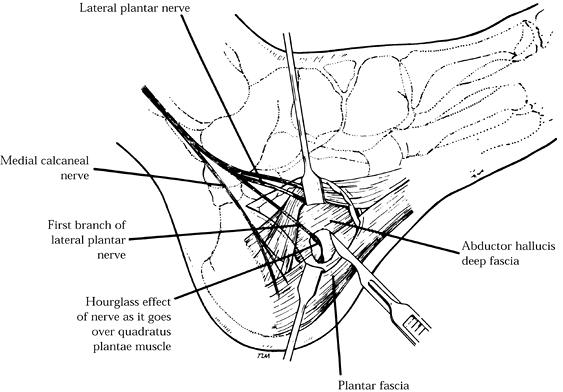 Figure 114.18.
Figure 114.18.
A section of deep fascia of the inferior abductor abductor hallucis
muscle is removed directly over the area where the nerve is compressed
between this taut fascia and the medial border of the quadratus plantae
muscle. -
Remove a heel spur, if present, taking
care to protect the nerve that runs superiorly with a Freer elevator.
Leave intact the abductor hallucis muscle belly and its superficial
fascia. -
Do not perform an extensive plantar
fascia release unless on direct examination there is evidence of
pathology in the entire proximal portion of the plantar fascia. -
Close the wound with interrupted sutures and apply a bulky dressing.
allow weight bearing for 3–4 days. Then begin gradual weight bearing.
Dress the wound daily with antibiotic ointment and apply gauze. One
week postoperatively, the patient can try to put on an athletic shoe
with a soft heel pad. The patient can resume light jogging by 4 weeks,
but some symptoms persist for 3 months.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
AL, Aszmann OC. Treatment of Superficial and Deep Peroneal Neuromas by
Resection and Translocation of the Nerves into the Anterolateral
Compartment. Foot Ankle 1998;19:300.
EJ, Quinn SF, Kneeland JB, et al. MR Imaging of the Tarsal Tunnel and
Related Spaces: Normal and Abnormal Findings with Anatomic Correlation.
AJR 1990;155:323.
R, DeLisa JA, Kraft GH. Motor Latencies through the Tarsal Tunnel in
Normal Adult Subjects: Standard Determinations Corrected for
Temperature and Distance. Arch Phys Med Rehabil 1980;61:243.
KA, Alman BA, Jevsevar DS, Morehead J. Digital Nerves of the Foot:
Anatomic Variations and Implications Regarding the Pathogenesis of
Interdigital Neuroma. Foot Ankle 1993;14:208.
MR, Kitaoka HB, Patzer GL. Nonoperative Treatment of Plantar
Interdigital Neuroma with a Single Corticosteroid Injection. Clin Orthop 1996;326:188.
MR, Kwong PK, Suthar M, et al. Morton’s Neuroma: Evaluation with MR
Imaging Performed with Contrast Enhancement and Fat Suppression. Radiology 1993;189:239.