Osteoporosis of the Spine
With the aging of populations worldwide, it is becoming more pervasive.
Similar to the intimal damage to arteries caused by hypertension, the
destruction of bone caused by osteoporosis is clinically silent until
an acute event occurs. With hypertension, the acute event is stroke or
myocardial infarction, whereas with osteoporosis the acute event is
fracture. Although a great deal of attention has been paid to
osteoporotic fractures of the hip, osteoporotic fractures of the spine
were thought to be benign, self-limited entities. With further study,
an increasing list of acute and chronic sequelae are being ascribed to
spinal insufficiency fractures, even in patients who never present to
their physician for evaluation.
patients with spinal osteoporosis. Osteoporosis may cause symptoms
directly through one of three common fracture types:
-
Vertebral compression fractures (VCF)
-
Osteoporotic burst fractures
-
Sacral insufficiency fractures
plan of other spinal interventions, such as instrumented stabilization
of a degenerative spondylolisthesis. Although fractures are the main
manifestation of spinal osteoporosis, the clinician must have a solid
understanding of the biology and biomechanics of osteoporosis in
treating any spinal condition in an osteoporotic patient population.
proteins, water, and cells. The exact composition of bone varies with
anatomic site, age, diet, and presence of disease. Osteoporosis results
from loss of the crystalline (inorganic) and collagenous (organic)
phases of bone. In general, the mineral phase represents 60% to 70% of
bone’s dry weight and is composed mainly of an analogue of the
naturally occurring mineral hydroxyapatite (Ca10(PO4)6(OH)2).
Loss of the mineral phase weakens the bone to compressive loading. The
organic matrix of bone represents approximately 30% of its dry weight,
and 90% of this matrix is collagen. Collagen is a protein of extremely
low solubility comprising three polypeptide chains of 1000 amino acids.
Loss of the organic matrix of bone makes it more brittle.
arrangement is modulated at the molecular level, and strain patterns of
the trabecular network are modulated at the organ level. The close
modulation of the molecular, cellular, and tissue properties of bone
result in a relatively lightweight tissue with a tensile strength close
to that of cast iron. At the microscopic level, bone consists of two
forms: woven (or primitive) and lamellar
bone. Lamellar bone begins to form 1 month after birth, and by age 4
years, most normal bone is lamellar. Lamellar bone is characterized by
highly organized, stress-oriented collagen, which gives it anisotropic
properties; that is, the mechanics of loading lamellar bone depend on
the direction of force application. Typically, bone is strongest
parallel to the long axis of the collagen molecules.
(dense or compact). Trabecular bone exhibits much greater metabolic
activity, with eight times greater turnover. This trabecular bone
represents 20% of the total bone mass and is found in the metaphyses
and epiphyses of long bones and in the cuboid bones (including the
vertebrae). In trabecular bone, spicules form a three-dimensional
branching lattice aligned to applied mechanical stresses. Conversely,
cortical bone has a fairly uniform density. Cortical bone forms the
“envelope” of cuboid bones and the diaphysis of long bones and
constitutes 80% of total bone mass.
Osteoblasts and osteocytes arise from the same lineage but differ in
location and function. Osteoblasts produce osteoid, or bone matrix;
line the surface of bone; and follow osteoclasts in cutting cones.
Osteocytes are osteoblasts encased in a mineralized matrix but are in
chemical contact with the osteoblasts on the bone surface by cellular
processes through canaliculi. Osteoblasts receive the endocrine
signals, then transmit them to the osteocytes. Strain-generated signals
within the bone are regulated by osteocytes and are passed on to the
osteoblasts. Osteoclasts are the major resorptive cells of bone and are
characterized
by
large size (20 to 100 µm) and multiple nuclei. These cells are derived
from pluripotent cells of bone marrow and bind to the bone surface
through cell attachment proteins (integrins).
removing old bone and creating new bone. In osteoporosis, there is a
decrease in the rate of bone formation relative to the rate of
destruction. In contradistinction, osteomalacia represents a
dysregulation of bone mineralization in the context of normal osteoid
production. Given the lower rates of formation in osteoporosis, the
overall mineral density of the bone decreases. With unbalanced
osteoclast activity, the normal connectivity of bone trabeculae is
lost. The bone is weakened in a material and in an architectural sense.
Although there are many environmental, genetic, and pharmacologic
factors affecting the development of osteoporosis, the root etiology of
this dysregulation is not yet understood and is probably multifactorial.
North America alone. This number is expected to triple over the next 3
decades with the aging of the population. VCFs are the most common
manifestation of spinal osteoporosis and are estimated to affect one
third of all North Americans at some point during their lifetime.
Outnumbering hip and wrist fractures combined, there are 700,000 VCFs
per year in the United States. In a population-based European study,
a12% prevalence of spinal fractures was recorded in men and women age
50 to 79 years. The direct medical costs associated with these
fractures have been estimated at $13.8 billion annually in the United
States alone. By 2030, annual direct costs are projected to exceed $60
billion, or $164 million per day. Indirect costs in lost productivity
are higher.
decrease in the organic and the inorganic phases of bone. With age,
everyone loses bone mass at approximately 0.5% per year, but not
everyone develops osteoporosis. The two most important determinants for
the development of osteoporosis are peak bone mass and the rate of bone
loss.
effective way to prevent the devastating complications of VCF is to
increase peak bone mass in pubertal patients. Disorders such as
anorexia and exercise-induced amenorrhea lead to profound osteoporosis.
Several studies have documented increasing rates of osteoporosis among
young women. Lack of weight-bearing exercise and changes in dietary
habits have been implicated.
The rate of loss is accelerated through decreased exposure to gonadal
hormones (i.e., menopause); genetic, environmental, and nutritional
conditions; and chronic disease states. Estrogen deficiency is
implicated directly in accelerated bone loss at 2% to 3% per year for
10 years. The mechanism of bone loss resulting from normal aging is
poorly understood, but its rate is equivalent in women and men. Other
endocrinopathies and risk factors associated with osteoporosis are
listed in Table 24-1.
|
TABLE 24-1 RISK FACTORS FOR OSTEOPOROSIS
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||
Stages of bone loss are classified by the t-score (see later). Bone
mineral density more than 1 SD below the mean young adult value is
defined as osteopenia. When the density is
more than 2.5 SD below the mean, the patient has osteoporosis. Bone
mineral density more than 2.5 SD below the mean with fragility
fractures is termed severe osteoporosis.
-
Type I (postmenopausal)—affects
women more often than men (hypogonadic men get this form of
osteoporosis as well). Patients are affected in their 50s and 60s, and
fractures of trabecular bone (wrist and spine) predominate. -
Type II (senile)—affects men and women equally, occurs in the 70s and 80s, and increasingly affects cortical bone.
-
Type III (secondary)—medications and disease states contribute.
-
Decreased calcium absorption across the intestinal lumen
-
Increased calcium loss from kidney
-
Direct inhibition of bone matrix formation (secondary hyperparathyroidism)
damage. Calcium, vitamin D, and antiosteoporotic medications counter
some of the deleterious effects.
morphologically. Just as osteoporotic fractures of the proximal femur
are divided anatomically into femoral neck and intertrochanteric
fractures
and are subdivided based on fracture pattern, axial skeleton injuries
first are described based on spinal level and then on fracture pattern.
The most common injury is VCF. There are a wide range of fracture
patterns, including failure of the superior, inferior, and both end
plates. Lateral compression deformities may worsen preexisting coronal
plane deformities (Fig. 24-1A).
In the lumbar spine, these fractures may include collapse of the
central portion of the superior and inferior end plates and has been
termed a biconcave or codfish vertebra.
In the thoracic spine, the anterior portion of the superior end plate
most typically is involved and leads to a wedge-compression fracture. A
senile burst fracture represents increased axial loading and failure of
the middle column (posterior vertebral body) with retropulsion of bone
into the spinal canal. Sacral insufficiency fractures may occur in the
context of insufficiency fractures of the pelvis with concomitant pubic
ramus fractures or as isolated injuries.
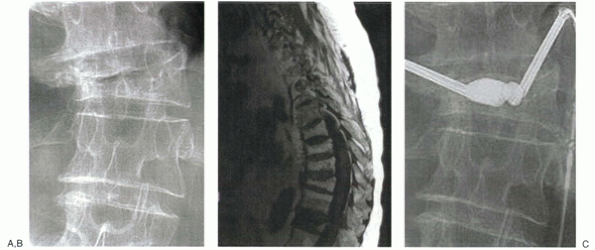 |
|
Figure 24-1
A 74-year-old man with secondary osteoporosis and degenerative scoliosis complained of severe midback pain and balance difficulties after a fall from a stepladder. Anteroposterior radiograph (A) shows osteopenia and a lateral vertebral compression fracture. Pain failed to respond to 4 weeks of management with narcotics and a Cash brace, and MRI was obtained. T1-weighted image (B) shows decreased signal intensity in T12. Increased signal was noted on T2 image (not shown). The patient was point tender over this level. Based on his continued pain and functional limitation, he was offered kyphoplasty. Anteroposterior fluoroscopic image during early balloon inflation (C) shows differential inflation on the more compressed side. The patient reported continued improvement for the next 2 weeks and ultimately returned to his baseline 3/10 visual analogue pain scale pain level. He was started on alendronate (Fosamax), calcium, and vitamin D. |
lowenergy (fragility) fractures occur. The most common sites for these
fractures are the spine, ribs, hips, and wrists. The patient reports
localized pain in these areas. Diffuse bone pain is a feature of
osteomalacia and is not seen with osteoporosis (Table 24-2).
Before fracture, osteoporosis is identified based on screening studies
in at-risk populations. All physicians, including orthopaedic and spine
surgeons, must ensure that their at-risk patients have been screened
and, if necessary, treated for osteoporosis (Table 24-3). Patient outcomes after primary prevention greatly exceed that of fracture stabilization.
osteoporosis, the work-up begins with a careful history. Fracture
occurs in the context of minimal to no trauma and leads to focal,
intense, deep midline spine pain. Fracture pain must be differentiated
from muscular pain, which more typically is diffuse pain or
paravertebral. Symptoms should be primarily mechanical and vary with
activity and loading. It is necessary to understand the time course of
the patient’s symptoms and the course of any previous fractures. The
physician should ask about associated thoracic or lumbar radicular
complaints. Past medical history, including a history of cancer,
tuberculosis, or systemic infection, is investigated to ascertain
appropriate treatment of underlying osteoporosis. Patients with night
pain, fevers, chills, unusual weight loss, or bowel or bladder changes
require more intense investigation.
and sagittal spinal balance are assessed. Body shape, difficulty
breathing, and obesity each affect the likelihood of effective bracing.
Associated rib tenderness should be sought because coexisting and
iatrogenic rib fractures are common. Acute VCFs and burst fractures
typically are point tender over the spinous process. A complete
neurologic exam should be conducted. Although major neurologic deficits
are rare (0.05%), many patients have significant stenosis or
neuropathic changes. Sacral insufficiency fractures cause pain over the
sacral body, in the sacroiliac joint regions, or in a bandlike
distribution across the low back. Maneuvers that stress the sacroiliac
joint, such as Gaenslen’s sign or Patrick’s test, increase this pain.
|
TABLE 24-2 COMPARISON OF OSTEOPOROSIS AND OSTEOMALACIA
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
exclude other causes of osteopenia, such as osteomalacia. Laboratory
studies may be abnormal in osteomalacia, which should be suspected when
the product of the serum calcium level multiplied by the serum
phosphate level remains chronically less than 25 mg/dL. Serum alkaline
phosphatase levels are elevated, and 24-hour urinary calcium excretion
may be less than 50 mg. Occasionally, serum blood tests alone are
insufficient to exclude the diagnosis of osteomalacia, at which time a
transiliac bone biopsy may be indicated.
|
TABLE 24-3 INDICATIONS FOR DUAL-ENERGY X-RAY ABSORPTIOMETRY SCREENING FOR OSTEOPOROSIS
|
|||||||
|---|---|---|---|---|---|---|---|
|
50 years old with idiopathic osteopenia, in patients when osteomalacia
is highly suspected, or in chronic renal failure patients with skeletal
symptoms. Two weeks before the biopsy, tetracycline is administered
twice each day for 3 days. This dose is repeated in the 3 days
immediately before biopsy. The tetracycline binds to newly mineralized
osteoid and permits the determination of mineralization rates. In
osteoporosis, a normal mineralization pattern of two distinct bands of
fluorescence, representing the tetracycline labels, is noted. With
impaired mineralization, a single band of fluorescence is encountered.
to provide complementary information to densitometry. Markers of bone
formation, such as bone-specific alkaline phosphatase (an osteoblast
enzyme) and osteocalcin (a bone matrix protein), and bone resorption,
such as urinary collagen degradation products (cross-linked
telopeptides and pyridinolines), offer improved prediction of future
fracture risk and a sensitive means to monitor therapy effectiveness.
In patients with unusual fracture patterns or histories suggesting
malignancy or infection, laboratory evaluation may include
sedimentation rates, differential blood counts, C-reactive protein
assays, tumor antigens, and protein electrophoresis.
assessing bone density. A decrease in bone mass of at least 30% is
necessary to detect osteopenia. More accurate measurements of bone mass
are crucial in the diagnosis and treatment of osteoporosis. Noninvasive
bone densitometry provides information about the specific site
measured, and density measurements in the lumbar spine correlate well
with the incidence of vertebral fracture. The first widely available
densitometry test was dual-photon absorptiometry, which measured axial
skeletal bone mineral density via soft tissue signal attenuation using
radioisotopes. Since the 1990s, dual-energy x-ray absorptiometry (DEXA)
has become the standard. This x-ray-based modality has significant
advantages over dual-photon absorptiometry, including:
-
Superior precision (1% to 2% at spine, 3% to 4% at femur)
-
Lower radiation dose
-
Shorter examination time
-
Higher image resolution
-
Greater technical ease
at-risk patients and to track response to therapy. The t-score compares
the patient’s bone mineral density with mean values for healthy
same-gender young adults. For each 1 SD below the norm, fracture risk
increases 1.5-fold to 3-fold. A t-score of -1 implies a 30% chance of
fracture. The z-score compares bone mineral density with age-matched
controls. A z-score less than -1.5 warrants a more extensive work-up
for underlying causes of the bone loss. DEXA values are falsely
increased with scoliosis, compression fractures, bone spurs,
extraosseous calcification, and vascular disease.
cross-sectional image of a vertebral body and allows preferential
measurement of trabecular bone density. Because the rate of turnover in
trabecular bone is eight times that in cortical bone, quantitative CT
is a sensitive indicator of bone density in highly vulnerable skeletal
areas. Quantitative CT involves the simultaneous scanning of tubes
containing standard solutions of a bone mineral equivalent. A standard
calibration curve is calculated, and vertebral trabecular bone density
is extrapolated. Measurements are taken from centers of vertebral
bodies, and values from T12 to L4 are averaged to yield a mean bone
density. This method allows exclusion of osteophytes and aortic
calcifications and is accurate to within 5% to 10%. Cost and radiation
dose are higher, however, than with DEXA. Ultrasound is an attractive
means of measuring bone density because it does not expose a patient to
ionizing radiation. Although these methods are rapid and inexpensive,
they are not as precise as DEXA and are useful mainly for initial
screening.
-
Extent of vertebral collapse
-
Location and extent of any lytic process
-
Visibility and degree of pedicular involvement
-
Presence of cortical destruction
-
Presence of epidural or foraminal stenosis
-
Age or acuity of the fracture
radiography. With standing radiographs, overall sagittal and coronal
spinal balance is apparent. Thoracolumbar fractures are discovered
readily, but sacral fractures are difficult to see. Determination of
the age or acuity of the fracture is more difficult. Comparison films,
including old chest radiographs, may be helpful, but apparent sclerosis
may represent healing or merely compressed bone. Spot films,
particularly at the thoracolumbar junction, aid visualization. During
early patient management for acute fracture, plain radiographs should
be followed serially over the short term to assess for further collapse.
posterior cortical compromise, such as widened pedicles and greater
than 50% height loss. End plate erosion suggests infection or pedicular
destruction (“winking owl” sign) as seen in malignancy. Fractures above
T6 are more likely to represent neoplasm.
The edema seen in acute fractures is reflected by increased signal on
T2 or short tau inversion recovery sequences. Acutely, fractures show
decreased T1 signal, and T1 and T2 marrow signal changes normalize over
time (see Fig. 24-1B). MRI may reveal several
key features that differentiate malignant from osteoporotic compression
fractures, including pedicular and soft tissue extension.
compression fractures. This continuing collapse of the vertebra after
minor trauma is particularly common in patients with known risk factors
for avascular necrosis, such as previous radiation therapy or long-term
corticosteroid use. On MRI, these fractures show the “double line sign”
of discrete fluid collections within a vacuum cleft with areas of
diminished T2 signal surrounding the cleft.
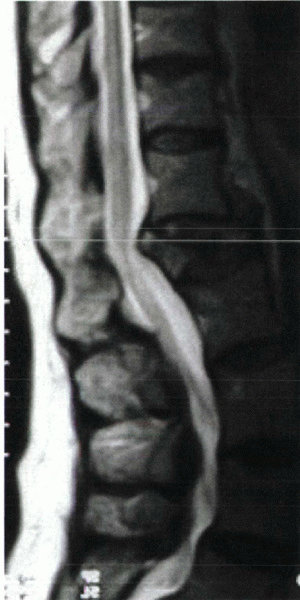 |
|
Figure 24-2
A 63-year-old woman with postmenopausal osteoporosis was admitted to the hospital with bladder incontinence and severe back pain after a slip and fall injur. MRI showed an L1 osteoporotic burst fracture with conus compression. |
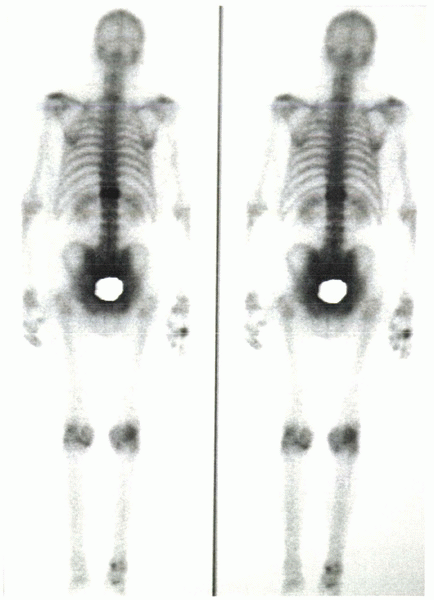 |
|
Figure 24-3 Bone scan shows intense uptake at T11 in a patient with 4 months of pain after sustaining a vertebral compression fracture.
|
and soft tissue contrast and clearly delineates posterior cortical
compromise. Fracture acuity may be determined by bone scan (Fig. 24-3).
MRI and CT show sacral insufficiency fractures. On bone scan, these
lesions may have the classic H configuration or may appear as a linear
band of increased uptake in the region of the sacral ala (Fig. 24-4C).
-
To identify decreased bone mineral density
-
To exclude the osteopenia of malignancy, osteomalacia, or secondary osteoporosis
-
To assess bone turnover state
In patients with t-scores less than -1.5, further evaluation is
undertaken. For younger patients and women with a questionable
menstrual history, a hormonal profile, including sex hormones,
thyroid-stimulating hormone, thyroxine, and parathyroid hormone (PTH),
should be ordered. Standard chemistries include serum calcium,
phosphate, alkaline phosphatase, creatinine, and a 24-hour urine
calcium excretion. If no clearly identifiable cause of osteopenia is
found, an iliac crest bone biopsy and marrow aspiration are indicated.
physician should obtain a good history, perform a thorough physical
exam, and evaluate plain radiographs. In patients with red flags,
neurologic involvement, or long-standing pain, MRI is recommended. In
patients unable to undergo MRI, CT and bone scan are ordered.
because of delayed and inaccurate diagnosis, insufficient understanding
of the disease process, and inadequate follow-up. The primary goal of
treatment is to decrease fracture risk (Table 24-4).
Optimal treatment begins before the first fracture and focuses on the
material and structural properties of bone by calcium and physiologic
vitamin D administration and mild weight-bearing exercise. These
measures are intended to decrease bone resorption and to mineralize
osteoid, but they do not increase total bone mass. Studies have shown
that individuals taking calcium supplements have a quarter of the hip
fractures of individuals with low calcium intake, but excess calcium
may be harmful. Other early management addresses fracture risk by fall
prevention. Household obstacles should be removed. Tai chi (an Asian
form of exercise) has been favored because it improves patient balance.
appropriate. Estrogen receptors have been identified in bone-forming
cells. Estrogen acts to block the action of PTH on osteoblasts and
marrow stromal cells, and estrogen supplementation decreases bone loss
by acting to counter the effect of unopposed PTH activity. Without
estrogen, osteoblasts and marrow stromal cells secrete increased levels
of interleukin-6, which stimulates the osteoclasts to resorb bone.
Estrogen does not alter bone formation rates appreciably, but it does
increase bone mass slightly by slowing resorption. More recent studies
seem to show increased rates of coronary artery disease, stroke,
pulmonary embolus, and cancer in women on hormone replacement therapy.
The potential of untoward side effects of estrogen has increased
interest in selective estrogen receptor modulators, such as raloxifene
(Evista). These agents seem to have similar bone-preserving effects as
estrogen without oncogenic or adverse cardiac effects.
with fractures or with femoral t-scores less than -2.5 should be
undertaken. Calcitonin, via subcutaneous
injection
or nasal spray, decreases osteoclastic bone resorption. Over the short
term, calcitonin enhances bone formation, leading to a slight net bone
accretion. With long-term treatment, osteoblastic activity slows, and
bone mass stabilizes.
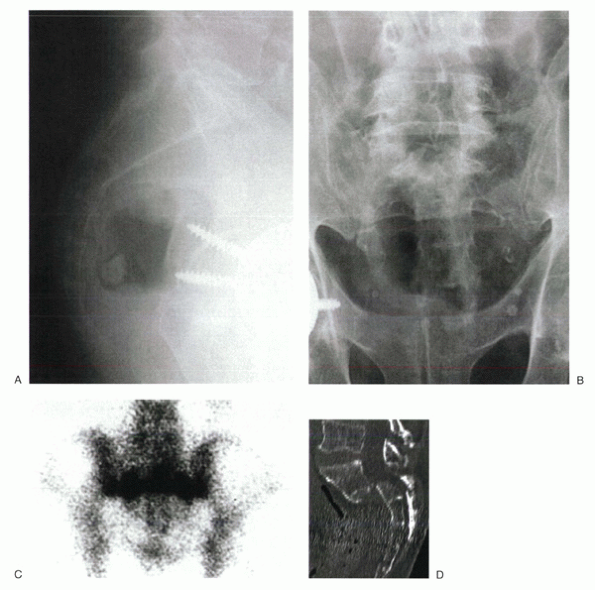 |
|
Figure 24-4
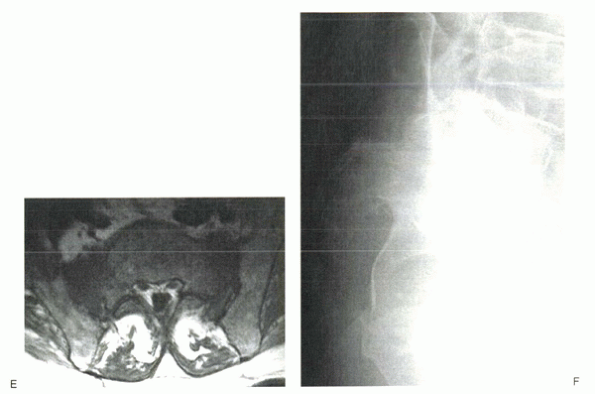
An 84-year-old man complained of severe sacral pain after “sitting down too hard.” He was point tender over the sacral body, and his pain increased with Patrick’s test. Initial antero-posterior (A) and lateral (B) radiographs show only osteopenia. Scintigraphy in patients with sacral insufficiency fractures generally shows intense uptake, often in an “H” pattern, over the sacrum (C). This patient was mobilized gradually, but sustained a second episode of falling into his hard kitchen chair. New lateral radiographs showed complete displacement of the S1-2 segment. CT scan with sagittal reconstructions shows a transverse fracture through S1-2 (D). MRI typically shows an abnormal signal pattern through the sacral ala (E). After his second fall, the patient reported urinary retention and ultimately was admitted to the hospital for pyelonephritis. He had toe flexor weakness. He underwent a laminectomy and noted improvement in neurologic symptoms (F). |
and decrease the risk for hip and spinal fractures. These agents
directly stabilize the bone crystal, making it more resistant to
osteoclastic bone resorption. They also inhibit osteoclast activity.
Bisphosphonates preserve bone architecture and overall density. Weekly
forms of these agents afford better compliance with no increase in
toxicity.
to early, dramatic increases in bone mass, especially in trabecular
bone. The long-term safety and efficacy of these protocols have not yet
been established, and clinical trials are under way. Ultimately, for
patients with severe osteoporosis, combination therapies linking an
anabolic agent with an antiresorptive agent along with calcium and
vitamin D supplementation may be ideal.
 |
|
Figure 24-4 (Continued)
|
-
Decreased pain
-
Early mobilization
-
Preservation of sagittal and coronal spinal stability
-
Prevention of late neurologic compromise
to their physicians typically are offered pain medications and braces.
Limited activity and often bed rest are advised or self-imposed. In
patients with osteoporosis, bed rest is associated with an additional
4% loss of bone mineral density.
|
TABLE 24-4 DETERMINANTS OF FRACTURE RISK IN OSTEOPOROSIS
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
in some circumstances pain persists beyond 3 months. Narcotic pain
medications may be continued until the patient can bear weight
comfortably. In elderly patients, use of narcotics may be associated
with as many functional problems as the underlying fracture. Nasal
calcitonin and bisphosphonates, useful in the treatment of
osteoporosis, also may be effective in decreasing fracture-related pain.
extension brace or Cash brace, is easy to fit, but compliance varies.
In short, obese, elderly patients, body habitus limits brace
effectiveness. Patients with concomitant shoulder problems have
difficulty donning and doffing the brace. Physical therapy may aid the
patient’s recovery to mobility.
refractory to nonoperative measures and require hospitalization, with
protracted periods of bed rest and narcotics. Fractures less likely to
improve with standard medical management include fractures of the
thoracolumbar junction (T11-L2), fractures with burst patterns, wedge
compression fractures with more than 30 degrees of sagittal angulation,
fractures with a radiographic vacuum shadow in fractured body (ischemic
necrosis of bone), and fractures with progressive collapse in office
follow-up.
indicated in some patients with continuing vertebral collapse or
intractable pain (Table 24-5). Vertebral
augmentation with polymethyl methacrylate (PMMA) variably restores
strength and stiffness to a fractured body. Strength reflects the
ability of the vertebral body to bear load and may protect against
future fracture of the treated segment. Stiffness limits micromotion
within the compromised vertebral body and is ostensibly the source of
symptom relief.
vertebral body through a 1-cm incision. With vertebroplasty the
vertebra is filled with liquid PMMA through an 11-gauge needle (Fig. 24-5). With kyphoplasty, a balloon tamp is used first to create a void in the bone and to attempt fracture reduction (see Fig. 24-1C)
These procedures may be performed under general anesthesia or with
local anesthesia and intravenous sedation. The patient is placed prone
on a radiolucent table or frame and bolstered to allow partial postural
reduction of the fracture. The crucial first step in either procedure
is to obtain true anteroposterior and lateral images with fluoroscopy.
Most typically, a transpedicular route to the vertebra is selected. In
some thoracic cases, the narrow and straight pedicle precludes
appropriate medialization, and an extrapedicular approach is required.
Kyphoplasty usually is performed through a bilateral approach.
Vertebroplasty may be performed unilaterally or bilaterally.
11 -gauge Jamshidi needle is positioned at the 10 o’clock or 2 o’clock
position on the pedicular ring. In contrast to pedicle screws, the goal
is not to proceed “straight down the barrel,” but rather to medialize
through the cylinder of the pedicle. The clinician starts lateral and
aims medial. When in bone, the clinician verifies the trajectory on the
lateral image. If the anteroposterior and lateral images do not show a
clearly intrapedicular position, an en face or oblique view is useful.
When the tip of the needle reaches the medial border of the pedicle on
the anteroposterior view, it must be at or anterior to the junction of
the pedicle and vertebral body on the lateral view.
|
TABLE 24-5 INDICATIONS AND CONTRAINDICATIONS FOR VERTEBRAL BODY AUGMENTATION
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
|
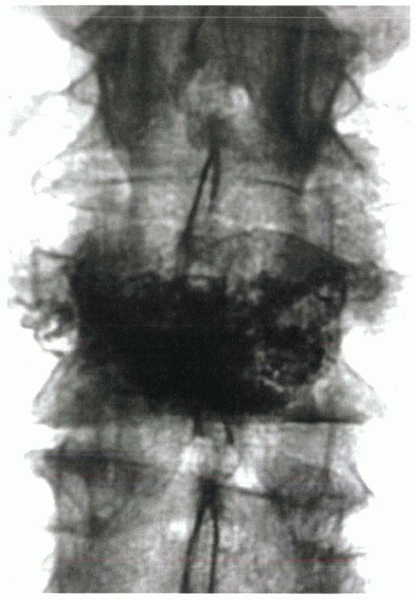
Figure 24-5 Postoperative radiograph after a midlumbar vertebroplasty performed through a unipedicular approach with a curved needle.
|
employed for vertebroplasty, most require advancement of the cannulae
into the central portion of the vertebral body. PMMA is mixed and
delivered under live fluoroscopy. In kyphoplasty, additional
instruments are employed. The Jamshidi needle is replaced with a
working cannula through which inflatable bone tamps are delivered.
These balloons are inserted to within 4 mm of the anterior vertebral
body cortex. When both balloons have been placed, they are inflated
sequentially in 0.5-cc increments until one of the following end points:
-
Realignment of vertebral end plates
-
Maximal balloon pressure (>220 psi) without decay (i.e., loss of pressure)
-
Maximal balloon volume—4 cc for 15/3 and 6 cc for 20/3
-
Cortical wall contact
with viscous PMMA. Sterile barium is added to the powder to increase
its radiopacity. For vertebroplasty, the PMMA is injected into the body
in a fairly liquid state to allow
it
to interdigitate between the crushed trabeculae of the fracture. For
kyphoplasty, the PMMA is inserted when it reaches a toothpaste-like
consistency. The balloons are removed, and the PMMA is implanted under
continuous fluoroscopy. The wound may be closed with a suture or
Steristrip. No braces or particular postoperative precautions are
needed. Newer resorbable calcium phosphate and hydroxyapatite cements
also are being tested.
insufficiency fractures. If there is a concomitant pubic ramus
fracture, limited weight bearing on the affected side and walker
ambulation are recommended. For bilateral fractures, a walker helps the
patient decrease weight bearing through the fracture to a degree. There
are no effective braces for these injuries. For fractures with
significant displacement or neurologic compromise, operative reduction
and stabilization may be required.
are more common than previously thought. In my experience, fractures
with more than 50% height loss usually have associated posterior
cortical compromise. In many cases, this compromise takes the form of
buckling of the cortex. If the canal occlusion is less than 33%, VBA
procedures may be entertained in select cases. If comminution exists,
VBA should not be undertaken because of the increased risk of cement
extravasation. In patients with neurologic compromise, open surgery may
be required.
in the context of significant or progressive neurologic deficit or
deformity. Operative intervention is associated with high morbidity and
mortality in this frail patient population. Similarly, spinal
instrumentation systems often fail in osteoporotic bone. Often,
combined anterior and posterior surgeries are required to achieve
adequate fixation. Screws can be augmented with PMMA to increase their
pull-out strength.
increasingly evident that any VCF can have significant functional and
physiologic effects, such as:
-
Acute and chronic pain
-
Recurrent fracture
-
Kyphotic deformity
-
Gastrointestinal dysfunction
-
Pulmonary dysfunction
-
Functional decline
-
Increased hospitalization rates
-
Increased mortality
note only mild and transient symptoms, others require hospitalization.
Although most patients report significant symptomatic improvement in
the first 4 weeks, the period of acute pain can persist for months.
When the acute pain subsides, chronic pain disorders can develop. Many
of these disorders seem to arise from the change in the sagittal
balance of the spine. Some patients report painful rubbing of the ribs
on the ilium. The risk of developing chronic pain increases with the
number of VCFs.
activities, such as standing, sitting, or bending. In many patients,
standing tolerance decreases to only a few minutes. Pain is relieved on
lying down, but the increase in bed rest, just as in patients with
acute fractures, serves only to accelerate bone loss.
truncal strength and with greater back-related disability, annual
number of bed days, and annual number of limited-activity days. This
loss of strength and decrease in activity level increases the risk of
additional fractures. Various studies have cited increased risks of
additional spinal fractures from 5 to 25 times baseline. Similarly the
risk of hip fracture increases five times in patients sustaining VCF.
In one study of physical function, common tasks, such as walking,
bending, dressing, carrying bags, climbing stairs, rising from supine
position, and rising from seated position, were assessed. Only 13% of
VCF patients were able to accomplish these activities without
difficulty, 40% had difficulty, and 47% required assistance.
types may have multiple physiologic implications. Taken together, the
osteoporotic body habitus is characterized by loss of height and
thoracic hyperkyphosis (the dowager’s hump). Abdominal protuberance and
loss of lumbar lordosis also may be noted. Many otherwise active,
elderly patients complain bitterly about the cosmetic effects. Women
sustaining osteoporotic vertebral compression fractures report many
debilitating psychological effects, including poor body image and
self-esteem, depression, and anxiety. Beyond the cosmetic effects,
compression on the abdominal viscera by the rib cage or by loss of
height through the lumbar spine leads to decreased appetite, early
satiety, and weight loss. Similarly, thoracic hyperkyphosis leads to
compression of the lungs, decreased pulmonary function, and an
increased risk of pulmonary death.
Although uncommon, deficits are not as rare as first thought. Tardy
neurologic decline may occur 18 months from the initial injury. These
late neurologic changes are thought to represent dysfunction of the
spinal cord as it drapes over the apex of kyphosis. The 5-year survival
after osteoporotic spinal fracture is significantly worse than for
age-matched peers (61% versus 76%) and is comparable to, or slightly
worse than, survival rates after hip fracture. Mortality risk increases
with the number of fractures.
cycle of decline seen in patients with VCF. There are no randomized
trials yet comparing nonoperative management with VBA. Many case series
have shown, however, that vertebroplasty and kyphoplasty procedures
tend to be well tolerated and associated with 70% to 95% pain relief.
outcomes of osteoporotic VCF treated by percutaneous vertebroplasty. On
a 100-mm visual analogue scale, pain decreased significantly from a
mean of 80 mm to 37 mm at 1 month. Results were stable over time. There
were no severe treatment-related complications. The vertebral deformity
did not progress in any of the injected vertebrae. A slight, but
significant, increase in adjacent segment fracture risk was reported. Garfin et al noted that 95% of patients treated with either kyphoplasty or vertebroplasty could expect significant
improvement in pain and functional status. Kyphoplasty conferred the additional advantage of 50% increase in vertebral height. Lieberman et al
reported the use of kyphoplasty inflatable bone tamp in the treatment
of symptomatic VCF in 70 consecutive procedures in 30 patients. There
were no major technique-related complications. A mean 47% height
restoration was encountered in 70% of the fractures. Bodily pain and
physical function scores showed significant improvement.
same types of complications. There are no unique complications reported
from use of the balloon tamp or from attempted reduction. Complications
can be categorized into medical, anesthesia, instrument placement, and
PMMA problems. Failure to improve, in most cases, is due to
inappropriate patient selection. In any spinal procedure, concordance
between the history, physical examination, and imaging findings
improves outcome. The more diffuse the patient’s pain, the less likely
the patient is to benefit from VBA. Placement of PMMA may increase the
risk of adjacent segment fracture. The correction of proper
weight-bearing axis with kyphoplasty may decrease the risk of
additional fracture.
elderly patient population, but VBA procedures are not significantly
physiologically taxing. Technical errors related to misplacement of the
vertebroplasty or kyphoplasty instrumentation are more likely. Quality
imaging and meticulous surgical technique with frequent evaluation of
anteroposterior and lateral fluoroscopy decrease these risks. The most
devastating complications of VBA procedures result from PMMA
extravasation. In vertebroplasty, a 6% leak risk per level has been
identified. Most of these leaks are asymptomatic. One potential benefit
of kyphoplasty is the placement of more viscous cement into a cavity of
known volume. High-quality, live image intensification during
placement, additional radiopacifying agent, avoidance of high-risk
fracture patients, and placement of viscous PMMA decrease these risks.
H, Maezawa Y, Kamitani K, et al. Osteoporotic vertebral collapse with
late neurological complications. Paraplegia 1995;33: 281-289.
SR, Yuan HA, Reiley MA. New technologies in spine: kyphoplasty and
vertebroplasty for the treatment of painful osteoporotic compression
fractures. Spine 2001;26:511-515.
DM, Browner WS, Palermo L, et al. Vertebral fractures and mortality in
older women: a prospective study. Arch Intern Med 1999;159:1215-1220.
IH, Dudeney S, Reinhardt MK, Bell G. Initial outcome and efficacy of
“kyphoplasty” in the treatment of painful osteoporotic vertebral
compression fractures. Spine 2001;26:1631-1638.
MC, Ettinger B, Black DM, et al. The association of radiologically
detected vertebral fractures with back pain and function: a prospective
study. Ann Intern Med 1998;128:793-800.
TW, Felsenberg D, Varlow J, et al. The prevalence of vertebral
deformity in European men and women. The European Vertebral
Osteoporosis Study. J Bone Miner Res 1998;11:1010-1018.
C, Minne HW, Bruckner T, et al. Reduced pulmonary function in patients
with spinal osteoporotic fractures. Osteoporos Int 1998;8:261-267.
