ORTHOPAEDIC MANAGEMENT OF LOWER EXTREMITY DYSFUNCTION FOLLOWING STROKE OR BRAIN INJURY
VII – NEOPLASTIC, INFECTIOUS, NEUROLOGIC AND OTHER SKELETAL DISORDERS
> Other Disorders > CHAPTER 123 – ORTHOPAEDIC MANAGEMENT OF LOWER
EXTREMITY DYSFUNCTION FOLLOWING STROKE OR BRAIN INJURY
Director, Neuro-Orthopaedics Program, Department of Orthopaedic
Surgery, Albert Einstein Medical Center, 19140; Professor of
Orthopaedic Surgery, Professor of Rehabilitation Medicine, Temple
University School of Medicine, Philadelphia, Pennsylvania, 19140.
injury (TBI) are distinct syndromes; however, the stroke patient and
brain-injured patient share many features (4,12,13,16,20,22,23,25,26,28,40,42,45,49,50,55,57,78,80,89,91,94,95 and 96,99,100 and 101,103,106,115,122,127,144,147,148,156,162,163,164 and 165,174,176,177,178,179 and 180,215,216,224,230,231,237,238,241,250).
Both patient groups exhibit upper motoneuron (UMN) syndromes with
impairment of motor control, spasticity, and stereotypic patterns of
movement (synergy). Cognitive, memory, and sensory deficits are also
commonly seen in these patients. Because of the similarities between
stroke and TBI, there is a great deal of overlap in terms of specific
surgical and nonsurgical techniques for treating the lower extremity
problems caused by these conditions.
peripheral nervous system, and the musculoskeletal system, as well as
lesions causing pain, may lead directly or indirectly to syndromes of
restricted or excessive motion of the limbs. Syndromes of restricted
limb motion are the most common types of movement impairment. Syndromes
of excessive motion are less common. A distinction between restricted
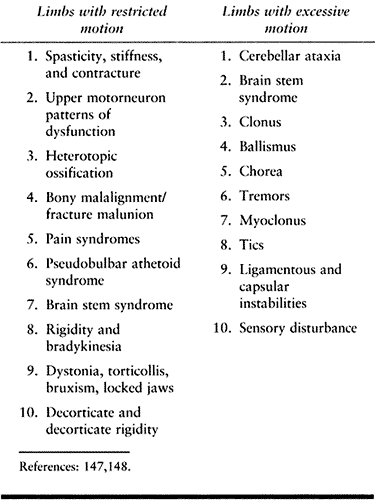
versus excessive motion is shown in Table 123.1 (147,148). The functional implications of each and the treatment of the problems they generate are very different.
 |
|
Table
123.1. Clinical Phenomena Associated with Impaired Movement that Functionally Lead to Restricted or Excessive Motion after TBI or CVA |
impaired access of the limb to targets in the environment during
voluntary movement. Limbs are unable or are poorly able to move toward
objects or places because motion across joints is restricted. An
example is a patient with spastic hip and knee flexors who attempts to
stand with a crouched posture. Another example seen after head injury
is heterotopic bone formation about the hip, which restricts joint
motion and impairs use of the lower extrem-ity even in the presence of
voluntary muscle action. Limbs with restricted motion lose their
operating range and are unable to be positioned adequately for
function. The general treatment strategy for limbs with restricted
motion is to identify and reduce sources of limb restriction.
impaired tolerances in the production of voluntary movement parameters
such as movement amplitude, accuracy, timing, and force. Clinical
conditions associated with excessive
motion
seen after head injuries include movement disorders such as
hemiballismus, athetosis, tremors, and cerebellar ataxia. Biomechanical
laxity in the musculoskeletal system may also be associated with
excessive motion. For example, posterior subluxation of the knee may
lead to excessive motion and instability during standing.
confines of the skull and the subsequent cognitive function of the
brain. The musculoskeletal system is profoundly affected by brain
dysfunction. Hypertonicity, the unmasking of primitive reflexes and
impaired motor control all contribute to the abnormal limb positions,
contractures, and impaired mobility that are so frequently encountered
in persons with brain injury.
affected by dysfunction of the musculoskeletal system. Just as the hip
and knee position of the foot for walking, the musculoskeletal system
gives mobility to the brain and positions it to interact with the
world. Mobility of the individual is a key element of human life and of
fundamental importance to our well-being.
stroke rehabilitation are knowledgeable about the cognitive and
behavioral deficits that accompany brain injury. It has been our
experience that less importance has been given to the musculoskeletal
impairment that results from brain trauma or stroke. The penalties of
musculoskeletal limitations for the individual can be devastating.
Improving an individual’s physical mobility is often therapeutic,
leading to increases in cognitive, behavioral, and emotional capacities.
care. This cannot mean the complete prevention of disease, injury, and
disability. In the physically disabled population, wellness promotion
means maximizing function and mobility in order to avoid the
complications of their chronic incapacity. Potential complications of
physical immobility include decubiti, infection, pain, social
isolation, physical dependence, and emotional dependence. For society,
this results in a costly loss of productivity for the patient and often
family members as well.
commonly arise regarding the indications for surgery, the cost, what
outcome to expect, and the practicality of this approach. These issues
should be considered on an individual basis for each patient. The
following general principles can serve as guidelines for decision
making.
treatment that will correct a limb deformity or improve function.
Surgery should not be considered a treatment of last resort when
“conservative” measures have failed. Physical and occupational therapy
cannot effect a permanent change in motor control. Drug therapy for
increased muscle tone has generalized effects and cannot be targeted to
specific offending muscles. Phenol blocks and botulinum toxin
injections provide only temporary modulation of muscle tone. When a
permanent treatment is needed to decrease muscle tone or redirect
muscle force, consider surgery. The results of surgical intervention
are improved when deformities are corrected early. Less muscle
lengthening is needed when deformities are mild and there is little or
no fixed contracture to overcome. Early surgery preserves maximum
muscle strength, joint capsule and ligament flexibility, and articular
cartilage integrity. In general, the patient will also be in better
physiologic condition to undergo surgery if there has not been a period
of several years of immobility.
Orthopaedic surgery cannot impart control to a muscle. Lengthening a
spastic muscle can improve its function by diminishing the overactive
stretch response and uncovering any control that is present. Successful
surgery depends on a careful evaluation preoperatively to determine the
amount of volitional control present in each individual muscle that is
affecting limb posture and movement.
scale, with the totally disabled at the lower end and the elite athlete
at the upper end. Infinitesimal improvements in the performance of
elite athletes distinguish between the winner and loser. Incremental
changes in limb function also result in performance improvements for
the disabled individual. Surgery should not be reserved only for
patients with severe impairment and deformity. Individuals with milder
degrees of impairment can benefit greatly from relatively simple
procedures such as lengthening of the Achilles tendon to regain a
plantigrade foot for standing, transfers, and ambulation. The amount of
improvement correlates best with the degree of underlying motor control
and not the severity of the deformity.
We commonly speak of “functional” and “nonfunctional” surgical
procedures. These terms refer to the expected outcomes for a limb but
do not indicate the outcome for the person as a whole. Surgical release
of a leg contracted in a flexed and adducted position in a
nonambulatory, quadriplegic patient often allows the person to sit
comfortably in a wheelchair and become interactive with others.
(EMG) is relatively modest for the benefits it provides. Dynamic EMG is
a one-time expense. The cost of performing an incorrect surgical
procedure that fails to correct or even worsens a limb deformity is
much greater. The cost of performing a surgical procedure is likewise
limited when compared with a lifetime of attendant care, spasticity
medications, repeated blocks, orthotics to control limb position,
complications such as skin ulceration and infection, and lost
productivity for the patient and caretakers.
It is also the third leading cause of death. Roughly 600,000 people
suffer a new or recurrent stroke each year. Management of the stroke
patient has become a major priority for physicians who treat the
elderly population. There are 4,000,000 stroke survivors today. The
average patient who survives beyond the first few months after a stroke
has a life expectancy of more than 6 years (12,82,83). In general, stroke victims survive long enough and achieve adequate function to justify aggressive rehabilitation.
oxygen. Any significant interruption of oxygenation by thrombosis,
emboli, or hemorrhage will result in neuron death and subsequent
deficits in cognitive, sensory, and motor functions. Thrombosis is the
most common cause of infarction and accounts for nearly three fourths
of all CVAs (82,83). Arteriosclerosis is the most significant predisposing factor.
of all CVAs, and includes spontaneous intracerebral hemorrhage and
subarachnoid hemorrhage. Hypertension is commonly present in these
patients. Isolated cerebral emboli account for less than 10% of CVAs.
-
atherosclerosis
-
increasing age
-
genetic predisposition
-
hypertension
-
hyperlipidemia
-
hypercholesterolemia
-
obesity
-
cardiac anomalies (arrhythmias, myocardial infarction, hypotension, mural thrombosis)
-
diabetes mellitus
-
collagen vascular disease (vasculitis, polyarteritis)
-
hyperviscosity states (polycythemia, sickle cell anemia)
-
oral contraceptive use
-
tobacco smoking
-
severe cerebrovascular spasm secondary to migraine headaches
-
septic vasculitis (tuberculosis, syphilis, and mucormycosis).
specific areas of the cerebral cortex. CVAs involving the middle
cerebral artery are the most common and produce the typical hemiplegic
picture of greater impairment in the upper extremity, face, and speech
compared with lower extremity involvement. The middle cerebral artery
supplies the largest area of cerebral cortex. This area controls
sensory and motor function of the trunk, upper extremity, and face, as
well as the functions of speech.
the sagittal plane. This area of cerebral cortex controls sensory and
motor function predominantly in the lower extremity. CVAs involving the
anterior cerebral artery result in a hemiplegic picture of sensory and
motor deficits chiefly involving the lower extremity.
in the occipital region. Involvement of this artery typically results
in visual impairment. Bilateral cortical involvement may lead to severe
mental impairment, frontal release signs, loss of short-term memory,
and inability to learn.
in balance and coordination arise from interruption of afferent and
efferent pathways between the brain and spinal cord. Balance reactions
also depend on limb control and proprioception (96,115,144,198).
mentation, decreased learning ability, and loss of short-term memory
may occur (12,16,20,22,25,28,33,42,50,80,82,83,93,104,106,144,151,160,161,164,179,217,228,251).
The ability to cooperate with treatment affects rehabilitation
potential. In patients with extensive frontal lobe deficits, these
deficits may be severe. Patients with extensive frontal lobe pathology
exhibit clinical features similar to those of senility, with lack of
attention span and little motivation for recovery. Their prognosis for
rehabilitation is poor.
receptive or expressive in nature; it usually involves both components.
Aphasia occurs with lesions of the left hemisphere, usually without
regard to hand dominance. A receptive aphasia hinders rehabilitation
most strongly because the patient cannot understand instructions. A
patient
with persistent receptive loss has a poor prognosis. On the other hand,
expressive aphasia may be compatible with rehabilitation, allowing a
patient to comprehend and follow instructions. Expressive aphasia may
resolve significantly.
characterized by a loss of ability to perform a previously learned
action, such as tying shoelaces or waving goodbye. Apraxia is not the
result of motor or sensory loss. It occurs more commonly with right
hemispheric involvement (left hemiparesis). The apraxia, however,
occurs on both sides of the body. The prognosis for patients with
severe apraxia is generally poor. Some improvement with practice and
repetition may occur. If impairment persists after 3 months, further
improvement is unlikely (12).
Sensory perception occurs in the cerebral cortex and is most often
affected by lesions of the middle cerebral artery. Sensory loss may be
manifest by impairment of touch, pinprick, two-point discrimination,
proprioception, discrimination of size, shape, texture, or point
localization, or the presence of astereognosis. Impairment of sensory
function in the extremities is a poor prognostic sign, even though
motor function may be intact or only minimally impaired (4,12,16,20,22,25,26,27 and 28,40,42,60,73,78,80,89,94,106,110,115,122,127,129,134,143,144,147,148,150,151,156,162,163,164 and 165,176,179,180,184,194,203,226,227,230,237,238,246,250,251).
Lesions of the parietal lobe of the nondominant hemisphere may result
in a lack of awareness of the involved side of the body (neglect). A
failure to recognize and use the involved side may occur despite
minimal motor involvement.
include hemianopia (blindness in one eye), disturbance of perception,
poor perceptual organization, loss of geometric sense, inability to
copy figures, and failure to perform tasks involving spatial analysis.
Hemianopia is likely to be permanent, but it usually has little impact
on rehabilitation potential. Disturbances in visual perception are more
significant and may result in failures of activities of daily living (209).
stroke. Recovery follows a fairly typical pattern. A period of flaccid
paralysis occurs lasting from 24 hours to several weeks. This is
followed by a period of increasing muscle tone. In general, the longer
the period of flaccidity, the poorer the prognosis for functional
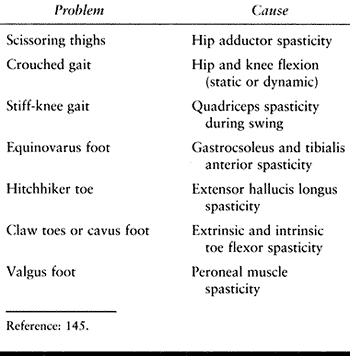
recovery. In the leg, either a flexor or externsor pattern may
predominate. With a flexor pattern, the hip and knee are flexed. With
an extensor pattern, the hip is adducted and extended, the knee is
extended, and the foot is in equinovarus (Table 123.2).
These changes are usually evident within 48 hours after the stroke. Any
paralysis remaining after 3 months will usually persist, although some
slight improvement may occur over a 6-month period (16,20,22,25,26,27 and 28,30,31,33,34,42,50,53,70,80,82,83,87,92,93,97,104,106,109,115,120,121,127,139,140,142,144,151,154,160,161,162,163 and 164,172,179,180,187,194,197,198,202,215,216,217 and 218,227,228,235,237,241,248,250,251,256,257).
Functional improvement may continue as a result of further sensorimotor
re-education. Increasing muscle tone usually leads to muscle
spasticity. Hyperactive deep tendon reflexes and clonus may appear.
 |
|
Table 123.2. Common Lower Extremity Limb Deformities
|
muscle groups of the limbs and follows in a proximal-to-distal
direction or pattern of recovery. Voluntary movement should be sought
and examined during the early recovery phase when flaccidity is present.
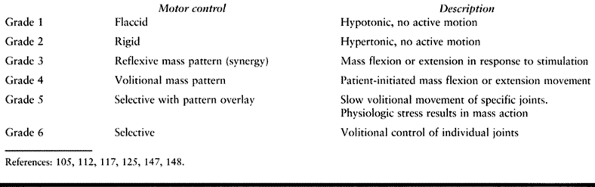
The extremity may be hypotonic or flaccid and without any volitional
movement. A spastic extremity may be held rigidly without any
volitional or reflexive movement. Patterned or synergistic motor
control is defined as a mass flexion or extension response involving
the entire extremity. Mass flexion in the lower extremity consists of
hip and knee flexion with dorsiflexion of the ankle. Mass extension in
the lower extremity consists of extension of the hip and knee with
equinovarus of the foot and ankle. Synergistic movement may be
reflexive and in response to a stimulus
but
without volitional control. Some patients can also volitionally
initiate the synergistic movement. Selective motor control with pattern
overlay is defined as the ability to move a single joint or digit with
minimal movement in the adjacent joints when performing an activity
slowly. Rapid movements or physiologic stress make the masspattern more
pronounced. Selective motor control is the ability to volitionally move
a single joint or digit independently of the adjacent joints.
Spasticity can mask underlying motor control.
 |
|
Table 123.3. Clinical Scale of Motor Control
|
a primitive form of motor control and of no functional use in the upper
extremity. The hand requires some selective control for functional use.
The lower extremity can more successfully use synergistic motions for
functional activities, such as transfers or walking. For example, the
patient can be taught to use the flexion movement to advance the limb
and the extension pattern to provide limb stability during stance.
cerebral cortex, where basic sensory information is integrated to
complex sensory phenomena such as proprioception, spatial
relationships, shape, sight, and texture. A patient with severe
parietal dysfunction and sensory loss may lack sufficient perception of
space and awareness of the involved segment of his or her body to
ambulate. A patient with severe perceptual loss may lack balance to
sit, stand, or walk.
of the patient. The orthopaedic surgeon is rarely involved in the acute
care of the stroke patient. In some situations, the orthopaedic surgeon
may be asked to assist with splinting extremities to prevent limb
deformities.
the first 6 months following a stroke. This is particularly true for
recovery of muscle function. During the subacute phase, limb flaccidity
changes to spasticity. The patient is commonly in a rehabilitation
facility for a portion of this time. Muscle weakness can result in
joint subluxation or ligamentous laxity if the limb is not protected.
When spasticity becomes pronounced, temporary measures are used to
prevent contracture formation until spontaneous neurologic recovery has
ceased. It is important to prevent the complications associated with
limb spasticity (Table 123.4) (121,122,147,148).
 |
|
Table 123.4. Complications of Spasticity
|
An epidemiologic study of physician-documented cases of TBIs occurring
in San Diego County, California, in 1981 determined an annual incidence
of 180 per 100,000 population (141).
population provides an estimate of 410,000 new cases of TBI cases each
year. Eleven percent of these patients will die shortly after the
injury. Approximately 80% of the survivors will have a good or moderate
neurologic recovery. Most traumatic injuries to the brain are in
individuals who are younger than 45 years of age, and those who survive
have a normal life span despite the injury.
The GCS is the total of the scores of a patient’s responses to eye
opening, motor responses, and verbal responses. Obtained within 24
hours of the patient’s admission to the hospital, a GCS of 11 or
greater is associated with an 82% probability of moderate or good
neurologic recovery. Lower scores have a significantly higher incidence
of severe sequelae. The Glasgow Outcome Scale (Table 123.6) is frequently used to grade long-term recovery following TBI.
 |
|
Table 123.5. Glasgow Coma Scale Components
|
 |
|
Table 123.6. Glasgow Outcome Scale
|
following brain injury regardless of the severity of injury. As a
group, patients younger than 20 years of age at the time of brain
injury experience a 62% chance of moderate or good neurologic recovery.
Patients between 20 and 30 years of age can expect a 46% chance of
moderate or good neurologic recovery. In a series of pediatric patients
with brain injury, overall 90% achieved a moderate or good neurologic
recovery and only 8% expired or remained in a persistent vegetative
state (95,96). Young
children with a GCS of 5 or better have a good prognosis for recovery.
In addition to having a poorer prognosis for recovery, the cost and
time required for rehabilitation of older patients is higher than that
for younger patients (40).
mortality accurately, recent studies have suggested the GCS as a single
variable may have limited value as a predictor of functional outcome,
and many trauma centers are now using the Revised Trauma Score (RTS) to
assist with triage of multitrauma patients. The RTS combines the GCS
with the systolic blood pressure and respiratory rate, and is used to
predict both mortality and disability (254,255).
emersion from coma occurs within the first 2 weeks of brain injury, 70%
of patients can be expected to achieve a good recovery. If the coma
persists beyond 4 weeks, the chance of good recovery is much
diminished. Brain stem involvement, as indicated by the presence of
decerebrate or decorticate posturing, has a poor prognosis for outcome.
If decerebrate posturing occurs and resolves within the first week
after injury, 40% of patients will receive a good neurologic recovery.
If decerebrate posturing persists beyond the first week, only 9% of
patients will achieve a good neurologic recovery. In a similar manner,
the duration of posttraumatic confusion can also be an
indicator
of prognosis. If the period of posttraumatic confusion persists for
more than 4 weeks, one third of patients will have a poor neurologic
outcome. It should be remembered, however, that prognosis is a
probability statement, and although various factors can be used as
guidelines, none is an absolute indicator in the individual patient.
-
the period of acute injury
-
the period of physiologic recovery
-
the period of functional adaptation to residual deficits
following the injury in the acute care hospital. The majority of TBIs
are the result of a motor vehicle accident. Multiple trauma is common.
The orthopaedic surgeon is a consultant with a critical role.
Aggressive treatment of orthopaedic injuries at an early stage is
important to functional outcome.
It is common for injuries such as fractures or major peripheral nerve
injuries to go undetected. Garland reported an 11% incidence of delayed
diagnosis of fractures, with an average time to diagnosis of 57 days (68,209).
In the comatose patient, obtain radiographs of all major joints and any
other areas suspected of injury. It is important not to assume that all
neurologic deficits present are from the CNS injury. Stone and Keenan (209)
reported that 34% of brain-injured patients have missed peripheral
nerve injuries. Especially in the presence of a limb fracture, suspect
a peripheral nerve injury (68,209).
sympathetic dystrophy, deep vein thrombophlebitis, spasticity, occult
fracture, and the formation of heterotopic ossification (HO) (37,63,64,84,85,166,204,209,210,224).
If pain is treated promptly (and this depends on accurate and early
diagnosis), prolonged restriction of motion may be prevented. HO,
fracture, and fracture malunion restrict motion due to lost structural
integrity. Many brain-injured patients who recover cognitive function
have residual spasticity and impaired balance, and consequently, are
less able to compensate for such structural impediments (63,156). Peripheral nerve injury produces weakness and pain, both potential causes of restricted motion.
the patient will make a good neurologic recovery. Treat all orthopaedic
injuries promptly and appropriately. When possible, internal fixation
is best. Spasticity develops, and casting a spastic joint in a flexed
position may result in a joint contracture or an unsatisfactory
reduction. Fracture healing is accelerated, presumably by the same
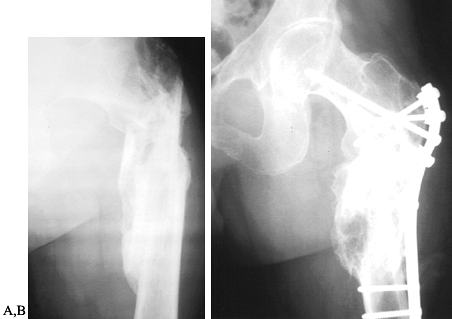
humoral factors that contribute to heterotopic bone formation (10,111,190,222). Fracture malunion is a common and potentially avoidable complication (Fig. 123.1).
 |
|
Figure 123.1. A:
Radiograph showing a malunited subtrochanteric fracture of the femur in a 27-year-old man. The fracture was not treated initially because the patient was not expected to survive. B: Radiograph showing the corrective osteotomy required to treat the malunion. |
cooperation. The patient emerging from coma may go through a period of
agitation and confusion. Fracture care should be made as foolproof as
possible because patient cooperation cannot be expected. In
anticipating a possible period of agitation, avoid, where possible,
traction and external fixators for treatment of extremity fractures (63,71,73,76,79,81,156,224,243,253).
prolonged period of time. The majority of improvement in motor control
occurs within the first 6 months following injury. Cognitive
improvement occurs most rapidly in the early phases following brain
injury but can continue for a very prolonged period of time, often
years (Table 123.7) (90).
Because the period of potential neurologic recovery following head
injury is prolonged, definitive surgical procedures are avoided during
the transitional stage. There is no exact time that must elapse before
considering surgery to improve musculoskeletal function. Consider the
rate of continued improvement in motor control when deciding at what
point to intervene surgically. If the additional improvement in motor
control will be overridden by the complications of contracture
formation, osteopenia, peripheral nerve compression, and muscle
atrophy, then early surgical intervention is appropriate.
 |
|
Table 123.7. Rancho Levels of Cognitive Functioning
|
is commonly in a rehabilitation facility. Serious head injury is
usually complicated by UMN syndrome (9,19,74,104,105,112,117,137,148).
Spasticity is commonly severe and prevents adequate joint range of
motion. Spasticity also interferes with the maintenance of limb
position despite the most conscientious and aggressive treatment. Even
in those situations in which joint motion can be maintained by a
knowledgeable therapist, it commonly requires much force, which is
painful for the patient, potentially harmful, and very time consuming.
Lesser degrees of spasticity can also impede a patient’s function or
require the use of positioning devices that interfere with the use of
an extremity.
Contractures are common. Limited positioning and myostatic contractures
combined with the patients diminished nutritional status can result in
pressure sores or hygiene problems. When fractures are present,
malunions can occur in the face of uncontrolled muscle tone and
accelerated fracture healing. Joint subluxation
can
also occur from prolonged spasticity or the attempts to range a joint
in the face of severe spasticity. If a ligamentous injury occurred at
the time of injury, frank dislocation of a joint can be caused by
hypertonicity. Spasticity also appears to be one of several etiologic
factors in the formation of HO in periarticular location (Fig. 123.2) (13,18,41,44,62,63,64 and 65,67,69,72,73,86,87,98,111,113,128,135,149,153,157,159,167,173,188,190,222,223). An-other common complication of spasticity is acquired peripheral neuropathy (39,47,51,61,68,166,209).
The most common peripheral neuropathies acquired with severe spasticity
and contracture formation are ulnar neuropathy at the elbow from severe
flexion and continuous pressure on the ulnar nerve and carpal tunnel
syndrome secondary to severe wrist flexion and pressure of the median
nerve against the leading edge of the transverse carpal ligament (166,209).
In the lower extremity, sciatic neuropathy can result from trauma such
as a hip dislocation or acetabular fracture. Sciatic neuropathy can be
caused by the pronounced inflammation associated with heterotopic bone
formation
posterior to the hip joint. In some cases, the bone can encase the
sciatic nerve. During the period of physiologic recovery, the temporary
control of spasticity is the major focus of treatment. Prevention of
additional complications, such as disuse muscle atrophy, joint
contractures, HO, and peripheral neuropathies, is critical to a good
functional outcome (5,13,18,36,37,39,41,44,47,48,54,58,61,62,63,64 and 65,67,69,72,73,77,86,87,98,111,113,128,135,149,153,157,159,173,174,181,185,188,190,191 and 192,199,204,205,206 and 207,209,210,212,213,222,223,243,245,253,256,257).
Early joint contractures are also best corrected during this phase of
treatment. This is accomplished by first reducing spasticity and then
correcting contractures by splinting, casting, and range-of-motion
therapy.
 |
|
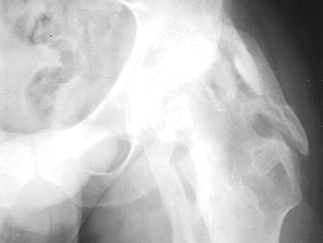
Figure 123.2. Radiograph of a hip showing periarticular heterotopic ossification posterior and lateral to the joint.
|
brain-injured patient is commonly left with residual limb deformities
from spasticity, contractures, and muscle imbalance. It is at this time
that definitive orthopaedic surgical procedures are performed to
rebalance the muscle forces and correct the residual deformities.
reaction about the joint seen as redness, warmth, severe pain, and
rapidly decreasing range of motion (5,13,18,36,37,39,41,44,48,54,61,62,63,64 and 65,67,69,72,73,77,86,87,98,111,113,128,135,149,153,157,159,173,188,190,191 and 192, 199,204,205,206 and 207,210,212,213,222,223,243,245) (see Fig. 123.2).Although
the time of initial occurrence is variable, HO is usually detected 2
months following the onset of TBI. Generally, radiographs show evidence
of the heterotopic bone in the form of spotty periarticular
calcification.
The exact etiology of HO is not known, and it is likely multifactorial.
It clearly has a predilection, however, for joints surrounded by
spastic or paretic muscle (63,64,69,72,111,222).
A dramatic increase in incidence to 85% is seen in patients who have
concomitant musculoskeletal injury. Because of this increased
incidence, consider prophylactic treatment. Several modalities have
been used with varying success. High-dose diphosphonates (Didronel) and
nonsteroidal anti-inflammatory drugs, particularly indomethacin, have
been used in the early postinjury period (5,65,68,136,193,207,212,243). Radiation (800 cGy limited field) can be used within several days following the injury (5,48,64,65,149,153,199). Joint manipulation has also been used, but the benefit of this is unclear if the heterotopic bone has already begun to form (77,212).
Formation of HO can be followed radiographically and by following
serial alkaline phosphatase measurements. The HO is mature when the
alkaline phosphatase has returned to a normal value and the radiographs
show a well-defined, corticated bone mass. Technetium bone scans show
increased uptake for many years and are of little value in this
situation. Studies have found that measuring the serum osteocalcin
levels is an unreliable method to diagnose HO or determine its
maturation (159). Surgical excision is thought
to carry a higher risk of recurrence if it is attempted before
maturation. This factor has been noted in work with HO in spinal
cord–injury patients and has not been shown to be applicable to TBI
patients (36,37,64,65,72,87,135,157,191,212).
Early excision should be considered in cases in which the HO is causing
progressive nerve or vascular compromise, or is threatening joint
ankylosis. Early excision of HO is technically more demanding because
the planes of dissection are less clear. There is also a much greater
blood loss when resecting immature HO.
“constant, spontaneous, severe, burning pain and is usually associated
with hypo- and hyperesthesia, hyperpathia, and allodynia, along with
vasomotor and sudomotor disturbances that, if persistent, result in
trophic changes” (85). RSD commonly develops
following CVAt (posthemiplegic dystrophy), TBI, and surgery. It may be
associated with trauma that occurred concurrently with TBI, although
the severity of the initial injury is unrelated to the severity of the
ensuing symptoms.
several weeks; however, with stroke and brain injury, the onset may be
delayed and atypical. Because of this, RSD may remain undiagnosed in
the stroke or brain-injured population until it becomes irreversible.
epiphyses even during the first phase suggest the diagnosis.
Subperiosteal resorption, tunneling of the cortex, and striation may be
evident on good-quality films. Unfortunately, none of these changes are
specific for RSD. A triple-phase bone scan may help with diagnosis. The
pattern seen varies with the phase of the disease. In the acute phase,
the flow (immediate), blood pool (early), and delayed (static) scan
patterns all show increased uptake, usually in a periarticular
distribution. False-negative results are frequent in the dystrophic
phase, in which the flow and blood pool scans are normal, and the
delayed scan shows a somewhat less prominent periarticular increase in
uptake. In the atrophic phase, both the flow and early scans show
decreased uptake, whereas the delayed phase is normal.
particularly active and active assisted range of motion, gentle muscle
strengthening and conditioning, massage, and heat therapy have been
effective. Tricyclic antidepressants (amitriptyline) may have an effect
because of their inhibition of serotonin uptake at pain-suppressing
neurons, prolonging the serotonin effect at the receptor. Narcotics
have a role in low-dose epidural infusions in combination with local
anesthetics. Systemic corticosteroids and adrenergic blocking agents
have both been advocated. Use of nerve blocks, including stellate
ganglion blocks, Bier blocks, surgical sympathectomy, and chemical
sympathectomy, have been reported resulting in improvement in a high
percentage of patients. At present, we use a regimen of amitriptyline,
physical therapy, and percutaneous sympathetic blockade.
flaccidity before the gradual onset of increasing muscle tone or
spasticity (resistance to quick stretch). Because these patients are
generally older than brain-injured patients, their muscles are also
weaker. This, combined with the shorter period of spontaneous
neurologic recovery (6 months), makes the temporary control of
spasticity an easier task in stroke patients. The use of phenol nerve
blocks or intramuscular botulinum toxin injection is therefore less
common than in the TBI patient.
intense level of spasticity (response to fast stretch), the presence of
rigidity (resistance to slow stretch), and the strong muscles found in
young TBI patients make the temporary control of spasticity much more
difficult. Phenol nerve blocks, botulinum toxin injections, and casting
are used more commonly and aggressively.
infarcts) have hemiplegic involvement with a nonfunctional upper
extremity and a lower extremity with greater potential for function.
The surgical procedures used to correct residual deformities in the
upper extremity are more likely to be for the correction of contractual
deformities than to improve function. Even when functional procedures
are employed, the gains from these procedures are more modest than
those in the brain-injured patient.
likely to have quadriplegic involvement, concomitant peripheral nerve
injuries, residual deformities from fractures, and joint limitation
from HO but better return of motor control. Functional surgical
procedures are more common in these patients.
injury and the prognosis for further recovery. In the period of
physiologic recovery, temporizing interventions are used because
permanent changes may result in chronic imbalance of forces across
joints (14,15,17,29,46,52,73,74 and 75,102,104,107,118,119,131,132 and 133,155,158,197,202,225,247,249).
Prevention of additional complications, such as disuse muscle atrophy,
joint contractures, HO, and peripheral neuropathies, is critical to a
good functional outcome. Several choices are available for treatment.
splinting techniques are commonly used to give temporary relief of
spasticity. Casting maintains muscle fiber length and diminishes muscle
tone by decreasing sensory input (14,73).
Local anesthetic nerve blocks are very helpful when they are
administered before cast application because relieving the spasticity
allows for easier limb positioning (Fig. 123.3). Casts are used primarily for the
correction of contractual deformities by applying a cast on a weekly
basis. Serial casting is most successful when a contracture has been
present for less than 6 months.
 |
|
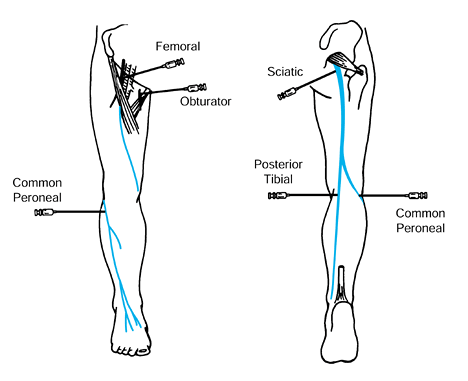
Figure 123.3.
The most commonly performed local anesthetic nerve blocks in the lower extremity. (From Keenan MAE. The Orthopaedic Treatment of Spasticity. The Journal of Head Trauma Rehabilitation 1987;2:62.) |
Antispastic agents that have sedating properties, such as baclofen,
diazepam, and clonidine, may compromise patients with attention
deficits or memory disorders. Even a drug such as dantrolene sodium,
which has a peripheral mechanism of action, may also cause drowsiness.
Other serious side effects such as hepatotoxicity can occur. Continuous
infusion of intrathecal baclofen has been reported to be useful in
managing spasticity secondary to spinal cord injury. Such delivery
avoids the cognitive side effects seen with oral delivery. Early
studies have shown that intrathecal bolus infusions of baclofen via a
catheter placed in the lumbar space may also be capable of reducing
spastic hypertonia associated with brain injury (2,3,152).
agents is the most suitable approach for treating restricted motion
secondary to spasticity. Neurolytic agents such as phenol and
chemodenervation agents such as botulinum toxin A are used during this
period because their effects are temporary, lasting only 3 to 5 months (15,17,29,46,73,74 and 75,101,102,104,107,118,119,131,132 and 133,155,158,225,249).
These agents are used when restricted motion occurs as a result of
focal spasticity. When these agents “wear off,” the patient is
re-evaluated to determine whether additional recovery has taken place
and whether there is further indication for reblocking. It is critical
that the functional problems of the patient be accurately ascribed to
specific muscles. This is done by evaluation using multichannel dynamic
EMG (60,105,109,112,117,125,136,137,147,148,176,183).
Even if many muscles in a limb are involved, a number of focal
injections are possible, and by doing so, CNS side effects can be
avoided. For patients with pathologies causing excessive motion,
environmental modification weights, bracing, and oral medications may
be considered during the period of physiologic recovery.
concentrations of 5% or more denatures the protein membrane of
peripheral nerves. When phenol is injected in or near a nerve bundle,
its neurolytic action on the myelin sheath or the cell membranes of
axons with which it makes contact serves to reduce neural traffic along
the nerve. The onset of the destructive process with higher
concentrations of phenol may begin to show effects several days after
injection. The denaturing process induced by phenol extends
biologically on the order of weeks but eventually regeneration occurs.
A phenol block is used as a temporizing measure rather than a permanent
intervention. In our clinical experience and the experience of others,
the effect of a phenol block typically lasts 3 to 5 months.
axons of all sizes in a patchy distribution but more so on the outer
aspect of the nerve bundle onto which the phenol is dripped. When
phenol is percutaneously injected, it is likely that the nerve block
will be incomplete. This is especially useful in situations in which a
spastic muscle also has volitional capacity, because under these
circumstances, it is desirable to reduce spasticity while still
preserving volitional capacity of a given muscle or muscle group.
stimulation. Motor branches are injected close to the offending muscle
or muscle group. These branches are referred to as motor points. A
surface stimulator is briefly used to approximate the percutaneous
stimulation site in advance. A 25-gauge Teflon-coated hypodermic needle
is advanced toward the motor nerve. Electrical stimulation is adjusted
by noting whether muscle contraction of the index muscle takes place.
As the electrode gets closer to the motor nerve, less current intensity
is required to produce a contractile response. The motor nerve is
injected when minimal current produces a visible or palpable
contraction of the muscle. Generally, 4 to 7 ml of 5% to 7% aqueous
phenol is injected at each site. As with any injection, care must be
taken not to inject the agent into a blood vessel; this is done by
aspirating before the injection (15,17,29,46,73,74 and 75,101,102,104,107,118,119,131,132 and 133,147,148,155,158,225).
treatment of spasticity. Ordinarily, an action potential propagating
along a motor nerve to the neuromuscular junction triggers the release
of acetylcholine (ACh) into the synaptic space. The released ACh causes
depolarization of the muscle membrane, activating a biochemical
sequence that leads to muscle contraction. Botulinum toxin type A is a
protein produced by Clostridium botulinum
that inhibits this calcium-mediated release of ACh at the neuromuscular
junction. Botulinum toxin A attaches to the presynaptic nerve terminal
and divides into a light and a heavy component. The light component
gets into the nerve cell and interferes with “fusion proteins”
affiliated with vesicles of ACh, thereby preventing the release of ACh
from their storage vesicles.
variety of dystonias and has been approved by the U.S. Food and Drug
Administration for the treatment of blepharospasm, facial spasm, and
strabismus. A number of studies have reported its use in treating
spasticity in individuals with cerebral palsy, stroke, head trauma, and
multiple sclerosis (38,52,60,147,148,197,202,247). The clinical benefit lasts 3 to 5 months but may be more variable.
Botulinum toxin is injected directly into an offending muscle and,
depending on the size of the muscle being injected, dosing has ranged
between 10 and 200 units (U). Current practice is to wait at least 12
weeks before reinjection and not to administer a total of more than 400
U in a single treatment session. Because this upper limit of 400 U may
be reached rather quickly, a different strategy is needed for the limb
requiring many proximal and distal injections. Botulinum toxin A and
phenol may be combined, with botulinum toxin A being injected into
smaller distal muscles and phenol aimed at larger proximal ones. A 3-
to 7-day delay between injection of botulinum toxin A and the onset of
clinical effect is typical. Effects are not seen immediately by the
patient, and usually a follow-up visit is arranged to check the result.
The amount of toxin given for a particular muscle is variable.
physicians inject through a syringe attached to a hypodermic needle
that doubles as a monopolar EMG-recording electrode. Patients may be
asked to make an effort to contract the targeted muscle, or the muscle
may contract involuntarily as in dystonia. After inserting the needle
electrode, injection is made when EMG activity is recorded. For deep or
small spastic muscles (e.g., extrinsic toe flexors), electrical
stimulation is preferred to localize the muscle before injection.
in the past several years. The advantages of botulinum toxin are its
ease of injection and the lack of residual scarring after injection.
The disadvantages of botulinum toxin are its high cost and the
stimulation of antibody formation, which requires higher doses for
repeated injections. Phenol, by contrast, requires more technical
expertise to localize the nerve or motor points for injection. Phenol
is caustic and causes localized scarring of the nerve and muscle. On
the other hand, phenol is inexpensive and readily available.
These include limited access to the perineum resulting in poor hygiene,
limited positioning of the patient, and the formation of decubitus
ulcers. In more functional patients, the increased adductor tone causes
scissoring of the legs when the patient is upright. Scissoring
interferes with balance, transfers, and ambulation by narrowing the
base of support (Fig. 123.4).
 |
|
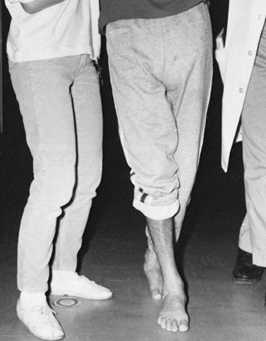
Figure 123.4.
Limb scissoring caused by spasticity of the hip adductor muscles produces a narrow base of support and difficulty with balance during ambulation. |
on a temporary basis in a patient who is still in the period of
physiologic recovery (Table 123.8). A phenol block of the obturator nerve can be performed percutaneously to
decrease the hip adductor tone. Because the sensory component of the
obturator nerve is small, direct injection of the nerve is not
problematic. It is advisable to administer an anesthetic nerve block
first to rule out a myostatic contracture. Locate the obturator nerve
as it emerges from the obturator canal by using a nerve stimulator and
insulated needle electrode. Then inject 2 to 5 ml of 5% phenol in
saline. The block will gradually take effect over the following 24
hours, and the effects will last for approximately 2 months. While the
hip adductor tone is decreased, use gentle range of motion exercises to
stretch out any contracture. Transfer and gait training can be
continued.
 |
|
Table 123.8. Techniques for the Temporary Management of Spasticity
|
can also be administered. If many large muscles in the lower extremity
require treatment for control of spasticity, phenol is a more practical
choice.
causes hip flexion contractures but may also contribute to knee flexion
deformities (Fig. 123.5). When hip flexor
spasticity cannot be controlled by the use of a long-leg cast, prone
lying, or positioning devices, then percutaneous paraspinal blocks of
the upper lumbar nerve roots can be performed to diminish hip flexor
spasticity.
 |
|
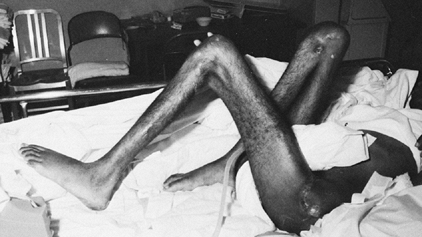
Figure 123.5.
Patient with severe, longstanding flexion contractures of both the hips and knees. The patient developed multiple pressure sores and had a flap procedure to cover a decubitus ulcer over the left greater trochanter. |
needle and nerve stimulator. Then inject 1 to 2 ml of 5% phenol in
saline. The hip flexor tone can be expected to decrease gradually over
the following 24 hours. Then begin a physical therapy program to
stretch out any fixed hip flexion contracture.
be administered but are technically difficult given the deep location
of the muscles and the proximity of the large femoral vessels.
with a brain stem or anoxic injury. Lack of adequate hip flexion range
of movement will greatly interfere with a patient’s ability to sit in a
chair (Fig. 123.6) (60,132,133,147,148).
Sitting is a crucial step in rehabilitation because it allows a person
to begin interacting with people and objects in the environment.
 |
|
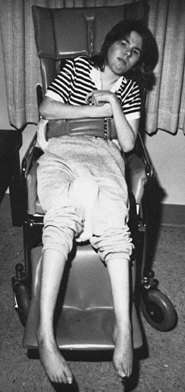
Figure 123.6.
Spasticity of the hip and knee extensor muscles in a brain-injured patient results in difficulty while sitting. This young patient requires the use of a seat belt and a semireclining wheelchair to maintain her position. |
nerve as it enters the muscle belly of the gluteus maximus is an
effective technique in reducing hip extensor tone and maintaining the
ability to flex the hip. The inferior gluteal nerve enters the gluteus
maximus muscle at the center of the muscle belly just proximal to the
piriformis tendon. Carefully localize the nerve with an electrode
needle and a nerve stimulator before injection. Inject 2 ml of 5%
phenol in saline into the muscle at the motor point. The muscle tone
can be expected to diminish over the following 24 hours. Then begin
passive range-of-motion exercises to increase hip flexion range. A
continuous passive motion machine is helpful to supplement the physical
therapy program. Botulinum toxin injections of the hip extensor muscles
can also be administered.
Because of the large sensory component of the sciatic nerve, direct
injection with phenol is undesirable. The dissection required to
identify the multiple motor branches of the hamstring muscles is
extensive and often impractical. Phenol motor point injections or
botulinum toxin injections of the hamstring muscles can be
administered. Prevention of knee flexion contractures can also be
achieved by performing repeated anesthetic nerve blocks of the sciatic
nerve and casting the limb in extension (73,78,80,88,101,103,120,122,127,147,148,169,174,178,194,201).
Take care to observe the knee very closely during the casting program
to avoid posterior subluxation of the knee joint. Using a sciatic nerve
block at the time of casting diminishes the likelihood of this
complication. An anesthetic
block
of the sciatic nerve will also temporarily eliminate calf spasticity
and allow for serial casting of an equinus contracture of the foot, if
present.
correct a knee flexion deformity or when posterior subluxation of the
tibia occurs secondary to the hamstring tightness, a lengthening of the
hamstring muscles can be performed even in the early stages following
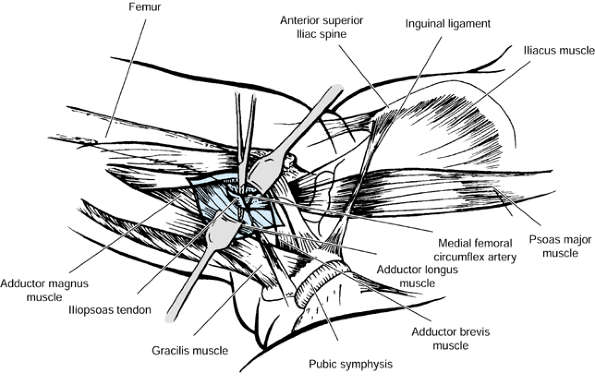
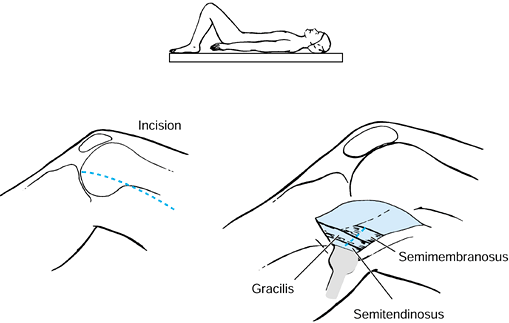
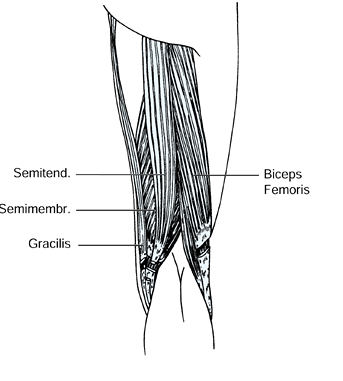
injury. The biceps femoris, gracilis, and the semimembranosus muscles
have myotendinous junctions that permit a fractional lengthening. The
semitendinosus muscle is either divided or Z lengthened (Fig. 123.7). Casting is still commonly needed following hamstring lengthening to correct the residual flexion deformity.
 |
|
Figure 123.7.
Lengthening of the biceps femoris, semimembranosus, and gracilis muscles at the musculotendinous junction. The semitendinosus is released. |
When knee range of motion cannot be maintained because of quadriceps
tone, a percutaneous phenol block of the femoral nerve can be performed
(15,38,46,60,73,101,102,131,132,147,148,155,158,247,249).
This block, in combination with the use of a continuous passive motion
machine, is then used to regain and maintain adequate knee range of
motion. Even in a severely injured patient, knee and hip flexion range
should be preserved to permit adequate sitting in a chair.
can therefore be directly injected using a percutaneous technique.
Localize the nerve in the groin lateral to the femoral vessels by using
an electrode needle and nerve stimulator. Then inject 2 ml of 5% phenol
in saline. Relaxation of quadriceps muscle tone can be expected to last
2 to 3 months. Use physical therapy and continuous passive motion to
regain knee flexion range.
individual muscles of the quadriceps and perform motor point blocks
using phenol. Use a surface stimulator to find the general vicinity
where the motor branches of the femoral nerve enter each muscle belly.
These are located at the proximal end of the muscles. Then use a needle
electrode and stimulator to localize the motor nerve more accurately.
can also be administered. Because the quadriceps are large muscles, a
large volume of botulinum toxin would be needed. It would also require
injections to be given on several occasions. For these reasons, phenol
is a more practical choice.
When the spasticity is mild, it can generally be contained and an
equinus contracture prevented by using a rigid ankle-foot orthosis
(AFO). At times the muscle tone is too severe to be adequately
controlled by a brace. The patient may exhibit ankle clonus while in
the upright position, causing the foot to piston up and down in the
brace.
injections of the posterior tibial nerve. We do not recommend this
procedure because of the large sensory component of the nerve. Phenol
is a caustic substance, and its injection into a mixed nerve can result
in severe painful dysesthesia and loss of sensation on the plantar
aspect of the foot. A phenol block of the motor branches of the
posterior tibial nerve can be performed as a surgical procedure to
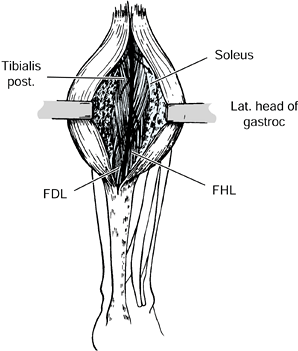
preserve sensation to the plantar aspect of the foot (Fig. 123.8) (73,75,78,103,108,122,127,147,148).
 |
|
Figure 123.8.
The branches of the posterior tibial nerve as exposed at the time of surgery. The individual motor branches of the nerve are injected with a 5% phenol and glycerin solution under direct vision. This procedure provides 6 months of relief of spasticity without interfering with sensation to the leg. FDL, flexor digitorum longus; FHL, flexor hallicus longus; gastroc, gastrocnemius. |
-
Make an incision on the posterior aspect of the calf at the midline beginning just distal to the popliteal crease.
-
Identify the posterior tibial nerve
between the heads of the gastrocnemius muscle and trace it distally.
Use a nerve stimulator to identify the motor branches. When all of the
motor branches have been identified to the gastrocnemius, soleus,
posterior tibialis, flexor hallucis longus, and flexor digitorum
muscles, protect each from the surrounding tissue with a moistened
gauze sponge. -
Then inject the motor branches with a 5%
phenol solution in glycerin. Glycerin is used for surgical phenol
blocks because it allows the phenol to be released more slowly and
therefore prolongs the effect of the block for 6 months. The glycerin
solution is too viscous for percutaneous use.
maintain the ankle at a neutral position to protect the calf muscles
while the block is in effect.
phenol blocks of the posterior tibial nerve are rarely needed. It is
much more practical to inject the gastrocnemius and soleus muscles with
botulinum toxin. It isusually necessary to inject the flexor hallucis
and flexordigitorum muscles simultaneously because they both contribute
to the equinus. Use an AFO after botulinum toxin injections because of
the muscle weakness caused by the blocks.
shown to be a primary muscle responsible for the varus deformity of the
foot in both stroke and brain-injured patients (Fig. 123.9 and Fig. 123.10) (16,20,22,25,26,28,43,59,60,78,80,103,105,106,108,109,122,127,138,147,148,163,174,175,176,177 and 178,180,184,194,195,226,227,230,241).
Most frequently, this deformity can be controlled initially with the
use of an AFO. In the event that a brace does not control the foot, a
phenol block of the motor branch of the deep peroneal nerve in the
proximal leg can be performed percutaneously to decrease the
spasticity. Localize the nerve by using a stimulator and needle
electrode before injection with 1 ml of 5% phenol in saline.
Alternatively, inject the muscle with botulinum toxin. The extensor
hallucis longus (EHL) muscle and the tibialis posterior muscle can
significantly contribute to a varus foot deformity. Phenol
motor
point or botulinum toxin injections of these muscles are often needed
as well. Use an AFO to maintain a neutral foot position after the
blocks.
 |
|
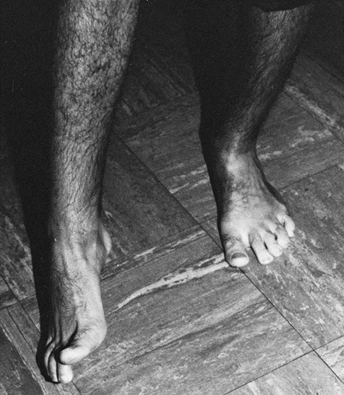
Figure 123.9. Patient with a spastic equinovarus deformity of the foot following a cerebrovascular accident.
|
 |
|
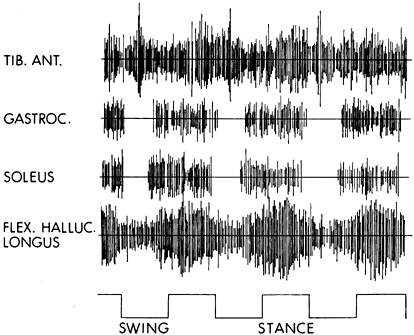
Figure 123.10.
Dynamic electromyogram (EMG) obtained during walking from a stroke patient with a spastic equinovarus foot deformity. The foot switches on the baseline indicate the swing and stance phases of gait. The tibialis anterior shows continuous activity in a nonphasic manner throughout (class IV EMG activity; see Table 123.9). The gastrocnemius shows class II EMG activity, which begins prematurely during the late swing phase and continues throughout the stance phase. The soleus muscle also shows class II activity with premature firing in late swing, continuing throughout the stance phase. The flexor hallucis longus exhibits class IIIC activity. The activity is continuous, but some phasic element can be seen with increased amplitude in midstance. |
 |
|
Table 123.9. Classification of EMG Activity
|
can occur. Phenol motor point or botulinum toxin injections of the
offending peroneal muscles temporarily control the deformity.
sophisticated mechanisms work together in unison. Improving lower
extremity function requires careful systematic evaluation before
surgery. The goals of surgery must be practical and clearly understood
by the patient and the family. Assessment includes an evaluation of
cognition and communication skills (4,20,22,25,26,28,78,80,101,105,106,109,115,122,138,147,148,150,151,156,178,184,194,195,226,241).
This is done during the physical examination. The patient must be
capable of following simple commands and should also be able to
cooperate with a postoperative therapy program. In addition, the
patient should have sufficient cognition to incorporate the improved
motor function into their use of the extremity. Adequate memory is
needed to retain what is taught during postoperative therapy.
adequate sensation in the foot and ankle. The basic modalities of light
touch and pain sensation are essential. Proprioception must be present
at the level of the ankle joint for good balance reactions. If
proprioception is impaired at the ankle, an AFO may be needed during
ambulation to prevent a compensatory knee extension thrust (115,175,178,180).
and diffuse hypoxic encephalopathy lead to a large variety of
posttraumatic motor phenomena, many of which are functionally
significant. Lesions affecting the corticospinal system, the cerebellum
and its pathways, and the extrapyramidal system are common. Hemiparesis
is the most common long-term residual of head injury. Many patients,
however, have a brain stem syndrome consisting of ipsilateral ataxia
and contralateral spastic hemiparesis. A small percentage have a
pseudobulbar athetoid type of picture. Patients can also have residuals
of bilateral hemiparesis, ataxia involving both sides of the body, and
severe dystonic decerebrate posturing or rigidity. Many patients,
especially during the early recovery stage from head injury, reveal
mixed signs such as spasticity combined with tremor and ataxia.
Peripheral neuropathy is common after head injury, and focal dystonia,
although unusual, is also seen. Because so many different aspects of
the motor control system may be affected by a head injury, we present a
way of organizing the unwieldy array of symptoms that emerge from a
damaged nervous system (147,148).
Our perspective is a practical one, namely taking into account the
impact of movement disorders on the patient’s ability to function in
real life.
functional problems are formulated and described in focal rather than
diffuse terms. Treatment of focal problems lends itself well to
surgical intervention, which can target particular muscles. Surgical
lengthening, transfer, or release of targeted muscles can provide very
effective solutions to problems of function that are clearly identified
from
the outset. The localizing approach is useful because it forces the
clinician to indicate the desired outcome in advance. The outcome is
based on an analysis that identifies the specific spastic muscles
responsible for the problem. For example, if the clinical problem is an
equinovarus foot that inhibits walking, surgically lengthening or
transferring the tibialis posterior will not solve the problem if
tibialis anterior and gastrocsoleus muscles are really the culprits
responsible for the problem. Identifying the specific offending muscles
is critically important to localized strategies of intervention (59,101,105,129,175,176 and 177,179,180,231,232,241).
difficult to distinguish between the many potential causes of limited
joint motion. The possibilities include increased muscle tone, a
myostatic contracture, the presence of periarticular HO, an undetected
fracture or dislocation, joint subluxation, pain, or the lack of
patient cooperation secondary to diminished cognition. Bony deformities
may not exhibit an obvious clinical deformity but can be detected by
radiography.
the role of muscle stiffness and contracture in relation to the
functional problem. For purposes of convenience, we identify seven
clinical patterns of lower extremity motor dysfunction that are most
commonly seen (Table 123.2).
common. Various muscles may contribute to motor dysfunction across
joints and limb segments in these clinical patterns. Evaluation focuses
on the following characteristics of the involved muscles: voluntary or
selective control, spastic reactivity, rheologic stiffness, and
contracture. Ask five specific questions:
-
Does the patient have voluntary control over a given muscle?
-
Is the muscle spastic to passive stretch?
-
Is the muscle, as an antagonist, activated during active movement generated by an agonist?
-
Does the muscle have increased stiffness when stretched?
-
Does the muscle have fixed shortening (contracture)?
each muscle may vary. Because each muscle may contribute to motion and
movement of the joint, information about each muscle’s contribution is
useful to the assessment as a whole. Treatment depends on such
information (60,147,148).
lower extremity, the most common pattern of spasticity is one of
flexion. First, establish passive range of motion of each joint. Test
by slow extension of the joint to avoid the velocity-sensitive response
of the muscle spindle. When spasticity is significant and passive joint
motion is incomplete, it is necessary and advisable to perform an
anesthetic nerve block to assess whether a myostatic contracture is
present (Fig. 123.3) (104,105,121,125,126).
To evaluate passive joint motion in the entire lower extremity, perform
combined femoral and sciatic nerve blocks by using a local anesthetic.
Alternatively, examine the patient while he or she is under general
anesthesia. This is usually done only when the patient is going to
surgery for another reason.
contributes to the motor impairment. Spasticity (hyperactive response
to quick stretch), rigidity (resistance to slow movement), or movement
dystonias may be present. The degree of spasticity within selected
muscles can be graded clinically in response to a quick stretch as
mild, moderate, or severe. There is surprising consistency between
observers using this simple grading system. Another method of
quantifying muscle tone, which is readily accessible and easily
performed at the bedside, is to measure the amount of intramuscular
pressure generated by a passive quick stretch or during functional use
of the limb. Intramuscular pressure can be measured by using a wick or
slit catheter technique. The pressure generated within the muscle is
proportional to the force of contraction (7,181).
The extremity may be hypotonic or flaccid and without any volitional
movement (grade 1). A spastic extremity may be held rigidly without any
volitional or reflexive movement (grade 2). Patterned or synergistic
motor control is defined as a mass flexion or extension response
involving the entire upper extremity. This mass patterned movement may
be reflexive in response to a stimulus but without volitional control
(grade 3). It is also possible for a patient to initiate mass patterned
movement volitionally (grade 4). Although patterned movement can often
be volitionally initiated, it is a neurologically primitive form of
motor control and of no functional use in the upper extremity.
Selective motor control with pattern overlay is defined as the ability
to move a single joint or digit with minimal movement in the adjacent
joints when performing an activity slowly (grade 5). Rapid movements or
physiologic stress make the mass pattern more pronounced. Selective
motor control is defined as the ability to move a single joint or digit
volitionally independently of the adjacent joints (grade 6). Observe
the patient clinically in a variety of functional tasks.
pain, increased muscle tone, and contracture to a limb deformity can be
difficult. Anesthetic nerve blocks are extremely useful in assessing
joint range of motion. The
blocks
can be easily performed without the use of special devices. By
temporarily eliminating pain and muscle tone, patient cooperation is
gained and the amount of myostatic contracture can be determined. By
using local anesthetic blocks, the strength and motor control of the
antagonistic muscle group can also be evaluated (Fig. 123.3).
the mainstay of evaluation. The clinical questions of interest
regarding a given muscle include the following: Does the patient have
selective voluntary control over the given muscle? Is the muscle
activated dyssynergically (i.e., in antagonism to movement) when the
patient attempts to move the relevant joint? Is the muscle resistive to
passive stretch (i.e., spastic)? Does the given muscle have fixed
shortening (i.e., contracture)? Given the degree of clinical effort,
patient morbidity, and procedural costs involved in treating
complicated movement dysfunction in patients with CVA and TBI, clinical
examination alone may not be sufficient to answer these questions with
a high degree of confidence.
formal gait and motion analysis, dynamic EMG studies, and nerve blocks
are helpful (7,11,24,25,26 and 27,35,43,52,59,60,89,94,108,109 and 110,114,115,127,129,138,143,147,148,175,176,177,178,179 and 180,198,203,214,220,229,231,232,233 and 234,236,239,240,241 and 242).
Perform laboratory gait analysis preoperatively on all patients using a
standard laboratory protocol. This consists of bidirectional,
slow-motion video recordings. Ground reaction forces are displayed
using a laser vector superimposed on the video. Ground reaction forces
are recorded from a force plate mounted into the walkway. The force
plate consists of a rigid platform suspended on strain gauge
transducers fitted with strain gauges. Each supporting corner has three
sensors set at right angles to one another, and the vertical load,
horizontal shear force, and forces in the mediolateral direction are
measured. Temporospatial parameters obtained include walking velocity,
cadence, stride time, and measurements of symmetry of gait. Dynamic
multichannel EMG is acquired with simultaneous measurements of joint
motion (kinematics) in the lower extremity while walking. Joint motion
is recorded using electrogoniometers. Surface electrodes are used for
the superficial lower limb muscles and wire electrodes for the deep
muscles such as tibialis posterior and long toe flexors.
clinician in interpreting whether voluntary function (effort-related
initiation, modulation, and termination of activity) is present in a
given muscle and whether that muscle’s behavior is also dyssynergic
(sometimes referred to as “out of phase” behavior). In addition,
responses to different rates of passive stretch of the muscle before
and after a local anesthetic nerve block can help the clinician
distinguish between the dynamic, velocity-sensitive reflex resistance
of spasticity versus passive muscle tissue stiffness and contracture.
Somatosensory-evoked potentials (SEPs) and motor evoked potentials
(MEPs) provide information on the integrity of the sensory and motor
pathways and may be helpful in predicting recovery of motor function
after stroke (93). Combined with clinical
information, laboratory measurements of muscle function often provide
the degree of detail and confidence necessary for making conservative
and surgical treatment decisions.
spastic patients from our institution the following classification of
EMG activity was devised to standardize terminology and may be used for
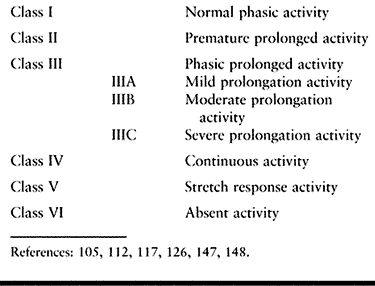
either the upper or lower extremity (Table 123.9) (105,112,117,125,126).
-
Class I constitutes a normal phasic pattern with appropriate on and off EMG activity.
-
Class II consists of EMG activity that,
although phasic, begins prematurely and continues for a short period
beyond the normal duration of activity for that muscle. This pattern is
more commonly seen in the lower extremity. -
Class III consists of phasic activity
with prolongation beyond the normal timing of the muscle. Class III
activity can be further subdivided into three patterns, depending on
the degree of prolongation. -
Class IIIA consists of phasic activity
with a short period of low-intensity EMG activity extending into the
next phase of the flexion-extension cycle secondary to mild spasticity. -
Class IIIB consists of phasic activity,
with prolongation extending for at least half of the next phase of
motion. This is indicative of a moderate amount of spasticity. -
Class IIIC represents a severely spastic
muscle and consists of phasic activity with severe prolongation in
which EMG activity is continued throughout the next phase of motion at
a high intensity but the underlying phasic nature of the muscle
activity is still distinguishable. -
Class IV consists of continuous EMG activity without phasic variations.
-
Class V consists of EMG activity seen
only in response to a quick stretch by the antagonist muscles. There is
no volitional activation of the muscle. This pattern is common in the
finger extensors. -
Class VI consists of absent EMG activity.
The gastrocnemius and soleus muscles are spastic, causing the equinus
posture of the foot. The tibialis anterior muscle is also spastic,
resulting in the varus position of the forefoot. In stroke and brain
injury, the tibialis posterior muscle is less commonly an offending
force. When the tibialis posterior is overactive, excessive heel varus
is seen (Fig. 123.11). The flexor digitorum
longus (FDL), flexor hallucis longus, and intrinsic muscles of the foot
are also spastic, resulting in curled toes.
 |
|
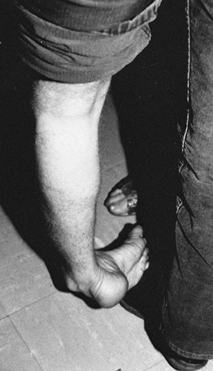
Figure 123.11.
Patient with an equinovarus deformity of the foot with pronounced inversion of the heel. This marked heel inversion usually indicates spasticity in the tibialis posterior muscle. Spasticity in the tibialis posterior muscle is seen in 10% of patients with UMN syndrome from stroke or brain injury. |
It may also occur during stance phase in combination with an
equinovarus deformity that occurs during the swing phase of gait.
 |
|
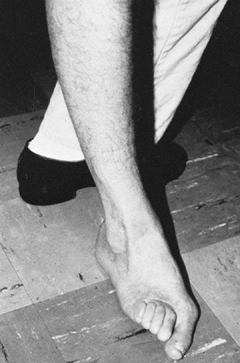
Figure 123.12. Patient with a spastic valgus deformity of the foot secondary to spasticity of the peroneus longus muscle.
|
 |
|
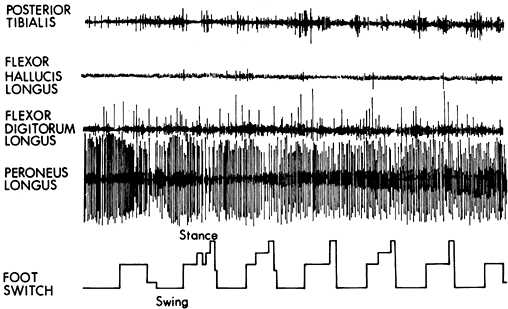
Figure 123.13.
Dynamic electromyographic record of a patient with a spastic valgus deformity of the foot. The foot switch tracing represents swing phase at the baseline of the tracing. Stance phase is indicated by the rise in the tracing. The tibialis posterior and flexor hallucis longus muscles show minimal activity throughout both swing and stance phase. The flexor digitorum longus muscle shows mild continuous activity throughout swing and stance. The peroneus longus muscle shows severe spasticity with no phasic pattern. All four muscles have class IV activity. |
causes the patient to hike the hip and circumduct the leg in order to
clear the foot. This is a very energy-consuming gait deviation. Because
equinus of the ankle will result in an extension thrust at the knee, it
is first necessary to rule out either static contracture or dynamic
equinus posturing as a cause.
of gait, which is marked by the point at which the opposite foot
contacts the ground and double stance begins. Knee
flexion
is a passive event caused by the forward momentum of the body rather
than by contraction of the knee flexor muscles. Dynamic EMG studies
during gait have shown that patients with a spastic stiff-legged gait
exhibit inappropriate activity of the quadriceps during early swing,
which blocks knee flexion (16,20,22,59,101,105,127,147,170,171,178,184,194,203,214,232).
In 13% of head-injured patients, there is isolated firing of the rectus
femoris during early swing. There is no clinical test to determine the
pattern of muscle activity within the quadriceps. Dynamic EMG gait
analysis is required for surgical decision making (Fig. 123.14).
 |
|
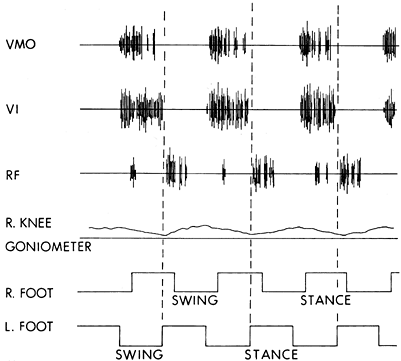
Figure 123.14.
Dynamic electromyogram of a patient with a stiff-knee gait abnormality. The muscle activity was recorded from the right leg. The knee goniometer baseline represents full knee extension, with upward deflections indicating knee flexion. Maximum knee flexion for this patient was 10°. The initiation of stance on the contralateral left foot represents the beginning of double limb support and the preswing stance period for the right leg. The vastus medialis obliqus (VMO) and the vastus intermedius (VI) show normal class I activity. The rectus femoris (RF) muscle shows activity in the preswing phase. This normal activity in the rectus femoris assists hip flexion and forward advancement of the limb. The spasticity in the leg triggers an overactive response that, in turn, blocks knee flexion. This patient is a candidate for transfer of the rectus femoris to the gracilis. |
and TBI are very similar. The same orthopaedic procedures can be used
in both patient populations. The orthopaedic treatment interventions
are described together. These procedures, however, are not applied
equally to both patient groups. The degree of spasticity, the timing of
neurologic recovery, and the pattern of spontaneous neurologic recovery
are different between stroke and brain-injured patients. These
differences account for the variation in the need for specific
treatments between the two groups.
challenges to the surgical team. The patients may have behavioral
deficits or cognitive limitations that would make them difficult to
manage with regional anesthesia and sedation. Therefore, general
anesthesia is preferred (70,208).
These patients have often previously had tracheostomy performed;
therefore, an anesthesia team familiar with airway difficulties caused
by this procedure is important. Take great care when positioning these
patients for long procedures because contractures of other portions of
their bodies may increase the risk of pressure ulcer formation.
owing to a combination of factors. Trauma concurrent with the brain
injury may cause complex fractures, which are predisposed to malunion.
Injuries may be missed in the initial resuscitation, leading to
malunion. The prognosis of the braininjury may be so poor that optimal
internal fixation offractures is not sought or obtained. Hemodynamic or
pulmonary instability may cause optimal fracture fixation to be
delayed. Poor patient compliance, agitation, and spasticity may alter
the initial reduction. If these problems are not anticipated, malunion
may result.
pelvic fractures. Patients usually present with rotational deformities,
leg-length discrepancy, pain, and gait abnormalities (23,63,145,253).
The need for surgical correction must be individualized to each
patient’s symptoms. For example, a small leg-length discrepancy without
pelvic pain may be best managed with a shoe lift.
hip fractures can be seen in TBI patients. They usually present with
leg-length discrepancy, gait abnormalities, and pain (see Fig. 123.1). These deformities can be managed with a corrective osteotomy after careful preoperative planning (196).
An anterior bow is better tolerated than either a varus or a valgus
alignment. Malalignment of fracture may lead to early degenerative
arthritis at the knee joint secondary to increased pressure along the
tibial plateau (130). Most deformities can be managed with a corrective osteotomy and intramedullary fixation.
the ankle and knee. This malalignment can lead to degenerative changes
at both the knee and ankle (130). Like the
femur, corrective osteotomies can be performed to restore balance.
External fixators can be employed for more complex deformities if
intramedullary fixation is not possible.
most common malunion of the ankle is shortening and malrotation of the
fibula (221). A corrective osteotomy can be performed to restore length and re-establish alignment (244).
Unidentified humoral factors that cause enhanced osteogenesis have been
demonstrated in the sera of patients with brain injury (10,66,190). Other contributing factors include soft-tissue trauma and spasticity (5,13,18,36,37,39,41,44,48,54,61,62,63,64 and 65,67,69,72,73,77,86,87,98,111,113,128,135,149,153,157,159,173,188,190,191 and 192,199,204,205,206 and 207,210,212,213,222,223,243,245).
clinical picture of ensuing HO is increasing pain and decreasing range
of motion about a joint. The bone forms in association with spastic
muscles. The alkaline phosphatase is elevated. Bone scans may aid in
early diagnosis, and radiographs confirm diagnosis. There is an intense
inflammatory reaction that occurs while the HO is forming. The swelling
and inflammation commonly impinge on adjacent nerves, causing severe
dysesthesia and pain. HO can also be associated with reflex sympathetic
dystrophy. Treatment of the nerve irritation using steroids,
amitriptyline, or gabapentin can be useful.
head-injured patients. It is virtually unheard of in children with
TBIs. Patients develop HO in one joint or at multiple sites. The
overall rate of joint ankylosis is 16% (69).
The heterotopic bone forms between the joint capsule and the overlying
muscle. Unlike myositis ossificans, HO does not form within the muscle
tissue itself.
inhibit calcium crystal deposition in the underlying collagen matrix.
Anti-inflammatory agents (indomethacin 50 mg tid) are used to control
the intense inflammatory process that occurs secondary to the formation
of HO. Spasticity is aggressively treated using medication, phenol
nerve blocks, or botulinum toxin injections. Physical therapy with
gentle range-of-motion exercises is used to maintain joint motion.
limbs. HO around the hip is seen in 44% of the patients who form
ectopic bone. When there has been a fracture or dislocation of the hip
or pelvis associated with TBI, the incidence of HO is 55% (63,69,72,79,81). Bilateral involvement and joint ankylosis are common. Heterotopic bone is less common about the knee joint but does occur (37).
heterotopic bone, but it should be delayed until the heterotopic bone
is fully mature to prevent recurrence. A true bone cortex should be
visible radiographically. The serum alkaline phosphatase level should
be normal. The patient should have sufficient cognitive recovery to
cooperate with the physical therapy program after surgery (Table 123.10) (72).
Surgical excision of functionally significant periarticular HO often
results in significant improvements in range of motion, independence,
and quality of life. Radiographically immature lesions have a higher
rate of recurrence (62,64,65,72,135,157,191).
It may not be feasible or desirable to allow the HO to reach maturity
before resection because although the risk of recurrence is higher,
ankylosis of the joint and subsequent contracture worsen the overall
functional result. HO may be detected earlier by obtaining a bone scan,
which can identify clinically silent lesions. After excision of the HO,
give prophylactic treatment to prevent recurrence. The drug therapy we
employ is diphosphonate 20 mg/kg/day combined with indomethacin 50 mg
tid. If there are contraindications to these medicines, low-dose
radiation therapy can be employed.
 |
|
Table 123.10. Functional Classes of the Brain-Injured Adult
|
tension caused by the spastic muscles. Anterior HO is seen to follow
the iliopsoas muscle. Anterior HO usually extends from the anterior
superior iliac spine (ASIS) and inner aspect of the pelvis to the
proximal femur in the region of the lesser trochanter.
-
When resecting anterior hip HO, perform a
standard anterior approach to the hip, but also dissect the femoral
triangle and its contents (see Chapter 2 and Chapter 3). -
Take care to identify all major divisions
of the femoral artery, nerve, and vein. It is prudent to place vascular
loops around all major vessels proximally to ensure easier control of
bleeding should a major vessel be damaged during the procedure. A wide
exposure and meticulous hemostasis are needed. -
Although all the neurovascular structures may be encased in HO, it is more common for the nerve to be entrapped (65).
The femoral nerve is already dividing into its many branches within the
femoral triangle. The motor branches are small in diameter; carefully
identify them. -
Because it is difficult to mobilize the
neurovascular structures, remove the heterotopic bone in small
segments. It is difficult to distinguish normal from abnormal bone. The
morphology of the normal bone and the HO must be closely observed.
Preoperative CT scans with three-dimensional reconstruction can be
extremely helpful in understanding the morphology. Also use
intraoperative radiographs or fluoroscopy to guide the resection (63,64 and 65,72,73,111,135,157,191).
follows the adductor muscles from the pubis and extends toward the
medial aspect of the femur.
-
Use a medial approach to the hip for
resection. If there is a significant hip adduction contracture, release
of the hip adductors may also be needed. -
Often, HO is seen both anterior and
medial to the hip. In these cases, it is necessary to make two separate
incisions and work between both.
Although posterior bone forms in conjunction with hip extensor
spasticity, it is usually associated with a flexion contracture of the
hip.
-
Use a posterior approach to the hip for resection.
-
Identify the sciatic nerve distally and
dissect it proximally. It is common for the sciatic nerve to be encased
within the mass of heterotopic bone. Significant scarring may also be
seen around the sciatic nerve secondary to the intense inflammatory
reaction that happens during
P.3224
the intermediate stages of bone formation. A neurolysis of the sciatic nerve may be needed. -
Once the sciatic nerve is identified, begin a more aggressive resection of the bone.
-
If a significant hip flexion contracture
persists after excision of the posterior HO, an anterior soft-tissue
release may be necessary (63,64 and 65,72,73,111,135,157,191).
-
Use a straight lateral or posterior incision.
-
It is important to separate the
heterotopic bone from the abductor muscles and preserve as much muscle
function as possible. The abductor muscles are usually very atrophic.
does occur, it usually is seen medially and may appear as a
Pellegrini-Stieda lesion. Even when the medial HO does not bridge the
joint, it commonly causes a knee flexion contracture by placing tension
on the surrounding tissues. When there has been knee extensor
spasticity, the HO can be found along the distal shaft of the femur
beneath the quadriceps muscle. It often wraps around both the medial
and lateral sides of the femur and greatly restricts knee motion (63,64 and 65,72,73,111,135,157,191). Posterior HO is uncommon after head injury but can occur in conjunction with hamstring spasticity (37,69,87,98,153,192,212).
limited. The choice of the surgical approach depends on the location of
the HO.
-
When a medial approach is needed, split
the collateral ligament longitudinally and strip it from its insertion.
Use a hinged brace postoperatively until the medial collateral ligament
has reattached and the knee is stable. -
When there is extensive HO beneath the
quadriceps, it is often necessary to make both medial and lateral
incisions and remove the ectopic bone from beneath the muscle. Preserve
the integrity of the quadriceps to allow for immediate mobilization
after surgery. -
If a posterior approach is chosen, first
identify the neurovascular structures proximally and dissect down to
the HO. Continuous passive motion devices are helpful postoperatively.
the patient a narrow base of support while standing and results in poor
balance. A preoperative obturator nerve block eliminates the adductor
spasticity and allows assessment of the adduction contracture (127).
Alternatively, the patient can be examined at the time of surgery while
under anesthesia to determine if a fixed myostatic contracture is
present. When no fixed adduction contracture is present, transection of
the anterior branches of the obturator nerve will denervate the
adductors and allow the patient a broader base of support (16,25,26,28,78,89,108,123,127,138,151,156,169,179,184,203,226,227). Commonly, a small contracture is found and the adductor longus muscle is released at the time of the obturator neurectomy.
-
Make a longitudinal incision directly over the adductor longus muscle.
-
Release and retract the adductor longus muscle.
-
Transect the anterior branch of the obturator nerve over the muscle belly of the adductor brevis.
care and hygiene in a nonambulatory patient or excessive limb
scissoring during attempted transfers and ambulation in a patient with
active function are indications for surgical release (16,25,26,28,78,89,108,123,127,138,151,156,169,179,184,203,226,227).
the hip and knee commonly occurs in conjunction with an adduction
contracture. As for any contracture, obtain preoperative radiographs
before performing soft-tissue releases to rule out the presence of HO
or an underlying bony deformity that would prevent correction.
-
With the patient in the supine position, make a longitudinal incision over the adductor longus muscle.
-
Place the incision distal to the groin crease to position the incision in a more hygienic location.
-
Use a longitudinal incision to decrease
tension on the wound edges as the leg is brought into a corrected
position after surgery. -
Dissect the adductor longus muscle free and transect it using electrocautery.
-
Identify and transect the anterior branches of the obturator nerve.
-
Release the adductor brevis and gracilis close to their pelvic origin (Fig. 123.15).
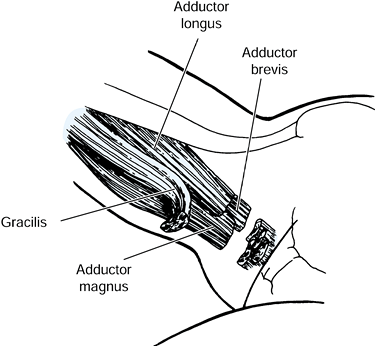 Figure 123.15.
Figure 123.15.
Release of the spastic hip adductor muscles. The adductor longus,
adductor brevis, and gracilis muscles are released through a medial
groin incision. The adductor magnus is rarely released. -
Close the wound over a drain.
this potentially contaminated area. Keep the hips in abduction for 4
weeks using casts or an abduction pillow splint to prevent recurrence
of the deformity during wound healing.
gait with compensatory knee flexion to maintain balance. This is a very
costly deformity because it requires constant use of the quadriceps,
hip extensor, and calf muscles
to
maintain upright posture. The energy requirement for the continuous
firing of these muscles is extremely high. Few patients are able to
remain ambulatory with this deformity.
during gait. Avoid complete release of the hip flexors in any patient
with the potential to ambulate. Because the iliopsoas has capsular
insertions, release of the iliopsoas tendon from the lesser trochanter
of the femur does not provide a complete release. Release of the tendon
from the lesser trochanter permits the iliopsoas to recess proximally,
thereby diminishing its pull but retaining its function.
-
Use a medial approach to the hip. Make a
longitudinal incision overlying the adductor longus tendon beginning 3
cm distal to the pubic tubercle. -
Bluntly develop a plane between the adductor longus and gracilis muscles.
-
Continue the dissection between the adductor brevis and adductor longus muscles.
-
Identify and protect the anterior division of the obturator nerve.
-
If an adduction deformity is also
present, perform division of the adductor longus muscle. This
facilitates exposure of the deeper muscles. -
Identify the pectineus muscle as it lies deep to the femoral vessels and medial to the adductor longus.
-
Divide the pectineus muscle using electrocautery. The lesser trochanter of the femur can be palpated in the depth of the wound.
-
Visualize the tendon of the iliopsoas by placing narrow reverse retractors above and below the lesser trochanter.
-
Divide the iliopsoas tendon from the trochanter and allow it to retract proximally (Fig. 123.16).
![]() Figure 123.16.
Figure 123.16.
Iliopsoas release from the lesser trochanter. The iliopsoas recedes but
remains attached to the anterior hip joint capsule. The pectineus is
released through its belly using electrocautery. (From Keenan MAE,
Kozin SH, Berlet, AC. Manual of Orthopaedic Surgery for Spasticity. New York: Raven Press, 1993, with permission.) -
Close the wound over a drain.
exercises and full weight-bearing gait training as tolerated. Prone
lying and electrical stimulation of the gluteus maximus muscle are
helpful to correct any residual hip flexion deformity.
nonambulatory patient that causes poor hygiene or pressure sores that
cannot be healed secondary to limited positioning of the patient are
indications for surgical release (16,25,26,28,78,89,108,123,127,138,151,156,169,179,184,203,226,227).
deformity of the knee are commonly associated with a hip flexion
contracture in the severely spastic patient. These deformities are
common in persons with TBI, stroke, cerebral anoxia, and multiple
sclerosis. As for any contracture, obtain preoperative radiographs
before performing soft-tissue releases to rule out the presence of HO
or an underlying bony deformity that would prevent correction.
present, it may be necessary to perform a percutaneous release of the
adductor longus tendon in the groin in order to position the patient
adequately and prep for further surgery. Simultaneously correct any
flexion contracture of the knee to prevent the leg from positioning in
flexion and causing a recurrent and more resistant contracture.
-
With the patient in the supine position,
make an anterior incision beginning 2.5 cm distal to the anterior
superior iliac spine. Carry it distally following the sartorius muscle
for a short distance. The incision should not extend over the iliac
crest because the patient will be expected to lie in the prone position
postoperatively to gain further correction of the deformity. -
Identify the lateral femoral cutaneous nerve as it passes distal to the anterior superior iliac spine and protect it.
-
Detach the sartorius muscle from its origin on the anterior superior iliac spine.
-
Release the rectus femoris muscle from its origin on the anterior inferior spine of the pelvis.
-
Gently retract the femoral nerve and vessels medially to expose the iliopsoas muscle on the anterior aspect of the hip.
-
Carefully divide the iliopsoas and
pectineus muscles over the pelvic brim by using electrocautery to
diminish postoperative bleeding. Because the iliopsoas has capsular
insertions, release of the iliopsoas tendon from the lesser trochanter
of the femur does not provide a complete release. The tensor fascia
lata and the anterior portion of the gluteus medius and gluteal
aponeurosis may be released from the iliac crest if necessary. The hip
joint capsule is not released (Fig. 123.17). Figure 123.17.
Figure 123.17.
Complete release of the hip flexor muscles for a severe spastic flexion
deformity in a nonambulatory patient. The sartorius, tensor fascia
lata, and rectus femoris muscles are released from their origins. The
iliopsoas and pectineus muscles are transected through their
midsubstance at the rim of the pelvis. -
In a severely contracted patient, take
care to identify all structures before release because the anatomy is
frequently distorted by the longstanding deformity. A large dead space
is left after releasing the hip flexor muscles, and a drain should be
used. -
Carefully close the wound and apply a compressive dressing, which is helpful in preventing postoperative infection.
approximately 50% of the deformity will be corrected at the time of
surgery. Daily wound care will help prevent infection in this area
where bacterial contamination is likely. Place the patient in a prone
position three times a day for increasing periods and institute gentle
stretching exercises to assist in correcting any residual hip flexion
deformity. When a release of a knee flexion contracture has been
performed simultaneously, the weight of the long-leg cast will also
provide a correcting force. Allow sitting in a wheelchair for short
periods.
subluxation or dislocation from severe and very longstanding hip
flexion and adduction spasticity. Both problems are most common with
anoxic encephalopathy. The hip joint has advanced osteoarthrosis and is
painful. The hip pain increases the spasticity, which, in turn, causes
more pain and so on in a circular fashion. In these cases, it is
usually necessary to resect the femoral head at the same time as
performing a complete muscle release. It is not advisable merely to
resect the femoral head because this approach will not treat the
spasticity and a more severe leg deformity can occur. After this
surgery, it is very important to maintain the leg in a neutral position
for at least 3 months while soft-tissue healing occurs. This can be
done with bilateral short-leg casts connected with a bar to hold the
legs
in neutral rotation and slight abduction. If the hip is allowed to
position in flexion, abduction, and external rotation while healing
occurs, the ability to sit in a chair may be severely compromised.
Although this problem is uncommon, an extension contracture will
interfere with a person’s ability to sit. When good sitting posture
cannot be obtained, a release of the proximal origin of the hamstring
muscles is indicated (56,123,200,201).
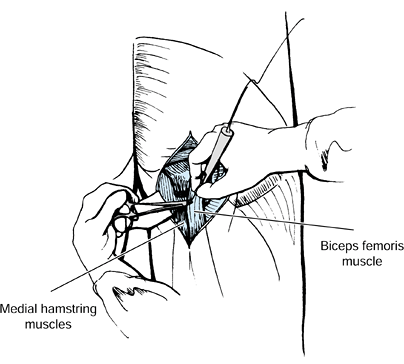 |
|
Figure 123.18. Proximal release of the hamstring muscles from the ischium. (From Keenan MAE, Kozin SH, Berlet, AC. Manual of Orthopaedic Surgery for Spasticity. New York: Raven Press, 1993, with permission.)
|
-
Place the patient in the prone position for surgery.
-
Make a longitudinal incision over the posterior thigh beginning at the gluteal fold.
-
Identify and protect the posterior femoral cutaneous nerve.
-
Lift the distal edge of the gluteus maximus proximally to expose the underlying hamstring muscles.
-
Then detach the biceps femoris,
semimembranosus, and semitendinosus muscles from their origins on the
ischial tuberosity and allow them to retract distally.
When the knee flexion deformity is less than 60° and the patient has
documented volitional activity in the hamstring muscles, perform a
lengthening procedure. This approach will correct the flexion deformity
while preserving the function of the hamstrings.
-
With the patient in the supine position,
make a longitudinal incision of approximately 8 cm on the lateral
aspect of the distal thigh just proximal to the knee joint (see Fig. 123.7). -
Isolate and protect the peroneal nerve
and divide the biceps femoris tendon obliquely as it overlies the
muscle belly. This allows the tendon to slide distally while still
maintaining continuity of the muscle. -
Also divide the portion of the iliotibial band that is posterior to the axis of knee flexion transversely.
-
Then make a longitudinal incision on the medial aspect of the distal thigh.
-
Isolate the tendons of the gracilis and
semimembranosus and fractionally lengthen them at the myotendinous
junction by making an oblique cut in the tendon as it overlies the
muscle belly (see Fig. 123.7). The
semitendinosus tendon has a very short myotendinous junction, which
does not permit fractional lengthening. Simply transect this tendon.
cast. Approximately 50% correction of the knee flexion deformity can be
expected at the time of surgery. Further correction is limited due to
tethering of the neurovascular structures. Cast the extremity in the
position of knee extension it assumes while being supported under the
heel, without attempting forced extension. Forced knee extension can
result in limb ischemia. Change the long-leg cast weekly until full
knee extension has been obtained. Use splints at night for an
additional 4 weeks to maintain correction.
Spasticity is frequently present. When severe spasticity of the
hamstring muscles or a knee flexion contracture of greater than 60° is
present, attempts to correct the knee position with casting or bracing
may result in posterior subluxation of the tibia. Distal release of the
hamstring tendons does not prevent a patient from becoming ambulatory.
If the hip flexion contracture or spasticity is not corrected at the
same time as the hamstring release, a recurrent knee flexion
contracture is likely to develop that is very resistant to surgical
correction.
-
With the patient in the supine position, make a longitudinal incision of approximately 8 cm on the lateral aspect
P.3228
of the distal thigh just proximal to the knee joint (Fig. 123.19).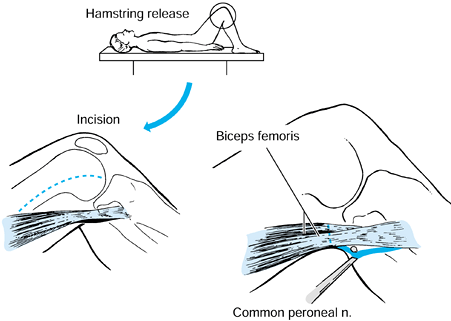 Figure 123.19. Release of the lateral hamstring muscles is performed through a lateral incision, with the patient in the supine position.
Figure 123.19. Release of the lateral hamstring muscles is performed through a lateral incision, with the patient in the supine position. -
Isolate and protect the peroneal nerve
and divide the biceps femoris muscle and tendon just proximal to its
insertion using electrocautery. -
Also, divide the portion of the iliotibial band that is posterior to the axis of knee flexion transversely.
-
Then make a longitudinal incision on the
medial aspect of the knee, and isolate and divide the tendons of the
gracilis, semimembranosus, and semitendinosus muscles (Fig. 123.20).
The semitendinosus tendon is usually surrounded by a layer of fat and
can closely resemble the posterior tibial nerve. Dissect the fatty
tissue from the tendon to confirm its identity before transection.![]() Figure 123.20. Release of the medial hamstring muscles is performed through a medial incision with the patient in the supine position.
Figure 123.20. Release of the medial hamstring muscles is performed through a medial incision with the patient in the supine position. -
In severe contractures, also perform
release of the sartorius muscle. By using electrocautery, the procedure
is easily performed without a tourniquet, which facilitates
localization and protection of the posterior tibial artery, vein, and
nerve. -
Following localization of the
neurovascular bundle, divide any remaining restricting bands as
required. The posterior fascia at the knee is often thickened, which
limits extension and may require release. The posterior joint capsule
may need to be released in longstanding contractures. -
Strip the joint capsule from the distal posterior femur using a periosteal elevator. Do not divide the cruciate ligaments.
-
If more correction is required, a closing
wedge osteotomy can be performed. A closing wedge will allow for some
shortening of the femur and release some tension from the neurovascular
structures and posterior skin.
cast. Approximately 50% correction of the knee flexion deformity can be
expected at the time of surgery. Further correction is limited due to
tethering of the neurovascular structures. Cast the extremity in the
position of knee extension it assumes while being supported under the
heel, without attempting forced extension. Forced knee extension can
result in limb ischemia. Postoperatively, change the long-leg cast
weekly until full knee extension has been obtained. Have the patient
use splints or a knee immobilizer at night for an additional 4 weeks to
maintain correction.
knee during the swing phase of gait. The deformity is a dynamic one,
meaning that it only occurs during walking.
There
is no restriction of passive knee motion, and the patient does not have
difficulty sitting. Usually, the knee is maintained in extension
throughout the gait cycle (Fig. 123.21).
Toe drag, which is likely in the early swing phase, may cause the
patient to trip; thus balance and stability are also affected (105,108,147,148,170,171,176,214,232).
The limb appears to be longer functionally. Circumduction of the
involved limb, hiking of the pelvis, or contralateral limb vaulting may
occur as compensatory maneuvers.
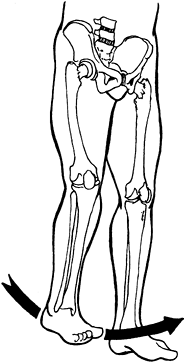 |
|
Figure 123.21.
A stiff-knee gait is caused by inappropriate activity in the quadriceps muscles, which inhibits knee flexion. The patient must hike the hemipelvis and circumduct the leg to clear the foot during swing. (From Keenan MAE, Kozin SH, Berlet, AC. Manual of Orthopaedic Surgery for Spasticity. New York: Raven Press, 1993, with permission.) |
Dyssynergic activity is commonly seen in the rectus femoris muscle from
preswing through terminal swing throughout the gait cycle. Abnormal
activity is also common in the rectus intermedius muscle. If knee
flexion is improved with a block of the rectus femoris or vastus
intermedius muscle, the rationale for surgical intervention is
strengthened. Any equinus deformity of the foot should be corrected
before evaluation of a stiff-knee gait because equinus causes a knee
extension force during stance. The amount of knee flexion during swing
is directly related to the speed of walking, so the patient should be
able to ambulate with a reasonable velocity in order to benefit from
surgery. Hip flexion strength is also needed for a good result because
it is the forward momentum of the leg that normally provides the
inertial force to flex the knee. In the past, a selective release of
the rectus femoris muscle or rectus and vastus intermedius muscles was
performed to remove their inhibition of knee flexion. On average, a 15°
improvement in peak knee flexion was seen after surgery. Transfer of
the rectus femoris to a hamstring tendon not only removes it as a
deforming muscle force, it also converts the rectus into a corrective
(flexion) force. This procedure provides improved knee flexion over
selective release.
-
Make a longitudinal incision on the anterior thigh from 10 cm above the patella to the middle aspect of the patella.
-
Dissect the rectus femoris muscle free from the other vasti muscles both proximally and distally.
-
Carry the dissection distally to the
patella and also remove a strip of periosteum from the patella to gain
additional length. If significant spasticity was seen in the vasti
muscle on the dynamic EMG study, these muscles are fractionally
lengthened by transecting their tendons as they overly the muscle belly. -
Make a second incision over the medial hamstrings just proximal to the knee.
-
Identify the gracilis tendon.
-
Then make a subcutaneous tunnel between the two incisions and through the intermuscular septum.
-
Pass the distal end of the rectus femoris
tendon through the tunnel to the medial wound, with the knee flexed 90°
and the femur externally rotated. -
Sew the rectus femoris tendon to the gracilis tendon (Fig. 123.22)
by means of a Pulvertaft weave. We prefer to use the gracilis as our
transfer site as opposed to the sartorius because of the strong tendon
repair possible.
P.3230
The gracilis also provides a more posterior attachment of the transfer, which, in turn, increases the flexor moment created.![]() Figure 123.22. The rectus femoris is transfered to the gracilis tendon to improve knee flexion during the swing phase of gait.
Figure 123.22. The rectus femoris is transfered to the gracilis tendon to improve knee flexion during the swing phase of gait. -
Transfer the rectus muscle under considerable tension.
-
Then gently extend the knee to be certain
that full knee extension can still be obtained. The location of the
transfer has not been shown to affect range of motion of the knee (170,171).
splint for 1 week to prevent a knee flexion deformity from occurring
secondary to the tension and to prevent discomfort of the transfer. The
patient can begin gait training in the immobilizer on the first
postoperative day. Because of the good fixation into the gracilis
tendon, start knee range of motion and ambulation without the splint 1
week later. Stress a marching gait pattern during therapy to facilitate
knee flexion during swing.
a knee extension contracture. This is usually seen in combination with
a hip extension contracture. Both deformities result in problems with
sitting. The knee extension deformity can be corrected by lengthening
the quadriceps. Quadriceps weakness is not a problem in these patients
because they are not ambulatory. In the past, V-Y lengthening of the
quadriceps tendon was routinely performed (123,169).
We have more recently been performing a fractional lengthening of the
individual muscles of the quadriceps. This procedure is more easily
performed and has a lower morbidity rate than the V–Y
lengthening. It also does not cause a patella baja deformity. Even
severe hyperextension knee deformities can be corrected with this
procedure.
-
Make a longitudinal incision over the anterior thigh at the junction of the middle and distal third of the quadriceps muscle.
-
Dissect the rectus femoris tendon free from the vasti at this level. This is easily done.
-
There is a long overlap of the rectus
muscle and tendon on the undersurface of the muscle belly. Sharply
transect the tendon over the muscle belly, leaving the underlying
muscle intact. The rectus tends to be the most contracted muscle and
two cuts are often made in the tendon. -
Beneath the rectus femoris muscle, locate
the myotendinous junctions of the vastus intermedius, medialis, and
lateralis muscles. Individually transect each tendon over the muscle
belly. -
Then gently and slowly flex the knee to at least 120°. The quadriceps muscles can be seen to lengthen as the knee is flexed.
-
Close the wound in a routine manner.
flexion several times daily to maintain knee flexion. Permit the
patient to sit as much as tolerated. Immobilization is not needed.
Equinus results from the overactivity or premature activity of the
gastrocnemius and soleus muscles. Surgical lengthening of the Achilles
tendon is indicated when the patient’s foot and ankle position is not
adequately controlled by an orthosis or when attempting to make the
patient brace free. Adequate lengthening of the Achilles tendon can be
performed using the Hoke triple hemisection technique percutaneously (Fig. 123.24).
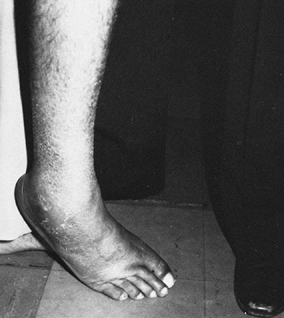 |
|
Figure 123.23. Patient with a spastic equinovarus deformity of the foot.
|
 |
|
Figure 123.24. Hoke technique for Achilles tendon lengthening. (From Keenan MAE, Kozin SH, Berlet, AC. Manual of Orthopaedic Surgery for Spasticity. New York: Raven Press, 1993, with permission.)
|
-
With the foot held in maximum
dorsiflexion, make three percutaneous hemitransections of the Achilles
tendon using a #11 knife blade. -
If a varus deformity of the foot is
present, make the proximal and distal cuts in the medial half of the
tendon, and place the center cut laterally. -
Then push the foot into dorsiflexion, allowing the tendon to lengthen.
cast for 2 weeks. We then use a cam walker boot for the next 4 weeks.
At this time, we allow the patient to remove the boot once daily for
bathing and skin care, provided that the patient does not stand or walk
without the boot. After this, the patient uses an AFO for an additional
6 weeks.
increased and innappropriate activity of the tibialis anterior muscle
(see Fig. 123.10). When the tibialis anterior
has been documented by dynamic EMG to be a cause of varus, the
deformity can be corrected by a split anterior tibial tendon transfer
(SPLATT). The SPLATT maintains the half of the tendon on the medial
aspect of the foot and transfers the other half of the tibialis
anterior tendon to the lateral side of the foot (Fig. 123.25) (4,101,105,109,127,184,203,226,230,241).
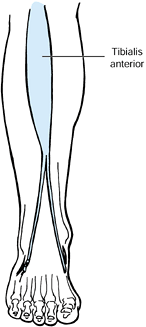 |
|
Figure 123.25. The split anterior tibialis tendon transfer for correction of varus caused by overactivity of the tibialis anterior muscle.
|
deformity, so a lengthening of the Achilles tendon should be performed
first and the toe flexor tendons divided.
-
Make an incision over the distal insertion of the tibialis anterior tendon on the medial aspect of the foot.
-
Sharply divide the lateral half of the
tendon from its insertion. Place a nonabsorbable suture in the cut end
of the tendon as a tag for ease of handling. The suture can be used
later to help secure the tendon in its new location in the cuboid bone. -
Make a second incision approximately 10
cm proximal to the ankle joint, just lateral to the crest of the tibia
over the tibialis anterior muscle. -
Make an opening in the fascia overlying the tibialis anterior.
-
We use a long twisted wire loop as a
tendon passer. Pass the wire loop under the fascia and follow the
tibialis anterior tendon to the incision on the medial foot. Pass the
suture in the free end of the tendon through the wire loop and pull it
into the proximal wound. Use this suture to pull the free end of the
tendon into the proximal wound, thereby splitting the tibialis anterior
tendon and a portion of the muscle belly. -
Wrap the tendon in a saline moistened sponge temporarily.
-
Then make a third incision on the lateral aspect of the foot. Expose the lateral face of the cuboid subperiosteally.
-
Use a drill to make a tunnel through the cuboid for anchoring of the lateral portion of the tendon.
-
Create a subcutaneous tunnel from the proximal wound to the lateral incision using a long forceps.
-
Pass the lateral arm of the tibialis tendon through this subcutaneous passage to protrude from the lateral incision.
-
Secure the tibialis anterior tendon on
the lateral side of the foot through the tunnel in the cuboid bone and
suture it to itself to hold the foot in a neutral position. When there
is marked osteoporosis, the cuboid bone may be too weak to use for the
transfer. In this situation, the tibialis anterior tendon can be
interwoven into the peroneus brevis using a Pulvertaft technique. -
Place the patient in a short-leg walking cast at surgery to hold the foot in a neutral position.
allowing the patient to bear full weight on the foot as tolerated. Keep
the cast on the foot for 2 weeks, then remove the cast and sutures.
Then place the patient in a cam walker boot for the next 4 weeks. With
reliable patients, we allow the cam boot to be removed once daily for
washing and skin care. The foot must be maintained in a neutral
position while the boot is off and no weight bearing is allowed. Six
weeks after surgery, use a nonarticulated, moderately rigid AFO for an
additional 6 weeks. The patient must sleep wearing the AFO during this
time to protect the foot from inadvertent stretch. At 3 months after
surgery, the brace can be removed for sleeping and gait training.
Discontinue the AFO if and when the patient has established sufficient
strength in the calf muscles to permit safe walking.
walking that is due to a moderate increase in tibialis anterior
activity. In this situation, a myotendinous lengthening is sufficient
to control the varus deformity.
-
Make an incision over the tibialis anterior muscle approximately 10 cm proximal to the ankle joint.
-
Open the fascial sheath of the tibialis
anterior and transect the tendon over the muscle belly of the tibialis
anterior, thereby allowing the muscle to lengthen fractionally.
immobilization is needed after surgery. Allow full weight-bearing
ambulation immediately.
patients the tibialis posterior muscle is also spastic and can
contribute to the varus deformity (105,109,231).
Clinically, this is evidenced by the increased heel varus in addition
to the forefoot varus caused by the tibialis anterior muscle (see Fig. 123.11).
present, perform a myotendinous lengthening by transecting the tendon
as it overlies the muscle belly, posterior and slightly proximal to the
medial malleolus. Complete release of the tibialis posterior tendon is
not recommended because a secondary planovalgus deformity may occur.
The EHL also contributes to the varus deformity of the forefoot. Many
patients with this condition complain of shoe wear problems from
pressure of the hallux against the shoe.
-
Make a 3 cm incision over the anterior
leg, beginning 10 cm proximal to the ankle joint. Identify the EHL
muscle and fractionally lengthen it by transecting the tendon as it
overlies the muscle belly.
immobilization is needed after surgery. Most commonly, the EHL is
lengthened in combination with a SPLATT procedure. In this situation,
follow the postoperative protocol of the SPLATT.
stroke and brain injury population. The deformity can result from
overactivity of the peroneus longus, peroneus brevis, or both (252). Use dynamic EMG to determine which muscles are causing the deformity.
-
Make an incision over the lateral leg, approximately 10 cm above the ankle joint.
-
Open the fascia of the lateral compartment and fractionally lengthen the offending peroneal muscles.
of the peroneus longus muscle. This problem can occur as an isolated
deformity but more commonly occurs in combination with spastic
equinovarus (123,252).
In the “spastic combination foot” deformity, equinovarus is observed
during swing phase from premature and prolonged firing of the tibialis
anterior and gastroc-soleus muscles. The planovalgus deformity occurs
during stance from the inappropriate activity of the peroneus longus
muscle. The pronation deformity may be accentuated by a premorbid
tendency to flat foot or by the presence of an equinus contracture.
tendon is transferred through the interosseous membrane to the tarsal
navicular bone to support the longitudinal arch of the foot during
stance.
-
Make a small incision on the lateral border of the foot
P.3233
just proximal to the base of the fifth metatarsal (Fig. 123.26).![]() Figure 123.26.
Figure 123.26.
Transfer of the peroneus longus tendon to the tarsal navicular for
correction of a severe spastic valgus foot deformity caused by
spasticity of the peroneus longus muscle. (From Keenan MAE, Kozin SH,
Berlet, AC. Manual of Orthopaedic Surgery for Spasticity. New York: Raven Press, 1993, with permission.) -
Identify the peroneus longus and brevis tendons.
-
Divide the peroneus longus tendon to obtain maximal length.
-
Make a second incision over the lateral leg approximately 10 cm above the ankle.
-
Identify the peroneus longus muscle and pull the distal end of the tendon proximally into this wound.
-
Make a third incision over the anterior leg 10 cm proximal to the ankle.
-
Carry the dissection down to expose the interosseus membrane.
-
Make a window in the interosseus membrane and pass the peroneus longus tendon through to the anterior leg wound.
-
Make a final incision over the navicular bone on the medial side of the foot.
-
Use a drill to create a tunnel through the navicular bone.
-
Then pass the peroneus longus tendon subcutaneously to the medial foot using a long forceps.
-
Pass the end of the tendon through the
tunnel in the navicular and secure it back to itself using
nonabsorbable sutures. Secure the tendon to hold the foot in a neutral
alignment. -
Place the patient in a short-leg walking cast at surgery, holding the foot in a neutral position.
anterior tibial tendon transfer along with an Achilles tendon
lengthening and toe flexor release to correct the swing phase
abnormalities.
allowing the patient to bear full weight on the foot as tolerated. Keep
the cast on the foot for 2 weeks, and then remove the cast and sutures.
Then place the patient in a cam walker boot for the next 4 weeks. With
reliable patients, we allow the cam boot to be removed once daily for
washing and skin care. The foot must be maintained in a neutral
position while the boot is off, and no weight bearing is allowed. Six
weeks after surgery, use a nonarticulated, moderately rigid AFO for an
additional 6 weeks. The patient must sleep wearing the AFO during this
time to protect the foot from an inadvertent stretch. At 3 months after
surgery, the brace can be removed for sleeping and gait training.
Discontinue the AFO if and when the patient has established sufficient
strength in the calf muscles to permit safe walking.
does not flatten with weight bearing. The deformity is probably a
result of muscle imbalance of both the intrinsic and extrinsic muscles
of the foot (146). If the foot is supple, a
soft-tissue procedure can be performed; however, if the foot is rigid,
a bony fusion must be performed. Steindler stripping is the release of
the plantar structures.
-
Expose the plantar fascia through a
medial foot incision. Take care to identify and preserve the medial
calcaneal nerve branches to the heel pad. -
Release the origin of the fascia under direct vision.
-
Identify the origin of the abductor hallucis and elevate it from the tuberosity of the os calcis.
-
Also release the origin of the flexor brevis and intrinsic muscles of the foot.
-
Then passively correct the foot and place it into a short-leg cast.
bearing on the first postoperative day. Because this procedure is
usually done in combination with the SPLATT operation, the SPLATT
protocol is generally followed.
Most commonly, this procedure is performed in combination with a
plantar release (Steindler stripping) and the SPLATT procedure.
-
Make an incision over the lateral foot from the tip of the fibula to base of the fourth metatarsal.
-
Elevate the extensor brevis and fat pad
to expose the calcaneocuboid joint and the sinus tarsi. Remove the
superior process of the distal calcaneus by using an oscillating saw to
fascilitate exposure. Save the bone to use as graft later. -
Insert a lamina spreader between the talus and calcaneus to expose the joint surfaces further.
-
Denude the posterior and middle facets of the subtalar joint of cartilage.
-
Also expose the calcaneal cuboid joint and remove the cartilage.
-
Expose the lateral talonavicular joint and prepare it through the same incision.
-
Make a small medial incision over the
talonavicular joint and remove the remaining cartilage. At this time,
fix the three joints with three large-diameter cannulated screws and
confirm their placement by fluroscopic guidance.
walking cast for 6 weeks. With the rigid fixation provided by the screw
fixation, weight bearing is permissable. If sufficient bony union is
seen at 6 weeks after surgery, allow the patient to use a rigid,
nonarticulated AFO for ambulation. Protect the fusion until full bony
healing is seen.
overactivity of the gastrocnemius muscles. Toe curling is caused by
overactivity of the flexor hallucis longus and flexor digitorum muscle
as well as the short toe flexor and occasionally the intrinsic muscles
of the foot (110).
-
Make a longitudinal incision on the
plantar surface of each toe at the metatarsal phalangeal joint level.
Identify the flexor tendons and release them under direct vision (Fig. 123.27). Also see Chapter 113.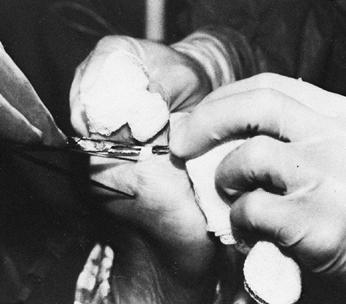 Figure 123.27. Release of the long and short toe flexor tendons at the base of each toe.
Figure 123.27. Release of the long and short toe flexor tendons at the base of each toe.
an Achilles tendon lengthening because bringing the foot into a
plantigrade position will worsen the toe curling. When an Achilles
tendon lengthening has been performed, immobilize the foot for 3 months
as described for that procedure.
syndrome. Lengthening the Achilles tendon to correct an equinus
deformity weakens the gastrocnemius-soleus muscle group, which was
already weak as a consequence of the underlying UMN syndrome. This calf
paresis generally results in the need for an AFO during ambulation.
Thus, transfer of the FDL muscle can be done to augment calf strength (Fig. 123.28). With this transfer, more patients eventually achieve brace-free ambulation (124).
In prior studies of treatment of a spastic equinovarus foot deformity,
30% of patients were able to walk safely without an AFO (109).
When the strength of the gastrocsoleus is augmented by transfer of the
FDL to the os calsis, 70% of patients achieve brace-free ambulation (124).
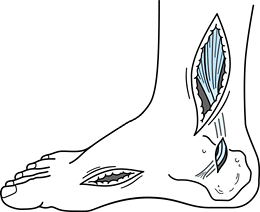 |
|
Figure 123.28.
Transfer of the flexor digitorum longus tendon to the os calcis to augment the strength of the gastrocnemius and soleus muscles. |
-
Make a 4 cm incision on the medial border
of the foot dorsal and parallel to the abductor hallucis muscle.
Reflect the abductor hallucis plantarward from the base of the first
metatarsal and isolate the flexor hallicus longus and FDL through the
deep fascia at the master knot of Henry. At this level, the FDL has not
yet split into four tendons and the FHL is easily dissected free. -
If the flexor brevis tendons are to be
released simultaneously to correct toe curling, release them before
transecting the FDL tendon. -
Dissect the FHL and FDL at the knot of Henry.
-
Then make a 5 cm incision at the medial
supramalleolar region, where the muscle belly of the FDL is isolated
with its tendon and delivered through the medial supramalleolar
incision. -
Make a 1 cm incision over the medial posterior superior os calcis.
-
Use a 3/8-inch drill to create a tunnel through the posterior superior calcaneus, exiting on the lateral side.
-
Make a small incision over the lateral malleolus at the exit site of the drill.
-
Place a suture ligature in the FDL and
pass the tendon subcutaneously to the medial heel wound. Then pass the
tendon medially to laterally through the tunnel created in the
calcaneus. -
Use a twisted wire loop to first pull the suture ligature through the tunnel.
-
Drill a Sta-tek (Zimmer; Warsaw, IN)
suture anchor into the lateral os calcis and securely tie the
transferred tendon with the foot in maximum dorsiflexion to prevent a
recurrent equinus deformity. -
Place the patient in a short-leg walking cast at surgery, holding the foot in a neutral position.
allowing the patient to bear full weight on the foot as tolerated. Keep
the cast on the foot for 2 weeks, then remove the cast and sutures.
Then place the patient in a cam walker boot for the next 4 weeks. With
reliable patients, we allow the cam boot to be removed once daily for
washing and skin care. The foot must be maintained in a neutral
position while the boot is off and no weight bearing is allowed. Six
weeks after surgery, the patient begins using a nonarticulated,
moderately rigid AFO for an additional 6 weeks. The patient must sleep
wearing the AFO during this time to protect the foot from an
inadvertent stretch. At 3 months after surgery, the brace can be
removed for sleeping and gait training. The AFO can be discontinued if
and when the patient has established sufficient strength in the calf
muscles to permit safe walking.
with spasticity. Even in the nonambulatory patient, these deformities
cause significant problems. These complications include pressure sores,
inability to wear shoes or protective footwear, and difficulty
positioning the feet on wheelchair supports for improved sitting
balance. Correct these deformities surgically to maintain a plantigrade
foot.
As in the more functional patient, muscle balance must be achieved by
performing the SPLATT, an Achilles tendon lengthening, and release of
the toe flexor tendons and lumbrical insertions. Because the spasticity
is often extreme and of long duration, it is commonly necessary to
perform a release of the plantar fascia to correct a cavus deformity as
well as a triple arthrodesis in conjunction with the tendon transfer.
to point out specific considerations to avoid complications and achieve
a maximal outcome. There are a few general guidelines to consider.
deformities from inappropriate muscle activity are release,
denervation, and lengthening or transfer. No surgical manipulation of
soft tissues will be successful if there is an underlying bony
restriction. Preoperative radiographs are obtained to assess joint
congruency, or to detect heterotopic bone or other deformity. Muscle or
tendon release removes the deforming force of that muscle. Release is
only used on muscles with no potential for function. A release corrects
both active deformity and a static contracture. Denervation of a muscle
removes the deforming force of that muscle and is useful when no fixed
contracture is present.
spastic response to quick stretch (spasticity) in a muscle that has
volitional use. By removing the overactive stretch response of the
muscle, its volitional use is significantly improved. Lengthening of a
muscle tendon corrects both static and dynamic deformities. Lengthening
is often performed exclusively to correct a dynamic deformity. When the
patient is under general anesthesia and the muscles are relaxed, a
purely dynamic deformity (e.g., an equinus foot deformity) appears
corrected. It is a mistake to alter the surgical plan at this time and
not lengthen the muscle in question. If a muscle exhibited dyssynergic
activity during functional activity, it should still be lengthened to
decrease its activity even in the absence of a fixed contracture.
force. It is not necessary for the muscle to have normal control. It is
critical that the transferred muscle has a predictable, nonvariable
action to achieve the desired result. Varying muscle activity, such as
in athetosis, is a contraindication to tendon transfer.
Clinical assessment alone is unreliable to determine the causes of a
spastic limb deformity. There are at least twenty different
combinations of muscle activity that can cause an equinovarus foot
posture. Dynamic poly-EMG studies or diagnostic nerve blocks are needed
to assess the problem fully. The outcome of surgical correction depends
on the accuracy of the preoperative assessment.
care of these patients follows standard, well-known orthopaedic
principles. Considering the specific limb problems individually and
then constructing a prioritization list is the most effective method of
dealing with patients who have multiple problems. As a starting point,
it is helpful to consider problems in functional categories and next to
consider whether or how correction of a specific limb deformity is
likely to improve the function. Examples of functional categories
include dressing, eating, transfers, and walking.
function refers to problems of passive manipulation of limbs to achieve
functional ends. Problems of passive function typically relate to
activities such as dressing, bathing, sitting, or transfers. Problems
of active function refer to a patient’s direct use of a limb to carry
out the functional activity. It is a useful construct to categorize
problems of function as being either passive or active in nature.
their caregivers. A patient with a severe equinovarus foot deformity
often is unable to walk. If the patient has some active hip flexion to
provide limb advancement and good sitting balance, then correction of
the foot deformity is likely to make the patient ambulatory. It may
also be necessary to correct a hand contracture for the patient to use
a cane or walker to achieve this goal. If the patient lacks active hip
flexion and has poor trunk balance, then correction of the foot
deformity will not allow walking. Correction of the foot may still be
useful to allow shoe wear or to improve sitting balance with the foot
resting on the leg support of a wheelchair.
into both functional and anatomic categories, it is easier to sort
through the numerous musculoskeletal issues faced by persons with
neurologic disorders. A major improvement in function and quality of
life is achieved for many, giving both the surgeon and the patient a
feeling of satisfaction and accomplishment.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
JU, Sutherland DH, Hanggi A. Intramuscular Pressure During Walking: An
Experimental Study Using the Wick Catheter Technique. Clin Orthop 1979;145:292.
MJ, Nakai RJ, Waters RL, et al. Motor Point Delineation of the Gluteus
Medius Muscle for Functional Electrical Stimulation: An In Vivo
Anatomic Study. Arch Phys Med Rehabil 1991;72:112.
MJ, Waters RL, Keenan MAE, et al. Orthopaedic Management of the Stroke
Patient: Part I: Pathophysiology, Limb Deformity, and Patient
Evaluation. Orthop Rev 1988;27:637.
MJ, Waters RL, Keenan MAE, et al. Orthopaedic Management of the Stroke
Patient: Part II: Treating Deformities of the Upper and Lower
Extremities. Orthop Rev 1988;27:891.
BR, Baker LL, Waters RL. Positional Feedback and Electrical
Stimulation: An Automated Treatment for the Hemiplegic Wrist. Arch Phys Med Rehabil 1979;60:497.
RM, Mooney V, Nickel VL. Flexor-Origin Release for Pronation-Flexion
Deformity of the Forearm and Hand in the Stroke Patient. J Bone Joint Surg [Am] 1970;52:907.
JR. Manual Stretch: Effect on Finger Movement Control and Force Control
in Stroke Subjects with Spastic Extrinsic Finger Flexor Muscles. Arch Phys Med Rehabil 1990;71:888.
G, Judet T, de Loubresse CG, Pirou P. Articulated Radial Head
Replacement and Elbow Release for Post Head-injury Heterotopic
Ossification. J Orthop Trauma 1996;10:68.
G, Judet T, Garreau de Loubresse C, Mollaret O. Excision of Heterotopic
Ossification Around the Knee Following Brain Injury. Injury 1996;27:125.
MK, Stacy M, Cooke DL, Stonnington HH. Comparison of Two Injection
Techniques Using Botulinum Toxin in Spastic Hemiplegia. Am J Phys Med Rehabil 1996;75:462.
HC, Tan CB, Tjia H. A Case of Bilateral Ulnar Nerve Palsy in a Patient
with Traumatic Brain Injury and Heterotopic Ossification. Singapore Med J 1997;38:447.
DX, Kreutzer JS, Marwitz JH, et al. Functional Outcomes of Older Adults
with Traumatic Brain Injury: A Prospective, Multicenter Analysis. Arch Phys Med Rehabil 1996;77:883.
TJ, Alexander MA, Steg NL. Early Detection of Heterotopic Ossification
in Young Patients with Traumatic Brain Injury. Arch Phys Med Rehabil 1992;73:258.
RB, Hahn GV, Tabas JA, et al. The Natural History of Heterotopic
Ossification in Patients Who Have Fibrodysplasia Ossificans
Progressiva. A Study of Forty-four Patients. J Bone Joint Surg [Am] 1993;75:215.
JL, Vargo M, Reidy ME. A Prospective Study of Peripheral Nerve Lesions
Occurring in Traumatic Brain-injured Patients [See Comments]. Am J Phys Med Rehabil 1989;68:15.
JP, Pellegrini VD Jr, Miller RJ, Jones JA. Treatment of Traumatic
Radioulnar Synostosis by Excision and Postoperative Low-dose
Irradiation. J Hand Surg [Am] 1994;19:394.
CH, Fardanesh L, Rubner D, et al. Profiles of Functional Recovery in
Fifty Traumatically Brain-injured Patients After Acute Rehabilitation. Am J Phys Med Rehabil 1997;76:213.
P, Filipetti P, Feve A, et al. Peripheral Selective Neurotomy of the
Brachial Plexus Collateral Branches for Treatment of the Spastic
Shoulder: Anatomical Study and Clinical Results in Five Patients. J Neurosurg 1997;86:648.
MM, Stokic DS, Wawro AW, Wun CC. Modification of Motor Control of Wrist
Extension by Mesh-glove Electrical Afferent Stimulation in Stroke
Patients. Arch Phys Med Rehabil 1996;77:252.
S, Meek RN, Snelling CF, et al. Range of Motion and Complications After
Postburn Heterotopic Bone Excision About the Elbow. J Trauma 1996;41:825.
P, Cotterill G, Kneale TA, et al. Outcome of Intensive Rehabilitation
after Severe Brain Injury: A Long-term Follow-up Study. Brain Inj 1996;10:631.
AS, O’Dell MW, Guiffra LJ, Sandel ME. Radial Nerve Injury Associated
with Traumatic Myositis Ossificans in a Brain Injured Patient. Arch Phys Med Rehabil 1993;74:770.
DE. Clinical Observations on Fractures and Heterotopic Ossification in
the Spinal Cord and Traumatic Brain Injured Populations. Clin Orthop 1988;233:86.
JK, Mueller BA, Rivara FA. Characteristics of Drivers and Driving
Record after Traumatic and Nontraumatic Brain Injury. Arch Phys Med Rehabil 1998;79:738.
HT, Hageman G, van Limbeek J. Prediction of Recovery from Upper
Extremity Paralysis after Stroke by Measuring Evoked Potentials. Scand J Rehabil Med 1997;29:155.
S, Bertelt C, Schaffrin A, et al. Restoration of Gait in Nonambulatory
Hemipareitc Patient by Treadmill Training with Partial Body-weight
Support. Arch Phys Med Rehabil 1994;75:1087.
SL, Sharpe MH, Metzer J. Outcomes 5 Years Post-traumatic Brain Injury
(with Further Reference to Neurophysical Impairment and Disability). Brain Inj 1997;11:661.
H, Maier-Lath ML, Eickhof C. The Functional Value of Electrical Muscle
Stimulation for the Rehabilitation of the Hand in Stroke Patients. Scand J Rehabil Med 1997;29:3.
EA, Mandac BR, Davidoff G, et al. Risk Factors for Heterotopic
Ossification in Children and Adolescents with Severe Traumatic Brain
Injury. Arch Phys Med Rehabil 1992;73:459.
MA, Creighton J, Garland DE, Moore T. Surgical Correction of Spastic
Equinovarus Deformity in the Adult Head Trauma Patient. Foot Ankle 1984;5:35.
MA, Gorai AP, Smith CW, De, G. Intrinsic Toe Flexion Deformity
Following Correction of Spastic Equinovarus Deformity in Adults. Foot Ankle 1987;7:333.
MA, Haider T. The Formation of Heterotopic Ossification after Traumatic
Brain Injury: A Biopsy Study with Ultrastructural Analysis. The Journal of Head Trauma Rehabilitation 1996;11:8.
MA, Peabody TD, Gronley JK, Perry J. Valgus Deformities of the Feet and
Characteristics of Gait in Patients Who Have Rheumatoid Arthritis. J Bone Joint Surg [Am] 1991;73:237.
MA, Romanelli RR, Lunsford BR. The Use of Dynamic Electromyography to
Evaluate Motor Control in the Hands of Adults Who Have Spasticity
Caused by Brain Injury. J Bone Joint Surg [Am] 1989;71:120.
MA, Todderud EP, Henderson R, Botte M. Management of Intrinsic
Spasticity in the Hand with Phenol Injection or Neurectomy of the Motor
Branch of the Ulnar Nerve. J Hand Surg [Am] 1987;12:734.
MA, Tomas ES, Stone L, Gersten LM. Percutaneous Phenol Block of the
Musculocutaneous Nerve to Control Elbow Flexor Spasticity. J Hand Surg [Am] 1990;15:340.
MAE, Lee GA, Tuckman AS, Esquenazi A. Improving Calf Muscle Strength in
Patients with Spastic Equinovarus Deformity by Transfer of the Long Toe
Flexors to the Os Calcis. Journal Head Trauma Rehabil 1999;14(2):163.
MAE, Perry J. Evaluation of Upper Extremity Motor Control in Spastic
Brain-injured Patients Using Dynamic Electromyography. The Journal of Head Trauma Rehabilitation 1990;5:13.
AA, Harmel MH, Forster S, Benton JG. Management of Spasticity by
Selective Peripheral Nerve Block with Dilute Phenol Solutions in
Clinical Rehabilitation. Arch Phys Med Rehabil 1964;45:513.
DJ, Katz SD, Keenan ME. Functional Outcome Following Surgical Resection
of Heterotopic Ossification in Pateints with Brain Injury. The Journal of Head Trauma Rehabilitation 1996;11:78.
SH, Keenan MA. Using Dynamic Electromyography to Guide Surgical
Treatment of the Spastic Upper Extremity in the Brain-injured Patient. Clin Orthop 1993;288:109.
NH, Esquenazi A, Keenan ME. Analysis and Management of Spasticity,
Contracture, and Impaired Motor Control. In: Horn LJ, Zasler ND, eds. Medical Rehabilitation of Traumatic Brain Injury. Philadelphia: Hanley & Belfus, 1996:411.
JM, DeVivo MJ, Hadley M. Prospective Study on the Use of Bolus
Intrathecal Baclofen for Spastic Hypertonia due to Acquired Brain
Injury. Arch Phys Med Rehabil 1996;77:461.
JM, Tuel SM, Cross LL, Mathew MM. Heterotopic Ossification of the
Extensor Tendons in the Hand Associated with Traumatic Spinal Cord
Injury. J Am Paraplegia Soc 1992;15:229.
H, Jorgensen HS, Raaschou HO, Olsen TS. Compensation in Recovery of
Upper Extremity Function after Stroke: The Copenhagen Stroke Study. Arch Phys Med Rehabil 1994;75:852.
H, Jorgensen HS, Raaschou HO, Olsen TS. Recovery of Upper Extremity
Function in Stroke Patients: The Copenhagen Stroke Study. Arch Phys Med Rehabil 1994;75:394.
MS, Muik E, Davis III RB, et al. Rectus Femoris Surgery in Children
with Cerebral Palsy. Part II: A Comparison Between the Effect of
Transfer and Release of the Distal Rectus Femoris on Knee Motion. J Pediatr Orthop 1993;13:331.
MS, Muik E, Davis III RB, et al. Rectus Femoris Surgery in Children
with Cerebral Palsy. Part I: The Effect of Rectus Femoris Transfer
Location of Knee Motion. J Pediatr Orthop 1993;13:325.
M, Balbi P, Russo G, Nilsson J. H-reflex Recovery Curve and Reciprocal
Inhibition of H-reflex of the Upper Limbs in Patients with Spasticity
Secondary to Stroke. Am J Phys Med Rehabil 1995;74:357.
J, Waters RL. Orthopaedic Evaluation and Treatment of the Stroke
Patient. Part III. Upper Extremity Management and Surgery. Instr Course Lect 1975;24:51.
A, Gauthier M, Wieler M, Kenwell Z. The Bionic Glove: An Electrical
Stimulator Garment that Provides Controlled Grasp and Hand Opening in
Quadriplegia. Arch Phys Med Rehabil 1997;78:608.
DJ Jr, Kellerman WC, Bonner FJ Jr. Heterotopic Ossification
Masquerading as Deep Venous Thrombosis in Head-injured Adult:
Complications of Anticoagulation. Arch Phys Med Rehabil 1986;67:339.
KJ, Banovac K, Hornicek FJ, et al. Evaluation of Serum Osteoblast
Mitogenic Activity in Spinal Cord and Head Injury Patients with Acute
Heterotopic Ossification. Spine 1994;19:740.
MA. Indomethacin: An Adjunct to Surgical Excision of Immature
Heterotopic Bone Formation in a Patient with a Severe Head Injury. A
Case Report. Orthopedics 1987;10:1379.
DM, Zasloff M, Peeper J, et al. Age- and Joint-specific Risk of Initial
Heterotopic Ossification in Patients Who Have Fibrodysplasia Ossificans
Progressiva. Clin Orthop 1994:243.
C, Ferreira JJ, Pinto AA, et al. Botulinum Toxin Type A for the
Treatment of Arm and Hand Spasticity in Stroke Patients. Clin Rehabil 1997;11:3.
CH, Clancy M, Steele HH. A Long Term Retrospective Study of Proximal
Hamsting Release for Hamstring Contracture in Cerebral Palsy. J Pediatr Orthop 1984;4:443.
DM, Alexander DN, O’Brien CF, et al. Botulinum Toxin Type A in the
Treatment of Upper Extremity Spasticity: A Randomized, Double-blind,
Placebo-controlled Trial. Neurology 1996;46:1306.
KM, Alexander MA, Harcke HT. Undetected Musculoskeletal Trauma in
Children with Traumatic Brain Injury or Spinal Cord Injury. Arch Phys Med Rehabil 1993;74:902.
G, Gennarelli TA, Rogers CR. Disodium Etidronate: Its Role in
Preventing Heterotopic Ossification in Severe Head Injury. Arch Phys Med Rehabil 1983;64:539.
DH, Santi M, Abel MF. Treatment of Stiff-Knee Gait in Cerebral Palsy: A
Comparison by Gait Analysis of Distal Rectus Femoris Transfer Versus
Proximal Rectus Release. J Pediatr Orthop 1990;10:433.
EL, McNeal DR, Kralj A, Waters RL. Electrical Stimulation and Feedback
Training: Effects on the Voluntary Control of Paretic Muscles. Arch Phys Med Rehabil 1976;57:228.
PG, Bray TJ, Simpson LA. Fractures and Soft Tissue Injuries of the
Ankle. In: Browner BD JJ, Levine AM, TrafLon PG, eds. Skeletal Trauma: Fractures, Dislocations, Ligamentous Injuries, Vol 2. Philadelphia: WB Saunders, 1992:1871.
A, Sazbon L, Latem M. Relationship Between Muscular Tone, Movement and
Periarticular New Bone Formation in Postcoma-unaware (PC-U) Patients. Brain Inj 1996;10:259.
RL, Frazier J, Garland DE, et al. Electromyographic Gait Analysis
before and after Operative Treatment for Hemiplegic Equinus and
Equinovarus Deformity. J Bone Joint Surg [Am] 1982;64:284.
RL, Hislop HJ, Perry J, Antonelli D. Energetics: Application to the
Study and Management of Locomotor Disabilities. Energy Cost of Normal
and Pathologic Gait. Orthop Clin North Am 1978;9:351.
RL, McNeal DR, Faloon W, Clifford B. Functional Electrical Stimulation
of the Peroneal Nerve for Hemiplegia. Long-term Clinical Follow-up. J Bone Joint Surg [Am] 1985;67:792.
LX, Bosse MJ, Mayo KA, et al. Results in Patients with Craniocerebral
Trauma and an Operatively Managed Acetabular Fracture. J Orthop Trauma 1990;4:376.
S, Grosswasser Z, Ohri A, et al. Histocompatibility (HLA) Antigens in
Heterotopic Ossification Associated with Neurological Injury. J Rheumatol 1979;6:88.
SL, Binder-MacLeod SA. Electromyographic Biofeedback Applications to
the Hemiplegic Patient. Changes in Upper Extremity Neuromuscular and
Functional Status. Phys Ther 1983;63:1393.
RD, Hammond FM, Mann NR, et al. Revised Trauma Score: An Additive
Predictor of Disability Following Traumatic Brain Injury? Am J Phys Med Rehabil 1996;75:456.
JP, Dugan SA, Young JL, et al. A Retrospective Review of the Incidence
and Rehabilitation Outcome of Concomitant Traumatic Brain Injury and
Ligamentous Knee Injury. Arch Phys Med Rehabil 1998;79:805.