Kyphosis
plane in which the convexity of the curve is directed posteriorly.
Lordosis is a curvature of the spine in the sagittal plane in which the
convexity of the curve is directed anteriorly. The thoracic spine and
the sacrum are normally kyphotic, and the cervical spine and the lumbar
spine normally are lordotic (1). Although
several authors have tried to define normal kyphosis of the thoracic
spine and normal lordosis of the lumbar spine, these studies have shown
much variability in what is considered normal (2,3,4,5,6,7,8).
The ranges of normal kyphosis and lordosis change with increasing age,
and vary according to gender and the area of the spine involved (2,3,4,5).
The degree of kyphosis or lordosis that is considered normal or
abnormal depends on the location of the curvature and the age of the
patient. For example, 30 degrees of
kyphosis is normal in the thoracic spine, but abnormal at the thoracolumbar junction.
The Terminology Committee of the Scoliosis Research Society has
expanded the normal range for thoracic kyphosis to 20 to 50 degrees,
and lumbar lordosis to 31 to 79 degrees. The measurement of thoracic
kyphosis from a lateral radiograph is the angle between the superior
endplate of the highest measurable thoracic vertebra, usually T2 or T3,
and the inferior endplate of T12. The thoracolumbar junction should
have no kyphosis or lordosis (10). Lumbar
lordosis begins at L1-2 and increases gradually until the L3-4 disc
space. The apex of normal thoracic kyphosis is the T6-7 disc space (10,11).
the entire spine is kyphotic. During the neonatal period the thoracic,
lumbar, and sacral portions of the spine remain in a kyphotic posture.
Cervical lordosis begins to develop when a child starts holding his or
her head up. When an upright posture is assumed, the primary and
secondary curves begin to develop. The primary curves are thoracic and
sacral kyphosis, and the secondary or compensatory curves in the
sagittal plane are cervical and lumbar lordosis. These curves balance
each other so that the head is centered over the pelvis (2,12,13).
showed that the ranges of normal thoracic kyphosis and lumbar lordosis
are dynamic, progressing gradually with growth. During the juvenile and
adolescent growth periods thoracic kyphosis and lumbar lordosis become
more pronounced and take on a more adult appearance. Differences also
exist between male and female spines (6). Thoracic kyphosis and spine mobility are different in boys and girls. Mellin and others (3,11)
have shown that during the juvenile and adolescent periods (ages 8 to
16 years), girls have less thoracic kyphosis and thoracic spinal
mobility than do boys of the same age. Thoracic kyphosis also tends to
progress with age. Fon et al. (15) showed that
from 30 to 70 years of age women have a progressive increase in
kyphosis, from a mean of 25 degrees to a mean of 40 degrees. Men also
show a definite progression with age, but at a lower rate.
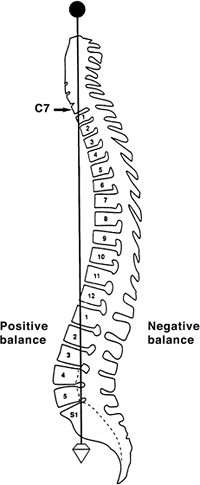
dropping from C7 and intersecting the posterosuperior corner of the S1
vertebral body (Fig. 20.1). Positive sagittal
balance occurs when the plumb line falls in front of the sacrum, and
negative sagittal balance occurs when the plumb line falls behind the
sacrum (16).
the presence of kyphosis or lordosis. In the upright position the spine
is subjected to the forces of gravity, and several structures maintain
its stability: the disc complex (nucleus pulposus and annulus), the
ligaments (anterior longitudinal ligament, posterior longitudinal
ligament, ligamentum flavum, apophyseal joint ligaments, and
interspinous ligament), and the muscles (the long spinal muscles, the
short intrinsic spinal muscles, and the abdominal muscles). Alteration
in function resulting from paralysis, surgery, tumor, infection, or
alteration in growth potentials can cause a progressive kyphotic
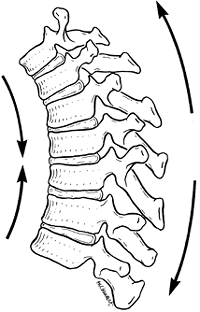
deformity in a child (17). Both compressive and tensile forces are produced by the action of gravity on an upright spine (Fig. 20.2).
In normal thoracic kyphosis, the compressive forces borne by the
anterior elements are balanced by the tensile forces borne by the
posterior elements. In a lordotic spine, the compressive forces are
posterior and the tensile forces are anterior. These forces of
compression and tension on the spinal physes can cause changes in
normal growth, and a growth deformity can be added to a biomechanical
deformity to cause a pathologic kyphosis (17,18).
 |
|
Figure 20.1
A plumb line is dropped from the middle of the C7 vertebral body to the posterosuperior corner of the S1 vertebral body. (From Bernhardt M. Normal spinal anatomy: normal sagittal plane alignment. In: Bridwell KH, DeWald RL, eds. The textbook of spinal surgery, 2nd ed. Philadelphia, PA: Lippincott-Raven, 1997:185.) |
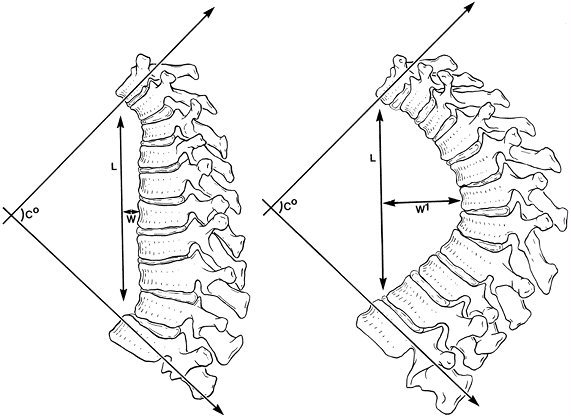
believe that relative differences in forces applied to the spine are
reflected more accurately by the length and width of a kyphotic curve
than by just the degree of the curve. For example, curves that are
longer and wider (farther from the center of gravity) are more likely
to cause deformity in an immature spine (Fig. 20.3). Winter and Hall (20) classified disorders that result in kyphosis of the spine. Only the more common causes are
presented in this chapter; the other causes are discussed elsewhere in this book (Table 20.1).
 |
|
Figure 20.2
Forces that contribute to kyphotic deformity of the thoracic spine. The anterior vertebral bodies are in compression, and the posterior vertebral elements are in tension. (From White AA III, Panjabi MM. Practical biomechanics of scoliosis and kyphosis. In: White AA, Panjabi MM, eds. Clinical biomechanics of the spine. Philadelphia, PA: JB Lippincott, 1990:127.) |
and is a common complaint seen in juvenile and adolescent patients.
Usually, the parents are more concerned about the postural roundback
deformity than the adolescent is, and these parental concerns typically
are what bring the patient to the physician’s office. The physician’s
role in this situation is to rule out more serious causes of kyphosis.
Postural kyphosis should be differentiated from pathologic types of
kyphosis, such as Scheuermann disease, and from congenital kyphosis.
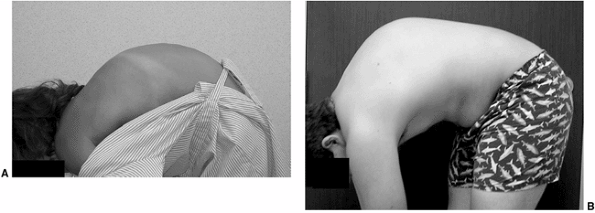
When observed from the side, patients with postural roundback have a
gentle rounding of the back while bending forward (Fig. 20.4).
Patients with Scheuermann disease and congenital kyphosis have a sharp
angular kyphosis or gibbus on forward bending when observed from the
side. Radiographs are usually necessary in order to rule out pathologic
types of kyphosis. Patients with postural kyphosis do not have
radiographic vertebral body changes, and the deformity is completely
correctable by changes in position or posture. This deformity is common
in patients who are taller than their peers, and in young adolescent
girls undergoing early breast development who tend to stoop because
they are self-conscious about their bodies (21).
indicated. Exercises have been suggested and may help maintain better
posture, but adherence to such a therapy program is difficult for
juveniles and young adolescents. This problem is best treated by
educating the patient and, more important, the parents, and by
observation (22).
despite its rare occurrence, neurologic deficits resulting from this
deformity are frequent.
 |
|
Figure 20.3
The two spinal curvatures represented by these drawings are different in magnitude; however, using Cobb’s method to measure the deformities, the degrees of curvature are identical. The differences in the curves are more accurately reflected when the length of the curves (L) and their respective widths (W and W1) are taken into consideration. (From Voutsinas SA, MacEwen GD. Sagittal profiles of the spine. Clin Orthop 1986;210:235.) |
|
TABLE 20.1 DISORDERS AFFECTING THE SPINE AND RESULTING IN KYPHOSIS
|
||
|---|---|---|
|
development of the vertebrae, including a failure of developing
segments of the spine to form or to separate properly (23). The spine may be either stable or unstable, or it may become unstable with growth (24).
Spinal deformity in congenital kyphosis usually progresses with growth,
and the amount of progression is directly proportional to the number of
vertebrae involved, the type of involvement, and the amount of
remaining normal growth in the affected vertebrae (24,25).
initially described two basic types of congenital kyphosis: a failure
of formation of part or all of the vertebral body, and a failure of
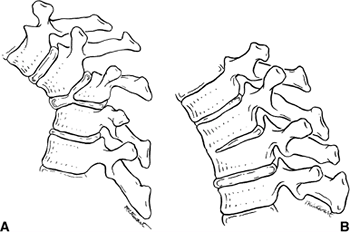
segmentation of part or all of the vertebral body. Winter et al. (23,28) developed the most useful classification of congenital kyphosis, which divides the deformity into three types (Table 20.2). Type I is failure of formation of all or part of the vertebral body (Fig. 20.5A); type II is failure of segmentation of one or multiple vertebral levels (Fig. 20.5B); and type III is a mixed form, with elements of both failure of formation and failure of segmentation.
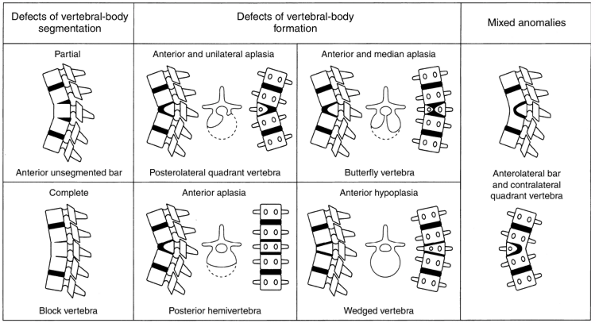
vertebral body segmentation consist of a partial (anterior unsegmented
bar) or a complete (block vertebrae) failure of segmentation. Defects
of vertebral body formation are divided into four types; (a)
posterolateral quadrant vertebrae, (b) butterfly vertebrae, (c)
posterior hemivertebrae, and (d) wedged vertebrae (Fig. 20.6) (29). A classification by Dubousset (30) and Zeller et al. (31) includes a rotary dislocation of the spine. Shapiro and Herring (32)
also described a type III congenital kyphosis, but further divided the
displacement into type A (sagittal plane only) and type B (rotary,
transverse, and sagittal planes). Any classification can be subdivided
further into deformities with or without neurologic compromise; this is
useful for making treatment decisions, because each type of congenital
kyphosis has a distinct natural history and risk of progression.
 |
|
Figure 20.4 A: Lateral view of normal spinal contour on forward bending. B:
Lateral view of a patient with Scheuermann disease on forward bending. Note the break in the normal contour and sharp angular nature of the spine. |
|
TABLE 20.2 WINTER’S CLASSIFICATION OF CONGENITAL DEFORMITY
|
||||||||
|---|---|---|---|---|---|---|---|---|
|
The somatic mesoderm, which is devoted to the formation of the
vertebral column and rib cage, undergoes segmentation into 38 to 44
pairs of discrete, bilateral somites. The formation of a vertebra
depends on contributions of cells from two separate and successive
pairs of sclerotomes. This condensation of the paired sclerotomes
occurs at approximately 5 weeks of gestation. If one side of the pair
of sclerotomes fails to develop, this will cause a hemivertebra to be
formed, resulting in congenital scoliosis (34,35).
 |
|
Figure 20.5 A: Congenital kyphosis caused by failure of formation of the vertebral body (type I). B: Congenital kyphosis caused by failure of segmentation (type II). (Courtesy of Robert Winter, MD, Minneapolis.)
|
congenital kyphosis and congenital scoliosis occur during different
periods of spinal development. He divided the development of the spine
into an embryonic period (the first 56 days) and a fetal period (from
57 days to birth). During the embryonic period, failure of segmentation
and aplasia of part of the vertebrae, resulting in hemivertebra
formation, cause scoliosis. He believes that the causes of congenital
kyphosis occur in the fetal period, during the cartilaginous phase of
development. Failure of formation occurs in this phase when the
cartilaginous centrum of the vertebral body forms a functionally
inadequate growth cartilage.
involves the pars and the facet joints and makes the spine unstable) to
involvement of only the anterior one third to one half of the vertebral
body. This abnormal development is thought to be the result of
inadequate vascularization of the vertebral body during the fetal
period, leading to hypoplasia or aplasia of the anterior vertebral
body. If one side of the vertebra is involved more than the other side,
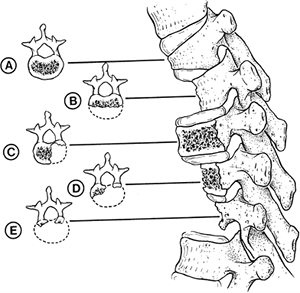
scoliosis also may occur (Fig. 20.7). Unlike
hemivertebral anomalies that occur in the embryonic period because of
maldevelopment of corresponding pairs of somites causing congenital
scoliosis, posterior arch anomalies are almost universally absent in
pure congenital kyphosis.
acting as a tether against normal growth and causing spinal deformity.
The height of the vertebral bodies is relatively normal, but the depth
of the ossification of the annulus fibrosus varies. Ossification may be
delayed, with a period of normal growth followed by spontaneous
ossification. Morin et al. (38) believe that
kyphosis caused by a “segmentation defect” represents a developmental
defect of the perivertebral structures (the annulus fibrosis, the ring
apophysis, and the anterior longitudinal ligament) rather than a true
intervertebral bar.
and based on the type of kyphosis: failure of formation (type I),
failure of segmentation (type II), or mixed anomalies (type III).
Congenital kyphosis tends to be progressive, with the greatest rate of
progression occurring during the time of most rapid growth of the spine
(birth to 3 years of age) and during the adolescent growth spurt.
Winter et al. found that failure of formation (type I deformity)
produces a much more severe kyphosis, with a rate of progression that
averages 7 degrees per year, whereas type II deformities progress an
average of 5 degrees per year (28). McMaster and Singh found the most rapid progression in type III kyphosis, followed by type I, because of
involvement of posterolateral quadrant vertebrae. In their study, a
type III kyphosis progressed at a rate of 5 degrees per year before 10
years of age and 8 degrees per year thereafter until the end of growth.
Type I (failure of formation) kyphosis progressed 2.5 degrees per year
before 10 years of age and 5 degrees per year thereafter (29).
Type I and type III deformities are associated with a much higher
incidence of neurologic involvement and paraplegia than type II
deformities are. Neurologic problems occur more frequently in patients
with type I and type III deformities because they tend to have an acute
angular kyphosis over a short segment, which places the spinal cord at
higher risk for compression at the level of acute angulation. Type II
deformities (failure of segmentation) rarely result in neurologic
problems because involvement of several segments produces a more
gradual kyphosis, and vertebral body height is usually maintained with
little or no vertebral body wedging. The most frequent location of
congenital kyphosis is T10–L1 (28).
 |
|
Figure 20.6
Drawings showing the different types of vertebral anomalies that produce congenital kyphosis or kyphoscoliosis. (From McMaster MJ, Singh H. Natural history of congenital kyphosis and kyphoscoliosis. J Bone Joint Surg 1999;81A:1367–1383.) |
 |
|
Figure 20.7
The five most common patterns of congenital vertebral hypoplasia and aplasia are illustrated in lateral and transverse views. Types B and E tend to produce pure congenital kyphosis. (From Tsou PM. Embryology of congenital kyphosis. Clin Orthop 1977;128:18.) |
anomalies. Intraspinal abnormalities have been reported to occur in 5%
to 37% of patients with congenital kyphosis and congenital scoliosis (39,40,41,42). A study by Bradford et al. (43)
indicates that this incidence may be even greater. They found that six
of eight patients with congenital kyphosis had spinal cord
abnormalities visible on magnetic resonance imaging (MRI). Although the
proposed time of development of the deformity may be different from
that of congenital scoliosis, other nonskeletal anomalies such as
cardiac, pulmonary, renal, and auditory disorders or Klippel-Feil
syndrome (44,45) can be associated with congenital kyphosis.
orthopaedist. The deformity may be detected before birth on prenatal ultrasonography (46)
or noted as a clinical deformity in the newborn. If the deformity is
mild, congenital kyphosis can be overlooked until a rapid growth spurt
makes the condition more obvious. Some mild deformities are found by
chance on radiographs that are obtained for other reasons. Clinical
deformities seen in the newborn tend to have a worse prognosis than
those discovered as incidental findings on plain radiographs. Physical
examination usually reveals a kyphotic deformity at the thoracolumbar
junction or in the lower thoracic spine. An attempt should be made to
determine the rigidity of the deformity by flexion and extension of the
spine. A detailed neurologic examination should be done, looking for
any subtle signs of neurologic compromise. Associated musculoskeletal
and nonmusculoskeletal anomalies should be sought on physical
examination.
radiographs provide the most information in the evaluation of
congenital kyphosis (Fig. 20.8). Failure of
segmentation and the true extent of failure of formation may be
difficult to detect on early films because of incomplete ossification.
Flexion and extension lateral radiographs are helpful in determining
the rigidity of the kyphosis and possible instability of the spine.
Computerized tomography (CT) with three-dimensional reconstructions can
identify the amount of vertebral body involvement and can determine
whether more kyphosis or scoliosis might be expected (Fig. 20.9).
CT scans can only identify the nature of the bony deformity and the
size of the cartilage anlage. They do not show the amount of growth
potential in the cartilage anlage, and therefore only an estimate of
possible progression can be made. MRI should be obtained in most cases
because of the significant incidence of intraspinal abnormalities. In
addition, the location of the spinal cord and any areas of spinal cord
compression caused by the kyphosis can be seen on MRI. The cartilage
anlage will be well defined by MRI in patients with failure of
formation (Fig. 20.10); however, as with CT
scans and plain radiographs, MRI cannot reveal how much growth
potential is present in the cartilage anlage, and can only help one to
estimate the probability of a progressive deformity.
 |
|
Figure 20.8
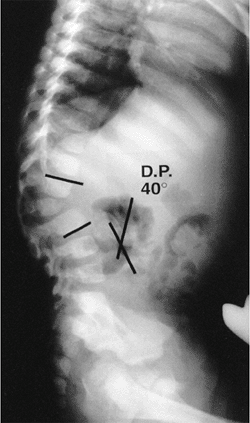
A 2-year-old child with type I congenital kyphosis measuring 40 degrees. Radiograph demonstrates failure of formation of the anterior portion of the first lumbar vertebra. |
problems, can be seen on routine prenatal ultrasonography at as early
as 20 weeks of gestation (46). Myelograms have
been used for documenting spinal cord compression but have been mostly
replaced by MRI. If myelography is used, images should be taken in the
prone and supine positions. Myelograms obtained in only the prone
position may miss information about spinal cord compression because of
pooling of dye around the apex of the deformity. Myelography can be
used in conjunction with CT scanning to add to the diagnostic
information obtained.
one of continued progression with an increased risk of neurologic
compromise, surgery is usually the preferred method of treatment (23).
If the deformity is mild or if the diagnosis is uncertain, close
observation may be a treatment option. However, observation of a
congenital kyphotic deformity must be used with caution, and the
physician must not be lulled into a false sense of security if the
deformity progresses only 3 to 5 degrees over a 6-month period. If the
deformity is observed over 2 to 3 years, it will have progressed 20 to
30 degrees and cannot thereafter be easily corrected. Bracing has no
role in the treatment of congenital kyphosis, unless compensatory
curves are being treated above or below the congenital kyphosis (23,44,47).
Bracing a rigid structural deformity, such as congenital kyphosis,
neither corrects the deformity nor stops the progression of kyphosis.
To document that there has been a significant change in kyphosis, the
radiographs should be taken by a standardized method, and the same end
vertebral bodies should be measured. This will ensure that any change
that has occurred since the previous radiograph is accurately measured.
of the deformity, the age of the patient, and the presence of
neurologic deficits. Procedures can include posterior fusion, anterior
fusion, both anterior and posterior fusions, and anterior osteotomy
with posterior fusion. Fusion can be performed with or without
instrumentation.
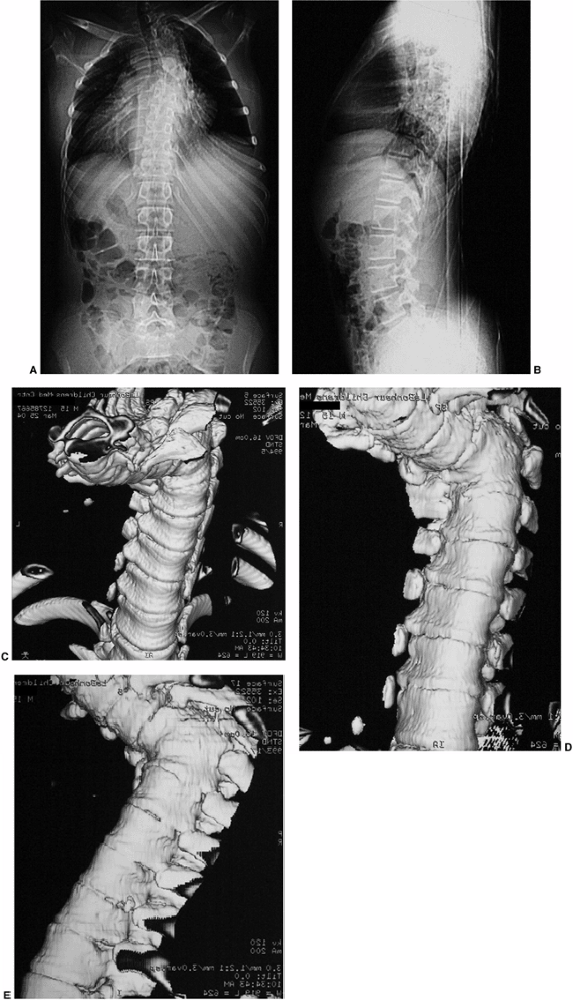 |
|
Figure 20.9 Congenital kyphosis. A and B: Anteroposterior and lateral radiographs. Note inadequate detail of kyphosis on lateral radiograph of spine. C to E:
Computerized tomography (CT) three-dimensional reconstruction views that clearly demonstrate the bony anatomy of congenital kyphosis. |
of the disease: early with mild deformity, late with moderate or severe
deformity, and late with severe deformity and spinal cord compression.
posterior fusion. If the deformity is less than 50 or 55 degrees and
the patient is younger than 5 years of age, posterior fusion alone,
extending from one level above the kyphotic deformity to one level
below, is recommended (23,28,44,48).
This may allow for some improvement in the kyphotic deformity because
of continued growth anteriorly from the anterior end plates of the
vertebrae one level above and one level below the congenital kyphotic
vertebrae that are included in the posterior fusion. Anterior and
posterior spinal fusions at least one level above and one level below
the congenital kyphosis are indicated in curves greater than 60 degrees
(49). Anterior and posterior fusion predictably
halts the progression of the kyphotic deformity but, because of
ablation of the anterior physes (31,48,49), it does not allow for the possibility of some correction of the deformity with growth.
posterior arthrodesis alone may be successful if the kyphosis is less
than 50 to 55 degrees (28,50).
If the deformity is more than 55 degrees (which usually is the case in
deformities detected late), anterior and posterior fusion produces more
reliable results (28,50).
Anterior arthrodesis alone will not correct the deformity. Any
correction of the deformity requires anterior strut grafting with
temporary distraction and posterior fusion, with or without posterior
compression instrumentation. The posterior instrumentation may allow
for some correction of the kyphosis but should be regarded more as an
internal stabilizer than as a correction device (23).
Correction by instrumentation should be used with caution in rigid,
angular curves because of the high incidence of neurologic
complications. If anterior strut grafting is performed, the strut graft
should be placed anteriorly under compression. If no correction is
attempted and the goal of surgery is just to stop progression of the
kyphosis, a simple anterior interbody fusion combined with a posterior
fusion can be performed. The use of skeletal traction (halo-pelvic,
halo-femoral, or halo-gravity) to correct the deformity is tempting,
but is not recommended because of the risk of paraplegia (51). In a patient with a
rigid gibbus deformity, traction pulls the spinal cord against the apex
of the rigid kyphosis and can lead to neurologic compromise (Fig. 20.11).
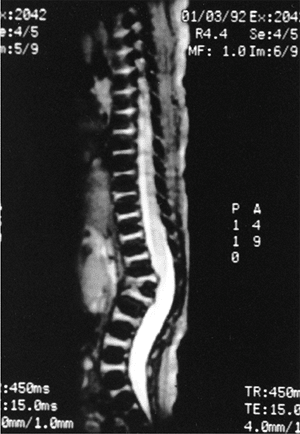 |
|
Figure 20.10
Magnetic resonance image (MRI) of type I congenital kyphosis. Failure of formation of the anterior vertebral body is demonstrated, but the growth potential of the involved vertebra cannot be determined. Note the pressure on the dural sac. |
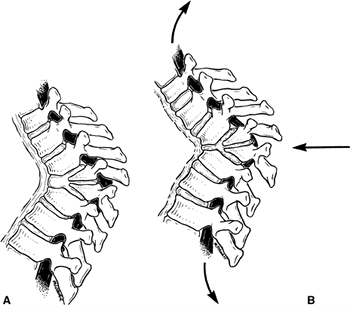 |
|
Figure 20.11 The effect of traction on a rigid congenital kyphosis. A: The apical area does not change with traction, but the adjacent spine is lengthened. B:
As the spine lengthens, so does the spinal cord, producing increased tension in the cord and aggravating existing neurologic deficits. (From Lonstein JE, Winter RB, Moe JH, et al. Neurologic deficit secondary to spinal deformity. Spine 1980;5:331.) |
congenital kyphotic deformity that is accompanied by spinal cord
compression. If congenital kyphosis causes spinal cord compression,
anterior decompression is indicated. The compression is created by bone
or disc material pressing into the front of the spinal cord, and this
can be decompressed only by an anterior procedure; laminectomy has no
role in the treatment of this condition (20).
If associated with scoliosis, the anterior approach for decompression
should be on the concavity of the scoliosis to allow the spinal cord to
move both forward and into the midline after decompression. After
adequate decompression has been achieved, the vertebrae involved are
fused with an anterior strut graft. This is followed by a posterior
fusion, with or without posterior stabilizing instrumentation.
Postoperative support with a cast, brace, or halo cast may be required.
early treatment of mild deformities and late treatment of severe
deformities as outlined by Mayfield et al. (52).
If a type II kyphosis is mild and detected early, posterior fusion with
compression instrumentation can be performed. The kyphosis should be
less than 50 degrees for a posterior fusion alone to have a good chance
of success. The posterior fusion should include all the involved
vertebrae, plus one vertebra above and one vertebra below the
congenital kyphosis.
type II deformities, because the kyphosis is more rounded and affects
several segments, instead of being sharply angular as in type I
deformities. If the deformity is severe and detected late, correction
can be obtained only by performing anterior osteotomies and fusion,
followed by posterior fusion and compression instrumentation (52).
congenital kyphosis are pseudarthrosis, progression of kyphosis, and
paralysis. Pseudarthrosis and progression of the kyphotic deformity can
be minimized by performing anterior and posterior fusions for
deformities of more than 50 degrees. The posterior fusion should extend
from one level above to one level below the involved vertebrae. This
may allow for some correction with growth.
spinal surgery. The risk of this complication can be lessened by not
attempting to maximally correct the deformity with instrumentation.
Instrumentation should be used only for stabilization of rigid
deformities. The use of halo traction in rigid congenital kyphotic
deformities has been associated with an increased risk of neurologic
compromise (51). Another long-term problem,
occurring in approximately 38% of patients with kyphosis, is low back
pain caused by increased lumbar lordosis, which is needed to compensate
for the kyphotic deformity (53).
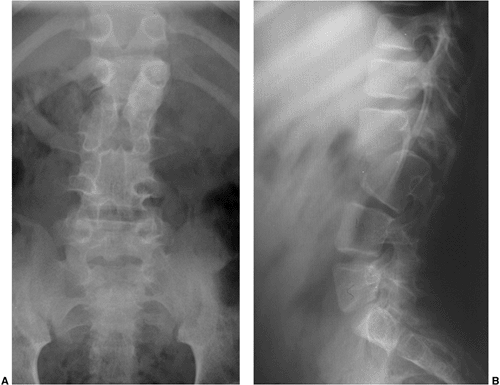
or dysgenesis of the spine, and resulting in severe spinal stenosis and instability (54).
A progressive kyphosis occurs at the site of segmental spinal
dysgenesis. This condition is often confused with other spinal
anomalies such as type I congenital kyphosis, sacral agenesis,
lumbosacral agenesis, and lumbar agenesis. Faciszewski et al. (55)
have given detailed radiographic and clinical definitions of this
condition. Segmental spinal dysgenesis is characterized by severe focal
stenosis of the spinal canal at the involved segment, and is associated
with significant narrowing of the thecal sac and absence of adjacent
nerve roots. At the involved level, a ring of bone encircles the
posteriorly positioned spinal canal, causing stenosis. The spinal canal
is hourglass-shaped with no neurocentral junctions. There is limited
potential for enlargement with growth because of the absence of
neurocentral junctions, where growth occurs (Fig. 20.12).
No pedicles or spinous or transverse processes are present at this
level. Anterior to the bony ring is a fat-filled space. The distal bony
anatomy and spinal canal are usually normal, although spina bifida has
been noted in a few cases (56). Neurologic
function can range from normal to complete paraplegia. Associated
anomalies are common, and there is a high incidence of neurogenic
bladder.
 |
|
Figure 20.12 Segmental spinal dysgenesis. Anteroposterior (A) and lateral (B) radiographs show narrowing of spinal canal and absence of L1 and part of L2 vertebral bodies.
|
The diagnosis can be made on the basis of plain radiographs, but MRI
and CT scans and three-dimensional reconstructions are usually needed
in order to fully show the extent of this condition. A progressive
kyphosis will occur with this condition. Progressive neurologic
deterioration has been noted by Flynn et al. (57) and Faciszewski et al. (55).
Early anterior and posterior fusion, with or without decompression, is
recommended. The use of spinal instrumentation is controversial because
of the small size of the patient. Hughes et al. (56)
believe that treatment should be directed toward the establishment and
maintenance of spinal stability first, and toward decompression of the
cord secondarily.
Rarely is it associated with absence of the most caudal segment of the
lumbar spine. The association with maternal diabetes has been well
documented (58,59,60,61). Kyphosis may occur with this condition, although it is usually not progressive and does not require treatment (62,63).
an uncommon cause of kyphosis in pediatric patients; however, if
discovered late it may be confused with type II congenital kyphosis.
Knutsson (64), in 1949, was the first to
describe PAVF in the English-language literature, and a total of 80
cases have since been reported. This condition is distinguishable from
type II congenital kyphosis because the disc spaces and vertebral
bodies are normal at birth and later become affected with an anterior
fusion. Although the etiology is unknown, PAVF is probably a distinct
clinical condition; however, consideration has been given to the
possibility that it may represent a delayed type II congenital
kyphosis. Dubousset (30) suggested that certain
forms of type II congenital kyphosis (failure of segmentation) may be
inherited. The patients have a failure of segmentation, with delayed
fusion of the anterior vertebral elements, which is not visible on
radiographs until 8 or 10 years of age. He described one family in
which three individuals had delayed ossification and congenital
kyphosis, and another family in which the grandmother, mother, and two
sisters had the deformity. Kharrat and Dubousset (65) also found this condition to be familial in 6 of 15 patients, and Van Buskirk et al. (66)
reported associated anomalies in 46% of 15 patients, including heart
defects, tibial agenesis, foot deformities, Klippel-Feil syndrome, Ito
syndrome, pulmonary artery stenosis, and hemisacralization of L5.
described five stages of PAVF: stage 1 is disc space narrowing, which
occurs to a greater extent anteriorly than posteriorly; stage 2 is
increased sclerosis of the vertebral end plates of the anterior and
middle columns; stage 3 is fragmentation of the anterior vertebral end
plates; stage 4 is fusion of the anterior and sometimes the middle
columns; and stage 5 is development of a kyphotic deformity.
anterior disc space fusing while part of the posterior disc space
remains open, allowing for continued growth in the posterior disc space
and the posterior column. Bollini et al. (68)
found that patients with thoracic PAVF had a relatively good prognosis,
whereas those with lumbar involvement had a poor prognosis. Involvement
of the thoracic spine is better tolerated by patients than is
involvement of the lumbar area because of the normal kyphotic posture
of the thoracic spine. Therefore, nonoperative treatment is recommended
for most thoracic PAVF deformities. For PAVF in the lumbar spine, a
posterior spinal fusion is indicated in stages 1, 2, and 3. In stages 4
and 5, the kyphotic deformity has already occurred in a normally
lordotic lumbar spine. Posterior fusion will only stop progression of
kyphotic deformity. If normal sagittal alignment is to be obtained, an
anterior osteotomy followed by posterior fusion and instrumentation is
recommended (64,65,66,67,68,69,70,71).
kyphosis in the thoracic, thoracolumbar, and lumbar spine. Scheuermann
originally described this rigid juvenile kyphosis in 1920; it is
characterized by vertebral body wedging that is believed to be caused
by a growth disturbance of the vertebral end plates (72,73) (Fig. 20.13).
groups: a typical form and an atypical form. These two types are
determined by the location and natural history of the kyphosis,
including symptoms occurring during adolescence and after growth is
completed. Typical Scheuermann disease usually involves the thoracic
spine, with a well-established natural history during adolescence and
after skeletal maturity (74). This classic form of Scheuermann kyphosis will have three or more consecutive vertebrae,
each wedged 5 degrees or more (Sorensen criteria), producing a
structural kyphosis. In contrast, atypical Scheuermann disease is
usually located in the thoracolumbar junction or in the lumbar spine,
and its natural history is well defined. The atypical type is
characterized by vertebral end-plate changes, disc space narrowing, and
anterior Schmorl nodes, but does not necessarily fulfill Sorensen’s
criteria of three consecutively wedged vertebrae of 5 degrees. Thoracic
Scheuermann is the most common form, with the atypical form less
frequently seen.
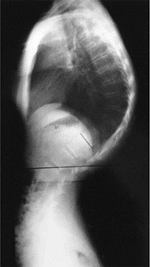 |
|
Figure 20.13
Lateral radiograph of a patient with Scheuermann disease and an 81-degree kyphotic deformity. Note the narrowing of the intervertebral disc spaces and the irregularity of the vertebral end plates. There is an associated increase in lumbar lordosis below the kyphotic deformity. |
kyphosis in a juvenile or adolescent spine. The apex of kyphosis is
located between T7 and T9 (10). The reported incidence of Scheuermann deformities in the general population ranges from 0.4% to 10% (75,76,77,78,79). Reported male-to-female ratios vary in the literature. Scheuermann originally reported a male preponderance of 88% (72). Most reports in the literature note either a slight male preponderance or an equal male-to-female ratio (35,77,78,79,80,81,82). Bradford et al. (76) has been the only one to report an increased incidence of Scheuermann disease in women.
described a Scheuermann prodrome in patients who had a lax, asthenic
posture from the age of approximately 4 to 8 years, and in whom, within
a few years, a fixed kyphosis developed. The clinical detection of
Scheuermann disease occurs at approximately 10 to 12 years of age.
Wedging of apical vertebrae has not been reported before 10 years of
age (83). Radiographic evidence of Scheuermann
disease is usually not detectable in patients younger than 10 years of
age because the ring apophysis is not yet ossified. Until the ring
apophysis ossifies, vertebral body wedging and irregularity of the end
plate are difficult to measure on radiographs.
Scheuermann disease, but the true cause remains unknown. Genetic,
vascular, hormonal, metabolic, and mechanical factors have been
suggested as causes of Scheuermann kyphosis. Sorensen (78) noted a high familial predilection, and Halal et al. (84), in a study of five families, and McKenzie and Sillence (85),
in a study of 12 families, suggested that the disease may be inherited
in an autosomal dominant fashion with a high degree of penetrance.
Additional support for a genetic basis for this condition is provided
by Carr et al. (86,87) in a report of Scheuermann disease occurring in identical twins. Halal et al. (84), McKenzie and Sillence (85), and Carr et al. (87) have reported possible autosomal dominant inheritance of Scheuermann kyphosis.
form of avascular necrosis of the ring apophysis, which led to a growth
disturbance resulting in a progressive kyphosis with growth (72,73).
The problem with this theory is that the ring apophysis contributes
little, if at all, to the longitudinal growth of the vertebrae (87,88). Bick and Copel (89)
demonstrated that the ring apophysis lies outside the true
cartilaginous physis and contributes nothing to the longitudinal growth
of the vertebral body. Therefore, a disturbance in the ring apophysis
should not affect growth of the vertebrae or cause vertebral wedging.
He believed that the herniation of disc material occurred because of a
weakened end plate. The disc herniation was thought to damage the
anterior end plate, resulting in abnormal growth, which in turn caused
the kyphosis. There is a definite increased incidence of Schmorl nodes
in patients with Scheuermann kyphosis, but the problem with this theory
is that Schmorl nodes are found outside the area of kyphosis, and are
also present in individuals who have asymptomatic, normal spines and do
not have a kyphotic deformity.
persistence of an anterior vascular groove altered the anterior growth
of the vertebral body, but Aufdermaur and Spycher (92,93) and Ippolito and Ponseti (94)
were unable to document growth disturbances around the anterior
vascular groove, and concluded that persistence of an anterior vascular
groove is a sign of immaturity of the spine. Lambrinudi (95)
postulated that Scheuermann disease resulted from upright posture and a
tight anterior longitudinal ligament. The fact that no cases of
Scheuermann disease have been found in quadruped animals lends support
to this theory (96). This has led to the more
popular belief that the anterior end-plate changes are caused by
mechanical forces in response to Wolff’s law or the Hueter-Volkmann
principle. Compression forces in the anterior growth plate cause a
decrease in growth in the area of the kyphosis. Indirect support for
this argument can be found in the changes in the wedging of the
involved vertebral bodies and the reversal of these changes when
bracing or casting is used in the immature spine. Scoles et al. (96)
also support this theory by demonstrating disorganized endochondral
ossification in the involved vertebrae, similar to that seen in Blount
disease. They conclude that the changes in endochondral ossification
are the result of increased pressure on the vertebral growth plate.
found that patients who have Scheuermann disease tend to be taller than
normal for their chronologic and skeletal ages, and that their bone age
tends to be more advanced than their chronologic age. Because they
found increased growth hormone levels in these patients, they suggested
that the increased height and the advanced skeletal age could be caused
by the increased growth hormone (97,98). The increased height and more rapid growth may make the vertebral end plates
more susceptible to increased pressure and result in the changes seen
in Scheuermann disease. The increased growth hormone levels noted by
Ascani et al. may also lead to a relative osteoporosis of the spine,
which, in turn, may predispose the spine to the development of
Scheuermann disease.
reported in the 1980s that Scheuermann kyphosis may be caused by a form
of juvenile osteoporosis. However, using quantitative CT scans, Gilsanz
et al. (102) found no evidence of osteoporosis
in patients with Scheuermann kyphosis compared with normal research
subjects. The authors suggested that the technique used to determine
osteoporosis might account for the differences between their report and
those that show osteoporosis. In a study using single-photon
absorptiometric analysis of cadaver vertebrae from patients with
Scheuermann kyphosis, Scoles et al. (96) also found no evidence of osteoporosis.
is that an alteration in endochondral ossification occurs. Whether this
altered endochondral ossification is the cause or result of kyphosis is
not known. Ippolito and Ponseti (94) found a
decrease in the number of collagen fibers, which were thinner than
normal, and an increase in proteoglycan content. Some areas of the
altered end plate showed direct bone formation from cartilage instead
of the normal growth-plate sequences for ossification. These studies
help support the belief that Scheuermann kyphosis is an underlying
growth problem of the anterior vertebral end plates.
lumbar kyphosis, is believed to be caused by trauma to the immature
spine, resulting in irregularities of the end plate (104).
history for Scheuermann disease and recommended early treatment to
prevent severe deformity, pain, impaired social functioning,
embarrassment about physical appearance, myelopathy, degeneration of
the disc spaces, spondylolisthesis, and cardiopulmonary failure.
Despite these reports, few long-term follow-up studies of Scheuermann
disease were performed until that of Murray et al. (77). Findings by Travaglini and Conti (35,105), Murray et al. (77), and Lowe (106) suggest that the natural history of the disease tends to be benign.
noted that, among the patients who required brace treatment, more than
half had progression of their deformities during this growth spurt
before brace treatment was begun. Little is known about progression of
the kyphosis after growth is completed, and whether it is similar to
that in scoliosis. It is not well documented whether the kyphosis will
continue to progress beyond a certain degree during adulthood.
that the kyphosis did progress during adulthood, but few patients
developed severe deformities. What is known is that patients with
Scheuermann kyphosis have more intense back pain, jobs that require
relatively little physical activity, less range of motion of the trunk
in extension, and different localization of back pain than the general
population that does not have Scheuermann kyphosis (77).
Even with these findings, when compared with normal individuals,
patients with Scheuermann kyphosis have no significant differences in
self-esteem, social limitations, or level of recreational activities.
The number of days they miss from work because of back pain is also
similar.
disease suggest that, although patients may have some functional
limitations, their lives are not seriously restricted, and they have
few clinical or functional problems. Pulmonary function actually
increases in these patients, probably because of the increased diameter
of the chest cavity, until their kyphosis is more than 100 degrees.
Patients with kyphosis of more than 100 degrees have restrictive
pulmonary function. Another finding in patients with Scheuermann
kyphosis was that disc degeneration was five times more likely to be
seen on MRI in patients with Scheuermann compared with controls (108). The clinical significance of this finding is not known (77).
but the curves tend to be small, approximately 10 to 20 degrees.
Scoliosis associated with Scheuermann disease usually has a benign
natural history. The scoliotic curve is rarely progressive, and usually
does not require treatment. Deacon et al. (109,110)
divided scoliotic curves found in patients with Scheuermann disease
into two types, based on the location of the curve and the rotation of
the vertebrae into or away from the concavity of the scoliotic curve.
In the first type of curves, the apices of scoliosis and kyphosis are
the same, and the curve is rotated toward the convexity. The rotation
of the scoliotic curve is opposite to that normally seen in idiopathic
scoliosis. Deacon et al. (109,110)
suggested that the difference in direction of rotation is caused by
scoliosis occurring in a kyphotic spine, instead of the hypokyphotic or
lordotic spine that is common in idiopathic scoliosis. In the second
type of curves, the apex of the scoliosis is above or below the apex of
the kyphosis, and the scoliotic curve is rotated into the concavity of
the scoliosis, more like idiopathic scoliosis. This type of scoliosis
seen with Scheuermann kyphosis is the more common, and it rarely
progresses or requires treatment.
The suggested reason for the increased incidence of spondylolysis is
that increased stress is placed on the pars interarticularis because of
the associated compensatory hyperlordosis of
the
lumbar spine in Scheuermann disease. This increased stress causes a
fatigue fracture at the pars interarticularis, resulting in
spondylolysis. Ogilvie and Sherman (111)
found a 50% incidence of spondylolysis in the 18 patients they
reviewed. Stoddard and Osborn reported a 54% incidence of spondylolysis
in their patients with Scheuermann kyphosis (112).
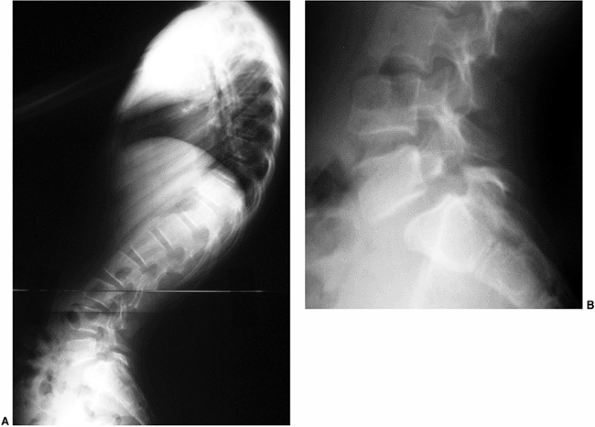 |
|
Figure 20.14 A and B: Lateral radiographs demonstrating spondylolisthesis with kyphosis.
|
time of puberty. The clinical feature that distinguishes postural
kyphosis from Scheuermann kyphosis is rigidity. Often, mild Scheuermann
disease is believed to be postural because the kyphosis may be more
flexible in the early stages than in later stages. Usually, the patient
seeks treatment because of a parent’s concern about poor posture.
Sometimes the poor posture has been present for several months or
longer, or the parents may have noticed a recent change during a growth
spurt. Attributing kyphotic deformity in a child to poor posture often
causes a delay in diagnosis and treatment.
than deformity. The pain is generally located over the area of the
kyphotic deformity, but also occurs in the lower lumbar spine if
compensatory lumbar lordosis is severe. Back pain is usually aggravated
by standing, sitting, or physical activity. The distribution and
intensity of the pain vary according to the age of the patient, the
stage of the disease, the site of the kyphosis, and the severity of the
deformity. Pain usually subsides with the cessation of growth, although
pain in the thoracic spine can sometimes continue even after the
patient is skeletally mature (77,116).
More commonly, after growth is completed patients complain of low back
pain caused by the compensatory or exaggerated lumbar lordosis.
during the rapid growth phase. During the growth spurt, pain is
reported by 22% of patients, but as the end of the adolescent growth
spurt approaches, this figure reaches 60%. Some authors believe that
when growth is complete the pain recedes completely, except for
well-circumscribed paraspinal discomfort (117,118,119). In adult patients with Scheuermann disease, pain may be located in and around
the posterior iliac crest. This pain is thought to result from
arthritic changes at T11 and T12, because the posterior crest is
supplied by this dermatome. Stagnara (120) believes that the mobile areas above and below the rigid segment are the source of pain.
noted that if the apex of kyphosis is in the upper thoracic spine,
patients have more pain with everyday activities. The degree of
kyphosis has also been correlated with symptoms. It seems logical that
the larger the kyphosis, the more likely it is to be symptomatic, but
Murray et al. (77) found that curves between 65
and 85 degrees produced the most symptoms, whereas curves of more than
85 degrees and less than 65 degrees produced fewer symptoms. However,
in patients with thoracolumbar or lumbar kyphosis (atypical Scheuermann
disease), activity decreased as the degree of kyphosis increased.
those with thoracic deformity. These patients usually have low back
pain but, unlike patients with the more common form of Scheuermann
disease, their kyphotic deformity is not as noticeable. Pain is
associated with spinal movement. Lumbar Scheuermann is especially
common in men involved in competitive sports and in farm laborers,
suggesting that the cause may be an injury to the vertebral physes from
repeated trauma (121).
examination of the back and a complete neurologic evaluation are
essential. With the patient standing, the shoulders appear to be
rounded and the head protrudes forward. The anterior bowing of the
shoulders is caused by tight pectoralis muscles. Angular kyphosis is
seen most clearly when the patient is viewed from a lateral position
and is asked to bend forward. Normally, the back exhibits a gradual
rounding with forward bending, but in patients with Scheuermann disease
an acute increase is evident in the kyphosis of the thoracic spine or
at the thoracolumbar junction. Stagnara et al. (122)
found cutaneous pigmentation to be common at the most protruding
spinous process at the apex of the kyphosis, probably the result of
friction exerted by the backs of chairs and clothing. Compensatory
lumbar and cervical lordosis, with forward protrusion of the head,
further increases the anterior flexion of the trunk. Associated
hamstring and hip flexor muscle tightness are often present.
correct completely with hyperextension. Larger degrees of kyphosis are
not necessarily more rigid, and the amount of rigidity will vary with
the age of the patient (77).
be overlooked. Spinal cord compression has been reported occasionally
in patients with Scheuermann disease (123,124,125,126,127). Three types of neural compression have been reported: ruptured thoracic disc (128),
intraspinal extradural cyst, and mechanical cord compression at the
apex of kyphosis. However, spinal cord compression and neurologic
compromise are rare (129). Bouchez et al. (70) found that only 1% of patients with a paralyzing disc herniation had Scheuermann disease. Ryan and Taylor (126)
suggest that the factors influencing the onset of cord compression in
patients whose cord compression is caused by the kyphosis alone are the
angle of kyphosis, the number of segments involved, and the rate of
change of the angle of kyphosis. This may be why neurologic findings
are rare in Scheuermann kyphosis: the kyphosis occurs gradually, over
several segments, and without acute angulation.
anteroposterior and lateral views of the spine with the patient
standing. The amount of kyphosis present is determined by the Cobb
method on a lateral radiograph of the spine. This is accomplished by
selecting the cranial- and caudal-most tilted vertebrae in the kyphotic
deformity. A line is drawn along the superior end plate of the most
cranial vertebra and the inferior end plate of the most caudal
vertebra. Lines are drawn perpendicular to the lines along the end
plates, and the angle they form where they meet is the degree of
kyphosis (130).
lateral radiograph is more than 5 degrees of wedging of at least three
adjacent vertebrae (78). The degree of wedging
is determined by drawing one line parallel to the superior end plate
and another line parallel to the inferior end plate of the vertebra,
and measuring the angle formed by their intersection. Bradford believes
that three wedged vertebrae are not necessary for the diagnosis, but
rather an abnormal, rigid kyphosis is indicative of Scheuermann disease
(131).
spaces are narrowed. The anteroposterior diameter of the apical
vertebra is frequently increased (96) (Fig. 20.15).
Associated Schmorl nodes are often seen in the vertebrae in the
kyphosis. Flexibility is determined by taking a lateral radiograph with
the patient lying over a bolster placed at the apex of the deformity,
to hyperextend the spine and maximize the amount of correction seen on
a hyperextension radiograph. On the lateral radiographs, most patients
will be in negative sagittal balance (132).
Sagittal balance is measured on the radiographs by dropping a plumb
line from the center of the C7 vertebral body and measuring the
distance from this line to the sacral promontory; a positive value
indicates that the plumb line lies anterior to the promontory of the
sacrum. Normal sagittal balance values are ± 2cm to the sacral
promontory. On a lateral radiograph of lumbar Scheuermann kyphosis,
irregular
end
plates, Schmorl nodes, and disc-space narrowing will be seen, but
vertebral-body wedging is not as common. MRI and CT scans are necessary
only if the patient has unusual symptoms or positive neurologic
findings. An anteroposterior or posteroanterior radiograph of the spine
should be obtained to look for associated scoliosis or vertebral
anomalies. The patient’s skeletal maturity can be estimated from a
radiograph of the left hand and wrist, or from the Risser sign on the
anteroposterior radiograph of the spine.
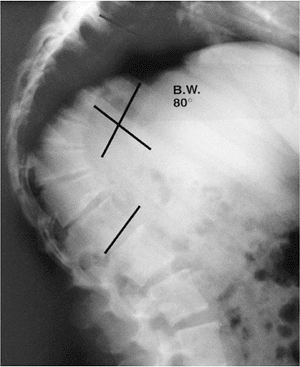 |
|
Figure 20.15
Lateral radiograph of a patient with Scheuermann disease demonstrates the kyphotic deformity seen in this disorder. Note the irregularity of the vertebral end plates and the anterior vertebral wedging. |
Scheuermann kyphosis can be grouped into five general categories: pain,
progression of deformity, neurologic compromise, cardiopulmonary
compromise, and cosmesis.
methods, and surgery. Observation is an active form of treatment. If
the deformity is mild and nonprogressive, the kyphosis can be observed
every 4 to 6 months with lateral radiographs. The parents and patient
must understand the need for regular follow-up visits. If the deformity
begins to progress, another form of treatment, such as bracing,
casting, or surgery, may be indicated.
physical therapy, bracing, and casting. Exercise and physical therapy
alone will not permanently improve kyphosis that is caused by skeletal
changes. The improvement seen with these methods is due to improved
muscle tone and correction of bad posture. The goals of physical
therapy are to increase flexibility of the spine, correct lumbar
hyperlordosis, strengthen extensor muscles of the spine, and stretch
tight hamstring and pectoralis muscles. The efficacy of this treatment
method has not been proven, and although it may improve the postural
component of Scheuermann disease, its effect on a rigid kyphosis is
questionable.
active correction systems (braces) and passive correction systems
(casts). For either a brace or a cast to be effective, the kyphotic
curve must be flexible enough to allow correction of at least 40% to
50% (83,98,133).
Milwaukee brace functions as a dynamic three-point orthosis that
promotes extension of the thoracic spine. The neck ring maintains
proper alignment of the upper thoracic spine, and the padded poster
uprights apply pressure over the apex of the kyphosis. The pelvic
girdle stabilizes the lumbar spine by flattening the lumbar lordosis. A
low-profile brace, without a chin ring and with anterior shoulder pads,
can be used for curves with an apex at the level of T9 or lower. The
indications for brace treatment are an immature spine (at least 1 year
of growth remaining in spine), some flexibility of the curve, and
kyphosis of more than 50 degrees. The brace is initially worn full-time
for an average of 12 to 18 months. If the curve is stabilized and no
progression is noted after this time, a part-time brace program can be
used until skeletal maturity is reached. Gutowski and Renshaw (135)
reported that part-time bracing (16 hours per day) is as effective as
full-time bracing, and is associated with improved patient compliance.
In this study, a Boston lumbar orthosis was used to treat the kyphosis.
The rationale for correction with this orthosis is that reduction of
the lumbar lordosis causes the patient to dynamically straighten the
thoracic kyphosis to maintain an upright posture. This presupposes a
flexible thoracic kyphosis, a normal neurovestibular axis, and the
absence of hip-flexion contractures.
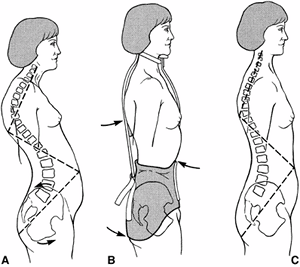 |
|
Figure 20.16 A:
Patient with Scheuermann kyphosis has thoracic kyphosis, compensatory lumbar lordosis, anterior protrusion of the head, and rotation of the pelvis. B: Patient with Scheuermann kyphosis in a Milwaukee brace. The placement of the pelvic girdle, posterior thoracic pads, occipital pads, and neck ring encourage correction of the kyphosis. C: Correction of kyphosis after Milwaukee brace treatment. (Courtesy of Robert Winter, MD, Minneapolis.) |
improvement, there is a significant loss of correction after the
discontinuation of brace treatment (50,136). Montgomery and Erwin (82)
believe that if permanent correction of kyphosis is possible, a change
in vertebral body wedging should be seen before bracing is
discontinued. Although some loss of correction can occur after bracing
is discontinued, it is still effective in obtaining some correction of
the kyphosis, and possibly in reversing vertebral body wedging, or at
least preventing progression of the kyphotic deformity (82) (Fig. 20.17).
Poor results with brace treatment have been reported in patients in
whom the kyphosis exceeded 75 degrees; in cases when the wedging of the
vertebral bodies was more than 10 degrees; or when the patient was near
or past skeletal maturity (131).
extensively in Europe for nonoperative treatment of Scheuermann
kyphosis, with good results (120,137,138,139). De Mauroy and
Stagnara (137)
developed a therapeutic regimen that uses serial casts for correction.
This method consists of three stages. First, a physical therapy program
is started in preparation for the casts. Next, three sequential
antigravity casts, changed at 45-day intervals, are applied in order to
obtain gradual correction of the deformity. The third stage involves
the use of a plastic maintenance brace that is worn until skeletal
maturity is reached. With this regimen the deformity was reported to
improve by 40%, and there was less loss of correction after this form
of nonoperative treatment was discontinued (120,138,139).
 |
|
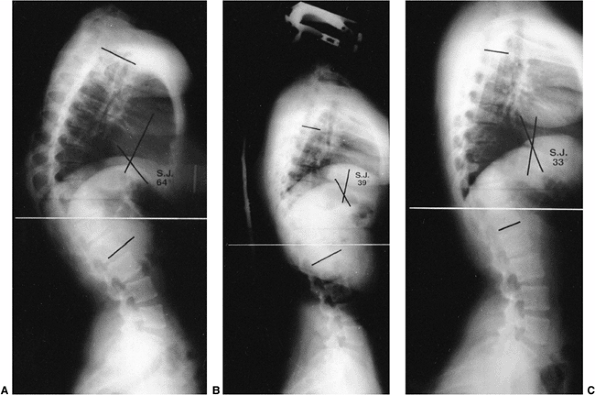
Figure 20.17 A: Lateral radiograph of a 15-year-old girl with a 64-degree thoracic kyphosis secondary to Scheuermann disease. B: Lateral radiograph of the patient in a Milwaukee brace with the kyphotic deformity improved to 39 degrees. C: Lateral radiograph obtained after the patient completed brace treatment; the kyphotic deformity has improved to 33 degrees.
|
because of various opinions about pain, disability, trunk deformity,
and importance of cosmesis. Therefore, the decision for surgery must be
made on an individual basis. The current indications for surgery are a
progressive kyphosis of more than 75 degrees, or significant kyphosis
associated with pain that is not alleviated by nonoperative treatment
methods. The biomechanical principles of correction of kyphosis
secondary to Scheuermann disease include lengthening the anterior
column (anterior release), providing anterior support (interbody
fusion), and shortening and stabilizing the posterior column
(compression instrumentation and arthrodesis) (140).
Surgical correction of kyphosis can be achieved by a posterior
approach, an anterior approach, or a combined anterior and posterior
approach. The combined anterior and posterior approach has been the
most frequently recommended and reliable procedure (141,142,143,144) (Fig. 20.18).
A posterior procedure alone can be considered if the kyphosis can be
corrected to, and maintained at, less than 50 degrees while a posterior
fusion occurs (136,137,138,145,146). The spine can be instrumented with Harrington compression rods (77,147) or a posterior segmental hook-and-screw type of instrumentation system (148).
If Harrington compression rods are used for posterior instrumentation,
¼-inch rods are used. Even when ¼-inch Harrington compression rods are
used, a brace or cast should be applied after surgery to prevent rod
breakage until a solid fusion is obtained. Prolonged immobilization is
necessary, however, and there are potential complications. With
posterior segmental instrumentation systems and the use of pedicle
screws, posterior-only surgery for flexible curves has become more
popular, but long-term outcome studies have yet to be published. When
this type of instrumentation system is used, postoperative
immobilization may not be required. Anterior instrumentation for
Scheuermann disease has been reported by Kostuik (149);
it consists of anterior interbody fusion and anterior instrumentation
with a Harrington distraction system augmented by postoperative
bracing. The single or dual rods and multiple bone screws that are
available in the present-day spine instrumentation systems may be used
instead of the Harrington distraction system. Although Kostuik has
reported good results with this technique, the anterior instrumentation
approach for treatment of Scheuermann kyphosis is not widely used (149).
performed for Scheuermann disease, the anterior release and fusion are
performed first. The anterior release can be done by an open anterior
exposure or by thoracoscopy. The posterior fusion and instrumentation
can be done on the same day as the anterior release and fusion, or they
can be done as a staged procedure. Harrington compression rods can be
used for posterior instrumentation, but usually a segmental
instrumentation system using multiple hooks or pedicle screws is used.
Lowe (143) and Coscia et al. (150)
have reported a high complication rate after using Luque rods and wires
for posterior fixation, because this system does not allow for any
compression. The posterior instrumentation should include at least
three fixation points above the apex and at least two fixation points
below the apex of the kyphosis. The fusion and instrumentation should
include the proximal vertebra in the measured kyphotic deformity and
the first lordotic disc distally (104,132,140,151).
If the fusion and instrumentation end in the kyphotic deformity, a
junctional kyphosis at the end of the instrumentation is likely to
develop.
He recommended that no more than 50% of the preoperative kyphosis be
corrected and that the final kyphosis should never be less than 40
degrees. He also found that patients with Scheuermann disease tend to
be in negative sagittal balance and become further negatively balanced
after surgery, which may predispose them to the development of
junctional kyphosis (132). Reinhardt and Bassett (152)
recommended fusion to the first square vertebra distally if the end
vertebra distally is wedged, so as to prevent junctional kyphosis. The
type of instrumentation used will determine whether postoperative
immobilization is needed. This immobilization can consist of a brace or
a Risser body cast. The patient’s activity is restricted for 6 to 9
months until a solid fusion is obtained.
often in children for the diagnosis and treatment of spinal cord
tumors, but may also be needed for other conditions such as
neurofibromatosis, Arnold-Chiari malformation, and syringomyelia (153,154).
Although deformity after laminectomy is unusual in adults, it is common
in children because of the unique and dynamic nature of the growing
spine (128,155,156,157,158,159). Postlaminectomy deformities usually result in kyphotic deformity, but a scoliotic deformity may also occur (156).
deformity can be multifactorial. Deformity of the spine after multiple
laminectomies can be caused by (a) skeletal deficiencies (facet joint,
laminae, and associated anterior column defects), (b) ligamentous
deficiencies,
(c) neuromuscular imbalance, (d) effects of gravity, and (e) progressive osseous deformity resulting from growth disturbances (153,160). Panjabi et al. (161)
showed that with loss of posterior stabilizing structures caused by
removal of the interspinous ligaments, spinous processes, and laminae,
the normal flexion forces placed on the spine will produce kyphosis.
Gravity places a flexion moment on the spine, producing compression
force on the anterior vertebrae and discs, and a tensile force on the
remaining posterior structures. This may explain why postlaminectomy
deformities occur most often in the cervical and thoracic spine and
less often in the lumbar spine. Gravity tends to cause a kyphosis in
the cervical and thoracic spine, whereas it accentuates the usual
lordosis of the lumbar spine.
 |
|
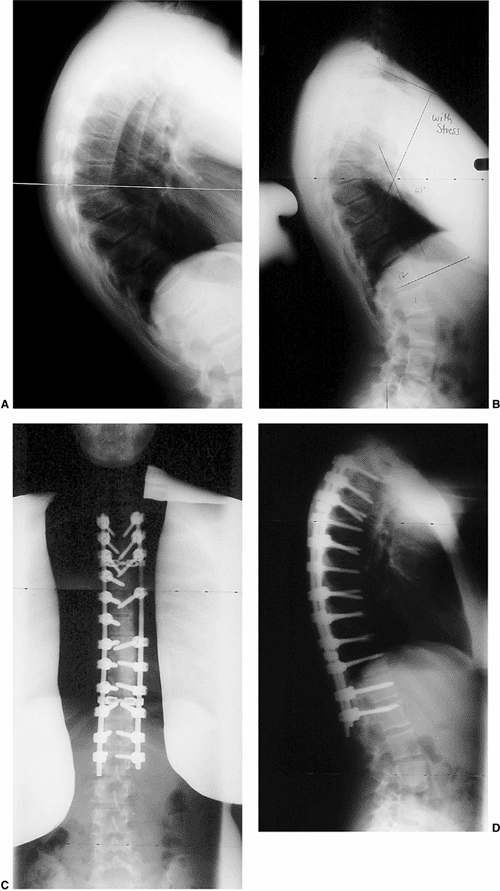
Figure 20.18 Thoracic Scheuermann kyphosis. A: Preoperative lateral radiograph. B: Stress lateral radiograph. C and D: Postoperative status of posterior instrumentation and fusion with pedicle screws. (Courtesy of Dr. Anant Kumar.)
|
factor noted as the most important one influencing the development of
postlaminectomy deformity is the integrity of the facet joint (156,161,162,163).
If the facet joint is removed or damaged during surgery, deformity is
likely to develop. In addition, any secondary involvement of the
anterior column, by tumor or surgical resection, adds to the risk of
instability and deformity after laminectomy. Also, multiple
laminectomies increase the risk of deformity when compared to
single-level laminectomies (164,165).
muscles that help stabilize the spine can also add to a postlaminectomy
deformity. The spine is unable to resist the normal flexion forces
placed on it by gravity and by the normal flexor muscles (166). Yasuoka et al. (167)
noted increased wedging of the vertebrae and excessive motion after
laminectomy in children, but not in adults. This increased wedging is
caused by increased pressure on the cartilaginous end plates of the
vertebral bodies. With time, the increased pressure will cause a
decrease in growth of the anterior portion of the vertebrae, according
to the Hueter-Volkmann principle (Fig. 20.19).
Excessive spinal motion in children after laminectomy can be attributed
to the facet joint anatomy in the cervical spine and the greater
ligamentous laxity of growing children. The orientation of the cervical
joint in a child is more horizontal than that seen in an adult. This
horizontal orientation offers less resistance to forces that tend to
cause kyphosis in the cervical spine.
scoliosis may also occur, either as the primary deformity or in
association with kyphosis. The incidence of postlaminectomy kyphotic
deformity ranges from 33% to 100% (168), and
depends on the age of the patient and the level of the laminectomy.
Generally, the deformity is more likely in younger patients, and after
more cephalad laminectomy. For example, Yasuoka et al. (167)
found that spinal deformity occurred in 46% of patients younger than 15
years of age, but in only 6% of patients 15 to 24 years of age. All the
patients between 15 and 24 years of age in whom deformity developed
were 18 years of age or younger. Yasuoka et al. (167) and Fraser et al. (169)
found that higher levels of laminectomy were associated with a greater
chance of deformity. In their studies, deformity occurred after 100% of
cervical spine laminectomies, after 36% of thoracic laminectomies, and
in none of the lumbar laminectomies. Hockley found that the greater the
number of laminae removed, the greater the risk is for developing
kyphosis (165,170).
 |
|
Figure 20.19
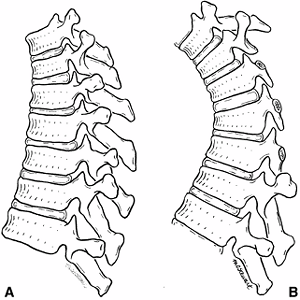
Drawings of the thoracic spine before and after repeated laminectomy demonstrate the effects on growth of the vertebral bodies. A: Before laminectomy, the anterior vertebral bodies are rectangular in configuration. B: The spine that has had multiple laminectomies will have increased compression anteriorly because of loss of posterior supporting structures. This compression results in less growth in the anterior portion of the vertebral body than in the posterior portion. In time, this will result in wedging of the vertebral bodies, causing a kyphotic deformity. (From Peterson HA. Iatrogenic spinal deformities. In: Weinstein SL, ed. The pediatric spine: principles and practice. New York: Raven, 1994:651.) |
common postlaminectomy deformity. The lumbar spine is normally in
lordosis, and this may protect it from developing kyphosis after
multiple lumbar laminectomies. Papagelopoulos et al. (170)
reported that hyperlordosis occurred in children who had lumbar
laminectomies for intraspinal tumors. If the laminectomies extended
into the thoracolumbar junction, kyphosis at the thoracolumbar junction
occurred in 33% of his patients. Peter et al. (171)
found that most of his patients did not develop a significant deformity
after multiple lumbar laminectomies for selective posterior dorsal root
rhizotomy; however, 9% developed spondylolysis. This may be the result
of increased lordosis in this patient population (172).
postoperative period or gradually over time. Kyphotic deformities have
been reported to occur as late as 6 years after surgery (155,172).
Progression can be either sudden or gradual, or the deformity may
progress significantly only during the adolescent growth spurt.
is varied and depends on the age of the patient at the time of surgery,
the location of the laminectomy or laminectomies, and the integrity of
the facet joint. Three types of postlaminectomy kyphosis have been
described in children: (a) instability after facetectomy, (b)
hypermobility between vertebral bodies associated with gradual rounding
of the spine, and (c) wedging of vertebral bodies caused by growth
disturbances (168).
sharp and angular and usually occurs in the immediate or early
postoperative period, causing associated loss of neurologic function (Fig. 20.20). Gradual rounding of the kyphotic deformity is seen more often when the facet
joints are preserved. Kyphosis increases gradually over time because of
the stress placed on the remaining posterior structures. If the spine
is immature when the laminectomy is performed, the resulting kyphosis
can inhibit the growth of the anterior growth plates of the involved
vertebrae. Unequal growth results in wedge-shaped vertebrae and a
progressive kyphotic deformity that is accelerated during the
adolescent growth spurt.
 |
|
Figure 20.20
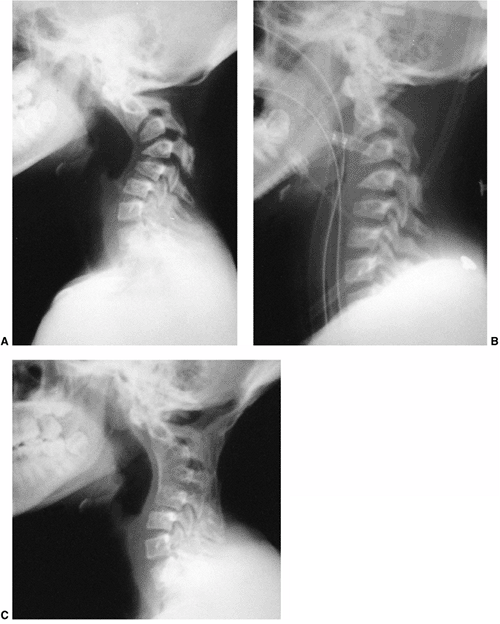
Radiographs of a 13-year-old girl treated for a low-grade astrocytoma. She underwent resection of the tumor, a portion of the occiput, and the laminae of C1-C4, followed by radiotherapy at a dose of 5400 cGy. A: A progressive cervical kyphosis developed. Note wedging of the anterior vertebral body. B: Radiograph in halo traction demonstrates partial reduction of the kyphosis. C: Postoperative radiograph after anterior and posterior fusion. |
cause kyphotic deformities include persistent spinal cord tumors,
neurologic deficits, intraspinal pathology (hydromyelia), and radiation
therapy (173,174).
focus on (a) the flexibility of the deformity, (b) loss of spinal
structures, and (c) determination of future deformity with growth. The
flexibility of a deformity can be estimated by flexion and extension
lateral radiographs. If these cannot be obtained, a lateral traction
film may be used. CT scans and three-dimensional reconstruction views
may better delineate which bony elements are missing. MRI may be used
but gives more information about the spinal cord, disc, and surrounding
soft tissue than about the bony elements. To aid in preoperative
planning, Lonstein recommends drawing the spine preoperatively (153).
The lines should represent the spinous processes and intact laminae and
facet joints. This may aid in predicting progression of a
postlaminectomy deformity.
The facet joints should be preserved whenever possible during
laminectomy. Localized fusion at the time of facetectomy or laminectomy
may help prevent progressive deformity (176).
Because of the loss of bone mass posteriorly, however, localized fusion
may not produce a large enough fusion mass to prevent kyphosis. Even
so, this approach is advocated because it may produce enough bone mass
posteriorly to stabilize what otherwise would be a severe progressive
deformity.
spinal cord may lessen the chance of progressive deformity. This
approach involves suturing the laminae back in place after removal, or
removing just one side of the laminae and allowing them to hinge open
like a book to expose the spinal cord, then suturing that side of the
laminae back in place (177,178,179).
This procedure may provide only a fibrous tether connecting the laminae
to the spine, but studies have shown a decreased incidence of
postlaminectomy kyphosis when it has been used (180,181).
Another technique is to hinge the laminae open in a lateral direction
after dividing the laminae in the midline. This provides a lateral
trough for the placement of bone graft for a lateral fusion (182,183). The use of these techniques has been reported to decrease the incidence of postlaminectomy deformity (165,184).
although no studies have documented the efficacy of this form of
treatment. After the deformity has occurred and started to progress,
bracing is ineffective in preventing further progression (153,156).
recommended, although the patient’s long-term prognosis should be
considered before making definitive treatment plans. If the prognosis
for survival is poor, spinal fusion may not be appropriate. However,
given the availability of effective treatment protocols for tumors and
the improved survival rates, fusion is usually indicated for
progressive deformity. Combined anterior and posterior spinal fusion is
preferred in most patients (187) because the frequency of pseudarthrosis is greater if either procedure is done alone.
pseudarthrosis in 57% of patients after posterior fusion and in 15% of
patients after anterior fusion. Anterior and posterior fusion can be
performed on the same day, or as staged procedures. When the anterior
procedure is performed, care must be taken to remove all the physes
back to the posterior longitudinal ligament. Leaving some of the physes
in the vertebral body can cause an increase in the deformity. When the
posterior procedure is performed, instrumentation of the involved spine
is desirable, but not always possible, because of the absence of
posterior elements. The development of pedicle screw fixation has been
helpful in allowing the use of posterior instrumentation for
postlaminectomy kyphosis. When it can be performed safely, this
procedure provides secure fixation while the spinal fusion is maturing.
Torpey et al. (188) recommend a posterior
fusion using titanium rod instrumentation at the time of laminectomy.
The instrumentation provides stability postoperatively, and the
titanium rods allow for postoperative MRI to evaluate spinal cord
tumors. In certain cases, anterior instrumentation with rod and bone
screws or plates may be used in order to obtain stability and
correction of the deformity (188). If the
deformity is severe or long-standing, anterior release followed by halo
traction, or a halo cast with an Ilizarov device, can be used for
obtaining gradual correction (189,190).
documented radiation-induced growth inhibition in growing cartilage and
bone. The longitudinal growth of a vertebral body takes place through
normal endochondral ossification, similar to the
longitudinal growth of the metaphyses of long bones. Bick and Copel (88,89)
demonstrated this on histologic sections in fresh autopsy specimens of
vertebral bodies, taken from research subjects ranging in age from 14
weeks of fetal development to 23 years. This endochondral ossification
at the physeal growth plate is radiosensitive (88,89,191,192,194,196,197). Engel (191,192) and Arkin and Simon (198) were able to produce spinal deformities in experimental animals using radiation. Arkin et al. (199)
were the first to report a case of spinal deformity in humans that was
caused by radiation. After these reports, it has become clear that
exposing an immature spine to radiation can produce spinal deformity,
including scoliosis, kyphoscoliosis, lordoscoliosis, and kyphosis.
which radiation therapy is part of the treatment regimen, and in which
the vertebral column is included in the radiation fields, are
neuroblastoma, Wilms tumor, and medulloblastoma. Early in the history
of radiation therapy, survival rates were poor and spinal deformities
were not as prevalent. With improved treatment protocols and survival
rates, the incidence of spinal deformities has increased. The degree of
growth inhibition of the spine is related to the accumulated radiation
dose and the age of the child when the spine is irradiated. Progression
is directly dependent on the remaining growth potential in the
irradiated vertebrae. The younger the child and the greater the
accumulated radiation dose, the greater the chance of deformity (200,201,202,203,204,205).
The most severe growth changes occur in patients who are 2 years of age
or younger at the time of irradiation. Initial vertebral changes
usually occur 6 months to 2 years after radiation exposure (206), but the deformity may not become apparent until years later, after a period of growth (201,204).
that an accumulated dose of less than 1000 cGy does not produce a
detectable inhibition of vertebral growth, whereas a dose of 1000 to
2000 cGy causes a temporary inhibiting effect on growth. Sometimes this
is manifested as a transverse growth arrest line in the vertebra, which
gives the appearance of a bone within a bone. A dose of radiation
between 2000 and 3000 cGy causes irregularity or scalloping of
vertebral end plates and diminution of axial height and sometimes leads
to a flattened, beaked vertebra (201,202,204,206,207,208,209,210). A dose of 5000 cGy causes bone necrosis (202).
The effect that radiation has on soft tissue also affects the
progression of spinal deformity. The soft tissue anterior to the spine
and the abdominal muscle can become fibrotic and act as a tether with
growth, adding to the deformity of the spine as the child grows (211).
These rates are decreasing because of shielding of growth centers,
symmetric field selection, and decreased total accumulated radiation
doses. The last of these changes has resulted from an increase in the
use and effectiveness of chemotherapeutic regimens that reduce the need
for large doses of radiation. Early reports showed an increased
incidence of scoliotic deformities with the use of asymmetric radiation
fields, and the incidence of kyphotic postirradiation deformities has
increased with the use of symmetric radiation fields (216).
should be observed carefully for the development of spinal deformity.
Because the development of deformity is related to the amount of
disordered growth in the vertebral bodies that were affected by
irradiation, it depends to a large extent on the amount of growth left
in the spine when the irradiation was started, and the amount of damage
to the physes caused by irradiation (which correlates directly with the
accumulated radiation dose). If the dose of radiation is large enough
to cause permanent damage to the physes, the deformity will be
progressive. Postirradiation scoliosis and kyphosis both progress more
rapidly during times of rapid growth such as the adolescent growth
period (204,205,208,216).
Before the adolescent growth spurt, the deformity may remain relatively
stable or progress at a steady rate. Severe curves can continue to
progress even after skeletal maturity, and these patients may require
continued observation.
should include standard posteroanterior and lateral radiographs of the
spine. Occasionally, CT scans with sagittal or coronal reconstruction
are needed for better delineation of the vertebral body deformities.
The spinal cord and surrounding soft tissue are evaluated best with
MRI. Neuhauser et al. (202) described the
radiographic changes seen in irradiated spines. The earliest changes
were alterations in the vertebral bodies within the irradiated section
of the spine caused by impairment of endochondral growth at the
vertebral end plates. Growth arrest lines produced a bone-within-a-bone
picture. This occurred in 28% of the 81 patients in the study by
Riseborough et al. (204). Other radiographic
changes were end-plate irregularity with an altered trabecular pattern,
and decreased vertebral body height. This pattern was the most common
radiographic change reported by Riseborough et al. (83%) (204).
Contour abnormalities causing anterior narrowing and beaking of the
vertebral bodies, much like those seen in patients with conditions that
affect endochondral ossification (e.g., Morquio syndrome,
achondroplasia), were the third type of radiographic change noted by
Neuhauser et al. (202).
progressive curves, but generally has been ineffective for
postirradiation kyphosis (204,216),
especially in patients with soft tissue contractures contributing to
the deformity. The irradiated skin may also be of poor quality, making
long-term brace wear difficult. If progression occurs, spinal fusion,
with or without instrumentation, should be performed
regardless
of the age of the patient. Because bone quality is poor, fusion can be
difficult to obtain after a single attempt. Anterior and posterior
fusion are recommended, and should extend at least one or two levels
above and below the end of the kyphosis (168,188,204,216,217,218).
The posterior fusion mass may require reexploration and repeated bone
grafting after 6 months, and immobilization may need to be prolonged
for 6 to 12 months. Posterior instrumentation should be used whenever
feasible, because it adds increased stability while the fusion mass is
maturing and may allow for some limited correction of the kyphotic
deformity (Fig. 20.21). Anterior
instrumentation can be used in certain cases. However, because of the
radiation, the vertebral bodies usually remain in an infantile form,
and instrumentation with bone screws may be difficult.
Typically, these curves are rigid, and soft tissue scarring and
contractures often further hamper correction. Healing can be prolonged,
and pseudarthrosis is common. Infection is a frequent complication in
these patients because of poor vascularity of the irradiated tissue (204). Riseborough et al. (204) reported a pseudarthrosis rate of 37% and an infection rate of 23% in his patients after surgery. King and Stowe (216)
also reported a high complication rate in patients who were treated
surgically. Because viscera also can be damaged by irradiation, bowel
obstruction, perforation, and fistula formation may occur after spinal
fusion. This can be difficult to differentiate from postoperative cast
syndrome, and the treating physician should be aware of this
complication (219). Radiation myelopathy may also occur in this patient population (220). King and Stowe (216)
reported postoperative paraplegia in 2 of 7 patients who had undergone
radiation treatment for neuroblastoma and surgery for correction of
their kyphotic spine deformity. King and Stowe believed that these two
patients had a subclinical form of radiation myelopathy and that spinal
correction compromised what little vascular supply there was to the
cord. Therefore, the surgeon should be aware of this possibility and
try to avoid overcorrection.
The natural history of spinal deformity varies with the type of
deformity and the type of dysplasia. Some sagittal plane deformities
that appear severe at birth or in infancy improve spontaneously with
growth, whereas others continue to progress and eventually can cause
paraplegia. A knowledge of the various skeletal dysplasias and the
natural history of sagittal plane deformities in each is necessary to
prevent overtreatment and undertreatment.
patients with achondroplasia. The most common sagittal plane deformity
in achondroplastic dwarfs is thoracolumbar kyphosis (223,224,225).
The kyphosis is usually detected at birth, and is accentuated when the
child is sitting because of the associated hypotonia in these infants (226).
Ambulation is delayed until approximately 18 months of age, but after
ambulation begins, the thoracolumbar kyphosis tends to improve. The
kyphosis usually does not resolve in children who have more hypotonia.
According to Lonstein (227), thoracolumbar
kyphosis resolves in 70% of achondroplastic dwarfs, and persists in
30%. In one third of these patients, or 10% of achondroplastic dwarfs,
the thoracolumbar kyphosis is progressive (227) (Fig. 20.22).
infancy shows anterior wedging of the vertebrae at the apex of the
kyphosis (225). In patients whose thoracolumbar
kyphosis resolves, the anterior vertebral body wedging also improves.
When the kyphosis is progressive, anterior vertebral body wedging
persists.
kyphosis should be corrected: (a) it may cause pressure on the conus
and result in neurologic symptoms; (b) it results in an increase in the
compensatory lumbar lordosis, which can increase problems from an
already stenotic lumbar spine; (c) it may increase significantly if
decompressive laminectomies are needed for lumbar stenosis in the
future (226).
evident once a child begins walking, a thoracolumbosacral orthosis
(TLSO) is recommended in order to try to prevent progression of the
kyphosis (228,229,230,231). Early treatment to prevent the development of a progressive kyphosis has been recommended by Pauli et al. (232).
They developed an algorithm for treatment of the young achondroplastic
patient, first counseling the parents against unsupported sitting and
continuing with close follow-up. If kyphosis develops and is greater
than 30 degrees, TLSO bracing is begun and continued until the child is
walking independently and there is evidence of improvement in vertebral
body wedging and kyphosis. Using this form of early intervention, Pauli
et al. (232) reported no occurrences of
progressive kyphosis. Sponseller recommends serial hyperextension
casting if the kyphosis does not respond to bracing. If there is a
satisfactory response to serial casting (50% or more correction in 3 to
4 months), brace treatment can be resumed (226).
kyphotic deformity, kyphosis of more than 40 degrees in a child older
than 5 or 6 years of age, and neurologic deficits relating to the
spinal deformity (224,226,233).
Distinguishing between neurologic deficits that result from a kyphotic
deformity and those associated with lumbar stenosis (which is common in
achondroplastic dwarfs) can
be
difficult. A thorough physical examination and diagnostic studies such
as CT scan and MRI may be necessary in order to determine appropriate
treatment. The infant should be evaluated for foramen magnum stenosis,
because this may be the underlying cause for the hypotonia and delayed
ambulation in achondroplastic patients with kyphosis. If present, the
stenosis should be treated by decompression of the foramen magnum (234).
Most patients with progressive thoracolumbar kyphosis require combined
anterior and posterior fusion. Instrumentation that uses hooks or wires
that go into the spinal canal is not recommended in these patients
because the small size of the spinal canal and the lack of epidural fat
make instrumentation hazardous. Ain has reported good results with
anterior fusion and instrumentation combined with posterior fusion.
This has the advantage of not entering an already stenotic spinal canal
and giving secure fixation to aid in spinal fusion (235).
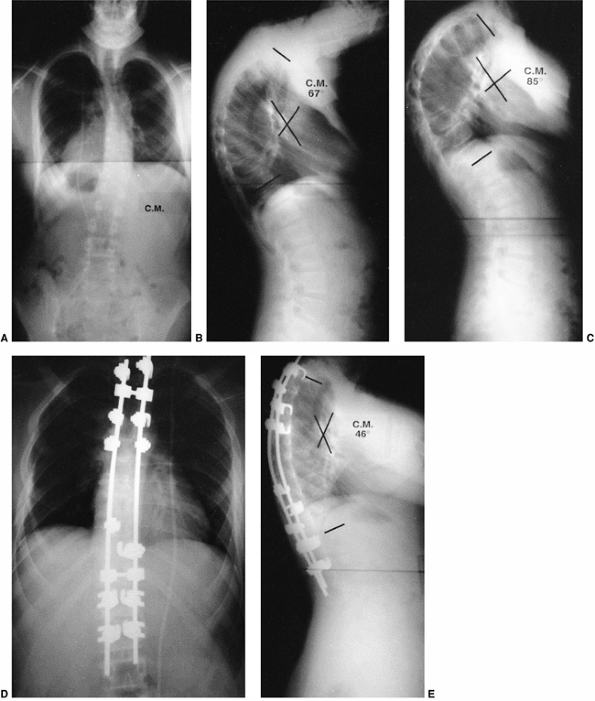 |
|
Figure 20.21 A and B:
Anteroposterior and lateral radiographs of a 16-year-old child with a suprasellar germinoma treated with resection and 3400 cGy of radiation to the base of the skull and the entire spine. Radiographs demonstrate a 67-degree kyphosis with associated scoliosis. C: The kyphosis progressed to 85 degrees over 18 months despite bracing. D and E: Anteroposterior and lateral radiographs after anterior and posterior fusion with posterior instrumentation. The kyphosis has been corrected to 46 degrees. |
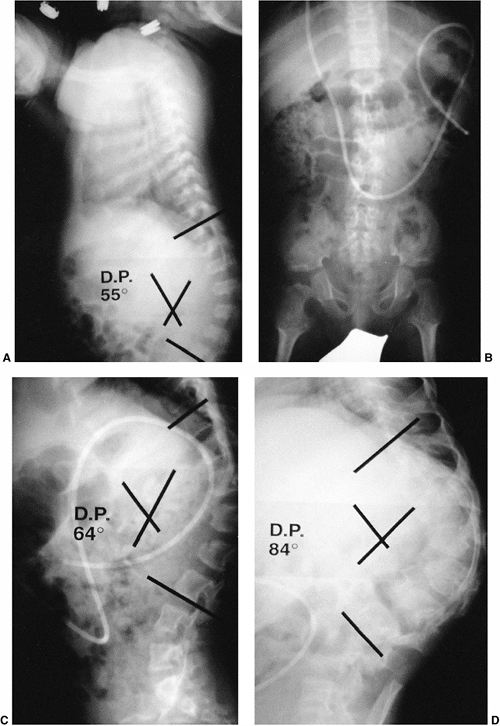 |
|
Figure 20.22 Achondroplastic dwarf with progressive thoracolumbar kyphosis. A: Lateral radiograph at 1 year of age shows a 55-degree thoracolumbar kyphosis. B:
Anteroposterior radiograph at 5 years of age shows narrowing of the lumbar interpedicular distance characteristic of achondroplasia. C: Lateral radiograph at 5 years of age reveals a 64-degree kyphosis. D: Lateral radiograph at 9 years of age shows an 84-degree thoracolumbar kyphotic deformity. |
pseudoachondroplasia and are caused by wedging of multiple vertebral
bodies in the thoracolumbar and thoracic spine. The kyphotic deformity
in patients with pseudoachondroplasia differs from that in patients
with achondroplasia. In patients with pseudoachondroplasia, the
kyphosis involves multiple levels, and is less acutely angular than the
deformity in patients with achondroplasia, which involves only one or
two levels. Bracing may prevent progression of this deformity, but
surgery is indicated if progression occurs despite bracing. Spinal
fusion with instrumentation can be performed safely in patients with
pseudoachondroplasia because there is no associated stenosis of the
spinal canal as in patients with achondroplasia (236,237).
approximately half of the patients with congenital spondyloepiphyseal
dysplasia; these deformities usually respond to a modified TLSO (226). If surgery is needed for a progressive kyphosis, anterior and posterior fusions are recommended (237).
These spinal deformities consist of cervical kyphosis, thoracic
kyphoscoliosis, and lumbar hyperlordosis. Midcervical kyphosis occurs
in 15% to 33% of patients with diastrophic dwarfism (226,239,240). However, Remes et al. and Herring have reported spontaneous improvement (239,240,241). Progressive cervical kyphosis (>60 degrees) can be stabilized with a spinal fusion (210,220,221).
If a posterior fusion is to be performed, the increased incidence of
cervical spina bifida in diastrophic dwarfism must be considered during
dissection (239,240,242).
disorders caused by deficiency of the enzymes that are necessary for
the degradation of glycosaminoglycans. There are at least 13 types of
mucopolysaccharidoses. The more common names of this condition are
Hurler, Hunter, Sanfilippo, Morquio, and Moroteux-Lamy syndromes. Bone
marrow transplantation has increased the life expectancy of these
patients. Before this treatment method became available, most children
did not survive long enough to require intervention for spinal
deformities. With increased survival, progressive kyphotic deformities
of the spine with neurologic compromise are being reported (243,244,245).
Despite bone marrow transplantation, the deposition of metabolites in
bone is not reversed to the same extent as that in soft tissue (246).
thoracolumbar kyphosis, with anterior beaking and flattening of the
vertebral bodies at the level of the kyphotic deformity. Swischuk
suggested a mechanical cause for the anterior beaking (247).
He postulated that hypotonia results in thoracolumbar kyphosis,
resulting in herniation of the nucleus pulposus into the anterior
vertebral body, which causes the anterior beaking of the vertebral
body. Field, however, examined two specimens at postmortem and found
that the end-plate formation was normal, but there was a failure of
ossification in the anterosuperior part of the vertebral body (246).
the kyphotic deformity, but the effectiveness of this form of treatment
has not been documented. Spinal fusion is recommended for a progressive
kyphosis in patients with mucopolysaccharidosis. Tandon reported good
results with posterior spinal fusion, and Dalvie has reported good
results with anterior fusion and instrumentation (243,244).
Further studies will be needed to determine which approach is best, but
the goal of surgery is to obtain a stable fusion of the involved area
to prevent any further progression of the kyphosis (226,237,248).
storage disorder characterized by the accumulation of glucocerebroside
in the lysosomes of macrophages of the reticuloendothelial system.
Splenomegaly with associated pancytopenia is the most common clinical
manifestation. The skeletal manifestations are caused by infiltration
of the bone marrow by Gaucher cells; they include bone crisis,
pathologic fracture, osteopenia, osteonecrosis, and osteomyelitis.
Progressive kyphosis of the spine has been reported in these patients.
The proposed etiology of the
kyphosis
is infiltration of the bone marrow by Gaucher cells, resulting in bone
crisis, osteopenia, and osteonecrosis that lead to vertebral body
collapse, often on multiple levels. Kyphosis can be progressive because
of continued vertebral body collapse or growth abnormalities secondary
to vertebral body collapse. If a progressive kyphosis develops,
surgical intervention is recommended. If the spine is still flexible,
posterior fusion and instrumentation are adequate, but if the deformity
is rigid, anterior and posterior fusion and instrumentation are needed (Fig. 20.23) (249,250).
tissue that affects the supporting structures of the body, especially
those in the musculoskeletal system. This syndrome is caused by
mutations in coding of the genes for the glycoprotein fibrillin (251,252).
Spinal deformity is the most common skeletal abnormality in Marfan
syndrome, and scoliosis is the most common of these spinal deformities (227,253,254,255,256,257,258). Thoracic lordosis has been traditionally reported as the most common sagittal plane deformity (259,260).
In some patients, the thoracic lordosis becomes severe enough to
compromise respiration. With the lordotic posture of the thoracic
spine, an associated kyphosis or relative kyphosis may develop in the
lumbar spine. A third common spinal deformity associated with Marfan
syndrome is thoracolumbar kyphosis, which affects approximately 10% of
patients (Fig. 20.24). These spinal deformities usually occur during the juvenile growth period, before the adolescent growth spurt (260).
Sponseller et al. found that 41% of their patients with Marfan syndrome
had a kyphotic deformity of more than 50 degrees, with a tendency
toward longer kyphoses extending through the thoracolumbar junction (261).
Thoracic lordosis is corrected by posterior segmental instrumentation
to correct the lordotic deformity, followed by posterior fusion (264).
Because dural ectasia corrodes pedicles, a CT scan of the pedicles
should be obtained to plan fixation. In addition, fusion should be
extended. Complications are more frequent after surgical correction of
spinal deformity in patients with Marfan syndrome than after spinal
surgery in other patients (262).
abnormalities are rare. Basilar impression and focal cervical kyphosis
are the most frequently reported cervical spine abnormalities. Focal
cervical kyphosis is usually associated with a lordotic thoracic spine (265).
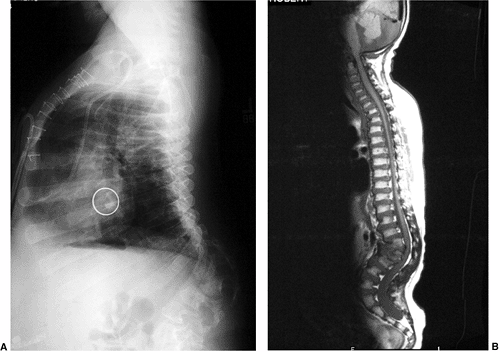 |
|
Figure 20.23 Lateral radiograph (A) and magnetic resonance imaging (MRI) (B) demonstrate progressive thoracolumbar kyphosis in a patient with Gaucher disease.
|
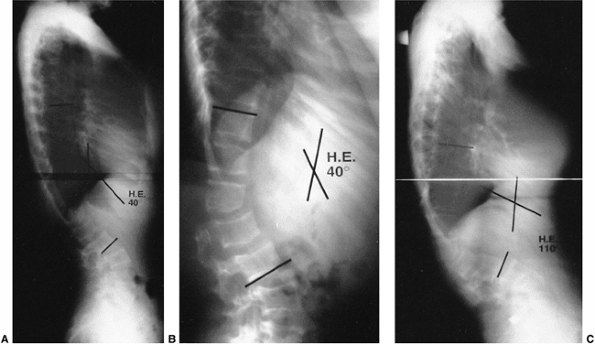 |
|
Figure 20.24 A and B: Lateral radiographs of a 17-year-old child with Marfan syndrome and a 40-degree progressive thoracolumbar kyphosis. C: Lateral radiograph of the same patient 3 years later shows that the thoracolumbar kyphosis has progressed to 110 degrees.
|
have recommended that patients with Marfan syndrome should avoid sports
that involve risks of high-impact loading of the cervical spine.
consisting of facial dysmorphism and hyperelasticity of the joints,
with congenital dislocation of the knees and frequent dislocation of
the hips and elbows (119,268,269,270,271).
Equinovarus or valgus foot deformities and ancillary calcaneal nuclei
are also characteristic features of this syndrome. Abnormalities of the
cervical spine, specifically cervical kyphosis, were not emphasized in
the original description, and often this life-threatening finding is
overlooked (189,268,272). Johnston et al. (267)
reported cervical kyphosis and vertebral body anomalies in five of nine
patients with Larsen syndrome. The apex of the kyphosis usually occurs
at the fourth or fifth cervical vertebra, with marked hypoplasia of one
or two of the vertebral bodies (Fig. 20.25).
Cervical kyphosis is present in infants with Larsen syndrome.
Developmental delay may be attributed to hypotonia and dislocation of
the knees or hips, but the underlying cause for developmental delay may
be a chronic myelopathy from the cervical kyphosis. Cervical kyphosis
and vertebral hypoplasia are easily demonstrated on lateral C-spine
radiographs. Flexion and extension views are usually not needed and may
be difficult to obtain safely in an infant. MRI scans will demonstrate
spinal cord compression or compromise.
Larsen syndrome is the use of an early posterior arthrodesis to
stabilize the spine. An in situ posterior
arthrodesis with autogenous iliac crest bone graft, followed by
immobilization in either a halo or Minerva cast or custom orthosis, is
recommended. Reduction of the kyphosis is obtained only in the
postoperative halo or Minerva cast or orthosis stage of the treatment.
Johnston et al. (267) found that, over a period
of time following a solid posterior arthrodesis, a gradual correction
of the kyphosis occurs because of continued anterior vertebral body
growth. Because the posterior arthrodesis is performed at a young age,
the patient must be followed for potential complications from continued
anterior growth, which would result in lordosis. Johnston and
Schoenecker (273) described a patient who developed neurologic symptoms from this growth-related lordosis.
fracture site from a malunion, chronic instability leading to
progressive deformity, paralysis after spinal cord injury, or from
anterior growth arrest (274,275,276,277,278).
Kyphosis at the fracture site is acute and spans a short segment of
vertebrae. Paralytic kyphosis is a long, C-shaped deformity that spans
many vertebral segments. Progressive kyphosis may also occur after
development of a posttraumatic syrinx (279).
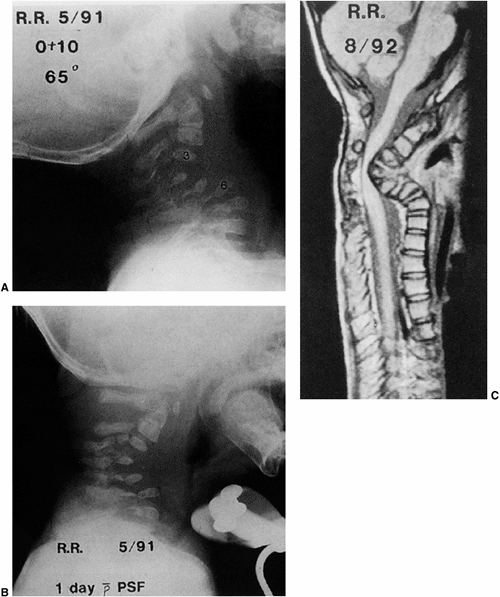 |
|
Figure 20.25 Larsen syndrome. A: Lateral radiograph of a 10-month-old patient showing kyphosis of 65 degrees, with correction to only 48 degrees in extension. B:
Lateral radiograph immediately after posterior arthrodesis, showing the patient with orthosis and correction of kyphosis to 39 degrees. C: T2-weighted magnetic resonance image 15 months postoperatively shows severe impingement on spinal cord. Kyphosis had progressed to 110 degrees, and the patient was quadriplegic after a fall. (From Johnston CE, Birch JG, Daniels JL. Cervical kyphosis in patients who have Larsen syndrome. J Bone Joint Surg Am 1996;78:538.) |
intervention for correction. Anterior, posterior, and combined anterior
and posterior procedures have been described for correction of
posttraumatic kyphosis (280,281,282,283,284). Brace treatment has been ineffective for progressive paralytic kyphosis (274),
and surgery is indicated for paralytic kyphosis of more than 60
degrees. If the kyphosis is flexible and can be reduced to less than 50
degrees, posterior fusion with segmental instrumentation can be
performed. If the kyphosis is rigid and cannot be reduced to less than
50 degrees on preoperative bending view x-ray films, anterior release
and fusion should be followed by posterior fusion and segmental instrumentation (274,278).
found that 50% of their patients with neurofibromatosis and spinal
deformity had an abnormal sagittal curve. The vertebral bodies are
frequently deformed and attenuated at the apex of the kyphosis.
Dystrophic vertebral body changes may develop over time (287,288). Crawford (287,289)
described this as modulation of the deformity, from a nondystrophic
curve to a dystrophic curve. The kyphosis is typically sharp and
angular over a relatively small number of vertebral segments. Severely
angular kyphosis can cause neurologic compromise (290,291). Lonstein et al. (292)
found that cord compression due to spinal curvature from
neurofibromatosis was second only to congenital kyphosis as a cause of
spinal cord compression. The kyphosis in patients with
neurofibromatosis typically involves the thoracic spine or upper
thoracic spine. Involvement of the cervical and cervicothoracic
vertebrae has also been reported (290,293,294,295,296,297).
neurofibromatosis begins with a thorough physical examination for
neurologic abnormalities. MRI scans should be obtained to demonstrate
any intraspinal lesions, such as pseudomeningocele, dural ectasia, or
neurofibroma, which may cause impingement on the spinal cord (298).
Any intraspinal lesions should be treated appropriately before spinal
fusion and instrumentation are undertaken. Because posterior fusion
done alone has resulted in a high rate of pseudarthrosis (65%) (299), combined anterior and posterior spinal fusion combined with posterior instrumentation is recommended (300).
Titanium instrumentation is preferred so as to allow for future MRI
studies of the spine. Abundant autogenous bone grafts and prolonged
immobilization may be required in order to obtain a solid fusion in
these patients, and repeated bone grafting may be required 6 months
after the initial surgery. Vascularized fibular or rib grafts may also
be used for anterior fusion and structural support (99,287,293,296,301,302,303).
Spinal tuberculosis is the most dangerous form of skeletal tuberculosis
because of its ability to cause bone destruction, deformity, and
paraplegia. In childhood spinal tuberculosis, the extent and degree of
abscess formation is greater than that seen in adult tuberculosis, but
paraplegia is less common in children than in adults with spinal
tuberculosis (305). The most frequent site of
spinal tuberculosis in children is the thoracolumbar junction and its
adjacent segments. Tuberculosis infection usually destroys the anterior
elements of the spine and results in a significant angular kyphosis at
the infected site. The involved anterior vertebral bodies usually fuse
once the infection is adequately treated. In young children, continued
growth of the intact posterior element can cause a late increase in
kyphosis in an already kyphotic spine (305).
a complete course of chemotherapy. First-line drugs are streptomycin,
isoniazid, and rifampicin, and second-line drugs are ethambutol and
pyrazinamide (306). Medical therapies for spinal tuberculosis will adequately treat the tuberculum infection in most cases (304,307,308,309,310).
Bracing or casting has been used along with medical therapy to try to
prevent progression of kyphosis during therapy. Rajasekaran found an
average increase of 15 degrees in deformity in all patients who were
treated nonsurgically (311). The greatest increase in deformity occurred during the first 6 months of treatment.
spinal instability, neurologic involvement, prevention or correction of
spinal deformity, drainage of significant abscesses, or diagnostic
biopsy (307). Neurologic involvement and
present or impending paraplegia are more obvious indications for
surgical intervention than the other indications. Rajasekaran described
four prognostic signs to predict spinal instability and late increase
in deformity. When more than two signs are present, this is a reliable
predictor of progressive deformity and spinal instability. These
prognostic signs include: (a) dislocation of the facets, (b) posterior
retropulsion of the diseased fragments, (c) lateral translation of the
vertebrae in the anteroposterior view, and (d) toppling of the superior
vertebra (Figs. 20.26 and 20.27) (312).
Other factors that lead to a significant increase in kyphosis in
children who are not treated surgically are involvement of three or
more vertebral bodies, initial kyphosis of more than 30 degrees, and
age younger than 15 years (313,314,315).
Anterior debridement and strut grafting, with or without a posterior
fusion and instrumentation, has the most consistent long-term results (306,321,322,323,324,325,326,327,328,329,330,331,332). Good results have been reported with the use of allografts for structural support anteriorly (333,334,335).
Anterior debridement and fusion with anterior instrumentation of the
spine has also had positive results in the treatment of spinal
tuberculosis (336,337).
Some correction of the kyphosis may be obtained at the time of surgery.
Kyphosis can also be a problem in patients with healed spinal
tuberculosis (338). The infected area of the
anterior spine usually fuses, and continued growth posteriorly causes
progressive kyphosis that can result in paraplegia. The presence of
neurologic symptoms is an indication for anterior decompression and
fusion, which can be followed by posterior fusion and instrumentation.
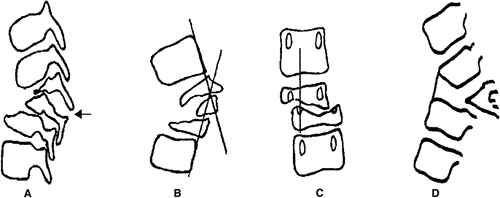 |
|
Figure 20.26 Radiologic signs for the spine at risk. A: Separation of the facet joint.
The facet joint dislocates at the level of the apex of the curve, causing instability and loss of alignment. In severe cases the separation can occur at two levels. B: Posterior retropulsion. This is identified by drawing two lines along the posterior surface of the first normal vertebrae above and below the curve. The diseased segments are found to be posterior to the intersection of the lines. C: Lateral translation. This is confirmed when a vertical line drawn through the middle of the pedicle of the first lower normal vertebra does not touch the pedicle of the first upper normal vertebra. D: Toppling sign. In the initial stages of collapse, a line drawn along the anterior surface of the first lower normal vertebra intersects the inferior surface of the first upper normal vertebra. “Tilt” or “toppling” occurs when the line intersects higher than the middle of the anterior surface of the first normal upper vertebra. (Reproduced with permission and copyright of the British Editorial Society of Bone and Joint Surgery. Rajasekaran S. The natural history of post-tubercular kyphosis in children. J Bone Joint Surg 2001;83B:954.) |
condition that consists of generalized osteoporosis in otherwise normal
prepubertal children (339). Although idiopathic
juvenile osteoporosis is uncommon, associated kyphosis and back pain
are common in patients with this condition.
this condition in 1939 and, since that time, other authors have
described its clinical findings and natural history (143,341,342,343,344,345,346,347).
The etiology of idiopathic juvenile osteoporosis is unknown. Laboratory
values of serum calcium, phosphorus, alkaline phosphatase, parathyroid
hormone, and osteocalcin are normal. The collagen type and ratios from
skin biopsy samples are also normal. There have been some reports of a
slight decrease in 1,25-dihydroxyvitamin D (345,348,349),
but the significance of this finding is not known. Low serum calcitonin
levels have also been reported, but treatment with calcitonin has not
proven to be beneficial (343,350). In contrast, Saggese et al. (346) noted normal serum calcitonin levels in their patients. Green (351)
believes that a mild deficiency of 1,25-dihydroxyvitamin D can explain
most of the findings in idiopathic juvenile osteoporosis. During rapid
growth phases the deficiency is discovered because growth requirements
cannot keep pace, causing a relative osteoporosis. When puberty occurs,
the increase in sex hormone overcomes the deficit in
1,25-dihydroxyvitamin D, and the relative osteoporosis improves. This
theory has yet to be proved.
Difficulty in walking may sometimes be the only finding. This condition
occurs during the prepubertal period, and is slightly more common in
boys than in girls. Vertebral collapse or wedging, with resulting
kyphosis, is common. Brenton and Dent (350)
classified idiopathic juvenile osteoporosis as mild, moderate, and
severe types. Patients with the mild type have only back pain and
vertebral fractures; those with the moderate type have back and
lower-extremity pain and fractures, with some limitation of activities
but eventual return to normal function; and those with the severe form
have back and lower-extremity pain and fractures. Both metaphyseal and
diaphyseal fractures can occur in the lower extremities. Patients with
severe disease improve clinically but do not return to normal activity
after puberty.
vertebral bodies. A “codfish” appearance of the vertebral bodies can
occur, with the superior and inferior borders of the vertebrae becoming
biconcave (Fig. 20.28). Other studies that can
be useful for following the progress of this disease are single-photon
absorptiometry, dual-photon absorptiometry, and quantitative CT
scanning (343,345,346). The problem with these tests is that normal ranges for adolescents and children are variable and have not been standardized.
exclusion. Other diseases that must be considered include metabolic
bone diseases, leukemia, Cushing syndrome, lysinuric protein
intolerance, type I homocystinuria, and
osteogenesis
imperfecta. The natural history of this condition is spontaneous
improvement or remission at the onset of puberty. Associated kyphosis
tends to improve after the onset of puberty.
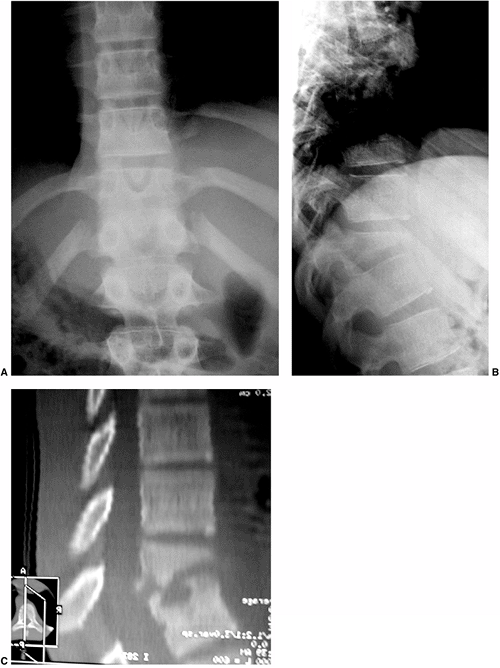 |
|
Figure 20.27 Anteroposterior and lateral radiographs (A and B) and a computed tomography (CT) scan (C) demonstrating vertebral body collapse secondary to tuberculosis.
|
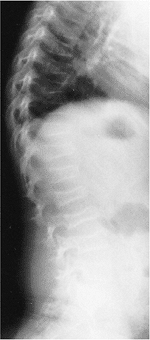 |
|
Figure 20.28
Lateral radiograph taken of a standing 10-year-old girl with idiopathic juvenile osteoporosis shows diffuse osteopenia, multiple “codfish” vertebrae in the thoracic and lumbar spine, and “coin” vertebrae in the upper thoracic spine secondary to extreme collapse. (From Green WB. Idiopathic juvenile osteoporosis. New York: Raven, 1994.) |
modification of activities, possible calcium and vitamin D
supplementation, and supportive treatment of spinal deformities. It
must be ensured that there is sufficient restriction of activities in
order to prevent fractures, but not so much restriction as to cause an
increase in osteoporosis. If a significant progressive kyphosis
develops, the Milwaukee brace is the treatment of choice (344).
The brace is to be worn until there is evidence of improvement of the
osteoporosis. Operative therapy for this condition has been associated
with a high complication rate because the poor bone quality makes
instrumentation and fusion difficult (355).
G, Harkonen H, Poussa M. Spinal mobility and posture and their
correlations with growth velocity in structurally normal boys and girls
aged 13 to 14. Spine 1988;3:152.
P, DeMauroy JC, Dran G, et al. Reciprocal angulation of vertebral
bodies in the sagittal plane: approach to references in the evaluation
of kyphosis and lordosis. Spine 1982;7:335.
M, Bridwell KH. Segmental analysis of the sagittal plane alignment of
the normal thoracic and lumbar spines and the thoracolumbar junction. Spine 1989;14:717.
RB, Moe JH, Wang JF. Congenital kyphosis: its natural history and
treatment as observed in a study of one hundred and thirty patients. J Bone Joint Surg Am 1973;55:223.
S-W, Sarwark JF, Vora A, et al. Evaluating congenital spine deformities
for intraspinal anomalies with magnetic resonance imaging. J Pediatr Orthop 2001;21:525–531.
RB, Moe JH, Lonstein JE. The surgical treatment of congenital kyphosis:
a review of 94 patients age 5 years or older, with 2 years or more
followup in 77 patients. Spine 1985;10:224.
J, Kalamchi A, MacEwen GD. Sacral agenesis: a clinical evaluation of
its management, heredity, and associated anomalies. Clin Orthop 1979;139:52.
J, Evans ER, Eggers GWN. Partial and complete agenesis or malformation
of the sacrum with associated anomalies. Etiologic and clinical study
with special reference to heredity. A preliminary report. J Bone Joint Surg Am 1959;41:497.
DS, Ahmed KB, Moe JH, et al. The surgical management of patients with
Scheuermann’s disease: a review of twenty-four cases managed by
combined anterior and posterior spine fusion. J Bone Joint Surg Am 1980;62:705.
DS, Moe JH, Montalvo FJ, et al. Scheuermann’s kyphosis. Results of
surgical treatment by posterior spine arthrodesis in twenty-two
patients. J Bone Joint Surg Am 1975;57:439.
P, Berkin C, Dickson R. Combined idiopathic kyphosis and scoliosis. An
analysis of the lateral spinal curvature associated with Scheuermann’s
disease. J Bone Joint Surg Br 1985;67:189.
M, Morris R. A study of the natural history of back pain. Part I:
development of a reliable and sensitive measure of disability in
low-back pain. Spine 1983;8:141.
SY, Dandawate AV. Progressive cord compression secondary to thoracic
disc lesions in Scheuermann’s kyphosis managed by posterolateral
decompression, interbody fusion, and pedicular fixation. A new approach
to management of a rare clinical entity. Eur Spine J 1994;3:66.
TG, Kasten MD. An analysis of sagittal curves and balance after
Cotrel-Dubousset instrumentation for kyphosis secondary to
Scheuermann’s disease. A review of 32 patients. Spine 1994; 19:1680.
CR, Caton J. Etude des resultants a long term d’une seie de cyphoses
regulieeres traitees pariorset de Livet a charniers. Presented at The Reunion of the Group d’Etude de la Scoliose, Aix-en-Provence, 1978.
A, Gebbia F, Eliseo F. Nonoperative treatment of adolescent
hyperkyphosis: a 30 years experience in over 3000 treated patients. Orthop Trans 1990;14:766.
MF, Bradford DS, Ogilvie JW. Scheuermann’s kyphosis: results in 19
cases treated by spinal arthrodesis and L-rod instrumentation. Orthop Trans 1988;12:255.
T, Yamamuro T, Shikata J, et al. Analysis and prevention of spinal
column deformity following cervical laminectomy I. Pathogenetic
analysis of postlaminectomy deformities. Spine 1991;16:494.
JS, Sgouros S, Walsh AR, et al. Spinal sagittal malalignment following
surgery for primary intramedullary tumours in children. Pediatr Neurosurg 2001;35:318.
S, Peterson HA, Laws ER Jr, et al. Pathogenesis and prophylaxis of
postlaminectomy deformity of the spine after multiple level
laminectomy: difference between children and adults. Neurosurgery 1981;9:145.
PJ, Peterson HA, Ebersold MJ, et al. Spinal column deformity and
instability after lumbar or thoracolumbar laminectomy for intraspinal
tumors in children and young adults. Spine 1997;22:442.
JC, Hoffman EB, Arens LJ, et al. Incidence of spinal deformity in
children after multiple level laminectomy for selective posterior
rhizotomy. Childs Nerv Syst 1990;6:30.
AJ, Gutierrez FA, Di Rocco C. Laminotomy and total reconstruction of
the posterior spinal arch for spinal canal surgery in childhood. J Neurosurg 1976;45:555.
J, Yamamuro T, Shimizu K, et al. Combined laminoplasty and
posterolateral fusion for spinal canal surgery in children and
adolescents. Clin Orthop 1990;259:92.
T, Kato S, Toba T, et al. Sagittal splitting laminoplasty for spinal
canal enlargement for ossification of the spinal ligaments (OPLL and
OLF). Semin Musculoskelet Radiol 2001;5:203.
A, Ikata T, Katoh S. Spinal deformity following surgery for spinal cord
tumors and tumorous lesions: analysis based on an assessment of spinal
functional curve. Spinal Cord 1996;34:536.
P, Boyd M, Cochrane D. Cervical spinal deformity following craniotomy
and upper cervical laminectomy for posterior fossa tumors in children. Childs Nerv Syst 1989;5:25.
BM, Dormans JP, Drummond DS. The use of MRI-compatible titanium
segmental spinal instrumentation in pediatric patients with intraspinal
tumor. J Spinal Disord 1995;8:76.
EA, Luigley JR, Hilken JA. Comparative experimental studies of 200
kilovolt and 1000 kilovolt roentgen rays. I. The biological effects on
the epiphyses of the albino rat. Am J Pathol 1940;16:605.
CL. The effect of roentgen rays upon the growing long bones of albino
rats. Quantitative studies of the growth limitation following
irradiation. Am J Roentgenol 1942;47:439.
JS, Lingley JR, Gall EA. The effect of roentgen irradiation on
epiphyseal growth. I. Experimental studies upon the albino rat. Am J Roentgenol 1943;49:104.
JA, Lingley JR, Gall EA, et al. The effect of roentgen irradiation on
epiphyseal growth. II. Experimental studies upon the dog. J Bone Joint Surg 1947;29:853.
CL. The effect of roentgen rays upon the growing long bones of albino
rats II. Histopathological changes involving endochondral growth
centers. Am J Roentgenol 1943;49:321.
WR, Butler MS, Robertson WWJ, et al. Late orthopaedic effects in
children with Wilms’ tumor treated with abdominal irradiation. Med Pediatr Oncol 1991;19:265.
EH, Grabias SL, Burton RI, et al. Skeletal alterations following
irradiation for Wilms’ tumor: with particular reference to scoliosis
and kyphosis. J Bone Joint Surg Am 1976; 58:526.
R, Daviddson JK, Flatman GE. Skeletal effects of ortho-voltage and
megavoltage therapy following treatment of nephroblastoma. Clin Radiol 1982;33:601.
A, Heikkila JT, Merikanto J, et al. Spinal deformity induced by
radiotherapy for solid tumors in childhood: a long-term followup study.
Eur J Pediatr 1993;152:197.
SE. Thoracolumbar kyphosis and lumbosacral hyperlordosis in
achondroplastic children. In: Nicoletti B, Kopits SE, Ascani E et al.,
eds. Human achondroplasia: a multidisciplinary approach. New York: Plenum, 1988:241.
AA, Kirby N, Hungerford DS. Orthotic correction of sitting abnormality
in achondroplastic children. In: Nicoletti B, Kopits SE, Acsani E et
al., eds. Human achondroplasia: a multidisciplinary approach. New York: Plenum, 1988:313.
RM, Horton VK, Glinski LP, et al. Prospective assessment of risks for
cervicomedullary-junction compression in infants with achondroplasia. Am J Hum Genet 1995:56:732.
Y, Winter RB, Lonstein JE. The spine in diastrophic dysplasia. The
surgical arthrodesis of thoracic and lumbar deformities in 21 patients.
Spine 1999;24:2325.
RE, Buchanan JAF, Copplemans MGJ, et al. Bone marrow transplantation in
Hurler’s syndrome: effect on skeletal development. J Bone Joint Surg 1994;76B:975.
L, Niggemeyer O, Kothe R, et al. Severe pathologic compression of three
consecutive vertebrae in Gaucher’s disease: a case report and review of
the literature. Eur Spine J 2003;12:97.
HC, Pyeritz RE, Hall BD, et al. The Marfan syndrome locus: confirmation
of assignment to chromosome 15 and identification of tightly-linked
markers at 15 q 15-921.3. Genomics 1991;9:355.
PR, Moe JH, Winter RB. Scoliosis in Marfan syndrome: its
characteristics and results of treatment in thirty-five patients. J Bone Joint Surg Am 1975;57:358.
RB. Thoracic lordoscoliosis in Marfan syndrome: report of two patients
with surgical correction using rods and sublaminar wires. Spine 1990;15:233.
BA, Gorlin RJ, Langer LD. The oto-palato-digital syndrome. A new
symptom-complex consisting of deafness, dwarfism, cleft palate,
characteristic facies, and generalized bone dysplasia. Am J Dis Child 1967;113:214.
PC, Bohlman HH, Yuan HA. Anterior decompression of traumatic
thoracolumbar fractures with incomplete neurological deficit using a
retroperitoneal approach. J Bone Joint Surg Am 1985;67:89.
T, Yamagishi M, Nemoto K, et al. Spinal fusion using a vascularized
fibular bone graft for a patient with cervical kyphosis due to
neurofibromatosis. J Spinal Disord 1997;10:537.
S, Ozawa H, Sakurai M, et al. One-stage anterior and posterior
correction of severe kyphosis of the cervical spine in
neurofibromatosis. A case report. Spine 1993;18:2332.
EA, van den Berg MP, Wuisman PIJM, et al. Correction of a dystrophic
cervicothoracic spine deformity in Recklinghausen’s disease. Clin Orthop Relat Res 1998;349:149.
BA, Harkey L, Parent AD, et al. Severe cervical kyphotic deformities in
patients with plexiform neurofibromas: case report. Neurosurgery 1994;35:960.
V, Doman I, de Jonge T, et al. Surgical treatment of spinal deformities
associated with neurofibromatosis type I. Report of 12 cases. J Neurosurg Spine 2002;97:310.
Regimens of Chemotherapy. Recommendations from the Committee on
Treatment of the International Union against Tuberculosis and Lung
Disease. Bull Int Union Tuberc Lung Dis 1988;63:60.
C, Valencia R. Sagittal alignment after anterior debridement and fusion
with or without additional posterior instrumentation in the treatment
of pyogenic and tuberculous spondylodiscitis. Spine 2003;28:1036.
KP, Kothe R, Leong JC, et al. Growth changes of solidly fused kyphotic
block after surgery for tuberculosis. Comparison of four procedures. Spine 1997;22:1150.
Research Council Working Party on Tuberculosis of the Spine. A
controlled trial of anterior spinal fusion and debridement in the
surgical management of tuberculosis of the spine in patients on
standard chemotherapy: studies in Hong Kong. Br J Surg 1974;61:853.
Research Council Working Party on Tuberculosis of the Spine. A
controlled trial of debridement and ambulatory treatment in the
management of tuberculosis of the spine in patients on standard
chemotherapy. J Trap Med Hyg 1974;77:72.
Research Council Working Party on Tuberculosis of the Spine. Five-year
assessments of controlled trials of ambulatory treatment, debridement
and anterior spinal fusion in the management of tuberculosis of the
spine: studies in Bulaway (Rhodesia) and in Hong Kong. J Bone Joint Surg Br 1978; 60:163.
Research Council Working Party on Tuberculosis of the Spine. A ten-year
assessment of a controlled trial comparing debridement and anterior
spinal fusion in the management of tuberculosis of the spine in
patients on standard chemotherapy in Hong Kong. J Bone Joint Surg Br 1982;64:393.
HL, Gabriel M, Hodgson AR, et al. Tuberculosis of the spine in
children: operative findings and results in one hundred consecutive
patients treated by removal of the lesion and anterior grafting. J Bone Joint Surg Am 1972;54:1633.
M-S, Woo Y-K, Lee K-S. Posterior instrumentation and anterior interbody
fusion for tuberculous kyphosis of dorsal and lumbar spines. Spine 1995;20:1910.
Research Council Working Party on Tuberculosis of the Spine. A
controlled trial of ambulant outpatient treatment and inpatient rest in
bed in the management of tuberculosis of the spine in young Korean
patients on standard chemotherapy: a study in Masan, Korea. J Bone Joint Surg Br 1973;55:678.
Research Council Working Party on Tuberculosis of the Spine. A ten-year
assessment of controlled trials of inpatient and outpatient treatment
and plaster-of-Paris jackets for tuberculosis of the spine in children
on standard chemotherapy: studies in Masan and Pusan, Korea. J Bone Joint Surg Br 1985;67:103.
SS, Saji MJ, Sell P, et al. The effect of age on the change in
deformity after anterior debridement surgery for tuberculosis of the
spine. Spine 1996;21:2356.
SS, Saji MJ, Sell P, et al. Spinal deformity after childhood surgery
for tuberculosis of the spine. A comparison of radical surgery and
debridement. J Bone Joint Surg Br 1994;76:91.
SS, Saji MJ, Sell P, et al. The effect of age on the change in
deformity after radical resection and anterior arthrodesis for
tuberculosis of the spine. J Bone Joint Surg Am 1994;76:701.
SS, Sell P, Saji MJ, et al. 17-year prospective study of surgical
management of spinal tuberculosis in children. Hong Kong operation
compared with debridement surgery for short- and long-term outcome of
deformity. Spine 1993;18:1704.
LCS, Cheng CL, Leong JCY. Pott’s paraplegia of late onset: the cause of
compression and results after anterior decompression. J Bone Joint Surg Br 1988;70:534.
K, Papapoulos SE, Peters ACB, et al. Characteristics and bisphosphonate
treatment of a patient with juvenile osteoporosis. J Clin Endocrinol Metab 1985;61:952.
GM, Cheng MY, Yeung CY. Back pain and vertebral compression: an
uncommon presentation of childhood acute lymphoblastic leukemia. J Pediatr Orthop 1987;7:175.
