Chondrosarcoma
II – Specific Bone Neoplasms and Simulators > 6 – Bone Sarcomas >
6.3 – Chondrosarcoma
human body and include a spectrum of lesions that range from low grade
to dangerously aggressive. Although cartilage bone lesions in general
have a characteristic radiographic appearance, differentiating between
benign and low-grade malignant cartilage lesions often presents a
diagnostic challenge (Table 6.3-1). Since treatment and outcome are based in large part upon an accurate diagnosis, this distinction is important (Fig. 6.3-1).
-
Unknown
-
Current speculation: may arise from monoclonal expansion of single chondrocyte
-
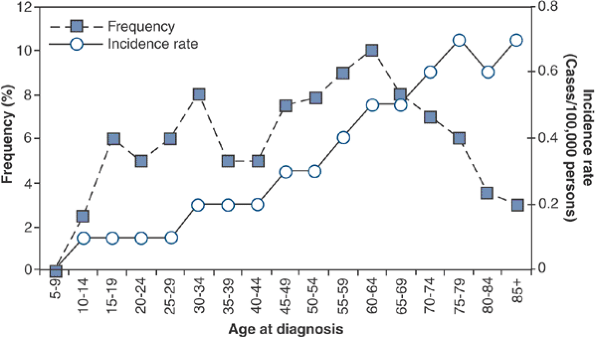
Peak age: third to sixth decades (Fig. 6.3-2)
-
Frequency of chondrosarcomas in the United States
-
~10% to 25% of primary bone tumors are chondrosarcomas.
-
Approximately 250 to 625 cases/year in United States
-
Second to osteosarcoma among bone sarcomas
-
|
Table 6.3-1 Differentiation Between Benign and Low-Grade Malignant Cartilage Lesions
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
-
Macroscopic appearance
-
Heterogeneous gross properties, including lobulated areas of chalky calcific admixture
-
Regions of firm translucent unmineralized gray cartilage with relatively low vascularity
-
Intermixed areas of necrosis and degeneration
-
-
Low-grade chondrosarcomas
-
Relatively acellular, heavily calcified areas
-
Regions of increased activity exhibiting immature cartilage cells with multiple nucleated lacunae
-
Permeation pattern of cartilage surrounding pre- existing bony trabeculae
-
-
High-grade chondrosarcomas
-
Densely packed, hyperchromatic, malignant-looking cells
-
May be difficult to determine that these cells are truly of cartilaginous origin
-
Myxomatous changes and highly degenerative areas common
-
 |
|
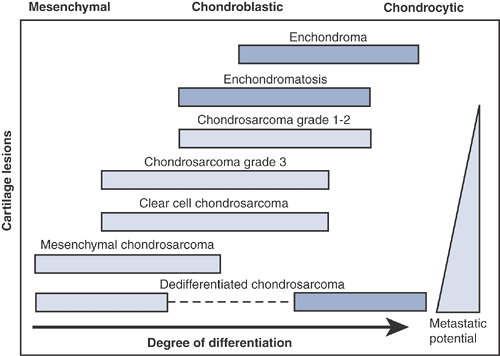
Figure 6.3-1
Degree of differentiation in cartilage tumors. (Adapted from Dorfman HD, Czerniak B. Malignant cartilage tumors. In: Dorfman HD, Czerniak B, eds. Bone Tumors. St. Louis: Mosby, 1998.) |
-
Several classification schemes
-
Histologic grade (I, II, III, dedifferentiated)
-
The most important factor in the
malignant potential of a chondrosarcoma is its histologic grade. Most
(~85%) chondrosarcomas are low-grade lesions.
-
-
Location within the bone and body
-
Peripheral (periosteal or juxtacortical
chondrosarcoma and secondary chondrosarcoma arising from
osteochondroma) versus central (intramedullary) -
Axial versus appendicular skeleton
-
-
Primary versus secondary
-
Primary: arises de novo
-
Secondary central: arises from enchondroma
-
Secondary peripheral: arises from osteochondroma
-
-
Specific histologic subtype
-
Conventional
-
Clear cell chondrosarcoma
-
Mesenchymal chondrosarcoma
-
Dedifferentiated chondrosarcoma
-
-
-
Histologic grades of conventional chondrosarcomas (Table 6.3-2)
-
Grade I (“low-grade”) tumors
-
Slow-growing and locally aggressive
![]() Figure 6.3-2
Figure 6.3-2
Age-specific frequency and distribution of chondrosarcoma, based on
SEER 1973–1987 data. (After Dorfman HD, Czerniak B. Bone cancers. Cancer 1995;75:223–227.)P.194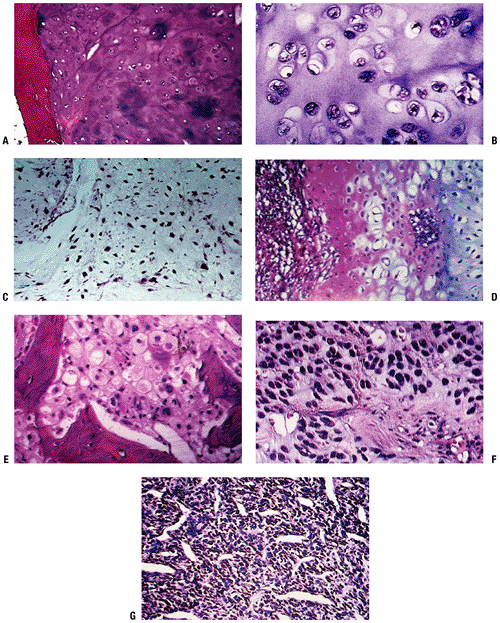 Figure 6.3-3 Representative histopathology specimens from typical chondrosarcomas. (A) Grade I chondrosarcoma demonstrates increased cellularity, perhaps some hyperchromatism but not necessarily atypia. (B) Grade II chondrosarcoma demonstrates increased cellularity and atypia. (C) Grade III chondrosarcoma demonstrates markedly increased cellularity and pleomorphism. (D)
Figure 6.3-3 Representative histopathology specimens from typical chondrosarcomas. (A) Grade I chondrosarcoma demonstrates increased cellularity, perhaps some hyperchromatism but not necessarily atypia. (B) Grade II chondrosarcoma demonstrates increased cellularity and atypia. (C) Grade III chondrosarcoma demonstrates markedly increased cellularity and pleomorphism. (D)
Dedifferentiated chondrosarcoma demonstrates area of low-grade
chondrosarcoma with abrupt transitions into a spindle cell sarcoma. (E)
Clear cell chondrosarcoma demonstrates bony trabeculae-intertwined
tumor cells containing clear cytoplasm with central atypical nuclei
admixed with benign giant cells. (F) Myxoid chondrosarcoma demonstrates round cells with myxoid features. (G)
Mesenchymal chondrosarcoma demonstrates nodules of chondroid tissue
surrounding vascular spaces, resembling a hemangiopericytoma.P.195Table 6.3-2 Clinicopathologic Features of Cartilaginous TumorsLesion Clinical Radiology Histopathology Treatment Enchondroma Mostly asymptomatic and incidentally recognized 9% have endosteal scalloping Enchondroma encasement pattern Surveillance, intralesional excision if symptomatic Chondrosarcoma Grade I 60% are painful Endosteal scalloping, calcifications in rings or spicules, uniform calcifications, eccentric lobular growth Chondrosarcomatous permeative pattern Controversial: Extended intralesional excision versus wide resection Grade II Up to 80% painful Endosteal scalloping, potentially more aggressive and adaptive changes Mixture of grade I and grade III characteristics Wide resection Grade III Up to 80% painful Endosteal scalloping, faint amorphous calcifications, large lucent areas, growing soft tissue mass Densely packed hyperchromatic
malignant-looking cells, cells of questionable cartilaginous origin,
myxomatous changes, highly degenerative areasWide resection
Possible adjunct radiation therapy and chemotherapy in selected cases -
Recurrence common, but very low metastatic potential
-
Histologically resemble normal hyaline
cartilage, may surround or permeate through areas of lamellar bone (a
feature not seen in benign lesions, which are more typically encased by
bone) -
Radiographically may show bone expansion, cortical thinning, endosteal scalloping, periosteal reaction, lytic areas
-
-
Grade II (“intermediate-grade”) tumors
-
Locally aggressive with higher potential for recurrence
-
~10% to 15% metastasize (lung > bone)
-
Histologically show increased cellularity
and cytological atypia (enlarged nuclei and often multinucleated cells)
with foci of myxoid changes -
Radiographically, grade II lesions show endosteal scalloping.
-
-
Grade III (“high-grade”) tumors
-
Highly aggressive and rapidly growing, with significant metastatic potential
-
More than 50% will metastasize.
-
Grade II lesions may recur as grade III.
-
Histologically: high cellularity, marked pleomorphism, and necrosis
-
Radiographically, these lesions show aggressive cortical destruction and a destructive pattern of growth.
-
-
-
Special histologic subtypes of chondrosarcoma
-
Clear cell chondrosarcoma
-
<5% of all chondrosarcomas
-
Low-grade malignant tumor with significant amounts of glycogen
-
Produces lytic defects in epiphysis that imitate chondroblastoma
-
Frequently extends to joint surface
-
Typically involves proximal portion of femur, tibia, or humerus
-
Histologically, displays tumor cells with
abundant clear cytoplasm embedded in a loose cartilaginous matrix and
infiltrative growth pattern -
Radiographically, produces lytic defect at end of long bones that is sharply demarcated with sclerotic margins
-
Peak age range 20 to 40
-
-
Mesenchymal chondrosarcoma
-
2% of all chondrosarcomas
-
Highly aggressive tumor that is
radiographically and histologically distinct from conventional and
dedifferentiated chondrosarcoma -
Noncartilaginous elements usually predominate.
-
Eccentrically located in bone
-
Prominent extension into soft tissues is common.
-
Usually affects young adults and teenagers
-
Usually affects axial skeleton; maxilla and mandible are frequent sites
-
Surgical excision is mainstay of treatment.
-
Ten-year survival rate ~28%
-
-
Dedifferentiated chondrosarcomas
-
~10% of all chondrosarcomas
-
Distribution in femur > pelvis > humerus > ribs > scapula
-
Two components on histologic analysis with clear demarcation
-
The dedifferentiated component likely arises from one of the other three histologic subtypes or from a benign precursor.
-
High-grade sarcoma with or without heterologous elements
-
-
-
Low-grade cartilaginous lesion
-
Radiographically, displays area of
punctuate opacities surrounded by lytic area with complete cortical
disruption and extension into the soft tissues
-
-
the histopathology and clinical behavior of chondrosarcomas. This is
reflected in the diversity of cytogenetic and molecular genetic
characteristics that have been described in these tumors.
Chondrosarcoma karyotypes may range from a few simple numerical changes
to complex numerical and structural abnormalities. A number of genes
have been investigated in chondrosarcoma. Table 6.3-3 offers a basic summary of current research on the genetics of chondrosarcoma.
to be investigated extensively. The understanding of these genetic
alterations will have profound implications on prognosis and may serve
as a foundation for the development of therapy unique to these tumors.
-
Pelvis > femur > ribs > humerus > scapula > tibia (Fig. 6.3-4)
-
Metaphysis > diaphysis
-
Most patients with a chondrosarcoma will have dull, aching pain.
-
Benign cartilage tumors less frequently produce pain.
-
Grade II or III chondrosarcomas present with pain in up to 80% of cases.
-
Rest pain and night pain are common in chondrosarcomas.
|
Table 6.3-3 Tumor Suppressor Genes and Oncogenes in Chondrosarcoma
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
-
Occurs in a relatively small number of cases (3% to 8%) of low-grade chondrosarcoma
-
Incidence much higher in high-grade tumors (36% of dedifferentiated chondrosarcomas in one series)
-
Local swelling and tenderness
-
Antalgic gait for lower extremity tumors (variable)
-
Decreased range of motion at adjacent joints (variable)
-
Palpable mass in tumors with associated soft tissue extension
features. Both can demonstrate the classic discrete stippled calcified
opacities indicative of hyaline cartilage matrix within the
intramedullary region(s) of typical long bones.
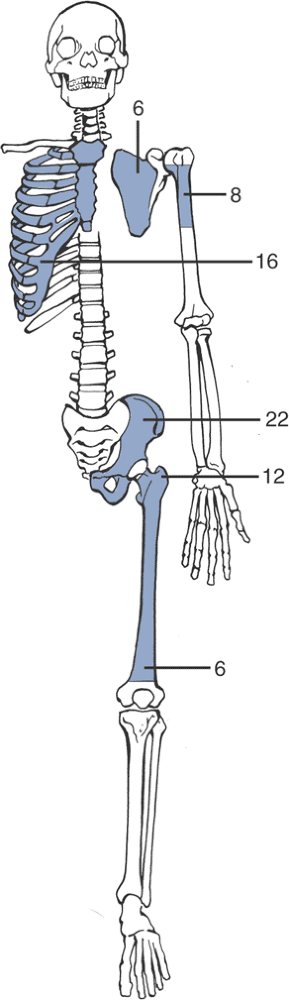 |
|
Figure 6.3-4 Distribution of chondrosarcoma by site of lesion (numbers represent percentage frequency).
|
-
Radiolucent area with varying numbers of punctate opacities
-
Endosteal scalloping can indicate malignant potential but is not confirmatory (Box 6.3-1).
-
Adaptive changes include cortical expansion or thickening.
-
Aggressive features include cortical disruption and soft tissue expansion.
-
Change in radiographic appearance with
time. Radiographs taken at different points in time (usually 3 months
apart) illustrating increases in endosteal scalloping and cortical
destruction or fewer intralesional calcifications suggest a malignant
lesion.
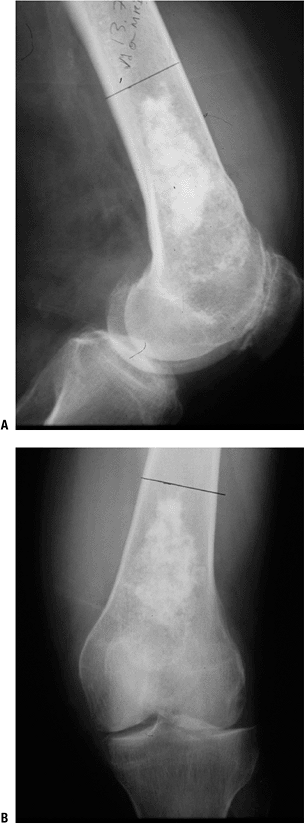 |
|
Figure 6.3-5 Plain radiographs of a low-grade cartilage lesion.
|
-
Axial computed tomography (CT) can assist in determining extent of endosteal scalloping and bony destruction.
-
Change in the nature of calcifications with sequential scanning can suggest malignancy.
|
-
Degree of medullary fill
-
Involvement of >90% of the medullary canal suggests chondrosarcoma.
-
<90% medullary involvement and noncontiguous foci of cartilage suggest a benign cartilage tumor.
-
-
Low-signal T1-weighted images, high-signal T2-weighted images
-
Septal enhancements on magnetic resonance
imaging (MRI) suggest intralesional fibrotic bands, a histologic
finding associated with grade I and II chondrosarcomas. -
Gadolinium enhancement delineates extent of tumor and proximity to neurovascular structures.
-
Identifies multifocal disease of multiple enchondromatosis (Ollier’s disease, Maffucci’s syndrome)
-
Lesions with radioisotope uptake are more likely to be chondrosarcoma than enchondroma, especially in long bones.
-
Bone scan may play a role in screening
malignant transformation of enchondroma into chondrosarcoma if serial
examinations are available. -
Some enchondromas can exhibit uptake of radioisotope.
-
May have a role in tumor grading in chondrosarcoma
 |
|
Figure 6.3-6 Axial CT of chondrosarcoma of vertebral elements (left) and distal femur (right).
|
successful means of treating chondrosarcomas. The decision regarding
the extent of surgical resection and adjuvant therapy depends on the
clinical and histologic characteristics of the lesion. This remains
somewhat controversial. To date, studies have not shown adjuvant
treatments such as chemotherapy or radiation to have any significant
impact on patient
morbidity
or mortality in most isolated primary lesions. Possible exceptions are
younger patients with a dedifferentiated subtype and those with
metastatic disease. As these adjunctive modalities are of no proven
benefit, the burden of a cure falls upon adequate initial surgical
resection.
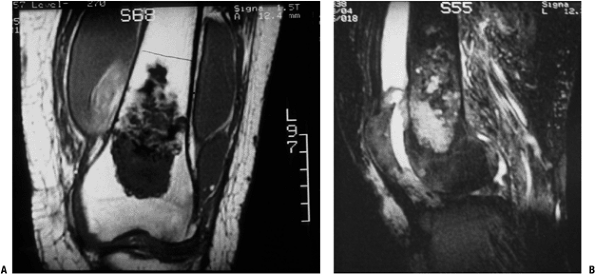 |
|
Figure 6.3-7 MRI images of distal femoral chondrosarcoma.
|
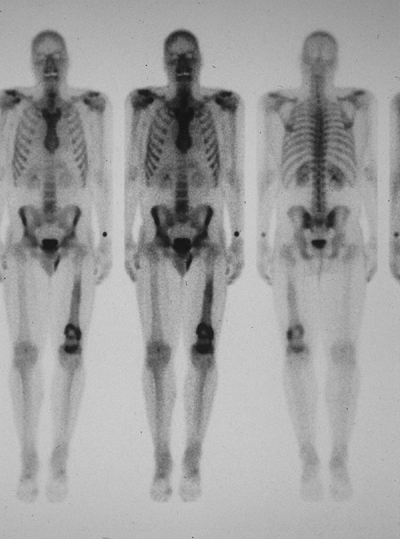 |
|
Figure 6.3-8 Bone scan of patient with left distal femoral chondrosarcoma.
|
of choice for all chondrosarcomas. Unfortunately, these tumors are
frequently found in regions such as the pelvis or proximal long bones,
where aggressive surgical management may endanger adjacent vital organs
and structures or compromise limb function. For low-grade tumors with
minimal metastatic potential, less aggressive approaches such as
marginal excision and extended intralesional excision with margin
expansion using adjuncts such as phenol or cryotherapy have received
increasing attention. While rigorous evidence-based criteria are
lacking at present, individual centers may have their own criteria and
algorithms for surgical decision making (see Algorithm 6.3-1).
In general, benign lesions should be treated conservatively, while
high-grade malignancies should be treated aggressively with complete
resection. Optimal treatment for low-grade chondrosarcoma remains a
diagnostic and therapeutic dilemma.
-
Primary indications for surgical treatment of cartilaginous tumors are for:
-
Symptomatic lesions
-
Aggressive appearance on radiographic studies
-
Change over time in radiographic appearance of lesion without initial aggressive appearance
-
-
Specific treatments
-
Low-grade lesions: wide resection versus extended intralesional curettage
-
Phenol and liquid nitrogen are commonly used adjuvants for extended intralesional curettage.
-
-
Intermediate grade: wide resection necessary
-
High-grade, aggressive tumors: wide resection necessary
-
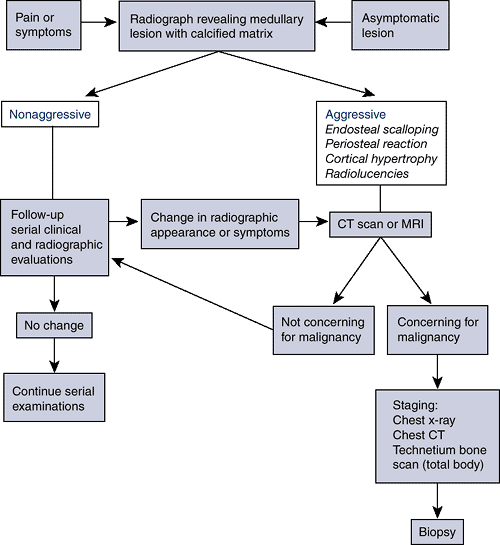 |
|
Algorithm 6.3-1. Diagnostic work-up of chondrosarcoma.
|
-
In general, the prognosis for
chondrosarcoma depends on the histologic grade of the lesion and the
attainment of complete excision of the tumor (Table 6.3-4). There is a limited role for adjuvant therapies (i.e., chemotherapy and irradiation).
-
Prognosis is excellent for low-grade tumors after adequate excision.
-
Low incidence of pulmonary metastasis in low- and intermediate-grade tumors if the primary lesion is widely resected
-
Recurrences can occur, even up to 15 years after treatment.
-
Secondary and peripheral chondrosarcomas have better prognosis than primary/central.
|
Table 6.3-4 Survival Features of Chondrosarcoma
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
-
Uniformly poor prognosis
-
Fair prognosis with wide resection and reconstruction
-
Overall recurrence rate 16%
-
Poor prognosis if treated with simple curettage and allografting
-
Often mistaken for chondroblastoma
-
Prognosis very poor despite aggressive treatment
-
High incidence of pulmonary metastatic disease
-
High mortality
-
Patients should be followed for serial
surveillance radiographic and clinical examinations to assess for
recurrence and/or metastatic disease.
JM, Gold R, Downs J, et al. A new histologic approach to the
differentiation of enchondroma and chondrosarcoma of the bones. A
clinicopathologic analysis of 51 cases. Clin Orthop Relat Res 1985;201:214–237.
MD, Andrews CL, Flemming DJ, et al. From the archives of the AFIP.
Primary tumors of the spine: radiologic pathologic correlation. Radiographics 1996;16:1131–1158.
AA, Bridge JA. Updates on the cytogenetics and molecular genetics of
bone and soft tissue tumors: chondrosarcoma and other cartilaginous
neoplasms. Cancer Genet Cytogenet 2003;143:1–31.