CHAPTER 65 –
Cole & Sekiya: Surgical Techniques of the Shoulder, Elbow and Knee in Sports Medicine, 1st ed.
Copyright ©
2008 Saunders, An Imprint of Elsevier
CHAPTER 65 – Anatomic Double-Bundle Anterior Cruciate Ligament Reconstruction
James S. Starman, BS,
Freddie H. Fu, MD DSc (Hon), DPs (Hon)
Anterior cruciate ligament (ACL) reconstruction remains one of the most common procedures performed by orthopedic surgeons in the United States, with approximately 100,000 reconstructions performed per year.[9] ACL surgery has evolved tremendously from the original open techniques to modern procedures focusing on endoscopic reconstruction of the anteromedial bundle with use of a variety of graft choices and fixation techniques. However, the success of single-bundle ACL reconstruction ranges from 69% to 90%. [6] [22]
Single-bundle ACL reconstruction is the “gold standard,” but some authors have noted persistent instability with functional testing of single-bundle ACL reconstruction. [18] [19] Thus, there is a growing trend toward a more anatomic ACL reconstruction that re-creates both the anteromedial and the posterolateral bundles. The double-bundle anatomy of the ACL was first described in 1938.[17] The terminology of the anteromedial and posterolateral bundles is chosen according to their tibial insertions. The tibial and femoral insertion sites of both the anteromedial and posterolateral bundles have been well described. [11] [16] Although the two bundles are intertwined, their functional tensioning pattern is independent throughout knee range of motion.[8]
The idea of reconstructing both bundles of the ACL was originated by Mott and Zaricznyj in the 1980s. [14] [23] They independently described a double-bundle technique with use of a single femoral tunnel and two tibial tunnels. Despite publication of their results, the technique did not become mainstream. There is recent biomechanical evidence to support that anatomic double-bundle ACL reconstruction more accurately re-creates the natural anatomy.[20] We present the senior author’s (F. H. F.) technique of anatomic double-bundle ACL reconstruction with two femoral and two tibial tunnels using two tibialis anterior allografts.
ACL injuries occur frequently in sports that involve running, jumping, and cutting movements. They can occur without contact when the foot is anchored to the playing surface—usually by way of cleats or a rubber sole—and the body rotates beyond the tolerance of the ligament as the knee buckles. Thus, it is important to ask how the injury occurred and the position of the knee during the injury, which may also allude to the ACL bundle injury pattern. This may be associated with an audible “pop.” Asking whether the athlete was able to continue to play will give you an idea of the severity of the injury. Knee pain and a hemarthrosis are usually present acutely. A complaint of instability is also common, especially when walking downhill or down stairs.
Inspect and palpate for an effusion. If a large effusion is present, consider aspiration for pain relief and inspection of the aspirate for any fat globules, which would suggest a fracture. Check the range of motion; if it is limited, a magnetic resonance imaging evaluation should be obtained to ensure that no displaced meniscal tear is present. The physical examination of an isolated ACL tear is usually significant for a side-to-side difference with regard to Lachman and pivot shift maneuvers. If a discrepancy between the Lachman and pivot shift maneuvers exists, this may signify a partial tear involving either the anteromedial or posterolateral bundle. The posterolateral bundle is mainly responsible for rotational stability, and a large pivot shift will be evident if it is torn. Similarly, the anteromedial bundle is mainly responsible for translational stability when the knee is flexed, and a large Lachman maneuver will be present if it is torn. KT-1000 testing can also confirm a side-to-side difference in anterior translation. A more prominent anterior drawer compared with a Lachman test result should clue the examiner to consider a concomitant posteromedial or posterior horn medial meniscal injury. Varus or valgus instability testing should be performed to ensure that no collateral injury is present. Dial testing and posterolateral drawer testing at 30 degrees should be performed to assess for a posterolateral knee injury. Gait analysis should be performed to inspect for any underlying varus laxity. Tests for possible meniscal disease should also be performed (i.e., joint line tenderness and McMurray maneuver), but it may be difficult to distinguish between a lateral meniscal tear and a bone contusion acutely. Thus, appropriate imaging is important.
A complete knee series consisting of weight-bearing anteroposterior and notch, lateral, and patellofemoral sunrise views should be obtained. The soft tissues can be inspected for an effusion. The bone anatomy should be inspected for any fractures or subtle signs of a rotational injury (i.e., Segond or reverse Segond capsular lesion). In addition, the status of the physes and any arthritic changes should be noted. Long-cassette films should be obtained for any patient with varus alignment on examination or if any arthritic changes are noted on the knee series obtained. This will help determine whether an osteotomy should be performed. Magnetic resonance imaging is essential not only to confirm an ACL tear but, more important, to assess for any concomitant ligamentous or cartilaginous injuries that will affect the operative plan. The posterolateral bundle is more easily visualized on coronal sectioning. Specifically, it may be seen at the level of the first cut that includes the posterior cruciate ligament.
Indications and Contraindications
The absolute indications for double-bundle ACL reconstruction are in evolution. Even though single-bundle ACL reconstruction is considered the gold standard, the technique can be improved. Gait analysis after single-bundle reconstruction has demonstrated that rotatory instability persists.[18] Furthermore, biomechanical cadaver studies have shown that even lowering of the femoral insertion site to the 3- or 9-o’clock position does not fully prevent rotatory instability.[4] Clinically, up to one fifth of the patients do not resume preinjury activities and usually complain of vague instability symptoms that objectively correspond to a mild persistent pivot shift.[2] In comparison, double-bundle ACL reconstruction does restore the rotational component in a cadaver model.[20] It has been suggested that a positive pivot shift result after ACL reconstruction is correlated with the development of later osteoarthrosis.[13]
In the young athlete with open physes, the double-bundle technique is contraindicated. Two tunnels would risk physeal arrest with subsequent malalignment and possible leg length discrepancy.
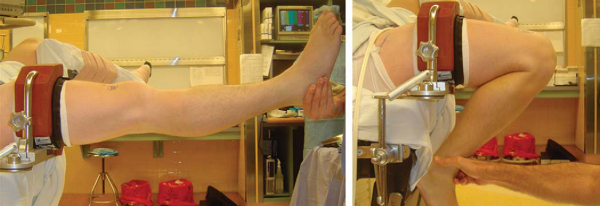
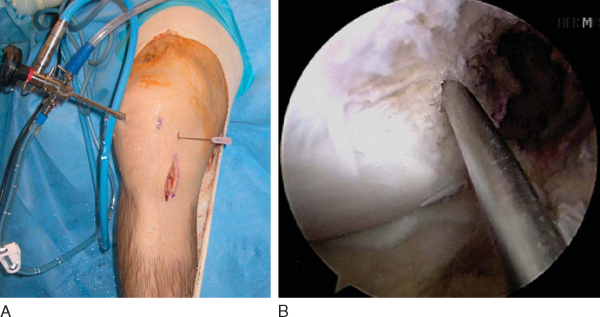
The operative extremity is identified by the patient and initialed by a member of the surgical team. All patients undergo a preoperative femoral nerve block in the holding area by our anesthesia colleagues. The patient is then placed in a supine position and given intravenous conscious sedation. A careful examination under anesthesia is performed and recorded to document the Lachman and pivot shift maneuvers. Once again, the senior author is interested in correlating the examination findings with the tear pattern of the individual bundles of the ACL. A tourniquet is applied to the proximal thigh. The extremity is then secured within a circumferential leg holder placed at the level of the tourniquet. The foot of the operating table is completely retracted to permit hyperflexion of the knee, which is crucial for later placement of the posterolateral femoral tunnel. The contralateral extremity is placed within a well-padded leg holder with the hip flexed approximately 90 degrees and abducted and externally rotated away from the surgical field to allow unobstructed access to the operative knee (
Fig. 65-1
). The leg is elevated for 5 minutes, and the tourniquet is then inflated. The knee is then prepared and sterilely draped.
|
|
|
|
Figure 65-1 |
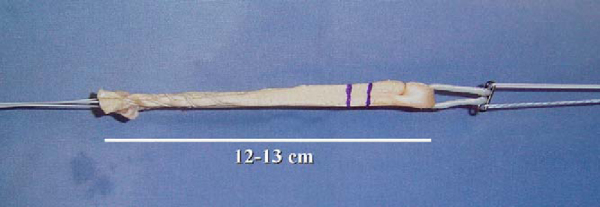
Two tibialis anterior allografts are individually fashioned as a double loop. The folded length of each graft should be approximately 12 cm for sufficient graft tissue. The grafts are trimmed to a folded diameter of 7 mm for the posterolateral bundle and 8 mm for the anteromedial bundle. A No. 2 braided suture is whipstitched up and down both ends of the graft for 3 cm. The stitch depth is alternated, and care is taken to avoid penetrating the suture and risking weakening or breaking. The graft is then passed through the closed-loop EndoButton (Smith & Nephew, Inc., Andover, Mass). Two FiberWire (Arthrex, Naples, Fla) sutures (one stripped and one nonstripped for later identification) are placed within the buttonholes. A 2-0 absorbable suture is tied through both strands of the folded graft to secure them once the graft is passed within the closed-loop EndoButton. Each graft is marked to alert the surgeon when to engage or to “flip” the EndoButton (
Fig. 65-2
).
|
|
|
|
Figure 65-2 |
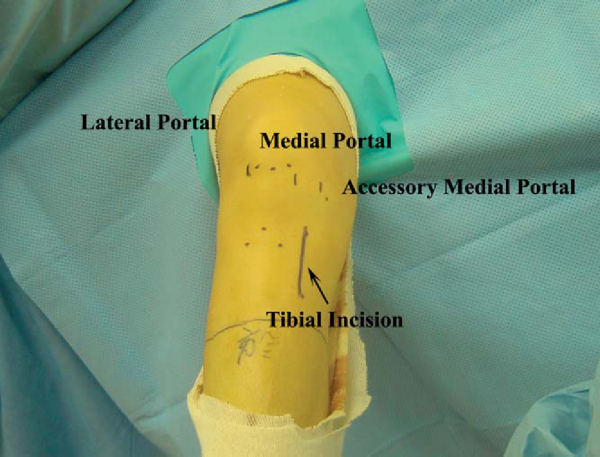
With the knee flexed approximately 45 degrees, the inferior pole of the patella is marked. The inferior extent of the lateral parapatellar portal begins at the level of the inferior pole of the patella and extends proximally for approximately 2 cm. The medial parapatellar portal begins at the level of the inferior pole of the patella and extends distally along the medial aspect of the patellar tendon for approximately 2 cm. The high placement of the portals will allow the arthroscopic instruments to enter the knee above the level of the fat pad. The No. 11 blade scalpel is angled approximately 45 degrees to the skin and distally toward the notch to safely enter the knee without harming the articular cartilage. A low anteromedial accessory portal will be placed with the assistance of a spinal needle to ensure proper trajectory for the posterolateral femoral tunnel (
Fig. 65-3
). The knee is flexed 90 degrees, and the No. 11 scalpel blade is angled upward as it enters the skin at the level of the spinal needle marking. The portal is extended proximally approximately 1 cm, avoiding injury to both the underlying meniscus and the articular cartilage.
The knee is flexed approximately 25 degrees, and the arthroscopic trocar is placed in the lateral parapatellar portal angled toward the notch and then redirected beneath the patella. The patellofemoral joint is visualized. Any cartilage defects are addressed as needed. The arthroscope is then dropped down over the trochlea and into the notch to view the ACL grossly. The knee is then extended, and a valgus stress is applied to open the medial compartment. The entire meniscus is visualized and probed for stability. Medial meniscal tears are usually seen with chronic ACL tears. Any meniscal disease is addressed with either repair or débridement. The knee is brought through a range of motion to inspect the femoral condylar articular surface for any defects. The tibial plateau articular cartilage is also inspected for any defects.
The knee is then placed in a figure-four position to view the lateral compartment. The posterolateral bundle is best visualized in this position. The entire meniscus is once again observed and probed for stability. Lateral meniscal longitudinal tears are commonly seen with acute ACL tears at the junction of the middle and posterior thirds. The posterior third of the lateral meniscus is more easily viewed with the knee flexed to 90 degrees; the middle third, with the knee flexed to 60 degrees; and the anterior third, with the knee flexed to 30 degrees. Medial meniscal tears are usually seen with chronic ACL tears. Any meniscal disease is addressed with either repair or débridement. The lateral femoral condylar articular surface is inspected for any defects as the knee is brought through an entire range of motion.
Specific Steps (
Box 65-1
)
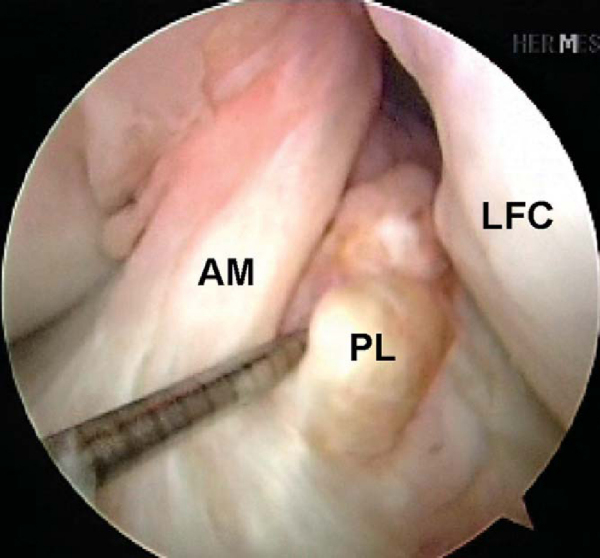
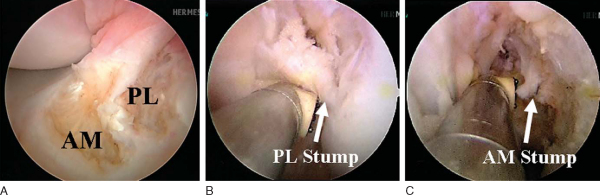
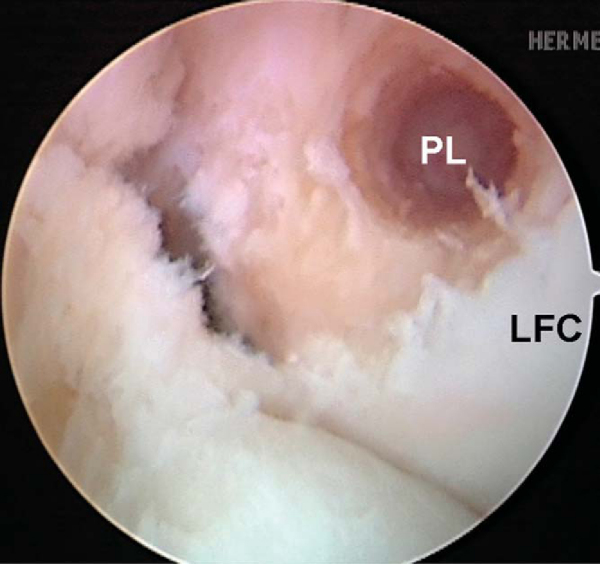
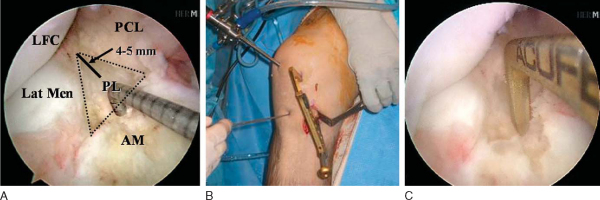
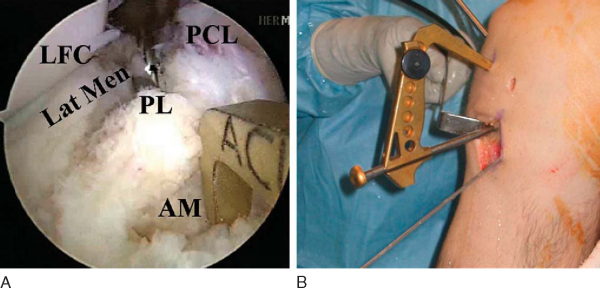
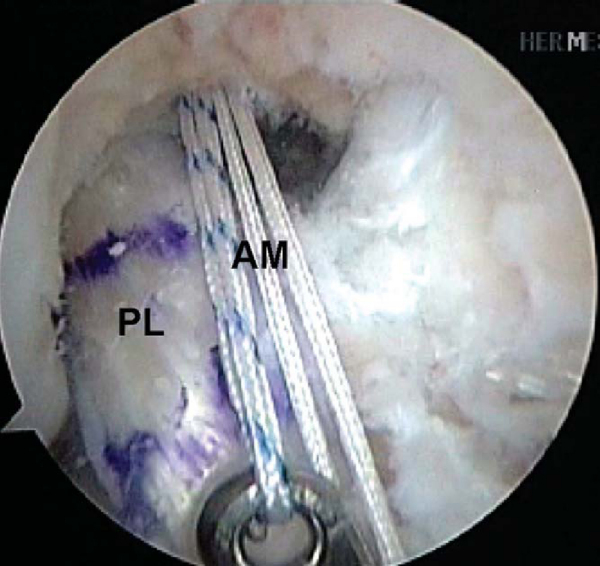
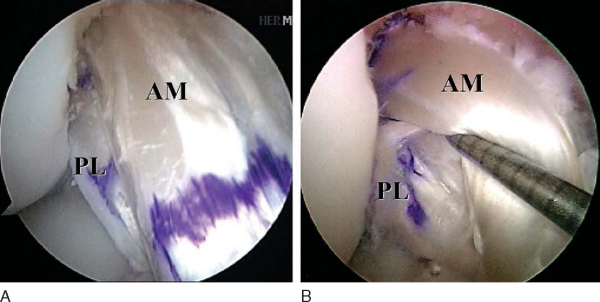
Attention is then redirected to the notch. Any obstructing fat pad or ligamentum mucosum is removed with the shaver or ArthroCare Coblation device. The bundles of the ACL are carefully dissected with a small ArthroCare Coblation device to fully appreciate the injury tear pattern. Because the two bundles are differentially tensioned according to the position of the knee at the time of injury, the tear patterns can be quite different for each bundle (
Fig. 65-4
).[7] The remnant of the ACL bundles is then débrided from both their femoral and tibial insertions. The ArthroCare Coblation device is used to mark the anteromedial and posterolateral femoral and tibial footprints (
Fig. 65-5
). No notchplasty is performed.
|
|
|
|
Figure 65-4 |
|
|
|
|
Figure 65-5 |
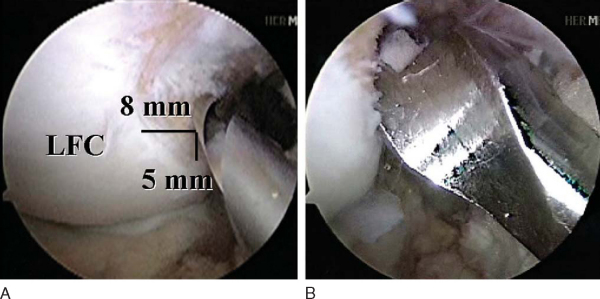
An 18-gauge spinal needle is directly visualized as it passes from the location of the low anteromedial portal onto the medial face of the lateral femoral condylar notch in the region of the previously marked posterolateral bundle (
Fig. 65-6
). Once satisfactory trajectory for the posterolateral bundle with the spinal needle is determined, the spinal needle is removed and the low anteromedial portal is made with a No. 11 scalpel blade angled upward to avoid cutting the anterior horn of the medial meniscus. A 3/32-inch Steinmann pin is then passed through the low anteromedial portal onto the medial face of the lateral femoral condylar notch. The pin is placed approximately 7 or 8 mm posterior to the anterior articular margin and approximately 3 to 5 mm superior to the inferior articular margin of the lateral femoral condyle. Once correct placement of the pin is obtained, the knee is brought into approximately 110 degrees of hyperflexion and the pin is tapped into place with a mallet (
Fig. 65-7
). Ensure that the pin did not penetrate the medial meniscus. Then slide the 7-mm acorn reamer over the Steinmann pin and have it rest against the medial face of the lateral femoral condylar notch. Ream to a depth of 25 mm. Then place the EndoButton drill within the previously reamed canal and drop your hand before starting the drill, which will maximize the posterolateral tunnel length. With the knee still flexed 110 degrees, have your assistant place a hand on the lateral aspect of the knee and push the biceps tendon inferiorly, which will deflect the common peroneal nerve away from the drill trajectory. Barely perforate the lateral femoral cortex with the EndoButton drill and quickly retract it backward to minimize the risk of injury to the common peroneal nerve. Measure the transcondylar length and choose the appropriately sized continuously looped EndoButton. If the length is greater than 35 mm, replace the 7-mm acorn reamer within the posterolateral tunnel and dilate the tunnel depth to 30 mm by hand. There should be at least 15 mm of graft tissue within the tunnel (
Fig. 65-8
).
|
|
|
|
Figure 65-7 |
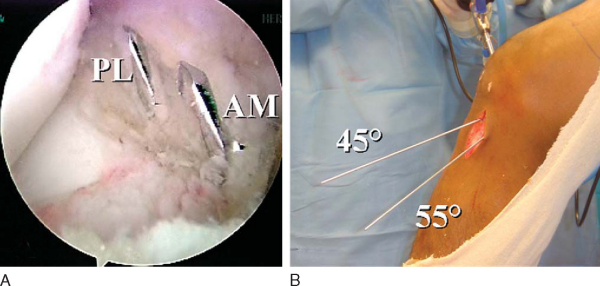
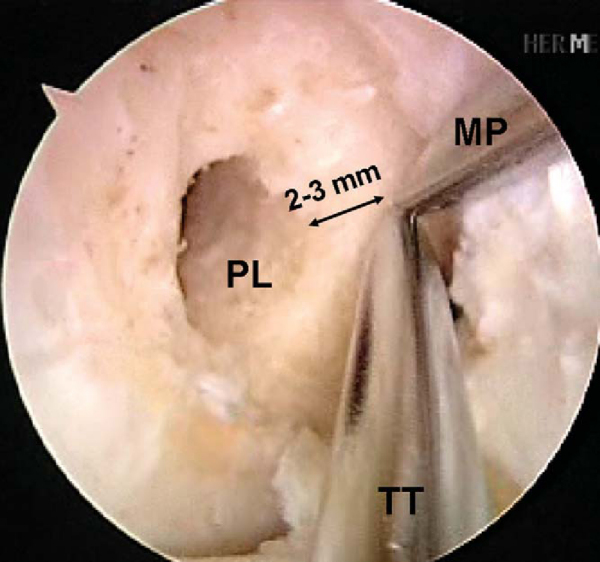
A 3- to 4-cm incision is made over the anteromedial surface of the tibia to drill the tibial tunnels and to pass the grafts. This is in a location midway between the tibial tubercle and the posteromedial border of the tibia. Dissect the soft tissues both medially and laterally for easy access to the future tibial anteromedial and posterolateral tunnels. Identify the tibial posterolateral footprint, which is just medial to the posterior horn of the lateral meniscus and anterior to the posterior cruciate ligament. The footprint should have been previously marked with the ArthroCare Coblation device. Place the ACL Director guide (Smith & Nephew) at 55 degrees with the tip centered within the posterolateral tibial footprint (
Fig. 65-9
). Drill a 3/32-inch Steinmann pin through the guide and within the joint so that a few millimeters are visible. With the ArthroCare Coblation device, identify the anteromedial tibial footprint, which is a couple of millimeters anterior to the ideal single-bundle reconstruction location—the posterior aspect of the anterior horn of the lateral meniscus, downward sloping side of the medial tibial eminence, and approximately 7 mm anterior to the posterior cruciate ligament. Reset the ACL Director guide to 45 degrees and place it within the previously marked anteromedial tibial footprint (
Fig. 65-10
). Drill a second 3/32-inch Steinmann pin through the guide and into the joint so that a few millimeters are visible. Note that the posterolateral tibial guide pin is quite vertical in comparison to the anteromedial tibial guide pin, which is quite horizontal. This pin attitude also prevents impingement within the notch because the posterolateral tunnel is centered within the knee and the anteromedial tunnel is horizontal. Once again, no notchplasty is necessary. The posterolateral tibial guide pin also enters the anteromedial surface of the tibia more posteromedially than the anteromedial tibial guide pin, which is more lateral and centered (
Fig. 65-11
). The skin on the tibia is retracted to allow placement of a 7-mm reamer over the posterolateral tibial guide pin, and a curet is placed within the joint overlying the posterolateral pin to protect the articular surfaces. The posterolateral tunnel is reamed, and debris is removed with a shaver. The 8-mm reamer is then placed over the anteromedial tibial guide pin, and a curet is once again placed overlying the pin to protect the articular surfaces. The anteromedial tunnel is then reamed, and debris is removed with a shaver. There should be an approximately 1-cm bone bridge between the two tibial tunnels.
|
|
|
|
Figure 65-9 |
|
|
|
|
Figure 65-10 |
|
|
|
|
Figure 65-11 |
Attention is then directed to the anteromedial femoral footprint. The anteromedial femoral tunnel is guided off the previously made femoral posterolateral tunnel and not off the back wall of the notch, which is traditionally done with over-the-top femoral tunnel drill guides. The 3/32-inch Steinmann pin is placed 3 mm posterior and slightly superior to the previously drilled femoral posterolateral tunnel. The Steinmann pin can be placed transtibially through the previously drilled anteromedial tunnel or through the low anteromedial portal, whichever one will allow the proper trajectory low enough within the notch (
Fig. 65-12
). The knee is hyperflexed as the Steinmann pin is then tapped into place. An 8-mm acorn reamer is then placed over the pin and passed within the joint to rest against the notch wall. The anteromedial femoral tunnel is then reamed to a depth of 40 mm. The EndoButton drill is then placed within the anteromedial tunnel. Your hand should be dropped as the knee is maintained in the hyperflexed position to maximize tunnel length and to ensure that the anterolateral femoral cortex will be perforated to allow passage of the Beath pin distal to the tourniquet. The transfemoral diameter is then measured, and the appropriately sized closed-loop EndoButton is chosen. Ideally, there should be at least 15 to 20 mm of graft tissue within the canal.
With the knee hyperflexed, place a Beath pin through the low anteromedial portal, through the posterolateral femoral tunnel, and out through the skin on the lateral aspect of the knee. Once again, you want to drop your hand and push the biceps tendon inferiorly as you pass the pin, to protect the common peroneal nerve from injury. A long looped suture is tied through the eyelet of the Beath pin, which is pulled intra-articularly and through the tibial posterolateral tunnel with arthroscopic suture retrievers. The 7-mm prepared posterolateral allograft is placed within the long looped suture attached to the Beath pin and pulled through the respective tibial and femoral tunnels. The EndoButton is flipped, and the graft is pulled to ensure proper engagement. The Beath pin is then passed through the tibial and femoral anteromedial tunnels with the knee hyperflexed to ensure that the pin exits the thigh distal to the tourniquet and remains sterile. Depending on the trajectory of the tunnels, the Beath pin may first need to be passed via the low anteromedial portal through the anteromedial femoral tunnel, and the long looped suture is pulled out through the anteromedial tibial tunnel with arthroscopic suture retrievers. The 8-mm prepared anteromedial allograft is placed within the long looped suture attached to the Beath pin and pulled through the respective tibial and femoral tunnels. The EndoButton is flipped, and the graft is pulled to ensure proper engagement (
Fig. 65-13
).
|
|
|
|
Figure 65-13 |
The knee is then cycled through a full range of motion from approximately 0 to 120 degrees for 20 to 30 times while tension is maintained on both graft ends to remove any slack and to check the isometry. The posterolateral bundle is tensioned first between 0 and 10 degrees of flexion. The anteromedial bundle is then tensioned with the knee in approximately 60 degrees of flexion. Tibial fixation is achieved with bioabsorbable interference screws that are the same diameter as the corresponding tunnel. One staple is used as adjunctive fixation for each graft on the tibial side (
Fig. 65-14
).
|
|
|
|
Figure 65-14 |
Postoperatively, the patient is placed in a hinged knee brace. Full weight bearing is allowed with the knee locked in extension. Continuous passive motion is started immediately from 0 to 45 degrees of flexion and increased by 10 degrees per day until maximum obtainable flexion permitted by the continuous passive motion machine is achieved for 2 consecutive days. This is usually after 1 to 2 weeks. The brace is unlocked at 1 week, and crutches are maintained until quadriceps control is re-established, typically 4 to 6 weeks. The accelerated rehabilitation protocol described by Irrgang[12] is implemented with return to contact sport at 6 months with a brace after successful function testing.
We have had three graft failures, all occurring after return to sport. Two were sustained during contact injuries in playing collegiate football. The third occurred in a noncompliant patient 3 months after reconstruction when she returned to playing high-school basketball without a brace. Four patients have undergone staple removal for symptomatic hardware. To date, after 186 double-bundle ACL reconstructions, we have had no fractures and no radiographic signs of femoral condylar avascular necrosis or tunnel widening.
| PEARLS AND PITFALLS | ||||||||||||||||||
|
According to Fithian et al,[5] 95% of patients who underwent single-bundle ACL reconstruction developed medial compartment degenerative radiographic changes after 7 years, and only 47% were able to return to their previous activity level. In addition, several in vivo functional biomechanical studies demonstrate that the kinematics are not completely restored with single-bundle reconstruction. [18] [19] Amis[4] has shown that even if the femoral tunnel is placed in a lower position than the traditionally described location (more “anatomic”) the kinematics are still not normal with regard to rotational stability. In contrast, Yagi et al[20] published their results of double-bundle ACL reconstruction in a cadaver model, showing that both translation and coupled rotation-translation were significantly less in the specimens with double-bundle ACL reconstruction. Clinical results of double-bundle ACL reconstruction surgery are still in evolution (
Table 65-1
). [1] [3] [10] [15] [21] [23]
| Author | Patients | Postoperative Anterior-Posterior Translation | Postoperative Pivot | Functional Outcome |
|---|---|---|---|---|
| Adachi et al[1] (2004) | 105 | KT: all <3 mm side-to-side difference | Not reported | 100% normal |
| Hamada et al[10] (2001) | 106 | KT: all <3 mm side-to-side difference | Not reported | IKDC score: 96% normal–nearly normal |
| Muneta et al[15] (1999) | 54 | KT: 2 patients >5 mm | Not reported | Lysholm score: 94.5 ± 5.3 |
| Yasuda et al[21] (2004) | 57 | KT: 49 < 3 mm | 0: 56 patients | Noyes score: 47.5 |
| 8, 3-5 mm | 1+: 1 patient | |||
| Zaricznyj[23] (1987) | 14 | Negative Lachman test result: 11 patients | 0: 14 patients | Marshall score: 12 good–excellent, 2 fair |
| 1+ Lachman score: 3 patients | ||||
| Aglietti (unpublished) | 50 | KT: all <3 mm side-to-side difference | 0: 40 patients | IKDC score: 96% normal–nearly normal |
| Fu (unpublished) | 192 | KT: average 1.2-mm side-to-side difference | 0: 105 patients | 93% normal, 6% nearly normal, 1% fair |
| 1+: 6 patients |
ACL reconstruction is one of the most common orthopedic procedures performed in the United States. Single-bundle ACL reconstruction, which focuses mainly on the anteromedial bundle, remains the gold standard that has enjoyed great success and returned many athletes to their sport. However, several authors have demonstrated that rotational instability persists. The goal of anatomic double-bundle ACL reconstruction is to address this issue and better restore kinematics to normal. It is hoped that this will decrease the rate of degenerative changes, but long-term clinical outcome studies are imperative. Regardless of whether a double-bundle reconstruction technique is chosen, knowledge of the underlying anatomy of the individual bundles will make one a better ACL reconstruction surgeon.
1.
Adachi N, Ochi M, Uchio Y, et al: Reconstruction of the anterior cruciate ligament. Single- versus double-bundle multistranded hamstring tendons.
J Bone Joint Surg Br 2004; 86:515-520.
2.
Aglietti P, Giron F, Buzzi R, et al: Anterior cruciate ligament reconstruction: bone–patellar tendon–bone compared with double semitendinosus and gracilis tendon grafts. A prospective, randomized clinical trial.
J Bone Joint Surg Am 2004; 86:2143-2155.
3.
Aglietti P. Double-bundle ACL reconstruction: single versus double incision. Personal communication.
4.
Amis AA. Persistence of the mini pivot-shift after anatomically placed ACL reconstruction. Personal communication.
5.
Fithian DC, Paxton EW, Stone ML, et al: Prospective trial of a treatment algorithm for the management of the anterior cruciate ligament–injured knee.
Am J Sports Med 2005; 33:335-346.
6.
Freedman KB, D’Amato MJ, Nedeff DD, et al: Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts.
Am J Sports Med 2003; 31:2-11.
7.
Fu FH. Rupture pattern of the anteromedial and the posterolateral bundle of the anterior cruciate ligament. Personal communication.
8.
Gabriel MT, Wong EK, Woo SL, et al: Distribution of in situ forces in the anterior cruciate ligament in response to rotatory loads.
J Orthop Res 2004; 22:85-89.
9.
Griffin LY, Agel J, Albohm MJ, et al: Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies.
J Am Acad Orthop Surg 2000; 8:141-150.
10.
Hamada M, Shino K, Horibe S, et al: Single- versus bi-socket anterior cruciate ligament reconstruction using autogenous multiple-stranded hamstring tendons with endobutton femoral fixation: a prospective study.
Arthroscopy 2001; 17:801-807.
11.
Harner CD, Baek GH, Vogrin TM, et al: Quantitative analysis of human cruciate ligament insertions.
Arthroscopy 1999; 15:741-749.
12.
Irrgang JJ: Modern trends in anterior cruciate ligament rehabilitation: nonoperative and postoperative management.
Clin Sports Med 1993; 12:797-813.
13.
Jonsson H, Riklund-Ahlstrom K, Lind J: Positive pivot shift after ACL reconstruction predicts later osteoarthrosis: 63 patients followed 5–9 years after surgery.
Acta Orthop Scand 2004; 75:594-599.
14.
Mott HW: Semitendinosus anatomic reconstruction for cruciate ligament insufficiency.
Clin Orthop Relat Res 1983; 172:90-92.
15.
Muneta T, Sekiya I, Yagishita K, et al: Two-bundle reconstruction of the anterior cruciate ligament using semitendinosus tendon with endobuttons: operative technique and preliminary results.
Arthroscopy 1999; 15:618-624.
16.
Odensten M, Gillquist J: Functional anatomy of the anterior cruciate ligament and a rationale for reconstruction.
J Bone Joint Surg Am 1985; 67:257-262.
17.
Palmer I: On the injuries to the ligaments of the knee joint.
Acta Chir Scand 1938; 91:282.
18.
Ristanis S, Stergiou N, Patras K, et al: Excessive tibial rotation during high-demand activities is not restored by anterior cruciate ligament reconstruction.
Arthroscopy 2005; 21:1323-1329.
19.
Tashman S, Collon D, Anderson K, et al: Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction.
Am J Sports Med 2004; 32:975-983.
20.
Yagi M, Wong EK, Kanamori A, et al: Biomechanical analysis of an anatomic anterior cruciate ligament reconstruction.
Am J Sports Med 2002; 30:660-666.
21.
Yasuda K, Kondo E, Ichiyama H, et al: Anatomic reconstruction of the anteromedial and posterolateral bundles of the anterior cruciate ligament using hamstring tendon grafts.
Arthroscopy 2004; 20:1015-1025.
22.
Yunes M, Richmond JC, Engels EA, Pinczewski LA: Patellar versus hamstring tendons in anterior cruciate ligament reconstruction—a metaanalysis.
Arthroscopy 2001; 17:248-257.
23.
Zaricznyj B: Reconstruction of the anterior cruciate ligament of the knee using a doubled tendon graft.
Clin Orthop Relat Res 1987; 220:162-175.