CHAPTER 14 –
Cole & Sekiya: Surgical Techniques of the Shoulder, Elbow and Knee in Sports Medicine, 1st ed.
Copyright ©
2008 Saunders, An Imprint of Elsevier
CHAPTER 14 – Treatment of Bone Defects of Humeral Head and Glenoid
Jon K. Sekiya, MD
Traumatic anterior glenohumeral dislocation typically results in disruption of the anteroinferior capsulolabral complex (Bankart lesion).[25] To date, anatomic Bankart reconstruction has represented the “gold standard” of treatment and has shown good to excellent results in most series. Although historic treatment protocols have not routinely addressed associated osteoarticular injury, the high prevalence of such lesions is well documented. Compression fractures of the posterior superior humeral head (Hill-Sachs lesion) have been found to occur in 32% to 51% of initial anterior dislocations, [5] [17] [18] [29] and anteroinferior glenoid deficiency has been reported in 22% of primary dislocations.[25] With recurrence rates after soft tissue Bankart reconstruction alone reported as high as 18% to 44% in some series, [2] [7] [12] [13] treatment protocols are evolving. More critical attention is being given to osseous defects of the glenoid and humeral head.
Burkhart and De Beer[4] reviewed 194 cases of arthroscopic Bankart repair, dividing patients into two groups—those with significant bone defects of the glenoid or humeral head and those without such defects. A remarkable difference was seen between the two groups with respect to recurrence rates. Specifically, patients without significant bone defects had a recurrence rate of 4% compared with a 67% failure rate in patients with significant bone defects. Other authors have also demonstrated a strong correlation between the presence of a Hill-Sachs lesion or anterior glenoid erosion and recurrent instability. [4] [11] [20] [25] [26]
Historically, the primary goal of treatment has been stable reduction and the prevention of recurrent dislocation. Nonanatomic reconstruction procedures have been employed to constrain the shoulder, including Bristow[16] or Latarjet[1] coracoid transfer, open capsular shift,[24] rotational proximal humeral osteotomy,[32] and transfer of the infraspinatus into the humeral head defect.[8] These procedures yield shoulder stability often at the great expense of motion. Some have been associated with significant long-term complications. [15] [33]
The surgical management of shoulder instability is evolving to include anatomic reconstruction of osseous defects of the glenohumeral joint. Success is no longer based on how well the shoulder is “blocked” from dislocation; rather, it is based on restoration of motion and strength with return to a high level of overhead function. Reconstruction of the glenoid has been described with iliac crest autograft or femoral head allograft. [14] [19] [31] Reconstruction of the humeral head has been performed with large, size- and side-matched allograft, [10] [23] a single allograft plug (osteochondral allograft transplantation),[22] and arthroscopic mosaicplasty.[6] Combined reconstructive procedure can be performed in the appropriate setting.
A complete history is obtained. This includes the mechanism of injury; associated injuries; prior treatments, if applicable; and nature of the instability. Specifically, the surgeon should elicit the timing of initial symptoms and the frequency of current symptoms, including pain and instability and their effect on the patient’s function. The typical patient initially suffers a high-energy injury secondary to a fall, motor vehicle trauma, or contact sports. Recurrent dislocations or a chronic sense of instability will have followed. Often, the patient will already have undergone multiple instability procedures without success.
Physical examination proceeds in a systematic fashion. Prior surgical scars should be noted. Active and passive range of motion and rotator cuff strength are tested and compared with the unaffected arm. Instability is assessed in anterior, posterior, and inferior directions and graded on a 3-point scale. Typically, the patient will experience clinically significant apprehension in varying degrees of abduction and external rotation. This factor coupled with significant pain may limit the in-office examination. Therefore, findings should be verified later with a complete examination under anesthesia.
| • | Anteroposterior view (internal and external rotation) | |
| • | True anteroposterior view of the glenohumeral joint | |
| • | Axillary lateral view | |
| • | Stryker notch view | |
| • | West Point view |
The computed tomographic (CT) scan proves best for assessment of the osseous defect. We have found that plain radiographs will often significantly underestimate the size of the Hill-Sachs or glenoid defect. Multiplanar CT scanning, often with three-dimensional reconstruction, is necessary for the extent of the defect to be truly appreciated. It is essential that CT scans be of high quality and allow accurate preoperative measurements. This allows one to confidently and consistently obtain an appropriately size-matched glenoid or proximal humerus allograft before surgery.
Magnetic resonance imaging with or without the administration of contrast material will aid in assessment of associated soft tissue injury. If possible, the study may prove more valuable if it is performed with the shoulder abducted and externally rotated (ABER view). This may provide clues as to the contribution of the bone defect when the arm is in the position of instability.
Indications and Contraindications
Patients with large osseous defects of the glenoid and humeral head who experience recurrent episodes of instability after anterior capsulolabral reconstruction require a secondary reconstruction procedure. Chronic dislocations and older patients who place less significant demands on the shoulder can probably be treated by more traditional methods (coracoid transfer, capsular shift, subscapularis advancement, or arthroplasty). Throwing athletes and younger patients with significant overhead demands will not tolerate such restricted motion and are ideal candidates for anatomic osteoarticular reconstruction of the glenoid or humeral head as indicated.
Diagnostic arthroscopy provides the most accurate assessment of which lesions will need to be addressed surgically. Significant osseous defects effectively shorten the normal “safe arc” of motion. In a position of athletic function, the humeral head translates anteriorly. With extensive glenoid erosion, the head will “run out” of glenoid and fall off or dislocate. [3] [9] [11] [21] [28] Likewise, with large Hill-Sachs lesions, the glenoid will “drop into” the humeral head defect in a similar position when the safe arc of motion is exceeded, causing an abrupt and unsettling sense of instability.[3]
Parameters are being defined to identify those patients who can benefit from osteoarticular reconstruction early at the time of the index procedure. The goal is to avoid foreseeable failure after soft tissue reconstruction and ultimately to spare the patient the repetitive trauma of recurrent instability and the need for revision surgery. Rowe[25] originally described glenoid lesions as significant if they involve 30% of the articular surface. Burkhart and De Beer[4] showed an exceptionally high likelihood of recurrent instability when the anterior-to-posterior glenoid diameter is less below the midglenoid notch than above it (“inverted pear”). Unfortunately, this method of assessment requires information obtained at the time of diagnostic arthroscopy. On the basis of the results of a biomechanical study, Gerber and Nyffeler[11] found that anteroinferior glenoid defects with a total length greater than half the maximum anterior to posterior diameter decreased the resistance to dislocation by 30%. Therefore, they and others have recommended reconstruction for defects of this size. [11] [30]
Hill-Sachs lesions that can be seen arthroscopically to engage the anterior glenoid rim in a position of function should be addressed. Conventional guidelines suggest that lesions greater than 25% be addressed, but biomechanical studies have shown that humeral head defects involving as little as 12.5% of the articular surface result in significant biomechanical changes across the glenohumeral joint. [3] [9] [28]
Contraindications to anatomic reconstruction include arthroscopic or radiographic evidence of advanced degenerative glenohumeral arthritis, avascular necrosis of the humeral head, unaddressed rotator cuff deficiency, and advanced age.
By use of CT or magnetic resonance imaging data, the size of the proximal humerus or glenoid allograft is determined. Fresh-frozen or cryopreserved size- and side-matched allograft is obtained from a certified tissue bank. A thorough discussion of potential risks associated with the use of allograft tissue is undertaken with the patient, and informed consent is obtained.
Osteoarticular Allograft Reconstruction of the Glenoid and Humeral Head
For the sake of brevity, we present combined anatomic allograft reconstruction of the glenoid and humeral head in this section. Isolated reconstruction of either defect can be performed in a similar fashion if it is warranted.
After general endotracheal anesthesia is induced, the patient is placed in the beach chair position with the head of the bed raised 30 degrees. A bump is placed under the medial border of the scapula, and the shoulder should be completely free to allow the maximal external rotation and extension that will be needed. An intrascalene block can be used at the discretion of the surgeon and anesthesiologist. The patient is prepared in the usual sterile fashion with the arm draped free.
Surgical Landmarks, Incisions, and Portals
| • | Anterior, lateral, and posterior borders of the acromion | |
| • | Coracoid process | |
| • | Distal clavicle | |
| • | Acromioclavicular joint | |
| • | Deltopectoral groove |
| • | Posterior portal | |
| • | Anterior portal | |
| • | Deltopectoral approach to glenohumeral joint |
Examination Under Anesthesia and Diagnostic Arthroscopy
Examination under anesthesia is performed, and findings are compared with the contralateral limb with specific attention to degree and position of instability. Instability should be graded anteriorly, posteriorly, and inferiorly and compared with the unaffected side.
Diagnostic arthroscopy is extremely useful in this setting. Soft tissue and chondral injury can be assessed, and the size and position of the glenoid or humeral head defect can be thoroughly inspected. In the most common clinical scenario, the surgeon may have underestimated the size of the defect. As a result, appropriate consent will not have been signed and allograft tissue will not be available. Therefore, it is not uncommon for diagnostic arthroscopy to be performed alone before the definitive procedure.
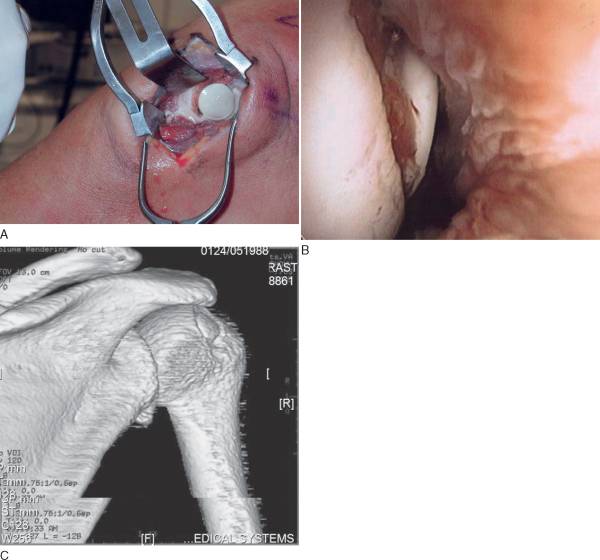
A 6- to 10-cm skin incision is made in line with the deltopectoral groove from the tip of the coracoid directed distally. Subcutaneous tissue is dissected sharply with meticulous hemostasis down to the level of the deltopectoral fascia. The deltopectoral interval is identified and developed, retracting the pectoralis major medially and the deltoid laterally. The cephalic vein can be taken medially or laterally, depending on the situation. If it is traumatized, the vein should be tied off before proceeding with deep dissection. The lateral border of the conjoint tendon is identified and retracted medially. The subscapularis tendon is then tagged with stay sutures and incised perpendicular to its fibers approximately 5 mm from its insertion point on the lesser tuberosity, ensuring that adequate tissue is left for later repair. When the subscapularis tendon is incised, it is important to externally rotate the humerus to minimize the risk of iatrogenic injury to the axillary nerve. A lateral humeral-based capsulotomy incision is made, again leaving a small cuff of tissue for later repair (
Fig. 14-1
).
|
|
|
|
Figure 14-1 |
2. Preparation of the Recipient Humeral Head Site
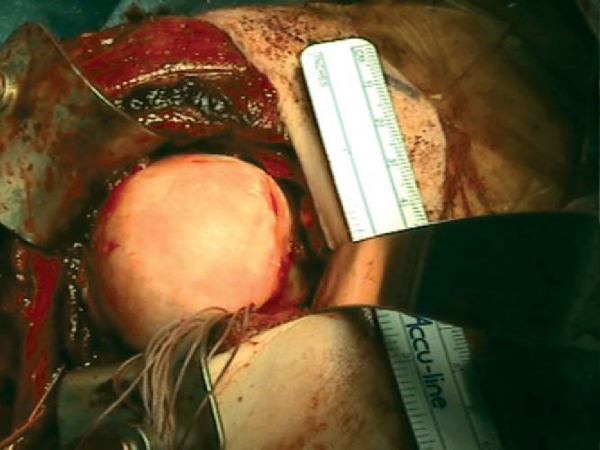
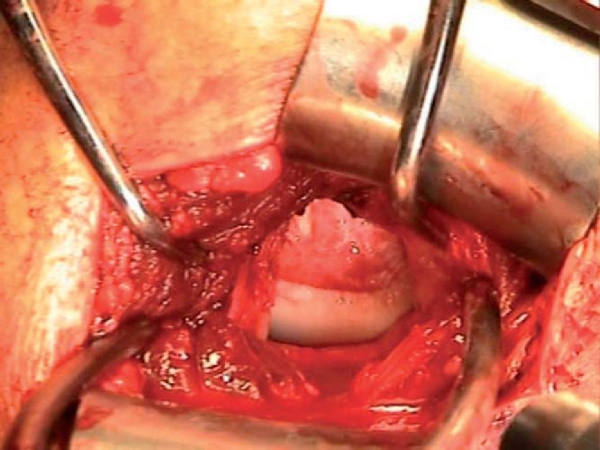
The humerus is maximally externally rotated and extended, exposing the Hill-Sachs lesion. Once it is exposed, a microsagittal saw or high-speed bur is used to reshape the defect and to make a bed of bleeding subchondral bone. Ultimately, a wedge-shaped defect needs to be made that can be press fitted with the allograft wedge. The final dimensions of the finished defect are measured with respect to length, width, and height and recorded.
The corresponding anatomic quadrant of the humeral head is identified and marked to best conform to the dimensions of the recipient defect (
Fig. 14-2
). A wedge is then cut from the allograft humeral head approximately 2 to 3 mm larger in all dimensions than the measured recipient defect (
Fig. 14-3
). It is best to err on the side of making too large an allograft plug as little can be done if the piece is too small. The allograft is then provisionally tried in the Hill-Sachs defect. Final adjustments in length, height, or width of the graft are made with a high-speed bur or microsagittal saw (
Fig. 14-4
). This is done in one dimension at a time with careful attention to detail, realizing that adjustments in one plane will affect the final size of the graft in all dimensions.
|
|
|
|
Figure 14-2 |
|
|
|
|
Figure 14-3 |
|
|
|
|
Figure 14-4 |
Once the surgeon is satisfied with the final size and configuration, the allograft is seated in the recipient defect and provisionally held with two 0.045 Kirschner wires (
Fig. 14-5
). The wires are then sequentially replaced with headless, variable pitch compression screws. Either way, it is absolutely essential that the screw heads be countersunk below the surface of the adjacent articular cartilage (
Fig. 14-6
).
|
|
|
|
Figure 14-5 |
|
|
|
|
Figure 14-6 |
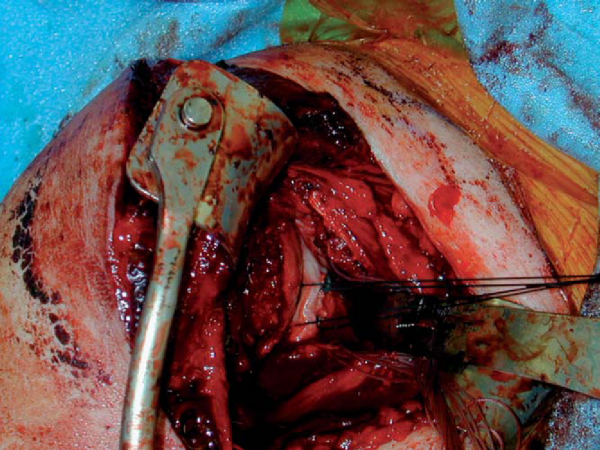
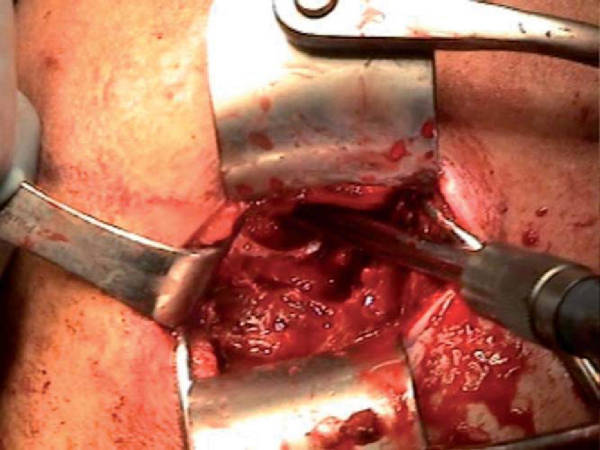
5. Preparation of the Glenoid Rim
The humeral head is retracted posteriorly with a humeral retractor (Fukuda), and the glenoid rim is exposed. A curved osteotome is used to subperiosteally strip the soft tissues medially off of the anteroinferior glenoid rim. The full extent of the anteroinferior glenoid rim defect can then be appreciated; a direct measurement is taken and correlated with the preoperative CT scan estimate of the defect. The glenoid defect is then prepared with a high-speed bur to make a surface of fresh bleeding bone.
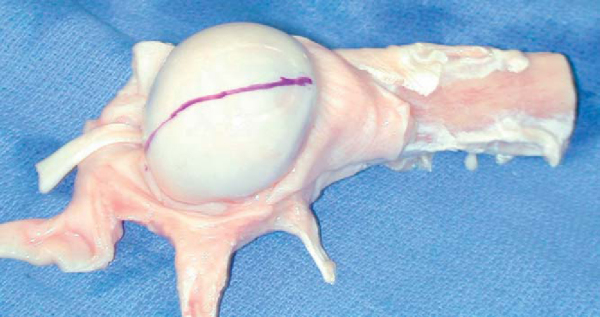
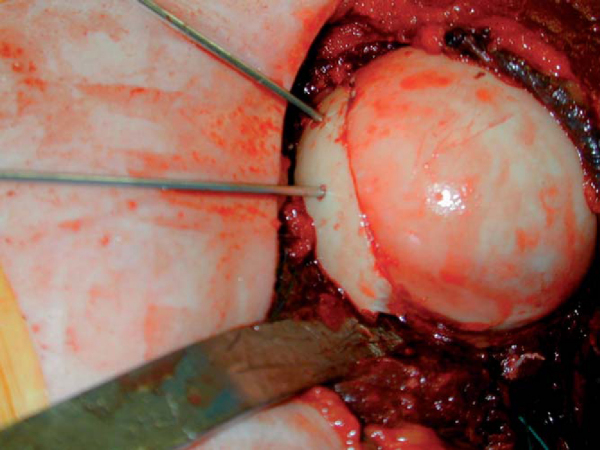
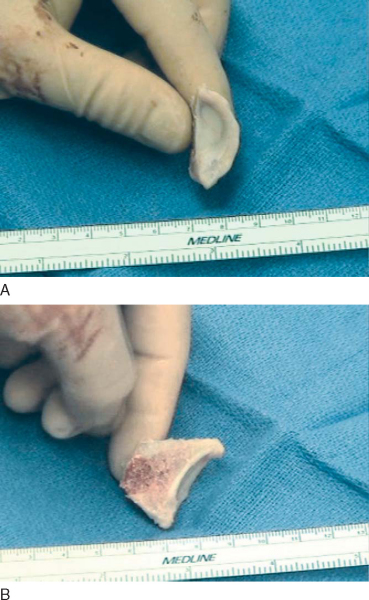
The appropriately size- and side-matched allograft glenoid or scapula with intact soft tissue attachments is obtained (
Fig. 14-7
). The in vivo measurement of the defect is compared with preoperative CT estimates of the glenoid rim defect. In the same anteroinferior position on the allograft glenoid, a piece is cut at least 2 or 3 mm larger than desired (
Fig. 14-8
). The glenoid is tried in the desired position and trimmed with a high-speed bur as necessary (
Fig. 14-9
). It is technically easier to perform subsequent capsulolabral repair if suture is provisionally passed through the allograft before implantation. We prefer to do so with two No. 0 Ethibond sutures (
Fig. 14-10
).
|
|
|
|
Figure 14-7 |
|
|
|
|
Figure 14-8 |
|
|
|
|
Figure 14-10 |
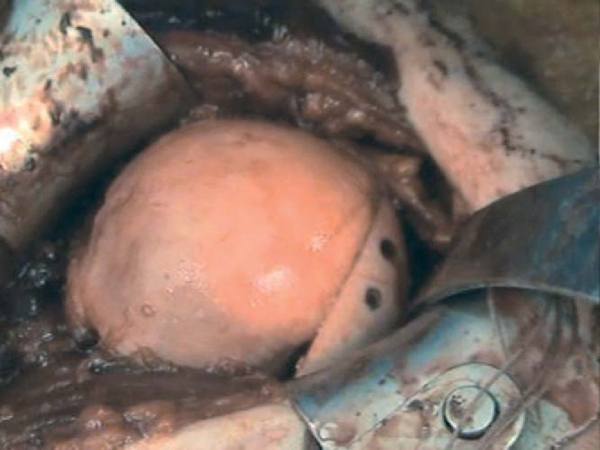
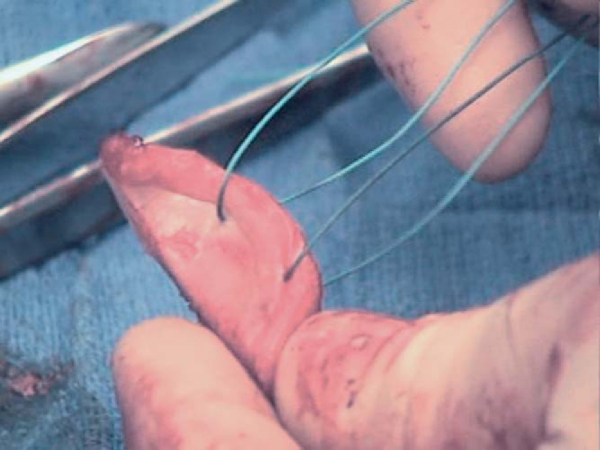
The prepared allograft is then placed in the appropriate anatomic position. When the surgeon is satisfied, it is held provisionally with two 0.045 Kirschner wires, which are sequentially exchanged for two AO 4.0 partially threaded cortical screws (
Fig. 14-11
).
The glenohumeral joint is reduced, and capsular redundancy is addressed as necessary by capsular shift. The previously passed Ethibond sutures in the glenoid allograft are passed through the intact capsule in a horizontal mattress fashion and tied on the outside of the capsule. This anchors the glenohumeral capsule to the newly secured glenoid allograft. The humeral-based shift is then completed in a standard fashion, with the arm placed in 30 degrees of abduction and external rotation. Often mere placement of the allograft will greatly reduce the size of the infraglenoid recess as the void has now been filled by graft.
The wound is then copiously irrigated with antibiotic solution. The shoulder is taken through a complete range of motion to ensure smooth articulation between the native humeral head, allograft, and glenoid. The joint capsule is closed with absorbable suture, and the subscapularis is repaired anatomically back to a cuff of tissue with a nonabsorbable suture. The conjoint tendon and deltopectoral interval are allowed to fall back into their native position. A 2-0 absorbable subcutaneous stitch and running 4-0 absorbable subcuticular suture are used to approximate the skin.
Immobilization, Followup, and Rehabilitation
The patient is placed immediately into a shoulder immobilizer. At the first postoperative visit, 6 to 8 days later, the wound is inspected and the patient is allowed to begin pendulum exercises only. At 1 month, active and passive range of motion is initiated under the guidance of an experienced physical therapist. Strengthening begins at 4 to 6 months. The patient is generally not cleared for return to sport or strenuous overhead activity until at least 7 to 12 months after the date of surgery.
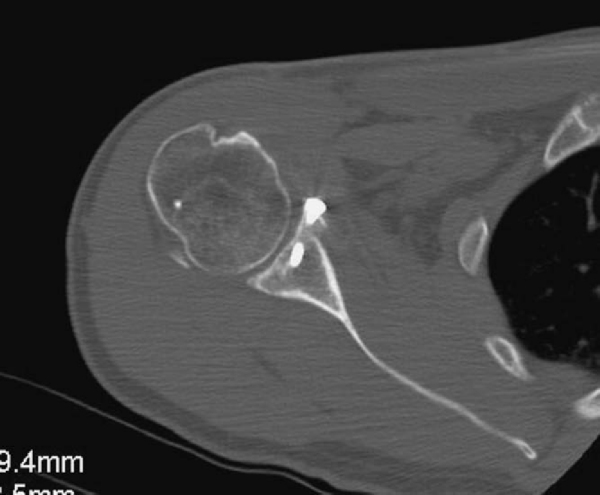
Radiographs are taken at 2, 6, 12, and 24 weeks postoperatively to ensure maintained position of the allograft. If it is clinically warranted, a CT scan can be performed to ensure incorporation of the allograft (
Fig. 14-12
).
|
|
|
|
Figure 14-12 |
Osteoarticular Allograft Reconstruction of Humeral Head Defects
The method of anatomic allograft reconstruction of the humeral head described in the preceding section requires an extensive deltopectoral approach and maximal external rotation of the humerus to present the Hill-Sachs lesion into the working field. If allograft reconstruction of the glenoid rim is planned concomitantly, such an approach is logical. However, a second population of patients exists—those with only a soft tissue Bankart lesion and an associated Hill-Sachs lesion. These patients will often be treated by open or arthroscopic Bankart repair yet remain symptomatic secondary to engagement of a large humeral head defect.[4] If the anterior soft tissue reconstruction is intact or if it is desired to perform an arthroscopic anterior capsulolabral repair, we believe that the humeral head defect can best be addressed through a limited posterior approach to the humeral head and osteochondral allograft transplantation.[22]
After general anesthesia is induced, an examination of the affected extremity is performed and findings are compared with the contralateral side. The patient is placed in the lateral decubitus position with the arm held in 10 pounds of traction. Diagnostic arthroscopy is performed and pathologic change is noted, confirming the size and location of the humeral head defect. If the glenoid does not show significant erosion, we choose to address the labral injury and capsular laxity arthroscopically. An anterior working portal is established by needle localization; arthroscopic labral repair and capsular shift by multi-pleated plication technique are then performed through a single working portal.[19]
Specific Steps (
Box 14-1
)
A skin incision is made from the posterolateral corner of the acromion directed distally in line with the deltoid fibers for approximately 6 cm (
Fig. 14-13
). Superficial dissection is carried down to the deltoid fascia and split in line with its fibers to the level of the upper border of the teres minor. The infraspinatus is split at the level of its tendinous raphe and retracted superiorly and inferiorly to expose the posterior joint capsule. Careful attention is given to protect the axillary nerve inferiorly. A vertical capsulotomy incision is made. The Hill-Sachs lesion can be easily visualized in its superior posterior position without the need to excessively rotate the humerus (
Fig. 14-14
).
| Surgical Steps | ||||||||||||
|
|
|
|
|
Figure 14-13 |
|
|
|
|
Figure 14-14 |
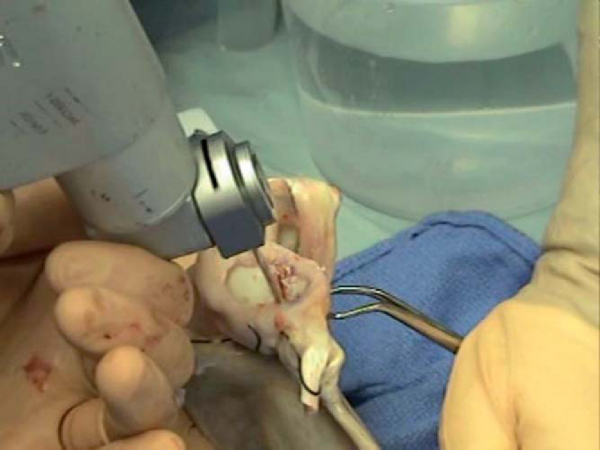
2. Preparation of the Recipient Bed
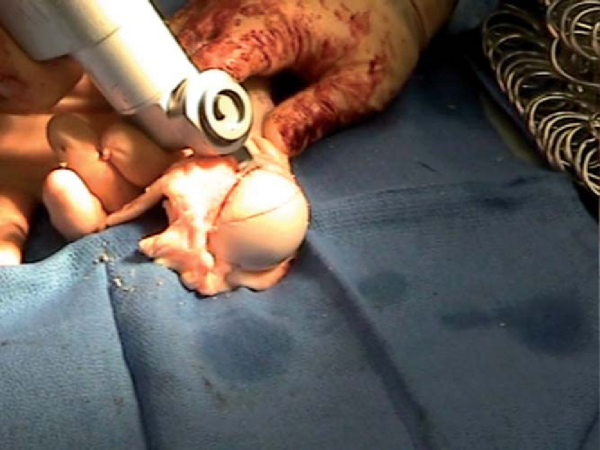
Once the humeral head is exposed, osteoarticular allograft transplantation is performed by use of the allograft Osteoarticular Transplantation System (OATS; Arthrex, Naples, Fla). A cannulated sizing guide is placed to size the lesion in a posterior inferior location, which will prevent “engagement” with the glenoid when it is filled with a single large allograft plug. The plug functionally serves as a block to engagement, so it is not necessary to completely fill the defect. While the sizer is held in the desired position, a drill tip guide pin is fired through the center, and orientation is marked. A calibrated cutting blade is passed over the guide wire, and the recipient site defect is reamed until a bleeding bed of subchondral bone is encountered (
Fig. 14-15
). The recipient socket has been made and should be measured in four quadrants.
|
|
|
|
Figure 14-15 |
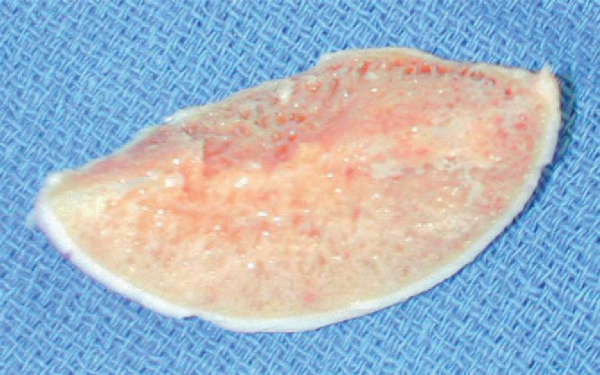
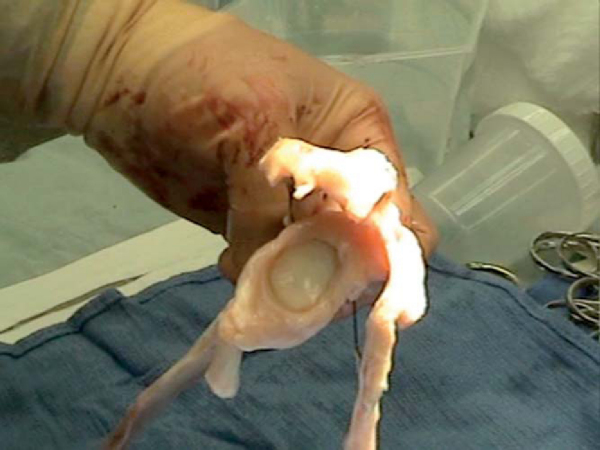
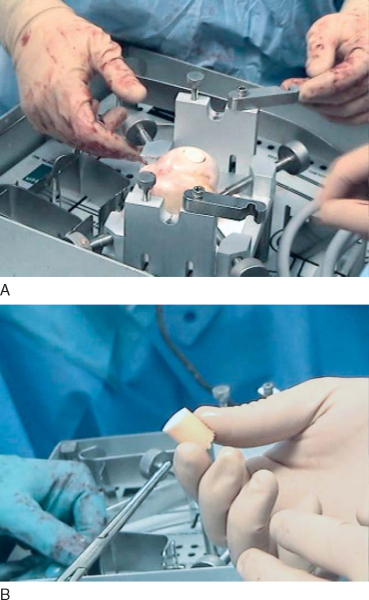
3. Preparation of the Allograft Plug
The allograft humeral head is secured in the allograft OATS workstation. The same sizer previously used to measure the Hill-Sachs lesion is placed over the corresponding location on the allograft and circumferentially marked, noting orientation. When the surgeon is satisfied with positioning, the donor harvesting drill is drilled through the entire length of the graft. It is important to drill the entire length of the allograft humeral head as it is relatively simple to trim the graft, but little can be done if the graft is too short. The harvested allograft is then measured and trimmed with an oscillating saw to mirror the depth of the defect (
Fig. 14-16
).
|
|
|
|
Figure 14-16 |
The allograft plug is then press fitted in the prepared socket and gently tapped into position until all edges are flush with the surrounding native cartilage rim (
Fig. 14-17
). No fixation is required. The posterior capsule is closed with absorbable suture, and subcutaneous tissue and skin are closed in a routine fashion.
|
|
|
|
Figure 14-17 |
Immobilization, Followup, and Rehabilitation
The shoulder is placed in a sling, and the sling is worn at all times including sleep. Pendulum exercises begin at 1 week. Passive and active-assisted range of motion begins at 1 to 2 months, avoiding stress on the posterior capsule (no adduction or internal rotation). The sling is discontinued at 2 months. Range of motion is gradually liberalized, and if the patient has full motion at 4 to 5 months, shoulder and periscapular strengthening is initiated. A functional training or throwing program begins at 6 to 7 months. Return to sport or full work duty is allowed when full functional pain-free range of motion and reasonable strength are achieved.
To date, long-term followup is not available for these treatment protocols, making prediction of potential complications difficult. Theoretical complications are those of allograft or autograft procedures, including nonunion, failed incorporation of the graft, and hardware failure. Long-term prospective data are needed to determine the rate of progression to degenerative arthritis.
Published reports of osteoarticular allograft reconstruction of osseous defects of the glenohumeral joint are limited. Most are case reports or small case series. The best followup data range from 2 to 5 years. Still, early biomechanical and clinical results are promising. To date, recurrent instability that follows these reconstruction procedures has not been reported. Satisfaction rates of patients and functional assessment scores are greatly improved and loss of motion is minimal compared with more historic procedures. Logic suggests that with greater stability, the progression of degenerative arthritis will be slowed, but more data and time are required to validate this claim. We believe that anatomic reconstruction of the humeral head and glenoid represents a viable alternative to Bristow and Latarjet procedures in the young, high-demand patient with a significant osseous lesion (Tables 14-1 and 14-2 [1] [2]).
| Author | Procedure | Followup | Outcome |
|---|---|---|---|
| Warner et al[31] (2006) | Iliac crest autograft | 33-month mean | No recurrence (N = 11) |
| 7-degree loss of flexion | |||
| 14-degree loss of external rotation | |||
| Hutchinson et al[19] (1995) | Iliac crest autograft (N = 6) | 32-month mean | No recurrence |
| Femoral head allograft (N = 9) | Average motion loss: 16 to 26 degrees of external rotation | ||
| Haaker et al[14] (1993) | Iliac crest autograft | 6 to 42 months | No recurrence (N = 24) |
| 10-degree loss of external rotation | |||
| Scheibel et al[27] (2004) | Anatomic screw fixation of large bony Bankart lesion | 30-month mean | No recurrence (N = 10) |
| 12-degree loss of external rotation |
| Author | Procedure | Followup | Outcome |
|---|---|---|---|
| Gerber[10] (1997) | Chronic locked anterior dislocation; large anatomic graft | 4 patients | No recurrence |
| Forward flexion of 145 degrees | |||
| Miniaci and Gish[23] (2004) | Large anatomic allograft | Average of 50 months | No recurrence (N = 18) |
| 2 of 18 required later hardware removal | |||
| Chapovsky and Kelly[6] (2005) | Arthroscopic mosaicplasty | Case report (1 year) | No recurrence |
| Return to sport (basketball) | |||
| Kropf and Sekiya[22] (2006) | Humeral head osteochondral allograft transplantation | Case report (1 year) | No recurrence |
| Return to active military duty |
1.
Allain J, Goutallier D, Glorion C: Long-term results of the Latarjet procedure for the treatment of anterior instability of the shoulder.
J Bone Joint Surg Am 1998; 80:841-852.
2.
Bacilla P, Field LD, Savoie FH: Arthroscopic Bankart repair in a high demand athletic population.
Arthroscopy 1997; 13:51-60.
3.
Burkhart SS, Danaceau SM: Articular arc length mismatch as a cause of failed Bankart repair.
Arthroscopy 2000; 16:740-744.
4.
Burkhart SS, De Beer JF: Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion.
Arthroscopy 2000; 16:677-694.
5.
Calandra JJ, Baker CL, Uribe J: The incidence of Hill-Sachs lesions in initial anterior shoulder dislocations.
Arthroscopy 1989; 5:254-257.
6.
Chapovsky F, Kelly JD: Osteochondral allograft transplantation for treatment of glenohumeral instability.
Arthroscopy 2005; 21:1007.
7.
Cole BJ, L’Insalata J, Irrgang J, Warner JJP: Comparison of arthroscopic and open anterior shoulder stabilization: a two to six-year followup study.
J Bone Joint Surg Am 2000; 82:1108-1114.
8.
Connolly JF: Humeral head defects associated with shoulder dislocations—their diagnostic and surgical significance.
AAOS Instr Course Lect 1972; 21:42-54.
9.
Flatow EL, Warner JJP: Instability of the shoulder: complex problems and failed repairs.
J Bone Joint Surg Am 1998; 80:122-140.
10.
Gerber C: Chronic locked anterior and posterior dislocations.
In: Warner JJP, Ianotti JP, Flatow EL, ed. Complex Revision Problems in Shoulder Surgery,
2nd ed.. Philadelphia: Lippincott; 2005:89-103.
11.
Gerber C, Nyffeler RW: Classification of glenohumeral joint instability.
Clin Orthop 2002; 400:65-76.
12.
Grana WA, Buckley PD, Yates CK: Arthroscopic Bankart suture repair.
Am J Sports Med 1993; 21:348-353.
13.
Green MR, Christensen KP: Arthroscopic Bankart procedure: two to five year followup with clinical correlation to severity of glenoid labral lesion.
Am J Sports Med 1995; 23:276-281.
14.
Haaker RG, Eickhoff U, Klammer HL: Intraarticular autogenous bone grafting in recurrent shoulder dislocations.
Mil Med 1993; 158:164-169.
15.
Hawkins RJ, Angelo RL: Glenohumeral osteoarthritis: a late complication of the Putti-Platt repair.
J Bone Joint Surg Am 1990; 72:1193-1197.
16.
Helfet AJ: Coracoid transplantation for recurring dislocation of the shoulder.
J Bone Joint Surg Br 1958; 40:198-202.
17.
Hill HA, Sachs MD: The groove defect of the humeral head. A frequently unrecognized complication of dislocations of the shoulder joint.
Radiology 1940; 35:690-700.
18.
Hovelius L: Anterior dislocation of the shoulder in teenagers and young adults.
J Bone Joint Surg Am 1987; 69:393-399.
19.
Hutchinson JW, Neumann L, Wallace WA: Bone buttress operation for recurrent anterior shoulder dislocation in epilepsy.
J Bone Joint Surg Br 1995; 77:928-932.
20.
Itoi E, Lee SB, Amrami KK, et al: Quantitative assessment of classic anteroinferior bony Bankart lesions by radiography and computed tomography.
Am J Sports Med 2003; 31:112-118.
21.
Itoi E, Lee SB, Berglund LJ, et al: The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study.
J Bone Joint Surg Am 2000; 82:35-46.
22.
Kropf EJ, Sekiya JK: Osteoarticular allograft transplantation for large humeral head defects in glenohumeral instability.
Arthroscopy 2007; 23(3):322.e1-322.e5.
23.
Miniaci A, Gish MW: Management of anterior glenohumeral instability associated with large Hill-Sachs defects.
Tech Shoulder Elbow Surg 2004; 5:170-175.
24.
Neer CS, Foster CR: Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder. A preliminary report.
J Bone Joint Surg Am 1980; 62:897-908.
25.
Rowe CR, Patel D, Southmayd WW: The Bankart procedure; a long-term end-result study.
J Bone Joint Surg Am 1978; 60:1-16.
26.
Rowe CR, Zarins B, Ciullo JV: Recurrent anterior dislocation of the shoulder after surgical repair.
J Bone Joint Surg Am 1984; 66:159-168.
27.
Scheibel M, Magosch P, Lichtenberg S, Habermeyer P: Open reconstruction of anterior glenoid rim fractures.
Knee Surg Sports Traumatol Arthrosc 2004; 12:568-573.
28.
Sekiya JK, Wickwire AC, Stehle, JH, Debski RE. Biomechanical analysis of Hill-Sachs lesions in a joint compression model: injury and repair using articular allograft transplantation. Arthroscopy Association of North America, unpublished data, 2006.
29.
Simonet WT, Cofield RH: Prognosis in anterior shoulder dislocation.
Am J Sports Med 1984; 12:19-24.
30.
Tjoumakaris FP, Knopf EJ, Sekia JK: Osteoarticular allograft reconstruction of a large glenoid and humeral head defect in recurrent shoulder instability.
Tech shoulder Elbow Surg 2007; 8(2):98-104.
31.
Warner JP, Gill TJ, O’Holleran JD, et al: Anatomical glenoid reconstruction for recurrent anterior glenohumeral instability with glenoid deficiency using an autogenous tricortical iliac crest bone graft.
Am J Sports Med 2006; 34:205-212.
32.
Weber BG, Simpson LA, Hardegger F: Rotational humeral osteotomy for recurrent anterior dislocation of the shoulder associated with a large Hill-Sachs lesion.
J Bone Joint Surg Am 1984; 66:1443-1446.
33.
Young CD, Rockwood Jr CA: Complications of a failed Bristow procedure and their management.
J Bone Joint Surg Am 1991; 73:969-981.