ANGULAR DEFORMITIES OF THE LOWER EXTREMITIES IN CHILDREN
IX – PEDIATRIC DISORDERS > CHAPTER 169 – ANGULAR DEFORMITIES OF THE
LOWER EXTREMITIES IN CHILDREN
are common and are a frequent reason for orthopaedic referral. They
predominantly occur in the tibia; the femur is much less frequently
involved. Angulation may occur in the frontal plane (varus and valgus),
the sagittal plane (anterior and posterior), or a combination of both
(anterolateral or posteromedial). Torsion may also be involved. It is
important to understand the various physiologic and pathologic causes
of angular deformities, the methods of evaluation, and the natural
histories of the abnormalities to determine appropriate treatment (50,115,287).
The classification for the differential diagnoses of genu varum
(bowleg), genu valgum (knock-knee), and congenital angular deformities
of the tibia and fibula are presented in Table 169.1, Table 169.2 and Table 169.3.
 |
|
Table 169.1. Classification of Genu Varum or Bowleg Deformities of the Lower Extremities in Children
|
 |
|
Table 169.2. Classification of Genu Valgum or Knock-knee Deformities of the Lower Extremities in Children
|
 |
|
Table 169.3. Differential Diagnosis of Congenital Angular Deformities of the Tibia and Fibula
|
common finding in infants and young children. It is the result of
molding of the lower extremities in utero.
The bowed appearance of the lower extremities is actually a combination
of external or lateral rotation of the hip (tight posterior capsule)
and internal or medial tibial torsion. This physiologic genu varum
tends to persist during the first year of life with only minimal
improvement. After a child begins to walk, the bowing corrects
spontaneously.
Complete correction may require up to 36 months of ambulation.
This is true genu valgum, not the result of a torsional combination
from in utero positioning. This deformity
also undergoes spontaneous correction with normal adult knee alignment
of mild genu valgum obtained by 5–8 years of age. Cahuzac et al. (55)
demonstrated that girls have a consistent genu valgum alignment by 10
years of age that remains constant as they finish musculoskeletal
growth. Boys, however, tend to have a decreasing valgus alignment until
approximately 16 years of age. Thus, men have less valgus at maturity
than do women.
They found a mean varus alignment of 15° in newborns. This decreased to
approximately 10° of varus alignment by age 1 year. Neutral alignment
occurred between 18 and 20
months
of age. The maximum valgus of approximately 12° was achieved by 3–4
years of age. The results were similar for boys and girls. By age 7
years, the children’s valgus alignments had corrected to those of
normal adults (8° in women, 7° in men). The researchers estimated that
in approximately 95% of the children physiologic genu varum or valgum
alignments resolved spontaneously with growth. In a follow-up study of
20 children between 1 and 4 years of age with pronounced physiologic
varus (16° to 33°) or valgus (15° to 20°) deformities of the knees,
Vankka and Salenius (279)
found that even these pronounced deformities resolved during growth,
although some did not completely correct until adolescence. They
recommended that surgical correction be cautiously considered for
children between 10 and 13 years of age, when corrective osteotomies
are usually performed.
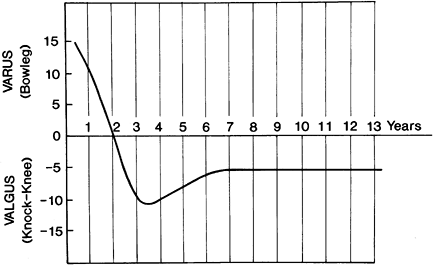 |
|
Figure 169.1.
Normal development of knee alignment from infancy through childhood. (Adapted from Salenius P, Vankka E. The Development of the Tibio-femoral Angle in Children. J Bone Joint Surg Am 1975;57:259.) |
and one of the most common causes of parental concern. In the majority
of cases, it will be physiologic in origin and will correct with normal
growth and development. However, there are pathologic genu varum
disorders that may progress and produce functional impairment (Table 169.1).
careful history and physical examination. The history will frequently
distinguish physiologic from pathologic genu varum. Obtain a birth
history, family history, the age at which developmental milestones
occurred, a nutritional history, and the previous percentiles for
height and weight. A family history of short stature or varus alignment
or progression of the deformity may indicate a pathologic process.
determine the percentile for age. Shortening of the extremities
relative to the trunk may indicate a skeletal dysplasia. In ambulatory
children, the appearance to the lower extremities during standing and
gait can provide important information. Determine the location of the
deformity, as well as whether there is a lateral knee thrust while
walking. Measure the range of motion of the hips, knees, and ankles.
Assess the presence of ligamentous laxity. Measure the degree of genu
varum in the standing and the supine positions. Measure and record the
distance between the medial femoral condyles in centimeters. In
addition, measure the torsional profile as described by Mosca and
Staheli (see Chapter 168). This includes the
foot progression angle, hip range of motion in extension, the
thigh–foot angle, and the shape of the foot. Torsional changes in the
femur and tibia are common in angular deformities of the lower
extremities. Obtain serial photographs, if possible, and place them in
the child’s chart as an aid in documentation of improvement or
worsening over time.
However, if the child is short, the deformity is asymmetric, there is a
history of progression, or the child is older than 3 years, obtain
radiographs consisting of a standing anteroposterior (AP) projection of
the lower extremities, including the hips, knees, and ankles. Position
the patellae pointing forward. Measure the femoro-tibial angle, the
mechanical axis, and the metaphyseal–diaphyseal angles. Assess the
physes of the femur and tibia, especially those about the knee.
Metaphyseal and physeal widening suggest an underlying metabolic
disorder.
evaluation then provide the basis for an accurate assessment of whether
the child has a physiologic or pathologic genu varum deformity. The
specifics of further evaluation and treatment are based on the
diagnosis.
positioning is a common finding in children between birth and 2 years
of age. It is usually associated with a toe-in gait due to medial
tibial torsion.
appear bowed. However, physical examination demonstrates excessive
lateral rotation of the extended hip and medial tibial torsion (Fig. 169.2).
Contracture of the posterior capsule is a normal finding in children up
to 1 year of age. It tends to improve during the first 3 years of life,
and ultimately medial rotation slightly exceeds lateral rotation (222).
Medial tibial torsion is the major component of physiologic genu varum.
The knees are normal, except for possibly a slight residual
knee-flexion contracture. A lateral knee thrust during gait is uncommon
and indicates a pathologic genu varum deformity (161,163).
The degree of varus can be measured by a goniometer (femoro-tibial
angle) or the distance between the medial femoral condyles (55,65,126).
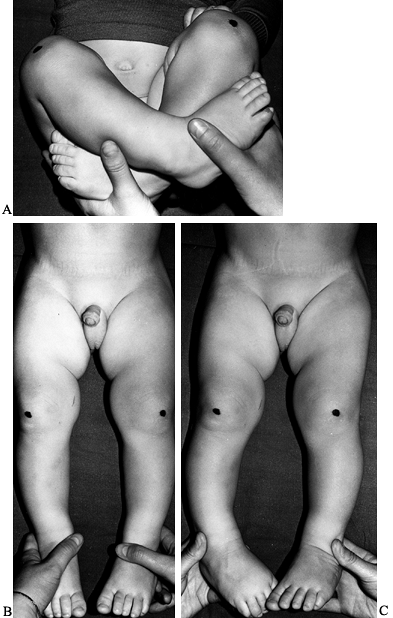 |
|
Figure 169.2. A: Physical examination of a 9-month-old boy with physiologic genu varum. The infant may still comfortably assume the in utero
position with the hips flexed, abducted, and laterally rotated. The knees are flexed, with the lower legs and feet medially rotated. This position results in a hip flexion contracture, a contracture of the posterior aspect of the hip capsule, knee flexion contracture, and medial tibial torsion. B: When the lower extremities are extended, the posterior hip capsule contracture results in increased lateral rotation (80° to 90°) and limited medial rotation (0° to 10°). When the patellae point laterally, the medial tibial torsion is not readily apparent. C: When the hips are maximally rotated medially and the patellae are directed anteriorly, the medial tibial torsion is more apparent. The medial tibial torsion can be measured by the thigh–foot angle or the transmalleolar axis. The medial tibial torsion may also produce in-toeing during ambulation. This can be assessed by measuring the foot progression angle. |
between physiologic genu varum and tibia vara (Blount’s disease) in
children younger than 3 years of age. Levine and Drennan (176) developed the metaphyseal–diaphyseal angle to aid in differentiating these two disorders (Fig. 169.3).
An angle of 11° or less indicates physiologic genu varum, and angles
greater than 11° suggest that progressive tibia vara is likely (Fig. 169.4). However, a later study by Feldman and Schoenecker (101)
indicated that angles greater than 16° are predictive of tibia vara,
whereas angles of 9° or less suggest physiologic genu varum and angles
between 10° and 15° are indeterminate. The metaphyseal–diaphyseal angle
has been shown to have good interobserver and intraobserver
reproducibility (104). However, it is important
that standing radiographs be obtained with the knees in a neutral
position, as rotational changes can alter measurements (265).
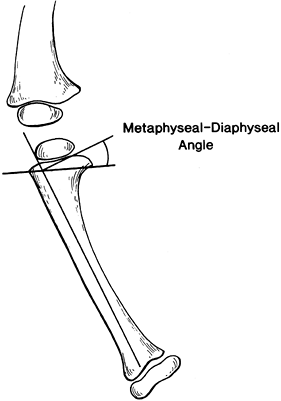 |
|
Figure 169.3.
Metaphyseal–diaphyseal angle. Draw a line between the radiographic corners of the medial and the lateral metaphyses of the proximal tibia, and another line parallel to the longitudinal axis of the tibial diaphysis. Then construct a line perpendicular to the diaphyseal line at the intersection of the metaphyseal and diaphyseal lines, and measure the angle between the right-angle line and the metaphyseal line. (Adapted from Levine AM, Drennan JC. Physiologic Bowing and Tibia Vara: The Metaphyseal–Diaphyseal Angle in the Measurement of Bowleg Deformities. J Bone Joint Surg Am 1982;64:1158.) |
 |
|
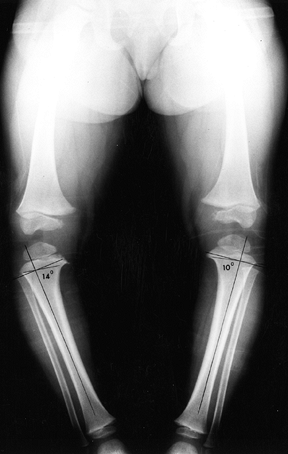
Figure 169.4.
Standing AP radiograph of an 18-month-old girl with asymmetric bowing of the lower extremities. The metaphyseal–diaphyseal angle is 14° on the right, indicating infantile tibia vara; it is 10° on the left, representing physiologic genu varum. There is already medial metaphyseal irregularity and beaking, as well as mild medial epiphyseal flattening on the right. |
calcium, phosphorus, and alkaline phosphatase levels. Obtain a
pediatric endocrinology evaluation to assist in diagnosis and
management.
Operative treatment is rarely indicated. Orthoses or corrective shoes
are not recommended because there is no evidence that they improve
alignment of the extremity. Follow infants and young children with
physiologic genu varum at 6-month intervals (Fig. 169.5). Recording accurate clinical measurements is useful in reassuring anxious parents that improvement is occurring.
 |
|
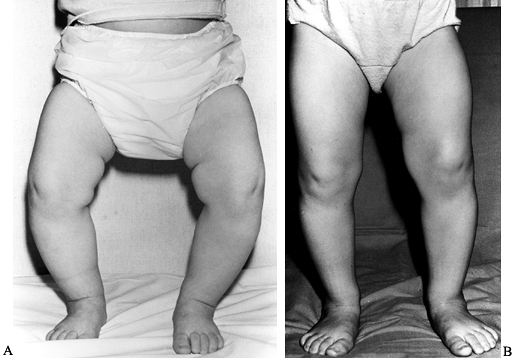
Figure 169.5. A:
A 16-month-old girl with physiologic genu varum. Observe the lateral rotation of the thighs and knees and the medial tibial torsion. B: One year later, with no treatment, there has been complete resolution of the physiologic genu varum. |
varum include a proximal tibial valgus derotation and diaphyseal
fibular osteotomies, proximal tibial hemiepiphyseal stapling, or
proximal tibial hemiepiphysiodesis. The latter two procedures are based
on adequate remaining growth to achieve complete correction (39). The graph developed by Bowen et al. (38) can be helpful in determining proper timing.
 |
|
Table 169.4. Surgical Options for Genu Varum Deformities
|
physiologic genu varum because it addresses both the varus and the
medial tibial torsion. A variety of techniques can be used, including
closing-wedge, opening-wedge, oblique, or dome osteotomies. These are
essentially the same procedures as for tibia vara or Blount’s disease.
A diaphyseal fibular osteotomy is performed concomitantly because the
fibula is usually too long and may be contributing to the deformity.
The technique of a closing-wedge proximal tibial and diaphyseal fibular
osteotomy is discussed in the section on tibia vara
(see below). Internal or external fixation to maintain alignment is
necessary and usually supplemented by a long-leg cast until complete
healing has occurred.
tibial epiphysis with staples is an effective method for correction of
persistent physiologic genu varum. If the deformity is severe or there
is limited remaining growth, a combined lateral stapling of the distal
femoral and the proximal tibial epiphyses may need to be performed.
This procedure will not correct any coexistent medial tibial torsion.
proximal tibial epiphysis can be effective in correcting persistent
physiologic genu varum in adolescents. The indications are essentially
the same as for stapling. However, once complete correction has been
achieved, a second procedure may be necessary on the medial side to
prevent overcorrection. The proximal fibular epiphysis is usually
closed concomitantly. This procedure will not correct any medial tibial
torsion.
valgus derotation and diaphyseal fibular osteotomies are managed
similar to patients undergoing the same procedure for tibia vara. In
children treated with a proximal tibial hemiepiphyseal stapling or
hemiepiphysiodesis, apply a knee immobilizer postoperatively for
approximately 2 weeks. This allows healing of the skin incision and
minimizes discomfort. Then begin active range of motion exercises, and
allow return to normal activities, typically at 4–6 weeks
postoperatively.
There is a risk for injury to the peroneal nerve as it passes around
the lateral aspect of the proximal fibula, and to the anterior tibial
artery as it passes into the anterior compartment through the hiatus
between the proximal tibia and fibula. Compartment syndromes have been
described, and a child must be carefully evaluated for the first 24–48
hours postoperatively. Perform prophylactic anterior compartment
fasciotomies at the time of surgery.
epiphysiodesis occur much less frequently. Physeal damage with
asymmetric closure and complete closure secondary to prolonged
compression are the most common problems but are fortunately rare.
that surgical intervention is necessary. There is a relationship
between this disorder and tibia vara (Blount’s disease). Persistent
varus deformity may progress to the latter disorder. It has been my
experience that the most common residual abnormality of physiologic
genu varum is persistent medial tibial torsion. This is a more common
indication for surgical treatment (see Chapter 168).
common pathologic genu varum deformity. It is characterized by abnormal
growth of the medial aspect of the proximal tibial epiphysis that
results in progressive varus angulation beneath the knee. This disorder
was first described by Erlacher (95) in 1922 and further analyzed by Blount (35) in 1937.
was initially classified into two broad groups, depending on the age at
clinical onset: infantile, with onset between 1 and 3 years of age; and
adolescent, with onset inconsistently described as occurring after 6–8
years of age or just before puberty (33,35,97,110,111,157,158,260). In 1984, Thompson et al. (275)
proposed a three-group classification based on the age at onset:
infantile (1–3 years), juvenile (4–10 years), and adolescent (11 years
or older). The juvenile and adolescent forms are commonly combined as
late-onset tibia vara. However, the incidence of recurrent deformity
after a corrective valgus osteotomy of the proximal tibia is much
higher in the juvenile group, justifying a three-group classification.
All three groups share relatively common clinical characteristics,
although the radiographic changes in the late-onset groups are less
pronounced. Although the exact cause of tibia vara remains unknown, it
appears to be secondary to growth suppression from increased
compressive forces across the medial aspect of the knee (22,29,33,35,59,60,69,111,157,168,171,172,274,275,286). Familial cases have been reported (23,111,173,250,253).
varus deformity. Infantile tibia vara can produce the greatest degree
of deformity because of the greater amount of growth time remaining. In
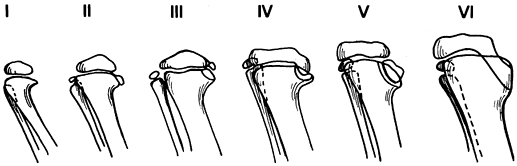
1952, Langenskiöld (172) described six stages
of progressive deformity in infantile tibia vara. Each grade advanced
the degree of physeal growth inhibition (Fig. 169.6).
It is possible to restore normal growth and development of the proximal
tibial physes in grades I and II and probable grades III and IV. Grades
V and VI represent severe damage to the medial proximal tibial physis
and probable premature or asymmetric closure. The rate of deformity in
grades V and VI is rapid, resulting in severe deformity and articular
malformation. There is relatively good interobserver agreement with the
use of this classification, especially for the early and late stages (264).
 |
|
Figure 169.6. Six grades of radiographic changes in infantile tibia vara as described by Langenskiöld (172). These represent a continuum of progressing deformity over time.
|
and late-onset (juvenile and adolescent) (29,46,129,167,274,275,286) forms of tibia vara shows similarities as well as distinct differences (Table 169.5). The infantile form is the most common (Fig. 169.7). However, the late-onset forms also occur frequently (Fig. 169.8). Anterior cruciate ligament incompetence may occur in severe deformities (28).
 |
|
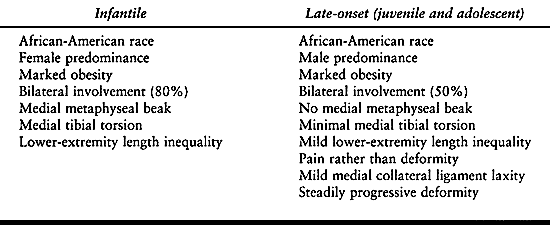
Table 169.5. Comparison of the Clinical Characteristics of Tibia Vara
|
 |
|
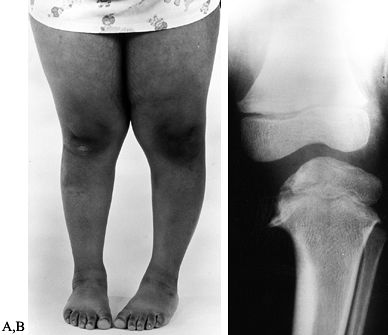
Figure 169.7. A:
Clinical photograph of a 5-year-old African-American girl with left infantile tibia vara. Observe the obesity, the unilateral left genu varum deformity, and the associated medial tibial torsion. B: Standing radiograph of the left knee demonstrates Langenskiöld grade III changes in the medial aspect of the proximal tibial epiphysis and metaphysis. Notice the metaphyseal beaking. |
 |
|
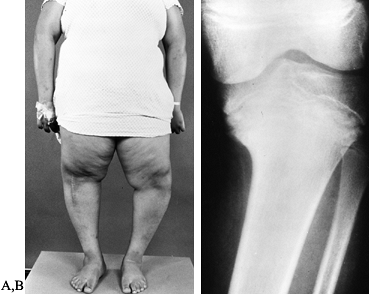
Figure 169.8. A:
Standing preoperative photograph of a 13-year-old African-American boy with bilateral adolescent or late-onset tibia vara. A previous proximal tibial osteotomy was performed on the right, producing only partial correction. Observe the marked obesity and the untreated left genu varum deformity and its medial tibial torsion. The patient subsequently underwent a laterally based closing-wedge proximal tibial osteotomy, including the physis, and a diaphyseal fibular osteotomy for correction of this deformity. B: On a standing AP radiograph of the left knee, the radiographic changes in adolescent tibia vara are less striking than in the infantile form. There is narrowing of the medial aspect of the proximal tibial epiphysis, physeal irregularity, and increased height of the lateral aspect of the epiphysis. |
deformity and beaking of the proximal medial tibial metaphysis are the
major features of infantile tibia vara (Fig. 169.7B) (54,116,151,260).
The changes in the proximal medial tibia are less conspicuous in the
late-onset forms and are characterized by wedging of the medial portion
of the epiphysis, a mild posteromedial articular depression, a
serpiginous cephalad-curved physis, and mild or no fragmentation or
beaking of the proximal medial metaphysis (Fig. 169.8B) (29,46,54,77,78,254,255 and 256,274,275,286).
The differences among the three tibia vara groups appear to be
primarily due to the age at onset, the amount of remaining growth, and
the magnitude of the medial compression forces on the involved side.
tibia vara is physiologic genu varum deformity. It is difficult to
differentiate these disorders in patients younger than 2 years.
Children with tibia vara are typically African-American and obese and
have a clinically apparent lateral thrust of the knee during the stance
phase of gait. The deformities are progressive and may be asymmetric.
Radiographic differentiation may be difficult. Standing radiographs of
the lower extremities in children younger than 18 months of age with
genu varum deformities are typically normal. However, normal knee
radiographs do not eliminate infantile tibia vara from consideration. A
metaphyseal–diaphyseal angle greater than 16° is an early
prognosticator of infantile tibia vara (Fig. 169.3) (101).
After 2–3 years of age, the radiographic characteristics become
apparent, and the condition can be classified according to the six
grades of Langenskiöld (172). The radiographic changes in late-onset forms of tibia vara are less dramatic but nevertheless diagnostic.
process for all three groups. Only a few biopsies of the proximal
medial tibial condyle have been obtained from patients with infantile
tibia vara (35,95,111,171,173).
Histopathologic abnormalities included islands of densely packed
chondrocytes exhibiting a greater degree of hypertrophy than would be
expected from their topographic position, areas of almost acellular
cartilage, and abnormal groups of capillaries. Langenskiöld (171) and Golding and McNeil-Smith (111), in studies of nine
and six biopsies, respectively, concluded that the abnormalities were
localized principally to the physes and that there was no evidence of
avascular necrosis.
and lateral aspects of the physes, although they are quantitatively
greater on the medial side. They are remarkably similar to the changes
observed in infantile tibia vara and in slipped capital femoral
epiphysis, suggesting a common cause (1,2). Lovejoy and Lovell (182) described two patients with late-onset tibia vara associated with slipped capital femoral epiphysis.
asymmetric compression and shear forces acting across the proximal
tibial physis result in suppression and deviation of normal
endochondral ossification, producing tibia vara. This concept, which
reflects the Heuter–Volkmann law, has been confirmed experimentally by
Arkin and Katz (13). Golding and McNeil-Smith (111)
concluded that children who had significant physiologic varus
deformity, walked early and stretched their knee ligaments. This
resulted in asymmetric compression with subsequent suppression of
posteromedial physeal growth and ultimate formation of an osseous
bridge, producing a permanent and progressive varus deformity. This
pathogenesis is consistent with Blount’s (33,35)
initial observations that infantile tibia vara is first recognized when
there is an increase of physiologic bowing during the first 3 years of
life. Langenskiöld (172,173)
emphasized that necrosis of the physeal cartilage is the principal
cause of growth disturbance, leading to varus deformity; he attributed
the abnormal cartilage to abnormal pressure or shear in overweight
children with physiologic bowleg. Others agreed that abnormal pressure
is probably the primary etiologic factor in infantile tibia vara (22,35,46,111,157,162).
minimal residual deformity after physiologic bowleg, rapid growth and
weight gain repetitively injure the posteromedial portion of the
proximal tibial physis, resulting in a cycle of varus-growth
suppression similar to the cycle described by Golding and McNeil-Smith (111) for the infantile form (127,129,274,275,286).
Progressive genu varum deformity is not due to an osseous bridge but is
caused by suppression of normal endochondral growth after repetitive
local injury (29,162,260,274,275,286). The concept of physeal growth suppression in tibia vara has been confirmed biomechanically by Cook et al. (69)
in a finite element analysis. As varus increases, the forces in the
proximal medial tibial physis increase. Obesity and mild varus (10°) in
older children create enough force to suppress growth. However,
Henderson and Green (127) reported a case of
late-onset tibia vara in an adolescent with previously documented
neutral mechanical alignment, suggesting that, at least in some cases,
preexisting varus alignment is not a prerequisite.
infantile tibia vara, begin treatment immediately. Orthotic management
may be considered for children 3 years of age or younger with
Langenskiöld’s grade II and possibly grade III involvement.
Approximately 50% to 65% of these in children can be corrected with an
orthosis (151,180,232,248).
Children with suspected infantile tibia vara with
metaphyseal–diaphyseal angles of 10° to 15° are more likely to benefit
from orthotic management (232). Use a
knee–ankle–foot orthosis with a single medial upright without a knee
hinge. Place pads, straps, or elastic webbing over the distal femur and
proximal tibia to apply a valgus force. The orthosis should be worn for
22–23 hours each day. Tighten the straps at 1–2-month intervals to
provide progressive correction. Obtain standing radiographs at 3-month
intervals to document correction of the tibia vara deformity. The
metaphyseal–diaphyseal angle should decrease (122).
After obtaining an absolute valgus mechanical axis, begin weaning from
the orthosis. Follow the child carefully thereafter to ensure
maintenance of correction.
currently recommended. If correction is not obtained after 1 year of
bracing, corrective osteotomy is indicated. Orthotic treatment is not
indicated after 3 years of age or for severe deformities. Bracing
children older than 3 years risks delaying performance of a corrective
osteotomy. Loder and Johnston (180) showed that
delay in performing corrective osteotomy, even by a few months, beyond
4 years of age risks failure to achieve lasting reversal of the physeal
inhibition of the proximal tibia. This predisposes a child to repeated
operative procedures to maintain a satisfactory result.
vara is contraindicated. The children are too large and the remaining
growth is too small to allow adequate
correction. Compliance with an orthosis in this age group is difficult to achieve.
tibia vara include age 4 years or less, failure of orthotic management,
and Langenskiöld grade III or higher. The possible procedures are
presented in Table 169.4. Proximal tibial
valgus osteotomy with fibular diaphyseal osteotomy is usually the
procedure of choice. Perform an anterior compartment fasciotomy
concomitantly. Tibial osteotomy techniques include closing-wedge,
opening-wedge, dome, and oblique osteotomies. The procedure selected
must correct the varus deformity and any associated medial tibial
torsion. Tibial length is usually not a problem in infantile tibia
vara. It is important that the selected osteotomy overcorrect the
mechanical axis of the knee to 5° or more of valgus. This ensures that
the supine correction obtained in the operating room is adequate.
Overcorrection compensates for the tendency of the knee to fall back
into varus after a patient resumes weight bearing because of the
depression in the posteromedial articular surface and the ligamentous
relaxation laterally. The goal is to transfer the line of weight
bearing to the lateral compartment of the knee. Schoenecker et al. (248) reported that correction within 5° of neutral usually proves satisfactory. However, others recommend overcorrection (168,173,180,229). Considering the physeal inhibition phenomena as proposed by Cook et al. (69), overcorrection to absolute valgus alignment is necessary to relieve the excessive compressive forces medially.
osteotomy of the proximal tibia for tibia vara. It is a single-plane
osteotomy that allows simultaneous correction of the varus and medial
tibial torsion deformities and permits postoperative cast wedging, if
necessary, to improve position. This ability to adjust the osteotomy
postoperatively is important because of the difficulty in achieving
satisfactory alignment intraoperatively.
those with Langenskiöld grade IV lesions or higher, a single osteotomy
of the proximal tibia is usually insufficient to restore normal
alignment and physeal growth. Langenskiöld grades IV and V act
effectively as medial physeal arrests. Possible procedures for these
children include the following (25,47,65,87,103,117,130,152,158,159,166,177,195,217,229,245,246,247 and 248,255,261,274,275):
surgical correction is necessary to restore the mechanical axis of the
knee. The same surgical options as those for older children with
infantile tibia vara are applicable in these groups. Correction to
physiologic genu valgum with careful preoperative biotrignometric
planning for the tibial osteotomy is the goal (57). Kline et al. (160)
demonstrated that distal femoral varus is a part of the deformity in
late-onset tibia vara. Evaluate this possibility, and perhaps consider
it in the treatment plan.
extension and with slight varus stress to ensure contact between the
medial femoral condyle and the posteromedial articular depression of
the proximal tibia. This technique can help minimize undercorrection of
the deformity. Aim to achieve at least 5° of valgus at the time of
osteotomy. The recurrence rate for the juvenile-onset group approaches
25% overall and is even higher in boys (274,275).
Evaluate all juvenile-onset patients with tomography or magnetic
resonance imaging (MRI) before surgery for evidence of premature
closure or impending closure of the proximal medial tibial physis. If
premature closure is not present, a simple closing-wedge metaphyseal
osteotomy or oblique proximal tibial osteotomy with correction to
physiologic valgus may be performed (229).
Correction using the Ilizarov ring fixation system and callotasis may
be applicable, especially if there is a significant lower-extremity
length discrepancy (227).
inhibition, then additional surgery is necessary; techniques include
physeal bridge resection and interposition graft, intraepiphyseal
osteotomy, elevation of the medial tibial plateau, and physeal excision
(117,152,174,177,185,245,248,255,289).
Internal or external fixation is usually necessary to maintain
alignment until satisfactory healing occurs. Physeal distraction with
an external fixator has also been used in Europe (86,88),
but it is not widely used. The procedure selected depends on the
patient’s age, the amount of growth remaining, and the severity of the
deformity. Proximal tibial physeal excision with proximal fibular
epiphysiodesis is usually recommended for recurrent deformities with
premature medial tibial physeal closure or for patients 12 years of age
or older (274). Healing is rapid and the
correction permanent. Measure any residual lower-extremity length
discrepancy with scanograms, and manage by contralateral
epiphysiodesis, when necessary.
results in nine children with late-onset tibia vara treated by
hemiepiphysiodesis of the lateral aspect of the proximal tibial
epiphysis. The average preoperative varus was 13° (range, 3° to 25°).
They
found a correction rate of 7° per year. Three patients required a
proximal tibial osteotomy because of incomplete correction. The authors
thought that hemiepiphysiodesis was an effective procedure with less
morbidity for managing varus deformities of the extremities of obese
children. Similar results can probably be anticipated with staples,
although they were not used in this study.
-
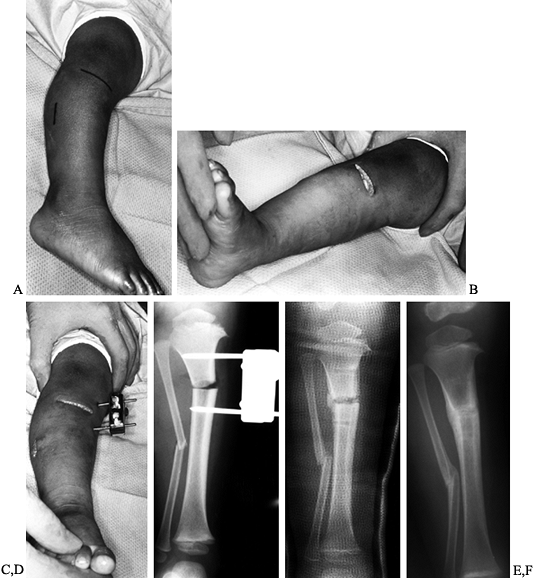
Make a 5 cm horizontal or transverse skin incision below the level of the tibial tubercle (Fig. 169.9).
![]() Figure 169.9. A:
Figure 169.9. A:
Intraoperative photograph shows the location and extent of the
incisions for a proximal tibial and diaphyseal fibular osteotomy. B:
After a transverse osteotomy of the proximal tibia, derotate the leg to
correct the associated medial tibial torsion. Remove an appropriate,
laterally based wedge to correct the residual varus deformity. C:
Insert a threaded Steinmann pin above and below the proximal tibial
osteotomy. Take care to ensure that the proximal pin is inferior and
posterior to the apophysis of the tibial tubercle. Secure the pins with
an external fixation clamp. D:
Postoperative radiograph after correction shows that there has been
some distraction at the osteotomy site. This is usually not a problem.
Ideally, the osteotomy should remain closed. The external fixation
clamp is incorporated into the cast, providing secure fixation. E: AP radiograph in a long leg cast shows that there is partial healing and the external fixation system has been removed. F:
AP radiograph obtained 3 months after surgery demonstrates satisfactory
healing of the osteotomy and correction of the tibia vara deformity.
The extremity is in approximately 5° to 7° of genu valgum. -
Expose the proximal tibia subperiosteally.
-
Release the fascia of the anterior
compartment. The fibers of the patellar tendon insertion are usually
visible in the proximal portion of the incision. -
Perform a fibular diaphyseal osteotomy
through a 3 cm vertical incision at the junction of the middle and
proximal thirds of the fibula. -
Identify the muscles of the lateral
compartment, and retract them anteriorly. Split the periosteum of the
fibula longitudinally, and reflect it circumferentially. -
Make an oblique osteotomy with a small oscillating saw.
-
With the fibular osteotomy completed,
proceed with the proximal tibial osteotomy. This may be a
closing-wedge, opening-wedge, or dome osteotomy. The procedure should
allow correction of the medial tibial torsion and the varus deformity.
I prefer a closing-wedge osteotomy. It is important to correct the
medial tibial torsion first and then perform the laterally based,
closing-wedge osteotomy. Excessive correction and unnecessary bone
excision may occur if the torsion is not corrected first. -
Fix the osteotomy with crossed Steinmann
pins, compression plate and screws, or an external fixation device. I
prefer the latter because the pins can be removed in the outclinic
without a separate operative procedure. -
Obtain intraoperative radiographs with
the knee in extension to confirm that approximately 5° of valgus
alignment have been obtained. -
After closure, immobilize the leg in a long-leg cast with the knee in extension and a slight valgus stress.
-
Make a transverse incision just beneath
the tibial tubercle. Make a Y-shaped incision in the periosteum, and
elevate it circumferentially -
Insert a small Steinmann pin at a
cephalad angle of approximately 45° 1 cm distal to the tibial tubercle,
and advance it under image-intensifier control until it passes through
the posterior cortex distal to the proximal tibial physis. The angle of
insertion determines the amount of correction for the varus and the
medial tibial torsion. Have a nomogram available to assist in
preoperatively determining the appropriate angle of guide-pin insertion. -
Carefully perform the osteotomy immediately beneath the Steinmann pin.
-
After completion of the tibial osteotomy,
perform a fibular diaphyseal osteotomy. This results in free mobility
at the tibial osteotomy. -
Drill a hole in the anteroposterior direction across the osteotomy and lateral to the tibial tubercle.
-
Insert a single 3.5 mm cortical or
cancellous lag screw, align the osteotomy, and loosely tighten the
screw to allow later adjustments, if necessary. -
After an anterior compartment fasciotomy, close the incisions. Insert a suction drain at the time of closure.
-
Apply a long-leg cast. Rab (229)
advised injection of contrast material into the knee to enhance the
ability to check the radiographic alignment after the dressings are
applied but before the long-leg cast is applied.
adolescent form of tibia vara, excision of the proximal tibial physis
may be advantageous.
-
This procedure is similar to the proximal
tibia osteotomy previously described. Make a similar incision, although
more proximally. -
Mobilize the patellar tendon on its medial and lateral sides.
-
Place a smooth Steinmann pin through the epiphysis just below the articular surface.
-
Excise the entire physis in a closing-wedge osteotomy. Insert a second pin distally, and apply an external fixator.
advantage of this procedure is that it is performed at the site of the
deformity and therefore allows maximal correction and physiologic
realignment of the tibia. Healing is usually rapid, and there is no
risk of recurrent deformity. A contralateral proximal tibia
epiphysiodesis may be performed at the same time. However, in most
cases, the degree of lower-extremity length discrepancy is followed
scanographically, and any residual leg-length discrepancy is corrected
by a contralateral distal femoral epiphysiodesis at the appropriate
time.
technique may be beneficial with either a cantilever or Ilizarov ring
fixation system. The advantage of this procedure is that it allows
slow, progressive correction.
Because
the patient is bearing weight, the precise degree of desired correction
can be achieved. This procedure is being used more frequently today
(see Chapter 171).
after any corrective osteotomy of the proximal tibia. Continue
immobilization until healing is complete; then place the children on a
physical therapy program at home for approximately 2 weeks. After
complete rehabilitation, allow them to return to normal activities.
Because of associated obesity, these children frequently benefit from a
dietary consultation.
osteotomy are common. They include peroneal nerve palsy, injuries to
the anterior tibial artery, and compartment syndromes (88,103,148,187,202,204,249,263).
Occurrence of a compartment syndrome may be minimized by performing an
anterior compartment fasciotomy at the time of surgery. If a
compartment syndrome occurs, temporarily reduce the correction, and
perform a four-compartment fasciotomy. Children with the infantile form
of tibia vara require long-term follow-up to assess the results of
surgery. The deformity may recur, especially in older children and
those with advanced Langenskiöld grades. Deformity persisting after
skeletal maturity predisposes to degenerative osteoarthritis (137,291).
diaphyseal osteotomy to correct infantile tibia vara. This allows
simultaneous correction of both components of the deformity. It is
important that the deformity be slightly overcorrected so that the
mechanical axis passes medial to the ankle joint. It can be difficult
to assess the degree of correction intraoperatively because radiographs
on a long cassette cannot be obtained. A helpful hint is to visualize
the iliac crest on the involved side. The cord from the
electrocoagulation knife can be used to measure the mechanical axis on
the fluoroscope. This can be stretched between the anterosuperior iliac
spine and the middle of the patella. Its distal extension can then be
judged with respect to the ankle joint. Undercorrection is a common
problem that prevents adequate alignment. Radiographic contrast
material in the knee joint at the time of surgery may also be helpful.
Manually manipulate the knee into varus after the osteotomy is
internally stabilized, as this gives a more realistic feeling for the
alignment of the extremity during weight bearing.
vara, especially the juvenile type, a preoperative MRI may be helpful
in assessing the integrity of the physis. However, even if the physis
appears open, it may still be abnormal and not respond to normalization
of the compressive forces postoperatively. Correction in these children
can be accomplished using an Ilizarov frame and callotasis. This allows
precise correction of the deformity. Weight-bearing radiographs may be
obtained during treatment.
bridge is suspected, I would suggest excision of the physis with
concomitant correction of both the residual tibia vara deformity and
any medial tibial torsion. Postoperatively, patients will need to be
evaluated for residual lower-extremity length discrepancy. An
appropriately timed contralateral epiphysiodesis will be necessary to
achieve relatively equal leg lengths at skeletal maturity.
dysplasia involving the medial aspect of the proximal tibial metaphysis
was first reported by Bell et al. (26) in 1985. Since then, additional cases have been reported (3,45,67,131,145,208,290). Tibia vara may also involve other areas of the body. Lincoln and Birch (178)
reported upper-extremity involvement. It is an uncommon cause of
pathologic genu varum but one that must be differentiated from Blount’s
disease because the natural histories of the two disorders are
distinctly different.
or for diagnostic purposes has shown consistent histopathologic
features. Grossly, there is a white cartilaginous lesion with well
defined margins deep to the insertion of the pes anserinus.
Histopathologic findings include acellular or sparsely cellular
collagenous tissue, inactive fibrocytes, plump cells resembling
chondrocytes in lacunae, and dense, nondescript fibrous tissue (26,45,155,192,208).
No giant cells, osteoid, or bone are found within these lesions. The
lesions suggest fibrocartilage centrally and tendinous tissues
peripherally. They do not involve the physis or epiphysis. Bell et al. (26)
observed that the tissue resembles that normally found at the site of
the insertion of tendons into cortical bone, as described by Cooper and
Misol (70) in 1970. They suggested that these
children had abnormal development of fibrocartilage at the insertion of
the pes anserinus. The exact mechanism of this abnormal growth is
unknown. The defect may be congenital.
fibrocartilaginous dysplasia present with unilateral bowing. There is
no apparent sex or side predilection. The onset is usually before age 1
year, and the deformity progresses until approximately age 2 years and
then begins to resolve. During the time of progression, the deformity
may become quite prominent, reaching 20° to 30° of varus. Medial tibial
torsion and mild tibial length discrepancy (0.5–1.0 cm) are common
associated findings (26, 45).
The lesions are characteristically not tender to palpation, and there
is no prominence of the proximal medial metaphysis, as seen in
infantile tibia vara.
medial metaphyseal region of the proximal tibia with an area of
surrounding sclerosis (Fig. 169.10). MRI will demonstrate dense fibroconnective tissue (192). Computed tomography shows similar findings, with an elliptical fibrous cortical defect but no soft-tissue mass (131,290).
On the basis of reported cases, it appears that the metaphyseal lesion
resolves spontaneously after age 2 years, followed by correction of the
tibia vara deformity. Significant improvement is usually evident by 4
years of age (26,45). Use of an orthosis does not increase the rate of improvement.
 |
|
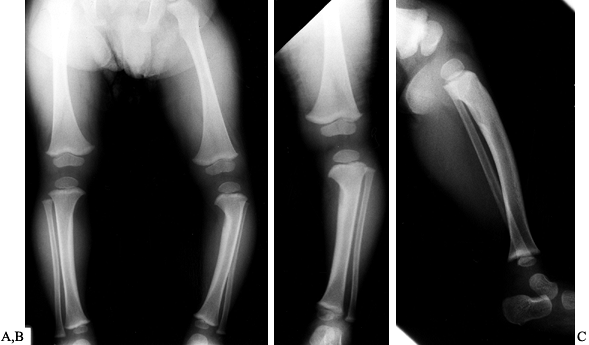
Figure 169.10. A: Standing AP radiograph of a 2-year-old Caucasian boy shows asymmetric genu varum involving the left lower extremity. B:
Observe the typical radiographic features of focal fibrocartilaginous dysplasia of the proximal tibia. There is a cortical defect involving the medial aspect of the proximal tibial metaphysis. There is associated sclerosis, as well as the mild tibia vara deformity. C: The lateral radiograph is relatively normal. |
with no evidence of spontaneous correction by 4 years of age are
candidates for corrective osteotomy (290). This
typically involves a proximal tibial osteotomy distal to the apophysis
of the tibial tubercle and a diaphyseal fibular osteotomy. Because of
the associated medial tibial torsion, the procedure of choice is a
laterally based closing derotation osteotomy or an oblique proximal
tibial osteotomy as described by Rab (229).
are the same as those for tibia vara. There is no intrinsic osseous
pathology that interferes with bone healing. Immobilization in a
long-leg cast or a one-and-one-half spica cast is necessary, depending
on the child’s age. A spica cast is usually advised for younger
children. After healing, there is usually rapid rehabilitation and
return to normal activities. Prolonged follow-up is necessary to assess
resolution of the lesion and subsequent growth and development of the
proximal tibia. Periodic scanograms are necessary to assess the length
of the extremities.
common in children with metabolic disorders such as vitamin D–resistant
rickets (hypophosphatemic rickets) or nutritional rickets. Vitamin
D–resistant rickets is an X-linked dominant disorder due to vitamin D
resistance that results in defective bone mineralization. Affected
children typically have bilateral symmetric genu varum; they are
relatively short, usually being in the tenth percentile. The varus
deformity is due to a combination of bowing and involvement of the
distal femur and the proximal tibia. Hematologic studies reveal normal
serum calcium and decreased phosphate values. In nutritional rickets,
the child has been receiving an unusual diet from the parents.
metaphyses, widening of the physes, and a cup-shaped relationship
between the physis and the metaphysis. The bowing is usually symmetric
throughout the femur and the tibia. Marked osteopenia and thinning of
the cortices are also common. Obtain serum calcium, phosphorus, and
alkaline phosphatase levels, as well as a pediatric endocrinology
consultation to confirm the diagnosis.
This typically includes oral phosphate supplementation and high doses
of vitamin D for vitamin D–resistant rickets, and dietary changes for
nutritional rickets. Surgical measures to correct genu varum
deformities are usually unsuccessful unless adequate medical control
has been obtained preoperatively. If such control cannot be obtained,
it is usually best to wait until skeletal maturity before attempting to
realign the mechanical axes.
young, observation is appropriate. Spontaneous improvement may occur in
children younger than 5 years. However, in older children or those who
are not improving spontaneously, surgical treatment is necessary (96,239).
This may consist of osteotomies of the distal femur, proximal tibia, or
both. If involvement is extensive, proximal femoral and distal tibial
osteotomies may be necessary to adequately realign the lower
extremities. Cast immobilization postoperatively may result in
immobilization-induced hypercalcemia and may require modification of
medical management. When osteotomies are done, healing time may be
twice normal. It is often advantageous to postpone major alignment
procedures until adolescence to minimize the recurrence that is common
in younger children.
renal osteodystrophy. The physes in these children show the same
pathologic changes found in tibia vara and slipped capital femoral
epiphysis. These include disorganized endochondral ossification at the
physeal–metaphyseal junctions. Because end-stage renal failure occurs
more commonly in older children who have achieved physiologic valgus
alignment, valgus deformities are much more common. Varus is more
likely when renal failure occurs at 3 years of age or younger. Renal
osteodystrophy has many of the same radiographic features as vitamin
D–resistant and nutritional rickets. There is physeal cupping and
widening at both the distal femoral and proximal tibial physes. Marked
osteopenia and thinning of the cortical bone are also present.
osteodystrophy is similar to that for vitamin D–resistant and
nutritional rickets. Surgical treatment is usually postponed until the
renal status has stabilized in response to medical treatment,
hemodialysis, or kidney transplantation. There will be a rapid
recurrence of the deformity if the underlying metabolic bone disease is
not corrected first.
Metaphyseal chondrodysplasia (both Jansen and Schmid types), which
results in abnormal chondroblast function and chondroid production, is
a common cause. Occasionally, these may be difficult to distinguish
from rickets. Although the physes are widened and cupped in the Schmid
type, the epiphyses are normal, and the presence of short stature may
be helpful in making the correct diagnosis.
rhizomelic dwarfing condition is due to abnormal endochondral bone
formation. Affected children have short stature and characteristic
craniofacial features. The genu varum deformity is due to asymmetric
growth of the proximal tibial epiphysis and overgrowth of the fibula.
These children rarely have knee pain.
deformities secondary to achondroplasia must be surgical because
orthotic management historically has not been effective. Their usual
procedure is a proximal tibial valgus osteotomy and proximal fibular
epiphysiodesis (Fig. 169.11). The latter must
be done early in childhood to prevent recurrence and progression of the
genu varum deformity. Others feel that genu varum in achondroplasia is
not associated with functional difficulty or increased risk of
osteoarthritis, and surgery may not be recommended (see Chapter 180).
 |
|
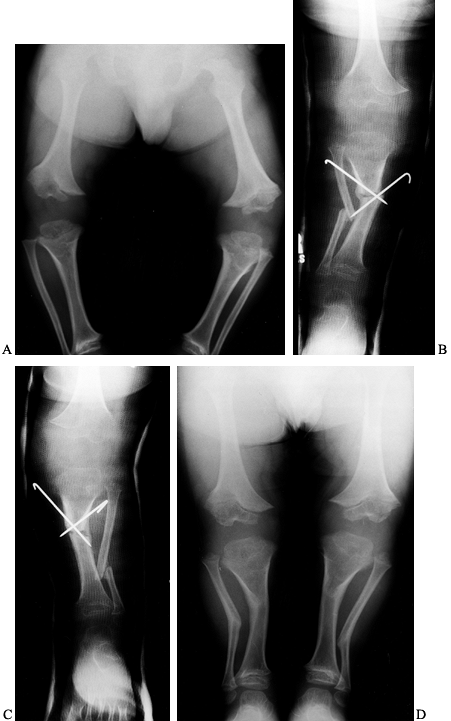
Figure 169.11. A:
Standing preoperative radiographs of a 6-year-old Caucasian boy with achondroplasia genu varum demonstrating the typical epiphyseal and metaphyseal changes, as well as overgrowth of the fibulae. B: Postoperative radiograph after proximal tibial and fibular diaphyseal valgus derotation osteotomies of the right leg shows that internal fixation was achieved with percutaneous smooth Steinmann pins. C: Postoperative radiograph of the left lower leg. D: Standing radiographs 6 months postoperatively demonstrates satisfactory healing and excellent correction of the genu varum deformities. |
collagen and produces varying degrees of skeletal fragility. Repeated
fractures often lead to bowing and torsional malalignment of the lower
extremities. The distal third of the femur is a common location for
these fractures, which frequently result in anterolateral angulation.
Residual deformities
after
fractures are common, and the varus angulation often increases as a
result of repeated fractures. Occasionally, in the more severe cases,
osteotomies with intramedullary fixation may be beneficial (see Chapter 180).
the physis may result in asymmetric growth and deformity. The distal
femur is the most common site of growth disturbance following a physeal
fracture. Physeal fractures of the proximal tibia occur much less
commonly. The management of genu varum deformities secondary to physeal
growth disturbance is complex. If an asymmetric physeal bar is present,
it may be resected and grafted with fat or Silastic. The deformity is
corrected concomitantly by an osteotomy. If the physeal damage is
extensive, complete physeal closure and management of the associated
leg-length discrepancy may need to be considered (see Chapter 164).
affecting the lower limbs in children and adolescents. Physiologic genu
valgum is the most common form, but pathologic genu valgum disorders
occur and may require treatment (Table 169.2). The most common pathologic causes of genu valgum are posttraumatic and renal osteodystrophy.
that for genu varum and includes a careful history and physical
examination. In the majority of children with genu valgum, the
femoro-tibial angles are within the physiologic range of two standard
deviations above or below the mean. Only those with an angle greater
than two standard deviations from the mean are considered to have a
deformity. Fat thighs, ligamentous laxity, and flat feet are often the
results of associated out-toeing, and this can accentuate the
appearance of the knock-knee, making physiologic genu valgum appear
more severe. Measurements of the femoro-tibial angle (with a
goniometer) and the intermalleolar distance are methods for assessing
and following genu valgum (55,65,126).
However, the intermalleolar distance may be misleading. The same
intermalleolar distance in an individual of short stature may be more
significant than the same distance in a taller individual. Torsional
malalignment is less common in genu valgum, but the combination of
femoral anteversion or torsion and compensatory external tibial torsion
gives the appearance of a valgus knee.
similar to those for genu varum. Short stature, asymmetry, history of
injury, or history of progression are indications. Standing AP
radiographs of the lower extremities, including the hip, knee, and
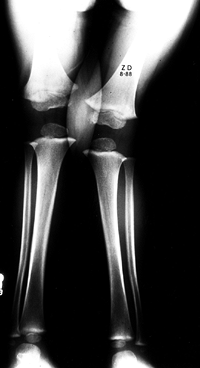
ankle, are the best method (Fig. 169.12). The
majority of children with genu valgum have physiologic genu valgum or
its persistence into later childhood and early adolescence. However,
there are other pathologic genu valgum disorders that may progress and
cause functional impairment.
 |
|
Figure 169.12.
Standing AP radiographs of the lower extremities of a 3-year-old boy with physiologic genu valgum show no radiographic abnormalities of the distal femoral or proximal tibial epiphyses or metaphyses. |
The maximal deformity occurs between 3 and 4 years of age. It rarely
causes symptoms or disability unless the deformity is severe. In these
cases, the knees may rub, and the child walks and runs with a
circumduction gait. With severe genu valgum deformities, the feet are
pronated. In older children or adolescents, malalignment of the
quadriceps mechanism may occur, resulting in patellar subluxation or
dislocation. Severe genu valgum occurs more frequently in obese
children.
The abnormal weight may produce a medial thrust that can result in
laxity of the medial collateral ligament and possibly early
degenerative osteoarthritis.
 |
|
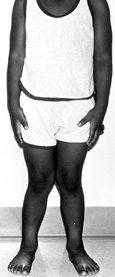
Figure 169.13.
Typical appearance of physiologic genu valgum in a 4-year-old boy. With the knees approximated, there is a wide separation between the ankles. There is approximately 15° of genu valgum bilaterally. |
Use of an orthosis is controversial and is not recommended. Even
significant deformities persisting into adolescence can be expected to
improve or resolve if slow, steady improvement can be documented.
Persistent deformities that are not improving may benefit from surgical
treatment.
physiologic genu valgum is a persistent, severe deformity (>15°) in
the immediate preadolescent years (ages 11 years in girls and 12 years
in boys). After this age, significant spontaneous improvement is not
likely to occur (141).
 |
|
Table 169.6. Surgical Options for Genu Valgum Deformities
|
proximal tibia, or both, depending on the patient’s age and the
severity and location of the deformity.
distal femur or proximal tibia by medial physeal stapling is a
relatively easy, reliable method of correcting a genu valgum deformity
if there is sufficient remaining growth to produce satisfactory
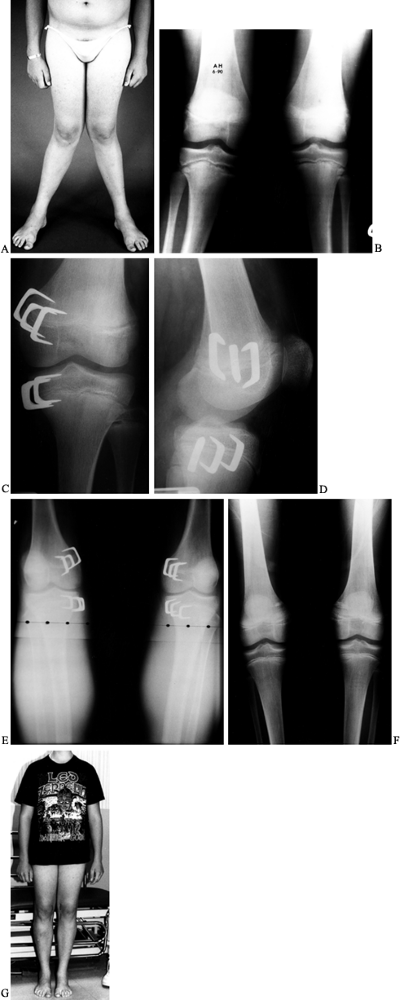
alignment (Fig. 169.14) (36,105,141,193,221,282,294).
If the deformity is pronounced or there is insufficient remaining
skeletal growth, a combined stapling of the medial aspect of the distal
femoral and the proximal tibial physes may be necessary (105,221).
 |
|
Figure 169.14. A: A 14-year-old boy with persistent severe physiologic genu valgum. B:
Standing AP radiograph of both lower extremities demonstrates a valgus deformity in the distal femora and proximal tibiae. Observe the physeal widening at the proximal tibial physes. A metabolic evaluation was normal. C: Postoperative radiograph of the left knee after stapling of the medial aspect of the distal femoral and proximal tibial epiphyses shows the three staples used to bracket each epiphysis. D: Lateral radiograph. E: Standing radiograph of both knees 9 months postoperatively shows excellent correction of the genu valgum deformities. F: On a standing radiograph 6 months after staple removal, physiologic alignment is being maintained. G: Clinical photograph. |
palpable at the junction of the maximal metaphyseal flare and the
medial femoral condyle. Stapling of the distal femoral epiphysis
proceeds as follows.
-
Approach the physis through a 4–5 cm
longitudinal incision between the anterior and posterior margins of the
medial femoral condyle. Begin the incision approximately 1–1.5 cm
distal to the physis, and extend it proximally. -
Divide the subcutaneous tissues, deep
fascia, and patellar retinaculum. If necessary, retract the medial
margin of the vastus medialis anteriorly. Identify the physeal plate
with a straight Keith needle or by fluoroscopy. Identification of the
physis and insertion of the staples occur more quickly and more
accurately with fluoroscopy. If the physeal plate is identified by
probing with
P.4306P.4307P.4308
a Keith needle, the plate is softer than the adjacent cancellous bone. -
Select Blount staples that are
rectangular or oblique, depending on the shape of the medial femoral
condyle and metaphysis. Vitallium staples cause less reaction, are
stronger, and are less likely to be extruded than stainless steel
staples. -
Avoid subperiosteal stripping to protect the perichondrial ring and the physeal plate.
-
With a staple holder, partially insert
three staples. Insert one directly medially and one each in the
anteromedial and the posteromedial aspects of the distal femur. Before
completely setting the staples, confirm their location and orientation
with radiographs or fluoroscopy. The physis should be in the mid
portion of each staple. Insert the ends of the staple parallel to the
physis to avoid physeal injury. If position and orientation are
satisfactory, drive the staples flush with the periosteum. Do not bury
the staples into the bone to avoid injury to the perichondrial ring. -
Close the patellar retinaculum and the
deep fascia separately. It is important that the patellar retinaculum
not be bound down by the staples because it can cause loss of knee
motion, local swelling, and pain. -
Close the subcutaneous tissues and skin
with absorbable sutures. A subcuticular closure of the skin gives the
best cosmesis. Reinforce the incision with adhesive closure strips, and
apply sterile dressings and a knee immobilizer.
-
Make a 4–5 cm longitudinal incision
directly over the medial aspect of the knee. The incision usually
begins just distal to the joint line and proceeds distally. -
Identify and retract anteriorly the
medial border of the pes anserinus, if possible. Occasionally, the pes
anserinus must be split. After the periosteum of the proximal tibia is
visualized, identify the physeal plate with a Keith needle or by
fluoroscopy. -
Insert one staple directly medially and
one each in the anteromedial and the posteromedial aspects of the
proximal tibia. The oblique or angulated Vitallium Blount staples are
quite useful in this location because they conform to the flare of the
medial aspect of the proximal tibia. Insert the staples parallel to the
physis and the articular surface. Center the staples over the physeal
plate. Before setting the staples, confirm the position
radiographically. -
Close the wound in layers. If the pes
anserinus is split, repair it with absorbable sutures. Close the
subcutaneous tissues and skin in a similar manner, and apply sterile
dressings.
approximately 2 weeks. Follow up at 2–3-month intervals, and assess
radiographically for correction. After the desired amount of correction
has been achieved, remove the staples. However, there is frequently
rebound overgrowth and slight recurrence of the deformity. Zuege et al.
(294) recommended allowing 5° of rebound.
Accomplish this by allowing the correction to proceed to slight
overcorrection before staple removal. However, Fraser et al. (105)
found that the amount of rebound overgrowth was minimal and
unpredictable. They also advised against leaving the staples in place
for longer than 1 year because of possible premature closure of the
physis. The amount of correction can be calculated mathematically on
the basis of the width of the physis and the amount of remaining growth
(38).
the distal femur or proximal tibia has been proposed as a method for
gradual correction of genu valgum deformity. The table devised by Bowen
et al. (38) can be used to determine the
appropriate time for epiphysiodesis. However, because of the
variability in the data necessary to make these determinations, a
second operative procedure is often required to close the remaining
lateral portion of the epiphysis.
were once popular. However, the percutaneous techniques of
epiphysiodesis using curet, drills, burrs, or a combination of these
are now preferred (37,39,58,207,276).
These are as accurate and much more cosmetic than the open bone graft
epiphysiodesis techniques. Although this technique is most commonly
used for lower-extremity length discrepancies, it can also be used
successfully in the correction of persistent angular deformities such
as genu valgum and genu varum (39).
-
Position the patient supine on a
fluoroscopy table. Identify and mark the mid portion of the medial
aspect of the distal femoral physis. -
Make a 2–3 mm incision directly over the physeal plate.
-
Enter the medial aspect of the physis
with a small curet or drill, and remove the medial portion of the
physis. This allows the formation of a medial bone bridge. Do not
extend the epiphysiodesis across the midline of the physis to avoid
symmetric closure. -
Only a single subcutaneous suture is usually necessary to close the wound.
tibia, use a knee immobilizer for approximately 2 weeks. This allows
skin and soft-tissue healing. The physis after epiphysiodesis is weak
and must be protected for a short
period
to prevent complete physeal separation. At the end of 2 weeks,
discontinue the knee immobilizer and allow the child active
range-of-motion exercises and full weight bearing. Continue restriction
of activities until 6 weeks postoperatively. Institute quadriceps and
hamstring strengthening exercises at that time, with a gradual return
to normal activities. After the desired correction has been achieved
with medial epiphysiodesis, a lateral epiphysiodesis of the distal
femur or proximal tibia is necessary to prevent overcorrection if the
lateral physes remain open.
or proximal tibial and fibular diaphyseal osteotomies allows full
correction of the deformity with a single operative procedure. However,
both procedures are extensive and require internal or external fixation
to maintain alignment until healing has occurred. In the correction of
a valgus deformity, attention must be given to the peroneal nerve
because neurapraxia or partial paralysis may occur if the nerve is
stretched. The osteotomy may be performed in early adolescence or after
skeletal maturity. Several techniques are available, including
opening-wedge, closing-wedge, and dome osteotomies. The choice of
technique is frequently based on the length of the lower extremity and
the individual bones. An opening-wedge or dome-shaped osteotomy adds
length to the extremity. DePablos et al. (87)
described a progressive opening-wedge osteotomy using an external
fixator; a fibular osteotomy is not required, and the osteotomy allows
progressive and adjustable correction. Both lower extremities can be
corrected simultaneously. Ordinarily, osteotomies are considered for
boys age 14 years or older and girls age 12 years or older.
osteotomy of the fibula are indicated if the valgus deformity is in the
proximal tibia below the knee joint and there is no associated lateral
tilt to the articular surface. The osteotomy is usually performed at
the junction of the metaphysis and the diaphysis, just distal to the
tibial tubercle. If there is associated lateral torsion of the tibia,
derotation may also be accomplished. If a closing-wedge osteotomy is to
be performed, perform the derotation initially because it frequently
decreases the amount of bone requiring resection to correct the angular
deformity. After satisfactory correction has been achieved, internal or
external fixation is required. Compression plate and screws, crossed
Steinmann wires, or an external fixator are suitable. In some cases,
the Ilizarov ring fixator and the callotasis technique may be
beneficial; this allows slow correction of the valgus and the
derotation. Hemichondrodiastasis, or asymmetrical physeal lengthening,
has been recommended by some, but it is not popular in the United
States. This procedure allows simultaneous correction of limb-length
inequality and correction of the genu valgum deformity. Because the
physeal plate closes after this procedure, it is best performed for
patients in late adolescence.
alignment of the distal femur with an osteotomy of the distal femur.
Deformities in this area are associated with a lateral tilt to the
joint line that cannot be corrected by a proximal tibial osteotomy. The
osteotomy may be performed through a medial or a lateral approach to
the distal femur. The medial approach is more complex because of the
proximity of the femoral artery, but it allows easier visualization of
the operative site. The lateral approach is simpler, and there is less
risk to the femoral artery as it passes posteriorly at the upper margin
of a medial incision. Opening- or closing-wedge osteotomies are
commonly performed because it is difficult to perform a dome osteotomy
of the distal femur. After the osteotomy is complete, internal or
external fixation is performed with a compression plate and screws,
crossed threaded Steinmann pins, or an external fixation device. See Chapter 30 and Chapter 31 for additional information on osteotomies of the femur and the tibia, respectively.
management depends on the type of internal or external fixation. If
rigid internal fixation has been achieved with a compression plate and
screws, immobilization is usually unnecessary, other than perhaps a
knee immobilizer for 1–2 weeks for comfort. Allow only toe-touch weight
bearing until early callus formation; then increase weight bearing,
although not to full weight bearing, until the osteotomy site is
completely healed. Remove the compression plate and screws 12–18 months
postoperatively.
a long-leg cast. Have the patient avoid weight bearing for 3–4 weeks,
until there is early radiographic callus formation. Then allow
toe-touch weight bearing. Usually, at 4–6 weeks after surgery, there is
sufficient healing to allow removal of the external fixation device in
the clinic. Apply a cylinder cast for an additional 2 weeks to allow
solid union. After this has been accomplished, institute
range-of-motion exercises. Failure to obtain a full range of motion at
the end of 2 weeks is an indication for a referral to physical therapy.
After full motion has been regained, begin strengthening exercises of
the quadriceps and hamstring muscles. Return the patient to full
activities after rehabilitation of the leg is complete.
unpredictability of growth after the staples have been removed,
possibility of asymmetric medial physeal closure, widening or loosening
of the staples with eventual
extrusion
requiring revision, irregular patterns of initial growth retardation
after stapling, the need for a second surgical procedure to remove the
staples or to perform a lateral epiphysiodesis, and long and frequently
wide operative scars due to stretching with knee motion. In 49 patients
with genu valgum treated with stapling by Pistevos and Duckworth (221),
there were no complications other than scarring, although six patients
did not obtain complete correction. Staples may be painful; however,
this resolves after removal.
result in peroneal nerve palsy, injury to the femoral or anterior
tibial arteries, and anterior compartment syndrome (148,187,202,204,249,263,268).
These severe complications are more common after proximal tibial
osteotomies. Monitor patients closely postoperatively so that immediate
intervention can be taken if a complication occurs. Postoperative wound
infection, delayed union, nonunion, overcorrection, and undercorrection
may occur after corrective osteotomies.
rotational component, medial physeal stapling is my procedure of
choice. This is usually performed on the proximal tibial epiphysis. In
severe deformities, however, the distal femur may be included. I have
not used the chart described by Bowen et al. (38).
Once slight overcorrection is achieved, I remove the staples, and I
have not encountered a case of premature physeal closure. This
procedure is simple and effective and requires minimal postoperative
immobilization.
relatively common and tend to occur most frequently in children between
3 and 6 years of age (range, 1–12 years) (79,146,150,205,237). Three times as many boys are affected as girls, which is typical for all tibial fractures (123). Skak et al. (258)
reported an incidence of 5.6 fractures per 100,000 children per year.
The fractures are usually the result of direct injury to the lateral
aspect of the extended knee. The primary injury patterns are
compression (i.e., torus fracture), incomplete tension–compression
(i.e., greenstick fracture), or complete fractures (235).
The fibula is typically intact but may be fractured or have a plastic
deformation. The incomplete tension–compression or greenstick fracture
is the most common pattern. The medial cortex on the tension side
fractures, whereas the lateral cortex on the compression side remains
intact or hinges slightly. The distal fragment may angulate into a
slight valgus deformity, but there is no displacement and the
apposition remains normal. However, most fractures are nondisplaced and
without angulation.
tibial metaphysis are valgus deformity and overgrowth of the tibia. In
1953, Cozen (72) reported on four patients with
valgus deformities after nondisplaced or minimally angulated fractures
of the proximal tibial metaphysis. Many other reports of this
complication have been published (16,18,21,27,30,44,51,66,71,76,79,109,113,133,146,147,150,153,183,184,226,258,273,281,283,292,293).
Similar valgus deformities were observed after other insults to the
immature proximal tibial metaphysis, such as osteomyelitis, bone-graft
harvest, osteochondroma excision, and osteotomy (18,243,280).
tibial metaphyseal fractures varies. It appears to occur in
approximately 50% of cases. Salter and Best (244)
reported on 21 patients with proximal tibial metaphyseal fractures,
observing the development of a valgus deformity of 11° to 22° in 13
(62%) of them. Robert et al. (235) reported the development of a genu valgum deformity in 12 (48%) of 25 patients. However, Skak et al. (258) reviewed 40 consecutive patients and found the development of deformity in only 4 (10%). Boyer et al. (44)
reported no valgus deformity in seven children 2–5 years of age who
sustained fractures while jumping on a trampoline with a heavier child
or adult. Valgus deformities occur predominantly in association with
greenstick or complete fractures and are uncommon after a torus
fracture (235,258).
injury to the lateral aspect of the proximal tibial physis, inadequate
reduction, premature weight bearing, hypertrophic callus formation,
dynamic muscle action, soft-tissue interposition, tethering from the
intact fibula, and asymmetric physeal growth stimulation (14,16,18,21,27,30,34,51,61,66,71,72,76,80,113,133,139,140,146,147,153,183,184,191,205,206,226,230,237,243,244,257,273,281,283,292,293).
measured the medial and the lateral metaphyseal–diaphyseal–metaphyseal
tibial distances in 17 children with 19 proximal tibial metaphyseal
fractures. They found four patients in whom the medial distance of the
injured tibia was longer than the lateral distance, which was the same
distance as the uninjured tibia. In 11 patients, there was overgrowth
on both the medial and the lateral sides of the injured tibia. This
indicates that a valgus deformity following a proximal tibial
metaphyseal fracture is usually due to eccentric proximal medial
overgrowth.
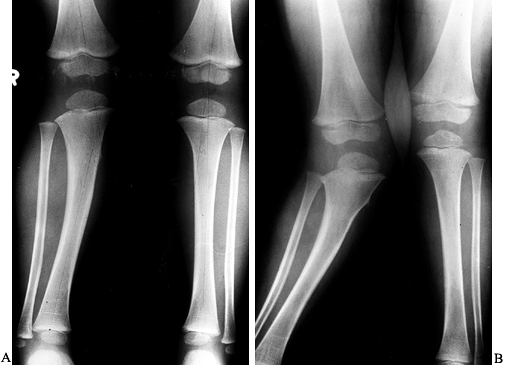
begins to improve by longitudinal growth through the proximal and distal physes (Fig. 169.15) (216). Unfortunately, there are no data indicating how much improvement can be anticipated. Salter and Best (244) found no improvement in 21 patients, and 13 later required proximal tibial varus osteotomies. Visser and Veldhuizen (281)
reported no spontaneous improvement in the valgus deformity from the
proximal tibial physis but observed some correction in alignment from
the distal tibial epiphysis. Taylor (273) found improvement in some patients but not all. Of the 12 children with valgus deformity described by Jordan et al. (153),
11 had documented improvement, although four subsequently required
corrective osteotomies. Two of these children had their deformities
recur, and two had compartment syndromes. Six children had complete
correction of their deformities.
 |
|
Figure 169.15. A:
Standing AP radiograph of a 5-year-old boy after treatment for a greenstick fracture of the right proximal tibial metaphysis. At the time of cast removal, there was already 22° of genu valgum on the right but only 5° on the left. B: One year later, there was increased genu valgum deformity. |
observed that deformities of 15° or less usually remodeled completely,
especially in young children. The more severe deformities, however, did
not completely correct. Bahnson and Lovell (16)
found some improvement in the valgus deformities in five children
followed for a minimum of 3 years after injury. Balthazar and Pappas (18)
reported that two of nine patients who were treated nonoperatively had
resolution of their valgus deformity in 1–3 years. Skak et al. (258)
found that valgus deformities tended to increase during the first year
after injury and then remained constant for 1–2 years and finally
improved. Only one of their six patients had residual deformity at
final follow-up.
followed seven children with posttraumatic tibial valgus deformities
for a mean of 39 months after injury. These children were 11 months to
6 years of age. The valgus deformities progressed most rapidly during
the first year after injury and then continued at a slower rate for as
long as 17 months. Overgrowth of the tibia accompanied the valgus
deformities. The mean overgrowth was 1 cm (range, 0.2–1.7 cm). Clinical
correction with subsequent growth occurred in six of their seven
patients. They recommended that the
alignment of the lower extremities be measured by the mechanical femoro-tibial angle as described by Visser and Veldhuizen (281) rather than the metaphyseal–diaphyseal angle of Levine and Drennan (176).
The latter measured only the alignment of the proximal tibia. Much of
the late correction of the deformity is due to distal realignment (183,258,281).
The distal epiphysis tends to realign itself perpendicular to the
applied forces, resulting in asymmetric growth and an S-shaped
appearance of the tibia radiographically (216).
must consist of correction of any associated valgus angulation by
manipulative reduction and immobilization in a long-leg cast with the
knee in extension for 4–6 weeks or until the fracture is well healed (238).
If closed reduction is required, it is best performed under general
anesthesia. Radiographic evaluation of fracture alignment may be
difficult unless radiographs of both lower extremities with the knee in
extension are obtained on a long cassette. Greenstick fractures with
slight valgus angulation may require that the intact hinged lateral
cortex be manually fractured. This usually allows correction of the
deformity. Slight overcorrection is desirable (205).
residual angulation. However, normal apposition is not always
necessary. There are limited indications for open reduction of these
fractures. Inability to correct a significant valgus deformity by
manipulation under general anesthesia rather than failure to close the
medial fracture gap is currently the major indication. Most angulated
displaced fractures are amenable to reduction by nonoperative methods.
family that although anatomic alignment of the fracture has been
obtained, the possibility of valgus angulation and tibial overgrowth
exist as a natural consequence of this fracture. This information
prepares the family for complications if they occur.
weekly during the first 3 weeks after injury. Correct any loss of
alignment. During this initial period, children should avoid bearing
weight to minimize compression forces and the possibility of valgus
angulation within the cast.
metaphyseal fractures is predominantly nonoperative with prolonged
observation. The use of orthoses has been suggested, but there is no
evidence to substantiate the efficacy of this method (89,133,146). MacEwen and Zionts (183)
recommended observation until early adolescence. If spontaneous
improvement fails to provide sufficient clinical correction, surgical
intervention may be necessary. McCarthy et al. (188) reported no difference in the long-term results between 10 patients treated nonoperatively and five managed operatively.
severe valgus deformities (>25°) and failure to achieve satisfactory
correction by the immediate preadolescent years. All children should be
allowed 2–4 years of growth after injury to allow spontaneous
correction to occur. Most deformities of 15° or less resolve, and those
that are 25° or more may not. Deformities between 15° and 25° must be
carefully followed. The development of a medial thrust during this
observation period is an indication for surgical intervention to
prevent laxity in the medial collateral ligament.
deformities after proximal tibial metaphyseal fractures are similar to
those for other valgus deformities and include the following:
tibia, these procedures are most commonly applicable to the proximal
tibia. They are described in the section on physiologic genu valgum.
If surgical intervention is contemplated, it is important to assess the
degree of tibial-length inequality. Surgery must address the valgus
deformity and residual tibial overgrowth. In young children with severe
genu valgum deformities that are not improving with spontaneous growth
and development, a corrective osteotomy may be indicated. This should
include shortening of the tibia by approximately 5 mm to allow
recurrent overgrowth. Similar consideration is necessary in the early
adolescent years when surgical intervention is planned.
phenomenon that led to the initial valgus deformity. Balthazar and
Pappas (18) reported that the valgus deformity
recurred, although lesser in magnitude, in six children undergoing a
proximal tibial varus osteotomy. Four of the six also had further
longitudinal overgrowth of the tibia. DalMonte et al. (80)
reported recurrent valgus deformities in 7 (44%) of 16 patients after
proximal tibial osteotomies. The recurrence rate for children younger
than 5 years was 60%, and for those between 5 and 10 years of age, it
was 36%. The authors concluded that the osteotomy is essentially a
second fracture and therefore has the same risks of deformity. If
surgery is undertaken, families should be advised that the deformity
can recur and that prolonged follow-up is required.
tibial metaphyseal fracture will undergo spontaneous correction during
growth, I now feel that these children should be observed for as long
as possible. Only a severe disabling deformity should be considered for
early surgical correction. Deformities persisting into adolescence can
be corrected with a proximal tibial or distal femoral medial
hemiepiphyseal stapling (or both). Typically, this allows for rapid
correction of any residual deformity. I try to avoid corrective
osteotomy because this may induce the same genu valgum deformity that
followed the initial fracture.
vitamin D–resistant rickets, nutritional rickets, and renal
osteodystrophy. These disorders are more likely to produce genu valgum
rather than genu varum. This is due to their later onset, at which time
the physiologic valgus alignment of the knee has already been achieved.
Renal osteodystrophy is the most common metabolic disorder producing
genu valgum (12,19,32,62,75,81,132,143,210). Oppenheim et al. (210)
described changes in the lateral proximal tibial epiphysis and
metaphysis in children with renal osteodystrophy similar to those seen
in the medial proximal tibia in Blount’s disease.
underlying metabolic disorder. Treatment before that time has a high
incidence of recurrence. After the metabolic condition has been
controlled, treatment may be by either osteotomy or physeal stapling of
the distal femur or proximal tibia. The latter is a particularly
effective method, provided there is sufficient remaining growth (Fig. 169.16).
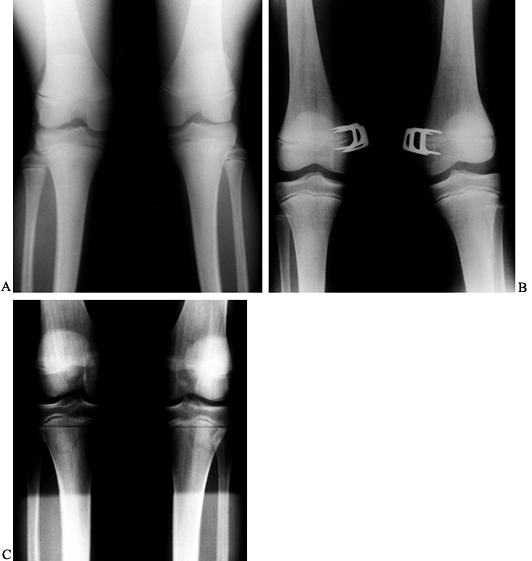 |
|
Figure 169.16. A:
Preoperative standing radiograph of a 15-year-old boy 2 years after renal transplantation. His genu valgum has not been improving, although the physes now appear normal. B: Postoperative radiograph after insertion of staples about the medial aspect of the distal femoral epiphyses. C: Standing radiograph at 18 months postoperatively and 9 months after staple removal demonstrates physiologic alignment and no evidence of growth disturbance. |
The deformities are progressive and require surgical treatment if there
is an asymmetric physeal bar or bridge. The extent of the bar can be
assessed by tomography or, preferably, MRI. Treatment options consist
of physeal bar excision and grafting with fat or Silastic (Langenskiöld
[169] procedure), together with a corrective osteotomy. If the bar is
extensive, then complete physeal closure and corrective osteotomy may
be performed, with delayed management of the leg-length discrepancy
(see Chapter 164).
as cerebral palsy, often have a pes valgus and excessive external
tibial torsion that may produce a progressive genu valgum. This is more
likely to be a torsional malalignment than a true genu valgum
deformity. Treatment may involve soft-tissue releases to restore muscle
balance and osteotomies to correct torsional and angular deformities
(see Chapter 177).
the physis or producing a reactive hyperemia and asymmetric growth
stimulation. Asymmetric physeal arrest is managed similarly to trauma
(see Chapter 176).
dysplasia, including multiple epiphyseal dysplasia, spondyloepiphyseal
dysplasia, metaphyseal dysplasia, and pseudoachondroplasia. Treatment
is based on the diagnosis. Orthotic management is usually ineffective
in skeletal dysplasia. Surgery with either stapling or corrective
osteotomies is usually necessary (Fig. 169.17) (see Chapter 180).
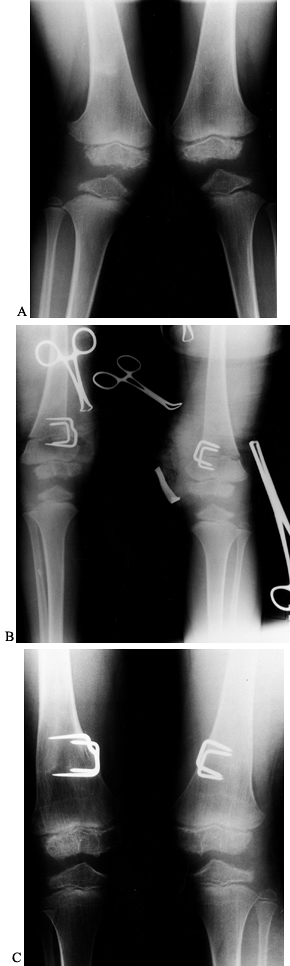 |
|
Figure 169.17. A: Preoperative standing radiograph of a 13-year-old boy with spondyloepiphyseal dysplasia and severe genu valgum. B: Intraoperative radiograph after closing-wedge distal femoral osteotomies. Staples were used to maintain alignment. C:
Standing radiograph 3 years postoperatively demonstrates that normal alignment is being maintained. Note the proximal migration of the staples with growth. |
Anterior and anterolateral angulation or bowing is the most common form
and is usually associated with other congenital anomalies, such as
congenital pseudarthrosis (8,11,15,120,125,135,165,200,212,231).
Congenital posteromedial angulation is less common, resolves
spontaneously, and is not associated with significant osseus pathology
other than residual lower-extremity length inequality.
The most common are congenital pseudarthrosis of the tibia, congenital
longitudinal deficiency of the tibia (paraxial tibial hemimelia), and
congenital longitudinal deficiency of the fibula (paraxial fibular
hemimelia) (see Chapter 174).
congenital malformation that includes all congenital fractures of the
tibia and pseudarthrosis of the tibia arising after pathologic fracture
in a tibia with congenital anterolateral angulation (4).
Usually, anterior or anterolateral bowing of the tibia is recognized
shortly after birth. Only occasionally are the fracture and
pseudarthrosis present at birth, and the pseudarthrosis is therefore
not truly congenital. Its incidence has been estimated to be 1 in
190,000 live births (149). The left side is affected slightly more often than the right (270); bilateral involvement is rare. Beals and Fraser (24,106)
reported cases with bilateral and familial involvement. Congenital
pseudarthrosis of the tibia is one of the most difficult and
challenging deformities confronting orthopaedic surgeons (120,213,270).
in a study of 17 children with a congenital pseudarthrosis, found that
eight had neurofibromatosis, three had fibrous dysplasia, and six had
no apparent disorder. Despite the clinical association with
neurofibromatosis, the cause remains obscure.
the pseudarthrosis shows a dense, cellular, fibrous connective tissue
with variable areas of cartilage formation (43,49,52,114).
Electron microscopy reveals the lack of a basement membrane, and the
cells resemble fibroblasts rather than Schwann cells or perineural
cells, even in children with known neurofibromatosis (49).
Only rarely is neurofibromatosis tissue observed in these specimens,
and these samples are usually from intraosseous neurofibromas (114). In some cases, the tissue does resemble fibrous dysplasia (42).
This dense, fibrous connective tissue with fibrocartilage and
occasional bone trabeculae fills a poorly vascularized gap between the
sclerotic bone ends to create the nonunion. This is not a true
pseudarthrosis. The defective tissue occurs within the bone itself, the
periosteum, the surrounding soft tissue, and possibly in the nerve and
vascular supply to the involved area.
the tibia characteristically presents with anterior or anterolateral
bowing of the tibia (Fig. 169.18). This rarely occurs
in conjunction with an acute fracture. The bowing is rarely
anteromedial. The angular deformity of the tibia is congenital. Unless
there is a fracture, the area is not tender, and a bony prominence is
palpable. With an acute fracture, the area is unstable and is usually
painful.
 |
|
Figure 169.18. A:
Photograph of a 3-month-old girl with anterolateral bowing of the left tibia shows numerous café-au-lait spots involving the left lower extremity. B: Lateral view shows anterior bowing of the tibia. C: On the posterior view, observe the café-au-lait spots. D: AP radiographs of the lower extremities demonstrate anterolateral bowing of the left tibia. The central portion of the tibia is sclerotic, and there is thinning of the fibula. This indicates a congenital prepseudarthrosis. E: Lateral radiograph demonstrates marked anterior bowing of the tibia. The apical sclerosis is more easily visualized in this view. |
these patients, the hallmarks of this disease must be sought. The
criteria used by Crawford (73) for diagnosis required at least two of the following:
the tibia, hemihypertrophy, or a short, sharply angulated spinal
curvature
presence of at least five spots measuring more than 0.5 cm in diameter
is considered diagnostic. The number of spots increases with the
patient’s age. Subcutaneous nodules (i.e., fibroma molluscum) are
uncommon until adolescence and are typical of chronic disease. Although
other bones may be involved in neurofibromatosis, involvement of more
than one bone is extremely rare. Isolated cases of congenital
pseudarthrosis of the fibula with an intact tibia have been reported (79,92,170).
They were usually associated with anterior bowing of the tibia and
ankle valgus. Curly and overlapping toes have been reported, as well as
congenital constriction bands (201,271).
anterior or anterolateral bowing of the tibia, there may be no clinical
evidence of neurofibromatosis. The clinical features of
neurofibromatosis usually become more apparent with growth and
development. Radiographs of the tibia before the establishment of a
pseudarthrosis may show an intact bowed tibia exhibiting sclerosis in
the area of angulation without a medullary canal. After a fracture has
occurred and a pseudarthrosis has been established, the proximal and
the distal ends of the fracture site become tapered. Both tapered bone
ends remain sclerotic.
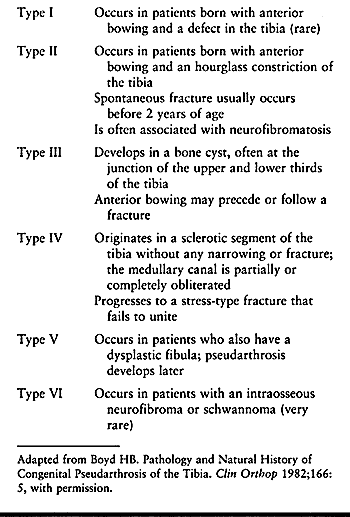 |
|
Table 169.7. Boyd Classification of Congenital Pseudarthrosis
|
|
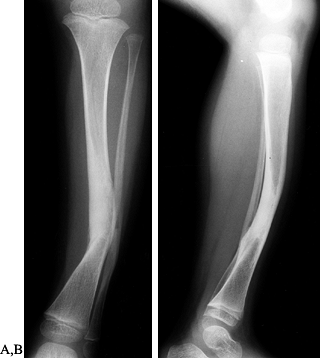 |
|
Figure 169.19. A:
AP radiograph of a 5-year-old boy with neurofibromatosis with a type II lesion of the left tibia. He has been managed in an ankle–foot orthosis since he began ambulation, and he has not had a fracture. B: Lateral radiograph demonstrates the apical sclerosis, which has been slowly improving. |
|
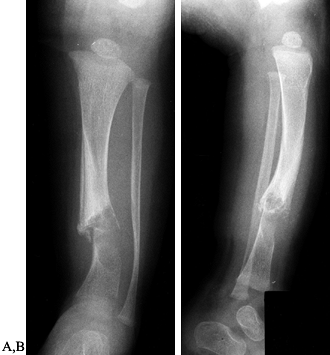 |
|
Figure 169.20. A:
AP radiograph of a 3-month-old child with a type IV congenital pseudarthrosis of the left tibia. Notice the cystic lesion and fracture. There is thinning of the distal aspect of the fibula. B: Lateral radiograph demonstrates anterior bowing. |
recommended treatment. In Crawford’s classification, a type I lesion
has the best prognosis, and the remaining three types have
progressively worse prognoses. However, the relation between the type
of pseudarthrosis and the clinical result is not always predictable (4,73,183,198).
The presence or absence of established neurofibromatosis makes no
difference in the classification and is not a factor in determining
treatment or prognosis. Cases in which bone-end resorption and
sclerosis are evident radiographically and bone graft rapidly resorbs
postoperatively have a poor prognosis. Those with a cystic lesion have
a more favorable prognosis (198).
of the tibia secondary to neurofibromatosis or fibrous dysplasia is a
fracture with the establishment of a pseudarthrosis. The treatment of
anterior and anterolateral bowing with an intact tibia is directed
toward prevention
of the fracture and pseudarthrosis. Congenital pseudarthrosis of the tibia is more than a mechanical problem (199); it represents a complex biologic problem because the established pseudarthrosis is extremely difficult to manage.
tibia without pseudarthrosis are best treated initially with a
total-contact plastic orthosis. This is usually an ankle–foot orthosis.
Prophylactic treatment may delay or prevent a fracture and subsequent
pseudarthrosis. These orthoses are worn for years. With growth and in
the absence of a fracture, the tibial bowing usually improves. There is
typically some residual shortening within the bone. The medullary canal
develops slowly over 5–10 years. It is possible, although unlikely,
that a fracture and pseudarthrosis can be avoided with the use of an
orthosis alone. If the tibia has straightened sufficiently, the
medullary canal has reconstituted, and there is adequate cortical
thickness, the orthosis may be discontinued as skeletal maturity is
approached. Vigorous physical activities should be avoided. There are
no long-term reports of successful orthotic management in adolescents
or adults.
occurred, the treatment is usually surgical. Casting alone rarely
results in healing. However, Roach et al. (234)
demonstrated that a late-onset fracture in a dysplastic tibia may heal
with prolonged immobilization. Six of 11 fractures healed, but four of
the six had a residual anterior bow susceptible to a stress fracture.
pseudarthrosis. The goals of treatment include obtaining union at the
pseudarthrosis site, maintaining union throughout growth and
development, and obtaining an acceptable limb length at maturity (213,278). Previously, surgery was advised only for children age 4 years or older (6,125,203).
Most physicians now recommend early surgical intervention and revision
if the first procedure does not result in union of the pseudarthrosis (186,199,213,214). Morrissey et al. (199) reported that a good result did not occur in any child whose tibia was not united by 6 years of age. Masserman et al. (186)
reported that union was more related to the pathologic process than the
age at surgery. Earlier union produces more normal growth of the distal
tibial epiphysis and less lower-extremity length discrepancy.
grafting alone, bone grafting and internal fixation, electrical
stimulation, microvascular bone grafting, Ilizarov external fixation
methods, and amputation.
This was thought to strengthen the deformed area and decrease the risk
for pathologic fracture. The technique described by McFarland (191)
is the most common procedure. A long corticocancellous graft from the
opposite tibia is placed posteriorly, spanning the deformity in the
normal biomechanical longitudinal axis of weight bearing. Lloyd-Roberts
and Shaw (179), however, reported success in only three of their seven patients, while Tachdjian (270) reported success in all five children. Recently, Strong and Wong-Chung (266) prevented fracture in six of nine children with a prepseudarthrosis secondary to neurofibromatosis. Paterson (213), however, felt that the procedure was indicated primarily for cystic prepseudoarthrosis. Tachdjian (270) suggested concomitant curettage and bone grafting of any cystic lesions.
reviewed 167 operations performed in 40 patients. The Farmer procedure,
using a composite bone graft from the opposite tibia, demonstrated the
best result, with a success rate of 53% (100).
Other procedures had lower success rates, including onlay grafts (13%),
bypass grafts (7%), Sofield procedure (25%), sliding grafts (33%), bone
allograft (17%), and autogenous grafts (10%).
the angular deformity of the tibia, and rigid internal fixation in
addition to bone grafting have improved the rate of primary union.
Stabilization has been achieved with compression plates and
intramedullary rods. The former is rarely used because of difficulties
involved in achieving adequate fixation (6,213).
The most common methods at this time are tibial or dual tibial and
fibular intramedullary rods. These techniques usually transfix the
ankle and subtalar joints to adequately stabilize the distal tibial
segment (4,9,17,63,108,278).
These joints are progressively freed with growth of the tibia and
proximal migration of the rod. This method does not result in
significant stiffness of the joints. Postoperatively, immobilize with a
unilateral hip spica cast followed by a long-leg cast and then a
knee–ankle–foot orthosis. Anderson et al. (9)
reported that 10 of 13 pseudarthroses healed with an intramedullary rod
technique. However, their mean follow-up time was short (6.9 years).
These rods extended with growth, decreasing the need for revision
surgery and protecting the union until skeletal maturity. They were not
inserted across the ankle or subtalar joint. Bitan et al. (31) reported satisfactory
extension of the rods in four of seven patients when these rods were used in revision surgery after primary union. Fern et al. (102)
recommended that the outer sleeve of an extendable rod be inserted
across the pseudarthrosis site to provide more strength and decrease
the risk of refracture. They reported primary union in all five
patients in whom extendable rods and bone grafting were used. All rods
expanded with growth up to a maximum of 6.4 cm.
stabilize the hind foot, and cancellous bone grafting increases the
rate of primary union. The correction of anterior or anterolateral
bowing undoubtedly enhances healing by allowing compression across the
pseudarthrosis. These rods are not removed until after skeletal
maturity because the tibia may undergo progressive bowing or refracture.
The various techniques include implanted direct-current bone growth
stimulators and external stimulation devices with pulsating
electromagnetic fields. The addition of electrical stimulation has
improved success rates after bone-grafting procedures (213,214).
Most reports recommend that electrical stimulation be used in
conjunction with internal fixation and bone grafting. In 1982, Kort et
al. (164) observed that the most important
variable in healing was the radiographic morphology of the nonunion.
Patients with spindled bone ends, a large gap, and gross mobility had a
poor prognosis, whereas those with a cystic or sclerotic transverse
fracture and a gap of less than 5 mm had better responses.
described a technique of excision of the pseudarthrosis and abnormal
tissue, fibular osteotomy, intramedullary rod fixation (i.e., large
Steinmann pin or Kuntscher nail), cancellous bone grafting, and an
implanted direct-current electrical bone growth stimulator. The leg was
protected in a long-leg plaster cast until clinical and radiographic
healing was achieved. Weight bearing was allowed and encouraged. They
reported primary union in 20 (74%) of 27 patients. The average time for
union was 7.2 months (range, 3–18 months). During a mean follow-up
period of 3.8 years (range, 6 months to 10 years), no refractures were
reported. The reasons for failure in seven patients included inadequate
correction of the anterior tibial bowing, poor internal fixation,
incorrect placement of the cathode, and extensively diseased bone.
Brighton et al. (48) reported that only one of
four patients with congenital pseudarthrosis of the tibia healed with
direct-current stimulation from an implanted single cathode. However,
they did not excise abnormal tissue, provide internal fixation, or use
bone grafting. The extremities were immobilized in a plaster cast, and
weight bearing was not allowed. They thought that the results did not
prove the efficacy of this technique for congenital pseudarthrosis of
the tibia.
reported the results in 34 patients with congenital pseudarthrosis of
the tibia treated with pulsed electromagnetic fields (PEMFs) by way of
external coils. They reported that 17 of 34 patients achieved complete
healing with reconstitution of the medullary canal. An additional seven
(21%) patients achieved union with function but required continued
protection with an orthosis. Healing of the pseudarthrosis occurred in
24 (71%) of 34 patients. Analysis of the failures demonstrated that
most occurred in male patients with a history of early fracture
(younger than 1 year) and with an atrophic, spindled, hypermobile
pseudarthrosis. The researchers did not employ any additional surgical
procedures in the initial treatment. However, after early healing was
demonstrated radiographically, surgical realignment, immobilization,
and bone grafting were combined with the PEMFs. This did not have an
adverse affect on the ultimate outcome. In 1982, Sutcliffe and Goldberg
(267) reported the results of 49 patients
treated for congenital pseudarthrosis of the tibia with PEMFs. The
definite end point of treatment was reached in 37 patients, and in 26
(70%) of them there was a successful outcome. Fifteen pseudarthroses
healed with PEMFs alone. The remaining 11 patients required subsequent
surgery, usually cancellous bone grafting, and a second course of PEMFs
before healing was obtained.
bone formation in the area of a pseudarthrosis and abnormal tissue.
Electrical stimulation alone is effective in approximately 50% of the
successful cases. In the remainder, additional procedures are necessary
before primary union can be achieved, including excision of the
pseudarthrosis and abnormal tissue, correction of existing deformity,
intramedullary fixation, and cancellous bone grafting. However, in
approximately 30% of cases, electrical stimulation with or without
surgical intervention results in failure. The incidence of refracture
appears to be low.
Vascularized rib, iliac crest, and fibula grafts have been used, and
the latter appears to be superior in congenital pseudarthrosis of the
tibia (64,68,84,85,92,108,121,154,175,219,220,284,285). The graft can be ipsilateral if it is of sufficient size (68,196,259,295).
The procedure consists of transferring the contralateral fibular
diaphysis on its vascular pedicle with a cuff of muscle to maintain the
periosteal blood supply into a defect created by resecting the
pseudarthrosis and abnormal soft tissue on the involved side (Fig. 169.21). Supplemental cancellous bone
grafting may be included to facilitate bone healing. The fibular graft
is advantageous because it is straight, a long segment can be
harvested, and it tends to hypertrophy after healing. Leung (175)
reported three successful microvascularized iliac crest grafts in
congenital pseudarthrosis of the tibia. He thought that it was easier
to harvest the iliac crest and there was a more rapid healing because
the graft was predominantly corticocancellous bone rather than cortical
bone alone. Iliac crest grafts ranging from 3 to 10 cm may be obtained
in children age 4 years or older. Rib grafts are less advantageous
because of their curvature. Hagan and Buncke (121)
reported that this curvature does not tend to correct with growth after
satisfactory incorporation and the curvature may increase. Use of
extensive corticocancellous grafting may prevent progressive bowing of
the vascularized rib graft. Donor site problems after vascularized
fibula transfers have been reported (209).
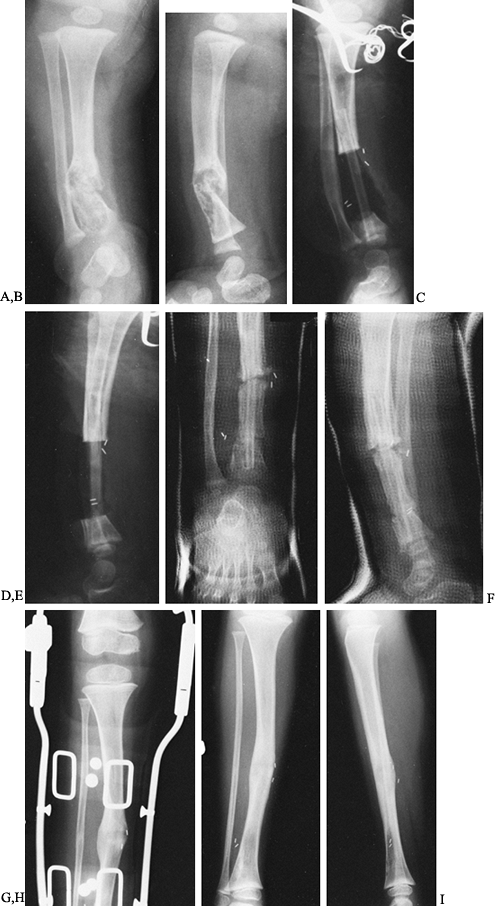 |
|
Figure 169.21. A:
AP radiograph of the right lower leg of a 6-month-old boy with neurofibromatosis and a congenital pseudarthrosis of the distal tibia. B: Lateral radiograph. C: A procedure using a vascularized fibula graft from the left leg was performed at 17 months of age. Internal fixation was not used, and the ends of the fibula graft were inserted into the medullary canal proximally and into the metaphysis distally. D: Lateral radiograph. E: Two months after vascularized-fibula grafting, there is extensive subperiosteal new bone formation and hypertrophy of the graft. F: Lateral radiograph. G: Twenty-two months later, the tibia is healed, but the leg is protected in a knee–ankle–foot orthosis. H,I: Thirty-three months postoperatively, the tibia has healed well, and the medullary canal is reforming in the area of the vascularized fibula. |
reported on the long-term results in 19 consecutive children with
congenital pseudarthrosis of the tibia treated with a vascularized
fibula graft. The mean age at surgery was 5.1 years (range, 1.4–11.4
years). The mean follow-up was 6.3 years (range, 2–11 years). They
reported that 18 (95%) of the 19 pseudarthroses healed. The
lower-extremity length discrepancy at follow-up was a mean of 1.6 cm
(range, 0–4 cm). Sixteen of the children had been treated with
electrical stimulation techniques, which failed, for at least 1 year
before surgery. However, the fibular graft hypertrophied rapidly, and
no graft fractured during follow-up. Five patients required secondary
procedures for nonunion and angulation. Only one child failed and
subsequently required an amputation. Four patients ultimately achieved
healing, although they required nine bone-grafting procedures. Two
children had fractures through normal bone distal to the vascularized
bone graft; they also required bone-grafting procedures to achieve
union. Morbidity of the donor site was minimal, but one patient
sustained a nondisplaced fracture of the tibia through a screw hole,
and a 20° valgus deformity requiring osteotomy developed in another.
Thirteen tibiae had residual deformity: valgus deformity (five
patients); anterior angulation (two patients), or both (six patients).
The mean valgus deformity was 25° (range, 5° to 45°), and the mean
anterior angulation was 24° (range, 10° to 30°). Two patients with a
valgus deformity required correction with an osteotomy. Four patients
had anterior bowing of more than 20°, but none required additional
surgery. All children
were treated with orthoses until skeletal maturity was achieved.
-
Harvest of the vascularized bone with an intact vascular pedicle
-
Excision of the tibial pseudarthrosis and abnormal tissue
-
Fixation of the vascularized bone in situ
-
Microvascular anastomosis
-
Skin closure
teams. One team harvests the vascularized bone, and the second prepares
the recipient site. Some form of internal fixation is usually necessary
to maintain alignment of the extremity. One advantage of
microvascularized bone grafts is the simultaneous correction of any
residual deformity and possible tibial lengthening, depending on the
mobility of the tibial segments. Prolonged immobilization is necessary
until healing occurs, with protected weight bearing allowed thereafter.
Weiland et al. (285) maintain their children in
hip spica casts for 2–3 months to allow healing. After healing occurs,
protected weight bearing with a knee–ankle–foot orthosis is allowed.
The orthosis is worn until skeletal maturity (see Chapter 36).
achieving union at the pseudarthrosis site and in simultaneously
correcting any associated angular deformity and lengthening of the
tibia to restore length (85,99,119,144,211,224).
The apparatus can be used in four ways: compression of the
pseudarthrosis, compression with metaphyseal tibial lengthening,
compression followed by distraction for hypertrophic nonunion, and
distraction alone for hypertrophic nonunion. Excellent short-term
results for union were reported. Whether the union is maintained in the
long term remains uncertain.
the tibia after previous surgical procedures, an amputation may be
advised. This should be a Boyd or Symes ankle-disarticulation
amputation of the foot (82,134,149,189). See Chapter 175 on principles of pediatric amputation and Chapter 120 and Chapter 122
on lower-extremity amputations and prostheses. Amputation with
appropriate prosthetic fitting allows rapid rehabilitation and return
to normal function. McCarthy (189) recommended
amputation for several criteria: failure to achieve bony union after
three surgical attempts, a significant lower-extremity length
inequality (usually 5 cm or greater), development of a deformed foot,
undue functional loss from prolonged hospitalizations, and high medical
costs.
choice. It preserves the heel pad and distal tibial epiphysis, which
allows end bearing on the stump. The bone and skin are lengthened as a
unit to avoid problems with overgrowth (82). A
below-knee amputation through the pseudarthrosis produces a poor
end-bearing stump for ambulation. The abnormal tissue and previous
surgical scar provides poor skin coverage and predisposes to breakdown.
There are also the problems of overgrowth and frequent revision.
Amputation above the pseudarthrosis site provides better skin coverage,
but there are problems with bony overgrowth.
the results of Symes amputation in eight children with pseudarthrosis
of the tibia. The average age at amputation was 8.2 years, and the mean
follow-up was 5.9 years. These children had a mean of 3.8 surgical
procedures performed before amputation. None of the pseudarthroses
healed, but with an appropriate Symes prosthesis, the children were
able to engage in normal activities, including sports. The
lower-extremity length inequality and some of the angular deformity
were corrected within the prosthesis. Herring et al. (134)
reported that 21 children (none with congenital pseudarthrosis) who had
23 Symes amputations had better psychological functioning than children
undergoing multiple corrective surgical procedures. The better
psychological function correlated with their better orthopaedic
function. The level of family stress influenced the child’s behavior,
self-perception, and intelligence. The physicians thought that an early
Symes amputation in a young patient was compatible with good athletic
and psychological functioning, which closely approached that of a
nonhandicapped child of the same age. Similar results were reported by
Davidson and Bohne (82) for 23 children, including one with a congenital pseudarthrosis of the tibia that did not heal.
treatment of pseudarthrosis of the tibia, it is recommended that Symes
amputation be discussed as an alternative method of treatment with the
parents and child from the outset. Discussion should not be delayed
until later in the treatment. Tell the family of the difficulties that
will be encountered in attempting to obtain primary tibial union and
satisfactory function.
regimen, but all share long-term orthotic management. The extremity
needs to be protected with a plastic ankle–foot orthosis. This helps
prevent recurrent refracture. Protection is required at least until
skeletal maturity and perhaps even longer. This decision is based on
the radiographic appearance of the tibia, the degree of residual
deformity,
and the presence or absence of a reconstituted medullary canal.
after healing of a congenital pseudoarthrosis of the tibia is very
important. Karol et al. (156) recently
performed gait analysis on 12 patients with healed lesions and four
patients treated by amputation. Gait and muscle strength were markedly
disturbed. Early onset of fracture, early surgery, and transankle
fixation lead to an inefficient gait compared with that of amputees.
and challenging deformity. Refracture, tibial and lower-extremity
length discrepancy, stiffness of the ankle and subtalar joints,
progressive anterior angulation of the tibia, and ankle valgus are the
major complications (270,285).
Surgical complications are also common. Most children have had multiple
surgical procedures and are at risk for infection and neurovascular
injury. Because of these problems, the true outcome of a congenital
pseudarthrosis cannot be fully assessed until skeletal maturity.
Crossett et al. (74) found that the clinical
results for their patients remained stable after skeletal maturity.
Neurofibromatosis does not increase the incidence of complications or
adversely affect the final clinical result (198).
children with congenital pseudarthrosis of the tibia is to minimize the
number of operative procedures and to maintain as normal function as
possible. Prevention of fractures in children with prepseudarthrosis
lesions is critically important. This can sometimes be achieved with a
clamshell ankle–foot orthosis. After a pseudarthrosis is established,
the best results with respect to union are achieved with a vascularized
fibula graft or intramedullary rod. I feel that the initial surgical
procedure should be the latter. This allows straightening of the tibia,
with weight bearing providing compression across the pseudarthrosis.
The results of a vascularized fibula transfer are also good, but I am
concerned about a major operative procedure on the uninvolved
extremity. Once it is apparent that a pseudarthrosis cannot be
satisfactorily healed, Symes amputation and prosthetic replacement
permit restoration of relatively normal function.
tibia and fibula is unknown. There is some evidence to indicate a
primary chondro-osseous defect in the embryologic development of the
distal tibial and fibular epiphyses (138,212). Pappas (212)
demonstrated delayed development of the secondary center of
ossification of the distal tibia and a relative reduction in the height
of the distal epiphysis. Other possibilities include intrauterine
fracture of the tibia and fibula with malunion, restriction of growth
from soft-tissue contractures, or intrauterine malpositioning with the
affected leg molded under the buttock (15,83,107,165).
and medially at the junction of the middle and distal thirds of their
shafts. The deformity, which is obvious at birth, is usually
unilateral. The right and left sides are equally affected, and there is
no sex predilection (138). Infants are typically normal, and there is no increased incidence of other congenital anomalies (288). Hofmann and Wenger (138)
reported on a child who had a contralateral talipes equinovarus
(clubfoot) deformity. Angulation can vary from 25° to 65°, with the
magnitude of deformity in the posterior and medial directions being
almost equal (241). The foot is
hyperdorsiflexed and has a marked calcaneovalgus posture. It appears to
fit into the anterior cavity of the lower leg. The anterior compartment
muscles appear shortened and limit plantar flexion of the foot. The
posterior bow of the shaft causes the distal portion of the tibia and
fibula at the ankle to angulate anteriorly. This makes the limitation
of plantar flexion seem even more severe. There is no true bone
deformity of the ankle or foot. The calf musculature is usually
slightly atrophic, and the foot is smaller than on the opposite, normal
side (8,138). There may be a dimple at the apex of the posteromedial angulation (8,53,135,138,212). Occasionally, an extra skin crease is associated with the dimple (212).
extremities of an affected child are necessary for complete assessment.
The proximal aspects of the tibia and fibula, including their
epiphyses, are normal. The degree of posteromedial angulation of the
distal aspect of the tibia and fibula can be measured directly from the
radiographs. The cortices in the concave aspect of the posterior and
medial bows are thickened, and the distal aspects of the tibia and
fibula are broader than the opposite, uninvolved side (212).
The intramedullary cavities at the apex of the bowing are usually
poorly developed or obliterated by sclerotic bone. The alignment of the
tarsal and metatarsal bones is relatively normal, although occasionally
there may be a
slight
valgus orientation. Radiographs of the femora and pelvis should be
obtained for thorough assessment of the lower extremities. Special
diagnostic studies, such as MRI, are rarely indicated.
However, the associated shortening of the tibia and fibula, which is
unrelated to the bowing, persists and progresses during growth (8,138,212,288).
The fibula is frequently slightly shorter than the tibia; there is
usually no shortening in the femur. The mean growth inhibition in the
involved tibia and fibula averages 12% to 13% (range, 5% to 27%). This
percentage of growth inhibition persists throughout growth and
development. There appears to be a direct correlation between the
degree of tibial shortening and the degree of posteromedial angulation:
The greater the angulation, the more severe is the lower-extremity
length discrepancy (135,136,138,165).
The mean tibial length difference is approximately 1.2 cm in the first
2 months of life, 2.4 cm by 5 years of age, 3.3 cm at 10 years, and 4.1
cm (range, 3.3–6.9 cm) at maturity (138,212).
It is possible to determine the percentage of inhibition and the
ultimate leg-length inequality by annual scanographic evaluation and
bone-age determination after the posteromedial angulation has resolved.
During the first 6 months of life, correction of the bowing is rapid,
and by 2 years of age approximately 50% of the angulation has undergone
spontaneous correction. After 3 years of age, improvement in the
deformity occurs at a much slower rate.
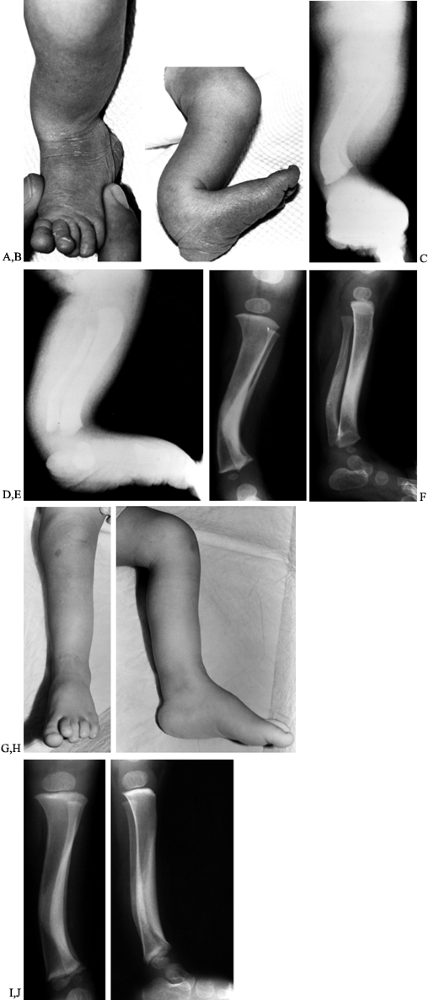 |
|
Figure 169.22. A:
Clinical photograph of a 1-month-old boy with congenital posteromedial bowing of the left tibia. Observe the medial bowing of the distal aspect of the tibia. B: The posterior bow of the distal tibia produces a calcaneovalgus appearance of the left foot. C: AP radiograph of the left leg confirms the severe medial bowing of the distal third of the tibia and fibula. D: Lateral radiograph demonstrates the posterior bowing and the calcaneovalgus appearance to the foot. The alignment of the foot is due to the dorsal angulation of the distal tibia and ankle. E: AP radiograph obtained at 1 year demonstrates decreased medial angulation of the distal tibia. F: Lateral radiograph shows a significant decrease in the posterior bow of the tibia and improved alignment of the ankle joint. G: Clinical photograph at 2 years of age shows marked improvement in the appearance of the left lower leg. H: There is only slight residual posterior angulation of the tibia in the sagittal plane. I: AP radiograph at 2 years of age shows further improvement in the medial angulation of the distal tibia. J: Lateral radiograph confirms further improvement in posterior angulation. |
growth and development. As the posterior bowing decreases, the degree
of plantar flexion improves. A pes planovalgus appearance of the foot
may persist. Hofmann and Wenger (138) found
mild loss of ankle dorsiflexion in older children. They thought that
this was due to mild equinus contracture from toe-walking to compensate
for the length discrepancy. It may also be due to the slightly shorter
fibula.
in children undergoes spontaneous resolution, treatment is
predominantly conservative. In newborn and young infants, passive
stretching exercises of the hyperdorsiflexed foot may be performed to
stretch the anterior compartment muscles and improve plantar flexion.
It is important to assess maximal plantar flexion of the foot on a
lateral radiograph because of the anterior angulation of the distal
tibia, fibula, and ankle joint. What appears to be limited plantar
flexion may only be secondary to the anterior tilt to the articular
surface of the distal tibia. The talus may be in full plantar flexion
in the ankle mortise, but the foot still may not appear plantigrade.
In 3–6 weeks, maximal stretching of the anterior compartment
musculature and anterior ankle capsule is usually achieved. Yadav and
Thomas (288) reported that six children with a
unilateral posteromedial bow of the tibia did well with serial casting,
although one patient underwent an anterior soft-tissue release before
initiation of casting. After complete correction has been obtained,
passive exercises may be continued to maintain alignment. In severe
cases, Tachdjian (269) recommended the use of
night splints to hold the foot in plantar flexion and inversion. After
2–3 years of age, a University of California Biomechanics Laboratory or
similar foot orthosis may be worn to support the planovalgus foot
deformity. However, in view of the natural history and management of
posteromedial bowing, the use of casts and orthoses is probably not
indicated. Heyman and Herndon (135) initially
thought that an orthosis was necessary to reduce the posterior thrust
at the apex of the deformity during weight bearing. However, in their
later report, they stated that the use of an orthosis was unnecessary (136).
-
Osteotomy to correct severe or persistent angulation
-
Equalization of lower-extremity length inequality
is rarely indicated. If severe medial bowing persists after 3 or 4
years of age, corrective osteotomy may be considered. Bone healing is
not a problem after a corrective osteotomy or a fracture because there
is no underlying bone disorder affecting healing (8,83,165,231). Hofmann and Wenger (138)
suggested corrective osteotomy in cases of severe bowing with
progressive shortening during the first 5 years of life. They thought
that an osteotomy would add length by correcting the deformity and
realigning the physes perpendicular to the axis of weight bearing,
stimulating growth. These concepts were not confirmed clinically.
Osteotomy can realign the tibia, but it has a minimal effect on the
ultimate lower-extremity length inequality. Krida (165) reported that all three patients treated by corrective osteotomies had significant residual leg-length discrepancies.
angulation of the tibia and fibula. Most affected children have enough
inequality (2 cm) to require equalization. The procedure performed to
equalize the leg length depends on the estimated tibial length
inequality at maturity and the predicted normal height of the child
(see Chapter 170).
I prefer prolonged observation. Casting of the foot is rarely
indicated, as the limitation of dorsiflexion is due primarily to the
alignment of the ankle. This will correct with growth. The associated
lower-extremity length inequality is followed by scanograms at 1- or
2-year intervals. An appropriately timed contralateral percutaneous
proximal tibial and fibular epiphysiodesis is the procedure of choice
for most patients. Leg-lengthening techniques are usually not
necessary, unless the discrepancy is severe or the patient has short
stature.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
DP, Weiner DS, Lloyd JK. Slipped Capital Femoral Epiphysis: A
Pathological Study. I. A Light Microscopy and Histochemical Study of 21
Cases. J Pediatr Orthop 1985;5:40.
DP, Weiner DS, Lloyd JK. Slipped Capital Femoral Epiphysis: A
Pathological Study. II. An Ultrastructural Study of 23 Cases. J Pediatr Orthop 1985;5:47.
DJ, Schoenecker PL, Sheridan JJ, Rich MM. Use of an Intramedullary Rod
for the Treatment of Congenital Pseudarthrosis of the Tibia. J Bone Joint Surg Am 1992;74:161.
I, Erken EHW, Molkin C. Progressive Valgus Deformity after
Juxta-epiphyseal Fractures of the Upper Tibia in Children. Injury 1987;18:169.
F, Rigault P, Padovani JP, Touzet P. Congenital Pseudarthrosis of the
Tibia in Childhood: Results of Treatment by Nailing and Bone Graft in
18 Cases. Fr J Orthop Surg 1987;1:331.
GA, Osebold WR, Ponseti IV. Congenital Pseudoarthrosis of Long Bones: A
Clinical, Radiographic, Histologic and Ultrastructure Study. Clin Orthop 1977;128:228.
JPH, Vardon D, Sales de Gauzy J. Development of the Clinical
Tibiofemoral Angle in Normal Adolescents: A Study of 427 Normal
Subjects from 10 to 16 Years of Age. J Bone Joint Surg Br 1995;77:729.
JR, Leeson MC, Thompson GH, et al. Late-onset Tibia Vara: A
Histopathologic Analysis: A Comparative Analysis with Infantile Tibia
Vara and Slipped Capital Femoral Epiphysis. J Pediatr Orthop 1988;8:187.
CW, Yu ZJ, Wang Y. A New Method of Treatment of Congenital
Pseudarthrosis Using Free Vascularized Fibular Graft: A Preliminary
Report. Ann Acad Med Singapore 1979;8:465.
SS, Coleman DA. Congenital Pseudarthrosis of the Tibia: Treatment by
Transfer of the Ipsilateral Fibula with Vascular Pedicle. J Pediatr Orthop 1994;14:156.
WP, David DS, Witsell J. Multiple Skeletal Complications in a Case of
Chronic Renal Failure Treated by Kidney Homotransplantation. Am J Med 1971;50:390.
HH, Verbout AJ, Nielsen HK, van der Eijken JW. Free Vascularized
Fibular Graft for Tibial Pseudarthrosis in Neurofibromatosis. Acta Orthop Scand 1988;59:425.
N, Della Corte S, Pempinello C, et al. Infantile Type of Blount’s
Disease: Complications concerning Etiopathogenesis and Treatment. J Pediatr Orthop B 1995;4:200.
WH Jr, Gross WL, Kirkpatrick JA Jr. Blount Disease (Tibia Vara):
Another Skeletal Disorder Associated with Childhood Obesity. J Pediatr 1982;101:735.
JP, Krajbich JL, Zucker R, Demuynk M. Congenital Pseudarthrosis of the
Tibia: Treatment with Free Vascularized Fibular Grafts. J Pediatr Orthop 1990;10:623.
GM, Staheli LT. The Natural History of Torsion and Other Factors
Influencing Gait in Childhood: A Study of the Angle of Gait, Tibial
Torsion, Knee Angle, Hip Rotation and Development of the Arch in Normal
Children. Clin Orthop 1974;99:12.
GA, Arulanantham K, Gage JR. Primary Hypophosphatemic Rickets: Effects
of Oral Phosphate and Vitamin D on Growth and Surgical Treatment. J Bone Joint Surg Am 1980;62:1130.
HL, Bailey RAJ, Price CHG. Infantile Pseudarthrosis of the Tibia: Three
Cases Treated Successfully by Delayed Autogenous Bypass Graft with Some
Comments on the Causative Lesion. J Bone Joint Surg Br 1969;51:604.
RK, Dickens DRV, Cole WG. Medial Physeal Stapling for Primary and
Secondary Genu Valgum in Late Childhood and Adolescence. J Bone Joint Surg Br 1995;77:733.
A, Brockman R. Congenital Pseudarthrosis of the Tibia: Long-term
Follow-up of 29 Cases Treated by Microvascular Bone Transfer. Clin Orthop 1995;314:37.
JSR, Bateson EM, McNeil-Smith JDG. Infantile Tibia Vara (Blount’s
Disease or Osteochondrosis Deformans Tibiae) In: Rang M, ed. The Growth Plate and its Disorders. Edinburgh: Churchill Livingstone, 1969:10.
A, Wosko I, Kandzierski G, Drabik Z. Double-elevating Osteotomy of
Tibiae in the Treatment of Severe Cases of Blount’s Disease. J Pediatr Orthop 1989;9:178.
G, Ingvarsson T, Ramgren B, Zayer M. Metaphyseal–Diaphyseal Angle in
Blount’s Disease: A 30-year Follow-up of 13 Unoperated Children. Acta Orthop Scand 1997;68:167.
CH, Herndon CH, Heiple KG. Congenital Posterior Angulation of the Tibia
with Talipes Calcaneus: A Long-term Report of Eleven Patients. J Bone Joint Surg Am 1959;41:476.
AC, Kooh SW, Fraser D, et al. Renal Osteodystrophy in Children with
Chronic Renal Failure: An Unexpectedly Common and Incapacitating
Complication. Pediatrics 1982;70:742.
GB, Poiakoff SJ, Bright RW. Surgical Correction of Severe Blount’s
Disease by Physeal Bridge Resection and Silastic Interposition. J Bone Joint Surg Br 1988;70:680.
LA, Haideri NF, Halliday SE, et al. Gait Analysis and Muscle Strength
in Children with Congenital Pseudarthrosis of the Tibia: The Effect of
Treatment. J Pediatr Orthop 1998;18:381.
O, Weintroub S. Serrated (W/M) Osteotomy: A New Technique for
Simultaneous Correction of Angular and Torsional Deformity of the Lower
Limb in Children. J Pediatr Orthop B 1995;4:204.
JS, Schink MM, Mitchell SN, Bassett CAL. Congenital Pseudarthrosis of
the Tibia: Treatment with Pulsating Electromagnetic Fields. The
International Experience. Clin Orthop 1982;165:124.
A. Pseudarthrosis of the Fibula with Progressive Valgus Deformity of
the Ankle in Children: Treatment by Fusion of the Distal Tibial and
Fibular Metaphysis: Review of 3 Cases. J Bone Joint Surg Am 1967;49:463.
AM, Drennan JC. Physiologic Bowing and Tibia Vara: The
Metaphyseal–Diaphyseal Angle in the Measurement of Bowleg Deformities. J Bone Joint Surg Am 1982;64:1158.
RL, Peterson HA, Bianco AJ Jr. Congenital Pseudarthrosis of the Tibia:
A Review of the Literature and 52 Cases at the Mayo Clinic. Clin Orthop 1974;99:140.
JF III, Moore R, Sekiya J, Koman LA. Congenital Pseudarthrosis of the
Tibia Treated with Free Vascularized Fibular Graft. J South Orthop Assoc 1997;6:227.
RWH, Levack B. Preliminary Observations of Epiphyseal Growth Rate in
Congenital Pseudarthrosis of the Tibia after Free Vascularized Fibular
Graft. Clin Orthop 1986;206:148.
RWH, Levack B, Satku K, Patradul A. Free Vascularised Fibula Graft in
the Treatment of Congenital Pseudarthrosis of the Tibia. J Bone Joint Surg Br 1985;67:64.
RB. External Rotation Contracture of the Extended Hip: A Common
Phenomenon of Infancy Obscuring Femoral Neck Anteversion and the Most
Frequent Cause of Out-toeing in Children. Clin Orthop 1975;110:139.
M, Khouri N, Carlioz H, Alain JL. Fractures of the Proximal Tibial
Metaphysis in Children: Review of a Series of 25 Cases. J Pediatr Orthop 1987;7:444.
M, Said SE, Glorieuz FH, et al. Principles and Results of Corrected
Lower Limb Osteotomies for Patients with Vitamin D–Resistant
Hypophosphatemic Rickets. Clin Orthop 1988;237:264.
RB, Best TN. Pathogenesis of Progressive Valgus Deformity Following
Fractures of the Proximal Metaphyseal Region of the Tibia in Young
Children. Instr Course Lect 1992;45:409.
RB, Best T. The Pathogenesis and Prevention of Valgus Deformity
Following Fractures of the Proximal Metaphyseal Region of the Tibia in
Children. J Bone Joint Surg Am 1973;55:1324.
T, Yagi T, Monji J, et al. Transepiphyseal Plate Osteotomy for Severe
Tibia Vara in Children: A Follow-up Study of Four Cases. J Pediatr Orthop 1986;6:61.
PL, Johnston R, Rich MM, Capelli AM. Elevation of the Medial Plateau of
the Tibia in the Treatment of Blount Disease. J Bone Joint Surg Am 1992;74:351.
HH, Sandrow RE, Sullivan PD. Complications of Tibial Osteotomy in
Children for Genu Varum and Valgum: Evidence That Neurologic Changes
Are Due to Ischemia. J Bone Joint Surg Am 1971;53:1629.
ML, Goldberg AAJ. The Treatment of Congenital Pseudarthrosis of the
Tibia with Pulsating Electromagnetic Fields: A Survey of 52 Cases. Clin Orthop 1982;166:45.
RW, Bowen JR, Guille JT, Choi IH. Prospective Evaluation of Fifty-three
Consecutive Percutaneous Epiphysiodeses of the Distal Femur and
Proximal Tibia and Fibula. J Pediatr Orthop 1991;11:350.
MP, Spaas FM, Fabry G. Progressive Valgus Deformity of the Knee after
Resection of an Exostosis at the Proximal Medial Tibial Metaphysis: A
Case Report. Acta Orthop Belg 1975;41:689.
L, Harcke TH, Brooks KM, MacEwen GD. Post-traumatic Tibia Valga: A Case
Demonstrating Asymmetric Activity of the Proximal Growth Plate on
Technetium Bone Scan. J Pediatr Orthop 1987;7:458.