Acetabular Fractures: Extended Iliofemoral Approach
reduction techniques, surgical implants, and preoperative and
postoperative evaluation of acetabular fractures have resulted in a
dramatic improvement in the outcomes in patients with acetabular
fractures. Nevertheless, the management of these injuries continues to
be a challenging problem for the orthopedic surgeon, in part, due to
the complex anatomy of the pelvis and acetabulum.
fractures is an accurate reduction of the articular surface such that a
congruent hip joint is obtained and normal joint mechanics are
restored. This is the primary tenet in the management of all
intra-articular fractures, and it is especially important in the weight
bearing joints of the lower extremity. In the case of the hip joint,
malreduction leads to abnormal loading of the articular cartilage and
subsequent painful posttraumatic arthrosis and loss of function.
fractures in general include displacement of the articular surface,
incongruence of the hip joint, unacceptable roof-arc measurements,
incarceration of an intra-articular fragment within the joint, and
subluxation of the femoral head. The timing of surgery is dependent
upon several factors including the availability of an experienced
surgeon; management of associated visceral, skeletal, and soft-tissue
injuries; and completion of all imaging studies necessary for
preoperative planning. Special situations arise such as in the case of
an incarcerated intra-articular fragment, an unreducible femoral-head
dislocation, or a femoral head fracture that mandate more urgent
intervention to prevent further damage to the articular cartilage or
minimize the risk of avascular necrosis of the femoral head.
Conversely, a Morel-Lavalle lesion deserves special attention and may
delay operative management of the acetabular fracture.
acetabular exposure may not be straightforward and is largely dependent
on the fracture pattern and the experience of the
surgeon.
Mayo identified five factors that affect the choice of surgical
approach: (a) the fracture pattern; (b) the local soft-tissue
conditions; (c) the presence of associated, major, systemic injuries;
(d) the age and projected functional status of the patient; and (e) the
delay from injury to surgery. Although elaborate, the Letournel-Judet
classification system is clinically useful in this regard. Injuries to
the pelvic ring must also be considered when determining the surgical
approach for an acetabular fracture.
the anterior and posterior columns for adequate visualization and
reduction. In these cases, an extensile approach will provide adequate
access to the roof of the acetabulum for anatomic restoration of its
articular surface. The extended iliofemoral approach was developed by
Letournel in 1974. It is one of the three most widely used surgical
approaches used to gain access to the acetabulum; the others are the
Kocher-Langenbeck and the ilioinguinal approaches.
columns of the acetabulum. It includes the lateral aspect of the iliac
wing, the internal iliac fossa, and the retroacetabular surface. It is
an anatomic approach that follows an internervous plane in which the
muscles innervated by the femoral nerve are reflected anteriorly and
the muscles supplied by the superior and inferior gluteal nerves are
reflected posteriorly. The posterior flap is mobilized as a unit
without damaging its neurovascular bundles (Fig. 43.1).
approach include (a) high (transtectal) transverse and T-type fracture
patterns with involvement of the weight bearing dome (Fig. 43.2);
(b) associated anterior column and posterior hemitransverse fractures;
(c) associated both-column fractures, with a posterior wall or a
comminuted posterior-column lateral-dome involvement (Fig. 43.3)
or extension into the sacroiliac joint; (d) transverse or associated
fractures where treatment has been delayed. Matta also considered this
approach in
certain
transverse posterior-wall fractures, such as those involving an
extended posterior-wall component where disruption of the
retroacetabular surface makes difficult the assessment of reduction
completed solely through a Kocher-Langenbeck approach.
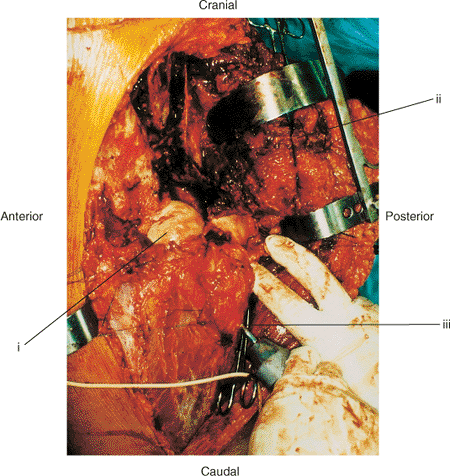 |
|
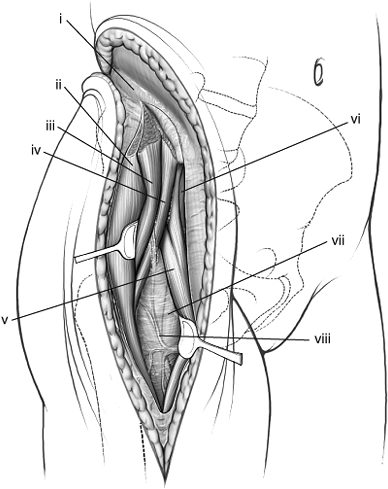
Figure 43.1. The extended iliofemoral approach for exposure of a comminuted left both-column acetabular fracture. (i) Femoral head. (ii) Abductor muscles and tensor fascia lata. (iii) Schanz pin in greater trochanter parallel with femoral neck.
|
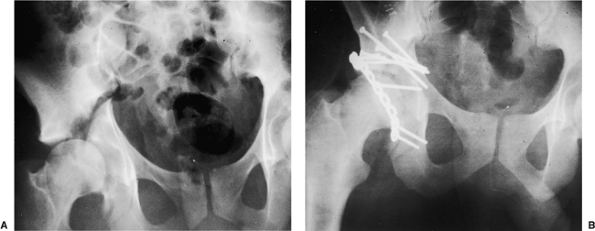 |
|
Figure 43.2. A 28-year-old man with right transtectal ischial T-type acetabular fracture. A. Preoperative AP pelvis. B. Postoperative AP pelvis.
|
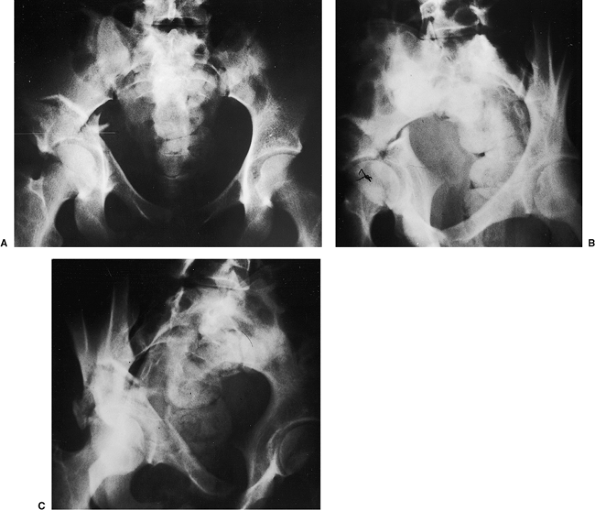 |
|
Figure 43.3. An 18-year-old woman with a both-column right acetabular fracture. A. AP pelvis. B. Iliac oblique view. C. Obturator oblique view.
|
approaches such as the ilioinguinal or Kocher-Langenbeck diminishes
because of limited joint visualization, organization of the hematoma,
increased formation and maturation of callus, and difficulty mobilizing
and reducing the fracture lines. Technical problems escalate at
approximately 2 weeks after injury with the ability to obtain an
anatomic reduction dropping from 75% to 62% of cases by the 3rd week.
This is due to the increasingly difficult task of taking down varying
amounts of callus, which will progressively interfere with the
anatomical reduction of all fractured segments of iliac crest and
acetabulum. Even in experienced of hands, late surgical reconstruction
of acetabular fractures results in excellent or good outcomes in only
65.5% of cases. Therefore, to improve the likelihood of achieving an
anatomic reduction, the extended iliofemoral method is the preferred
surgical approach for the most complex acetabular-fracture cases in
which surgery has been delayed more than 2 to 3 weeks.
Dissection medial to the iliopectineal eminence becomes more difficult
where the psoas muscle and iliopectineal fascia block exposure. While a
psoas tenotomy can increase visualization, the risk of injury to the
femoral artery and nerve must be considered.
extended iliofemoral approach. Blunt trauma to the gluteal muscle mass
and peritrochanteric region is probably the most common cause for
concern. Contusions and abrasions in this area are often associated
with the Morel-Lavalle lesion, an area of fluctuance secondary to fatty
necrosis and a hematoma that develops under the degloved skin and
subcutaneous tissues around the hip. The Morel-Lavalle lesion requires
surgical debridement and drainage before internal fixation and is
associated with a higher infection rate.
iliofemoral approach include the presence of a closed-head injury,
which may lead to massive heterotopic ossification. Extensile
approaches are generally avoided in the elderly because of the
prolonged operative
time,
extensive blood loss, prolonged rehabilitation, and increased risk of
infection and heterotopic ossification. Finally, the presence of
superior–gluteal vascular injury makes this approach less desirable
because ligation of the lateral femoral-circumflex artery during the
dissection removes a major source of collateral circulation to the
abductor musculature; however, this stance on the procedure remains
controversial.
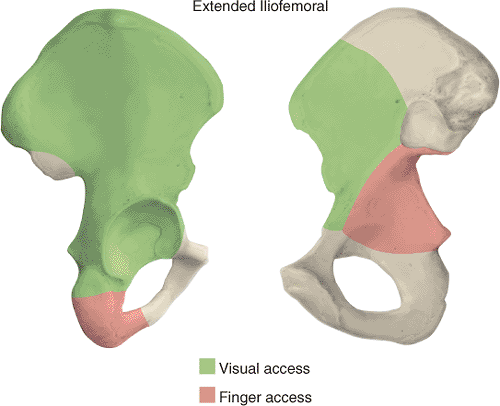 |
|
Figure 43.4. Access to the right pelvis via the extended iliofemoral approach. A. Lateral (outer) bony pelvis. B. Medial (inner) bony pelvis.
|
 |
|
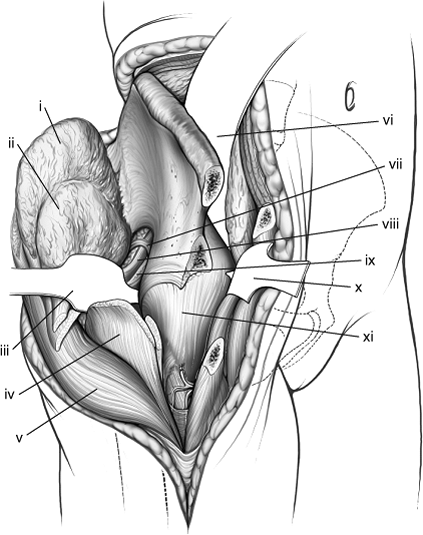
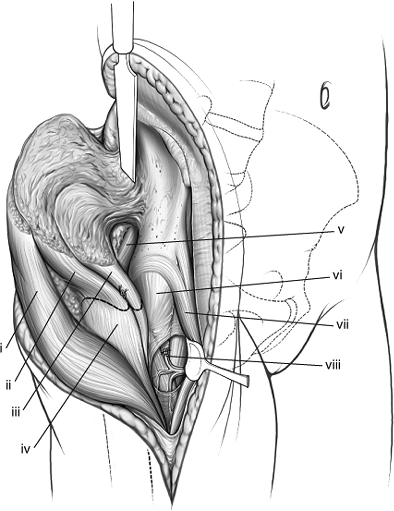
Figure 43.5. Maximal exposure of right acetabulum via the extended iliofemoral approach. (i) Gluteus medius muscle. (ii) Gluteus minimus muscle. (iii) Blunt Homan in lesser sciatic notch. (iv) Greater trochanter. (v) Tensor fascia-lata muscle. (vi) Malleable retractor under the iliacus muscle. (vii) Superior–gluteal neurovascular bundle. (viii) Piriformis muscle. (ix) Sciatic nerve. (x) Pointed Homan retractor over the anterior capsule of the hip. (xi) Hip-joint capsule.
|
history and physical examination, as well as an appropriate trauma
workup, to identify any associated skeletal and visceral injuries. An
accurate neurologic examination is mandatory, as the incidence of
sciatic nerve injury after acetabular fractures ranges from 12% to 38%.
classification can be accomplished with three basic roentgenograms
described by Judet et al and include an anteroposterior (AP) view of
the pelvis, an iliac oblique view, and an obturator oblique view of the
acetabulum. These three roentgenographic views provide sufficient
information to allow the surgeon to outline the fracture pattern on a
pelvic model as part of the preoperative plan.
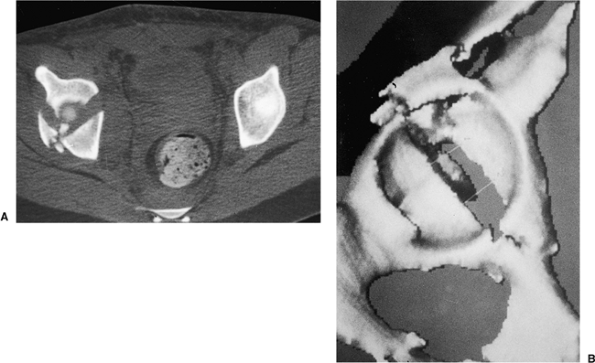
views provide additional information about the extent of injury to the
acetabulum (Fig. 43.6A), especially
identification of posterior-wall fractures, rotation of the columns,
the presence of intra-articular fragments or femoral head fractures,
and the assessment of articular displacement. Axial CT
scans
also can identify associated injuries to the posterior aspect of the
pelvis, such as a sacroiliac-joint disruption and sacral fractures.
Thin (1 to 2 mm) cuts should be used, along with sagittal and coronal
reformatting, to evaluate thoroughly the fracture pattern
preoperatively. Advances in imaging software technology have led to the
development of three-dimensional CT, which provides an even better
understanding of the spatial relation of the fracture pattern relative
to the pelvis (see Fig. 43.6B).
 |
|
Figure 43.6. CT of pelvis of patient in Figure 43.3. A. Axial view revealing significant dome comminution. B. Three-dimensional reconstruction facilitating perception of configuration.
|
or pelvic injuries, are at extremely high risk for developing deep vein
thrombosis (DVT); in some series, the DVT cases are at 60%. We screen
all of our acetabular fracture patients for DVT and treat them with
compression boots and subcutaneous low-molecular-weight heparin if a
delay in surgery is anticipated. Our preferred method of screening is
magnetic resonance venography, which we have found to be extremely
sensitive and reliable. Patients with an increased risk of DVT or those
with documented, preoperative DVT are managed with a vena cava filter
and intravenous heparin before surgery.
elevation of all the gluteal muscles with the tensor fascia lata, (b)
division of the external rotators of the hip, and (c) an extended
capsulotomy along the lip of the acetabulum. The end result is complete
exposure of the outer aspect of the ilium and the whole posterior
column inferiorly to the upper part of the ischial tuberosity.
Furthermore, the approach may be extended to allow a limited exposure
of the internal iliac fossa and the anterior column to the level of the
iliopectineal eminence. This allows simultaneous exposure of both
columns and permits direct visualization of the reduction and fixation
of the anterior and posterior columns (see Fig. 43.4).
The articular surface of the acetabulum along with the femoral head may
also be visualized if this approach is combined with a surgical
dislocation of the hip.
approach requires special training and a familiarity with the complex
anatomy of the pelvis, particularly the many neurovascular structures
that are encountered. Those structures that require identification are
listed below.
posterior column and must be identified, as in the Kocher-Langenbeck
approach, along the belly of the quadratus femoris muscle. Traction
along the nerve should be minimized by maintaining the hip in extension
with the knee flexed at all times.
exposure of the anterior–superior iliac spine. It is also very
susceptible to a traction injury during mobilization of the soft
tissues. Patients should be warned preoperatively of the significant
risk of numbness in the anterolateral thigh after this exposure.
during exposure of the greater sciatic notch. Therefore, it must be
protected from undue traction or penetration by retractors.
is the iliopsoas muscle and the iliopectineal eminence. Further medial
dissection without an ilioinguinal incision places the femoral
neurovascular structures at risk.
through the greater sciatic notch, wraps around the ischial spine, and
travels back into the pelvis through the lesser sciatic notch.
the continuous epidural anesthesia as it provides improved
postoperative pain relief. A Foley catheter is placed in the patient’s
bladder. The patient is supported on a beanbag and placed in the
lateral decubitus position on a radiolucent operating table or fracture
table, depending on the surgeon’s preference.
catheters is important for these lengthy procedures in which
significant blood loss is common. The patient’s age and medical
condition often dictate placement of an arterial or central line.
minimize transfusion requirements. This permits recycling of about 20%
to 30% of the effective blood loss and is best used when blood loss of
more than 2 L is expected.
the procedure to minimize sciatic nerve injury. In addition,
intraoperative sciatic-nerve monitoring with spontaneous
electromyography (EMG) and somatosensory evoked potentials (SSEP) is
used in all cases. The entire pelvis, hip, abdomen, and involved
extremity are prepped free, and sterile subdermal electrodes are
inserted. The sensory electrodes are inserted adjacent to the common
peroneal and posterior tibial nerves and the motor adjacent to the
tibialis anterior, peroneus longus, abductor hallucis, and flexor
hallucis brevis. The ground is inserted in the heel.
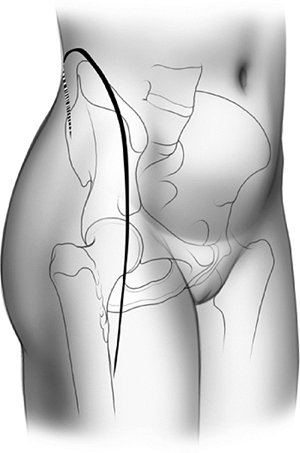
and begins at the posterior–superior iliac spine, extending along the
iliac crest toward the anterior–superior iliac spine (ASIS). From here,
the distal arm of the incision proceeds along the anterolateral aspect
of the thigh for a distance of 15 to 20 cm (Fig. 43.8).
The surgeon has a tendency to make this arm more medial than is
desired. To avoid this, one should visualize a point 2 cm lateral to
the superolateral pole of the patella. With the leg held in neutral
rotation, this location is generally in line with the desired incision.
Furthermore, a gentle posterior curve may be helpful in obese patients.
and divided sharply along its avascular “white line,” where bleeding
will be minimized. Often it is easiest to start in the area of the
gluteus medius tubercle where landmarks are more obvious and to
progress posteriorly and anteriorly from this point. Posteriorly, the
strong fibrous origins of
the
gluteus maximus should be sharply released from the crista glutei.
Depending on the starting location, the tensor fascia-lata muscle and
the gluteus medius are subperiosteally released in a stepwise fashion
from the outer aspect of the iliac crest (Fig. 43.10).
Using an elevator, the musculature along the external surface of the
iliac wing is released up to the superior border of the greater sciatic
notch and anterosuperior aspect of the hip joint capsule (Fig. 43.11).
During this segment of the exposure, care must be taken to identify the
superior-gluteal neurovascular bundle, which is at risk as it exits
from the notch.
 |
|
Figure 43.7. Inverted “J” skin incision, right side.
|
 |
|
Figure 43.8. Anterolateral view, right side. The inverted-J skin incision with distal extension for the extended iliofemoral approach.
|
 |
|
Figure 43.9. Subfascial exposure of right iliac crest and anterior distal limb. (i) Avascular white line. (ii) Fascia covering tensor fascia-lata muscle. (iii) Fascia covering sartorius muscle.
|
The distal limb of the incision is carried over the fascia covering the
tensor fascia-lata muscle, and the muscle sheath is entered. The
surgeon must stay within the bounds of the sheath, as this will keep
the dissection lateral to the lateral femoral-cutaneous nerve, sparing
the majority of its branches. It is often helpful to open the sheath
from distal to proximal.
fascia and retracted laterally and upward to expose the floor of the
sheath and fascia overlying the rectus femoris muscle (see Fig. 43.10).
Small vessels from the superficial circumflex artery are divided and
coagulated close to the bone between the superior and inferior spines.
Distally, the incision must be long enough to expose the inferior
aspect of the muscle belly. This facilitates further release of the
gluteal muscles from the crest.
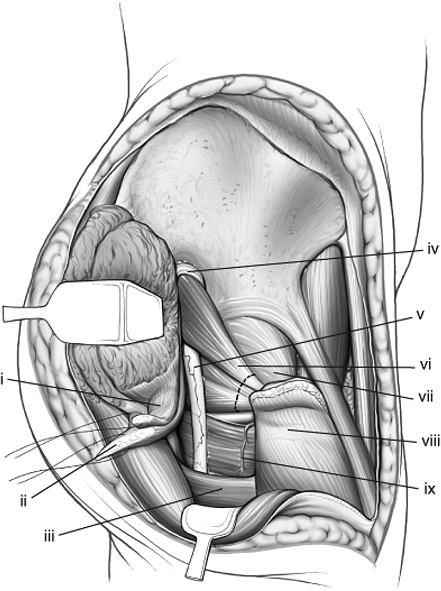
 |
|
Figure 43.10. Subfascial reflection of tensor fascia lata and abductor muscle origins from right iliac crest. (i) Avascular white line. (ii) Tensor fascia-lata muscle. (iii) Gluteus medius muscle. (iv) Gluteus minimus muscle. (v) Rectus femoris muscle. (vi) Sartorius muscle. (vii) No-name fascia covering vastus lateralis. (viii) Ascending branch of the lateral, femoral, circumflex artery.
|
 |
|
Figure 43.11.
Proximally, the abductor and tensor fascia-lata muscles have been stripped subperiosteally from the outer table of the right ileum. Distally, the ascending branch of the lateral circumflex artery has been ligated. The abductor insertions have been marked for release. (i) Tensor fascia lata muscle. (ii) Gluteus medius muscle. (iii) Gluteus minimus muscle. (iv) Greater trochanter. (v) Piriformis muscle. (vi) Hip-joint capsule. (vii) Two heads of the rectus muscle. (viii) Ligated ascending branch of the lateral, femoral, circumflex artery. |
longitudinally and horizontally, and its reflected head and direct
heads retracted downward and medially to expose a very strong
aponeurosis (the “no name” fascia) over the vastus lateralis muscle
(see Fig. 43.10). When the rectus is
retracted, a constant small vascular pedicle reaching the lateral
border of the muscle always requires coagulation. The aponeurosis can
be divided longitudinally to expose the ascending branches of the
lateral circumflex vessels, which must be isolated and ligated (see Fig. 43.11). Should the upper portion of this exposure be unnecessary, these vessels can occasionally be spared.
and longitudinally incised. This allows the use of an elevator to strip
the fibers of the psoas from the anterior and inferior aspects of the
hip capsule. The exposure of the iliac wing is complete when the
reflected head of the rectus femoris is sharply released from its
insertion.
into the anterior edge of the greater trochanter and tagged and
transected, leaving a 3- to 5-mm cuff for repair (Figs. 43.11 and 43.12).
The gluteus minimus muscle also has extensive attachments to the
superior aspect of the hip capsule that may need to be released.
Posteriorly and superiorly, the gluteus medius tendon, measuring 15 to
20 mm in length, is also isolated, tagged, and transected, leaving a 3-
to 5-mm cuff (see Figs. 43.11 and 43.12).
The surgeon must transect and tag these structures sequentially and
carefully for subsequent reattachment. The tensor fascia-lata and
gluteal muscles are held in continuity as a flap and reflected
posteriorly to expose the external rotators and sciatic nerve (see Fig. 43.12).
muscle, and the inferior and superior gemelli muscles are tagged and
transected as in the Kocher-Langenbeck approach (Figs. 43.12 and 43.13). The tendinous femoral insertion of the gluteus maximus is identified, tagged,
and released with a cuff for repair (see Fig. 43.13).
It cannot be overemphasized that the quadratus femoris and its blood
supply to the femur via the ascending branch of the medial femoral
circumflex artery must be preserved. The dissection is now complete
(see Fig. 43.1).
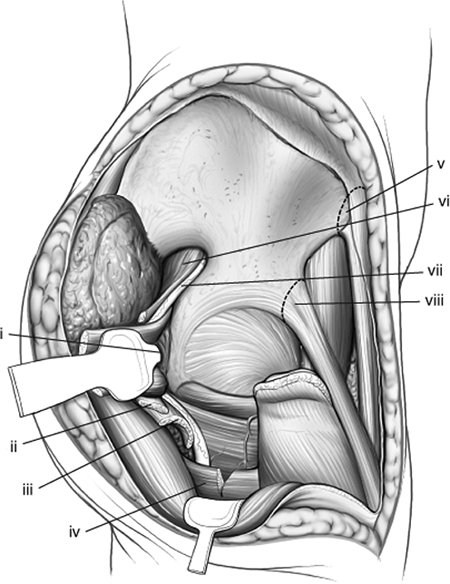
 |
|
Figure 43.12.
Abductors of the right hip have been tagged and their insertions into the greater trochanter released, allowing their muscle pedicle to be retracted to expose the sciatic nerve. The external rotators also have been marked for release. (i) Gluteus minimus tendon. (ii) Gluteus medius tendon. (iii) Gluteus maximus tendon. (iv) Superior–gluteal neurovascular bundle. (v) Sciatic nerve. (vi) Piriformis and conjoint tendons. (vii) Hip-joint capsule. (viii) Greater trochanter. (ix) Quadratus femoris. |
 |
|
Figure 43.13.
Retraction of right-hip external-rotator muscles and release of gluteus maximus insertion distally. Medially, the anterior–superior and anterior–inferior iliac spines have been marked for either release or osteotomy. (i) Blunt Homan in lesser sciatic notch. The conjoint tendons have been positioned between the retractor and the sciatic nerve. (ii) Gluteus minimus tendon. (iii) Gluteus medius tendon. (iv) Partial release of gluteus maximus tendon. (v) Anterior–superior iliac spine and sartorius muscle origin. (vi) Piriformis muscle. (vii) Sciatic nerve. (viii) Anterior–inferior iliac spine and reflected head of rectus femoris muscle. |
sciatic notch and the obturator internus muscle to the lesser sciatic
notch. A Homan or sciatic nerve retractor is then placed into the
lesser notch, allowing complete exposure to the posterior column of the
acetabulum. The surgeon must ensure that the tendon of the obturator
internus maintains its position in the lesser notch between the sciatic
nerve and the retractor. Should additional retraction be required, a
blunt Homan is gently placed into the greater sciatic notch, with the
surgeon aware that no structure is protecting the nerve. The distal
portion of the posterior column can be visualized to the ischial
tuberosity through sharp dissection of the origin of the hamstring
muscles proximally, if necessary.
further access to the internal iliac fossa and acetabulum is possible.
This access is obtained by subperiosteal dissection beneath the
sartorius and direct head of the rectus or by osteotomy of the superior
and inferior iliac spines, which will, respectively, release these
muscles (see Figs. 43.5 and 43.13).
The insertion of the external oblique muscle onto the crest can also be
subperiosteally released to reveal the inner table of the pelvis, which
is further exposed by the surgeon stripping off the iliacus muscle with
a periosteal elevator. However, extensile exposure of the outer and
inner tables of the iliac wing, especially in the presence of local
fractures, will create a risk of iliac-bone devascularization.
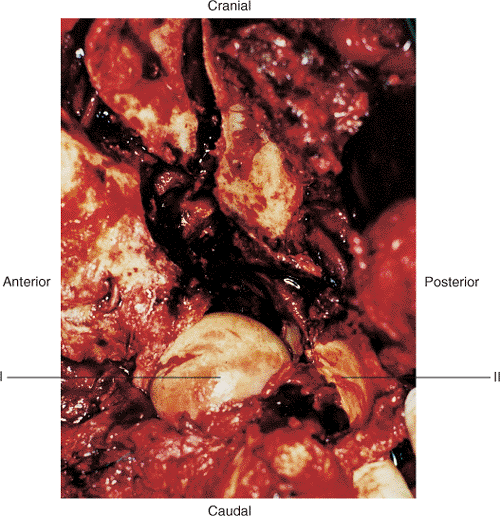 |
|
Figure 43.14. Close-up of acetabular-joint exposure of patient in Figure 43.1. (I) Femoral head. (II) Loose articular fragments.
|
Matta warned of its occurrence, especially in associated both-column
fractures. To avoid devascularization of the iliac bone in this case,
he suggested leaving, at a minimum, the direct head of the rectus
femoris and anterior hip capsule attached to the anterior column. Also
of concern with this exposure is the blood supply to the dome of the
acetabulum, which is at risk during dissection of the anterior–inferior
iliac spine.
capsule. If not present, exposure of the acetabular articular surface
can be obtained with a marginal capsulotomy, leaving a cuff of tissue
for repair. Once the hip joint is exposed, distraction with either a
Schanz screw placed into the femoral head or a femoral distractor will
facilitate visualization (Figs. 43.1 and 43.14).
The visualization is important for evaluating the articular reduction,
ruling out any intra-articular hardware, and removing any incarcerated
osteochondral fragments.
has been completed, the fracture can be reduced according to the
preoperative plan. The soft-tissue flaps must be kept moist with wet
sponges and periodic irrigation throughout the procedure.
These include the iliac crest, the superogluteal ridge, the greater
sciatic buttress (above the sciatic notch and to the anterior–inferior
iliac spine), the anterior column, and the posterior column. Extra-long
screws, ranging from 50 to 120 mm, should be available.
inferior portion of the acetabulum may be found. In the T-type fracture
patterns, the anterior and posterior fragments may be
separate
and both columns may have become displaced and malrotated. Usually, the
anterior segment has medial displacement of its inferior portion so
that the radius of curvature of the acetabulum is greater than that of
the femoral head. In both transverse and T-type acetabular fractures,
reduction is achieved with a pelvic-reduction clamp attached to 4.5-mm
screws placed proximal and distal to the posterior-column fracture. The
pelvic-reduction clamp initially allows distraction for debriding of
the fracture surfaces, and then facilitates manipulative reduction of
the fracture. A bone spreader in the fracture site can also facilitate
exposure of the fracture or the joint (Fig. 43.15A).
Additional control of rotation is provided by a Schanz screw placed
into the ischium and a pelvic clamp in the greater sciatic notch.
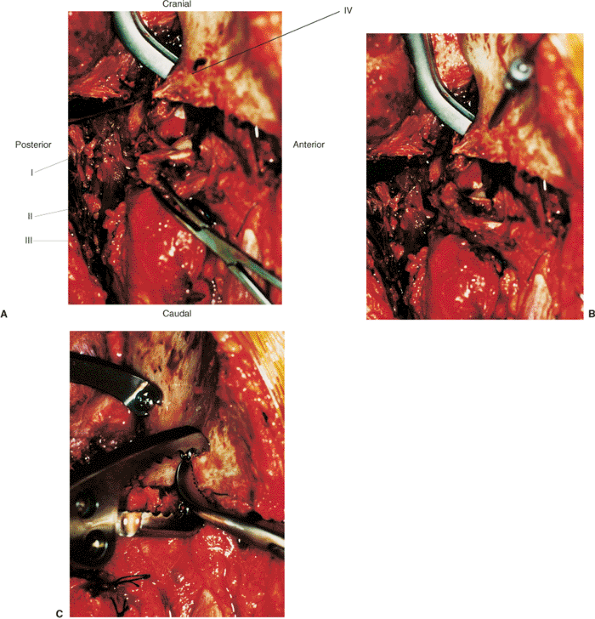 |
|
Figure 43.15. Steps to fracture reduction of the right both-column acetabular fracture in Figures 43.4 and 43.5. A. Laminar spreader in fracture site, exposing joint to allow debridement of loose intra-articular fragments and callus. (I) Femoral head in joint. (II) Superolateral dome fragment with capsular attachments. (III) Greater trochanter. (IV) Intact iliac wing. B. Predrilling the gliding hole for the anterior-to-posterior column screw. C.
Use of a Farabeuf clamp affixed to screws to reduce the anterior column to the superolateral fragment and a pelvic-reduction clamp affixed to screws to reduce the anterior-to-posterior column (posterior-column portion not shown). |
variants, the anterior column should be reduced first with respect to
the residual acetabular roof portion of the ilium. The adequacy of
reduction of the posterior column can be visualized by direct
assessment of the articular surface and also with digital palpation
through the greater and lesser sciatic notches.
inserted into the proximal aspect of the posterior column from superior
to inferior (see Fig. 43.15B). This hole helps the surgeon assure that the gliding hole is in the middle of the posterior column.
aspect of the iliac wing into the anterior column distal and medial to
the articular surface. Generally, this requires the insertion of a lag
screw 6 cm proximal to the superior aspect of the articular surface and
2 cm posterior to the gluteal ridge. The lag screw is then angled from
posterosuperior to anteroinferior directly down the superior pubic
ramus to secure the anterior column of the acetabulum. In large
individuals, this can be accomplished with a 4.5-mm cortical screw. In
small individuals, including most women, a 3.5-mm cortical screw is
preferred. Care must be taken to assure that this screw remains
extra-articular and also does not penetrate the anterior aspect of the
superior ramus in the area of the iliopectineal eminence where the
femoral vessels are in close proximity. The use of intraoperative
fluoroscopy for the insertion of this screw is highly recommended.
respect to the plane of the fracture and geometry of the osseous
surfaces is crucial for an adequate reduction. A variety of instruments
is available to facilitate reduction: narrow curved osteotomes, bone
hooks, ball spike pushers, King-Tong and Queen-Tong forceps, and the
Farabeuf and pointed reduction clamps (see Fig. 43.15C).
by reducing each fracture fragment sequentially. Once the iliac wing is
stabilized with lag screws, by 3.5-mm laterally applied reconstruction
plates, or both, the posterior column is reduced to the iliac wing as
the surgeon has direct visualization of the acetabular articular
surface.
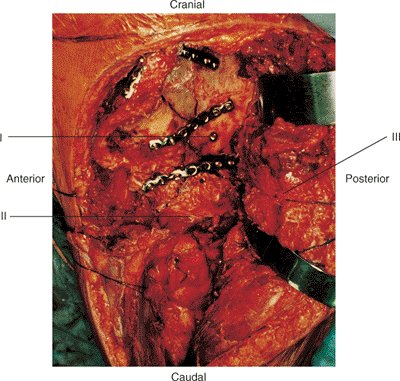 |
|
Figure 43.16. Reconstruction of comminuted left both-column acetabular fracture shown in Figures 43.1 and 43.14. Reconstruction proceeds centripetally from the periphery. (I) Posterior-to-anterior column lag screw. (II) Greater trochanter. (III) Abductor muscles and tensor fascia lata.
|
plate fixation is utilized for transverse and T-type fractures. The
anterior column is then reduced to the intact posterior column. This
reduction can be accomplished with anterior to posterior 4.5-mm lag
screws inserted from the anterior–superior spine into the sciatic
buttress, or anterior-column lag screws from the lateral aspect of the
iliac wing (as described previously), or both. The adequacy of the
reduction is assessed, both by direct visualization of the acetabulum,
with finger palpation of the greater and lesser sciatic notches and
quadrilateral plate, and if necessary, in the internal iliac fossa. The
use of fluoroscopy is essential to assure the adequacy of reduction and
the position of the fixation (Fig. 43.17).
chondrolysis, the surgeon must confirm hardware position before
closure. This confirmation is best achieved radiographically through
use of intraoperative fluoroscopic Judet views (especially the
obturator oblique) and clinically by rotating the hip back and forth
while a finger, to detect any crepitus, is
placed along the quadrilateral surface.
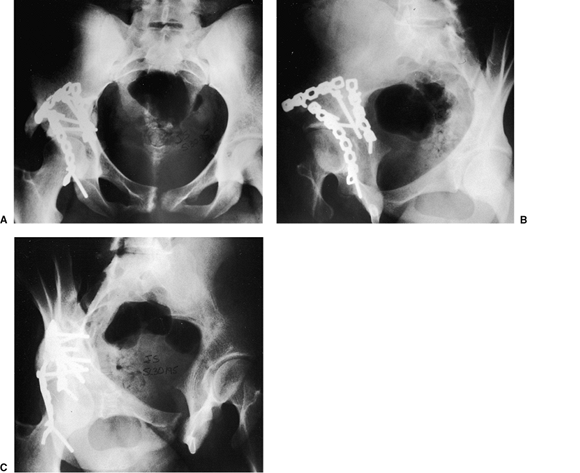 |
|
Figure 43.17. Patient from Figures 43.3 and 43.6 at 1-year follow-up. Congruent reduction and maintenance of joint space is shown. A. AP pelvis. B. Iliac oblique view. C. Obturator oblique view.
|
placed along the external surface of the iliac wing in the vicinity of
the posterior column and vastus lateralis muscle. If the internal iliac
fossa has been exposed, a third drain is placed here. All drains should
exit anteriorly.
reattachment of the tendinous insertions of the short external rotators
to the greater trochanter through drill holes, and femoral insertions
of the gluteus maximus. Next, the trochanteric insertions of the
gluteus medius and minimus muscles are repaired, through use of five or
six sutures for each tendon, as recommended by Letournel. Finally, the
tensor fascia-lata and gluteal muscles are reattached to their origins
on the iliac crest.
origins of the sartorius and direct head of the rectus femoris muscles
are reattached through drill holes. If osteotomies have been performed,
lag screws should be used.
muscle is repaired. Then a subcutaneous suction drain is placed and the
skin closed.
cefazolin for 48 to 72 hours. Our postoperative anticoagulation regimen
includes 6 weeks of warfarin in conjunction with compression boots.
Heterotopic ossification prophylaxis is also mandatory, preferably with
indomethacin 75 mg daily for 6 weeks. Drains are not removed until
output has tapered to 10 to 20 ml per 8-hour shift and the patient has
begun mobilizing, usually over the first 48 to 72 hours.
period, allowing patients to sit at the edges of their beds, dangle
their legs, and progress to chairs within the first 24 to 48 hours
after surgery. We do not use continuous passive motion as we have not
had difficulty regaining hip motion in this patient population.
undertake toe-touch weight bearing up to 20 pounds with crutches.
Strengthening exercises along with gait training are initiated by the
physical therapist. Weight bearing is not advanced and active
abduction, any adduction, and flexion of the hip past 90 degrees are
avoided for 6 to 8 weeks.
injury can pose a difficult rehabilitation problem because of lack of
muscle activity or neurogenic pain. These frequently require
consultation with a neurologist and the pain management service.
pelvis and 45-degree oblique Judet views) and a CT scan to assess
critically the fracture reduction and hardware position. The CT scan is
usually obtained on postoperative day 5, just before discharge. At the
time of discharge, home physical therapy is arranged.
sutures are removed. At the 6-week follow-up, new roentgenograms are
obtained, and generally, the abduction/adduction/flexion precautions
are discontinued. The patient returns at 8 to 10 weeks, and depending
on the roentgenographic findings, progression to full weight bearing is
allowed, as tolerated, over the ensuing 4 weeks. An aggressive
outpatient rehabilitation program should be initiated at this stage.
and is expected to be weight bearing as tolerated with the assistance
of a cane. In the absence of any contraindications, rehabilitation
becomes more aggressive with the initiation of strengthening exercises.
At 6 months follow-up the patient should be back to full activity.
Additional evaluation with radiographs are done at 1 year after the
surgery and then annually.
long-term clinical outcome following surgical fixation of acetabular
fractures is the quality of the reduction. Rowe and Lowell reviewed 93
acetabular fractures treated nonoperatively and noted poor results for
all 10 patients in whom the weight-bearing dome was not anatomically
reduced.
investigators have shown that long-term clinical outcomes correlate
closely with the quality of reduction achieved during surgery. In his
review of 569 acetabular fractures treated within 3 weeks of injury,
Letournel achieved an anatomic reduction (a maximum of 1 mm of
displacement on any of three views) in 74% of cases, with 82% of these
patients having very good clinical outcomes in follow-ups that were
conducted as late as 33 years after the surgery. Of the 26% with an
imperfectly reduced acetabulum, very good results were obtained in 54%
of cases if the femoral head was centered under the dome and in only
23% of cases where residual subluxation of the femoral head was
present. In patients treated within 3 weeks of injury, Letournel noted
osteoarthritis (OA) in only 10.2% of those with perfect reductions, as
opposed to 35.7% with imperfect reductions. In an interesting finding,
when treatment was delayed past 3 weeks, these rates were 24% and 23%
respectively.
results in 429 acetabular fractures. Like Letournel, they found that
clinical outcomes correlated well with the quality of reduction. In
their study, 89% of the patients with anatomic reductions had good or
excellent clinical results based on Harris Hip Scores. In 77% of
patients with fair or poor results, at least one of the following
predisposing factors was present: femoral head or neck injury,
acetabular impaction, marked displacement, preexisting arthritis, or
delayed presentation. In their study, 53% of their patients with morbid
obesity also had fair or poor clinical outcomes.
patients with articular surface incongruity or residual subluxation of
the hip joint. In the study of Mears et al, 12% of patients underwent
total hip arthroplasty or arthrodesis at an average of 5 years 2 months
postoperatively.
acetabular fractures, showed superior long-term results in their
patients when the articular surface was restored to within 4 mm. In a
similar retrospective study, Matta et al demonstrated satisfactory
clinical outcomes if the femoral head remained congruous within the
weight-bearing dome and if articular surface incongruity did not exceed
3 mm. However, in a subsequent prospective study, Matta and Merritt
suggested that 3 mm is probably unacceptable. He reported that an
anatomic reduction was achieved in 71% of 262 acetabular fractures,
with 83% of these patients having good or excellent outcomes at an
average follow-up of 6 years. Of the 29% with an imperfectly reduced
acetabulum, good or excellent results were obtained in 68% of cases if
the defect measured 2 to 3 mm, but these positive results were found in
only 50% of those with defects that measured more than 3 mm. The most
clear, predictive, initial factor for a poor result was damage to the
femoral head.
of Helfet and Schmeling who previously noted that an articular step-off
of more than 2 mm or a gap of more than 3 mm were associated with a
fourfold increase in joint space narrowing at early follow-up. Alonso
et al noted an 81% rate of good or excellent results in 21 patients
treated with an extended iliofemoral approach, in which all cases
achieved a reduction within 2 mm. Finally, Malkani et al and Hak et al
used cadaver models to further support 2 mm or less as the appropriate
criterion for an acceptable reduction.
postoperative period. It is more likely in elderly patients with
osteopenic bone where fractures must be adequately buttressed. The loss
of accuracy of reduction and the increased incidence of intra-articular
damage in the elderly population further compromise the outcomes in
this population.
acetabular fractures are best divided in three groups: intraoperative,
early, and late. Intraoperative complications include neurovascular
injury, malreduction, articular penetration of hardware, and death.
Early postoperative complications include DVT, pulmonary embolism (PE),
skin necrosis, infection, loss of reduction, arthritis, and death. The
late group includes heterotopic ossification (HO), chondrolysis,
avascular necrosis, and posttraumatic arthrosis.
preexisting deficit is a significant problem. In our experience,
patients at increased risk include those with preoperative sciatic
nerve compromise and those with fracture patterns that involve the
posterior wall or column. Other authors have identified patients
treated via an ilioinguinal approach to be at increased risk, possibly
related to indirect reduction of the posterior column with the hip
flexed. The peroneal nerve division is most commonly involved. The most
significant factor in reducing the incidence of iatrogenic
sciatic-nerve injury appears to be the experience of the surgical team.
postoperative, iatrogenic, sciatic-nerve injury using the
Kocher-Langenbeck approach, which he subsequently reduced to 3.3%.
However, he also noted that none of his 114 patients treated with an
extensile approach developed this complication. Matta initially
reported a 9% incidence of iatrogenic nerve palsy, which he reduced to
3.5% after gaining further experience (3.4% of 59 extended iliofemoral
approaches). The incidence of iatrogenic nerve palsy remained elevated,
with an overall incidence rate of 12%, when open reduction and internal
fixation (ORIF) was delayed longer than 3 weeks. The majority of these
injuries involved the sciatic nerve. Alonso et al found postoperative
sciatic-nerve palsy in only 1 of their 21 patients treated with an
extended iliofemoral approach.
use of SSEP remains controversial. In the studies of Helfet et al,
intraoperative nerve monitoring reduced the incidence of iatrogenic
sciatic nerve injury to 2%. However, more recent studies have
questioned the value of intraoperative SSEPs because the monitoring has
failed to demonstrate a reduction in the rate of iatrogenic nerve
palsies. A high false-positive rate makes unclear the extent to which
intraoperative SSEP changes predict functional outcome. Intraoperative
monitoring of motor pathways with EMG allows for earlier detection of
neurologic compromise and removal of noxious stimuli, and in theory,
decreasing the risk of neurologic sequelae. In one of Helfet’s studies,
the addition of spontaneous EMG to intraoperative SSEP monitoring was
superior to SSEP alone. Because of the significant learning curve that
exists in the treatment of acetabular fractures, most authors agree
that intraoperative monitoring may prove most beneficial among
relatively less-experienced surgeons.
and is caused by either the fracture or an iatrogenic insult during
surgery. Letournel reported an incidence of 3.5% in his series. This
potentially lethal occurrence is more likely to occur with severe
displacement of the sciatic notch (e.g., in high-transverse fractures
with marked medial rotation). Acutely, hemodynamic instability with an
arterial injury must be addressed during the initial evaluation and
resuscitation, and it is usually done with arteriography and
embolization. However, once the bleeding has been stopped, concerns may
arise with regard to muscle flap viability.
the superior gluteal vessels are the only blood supply to the flap. If
they are compromised, then complete ischemic necrosis is, in theory,
likely. Mears and Rubash developed their triradiate approach partly in
response to reports of flap necrosis following the extended iliofemoral
approach; however, it has not been established whether the superior
gluteal artery is to blame in these cases. In fact, the incidence of
this complication is relatively low. In over 400 acetabular fractures
addressed with an extended iliofemoral approach by Letournel, Mast,
Martimbeau, and Matta, no one reports abductor flap necrosis. Alonso et
al did not observe this complication when they used either an extended
iliofemoral or a triradiate approach in 59 cases of complex acetabular
fracture.
superior–gluteal artery injury combined with an extended iliofemoral
approach was postulated based on early animal and cadaver studies
alone. Canine studies by Tabor et al showed that although necrosis of
muscle
and
loss of mass occurs after the extended iliofemoral approach in the
presence of gluteal vessel injury, it does not appear to be
functionally significant. In their study, none of the gluteal muscle
flaps sustained complete ischemic necrosis. Thus, some collateral flow
to the abductor muscles must be present and appears to increase in the
presence of superior–gluteal vessel injury.
integrity of the superior gluteal artery, a preoperative angiogram be
completed prior to performing an extended iliofemoral approach.
However, a more recent study, based on intraoperative Doppler
examination by Reilly et al, demonstrated only a 2.3% incidence of
absent flow on the superior gluteal artery. No evidence of abductor
muscle ischemia was found in any patient of Reilly et al. This result
does not support the use of routine preoperative angiography in the
management of these injuries.
following operative fixation of acetabular fractures; the majority of
the deaths occurred in patients older than 60 years. Although DVT
probably plays a major role, its true incidence after an acetabular
fracture is unknown. However, patients with lower extremity trauma are
particularly a risk. By venography, Kudsk et al demonstrated a 60%
incidence of silent DVT in patients with multiple trauma immobilized 10
days or more. In a prospective study, Geerts et al also demonstrated a
60% incidence of DVT in patients with primary lower-extremity
orthopedic injuries. Letournel reported a 3% incidence of clinically
evident DVT with four fatal and eight minor pulmonary emboli in a
series of 569 patients, most of whom had received anticoagulant
prophylaxis.
prophylaxis and postoperative anticoagulation prophylaxis, venous
thrombosis and PE rates of less than 3% and 1% respectively, have been
achieved. Improved detection of venous thromboembolism through use of
magnetic resonance venography has also led to a lower incidence of PE
because it has inspired aggressive treatment of asymptomatic DVTs in
the pelvis and proximal thigh. However, other data suggest that
magnetic resonance venography has a high false-positive rate for the
detection of thrombi in the pelvic veins, and its usefulness as a
screening tool is still debated. Borer et al recently reviewed 973
patients with pelvis or acetabular fractures and found that the overall
rate of PE was 1.7%, and the overall rate of fatal PE was 0.31%.
Routine preoperative screening for DVT had no effect on the incidence
of PE in this study.
high as 19% but probably lies between 4% and 5%. Matta noted a 5%
incidence of postoperative wound infection in 262 patients. Of 59
patients who received extended iliofemoral approaches, 5 (8.5%)
developed deep infection. Mayo found a 4% overall infection rate, which
was 19% in 26 patients who underwent an extended iliofemoral approach.
Letournel reported 24 postoperative infections in 569 patients (4.2%)
with nine superficial, ten early deep, and five delayed or late
infections. Furthermore, he observed skin necrosis in 1.8% (10.2% of
extended iliofemoral approaches) and hematomas in 6.7% of cases. To
minimize wound problems, he advocated the use of prophylactic
antibiotics, multiple suction drains in all recesses to prevent
hematoma formation, surgical evacuation of hematomas, and if present,
debridement of the Morel-Lavalle lesion over the greater trochanter.
Other factors such as morbid obesity and burns must also be taken into
consideration as they may render the patient more susceptible to
infection.
fixation of acetabular fractures through the extended iliofemoral
approach is HO (Fig. 43.18), with an incidence ranging from 18% to 90%. However, functional limitation in patients with HO occurs in only 5% to 10%
of cases. Nevertheless, heterotopic bone formation is more common and
severe with the extended iliofemoral approach because of the external
surface of the iliac wing is stripped. Letournel reported its
occurrence in 46% of his extended iliofemoral approaches performed
within 4 months of injury; a 21% incidence was found in 635 other
approaches. Prior to his use of prophylaxis, these rates were 69% and
24% respectively. Matta noted a significant loss of motion in 20% and
Letournel observed severe HO (Brooker III and IV) in 35% of patients
treated with this approach within 3 weeks of injury. Both indomethacin
and low-dose radiation therapy (single or multiple fractions) have been
shown to decrease the incidence and severity of HO in patients with
acetabular fractures. However, concerns remain about the cost and the
long-term effects of radiotherapy, particularly in the younger trauma
population.
 |
|
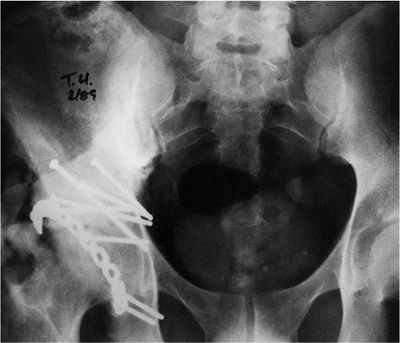
Figure 43.18. AP pelvis of patient in Figure 43.3,
at 5 months after extended iliofemoral approach. Significant (Brooker grade III) HO is present in the soft tissues of the right hip. |
by Johnson et al have reported rates of HO ranging from 86% to 88% in
patients treated with an extended iliofemoral approach. Of these
patients, Brooker class III or IV ossification was present in 14% and
13%, respectively. In the Johnson et al study, the majority of patients
in the treated group had Brooker class 0-II ossification, with the
untreated group having mostly Brooker III and IV ossification. A more
recent study by Moed et al showed a 50% incidence of HO after extensile
exposures in patients treated with indomethacin. Only one patient in
the treated group had severe (Brooker III-IV) ossification.
Indomethacin clearly does not eliminate the occurrence of HO, but it
significantly decreases its severity.
operative treatment of acetabular fractures has generally ranged from
3% to 9%, with the majority of cases identified between 3 and 18 months
after surgery. However, an increased incidence of AVN of the femoral
head is found in cases presenting after 3 weeks and those associated
with a posterior fracture/dislocation. In all probability, the fate of
the femoral head is determined at the time of the injury.
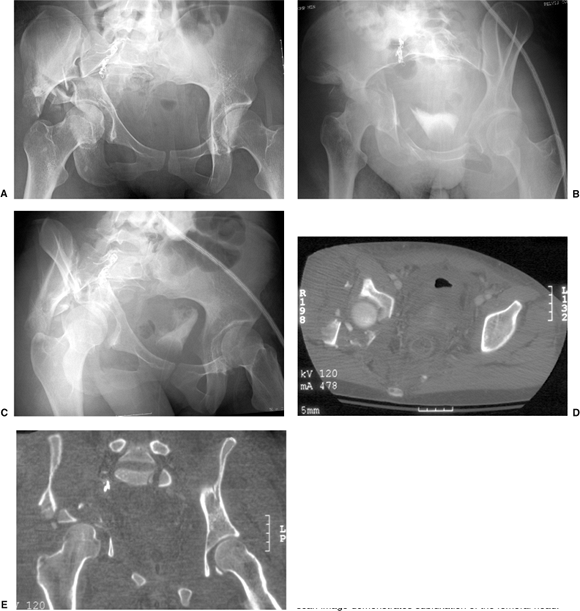
accident, sustaining a right associated both-column acetabular fracture
and extensive burns on the left side of her body. She also had a
Morel-Lavalle degloving injury involving her right thigh and buttock
and a preoperative,
right, sciatic-nerve injury with a foot drop. AP pelvis and Judet view radiographs and selected CT scan images, are shown in Figure 43.19A–E.
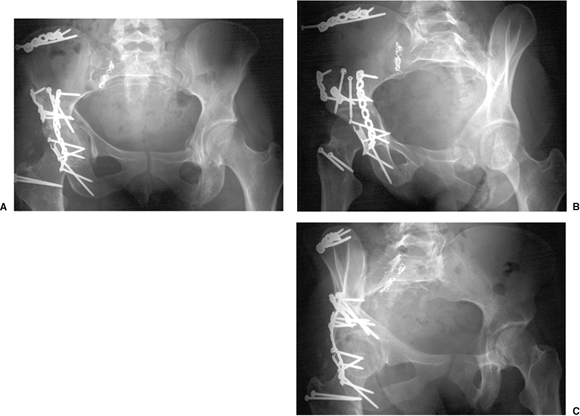
At 3 days postinjury, the patient underwent ORIF through an extended
iliofemoral approach. Her postoperative course was complicated by an
infection of the iliac crest wound which necessitated surgical
debridement and 6 weeks of intravenous antibiotics. Postoperative
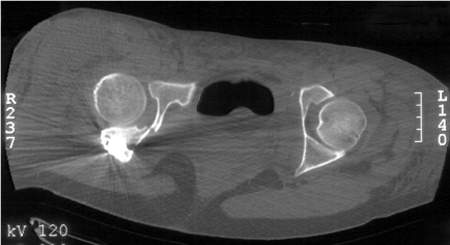
radiographs are shown in Figure 43.20. Her postoperative CT scan shows congruent reduction of the hip joint (Fig. 43.21).
At 5 months follow-up she has a healed acetabular fracture, is full
weight bearing, and has progressively improving sciatic-nerve function.
 |
|
Figure 43.19. Both-column acetabular fracture. A. AP pelvis. B. Iliac oblique view. C. Obturator oblique view. D. Axial CT-scan image showing extensive comminution. E. Coronal CT-scan image demonstrates subluxation of the femoral head.
|
 |
|
Figure 43.20. Postoperative radiograph. A. AP pelvis. B. Iliac oblique view. C. Obturator oblique view.
|
 |
|
Figure 43.21. Postoperative axial CT-scan image demonstrates congruent reconstruction of the hip joint.
|
JE, Davila R, Bradley E. Extended iliofemoral versus triradiate
approaches in management of associated acetabular fractures. Clin Orthop 1994;305:81–87.
DS, Starr AJ, Reinert CM, et al. The effect of screening for deep vein
thrombosis on the prevalence of pulmonary embolism in patients with
fractures of the pelvis or acetabulum: a review of 973 patients. J Orthop Trauma 2005;19(2):92–95.
J, Goldfarb C, Catalano L, et al. Assessment of articular fragment
displacement in acetabular fractures: a comparison of computerized
tomography and plain radiographs. J Orthop Trauma 2002;16(7):449–456.
MJ, Poka A, Reinert CM, et al. Heterotopic ossification as a
complication of acetabular fracture: Prophylaxis with low-dose
irradiation. J Bone Joint Surg 1988;70(8):1231–1237.
MJ, Poka A, Reinert CM, et al. Preoperative angiographic assessment of
the superior gluteal artery in acetabular fractures requiring extensile
surgical exposures. J Orthop Trauma 1989;2(4):303–307.
TA, Lowry KJ, Anglen JO. Indomethacin compared with localized
irradiation for the prevention of heterotopic ossification following
surgical treatment of acetabular fractures. J Bone Joint Surg 2001;83(12):1783–1788.
MW. Effect of surgical approaches on the blood supply to the
acetabulum. Paper presented at: The First Annual International
Consensus on Surgery of the Pelvis and Acetabulum; October 11–15, 1992;
Pittsburgh, Pennsylvania.
AJ, Greeno RA, Brooks LR, et al. Prevention of deep vein thrombosis and
pulmonary embolism in acetabular and pelvic fracture surgery. Clin Orthop 1994;305:133–137.
R, Gill TJ, Gautier E, et al. Surgical dislocation of the adult hip: a
technique with full access to the femoral head and acetabulum without
the risk of avascular necrosis. J Bone Joint Surg 2001;83(8):1119–1124.
DE. Clinical observations on fractures and heterotopic ossification in
the spinal cord and traumatic brain injured populations. Clin Orthop 1988;233:86–101.
N, Matta JM, Bernstein L. Heterotopic ossification following operative
treatment of acetabular fracture: an analysis of risk factors. Clin Orthop 1994;305:96–105.
GJ, Scaduto J, Herscovici D, et al. Iatrogenic nerve injury in
acetabular fracture surgery: a comparison of monitored and unmonitored
procedures. J Orthop Trauma 2002;16(5):297–301.
DJ, Olson SA, Matta JM. Diagnosis and management of closed internal
degloving injuries associated with pelvic and acetabular fractures: the
Morel-Lavallee lesion. J Trauma 1997;42(6):1046–1051.
M, Ostvogel H, Klasen H. Conservative treatment of acetabular
fractures: the role of the weightbearing dome and anatomic reduction in
the ultimate results. J Trauma 1987;27(5):555–559.
DL. Invited commentary on “incidence of sciatic nerve injury in
operatively treated acetabular fractures without somatosensory evoked
potential monitoring” by Middlebrooks et al. J Orthop Trauma 1997;11(5):329.
DL, Schmeling GJ. The management of acute, displaced complex acetabular
fractures using indirect reduction techniques and limited surgical
approaches. Orthop Trans 1991;15:833–834.
DL, Schmeling GJ. Somatosensory evoked potential monitoring in the
surgical treatment of acute, displaced acetabular fractures: results of
a prospective study. Clin Orthop 1994;301:213–220.
DL, Anand N, Malkani AL, et al. Intraoperative monitoring of motor
pathways during operative fixation of acute acetabular fractures. J Orthop Trauma 1997;11(1):2–6.
DL, Hissa EA, Sergay S, et al. Somatosensory evoked potential
monitoring in the surgical management of acute acetabular fractures. J Orthop Trauma 1991;5(2):161–166.
R, Judet J, Letournel E. Fractures of the acetabulum: classification
and surgical approaches for open reduction: preliminary report. J Bone Joint Surg 1964;46(8):1615–1646.
FA, Bone L, Border JR. Open reduction and internal fixation of
acetabular fractures: heterotopic ossification and other complications
of treatment. J Orthop Trauma 1991;5(4):439–445.
AL, Voor MJ, Rennirt G, et al. Increased peak contact stress after
incongruent reduction of transverse acetabular fractures: a cadaveric
model. J Trauma 2001;51(4):704–709.
JM. Fractures of the acetabulum: accuracy of reduction and clinical
results in patients managed operatively within three weeks of the
injury. J Bone Joint Surg 1996;78(11):632–645.
ES, Sims SH, Kellam JF, et al. Incidence of sciatic nerve injury in
operatively treated acetabular fractures without somatosensory evoked
potential monitoring. J Orthop Trauma 1997;11(5):327–329.
BR, Karges DE. Prophylactic indomethacin for the prevention of
heterotopic ossification after acetabular fracture surgery in high risk
patients. J Orthop Trauma 1994;8(1):34–39.
KD, Potter HG, Helfet DL. Magnetic resonance venography to evaluate the
deep venous system of the pelvis in patients who have an acetabular
fracture. J Bone Joint Surg 1995;77(11):1639–1649.
KD, Potter HG, Helfet DL. The detection and management of proximal deep
venous thrombosis in patients with acute acetabular fractures: a
follow-up report. J Orthop Trauma 1997;11(5):330–336.
KD, Goss K, Anglen JO. Indomethacin versus radiation therapy for
prophylaxis against heterotopic ossification in acetabular fractures: a
randomised, prospective study. J Bone Joint Surg 1998;80(2):259–263.
CM, Bosse MJ, Poka A, et al. A modified extensile exposure for the
treatment of complex or malunited acetabular fractures. J Bone Joint Surg 1988;70(3):329–337.
ML Jr, Swiontkowski MF. Operative treatment of complex acetabular
fractures: combined anterior and posterior exposures during the same
procedure. J Bone Joint Surg 1990;72(6):897–904.
U, Hoffmann R, Sudkamp NP, et al. Treatment of complex acetabular
fractures through a modified extended iliofemoral approach. J Orthop Trauma 2002;16(4):220–230.
MD, Morgan SJ, Bosse MJ, et al. Prospective comparison of
contrast-enhanced computed tomography versus magnetic resonance
venography in the detection of occult deep pelvic vein thrombosis in
patients with pelvic and acetabular fractures. J Orthop Trauma 2002;16(9):613–621.
OB, Bosse MJ, Greene KG, et al. Effects of surgical approaches for
acetabular fractures with associated gluteal vascular injury. J Orthop Trauma 1998;12(2):78–84.
M, Gordon RG, Mears DC, et al. Intraoperative somatosensory evoked
potential monitoring of pelvic and acetabular fractures. J Orthop Trauma 1992;6(1):50–58.
