Brachial Plexus Blocks
The patient lies supine, the head slightly to contralateral side, the
arm extended comfortably along the body; a roll sheet is placed under
both shoulders.
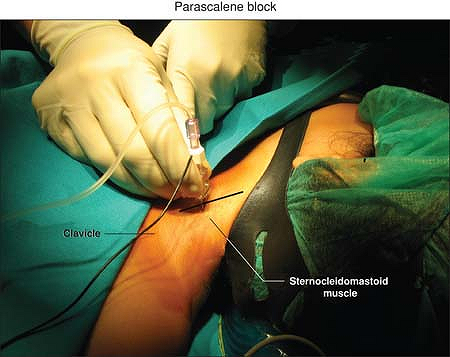
The clavicle, the lateral border of the sternocleidomastoid muscle, and
the transverse process of C6 (Chassaignac tubercle). The puncture is
made at the junction of the upper two-thirds with the lower third of
the line joining the C6 transverse process to the midpoint of the
clavicle (Fig. 47-1).
Find the superficial projection of the Chassaignac tubercle (insertion
of the transverse line at the level of the cricoid cartilage and the
lateral border of the sternocleidomastoid muscle). The site of
introduction of the needle is the junction of the upper two-third and
the lower one-third of the line joining the midpoint of the clavicle
and the Chassaignac tubercle. Set the nerve stimulator at a frequency
of 2 Hz and a current of 2.5 mA. Connect this to the pen dedicated for
the transcutaneous technique (instead of the pen it is possible to use
the negative electrode of the ENS) and point it perpendicularly to the
skin in an anteroposterior direction until a motor response
(contraction of biceps and/or brachial muscle) is elicited. Then insert
the needle connected to the nerve stimulator set at 1 mA and 2 Hz,
exactly at the point evidenced via transcutaneous in an anteroposterior
direction until a motor response is again elicited. Adjust the position
of the needle to maintain the appropriate muscle response with a
current of 0.4 to 0.5 mA. Then, after negative aspiration, slowly
inject the local anesthetic solution.
 |
|
Figure 47-1. The puncture point for the parascalene block.
|
-
The parascalene approach is the safest
supraclavicular approach to the brachial plexus, aiming at penetrating
the interscalene space at a distance from the apical pleura, the great
vessels and nerve of the neck, the stellate ganglion, and the spinal
canal. In children the use of the parascalene block is safer and has
lower incidence of complications than the other blocks (interscalene
and infraclavicular block). -
The brachial plexus is located at a depth of 7 to 20 mm from the skin.
-
This technique provides excellent
analgesia to the upper part of the arm, but in 50% of patients the
lower branches of the cervical plexus are also blocked. -
Complications include:
-
Horner syndrome (ptosis of the eye,
miosis, anophthalmos, hyperemia of the conjunctiva, hyperthermia,
anhidrosis of the face) for the stellate ganglion block. -
Because of the risk of respiratory
failure linked to bilateral phrenic paralisys given by bilateral block,
this block is contraindicated in cases of acute or chronic respiratory
insufficiency or whenever it is necessary a bilateral block. -
Vessel puncture of the large blood vessel of the neck (carotid artery and internal jugular vein) or of the vertebral artery.
-
Pneumothorax.
-
-
Epidural and intrathecal injections are avoided by using this technique.
-
If the position of the needle is too
lateral, causing the stimulation of the suprascapular nerve (levator
scapulae muscle), retract the needle and advance more ventral; if the
position is too ventral, causing the stimulation of the phrenic nerve
(unilateral singultus), retract the needle and advance more lateral.
B, Vanneuville G, Tanguy A. A new parascalene approach to the brachial
plexus in children: comparison with the supraclavicular approach. Anesth Analg 1987;66:1264–1271.
The patient lies supine, the arm on the side to be injected abducted at
the shoulder and flexed at a right angle at the elbow so that the wrist
is at the same level as the patient’s head.
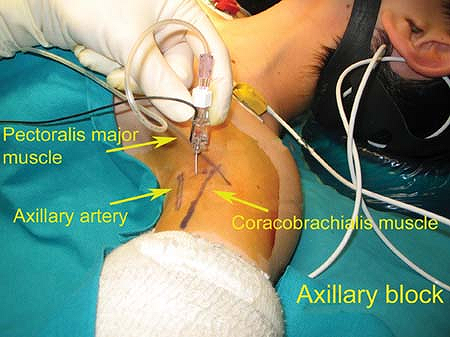
The axillary artery should be palpated and followed as high as possible
up into the axilla. The site of introduction of the needle is just
above the axillary artery which is firmly held by finger compression at
the crossing of the medial border of the coracobrachialis with the
lower border of the pectoralis major muscle. Set the nerve stimulator
at a frequency of 2 Hz and a current of 1.5 mA, then point the needle
in an anteroposterior direction until the motor response is elicited
(e.g., contraction of the hand—it is usually easier to stimulate the
median nerve with a flexion of the thumb and the first three fingers).
Adjust the position of the needle to maintain the appropriate muscle
response with a current of 0.4 to 0.5 mA. Then, after negative
aspiration, slowly inject the local anesthetic solution.
 |
|
Figure 47-2. Anatomic landmarks for an axillary block.
|
-
In infants and children, it is enough to block one of the components of the plexus to obtain a complete anesthesia of the hand.
-
The complication rate of the axillary
block is virtually nil, whatever the technique used. The single
described complication is hematoma if the axillary artery is injured
from the puncture being too deep. -
There are no specific contraindications except severe lymphadenopathy.
intraabdominal organs Sellheim and Läwen in the beginning of the
nineteenth century were the first to inject local anesthetics in the
paravertebral space (PVS) and the technique was later used by Kappis
(1919) to provide surgical analgesia for abdominal surgery. Deposition
of local anaesthetics in the PVS will lead to strict unilateral
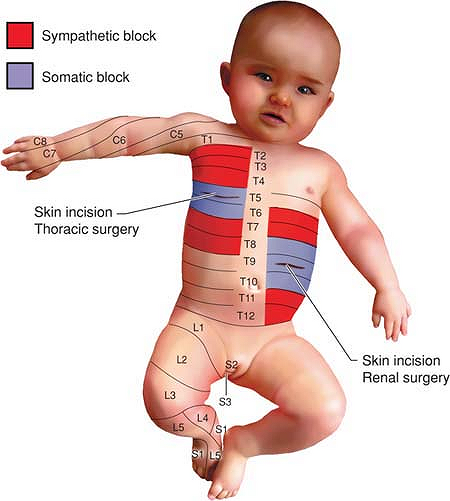
anaesthesia of one or more adjacent dermatomes (Fig. 47-3) and the main indications for paravertebral nerve block (PVB) are unilateral thoracic or abdominal surgical procedures (Table 47-1).
Sabanathan reported a method for intraoperative placement of a catheter
in the PVS during thoracic surgery and Lönnqvist described a
modification of the Eason-Wyatt technique for percutaneous preoperative
cannulation of the PVS.
 |
|
Figure 47-3. Distribution of somatic and sympathetic blockade after thoracic PVB for thoracotomy (right) and renal surgery (left), respectively; blue, somatic blockade, red,
sympathetic blockade. Recommended level of injection: thoracotomy, T5 or T6; renal surgery, T9. Recommended volume of local anesthetic, 0.5 mL/kg at all levels. |
|
Table 47-1. PVB: summary
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
angle between the lateral border of the vertebral body and the anterior
surface of the transverse process. The PVS only exists between Th 1-12.
Below Th 12 the space is sealed off by the origin of the psoas muscle
from the vertebral body and the transverse process. Cranially the space
appears to communicate with fascial planes in the neck since an upper
thoracic PVB may cause Horner syndrome. The different thoracic levels
of the PVS communicate, which is the foundation for spread of an
injection of local anesthetics to multiple segments (Fig. 47-4).
The medial boundary of the PVS is the lateral part of the vertebral
body and disc, the dorsal limitation is the transverse
process/costotransverse ligament, and the anterolateral boundary is the
parietal pleura. Significant structures that pass through the PVS are
the spinal nerve root/intercostal nerve, the sympathetic chain, and the
intercostal vessels. The PVS is not like
the
epidural space, since the pleura is very adhesive to the other
structures, but should instead be viewed as a potential space. This
fact accounts for the slight difficulty in introducing a percutaneous
catheter into the PVS.
level each individual level will have to be blocked separately since
the lumbar nerves exit through separate pockets within the psoas
muscle, which is the reason why there is no communication in-between
adjacent lumbar levels. This is in clear contrast to the situation at
the thoracic level.
and the needle is advanced in a perpendicular manner until contact is
made with the transverse process. The Tuohy needle (19 to 20 gauge if
≤1 y, 18 gauge if >1 y) is then “walked” below or underneath the
transverse process and by means of a loss-of-resistance technique the
costotransverse ligament is pierced and the PVS located. Alternatively,
the needle can be “walked” above or over the top of the transverse
process but by using this approach there is the risk of hitting the
neck of the rib before entering the PVS. If so, the needle will have to
be re-angled and it can occasionally be virtually impossible to get at
a reasonable approach to the PVS. Thus, to go below the transverse
process is clearly advocated by the author.
be injected after careful aspiration to exclude the presence of blood
or air. If a continuous technique is preferred, a catheter can be
introduced approximately 1 to 2 cm into the PVS through the Tuohy
needle. The insertion of the catheter frequently needs some
manipulation of the Tuohy needle in order to be successful and
occasionally one will have to make the injection of the bolus dose in
order to “open up” or “create” a space wide enough to allow catheter
insertion. One should not insert more than 1 to 2 cm of the catheter
into the PVS since further advancement may cause the catheter to go
into the spinal canal through the intervertebral foramen (causing an
epidural distribution of the block) or to go laterally, following the
path of the intercostal nerve (giving a dense block of only one single
dermatome).
skin puncture site (SP–PVS distance) and an estimate of the distance
from the skin to the PVS (S–PVS depth) can be calculated by the
following equations:
intervention but for a thoracotomy, the puncture should be performed at
Th 5-6 and for renal surgery at Th 9-10.
dermatomes are determined by manual palpation. The injection site is
marked 1 to 2 cm laterally to the midline on the intervertebral line
according to patient weight.
attached to a nerve stimulator (initial stimulating current: 2.5 to 5
mA, 1 Hz), is thereafter introduced perpendicularly to the skin in all
planes. A contraction of the paraspinal muscles is initially observed,
and the needle is subsequently advanced until the costotransverse
ligament is reached. At this point the contraction of the paraspinal
muscles will disappear.
muscular response of the corresponding level is sought and the needle
tip is manipulated into a position allowing a muscular response while
reducing the stimulating current to 0.4 to 0.6 mA. At this point the
local anesthetic is injected at the appropriate dose and volume.
paravertebral space is not an in-and-out movement but is rather an
angular manipulation and circumferential rotation around the axis of
the needle in order to reach an optimal position of the needle tip with
regard to the nerve within the paravertebral space.
By use of a curved haemostat the surgeon creates an extrapleural tunnel
from the medial angle of the parietal pleura incision to the PVS. At
this point a pocket stretching over 3 to 4 paravertebral segments is
made. An epidural catheter is then introduced into the paravertebral
pocket with the aid of the haemostat and the catheter is then secured
in place by one or two sutures that also will help to close the
extrapleural tunnel, so that the local anesthetic will not leak
backward into the pleural space. The catheter is then tunneled through
the thoracic wall with the use of a Tuohy needle or similar tunneling
device.
transverse processes and the depth to the PVS can easily be determined,
something that is quite helpful regardless of whether a
loss-of-resistance or nerve-stimulator guided technique is used. A more
specialized ultrasound guided technique has so far not been described
but is currently under development by the author.
block in association with orchidopexy. Adequate nerve block including
both the inguinal surgical field as well as testicular innervation is
accomplished by injection of local anesthetic at two separate
paravertebral levels. The injection made at one lower thoracic level
(Th10, 11, or 12) will block the testicular innervation as well as
parts of the surgical field in the groin. However, to get a complete
block of the inguinal region a separate PVB of L1 (below the transverse
process of L1) will have to be performed. This will result in two
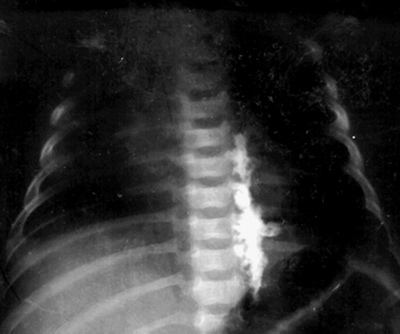
different types of spread as seen in Figure 47-4.
 |
|
Figure 47-4. Radiograph after injection of a mixture of local anesthetic and radio-opaque dye.
|
through just one single skin puncture. In this instance the skin
puncture is made lateral to the L1 spinous process and contact with the
transverse process of L1 is made. The needle is then walked over the
top of the transverse process and 0.375 ml kg-1 is injected.
This injection is thus made in the most caudal part of the thoracic PVS
and the local anesthetic will spread cranially to include at least the
Th12–10 levels. The needle is then withdrawn and walked below the
transverse process and the L1 level is blocked separately by the
injection of 0.125 ml kg-1.
contractions of the abdominal wall are clearly seen when the thoracic
level is approached and movement of the hip is seen when the L1 root is
simulated.
of a test dose, 0.5 mL/kg of the local anesthetic (levobupivacaine
0.25%, bupivacaine 0.25% with adrenaline or lidocaine 1% with
adrenaline) is injected in toddlers and older children. This dose will
usually cover at least five segments. Following the bolus injection, a
continuous infusion of the same local anesthetic solution (0.25 mL kg-1 h-1)
is started and can be used for approximately 48 hours. A typical
distribution of the block will be unilateral analgesia of the trunk
ranging from Th4 to Th12.
incision (e.g., thoracotomy, breast surgery, subcostal abdominal
incisions, renal surgery, inguinal hernia repair, and appendectomy).
The methods can also successfully be used for multiple rib fractures.
In children the main indications are thoracotomy, subcostal abdominal
incisions, renal surgery, and inguinal hernia repair. Anecdotal use of
PVB for postoperative pain relief following ductus ligation in
premature children and congenital diaphragmatic hernia repair has also
been reported.
analgesia after orchidopexy. The reason for the efficacy of PVB in this
context is that it (contrary to, for example,
ilioinguinal-iliohypogastric nerve block) is able to block the pain
transmission caused by testicular traction/dissection of the testicular
vessels. The pain fibers responsible for this type of pain travel
together with the testicular vessels to the Th10 level and can thus be
blocked by a low thoracic paravertebral injection of local anesthetic.
to learn this technique since the risk for serious complications at the
Th12/L1 level is minimal.
aortic aneurysm repair, laparoscopic cholecystectomy, and ventral
hernia repair, but in children this approach has so far only been
reported for surgical placement in association with bilateral
thoracotomy.
in a mixed adult and pediatric population was found to be associated
with an overall failure rate of approximately 10%; the complications
experienced were: hypotension 5% (only adults), vascular puncture 4%,
inadvertent pleural puncture 1%, and pneumothorax 0.5%.
nerve stimulator guided technique is used; this technique also appears
to be associated with a reduced risk for complications. Further
improvement can be expected when an ultrasound guided technique has
been refined.
be of benefit following thoracic surgery in babies and infants. In a
retrospective study the use of continuous PVB provided better
postoperative analgesia and lower morphine consumption compared with
continuous epidural analgesia. Two recent prospective randomized
clinical trials have demonstrated that PVB is superior compared with
both regular systemic postoperative analgesia as well as
ilioinguinal/iliohypogastric nerve blocks following inguinal hernia
repair.
-
Analgesia is limited to the area of surgery.
-
More complete afferent blockade.
-
Limitation of the sympathetic block; however, the block will include the sympathetic chain contrary to epidural blocks.
-
No risk for lower limb weakness or
paralysis and no risk for urinary retention, making routine Foley
catheterization unnecessary. -
No risk for unintentional damage to the spinal cord compared with thoracic epidurals.
-
No risk for significant block of the cardio-accelerator fibers.
-
Hemodynamic stability caused by a combination of points 3 and 6.
-
Presence of coagulopathy only constitutes a relative contraindication to the technique.
block also appears to posses some preemptive analgesic qualities,
something that has not been possible to demonstrate for epidural
analgesia.
postoperative analgesia in pediatric patients undergoing thoracotomy or
abdominal surgery with a unilateral incision. The block is no more
difficult to learn compared with other regional techniques and the use
of PVB therefore deserves much more widespread use than has been the
case previously.
SL, Booker PD, Franks R, Pozzi M. Serum concentrations of bupivacaine
during prolonged continuous paravertebral infusion in young infants. Br J Anaesth 1997; 79:9–13.
MK, Booker PD, Franks R. Bilateral continuous paravertebral block used
for postoperative analgesia in an infant having bilateral thoracotomy. Paediatr Anaesth 1997;7(6):469–471.
MK, Booker PD, Franks R, Pozzi M. Continuous extrapleural paravertebral
infusion of bupivacaine for post-thoracotomy analgesia in young
infants. Br J Anaesth 1996;76(6):811–815.
PA, Hesser U. Location of the paravertebral space in children and
adolescents in relation to surface anatomy assessed by computed
tomography. Paediatr Anaesth 1992;2:285–289.
PA, Olsson GL. Paravertebral vs. epidural block in children: effects on
postoperative morphine requirement after renal surgery. Acta Anaesthesiol Scandinavica 1994;38:346–349.
MZ, Ziade MF, Lönnqvist PA. General anaesthesia combined with bilateral
paravertebral blockade (T5-6) vs. general anaesthesia for laparoscopic
cholecystectomy: a prospective, randomized clinical trial. Eur J Anaesthesiol 2004;21(6):489–495.
ZM, Raf M, El Rajab M, et al. Comparison between nerve stimulator
guided paravertebral block vs. ilioinguinal nerve block for
postoperative analgesia following inguinal herniorrhaphy in children. Anaesthesia 2006, in press.
ZM, Raf M, El Rajab M, et al. Nerve stimulator-guided paravertebral
blockade combined with sevoflurane sedation versus general anesthesia
with systemic analgesia for postherniorrhaphy pain relief in children:
a prospective randomized trial. Anesthesiology 2005;103(3):600–605.
F, Wildling E, Klimscha W, Weinstabl C. Sonographic measurement of
needle insertion depth in paravertebral blocks in women. Br J Anaesth 2000;85(6):841–843.
J, Vowden P, Sabanathan S. Bilateral paravertebral analgesia for major
abdominal vascular surgery: a preliminary report. Anaesthesia 1995;50:995–998.
