Biological and Biophysical Technologies for the Enhancement of Fracture Repair
One – General Principles: Basics > 5 – Biological and Biophysical
Technologies for the Enhancement of Fracture Repair
biological process that includes multiple signaling pathways and is
regulated by both local and systemic factors. Despite this physiologic
control, it is estimated that between 5% and 10% of the fractures
occurring annually in the United States exhibit some degree of impaired
healing.67 In many instances, the cause of the impairment is unknown and may be related to inadequate reduction, instability,48 the systemic state of the patient,68,151 or the nature and extent of energy associated with the traumatic insult itself.170,228
In addition, there are certain areas within the appendicular skeleton
that have a predilection to impaired healing because of aspects of the
local biomechanical environment or anatomy of the blood supply.
Examples include open fractures of the tibia that have delayed union
rates of between 16% and 100% depending on the grade of injury92; the scaphoid and femoral neck, where the repair processes are influenced by the anatomy of arterial blood flow60,93,177; and the subtrochanteric region of the femur, where mechanical loads are among the highest in the appendicular skeleton.114
incident, complications related to delay in union or nonunion can be
severe with regard to patient morbidity and medical treatment costs.
Direct costs for treatments of tibia nonunions have been estimated to
be approximately $7500, and these can escalate to $17,000 when indirect
costs such as loss of work productivity are taken into account.38
To improve and expedite repair, surgeons may consider the use of bone
grafts or orthobiologic agents. This chapter will review the current
use and development of these materials in the restoration of skeletal
function.
is increasing, and the indications are growing with rising numbers of
spinal fusions, primary and revision arthroplasties, and periprosthetic
fractures.10,154,198,236
It is estimated that more than 2.2 million bone grafts are performed
worldwide each year, with 450,000 performed in the United States.140
In addition to the treatment of musculoskeletal injuries and
conditions, a significant number of grafts are used in the repair and
reconstruction of the craniofacial bones.218
bone graft because it provides the basic components required to
stimulate skeletal repair, including osteoinductive factors, an
osteoconductive extracellular matrix, and osteogenic stem cells present
in the form of bone marrow elements. Osteoinduction
refers to the process by which pluripotent mesenchymal stem cells are
recruited from the surrounding host tissues and differentiate into
bone-forming osteoprogenitor cells. This is mediated by graft-derived
growth factors such as bone morphogenetic proteins and other peptide
signaling molecules.211,229 Osteoconduction
is a process in which the macroscopic and microscopic architecture of
bone, as well as its surface chemistry and charge, serves as a scaffold
to support the ingrowth of blood vessels and the attachment of
osteoprogenitor cells. This occurs in an ordered sequence determined by
the three-dimensional structure of the graft, the local blood supply,
and the biomechanical forces exerted on the graft and surrounding
tissues.211 Osteogenesis
refers to the process of bone formation and is conducted by fully
mature osteoblasts. With regard to bone grafting, an osteogenic
material is one that contains live donor osteoblasts capable of
producing bone or osteoprogenitor cells that have the ability to
differentiate into osteoblasts in the host.
however, the morbidity associated with graft harvesting, such as donor
site pain, nerve or arterial injury, and infection rates of between 8%
and 10%,15,77,91,219,242
have prompted extensive research into alternatives. One alternative
that has gained acceptance for a variety of procedures is allograft
bone.35,65,94,108,200
While the problems related to autogenous graft harvesting are avoided,
limitations such as decreased or absent osteoinductive potential20 and increased cost have restricted its use.176
In addition, although current methods of donor selection and screening
have greatly reduced the risk, the issue of disease transmission
remains a concern for many patients and surgeons.16,110
For these reasons, the development of effective bone graft substitutes
and strategies for tissue engineering of bone have led to a new field
of study for the future of fracture management.
materials and technologies to enhance bone healing are compared. It
provides the ideal graft requirements in terms of osteoinductivity,
osteoconductivity, and osteogenicity. The most common and
best-described sources of autologous bone include the pelvis, the
distal radius,216 the fibula,137 the proximal tibia,171 and the ribs.145
potential for a graft-versus-host reaction is eliminated, as is the
risk of disease transmission. Based on the type of graft needed, either
cancellous or cortical bone can be harvested. There is also the
potential to harvest a vascularized graft, particularly from the fibula
or the rib. Careful planning is needed to ensure that the proposed
harvest site will contain both the correct type and amount of graft.
For example, a large segmental defect in the tibia would need a large
structural graft,73,159 whereas a tibia plateau fracture with a depressed fragment may just require a small amount of cancellous graft.
are similar to those involved in the normal repair process and include
hematoma formation and recruitment of circulating progenitor cells in
response to the release of proinflammatory and proangiogenic factors.24
The recruited cells then begin the process of graft incorporation, and
osteoclasts begin resorption of necrotic graft material. Pluripotential
mesenchymal cells respond to local growth factors and differentiate
into osteoblasts that produce osteoid. While osteoblasts and endosteal
lining cells on the surface of the graft may survive the
transplantation and contribute to the healing, it is likely that the
main contribution of the graft is to act as an osteoinductive and
osteoconductive substrate. These properties provide the necessary
physical and chemical requirements to support the attachment,
spreading, division, and differentiation of the cells that form bone.
The final stages in the process involve mineralization of the osteoid,
remodeling of the callus, and incorporation of the remaining graft. The
process of remodeling of the callus (composed of woven bone) involves
the coordinated activities of osteoblastic bone formation and
osteoclastic bone resorption, with woven bone ultimately being replaced
by lamellar bone.
specific types of fractures, particularly those that do not require
immediate structural support from the graft. Its main function is to
act as a scaffold for the attachment of host cells and to provide the
osteoconductive and osteoinductive functions required for the laying
down of new bone. The process by which the graft is replaced by new
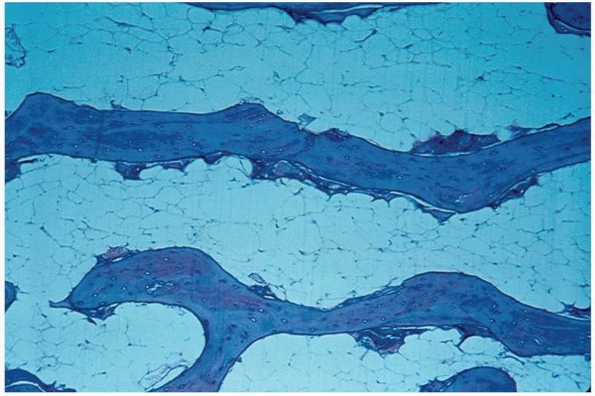
bone is known as “creeping substitution”210 and is usually complete within 1 year (Fig. 5-1, Table 5-1).
 |
|
FIGURE 5-1
Low-power photomicrograph showing creeping substitution. Newly formed woven bone, containing osteoblasts with basophilic-staining nuclei, is laid down upon dead lamellar bone identified by the presence of empty osteocytic lacunae (hematoxylin and eosin stain, original magnification ×10). |
|
TABLE 5-1 Properties of Types of Autologous Bone Grafts
|
||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||
iliac crest, distal radius, greater trochanter, and proximal tibial and
distal femoral metaphyses.130,171
While cancellous graft does not provide structural support by itself,
it can be impacted into skeletal defects and, in conjunction with
internal fixation devices, support areas of bone loss. Examples of this
use are in the treatment of depressed fractures of the tibial plateau
and in revision hip and knee arthroplasty where there is bone loss.224,225
osteoconductive and osteoinductive properties. Cortical bone grafts are
usually harvested from the ribs, fibula, or crest of the ilium (as a
so-called tricortical graft) and can be transplanted with or without a
vascular pedicle. Nonvascularized grafts are mostly osteoconductive and
possibly provide some osteoinductive properties but possess little or
no osteogenic properties because they contain very few osteoblasts or
osteoprogenitor cells (Table 5-1). Diffusion of
nutrients is limited by the thickness of the cortical matrix, and, as
such, the survival of transplanted osteocytes is limited.57
The density of the graft also plays a role in the incorporation and
remodeling process. Revascularization of the graft is slow as the dense
cortical bone must be resorbed. Remodeling proceeds as it does for
cancellous bone but can require up to 2 years for completion.37,72,203
nonvascularized counterpart, not only in terms of the rate of repair
but also in the way in which remodeling occurs.57
Once implanted with its viable vascular pedicle, there is the provision
of an immediate blood supply that is independent of the surrounding
bone. Using a canine model, Shaffer et al.203
demonstrated that at 1 week after transplantation, six of eight
vascularized grafts contained patent cortical vessels, while the
cortices of contralateral nonvascularized grafts did not show evidence
of vascularity until 6 weeks. In addition, osteocyte survival was
greater after vascularized transplantation. Dell et al.57
examined vascularized and nonvascularized grafts histologically and
graded the amount of necrosis based on the presence or absence of
osteocytes. At 2 weeks, the vascularized graft remained mostly viable,
with the only area of necrosis noted at the periphery. In comparison,
conventional nonvascularized grafts showed diffuse necrosis of the
medullary cavity and it was not until 24 weeks that the histologic
appearance of the two became similar. The increase in osteocyte
survival and the early vascularity seen in vascularized grafts are
consistent with the observation of more rapid incorporation of
vascularized bone graft compared with nonvascularized grafts.86,203
vascularized grafts are the result of the vascular source.
Nonvascularized grafts are incorporated from the outside in through
creeping substitution, resulting in substantial callus formation. In
contrast, vascularized grafts do not induce the vast angiogenic
response seen at the cortex of nonvascularized grafts and most of the
early mechanical strength is derived from the graft itself. By 6 weeks,
in a canine model, both grafts demonstrate comparable strength but
through vastly different processes.57
without grafting, also known as critical-sized defects, both
vascularized and nonvascularized grafts are indicated. For defects up
to 6 cm in length where immediate structural support is desired,
nonvascularized grafts can be used.75
Controversy exists regarding the best alternative for defects between 6
and 12 cm, while defects greater than 12 cm are good candidates for
vascularized grafting procedures.57,73
Vascularized grafts are also indicated for reconstruction of defects
where the microenvironment of the host is inadequate to initiate an
effective biological response. Examples of this include acute traumatic
injuries with extensive soft tissue damage and impairment of blood
supply, atrophic nonunions, and irradiated or severely scarred tissue.57,73
present or recruited to the site of injury to provide a source of cells
to differentiate into chondroblasts and osteoblasts during endochondral
and intramembranous bone formation. Progenitor cells have the
capability of differentiating into different cell types. At first these
cells are totipotent, in which case they have the ability to form any
cell type in the body, and then they progress toward more committed, or
monopotent cells. In contrast, multipotent cells, such as mesenchymal
stem cells (MSCs), can be directed toward cells of a specific germ
layer only.185
may be diminished, leading to delayed or possibly impaired fracture
healing.95,214 The aging process affects the available pool of stems cells, specifically the endothelial progenitor cells (EPCs) and MSCs.55,222
to be a source of autologous graft material. When Muschler et al.163
aspirated MSCs from the iliac crest, they noted that the mean
prevalence of colony-forming units expressing alkaline phosphatase
(CFU-APs), a marker of osteoblast progenitors, was 55 per 1 million
nucleated cells (57 patients; 31 men and 16 women; age range, 15 to 83
years and 13 to 79 years). The investigators also demonstrated an
age-related decline in the number of progenitor cells for both men and
women. When considered as graft material, these investigators showed
that the volume of aspirate used for grafting can affect the number of
CFU-APs. As the aspirate volume increases, so does the number of
CFU-APs. Contamination of the sample by peripheral blood, however,
increases as the aspiration volume increases. Increasing the aspirate
volume from 1 to 4 mL caused an approximate 50% decrease in the final
concentration of CFU-APs, resulting in the need to aspirate multiple
sites to obtain the needed number of progenitor cells.162
These and other findings have resulted in the search for alternatives
to standard autogenous bone marrow grafting, including the use of
allogeneic MSCs and expansion of autogenous cells in vitro.
demonstrate improved bone healing with grafts of autogenous bone marrow
containing MSCs.33,174,180 Early reports in patients with the use of unconcentrated bone marrow showed promising results. Healy et al.100
treated eight patients with nine nonunions using injections of freshly
harvested, unconcentrated autologous bone marrow. The nonunions were
the result of failed bone grafting with internal fixation following en
bloc resection of lower extremity sarcomas of bone. The results showed
that five of nine constructs had achieved union, with new bone
formation evident in seven of the patients.
studied percutaneous injection of concentrated autologous bone marrow
aspirated from the iliac crests in 60 patients with established
nonunions of the tibia. Analysis of the patients at 6 months found that
bony union had occurred in 53 of the patients as determined by clinical
and radiographic criteria. A retrospective analysis of the composition
of the graft found that osteogenic progenitor cell concentration was
significantly lower (<1000 cells/cm3)
in the seven patients who failed to achieve union in comparison to the
53 who did. In light of these findings, the authors recommend the use
of greater than 1000 progenitors/cm3 in the treatment of tibial nonunions.
The ability to concentrate stems cells and to culture and expand them
in vitro were two major accomplishments in the development of this
technology. Work in several laboratories demonstrated that MSCs could
also be isolated, cryopreserved, and expanded without the loss of
osteogenic potential.32,99,117
An important breakthrough in the use of MSCs as a bone graft substitute
occurred through a series of experiments in both humans and animals. It
was shown that allogenic MSC xenografts placed in utero would
differentiate and persist for up to 13 months. Others found, in humans,
autologous culture expanded MSCs could be infused without clinically
significant adverse events.136,145
Le Blanc et al. studied this phenomenon by combining MSCs with
allogenic mixed lymphocyte cultures and found that the lymphocytes
actually inhibited proliferation of the MCSs and that this suppression
was most significant for MSCs that had differentiated into the
osteoblastic lineage.137
Critical-sized defects in dogs treated with allogenic stem cells loaded
onto ceramic carriers have demonstrated healing similar to those
treated with autologous cells, and no immunologic responses were
observed at any time points.9
hypothesized that embryonic stem cells are deposited during
embryogenesis in various organs, including bone marrow, and may persist
in these locations into adulthood as pluripotent stem cells.85,132
These cells have the capability to both respond to a normal repair
process in the body and participate in the repair of soft tissue and
bone. Examples of such cells include very small embryoniclike (VSEL)
cells, multipotent adult progenitor cells (MAPCs), MSCs, and
marrow-isolated adult multilineage inducible (MIAMI) cells.184 These cells express the embryonic specific gene Oct-4+,
a gene that is downregulated during development. Although there is
little known about the use of these cells for skeletal grafting, there
is currently great interest in gaining a better understanding of their
potential because the use of embryonic stems cells in other organ
systems has yielded impressive results.186
grafts, as well as the limited amount of bone available to fill large
defects, has led to the use of alternative methods of treating skeletal
defects and promoting bony union. A popular alternative is the use of
allograft or allogeneic bone, because it is relatively abundant and has
shown good healing potential in several studies.46,108,274 Allografts are frequently used in spinal surgery64 and in joint arthroplasty71,160 and account for approximately one third of the bone grafts performed in the United States.28
Despite their widespread use in elective procedures, considerably less
is known about their use in the repair of fresh fractures or nonunions.
graft material may be attributed to its storage and sterilization
procedures such as freeze-drying or freezing that are used to lower the
rate of disease transmission. Freeze-drying, or lyophilization,
involves removal of water and vacuum packing of the tissue and has been
shown to significantly reduce immunogenicity, including the expression
of the major histocompatibility complex (MHC) class I antigen in
osteoblasts.80,243 Conversely, Pelker et al.178
demonstrated that such treatment of the graft reduces its mechanical
integrity, thereby diminishing its loadbearing properties. In addition,
freeze-drying reduces the osteoinductive potential of the allograft by
inducing the death of its osteogenic cells. Allogeneic bone is
available in many preparations including morselized and cancellous
chips, corticocancellous and cortical grafts, osteochondral segments,
and demineralized bone matrix.75
similar to those seen with nonvascularized autografts, except that they
occur much more slowly, particularly when large grafts are used.87
This is in part related to the lack of viable donor cells that
contribute to healing and the immune response that occurs during the
inflammatory process of allograft incorporation. This results in
limited revascularization, creeping substitution, and remodeling of the
graft.36,212
Studies have suggested that this lack of vascularization may account
for the high incidence of fractures seen with these grafts, which has
been reported to occur in between 16% and 50% of cases.74,221 Histologically, mononuclear cells invade the graft and surround newly developing
blood vessels. Necrotic graft bone remains in the host tissue much
longer compared with autograft bone and may be seen for many years
after implantation depending on the size of the graft and its anatomic
location.88,211
reported the histologic evaluation of 73 retrieved allografts. Of these
specimens, 24 (33%) were obtained at autopsy or after amputation. The
investigators were able to study the incorporation of grafts over time
and found that, overall, vascular penetration of the graft and healing
were poor. During the first 2 years, new vessel penetration rarely
exceeded a depth of 5 mm, and new bone apposition occupied no more than
20% of the graft. The depth of penetration after 2 years was typically
less than 10 mm, although 80% of the surface area of the graft was
found to be attached to the local soft tissues. Overall, necrotic
tissue remained in the central aspects of the allograft, and these
areas appeared to be isolated from the remodeling process.
critical factor in facilitating allograft incorporation. A
well-vascularized bed aids in the incorporation of the allograft
through a combination of revascularization, osteoconduction, and
remodeling.123
including the pelvis, ribs, and fibula. They are available as whole
bone segments for limb salvage procedures or they may be cut
longitudinally to yield struts that can be used to fill bone defects or
reconstitute cortical bone after periprosthetic fractures.97
The relative inertness of cortical allografts limits their potential to
achieve graft-host union. To improve this, autogenous graft harvested
from the iliac crest can be placed at the allograft-host bone
interface. This technique was described by Wang and Weng235
in the treatment of distal femoral nonunions. Thirteen patients with
femoral nonunions were treated with open reduction and internal
fixation with deep-frozen cortical allograft struts. Seven unicortical,
five bicortical, and one tricortical allograft, with an average length
of 10 cm, were used. Autogenous bone grafts were inserted into the
defect between the allograft and host femur. All nonunions united at an
average of 5 months.
The bioavailability of the growth factors contained in DBM results in
its greater osteoinductive potential than conventional allografts.76
These properties can be affected by different storage, processing, and
sterilization procedures. Donor-to-donor variability in the
osteoinductive capacity of DBM exists, resulting in the requirement by
the American Association of Tissue Banks and the U.S. Food and Drug
Administration (FDA) that each batch of DBM be obtained from a single
human donor.12
followed by hematoma formation and an inflammatory process
characterized by polymorphonuclear cell migration into the implant
within 18 hours. MSCs differentiate into cartilage-producing
chondrocytes by day 5. The cartilage becomes mineralized and is then
invaded by new blood vessels by 10 to 12 days. The accompanying
perivascular cells differentiate into osteoblasts, leading to new bone
formation. Remodeling then occurs with all implanted DBM being
eventually resorbed and replaced by host bone.211
reported a case series on the use of DBM in conjunction with bone
marrow in the treatment of 39 patients with either fresh fractures
associated with bone loss or comminution, nonunions, joint arthrodesis,
or cavitary lesions resulting from tumor or joint revisions. All 39
patients were available for follow-up and review, and 30 demonstrated
bony union. Patients with fracture nonunion represented the most
recalcitrant group clinically, with union being achieved in only 11 of
18 patients. Because no control patients were included in the study,
the efficacy of the DBM-bone marrow composite could not be determined.
Ziran et al.244 followed 107
patients treated with DBM and cancellous allograft bone chips for the
treatment of acute fractures with bone loss or atrophic nonunions, the
majority (18 of 25) of which occurred in smokers. They found that 87
fractures healed at a mean of 32 months.
the manufacturing processes. They are available as a freezedried
powder, granules, gel, putty, or strips. All have osteoinductive
effects in animal studies, while human studies have shown mixed
results. A prospective nonrandomized study comparing the use of
autograft and human DBM (Grafton, Osteotech, Inc., Eatontown, NJ) in
anterior cervical spine fusion found higher rates of pseudarthrosis and
graft collapse with DBM, although the differences did not reach
statistical significance.4 Ziran et al.245
retrospectively reviewed 41 patients with atrophic and oligotrophic
nonunions treated with human DBM (AlloMatrix; Wright Medical
Technologies, Memphis, TN). Postoperative complications were high, with
51% experiencing wound complications, of which 32% required operative
debridement. Of the 41 treated patients, only 22 went on to heal
without the need for additional bone grafting. Bibbo et al.23
studied the use of human DBM and calcium sulfate compound (AlloMatrix
Wright Medical, Arlington, TN) combined with vancomycin for the
treatment of calcaneal fractures. Their results demonstrated that
fractures treated with AlloMatrix and vancomycin healed at a mean of
8.2 weeks, compared with 10.4 weeks needed for those that were not
grafted. It is interesting to note that while the study was not
randomized, the fractures that received DBM and calcium sulfate
represented more significant injuries in that they had substantial bone
loss and included six open fractures (Gustilo grade 1). Hierholzer et
al.106 retrospectively reviewed the
results of the treatment of 45 aseptic nonunions of the humerus treated
with either autograft or DBM allograft (Grafton; Osteotech, Inc.,
Eatontown, NJ). The union rate in the 45 patients treated with
autograft was 100%, which was similar to the 97% union rate in 33
patients treated with DBM. Donor site pain was a significant problem in
the patients treated with autograft, with 44% of the patients
experiencing prolonged pain or paresthesias and one patient having a
superficial infection requiring operative debridement.
for the augmentation of fractures associated with bone loss, nonunions,
and small bone defects requiring grafting (e.g., a metaphyseal or
middiaphyseal cyst that has undergone curettage). Diaphyseal defects up
to 12 cm in length can be treated with nonvascularized cortical
autografts. For defects
of
more than 12 centimeters, vascularized cortical autografts are
recommended. We do not believe there is sufficient information, nor
have there been sufficient studies providing good evidence, to support
the use of freshly harvested, unconcentrated autologous bone marrow in
traumatic or reconstructive orthopaedic surgery. Because the number of
osteoprogenitor cells in any human bone marrow aspirate is very small,
it is unclear if this complement of cells can support a robust
osteogenic response. However, freshly harvested autologous bone marrow,
obtained by multiple aspirations of no more than 5 mL each, in
conjunction with the use of so-called selective retention methods or
methods involving centrifugation of the freshly harvested bone marrow
may optimize the concentration of osteoprogenitor cells and serve as an
effective graft material.103,105
bone to enhance the healing of fresh fractures or nonunions. We suggest
that allogeneic cancellous bone chips be used to augment the healing of
fresh fractures associated with bone loss or to treat nonunions when
used in conjunction with autologous bone to make up a sufficient volume
of graft material. Incorporation of allogeneic strut grafts may also be
enhanced by the use of autogenous cancellous bone at the junction with
the host bone.
unclear. Although widely available and known to contain bone
morphogenetic protein (BMP), we do not believe there is sufficient
evidence demonstrating its efficacy when used alone in the treatment of
fresh fractures or nonunions or the reconstruction of bone defects.
However, we and others have used DBM in conjunction with autologous
cancellous bone to increase the volume of graft material. We have also
used DBM as a delivery vehicle for bone marrow aspirate concentrate. In
these settings, we believe that DBM provides an osteogenic advantage
and may enhance the ability of a fixed volume of autologous graft or
bone marrow to be effective.
elements: scaffolding for osteoconduction, growth factors for
osteoinduction, and progenitor cells for osteogenesis.233
The currently available materials, including calcium phosphate
ceramics, calcium sulfate, bioactive glass, biodegradable polymers,
recombinant human BMPs (osteogenic protein 1 [OP-1] and BMP-2), and
autologous bone marrow cells, each fulfill only some of these criteria.139
appropriate three-dimensional structure to allow for osteointegration
and invasion by cells and blood vessels. It should also be
biocompatible and biodegradable with biomechanical properties similar
to those of the surrounding bone. Many of the ceramics used as bone
grafts enable osteoconduction to occur.69,234 Despite this, their brittleness and poor tensile strength limit their use as bone graft materials.
Since then, several animal studies have reported favorable results.
Despite these early experiments, it was not until the 1970s that
calcium phosphates, and in particular, hydroxyapatite (HA), were
synthesized, characterized, and used clinically.118,156,191
Calcium phosphate ceramics are osteoconductive materials produced by a
sintering process in which mineral salts are heated to over 1000°C.
Sintering reduces the amount of carbonated apatite, an unstable and
weakly soluble form of HA.
divided into slow and rapid resorbing ceramics, and this difference is
important with regard to whether the compound will need to provide
long-term structural support or is acting as a void filler that will be
quickly replaced.76 HA is a slow resorbing compound that is derived from several sources, both animal153 and synthetic.102,187
Interpore (Interpore International, Irvine, CA) is a coralline
hydroxyapatite and was the first calcium phosphate-based bone graft
substitute approved by the FDA. A simple hydrothermal treatment process
converts it from its native coral state to the more stable HA form with
pore diameters of between 200 and 500 µm, a structure very similar to
human trabecular bone. Bucholz et al.34
investigated its use to treat tibial plateau fractures. Forty patients
with metaphyseal defects needing operative reduction were randomized
into a control group treated with autogenous bone graft or a group
treated with Interpore HA. Indications for surgery included valgus
instability of the knee secondary to a lateral tibial plateau fracture,
varus instability because of a medial plateau injury, articular
incongruence of 10 mm or greater, and translation of the major condylar
fragment of greater than 5 mm. After insertion of the graft, cortical
fracture fragments were reduced, and a standard AO interfragmentary
screw and plate fixation device was used to stabilize the reduction.
With an average of 15.4 months for the autograft and 34.5 months for
the Interpore-treated groups, radiographic and functional knee joint
assessments revealed no differences between the two groups. No evidence
of ceramic resorption was found in the radiographic follow-up 3 years
after implantation, highlighting the potential use of HA as a bone
filler. Attempts at using HA as a stand-alone implant for fixation in
distal radius fractures did not show such promising results.119
Compared with Kapandji wiring, those fractures treated with HA only
showed substantial loss of reduction at 6, 12, and 26 weeks. Clinical
parameters were also decreased for the HAtreated patients with regard
to decreased grip strength and palmar flexion.
undergoes partial resorption and some of it may be converted to HA once
implanted in the body. The composition of TCP is very similar to the
calcium and phosphate phase of human bone. This combined with its
porous nature appears to facilitate incorporation with host bone in
both animals and humans by 24 months.11,84
investigated the suitability of TCP to treat bony defects in a case
series of 43 patients with 33 acute fractures and 13 nonunions.
Patients were followed for an average of 1 year. Healing was
demonstrated in 90% of the fracture patients and 85% of those with
nonunions. Radiographic analysis showed complete resorption of TCP
between 6 and 24 months after implantation.
and located in the lower extremity. The average defect size was 43 cm3,
and the patients were followed for an average of 10 months. Full weight
bearing in patients with a lower extremity defect occurred at a mean of
7 weeks, and radiographic follow-up showed that the graft had
completely resorbed in all but except patient at 6 months.
most abundant protein in the extracellular matrix of bone and promotes
mineral deposition by providing binding sites for matrix proteins.
Types I and III collagen have been combined with HA, TCP, and
autologous bone marrow to form a graft material devoid of structural
support but able to function as an effective bone graft substitute or
bone graft expander to augment fracture healing. This was demonstrated
by Chapman et al.,45 who conducted a
multicenter prospective randomized controlled study comparing
autogenous bone graft and a composite of bovine collagen, calcium
phosphate, and autogenous bone marrow (Collagraft; Zimmer, Inc.,
Warsaw, IN) in the treatment of acute long bone fractures. Two hundred
forty-nine fractures were grafted and followed for a minimum of 2
years. The authors observed no significant differences between the two
treatment groups in terms of union rates, functional outcomes, or
impairment of activities of daily living. The prevalence of
complications was similar in the two groups except for higher infection
rates in patients receiving autogenous bone grafts. Antibodies to the
bovine collagen developed in 12% of the patients in the
Collagraft-treated group but no specific allergic problems were
identified. Similar results using this material have been reported by
others.52,128,134
It acts as an osteoconductive material, which completely resorbs as
newly formed bone remodels and restores anatomic features and
structural properties.
investigated the use of calcium sulfate as a bone graft substitute in a
prospective nonrandomized clinical study for the treatment of
acetabular fractures with intra-articular comminution, marginal
impaction, or both. Thirty-two fractures were treated with calcium
sulfate pellets. Radiographic analysis demonstrated that the majority
of fractures healed successfully with most of the pellets being
replaced by bone. Two groups of investigators reported the use of
calcium sulfate as a material that augments or extends the use of
autologous bone graft. In a prospective nonrandomized multicenter
study, Kelly et al.121 treated 109
patients with bone defects with calcium sulfate pellets alone or mixed
with unconcentrated bone marrow aspirate, demineralized bone, or
autograft. After 6 months, the radiographic results showed that 99% of
the pellets were resorbed and 88% of the defects were filled with
trabeculated bone. Borrelli et al.26
treated 26 patients with persistent long bone nonunions or osseous
defects after an open fracture, with a mixture of autogenous iliac
crest bone graft and medical-grade calcium sulfate. Twenty-two patients
achieved healing after the primary surgery, while an additional two
demonstrated union after a second procedure. Persistent nonunions were
seen in two patients.
with the thought that they would be both osteoconductive and
osteoinductive, as well as provide structural support. Early results in
animals indicate that this combination may have benefits.23
Despite these encouraging reports, there have been no randomized
controlled trials to study the efficacy of calcium sulfate in the
treatment of skeletal injuries.
bone-void fillers in the treatment of bony defects associated with
acute fractures. Inorganic calcium and phosphate are combined to form
an injectable paste that can be delivered into the fracture site.
conducted a prospective randomized controlled study examining the use
of a commercially available CPC, Norian SRS (Norian Corporation,
Cupertino, CA), in the treatment of distal radius fractures. Under
physiologic conditions, this material begins to harden within minutes,
forming a mineral known as dahllite. By 12 hours, dahllite formation is
nearly complete, providing the cement with an ultimate compressive
strength of 55 megapascals (MPa).89 In comparison, proximal tibia trabecular bone from human cadavers has an ultimate stress that varies with age.63
Younger patients (16 to 39 years) had an average ultimate stress of
10.6 MPa, while older individuals (60 to 83 years) had significantly
lower values at 7.27 MPa. Studies in animals have shown that it is
remodeled in vivo and, in some cases, is completely resorbed and
replaced by host bone.50 One hundred
ten patients, who were between 50 and 85 years of age and who had
sustained either an AO type A3 or C2 distal radius fracture, were
enrolled. Patients were prospectively randomized to receive either
closed reduction with a short arm cast for 6 weeks or closed reduction
and stabilization with Norian SRS for 2 weeks. They were followed for a
12-month period and assessed by radiography, range of motion, and grip
strength. The results showed improved functional and radiographic
outcomes in the patients treated with Norian SRS. In a subsequent
randomized controlled study, Cassidy et al.44
compared the use of Norian SRS and closed reduction to closed reduction
and the application of a cast or external fixation in 323 patients with
fractures of the distal radius. Significant clinical differences were
seen at 6 to 8 weeks postoperatively, with better grip strength, wrist
and digit range of motion, and hand function and less swelling in the
patients treated with Norian SRS. By 1 year, these differences had
disappeared.
radius fractures, Norian SRS has been used to treat other fractures.
Schildhauer et al.197 reported its
use in the treatment of complex calcaneal fractures. Thirty-six joint
depression fractures were treated with Norian SRS after standard open
reduction and internal fixation. Patients were allowed to bear weight
fully as early as 3 weeks postoperatively. Results demonstrated no
statistical difference in clinical outcome scores in patients who bore
full weight before or after 6 weeks postoperatively, suggesting that
this cement may permit early full weight bearing after treatment of
this fracture.
found improved function of calcium phosphate cements compared with the
gold standard of autograft with regard to structural support in tibial
plateau fractures. Yetkinler et al.241
evaluated the compressive strength of TCP compared with autograft in
experimentally created centrally depressed tibial plateau fractures
treated with two screws. The cadaveric tibia were then subjected to
10,000 cycles of load, after which they were loaded
to
failure. Results showed no difference in the load to failure; however,
the TCP-treated specimens showed significantly less displacement than
control subjects. Welch et al.237
created bilateral subchondral defects that were 8 mm in diameter and 10
mm deep beneath the subchondral bone of the articular cartilage in the
lateral tibial plateau of goats. These defects were filled with
cancellous autograft or TCP and the tibias were harvested at varying
time points during the healing process. At all times, the subsidence at
the fracture site was significantly less in those treated with TCP,
with a mean subsidence of 0.3 mm at 6 months compared with 3.7 mm in
the control group.
used Norian SRS in the treatment of 26 tibial plateau fractures (OTA
types B2, B3, and C3) followed for a mean of 19.7 months. Successive
radiographs were obtained and clinical parameters were measured using
the Lysholm knee score and Tegner activity scale. Twenty-two fractures
healed without any displacement or complications. Two cases required
early wound revision secondary to sterile drainage, and two cases
developed partial loss of fracture reduction between 4 and 8 weeks
postoperatively requiring revision surgery. The high mechanical
strength of the cement allowed earlier weight bearing after a mean
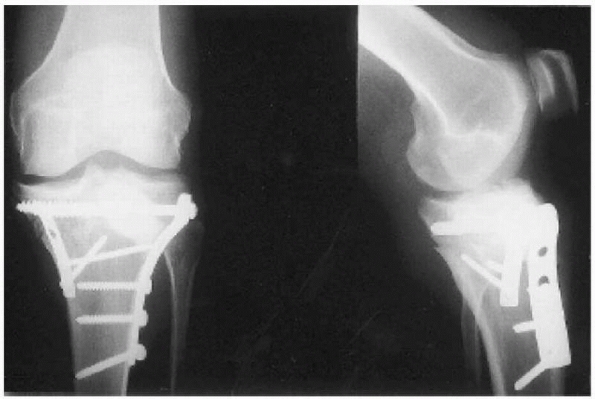
postoperative period of 4.5 weeks (Fig. 5-2).
Similar results supporting the use of Norian SRS for filling
metaphyseal defects in the treatment of displaced tibial plateau
fractures have been reported by others.109,237 Simpson et al.206
followed 13 tibial plateau fractures treated with either limited
internal fixation and injectable Norian SRS or buttress plating and
cancellous autograft. At 1-year follow-up, the mean subsidence of the
autograft-treated group was 4 mm, while the SRS-treated group had only
subsided 0.7 mm.
reviewed 14 randomized controlled trials that evaluated calcium
phosphate cement. They found that the use of calcium phosphate cement
was associated with a lower incidence of pain compared with control
subjects. They also found a 68% relative risk reduction in the loss of
fracture reduction compared with fractures supplemented with autograft.
Despite this, sterile serous drainage was reported in at least three of
the papers.146,155,232
The exact cause for the sterile drainage is not known but may be
related to local reaction to cement particles or loose bodies secondary
to hematoma formation before complete curing of the cement.
 |
|
FIGURE 5-2
Postoperative radiographs after open reduction and internal fixation and injection of 19.5 mL of the calcium phosphate cement Norian SRS in the lateral tibail plateau defect. The patient used crutches and walked bearing partial weight for 6 weeks. (Reprinted with permission from Lobenhoffer P, Gerich T, Witte F, et al. Use of an injectable calcium phosphate bone cement in the treatment of tibial plateau fractures: a prospective study of 26 cases with 27 mean follow-up. J Orthop Trauma 2002;16:143-149.) |
as bone void fillers when it is possible to implant them such that they
are surrounded by host bone on all sides. It is preferable to use them
in parts of the skeleton where tensile strains are low or nonexistent.
Calcium sulfate, which is much more rapidly resorbed than the other
calcium-based materials, must be used in parts of the skeleton where
compressive strength is required for only short periods. These
materials should not be used to bridge segmental diaphyseal defects or
as onlay grafts where the majority of the surface is exposed to soft
tissues.
several randomized controlled clinical trials. Based on these data, its
use to shorten the time in a cast during treatment of distal radius
fractures or to shorten the time to weight bearing in the augmentation
of tibial plateau and calcaneal fractures is supported by clinical
evidence and this is a viable treatment options for these indications.
It may be useful in other applications such as acetabular fractures and
fractures of the hip, but sufficient evidence is not yet available for
its use in these settings.
through a host of signaling molecules, including systemic hormones,
peptide growth factors, and proinflammatory cytokines. These molecules
have autocrine, paracrine, or endocrine effects through actions on
appropriate target cells. In addition to promoting cell
differentiation, some have direct effects on cell adhesion,
proliferation, and migration by modulating the synthesis of proteins,
other growth factors, and receptors.120
BMPs are a group of noncollagenous glycoproteins that belong to the
transforming growth factor beta (TGF-β) superfamily. They are
synthesized locally and predominantly exert their effects by autocrine
and paracrine mechanisms. Fifteen different human BMPs have been
identified and their genes cloned.54 For clinical applications, the most extensively studied among these are BMP-2 and BMP-7 (also called OP-1).
characterized the temporal expression of BMPs during fracture healing
in mice, defining specific periods when individual BMPs may exert
important roles in normal skeletal repair. BMP-2 showed maximal
expression
on
day 1 after fracture, suggesting its role as an early response gene in
the cascade of healing events. BMP-3, -4, -7, and -8 exhibited a
restricted period of expression from day 14 through day 21, when the
resorption of calcified cartilage and osteoblastic recruitment were
most active. BMP-5 and -6 were constitutively expressed from day 3 to
day 21.
BMPs were likely to play a key role during fracture healing in
patients. Kloen et al.126
demonstrated the presence of BMPs and their various receptors in human
fracture callus. Tissue was obtained from the fracture site of
malunions in five patients undergoing a corrective osteotomy.
Immunohistochemical analysis was performed and results demonstrated
consistent positive staining for all BMPs and BMP receptors, with
immunoreactivity most intense for BMP-3 and -7. More recently, Rosen et
al. demonstrated the importance of BMP-2 in the fracture repair
cascade. Tibia fractures were produced in transgenic mice in which
BMP-2 was deleted in a limb-specific manner, before the onset of
skeletal development. Mice heterozygous for this mutation were shown to
have impaired healing during the earliest stages of repair with reduced
periosteal reaction and decreased formation of other BMPs involved in
the repair process (e.g., BMP-4 and BMP-7). However, in mice homozygous
for this mutation, fracture healing was completely abolished. This
study demonstrated that BMP-2 is essential for fracture healing.226
recombinant human BMPs (BMPs synthesized by recombinant gene technology
using human BMP DNA) in the treatment of fractures and nonunions. In a
large prospective randomized controlled, partially blinded, multicenter
study, Friedlaender et al.81
assessed the efficacy of recombinant human (rh)BMP-7 (OP-1) versus
iliac crest bone graft in the treatment of 122 patients with 124 tibial
nonunions. All of the nonunions were treated with reduction and
fixation with an intramedullary nail and were randomized to receive
either autologous bone graft or implantation of rhBMP-7 (OP-1) in a
type I collagen carrier. Clinical assessment at 9 months indicated
equivalent rates of union, with 81% of the 63 patients treated with
BMP-7 and 85% of the 61 control patients demonstrating evidence of
healing. Radiographic assessments showed bridging callus in 75% and 84%
of these patients, respectively. As these results showed equivalent
efficacy between OP-1 and autogenous bone graft, the authors concluded
that OP-1 was a safe and effective alternative to bone graft in the
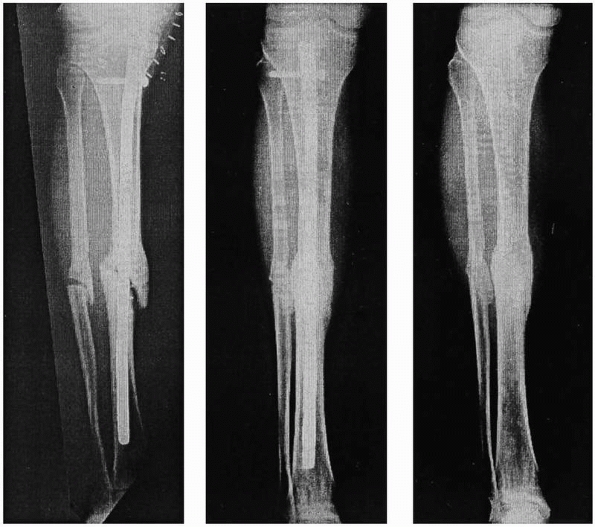
treatment of tibial nonunions (Fig. 5-3).
 |
|
FIGURE 5-3
Sequential radiographs of a tibial nonunion treated with recombinant human OP-1 immediately postoperatively and at 9 months and 24 months after intramedullary nailing. Note the bridging callus and subsequent tibial union. [Reprinted with permission from Friedlaender GE, Perry CR, Cole JD, et al. Osteogenic protein 1 (bone morphogenetic protein 7) in the treatment of tibial nonunions. J Bone Joint Surg Am 2001;83:S151-S158]. |
prospectively followed 23 patients with humeral nonunions treated with
plate and screw or intramedullary nail fixation in conjunction with
various combinations of autograft, allograft, or DBM. In addition,
patients were treated with recombinant human (rh)OP-1 contained within
a type I collagen matrix implant. The investigators found that all
patients had healed at an average of 144.3 days. They concluded that
OP-1 used in conjunction with allograft and/or DBM was an effective
alternative to autograft for the treatment of humeral nonunions. A
similar study was performed in 26 fracture nonunions in 25 patients
treated with OP-1 and followed to union.62
Of the 26 fractures, 17 also received autologous bone graft at the time
of final fixation. Radiographic union occurred in 24 of the 26
fractures at an average of 5.6 months. The two cases of persistent
nonunion occurred in open fractures that were complicated by infection
prior to the application of OP-1.
the treatment of acute fractures in several human studies. The BMP-2
Evaluation in Surgery for Tibial Trauma (BESTT)
Study
Group reported on a large prospective randomized controlled multicenter
trial evaluating the effects of rhBMP-2 in the treatment of open tibial
fractures.92
Four hundred fifty patients with these injuries were randomized to
receive either initial irrigation and debridement followed by treatment
with intramedullary (IM) nail fixation alone or IM fixation plus an
implant containing either 0.75 mg/kg or 1.5 mg/ kg rhBMP-2. The implant
was placed over the fracture site at the time of wound closure. After 1
year, there were fewer secondary interventions (returns to the
operating room for additional treatment) in the group treated with 1.5
mg/kg rhBMP-2. In addition, those patients treated with 1.5 mg/kg
rhBMP-2 had accelerated times to union, improved wound healing, and
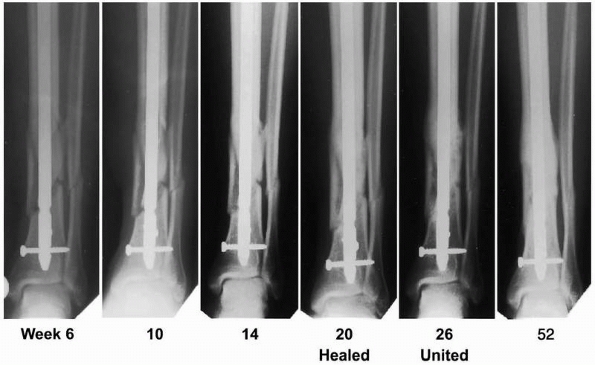
reduced infection rates (Fig. 5-4). A subgroup analysis was performed on this cohort by Swiontkowski et al.,215
who also included results in 60 additional patients treated in a
similar manner. The investigators analyzed 113 patients with either
type IIIA or IIIB open fractures and included only patients who
received placebo (65 patients) or 1.5 mg/ml of rhBMP-2 (66 patients).
The results showed that the treatment group required significantly
fewer bone grafts to achieve union and had a lower incidence of
infection.
 |
|
FIGURE 5-4
Radiographs of a patient who had sustained an open fracture of the left tibia (Gustilo and Anderson type IIIB) and was treated with an unreamed intramedullary nail and 1.50 mg/mL recombinant human BMP-2. The fracture was considered to be clinically healed by 20 weeks and healed radiographically by 26 weeks. (Reprinted with permission from Govender S, Csimma C, Genant HK, et al. Recombinant human bone morphogenetic protein 2 for treatment of open tibial fractures. A prospective, controlled, randomized study of 450 patients. J Bone Joint Surg Am 2002;84:2123-2134). |
in animal models of fracture healing and critical-sized defect repair,
results of the use of BMPs in patients have been less impressive.
Diefenderfer et al.61 noted that one
of the reasons may be a differential response of human bone marrow
stromal cells to BMPs. Bone marrow cells isolated from patients
undergoing hip replacement were cultured and grown to confluence with
or without dexamethasone and treated with BMPs. The results
demonstrated no significant osteogenic response to BMP-2, -4, or -7 as
determined by alkaline phosphatase induction, unless the cells were
pretreated with dexamethasone. Moreover, even when the cells were
pretreated, the alkaline phosphatase response to BMPs was only about
50% of that measured in mouse bone marrow cell cultures. The authors
concluded that the ability of human bone marrow cells to respond to
BMPs may differ substantially from that which exists in lower mammalian
species.
both the progenitor cells and local inflammatory cells that create the
rich vascular network at the site of fracture repair. Some of the
factors directly enhance the effects of local BMPs, whereas others
stimulate local inflammation and angiogenesis, both of which are
prerequisites for bone healing. While none of these factors are
currently available for clinical treatment of fractures, each has shown
promise in animal models and in the treatment of other disease
processes.
similar functions. It is known to influence a number of cell processes
including the stimulation of MSC growth and differentiation,
enhancement of collagen, and other extracellular matrix protein
synthesis, and it functions as a chemotactic factor for fibroblast and
macrophage recruitment.124
TGF-β on fracture healing, with high and low doses having different
effects. Lind et al.146 tested two
doses of TGF-β in rabbits in which tibial defects had undergone
unilateral plate fixation. After 6 weeks of healing, the investigators
found that bending stiffness was only improved in the group treated
with the low dose, while callus formation was significantly improved
for both doses. Critchlow et al.53
performed a study of tibial defect healing in rabbits to test the
hypothesis that the anabolic effects of TGF-β on bone repair are
dependent on mechanical stability at the fracture site. The results
showed that under stable mechanical conditions, a low dose of TGF-β2
had an insignificant effect on callus development, whereas the higher
dose, which was closer to the low dose used by Lind et al.,170 led to a larger callus.
in augmenting fracture healing; however, the effects are highly dose
dependent and not especially robust. Although its application to
directly influence fracture repair has not been as promising as those
of several of the BMPs, a recombinant fusion protein with TGF-β,
containing a collagen binding domain, has been shown to induce
osteogenic differentiation of bone marrow cells in rats.5 Becerra et al.18
presented a case report of a 69-year-old man with a proximal tibial
defect from resection of longstanding osteomyelitis. Bone marrow cells
were cultured in the presence of the TGF-β fusion protein after they
were obtained
from
the iliac crest. Expanded cells were then placed in the tibial defect
in conjunction with an HA carrier. Imaging at 90 days was consistent
with new bone formation including bridging callus, and biopsy samples
taken at the 8 weeks showed new bone formation.
this is the way cells receive nutrients and oxygen. Early in the
fracture repair process, vascular endothelial growth factor (VEGF) has
been shown to be upregulated.213 Eckhart et al.66
tested the ability of recombinant human VEGF (rhVEGF) to heal
criticalsized defects in rabbits. They compared the healing at 7 weeks
with autograft and vehicle-treated controls. Biomechanical testing of
the treated bones found that the amount of torque required to failure
and the stiffness were significantly greater in the rhVEGF-treated
animals compared with controls and equivalent to autograft treatment.
Micro-computed tomography analysis showed abundant callus in both the
rhVEGF- and autograft-treated groups, and this callus was absent in the
control groups.
slow process that occurs through creeping substitution. Surface healing
can leave large central areas of necrotic bone that contributes to the
25% to 35% failure rate with this type of grafting.22,148 Ito et al.115
found that VEGF and receptor activator of nuclear factor-κB ligand
(RANKL) were downregulated during allograft healing. They developed a
method by which RANKL and VEGF were combined with a viral vector and
attached to the surface of allografts. Theses allografts were then used
in a mouse fracture model, where histologic analysis at 4 weeks showed
periosteal resorption with new bone formation and medullary
neovascularization that was not seen in untreated controls. These
preliminary results demonstrate a novel way to increase allograft
healing and warrant further study.
FGF-2, belongs to a class of growth factors that have an affinity for
heparin and of which at least 22 members have been described.116
It is one of the most potent stimulators of angiogenesis, partially
through its influence on endothelial cell migration and upregulation of
integrin expression.125 During growth, wound healing, and fracture repair, it acts as a mitogen for fibroblasts, chondrocytes, and osteoblasts.111,114
In the early stages, FGF-1 and -2 are localized to the proliferating
periosteum. This expression is then limited to osteoblasts during
intramembranous bone formation and to the chondrocytes and osteoblasts
during endochondral bone formation. In light of their active
involvement during fracture repair, investigators have studied the
potential therapeutic roles of FGFs in bone formation. Nakamura and
associates165 studied these effects
by injecting bFGF into middiaphyseal transverse tibial fractures in
dogs. Controls were injected with carrier molecules. Results showed
that bFGF enlarged the callus area at 4 weeks and increased the callus
bone mineral content at 8 weeks. Subsequent to the reporting of these
findings in animals, at least one biotechnology company initiated
preliminary studies in humans to set the stage for a multicenter
randomized controlled trial in patients with closed tibia fractures.
Those preliminary results have not been reported and the multicenter
clinical trial has not been conducted. At this time, the status of the
FGFs in the enhancement of fracture healing in patients is unknown.
polypeptide that consists of two chains that share 60% amino acid
sequence homology.208 Its potential role in bone healing is related to its mitogenic and chemotactic properties for osteoblasts.39,42
A positive effect of PDGF on fracture healing was demonstrated in a
rabbit tibial osteotomy model in which the fractures were injected with
either 80 µg of PDGF in a collagen carrier or collagen alone.167
Results showed an increase in callus formation and a more advanced
stage of endosteal and periosteal osteogenic differentiation in the
PDGF-treated group compared with the controls. However, the treatment
had no effect on the mechanical properties of the calluses compared
with controls.
in a geriatric, osteoporotic rat model found significant gains in
mechanical strength in fractures treated with PDGF combined with an
injectable beta-tricalcium phosphate-collagen matrix. At 5 weeks after
the initial injury, the torsion to failure in the PDGF-treated tibias
was comparable to that of the uninjured extremity, while control and
untreated fractures remained unhealed. These preclinical data and
encouraging results from clinical studies of PDGF treatment of dental
implants169 and diabetic foot ulcers238 suggest a potential role for PDGF in skeletal trauma.
longchain fatty acids that are known to have profound osteogenic
effects when implanted into skeletal sites142,175 or infused systemically.227
The release of arachidonate from alkyl-arachidonyl phospholcholine
produces the precursor of several potent proangiogenic and
proinflammatory mediators. Arachidonate is converted to several types
of PGs by either of two known prostaglandin synthases
(cyclooxygenases): COX1 or the inducible COX2. In a study of rabbit
tibial fractures, Dekel et al.56 demonstrated that PGE2
caused a dose-dependent stimulation of callus formation and an increase
in total bone mineral content. Its effects were also shown to be
greatest during the latter stages of fracture healing, suggesting that
the primary effect may be to stimulate osteoblasts and osteoprogenitor
cells as opposed to undifferentiated MSCs.
treatment of fractures is its undesirable systemic effects. These
include nausea, pyrexia, diarrhea, lethargy, and flushing, and they are
mitigated by the binding of PGs to all of its four receptors (EP1, EP2,
EP3, and EP4). Recently, Li and coworkers142 reported on enhanced fracture repair by the nonprostanoid PGE2
agonist CP-533,536. Using this more selective approach, binding of the
synthetic PG agonist to its EP2 receptor was shown to lessen the
systemic effects while targeting the receptor that primarily regulates
bone anabolic activity. In models of both rat and canine fracture
healing, they delivered CP-533,536 in a poly(DLlactide-coglycolide)
matrix to fractures sites in a dose-dependent fashion. Each dose
increased callus size, density, and strength compared with the
controls. Histologic examination showed extensive endochondral and
intramembranous ossification. These data suggest that a selective
EP2-receptor agonist
may
have a therapeutic role in the augmentation of the fracture repair
process. Clinical trials are currently under way to investigate this
application.
of recalcitrant nonunions of long bones and BMP-2 for the treatment of
open tibia fractures. The other molecules discussed in this section
have not been fully tested for their clinical efficacies, and therefore
it is not possible to consider their use at this time.
that involves multiple signaling pathways and organ systems. The
largest storage site of calcium and phosphate is the skeletal system,
and the release of these ions and their accumulation within this system
are largely regulated by the coordinated stimulation and suppression of
osteoblasts and osteoclasts. Parathyroid hormone (PTH) is a major
regulator of mineral homeostasis and exerts its effects by binding to
its receptor on osteoblasts.189,190
PTH is an 84-amino acid peptide that is produced in response to
depressed serum calcium levels. Its major effects are in the kidneys,
where it regulates phosphate diuresis and 1,25-dihydroxyvitamin D
synthesis with its subsequent enhancement of gastrointestinal calcium
and phosphate absorption.181 The
actions of PTH on bone metabolism can be both stimulatory and
inhibitory. It has been found that continuous release of PTH leads to
an increase in osteoclast numbers and activity,143 while intermittent exposure results in increased bone formation in both rats and humans.59,168
in bone mass in osteoporotic men and an increase in bone mineral
density and a reduction of vertebral and other osteoporotic related
fractures in postmenopausal women.58,168 Neer et al.168
assessed the efficacy of PTH(1-34) for improving bone mineral density
in a clinical trial involving 1673 postmenopausal women with prior
nontraumatic vertebral fractures. Results demonstrated that PTH
increased bone mineral density and reduced the risk of fracture.
several animal studies have been conducted examining the effects of PTH
on the repair of bone. All have demonstrated an enhancement of fracture
healing when doses where given intermittently.164,166 Manabe et al.152
studied PTH in a primate model of fracture repair. Seventeen female
cynomolgus monkeys underwent a femoral osteotomy with plate fixation.
Treatment groups consisted of either low-dose (0.77 µg/kg) or high-dose
(7.5 µg/kg) PTH or placebo for control, and injections were given twice
weekly for 3 weeks. All groups healed by 26 weeks, at which time the
animals were killed. Ultimate stress and elastic modulus of the healing
osteotomy were significantly higher in PTH-treated animals, while the
callus size was larger but had lower density in control animals.
Alkhiary et al.3 reported on the use
of PTH(1-34) in the treatment experimental femur fractures in
Sprague-Dawley rats. Animals were treated with either 5 or 30 µg/kg/day
PTH(1-34) for a total of 35 days beginning at the time of fracture
creation. Animals were killed at various time points, and the fracture
callus was analyzed for bone mineral content and density as well as
undergoing mechanical testing to failure. Results compared with control
animals demonstrated significant increases in strength and bone mineral
content for the 30 µg/kg group as early as 3 weeks, and these
differences where sustained at 85 days.
treatment of fractures of the distal radius in humans showed that time
to fracture healing was shorter in the PTH-treated patients.1 These findings suggest that PTH(1-34), as well as other PTH fragments, may have role in the clinical treatment of fractures.
(IGFs) play an important role in skeletal development and remodeling.
GH is currently used clinically to treat patients with short stature172
because it stimulates endochondral ossification, periosteal bone
formation, and linear growth. It mediates these effects through the IGF
system including the ligands, receptors, IGF binding proteins (IGFBPs),
IGFBP proteases and activators, and inhibitors of IGFBP proteases.
IGF-II. Although IGF-II is the most abundant growth factor in bone,
IGF-I has the greater potency for promoting growth and has been
localized in healing fractures of humans.7,40,41
IGF-I and IGF-II promote bone matrix formation (type I collagen and
noncollagenous matrix proteins) by fully differentiated osteoblasts,
inhibit collagen degradation, and promote osteoblast maturation and
replication.43 Expression of the IGF-I increases with expression of GH,183 and it is likely responsible for the anabolic effects of GH.
showed that GH significantly improves the mechanical properties of
fracture callus in minipigs. A recent randomized clinical trial was
presented by Raschke et al.183 in
which 406 patients with tibia fractures were treated daily with varying
concentrations of GH or placebo for up to 26 weeks. Dosages were
gradually increased to the assigned dose over a 3-week period in an
attempt to decrease adverse events such as water retention. With regard
to the primary outcome of radiographic union, no significant difference
was seen in the open fractures between control and GH-treated patients.
On the other hand, the relative risk for healing a closed fracture was
greatest in the group treated with the highest dose of GH (60 µg/day;
relative risk [RR], 1.44; 95% confidence interval [CI], 1.01-2.05; P
= 0.045). Patients treated with 60 µg/day GH were able to bear full
weight at an average of 87 days versus 108 days in the placebo-treated
controls. However, the benefits of GH might have been overshadowed by
the high number of adverse events: 58% in the 60 µg/day GH-treated
group compared with 35% in placebo-treated controls. These adverse
events included arthralgias, edema, and, to a lesser extent, wound
infection.
mevalonic acid production. The conversion of HMG-CoA to mevalonic acid
occurs early in the pathway and also inhibits the production of
farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP).
Small GTP-binding proteins such as Rho and Ras require GGPP and FPP,
respectively, for translocation to the plasma membrane.134 Inhibition of this process by statins may block osteoclast maturation and subsequent bone catabolism.203 In addition, studies have shown that statins stimulate the BMP-2 promoter in osteoblasts leading to enhanced bone formation.161 In mice, Skoglund et al.207
showed that daily injections of simvastatin had no effect on fracture
healing, while a continuous systemic infusion and continuous local
delivery improved the force to failure by 160% and 170%, respectively.
The large systemic doses were likely required because commercially
available statins target the liver, and little is available to the
skeletal tissues at standard doses. Garret el al.84
addressed this by developing poly(lactic-coglycolide acid) nanoparticle
beads containing various concentrations of lovastatin. They then
created femur fractures in Sprague-Dawley rats that were treated
immediately with injection of nanoparticles that eluted either 0, 0.2,
1, 1.5, or 7.5 µg/day lovastatin. Their results showed at 4 weeks that
doses of 1 and 1.5 µg/day significantly accelerated fracture healing as
measured by size of the fracture gap and biomechanical strength.
fracture healing. Direct mechanical perturbation and biophysical
modalities such as electrical and ultrasound stimulation have been
shown to affect fracture healing. To enhance fracture repair by these
mechanical measures, it is necessary to develop a fundamental
understanding of the ways by which the mechanical environment impacts
cellular and molecular signaling.
found that early weight bearing accelerates the fracture-healing
process. Standardized femoral fractures were produced in rats and
stabilized by nonrigid intramedullary fixation. The animals were either
allowed to bear weight at an early stage or were kept non-weight
bearing by cast immobilization. Histologic, radiographic, and
mechanical differences were present by the second week after fracture.
These differences became progressively greater during the next 3 weeks.
The authors attributed these findings to early mobilization
facilitating the maturation of callus tissue produced by endochondral
ossification.
Using a standardized, bilateral tibial canine osteotomy model,
compression plating of the fracture was compared with the less stable
external fixation performed on the opposite side. At 120 days after
injury, bone formation was biomechanically less mature on the external
fixator side. These tibias had significantly less intracortical
new-bone formation and more bone porosity compared with those that had
been treated with compression plates. Endosteal new bone formation was
greater on the plated side. Because the in vitro stiffness of the
external fixator was less in all modes tested (compression,
distraction, torsion, and anteroposterior bending) except lateral
bending, the authors concluded that the rigidity of the fixation may be
an important factor in early remodeling of a healing osteotomy.
fracture healing by altering the mechanical strain environment. In a
prospective randomized clinical trial, Kenwright et al.122
compared the effects of controlled axial micromotion on tibial
diaphyseal fracture healing in patients who were treated with external
fixation and stratified according to fracture severity and extent of
soft tissue injury. A specially designed pneumatic pump was attached to
the unilateral external fixation frame of one group of patients and
delivered a cyclic axial displacement of 1.0 mm at 0.5 Hz for 20 to 30
minutes a day. Fracture healing was assessed clinically,
radiographically, and by measurement of the mechanical stiffness of the
fracture. Both clinical and mechanical healing was enhanced in the
group subjected to micromovement, compared with those treated with
frames without micromotion. The differences in healing times were
statistically significant and independently related to the treatment
method. There was no difference in complication rates between treatment
groups.
the application of tensile forces to developing callus via a controlled
osteotomy.141,157
The controlled distraction of bone fragments results in the expression
of various growth factors, including those involved in angiogenesis.
Pacicca et al.173 demonstrated the
expression of several of these molecules localized to the leading edge
of the distraction gap, where nascent osteogenesis was occurring. The
greatest levels were seen during the active phase, consistent with the
apposition of new bone matrix. Others have shown that robust
angiogenesis, under VEGF control, occurs during the active and
consolidation phase and is supported by the recruitment of endothelial
progenitor cells.138
distraction osteogenesis to stimulate new bone formation in the
clinical setting. Kocaoglu et al.127
treated 16 patients with hypertrophic nonunions with the Ilizarov
distraction method. All patients had at least 1 cm of shortening, three
patients had a deformity in one plane, and the remainder had a
deformity in two planes. Distraction was begun on the first
postoperative day at the rate of 0.25 mm/day divided into four equal
increments. Once the desired length had been obtained, the fixator was
left in place until at least three of four cortices showed bridging
callus. All nonunions had healed at an average follow-up of 38.1
months, with correction of all preoperative length inequalities and
limb angulation to normal alignment. A similar study of 17 patients
with tibial nonunions with bone loss found an average treatment time of
8 months, with functional results being reported as excellent in 15 and
good in 2.201
compression-distraction osteogenesis using the Ilizarov-type circular
external fixator. After an average of 30-month follow-up, results were
excellent in 21 and good in 3 patients. Functional assessment scores
were excellent in 19, good in 4, and fair in 1 patient (Fig. 5-5).
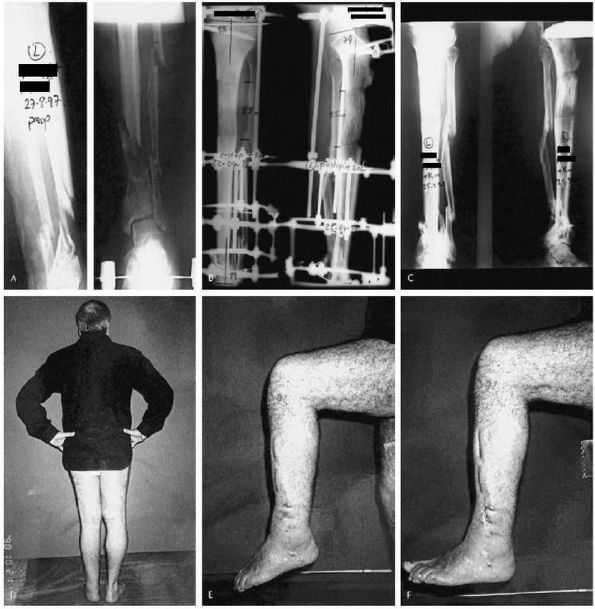 |
|
FIGURE 5-5 A 53-year-old man sustained a grade IIIB open fracture of his left distal tibia with 8.5 cm of bone loss. A. Preoperative anteroposterior and lateral radiographs. B. Late postoperative radiographs taken at the end of the distraction period. C.
Radiographs after frame removal displaying complete union of the fracture and completed lengthening through the proximal tibia. D. Leg length equality at the end of treatment. E,F. Ankle range of motion during the last follow-up examination. (Reprinted with permission from Sen C, Kocaoglu M, Levent E, et al. Bifocal compression-distraction in the acute treatment of grade III open tibia fractures with bone and soft-tissue loss-a report of 24 cases. J Orthop Trauma 2004;18:150-157.) |
in 1957. With this discovery, investigators began to study the
influence that electrical current might have on the healing of bone. In
1971, Freidenberg et al.79 found
that the healing of nonunions could be affected by the use of direct
current. Within 5 years, more than 119 articles had been published
highlighting the use of electrical stimulation on bone growth and
repair.29
stimulation of bone healing: (i) constant direct-current (DC)
stimulation with the use of percutaneous or implanted electrodes
(invasive), (ii) capacitive coupling (noninvasive), or (iii)
time-varying inductive coupling produced by a magnetic field
(noninvasive; also known as pulsed electromagnetic field [PEMF]
stimulation). DC stimulation uses stainless steel cathodes placed in
the tissues near the fracture site. New bone formation is directly
proportional to the level of applied current, with a threshold level
above which cellular necrosis may occur.78
With pulsed electromagnetic stimulation, there is an alternating
current that is produced by externally applied coils. This produces a
timevarying magnetic and electrical field within the bone. In
capacitively coupled electric fields (CCEFs), an electrical field is
induced in bone through the use of an external capacitor—that is, two
electrically charged metal plates placed on either side of a limb.30
used DC for the treatment of 178 nonunions in 175 patients at a single
center. Union was achieved in 84% of the patients. Interestingly, the
investigators found that even in the presence of osteomyelitis the
healing rate was nearly 75%. The presence of previously inserted
metallic fixation devices did not affect the healing rate. The study
began with two treatment groups,
one
receiving 10 microamperes and the other 20 receiving microamperes of
current. The first 11 patients failed to heal by 12 weeks, and
thereafter all patients received the higher dose of current. When this
study was expanded to include other centers, an additional 58 of 89
nonunions achieved similar results. Treatment failures were attributed
to inadequate electricity, the presence of a synovial pseudarthrosis or
infection, and dislodgment of the electrodes. Complications were minor
and, with the exception of patients with previous osteomyelitis, no
deep infections resulting from this treatment were noted. The authors
concluded that given proper electrical parameters and cast
immobilization, a rate of bone union comparable to that seen with
bone-graft surgery could be achieved.
reported similar results in a prospective, double-blind trial using
capacitive coupling in patients with established nonunions. In a
population of 21 patients, 10 were actively managed and 11 were treated
with a placebo unit. Healing was achieved in 60% of the patients who
received electrical stimulation (Fig. 5-6). Patients managed with the placebo unit showed a complete lack of bone formation.
reported on the use of PEMF in the treatment of nonunited tibial
diaphyseal fractures. One hundred twenty-five patients with 127
nonunions underwent long-leg plaster cast immobilization. Patients were
treated with non-weightbearing ambulation and a total of 10 hours of
PEMF stimulation daily. Bony union occurred in 87% of the patients and
was independent of patient age or sex, the number of previous
operations, and the presence of infection or metal fixation.
a double-blind, multicenter trial of the use of PEMFs in patients who
had developed delayed union of tibial fractures. Forty-five tibial
fractures that had not united for more than 16 weeks but less than 32
weeks were treated with immobilization in a plaster cast that
incorporated the coils of an electromagnetic stimulation unit. The unit
was activated for 20 of these fractures and was not active for 25.
There was radiographic evidence of union in nine of the fractures that
had been subjected to electromagnetic stimulation compared with only
three of the fractures in the control group.
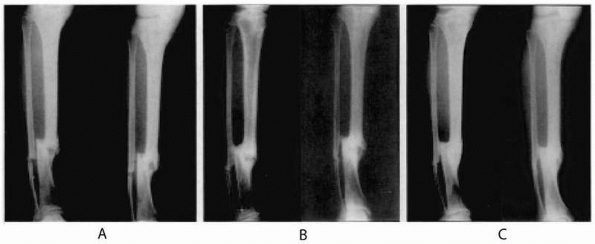 |
|
FIGURE 5-6
Anteroposterior and oblique radiographs of the tibia of a 23-year-old man who had sustained a closed fracture of the tibial diaphysis while playing soccer. He underwent closed intramedullary nailing of the tibia on the day of the injury, but an infection developed at the site of the fixation within a few months. The fixation device was removed but a low-grade infection with wound drainage persisted despite antibiotic treatment. The fracture was mobile and tender. The patient was entered into a placebo-controlled study of electrical capacitive coupling. A. Radiographs made at the beginning of treatment with a placebo unit. B. After 9 months of treatment with the placebo unit, the fracture site was still mobile, clinically painful, and discharging pus. No progress toward healing was seen. C. Six months after a 14-week period of electrical capacitive coupling, there was no longer any drainage and the fracture had united. (Reprinted with permission from Scott G, King JB. A prospective, double-blind trial of electrical capacitive coupling in the treatment of nonunion of long bones. J Bone Joint Surg Am 1994;76A:820-826.) |
nonunions and delayed unions, the application of this technology to the
treatment of fresh fractures has not been clearly defined. Although
some studies have shown that PEMFs favorably influence fracture healing
in experimental animals78 and osteotomies in patients,27,151 other studies have failed to demonstrate clinically significant effects.17 Beck et al.19
found no difference in healing time in 44 patients who were randomly
assigned to either CCEF or placebo. At present, there continues to be a
paucity of published clinical studies showing that electrical
stimulation enhances the healing of fresh fractures.
to promote fracture repair and increase the mechanical strength of
fracture callus in both animal179,240 and clinical101,131 studies. In a prospective randomized double-blind trial, Heckman et al.101
examined the use of LIPUS as an adjunct to conventional treatment with
a cast in 67 patients with closed or open grade I tibial shaft
fractures. Thirty-three fractures were treated with the active device
and 34 with the placebo. Using clinical and radiographic criteria, the
authors noted that there was a statistically significant decrease in
the time to union (86 ± 5.8 days in the LIPUS treatment group versus
114 ± 10.4 days in the control group) and in the time to overall
healing (96 ± 4.9 days in the ultrasound treatment group versus
154±13.7 days in the controls). There were no issues with patient
compliance in the treatment group and no serious complications reported
with its use.
evaluated the efficacy of LIPUS in the treatment of dorsally angulated
distal radius fractures that had been treated with closed reduction and
a cast.
The
time to union was significantly shorter for the fractures that were
treated with LIPUS compared with the controls (61 ± 3 days versus 98±5
days). The authors further noted that treatment with LIPUS was
associated with significantly less loss of reduction (20% ± 6% versus
43% ± 8%) as determined by the degree of volar angulation as well as
with a significant decrease in the mean time until the loss of
reduction ceased (12 ± 4 days versus 25 ± 4 days).
studied LIPUS for the treatment of acute tibial and distal radius
fractures in smokers. Healing time in this patient population is
typically delayed, with tibial and distal radius fractures requiring
175 ± 27 days and 98 ± 30 days, respectively, to achieve bony union.
Treatment with LIPUS was able to reduce this time to 103 ± 8.3 days in
the tibial fracture group and 48 ± 5.1 days in the patients with distal
radius fractures. Treatment with LIPUS also substantially reduced the
incidence of delayed unions in tibias in smokers and nonsmokers. These
results are important because they suggest that LIPUS can over-ride
some of the detrimental effects that smoking has on fracture healing.
Rutten et al.193 prospectively
analyzed 71 cases of tibial nonunion and found that treatment with
LIPUS resulted in a healing rate of 73%, and that this was
significantly higher than the rate of spontaneous healing. Within the
subgroups analyzed, the rate of healing in smokers and nonsmokers was
not found to be statistically significant.
fracture healing may be affected by the presence of fixation devices.
Emami et al.70 noted that ultrasound
did not appear to have a stimulatory role on tibial fracture repair in
a prospective randomized controlled double-blinded study to evaluate
its effects in patients with fresh tibial fractures who were treated
with a reamed and statically locked intramedullary rod. Patients were
divided into an ultrasound group and placebo group. They all underwent
treatment with an ultrasound device for 20 minutes daily for 75 days
without knowing whether it was active. Standardized radiographs were
taken every third week until healing and at 6 and 12 months. Results
showed that low-intensity ultrasound treatment did not shorten the
healing time.
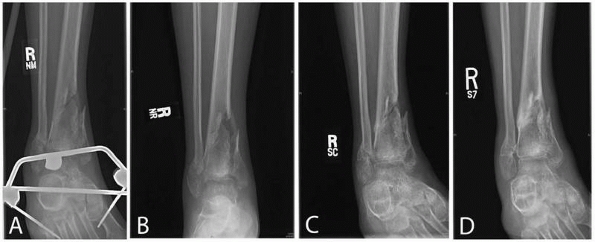 |
|
FIGURE 5-7
Sequential radiographs of a patient who sustained a grade II distal tibia fracture that underwent irrigation and debridement with placement of an external fixator. A. At 4 months, the external fixator was removed and the patient was believed to have a delayed union. Daily treatments with Exogen (Smith and Nephew, Memphis, TN) ultrasound stimulation was started. B. At 1 month of treatment, the patient progressed to partial weight bearing. C. At 2 months, radiographs showed continued progression of healing. D. At 6 months, the patient was bearing full weight without pain. (Courtesy Paul Tornetta III, MD.) |
has not been widely used and we have no experience with this method.
The use of appropriately applied distraction osteogenesis for the
treatment of nonunions is recommended for surgeons who are experienced
with the use of small-pin, ring fixation.
electrical stimulation for the treatment of nonunions and delayed
unions. DC, capacitive coupling, and PEMFs have all been demonstrated,
in randomized controlled trials, to enhance the healing of nonunions.
PEMFs can also be used for the treatment of delayed unions. There is no
evidence that electrical stimulation of any type enhances the healing
of fresh fractures.
fresh closed fractures of the distal radius and tibia when treated in a
cast or external fixation device. We also recommend this treatment as
an adjunct to the management of closed fractures of long bones in
patients who smoke. We have also had good results in the treatment of
tibia fractures that show delayed union (Fig. 5-7).
Until there is evidence to support the use of LIPUS in patients treated
with fixation devices, we do not recommend the use of ultrasound in the
treatment of fractures of patients who have undergone an operation in
which fixation devices have been implanted.
skeletal injuries. There are, however, instances when fracture repair
is delayed or fails to occur. With improved understanding of the
intracellular and extracellular pathways involved in bone healing,
our ability to successfully augment this repair process continuously evolves.
limited. Accepted options include returning to the operating room to
perform an open procedure supplemented with some type of physiologic or
synthetic graft, recombinant human OP-1 or BMP-2, or the use of LIPUS.
Systemic treatments, such as the use of statins or hormones, are still
in the development stages, but these types of treatments may allow
earlier interventions, especially in at-risk patients. Current
advances, including improved methods for obtaining autogenous and
allogenic MSCs, development of delivery mechanisms for gene therapy,
and improvements in synthetic bone graft materials, will greatly
enhance the ability to improve fracture repair.
YM, Gerstenfeld LC, Krall E, et al. Enhancement of experimental
fracture-healing by systemic administration of recombinant human
parathyroid hormone (PTH 1-34). J Bone Joint Surg Am 2005;87:731-741.
HS, Simpson JM, Glover JM, et al. Comparison between allograft plus
demineralized bone matrix versus autograft in anterior cervical fusion.
A prospective multicenter study. Spine 1995;20:2211-2216.
JA, Han B, Becerra J, et al. A recombinant human TGF-beta1 fusion
protein with collagen-binding domain promotes migration, growth, and
differentiation of bone marrow mesenchymal cells. Exp Cell Res
1999;250:485-498.
TT, Oxlund H. Local anabolic effects of growth hormone on intact bone
and healing fractures in rats. Calcif Tissue Int 2003;73:258-264.
JG, Hoyland J, Freemont AJ, et al. Insulin-like growth factor gene
expression in human fracture callus. Calcif Tissue Int 1993;53:97-102.
CJ, Holdridge SP, Baird B, et al. Ultraporous beta-tricalcium phosphate
is well incorporated in small cavitary defects. Clin Orthop Relat Res
2005;May:251-257.
TL, Peter SJ, Archambault MP, et al. Allogeneic mesenchymal stem cells
regenerate bone in a critical-sized canine segmental defect. J Bone
Joint Surg Am 2003;85A: 1927-1935.
JJ, Verdonschot N, Buma P, et al. Larger bone graft size and washing of
bone grafts prior to impaction enhances the initial stability of
cemented cups: experiments using a synthetic acetabular model. Acta
Orthop 2006;77:227-233.
Z, Weinreb M, Givol N, et al. Biomaterial resorption rate and healing
site morphology of inorganic bovine bone and beta-tricalcium phosphate
in the canine: a 24-month longitudinal histologic study and
morphometric analysis. Int J Oral Maxillofac Implants 2004;19:357-368.
HW, Zhao L, Kanim LE, et al. Intervariability and intravariability of
bone morphogenetic proteins in commercially available demineralized
bone matrix products. Spine 2006;31:1299-1306.
SS, Zlowodzki M, Schmitz-Lelwica AE, et al. The use of calcium
phosphate bone cement in fracture treatment: a meta-analysis of
randomized trials. Orthop Trauma Assoc 2007.
B, Jorgensen PH, Andreassen TT. The stimulating effect of growth
hormone on fracture healing is dependent on onset and duration of
administration. Clin Orthop Relat Res 1991;March:295-301.
JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site
morbidity. A statistical evaluation. Spine 1995;20:1055-1060.
A, az-de-Rada P, Barroso JL, et al. Frozen cancellous bone allografts:
positive cultures of implanted grafts in posterior fusions of the
spine. Eur Spine J 2004;13: 152-156.
CA, Mitchell SN, Gaston SR. Treatment of ununited tibial diaphyseal
fractures with pulsing electromagnetic fields. J Bone Joint Surg Am
1981;63A:511-523.
J, Guerado E, Claros S, et al. Autologous human-derived bone marrow
cells exposed to a novel TGF-beta1 fusion protein for the treatment of
critically sized tibial defect. Regen Med 2006;1:267-278.
BR, Matheson GO, Bergman G, et al. Do capacitively coupled electric
fields accelerate tibial stress fracture healing? A randomized
controlled trial. Am J Sports Med 2008; 36:545-553.
W, Becker BE, Caffesse R. A comparison of demineralized freeze-dried
bone and autologous bone to induce bone formation in human extraction
sockets. J Periodontol 1994;65:1128-1133.
PK, Hotchkiss RN, Athanasian EA, et al. Recalcitrant nonunion of the
distal humerus: treatment with free vascularized bone grafting. Clin
Orthop Relat Res 2005:134-139.
BH Jr, Lord CF, Gebhardt MC, et al. Fractures of allografts. Frequency,
treatment, and end-results. J Bone Joint Surg Am 1990;72:825-833.
C, Patel DV. The effect of demineralized bone matrix-calcium sulfate
with vancomycin on calcaneal fracture healing and infection rates: a
prospective study. Foot Ankle Int 2006;27:487-493.
MR, Capla EL, Egol KA, et al. Osteogenic protein-1 (bone morphogenic
protein-7) combined with various adjuncts in the treatment of humeral
diaphyseal nonunions. Bull Hosp Jt Dis 2005;63:20-23.
J Jr, Prickett WD, Ricci WM. Treatment of nonunions and osseous defects
with bone graft and calcium sulfate. Clin Orthop Relat Res
2003;June:245-254.
G, Bagnacani M, Bettati E, et al. Electrical stimulation of human
femoral intertrochanteric osteotomies. Double-blind study. Clin Orthop
Relat Res 1988;December: 256-63.
T, Edwards J, Scarborough N. Allograft bone. The influence of
processing on safety and performance. Orthop Clin North Am
1999;30:571-581.
CT, Black J, Friedenberg ZB, et al. A multicenter study of the
treatment of nonunion with constant direct current. J Bone Joint Surg
Am 1981;63A:2-13.
SP, Fink DJ, Caplan AI. Mesenchymal stem cells in bone development,
bone repair, and skeletal regeneration therapy. J Cell Biochem
1994;56:283-294.
SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the
osteogenic potential of purified human mesenchymal stem cells during
extensive subcultivation and following cryopreservation. J Cell Biochem
1997;64:278-294.
SP, Kraus KH, Goldberg VM, et al. The effect of implants loaded with
autologous mesenchymal stem cells on the healing of canine segmental
bone defects. J Bone Joint Surg Am 1998;80:985-996.
RW, Carlton A, Holmes R. Interporous hydroxyapatite as a bone graft
substitute in tibial plateau fractures. Clin Orthop Relat Res
1989;March:53-62.
PJ, Gebhardt MC. Are fibula strut allografts a reliable alternative for
periarticular reconstruction after curettage for bone tumors? Clin
Orthop Relat Res 2007;461: 170-174.
H, Busbee GA III, Enneking WF. Repair of experimental autologous grafts
of cortical bone. J Bone Joint Surg Am 1975;57A:814-819.
JW, Bhandari M, Sprague S, et al. An economic analysis of management
strategies for closed and open grade I tibial shaft fractures. Acta
Orthop 2005;76:705-712.
E. Effect of platelet-derived growth factor on DNA and protein
synthesis in cultured rat calvaria. Metabolism 1981;30:970-975.
E, Centrella M, McCarthy TL. Regulation of insulin-like growth
factor-II production in bone cultures. Endocrinology 1991;129:2457-2462.
E, McCarthy T, Centrella M. Isolation and characterization of
insulin-like growth factor I (somatomedin-C) from cultures of fetal rat
calvariae. Endocrinology 1988;122:22-27.
E, McCarthy TL, Centrella M. Effects of platelet-derived growth factor
on bone formation in vitro. J Cell Physiol 1989;140:530-537.
E, Pash J, Gabbitas B, et al. Growth factors regulate the synthesis of
insulinlike growth factor-I in bone cell cultures. Endocrinology
1993;133:33-38.
C, Jupiter JB, Cohen M, et al. Norian SRS cement compared with
conventional fixation in distal radial fractures. A randomized study. J
Bone Joint Surg Am 2003; 85A:2127-2137.
MW, Bucholz R, Cornell C. Treatment of acute fractures with a
collagencalcium phosphate graft material. A randomized clinical trial.
J Bone Joint Surg Am 1997;79A:495-502.
MJ, McAndrew MP, Thomas R, et al. Structural allografts for
reconstruction of lower extremity open fractures with 10 centimeters or
more of acute segmental defects. J Orthop Trauma 1995;9:222-226.
TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of
members of the transforming growth factor beta superfamily during
murine fracture healing. J Bone Miner Res 2002;17:513-520.
L, Augat P, Suger G, et al. Influence of size and stability of the
osteotomy gap on the success of fracture healing. J Orthop Res
1997;15:577-584.
A. On the means of lengthening, in the lower limbs, the muscles and
tissues which are shortened through deformity. 1904. Clin Orthop Relat
Res 1994;April:4-9.
BR, Ison IC, Fulmer MT, et al. Skeletal repair by in situ formation of
the mineral phase of bone. Science 1995;267:1796-1799.
SD, Ryaby JP, McCabe J, et al. Acceleration of tibia and distal radius
fracture healing in patients who smoke. Clin Orthop Relat Res
1997;April:198-207.
MA, Bland YS, Ashhurst DE. The effect of exogenous transforming growth
factor-beta 2 on healing fractures in the rabbit. Bone 1995;16:521-527.
S, Rauch F, Silvestri A, et al. Bone morphogenetic proteins in
orthopedics: from basic science to clinical practice. Orthopedics
1999;22:686-695.
G, Schiller PC, Ricordi C, et al. Age-related osteogenic potential of
mesenchymal stromal stem cells from human vertebral bone marrow. J Bone
Miner Res 1999; 14:1115-1122.
S, Lenthall G, Francis MJ. Release of prostaglandins from bone and
muscle after tibial fracture. An experimental study in rabbits. J Bone
Joint Surg Br 1981;63B: 185-189.
PC, Burchardt H, Glowczewskie FP Jr. A roentgenographic, biomechanical,
and histological evaluation of vascularized and nonvascularized
segmental fibular canine autografts. J Bone Joint Surg Am
1985;67A:105-112.
DW, Cosman F, Kurland ES, et al. Effects of daily treatment with
parathyroid hormone on bone microarchitecture and turnover in patients
with osteoporosis: a paired biopsy study. J Bone Miner Res
2001;16:1846-1853.
JJ, Wildin CJ, Bhowal B, et al. Should acute scaphoid fractures be
fixed? A randomized controlled trial. J Bone Joint Surg Am
2005;87A:2160-2168.
DL, Osyczka AM, Garino JP, et al. Regulation of BMP-induced
transcription in cultured human bone marrow stromal cells. J Bone Joint
Surg Am 2003; 85A(suppl 3):19-28.
R, Dahabreh Z, Katsoulis E, et al. Application of recombinant BMP-7 on
persistent upper and lower limb nonunions. Injury 2005;36(suppl
4):S51-S59.
M, Dalstra M, Danielsen CC, et al. Age variations in the properties of
human tibial trabecular bone. J Bone Joint Surg Br 1997;79B:995-1002.
CA, Fergusson CM, Freedman L, et al. Allograft versus autograft bone in
scoliosis surgery. J Bone Joint Surg Br 1988;70B:431-434.
CM, Henning JA, Anderson JG, et al. Randomized prospective study
comparing tricortical iliac crest autograft to allograft in the lateral
column lengthening component for operative correction of adult acquired
flatfoot deformity. Foot Ankle Int 2007;28: 8-12.
H, Ding M, Lind M, et al. Recombinant human vascular endothelial growth
factor enhances bone healing in an experimental nonunion model. J Bone
Joint Surg Br 2005;87:1434-1438.
TA, Bonnarens F, Burstein AH. The contributions of dietary protein and
mineral to the healing of experimental fractures. A biomechanical
study. J Bone Joint Surg Am 1986;68A:1389-1395.
LG, Nelson DG, Featherstone JD. Crystallographic structure and surface
morphology of sintered carbonated apatites. J Biomed Mater Res
1988;22:541-553.
A, Petren-Mallmin M, Larsson S. No effect of low-intensity ultrasound
on healing time of intramedullary fixed tibial fractures. J Orthop
Trauma 1999;13:252-257.
GA, Ammeen DJ. Use of structural allograft in revision total knee
arthroplasty in knees with severe tibial bone loss. J Bone Joint Surg
Am 2007;89:2640-2647.
WF, Burchardt H, Puhl JJ, et al. Physical and biological aspects of
repair in dog cortical-bone transplants. J Bone Joint Surg Am
1975;57:237-252.
WF, Eady JL, Burchardt H. Autogenous cortical bone grafts in the
reconstruction of segmental skeletal defects. J Bone Joint Surg Am
1980;62:1039-1058.
JE Jr, Cornell CN, Muschler GF. Bone cells and matrices in orthopedic
tissue engineering. Orthop Clin North Am 2000;31:357-374.
BL, Dall BE, Rowe DE. Complications associated with harvesting
autogenous iliac bone graft. Am J Orthop 1995 December;24:895-903.
ZB, Andrews ET, Smolenski BI, et al. Bone reaction to varying amounts
of direct current. Surg Gynecol Obstet 1970;131:894-899.
ZB, Harlow MC, Brighton CT. Healing of nonunion of the medial malleolus
by means of direct current: a case report. J Trauma 1971;11:883-885.
GE. Immune responses to osteochondral allografts. Current knowledge and
future directions. Clin Orthop Relat Res 1983;April:58-68.
GE, Perry CR, Cole JD, et al. Osteogenic protein-1 (bone morphogenetic
protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am
2001;83A(suppl 1, pt 2):S151-S158.
RD, Toonen HG, van Heerwaarden RJ, et al. Mechanism of bone
incorporation of beta-TCP bone substitute in open wedge tibial
osteotomy in patients. Biomaterials 2005;26:6713-6719.
IR, Gutierrez GE, Rossini G, et al. Locally delivered lovastatin
nanoparticles enhance fracture healing in rats. J Orthop Res
2007;25:1351-1357.
K, Feschuk C, Sharp JG, et al. Does the number or quality of
pluripotent bone marrow stem cells decrease with age? Clin Orthop Relat
Res 2007;465:202-207.
SB, Bauer TW, Carter D, et al. Norian SRS cement augmentation in hip
fracture treatment. Laboratory and initial clinical results. Clin
Orthop Relat Res 1998; March:42-50.
AE, Cunningham JL, Kenwright J. Strain rate and timing of stimulation
in mechanical modulation of fracture healing. Clin Orthop Relat Res
1998;355(suppl): S105-S115.
JA, Senunas LE, DeSilva GL, et al. Autogenous iliac crest bone graft.
Complications and functional assessment. Clin Orthop Relat Res
1997;June:76-81.
S, Csimma C, Genant HK, et al. Recombinant human bone morphogenetic
protein-2 for treatment of open tibial fractures: a prospective,
controlled, randomized study of 450 patients. J Bone Joint Surg Am
2002;84A:2123-2134.
DP, Kalen V, Ross TI, et al. Use of allograft bone for posterior spinal
fusion in idiopathic scoliosis. Clin Orthop Relat Res
1999;December:273-278.
RB, Anderson JT. Prevention of infection in the treatment of 1025 open
fractures of long bones: retrospective and prospective analyses. J Bone
Joint Surg Am 1976;58: 453-458.
FS, Duncan CP. Cortical onlay allograft struts in the treatment of
periprosthetic femoral fractures. Instr Course Lect 2003;52:291-300.
GJ, Berry DJ. Nonunion of fractures of the subtrochanteric region of
the femur. Clin Orthop Relat Res 2004;February:185-188.
SE, Goshima J, Goldberg VM, et al. Characterization of cells with
osteogenic potential from human marrow. Bone 1992;13:81-88.
JH, Zimmerman PA, McDonnell JM, et al. Percutaneous bone marrow
grafting of delayed union and nonunion in cancer patients. Clin Orthop
Relat Res 1990;July: 280-285.
JD, Ryaby JP, McCabe J, et al. Acceleration of tibial fracture-healing
by noninvasive, low-intensity pulsed ultrasound. J Bone Joint Surg Am
1994;76A:26-34.
SL, Nik Intan NI, Fazan F. Comparison of hydroxyapatite powders derived
from different resources. Med J Malaysia 2004;59(suppl B):77-78.
P, Mathieu G, Poignard A, et al. Percutaneous autologous bone-marrow
grafting for nonunions. Surgical technique. J Bone Joint Surg Am
2006;88(suppl 1, pt 2):322-327.
P, Poignard A, Beaujean F, et al. Percutaneous autologous bone-marrow
grafting for nonunions. Influence of the number and concentration of
progenitor cells. J Bone Joint Surg Am 2005;87A:1430-1437.
P, Poignard A, Manicom O, et al. The use of percutaneous autologous
bone marrow transplantation in nonunion and avascular necrosis of bone.
J Bone Joint Surg Br 2005;87B:896-902.
C, Sama D, Toro JB, et al. Plate fixation of ununited humeral shaft
fractures: effect of type of bone graft on healing. J Bone Joint Surg
Am 2006;88A:1442-1447.
JO, Onikepe AO, MacKrell J, et al. Accelerated fracture healing in the
geriatric, osteoporotic rat with recombinant human platelet-derived
growth factor-BB and an injectable beta-tricalcium phosphate/collagen
matrix. J Orthop Res 2008;26:83-90.
FJ, Zych GA, Hutson JJ, et al. Salvage of humeral nonunions with onlay
bone plate allograft augmentation. Clin Orthop Relat Res
2001;May:203-209.
WG, Verheyen CC, Leemans R. An injectable calcium phosphate cement as a
bone-graft substitute in the treatment of displaced lateral tibial
plateau fractures. Injury 2003;34:141-144.
MM, Abreu C, Harrison JR, et al. Basic fibroblast growth factor
inhibits type I collagen gene expression in osteoblastic MC3T3-E1
cells. J Biol Chem 1993;268: 5588-5593.
GA, Khelimskii AM, Saks RG. [Characteristics of systemic growth
regulation of the limbs under the effect of various factors influencing
their growth and length]. Ortop Travmatol Protez 1978;August:37-41.
GA, Pereslitskikh PF, Barabash AP. [Closed directed longitudino-oblique
or spinal osteoclasia of the long tubular bones (experimental study)].
Ortop Travmatol Protez 1978;November:20-23.
DE, Folkman J. Mechanochemical switching between growth and
differentiation during fibroblast growth factor-stimulated angiogenesis
in vitro: role of extracellular matrix. J Cell Biol 1989;109:317-330.
H, Koefoed M, Tiyapatanaputi P, et al. Remodeling of cortical bone
allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat
Med 2005;11:291-297.
N, Haynesworth SE, Caplan AI, et al. Osteogenic differentiation of
purified, culture-expanded human mesenchymal stem cells in vitro. J
Cell Biochem 1997;64: 295-312.
M, Kay JF, Gumaer KI, et al. Tissue, cellular, and subcellular events
at a boneceramic hydroxylapatite interface. J Bioeng 1977;1:79-92.
M, Andrew JG, Muir LT, et al. Controlled trial of distal radial
fractures treated with a resorbable bone mineral substitute. J Hand
Surg [Br] 2002;27:146-149.
EE, Urist MR, Finerman GA. Repair of segmental defects of the tibia
with cancellous bone grafts augmented with human bone morphogenetic
protein. A preliminary report. Clin Orthop Relat Res
1988;November:249-257.
CM, Wilkins RM, Gitelis S, et al. The use of a surgical grade calcium
sulfate as a bone graft substitute: results of a multicenter trial.
Clin Orthop Relat Res 2001; January:42-50.
J, Richardson JB, Goodship AE, et al. Effect of controlled axial
micromovement on healing of tibial fractures. Lancet 1986;2:1185-1187.
S, Giancotti FG, Presta M, et al. Basic fibroblast growth factor
modulates integrin expression in microvascular endothelial cells. Mol
Biol Cell 1993;4:973-982.
M, Eralp L, Sen C, et al. Management of stiff hypertrophic nonunions by
distraction osteogenesis: a report of 16 cases. J Orthop Trauma
2003;17:543-548.
A, Wallace WA, Prince HG. Clinical experience with a new artificial
bone graft: preliminary results of a prospective study. Injury
1990;21:142-144.
S, Bail H, Schmidmaier G, et al. Homologous growth hormone accelerates
bone healing: a biomechanical and histological study. Bone
2003;33:628-637.
TK, Ryaby JP, McCabe J, et al. Accelerated healing of distal radial
fractures with the use of specific, low-intensity ultrasound. A
multicenter, prospective, randomized, double-blind, placebo-controlled
study. J Bone Joint Surg Am 1997;79:961-973.
M, Machalinski B, Ratajczak MZ. The developmental deposition of
epiblast/germ cell-line derived cells in various organs as a
hypothetical explanation of stem cell plasticity? Acta Neurobiol Exp
(Wars) 2006;66:331-341.
J, Tulikoura I, Konttinen YT, et al. Treatment of infection and
nonunion after bilateral complicated proximal tibial fracture. Ann Chir
Gynaecol 2000;89:325-328.
HM, Haynesworth SE, Gerson SL, et al. Ex vivo expansion and subsequent
infusion of human bone marrow-derived stromal progenitor cells
(mesenchymal progenitor cells): implications for therapeutic use. Bone
Marrow Transplant 1995;16: 557-564.
BK, Tammik C, Rosendahl K, et al. HLA expression and immunologic
properties of differentiated and undifferentiated mesenchymal stem
cells. Exp Hematol 2003;31: 890-896.
CM, Rizzo M, Gunneson EE, et al. Free vascularized fibular bone
grafting in the management of femoral neck nonunion in patients younger
than 50 years. J Orthop Trauma 2002;16:464-472.
DY, Cho TJ, Kim JA, et al. Mobilization of endothelial progenitor cells
in fracture healing and distraction osteogenesis. Bone 2008;January 26.
DG, Chao EY, Kasman RA, et al. Comparison of the effects of compression
plates and external fixators on early bone-healing. J Bone Joint Surg
Am 1984;66A: 1084-1091.
KU, Gresser JD, Wise DL, et al. Bioresorbable bone graft substitutes of
different osteoconductivities: a histologic evaluation of
osteointegration of poly(propylene glycol-co-fumaric acid)-based cement
implants in rats. Biomaterials 2000;21: 757-764.
D, Maor G, Rozen N, et al. Expression of vascular antigens by bone
cells during bone regeneration in a membranous bone distraction system.
Histochem Cell Biol 2001;116:381-388.
M, Ke HZ, Qi H, et al. A novel, nonprostanoid EP2 receptor-selective
prostaglandin E2 agonist stimulates local bone formation and enhances
fracture healing. J Bone Miner Res 2003;18:2033-2042.
X, Qin L, Bergenstock M, et al. Parathyroid hormone stimulates
osteoblastic expression of MCP-1 to recruit and increase the fusion of
pre/osteoclasts. J Biol Chem 2007; 282:33098-33106.
KW, MacKenzie TC, Shaaban AF, et al. Human mesenchymal stem cells
engraft and demonstrate site-specific differentiation after in utero
transplantation in sheep. Nat Med 2000;6:1282-1286.
CH, Wei FC, Levin LS, et al. Free composite serratus anterior and rib
flaps for tibial composite bone and soft-tissue defect. Plast Reconstr
Surg 1997;99:1656-1665.
M, Schumacker B, Soballe K, et al. Transforming growth factor-beta
enhances fracture healing in rabbit tibiae. Acta Orthop Scand
1993;64:553-556.
P, Gerich T, Witte F, et al. Use of an injectable calcium phosphate
bone cement in the treatment of tibial plateau fractures: a prospective
study of 26 cases with 20-month mean follow-up. J Orthop Trauma
2002;16:143-149.
CF, Gebhardt MC, Tomford WW, et al. Infection in bone allografts.
Incidence, nature, and treatment. J Bone Joint Surg Am 1988;70A:369-376.
SC, Boden SD. Osteoinductive bone graft substitutes for spinal fusion:
a basic science summary. Orthop Clin North Am 1999;30:635-645.
LR, Kana SM, Jingushi S, et al. Defects of early fracture-healing in
experimental diabetes. J Bone Joint Surg Am 1989;71A:722-733.
GI, Rocchi R, Cadossi R, et al. The electrical stimulation of tibial
osteotomies. Double-blind study. Clin Orthop Relat Res
1993;March:246-253.
T, Mori S, Mashiba T, et al. Human parathyroid hormone (1-34)
accelerates natural fracture healing process in the femoral osteotomy
model of cynomolgus monkeys. Bone 2007;40:1475-1482.
I, Sivakumar M, Sampath Kumar TS, et al. Synthesis and characterization
of functional gradient materials using Indian corals. J Mater Sci Mater
Med 2000;11: 705-709.
A, Kusuzaki K, Matsubara T, et al. Calcium phosphate cement in
musculoskeletal tumor surgery. J Surg Oncol 2006;93:212-220.
MP, Gorman PW, Lange TA. Tricalcium phosphate as a bone graft
substitute in trauma: preliminary report. J Orthop Trauma
1988;2:333-339.
U, Meyer T, Wiesmann HP, et al. Mechanical tension in distraction
osteogenesis regulates chondrocytic differentiation. Int J Oral
Maxillofac Surg 2001;30:522-530.
BR, Willson Carr SE, Craig JG, et al. Calcium sulfate used as bone
graft substitute in acetabular fracture fixation. Clin Orthop Relat Res
2003;May:303-309.
JR, Weiland AJ, Daniel RK. Use of free vascularized bone grafts in the
treatment of bone tumors. Clin Orthop Relat Res 1983;May:37-44.
CS, Einhorn TA. Enhancement of skeletal repair. In: Browner BD, Jupiter
JB, Levine AM, eds. Skeletal Trauma. Basic Science, Management, and
Reconstruction. 3rd Ed. Philadelphia: Saunders; 2003:639.
G, Garrett R, Harris S, et al. Stimulation of bone formation in vitro
and in rodents by statins. Science 1999;286:1946-1949.
GF, Boehm C, Easley K. Aspiration to obtain osteoblast progenitor cells
from human bone marrow: the influence of aspiration volume. J Bone
Joint Surg Am 1997; 79A:1699-1709.
GF, Nitto H, Boehm CA, et al. Age- and gender-related changes in the
cellularity of human bone marrow and the prevalence of osteoblastic
progenitors. J Orthop Res 200119:117-125.
A, Shimoji N, Shiomi K, et al. Mechanisms for the enhancement of
fracture healing in rats treated with intermittent low-dose human
parathyroid hormone (1-34). J Bone Miner Res 2002;17:2038-2047.
T, Hara Y, Tagawa M, et al. Recombinant human basic fibroblast growth
factor accelerates fracture healing by enhancing callus remodeling in
experimental dog tibial fracture. J Bone Miner Res 1998;13:942-949.
T, Nakajima A, Shiomi K, et al. Effects of low-dose, intermittent
treatment with recombinant human parathyroid hormone (1-34) on
chondrogenesis in a model of experimental fracture healing. Bone
2005;37:711-719.
TJ, Howlett CR, Martin C, et al. Effect of platelet-derived growth
factor on tibial osteotomies in rabbits. Bone 1994;15:20320-20328.
RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone
(1-34) on fractures and bone mineral density in postmenopausal women
with osteoporosis. N Engl J Med 2001;344:1434-1441.
M, Camelo M, Nevins ML, Schenk RK, Lynch SE. Periodontal regeneration
in humans using recombinant human platelet-derived growth factor-BB
(rhPDGF-BB) and allogenic bone. J Periodontol 2003;74:1282-1292.
RM Jr, Riemer BL, Butterfield SL. Harvesting of autogenous cancellous
bone graft from the proximal tibial metaphysis. A review of 230 cases.
J Orthop Trauma 1991;5:469-474.
D, Young MC, Wiley AM, et al. Percutaneous bone marrow grafting of
fractures and bony defects. An experimental study in rabbits. Clin
Orthop Relat Res 1986;July: 300-312.
VM, Borovecki F, Ke HZ, et al. An EP2 receptor-selective prostaglandin
E2 agonist induces bone healing. Proc Natl Acad Sci U S A
2003;100:6736-6740.
MJ, Raghavan R, Gurusamy K. Incidence of fracture-healing complications
after femoral neck fractures. Clin Orthop Relat Res 2007;458:175-179.
RR, Friedlaender GE, Markham TC, et al. Effects of freezing and
freeze-drying on the biomechanical properties of rat bone. J Orthop Res
1984;1:405-411.
AA, Mont MA, Nasser PR, et al. Noninvasive low-intensity pulsed
ultrasound accelerates bone healing in the rabbit. J Orthop Trauma
1990;4:246-253.
H Jr, Hollmann K, Wilfert KH. Experimental bridging of osseous defects
in rats by the implantation of Kiel bone containing fresh autologous
marrow. J Bone Joint Surg Br 1972;54B:735-743.
M, Kolbeck S, Bail H, et al. Homologous growth hormone accelerates
healing of segmental bone defects. Bone 2001;29:368-373.
M, Rasmussen MH, Govender S, et al. Effects of growth hormone in
patients with tibial fracture: a randomized, double-blind,
placebo-controlled clinical trial. Eur J Endocrinol 2007;156:341-351.
MZ, Machalinski B, Wojakowski W, et al. A hypothesis for an embryonic
origin of pluripotent Oct-4(+) stem cells in adult bone marrow and
other tissues. Leukemia 2007;21:860-867.
MZ, Zuba-Surma EK, Machalinski B, et al. Bone marrow-derived stem
cells: our key to longevity? J Appl Genet 2007;48:307-319.
MZ, Zuba-Surma EK, Shin DM, et al. Very small embryonic-like (VSEL)
stem cells in adult organs and their potential role in rejuvenation of
tissues and longevity. Exp Gerontol 2008;June 14.
U, Duneas N. Tissue morphogenesis and regeneration by bone
morphogenetic proteins. Plast Reconstr Surg 1998;101:227-239.
MF, Mitchell J, Goltzman D. In vivo distribution of parathyroid hormone
receptors in bone: evidence that a predominant osseous target cell is
not the mature osteoblast. Endocrinology 1988;123:187-191.
MF, Mitchell J, Goltzman D. Characterization of the major parathyroid
hormone target cell in the endosteal metaphysis of rat long bones. J
Bone Miner Res 1990; 5:1043-1053.
CH, Miyakoshi N, Ramirez E, et al. Expression of the fibroblast growth
factor receptor genes in fracture repair. Clin Orthop Relat Res
2002;October:253-263.
S, Nolte PA, Guit GL, et al. Use of low-intensity pulsed ultrasound for
posttraumatic nonunions of the tibia: a review of patients treated in
the Netherlands. J Trauma 2007;62:902-908.
J, Munuera L, Madero R. Treatment of fractures of the distal radius
with a remodellable bone cement: a prospective, randomized study using
Norian SRS. J Bone Joint Surg Br 2000;82:856-863.
RA, Sackett JR. Open reduction and internal fixation of delayed union
and nonunion of the distal humerus. J Orthop Trauma 1990;4:254-259.
A, Schaeffer JF, Beckerman L, et al. Fracture healing in rat femora as
affected by functional weight-bearing. J Bone Joint Surg Am
1977;59A:369-375.
TA, Bauer TW, Josten C, et al. Open reduction and augmentation of
internal fixation with an injectable skeletal cement for the treatment
of complex calcaneal fractures. J Orthop Trauma 2000;14:309-317.
BW, Arts JJ, Verdonschot N, et al. Femoral component revision with use
of impaction bone-grafting and a cemented polished stem. Surgical
technique. J Bone Joint Surg Am 2006;88(suppl 1, pt 2):259-274.
G, King JB. A prospective, double-blind trial of electrical capacitive
coupling in the treatment of non-union of long bones. J Bone Joint Surg
Am 1994;76A:820-826.
JM, Torner P, Garcia S, et al. Use of bone allograft in tibial plateau
fractures. Arch Orthop Trauma Surg 1998;117:357-359.
C, Eralp L, Gunes T, et al. An alternative method for the treatment of
nonunion of the tibia with bone loss. J Bone Joint Surg Br
2006;88B:783-789.
C, Kocaoglu M, Eralp L, et al. compression-distraction in the acute
treatment of grade III open tibia fractures with bone and soft-tissue
loss: a report of 24 cases. J Orthop Trauma 2004;18:150-157.
JW, Field GA, Goldberg VM, et al. Fate of vascularized and
nonvascularized autografts. Clin Orthop Relat Res 1985;July:32-43.
AS. Use of bisphosphonates to improve the durability of total joint
replacements. J Am Acad Orthop Surg 2006;14:215-225.
WJ. A double-blind trial of pulsed electromagnetic fields for delayed
union of tibial fractures. J Bone Joint Surg Br 1990;72B:347-355.
WA. Autogenous cancellous strip grafts in the treatment of delayed
union of long bone fractures. J Bone Joint Surg Br 1969;51B:63-75.
S, Li XQ, Martin B. The fate of cancellous and cortical bone after
transplantation of fresh and frozen tissue-antigen-matched and
mismatched osteochondral allografts in dogs. J Bone Joint Surg Am
1991;73:1143-1156.
J, Winter D, Wang JH, et al. Is human fracture hematoma inherently
angiogenic? Clin Orthop Relat Res 2000;September:224-237.
JT, Wang JH, Wu QD, et al. The angiogenic response to skeletal injury
is preserved in the elderly. J Orthop Res 2001;19:1057-1066.
MF, Aro HT, Donell S, et al. Recombinant human bone morphogenetic
protein-2 in open tibial fractures. A subgroup analysis of data
combined from two prospective randomized studies. J Bone Joint Surg Am
2006;88A:1258-1265.
AD, Cutler L, Murali SR, et al. In scaphoid nonunion, does the source
of graft affect outcome? Iliac crest versus distal end of radius bone
graft. J Hand Surg [Br] 2006;31:47-51.
BK, Patel VV, Bradford DS. Calcium sulfate- and calcium phosphate-based
bone substitutes. Mimicry of the mineral phase of bone. Orthop Clin
North Am 1999;30: 615-623.
P, Kawamoto H, Matthews D, et al. Autogenous bone grafts and bone
substitutes—tools and techniques: I. A 20,000-case experience in
maxillofacial and craniofacial surgery. Plast Reconstr Surg 2005;116(5
suppl):6S-24S.
P, Kawamoto H, Posnick J, et al. Complications of harvesting autogenous
bone grafts: a group experience of 20,000 cases. Plast Reconstr Surg
2005;116(5 suppl): 72S-3S.
SR, Dart A, Tesluk H. The effects of insulin-like growth factor-1 on
criticalsize calvarial defects in Sprague-Dawley rats. Ann Plast Surg
1993;31:429-433.
T, Hoeber S, Froese S, et al. Age-dependent impairment of endothelial
progenitor cells is corrected by growth-hormone-mediated increase of
insulin-like growth-factor-1. Circ Res 2007;100:434-443.
JJ, Garvin KL, Kile TA, et al. The role of a composite, demineralized
bone matrix and bone marrow in the treatment of osseous defects.
Orthopedics 1995;18: 1153-1158.
AD, Barker RL, Jones RS, et al. Impaction bone-grafting in revision
joint replacement surgery. J Bone Joint Surg Am 2004;86A:2050-2060.
AD, McClelland D, Chua L, et al. Mechanical testing of impaction bone
grafting in the tibia: initial stability and design of the stem. J Bone
Joint Surg Br 2005;87B: 656-663.
K, Bandyopadhyay A, Harfe BD, et al. BMP2 activity, although
dispensable for bone formation, is required for the initiation of
fracture healing. Nat Genet 2006;38: 1424-1429.
K, Saito A, Nakano H, et al. Cortical hyperostosis following long-term
administration of prostaglandin E1 in infants with cyanotic congenital
heart disease. J Pediatr 1980;97:834-836.
MR. Osteoinduction in undemineralized bone implants modified by
chemical inhibitors of endogenous matrix enzymes. A preliminary report.
Clin Orthop Relat Res 1972;87:132-137.
F, Ulkur E, Pehlivan O, et al. Soft tissue necrosis following using
calcium phosphate cement in calcaneal bone cyst: case report. Arch
Orthop Trauma Surg 2007; December 4.
G. Cellular biology and biochemical mechanism of bone resorption. A
review of recent developments on the formation, activation, and mode of
action of osteoclasts. Clin Orthop Relat Res 1988;June:239-271.
JW, Weng LH. Treatment of distal femoral nonunion with internal
fixation, cortical allograft struts, and autogenous bone-grafting. J
Bone Joint Surg Am 2003; 85A:436-440.
PM, Stewart SL. Surgical treatment of nonunion and avascular necrosis
of the proximal part of the scaphoid in adolescents. J Bone Joint Surg
Am 2002;84A:915-920.
RD, Zhang H, Bronson DG. Experimental tibial plateau fractures
augmented with calcium phosphate cement or autologous bone graft. J
Bone Joint Surg Am 2003; 85A:222-231.
TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation
of recombinant human platelet-derived growth factor-BB (becaplermin) in
patients with chronic neuropathic diabetic ulcers. A phase III
randomized placebo-controlled double-blind study. Diabetes Care
1998;21:822-827.
GN, Cocking MR. Union of medial opening-wedge high tibial osteotomy
using a corticocancellous proximal tibial wedge allograft. Am J Sports
Med 2008;January 28.
KH, Parvizi J, Wang SJ, et al. Exposure to low-intensity ultrasound
increases aggrecan gene expression in a rat femur fracture model. J
Orthop Res 1996;14: 802-809.
DN, McClellan RT, Reindel ES, et al. Biomechanical comparison of
conventional open reduction and internal fixation versus calcium
phosphate cement fixation of a central depressed tibial plateau
fracture. J Orthop Trauma 2001;15:197-206.
BH, Hendi P, Smith WR, et al. Osseous healing with a composite of
allograft and demineralized bone matrix: adverse effects of smoking. Am
J Orthop 2007;36: 207-209.
BH, Smith WR, Morgan SJ. Use of calcium-based demineralized bone
matrix/ allograft for nonunions and posttraumatic reconstruction of the
appendicular skeleton: preliminary results and complications. J Trauma
2007;63:1324-1328.
