ARTHRODESIS OF THE HAND AND WRIST
III – THE HAND > Reconstructive Procedures > CHAPTER 72 –
ARTHRODESIS OF THE HAND AND WRIST
functional and productive living. There are numerous medical conditions
that limit the use of the small joints of the hand because of pain,
deformity, dysfunction, and instability. Among these are trauma,
infection, connective tissue disease, arthritides, osteoarthrosis,
paralytic disorders, failed surgical procedures, and congenital
deformities. Many medical and surgical advances have been made to
improve the hand function of affected patients. One of the mainstays
has been to perform arthrodeses of selected joints of the hand and
wrist. Arthrodesis, or joint fusion, remains one of the most
time-tested, reliable, and useful hand surgery procedures (39,62). Reasons to perform small-joint arthrodesis are to correct deformity, to relieve
pain, to control instability, and to improve loss of function caused by neurovascular disease (9,48,55,74,89).
A properly performed small-joint arthrodesis can markedly improve hand
function and the overall quality of life for an afflicted patient.
interphalangeal (DIP) joints of the fingers and, to a lesser extent,
the interphalangeal (IP) joint of the thumb. Other diseases that less
commonly affect the DIP joints are psoriatic arthritis, rheumatoid
arthritis, and infection. In all these diseases, hand function is
limited by painful motion of the joints. Arthrodesis of the DIP joint
is a successful way to eliminate the pain associated with these
disorders.
commonly involved in rheumatoid arthritis as well as osteoarthritis.
Posttraumatic arthrosis of a single PIP joint is a common problem. The
disability for the rheumatoid patient is the associated swan-neck or
boutonnieére deformity, while for the posttraumatic arthritic patient
it is usually pain. Arthrodesis of the PIP joints is a reliable method
of returning function to these fingers, and it is especially
appropriate for the index and small fingers because of their unique
position as border digits.
multiple fingers in rheumatoid arthritis, or singly in posttraumatic
arthritis. Arthroplasty is usually preferred for the MCP joints of the
fingers to preserve good motion and function. Arthrodesis is useful for
joints when arthroplasty is not indicated, or when a previous
arthroplasty has failed.
performed, can also improve functional outcomes, particularly in the
fourth and fifth carpometacarpal joints, which may have osteoarthrosis
as a late sequela of fracture dislocations (15,17). Elimination of pain and increased stability improves hand function.
IP joints of the digits. It is important for stable power pinch, which
may be compromised in osteoarthritis because of pain or connective
tissue disease caused by joint laxity.
joints in that significantly more stress is placed on the ligamentous
restraints than in the finger joints. This is because of the unique
position of the thumb and its role in pinch and grasp. Instability of
this joint is common because of chronic laxity after a missed acute
tear of the ulnar collateral ligament (gamekeeper’s thumb) and
rheumatoid arthritis (19,48).
While ligament reconstruction and soft-tissue procedures are usually
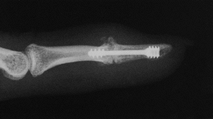
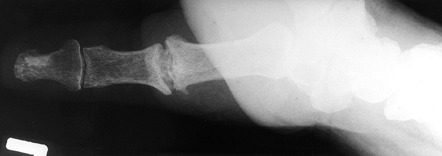
preferred, in the arthritic thumb MCP joint, arthrodesis (Fig. 72.1, Fig. 72.2) provides a more lasting option and is generally preferred to arthroplastic techniques for a number of different disorders (36).
 |
|
Figure 72.1. Thumb metacarpophalangeal joint fusion, anteroposterior view.
|
 |
|
Figure 72.2. Metacarpophalangeal joint fusion, lateral view.
|
This is perhaps the most commonly involved joint in postmenopausal
women with early osteoarthritis. It has been postulated to be secondary
to laxity of the metacarpal volar beak ligament, allowing enough
subluxation and incongruity of the joint to become pathologic. A second
commonly affected group is young people with high demands on their
hands (e.g., manual laborers) who have posttraumatic arthritis after an
old intraarticular fracture of the base of the metacarpal (Bennett’s or
Rolando’s fracture). Eaton et al. (28a) have
classified this pattern of osteoarthrosis of the first CMC joint into
four stages. Stage I has normal intraarticular cartilage with
joint-space widening. In stage II, there is narrowing of the joint
space but the articular contours are normal. Stage III disease has
significant
destruction of the thumb CMC joint, but the scaphotrapeziotrapezoidal
(STT) joint is normal. In stage IV disease, there is destruction of the
STT joint in addition to the first CMC joint. Arthrodesis of the thumb
CMC joint is indicated for stage III disease but is contraindicated in
the presence of any scaphotrapeziotrapezoidal disease (stage IV).
thumb CMC joint are usually young, active people who require a strong,
stable, pain-free thumb to perform work activities (6,20). House et al. (43) found that arthrodesis of the first CMC improved hand function in patients with tetraplegia following spinal cord injury (see Chapter 68).
Arthrodesis is the best salvage procedure for failed arthroplasty or
previous infection of the first CMC joint. Older patients and patients
whose demands for strength of pinch and grip are not high are better
served with an arthroplasty (see Chapter 70). Moore et al. (72)
reported successful use of arthrodesis for a rare problem, laxity of
the thumb CMC joint in patients with Ehlers-Danlos syndrome. Thumb CMC
arthrodesis significantly limits thumb motion, although some
compensatory motion occurs at the STT and the MCP joints; therefore, in
patients who require motion of the thumb, arthrodesis is
contraindicated.
approach to the soft-tissue envelope about a joint; the manner in which
the joint surfaces are prepared; whether to use bone graft or bone
substitutes, or no graft; fixation methods; and postoperative
management, including rehabilitation (27). The
goals of arthrodesis in the small joints of the hands are uncomplicated
soft-tissue and skin healing, appropriate joint position, and bony
union in the shortest possible time (15). The
best techniques are simple, straightforward, and reliable, and they
allow early motion of the remainder of the hand. Successful fusion
requires a good soft-tissue envelope about the joint as well as
well-vascularized bone at the fusion site. Address any deficiencies
prior to undertaking a fusion.
-
Straight longitudinal incisions are the
best incisions for fusions in the small joints of the hand. Use gentle
curved incisions, and H, Y, V, and other types of incisions, only in
good-quality, well-vascularized skin and soft tissue. Expose the thumb
carpometacarpal joint through a Wagner type of anterior incision or
through a dorsal incision directly over the joint (see Chapter 37). -
Approach the DIP joints of the fingers
and the IP joint of the thumb by transversely dividing the extensor
mechanism and capsule. Debride the soft tissues as needed. We prefer
excision of the radial and ulnar collateral ligament complexes prior to
arthrodesis. -
Remove all marginal and dorsal osteophytes.
-
Approach the digital PIP joints by
dividing the extensor tendon mechanism longitudinally. Careful
dissection preserves the interval between the extensor mechanism and
the dorsal joint capsule. Preserve the joint capsule if possible. -
Approach the MCP joints of the fingers by
dividing the ulnar sagittal fibers. Retract the entire extensor
mechanism to one side and visualize the joint capsule. Then make a
direct longitudinal approach through the capsule. -
Approach the MCP joint of the thumb by
dividing the radial sagittal fibers, detaching the extensor pollicis
brevis insertion, and pulling the extensor pollicis longus (EPL)
ulnarly. Incise the joint capsule longitudinally and debride as
indicated. -
For the carpometacarpal joint of the thumb, make a volar anterior approach.
-
Elevate the origin of the thenar musculature from the thumb metacarpal and trapezial area.
-
You may partially detach the thenar
muscle and bone insertions of the abductor pollicis longus if necessary
for exposure of the carpometacarpal joint. Pay careful attention to
preserving the joint capsule for closure.
the hand will generally be better served with an arthroplasty of the
finger MCP joints and arthrodeses of the PIP and DIP joints. The
position for arthrodesis in the fingers is critical to hand function.
In general, there should be a gentle cascade from radial to ulnar with
more flexion of the ulnar digits, as can be appreciated in the normal
hand at rest.
-
Fuse the MCP joint of the index finger in
25° to 30° of flexion, adding another 5° of flexion at each joint,
moving ulnarly, to end at 40° to 45° of flexion in the small-finger MCP
joint. -
There should be no radial or ulnar
deviation at the MCP joints. Ensure that there is no rotational
deformity, although some have suggested that gentle supination may help
with thumb-pad pinch. There should be more flexion at the PIP joints
than at the MCP joints in each digit. -
Fuse the index-finger PIP joint at 40° to
45° of flexion, with an additional 5° of flexion added at each PIP
joint to end at 55° to 60° of flexion at the small-finger PIP joint. -
The DIP joints are very important to hand
function. Fusion in too much flexion is disabling and cosmetically
undesirable. Our experience is that too much extension of these joints
is tolerated better than too much flexion. Fuse the DIP joint at 0° to
15° of flexion. -
The thumb position is most critical to
hand function because of its unique role. Fuse the thumb MCP joint in
approximately 10° flexion with no radial or ulnar deviation. -
Fuse the thumb IP joint in a position of 0° to 15° of flexion.
-
Fuse the thumb carpometacarpal joint in 15° to 20° of extension, 45° of palmar abduction, and 5° to 10° of pronation.
-
Check the positioning of all joint
arthrodeses intraoperatively by temporarily fixing the joints with
Kirschner wires (K-wires) prior to permanent fixation.
-
Remove the articular cartilage and subchondral bone using a small curet or rongeur and expose the subchondral cancellous bone.
joint; therefore, minimal shortening is required and positioning is
easy in flexion/extension, radial/ulnar deviation, and rotation.
systems. It is considered advantageous because it presents large,
opposing cancellous surfaces for fusion (19,66),
but it does increase digital shortening. Recently, cup and cone reamers
have been made available commercially that provide matching surfaces in
the joint. This method is generally used more commonly at the MCP
joints. The CMC joint of the thumb can be fused using this method, with
the cone being made from the first metacarpal base and the cup being
fashioned in the trapezium. Carroll (16) has shown excellent results.
Although precise transverse or straight cuts are difficult to achieve,
this is our preferred method, and it provides excellent arthrodesis
rates (9).
Cancellous bone area for fusion is improved, but the technique causes
some digital shortening and is technically demanding. Cuts may be made
with the apex of the chevron pointing either distally or proximally.
have been used, and multiple studies have described consistent success
using different techniques (5,9,12,15,16,17,19,20,27,31,36,45,48,49 and 50,54,55,58,66,67,74,82,83,85,86,88,89,93,96,102,103,107).
They differ with regard to the degree of difficulty of using the
instrumentation, whether bone grafts are harvested from other surgical
sites, and technical difficulty.
compared crossed K-wires, tension band wiring, and an interosseous loop
supplemented by a K-wire in a PIP arthrodesis model. Tension band
wiring was found to be the strongest. In a
comparison
study of four methods of fixation for CMC arthrodesis using crossed
K-wires, cerclage wiring, and cup and cone with single K-wire and
tension band wiring, Stokel et al. (86) found that tension band wire and cerclage techniques provided the most stable construct. Bamberger et al. (6) have used the cup and cone method originally described by Carroll and Hill (19)
with staple or K-wire fixation. They found a 42% delayed union/nonunion
rate for the staple method compared to an 11% rate of delayed union for
the K-wire group.
They provide stable fixation, are relatively uncomplicated technically,
and may be used in conjunction with cup and cone, miter, or straight
cut techniques. They allow easier adjustment of the arthrodesis site
than many other techniques. Both techniques are excellent for the
patient with rheumatoid arthritis, in whom inadequate bone stock may
not allow screw techniques.
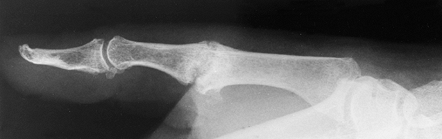
 |
|
Figure 72.3. Metacarpophalangeal joint fusion with Kirschner wire fixation, lateral view.
|
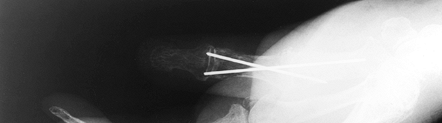 |
|
Figure 72.4. Metacarpophalangeal joint fusion with Kirschner wire fixation, AP view.
|
-
Prepare both bone ends at the joint for
arthrodesis and ensure good bone-to-bone contact. Then stabilize the
arthrodesis by driving two crossed K-wires from distal to proximal
across the joint. Pins driven from proximal to distal may distract the
arthrodesis. -
Drive one pin first, and then check the
arthrodesis position by intraoperative radiographs or imaging on a
fluoroscope. If the position is acceptable, drive the second pin and
check the position once more. Position the pins to avoid prominence
that might cause soft-tissue damage. -
We do not leave K-wires exposed but cut
them off below the level of the skin. Remove the pins when fusion is
healed, which is usually in 4–6 weeks. -
For the tension band technique, prepare the joint surfaces and position the joint.
-
Drill two K-wires parallel to each other,
leaving a dorsal wire protruding from the proximal portion of the
distal bone, 6 mm distal to the cut surface. -
Drill a transverse hole through the
proximal fragment and pass a malleable monofilament stainless steel
wire of appropriate size through the hole. -
Tightly coapt the fusion site and pass
the monofilament wire in a figure-eight fashion dorsally. Pass it
beneath and tighten it around the ends of the K-wires. The arthrodesis
site will be compressed as the figure-eight wire is tightened. -
Cut the pins as low as possible and contour them to fit closely to the bone dorsally. (See Chapter 11 for more details.)
be removed as they are superficial and tender. Do not use tension band
wire techniques at the DIP joint because of the possibility of injury
to the germinal matrix of the nail.
with high activity demands. Screw fixation usually permits early motion
of the hand with a reduced risk of loss of fixation when compared to
K-wire fixation. Many varieties of screws are currently available and
all of them can be used to provide compression across the arthrodesis.
Screw techniques require careful attention to technical detail.
-
To perform an arthrodesis on a PIP joint utilizing the Herbert screw, prepare the joint surfaces as previously described.
-
Drill a pilot hole with an 0.045 K-wire.
Drill from the dorsal surface of the proximal phalanx into the
medullary canal of the middle phalanx. We have found that starting the
hole relatively proximal makes the dorsal cortical bridge larger,
preventing fracture. -
Use a small-diameter Herbert screw drill
bit to enlarge the drill hole through its entire length, from proximal
to distal. Avoid breaking the dorsal cortical bridge between the entry
hole and the fusion site. -
Enlarge the cortical opening with a small rongeur.
-
Using a large-diameter Herbert screw
drill, overdrill the hole in the proximal phalanx. Then insert a
Herbert screw tap through the arthrodesis site. -
Use intraoperative radiography or
fluoroscopy to help determine the size of the screw to be chosen. The
screw should be at least 2 mm shorter than the measured length to allow
the screw head to sink into the proximal phalanx and not cause
soft-tissue irritation. -
Place the Herbert screw of appropriate
size. It is important to hold the arthrodesis site compressed in
appropriate position as the screw is tightened.
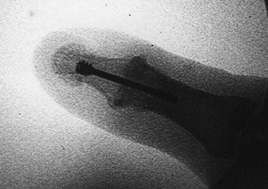 |
|
Figure 72.5. Intraoperative confirmation of distal interphalangeal joint arthrodesis with Herbert screw fixation.
|
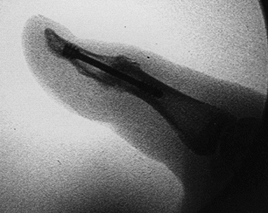 |
|
Figure 72.6. Intraoperative confirmation of position, Herbert screw fixation, lateral view.
|
-
After preparing the joint surfaces, make
a K-wire pilot hole drilling from proximal to distal through the center
of the distal phalanx. The wire exits just under the hard nail, through
the distal skin. -
Make a transverse skin incision at this
level and enlarge the pilot hole, using the small-diameter drill bit,
from distal to proximal. -
Position the arthrodesis site, and pass the small-diameter drill across the joint from distal to proximal.
-
Tap the drill hole from distal to proximal with the Herbert screw tap.
-
Use intraoperative fluoroscopic imaging to determine the length of the screw.
-
Place an appropriate-length Herbert screw
from distal to proximal, countersinking the proximal threads of the
Herbert screw deep enough to keep it from irritating the tip of the
finger (84). Take care, as germinal matrix
injuries can occur. Note that this technique at the DIP joint requires
more extension than other techniques (Fig. 72.7, Fig. 72.8).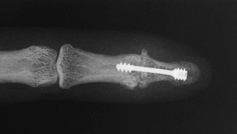 Figure 72.7. Distal interphalangeal joint arthrodesis with Herbert screw fixation, AP view.
Figure 72.7. Distal interphalangeal joint arthrodesis with Herbert screw fixation, AP view.![]() Figure 72.8. Distal interphalangeal joint arthrodesis with Herbert screw fixation, lateral view.
Figure 72.8. Distal interphalangeal joint arthrodesis with Herbert screw fixation, lateral view.
proceeds as described for the Herbert screw, with the following
differences (88). The AO 2.7 mm screw is a
standard screw with threads on one end and a head on the other;
therefore, compression requires lag technique. Overdrill the proximal
phalanx with a 2.7 mm drill bit to gain compression. Countersink the
drill hole before you place the screw, which allows the screw to sit
flush with the bone.
fusion of the DIP joint. In our experience, the AO screw head is too
prominent, causing too much pain in the fingertip; therefore, we
currently do not use it for DIP joint fusion.
available for performing an arthrodesis in the hand. Plating is an
excellent technique when bone grafting is required. It requires
significant soft-tissue stripping and is more complex, so it is used
less commonly for a simple primary arthrodesis. Plate fixation is used
more commonly for salvage of failed previous procedures.
-
After the joint ends have been exposed
and prepared for arthrodesis, expose the dorsal aspects of the proximal
and distal bone surfaces as necessary for plate application. -
Once the bone ends are prepared and any
bone graft has been selected, tailored, and placed, select a
mini-semitubular plate that allows for at least two screws in the
middle phalangeal shaft and two screws in the proximal phalangeal
shaft. Generally, a four-hole plate will not bridge a significant
defect, so a five- or six-hole plate may be required. -
Contour the plate to fit over any bone graft dorsally.
-
Fix the plate with screws to the dorsal
aspect of the distal bone first. Check the screw lengths using
intraoperative fluoroscopy or radiographs. -
Use the dynamic compression principle (Chapter 11) to apply compression between the bone ends and across any graft that is needed.
prominence of the plate and screws, removal and/or extensor tenolysis
may be required as a second-stage procedure.
Fusion rates are excellent but we feel that most external fixation
devices are too bulky and limit the motion of other digits. The
transverse wires and compression devices may cause scarring of the
extensor mechanism. External fixators are most appropriate for fusions
after severe articular surface bone and soft-tissue injuries
complicated by joint infection and osteomyelitis (102). We do not recommend external fixation for uncomplicated phalangeal and MCP joint arthrodeses.
-
We prefer absorbable sutures for closure
of the joint capsule and extensor tendon mechanism because the lack of
joint motion after fusion takes tension off the tendon repair.
Nonabsorbable sutures may cause patient discomfort after surgery by
irritating the skin edges. -
After closure of the deep soft tissues, injection of 0.5% bupivacaine may diminish postoperative discomfort.
-
Close the skin with interrupted nonabsorbable sutures.
-
Apply a well-padded bulky dressing, and
splint the fused joints for comfort and to protect the arthrodesis. At
10–14 days, change the dressings and encourage motion at joints other
than the fusion. Fashion an orthoplast splint to the fused joint and
keep it in place until the fusion is radiographically solid. Protect
thumb MCP and carpometacarpal fusions in thumb spica casts for 4–6
weeks.
Management of complications begins with understanding the potential for
them prior to surgery, and making the patient aware of them. Detect
vascular compromise by carefully observing capillary refill in the
operated digit after tourniquet deflation. Any impairment requires
immediate intervention to prevent loss of the digit. Pin track
infection can be minimized by keeping pins under the patient’s skin.
Diminish wound
problems
by using straight longitudinal dorsal incisions, especially in patients
with immunocompromised status, such as those with diabetes mellitus or
connective tissue diseases. Prevent nonunion by establishing broad bone
contact at the arthrodesis site and stable fixation.
in any arthrodesis because stress is added across these joints. Carroll
(16) found no evidence of STT arthritis after
CMC joint fusion in patients less than 50 years old who were followed
for 3–25 years. More recently, Bamberger et al. (6)
had radiographs of their series of patients reviewed independently for
progression of STT arthrosis after CMC arthrodesis. They found
progression of STT arthritis in 2 of 12 patients but attributed this to
error in technique. Guiral et al. (38) reported
a rare complication of arteriovenous fistula with venous aneurysm after
thumb carpometacarpal joint arthrodesis. This was treated by ligation
and resection of the aneurysm. A painful scar from injury to the
branches of the radial sensory nerve is avoided by careful technique.
Problems with painful implants can be resolved by removal.
based on the biomechanical principle of load transfer from one side of
the carpus to another. The intercarpal mobility that is preserved
compensates for the motion lost to arthrodesis.
fusion procedures include STT fusion; scaphocapitate (SC) fusion;
lunotriquetral (LT) fusion; and fusion of the capitate, hamate, lunate,
and triquetrum, which is known as a four-bone fusion.
showed that the range of motion of the wrist required for ADL is 5° of
flexion, 30° of extension, 10° of radial deviation, and 15° of ulnar
deviation. Brumfield and Champoux (14) showed that 10° of flexion and 35° of extension are required. Ryu et al. (80) showed that 40° of extension, 40° of flexion, and 40° of combined radial and ulnar deviation are needed to perform ADL.
proximal and the distal carpal rows. Because of its unusual anatomy,
deformity of the wrist follows well-delineated patterns when the
scaphoid or its ligamentous restraints are injured (see Chapter 41 and Chapter 42).
One of these patterns, the scapholunate advanced collapse (SLAC)
pattern of wrist arthritis, accounted for 57% of degenerative wrist
arthritis when Watson and Ballet (98) reviewed
4,000 radiographs. These authors also reported primary triscaphe joint
arthritis in 27% of these patients and a combination of both in 15% of
patients. The primary disorder in the SLAC wrist is that of
scapholunate dissociation secondary to scapholunate interosseous
ligament rupture (3). This allows for unopposed volar flexion of the scaphoid and the dorsal intercalated segmental instability (DISI) pattern (26).
Lateral radiographs may show the scapholunate angle to be increased
beyond 60°, which is felt to be the upper limit of normal. On an
anteroposterior (AP) radiograph, the scaphoid appears foreshortened,
has a “cortical ring” sign and there is a scapholunate gap of greater
than 3 mm.
stage, stage 1 arthritis, with destruction of the distal aspect of the
radioscaphoid joint (2). This is caused by the
incongruity between the scaphoid and the scaphoid facet of the radius
when the scaphoid is extremely volar flexed. In stage 2, the entire
radioscaphoid joint is arthritic. Stage 3 is characterized by further
separation between the scaphoid and the lunate, allowing the capitate
to migrate proximally. When this occurs, the SC and capitolunate joints
become arthritic. Stage 4, in which the radiolunate joint becomes
arthritic, is rarely seen because the spherical shape of the proximal
lunate and the lunate fossa of the radius make incongruity unlikely
except in the most severe cases.
predictable pattern of wrist arthritis when not treated. Because of its
similarity to the SLAC pattern in both progression and treatment, it
has been called the scaphoid nonunion advanced collapse (SNAC) pattern.
In it, volar flexion of the distal pole of the scaphoid leads to
radioscaphoid arthritis (101). The proximal
pole, restrained by the scapholunate interosseous ligament, remains in
normal alignment with the lunate. Given the dissociation between the
proximal and distal rows as a result of the nonunion, a DISI pattern of
deformity results. If left untreated, the
wrist will undergo the same degenerative pattern seen in a SLAC wrist.
for stage 2 or 3 SLAC/SNAC disease, it is clear is that scaphoid
excision and four-corner fusion are preferred to proximal row
carpectomy when the capitate head is arthritic.
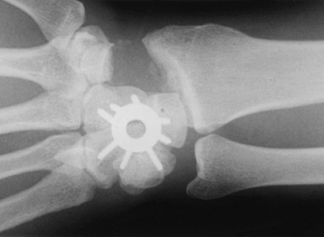 |
|
Figure 72.9. Capitohamate triquetrolunate intracarpal arthrodesis with spider plate fixation.
|
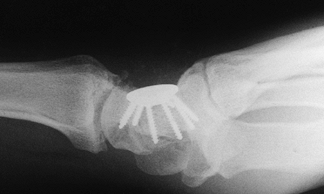 |
|
Figure 72.10. Capitohamate triquetrolunate intracarpal arthrodesis with spider plate fixation, lateral view.
|
symptoms are predictable. In patients with stage 2 or 3 SLAC/SNAC
disease, when strength is important and the patient’s activities do not
require a full range of wrist motion, we perform scaphoid excision and
four-corner fusion, as described later.
arthritis. The pain associated with this disorder can be surprisingly
debilitating. The disability associated with STT arthritis can be best
appreciated in activities that axially load the thumb (e.g., strong
pinch, key turning). Watson et al. (99)
attributed rotatory subluxation of the scaphoid as a possible cause of
isolated STT arthritis. They noted early degenerative changes in these
joints when performing rotary subluxation of the scaphoid (RSS
surgery). For isolated STT arthritis, STT arthrodesis has been found to
give good function and excellent pain relief and is preferred by other
authors (53,97).
of arthritis in the wrist occurs between the lunate and the triquetrum (24,42,51).
This pattern of arthritis is most probably a result of a chronic
lunotriquetal ligament tear that has not healed. Patients typically
have the radiographic findings associated with volar intercalated
segmental instability (VISI). The scapholunate angle on a lateral
radiograph is less than 30°, the lower limit of normal. This indicates
that the ligamentous complex between the lunate and the triquetrum is
disrupted, allowing the lunate to volar-flex and align with the
scaphoid. Another cause of isolated LT arthritis is incomplete carpal
coalition of the lunate and triquetrum (1).
nonunion is left untreated, and in the SLAC wrist deformity. Watson and
Weinzweig (101) described the use of STT
arthrodeses to manage scaphoid nonunion for three specific indications:
scaphoid fractures with a very small proximal fragment, a distal
scaphoid nonunion causing malalignment of the triscaphe joint, and
scaphoid fracture in association with scapholunate dissociation. With
small scaphoid proximal fragments, Watson prefers a dorsal approach
with bone grafting of the nonunion, with a simultaneous triscaphe
arthrodesis. STT arthrodesis is used in distal scaphoid nonunions to
improve alignment in the STT joint. Scapholunate dissociation may be
treated by intercarpal arthrodesis when it is associated with scaphoid
fracture.
Static instabilities have malalignments seen on standard radiographs.
Dynamic instabilities may appear normally aligned on standard
radiographs but are often exhibited on stress views or other special
projections. Acute injuries (up to 1 week old) have the maximum
potential to go on to primary healing of the ligaments. Subacute
injuries (1–6 weeks old) still can heal and do not display fixed
deformity or arthrosis. Chronic injuries older than 6 weeks have the
least potential for healing, may have fixed deformities or
arthrosis,
and often require surgical repair and reconstruction. It is apparent
that early detection is most important. The position of the lunate as
seen on lateral radiographs is one of the key elements used to
determine loss of normal carpal alignment. The terms dorsal intercalated segmental instability and volar intercalated segmental instability describe the malaligned dorsal or volar tilted lunate of unstable wrists (61).
Although many instability classifications have been proposed, none have
gained universal acceptance, which underscores the complexities of
these injuries and the lack of our present comprehension of this
subject.
kinematics is not well understood. When choosing the surgical
procedure, consider the amount of wrist motion that will remain and to
what extent compensatory increases in wrist motion will occur over
time. Although the proximal and distal rows function separately, an
intercarpal arthrodesis that links these rows will have long-term
effects on wrist motion and on radiocarpal and ulnocarpal loading that
could lead to degenerative arthritis in the long term. Gellman et al. (35)
showed that fusion within a carpal row has minimal effect on wrist
motion in all planes. Their study demonstrated that two thirds of
flexion occurs at the radiocarpal joint and one third occurs at the
mid-carpal joint. It also showed that capitolunate fusion caused the
greatest loss of motion in a flexion/extension arc, with STT fusion
causing the greatest loss of motion in the radioulnar plane.
the effects of intercarpal arthrodeses on wrist range of motion. They
showed that STT fusions had a greater loss of flexion than SC fusion,
which resulted in a greater loss of extension and radial deviation.
Shear stress was noted to be increased at the radiolunate joint. Viegas
et al. (95) reported that STT and SC fusions
decrease axial load through the radioscaphoid fossa, while
scapholunocapitate and capitolunate fusions distribute load through
both the radioscaphoid and radiolunate fossae.
-
Unaffected joints must be left unfused.
-
Normal external dimensions of carpal bones included in the arthrodesis must be preserved.
-
Bone fixation must include only those bones that are involved in the arthrodesis.
(triscaphe) fusion results in a single bony unit that preserves the
external dimensions of these three carpal bones. Current indications
for STT fusion are STT arthrosis, Kienböck’s disease, and carpal
instability, including static or dynamic rotary subluxation of the
scaphoid.
-
Make a dorsal transverse incision on the wrist just distal to the radial styloid (10).
-
Expose the radial styloid through an incision in the capsule overlying the radial styloid and scaphoid junction.
-
Remove the distal 5 mm of the styloid with a rongeur, sloping in a palmar direction from distal to proximal.
-
Make a transverse dorsal capsular incision and evaluate the radioscaphoid interval.
-
Watson and Ashmead (97)
recommend performing a SLAC wrist reconstruction rather than a
triscaphe arthrodesis if there is any articular cartilage damage. It is
critical in an STT fusion to have normal articular cartilage between
the distal radius and the proximal scaphoid. -
Remove the articular surfaces between the
scaphoid, trapezium, and trapezoid with a small rongeur, taking only
the proximal half of the trapezium and trapezoid articulations. Remove
the hard subchondral bone down to softer cancellous surfaces. -
Remove the dorsal cortex of the trapezium and trapezoid to provide a broader surface area for bone graft.
-
Use the distal radius as a source of cancellous bone for grafting (71).
To harvest the graft, make a second, transverse incision 3 cm proximal
to the radial styloid, extending from Lister’s tubercle to just palmar
to the first dorsal compartment. A flat surface on the radius can
always be identified between the first and second extensor
compartments, and a constant periosteal artery is seen in this area. -
Incise the periosteum and make a cortical
window. Remove cancellous bone from the distal radius and replace the
cortical window after harvesting the graft. -
The most important part of this procedure is the reduction of the scaphoid. Watson and Ashmead (97)
do this by placing a 5 mm spacer, which is usually the handle of a
small bone hook, into the scaphotrapezoid space to maintain the
original external dimensions of the triscaphe joint and manipulate the
scaphoid into proper position. Then drive one or two K-wires from the
trapezium and trapezoid into the scaphoid, avoiding impingement of the
radioscaphoid joint. -
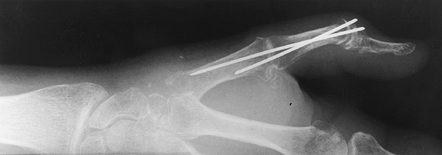
Remove the spacer and pass a second K-wire on the ulnar side of fusion construct (Fig. 72.11).
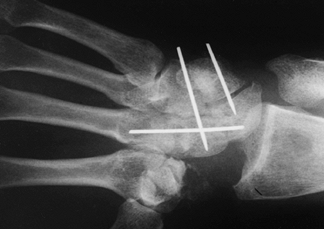 Figure 72.11. Capitohamate triquetrolunate arthrodesis with Kirschner wire fixation, AP view.
Figure 72.11. Capitohamate triquetrolunate arthrodesis with Kirschner wire fixation, AP view. -
Densely pack cancellous bone into the spaces between the trapezium, the trapezoid, and the scaphoid.
-
Cut the K-wires short and leave them under the skin.
-
Close only the skin and subcutaneous tissues.
long-arm plaster splint to place the hand in a functional position,
with the wrist in slight extension and radial deviation, the forearm in
neutral position, and the elbow at 90° of flexion. Three to 5 days
later, change this and apply a long-arm thumb spica cast. At 4 weeks,
exchange the cast for a short-arm thumb spica cast. At 6 weeks after
surgery, if there is radiographic evidence of healing, remove the
K-wires. Begin range-of-motion exercises once fusion is ensured.
rotary subluxation of the scaphoid and scaphoid instability, resistant
scaphoid nonunion, and Kienböck’s disease.
-
Make a longitudinal skin incision of sufficient length over the third dorsal compartment, and open the retinaculum.
-
Free the EPL tendon proximally and
distally, and transpose it radially. This allows retraction of the
radial wrist extensors, the EPL, and the digital extensors. -
Make a longitudinal incision in the capsule and develop radial and ulnar flaps.
-
Inspect the proximal scaphoid and distal
radial articular cartilage as for the STT arthrodesis. If there is any
indication of degenerative arthritis in the radiocarpal joint, do a
SLAC wrist reconstruction instead. -
Remove the articular surface between the scaphoid and the capitate, using rongeurs and curets.
-
Harvest a bone graft from the radius, as described previously for the STT fusion.
-
After aligning the scaphoid, drive two 0.045 K-wires from the scaphoid into the capitate.
-
Pack bone graft into the
scaphoid–capitate gap. Cut off the K-wires under the skin, and close
the capsule with absorbable suture. Close the third dorsal compartment
with absorbable suture. -
Close the skin and subcutaneous fat with nonabsorbable suture and inject 0.5% bupivacaine for postoperative pain control.
-
Postoperative immobilization is identical to that of the STT fusion, as is the postoperative management.
scaphoid is an excellent method to treat radioscaphoid degenerative
arthritis and chronic nonunion of the scaphoid, as well as advanced
destruction from scapholunate dissociation or idiopathic avascular
necrosis of the scaphoid.
-
Make a longitudinal incision dorsally, over the third and fourth dorsal compartments.
-
Open the third dorsal compartment, and
transpose the EPL tendon radially. Then retract the radial wrist
extensors radially and the digital extensors ulnarly. -
Then open the capsule with a longitudinal incision and elevate the capsule to create radial and ulnar flaps.
-
Excise the scaphoid.
-
Prepare the articular surfaces between
the capitate, lunate, triquetrum, and hamate by removing the articular
cartilage and subchondral bone between them. -
Harvest cancellous bone proximally from the distal radius, as previously described.
-
Anatomic alignment of the four carpal
bones is important to provide an excellent outcome. The position of the
lunate is critical. -
Flex the lunate from its extended
position into a neutral position, generally by using a “joystick,” such
as a 0.045 K-wire inserted into the lunate. -
Fix the remaining four carpal bones. Confirm proper position with a radiograph. K-wires (Fig. 72.11),
Herbert and other types of screws, and, recently, new plate–screw
systems have been used to provide fixation and allow early mobilization
(Fig. 72.9, Fig. 72.10). After
P.1980
fixation, pack the intercarpal spaces with cancellous bone. -
Cut off any K-wires beneath the patient’s
skin and close the capsule with absorbable suture. Close the skin with
nonabsorbable suture, and inject the operative site with 0.5%
bupivacaine.
5–7 days later for a short-arm thumb spica cast. Generally, leave wires
in for 6 weeks. At that time, remove the K-wires if early union is seen.
-
Make a longitudinal incision between the fifth and sixth dorsal compartments.
-
Protect the dorsal sensory branch of the ulnar nerve throughout the procedure.
-
Open the joint capsule between the lunate and the triquetrum.
-
Remove the articular cartilage between these two bones down to subchondral bone.
-
Place three 0.045 K-wires or compression screws across this joint.
-
Pack the gap between the two bones with bone graft harvested from the radius as described previously.
-
Close the capsule with absorbable sutures and the skin with nonabsorbable sutures.
5–7 days, then exchange it for a long-arm cast. Keep this in place for
6 weeks to allow for adequate healing, as evidenced clinically and
radiographically. Then remove pins and use a short-arm splint for 4–6
weeks.
into two categories: intraoperative complications and problems with
long-term outcomes (13,53,106).
Major complications from STT arthrodesis include radiocarpal arthrosis,
trapeziometacarpal arthrosis, nonunion, osteomyelitis, and radial
styloid scaphoid impingement (79). This last
complication has been diminished by radial styloidectomy at the time of
surgery. Inadequate reduction of the scaphoid in this procedure leads
to a predictable progression of radiocarpal arthritis. The proximal
pole of the scaphoid must be reduced anatomically into the scaphoid
fossa of the radius, and the radioscaphoid angle must be approximately
45° to 55°.
arthrodesis. Long-term follow-up has shown a greater loss of wrist
flexion with the latter procedure. Relatively few complications have
been reported with the SLAC wrist reconstruction or four-bone
arthrodesis. Nonunion has been rare, and the development of radiolunate
arthritis and impingement between the fusion mass and the distal radius
have been reported. The most common complication of LT joint
arthrodesis is nonunion, with a reported incidence of 10% to 50% (13,52,53,91,100,106).
radiocarpal and mid-carpal joints, including the radiolunate,
radioscaphoid, and radiocapitate joints. It is one of the oldest, most
common, longest-used, and most successful reconstruction procedures for
the wrist (39,40 and 41,44,59). It predictably relieves pain, but it does eliminate all radiocarpal motion.
arthrodesis is posttraumatic degenerative arthritis of the radiocarpal
and midcarpal joints (41). This includes
chronic carpal dissociations as well as complex intracarpal and distal
radius intraarticular fractures. Other indications for arthrodesis
include the following:
-
Chronic infection unresponsive to limited surgical debridement (29,30)
-
Paralysis about the wrist and hand
(arthrodesis provides the stability required for tendon transfers about
the thumb and digits) (87) -
Rheumatoid arthritis and other inflammatory disorders involving the radiocarpal joint (18,28,63,64)
-
Limited arthrodeses that have not provided stability or pain relief
-
Loss of soft tissue and bone as a result of severe trauma or tumor resection
extremity and loss of hand function. Stabilization of the wrist makes
the flexor and extensors of the wrist available as transfers to restore
power and function to the fingers. Pomerance and Keenan (76)
demonstrated that by performing total wrist fusion, tendon transfers,
and muscle releases in a single staged procedure, they were able to
correct the severe contractures of the hand and wrist with resolution
of the preoperative hygiene problems. (See Chapter 66, Chapter 67 and Chapter 68 for more details.)
undergone failed attempts at both conservative and surgical treatments.
Hastings et al. (41) reported that 71% of the
112 wrists that underwent arthrodeses for posttraumatic arthritis had
undergone 137 prior surgical procedures, for an average of 2.3
procedures each. Field et al. (32) found a mean
of three operations (mostly limited carpal fusions) performed on the
wrist in their 20 patients undergoing arthrodesis for posttraumatic
conditions. By the time many of these patients present for arthrodesis,
they have so much pain in the wrist that they have little or no motion
remaining. For these patients, a total wrist fusion improves stability
and function, decreases deformity, and relieves pain.
simulated wrist arthrodesis in varying degrees of flexion and extension
in 20 normal volunteers by immobilizing them in leather gauntlets. This
study showed that from 15° of flexion to 30° of extension, the grip
strength was equal in all positions except for 15° flexion, where it
was decreased. Their overall recommendation was to avoid fusing the
wrist in flexion. They recommended placement of the wrist in palmar
flexion only when there is bilateral involvement and one wrist is
placed in extension, the other in flexion to improve independent
feeding and perineal care.
found that most patients functioned well after wrist arthrodesis for
posttraumatic conditions and that the most difficult tasks were
perineal care and manipulating the hand in tight spaces. Rayan et al. (78)
reviewed the function of nine rheumatoid arthritis patients and found
that even among those who underwent bilateral radiocarpal arthrodesis,
seven of nine had improvement of subjective function, two of nine
remained the same, and no patient was made worse.
the results of arthroplasty versus radiocarpal arthrodesis in
rheumatoid arthritis patients. They found that the arthrodesis group
had overall 97% good results compared to 75% good results in the
arthroplasty group. The arthrodesis group had an 18% complication rate,
while the arthroplasty group had a 25% complication rate, 4 of 37
requiring revision, at an average follow-up of 51 months.
choice for the most severe wrist deformities, but efforts to perfect
wrist arthroplasty and motion-preserving operations continue. While it
is likely true that most patients would prefer a motion-sparing
procedure to radiocarpal arthrodesis, function after complete wrist
fusion is surprisingly good and therefore this remains the gold
standard to which all other procedures should be compared. With the
right indications, radiocarpal arthrodesis can salvage function for an
otherwise debilitated patient.
to the final function desired, and although a wrist fused in a poor
position may be pain free, function suffers. The ideal position for
radiocarpal arthrodesis is controversial. Several studies recommended
fusion in 20° to 30° of extension (11,18,21,22,32,41,44,59,62,64,68,70,81,104) and Clayton and Ferlic (21)
recommended neutral position. Although the ideal position for
radiocarpal fusion likely will continue to be debated, functional
outcome studies show that a position in 0° to 10° of extension and 0°
to 10° of ulnar deviation seems to give the best results.
diagnosis underlying the wrist deformity in patients with systemic
disease before performing surgery. This is most important in evaluating
patients with rheumatoid arthritis.
Surgical procedures performed on any of these joints affect the more
distal or proximal joints. In patients with rheumatoid arthritis, keep
procedures minimal and simple. Wrist fusion using a Steinmann pin for
intramedullary fixation is better than using a dorsal plate, as it is
less likely to cause problems with the skin and extensor tendons.
Stability is adequate, immobilization is shortened, an iliac
crest graft is usually not required, and splinting and partial weight bearing early is possible. Millender and Nalebuff (68)
reported that patients were able to walk with platform crutches 1 week
after surgery using this technique. Another advantage of this procedure
is that it can be performed rapidly enough to allow other surgical
procedures to be performed concomitantly, as is necessary in many
patients with severe rheumatoid arthritis (69). (See Chapter 70 for more details on treatment of the rheumatoid hand.)
loads and higher demands on their hands and wrists than do patients
with rheumatoid arthritis. Rigid internal fixation is crucial. Recent
studies have shown that plate fixation with the application of local
bone graft provides reliable fusion and early rehabilitation (11,104).
antibiotics and axillary block or general anesthesia. All patients with
connective tissue diseases or in whom there is concern of cervical
spine instability are prescreened with cervical spine radiographs.
Perform all surgery under tourniquet control, through a dorsal
longitudinal skin approach to the extensor retinaculum.
-
Use a longitudinal skin incision just ulnar to Lister’s tubercle.
-
Make a longitudinal incision through the fascia of the third dorsal compartment, and transpose the EPL tendon.
-
Incise the floor of the third compartment
and extend it distally to the base of the third metacarpal, staying
ulnar to the extensor carpi radialis brevis (ECRB) tendons. -
Using subperiosteal dissection, raise medial and lateral flaps to expose the entire distal radius, ulna, and carpus.
-
We resect the posterior interosseous
nerve in the floor of the fourth compartment to provide lasting pain
relief. Because of the commonly associated disease of the distal
radioulnar joint (DRUJ) in patients with rheumatoid arthritis, we
prefer to perform a Darrach resection of the distal ulna by transecting
the distal ulnar shaft just proximal to the ulnar head, using an
oscillating saw (see Chapter 43). -
Use a subperiosteal approach to excise the entire distal ulna. Preserve the ulnar head for use as autogenous bone graft.
-
Using a small rongeur, resect the
remaining radiocarpal and intercarpal articular surfaces, including the
third CMC and intercarpal joints, until you expose cancellous bone. Use
the harvested distal ulna to fill the defects between the intercarpal
joints. If more bone graft is needed, use a curet to harvest any needed
bone from the distal radius through the base of Lister’s tubercle. -
Insert a 3.2 or 3.6 mm Steinmann pin
either down the medullary canal of the third metacarpal shaft or
between the second and third metacarpal bases for more ulnar deviation,
and bring it out distally through the skin. -
Reduce the wrist into final position, and
slip a drill guide over the distal end of the pin. Slide a similar-size
pin into the guide and tap the pin across the wrist into the radius in
a retrograde fashion. Bury the pin distally under the skin. -
Confirm adequate pin position with intraoperative radiographs or insert the pin under fluoroscopic control.
-
Transpose approximately one third to one
half of the extensor retinaculum palmar to the extensor tendons, and
suture it into place with absorbable sutures. Place a suction drain
deep into the wound and bring it out distally for ease of removal the
following day. Place subcutaneous sutures and then close the skin with
nonabsorbable sutures in an interrupted fashion.
rotation. Remove the drain in 24 hours. After 1 week, remove the
dressing and inspect the wound. Generally leave sutures in for 2 weeks
and keep the patient in a splint or cast until their removal. At 2
weeks postoperatively, remove the sutures and place the patient into a
short-arm cast for an additional 2–4 weeks, or until clinical and
radiographic union is achieved. Encourage the patient to mobilize his
or her fingers as much as possible until the cast is removed. At that
time, evaluate the patient and treat any remaining stiffness with
aggressive hand therapy.
other than rheumatoid arthritis, we prefer to use the AO method of
plate fixation popularized by Hastings (39) and others (81) (Fig. 72.12 and Fig. 72.13).
Most commonly, the indication for this type of arthrodesis is
posttraumatic arthritis of the wrist or failed wrist arthroplasty.
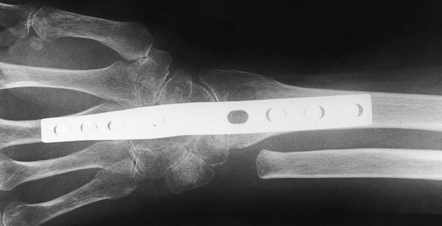 |
|
Figure 72.12. Radiocarpal arthrodesis using wrist fusion plate, AP view.
|
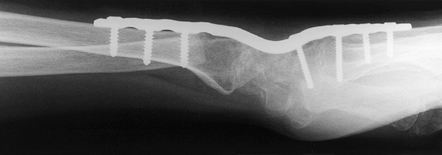 |
|
Figure 72.13. Radiocarpal arthrodesis using wrist fusion plate, lateral view.
|
-
Make a midline dorsal longitudinal skin incision.
-
Open the third dorsal compartment and
transpose the EPL tendon. Expose the entire carpus and distal radius,
as well as the base of the third metacarpal subperiosteally. Do not
enter the DRUJ unless there is concomitant disease that is being
treated surgically. The best
P.1983
way
to avoid the problem of DRUJ instability after arthrodesis is to keep
the volar and dorsal radioulnar ligament complex intact. -
Decorticate the dorsal 50% of the joint
surfaces of the radioscapholunate, the radioscaphocapitate, the
scapholunate, the lunocapitate, and the capitate, and the third
metacarpal base until you expose cancellous bone. -
Arthrodesis of the remaining joints of
the carpus and the second carpometacarpal joint can be performed, but
we add these joints only if concomitant arthrosis exists. Keeping the
volar 50% of the articular surface intact maintains alignment of the
carpus better than if the entire joint is taken. -
Harvest cancellous bone graft from the
distal radius by removing Lister’s tubercle and widening the cortical
defect with a curet. -
Contour the dorsal surfaces of the distal
radius, lunate, capitate, and third metacarpal base with an osteotome
so that the plate fits against the bone without gaps or defects. -
Before fixing the plate to the wrist,
pack cancellous bone graft between the joint surfaces to be fused.
Apply the plate (we prefer the precontoured AO wrist fusion plate,
which provides 10° to 15° of wrist extension) first to the third
metacarpal with at least three 2.7 mm cortical screws. Stabilize the
distal radius with at least three (usually four) 3.5 mm cortical
screws. Then use a cancellous screw to affix the plate to the carpus
(usually the capitate). -
Ensure that the position of the hardware
is adequate with intraoperative radiographs or fluoroscopy, and make
changes as necessary. -
Close the wound by transposing one third to one half of the extensor retinaculum volar to the extensor tendons over the plate.
-
Place a suction drain deep into the wound and bring it out distally for removal the next day.
-
Close the skin with interrupted horizontal mattress sutures of 4-0 nylon.
After 1 week, remove the bandage and splint; inspect the wound and
remove the sutures if it is adequately healed. Apply a custom-molded
plastizote splint and begin a controlled-motion hand therapy protocol.
Do not permit active exercises
against resistance until clinical and radiographic union is achieved, usually in 6–10 weeks.
have reported complications of radiocarpal arthrodesis. Clendenin and
Green (22) divided these into major
complications (requiring revision surgery or prolonged hospitalization)
and minor (when morbidity is prolonged but resolves without further
hospitalization). Major complications include nonunion, wound
infection, painful neuromas, fracture of a previously healed fusion,
iliac crest bone graft site complications, acute carpal tunnel
syndrome, plate failure, DRUJ and ulnocarpal impingement (33,77,92),
and chronic pain syndromes. Minor complications include postoperative
pain caused by tight dressings, sensory neurapraxias, minor skin
irritations, and necrosis. Hastings (39) noted
that major complications are less common in internal fixation with a
wrist fusion plate than in other surgical methods. Bone graft donor
site morbidity can be diminished by using local bone graft for
radiocarpal arthrodesis.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
A, Ebraheim NA, Lu J, Yeasting RA. A Modified Dorsal Approach to the
Wrist for Arthrodesis of the Non-Rheumatoid Wrist. An Anatomical Study.
J Hand Surg [Br] 1996;21:434.
RG, Lane LB, Littler JW, Keyser JJ. Ligamentous Reconstruction for the
Painful Thumb Carpometacarpal Joint: A Long-term Assessment. J Hand Surg [Am] 1984,9:692.
H, Kauffmann D, Lenihand M, et al. An In Vitro Analysis of Wrist
Motion: The Effect of Limited Intercarpal Arthrodesis and the
Contributions of the Radiocarpal and Midcarpal Joints. J Hand Surg [Am] 1988;13:378.
JL, Koman LA, Gelberman R, et al. Arthrodesis of Metacarpophalangeal
Joint of the Thumb in Children and Adults: Adjunctive Treatment of
Thumb-in-Palm Deformity in Cerebral Palsy. Clin Orthop 1990;253:75.
J, Ortega M, Manzanares J. Arteriovenous Fistula with Venous Aneurysm
as a Complication of the Trapeziometacarpal Arthrodesis. Acta Orthop Belg 1993;59:404.
CB, VanEgmond DB, Hovious SER, van der Meulen JC. Results of Small
Joint Arthrodesis: Comparison of Kirschner Wire Fixation with Tension
Band Wire Technique. J Hand Surg [Am] 1992;17:952.
D, Schneider LH, Kirkpatrick WH, et al. Scaphoid Excision and
Capitolunate Arthrodesis for Radioscaphoid Arthritis. J Hand Surg [Am] 1993;18:780.
WB, Carroll C IV. Scapho-trapezoid Arthrodesis for Treatment of Chronic
Static and Dynamic Scapholunate Instability: A 10-year Prospective on
Pitfalls and Complications. J Hand Surg [Am] 1990;15:408.
JC, Werner FW, Palmer AK, et al. Biomechanical Analysis of Internal
Fixation Techniques for Proximal Interphalangeal Joint Arthrodesis. J Hand Surg [Am] 1986;11:562.
SJ, Strickland JW. Arthrodesis of the Proximal Interphalangeal Joint of
the Finger: Comparison of the Use of the Herbert Screw with Other
Fixation Methods. J Hand Surg [Am] 1994;19:181.
HH, Stern PJ. “Salvage” Procedures in the Treatment of Kienböck’s
Disease: Proximal Row Carpectomy and Total Wrist Arthrodesis. Hand Clin 1993;9:521.
RI, Dobyns JH, Beabout JW, Bryan RS. Traumatic Instability of the
Wrist: Diagnosis, Classifications and Pathomechanics. J Bone Joint Surg Am 1972;54:1612.
LH, Nalebuff EA. Arthrodesis of the Rheumatoid Wrist: An Evaluation of
Sixty Patients and a Description of a Different Surgical Technique. J Bone Joint Surg Am 1973;55:1026.
EA, Tencer AF, Driscol HL, Trumble TE. A Biomechanical Comparison of
Four Methods of Fixation of the Trapeziometcarpal Joint. J Hand Surg [Am] 1994;19:86.
SF, Patterson RM, Peterson PD, et al. Evaluation of the Biomechanical
Efficacy of Limited Intercarpal Fusion for the Treatment of
Scapholunate Dissociation. J Hand Surg [Am] 1990;15:120.
P, Merle M, Membre H, Fockens W. Bioabsorbable Rods and Pins for
Fixation of Metacarpophalangeal Arthrodesis of the Thumb. J Hand Surg 1995;6:1032.
JD, Stern PJ, Kiefhaber TR. Motion-Preserving Procedures in the
Treatment of Scapholunate Advanced Collapse Wrist: Proximal Row
Carpectomy versus Four-Corner Arthrodesis. J Hand Surg [Am] 1995;20:965.