Adolescent Deformity
lateral curvature and rotation of the spine that occurs in patients
just before or during puberty. If congenital, neuromuscular,
infectious, and pathologic conditions (discussed in other chapters)
have been ruled out, the curvature is considered to be idiopathic, or
of unknown origin. Curves can progress to cause significant trunk
deformity and eventually cardiorespiratory compromise. Nationwide
school screening programs have been started to identify scoliosis in an
early phase so that progression can be prevented with bracing or
corrected with surgery before the development of these late
complications.
frequently is a positive family history; however, the expression is
variable, and a specific gene has not been identified yet. Harrington
showed that there was a 27% incidence of scoliosis in girls whose
mother had a curve greater than 15 degrees. A genetic survey of
patients with AIS indicated that 11% of first-degree relatives have
scoliosis; this decreases to 2.4% of second-degree relatives and 1.4%
of third-degree relatives. Studies of monozygotic twins have shown
concordance of 73%.
factors, such as melatonin, growth hormone, and calmodulin, may play a
role. Studies also have looked at disorders of connective tissue;
neurologic abnormalities; and proprioception, muscle, and growth
differences as the cause. No study has shown conclusive evidence as to
the underlying etiology of AIS, however, and the term idiopathic remains appropriate.
have AIS. Small curves (<10 degrees) occur equally in adolescent
boys and girls. The female-to-male ratio is 4:1 for large curves,
however, and females have a 10 times greater risk of curve progression
compared with males. Approximately 10% of cases that are detected with
school screening require eventual treatment with a brace or surgery.
Most adolescent idiopathic curves have their apex to the right. A
thoracic curve with the apex to the left should undergo further work-up
(i.e., magnetic resonance imaging) to rule out other causes of
scoliosis.
-
Skeletally immature patients with large curves are at the greatest risk of curve progression.
-
Double-curve patterns have a higher risk of progression than single-curve patterns.
-
Curves with loss of thoracic kyphosis
also are at increased risk of progression and are more likely to
develop pulmonary compromise.
curves can continue to progress at a slower rate when the patient is
skeletally mature. In adults, small curves (<30 degrees) are
unlikely to progress. Curves of 30 degrees to 50 degrees may progress
an additional 10 degrees to 15 degrees, and large curves (>50
degrees) continue to progress at a rate of approximately 1 degree/yr.
|
TABLE 20-1 PROBABILITY OF CURVE PROGRESSION BASED ON RISSER SIGN AND MAGNITUDE OF CURVE
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||
apical vertebra, which is the most horizontal and laterally placed
vertebral body or disc. The King and Moe (Table 20-2)
classification was the standard for curve evaluation and management
decisions before the advent of modern imaging and surgical treatment
options. This classification is limited because it evaluates the curve
in only one dimension and because it has been shown to have poor
interobserver and intraobserver reliability. There are four main
patterns:
-
Thoracic
-
Lumbar
-
Thoracolumbar
-
Double major
classification system for AIS that has improved interobserver and
intraobserver reliability. This system is treatment based and evaluates
the spine in two dimensions—coronal and sagittal (Table 20-3).
Six curve patterns (1 through 6) are modified further based on
characteristics of the lumbar curve (A, B, or C) and the presence or
absence of thoracic kyphosis (-, N, or +).
|
TABLE 20-2 CURVE PATTERNS AS DESCRIBED BY KING AND MOE
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||
|
TABLE 20-3 CURVE PATTERNS AS DESCRIBED BY LENKE et al
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-
The lumbar curve modifier is determined by where the central sacral vertical line intersects the lumbar apical vertebral body.
-
The stable vertebra is the most proximal
lower thoracic or lumbar vertebra that is bisected most closely by the
central sacral vertical line (Fig. 20-1). -
Curves are considered structural if the Cobb angle is greater than 25 degrees on side bending films.
-
Each patient is described by a designation such as 1B + (curve type/lumbar modifier/thoracic sagittal modifier) that thoroughly describes the curve.
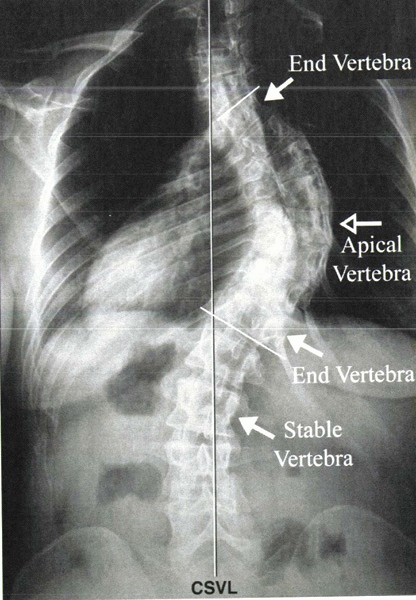 |
|
Figure 20-1
Radiograph showing the central sacral vertical line (CSVL), apical vertebra, stable vertebra, and end vertebrae. The stable vertebra is the most proximal lower thoracic or lumbar vertebra that is bisected most closely by the CSVL. |
type is main thoracic, comprising 51% of curves, followed by double
thoracic (20%), thoracolumbar/lumbar (12%), and double major curves
(11%). Common usage of this more comprehensive classification system
may be limited to scoliosis centers because of its relative complexity.
adolescents for scoliosis refer many patients to orthopaedic surgeons.
Screening is performed using the Adams forward bending test. With the
patient bending forward from the waist, the examiner views the patient
from behind for thoracic asymmetry, from in front for lumbar asymmetry,
and from the side for assessment of kyphosis. A rib prominence seen
with the forward bending test is from rotation of the spine and can be
measured with a scoliometer. An angle of trunk rotation measurement of
7 degrees with the scoliometer is the current recommendation for
further work-up. With this level of screening, approximately 10% of
children referred require treatment. There is concern that this cutoff
leads to overreferral and unnecessary x-rays; however, the 7-degree
cutoff prevents missing children with curves that are likely to
progress.
possible scoliosis, in addition to routine history questions (age,
gender, past medical history, family and social history), it is
important to determine the following: age at onset of menarche in
girls, family history of scoliosis, and current height of other
first-degree family members (to determine potential for growth
remaining). Any history of neuromuscular conditions, presence of skin
lesions (neurofibromatosis), back pain, or neurologic symptoms such as
bowel and bladder dysfunction should prompt further diagnostic work-up.
presence of axillary or pubic hair, and breast development. The skin
should be examined for café au lait spots, which may indicate
neurofibromatosis, and for signs of dysraphism (patch of hair or dimple
over spine). Leg lengths need to be measured because leg-length
discrepancy is a frequent cause of spinal asymmetry. Hamstring
tightness can indicate a spinal cord problem, such as tethering, and
should be checked during the forward bending test. A screening
neurologic exam includes strength testing, deep tendon reflexes,
Babinski’s sign, abdominal reflexes, and examination for clonus.
height asymmetry, scapular prominence, breast and flank fold asymmetry,
and the presence of a kyphotic deformity. The forward bending test
allows the examiner to evaluate the degree and severity of rotational
deformity. The combination of these measurements allows the examiner to
begin to understand the curve in three dimensions.
posteroanterior x-ray (which protects the breasts from the excess
radiation exposure of an anteroposterior x-ray) on a 36-inch cassette
should include the cervical spine and the pelvis. Including the iliac
apophyses allows for evaluation of the Risser sign, which is an
indicator of growth remaining (Fig. 20-2). A
lateral x-ray of the spine is obtained if there is concern for a
round-back deformity or spondylolisthesis, but it is not necessary for
routine scoliosis screening. Magnetic resonance imaging should be
obtained if the curve is atypical (apex to the left), if there is
associated back pain (concern for infection or malignancy), or if there
is any neurologic abnormality (concern for intraspinal process, such as
syrinx, tethering, or tumor).
to describe the location of the curve. The Cobb method is used to
measure the curve: The end plates of the most tilted vertebrae at the
top and bottom of the curve are marked, and lines perpendicular to
these end plates are drawn. The angle of intersection of these two
lines is the Cobb angle (Fig. 20-3). Curves
less than 10 degrees are considered spinal asymmetry, which is a normal
variation. Curves greater than 10 degrees are considered scoliosis and
should be followed to monitor for progression. Frequency of follow-up
is
determined by level of maturity and the degree of curve; immature
patients with larger curves need to be followed more closely (every 4
to 6 months), whereas older patients with small curves can be followed
yearly until they are past their period of peak growth velocity. If
surgery is considered, a lateral x-ray of the spine is obtained to
assess the lateral contour, and bending films are obtained to assess
the stiffness of the curves. On bending films, a curve that remains
greater than 25 degrees is considered structural.
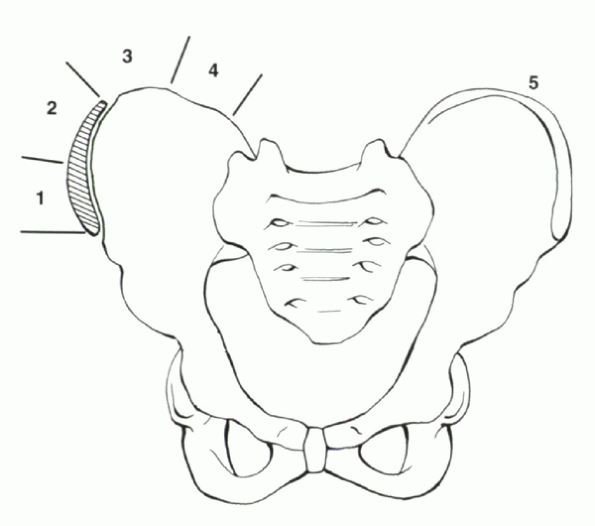 |
|
Figure 20-2
Risser sign—progressive ossification of the iliac apophyses is a soft indicator of skeletal maturity. Risser 1 (beginning of ossification) usually is visualized around the time of menarche. Risser 4 (entire apophysis visualized but not fused to crest) signifies the patient is past peak height velocity and is nearing the end of spinal growth. Risser 5—the entire apophysis has fused to the crest. |
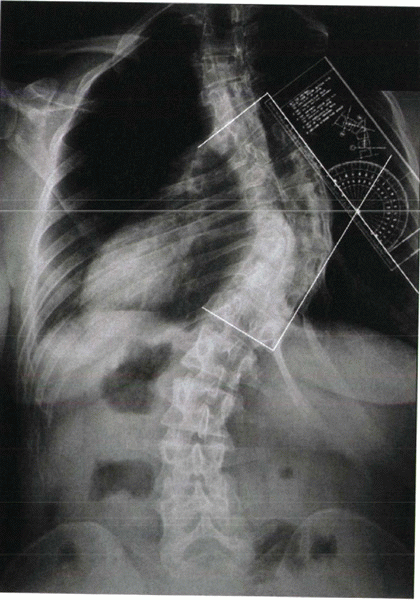 |
|
Figure 20-3 Cobb technique of measuring a scoliotic curve.
|
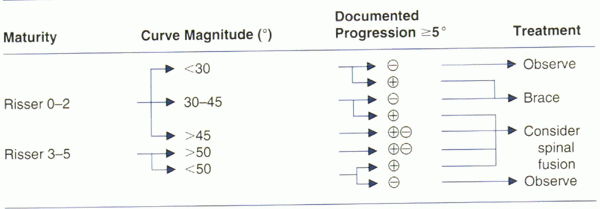
magnitude of the curve, and the progression of the curve over time.
Options for treatment include the following:
-
Observation
-
Bracing
-
Surgery
stimulation have been shown to have little or no measurable effect on
curve progression. A general treatment algorithm is presented in Table 20-4.
be monitored clinically by the primary care physician and referred for
x-ray only if there is curve progression based on the forward bending
test. Patients with curves measuring 10 degrees to 20 degrees can be
monitored with an exam and a posteroanterior x-ray every 6 to 12 months
until skeletal maturation occurs. Skeletally immature patients with
curves of 20 degrees to 30 degrees should be followed every 4 to 6
months to monitor closely for curve progression. Of patients referred
for scoliosis, 90% require only observation.
greater than 25 degrees with documented progression of greater than 5
degrees, bracing should be considered in a skeletally immature patient.
Patients who have reached skeletal maturity are not candidates for
brace wear because braces help to prevent progression but in general do
not correct scoliosis.
custom molded with pads that provide an external force on the ribs and
trunk to correct rotation and curve of the spine. The current standard
is a thoracolumbosacral orthosis (TLSO) (Boston brace, Wilmington
brace, or Miami brace) for curves with the apex below T7 and a
cervicothoracolumbosacral orthosis (CTLSO) (Milwaukee brace) for more
proximal curves. It is much more difficult to convince a teenager to
wear a CTLSO because the chin extension makes the brace difficult to
hide under clothes. The psychological impact of bracing is difficult to
measure, but it can have an adverse effect on self-esteem at a time
when social acceptance is important.
progression of the curve. Some authors have suggested a 16-hour per day
schedule. The Charleston bending brace, which is designed to be worn
only at night to improve compliance, has been shown to be effective in
some studies. Most experts
believe,
however, that brace efficacy is dose related: The more hours per day
the brace is worn, the greater the chance for curve control.
 |
mature, which is approximately 2 years postmenarchal for girls and when
boys are Risser 4 to 5 by x-ray. X-rays should be checked every 4 to 6
months during brace wear. We recommend alternating x-rays in and out of
the brace to assess for curve progression and adequacy of brace fit
with a minimum of radiation exposure. The brace may need to be adjusted
or remade as the adolescent grows. If the curve progresses beyond 40
degrees despite brace wear, surgical stabilization should be considered.
-
Progression of the curve despite use of brace
-
Curve greater than 40 degrees in a skeletally immature patient
-
Curve greater than 50 degrees in a skeletally mature patient
appearance and emotional acceptance, which are not based on x-ray curve
magnitude. Some patients with smaller curves are unwilling to accept
the cosmetic deformity of scoliosis or the social stigma of wearing a
brace. These patients may be candidates for surgery before the curve
reaches 45 degrees. This is particularly true for lumbar curves, in
which a 35-degree curve may produce a marked trunk shift. Likewise,
some patients with large curves refuse surgery. Each patient must be
treated individually to obtain the best outcome emotionally and
physically. When the surgeon and patient have agreed that surgical
intervention is warranted, many options are available. The surgical
approach and fusion can be posterior, anterior, or anterior and
posterior.
standard for most scoliosis curves. It has the longest track record,
going back to 1960 when Harrington introduced metal implants for curve
correction. Cotrel and Dubousset developed a modern universal
instrumentation system (CD) that uses rods, hooks, and screws to
correct scoliosis mechanically through translation, rotation, and
distraction in three dimensions (Fig. 20-4).
The CD system has been modified, and many variations now are available.
This mechanical correction must be maintained ultimately by spinal
fusion produced by decortication, facet excision, autograft, or
allograft.
sacrum if necessary; however, it is rare that patients with IAS require
fusion below L4. Every effort should be made to stop the fusion at L3
if possible to leave lumbar motion segments. Fusion to L4 or L5 has led
to an increased incidence of late low back pain. The levels fused
during posterior spinal surgery depend on the curve and the flexibility
of curves above or below. The structural curves always should be fused;
compensatory curves often can be left out of the fusion mass. Moe
recommended that the neutral vertebra (vertebra with symmetric pedicles
on posteroanterior x-ray) should be included in the fusion.
but cannot be used for double structural curves. Anterior spinal fusion
can be done through an open incision or endoscopically. The
video-assisted endoscopic approach is becoming more common as
techniques and instrumentation improve, but this technique has the
steepest learning curve for the surgeon.
should not be used for patients with excessive preoperative thoracic
kyphosis, and it must be used cautiously when trying to preserve or
enhance lordosis. All levels within the curve should be fused;
instrumentation and fusion should extend from the transitional neutral
vertebra above to the transitional neutral vertebra below the curve.
This extension may allow a shorter segment of fusion than posterior
spinal fusion. Anterior spinal fusion can be accomplished through an
open thoracotomy, retroperitoneal approach, anterior thoracolumbar
approach, or endoscopically (either laparoscopically or
thoracoscopically). After disc removal and structural bone grafting at
each level (with autograft ribs, allograft, or cages), a bicortical
screw is placed in each vertebral body, and a single rod is used for
correction. Newer systems use double screws at each level and double
rods to improve stability and possibly to decrease the pseudarthrosis
rate, which has been shown to be higher with anterior
fusions. A double-rod system also may obviate the need for postoperative bracing.
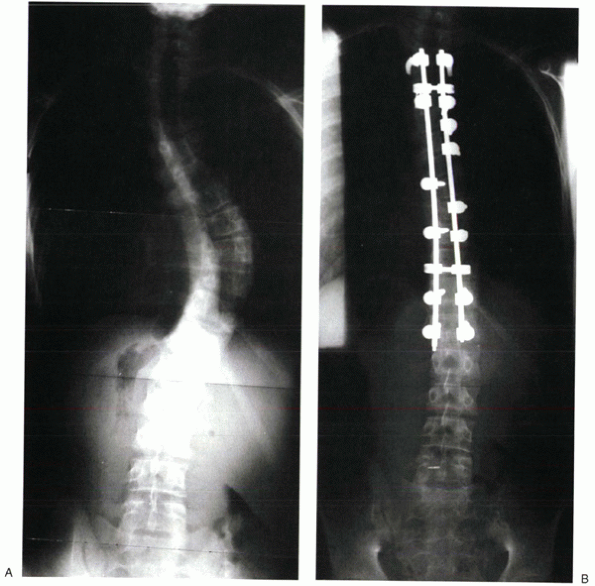 |
|
Figure 20-4 Preoperative (A) and postoperative (B) x-rays of a patient treated with segmental posterior spinal instrumentation and fusion for scoliosis.
|
posterior fusion. This procedure can be done through an open or
thoracoscopic approach and involves removing the disc at each segment
within the curve to be fused. The stiffness of the curve is determined
on the preoperative bending films. Curves that do not change or change
very little with bending are unlikely to correct adequately with
posterior fusion alone; anterior release and sometimes anterior
instrumentation improve the correction.
spinal fusion, the anterior spine can continue to grow and create a
crankshaft phenomenon as the anterior spine rotates around the
posterior fusion mass. Young patients (Risser 0 or 1) who are at risk
of developing problems from the crankshaft phenomenon frequently
undergo a combined anterior and posterior spinal fusion. This procedure
can be done in a single setting or staged days to months apart,
depending on the condition of the patient and surgeon preference.
After any type of spinal fusion, thoracoplasty or partial rib
resections can be done on the convex side of the curve to reduce a
prominent rib hump and to improve the associated deformity.
scoliosis surgery is neurologic damage, ranging from a mild neuropathy
to paraplegia. Intraoperative monitoring during surgery can help
protect a child from permanent injury. Many centers use neuromotor
evoked potential (NMEP) or somatosensory evoked potential (SSEP)
monitoring intraoperatively. NMEPs monitor the motor pathways and are
more sensitive and reliable than SSEPs, which monitor only the sensory
pathways in the dorsal columns of the spinal cord. The Stagnara wake-up
test is the gold standard for monitoring motor function if there is any
intraoperative concern; this allows alteration of hardware or curve
correction with the patient still under anesthesia.
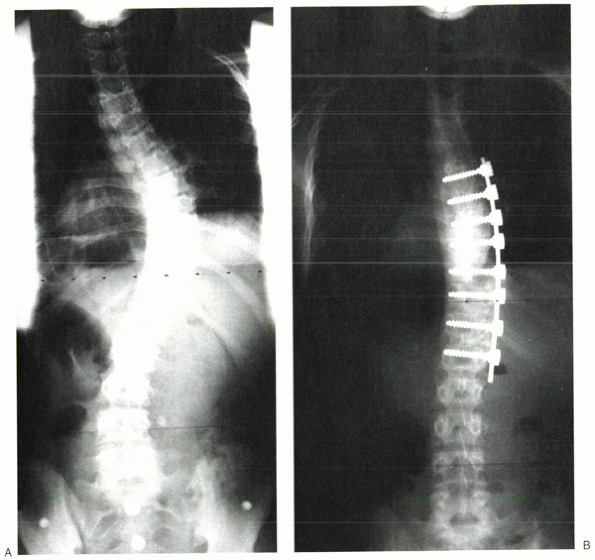 |
|
Figure 20-5 Preoperative (A) and postoperative (B)
x-rays of a patient treated with anterior instrumentation and fusion for scoliosis. This thoracic curve showed hypolordosis, which was corrected by the kyphogenic anterior instrumentation. |
|
TABLE 20-5 SURGICAL TREATMENT BASED ON CURVE TYPE
|
||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||
There also is a risk of pseudarthrosis, hardware failure, and
postoperative back pain. Later in life, there is a concern for
degenerative changes above and below the levels fused.
and surgeon. After posterior fusion, with good fixation and bone
quality, most patients do not require bracing. Anterior spinal fusion
alone frequently requires external support with a custom-molded TLSO
for 6 to 12 weeks postoperatively. In our institution, patients are
mobilized the day after surgery. If bracing is necessary, the patient
is molded and fitted with the brace before discharge from the hospital.
Patients with spine fusion for AIS typically are discharged by
postoperative day 4 to 7. They are encouraged to walk as much as
tolerated for the first 3 months, with no lifting, twisting, or bending
permitted. After 3 months, activity usually is progressed to jogging
and bicycling. After 12 months, if x-rays show adequate fusion,
patients are allowed to return to noncontact sports. It is recommended
that patients avoid contact sports for at least another year.
adolescents, ranges from flexible or postural round-back deformity to a
true rigid deformity with bony and soft tissue changes. In 1920,
Scheuermann described the deformity as having characteristic changes on
x-ray, including vertebral wedging and end plate irregularity. In
addition to trunk deformity, Scheuermann’s kyphosis in the thoracic
spine frequently causes back pain that is modest early in the disease
process but tends to increase as patients approach skeletal maturity.
The less common lumbar form of Scheuermann’s disease often causes
severe back pain but little deformity. Adults also may experience back
pain; Bradford reported that adults with Scheuermann’s kyphosis have a
higher incidence of disabling back pain than the normal population.
Scheuermann’s kyphosis is known to progress during growth periods, but
it also can progress during adult life, and it is worsened by senile
progression into kyphosis with advanced age. Physicians should
recognize and manage Scheuermann’s kyphosis appropriately to prevent
progression, limit deformity, and minimize pain or discomfort.
Scheuermann’s kyphosis have been suggested, yet the true etiology
remains unknown. Scheuermann originally hypothesized that aseptic
necrosis of the ring apophyses led to an arrest of growth and anterior
wedging of the vertebrae, but this hypothesis has not been confirmed.
Scheuermann later noted an increased incidence of Scheuermann’s
kyphosis in patients carrying heavy loads, suggesting a mechanical
disruption—a theory supported by several other authors. Schmorl and
Junghans noted the radiographic finding of Schmorl’s nodes and
hypothesized that a weakening of the cartilaginous end plate allowed
the intervertebral disc to penetrate the bone and disrupt normal
growth. It since has been shown, however, that Schmorl’s nodes are
present in many different types of spinal deformity and are not limited
to Scheuermann’s kyphosis.
Scheuermann’s kyphosis, Lambrinudi proposed that the hamstring
tightness is the primary factor in the etiology owing to the creation
of posterior pelvic tilt and resultant increased spine flexion above to
maintain sagittal balance.
disease processes, including endocrine abnormalities, hypovitaminosis,
inflammatory disease, neuromuscular disorders, dural cysts, and
spondylolysis, but no direct cause-and-effect relationship between
these conditions and Scheuermann’s kyphosis has been established.
Scheuermann’s kyphosis has shown grossly disorganized endochondral
ossification. Bradford et al suggested that a change in calcium
metabolism and resultant osteoporosis is the primary cause of the
vertebral wedging.
studied five families with a high incidence of this deformity and
suggested that it is inherited in an autosomal dominant fashion with a
high degree of penetrance and variable expressivity. Ascani and
Montanaro reported that affected patients have elevated levels of
growth hormone and advanced bone age compared with chronologic age. A
report of identical twins with thoracolumbar Scheuermann’s kyphosis
suggested that genetics and mechanical factors play a role; the twin
who was more active in strenuous activities had a worse deformity.
hereditary and environmental factors that result in kyphotic deformity.
Further studies need to be done to determine more definitively the
etiology and biology of Scheuermann’s kyphosis.
10 and 14 years of age with a prevalence of 0.4% to 8% of the general
population. Generally, boys and girls seem to be affected equally,
although some studies show a slight gender bias toward either boys or
girls. An associated mild scoliosis is noted in 20% to 30% of patients,
but this lateral curve rarely progresses to require treatment.
Progression of kyphosis has been documented during the growth spurt and
later in adult life. In contrast to idiopathic scoliosis, the risk for
kyphosis progression currently is unknown and warrants further study.
There have been rare reports of acute paraparesis in patients with
severe Scheuermann’s kyphosis, which is thought to be secondary to
thoracic disc herniation, dural cysts, or the canal compromise from the
deformity.
type II. The classic thoracic type (type I) has an apex between T7 and
T9 and is associated with increased lumbar lordosis. The thoracolumbar
or lumbar type (type II) has a lower apex, which frequently is
associated with reduced upper thoracic kyphosis or thoracic lordosis.
Type II Scheuermann’s kyphosis occurs more frequently in males
in a slightly older age group (15 to 18 years). This form tends to be more painful but rarely leads to progressive deformity.
medical history, and family history), one should determine maturity:
age at menarche for girls, presence of pubertal hair, history of a
growth spurt, current patient height, and the height of first-degree
family members. Further assessment includes evaluation of pain or any
neurologic symptoms.
be evaluated. The skin should be examined for café au lait spots,
axillary and inguinal freckling (which may indicate neurofibromatosis),
and signs of dysraphism (skin markings, dimpling, hair on the back).
The adolescent should be examined for asymmetry of shoulders, pelvis,
and muscular development. Tightness of the hamstrings is common and can
be evaluated with the straight-leg raise test or forward bending test.
The patient should be examined for hip flexion contracture or fixed
pelvic tilt with the Thomas test. A neurologic exam needs to be done on
every patient with a spinal deformity.
dimensions. In a forward bending test, the kyphotic deformity is
accentuated, and the apex appears as a sharp angulation, in contrast to
the smooth curve of a patient with postural kyphosis. A forward bending
test also exposes any associated scoliosis. The hyperextension test
helps the examiner understand the rigidity of the curve. A curve that
is flexible or reduces significantly with hyperextension is typically
postural and not Scheuermann’s kyphosis, although in younger children a
flexible round-back deformity may be the first sign of evolution to
true Scheuermann’s kyphosis.
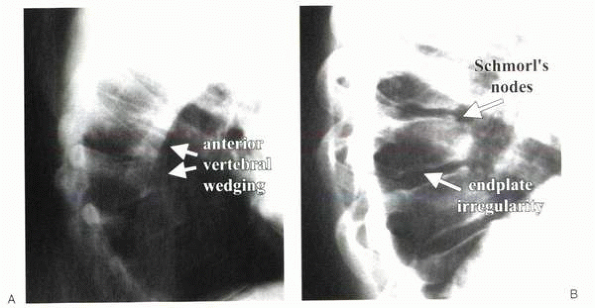 |
|
Figure 20-6 (A and B) Radiographs showing typical bone changes associated with Scheuermann’s kyphosis.
|
thoracic kyphosis as 20 degrees to 40 degrees. Standing radiographs of
the entire spine of a patient with Scheuermann’s kyphosis typically
have the following characteristic features (Fig. 20-6):
-
Anterior wedging (>5 degrees) of three or more consecutive vertebral bodies
-
Increased kyphosis (>45 degrees), which can be measured by the modified Cobb method on the lateral x-ray
-
Irregularity of vertebral end plates
-
Schmorl’s nodes—depressions in the vertebral bodies that represent disc herniation into the end plate
shows an associated mild scoliosis in the area of the kyphosis. The
scoliotic apex usually corresponds with the kyphotic apex. A lateral
x-ray should be examined for spondylolisthesis in addition to kyphosis.
In the later stages of Scheuermann’s kyphosis, x-rays often reveal
changes consistent with degenerative arthritis, including decreased
intervertebral disc spaces, marginal osteophytes, and ankylosis.
physical therapy, bracing, and, rarely, surgery, depending on the
degree of deformity and patient acceptance. It is important to
understand how each patient is affected mentally and physically by a
kyphotic deformity. This assessment should be made over time because
the symptoms of Scheuermann’s kyphosis are inconsistent, and there are
no certain rules regarding who should have bracing or surgery.
problem until severe deformity is present, all patients with
mild-to-moderate kyphosis (<75 degrees) should have a trial of
conservative treatment to control symptoms and
minimize
deformtiy. Physical therapy should emphasize hamstring stretching,
trunk strengthening exercises, and postural improvement. Ideally the
surgeon and family should find a physical therapist who has a special
interest in posture and the relationship between trunk appearance and
self-esteem.
decrease thoracic kyphosis. In skeletally immature patients with
thoracic kyphosis greater than 45 degrees and less than 75 degrees,
bracing can be considered. Attempts to brace curves greater than 75
degrees have led to a high failure rate, and this is not recommended.
To influence a type I deformity, a CTLSO or modified Milwaukee brace
provides the best extension force, but wear compliance with this brace
tends to be poor. More cosmetically acceptable underarm braces commonly
are used now, but these require expert orthotic design and alteration
to be effective.
literature. Acute application of a brace can influence the deformity
and improve kyphosis by 40% to 50%; however, several articles have
shown at least partial loss of this correction when brace wear is
stopped. It is recommended that a brace be worn full-time (23 hours per
day) for 12 to 18 months, then part-time to full-time (depending on the
severity of the kyphosis) until the patient reaches skeletal maturity.
If a teenager strongly refuses to wear a brace out of the house (a
frequent reaction in a peer-conscious age group), a vigorous exercise
program combined with nighttime brace wear can be considered. All
kyphosis braces require careful orthotist attention to ensure fit and
to recontour the posterior bars and pads every 2 months to gain further
correction progressively. There is no indication to brace skeletally
mature patients.
-
Pain
-
Progressive curve
-
Neurologic compromise
-
Cardiopulmonary compromise
-
Trunk deformity
severe kyphosis (>100 degrees) can cause neurologic deficit in
patients (usually adults) with Scheuermann’s kyphosis. Neurologic
deficits are the only absolute indication for surgery. Relative
indications include kyphosis greater than 75 degrees and kyphosis
greater than 60 degrees associated with pain that is not alleviated by
nonoperative measures. The goals of surgery include correction of the
deformity and relief of pain. Surgical correction of the deformity
always includes spinal fusion.
approach and Harrington compression instrumentation. With larger rigid
curves, however, this approach led to a high pseudarthrosis rate,
frequent loss of correction, and unacceptable hardware failures,
including broken rods and hooks pulling through the lamina.
CD system, provides much better fixation. Modern instrumentation
includes cross-linked rods attached to hooks and screws that can
correct the deformity in three dimensions; this allows correction of
any associated scoliosis in addition to the kyphosis. There still are
occasional problems with junctional kyphosis developing at the ends of
the instrumented segment. Otsuka recommends posterior fusion alone only
if the curve bends out to less than 50 degrees on a hyperextension
lateral radiograph.
in addition to posterior fusion has provided significantly better
results with regard to immediate and long-term correction of large
kyphotic deformities and now is the standard of care for large, rigid
deformities (Fig. 20-7). The anterior procedure can be done open or endoscopically before the posterior instrumentation.
Scheuermann’s kyphosis. It is clear from the literature that too short
a fusion leads to junctional kyphosis developing at the proximal and
distal ends of the rods. The criteria to determine the fusion levels in
Scheuermann’s kyphosis are not as well established as they are for
scoliosis. Current recommendations are to include the proximal end
vertebra (determined by the modified Cobb method) and to extend the
fusion past the transitional zone to the first lordotic disc distally.
Lowe also recommended limiting correction of the kyphosis to 50% of the
original deformity or less to prevent junctional kyphosis.
Overcorrection should be avoided. Patients with type II Scheuermann’s
kyphosis almost never require surgery.
Scheuermann’s kyphosis include death, gastrointestinal obstruction,
hardware failure, pseudarthrosis, progression of the deformity,
hemothorax, pneumothorax, pulmonary emboli, and persistent
postoperative back pain. The most feared complication is neurologic
injury, including paralysis. Vascular insult to the cord and mechanical
damage have led to paraplegia. Correction of kyphosis carries a higher
than usual risk of neurologic injury, which is related directly to the
amount of correction. Intraoperative neurologic monitoring is crucial
during any surgery to correct kyphosis because the thoracic cord is at
risk during correction and instrumentation. NMEPs and SSEPs are used in
most spine centers. The Stagnara wake-up test is the gold standard for
motor monitoring if there are any concerns during surgery. If
monitoring or the wake-up test indicates a neurologic deficit, any
corrective maneuvers should be reversed.
thoracic kyphosis that must be weighed carefully when considering
surgery because the natural history of Scheuermann’s kyphosis still is
not yet well defined. Corrective kyphosis surgery has benefited
immensely from new developments in spine instrumentation (e.g., pedicle
screws, in situ bending). Perhaps even more than idiopathic scoliosis,
surgical management of Scheuermann’s kyphosis requires treatment by
experienced spinal deformity surgeons.
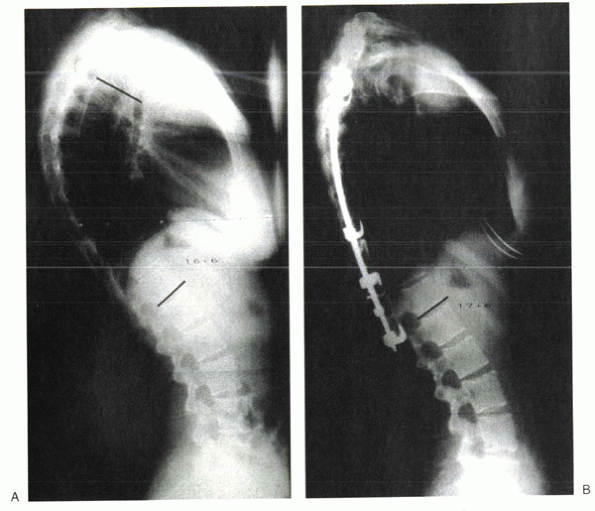 |
|
Figure 20-7 Preoperative (A) and postoperative (B) x-rays of a patient treated with posterior instrumentation and fusion.
|
Scheuermann’s kyphosis require postoperative bracing for approximately
3 to 6 months. Patients typically are mobilized the day after surgery
to help prevent atelectasis and gastrointestinal obstruction. They
usually are hospitalized for 4 to 7 days and are encouraged to ambulate
several times a day for the first 3 months. No bending, stooping, or
lifting is allowed for at least 3 months. No sports are allowed for the
first year after surgery. After 1 year, patients may return slowly to
normal activities if x-rays show consolidation of the fusion and
minimal progression of the kyphosis.
E, Montanaro A. Scheuermann’s disease. In The Pediatric Spine, DS
Bradford, RM Hensinger, eds. New York: Thieme, 1985: 307-324.
KM, Raso VJ, Hill DL, et al. Melatonin levels in idiopathic scoliosis.
Diurnal and nocturnal melatonin levels in girls with adolescent
idiopathic scoliosis. Spine 1996;21:1974-1978.
RL, Wyatt MP, Whitecloud TS, 3rd, et al. Vibratory hypersensitivity in
idiopathic scoliosis. J Pediatr Orthop 1988;8:389-395.
DS, Ahmed KB, Moe JH, et al. The surgical management of patients with
Scheuermann’s disease: a review of twenty-four cases managed by
combined anterior and posterior spine fusion. J Bone Joint Surg Am
1980;62:705-712.
DS, Moe JH, Montalvo FJ, et al. Scheuermann’s kyphosis and roundback
deformity. Results of Milwaukee brace treatment. J Bone Joint Surg Am
1974;56:740-758.
DS, Moe JH, Montalvo FJ, et al. Scheuermann’s kyphosis. Results of
surgical treatment by posterior spine arthrodesis in twenty-two
patients. J Bone Joint Surg Am 1975;57:439-448.
P, Jansson E, Dahlberg E, et al. Muscle fiber types in erector spinae
muscles. Fiber types in idiopathic and other forms of scoliosis. Clin
Orthop 1987;214:222-228.
AJ, Ogilvie DJ, Wordsworth BP, et al. Segregation of structural
collagen genes in adolescent idiopathic scoliosis. Clin Orthop 1992;
274:305-310.
JR. Outline for the study of scoliosis. Instructional Course Lectures,
The American Academy of Orthopedic Surgeons 1948;5: 261-275.
T, Irstam L, Nachemson A. Long-term anatomic and functional changes in
patients with adolescent idiopathic scoliosis treated by Harrington rod
fusion. Spine 1983;8:576-584.
P, Flood BM, Dickson RA. Idiopathic scoliosis in three dimensions. A
radiographic and morphometric analysis. J Bone Joint Surg Br
1984;66:509-512.
RA, Lawton JO, Archer IA, et al. The pathogenesis of idiopathic
scoliosis. Biplanar spinal asymmetry. J Bone Joint Surg Br 1984;66:8-15.
JW, Moskowitz A, Whitney J. Surface electrical stimulation versus brace
in treatment of idiopathic scoliosis. Spine 1990;15: 888-892.
N, Mims B, Milewicz DM. the potential role of the elastic fiber system
in adolescent idiopathic scoliosis. J Bone Joint Surg Am
1994;76:1193-1206.
WA, Emans JB, Micheli LJ, et al. Combined anterior and posterior fusion
for Scheuermann’s kyphosis. Spine 2000;25: 1028-1035.
AS, Blakemore LC, Loder RT, et al. The role of melatonin in the
pathogenesis of adolescent idiopathic scoliosis. Spine 1996;
21:1147-1152.
HA, Moe JH, Bradford DS, et al. The selection of fusion levels in
thoracic idiopathic scoliosis. J Bone Joint Surg Am 1983;65: 1302-1313.
DM, Weiss RL, Allen JE. Scheuermann’s dorsal kyphosis and spinal cord
compression: case report. Neurosurgery 1986;18: 628-631.
LG, Betz RR, Clements D, et al. Curve prevalence of a new
classification of operative adolescent idiopathic scoliosis: does
classification correlate with treatment? Spine 2002;27:604-611.
LG, Betz RR Harms J, et al. Adolescent idiopathic scoliosis: a new
classification to determine extent of spinal arthrodesis. J Bone Joint
Surg Am 2001;83-A:1169-1181.
JE, Carlson JM. The prediction of curve progression in untreated
idiopathic scoliosis during growth. J Bone Joint Surg Am
1984;66:1061-1071.
TG. Double L-rod instrumentation in the treatment of severe kyphosis
secondary to Scheuermann’s disease. Spine 1987;12: 336-341.
TG, Kasten MD. An analysis of sagittal curves and balance after
Cotrel-Dubousset instrumentation for kyphosis secondary to
Scheuermann’s disease. A review of 32 patients. Spine 1994;19:
1680-1685.
TG, Peters JD. Anterior spinal fusion with Zielke instrumentation for
idiopathic scoliosis. A frontal and sagittal curve analysis in 36
patients. Spine 1993;18:423-426.
M, Dubousset J, Imamura Y, et al. Melatonin. A possible role in
pathogenesis of adolescent idiopathic scoliosis. Spine 1996;21:
1147-1152.
S, Ponseti IV, Samaan N, el al. Growth hormone blood levels in patients
with idiopathic scoliosis. Clin Orthop 1971;81:122-125.
F, Willner S. The natural history of idiopathic scoliosis: incidence of
treatment in 15 cohorts of children born between 1963 and 1977. Spine
1997;22:772-774.
PM, Weinstein SL, Spratt KF. The natural history and long-term
follow-up of Scheuermann kyphosis. J Bone Joint Surg Am 1993;75:246-248.
PO, Shea KG, Granlund KF. Defining the pediatric spinal thoracoscopy
learning curve: sixty-five consecutive cases. Spine 2000;25:1028-1035.
PJ, Klassen RA, Peterson HA, et al. Surgical treatment of Scheuermann’s
disease with segmental compression instrumentation. Clin Orthop
2001;386:139-149.
CT, Scott DS, Reed FR Jr, et al. Nighttime bracing for adolescent
idiopathic scoliosis with the Charleston Bending Brace. Preliminary
report. Spine 1990;15:1294-1299.
CT, Scott DS, Reed FR Jr, et al. Nighttime bracing for adolescent
idiopathic scoliosis with the Charleston Bending Brace: long-term
follow-up. J Pediatr Orthop 1997;17:703-707.
EJ, Wynn-Davies R. A genetic survey of idiopathic scoliosis in Boston,
Massachusetts. J Bone Joint Surg Am 1973;55: 974-982.
EJ, Drummond DS, Gurr J. Scoliosis: incidence and natural history: a
prospective epidemiological study. J Bone Joint Surg 1978;60A:173-176.
B, Bradford D, Winter R, et al. Scheuermann kyphosis. Follow-up of
Milwaukee brace treatment. J Bone Joint Surg Am 1987;69: 50-57.
B, Beekman C, Hall V, et al. The effect of an exercise program on
change in curve in adolescents with minimal idiopathic scoliosis. A
preliminary study. Phys Ther 1979;59:759-763.
TC, Wenger DR, Stephen J, et al. Surgical management of thoracic
kyphosis in adolescents. J Bone Joint Surg Am 1979;61: 496-503.
Linthoudt D, Revel M. Similar radiologic lesions of localized
Scheuermann’s disease of the lumbar spine in twin sisters. Spine
1994;19:987-989.
SL, Zavala DC, Ponseti IV. Idiopathic scoliosis: long-term follow-up
and prognosis in untreated patients. J Bone Joint Surg Am
1981;63:702-712.
RB, Lovell WW, Moe JH. Excessive thoracic lordosis and loss of
pulmonary function in patients with idiopathic scoliosis. J Bone Joint
Surg Am. 1975;57:972-977.
