Open Carpal Tunnel Release
compressive neuropathy in the upper extremity. It is not a single
disease entity but rather a constellation of symptoms. Although some of
these symptoms were reported in 1854 by Sir James Paget in a patient
who had sustained a fracture of his distal radius, it was not until the
1930s that numbness following distal radius fractures was associated
with median nerve compression. The term carpal tunnel syndrome
was coined by Moersch in 1938, but it was not until Phalen published a
series of articles beginning in 1950 that the condition was
popularized. It is estimated that approximately 1 million adults in the
United States are diagnosed each year with carpal tunnel syndrome.
that topographically extends from the wrist flexion crease to the
midpalm, a distance of about 4 cm. It is a semirigid channel whose
floor and sides are formed by a concave arch of carpal bones and whose
roof or palmar surface is the transverse carpal ligament (Fig. 13-1).
The ligament, whose proximal margin blends into the antebrachial fascia
of the forearm, measures 3 to 4 cm in width and 2.5 to 3.5 cm in
thickness. It attaches radially to the scaphoid tubercle and trapezial
ridge and ulnarly to the hook of the hamate and pisiform. The carpal
tunnel is the conduit for the median nerve and digital flexor tendons
from the forearm into the hand. Nine flexor tendons pass through the
tunnel, the flexor superficialis and profundus to each finger and the
flexor pollicis longus to the thumb. The median nerve lies immediately
beneath the transverse carpal ligament and at this site is comprised of
30 to 35 fascicles. The sensory fascicles to the middle finger are
usually the most superficial (volar), and the motor fascicles to the
thenar intrinsic muscles are situated volar and radial.
nerve is variable and is classified according to its relationship to
the transverse carpal ligament. In most cases (47%) the nerve branches
just distal to the ligament and takes a recurrent or extraligamentous course to the muscles. Less often (31%), it branches within the tunnel and takes a subligamentous course to the muscles, and in 23% of cases it actually penetrates the ligament and takes a transligamentous
course. The median artery travels on the volar surface of the median
nerve. It is usually a thin vestigial structure but occasionally it is
sizable and makes a significant contribution to the superficial palmar
arterial arch.
the most commonly encountered compressive neuropathy in the upper
extremity. It generally worsens with time and can,
therefore,
be classified into early, intermediate, and advanced stages based on
symptoms, physical findings, and electrodiagnostic studies. The early
stage of the disease is when symptoms have been present for less than
one year. There is rarely any intrinsic muscle weakness during this
stage. Electrodiagnostic studies usually show some delay in sensor
conduction, although the studies are often negative. Treatment for the
early stage is generally nonoperative. If the patient has nighttime
paresthesias, the use of a wrist splint while sleeping is usually
effective. In
the absence of thenar muscle weakness, the indication for surgery
depends solely on the magnitude of discomfort that only the patient can
determine. If that discomfort interferes with the patient’s work and/or
leisure time activities, surgery is recommended.
 |
|
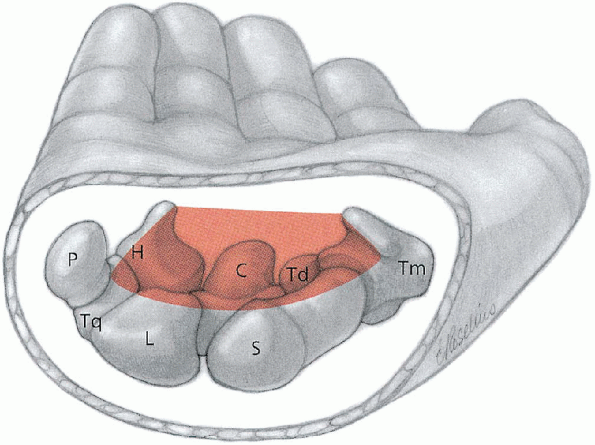
FIGURE 13-1.
The carpal tunnel is a fibroosseous canal. Associated bones which contribute to its boundaries include the hook process of the hamate (H), capitate (C), trapezoid (Td), pisiform (P), triquetrum (Tq), lunate (L), scaphoid (S), and trapezium (Tm). The roof is formed by the flexor retinaculum. (From Berger RA, Doyle JR, Botte MJ. Wrist. In: Doyle JR, Botte MJ, eds. Surgical anatomy of the hand and upper extremity. Philadelphia: Lippincott Williams & Wilkins, 2003:486-531, with permission.) |
decrease in strength of these muscles, as demonstrated on physical
examination, is the key indication for surgery even when the patient’s
sensory complaints are not disabling or even when nighttime
paresthesias are eliminated with the use of a wrist splint. A wrist
splint provides only symptomatic improvement; it is not therapeutic and
does not reduce or reverse the deleterious effects of compression on
the motor fascicles.
thenar muscle weakness but also by atrophy of these muscles. In some
cases, the atrophy is so severe that there is complete wasting of the
thenar eminence and there is no longer any muscle mass covering the
radial side of the thumb metacarpal. Although surgery is unlikely to
improve this situation, especially in elderly patients, it usually
relieves some of the sensory complaints, particularly nighttime
paresthesias. Numbness, however, often persists. Paresthesias
and numbness are separate and distinct symptoms, and patients often
have difficulty distinguishing between them, particularly
preoperatively. It is not uncommon for patients to report that
following surgery their paresthesias were relieved but that their
fingers remained “numb.” Consequently, they believe that the operation
was unsuccessful or, worse, that the surgeon was negligent and failed
to decompress the carpal tunnel. Actually, the persistence of numbness
is related to the severity and chronicity of median nerve compression
that has resulted in permanent damage to the sensory fascicles. Before
surgery it is important to explain to patients the limited objectives
of the operation. They should be informed that the primary objective is
to prevent further nerve damage and that, although paresthesias will
probably diminish, any improvement in sensibility and/or thenar muscle
strength depends on the ability of the nerve to recover. In all
likelihood, that improvement will be incomplete and in advanced cases
there may not be any improvement. Surgery should, therefore, be
performed before irreversible nerve changes develop. It is for that
reason that patients whose carpal tunnel is in the intermediate stage
are advised to undergo surgery without any undue delay. Although delaying surgery for weeks is not detrimental, deferring it for months is ill-advised.
and physical examination are important. A history of an endocrine
disorder such as diabetes mellitus or thyroid dysfunction may be
indicative of a demyelinating polyneuropathy rather than a localized
compressive neuropathy. Electrodiagnostic studies are useful to
differentiate between the two types of neuropathies. Even in the
absence of a polyneuropathy, endocrine disorders can “sensitize” nerves
to the effects of even mild compression. Carpal tunnel syndrome,
therefore, tends to be more common in diabetic than nondiabetic
patients. Patients with carpal tunnel syndrome
are also prone to develop compressive neuropathies at other sites
(e.g., ulnar nerve at the elbow). They are also likely to develop
trigger fingers. The frequency of these associations gives added
importance to obtaining a complete history and conducting a
comprehensive physical examination each time that the patient is seen.
Even in the absence of any concomitant problem, the patient should be
informed that other compressive neuropathies and/or trigger fingers
could develop at some future time. It is important to emphasize that
these problems are not a complication of surgery and that they are as
likely to develop in patients who opt not to have surgery.
armboard. The greater width of a hand table facilitates better
instrument control by the surgeon and surgical assistant because they
are able to rest their forearms on the table. Surgical instruments are
standard hand instruments.
operating room table with his or her arm outstretched on the operating
hand table. The hand table is positioned so that the patient’s arm is
abducted about 70 degrees at the shoulder. Abduction
greater than 90 degrees should be avoided because it can result in
postoperative shoulder discomfort and possibly a traction injury to the
brachial plexus. The operation is usually performed under
regional block anesthesia, either an axillary block or infraclavicular
block, and tourniquet control (Figs. 13-2 and 13-3).
 |
|
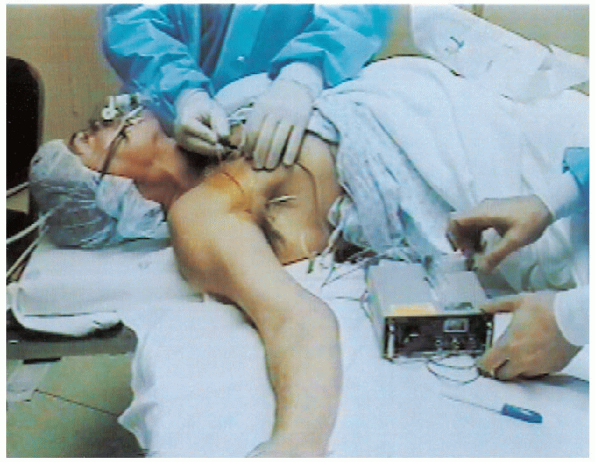
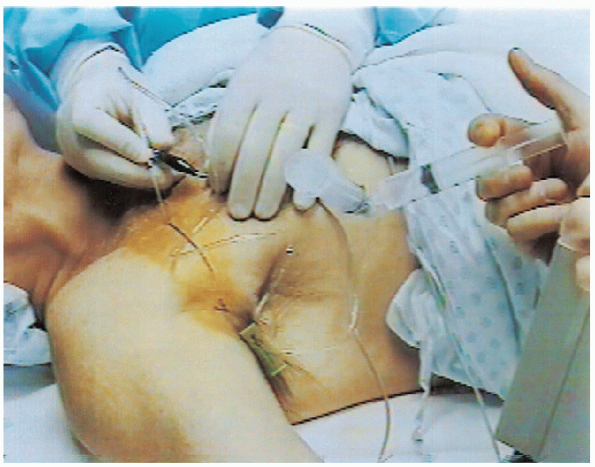
FIGURE 13-2.
With the patient in the supine position and the arm on the operating hand table, the anesthesiologist administers an infraclavicular anesthetic block. A nerve stimulator facilitates localization of the brachial plexus. |
incision is made directly in the crease and not adjacent to it, and it
is carried proximally to stop at the wrist flexion crease (Fig. 13-4).

In most cases, this surgical approach provides excellent visualization
of the entire carpal tunnel. However, in the patient whose carpal
tunnel syndrome is secondary to a proliferative tenosynovitis of the
flexor tendons, as seen in rheumatoid arthritis for which a
tenosynovectomy is required, the incision is carried more proximally
into the distal forearm. When the incision is
extended proximally, it should not cross the wrist flexion crease at a
right angle because it will likely result in a hypertrophic, unsightly
scar. Instead, the incision is continued ulnarly within the wrist
flexion crease for a distance of 1 to 2 cm and it is then curved
proximally. A similar surgical approach is sometimes necessary to
achieve satisfactory exposure in nonrheumatoid patients who have thick,
beefy hands.
 |
|
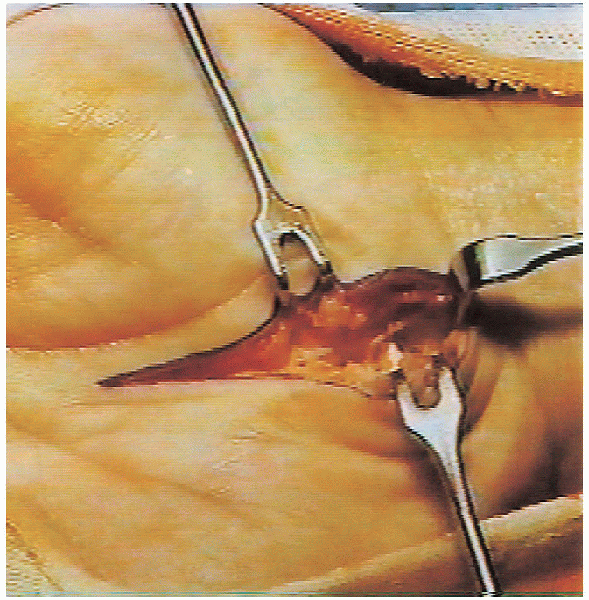
FIGURE 13-3. With the needle in the correct position, the anesthetic is injected.
|
 |
|
FIGURE 13-4.
The skin incision is made within a skin crease adjacent to the thenar crease and is carried proximally to stop at the wrist flexion crease. |
the skin incision is completed, the subcutaneous tissues are incised
and care is taken to avoid injury to the palmar cutaneous nerve that
branches from the main body of the median nerve proximal to the wrist
flexion crease (Fig. 13-4).

Usually, the palmar cutaneous nerve is not seen because it is radial to
the incision. However, it sometimes crosses the incision site and
should be protected. The palmar fascia is then incised, and in the
proximal portion of the operative field the palmaris longus tendon is
retracted radially. Cutting the palmaris longus
tendon should be avoided because it can retract proximal to the wrist
flexion crease and leave a ball of tissue that the patient may complain
is unsightly. Following incision of the palmar fascia, the
proximal portion of the transverse carpal ligament is usually obscured
by fat tissue extending from the hypothenar area. Rather
than cutting through this fat tissue, it is mobilized by sectioning one
or two vertical septa of the palmar fascia. The fat tissue can then
easily be retracted ulnarly, which permits excellent visualization of
the entire length of the transverse carpal ligament.
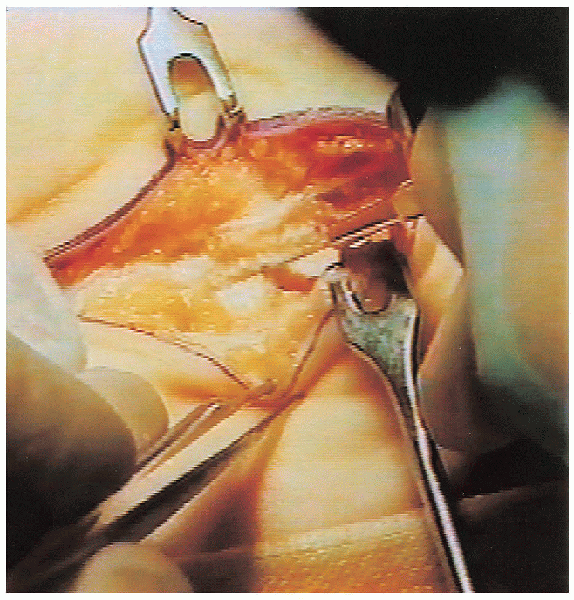
 The transverse carpal ligament is then incised (Fig. 13-5);
The transverse carpal ligament is then incised (Fig. 13-5);this should be done slowly to avoid injury to the median nerve and its
motor branch. A motor branch that takes a transligamentous course to
the thenar muscles is especially vulnerable to injury. A
clue to the presence of a motor branch that takes this route is a small
clump of fat tissue at the site where the branch penetrates the
ligament. It is important to ensure that the entire length of the
transverse carpal ligament has been divided. It is also advisable to release the distal portion of the antebrachial fascia (Fig. 13-6). 
 |
|
FIGURE 13-5.
The transverse carpal ligament is divided and care is taken to avoid injury to a motor branch that has a transligamentous approach to the thenar muscles. |
median nerve usually lies immediately beneath the divided ligament.
However, in some cases it is adherent to the radial side of the
ligament and is not immediately visualized. The
median nerve must be mobilized from this position; in doing so the
surgeon rather than the surgical assistant should position the
retractor to avoid injuring the nerve by inadvertently placing the
retractor, particularly a rake retractor, directly into or around the
nerve (Figs. 13-7 and 13-8).

The motor branch is then visualized; this is facilitated by blunt
dissection along the radial border of the median nerve. The first
branch is usually the motor branch. When dissecting along the ulnar
border of the median nerve, a thin nerve branch is sometimes
encountered. This is a sensory branch that connects with the common
digital nerve from the ulnar nerve and should be protected. As
with a transligamentous motor branch of the median nerve, a clue to the
presence of this thin sensory branch is some fat tissue along the ulnar
border of the median nerve.
 |
|
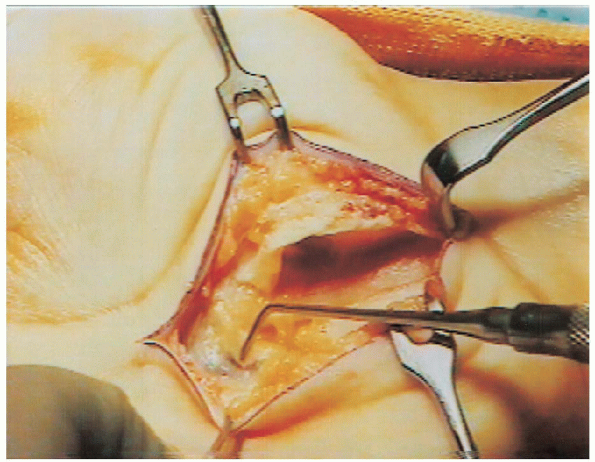
FIGURE 13-6. With the transverse carpal ligament divided, the superficial arterial palmar arch is visualized.
|
 |
|
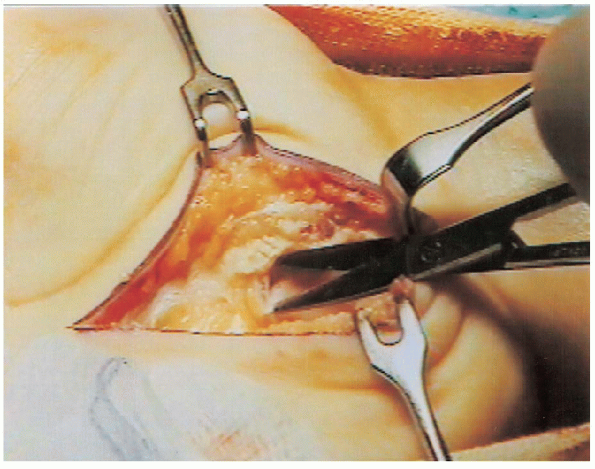
FIGURE 13-7. Thickened fibrotic tenosynovium tissue is excised to visualize the compressed median nerve.
|

The indication for an epineurolysis (epineurotomy or epineurectomy) at
the site of nerve compression is controversial. Although some believe
it is unnecessary, it is probably beneficial when there is a severe
compression
and the epineurium is thickened. The entire carpal tunnel, including
the floor of the tunnel deep to the tendons, should be inspected.
Occasionally, a mass such as a ganglion arising from the floor of the
tunnel is the cause for nerve compression. At the conclusion of
surgery, the tourniquet is released and any bleeding points cauterized.
It is unnecessary to lengthen the transverse carpal ligament by any
Z-plasty procedure, and only the skin incision must be sutured (Fig. 13-11).
A gauze dressing is applied to the hand and wrist. A bulky dressing
that interferes with digital flexion or extension should be avoided (Fig. 13-12).
The use of a wrist splint depends on the personal preference of the
surgeon. It is rarely necessary, but if one is applied it should be
discontinued after a week or two.
 |
|
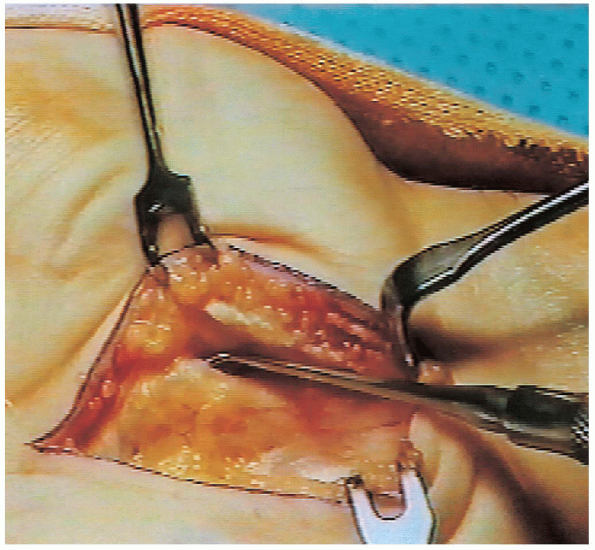
FIGURE 13-8.
The motor branch is identified. It is situated just distal to the transverse carpal ligament, which is the most common location (recurrent or extraligamentous) for a motor branch. |
 |
|
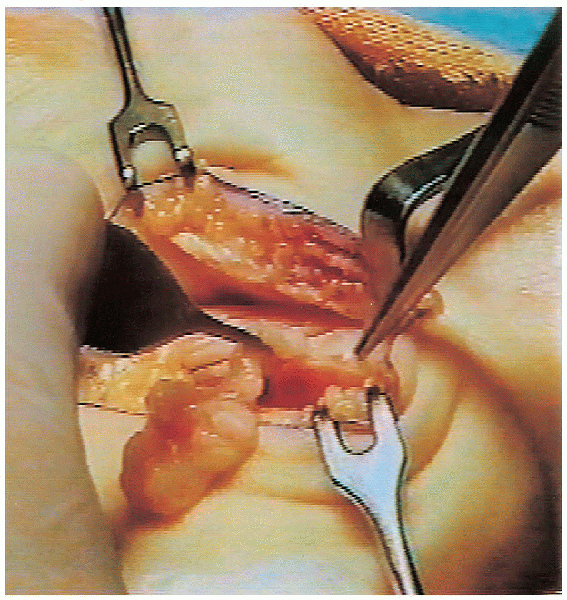
FIGURE 13-9. Thickened fibrotic tenosynovium is excised.
|
 |
|
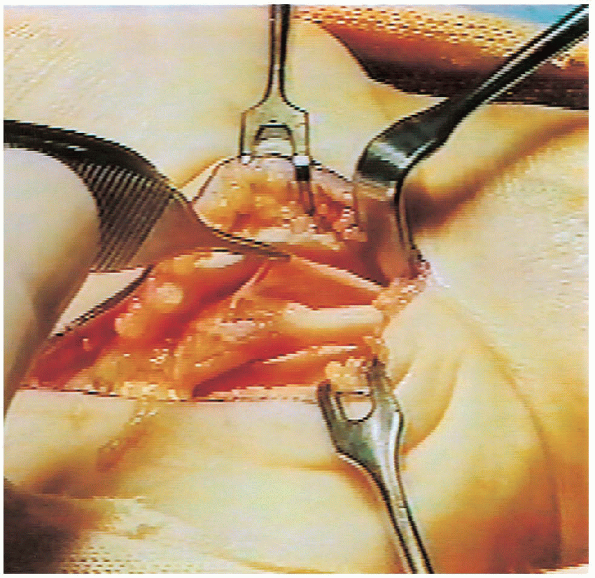
FIGURE 13-10. In this patient the epineurium around the compressed portion of the nerve was abnormally thick and it was also excised.
|
 |
|
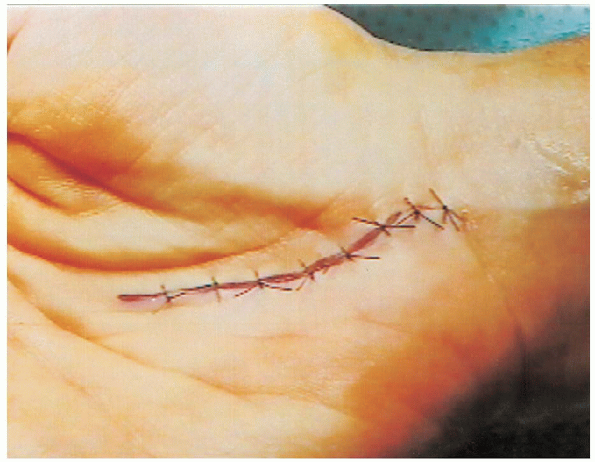
FIGURE 13-11. The skin is closed with interrupted 4-0 nylon sutures.
|
be effective, elevation must be constant; to emphasize this point,
patients are told that keeping their hand in a dependent position for 1
minute negates keeping it elevated for hours. Digital exercises
are encouraged as soon as the effects of the anesthetic block have worn
off. Patients are instructed to actively flex and extend their fingers
and thumb on a regular basis. They are encouraged to perform these
exercises 10 times each hour. The frequency of exercising is much more
important than the duration of
each
session. Patients who exercise effectively usually regain complete
digital mobility within the first week. Active range of motion
exercises are also encouraged for the shoulder, elbow, and wrist.
 |
|
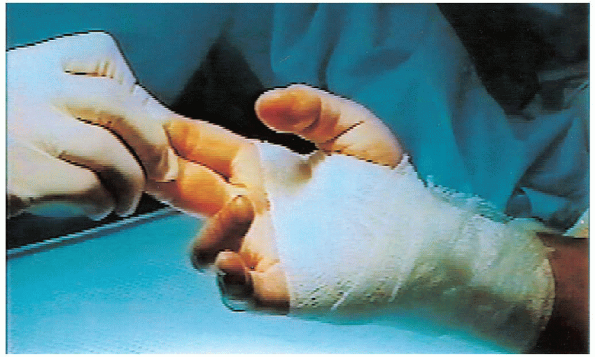
FIGURE 13-12. The postoperative bandage is thin and only around the palm and wrist to avoid interfering with digital motions.
|
 |
|
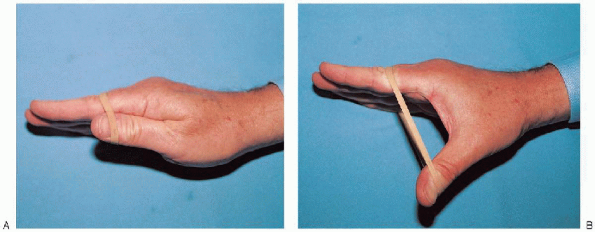
FIGURE 13-13. A and B: Resistive exercises are used to improve thenar strength.
|
exercises for the thenar muscles involve abducting the thumb against
resistance. This is best achieved by wrapping a rubber band around the
palm and thumb, at the level of the interphalangeal joint, and
abducting the thumb against the resistance of the rubber band (Fig. 13-13).
Similar to digital exercises, 10 repetitions are encouraged each hour.
As thenar muscle strength improves, the exercises are performed against
greater resistance using thicker rubber bands or multiple rubber bands.
Swelling and induration at the operative site are common but gradually
diminish over the ensuing weeks. Application of a thin silicone pad to
the area hastens the normal maturation process of the scar. Patients
can usually resume light work activities 2 to 3 weeks after surgery and
strenuous activities, including sports activities, within 6 to 8 weeks.
Serious amateur and professional athletes often return to their sport
as early as 1 week after surgery.
