Cervical Spine
spine mandates careful preoperative planning and an appreciation of the
limitations inherent in each surgical approach. Such planning can
significantly reduce the technical demands of the surgery, and reduce
the likelihood of complications.
positioning prior to an incision will lead to a smoother procedure, and
may improve the result of the operation. This includes the use of
imaging modalities after positioning to ensure adequacy of the image,
which can be adjusted much more easily prior to final preparation and
draping of the patient.
the anatomic levels requiring surgical management, as well as the
techniques planned for any reconstruction. Table 12-1
lists the more common anterior and posterior approaches, and the
cervical levels exposed most effectively by each approach. With the
advent of fluoroscopically guided techniques (i.e., transarticular C1-2
screws or anterior odontoid screws), one must also consider the
trajectories required for placing instrumentation when choosing the
surgical approach.
anterior processes, such as the odontoid with basilar invagination,
infection, tumors, irreducible odontoid fractures in chronic
dislocations, and congenital disorders of the anterior axis or atlas.
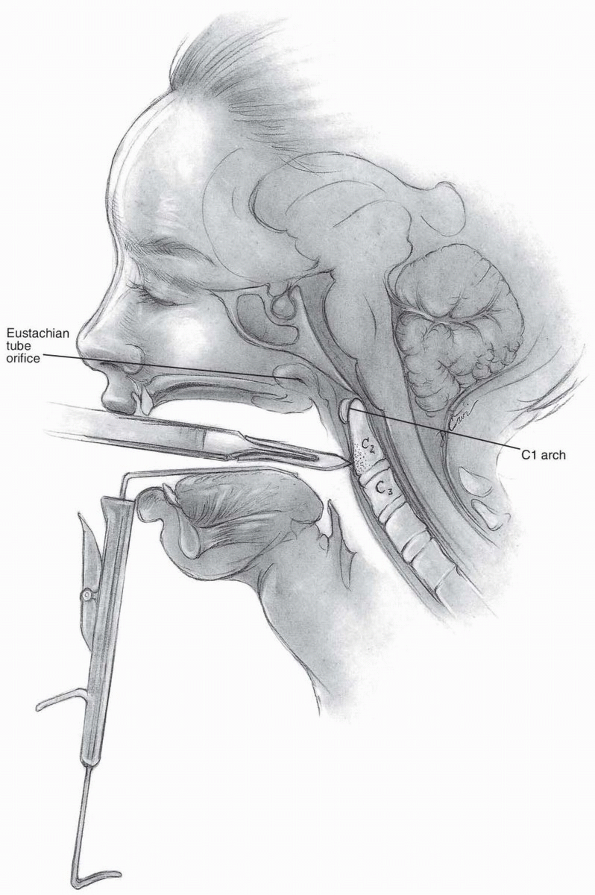
as its pathway to the upper cervical spine. Visualization can be
difficult due to the constraints of the jaw and oral cavity, but it can
allow direct access from the clivus to the upper portion of C3.
Although there is little vascularity encountered when approaching the
spine through this midline approach, infection and cerebrospinal fluid
leakage have been considerable problems in the past. The heightened
chance of infection and historically poor outcome of bone grafting with
the transoral approach make it a questionable choice for use during
anterior cervical fusions (1).
The limitations in exposure with this approach can be reduced by the
use of mandibular osteotomies. Morbidity from these techniques can be
substantial; however, the approach does allow unique anterior exposure
from the clivus to C6. It is typically useful for lesions within the
bodies of C2-3, and when the oral cavity cannot be easily traversed,
such as with severe temporomandibular joint arthrosis or an interdental
distance less than 25 mm.
|
TABLE 12-1. Common Approaches to the Cervical Spine
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
be ascertained, but particular focus must be paid to nutritional
status. Total lymphocyte count and albumin are generally useful markers
of nutritional status. Dental evaluations should be performed
preoperatively to ensure an oral cavity that is free from ongoing
infection. Standard preoperative antibiotic recommendations currently
include an intravenous cephalosporin and metronidazole.
are used to stabilize the head with the patient in the supine position.
Awake, nasotracheal intubation may be performed with or without
fiberoptic assistance depending on the perceived stability of the
cervical spine. Once a neurologic examination is performed, anesthetic
can be administered, and then the patient can be placed in a mild
reverse-Trendelenberg position to prevent aspiration (2).
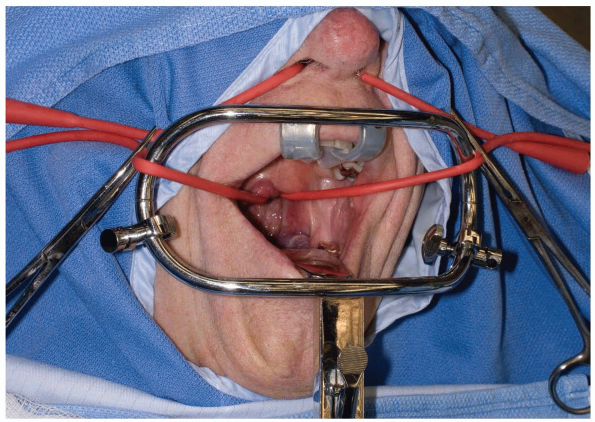
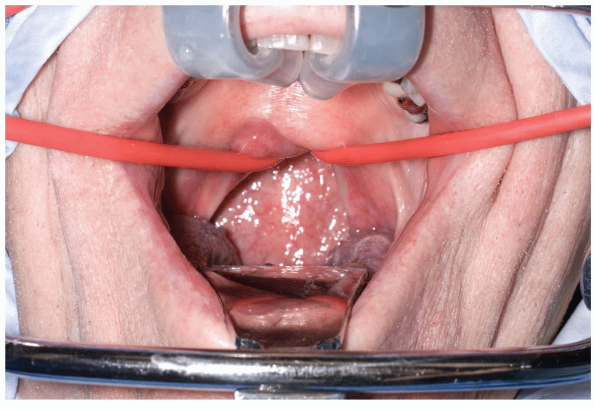
Various retractors can be used for widening the interdental gap, such
as the Codman Crawford or the Spetzler-Sonntag transoral retractors (Figs. 12-1, 12-2 and 12-3).
The soft palate can often be elevated with a malleable self-retainer.
Alternatively, two Red Robin catheters can be fed through the nares
(one on each side) and brought out through the mouth. Once both
catheters have been placed, the two ends on each side are tensioned
superolaterally and secured to the drapes. Prepare the oropharyngeal
area with betadine solution, and place a throat packing to prevent
debris from falling into dependent laryngeal spaces or the trachea.
Obtain localizing radiographs once the posterior pharyngeal area is
exposed, and then inject epinephrine solution along the site of planned
incision. Although surgical loupes with overhead illumination can be
used in these cases, an operating microscope provides a clearer image
and allows for easier assistant participation.
 |
|
FIGURE 12-1
|
 |
|
FIGURE 12-2
|
 |
|
FIGURE 12-3
|
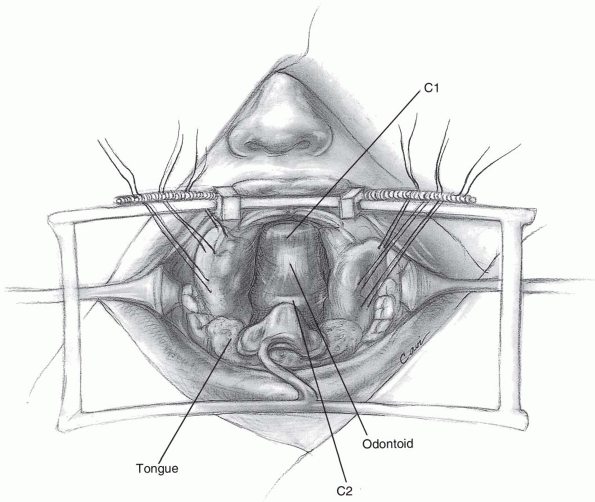
midline, and a vertical incision is carried out 1 to 2 cm above and
below the tubercle in the midline (Fig. 12-4).
-
Incision: the incision is made full
thickness to bone through the mucosa and pharyngeal musculature.
Subperiosteal elevation should be carried out from the midline, which
will bring the longus colli muscles and anterior longitudinal ligaments
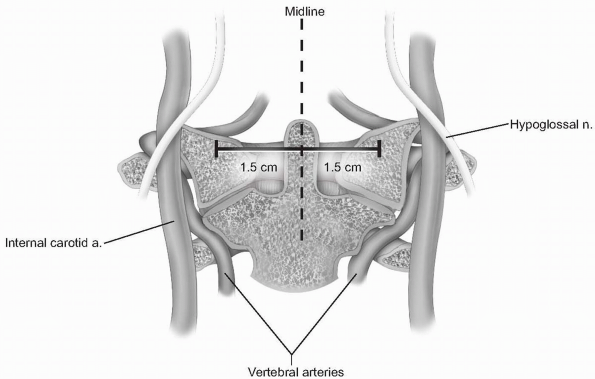
laterally. Lateral elevation on the atlas should be restricted to 1.5
cm from the midline, as the vertebral arteries, internal carotid
arteries, and hypoglossal nerves are at risk with more aggressive
lateral exposure (3) (Fig. 12-5).
The internal carotid artery can be quite close to the midline, as
suggested by Currier et al, who found it more than 7.5 mm medial to the
foramen transversarium in 6% of their subjects (4). The hypoglossal nerve lies approximately 2 to 3 mm lateral to the middle of the anterior aspect of the C1 lateral mass (5) (see Fig. 12-5). -
If access to the odontoid is desired, the
central 1 to 1.5 cm of the atlas may be resected to expose the upper
portion of C2. Should the anterior portion of the atlas be removed,
attempt to preserve the transverse ligament, if possible. The
transverse ligament prevents lateral displacement of the atlas lateral
masses, which can lead to craniocervical instability (6).
If it cannot be preserved, a posterior occipitocervical arthrodesis may
be necessary. Soft tissues, such as pannus, can be resected from the
areas surrounding the bony anatomy, but residual amounts adherent to
the dura may best be left in place to avoid cerebrospinal fluid (CSF)
leakage. The tectorial membrane can be decompressed to allow
improvement in CSF flow.
 |
|
FIGURE 12-4
|
 |
|
FIGURE 12-5
|
completed, CSF containment should be confirmed with a Valsalva
maneuver, and then reconstruction or closure can proceed. If possible,
the longus colli is reapproximated in the midline, followed by
absorbable suture approximation of the pharyngeal layer. Intubation is
typically continued for 1 to 2 days due to the risk of swelling
postoperatively. Antibiotic regimens vary in the literature, but we
typically use cefazolin and metronidazole for 5 to 7 days starting
immediately preoperatively. The metronidazole can be administered
through a nasogastric feeding tube placed intraoperatively after wound
closure. Clear liquids are begun approximately one week after surgery,
but a regular diet should be withheld until 3 weeks postoperatively (6).
been quoted between 18% and 26%, including a mortality rate of 6%,
according to one source (7). Although early use
of the approach led to very high infection rates (above 50%), more
recent studies demonstrate infection rates below 3% (8, 9, 10).
CSF leakage during the procedure should be addressed with attempted
closure, grafting, fibrin glue, and a meticulous closure, as the
persistence of leakage can result in fistula formation (11).
A lumbar drain should be strongly considered in this situation, as the
persistence of CSF leakage can be exceptionally troublesome. Other
potential complications include bleeding from injury to the vertebral
or internal carotid arteries, craniocervical instability, lingual
swelling, and postoperative infection.
anterior upper cervical spine, and may be indicated for debridement of
tumors or infection, and stabilization of the atlantoaxial segment. It
is possible to expose the C1-2 facet articulations at the upper end of
the approach, while the most caudal access afforded by the primary
incision is the upper end of C3. An extensile vertical incision can be
employed to allow access to the mid and lower cervical regions.
(anterior approach) or lateral (anterolateral approach) to the carotid
sheath, we favor the use of the method described by McAfee and
colleagues, termed the anterior retropharyngeal approach (12). It is an extension of the Southwick and Robinson approach initially described for use in the lower cervical spine (13).
By accessing the retropharyngeal space medial to the carotid sheath,
one avoids the risk of injuring the carotid vessels or cranial nerves
at the skull base, but there is a higher risk of injury to the superior
laryngeal or glossopharyngeal nerves than when approached lateral to
the carotid sheath. Additionally, the anterior approach allows access
to both vertebral arteries, while exposing lateral to the carotid
sheath compromises access to the contralateral vertebral artery.
Finally, anterior decompression and reconstructive measures may be more
easily performed when visualizing the spine from the anterior approach
than the anterolateral approach.
of swallowing or respiratory function. In the revision setting, it may
be necessary to have the patient undergo vocal cord evaluation. A
preoperative tracheostomy may be warranted to avoid airway problems,
and an otolaryngologist can assist with this determination. When
attempting tumor resections, angiography or a CT angiogram should be
obtained to define the locations of the vertebral arteries.
traction should be strongly considered. If postoperative halo-vest
immobilization is planned, the use of a halo ring can be substituted
for Gardner-Wells tongs. Before traction is applied, baseline evoked
potentials from neuromonitoring should be obtained (if used). The
patient should be awake for fiberoptic nasotracheal intubation, so
neurologic assessment can be performed following the intubation and
positioning. Oral placement of tubes or devices should be avoided, as
they may inferiorly displace the mandible and compromise maximum
exposure of the upper-most aspects of the cervical spine. Finally,
place the patient in a slight reverse-Trendelenberg position to aid in
visualization and improve venous drainage.
approach need not be dictated by underlying anatomical variations. A
right-sided approach may be easier for those surgeons with right-handed
dominance, but if there is a foreseeable need to extend below C5 then
one should consider a left-sided approach to avoid injury to the
recurrent laryngeal nerve. Tumor location predominantly on one side may
also influence the need for approach on a particular side. Mild head
rotation can facilitate the exposure. If performing the anterolateral
high retropharyngeal approach, the ipsilateral earlobe should be
prepped and then sutured to the cheek anteriorly. Otherwise, it will be
an obstruction during the exposure.
-
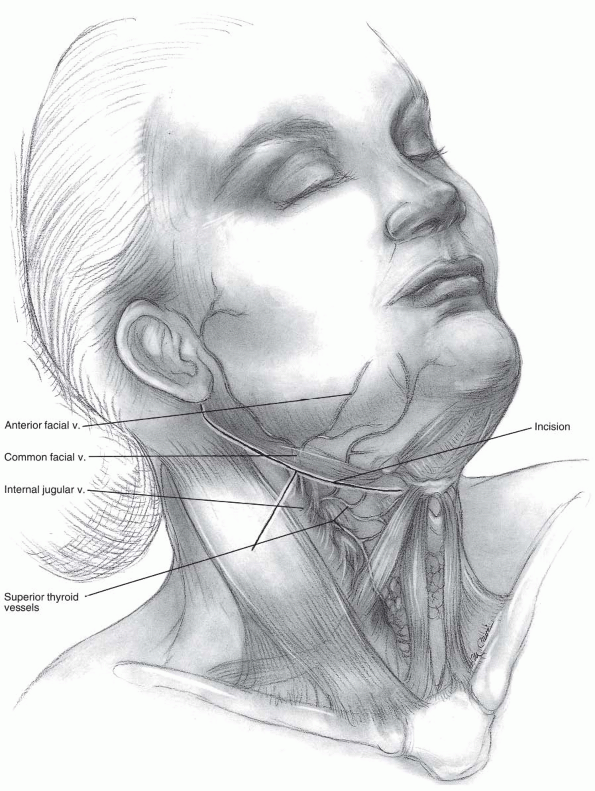
Incision: the submandibular incision for
the anterior approach begins at the tip of the mastoid process and
traverses medially to the level of the hyoid bone (modified Schobinger
incision) (14). When more caudal exposure is
desirable a vertical incision can be made along the sternocleidomastoid
to intercept the submandibular incision (Figs. 12-6 and 12-7).
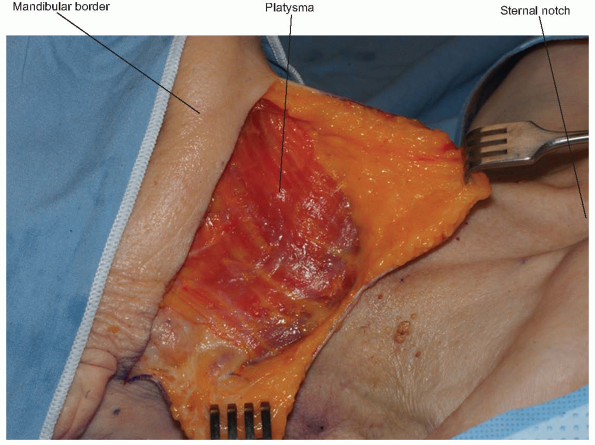
Once the superficial fascia and platysma are divided, skin flaps can be
raised deep to the platysma. Before proceeding more deeply, a nerve
stimulator can be used to find the mandibular branch of the facial
nerve (Fig. 12-8).
It generally courses superiorly above the retromandibular vein and
should be preserved due to its innervation of the orbicularis oris
muscle. The retromandibular vein can be sacrificed by ligation adjacent
to its junction with the internal jugular vein. -
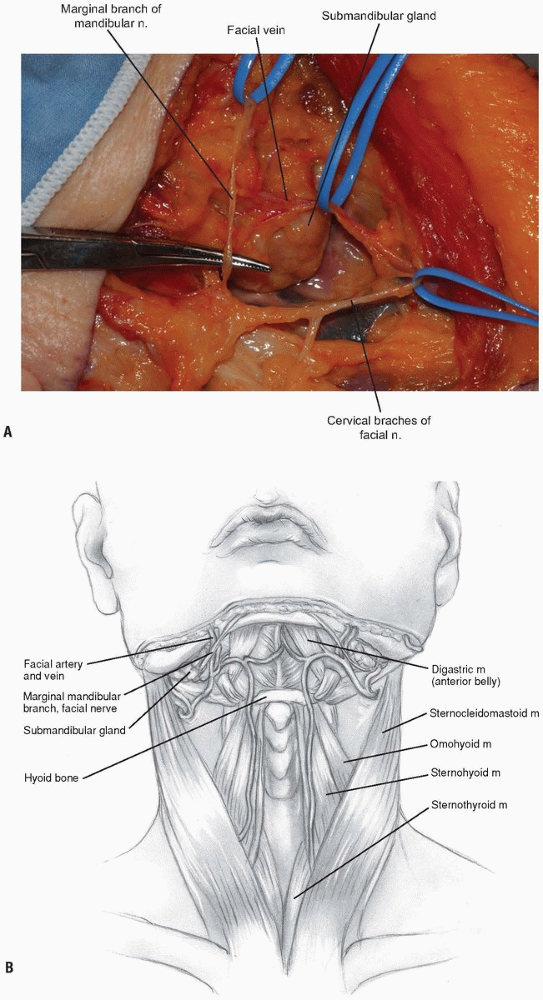
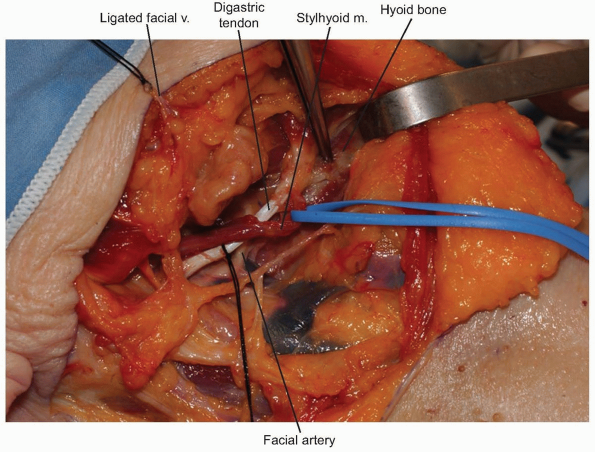
The facial vein can be found and ligated just inferior to the submandibular gland (Fig. 12-9).
It typically courses superficial to the gland and is oriented
cephalocaudally. By leaving the ligature on the superior stump of the
vein, it can be used to retract the superficial fascia of the
submandibular gland and protect the marginal branch of the mandibular
nerve as it courses within the fascia. The submandibular gland is then
mobilized superiorly, exposing the intersection of digastric and
stylohyoid muscles. These muscles are divided at their confluence near
the hyoid bone and reflected proximally (Fig. 12-10).
Occasionally, the submandibular gland must be resected to allow
adequate exposure; however, its corresponding salivary duct must then
be ligated to prevent fistula formation. -
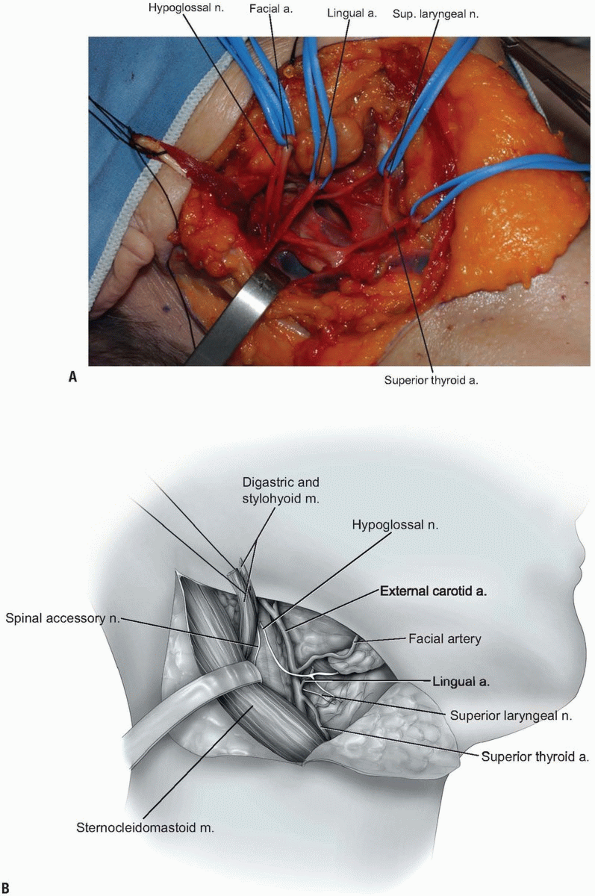
Next, the superior thyroid, lingual, and facial arteries and associated veins are ligated and divided (Fig. 12-11).
It is helpful to proceed from inferior to superior during this process,
using the hyoid bone as a marker for localizing each artery. The
superior thyroid artery is just below the hyoid bone, while the lingual
and facial arteries are at and just above it, respectively. The
superior laryngeal nerve often travels close to the superior thyroid
artery, and injury to it must be avoided. -
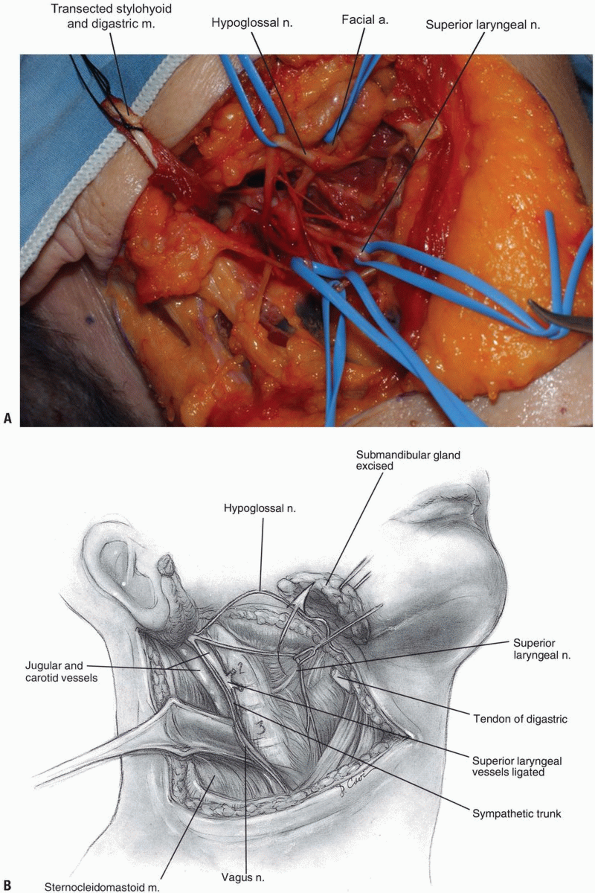
The deep fascia can now be divided along
the border of the sternocleidomastoid muscle, and the carotid sheath is
localized by palpating the internal carotid pulse. Once the digastric
and stylohyoid muscles have been transected, the hyoid bone and
accompanying pharyngeal structures can be more easily mobilized
medially. Care should be taken not to retract these muscles too
vigorously near the mastoid, as the facial nerve courses through this
area and may sustain a neuropraxic injury. -
The hypoglossal nerve is found more
superior than the superior laryngeal nerve; both should be protected by
careful dissection and mobilization. Once the hypoglossal nerve is
dissected out from its exiting site at the skull base to its insertion
near the tongue, it can be retracted superiorly (Fig. 12-12, see Fig. 12-10B).
The carotid sheath can then be retracted posterolaterally. Although
medial retraction of the pharynx is helpful, excessive force can cause
iatrogenic injury to the laryngeal and pharyngeal branches of the vagus
nerve. Use blunt finger dissection to separate the carotid sheath from
the medial pharynx and larynx, and the retropharyngeal space can then
be safely entered. -
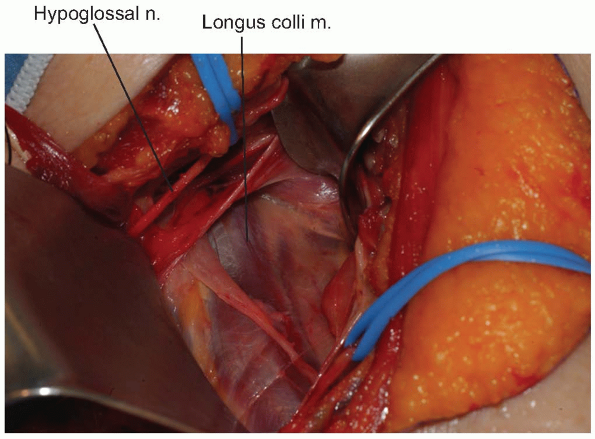
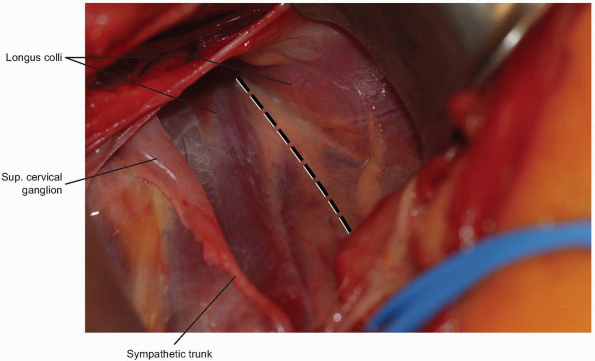
A peanut or Kittner dissector can now be
used to clear alar and prevertebral fascia away from the longus colli
muscle, which is then divided longitudinally in the midline (Fig. 12-13).
The longus colli muscles insert on the anterior arch of the atlas
bilaterally, so they can help define the midline of C1 if adequately
exposed. When starting the midline incision, avoid extending beyond the
cephalad margin of the atlas, which could violate the anterior
occipitoatlantal membrane. The incision is carried down to bone and
subperiosteal flaps are elevated laterally. The anterior longitudinal
ligament underlying the longus colli should be reflected within these
flaps. Limit lateral elevation of these flaps on the atlas to 1.5 cm
from midline, as the vertebral arteries can be in significant danger
beyond this distance. -
If decompression is planned, it should
generally be performed in a cephalad-to-caudal direction. This reduces
difficulty with visualization, as bleeding tends to run in a caudal
direction. The posterior longitudinal ligament and uncovertebral joints
help identify the posterior and lateral safety margins of the
decompression. If reconstruction is to be performed, the head should be
carefully repositioned to neutral alignment prior to graft placement
and fixation. -
The transected digastric and stylohyoid
muscles should be reapproximated with sutures. Similarly, the origin of
the sternocleidomastoid muscle must be repaired at the mastoid process.
Before occlusion of the retropharyngeal space, a drain should be placed
in this deeper area of the wound. The platysma can be reapproximated
with a running absorbable suture, followed by the skin in a routine
fashion according to the surgeon’s preference.
 |
|
FIGURE 12-6
|
 |
|
FIGURE 12-7
|
 |
|
FIGURE 12-8
|
 |
|
FIGURE 12-9
|
 |
|
FIGURE 12-10
|
 |
|
FIGURE 12-11
|
but longer cases or those with greater likelihood of significant edema
should be considered for a day or two of continued intubation. Less
commonly, tracheostomy might be required immediately or in a delayed
fashion if there is more serious airway compromise or concern. Drains
are typically left in for approximately 24 hours, but higher outputs
may necessitate longer durations or exploration in cases of persistent
heavy bleeding. It is generally advised to keep the head of the bed
elevated to encourage more rapid resolution of edema. Dietary
advancement should begin with sips of water or clear liquids, and then
slow progression may ensue if tolerated on the first postoperative day.
-
Neurapraxias or lacerations of various
nerves may occur during the high retropharyngeal exposures. Injury to
the mandibular branch of the facial nerve may cause drooping of the
ipsilateral mouth corner, but this usually resolves spontaneously over
the first few postoperative months in cases of neuropraxia. The
hypoglossal nerve typically recovers from neuropraxic injury within
several months after surgery (6,15).
The superior laryngeal nerve has a role in voice physiology, and injury
may result in high-pitch phonation and diminished supraglottic
sensation (6). Its recovery from neuropraxia is
less predictable. Finally, the spinal accessory nerve can suffer injury
during mobilization of structures around the mastoid process, and may
result in ipsilateral paralysis of the trapezius and
sternocleidomastoid muscles. -
Inadvertent entry into the pharynx or
esophagus warrants immediate placement of a nasogastric tube, if not
already present. This should be done under direct visualization, and
the disruption repaired in two absorbable layers. Postoperatively, the
nasogastric (NG) tube should be left in for 7 to 10 days to prevent
fistula formation, and an esophagram and/or esophagoscopy should then
be performed prior to an oral diet (16).
Parenteral or NG tube nutrition will be required during the period of
oral dietary restriction. Intravenous antibiotics that cover both
aerobic and anaerobic pathogens should be employed for approximately 5
days postoperatively. In the case of delayed discovery or presentation
of the perforation, a pedicled sternocleidomastoid muscle flap may be
required for reinforcement (16). -
Retraction against the medial pharyngeal
structures may induce enough edema to result in airway obstruction.
Sustained intubation or short-term tracheostomy may be employed while
the edema resolves. Hematoma formation is another possible cause of
airway obstruction, so postoperative bulb drainage is generally
encouraged.
 |
|
FIGURE 12-12
|
 |
|
FIGURE 12-13
|
approached through two skin incisions, depending on the extent of
exposure required. A transverse incision can be employed for exposure
of three disc levels or two vertebral body resections. When more
extensive visualization is required, a longitudinal incision should be
used. These anterior approaches typically allow access from C3 to T1,
but anatomic variabilities may reduce the extent of this range. By
employing the interval between the sternocleidomastoid muscle laterally
and pharyngeal structures medially, one is afforded a fairly direct
anterior visualization of the cervical spine. This vantage point is
ideal for most standard anterior spinal procedures.
swallowing abnormalities, phonation problems, or aberrant anatomy. In
the revision setting, preoperative endoscopic assessment of vocal cord
function should be strongly considered. If the vocal cord ipsilateral
to the original surgical approach is dysfunctional, surgery should
proceed through the same side, so inadvertent injury to the remaining
functional vocal cord does not occur. If both vocal cords appear to be
functioning well, some authors have advocated using an approach from
the contralateral side (17). In most cases, a
left-sided approach should be employed, as the left recurrent laryngeal
nerve takes a less variable course within the tracheoesophageal groove
than the nerve on the right side.
the carotid pulses and listen for bruits with a stethoscope.
Abnormalities should be evaluated with a carotid ultrasound (Duplex)
study, and an appropriate consultation as indicated. Thyromegaly can
compromise wound closure, or cause increased external airway pressure
following closure. A partial thyroidectomy may be required prior to
wound closure in these circumstances.
scrutinized, so that any unique anatomic features are anticipated and
accounted for during the surgical exposure. In the case of odontoid
fractures for which anterior screw placement is being considered,
pectus carinatum (barrel-chest) may be a contraindication to using the
technique. The acute angle required for drilling and placing the screw
may be impossible to achieve. However, newer equipment designs such as
systems incorporating flexible drill bits have minimized these early
problems.
table, and if iliac crest bone is to be taken, place a bump under the
ipsilateral hip. Standard preoperative antibiotics are adequate for the
majority of anterior cervical procedures. Intubation in patients with
spinal cord compression or myelopathic findings should be performed
awake and/or with fiberoptic assistance. If neuromonitoring is planned,
the set-up should begin prior to patient positioning and intubation. If
the spine is stable, position the neck in a safe degree of extension
(as determined by preoperative examination). If there are any changes
with the electrophysiologic monitoring, reposition the neck to the
neutral position and do not proceed unless the changes revert to
baseline. A rolled towel, intravenous saline bag (1 L), or other
similar-sized bump between the shoulder blades will help maximize
cervical extension. Slight cervical rotation away from the chosen side
of approach can facilitate visualization during exposure, as the
mandible can otherwise partially obstruct an anteroposterior view. The
head should be well-padded at its contact point with the table,
especially during longer cases.
traction with Gardner-Wells tongs can be used in place of operative
site distraction (i.e., Caspar distractor). Using external traction
rather than local distraction should be more strongly considered in
multilevel decompressions, or in cases with poor bone quality. If
Gardner-Wells tongs are used for traction, be mindful of the placement
of the tongs relative to the axis of occipitocervical rotation in the
sagittal plane, as well as the vector of pull. When extension of the
cervical spine is desirable, tong position should be slightly anterior
within the “safe window” above the ear, and the traction vector from
anterior to posterior.
patient’s torso. Gel or foam pads are rolled around each arm, ensuring
that the patient’s skin is well-padded from any prominent intravenous
tubing, connections, or neuromonitoring leads. Cotton padding is placed
around each wrist underneath straps that can be used for intermittent
caudal upper extremity traction during intraoperative radiographs.
Avoid traction if the patient has upper extremity deformities or
injuries. The pre-positioned sheet can now be folded over the patient’s
torso and taped in position to secure the arms. The width of the sheet
must allow access to both the iliac crest and the upper chest.
area from the clavicles to the mandibular prominences, as well as
lateral to the sternocleidomastoid muscles. Ideally, both sides should
be draped out and prepped, so that any emergent vascular or respiratory
issues can be managed without the need for additional redraping or
preparation.
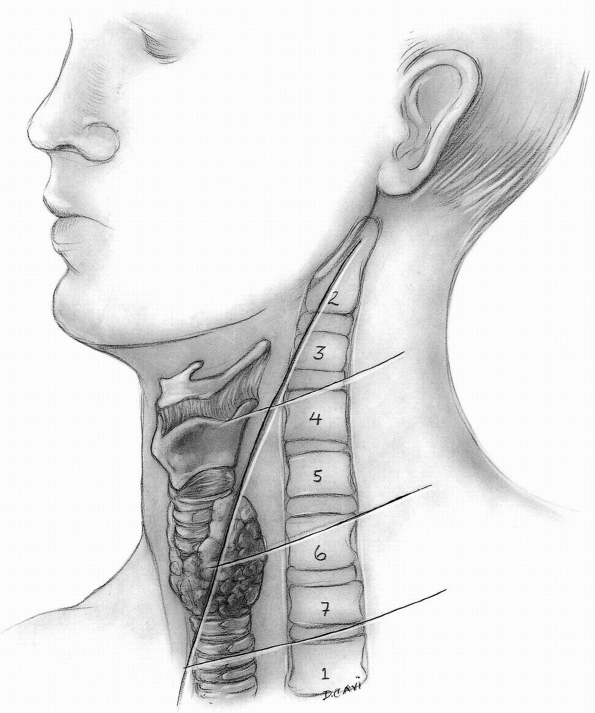
than the longitudinal incision, and must therefore be placed in the
neck according to the desired levels of visualization. Palpable
landmarks within the neck usually provide adequate guidance for
incision placement (Table 12-2),
however it must be noted that cervical extension may shift the
superficial landmarks (hyoid bone, thyroid cartilage, cricoid
cartilage) slightly more cephalad relative to their corresponding
vertebral levels at a neutral position (Fig. 12-14).
The hyoid is at the level of C3, the thyroid is at the level of C5 to
C6, the cricoid cartilage is at the level of C5 to C6 interspace, and
the carotid tubercle is at the level of C6. This requires a mild shift
of the incision caudally relative to the palpable landmarks when the
neck is extended. Attempt to place the transverse incision within a
skin crease, or parallel to Langer lines. It is helpful to mark the
planned incision with a marking pen, and then place a sheet of Ioban
over the neck region. Attempting to discern skin creases after
placement of the Ioban can often be difficult. We also recommend
pre-incision subdermal infiltration of epinephrine solution, with or
without local anesthetic to diminish bleeding.
|
TABLE 12-2. Palpable Landmarks in the Neck
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
 |
|
FIGURE 12-14
|
-
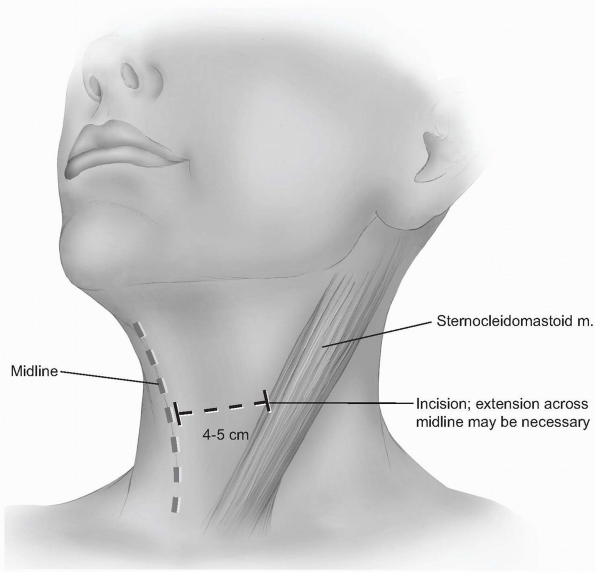
Incision: the incision should begin
around the midline of the neck and extend laterally to the anterior
border of the sternocleidomastoid. Although one can extend beyond the
midline without significant problems, carrying the incision too far
laterally can cause cosmetically displeasing adhesions to the
underlying sternocleidomastoid. Typically, a 4 to 5 cm incision is
adequate for two-level visualization, but may require extension across
the midline of the neck when more exposure is desired (Fig. 12-15).
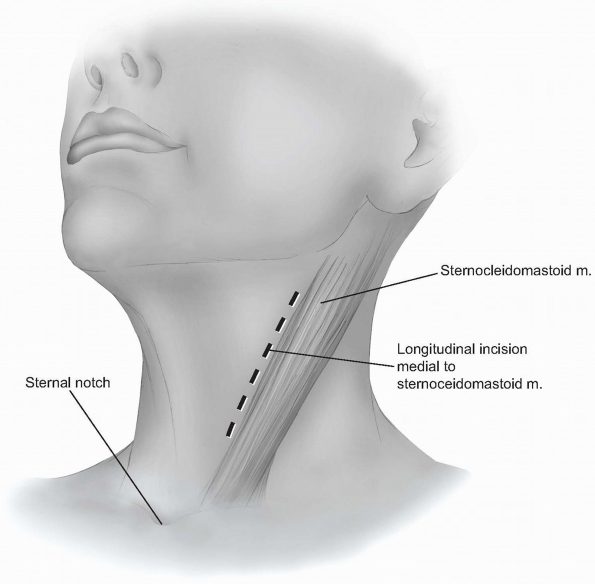
A longitudinal incision can be used to provide more extensile access to
the anterior cervical spine. This incision is carried out along a line
drawn between the sternal notch and the mastoid process, just medial or
anterior to the sternocleidomastoid muscle (Fig. 12-16).
It should be placed over the target vertebrae using the palpable
landmarks, and its length will vary according to the extent of exposure
required. -
Because the skin is thin and cosmesis is
of greater concern in this area, dermal bleeding is best managed with
bipolar electrocautery following the initial skin incision. Skin hooks
or rakes are placed for retraction, and the skin edges are lifted away
from the neck to facilitate separating the dermis and subcutaneous
layer from the platysma with Metzenbaum scissors or electrocautery. If
the external jugular vein is encountered, it can be swept aside or
ligated as needed. The platysma is identified by noting its
longitudinally oriented fibers as seen in Figure 12-17,
and it (as well as the investing fascia) can be divided parallel to its
fibers. Elevate the platysma with two sets of tissue forceps and snip
between them through the full thickness of the muscle. While
maintaining upward tension with the forceps, the plane deep to the
platysma can be developed by spreading with the Metzenbaum scissors,
allowing the muscle to be incised without damage to underlying
structures. A self-retainer is placed deep to the platysma, exposing
the superficial layer of the deep cervical fascia. -
Dissecting scissors can now be used to
divide the investing superficial layer of the deep fascia from one end
of the incision to the other (Fig. 12-18).
The superficial jugular branches may be encountered and ligated, if
they represent obstruction to further dissection. Enter the next layer
of deep fascia along the anterior border of the sternocleidomastoid
muscle. This can be accomplished with careful spreading with scissors,
as well as blunt finger dissection, until a plane is developed below
the muscle. Take care to incise the deep cervical fascia just medial to
the medial border of the sternocleidomastoid muscle. Entering the
fascia over the substance of the muscle requires dissection through
both layers of the investing fascia, as well as the muscle itself (Fig. 12-19). -
At this point gently palpate for the
carotid artery pulse just medial and deep to the sternocleidomastoid.
Once the carotid sheath has been identified in this manner, continue
with gentle finger dissection medial to the sheath (Fig. 12-20).
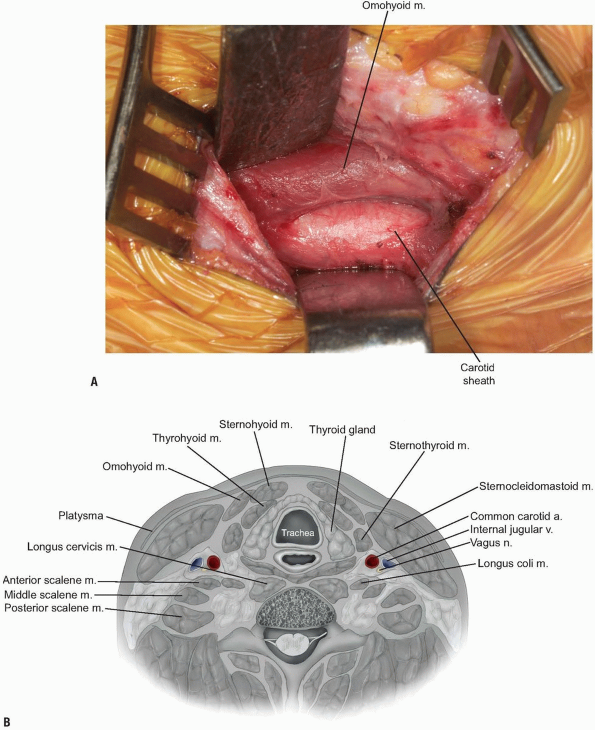
A smooth Meyerding or Richardson retractor can then be placed medial to
the dissecting finger, so that it can be used to retract the trachea
and esophagus (Fig. 12-21).
In the upper cervical levels, the superior thyroid artery may be
identified during this maneuver. The superior laryngeal nerve
accompanies the artery and protection of the artery will help protect
the more friable and less visible nerve. Gentle rostral retraction of
both structures will generally afford adequate exposure. In the
mid-levels, the middle thyroid artery and accompanying veins may be
identified, and may be transected to allow continued approach to the
spine, if necessary. In the lower levels, the recurrent laryngeal nerve
can occasionally be seen traversing from caudal to cephalad as it moves
medially into the tracheoesophageal groove. The ansa cervicalis may
also be appreciated running along the anteromedial aspect of the
carotid sheath. -
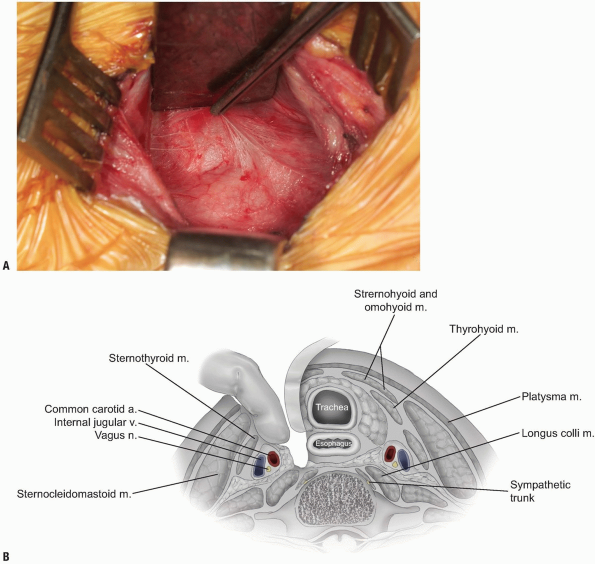
The middle layer of the deep cervical fascia should now be visible at the base of the exposure (see Fig. 12-21),
and it may invest the omohyoid muscle within the C5 and C7 region.
Occasionally, the omohyoid muscle must be transected, but generally it
can be retracted rostrally or caudally to provide adequate exposure.
Palpate the anterior spine and enter the middle fascial layer over the
vertebral bodies with blunt finger dissection while protecting the
medial structures with the retractor. The prevertebral fascia (also
known as alar fascia) overlying the anterior longitudinal ligament and
vertebral bodies should now be in view. -
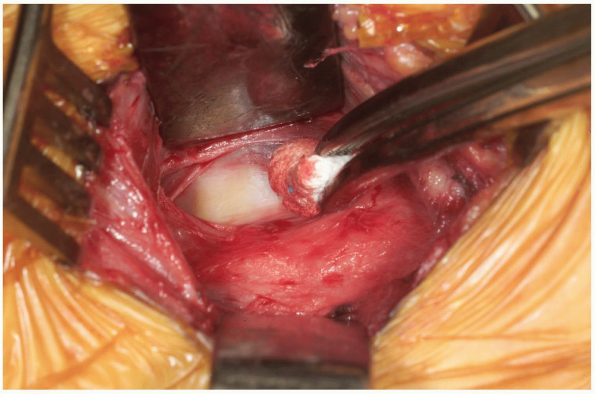
Palpate the vertebral bodies through the
fascia to determine the approximate location of the midline, and then
use a Kittner or peanut dissector to clear the fascia away from the
midline of the spine (Fig. 12-22).
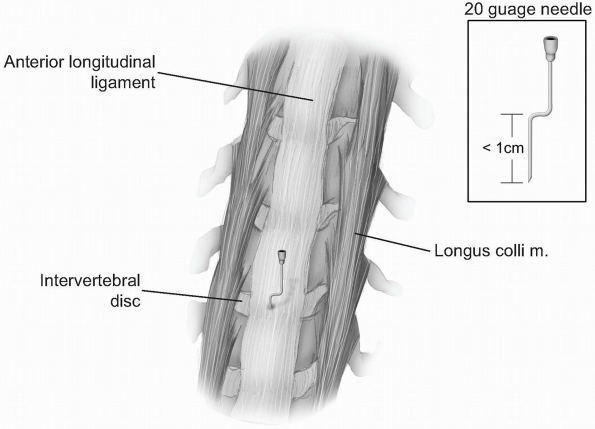
Once a vertebral segment and disk have been exposed by this maneuver,
place a radiopaque marker into the disc space. This can be accomplished
with a specially designed marking needle or by bending a standard
20-guage needle with two oppositely-directed 90 degree bends, such that
the tip of the needle is no more than 1 cm from the first bend (Fig. 12-23).
A localizing radiograph should then be obtained. Concurrent caudal
traction on the patient’s arms may be necessary to visualize the lower
cervical segments on lateral radiographs. Usually, lateral radiographs
or fluoroscopy is adequate; however, lower cervical regions may be
obscured by the shoulders and necessitate use of an anteroposterior
image. If plain radiography is used and iliac crest bone autografting
is planned, the graft harvesting can be performed while waiting for the
film to be developed. -
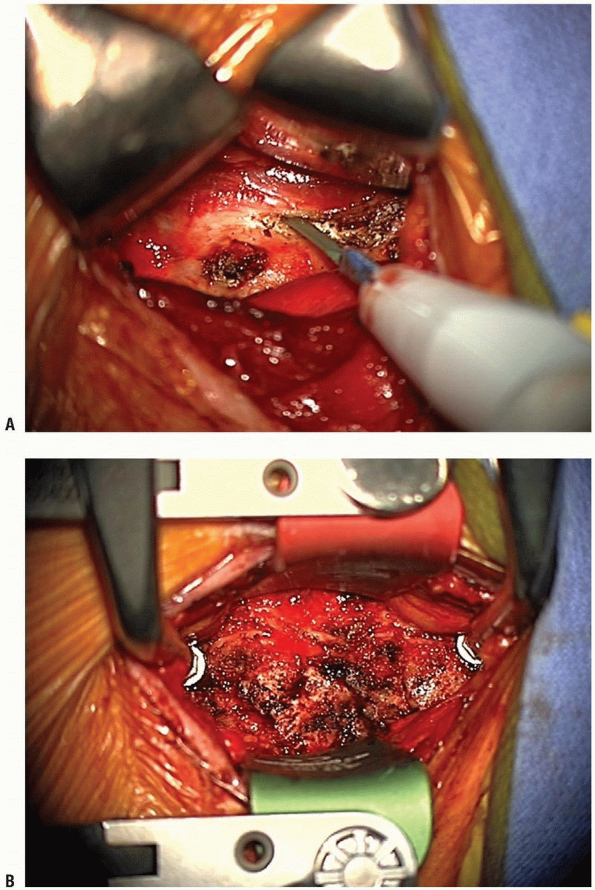
Once the proper levels are confirmed with
imaging, handheld retractors are replaced medially and laterally, and
the localizing needle is removed. Additional fascial clearing may be
necessary above and below the localized segment using the peanut to
uncover the anterior longitudinal ligament. Mark the midline using the
longus colli muscles for orientation. Using the bovie electrocautery,
elevate the longus colli muscles laterally, superficial to the anterior
longitudinal ligament and the annulus. Take care not to use the cautery
aggressively over discs that are not planned for removal, and not to
damage more superficial structures with the cautery. These flaps must
be elevated laterally enough to allow uncovertebral joint
visualization, but do not overexpose laterally or the vertebral
arteries can be violated, especially at the level of the disc. A small
Cobb elevator can be used to elevate the longus colli muscles. Bleeding
can be controlled with bipolar cautery or gel foam. Elevation of the
longus colli in this manner allows self-retaining retractors to be
placed beneath them (Fig. 12-24).
This maneuver protects the sympathetic chain overlaying the lateral
aspect of the longus colli, and also shields the esophagus and trachea
from direct retraction forces. -
Individually place retractor blades
medially and a blunt-tooth blade laterally, with blade tips below the
longus colli, and then attach the self-retractor apparatus. The
anesthesiologist should be asked to deflate the cuff of the
endotracheal tube at this point, and then reinflate it to the lowest
pressure necessary for maintaining a seal. This may help prevent
excessive pressure on the recurrent laryngeal nerve between the
retractor and tracheal cuff. Handheld or self-retractors with smooth
blades can be used in cephalad and cranial positions to improve
exposure. Alternatively, distraction screws may be inserted into the
vertebral bodies in the midline. They may be used to apply segmental
distraction as needed, and they also facilitate the rostral and caudal
exposure. -
Following the decompressive or
reconstructive portion of the procedure, copiously irrigate the wound,
and carefully inspect the medial structures for maintenance of
integrity. If there is any suspicion of esophageal perforation,
methylene blue or indigo Carmen solutions can be injected into the
esophagus by the anesthesiologist. Any leakage should be addressed by
repair with absorbable suture, copious irrigation, and administration
of intravenous antibiotics covering aerobic and anaerobic organisms for
5 to 7 days. If available, consider asking an ENT colleague to inspect
the defect intraoperatively and help manage the patient postoperatively. -
A drain should be placed in the deeper
areas of the wound, and may be brought out through the incision or a
separate site. The platysma is reapproximated with a running 2.0
absorbable suture through its investing fascia, and the subcutaneous
and skin tissue can be approximated according to the surgeon’s
preference. We typically use 2-0 or 3-0 absorbable suture in the
subcutaneous/dermal layer, followed by a subcuticular 4-0 absorbable
suture in the skin and steri-strips. A dry, sterile dressing is applied
and any external immobilization can then be placed.
 |
|
FIGURE 12-15
|
 |
|
FIGURE 12-16
|
 |
|
FIGURE 12-17
|
 |
|
FIGURE 12-18
|
 |
|
FIGURE 12-19
|
 |
|
FIGURE 12-20
|
 |
|
FIGURE 12-21
|
 |
|
FIGURE 12-22
|
 |
|
FIGURE 12-23
|
 |
|
FIGURE 12-24
|
but longer cases or those with greater likelihood of significant edema
should be considered for a day or two of continued intubation. Less
commonly, tracheostomy might be required immediately or in a delayed
fashion if there is more serious airway compromise or concern. Although
there is no direct evidence to suggest antibiotics are effective at
reducing infections beyond the initial preoperative dose, they are
typically given for 24 hours postoperatively. Drains are typically left
in place for approximately 24 hours, but higher outputs may necessitate
longer durations or exploration in cases of persistent bleeding. The
head of the bed can be elevated to 30 to 45 degrees, which can
encourage resolution of edema and hematoma drainage. Dietary
advancement should begin with sips of water or clear liquids, followed
by slow progression of the diet on the first postoperative day.
anterior odontoid screw placement requires a unique trajectory of
insertion. The position of the patient’s head may need to be altered
during positioning to allow for the appropriate screw trajectory. If
the patient has a barrel-chest or large breasts, it may be impossible
to achieve the correct trajectory without compromising the alignment of
the odontoid fragments. The surgical approach must address this
unusually sharp angle of insertion by beginning an anterior approach at
the C5 level. The dissection through the anterior neck structures will
therefore be no different than as described above, however, once the
prevertebral fascia is identified, it will be cleared away from the
C2-3 disc level. The caudal portion of the C2 body must be visualized
and confirmed with lateral imaging using the marker technique described
above.
are uncommon, many patients complain of temporary hoarseness and
swallowing dysfunction. Injury to the recurrent laryngeal nerves (RLN)
can cause major problems with phonation or swallowing, and spontaneous
recovery from neuropraxia is not a certainty. It had been thought that
a left-sided approach may be less likely to result in injury to the
RLN; however, a recent series demonstrated similar rates of RLN
dysfunction in both right and left-sided approaches (18).
A prospective study of RLN function following anterior cervical surgery
suggests that clinically symptomatic injury (hoarseness) occurred with
an incidence of 8.3%. Laryngoscopy demonstrated that the overall
incidence of RLN injury was 24.2%, including those patients with
laryngoscopy-evident asymptomatic dysfunction. Fortunately, at 3 months
postoperatively only 2.5% of subjects continued to have symptomatic
dysfunction (19).
a recent prospective analysis of swallowing after anterior and
posterior cervical surgery, nearly one-half of patients undergoing
anterior surgery suffered clinically significant dysphagia
postoperatively. Most of these patients recovered by 2 to 3 months
postoperatively, but 4 of the 38 patients in the anterior approach
group required up to 10 months of dysphagia treatment (20).
longus colli, and is at risk of injury with aggressive dissection or
retraction above the muscle. It is most vulnerable around C6 as it
courses around the carotid tubercle, and injury can lead to ipsilateral
postoperative Horner’s syndrome (ptosis, meiosis, and anhydrosis).
in close proximity to the exposure and must be carefully protected at
all times. Lacerations should be promptly repaired. Arterial occlusion
can be devastating if the contralateral ICA or vertebral arteries have
high-grade obstruction or the Circle of Willis is incomplete.
Manipulation of these vessels can also cause loosening of
arteriosclerotic plaques, and lead to thromboembolic strokes.
abscesses or mediastinitis if they are not addressed at the time of the
index operation. Subsequent surgical debridements and repairs are
wrought with difficulty and further complications, so scrutiny for
tears during the initial operation is critical.
excessive dissection lateral to the vertebral bodies may cause this
unfortunate event. Pressure at the site of injury can typically control
the bleeding, and collateral flow will often be adequate to prevent
stroke. If it is known that the opposite vertebral artery is occluded,
however, vascular repair or shunting should be considered.
significant airway obstruction, and require the replacement of an
endotracheal tube. Reintubation is difficult in this setting,
especially when the neck cannot be extended. In-line traction, or
occasionally, fiberoptic visualization may be necessary. If these are
unsuccessful, an emergency tracheostomy may be required. Recognizing
and treating the problem early in its course is essential.
fusion procedures, and may serve as the extensile rostral end of a
longer posterior spinal exposure.
occipitocervical region begins with similar principles as utilized in
the more caudal areas of the spine, the unique anatomy requires
distinct technical considerations. Careful attention must be paid to
preoperative positioning so that deeper structures are accessible, and
alignment is optimal in cases planned for arthrodesis.
underlying abnormality, as well as the particular plan for correction.
Standard plain radiographs of the cervical spine should be obtained,
and flexion-extension views can play a critical role in determining
which levels below the occipitocervical junction require additional
exposure. If the possibility of instrumentation exists, preoperative
computed tomography is advised. Using contrast to elucidate the
vascular anatomy provides invaluable guidance for safely placing
hardware into the bony elements.
characteristics of the patient’s upper neck. Skin conditions that may
predispose the patient toward increased infection risk need to be
recognized and treated before proceeding with surgery. Rarely, abnormal
body habitus can complicate the feasibility of performing an adequate
posterior exposure of the occipitocervical junction. This may require
consideration of alternative treatment options or anterior surgical
approaches.
this region of the spine. Once baseline monitoring has been
established, intubation can proceed. Fiberoptic intubation may be
required when strict cervical immobilization must be maintained. Awake
intubation can also be employed for situations in which baseline
neuromonitoring is not available. The pinion is placed so that it
resides a minimum of 1 to 2 cm above the surfaces of the face or nose.
Facial edema that occurs during long surgery in the prone position can
otherwise cause encroachment onto the pinion, and subsequent skin
breakdown may occur. If postoperative halo vest immobilization is
anticipated, use a halo ring in place of the pinion. For procedures in
the posterior cervical spine, it is helpful to have the arms located at
the sides during surgery. A folded sheet can be used to secure the arms
to the body once they are wrapped in gel sheets. The patient is then
placed in the prone position on an operating table, with dependent
areas of the body well-padded. The alignment of the spine must be
adjusted and checked with lateral x-rays or the image intensifier
before prepping the wound. The relationship of the occiput to the upper
cervical spine should be optimized to prevent postoperative swallowing
dysfunction, which may result from fusing the occiput cervical area in
a nonphysiologic position. Subluxed vertebrae can often be realigned
and the orientation of the spine can be manipulated to facilitate
exposure and instrumentation. If cables or wires are to be passed under
the arch of C1, opening the gap between the occiput and atlas will make
the operation easier. If C1-2 transarticular screws are planned, the
trajectory of the screws must be checked during positioning to be sure
the procedure is feasible. Positioning should be performed with the
patient under light sedation to allow a wake-up test following
positioning, to assure maintenance of neurologic integrity.
Reverse-Trendelenberg position of the operating table facilitates
visualization of the occipitocervical junction, and may also reduce
venous congestion and bleeding during surgery.
the external occipital protuberance (EOP), and then laterally out to
the ears. Widely prepare and drape the patient from the mid-thoracic
level to just above the EOP by 2 cm, and to the mid-lateral aspects of
the neck on either side. Tape placed transversely across the back of
the head at the new hairline helps keep longer hair from violating the
sterile area during preparation. The incision is marked in the midline
from just below the EOP to just below the often palpable and prominent
spinous process of C2. We prefer the use of an Ioban sheet for the
operative area at this point.
-
Incision: infiltrate the line of incision
dermally using epinephrine solution with or without local anesthetic,
and make an incision down to subcutaneous fat. Obtain hemostasis with
Bovey electrocautery or self-retractor pressure, and use the cautery to
dissect through the subcutaneous fat in the midline. The nuchal fascia
should come into view during this maneuver, and finger palpation may be
utilized to assure that the incision is in the midline. The C2 spinous
process should be readily apparent as the most prominent upper cervical
vertebra at this point. It is sometimes helpful to gently clear
subcutaneous fat off of the midline nuchal fascia, to aid in
identifying the proper planes during closure. -
Incise the fascia at the midline with
electrocautery, taking care to prevent violation of the C2-3
interspinous ligament, unless this segment is planned for inclusion in
decompression or fusion. Once division of the fascia has been carried
out to the EOP, discern the avascular plane found in the midline.
Finger palpation may help if the plane is otherwise unidentifiable.
Although access to the deeper structures through this avascular plane
is not critical, the reduction in bleeding afforded by this technique
facilitates hemostasis and visualization during surgery. -
Gently palpate the bony anatomy once
again and note the depth of the posterior arch of C1 for orientation.
Electrocautery can be used to incise down to bone on the occiput, as
well as to perform a subperiosteal elevation from the laminae of C2.
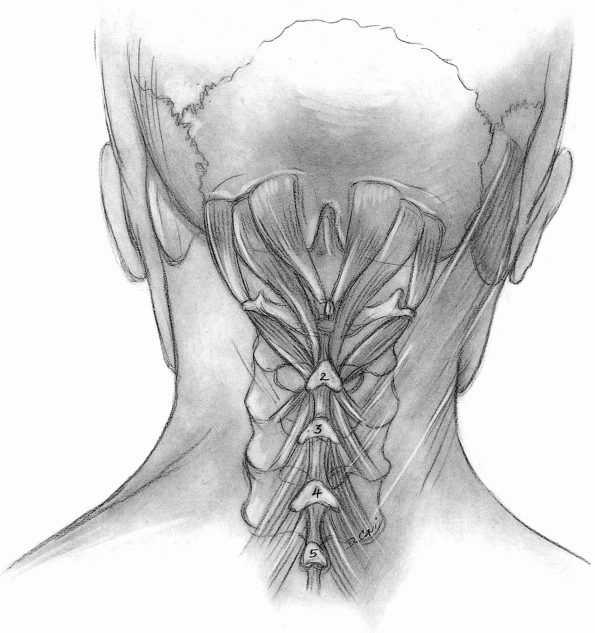
Muscles released from C2 include the rectus capitis major and the
obliquus capitis inferior, but muscles directed caudally from the C2
spinous process (semispinalis cervicis) should be preserved unless
access to the caudal aspect of the C2 spinous process is critical (Fig. 12-25).
Unless the C2-3 segment must be fused, the C2-3 articular capsules
should be preserved. This capsular layer is often wispy and difficult
to recognize, but it plays an important role in posterior stability. If
the semispinalis cervicis must be released, elevate the insertion of
the muscle with a thin piece of bone using an osteotome. The muscle
attachment can then be repaired anatomically and securely at the end of
the case. -
Sharp dissection with a scalpel can now
be carried down to the posterior arch of C1 in the midline. This
maneuver should only be performed if adequate preoperative computed
tomography or intraoperative palpation confirms an intact posterior
ring. Rarely, the posterior ring of C1 can be nonconfluent or absent.
Use a Cobb elevator to raise subperiosteal flaps off of the occipital
bone, and gently from the C1 posterior arch. Extreme caution should be
employed when elevating laterally off of the C1 arch, limiting the
dissection on the cephalad aspect of C1 to 0.8 cm from the midline, as
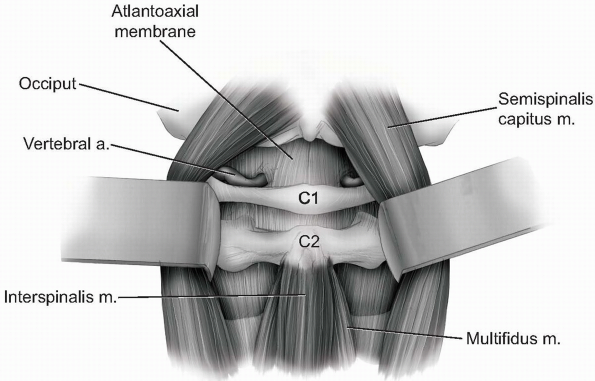
the vertebral arteries can be easily injured beyond this point. -
Continue gentle blunt dissection to lift
paraspinal musculature away from base of the occiput and off of the
atlantoaxial junction superficial to the level of the lamina (Fig. 12-26).
If necessary, the atlantoaxial membrane may be carefully elevated from
the arch of C1 and lamina of C2 with a curette to expose the dura. The
venous plexus surrounding the C2 nerve roots and ganglions become
visible as the C1-2 articulation is approached. -
Under most circumstances we prefer the
use of a drain placed in the subfascial lateral recesses of the wound.
Closure is performed by approximating the nuchal fascia with absorbable
sutures to create a watertight seal. The greater occipital nerve
courses through the fascia and can be injured if the exposure strayed
from the midline or if fascial sutures are placed too far laterally.
Absorbable 2-0 sutures are then applied as a buried dermal layer,
followed by a subcuticular stitching with absorbable 3-0 suture. In
revision cases, we prefer the use of 3-0 nylon sutures for the skin.
 |
|
FIGURE 12-25
|
 |
|
FIGURE 12-26
|
significant facial and cervical edema, which may require continued
intubation following surgery. Depending on the need for additional
postoperative immobilization, the halo ring may be secured to a vest.
If a pinion was used, it should be removed after the patient is
transferred to the supine position. A collar may be applied before the
patient awakens from anesthesia. If necessary, maintaining elevation of
the head above the feet during the first 24 to 48 hours after surgery
can facilitate edema reduction.
posterior cervical or occipitocervical approach, with rates ranging
from 2% to 5% in most series (22, 23, 24, 25).
However, underlying neural and vascular structures are at slightly
greater risk during occipitocervical exposure. The vertebral arteries
are in close proximity to the posterior arch of C1, and can be injured
during subperiosteal elevation of soft tissues from the posterior
surface of the arch. The spinal cord has more limited bony protection
between the occiput and C2, and can be more easily injured during
exposure. Finally, the exiting C2 nerve root does not have overlying
posterior bony protection, and may be at more substantial risk during
mobilization of superficial tissue. In general, complications for the
occipitocervical approach tend to be related to instrumentation
placement, rather than the exposure.
is simply an extension of a similar approach used both rostrally and
caudally in the spine. Indications typically include conditions that
require posterior decompression and/or arthrodesis, such as spondylotic
myelopathy or radiculopathy, and rarely infectious or neoplastic
processes.
may include flexion and extension views to assess for segmental
instability prior to decompression or fusion procedures. In general,
when instrumentation of the cervical spine is planned, we favor
obtaining a CT scan to provide more accurate detail of the bony anatomy.
condition must be carefully assessed preoperatively, so adjustments to
the planned approach can be made accordingly.
changes are present, we prefer to use neuromonitoring during the case.
Neuromonitoring ideally begins with baseline potentials prior to
intubation or postioning of the patient. The patient can then be
intubated, with proper precautions taken in cases of possible spinal
cord compression. Pathologic spinal cord compression may occur under
various circumstances, such as with destabilizing traumatic injury or
spondylotic narrowing of the canal. In these more worrisome situations,
fiberoptic and/or awake intubation should be considered. Patients with
advanced degenerative change and limited preoperative cervical motion
should not be manipulated beyond what they’ve demonstrated possible in
the preoperative clinical setting, unless neuromonitoring is employed
during the positioning. Such caution should be heeded during prone
positioning, as well. Another option for neurologic assessment during
intubation, positioning, or surgery is the wake-up test. In most cases
a pinion can be applied after intubation and prior to prone positioning
on an operating table. If decompression is planned, the patient’s neck
should be placed in the position that provides the greatest space for
the neural elements. The neck position can be carefully changed to
physiologic lordosis after the decompression if a fusion is required.
Slight cervical flexion during the initial portion of the procedure
also tends to decrease the creasing of the skin at the posterior neck
and gives more room for the exposure. All dependent areas of the
patient’s body in contact with the operating table should be well
padded, and for longer cases a urinary catheter is placed. As covered
in the occipitocervical section, the arms are usually kept to the sides
of the patient with well-padded traction straps extending from the
wrists to the foot of the bed. These facilitate intermittent traction
on the arms, which provides radiographic access to the lower cervical
spine on lateral imaging. Reverse-Trendelenberg position of the
operating table reduces venous congestion of neck and epidural
venous
systems, which may decrease bleeding. Because of the anterior position
of the lower cervical spine relative to the upper thoracic spine (as it
begins the lordotic curve), the reverse-Trendelenberg position can also
provide better visualization of C6-T1 from a posterior vantage point.
cephalad direction, the hair should be shaved accordingly. The surgical
field should include the posterior iliac crest sites in the event
autograft bone is planned, and we prefer the use of iodine-impregnated
adhesive sheets over the exposed skin after draping. Palpation of the
midline cervical structures can assist in localizing the incision; the
spinous processes of C2, C7, and T1 tend to be the most prominent. When
small incisions or very limited exposure is desired, fluoroscopy and a
needle can be used to localize the area of interest.
-
Incision: epinephrine solution can be
injected in the subdermal layer along the line of the planned midline
incision to decrease bleeding from the skin edges. Once the nuchal
fascia is identified, sweep the overlying subcutaneous tissue laterally
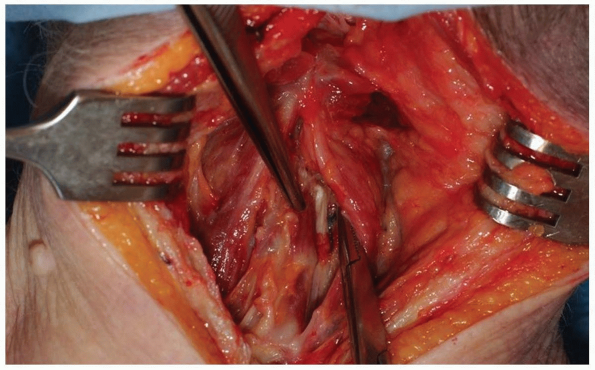
to expose a centimeter-wide stripe of the fascia in the midline. -
Use electrocautery to divide the nuchal
fascia in the midline and elevate the flaps slightly in both lateral
directions from the underlying paraspinal musculature. This will expose
an avascular plane lateral to the interspinalis cervicis muscles on
either side of the spinous process to aid in preserving the
interspinous ligaments (Fig. 12-27).
Once the nuchal fascia has been divided, palpate the spinous processes
for orientation. The most prominent spinous process rostrally is C2 and
the most distal bifid spinous process is usually C6. An intraoperative
radiograph is essential to confirm the level because anatomic
variations are common. Lateral imaging may be satisfactory for
visualizing the upper cervical spine, but can be problematic below C5
in some individuals. It may be necessary to acquire anteroposterior
imaging under these circumstances, using the first thoracic vertebra as
a landmark for discerning the vertebral levels. -
If surgical exposure need only be
performed below C2, the muscular origins of the rectus capitis
posterior major and obliquus capitis inferior should be left intact on
the spinous process of C2. Likewise, the interspinous ligaments between
C7 and T1 should also be left intact, as they are important
biomechanical restraints to subsequent kyphotic deformity. -
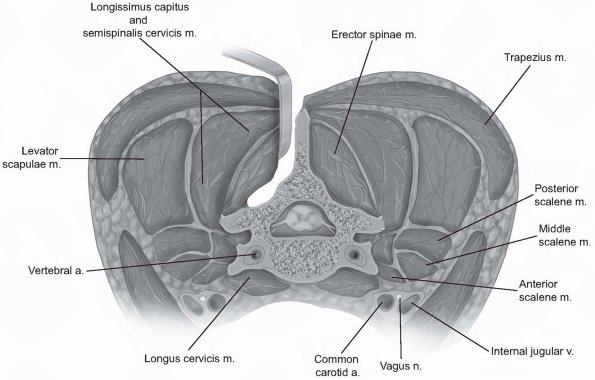
For decompressive procedures alone,
including laminoplasty, the subperiosteal elevation should be carried
laterally only to the facet capsules. The ligamentous capsular tissue
in the cervical region is generally less robust than lower in the
spine; however, maintaining capsular integrity may help prevent
postoperative kyphotic progression. Otherwise, subperiosteal elevation
can be carried out to the lateral margins of the facets/lateral masses.
Gentle probing around the lateral mass edge can be used to assess the
true lateral margin, rather than continuing subperiosteal
electrocautery dissection “around the corner” (Fig. 12-28).
If one persists with elevation beyond the superficial lateral margin,
perforating vessels become more prevalent and bleeding can be
problematic. Dissecting anterolateral to the lateral aspect of the
facets is rarely necessary and may place the exiting nerve roots at
risk of injury. -
Closure of posterior cervical approaches
can be performed with 0-0 or 1-0 absorbable sutures in the nuchal
fascia, followed by 2-0 absorbable suture for dermal and subcutaneous
approximation. The skin can be closed with subcutaneous absorbable
sutures and steristrips, or with nylon sutures. For compromised tissue
following infection or radiation, we prefer to use nonabsorbable
sutures in all layers. A drain may also be placed prior to closure
based on surgeon preference, but avoid placing the drain over any
exposed dura or neural elements.
 |
|
FIGURE 12-27
|
 |
|
FIGURE 12-28
|
procedure may necessitate continued intubation following surgery.
Elevating the head of the patient’s bed 30 to 45 degrees can reduce
excess facial or cervical edema that occurs during prolonged prone
positioning. Postoperative immobilization is individualized.
unintentional damage to the posterior ligamentous complex can lead to
debilitating postoperative deformity, which underlines the importance
of preserving adjacent ligamentous and muscular restraints to such
deformity.
stripping of the paraspinal musculature from the underlying osseous
elements. Such stripping of soft tissue causes more devitalization than
one typically must perform during anterior surgery, which may explain
the relatively higher wound infection rates seen with posterior
approaches. The actual rate of infection with posterior approaches is
difficult to ascertain given the variety of surgeries performed with
them, and the frequently immunocompromised patients in whom they are
often performed. Studies of immunocompetent patients have not
demonstrated marked differences between anterior and posterior
approaches, however (22,24, 25, 26, 27, 28). Several studies of posterior cervical arthrodesis demonstrate infection rates of <5%.
the occipitocervical musculature and ligamentous structures and the
semispinalis cervicis insertion on C2, can lead to development of
post-operative flexion deformity (29). As
mentioned previously, kyphosis may also occur at the cervicothoracic
junction if the interspinous ligament between C7 and T1 is transected,
and the segment is not included in the fusion construct (22,29, 30, 31).
HA, Sen CN. The transoral approach for the management of intradural
lesions at the craniovertebral junction: review of 7 cases. Neurosurgery 1991;28(1):88-97.
MN, Spetzler RF, Sonntag VK. The transoral approach to the superior
cervical spine: A review of 53 cases of extradural cervicomedullary
compression. J Neurosurg 1989;71(1):16-23.
A, McAfee PC, Gastein CD. Anterior retropharyngeal approach to the
upper cervical spine. In: Bridwell KH, DeWald RL, eds. The Textbook of Spine Surgery, 2nd ed. Philadelphia: Lippincott-Raven Publishers, 1997:227-236.
DK, Grevitt MP, Mehdian SM. Hypoglossal nerve injury as a complication
of anterior surgery to the upper cervical spine. Eur Spine J 1999;8(1):78-80.
ER, Caroli E, Ferrante L. Management of the cervical esophagus and
hypofarinx perforations complicating anterior cervical spine surgery. Spine 2003;28(15):E290-295.
WJ, Sweeney CA, Connolly PJ. Recurrent laryngeal nerve injury with
anterior cervical spine surgery risk with laterality of surgical
approach. Spine 2001;26(12):1337-1342.
A, Schramm J, Lehnerdt K, Herberhold C. Recurrent laryngeal nerve palsy
during anterior cervical spine surgery: a prospective study. J Neurosurg Spine 2005;2(2):123-127.
CA, New KC, Pietrobon R, et al. Prospective analysis of incidence and
risk factors of dysphagia in spine surgery patients: comparison of
anterior cervical, posterior cervical, and lumbar procedures. Spine 2004;29(13):1441-1446.
BJ, Follett KA, Traynelis VC. Complications of posterior articular mass
plate fixation of the subaxial cervical spine in 43 consecutive
patients. Spine 1998;23(2): 193-200.
MG, Cooper PR, Errico TJ. Posterior plates in the management of
cervical instability: Long-term results in 44 patients. J Neurosurg 1994;81(3):341-349.
JP, Kitchen ND, Moore AJ, et al. Results of posterior cervical
foraminotomy for treatment of cervical spondylitic radiculopathy. Br J Neurosurg 2000;14(1):40-43.
K, Mehmet T, Ufuk T, et al. Results of surgical treatment for
degenerative cervical myelopathy: Anterior cervical corpectomy and
stabilization. Spine 2004;29(22):2493-2500.
