Slipped Capital Femoral Epiphysis
the displacement of the femoral head relative to the femoral neck and
shaft. The term slipped capital femoral epiphysis
is actually a misnomer. The femoral head is stabilized in the
acetabulum, whereas the femoral neck and shaft move relative to the
femoral head and acetabulum. In almost all cases of SCFE, the proximal
femoral neck and shaft move anteriorly and rotate externally relative
to the femoral head (1). If progression occurs
to the point at which the femoral neck is completely anterior to the
femoral head, then proximal migration of the femoral neck occurs as
well.
the last century. The male population with SCFE outnumbers the female
population by 1.4 to 2.0 in most studies (2,3,4,5,6,7,8,9,10,11).
The annual incidence is 2 to 13 per 100,000 and the cumulative risk is
between 1 per 1000 and 1 per 2000 for the male population and is
between 1 per 2000 and 1 per 3000 for the female population (8,12,13,14). Incidence of SCFE varies significantly among different populations,
with higher incidences in those groups with higher mean body weights (15).
Loder has noted more than a 40-fold difference in the incidence among
differing races, with the highest rate being found in Polynesian
children and the lowest rate being found in children from the
Indo-Mediterranean region (15).
an average age of 12±1.5 years for girls and 13.5±1.7 years for boys in
an international study carried out with more than 1600 patients (15).
At the time of presentation, approximately 80% of the boys are reported
to be between 12 and 15 years, and 80% of the girls between 10 and 13
years (8). Onset of SCFE is unusual for
children of either sex less than 10 years old and for girls older than
14 and boys older than 16. Diagnosis of SCFE in such patients should
raise the orthopaedist’s suspicion that an underlying metabolic or
systemic condition may have played a causative role.
been reported to be significantly narrower than the range of their
chronologic age (11,16,17). Most of the children with SCFE have open triradiate cartilage and are Risser 1 (18).
SCFE at the time of presentation, with left hip involvement in most
unilateral cases (12,13,15,25,26).
In addition to the 20% who initially present with bilateral SCFE, 10%
to 20% develop a symptomatic contralateral slip in adolescence (6,13,27,27,28,29).
Long-term studies have reported radiographic evidence of a long-term
bilateral involvement in as many as 80% of the patients (30), although most series report bilateral involvement at long-term follow-up in the 60% range in adulthood (13,28).
in the incidence of SCFE at latitudes above 40 degrees, but not in
lower latitudes (31,32,33). Others have not noted any seasonal variation (13). Such data appear to have little impact on the diagnosis and treatment of children with SCFE.
peripubertal children. Although any child presenting with hip, groin,
thigh, or knee pain must be evaluated for possible hip pathology, the
orthopaedist should be particularly suspicious of the possibility of
SCFE in overweight, peripubertal children.
unknown. Regardless of the underlying etiology, the final common
pathway appears to be a mechanical insufficiency of the proximal
femoral physis to resist the load across it (34).
SCFE may be thought of as occurring because of physiologic loads across
an abnormally weak physis or abnormally high loads across a normal
physis.
abnormalities, systemic diseases (such as renal osteodystrophy), and
previous radiation therapy in the region of the proximal femur (35–40).
Multiple mechanical factors have been postulated to account for
abnormally high loads across the proximal femoral physis in children
with SCFE, including obesity and anatomic variations in the proximal
femoral and acetabular morphology.
These effects appear to be secondary to the impact that these hormones
have on physeal width since mechanical strength of the physis varies
inversely with physeal width (41,43,44).
SCFE cases, and SCFE has been estimated to be six times more common in
patients who have an endocrinopathy than in those who do not (35,36,37,38,39,40,45,46,47,48,49,50,51,52).
Although one recent study showed frequent endocrine abnormalities, most
investigators have been unable to demonstrate consistent abnormalities
in most children with SCFE (22,24,53,54).
are hypothyroidism, panhypopituitarism, growth hormone (GH)
abnormalities, and hypogonadism (35,36,37,38,39,40,45,46,47,48,49,50,51,52). Other endocrine causes of SCFE include hyperparathyroidism or hypoparathyroidism (35,38,55).
The increased prevalence of hypothyroidism in children with Down
syndrome is a likely explanation for the increased risk of SCFE in
these children (56,57,58).
The initial diagnosis of hypothyroidism is often made after the
diagnosis of SCFE; in most children with SCFE and GH deficiency, the
endocrine abnormality is known prior to the diagnosis of SCFE (35).
the time of puberty. It may be that the abnormalities in the complex
interplay of hormones at puberty puts their hips at risk for SCFE (24,62). Laboratory studies in rats have also shown a decreased physeal strength at puberty (63).
is relatively low, previous authors have recommended against the
routine screening of patients with SCFE without clinical evidence of an
endocrinopathy (53). Burrow et al. reported
that a person’s height below the 10th percentile was the only useful
screening characteristic for endocrine abnormalities; the sensitivity
and the negative predictive value of using height below the 10th
percentile as a cutoff were each reported to be at least 90% (46).
screening of all patients with SCFE for any potential endocrine disease
is not warranted. For children with suspected endocrine disease
(including those who are younger than 10 years or older than 15 years
and those who are of short stature), thyroid function tests should be
carried out. GH levels should be checked for children of short stature.
It is important to remember that most children with SCFE and thyroid
dysfunction have no known history of any thyroid dysfunction at the
time of presentation with SCFE. Among other children with both
endocrinopathies and SCFE, the underlying endocrine disorder is often
known prior to the diagnosis of SCFE.
The absolute risk of SCFE in patients with previous radiation therapy
is unknown, although a risk as high as 10% has been cited (64).
Unlike the typical patient with SCFE, children with SCFE following
previous radiation therapy have been reported to have a median weight
at the 10th percentile (65).
The incidence of SCFE has been reported as 0.03 to 0.64 per 1000
person-years among patients with end-stage renal disease receiving GH,
with the highest rates in those patients who were on dialysis and
receiving GH (66). Patients with renal osteodystrophy and SCFE are noted to be small in both weight and height (67).
osteodystrophy is due to secondary hyperparathyroidism in these
children, and medical management of the secondary hyperparathyroidism
is of primary importance (67). If the
hyperparathyroidism is controlled, slip progression will become rare,
and surgical stabilization may not be necessary (67).
Unlike the situation in other causes of SCFE, the displacement in
patients with renal osteodystrophy is often through the metaphysis (35%
of reported SCFE in one series), and other epiphyses have also been
known to displace (67,68,69).
Bilateral involvement has been reported in 82% to 95% of the patients
with SCFE and renal osteodystrophy in large series studies (67,69).
That many of these so-called SCFE cases do not occur through the physis
may partly be the reason for the poorer results in the treatment of
SCFE in children with renal osteodystrophy.
component of complement have previously been reported in patients with
SCFE (70). In patients with chondrolysis, serum immunoglobulin M (IgM) level was elevated as well (70).
More recent studies have failed to show such abnormalities in serum
levels, although synovial fluid abnormalities were noted in patients
with SCFE (71,72). One study reported that plasma cells were a significant component of the synovitis in SCFE (71). In the same study, two of three patients with IgG and C3 present on synovial immunofluorescence developed chondrolysis (71).
A later study revealed the presence of immune complexes in the synovial
fluid in 10 of the 11 hips with SCFE (91%), but not in 2 of the 21
joints without SCFE (10%) (71,72). The role of these immune complexes in SCFE has not been defined.
established. Among the patients with SCFE, a second member of their
family has been reported to be affected in 3% to 7% of the cases in
most series of studies carried out (11,21,29,73,74,75,76,77,78,79,80). SCFE has been reported in identical twins (73,75,81), and has been found to have autosomal dominant inheritance with variable penetrance in familial cases (79,80).
Whether this is due simply to a genetic predisposition for SCFE or due
also to a tendency toward other risk features (such as obesity) remains
unclear (79,82).
the etiology of SCFE. Anatomic risk factors in the proximal femoral and
acetabular morphology have been described. The high incidence of
obesity in this patient population also suggests a mechanical role in
the etiology of SCFE.
ante-version has been reported, and this has been attributed to
increased shear force across the proximal femoral physis in such
patients (85,86). Anteversion values of the unaffected hips in the same patients were closer to normal (85).
patients with SCFE compared to the hips of unaffected persons has also
been reported (86). Such a decrease in the
neck–shaft angle results in a more vertical physis, which may increase
the shear force across the physis. Proximal femoral physeal inclination
has previously been shown to change significantly between the ages of 9
and 12 years in humans, which is a potential contributing factor for
SCFE (88). In the laboratory, the shear strength has also been shown to vary with physeal inclination (44).
forces across the physis may be exaggerated, especially at the extremes
of the range of motion. Variability in acetabular depth has been
suggested as a potential cause for differences in the incidence of SCFE
among different races. A recent study of acetabular morphology in
patients with trauma calls this finding into question (90).
It is possible that this study did not find such a correlation either
because of limited sample size and/or because SCFE may simply be
occurring in a small subset of the population who are outliers
regarding such measures as acetabular depth.
acetabular morphology in the affected and unaffected hips of children
with SCFE (91). The lack of such acetabular
differences is likely because SCFE generally occurs at an age at which
little potential remains for acetabular remodeling, and this may help
explain the high incidence of bilateral SCFE. Such bilateral acetabular
symmetry in those with unilateral SCFE suggests that even if increased
acetabular depth is a risk factor, there must be other etiologic
factors involved as well.
the femoral head during gait can be 6.5 times body weight and that such
forces may be enough to cause a SCFE in an obese patient with a normal
physis (92). Other authors have confirmed that
mechanical forces across the hip during normal activities such as
running are great enough to potentially cause SCFE (93).
and is likely to be multifactorial. Endocrinopathies, other systemic
diseases and local abnormalities (such as those caused by previous
radiation exposure) have been noted to result in an increased risk of
SCFE. Studies carried out on humans and animals indicate that such an
increased risk of SCFE appears related to the impact that these
maladies have on the strength of the growth plate. The association of
hypothyroidism in children with Down syndrome and of secondary
hyperparathyroidism in those with renal osteodystrophy explains the
sometimes unclear risk profile of SCFE in certain groups of patients.
Subtle abnormalities of hormonal balance at the time of puberty may
also be partially responsible for SCFE in children without any definite
systemic or hormonal abnormalities.
in the development of SCFE. Clearly, systemic and local factors alone
cannot explain all the cases of SCFE because many patients with the
aforementioned abnormalities do not develop a SCFE. In addition, most
patients with SCFE provide evidence of increased forces across the
proximal femoral physis due to one or more potential causes, including
obesity and variations in the proximal femoral and/or acetabular
morphology.
temporal basis. Chronic slips are those causing symptoms for a period
of at least 3 weeks, whereas acute slips are those that are symptomatic
for less than 3 weeks. Acute-on-chronic slips are those with an acute
exacerbation of the symptoms following a prodrome of symptoms of at
least 3 weeks’ duration. Chronic slips appear to account for 80% to 90%
of all SCFE (2,15,94,95,96).
Although not part of the preceding scheme, a “pre-slip” has been
defined as a symptomatic hip with evidence of physiolysis prior to true
movement of the femoral neck relative to the femoral head.
An unstable SCFE was defined as occurring in an extremity upon which
the patient could not bear weight either with or without crutches. With
a stable slip, the child is able to bear weight on the involved
extremity. Unstable SCFE account for 50% to 60% of acute SCFE and for
5% to 10% of all SCFE (95,97,98,99).
This classification of SCFE based on stability has largely supplanted
the aforementioned temporal classification scheme because of its
improved ability to predict both osteonecrosis (ON) and poorer
outcomes. Whereas ON is usually reported in 10% to 15% of acute SCFE,
Loder et al. reported ON in 47% of unstable SCFE and 0% stable SCFE in
their landmark paper (97). Even in cases of acute SCFE, only the unstable subset appear to be at significant risk for ON and a poor outcome (97,100).
pain, limp, and decreased range of motion of the hip. Hip or groin pain
in an obese, peripubertal child is highly suggestive of SCFE. However,
hip pain is absent in as many as 50% of the children with SCFE,
including up to 8% with a painless limp (101). Pain is localized to the knee and/or distal thigh in 23% to 46% of cases (4,6,101,102).
Previous studies have noted that distal thigh and/or knee pain often
result in significant misdiagnosis of SCFE, delay in diagnosis,
unnecessary radiographs, increased slip severity, and sometimes in
unnecessary knee arthroscopy (4,6,20,76,101,102,103). These findings indicate the importance of examining the hip in all children presenting with distal thigh and/or knee pain.
Although patients report a specific inciting event as the cause of pain
in approximately 50% of cases, severe trauma is rarely reported (101).
Even when trauma is reported, further questioning often reveals a
history of pain for weeks or months preceding the inciting event.
with unstable SCFE present with an acute onset of severe hip pain in
the absence of prodromal symptoms (15,96,105). Such SCFE often follow mild trauma.
Short stature (height less than the 10th percentile) has been reported
to be an indicator of increased risk for underlying systemic disease in
children with SCFE (46). Loder and Greenfield
noted that SCFE due to an underlying cause (such as underlying systemic
disease or previous radiation exposure) was much greater in children
older
than 16 years and/or those who were below the 50th percentile for weight at the time of presentation (106).
pain, care must be taken to evaluate both hips. The physician needs to
be persistent when asking about symptoms in both hips, because a child
often initially complains of only the more symptomatic hip in cases of
bilateral SCFE.
the observational gait analysis when the child walks into the examining
room. The limp in children with SCFE is due to several gait deviations.
Hip abductor weakness commonly manifests as a trunk lean to the
affected limb in stance (Trendelenburg gait). If there is marked pain,
an antalgic gait (decreased stance phase on the affected limb) will be
present as well. Finally, because of the external rotation of the
femoral neck and shaft (relative to the femoral head), the foot and
knee progression angles on the affected side are often markedly
external. Children with unilateral involvement have significant
asymmetry of foot and knee progression angles with a unilateral
Trendelenburg gait, whereas children with bilateral SCFE present with a
more “waddling” gait bilaterally, and bilateral external foot and knee
progression.
hips—including the rotational profile of the hips—should be measured
and compared. Hip flexion to 90 degrees is unusual, and hip flexion
contractures are common. Because hip flexion and extension are both
lost, there is significant diminution of the sagittal arc. Hip
abduction is significantly limited both actively and passively, and the
hip abductors are weak.
anatomy and the synovitis that accompany SCFE. Loss of the hip internal
rotation is combined with preservation of (or even an increase in)
external rotation. With a SCFE, the hip will automatically fall into
external rotation (so-called obligate external rotation) as it is
progressively flexed. Obligate external rotation of the hip(s) is
essentially pathognomonic for SCFE. In cases of unilateral SCFE,
comparison with the rotation of the contralateral hip clearly
demonstrates this change in the arc of motion. In bilateral SCFE, both
hips will demonstrate this shift toward external rotation.
years who presents with a limp and pain in the groin, hip, thigh, or
knee should be considered to have a SCFE until proven otherwise.
Diagnoses such as pulled groin muscles are rarely correct in children,
although such misdiagnoses are still commonly made in children with
SCFE. The index of suspicion for the diagnosis of SCFE is markedly
increased in obese, peripubertal children with a limp, external foot
progression, and pain in the groin, hip, thigh, or knee. The index of
suspicion is also very high in patients with a known history of
endocrine abnormalities and in those with underlying diseases
associated with endocrine abnormalities, such as Down syndrome and
renal osteodystrophy.
each hip should be obtained to confirm the diagnosis of SCFE. Because
of the high frequency of bilateral SCFE, bilateral imaging has been
recommended for decades (20,107,108).
In an unstable, acute SCFE, a lateral view is not obtained
preoperatively in order to avoid causing pain and because of the
potential for displacement of the SCFE.
of the physis may be the only radiographic findings prior to, or with
minimal, displacement of the femoral neck and shaft relative to the
femoral head. Cowell noted that the displacement may not be evident in
14% of the anteroposterior views (101). Another
common finding on the anteroposterior view is a decreased height of the
capital femoral epiphysis when the epiphysis lies posterior to the
femoral neck. As slipping progresses, the metaphysis appears
progressively more lateral relative to the acetabular teardrop, and an
increased radiodensity of the proximal metaphysis (the so-called
“metaphyseal blanch”) may be noted (109). Osteopenia of the affected hip is common as well.
degrees of slip. With increased magnitude of slipping, the SCFE becomes
evident on the anteroposterior view as well. Normally, a portion of the
femoral head lies lateral to Klein’s line (a line drawn along the
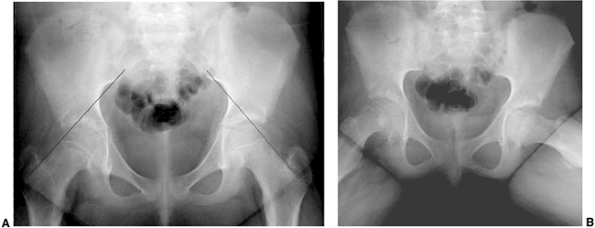
lateral border of the femoral neck) (108) (Fig. 26.1 A,B).
A SCFE is present if the Klein’s line lies cephalad to the femoral
head, or if the amount of femoral head cephalad to the Klein’s line is
less than is seen for the contralateral hip.
reliable than frog lateral views in the assessment of SCFE, which may
be due to difficulties with the positioning of these children (110,111).
However, using a femoral model, Loder reported that an accurate
representation of the SCFE was obtained with either cross-table or frog
lateral views when the femur is rotated externally by 30 degrees or
less (112). The value of other specialized views, such as the Billing lateral, is still being debated (112,113).
of femoral head displacement as a percentage of the femoral neck
diameter, and was first described by Wilson in 1938 (20). Slips have been categorized as mild (less than 33%), moderate (33% to 50%), and severe (more than 50%) (6,21).
Although frequently used, this measurement can be inconsistent because
of variations in patient positioning and can change over the passage of
time because of proximal femoral remodeling. This measurement should
therefore be used only in the evaluation of SCFE prior to remodeling (114).
proximal femoral physis and the femoral shaft, the so-called
“head–shaft” angles, on both anteroposterior and
lateral radiographs (115).
The difference between these two angles obtained at the affected and
unaffected sides determines the degree of abnormal alignment, and are
often referred to as Southwick angles. The
lateral view gives an indication of posterior angulation. A difference
of less than 30 degrees has been deemed mild, a difference of 30
degrees to 50 degrees moderate, and more than 50 degrees is deemed as
severe (116).
 |
|
Figure 26.1
Radiographs of a 12-year-old boy with 3 months of hip pain show typical findings of a slipped capital femoral epiphysis (SCFE). A: Anteroposterior view demonstrates physeal widening, osteopenia, decreased epiphyseal height, increased metaphyseal-teardrop distance, and asymmetry of Klein’s line. B: Although many of these features are seen on the anteroposterior view, the most striking feature is how much more easily the displacement is seen on the frog lateral view. The importance of obtaining lateral views when evaluating for SCFE cannot be overemphasized. |
femoral neck, the so-called “head–neck” angle, may be measured but is
less reliable because remodeling adjacent to the SCFE may artificially
decrease this number in the absence of clinically significant changes
in femoral version.
children with SCFE. However, additional imaging may be warranted in
special circumstances, such as in the evaluation of a presumed
“pre-slip” in a child with normal radiographs, or in the early
evaluation of a patient with SCFE at risk for ON.
If a child presents very late in the course of SCFE, a CT scan may be
useful in determining whether sufficient physeal closure has already
occurred, thereby potentially precluding the need for an in situ
fixation. A CT scan may also be helpful postoperatively in determining
whether any hardware used during surgery has accidentally penetrated
the joint surface. This is particularly true in the case of femoral
head collapse in association with ON of the femoral head.
currently appears to have little use in the routine evaluation of
patients with SCFE (119,120,221,122). Previous studies using ultrasound images have indicated the presence of effusion in 42% to 60% of patients with SCFE (121,122).
In experienced hands, ultrasound may have a role in confirming a
suspected case of SCFE in the absence of any radiographic findings, but
magnetic resonance imaging (MRI) is more commonly used in such
situations.
hips of patients who are presumed to have SCFE but have normal
radiographs, and MRI may also be used for the early detection of ON.
The MRI findings in SCFE have been well described (118,123,124,125).
Physeal widening, osseous edema adjacent to the physis, and the
anatomic deformity associated with SCFE are typically seen, with the
findings of physeal widening and irregularity as well as osseous edema
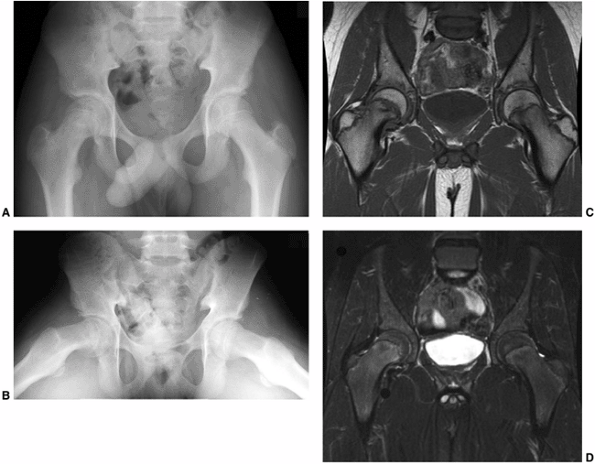
adjacent to the physis seen in cases of “pre-slips” (125). In a child with suspected SCFE and normal radiographs, MRI is useful in determining whether a pre-slip is present (Fig. 26.2). Currently, MRI scanning is rarely used in evaluating patients with evident SCFE.
order to evaluate for the presence of ON, as well as its extent and
distribution if present. Unfortunately, metal artifact may
significantly interfere with MRI signals. The findings of ON seen on
MRI scans have not been correlated with subsequent radiographic
findings and the clinical course of the affected hips.
 |
|
Figure 26.2
A 12-year-old boy presented with pain in the right hip for two months. On further questioning, he reported some vague, intermittent symptoms in the left hip. Physical examination revealed pain in the right hip and obligate external rotation, but no such findings on the left. A, B: Anteroposterior and frog pelvis views at the time of presentation. A right slipped capital femoral epiphysis (SCFE) is evident, without definite plain radiographic changes on the left. C, D: Because of the vague left hip symptoms, magnetic resonance imaging (MRI) was done to rule out a left SCFE. MRI demonstrated physeal widening and irregularity (T1: flip angle 90, TR 700, TE 18) (seen best in C) and signal change on the right, mostly in the metaphysis in this case (fat saturation: flip angle 90, TR 4500, TE 75.37) (best seen in D), without any definite abnormalities on the left. Only the right hip underwent in situ fixation because of the normal physical examination and the lack of considerable MRI findings in the left hip. The patient denied ongoing pain in the left hip until nine months following in situ pinning of the right hip. He then had progressive pain in the left hip and re-presented to the orthopaedist one month later, at which time a mild left SCFE was noted and in situ fixation of the left hip was performed. |
in potential cases of ON of the femoral head, with decreased uptake
being evident in cases of ON. Multiple studies have reported the
utility of bone scanning in the detection of ON in SCFE (121,126,127).
Sensitivity in detecting ON has been 100% in several series, although a
false negative bone scan has been reported in a child who went on to
develop mild ON (121,126,127,128).
they are also associated with false positive results (i.e., an abnormal
bone scan in a hip that does not develop ON). In two series,
false-positive bone scans have been reported in one of the six (17%)(127) and two of three (67%) hips that were imaged (121).
acetabulum, the slip is best thought of as a slip of the proximal
femoral neck and shaft relative to the femoral head. In children
younger than 3 years, the perichondral ring imparts significant physeal
stability, whereas the mammillary processes of the physis are primarily
responsible for increasing physeal shear strength thereafter (92).
The mechanical patterns of physeal fracture and the zone through which
physeal shear causes fractures has been shown in rabbits to vary with
increasing age and with the direction of loading (129,130).
In most cases, the proximal femoral neck and shaft migrate anteriorly
and rotate externally, although slips have been noted to occur in other
directions (131,132). Previous authors have confirmed this anatomy and suggested a torsional force as the cause of acute SCFE (133).
With progression of the slip, the femoral neck may come to lie
completely anterior to the femoral head. When this occurs, proximal
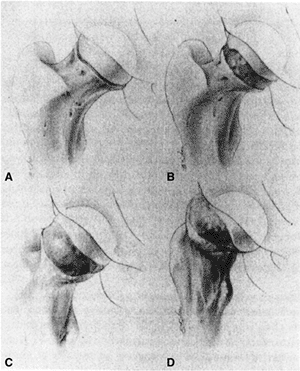
migration of the proximal femur is possible (Fig. 26.3).
However, most SCFE do not appear to progress to this point, and the
apparent varus seen radiographically has been attributed to parallax (134,135).
Degenerative changes, including cyst formation, may be seen in the
anterior femoral neck and/or acetabulum because of impingement of the
anterior femoral neck against the acetabulum during hip flexion, and
such changes may be evident within years of the diagnosis of SCFE.
He reported that as the slip angle increases, progressively greater
external hip rotation is necessary to avoid anterior impingement of the
proximal femoral metaphysis against the acetabulum during gait. Such
levering can damage the anterosuperior acetabular cartilage and/or
cause posterolateral labral injuries (136,137,138,139).
Intraoperative evaluation by other authors has confirmed the mechanical
impingement of the metaphysis against the superomedial acetabulum, with
resulting cartilage and labral damage (140). Femoracetabular impingement has been suggested as a cause of idiopathic arthritis as well (141).
As noted by Rab, as the proximal femur remodels and motion returns
toward normal, an increasing portion of the remodeled metaphysis
becomes an intraarticular weight-bearing surface, potentially
contributing to late osteoarthritis (OA) (136).
 |
|
Figure 26.3 Pathoanatomy of SCFE is demonstrated.A: No displacement is seen. B:
Rotation of the proximal femoral neck, with the femoral head (which is anchored in the acetabulum) posterior relative to the femoral neck. C: Progressive external rotation, with progressive posterior relation of the femoral head to the femoral neck. D: Proximal migration of the femoral neck due to the markedly posterior relation of the femoral head to the femoral neck. (From Morrissy RT. Principles of in situ fixation in chronic slipped capital femoral epiphysis. Instr Course Lect 1989;38:257–262, with permission.) |
Subsequent authors have confirmed the columnar disorganization with
cartilage cell clumping in the physis, metaphysis, and epiphysis (148,150). Groups of cartilage cells have been noted between metaphyseal trabeculae (146,148,150). Collagen fibrils are markedly diminished in the hypertrophic zone (148). The resting zone is essentially normal (146,148).
The proliferative zone has less densely packed collagen and increased
disorganization, with ground substance replacing the normal
chondrocytes. The hypertrophic zone is much larger than usual (up to
80% of the physeal width in comparison to 15% to 30% in normal physes)
with marked disorganization, increased ground substance, and
significant staining for glycoproteins (146,148).
Cell degeneration and death have been noted in the proliferative and
hypertrophic zones (142–144). The slip occurs through the proliferative
and hypertrophic zones of the physis in an irregular pattern (62,146,148). Histologic sections of the physis in SCFE before and after in situ
fixation demonstrate a return to a more normal architecture following
fixation; such findings have been postulated to indicate that
mechanical stabilization of the physis, with removal of the abnormal
shear forces across the physis, allows at least a partial reversal of
the pathology seen with SCFE (145).
complications associated with SCFE, and understanding the proximal
femoral blood supply is important in attempting to minimize the
frequency of this complication. The blood supply of the proximal femur
can be divided into the intraosseous and extraosseous components, as
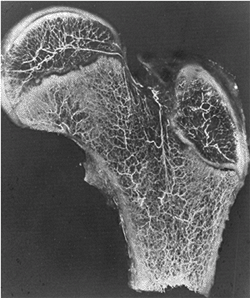
has been well documented by Crock and subsequently by Chung (151,152) (Fig. 26.4).
Chung noted that these components are present in an individual at birth and persist without significant change into adulthood (151).
In cases of SCFE, the blood supply can be disrupted because of the SCFE
itself (especially in cases with unstable SCFE), and it may also be
compromised at the time of surgery.
 |
|
Figure 26.4
A coronal section demonstrating vascularity of the proximal femur in a 13-year-old boy. Part of the vascular ring is visible at the base of the femoral neck, giving rise to the ascending cervical arteries which then enter the femoral head and supply blood to the superior head. (From Crock HV. A revision of the anatomy of the arteries supplying the upper end of the human femur. J Anat 1965;99:77–88, with permission.) |
disruption of the blood supply, which may occur because of displacement
at the time of injury or at any time prior to operative fixation.
Angiography performed in 12 patients with SCFE preoperatively showed
filling of the superior retinacular artery in all seven stable slips
and in only two of the five unstable slips (153).
In one of the three unstable SCFE without preoperative filling of the
superior retinacular artery, postoperative angiography demonstrated
appropriate filling (153).
be disrupted in acute SCFE and has been well described. An arterial
ring at the base of the femoral neck gives rise to ascending cervical
arteries which penetrate the hip capsule and provide circulation to the
femoral head, neck, and greater trochanter (151,152).
The arterial ring at the base of the femoral neck consists of the
lateral femoral circumflex artery, which runs anteriorly and
constitutes the anterior portion of the arterial ring, and the medial
femoral circumflex artery, which travels posteriorly and constitutes
the medial, lateral, and posterior portions of the ring. The ring is
most commonly incomplete, without communication between the branches
from the medial and lateral circumflex arteries.
vessels) arise from each portion of this extracapsular arterial ring
and penetrate the hip capsule to enter the hip joint. The numerous
branches from the lateral ascending cervical artery (which branch from
the medial femoral circumflex artery) provide circulation to the
greatest portion of the femoral head and neck. After penetrating the
hip capsule, the ascending cervical arteries form a second arterial
ring which is also usually incomplete. This intraarticular, subsynovial
ring is smaller than the extracapsular ring and is located at the
border between the articular surface of the femoral head and the
femoral neck. These subsynovial vessels are consistently present
medially and laterally and less commonly present anteriorly and
posteriorly. The epiphyseal branches of these vessels cross the physis
on the surface of the femoral head, enter the perichondral ring, and
then cross into the epiphysis.
proximal femoral osteotomies or the internal fixation of SCFE. The
ascending cervical arteries penetrate the intracapsular femoral neck,
with different vessels supplying the metaphysis and epiphysis (151,152,154).
The intraosseous blood supply of the femoral head is mainly located in
its posterior and superior portions, with potential implications for
the positioning of hardware (151). The extent
of anastomoses between these vessels and the arterial branches of the
ligamentum teres (which supply the medial third of the femoral head)
appears to be quite limited (151,152,154).
hip is one of progressive displacement, followed ultimately by
stabilization of the slip and physeal closure. Although all slips must
eventually cease progressing, the timing of cessation and the degree of
the slip prior to cessation and physeal closure are unpredictable. Most
slips progress slowly, although some may have significant, acute
progression. The hips with such acute progression are the ones at the
highest risk for significant complications.
It is probable that this frequency will further increase with the
increased awareness of the frequency of bilateral involvement and with
the ongoing improvements in the imaging of SCFE.
SCFE cases are diagnosed within 18 months of the diagnosis of the first
slip, with 66% to 81% being diagnosed in the first year (15,25,116,155).
The average duration between the diagnosis of the first and second
slips in metachronous bilateral SCFE has been reported as 1.0 ± 0.8
years (15). Contralateral slips have been reported as late as 4 to 5 years following the initial SCFE (6,15,21).
These data suggest that if 20% of the patients present with bilateral
SCFE, then half of the 80% who present with unilateral SCFE will
ultimately have a contralateral SCFE.
endocrinopathies and SCFE have bilateral slips, although metachronous
involvement is common (35,52).
Because of this significant short-term risk, prophylactic pinning of
the contralateral hip is recommended in patients with SCFE and
endocrine disease (35,52).
of OA, poorer results being associated with an increasing degree of
SCFE (9,116,156,157,158,159).
Hagglund et al. reported radiographic evidence of OA in 27% (28 of 104)
of hips with SCFE at long-term follow-up (mean follow-up: 33 years)
compared with 9% of control hips (9 of 101) (28).
Carney and Weinstein reported a long-term follow-up (mean follow-up, 41
years) of 28 patients with 31 untreated SCFEs (between 1915 and 1952)
and correlated the degree of the slips with radiographic and clinical
scores (157). Patients with mild slips fared
better than did those with moderate and severe slips with regard to
radiographic changes and Iowa hip scores. At long-term follow-up, Iowa
hip scores were at least 80 in all 17 hips with mild slips and in 9 of
the 14 hips (64%) with moderate or severe slips. There was radiographic
evidence of OA in 64% (9 of 14) of the mild slips and in 100% (13 of
13) of the moderate and severe slips.
(mean follow-up, 37 years) of 49 cases of SCFE that did not undergo
primary treatment (159). They reported that
only “a few” patients had restrictions regarding their work or social
lives and that only 2 of 49 (4%) had required surgery for arthritis.
Limb length discrepancy (LLD) of at least 2 cm was noted in 31% of the
cases. The authors also noted that these results were far superior to a
comparable group of patients treated with closed reduction and casting.
Jerre noted superior results in untreated patients in Sweden as well (29).
A cadaveric study noted “post-slip” morphology in 8% of the skeletons
and showed that OA was associated with such morphology (165).
have been reported as having a stigma of pediatric hip disease, such as
a “pistol grip” deformity. Murray reported an apparent association with
SCFE in 40% of the adult hips thought to have degenerative arthritis as
evidenced by the so-called “tilt deformity” of the femoral head (166). Stulberg et al. reported such deformity in 40% of patients with hip OA and no previously diagnosed hip disease (167). Stulberg et al., however, noted that the “tilt deformity” did not appear to be unique to SCFE (167). Resnick has suggested that the “tilt deformity” is not due to SCFE, but is due to the remodeling of the osteoarthritic hip (168).
unilateral disease, an additional 10% to 20% develop a contralateral
slip during adolescence, and 60% of the patients have bilateral SCFE
which is evident at long-term follow-up. In all the cases of SCFE, OA
appears to result, with worse slips being associated with increased
rates and severity of the OA. Although SCFE leads to late degenerative
changes, most hips function well into their 5th decade or later.
admitted to the hospital and is confined to bed until surgery is
performed, as has been recommended for decades (20).
Under no circumstances should the child be allowed to bear weight once
the diagnosis of an acute/unstable SCFE is made, as it may result in ON.
prevention of further slipping, and avoidance of complications.
Although attention is often focused on the affected hip, care of the
unaffected hip (either through careful observation or through
prophylactic treatment) cannot be forsaken.
with our understanding of this disease. Increased vigilance and
enhanced imaging allow the early detection of SCFE, and percutaneous
fixation techniques allow for short hospital stays (or even outpatient
surgery). With these enhancements in care, one recent study comparing
treatment of children with SCFE at a pediatric hospital to the
treatment given at a general hospital reported shorter hospital stays
and lower hospital charges at the children’s hospital (169).
risk of OA, with the risk increasing along with the increase in the
degree of slip. In some cases, the outcomes of SCFE (treated or
untreated) are so poor that salvage treatment by arthrodesis or
arthroplasty may be needed.
femoral deformity. In the past, use of manipulation in the case of a
SCFE has been described with a variety of treatments, including spica
casting and internal fixation. There is no role for any forceful
manipulation in the treatment of SCFE, and many authors have long
cautioned against forceful
manipulation (1,128,170).
In a study of four patients with SCFE and treated with manipulation,
Jerre et al. noted poor results at long-term follow-up in all four; two
had to undergo salvage surgery, and the other two had poor clinical hip
scores (170).
following the forceful manipulation/reduction of SCFE. Casey reported
ON in 14% of acute cases of SCFE, with ON in 42% of those treated with
only manipulation and casting and in none of those treated with
traction and internal fixation, with or without supplemental reduction (95).
Aadalen reported ON in 15% of the acute cases of SCFE, with a rate of
5% (1 of 19) among those treated with manipulation, epiphysiodesis, and
casting; 19% (3 of 16) among those treated with manipulation and
internal fixation; and 25% (3 of 12) among those treated with
manipulation and epiphysiodesis 171). Hall noted ON in 5% of the cases
of SCFE treated with in situ fixation
using a Smith-Peterson nail and a 37.5% incidence among those treated
with fixation using a Smith-Peterson nail following manipulation,
although these results may have been influenced by selection bias (172).
indicated because of the increased risks of complications including ON.
A serendipitous reduction, which may occur with patient positioning on
the operating table, does not appear to negatively affect patient
outcome.
of a SCFE. Although used in the treatment of SCFE for much of the last
century, spica casting is now rarely used in the treatment of SCFE.
Because most children with SCFE are obese adolescents, use of a spica
cast for these children holds little appeal for most patients, their
families, and physicians.
Meier et al. reported complication in 14 of 17 hips in which a SCFE
(82%) had been treated with spica casting, including nine cases of
chondrolysis (53%), three cases of further slip after cast removal
(18%), and two cases in which a total of three pressure sores developed
(12%) (175). Chondrolysis has been reported in
14% to 53% of the cases of SCFE treated with spica casting, and it has
also been reported in the uninvolved hip following immobilization (29,94,175,176,177).
ON has commonly been reported with the use of spica casting as well,
although most cases of ON appear to be due to the forceful manipulation
of the SCFE rather than to the spica cast itself.
Although Betz et al. cited only a 3% incidence (1 per 37 hips), the
true rate is 5% in their study because they excluded the progression of
one additional hip that had been followed up for less than 2 years (176).
absence of any operative intervention, most proximal femoral physes do
not close for a year or more following the diagnosis of SCFE. Most
children treated with casting are immobilized for 3 to 4 months (175,176).
Betz et al. noted that spica casts could safely be removed when the
juxtaphyseal metaphyseal radiolucency was no longer visible, and that
this occurred by 16 weeks in their patients (176).
Although all patients were immobilized in a cast for periods ranging
from 117 to 124 days, Meier reported progressive slips in 18% (3 of 17)
of the hips after cast removal (175).
techniques, cannulated screw systems, and the decrease in operative
morbidity, there is little role for nonsurgical treatment in children
with SCFE.
fixation is currently the preferred initial treatment for most cases of
SCFE, both stable and unstable, although the outcome of such treatment
differs depending on the slip stability.
Large nail-type devices gave way to pins, which have given way to
cannulated screw systems in most centers. Because of the wide
availability of fluoroscopic imaging, the ability to optimally position
the fixation devices has improved as well. Cannulated screw systems now
allow these procedures to be performed percutaneously.
Use of a fracture table allows a true lateral radiograph to be
obtained, although the quality of such images in obese patients is
often suboptimal and this setup requires the presence of a technician
to rotate the fluoroscope. In contrast, with the patient on a
radiolucent table a technician is not needed, as the fluoroscope may be
left in one position and it is easy to obtain a higher quality frog
lateral radiograph; however, a true lateral can only be obtained by
moving the patient. In addition, the guide wire for percutaneous
fixation may be bent as the hip is rotated.
performed nearly universally for stable SCFE, an inadvertent reduction
of an unstable SCFE sometimes occurs simply with patient positioning.
This is particularly true in cases of markedly displaced, unstable
SCFE. Most authors agree that such inadvertent reductions do not appear
to cause ON (180,181). The risk of ON appears to be due to the disruption of the blood supply at the time of injury or with subsequent displacement
prior to surgical fixation rather than to an inadvertent reduction in the operating room (128,153).
pinning an unstable SCFE. First, fluoroscopic imaging is helpful in
assessing the degree of reduction as well as aiding in pin positioning.
Gentle adjustments (such as increasing hip internal rotation) in limb
positioning may be indicated in order to reduce marked displacement, if
persistent, following patient positioning (182).
Second, if a radiolucent table is used while an unstable SCFE is being
pinned, a provisional guide wire should be placed across the physis and
into the femoral head percutaneously before a lateral image is
obtained, in order to prevent ongoing motion between the femoral head
and neck.
SCFE is essential for understanding how to position the hardware
optimally and minimize complications. As noted previously, the proximal
femoral neck and shaft migrate anteriorly and rotate externally in most
SCFE. As a result, a greater portion of the femoral head is located
posterior to the femoral neck as the SCFE progresses. In very severe
cases of SCFE, the entire femoral head is posterior to the femoral neck.
the three-dimensional interpretation of intraoperative radiographic
images. Walters and Simon alerted the orthopaedic community to the risk
of unrecognized pin penetration in cases of SCFE treated with in situ fixation, and the associated risk of chondrolysis (183).
They demonstrated that a “blind spot” can exist radiographically, since
a protruding pin may appear to be located within the femoral head on
both anteroposterior and lateral views (183). Other authors have described a geometric analysis of the blind spot, although this technique is rarely used (184).
center of the proximal femoral epiphysis on both the anteroposterior
and lateral views and should be perpendicular to the physis in both
views as well (185,186).
This so-called “center-center” position of the fixation device
minimizes the “blind spot,” and thus the risk of pin penetration and
complications (183,187).
inserted from the anterior femoral neck in most cases in order to allow
fixation perpendicular to the physis and to prevent hardware
penetration through the posterior femoral neck (135,188) (Fig. 26.5). In fact, in very severe cases, the hardware may need to be inserted in a directly anterior-to-posterior direction (Fig. 26.6).
done in the pinning of adult hip fractures) will generally result in
one or more of the following problems: poor biomechanical alignment of
the hardware (very oblique rather than perpendicular to the physis),
purchase of the hardware in only a small portion of the femoral head,
joint penetration, hardware exiting the posterior femoral neck before
entering the femoral head, and creation of stress risers on the tension
side of the proximal femur. Common sequelae with a lateral starting
point are that the hardware either entirely misses or engages only a
small portion of the anterior femoral head, and that such hardware also
often penetrates the joint surface. If the hardware exits the posterior
femoral neck before entering the femoral head, as has been reported in
up to 6% of cases (97,189), the extraosseous and intraosseous blood supplies to the femoral head are at risk, thereby increasing the risk of ON.
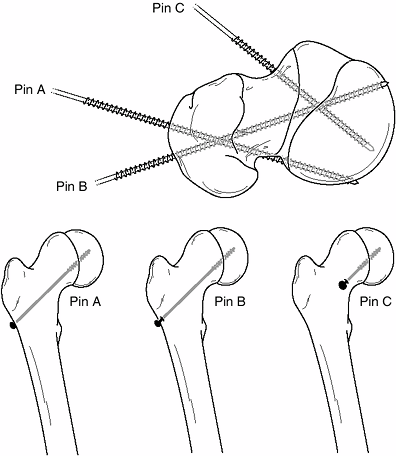 |
|
Figure 26.5 Two common problems associated with lateral-entry pins (pins A and B) in slipped capital femoral epiphysis (SCFE) are contrasted with correct pin positioning (pin C) using an anterior entry point. Top:
Because of their lateral starting points, pins A and B are both eccentric in the femoral head and oblique to the physis. In addition, pin A is shown exiting the posterior femoral neck before entering the epiphysis. Bottom: How pins A, B, and C will look on an anteroposterior radiograph, and how a potential blind spot exists in which a protruding screw may be missed radiographically. This reinforces the importance of imaging a pinned hip as the hip is rotated through a complete range of motion. |
amount of space in the femoral head and neck for appropriate hardware
positioning. Multiple clinical studies have confirmed increasing rates
of pin penetration and complications with an increasing number of
implants (27,174,189,190,191,192,193,194).
In 1984, Lehman et al. reported a 37% incidence of unrecognized pin
penetration in cases of SCFE undergoing treatment with implants and
noted that some areas of the head may not be well visualized
fluoroscopically (195). In a 1990 study of the
cases of SCFE fixed with multiple pins or screws, Riley et al. reported
hardware-related complications in 26% of the treated hips, which
included pin penetration in 14% (196) (Fig. 26.7).
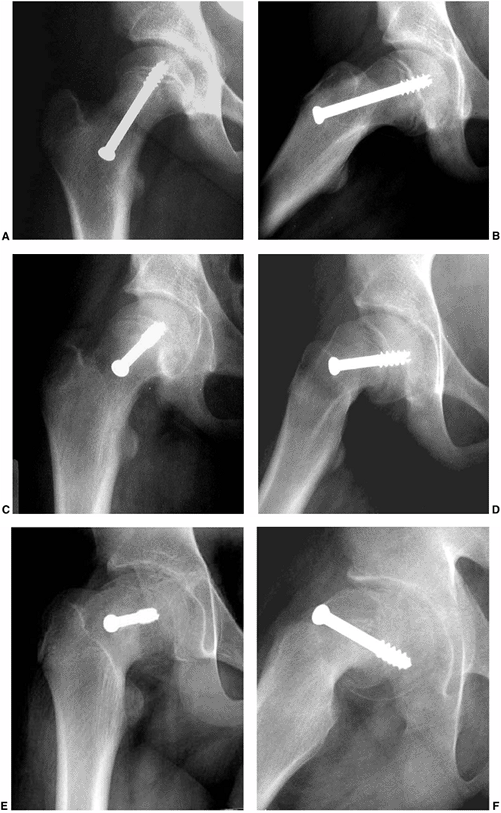 |
|
Figure 26.6 Proper screw locations in slips of varying severity in three different cases: (A, B), (C, D), and (E, F).
In all three cases, the screws enter the anterior femoral neck, are perpendicular to the physis, and are located in the center of the femoral head. The starting point is more proximal and the screw is angled progressively more posteriorly as the magnitude of slip progresses from least (A, B) to most (E, F) severe. |
using fluoroscopy. This can be done throughout the procedure if a
radiolucent table is used. If a fracture table is used, this can only
be done following removal of traction on the operated leg. The
“approach-withdraw phenomenon” described by Moseley is checked (197). This is the fluoroscopic appearance of the implanted hardware approaching the subchondral bone and then moving away from it (197).
When the hardware reaches the apex of this arc and then begins to
recede, the point of maximal proximity to the subchondral bone has been
reached, and this distance should be measured. Center-center pins are
left 5 to 6 mm from the subchondral bone (corrected for magnification)
while other pins are left 10 mm from the subchondral bone (183).
The posterior and superior portions of the femoral neck should be
avoided, because the hardware implanted in such locations may
compromise the intraosseous blood supply to the femoral head and
increase the risk of ON. Poor hardware position has been noted to
correlate with poor clinical outcomes (188,193).
under fluoroscopic control and bone endoscopy are two ways that have
been reported for checking for pin penetration when high-quality
radiographic images cannot be obtained intraoperatively (198,199).
However, imaging quality is almost always sufficient to obviate the
need for either of these techniques. In addition, each of these
techniques has the potential risk of flushing bone chips into the hip
joint. If radiographic imaging is deemed insufficient intraoperatively,
then a hip arthrogram through a
standard anterior approach may be performed to better ascertain the relation of the hardware to the femoral head.
techniques have been studied. Two previous biomechanical studies of
acute physeal disruptions in animal femora stripped of soft tissue
attachments have demonstrated an increased rigidity for two-pin or
two-screw constructs compared to those using only one comparable
fixation device (200,201),
and another found no statistically significant difference in resistance
to creep in between single- and double-screw constructs in bovine
femora (202). The authors of the two bovine
studies stated that the biomechanical advantages of two-screw
constructs were insufficient to justify the increased risk of pin
penetration when two screws are used instead of one (200,202).
One additional study using bovine femora with acutely created physeal
disruptions indicated that compression across the physis may be
obtained if screw threads do not cross the physis, although there was
no significant difference in the ultimate strength or the energy
absorbed or in the
degree of failure as compared to the results with a standard screw (203).
Because all these studies involve acute physeal disruptions, their
applicability to stable SCFE is limited. Their applicability to even
acute SCFE in humans is unclear as well.
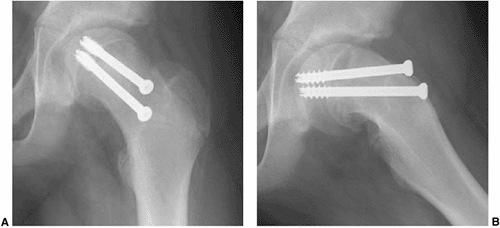 |
|
Figure 26.7 An 11.5-year-old boy presented with hip pain 1 month following in situ fixation of a stable slipped capital femoral epiphysis (SCFE). Anteroposterior radiograph (A) demonstrates what appears to be adequate alignment of the hardware, although the frog lateral view (B)
is suggestive of pin penetration. The proximity of the hardware to the joint surface had not been recognized at the time of surgery, and demonstrates the importance of leaving the pin at least 5 mm from subchondral bone, even if the hip is imaged through a range of motion at the time of surgery. This case also illustrates that only one implant can be in both a center-center position and perpendicular to the physis. |
Whether the rapid physeal closure is due to the SCFE itself or to the
fixation across the physis is not known. In a young child with
unilateral SCFE, rapid unilateral physeal closure has the undesired
effect of causing a potential limb length discrepancy (LLD). Multiple
studies carried out in Europe have touted the use of fixation devices
without threads crossing the physis (including smooth wires, hook pins,
and partially threaded screws) as a way to avoid physeal closure and to
allow further growth of the proximal femur (207,208).
In young patients with underlying causes of SCFE, some authors have
noted that epiphysiodesis may be needed in combination with in situ fixation (36).
acute-on-chronic, and chronic cases of SCFE (with 75% to 86% chronic
cases of SCFE) reported good or excellent results in 90% to 95% of the
patients (2,209).
Another recent series, limited to 21 hips with acute or
acute-on-chronic SCFE treated with single screws, reported 95% good to
excellent results, with no cases of ON or chondrolysis (187).
In series of studies with worse results, it is seen that the results
are better in milder slips than in the more severe slips (188).
Aronson et al. reported good or excellent results in 70% of the overall
cases, with 86% good or excellent results in cases of mild SCFE, 55% in
cases of moderate SCFE, and 24% in cases of severe SCFE (188).
This remodeling typically involves resorption of a portion of the
prominent superior femoral neck, and has also been reported to result
in changes in the proximal femoral head–neck and head–shaft angles.
Studies that report proximal femoral remodeling typically report
angular changes in the range of 7 degrees to 14 degrees (26,206,209,210).
Remodeling is most commonly reported in more severe slips and has been
reported in 68% to 83% of moderate to severe cases of SCFE at long-term
follow-up (19,116,209,210). An open triradiate cartilage has been reported to be an indicator of more potential for such remodeling (210,211). However, some authors have even reported remodeling after proximal femoral physeal closure (194).
limitations. One such limitation is the inherent error in radiographic
measurements. Another limitation is the variability in patient
positioning, especially when a painful hip with synovitis is imaged at
the time of presentation and a painless hip is imaged on subsequent
evaluations. Finally, significant remodeling in the slowly growing
peripubertal proximal femur with a fixation device across the physis
seems intuitively unlikely.
hip motion have been noted postoperatively, especially in the first 6
months (26). Siegel et al. reported such rapid improvement prior to significant remodeling, even in hips with severe deformity (26).
At 2-year follow-up, by which time the average slip angle had decreased
from 44 degrees to 30 degrees, mean hip flexion had improved by 22
degrees (to 118 degrees), hip abduction by 11 degrees (to 40 degrees)
and hip internal rotation in flexion by 19 degrees (to 11 degrees) (26).
Other authors have noted similar improvement in the range of hip motion
postoperatively, with improvements of 31 degrees for hip flexion, 25
degrees for internal rotation, 19 degrees for external rotation, and 21
degrees for abduction (193). However, a
decreased range of motion was still noted relative to the unaffected
hip in 40% of the patients, with flexion decreased by 15 degrees,
internal rotation decreased by 17 degrees, and external rotation
decreased by 10 degrees in this same study (193). O’Brien and Fahey noted painless hips in 83% (10 of 12) of moderate to severe cases of SCFE 2 to 17 years following in situ
pinning, with seven of these ten hips having “essentially normal”
motion except for a loss of 5 degrees to 20 degrees of internal
rotation (210).
There were no significant differences between the range of motion of
normal hips and those that had not been treated for SCFE or those
treated with in situ fixation. The only
significant loss of range was the loss of external rotation of hips
treated previously with osteotomy. The hips without treatment (slip
angle 18.8 degrees) or treated with in situ
fixation (slip angle 25.4 degrees) had markedly lower slip angles than
did those with osteotomy (slip angle 73.7 degrees). Although this study
has obvious selection bias, it does demonstrate that in cases of hips
without OA, there is no inexorable loss of motion.
fixation may be either iatrogenic or may be due to the natural history
of SCFE. The two most severe complications are ON and chondrolysis. ON
may be the natural sequela of an unstable SCFE or may result from pin
placement problems (with superior and/or posterior femoral head
placement), whereas chondrolysis may occur because of unrecognized pin
penetration. Other complications include further slipping, growing off
the screw, loosening or failure of screw fixation, proximal femoral
fracture, and LLD.
ON with stable SCFE than with unstable SCFE. Many series of studies
have reported 0% ON in stable SCFE, with the rates of ON in unstable
SCFE ranging from 12.5% to 58% (2,97,173,174,213).
In a series of 55 acute cases of SCFE treated with internal fixation,
Loder et al. reported ON in 14 cases (25%) with a rate of 47% in
unstable slips (14 of 30) and 0% in stable slips (n = 25) (97).
In another series with a 10% rate of ON in cases of acute SCFE, Dietz
et al. reported a 21% incidence in unstable SCFE and none in stable
SCFE (214).
due to the SCFE itself, although it is likely that the intraosseous
blood supply to the femoral head may be disrupted if internal fixation
devices are located in the superior or posterosuperior regions of the
femoral head (151,152,154,180,182).
Difficulty in avoiding these areas with any implants may be the reason
that the rate of ON is greater when multiple implants are used to fix a
SCFE (174,189).
filling of the superior retinacular artery in only two of five unstable
slips (153). One of the three hips without
filling of the superior retinacular artery preoperatively was studied
postoperatively, at which stage postoperative restoration of the
filling of the artery was evident (153).
Preoperative bone scans are quite sensitive in detecting ON, although
both false positives and false negatives have been reported (128).
Because almost all cases of ON were noted to have abnormal tracer
uptake preoperatively, the surgery does not appear to be the main cause
of ON in these patients.
the treatment of cases of unstable SCFE is undetermined. Clinical and
laboratory studies have suggested a potential benefit, with capsulotomy
reducing the rate of ON in adults and children with proximal femoral
fractures; studies have also shown an increase in intracapsular
pressure when the hip is maintained in internal rotation (215,216,217,218,219,220).
In the laboratory, Woodhouse documented ON in dogs with intracapsular
pressures of at least 50 mm of mercury for at least 12 hours (220). As seen from this and other studies (221,222),
the amount of pressure required to cause a significant decrease in the
femoral head perfusion seems to greatly exceed the increased
intracapsular pressure present in human hips with SCFE.
capsulotomy is beneficial remains unresolved. Some authors have
recommended capsulotomy at the time of SCFE fixation in an attempt to
decrease the rate of ON (128). Such
recommendations are based on inconclusive data from a small number of
cases. Gordon et al. advocate the importance of performing a
capsulotomy at the time of reduction and fixation of unstable SCFE,
although examination of their data demonstrates that this
recommendation is based on a single case of “mild” ON out of a total of
five patients who underwent early reduction without capsulotomy, in
comparison to no ON in six cases treated early with capsulotomy (128).
Even in this case of “mild” ON, the authors reported that the child
with mild ON was asymptomatic at 5-year follow-up. The supposition with
recommending capsulotomy is that there is a significant hemarthrosis
under pressure, which should be decompressed, although the pressures
that appear necessary to cause vascular embarrassment to the proximal
femur do not likely occur in most children with SCFE. At the current
time, there is insufficient evidence to conclude whether capsulotomy is
beneficial in reducing the rate of ON following acute/ unstable SCFE.
The timing of SCFE reduction has also been suggested as a causative
factor for ON. Several series have reported ON in 0% to 9% of hips
treated within 24 hours of symptom onset and in 18% to 20% of cases
treated thereafter (99,128,171,223).
Loder et al., however, did not demonstrate any benefit to early
reduction in a series of 55 acute cases of SCFE, 30 of which were
unstable (97).
scan or MRI. If ON is present, consideration may be given to free the
vascularized fibular graft prior to femoral head collapse in order to
maximize patient outcome (224). If vascularity
of the femoral head appears normal, then the child, family, and
physician may be reassured and earlier resumption of normal activities
allowed.
Aprin et al. have demonstrated that pin penetration in rabbits can lead
to chondrolysis, and that the severity of chondrolysis is related to
the duration of pin penetration (225). In
another study carried out in rabbits, Sternlicht et al. demonstrated
that pin protrusion caused mechanical destruction of the cartilage and
loss of proteoglycans in the articular cartilage, but did not result in
decreased joint space (226).
pinning varies from 0% to 9% in most of the reported series and appears
to be due to unrecognized pin penetration at the time of surgery (2,21,94,116,187,188,189,190,193,196,205,227,228).
Multiple series of studies carried out recently, each with more than 50
cases of SCFE treated using current fixation techniques with a single
screw, have reported no cases of chondrolysis (2,205,229).
Rates of chondrolysis appear to be higher when multiple fixation
devices are used because of the increased risk of unrecognized pin
penetration with the use of multiple fixation devices (189). Pin penetration with single cannulated screws appears to be quite low. Ward et al. (205) reported pin penetration in 1.7% (1 of 59 hips) fixed with one screw, and others (187) have reported a 0% rate.
are many cases of unrecognized pin penetration in the treatment of SCFE
without any resultant chondrolysis (189,228).
Previous authors have reported chondrolysis to occur in 11% to 51% of
the cases with unrecognized pin penetration. In one study with pin
penetrations reported in 28 cases, chondrolysis resulted in only three
of these 28 hips (11%) (189). The location of pin penetration is important (193), with less apparent risk if the penetration occurs in the
inferior head or fovea. Several studies have reported that if pin
penetration is recognized at the time of surgery and the protruding pin
is removed, there does not appear to be an increased risk of
chondrolysis or other complication (230,231).
fixation if the progressive growth of the proximal femur results in
loss of fixation across the physis, or if a properly located screw
loses fixation. Slip progression following in situ pinning has been reported in 0% to 3% of the cases in most series (2,94,97,205,209,232,233), although one series reported a rate of 20% (234).
This high rate reported by Carney et al. is likely due to femoral neck
resorption and changes in patient positioning rather than to true slip
progression. In another series, the proximal femur was noted to grow
off 29% of hips fixed with Steinmann pins, 18% of hips fixed with
Knowles pins, and 0% of hips fixed with cannulated screws (235). Growing off a screw appears much less common than growing off wires (229,236). Previous authors have noted the risk of progressive slip if hardware is removed prior to physeal closure (237).
of 202) without any evident cause, and progression in an additional 5%
of the hips after the fixation device(s) no longer engaged the
epiphysis (236). By far the highest rate of progression following in situ fixation was recently reported by Carney et al., who found progression of SCFE in 20% of hips following in situ fixation with a single screw (234).
In a series of seven progressive slips with appropriate hardware
positioning, fixation in the epiphysis remained good, but metaphyseal
loosening with “windshield wipering” was noted in each case (233).
Such fractures often follow relatively minor trauma. Many reports have
focused on subtrochanteric fractures following insertion of the
hardware from the lateral aspect of the femur, which is the side of the
bone with more tension (193,196,229,238). Most such fractures occur through the used or unused drill holes at or distal to the lesser trochanter (238,239) (Fig. 26.8). Fracture has also been reported following hardware removal (2).
Previous reports have focused on the importance of minimizing the
number of drill holes (and, therefore, stress risers) in the proximal
femur. Local bone death due to the high temperatures associated with
reaming through dense bone has been suggested as a possible etiology in
some cases as well (240). Stress fracture of the femoral neck has also been reported (229).
It appears that the way to minimize the risk of proximal femoral
fractures is to use an anterior starting point in the femoral neck and
to avoid drilling into the proximal femur until the precise insertion
site is localized.
To prevent significant LLD in children with unilateral SCFE,
prophylactic pinning of the contralateral hip should be considered if
such children are younger than 10 years at presentation. If a projected
LLD is the only concern, then an alternative would be to perform a
contralateral distal femoral epiphysiodesis at a later stage.
Complications of hardware removal include hardware breakage, inability
to retrieve the hardware, difficulties requiring extensive bone
removal, and fracture. Bellemans et al. reported inability to remove
the hardware in 30% of the cases and the need for major decortication
to remove hardware in 20% of the cases in the same series (209).
Greenough et al. reported two subtrochanteric fractures in a study of
57 hips following hardware removal, presumably due to significant bone
removal at the time of hardware removal (243).
Crandall et al. reported lower complication rates with cannulated screw
removal compared to pin removal, although the screws were noted to be
buried and difficult to remove in 36% of the cases (245). Screw breakage during attempted removal has been reported in 6% of the cases in one series (2).
Removal of titanium screws has been reported to be more difficult than
removal of stainless steel screws, possibly due to the significant
amount of osseous integration seen with titanium screws (246).
fixation in cases of SCFE. If hardware removal is necessary (for later
surgery, for example), then removal of the screws with reverse-cutting
threads may prove easier.
Hagglund noted that no patient who had a hip with a mild or moderate
slip in childhood or adolescence and who had been treated with in situ pinning developed arthritis before the age of 50 years (247).
Hansson et al. reported that at 30.9 years mean follow-up, OA was seen
in 22% of mild slips (30 degrees or less) and in 50% of moderate slips
(30 degrees to 50 degrees) and that Harris hip scores were at least 90
in 93% of the cases with mild slips and in 78% of the cases with
moderate slips (158). They also noted that
radiographic findings correlated with Harris hip scores, with hips with
mild OA having a mean score of 96.5 and hips with severe OA having a
mean score of 74.3 (158).
fixation, and that SCFE reduction or realignment resulted in higher
rates of complications (including ON and chondrolysis). Carney et al.
also noted that Iowa hip scores decreased with the increase of every
decade in follow-up
studies (94).
However, even those with late OA often function relatively well into
their fifties in the absence of any significant complications of the
initial treatment. Carney et al. stated that in situ
fixation is the procedure of choice, regardless of slip magnitude,
because of its long-term functional and radiographic outcomes and low
risk of complications. Despite the presumed accuracy of the data, their
interpretation may be incorrect because of an inherent bias involved in
the selection of cases: in situ fixation was used to treat milder cases of SCFE and realignment procedures used to treat more severe cases of SCFE. In situ fixation cannot be recommended over realignment in severe cases of SCFE unless in situ fixation has demonstrably better results in the treatment of such severe cases.
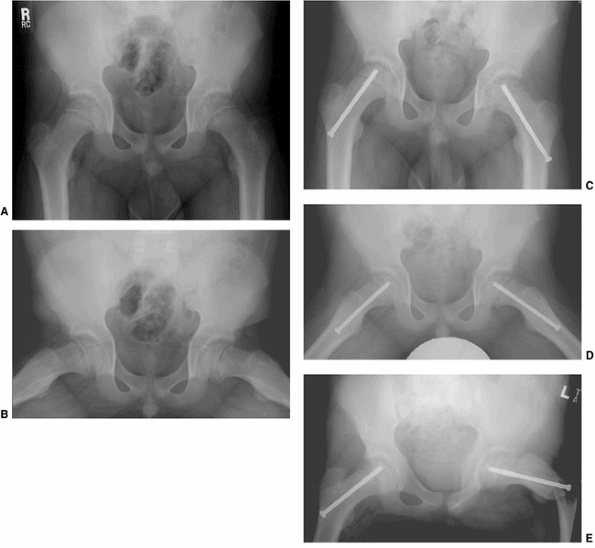 |
|
Figure 26.8
A 12-year-old boy presented with bilateral stable slipped capital femoral epiphysis (SCFE). Anteroposterior and lateral radiographs demonstrate mild bilateral SCFE (A, B). Postoperative radiographs demonstrate that both screws were inserted through the lateral cortex at or distal to the lesser trochanter (C, D). Six weeks postoperatively the boy had acute onset of pain while playing baseball, due to a left subtrochanteric fracture (E). Proximal femoral fractures occur most commonly when hardware enters the lateral cortex of the femur at or distal to the lesser trochanter, and may also occur through unused drill holes at this level. Because of the posterior direction of slip, an anterior femoral neck starting point would have been feasible in this case, and preferable both for biomechanical reasons and the lower risk of fracture associated with an anterior starting point. |
fixation. Ross et al. reported good or excellent results in patients
without intraoperative complications at 10- to 20-year follow-up, but
fair to poor results in 10 of 15 hips (67%) at more than 20-year
follow-up. One potential reason for this difference, in addition to
increased duration of follow-up and potential bias in selection, is
that moderate and severe slips accounted for 40% of the
hips
followed up for less than 20 years and 53% of the hips followed up for
more than 20 years. Ross et al. also noted that this deterioration
seemed related to bilateral SCFE (248).
fixation of cases with SCFE have decreased considerably. Much of this
decrease is due to a reduction in the rates of ON and chondrolysis
because of the recognition of the importance of proper pin or screw
placement. In situ fixation is considered
the treatment of choice for cases of SCFE of all degrees in most
centers because of the relative simplicity of this extensively studied
and well-documented technique (94,156,249,250).
treatment of all cases of SCFE, regardless of the slip stability and
degree of displacement. I prefer to use a radiolucent table to pin a
SCFE because I find the frog lateral image on a radiolucent table to be
of superior quality to the true lateral obtained on a fracture table. I
also find it easier and quicker to reposition the patient’s leg (as is
done with a radiolucent table) to obtain a lateral view than to move
the fluoroscopy machine (as is necessary with a fracture table).
taken to rotate the affected hip internally until the patella is facing
forward before obtaining an anteroposterior image of the hip when
choosing a pin insertion point and directing the guide wire. Failure to
do so will result in the pin being inserted with the hip in a degree of
external rotation; as a result, when a true anteroposterior view is
obtained, the screw will be seen to be located in the superior portion
of the femoral head. Other potential disadvantages of using a
radiolucent table are that obtaining a true lateral is more difficult,
and that the guide wire may be bent as the hip is moved into the frog
position. In order to obtain a true lateral radiograph on a radiolucent
table, in addition to rotating the patient’s hip, the patient’s body
must be tilted (rotated) toward the affected hip.
with reverse-cutting threads. The starting point is on the anterior
femoral neck, and I attempt to place the screw so that it is
perpendicular to the physis on all views, is in a center-center
position in the femoral head, and is 5 to 6 mm from the subchondral
bone at its closest location when the hip is taken through a full range
of motion intraoperatively.
SCFE, as noted in the preceding text. It is important to remember,
however, that there may be some differences in pin position and results
depending on the duration of symptoms and the magnitude of the slip. As
a rule, stable slips will be only mildly displaced in patients with
only a few days or weeks of symptoms, and will be more displaced in
patients with many months of symptoms. For a mild slip, the starting
point on the anterior femoral neck will be more distal and the screw
will be inserted more horizontally than for a more displaced slip
because of the posterior direction of the slip. Because the screw in a
more severe slip has a more proximal starting point and often has to
traverse a shorter distance in order to fix the physis, a more severe
SCFE is often less difficult to pin than a very mild slip, if the
correct starting point is used.
I generally use a single, cannulated 7.3-mm screw as well. However, if
a slip is noted to be markedly unstable at the time of surgery—with
gross movement or a significant change in alignment—I prefer to use two
screws. The decision to use two screws is arbitrary, although I believe
that the added stability is worthwhile in markedly unstable slips,
despite the increased risk of pin penetration.
SCFE, I place a temporary guide wire across the physis before checking
a lateral x-ray film in order to assess the proximal femoral alignment
before definitive fixation. Frequently, simply positioning the child on
the radiolucent table with the patella directed anteriorly results in
satisfactory alignment. However, if there is still marked displacement
following this positioning, I will return the hip to an anteroposterior
position on the fracture table, back the temporary pin out the
epiphysis, and apply gentle traction and internal rotation.
Fluoroscopic images are checked following this repositioning. Following
stabilization of the epiphysis with the guide wire, the frog view may
be rechecked. Once the alignment is deemed appropriate, then two screws
are inserted with avoidance of the posterosuperior head.
support hip capsulotomy, although I do attempt an aspiration of the hip
joint with an 18-gauge spinal needle for markedly unstable slips, since
the risks of aspiration are small, although this is an arbitrary
decision. Usually, only a few milliliters of blood can be aspirated
without any clear evidence of significant pressure.
considered following unstable SCFE to evaluate for ON. If ON is
detected prior to radiographic evidence of collapse, then treatment
with a vascularized fibula graft may be considered.
bear weight as tolerated. They are given crutches that are generally
discarded by the time of the 1-week postoperative office visit.
Sporting activities are allowed at 3 to 6 months postoperatively. I
arbitrarily preclude children with unstable SCFE from full weight
bearing for 3 to 4 weeks. In the absence of any evidence of ON, they
resume sporting activities at 6 months postoperatively.
fixation, is the prevention of slip progression. However, the way in
which this is achieved with the two methods defer. Slip progression is
prevented with bone graft epiphysiodesis primarily by hastening physeal
closure, whereas in situ fixation prevents
slip progression primarily by stabilizing the physis. Indications for
bone graft epiphysiodesis include acute/unstable or chronic/stable SCFE
of any magnitude, although some authors have conceded that cases of
mild SCFE are better treated with in situ fixation (251).
across the physis into the epiphysis with placement of bone graft (most
commonly autologous bone pegs), was first described in 1931 by Ferguson
and Howorth (252). Although reported results have often been good (213,251,253,254,255,256,257,258,259,260), this operation has been abandoned at many institutions because of potential for morbidity and technical difficulties (261,262,263).
anterolateral approach and may be combined with osteoplasty of the
anterior femoral neck (251,260,264).
A 50-year experience with bone graft epiphysiodesis in 318 cases of
SCFE presents this procedure as a “reasonable alternative” for the
treatment of SCFE (253). Patients with acute
SCFE are placed in a spica cast or brace postoperatively and kept
without bearing weight for 6 to 8 weeks. Patients with chronic slips
begin touch-down weight bearing 2 to 3 days postoperatively and bear
weight progressively as the physeal closure progresses. Some authors
have reported the time required until full weight bearing as averaging
10 weeks (259).
Weiner et al. have reported estimated blood loss (EBL) for autologous
bone peg epiphysiodesis of at least 200 mL in 52% (25 of 48) patients (259), and other authors have reported mean EBL ranging from 426 to 800 mL (261,262,265). When allograft is used instead of autograft, mean EBL has been reported as 360 mL (213).
In a series of bone peg epiphysiodesis with allograft, a partial
physeal closure was noted radiographically after an average of 11 weeks
and complete closure after an average of 28 weeks, with physeal closure
occurring in the operated hip before it occurred in the unoperated hip
in all of the 16 unilateral cases (213).
failure to achieve physeal closure, slip progression, heterotopic
ossification, lateral femoral cutaneous nerve (LFCN) palsy, donor site
morbidity, chondrolysis, and ON. Heterotopic ossification has been
reported in up to 69% of patients (262).
Despite intraoperative protection of the nerve, Ward et al. reported
LFCN palsy in 10 out of 14 patients (71%) specifically examined for
this finding postoperatively (265). Rao et al. reported transient LFCN palsy in 11% of their patients (262).
are well described. In two large series with very good results,
Adamczyk et al. reported graft resorption with failure of
epiphysiodesis in a period of 1 year in 4% of cases (12 of 318 hips) (253), and Howorth reported graft resorption in 2% of cases (4 of 200 hips), with no cases of progressive slip (254).
In a series of 17 cases of SCFE, Ward and Wood reported “graft
insufficiency,” defined as graft movement, resorption, or fracture, in
eight hips (47%) (265). Protrusion of the graft into the hip joint has also been reported (261).
has generally been low, with most reports in the range of 0% to 6%,
with higher rates in acute/unstable SCFE (213,253,254,262,264,265).
Adamczyk reported an overall rate of 2%, with a risk of 7% in acute
slips (3 of 45 cases) and 1.5% in chronic slips (4 of 273 cases) (253).
The low rate of ON in bone peg epiphysiodesis is likely due to
placement of the grafts from the anterolateral neck and into the center
of the epiphysis, thereby avoiding the intraosseous blood supply.
following bone peg epiphysiodesis, with the highest risk being in acute
SCFE (213,253,254).
Although Rao et al. noted a change in the femoral head–shaft angle of
at least 5 degrees in 42% of patients (27 of 64), the angle increased
in 19% and decreased in 23% of the cases (262).
One presumed reason for slip progression is that the bone graft does
not stabilize (and may actually destabilize) the proximal femur as well
as does a screw. Another potential cause of progressive slip in these
patients is the delayed or incomplete physeal closure.
fixation of SCFE, although there are significant drawbacks to its use.
Children treated with bone graft epiphysiodesis have greater blood
loss, increased donor site morbidity, increased risk of nerve palsy,
increased risk of slip progression, and are not allowed to bear weight
as early as do those children undergoing in situ fixation. Bone graft epiphysiodesis does not appear to have a significant role in the treatment of SCFE in the 21st century.
both temporal and anatomic terms. Temporally, osteotomies can be
thought of as either early or delayed. Early osteotomies are undertaken
as part of the primary treatment of SCFE in an attempt to restore a
more normal anatomy as well as to prevent further slipping. These
osteotomies require fixation across the physis in order to prevent
progression. Late osteotomies are generally undertaken to correct
residual deformity after physeal closure. Usually these are performed
at least 1 year after the initial treatment if significant symptoms
persist or if the anatomic derangement is felt to be severe enough to
require treatment.
The ability to correct the deformity is greatest with a subcapital
osteotomy, least with a femoral neck osteotomy, and intermediate with
an intertrochanteric osteotomy. The risk of ON is inversely related to
the distance from the physis to the osteotomy, with subcapital
osteotomies having the highest risk and intertrochanteric osteotomies
having the lowest. Frymoyer reported ON in 30% of the femoral neck
osteotomies compared to 0% of intertrochanteric osteotomies (266).
Interestingly, Jerre et al. reported lower short-term complications but
poorer long-term outcomes with intertrochanteric osteotomies compared
to subcapital osteotomies (170).
lower intraarticular contact stress in femoral neck osteotomy compared
to intertrochanteric osteotomy (267). Although
clearly still experimental, preoperative computer simulation of
osteotomies has been suggested as an option to optimize the surgical
planning for patients with SCFE (268).
have demonstrated the potential risks of femoroacetabular impingement,
with resultant cartilage damage and/or labral tears, in hips with SCFE,
especially in hips with more severe slips. After remodeling, as the
range of motion increases, an increasing portion of the remodeled
metaphysis becomes an intraarticular weight-bearing surface,
potentially contributing to late OA (136).
Although theoretical reasons and indications from some medium-term
clinical studies argue that restoring a more normal osseous alignment
may be beneficial to the hip in the long term (269,270),
there are no clinical data in the literature to prove that such
realignment results in enhanced long-term hip function or durability.
commonly been described as a primary treatment of moderate to severe
SCFE, with the goals of deformity correction and prevention of slip
progression. Because they are performed at the level of deformity,
subcapital osteotomies are the most powerful osteotomies for deformity
correction. These are very technically demanding operations, are
associated with high rates of ON, and are rarely performed. As early as
1948, Martin noted the importance of avoiding tension on the posterior
periosteal vessels in order to minimize the risk of ON (271). Subcapital osteotomies have been referred to as orthopaedic roulette because of their risky nature (272).
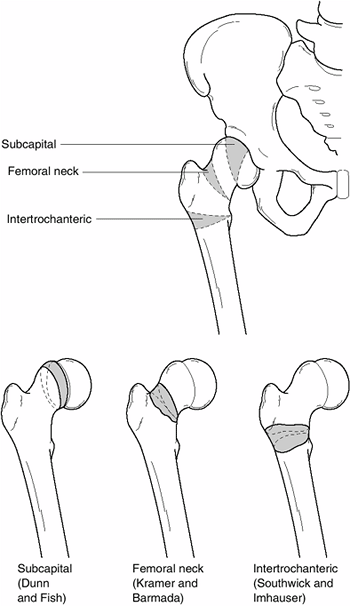 |
|
Figure 26.9
The three levels of osteotomy to correct the proximal deformity following slipped capital femoral epiphysis (SCFE). The ability to correct the deformity is greatest with a subcapital osteotomy, least with a femoral neck osteotomy, and intermediate with an intertrochanteric osteotomy. The risk of osteonecrosis (ON) is inversely related to the distance from the physis to the osteotomy. Intertrochanteric osteotomies are currently the most commonly performed osteotomies because of the low rate of ON and the ability to obtain good correction. |
patients following cuneiform subcapital osteotomy at a mean follow-up
of 13 years (273,274). This surgery was limited to those with slips exceeding 30 degrees (273,274).
He noted the importance of removing all callus and physis in order to
avoid tension on the posterior periosteum as the epiphysis is reduced
onto the femoral neck, and also noted that this osteotomy should only
be performed in hips with an open proximal femoral physis. He
demonstrated excellent corrections with low rates of complications.
Other reports of such excellent results following subcapital osteotomy
are rare, although Nishiyama et al. reported 93% excellent results at a
mean follow-up of 10 years in the cases of 15 patients with 18 SCFEs
treated with cuneiform osteotomies (275).
also described a transtrochanteric subcapital osteotomy which shortened
the femoral neck and preserved the posterior blood supply to the
femoral head. Even in Dunn’s hands results were mixed, with good
clinical results in only 55 of 73 hips (75%) and good radiographic
results in only 41 (56%) of the hips at a mean follow-up of less than 9
years (277). Other authors following Dunn have reported mixed results and high rates of complications (170,263,278,279,279,280,281). Average EBL exceeding 500 mL has been reported (279).
following subcapital osteotomies, with most authors reporting rates in
the range of 3% to 18% (274,275,276,278,279,280,281,283).
Dunn reported chondrolysis in 18% of his cases (13 of 73), with a rate
of 17% in 24 in acute-on-chronic cases and 18% in 49 in chronic cases (276).
Fish reported an LLD of at least 1 cm in 35% of patients (23 of 66) and
of at least 2 cm in 6% (4 of 66) of patients treated with cuneiform
subcapital osteotomy, with a maximum difference of 5 cm (274). Nishiyama et al. reported an average LLD of 1.5 cm in their series of subcapital osteotomies, ranging from 1 to 2 cm (275). Velasco et al. noted LLD of at least 1 cm in 6% and at least 2 cm in 3% of their patients (281).
because of the powerful scope for correction they afford. However,
because the learning curve is steep, complications are frequent and
severe, and experience is necessary for good results, subcapital
osteotomies are rarely used currently.
osteotomies have less power to correct deformity but are also
associated with a somewhat lower risk of ON. These osteotomies can be
performed in the middle of the neck or at the base of the neck and may
be performed as either a primary or a secondary treatment of SCFE. As
with other proximal femoral osteotomies, the goal of femoral neck
osteotomy is to restore a more normal proximal femoral alignment.
deformity, maximum correction may be incomplete in moderate to severe
slips. Despite the incomplete correction of the underlying deformity,
proponents note that sufficient correction can be obtained to
significantly improve hip alignment and biomechanics (284,285,286).
intracapsular or extracapsular (284–286). Care must be taken to
preserve the posterior blood supply to the femoral head. Extracapsular
osteotomies have the theoretical benefit of a decreased risk of ON,
although they are less able to correct the underlying deformity because
of their more distal location (284,285).
In a series of 36 extracapsular osteotomies, Abraham et al. reported
89% good to excellent results and no cases of ON at an average
follow-up of 9 years (284).
unrecognized pin penetration, chondrolysis, ON, hardware failure, LLD,
joint space narrowing, and OA. Gage et al. have noted decreasing rates
of both ON and chondrolysis with more distal osteotomies (283).
Even with attempts to preserve the blood supply, ON has been reported
in up to 10% of cases following femoral neck osteotomy (283). Chondrolysis has been reported in 2% to 10% of the base-of-neck osteotomies for SCFE (284,285).
Barmada et al. reported one case each of loss of fixation and joint
penetration by hardware in their study of a series of 20 hips (285). Joint space narrowing has been noted in 10 of 11 hips (91%) followed up for at least 13 years in one series (284).
of at least 1 cm in 61% of patients and at least 2 cm in 42% (15 of 36
patients) (284). Three patients in the same
series (8%) had LLD of at least 4 cm, and in male adolescents with at
least 3 years of growth remaining, unilateral SCFEs were noted to lead
to LLD of 3 to 5 cm (284).
osteotomies and toward intertrochanteric osteotomies because of the
technical difficulties of femoral neck osteotomies, their risk of
complications, and the limited ability to completely correct the
deformity.
commonly performed osteotomy for SCFE. Such osteotomies are generally
performed after physeal closure in patients with significant
limitations in the range of motion, significant pain, and/or marked
proximal femoral deformity. The most common intertrochanteric
osteotomies are angular osteotomies described by Southwick and by
Imhauser (115,287,288,289,290,291). These osteotomies are generally fixed with plates, but use of external fixation has also been reported (292,293).
triplanar. The three common components of the osteotomy are valgus,
flexion, and internal rotation. The degree of correction is based on
anatomic alignment, range of motion deficit, and patient complaints.
The Southwick osteotomy is the most commonly performed
intertrochanteric osteotomy in North America, and the Imhauser
osteotomy is more popular in Europe. Southwick described a valgus and
flexion osteotomy to which internal rotation of the distal fragment is
generally added (115,287,288,289,290).
Therefore, Southwick osteotomies are generally triplanar osteotomies.
Imhauser described a biplanar flexion and internal rotation osteotomy
without valgus. A “reverse” Imhauser osteotomy has been reported for
the uncommon valgus SCFE (294). With either
type of intertrochanteric osteotomy, significant internal rotation of
the distal fragment must usually be performed in order to restore both
proximal femoral anatomy and a more normal rotational arc of motion.
made on the basis of clinical signs and symptoms or on a biomechanical
basis in an attempt to normalize proximal femoral anatomy with the
theoretical decrease in the long-term risk of OA. Clinical indications
for intertrochanteric osteotomy may include hip and/or groin pain with
prolonged sitting (owing to femoral neck impingement on the acetabulum)
and difficulty in performing activities because of the abnormal arc of
hip motion. Lack of hip flexion and internal rotation may make routine
activities such as sitting in a chair, climbing stairs, riding a
bicycle or scooter, donning and doffing socks, and cutting one’s
toenails difficult or impossible. A significant varus deformity of the
proximal femur may result in significant abductor weakness, with a
persistent Trendelenburg gait and fatigue pain with ambulation. If the
recommendation for intertrochanteric osteotomy is based on clinical
signs and symptoms, the physician should wait at least one year
following in situ fixation to be certain that such signs and symptoms do not spontaneously improve.
osteotomy is being made on a biomechanical basis regardless of the
patient’s symptomatology. The argument for such surgery is the
increasing body of knowledge that OA is more common and more severe in
the more severe cases of SCFE, even in the absence of short-term
complications (94,96,116,156,158,159).
In addition, biomechanical modeling studies have shown that the
deformity associated with SCFE would place patients with SCFE at
long-term risk of OA (136). A SCFE can result
in the anterior femoral metaphysis articulating with acetabular
cartilage and can also cause impingement of the femoral neck against
the anterior acetabulum (136). Two recent
studies have reported an apparent decrease in the expected rate of OA
following a realignment at a follow-up of more than 20 years (269,270).
Despite these results there are currently no long-term clinical data
that conclusively demonstrate advantages of routine proximal femoral
osteotomy in patients followed into middle and old age (86,295,296).
of deformity and the clinical arc of motion. As a result, the amount of
correction noted at follow-up may vary with patient selection criteria.
In one series of Southwick osteotomies, Salvati et al. noted a mean
increase in internal rotation of 33 degrees and an abduction of 17
degrees at follow-up (297).
have been good, with acceptable rates of complications. At a follow-up
at least 5 years after the surgery, Southwick reported excellent or
good results in 93% of the patients (115), and
13 years later the long-term follow-up results were reported as good or
excellent in 87%, although the precise duration of the follow-up was
not specified (287). Other authors have
reported good to excellent clinical results in 80% to 85% of patients
who were followed up for 5 to 10 years, with good radiographic results
in about 60% (298,299).
Other authors who reported poor results noted that the poor results
were seen in conjunction with insufficient surgical correction of the
underlying deformity (300).
Imhauser osteotomies, with Parsch et al. reporting good or very good
results in 92% of the hips operated on from 1975 to 1982, and an
average Iowa hip score exceeding 90 in patients operated on
subsequently (301).
ON, are noted to have other significant complications including
chondrolysis, delayed union, need for reoperation, late arthritis, LLD,
and fracture. Because these osteotomies are typically performed after
physeal closure, progressive slip does not occur.
following intertrochanteric osteotomies, although rates up to 59% have
been reported in small series (115,228,266,287,297,298,301). Delayed unions have been reported in up to 3% or 4% of the hips following intertrochanteric osteotomy in several series (270,297,299).
Loss of fixation has been reported in 4% to 6% of cases in some series
297,300). Fractures are not reported in most series following
osteotomy, but were reported in 6% of 130 cases of intertrochanteric
osteotomies in one series (301).
described. However, because a Southwick intertrochanteric osteotomy
includes a valgus component, it is believed to lead to less LLD than
other osteotomies. LLD following Southwick osteotomy has been reported
in 19% to 26% of the cases, with a maximum LLD of 2 cm (297,299,300). The operated leg was short in 15% to 19% of patients in these series and was long in 0% to 11% (297,299,300).
Schai et al. reported LLD in 81% of patients (38 of 47) following
Imhauser osteotomy, with the affected leg being an average of 0.9 cm
short in 35 patients and being 0.5 to 2.0 cm long in the other three
patients (270).
intertro-chanteric osteotomy, Schai et al. reported moderate OA in 28%
of the hips and severe OA in 17% (270). Jerre et al.
reported 36% good to excellent results in 11 hips at long-term
follow-up averaging 36.1 years, although radiographic and surgical
techniques have advanced significantly in the interim (170). One of these 11 patients (9%) was found to have undergone salvage surgery by the time of long-term follow-up (170).
common osteotomies performed on children with SCFE. These osteotomies
can be challenging in these very large, heavy patients with significant
deformity. Intertrochanteric osteotomies are also somewhat limited in
their ability to correct the deformity because of their considerable
distance from the site of deformity. Despite incomplete correction,
there is generally sufficient correction to allow for good clinical
outcomes with an acceptably low rate of complications (Fig. 26.10).
the role of osteotomy is unclear. The real question to consider in such
patients is whether they are best served by an osteotomy in
adolescence, with its attendant risks, in an attempt to delay or avoid
total hip arthroplasty (THA). One of the confounding variables in such
an evaluation is that it is impossible to be certain what impact the
advances in THA or basic science (such as gene manipulation) may have
on the long-term outcomes of SCFE and OA in the coming decades.
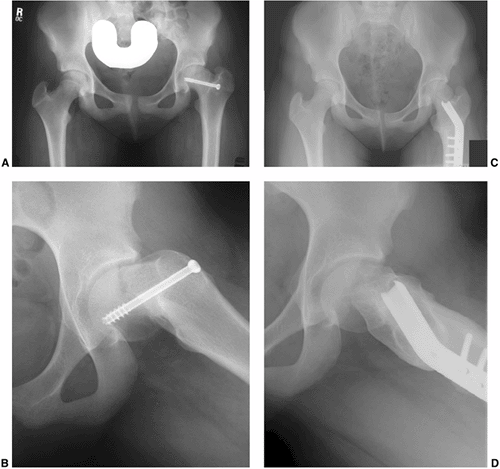 |
|
Figure 26.10
A 13-year-old girl presented with pain on sitting and difficulty riding a bike because of external rotation of the left hip 16 months following in situ fixation. Anteroposterior pelvis (A) and lateral (B) radiographs of the left hip show the residual deformity 16 months following pinning. Lateral x-ray film shows the proximal femoral metaphysis articulating with the acetabulum preoperatively. Anteroposterior (C) and lateral (D) radiographs show the alignment 1 year after triplanar (flexion-valgus-internal rotation) osteotomy. Following redirectional osteotomy, there is an increased neck–shaft angle, with distal and slightly lateral translation of the greater trochanter (with a resultant increase in the articulotrochanteric distance), and the relation of the femoral head and acetabulum has changed. The metaphysis is no longer intraarticular. A downside of the surgery is that if total hip arthroplasty is necessary in the future, distortion of the proximal femoral anatomy will make such a replacement more difficult. |
osteotomy for correction of significant residual proximal femoral
deformity following SCFE (302,303).
Sugioka states that such an osteotomy is indicated for SCFE with a
displacement greater than 45 degrees, based on the head–shaft angle
measurement (303). Although this osteotomy has
the potential to significantly enhance the anatomic alignment, it is
quite demanding technically and is rarely used.
SCFE treated with this method, with an improved range of motion of the
hip in 90% of the patients (303). One case of
ON was reported among these 10 hips. Sugioka noted postoperative valgus
in three hips that had marked deformity preoperatively.
One patient had ON and the other had loss of fixation. At less than 3
years mean follow-up, four hips were clinically asymptomatic. No cases
of chondrolysis have been reported in the literature in the two small
series (a total of 15 patients) of transtrochanteric rotational
osteotomies in children with SCFE (303,304).
promising, the technique is demanding, complications are common, and
its use has been reported in only a few centers.
those patients with significant signs and/or symptoms that persist at
least 1 year following in situ fixation.
Most commonly, these include hip and/or groin pain with prolonged
sitting (owing to femoral neck impingement on the acetabulum) and
functional limitations due to loss of the hip range of motion. Although
such signs and symptoms are common in the first few months following in situ
fixation, they often improve within 1 year of pinning. If such
limitations persist and affect a patient’s quality of life, I will
perform an osteotomy.
because I believe that an osteotomy at this level provides the best
ratio of deformity correction to risk. I generally perform a triplanar
proximal femoral osteotomy using a blade plate with correction in the
planes of valgus, flexion, and internal rotation.
asymptomatic patients with moderate or severe slips. I currently think
that the risks of such osteotomies in asymptomatic patients are too
great relative to the uncertain potential for long-term gains,
especially given the anticipated ongoing advances in the fields of
orthopaedics and basic science during the lifetime of these children.
In addition, asymptomatic patients who undergo proximal femoral
osteotomy are generally made clinically worse for at least the first 6
months following a proximal femoral osteotomy.
area of significant controversy in the management of children with
SCFE. Recent authors have attempted to weigh the risks and benefits and
their recommendations are conflicting. Proponents of prophylactic
pinning cite the high rates of bilateral SCFE, the increased risk of OA
in patients with SCFE at long-term follow-up, and the decreased risks
of prophylactic pinning as technology and techniques improve (10,247,305,306).
Schultz et al. indicated that prophylactic pinning of the contralateral
hip in cases with unilateral SCFE would appear to be beneficial in
terms of long-term Iowa hip scores, but cautioned that clinical
judgment and patient preferences should be used on a case-by-case basis
(307).
of prophylactic treatment, noting the potential risks of pinning
numerous hips that will never slip, and also pointing out that with
appropriate patient counseling and close follow-up most subsequent
slips will be detected while still mild. Previous authors have reported
complication rates of up to 34% with prophylactic pinning of possible
SCFEs, although the techniques and results in these studies would not
be considered acceptable by current standards (209,243,308). In fact, in one recent study of 94 hips treated with prophylactic pinning, there were no complications (10).
pinning in unilateral SCFE, it is important to consider the data
regarding the risk of contralateral SCFE, the anticipated severity and
stability of such a slip, and the risks and benefits of observation and
of prophylactic treatment. An important consideration in prophylactic
pinning is that because the distance the screw must traverse in an
unslipped hip is much greater than the distance in a moderate to severe
SCFE, there is actually less room for error in selecting the starting
point and the angle of screw insertion. This geometry dictates that for
every few degrees of deviation from the optimal path, the tip of the
screw will be more eccentric in the epiphysis in a mild slip (or in a
hip that has not slipped) than it would be in a severe slip (Fig. 26.11).
systemic causes of SCFE, bilateral SCFE is present in approximately 20%
at the time of initial presentation, is identified in another 15% to
20% in adolescence, and is present at long-term follow-up in
approximately 60% (9,10,11,12,13,19,25,27,28,29,30,76,94,95,96,101,107,116,156,235,305,309,310).
These data indicate that in the 80% of the patients who present with
unilateral SCFE, 20% to 25% will develop a contralateral SCFE in
adolescence, and half of the 80% will develop a second SCFE by the time
of long-term follow-up. It can be inferred from these data that the
probability of a contralateral slip first being recognized after
adolescence is 25% to 30%. It is likely that these represent minimal,
asymptomatic slips that were not recognized during adolescence.
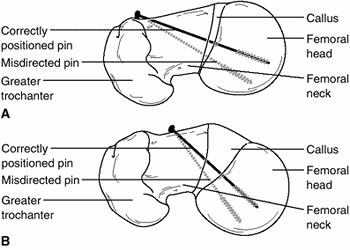 |
|
Figure 26.11
Despite an accurate starting point on the anterior femoral neck, if the angle of insertion varies from the optimal angle, the hardware will not be perpendicular to the physis and will be eccentric in the femoral head. For a given degree of misdirection, the biomechanical alignment and eccentricity in the femoral head will be worse for a mild slip (A) than it will be for a more severe slip (B) because of the longer distance the screw must traverse in a mild slip. These problems are most pronounced for a pre-slip, or a hip that is pinned prophylactically. |
to the stability of the SCFE, it is also important to estimate the
frequency of unstable contralateral metachronous slips. Because 20% to
25% of those presenting with unilateral SCFE will develop a
contralateral slip during adolescence, and because 5% to 10% of the
SCFEs are unstable, the risk of a contralateral, unstable SCFE
occurring during adolescence is 1% to 2%. With appropriate counseling
about the risk of contralateral SCFE and potential signs and symptoms
thereof, the risk of unstable contralateral SCFE may be even less.
or severe contralateral SCFE during adolescence should also be low if
there is no underlying systemic cause of SCFE and the child is
compliant with follow-up (Fig. 26.12). Most contralateral SCFEs initially noted at the time of long-term radiographic follow-up are also quite mild.
pinning of all hips will potentially prevent two complications: ON due
to unstable SCFE and late OA. Given the data provided in preceding
text, prophylactic pinning will only prevent ON in 1% or fewer of all
contralateral hips even if the rate of ON in unstable SCFE is as high
as 50%. Prophylactic pinning of contralateral hips in children with
unilateral SCFE would potentially decrease OA by 9% in the
contralateral hips of these patients, based on Hagglund’s report of
radiographic evidence of OA in 27% of hips (28 of 104) with SCFE
compared with 9% of control hips (9 of 101) at a mean follow-up of 33
years and based on his report that the risk of metachronous
contralateral SCFE is approximately 50% (28). Further, Hagglund noted that no hip with a mild or moderate slip treated with in situ
pinning developed arthritis before the age of 50 years. Because most
patients with appropriate follow-up would likely be diagnosed before a
severe contralateral slip develops, OA occurring before 50 years of age
would be expected to be unusual in contralateral hips (247). Chondrolysis will not be prevented because chondrolysis occurs almost exclusively in treated hips.
be weighed against the frequency of its potential complications,
including those of chondrolysis (2%), ON (1%), and proximal femoral
fracture (1%). It is also important to recognize that slip progression
occurs postoperatively in 0% to 3% of the cases of SCFE in most of the
series of SCFE treated with in situ fixation (2,94,97,205,209,232,233).
patient groups. Prophylactic pinning should be performed for children
with underlying endocrine disease because of their high rate of
contralateral slip. Previous pelvic radiation, which included the
contralateral hip in the field, is another indication for prophylactic
pinning. Prophylactic pinning should be strongly considered in children
younger than 10 years at the time of presentation because of potential
LLD following unilateral pinning and the high risk of bilateral
involvement in such young children (25,311).
In addition, children residing in more remote areas, who do not have
easy access to medical care, should be considered for prophylactic
fixation of the contralateral hip. In children with renal disease,
medical management rather than prophylactic pinning is recommended.
SCFE has been cited as a cause for decreased hip flexion, abduction,
and internal rotation (312). Femoral neck
osteoplasty involves removal of the prominent anterosuperior femoral
neck and may be performed alone or in combination with other
procedures, such as proximal femoral osteotomies (115,312,313).
The goals of such treatment are to enhance hip range of motion and/or
to potentially prevent anterior femoroacetabular impingement and OA (141).
anterosuperior femoral neck which abuts the acetabulum in cases of
chronic SCFE (264,313).
Symptoms that may suggest the potential benefit of osteoplasty include
pain on sitting caused by the impingement with hip flexion.
the abnormal relation between the femoral head, neck, and shaft, with
relative retroversion, extension, and varus. Because the anatomic
relation between the femoral head, neck, and shaft are not changed by
osteoplasty, isolated osteoplasty will still result in impingement of
the anterior femoral neck against the acetabulum and persistent range
of motion deficits. Previous authors have noted that
osteoplasty may further enhance hip range of motion following intertrochanteric osteotomies (115,312,313).
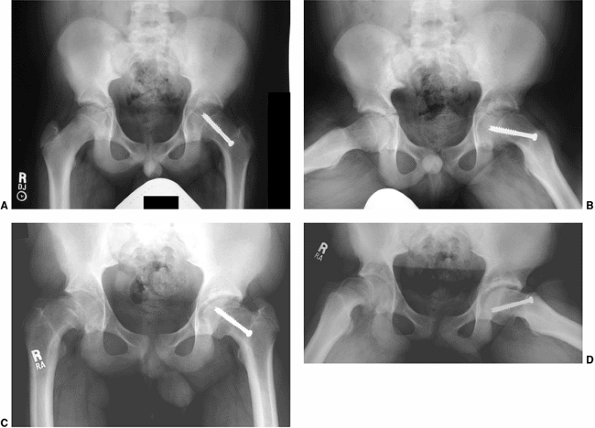 |
|
Figure 26.12 Anteroposterior (A) and frog lateral (B) radiographs show a left slipped capital femoral epiphysis (SCFE) following in situ
fixation with a single cannulated screw. The hardware is aligned adequately. Anterior metaphyseal prominence is evident. Right hip pain began several months later, although the child did not return to the orthopaedist for more than 1 year. At the time of re-presentation, Anteroposterior (C) and frog lateral (D) radiographs demonstrate marked slip of the previously normal right hip. Significant remodeling of the left hip (including resorption of the metaphyseal prominence) is visible, although there is no significant change in the femoral head–neck angle. This case exemplifies the importance of expert clinical and radiographic follow-up of children with SCFE until closure of the proximal femoral physes. |
performed for patients with symptomatic anterior impingement and/or
significantly distorted proximal femoral anatomy, proximal femoral
osteotomies are much more commonly recommended in an attempt to restore
more normal proximal femoral alignment and range of motion.
cases of SCFE is avoidance of complications. Hall noted that
complications were the only factor that seemed to lead to an early poor
result (172). Previous authors have reported
that the results of treated SCFE were often worse than those of
untreated SCFE because of the lack of catastrophic complications in
untreated hips (29,159,314).
have an uncomplicated course following a SCFE function well until at
least the 5th decade. At a mean follow-up of 41 years, Carney and
Weinstein reported Iowa hip scores of at least 80 in 26 of 31 hips
(84%) (157). At a mean follow-up of 37 years,
Ordeberg et al. reported that, of 49 cases of SCFE, only “a few”
patients had restrictions regarding their work or social lives, and
that only 2 of the 49 (4%) had required surgery for arthritis (159).
The late complications include OA, ON, and chondrolysis. OA is a nearly
inevitable late sequela of SCFE whether treated or not, whereas ON and
chondrolysis are often devastating complications that occur almost
exclusively following treatment of SCFE.
both treated and untreated SCFE because any significant biomechanical
derangement of the hip joint can lead to OA if the affected individual
reaches old age. The prevalence and severity of OA increases with the
increased time to follow-up and increased slip severity. The
complications of chondrolysis and ON markedly accelerate the
development of OA, with OA occurring in adolescents and young adults
who have a history of these serious complications (96).
deformity associated with SCFE would place the patients with SCFE at
long-term risk of OA (136). A SCFE can result
in the anterior femoral metaphysis articulating with acetabular
cartilage and can also cause impingement of the femoral neck against
the anterior acetabulum (136). As noted
earlier, in an attempt to potentially decrease this long-term risk of
arthritis from such mechanical malalignment, some authors use such
biomechanical studies to advocate early osteotomies in children with
significant residual deformity following SCFE (86,269,270,295,296).
persons, only 2% to 9% of those with end-stage OA have been reported to
have a history of SCFE (160,161,162,163,164).
Studies of adults undergoing THA have shown that up to 40% of these
patients have evidence of pediatric hip disease, including SCFE, at the
time of joint arthroplasty (167). Radiographic
studies of adult hips also demonstrate the stigmata of pediatric hip
disease in up to 40% of those with findings of OA (166).
Not all authors agree with these studies, and some have noted that
these radiographic findings are common in end-stage OA of various
etiologies (168).
results in significant biomechanical changes in the hip because a
portion of the proximal femoral metaphysis articulates with the
acetabulum and leads to accelerated degenerative changes in the hip.
Recent authors have sought to prevent late arthritis by restoring more
normal proximal femoral anatomy by performing proximal femoral
redirectional osteotomies (269,270). Although such authors report an apparent decrease in OA following realignment at follow-up beyond 20 years in each study (269,270),
longer follow-up will be needed to know if these apparently superior
results continue in the ensuing decades, and to decide whether such
procedures are indicated in patients who are asymptomatic despite
significant residual deformity.
sequela of both treated and untreated SCFE, with earlier onset and more
severe degeneration in high-degree slips. The complications of ON and
chondrolysis greatly accelerate the development of OA and often lead to
end-stage OA in adolescence.
serious complications encountered in the treatment of children with
SCFE. ON is reported to occur in 4% to 25% of the cases of SCFE in most
series and is found almost exclusively in hips classified as acute on a
temporal basis or unstable (as classified by Loder). The rate of ON is
most commonly reported as 10% to 15% in acute or acute-on-chronic SCFE (95,96,133,214).
children with SCFE: disruption of the blood supply preoperatively and
disruption of the blood supply due to the surgery itself. With current
techniques, including recognition of the posterosuperior blood supply
to the femoral head and the importance of accurate hardware placement,
the risk of iatrogenic ON should decrease.
ON in unstable SCFE is debated. In two series in which ON was noted to
occur only in unstable SCFE, Kennedy et al. (173) reported that the degree of slip does not appear to be an independent predictor of ON, whereas Tokmakova et al. (174)
reported that the degree of slip is a risk factor for developing ON.
When considering this, however, the degree of displacement evident
radiographically at the time of presentation may have no relation to
the true amount of maximal displacement that has already occurred or
will occur prior to operative stabilization.
method of treatment affects the rate of ON. The incidence of ON
following proximal femoral osteotomy is greatest with subcapital
osteotomies and progressively decreases with more distal osteotomies (21,172,266,271,274,275,277,278,279,280,282,283). Most authors report ON rates of 5% to 35% following subcapital osteotomy, with rates at times as high as 42% (21,271,274,275,277,278,279,280,282,283). Base-of-neck osteotomies result in ON in 0% to 5% of the cases (284,285,286). Most authors do not report any cases of ON following intertrochanteric osteotomy for SCFE (115,266,298,299), although rates of up to 6% have been reported (270,297,300,301).
of ON previously associated with spica casting were likely due to a
manipulative reduction prior to cast application and/or positioning in
the cast.
In a series of 22 patients with 24 cases of SCFE complicated by ON, who
were followed an average of 31 years, nine of the hips (38%) had
required salvage treatment and the other 15 hips had osteoarthritic
changes that were evident radiographically (316).
treatment is aimed at maintenance of the range of motion, prevention of
progressive femoral head collapse, and joint preservation when
possible. The combination of antiinflammatory medications, physical
therapy, and protected weight bearing may be helpful in maintaining the
range of motion and preventing progressive femoral head collapse. When
femoral head collapse occurs in the area of previously placed screws,
the screws must often be backed out or removed in order to prevent
joint penetration and chondrolysis.
have been reported following ON in children, although no large series
has been reported specifically addressing ON following SCFE.
collapse, the long-term prognosis is significantly worse. With
progressive collapse and joint degeneration, salvage procedures are
often necessary.
of SCFE. With the passage of time, hips with ON complicating SCFE will
inexorably develop arthritic changes if left untreated. Even if ON is
detected early, salvage procedures are often necessary for these hips.
As a result, one of the prime goals in the treatment of SCFE should be
the avoidance of ON.
present with markedly displaced, unstable slips, and those with
evidence of gross instability at the time of surgery—I recommend
routine MRI screening for ON approximately 1 month following operative
stabilization. Following MRI, those patients without ON can be allowed
to bear weight as tolerated and return to sporting activities by 3 to 6
months postoperatively, as do those children with uncomplicated stable
slips. For those with ON, the distribution and extent of femoral head
involvement must be determined and further treatment (such as free
vascularized fibular grafting or proximal femoral osteotomy) considered.
However, if a child initially does well in the weeks or months
immediately after surgery and then begins to have recurrent hip
symptomatology, ON must be considered. Alternatively, ON may first be
evident months after fixation on routine follow-up radiographs. In
either suspected or documented ON, MRI is a critical part of a thorough
evaluation.
treatment of ON carries a better prognosis when undertaken prior to
femoral head collapse. I believe that free vascularized fibular
grafting, despite donor site morbidity, is the most appropriate option
for a hip with segmental ON involving the weight-bearing portion of the
femoral head before femoral head collapse. If ON is not detected until
after femoral head collapse occurs, then I would consider a
redirectional femoral osteotomy if a sufficient pillar of viable bone
can be moved into a weight-bearing position.
to remove any hardware that is protruding into the joint and to replace
screws into another area of the head so long as the physis remains open.
with ON, either because it is not indicated (as with severe collapse)
or because such treatment is declined by the patient’s family,
conservative measures should be undertaken in an attempt to delay
salvage treatment. Impact activities such as running, jumping, and ball
sports should be avoided, whereas swimming and bicycling may be
undertaken to maintain cardiovascular fitness, strength, and range of
motion. Antiinflammatory medications and ambulatory aids may be
beneficial as well, although these are often rejected by otherwise
healthy adolescents and young adults.
Chondrolysis involves cartilage destruction of both the femoral head
and the acetabulum, and is defined as the triad of pain, decreased hip
range of motion, and radiographic joint space narrowing (Fig. 26.13).
Normal cartilage thickness of the pediatric hip has been reported to
decrease from a mean of 6 mm in children aged 1 to 7 years, to 5 mm in
those aged 8 to 12 years, and to 4 mm in those aged 13 to 17 years (324). Chondrolysis has been reported to occur in 0% to 28% of patients with SCFE (4,94,100,187,189,228,325,326,327,328).
range of motion are not improved in the first 2 to 3 weeks following
surgery, and any child with decreasing range of motion postoperatively
must be suspected of having chondrolysis. Unlike many other hip
maladies, chondrolysis causes the hip to be held in abduction and
ultimately results in a fixed abduction contracture.
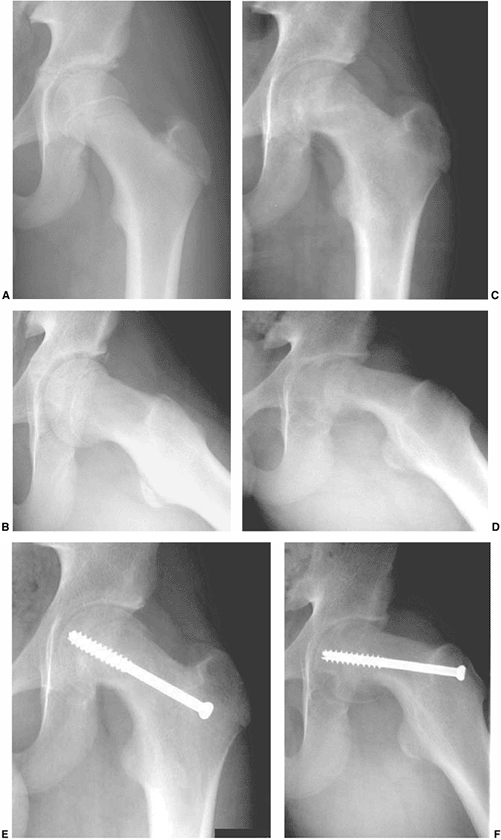 |
|
Figure 26.13 Left hip chondrolysis in a 13-year-old boy. A,B:
Normal joint space of the left hip when the patient presented with a right slipped capital femoral epiphysis (SCFE). Ten months later, the patient presented to the office with a one-month history of left hip pain. C, D: Radiographs at that time demonstrated a left SCFE and joint space narrowing. The left hip was pinned in situ with prompt symptom resolution. However, 2 months postoperatively the patient began to have increased hip pain, difficulty walking, and decreased hip range of motion. E, F: Radiographs at that time revealed mild additional joint space narrowing. This case demonstrates that joint space narrowing can occur without treatment, but that chondrolysis is not simply joint space narrowing. Rather, it is the triad of joint space narrowing, pain, and decreased range of motion. |
and has also been reported to be present at the time of initial
presentation in some patients (228,230).
Maurer suggested that chondrolysis was more common with severe slips
and with spica casting, open reduction, or prolonged casting (327).
Ingram et al. noted that chondrolysis was more common in
acute-on-chronic slips, and that the highest rates occurred with
osteotomies and the lowest rates with in situ fixation (228). Chondrolysis in the unaffected hip has been reported following immobilization (29,177).
SCFE with spica casting, with its incidence reported as 14% to 53% of
the cases (94,175,176). Rates of chondrolysis are commonly reported as 3% to 18% following subcapital osteotomy (274,275,276,278,279,280,281,283), 2% to 10% following base-of-neck osteotomy (284,285), and 2% to 25% following intertrochanteric osteotomy (115,228,266,287,297,298,301). Reported rates of chondrolysis following in situ pinning and bone peg epiphysiodesis are most commonly less than 5% (2,21,94,116,187,188,189,190,193,196,205,213,227,228,253,254,262,265).
may also reveal premature closure of the apophysis of the greater and
lesser trochanters (228,332).
Bone scan has been noted to demonstrate decreased activity in the
apophysis of the greater trochanter in 47% of hips affected with
chondrolysis, a finding that may precede radiographic changes (333). In cases with an unusual presentation, workup for a septic hip including joint aspiration may be indicated.
self-limited condition from which full recovery may occur to the rapid
destruction of a joint necessitating salvage treatment in teenagers (230,328,334).
Despite decades of experience in treating children with chondrolysis,
the reasons for such disparate prognoses remain unclear. When an
individual patient presents with chondrolysis, it is still impossible
to accurately predict the child’s prognosis.
with a combination of protected weight bearing, physical therapy (for
range of motion and attempted strengthening of the hip musculature),
and oral antiinflammatory medications. Distraction of the hip joint
with external fixation has been reported to be of value in selected
cases (335). Failure of such conservative measures may require surgical intervention such as arthrodesis or arthroplasty.
complications of SCFE. Early recognition and treatment are indicated,
but the prognosis following this complication is guarded. In patients
unresponsive to conservative measures, salvage procedures may be
necessary.
7th decades of life if the complications of chondrolysis and ON are
avoided. Unfortunately, if treatment is complicated by chondrolysis
and/or ON, rapid clinical deterioration may occur in adolescence or
early adulthood. Significant symptomatology such as pain with sitting,
with sleeping, and with activities of daily living may necessitate
salvage treatment. In the child with such significant symptomatology
following SCFE that has been complicated by ON, joint-preserving
procedures including osteotomies, vascularized fibular grafting, and
bone grafting procedures are sometimes possible, although such
procedures are not beneficial in children with chondrolysis following
SCFE because of their diffuse joint destruction.
are not good candidates for joint-sparing procedures, then salvage
treatment with hip fusion or hip arthroplasty should be considered. The
extent of hip disease in both hips is important in making the decision
regarding hip arthrodesis versus arthroplasty. Patients can be thought
of as falling into one of three categories: a unilateral salvage hip
with a normal contralateral hip, a unilateral salvage hip with a mild
contralateral SCFE, or bilateral salvage hips. In an adolescent with a
unilateral salvage hip and either a normal contralateral hip or a mild
contralateral
SCFE,
either hip fusion or arthroplasty may be considered. Hip fusion should
never be considered in a case with bilateral salvage hips.
choice for a degenerated hip in adolescents with unilateral hip disease
because of the poor long-term results of THA in heavy, young, active
patients (336,337,338,339).
Currently, many teenagers and their families are reluctant to accept
the physical limitations associated with hip arthrodesis, despite the
less than stellar results of hip arthroplasty in young patients.
ultrahigh-molecular-weight polyethylene has been the gold standard
since the earliest total joint replacements. The long-term results in
young patients have been inferior to those in older patients, with much
of the loosening attributable to the generation of particulate debris
and component loosening (161,338,339,340,341).
New bearing surfaces such as those including highly cross-linked
polymers, metal-on-metal, and ceramics have given hope to a new
generation of surgeons and patients about the potential for hip
arthroplasty in the young and active population. However, such promise
is currently just that, and the current reality is that THA is expected
to have a limited lifespan in this patient population and that multiple
revisions will likely be necessary throughout adulthood.
considered if the contralateral hip is normal or has only a mild SCFE
due to the increased demand that would be placed on the contralateral
hip following hip fusion of the affected hip. Many procedures have been
described for hip fusion including intraarticular and extraarticular
fusion using a variety of fixation devices including screws, plates,
and external fixation (342,343,344,345,346,347).
The most common hip fusion technique in children currently is an
intraarticular fusion with subtrochanteric osteotomy, which has been
reported by multiple authors since it was first reported by Farkas in
1939 (344,348,349,350).
This technique is felt to promote fusion because contact between the
femoral head and acetabulum can be maximized and the long lever arm of
the leg is avoided by performing the subtrochanteric osteotomy. With
the typical deformity following SCFE and ON, optimizing the fit between
femoral head and acetabulum would result in unacceptable positioning of
the leg without subtrochanteric osteotomy. Further, the alignment of
the leg can be readjusted postoperatively if needed.
the fusion and the ability to return to full activity, including manual
labor. Long-term results in studies with mean follow-up exceeding 35
years have been quite satisfying, although reported findings include
back pain in 57% to 61%, ipsilateral knee pain in 45% to 57%, and
contralateral hip pain in 17% to 27% (343,347). Conversion to total joint arthroplasty was reported in 13% to 21% in the two studies (343,347).
There is reliable relief of back, hip, or knee pain, although the
results are not comparable to those with primary joint replacements.
The results are also better in hips that have fused spontaneously than
in those which have undergone surgical fusion. Technically, the
conversion procedure is much easier and the results are better if the
abductor musculature was not disturbed with the initial procedure.
not an option and arthroplasty should be considered if symptoms are
severe. The advantages of THA are the rapid restoration of motion and
function without added stress across the contralateral hip, ipsilateral
knee, and the spine. Because many of these patients are rather active,
once the THA renders them essentially asymptomatic early failure is a
frequent result. In a recent series of primary THA in patients 50 years
and younger at implantation, THA survivorship was only 54% at 15-year
follow-up (339). Others have reported actual or
potential loosening in 57% of the prostheses at the 5-year follow-up in
patients who had undergone THA prior to 30 years (337).
One study of THA in patients aged less than 50 years reported more
encouraging results, noting that the survivorship of the original
prosthesis was 63% in patients living at least 25 years postoperatively
(161).
amenable to redirectional osteotomy needs salvage treatment, the two
options that remain are THA and hip arthrodesis. Neither of these
treatments has outstanding long-term results for the hip and the other
joints of the lower extremity or for the spine. Currently, decisions
continue to be made on a case-by-case basis to choose between THA (with
better short-term hip function and less risk to other joints) and hip
arthrodesis (with better long-term durability but more risk to the
remainder of the lower extremities and spine). Promising technologic
advances in bearing surfaces hold hope for the future of THA in young,
active patients.
the most appropriate salvage treatment of a given hip, as well as the
current and future demands that would be placed upon the hip. As noted,
hip arthrodesis or arthroplasty are both reasonable options in an
adolescent with a unilateral salvage hip and no or mild contralateral
hip disease, and arthroplasty is the only reasonable choice in a child
with bilateral salvage hips.
suboptimal options for treating a unilateral salvage hip in adolescents
with SCFE. In the short term, the range of motion and rehabilitation
benefits clearly favor arthroplasty. Although the patients can walk
extremely well following arthrodesis, within several years of an
arthrodesis they may have back and ipsilateral knee pain at the end of
the day. These adolescents also often struggle with routine activities
including donning and doffing socks, cutting toenails, riding a
bicycle, and climbing stairs.
define precisely. The risks of arthroplasty involve the affected hip
itself, and can be marked and repetitive because of wear, loosening,
and dislocation. In contrast, the complications of arthrodesis most
frequently are resultant degeneration in the lumbar spine, ipsilateral
knee, and contralateral hip. An additional confounding variable is the
uncertainty of the future changes in orthopaedics and basic science,
which may dramatically alter the implications of these long-term risks.
adolescents exceeding a weight of 200 pounds, given the high demands
placed on such a joint. For those weighing less than 200 pounds, I
discuss the options of both hip fusion and hip replacement, with the
understanding that impact-type sporting activities must be avoided
following either surgery. Some young women are not interested in
arthrodesis because of concerns regarding sexuality and childbearing.
Only the patient and family can make the ultimate decision between
arthroplasty and arthrodesis.
CR, Knight D, Mainds CC, et al. Slipped upper femoral epiphysis—a
review of 12 years of experience in Glasgow (1972–1983). J Pediatr Orthop 1987;7:283–287.
R, Billing L, Hansson G, et al. Bilaterality in slipped capital femoral
epiphysis: importance of a reliable radiographic method. J Pediatr Orthop B 1996;5:80–84.
V. Epiphysiolysis of the head of the femur; a follow-up examination
with special reference to end results and the social prognosis. Acta Orthop Scand 1953;23:100–120.
JL, Keggi KJ, Southwick WO. The incidence and distribition of slipped
capital femoral epiphysis in Connecticut and Southwestern United
States. J Bone Joint Surg Am 1970;52: 1203–1216.
D, Dimeglio A, Bentahar T. Staging puberty in slipped capital femoral
epiphysis: importance of the triradiate cartilage. J Pediatr Orthop 2004;24:144–147.
RT, Aronson DD, Greenfield ML. The epidemiology of bilateral slipped
capital femoral epiphysis. A study of children in Michigan. J Bone Joint Surg Am 1993;75:1141–1147.
DB, Kasser JR, Sponseller P, et al. Slipped capital femoral epiphysis.
A quantitative analysis of motion, gait, and femoral remodeling after
in situ fixation. J Bone Joint Surg Am 1991;73: 659–666.
JS, Taylor B, Johnston CE II. Comparison of single pin versus multiple
pin fixation in treatment of slipped capital femoral epiphysis. J Pediatr Orthop 1992;12:384–389.
DP. The John Charnley Award paper. Experimental epiphysiolysis:
etiologic models slipped capital femoral epiphysis. In: Nelson JP, ed. The Hip: Proceedings of the Tenth Open Scientific Meeting of the Hip Society. St. Louis, MO: CV Mosby Company, 1982: 68–88.
PC, Cady RB. Endocrinologic and metabolic factors in atypical
presentations of slipped capital femoral epiphysis. Report of four
cases and review of the literature. Clin Orthop 1983;180:188–197.
GA, Hughston JC. Slipped capital femoral epiphysis in a true
hypogonadal male (Klinefelter’s mosaic XY-XXY). A case report. J Bone Joint Surg Am 1971;53:597–601.
L, Al-Quaimi M, Ahmad A. Slipped capital femoral epiphysis associated
with primary hyperparathyroidism and severe hypercalcemia. Clin Pediatr (Phila) 2003;42:439–441.
JL, Vani JN, Eick JD, et al. Shear strength of the physis varies with
anatomic location and is a function of modulus, inclination, and
thickness. J Orthop Res 1999;17:214–222.
FW, Greenwood RH, Boase DL. Slipping of the upper femoral epiphyses in
patients with intracranial tumours causing hypopituitarism and chiasmal
compression. J Bone Joint Surg Br 1976;58:169–175.
WG Jr, Urbaniak JR, Ogden WS, et al. Acquired hypothyroidism and
slipped capital femoral epiphysis. Report of three cases. J Bone Joint Surg Am 1976;58:705–708.
RD, Grasemann H, Oberste-Berghaus C, et al. Serum insulin-like growth
factors IGF-I and IGFBP-3 in children with slipped capital femoral
epiphysis. J Pediatr Orthop B 1999;8: 103–106.
DC, Weddington J, Richton S. Hormonal studies in patients with slipped
capital femoral epiphysis without evidence of endocrinopathy. J Pediatr Orthop 1988;8:543–545.
S, Hara T, Sugioka Y. Deficiency of a parathyroid hormone fragment
containing the midportion and 1,25-dihydroxyvitamin D in serum of
patients with slipped capital femoral epiphysis. J Pediatr Orthop 1997;17:216–219.
SL, Rundle AC. Slipped capital femoral epiphysis in children treated
with growth hormone. A summary of the National Cooperative Growth Study
experience. Horm Res 1996; 46:113–116.
RN, Ho M, Tejani A, et al. Adverse events with rhGH treatment of
patients with chronic renal insufficiency and end-stage renal disease. J Pediatr 2003;142:539–545.
J, Millis M, Jolesz FA, et al. Three-dimensional analysis of the
proximal femur in patients with slipped capital femoral epiphysis based
on computed tomography. J Pediatr Orthop 2001;21:179–182.
N, Weiner DS, Askew M. The evolving slope of the proximal femoral
growth plate relationship to slipped capital femoral epiphysis. J Pediatr Orthop 1988;8:268–273.
RT, Mehbod AA, Meyer C, et al. Acetabular depth and race in young
adults: a potential explanation of the differences in the prevalence of
slipped capital femoral epiphysis between different racial groups? J Pediatr Orthop 2003;23:699–702.
J, Richolt JA, Millis M, et al. Development of the acetabulum in
patients with slipped capital femoral epiphysis: a three-dimensional
analysis based on computed tomography. J Pediatr Orthop 2001;21:174–178.
MD, Weiner DS, Green NE, et al. Acute slipped capital femoral
epiphysis: the value and safety of urgent manipulative reduction. J Pediatr Orthop 1997;17:648–654.
MJ, Patton CM, Luhmann S, et al. Knee pain as the initial symptom of
slipped capital femoral epiphysis: an analysis of initial presentation
and treatment. J Pediatr Orthop 1999;19: 455–460.
DL, Roy AK, Vitale MG, et al. Quality of evaluation and management of
children requiring timely orthopaedic surgery before admission to a
tertiary pediatric facility. J Pediatr Orthop 2002;22:265–267.
RT, Greenfield ML. Clinical characteristics of children with atypical
and idiopathic slipped capital femoral epiphysis: description of the
age-weight test and implications for further diagnostic investigation. J Pediatr Orthop 2001;21:481–487.
MS, Gelberman RH, Griffin PP, et al. Slipped capital femoral epiphysis:
assessment of epiphyseal displacement and angulation. J Pediatr Orthop 1986;6:259–264.
L, Bogren HG, Wallin J. Reliable X-ray diagnosis of slipped capital
femoral epiphysis by combining the conventional and a new simplified
geometrical method. Pediatr Radiol 2002;32:423–430.
DW, Mickelson MR, Ponseti IV. Slipped capital femoral epiphysis.
Long-term follow-up study of one hundred and twenty-one patients. J Bone Joint Surg Am 1981;63:85–95.
V, Falciglia F. Slipped capital femoral epiphysis: comparison of a
roentgenographic method and computed tomography in determining slip
severity. J Pediatr Orthop 1991;11:6–12.
H, Liebling MS, Moy L, et al. Slipped capital femoral epiphysis: a
physeal lesion diagnosed by MRI, with radiographic and CT correlation. Skeletal Radiol 1998;27:139–144.
PE, Lequesne GW, Paterson DC, et al. Ultrasonography in slipped capital
femoral epiphysis. Diagnosis and assessment of severity. J Bone Joint Surg Br 1991;73:884–889.
PE, Mah ET, Foster BK, et al. Slipped capital femoral epiphysis.
Incidence and clinical assessment of physeal instability. J Bone Joint Surg Br 1995;77:752–755.
PE, Paterson DC, Foster BK, et al. Classification in slipped capital
femoral epiphysis. Sonographic assessment of stability and remodeling. Clin Orthop 1993;294:196–203.
K, Vade A, Lomasney LM, et al. Radiologic case study. Bilateral slipped
capital femoral epiphysis, acute on the left and preslip on the right. Orthopedics 2001;24:737, 808–809, 811–812.
T, Suzuki S, Seto Y, et al. Sequential magnetic resonance imaging in
slipped capital femoral epiphysis: assessment of preslip in the
contralateral hip. J Pediatr Orthop B 2001;10:298–303.
B, Chotel F, Vargas Barreto B, et al. The value of early postoperative
bone scan in slipped capital femoral epiphysis. J Pediatr Orthop B 2001;10:51–55.
JE, Abrahams MS, Dobbs MB, et al. Early reduction, arthrotomy, and
cannulated screw fixation in unstable slipped capital femoral epiphysis
treatment. J Pediatr Orthop 2002;22: 352–358.
KE, Pelker RR, Rudicel SA, et al. Histologic patterns of capital
femoral growth plate fracture in the rabbit: the effect of shear
direction. J Pediatr Orthop 1985;5:32–39.
M, Casillas MM, Hamlet M, et al. Slipped capital femoral epiphysis:
early mechanical damage to the acetabular cartilage by a prominent
femoral metaphysis. Acta Orthop Scand 2000;71: 370–375.
DP, Weiner DS. Slipped capital femoral epiphysis: a pathologic
investigation into light microscopy, histochemistry and ultrastructure.
Orthop Trans 1985;9:496–497.
DP, Weiner DS, Lloyd JK. Slipped capital femoral epiphysis: a
pathological study. I. A light microscopic and histochemical study of
21 cases. J Pediatr Orthop 1985;5:40–46.
DP, Weiner DS, Lloyd JK. Slipped capital femoral epiphysis: a
pathological study. II. An ultrastructural study of 23 cases. J Pediatr Orthop 1985;5:47–58.
V, Falciglia F, Stanitski CL, et al. Slipped capital femoral epiphysis:
physeal histologic features before and after fixation. J Pediatr Orthop 2003;23:571–577.
G, Billing L, Hogstedt B, et al. Long-term results after nailing in
situ of slipped upper femoral epiphysis. A 30-year follow-up of 59
hips. J Bone Joint Surg Br 1998;80:70–77.
G, Hansson LI, Sandstrom S. Slipped capital femoral epiphysis in
southern Sweden. Long-term result with no treatment or symptomatic
primary treatment. Clin Orthop 1984;191:95–104.
JD, Callaghan JJ, Goetz DD, et al. Twenty-five-year results after
Charnley total hip arthroplasty in patients less than fifty years old:
a concise follow-up of a previous report. J Bone Joint Surg Am 2003;85-A:1066–1072.
CS, Atkinson RE, Salvati EA, et al. Conventional total hip arthroplasty
for degenerative joint disease in patients between the ages of forty
and sixty years. J Bone Joint Surg Am 1984;66:745–752.
DA, Feighan JE, Smith AD, et al. Subclinical slipped capital femoral
epiphysis. Relationship to osteoarthrosis of the hip. J Bone Joint Surg Am 1997;79:1489–1497.
SD, Cordell LD, Harris WH, et al. Unrecognized childhood hip disease: a
major cause of idiopathic osteoarthritis of the hip. In: Amstutz HC,
ed. The hip: Proceedings of the Third Open Scientific Meeting of the Hip Society. St. Louis, MO: CV Mosby Company, 1975:212–228.
KP, Stanton RP, Mason DE. Factors influencing the development of
osteonecrosis in patients treated for slipped capital femoral
epiphysis. J Bone Joint Surg Am 2003;85-A:798–801.
R, Simon SR. Joint destruction: a sequel of unrecognized pin
penetration in patients with slipped capital femoral epiphysis. In:
Riley LH Jr., ed. The Hip: Proceedings of the Eighth Open Scientific Meeting of the Hip Society. St. Louis, MO: CV Mosby Company, 1980:145–164
WW, Johnson JT, Robertson WW Jr. Single screw fixation for acute and
acute-on-chronic slipped capital femoral epiphysis. Clin Orthop 1996;322:86–90.
G, Carsi B, Abril JC, et al. Results after preoperative traction and
pinning in slipped capital femoral epiphysis: K wires versus cannulated
screws. J Pediatr Orthop B 1998;7:53–58.
Sanctis N, Di Gennaro G, Pempinello C, et al. Is gentle manipulative
reduction and percutaneous fixation with a single screw the best
management of acute and acute-on-chronic slipped capital femoral
epiphysis? A report of 70 patients. J Pediatr Orthop B 1996;5:90–95.
JL, Davidson RS, Ellis RD, et al. Slipped capital femoral epiphysis: an
analysis of 80 patients as to pin placement and number. J Pediatr Orthop 1986;6:265–273.
WB, Menche D, Grant A, et al. The problem of evaluating in situ pinning
of slipped capital femoral epiphysis: an experimental model and a
review of 63 consecutive cases. J Pediatr Orthop 1984;4:297–303.
WB, Grant A, Rose D, et al. A method of evaluating possible pin
penetration in slipped capital femoral epiphysis using a cannulated
internal fixation device. Clin Orthop 1984; 186:65–70.
LA, Doane RM, Cornicelli SF, et al. Single versus double screw fixation
for treatment of slipped capital femoral epiphysis: a biomechanical
analysis. J Pediatr Orthop 1992;12:741–745.
DM, Herzenberg JE, Viviano DM, et al. Biomechanical comparison of
single- and double-pin fixation for acute slipped capital femoral
epiphysis. Clin Orthop 1990;259:277–281.
LJ, Doane RM, Karol LA, et al. Biomechanical analysis of single- versus
double-screw fixation in slipped capital femoral epiphysis at
physiological load levels. J Pediatr Orthop 1994;14:627–630.
SD, Hedman TP, Reynolds RA. Biomechanical analysis of compression screw
fixation versus standard in situ pinning in slipped capital femoral
epiphysis. J Pediatr Orthop 2001;21: 183–188.
J, Fabry G, Molenaers G, et al. Slipped capital femoral epiphysis: a
long-term follow-up, with special emphasis on the capacities for
remodeling. J Pediatr Orthop B 1996;5: 151–157.
R, Billing L, Karlsson J. Loss of hip motion in slipped capital femoral
epiphysis: a calculation from the slipping angle and the slope. J Pediatr Orthop B 1996;5:144–150.
GPK, Cole WG. Effect of early hip decompression on the frequency of
avascular necrosis in children with fractures of the neck of the femur.
Injury 1996;27:419–421.
MF, Tepic S, Perren SM, et al. Laser Doppler flowmetry for bone blood
flow measurement: correlation with microsphere estimates and evaluation
of the effect of intracapsular pressure on femoral head blood flow. J Orthop Res 1986; 4:362–371.
SA, Griffiths WE, Clarke NM. The timing of reduction and stabilisation
of the acute, unstable, slipped upper femoral epiphysis. J Bone Joint Surg Br 2001;83:1046–1049.
GS, Kime RC, Fitch RD, et al. Treatment of osteonecrosis in the hip of
pediatric patients by free vascularized fibula graft. Clin Orthop 2001;386:106–113.
AL, Ehrlich MG, Armstrong AL, et al. Role of pin protrusion in the
etiology of chondrolysis: a surgical model with radiographic,
histologic, and biochemical analysis. J Pediatr Orthop 1992;12:428–433.
LE, Simonian PT, Harvey JP Jr. Transient penetration of the hip joint
during in situ cannulated-screw fixation of slipped capital femoral
epiphysis. J Bone Joint Surg Am 1991;73: 1054–1060.
BT, Birnbaum P, Minter C. Slip progression after in situ single screw
fixation for stable slipped capital femoral epiphysis. J Pediatr Orthop 2003;23:584–589.
R, Karlsson J, Romanus B, et al. Does a single device prevent further
slipping of the epiphysis in children with slipped capital femoral
epiphysis? Arch Orthop Trauma Surg 1997;116:348–351.
GM, Prymka M, Hassenpflug J. The role of prophylactic pinning in the
treatment of slipped capital femoral epiphysis—a case report. Acta Orthop Scand 1999;70:631–634.
ST, Azar F, Young J, et al. Subtrochanteric fracture after fixation of
slipped capital femoral epiphysis: a complication of unused drill
holes. J Pediatr Orthop 1994;14:623–626.
GC, Lucie RS, Cummings RJ. Femoral neck fracture secondary to in situ
pinning of slipped capital femoral epiphysis: a previously unreported
complication. J Pediatr Orthop 1991;11:187–190.
ST, Casillas M, Banta JV. Displaced femoral neck fractures at the
bone-screw interface after in situ fixation of slipped capital femoral
epiphysis. J Pediatr Orthop 1997;17:212–215.
MJ, Weiner DS, Hawk D. A 50-year experience with bone graft
epiphysiodesis in the treatment of slipped capital femoral epiphysis. J Pediatr Orthop 2003;23:578–583.
DS, Weiner S, Melby A, et al. A 30-year experience with bone graft
epiphysiodesis in the treatment of slipped capital femoral epiphysis. J Pediatr Orthop 1984;4:145–152.
DS, Weiner SD, Melby A. Anterolateral approach to the hip for bone
graft epiphysiodesis in the treatment of slipped capital femoral
epiphysis. J Pediatr Orthop 1988;8:349–352.
RN, Rosenzweig AH, Cotler HB, et al. Epiphysiodesis in slipped capital
femoral epiphysis: a comparison of various surgical modalities. J Pediatr Orthop 1985;5:661–664.
CH, Heyman CH, Bell DM. Treatment of slipped capital femoral epiphysis
by epiphyseodesis and osteoplasty of the femoral neck. A report of
further experiences. J Bone Joint Surg Am 1963;45:999–1012.
O, Antolic V, Iglic A, et al. The assessment of contact stress in the
hip joint after operative treatment for severe slipped capital femoral
epiphysis. Int Orthop 2001;25:9–12.
K, Cordier W, Katthagen BD. Long-term follow-up study after corrective
Imhauser osteotomy for severe slipped capital femoral epiphysis. J Pediatr Orthop 2000;20:749–756.
PA, Exner GU, Hansch O. Prevention of secondary coxarthrosis in slipped
capital femoral epiphysis: a long-term follow-up study after corrective
intertrochanteric osteotomy. J Pediatr Orthop B 1996;5:135–143.
K, Sakamaki T, Ishii Y. Follow-up study of the subcapital wedge
osteotomy for severe chronic slipped capital femoral epiphysis. J Pediatr Orthop 1989;9:412–416.
DM, Angel JC. Replacement of the femoral head by open operation in
severe adolescent slipping of the upper femoral epiphysis. J Bone Joint Surg Br 1978;60-B:394–403.
D, Forgues D, Mayrargue E, et al. Follow-up study of severe slipped
capital femoral epiphysis treated with Dunn’s osteotomy. J Pediatr Orthop 2000;20:320–325.
R, Schai PA, Exner GU. Slipped capital femoral epiphysis: a long-term
follow-up study after open reduction of the femoral head combined with
subcapital wedge resection. J Pediatr Orthop B 1998;7:43–52.
JR, Sundberg AB, Nolan DR, et al. Complications after cuneiform
osteotomy for moderately or severely slipped capital femoral epiphysis.
J Bone Joint Surg Am 1978;60:157–165.
E, Garst J, Barmada R. Treatment of moderate to severe slipped capital
femoral epiphysis with extracapsular base-of-neck osteotomy. J Pediatr Orthop 1993;13:294–302.
R, Bruch RF, Gimbel JS, et al. Base of the neck extracapsular osteotomy
for correction of deformity in slipped capital femoral epiphysis. Clin Orthop 1978;132:98–101.
WO. Compression fixation after biplane intertrochanteric osteotomy for
slipped capital femoral epiphysis. A technical improvement. J Bone Joint Surg Am 1973;55: 1218–1224.
RA. Compression external fixation after biplane femoral trochanteric
osteotomy for severe slipped capital femoral epiphysis. J Bone Joint Surg Am 1980;62:557–560.
MA, Sweet MB, Jakim I. Acute-on-chronic bilateral reversed slipped
capital femoral epiphysis managed by Imhauser-Weber osteotomy. Arch Orthop Trauma Surg 1989;108:336–338.
EA, Robinson JH Jr, O’Down TJ. Southwick osteotomy for severe chronic
slipped capital femoral epiphysis: results and complications. J Bone Joint Surg Am 1980;62:561–570.
K, Zehender H, Buhl T, et al. Intertrochanteric corrective osteotomy
for moderate and severe chronic slipped capital femoral epiphysis. J Pediatr Orthop B 1999;8:223–230.
Y. Transtrochanteric anterior rotational osteotomy of the femoral head
in the treatment of osteonecrosis affecting the hip: a new osteotomy
operation. Clin Orthop 1978;130:191–201.
Y. Transtrochanteric rotational osteotomy in the treatment of
idiopathic and steroid-induced femoral head necrosis, Perthes’ disease,
slipped capital femoral epiphysis, and osteo-arthritis of the hip.
Indications and results. Clin Orthop 1984; 184:12–23.
T, Matsuno T, Hasegawa I, et al. Transtrochanteric anterior rotational
osteotomy for slipped capital femoral epiphysis: a report of five
cases. J Pediatr Orthop 1986;6:18–23.
FP Jr, Bennett JT, Doulens K. Epidemiological perspective on
prophylactic pinning in patients with unilateral slipped capital
femoral epiphysis. J Pediatr Orthop 2000;20:745–748.
DA, Schmidt J, Eisenburger SH, et al. Prophylactic dynamic screw
fixation of the asymptomatic hip in slipped capital femoral epiphysis. J Pediatr Orthop 1996;16:249–253.
WR, Weinstein JN, Weinstein SL, et al. Prophylactic pinning of the
contralateral hip in slipped capital femoral epiphy-sis: evaluation of
long-term outcome for the contralateral hip with use of decision
analysis. J Bone Joint Surg Am 2002;84-A:1305–1314.
LS, Davidson RS, Robertson WW Jr, et al. Growth disturbances of the
proximal femur after pinning of juvenile slipped capital femoral
epiphysis. J Pediatr Orthop 1991;11:631–637.
LA, Schoenecker PL. Combined valgus derotation osteotomy and cervical
osteoplasty for severely slipped capital femoral epiphysis: mechanical
analysis and report of preliminary results using compression screw
fixation and early weight bearing. Clin Orthop 1978;132:88–97.
H, Vogt JC, Barba L, et al. Treatment of slipped upper femoral
epiphysis: 80 cases operated on over 10 years (1968–1978). J Pediatr Orthop 1984;4:153–161.
B, Hoffer M, Weinert C, et al. Percutaneous in situ fixation of slipped
capital femoral epiphysis using two threaded Steinmann pins. J Pediatr Orthop 1996;16:56–60.
TH, Canale ST, Beaty JH, et al. Long-term follow-up of patients with
avascular necrosis after treatment of slipped capital femoral
epiphysis. J Pediatr Orthop 1993;13:154–158.
HP, Chou LB, Ganz R. Open-reduction and inter-trochanteric osteotomy
for osteonecrosis and extrusion of the femoral head in adolescents. J Pediatr Orthop 1995;15:16–20.
Y, Hotokebuchi T, Tsutsui H. Transtrochanteric anterior rotational
osteotomy for idiopathic and steroid-induced necrosis of the femoral
head. Indications and long-term results. Clin Orthop 1992;277:111–120.
RL. The pathology of acute necrosis of cartilage in slipping of the
capital femoral epiphysis. A report of two cases with pathological
sections. J Bone Joint Surg Am 1963;45:1013–1024.
C, Caterini R, Farsetti P, et al. Chondrolysis of the hip complicating
slipped capital femoral epiphysis: long-term follow-up of nine
patients. J Pediatr Orthop B 1999;8:107–111.
MR, Rosenberg AG, Kull L, et al. Primary total hip arthroplasty using
noncemented porous-coated femoral components in patients with
osteonecrosis of the femoral head. J Arthroplasty 1994;9:457–468.
HP, Reineck FT, Wixson RL, et al. Total hip replacement in patients
younger than thirty years old. A five-year follow-up study. J Bone Joint Surg Am 1981;63:1426–1434.
GP, Berry DJ, Rowland C, et al. Primary uncemented total hip
arthroplasty in patients, 40 years old: 10- to 14-year results using
first-generation proximally porous-coated implants. J Arthroplasty 2001;16:140–144.
JD, Lachiewicz PF. Survival and polyethylene wear of porous-coated
acetabular components in patients less than fifty years old: results at
nine to fourteen years. J Bone Joint Surg Am 2002;84-A:729–735.
RE, Rodriguez JA, Deshmukh RG, et al. Polyethylene wear and
periprosthetic osteolysis in metal-backed acetabular components with
cylindrical liners. J Arthroplasty 1998;13:1–7.
TE, Richards BS, Haideri N, et al. Intermediate follow-up of a simple
method of hip arthrodesis in adolescent patients. J Pediatr Orthop 1996;16:30–36.
PL, Johnson LO, Martin RA, et al. Intra-articular hip arthrodesis
without subtrochanteric osteotomy in adolescents: technique and
short-term follow-up. Am J Orthop 1997; 26: 257–264.
