Osteomyelitis and Septic Arthritis
present itself in a myriad of clinical situations in all regions of the
musculoskeletal system. Bone and joint infection may cause rapid
destruction and permanent impairment of the musculoskeletal system if
not treated urgently, so prompt diagnosis is imperative. Unfortunately,
there is not a single test or finding that consistently allows rapid
diagnosis. Trauma, neoplasm, inflammatory arthropathy, or synovitis may
all present with a clinical picture similar to infection.
In 20% or more of musculoskeletal infection cases, no organism is identified, making diagnosis and even definition challenging (1).
To complicate the situation further, as a disease entity,
musculoskeletal infection is continually changing. Over relatively
short periods, as immunization, antibiotics, and living conditions
change, new infectious organisms causing clinically significant disease
arise, and organisms previously responsible for infection become less
prevalent. This would all be of little interest to the orthopaedist if
musculoskeletal infection in children were a rare condition, but in
fact, it is a relatively common disorder. These varied factors ensure
that musculoskeletal infection will remain an important and challenging
pediatric orthopaedic disorder.
it is not possible to identify a causative organism in a significant
percentage of patients with the condition. Therefore, the presence of
an identifiable organism is not essential for definition and diagnosis
of the disease. Morrey and Peterson proposed a definition that
classified osteomyelitis as being definite, probable, or likely (2).
Definite osteomyelitis is present when an organism is recovered from
bone or adjacent soft tissue or when there is histologic evidence of
infection. Osteomyelitis is probable when there is a positive blood
culture in addition to clinical and radiographic features of
osteomyelitis, and osteomyelitis is likely to be present when there are
typical clinical and radiographic features of osteomyelitis along with
a response to antibiotics in the absence of a positive culture. Peltola
and Vahvanen also suggested a definition of osteomyelitis (3),
considering the diagnosis to be firm when two of the following four
criteria are present: pus aspirated from bone; positive bone or blood
culture; classic symptoms of localized pain, swelling, warmth, and
limited range of motion (ROM) of the adjacent joint; and radiographic
changes typical of osteomyelitis.
percentage of patients with septic arthritis have negative cultures,
and therefore it is also important to establish diagnostic criteria for
septic arthritis which do not mandate positive cultures. Morrey et al.
included patients with negative cultures who experience five of the
following six criteria: temperature greater than 38.3°C, pain in the
affected joint made worse by motion, swelling of the affected joint,
systemic symptoms, absence of other pathologic processes, and
satisfactory response to antibiotic therapy (4).
causative organism, duration of symptoms, and route of infection.
Infection in the neonate has distinct characteristics that differ from
childhood osteomyelitis, which in turn differs from osteomyelitis in
the adult population. Pyogenic organisms are the most common causative
organisms, but granulomatous osteomyelitis is being encountered more
frequently with increased international travel and immigration. The
most common route of infection in osteomyelitis is hematogenous, but
direct inoculation is frequently the route of infection in the foot.
annual rate of acute hematogenous osteomyelitis (AHO) in children
younger than 13 years estimated to be 1 in 5000 in the United States (5). Worldwide incidence estimates range from 1 in 1000 to 1 in 20,000 (6), and half of all cases of osteomyelitis occur in children younger than age 5 (7).
In childhood, septic arthritis occurs about twice as often as
osteomyelitis and also tends to have its peak incidence in the early
years of the first decade (8).
infection are continually changing. Fulminate infection is seen less
frequently, atypical forms of infection such as subacute osteomyelitis
are becoming more common, and musculoskeletal infection incidences may
be declining (9,10).
These changes may be due to a variety of factors, including increased
awareness, immunization patterns, and modification of clinical course
by antibiotics. At the Royal Hospital for Sick Children in Glasgow,
Scotland, researchers noted a 44% decline in incidences of AHO when the
period from 1970 to 1990 was compared to the period from 1990 to 1997 (11). Annual incidence dropped to a rate of 2.9 new cases per 100,000 population per year. Staphylococcus aureus remains the most common causative organism, occurring in 40% to 90% of cases of musculoskeletal infection (11, 12, 13, 14, 15, 16). Other organisms commonly causing osteomyelitis or septic arthritis include coagulase-negative Staphylococcus, group A β-hemolytic Streptococcus, Streptococcus pneumoniae, group B Streptococcus, and Salmonella (16).
has dramatically decreased. In 1982, the University of Helsinki
organized a prospective multicenter study of orthopaedic infection. In
1986, Finland began a large-scale immunization program against H. influenza. From 1982 to 1988, 36% of orthopaedic infections treated by the study group were caused by H. influenza, whereas from 1988 to 1998, there was not a single orthopaedic case of H. influenza infection, and the total number of childhood septic arthritis cases decreased by 30% (14). This change in epidemiology has resulted in modification of initial empiric antimicrobial therapy recommendations to cover
primarily gram-positive cocci. Howard et al. reported similar results following immunization for H. influenza in eastern Ontario, where H. influenza septic arthritis dropped from 41% of cases to 0% of cases following initiation of an H. influenza immunization program (17). Dramatic reduction in H. influenza infection following immunization has been confirmed by authors at other centers from around the world (12,18).
are now recognized as being responsible for a greater percentage of
musculoskeletal infections. In a study by Yagupsky and Dagan, K. kingae was the most common organism responsible for septic arthritis in children younger than 24 months (19). K. kingae
is a fastidious, gram-negative bacillus that until relatively recently
was thought to rarely cause clinical infection in children. Residing in
the oropharynx of young children, K. kingae
appears to be an opportunistic pathogen that gains access to the
bloodstream during the course of upper respiratory infection. Once in
the bloodstream, K. kingae has a predilection for the heart and musculoskeletal system. Our greater appreciation of K. kingae
as a clinically significant causative organism for musculoskeletal
infection may in part be due to our improved understanding of how to
culture this organism. Inoculation of a specimen into enriched blood
culture media has considerably improved recovery rate (20). K. kingae is usually sensitive to β-lactam antibiotics and typically responds well to antibiotic treatment with few sequelae.
decade. Musculoskeletal infection is much more likely to affect the
lower extremity than the upper extremity or axial skeleton. In a recent
study performed in Taiwan, 90% of septic arthritis cases occurred in
the lower extremity. The hip was the most commonly involved joint, with
hip infection occurring in 54% of patients (16).
Newton et al. reported the hip and knee to be the joints most commonly
affected in their series of 186 patients with septic arthritis (21).
In a study by Khachatourians et al. of 50 patients with septic
arthritis and/or osteomyelitis, 70% of infections occurred in the lower
extremities (13). Peltola et al. reported 72% of osteomyelitis cases in their series to occur in the lower extremities (22).
simultaneously. Patients younger than 18 months have a blood supply to
the chondroepiphysis, which predisposes infants to develop
osteomyelitis and septic arthritis. These diseases can also occur in
four locations in older children where the metaphysis lies within the
joint: in the proximal femur, proximal humerus, distal lateral tibia,
and proximal radius. Septic arthritis results when bacteria breach the
metaphyseal periositium and enter the joint. Perlman et al. reported
that signs of adjacent joint septic arthritis may be as high as 40%,
and therefore careful evaluation of neighboring joints is important (23).
musculoskeletal system daily and yet rarely cause clinical infection.
For musculoskeletal infection to occur, several circumstances must be
present. A virulent organism capable of causing infection must be
present, sufficient numbers of that organism for multiplying and
reaching a critical mass must be present, and the species and number of
bacteria present must overwhelm host defenses in the particular
anatomic site in question. Although random chance may have a role in
determining where and when bone and joint infection occurs, specific
patterns of infection have been observed that can lead to no other
conclusion than that specific factors influence where and in whom
musculoskeletal infection occurs.
common site for AHO to develop. Hobo described vascular loops present
in the long bone metaphysis that take sharp bends and empty into venous
lakes, creating areas of turbulence where bacteria accumulate and could
cause infection (24). Relative absence of
tissue macrophages in metaphyseal bone adjacent to the physis appears
to contribute to the predilection of osteomyelitis for this location.
Others have suggested that gaps in the endothelium of growing
metaphyseal vessels allow passage of bacteria (25) that may adhere to type I collagen in the hypertrophic zone of the physis. S. aureus surface antigens may play a key role in this local adherence (26).
The best evidence confirming the role of trauma has been established in
an animal model by Morrissy and Haynes, who noted that intravenous
injection of S. aureus caused infection in the metaphysis of an injured rabbit (32,33).
Interestingly, infection did not develop in fractures of the fibula
diaphysis, indicating that fracture hematoma cannot be the explanation.
Although rare, acute infection of fracture hematoma has occurred
clinically. There are no similar clinical data for septic arthritis,
but experimental models demonstrate the role of trauma in the
production of the disease (34,35).
Therefore, the precise mechanism by which trauma reduces local host
defenses and predisposes a particular location for infection has not
been conclusively determined.
host defense mechanisms preventing bone and joint infection. Patients
with conditions associated with decreased or altered immune response,
such as the neonate, are known to be susceptible to infection.
Varicella infection provides a portal for bacteria to enter the
musculoskeletal system and also lowers the host immune system, making
the host more susceptible to infection (36,37). Other
aspects of musculoskeletal infection etiology, such as the predilection
for men and the lower extremity and peak age incidence, are less well
understood and are yet to be explained.
 |
|
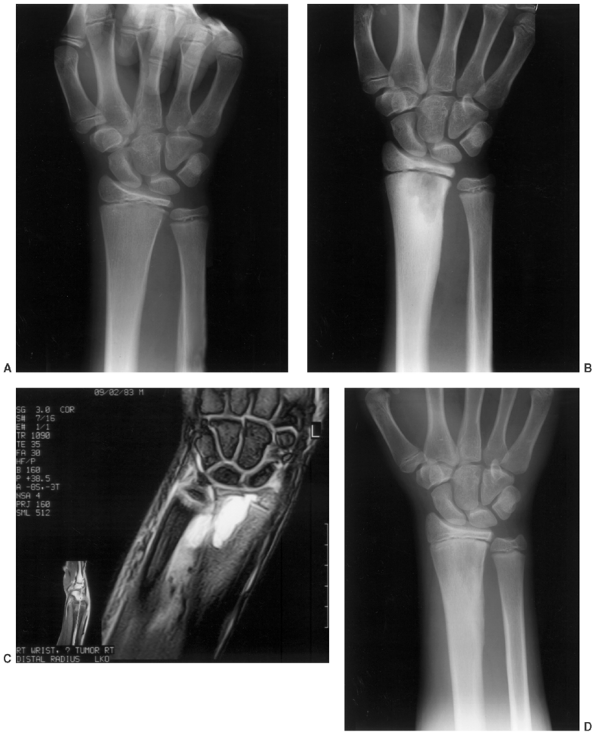
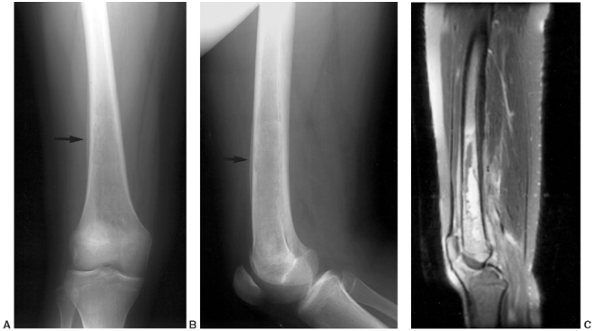
Figure 13.1 A:
12-year-old boy was struck in the distal radius by a hockey puck. Initial radiographs were negative, and the patient’s symptoms completely resolved over 2 weeks. B: Two months later, the patient experienced increasing pain and swelling. Radiographs were repeated and demonstrated a lytic lesion with a sclerotic margin that appeared to cross the physis consistent with osteomyelitis. C: T2-weighted magnetic resonance imaging (MRI) suggested the diagnosis of infection that crossed the distal radial physis with cortical breach and adjacent soft-tissue abscess. D: Irrigation and debridement of purulent material was performed, and cultures obtained at surgery confirmed S. aureus osteomyelitis. To reduce risk of persistent infection and to reduce the likelihood of physeal arrest, no bone graft was placed. Two years after surgery, the bone defect has healed, there is no evidence of infection, and the distal radial physis is growing normally. |
facilitate better understanding of osteomyelitis pathophysiology. The
diaphyseal region of long bones consists of a dense lamellar cortex,
which is relatively acellular, and a medullary cavity, which contains
little bone but is filled with a rich reticuloendothelial system. In
contrast, the metaphyseal region is composed of a cortex which is
little more than compact cancellous bone, and a medullary cavity that
has greater bone content arranged in a trabecular pattern but
relatively few reticuloendothelial cells. Covering metaphyseal and
diaphyseal cortical bone is the periosteum, which in children is thick,
easily separated from bone, but not easily penetrated. Periosteal blood
supply comes from the outside so that it remains viable, producing
osteoid and bone even when elevated off of the bone surface.
described his experiments on the localization of both India ink
particles and bacteria in bone after intravenous injection. Hobo noted
that although most bacteria lodged in the diaphyseal medullary cavity,
they were rapidly phagocytosed and no infection resulted. In contrast,
few bacteria were localized to the area beneath the epiphyseal plate,
but because of the absence of phagocytic cells in this region of the
bone, infection subsequently developed. Hobo proposed that the vessels
beneath the physeal plate were small arterial loops that emptied into
venous sinusoids and that the resulting turbulence was the cause of
localization. Subsequently, electron microscopic studies have shown
these to be small terminal branches (38). In
addition, it has been demonstrated that the endothelial wall of new
metaphyseal capillaries have gaps that allow the passage of blood cells
and, presumably, bacteria (25).
the most rapidly growing end of the large long bones, especially those
of the lower extremity. This predilection may be explained by the
observation that, in rapidly growing bones, the phagocytic cells are
further from where the bacteria localize because of the structure of
these bones. Therefore, the inflammatory response takes longer to reach
the bacteria, allowing a clinical infection to become established.
adjacent to the physis, a process of net bone resorption begins.
Osteoblasts die and bone trabeculae are resorbed by numerous
osteoclasts within 12 to 18 hours. Lymphocytes may release osteoclastic
activating factor, and macrophages, monocytes, and vascular endothelial
cells may all directly resorb both the crystalline and matrix
components of bone. In response to toxins and bacterial antigens,
interleukin-1 is produced by macrophages and polymorphonuclear
leukocytes (39). Prostaglandin E2 is also produced, which stimulates further bone resorption (40).
These stimuli cause inflammatory cells to migrate and accumulate to the
area of bacterial localization beneath the physis. As inflammatory
cells migrate to the site of accumulating bacteria, the bone in the
path of this migration is resorbed.
causes thrombosis of medullary vessels, further reducing the host’s
ability to fight infection. A purulent exudate is formed that may exit
the porous metaphyseal cortex to create a subperiosteal abscess. As the
periosteum is elevated, the cortical bone is deprived of its blood
supply and may become necrotic, forming a sequestrum. Because the
periosteum retains its blood supply, it remains viable and produces
osteoid. The new bone forming around the necrotic sequestrum is known
as involucrum. If the metaphysis is
intraarticular at the site where infection breaches the metaphyseal
cortex, septic arthritis results. This occurs in four locations in the
older child: proximal femur, proximal humerus, distal lateral tibia,
and proximal radius. Infection generally does not spread down the
medullary cavity because the well-developed reticuloendothelial system
of the diaphysis is able to prevent its expansion in this direction.
interosseous blood supply, osteomyelitis pathophysiology in the infant
may vary from the pattern described in preceding text. Trueta first
noted that before the ossific nucleus forms, the vessels from the
metaphysis penetrate directly into the cartilaginous ephysis analog (41).
Because of this blood supply pattern, the initial bacterial
localization may occur in the cartilage epiphysis precursor. Infection
of the epiphysis precursor may spread to the joint, causing septic
arthritis as well as physeal injury and growth alteration. As the
ossific nucleus develops, a separate blood supply to this epiphysis
develops and the metaphyseal vessels crossing the developing physeal
plate disappear. When the physeal plate is formed, it provides a
temporary barrier to the spread of infection into the epiphysis.
synovial joint affect the pathophysiology of septic arthritis. Joint
synovium is a unique tissue that does not have a basement membrane and
secretes fluid that is essentially a transudate of serum. The remaining
interior joint surface is covered with articular cartilage, creating an
environment favorable to bacterial proliferation, similar to a culture
tube. Just as in bone, it is likely that transient bacteremia results
in bacteria entering the joint, but, in
almost all cases, the joint has the ability to clear itself of bacteria and avoid infection (42). However, when the inoculum is large, or when virulent pathologic bacteria such as S. aureus are less effectively cleared, clinical infection may result.
understood than osteomyelitis. Although trauma has been implicated as a
causative factor (35), trauma cannot completely
explain the tendency for infection to involve large joints and those of
the lower extremities. What is known is that when septic arthritis
occurs, bacteria rapidly gain access to the joint cavity and within a
matter of hours cause synovitis and formation of fibrinous exudate
followed by areas of synovial necrosis.
and reverse the process of articular cartilage destruction, and an
understanding of this process will facilitate optimal treatment.
Proteases, peptidases, and collagenases are released from leukocytes,
synovial cells, and cartilage. These enzymes catalyze reactions that
break down the cellular and extracellular structure of cartilage (42, 43, 44, 45, 46, 47, 48).
The loss of glycosaminoglycans is the first measurable change in
articular cartilage, occurring as early as 8 hours after bacteria are
introduced into the joint (49). Loss of
glycosaminoglycans softens the cartilage and may cause it to be
susceptible to increased wear. Collagen destruction follows and is
responsible for visible change in cartilage appearance (50, 51, 52).
Once catalytic enzymes are released into the joint, the presence of
living bacteria is not necessary for cartilage destruction to continue.
critical to the diagnosis of musculoskeletal infection in children.
Pain is the most common symptom in patients with bone or joint sepsis (53,54),
but children are not always able to verbalize this common symptom.
Instead, children may refuse to walk, refuse to bear weight, limp, or
refuse to use or move a limb. Frequently, the physician obtains the
history indirectly from a parent or caregiver instead of obtaining it
directly from the patient. Careful questioning can provide important
information about the infection location, likely causative organisms,
and the duration of the infectious process.
willing to bear weight on the thigh, and the clinician can focus on the
leg distal to the knee as the possible infection location. Patient age,
recent activity, and exposure can all provide clues to the causative
organism; the neonate is more likely to have infection caused by group
B Streptococcus or gram-negative rods, and patients with sickle cell disease are predisposed to Salmonella infection.
relatively closely with stages of infections observed in experimental
animals. Fever, malaise, anorexia, and night pain are common symptoms
of musculoskeletal infection but are not always present. Temperature
greater than 38°C has been reported to occur in only 36% to 74% of
patients (16,54,55).
A shrinking minority of patients fit the stereotype of an ill-appearing
child who has experienced symptoms for a week and who presents with an
obviously infected bone or joint. More frequently, children may present
within 12 hours of onset of limp, with normal or mildly elevated
laboratory values and a positive bacterial aspirate from bone or joint.
musculoskeletal infection or may affect the type of musculoskeletal
infection present. Care must be taken to consider recent antibiotics
when interpreting patient symptoms, and greater vigilance should be
assumed for subacute osteomyelitis.
information to consider when evaluating a patient for possible
musculoskeletal infection, and such illness may be present in one third
to one half of patients. Recent upper respiratory symptoms may suggest
a noninfectious cause for patient symptoms such as toxic synovitis or
poststreptococcal reactive arthritis. Rashes or swollen lymph nodes are
important for their association with conditions such as Lyme disease,
rheumatoid arthritis, and leukemia. Concurrent chickenpox is notable
for creating a portal of entry into the circulatory system as well as
for lowering host immunity, predisposing a patient to musculoskeletal
infection caused by group A Streptococcus in particular.
present with a history of local trauma, and, as noted previously, local
trauma can contribute to the development of bone or joint sepsis. The
crucial and difficult issue for the clinician to determine is whether a
patient’s pain is caused by trauma or by infection. Close attention to
the clinical course following a traumatic event is very helpful;
symptoms caused by trauma tend to improve, whereas symptoms caused by
sepsis generally worsen. Physical examination, laboratory tests, and
imaging studies may also be helpful.
observing the child in the examination room while obtaining the history
as part of the evaluation. If the child does not appear acutely ill or
moribund, encourage the child to play independently while you are
interviewing the parent. Unaware that he or she is being observed, the
young child will often be more active than later in the structured
segment of the physical examination. Refusal to bear weight on a lower
extremity, a limp, or the disuse of an upper extremity give important
clues about the location of pathology.
proven otherwise. The importance of palpable bone pain in establishing
the diagnosis of osteomyelitis cannot be overemphasized. Gentle,
systematic palpation is often the best means available on physical
examination to localize pathology in an irritable, uncooperative
2-year-old child who refuses to use an extremity. Allowing the child to
remain in the arms of the parent and watching the child’s face, not the
limb, while systematically palpating the limb often reveals the
location of pathology. In the case of small children who cry at the
mere presence of a stranger and panic at being touched, it is often
beneficial to instruct the parent how to elicit the tender area. After
showing the parent how to palpate the area, the physician should leave
the room and allow the parent to first examine the unaffected part,
then the affected part, and report the results.
examines for increased warmth, erythema, or other skin changes.
Erythema and swelling may appear as early as 24 to 36 hours following
onset of pain and can progress rapidly. Skin changes are detectable
earliest in bones or joints that are not covered by muscle. Visual
comparison of the normal and affected limb, symmetrically positioned,
should always be done. Loss of normal concavities and loss of normal
skin wrinkles are other subtle clues that may be present. Severe limb
swelling may indicate extensive underlying infection or deep venous
thrombosis (56).
osteomyelitis but should not cause substantial joint irritability. Pain
with passive joint motion is a hallmark sign of septic arthritis and is
usually associated with decreased range of motion (ROM) as well.
Palpation of joints often elicits tenderness, and joint effusion can
frequently be demonstrated in joints that are not covered by large
amounts of tissue. Joints of the axial skeleton, including the spine
and pelvis, are less accessible for examination, and diagnosis is more
dependent on findings such as pain with motion, percussion, and
compression. The hip joint is also inaccessible to direct observation,
but noting the position of thigh relative to the pelvis may be helpful.
The patient often lies with the hip flexed, abducted, and externally
rotated because internal rotation, extension, and adduction all tighten
the hip capsule, causing pain in a distended and inflamed joint.
present with similar signs, but knowledge of characteristic patterns is
helpful in establishing a diagnosis. Rheumatoid arthritis often
presents as a joint that looks worse than it feels. The joint may be
warm and markedly swollen with inflamed synovium and effusion but not
be especially painful. Rheumatic fever has a tendency to appear just
the opposite, with exquisite pain and markedly restricted motion in a
joint having minimal effusion or swelling.
infection should include complete blood count (CBC) with differential,
blood culture, erythrocyte sedimentation rate (ESR), and C-reactive
protein (CRP). None of these tests are specific for musculoskeletal
infection. White blood cell (WBC) count is the least sensitive, being
elevated in 25% to 73% of patients with osteomyelitis (2,16,22,54,57). Similar sensitivity has been reported for patients with septic arthritis (4,27,53,54).
Occasionally, patients with apparent AHO will have a low WBC or
platelet count, which may indicate systemic sepsis or leukemia. If the
diagnosis of musculoskeletal infection is in question, a manual
differential count should be performed to look for atypical leukocytes
and leukemia.
more useful. Acute-phase response is the increase or decrease in the
levels of a variety of plasma proteins in response to cytokine
production that occurs in acute or chronic inflammation. These proteins
are responsible for many of the systemic symptoms seen in infection,
such as fever, anorexia, lethargy, and anemia, and an increase in the
levels of many of these proteins can be measured in the blood. The two
most common tests to measure acute-phase response today are the ESR and
CRP.
in response to inflammation. The test measures the rate at which an
erythrocyte falls through plasma and is dependent on the concentration
of fibrinogen. The ESR result can be affected by the size, shape, and
number of erythrocytes present, as well as by other proteins in plasma.
The ESR is unreliable also in the neonate, in the presence of anemia,
in patients with sickle cell disease, or when the patient is taking
steroids (54,55).
of the onset of infection and returns to normal over a period of 2 to 4
weeks after elimination of infection. The ESR is less reliable in the
first 48 hours of infection than after 48 hours. The clinician can
expect the ESR to be elevated in 85% to 95% of cases of septic
arthritis (4,13,16) and in 90% to 95% of osteomyelitis cases (53,54).
Although noted to be elevated just as often in patients with
osteomyelitis, the ESR was significantly higher in those with septic
arthritis (4).
that it continues to rise for 3 to 5 days after institution of
successful therapy. Although a continuing rise beyond the fourth to
fifth day of treatment is an indication of failure to eradicate the
infection, it is because of this delayed response that the ESR is not a
good means of assessing the resolution of sepsis during the first week
of treatment (58).
inflammation and also trauma. The CRP may begin to rise within 6 hours
of the triggering stimulus, then increases several hundredfold,
reaching a peak within 36 to 50 hours.
Because
of the short half-life of the protein (47 hours), it also falls quickly
to normal with successful treatment, in contrast to the ESR. This makes
the CRP of greater value than the ESR, not only for earlier diagnosis
of infection but also for determining resolution of the inflammation (59).
evaluation of musculoskeletal infection, its level being elevated in as
many as 98% of patients with osteomyelitis compared to 92% of patients
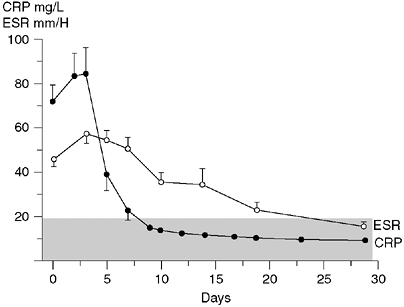
having elevated ESR (60). Peak CRP was noted on day 2 compared to peak ESR measured on days 3 through 5 (Fig. 13.2).
Following initiation of treatment, it may take the ESR approximately 3
weeks to normalize, whereas the CRP typically returns to normal within
1 week. Failure of the CRP to rapidly normalize after initiation of
treatment has been predictive of long-term sequelae (61).
Therefore, the CRP is more likely to be helpful in diagnosing an early
case of infection and is more useful in monitoring its resolution.
that a patient does not have musculoskeletal infection. Levine et al.
reported that if the CRP is less than 1.0 mg per dL, the probability
that a patient does not have septic arthritis is 87% (62).
The presence of both osteomyelitis and adjacent septic arthritis also
increases the likelihood that serologic testing will be abnormal.
Khachatourians et al. reported the ESR and CRP being elevated 100% of
the time and the WBC count being elevated in 87% of patients with both
septic arthritis and adjacent osteomyelitis (13).
Twenty-five patients were treated with surgery and 25 patients were
treated with antibiotics alone. In the surgery group, it took twice as
long for the CRP and ESR to reach peak values and then twice as long to
normalize after initiation of treatment.
 |
|
Figure 13.2
C-reactive protein reaches a peak value more precipitously and has a more rapid return to normal than does the erythrocyte sedimentation rate (ESR). The stippled area denotes the normal range of values. (Reproduced from Unkila-Kallio L, Kallio MJT, Eskola J, et al. Serum C-reactive protein, erythrocyte sedimentation rate, and white blood cell count in acute hematogenous osteomyelitis of children. Pediatrics 1994;93:59–62, with permission.) |
CRP is useful in separating a musculoskeletal infection from an otitis
media, which is commonly seen in children. Elevated CRP values are
reported in 22% of patients with a bacterial otitis media and in 65% of
those with a viral otitis media (63). Therefore, it would seem that an elevated CRP may be due to otitis media.
identification associated with standard bacterial cultures has
stimulated significant interest in molecular techniques for detection
and speciation of bacterial and viral infections (15,64).
Molecular testing can be performed in an hour and does not depend on
the presence of live bacteria for culture. Molecular test results
should not be affected if antibiotic treatment has already begun. These
techniques fall into two broad categories: nonamplified and amplified.
In nonamplified techniques, direct binding of a target molecule is done
with a labeled oligonucleotide probe or monoclonal antibody, followed
by the detection of the probe agent with radiolabeling, enzyme-linked
immunosorbent assay, or chemoluminescence. The nonamplified technique
is specific and appropriate when one is looking for a particular
organism. Using amplification techniques, geometric amplification of
the target molecule is achieved through enzyme-driven reactions.
(PCR). A target segment of bacterial DNA or RNA is chosen that is not
present in human cells. A probe or primer specific to that segment of
DNA or RNA is introduced, which promotes binding of a polymerase that
replicates the target segment in a series of temperature-dependent
cycles. The amplification products are then identified by gel
electrophoresis. PCR has produced some promising results in the
diagnosis of periprosthetic infections and septic arthritis, but a high
false-positive rate has been reported (65).
This and other limitations make PCR and other molecular testing methods
impractical for routine diagnosis of musculoskeletal infection at this
time, but refinement of molecular testing methods may significantly
increase their future application.
battery of tests obtained when one suspects musculoskeletal infection
because, in both osteomyelitis and septic arthritis, blood cultures
yield organisms in 30% to 60% of patients (5,12,54),
allowing organism identification and facilitating optimal antibiotic
therapy. The yield from both blood culture and aspirated material
decreases with previous antibiotic therapy (4).
Even with previous antibiotic treatment, however, the chances of
obtaining positive cultures, when all sources (i.e., blood, bone, and
joint fluid) are cultured, remain high (54).
sensitivity and specificity of radiographs range from 43% to 75% and
from 75% to 83%, respectively (66). The role of
radiography in the diagnosis of early bone and joint sepsis is often
undervalued because clinicians often look only for changes seen in
bone. Plain radiographs may show soft-tissue swelling and loss of
tissue planes within 3 days of infection onset, whereas bone changes
may not appear for 7 days or more (57,67).
Because the inflammation in the bone or joint produces edema in the
soft tissues adjacent to the area of inflammation, there is swelling in
this region, and enlargement of this muscle layer is detectable on the
radiograph. In addition, the edema obliterates the normal fat planes
that can be seen between the muscle layers. Radiographs to detect deep
soft-tissue swelling are of most value in suspected sepsis of the long
bones. Symmetrically positioned views of the contralateral extremity
may be helpful for comparison.
easily seen in peripheral joints such as the knee or elbow. At the hip,
there may be asymmetric widening of the joint space compared to the
uninvolved hip (Fig. 13.3). Although this may
be seen frequently in the neonate, hip joint space widening is often
lacking in older children. It is a late sign, and its absence is not to
be interpreted as lack of sepsis (68).
Untreated septic arthritis may result in joint destruction, narrowing
of the joint space, or pathologic bone changes on both sides of the
joint. Additional sequelae such as osteonecrosis of the femoral head
may also be seen.
plain radiographs suggests that by the time bone changes are seen,
osteomyelitis is already well established. While not entirely reliable,
it is fair to suggest that when radiographic changes are present,
surgical treatment of osteomyelitis is more likely to be necessary than
if radiographic changes are not present. Although infection can appear
in any bone and in any location, the most common radiographic
presentation for osteomyelitis is a destructive, lytic, eccentric
metaphyseal lesion, often associated with periosteal elevation and new
bone formation. Bone destruction caused by osteomyelitis may appear
aggressive, infiltrative, and ominous in appearance and may be mistaken
for neoplasm (69, 70, 71).
scanning is sensitive in localizing suspected musculoskeletal
infection, detecting osteomyelitis in as many as 94% of patients (12).
In a separate report, Howie et al. demonstrated a sensitivity of 89%
and a specificity of 94%, with an overall accuracy of 92% for this
method (72). The bone scan consists of three
phases: an angiogram, performed immediately after injection;
immediately followed by the second or “blood pool” phase; and 2 to 3
hours later, the mineral phase, which reflects uptake in the bone. All
three phases are helpful, especially in distinguishing cellulitis from
osteomyelitis. The mechanism by which technetium-99m bone scanning
works is isotope uptake, which depends on vascularity and calcium
phosphate deposition (73).
the bladder should be empty at the time of the scan to prevent
accumulated isotope from obstructing the sacrum and sacroiliac (SI)
joints. Symmetrically positioned views of both sides should be
obtained. Technetium scanning using pinhole-collimated views and
single-photon emission computerized tomography (SPECT) can increase
both sensitivity and specificity (74). Because
most AHO occurs in the metaphysis adjacent to the physeal plate, such
views are necessary to separate early metaphyseal changes from the
large amount of uptake found in the physeal plate. These images are
time consuming to obtain and may require that the child be sedated. It
is therefore important that the physician communicate the desired areas
of interest to the radiologist.
patients in whom the site of suspected musculoskeletal infection is
unclear (Fig. 13.4) or when looking for multiple foci of bone involvement (75).
Bone aspiration and initiation of treatment should not be delayed for
fear of affecting bone scan results. Using an animal model, Canale et
al. demonstrated that if a bone scan is performed within 48 hours after
bone aspiration, the bone aspiration does not cause a false-positive
scan result (76).
osteo-myelitis when “hot” or showing increased uptake, a “cold” bone
scan may provide evidence of severe osteomyelitis and has been reported
to have a positive predictive value of 100% (74,77).
Pennington et al. at the Medical College of Wisconsin reviewed 81
patients evaluated with technetium bone scan for osteomyelitis (78).
Seven of the 81 patients had a photopenic region defect, or cold scan,
consistent with osteomyelitis. A control group of matched patients with
hot scan osteomyelitis was compared to the cold scan group. Patients
with cold scan osteomyelitis had statistically increased temperature,
resting pulse rate, ESR, length of hospital stay, and rate of surgical
intervention compared to patients with hot scan osteomyelitis.
uptake on both sides of a joint. Although bone scanning may correctly
identify the site of joint sepsis in approximately 90% of infected
joints, it does not separate bone from joint sepsis or differentiate
infectious from noninfectious arthritis (72,79).
This is a particular problem in the hip, in which the differential
diagnoses may include transient synovitis, septic arthritis, or
osteomyelitis of the femoral neck.
 |
|
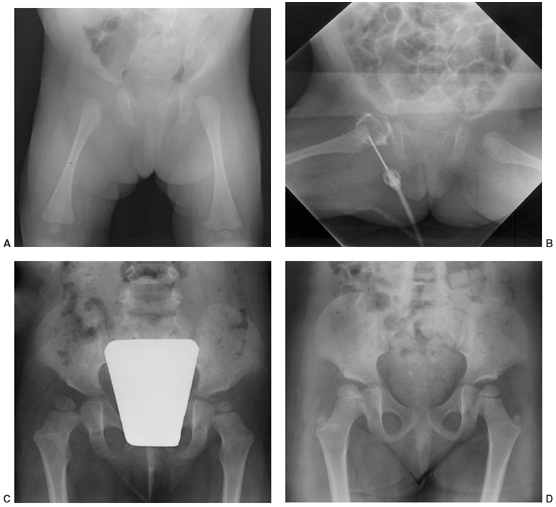
Figure 13.3 A:
A 2-month-old infant presents following 3 days of increasing irritability, fever, and pseudoparalysis of the right leg. Anteroposterior pelvis radiograph demonstrates widening of the right hip joint space. B: The patient was rushed to the operating room, where the right hip was aspirated and an arthrogram was performed to document intraarticular position of the needle. Cell count of the hip joint aspirate was 65,000 per mL; open joint irrigation and debridement of septic arthritis was performed. C: Two years following open surgical irrigation and drainage, the patient is asymptomatic but on performing radiography is found to have mild hip dysplasia on the right with acetabular index of 25 degrees compared to 22 degrees on the left, 50% femoral head coverage on the right compared to 70% coverage on the left, and widening of the right femoral neck. D: Four years following irrigation and debridement, the right hip dysplasia has improved, with the right acetabular index now measuring 21 degrees and with a femoral head coverage of 70%. Mild coxa magna and femoral neck widening persists. |
Technetium scanning is relatively nonspecific, and increased uptake may
be caused by any process that increases vascularity or deposition of
calcium phosphate. Tumor, trauma, and bone resorption due to disuse may
cause increased uptake. The scans may be negative in the first 24 hours
of infection before stimulation of bone turnover, and there may be a 4%
to 20% false-negative rate with technetium scanning (54).
In neonatal infection, the reported sensitivity for technetium scanning
has ranged from 30% to 86%, and standard radiography may be more
helpful (6,7,80).
Overall specificity and sensitivity are improved when the scan is
interpreted with knowledge of the clinical findings and initial
laboratory studies, compared to when the interpretation was a blind
reading of the scan (81).
 |
|
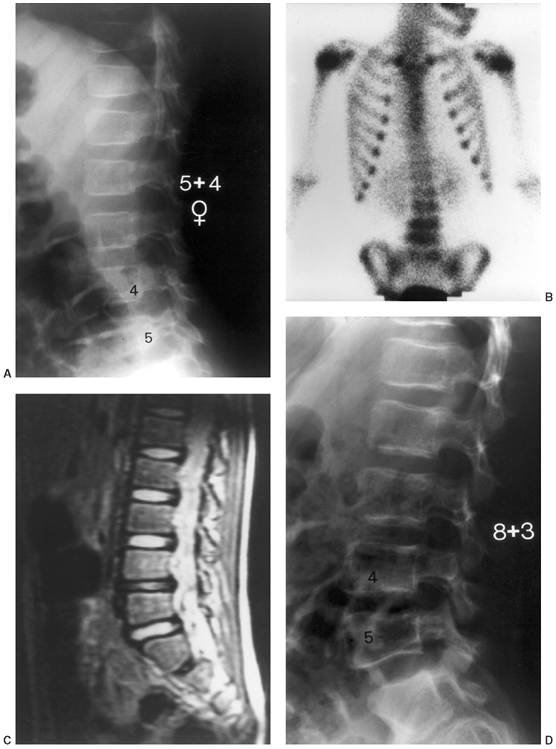
Figure 13.4
A 5-year-old child presents with an increasing limp over 48 hours and with suspected musculoskeletal infection. History and physical examination do not localize the process. Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are elevated. A: The lateral (as well as the anteroposterior) radiograph of the spine is normal. B: Technetium bone scan shows increased isotope uptake in the L4 and L5 vertebral bodies suggestive of discitis, but neoplasm cannot be excluded. C: T2-weighted MRI helps confirm the diagnosis of discitis, demonstrating that the process is centered in the L4-L5 disc with no evidence of neoplasm, bone, soft tissue, or epidural abscess. Intravenous followed by oral antibiotic treatment was initiated, with complete resolution of symptoms after a total antibiotic therapy duration of 3 weeks. D: Final follow-up 3 years later demonstrates a normal lumbar spine radiograph in the asymptomatic patient. |
helpful in evaluating osteomyelitis in children. Gallium-67 citrate and
indium-labeled leukocytes are more expensive, result in more radiation
exposure, take longer to complete, and are not often useful in the
evaluation of musculoskeletal infection in children (9).
Indium-111–labeled WBC scanning may be helpful in the rare circumstance
when infection is suspected but the technetium scan is normal. However,
indium scanning requires preparation time and may take as long as 24
hours to perform (15). Granulocyte scintigraphy
is an imaging technique performed with a Tc-99m-labeled monoclonal
murine antibody (MoAb) against granulocytes and has been shown to be an
effective and specific method of imaging infection in adults.
Unfortunately, in children the same imaging technique was neither
sensitive nor specific (82).
antibiotic that binds bacteria, is felt to have promise for localizing
deep infection in bone and soft tissues. Although the mechanisms for
the uptake of the radiolabeled ciprofloxacin have not been fully
established, it is considered to be a specific tracer for bacterial
infections because it binds to the DNA gyrase enzyme of living
bacteria. In a prospective multicenter, international study, 897
patients were imaged with Tc-99m ciprofloxacin, resulting in an overall
sensitivity of 85.4% and a specificity of 81.7% for detecting deep bone
or soft-tissue infections (83). Additional
trials of Tc-99m ciprofloxacin have been performed looking specifically
for orthopaedic infection. Malamitsi et al. compared Tc-99m
ciprofloxacin with conventional scans (84). The
sensitivity and specificity of Tc-99m ciprofloxacin scans were found to
be 97% and 80%, respectively, with positive predictive value of 95% and
negative predictive value of 89%. False positive results tended to
occur in patients with conditions that cause abundant new bone
formation and primary bone tumors.
helpful. Appelboom et al. studied Tc-99m ciprofloxacin in patients with
osteoarthritis and inflammatory arthropathy and found that Tc-99m
ciprofloxacin uptake was increased in inflamed joints independent of
the pathology (85). Dumarey et al. noted Tc-99m
ciprofloxacin uptake in growth cartilage, thyroid tissue, lungs, liver,
and the gastrointestinal (GI) tract (86).
Considering ciprofloxacin’s affinity for cartilage in children, Tc-99m
ciprofloxacin may have limited value for imaging musculoskeletal
infection in patients who are skeletally immature. Additional testing
is required before Tc-99m ciprofloxacin is considered for clinical use
in pediatric and adolescent patients.
evaluation of musculoskeletal infection, with reported sensitivity
ranging from 88% to 100%, specificity from 75% to 100%, and a positive
predictive value of 85% (87, 88, 89).
MRI provides better soft-tissue resolution and can be used to identify
abscesses as well as to help differentiate cellulitis from
osteomyelitis. MRI is useful in visualizing marrow involvement and
differentiating between malignant neoplasm and infection (Figs. 13.5 and 13.6).
MRI findings of osteomyelitis include a decrease in the normally high
marrow signal intensity on T1-weighted images caused by replacement of
marrow fat by inflammatory cells and edema. The inflammatory cells and
edema appear as increased signal intensity on T2-weighted images (9).
Acute infarcts demonstrated thin, linear rim contrast enhancement,
whereas osteomyelitis caused more geographic and irregular marrow
enhancement. Osteomyelitis cases may also demonstrate subtle cortical
defects with abnormal signal crossing marrow and soft tissue.
musculoskeletal infection for those cases in which there is confusion
with possible neoplasia; in cases that have been previously treated; in
suspected sepsis in the axial skeleton; or when there is conflicting
information, but the location of the disease process is known. Its
actual use, however, is mitigated by its cost and the frequent
necessity for sedation or general anesthesia in small children. It is
simply not necessary for the diagnosis and treatment of the usual case
of osteomyelitis or septic arthritis.
extent of bone destruction as well as in detecting soft-tissue
abnormalities and is most sensitive at detecting gas in soft tissues (15,75).
Especially in infection of the axial skeleton such as the spine and
pelvis, CT is invaluable in localizing the infection within the
skeleton and can assist in the planning of the surgical approach if
surgical debridement is indicated. CT scanning can be used to guide
needle localization prior to surgical biopsy or debridement, to direct
aspiration of bone or soft tissue, and to guide percutaneous placement
of drainage tubes. Compared to MRI, its advantages are its greater
availability and lower cost, which must be weighed against the
disadvantages of its being unable to detect changes within the marrow
in early cases and being less sensitive at detecting soft-tissue
changes.
musculoskeletal infection has been studied extensively, especially with
regard to septic arthritis of the hip. Ultrasonography is attractive
because of its low cost, relative availability, noninvasive nature,
absence of ionizing radiation, and the lack of need for sedation.
However, ultrasound as a noninvasive means of evaluating
musculoskeletal infection has been disappointing. The lack of
specificity, the dependence
on operator skill, and the inability to image marrow or show cortical detail have limited ultrasound’s usefulness.
 |
|
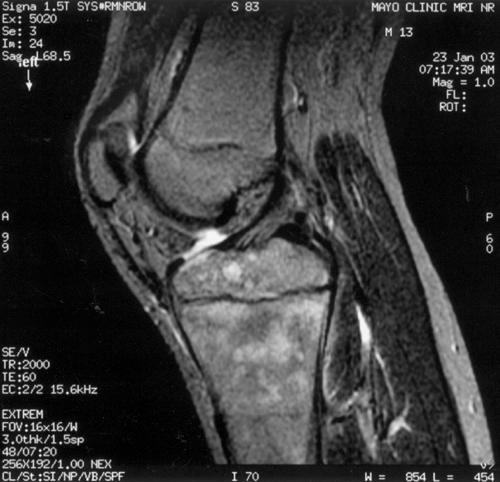
Figure 13.5 Magnetic resonance imaging (MRI) may be very helpful when differentiating between osteomyelitis and primary bone malignancy. A, B:
This 12-year-old female patient was referred for evaluation of femoral osteosarcoma. The standard anteroposterior and lateral radiograph shows periosteal reaction along the distal one third of the femur, consistent with primary bone sarcoma or osteomyelitis (arrows). C: T2-weighted MRI without contrast demonstrates preservation of some normal marrow fat within the intramedullary canal and a fluid-filled abscess cavity diagnostic of osteomyelitis. Diffuse inflammation is present in adjacent soft tissues without a discrete solid mass. Osteomyelitis was confirmed at surgery. |
They found a false-negative rate of 5% in patients who were determined
by ultrasonography to have no effusion but were subsequently diagnosed
with septic arthritis. Children with onset of symptoms less than 24
hours prior to hip ultrasonography and children who had bilateral hip
effusions were more likely to have a false-negative result.
reliably differentiated from septic arthritis by ultrasound alone.
Toxic synovitis may have a slightly higher incidence of bilateral hip
effusions than septic arthritis, and late septic arthritis may have an
effusion that is more echo dense and appears fibrinous compared to
toxic synovitis, but these findings are not accurate enough to be
diagnostic (92,93).
Ultrasonography may be used to guide hip aspiration when performed in
the radiology department for patients where septic arthritis is
suspected and in evaluation of the irritable hip that may be secondary
to extracapsular irritation (e.g., early pelvic osteomyelitis with
irritation of the surrounding muscles).
osteo-myelitis, primarily on the basis of detection of subperiosteal
abscess, thickening of the periosteum, and changes in the surrounding
soft tissues. Sadat-Ali et al. recently reported that ultrasonography
can be very helpful when differentiating between vasooclusive crisis
and osteomyelitis in patients with sickle cell disease (94).
Ultrasonography scan showed that six patients had periosteal thickening
and elevation with hypoechogenic regions, eight had abscesses, and
three patients had cortical destruction. All patients were found at
surgery to have osteomyelitis. These changes are all relatively late
findings of osteomyelitis. Ultrasonography is of limited value when
attempting to detect early changes within bone.
limp and suspected musculoskeletal infection presents an imaging
dilemma that illustrates the importance of all four components of
patient evaluation for infection: history, examination, laboratory
evaluation, and imaging studies. If the 5-year-old can localize the
source of pain, and localization is confirmed by physical exam, plain
film radiographs are an appropriate first imaging studyin addition
to
CBC, ESR, and CRP. If examination is not suggestive of septic
arthritis, plain film radiographs are normal, and all laboratory values
are normal, then close observation with follow-up examination in 1 to 3
days is appropriate. If history and examination suggest an accessible,
localized process and laboratory values suggest infection, but plain
film radiographs are normal, then aspiration of the localized bone or
joint is appropriate. If patient history, exam, or standard radiographs
do not allow localization of the process, then technetium bone
scintigraphy is an appropriate next imaging study (Fig. 13.4).
Once the process has been localized—at any point in the evaluation
described in preceding text—if additional imaging is needed to
establish a diagnosis or characterize a pathologic process, then MRI is
the imaging study of choice to provide maximal information about the
bone and soft-tissue pathology. For straightforward musculoskeletal
infection in the appendicular skeleton, MRI is not necessary; but for
patients whose history, examination, laboratory evaluation, and plain
film radiographs are not concordant, or for patients with suspected
infection of the pelvis or axial skeleton, MRI is a very helpful
imaging study.
 |
|
Figure 13.6
This 13-year-old male presents with a 4-month history of proximal tibial pain and normal plain film radiographs. Lateral T2 magnetic resonance imaging (MRI) without contrast lacks the high signal intensity associated with marrow edema caused by osteomyelitis and suggests a more indolent cause. MRI is the only imaging modality that can provide such detailed information. Biopsy established the diagnosis of a diffuse, large B-cell lymphoma. |
possible and as soon as possible when musculoskeletal infection is
suspected, because it serves two important purposes: (a) aspiration may
confirm the presence of a bone/subperiosteal abscess or septic joint
that requires urgent surgical drainage and (b) aspiration often allows
determination of the specific bacteri responsible for infection.
Whenever possible and when safe to do so, initiation of antibiotic
treatment should be held until all initial cultures are obtained.
location for osteomyelitis is fortuitous and makes bone aspiration a
relatively easy task to accomplish in the emergency department.
Depending on the age and cooperation of the child, sedation may be
beneficial. Fluoroscopy is not necessary for bone or joint aspiration
but is now available in many emergency departments and can be helpful
in guiding and documenting needle placement. At the point of maximal
tenderness, the skin is sterilely prepped. Avoidance of cellulitic skin
when possible is reasonable but not mandatory; aspiration of bone
through cellulitis has not been shown to cause osteomyelitis, and
direct culture of cellulitic areas yields a positive culture in less
than 10% of cases (95). Local anesthetic is
used to anesthetize the skin and the underlying periosteum with its
abundant nerve supply. Using a large-bore trocar needle, such as an 18-
or 20-gauge spinal needle, the area at and beneath the periosteum is
aspirated for possible subperiosteal abscess.
the needle is passed through the thin metaphyseal cortex by rotating
the needle back and forth with gentle pressure directed toward the
center of the bone. A spinal needle with its solid trocar facilitates
passage through bone and prevents the needle lumen from being plugged
with bone fragments. Once inside the cortex, aspiration may yield
purulent material but, more commonly, and especially in early
osteomyelitis, sanguinous fluid returns. The purulent or sanguinous
fluid is then placed in appropriate media and sent for aerobic and
anaerobic culture as well as for microscopic Gram stain analysis.
Depending on the clinical situation, the aspirate may be sent for
fungal and mycobacterial culture. Sending bone aspirate for cell count
is less helpful than sending joint fluid, but if adequate aspirate
fluid is available, elevated WBC count can support the diagnosis of
infection. Bone aspirate cultures yield organisms in 50% to 85% of
patients with osteomyelitis (12,15,54,58).
information than does bone aspiration and is performed in a similar
fashion. Using an 18- or 20-gauge needle, the joint is aspirated and
fluid is placed in appropriate culture media and tubes for fluid
analysis. Hip aspiration should typically be performed under general
anesthesia in the operating room using a spinal needle and accompanied
by an arthrogram to document the presence of the needle within the hip
joint (Fig. 13.3). The most important tests for
joint fluid aspirate are Gram stain, culture, leukocyte count, and
determination of the percentage of
polymorphonuclear
cells. If Lyme disease is suspected, synovial fluid should be sent for
PCR testing as well. Routine use of other synovial fluid tests are of
little value (96,97).
Because fluid from an infected joint frequently clots, it may be
helpful to rinse the syringe with heparin before aspirating the joint.
Because only a small amount of fluid may be obtained, care must be
taken not to leave any significant volume of heparin in the syringe,
which may alter the cell count.
aspirate cell count greater than 50,000 per mL. Although the most
likely cause for a joint aspirate cell count to be greater than 50,000
per mL is bacterial septic arthritis, it is neither 100% sensitive nor
100% specific (Table 13.1). In a series of 126 bacteriologically proven cases of septic arthritis, Fink and Nelson (96)
found leukocyte counts of 50,000 per mL or less in 55%, with 34% having
counts less than 25,000 per mL. At the same time, inflammatory diseases
(e.g., rheumatoid arthritis) may have counts in excess of 80,000 per mL
(59). A joint aspirate with a percentage of polymorphonuclear cells greater than 75% is highly suggestive of joint sepsis (98).
aspirate cell count to approach 50,000 per mL. Nine patients with
brucellar arthritis treated at Ben-Gurion University had a median
synovial fluid cell count of 9500 WBC per mm3 (range 300 to 61,500 WBC per mm3) and only one patient had a cell count of greater than 50,000 per mL. Brucella melitensis was recovered from the synovial fluid culture in all patients (99).
seems to be slightly higher with open biopsy than with needle biopsy,
but the difference is not great. In addition, the positive yields are
generally not as high as in osteomyelitis, ranging in various reports
from 30% to 80% (53,58,100). The importance of obtaining material from blood and bone or joint aspiration is emphasized in a report by Vaughan et al. (101), in which many children with osteomyelitis had only positive blood cultures, whereas others had only positive bone cultures.
identification of the organism within a few hours of initial patient
contact and is therefore a valuable test that should not be ignored. It
appears from reports of both septic arthritis and osteomyelitis that
the Gram stain demonstrates an organism in about one third of the bone
or joint aspirates (53,54,96).
hours of specimen collection. However, fastidious organisms may take as
long as 7 days to become positive. S. aureus remains the most common causative organism, causing musculoskeletal infection in 60% to 90% of patients (57,102). Streptococci, pneumococci, Kingella kingae, and gram-negative bacteria are also potential causative organisms. Streptococcus infections have been associated with skin lesions associated with measles and varicella. Salmonella is specifically seen in association with sickle cell disease.
culture-positive and culture-negative septic arthritis. Lyon and
Evanich reviewed 76 children treated at Medical College of Wisconsin
and Children’s Hospital of Wisconsin for isolated joint infection
between 1990 and 1997 (103). All patients
underwent joint aspiration with fluid analysis, including culture, and
a causative organism was identified in only 30% of cases. There were no
significant clinical or laboratory differences between the
culture-positive and culture-negative groups, and all patients were
treated similarly with joint drainage and antibiotic therapy. All
patients had complete resolution of infection following treatment. Lyon
and Evanich concluded that, with regard to clinical presentation and
response to treatment, culture-negative septic arthritis did not differ
significantly from culture-positive septic arthritis and therefore
warranted a similar diagnostic and treatment approach.
several differences in the clinical presentation of culture-positive
septic arthritis compared to culture-negative arthritis (1).
Patients whose cultures were positive were more likely to have
antecedent trauma, overlying skin changes, and a shorter duration of
symptoms prior to diagnosis. However, treatment and treatment results
did not differ significantly between groups. In summary,
culture-negative septic arthritis can be treated empirically as
presumed staphylococcal disease with excellent long-term results.
be mistaken for infection. Trauma is the most common, made more
confusing because of the history of trauma often associated with
osteomyelitis. Similar features are typically present, including pain,
tenderness, swelling, and soft-tissue swelling on radiographs. However,
several distinguishing features may be present. Traumatic symptoms are
usually sudden in onset with gradual improvement, compared to symptoms
of infection, which are more likely to be gradual in onset and
progressive in nature. Trauma may be associated with elevation of the
CRP but not the ESR, whereas both are usually elevated in osteomyelitis.
Approximately 40% of children with leukemia present with constitutional
symptoms such as lethargy, 18% present with fever, and 60% have an
elevated leukocyte count and elevated ESR (105).
Although lucent metaphyseal bands are said to be characteristic of
leukemia, other bone changes are also seen. One study found lytic
lesions in 19%, sclerotic lesions in 4%, and periosteal new bone in 2% (105). A purely lytic lesion without uptake on bone scan is also characteristic of leukemia as well as eosiniphilic granuloma (106).
should raise suspicion of leukemia. A low leukocyte count may be
present in 35% of patients with leukemia, although this can also be a
sign of serious systemic sepsis. Anemia and an abnormally low platelet
count should also raise suspicion. Abnormal WBC forms seen on manual
differential is often diagnostic.
In the young child, metastatic neuroblastoma or eosinophilic granuloma
should be considered. Older children are more likely to have Ewing or
osteogenic sarcoma. Lymphoma may also occasionally arise primarily from
bone (Fig. 13.6). These lesions should be
approached as a malignancy with complete staging studies and diagnosis
confirmed by biopsy using an approach that will not jeopardize limb
salvage surgery.
disease is a glycogen storage disorder in which bone infarction occurs
and can cause symptoms similar to osteomyelitis. Similar to sickle cell
disease, patients with Gaucher disease may develop osteomyelitis, so
the physician should not simply assume symptoms to be caused by bone
infarction (107).
more challenging than for osteomyelitis for several reasons. There is
greater urgency because septic arthritis can cause permanent articular
cartilage changes within 8 hours if untreated (49),
and for septic arthritis there are more diagnostic alternatives than
for osteomyelitis. Interestingly, specific joints appear to be
especially susceptible to permanent injury following septic arthritis.
For example, the hip is more likely than the knee to progress to joint
destruction following septic arthritis. The physician should always
consider what must be diagnosed today, what can be diagnosed tomorrow,
and what can be diagnosed next week. For example, septic arthritis,
particularly of the hip, should be diagnosed as soon as possible,
whereas there is little harm to the patient if juvenile rheumatoid
arthritis (JRA) is diagnosed next week.
differentials is between septic arthritis of the hip and toxic
synovitis, a condition thought to be caused by a postinfectious
arthritis. The importance of this diagnosis is the need for immediate
drainage of the hip in the presence of bacterial sepsis, whereas toxic
synovitis need only be observed. Both may present with a history of a
few to several days of hip pain, with limp progressing to the inability
to walk. The physical signs are similar in both, with limited and
painful internal rotation, abduction, and extension. A longer history
of symptoms, with cyclic improvement and worsening, suggests toxic
synovitis. The pain is usually worse and the motion more restricted in
septic arthritis.
such as transient synovitis may be challenging. Kocher et al. reviewed
the cases of all children treated at Boston Children’s Hospital from
1979 to 1996 for an acutely irritable hip and developed a clinical
prediction algorithm to differentiate between septic arthritis and
toxic synovitis (108). Although several
variables differed significantly between septic arthritis and toxic
synovitis, there was considerable overlap, making diagnosis based on
individual variables alone difficult. However, four independent
multivariate clinical predictors—history of fever, non–weight bearing,
ESR of at least 40, and serum WBC count of more than 12,000 per mL—were
identified that, when combined, improved diagnostic accuracy. The
predicted probability of septic arthritis was 3.0% if one predictor was
present, 40.0% for two predictors, 93.1% for three predictors, and
99.6% if all four predictors were present. Although the presence of
three or more predictors was very specific for septic arthritis, it was
not highly sensitive.
attempting to validate the clinical algorithm proposed by Kocher et al.
At the same institution where the clinical algorithm was initially
formulated, Kocher et al. prospectively applied the algorithm to
children presenting with an acutely irritable hip (109).
The predicted probability of septic arthritis in the follow-up study
was 9.5% if one predictor was present, 35.0% for two predictors, 72.8%
for three predictors, and 93.0% if all four predictors were present.
The authors concluded that the four clinical predictors of septic
arthritis demonstrated diminished, but nevertheless good, diagnostic
performance
in
a new patient population. At a different institution, Luhmann et al.
applied Kocher’s clinical algorithm retrospectively to 163 patients who
presented with an acutely irritable hip and found that if all four of
the clinical variables in the algorithm were present, the predicted
probability of their patients having septic arthritis was 59%, in
contrast to the 99.6% predicted probability reported in Kocher’s
original article (110).
Although the proposed algorithm may be helpful, differentiating between
septic arthritis and toxic synovitis of the hip in an acutely ill child
will continue to rely on the clinical acumen of the orthopaedist.
diagnosis with septic arthritis, but several clinical features may be
used to distinguish between the two disorders. The hip joint is rarely
the initial joint affected in JRA. Symptoms in JRA are typically more
gradual in onset than septic arthritis, and the patient almost always
remains ambulatory. A joint affected by JRA typically looks worse than
it functions, with relatively good motion and modest pain despite the
large amount of swelling and synovitis that is typically present.
Initial laboratory values are often of little help in distinguishing
between septic arthritis and JRA. Joint fluid cell count typically
contains fewer than 100,000 leukocytes per mL in JRA, but leukocyte
counts of greater than 100,000 per mL have been reported (111).
In such cases, the treating physician has little choice but to begin
treatment of septic arthritis while continuing to work to determine the
diagnosis.
appearance than JRA, typically causing exquisite joint pain that seems
out of proportion to the normal-appearing joint. A sequela of group A
streptococcal infection, rheumatic fever most often causes pain in the
knees, ankles, elbow, and wrists that is evanescent and migratory.
Detailed questioning of the patient may unearth a history of untreated
pharyngitis, febrile illness, or rash caused by group A Streptococcus
approximately 2 weeks before onset of symptoms. Involvement of multiple
joints strongly directs the investigator away from septic arthritis.
Diagnosis of rheumatic fever is based on the Jones criteria. Major
criteria include carditis, arthritis, chorea, subcutaneous nodules, and
erythema marginatum. The minor criteria are arthralgia, elevated ESR or
CRP, heart block on electrocardiogram (EKG), and a history of previous
rheumatic fever. The diagnosis is made when a patient has two major
criteria, or one major and two minor criteria.
exposure, do not meet the Jones criteria, but have significant
arthralgia without other identifiable cause, the diagnosis of
poststreptococcal reactive arthritis (PSRA) has been used (112,113). A recent streptococcal infection may be documented by the presence of an antibody response to group A Streptococcus
or positive throat culture. Patients with acute rheumatic fever are
treated with long-term prophylactic antibiotics to prevent recurrent
rheumatic fever and associated carditis. The risk of carditis to
children with PSRA is unclear, and the role of long-term prophylactic
antibiotics following PSRA is controversial.
mimic septic arthritis include Henoch-Schonlein purpura and
enteroarthritis secondary to Salmonella or Yersinia
infection. Kawasaki disease and serum sickness are two additional
conditions also characterized by a rash and arthritis. Although the
joint symptoms do not require treatment and usually disappear within
days, patients with any of these conditions may require medical
management for the other, sometimes more serious, manifestations of the
disease.
musculoskeletal infection consist of antibiotics and surgery. The goal
of treatment should be to select the safest, least morbid, and most
cost-effective treatment that provides the highest likelihood for
complete and permanent elimination of infection. The treatment best
able to accomplish this goal depends on multiple factors, including
whether the infection is septic arthritis or osteomyelitis, its
location, the extent of involvement, the duration of symptoms, and the
specific causative organism.
following blood culture and culture of bone or joint. Understanding the
relative incidence of causative organisms in particular clinical
situations is very important because it allows for selection of an
effective antibiotic before an organism is positively identified by
culture. Neonates are at risk for septic arthritis caused by group B Streptococcus, gonococci, S. aureus,
and coliform bacteria; thus initial therapy should consist of
ceftriaxone or cefotaxime and oxacillin. For unimmunized infants
younger than 2 years, H. influenzae, Group A Streptococcus, and S. aureus
are likely pathogens, and initial therapy should consist of cefuroxime,
ceftriaxone or cefotaxime, and oxacillin. Septic arthritis in immunized
infants and older children is most likely caused by Staphylococcus, pneumococcus, or group A Streptococcus species and can be treated initially with oxacillin or cefazolin. Table 13.2 lists antibiotics and dosages commonly used in the treatment of pediatric bone and joint sepsis.
immediate and elevated serum antibiotic levels. The timing for
transition to oral medication remains controversial. Ampicillin,
cephalexin, cloxacillin, dicloxacillin, and penicillin G all penetrate
into pus and synovial fluid in
children
with septic arthritis in concentrations several times greater than the
mean inhibitory and mean bactericidal concentrations for S. aureus (114).
However, because toxic products of septic arthritis may cause
irreversible damage to articular cartilage within 8 hours of infection
onset, septic arthritis should be treated with joint irrigation and
debridement in addition to antibiotic treatment.
|
TABLE 13.2 ANTIBIOTICS COMMONLY USED IN THE TREATMENT OF BONE AND JOINT SEPSIS
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
standard for treatment of musculoskeletal infection. Although
parenteral administration of antibiotics ensures an immediate and high
serum concentration, this is achieved with some risk, inconvenience,
and expense. Outpatient parenteral antibiotic therapy (OPAT) has
reduced hospital stays and expense, but not without potential problems (115).
In a study of 184 patients with musculoskeletal infection treated using
OPAT, investigators at the University of Florida Health Science Center
identified several problems associated with OPAT (116).
Only 64% of patients completed their OPAT course without interruption,
and rehospitalization occurred in 26% of patients. Early
discontinuation of parenteral antibiotics because of adverse drug
reactions occurred in 24% of patients. There were 128
complications,
approximately half of which were related to catheter malfunction, and
catheter malfunction was more common in peripheral intravenous central
catheters (PICC) than in central catheters.
have examined the efficacy of oral antibiotic therapy. In the 1970s,
Nelson and others demonstrated that adequate bactericidal activity in
bone and joint tissue of children could be obtained using oral
antibiotics (114,117).
The serum bactericidal titer is often used as a marker to determine if
an adequate serum antibiotic concentration has been achieved using oral
antibiotics, with a peak titer of 1:8 as the level of serum activity
considered clinically effective. The ability to confirm adequate serum
antibiotic concentrations has led many physicians to use high-dose oral
therapy following initial intravenous antibiotic therapy as standard
practice, with excellent results.
and Case Western Reserve in Cleveland, investigators reviewed records
of 186 children treated for septic arthritis initially with parenteral
followed by oral antibiotics (21). Initial
parenteral therapy consisted of cefazolin administered at 75 to 100
mg/kg/day divided every 8 hours. Children who demonstrated clear
improvement on parenteral therapy—decreased swelling, tenderness, and
erythema and decreasing or absent fever—and who had families judged to
be compliant with oral therapy were placed on an oral antibiotic.
positive for staphylococci were administered cephalexin or cloxicillin
at a dose of 100 to 150 mg/kg/day or dicloxacillin at 75 to 100
mg/kg/day divided q.i.d. For streptococcal or pneumococcal infections,
penicillin V or amoxicillin at 75 to 100 mg/kg/day was used.
Bactericidal titers were drawn 60 to 90 minutes following the second or
third oral dose of antibiotic. If the titer was less than 1:8, the oral
dose of the β-lactam antibiotic was increased to a maximum of 150
mg/kg/day, and repeat bactericidal testing was performed to ensure that
a titer of at least 1:4 to 1:8 was achieved. Using this protocol, no
child required readmission for parenteral therapy due to inadequate
serum bactericidal activity. Average total duration of therapy was 30
days, with normalization of ESR at a mean of 33 days. Infection was
eradicated without sequelae in all except one patient for a
complication rate of 0.5%. Additional authors report similar results
with conversion to oral antibiotic after clinical response to a short
duration of parenteral antibiotics in the treatment of acute septic
arthritis or acute osteomyelitis (118, 119, 120).
osteomyelitis performed at eight tertiary pediatric hospitals in
Finland, peak bactericidal titers were not measured, utilizing instead
empiric high-dose clindamycin or cephalosporin and monitoring clinical
response to treatment, leukocyte count, ESR, and CRP. All patients
received initial parenteral antibiotic therapy and switched to
high-dose oral therapy within 5 days. No treatment failures were
detected at 1-year follow-up, suggesting that early conversion to oral
antibiotic treatment without monitoring serum bactericidal levels can
simplify treatment and reduce laboratory costs.
and vancomycin) can and should be monitored in all cases. These
antibiotics can be measured directly in the blood. Not only does the
blood level of these intravenous antibiotics vary significantly between
individuals, but their toxic side effects are significant. Both the
peak and trough levels need to be measured and monitored. For
gentamicin, blood is drawn approximately 30 minutes after
administration and just before the next dose. The peak level should be
between 5 and 10µg per mL, and the trough should be 1.9 µg per mL or
less. For vancomycin, blood is drawn 1 hour after administration and
just before the next dose. The peak level should vary between 20 and 40
µg per mL and the trough between 5 and 10 µg per mL.
should be measured every 3 to 4 days, as should the levels of blood
urea nitrogen (BUN) and creatinine. For prolonged (longer than 3 weeks)
or recurrent therapy with these drugs, it is wise also to monitor the
patient for ototoxicity. Vancomycin should be infused over no less than
1 hour to avoid the release of histamine by the drug (red man syndrome)
or serious hypotension. If a rash occurs, it usually can be
circumvented by administering the drug over 90 to 120 minutes or by the
use of intravenous diphenhydramine (Benadryl) 1 mg per kg (total dose
not to exceed 50 mg) just before the infusion.
cost while maintaining a high treatment success rate for patients with
septic arthritis. Utilizing early conversion to oral antibiotics,
Kocher et al. created a clinical practice guideline for treatment of
septic arthritis of the hip in children (121) (see Appendix).
Thirty consecutive patients with septic arthritis of the hip managed
before utilization of the guideline were compared with 30 consecutive
patients treated according to the guideline. There were several
statistically significant differences noted between groups. Patients
treated according to the guideline were much more likely to have a
follow-up CRP performed, had a lower rate of initial bone scanning, a
lower rate of presumptive surgical hip drainage, a greater compliance
with recommended antibiotic therapy, a faster change to oral
antibiotics, and a shorter hospital stay. There were no significant
differences with regard to outcome variables, including readmission to
the hospital, recurrent infection, recurrent drainage, development of
osteomyelitis or septic osteonecrosis, and limitation of motion.
Patients treated according to the clinical practice guideline had less
variation in the process of care and improved efficiency of care
without adverse outcome.
The choice of antibiotics for treatment of MRSA is critical and
limited. Vancomycin has been the antibiotic of choice for MRSA but
there is the risk of encountering S. aureus
strains resistant to vancomycin. Martinez-Aguilar et al. examined the
effectiveness of clindamycin treatment in 46 children with MRSA
infection of bone, joint, and sites outside the musculoskeletal system (123).
No significant difference could be detected between the patients with
MRSA infection and the group of patients with methicillin-sensitive S. aureus
infection, with successful eradication of infection in all groups
allowing the authors to conclude that clindamycin is an acceptable
antibiotic choice for the treatment of MRSA. Recent emergence of
vancomycin-resistant S. aureus in Japan
and in parts of the United States has raised the possibility of
musculoskeletal infection caused by bacteria for which there is no
known effective antibiotic treatment (124).
side effects. Pancytopenia, leukopenia, impaired liver function, or
impaired renal function may occur and should be monitored by weekly
determination of levels of creatinine, alanine aminotransferase (ALT),
aspartate aminotransferase (AST), and CBC. ESR and CRP should also be
checked weekly to monitor response to treatment. Antibiotic choice and
dosage are adjusted on the basis of side effects that may arise.
of infection and to achieve serum concentrations that should be
sufficient to kill bacteria, antibiotic treatment alone is not
universally successful. Factors that may reduce antibiotics’
effectiveness include possible interference of purulent material from
gram-negative organisms with the action of certain antibiotics (125,126), as well as the production of large amounts of β-lactamase by bacteria, rendering semisynthetic penicillins and cephalosporins ineffective (127, 128, 129).
These factors suggest that the local environment is important to the
effective action of the antibiotic. It makes sense, then, that altering
the local environment by irrigation and debridement improves antibiotic
effectiveness.
evacuation and elimination of bone or joint abscess, and for stopping
tissue destruction. Antibiotic therapy is always used in addition to
surgery when musculoskeletal infection is confirmed. By eliminating
dead space, nonviable tissue, and bacterial and host by-products,
abscess debridement and evacuation facilitates antibiotic delivery and
effectiveness. By removing harmful bacterial and host by-products,
debridement prevents further cartilage and tissue damage as well (130, 131, 132).
urgent indication for surgery, and in septic arthritis of the hip, a
true surgical emergency. Toxic lysosomal enzymes and by-products of
host defense response erode and destroy articular cartilage. Antibiotic
treatment alone will not remove the caustic by-products, and articular
cartilage destruction occurs despite appropriate antibiotic treatment
unless the corrosive material is evacuated.
rabbits that were treated with antibiotics, the beneficial effect of
surgical lavage has been demonstrated (52).
During the first arthrotomy at 4 days, the material generated by
infection in the knee could be washed out; at 7 days, it had to be
removed manually. All cultures were negative at 7 days. Both the
surgically treated and nonsurgically treated animals showed loss of
glycosaminoglycan. There was no collagen degradation in those treated
by surgical lavage, however. A similar study has shown that arthrotomy
and irrigation may be more effective than repeated aspirations, as the
data given in preceding text suggest (133).
child or adolescent. Erring on the side of surgical joint debridement
is appropriate. At the time the decision is made whether or not to
treat septic arthritis, culture results are rarely available. The
decision to treat (and usually to operate) is based primarily on
history, examination, and several laboratory tests, as discussed in
preceding text. The most helpful test is synovial fluid cell count (Table 13.1).
Although rheumatoid arthritis may cause a WBC count to become elevated
to more than 50,000 per mL, in most patients a synovial fluid WBC of
more than 50,000 per mL is caused by septic arthritis and warrants
irrigation and debridement.
treatment of septic arthritis. It is a procedure with which most
orthopaedists are familiar and that can be performed on short notice,
in a short amount of time, through a small incision with little
specialized equipment, yielding a high success rate. Debridement of all
nonviable, grossly infected tissue is indicated. Acute septic arthritis
does not require synovectomy and often can be closed over drains after
a single surgical procedure, whereas more chronic infections may
benefit from serial debridement. Chronic infection or recurrent septic
arthritis may result in a thick rind of tissue that lines the joint
cavity and must be removed. Several tissue cultures should be sent
during surgery before antibiotics are administered. Sending specimens
to pathology for microscopic examination may also be helpful in
establishing a diagnosis, especially if all cultures turn out to be
negative.
The specific joint involved, chronicity of the infection, experience of
the surgeon, and experience of the allied health staff available at the
time of surgery should be considered before deciding to perform
arthroscopic joint
debridement.
In the hands of an experienced arthroscopist, debridement of relatively
inaccessible areas such as the posterior compartment of the knee can be
performed, and the joint can be irrigated with a very large volume of
fluid. One distinct disadvantage of arthroscopic debridement is that
because the arthroscope is looking out from the inside of the joint, it
may be very difficult to accurately assess the depth of the purulent
material and the extent of infection, especially in chronic cases. This
may lead to inadequate debridement and persistent infection.
open surgery or arthroscopically, drains should be placed at the
conclusion of the procedure and appropriate empiric parenteral
antibiotic therapy initiated.
osteomyelitis. Acute osteomyelitis without abscess formation can
typically be managed successfully with antibiotics alone (137),
whereas chronic osteomyelitis is almost always most appropriately
treated with surgical debridement. One situation in which surgical
treatment of acute osteomyelitis is indicated is when subperiosteal or
bone abscess is present. Subperiosteal or bone abscess may form within
3 to 5 days following the onset of infection. Although there is not any
absolute or uniformly accepted definition, it is reasonable to consider
osteomyelitis chronic if the patient has been experiencing symptoms
more than 3 weeks or there is radiographic evidence of long-standing
infection.
point tenderness over bone in a febrile child should be considered AHO
until proven otherwise. Blood cultures and appropriate lab tests should
be obtained and bone aspiration performed urgently. As soon as cultures
are obtained, empiric high-dose intravenous antibiotic treatment should
be initiated with the antibiotic choice based on patient age and
clinical circumstances. The most common organism causing AHO in
patients of all ages is S. aureus, which
must be adequately covered. For neonatal osteomyelitis, treatment
targeting group B streptococci and gram-negative rods should be added.
Children younger than 4 years should be covered for H. influenza type b if not immunized. For fully immunized children, the most likely pathogens are S. aureus, Streptococcus pyogenes, and S. pneumoniae (15). If bone aspirate or blood cultures are positive for a specific bacteria, the antibiotic choice is adjusted accordingly. Table 13.2 lists antibiotics and dosages commonly used in the treatment of pediatric osteomyelitis.
semisynthetic penicillin may be chosen. Methicillin may cause
interstitial nephritis, and nafcillin may cause skin sloughing if
subcutaneous infiltration occurs, so oxacillin is a good initial
choice. At the time of conversion to an appropriate oral antibiotic, an
acceptable choice would be cephalexin at a dose of 100 to 150 mg/kg/day
or dicloxacillin at 75 to 100 mg/kg/day divided q.i.d. For patients
allergic to penicillin, clindamycin is an appropriate oral antibiotic
choice, and vancomycin is an appropriate intravenous antibiotic.
Similar to the antibiotic treatment of septic arthritis, the
administration route and duration of treatment of AHO is controversial
and depends upon the clinical situation of each patient. At one time,
all children with AHO were routinely treated with 6 weeks of
intravenous antibiotics as a hospital inpatient. Over the last two
decades, several trends have developed: (a) treatment has moved from
inpatient to an outpatient setting and (b) treatment has shifted from
entirely parenteral to a parenteral then oral antibiotic regimen.
Intravenous therapy is initiated in the hospital setting, but once
therapeutic response to treatment is confirmed, conversion to oral
antibiotic treatment is made and continued on an outpatient basis.
not known and varies with clinical circumstances. Previous authors have
reported that a treatment duration of less than 3 weeks is associated
with an increased likelihood of recurrence (27), and antibiotic treatment of at least 3 weeks has now become accepted (12,22,54,60).
The route and duration of antibiotic treatment is individualized for
each patient, considering factors such as the age and overall health of
the patient, duration of infection, whether a bacterial organism has
been isolated, the susceptibility of the organism, the amount of tissue
destruction present, previous surgery, adequacy of debridement, and the
site of involvement. If a susceptible organism is cultured and the
patient experiences a good clinical response to treatment, then
conversion to an appropriate high-dose oral antibiotic as early as 3 to
5 days after initiating treatment is appropriate. If there is no
clinical response to medical management within approximately 48 hours,
the presence of an abscess becomes a possibility. Reevaluation of the
patient with consideration of additional imaging studies such as CT or
MRI (if not already obtained) and contemplation of surgical treatment
is appropriate.
is the most important factor to consider when making the decision to
convert to oral antibiotics; it may be defined as the absence of fever
with improvement in symptoms such as tenderness, limp, malaise,
anorexia, and night pain, and reduction in CRP. Ultimately, it is the
antibiotic serum concentration rather than the route administration
that correlates with treatment success (117).
To achieve adequate serum antibiotic levels with oral therapy, several
prerequisites have been suggested including the patient’s ability to
swallow and absorb oral antibiotics, the ability to follow serum
bactericidal levels, and the resistance or susceptibility of the
organism to available oral antibiotics. (57,117). Peak antibiotic serum levels are obtained by drawing a blood sample 1 hour after oral administration of the drug.
A bactericidal level of 1:8 has been recommended (102).
Recent literature suggests that if high recommended dosages are
followed, monitoring bactericidal levels is not necessary to achieve
excellent treatment results (22). More chronic
infections caused by virulent or resistant organisms in patients
experiencing a slow clinical response warrant a longer duration of
intravenous antibiotics.
Because ESR may require 4 to 8 weeks before normalization, antibiotic
therapy may be unnecessarily prolonged if ESR normalization is a
prerequisite for discontinuation of antibiotic therapy. Peltola and the
Finnish Study Group noted that CRP responds more rapidly than ESR to
effective treatment, and they often discontinued antibiotic therapy
before ESR completely normalized with no increase in infection
recurrence rate (22).
treatment is begun using intravenous cefazolin at a dose of 100 to 150
mg/kg/day divided every 8 hours, after obtaining local bone and blood
cultures (15). Serial values for CRP are
monitored daily (or every other day) and in uncomplicated AHO should
normalize within 5 to 7 days following initiation of parenteral
treatment. Good clinical response, as demonstrated by absence of fever,
lack of tenderness, improvement in swelling, resolution of limp, return
of appetite, and absence of night or rest pain, should coincide with
normalization of CRP. Once CRP normalizes and clinical improvement is
seen, oral cephalexin therapy is begun at a dosage of 150 mg/kg/day
divided every 6 hours. For uncomplicated AHO that responds rapidly to
the regimen described in preceding text, a total duration of 3 weeks of
antibiotic therapy is appropriate.
therapy, we recommend weekly laboratory testing, including CBC, ESR,
CRP, AST, and ALT, to monitor for continued response to treatment and
antibiotic-related side effects such as neutropenia or alteration in
liver function tests.
Surgery for osteomyelitis should remove the purulent material as well
as necrotic or avascular bone and all possible grossly infected and
nonviable soft tissue. When the bacterial mass and necrotic tissue are
dramatically reduced, host defense mechanisms work more effectively and
antibiotic delivery to the region is facilitated. When possible,
surgical debridement is performed before antibiotics are administered,
and bone cultures taken at the time of surgery provide another
opportunity to identify the causative organism.
importance of routine histologic examination of material from the bone
is twofold. Some tumors have a tendency to become necrotic and, when
surgically explored, may look similar to pus; the most common is
metastatic neuroblastoma, followed by Ewing sarcoma. In addition, if
positive identification of the organism is not obtained, it is
reassuring to have a histologic diagnosis of osteomyelitis.
antibiotic therapy is a second and important indication for surgical
treatment of acute osteomyelitis. Before proceeding immediately to
surgical exploration and debridement, the treating physician should
evaluate more carefully why antibiotic treatment alone was not
sufficient. The most likely causative organism should be reexamined,
antibiotic choice and dose reviewed, possible alternative source of
infection considered, and evaluation for bone or soft tissue abscess
performed. If no explanation other than insufficiently treated local
musculoskeletal infection can be found, local imaging with CT or MRI is
often helpful to identify possible abscess and to plan surgical
debridement. Because it provides the greatest soft-tissue detail and
marrow edema imaging, MRI is becoming the study of choice if readily
available.
primarily over drains. Intravenous antibiotic treatment is initiated
immediately after cultures are obtained, and conversion to oral
antibiotics is made after clinical response to treatment has been
documented.
chronic if the patient has been experiencing symptoms for more than 3
weeks or there is radiographic evidence of long-standing infection.
Aggressive osseous debridement is the most important aspect of chronic
osteomyelitis surgical treatment (9). Whether
to perform a single debridement or multiple debridements is a decision
made based on clinical circumstances and surgeon judgment at the time
of the operation. A small area of infection that is aggressively
debrided can be closed primarily over drains. Whenever in doubt, the
safe course is to return for a second look with repeat debridement 2 to
3 days later. A common mistake and natural tendency is to perform
inadequate debridement. The status of the periosteum overlying infected
bone is significant (134). The presence of an
involucrum confirms periosteum viability and its ability to form new
bone. Involucra form when infection elevates the periosteum off of
underlying infected bone, and the periosteum begins to form bone in its
new position. Infected, with its periosteal blood supply no longer
intact, the underlying cortical bone quickly becomes a necrotic
sequestrum. A valuable general treatment principle is to aggressively
debride the necrotic sequestrum but to leave in place the viable
involucrum.
remarkable, such that large defects created by debridement will
reossify without bone grafting. Unfortunately, the older patients are
less able to reossify extensive bone defects created by chronic
osteomyelitis and debridement. Depending on the size and location of
the lesion, children younger than 10 years will occasionally benefit
from bone grafting,
children
between ages 10 and 15 will often require bone grafting, and patients
16 years and older will almost always benefit from bone grafting,
especially in weight-bearing bones. The timing and source of bone graft
is a matter for discussion. Bone grafting at the time of initial
debridement risks persistent infection by placing nonviable tissue into
an area where high bacteria counts are known to exist. Primary bone
grafting may be acceptable in situations where radical debridement of
infection caused by a susceptible organism is performed. In most
situations, bone grafting is best performed at a second operation. In
favorable clinical situations, bone grafting may be done 2 to 3 days
following the initial debridement.
allogenic bone graft, or another material to stimulate bone formation
in large osseous defects continues to evolve and is beyond the scope of
this chapter. Factors that should be considered when choosing a
material include safety, effectiveness, availability, and cost.
Unfortunately, there currently is no manufactured substance that
clearly surpasses autologous or allograft bone material, and therefore
bone grafting remains the most appropriate means of filling large
osseous defects following osteomyelitis debridement. Powdered
antibiotic such as gentamicin, tobramycin, or vancomycin may be mixed
with bone at the time of grafting.
delayed a full month while clinical and laboratory parameters confirm
successful treatment by continued parenteral antibiotics. If delayed
bone grafting is anticipated, placement of antibiotic-impregnated
polymethylmethacrylate (PMMA) at the conclusion of the initial series
of debridements may be considered. The antibiotic impregnated PMMA is
removed at the time of bone grafting.
have not been clearly established, but the use of
antibiotic-impregnated PMMA is increasing. Antibiotic impregnated-PMMA
should not be used for uncomplicated metaphyseal osteomyelitis.
Adequate surgical debridement and appropriate parenteral and/or oral
antibiotic therapy has such a high success rate that
antibiotic-impregnated PMMA adds no significant benefit. Relative
indications for antibiotic-impregnated PMMA include
-
Recurrent/persistent infection after reasonable and appropriate treatment)
-
Extensive chronic infection that cannot be completely débrided surgically)
-
Extensive osseous defects and dead space to be treated by delayed bone grafting (Fig. 13.7)
high concentrations of antibiotic locally while maintaining low
systemic levels. An acceptable antibiotic cement mixture consists of
4.8 g of gentamicin (1.2 g per vial) or 4.8 g of tobramycin (1.2 g per
vial), 4.0 g of vancomycin (1.0 g per vial), and one full batch of
PMMA. Methylene blue may be added to facilitate mixing the large volume
of powdered antibiotic and PMMA as well as to aid in visualization of
PMMA at the time of removal. In situations where antibiotic-impregnated
PMMA is used, patients typically receive 6 to 8 weeks of parenteral
antibiotics. A reasonable protocol is to place antibiotic-impregnated
PMMA into the infection bed at the time of initial closure. Four weeks
after closure, the PMMA is removed, and bone grafting is performed if
necessary. This protocol allows for 2 to 4 weeks of parenteral
antibiotics to continue after antibiotic PMMA removal.
to treat large osseous defects. Kucukkaya et al. used the Ilizarov
method in seven children, ages 6 to 8, with tibial defects averaging
7.4 cm in length following osteomyelitis debridement (135). All patients had complete healing without bone grafting.
surgical debridement and initial parenteral antibiotics with conversion
to oral antibiotics after favorable treatment response. Total treatment
duration of approximately 3 weeks is appropriate, depending on clinical
and laboratory response to treatment.
no abscess is present, then surgical debridement is not indicated.
Parenteral antibiotics should be initiated, with conversion to oral
antibiotics after favorable clinical response for total treatment
duration of approximately 3 weeks, depending on clinical and laboratory
response to treatment.
history of acute infection (e.g., 4 days of increasing symptoms) and
aspiration demonstrates subperiosteal or bone abscess. Surgical
debridement of the abscess is indicated; extensive debridement of
surrounding bone is not. Closure over drains and initiation of
parenteral, then oral, antibiotic treatment for AHO, as described in
preceding text, is appropriate.
been present for more than 3 weeks, in association with bone abscess or
destruction, surgical debridement is indicated. Preoperative MRI, CT,
and often both are helpful for determining the extent of infection and
planning surgical debridement. Tissue cultures are obtained, and
depending on culture results, extent of infection, and response to
treatment, a long-term course of parenteral antibiotics for 6 to 8
weeks is often appropriate. If the infection is recurrent or cannot be
completely debrided, the use of antibiotic-impregnated PMMA is
acceptable. Bone grafting or bone transport may be used to fill large
bone defects and can be performed 3 to 4 weeks after initiating
parenteral antibiotic treatment.
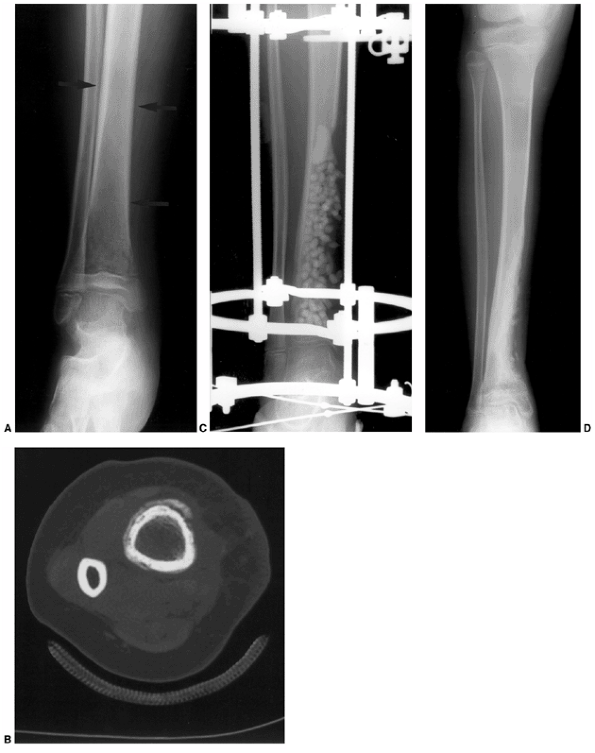 |
|
Figure 13.7 A:
An 8-year-old girl is placed in a cast after an ankle sprain. Multiple trips to the local emergency department for increasing pain were treated with narcotic pain medication. Three weeks following the injury, the cast was removed to reveal a swollen, erythematous leg. The anteroposterior radiograph demonstrates an infiltrative, destructive process in the distal tibial metaphysis suggestive of osteomyelitis. Periosteal reaction can be seen over the distal half of the tibia (arrows). B: A computed tomography (CT) image at the metaphyseal-diaphyseal junction shows the infection eroding through the cortex and elevating the periosteum. The elevated periosteum forms an involucrum as the distal tibia becomes a sequestrum. C: Thorough debridement of osteomyelitis created a very large segmental defect that was filled with antibiotic-impregnated polymethylmethacrylate beads to deliver local antibiotics, fill dead space, and prepare for future bone grafting. A circular fixator was placed to maintain stability, length, and alignment. D: One year after initial debridement, the patient is free from evidence of infection, and bone graft continues to consolidate, but varus deformity of the distal tibia caused by partial physeal arrest is present. |
Diarrhea, nausea, rash, thrombocytopenia, transient changes in liver
enzymes, and antibiotic-induced neutropenia have been observed (117).
Checking weekly ESR, CRP, CBC, and liver function tests ALT and AST can
monitor for adequate response to treatment and for adverse side effects
associated with antibiotic treatment.
destruction, joint contracture, gait abnormality, and abnormalities of
bone growth. Avascular or osseous necrosis of the femoral head is a
known complication of hip septic arthritis. Most likely caused by
occlusion of intracapsular ascending vessels by increased intracapsular
pressure or involvement by infection itself, osseous necrosis of the
femoral head can be a devastating complication for which there is no
good treatment. Femoral head osseous necrosis typically results in
collapse, fragmentation, and growth retardation of the femoral head.
Both the femoral head and acetabulum may become dysplastic with
subluxation and dislocation possible. These events lead to limb-length
discrepancy, stiffness, and endstage joint destruction.
places infection in close proximity to the physis. Physeal involvement
by osteomyelitis may injure a region of the physis or destroy the
physis completely, causing growth inhibition and angular deformity or
limb-length discrepancy. Prompt, appropriate treatment of infection and
avoidance of bone grafting across the physis may prevent growth
disturbance (Fig. 13.1). Children who
experience systemic sepsis such as meningococcemia or purpura fulminans
may develop multiple physeal arrests and extensive soft-tissue injury
with distressing consequences. Failure to completely eradicate
osteomyelitis may result in chronic pain or draining sinus tract.
reported a case in which septic arthritis of the sacroiliac joint led
to arterial and venous thrombosis associated with hereditary protein C
deficiency (138). Walsh and Phillips reported four children with musculoskeletal sepsis who developed deep venous thrombosis (56).
One child died as a result of the thrombosis; the remaining three
children were screened, but had no evidence of, an underlying
thrombolic disorder. While rare, clinicians should consider vascular
thrombosis in children with musculoskeletal sepsis who experience
severe limb swelling.
Over the past several decades, various descriptions in the literature
of vertebral osteomyelitis and discitis reflect the uncertainty that
these are indeed two separate conditions (141,142).
Modern imaging modalities, such as scintigraphy, CT, and MRI, have
helped resolve the confusion by demonstrating evidence of bone
involvement in children with the clinical presentation of discitis (143,144).
It therefore appears that both vertebral osteomyelitis and discitis are
the result of a hematogenous infection beginning in the bone adjacent
to the cartilaginous vertebral end plate.
demonstrated that the blood supply to the disc comes from the
contiguous bone of the vertebral bodies (145, 146, 147, 148).
In the young child, vessels can be identified traversing the
cartilaginous vertebral endplate and entering the annulus. By the age
of 8, these vessels have largely disappeared. It is likely that
discitis and vertebral osteomyelitis represent two slightly different
clinical manifestations of a similar disease process affected by
changes in vascular anatomy with growth and development. Immature
vertebral endplate and disc-space vascular anatomy result in a clinical
focus of infection within the discs of young children, whereas older
children are more likely to have a primary focus of infection within
the vertebral body. This is consistent with the observation that the
average age at onset in patients with discitis is 2.5 years compared to
the mean age at presentation for vertebral osteomyelitis, which is 7.5
years of age (149).
systemic signs of illness such as fever, malaise, anorexia, or sleep
disturbance (150). Patients often refuse to
ambulate but have a normal lower extremity exam. Back pain or abdominal
pain may also be present. Spine exam frequently demonstrates decreased
ROM and discomfort to palpation and percussion. Laboratory parameters,
including ESR and CRP, are usually, but not always, elevated. Plain
film radiographs are usually normal but may show subtle vertebral
endplate irregularity and, later, slight reduction in disc height at
the suspected level. In confusing clinical situations, bone scan is
often the most helpful test to localize infection of the axial skeleton
(Fig. 13.4). If the clinical situation strongly
suggests a pathologic process of the spine, but additional imaging is
necessary to confirm the diagnosis, MRI is recommended as it provides
the greatest amount of information in a single imaging study (143,144).
is the result of bacterial infection. Blood cultures are usually
negative, disc aspiration and culture are rarely performed, and there
have been several reports of patients recovering completely after
treatment with immobilization alone. When positive, the biopsy results
show a preponderance of S. aureus as the causative organism (150, 151, 152, 153). Most agree that discitis is a bacterial infection that is best treated with
high-dose intravenous followed by oral antibiotics and immobilization
as necessary to control symptoms. Empiric intravenous antibiotic
therapy is directed at the most common offending organism, S. aureus.
Following clinical response to treatment, transition to an appropriate
oral antibiotic is made, and treatment is continued for approximately 3
to 5 weeks. Weekly lab testing is performed to monitor for antibiotic
side effects and response to treatment.
72 hours. If this is not the case, the physician should begin to
question the diagnosis or the specific bacterial etiology. Further
imaging studies, such as CT or MRI, may be justified in such
circumstances to search for tumor or abscess formation. Biopsy may be
indicated in a patient who fails to respond to antibiotics and bed rest
and is indicated in any child whose imaging studies suggest a diagnosis
other than typical discitis.
Compared to discitis, children with osteomyelitis are older, are more
often febrile and ill-appearing, and have a longer symptom duration (149).
Standard radiographs are often normal during the first few weeks of
symptoms in patients with vertebral osteomyelitis, and either bone scan
or MRI is often helpful in establishing the diagnosis. The abundant
vertebral blood supply provides outstanding antibiotic delivery.
Vertebral osteomyelitis can almost always be eradicated by antibiotics
alone unless abscess formation occurs.
Usually presenting with back pain, patients can present with neurologic
deficit. MRI is the preferred imaging modality for evaluation of
suspected epidural abscess. When present and correlative with
neurologic deficit, emergent decompression of the epidural abscess is
indicated. If there is no neurologic deficit or displacement of the
spinal cord caused by the epidural abscess, antibiotic treatment alone
can be effective.
with vague hip or back pain and difficulty localizing their symptoms,
and their physical exam is often nonspecific. Difficulty in diagnosis
can lead to unnecessary procedures such as appendectomy (139).
Perhaps the most common diagnosis that is confused with SI joint sepsis
is septic hip. SI joint infection is generally seen in older children,
with the mean age being 10 years, whereas septic hip is more common in
the younger child (155). Despite the complaint
of pain around the hip, children with SI joint infection often remain
ambulatory and have relatively free internal rotation of the hip, in
contrast to those with a septic hip. Conversely, patients with SI joint
infection frequently experience greater pain on external rotation of
the hip than internal rotation. If the FABER test (flexion, abduction,
external rotation) is performed, it usually elicits pain in the
presence of SI joint sepsis, as does compression of the pelvis
(Gaenslen test). Tenderness almost always is found over the SI joint,
if sought. Other areas (e.g., the ischium, pubis, ilium) should always
be palpated for tenderness in children with gait disturbance or hip
pain.
radiographic views demonstrating the SI joint may be obtained, but
their value in making an early diagnosis today, when better imaging
techniques are available, is doubtful. In most cases of pelvic
osteomyelitis, the initial radiographs are normal. This is especially
true when symptoms have been present for fewer than 1 or 2 weeks. The
earliest sign of infection on the radiograph is disappearance of the
subchondral margins and erosion; however, this should be considered to
be a late finding. If radiographic changes are present within less than
1 week of symptoms, careful consideration should be given to other
disorders, such as tumor or chronic inflammatory SI disease.
Radionucleotide bone scan is most helpful in localizing the infection,
followed by CT or MRI to more clearly determine its extent. Because of
the complicated three-dimensional pelvic anatomy, CT or MRI should be
performed in all patients with pelvic osteomyelitis to determine the
extent of bone and soft tissue involvement, as well as to detect
possible abscess formation (Fig. 13.8).
established in 57% of the cases they studied from their own patients,
and in a literature review (155). In most cases, S. aureus is the organism that is cultured from blood, direct aspiration, or biopsy (155, 156, 157, 158, 159). Staphylococcus epidermidis and Streptococcus species are also reported but, in many cases, may be contaminants (158). An occasional Salmonella species may be isolated in patients who are not otherwise predisposed (155,159,160); therefore, stool cultures should be obtained.
Complex anatomy often makes surgical debridement difficult. In a
comprehensive recent review, Davidson et al. described the most
frequent locations for osteomyelitis to occur within the pelvis and
reported treatment results in 64 children (161).
The most common pelvic sites of infection were the ilium in 21 and the
acetabulum in 20 patients, followed by the pubis and ischium in 11 and
10, respectively. Sixty-two patients were treated with antibiotics
alone, and five were treated with antibiotics and surgical debridement,
confirming that surgery is indicated in a small minority of patients
with pelvic osteomyelitis.
of suspected tumor, an unusual presentation, or failure to achieve
resolution of the symptoms in a reasonable amount of time. Drainage of
a large abscess may be necessary, especially in the presence of
systemic symptoms. Abscess drainage can often be performed
percutaneously under image guidance, with a reported success rate in
children of 85% to 90% (162).
as used in the treatment of AHO (Table 13.2).
If symptoms resolve and the CRP begins to fall, the patient may be
switched to oral antibiotics in 5 to 7 days, provided adequate blood
levels are obtained with oral administration. Initial and subsequent
antibiotics should be adjusted to reflect information from blood and
stool cultures as well as from biopsy material if that has been
obtained. Failure of a response suggests that the antibiotic is not
effective against the causative organism, a large abscess persists, or
the etiology is not infectious.
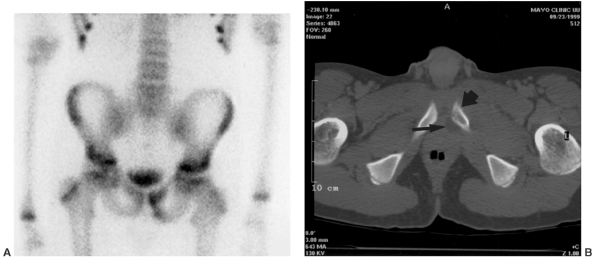 |
|
Figure 13.8 A:
A 15-year-old boy presents with groin pain, fever, chills, and malaise. Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are elevated, but plain film radiographs of pelvis and spine are normal. Bone scan demonstrates increased uptake in the left inferior pubic ramus. B: Computed tomography (CT) shows destructive changes within the pubic ramus (large arrow) and adjacent abscess formation (small arrow) suggestive of osteomyelitis, which was confirmed at the time of surgical biopsy, culture, and debridement. |
-
Localize the infection. If history, exam,
and standard radiographs do not allow localization of the infection,
technetium bone scan should be performed. -
Determine the extent of infection. Once
localized, CT or MRI should be performed in virtually all patients with
suspected osteomyelitis of the pelvis and most infections of the spine.
Discussion with your radiologist is often helpful when making the
decision whether to use MRI or CT. MRI provides the greatest amount of
information regarding bone and soft-tissue pathology, but it may
require general anesthesia for young children, is more expensive than
CT, and is not as readily available as CT is at some institutions. CT
may allow aspiration or placement of percutaneous drains under image
guidance. -
Treat the infection. Although epidural
abscess with neurologic compromise or large abscess with systemic
sepsis require immediate surgical treatment, almost all other infection
of the axial skeleton can be treated effectively with parenteral
antibiotics. Failure to respond to parenteral antibiotic therapy
warrants abscess drainage. This may be performed percutaneously or
surgically, depending on abscess size, location, and availability of
interventional radiology.
with the earth, it is susceptible to trauma and puncture that can
result in infection (Fig. 13.9). The foot is
more likely to be inoculated with bacteria from the local environment
and therefore is more likely to have infection caused by a spectrum of
bacteria different from those causing the hematogenous osteomyelitis
seen in long bones. Since Johanson’s 1968 report, orthopaedic surgeons
have become increasingly aware of the association between puncture
wounds of the foot and Pseudomonas aeruginosa as the causative organism of deep infections that follow (163). It was subsequently demonstrated that Pseudomonas can be recovered from the inner spongy sole of well-worn tennis shoes (164). P. aeruginosa
is a gram-negative aerobic organism with anaerobic tolerance, which is
found widely in soil, water, and on the skin. As a human pathogen seen
in orthopaedic conditions, it seems to have an affinity for cartilage.
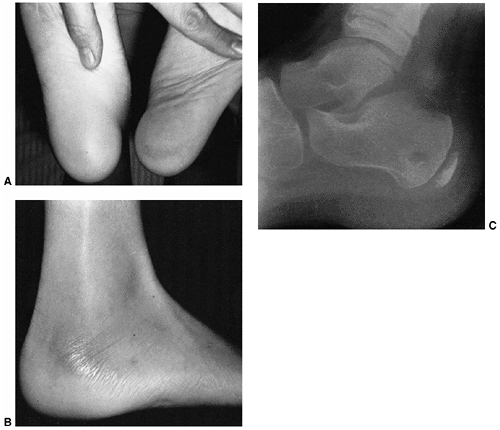 |
|
Figure 13.9
This patient was seen with pain 2 weeks after a puncture wound of the heel. He initially came to the emergency department 3 days after the puncture wound because of increasing pain and swelling. Therapy was begun with a first-generation cephalosporin antibiotic. He experienced temporary improvement, but later the pain became worse. A: Note the swelling of the affected heel, compared with the opposite contralateral heel side. B: Because of the dense septated tissue in the heel, osteomyelitis of the calcaneus usually is seen laterally. The swelling and erythema on the lateral side of the heel indicates deep infection. C: A radiograph demonstrates a lytic lesion of the heel, in addition to the soft-tissue swelling. Pseudomonas aeruginosa was cultured from the infected site. |
to the foot do not develop bone or joint infection. Fitzgerald and
Cowan (166) identified puncture wounds of the
foot as the reason for an emergency room visit in 0.8% of children
younger than the age of 15. Only 0.6% of those who were not referred to
the emergency room for an established infection subsequently developed
osteomyelitis. Of 132 patients seen with soft-tissue infection after
puncture wound of the foot, 112 had a prompt response to soaks, rest,
elevation, and antibiotics.
soft-tissue infection, a reasonable approach to the initial management
of a puncture wound is warranted. Superficial cleaning and debridement
of the skin and inspection for a foreign body is appropriate. Tetanus
prophylaxis is important. There does not seem to be any solid evidence
either for or against the routine use of antibiotics in the initial
management. Prompt return for reevaluation at the first sign of
infection is recommended, and treatment with soaks, elevation, and an
oral antistaphylococcal antibiotic is warranted. If the patient has
cellulitis, this regimen usually results in a cure.
soaks, rest, elevation, and oral antibiotic treatments suggests the
presence of septic arthritis or osteomyelitis. Careful clinical
examination is helpful in establishing the presence and location of
infection. Comparison with the contralateral foot is helpful, looking
for focal swelling, skin changes, or tenderness on palpation. Pain on
motion of a specific metatarsophalangeal joint is usually indicative of
a septic arthritis in that joint. Dorsal swelling on the forefoot, or
swelling laterally and medially around the heel, is often an additional
sign of a serious deep infection.
osteomyelitis or septic arthritis, but should be obtained to look for
bone changes or a metallic foreign body. MRI may be the most
cost-effective way of identifying deep infection of the foot following
puncture wounds (167). If deep infection is
identified, surgical debridement is indicated to evacuate infected
material and search for a foreign body.
bone or joint is a surgical disease; the failure of antibiotics alone
to resolve these infections has been adequately demonstrated (168). The surgical approach may be either dorsal or volar, but must
give adequate access to both the bones and joints in the region of the puncture, because P. aeruginosa
is a cartilage-seeking organism. Some surgeons believe that the volar
approach leaves a potentially painful scar. When properly placed,
however, this should not be the case. This approach has the advantage
of directly exposing the puncture track, which is an essential part of
the surgery, because of the high incidence of foreign material found at
surgical debridement (166,169).
The dorsal approach allows direct access to the joints and bones
through a more anatomic and extensile approach, which is not limited by
the considerations of placement on the sole of the foot. This can be
combined with a limited debridement of the volar puncture wound. The
calcaneus should be approached from a medial or lateral incision, or
from both.
is usually one of them. For this reason, it makes sense to begin
antibiotic therapy with a combination of antibiotics effective against
both gram-positive organisms and gram-negative organisms, including P. aeruginosa. An initial choice may be ceftazidime (Fortaz) and gentamicin or oxacillin and gentamicin (Table 13.2). Jacobs et al. (168,169)
suggest that 7 days of intravenous antibiotics after adequate surgical
debridement is sufficient, although others recommend longer treatment,
from 10 days to 2 weeks.
However, its use in children has been limited by reports of interfering
with the growth plate in animal studies. Despite this, it has been used
in cystic fibrosis and in other serious infections in children, without
reports of ill effects on cartilage or growth.
foot as well and may have a predilection for the calcaneus.
Puffinbarger et al. reported a series of 11 cases of osteomyelitis of
the calcaneus and noted that cases of hematogenous osteomyelitis were
most commonly caused by S. aureus, whereas all puncture-related cases were positive for P. aeruginosa (170).
Several factors make treatment of calcaneal osteomyelitis especially
challenging. The calcaneus does not have investing musculature to
provide blood supply and antibiotic delivery or to provide soft-tissue
coverage following surgery. Eggshell-like bone and limited ability to
regenerate itself following debridement limit surgical options for
calcaneus debridement. These factors do not limit what needs to be done
but may cause increased morbidity with surgery.
osteo-myelitis allows effective treatment with antibiotics alone.
Jaakkola and Kehl reported successful antibiotic treatment of
hematogenous calcaneal osteomyelitis without surgical intervention (171).
If the diagnosis of calcaneal osteo-myelitis is delayed, significant
complications may result including shortening of the foot, tarsal bone
fusion, adjacent bone osteomyelitis, and avascular necrosis that can
require radical surgery such as calcanectomy (172, 173, 174).
8 weeks of age, is susceptible to a variety of musculoskeletal
infections unique to this age group. Their immature immune system makes
neonates susceptible to a wide range of organisms that are less
virulent under normal circumstances and prevents them from expressing
symptoms and signs that allow early diagnosis.
neonate: that recognized in the hospital in premature infants and that
which becomes apparent after discharge from the nursery in otherwise
healthy, full-term neonates. The type manifest in the hospital usually
occurs in premature infants undergoing invasive monitoring. These
neonates remain in the intensive care unit in the presence of
nosocomial pathogens, coupled with invasive monitoring, intravenous
feeding, drug administration, and blood sampling. Indwelling vascular
catheters, particularly those in the umbilical vessels, have long been
recognized as one of the main sources of infection (175). These infants are more likely to have infection caused by S. aureus
or gram-negative organisms, to have multiple sites of involvement, and
to be systemically ill. More than 40% of affected infants have more
than one site of involvement (176,177).
of life (sometimes as late as 8 weeks), in infants who are not
systemically ill and are developing and feeding normally. These
infections are more likely to be due to group B Streptococcus
and involve a single site. Infants delivered by vaginal delivery are
exposed to potential pathogenic bacteria during delivery. Before
vaginal delivery, women undergo culture of the birth canal for group B Streptococcus.
If positive, women are treated prophylactically with antibiotic
coverage at the time of delivery to prevent transmission of group B Streptococcus infection to the newborn. Should transmission of group B Streptococcus to the newborn occur, the newborn is at risk of developing osteomyelitis or septic arthritis.
ossification appear, metaphyseal vessels penetrate directly into the
chondroepiphysis. Osteomyelitis originating in the metaphysis can
spread into the epiphysis and joint, with a reported association as
high as 76% (176,177).
The transphyseal vessels persist until 6 to 18 months of age, when
secondary ossification centers begin to form and the physis becomes a
mechanical barrier to infection. Permanent growth arrest, physeal
injury, and joint destruction can result. The proximal femur is the
most commonly involved site, but in up to 40% of neonates with AHO,
multiple sites may be involved (102). The lesson for the physician is that when a septic joint is diagnosed in the neonate, a thorough
search for osteomyelitis in an adjacent metaphysis or epiphysis is mandatory.
infection, so typical symptoms, signs, and laboratory indicators of
infection are often absent, and multiple sites of infection are common.
Physical examination often demonstrates swelling and pain or
irritability with movement, but findings are often unremarkable,
compared to what they would be in an older child. The leukocyte count
and differential leukocyte count are not reliably elevated. The blood
cultures are positive in approximately 50% of patients with proven
infection. In a study by Klein examining the sensitivity of objective
parameters in patients with septic arthritis of the hips, none of the
neonates in the study group were febrile or had an elevated leukocyte
count, but all did have an elevated ESR (55).
multiple sites of infection, but false-negative studies sometimes
occur, and bone scan may not reveal all infected sites. Reports vary as
to the sensitivity of technetium-99 scanning in neonates. In one report
on the value of bone scintigraphy in detection of neonatal
osteomyelitis, the sensitivity for diagnosing focal disease by clinical
findings was 20%, radiography 65%, and bone scintigraphy 90% (179), but other investigators have found the bone scan to be less sensitive. Ash and Gilday (180)
found that only 32% of proven sites of osteomyelitis in 10 neonates
were positive on bone scan. Higher-resolution scintigraphy equipment
and magnification views of all suspected areas appear to provide
improved results (181).
sepsis should be performed, and fluid aspirate sent for analysis
including Gram stain and culture. One can make a strong case for
aspirating both hips in any neonate with known osteomyelitis or septic
arthritis because
-
Multiple sites of involvement are common.
-
The proximal femur and hip joint are frequently involved.
-
Symptoms and signs are often subtle or lacking.
-
The hip is the most difficult joint to examine.
-
The window of opportunity for effective treatment is small.
-
The hip joint is the most frequent site of permanent sequelae.
and should be managed in conjunction with a neonatologist or pediatric
infectious disease consultant whenever possible. Most importantly,
initial antibiotic selection must cover bacteria unique to neonatal
musculoskeletal infection (Table 13.2). In this
age group, choices may include oxacillin along with gentamicin or a
third-generation cephalosporin such as cefotaxime (Claforan).
Ceftriaxone (Rocephin) is also a good choice in a child without
jaundice. Because neonates are more prone to generalized sepsis, have
less consistent oral antibiotic absorption, and have less predictable
radiographic and serologic response to treatment, it has been generally
recommended that the entire course of treatment be intravenous (15,80).
abscess is appropriate. To reduce the risk of permanent growth
alteration or joint abnormality, cautious debridement of the
chondroepiphysis is indicated.
In the newborn, the disease is contracted from the mother during
passage through the birth canal and results most commonly in
conjunctivitis and scalp abscesses (182,183).
Gonococcal infection is also frequently seen in the adolescent age
group. Although gonococcal infection can take many forms, the
orthopaedist is most likely to encounter this infection as septic
arthritis following dissemination of genitourinary disease. The delay
between the genitourinary infection and the arthritis is variable,
ranging from a few days to several weeks (184).
before puberty, and in sexually inactive adolescents, sexual abuse
should be suspected. Sexual abuse may occur in as many as 10% of all
abuse cases, and it is estimated that between 5% and 20% of sexually
abused children have a sexually transmitted disease, most commonly
gonococcal infection (185,186).
Children who are identified with or suspected of having a gonococcal
infection should have cultures of all mucous membranes, including
pharynx, vagina, and rectum, before the administration of antibiotics.
These cultures should be handled in a manner that permits them to be
used as evidence in court. In addition, reporting of suspected cases is
mandated by the Child Abuse Reporting Law. For all of these reasons,
the orthopaedist should involve a knowledgeable pediatrician in the
evaluation of these patients.
a small erythematous macule present on the genitalia. This may
disappear or develop a small vesicle, followed by a necrotic center
that may form a pustule. Approximately one third of patients develop a
distinctive rash that is the result of gonococcal septicemia.
Additional associated systemic symptoms include fever, tenosynovitis,
and migratory polyarthralgia (187).
knee is most often affected, but it is important to remember that any
joint, large or small, can be involved. The size of the effusion may
vary widely and may even be absent. The involved joints are usually
painful. The nature of the arthritis does not appear to have changed
over the past several decades, although treatment with antibiotics has
resulted in the virtual elimination of joint destruction (188, 189, 190, 191, 192). Osteomyelitis still may be seen as an occasional complication (193).
Culture and Gram staining of joint fluid, and of the cervix of
postpubertal girls and the vagina of prepubertal girls, should be
performed. Any urethral or prostatic discharge in boys should also be
cultured and examined by Gram staining. Blood cultures should be
routine. The organism may occasionally be isolated from skin lesions,
but Gram staining gives a higher yield.
difficult organism to grow, and special care is needed in the handling
of the material for culture. Because the organism is sensitive to cold,
material for culture should be plated directly onto a warm medium
whenever possible (194). Special culture tubes
for transport of gonococcal cultures are available and should be used;
specimens should be delivered promptly to the bacteriology laboratory
when direct plating is not feasible. Cultures from sterile sites (e.g.,
blood, synovial fluid) are plated on chocolate blood agar. Cultures
from nonsterile sites (e.g., the vagina, skin lesions) should be plated
on selective media (e.g., Thayer-Martin agar) that contains antibiotics
to inhibit the growth of other organisms. Cultures are grown in a 5% to
10% CO2 atmosphere.
to penicillin and tetracycline makes parenteral administration of a
third-generation cephalosporin (e.g., ceftriaxone, 50 mg/kg/day
intramuscularly or intravenously, once daily) the initial drug of
choice (194) (Table 13.2).
If the organism is demonstrated to be sensitive to it, penicillin can
be used. Involvement of the hip joint requires surgical drainage.
Recommendations for drainage of other joints remain variable. In other
large joints with large amounts of purulent fluid, surgical drainage
may be preferable to repeated needle aspiration. If surgical drainage
is used, it is wise to leave a closed suction drain in the joint,
because the tendency to reaccumulate fluid in gonococcal infection is
greater than with other forms of septic arthritis.
Fortunately, there is no information to date suggesting that the
presenting signs or symptoms or recovery from infection are affected by
HIV coinfection; however, broad-spectrum antibiotic coverage is
recommended due to the wide range of causative organisms that have been
reported in children with HIV infection (6).
HIV infection can have several musculoskeletal manifestations. Patients
may experience rheumatologic manifestations of HIV as well as
susceptibility to atypical musculoskeletal infection (195).
Rheumatologic manifestations including Raynaud phenomenon, vasculitis,
and arthralgia were identified by Martinez-Rojano in 5 of 26
HIV-infected children (195). All rheumatologic changes were seen in advanced stages of HIV disease.
The organism is spread to humans through the bite of the deer tick,
endemic to northern states in the upper Midwest and New England. The
hallmark clinical feature of Lyme disease is the appearance of single
or multiple expanding skin lesions, erythema migrans, which expand to
at least 5 cm and may have partial central clearing. The most common
orthopaedic manifestation caused by Borrelia burgdorferi
is an intermittent reactive arthritis that does not cause the joint
destruction seen in bacterial septic arthritis but causes recurrent
intermittent swelling, stiffness, and pain. Arthralgia can occur in the
acute or late phases of Lyme disease and typically affects the knee (196).
The clinical picture is often more similar to JRA than to bacterial
septic arthritis, with swelling and stiffness and less severe pain.
most often made by positive blood serology in a patient with a history
of possible exposure and in whom other causes have been excluded.
Almost all untreated patients have high levels of serum immunoglobulin
G antibodies and sometimes low levels of immunoglogulin M antibodies to
Borrelia burgdorferi. At many centers,
screening is done by enzyme-linked immunosorbent assay (ELISA) and
confirmed by Western blot. Serologic immunoglobins may persist after
effective antibiotic treatment and do not accurately distinguish
between active or past infection. Joint aspirate fluid can also be
tested for Lyme disease but is not necessary to make the diagnosis.
Treatment in children is simply amoxicillin 25 to 50 mg/kg/day divided
b.i.d. for 3 weeks and is very effective at completely eliminating the
organism and all musculoskeletal symptoms (197). A small percentage of patients have persistent knee synovitis that may be caused by intrasynovial autoimmunity (196).
that mimics septic arthritis. Willis et al. reported a series of ten
cases in which Lyme arthritis presented as acute painful arthritis (198).
None of these ten patients had a known history of a tick bite or had
evidence of erythema migrans. Five patients were febrile at
presentation, and all patients had limited joint motion with pain at
motion endpoints. All the patients were able to ambulate independently
at home, although two patients refused to bear weight at initial
presentation to the orthopaedic surgeon. One third of patients had an
elevated serum WBC, but only one had a significant left shift. The ESR
was elevated beyond 40 mm per hour in 60% of the cases and beyond
normal (20 mm per hour) in 90% of cases. The CRP was elevated in nine
of ten patients.
cell count and differential as well as culture. The joint WBC count in
this series of ten patients with Lyme arthritis averaged 82,900 cells
per mm3 (range 40,900 to 140,500), and
60% of the aspirates had more than 80,000 cells per mm3.
In all patients, the percentage of polymorphonuclear cells was more
than 80%. All patients had positive screening and confirmatory
serologic testing for Borrelia burgdorferi.
Based on initial history, examination, and laboratory and joint
aspirate data, seven patients underwent surgical joint irrigation. The
factors that best differentiated cases of Lyme arthritis from septic
arthritis in this series were the ability to ambulate and normal serum
polymorphonuclear cell count. Rapid 1-hour Lyme immunoassays are now
becoming available, which will greatly facilitate prompt diagnosis.
endemic, children who present with monoarticular arthritis should have
an urgent evaluation for possible septic arthritis with the addition of
Lyme serology. If the patient is ambulatory and has a normal serum WBC
and differential, and the rapid Lyme enzyme immunoassay (EIA) results
are available and positive, the physician may choose to initiate
Lyme-specific antibiotic therapy while awaiting final joint aspiration
cultures and confirmatory Lyme Western blot serologic testing. If the
patient has rapid clinical improvement and the joint cultures remain
negative, surgical irrigation and debridement of the joint may be
avoided. However, if rapid Lyme EIA results are not available or if
there remains significant clinical suspicion regarding the diagnosis of
septic arthritis, we recommend proceeding with immediate irrigation and
debridement of the affected joint, and later altering the treatment
regimen appropriately based on the final results of preoperatively
obtained bacterial cultures and two-stage Lyme serology.
musculoskeletal infection in children. Between 1985 and 1995, 84
children were hospitalized at Texas Southwestern Medical Center for
bacterial complications of primary varicella (199). Skin infection was the most common type of infection, and group A β-hemolytic Streptococcus was the predominant isolate. Schreck et al. examined musculoskeletal infection associated with varicella (200).
Twenty-seven (6%) of 417 admissions for varicella at San Diego
Children’s Hospital were for musculoskeletal complications requiring
operative treatment. There were seven admissions for osteomyelitis,
four for septic arthritis, five for necrotizing fasciitis, ten for a
deep-tissue abscess, and one for toxic shock syndrome leading to
multiple limb amputations. Bacterial pathogens were identified as the
cause of 25 of the 27 complications that led to operative treatment. Of
these 25, 21 (84%) were found, on culture, to be caused by group A
β-hemolytic Streptococcus. This pathogen was the cause of the infection in five of the seven patients who had osteomyelitis, whereas S. aureus was the cause in only one. Group A β-hemolytic Streptococcus
was also the causative organism in two of the four patients who had
septic arthritis, three of the five who had necrotizing fasciitis, and
all ten who had a deep-tissue abscess.
may have a selective advantage in patients who have varicella because
of its ability to gain access to deeper tissue through the varicella
vesicle itself. The vesicle is created by a split in the epidermis that
fills with a proteinaceous fluid. This may provide a route from the
surface of the skin to the subcutaneous tissues, with subsequent
bacteremia, or local spread, and musculoskeletal infection. Trauma to
the skin from scratching may contribute to bacterial contamination of
the varicella vesicle. Group A β-hemolytic Streptococcus
possesses tissue-dissolving enzymes such as hyaluronidase and
streptolysin, which facilitate penetration of the tissue. In addition
to local factors, varicella appears to cause a transient systemic
suppression of immunity, making patients susceptible to infection (201).
varicella may lead to a decrease in musculoskeletal infection
associated with varicella, but no studies have been published to
confirm this hypothesis. Physicians should have a high level of
suspicion of musculoskeletal infection when examining children with
varicella who have localized warmth and erythema, swelling, or pain or
who refuse to bear weight. Prompt evaluation, appropriate operative
intervention, and adjunctive systemic antibiotic therapy may prevent
the spread of infection and the loss of life or limb. Because of
transient immune suppression and susceptibility to infection, it is
prudent to avoid elective surgery within 1 month of varicella infection
or definite exposure.
Patients with sickle cell disease have significant impairment in
splenic function and are also at increased risk for infection caused by
encapsulated organisms such as H. influenzae and S. pneumoniae. Musculoskeletal infection caused by such encapsulated organisms, however, is not as prevalent as Salmonella infection and typically occurs in children younger than 3 years compared with infection caused by Salmonella, which has a peak onset between 5 and 10 years of age (201,204,206).
In addition to osteomyelitis, patients with sickle cell anemia are also
at increased risk of developing septic arthritis, but the association
with Salmonella as a causative organism has not been as strong.
function and decreased specific antibiotic production may contribute to
susceptibility. Preceding episodes of bone avascular necrosis have been
found to be more frequent in patients with osteomyelitis, suggesting
that ischemic bone may provide a favorable environment for localized
infection (207). Despite these clues, there is not a clear understanding why sickle cell disease causes susceptibility to Salmonella infection.
cell anemia in the United States is low, despite the attention it
receives in the literature. In 1971, Specht (208)
found only 82 cases in the literature, and the relatively few cases
reported over several years in other large centers attests to the
infrequent occurrence (209,210).
This low incidence is important to the orthopaedist when considered
relative to the number of admissions for sickle cell crisis (210).
It is important to remember that patients presenting with sickle cell
ischemic crisis outnumber those with osteomyelitis associated with
sickle cell disease 50:1.
diagnostic and treatment dilemma. Sickle cell disease causes patients
to experience vasoocclusive crises and predisposes patients to
musculoskeletal infection. Osteomyelitis almost certainly begins in an
area of bone infarction. Bone infarction and bone infection may have
identical clinical presentations but are treated with very different
therapy; vasoocclusive crisis is treated with hydration and analgesia,
whereas musculoskeletal infection requires antibiotics and often
emergent surgical debridement.
reliable means of differentiating between these two events. Clinical
examination provides little help in differentiating between infarction
and infection, because both patient groups tend to present with focal
tenderness to palpation, swelling, and severe limp. Fever, elevated
ESR, and elevated CRP are also commonly found with both conditions.
pathophysiology may be the most powerful tool in helping to
differentiate between infarction and infection. Clinically, the
exquisite pain of ischemia associated with fever, swelling, and limp
may show signs of improvement within 48 hours of initiating hydration
therapy and appropriate analgesia, whereas clinical symptoms may
continue to worsen, and ESR and CRP continue to rise, if the patient is
experiencing musculoskeletal infection.
ischemic phase of a sickle cell crisis, whereas osteomyelitis is much
more likely to immediately show increased uptake in the early stages of
infection. As the sickle cell crisis evolves and local inflammation
surrounds the area of infarction, bone scanning will show increased
uptake within 36 to 48 hours, making serial scanning more helpful than
a single isolated scan.
infarct and osteomyelitis but is not a perfect diagnostic test. Marrow
infarction and soft-tissue changes seen with sickle cell infarction are
not reliably differentiated from sepsis on MRI (90,211).
Still, as our knowledge and interpretation skills improve, MRI has the
greatest promise to allow early identification of osteomyelitis in
patients with sickle cell disease.
Ultrasonographic scans in patients with vasoocclusive disease were
totally normal, whereas those with osteomyelitis showed a variety of
changes such as periosteal elevation, subperiosteal or intramedullary
abscess, and cortical erosions. However, these are relatively late
changes caused by osteomyelitis, and ultrasound may be less helpful in
the early stages of infection. Ultrasono-graphy is attractive because
of its availability, relatively low cost, safety, and the short time
needed to complete the evaluation.
The condition occurs in infants and young children, usually those
younger than 4 years. No case of a child older than 7 years has been
reported. It may precede the diagnosis of sickle cell disease. The
actual incidence is probably between 10% and 20% of children with
sickle cell disease, and it seems to be more common in Africa. Patients
present with acute, painful swelling of the hands and feet. Although
sickle cell dactylitis is considered to be a benign condition requiring
no further evaluation (213), Salmonella osteomyelitis has been associated with this condition (214,215).
Laboratory tests do not help in the differential diagnosis.
Radiographic findings in the hand-foot syndrome at first demonstrate
only soft-tissue swelling, but after 7 to 14 days, periosteal new bone
formation is visible, followed by medullary resorption, coarsening of
trabeculae, and cortical thinning. The changes revert to normal in
weeks to months. Chronic radiographic changes associated with sickle
cell disease include biconcave deformity of the vertebral end plates
and avascular necrosis of the femoral and humeral heads.
differentiate between bone infarction and osteomyelitis, the ability to
isolate a causative organism and confirm the diagnosis of infection
becomes of great importance. Blood cultures should always be obtained,
and aspiration of bone should be performed whenever infection is
suspected. Because sickle cell osteomyelitis has a predilection for
diaphyseal location, it requires aspiration through cortical bone,
which is a difficult procedure best performed in the operating room
under sterile conditions.
specific indications (218,219).
A close look at the outcomes and complications of this disease lead the
modern orthopaedist to conclude that the most predictable treatment
with highest likelihood of rapid and complete eradication of infection
and the lowest chance of late sequelae utilizes surgical drainage in
addition to antibiotic therapy. Early diagnosis and prompt drainage of
an abscess, especially in an area of infarction, may result in outcomes
comparable with normal children having the usual course of pyogenic
osteomyelitis.
sickle cell disease who are undergoing extremity surgery is frequently
raised because of the possibility that the ischemia may provoke
thrombosis. This does not seem to be a problem; when the patient is
properly prepared for surgery, no complications from the use of a
tourniquet should result (220,221).
Appropriate precautions to reduce the incidence of vasoocclusive events
include adequate preoperative hydration, avoidance of intraoperative
hypothermia, and maintenance of adequate blood volume and oxygenation.
Recent evidence suggests that preoperative transfusion to achieve a
hemoglobin of at least 10 g per dL is recommended and is as effective
at preventing vasoocclusive events as more aggressive exchange
transfusion protocols that attempt to lower the sickle cell hemoglobin
to a specific percentage (222).
but this question has little clinical significance because both
organisms must be covered by antibiotic therapy. A recent article
reviewing the world literature since 1959 found Salmonella to be the most common (223). Initial antibiotic choices are cefotaxime (Claforan) or ceftriaxone (Rocephin), each of which covers both S. aureus and Salmonella species, including those Salmonella resistant to ampicillin, chloramphenicol, or trimethoprim-sulfamethoxazole (Bactrim) (Table 13.2).
Initial antibiotics are administered intravenously, and conversion to
oral antibiotic is made after clinical and laboratory signs of
effective treatment response for a total duration of therapy of
approximately 4 to 6 weeks.
The most common is an aseptic arthritis, most likely due to the sickle
cell disease. It may be seen during crisis but is more often a
transient synovitis, usually involving the knee, which resolves within
5 days (225,226). A second form of aseptic arthritis is that associated with a remote Salmonella
infection. This may be seen with other organisms, and the exact
mechanism is not clear. Finally, the patient with sickle cell disease
may have a septic arthritis. When this is the case, Salmonella is not the most likely organism. Salmonella is a rare organism in septic arthritis (226); when it occurs, it is most often in patients without sickle cell disease. When Salmonella
septic arthritis occurs in patients with sickle cell anemia, it is most
often from contiguous spread of osteomyelitis. More likely organisms in
septic arthritis are Staphylococcus species (216,227). As with osteomyelitis, there is a difference of opinion on the advisability of arthrotomy for drainage (216,227).
should include CBC with differential, ESR, CRP, blood cultures, plain
film radiographs, technetium bone scan, and MRI. Admission to the
hospital is recommended with initiation of hydration therapy,
appropriate analgesia, and transfusion if necessary to achieve a
hemoglobin of 10 g per dL. If the patient’s symptoms improve
significantly with initial therapy, management of the ischemic episode
is continued. If symptoms are unchanged or worsen, laboratory studies,
technetium bone scan, and MRI are repeated approximately 48 hours after
admission. If laboratory values or imaging studies are equivocal or
suggest osteomyelitis, bone aspiration for culture and Gram stain is
performed. Bone aspiration is performed in the operating room because
it is very difficult to aspirate through diaphyseal bone where
osteomyelitis associated with sickle cell disease is most likely to
reside. Empiric antibiotic therapy covering Salmonella and S. aureus
is initiated and continued until culture results are available. If
subperiosteal or bone aspirate is encountered, surgical debridement is
performed and closed over drains.
resurgence of musculoskeletal infection caused by tuberculosis in
developed countries as well (228). These data,
which include patients of all ages, found 1985 to be the year with the
lowest number of reported tuberculosis cases since the reporting began
in 1953. However, since 1985 the incidence of tuberculosis has risen
sharply. The largest increase has been reported for patients born
outside of the United States and its territories. In 1993, these
patients comprised almost 30% of the reported cases. California, New
York, and Texas saw the largest increases. The increased incidence has
been accompanied by HIV infection and multidrug-resistant organisms.
children, particularly those younger than 5 years, the orthopaedic
surgeon must again become aware of this possibility when evaluating
chronic joint inflammation or chronic bone lesions. Patients who are
exposed to tuberculosis may or may not become infected, and those who
are
infected
may or may not become diseased. There is a time lag between infection
and diagnosis of the extrapulmonary disease of approximately 1 year.
In developing countries, bovine tuberculosis may occur following the
ingestion of unpasteurized milk. The tubercle bacilli may disseminate
to bones or joints during the lymphatic and hematogenous spread of the
initial infection. If the initial lung infection remains untreated,
involvement of the bones and/or joints occurs in 5% to 10% of children (228,229).
The development of the lesions in bone is time- and location-related.
Dactylitis may occur within a few months in younger children. Long-bone
involvement may occur in 1 to 3 years.
experiencing tuberculous osteomyelitis have a less acute onset with
less severe symptoms, and delayed diagnosis is common. Patients with
tuberculosis osteomyelitis are typically afebrile, have less pain, and
may have normal laboratory values. Patients often experience local
swelling, and initial radiographs may be normal (230).
Osteolytic lesions develop with the focus of osteomyelitis usually in
the metaphysis, occasionally in the epiphysis, and rarely in the
diaphysis of long bones. Involvement of virtually any bone can occur (173,231).
bone destruction in a centrifugal fashion, producing a characteristic
round lytic lesion with ill-defined margins. These lesions are filled
with an inflammatory granulation tissue, creating a reactive hyperemia,
which produces a wide area of osteopenia surrounding the lesion. This
process is almost purely destructive or lytic, with little or no bone
reaction, no sclerotic margins, and no periosteal response. Because of
the chronicity and hyperemia, widening and accelerated growth of the
epiphysis may be seen. The physeal plate offers little resistance to
the spread of the infection as it does in other pyogenic infections.
usually in the anterior third of a vertebral body in the lower thoracic
or upper lumbar spine. The first lumbar vertebra is most commonly
involved, whereas T10 infection is most commonly associated with
neurologic deficit (233). Paravertebral abscess
formation is characteristic, and calcification developing within the
abscess is almost diagnostic of a tuberculous abscess. The discs become
involved when two adjacent vertebral bodies are affected. The bone
lesions in the vertebral bodies are mainly destructive. This frequently
leads to kyphotic deformity, which becomes rigid when chronic. Patients
with significant kyphosis often present with neurologic deficit (234).
Isolated joint infections, unusual in childhood, are initially
characterized by effusion in addition to synovial proliferation and
thickening. In the early stages, there are no radiographic
characteristics that
separate
tuberculous arthritis from any chronic inflammation of the joint. As
with the bone lesions, the hyperemia causes widespread osteopenia and
may cause overgrowth of the epiphyses. The infection proceeds both by
pannus formation over the articular cartilage and by erosion of the
subchondral bone, beginning at the synovial margins (236,237).
The result is joint space narrowing and subchondral cystic erosion. MRI
may demonstrate central and peripheral erosions, active and chronic
pannus, abscess, bone chips, and synovial changes (238).
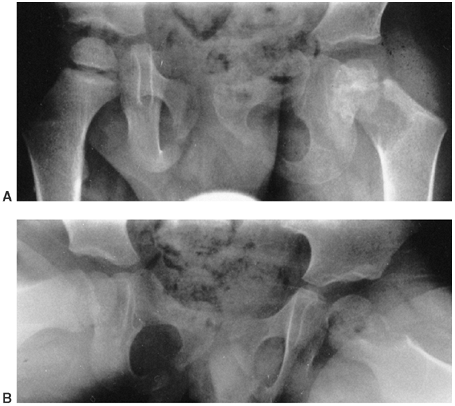 |
|
Figure 13.10 A and B:
Radiographs of a 3-year-old boy who recently moved to the United States from Mexico. The child had complaints of increasing limp on the left, pain that worsened at night, but no significant limitation of activity. Examination demonstrated limited motion with irritability. Laboratory studies showed a normal complete blood count, erythrocyte sedimentation rate of 25 mm per hour, and a positive purified protein derivative test. Open biopsy confirmed the diagnosis of tuberculosis by histology and culture. (Courtesy of Hugh Watts, MD) |
caseous material and pus accumulate and dissect along normal tissue
planes. Eventually, a sinus track to the surface is formed—a hallmark
of a long-standing neglected case. The abscess formed by tuberculous
infection is called a cold abscess because of the lack of any signs of acute inflammation.
tuberculous dactylitis, may resemble sickle cell dactylitis, with
swelling of the phalanges, metacarpals, and metatarsals. Tuberculous
dactylitis is usually not very painful, however, and onset is usually
consecutive rather than simultaneous. Before the availability of
radiographs, this was called spina (Latin for “a short bone”) ventosa (meaning “inflated with air”). The radiographs show a cystlike expansion of the tubular bones, with thinning of the cortex (239).
A second presentation is with multifocal cystic involvement of the
bone. This is characterized by areas of simultaneous destruction in the
shafts of long bones and in flat bones, with a strong tendency to
symmetry (240).
tuberculous infection of the bone or joint is to consider it as a
possibility. In addition, when tuberculosis is diagnosed, underlying
HIV infection must also be considered. Tuberculosis should be
considered whenever a chronic-appearing bone lesion is encountered.
Early diagnosis is important to prevent spread to a contiguous joint.
The clinical picture is variable, depending on the location and the
stage of the disease. It is characterized by its insidious onset; lack
of characteristic inflammatory features, such as erythema; and bone
destruction or joint involvement greater than the symptoms would
suggest.
The purified protein derivative skin test usually is positive.
Radiographic changes are usually present at the time of presentation.
The diagnosis depends on the identification of the organism M. tuberculosis.
Positive cultures are obtained in 85.5% of patients who have both
pulmonary and extrapulmonary disease, in 83.5% of those with only
pulmonary disease, and in 76.5% of those who have only extrapulmonary
disease (241). M. tuberculosis is one organism that can be reliably diagnosed by PCR (242, 243, 244), and tissue should be sent for PCR testing whenever possible.
inflammation, which may lead the surgeon to obtain biopsy material that
does not contain mycobacteria and results in negative cultures. In
tuberculosis arthritis without bone involvement, the biopsy should be
taken from the peripheral junction of the synovium with the bone, or
preferably from the junction of the synovium with a cyst (245). In cases with bone lesions, the granulation tissue filling the destructive bone lesion is the best material for biopsy.
Surgical debridement of the bone lesions is not necessary for a cure,
although drainage of large abscesses often improves the patient’s
overall constitutional symptoms (240,245,246).
In addition, open surgical biopsy is often necessary. Surgical
treatment of the knee for early disease has been reported to achieve
favorable results (247), whereas later stage
disease with joint space narrowing at presentation did not benefit from
surgical treatment. Because of the effectiveness of drug therapy, there
is little chance that surgical biopsy will lead to sinus formation. It
is important to always be aware that superinfection with pyogenic
organisms can occur, and this may be a reason for apparent treatment
failure with antitubercular drugs. This is particularly true when a
sinus has formed (246).
Although patients with neurologic involvement can recover with medical
management, they seem to do so faster with surgical management (248).
Surgical treatment of the kyphosis produces a higher rate of union and
less deformity than regimens without surgical stabilization (249,250).
Therefore, it appears that with contemporary surgical and anesthetic
techniques, tuberculous kyphosis is best treated early with anterior
surgery for debridement and strut grafting if indicated, as well as
posterior instrumentation. The treatment of spinal instability,
especially that spanning more than two disc spaces, is difficult and
requires both anterior arthrodesis with strut grafting and posterior
arthrodesis with instrumentation (251).
Surgical treatment must be accompanied by antituberculous treatment for
at least a year, as shorter treatment duration has been associated with
recurrent disease (234).
controversial and has shown inconsistent benefit over antituberculous
treatment alone. Many cases do well with medical management only (248, 249, 250).
there is evidence of an increasing incidence of resistant strains, due
most likely to inadequate treatment of the initial infection (241).
This emphasizes both the need for constant surveillance for drug
resistance and the importance of careful supervision of outpatient oral
therapy to be certain that compliance is optimal. Antimicrobial therapy
should be of at least 9 months’ duration, longer in children and
immunocompromised hosts.
likelihood of drug-resistant organisms, whereas long-term selection
should be guided by susceptibility testing. In those who are not at
high risk for drug-resistant organisms, various regimens of isoniazid,
rifampin, and pyrazinamide are recommended (252).
In children who come from areas where antibiotics are sold over the
counter, where high rates of drug-resistant tuberculosis occur, and
when incomplete treatment may have resulted in multidrug-resistant
strains, ethambutol or streptomycin should be added to the standard
three-drug regimen. Treatment of bone and joint tuberculosis in
children should be continued for 1 year.
onset of pain, absence of systemic signs, and radiographic presence of
a bone lesion at the time of presentation with no previous acute attack
to suggest evolution of an acute osteomyelitis to a chronic form (253).
Subacute osteomyelitis is becoming an increasingly prevalent form of
musculoskeletal infection and often results in a diagnostic and
treatment dilemma (10). Because symptoms are often insidious in onset and less severe than in acute osteomyelitis, diagnosis is often delayed (254).
presumed to result from an increased host resistance, decreased
virulence of the causative organism, and possible antibiotic exposure,
causing alteration in the host-pathogen relationship. As a result,
children typically present without the typical features of
osteomyelitis, having only a mild limp or intermittent pain of at least
1 to 2 weeks duration. Systemic symptoms such as fever, malaise, and
anorexia are absent. Supportive laboratory data are often inconsistent.
The leukocyte count is usually normal or only slightly elevated. The
ESR is usually elevated, although usually not as high as in AHO, and
CRP is often normal (255). Blood cultures are usually negative (256), although curettings from the lesions are frequently culture positive, usually for S. aureus. Histology is compatible with acute and chronic inflammation.
extremity long bones, but the upper extremity, axial skeleton, hand, or
foot may be involved (174, 256, 257, 258).
Technetium 99 bone scintigraphy is very sensitive for subacute
osteomyelitis and can be helpful when the location of a suspected
lesion is unclear.
patients, but the lesions often appear similar to neoplasm or other
diagnoses. The radiographic classification system initially proposed by
Gledhill (259) and modified by Roberts et al. (257) (Fig. 13.11)
facilitates establishing a diagnosis. The most common type of subacute
osteomyelitis in the pediatric age group is the metaphyseal lesion
(types IA and IB) (260). This represents a true
Brodie abscess, a localized abscess of bone without previous acute
illness. The lesion is located eccentrically in the metaphysis, with
frequent visible extension into the epiphysis. The second most common
type is the epiphyseal lesion (type V) (261, 262, 263).
The radiographic appearance is similar to the lesion in the metaphysis,
and it also may extend across the physis into the metaphysis. Despite
crossing the physis, subacute osteomyelitis rarely causes permanent
growth alteration (255).
dependent upon the radiographic appearance and subtype of the lesion.
The differential diagnoses for type I lesions include eosinophilic
granuloma and, rarely, giant cell tumor. Type II metaphyseal lesions
can mimic osteosarcoma, if aggressive, or metastatic neuroblastoma.
Type III lesions cause cortical reaction and thickening and can be
mistaken for an osteoid osteoma. The periosteal reaction seen in type
IV lesions is a finding also seen with Ewing sarcoma. The differential
diagnoses for type V lesions in the epiphysis include chondroblastoma,
osteoid osteoma, eosinophilic granuloma, or enchondroma.
lesion. CT has the advantage of being relatively inexpensive and more
readily available, whereas MRI provides greater information about soft
tissues, bone, and marrow edema. A characteristic “penumbra sign,”
which is reportedly helpful in differentiating subacute osteomyelitis
from neoplasm, has been described on T1-weighted MR images (264).
lesions into two categories: aggressive lesions and more
benign-appearing cavities in the region of the metaphysis and
epiphysis. All of the lesions in the aggressive group that were in the
diaphysis or metaphysis demonstrated onionskin periosteal new bone. The
other lesions were all in the metaphysis or epiphysis and had the
typical radiologic features of type I and V lesions described in the
preceding text. Benign-appearing epiphyseal and metaphyseal cavities
were treated with 48 hours of intravenous semisynthetic penicillin or
first-generation cephalosporin followed by 6 weeks of oral antibiotic.
Eighty-seven percent of the children treated with antibiotics alone
healed their lesion. Antibiotic treatment failure was associated with
increased patient age. Hamdy reported similar successful results with
antibiotic treatment alone for benign-appearing lesions (256). Aggressive lesions, where the diagnosis cannot be conclusively determined, should be
biopsied and treated with curettage if osteomyelitis is confirmed (266).
 |
|
Figure 13.11 The variety of presentations of subacute hematogenous osteomyelitis in the classification of Roberts et al. A: Type IA is a punched-out metaphyseal lesion resembling an eosinophilic granuloma. B: Type IB is similar to type IA, but has a sclerotic cortex. C: Type II lesions erode the metaphyseal bone, often including the cortex, and appear as aggressive lesions. D: Type III lesions are localized cortical and periosteal reactions, simulating osteoid osteoma. E: Type IV lesions produce onionskinlike periosteal reactions in the diaphysis and resemble Ewing sarcoma. F: Type V lesions are epiphyseal erosions. G:
Type VI lesions involve the vertebral bodies. (From Roberts JM, Drummond DS, Breed AL, et al. Subacute hematogenous osteomyelitis in children: a retrospective study. J Pediatr Orthop 1982;2:249–254.) |
different from acute or chronic osteomyelitis in that it responds
predictably to antibiotic therapy, and surgical debridement is often
unnecessary (10,174,255,256,265). Recommendations for route and duration of antibiotic treatment may vary, but the choice of antibiotic should cover S. aureus
and typically consists of 2 to 7 days of parenteral antibiotics
followed by oral antibiotics for a total treatment duration of 4 to 6
weeks. Surgery should be reserved for aggressive lesions and cases that
do not respond to antibiotic therapy.
the developing world, accounting for up to 3% to 5% of hospital
admissions (10,267). Because pyomyositis is
being reported with increased frequency in developed countries and
commonly affects the musculature about the hip, where it may confound
the diagnosis of septic arthritis, all orthopaedists should be aware of
the condition (268, 269, 270, 271, 272).
bacterial infection, pyogenic muscle abscesses are infrequent. The
development of pyomyositis, in the absence of penetrating trauma,
presumably requires the coexistence of bacteremia and alterations in
the microenvironment that facilitate the sequestration and
proliferation of organisms. Once bacterial proliferation begins,
pyomyositis may progress through three stages during which clinical
findings parallel the progression from diffuse inflammation to focal
suppuration. The initial, invasive stage involves the insidious onset
of dull, cramping pain, with or without low-grade fevers, that
progresses over 10 to 21 days. An increase in the magnitude of
symptoms, associated with systemic signs, heralds the suppurative phase
of the disease. Most patients present during this stage, and physical
findings are more focal. The late stage includes fluctuance and more
profound systemic manifestations that require urgent treatment (273).
involvement of muscles in the thigh and hip regions, other sites of
involvement in children have included the chest wall, psoas, glutei,
adductors, obturator internus and externus, quadriceps, hamstrings,
gastrocsoleus, paraspinals, infraspinatus, subscapularis, biceps,
triceps, and forearm muscles.
typically include neoplasm, osteomyelitis, hematoma, deep muscle
contusion, and others. The diagnosis is often delayed, and treatment
directed at other spurious problems is common (267).
Sedimentation rate is almost always elevated, and WBC count often is
abnormal. Clinicians may have a strong sense of the presence of an
infection but have difficulty localizing the source. Blood cultures are
occasionally positive, and wound cultures frequently grow a causative
organism, most often S. aureus (274,275). Streptococcus pyomyositis has been associated with varicella infection in children (276).
and provide guidance for placement of a drainage catheter. MRI provides
excellent soft-tissue detail and can identify abscesses and coexisting
regional pathology such as septic arthritis and osteomyelitis and
therefore is the imaging modality most ideally suited for evaluation of
myositis (275,277). MRI
with gadolinium enhancement may be able to differentiate between the
invasive and purulent stages of the disease. This distinction is
important because nonsuppurative myositis can usually be treated with
antibiotic therapy alone, while abscess formation is typically an
indication for surgical or percutaneous drainage (267).
which it is diagnosed. Early stages of disease may be treated
successfully with antibiotics. Later stages in which abscesses have
formed require drainage. Although historically open surgical drainage
has been used, recent reports have suggested that percutaneous drainage
in conjunction with appropriate antibiotic therapy is efficacious (267). Empiric intravenous antibiotic therapy with good Staphylococcus
coverage is recommended. Conversion to oral antibiotic is appropriate
following clinical response to treatment. Because of the excellent
healing potential of skeletal muscle, antibiotic therapy should not
need to be continued for as long as it would be for osteomyelitis.
Duration of antibiotics should range from 2 to 4 weeks. With
appropriate drainage and antibiotic treatment, persistent or recurrent
infection is uncommon.
or on the surface of the iliopsoas muscle. Psoas abscess may arise
primary or secondary to associated conditions such as appendicitis or
inflammatory bowel disease. Differentiation of a psoas abscess from
septic arthritis of the hip can be a diagnostic challenge made
difficult by the wide variability in clinical presentation of children
with psoas abscess and the uncommon occurrence of the condition.
clinical data at two major pediatric hospitals and identified 11
children with an average age at presentation of 8 years who were
treated for psoas abscess (278). All 11
patients had pseudoparalysis of the hip with apparent flexion
contracture and pain with active or passive motion in all planes.
Additional symptoms may include limp, groin pain
and swelling, back pain, abdominal pain, genitourinary pain, and thigh pain (279).
differentiating septic arthritis from psoas abscess. It is performed by
determining hip pain during ROM when the hip is in a flexed versus an
extended position. When the hip is flexed, tension on the psoas is
relaxed, and the patient with a psoas abscess may have minimal pain
with hip internal and external rotation, whereas the patient with
septic arthritis will have significant pain with the same motion.
Extending the hip places the psoas muscle and the hip capsule under
tension, resulting in severe pain with internal and external rotation
in both patient groups. This sign is useful when present but is not
very specific, with many patients ultimately diagnosed with psoas
abscess having significant hip irritability in all positions, including
flexion. Other patients may have no hip pain whatsoever. Atypical
features such as femoral nerve neuropraxia or bladder irritability
associated with hip pain are signs that may assist clinicians in
differentiating between septic arthritis of the hip and psoas abscess.
Plain film radiography is rarely useful in establishing a diagnosis;
CT, MRI, and ultrasound are most helpful. Frequently the hip is
aspirated, and occasionally debridement is performed before the
diagnosis of psoas abscess is made.
antibiotic treatment and, typically, drainage. The most common
infecting organism is S. aureus (279).
Traditionally drainage has been performed surgically, but more recently
CT or ultrasound guided percutaneous drainage has achieved equally
successful results (280). Duration of
antibiotic therapy has varied from 3 weeks to 6 months depending on
clinical response and normalization of sedimentation rate. Typically,
most psoas abscesses can successfully be eradicated with adequate
drainage and 3 to 6 weeks of appropriate antibiotic therapy.
inflammatory bone disease of unknown etiology characterized by an
unpredictable and prolonged course with exacerbations and spontaneous
remissions occurring over a period of at least 6 months. It is a
nonpyogenic inflammatory process with a lack of demonstrable causative
agent, occurring predominantly during childhood and adolescence (281).
At initial presentation, CRMO is often indistinguishable from bacterial
osteomyelitis. The most common presenting symptom is local bone pain at
one or more sites, often associated with fever. Girls are affected in
approximately 70% of the cases (282). WBC is
typically normal, but ESR and CRP are often elevated, and radiographs
frequently show a lytic destructive lesion in a long bone metaphysis (281).
developed. These lesions consist of poorly delimited eccentric
metaphyseal lucencies along the physeal border (Fig. 13.12). The lesions have been shown to cross into the epiphysis (193,283).
As healing occurs, sclerosis surrounds the lesion. When the lesion
extends into the cortex, periosteal reaction may occur. This is more
likely to be seen early in the course in the small tubular and flat
bones. This picture can be confused with bony neoplasm, such as
leukemia, Ewing sarcoma, or eosinophilic granuloma.
and proximal metaphyses of the tibia and femur, and there may be a
tendency for symmetric involvement. Other affected sites are the distal
radius and ulna, the distal fibula, and the metatarsals, as well as the
medial aspect of bones in the anterior chest wall. When the clavicle is
involved, it typically presents as a chronic sclerosing osteomyelitis
originating at the medial end and may demonstrate both lucencies and an
onionskin periosteal reaction (284) (Fig. 13.13). Multifocal involvement is present in over 90% of patients but is often not simultaneous (281).
Frequently, patients may experience a single symptomatic lesion but
have other asymptomatic lesions that are identifiable with bone scan,
making technetium 99 bone scintigraphy very helpful at establishing
multifocal involvement and the diagnosis of CRMO, sometime before
lesions are visible on plain film radiographs (Fig. 13.13).
CRMO is associated with a variety of other curious disorders of bone
and skin, including chronic sclerosing osteomyelitis of Garré,
hyperostosis of the clavicle (285), sternocostoclavicular hyperostosis (286), and palmoplantar pustulosis (287, 288, 289).
osteomyelitis at initial presentation, bone culture and biopsy are
often performed. Histopathologic features include chronic inflammation
with a variety of cell types, occasional necrotic bone fragments, and
fibrosis without the acute inflammation associated with bacterial
osteomyelitis. Infiltration with fibrovascular tissue and inflammatory
cells, followed by osteoblast proliferation and trabecular thickening,
have been noted in later stages of the disease (193,282,287).
The mainstay of CRMO treatment is scheduled nonsteroidal
antiinflammatory drug (NSAID) use during periods of exacerbations. The
time from onset of illness to remission of symptoms is 3 to 5 years, by
which time CRMO seems to burn itself out. No association has been
observed between the number of lesions and the response to treatment or
outcome. Long-term sequelae are rare, but premature
closure of a physis, bone deformity, kyphosis, chronic pain, and thoracic outlet syndrome have been reported (279, 291, 292, 293).
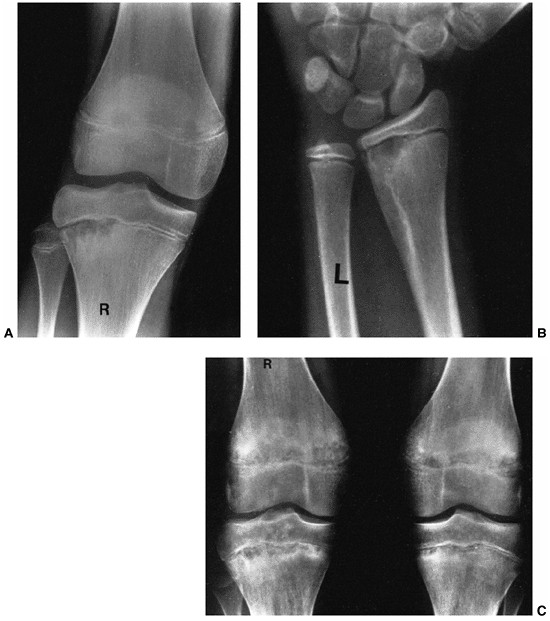 |
|
Figure 13.12 A:
A 12-year-old girl presented with a recurrent limp over a period of 18 months. She complained of pain in the right knee. Examination demonstrated tenderness about the right knee, but no other signs of inflammation. Radiograph of the right knee showed metaphyseal irregularity of the proximal tibia. B: Skeletal survey demonstrated additional similar lesions in the opposite knee, distal tibia, and radius. These lesions were asymptomatic. C: Radiographs 1 year later show diffuse metaphyseal changes of the distal femur and proximal tibia of both legs. No antibiotics were administered, and the symptoms resolved over the next several months. |
Like CRMO, SAPHO etiology has not been determined. There is speculation
of a genetic predisposition, with immunologic response to an infective
agent. Propionibacterium acnes, a skin
saprophyte, has been detected in the cutaneous lesions of severe acne
and in the articular and osseous lesions associated with pustulosis.
However, most biopsies of involved areas are negative, demonstrating
nonspecific inflammatory infiltrate. CRMO has also been associated with
psoriasis and inflammatory bowel disease, lending further
circumstantial evidence for an autoimmune-mediated cause (295,296).
disease, SAPHO clinical course is similar to CRMO, characterized by
recurrences and remissions; it is benign and self-limiting, with NSAID
treatment usually effective at controlling symptoms. Chronic bone
changes may persist, with the initial inflammatory changes being
replaced by Paget-like features, including hypertrophic but inactive
bone and fibrosis of the bone marrow (297).
unifocal form of CRMO and typically presents as an enlarged, painful
segment of bone. The metaphysis of long bones and the mandible are the
most commonly involved sites (10). Symptoms may
fluctuate over time, resolving and then reappearing periodically over
several years. Treatment typically consists of the symptomatic use of
NSAIDs.
 |
|
Figure 13.13 A:
This 16-year-old female patient presents with a 6-month history of increasing pain and swelling over the medial right clavicle. Standard radiographs demonstrate a lesion of mixed density with hyperostosis and enlargement of the medial clavicle. B: On the basis of the patient’s atypical history and radiographs, chronic recurrent multifocal osteomyelitis (CRMO) or a similar variant was suspected, and a technetium-99 bone scan was obtained to search for polyostotic lesions. Bone scan reveals increased uptake in the proximal left tibia and right calcaneus, although plain films remain normal. One year later, the patient developed palmar pustules resulting in a final diagnosis of synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome. |
This Clinical Practice Guideline is designed to provide clinicians an
analytical framework for evaluation and treatment of a particular
diagnosis or condition. It is not intended to establish a protocol or
to identify all patients with a particular condition, nor is it
intended to replace a clinician’s clinical judgment. A clinician’s
adherence to this Clinical Practice Guideline is voluntary. It is
understood that some patients will not fit into the clinical conditions
contemplated by this Clinical Practice Guideline and that the
recommendations contained in this Clinical Practice Guideline should
not be considered inclusive of all proper methods or exclusive of other
methods of care reasonably directed to obtaining the same results.
Decisions to adopt any specific recommendation of this Clinical
Practice Guideline must be made by the clinician in light of available
resources and the individual circumstances presented by the patient.
 |
|
Figure. No caption available.
|
 |
|
Figure. No caption available.
|
 |
|
Figure. No caption available.
|
 |
|
Figure. No caption available.
|
 |
|
Figure. No caption available.
|
H, Vahvanen V. A comparative study of osteomyelitis and purulent
arthritis with special reference to aetiology and recovery. Infection 1984;12:75–79.
MJ, Kincaid R, Craigen MA, et al. The changing epidemiology of acute
and subacute haematogenous osteomyelitis in children. J Bone Joint Surg 2001;83B:99–102.
H, Kallio MJ, Unkila-Kallio L. Reduced incidence of septic arthritis in
children by Haemophilus influenzae type-b vaccination: implications for
treatment. J Bone Joint Surg 1998;80B: 471–473.
CL, Wang SM, Yang YJ, et al. Septic arthritis in children: relationship
of causative pathogens, complications and outcomes. J Microbiol Immunol Infect 2003;36:41–46.
AW, Viskontas D, Sabbagh C. Reduction in osteomyelitis and septic
arthritis related to Haemophilus influenzae type B vaccination. J Pediatr Orthop 1999;19:705–709.
R, Cockayne A, Humphreys H. Clinical and molecular aspects of the
pathogenesis of Staphylococcus aureus bone and joint infections. J Med Microbiol 1996;44:157–164.
E, Rombouts-Godin V, Rombouts JJ. Acute hematogenous osteomyelitis due
to ordinary germs in children with closed injuries: study of a series
of 44 cases. Acta Orthop Belg 1991;57:91–96.
JL, Fitzgerald RH Jr, Morrissy RT. A histological study of acute
hematogenous osteomyelitis following physeal injuries in rabbits. J Bone Joint Surg 1988;70A:1383–1392.
DJ, Mirra J, Ding A, et al. E. coli arthritis in the rabbit: a model of
infectious and post-infectious inflammatory synovitis. J Rheumatol 1977;4:118–128.
WJ, Mosca VS, Nizet V. Orthopaedic manifestations of invasive group A
streptococcal infections complicating primary varicella. J Pediatr Orthop 1996;16:522–528.
AH, Campbell WG, Callahan BC. Infection of rabbit knee joints after
intra-articular injection of Staphylococcus aureus. Am J Pathol 1970;60:165–202.
ED, McCroskery PA. The influence of temperature and fibril stability on
degradation of cartilage collagen by rheumatoid synovial collagenase. N Engl J Med 1974;290:1–6.
L, Schurman DJ, Kajiyama G, et al. The effect of antibiotics on the
destruction of cartilage in experimental infectious arthritis. J Bone Joint Surg 1987;69A:1063–1068.
H, Vahvanen V, Aalto K. Fever, C-reactive protein, and erythrocyte
sedimentation rate in monitoring recovery from septic arthritis: a
preliminary study. J Pediatr Orthop 1984;4:170–174.
L, Kallio MJT, Eskola J, et al. Serum C-reactive protein, erythrocyte
sedimentation rate, and white blood cell count in acute hematogenous
osteomyelitis of children. Pediatrics 1994;93:59–62.
I, Faingezich I, Arguedas A, et al. Serial serum C-reactive protein to
monitor recovery from acute hematogenous osteo-myelitis in children. Pediatr Infect Dis J 1995;14:40–44.
MJ, McGuire KJ, McGowan KL, et al. Assessment of the test
characteristics of C-reactive protein for septic arthritis in children.
J Pediatr Orthop 2003;23:373–377.
N, Chonmaitree T, Rassin DK, et al. Use of C-reactive protein in
differentiation between acute bacterial and viral otitis media. Pediatrics 1995;95:664–669.
M, Isaacs D, Howman-Giles R, et al. Clinical and diagnostic features of
osteomyelitis occurring in the first three months of life. Pediatr Infect Dis J 1995;14:1047–1053.
S, Jacobsson H, Hirsch G. Specific or superfluous? Doubtful clinical
value of granulocyte scintigraphy in osteomyelitis in children. J Pediatr Orthop 2001;10B:109–112.
J, Giamarellou H, Kanellakopoulou K, et al. Infection: a
99mTc-ciprofloxacin radiopharmaceutical for the detection of bone
infection. Clin Microbiol Infect 2003;9:101–109.
T, Emery P, Tant L, et al. Evaluation of technetium-99m-ciprofloxacin
(Infecton) for detecting sites of inflammation in arthritis. Rheumatology 2003;42:1179–1182.
D, Trevers ST, Kasser JR, et al. Osteomyelitis and septic arthritis in
children: appropriate use of imaging to guide treatment. Am J Roentgenol 1995;165:399–403.
JM, Ross G, Cummings RJ, et al. Usefulness of magnetic resonance
imaging for the diagnosis of acute musculoskeletal infections in
children. J Pediatr Orthop 1995;15:144–147.
H, Harameti N, Flusser G. The diagnostic role of gadolinium enhanced
MRI in distinguishing between acute medullary bone infarct and
osteomyelitis. Magn Reson Imaging 2000;18:255–262.
JE, Huang M, Dobbs M, et al. Causes of false-negative ultrasound scans
in the diagnosis of septic arthritis of the hip in children. J Pediatr Orthop 2002;22:312–316.
M, al-Umran K, al-Habdan I, et al. Ultrasonography: can it
differentiate between vasoocclusive crisis and acute osteomyelitis in
sickle cell disease? J Pediatr Orthop 1998;18: 552–554.
IM, Gupta S, Palmer MK, et al. The prognostic significance of
radiological and symptomatic bone involvement in childhood acute
lymphoblastic leukemia. Med Pediatr Oncol 1979;6:51–55.
N, Gotze H, Pedersen A, et al. Skeletal scintigraphy and radiography at
onset of acute lymphocytic leukemia in children. Med Pediatr Oncol 1983;11:291–296.
MS, Zurakowski D, Kasser JR. Differentiating between septic arthritis
and transient synovitis of the hip in children: an evidence-based
clinical prediction algorithm. J Bone Joint Surg 1999;81A:1662–1670.
MS, Mandiga R, Zurakowski D, et al. Validation of a clinical prediction
rule for the differentiation between septic arthritis and transient
synovitis of the hip in children. J Bone Joint Surg 2004;86A:1629–1635.
SJ, Jones A, Schootman M, et al. Differentiation between septic
arthritis and transient synovitis of the hip in children with clinical
prediction algorithms. J Bone Joint Surg 2004;86A:956–962.
AR, Chang F, Zuckner J. Markedly raised synovial fluid leukocyte counts
not associated with infectious arthritis in children. Ann Rheum Dis 1978;37:404–409.
JD, Howard JB, Shelton S. Oral antibiotic therapy for skeletal
infections of children. I. Antibiotic concentrations in suppurative
synovial joint. J Pediatr 1978;92:131–134.
JD, Bucholz RW, Kusmiesz H, et al. Benefits and risks of sequential
parenteral-oral cephalosporin therapy for suppurative bone and joint
infections. J Pediatr Orthop 1982;2:255–262.
FM, Shahcheraghi GH, Ahadzadeh M. Short-term intravenous antibiotic
treatment of acute hematogenous bone and joint infection in children: a
prospective randomized trial. J Pediatr Orthop 2002;22:317–320.
MS, Mandiga R, Murphy JM, et al. A clinical practice guideline for
treatment of septic arthritis in children: efficacy in improving
process of care and effect on outcome of septic arthritis of the hip. J Bone Joint Surg 2003;85A:994–999.
DP, Stott NS. Community-acquired methicillin-resistant Staphylococcus
aureus: a cause of musculoskeletal sepsis in children. J Pediatr Orthop 1999;19:413–416.
G, Hammerman WA, Mason EO Jr. Clindamycin treatment of invasive
infections caused by community-acquired, methicillin-resistant and
methicillin-susceptible Staphylococcus aureus in children. Pediatr Infect Dis J 2003;22:593–598.
LE, Van den Elzen HM. Streptomycin accumulation in susceptible and
resistant strains of Escherichia coli and Pseudomonas aeruginosa. Antimicrob Agents Chemother 1976;9:928–938.
WE Jr, O’Dell NM. Comparative f3-lactamase resistance and
antistaphylococcal activities of parenterally and orally administered
cephalosporins. J Infect Dis 1978;137:490–493.
LD, Garner C, Wilcox C, et al. Effect of inoculum and of beta-lactamase
on the anti-staphylococcal activity of thirteen penicillins and
cephalosporins. Antimicrob Agents Chemother 1975;8:344–349.
I, Sela MN. The role of leukocytes and their hydrolases in the
persistence, degradation, and transport of bacterial constituents in
tissues: relation to chronic inflammatory processes in staphylococcal,
streptococcal, and mycobacterial infections and in chronic periodontal
disease. CRC Crit Rev Microbiol 1976;4:249–322.
J, Goultchin A, Stabholtz N, et al. Streptococcal and staphylococcal
arthritis: can chronic arthritis in the human be caused by highly
chemotactic degradation products generated from bacteria by leukocyte
enzymes and by the deactivation of leukocytes by inflammatory exudates,
polyelectrolytes, leukocyte hydrolases and by cell sensitizing agents
derived from bacteria? Agents Actions 1980;7:260–270.
WM, Gleason TF, Barmada R. A comparison between arthrotomy and
irrigation and multiple aspirations in the treatment of pyogenic
arthritis. Orthopaedics 1983;6:1309–1314.
A, Saighi-Bouaouina A. Treatment of sequestra, pseudoarthroses and
defects in the long bones of children who have chronic hematogenous
osteomyelitis. J Bone Joint Surg 1989;71A:1448–1468.
M, Lalonde F, Davidson D, et al. Atrial and venous thrombosis secondary
to septic arthritis of the sacroiliac joint in a child with hereditary
protein C deficiency. J Pediatr Orthop 1999;19:156–160.
D, Johnston CE, Wenger KR. Pyogenic infectious spondylitis in children:
the convergence of discitis and vertebral osteomyelitis. J Pediatr Orthop 1995;15:652–660.
MB, Ghormley RK, Kernohan JW. The intervertebral discitis microscopic
anatomy and pathology. Part I. Anatomy, development and physiology. J Bone Joint Surg 1945;27:105–112.
AG, Espersen F, Skinhoj P, et al. Increasing frequency of vertebral
osteomyelitis following Staphylococcus aureus bacteraemia in Denmark
1980–1990. J Infect 1997;34:113–118.
MC, Goldsmith JF, Gilligan PH. Sneakers as a source of Pseudomonas
aeruginosa in children with osteomyelitis following puncture wounds. Pediatrics 1985;106:607–609.
LS, Bin G, Jaovisidua S, et al. Cost effectiveness of magnetic
resonance imaging in diagnosing Pseudomonas aeruginosa infection after
puncture wound. J Foot Ankle Surg 1997;36:36–43.
RF, McCarthy RE, Elser JM. Pseudomonas osteochondritis complicating
puncture wounds of the foot in children: a 10-year evaluation. J Infect Dis 1989;160:657–661.
MS, Baker CJ, Wagner ML, et al. An etiologic shift in infantile
osteomyelitis: the emergence of the group B streptococcus. J Pediatr 1978;93:578–583.
AT, Eisenstein BI. Disseminated Gonococcal Infection (DGI) and
Gonococcal Arthritis (GCA): II. Clinical manifestations, diagnosis,
complications, treatment and prevention. Sem Arth Rheum 1981;10:173–197.
CM, Morris CR, Wasilauskas BL, et al. Gonococcal arthritis in an era of
increasing penicillin resistance: presentations and outcomes in 41
recent cases (1985–1991). Arch Intern Med 1994;154:2690–2695.
JB, Forsythe DA, Bertrand SL, et al. Retrospective review of
osteoarticular infections in a pediatric sickle cell age group. J Pediatr Orthop 2000;20:682–685.
DP, Heyneman LE, Ware RE, et al. MR features of soft tissue
abnormalities due to acute marrow infarction in five children with
sickle cell disease. Am J Roentgenol 1999;173:989–993.
MM, Hariharan V, Aradi AJ, et al. The value of ultrasound and
aspiration in differentiating vaso-occlusive crisis and osteomyelitis
in sickle cell disease patients. Clin Radiol 1999; 54:636–639.
M, Pavone V, Polizzi A, et al. Tuberculosis of the ankle in childhood:
clinical, roentgenographic and computed tomography findings. Clin Pediatr 1997;36:529–534.
A, Gicquel B, Lecosiier D, et al. Rapid diagnosis of tuberculosis by
amplification of mycobacterial DNA in clinical samples. Lancet 1989;334:1069–1071.
RH, Yazici M, Atabey N, et al. Detection of mycobacterium tuberculosis
in formaldehyde solution-fixed, paraffin-embedded tissue by polymerase
chain reaction in Pott’s disease. Spine 1996;21:1991–1995.
Research Council Working Party on Tuberculosis of the Spine. Five-year
assessment of controlled trials of inpatient and outpatient treatment
and of plaster-of-Paris jackets for tuberculosis of the spine in
children on standard chemotherapy. Studies in Masan and Pusan, Korea. J Bone Joint Surg 1976;58B:399–411.
Research Council Working Party on Tuberculosis of the Spine. Five-year
assessment of controlled trials of ambulatory treatment, debridement
and anterior spinal fusion in the management of tuberculosis of the
spine; studies in Bulawayo (Rhodesia) and in Hong Kong. J Bone Joint Surg 1978;60B:163–177.
E, Thompson G, Salter RB. Foci of chronic circumscribed osteomyelitis
(Brodie’s abscess) that traverse the epiphyseal plate. J Pediatr Orthop 1984;4:162–169.
TS, Hedeboe J, Christensen ER. Primary epiphyseal osteomyelitis in
children: report of three cases and review of the literature. J Bone Joint Surg 1988;70B:818–820.
AC, Davies AM, Mangham DC, et al. The “penumbra sign” on T1-weighted MR
imaging in subacute osteomyelitis: frequency, cause and significance. Clin Radiol 1998;53:587–592.
DA, Myer JS, Dormans JP, et al. Pyomyositis in children and
adolescents: report of 12 cases and review of the literature. J Pediatr Orthop 1999;19:143–150.
Jesus FR, Mendiola-Segura I. Clinical stage, age and treatment in
tropical pyomyositis: a retrospective study including forty cases. Arch Med Res 1996;27:165–170.
DJ, Peterson CL, Meyers HB, et al. Invasive group A streptococcal
infections in children with varicella in Southern California. Pediatr Infect Dis J 1996;15:146–150.
AG, Moller BN. Chronic sclerosing osteomyelitis of the clavicle: a
manifestation of chronic recurrent multifocal osteomyelitis. Arch Orthop Trauma Surg 1987;104:144–151.
K, Doita M, Tateishi H, et al. Bone and joint lesions associated with
pustulosis palmaris et plantaris: a clinical and histological study. J Bone Joint Surg 1988;70B:117–122.
K, Bjorksten B, Gustavson KH, et al. Pustulosis palmoplantaris and its
relation to chronic recurrent multifocal osteomyelitis. Dermatologica 1979;159:37–45.
C, Reed MH, Black GB. Premature epiphyseal fusion and degenerative
arthritis in chronic recurrent multifocal osteo-myelitis. Skeletal Radiol 2000;29:94–96.
AM, Lam PY, Duffy CM, et al. Chronic recurrent multifocal
osteomyelitis: clinical outcomes after more than five years of
follow-up. J Pediatr Orthop 2002;141:198–203.
CM, Lam PY, Ditchfield M, et al. Chronic recurrent multifocal
osteomyelitis: review of orthopaedic complications at maturity. J Pediatr Orthop 2002;22:501–505.
RM, Shore SD, Manson D, et al. Chronic recurrent multifocal
osteomyelitis and psoriasis: a report of a new association and review
of related disorders. Semin Arthritis Rheum 1988; 17:260–270.
A, Marcon M, Treem W, et al. Chronic recurrent multifocal osteomyelitis
associated with chronic inflammatory bowel disease in children. Dig Dis Sci 1999;44:2500–2507.
Y, Taguchi A, Tanimoto K. Diagnostic points and possible origin of
osteomyelitis in synovitis, acne, pustulosis, hyperostosis, and
osteitis (SAPHO) syndrome: a radiographic study of 77 mandibular
osteomyelitis cases. Rheumatology 2003;42: 1398–1403.
Kocher MS, Mandiga R, Murphy JM, et al. A clinical practice guideline
for treatment of septic arthritis in children: efficacy in improving
process of care and effect on outcome of septic arthritis of the hip. J Bone Joint Surg 2003;85A:994–999. with permission.
