USE OF LASERS IN ORTHOPAEDIC SURGERY
developed for endoscopic applications in many surgical disciplines.
More recently, arthroscopic laser systems have been introduced in
orthopaedic surgery as low-profile cutting tools. Initial orthopaedic
studies used existing commercial and surgical laser systems, which were
poorly suited for arthroscopic use. These lasers resected torn meniscal
tissue but increased perioperative morbidity and required changes in
surgical technique. Pulsed near-infrared systems later allowed delivery
of high-power laser light to the joint tissues through small fiber
optics and more convenient instrumentation. These near-infrared systems
are now used in shoulder, knee, and spinal surgery. Several recent
cases of osteonecrosis in laser-treated knees, however, have generated
controversy regarding the safety of these devices (21,23,34,59).
for light amplification by stimulated emission of radiation. The laser
is a device that produces high-intensity light energy with three unique
properties. It is (a) monochromatic—the light has a single wavelength,
(b) coherent—the light waves are in phase with one another, and (c)
directional. These characteristics allow laser light, at high energies,
to be transmitted through fiber optics, waveguides, or articulated arms
to tissue. The light energy, for surgical applications, can then cut,
coagulate, or ablate tissue.
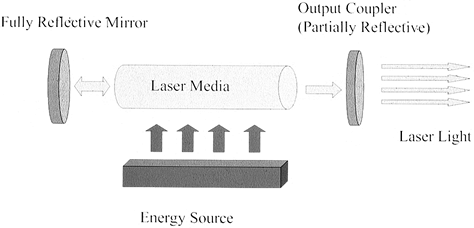
The laser medium—a solid, liquid, or gas—determines the wavelength of
the laser light. The resonator cavity sandwiches the medium between two
mirrors—one partially reflective and the other fully reflective. The
excitation source pumps high-energy electric or optical energy into the
laser medium. A cooling system removes excess thermal energy from the
resonator cavity to prevent damage to optical components.
 |
|
Figure 98.1.
The laser system is composed of a laser medium placed between two mirrors, one of which is partially reflective. Energy delivered to the medium produces light energy that is amplified between the two mirrors. Light escaping the output coupler has the unique properties of being monochromatic, directional, and in phase. |
laser medium, the absorbed photons are stimulated into higher energy
levels. The collapse of the electrons to their ground states releases
photons of a specific wavelength. These photons are reflected between
the two mirrors of the resonator cavity. High-intensity light
eventually escapes through the partially reflective mirror.
laser medium characterize surgical laser systems. In the United States,
most surgical systems produce visible, near-infrared or infrared laser
energy. Ultraviolet systems have been approved for ophthalmic use but
are not currently Food and Drug Administration (FDA)-approved for
surgical applications. The standard media include CO2 and
argon gases, and yttrium alluminum garnet (YAG) crystals doped with
heavy metals including holmium (Ho), erbium (Er), thulium, and
neodynium (Nd).
wave (CW) and pulsed. CW lasers produce a continuous beam of light
energy similar to a flashlight. The conduction of heat that occurs
during the use of a CW system may produce large peripheral zones of
thermal damage. Pulsed laser systems produce high-intensity light over
short time intervals. The high peak power of each laser pulse may
produce explosive ablation of target tissues. The time between pulses
allows for dissipation of thermal energy and prevents excessive damage
due to thermal conduction.
be delivered through the handpiece, in a manner similar to that seen in
electrocautery. For a pulsed laser system, the surgeon sets the energy
per pulse and the repetition rate. The flow of light is controlled by a
hand- or foot-switch. The focus (or defocus) of the light energy at the
tissue level controls the light intensity and affects the zone and
depth of laser-induced damage.
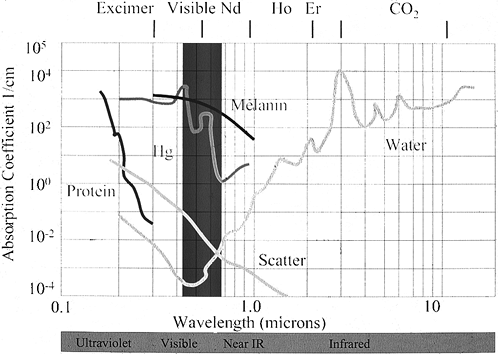
the laser light are major determinants in controlling the effect of the
laser light on the treated tissues. Light striking tissue reflects,
refracts, scatters, or is absorbed by the tissue. All tissues have
characteristic properties for each of these parameters, which vary as a
function of wavelength. Most existing surgical laser systems target
either the water within the tissue or selected chromophores—tissue
pigments such as hemoglobin, hemosiderin, or melanin.
and heat. Heating water above 100°C causes vaporization. Pulsed
infrared lasers deliver high peak powers of laser light in short time
periods of less than a fraction of a second. Tissue ablation occurs by
the explosive release of the vaporized tissue contents (Fig. 98.2).
Heating tissues below the vaporization threshold produces thermal
changes if collagen is heated above its phase transition temperature of
62°C.
 |
|
Figure 98.2.
Absorption spectra for tissue components. Near-infrared lasers are absorbed by the water within tissue, whereas visible lasers are absorbed by pigments such as hemoglobin or melanin. |
dissociate molecular bonds. If the energy of ultraviolet photons is
greater than the energy of molecular bonds, photon absorption may
directly disrupt the molecules for nonthermal cutting of tissue. The
so-called cold excimer lasers operate by this mechanism.
thermal energy and rapid expansion of vaporization contents creates
high shear stresses within the tissue. Photoacoustic shock waves may
create damage at large distances from the site (73). The process may occur for any pulsed laser system operating throughout the ultraviolet, visible, or infrared spectrum (16,60).
determined by availability. Modification of existing surgical systems
allowed passage of light energy into joint cavities. The earliest
systems, using CO2 laser media, varied considerably in their
configurations. Both CW and pulsed systems were evaluated, with
variable tissue effects. Ablation of cartilage produced excessive
charring and thermal damage with all devices. As 10.6-micron light
energy cannot pass through standard optical fibers, all CO2
systems required large, cumbersome devices for light delivery to the
joints. The requirement for joint distention with gas, the lack of
proven benefit, and the limited availability of awkward instrumentation
led to the early abandonment of the CO2 laser arthroscopic devices.
poorly suited for arthroscopic use. Solid state Nd:YAG surgical lasers
are used in both CW and pulsed formats. The Nd:YAG lasers produce light
(at 1.06 and 1.32 microns) that is readily transmitted through standard
silica optical fibers. The Nd:YAG light is strongly absorbed only by
pigmented or darkly stained tissue but not well absorbed by most
intra-articular tissues. Because of its low tissue absorption, at high
energies the light deeply coagulates tissue before producing changes at
the tissue surface. Cutting effects vary significantly as desiccation
or charring of the tissue surface substantially increases the light
absorption characteristics.
necrosis of tissue to a depth greater than 1 cm before any apparent
cutting effect occurs at the tip of the instrument. A contact sapphire
tip Nd:YAG laser system received limited use as an arthroscopic cutting
tool for meniscectomy and occasional chondroplasty (44,49). Multiple cases of osteonecrosis have been reported with this laser system (23,34). However, it has been largely abandoned because of concerns about effectiveness and safety.
ablates and cuts tissues via nonthermal mechanisms. Tissue effects
occur when ultraviolet light energy is absorbed by carbon–carbon double
bonds of organic chromophores. Efficient light absorption limits the
light penetration to within microns of the tissue surface. The high
power density of the absorbed light generates precise and effective
tissue-cutting effects with minimal thermal damage (3,8,17,22,53).
Unfortunately, ultraviolet light energy cannot be transmitted down
standard optical fibers, and development of convenient arthroscopic
delivery systems has proven difficult. Only the 308-nanometer xenon
chloride excimer laser can be delivered fiberoptically through
expensive, nonflexible zirconia fluoride fibers.
Excimer arthroscopic systems have received limited use, and that has
been largely in the European market (24,29). Nuss et al. (48)
reported on the use of the 308 nm excimer laser in a prospective,
randomized clinical trial of 70 patients with chondral degeneration.
After a 6-month follow-up, the authors found a significant reduction in
pain and in reactive synovitis in laser compared with control groups.
No differences in level of disability or function were noted between
groups.
laser system. It was specifically developed for arthroscopic use and
has now been adapted for use in multiple surgical disciplines. The
device produces pulsed 2.1-micron-wavelength laser light, which is
readily transmitted down conventional optical fibers into low-profile
handpieces. The Ho:YAG laser effectively cuts, coagulates, and ablates
well-hydrated intra-articular tissues (72,75,76 and 77). The laser is used in contact or near contact mode.
for meniscectomy was granted in the late 1980s. Fanton et al. reported
decreased postoperative symptoms and recuperation time following laser
meniscectomy (19). No prospective, double-blind, randomized clinical studies have validated the original claims.
pulsed 2.1-micron light energy is absorbed by the water within the
well-hydrated intra-articular tissues. Rapid absorption, heating, and
vaporization of tissue creates cavitation—an explosive expansion of the
vaporized water. The amount of tissue ablated per pulse at low
repetition rates correlates with the amount of energy delivered per
pulse. Approximately 10 joules (J) of energy are required to remove 1
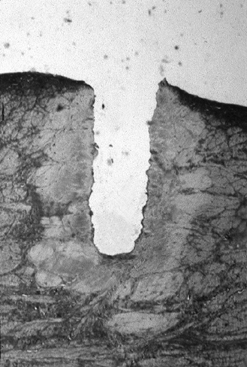
cubic millimeter of articular cartilage or meniscus (72). Each individual pulse creates a crater surrounded by a zone of thermal damage (40) (Fig. 98.3; see COLOR FIG. 98.3).For low repetition rates, the damage extends approximately 350 microns laterally and 500 microns deep within the tissue (65,72,77).
Clinically, the laser is used at repetition rates ranging from 10 to 30
hertz. At these rates, thermal diffusion may occur between
pulses, and thermal damage may extend significantly greater distances within the tissue. In vivo,
photoacoustic effects may produce damage in tissue remote from the
laser site, especially if the laser is used in confined spaces (73).
 |
|
Figure 98.3. (See COLOR FIG. 98.3).
Thermal damage in meniscus after three shots at 1.5 J per pulse at a repetition rate of 1 Hz. Damage extends approximately 350 microns from the edges of the crater (trichrome stain, 40×). |
response of articular cartilage following laser irradiation is
consistent with that following mechanical instrumentation (13,54,75).
Partial-thickness defects do not heal, and full-thickness defects are
characterized by fibrocartilaginous repair, which is less durable than
the host tissue (75). Loss of cellularity and
necrosis in subchondral bone is found consistently at the border of
treated regions in an equine model (13).
which the laser was originally developed. The laser offers the
theoretical advantage of less iatrogenic damage to articular surfaces
when treating poorly accessible, posterior horn meniscal tears. Whereas
mechanical instruments may abrade or inadvertently gouge chondral
surfaces, the low-profile laser handpieces may be used in limited
noncontact mode to ablate and cut the damaged posterior structures.
Limited literature is available to substantiate these claims (19,66).
Rather than minimizing damage, the laser, when used in an unsafe
manner, may play a role in producing osteonecrosis in subchondral bone.
of osteonecrosis following laser arthroscopy with either the Nd:YAG or
the Ho:YAG laser systems (21,23,34,59).
Early laser tissue interaction studies suggested that thermal damage
would be limited to the optical penetration of the tissue, which is
approximately 300–500 microns. The studies, performed with low laser
repetition rates on cartilage (13,54,63,72,75,77),
did not address the tissue effects at the clinically relevant higher
repetition rates. Studies suggest that osteonecrosis, in many cases,
may be preexisting and may not relate to the use of the laser (33).
produces extensive subchondral damage if used to drill into articular
surfaces (73). If the laser energy penetrates
subchondral bone, the expansion of the vaporation bubble may produce a
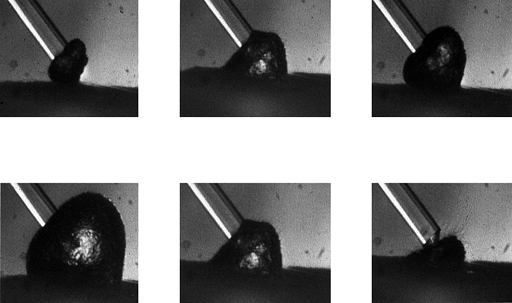
fluid wave within the confined space (Fig. 98.4).High
shear stresses may tear blood vessels and produce hemorrhage several
centimeters deep within the subchondral bone. Additional data suggest
that less than 25 J of energy may induce this deleterious effect. These
studies suggest that inadvertent direction of the laser energy into the
articular cartilage with penetration of the calcified cartilage can
cause damage, which may lead to osteonecrosis. Avoid drilling articular
cartilage with any laser system.
 |
|
Figure 98.4.
Expansion and collapse of a cavitation bubble in saline during a 0.5 J Ho:YAG laser pulse. These six images were obtained sequentially over 250 microseconds. The optical fiber has a diameter of 400 microns. Explosive cavitation may produce photoacoustic effects in confined spaces. |
articular cartilage. The laser can thermally alter tissue if the energy
striking the tissue is less than the tissue ablation threshold. If held
remotely from the tissue, the laser can deliver sufficiently low
amounts of light energy to thermally alter the tissue without
vaporization. At 62°C, collagen undergoes a phase transition from a
triple helix to an amorphous gel. Due to gross structural changes,
ordered collagenous tissues such as capsule or tendon “shrink” in
length, whereas surfaces of meniscus or articular cartilage coagulate
or “anneal.” In using the laser for meniscectomy, surgeons routinely
produce smooth residual rims of meniscus (Fig. 98.5; see COLOR FIG. 98.5).The
durability and the long-term clinical value of the annealing treatment
is unknown. Tissue tensile properties, long-term healing response, and
short-term histopathologic effects of the annealing treatments have not
been adequately characterized. Annealing should be considered
experimental at this time.
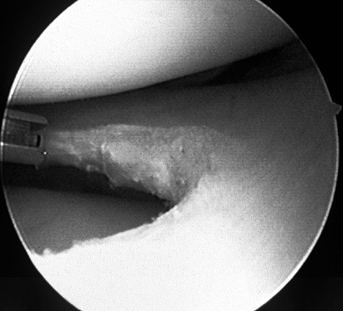 |
|
Figure 98.5. (See COLOR FIG. 98.5).
Application of laser energy to the edge of a debrided meniscal tear. The long-term effects of this “annealing” treatment remain controversial. |
limited hemostasis when performing cutting or ablative procedures. Two
investigators have evaluated the Ho:YAG laser for lateral retinacular
release. In a retrospective review, Shapiro et al. suggested that
laser-treated patients have lower morbidity than patients treated with
electrocautery (61). A prospective study by Carter and Edinger demonstrated no clinical difference between laser and cautery groups (9).
shoulder joint capsule has led to the development of arthroscopic laser
and radiofrequency techniques for treating excess capsular laxity (20,27,30,55,70).
Symptomatic inferior and multidirectional instability is based on two
factors in varying proportions: (a) the inherent laxity of the shoulder
capsule, and (b) the activities of the patient (45). In cadaveric specimens, temperatures at or above 65°C cause significant shrinkage of glenohumeral joint capsular tissue (28).
The shrinkage is achieved with use of nonablative laser energy without
detrimental effects to the viscoelastic properties of the tissue (25).
architecture caused by the thermal effect of laser energy is probably
the dominant mechanism of laser-induced tissue shrinkage (27). Studies have demonstrated that remodeling of capsule is unimpeded following treatment (26). In cadaveric specimens, laser anterior capsuloplasty acutely reduces anterior and posterior translation (70). Studies have demonstrated variable consistency and durability of the thermal effects (55). The depth of the thermal effect and potential damage to surrounding structures has been questioned.
Bipolar (Mitek, Arthrotec) and radiofrequency devices (Oratec) function
by applying electrical or microwave energy to the collagenous tissues
by direct contact. The radiofrequency device also incorporates
temperature control in an attempt to limit the thermal damage. The
bipolar devices pass electrical current from leads positioned at the
tip to a grounding surface on the edge of the handpiece, whereas the
radiofrequency device transmits energy directly into the tissue. The
radiofrequency-induced thermal effects vary with the impedance of the
tissues. Equivalent tissue effects have been reported for treating
capsular tissues with laser and with nonlaser thermal devices (50).
development of laser discectomy techniques. The first discectomy study
used the CO2 laser on a canine model (17). This study and others using multiple animal models (4,12,80) demonstrated equivalent healing following cervical discectomy performed with either mechanical or CO2 laser tools. Laser and mechanical tools both produced lower mean intradiscal pressures (11,12,52,80) due to a bulk modulus effect (78).
Ho:YAG, Nd:YAG, and potassiumitanyl-phosphate (KTP) all create defects
greater than 1.5 cm in diameter with up to 1,200 J of energy. The
greatest temperature elevations occur with the Nd:YAG laser (39). Temperatures remain within safe limits, however, in the
region of the posterior longitudinal ligament adjacent to the nerve root.
are FDA-approved for discectomy procedures in the United States. No
clinical results with the KTP system have been reported. The Ho:YAG
system demonstrated no difference between treatment and nonoperative
control groups in a nonrandomized study of lumbar discectomy (64).
performed in Europe. Choy et al. reported clinical results of
outpatient laser discectomy performed with continuous-wave Nd:YAG laser
systems (10). Laser energy was delivered by
400-micron optical fiber through a fluoroscopically placed 18-gauge
spinal needle. Fair or good results were noted in only 78% of patients,
with gross failure in 22% and one complication of discitis. The system
is not currently available for use in spinal procedures in the United
States.
controversial. Results have been comparable to other percutaneous
techniques such as chymopapain injection (41).
Use has been restricted to prolapsed or extruded discs but not
sequestered discs. Laser ablation of subligamentous extruded nucleus
pulposus has been noted to be ineffective. Indications for use remain
restricted until further clinical and basic science studies can
demonstrate spinal laser use to be both safe and effective.
instrumentation may increase the indications for laser use. Lasers are
currently being used experimentally in Europe for foraminal
decompression and debridement of extruded discs. While lasers offer
promise, future controlled clinical trials will be required before they
attain an expanded role in spinal surgery.
manufacturers, with either rigid or flexible handpieces. Check the
laser system for proper function before starting the procedure.
-
Prepare and drape the knee in the usual
fashion. After creation of standard arthroscopic portals and
introduction of the arthroscope, carry out diagnostic arthroscopy in
standard fashion. -
Identify intra-articular pathologies
including meniscal tears. Choose a laser handpiece. I prefer to use a
30°-bend, recessed-tip device, which usually provides access to all
regions of the joint. Plug the optical coupler attached to the
handpiece into the laser system. Turn the system on and place it into
standby mode. During laser use, wear protective laser goggles. Program
laser parameters into the system. For meniscectomy, a power of 2 J per
pulse and a repetition rate of 10 Hz are recommended. -
Place the laser handpiece into the joint
with the tip in contact with the meniscal tear. Activate the laser with
a foot pedal. Debride flap tears. If desired, also debride or anneal
remnants of frayed meniscus. Conventional mechanical instruments may
supplement laser treatments. -
The laser may also be used to coagulate
bleeding vessels in a noncontact mode. After completion of debridement,
place the laser in standby mode. Remove the instruments and close the
wounds in the usual fashion.
arthroscopic debridement of hypervascular rheumatoid synovitis. The
procedure is indicated for patients with medically refractory
monoarticular synovitis with no radiographic evidence of joint
degeneration. Treatment of synovitis secondary to hemophilia has also
been advocated (46).
-
For synovectomy, set the laser at 2 J per
pulse and a repetition rate of 10–20 Hz. Hold the laser 2–3 mm from the
tissue to thermally necrose and coagulate synovium in a noncontact
manner. With use of accessory posterior portals, extensive debridement
of posterior compartment synovitis can be performed with minimal
bleeding and improved visualization.
properties, the laser has been used extensively for debridement of torn
intra-articular tissues including superior labral anterior and
posterior (SLAP) tears and other labral lesions. The use of the Ho:YAG
laser for the debridement of rotator cuff lesions and biceps tendon
tears is contraindicated because of the risk of damage to adjacent
normal tissues.
-
Place the patient in either a beach-chair
or lateral decubitus position. Examination under anesthesia is
performed preoperatively. Sterilely prep and drape the shoulder. Create
standard anterior, mid-lateral and posterior portals and carry out
diagnostic arthroscopy on the glenohumeral joint and subacromial bursa. -
If you identify a labral tear that
requires debridement, turn the laser on and insert a 30° angled laser
handpiece through the anterior or posterior operating portal that
affords best access to the lesion. Laser parameters of 2 J per pulse at
a repetition rate of 20 Hz (20 watts) are routinely used for
debridement. -
Place the laser in active mode. Put the
laser tip in contact or near contact with the tissue and activate the
laser by foot pedal. Ablate the damaged tissues. As the energy is
delivered, you can focus and defocus the beam by controlling the
distance between the probe and the tissue. -
After completion of the debridement,
place the laser in standby mode before removal of handpieces from the
joint. Close wounds in routine fashion.
decompression, substantial bleeding is frequently a problem. It limits
visualization, prolongs the procedure, increases fluid extravasation,
and leads to increased perioperative morbidity. Use of the arthroscopic
pump often is supplemented with epinephrine within the irrigation
solution to limit bleeding. The laser’s hemostatic properties in a
“defocused” mode make it useful in subacromial decompression procedures.
-
After completion of glenohumeral
arthroscopy as previously described, visualize the subacromial space
with the arthroscope placed in the posterior portal. Place cannulas in
the anterior and lateral mid-deltoid portals. -
Debride the subacromial bursa with a
mechanical shaver according to standard technique. Use of the
mechanical tools is faster for removal of larger tissue volumes and can
be supplemented with use of the laser in defocused, noncontact mode to
achieve hemostasis. -
Set the laser initially at 2 J per pulse
and a repetition rate of 20 Hz (40 W), and use it after completion of
the bursal resection to create a hemostatic field. Deliver the laser
probe through the lateral portal. Expose the anterior and lateral
margins of the acromion by laser debridement of the undersurface
periosteum. Then reset the laser to a higher repetition rate of 30 Hz
at a total average power of 60 W. Identify the coracoacromial ligament
and resect it off the anterior edge of the acromion with the laser.
Bleeding that is frequently encountered from the acromial branch of the
thoracoacromial artery can be coagulated in noncontact mode with the
laser. A mechanical shaver may complete the debridement and remove the
remnant of the ligament. The subacromial periosteum is coagulated with
the laser. -
Perform acromioplasty with standard burr
to the desired level; additional bleeding may be coagulated with the
laser as previously described. Maintain the laser in standby mode when
the handpiece is removed from the joint. Close wounds in routine
fashion. Postoperative rehabilitation is identical to that of nonlaser
procedures.
-
Perform examination under anesthesia to
confirm the magnitude and direction of the glenohumeral instability.
Position, prep, and drape the patient, and perform diagnostic
arthroscopy as previously described. After treating the intra-articular
pathology according to standard arthroscopic technique, turn attention
to the capsule. -
For the capsular treatments, use the 30°
angled laser probe with laser settings of 1 J per pulse and a
repetition rate of 10 Hz. For patients with anterior instability,
introduce the laser through the anterior portal, with the arthroscope
placed posteriorly. The laser is activated 5 mm from the tissue and
advanced until shrinkage is observed without tissue ablation (Fig. 98.6).The laser probe energy is applied radially in brushstroke fashion
P.2544
from the edge of the glenoid laterally. The process is repeated sequentially.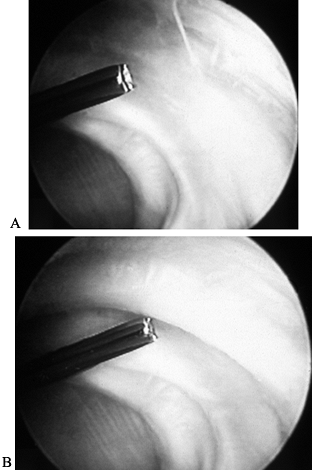 Figure 98.6.
Figure 98.6.
Capsular shrinkage procedure. Ho:YAG laser energy is applied to
anterior shoulder capsule in a noncontact mode. The two figures
demonstrate the capsule pretreatment (A) and after application of laser energy (B). Note the contracted band of tissue at the tip of the device that appears after application of laser energy. -
Do not make repeat passes over treated
areas; nearly all shrinkage occurs with the initial laser treatment.
Additional laser energy may increase thermal damage beyond the capsule.
Initiate the treatment posteroinferiorly, bring it into the axillary
region, then to the region of the anterior inferior glenohumeral
ligament. Advance the arthroscope for improved anterior visualization.
Then treat the anterior capsule, and the anteroinferior, middle, and
superior glenohumeral ligaments. -
Treat unidirectional posterior
instability with similar technique, but place the arthroscope in the
anterior portal with the laser probe in the posterior portal. The
posterior structures are thermally treated starting in an
anteroinferior location and advancing posteriorly. -
In the case of multidirectional
instability, the capsule and ligaments are treated circumferentially.
Visualization is improved if the posterior structures are treated
first, in a manner similar to that described for posterior instability.
The arthroscope and laser probe then exchange portals prior to
treatment of anterior tissues. Close wounds in routine fashion. -
Tailor rehabilitation to the age of the
patient and the direction of instability. For unidirectional anterior
instability, keep older patients in a sling for 1 to 2 weeks, and
younger patients, 2 to 3 weeks. Encourage patients to attain 90° of
abduction and limit them to 45° of external rotation by 6 weeks.
Rotation and abduction are then advanced as tolerated, limiting motion
to the final 15° of abduction and external rotation until 12 weeks.
Allow patients unrestricted return to sports and overhead throwing at
3–4 months, depending on strength, conditioning, and endurance. -
After posterior stabilization, stabilize
the arm in 30° of external rotation for 4 weeks. Start glenohumeral and
scapulothoracic exercises at that time, avoiding full internal rotation
for 12 weeks.
difficult challenge for revision total joint surgeons. The laser offers
the potential advantage of selective ablation of retained cement
without damage to host bone (5,6,62,68).
PMMA strongly absorbs infrared and near-infrared light energy. In the
infrared region, the water within tissue absorbs light energy and the
dry components of bone have minimal optical absorption. By using
wavelengths of light that do not correlate with the absorption peaks
for water, many researchers have documented efficient cement removal
with infrared laser systems.
been widely adapted or developed because of concerns about safety,
efficacy, and convenience. Vaporization of PMMA produces highly toxic
byproducts that must be removed from the operating room with
high-powered evacuation systems to prevent injury to personnel.
Vaporization products are flammable and may ignite during the laser
process. The infrared laser light cannot be transmitted through fiber
optics, and cumbersome delivery systems are required.
effective at ablating cement, is nonselective and may also perforate
and ablate host bone. Desiccation of the tissues in the surgical field
may produce extensive charring. Visualization within the femoral canal
is difficult and the interface between host bone and PMMA becomes
difficult after use of the laser has been initiated. Access to the
distal canal is often difficult with the free-beam CO2 laser.
gas into the laser field. The gases reduce the oxygen content in the
plume and dilute the vaporization contents, reducing the risk of
ignition. The waveguide is also less cumbersome than the articulated
arm handpieces for the CO2 laser. Distal removal of cement
from the femoral canal has proven difficult with these devices. Few
surgeons are currently using the laser clinically or experimentally for
cement removal in revision joint arthroplasty.
easily through calcified tissues with minimal trauma to the host
tissues. Multiple laser systems, both CW and pulsed, at high peak
powers efficiently ablate bone (32,35,42,47,48).
Cutting through bone at rates comparable to mechanical tools requires
larger, more expensive laser systems. In contrast to mechanical tools,
the lasers can thermally damage the osteotomized bone. In vivo studies demonstrate delayed healing after ablation with near-infrared Er and Ho systems in rat tibial and calvarial bone (7,18).
The lack of a readily adaptable high-powered lasers for osteotomy, the
increased cost, the effectiveness of current mechanical tools, and
concerns about impaired healing result in limited indications for laser
osteotomy at this time.
collagenous connective tissues. The two primary welding mechanisms
under investigation are based on thermal and photochemical mechanisms.
The laser-based techniques use light energy to “melt” the end of the
collagenous structures. The melting occurs as light disrupts collagen
crosslinks
and,
in turn, the organized collagen triple helical structure. The collagen
phase transition, and resulting “molten” mass, occurs within a narrow
temperature range bridging 62°C (38).
Tissue welding occurs when the molten uncrosslinked tissues are
mechanically apposed, then recrosslinked. Limited success has also been
achieved with use of fibrin solders (67).
to directly crosslink collagen of severed tissues. Experimental
naphthalimide dyes have produced high-strength welds of menisci and
articular cartilage. Preclinical studies with Barbados sheep have
demonstrated healing and retention of articular cartilage welds for
longer than 12 months (31,36,37).
Photochemical techniques are early in development. Toxicity, safety,
and efficacy studies will need to be done before the techniques are put
to clinical use in humans.
which low-level light energy is used to induce therapeutic effects. The
theory is based on observations that low-level light energy increases
cellular metabolism. Multiple endogenous chromophores within cells
produce multiple complex in vitro cellular effects. The cellular effects are dependent on both wavelength and light dose (1,51).
benefit from low-level light treatments if the metabolic effects can be
induced broadly throughout tissues. Studies have assessed the influence
of low-level light on both fracture and wound healing in animals (2,14,81).
Clinical trials in the United States currently evaluate biostimulation
for the treatment of low back pain and carpal tunnel syndrome. No
systems are currently approved for use in the United States.
in which light-activated chemicals are used to treat a variety of
pathologic conditions. The FDA has currently approved the
photosensitizer Photofrin for the treatment of lung cancer and multiple
other applications. Additional dyes are in later-stage clinical trials.
As both drug and light must reach the target tissue to induce
therapeutic effects, PDT offers the potential for selective drug
toxicity. The difficulty in delivering light energy to opaque solid
musculoskeletal tumors has prevented the adoption of PDT techniques in
orthopaedic oncology.
The hypervascular proliferative synovium of rheumatoid arthritis can be
targeted and destroyed with PDT. Photosensitizers are delivered
systemically or via intra-articular injection.
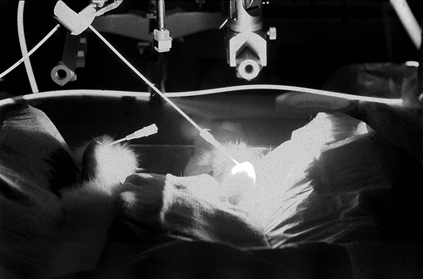 |
|
Figure 98.7. (See also COLOR FIG. 98.7)
Photochemical treatment of a rabbit model of rheumatoid arthritis. Light delivered percutaneously to the anterior knee joint activates photochemicals that destroy the proliferative synovium. |
deliver light energy to the inflamed synovium. The light-activated
dyes, or photosensitizers, are large multiconjugated structures that
produce reactive oxygen species after absorbing the light energy of a
specific wavelength. The singlet and triplet oxygen reacts locally to
disrupt cellular and nuclear membranes inducing both necrosis and
apoptosis. Direct occlusion of tissue neovasculature indirectly
produces synovial necrosis. Activation of multiple photosensitizers
with a variety of light-delivery systems produces synovial necrosis
without apparent damage to the adjacent chondral or soft tissues.
Development of these techniques remains at the preclinical research
level.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
M, Usuba M, Maeshima T, et al. Laser’s Effect on Bone and Cartilage
Change Induced by Joint Immobilization: An Experiment with Animal
Model. Lasers Surg Med 1997;21(5):480.
M, Grothues-Spork M, Hertel P, Moazami- Goudarzi Y. Reactions of
Meniscal Tissue after Arthroscopic Laser Application: An in Vivo Study
Using Five Different Laser Systems. Arthroscopy 1996;12(4):441.
J, Rhodes A, Lane GJ, et al. The Chronic Effects of Anterior Cervical
and Percutaneous Lumbar Discectomy Using the Holmium:YAG Laser: An
Animal Model. State Art Rev (Spine) 1993;7:31.
M, Kutschera HP, Katterschafka T, et al.Erb:YAG and Hol:YAG Laser
Osteotomy: The Effect of Laser Ablation on Bone Healing. Lasers Surg Med 1994;15(4):373.
T, Edinger S. Arthroscopic Lateral Release of the Knee. Presented at
American Orthopaedic Society for Sports Medicine Specialty Day,
Atlanta, GA, 1996.
DSJ, Altman PA, Case RB, Trokel SL. Laser Radiation at Various
Wavelengths for Decompression of Intervertebral Disk. Experimental
Observations on Human Autopsy Specimens. Clin Orthop 1991;267:245.
MA, Haugland LM, Bellamy J, et al. Effects of Holmium:YAG Laser on
Equine Articular Cartilage and Subchondral Bone Adjacent to Traumatic
Lesions: A Histopathological Assessment. Arthroscopy 1993;9(5):536.
M, Jahn R, Neu W, Jungbluth KH. Studies in Fiber Guided Excimer Laser
Surgery for Cutting and Drilling Bone and Meniscus. Lasers Surg Med 1991;11(6):569.
Montaser MA, Devlin H, Sloan P, Dickinson MR. Pattern of Healing of
Calvarial Bone in the Rat Following Application of the Erbium-YAG
Laser. Lasers Surg Med 1997;21(3):255.
JP, Lotke PA, Sapega AA, et al. Osteonecrosis of the Knee Following
Laser-assisted Arthroscopic Surgery: A Report of Six Cases. Arthroscopy 1995;11(4):467.
K, Markel MD, Thabit G 3rd, et al. The Effect of Nonablative Laser
Energy on Joint Capsular Properties. An in Vitro Mechanical Study Using
a Rabbit Model. Am J Sports Med 1995;23(4):482.
K, Nieckarz JA, Thabit G 3rd, et al. Effect of Nonablative Laser Energy
on the Joint Capsule: An in Vivo Rabbit Study Using a Holmium:YAG
Laser. Lasers Surg Med 1997;20(2):164.
K, Thabit G 3rd, Bogdanske JJ, et al. The Effect of Nonablative Laser
Energy on the Ultrastructure of Joint Capsular Collagen. Arthroscopy 1996;12(4):474.
K, Thabit G 3rd, Massa KL, et al. The Effect of Thermal Heating on the
Length and Histologic Properties of the Glenohumeral Joint Capsule. Am J Sports Med 1997;25(1):107.
AB, Roscher E, Konig U. Arthroscopic Shoulder Stabilization.
Differentiated Treatment Strategy with Suretac, Fastak, Holmium:
YAG-laser and Electrosurgery [Review] [in German]. Orthopade 1998;27(8):518.
R, Judy M, Matthews J, Nosir H. Photochemical Tissue Welding with
1,8-Naphthalimide Dyes: In Vivo Meniscal and Cartilage Welds. Orthop Trans 1999;22(3):992.
DL, Kosarek FJ, Helms CA, et al. Osteonecrosis after Contact
Neodymium:Yttrium Aluminum Garnet Arthroscopic Laser Meniscectomy. Am J Roentgenol 1997;169(3):855.
MM, Chen L, Fuh L, et al. Photochemical Cross-linking of Type I
Collagen with Hydrophobic and Hydrophilic 1,8-naphthalimide Dyes. In: SPIE Proceedings, San Jose, CA, 1996.
MM, Matthews JL, Boriak RL, et al. Heat-free Photochemical Tissue
Welding with 1,8-naphthalimide Dyes Using Visible (420 nm) Light. In: SPIE Proceedings, San Jose, CA, 1993.
FX, Choma TJ, Popovic N, et al. Tendon Repair by Laser Welding: A
Histologic and Biomechanical Comparison and Suture Repair with CO2 and Argon Lasers. Lasers Surg Med 1996;19(4):487.
JG, Amiel ME, Monosov AZ, Amiel D. Matrix Assessment of the Articular
Cartilage Surface after Chondroplasty with the Holmium:YAG Laser. Am J Sports Med 1997;25(4):560.
KU, Lorente C, Schomacker KT, et al. Use of the Er:YAG Laser for
Improved Plating in Maxillofacial Surgery: Comparison of Bone Healing
in Laser and Drill Osteotomies. Lasers Surg Med 1996;19(1):40.
DV, SJ O’Brien, Arnoczky SS, et al. The Use of the Contact Nd:YAG Laser
in Arthroscopic Surgery: Effects on Articular Cartilage and Meniscal
Tissue. Arthroscopy 1989;5(4):245.
CSD, Foster CR. Inferior Capsular Shift for Involuntary Inferior and
Multidirectional Instability of the Shoulder. A Preliminary Report. J Bone Joint Surg Am 1980;62(6):897.
C, Dechavanne M, Lalain JJ, Delacroix P. Successful Arthroscopic
Synovectomy Using Holmium : Yag Laser and FEIBA in a High Responder
Hemophiliac [letter]. Am J Hematol 1993;44(1):74.
SL, Hecht P, Hayashi K, et al. The Effect of Radiofrequency Energy on
the Length and Temperature Properties of the Glenohumeral Joint
Capsule. Arthroscopy 1998;14(4):395.
MA, Chen JW, Zhang K. Effects of Low-energy Gallium-aluminum-arsenide
Laser Irradiation on Cultured Fibroblasts and Keratinocytes. Lasers Surg Med 1997;20(4):426.
JG, Collier MA, Das P, et al. Effects of Holmium:YAG Laser Energy on
Cartilage Metabolism, Healing, and Biochemical Properties of Lesional
and Perilesional Tissue in a Weight-bearing Model. Arthroscopy 1996;12(1):15.
JG, Collier MA, Johnson LL, et al. Holmium:YAG Laser-assisted Capsular
Shift in a Canine Model: Intraarticular Pressure and Histologic
Observations. J Shoulder Elbow Surg 1997;6(3):272.
LG, Chowdhary RK, Neyndorff HC, et al. Photodynamic Therapy: A
Comparison with Other Immunomodulatory Treatments of Adjuvant-enhanced
Arthritis in MRL-lpr Mice [see comments]. Clin Exp Immunol 1994;95(3):373.
W, Vari SG, van der Veen MJ, et al. Effect of Varying Laser Parameters
on Pulsed Ho:YAG Ablation of Bovine Knee Joint Tissues. Arthroscopy 1993;9(1):96.
W, Saunier J, Gerber B. Two-year Follow-up Results of Arthroscopic
Laser Surgery of the Knee: European Multicenter Study. Tech Orthop 1995;10:309.
JE, McMahon PJ, Shrader TA, et al. Glenohumeral Joint Translation after
Arthroscopic, Nonablative, Thermal Capsuloplasty with a Laser. Am J Sports Med 1998;26(4):495.
K, Gandour-Edwards R, Bamberg M, et al. Influence of Light Delivery on
Photodynamic Synovectomy in an Antigen-induced Arthritis Model for
Rheumatoid Arthritis. Lasers Surg Med 1998;22(3):147.
K, Nishioka N, Patel D. Pulsed Holmium:Yttrium-aluminum-garnet (Ho:YAG)
Laser Ablation of Fibrocartilage and Articular Cartilage. Am J Sports Med 1990;18(3):316.
KB, Gandour-Edwards R, Bamberg M, et al. Photodynamic Synovectomy Using
Benzoporphyrin Derivative in an Antigen Induced Arthritis Model for
Rheumatoid Arthritis. Photochem Photobiol 1998;67(1):133.
CT Jr, Ghaderi B, Brustein M, et al. Ablation Rates of Human Meniscal
Tissue with the Ho:YAG Laser: The Effects of Varying Fluences. Arthroscopy 1997;13(2):148.
CT Jr, Watson T, Saadatmanesh V, Moran K. Pulsed Ho:YAG Laser
Meniscectomy: Effect of Pulsewidth on Tissue Penetration Rate and
Lateral Thermal Damage. Lasers Surg Med 1995;16(1):61.
