ADOLESCENT SPORTS INJURIES
Staff, Peachtree Orthopaedic Clinic, Piedmont Hospital; Team Physician,
Georgia State University and the Atlanta Glory Basketball Team,
Atlanta, Georgia.
All in all, sports account for 36% of the injuries of all kinds seen in
children and adolescents in the United States yearly. Injuries are more
frequent in boys than girls at a ratio of 1.8:1 and increase with age (26), a statistic that reflects lower levels of training intensity in the younger age group (76,193). Injuries tend to be sport specific and are more common in collision sports (26,182,223), with 40% to 80% of football players sustaining an injury yearly (196). Although catastrophic injuries do occur (156) the majority of injuries are minor (77,196). A full 25% of youth football players, however, require five days off during the season for sports-related injuries (77). Unorganized sports are associated with a higher rate of injury than organized athletics (49,223), a
fact that underscores the importance of adult supervision in youth athletics.
trauma that overwhelms the individual’s healing capacity. Two scenarios
have been blamed for the occurrence of overuse injuries in children: an
inadequately trained athlete experiences high musculoskeletal demands (63) or an extremely fit athlete overtrains (197).
Children rarely develop stress injuries during unstructured play, but
under the pressures of coaches, parents, or occasionally, peers, they
will press past pain into injury (192). With
greater emphasis on excelling in a single sport, children have begun
training for many hours a day at very young ages. The young runner runs
10 to 15 miles a day, an elite swimmer strokes up to 400,000 times in a
10-month season (161), a gymnast spends 4 to 6
hours a day in the gym, and a dancer dances a similar amount of time.
Few studies have explored the ability of immature musculoskeletal
tissue to adapt to increased physical stresses or the influence of
factors such as psychological stress, hormonal fluctuations, and
nutrition. Table 97.1 lists commonly reported risk factors for overuse injuries in children and adolescents.
 |
|
Table 97.1. Risk Factors for Overuse Injuries in Children and Adolescents*
|
encouraged to limit practice hours; use good-quality, properly fitted
equipment; cross train; and participate in conditioning programs to
develop strength and flexibility. Overuse injuries in children most
commonly affect the bone or its physis (stress fractures), cartilage at
the attachment of tendons (nonarticular osteochondroses), or cartilage
and bone at the joint surfaces (primary and secondary osteochondroses).
Before the advent of organized sports for children, stress fractures in
this age group were rare; however, with emphasis on serious sports
training at earlier ages, the incidence has increased (54). These fractures are most commonly reported in the tibia (50%), fibula (20%), pars interarticularis (15%), and femur (3%) (183).
Sports most commonly associated with stress fractures are running
(24%), basketball (13%), gymnastics (21%), football (9%), and ice
skating (15%) (132). Upper extremity stress fractures are seen in baseball, tennis, swimming, and gymnastics.
fracture is similar to that of an adult. Unlike the pain associated
with soft-tissue overuse injuries, stress fracture pain is minimal or
absent on arising in the morning, progressively gets worse with
activity, and is relieved with rest. In certain sites where the bone is
palpable directly beneath the skin, such as the tibia, fibula, or
distal radius, the athlete may be able to localize the pain to the
specific site of involvement. At other sites—hip, femur, or
humerus—pain localization may not be possible; the athlete vocalizes
only that the limb “hurts” with increased activity. On physical
examination, stressing the injured bone either by taping or applying
digital pressure or angular or rotational stresses generally reproduces
symptoms. There may be localized tenderness, warmth, or discoloration
at the fracture site, but these findings are more typically seen after
the athlete has been symptomatic for a while and may not be present in
the early stages of injury. Young children may have a low-grade fever.
be normal. Radiographic findings may take several weeks or months to
develop. In fact, if the athlete ceases activity when the symptoms
first begin, the injury may never be seen radiographically (132). However, technetium-99M phosphate bone scans are positive within 6 to 72 hours of injury (85,132).
The degree of uptake depends on the rate of bone repair and local blood
flow. Uptake is typically localized, but diffuse uptake patterns may
occasionally occur. Although they are highly sensitive, bone scans are
not specific because other conditions, including infection and tumor,
result in positive scans. Periostitis associated with inflammation at
the origin of the posterior tibial muscle (shin splints) may be
associated with a diffuse linear
uptake
pattern, which can be confused with a stress fracture. Imaging studies
must be correlated with the clinical history and physical exam.
Computed tomography (CT) scanning is less sensitive. Magnetic resonance
imaging (MRI) is extremely sensitive to early stress reactions
presenting as edema—that is, areas of low-intensity signal on T-1
weighted images that increase on T-2 weighted images. In advanced
stress reactions, T-2 weighted images demonstrate low intensity bands
continuous with the cortex, presumably representing fracture lines (10). Figure 97.1 presents Anderson and Greenspan’s imaging algorithm (10) for suspected stress fractures.
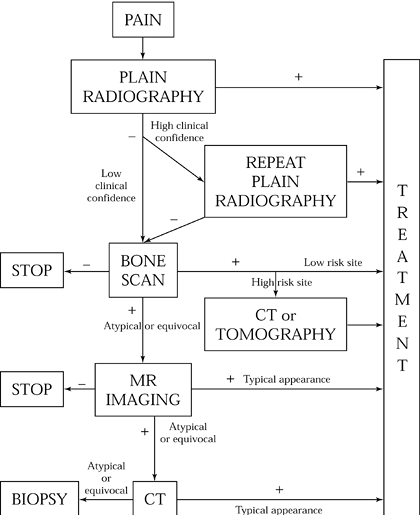 |
|
Figure 97.1. Imaging algorithm for suspected stress fractures of bone. (From Anderson MW, Greenspan A. Stress Fracture. Radiology 199:1;1996.
|
is to decrease stress to the injured area while maintaining a
conditioned state. The intact soft-tissue envelope (skin, muscle,
tendon, and ligament) provide support for the injured bone. Therefore,
crutches or a cane to decrease weight-bearing stress to the injured
extremity rather than a cast may be all that is needed. The key phase
in the treatment of stress fractures is to increase the stresses to the
bone gradually within the limits of comfort, advancing from
non-weight-bearing, nonimpact activities to weight-bearing, nonimpact
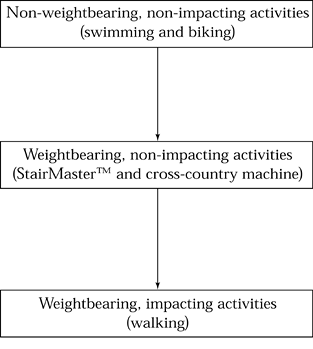
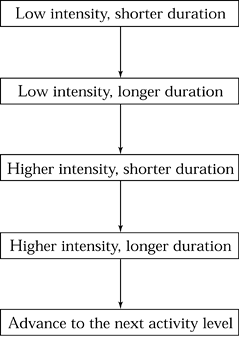
activities, and finally, to weight-bearing impact activities (Fig. 97.2). Progression of each new activity should be done as in Figure 97.3. As part of the treatment for a stress fracture, try to identify and correct any causative factors.
 |
|
Figure 97.2. Recovering from a stress fracture: progression of activities.
|
 |
|
Figure 97.3. Recovering from a stress fracture: progression within each activity
|
interarticularis fractures of the spine may take a prolonged time to heal (86). The former occur primarily in jumping sports (86,161). Some have recommended debridement and bone grafting for these fractures if union is delayed (10), whereas others have recommended electrical or ultrasound stimulation (172).
considered in the differential diagnosis of the young growing athlete
with low back pain, especially if the athlete is involved in sports
requiring repetitive hyperextension of the lumbar spine, such as
football, dancing, skating, and gymnastics (183).
On clinical examination, the athlete may not have pain on palpation of
the area of injury despite complaining of unilateral low back pain with
lumbar hyperextension. If radiographs are normal at the time of
diagnosis, early prolonged protection in an antilordotic orthosis may
result in healing in 4 to 6 months (54). This isthmic spondylolysis infrequently progresses to severe spondylolisthesis (183).
The diagnosis is made on the basis of history and physical examination.
For distal radial fractures, the most common physeal stress fractures,
the patient is typically a young male or female gymnast performing at a
high intensity with no history of a single traumatic event but
gradually increasing wrist pain. There is tenderness to palpation over
the distal radius. Initially, range of motion may be full, but as the
symptoms progress, range of motion decreases.
widening of the growth plate with cystic changes and marginal
irregularities, typically seen on the metaphyseal side of the plate,
can be seen in those athletes who continue to participate despite pain (41,127).
wrist when symptoms first begin and radiographs are normal, complete
recovery follows. If symptoms persist and treatment is delayed until
radiographic changes occur, time for healing is prolonged. Cast
immobilization may be needed for rest compliance. In cases in which
participation continues despite pain, a decrease in the growth of the
distal radius with continued growth of the ulna can result in a
positive ulna variance of between +0.35 and +0.68 mm (41,127).
stress fractures of the proximal humerus in Little League pitchers who
presented with vague pain during pitching. Radiographs, which were
initially normal, soon demonstrated a widened proximal humeral physis
and finally show callus with progressive healing. Complete healing took
approximately 6 weeks.
abnormalities that occur at the apophysis, at the joint surface, and at
the physeal plate. They have been defined by Siffert (189)
as “idiopathic conditions characterized by disorderliness of
endochondral ossification, including both chondrogenesis and
osteogenesis that comes upon a formerly normal growth mechanism” (Table 97.2). Some researchers believe that repetitive stress (microtrauma) is the primary precipitating factor (145) or at least a major contributing factor (64).
However, the etiology of the osteochondroses is still debatable;
vascular, hormonal, genetic, and metabolic factors as well as
infections have all been proposed as precipitating or contributing
causes. These conditions may merely represent abnormalities of
maturation. Because diagnosis typically depends on radiographic
confirmation (64,159,169), many of the osteochondroses carry the name of the individual who first described their radiographic appearance.
 |
|
Table 97.2. Siffert’s Classification of the Osteochondroses189
|
growth, are generally self-limited, and resolve without residual impairment (107).
Symptomatic treatment is appropriate in young athletes to try to
minimize morbidity and enhance recovery. Outcome studies justifying the
expense of such treatment are lacking, and one could argue, as did
Voltaire, that “the physician entertains the patient while nature cures
the disease.”
|
Table 97.3. The Nonarticular Osteochondroses (“The Apophysites”)
|
 |
|
Table 97.3A. Treatment of a Nonunion of an Olecranon Apophyseal Stress Fracture
|
“diseases of the growth or ossification centers in children that begin
as a degeneration or necrosis followed by regeneration or
recalcification” (25).
Stage 1 is reactive edema of the articular tissues and active hyperemia
of the metaphysis leading to decreased diffusion of nutrition to cells
of the epiphysis. Thrombosis and trabecular microfracture further
compromise the epiphysis, resulting in epiphyseal irregularities and
thinning at the subcortical zone (stage 2). Peripheral ingrowth of the
capillaries and mesenchymal cell differentiation are responsible for
repair with gradual replacement of necrotic tissue (stage 3).
protective immobilization of the injured area until symptoms resolve
and radiographic recovery is evident. In some cases, surgery is needed
to correct residual anatomic abnormalities that prevent normal
function. Two of the more common primary articular osteochondroses seen
in young athletes are Freiberg’s and Panner’s diseases (Table 97.4).
 |
|
Table 97.4. The Primary Articular Osteochondroses
|
involvement of the articular epiphyseal cartilage as a consequence of
loss of support from destroyed subchondral bone (189).
Included in this category are Legg-Calvé-Perthes syndrome, Köhler’s
disease, and osteochondritis dissecans, which in adolescent athletes
occurs most commonly in the knee, ankle, and elbow (184)
and can significantly impact athletic participation. Osteochondritis
dissecans (OCD) has been characterized as an “isolated segment of
epiphyseal and articular cartilage–covered necrotic bony centrum” (189)
occurring in diarthroidal joints. The etiology remains obscure, but OCD
is believed to be unlikely to be an inflammatory condition, even though
its name implies that it is (164). Many authors
favor a traumatic etiology, either a single traumatic event or
repetitive stress, resulting in either vascular compromise to a bony
segment or a subchondral fracture that fails to heal and therefore goes
on to develop avascular necrosis (39,52,75). Some believe that a vascular insult in the absence of trauma may occur (65).
Metabolic, hormonal, or genetic factors may enhance the vulnerability
of certain individuals to OCD. Age may also be a predisposing factor,
and a familial tendency has been found (74,155,208). Bilaterality has been reported as high as 33% in one series (87).
Although the etiology of this entity remains obscure, there is
agreement that the reparative process is more likely to occur before
cessation of skeletal growth, and in fact, repair is the likely outcome
in children; it may occur in adolescence, but it is rare in adults.
superficial joint surface. Focal avascular necrosis is followed by
reparative osteogenesis consisting of revascularization, granulation
tissue invasion, osteoclasis of necrotic fragments, ingrowth of
osteoid, and finally, remodeling of new bone. In the early stages of
the lesion, the overlying articular cartilage is normal, but later it
becomes softened, discolored, fibrillated, and fissured. Finally, from
lack of support of the underlying subchondral bone, it eventually
separates, leaving a bare area of bone that will ultimately fill with
fibrous tissue. Even before the fragment separates completely, if the
subchondral bone fails to heal but instead remains avascular, fibrous
tissue may develop beneath the fragment. If a healing response occurs
before cartilage collapse, the subchondral bone is characterized by
active cellular processes with vascular ingrowth and active
osteogenesis, providing once again a viable structural foundation for
the overlying articular cartilage.
was really first described in 1866 by Sir James Pagent, who termed it
“quiet necrosis,” a name that perhaps more typifies its pathophysiology
than does Koenig’s term.
knee is variable and nonspecific. The lesion may be noted in
asymptomatic athletes on review of radiographs taken for an unrelated
problem, may cause minor symptoms such as an ache in the knee
aggravated by activity, may result in a slight effusion, or if the
fragment separates, may cause frank locking or catching. The
symptomatic individual may recall a precipitating traumatic event,
often minor and not resulting in loss of time from sports or may recall
no such event, but typically patients have been active in sports and
exercise (36,175). In fact, participation
in sports at a young age has been implicated as an etiologic factor in the development of OCD (4,36,119).
the knee 90° and palpating for tenderness along the condyle. There may
be slight swelling, but no instability is present. Typically there is a
near full range of motion unless a fragment has become loose, resulting
in mechanical locking of the knee. Thigh muscle atrophy and externally
rotated gait may be noted. Pain may be elicited by flexing the knee to
90° and, with the tibia externally rotated on the femur, slowly
extending the knee to approximately 30°. Internally rotating the tibia
rapidly relieves this pain (Wilson’s sign) (219).
-
Age or, more specifically, status of the physis at the time of diagnosis (164)
-
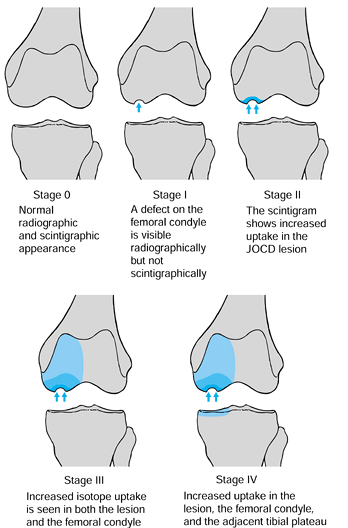
Scintigraphic appearance of the lesion, as correlated with radiographs (Fig. 97.4) (36)
![]() Figure 97.4.
Figure 97.4.
Classification of osteochondritis of the knee: scintigraphic
appearance, as correlated with radiographs. JOCD: Juvenile
osteochondritis dissecans. (Redrawn from Cahill BR, Berg BC.
99M-Technetium Phosphate Compound Joint Scintigraphy in the Management
of Juvenille Osteochondritis Dissecans of the Femoral Condyle. Am J Sports Med 11:329;1983.) -
Location on the femoral condyle (Fig. 97.5) (39)
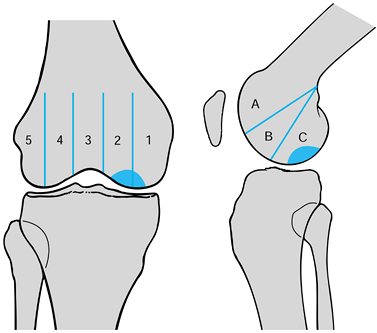 Figure 97.5.
Figure 97.5.
Classification of osteochondritis dissecans by its location on the
femoral condyle, as seen radiographically. (Redrawn from Cahill BR,
Berg BC. 99M-Technetium Phosphate Compound Joint Scintigraphy in the
Management of Juvenile Osteochondritis Dissecans of the Femoral
Condyle. Am J Sports Med 11:329;1983.) -
Appearance on imaging studies with contrast (MRI or CT) (112)
-
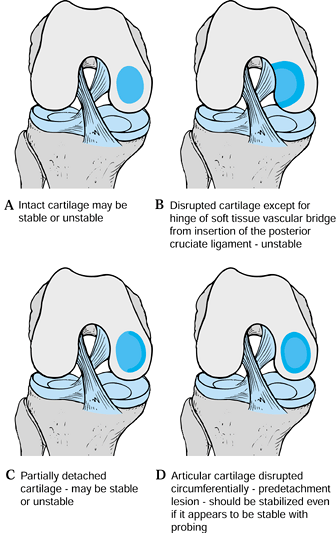
Intactness of the articular mantle and mobility of the fragment, as noted surgically (Fig. 97.6).
![]() Figure 97.6.
Figure 97.6.
Classification of osteochondritis dissecans of the knee by the
appearance of the articular cartilage and the mobility of the fragment.
(Redrawn from Cahill BR. Osteochondritis Dissecans of the Knee:
Treatment of Juvenile and Adult Forms. J Am Acad Ortho Surg 3:237;1995, with permission.)
OCD seems most critical in predicting outcome. Juvenile OCD, which
occurs in children or young adolescents with open growth centers, has a
higher probability of healing without the development of degenerative
changes than does adult-onset OCD (36,120). The prognosis and course of OCD diagnosed in late adolescence is similar to that in adults.
visible on routine radiographs of the knee. Tunnel views may be
required to enhance visualization. The lesion’s appearance is that of a
well-circumscribed area of subchondral bone, which may be sclerotic or
fragmented and is often separated from the femoral condyle by a
crescent-shaped radiolucent line.
Bone scans that measure osteoblastic activity and regional blood flow
can be used to evaluate the potential for fragment healing (39,124). However, Paletta et al. (162)
demonstrated that although quantitative bone scanning with
technetium-99M has a 100% predictive value for prognosis in patients
with a open physis, it is less reliable in predicting healing in
adolescents with a closed physis. Unfortunately, it is in the group of
adolescents with a closed physis in whom outcome is unpredictable, and
predictability by bone scans would be extremely helpful were it more
reliable.
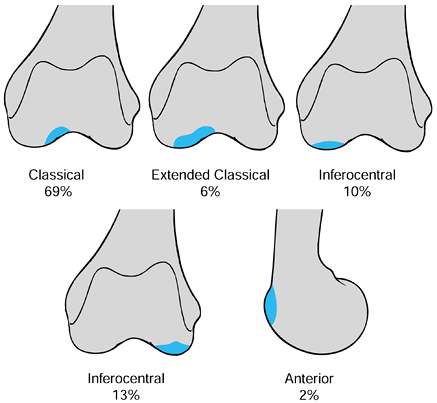 |
|
Figure 97.7.
Osteochondritis dissecans of the knee: common sites of occurrence. (From Linden B. The Incidence of Osteochondritis Dissecans in the Condyles of the Femur. Acta Orthop Scand 1976;47:664, with permission.) |
outline the lesion and to determine the intactness of the overlying
articular cartilage (112). Some authors argue
that loose bodies within the joint are better visualized with
arthrotomography rather than with MRI, although Mesgarzadeh et al. (140) reported adequate visualization of loose bodies with MRI.
remains controversial. Most would agree, however, that the ultimate
goal is to promote recovery of the subchondral bony defect while
preserving the intactness of the overlying cartilage. Early literature
is confusing because outcomes were not related to the age of the
patient at the time of onset (a major determinant of prognosis).
extremely helpful in trying to formulate a treatment plan. Many agree
that for symptomatic children with an open physis (juvenile
osteochondritis dissecans [JOCD]) in whom contrast arthrography
demonstrates an intact articular surface, nonoperative care is
reasonable (87,119,176,198).
For this group of patients, Cahill (36)
recommends activity modification initially using crutches for
non-weight bearing or partial weight bearing. He does not recommend
braces or casts. When acute symptoms subside, discard crutches and
institute recreational cycling, swimming, and lower extremity
strengthening exercises. Perform serial bone scans every 4 months; the
lesion is considered healed when bone scans revert to stage II lesions (Fig. 97.4). Cahill’s reported average time to healing is 10 months. Schenck and Goodnight (184)
support this scheme but would recommend splint, cast, or an immobilizer
during the early stages of treatment to increase compliance. They
suggest following healing with plain radiographs or occasionally serial
MRI in lieu of bone scans (36). The success rate with JOCD treated conservatively has been reported to be 50% (36). However, Pappas (164) stated that if all JOCD patients are subclassified into Pappas category I and II (Table 97.5), successful treatment in category I (children and young adolescents) is usually ensured without surgery.
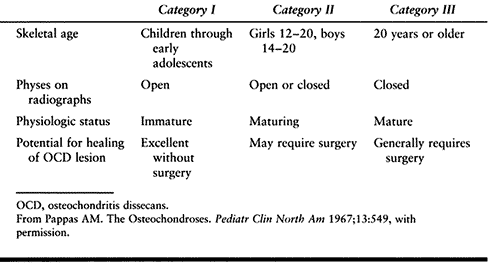 |
|
Table 97.5. Pappas’s Classification of Osteochondritis Dissecans of the Knee
|
conservative care fails to show progressive healing of the lesion on
serial bone scans or when the cartilage mantle becomes disrupted,
indicating instability of the fragment. How long conservative care
should be pursued if symptoms persist without improvement or
radiographs remain unchanged is debatable. Schenck and Goodnight (184) suggest 6 to 12 months of conservative care before considering surgical intervention.
noting “with the exception of earlier surgical intervention, the
principles of surgical treatment of OCD are identical of those for
JOCD”:
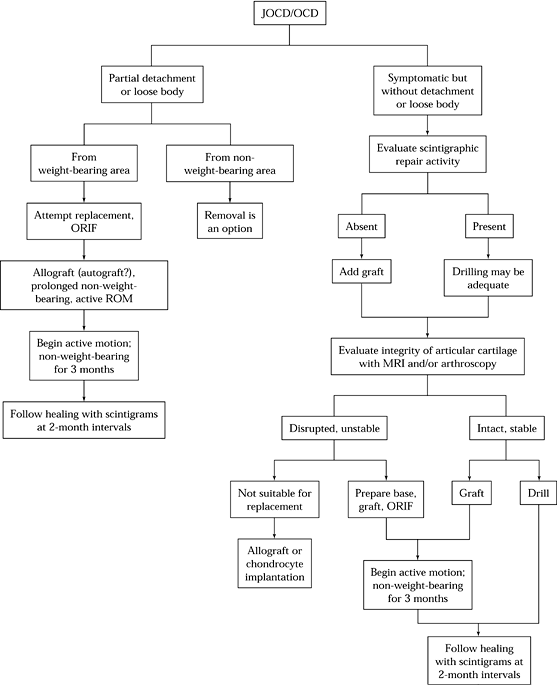 |
|
Figure 97.8. Algorithm for surgical treatment of osteochondritis dissecans (JOCD/OCD) of the knee. ORIF, open reduction and internal fixation; ROM, range of motion. (From Cahill BR. Osteochondritis Dissecans of the Knee: Treatment of Juvenile and Adult Forms. J Am Acad Orthop Surg 3:237;1995, with permission.)
|
-
Visualize the lesion, and remove or flip-up the fragment on a tissue hinge.
-
Clean and drill the base of lesion and fill the base with graft as needed.
-
Replace the lesion.
-
Bend the articular end of a smooth
0.062-inch pin to a right angle 1 mm from the end. Insert the pin
through the lesion and the condyle so it exits from the epicondylar
region. Insert the pin antegrade through the lesion and the condyle so
it exits from the epicondyle. Place the drill on the pin as it exits
from the epicondyle and drill until only a few millimeters of the pin
are visible on the articular distal end. -
Place a clamp on the proximal end of the
pin, and with a small slap hammer, seat the bent distal end into the
articular cartilage.
described a technique similar to that of Cahill but used 0.062-inch
K-wires threaded on one end. He inserted the pin antegrade with the
smooth end and drove the smooth end through the defect into the
condyle, exiting in the epicondylar region. The pin was then pulled
retrograde until the threaded end was flush with the articular surface.
Pins were removed in 4 to 6 weeks.
the blood supply; and (3) ensure stability of the bony fragment.
-
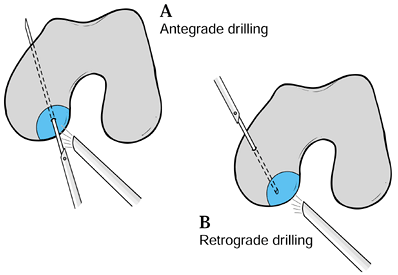
If a lesion is stable at the time of surgery and has an intact cartilage mantle, perform antegrade (7) or retrograde drilling under image visualization (90,198) to enhance the vascularity of the fragment (Fig. 97.9). Cahill (36)
believes that the intactness of the cartilage mantle is not critical,
and even if there is a partial articular defect, if the fragment is
stable with probing, no internal fixation is needed. Postoperatively,
with stable lesions that are drilled, progress weight bearing as
tolerated.![]() Figure 97.9. Schematic diagram of (A) antegrade and (B) retrograde drilling of osteochondritis lesions of the medial femoral condyle.
Figure 97.9. Schematic diagram of (A) antegrade and (B) retrograde drilling of osteochondritis lesions of the medial femoral condyle. -
If the lesion is unstable or partially
detached, remove the fragment from its base and curet the base of the
fragment as well as the base of the lesion to debride all fibrous
tissue. -
If there is a soft-tissue bridge at the
insertion of the posterior cruciate ligament on the femoral condyle,
preserve this potential vascular bridge by elevating the fragment on
this as a hinge rather than removing the fragment. -
Drill the dense bone of the crater to
enhance vascularity before replacing the fragment. In a partial or
totally detached lesion, crater fragment mismatch may exist either
because the fragment, nourished by joint fluid, grows larger than the
crater or, because with the loss of subchondral bone, the fragment is
too small. -
If the fragment is too large, sculpt it
to fit the crater; if the crater is too large, graft the crater’s base
with local bone from the metaphyseal area of the tibia to provide
support for the fragment (36,184,187).
Take care to match the crater to the fragment as accurately as possible
because inadequate reductions of the articular surface yield poor
results (87).
believes that metallic fixative devices may adversely affect the
cartilage surface and prefers smooth pins over screws. Initially a
sterile synovitius was observed with biodegradable self-reinforced
polyglycolide rods and polylactic acid pins. This has not been seen
with the newer biodegradable pins and screws made of the slower
absorbing polylactide (133).
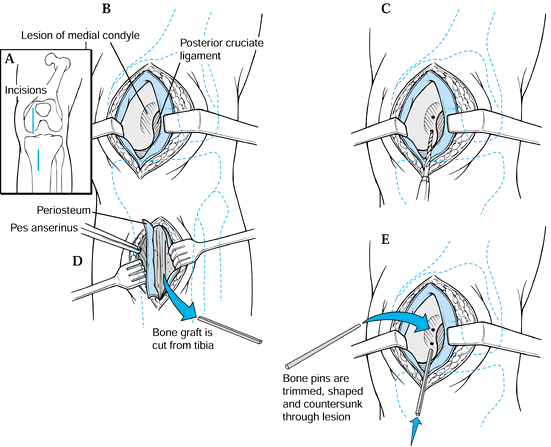 |
|
Figure 97.10. Bone peg fixation of OCD lesion. A: Make two anteromedial skin incisions. B: Expose the lesion on the medial condyle. C: Place drill holes in the fragment across the lesion. D: Harvest bone graft from the tibia and fashion bone pins. E:
Insert bone pins into the fragment to fix it. (Redrawn with permission from Gillespie HS, Day MD. Bone Peg Fixation in the Treatment of Osteochondritis Dissecans of the Knee Joint. Clin Orthop 143:126;1979, with permission.) |
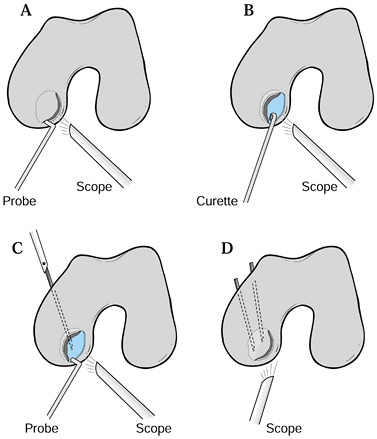 |
|
Figure 97.11. Authors’ preferred method for treating unstable partially detached lesions of the medial femoral condyle. A: Visualize and probe lesion. If unstable, hinge open lesion, preserving intact cartilage rim. B:
Curet base of lesion and drill or pierce retrograde with arthroscopic awls or picks, taking care to perforate but not destroy subchondral plate. C: Replace lesion using bone if needed from femoral condylar region obtained percutaneously or with a hollow core drill from proximal tibial metaphysis. D: Stabilize the lesion with K-wire drilled antegrade until just piercing the articular cartilage surface, cutting pins beneath the skin or stabilized with PDS pegs by Johnson & Johnson placed retrograde under arthroscopic visualization.Postoperative care: Immobilize the knee for 3 to 5 days and then brace to allow full range of motion and begin strengthening exercises. No weight bearing is permitted for 8 to 10 weeks until radiographic healing is apparent. |
open procedures. The decision of which technique to use depends on the
ability of the surgeon to approach the fragment arthoscopically.
-
If nonbiodegradable pins are used for fixation, they are generally removed at 3 to 8 weeks postoperatively (36,123). Postoperative care includes early range of motion, with strict adherence to non-weight bearing until the fragment is healed.
-
Consider removal of only small fragments (184), fragments on the non-weight-bearing articular surface (36),
or fragments that pose a significant fragment-crater mismatch.
Treatment of the crater remains controversial. Doing nothing but
fragment removal has yielded poor long-term results, even though
short-term outcomes are generally good (8). -
The defect can be drilled or “picked” with arthroscopic awls (28)
used to create microfractures in the subchondral bone to enhance
vascularity of the bed and thus promote the development of type I
fibrocartilage. -
Stabilize the edges of the defect by trephining (90) or spongialization (187) rather than saucerization (Fig. 97.12).
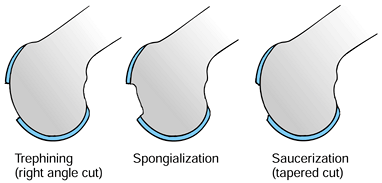 Figure 97.12.
Figure 97.12.
Methods of dealing with articular cartilage defects. (Redrawn from
Clanton TO, DeLee JC. Osteochondritis Dissecans—History,
Pathophysiology, and Current Treatment Concepts. Clin Orthop Rel Res 167:50;1982, with permission.) -
Other options include filling the defect
with a paste of mortilized autologus cartilage and bone taken from the
joint (Construct Chondral Repair System by DePuy [207]), core autografts harvested with one of the several commercially available systems (MosiacPlasty by Smith and Nephew [92], OATS by Arthex [30], and COR System by Innovasive), or autologous cell culture grafting using the Genzyme technique (34).
Stone was the first to use the term OCD for lesions in the ankle.
Whether the ankle lesion is the same pathologically as that in the knee
is debatable.
reviewed the literature on osteochondral lesions of the talus (191
lesions in 183 patients) and concluded that these lesions, whether they
appeared
on the medial (57%) or the lateral (43%) side of the talus, were
trauma-induced transchondral fractures. Canale and Belding (42),
in their survey of 31 lesions, found all lateral lesions were secondary
to trauma, whereas only 9 of 14 medial lesions were associated with
trauma.
symptoms and a greater association with later degenerative changes.
Roden et al.’s (179) data reinforced Canale and
Belding’s observation that medial talar lesions can heal spontaneously,
have few symptoms, typically are not associated with degenerative
changes, and may not all be trauma related, whereas lateral lesions are
by and large all traumatic (69,131).
and concluded that the radiographic stage did not necessarily correlate
with the severity of hyalin cartilage injury (arthroscopic grading).
They recommended using the arthroscopic rather than radiographic
appearance for treatment decisions.
 |
|
Table 97.6. Treatment of Osteochondritis Dissecans of the Talus Based on Berndt and Harty’s Classification System21 *
|
 |
|
Figure 97.14. Pritsch’s classification of osteochondritis dissecans of the talus based on the intactness of the articular surface.
|
radiograph. A mortise view taken in plantar flexion highlights
posteromedial lesions, whereas anterolateral lesions are best seen if
the mortise view is done in dorsiflexion (206). Anderson et al. (9) recommends bone scans on patients in whom osteochondral lesions are suspected but routine films are negative.
provide accurate information on fragment size and location, and
correlate fairly well with arthroscopic evaluation of cartilage
congruity and fragment stability (9,66).
intact lesions; open reduction internal fixation of detached lesions;
and fragment excision and curettage, abrasion, or drilling of the base.
Recently, autologous bone grafting techniques developed for
osteochondritis of the knee have been tried for OCD lesions of the
ankle. Lateral lesions are fairly easy to approach arthroscopically,
whereas approaching medial lesions arthroscopically may pose some
difficulty (66,170). Some authors prefer a medial malleolar osteotomy for visualization of medial lesions.
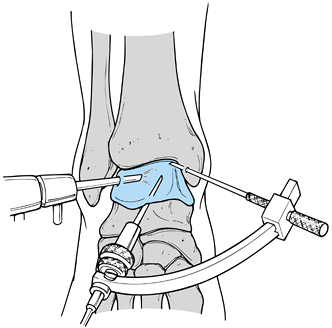 |
|
Figure 97.13. Ankle arthroscopy for osteochondritis dissecans of the talus. (From Stone JW. Osteochondral Lesions of the Talar Dome. J Am Acad Orthop Surg 1996;4:63, with permission.)
|
-
Inspect the joint, looking in all gutters as well as anteriorly and posteriorly for loose bodies.
-
For anterolateral lesions, place the
scope in the anteromedial portal, inserting instruments through the
anterolateral portal. Probe the lesion to examine the intactness of its
edges and base. Trephine the edges and debride the base of the crater
with a ring curet. Use 0.062-inch smooth K-wires to drill the lesion.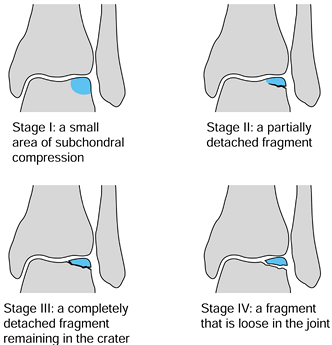 Figure 97.15. Berndt and Harty Classification of OCD of the talus (see Table 97.6).
Figure 97.15. Berndt and Harty Classification of OCD of the talus (see Table 97.6). -
For anteromedial lesions, place the scope
through the anterolateral portal and visualize the lesion through the
anteromedial portal. The lesion may be difficult to reach for curetting
and drilling if it is located far posteriorly. However, with plantar
flexion of the foot, most lesions are accessible. -
If the lesion cannot be reached through
the anteromedial portal for drilling, visualize the lesion
arthroscopically, but insert the K-wires through the medial malleolus
down into the lesion while plantarflexing and dorsiflexing the foot to
aid in visualizing and drilling the lesion. This technique does violate
the intactness of the ankle’s articular surface. -
Alternatively, while watching
arthroscopically, use a drill guide to drill transtalarly into the
lesion, entering from the sinus tarsi into the talus in its
nonarticular portion anterior to the anterior talofibular ligment
insertion area.
advises immobilizing the ankle for 6 weeks and then beginning active
and passive motion exercises. Others immobilize the ankle for only 7 to
10 days and then start active range of motion and strengthening
exercises, with weight bearing being delayed for 6 to 8 weeks. See
section on ankle arthroscopy for additional procedures.
capitellum but occasionally also seen in the radial head) is part of
the injury complex popularly termed “Little League elbow.” This term
was originally used by Brodgon and Crowe (35)
to describe clinical and radiographic changes on the medial side of the
elbow in young baseball pitchers, but was expanded by Adams (3) to all the pathologic processes that occur about the elbow secondary to the stresses of pitching or throwing (Fig. 97.16),
including not only medial tension injuries but also lateral compression
and posterior fossa injuries. Repetitive medial tension forces on the
growing youth’s elbow include medial apophysitis, avulsions of the
medial condyle, accelerated growth, or fragmentation of the medial
epicondyle. Repetitive lateral compression forces are thought to be
responsible for the development of OCD of the capitellum and radial
head. The valgus position of the elbow as it is forcefully propelled
forward by the shoulder with the wrist in a supinated position during
the acceleration phase of pitching is the mechanism blamed for the
development of these tension and compression loads (5) (Fig. 97.17). Children who pitch with a side-arm delivery are therefore more likely to develop symptoms (197).
 |
|
Figure 97.16. The five phases of pitching.
|
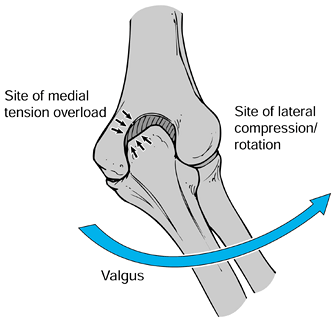 |
|
Figure 97.17. Tension and compression loads on the elbow during acceleration phases of pitching. Right elbow, posterior aspect.
|
the posterior medial sheer force, which develops during late cocking
and from the hyperextension that develops during follow-through. Such
forces result in posterior medial spurs, olecranon spurs, stress or
avulsion fractures of the olecranon apophysis (see the section on nonarticular osteochondroses), and traction spurs of the coronoid process.
Changes similar to those that occur in Little League elbow have been
found in other throwing athletes (javelin throwers and tennis players)
as well as gymnasts, the latter presumably from weight bearing on an
extended valgus elbow.
11 and 16 years of age and may complain only of decreased throwing
performance but more typically complains of pain with activity that
improves with rest. If the athlete continues to play with pain, the
pain may last after playing hours. Night pain is rare. The pain may be
diffuse but is generally limited to medial, lateral, or posterior
aspects of the elbow. There may be a lack of full extension, and this
may be the only complaint. Flexion is rarely limited.
extension, there may be a mild effusion or localized swelling over the
medial epicondyle. Occasionally, muscle atrophy is apparent.
Crepitation with motion can be present if there are loose bodies.
traction on the ulnar nerve may result in altered sensation in the ring
or little fingers. Rarely is there a lack of strength in the intrinsic
muscle.
 |
|
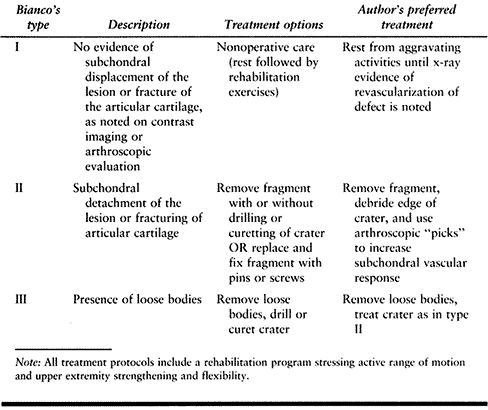
Table 97.7. Treatment Options for Osteochondritis Dissecans of the Capitellum Based on Bianco’s Classification System25,152
|
well-defined sclerotic border (Fig. 97.18).
Loose bodies may or may not be identified. In the symptomatic athlete
with normal radiographs or in the athlete whose lesion is seen on
routine radiographs but in whom there is a question regarding the
integrity of the cartilage mantle or the presence of loose bodies,
arthrotomography or contrast MRI may be extremely helpful. In chronic
cases of Little League elbow, routine films may also show an enlarged
or beaked medial epicondyle, fragmentation or separation of the medial
apophysis, an enlarged radial head, or fragmentation or widening of the
olecranon epiphysis.
 |
|
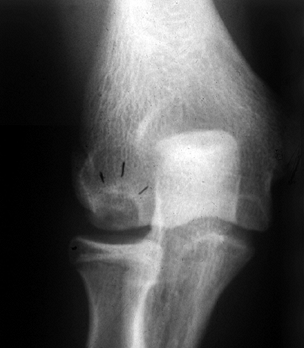
Figure 97.18. Radiograph of 15-year-old pitcher. Note osteochondritis lesion of the capitellum.
|
demonstrates an intact lesion, treat with cessation of the offending
activity; range-of-motion exercises; and stretching and strengthening
the muscles of the shoulder, arm, forearm, and wrist. Give particular
attention to stretching the anterior elbow capsule. A cast or a splint
may be needed for several weeks to enhance compliance with ceasing
activity. Limit resistance weight training for 6 to 12 weeks. When
radiographic revascularization and healing is evident on repeat serial
x-ray studies, begin a gradual return to a
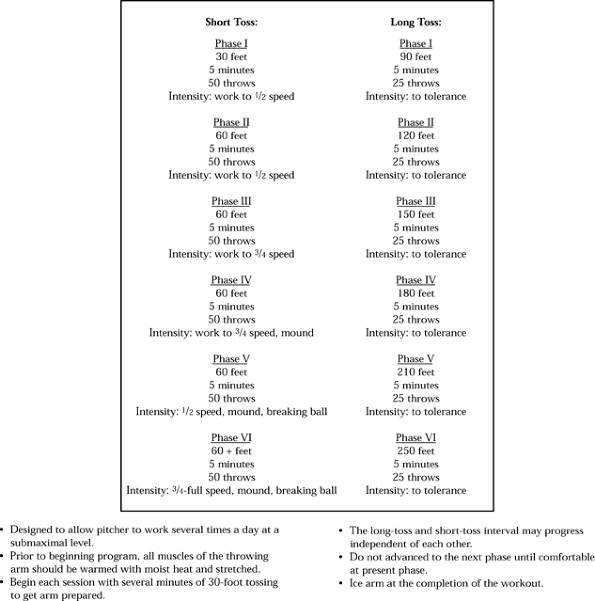
throwing program, with emphasis on proper technique (Fig. 97.19).
 |
|
Figure 97.19. An example of gradual return to throwing program.
|
place limits on the number of pitches thrown both in practices and
games. A change of position for pitchers is advised, even if only on a
temporary basis. The prognosis is favorable for adolescents who have
lucent subchondral defects with intact overlying cartilage. Return to
sports typically occurs within 6 months to 1 year but may be earlier
based on resolution of symptoms and radiographic healing (60,190).
using unthreaded K-wires placed antegrade into the lesion and through
the lateral condyle. The K-wires are left either under the skin or just
above it to be removed in 6 to 8 weeks. Others (152,190) recommend surgery
only for loose fragments that cause locking or catching. The three options available for treating loose fragments are (1) to replace the fragment using bone graft if needed, (2) to remove the fragment and either drill (or drill and curet) the base (most authors [136,164,190] recommend preservation of the subchondral plate when curetting), and (3) to simply remove the fragment (152,221).
the lesion yields poor results and 85% of those treated with a
well-structured rehabilitation program following excision and drilling
(or drilling and curetting) are able to throw successfully in 6 months
to a year (136). Younger patients typically
have a more favorable outcome no matter what stage of the lesion they
have at the time of diagnosis.
 |
|
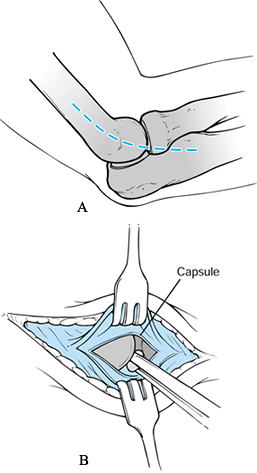
Figure 97.20. McManama et al.’s treatment of osteochondritis dissecans of the capitellum. A: Superficial incision. B:
Deep incision exposing joint. (From McManama GR, Micheli LJ, Beary VM. The Surgical Treatment of Osteochondritis of the Capitellum. Am J Sports Med 1985;12:11, with permission.) |
-
Make a lateral incision by beginning 1 to
2 cm proximal to the lateral epicondyle of the humerus. Extend the
incision distally over the epicondyle and along the posterolateral
surface of the forearm for 4 to 5 cm. Proximally, the deep incision
should enter through the triceps posteriorly and the brachioradialis
and extensor carpi ulnaris anteriorly to expose the lateral condyle and
the capsule covering the radial head. Distally, the deep incision
should separate the extensor carpi ulnaris from the anconeus. -
Reflect the periosteum off the anterior and posterior surfaces of the humerus.
-
Subperiosteally, reflect the common origin of the extensor muscle anteriorly off the lateral epicondyle.
-
Open the joint capsule longitudinally. Explore the joint for loose bodies.
-
Debride the capitellar chondral defects down to subchondral bone.
-
Use a smooth K-wire to make multiple drill holes into the subchondral bone to obtain bleeding.
lesion is drilled arthroscopically, use a sling for approximately 3
weeks. Begin range-of-motion and strengthening exercises at about 10 to
14 days. If an open procedure is performed, immobilization in a splint
may be needed for several weeks before beginning the rehabilitation
program. The decision to approach the lesion arthroscopically or open
depends on the surgeon’s ability to see and treat the lesion
arthroscopically.
previously thought to be rare but are now more common and occur almost
exclusively during athletic activity (171).
Secondary ossification centers remain open in different parts of the
skeleton between 14 and 25 years of age, and the unification time for
each particular apophysis correlates with the average age of occurrence
of avulsion fractures at their respective locations (216). Longitudinal bone growth stimulates muscle tendon growth (38,114),
but there is often a lag time for muscle tendon unit growth, resulting
in a period of relative inflexibility of these soft tissues relative to
the adjacent skeletal tissue. This occurs during or immediately after
periods of rapid growth and also coincides with periods of relative
weakness at the physis. This relative inflexibility is particularly a
problem for muscle-tendon units that cross two joints, such as the
rectus femoris, sartorius, hamstrings, gastrocnemius, iliotibial
band,
and iliopsoas. The great majority of avulsion fractures occur in
children who are constitutionally tight and have just finished a growth
spurt (146,149).
Avulsion fractures are seen most commonly in ballistic sports such as
soccer, track and field, and jumping sports in which a sudden violent
contracture of a muscle tendon unit can lead to avulsion of the
apophysis (118,146,149).
Adolescent athletes who participate in running or jumping sports tend
to be less flexible than age-matched controls, and flexibility is
gained slowly (110). The mechanism of avulsion tends to result from either excessive stretching or a violent contraction (45)
of the adjacent muscle tendon unit. Although conservative management is
often successful, these fractures must be differentiated from neoplasm
or normal sesamoid bones.
The amount of displacement seen in these avulsion fractures is related
to the degree of initial injury and to the associated soft-tissue
attachments that may tether the avulsed fragment. The anterior superior
iliac spine avulses secondary to extensive pulling of the sartorius,
usually with the hip extended and the knee flexed (177). There is pain and swelling over the anterior superior iliac spine, and the average age of presentation is 15 years (178).
Radiographs show displacement of the anterior superior iliac spine
apophysis. Treat with bed rest, followed by progressive weight bearing
on crutches for approximately 4 weeks. Surgery is rarely required, even
if displacement is noted, and the athlete may return to sports after 2
to 3 months as pain allows (45).
 |
|
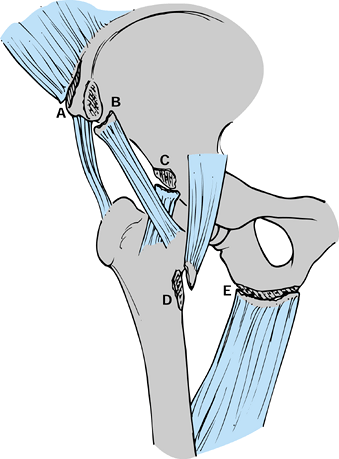
Figure 97.21.
Pelvic avulsion fractures in the growing athlete include the anterior superior iliac spine (A), anterior inferior iliac spine (B), ischial tuberosity (E) and more rarely the anteroinferior iliac spine (C) and lesser trochanter (D). These injuries occur around periods of rapid growth, but displacement of these fragments is often limited by adjacent soft tissues. |
to excessive traction by the direct head of the rectus femoris, usually
with the hip hyperextended and the knee flexed (Fig. 97.21). This injury typically occurs in kicking sports such as soccer, rugby, and football (215).
This avulsion is much less common than that of the anterior superior
iliac spine and occurs in a younger age group, averaging 13 years,
reflecting the earlier age of fusion of this apophysis (171,216). Pain from this lesion is often difficult to localize (67),
but patients will commonly be most comfortable in a position that
relaxes the rectus femoris by keeping the hip flexed and knee extended.
Radiographs may reveal minimal displacement of the anterior inferior
iliac spine, and this fragment must be differentiated from an os
acetabuli or acetabular epiphysis (215).
Further displacement of this fragment is typically limited by the
tethering effect of the reflected head of the rectus femoris. These
respond well to bed rest, followed by progressive mobilization on
crutches, with a fairly rapid return to sports as pain allows (44,45).
rapid traction on the hamstring muscles either with violent contraction
or a sudden excessive passive stretch (1,22,55,108,149) (Fig. 97.21). This epiphysis is not ossified until 15 years of age and often is not united with the pelvis until 25 years of age (105).
This fracture occurs in an older age group averaging 15 years of age
and is usually sports related. Patients present complaining of
acute-onset of pain in the ischium or chronic pain with a sudden
exacerbation of symptoms (180). On physical
examination, pain can be elicited by passive straight leg raising or
abduction of the hip. Patients often notice the pain while sitting, and
radiographs show a variable amount of displacement of
the
ischial apophysis. Wide displacement tends to be limited by the
sacrotuberous ligament. Treatment of this lesion is controversial
because wide displacement of the ischial tuberosity can lead to
excessive callous formation, which causes pain when sitting (29). This problem rarely requires excision (180) but has led some authors (145,148,149)
to recommend open reduction and internal fixation to avoid fibrous
union, pain, and prominence. The vast majority of these cases can be
treated successfully with conservative management with a period of bed
rest, followed by progressive ambulation on crutches and slow
advancement to sport as pain allows (108). In
the absence of a characteristic history, periosteal reaction and
callous formation in the area of the ischium must be differentiated
from neoplasm or infection, and biopsy must be considered (94,98).
and avulsion fractures of the medial epicondyle represent almost 12% of
all fractures about the elbow in children. The peak age of occurrence
is between 11 and 12 years, and boys outnumber girls 4 to 1 (6,18).
This injury can follow a prodrome of medial elbow pain from apophysitis
and overuse or may follow a single violent injury to the elbow.
Ossification of the medial epicondyle occurs between 5 and 7 years of
age, and the epicondyle continues to enlarge and finally fuses to the
humerus at approximately 14 years of age in girls and 17 in boys (165).
This is the last secondary ossification center to fuse in the distal
humeral metaphysis. Two mechanisms for medial epicondylar avulsion have
been proposed: Avulsion can occur by (1) violent contraction of the flexor pronator mass or (2)
elbow dislocation associated with valgus stress in which the ulnar
collateral ligament provides the avulsion force. Fracture by direct
blow to the elbow is thought to be extremely rare. In younger children,
a fall on the outstretched hand with the wrist extended tightens the
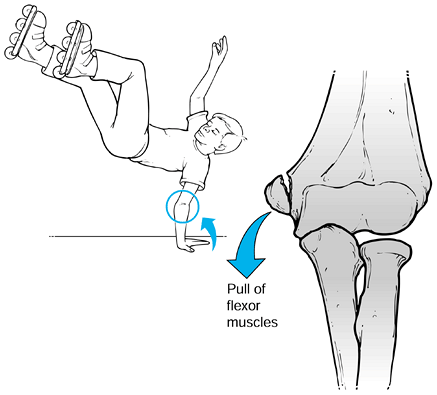
flexor pronator mass and may result in this avulsion (Fig. 97.22). In the older adolescent athlete, the most common cause is throwing a baseball with sudden contracture of the forearm muscles.
 |
|
Figure 97.22.
The usual mechanism for avulsion of the medial epicondyle in the child is a fall on the outstretched hand with the elbow in extension and wrist held in hyperextension. The flexor pronator mass, which inserts on the medial epicondyle, avulses this fragment of bone. Adapted from ref. 178. |
and they are age dependent. Type I fractures with avulsion of a large
fragment involving the entire epicondyle occur in younger children.
This fragment may be displaced and malrotated. In the older adolescent
patient, a small fracture fragment of medial epicondyle occurs and may
be associated with avulsion of the anterior oblique portion of the
medial collateral ligament. This fragment can be displaced distally and
incarcerated in the joint. Medial epicondylar fractures can be either
nondisplaced, minimally displaced (less than 5 mm displaced),
significantly displaced (greater than 5 mm displaced), or entrapped, in
which case the fracture fragment is typically seen at the joint line on
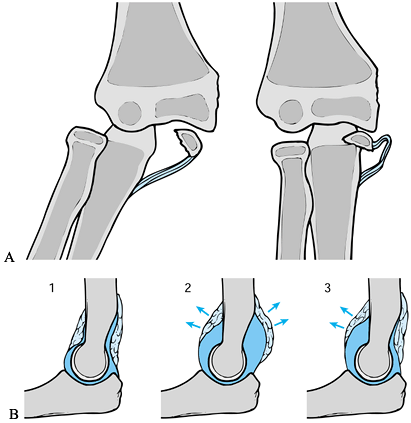
x-ray study (178) (Fig. 97.23A).
 |
|
Figure 97.23. A: Intraarticular displacement of the medial epicondyle and entrapment within the joint after reduction of dislocation of elbow. B:
Intraarticular effusion at the elbow joint will lead to displacement of anterior and posterior fat pads, which is often easily visible on the lateral x-ray study of the elbow.1. Normal fat pads. 2. Displacement of anterior and posterior fat pads. 3. Isolated displacement of anterior fat pad. |
and swollen with ecchymosis over the medial epicondylar region and
decreased motion due to pain and effusion. Point tenderness is noted at
the flexor origin over the medial epicondyle, and significant valgus
instability may be present. Check for function of the ulnar nerve (136,220).
humerus may reveal only widening or irregularity of the apophyseal
line. A displaced fragment is difficult to visualize on radiographs but
may be missing from its usual location. The fat pad (Fig. 97.23B)
sign is typically not seen because in older children this fragment is
extra-articular, and in younger children this fracture is associated
with rupture of the capsule and the dissemination of the joint effusion
into the soft tissues. Fracture of the medial condyle, which is an
intraarticular injury, is less common and can be ruled out on
radiographs. Radiographs taken with the arm held in an abducted
supinated position, which
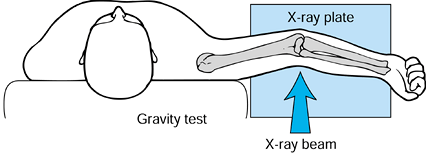
causes a valgus force at the elbow, will show any instability (186) (Fig. 97.24).
 |
|
Figure 97.24.
The weight of the forearm exerts a gentle valgus stress on the injured elbow and reliably demonstrates valgus instability secondary to ulnar collateral ligament injury or medial epicondylar avulsion. |
fractures of the medial epicondyle consists of a period of
immobilization for several weeks, followed by gradual resumption in
range of motion and return to throwing as pain allows (23,136).
Incarcerated fragments require open reduction and internal fixation.
This surgery usually results in closure of the growth plate, but
because this epiphysis does not contribute to longitudinal growth of
the humerus, growth disturbances are not significant. Treatment of
displaced fractures of the medial epicondyle are controversial, and
operative management is recommended primarily in throwing athletes (220). Nonoperative management in other children usually has good results despite the fact that fibrous union may occur (17,23,71).
Ulnar nerve symptoms represent another indication for operative
exploration. Even patients with minimally displaced fragments should be
reevaluated for valgus instability 6 weeks following injury because
fixation may be required in cases in which valgus stability is
important.
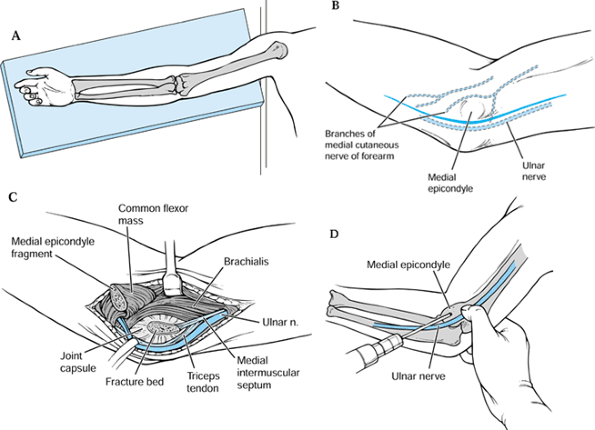 |
|
Figure 97.25. Open reduction and internal fixation of medial epicondyle fractures. A: Position arm on a radiolucent table. B: Expose the medial epicondyle through a longitudinal incision. C: Identify the fracture and reduce it. D: Fix the fracture with one or more K-wires.
|
-
Place the patient supine on the operating table, with the affected arm abducted onto a hand table (Fig. 97.25A).
-
Incise the skin along the medial aspect of the elbow centered at the elbow joint (Fig. 97.25B).
-
Identify the fracture fragment and remove fracture hematoma (Fig. 97.25C).
-
Reduce the fragment and fix temporarily
with K-wires. Fixation with one 3.5 mm screw is adequate if the bone
fragment is large enough. Multiple K-wires can be used for smaller
fragments. Lysis of adhesions around the ulnar nerve may be performed,
but ulnar nerve transposition is typically not required (Fig. 97.25D).
The average age of presentation of tibial tubercle avulsion is 14
years, with a strong male predominance. Because this injury may occur
near the age of physiologic epiphysiodesis, growth problems following
treatment are rare. Whereas Osgood-Schlatter disease is usually
insidious, with mild symptoms creating partial disability, tibial
tubercle avulsion typically occurs as an acute injury with marked pain
and swelling and inability to stand or walk. Fractures are usually
displaced, requiring surgical intervention. Avulsion of the tibial
tubercle by the patellar ligament occurs with violent contraction of
the quadriceps against the fixed tibia, as in jumping, or by acute
passive flexion, as in landing, and accounts for the fact that this
injury is most commonly seen with jumping or running sports (57,72,109). Children with tight hamstrings, pre-existing tibial apophysitis, and patella baja are at risk for this injury.
-
Acute swelling and tenderness is present, often associated with a kind effusion or tense hemarthrosis.
-
A freely movable fragment at the tibial tubercle, and proximal patellar displacement is usually present.
-
With a type I fracture, active knee
extension may be possible, but in more displaced fragments, straight
leg raising is not possible. -
A lateral radiograph of the knee is diagnostic and guides both classification and further treatment.
in extension and molding over the proximal pole of the patella to
decrease stress on the tibial tubercle. Minimal displacement, however,
is rare, and displaced fractures of the type II or type III variety
require open reduction and internal fixation, usually through a
vertical incision. Larger fragments require the use of a screw, and
small fragments can be treated with multiple pins with repair of the
periosteal rupture (Fig. 97.26).
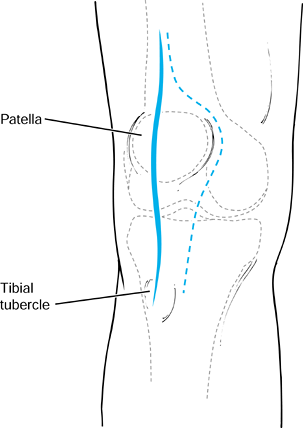 |
|
Figure 97.26. Incision for open reduction and internal fixation of tibial tubercle avulsion fractures.
|
-
Place the patient supine on the operating
table and make a vertical incision centered over the tibial tubercle
along the medial or lateral border. -
Remove any soft-tissue interposition at the fracture site and reduce the fracture fragment.
-
If the fragment is large enough, fix this
fragment with a cancellous screw extending through the tubercle and
into the metaphysis. -
If comminution of the fragment is
encountered, use multiple threaded Steinmann pins and reinforce these
using periosteal sutures.
molded on either side of the tibial tubercle and proximal patella to
reduce tension at the fracture site. Allow the patient to walk full
weight bearing in a cylinder cast for the first month. Allow gentle
range-of-motion exercises and quadriceps-strengthening exercises after
a month with continued protected ambulation, and add resistive
exercises when the tibial tubercle is no longer tender and healing is
noted on x-ray study.
with a return to sports possible in the vast majority of patients. No
significant deformity usually results in acute cases (24,109,128).
Although genu recurvatum can occur in skeletally immature athletes,
tubercle avulsion usually occurs close to skeletal maturity, so this
complication is rare. Loss of knee flexion and persistent quadriceps
atrophy occurs rarely (178).
but considered rare. Reports of the incidence of anterior cruciate
ligament (ACL) injuries in those younger than 14 to 15 years of age
vary from 3.4% (122) to 10% (59),
but the number or injuries appears to be increasing as children and
young adolescents become more competitive in sports. The reported
incidence of meniscal lesions associated with ACL injuries in children
and adolescents is also variable. McCarroll et al. (134) reported a 60% rate of meniscal injury, and Staninski (203) reported a 47% incidence, but others (12,58,83) have found the meniscus less frequently injured in ACL injuries in children than in adults.
evaluation in children, some authors have recommended early evaluation
under anesthesia. However, others believe that if adequate time is
taken to win the confidence of the injured adolescent or child, a
reasonable evaluation can be accomplished.
associated with a pop and severe pain followed by knee swelling and
lack of extension can also be consistent with an episode of patella
subluxation. Therefore, palpate the medial patella retinaculum for
tenderness and manipulate the patella in the trochlear groove, noting
its degree of movement and the patient’s apprehension during the
examination.
youngsters with an ACL injury for maturity by noting chronologic age,
bone age as determined by left-hand anteroposterior (AP) radiographs in
comparison to known standards, sexual development (e.g., Tanner
classification; Table 97.8), growth remaining
via assessment of parents’ and siblings’ heights, patient’s projected
height on basis of prior growth chart data (if available), and the
extent of physeal closure on routine radiographs.
 |
|
Table 97.8. Tanner’s Stages of Sexual Development*
|
physis and to exclude associated fractures or osseous congenital
variations. Obtain stress radiographs as needed to rule out physeal
injuries. MRI may be helpful in determining the extent of injury to the
ligament and the status of other intraarticular structures (58,82).
Take care in interpreting meniscal tears on MRI because a high
incidence of false-positive results in children has been described (201).
a difficult management problem. Most natural history studies of ACL
injuries in children have deficiencies (12,32,83,104,168,195). Associated meniscal injuries are frequently not documented, and the maturity of the child is infrequently noted (14,83). Some authors have reported reasonable results following conservative measures (12,50),
but most authors have reported a high incidence of reinjury (83,106,195).
restraints and areas of cartilage contusion to heal. Identify
associated meniscal injuries and treat as in adults with repair or
resection. Implement a well-structured rehabilitation program to
restore range of motion and strength as well as develop balance skills
and proprioceptive feedback pathways. Teach youngsters proper jumping
and landing techniques to prevent future reinjuring (19). Although it has not been well documented to eliminate instability, bracing can be clinically useful.
reconstruction of the anterior cruciate ligament and the type of
procedure chosen for the adolescent who is nearing growth completion
(Tanner’s stages 3, 4, or 5 with closing physes) follows guidelines
established for adults. In the child or preadolescent (Tanner’s stages
1 or 2 with widely open physes), the decision becomes more difficult
because premature femoral or tibial physeal closure can result in a
significant leg length discrepancy, especially in a young child.
unstable ACL-deficient knee in the child is often poor. Recurrent
episodes of pivoting result in meniscal and articular cartilage injury,
often leading to early degenerative changes (83,135,134,150,151,167). However, placing hardware or even drilling tunnels across an open physis carries the risk of causing premature closure (52,122,134).
Bergfeld, in an attempt to use an intraarticular reconstruction but
avoid the physeal plate, developed what has been called the “tomato
stake” procedure (167), which is performed as follows (Fig. 97.27):
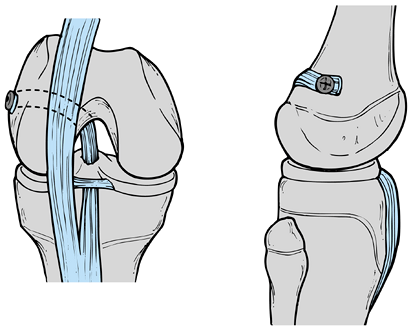 |
|
Figure 97.27. Bergfeld’s “tomato stake” procedure. (Adapted from ref. 58.)
|
-
Make a midpatella tendon incision.
-
Isolate the middle third of the patella tendon, leaving it attached at the proximal tibia.
-
Pass the tendon over the front of the tibia, beneath the transverse ligament, through the knee, and around the top of the femur.
-
Fasten the tendon to the femur above the physis with a staple; do not violate the physis with the staple.
-
The stump of the ACL can be used to augment the reconstruction.
this technique by creating a groove in the tibia and femur to make the
tunnel more isometric and also added an extraarticular iliotibial band
tenodesis. Brief (32) developed a procedure similar to that of Parker et al. but prepared the groove and passed the graft arthroscopically (Fig. 97.28).
He reported using hamstring graft, but his technique can also be
adapted for patella tendon graft. Brief’s technique, incorporating the
“groove” in the tibia suggested by Parker et al. (167) to improve isometry, follows.
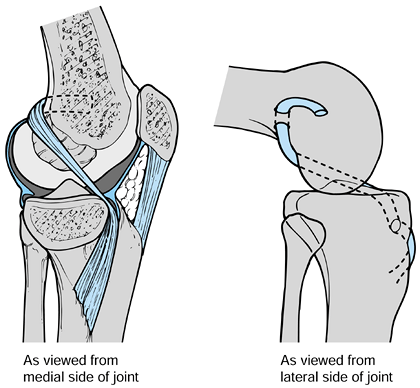 |
|
Figure 97.28. Brief’s arthroscopic modification (32) of the Parker and Drez’s modified “tomato stake” procedure (167). Redrawn from ref. 32.
|
-
Arthroscopically inspect the knee and correct intraarticular pathology.
-
Create a groove in the anterior tibia
through which a graft composed of gracilis and semitendinous tendons
harvested through an oblique proximal tibial incision is brought up
through the joint and around and over the top position of the femur. -
Fasten the graft to the distal lateral
femur above the physis through a distal lateral thigh incision. (The
middle third of the patella tendon can be substituted for the hamstring
in this procedure.)
reported no complications with drilling across the physis, as long as
the fixation device does not cross the physis. In their series, only
one patient had premature physeal closure, and this was attributed to
placement of a fixation staple across the physis.
hamstring grafts put through standard tibial and femoral tunnels for
adolescents within 1 to 2 years of skeletal maturity, reported no
complications. For younger children who failed a well-structured
conservative program, hamstring tendon grafts placed through standard
tibial holes in the over-the-top femoral position was advised.
McCarroll et al. (134) recommend activity
modification, functional bracing, frequent follow-up, and delayed
definitive hamstring reconstruction for the child with wide open physes
who is several years from skeletal maturity. In contrast, Fowler (70)
recommends a hamstring or patella-quadriceps tendon graft placed
through small (6 mm) tibial tunnels and in the over-the-top position on
the femur. In reviewing 29 young adolescents treated in this manner, he
found no leg length discrepancies. All youngsters returned to their
preoperative activity levels. The use of Achilles tendon or fascia lata
allografts (7 mm) placed through a tibial tunnel and in the
over-the-top position in the femur to replace the ACL in skeletally
immature patients has been reported (11) on a limited series (eight patients) with good early results (58 months).
the child with widely open growth centers who is in Tanner’s stage 1 or
2 and who has taller siblings and parents. We use MRI to help evaluate
intraarticular structures, provide early protection until acute
symptoms subside, and correct meniscal pathology if such exists. We
then prescribe a well-structured rehabilitation program stressing range
of motion, strength, proprioceptive skills, and guided return to
functional activities.
and 5 with radiographs demonstrating little growth remaining in their
physes. We prefer quadruple hamstring tendon grafting with fixation
above and below the physes.
significant growth remaining, we use a modification of an
extraarticular reconstruction (either DeLee’s or a modified Andrew’s),
until the physes are closing and the athlete is in Tanner’s stage 3, at
which time an intraarticular reconstruction procedure can be done.
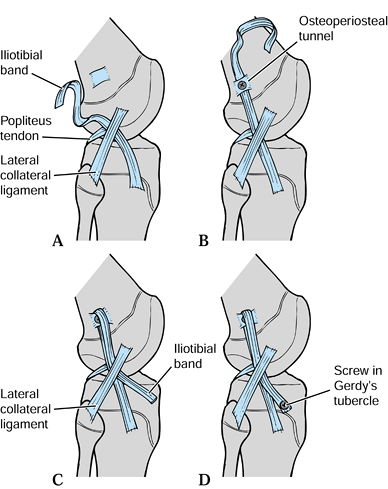
DeLee’s reconstruction (Fig. 97.29) is as follows:
 |
|
Figure 97.29.
DeLee’s extraarticular reconstruction for the anterior cruciate ligament in children with mild to moderate laxity and significant growth remaining (more than 2 cm) in whom conservative care has failed. Adapted from ref. 58. |
-
Isolate a 2.5 cm wide strip of iliotibial band, 15 cm in length. Leave the iliotibial band attached to Gerdy’s tubercle (Fig. 97.29A).
-
Pass the strip beneath the fibular collateral ligament.
-
Create an osteoperiosteal tunnel proximal to the physeal plates.
-
Fix the graft in the tunnel with a ligament screw and washer (Fig. 97.29B).
-
Next, pass the iliotibial band back distally beneath the fibular collateral ligament (Fig. 97.29C) and fix the ligament with a screw and washer to Gerdy’s tubercle (Fig. 97.29D).
-
Place a tourniquet high on the thigh and
the knee in a leg holder immediately below the tourniquet, leaving most
of the distal thigh exposed. -
Prep and drape the extremity in the typical fashion for arthroscopy.
-
Inflate the tourniquet, tighten the knee
holder, and perform a diagnostic arthroscopy, correcting any
intraarticular pathology found. -
With the knee bent 90° in the knee holder
and the foot hanging free, make a lateral incision in the distal thigh
beginning approximately 8 cm above the knee joint and extending down to
just above Gerdy’s tubercle. Extend the incision through the
subcutaneous tissue, exposing the iliotibial band. -
Longitudinally divide the iliotibial band
to create a strip approximately one third the width of the band,
situated 2 to 3 cm from its posterior border. -
Retract the remaining iliotibial band
anteriorly and, using a periosteal elevator, denude the soft tissue and
periosteum from the area of the femur at the lateral intermuscular
septum just proximal to the beginning of the femoral condyle and above
the physis. Use an osteotome to fish scale this area. -
Place a modified Kessler stitch with a 2-0 Polydec suture through the strip of iliotibial band, going distal to proximal.
-
With tension on the iliotibial band
through traction on the previously placed suture, staple the iliotibial
band to the previously prepared bed on the femur. -
Close the wound in layers. Apply a sterile dressing and a limited motion brace from 30° to 90°.
described earlier, advancing from 30° to full flexion within 2 weeks.
Begin close kinetic strengthening exercises in several days and advance
over the next 6 to 8 weeks. Do not permit running for 3 to 4 months,
and require brace protection for return to low-risk pivotal activities.
This extraarticular repair is not intended to create a stable knee for
high-risk activities but is merely used as a time-buying procedure for
those who have failed conservative care in order to enhance stability
for low-risk activities. The rehabilitation protocol is much like that
used for nonoperative management.
arthroscopic modification of Bergfeld’s “tomato stake” procedure may be
a reasonable alternative; however, this reconstruction is not ideal
because it uses a prime source of autologous graft material (patellar
or hamstring tendon), which is placed in a nonisometric position,
thereby subjecting it to failure over time. One needs to take care in
grooving the over-the-top femoral tunnel not to groove the physis,
which is only 2.5 cm proximal to the ACL’s attachment (19).
physis, the intercondylar eminence offers less resistance to traction
forces than the ACL, making it more commonly injured than the ACL in
children (173,210).
Recently, with the increased emphasis on competitive sports in
children, increasing numbers of ACL midsubstance tears are being seen.
In the athletic population, such injuries may be more frequent than
eminence fractures (135,194).
collision with a car are the most likely events resulting in a tibial
eminence fracture (84). The mechanism of injury has not been well defined. Wiley and Baxter (218)
suggest that injuries occur when, with the foot planted on the ground,
the extended knee is axially loaded simultaneously with outward
rotation of the femur on the tibia. Hyperflexion is another potential
mechanism of injury.
reported that tibial eminence fractures in children were often the
result of less violent injuries than those resulting in proximal tibial
fractures in adults and were typically an isolated injury. In contrast,
DeLee’s (58) more recent review of this injury
led him to conclude that this fracture was often associated with
collateral or meniscal injury.
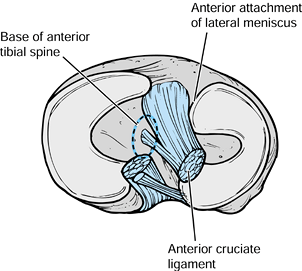
plateaus, consisting of the two tibial spines and the bony attachments
for the anterior and posterior horns of the medial and lateral menisci (218). The ACL is partially attached to the medial spine (Fig. 97.30).
Spine fractures in children occur through the cancellous bone beneath
the subcortical plate. The fracture fragment typically includes the ACL
and a variable-sized portion of the intermeniscal area (208).
The ACL receives its blood supply from the middle genticular artery but
can atrophy if it remains detached at one end due to a nondisplaced
avulsion fracture (143).
 |
|
Figure 97.30. Anatomy of the tibial eminence. (Redrawn from DeLee JC. Ligaments of the Knee. In: Stanitski CL, DeLee JC, Drez D Jr. eds. Pediatric and Adolescent Sports Medicine. Philadelphia: W.B. Saunders, 406;1994, with permission.)
|
 |
|
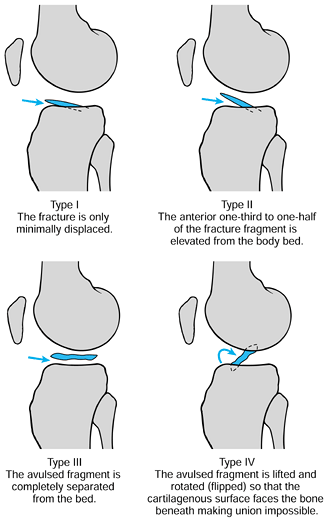
Figure 97.31.
Meyer and McKeever’s classification of tibial eminence fractures.(From Meyers HH, McKeever FM. Fracture of the Intercondylar Eminence of the Tibia. J Bone Joint Surg 1959;41A:209, with permission.) |
standard radiographs of the knee, although tunnel and oblique views in
nondisplaced fractures may be helpful. The avulsed bony fragment may be
small compared with the cartilaginous fragment, making radiographic
identification more difficult.
with a cast or a hinged knee brace locked in extension. Type II
fractures can frequently be reduced by aspirating the joint, instilling
local anesthesia, and then gently extending the knee. As the knee comes
into full extension (the last 5° are critical [58,174]),
the femoral condyle impinges on the fragment, resulting in its
reduction. Following reduction, immobilize the knee in full extension
by cast or brace until healing at 6 to 8 weeks. Note that Meyers and
McKeever (143) and Medler and Jammason (138) recommend immobilization in 20° of flexion because in full extension, the ACL can exert tension on the fragment.
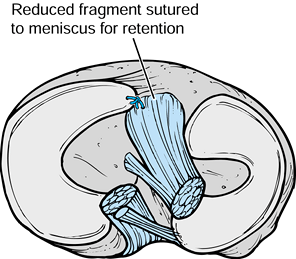
fractures, we recommend arthroscopic or open reduction of the fragment
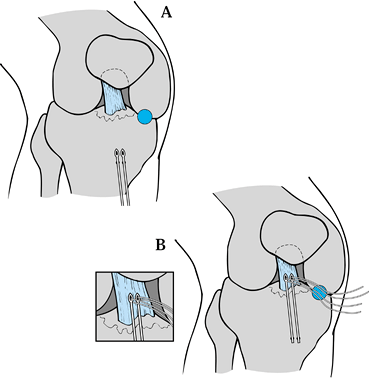
with fixation using absorbable or nonabsorbable sutures, pins, or
screws (Fig. 97.32 and Fig. 97.33). If left displaced, the eminence will likely block full extension and result in laxity of the ACL.
 |
|
Figure 97.32.
Meyer and McKeever’s method of fixation of tibial eminence fractures. Note the use of absorbable sutures placed through the fragment and sutured to the anterior horn of the medial meniscus, which are used in lieu of screws, pins, or transitional sutures (52,142,43). |
 |
|
Figure 97.33.
Arthroscopic treatment of fractures of the tibial spine. (From Medlar RG, Jansson KA. Arthroscopic Treatment of Fracture of the Tibial Spine. Arthroscopy 1994;10:292, with permission.) |
-
Expose the knee joint through a distal portion of an anterior medial parapatella incision.
-
Examine the joint for other associated pathology.
-
Inspect the anterior horn of the menisci to make certain that they are not trapped beneath the fragment.
-
Clear the fracture of any debris. Place the knee joint in extension to reduce the fragment.
-
Drill two holes from distal to proximal
through the tibial physis, entering the knee joint just medial and
lateral to the fracture fragment or piercing the fragment itself. Take
care to place these holes proximal to the physis. -
Place an 18- or 19-gauge wire or a 1-0
nonabsorbable suture through the distalmost portion of the ACL, just
proximal to the fragment. -
Pass the suture through the previously drilled holes and tie them after the fragment is reduced.
-
Flex and extend the knee to check for stability.
-
After examination of the knee under
anesthesia, elevate the leg and inflate tourniquet. Place the leg in
leg holder and prep and drape the extremity in the usual sterile
fashion. -
Examine the joint arthroscopically, correcting any associated injuries.
-
Clean the fracture area of all loose and unstable tissue.
-
Perform a trial fracture reduction using the probe to guide the fragment into position.
-
Make an anteromedial proximal incision over the tibia and carry it down to bone.
-
Place two drill-tipped guide wires through the tibia and through the fracture fragment (Fig. 97.33A).
-
Insert spade-tipped guide pins through
the drill holes and through the bony fragment using a probe or small
rasp to hold the fragment reduced while passing the guide wires and
pins. -
Use the Accufex suture passed from the shoulder set to pass a #1 PDS through a 7 mm cannula in the anteromedial portal (Fig. 97.33B).
-
Thread the suture through the islet of
the spade-tipped pins and bring it back through the same portal with a
grasping instrument. Repeat this procedure for the other pins. -
Remove both pins from the knee, bringing the double strands of sutures with them.
-
Tie the sutures protruding through the
anteromedial portal and pull them back into the joint, creating a loop
that snugly ties down the avulsed fragment. -
Tie the two loops together over the small bony bridge as they exit from the anterior tibia.
-
Close wounds in a routine fashion.
report good to excellent results following open reduction and internal
fixation with no laxity after healing of the fracture, others (218,224)
report mild laxity without functional disability. The lack of
significant functional disability despite evidence of mild to moderate
clinical laxity has been attributed to either intact proprioceptive
mechanisms or increased stability from enlargement of the reduced
fragment secondary to the increase in its blood supply during healing (218). In contrast, Smith (195) and Gronkvist et al. (88)
found residual symptoms and clinical instability in a significant
number of patients, indicating that a reevaluation of treatment
protocols was needed.
meniscal tears must be suspected in adolescents older than 12 years of
age with signs of internal derangement of the knee following a twisting
injury in sports (106). Menisci perform several very important functions in the knee (178). The emphasis of treatment is meniscal preservation (202). The menisci in children are more vascular, suggesting a greater ability to heal following injury (53).
In adults, vascular channels are present in the peripheral third of the
menisci, whereas in children, these vascular channels often extend up
to or even beyond the halfway mark (78). The
pattern of meniscal tearing in children also differs from that in
adults in that the younger the patient, the more peripheral the
meniscal tear tends to be (111).
more difficult than in adults because they are poorer historians, but
typically a more significant injury is required to tear the meniscus
and it is often associated with tears of the ACL. Radiographs are
typically normal. Consider MRI if tenderness and pain persist beyond 2
weeks. Beware of false-positive results (79).
is poor, with a large number of patients developing advanced
degenerative changes in the knee (98,128,223). On the other hand, healing of meniscal repairs in adolescents is superior to that in adults (79,91,96,153).
Type I, the most common variety, is a complete disk-shaped semilunar
type with a thinner center. Type II consists of an incomplete semilunar
type with a concave or convex free edge. Type III is a hypermobile or
Wrisberg-type, which lacks a posterior tibial
attachment.
Although the Wrisberg-type is the most rare, it is most likely to lead
to symptoms because of its hypermobile nature. Types I and II discoid
menisci are often asymptomatic, although athletes with these types are
susceptible to a greater frequency of tears because of the relatively
poor vascularity and thickness of the discoid meniscus (93).
In the Wrisberg-type discoid meniscus, there is no posterior attachment
to the tibial plateau, resulting in a hypermobile posterior horn that
displaces into the intracondylar notch with extension because of
traction on the lateral meniscal femoral (Wrisberg) ligament. This
results in the often-symptomatic “snapping knee syndrome,” which is
notable due to the audible clicking when the knee is moved (79).
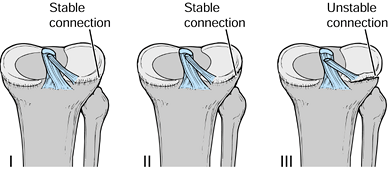 |
|
Figure 97.34.
Watanabe classification of discoid menisci. Type I, stable complete discoid meniscus. Type II, stable incomplete discoid meniscus. Type III, unstable discoid meniscus secondary to lack of a complete meniscotibial ligament. |
between 12 and 15 years of age and often complain of the classic
meniscal symptoms of limited extension, snapping, locking, and pain.
Physical examination may reveal loss of extension, quadriceps atrophy,
and a variable effusion.
lateral joint space and squaring of the lateral femoral condyle. In
addition, hypoplasia of the lateral tibial spine with tilting of the
lateral tibial articular surface and apparent elevation of the fibular
head may be noted. MRI reveals the presence of abnormally thickened
meniscal tissue interposed into the central intercondylar zone, which
may be limited to only one portion of the meniscus (79).
children and adolescents, the focus in treatment of symptomatic discoid
lateral menisci is preservation of as much normal meniscal tissue as
possible. A course of conservative management is indicated initially,
but if symptoms persist, arthroscopy is indicated. Small loose
fragments can be removed and peripheral tears may be considered for
repair. Meniscoplasty has been recommended to reduce the thickness of
the discoid meniscus and prevent recurrent tears. Hayashi et al. (93)
advise leaving a rim of 6 mm in the complete type discoid meniscus and
8 mm in the incomplete type. This allows the remaining meniscus to
participate in weight bearing (Fig. 97.35). The pattern of the meniscal tear will sometimes dictate the amount of meniscus to be resected. Several reports (20,62,93)
have documented good results following total meniscectomy for discoid
lateral meniscus. Total excision, however, should be the last resort to
avoid the possibility of progressive degenerative arthritis.
 |
|
Figure 97.35.
Meniscoplasty by resection of the central portion of the discoid meniscus reduces the thickness of the meniscus to prevent recurrent tears. |
child and adolescent athlete are extremely common and can be
categorized as either patellofemoral stress syndrome, characterized by
pain, or patellofemoral instability, with symptoms of subluxation and
recurrent dislocation. Although laxity does play a part in both of
these problems, it is one small part of an overall malalignment
syndrome, which may include tissue imbalance, muscle weakness, angular
and rotational deformities, and dysplastic bone (Fig. 97.36).
Evaluation and treatment of these problems therefore must take into
account the overall configuration of the patellofemoral joint so that
treatment can be directed toward the specific pathology (217).
 |
|
Figure 97.36.
Patellofemoral biomechanics. The patella depends on muscular, soft-tissue, and bony factors to maintain stable aligment in the patellofemoral joint. |
decrease the medial restraining mechanism will lead to lateral tracking
of the patella with associated pain and instability (79,129,168,218) (Table 97.9). The cause of pain appears to be excessive tension and rotational forces on the peripatellar soft tissues (102) as well as excessive pressure on the lateral patellar facet (68).
Patients typically present reporting diffuse generalized anterior knee
pain that is exacerbated when weight bearing is performed on the bent
knee with such activities as descending stairs and squatting. Patients
may complain of associated instability, but true locking of the knee is
uncommon, although a
catching sensation is reported (79).
Pain is often present after a period of prolonged sitting with the knee
flexed. A history of sudden increase in training or in training errors
is commonly obtained from these patients (102).
 |
|
Table 97.9. Miserable Malalignment Syndrome
|
quadriceps angle; hamstring and iliotibial band tightness; patellar
tilting; and rotational deformities of the tibia and femur; and
carefully evaluate for pronation of the foot. Decreased thigh
circumference indicates chronicity. Patients typically exhibit pain
with patellar compression and tenderness to palpation of the patellar
facets and retinaculum. Take AP, lateral, and tangential radiographic
views of the patella to rule out other bony abnormalities, including
bipartite patella, Sinding-Larsen-Johansson disease, and OCD. Evaluate
for patella alta or baja on the lateral views. Tangential views such as
the Merchant view (139) allow evaluation of
patella maltracking or subluxation. Nonoperative management is
successful in the vast majority of patients (59,222).
Initially, reduce pain with physical therapeutic modalities, followed
by a stretching program for the iliotibial band and hamstring muscles.
Strengthen the vastus medialis obliquus (VMO) to increase the medial
pull on the patella and improve patellar tracking. Foot orthotics may
be helpful, and knee bracing with a Palumbo style brace may also reduce
patellar stress. Slowly allow resumption of sports when flexibility and
strength have been regained and pain is under control. Surgical
treatment is rarely indicated, and although it is usually successful,
failures of surgical management tend to lead to a significant
disability (79).
tracking. Lateral retinacular release may be indicated in patients with
contracted lateral tissues but is contraindicated in the hypermobile
patella. The articular surface of the patella is rarely a cause of
symptoms in adolescent athletes, and therefore, arthroscopic chondral
shaving is rarely indicated. Bony realignment is contraindicated with
open physes, and any type of surgical intervention is contraindicated
in patients who are not cooperative with rehabilitation, exhibit a
psychosomatic component to their report, or have reflex sympathy
dystrophy (79).
syndrome, adolescent athletes who present with patellar dislocation
often exhibit anatomic features that render them susceptible to this
problem (79) (Table 97.10).
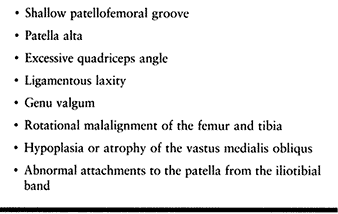 |
|
Table 97.10. Risk Factors for Patellar Dislocation
|
inversely related to the degree of malalignment and dysplasia
encountered. Patients present usually between 16 and 20 years of age (31,48,97,116)
following a single twisting maneuver involving the knee. Football,
baseball, and basketball are commonly involved, but cases have been
reported in gymnastics as well as cheerleading. Just as with
patellofemoral stress syndrome, girls are more commonly affected than
boys, and in almost one quarter of cases, a positive family history for
patellofemoral instability is elicited (31).
Dislocations frequently reduce spontaneously, and the adolescent
athlete presents with diffuse knee pain and swelling. Physical
examination may reveal an effusion, diffuse parapatellar tenderness, a
positive apprehension test for patellar dislocation, and a palpable
defect
in the VMO insertion into the patella. Patients should be evaluated for
signs of other ligamentous injury about the knee, which can occur with
the same mechanism. Look for other sources of pain referred to the
knee. Reduce persistent dislocations of the patella immediately with
intravenous sedation and an intraarticular anesthetic if needed. As
hamstring spasm resolves, the knee can be brought gently into
extension, and spontaneous reduction usually occurs. Radiographic
evaluation of the acutely injured knee includes AP and lateral x-ray
studies, and this can be supplemented with a CT scan if these studies
are nonconclusive. Osteochondral fragments may be noted in the joint,
and bone fragments along the medial patellar margin, which are not free
floating, may be evident.
During this time, straight-leg raises for progressive strengthening are
beneficial in reestablishing the pull of the VMO. Osteochondral
fractures, which occur in approximately 10% of cases of patellar
dislocation, require immediate surgery. Fragments larger than 5 mm may
require repair, whereas smaller fragments can be excised (79). Approximately 75% of patients obtain acceptable results following closed treatment of patellar dislocation (48),
but only 50% become asymptomatic. Risk factors for redislocation
include osteochondral fragment, age younger than 14 years,
participation in athletics, palpable medial defect, contralateral
evidence of knee dysplasia, patellar hypermobility, prior dislocations,
positive family history for dislocations, and patella alta (48).
Recurrent dislocations can be treated conservatively as well with
vigorous quadriceps strengthening, but patients with high-risk factors
may benefit from early surgery. Surgery should be tailored to the
pathology encountered, and the previously mentioned dysplastic features
should guide the surgical approach (99). Options include lateral retinacular release and distal realignment procedures (15,48,95,127,141).
Bony distal realignment procedures such as the Elmslie-Trillat
procedure lead to an overall good result in more than 80% of cases (79) but are contraindicated in younger patients with open growth plates (95,126).
Soft-tissue procedures such as medial plication, hemitransfer of the
patella tendon, and semitendinosus transfer to the distal pole of the
patella (2,15) have
also enjoyed success. Results tend to be worse in children and
adolescents with ligamentous laxity, and in younger children, surgery
may be unpredictable and require a tibial tubercle transfer after
skeletal maturity has been reached. Figure 97.37
shows the potential steps in patellar realignment in the growing child.
Lateral release is nearly always necessary. Medial plication is usually
required. Medial transfer to the lateral half of the patellar tendon is
sometimes required. Tenodesis of the semitendinosis tendon to the
patella is required for severe deformity in which the other three steps
are inadequate.
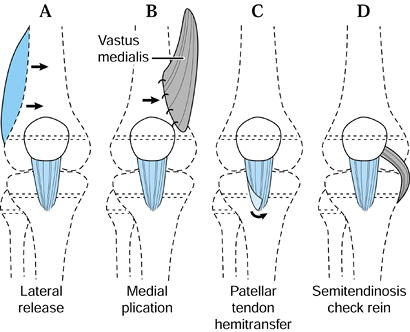 |
|
Figure 97.37. The steps in patellar realignment in the growing child. Lateral release (A) is almost always required and medial plication (B) is usually required. Medial transfer of the lateral half of the patellar tendon (C) may also be required and tenodesis of the semitendinosis (D) is usually required only for severe deformity. (Adapted from Staheli LT. The Lower Limb. In: Morrissy RT, ed. Lovell and Winter’s Pediatric Orthopaedics, 3rd ed. Philadelphia: JB Lippincott, 1990:741.
|
adolescent are no different from those of an adult, although generally
the child progresses through these phases more
rapidly. A typical program may be divided into five phases:
-
phase I—decreasing inflammation
-
phase II—improving range of motion
-
phase III—improve strength and proprioception
-
phase IV—return to functional skills
-
phase V—return to sport
before progressing to the next phase. Providing youngsters with
short-term and intermediate goals will help maintain their interest and
enthusiasm. One must also remember that children have short attention
spans, and therefore, exercises need to be brief, simple, and fun.
Incorporate range-of-motion, proprioception, and strengthening
exercises into games using a variety of colorful equipment. During
adolescence, exercising with peers often results in greater compliance.
adolescents, especially those involved in highly competitive sports, as
it is for adults, and just as in adults, proper conditioning in
children and adolescents is associated with a decrease in injury rates (118).
adolescents is appropriate provided the equipment is sized
appropriately. (Hence free weights are frequently better than machines
built for adults.) Although this age group lacks adequate androgen to
develop muscle bulk, weight-training programs have been found to
develop tone and strength and enhance performance (118).
all youngsters are flexible and do not need to stretch before and after
practice and play. Particularly during periods of rapid growth, when
young bones may be growing faster than the surrounding muscles, warming
up and stretching is important in preventing overuse injuries such as
the traction apophysites. (See section on nonarticular osteochondroses in this chapter.)
with sports activity, one must always keep in mind that other systemic
problems can masquerade as sport injuries. For example, a persistently
swollen ankle in an 11-year-old may not be secondary to landing wrong
while practicing a basketball drill but may be the first sign of
juvenile rheumatoid arthritis; a swollen tender tibial tubercle or
iliac crest may not be an acute traumatic apophysitis, but the
inflammation may be secondary to infection (56);
the first sign of leukemia may be fatigue with activity; and the early
stages of osteogenic sarcoma may be confused radiographically with a
healing stress fracture.
far more severe than the objective findings or who frequents the
physician’s office with a variety of minor problems over a brief period
of time. This adolescent, although strongly verbalizing his desire to
“play through any pain,” may in reality be trying to use his “injury”
as an “acceptable way out” of sports. Such a scenario is not infrequent
during teenage years, when adolescents may no longer wish to continue
to spend the long hours in training that has been required of them but
instead may wish to spend more time socializing with friends. However,
these adolescents fearing rejection and chastisement from disappointed
parents, cannot admit to them that they just do not want to participate
anymore. Often the physician must “read between the lines,” gain the
athlete’s confidence, and help the athlete deal with the situation in
an acceptable manner.
scheme: 01, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
JA, Jokl P, Shaw R, et al. Clinical Study of Baseball Pitchers:
Correlation of Injury to the Throwing Arm with Method of Delivery. Am J Sports Med 1978;6:15.
CT, Paletta GA Jr, Potter HG, Wickiewicz TL. The Relationship of the
Femoral Attachment of the ACL and the Distal Femoral Physeal Plate in
the Skeletally Immature Knee: An Anatomic Study. Presented at the
American Orthopaedic Society for Sports Medicine Annual Meeting,
Vancouver, Canada, July 1998.
F, Steadman JR, Rodrigo JJ, Stillman J. Treatment of Articular
Cartilage Defects in Athletes: An Analysis of Functional Outcome and
Lesion Appearance. Orthopaedics 1997;21:761.
V. An Update on Arthroscopic Osteochondral Autograph Transplantation.
American Academy of Orthopaedic Surgery 65th Annual Meeting. New
Orleans, March, 1998.
N, Lindahl A, Nilsson A, et al. Treatment of Deep Cartilage Defects in
the Knee with Autologous Chondrocyte Transplantation. N Engl J Med 1994;331:889.
BR, Berg BC. 99m-Technetium Phosphate Compound Joint Scintigraphy in
the Management of Juvenile Osteochondritis Dissecans of the Femoral
Condyle. Am J Sports Med 1983;11:329.
D, Roy S, Singer K, Broekhoff J. Stress Change of the Distal Radial
Growth Plate: A Radiographic Survey and Review of the Literature. Am J Sports Med 1992;20:290.
JW. Arthroscopic Management of Transchondral Talar Dome Fractures
(Osteochondritis Dissecans) and Anterior Impingement Lesion of the
Ankle. Clin Sports Med 1991;10:677.
AB, Gould N. Osteochondritis Dissecans of the Talus (Transchondral
Fracture of the Talus): Review of the Literature and New Surgical
Approval for Medial Dome Lesions. Foot Ankle 1985;5:165.
PJ. Management of the Acute Knee Injury in the Skeletally Immature
Athlete. Instructional Course Presented at the 22nd AOSSM Mtg. Lake
Buena Vista, FL, 1996.
BK, Lange RH, Fujisaki CK, et al. Anterior Cruciate Ligament Tears in
Skeletally Immature Patients: Meniscal Pathology at Presentation and
After Attempted Conservative Treatment. Arthroscopy 1992;8:229.
L, Kish G, Karpati Z, et al. MosaicPlasty for the Treatment of
Articular Cartilage Defects: Application in Clinical Practice. Orthopaedics 1998;21:751.
DW, Runion PR, Morrison DS, et al. Osteochondritis Dissecans in the
Female Gymnasts Elbow. AANA Annual Meeting Washington DC, 1988.
KG. Reconstruction of the Anterior Cruciate Ligament Using the Central
One-third of the Patellar Ligament: A Follow-up Report. J Bone Joint Surg 1970;42A:1302.
WB, Chandler TJ. Musculoskeletal Adaptation and Injuries Associated
with Intense Participation in Youth Sports. In: Cahill BR, Pearl AJ,
eds. Intensive Participation in Children Sports. Champaign, IL: Human Kinetic, 1993:203.
PR Jr, Lipscomb PR Sr, Bryan RS. Osteochondritis Dissecans of the Knee
with Loose Fragments Treatment by Replacement and Fixation with Readily
Removed Pins. J Bone Joint Surg 1978;60A:235.
HM, McCullough RW, Grandsman EJ, Schatz SL. Computerized Blood Flow
Analysis for Decision Making in Treatment of Osteochondritis Dissecans.
J Pediatr Orthop 1988;8:208.
BR, Bartolozzi AR, Davis CA, et al. Wrist Pain Syndrome in the Gymnast.
Pathogentic, Diagnostic, and Therapeutic Considerations. Am J Sports Med 1989;17:305.
JR, Shelbourne KD, Porter DA, et al. Patellar Tendon Graft
Reconstruction for Midsubstance Anterior Cruciate Ligament Rupture in
Junior High School Athletes. Am J Sports Med 1994;22:478.
M, Sapega A, Bonakdarpone A. Osteochondritis Dissecans Analyses of
Mechanical Stability with Radiography, Scentigraphy and MR Imaging. Radiology 1987;165:775.
H, Kubota K, Shiraishi M, et al. The Conservative Treatment of Complete
Tears of the Anterior Cruciate Ligament in Skeletally Immature
Patients. J Bone Joint Surg 1995;77B:890.
WM, Matsuura PA. Current Concepts and Review of the Literature.
Management of Complete Traumatic Anterior Cruciate Ligament Tears in
the Skeletally Immature Patient. Arthroscopy 1994;10:569.
GA Jr, Bednarz PA, Stanitski CL, et al. The Prognostic Value of
Quantitative Bone Scan in Knee Osteochondritis Dissecans. Am J Sports Med 1998;26:7.
AC, Shelbourne KD, McCarroll JR, et al. The Natural History and
Treatment of Delayed Union of Stress Fractures of the Anterior Cortex
of the Tibia. Am J Sports Med 1988;16:250.
CL. Arthroscopic and Clinical Correlation of Knee Magnetic Resonance
Imaging Findings in Children and Adolescents. Oral Presentation, AAOS
65th Annual Meeting, New Orleans 1998.
K, Walgenback. A Surgical Technique for Articular Cartilage
Transplantation to Full Thickness Cartilage Defects in the Knee
Joint—Operative Techniques. Orthopaedics 1997;7:305.
JS, Moyer RA. Nonunion of the Olecranon Stress Fractures Through the
Olecranon Epiphyseal Plate Observed in an Adolescent Baseball Pitcher:
10 Case Reports. J Bone Joint Surg 1977;59:264
JS, Pollack H, Sweterlitsch P. The Effect of Competitive Pitching on
the Shoulder and Elbows of Pre-adolescent Baseball Players. Pediatrics 1072;49:267.
AH, Andrews JR, Schob CK, et al. Fractures of Unfused Olecranon Physes:
A Re-evaluation of this Injury in Three Athletes. Orthopaedics 1995;18:390.