Spine
plane greater than 10 degrees associated with a variable degree of
rotational deformity. A measurable curve less than 10 degrees is
referred to as a spinal asymmetry and is
of little consequence. Idiopathic scoliosis refers to scoliosis without
a known etiology. It is a diagnosis of exclusion.
described based on the patient’s age at onset: infantile (0 to 3
years), juvenile (4 to 10 years), and adolescent (older than 10 years).
Prognosis and treatment for each type differ.
form of idiopathic scoliosis with a prevalence of approximately 3%.
Though various potential etiologies have been examined, to date
available evidence does not support any single causative factor. The
role of genetic factors is widely documented but a specific mode of
inheritance remains undetermined. Boys and girls are affected equally
by AIS, but the risk of curve progression is seven times greater in
girls than in boys.
or school screening programs. Occasionally parents notice that their
child’s clothing does not hang correctly or that the child’s posture is
awkward. Pain is not characteristic of AIS. The history should always
include questioning for pain and neurologic complaints. A painful
scoliosis should prompt further evaluation for other conditions
including neoplasm and infection.
patient is standing erect. Physical exam should document uneven
shoulder elevation and waistline asymmetry, even though these findings
may be present in the absence of scoliosis. The Adams forward bending
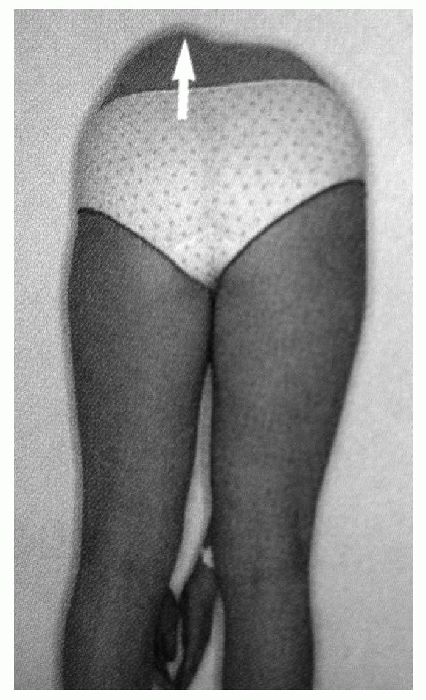
test is the most sensitive clinical exam to screen for scoliosis (Fig. 7.1-1).
The examiner should observe the patient from the back as he or she
bends forward with feet together, knees straight, and arms hanging
free. This test accentuates the rotational component of scoliosis and
allows the examiner to appreciate rib or lumbar paravertebral
prominences. Inability to flex forward in a supple manner should alert
the examiner and prompt further workup. Assessment of leg lengths,
trunk shift, sagittal plane balance, and complete neurologic
examination including abdominal reflexes are mandatory. Positive
neurologic findings should prompt further workup for intraspinal
pathology including syringomyelia and neoplasm. Left-sided thoracic
curves have a significant association with spinal cord pathology and
again should prompt further diagnostic workup, including screening
magnetic resonance imaging (MRI) of the spinal cord.
full-length posteroanterior and lateral views of the spine. A
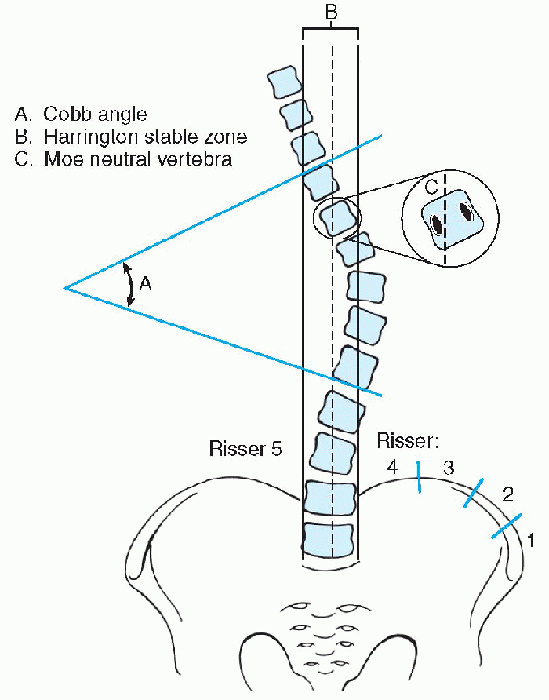
determination of skeletal maturity should be based on the Risser sign (Fig. 7.1-2). The Cobb technique is the standard method used to quantify the degree of curvature in scoliosis.
The Cobb angle for a particular curve is the angle formed by the
intersection of a line parallel to the superior end plate of the most
cephalad vertebra of the curve to a line parallel to the inferior end
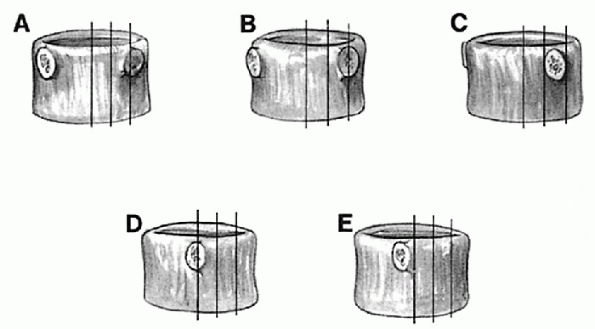
plate of the most caudad vertebra of the curve. Vertebral rotation
should also be noted (Fig. 7.1-3). Curves are described by the location and direction of their apical convexity (Table 7.1-1).
The most common curve types in AIS, in order of frequency, are isolated
right thoracic, right thoracic/left lumbar, left lumbar, and right
lumbar.
 |
|
Figure 7.1-1 The Adams forward bending test gives the best visualization of truncal asymmetries.
|
 |
|
Figure 7.1-2
Measurements for scoliosis. (From Stephens RM, Fridian MA. Pediatric orthopaedics. In: Miller X, ed. Review of orthopaedics, 3rd ed. Philadelphia, WB Saunders, 2000:165.) |
 |
|
Figure 7.1-3
Nash-Moe method of determining vertebral body rotation. Grade of rotation is determined by the location of the convex pedicle. As more rotation occurs, the concave pedicle disappears, and the convex pedicle moves into the apparent midpoint of the vertebral body. (From Bridwell KH. Adolescent idiopathic scoliosis: surgery. In: Weinstein SL. The pediatric spine: principles and practice, 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2001:386.) |
functional limitations. Approximately 10% of patients require treatment
other than observation. Risk factors for curve progression include
female sex, younger age at presentation (less than 12 years), relative
skeletal immaturity (Risser less than 2), premenarchal status, and
curve magnitude at presentation (more than 20 degrees) (Tables 7.1-2 and 7.1-3).
Progression of curves after skeletal maturity is possible. Curves less
than 50 degrees at skeletal maturity usually remain static while curves
greater than 60 degrees have a significant risk of progression with
subsequent cardiopulmonary compromise and early death. Cardiopulmonary
function is not usually impaired in otherwise healthy patients until
the curve measures 90 degrees. The risk of disabling back pain in
adulthood in patients with modest curves (40 to 50 degrees) is similar
to that of the general population.
|
TABLE 7.1-1 CURVE DESCRIPTION BY APICAL VERTEBRA
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
TABLE 7.1-2 INCIDENCE OF PROGRESSION BY RISSER SIGN AND CURVE MAGNITUDE AT PRESENTATION
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||
|
TABLE 7.1-3 PROBABILITY OF PROGRESSION BY CURVE MAGNITUDE AND AGE AT PRESENTATION
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||
Options include observation, bracing, and surgery. Curves less than 20
degrees in skeletally immature patients should be followed regularly by
clinical and radiographic examination. Documented progression of
greater than 5 degrees warrants consideration of bracing in the
skeletally immature. Children presenting with curves greater then 30
degrees at first visit should also be considered for bracing. Bracing
is not considered efficacious in curves more than 40 degrees. Surgery
may be considered for curves over 45 to 50 degrees, as curves over 50
degrees at maturity have a risk of significant progression over the
patient’s lifetime.
|
TABLE 7.1-4 TREATMENT RECOMMENDATIONS
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
most surgeons who treat scoliosis believe that bracing has a role in
the prevention of curve progression in the immature patient. The
ultimate goal of bracing is to halt curve progression until the patient
reaches skeletal maturity, at which time the risk of progression of
curves less than 50 degrees is low. Bracing does not permanently reduce
the magnitude of an established curve. Its effectiveness at halting
progression seems to be “dose-related” (i.e., related to the extent of
brace use), although there are reports showing comparable results
between full-time use (23 hours per day) and part-time use (16 hours
per day).
useful for curves with the apex at T8 or below. Curves with the apex
cephalad to T8 are uncommon, and require the Milwaukee type brace,
which has a chin extension and is less well accepted by children. The
Charleston nighttime brace holds the patient bent in a position
opposite to the major curve. Its efficacy has been reported to be
greatest in single curves less than 35 degrees. To evaluate a brace,
in-brace radiographs are taken. An in-brace x-ray showing more than 50%
reduction of curve magnitude correlates with successful outcomes. Brace
treatment should continue until a girl is 2 years’ postmenarcheal and
is Risser 4 and a boy is Risser 5. Whenever bracing is discontinued, a
“rebound” phenomenon of an increase in curve magnitude may be expected.
than 45 to 50 degrees in skeletally immature patients. There are
multiple spinal instrumentation systems available that provide
excellent segmental fixation of the vertebral column. Whatever system
is used, the goal of surgery is to halt progression and partially
correct the curve while maintaining acceptable sagittal and coronal
balance. The basic options for the surgical treatment of AIS are
anterior spinal fusion with instrumentation, posterior spinal fusion
with instrumentation, or both anterior and posterior approaches. The
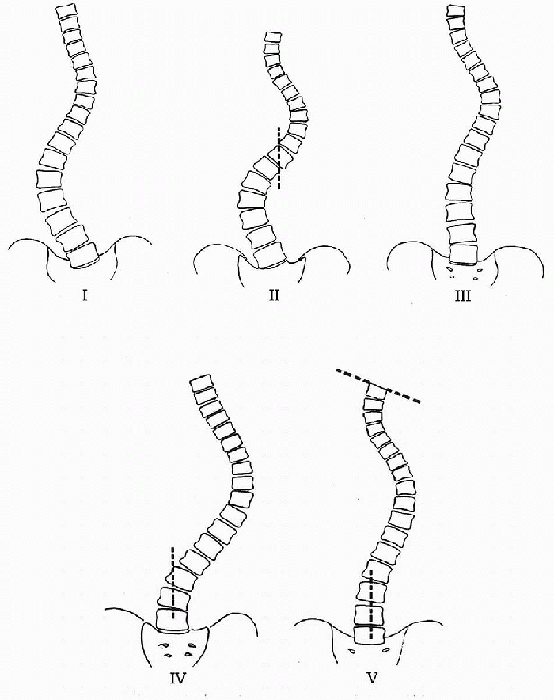
type of curve determines the approach used. King and Moe identified
five patterns of scoliosis and proposed a method for the selection of
fusion levels in thoracic idiopathic curves using first and second
generation segmental spinal fixation systems (Fig. 7.1-4). The choice of fusion levels shows some variability and is generally based on the stable and neutral
vertebrae. Stable vertebrae are defined as those vertebrae bisected by
the central sacral line. Bending films identify the neutral disc as
that disc which opens both to the right and left on supine bending
films. Historically, Harrington
instrumentation
involved fusion from stable vertebra to stable vertebra. With newer,
more powerful instrumentation including the use of pedicle screws, it
may be possible to successfully end the fusion on the vertebrae above
the neutral disc. Anterior instrumentation also provides more powerful
correction than Harrington instrumentation and allows for potentially
shorter fusions. Long-term follow-up has revealed an increased
incidence of late, low back pain with fusion to L5, though this is
controversial. Some believe that low back pain in spinal fusions to the
lower lumbar regions is more related to loss of lordosis with earlier
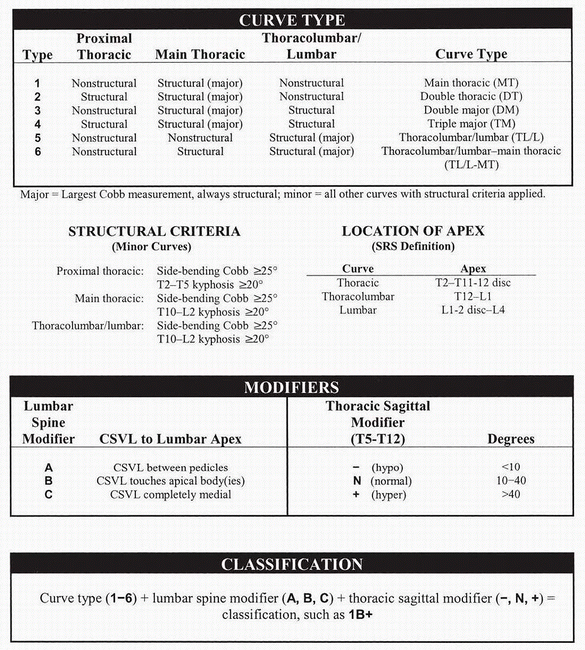
systems than to the level of fusion. A new classification has been
proposed to attempt to better guide treatment options given the
development of newer, more powerful segmental instrumentation systems (Fig. 7.1-5).
 |
|
Figure 7.1-4
King classification of scoliotic curves. (From King HA, Moe JH, Bradford DS, et al. The selection of fusion levels in thoracic idiopathic scoliosis. J Bone Joint Surg (Am) 1983;65:1302-1313.) |
spinal fusion. The traditional iliac crest bone grafting is associated
with high rates of long-term pain, and has become less popular.
Allograft bone grafting has been shown to be safe and efficacious in
multiple series. A rib resection of the most prominent concave ribs may
provide autograft, with significant cosmetic improvements as well.
infection, hardware failure, and pseudoarthrosis, and the overall
reoperation rate has been described by numerous authors as between 5%
to 15%. The most devastating complication of surgery is irreversible
neurologic impairment, with an incidence of about 1 per 1,000 patients.
The widespread use of intraoperative somatosensory and motor evoked
potential monitoring minimizes this complication and has largely
replaced the use of the Stagnara wake-up test. Excessive blood loss and
the need for homologous transfusion are minimized by the use of
preoperative recombinant erythropoietin, intraoperative cell saver,
hypotensive anesthesia, and autologous blood donation. The crankshaft phenomenon
occurs in skeletally immature patients after posterior spinal fusion,
as anterior spinal growth progresses without restraint. The result is
rotational and sagittal deformity over the fused levels. Anterior
fusion should be considered with or without posterior fusion and
instrumentation for children with more than 3 years of remaining
growth. Late complications such as the crankshaft phenomenon and
decompensation below fused levels are best avoided by careful
preoperative planning.
 |
|
Figure 7.1-5 Synopsis of all necessary criteria for curve classification. SRS, Scoliosis Research Society; CSVL,
center sacral vertical line. (From Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis, a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg [Am] 2001;83A:1169-1181.) |
AIS. It accounts for less than 1% of all cases of idiopathic scoliosis
in the United States. It occurs in children younger than 3 years, but
deformity is usually noticed during the first 6 months of life.
unknown. Significant geographic variability in its incidence has led
some to suggest pressure molding from postnatal positioning as a
possible etiology. In Europe, where the incidence of infantile
idiopathic scoliosis has been higher, children are customarily placed
in the supine or lateral decubitus position. In the United States the
earlier trends had been to place children in the prone position. This
difference may account for the geographic variation in prevalence and
play a role in the etiology of the disease. If this theory is correct,
as more children in the U.S. are placed supine because of the fear of
sudden infant death syndrome, an increased incidence may be seen.
are left-sided. In addition, there is a strong male predominance. The
curves are generally located in the thoracic and thoracolumbar regions.
Associated anomalies include plagiocephaly, congenital muscular
torticollis, and developmental hip dysplasia. It is imperative to rule
out other causes of scoliosis in this age group, including congenital
scoliosis, neuromuscular scoliosis, and scoliosis secondary to
intraspinal pathology.
entire spine and pelvis, including both hips, should be examined
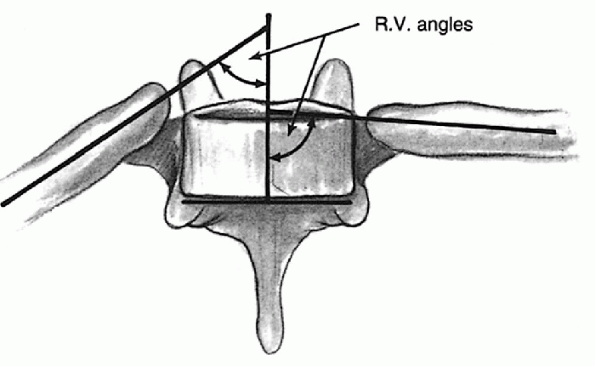
carefully for the presence of congenital abnormalities. Cobb angle
measurements and the apical rib—vertebra angle difference (RVAD) should
be recorded at each evaluation (Fig. 7.1-6).
Risk of curve progression depends on the patient’s age at time of
presentation, curve magnitude at presentation, and the RVAD. Older
children (more than 1 year of age) with larger curves have a greater
risk of progression. Most curves less than 25 degrees with an RVAD less
than 20 degrees will tend to resolve sponta-neously.
greater than 20 degrees, or documented progressive curves require
screening MRI of the brainstem and spinal cord. Serial casting or
bracing is traditionally considered the first line of treatment for
these patients. If the correction is maintained, the patient may be
gradually weaned from the brace with close observation to maturity.
Surgery should be considered in children who progress despite bracing.
Surgery is problematic in such young children, as significant growth
retardation is possible. Traditional surgical options include
instrumentation without fusion (growing rod) and limited short segment
circumferential fusion. Instrumentation without fusion involves
exposing the cephalad and caudad ends of the curve, achieving fixation
at these sites and distracting through these sites with a subcutaneous
rod. The rod is lengthened or replaced every 6 months, as additional
length is needed to accommodate growth. This theoretically allows
continued growth of the spine with delay of formal fusion until the
patient is closer to skeletal maturity, though actual growth reported
in many series is disappointing.
 |
|
Figure 7.1-6
Measurement of rib—vertebra angle difference. (From Warner WC Jr. Juvenile idiopathic scoliosis. In: Weinstein SL. The pediatric spine: principles and practice, 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2001:333.) |
rib prosthesis, with subsequent lengthening two to three times each
year, is a new and promising technique. Initial reports demonstrate
significant improvement of scoliosis, acceleration of spinal growth on
the concave side, and chest expansion, which encourages lung growth in
the collapsed hemithorax during the critical period of alveolar
development.
scoliosis detected between the ages of 3 to 10 years. It accounts for
12% to 21% of patients with idiopathic scoliosis. Its etiology is
unknown and may differ depending on the age of presentation. Patients
classified as having juvenile idiopathic scoliosis may actually have
late-onset infantile idiopathic scoliosis or early onset AIS but fall
into the juvenile category based on age. In children between 3 and 6
years, the female-to-male ratio is 1:1. In children between 6 and 10
years, girls are more frequently affected and often present with larger
curves.
resemble AIS with a right thoracic and right thoracic/left lumbar
double major predominance. The left thoracic curves common in infantile
idiopathic scoliosis are not typical in this age range and, as in AIS,
should prompt further workup to rule out intraspinal pathology.
more aggressive. Approximately 70% of juvenile curves progress and
require some form of treatment. A thorough history and physical
including neurologic examination is important to rule out any
underlying cause of the curvature. The loss of abdominal reflexes is
sometimes the only clinical finding in a patient with scoliosis with an
underlying dysraphism. Factors associated with curve progression
include curves greater than 45 degrees at presentation and thoracic
kyphosis of less than 20 degrees. The RVAD is less useful in
determining prognosis in juvenile idiopathic scoliosis than in
infantile idiopathic scoliosis.
curves less than 20 degrees at initial presentation. Curves larger than
20 degrees or smaller curves with documented
progression
of 5 degrees or more should be braced because of a high risk of
progression. Children younger than 6 years should be treated in the
same manner as infantile curves discussed previously. When curves
continue to progress despite bracing, bracing may still be continued to
allow for further spine growth prior to fusion. Once a curve reaches 50
degrees, surgery should be considered.
child and the amount of spinal growth available. Options are similar to
those discussed with infantile idiopathic scoliosis. Spinal
stabilization without fusion may be considered in children younger than
8 years. This may involve a growing rod, a Luque trolley construct, or
a titanium rib implant. Combined anterior/posterior fusion should be
considered in children over 8 years of age who have a Risser 0 sign
with open triradiates cartilages. The following shortening formula
provides a quick and easy way to predict spinal shortening after spinal
fusion, though height may be gained with a decrease in the spinal
curvature after surgery:
RG, Cole AA, Cook TA, et al. Pathogenesis of idiopathic scoliosis: the
Nottonhham concept. Acta Orthop Belg 1992;58 [Suppl]:33.
HA, Moe JH, Bradford DS, et al. The selection of fusion levels in
thoracic idiopathic scoliosis. J Bone Joint Surg (Am) 1983;65:
1302-1313.
GC, Pilcher MF. Structural idiopathic scoliosis in infancy: a study of
the natural history of 100 patients. J Bone Joint Surg (Br)
1965;47:520-523.
JE, Carison JM. The prediction of curve progression in untreated
idiopathic scoliosis during growth. J Bone Joint Surg (Am) 1984;66:1067.
JO, Herring JA, Browne RH. Posterior arthrodesis and instrumentation in
the immature spine in idiopathic scoliosis. J Bone Joint Surg (Am)
1995;77:39.
SL, Zavala DC, Ponseti IV. Idiopathic scoliosis: long-term follow-up
and prognosis in untreated patients. J Bone Joint Surg (Am)
1981;63:702-712.
disorders of the neuromuscular system. The diseases associated with
neuromuscular scoliosis are quite diverse but share many common
features. They can be classified into either neuropathic, myopathic, or
mixed disease states (Box 7.2-1). Although the
specific disorder dictates the type of curve encountered and its risk
of progression, the classification simplifies patterns of natural
history, evaluation, and management.
onset with rapid progression during growth and continued progression
after skeletal maturity. The curves are generally long, extend into the
sacrum, and are associated with pelvic obliquity. Kyphosis is an
important part of the neuromuscular deformity pattern and must be
carefully considered in the evaluation of these deformities. Severe
deformities commonly compromise sitting ability and pulmonary function
in patients with baseline dysfunction. Characteristically, these
patients have poor head control, lack of neck and trunk balance, and
poor coordination. Disuse osteopenia and medication-associated
osteomalacia frequently present treatment challenges in this patient
population.
-
Cerebral palsy
-
Syringomyelia
-
Spinal cord tumor
-
Spinal cord trauma
-
Spinocerebellar degeneration
-
Poliomyelitis
-
Spinal muscular atrophy
-
Dysautonomia
-
Muscular dystrophy
-
Arthrogryposis
-
Congenital hypotonia
-
Myotonia dystrophica
neuromuscular scoliosis is to maintain the spine in a balanced position
in both the coronal and sagittal planes over a level pelvis. Bracing is
sometimes effective at controlling structural curves prior to the
adolescent growth spurt, delaying the need for surgical stabilization.
With the onset of puberty, control is often lost necessitating
operative intervention. Prior to that, wheelchair seating adaptations
allow for upright trunk positioning and provide some control of
pathologic reflexes. Additionally, they have the advantage of not being
as restrictive as spinal orthoses. In general however, bracing in the
patient with neuromuscular scoliosis should be viewed as slowing the
inevitable progression of the curve until the child can safely undergo
definitive spinal surgery.
scoliosis is associated with much higher rates of complication than
those encountered with the surgical treatment of AIS. The general state
of the patient’s health, poor bone quality, poor nutritional status,
impaired respiratory function, and susceptibility to infection all may
adversely affect outcome. Prior to embarking on surgical stabilization
patients with neuromuscular scoliosis should have a thorough evaluation
of respiratory and cardiac function, nutritional status, and risk for
seizure. Patients with preoperative vital capacity less than 30% of the
predicted normal value may require prolonged postoperative respiratory
support. An albumin of less than 3.5 mg/dL is associated with a higher
rate of infection, prolonged intubation, and longer hospitalization.
Evaluation and treatment of low-grade urosepsis and metabolic bone
disease is equally important.
neuromuscular scoliosis is to produce a stable, balanced spine over a
stable, balanced pelvis. This maximizes pulmonary function, sitting
ability, functional independence, and ease of handling. Usually this
involves fusion from the thoracic spine to the pelvis with segmental
spinal instrumentation.
is most prevalent in patients with quadriplegia and total body
involvement. In general, the severity of the scoliosis parallels the
severity of the neurologic involvement. Functional decline in these
patients parallels progression of the spinal deformity. Progressive
functional impairment may limit walking tolerance in ambulatory
patients and sitting tolerance in nonambulatory patients. Patients may
even entirely lose the ability to sit or stand.
are sometimes difficult to gauge. In general, if progression
compromises function, causes pain, or increases nursing care demands,
the deformity should be treated. Benefits from adaptive seating
devices, frequent positional changes by caregivers, and the judicious
use of braces should be maximized prior to surgical intervention.
Surgery should be considered in patients with severe (more than 50
degrees) progressive curves that cause problems with seating. Medical
comorbidities, including poor nutritional status, must be addressed
prior to surgery. This may involve a nutritional improvement schedule
or placement of a gastrostomy or gastrojejunostomy feeding tube prior
to spinal surgery.
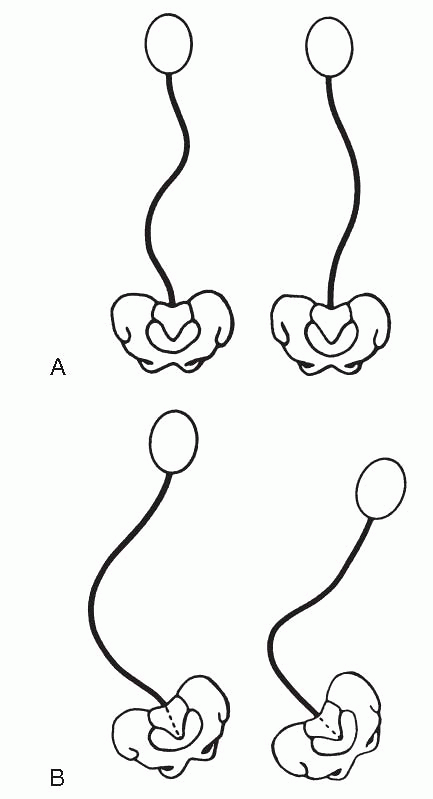
Group I curves are single or double curves without pelvic obliquity.
They usually occur in ambulatory patients and resemble curve patterns
seen in idiopathic scoliosis. Group II curves are long thoracolumbar or
lumbar curves with pelvic obliquity. They occur most often in
nonambulatory patients. While fusion to the pelvis should be avoided in
group I patients, it is advocated in patients with group II curves. In
both types of patients fusion should extend proximally above the
thoracic kyphosis, to reduce the tendency toward thoracic
hyperkyphosis. Preoperative bending or traction radiographs help
determine the flexibility of the curve and the necessity of anterior
fusion. When traction radiographs fail to balance the thorax over the
pelvis, an anterior/posterior fusion is usually indicated. Combined
anterior/posterior fusion should also be considered in patients with
large lumbar curves, rigid pelvic obliquity, and significant
anterior
growth potential. Data suggest that combined anterior/posterior
procedures facilitate spinal correction and a higher fusion rate in the
neuromuscular population. Whether to perform combined
anterior/posterior surgery in one sitting or as a staged procedure is
subject to debate.
 |
|
Figure 7.2-1 Curve patterns of cerebral palsy scoliosis. Group I curves (A) are double curves with thoracic and lumbar components. There is little pelvic obliquity. Group II curves (B)
are large lumbar or thoracolumbar curves with marked pelvic obliquity. (Lonstein JE. Spine deformities due to cerebral palsy. In: Weinstein SL. The pediatric spine: principles and practice, 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2001:800.) |
multidiscipline intensive care specialists. Short-term goals include
stabilization of pulmonary function, reinstitution of nutritional
supplementation, and achieving upright posture.
progressive diseases with unique phenotypic and genetic features.
Duchenne muscular dystrophy is most common, with an incidence of 30 per
100,000 liveborn males. It is inherited as a sex-linked recessive trait
characterized by a mutation of the gene coding for the structural
protein dystrophin. The pathogenesis involves rapid deterioration and
regeneration of muscle fibers until the repair capacity is overrun.
Eventually irreversible degradation occurs and fat and connective
tissue replace muscle cells. The disorder is present at birth, but only
becomes apparent between ages 3 and 5. By age 12, most patients are
confined to a wheelchair. It is universally fatal by early adulthood.
dystrophy develop a progressive scoliosis. Treatment objectives include
maintaining the patient in an upright, ambulatory status for as long as
possible. This involves judicious use of lower extremity surgery and
bracing. Scoliosis progresses rapidly once the child is
wheelchair-bound and spinal orthoses are ineffective in preventing
progression. The spinal deformity and the myopathy itself result in a
progressive restrictive lung disease. By 14 years of age, the
functional vital capacity is usually less than 50% of predicted normal
values. With decreasing pulmonary function, respiratory complications
rise. Surgery is indicated for curves 25 degrees or greater in patients
with a functional vital capacity of at least 20% to 30% of the normal
predicted value. The goals of surgery are to maintain the patient’s
sitting ability, relieve pain, and improve quality of life. Standard
surgical procedure involves posterior spinal fusion with
instrumentation from T2 to L5 or the pelvis if there is significant
pelvic obliquity. Postoperative goals include aggressive weaning of
ventilatory support and rapid mobilization to an upright posture.
J, Akbarnia B. Operative treatment of spinal deformities in patients
with cerebral palsy or mental retardation. J Bone Joint Surg (Am)
1983;65:43.
JE, Winter RB, Moe JH, et al. Neurological deficits secondary to spinal
deformity: a review of the literature and report of 43 cases. Spine
1980;5:331.
I. Scoliosis in muscular dystrophy: some comments about diagnosis,
observations on prognosis, and suggestions for therapy. Clin Orthop
1973;93:235.
elements begins with the migration of ectodermal cells between the
endoderm and ectoderm to form the mesoderm at the end of the second
week of development. The mesoderm forms pairs of somites that line both
sides of the notochord and develop into the dermatomes, myotomes, and
sclerotomes. Sclerotomal cells further differentiate to ultimately
surround the notochord to form the vertebral bodies and posterior
elements. Each sclerotome contributes the intervertebral disc and the
caudal half of the vertebra above and the cephalad half of the vertebra
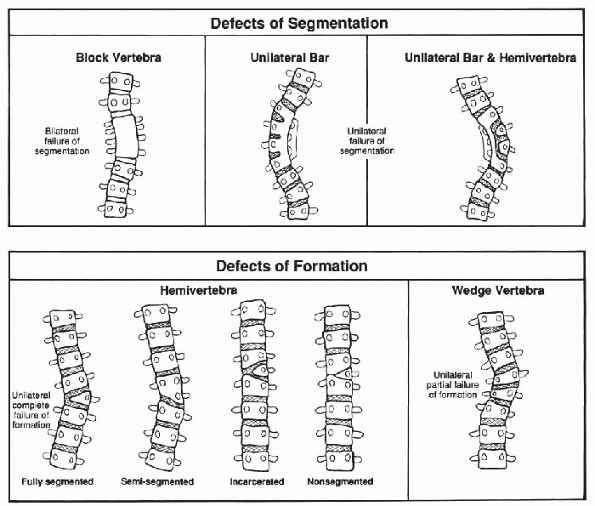
below. Congenital spinal disorders result from a developmental defect
in the formation and differentiation of the sclerotome during the
fourth to sixth weeks of development. The resulting defects are
classified as errors of segmentation, errors of formation, or mixed
types (Fig. 7.3-1).
congenital anomalies involving other organ systems. The genitourinary
tract is most commonly affected with an incidence between 20% and 33%.
Many of the genitourinary anomalies do not demand treatment because
they are anatomic variants with normal renal function, but obstructive
uropathy is not uncommon. Thus, all patients with congenital spine
anomalies should have an evaluation of the genitourinary system by
ultrasound or MRI. Cardiac anomalies are present in 10% to 15% of
patients and may go undiagnosed until the spine anomalies are
discovered.
congenital spine anomalies has been observed. Careful neurologic
examination, including abdominal reflexes, may uncover subtle deficits
resulting from tethered cord, diastematomyelia, or syringomyelia.
Examination of the skin overlying the vertebral column is important.
Hair patches, dimples, and subtle pigmentation often herald underlying
spinal dysraphism. Radiographs should be examined for interpedicular
widening and midline bony
speckles. Screening MRI should be obtained in all patients being treated for congenital spinal anomalies.
 |
|
Figure 7.3-1
Congenital scoliosis. (From McMaster MJ. Congenital scoliosis. In: Weinstein SL. The pediatric spine. New York: Raven Press, 1994:229.) |
vertebral development. It is classified into errors of formation,
errors of segmentation, or mixed deformities (Fig. 7.3-1).
Errors of formation include hemivertebra and wedge vertebra. Errors of
segmentation include unilateral bars and block vertebrae. Associated
sagittal plane deformity is common.
One must accurately document the type of anomaly and its magnitude from
the initial radiographs. All congenital curves must be examined
periodically to determine whether they are progressive or not. Some
curves never progress while others that have been stable for many years
suddenly progress with the adolescent growth spurt. The majority of
congenital curves, however, are progressive.
anomalies is essential as it guides prognosis and treatment. The rate
of progression depends on the area of spine involved and the type of
anomaly. Thoracic curves have a poor prognosis. Unilateral thoracic
unsegmented bars with contralateral, fully segmented vertebrae carry
the worst prognosis and have the greatest likelihood of progression.
Other anomalies with high rates of progression include unilateral
unsegmented bar, double ipsilateral hemivertebrae, and single fully
segmented hemivertebra. Block vertebrae have the best prognosis. The
most common anomaly is a single hemivertebra. Its risk of progression
is unpredictable. Hemivertebra at the lumbosacral junction should be of
particular concern because there is no room for natural compensation to
occur below the anomaly. These patients often have a progressive list
to one side. Similarly, unbalanced hemivertebrae at the cervicothoracic
junction often result in a progressive head tilt.
reliably progressive should have early fusion before the deformity
becomes rigid and more difficult to treat successfully with surgery.
Such anomalies include the unilateral unsegmented bar with or without
contralateral hemivertebra. Close observation is essential in care of
patients with anomalies whose natural history is less reliable, such as
hemivertebrae and mixed deformities. Such patients must be examined
both clinically and radiographically every 4 to 6 months, especially
during the first 4 years of life and the adolescent growth spurt. Close
comparisons of films at successive visits is essential.
of congenital scoliosis. Braces should not be used with short rigid
curves, unsegmented bars, and congenital kyphosis. Their role should be
limited to mixed flexible anomalies with progressive secondary curves.
They will always fail if used to treat curves whose natural history
dictates surgical intervention.
congenital scoliosis. Any intraspinal pathology must be addressed prior
to instrumentation and fusion. There is no single algorithm to
determine what procedure to perform or at what age it should be done.
Patients with unilateral unsegmented bars with or without contralateral
hemivertebrae and those with two ipsilateral hemivertebrae have
reliably progressive curves and should undergo early operative
intervention. Documented progressive curves should also be treated
surgically. Options include hemivertebra excision, convex growth arrest
(hemiarthrodesis/hemiepiphysiodesis), posterior fusion in situ,
instrumentation without fusion, and combined anterior and posterior
fusion. In immature patients circumferential fusion should be
considered to avoid the crankshaft phenomenon. If fusion without
instrumentation is performed, postoperative casting may favorably
affect correction. Instrumentation generally is used to stabilize the
spine rather than to impart corrective forces. In general, treatment
should be directed at early intervention to avoid severe rigid curves
that are technically more difficult to treat.
caused by errors of formation or errors of segmentation of the
vertebral elements. Pure anterior failure of formation results in pure
kyphosis while anterolateral errors of formation result in
kyphoscoliosis. Complex errors of formation may even result in sagittal
translation of the vertebral column or narrowing of the spinal canal.
Such deformities are known as congenital spondylolisthesis and
segmental spinal dysgenesis, respectively.
scoliosis, they are complicated more often by neurologic compromise
including paraplegia. This is of particular concern with errors of
formation in the upper thoracic area where the blood supply to the cord
is tenuous. The paraplegia may occur early in life but is more common
during the adolescent growth spurt with rapid progression of the
kyphosis. Errors of segmentation causing kyphosis may involve single or
multiple levels resulting in a rounded kyphosis with little risk of
neurologic compromise. The rate of progression in deformity due to
errors of segmentation is usually less than that due to errors of
formation because the bars form late in the juvenile years, and the
growth discrepancy is not as great.
universally poor, especially for errors of formation. There is usually
no role for nonoperative treatment, including bracing. Surgical options
include posterior fusion and combined anterior and posterior fusion.
Patients with neurologic findings also often require anterior
decompression.
MJ, Ohtsuka K. The natural history of congenital scoliosis: a study of
two hundred and fifty-one patients. J Bone Joint Surg (Am) 1982;64:1128.
RB, Moe JH, MacEwen GD, et al. The Milwaukee brace in the nonoperative
treatment of congenital scoliosis. Spine 1986;1:85.
requires an understanding of distinctive anatomic and biologic
characteristics that differ significantly from adults. While cervical
spine disorders in adults usually present with pain, infants and
children usually present with deformity as the primary symptom. The
pediatric cervical spine approaches the adult configuration at 8 years
of age. Hypermobility, incomplete ossification, and hyperlaxity of
ligamentous and capsular structures are all characteristics of the
normal immature cervical spine. The relative laxity and horizontal
orientation of the facet joints allow for increased mobility in
children. Congenital abnormalities and developmental alterations
further complicate evaluation and treatment. Connective tissue
disorders and syndromes associated with ligamentous laxity may
accentuate the baseline hypermobility and cause progressive
instability and serous neurologic injury or sudden death.
 |
|
Figure 7.4-1
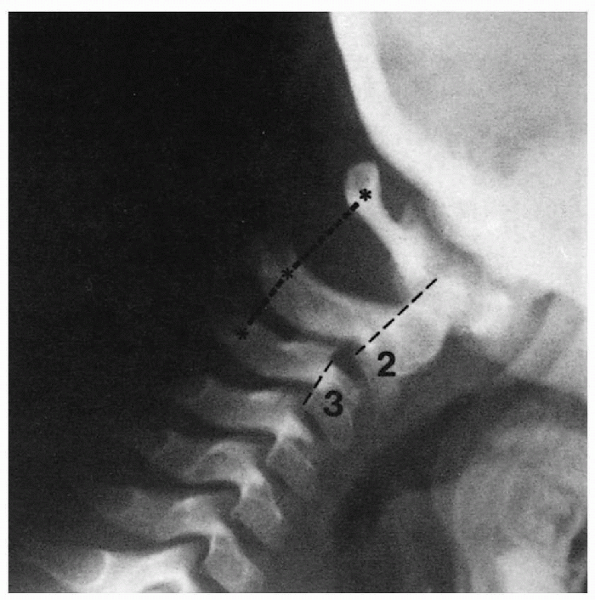
Lateral cervical radiograph of a child with pseudosubluxation at C2-C3. The step-off at C2-C3 is present, but the posterior cervical line of Swischuk is normal. (From Loder RT, Hensinger RN. Developmental abnormalities of the cervical spine. In: Weinstein SL. The pediatric spine: principles and practice, 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2001:317.) |
children should include anteroposterior, lateral, and open-mouth views.
Misinterpretation of normal findings as pathologic is not uncommon. Pseudosubluxation is commonly misdiagnosed as pathologic instability (Fig.7.4-1).
Persistence of the basilar synchondrosis of C2, loss of normal cervical
lordosis, and variations in prevertebral soft tissue appearance may be
normal findings in some children. Instability is best evaluated by
flexion-extension lateral views.
 |
|
Figure 7.4-2
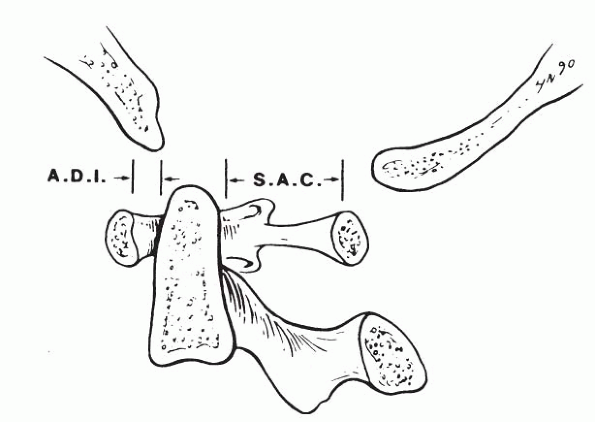
Atlantoaxial joint demonstrating the normal atlasdens interval (ADI) and the normal space available for the spinal cord (SAC). (From Moskovitch R. Cervical instability [rheumatoid, dwarfism, degenerative, others]. In: Bridwell KH, DeWald RL, eds. The textbook of spinal surgery, 2nd ed. Vol 1. Philadelphia: Lippincott-Raven, 1997:970.) |
The ADI is used to measure the stability of the atlantoaxial
articulation and is measured on a flexion-lateral view of the C-spine.
Normal is 4.5 mm or less in a child, and 3.5 mm or less in an adult. An
increased ADI indicates potential C1-C2 instability. In this setting, a
decreased SAC signals possible catastrophic encroachment of the cord.
Computed tomography (CT) scanning is indicated to more clearly define
complex anomalous bony anatomy. MRI is useful for evaluating
paracervical soft tissues and spinal cord pathology.
in 1 in 700 live births. It is the result of a nonsegregation of
chromosome 21 during meiosis, causing trisomy 21. Phenotypic features
of affected individuals include characteristic facies, mental
retardation, congenital heart disease, and ligamentous laxity. Cervical
instability is common in those with Down syndrome. Progressive
instability with neurologic deficits is seen more commonly in boys than
in girls. Patients with instability may present with gait
abnormalities. Careful regular neurologic examination is mandatory.
Surveillance cervical spine flexion-extension views should be obtained
at least every 3 years. Recommendations for asymptomatic patients with
an ADI greater than 5 mm include avoidance of activities that stress
the cervical spine including contact sports and diving. Symptomatic
patients and patients with an ADI greater than 10 mm regardless of
symptomatology should undergo C1-C2 fusion. Surgical complications,
including pseudoarthrosis and high mortality occur more frequently in
patients with Down syndrome.
visceral anomalies with a variable phenotypic presentation. The classic
triad of fusion of cervical vertebra, shortening of the neck, and a low
hairline is only present in 50% of patients. The term Klippel-Feil syndrome
is used for any patient with fusion of the cervical vertebrae.
Associated findings include a 70% incidence of scoliosis, deafness,
Sprengel deformity, synkinesis, cervical ribs, and congenital heart and
renal disease. Children with Klippel-Feil usually present with concerns
relating to the appearance and motion of the neck or complaints of neck
pain. Physical examination is usually notable for decreased neck range
of motion, especially lateral bending and rotation. A thorough
neurologic examination is mandatory. Positive findings may include
cranial nerve abnormalities, cervical radiculopathy, or even
myelopathy. Radiographic analysis should include standing full-length
posteroanterior and lateral views of the entire spine as well as
flexion-extension lateral views of the cervical spine. It is believed
that children with multiple fused segments are at greater risk for
instability through
the
unfused segments, and should be kept out of contact sports to prevent
catastrophic injury. Patients with radiographic evidence of progressive
instability clearly are at risk for early neurologic injury. Treatment
recommendations include regular flexion-extension radiographic
examination in asymptomatic children. The treatment of symptomatic
patients includes activity limitations, use of cervical orthoses, and
traction. Posterior cervical fusion is recommended for progressive
symptomatic segmental instability or neurologic compromise.
disorders of bone and cartilage growth with variable phenotypic
presentations of dwarfism. Angular deformities of the extremities,
premature degenerative joint disease, and spinal disorders including
cervical instability are common clinical features of these disorders.
The diagnosis is often made during early growth when disproportion of
the trunk and limbs becomes apparent. Any child below the fifth
percentile in growth should be evaluated for a skeletal dysplasia or
metabolic disorder. Identification of the exact skeletal dysplasia is
often difficult and should be made in consultation with a geneticist.
(SED) refers to a subgroup of osteochondrodysplasias that are
characterized by primary epiphyseal and vertebral involvement. Despite
some clinical heterogeneity, they all result in short-trunk dwarfism.
Associated anomalies include pectus carinatum, scoliosis, genu valgum,
and hip flexion contractures. Radiographic features include delayed
vertebral body and epiphyseal ossification, platyspondyly, and coxa
vara. Up to 40% of children with SED develop atlantoaxial instability.
Odontoid hypoplasia and os odontoideum are common features that
predispose to this instability. Persistent hypotonia and delay in motor
milestones are early manifestations of neurologic compromise caused by
the instability. Lateral cervical spine flexion-extension radiographic
examination is mandatory for all patients with SED. Surveillance
radiographs should be obtained at least every 3 years. Surgical
stabilization is recommended for children with instability with
neurologic compromise. Prophylactic stabilization should be considered
for asymptomatic children with instability greater than 5 mm.
are another subgroup of osteochondrodysplasias with a high incidence of
significant cervical spine involvement. Although they share many
features, specific clinical features and the natural history of these
disorders vary depending on the specific enzyme deficiency. These
disorders produce a proportionate dwarfism caused by the accumulation
of mucopolysaccharides due to an inherited hydrolase enzyme deficiency.
The diagnosis is made by urine screening for mucopolysaccharides and
confirmed by serum testing for the specific sugar abnormality. Morquio
syndrome is the most common form and is characterized by the inability
to metabolize keratin sulfate. Clinical manifestations include coarse
facial features, abnormal dentition, kyphosis, corneal clouding,
ligamentous laxity, and joint stiffness. Radiographic features include
oval-shaped vertebral bodies with anterior beaking, coxa vara with
unossified femoral heads, bullet-shaped metacarpals, and wide, flat
ilia. Odontoid hypoplasia is common in children with Morquio syndrome.
Atlantoaxial instability with progressive myelopathy is one of the most
disabling features. Lateral cervical spine flexion-extension
radiographic examination is mandatory for all patients with
mucopolysaccharidoses. Surveillance radiographs should be obtained at
least every 3 years. Surgical stabilization is recommended for children
with instability with neurologic compromise. Prophylactic stabilization
should be considered for asymptomatic children with instability greater
than 5 mm.
common osteochondrodysplasias. It results in disproportionate
short-limb dwarfism. It is an autosomal dominant condition caused by
abnormal endochondral bone formation. Clinical features include frontal
bossing, flattened nasal bridge with midface hypoplasia, trident hands,
kyphosis, and lumbar stenosis. Atlantoaxial instability is uncommon but
stenosis of the foramen magnum may present with sleep apnea or sudden
death. Foramen magnum decompression should be performed for repeated
apneic episodes or evidence of neurologic compromise. Prophylactic
decompression is not recommended.
deformity of the cervical spine. Its presence is usually indicative of
an atlantoaxial problem, as 50% of cervical spine rotation occurs
through this joint. A number of conditions are associated with
torticollis (Box 7.4-1) but congenital muscular torticollis and atlantoaxial rotary subluxation are the most common causes in children.
is the most common type of torticollis in the infant and young child.
The deformity is usually discovered within the first 2 months of life
and is caused by a contracture of the sternocleidomastoid muscle. The
exact etiology is unknown but current theory suggests that it is the
result of a compartment syndrome of that muscle. The head tilts toward
the side with a tight sternocleidomastoid and the chin rotates toward
the opposite shoulder. The right sternocleidomastoid muscle is involved
75% of the time. Occasionally an “olive-like” mass is palpable within
the muscle belly. Cervical spine radiographs should be obtained to rule
out congenital vertebral
anomalies.
Careful examination of the hips, including a screening ultrasound, is
mandatory since 20% of children with congenital muscular torticollis
also have developmental dysplasia of the hip. Initial treatment is
nonoperative and includes a home stretching program supervised by a
physical therapist. This is effective in 90% of cases. Surgical release
is indicated if the deformity persists after 1 year of age, since
stretching is usually ineffective in older children. If untreated,
skull and facial flattening (plagiocephaly) may result during the first
year of the child’s life.
is the most common cause of acquired childhood torticollis. The patient
generally presents with discomfort and a torticollis that has developed
following trauma, an upper respiratory infection, or following head and
neck surgery. Even routine events, like vomiting, may cause
subluxation. Grisel syndrome is
spontaneous atlantoaxial subluxation that follows an upper respiratory
infection. It is caused by an inflammatory ligamentous laxity following
the infectious process.
differentiate which sternocleidomastoid muscle is tight. In torticollis
associated with atlantoaxial rotatory subluxation the
sternocleidomastoid muscle opposite to the head tilt is stretched
causing the muscle to go into spasm. In congenital muscular torticollis
the head tilts toward the shoulder on the same side as the involved
contracted muscle. Plain radiographs are often difficult to obtain
because the child cannot be positioned properly. The open-mouth
odontoid view may show a medial and lateral offset of the lateral
masses. A dynamic CT scan of C1-C2 in which the head is rotated
maximally to both sides should be obtained in all suspected cases. A
fixed relationship of C1 to C2 is diagnostic.
on the duration and severity of symptoms. Patients who have had the
symptoms for less than 1 month should be hospitalized and treated with
cervical traction. This usually reduces the subluxation and corrects
the torticollis. After confirmation of reduction by CT scan, the
patient should be immobilized in a soft collar for 4 to 6 weeks. For
subluxations that have been present for more than 1 month, posterior
spinal fusion of C1 and C2 is usually required because the deformity is
either irreducible or persistently recurs when the patient is removed
from traction.
R, Lang JE, MacEwen GD. Klippel-Feil syndrome: a constellation of
associated anomalies. J Bone Joint Surg (Am) 1974; 56:1246-1253.
adolescents is unknown. The literature suggests that significantly more
children have back pain than the number who actually seek medical
attention. The differential diagnosis of back pain in children, unlike
adults, more often includes neoplastic, developmental, and inflammatory
conditions (Box 7.5-1). Overuse and lumbar
strain should be diagnoses of inclusion after all other causes for back
pain have been excluded. The evaluation of a child with back pain must
include a detailed history, physical examination, radiographic
evaluation, and appropriate laboratory and diagnostic imaging studies
when indicated.
-
Overuse syndrome
-
Herniated disc
-
Spondylolysis/spondylolisthesis
-
Scheuermann disease
-
Benign
-
Malignant
-
Discitis
-
Osteomyelitis
-
Rheumatologic disorders
regarding the onset, location, frequency, severity, and duration of
symptoms. Alleviating and aggravating factors must be addressed
specifically. Pain that is promptly relieved by nonsteroidal
antiinflammatory drugs (NSAIDs) may be related to an osteoid osteoma.
Back pain associated with pain in other joints relieved by NSAIDs is
usually related to an underlying rheumatologic disorder.
level and degree of sport participation. Competitive gymnasts, for
example, have a relatively high incidence of spondylolisthesis.
Traumatic events and changes in activity or athletic participation
require careful exploration. The examiner
must
learn from both the patient and the parent how the pain is affecting
the child’s normal activities and routines. A child who self-limits
enjoyable activities because of pain requires a thorough evaluation.
who wakes in the middle of the night with pain and is unable to return
to sleep must be considered to have a neoplastic or inflammatory
condition until proven otherwise. Other common causes of back pain in
children including overuse syndromes, spondylolysis, and Scheuermann’s
disease typically improve at night with rest.
legs and changes in bowel and bladder habits must be carefully
documented. Similarly, it is important to note symptoms of weight loss,
fever, chills, lethargy, rashes, and infections.
orthopedic screening examination including assessment of both upper and
lower extremities and the patient’s gait. Subtle changes in gait may
reflect an underlying neurologic problem. The evaluation of the spine
should include the Adams forward bending test. Inability to flex
forward in a supple manner should alert the examiner as such patients
may have a spondylolysis or spinal cord neoplasm. Similarly,
lumbosacral pain that worsens with hyperextension seems to correlate
well with spondylolysis and spondylolisthesis. Assessment of leg
lengths, trunk shift, sagittal plane balance, and complete neurologic
examination including abdominal reflexes are mandatory.
anteroposterior and lateral views of the spine in all children
presenting with pain. Thereafter, the working differential diagnosis
should guide additional radiographic and laboratory tests. Oblique
views are useful in children with suspected spondylolysis. Children
with normal radiographs and significant pain should have a technetium
bone scan. Singlephoton emission computed tomography (SPECT) bone scans
have shown an increased sensitivity and specificity in finding
fractures in the lumbar spine as compared to traditional bone scans.
SPECT may provide further insight in patients with suspected
spondylolysis and negative plain bone scans. CT scans are useful to
more clearly define bone pathology detected by bone scan. MRI is useful
for patients with neurologic signs or symptoms. It is invaluable in
diagnosing spinal cord tumors, syringomyelia, tethered cord, and disc
herniations.
is relatively uncommon in children and adolescents. Children with disc
herniations may present with back pain with or without leg pain.
Symptoms are often related to traumatic events. On physical examination
patients usually demonstrate a positive straight leg raise test but
neurologic deficits are uncommon. Most disc herniations occur at the
L4-L5 and L5-S1 levels. MRI is the best test to demonstrate a herniated
disc. Treatment is the same as in adults. Rest, NSAIDs, and physical
therapy comprise the initial line of treatment. Patients who fail
nonoperative treatment usually improve with disc excision without
fusion.
 |
|
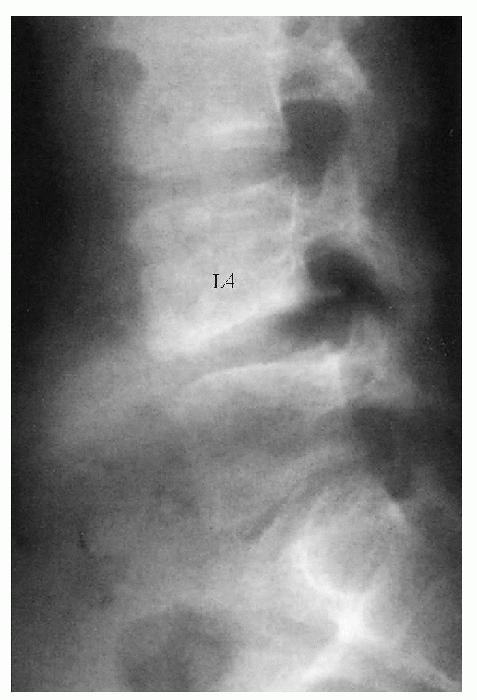
Figure 7.5-1
A 15-year-old patient complained for several months of severe right sciatica after having lifted a heavy weight. The spot lateral lumbar spine shows a localized kyphosis at L4-L5 and an avulsed piece from the inferior border of L4. (From Arlet V, Fassier F. Herniated nucleus pulposis and slipped vertebral apophysis. In: Weinstein SL. The pediatric spine: principles and practice, 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2001:461.) |
It most often occurs in adolescents following trauma and presents
similar to a disc herniation. It can be visualized as a bony fleck
within the spinal canal but is best visualized by CT. Treatment is
surgical excision.
the area between the superior and inferior articular facets of the
posterior vertebral arch. The defect may be unilateral or bilateral.
Spondylolisthesis occurs in the setting of a spondylolysis when one
vertebral body slips forward in relation to the vertebral body below.
It is graded by the percentage of uncovered length of the superior
border of the vertebral body below the slip measured on standing
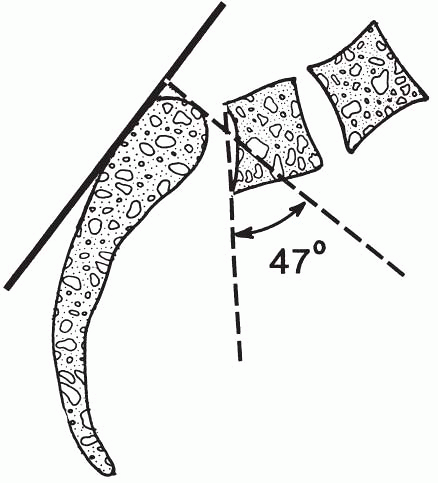
lateral radiographs (Fig. 7.5-2). The slip angle is used to quantify the resulting lumbosacral kyphosis in high-grade slips (Fig.7.5-3). Spondylolysis and spondylolisthesis are common causes of back pain in children and adolescents older than 10 years.
dysplasia of the L5-S1 facet joint that allows forward slippage of L5
on the sacrum. Isthmic spondylolysis is characterized by a
developmental defect of the pars interarticularis. The defect is
usually in L5 or the sacrum. The etiology is believed to be a stress
fracture of the pars interarticularis caused by repetitive lumbar
hyperextension. It is common in young gymnasts, wrestlers, and football
linemen. The defect generally develops between the ages of 5 and 8. Its
incidence is 6% in the general population.
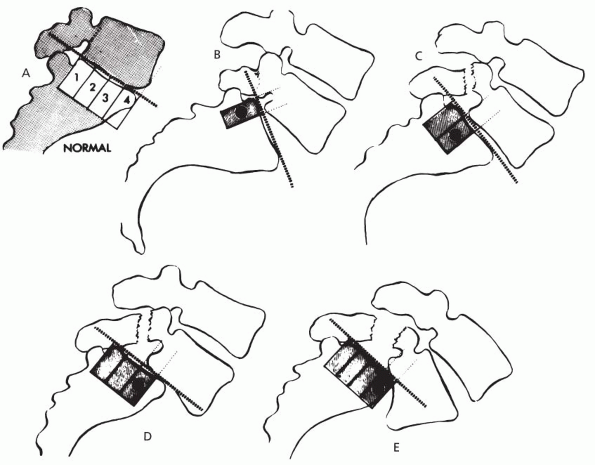 |
|
Figure 7.5-2 Meyerding classification of spondylolisthesis. (A) Normal or grade 0. (B) 1% to 25% slippage is grade I. (C) Up to 50% is grade II. (D) Up to 75% is grade III. (E)
76% to 100% slippage is grade IV. (From Hu SS, Bradford DS. Spondylolysis and spondylolisthesis. In: Weinstein SL. The pediatric spine: principles and practice, 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2001:436.) |
spondylolisthesis are asymptomatic. Patients with symptomatic
spondylolysis present with complaints of activity-related back pain.
Symptoms can be acute and associated with trauma but usually are
insidious in onset. They may be mild or quite severe, causing the child
to limit sporting activities. The severity of the slip may not
correlate with the severity of symptoms. Patients may present with
hamstring tightness and a knee-flexed, hip-flexed gait (Phalen-Dickson sign). Neurologic signs and symptoms are rare, but can occur with severe slips.
 |
|
Figure 7.5-3
The slip angle (47 degrees shown) is determined by drawing a line along the posterior cortex of the sacrum and measuring the angle between its perpendicular and a line drawn along the inferior border of L5. (From Hu SS, Bradford DS. Spondylolysis and spondylolisthesis. In: Weinstein SL. The pediatric spine: principles and practice, 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2001:436.) |
spondylolysis and negative plain radiographs. It is also useful in
determining the acuity of the fracture. CT imaging can differentiate a
pars defect from other entities that may result in a positive bone
scan, including neoplasms.
 |
|
Figure 7.5-4
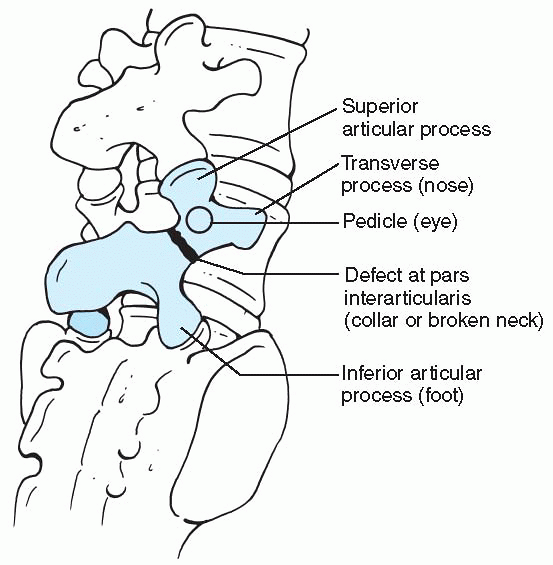
“Scotty dog” seen on oblique views of the lumbar spine. (From Smith JA, Hu SS, et al. Management of spondylolysis and spondylolisthesis in the pediatric and adolescent population. Orthop Clin North Am 1999;30:488.) |
therapy comprise the initial line of treatment for patients with a
symptomatic spondylolysis or low-grade spondylolisthesis. Brace
immobilization with a modified TLSO to reduce lumbar lordosis is also
useful in alleviating symptoms, and may even heal early defects. The
risk of progression of a spondylolisthesis is related to the patient’s
age and the grade of slip at presentation.
nonoperative treatment and patients who present with high-grade slips.
Young patients with a spondylolysis but no spondylolisthesis may
undergo direct repair of the pars defect. This involves debridement of
the defect, autogenous bone grafting, and internal fixation with either
screws or wires. Alternatively, such patients may be treated with
lateral in situ fusion with or without
instrumentation. The addition of pedicle screw fixation enables rapid
patient mobilization and obviates the need for prolonged postoperative
bracing. Skeletally immature patients with slips greater than 50% or
with slip angles greater than 45 degrees have a high rate of
progression and should undergo surgery regardless of their
symptomatology. Patients with nerve root or thecal sac compression
require decompression in addition. There is considerable debate
regarding the role of reduction of high-grade slips. Spondyloptosis is
usually treated with fusion in situ with a
fibula strut graft or vertebral column shortening with a L5
vertebrectomy. Complications include slip progression, pseudoarthrosis,
and neurologic compromise.
thoracic or thoracolumbar spine. It is the most common cause of
structural kyphosis in adolescence. Its prevalence is reported to be
between 4% and 8%, affecting boys and girls equally. Its etiology is
unknown although there appears to be underlying genetic factors.
 |
|
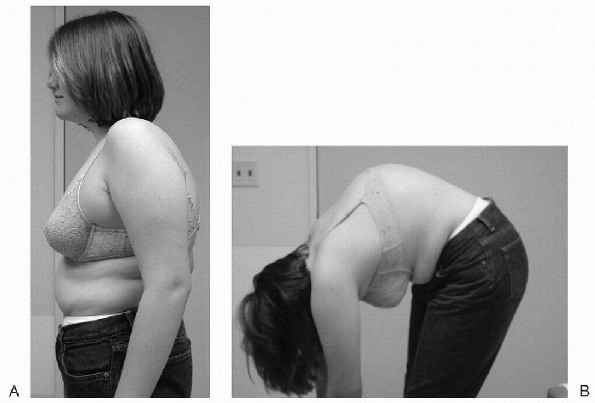
Figure 7.5-5 (A) A 14-year-old patient with Scheuermann kyphosis. (B) Note the “A-frame” deformity on forward bending.
|
Cosmetic concerns usually cause the patient’s parents to seek medical
attention. Occasionally, the deformity is attributed to poor posture
delaying the diagnosis and treatment. Pain and fatigue are common
complaints but are usually mild and related to exercise or prolonged
sitting. When present, the pain is usually localized to the apex of the
deformity and disappears with skeletal maturity. Severe,
activity-limiting pain is uncommon with Scheuermann’s disease.
Persistent or severe low back pain in patients with Scheuermann’s
disease may be related to spondylolisthesis, which is noted with an
increased incidence in these patients.
disease is readily apparent on physical examination. Observation from
the side with the patient standing erect reveals a thoracic or
thoracolumbar kyphosis (Fig. 7.5-5). It is
sharply angulated and fixed, even with hyperextension of the spine. The
kyphosis becomes more prominent in the position of the Adams forward
bending test and is likened to an “A-frame” deformity. Typically, the
cervical spine and the lumbar spine display increased flexible
lordosis, while the overall sagittal balance is well maintained. This
lumbar hyperlordosis and subsequent degenerative disc and facet
arthropathy predispose adults to low back pain. The shoulder girdles
are often rotated anteriorly, which in combination with the kyphosis
can produce an awkward stooped appearance. The arms and legs will
appear relatively long compared with the shortened truck. Concomitant
scoliosis occurs in about one-third of patients. Hamstring tightness is
a common finding. Neurologic dysfunction has been reported but is rare
and, if present, requires a thorough evaluation including MRI of the
spinal cord. Cardiopulmonary complaints are extremely rare on initial
presentation,
although restrictive pulmonary disease can be seen in patients with kyphosis measuring greater than 100 degrees.
 |
|
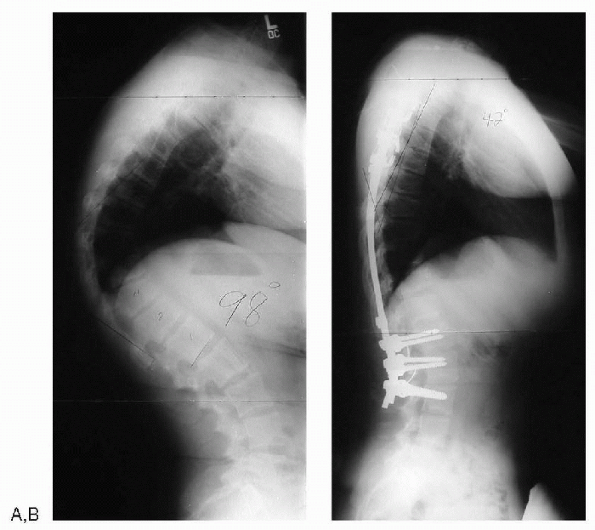
Figure 7.5-6 (A) Standing lateral radiograph of a patient with Scheuermann’s disease. (B) After anterior releases and posterior spinal fusion with instrumentation.
|
long cassette posteroanterior and lateral views as well as a supine
hyperextension lateral view over a bolster. Normal thoracic kyphosis in
adolescents is 20 to 45 degrees, although it varies with age and is
slightly greater in women than in men. The radiographic criteria for
Scheuermann’s disease, include increased thoracic kyphosis with
anterior wedging of 5 degrees or more of at least three adjacent
vertebral bodies. Both the vertebral wedging and the kyphosis should be
measured by the Cobb technique. When evaluating serial radiographs,
care should be taken to ensure that the same levels are being measured.
Associated radiographic findings include Schmorl nodes, irregularity
and flattening of the vertebral end plates, narrowing of the
intervertebral disc spaces, and anteroposterior elongation of the
apical vertebral bodies. Radiographic examination with forced extension
films is the most helpful tool in differentiating Scheuermann’s disease
from postural kyphosis (Fig. 7.5-6).
nonoperative and based on the magnitude of the deformity, the presence
of pain, and the age of the patient. Adolescents with a thoracic
kyphosis of less than 60 degrees should be treated with NSAIDs and a
thoracic extension exercise program. Skeletally immature patients with
kyphosis measuring greater than 60 degrees should also undergo brace
treatment. The brace should be a Milwaukee type with a neck ring. It
can be weaned with the onset of skeletal maturity. Surgical treatment
is reserved for patients with severe deformity (more than 75 degrees)
and those with neurologic compromise. Surgical options include
posterior spinal fusion with instrumentation and combined anterior
release with posterior fusion with instrumentation. The posterior-only
approach is generally reserved for skeletally immature patients or
skeletally mature patients with a kyphosis that corrects to at least 50
degrees on hyperextension lateral views. The anterior approach may be
performed either open or thoracoscopically. Overcorrection should be
avoided. Complications include the development of a junctional
deformity either above or below the fusion and neurologic compromise.
RJ, Heyman S, Drummond DS, et al. The use of single photon emission
computed tomography (SPECT) in the diagnosis of low-back pain in young
patients. Spine 1988;13:1155-1160.
JA, Epstein NE, Marc J, et al. Lumbar intervertebral disc herniation in
teenage children: recognition and management of associated anomalies.
Spine 1984;9:427.
TL, Anderson RL, Dearwater SR, et al. The epidemiology of low back pain
in adolescent population. Am J Public Health 1992;82: 606-609.
DL, Samuelson MA, Hale JM, et al. Complications of posterior iliac
crest bone grafting in spine surgery in children. Spine
2000;25:2400-2402.
MG, Stazzone EJ, Gelijns AC, et al. The effectiveness of preoperative
erythropoietin in avoiding allogeneic blood transfusion among children
undergoing scoliosis surgery. J Pediatr Orthop (Br) 1998;7: 203-209.
L. Spondylolisthesis: classification and etiology. Symposium on the
spine. American Academy of Orthopedic Surgeons. St. Louis: Mosby,
1969:143-166.
