Thoracolumbar Spine Fractures and Dislocations
the spinal column, with approximately 90% occurring within the thoracic
or lumbar regions.43 The vast
majority of these injuries have been shown to affect the motion
segments between T11 and L2 that comprise the thoracolumbar junction.54
These levels connect the relatively rigid, kyphotic thoracic spine,
which is stabilized by the rib cage to the more mobile, lordotic lumbar
vertebrae; consequently, this transitional zone may experience
substantial biomechanical stresses during traumatic incidents, which
generally make it more susceptible to fracture.
distribution, with peaks among males under 30 years of age and in the
geriatric population.54 In younger
patients, these fractures normally arise from high-energy blunt trauma
such as motor vehicle accidents, falls from a height, and
sports-related injuries. In particular, motorcycle riders are more
likely to sustain severe spinal fractures following a collision than
are occupants inside an automobile.114
Nevertheless, in recent years the incidence of thoracolumbar injuries
resulting from gunshot wounds and other projectiles has been increasing.21
Because of their poor bone density and declining mental status, the
elderly are also at risk for these fractures after a fall from a
standing position and other minor traumatic episodes. Unfortunately, as
many as 20% of thoracolumbar fractures will be accompanied by some type
of neurologic deficit, which corresponds to nearly 1 of every 20,000
individuals living in the United States.40 In addition
to the extensive morbidity sustained by these patients, the 1-year
mortality rate of patients with paraplegia or other catastrophic spinal
cord injuries approaches 7%.122
studies may all be useful for understanding the mechanism of injury for
a specific thoracolumbar fracture. In these situations, the spinal
column may be subjected to either a single or more often a combination
of forces including axial, flexion, extension, shear, and rotational
moments; the injury pattern is largely determined by the overall
direction and magnitude of these vectors. This information is not only
obligatory for elucidating the pathogenesis of these injuries but it
may also be critical for assessing their stability and directing
subsequent treatment. Unfortunately, even with a detailed history and
supporting imaging studies, the mechanism of injury is often impossible
to determine precisely and is often debated among spinal care providers.
generate compression fractures in which there is disruption of the
anterior vertebra with sparing of the middle and posterior portions of
the body (Fig. 43-1). These end plate or
wedge-shaped fractures are usually considered to be stable, but any
evidence of damage to the posterior ligamentous tension band may be
indicative of a more serious injury. With more significant axial
forces, the fracture line may extend posteriorly through the entire
body, which is characteristic of a burst injury (Fig. 43-2).
By definition, these fractures are more unstable than compression
injuries and frequently bring about compression of the neural elements
secondary to the retropulsion of bony fragments into the spinal canal.
Although in the past burst fractures were attributed primarily to axial
loading of the spine, the authors of a recent in vitro biomechanical
study reported that at least some degree of extension was also required
to reproduce the interpedicular widening and canal compromise that are
regularly observed in conjunction with these injuries.84
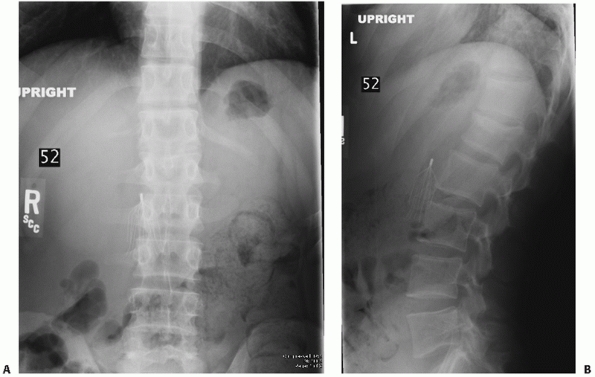 |
|
FIGURE 43-1 Anteroposterior (A) and lateral (B) radiographs depicting a compression fracture of the L1 vertebral body.
|
patterns depending on the position of the rotational axis and the
anatomic structures through which it passes (i.e., bone or disc space) (Fig. 43-3).
When the center of rotation is located within the spinal column in
close proximity to the posterior longitudinal ligament, the vertebra
will undergo compression as the posterior elements are distracted.
However, if this point is situated more anteriorly as is the case with
“seatbelt” fractures, the entire spine will fail in tension. In
contrast, a hyperextension mechanism has been implicated in the
development of shear fracture-dislocations, also referred to as
“lumberjack” injuries, in which the body is distracted while the
posterior spine is exposed to either compressive or tensile forces42 (Fig. 43-4).
setting of polytrauma until proved otherwise, especially when the
patient may be distracted by injuries to other organ systems; in one
series, approximately 24% of all thoracolumbar fractures were initially
missed in these cases.12 The
emergent care of these individuals should commence immediately in the
field where appropriate measures should be initiated to immobilize the
entire spinal column and minimize the risk of further injury as they
are extricated and transported to a hospital facility. Because these
fractures are routinely associated with other life-threatening
injuries, the basic treatment principles set forth in the Advanced
Trauma Life Support (ATLS) protocol should be meticulously adhered to
to ensure that the airway, breathing, and circulation are adequately
maintained. Resuscitation with supplemental
oxygen,
intravenous fluids, or cardiac pressors may also be warranted in
certain clinical scenarios, although it is important to differentiate
patients with hypovolemia who are tachycardic and hypotensive from
those with neurogenic shock where sympathetic dysfunction leads to both
decreased blood pressure and a paradoxical bradycardia.139
Strict spinal precautions including logrolling techniques and the use
of a backboard for all transfers must be followed until the existence
of any unstable injuries have been clearly ruled out by clinical
examination or imaging modalities, especially in trauma victims who
remain unconscious.
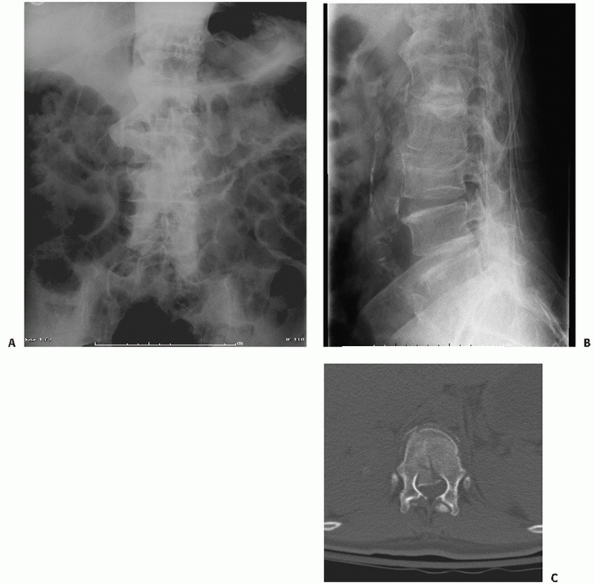 |
|
FIGURE 43-2 Anteroposterior (A) and lateral (B)
radiographs demonstrating a L3 burst fracture with retropulsion of bony fragments into the spinal canal noted on an axial CT image (C). |
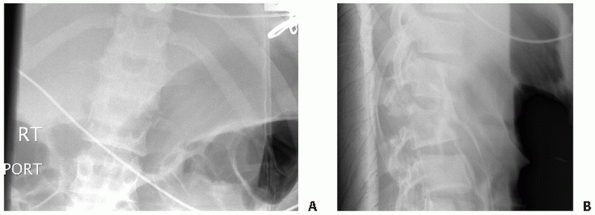 |
|
FIGURE 43-3 Anteroposterior (A) and lateral (B) radiographs revealing a Chance fracture of L3.
|
reasonable candidates for the administration of corticosteroids. The
initial mechanical contusion to the neural elements has been found to
precipitate a complex biochemical cascade, which ultimately leads to
tissue edema, microvascular changes with ischemic damage, and the
production of inflammatory factors.39 The primary objective of steroids and other pharmacologic
strategies is to limit the extent of any secondary neurologic insults
by inhibiting the release of neurotoxic molecules, preserving membrane
integrity, scavenging free radicals, and correcting electrolyte
imbalances.14
According to a prospective, randomized multicenter trial known as the
second National Acute Spinal Cord Injury Study (NASCIS II), a steroid
regimen consisting of a 30 mg/kg bolus of methylprednisolone that is
continued at a rate of 5.4 mg/kg/hr resulted in superior long-term
neurologic outcomes.22 The ensuing
NASCIS III trial demonstrated that patients who received steroids
within 3 hours of the time of their injuries may necessitate only 24
hours of therapy, while those whose infusions were started between 3
and 8 hours should be treated for 48 hours.23
Despite the potential complications of high-dose steroids and the
growing controversy surrounding the validity of these findings, these
guidelines are still widely accepted as the “standard of care,” which
in this litigious environment may compel practitioners to follow these
recommendations even though their efficacy has yet to be definitively
established.44,62,69
Other preparations that have been investigated as neuroprotective
agents for spinal cord injuries include lipid peroxidase inhibitors,
calcium channel blockers, glycolipids, and opiate receptor antagonists.14
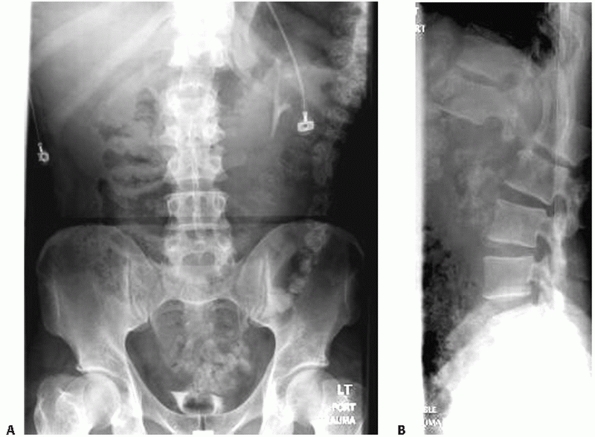 |
|
FIGURE 43-4 Anteroposterior (A) and lateral (B) radiographs of a T12-L1 fracture-dislocation.
|
disrupt the integrity of the spinal column and the close proximity of
numerous vital thoracic and abdominal viscera, it is not surprising
that more than 50% of individuals with thoracolumbar fractures will be
diagnosed with a variety of nonspinal injuries (involvement of one
other organ system, 30%; two systems, 20%; three or more systems, 5%).118
For instance, up to 45% of patients with “seatbelt” fractures will also
sustain some type of intraabdominal injury such as a laceration of the
spleen or liver.11 Moreover, the incidence of noncontiguous spinal fractures in this population has been shown to be approximately 17% to 20%.5,128
Many victims of motor vehicle accidents or falls from a significant
height may also present with head injuries or fractures of the
extremities.
the patient and any other individuals who may have witnessed the trauma
because the specific details of the event may alert the treating
physicians to the possibility that a thoracolumbar fracture may be
present as well as other spinal or appendicular injuries. Relevant
information includes the speed of the vehicle and the use of restraints
during automobile collisions as well as the distance and point of
impact of those who have fallen. In addition to axial spinal pain and
decreased range of motion, patients with thoracolumbar injuries may
describe a spectrum of neurologic symptoms depending on the status of
the spinal cord and other neural elements.
be carefully logrolled so that the posterior spine may be visually
inspected for ecchymoses, abrasions, lacerations, or swelling; it is
also important to note any “seatbelt” contusions across the anterior
abdomen, which are frequently observed with flexion-distraction
injuries.27,55,60
Palpation of this region may elicit focal tenderness at the fracture
site and reveal an obvious stepoff between the spinous processes,
crepitus, soft tissue defects, or other signs of malalignment.
before moving the patient to establish the baseline level of function
and serial assessments must be repeated over time to ensure that any
further deterioration does not escape detection. The presence of any
neurologic injury is verified by performing motor, sensory, and reflex
testing, although this may not be feasible in those who are
unresponsive or otherwise unable to cooperate with the examination. In
adults the transition between the central and peripheral nervous
systems usually occurs
behind
the L1 vertebral body so that individuals with fractures localizing to
the thoracolumbar junction may exhibit a variety of abnormalities based
upon the anatomic structures that are affected. An isolated
radiculopathy may manifest as a dermatomal pattern of altered
sensation, myotomal weakness, or hyporeflexia, while injuries to the
spinal cord, conus medullaris, or cauda equina may give rise to any
number of deficits ranging from diffuse sensory and motor changes in
the lower extremities to dense paraplegia and sphincter incontinence. A
complete spinal cord injury must also be distinguished from spinal
shock, which represents a transitory block of neurologic impulses that
ordinarily lasts no longer than 48 hours. The bulbocavernosus reflex is
elicited by stimulating the glans penis or clitoris, which should bring
about an involuntary contraction of the anus; the long-term prognosis
of a patient cannot be reliably ascertained until this reflex arc
returns, which signifies that the episode of spinal shock has resolved.
Furthermore, the sparing of sacral sensation and maintenance of rectal
tone are also signs of an incomplete injury because they affirm that at
least some neural pathways are still intact. Spinal cord injuries may
be classified using either the Frankel system or the more elaborate
American Spinal Injury Association (ASIA) scoring method in which
muscle strength is graded from 0 to 5 and sensation to both pin prick
and light touch is recorded throughout the entire spine49,92 (Fig. 43-5).
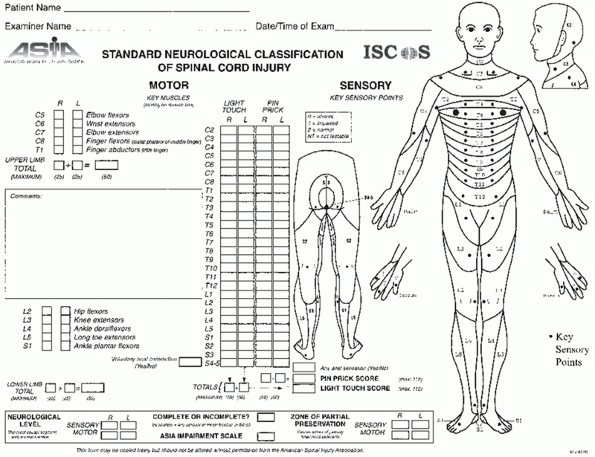 |
|
FIGURE 43-5
American Spinal Injury Association (ASIA) worksheet for classifying spinal cord injuries. (Reproduced with permission from ASIA.) |
findings are generally suggestive of a spinal fracture, a complete
battery of imaging studies may be required to confirm the diagnosis of
a thoracolumbar injury. In most clinical centers, conventional
radiography is still the most accessible and expedient method for
visualizing the spinal column. In addition to displaying any
irregularities in coronal alignment, anteroposterior (AP) views may
reveal interpedicular widening that occurs when the fragments of burst
fractures are laterally displaced or an increased interspinous process
distance characteristic of damage to the posterior ligamentous complex
(PLC).53,68
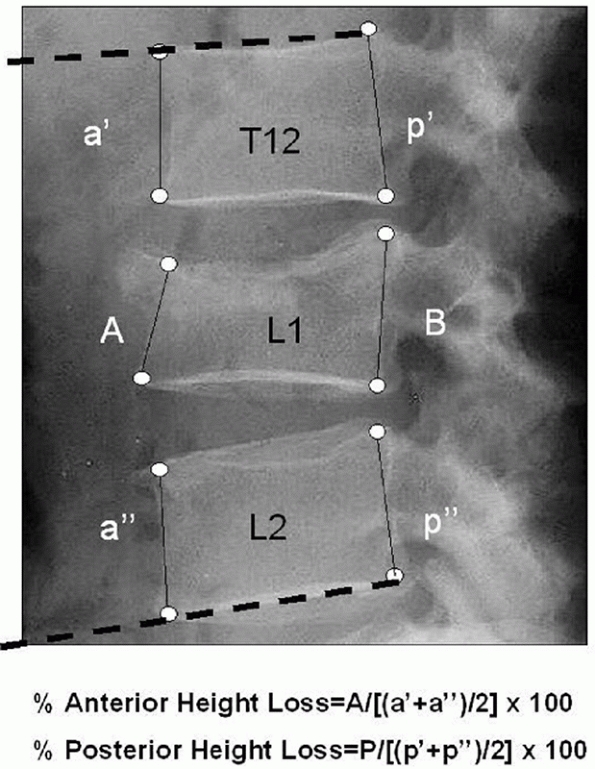
A lateral radiograph may be used to quantify any kyphotic deformities
that may be present by assessing the Cobb angle, which is delineated by
the lines corresponding to the superior and inferior endplates of the
cranial and caudal vertebrae adjacent to the site of injury,
respectively32 (Fig. 43-6).
The amount of vertebral collapse may be determined by calculating the
height of the fractured body and expressing it as a percentage of the
values acquired from the uninjured levels of the spine. For certain
fractures, the magnitude of compression may be more precisely described
by measuring both the anterior and posterior margins of the body. The
intersection of lines drawn parallel to the end plates and the
posterior cortex of the fractured body is known as the posterior
vertebral angle, which may be used to differentiate compression fractures from more unstable burst injuries.99
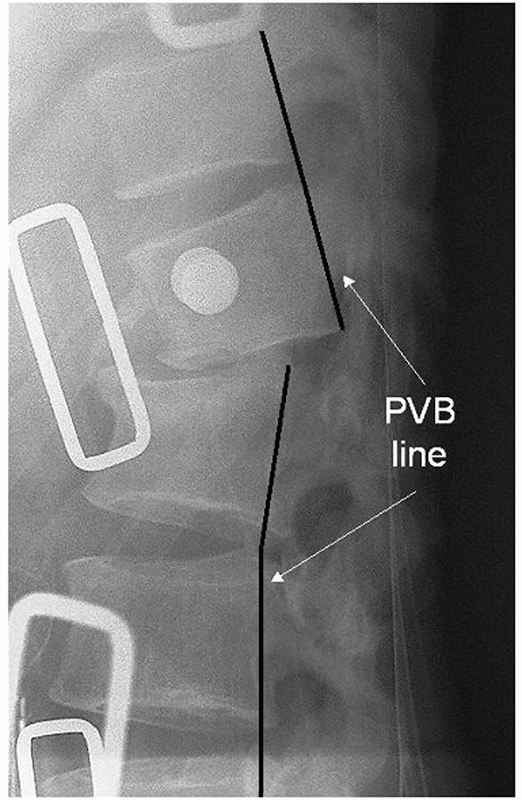
While translation in the sagittal plane may normally be recognized by
scrutinizing the anterior and posterior vertebral lines, the bony
contours are often obscured by preexisting spondylosis, which will
decrease the utility of these anatomic markers (Fig. 43-7).
 |
|
FIGURE 43-6
Radiographic method for evaluating the deformity associated with a thoracolumbar fracture. The kyphosis may be assessed by determining the Cobb angle, defined by the angle of intersection between the superior endplate of the vertebra cephalad to the fracture (T12, dashed line) and the inferior endplate of the caudal vertebra (L2, dashed line). Similarly, the amount of collapse may be calculated by measuring the heights of both the anterior and posterior margins (A, B) of the fractured body and expressing them as percentages of the corresponding values derived from the adjacent, uninjured segments using the formulas listed above. |
larger than 30 degrees may reflect PLC disruption, particularly at the
thoracolumbar junction.70,145
Similarly, translation greater than 2.5 mm in any plane or vertebral
body height loss of 50% or greater may also be consistent with failure
of the posterior ligaments.108,147
Even though these guidelines have become well accepted by many
physicians, their reliabilities for predicting posterior instability
have not been supported by any Level I evidence from randomized,
controlled studies.
injuries that are being managed nonoperatively, it may be important to
obtain standing radiographs in an orthosis to ensure that there is no
further collapse or progressive kyphosis once the patient is mobilized.
In one investigation, 28 patients with fractures located between T11
and L2 that were initially thought to be amenable to conservative
treatment ultimately underwent surgical intervention because of the
findings noted on weight-bearing radiographs.102
 |
|
FIGURE 43-7
Any interruptions in the lines drawn along the anterior or posterior aspects of the verterbral bodies may be suggestive of a thoracolumbar injury with translation in the sagittal plane. Note the break in the posterior vertebral line (PVB) observed on this lateral radiograph of a fracture-dislocation. |
multiplanar reconstructions of the spinal column that frequently
provide more information about the extent of a thoracolumbar injury
than radiographs alone, which may yield an incorrect diagnosis in as
many as 25% of individuals with burst fractures and underestimate their
amount of canal compromise by 20%.13,76
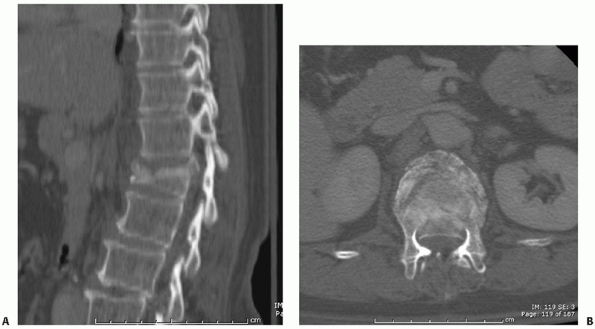
Thin-cut CT images (less than 2 mm) are able to depict comminution of
the vertebral body as well as the size and location of any retropulsed
fragments, all of which may influence the manner in which these
injuries are addressed94,95 (Fig. 43-8).
Once again, the shape of the canal as defined by the
sagittal-to-transverse diameter ratio derived from axial views of the
spine has been reported to be predictive of neurologic function.137
CT is also the best modality for identifying fractures of the posterior
elements that may otherwise be missed on biplanar radiographs. Because
of its greater sensitivity and efficiency, a single helical CT scan has
been shown to be preferable to a series of plain radiographs for
screening polytrauma patients who may have spinal injuries.66,150
acknowledged to be the “gold standard” technique for visualizing the
soft tissue component of these fractures including disc herniations,
epidural hematoma, ligamentous injury, or any intrasubstance
alterations of the spinal cord itself. These pathologic findings may
play a crucial role in determining the specific treatment and the
long-term prognosis of these individuals; for example, evidence
of myelomalacia on MRI studies is associated with poorer motor recovery.133
Although MRI of the thoracolumbar spine is not mandatory in the absence
of any neurologic deficits, these scans may be necessary for patients
who demonstrate clinical signs or symptoms of neural element
compression. MRI is considered to be the optimal noninvasive strategy
for judging whether the PLC is intact, especially when radiographs are
normal.87 On sagittal views of the
spine, any edema involving the posterior supporting structures may be
interpreted as a sign of a traumatic insult to the PLC, which has been
incorporated by many contemporary thoracolumbar injury classification
algorithms86,136,138;
whereas a strain-type injury usually gives rise to diffuse signal
changes within the ligaments, the presence of a discrete stripe of
fluid extending through these tissues on fat suppressed T2-weighted
images is indicative of a frank disruption of the posterior tension
band (Fig. 43-9). The use of MRI for gunshot
wounds involving the spine is somewhat controversial; some groups have
proposed that the magnetic forces may lead to further harm as the
bullet moves within the tissues, while others maintain that this
modality is safe for these cases.47,129
Nevertheless, it should be anticipated that the metal artifact from
these fragments will adversely affect the quality of the images.15
 |
|
FIGURE 43-8 Sagittal (A) and axial (B)
computed tomography images of a burst fracture at the thoracolumbar junction with approximately 50% canal compromise secondary to the retropulsion of bony fragments posteriorly into the spinal canal. |
important to image the entire spinal column to rule out any other
noncontiguous fractures. Furthermore, diagnostic studies of other
anatomic structures should be performed based on the fracture pattern
or presumed mechanism of injury. Since this patient population often
experiences attendant trauma to their extremities or other organ
systems, it may be prudent to request radiographs of the lower
extremities for those with burst fractures and order an abdominal CT or
ultrasound when encountering “seatbelt”-type injuries.
thoracolumbar fractures have been introduced over the past several
decades, given the inherent complexity of normal spinal anatomy and
biomechanics as well as the ongoing uncertainties surrounding the
factors that define “stability,” there is still no consensus among
trauma experts regarding the best method for categorizing these
injuries.40,68,77,91,95,106
As with any heterogeneous collection of pathologic conditions, the
ideal method for classifying spinal injuries should be comprehensive
yet easy to apply so that it possesses sufficient reliability and
reproducibility. By integrating modern imaging technology and taking
advantage of recent advances in the understanding of the natural
history of thoracolumbar fractures, it should be possible to stratify
these injuries according to their severity, which may be helpful for
directing treatment and predicting the outcomes of these patients. An
effective classification algorithm also successfully minimizes any
confusion among different practitioners participating in the care of
these individuals and facilitates prospective clinical research in the
field of spinal trauma.
 |
|
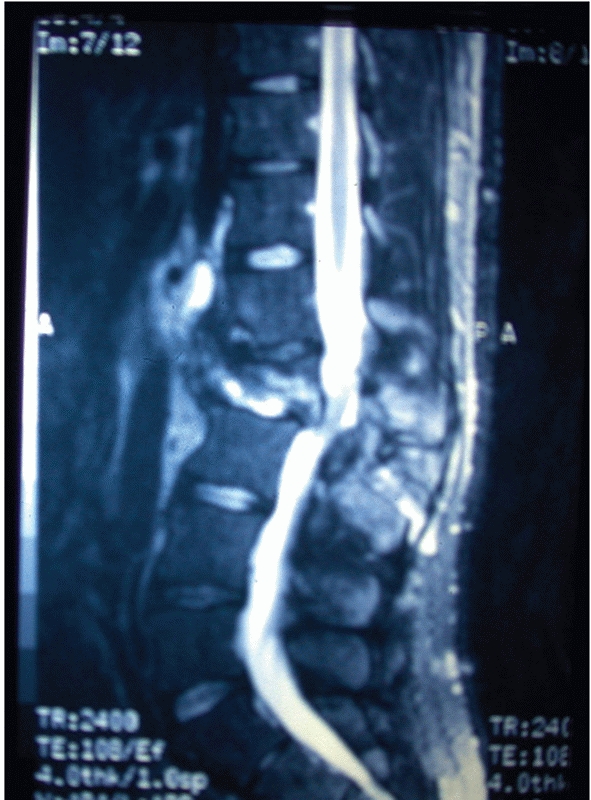
FIGURE 43-9
Sagittal T2-weighted magnetic resonance image of a L2 Chance fracture with edema evident within the posterior soft tissue structures indicative of an associated ligamentous injury. |
 |
|
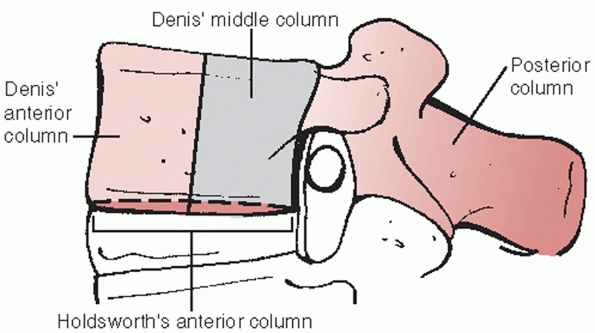
FIGURE 43-10
Denis three-column model of spinal stability which involves anterior (anterior ½ of vertebra/disc and anterior longitudinal ligament), middle (posterior ½ of vertebra/disc and posterior longitudinal ligament), and posterior (posterior elements including the pedicles and facet joints and the remaining ligaments) columns. According to this paradigm, any injury extending into the middle column is largely considered to be unstable. |
 |
|
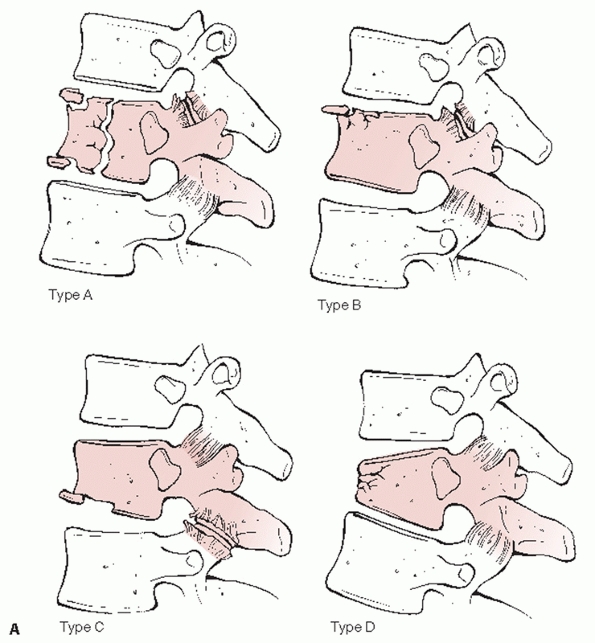
FIGURE 43-11
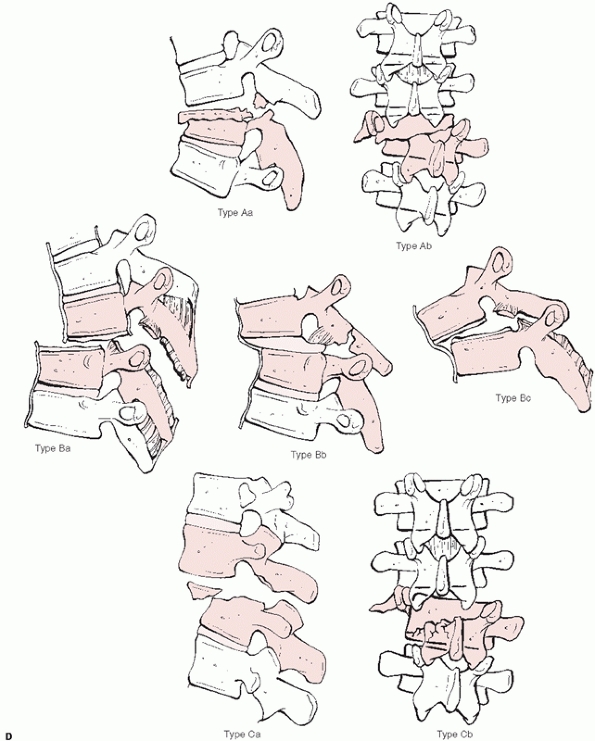
Denis classification system for thoracolumbar fractures categorizes these injuries into 4 major categories with multiple subgroups based on the three-column theory of spinal stability: A. compression (type A, both endplates; type B, superior endplate; type C, inferior endplate; type D, anterior body). (continues) |
was conceived by Denis, who attempted to elucidate the concept of
spinal stability by assigning osseous and soft tissue structures into
one of three columns: anterior (anterior half of vertebra/disc and
anterior longitudinal ligament), middle (posterior half of
vertebra/disc and posterior longitudinal ligament), and posterior
(posterior elements including the pedicles and facet joints and the
remaining ligaments)40 (Fig. 43-10).
This system divides thoracolumbar injuries into four principal
categories —compression, burst, Chance, and fracture-dislocations—with
an additional 16 subgroups (Fig. 43-11). With
this paradigm, the greatest emphasis is placed on the middle column
such that any injury extending into this portion of the spine is
generally assumed to be unstable. However, because it does not take
into account the results of advanced imaging modalities or the status
of the PLC, the Denis algorithm may be overly simplistic and does not
appear to be beneficial for directing the management of these fractures.107,152
primary vector forces that are applied to the spinal column as the main
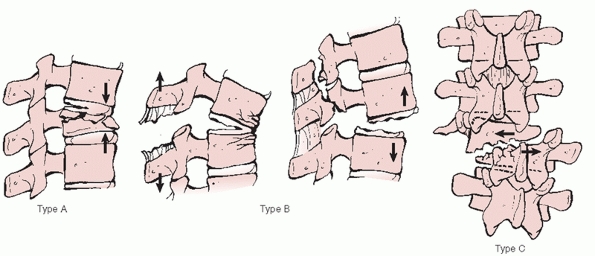
criterion for segregating thoracolumbar injuries.91
In this format, groups A, B, and C are composed of fractures generated
by compression, distraction, and torsional/rotational loads,
respectively (Fig. 43-12). The AO algorithm features multiple
levels of organization, which are not only designed to specify the
location, morphology, and direction of displacement for each fracture
but also allow for a distinction to be made between bony and soft
tissue injuries. The interobserver and intraobserver reliabilities of
the Denis and AO systems have been reported by a number of independent
investigators. In one study, the most basic subcategory of the Magerl
scheme (i.e., A, B, or C) exhibited only a fair amount of agreement (κ
= 0.33) with an interobserver reliability of 67%.18 In their comparisons of these classification systems, Oner et al.107 and Wood et al.153
both concluded that the Denis scheme was more reproducible than the AO
method. However, from these analyses it is clear that neither algorithm
is without its flaws. The AO system is certainly more inclusive but is
not as practical to implement because its complicated alphanumeric
scoring protocol, which undoubtedly reduces its overall reliability.
Conversely, the more straightforward Denis paradigm is associated with
improved interobserver agreement, but it may be too simplistic so that
unusual fracture configurations may be inadvertently excluded.
 |
|
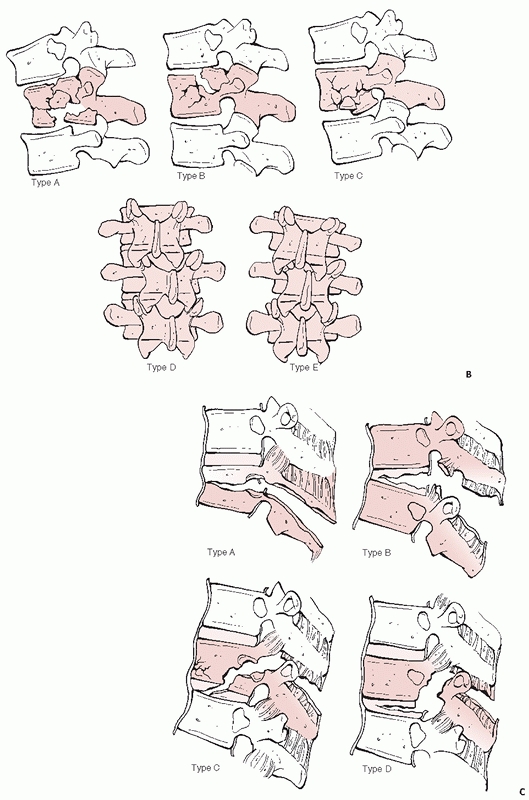
FIGURE 43-11 (continued) B.
Burst (type A, both endplates; type B, superior endplate; type C, inferior endplate; type D, rotational deformity; type E, lateral translation). C. Flexion-distraction (type A, bony involving one segment; type B, soft tissues of one segment; type C, bony involving two segments; type D, soft tissues of two segments). (continues) |
 |
|
FIGURE 43-11 (continued) D. Fracture-dislocations (type A, bony involving one segment; type B, soft tissues of one segment; type C, two level injuries).
|
 |
|
FIGURE 43-12
AO/Magerl classification system for thoracolumbar injuries categorizes these injuries into three primary types according to the vector forces applied to the spine: A, compression; B, distraction; C, rotation. |
because of their potential to guide treatment and provide prognostic
information about these injuries. After reviewing the radiographs and
CT scans of 100 thoracolumbar fractures, McAfee et al. separated these
injuries into 6 discrete groups: wedgecompression, stable and unstable
burst, Chance, flexion-distraction, and translational.94
With its emphasis on the mechanism by which the middle column failed,
this scheme was able to determine which type of instrumentation (i.e.,
distraction or compression) was most suitable for each fracture.
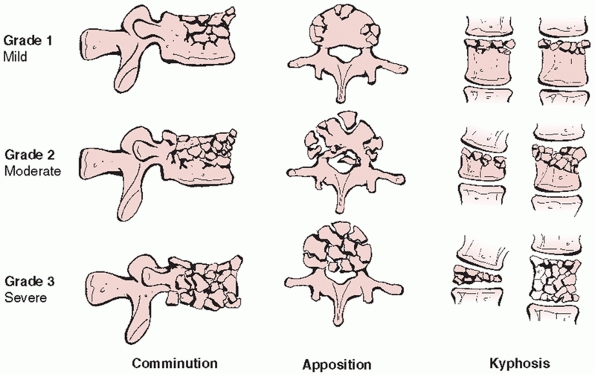
McCormack et al.95 later devised the
“load sharing classification,” which uses a grading system to assess
vertebral body comminution, spread of the bony fragments, and
posttraumatic kyphosis as a means of establishing which injuries may be
appropriately managed with immobilization alone or short-segment
transpedicular constructs limited to only the levels immediately above
and below the fracture site (Fig. 43-13). By
identifying cases that were complicated by implant breakage, the
authors suggested that a point total greater than 6 justified a
concomitant anterior arthrodesis with a strut graft. The load sharing
classification algorithm has since been validated by both in vitro
biomechanical experiments and other clinical series.6,34,109,142
developed the Thoracolumbar Injury Severity Score (TLISS), an
innovative paradigm that focuses on three key parameters that reflect
the global stability of the disrupted spinal column: (a) mechanism of
injury as interpreted from imaging studies, (b) integrity of the PLC,
and (c) neurologic status. The designated point values for these
categories are combined to calculate a total score that may assist in
the clinical decision-making process for each of these fractures.
Despite its excellent reliability as demonstrated in previous
prospective investigations,65,134
the TLISS scheme was revised so that the mechanism category was
replaced with fracture morphology, which was thought to be a more
objective variable for surgeons to evaluate; once these changes were
instituted, this modified algorithm was renamed the Thoracolumbar
Injury Classification and Severity Score (TLICS)136 (Table 43-1).
In a recent prospective, comparative study of these two systems, the
interrater reproducibility of the TLISS scheme was found to be superior
to that of the TLICS method, which implies that a retrospective
reconstruction of the pathomechanisms underlying a fracture may
actually be more informative than a description of its morphology.146
At any rate, since mechanism and morphology are closely related and
each would be expected to contribute to spinal instability following an
injury, it is likely that both of these factors will need to be
addressed during the classification and subsequent treatment of
thoracolumbar fractures.
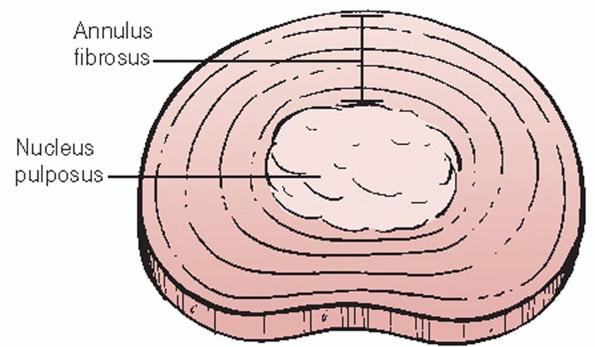
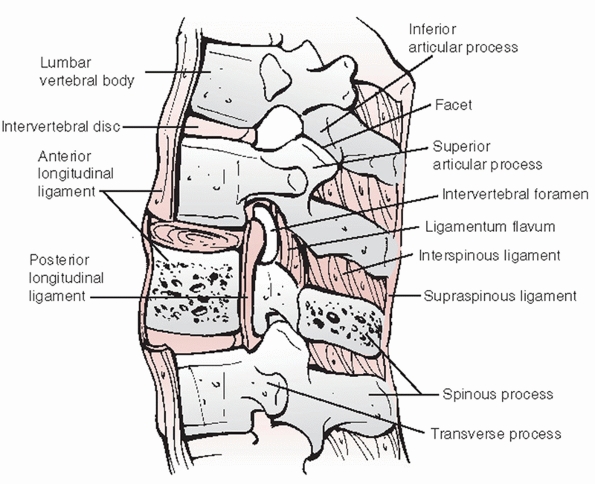
superiorly and inferiorly. Intervertebral discs are composed of an
outer circumferential layer of collagen known as the annulus fibrosis
that surrounds a soft, hydrophilic central core called the nucleus
pulposus (Fig. 43-14).
The purpose of the annulus fibrosis is to counteract torsional,
tensile, and axial forces while the nucleus pulposus resists
compressive loads. The vertebrae are spanned by the anterior and
posterior longitudinal ligaments (i.e., ALL and PLL), which stabilize
the spinal column during extension and flexion, respectively (Fig. 43-15).
 |
|
FIGURE 43-13
The load sharing classification system for thoracolumbar fractures identifies injuries that may be appropriately treated with short-segment posterior instrumentation constructs by assigning points based on the extent of vertebral body comminution, apposition of the bony fragments, and the degree of focal kyphosis. |
|
TABLE 43-1 Thoracolumbar Injury Classification and Severity Score
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Total Score |
Treatment |
|
≤3 |
Nonoperative |
|
4 |
Indeterminate (nonoperative vs. operative) |
|
≥5 |
Operative |
|
From |
|
neural arch include the pedicles, laminae, transverse and spinous
processes, and facet (zygoaphophyseal) joints. The pedicles are short,
tubular protuberances projecting from the vertebral bodies whose
dimensions have been shown to vary throughout the thoracolumbar spine.100
The pedicles between T3 and T9 typically exhibit the smallest
diameters, measuring less than 5 mm at certain levels; in contrast, the
average widths of the lumbar pedicles range between 9 mm at L1 to 18 mm
for L5.126 The remainder of the
neural arch consists of bilateral laminae, which coalesce in the
midline to form the spinous process. The zygoapophyseal joints involve
the superior and inferior facets, which arise from the laminae of two
adjacent vertebrae. In the upper thoracic spine, these synovial
articulations are aligned in the coronal plane, which restricts
anteroposterior translation, whereas the sagittal orientation of those
in the lower thoracic and lumbar regions minimizes medial-lateral
displacement. The transverse process marks the junction between the
pedicle, pars interarticularis, and superior facet. These protuberances
serve as attachment sites for numerous paraspinal muscles and
supporting ligaments, especially in the thoracic spine, where they also
articulate
with
the rib head anteriorly, which confers greater stability to this
portion of the spine relative to cervical and lumbar motion segments.
 |
|
FIGURE 43-14
Schematic drawing of the intervertebral disc, which is composed of an outer circumferential layer of fibrous tissue referred to as the annulus fibrosis surrounding a gelatinous, hydrophilic core known as the nucleus pulposus. |
ligamentum flavum, a flexible connective tissue that covers the
posterior aspect of the dura. Successive spinous processes are linked
by interspinous and supraspinous ligaments, which are collectively
referred to as the posterior ligamentous complex (PLC). Following a
traumatic insult to the thoracolumbar spine, it is essential that the
integrity of these ligaments be assessed because disruption of this
fibrous tension band may be indicative of an unstable injury.
the vertebral body, intervertebral disc and PLL anteriorly, the
pedicles and facet joints laterally, and the laminae and ligamentum
flavum posteriorly. With these fractures, any encroachment of the
spinal cord or nerve roots may usually be attributed to the posterior
retropulsion of bony fragments; however, injury to any of these
structures may diminish the cross-sectional area of the spinal canal
and bring about clinically significant compression of the neural
elements.
 |
|
FIGURE 43-15 Sagittal cross-sectional anatomic diagram depicting the osseous and soft tissue structures of the thoracolumbar spine.
|
by the spinal cord, which is composed of specialized arrangements of
neurons and axons corresponding to its gray and white matter,
respectively. For instance, motor signals from the contralateral
cerebral hemisphere shift within the brain and travel distally in the
lateral corticospinal tracts. The axons to the upper extremities are
located more centrally than those destined for the lower extremities so
that patients with central cord injuries will often display more
pronounced weakness in their arms compared to their legs. Likewise,
sensory impulses ascend proximally within segregated zones of the
spinal cord depending upon the type of neuron that has been activated.
Pressure, vibration, and proprioception stimuli are transmitted in the
ipsilateral dorsal columns before being received by the opposite
cerebral cortex, while information about pain and temperature contained
within the spinothalamic pathways crosses over at the level of the
spinal cord. Thus, individuals with a Brown-Sequard lesion, which
results in a functional hemisection of the spinal cord, will
classically demonstrate deficits in ipsilateral light touch,
proprioception, and motor strength with concurrent loss of
contralateral pain and temperature sensation. The spinal cord is
coupled to the peripheral nerve rootlets of the cauda equina by the
conus medullaris, which is situated at the thoracolumbar junction
anywhere from T11 to L3.
stabilizing effects of the rib cage, fractures involving this portion
of the spine are associated with a high risk of catastrophic neurologic
injury for a variety of reasons. The ratio of the canal to cord
dimensions is smallest in the thoracic spine so that there is a greater
risk of compression with any degree of compromise. Furthermore, the
thoracic spine represents a vascular watershed area whose circulation
is predominantly supplied by the artery
of
Adamkiewicz, which perfuses the cord in a retrograde fashion after
entering a single intervertebral foramen between T9 and L2, most
commonly on the left side.
a midline skin incision based over the fracture site. The subcutaneous
tissues and deep fascial layer are split so that the paravertebral
muscles may be elevated off the posterior elements. The subperiosteal
exposure proceeds laterally until the facet joints and the transverse
processes of the segments of interest are fully exposed in preparation
for an instrumented spinal arthrodesis.
the right or the left sides, but injuries at the thoracolumbar junction
or in the lumbar spine are best treated with left-sided
thoracoabdominal and retroperitoneal exposures, respectively, because
these approaches avoid the liver and do not require as much
manipulation of the more delicate vena cava. An oblique skin incision
is centered over the rib one or two levels cephalad to the fracture,
extending from the umbilicus to the lateral paraspinal musculature. A
subperiosteal dissection is performed to detach the intercostal muscles
from the rib without disturbing the neurovascular bundle along its
inferior aspect. At this point, the intercostal space may be opened
with or without removing the rib; if the surgeon elects to proceed with
excision, an osteotome is used to cut the rib anteriorly at the
costochondral junction and posteriorly where it articulates with the
transverse process. The ribs are spread with a self-retaining
thoracotomy retractor and the lung is selectively deflated by the
anesthesia team. For fractures of the thoracolumbar junction or other
injuries for which the diaphragm must be released, it is recommended
that a cuff of tissue measuring at least 1 cm should be left in
continuity with the chest wall and marked with sutures to permit an
anatomic repair at the conclusion of the surgery. An incision is made
through the parietal pleura of the thoracic cavity so that it may be
dissected off the spinal column above and below the injury. When
exposing the T9-T12 levels, it may be useful to temporarily clamp the
segmental arteries and verify that there are no subsequent changes in
neuromonitoring potentials before sacrificing them in an effort to
decrease the risk of injury to the artery of Adamkiewicz and possibly
avoid precipitating an ischemic insult to the spinal cord.
be extended distally along the anterolateral portion of the flank
toward the abdomen and the lateral border of the rectus abdominis
muscle; the subcutaneous tissues and the musculature of the abdominal
wall (i.e., external and internal obliques, transverses abdominis) are
divided in line with this incision. With blunt dissection, the
peritoneal contents are mobilized and retracted medially along with the
great vessels to gain access to the retroperitoneal space and the
spinal column.
completed, the “hills” of the convex discs and the “valleys”
corresponding to the concave vertebrae should be apparent. Care must be
taken to carefully ligate the segmental vessels overlying the vertebral
bodies to minimize any bleeding during the remainder of the procedure.
A fracture routinely creates an obvious deformity of the spine, but it
is always recommended that a needle be placed in one of the discs
adjacent to the vertebral body presumed to be injured so that an
intraoperative radiograph may be obtained to visualize the segment of
interest. Once the level of the fracture has been identified, the
iliopsoas muscle is cleared off the vertebra and retracted away from
the bony fragments.
is governed by both biomechanical and clinical considerations such as
the ability of the spine to withstand physiologic stresses as well as
the presence of any neurologic deficits, associated traumatic injuries,
or other relevant medical issues. The objectives of every therapeutic
strategy are the same regardless of whether a fracture is addressed
surgically or with nonoperative measures: maintain or restore spinal
stability, correct any deformities in the sagittal or coronal planes,
maximize neurologic recovery, improve pain, and allow for prompt
rehabilitation. In general, many patients with thoracolumbar injuries
who do not demonstrate any clinical or radiographic findings consistent
with neural compression or instability may be treated nonsurgically
with immobilization and early ambulation.
past to support the fractured spinal column, these techniques have
largely been supplanted by a number of different external braces. A
Jewett device and other hyperextension appliances are designed to
resist flexion but are less apt to control rotation or lateral bending.
A prefabricated or custom-fit “clamshell” thoracolumbosacral orthosis
(TLSO) reduces motion in multiple planes and may be better suited for
more unstable fractures.85 Miller et al.103
compared the effects of a simple corset, a Jewett brace, and a TLSO on
the mobility of the lumbar spine. While both the Jewett orthosis and
the TLSO limited motion in the lower lumbar spine, none of these braces
were able to completely eliminate all movement. Moreover, the L5-S1
segment was not sufficiently stabilized by any of these appliances and
some of these individuals actually exhibited hypermobility at this
level while in these devices. There are some data to suggest that the
supplementation of these braces with a leg cuff may impart even greater
stability to the spine.140
Similarly, a cervical extension may also be beneficial for inhibiting
any pathologic motion that may occur with fractures of the upper
thoracic spine (i.e., above T5).
majority of patients with compression fractures may experience bony
healing and symptomatic relief with approximately 12 weeks of
immobilization.135 Nevertheless,
compression injuries that demonstrate greater than 50% height loss or
25 degrees of focal kyphosis, disruption of the PLC, or any other signs
of obvious instability on imaging studies should be followed closely
because these injuries are predisposed to further collapse and
progressive deformity despite the initiation of a suitable bracing
regimen. Folman and Gepstein48 noted
in their retrospective review of 85 patients with traumatic
thoracolumbar wedge fractures that even though most of these injuries
were adequately managed with conservative measures alone, a large
proportion of these individuals continued to have chronic low back pain
whose intensity correlated with the amount of segmental kyphosis.
to more substantial axial forces and are frequently observed with
higher energy trauma. Because they characteristically involve the
middle and posterior aspects of the vertebral body, burst fractures are
by definition more unstable than compression injuries and any
retropulsed fragments may lead to significant canal stenosis with
ensuing neurologic decline. In addition to espousing the same
radiographic criteria that have been used to determine which
compression fractures are most appropriate for nonoperative treatment,
other authors have reported that burst fractures with less than 50%
canal compromise may also be addressed without surgery130;
unfortunately, these guidelines have not been properly validated by the
existing literature. A period of immobilization lasting up to 3 months
may be acceptable for patients with stable burst fractures who have a
normal neurologic examination and an intact PLC as well as those with
unstable injuries who are not able to tolerate an operative procedure.67,98,113
Individuals who present with complete spinal cord injuries secondary to
burst fractures may also be candidates for conservative care. Even
without surgery, the displaced fragments of burst injuries have been
shown to undergo spontaneous remodeling, which may increase the patency
of the spinal canal and perhaps relieve any impingement of the neural
elements.38 Any burst fracture that
is being braced should be regularly assessed by repeating standing
radiographs in the orthosis to ensure that there is no further collapse
of the vertebral body or increase in segmental kyphosis over time.50
long-term outcomes may be achieved with the closed management of
thoracolumbar burst fractures in the absence of any instability or
neurologic abnormalities.6,9,104,105,144 Chow et al.31
also recorded excellent results with hyperextension casting of 24 burst
injuries that were categorized as being unstable according to the Denis
classification system, but only 10 of these fractures actually
demonstrated interspinous widening or any other radiographic findings
indicative of PLC disruption.
anterior and posterior spinal elements, flexion-distraction injuries
and fracture-dislocations are rarely managed without surgery with the
exception of certain purely osseous Chance fractures.58,88
In certain circumstances, patients with these injuries who have a
normal neurologic examination and less than 15 degrees of kyphosis may
be braced in hyperextension as long as a satisfactory reduction is able
to be maintained for 3 months or more until the bony fragments have
consolidated.10 Conversely, reliable
healing of soft tissue flexion-distraction injuries and virtually all
fracture-dislocations is unlikely to be attained if internal fixation
is not used to reestablish the normal alignment and biomechanical
integrity of the spine.
may be treated with a rigid orthosis and prompt mobilization, a large
subset of these injuries may be more effectively addressed with
surgery. Operative intervention is intended to convey immediate
stability to the spine, allow for the correction of deformities, and
optimize neurologic improvement by directly or indirectly relieving any
residual impingement of the neural elements; for these reasons,
surgical techniques may have the potential to enhance clinical
outcomes, facilitate rehabilitation, and eschew many of the adverse
consequences of nonoperative therapies.19,149
several different factors such as the morphology of the fracture, the
status of the posterior ligamentous complex, the neurologic status of
the patient, and any other traumatic injuries or medical comorbidities.
Even so, it is widely believed that individuals with unstable fractures
who present with worsening kyphosis or intersegmental translation,
incomplete neurologic deficits in the setting of persistent compression
of the spinal cord, or radiographic evidence of damage to the posterior
ligamentous structures will presumably benefit from surgery.98
Operative strategies may also be preferable for patients who cannot
tolerate external immobilization because of their body habitus or major
injuries involving the extremities or other organ systems.
an attempt to stabilize and decompress the injured thoracolumbar spine,
all of which are performed either through a posterior, anterior, or
circumferential approach. The ideal method for treating these fractures
is still a matter of some debate, and in most cases the operative plan
is influenced by both clinical and radiographic concerns including the
neurologic examination, other critical traumatic injuries or pertinent
medical conditions, amount of sagittal plane deformity, encroachment of
the thecal sac on axial images, and any signs of spinal instability.
techniques are regularly used as a means of effecting fracture
reduction and realignment of the spine, relieving compression of neural
structures, and generating a solid arthrodesis of the vertebral column.
One of the principal advantages of these surgeries is that they avoid
the morbidity inherent to anterior thoracolumbar exposures, which may
decrease blood loss and operative times. Although a combination of
hooks or sublaminar wires may be used in the thoracolumbar spine, these
constructs have been replaced to a large extent by contemporary
transpedicular fixation systems. Since pedicle screws are inserted
through the posterior elements into the vertebral body, this
instrumentation confers greater stiffness to the fused segment so that
larger axial and rotational forces are able to be applied to the spine.
The increased pull-out strength of these implants has served as the
rationale for short-segment constructs, which only incorporate the
segments contiguous to the injury. Previous reports have documented
that this strategy may lead to higher rates of hardware failure with
kyphosis83,101,119,143 (Fig. 43-16). In response to these suboptimal results, transpedicular bone grafting4,79,89 and cement augmentation of the fractured vertebra1,2,30,80,81,141
have been proposed as adjunctive interventions, which may be
implemented to reinforce these more limited fusions. However,
short-segment fixation may be inadequate for patients with osteoporosis
injuries located at the thoracolumbar junction or highly comminuted
fractures7; in these situations, it
may be prudent to extend the arthrodesis two levels above and below the
injury to decrease the rates of pseudarthrosis and postsurgical
deformity (Fig. 43-17).
anterior corpectomy, but a decompression may also be accomplished through a posterior approach.8
Through a simple laminectomy, it is also possible to remove symptomatic
epidural hematomas, repair traumatic dural tears, and extricate nerve
roots that may have become trapped within the ends of a vertically
oriented lamina fracture25,41 (Fig. 43-18).
Alternatively, other posterolateral techniques have been described such
as the transpedicular, costotransversectomy, lateral extracavitary, and
lateral parascapular extrapleural exposures, all of which may be used
to gain access to the spinal canal and relieve any encroachment of the
neural elements.3,46,52,59,63,75
By further attenuating an already destabilized spine, these posterior
decompressive procedures are normally performed in conjunction with an
instrumented arthrodesis to prevent the development of any iatrogenic
deformities or deterioration in neurologic function.
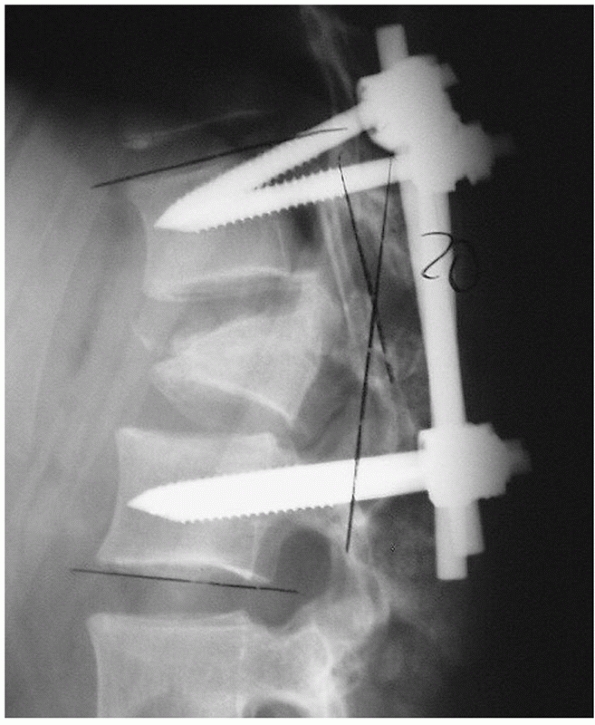 |
|
FIGURE 43-16
Lateral radiograph demonstrating failure of a short-segment posterior instrumentation construct used to stabilize a burst fracture continued to develop progressive collapse and kyphosis despite operative stabilization. |
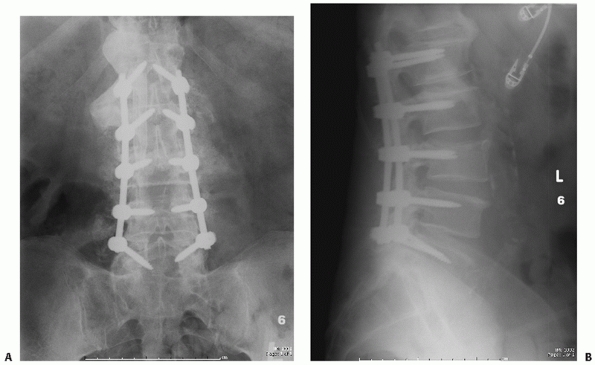 |
|
FIGURE 43-17 Anteroposterior (A) and lateral (B)
radiographs of a L3 burst injury obtained 6 months after treatment with a posterior instrumented arthrodesis extending two levels above and below the fractured vertebra. On the anteroposterior view, exuberant bone formation is evident posterolaterally, indicative of a solid fusion. |
of promoting a successful fusion, posterior instrumentation may also be
used to distract across the injury to indirectly decompress the thecal
contents by taking advantage of ligamentotaxis. While the spinal canal
may be expanded by up to 50% with this maneuver, in most instances the
amount of occlusion will improve to less than 20% of the original
cross-sectional area.33,64,74,124,152
This method of reduction is only feasible if the extruded bone is still
attached to the posterior annulus via Sharpey’s fibers. Gertzbein et al.56
suggested that distraction and ligamentotaxis may be less useful for
individuals with canal compromise measuring greater than 67% because
the retropulsed bony fragments may no longer be in continuity with the
soft tissues. Furthermore, this process must be completed in a timely
fashion since the efficacy of this intervention has been shown to
diminish as early as 3 days after the traumatic injury.33,148,154
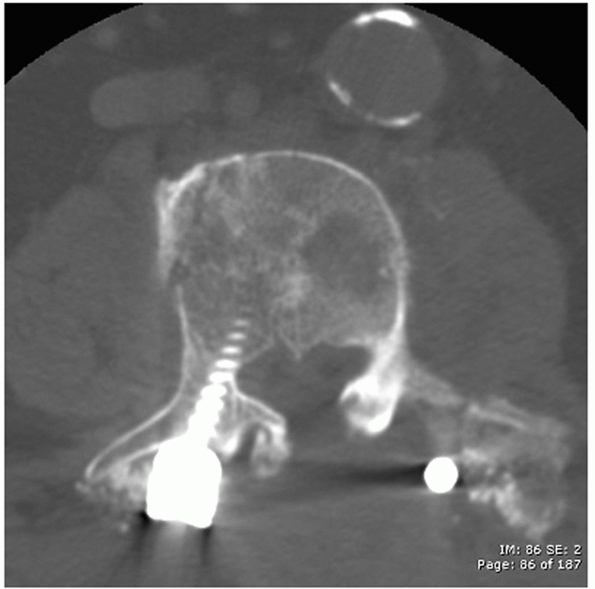 |
|
FIGURE 43-18
Axial computed tomography image demonstrating a lumbar burst injury resulting in symptomatic compression of the neural elements that was treated with a posterior laminectomy performed at the level of the fracture. |
with an anterior operation, are usually selected for most unstable
burst, flexion-distraction (bony and soft tissue), and
fracture-dislocation injuries where there is disruption of the PLC
because this approach allows for the reduction and secure fixation of
the spine, thereby reconstituting the incompetent posterior tension
band. A posterior arthrodesis is generally sufficient for patients with
burst fractures who are neurologically intact and do not require a
formal decompression; an instrumented fusion may also be appropriate
for acute burst injuries associated with a moderate degree of canal
compromise or a neurologic decline where distraction and ligamentotaxis
may bring about an indirect reduction of any retropulsed fragments.
However, certain methods for posterior stabilization such as
short-segment fixation may lead to delayed hardware failure, especially
in the setting of significant vertebral comminution, so that anterior
column support is often required to restore normal sagittal plane
alignment.
injury who has a poor prognosis for any meaningful neurologic recovery
may benefit from a posterior arthrodesis to minimize the risk of
subsequent deformity and facilitate rehabilitation. For posterior
element fractures producing a neurologic deficit, a posterior operation
may be necessary to address symptomatic durotomies or free entangled
nerve roots. A laminectomy may also be indicated for certain injuries
involving the proximal thoracic spine (e.g., T2 burst fracture) that
are not amenable to a high thoracotomy exposure. Finally, considering
that the morbidity of an anterior approach is not inconsequential,
medical comorbidities, including pulmonary disorders and morbid
obesity, as well as other serious injuries to the thoracic or abdominal
viscera, may ultimately dictate that a thoracolumbar fracture be
treated posteriorly.
surgical management of a wide range of thoracolumbar injuries has been
well documented in the literature. In a consecutive series of 32
patients with unstable thoracic fractures, transpedicular screw
fixation was found to be an expedient method for fusing the spine.156
Multiple studies have reported excellent clinical and radiographic
outcomes following the use of posterior operative techniques for burst
fractures.16,36,45,151
Several authors have also recorded significant reductions in back pain
and kyphosis with open reduction and short-segment instrumented
posterior arthrodesis for flexion-distraction injuries.90,132
These more limited constructs may not be rigid enough for
fracture-dislocations so many practitioners have advocated longer
fusions for these grossly unstable injuries in an effort to decrease
the incidence of implant failure.42,111,155
in neurologic abnormalities, the majority of compression may be
attributed to encroachment of the canal from retropulsed fragments
arising from the posterior aspect of the damaged vertebral body. An
anterior exposure affords unparalleled visualization of the dura and
allows for a more meticulous decompression of the neural elements45;
for these reasons, anterior procedures may be more desirable than
posterior-based strategies for individuals with incomplete spinal cord
injuries whose imaging studies depict severe stenosis in the axial
plane. Anterior column support with a strut graft or other interbody
device may also be advisable for a thoracolumbar injury with
considerable comminution, as noted above, which predisposes this
segment to additional collapse and deformity. These load-sharing
constructs are routinely augmented with anterior instrumentation
consisting of any combination of screws, staples, plates, or rods,
which may enhance their biomechanical properties8 (Fig. 43-19).
with canal compromise greater than 67% or focal kyphosis measuring at
least 30 degrees.96,120
Moreover, anterior interventions are often obligatory for subacute
injuries occurring more than 5 days in the past, which may no longer be
able to be indirectly reduced with posterior distraction and
ligamentotaxis. It may also be more practical to extirpate any
symptomatic intervertebral disc herniations through an anterior
approach. Posterior element fractures and other anatomic constraints
(e.g., pedicles that are too small to safely accommodate screws) that
preclude the placement of posterior implants may also justify the use
of anterior techniques.
specific types of thoracolumbar injuries have been established by the
findings of numerous investigations. According to various retrospective
reviews, patients with burst fractures who were managed with anterior
decompression, strut grafting, and instrumented arthrodesis exhibited
high rates of fusion with relatively few complications and nearly all
of those with incomplete neurologic injuries demonstrated at least
partial resolution of their deficits.36,57,61,71,72,82,93,97
In a large series of 150 consecutive burst injuries with concomitant
neurologic involvement that were addressed in this fashion, solid
fusions were observed in 93% of cases with a mean percentage of canal
clearance of 98%; postoperatively, 95% of these individuals improved by
a minimum of one neurologic category on the Frankel scale, and 96%
eventually returned to work.72 Sasso et al.115
also achieved similar success when using this surgical approach for
more unstable thoracolumbar fractures that included both the anterior
and posterior spinal elements. At the time of final follow-up, 95% of
these subjects displayed
radiographic evidence of fusion and 91% had regained one or more Frankel grade.
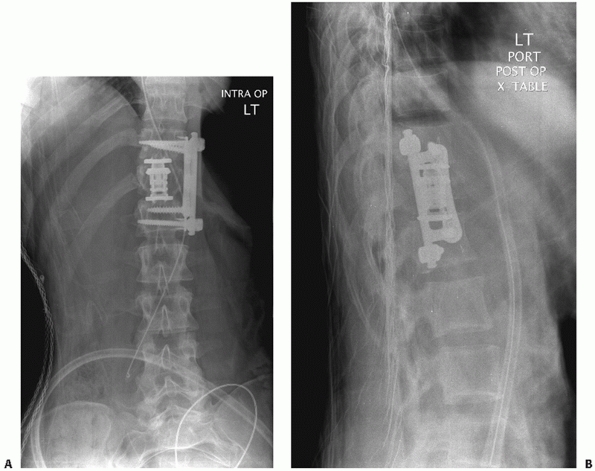 |
|
FIGURE 43-19 Postoperative anteroposterior (A) and lateral (B)
radiographs of a fracture located at the thoracolumbar junction that was addressed anteriorly with a T12 corpectomy, introduction of a titanium expandable cage filled with local autograft, and placement of instrumentation. |
performed through a single approach have been favorable, a combination
of anterior and posterior techniques may be indicated for certain
thoracolumbar injuries. Fractures with extensive vertebral body
comminution that are treated solely with posterior instrumentation may
be susceptible to implant failure and sagittal plane deformity if the
spinal column is not stabilized anteriorly as well116,125 (Fig. 43-20).
Stand-alone anterior constructs may also be at greater risk for
nonunion if the PLC is ruptured so that it is no longer able to serve
as a posterior tension band to counteract any distractive forces.57
Isolated anterior reduction and fixation is also not a viable option
for fracture-dislocations or other translational or rotational
deformities, which ordinarily necessitate an initial reduction and
arthrodesis through a posterior exposure of the spine. Because of their
poor bone quality, patients with osteoporosis are susceptible to graft
subsidence, segmental collapse, and pseuduarthrosis with an
anterior-only strategy, making them ideal candidates for a
circumferential procedure.
it may be prudent to stage these interventions until it is confirmed
that a second operation is warranted. To this end, interval imaging
studies may be obtained after the first surgery to assist in this
decision-making process. For example, a CT scan may reveal the amount
of residual encroachment of the neural structures that remains
following an indirect reduction of the canal using posterior
distraction and ligamentotaxis, which is useful for determining whether
a subsequent anterior decompression should be completed. In fact,
anterior techniques may be implemented in a delayed fashion such that
individuals with ongoing occlusion of neural tissues have been found to
experience superior pain relief and at least partial return of their
neurologic function up to several years after an initial posterior
procedure.20,131
Likewise, any structural grafts or interbody devices that demonstrate
any evidence of delayed or nonunion on plain radiographs may merit
supplementation with transpedicular screws, which are known to be a
reliable method for managing anterior constructs that do not heal.72
plain radiography, CT, or MRI should be considered before surgery
because a number of radiographic findings have been shown play a
critical role in determining which operative
approach
should be used (e.g., sagittal alignment, amount of canal compromise,
integrity of the posterior ligaments, etc.). If the arthrodesis is to
be augmented with instrumentation, the dimensions of the bony pedicles
should be assessed on sagittal and axial CT views of the spine to
ascertain whether they are large enough to safely accommodate screws.
Fluoroscopy or plain radiography should be readily available during
these cases to identify the fractured segment and guide the placement
of any implants. With the exception of individuals with complete spinal
cord injuries, we typically use intraoperative neuromonitoring
strategies that entail the recording of somatosensory and transcranial
electric motor-evoked potentials (SSEPs and MEPs, respectively) as well
as spontaneous and triggered electromyographic data, because any acute
changes in this real-time electrophysiologic feedback may alert the
practitioner to the possibility of impending neurologic deterioration.
Even in the setting of a complete neurologic deficit,
electrophysiologic monitoring is still an effective method for ensuring
that the brachial plexus is not subjected to any excessive stretching
during the case.
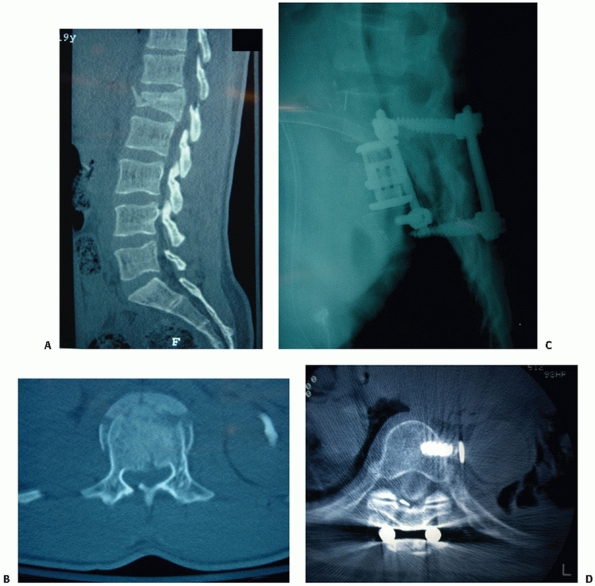 |
|
FIGURE 43-20 Sagittal (A) and axial (B)
computed tomography images demonstrating an L1 burst injury with evidence of significant vertebral body collapse and retropulsion of fracture fragments into the spinal canal. Postoperative lateral radiograph (C) and axial computed tomography image (D) obtained following a circumferential procedure consisting of an L1 corpectomy with reconstruction using an expandable cage and anterior instrumentation followed by the insertion of short-segment posterior fixation. |
arthrodesis remains a controversial issue. Extending the fusion so that
it comprises at least the two vertebrae above and below the injury may
increase the stability of the spinal column, which is essential for the
treatment of fractures in which there is great deal of comminution or
kyphosis. Although short-segment constructs may be appropriate for
circumferential procedures or injuries involving the lower lumbar
spine, this technique is not always recommended for patients with
osteoporosis or those with fractures of the thoracolumbar junction who
generally require more rigid fixation of the spine.
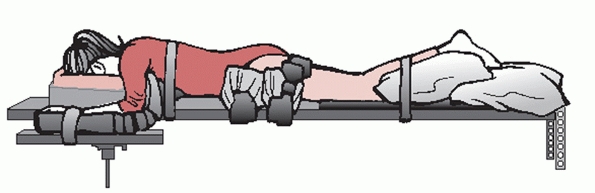 |
|
FIGURE 43-21 Schematic drawing of a patient who has been placed in the prone position on a Jackson spinal table.
|
of anesthesia has been attained, the patient is positioned prone on a
radiolucent operating room table such as a Jackson frame, taking care
to adhere to strict spinal precautions at all times during the transfer
(Fig. 43-21). In addition to making certain
that all bony prominences are cushioned so that they are not subject to
any undue pressure, the shoulders and other upper extremity joints
should be maintained at angles less than 90 degrees to avoid a brachial
plexopathy or any other neuropraxias; similarly, the hips are extended
and the knees are slightly flexed to minimize the risk of sustaining a
traction injury to the sciatic nerves. It is also important to ensure
that the abdomen is free of any restrictive pads because any
compression of visceral or vascular structures may lead to elevated
intraspinal pressures and increase the amount of epidural blood loss.
These maneuvers may be performed in a patient who is awake but sedated
to minimize the risk of injury during positioning; alternatively, this
somewhat onerous process may be avoided altogether if
electrophysiologic monitoring is available to acquire potentials both
at baseline and after the individual has been transferred.
thoracolumbar spine is sterilely prepped and draped. The iliac crests
should also be included within the surgical field if autogenous bone is
to be procured as graft material for an arthrodesis. At this point, a
standard midline posterior exposure of the thoracolumbar spine is
performed as discussed previously.
joint, pars interarticularis, and transverse process as anatomic
landmarks (Fig. 43-22). The entry site for
thoracic pedicle screws is located immediately lateral to the midpoint
of the facet joint along the superior third of the transverse process.
In the lumbar spine, this point is demarcated by the intersection of a
vertical line that passes along the lateral aspect of the facet joint
and a horizontal line that bisects the transverse process. After a
cortical window has been created with a Midas Rex drill or other
high-speed burr, an awl or curette is advanced through the pedicle into
the vertebral body with the assistance of fluoroscopic visualization in
multiple planes. The integrity of the channel is verified by examining
the walls of the pedicle with a probe to confirm that there is a firm
end point with no bony perforations. The proper length of each implant
is estimated with a depth gauge and the correct diameter may be derived
from preoperative CT or MR axial images, ranging from 4 mm in the upper
thoracic region to as large as 7 mm in the lower lumbar spine and
sacrum. The tract may be tapped if needed and the screw is inserted
into the spine.
be observed as the patient is positioned prone on the operating room
table. For flexion-distraction injuries or fracture-dislocations,
manipulation of perched facets and correction of any sagittal or
coronal plane deformities are best accomplished through a posterior
approach in conjunction with towel clamps, lamina spreaders, or Cobb
elevators. Alternatively, many thoracolumbar injuries may be
successfully reduced by taking advantage of any segmental
instrumentation. Following the introduction of transpedicular fixation,
connecting rods of the appropriate size are cut and fashioned such that
they are not only straight in the coronal plane but replicate the
physiologic sagittal curvature of the spine as well. The rods are first
linked to the proximal implants before being delivered to the distal
anchors to restore normal alignment (Fig. 43-23).
Locking the rods within the heads of the screws at only one end also
allows distractive forces to be applied across a burst injury with
moderate canal compromise to increase the height of the collapsed
vertebra and tension the ligamentous attachments to any retropulsed
fragments in an attempt to bring about an indirect reduction of the
fracture through ligamentotaxis (Fig. 43-24). Other salient surgical pearls and pitfalls related to these posterior techniques are listed in Tables 43-2 and 43-3.
of the caps are tightened to maintain the reduction that has been
achieved. Final AP and lateral C-arm images or intraoperative plain
radiographs are acquired to visualize the implants and evaluate the
overall alignment of the spinal column. The construct may also be
supplemented with transverse cross connectors to further enhance its
rigidity. The facet joint capsules and any other residual soft tissues
are eradicated so that the posterior elements may be adequately
decorticated with gouges or a power-driven burr. The wound is copiously
irrigated with antibiotic-containing solution and
any
active bleeding is addressed with electrocautery or thrombogenic
agents. As a final step, autogenous cancellous bone is placed over the
bleeding surfaces of the laminae and in the lateral gutters to promote
the formation of a solid arthrodesis. A drain may be left above or
below the fascia and the various layers of the incision are
approximated. The preoperative and postoperative imaging studies of a
patient with an unstable thoracolumbar fracture-dislocation that was
treated with a posterior reduction and instrumented arthrodesis are
included in Figure 43-25.
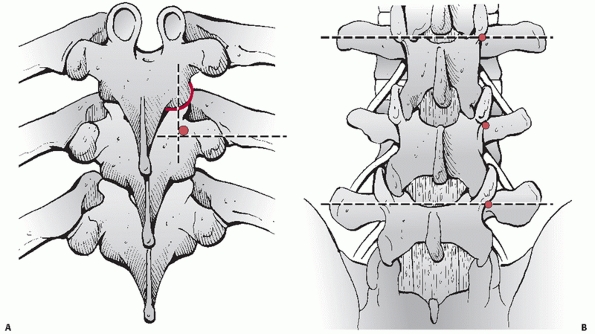 |
|
FIGURE 43-22 Schematic drawings identifying the anatomic landmarks commonly used for the placement of thoracic (A) and lumbar (B) pedicle screws.
|
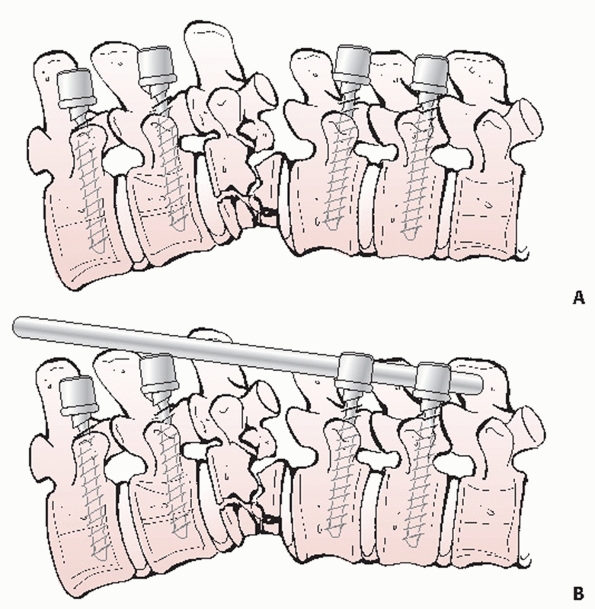 |
|
FIGURE 43-23
Schematic drawings showing the steps involved in achieving an open reduction of a thoracolumbar fracture with correction of any resultant kyphosis. After transpedicular fixation is inserted above and below the level of the injury (A), a precontoured rod is secured within the upper screws and is delivered to the lower instrumentation as part of the reduction maneuver (B). |
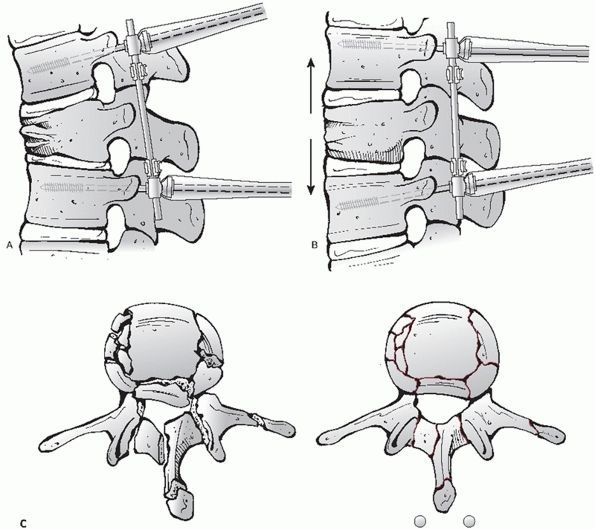 |
|
FIGURE 43-24
Schematic drawings depicting the indirect reduction of a thoracolumbar fracture. Once transpedicular fixation has been placed in the vertebrae adjacent to the level of the fracture (A), a reduction may be indirectly achieved by applying distraction across the injured segment (B) and taking advantage of ligamentotaxis to decrease the amount of canal compromise as seen in these cross-sectional images (C). |
postoperative bracing regimens give rise to superior fusion rates or
improved outcomes, especially if the fracture has been stabilized with
internal fixation. Nevertheless, we regularly immobilize these patients
with a corset or thoracolumbosacral orthosis (TLSO) for up to 3 months
depending upon the nature of the injury and the healing response of the
patient. Patients are encouraged to ambulate as soon as possible to
reduce the risk of complications that commonly occur with prolonged
recumbency. Standing radiographs should also be reviewed at regular
intervals to evaluate for any radiographic signs indicative of
pseudarthrosis such as worsening kyphosis or collapse of the fractured
vertebra.
review of the preoperative images is equally as important for
thoracolumbar injuries that are to be managed through an anterior
approach. Besides depicting the amount of canal occlusion and the
degree of fracture comminution, CT scans are also be indispensable for
quantifying the dimensions of the adjacent vertebral bodies, which will
determine the size of the strut graft and the length of the implants
that may be safely placed to reconstitute the anterior spinal column.
MRI studies may
display
a variety of pathologic conditions affecting the soft tissues such as
epidural hematomas, cerebrospinal fluid leaks, traumatic disc
herniations, and any intrinsic damage to spinal cord. In particular,
MRI is the most sensitive modality for detecting any disruption of the
posterior ligaments, which may be a contraindication for a stand-alone
anterior operation. Once again, we strongly advocate the use of
electrophysiologic monitoring during anterior interventions as reliable
methods for decreasing the incidence of intraoperative injuries to the
neural elements.
|
TABLE 43-2 Pearls and Pitfalls for Posterior Surgical Techniques—Fracture Reduction
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||
fracture, strict compliance with spinal precautions is absolutely
mandatory to prevent any iatrogenic neurologic insults. General
endotracheal intubation is performed with the patient lying supine; if
a thoracotomy is required, a double-lumen tube may be used to deflate
the ipsilateral lung so that the injured segment is more accessible.
The patient is transferred to the lateral decubitus position and is
buttressed with a bean bag or other bolsters (Fig. 43-26).
It may be advantageous to shift the patient so that the fracture is
situated over the break in the operating room table because any
increase in flexion would be expected to expand the intercostal space
to improve visualization of the spinal column and expedite placement of
a strut graft. The upper arm is maintained on a Mayo stand and an
axillary roll is inserted underneath the trunk to support the shoulder
girdle. Both hip joints are flexed so that the iliopsoas muscles are
less prominent within the surgical field. The extremities are also
padded with foam or gel material to protect the skin and peripheral
nerves during the case.
prepped and draped in a sterile manner from the midline of the chest
wall and abdomen to the spinous processes, making sure to incorporate
the iliac crest if a structural autograft is to be harvested. A
standard anterior thoracolumbar exposure is completed as described in a
preceding section of the text.
head where it articulates with the transverse process are detached to
reveal the posterior elements (i.e., the pedicle) corresponding to the
level of the fracture (Fig. 43-27). A Penfield
dissector is introduced into the neuroforamen, where it may not only be
used as both a retractor but also as a tool for palpating the bony
margins of the pedicle, which is subsequently skeletonized with a burr
so that it may be easily extracted with a Kerrison rongeur. The
excision of this structure proceeds ventrally to the point where it
joins the posterior vertebral body until the anterior and lateral
portions of the thecal sac are clearly visible. The intervertebral
discs adjacent to the fracture are sharply divided with a scapel and
the nucleus pulposus tissue is extracted with a pituitary rongeur or
curettes.
larger fracture fragments are removed with osteotomes, rongeurs, or a
burr and saved as bone graft material. An angled curette may then be
used to deliver any smaller pieces of bone out of the canal and into
the cavity that has been formed anteriorly. If possible, the anterior
longitudinal ligament with its bony attachments should be preserved
because their presence
may
maximize spinal stability and impede any anterior displacement of the
graft. The corpectomy is expanded until the contralateral pedicle is
encountered, which signifies that a satisfactory decompression has been
achieved, at which time the normal contours of the dural tube will be
evident.
|
TABLE 43-3 Pearls and Pitfalls for Posterior Surgical Techniques—Instrumentation
|
||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||
vertebrae are scraped off with an elevator in preparation for an
arthrodesis, it is important not to violate the subchondral bone, which
may diminish the biomechanical properties of the construct and lead to
graft subsidence. The spinal column may either be reduced by opening a
lamina spreader within the corpectomy defect, having an assistant
manually apply an anterior vector force to the patient’s spine, or by
distracting through anterior instrumentation; direct pressure on the
apex of a kyphosis may yield even greater correction of any sagittal
plane deformities.
factors required for a successful fusion—namely, osteoblastic cells,
osteoinductive signaling proteins, and an osteoconductive
scaffold—autograft is still considered to be the “gold standard” for
anterior reconstructions of the thoracolumbar spine. Given that the
morbidity of these grafting procedures is not trivial, it is not
surprising that several different strategies have been developed as
potential replacements for autogenous bone such as humeral or tibial
allografts as well as metal cages and other synthetic devices composed
of polyetheretherketone (i.e., PEEK) or carbon fiber packed with a bone
graft extender or local autologous bone. Osteogenic fillers that may be
beneficial for eliciting a solid arthrodesis of the anterior column
include demineralized bone matrices and recombinant human bone
morphogenetic proteins (e.g., rhBMP-2). Unfortunately, the relative
superiority of any one of these methods for this specific application
has not been corroborated by comparative investigations.
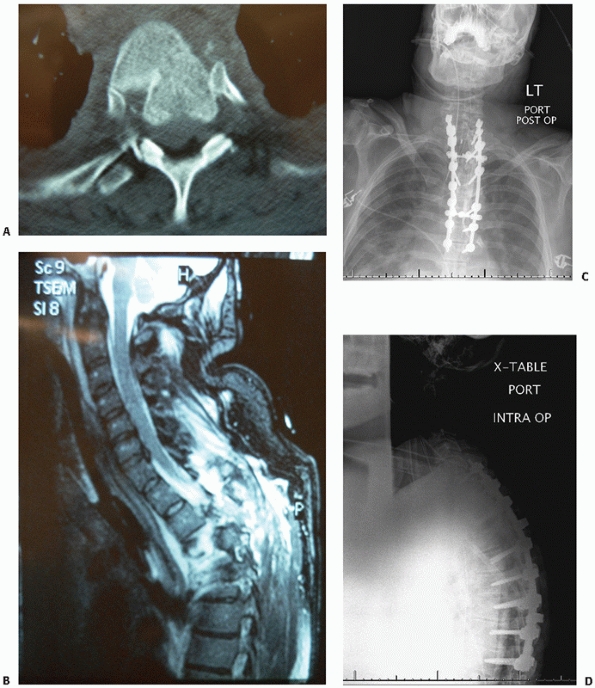 |
|
FIGURE 43-25
Imaging studies of a 67-year-old man who presented with a complete spinal cord injury following a motor vehicle collision. Axial computed tomography (A) and sagittal T2-weighted magnetic resonance (B) images reveal a fracture-dislocation at T2-T3. Postoperative anteroposterior (C) and lateral (D) radiographs obtained following an open reduction and posterior instrumented fusion extending from C7 to T8. |
cancellous bone and carefully introduced into the corpectomy site
without encroaching on the spinal cord, nerve roots, or blood vessels
that are in close proximity to the anterior column (Fig. 43-28). Many surgeons elect to score the vertebral end
plates so that the strut graft may be keyed into these grooves, but
this technique may result in a greater degree of settling. This segment
may be provisionally compressed by straightening the operating room
table and discontinuing any distraction of the spinal column.
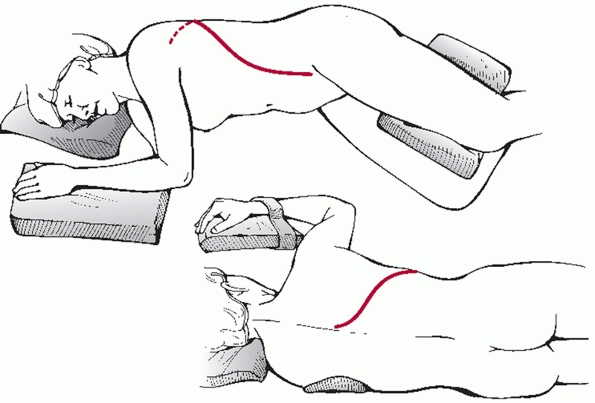 |
|
FIGURE 43-26 Schematic drawing of a patient who has been placed in the lateral decubitus position in preparation for a thoracotomy.
|
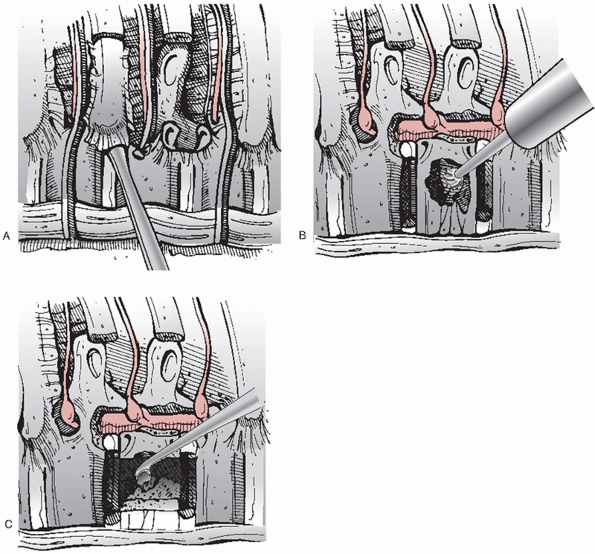 |
|
FIGURE 43-27
Schematic drawings illustrating the steps involved in performing a corpectomy for the purpose of decompressing the neural elements. |
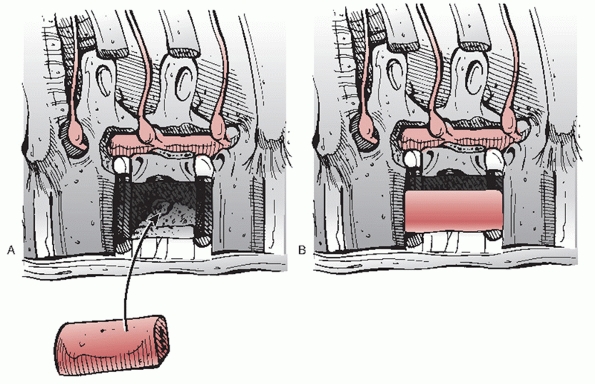 |
|
FIGURE 43-28
Schematic drawings demonstrating the steps involved in the implantation of a strut graft for the subsequent reconstruction of the anterior column. |
dramatically in recent years because of their well-documented capacity
for increasing the rigidity of the spine, which may translate into
improved fusion rates and fewer graft complications; consequently, with
contemporary screw and rod systems it is now reasonable to consider
anterior-only constructs for certain thoracolumbar injuries. To avoid
impinging on the great vessels located anterior to the spine, the “safe
zone” for any internal fixation is limited to the posterolateral
portion of the vertebral bodies (Fig. 43-29).
Prominent osteophytes and other bony protrusions are leveled with a
pituitary rongeur or burr, which permits the hardware to lie flush
against the spinal column. After the screw holes are created across
both the superior and inferior vertebrae, these implants are advanced
with or without the aid of fluoroscopic guidance with the goal of not
perforating the uninvolved disc spaces or projecting more than 1 to 2
mm beyond the far cortex if bicortical purchase is desired. Before
securing the screws to a spanning plate or rod, additional compressive
forces may be generated through the instrumentation, which may
stimulate bony healing at the interface between the graft and the end
plates. A number of pearls and pitfalls associated with anterior
surgical protocols are listed in Tables 43-4 and 43-5.
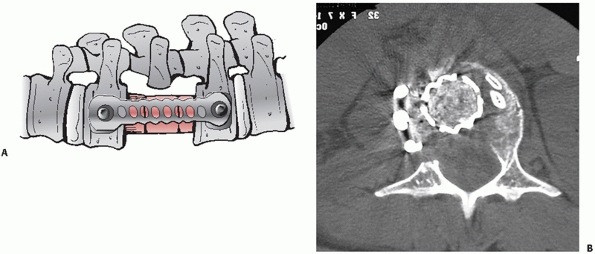 |
|
FIGURE 43-29 A.
Schematic drawing of an anterior construct consisting of an interbody graft within the corpectomy defect, which is spanned by a plate. B. Axial CT image demonstrating a metal cage filled with bone graft with instrumentation positioned on the lateral aspect of the spinal column. |
|
TABLE 43-4 Pearls and Pitfalls for Anterior Surgical Techniques—Preoperative Planning and Decompression of the Neural Elements
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
tissues must be controlled with bipolar electrocautery or hemostatic
substances before suturing together the ends of the parietal pleural.
If necessary, an anatomic repair of the diaphragm is performed and a
thoracostomy tube is ordinarily inserted to drain out any postoperative
air or fluid collections. Once the ipsilateral lung is reinflated and
the intercostal space has been sealed, the individual layers of the
wound are closed separately. The preoperative and postoperative images
of a thoracolumbar burst fracture that was addressed with an anterior
corpectomy and arthrodesis with an interbody implant and supplementary
instrumentation are presented in Figure 43-30.
there is minimal drainage, and it is only pulled when there are no
clinical or radiographic signs of a pneumothorax or air leak. Oral
intake should be prohibited until flatus or bowel sounds have been
recorded. As with those who have undergone posterior spinal procedures,
patients whose thoracolumbar fractures have been treated through an
anterior approach are managed with a regimen consisting of rapid
mobilization and variable periods of bracing. Likewise, standing
radiographs must be obtained at multiple time points so that any
evidence of progressive deformity, graft subsidence or other findings
consistent with a nonunion are not missed.
no surprise that thoracolumbar fractures may give rise to a wide range
of complications. Although patients with obvious neurologic
deterioration are usually treated with surgery in the acute setting, it
is not uncommon for individuals who have sustained spinal trauma to
experience increasing impairment secondary to other pathologic
conditions such as a synringomyelia. Whether surgical stabilization is
performed, the inherent instability of many thoracolumbar injuries also
predisposes many of these patients to the development of spinal
malalignment in any plane. In most cases, fractures that demonstrate
kyphosis greater than 30 degrees, height loss greater than 50%,
translation greater than 2.5 mm, canal compromise greater than 50%, or
signs of posterior ligamentous disruption on MRI studies may be
particularly susceptible to progressive deformity. Because these
fractures are frequently associated with injuries to the spinal cord
and abdominal viscera, there is also a fairly high incidence of
gastrointestinal abnormalities including ileus, gastroesophagel reflux
disease,
and constipation, all of which must be managed with appropriate
interventions. Individuals with thoracolumbar fractures, especially
those with concomitant spinal cord injuries, are clearly at risk for
thromboembolic events; in one investigation, the rate of symptomatic
deep vein thrombosis or pulmonary embolism was reported to be
approximately 2%, with the incidence of asymptomatic clots estimated to
be even higher.110
Consequently, some form of prophylaxis is indicated for these patients
such as chemical anticoagulation, intermittent pneumatic compression
devices, or vena cava filters. Despite the appearance of a healed
fracture, a large proportion of this population will continue to
complain of intractable pain, which may be refractory to both
conservative and operative measures. Finally, these types of injuries
regularly necessitate prolonged hospitalizations, which may lead to
problems related to pneumonia, pressure ulceration of the skin, or
malnutrition.
|
TABLE 43-5 Pearls and Pitfalls for Anterior Surgical Techniques—Arthrodesis and Instrumentation
|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
decompress and stabilize thoracolumbar fractures are considered to be
reasonably safe, these procedures may still subject the patient to a
number of potential complications. Iatrogenic neurologic insults have
been shown to occur in 1% of posterior operations secondary to the
erroneous placement of pedicle screws.73
Malpositioned implants may also bring about serious injuries to any
number of visceral or vascular structures, which are often
life-threatening, especially when they are not diagnosed and addressed
expediently (Fig. 43-31). Up to 10% of
instrumented fusions for thoracolumbar fractures may become infected,
all which require either long-term antibiotic therapy directed toward
the causative organism or possibly open irrigation and débridement if
indicated.112 Symptomatic durotomies
should be repaired primarily if possible, but individuals with
unrelenting leakage of cerebrospinal fluid from fistulas are routinely
managed with protracted bed rest or even insertion of a lumbar
subarachnoid drain to decrease intradural pressure enough so that a
watertight closure may be obtained. Any remaining intersegmental
instability at the arthrodesis site may result in the formation of a
pseudarthrosis with hardware failure, recurrent deformity, and
unrelenting pain (Fig. 43-32). Unfortunately,
these surgical approaches are also not without their morbidity; that
patients undergoing anterior or posterior procedures may experience
substantial blood loss from a corpectomy or the epidural veins,
compromised pulmonary function, or poor wound healing.
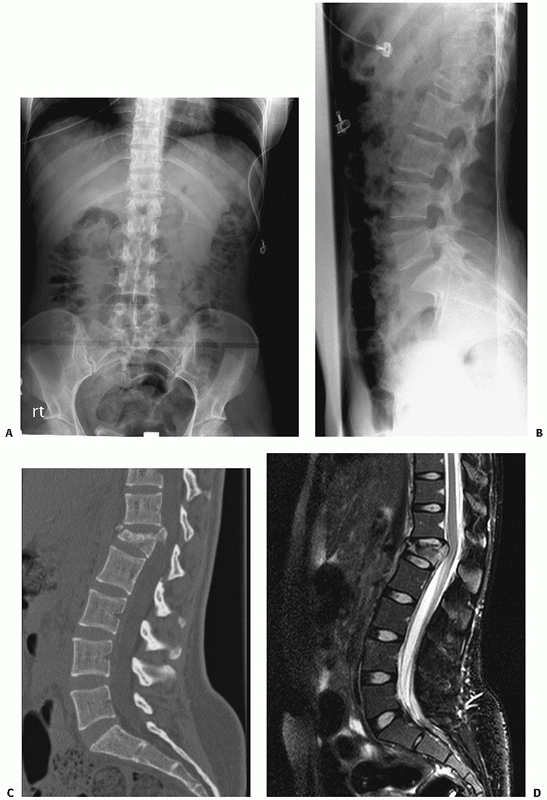 |
|
FIGURE 43-30
Imaging studies of a 27-year-old man who presented with progressive numbness and weakness in both lower extremities following a fall from a second-story window. An injury to the L1 vertebral body is evident on the initial anteroposterior (A) and lateral (B) radiographs. Sagittal computed tomography (C) and magnetic resonance (D) images demonstrate a burst injury with compression of the conus medullaris secondary to retropulsion of a bony fragment into the spinal canal. (continues) |
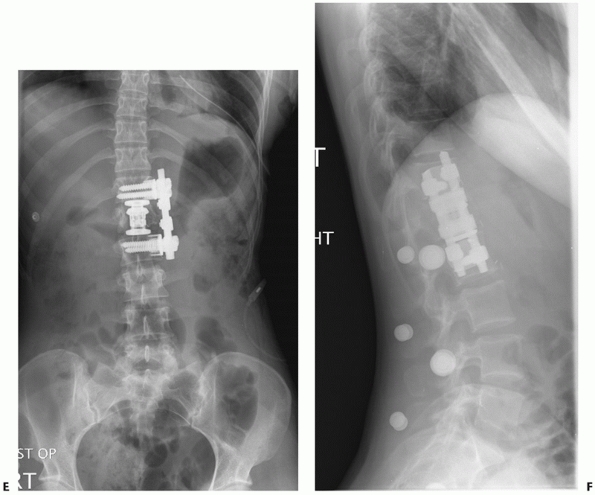 |
|
FIGURE 43-30 (continued) Postoperative anteroposterior (E) and lateral (F)
views of the thoracolumbar junction obtained following a L1 corpectomy, insertion of an expandable titanium cage, and placement of anterior instrumentation. |
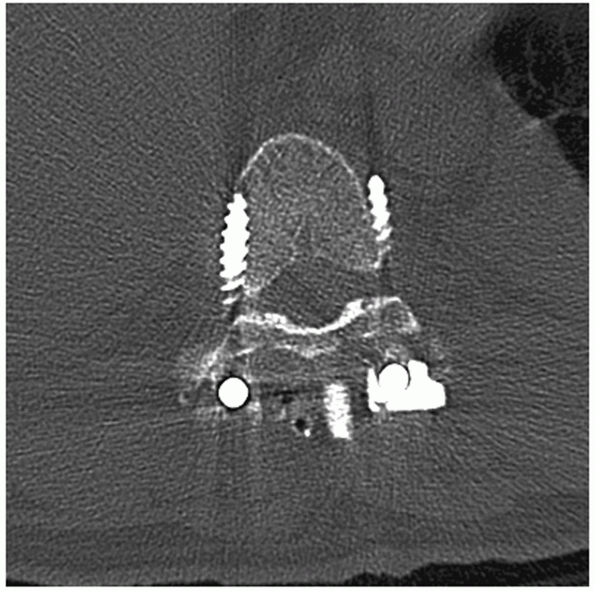 |
|
FIGURE 43-31
Axial computed tomography image showing pedicle screws that have been directed too laterally so that their tips protrude beyond the vertebral body, increasing the risk of a potentially life-threatening injury to vascular or visceral structures. |
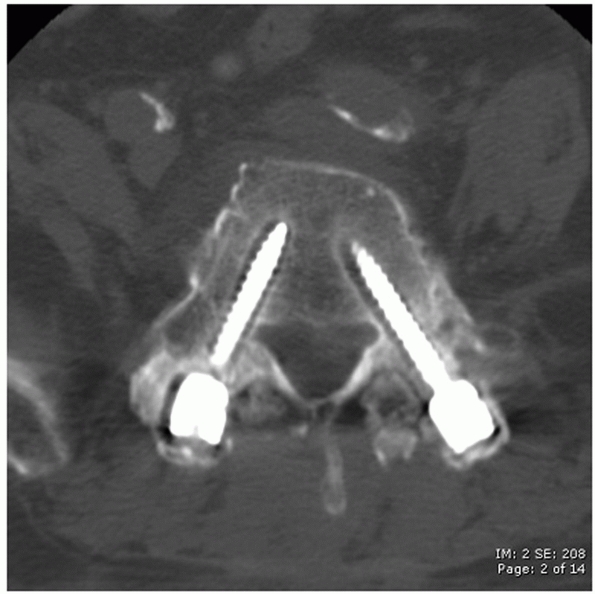 |
|
FIGURE 43-32
Axial computed tomography image of a patient with a lumbar fracture treated with a posterior instrumented fusion construct, which reveals obvious lucencies around the pedicle screws indicative of implant loosening and pseudarthrosis. |
retrospectively reviewed a large series of 147 thoracolumbar fractures
that occurred in conjunction with multiple other injuries. According to
their analysis, the group that had been immobilized was significantly
more likely to contract pneumonia and required longer hospital stays
than the subjects who were managed with operative fixation. Shen et al.121
prospectively evaluated 80 neurologically intact patients with
single-level burst injuries at the thoracolumbar junction who were
either treated with a course of bracing or short-segment instrumented
posterior arthrodesis. In this series, surgery brought about greater
initial correction of kyphosis and earlier pain relief but at 24 months
there were no significant differences between these two cohorts. Wood
et al.152 also performed a
randomized, prospective study comparing surgical and conservative
treatments for stable burst fractures without any accompanying
neurologic impairment. At an average follow-up of 44 months, operative
stabilization of these injuries did not appear to provide any
meaningful advantages to these individuals; in fact, those who were
placed in a body cast or orthosis actually reported less disability,
greater pain relief, and fewer complications. However, another recent
prospective, randomized, multicenter trial supported the use of
short-segment posterior stabilization for these types of fractures by
showing that these procedures gave rise to decreased kyphosis, better
functional scores, and a faster return to work relative to
immobilization in a Jewett device.123
over the others has yet to be borne out in the current literature. Two
centers independently proposed that anterior decompression of burst
fractures may generate more substantial improvements in neurologic
status compared to indirect reduction of these injuries through a
posterior approach.24,93
In another series of unstable burst fractures, an anterior corpectomy
and instrumented arthrodesis were found to be more efficacious than
posterior short-segment fixation for restoring normal sagittal
alignment.117 While Been and Bouma16
also observed better deformity correction with circumferential
surgeries than with posterior implants alone, the neurologic outcomes
of these cohorts were equivalent. However, Danisa et al.36
identified no significant differences between patients with unstable
burst injuries addressed anteriorly, posteriorly, or with a combined
technique. Esses et al.45 conducted
a prospective, randomized investigation in which subjects with acute
thoracolumbar burst fractures were alternately treated with posterior
distraction and pedicle screws or direct anterior decompression in
conjunction with a fusion construct composed of an autogenous
tricortical iliac crest strut graft and a screw-rod device. Although
the authors noted no obvious disparities between the clinical or
radiographic results of these two strategies, they emphasized that
anterior operations permitted a more thorough decompression of the
spinal canal. In a more recent prospective clinical trial, 38 patients
with stable burst injuries located at the thoracolumbar junction and no
attendant neurologic deficits were randomized so that they either
underwent a posterior instrumented arthrodesis or an anterior
reconstruction with an allograft strut and lateral fixation.151
At a minimum of 2-year follow-up, the fusion rates and functional
outcomes of these two cohorts were comparable but there was a trend
toward more frequent complications and a higher incidence of revision
surgeries with a posterior approach.
generally accepted that prompt operative intervention may yield
superior clinical outcomes.28 In
animals, rapid decompression appears to correlate with enhanced
neurologic recovery but these benefits have not necessarily been
recorded in humans.26 However, the
optimal time to perform surgical procedures in individuals who are
neurologically intact is still a controversial issue. The authors of
one retrospective study observed a trend toward greater return of
neurologic function among a group of thoracolumbar fractures that were
addressed surgically within 8 hours of the traumatic event compared to
injuries in which these operations were completed in a delayed fashion,
but otherwise there were no significant time-dependent effects
identified in this series.51 In similar investigation, Chipman et al.29
concluded that even though early surgery did not provide any additional
improvements in terms of postoperative neurologic status, patients that
were treated less than 3 days after sustaining their fractures
exhibited significantly fewer complications and shorter
hospitalizations than others whose interventions occurred after this
point. Based on this limited data, patients with thoracolumbar
fractures who do not exhibit neurologic compromise requiring emergent
decompression may be carefully evaluated before any type of operation;
for instance, it may be prudent to postpone definitive surgical
stabilization until any hemodynamic instability has been corrected and
traumatic injuries to all other organ systems have been thoroughly
ruled out (e.g., cardiac, pulmonary).
increasing popular in recent years because of its potential for
decreasing operative morbidity and preserving the stability of the
vertebral column, which many advocates claim may translate into
improved long-term functional outcomes. Nevertheless, many of these
purported advantages are still largely theoretical and have not been
supported by any randomized, prospective, controlled clinical trials
comparing MISS with open techniques. Nevertheless, the rationale for
MISS is undoubtedly intuitively pleasing to surgeons and patients
alike. While MISS may be inappropriate for many thoracolumbar
fractures, such as those that are associated with a substantial amount
of canal occlusion and an incomplete neurologic injury, that would be
best served with a wide decompression through an open approach, this
feasibility
of
MISS for this application has already been preliminarily established.
One center has reported their considerable experience with
endoscopically assisted anterior corpectomy, interbody reconstruction,
and instrumentation for thoracolumbar fractures.17,78
While the initial results have been favorable, the authors concede that
this strategy is very difficult and entails a very steep learning curve.
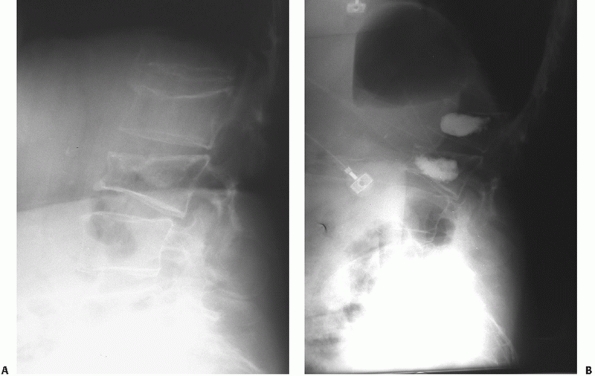 |
|
FIGURE 43-33 Preoperative (A) and postoperative (B)
lateral radiographs demonstrating multiple compression injuries of the lumbar spine that were addressed with vertebroplasy, a technique in which cement is injected percutaneously into the void created by the fracture. |
investigation includes the use of vertebroplasty/kyphoplasty for
traumatic thoracolumbar injuries, either with1,2,31,80,81,141 or without supplemental fixation37,127 (Fig. 43-33).
One major concern regarding the injection of cement into a fractured
vertebral body with disruption of the posterior cortex is the
possibility that this material may extrude into the canal and produce
an iatrogenic neurologic deficit. Clearly, multiple well-designed
studies are warranted to confirm the safety and efficacy of this and
other surgical techniques for the treatment of the entire spectrum of
thoracolumbar spinal injuries.
FL Jr, Aryan HE, Taylor WR, et al. Kyphoplasty-augmented short-segment
pedicle screw fixation of traumatic lumbar burst fractures: initial
clinical experience and literature review. Neurosurg Focus 2005;18:e9.
S, Akbar S, Dhar SA. Short segment pedicle screw instrumentation and
augmentation vertebroplasty in lumbar burst fractures: an experience.
Eur Spine J 2008;17: 336-341.
A, Acaroglu E, Yazici M, et al. The effect of transpedicular
intracorporeal grafting in the treatment of thoracolumbar burst
fractures on canal remodeling. Eur Spine J 2001;10:512-516.
A, Katonis P, Stergiopoulos K, et al. Functional outcome of burst
fractures of the thoracolumbar spine managed nonoperatively, with early
ambulation, evaluated using the load-sharing classification. Acta
Orthop Belg 2002;68:2789-287.
M, Ozkurt B, Aktekin CN, et al. Treatment of unstable thoracolumbar
junction burst fractures with short- or long-segment posterior fixation
in Magerl Type A fractures. Eur Spine J 2007;16:1145-1155.
HS, Lim TH, You JW, et al. Biomechanical evaluation of anterior
thoracolumbar spinal instrumentation. Spine 1995;20:1979-1983.
HS, Vaccaro AR, Cotler JM, et al. Low lumbar burst fractures.
Comparison among body cast, Harrington rod, Luque rod, and Steffee
plate. Spine 1991;16:S440-S444.
PA, Henley MB, Rivara FP, et al. Flexion distraction and chance
injuries to the thoracolumbar spine. J Orthop Trauma 1991;5:153-160.
S, Biros MH, Reardon RR. Delayed diagnosis of thoracolumbar fractures
in multiple-trauma patients. Acad Emerg Med 1996;3:832-839.
RT, Mackersie R, Abitbol JJ, et al. Can burst fractures be predicted
from plain radiographs? J Bone Joint Surg Br 1992;74B:147-150.
E, Cybulski G, Chaudhri K, et al. Magnetic resonance imaging and
computed tomography in the evaluation of penetrating gunshot injury.
Case report. Spine 1993; 18:772-773.
HD, Bouma GJ. Comparison of two types of surgery for thoraco-lumbar
burst fractures: combined anterior and posterior stabilization vs.
posterior instrumentation only. Acta Neurochir (Wein) 1999;141:349-357.
R, Muckley T, Schmidt MH, et al. Surgical technique and results of
endoscopic anterior spinal canal decompression. J Neurosurg Spine
2005;2:128-136.
M, Bastian L, Knop C, et al. Interobserver reliability in the
classification of thoraco-lumbar spinal injuries [German]. Orthopade
1999;28:662-681.
HH, Freehafer A, Dejak J. The results of treatment of acute injuries of
the upper thoracic spine with paralysis. J Bone Joint Surg Am
1985;67A:360-369.
HH, Kirkpatrick JS, Delamarter RB, et al. Anterior decompression for
late pain and paralysis after fractures of the thoracolumbar spine.
Clin Orthop Relat Res 1994;300:24-29.
MB, Shephard MJ, Collins WF, et al. A randomized, controlled trial of
methylprednisolone or naloxone in the treatment of acute spinal cord
injury: results of the Second National Acute Spinal Cord Study. N Engl
J Med 1990;322:1405-1411.
MB, Shepard MJ, Horford TR, et al. Administration of methylprednisolone
for 24 to 48 hours or tirilized mesylate for 48 hours in the treatment
of acute spinal cord injury. Results of the Third National Acute Spinal
Cord Injury Randomized Controlled Trial. JAMA 1997;277:1597-1604.
DS, McBride GG. Surgical management of thoracolumbar spine fractures
with incomplete neurologic deficits. Clin Orthop Relat Res
1987;218:201-216.
FP Jr, Eismont FJ, Green BA. Dural laceration occurring with burst
fractures and associated lumbar fractures. J Bone Joint Surg Am
1989;71A:1044-1052.
GD, Minato Y, Okada A, et al. Early time-dependent decompression for
spinal cord injury: vascular mechanisms of recovery. J Neurotrauma
1997;14:951-962.
JR, Agel J, Jurkovich GJ, et al. Thoracolumbar flexion-distraction
injuries: associated morbidity and neurological outcomes. Spine
2008;33:648-657.
JG, Deuser WE, Beilman GJ. Early surgery for thoracolumbar spine
injuries decreases complications. J Trauma 2004;56:52-57.
DY, Lee WY, Sheu PC. Treatment of thoracolumbar burst fractures with
polymethyl methacrylate vertebroplasty and short-segment pedicle screw
fixation. Neurosurgery 2003;53:1354-1360.
GH, Nelson BJ, Gebhard JS, et al. Functional outcome of thoracolumbar
burst fractures managed with hyperextension casting or bracing and
early mobilization. Spine 1996;21:2170-2175.
JP Jr, Anderson PA, King HA, et al. Indirect spinal canal decompression
in patients with thoracolumbar burst fractures treated by posterior
distraction rods. J Spinal Disord 1991;4:39-48.
LY, Jin WJ. Interobserver and intraobserver reliability in the
load-sharing classification of the assessment of thoracolumbar burst
fractures. Spine 2005;30:354-358.
LY, Yao WF, Cui YM, et al. Thoracolumbar fractures in patients with
multiple injuries: diagnosis and treatment: a review of 147 cases. J
Trauma 2004;56:348-355.
OA, Shaffrey CI, Jane JA, et al. Surgical approaches for the correction
of unstable thoracolumbar burst fractures: a retrospective analysis of
treatment outcomes. J Neurosurg 1995;83:977-983.
Falco R, Scarano E, Di Celmo D, et al. Balloon kyphoplasty in traumatic
fractures of the thoracolumbar junction. Preliminary experience in 12
cases. J Neurosurg Sci 2005;49:147-153.
Klerk LW, Fontijne WP, Stijnen T, et al. Spontaneous remodeling of the
spinal canal after conservative management of thoracolumbar burst
fractures. Spine 1998;23: 1057-1060.
RB, Sherman J, Carr JB. Pathophysiology of spinal cord injury: recovery
after immediate and delayed decompression. J Bone Joint Surg Am
1995;77A: 1042-1049.
F. The three columns of the spine and its significance in the
classification of acute thoracolumbar spine injuries. Spine
1983;8:817-831.
F, Burkus JK. Diagnosis and treatment of cauda equina entrapment in the
vertical lamina fracture of lumbar burst fractures. Spine
1991;16:S433-S439.
F, Burkus JK. Shear fracture-dislocations of the thoracic and lumbar
spine associated with forceful hyperextension (lumberjack paraplegia).
Spine 1992;17:156-161.
JC, Nachtigall D, Humphreys SC, et al. Questionnaire survey of spine
surgeons on the use of methylprednisolone for acute spinal cord injury.
Spine 2006;31:E250-E253.
RG, Dietze DD Jr, Millan MM, et al. Lateral parascapular extrapleural
approach to the upper thoracic spine. J Neurosurg 1991;75:349-355.
S, Falcone S, Green B. MR of the spine in the presence of metallic
bullet fragments: is the benefit worth the risk? Am J Neuroradiol
1999;20:354-356.
Y, Gepstein R. Late outcome of nonoperative management of thoracolumbar
vertebral wedge fractures. J Orthop Trauma 2003;17:190-192.
HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the
initial management of closed injuries of the spine with paraplegia and
tetraplegia. Paraplegia 1967;7:179-192.
C, Maier R, Kutscha-Lissberg F, et al. Results of spinal cord
decompression and thoracolumbar pedicle stabilization in relation to
time of injury. Spinal Cord 1999; 37:33-39.
SR, Mowery CA, Guerra J Jr, et al. Confirmation of the posterolateral
technique to decompress and fuse thoracolumbar spine burst fractures.
Spine 1985;10:218-223.
JA, Daffner RH, Osborne RL. Relevant signs of stable and unstable
thoracolumbar vertebral column trauma. Skeletal Radiol 1981;7:179-183.
SD, Court-Brown CM. Flexion-distraction injuries of the lumbar spine.
Mechanisms of injury and classification. Clin Orthop Relat Res
1988;227:52-60.
AJ, Zdeblick TA. Anterior instrumentation in the management of
thoracolumbar burst fractures. Clin Orthop Relat Res 1997;335:89-100.
AW 3rd, MacMillan M, Fessler RG. Lateral extracavitary approach to the
thoracic and thoracolumbar spine. Orthopedics 1997;20:605-610.
N, Blauth M, Tscherne H. Anterior plating in thoracolumbar spine
injuries. Indication, technique, and results. Spine 1991;16:S100-111.
WT Jr, Cook WA Jr, Friedman AH, et al. Bilateral transpedicular
decompression and Harrington rod stabilization in the management of
severe thoracolumbar burst fractures. Spine 1992;17:162-171.
RM, Budorick T, Hoyt J, et al. Biomechanics of indirect fracture
reduction of bone retropulsed into the spinal canal in vertebral
fracture. Spine 1993;18: 692-699.
JS, Vaccaro AR, Hurlbert RJ, et al. Intrarater and interrater
reliability and validity in the assessment of the mechanism of injury
and integrity of the posterior ligamentous complex: a novel injury
severity scoring system for thoracolumbar injuries. Invited submission
from the Joint Section Meeting on Disorders of the Spine and Peripheral
Nerves, March 2005. J Neurosurg Spine 2006;4:118-122.
CJ, Visvikis G, Henrichs C, et al. Prospective validation of computed
tomographic screening of the thoracolumbar spine in trauma. J Trauma
2003;55:228-234.
RJ, Moulton R. Why do you prescribe methylprednisolone for acute spinal
cord injury? A Canadian perspective and a position statement. Can J
Neurol Sci 2002; 29:236-239.
RR, Asher MA, Snider RK. Thoracolumbar spinal injuries: a comparative
study of recumbent and operative treatment in 100 patients. Spine
1980;5:463-477.
K, Abumi K, Fujiya M. Burst fractures with neurologic deficits of the
thoracolumbar-lumbar spine. Results of anterior decompression and
stabilization with anterior instrumentation. Spine 1984;9:788-795.
K, Taneichi H, Abumi K, et al. Anterior decompression and stabilization
with the Kaneda device for thoracolumbar burst fractures associated
with neurological deficits. J Bone Joint Surg Am 1997;79A:69-83.
P, Christoforakis J, Kontakis G, et al. Complications and problems
related to pedicle screw fixation of the spine. Clin Orthop Relat Res
2003;411:86-94.
PG, Kontakis GM, Loupasis GA, et al. Treatment of unstable
thoracolumbar and lumbar spine injuries using Cotrel-Debousset
instrumentation. Spine 1999;24: 2352-2357.
RA, Aydin Y. Modified transpedicular approach for the surgical
treatment of severe thoracolumbar or lumbar burst fractures. Spine J
2004;4:208-217.
J, Fischer S, Vanderby R, et al. Significance of acute posttraumatic
bony encroachment of the neural canal. Spine 1989;14:799-802.
LT, Beisse R, Potulski M. Thoracoscopic-assisted treatment of thoracic
and lumbar fractures: a series of 371 consecutive cases. Neurosurgery
2002;51:S104-S117.
C, Fabian HF, Bastian L, et al. Late results of thoracolumbar fractures
after posterior instrumentation and transpedicular bone grafting. Spine
2001;26:88-99.
P, Hadjipavlou A, Repantis T. Minimal invasive short posterior
instrumentation plus balloon kyphoplasty with calcium phosphate for
burst and severe compression lumbar fractures. Spine 2008;33:658-667.
P, Repantis T, Petsinis G, et al. Direct reduction of thoracolumbar
burst fractures by means of balloon kyphoplasty with calcium phosphate
and stabilization with pedicle-screw instrumentation and fusion. Spine
2008;33:E100-E108.
JP. Anterior fixation for burst fractures of the thoracic and lumbar
spine with or without neurological involvement. Spine 1988;13:286-293.
DL, Rodgers WB, Mansfield FL. Transpedicular instrumentation and
short-segment fusion of thoracolumbar fractures: a prospective study
using a single instrumentation system. J Orthop Trauma 1995;9:499-506.
NA, Harten RD, Lin DC, et al. Acute thoracolumbar burst fractures: a
new view of loading mechanisms. Spine 2002;27:498-508.
HM, Kim HS, Kim DJ, et al. Reliability of magnetic resonance imaging in
detecting posterior ligament complex injury in thoracolumbar spinal
fractures. Spine 2000;25: 2079-2084.
JY, Vaccaro AR, Schweitzer KM Jr, et al. Assessment of injury to the
thoracolumbar posterior ligamentous complex in the setting or
normal-appearing plain radiography. Spine J 2007;7:422-427.
DA, Petrie DP, Alexander DI. Flexion-distraction injuries of the lumbar
spine and associated abdominal trauma. J Trauma 1990;31:436-444.
KC, Hsieh CH, Lee CY, et al. Transpedicle body augmenter: a further
step in treating burst fractures. Clin Orthop Relat Res
2005;436:119-125.
F, Aebi M, Gertzbein SD, et al. A comprehensive classification of
thoracic and lumbar injuries. Eur Spine J 1994;3:184-201.
FM Jr, Bracken MB, Creasey G, et al. International standards for
neurologic and functional classification of spinal cord injury.
American Spinal Injury Association. Spinal Cord 1997;35:266-274.
PC, Bohlman HH, Yuan HA. Anterior decompression of traumatic
thoracolumbar fractures with incomplete neurological deficit using a
retroperitoneal approach. J Bone Joint Surg Am 1985;67A:89-104.
PC, Yuan HA, Fredrickson BE, et al. The value of computed tomography in
thoracolumbar fractures. An analysis of 100 consecutive cases and a new
classification. J Bone Joint Surg Am 1983;65A:461-473.
G, Vaccaro AR, Garfin SR. Thoracic and lumbar trauma: rationale for
selecting the appropriate fusion technique. Orthop Clin North Am
1998;29:813-828.
PW, Davis R, Tribus C, et al. The management of acute thoracolumbar
burst fractures with anterior corpectomy and Z-plate fixation. Spine
2004;29: 1901-1908.
RD, Bradford DS. The management of burst fractures of the thoracic and
lumbar spine. Experience in 53 patients. Spine 1985;10:631-637.
BJ, VanderWilde RS, Currier BL, et al. Diagnosis of subtle
thoracolumbar burst fractures. A new radiographic sign. Spine
1993;19:2282-2285.
RF, Ferrera L, Kabins M. Pedicle morphology in the upper thoracic
spine: limits to safe placement in older patients. Spine
2002;27:2467-2471.
RF, Sparling E, Benson DR. Early failure of short-segment pedicle
instrumentation for thoracolumbar fractures. A preliminary report. J
Bone Joint Surg Am 1993; 75A:162-167.
JS, Reed MR, McVie JL, et al. Weight-bearing radiographs in
thoracolumbar fractures: do they influence management? Spine
2004;29:564-567.
RA, Harcastle P, Renwick SE. Lower spinal mobility and external
immobilization in the normal and pathologic condigion. Orthop Rev
1992;21:753-757.
A, Hasserious R, Redlund-Johnell I, et al. Nonoperatively treated burst
fractures of the thoracic and lumbar spine in adults: a 23- to 41-year
follow-up. Spine J 2007; 7:701-707.
J, Weinstein JN, Spratt KF, et al. Thoracolumbar burst fractures. The
clinical efficacy and outcome of nonoperative management. Spine
1993;18:955-970.
FC, Ramos LM, Simmermacher RK, et al. Classification of thoracic and
lumbar spine fractures: problems of reproducibility. A study of 53
patients using CT and MRI. Eur Spine J 2002;11:235-245.
MM, Hausefled JN, White A. A biomechanical study of the ligamentous
stability of the thoracic spine in man. Orthop Scand 1981;52:315-326.
JW, Lane JR, Karaikovic EE, et al. Successful short-segment
instrumentation and fusion for thoracolumbar spine fractures: a
consecutive 41/2-year series. Spine 2000; 25:1157-1170.
P, Thalhammer G, Jaindl M, et al. Thromboembolic complications after
spinal surgery in trauma patients. Acta Orthop 2006;77:755-760.
M, Mahmud M, Mokhtar SA, et al. Thoracolumbar fracture-dislocation
results of surgical treatment. Med J Malaysia 2000;55:C14-C17.
GR, Bono PL, Cahill D, et al. Postoperative wound infection after
instrumentation of thoracic and lumbar fractures. J Orthop Trauma
2001;15:566-569.
DC, Hu R, Davis LA, et al. The nonoperative treatment of burst
fractures of the thoracolumbar junction. J Trauma 1988;28:1188-1194.
A, Branfoot T, Barlow IF, et al. Spinal injury patterns resulting from
car and motorcycle accidents. Spine 2002;27:2825-2830.
RC, Best NM, Reilly TM, et al. Anterior-only stabilization of
three-column thoracolumbar injuries. J Spinal Disord Tech
2005;18:S7-S14.
RC, Cotler HB. Posterior instrumentation and fusion for unstable
fracatures and fracture-dislocations of the thoracic and lumbar spine.
A comparative study of three fixation devices in 70 patients. Spine
1993;18:450-460.
RC, Renkens K, Hanson D, et al. Unstable thoracolumbar burst fractures:
anterior-only versus short-segment posterior fixation. J Spinal Disord
Tech 2006; 19:242-248.
CL, Ansell LV. Selection criteria and outcome of operative approaches
for thoracolumbar burst fractures with and without neurological
deficit. J Neurosurg 1997;86: 48-55.
WJ, Liu TJ, Shen YS. Nonoperative treatment versus posterior fixation
for thoracolumbar junction burst fractures without neurologic deficit.
Spine 2001;26:1038-1045.
J, Yamamuro T, Lida H, et al. Surgical treatment for paraplegia
resulting from vertebral fractures in senile osteoporosis. Spine
1990;15:589-591.
J, Leferink VJ, Segers MJ, et al. Treatment of traumatic thoracolumbar
spine fractures: a multicenter prospective randomized study of
operative versus nonsurgical treatment. Spine 2006;31:2881-2890.
L, Karlstrom G, Pech P, et al. Indirect spinal canal decompression in
burst fractures treated with pedicle screw instrumentation. Spine
1996;21:113-123.
PJ Jr, Patwardhan AG, Lorenz M, et al. Instability of the lumbar burst
fracture and limitations of transpedicular instrumentation. Spine
1995;20:1452-1461.
JM, Connolly PJ, eds. Orthopaedic Knowledge Update: Spine 3. Rosemont,
IL: American Academy of Orthopaedic Surgeons, 2006.
M, Wolf I, Ringel F, et al. Treatment of painful osteoporotic
compression and burst fractures using kyphoplasty: a prospective
observational design. J Neurosurg Spine 2007;6:313-319.
EE, White D, Bradford DS, et al. Delayed anterior decompression in
patients with spinal cord and cauda equine injuries of the
thoracolumbar spine. Spine 1990; 15:953-957.
K, Fujikawa A, Honya K, et al. Value of diffusion-weighted MR imaging
in acute cervical cord injury as a predictor of outcome. Neuroradiology
2006;48:803-808.
AR, Baron EM, Sanfilippo J, et al. Reliability of a novel
classification system for thoracolumbar injuries: the Thoracolumbar
Injury Severity Score. Spine 2006;31: S62-S69.
AR, Kim DH, Brodke DS, et al. Diagnosis and management of thoracolumbar
spine fractures. J Bone Joint Surg Am 2003;85A:2456-2470.
AR, Lehman RA Jr, Hurlbert RJ, et al. A new classification of
thoracolumbar injuries: the importance of injury morphology, the
integrity of the posterior ligamentous complex, and neurologic status.
Spine 2005;30:2325-2333.
AR, Nachwalter RS, Klein GR, et al. The significance of thoracolumbar
spinal canal size in spinal cord injury patients. Spine 2001;26:371-376.
AR, Zeiller SC, Hurlbert RJ, et al. The Thoracolumbar Injury Severity
Score: a proposed treatment algorithm. J Spinal Disord Tech
2005;18:209-215.
FL, Burns J, Jackson AB, et al. Combined medical and surgical treatment
after acute spinal cord injury: results of a prospective pilot study to
assess the merits of aggressive medical resuscitation and blood
pressure management. J Neurosurg 1997; 87:239-246.
Kooi D, Abad G, Basford JR, et al. Lumbar spine stabilization with a
thoracolumbosacral orthosis: evaluation with video fluoroscopy. Spine
2004;29:100-104.
JJ, Dhert WJ, Verbout AJ, et al. Balloon vertebroplasty in combination
with pedicle screw instrumentation: a novel technique to treat thoracic
and lumbar burst fractures. Spine 2005;30:E73-E79.
XY, Dai LY, Xu HZ, et al. The load-sharing classification of
thoracolumbar fractures: an in vitro biomechanical validation. Spine
2007;32:1214-1219.
XY, Dai LY, Xu HZ, et al. Kyphosis recurrence after posterior
short-segment fixation in thoracolumbar burst fractures. J Neurosurg
Spine 2008;8:246-254.
JN, Collalto P, Lehmann TR. Thoracolumbar “burst” fractures treated
conservatively; a long-term follow-up. Spine 1988;13:33-38.
PG, Vaccaro AR, Poelstra KA, et al. The influence of fracture mechanism
and morphology on the reliability and validity of two novel
thoracolumbar injury classification systems. Spine 2007;32:791-795
J, Lindahl S, Irstam L, et al. Unstable thoracolumbar fractures. A
study by CT and conventional roentgenology of the reduction effect of
Harrington instrumentation. Spine 1984;9:214-219.
M, Mouhsine R, Theumann N, et al. Thoracolumbar spine fractures in
patients who have sustained severe trauma: depiction with multidetector
row CT. Radiology 2003;227:681-689.
K, Buttermann G, Mehbod A, et al. Operative compared with nonoperative
treatment of a thoracolumbar burst fracture without neurologic deficit.
A prospective, randomized study. J Bone Joint Surg Am 2003;85A:773-781.
KB, Bohn D, Mehbod A. Anterior versus posterior treatment of stable
thoracolumbar burst fractures without neurologic deficit: a
prospective, randomized study. J Spinal Disord Tech 2005;18:S15-S23.
KB, Khanna G, Vaccaro AR, et al. Assessment of two thoracolumbar
fracture classification systems as used by multiple surgeons. J Bone
Joint Surg Am 2005;87A: 1423-1429.
M, Gulman B, Sen S, et al. Sagittal contour restoration and canal
clearance in burst fractures of the thoracolumbar junction in T12-L1:
the efficacy of timing of the surgery. J Orthop Trauma 1995;9:491-198.
SW, Fang KF, Tseng IC, et al. Surgical outcomes of short-segment
fixation for thoracolumbar fracture dislocation. Chang Gung Med J
2002;25:253-259.
JJ, Sossan A, Selgrath C, et al. The treatment of unstable thoracic
spine fractures with transpedicular screw instrumentation: a 3-year
consecutive series. Spine 2002; 27:2782-2787.
