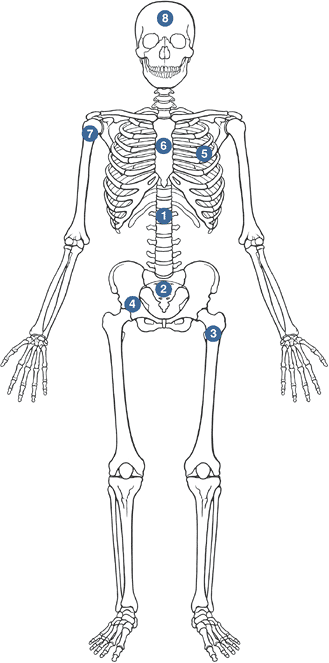
Metastatic Disease
number of bone lesions in patients greater than 40 years of age. In
fact, the differential diagnosis for an aggressive bone lesion in an
adult is “mets, mets, mets, myeloma, lymphoma, and sarcoma,” in
decreasing order of frequency. Even solitary bone lesions in this age
group are most commonly due to metastatic carcinoma. The role of the
orthopaedist is to establish or confirm the diagnosis, evaluate for
risk of fracture, and stabilize or otherwise surgically treat
pathological fractures and impending fractures. Pathologic fractures
are discussed in Chapter 4, Treatment Principles.
cause of bone lesions in this age group, bone lesions should never be
assumed to be due to metastatic disease without compelling evidence.
One of the most important pitfalls in the evaluation and treatment of
bone lesions in adults is the erroneous treatment of a sarcoma under
the mistaken assumption that the lesion is due to metastatic carcinoma.
Because the principles for treatment differ so greatly between sarcomas
and metastatic carcinomas, the specific diagnosis must be established
before initiation of care. Hence, an aggressive bone lesion in an
adult, while frequently due to metastatic disease, should be considered
a sarcoma until proven otherwise.
-
Common sources of bone carcinomas metastasizing to bone in adults
-
Most common “osteophilic” carcinomas
-
Breast, prostate, lung, kidney, thyroid (the “big 5”)
-
Melanoma: emerging osteophilic tumor
-
Common sources of metastatic carcinoma (Fig. 8-1)
-
-
Most common when there is an established history of cancer:
-
Breast and prostate
-
-
Most common when there is no established history of cancer
-
Lung and kidney, respectively
-
-
-
Common sources of metastases to bone in pediatric patients
-
Neuroblastoma (<5 years old) > rhabdomyosarcoma > retinoblastoma
-
-
Common sources for unusual patterns of distribution (any primary may)
-
Most common metastases distal to the elbows and knees (“acral” mets)
-
Lung and kidney, respectively
-
50% of acral mets due to lung carcinomas
-
-
Most common metastases to soft tissue
-
Lung and kidney, respectively
-
-
-
Frequency of common sources of bone carcinoma metastases (Table 8-1)
-
New cases: prostate > breast > lung > kidney > thyroid
-
Frequency of metastases and survival according to primary source (Table 8-2)
 |
|
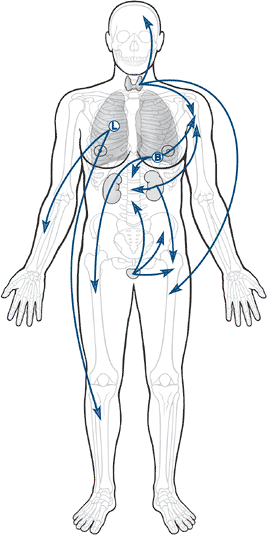
Figure 8-1 Body diagram showing breast, prostate, lung, kidney, and thyroid with arrows to bone.
|
|
Table 8-1 Epidemiology of The “Big Five” Most Common Primary Sources of Metastases to Bone
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||
is very important to allowing the establishment of a tumor growth, the
distribution of metastases is intimately related to the route that
metastases take from their site of origin to get to their ultimate
destination (Fig. 8-2 and Table 8-3).
Most metastases escape the circulation by traversing thin-walled venous
structures, so they follow the distribution of the systemic (caval),
portal, or vertebral venous circulation (Batson’s plexus; Box 8-1) to arrive within the lung, liver, or bone, respectively (see Table 8-3).
The axial pattern of distribution of the vertebral venous circulation
is reflected in the predominance of axial locations for the most common
sites of bone metastases (see Fig. 8-2). Lung
carcinomas, given their location, have a relatively greater frequency
of accessing the arterial circulation by means of eroding the pulmonary
venous vasculature within the lungs. Hence, lung carcinomas are the
most common source of bone metastases to the acral skeleton (distal to
the elbow and knee).
-
Common sites
-
Thoracolumbar spine > sacrum > proximal femur > pelvis > ribs > sternum > proximal humerus > skull
-
-
“Soil and seed” versus circulation controversy
-
Classic argument: idea that certain
tissues are better “soil” for cancer cells versus idea that the
circulation determines the patterns of metastases -
“Soil hypothesis” from Paget (1889) with modern considerations
-
Metastatic tumors have a predilection for red marrow (proximal sites) over yellow marrow.
-
Adhesion molecules favor recruitment of tumor cells.
-
For example, stromal cell–derived
factor-1 (SDF-1) recruits prostate cancer cells via interaction with
chemokine receptor (CXCR4) on tumor cells.P.231Table
8-2 Fraction of Patients with Each Primary Source Who Will Develop
Metastases, Fraction of Those That Will Involve Bone, and Survival With
MetastasesPrimary Tumor Pts. with Metastatic Disease (%) Pts. with Metastases who Have Bone Involvement Clinically (%) Median Survival After Diagnosis of Metastases (mo) Mean 5-year Survival Rate (%) Breast carcinoma 65–75 24 20 Prostate carcinoma 65–75 30–40 40 25 Lung carcinoma 30–40 20–40 <6 <5 Renal carcinoma 20–25 15–25 6 10 Thyroid carcinoma 60 20–40 48 40 Multiple myeloma 95–100 100 20 10 Data from Coleman RE. Skeletal complications of malignancy. Cancer 1997;80:1588–1594. ![]() Figure 8-2 Common sites of metastases to bone, in order of relative frequency.Table 8-3 Circulation Theory of Metastatic Distribution
Figure 8-2 Common sites of metastases to bone, in order of relative frequency.Table 8-3 Circulation Theory of Metastatic DistributionCirculatory Component Consequent Site of Metastases Systemic (caval) venous circulation Lung Portal venous circulation Liver Batson’s vertebral venous plexus Bone Pulmonary venous circulation All organs, bones P.232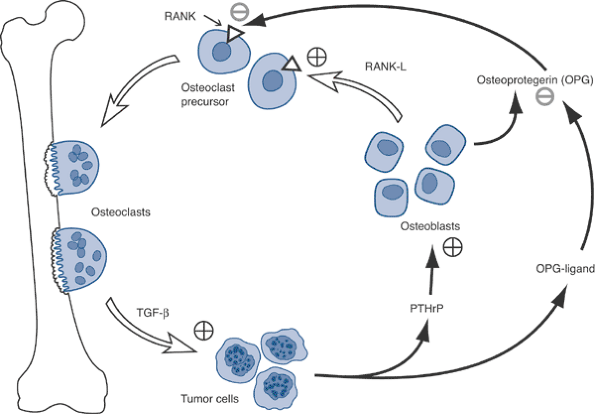 Figure 8-3 Disruption by tumor cells of normal osteoblast–osteoclast interaction via OPG-ligand.
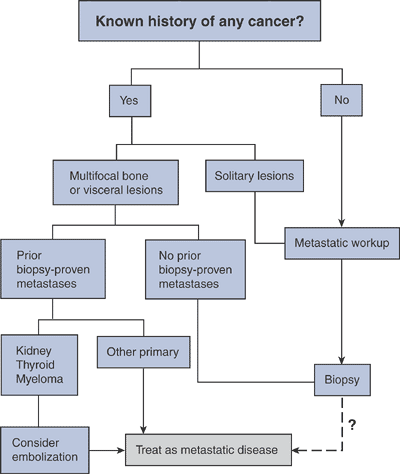
Figure 8-3 Disruption by tumor cells of normal osteoblast–osteoclast interaction via OPG-ligand.![]() Algorithm 8-1 Diagnostic work-up of metastatic tumors.
Algorithm 8-1 Diagnostic work-up of metastatic tumors.
-
-
Growth factors capable of stimulating tumor proliferation are replete in bone.
-
Insulin-derived growth factor (IGF)
-
Bone morphogenic proteins (BMP)
-
Transforming growth factor-beta (TGF-β)
-
P.233 -
-
“Circulation theory” from Ewing (1928) (see Table 8-3)
-
-
Network of interconnected veins with thin, low-pressure walls
-
Extends longitudinally from skull to sacrum, supplying segmental vertebra
-
Location outside thoracoabdominal cavity: unaffected by Valsalva maneuvers
-
Retrograde movement possible via valveless veins
-
Connections to common osteophilic primaries (breast, prostate, lung, kidney, thyroid)
-
Osteoclasts, not tumor cells, destroy bone.
-
Osteoclasts mediate tumor cell attachment to bone.
-
Breast cancer cells recruit osteoclasts via parathyroid hormone–related protein (PTHrP) (Fig. 8-3).
-
Tumor cells produce PTHrP and osteoprotegerin ligand (OPG-L).
-
PTHrP stimulates osteoblasts to release osteoprotegerin (OPG) and receptor activator of nuclear factor kappa ligand (RANK-L).
-
RANK-L stimulates the RANK receptor on
osteoclast precursors to cause differentiation into osteoclasts, which
leads to resorption. -
Osteoprotegerin inhibits the interaction of RANK-L with RANK, decreasing the bone resorption.
-
-
Myeloma cells stimulated by interleukin-6 (IL-6) released by osteoclasts
 |
|
Algorithm 8-2
Evaluation for metastatic disease of unknown primary site. (Adapted from Rougraff BT, Kneisl JS, Simon MA. Skeletal metastases of unknown origin: A prospective study of a diagnostic strategy. J Bone Joint Surg [Am] 1993;75:1276–1281.) |
stage of any primary malignancy and carries a similar weight in staging
systems for these cancers as metastases to other distant sites. While
the TLM (tumor, lymph node, metastasis) classification systems differ
in their details between sarcomas and the common carcinomas that
metastasize to bone, spread to bone in all cases shifts the disease to
the highest level.
However, in many cases in which the orthopaedic surgeon will be
involved, the diagnosis will require a more comprehensive evaluation
and biopsy for confirmation.
-
Key examinations search for common sources of bone metastases:
-
Breast, prostate, lungs, flank (kidney), thyroid
-
-
Comprehensive evaluation for other less common sources of bone metastases and sites of concurrent metastases (Table 8-4):
-
Skin (melanoma), lymphatics (lymphoma), guaiac examination (colon)
-
-
Pain
-
The most common presenting clinical symptom of bone metastases
-
Night pain characteristic
-
-
Less common presentations of metastases
-
Soft tissue mass (from soft tissue extension or soft tissue metastasis)
-
Incidental findings on staging studies coincident with primary tumor diagnosis
-
Paraplegia (spine metastases with or without pathologic spinal fracture)
-
Pathological fracture (usually preceded by pain, even of short duration)
-
-
Soft tissue metastases: more frequently painful rather than painless, whereas most soft tissue sarcomas are painless
|
Table
8-4 Comprehensive Systemic Approach for Physical Examination and Review of Systems Relative to Patients with Potential Metastatic Disease |
|||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||
-
Most common radiographic appearance: aggressive features (Box 8-2)
-
Typical margins: moth-eaten to permeative
-
Typical effects on bone: cortical destruction, soft tissue extension (variable)
-
Typical bone reactions: nonlaminar periosteal reaction (variable), Codman’s triangle (unusual) (Fig. 8-4, onion-skinning (rare), sunburst (rare)
-
Matrix: lytic (Fig. 8-5), sclerotic (Fig. 8-6), or mixed (Fig. 8-7)
-
Bone scan: increased uptake (see Fig. 8-4), except in very aggressive lytic tumors (e.g., renal, thyroid, myeloma)
-
Radioiodide nuclear medicine study: increased uptake for thyroid carcinoma
-
-
Differentiation from myeloma
-
Myeloma classic features not typical in metastases
-
Myeloma classic features: multiple punched-out lytic defects, raindrop pattern, diffuse osteopenia (Fig. 8-8)
-
Only 20% to 30% of lesions “hot” on bone scan
-
-
Differentiation from lymphoma
-
Paucity of radiographic changes typical for lymphoma
-
Magnetic resonance imaging (MRI) marrow replacement with subtle plain radiographic findings
-
Predilection for ends of long bones
-
Confluent epiphyseal/metaphyseal/adjacent diaphyseal uptake on bone scan (Fig. 8-9)
-
-
Differentiation from Paget’s disease
-
Paget’s disease shows classic features of expansion with coarsening of trabeculae and cortical thickening in blastic phase (Fig. 8-10), which are atypical for metastases.
-
Paget’s disease lytic phase classic features: blade-of-grass advancing edge sharply delineated from normal bone
-
Predilection: ends of long bones, pelvis, spine
-
Bone scan: increased uptake on bone scan if untreated
-
A limited number of specific laboratory tests are helpful in seeking a
specific diagnosis, while others seek information that may be related
to visceral involvement and potential complications of the malignancy.
-
Role of specific serum tumor markers in primary work-up: unclear
-
Examples: prostate specific antigen (PSA) for prostate cancer, carcinoembryonic antigen (CEA) for colon carcinoma
-
-
Tests to seek potential visceral involvement and/or complications
-
Complete blood count (CBC) and platelets:
marrow replacement by tumor or the adverse effects of tumor treatment
(radiation, chemotherapy) may result in anemia, thrombocytopenia, or
neutropenia -
Metabolic panel: either visceral
involvement or the toxicities of treatment may result in electrolyte
disturbances such as hypercalcemia and/or alterations in renal or
hepatic function -
Erythrocyte sedimentation rate (ESR):
elevations in ESR are frequently seen in lymphoma and myeloma, but only
uncommonly in metastatic carcinoma
-
-
Hypercalcemia is common in patients with bone metastases.
-
It is important to recognize for treatment and as a prognostic factor.
-
Hypercalcemia can be seen in primary or
secondary hyperparathyroidism, which can create multifocal bone lesions
that may be confused with metastatic carcinoma. -
Two types of hypercalcemia of malignancy
-
Local osteolytic hypercalcemia: effect of bone metastases
-
Humoral hypercalcemia: systemically mediated effect without bone metastases
-
Common tumors: squamous cell carcinoma of lung, head and neck carcinoma, kidney cancer, ovarian cancer
-
-
Mediators of hypercalcemia of malignancy
-
PTHrP, IL6, TNF-β, vitamin D
-
-
Interpreting serum calcium levels
-
Ionized calcium is the portion of the total (“uncorrected”) calcium not bound to albumin.
-
Serum calcium reflects calcium both bound and not bound to albumin.
-
If serum albumin is low, an elevated ionized calcium level may be masked by a normal total uncorrected calcium level.
-
-
Symptoms of hypercalcemia: weakness, urinary frequency, thirst, nausea, constipation
-
Treatment of hypercalcemia of malignancy: hydration, bisphosphonates
-
Prognostic importance: hypercalcemia in metastatic cancer is a poor prognosticator, especially for metastatic lung cancer
-
Hypercoagulable states are much more common in malignancy than are coagulopathies.
-
Hypercoagulability may be due to
paraneoplastic erythrocytosis or thrombocytosis from tumor-produced
circulating erythropoietin and thrombopoietin, respectively.
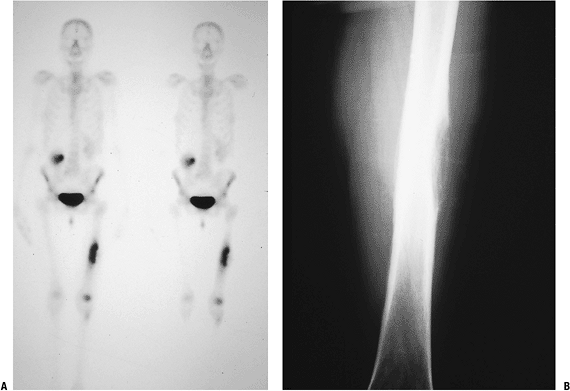
P.236![]() Figure 8-4 (A)
Figure 8-4 (A)
An isolated left distal femoral bone lesion in an elderly woman without
a history of cancer demonstrates increased uptake on bone scan,
permeative borders, Codman’s triangle, and soft tissue extension.
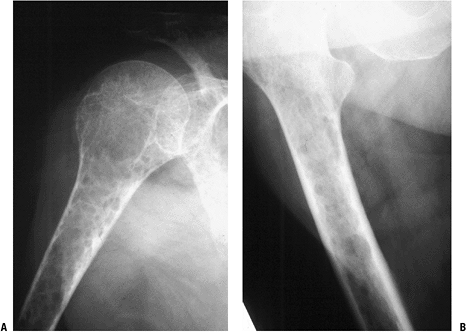
Biopsy showed metastatic adenocarcinoma. (B) Computed tomography scan of the lung revealed a mediastinal mass consistent with a lung carcinoma primary.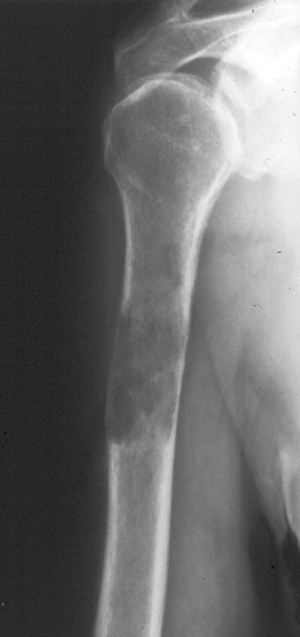 Figure 8-5 A lytic, relatively well-defined metastatic lesion of the right humerus in a patient with renal cell carcinoma.
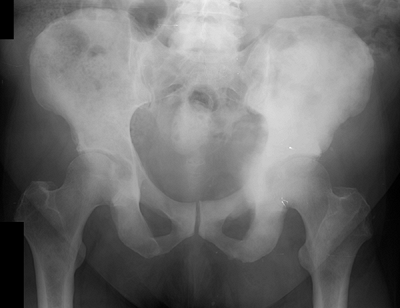
Figure 8-5 A lytic, relatively well-defined metastatic lesion of the right humerus in a patient with renal cell carcinoma.![]() Figure 8-6 A pelvic radiograph shows typical blastic metastases from prostate cancer.P.237
Figure 8-6 A pelvic radiograph shows typical blastic metastases from prostate cancer.P.237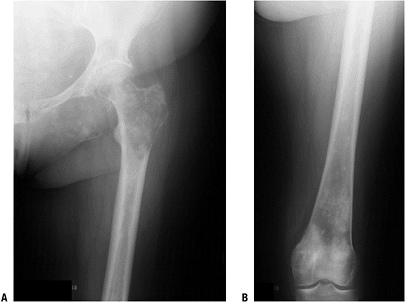 Figure 8-7
Figure 8-7
Multiple bone lesions in a woman with breast cancer show the wide
variation in appearance of breast cancer metastases to bone, from the
predominately lytic appearance in the ischium, the mixed lytic and
blastic proximal femur metastases, and the sclerotic distal femur
metastases.![]() Figure 8-8
Figure 8-8
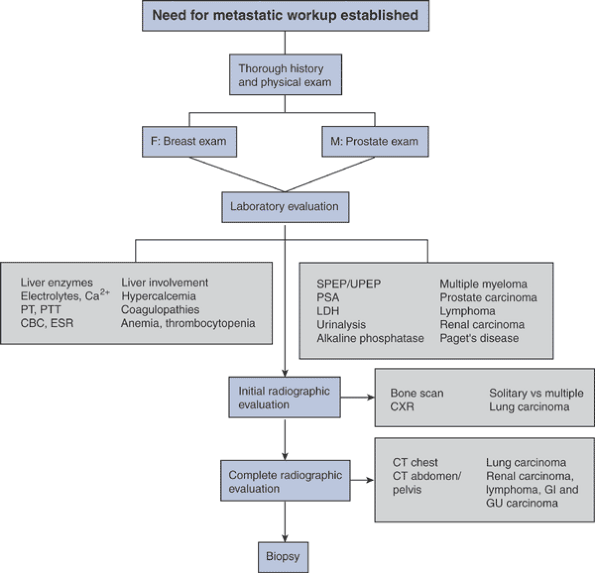
Radiographs from humerus and femur of a patient with multiple myeloma
show classic raindrop pattern of punched-out lytic defects throughout
the bones.P.238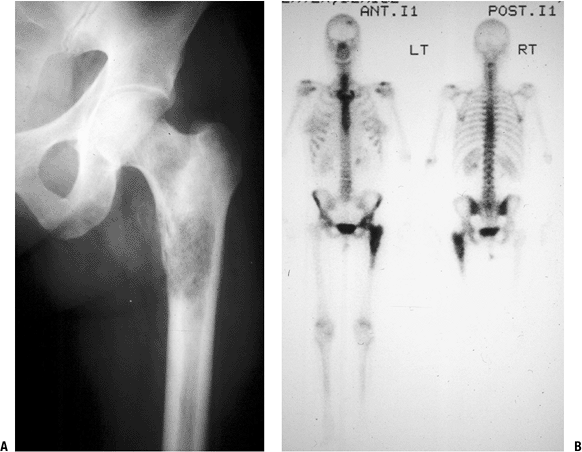 Figure 8-9
Figure 8-9
An isolated left proximal femoral bone lesion shows a permeative lytic
process involving the proximal femur with confluent uptake on bone scan
involving the femoral epiphysis, metaphysis, and proximal diaphysis.
Biopsy showed lymphoma.![]() Figure 8-10
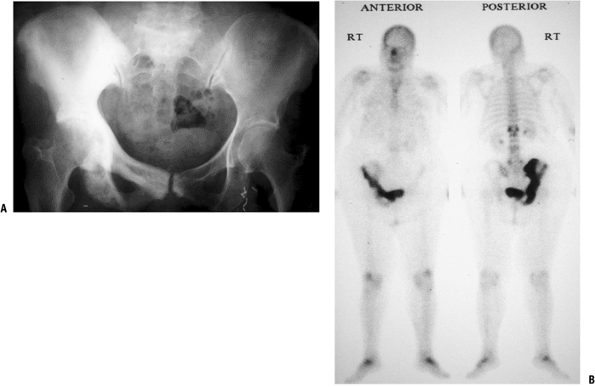
Figure 8-10
Paget’s disease of bone in its sclerotic phase can be confused with
prostate cancer. This patient with Paget’s disease of the entire right
hemipelvis shows the typical coarsening of trabeculae and bony
expansion (both best seen in the pubic rami) as well as confluent
uptake on the bone scan.P.239Table 8-5 Laboratory Tests Important in The Evaluation of Metastatic DiseaseLaboratory Test Positive Finding Disease Association Serum protein electrophoresis Monoclonal spike (monoclonal gammopathy) Multiple myeloma Urine protein electrophoresis Bence Jones proteins Multiple myeloma Lactate dehydrogenase Elevated Lymphoma (nonspecific; depends on disease load; also with osteogenic sarcoma, Ewing sarcoma) Prostate specific antigen Elevated Prostate carcinoma Urinalysis Hematuria Renal cell carcinoma Alkaline phosphatase Elevated Paget’s disease of bone (nonspecific; also with osteogenic sarcoma) -
-
Common tumors: adenocarcinomas, especially lung, pancreas, and stomach
-
Diagnosis: no helpful screening laboratory tests available
metastatic in origin should follow the same principles as those for a
sarcoma. If a biopsy is to be done prior to the stabilization of an
impending or actual pathologic fracture, care should be taken to avoid
contaminating tissue or bone regions not already involved by the tumor.
Do not send reamings from the femur or
humerus as a biopsy specimen to establish the diagnosis; in addition to
being histologically inferior to a standard biopsy, if the patient has
a sarcoma, the lesion will then have been unnecessarily spread
throughout the reamed region of bone and possibly disseminated into the
caval venous system as well. The best solution in the case of an
impending or pathologic fracture is to establish the diagnosis first by
way of a direct approach to the tumor for an incisional biopsy,
contaminating the fewest tissue planes and compartments possible. In
the proximal femur, this is done through a lateral approach at or near
the ridge between the gluteus maximus insertion and the vastus
lateralis origin. In the proximal humerus, a proximal biopsy should be
performed through the anterior aspect of the deltoid but not exposing
the cephalic vein.
|
Table 8-6 Metastatic Tumors with Histopathology of Specific Origin
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
reveal the specific site of origin. There are a limited number of
exceptions to that rule (Table 8-6 and Fig. 8-11).
More importantly, when an appropriate metastatic work-up has been done
in advance, the pathology only needs to confirm the presence of
metastatic carcinoma consistent with the identified probable source to
allow surgical treatment to
proceed.
Since many of these cases rely upon frozen section for diagnosis, the
pathologist does not have the luxury of waiting for
immunohistochemistry staining results, which can be very helpful (Table 8-7).
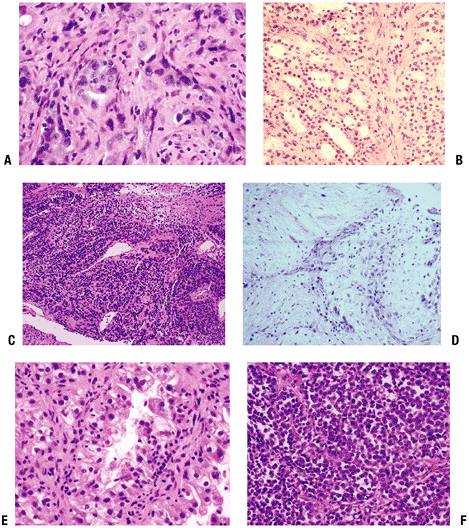 |
|
Figure 8-11 Representative pathology sections from typical metastatic lesions. Metastatic adenocarcinoma of lung (A), follicular thyroid carcinoma (B), malignant melanoma (low power) (C), squamous cell carcinoma (D), clear cell renal carcinoma (E), and melanoma (high power) (F).
|
|
Table 8-7 Immunohistochemistry for Metastatic Carcinomas and Tumors in Their Differential
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
-
Histopathology that identifies the specific source of origin (see Table 8-6)
-
Histopathology suggestive of source of origin
-
Adenocarcinoma of breast
-
Adenocarcinoma of colon
-
-
Histopathology of nonspecific origin (Table 8-8)Table 8-8 Tumors With Histopathology of Nonspecific Origin
Tumor Potential Sources Metastatic squamous cell carcinoma Lung, head and neck, esophagus, cervix Metastatic adenocarcinoma without differentiating features Breast, colon, prostate, ovary, endometrium, stomach, lung Metastatic clear cell adenocarcinoma Ovary, endometrium, lung Poorly differentiated carcinomas Lung, prostate, pancreas, thyroid, breast, colon, ovary, endometrium, stomach, etc. -
Differential pathology diagnoses
-
Anaplastic large cell lymphoma
-
Poorly differentiated angiosarcoma
-
Primary and metastatic sarcoma
-
patients with metastases are for diagnosis, prophylactic fixation of
impending fractures, and stabilization of pathologic fractures. Many
patients with bone involvement by metastatic disease or myeloma can be
treated nonoperatively. Both surgical and nonsurgical options are
discussed in more detail in the chapter on pathologic fractures (Chapter 4).
-
Solitary new destructive lesion with or without history of cancer
-
Biopsy first before proceeding with treatment!
-
Other processes seen in adults that
should not be treated the same as a metastatic lesion and therefore
must be considered in the differential diagnosis-
Bone sarcomas: chondrosarcomas, malignant fibrous histiocytoma, fibrosarcoma, postradiation sarcoma, Paget’s sarcoma
-
Most common mistake: placement of an intramedullary nail through a bone sarcoma without biopsy
-
Most common result: unnecessary amputation
-
-
Other processes seen in adults that may require medical intervention prior to surgical procedure
-
Hyperparathyroidism
-
Paget’s disease
-
-
-
Multiple bone lesions without biopsy-proven metastatic bone disease (even with history of cancer)
-
Biopsy first before proceeding with treatment!
-
Multiple other scenarios mimic metastatic carcinoma! (Box 8-3)
-
-
Situation that does not require biopsy of new bone lesion
-
Established histologically proven mets to bone and/or viscera
-
-
Paget’s disease
-
Hyperparathyroidism
-
Vascular bone sarcomas: hemangiopericytoma, hemangioendothelioma, angiosarcoma
-
Multicentric chondrosarcomas
-
Multiple hereditary exostoses
-
Ollier’s disease
-
Polyostotic fibrous dysplasia
-
Angiomatosis (lymphangiomatosis)
-
The site and type of pathologic fracture
determine to a large extent the need for surgical stabilization, and
those specific details are discussed in Chapter 4.5, Pathologic Fractures.-
Sites that frequently require stabilization
-
Upper extremity: humerus
-
Lower extremity: femur, tibia
-
-
-
Patient factors should be considered as well.
-
Moribund, immediately preterminal patients are a contraindication to surgery.
-
Minimum expected survival: 6 to 12 weeks
-
-
These factors are discussed in detail in Chapter 4.5.
modification of the technique for anesthesia and/or difficulty with
postoperative care. These lesions and the potential problems they may
create should be investigated. Review of the most recent bone scan is
useful in this regard.
-
Cervical spine disease: an anesthesia concern
-
History and examination: Does patient have neck pain or tenderness?
-
Radiologic investigation: Review most recent bone scan, consider new plain cervical spine radiographs.
-
-
Impending upper extremity pathologic
fractures: may cause postoperative pain and/or fracture if recovery
period requires ambulatory aids for weight bearing.-
History and examination: Does patient have shoulder or arm pain and/or tenderness?
-
Radiologic investigation: Review most recent bone scan; consider new shoulder or humeral radiographs.
-
-
Anemia management guidelines
-
Consider preoperative transfusion if hemoglobin is <10.0 mg/dL or large amount of blood loss anticipated.
-
Use leukodepleted, irradiated red blood cells if patient has been on chemotherapy.
-
-
Thrombocytopenia management guidelines
-
Preoperative platelet transfusion if platelet count is <50,000/mm3
-
-
Leukopenia management guidelines
-
Consider canceling major operation if absolute neutrophil count (ANC) is <1,000/mm3.
-
Avoid major operations at time of typical nadir in white blood cell count following chemotherapy.
-
-
Highest risk of bleeding during intralesional procedures: renal carcinoma metastases
-
Increased risk of bleeding (others): myeloma and thyroid carcinoma metastases
-
Consider embolization.
-
Especially for renal carcinoma intralesional procedures
-
Also myeloma and thyroid carcinoma of spine, pelvis
-
limited ability to heal fractures, shortened life spans, and the
potential to develop other sites of bone involvement, which may
compromise prior surgery. Therefore, these patients cannot be treated
the same as those with routine fractures. The specific approaches to
achieving these goals are discussed in Chapter 4.5.
-
Achieve immediately rigid fixation to allow early use.
-
Achieve durable fixation to last the patient’s expected shortened lifetime.
-
Protect the entire bone when feasible.
-
Intramedullary fixation
-
Diaphyseal fractures and impending fractures
-
Metaphyseal fractures with adequate epiphyseal bone
-
Consider bone cement supplementation.
-
-
Plate fixation
-
Metaphyseal and diaphyseal fractures
-
Supplement with bone cement in most cases.
-
-
Indications
-
Failure of or inability to achieve goals
by internal fixation (e.g., extensive peritrochanteric destruction not
necessarily involving femoral head) -
Joint surface destruction (e.g., femoral head, femoral condyles, humeral head)
-
Need for resection and reconstruction, such as sometimes indicated for the solitary renal carcinoma metastasis
-
metastatic disease and myeloma entails all of the usual risks. Only
those with specifically increased or unique risks are detailed below.
-
Infection
-
Administer prophylactic cephalosporin antibiotics in all cases.
-
Consider adding aminoglycoside for patients with neutropenia or large prosthetic reconstructions.
-
-
Failure of internal fixation
-
Avoid by achieving goals of surgical intervention in operating room.
-
Administer postoperative radiotherapy to avoid disease progression that may compromise result.
-
-
Cardiovascular collapse from cementing
-
Especially with long-stem cemented femoral stems
-
Communicate with anesthesia prior to cementing.
-
patient with metastatic disease should not be longer than the patient’s
expected survival. However, prediction of survival is difficult at best.
-
Nonspinal sites postoperative survival
(patients undergoing surgical intervention for metastatic bone disease
of any site other than spine)-
Postoperative survival rates over time
-
1 year: 40%
-
2 years: 30%
-
3 years 20%
-
-
1-year survival rates (Table 8-9)
-
Negative prognostic factors (multivariate analysis)
-
Pathologic fracture
-
Visceral metastases
-
Hemoglobin <7 mmol/L
-
Lung cancer
-
-
Positive prognostic factors (multivariate analysis)
-
Myeloma
-
-
-
Proximal femur (patients undergoing hip arthroplasty for femoral and/or acetabular metastatic bone disease)
|
Table 8-9 Postoperative 1-Year Survival Rates
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||
-
Metastatic breast cancer
-
Poor prognostic factors
-
Caudal metastases: 5-year survival rate
is 16% for those with bone mets below lumbosacral junction versus 36%
for those above lumbosacral junction -
Extraosseous metastases: median survival 5 months after identified
-
Lytic metastases: 5-year survival 23% versus 42% with blastic metastases
-
Hypercalcemia: harbinger of subsequent visceral metastases
-
-
Good prognostic factors
-
Cranial metastases (above lumbosacral junction)
-
No extraosseous metastases
-
Blastic metastases
-
No hypercalcemia
-
-
-
Metastatic prostate cancer
-
Number of metastatic sites inversely proportional to expected survival duration
-
Best prognoses: (1) pelvis only, (2) thoracic vertebra only
-
Worst prognoses: (1) skull combined with other sites, (2) sternum combined with other sites
-
-
Antibiotics
-
Routine cases: 24 to 48 hours intravenous cephalosporin coverage or until drain discontinued
-
-
Mobilization
-
Strive for goal of early mobilization with full weight bearing when feasible.
-
-
External-beam radiotherapy
-
Radiotherapy as an adjunct to operative treatment
-
There are documented benefits in metastatic disease patients treated surgically for bone disease.
-
Reduces need for subsequent surgical procedures (3% versus 15%)
-
Improves postoperative functional status
-
-
Postoperative timing
-
After wound healing completed
-
Typically 2 to 4 weeks postoperatively
-
-
Doses
-
3,000 cGy in 10 fractions or 2,000 cGy in 5 fractions
-
4,500 cGy in 180-cGy fractions for patients with renal and thyroid carcinoma and life expectancy of 12 months or more
-
-
-
PW, Rosenthal HG, Smalley SR, et al. Impact of postoperative radiation
therapy and other perioperative factors on outcome after orthopedic
stabilization of impending or pathologic fractures due to metastatic
disease. J Clin Oncol 1994;12:2345–2350.