MANAGEMENT OF ACUTE AND CHRONIC OSTEOMYELITIS
VII – NEOPLASTIC, INFECTIOUS, NEUROLOGIC AND OTHER SKELETAL DISORDERS
> Infection and Hemophilia > CHAPTER 133 – MANAGEMENT OF ACUTE
AND CHRONIC OSTEOMYELITIS
of the bone, can result from hematogenous seeding, from direct
inoculation (i.e., following open fractures or open reduction and
internal fixation of fractures), or from the contiguous spread of
bacteria from infected structures. Early diagnosis and effective
surgical and antibiotic management can control the infection;
suppression of infection may last a lifetime.
outcome has been enhanced by the addition of microvascular and local
muscle transfers, bone grafting techniques, bone transport for large
bone defects, newer antibiotics, and local antibiotic delivery systems.
The treatment of osteomyelitis requires the work of a team,
consisting
of the orthopaedic surgeon, an infectious disease physician, and, in
complex cases involving soft-tissue defects or inadequate soft-tissue
coverage, a plastic surgeon. In many institutions, some orthopaedic
nurses are specially trained in the care of patients with orthopaedic
infections.
the portal of entry and the type, age, and associated medical
conditions of the host. Unfortunately, it is usually not possible to
accurately predict the specific pathogen on the basis of clinical
presentation alone. In the past, the most common causes of
osteomyelitis were Staphylococcus aureus and various streptococcal species. Gustilo et al. (22) and Patzakis et al. (48) reported that S. aureus
and coagulase-negative staphylococci were the most common organisms
causing infections in open fractures. However, the microbiology of
musculoskeletal sepsis has been changing in recent years. While S. aureus
remains the predominant cause of osteomyelitis, there has been an
increase in the frequency of infections caused by gram-negative
organisms (52,53).
determination of the microbiology. Hematogenous osteomyelitis is most
commonly caused by S. aureus. In the past, Haemophilus influenzae type B (HiB) was a common organism in children, but the advent of HiB vaccine has made it a rare cause (3).
Causes of osteomyelitis due to direct inoculation, such as into an open
fracture, will be influenced by the environment in which the injury
occurred. For example, injuries occurring in water may lead to
infection with Aeromonas or Plesiomonas. Nail puncture wounds of the foot, particularly through a tennis shoe, are associated with infection with Pseudomonas aeruginosa (28,50).
osteomyelitis. Osteomyelitis caused by fungal infections is more likely
to be seen in immunocompromised hosts. Diabetic foot infections leading
to osteomyelitis often are polymicrobial, often involving anaerobes and
gram-positive and gram-negative aerobic bacilli. Patients infected with
the human immunodeficiency virus (HIV) are at risk for a variety of
unusual infections, including atypical mycobacteria.
growing frequency of antimicrobial resistance in bacteria.
Methicillin-resistant S. aureus has been
known for decades, but its incidence is growing, even among
nonhospitalized patients. Multidrug-resistant gram-negative bacteria
are increasingly becoming a problem, limiting treatment options.
Vancomycin-resistant Enterococcus faecium
has become a major problem in nosocomial infections in the United
States, but thus far has been rarely seen in osteomyelitis. The
greatest concern is the development of vancomycin-resistant S. aureus,
which has now been identified in patients in Japan, the United States,
and Europe. Although this has not yet been reported as a cause of
osteomyelitis, bone infections with this organism could be
catastrophic, as no effective antibiotics for this infection are yet
available.
management, which includes adequate debridement and wound management.
There has been a recent surge in the number of antibiotics available
for the treatment of osteomyelitis. The beta-lactam antibiotics, such
as penicillin and cephalosporin, are effective and relatively safe
antimicrobials that can cover a wide range of gram-positive and
gram-negative infections. The quinolones, however, have changed the
treatment of osteomyelitis by providing oral drugs that achieve
excellent serum levels and have good bone penetration.
antimicrobials concern the choice of agent, the optimal route
(intravenous, oral, or local), and the length of administration. The
Gram stain, culture, and antimicrobial sensitivity tests are important
guides in determining the specific antimicrobials to be used.
Sensitivity testing not only helps to identify resistant organisms but
also to guide the selection of the most active antimicrobial for the
specific infection.
remains the most common cause of osteomyelitis. A semisynthetic
penicillin (such as nafcillin) is the drug of choice for most
staphylococcal infections. Alternative choices could include a
first-generation cephalosporin [such as cefazolin (Ancef)],
clindamycin, or a quinolone. Vancomycin is less active against S. aureus
than the penicillins and should not be used except in the case of
patient allergy to the beta-lactams or for the treatment of
methicillin-resistant S. aureus. Rifampin may be used in combination with one of the other agents for synergy.
with a penicillin plus beta-lactamase inhibitor combination [such as
piperacillin-tazobactam (Zosyn)], a cephalosporin [such as cefotaxime
(Claforan) or ceftriaxone (Rocephin)], an aminoglycoside, or a
quinolone. For infections with P. aeruginosa,
treatment with an antipseudomonal beta-lactam (such as piperacillin,
ceftazidime, or cefipime) together with an aminoglycoside (such as
tobramycin,
which is the most effective against Pseudomonas)
still represents the gold standard of therapy. Although successful
treatment with a single agent, such as ceftazidime, has been reported (1,16),
treatment failures with single-agent therapy have been noted, including
some cases associated with the emergence of resistance.
antimicrobials available for the treatment of osteomyelitis. Currently
available quinolones that may be useful in the treatment of
osteomyelitis include ciprofloxacin, ofloxacin, and levofloxacin. They
have a broad spectrum of activity including S. aureus
and streptococci (with the newer quinolones such as levofloxacin having
much better activity against the gram-positive organisms than
ciprofloxacin), the aerobic gram-negative enteric bacilli, and P. aeruginosa.
is the fact that oral administration results in levels similar to those
obtained with intravenous administration, suggesting that oral therapy
can replace intravenous therapy, at least in some instances (17,58).
Since the incidence of adverse reactions to these drugs is low, they
may be used in place of more toxic antimicrobials such as the
aminoglycosides.
has been influenced by the rise in drug-resistant organisms. The
current treatment recommendations are starting with three or four drugs
(isoniazide, rifampin, pyrazinamide, and ethambutol), depending on the
level of isoniazide resistance in the community. If cultures show that
the organism is sensitive to isoniazide and rifampin, therapy can
continue with isoniazide and rifampin for a minimum of 6 months,
although longer regimens (9–12 months) are probably required (55).
amphotericin B, which was the only agent effective against deep fungal
infections. Although it is often effective, there are significant
adverse effects associated with the administration of amphotericin B,
including fever, chills, and nausea during administration, and renal
insufficiency or failure with potassium and magnesium wasting. Newer
agents such as fluconazole and itraconazole have excellent activity
against many of the fungal organisms and far fewer side effects.
available, the problem of increasing resistance among organisms, and
the potential for significant adverse reactions with many of the drugs,
it is imperative that an infectious disease consultant help with the
selection of specific antibiotic therapy and the monitoring of the
patient for adverse reactions. Table 133.1 lists commonly used antimicrobials (also see Chapter 132).
 |
|
Table 133.1. Commonly Administered Antimicrobials
|
Hippocrates and later Galen used medicinal ointments that included
honey, a bactericidal agent, in the treatment of open fractures. In the
early 1900s, Lord Lister, with good success, used phenol-soaked
compresses to combat the microorganisms believed to be responsible for
infection (35). He later introduced the use of
carbolic acid as an antiseptic. Following the discovery of the
sulfonamides, sulfa powder in wounds was popular among war surgeons.
The major
changes of today are the choice of the agent used and the development of new vehicles for delivery.
solution for irrigation of infected wounds at the time of surgery.
Although any antimicrobial could be used, the most commonly used agents
are polymyxin (1 million units/L of saline) and bacitracin (50,000
units/L of saline).
systems have been used for ingress and egress, usually administered for
a period of 3 to 21 days. The main deterrent to this mode of
administration has been the emergence of new organisms, usually
hydrophilic gram-negative organisms (10,29,47).
The overall success for antibiotic irrigation has been shown to be the
same as for antibiotic-impregnated polymethylmethacrylate (PMMA) beads (62).
Therefore, tube-suction irrigation has been abandoned by many surgeons.
If this system is used, adhere to every means possible to prevent
contamination. The risk of secondary contamination increases with the
length of usage of the irrigation system. It is advisable to use
Silastic drains and to place the tubes on suction or egress for 24
hours before removing the system. This procedure will remove all of the
fluid and hematoma present and will collapse the dead space, thereby
precluding the possibility of abscess formation. I do not recommend the
use of suction-irrigation systems.
delivery of high concentrations of antibiotics. Among the advantages of
antibiotic beads are low systemic levels of antibiotics, resulting in a
lower potential of systemic toxicity, decreased need for systemic
intravenous therapy, and decreased length of hospital stay. The
disadvantages are that it requires a closed wound for the development
of high local levels of antibiotics. Moreover, it may act as a foreign
body, especially in the presence of resistance to the antibiotic in the
vehicle, such as gentamicin or tobramycin.
Klemm and other investigators have reported good results with the use
of gentamicin-impregnated methylmethacrylate beads (Septopal) for the
treatment of chronic osteomyelitis (30,31).
Unfortunately, the United States Food and Drug Administration (FDA)
approval of factory-made gentamicin beads (Septopal) has been
indefinitely delayed, leading many surgeons to make their own beads
using bead molds (13).
majority of studies have evaluated gentamicin, but as it is no longer
commercially available in powder form for injection in the United
States, physicians now use tobramycin and vancomycin since they are
active against the most common organisms leading to prosthetic joint
infections and osteomyelitis. Antibiotic elution from
antibiotic-impregnated PMMA is proportional to the surface area of the
cement (68,69)
and affected by the type and concentration of the antibiotic used, the
brand of PMMA cement, and the amount and turnover of the surrounding
fluid (66). Studies have shown that antibiotics
elute better from beads than from spacers, and better from palacos
cement than from simplex cement, and tobramycin achieves better levels
than vancomycin (20,33,36).
antibiotic-impregnated PMMA beads are encouraging, so the use of these
in selected cases seems appropriate (Fig. 133.1).
A different vehicle for the delivery of local antibiotic therapy may
improve results and lessen the disadvantages associated with this
therapy. Various biodegradable antibiotic delivery systems are now
being evaluated but are still in the investigational stage.
 |
|
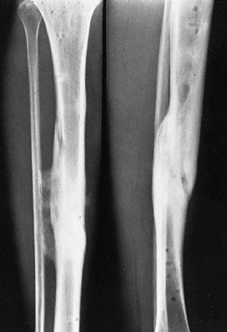
Figure 133.1.
Anteroposterior roentgenograms of a 46-year-old man with chronic osteomyelitis. The patient was treated with debridement followed by insertion of aminoglycoside-impregnated methylmethacrylate beads and a local muscle flap. |
The four anatomic types are type I, medullary osteomyelitis; type II,
superficial osteomyelitis; type III, localized osteomyelitis; and type
IV, diffuse osteomyelitis.
the host. An “A” host has good systemic defenses with good local
vascularity and a normal physiologic response to infection and surgery.
A “B” host is a compromised host with either local, systemic, or
combined deficiency in wound healing and infection response. A “C” host
is a patient who is not a surgical candidate, requires suppressive or
no treatment, who has minimal disability, or for whom the treatment or
results of treatment are more compromising than the disability caused
by the disease itself.
an emergency. The presenting signs and symptoms may vary with the
severity of the infection. Symptoms include fever and chills, general
malaise, irritability, pain, and swelling. With lower extremity
involvement, there is either a limp or an inability to bear weight. An
infant with upper extremity involvement may exhibit pseudoparalysis;
older children and adults with upper extremity involvement complain of
pain on movement or use of the extremity.
tenderness, which is usually warm and swollen. In children it is
generally in the metaphyseal region. It is also important to evaluate
the adjoining joint for evidence of septic arthritis, which can occur
as an extension of the adjoining osteomyelitis. Osteomyelitis involving
the neck of the femur, talus, and humeral head often leads to sepsis of
the joint because these foci are located within the joint capsule.
aspirate the area, and send the pus or fluid obtained for Gram stain,
culture, and sensitivity studies. If tuberculosis or a fungal infection
is suspected, obtain an acid-fast stain and tuberculosis and fungal
cultures. When a joint effusion is present or joint involvement is
suspected, aspirate the joint and confirm with an arthrogram. The
arthrogram helps document the location of the aspiration and is useful
for positive as well as for negative aspirations, since it may also
identify joint capsule rupture. Aspiration of the hip joint under
ultrasound guidance is very helpful in children and adults.
depending on the severity of the infection, with an increase in
immature or band cells. The erythrocyte sedimentation rate and the
C-reactive protein are usually elevated. Obtain blood cultures in all
cases of acute hematogenous osteomyelitis and in chronic osteomyelitis
exacerbated by fever and bacteremia.
generally show soft-tissue swelling. Bony changes are not present until
7–10 days after the onset of infection. Radionuclide bone scanning
using radioactive-labeled isotopes, such as technetium-99m and, more
specifically, gallium citrate-67 and indium-111-labeled white blood
cells, is helpful in localizing the area of involvement and in helping
diagnose the condition.
and indium-labeled leukocyte scintigraphy are the studies most commonly
used. Indium 111-labeled leukocytes have been reported by a number of
investigators as being more useful than other imaging in osteomyelitis
complicated by fractures and nonunions (11,38,40,63) (see Chapter 132).
Aspiration is used for the diagnosis, especially when there is no
drainage or sinuses, and in some cases bone biopsy may be necessary.
dimension to the diagnosis, localization, and characterization of the
extent of infection. MRI has been reported by Modic et al. to have a
94% accuracy in diagnosing spine infections (40).
T1- and T2-weighted images are the initial screening techniques for
diagnosing osteomyelitis. The MR finding in osteomyelitis on
T1-weighted image sequences is a low signal intensity due to a dark
marrow signal and an increased signal intensity due to a bright marrow
signal on the T2-weighted image sequences (Fig. 133.2, Fig. 133.3).
MRI has decreased the need for bone scanning and provides more useful
information. Do sinograms whenever there is a sinus tract or open
draining area from which the depth and extent of the infection can be
determined (Fig. 133.4).
Do abscessograms whenever an abscess is present and frank pus is
aspirated. The abscessogram will help outline the extent of the abscess
cavity (Fig. 133.5). MRI, computed tomographic scanning, and radiographic tomogram are all useful tools in evaluating osteomyelitis.
 |
|
Figure 133.2.
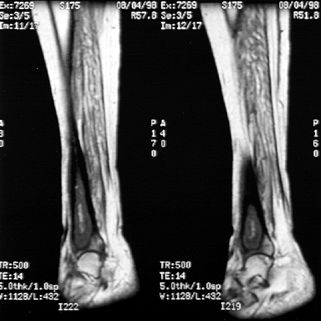
These are T1-weighted images of a 44-year-old woman with systemic lupus erythematosis and hematogenous osteomyelitis. There is decreased signal within the distal third of the tibia and a serpiginous area of low intensity consistent with osteonecrosis. A biopsy had been performed previously. At surgery, she had an abscess with sequestra. |
 |
|
Figure 133.3.
These T2-weighted images of a 15-year-old boy show increased signal from the ankle to the mid tibia. There is increased signal in the adjacent soft tissues about the ankle and tibia. There is a minimal ankle effusion present. At surgery, the patient had an intramedullary abscess of the metaphysis and distal tibia. |
 |
|
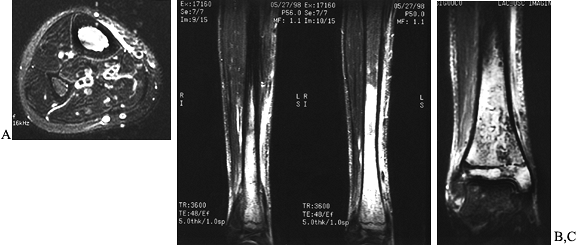
Figure 133.4. Anterior (A) and posterior (B) roentgenograms of a sinogram in a 21-year-old man with chronic osteomyelitis that tracks into the intramedullary cavity.
|
 |
|
Figure 133.5. Lateral radiograph (A) of a 15-year-old boy shows a subperiosteal abscess that was aspirated and injected with contrast material. Anteroposterior (B) and lateral (C)
radiographs of a 9-year-old child show contrast material in the subperiosteal area and within the medullary cavity after pus had been aspirated. The contrast material demonstrates the extent of the abscess. |
stabilization of the fracture, soft-tissue coverage, and bone graft of
ununited fractures and large bone defects.
culture and sensitivities, and antibiotic therapy. In chronic
osteomyelitis, obtain aerobic, anaerobic, and fungal cultures. Recent
studies have advocated taking of multiple deep cultures from purulent
material, soft tissue, and bone (51,56).
Marrie and Costerton postulated that different organisms may be growing
in isolated microenvironments. Sampling differences and bacterial
viability may influence the culture results (37).
essential. Soft-tissue coverage may require the use of local muscle
flaps and free vascularized muscle flaps for soft-tissue defects or an
inadequate soft-tissue envelope after control of the osteomyelitis.
Local muscle flaps and free vascularized muscle transfers also help by
bringing in a new blood supply, which is important in host defense
mechanisms, antibiotic delivery, and osseous and soft-tissue healing (46).
For the tibia, use the gastrocnemius muscle for proximal-third defects,
the soleus for middle-third, and for the distal-third, free
vascularized muscle transfers.
the muscle preoperatively and not to transfer damaged muscle. Also
avoid using crushed or badly damaged muscle, as flap necrosis or flap
complications may result. In these cases, use free vascularized tissue
transfer. Do preoperative angiograms on patients whose vascular status
has been altered from any cause.
with sickle cell disease or significant peripheral vascular disease.
The tourniquet improves hemostasis and thus facilitates identification
of the infection process. In acute cases with swelling, cellulitis, or
abscess formation, elevate the extremity for several minutes before
inflating the tourniquet. In chronic osteomyelitis without significant
cellulitis or abscess formation, use an elastic bandage to extravasate
the extremity before inflating the tourniquet.
desiccated bone is essential. Do not remove viable infected bone, so as
not to create large bony defects. It is not necessary to debride viable
infected bone. Dyes and tetracycline labeling have been used as a means
of identifying necrotic bone, but I have not found these techniques to
be useful.
than normal and should be debrided. Necrotic bone that has not been
exposed may appear at surgery more yellowish than viable bone, which is
whitish. The main finding is that viable bone bleeds, whereas necrotic
bone does not.
cortex of the questionable bone results in small areas of punctate
bleeding. Some bone that may have been exposed to air may be viable; in
these cases, the exposed outer cortex should be debrided with an
osteotome down to good bleeding bone. Evacuate all pus and abscess, and
remove all necrotic and infected soft tissue.
the area of purulent exudate, loose soft tissue, and bony fragments,
and decreases the bacterial count. I use 10 L of irrigating fluid for
most infected wounds. I use 2 L of antibiotic solution containing
50,000 units of bacitracin and 1 million units of polymyxin per liter
as the final irrigating solution. Other antibiotics can be used for
topical irrigation as well.
requires careful judgment. In the majority of acute infections, and in
all cases in which there is associated abscess formation with
cellulitis and swelling, the wound should be left open. In some cases
of early postoperative infection, the wound may be closed over drainage
tubes, as long as the wound is thoroughly clean and the infection is
not anaerobic.
significant cellulitis or abscess formation and in which the wound has
been adequately debrided and converted to a clean wound, the wound may
be closed over drainage tubes. In some cases in which bone or metal
will be exposed if the wound is left open, a partial closure over the
bone or metal may be desirable, as long as an adequate pathway has been
provided for drainage. When there is any doubt, it is safest to leave
the wound open. If the wound is closed, the wound site must be examined
daily for any signs of infection; if such signs appear, the wound must
be opened.
case of large wounds or when delayed closure is preferable, do not
attempt closure until two criteria are met. First, the wound should
appear clinically healthy, with clean granulating tissue and without
any purulent exudate or necrotic tissue. If infected necrotic tissues
are present, redebride the wound until it appears healthy. Second, once
the
clinical appearance of the wound is clean, take quantitative tissue
cultures and do Gram stains. Wounds with either a positive Gram stain
or quantitative tissue cultures with a bacterial count greater than 10-5 organisms should never be closed. (A positive Gram stain implies a bacterial count of greater than 10-5 organisms.)
reassessed for further surgical debridement and the appropriateness of
the systemic antibiotic therapy. With experienced surgical teams,
tissue cultures are not routinely performed. It has been our practice
to do a thorough initial debridement followed by an en bloc excision of the wound at closure or muscle transfer.
resection of the granulating tissue for several reasons. First,
although the bacterial count is low, these tissues should still be
considered contaminated; debridement will further reduce the bacterial
count and thereby diminish the chance of infection. Second, debridement
allows for cleaner, healthier tissue to be approximated by wound
closure or covered by muscle transfer.
polyethylene (Hemovac) drains may be used. I prefer the Jackson-Pratt
drains. Penrose drains, made of rubber, are the most reactive, and if
left in for long periods can cause foreign-body granulomas. Do not use
Penrose drains in orthopaedic infection management.
allows the removal of all hematoma and tissue fluid, and the collapse
of the potential dead space. The drains should be removed under sterile
conditions and the tip cut off and sent for culture and sensitivity
tests.
because it is a foreign body and because tissue fluids are removed
through it. In general, a positive culture of the drain tip is a bad
prognostic sign: It means that bacteria remain behind. Monitor the
clinical course and wound site closely; if any clinical signs of wound
infection reappear, it may be necessary to consider redebridement and
reassessment of antibiotic therapy.
drainage. Make certain, therefore, when packing wounds with gauze or
other materials, that packing does not obstruct drainage. If it does,
the purulent exudate will be retained in the wound, possibly causing
tissue breakdown and necrosis with secondary cellulitis or even abscess
formation. It is best to put wicks perpendicular to the open wound to
allow free drainage. Wicks can be either povidone-iodine
(Betadine)-soaked gauze, plain gauze, or fine-mesh gauze. The size
varies with the size of the wound. The ends of the wicks should always
protrude through the skin edges to allow easy access and removal and to
prevent retention.
This concept has been expanded, and I now use a physician-made
antibiotic bead pouch during the interval between the initial
debridement and the time of muscle transfer, a median of 4 days.
Microbe-specific antibiotic(s) can be added to either Palacos or
Simplex PMMA. I prefer Palacos: The antibiotic elution has been
reported by some investigators to be better them Simplex. Although most
antibiotics may be added to PMMA depending on the microbial sensitivity
results, tobramycin and other aminoglycosides are the most commonly
used antibiotics.
sufficient to make enough bead chains to fill large defects. I use a
mold to make 6- or 7-mm beads strung on 24- or 26-mm wire. For those
surgeons who make beads without a mold, the bead size should be small
because increased surface area allows for better antibiotic elution.
The beads are then covered by Tegaderm, Opsite, or an equivalent
material. The advantages of the antibiotic bead pouch used in this
fashion are high local antibiotic levels with low systemic toxicity and
less chance for secondary contamination because the wound is covered.
Also important is patient comfort, as dressing changes are not
required, and there are decreased requirements for wound care.
infants, children, drug abusers, and immunosuppressed hosts. In 1894,
Lexer injected laboratory animals with S. aureus organisms and then traumatized a bony area, causing infection to appear at that site (34).
Hobo explained that the predilection for the metaphysis in acute
osteomyelitis was due to the fact that the arteries in this location
are end arteries, that there is slowing of the venous flow in the
sinusoids, and that phagocytosis is defective in this area (27).
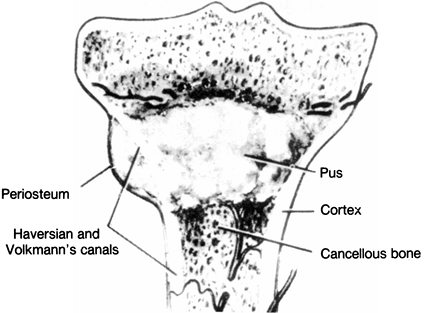
through the Volkmann canals into the subperiosteal region, extend
itself within the medullary cavity, or spread into the epiphysis (Fig. 133.6).
It is not uncommon for the joint to be involved secondarily, especially
a joint in which the metaphysis is intra-articular, such as the hip or
shoulder. Often the abscess extends into the soft tissues as well (42,67).
 |
|
Figure 133.6.
Outline of the pathophysiology of hematogenous seeding. When under pressure, the exudate or abscess can extend through the Volkmann canals into the subperiosteal region, and from there into the intramedullary cavity or the epiphysis. (Modified from Hobo T. Zur Pathogenese der Akuten Haematogene Osteomyelitis, mit Berucksichtigung der Vitalfarbungslehre. Acta Sch Med Univ Imper Kioto 1921;4:1.) |
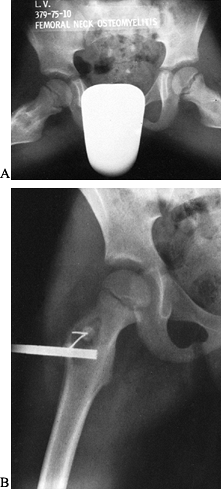
or in which either inadequate or no treatment is given, the disease
enters the subacute stage (Fig. 133.7). Because
of the introduction of antibiotics and improved diagnostic and surgical
techniques, the chronic stage and its sequelae are no longer seen as
frequently as they used to be.
 |
|
Figure 133.7. A: A frog-leg lateral view shows a lytic area in the metaphysis of a 7-year-old boy with a 6-month history of pain and a limp. B: Anteroposterior radiograph taken at the time of biopsy. The lesion was found to be a subacute osteomyelitis caused by a Staphylococcus aureus organism.
|
hematogenous osteomyelitis is whether to treat all patients surgically.
Unless clinical evidence of an abscess is present, I treat patients
with systemic antibiotics and without surgery. However, the clinical
situation must be repeatedly assessed during treatment. Many
osteomyelitic processes are seen early in the disease process or
represent a cellulitic process of the bone, and pus or the abscess
phase may not occur.
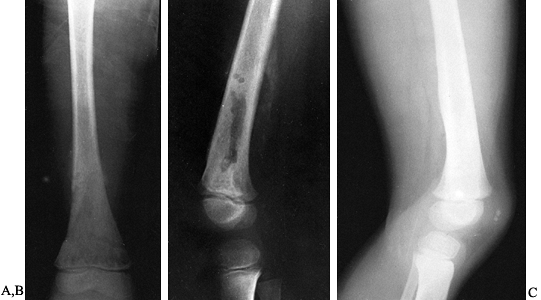
drill a window in cases of subperiosteal or soft-tissue-extended
osteomyelitis, I routinely window the cortical bone for better
debridement of the residual intramedullary abscess and necrotic bone
and tissue (Fig. 133.8) (see Chapter 176).
 |
|
Figure 133.8. A: Anteroposterior radiograph in a child shows destructive lytic changes in the metaphyseal region with periosteal reaction. B: Lateral film shows the extent of the cortical window made for debridement of the medullary abscess. C: Lateral film taken 7 months later shows healing of both the cortical window and the osteomyelitic process.
|
-
Using tourniquet control, make a
longitudinal incision along the posterior border of the medial tibia
and over the affected part of the tibia. If a subperiosteal abscess is
present (more likely in a child than in an adult), incise the
periosteum longitudinally over the abscess. -
If no subperiosteal abscess is found,
observe the status of the cortex. The infected area is often soft and
may be pitted, with or without obvious cortical destruction. -
Outline with a drill an elongated
cortical window extending along the extent of the intramedullary
abscess. The outlined elongated window should be centered in the
posterior half of the anteroposterior diameter of the bone to allow for
dependent drainage. The length of the window depends on the extent of
the intramedullary abscess, and the width depends on the diameter of
the bone. For children, use a 1- to 2-cm-wide window. After debridement
of any obvious sequestrum and copious irrigation, insert a Betadine
gauze into the wound and leave it open. A similar type of window of the
femur is made for osteomyelitis of the femur.
is important to assess the extent of the infection and to obtain a Gram
stain, culture, and sensitivity test. Start appropriate systemic
antibiotics, and take the patient to surgery for irrigation,
debridement, and stabilization of the fracture if it is not already
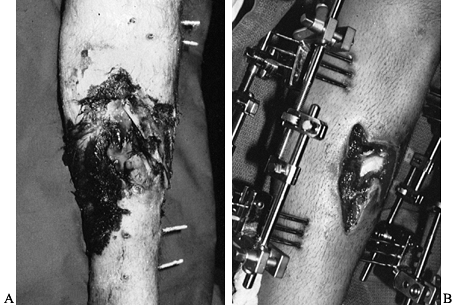
adequately stabilized (Fig. 133.9). The
majority of fractures in the tibia can be stabilized with half-pin
external fixation devices. Use a biplanar or delta frame for more
stability if required.
 |
|
Figure 133.9. A: A 28-year-old man with an infected open fracture. B:
There is a clean granulating wound 12 days after the fracture had been irrigated, debrided, and stabilized with an external fixator and the patient given systemic antibiotic. At this time, biplanar fixation with half-pins rather than full-length pins would be performed. |
the fracture stabilized, perform cancellous autogenous bone grafting,
particularly for fractures with bone deficiency and type III open
fractures. Fracture healing, which facilitates infection control, is an
important principle in the management of infected nonunited fractures
and nonunions. Although many fractures heal in the presence of
infection, infection interferes with the osteogenic process and may
lead to nonunion by a number of mechanisms: The bacteria compete with
osteogenic cells for oxygen and nutrients, activate enzymes deleterious
to the osteogenic process, lower the pH, affect oxygen potential,
interfere with the differentiation of osteogenic cells, and retard
tissue maturation.
type III infected open tibial fractures, I recommend early autogenous
cancellous bone grafting through a posterolateral approach.
Alternatively, if a muscle flap has been made anteriorly for
soft-tissue coverage, an autogenous
cancellous bone graft can be done anteriorly 6 weeks later, provided there is no evidence of recurrent infection.
-
Avoid making a cortical window directly
under the skin incision; the soft tissues will not granulate over a
cortical defect, and skin coverage therefore will not occur. A muscle
transfer may then be necessary. -
Make the cortical window in the dependent one half of the anteroposterior diameter of the bone to allow for adequate drainage.
-
Avoid making too large a cortical window, which can lead to pathologic fractures, especially if the width is excessive.
-
In children, in whom the usual location
for hematogenous osteomyelitis is the metaphysis, be careful not to
damage the epiphyseal plate. Use roentgenograms intraoperatively to
help locate the physis. -
If follow-up cultures are positive or if pus continues to drain, consider performing a second irrigation and debridement.
-
Postoperatively, apply a posterior splint. A wound left open generally closes by secondary intention within 3 weeks.
-
Recommend protected weight bearing in a cast or orthosis for 6–12 weeks whenever a cortical window is used.
achieving union of an infected nonunion or an infected fracture of the
tibia. Freeland and Mutz have reported a 100% union rate in 26 patients
with infected nonunions treated in this manner (14). I have achieved a union rate of approximately 91% in the treatment of 61 infected tibial nonunions (49).
tibia. It was described by Harmon and is also used for tibia-pro-fibula
grafting (23) (Also, see Chapter 3).
-
Place the patient in either a prone or a
lateral decubitus position. Identify the posterior border of the fibula
and the lateral border of the gastrocnemius muscle. Using tourniquet
control, begin an incision of appropriate length along the lateral
border of the gastrocnemius and posterior to the fibula. -
Now reflect the soleus and flexor hallucis longus posteriorly and medially to expose the posterior aspect of the fibula.
-
Elevate the origin of the tibialis
posterior muscle from the posterior aspect of the interosseous
membrane. Locate the posterolateral border of the tibia and, using
sharp dissection, expose the posterior surface of the tibia by
stripping the muscles subperiosteally off the tibia. In the distal one
third of the tibia, approximately four to five fingerbreadths above the
tip of the lateral malleolus, an interosseous arteriole branch from the
peroneal artery perforates the interosseous membrane and travels
anteriorly to anastomose with the anterior tibial artery. Take care to
protect this vessel. The posterior tibial neurovascular bundles lie
between the tibialis posterior and flexor hallucis longus muscles and
are not visible. The muscular branches of the peroneal artery lie
within the peroneal muscles. -
Once the posterior aspect of the tibia is exposed (Fig. 133.10),
prepare the tibia and the posterior aspect of the fibula for bone
grafting by roughening up the cortex with either a burr or an
osteotome. Be extremely careful not to disturb the fracture site so as
to avoid contamination posteriorly.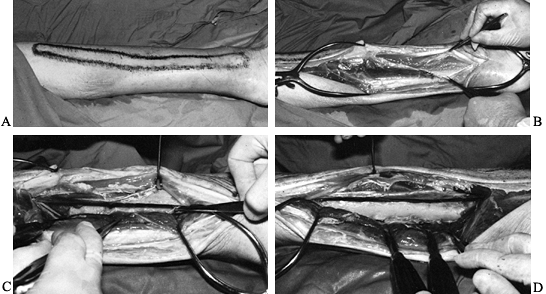 Figure 133.10. A:
Figure 133.10. A:
A cadaveric specimen outlining the fibula. A skin incision varying with
the exposure desired is made just posterior to the fibula along the
lateral border of the gastrocnemius and soleus muscles. B,C:
The peroneal muscles are retracted anteriorly and the border of the
fibula is exposed. The tibialis posterior and the flexor hallucis
muscles are dissected from the interosseus membrane and the posterior
tibia and retracted posteriorly. The posterior tibialis neurovascular
bundle is protected by the tibialis posterior and flexor hallucis
longus muscles, which it lies between. D:
The posterior aspect of the fibula is now exposed, and the posterior
border of the fibula and interosseous membranes are visible. -
Lay cancellous bone grafts posteriorly
several inches above and below the fracture site and between the tibia
and fibula across the interosseous membrane. The objective is to
achieve not only union of the fracture site, but also a tibial–fibular
synostosis (Fig. 133.11). A 1.5–2 oz medicine glass filled with cancellous bone is generally sufficient to repair a nonunion without bone loss.![]() Figure 133.11.
Figure 133.11.
Anteroposterior and lateral films show healing of the fracture
following the posterolateral bone graft. Note the synostosis between
the tibia and fibula. -
At this point, release the tourniquet and
achieve hemostasis. Insert a Silastic drain. Allow the peroneal muscles
and posterior muscle mass to return to their anatomic positions. Do not
close the deep fascia. -
Close the subcutaneous tissues and skin with interrupted sutures.
-
Remove the Silastic drains in approximately 48 hours and elevate the leg for 72 hours.
-
Start full weight bearing to tolerance 7 days postoperatively if the patient is immobilized in a cast.
-
If an external fixator is used for an unstable fracture, it can generally be removed at 6–8 weeks and a walking cast applied.
-
Union generally occurs in 4–7 months, median time being 6 months.
-
An arteriogram obtained before this procedure is performed can help determine what vessels are patent and where they are.
-
Be aware of the location of the neurovascular structure.
filled to reduce the chance of continued infection and loss of
function. Advances in microvascular techniques have made possible the
transfer of muscle, myocutaneous, osseous, and osteocutaneous flaps to
the soft-tissue and bony defects. Fitzgerald et al. reported a 93%
success rate in the treatment of chronic osteomyelitis with local
muscle flaps combined with thorough debridement and specific
antimicrobial therapy (12). Our results using this technique have also been encouraging (46).
proximal third of the tibia, use the gastrocnemius muscle; for those
involving the middle third, use the soleus muscle; and for those
involving the distal third, use a free-tissue transfer.
bone grafting six or more weeks later. The muscle flap can be elevated
and cancellous bone grafting performed underneath, provided there are
no signs of infection (Fig. 133.12).
 |
|
Figure 133.12. Anteroposterior (A) and lateral (B)
radiographs of the tibia and fibula in a 21-year-old man who had a previous posterior bone graft and now shows the presence of a large sequestrum. Lateral film (C) shows debridement of the infection and sequestra. Lateral film (D) taken 10 months after a local flap was performed and an autogenous cancellous bone graft was performed. |
recurrence of infection in free-tissue transfer in osteomyelitis is
found in cases associated with a segmental bone defect (70).
It has been our experience that the fibula is the key factor in
treating osteomyelitis with segmental bone loss of the tibia. If there
is a bony defect or segmental loss of both the fibula and tibia with
chronic osteomyelitis, an amputation is advisable. If the fibula is
intact or without a bony defect, reconstruction of the bony defect is
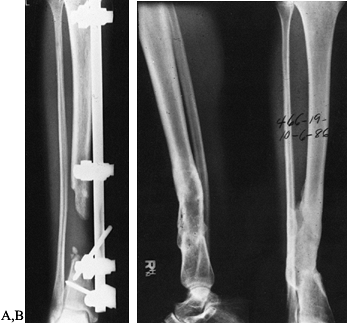
more likely to be successful. For defects up to 6 cm, autogenous
cancellous bone grafting can be done (Fig. 133.13).
 |
|
Figure 133.13. A: Anteroposterior radiograph of a 34-year-old woman with approximately a 6 cm defect. B:
Anteroposterior and lateral films taken 1 year following performance of an autogenous cancellous bone graft to fill dead space caused by bony defects. |
graft. A tibia-pro-fibula synostosis using the previously described
posterolateral approach can be done when a free vascularized osseous
graft or autogenous cancellous bone grafting of the defect is not
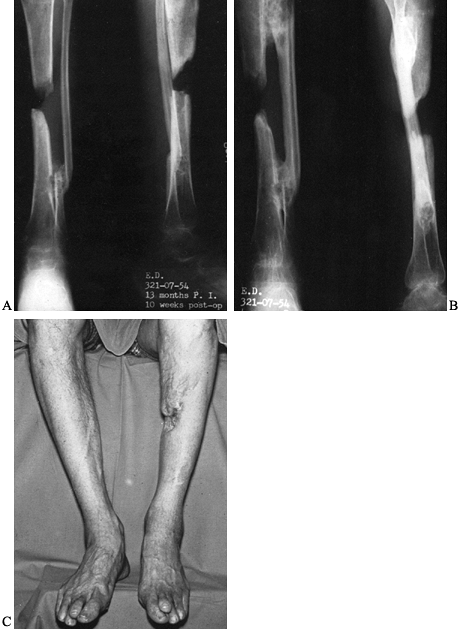
possible (Fig. 133.14). The fibula hypertrophies with time, allowing for functional weight bearing.
 |
|
Figure 133.14. A:
Anteroposterior and lateral radiographs of a 48-year-old man who developed clostridial myonecrosis after an open fracture of the tibia and treatment with a tibia-pro-fibula synostosis. The distal synostosis site was bone grafted first and is seen here 10 weeks postoperatively. B: Radiographs taken 4 years after the tibia-pro-fibula synostosis for the segmental defect. Note the synostosis proximally and distally, and the hypertrophy of the fibula. C: Healed wounds show no evidence of recurrence of infection. Notice the area of defect in the middle calf area. |
have all reported favorable results. However, because of the associated
long hospitalization time, long healing time, high complication rate,
meticulous care required, and resulting unstable scar skin, I use it
only if local muscle transfers or free-vascular tissue transfers with
secondary cancellous bone grafting cannot be done.
-
Thorough debridement of all infected
tissues, repeated as necessary; stabilization of the fracture with an
external skeletal fixator -
Cancellous autogenous bone grafting into a defect lined with clean uninfected granulation tissue
-
Skin coverage either by secondary epithelialization or, in larger defects, by split-thickness skin grafting
-
Debride all infected soft tissue and
sequestra, and debride all necrotic bone to bleeding osseous tissue.
Perform stabilization using an external skeletal fixator. -
When exposed surfaces are covered with
clean granulation tissue, pack finely morcelized autogenous cancellous
bone into the defect created by the bone debridement or previous bone
loss. The diameter of the graft should be slightly larger than the
diameter of the bone being replaced, since the graft will tend to
contract. Rhinelander recommends that the maximum graft thickness be
1.5 cm from the nearest granulation surface (57). -
Dress the wound with gauze and keep it
moist with a physiologic irrigating solution such as Ringer’s lactate,
either by intermittent soaking of the dressings or by a slow
intravenous drip. The dressing, which should be changed daily, is to be
soaked with physiologic solution until the wound is covered by
epithelialization or, in some cases, by secondary split-thickness skin
grafting (Fig. 133.15).![]() Figure 133.15. A:
Figure 133.15. A:
Lateral radiograph of the tibia and fibula in a 37-year-old woman with
loss of the tibia following an infection that developed after the
patient sustained a type III open fracture. B: Anteroposterior photograph shows the soft-tissue and bone loss and exposed tibial shaft. C: Photograph taken at the time of autogenous cancellous bone grafting of the dead space. D,E: Anteroposterior and lateral radiographs, taken after the grafts had consolidated, show healing of the fracture. F:
Lateral photograph, taken 3 years after the procedure, shows knee
flexion and the appearance of the leg. The patient has been free of
infection.
-
Make sure all necrotic soft tissue and bone are debrided.
-
Stabilize the fracture.
-
There must be a clean granulating base
before autogenous cancellous bone grafting is performed. Do a
quantitative tissue culture and Gram stain. If the quantitative tissue
culture yield is greater than 10-5 organisms, or if the Gram stain is positive (implying the presence of more than 10-5 organisms), do not perform the cancellous bone grafting. A count greater than 10-5 organisms is consistent with infection, in which case redebridement is necessary.
fracture stabilization poses a special problem for the surgeon. Should
the metal be removed or left in? The answer to this question is guided
by such factors as the stage of fracture healing, the amount of
stability provided, the amount of time since surgery, and the location
of the fracture.
prosthesis-related infections, microorganisms grew in a biofilm or
glycocalyx that adhered to the surfaces of the biomaterials present (21).
They suggested that infections in the presence of orthopaedic implants
may be more resistant than normal to host defense mechanisms and to
antimicrobial therapy.
a healed fracture, remove the metal. If the fracture is not united and
the metal is not providing stability, remove the metal and restabilize
the fracture. In the immediate
postoperative period (within the first 1–6 weeks), retain the internal fixation device if it is stable and is required.
In general, for late infected nonunion of tibial shaft fractures with
internal fixation, I recommend removal of the internal fixation device
and stabilization with an external fixator. For late nonunion
infections of the femur with plate fixation, I recommend removal of the
plate and eventual stabilization with an intramedullary rod.
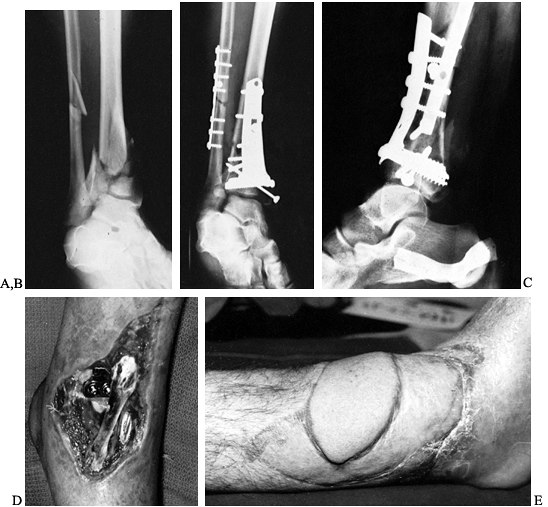 |
|
Figure 133.16. A: Anteroposterior radiograph of the distal tibia and fibula showing a comminuted fracture of the tibia and plafond. B,C:
Anteroposterior and lateral films taken following open reduction and internal fixation show fractures nonunited at the time of infection. D: Anterior photograph of the ankle shows exposed metal and ankle joint with a necrotic anterior tibialis tendon after an acute postoperative infection. E: There is no evidence of infection 9 months following removal of metal and a free-vascularized latissimus dorsi muscle transfer. |
abscess with cellulitis is present, it may be necessary to insert
antibiotic beads into the medullary canal, treat the patient in
traction for several days, and then redebride the femur and stabilize
the fracture with an intramedullary locked nail. I prefer
intramedullary nail stabilization over external fixation in most cases
because of the propensity for pin loosening, infections, and binding
down of muscles with external fixation.
the infected membrane from within the canal by reaming followed by
copious pulsatile irrigation. In the case of infected intramedullary
nails involving the femoral shaft (unless there is an extensive
associated intramedullary abscess), the metal does not adversely affect
eradication of the infection, provided the infection has been
appropriately treated with surgical debridement and parenteral
antibiotics followed by fracture healing with or without autogenous
cancellous bone grafting (54).
reported to be the most common organism causing infection following
nail puncture wounds of the foot. The treatment that produces the best
results consists of surgical drainage, debridement, and curettage of
the puncture wound in the bone, and debridement of all necrotic bone,
along with specific antibiotic therapy. The antibiotic of choice for Pseudomonas
infections is generally tobramycin, in combination with either
piperacillin or a third-generation cephalosporin, depending on the
sensitivity reports. Continue systemic antibodies for approximately 3
weeks (Fig. 133.17, Fig. 133.18).
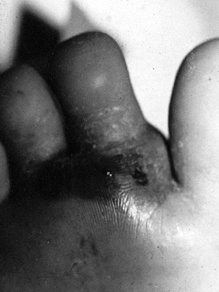 |
|
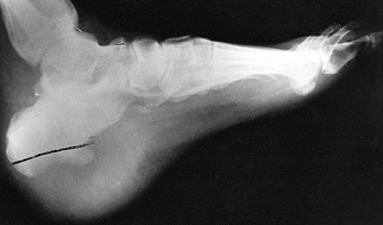
Figure 133.17.
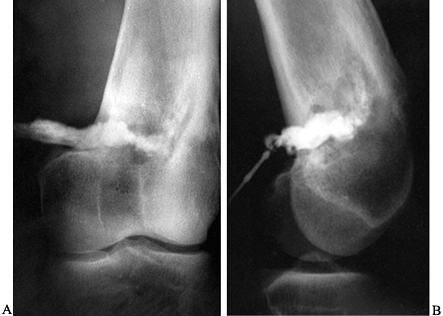
The plantar aspect of the foot of a 5-year-old child has swelling, erythema, and abscess formation following a nail puncture wound to proximal phalanx of the second toe. |
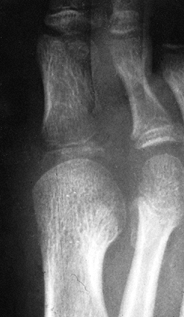 |
|
Figure 133.18.
Anteroposterior radiograph of a 6-year-old child shows osteomyelitic changes of the proximal metaphysis with involvement of the first metatarsal phalangeal joint. |
can be used when there is joint involvement, provided that debridement
of the plantar surface of the metatarsal head or proximal phalanx is
accomplished.
-
Make a plantar transverse incision distal
to the weight-bearing area of the metatarsal heads but proximal to the
web space of the toes. Carry the incision down through the subcutaneous
tissues and identify the flexor tendons, which should be retracted to
expose the involved metatarsal head. -
Incise the periosteum and elevate it to
expose the proximal metatarsal; generally, the periosteum has been
destroyed and this latter step is not necessary. -
Identify the area of osteomyelitis, and
curet and debride the lytic infected bone. If there has been extensive
destruction of the metatarsal head, the head can be excised through
this approach.
-
Be certain to debride all necrotic tissue.
-
Always curet the bony puncture site when it is identifiable because this site could be a source of continued infection.
-
Make a longitudinal incision over the
affected joint. Retract the extensor tendons. Identify the joint
capsule and incise it longitudinally. Debride the joint of any necrotic
tissue. -
Next, free the soft tissue from the
plantar surface of the metatarsal, debride any necrotic bone and
tissue, and curet the puncture site. -
Finally, irrigate the wound with copious amounts of irrigating fluid, and leave a small Betadine gauze or wick in the wound.
diabetic patients or persons who have open fractures. Depending on the
extent of the infection, local debridement of all soft tissue and bone
may be satisfactory. In more extensive infections in which the entire
metatarsal is necrotic, it may be necessary to remove it.
-
Make a longitudinal incision over the
involved bone extending from just distal to the distal row of tarsal
bones to the middle of the proximal phalanx of the involved toe. -
Identify the extensor tendons and retract them.
-
Identify the involved metatarsal, incise the periosteum longitudinally, and strip it and all soft tissues from the bone.
-
Resect the entire shaft or part of the shaft as indicated. In children, avoid injury to the physis.
infections, there may be extensive soft-tissue loss with exposed viable
bone. In such a case, it may be necessary to perform tissue transfers,
including free-vascularized tissue transfers, for coverage.
-
Irrigate the wound with copious amounts
of irrigating fluid. Pack the wound loosely with a Betadine gauge or a
fine-mesh gauze and leave a wick protruding from the wound. When the
infection is more localized, use a smaller longitudinal incision over
the involved metatarsal. -
Apply a below-the-knee splint and dress the wound.
difficult to eradicate and requires adequate, thorough debridement. In
early involvement, extensive local debridement and curettage are
usually sufficient. With extensive involvement of the calcaneus,
resection of the diseased area is necessary to eradicate or control the
infection. Osteomyelitis occurring either medially or laterally in the
calcaneus usually follows pin-track infections, gunshot wounds, or open
fractures.
useful in draining a localized abscess, curetting the infected bone
tissue, doing a local resection, and windowing the bone cortex for an
osteomyelitic abscess.
with modifications. Gaenslen divided the calcaneus with an osteotome
from posterior to anterior, thereby exposing the inside of the bone. He
used this technique primarily to treat hematogenous osteomyelitis.
that it creates a fracture, which could cause an infection to continue,
leading to extensive bone loss. Therefore, avoid splitting the
calcaneus when you plan to preserve it. Excision of the calcaneus
through this approach, especially when there is a wound or fistula on
the weight-bearing surface of the heel, works well.
-
With the patient prone, make a
longitudinal incision centered in the midline of the heel. Start the
incision just inferior to the insertion of the Achilles tendon on the
tuberosity, and extend it plantarward approximately to the level of the
base of the fifth metatarsal. -
Incise the plantar aponeurosis in a plane
between the abductor digiti quinti and flexor digitorum brevis muscles.
Visualize the lateral plantar artery and nerve in the distal aspect of
the wound and retract them medially. -
Expose the quadratus plantar muscle and
split both it and the long plantar ligament longitudinally. The plantar
surface of the calcaneus is now exposed. For localized infection use a
curet to remove sequestra, infected tissue, and sinuses. For more
extensive involvement use an osteotome to resect the involved bone
parallel to the plantar surface of the foot (Fig. 133.19). After debridement and copious irrigation, place a Betadine-soaked gauze dressing in the wound and leave the wound open.![]() Figure 133.19.
Figure 133.19.
Lateral radiograph of a 42-year-old man demonstrates extensive
destruction of the calcaneus by osteomyelitis. A marking pen outlines
the area of bones to be resected. -
Postoperatively, change dressings daily. Permit weight bearing after the incision has healed.
excise the proximal three fourths of the fibula, provided the fibular
collateral ligament and the biceps femoris tendons are inserted into
the tibia when this area of bone is sacrificed. The distal one fourth
should not be excised, because excision would lead to impaired function
of the ankle, and deformity. If it is necessary to remove this part, a
tibial–talar fusion will be necessary later.
-
Make a longitudinal incision, starting on
the posterior border of the fibula from the head of the fibula and
extending distalward as needed. This incision can be carried down the
entire length of the fibula. -
Identify the common peroneal nerve in the
proximal part of the wound; it crosses under the peroneus longus just
distal to its attachment to the lateral surface of the head of the
fibula. Identify the fascial plane between the soleus muscle and the
peroneal muscles anteriorly, and carry the dissection down to the
fibula. -
Retract the peroneal muscles anteriorly,
and expose the fibula by incising the periosteum. Take care not to
injure the branches of the deep peroneal nerve, which lie on the deep
surfaces of the peroneal muscles at the neck of the fibula and proximal
5 cm of the fibula. Now expose the fibula, and debride or resect the
infected area.
-
Debridement is the key. Don’t be hesitant to resect all infected necrotic bone.
-
Be careful of the lateral plantar neurovascular structures.
-
The distal one fourth of the fibula is
subcutaneous and can be easily exposed by a longitudinal incision along
the posterior border of the fibula. -
A Betadine-soaked gauze strip can be loosely placed in the wound.
-
Apply a long posterior molded splint. Dress the wound daily.
-
Expose the patella through a longitudinal
skin incision that starts just proximal to the patella and extends
distally to the inferior pole. Carry the incision down through the
subcutaneous tissue and fascia to the periosteum. -
Incise the periosteum and elevate it with a periosteal elevator. Curette and debride the involved area of bone.
-
In cases of extensive involvement of the
patella, either part or all of the patella can be removed. In cases in
which the knee joint is involved, irrigate the knee with 10 L of
irrigating fluid. Close the joint capsule over Silastic drainage tubes,
which are removed in 48–72 hours.
femur are very important in its management. For hematogenous
osteomyelitis, windowing of the cortex as described in this chapter is
the preferred method of treatment.
-
Use either a lateral decubitus position
or turn the patient slightly to elevate the involved extremity. This
approach provides access to the entire femoral shaft. -
Make an incision from the base of the
greater trochanter distally to the lateral femoral condyle, depending
on the desired length of exposure. Incise the superficial fascia and
the fascia lata along the anterior border of the iliotibial band. -
Expose the vastus lateralis muscle and
retract it anteriorly. Continue along the anterior surface of the
lateral intermuscular septum, which attaches to the linea aspera. -
Expose the periosteum and incise it
longitudinally. Then use a periosteal elevator to expose the bone.
Debride the area of infected necrotic tissue and bone. -
In cases of nonunion in which a plate is
present and the infection is seen late, remove the plate and apply an
external fixation device. Follow with an autogenous cancellous
bone-grafting procedure when the infection is controlled. -
In the middle third of the thigh, take
care to identify and ligate the second perforating branch of the
profunda femoris artery and vein, which travel transversely from the
biceps femoris to the vastus lateralis. Avoid damaging the sciatic
nerve and profunda femoris artery and vein by not dividing the long and
short heads of the biceps femoris muscle.
or inner, cortex and on the lateral, or outer, cortex, or on both.
Hematogenous seeding is more common in young persons. In adults,
osteomyelitis usually occurs following open fractures, bone-grafting
procedures, or, in debilitated patients confined to bed, secondary to
pressure sores.
-
Make an incision along the crest of the
ilium extending the length of the infected area. Extend the dissection
through the subcutaneous tissues to the crest. -
Incise the periosteum over the top of the
crest and subperiosteally strip the muscles from the lateral cortex of
the iliac wing. Identify the abscess, if one is present, and the extent
of bone involvement. Drain the abscess and debride all necrotic bone.
Window the cortex as necessary. Be careful of the lateral femoral
cutaneous nerve, which normally lies just medial to the anterior
superior iliac spine. Perform local resection of the ilium for
extensive local involvement, especially in chronic osteomyelitis (Fig. 133.20).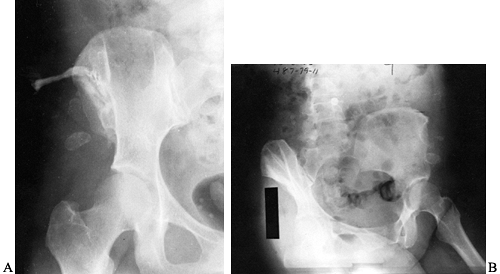 Figure 133.20. A:
Figure 133.20. A:
Anteroposterior view of the ilium and hip showing a sinogram tract down
to the ilium. This 44-year-old woman developed chronic osteomyelitis of
the ilium following an open fracture. She had undergone multiple
previous procedures. B: Radiograph after local resection of the ilium.
and develop pressure sores with secondary infection, necrosis, and
osteomyelitis. In these infections, it is necessary to debride all
necrotic tissue, after which the infected ischial tuberosity can be
resected with an osteotome and mallet. Irrigate the wound copiously and
leave it open, with Betadine-soaked gauze inserted into it to allow for
drainage. In paraplegic patients, soft-tissue transfers are often
necessary following infection control.
obturator externus or internus often develop with osteomyelitis of the
pubis and ischium.
-
Because the posterior surface of the ulna
lies subcutaneously essentially throughout its length, expose the ulna
by a skin incision carried down through the fascia and periosteum. -
Approach the radius in its distal third by the anterior approach, as described by Henry (24). Expose the proximal fourth of the radius with an anterior Henry incision or an extended Thompson approach.
-
The middle two thirds of the radius can then be exposed by the Thompson approach (64) (see Chapter 1).
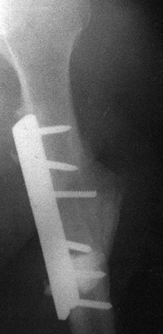
follows internal fixation of the humerus. Often, draining sinuses are
present (Fig. 133.21).
 |
|
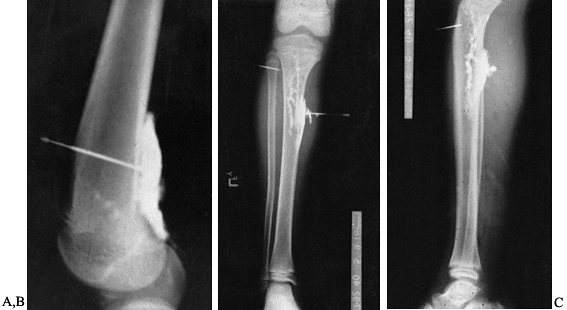
Figure 133.21.
A sinogram tract down to metal and bone. The patient had persistent drainage for 7 months following open plating of the humerus. Notice that the fracture is healed. |
-
After assessing the extent of the infection, expose the humeral shaft through an anterolateral approach (see Chapter 1).
Make a skin incision along the anterior border of the deltoid muscle,
extending it distally along the lateral border of the biceps muscle to
within several inches of the elbow joint. Identify the cephalic vein
and ligate it. -
Retract the deltoid laterally and the
biceps medially to expose the proximal shaft. Distal to the deltoid
insertion, identify the brachialis and split it longitudinally to the
bone, retracting the lateral half laterally and the medial half
medially. The radial nerve is protected by the lateral half of the
brachialis muscle. In the distal third of the exposure, identify the
radial nerve, which lies between the brachioradialis and brachialis
muscles, and protect it. Avoid injury to the musculocutaneous nerve. -
Debride all infected and necrotic bone
and soft tissue. If the humerus is unstable, remove the internal
fixation and stabalize with external fixation. Leave the wound open.
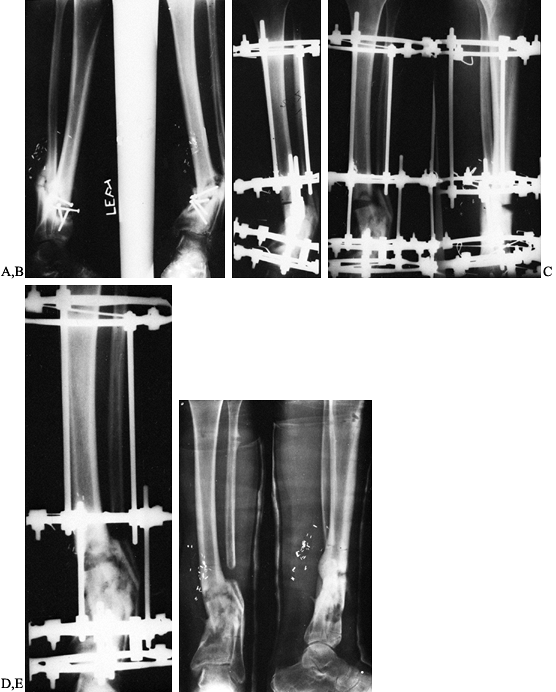
to be useful for the treatment of extensive bone loss problems or for
angulatory nonunions (44) (Fig. 133.22). A more
extensive discussion of the Ilizarov technique is presented in Chapter 32.
 |
|
Figure 133.22.
Correction of deformity and subsequent healing process in a 29-year-old man with an infected nonunion of the tibia in marked varus, treated with the Ilizarov technique. |
Clinical Medicine and Orthopaedic Surgery, section of Infectious
Disease, University of Southern California School of Medicine, for his
technical assistance in the antibiotic section of this chapter.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical outcome study.
JL, Goll SR, McCarthy KE, et al. Indium-111 Leukocyte Scintigraphic
Detection of Subclinical Osteomyelitis Complicating Delayed and
Nonunion of Long Bone Fractures: A Prospective Study. J Orthop Res 1987;5:1.
AB, Herbert JC, Goodell J, Kristiansen T. Noncommercial Fabrication of
Antibiotic-impregnated Polymethylmethacrylate Beads. Clin Orthop 1987;223:282.
N, Holtom PD, Warren CA, et al. In Vitro Elution of Tobramycin and
Vancomycin Polymethylmethacrylate Beads and Spacers from Simplex and
Palacos. Am J Orthop 1998;27:201.
K, Brown M, Dewanjee M, Fitzgerald R. Comparison of
Indium-labelled-leukocyte Imaging with Sequential Technetium-gallium
Scanning in the Diagnosis of Low-grade Musculoskeletal Sepsis. J Bone Joint Surg Am 1985;67:465.
LJ, Alfageme A, Delcourt JP, Pilon BL. Chronic Osteomyelitis of Long
Bones—Resection and Bone Grafting with Delayed Skin Closure. J Bone Joint Surg Br 1976;52:138.
MJ, Wilkins J, Brien WW, Carter VS. Wound Site as a Predictor of
Complications Following Deep Nail Punctures to the Foot. West J Med 1989;150:545.
MJ, Wilkins J, Kumar J, et al. Comparison of the Results of Bacterial
Cultures from Multiple Sites in Chronic Osteomyelitis of Long Bones. J Bone Joint Surg Am 1994;76:664.
CR, Pearson RL, Miller GA. Accuracy of Cultures of Material from
Swabbing of the Superficial Aspect of the Wound and Needle Biopsy in
the Preoperative Assessment of Osteomyelitis. J Bone Joint Surg Am 1991;73:745.
FL, Montgomerie JZ. Vertebral Osteomyelitis in Intravenous Drug
Abusers: Report of Three Cases and Review of the Literature. Rev Infect Dis 1980;2:196.
J, Hackenbroch MH, Kumm D, Taravati V. Is Instillation Drainage for the
Treatment of Infected Joints, Bones and Soft Tissues Still up to Date? Arch Orthop Trauma Surg 1996;115:149.
JE, Nepola JV, Conrad GR, et al. Detection of Osteomyelitis at Fracture
Nonunion Sites. Comparison of Two Scintigraphic Methods. Am J Roentgenol 1989;152:1021.
FA, Medoff G, Swartz MN. Osteomyelitis: A Review of Clinical Features,
Therapeutic Considerations and Unusual Aspects. N Engl J Med 1970;282:198, 316.