MANAGEMENT OF PYARTHROSIS
VII – NEOPLASTIC, INFECTIOUS, NEUROLOGIC AND OTHER SKELETAL DISORDERS
> Infection and Hemophilia > CHAPTER 134 – MANAGEMENT OF
PYARTHROSIS
Therefore, pyarthrosis by definition denotes a suppurative arthritis.
In common medical usage, the term refers to pyogenic infections
involving the synovial joints. Septic arthritis accounts for 0.2% to
0.7% of hospital admissions and continues to be a cause of an
orthopedic emergency that can lead to morbidity and mortality (83).
mechanisms, including hematogenous spread from a distant source and
spread from an adjacent focus of infection, for example contiguous
osteomyelitis (6). Direct inoculation through a
penetrating injury is another means, as is intra-articular injection
and or a surgical procedure.
the host and the infecting organism. Factors important to this
interaction include general host factors, local host factors, and the
quantity and virulence of the infecting organism. General host factors
that predispose individuals to septic arthritis include defects in the
immune system (e.g., hypogammaglobulinemia), quantitative and
qualitative white blood cell deficiencies, cancer, immunosuppression
caused by chemotherapeutic agents, and severe chronic illness such as
liver and kidney disease, rheumatoid arthritis, systemic lupus
erythematous, diabetes mellitus, sickle cell anemia, and alcoholism (5,20,71,78,86,87,117).
Any distant focus of infection that induces recurrent bacteremia may
predispose a patient to joint sepsis; sources include chronic
sinusitis, bronchiectasis, and intravenous drug abuse (91).
is more common after previous joint trauma, and a history of prior
arthritis increases the risk of septic arthritis in the same joint (31,71,87,103).
In rheumatoid arthritis, the increased risk of superimposed pyogenic
arthritis is related to local factors (e.g., chronic hyperemia, a
protein-rich inflammatory exudate within the joint, or local steroid
injection), as well as generalized host factors (e.g., humoral and
white blood cell deficiencies, the use of systemic
immunosuppressive agents, and general debility) (5,36,66,69,71,85,114,117).
with an increased incidence of septic arthritis include degenerative
joint disease, Charcot arthropathy, and crystal-induced arthritis (gout
and pseudogout) (22,61,64).
The incidence of local infection is also increased after the
implantation of various biomaterials commonly used in orthopaedic
surgery (82). In particular, methylmethacrylate
has been shown to have profound effects on local polymorphonuclear
leukocyte chemotaxis and phagocytosis (81,82).
also on the quantity and virulence of the infecting organism. Important
factors in this respect include exotoxin, endotoxin, and enzyme
production by the bacteria and the synovial membrane. These
by-products, as well as bacterial debris, may also play a role by
stimulating the host’s immune system to produce the postinfectious
arthritis syndrome (31,37).
inflammatory response ensues. Proteolytic enzymes are produced that
degrade the proteoglycan matrix of the cartilage ground substance and
the collagen. These enzymes are released by the polymorphonuclear
leukocytes and the lysosomes within the synovium (18,19,113,118). The bacteria themselves may also contribute to this depletion of cell matrix, even in the absence of inflammation (108).
loss has begun, the proteoglycan losses may be reversed. Enzymes
released by the polymorphonuclear leukocytes and synovium, including
collagenase, elastase, cathepsins, and other proteases, remove the
proteoglycan matrix from the cartilage, destroy the collagen
superstructure, and subject
the
chondrocytes to increased mechanical stress. As cartilage cells die,
matrix formation is decreased, and a vicious circle ensues. The
articular cartilage is eroded away, and the synovium becomes
hyperplastic. A pannus of chronic granulation tissue may cover the
joint surface. Fibrous or bony ankylosis may be the final result (46) (Fig. 134.1).
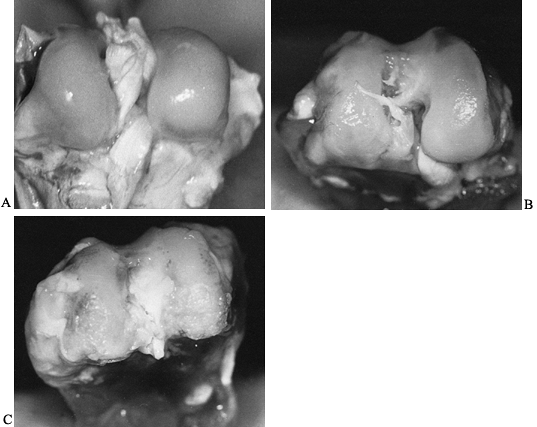 |
|
Figure 134.1. A: Photograph of the distal femoral articular surface in a normal rabbit. B: Photograph of distal femur of rabbit infected with Staphylococcus aureus
and having antibiotic treatment started at 48 hours. Notice the irregularity and pitting in the weight-bearing portion of the articular cartilage. C: Distal femoral articular surface of a rabbit that was infected with S. aureus but did not receive antibiotics. Notice the severe degenerative arthritis that has developed. |
propensity for neonates and infants, older adults, and patients who
have a chronic, systemic disease, or a compromised immune system.
Although virtually any joint may be involved, the large weight-bearing
joints of the lower extremity are most at risk (5,6,29,31,46). The joints most commonly involved are the knee, hip, ankle, shoulder, wrist, and elbow (32). Usually, only a single joint is involved, but multiple joint involvement has been documented (5,31,52,74).
joint that demonstrates the signs of acute inflammation: pain,
swelling, heat, erythema, and loss of function. In patients with
chronic arthritis (e.g., rheumatoid arthritis), the diagnosis may be
delayed because either the patient or the physician assumes that the
clinical picture is a manifestation of an acute exacerbation of the
chronic disease. Such was the case in 4 of 13 rheumatoid patients
reported by Gristina et al. (36) In these
instances, the physician must maintain a high index of suspicion of
septic arthritis superimposed on a chronic illness.
Therefore, signs of systemic sepsis, such as fever and tachycardia, may
be present. In one study, during the first 24 hours of hospitalization,
78% of patients with septic arthritis were febrile. Chills and rigors
are uncommon, and the temperature infrequently goes above 39°C
(102.2°F) (31,70). The
original focus of infection may be discovered after a careful history
and physical examination. Special attention should be paid to the ears,
nose, throat, and chest, as well as the integumentary, genitourinary,
and gastrointestinal systems.
Whereas nongonococcal septic arthritis affects the very young, the
elderly, or the immunocompromised, gonococcal arthritis affects healthy
persons, usually younger than 40 years of age. The increasing female
preponderance appears to be related to the asymptomatic carrier status.
Women are often seen during pregnancy or just after beginning the
menstrual period, implicating local gynecologic and physiologic factors
in the production of disseminated disease (70). Systemic signs and symptoms are variable (10,17,42,51,109).
polyarthritis, which may eventually become monoarticular. The knees,
wrists, ankles, and hands are most often affected, and there may be an
associated tenosynovitis. This classic clinical presentation of a
migratory polyarthritis, tenosynovitis, and the characteristic
vesicopapular skin lesions of gonorrhea is rarely seen in cases of
nongonococcal septic arthritis. Look for other manifestations of
gonorrhea, including signs of heart (endocarditis or myocarditis),
central nervous system (meningitis), and liver involvement and
conjunctivitis. Undertake an examination and cultures of the genitalia,
rectum, oral pharynx, and other possible lesions (e.g., of the skin).
Whereas joint aspiration and blood cultures are frequently positive in
nongonococcal arthritis, this is not so in the case of gonorrhea.
problem. Whereas adults with septic arthritis can verbalize their pain,
neonates and infants cannot. The child with a septic joint is generally
systemically ill (34,35).
Pseudoparalysis of the limb and resistance to passive range of motion
are frequently present and often suggest to the unwary that the child
has a fracture. It is only after a more in-depth examination that the
signs of inflammation are noted and focused to a single joint. The
physician must always be suspicious of septic arthritis, especially in
the immunocompromised child, the neonate who is small for gestational
age, and at any age when invasive monitoring techniques are being used (34,35,68).
monoarticular inflammation should suggest septic arthritis. A white
blood cell count from the peripheral blood is greater than 10,000
cells/mm3 in only 50% of patients and is occasionally helpful. The key diagnostic study is analysis and culture of the synovial fluid (31,46,70).
Aspirate the fluid under strictly sterile conditions, and perform the
following tests: gross examination of appearance, viscosity, and color;
a white blood cell count; the percentage of polymorphonuclear
leukocytes; the glucose concentration of the aspirate; Gram’s stain;
and culture. Tables have been published to differentiate the
characteristics of normal synovial fluid from those associated with
degenerative joint disease, inflammatory arthritis, and septic
arthritis (91). Send all specimens to the
laboratory promptly for immediate analysis. Culture the arthrocentesis
fluid aerobically and anaerobically, and incubated in 5% to 10% CO2
on chocolate agar plates if gonococcal arthritis is suspected. If
tuberculous arthritis is a possibility, order a Ziehl-Neelsen stain and
appropriate culture. Fungal infections may be viewed microscopically
with potassium hydroxide preparations and cultured on Sabouraud’s
medium. If the synovial
fluid is centrifuged, the concentrated sediment often improves the yield of a Gram stain or other stain.
The viscosity of the fluid is variable; however, the mucin clot is
usually very friable. The synovial fluid white blood cell count is
usually more than 50,000 cells/mm3 and is often over
100,000. Polymorphonuclear leukocytes constitute at least 75% of the
cellular population and frequently make up more than 90%. Synovial
fluid glucose concentration is usually lower than the blood glucose
level. Frequently, the fasting blood minus synovial fluid glucose level
is more than 50 mg/dl (97). In tuberculous and
fungal arthritis, the findings are similar, except there is usually a
higher proportion of mononuclear cells (30% to 50%). Look for the
presence of crystals under polarized light to rule out gout or
pseudogout in afebrile adults. See Chapter 99 for more details on synovial fluid analysis.
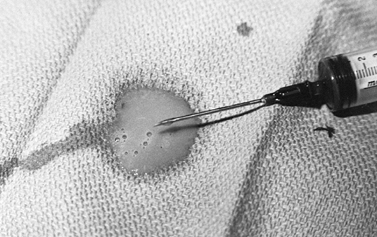 |
|
Figure 134.2. Pus aspirated from a knee joint infected with Staphylococcus aureus.
|
entry. This may include any suspicious skin lesion or wound, the nasal
pharynx, sputum, urine, and stool. Approximately 50% of patients with
nongonococcal bacterial arthritis have a positive blood culture,
whereas the frequency of positive synovial fluid cultures ranges from
25% to 97% (38). If the patient was treated with antibiotics before the synovial culture was obtained, the yield is much lower.
in the synovial fluid in cases of septic arthritis. These procedures
are sometimes costly and in practice have had only limited application.
Immunodiagnosis employs monoclonal antibodies in patients with
culture-negative infections, especially those who have been previously
treated with antibiotics. The method employs immunoassays with
counterimmunoelectrophoresis or latex particle agglutination to detect
antibodies or bacterial antigens in synovium, including Streptococcus pneumoniae, Neisseria meningititis, and Haemophilus influenzae type b.1 (38,111).
If the antibody to a bacterial antigen is found, then the patient has
been previously exposed and the immune response has been activated;
however, the test does not differentiate between a recent exposure or a
remote one. These tests have been shown to be positive in 75% of
patients with H. influenzae infection (63).
may show evidence of pre-existing disease and may alert the physician
to the possibility of a superimposed septic arthritis. Usually,
however, the radiographs show soft-tissue swelling and synovial
distention and little more. Later radiographs (1 to 2 weeks) may show
osteopenia in the subchondral area or evidence of a coexisting
osteomyelitis. Subluxation or dislocation of the joint may be seen
early or late. It is more common in the hip of the infant and may be
accompanied by exuberant new periosteal bone formation (57). Septic arthritis associated with subluxation of the glenohumeral joint has been documented in the adult (62).
If a gas-forming organism (e.g., some gram-negative organisms and
anaerobes) is the cause of septic arthritis, the radiograph may
disclose gas in the joint and surrounding tissues (9,65).
Late radiographic changes in untreated cases of septic arthritis
include progressive joint narrowing and destruction. Fibrous or bony
ankylosis may be the final result.
because the appearance of the “classic” radiographic signs may be
altered (54). In a series of proven hip infections after surgical treatment of hip fractures with internal fixation,
Lewis and Norman found that pericapsular edema was absent in 50% of
patients. Joint space narrowing was the initial and most reliable
radiographic sign and was recognized as early as 4 weeks after surgery.
Later acetabular destruction superiorly in the weight-bearing area was
often accompanied by subluxation. If septic arthritis is suspected in a
prosthetic joint, radiographs should be taken to assess whether there
is evidence of new periosteal bone formation, bone lysis, or loosening
of the arthroplasty (88). This subject is discussed in more detail in Chapter 135.
citrate, indium, or other radioisotopes is sometimes helpful in the
diagnosis of difficult cases of septic arthritis (55,93).
The scan may localize septic areas that are difficult to examine
clinically and may help differentiate cellulitis from septic arthritis
or osteomyelitis. Further localization of difficult joints (e.g., the
sacroiliac or sternoclavicular joints) may also be afforded by plain or
computed tomography (CT) (67).
to help detect infection. This test is 95% sensitive, but the
specificity is lower at 85% because the test cannot distinguish between
infection and inflammation (3).
of infection earlier than radiographs, because MRI is sensitive for
detecting fluid in joints and is able to show abnormalities within 24
hours (13,49,60).
MRI has low specificity because it cannot distinguish fluid from
infectious, inflammatory, or hemorrhagic causes. MRI is useful in
showing infection of the soft-tissue and bony involvement of the spine
and pelvis or chronic osteomyelitis. It also shows marrow changes,
contrast between bone and soft tissue, and anatomic detail. Generally,
T1-weighted images show decreased signal intensity, whereas increased
signal intensity is seen on T2-weighted imaging because of marrow
edema, ischemia, or exudation. Chronic cases show well-defined
soft-tissue abnormalities, and thickened cortices, whereas an acute
infection shows poorly defined soft-tissue planes, no cortical
thickening, and poor distinction between normal and diseased marrow.
osteomyelitis and the presence of an abscess. MRI is also useful in
cases that may need surgical treatment, especially of the spine or
pelvis. A study by Hovi et al. (43) found that
persistent pathologic findings on MRI were seen in some patients
despite normal C-reactive protein (CRP) levels and a low erythrocyte
sedimentation rate (ESR). The investigators suggested that
antimicrobial therapy continue even in the absence of clinical signs of
infection and normal CRP levels and ESR if there are MRI abnormalities
consistent with infection.
case of infectious from noninfectious arthritis or to provide material
for culture and histologic analysis in difficult, perplexing cases (30).
is the most common cause of septic arthritis in the healthy adult
population younger than 40 years of age. It has been estimated that two
to three cases of gonococcal arthritis are seen for every case of
bacterial arthritis (24).
Various streptococcal species constituted 27% of infections. Recently,
gram-negative bacillary septic arthritis has become more prevalent,
constituting 23% of cases in the above-mentioned series. Diplococcus pneumoniae (6%) and S. epidermidis
(4%) accounted for the remainder. In two other large series,
staphylococcal infections played an even more prominent role.
Staphylococci made up 77% (108 of 141, and 80 of 104 cases) of all
bacteria isolated in nongonococcal septic arthritis in adults (52,74). These bacteria are showing increasing penicillin resistance (103), necessitating the use of alternative chemotherapeutic agents.
(pneumococcus), almost always cause septic arthritis through
hematogenous spread from the upper or lower respiratory tracts or skin;
other rare sources have been noted (12,32,53). Gram-negative bone and joint infections appear to be on the increase (51),
a fact related to the rise in intravenous drug abuse and to chronic
medical problems such as diabetes, cancer, and the use of
chemotherapeutic agents (28,90,104,117). Whereas Pseudomonas aeruginosa and Serratia marcescens septic arthritis are usually associated with intravenous drug abuse, Escherichia coli and Proteus mirabilis infections often stem from urinary sepsis (28,90,104,116). Many other gram-negative organisms have also been implicated in cases of septic arthritis (28,103).
a slightly different bacteriology. For the first few months of life,
the neonate attains passive immunity from maternal antibodies. In this
age group, the most common bacteria causing septic arthritis include
gram-positive cocci (staphylococcal and β-streptococcal species) and
gram-negative rods (70,102).
These bacteria may emanate from the maternal vaginal flora or from
invasive procedures such as intravenous catheters introduced in the
hospital nursery. Gonococcal arthritis should always be kept in mind in
this age group. Infants older than 6 months of age and children up to
several years of age have an increased incidence of H. influenzae infections in addition to the common neonatal
organisms. Frequently, septic arthritis due to H. influenzae is resistant to ampicillin. In children older than 2 years of age, S. aureus is the usual bacterial organism cultured; however, H. influenzae, streptococcal, gonococcal, and other organisms may be the cause (34,35,68,70). In older children, infection associated with a foreign body in the joint (e.g., the knee) should always be suspected.
forms of arthritis are rare. In general, appropriate cultures should be
made to exclude these agents, especially in the case of an
immunocompromised host (33,56). The atypical mycobacteria, including Mycobacterium kansasii, Mycobacterium marinum, Mycobacterium intracellulare, and others, usually cause a monoarticular-pauciarticular arthritis of the hands or knees (41). Mycotic septic arthritis may include infection with Sporothrix schenckii, Candida species, the maduromycoses, Cryptococcus neoformans, Coccidiodes immitis, Blastomyces dermatitidis, Aspergillus fumigatus, and others (41).
It frequently appears with infection due to rubella, hepatitis B, and
alphavirus, and less commonly with infection due to mumps, adenovirus,
herpesvirus, and enterovirus. Joint symptoms are transient, often
polyarticular, and nondestructive. The etiology of the arthralgia or
arthritis may be due to direct synovial invasion of the virus or to a
virus–host interaction involving stimulation of the immune system (96).
nongonococcal bacterial arthritis, with the most important being total
joint arthroplasty. The rate of infection following total joint
arthroplasty of the hip and knee is 1% to 5%, with S. aureus being the most common cause (15,75).
convalescence from an illness or surgery, urinary tract infection,
failure of fixation of a hip fracture, and diabetes mellitus. Factors
associated with knee joint infections include antibiotics given for
wound infections, increased pain and limited motion, and rheumatoid
arthritis (26). Sickle cell disease continues
to be an important predisposing factor for pyarthrosis, especially due
to salmonella. Human immunodeficiency virus (HIV) infection is becoming
a more common predisposing factor, with S. aureus as the most common organism and the knee the most common site (83).
are usually due to one predominant organism. Gram-positive bacteria
(i.e., S. aureus and S. epidermidis) comprise 60% to 80% of the organisms cultured (1,2,18,93).
Gram-negative and multiple organisms occur less frequently. After joint
replacement, staphylococcal infections take on a new significance (96,105). S. epidermidis infection becomes as common as S. aureus and appears to be more difficult to eradicate (105).
In one large series of 137 infected total hip replacements, 68% of
cultures grew gram-positive organisms, 18% grew gram-negative
organisms, one hip was infected with multiple organisms, and 11% of
cultures were sterile (results in two cases were not available) (45). Most authors agree that infection with gram-negative organisms or mixed organisms yield the poorest results (14,44,45,105). This subject is discussed in greater detail in Chapter 135.
demands an immediate thorough workup, including history, physical
examination, appropriate blood work, radiography, joint aspiration with
Gram’s stain and culture, and an intense search for a primary infective
focus.
-
Sterilize the joint by providing adequate drainage and appropriate antibiotics in sufficient dosages to kill the pathogen.
-
Prevent the occurrence of deformity.
-
Fully rehabilitate the joint and limb (37).
of septic arthritis is the method of drainage. All agree that adequate
drainage is of paramount importance. It mitigates the ongoing
degradation of the cartilage ground substance by the enzymes of the
polymorphonuclear leukocytes and lysosomes within the synovium (18,19,118).
The debate centers around the method of drainage–in other words,
whether repeated aspiration or surgical drainage is indicated.
arthritis of the hip and other less accessible joints requires surgical
drainage, the method of treatment in other joints is less clear. In
general, internists and pediatricians favor repeated aspiration of an
infected joint; most surgeons favor surgical drainage (5,27,31,35,52,57,74,79,91).
Arthroscopy is the method of choice for the knee, and when the surgeon
is experienced, it is also the method of choice for the shoulder,
elbow, wrist, and ankle (23,41).
Indeed, Paterson has stated, “It is suggested that aspiration has no
place in the treatment of suppurative arthritis of the hip, and it is
not considered a safe method for any other joint” (79).
However, there have been no randomized, prospective studies to document
the superiority of one form of treatment over the other. Several
retrospective studies, including those by Goldenberg et al. (27) and Rosenthal et al. (92) compared the two methods of treatment
and concluded that patients undergoing needle aspiration did better
than those undergoing surgical drainage and debridement. However, the
controversy has not been settled. Other issues are also involved; for
example, consider how difficult it is to convince a pleading, crying
child with septic arthritis that he or she must undergo aspiration once
(or more) per day. See Chapter 176 for more details about pyarthrosis in children.
joint frequently enough to prevent the stagnation and loculation of
pus, initially, aspirate once or twice each day (28,31,91). Careful sterile technique is mandatory.
-
Use a large-bore (at least an 18-gauge)
needle for larger joints. Culture the aspirate every 1 to 2 days after
antibiotic treatment has commenced to assess the efficacy of therapy.
Continue aspirations until little exudate is retrieved and repeat joint
fluid cultures are negative. -
Monitor the clinical status closely. It
is sometimes useful to lavage the joint with saline after the
aspiration to wash the debris and chondrolytic enzymes from the joint. -
The specific location of the needle
insertion for aspiration is not important, as long as adequate drainage
is attained and the joint and important structures around it (i.e.,
vessels, nerves, tendons) are not injured (27,77,91). -
We find it easiest to aspirate the
shoulder either anteriorly or posteriorly. In the anterior approach,
direct the needle posteriorly, superiorly, and laterally from a point
slightly inferior and lateral to the coracoid process. This maneuver is
often facilitated by external rotation of the shoulder. For the
posterior approach, enter the shoulder joint 1.5 inches (3.75 cm)
inferior and medial to the acromial angle. -
Approach the elbow joint
posterolaterally, just below the midpoint connecting the lateral
epicondyle and the lateral edge of the olecranon. Keep the elbow flexed
90° during the aspiration. -
Approach the wrist dorsally. This provides excellent access while avoiding many of the critical structures in the area.
-
Enter the finger joints through a
posteromedial or posterolateral stab, just volar to the extensor
mechanism using a smaller bore needle. -
The carpometacarpal joint of the thumb is
more difficult because of the large number of tendons, nerves, and
vessels passing in the vicinity. It is approached most safely by
flexing the thumb across the palm and directing the needle at the base
of the first metacarpal volar to the radial artery and anatomical snuff
box, aiming for the fourth metacarpal base. -
The hip joint is one of the most
difficult to aspirate. All physicians involved in the treatment of
pyarthrosis must know the proper technique. Aspiration of the hip is
best performed in the radiology suite or operating room, where good
radiographic imaging with fluoroscopy is available. Place the patient
in the supine position on a radiolucent table. In the anterior
approach, extend and externally rotate the hip. Introduce the needle 1
inch (2.5 cm) below the anterosuperior iliac spine and 1 inch (2.5 cm)
lateral to the palpable pulsations of the femoral artery. Direct the
needle into the joint posteromedially at a 60° angle. For the lateral
approach to the hip, position the hip in extension and internal
rotation. Identify the greater trochanter. Direct the aspiration needle
along the anterior portion of the greater trochanter, parallel to the
femoral neck, then medially and cephalad toward the middle of the
inguinal ligament. -
The knee
joint is the easiest joint to aspirate because it is superficial and
contains the largest synovial cavity in the body. With the patient in
the supine position and the knee fully extended, insert the needle from
a midlateral or midmedial parapatellar location and direct it
posteriorly and inferior to the patella. Take care not to scratch or
scuff the articular surfaces. -
The ankle may
be approached easily either anteromedially or anterolaterally. In the
anteromedial approach, insert the needle just distal to the tibial
plafond, between the lateral edge of the medial malleolus and the
medial edge of the tibialis anterior tendon. For the anterolateral
approach, enter the ankle joint just lateral to the extensor digitorum
communis tendons. -
Enter the subtalar joint either just
below the tip of the lateral malleolus or at the level of the sinus
tarsi. The sinus tarsi is found just below and anterior to the lateral
malleolus. If a septic arthritis of this joint is noted, the normal
“pitting” landmark of the sinus tarsi may be replaced by bulging
synovium. -
Approach the small joints of the foot and toes in a fashion similar to that used for the small joints in the hand.
various joints remain controversial, there is general agreement that
deeper joints such as the hip and sacroiliac joints, which are less
accessible to aspiration, should be treated surgically. Another
indication for surgical drainage is thick purulence or loculations
within the joint. Also, drain surgically any joint that does not
respond to repeated aspirations and appropriate systemic bactericidal
antibiotics within 48 hours.
recommended in the child because of the difficulty in aspiration of
this deeply seated joint, the risk of inadvertent damage to the joint
surface during the procedure, and the risk of avascular necrosis to the
head of the femur. The
avascular
necrosis of the head of the femur may result from compromise of the
retinacular vessels caused by increased intracapsular pressure (35,57,79).
indicated, perform the operation promptly. The aims of surgery are to
debride the joint thoroughly; excise all dead, infected, nonviable
tissue; and assess the articular surface with respect to the possible
need for future reconstructive procedures. Some investigators believe
that a concomitant synovectomy is indicated, especially if there has
been any delay in diagnosis or a poor response to antibiotic treatment
and drainage by closed means (25,112).
In one study, synovectomy helped prevent late joint destruction in
cases of septic arthritis involving the knee joint but not the hip (112).
At a minimum, the joint should be thoroughly debrided of all nonviable
tissue. During arthrotomy, always take appropriate specimens for
microbiologic and pathologic studies.
complete inspection of the joint. We believe that the joint should not
be left open to the environment for fear of colonization of the
dressings (and joint) with other organisms. We close the debrided joint
over suction tubes, which are removed after several days, depending on
the patient’s clinical response and the amount and type of drainage.
-
Occasionally, suction drainage has been
combined with an irrigation system, particularly when a great deal of
thick, purulent material has been excised (16,50). -
Depending on which joint is involved,
establish a suitably sized inflow catheter on one side of the joint,
and one or two larger bore outflow catheters on the opposite side of
the joint. (For example, a central venous pressure tubing or small
Hemovac drain may be used for inflow irrigation of the knee joint, and
a larger bore Hemovac drain may be used for outflow.) Place the drains
far enough apart so that distention of the joint and circulation of the
fluid will be accomplished. -
Keep the system entirely closed, and secure all tubing joints with adhesive tape.
-
A physiologic saline solution may be
infused at a rate sufficient to provide a constant outflow. Antibiotics
need not be added to the irrigant because sufficient joint levels are
attained with systemic antibiotics (73,98,99,100,101 and 102) -
Leave the irrigation tubes in place for 2
to 3 days and then remove them. Leave the effluent tubes in place for
approximately 24 hours after the inflow tubes are removed. -
These time periods have been selected
because of experimental evidence that has demonstrated changes in the
articular cartilage glycosaminoglycan staining after 3 days of constant
saline irrigation (50); these changes reflect
deprivation of the nutrients in the synovial fluid that normally bathes
the articular cartilage. Furthermore, if drains are left in much longer
than several days, they may serve as a portal of entry for bacteria and
may contribute to the formation of a synovial fistula (59,115).
with systemic antibiotics to debride infected joints, most notably the
knee. Although most of the reported series are small, this method
appears to allow adequate drainage and visualization of the joint with
minimal morbidity (23,47,48).
If the joint is to be left open, then dependent drainage can be
facilitated by the surgical approach selected and appropriate
postoperative positioning techniques. We routinely close arthrotomy
incisions and use suction (with or without irrigation), so the actual
approach may be of less importance because joint dependency is not
necessary.
-
The shoulder
joint may be decompressed either anteriorly or posteriorly; we usually
select the anterior approach. We use a deltopectoral approach and
identify the shoulder joint beneath the subscapularis muscle, which is
reflected medially, allowing a cuff of tendon to remain on the lesser
tuberosity for closure. -
The elbow
joint is most easily debrided posterolaterally through an oblique
incision between the extensor carpi ulnaris and anconeus muscles. If a
posteromedial approach is selected, identify and protect the ulnar
nerve. -
Approach the wrist joint and carpus
dorsally, with the forearm pronated. Make a central incision and enter
the wrist joint between the third (extensor pollicis longus) and fourth
(extensor digitorum communis) compartments. Reconstruct the extensor
retinaculum before closure. -
Use a midlateral or midmedial longitudinal approach for the finger joints. Avoid injury to the digital nerves and vessels.
-
Arthrotomy of the hip
joint may be performed through a number of different approaches. We
prefer the standard anterior iliofemoral approach in children and the
posterolateral, gluteus maximus–splitting approach in adults. If the
hip joint is to be left open, we recommend the posterolateral approach
because it allows dependent drainage with the patient supine. A child
may be placed in the prone position after an iliofemoral approach. -
For the knee,
either the anteromedial or anterolateral parapatellar approach allows
excellent surgical decompression and visualization of the knee joint.
The anteriomedial approach is most often used. Use the posteromedial
approach just posterior to the medial collateral ligament if dependent
drainage is required. -
The ankle
joint may be exposed by numerous approaches (anteromedial,
anterolateral, posteromedial, or posterolateral). We use the
anterolateral exposure lateral to the extensor digitorum longus) or
posterolateral exposure (between the fibula and the Achilles tendon)
most often. In the posterolateral approach, identify and protect the
sural nerve. This approach allows dependent drainage in the supine
position if the wound is left open. -
Approach the small joints in the foot and toes dorsally (talus) or midlaterally or midmedially (toes), through exposures similar to those used in the hand.
-
In each case, thoroughly irrigate,
debride, inspect, and loosely close the joint over suction tubes.
Irrigation tubes may be added if the purulent material is thick. Close
skin and subcutaneous tissues loosely. If the infection is particularly
worrisome (loculated with thick pus, severe joint destruction), leave
the joint open to granulate. Change dressings three to four times per
day for open wounds, using hydrogen peroxide or another antiseptic
solution.
position and begin active assisted and gentle passive range-of-motion
exercises when the inflammatory response subsides, usually after 24 to
48 hours. We have not used postoperative continuous passive motion, but
experimental evidence suggests that this modality may prove useful in
maintaining joint range of motion and minimizing loss of cartilage
glycosaminoglycan (95). Certainly, prolonged
immobilization appears to be contraindicated because it leads to
intra-articular adhesions, more cartilage destruction, stiffness,
atrophy, and poor rehabilitation.
weight bearing until inflammation has ceased, range of motion is pain
free, and rehabilitation is well under way. In general, do not perform
extensive bony or soft-tissue reconstructive procedures during the
acute phase of septic arthritis; postpone these procedures until the
infection has resolved and the joint has been fully rehabilitated.
The immediate Gram stain of the synovial fluid aspirate may identify
specific microorganisms, which will determine the choice of
antibiotics. If no organisms are seen on Gram’s stain but pus cells are
identified in the aspirate, administer antibiotics according to the
“best guess principle,” as described later. Continue them until the
results of the synovial aspirate and blood cultures become available,
which is usually within 24 to 48 hours.
antibiotics are generally preferred over bacteriostatic agents.
Intra-articular injection of antibiotic is not necessary because
adequate levels are achieved in synovium and bone with parenteral use;
also, a chemical synovitis may result from direct intra-articular
inoculation (31,71,73,98,99,100,101 and 102).
In difficult cases, the blood and synovial fluid may be monitored to
ensure that antibiotic levels are above the minimal inhibitory
concentration for a specific microorganism.
patient with septic arthritis with antibiotics. In general, we treat
with parenteral antibiotics until systemic toxicity and local swelling
are under control. We monitor the white blood cell count and the ESR in
peripheral blood, and perform repeated synovial fluid aspirations in
patients treated with needle drainage. We culture the aspirate every 1
to 3 days and perform a Gram stain. A glucose concentration, white
blood cell counts, and polymorphonuclear leukocyte counts may be
performed as well. We continue antibiotics orally an additional 2 to 3
weeks after the course of parenteral antibiotics. This may be done on
an outpatient basis with close supervision. Longer courses are
recommended for patients with a concomitant osteomyelitis, for
gram-negative infections, multiple microorganisms, or in the
immunocompromised patient. We frequently confer with our infectious
disease colleagues, especially in the more complex situations.
stain, staphylococcal or streptococcal species are the usual
microorganism. We use a semisynthetic, penicillinase-resistant
penicillin (e.g., nafcillin, oxacillin, cloxacillin; 8 to 12 g/day,
given every 4 to 6 hours in adults; 150 to 200 mg/kg daily, given in
four to six divided doses in children) until cultures are available.
Alternate choices include a cephalosporin (e.g., cefazolin, 1 to 2 g
intravenously every 6 hours in adults; 100 to 200 mg/kg daily in
children), or vancomycin (2 g/day in divided doses in adults; 40 mg/kg
daily in two divided doses in children). We continue the
above-mentioned regimen if penicillinase-producing staphylococci are
cultured. In penicillin-sensitive cases (e.g., some S. aureus
and streptococcal infections), we switch to penicillin G (10 million
units, given in four divided doses in adults; 100 to 200 mg/kg daily,
given in six divided doses in children). Vancomycin may be substituted
in patients who are allergic to penicillin.
usually signify gonorrheal infection. We recommend the use of
parenteral penicillin therapy (penicillin G, 10 million units daily, in
four divided doses). The patient can be switched to oral penicillin
therapy when signs and symptoms abate, for a 2-week course.
Spectinomycin is recommended in patients with penicillinase-producing
gonococcal infection (89). Third-generation
cephalosporins can be extremely effective, and long-acting agents such
as ceftriaxone are being used with increasing frequency.
infection. Whereas we used to give a combination of ampicillin and
chloramphenicol, these drugs are presently less used because of
ampicillin resistance and the very close monitoring of serum
concentrations and side effects that is necessary with chloramphenicol.
At present, we use cefuroxime, a second-generation cephalosporin (100
mg/kg daily, given every 8 hours). Other alternatives include
cefotaxime and ceftriaxone. The clinical response is monitored closely,
and a final choice is made when cultures are available.
aspirate require immediate treatment and close observation. Look for
infection of the urinary tract and biliary tract, for generalized
debilitating disease, and for the possibility of drug abuse (28,90,103,104,116).
We use tobramycin (5 mg/kg daily, given in three divided doses),
gentamicin (same dose), or amikacin (15 mg/kg daily) until the cultures
are returned. Ticarcillin (300 mg/kg daily given every 4 to 6 hours) is
frequently prescribed by our infectious disease colleagues in addition
to one of the above-mentioned drugs, especially for Pseudomonas
infections. If aminoglycosides are used, monitor renal and
vestibuloauditory function, at least on a weekly basis. Obtain
antibiotic peak and trough blood levels in the first few days and
repeat later if clinical response is not appropriate, or if
aminoglycosides are used.
bacteria, a “best guess” must be made until the cultures return. The
physician must consider the patient’s age, associated disease, and
immunocompetence. In the neonate, the most common bacteria causing
septic arthritis include gram-positive cocci (staphylococcal and
β-streptococcal species) and gram-negative rods (70,104).
Until the cultures are available, a semisynthetic
penicillinase-resistant penicillin should be combined with tobramycin,
gentamicin, or amikacin. In infants and children from 6 months to 3
years of age, S. aureus and H. influenzae
are the most likely pathogens. In such cases, we generally use
cefuroxime either alone or in combination with nafcillin. The results
of the cultures and sensitivities will dictate which drug will be
continued. In older children and adults, a semisynthetic
penicillinase-resistant penicillin active against S. aureus
or a cephalosporin may be used. In the immunocompromised host, we
combine an anti-staphylococcal agent with tobramycin, gentamicin, or
amikacin. A consultation with an infectious diseases specialist can be
very helpful.
may prove useful for the continuation of oral therapy, after a course
of intravenous antibiotics with another drug. Ciprofloxacin is
bactericidal against most gram-negative aerobic bacteria (including P. aeruginosa), some strains of S. aureus and S. epidermidis,
and enterococcus. Ciprofloxacin has poor activity against anaerobes.
Perform sensitivity testing. This drug is a useful adjunct because it
requires oral administration only twice per day. It is not recommended
for children. Absorption of ciprofloxacin is markedly decreased by
aluminum- and magnesium-containing antacids. Ciprofloxacin
administration also increases the serum theophylline level in patients
taking these drugs simultaneously. Monitor serum theophylline levels
closely.
combination therapy is most commonly prescribed, including two or more
of the following: isoniazid (300 mg/day in adults; 3 to 5 mg/kg daily
in children), ethambutol (1.5 to 2 g/day in adults; 15 to 25 mg/kg
daily in children), and rifampin (600 mg/day in adults; 10 mg/kg daily
in children). Pyridoxine is given to prevent neuritis if isoniazid is
used. Liver function should be closely monitored with isoniazid, and
one should be aware of the lupus syndromes associated with its use.
Thrombocytopenia, hepatitis, and flulike syndromes may be associated
with the use of rifampin, and visual disturbances may be associated
with ethambutol. Continue therapy for at least 18 months. Other drugs
that may be useful include streptomycin and aminosalicylic acid.
(0.6 to 1.0 mg/kg daily) must be performed with great caution because
of the toxicity of this drug. Monitor renal, hematologic,
gastrointestinal, and other side effects closely.
accompanies the signs of inflammation, which may lead to pathologic
subluxation or dislocation, or the development of contractures (79).
After adequate drainage and the institution of appropriate antibiotic
therapy, position the limb to encourage retention of the joint in a
position of function while avoiding subluxation or the development of
contractures. Position the hip joint in abduction and neutral rotation.
We have used skin traction in a Thomas splint or a hip spica cast to
maintain this position. After drainage, position the knee in full
extension and place the ankle in neutral dorsiflexion and plantar
flexion. A long- or short-leg plaster cast works well for the knee and
ankle. After drainage of the shoulder, a sling, a collar and cuff, or
Velpeau dressing provides adequate immobilization. We recommend
splintage of the elbow in a 90-degree cast, with the forearm in neutral
or mild supination. Splint the wrist in slight dorsiflexion and place
the hand in the functional “apple-holding position.”
septic arthritis is not a new one. Before the use of antibiotics,
Willems in 1919 advocated surgical decompression
and early active motion for septic joints (116). This concept was further put to use by Ballard et al. (8),
who combined arthrotomy, systemic antibiotics, and early, active
range-of-motion exercises in the treatment of septic arthritis of the
knee. They demonstrated an 82% fair or good result rate despite a
difficult population composed primarily of prior treatment failures.
Perhaps the most elegant experimental study was performed by Salter’s
group (95). Using a model of staphylococcal
septic arthritis of the rabbit knee, they combined arthrotomy and
antibiotic treatment with either plaster immobilization of the knee
joint, cage activity, or continuous passive motion (CPM) on a specially
designed machine. The CPM group fared the best, showing decreased
cartilage ground substance losses compared with the other treatment
methods. Our current treatment protocol emphasizes proper splintage
during the early stages of treatment, with the institution of active
assisted and gentle passive range-of-motion exercises after 24 to 48
hours, when the inflammation and pain subside. We delay weight bearing
on septic joints of the lower extremity until range of motion and
strength are virtually normal.
emphasized that “every hour that an acute suppurative process continues
within a joint is of urgent significance to prognosis” (79).
Lloyd-Roberts agreed that Paterson did “not exaggerate the sense of
urgency required of us when confronted by either the certainty,
probability or even the possibility of this affection” (57).
correlation between the length of time from onset of symptoms to
documentation of a sterile joint and the quality of the outcome (7,29,31,39,58).
Other factors associated with a poor outcome include immunodeficiency
in the host (e.g., malignancy, rheumatoid arthritis, or prematurity in
neonates), concomitant osteomyelitis, infection involving the hip joint
or any prosthetic joint, the presence of positive blood cultures, and
infection with S. aureus or multiple organisms, especially anaerobes or gram-negative rods (40),
symptoms greater than 1 week before treatment, involvement of more than
four joints, and positive cultures with repeat aspiration after 7 days
of antibiotic treatment (107). In general, gonococcal arthritis has a much better prognosis if treatment is instituted promptly (5,17,24,42,51,76,109).
death, variable destruction of the joint with residual joint stiffness
and functional limitation, subluxation and dislocation, avascular
necrosis, local growth disturbance (Fig. 134.3), osteomyelitis, and postinfection synovitis (5,7,21,29,39,57,58,72,79,92,106).
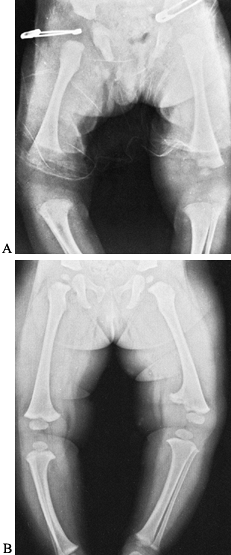 |
|
Figure 134.3. A:
Radiograph of lower extremities in a 2-year-old child with a painful left knee of 2 months’ duration. The child was febrile. Notice the soft-tissue swelling over the left distal thigh. Aspiration of the knee joint demonstrated Staphylococcus aureus. B: This septic arthritis of the left knee led to premature closure of the medial half of the left distal femoral epiphysis, resulting in growth abnormality and a varus deformity of the left knee. |
Avascular necrosis, sequestration and absorption of the femoral capital
epiphysis, chondrolysis of the articular cartilage, destruction of the
epiphyseal plate with growth disturbance or arrest, and subluxation or
dislocation of the epiphysis or entire hip joint can occur. These
complications have a profound effect on a child of any age and may
herald a long course of repeated reconstructive surgeries. In the
adult, degenerative arthritis with fibrous or bony ankylosis may be the
result. These complications may occur in virtually any septic joint but
are most profound in the hip (Fig. 134.4).
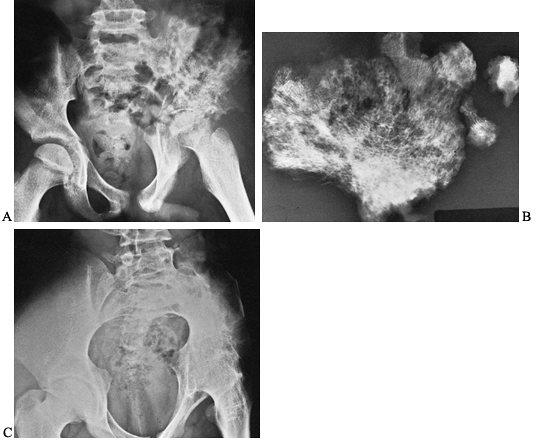 |
|
Figure 134.4. A 16-year-old patient developed severe pain in the left hip girdle and a temperature of 104°F (40°C). A: He developed osteomyelitis of the left iliac wing, as well as septic arthritis of the left hip joint. B: A sequestrectomy of the left ilium was performed and the wound was packed open. C:
The patient’s extremity was placed in a hip spica cast, which resulted in bony ankylosis of the left hip. (Note: This case is from 1939, the preantibiotic era). (Radiographs courtesy of Dr. Henry Jones, Radiology Department, Stanford University Medical Center.) |
of microorganisms from the joint. This complication is most common
after intra-articular antibiotic injection (5). Nonsteroidal anti-inflammatory drugs may aid in the treatment of this puzzling residuum.
emphasizes the need for a heightened index of suspicion of this disease
and the immediate institution of appropriate diagnostic and treatment
procedures.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
SG, Ward LR. Acute Hematogenous Osteomyelitis Progressing to Septic
Synovitis and Eventual Pyarthrosis: The Vascular Pathway. Arthritis Rheum 1978;21:968.
I, Reza MJ, Bricknell KS, et al. Abnormalities in Synovial Fluid of
Patients with Septic Arthritis Detected by Gas Liquid Chromatography. Ann Rheum Dis 1980;39:168.
EL, Metyger WI, Mitra RN. The Treatment of Pyogenic Bone and Joint
Infections by Closed Irrigation (Circulation) with a Non-toxic
Detergent and One or More Antibiotics. J Bone Joint Surg 1967;49-A:614.
A, Masai AT. Acute Infectious Agent Arthritis (IAA): A Detailed
Comparison of Proved Gonococcal and Other Blood Borne Bacterial
Arthritis. J Rheumatol 1974;1:13.
DL, Brandt KD, Cohen AS, Cathcart ES. Treatment of Septic Arthritis.
Comparison of Needle Aspiration and Surgery as Initial Modes of Joint
Drainage. Arthritis Rheum 1975;18:83.
DL, Cohen AS. Acute Infectious Arthritis: A Review of Patients with
Non-gonococcal Joint Infections (with Emphasis on Therapy and
Prognosis). Am J Med 1976;60:369.
DL, Cohen AS. Synovial Membrane Histopathology in the Differential
Diagnosis of Rheumatoid Arthritis, Gout, Pseudogout, Systemic Lupus
Erythematosus, Infectious Arthritis and Degenerative Joint Disease. Medicine 1978;57:239.
I, Valtonen M, Korhola O, Hekali P. Low-field MR Imaging for the
Assessment of Therapy Response in Musculoskeletal Infections. Acta Radiol 1995;36:220.
D, Treves ST, Kasser JR, et al. Osteomyelitis and Septic Arthritis in
Children: Appropriate Use of Imaging to Guide Treatment. Am J Roent Ray Soc 1995;165:399.
RG, Herbert MA, Wright S, et al. The Response of Articular Cartilage to
the In Vitro Replacement of Synovial Fluid with Saline. Clin Orthop 1983;174:285.
R, Rosenthal L. Observations on the Sequential Use of 99m Tc-Phosphate
Complex and 67Ga Imaging in Osteomyelitis, Cellulitis, and Septic
Arthritis. Radiology 1977;123:123.
J, Peterson PK, Simmons RL, Najarian JS. Mycobacterial Infections in
Renal Transplant Recipients: Seven Cases and a Review of the
Literature. Arch Intern Med 1982;142:888.
JR, Root HS, Kim SO, Johnson LG. Staphylococcus Suppurative Arthritis
Occurring in Neuropathic Knee Joints: A Report of Four Cases with a
Discussion of the Mechanisms Involved. Arthritis Rheum 1965;8:389.
GJ Jr, Schlegelmilch JG, Spiegel PK. Early Diagnosis of Septic
Arthritis of the Sacroiliac Joint by the Use of Computed Tomography. J Rheumatol 1981;8:979.
JP, Goldenberg DL, Rice PA. Disseminated Gonococcal Infection: A
Prospective Analysis of 49 Patients and a Review of Pathophysiology and
Immune Mechanisms. Medicine 1983;62:395.
RA, Kennett R. Use of Monoclonal Antibodies in an Enzyme-linked
Inhibition Assay for Rapid Detection of Streptococcal Antigen. J Pediatr 1980;97:540.
W, Leers WD, Wardlow AC. Bacteriolytic and Bactericidal Activity of
Sera and Synovial Fluid in Rheumatoid Arthritis and Osteoarthritis. Arthritis Rheum 1974;17:207.
RB, Bell RS, Keeley FW. The Protective Effect of Continuous Passive
Motion on Living Articular Cartilage in Acute Septic Arthritis: An
Experimental Investigation in the Rabbit. Clin Orthop 1981;159:223.
