INSTABILITY AND TENDON DISORDERS OF THE ELBOW
IV – SPORTS MEDICINE > Elbow > CHAPTER 81 – INSTABILITY AND
TENDON DISORDERS OF THE ELBOW
flexion and extension, the ligament restraints and tendon insertions
are subject to significant stress during the course of athletic
activities. Whereas daily activities depend on the upper extremity for
lifting and positioning, athletic activities require specific and
precise motions for propulsive activity. Interaction between static
restraints and the dynamic muscle unit allows for the versatile motion
and power required in competitive performance. Most injuries to the
elbow in the athletic population occur with overuse and repetitive
activities, though acute traumatic injuries
may occur (4,9,12,31,56).
Typically, chronic repetitive stress injuries to the elbow occur during
the valgus stresses imparted to the elbow in the course of athletic
endeavors. The valgus forces seen during athletics produce tension
overload to the medial soft-tissue restraints, compression injuries to
the lateral radiocapitellar joint, and extension overload injuries
within the posterior compartment (1,2,51,61).
While less frequent in incidence, soft-tissue instability of the
lateral capsule (lateral and posterior lateral instability) may occur (49).
source of elbow pain in both the athletic and the nonathletic
populations (23,24,30,54,55). Although the term tennis elbow
has become accepted as a descriptive and diagnostic term, it is
something of a misnomer as it occurs more commonly in the nonathletic
population.
radial tuberosity, and avulsion of the triceps from the olecranon are
relatively rare (3,6,7,11,16,40,45).
Failure to diagnose and treat these two injuries may lead to
significant disability, not only during athletic activity but with
activities of daily living.
understanding of the anatomy, pathophysiology, and clinical evaluation
are necessary for an accurate diagnosis. While rehabilitation is
usually successful in treating elbow tendon and ligament disorders,
surgical alternatives now exist for those cases not responsive to an
appropriate nonoperative treatment program.
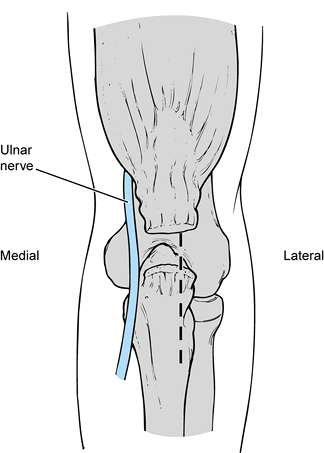
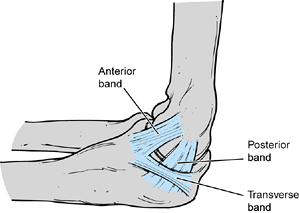
The anterior band, which is composed of two distinct bundles, is the
most important restraint to valgus stress across the elbow. The ulnar
collateral ligament is uniquely configured to provide stability
throughout the full range of motion (ROM) of the elbow. The weaker
posterior band, which is fan shaped, provides stability primarily
beyond 90° of elbow flexion. The transverse ligament does not cross the
ulnohumeral joint but exists as a thickening of the inferior joint
capsule. The lateral collateral ligament complex is less consistent and
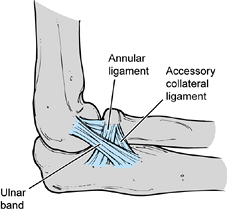
not as well understood as the ulnar collateral ligament (Fig. 81.2).
The annular part of the lateral collateral ligament originates from the
lateral epicondyle and blends with the fibers of the annular ligament.
The ulnar band of the lateral collateral ligament originates from the
anteroinferior portion of the lateral epicondyle, crosses the
radiocapitellar joint, and inserts on the tubercle of the supinator
crest of the ulna.
 |
|
Figure 81.1. Medial collateral ligament.
|
 |
|
Figure 81.2. Lateral collateral ligament.
|
By inserting on the radius, the biceps acts as a supinator first and,
second, flexes the elbow. With the arm pronated, the biceps is weaker
because its functional leverage is less. The biceps also acts to
decelerate elbow extension. The medial head of the triceps is most
active during elbow extension, with the lateral and long head of the
triceps supplementing the extension force. There is increased activity
of the triceps with increasing elbow flexion resulting from its
secondary action as a decelerator and from an increasing stretch reflex.
and the pattern of responding muscles. Maximal elbow flexion strength
occurs at 90°, with decreasing force during extension (49,61). Because the flexor muscles have
such a poor mechanical advantage with the elbow in relative extension,
the isometric forces have to be greatest in this position. Flexor
muscles introduce a posterosuperior compressive force across the distal
humerus.
 |
|
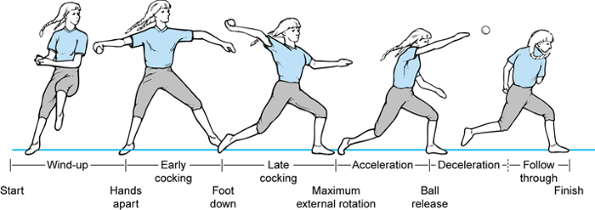
Figure 81.3. Phases of the baseball pitch.
|
abduction and external rotation that begins as the ball is released
from the nondominant hand and terminates with foot–ground contact.
until maximal external rotation at the shoulder is attained. Maximal
valgus forces occur across the elbow during this stage.
In early and late cocking, muscle activity is of moderate intensity.
Acceleration is an explosive, short stage with reduced muscle activity
in follow-through. The low to moderate activity indicates that the
muscle groups act primarily to position the arm to accept energy
transfer from the upper arm and trunk.
ulnar collateral ligament insufficiency has been compared to that in
pitchers without injury (32). Differences were
seen during the late cocking and acceleration phases, which place the
greatest stress on the ulnar collateral ligament. The extensor carpi
radialis brevis and longus showed increased activity in the injured
pitchers. The triceps, flexor carpi radialis, and pronator teres all
showed less activity in the injured pitchers. One would expect enhanced
muscle activity if the flexor carpi radialis and pronator teres were
substituting for the deficient ulnar collateral ligament and
functioning as dynamic stabilizers. However, the opposite was found.
This pattern of asynchronous muscle activity may predispose the joint
to further injury and attenuation of the ulnar collateral ligament.
ulnar collateral ligament, the anterior joint capsule, and the bony
articulation of the radiocapitellar joint when the elbow is in full
extension (2,61,62,65).
When the elbow is in 90° of flexion, the anterior bundle of the ulnar
collateral ligament serves as a primary constraint of the elbow during
valgus stress. Release or attenuation of the anterior portion of the
ulnar collateral ligament leads to valgus laxity even when the radial
head is present. The radial head is a secondary stabilizer that resists
valgus and rotatory stresses when the anterior bundle of the ulnar
collateral ligament is absent.
is provided by the bony architecture, the anterior joint capsule, and
the lateral collateral ligament complex (2,49).
The ulnar band of the lateral collateral ligament is the major
stabilizer both in varus stress and in posterior lateral rotatory
stress across the elbow joint.
Overhead athletic activities generate significant valgus forces that
are transmitted to the stabilizing soft tissues about the medial aspect
of the joint. These stresses are transmitted primarily to the ulnar
collateral ligament complex, the flexor carpi ulnaris, and, to a lesser
extent, the flexor–pronator muscle group. Poor throwing mechanics
and/or muscle fatigue may lead to muscular dysfunction, allowing
further stresses to be transmitted to the ulnar collateral ligament (4,37).
When these forces are applied at a rate that exceeds tissue repair,
progressive microscopic tearing and attenuation occurs within the
ligament. Initially, this produces swelling and inflammation associated
with pain and tenderness about the medial aspect of the joint. Further
stresses from throwing may attenuate the ligament or cause further
degeneration of the ligament fibers.
typically continues to attenuate, yielding clinically significant
instability. With time, recurring and progressive medial tension
overload creates strong compressive forces over the lateral and
posterior aspects of the joint. This may produce transient synovitis
and osteochondral injuries, including osteochondritis dissecans and
frank osteochondral fracture. Lateral compression stresses typically
injure the capitellum, although radial head injuries may occur.
throwing motion, extension injuries may occur within the posterior
compartment (5,38).
Repeated stresses may lead to a triceps muscle strain, avulsion of the
tip of the olecranon process, hypertrophic olecranon spur formation,
and posterior impingement.
complex are uncommon and usually the result of an acute traumatic
injury such as an elbow dislocation or a hyperextension injury (49,51,57,59).
Lateral collateral ligament laxity rarely occurs after isolated varus
stress to the elbow. A complete elbow dislocation is by far the most
common cause for chronic lateral ligamentous insufficiency. It may also
develop from elbow subluxations that may or may not be associated with
fractures of the radial head. Iatrogenic lateral collateral ligament
laxity may occur during release of the common extensor tendon for
lateral epicondylitis or radial head excision with injury of the
lateral collateral ligament complex.
With repetitive trauma, this process results in mucoid degeneration and
reactive granulation within the tendinous origin of the extensor carpi
radialis brevis. It has been shown that this reactive granulation
tissue contains a large number of free nerve endings and may lead to
pain production with tension or compression. Nirschl and Pettrone (54)
have described the histology as “angiofibroblastic hyperplasia.” The
normal parallel orientation of the collagen fibers is disrupted by an
invasion of fibroblastic and vascular granulation tissue. The overall
intensity and duration of arm use will influence this tendinitis and
degeneration. Lateral epicondylitis is directly related to activities
that increase the tension and the stresses along the course of the
wrist extensors and supinator muscles.
elbow may cause inflammation of the flexor–pronator muscle mass. The
pronator teres and flexor carpi radialis are the most common sites of
pathologic changes that include tissue overload with resultant
microscopic tearing, tendinitis, and degeneration.
The majority of ruptures involve the dominant extremity and usually
result from a single traumatic event with an intense biceps contraction
overwhelmed by an unanticipated extension force. Most injuries occur
when lifting, pulling, or straining with heavy objects. Chronic
degeneration of the tendon of the radial tuberosity has been seen with
both partial and complete rupture, and it has been postulated that
chronic inflammation of the bursa at the radial tuberosity may
contribute to degeneration of the biceps tendon, making the tendon more
susceptible to rupture.
This occurs as a result of deceleration stresses superimposed on a
contracting triceps muscle with or without a concomitant blow to the
posterior aspect of the elbow. Most commonly, rupture occurs as the
result of an abrupt, forceful, eccentric contraction of the triceps.
-
Ulnar collateral ligament tendinitis and/or insufficiency
-
Medial epicondylitis with a strain of the flexor–pronator muscle mass
-
Ulnar neuritis
pain with overhead activities and valgus stress will affect the ulnar
collateral ligament. As the ulnar collateral ligament attenuates, there
may be traction on the cubital tunnel producing secondary symptoms of
ulnar neuritis. Primary ulnar neuritis in the overhead athlete is rare
but can occur, especially if there is a bony spur in the cubital tunnel
or a subluxating ulnar nerve that causes the nerve to become scarred
and irritated over time (15). Patients with
medial epicondylitis tend to exhibit pain primarily over the medial
epicondylar origin rather than over the ulnar collateral ligament,
which will tend to be extenuated with resisted palmar flexion of the
wrist and fingers and typically not affected by valgus stress to the
elbow. Patients with medial epicondylitis tend to have a history of
pain associated with forearm and wrist activities such as pushing a
heavy object, or with overuse of the flexor–pronator muscle mass in
athletic activities such as golf. While symptomatic cervical
radiculitis typically is not confused with lateral epicondylitis, the
cervical spine must also be evaluated
to rule out referred radicular pain that occurs isolated to the lateral epicondylar region.
depends on the patient’s history and physical findings. Radiographic
evaluation may provide correlative information but is not the basis by
which a diagnosis is made. Patients with elbow instability typically
give a history of repetitive stress to the elbow, though on occasion a
single event may be causative.
ulnar collateral ligament will describe an insidious but progressive
onset of medial elbow pain. The pain typically occurs during an
athletic endeavor such as throwing a baseball, serving a tennis ball,
or spiking a volleyball. Pain tends to be most noticeable during the
late cocking and acceleration phases of the overhead motion but may
progress over time to include pain during the entire activity, and it
may even progress to include pain with activities of daily living.
Athletes who have had ulnar collateral ligament insufficiency for a
period of time often present with symptoms of associated ulnar neuritis
secondary to traction stresses placed on the ulnar nerve during the
overhead activity (31). Rest from activity will typically relieve the symptoms.
an athletic activity, when the athlete senses a pop or sudden sharp
pain along the medial aspect of the elbow joint. This may be the result
of a valgus stress applied to a previously asymptomatic attenuated
ligament. Acute injuries are typically associated with traumatic events
such as falling on the outstretched elbow and may rarely present as a
postreduction complication following an elbow dislocation.
insufficiency typically occurs along the course of the ulnar collateral
ligament. An athlete may complain of fatigue over time and lack of
normal strength associated with athletic activities. As the
inflammation along the ulnar collateral ligament progresses, athletes
may notice pain and tenderness beyond the ulnar collateral ligament,
extending into the cubital tunnel and flexor–pronator muscle mass. When
the inflammation has progressed to include not only the ulnar
collateral ligament but also the flexor–pronator muscle mass and ulnar
nerve, a very careful assessment is required to help differentiate
between the primary source of the pathology and the secondary problems.
ligament insufficiency typically reveals tenderness along the course of
the ulnar collateral ligament, especially at the mid and distal
portions rather than the proximal origin at the medial epicondyle.
Typically, the examiner will not note tenderness at the medial
epicondyle itself unless there has been an associated inflammation of
the flexor–pronator muscle mass.
unless there has been repeated ulnar neuritis secondary to traction
stresses. When the ulnar nerve has been inflamed repeatedly, there may
be a positive Tinel sign, and in addition there may be other focal
ulnar nerve findings such as intrinsic muscular atrophy and loss of
sensation in the ulnar distribution of the hand (27).
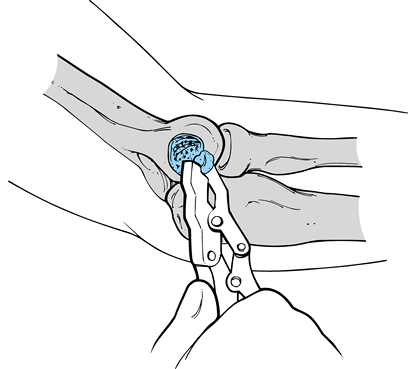
Testing for valgus instability is best performed with the patient
seated and his hand and wrist securely held between the examiner’s
elbow and trunk. The examiner firmly grasps the patient’s elbow and
proximal forearm while simultaneously palpating the ulnar collateral
ligament at the medial joint line. Varus and valgus stress can then be
applied to determine the presence of an end point and the degree of
laxity (Fig. 81.4). It is important during
stress testing that the elbow be flexed beyond 25° to eliminate the
stabilizing effect of the olecranon within the olecranon fossa. It is
also critical to compare the involved with the uninvolved elbow to
differentiate between normal laxity and pathologic instability. Even
asymptomatic overhead athletes tend to have increased medial laxity,
and therefore the physical findings of laxity must be correlated with
the areas of tenderness and the characterization of pain that occurs
with activities. When ulnar collateral ligament insufficiency has been
present over time, there may also be findings consistent with lateral
compression injuries, including swelling,
tenderness,
or even crepitation around the radiocapitellar joint. Chronic ulnar
collateral ligament insufficiency may cause or contribute to valgus
extension overload that affects the posterior compartment, with
findings of tenderness, crepitation, and even loose bodies within the
posterior compartment. Decreased ROM and synovitis may result from the
chronic secondary effects of ulnar collateral ligament insufficiency.
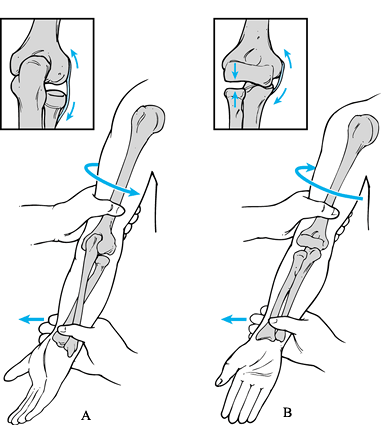 |
|
Figure 81.4. Varus and valgus instability.
|
have symptoms that vary from frank recurrent dislocations to minor
mechanical symptoms from occult subluxations. Patients may complain of
pain, tenderness, catching, snapping, or frank symptoms of instability.
Patients who have recurrent lateral subluxation can be mistakenly
diagnosed as having a recurrent dislocation of the radial head, which
may lead to unsuccessful and improper treatment. The typical patient
has had an initial traumatic injury, such as a sprain, dislocation, or
fracture of the elbow, or she has had surgery involving the lateral
aspect of the elbow. Following this, she will have symptoms of
recurring subluxation that can include clicking, catching, snapping, or
even locking.
to identify these patients. This test involves flexion of the elbow
from an extended position and supination of the forearm while the
valgus stress and axial load is applied. This maneuver subluxates or
dislocates the radial head posteriorly with rotatory subluxation of the
ulnohumeral joint. When the lateral pivot-shift test is positive, there
is a palpable and visible reduction of the radiohumeral joint together
with the ulnohumeral joint when the elbow is flexed beyond 40°. The
pivot-shift test may fail to cause apprehension in patients or may
appear falsely negative in patients with severe instability when the
elbow does not reduce with flexion. Be aware that the varus stress test
may be normal in the presence of a positive pivot-shift maneuver and
clinically significant lateral insufficiency.
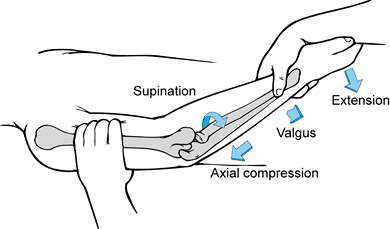 |
|
Figure 81.5. Pivot-shift test.
|
is typically used for this syndrome, most patients develop the symptoms
from activities such as lifting at work, carrying luggage and
briefcases, and typing (30,52,55).
Pain originates over the lateral epicondyle but may radiate into the
proximal forearm overlying the proximal extensor musculature. As the
condition progresses, even light daily activities such as shaking
hands, picking up a bottle, or grasping for a carton of milk may cause
symptoms. During tennis, the lateral epicondylar pain typically occurs
at the point of ball contact during the ground strokes and is more
often noted with backhand than forehand strokes. Differential diagnosis
of lateral epicondylitis includes the supinator syndrome with
entrapment of the posterior interosseous nerve. Typically, these
patients have pain overlying the proximal supinator musculature
exacerbated with activities that include pronation and supination of
the forearm. Usually there will be absence of tenderness along the
lateral epicondyle unless there is combined lateral epicondylitis and
radial nerve entrapment. Differential diagnosis may be difficult
because posterior interosseous nerve entrapment may coexist with
lateral epicondylitis in about 5% of patients. A selected injection of
lidocaine in the lateral epicondyle or the proximal supinator may help
differentiate these two diagnoses. Other causes of lateral elbow pain
include arthrosis, osteochondral injuries to the capitellum, and
lateral collateral ligament insufficiency.
tendon origin at the lateral epicondyle. Resisted wrist and finger
extension will reproduce the patient’s pain along the lateral
epicondyle, and resisted long-finger extension often produces the most
pain. ROM of the elbow is usually normal unless there are other
conditions such as arthrosis or bony elbow impingement. After repeated
cortisone injections (19,20),
there may be atrophy of the skin and subcutaneous tissue overlying the
lateral epicondyle, causing a very prominent bony epicondyle.
medial epicondyle, which is aggravated with resisted palmar flexion of
the wrist and fingers, and pronation with the forearm. The onset of
pain typically occurs in an insidious fashion, although there are cases
that are traumatic in origin and present with acute onset of pain. The
medial epicondyle pain is typically aggravated with activities that
involve stress across the flexor–pronator muscle mass; it usually is
not associated with valgus stress across the joint unless there is an
associated inflammation of the ulnar collateral ligament. Pain along
the cubital tunnel and
ulnar nerve symptoms typically are absent unless there is an associated ulnar neuritis.
origin of the flexor–pronator muscle mass at the medial epicondyle, and
possibly radiation of tenderness into the substance of the
flexor–pronator muscle mass. Resisted wrist flexion and forearm
pronation will recreate the patient’s pain. Elbow ROM is not affected
by this condition unless there are unrelated entities such as
osteoarthritis. While medial epicondylitis usually occurs as an
isolated entity, associated ulnar neuritis may occur and, if it does,
there will be tenderness along the cubital tunnel with a positive Tinel
sign.
of a sudden sharp tearing sensation or pop within the anterior
antecubital fossa. Subsequently, antecubital swelling and ecchymosis
may occur, with pain aggravated by any flexion and supination of the
forearm. The intense pain will change to a persistent dull ache, and
the biceps musculature will retract proximally, yielding an
asymmetrical appearance compared to the opposite extremity. The patient
will complain of weakness and fatigue of the arm, especially with those
activities that require elbow supination (such has using a screw
driver) and repetitive elbow flexion and supination (such as hammering).
reveal deformity of the proximal biceps, but this deformity can be
masked by antecubital swelling and ecchymosis. With palpation, there
may be tenderness along the course of the distal biceps and a defect
may be palpated. An intact lacertus fibrosus can be mistakenly
interpreted as the biceps tendon, but comparison to the opposite
extremity will reveal the differences between the injured arm and the
normal intact biceps tendon. Elbow ROM will not be changed except as a
result of pain at the extremes of motion. There will be decreased
flexion strength and weakness in supination.
history of falling on an outstretched arm with acute pain and a tearing
sensation. Examination reveals loss of the normal posterior contour of
the arm and a palpable defect in a complete disruption. In a partial
tear, tenderness typically is present at the muscle–tendon junction,
but a defect is not palpable. Extension strength of the elbow is
diminished or absent. Assess extension power with the patient seated
and her arm draped over the back of a chair, or with the patient in a
prone position with her forearm hanging over the table. In the normal
extremity, squeezing the triceps muscle belly will produce some elbow
extension, but no motion will occur if there is a complete rupture. If
a traumatic event has not been eliminated as the cause of the triceps
tendon injury, look for other sources such as referred cervical nerve
root lesions.
joint arthrosis, posterior elbow osteophytes, or loose bodies in
particular. Radiographs may identify ossification within the ulnar
collateral ligament, which typically is a sign of chronic inflammation
rather than of an acute injury to the ligament (Fig. 81.6).
Stress radiographs may reveal excessive laxity and medial joint line
opening, especially compared to the opposite elbow. In patients with
ulnar collateral ligament instability, radiographic valgus stress
testing may be used to document the degree of instability when the
diagnosis is in question. MRI may be used as an adjunctive diagnostic
tool, as it will clearly delineate pathology within the ulnar
collateral ligament (Fig. 81.8) (43).
Unfortunately, the magnetic resonance imaging (MRI) scan is not always
capable of delineating the difference between degenerative tendinitis
that is otherwise asymptomatic, and symptomatic tendinitis or a tear
with associated laxity. An MRI scan revealing a normal ulnar collateral
ligament (Fig. 81.7) can provide impetus to search for a different diagnosis. Arthrography is not useful
because of frequent false negative findings in cases of chronic ulnar collateral ligament insufficiency with an intact capsule.
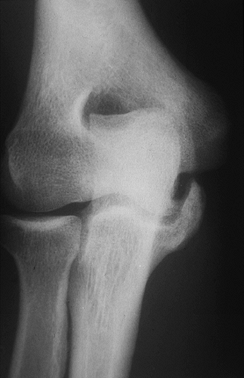 |
|
Figure 81.6. Ulnar collateral ligament calcifications.
|
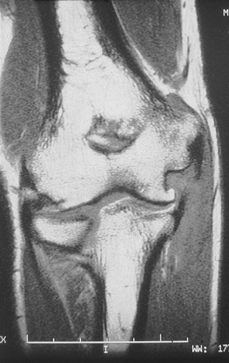 |
|
Figure 81.7. MRI showing normal ulnar collateral ligament.
|
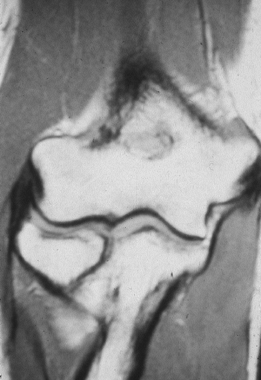 |
|
Figure 81.8. MRI showing a tear in an ulnar collateral ligament.
|
instability is typically unrevealing except for associated and
unrelated bony pathology. Varus stress to the elbow typically does not
reveal insufficiency, even when present. The presence of asymmetrical
joint opening under radiographic varus stress, when present, may help
to confirm the findings of examination.
typically are normal, though approximately 20% to 25% may show
calcification within the soft tissues about the lateral epicondyle (17,44).
The occurrence of this calcification has no prognostic implication.
These and other radiographic modalities such as MRI do not appear to
add further diagnostic information (Fig. 81.9).
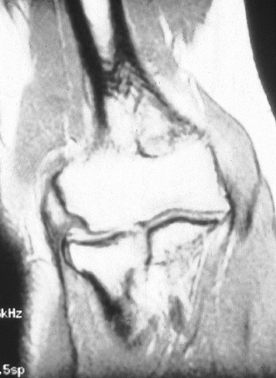 |
|
Figure 81.9. MRI demonstrating lateral epicondylitis.
|
epicondylitis typically are normal. Throwing athletes may have an
associated ulnar traction spur and calcification within the ulnar
collateral ligament.
irregularity of the biceps tuberosity, suggesting an underlying
degenerative etiology. Elbow radiographs in those with a triceps
avulsion may reveal a small avulsion fracture from the olecranon. It
has been suggested that the
“flake sign” will be present in two thirds of cases with a complete triceps rupture (48,64).
When the physical examination is not revealing or is confusing due to
significant swelling, an MRI scan may be valuable in differentiating
between an incomplete and a complete tear of the triceps or biceps
muscle tendon unit (Fig. 81.10). MRI is not necessary when complete rupture of either the biceps or the triceps tendon is clinically obvious.
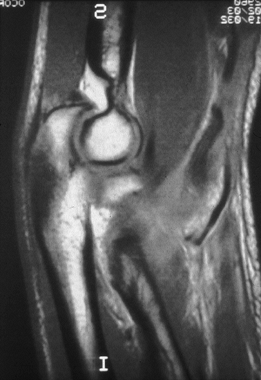 |
|
Figure 81.10. MRI showing a torn distal biceps tendon.
|
activities), nonsteroidal anti-inflammatory drugs (NSAIDs), and other
modalities (e.g., ice) are the main focus of the initial treatment.
Steroid injections of the ulnar collateral ligament can decrease the
biomechanical strength of the ligament as well as mask clinical
symptoms allowing too early a return to activity. After swelling and
pain have subsided, a flexibility and strengthening program is
completed before return to throwing. While most athletes with ulnar
collateral ligament tendinitis and insufficiency will respond to
conservative treatment, failure to do so after 3 to 6 months of
treatment necessitates surgical intervention (13,22).
Avoidance of valgus stresses avoids aggravation and possible
attenuation of the ligament. Bracing, while theoretically feasible, has
not proved efficacious. Athletes involved in valgus stress activities
such as throwing, tennis, and volleyball are the most likely to fail
nonoperative treatment.
have found that recurrent elbow instability due to lateral collateral
ligament laxity does not improve with nonoperative treatment. As
lateral collateral ligament insufficiency typically interferes with
daily functional activities, early surgery is recommended in most
cases. Less active patients who do not have significant instability may
avoid surgery by modifying activities and using a hinge brace with an
extension block.
with surgery reserved for the minority who do not respond to
conservative treatment (14). Advise
modification or avoidance of all athletic and daily activities that
aggravate symptoms. Useful measures include cryotherapy, NSAIDs, and
counterforce bracing to reduce tensile stress along the epicondyle (55).
A counterforce brace may inhibit full musculature expansion and
therefore decrease the tension developed by the inflamed tendon
proximal to the brace (29,35).
Mix the steroid with a local anesthetic. Avoid direct injection into
the tendon, and inject over the inflamed tendon. If the injection has
been efficacious but symptoms recur, administer a maximum of two to
three injections over a 3-month period. If the etiology results from
athletics, evaluate the biomechanics and equipment used during the
sport. For example, a tennis racquet should be assessed for proper grip
size. Lighter racquets made of low vibration materials and strung less
tightly may dampen the impact forces on the extensor origin. Upon
improvement in symptoms, institute a rehabilitation program to regain
strength and flexibility. As strength, endurance, and flexibility
improve, prescribe progressive eccentric and concentric resisted
exercises. Indications for surgery include failure of a well-managed
conservative program over a period of 3 to 6 months and impairment of
athletic or daily living activities.
some recommend conservative treatment (18).
Patients treated nonoperatively gain improvement in elbow flexion and
supination over time, but they typically have significant loss of both
elbow flexion and supination strength, as well as endurance (7,48).
Isokinetic muscle testing has documented deficits in elbow flexion of
30% to 36% and supination deficits of 40% to 45% following conservative
treatment (50).
accomplished as late as 6 weeks following injury. Mobilization of the
retracted tendon may be required. Late reconstruction has been used for
those who have persistent symptoms, although it is not possible to
reattach the distal biceps tendon to the radial tuberosity. Options for
late reconstruction include direct repair of the biceps tendon to the
brachialis muscle, which helps to restore elbow flexion strength but
will not change the supination strength deficit. Late reconstruction
can also involve an interposition graft such as a facial autograft (36).
do exist and are best diagnosed with an MRI scan. Provided that there
is continuity of tendon to the radial tuberosity, partial tears may be
treated conservatively. In the face of persistent symptoms, especially
with resisted flexion and supination activities, the patient is at
increased risk for eventual rupture of the tendon. Clinical judgment,
with consideration of the severity of the patient’s symptoms and
functional status, dictates whether early surgical intervention or
continued conservative treatment best suits the individual patient.
triceps tendon ruptures. When there is complete rupture of the triceps
tendon mechanism, significant disability occurs without surgical repair
(6,28,63).
Triceps function is also important for the elderly who require the use
of ambulatory aides and transfers because of lower extremity
impairment. In the presence of a partial tear, conservative treatment
may be employed. An MRI scan may be helpful in evaluating the degree of
a partial tear. Persistent symptoms, such as pain and weakness with
attempted elbow extension, despite conservative treatment, are
indications for surgical treatment.
the ulnar nerve was always transferred anteriorly in association with
an ulnar collateral ligament reconstruction. It has been shown,
however, that there is an increased complication rate with
transposition of the ulnar nerve, and therefore I now transfer the
nerve only when clinically indicated. When the patient does not have
chronic ulnar nerve symptoms, leave the ulnar nerve within the cubital
tunnel. This requires a change in the technique for transfixing the
ulnar collateral ligament graft proximally at the medial humerus. When
the patient has clinically significant ulnar neuritis, transfer the
ulnar nerve anteriorly in a submuscular fashion in conjunction with the
ulnar collateral ligament reconstruction.
-
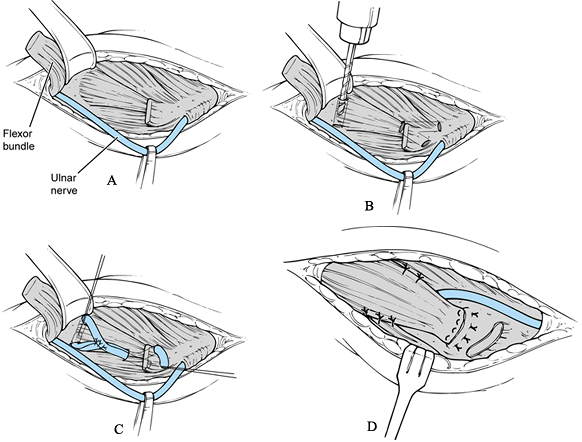
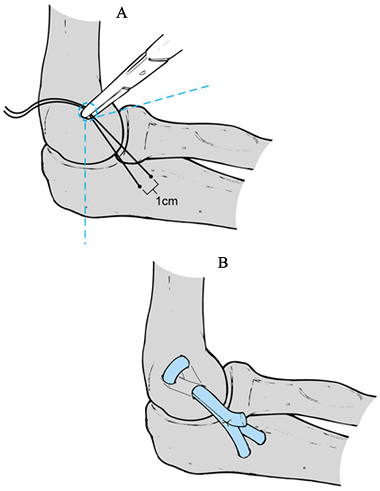
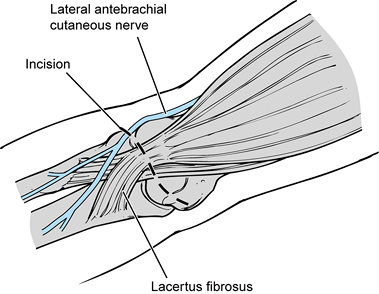
Make an incision just above the medial epicondyle extending proximally 3 cm and distally 5 cm (Fig. 81.11A). Carry the dissection down to the fascia overlying the flexor–pronator muscle mass and overlying the cubital tunnel.
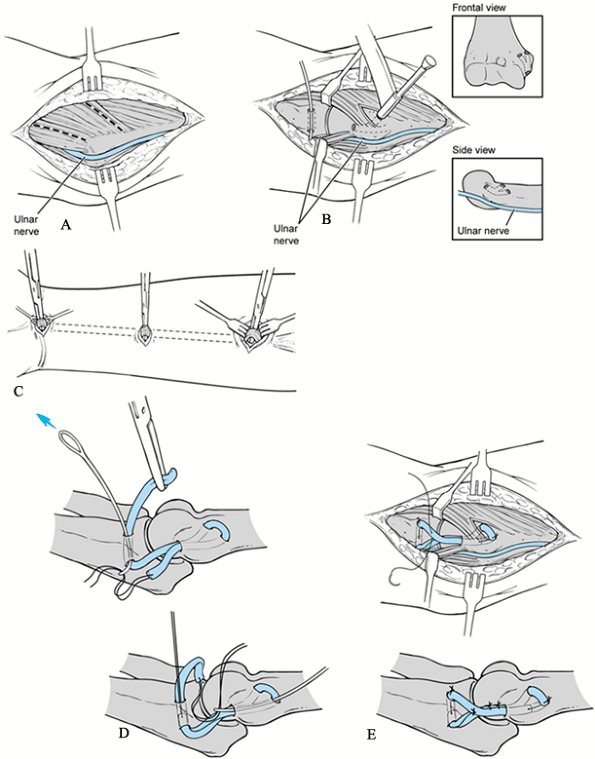 Figure 81.11. Ulnar collateral reconstruction. A: Approach. B: Bone tunnel placement. C: Harvesting of palmaris longus tendon. D: Passage of graft. E: Fixation of graft.
Figure 81.11. Ulnar collateral reconstruction. A: Approach. B: Bone tunnel placement. C: Harvesting of palmaris longus tendon. D: Passage of graft. E: Fixation of graft. -
Protect and identify the underlying
cutaneous branches of the medial antebrachial cutaneous nerve. Injury
of this nerve will cause paresthesias or complete loss of sensation
along the proximal medial forearm. In addition, there is the risk of
developing an underlying neuroma, which could require further surgery.
The cutaneous nerves tend to lie just above the flexor–pronator muscle
fascia and to run in an oblique direction, from proximal lateral to
distal medial, often parallel to the basilic vein. -
Identify the cubital tunnel and, using
palpation, visualize the course of the ulnar nerve along the posterior
aspect of the distal intermuscular septum and through the cubital
tunnel, and its passage into the flexor carpi ulnaris. -
Identify the flexor–pronator muscle mass
and its origin on the medial epicondyle. Split the muscle horizontally
in line with its fibers overlying the ulnar collateral ligament.
Bluntly dissect through the muscle down to the ulnar collateral
ligament and surrounding capsule. Inspect the ulnar collateral ligament
both by observation and palpation. There may be signs of obvious tissue
degeneration, attenuation, or complete disruption of the ligament. Make
a longitudinal incision in the ligament to inspect its substance.
Changes may be revealed that were not apparent from the surface. -
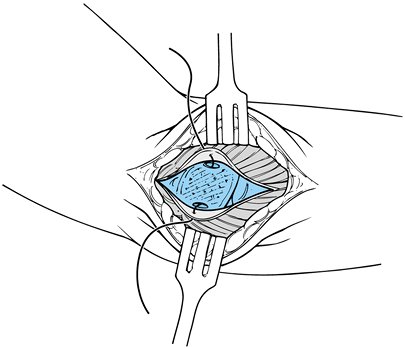
Inspect the joint and determine the
degree of valgus opening. If the ligament is lax, elevate the ligament
and capsule in an anterior and posterior direction in subperiosteal
fashion to gain exposure for subsequent placement of the ulnar
collateral ligament graft into bony tunnels. -
Harvest the palmaris longus tendon graft (Fig. 81.11B). Previous studies (22)
have not demonstrated any clinical difference with respect to the
graft, including ipsilateral arm, contralateral arm, or tendon graft
from the
P.2217P.2218
leg,
such as plantaris or strip of Achilles tendon. When the palmaris longus
tendon graft is present, it is the graft of choice; it is typically
harvested from the ipsilateral extremity unless it is considered to be
significantly smaller then the palmaris longus tendon graft from the
opposite extremity. To harvest the palmaris longus graft, create a 2 cm
transverse skin incision within the distal flexor crease of the wrist.
Protect the median nerve and palmar cutaneous branches as the tendon is
isolated at its insertion into the palmar fascia. Make two additional
transverse incisions overlying the palmaris longus tendon, the most
proximal incision over the muscle–tendon junction and an additional
incision half-way between the muscle–tendon junction and the wrist
crease. Free the tendon from its origin into the palmar fascia and
place a #0 nonabsorbable braided suture through the end of the free
tendon graft. Retrieve the tendon through the middle incision and then
proximally at the muscle tendon junction. Finally, place a #0
nonabsorbable braided suture through the proximal end of the tendon. -
Create bone tunnels proximally and distally (Fig. 81.11C)
with the tunnels having adequate size for the tendon graft. Typically,
a 3.2 mm drill bit makes an adequate bony tunnel for passage of the
graft. Place the converging drill holes at the proximal ulna orientated
in a vertical direction and at the level of the coronoid tubercle just
distal to the articular surface of the proximal ulna. Make the drill
holes proximally at the medial epicondyle at the level of the anatomic
origin of the anterior bundle of the ulnar collateral ligament. Begin
the drill hole midway between the base and the tip of the medial
epicondylar prominence. The holes are fashioned with a single entrance
hole at the medial epicondyle, which then diverges into two separate
anterior-superior and posterior-superior holes more proximally. Drill
the posterior-superior hole to exit posterior to the first
anterior-superior hole, but not in the cubital tunnel. Split the
flexor–pronator muscle mass at a second location just proximal to the
medial epicondyle in a longitudinal fashion to facilitate placement of
the anterior-superior and posterior-superior drill holes. -
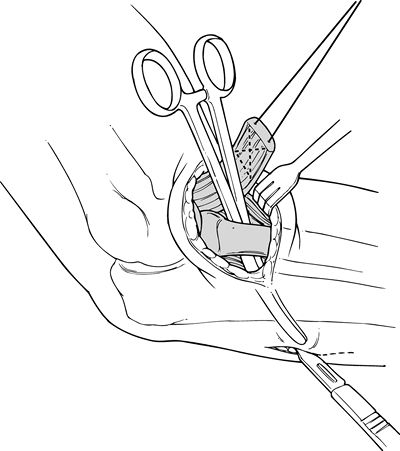
Pass tendon graft through the respective proximal and distal bone tunnels (Fig. 81.11D).
Pull the graft taut and move the joint through a ROM to assess
isometricity and valgus stability. Excess length of the tendon graft
can be routed back through a bone tunnel upon itself to provide more
tissue to overlie the joint. Avoiding valgus stress, pull the graft
taut and suture it to itself with #0 nonabsorbable sutures (Fig. 81.11E) at 45° of elbow flexion. -
Close the remnant of the original medial
collateral ligament and capsule in a side-to-side manner overlying the
tendon graft, imbricating the overlying capsule with a #0 or #1
nonabsorbable suture. -
Release the tourniquet and achieve
hemostasis. Repair the flexor–pronator muscle mass in a side-to-side
manner. Close the wound with careful attention to avoid injury to the
cutaneous nerve. Apply a long-arm posterior plaster splint to
immobilize the elbow in 90° of flexion and neutral forearm rotation
avoiding any valgus stress.
neuritis associated with instability, a decompression of the ulnar
nerve and submuscular ulnar transposition may be performed in
conjunction with the ulnar collateral ligament reconstruction. The
difference in technique requires removal and subsequent repair of
flexor–pronator muscle mass origin at the medial epicondyle.
-
Make a similar skin incision, except that
2–3 cm of proximal extension may be needed to expose the ulnar nerve
and intermuscular septum. Again, protect the branches of the medial
antebrachial cutaneous nerve. -
Unroof the cubital tunnel and free the
ulnar nerve, tracing it proximally to the arcade of Struthers, which
must be released. Mobilize the ulnar nerve using a Penrose drain for
retraction. Dissect the ulnar nerve from the epicondylar groove. Divide
the arcuate ligament and follow the nerve to the interval between the
two heads of the flexor carpi ulnaris. The articular branches will be
sacrificed but preserve the motor branches to the flexor carpi ulnaris
during the distal dissection. Excise the medial intermuscular septum
for 5–8 cm proximal to its attachment to the medial epicondyle to
ensure that the transposed nerve will not rest against its edge and
cause a new site of compression or impingement. Avoid the numerous
vessels that underlie the intermuscular septum and are part of the
extensive collateral circulation of the elbow. -
Detach the flexor–pronator muscle group
1–2 cm distal to the medial epicondyle, leaving the proximal tendon
origin for later reattachment (Fig. 81.12A).
Bluntly elevate the flexor–pronator muscle mass, avoiding injury to the
median nerve and its branches. At this point, palpate and inspect the
underlying ulnar collateral ligament.![]() Figure 81.12. Ulnar collateral reconstruction with ulnar nerve transfer. A: Exposure. B: Bone tunnels. C: Fixation of graft. D: Submuscular ulnar nerve transfer.
Figure 81.12. Ulnar collateral reconstruction with ulnar nerve transfer. A: Exposure. B: Bone tunnels. C: Fixation of graft. D: Submuscular ulnar nerve transfer. -
Incise the ligament and capsule in a
longitudinal fashion as previously described. Explore the joint and
note the degree of valgus laxity and ligamentous pathology. Obtain a
tendon graft in the manner previously described. -
Make converging drill holes in the
proximal ulna (as previously described) at the level of the coronoid
tubercle. The bone tunnels within the medial epicondyle may
P.2219
be made in the manner noted previously, or they may be made to exit within the cubital tunnel (Fig. 81.12B).
If the proximal holes exit within the cubital tunnel, drill the hole
again to create a single entrance hole anteriorly at the level of the
anatomic origin of the anterior bundle of the ulnar collateral ligament
between the base and tip of the medial epicondylar prominence. Make two
divergent drill holes within the cubital tunnel, allowing a bone bridge
within the cubital tunnel between the two posterior holes. -
Pass the graft in figure-eight fashion.
Apply tension and observe the graft during ROM and valgus stress. If
there is extra length of the graft, it may be passed back upon itself
through one of the bony tunnels. Secure the graft by suturing it to
itself with a nonabsorbable suture (Fig. 81.12C). Close the overlying capsule in a side-to-side imbricating manner with a #0 or #1 nonabsorbable suture. -
At this point, transpose the ulnar nerve anteriorly (Fig. 81.12D).
Place the nerve anterior to the medial epicondyle and beneath the
flexor–pronator muscle mass. It is important to take the elbow through
a ROM to ensure that there are no areas of impingement along the new
course of the ulnar nerve: Look for any impingement on sutures or on a
portion of the underlying ulnar collateral ligament repair. -
Place the sutures for reattachment of the
flexor–pronator muscle mass under direct vision to avoid injury to the
underlying ulnar nerve. Do not tie the sutures until all have been
placed. This repair is by nonabsorbable suture to the tendinous cuff of
tissue left at the medial epicondyle or through drill holes made within
the medial epicondylar bone. Tie the sutures and observe that the ulnar
nerve glides freely without any impingement or entrapment from the
flexor–pronator muscle mass repair. Assess the integrity of the
flexor–pronator repair during elbow ROM. Wound closure, postoperative
immobilization, and rehabilitation are then performed as for patients
without ulnar nerve transposition.
-
Approach the elbow through a modified
lateral Kocher’s incision. Expose the joint capsule by reflecting the
anconeus muscle posteriorly. Make a longitudinal incision through the
extensor tendon origin to the lateral epicondyle and reflect the tendon
anteriorly from the lateral epicondyle to expose the origin of the
radial collateral ligament. -
Palpate the ulnar attachment of the
lateral ulnar collateral ligament at the tubercle of the supinator
crest deep to the fascia over the extensor carpi ulnaris and the
supinator muscles. Reflect the triceps and anconeus from the posterior
margin during reflection of the common extensor tendon origin. It is
important to preserve the underlying capsule and the lateral ulnar
collateral ligament. -
Findings may reveal a lax ulnar band of
the lateral collateral ligament proximal to the annular ligament. The
lateral pivot-shift maneuver can demonstrate subluxation of the radial
head and ulnohumeral joints, as well as attenuation of the capsule.
Make an arthrotomy at the level of the radiocapitellar and ulnohumeral
joints. Treat any associated loose bodies, synovitis, and osteochondral
injuries. Place plication sutures within the anterior and posterior
aspect of the joint capsule, tying the sutures at the completion of the
procedure. -
At this point, decide whether to do a
ligamentous repair or a ligamentous reconstruction. When the lateral
ligament is attenuated, reinforce the repair with an autogenous free
tendon graft, typically using the palmaris longus, although other
sources such as a plantarus or a medial strip of Achilles tendon may be
used. -
If the lateral collateral ligament has
become detached from the humerus, treat it with two #1 nonabsorbable
sutures placed in Bunnell fashion in the lateral ligamentous complex
including the radial collateral ligament and lateral ulnar collateral
ligament. Bring these sutures through drill holes placed in the
anatomic origin of the ligament at the midportion of the lateral
epicondyle. Address further capsular redundancy by plicating the
anterior and posterior capsule (Fig. 81.13).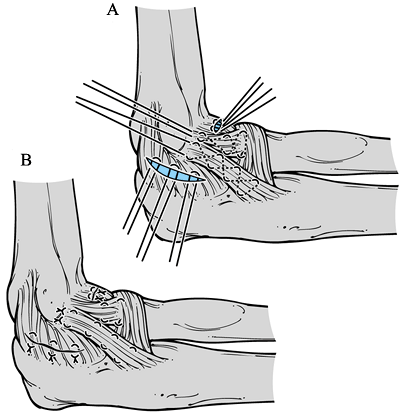 Figure 81.13. Lateral collateral ligament repair. A:Placement of sutures for repair. B:Completion of the repair.
Figure 81.13. Lateral collateral ligament repair. A:Placement of sutures for repair. B:Completion of the repair. -
Obtain a tendon graft as previously
described for the ulnar collateral ligament. As with the ulnar
collateral ligament reconstruction, an osseous tunnel must be created
in the correct anatomic position (Fig. 81.14).
Identify the insertion site of the lateral ulnar collateral ligament on
the tubercle of the supinator crest of the ulna by palpation or by
stressing the elbow in supination and varus. Make one hole in the ulna
just posterior to this point, and another proximally near the annular
ligament, so that a bridge of bone of 1–1.25 cm spans the holes.![]() Figure 81.14. Lateral collateral ligament (LCL) reconstruction. A.Determine the isometric point for the LCL. B.Drill holes and insert the polmaris longus tendon as described in the text.
Figure 81.14. Lateral collateral ligament (LCL) reconstruction. A.Determine the isometric point for the LCL. B.Drill holes and insert the polmaris longus tendon as described in the text. -
At the chosen site, drill the hole
eccentrically in a posterior and proximal direction with reference to
the isometric point. Exit posterior to the supracondylar ridge and 2 cm
proximally. With a bone bridge of 1–1.5 cm, create a reentry tunnel
from this site back to the original entry at the epicondyle. -
Pass the graft though these osseous
tunnels using a suture passer, and flex the elbow to 30° with full
pronation. Close the joint capsule so that the tendon graft becomes
extraarticular and does not rub against the lateral margin of the
capitellum. -
With tension placed on the tendon graft,
use #0 nonabsorbable sutures to anchor the tendon to itself and to the
surrounding soft tissues. Reapproximate the anconeus and extensor
muscles along with the common extensor origin, and complete routine
closure.
-
Place the patient in a supine position
with the upper extremity supported by an arm board. Drape the involved
extremity free to allow for control of elbow and arm position during
the procedure. Begin an incision just proximal and posterior to the
lateral epicondyle, extending distally to the radiocapitellar joint (Fig. 81.15).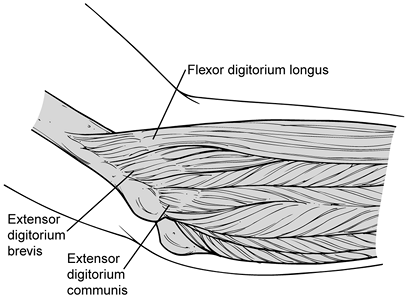 Figure 81.15. Anatomy of extensor mechanism.
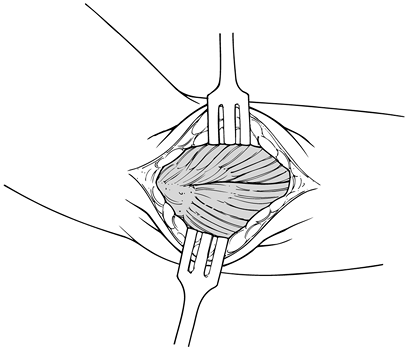
Figure 81.15. Anatomy of extensor mechanism. -
While exposing the deep fascia through
mobilization of subcutaneous flaps, take care to avoid any distal
branches of lateral cutaneous nerves. After complete exposure of the
extensor tendon and its aponeurosis (Fig. 81.16), palpate the extensor mechanism as it inserts into the lateral epicondyle.![]() Figure 81.16. Exposure of extensor mechanism laterally.
Figure 81.16. Exposure of extensor mechanism laterally. -
Incise the deep antebrachial fascia
directly over the epicondyle, and subsequently divide it distally
toward the radiocapitellar joint, thus exposing the fibrinous origin of
the common extensor tendon and the superior and inferior margins of the
lateral epicondyle (Fig. 81.17). Elevate the
superficial and distal fibers of the extensor carpi radialis longus
anteriorly. Continue the incision subperiosteally and distally to the
extensor carpi radialis brevis.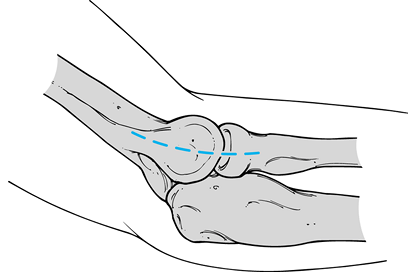 Figure 81.17. Lateral approach over the lateral epicondyle and extending distally to the radial head.
Figure 81.17. Lateral approach over the lateral epicondyle and extending distally to the radial head. -
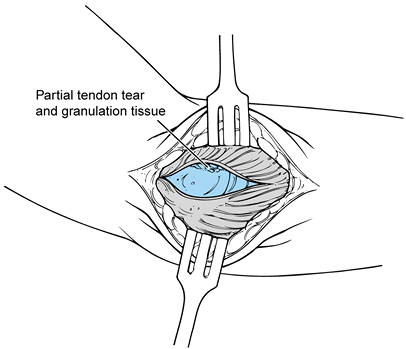
Inspect the underlying extensor tendon mechanism that has been released subperiosteally (Fig. 81.18).
Note any evidence of tears, granulation tissue, or fibrinous
replacement of the tendon. Inspect the radiocapitellar joint and the
overlying synovium.![]() Figure 81.18. Lateral exposure of the pathology in the ECRB and joint.
Figure 81.18. Lateral exposure of the pathology in the ECRB and joint. -
Tears of various degrees and magnitudes
are commonly found on the underside of the extensor carpi radialis
brevis and frequently may penetrate distally to the level of the
lateral compartment of the elbow joint. Therefore, follow the tendon to
the level of the radiocapitellar joint to be sure that the entire
pathology has been observed (Fig. 81.19). If a
tear, granulation tissue, or fibrinous material is not identified in
the extensor carpi radialis brevis, inspect the extensor carpi radialis
longus proximally and the extensor digitorum distally.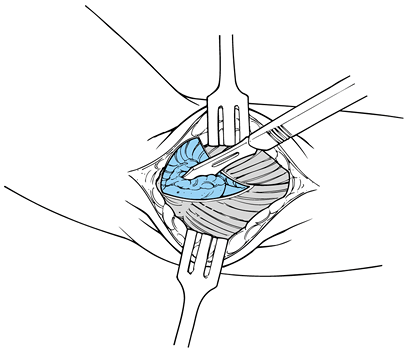 Figure 81.19. Excision of the degenerative portion of the ECRB tendon.
Figure 81.19. Excision of the degenerative portion of the ECRB tendon. -
Elevate the extensor origin in a
subperiosteal fashion both anteriorly and posteriorly until the entire
pathologic area of tendon tear and degeneration has been exposed. It is
not necessary to elevate the entire extensor origin but merely that
portion of the extensor tendon involved with disease. The normal muscle
length should be preserved; this is accomplished by continuing the
dissection in a longitudinal subperiosteal fashion, beginning with a
longitudinal incision. -
The degenerative portion of the tendon
typically occurs along its undersurface at its insertion into the
lateral epicondyle. A nerve hook can be introduced for palpation of the
partial-thickness tear within the tendon. Excise the degenerative
portion of the tendon, as well as any abnormal granulation tissue and
fibrillated torn edges. Typically this requires removal of a portion of
the undersurface of the extensor carpi radialis brevis tendon. -
Using a rongeur, superficially decorticate the area of the lateral epicondyle that has been exposed (Fig. 81.20). This helps provide a cancellous bleeding surface for later reattachment of the extensor mechanism.
![]() Figure 81.20. Debridement of the lateral epicondyle.
Figure 81.20. Debridement of the lateral epicondyle. -
Use a 5/64-inch drill bit (Fig. 81.21) to create a V-shaped tunnel in the lateral epicondyle, allowing passage of a slow-absorbing #0 suture through the bone (Fig. 81.22)
for improved fixation, and eliminating any potential dead space between
the tendon and the lateral epicondyle at the completion of the
procedure.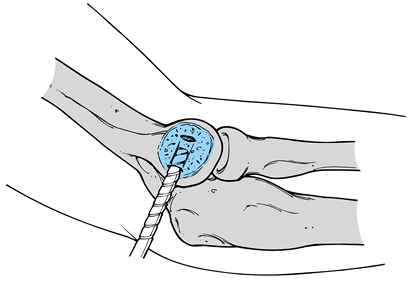 Figure 81.21. Drilling of the lateral epicondyle.
Figure 81.21. Drilling of the lateral epicondyle.![]() Figure 81.22. Passage of suture through bone.
Figure 81.22. Passage of suture through bone. -
Finally, perform a side-to-side repair
using absorbable #0 suture, replacing the extensor tendon mechanism
anatomically to the lateral epicondyle. -
Closure typically begins at the extensor carpi radialis longus (Fig. 81.23)
and proceeds distally to include any remaining portion of the extensor
carpi radialis brevis and synovial layer of the elbow joint. Release
the tourniquet and obtain hemostasis. Close the subcutaneous tissue,
and follow with a running subcuticular skin closure. Apply a soft
dressing and place the elbow in a sling for comfort.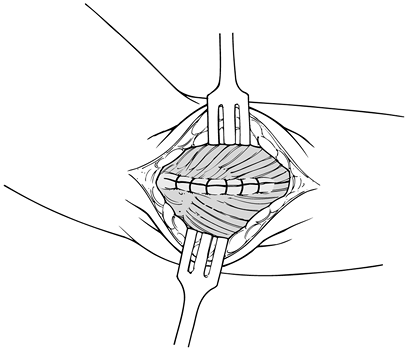 Figure 81.23. Closure of extensor mechanism.
Figure 81.23. Closure of extensor mechanism.
-
Place the patient supine with the
extremity resting on an arm board and the limb draped free. Incise
anterior to the prominence of the medial epicondyle, extending distally
approximately 4 cm. Identify and protect the underlying cutaneous
nerves. -
Incise the common flexor at its origin on
the medial epicondyle, extending the dissection through the central
portion of the flexor–pronator muscle mass. Elevate the flexor–pronator
muscle mass tendon origin in a longitudinal subperiosteal fashion off
the medial epicondyle. Avoid inadvertent entry into the cubital tunnel. -
Inspect the underlying flexor–pronator
tendon for any tear, granulation tissue, or fibrinous replacement. The
degenerative portion of the tendon typically occurs along its
undersurface at its insertion into the medial epicondyle. Use a nerve
hook for palpation of partial thickness tears. Excise the degenerative
portion of the tendon as well as any abnormal granulation tissue. -
Using a rongeur, decorticate the area of
the medial epicondyle that has been exposed, creating a cancellous
bleeding surface for later reattachment of the flexor tendon. Use a 5/64-inch
drill bit to create a V-shaped tunnel within the medial epicondyle to
allow passage of a slow-absorbing suture through the bone for improved
fixation and to eliminate dead space between the tendon and epicondyle
postoperatively. Use caution while drilling, to avoid any entry into
the cubital tunnel. -
Reapproximate the flexor tendon to
itself, overlying the medial epicondyle in a side-to-side manner. Use
simple interrupted #0 absorbable sutures to reapproximate the tendon.
Release the tourniquet and obtain hemostasis. Close the subcutaneous
tissue, and follow with a running subcuticular skin closure.
tuberosity through a modification of the two-incision technique
described by Boyd and Anderson (16).
-
Place the patient supine with the elbow
extended and the forearm supinated on an arm board. Make an oblique
incision beginning medially and proximally to the elbow crease, and
extending distally and laterally (Fig. 81.24).
The lacertus fibrosis may be intact and should be released to reveal
the underlying hematoma and biceps tendon. Protect the underlying
lateral antebrachial cutaneous nerve, which runs lateral to the biceps
tendon. Identify the end of the tendon. Place two different-colored,
nonabsorbable #2 sutures within the biceps tendon in a modified Kessler
fashion (Fig. 81.25).![]() Figure 81.24. Anterior incision for repair of a bicep tendon.
Figure 81.24. Anterior incision for repair of a bicep tendon.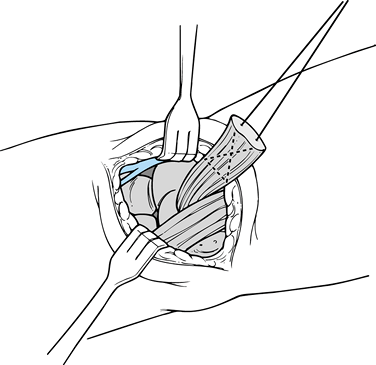 Figure 81.25. Insertion of a suture into the end of the biceps tendon.
Figure 81.25. Insertion of a suture into the end of the biceps tendon. -
Insert a curve clamp between the radius and the ulnar, down the tunnel vacated by the biceps tendon (Fig. 81.26).
Flex the elbow and direct the curved clamp posterolaterally, tenting
the skin on the forearm. Make a second 4–6 cm incision, paralleling the
radial border of the ulnar. Split the extensor muscle mass carefully,
avoiding exposure of the ulna. Avoidance of the ulna and its
subperiosteum minimizes the postoperative risk of radioulnar synostosis.![]() Figure 81.26. Posterior incision.
Figure 81.26. Posterior incision. -
Identify the radial tuberosity and clear it of soft tissue. Make a 1/8-inch
drill hole within the radial tuberosity, enlarging it with a ¼-inch
drill bit. Enlarge the cavity with a high-speed burr or curet. It is
important to remove all bony debris after drilling, with copious
irrigation. Drill two smaller holes through the edges of the cavity in
the tuberosity using a 5/64-inch drill bit. -
Pass the tendon from anterior to posterior through the soft-tissue tunnel between the radius and the ulna.
P.2225
Using a 24-gauge wire, a commercial suture passer, or a free needle,
pass the nonabsorbable sutures through the cavity in the radial
tuberosity, exiting through the smaller side-drilled holes. Using the
different-colored sutures, bring one limb through each of the two
side-drilled holes. Pull the sutures firmly with the arm in flexion and
supination, and be sure by direct palpation that the tendon is seated
within the bony defect created within the radial tuberosity. Before
tying the suture, release the tourniquet and thoroughly irrigate and
close the anterior incision. It is critical to close the anterior
incision before tying the sutures of the biceps tendon through the
posterior incision, as the elbow will remain in a flexed position
following closure of the biceps tendon repair. -
Tie each of the sutures from the biceps
tendon, and again inspect the distal tendon of the biceps to make
certain that it is secured within the radial tuberosity. Tie the
sutures securely with the elbow flexed 90° and the forearm supinated (Fig. 81.27).
Irrigate the posterior wound thoroughly. Close the deep fascia with
interrupted #0 absorbable suture, and close the subcutaneous tissue
with a running subcuticular closure.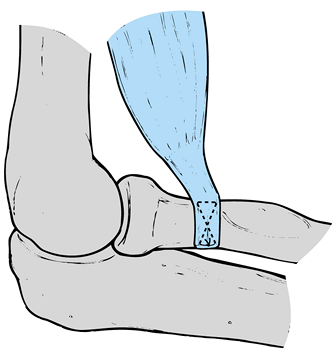 Figure 81.27. Biceps repair.
Figure 81.27. Biceps repair.
-
Use a posterior approach to the distal arm (Fig. 81.28)
in either a supine lateral or prone position. Make the skin incision
over the distal triceps mechanism and develop subcutaneous flaps,
protecting any underlying cutaneous nerves. While the ulnar nerve does
not need to be freed or transposed, it is critical to identify the
course and to be aware of its position throughout the surgical repair.
Isolate the avulsed triceps tendon and at this point inspect the
posterior aspect of the joint. Excise any bone flecks accompanying the
triceps tendon avulsion. If there has been a large fragment avulsed
with the triceps tendon (often seen in the adolescent athlete),
P.2226
repair this fragment with either a tension band technique or screw fixation.![]() Figure 81.28. Biceps repair.
Figure 81.28. Biceps repair. -
Place two different-colored #2
nonabsorbable sutures through the tendon in a modified Kessler fashion.
Use crisscrossed bone tunnels in the olecranon as described by Morrey (48) to secure the sutures to bone (Fig. 81.29).
Make sure that, as the sutures are placed through the bone and tension
is placed on the tendon, the overlying fascia is not tethered, which
would compromise the ulnar nerve.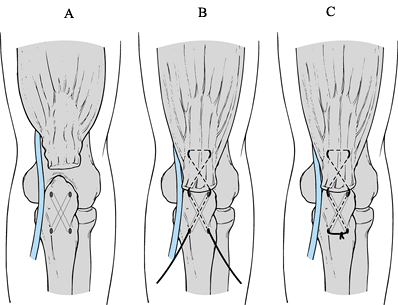 Figure 81.29. Triceps repair.
Figure 81.29. Triceps repair. -
Deflate the tourniquet prior to closure
and obtain hemostasis. Tie the sutures and check the integrity of the
repair during ROM of the elbow. After routine closure, splint the elbow
at 45° of flexion.
in a soft dressing and allow gentle active-assisted ROM. In 7–10 days,
remove the sutures. Encourage gentle passive and active flexion,
extension, supination, and pronation. Initiate ROM exercises for the
wrist and fingers, including squeezing a light rubber ball with the
hand. Discourage activities that require lifting with the involved
extremity, and avoid any resisted dorsiflexion of the wrist or fingers.
At 6 weeks, start a strengthening program, including isometric and
isotonic exercises, that progresses on a graduated basis. Return to
normal activities such as heavy lifting or athletics at 3 months.
elbow after surgical treatment of medial epicondylitis. Adopt a program
similar to that described for lateral epicondylitis, with a similar
time frame for return to sports and heavy activity.
forearm in supination with the elbow flexed 90°. Remove the sutures at
1 week while continuing to immobilize the elbow for 2 more weeks. At 3
weeks, initiate gentle active extension and passive flexion exercises,
as well as gentle active pronation and passive supination exercises. At
6 weeks, continue ROM exercises and introduce isometric strengthening
exercises in all planes. Initiate a progressive strengthening program
that includes isotonic exercises at 12 weeks, and progressively
continue until full ROM strength and endurance is restored. Do not
recommend full activities until full ROM strength and endurance have
been achieved, which typically takes 4 months following surgical repair.
immobilize the arm in a plaster splint at 45° of elbow flexion for 3
weeks. Remove sutures at 7–10 days postoperatively. Next, begin gentle
passive extension and active flexion. At 6 weeks, initiate progressive
resisted exercises beginning with isometric exercises, and progress to
isotonic exercises with a progressive strengthening program.
Unrestricted activity typically can be initiated 4 months after
surgery, when full ROM, strength, and endurance have been achieved.
for medial elbow instability, all the complications except one
postoperative hematoma were related to either cutaneous nerves or the
ulnar nerve (22). Cutaneous nerve complications
included a transient paresthesia, cutaneous neuroma, and residual pain.
In the reconstruction group, 21% had ulnar nerve symptoms
postoperatively. One third of this group had transient paresthesias
that subsided after 4 months. The remaining two thirds required a
revision procedure on the ulnar nerve, with the majority having
residual paresthesias of the ulnar nerve during long-term follow-up.
When performing an ulnar collateral ligament reconstruction, it is
critical to be mindful of the potential iatrogenic neurogenic injuries
that may occur. The cutaneous nerves off the medial antebrachial
cutaneous nerve tend to be robust, and they are easily injured.
Traction on the cutaneous nerves may result in transient postoperative
paresthesias that typically resolve. It is the residual neuroma from an
accidental transection of the nerve that results in the most difficulty
in the postoperative course for these patients. Ulnar nerve problems
are common, but their incidents may be reduced by separating those
patients who require an ulnar nerve transfer from those who do not.
of ulnar collateral ligament reconstruction from the Kerlan-Jobe
Orthopaedic Clinic were limited to patients who uniformly had
submuscular ulnar nerve transpositions performed as a part of their
reconstructive procedure. The majority of patients with ulnar
collateral ligament insufficiency do not require ulnar nerve transfer.
It is critical in that group to be sure that, during the course of the
surgical procedure, the cubital
tunnel
is not violated. For those patients who require an ulnar nerve
transposition, strict attention to detail is required, as for any
submuscular ulnar nerve transfer. It is imperative that any residual or
new site of compression or impingement of the nerve be eliminated and
that the nerve is noted to lie free underneath the flexor–pronator
muscle mass following its repair at the conclusion of the procedure.
due to impingement of the posterior medial olecranon in the olecranon
fossa. Termed valgus extension overload, this may result in the
development of osteophytes on the posteromedial margin of the olecranon
and loose fragments of bone within the olecranon fossa. Although in
many patients, impingement may be eliminated by restoration of the
ulnar collateral ligament, I believe that it is important to remove any
posterior osteophytes and loose bodies at the time of surgery.
athletes must rehabilitate the shoulder along with the elbow and
maintain good overall physical condition to minimize the risk of injury
during the early postoperative period. Proper throwing mechanics,
balance, and coordination must be restored before returning to
competition.
improper placement of the osseous tunnels in a nonisometric position.
Other problems include penetration of the articular surface during
drilling of the osseous tunnel, and loss of the bone bridge between the
two surfaces of the osseous tunnel if forceful placement of the graft
occurs without having previously ensured adequate communication between
the two tunnels. Salvage of a disrupted bone tunnel may require the
need for a bone anchor and fixation of the tendon graft into a trough.
I have had no experience with this and cannot predict the difference in
fixation strength between a tendon graft fixed within a bony tunnel and
one fixed into a trough secured by bone anchor and sutures.
It is critical at the time of the procedure to determine the presence
or absence of competency of the lateral ulnar collateral ligament, in
addition to that of the remaining posterior and anterior lateral
capsule. When a repair or reconstruction is being performed for the
lateral ulnar collateral ligament, establish the isometric points
proximally and distally to ensure stability in the postoperative
period. In addition, at surgery, assess other pathologically lax
sources for instability, such as the anterior and posterior capsule,
which will require plication sutures as part of the repair. Injury to
the cutaneous nerves, while less common than after a medial approach,
may occur and can be a significant source of impairment for the
patient. Careful attention to avoid any injury to the cutaneous nerves
is imperative. In addition, be aware of the proximity of the radial
nerve while dissecting and immobilizing the anterior capsule.
may be caused by improper patient selection or improper diagnosis. The
differential diagnosis of a patient with lateral epicondylitis includes
degenerative arthrosis, forearm extensor tendinitis, lateral collateral
ligament instability, and entrapment of the radial nerve. A
preoperative assessment must be completed to distinguish between these
various entities. An incomplete release or excision of the pathologic
tissue may also cause persistent symptoms after the surgical procedure,
so pay careful attention to technique.
that injury to the radial nerve be avoided when elevating the lateral
epicondylar tendon anteriorly and exploring the radiocapitellar joint.
During the initial dissection, when the longitudinal incision is made
through the extensor tendon and carried distally to the radiocapitellar
joint, iatrogenic injury to the radial collateral ligament may occur.
The dissection must be carried out in a longitudinal fashion, and the
lateral capsule must not be transected with a transverse incision. It
is also critical that the lateral ulnar collateral ligament not be
violated during the course of the dissection and repair.
treatment of medial epicondylitis to avoid any injury to the underlying
cutaneous nerves, as noted previously. A common source of persistent
postoperative pain is failure to adequately diagnose the underlying
condition. Those patients with symptomatic collateral ligament
insufficiency or ulnar neuritis will have persistent symptoms if their
underlying disease is not recognized. During the surgical approach,
avoid injury to the ulnar nerve at the cubital tunnel, and to the ulnar
collateral ligament during the reflection and exploration of the
tendinous origin of the flexor–pronator muscle mass. The origin of the
ulnar collateral ligament lies just underneath the flexor–pronator
muscle mass attachment to the medial epicondyle.
tendon reattachment is significantly reduced since the development of
the two-incision technique. The radial nerve
was
vulnerable when we previously used an extended anterior approach.
However, the two-incision technique has raised the incidence of
radioulnar synostosis. When creating the posterior incision, a
muscle-splitting approach must be performed rather than a subperiosteal
dissection along the course of the ulna. It addition, the presence of
the hematoma communicating with the radius and the ulna may also
increase the risk of a postoperative synostosis.
tuberosity may be used through a single anterior incision, avoiding the
potential risks of radioulnar synostosis. We have no experience with
this technique and do not know if this will again initiate an increased
incidence of radial nerve injuries, nor do we know if the fixation
strength of the biceps tendon to bone will be altered.
mobilization of the triceps may occur, but it is not commonly reported.
Careful attention of the anatomic location to the ulnar nerve and
avoidance of the cubital tunnel is imperative.
overhead and throwing athletes unable to compete prior to surgery has
been successful in returning the majority of them to their previous
level of participation. Of 71 surgical procedures with valgus
instability treated at the Kerlan-Jobe Orthopaedic Clinic, 14 had a
direct repair and the remainder had a reconstruction using a free
tendon graft (22). These patients were followed
for 6.3 years postoperatively, and 68% of the reconstruction group
returned to their previous level of participation. The mean time to
return to competition was 12 months. The negative prognostic factors
included previous operations on the elbow and being a baseball pitcher
(as opposed to other throwing positions). Postoperative problems
involving the cutaneous nerves or the ulnar nerve itself accounted for
the major complications following this procedure. There were no
differences in results when the source of graft material was evaluated,
which included the ipsilateral palmaris longus tendon, the
contralateral palmaris longus tendon, and the plantaris or partial
Achilles tendon graft.
is not common in the general population, but it is quite frequent in
the athletic population engaged in athletic activities that place
valgus stress across the medial aspect of the joint. Most athletes with
ulnar collateral ligament tendinitis or instability improve with
conservative treatment and do not require surgical intervention. Those
athletes not responding to conservative care, and those who wish to
continue to pursue athletic endeavors, are excellent candidates for a
surgical reconstruction. The results of surgical repair, especially in
the chronic setting, are unpredictable. When addressing the problems of
ulnar collateral ligament insufficiency surgically, it is recommended
that a reconstruction be performed with the use of a free tendon graft.
Postoperative complications may be minimized (a) by paying meticulous
attention to avoid injury to the cutaneous nerves and (b) by limiting
transposition of the ulnar nerve to those cases with preoperative
clinical indications.
with 11 cases that were followed a minimum of 1 year. Six cases were
rated as excellent, which indicated that the patients perceived the
elbow to be normal. One case was considered to be good, which indicated
no objective or subjective elements of instability, with residual mild
pain or apprehension. Three patients were rated as fair, which
indicated no objective instability but the patient did have subjective
symptoms including an apprehension sign. Mild or moderate pain with the
loss of more then 10° of motion was also present. There was one poor
result with a recurrence of instability that was perceived and could be
demonstrated by a positive pivot-shift maneuver.
posterior lateral instability are not common. Keep these diagnoses in
mind when evaluating any patient with lateral elbow pain. Many patients
who failed treatment for lateral epicondylitis and other diagnoses for
lateral elbow pain, in fact had lateral collateral ligament laxity that
was not recognized. When the diagnosis is made and clinical symptoms
are sufficient, the treatment is primarily surgical; there is little
role for the nonoperative management.
noted that 85% to 90% of patients returned to full activity without
pain after surgical treatment of lateral epicondylitis. Approximately
10% to 12% were noted to have improvement, but with some residual pain,
and 2% to 3% were felt to have no appreciable improvement
as
a result of the surgical procedure. At the Kerlan-Jobe Orthopaedic
Clinic, 1,200 patients with a diagnosis of lateral epicondylitis were
treated over a 10-year period (20).
Of the 1,200 patients, 60 subsequently underwent surgical treatment for
persistent symptoms. Of those 60, 39 were available for long-term
follow-up. Although 94% had dramatic improvement in their symptoms,
this did not correlate with the objective findings, which demonstrated
that 36% had residual limitations from heavy lifting. Grip-strength
deficits were found in 15%, and 100% had some degree of isokinetic
strength deficit. It was concluded that while the procedure provides
excellent subjective postoperative relief, the subjective results do
not necessary correlate with objective findings of persistent weakness.
common diagnoses made by musculoskeletal specialists dealing with elbow
problems. The results of conservative treatment are uniformly
excellent. However, those patients who have associated entrapment of
the radial nerve or other causes for lateral elbow pain must be
identified. For those who fail conservative treatment, lateral
epicondylitis surgery is predictable and excellent, but careful
attention to detail is critical at surgery to avoid any injury to the
lateral collateral ligament complex.
of 35 patients with surgically treated medial epicondylitis revealed
that subjective function improved from 38% to 98%. Excellent or good
results were obtained in 97% and isokinetic grip-strength testing
revealed no significance between the involved and uninvolved elbows.
epicondylitis may be treated conservatively with excellent results, the
results of surgical treatment are uniformly excellent. The hallmark of
an excellent outcome is an accurate diagnosis. In the athletic
population, rule out underlying ulnar collateral ligament insufficiency
and symptoms associated with ulnar neuritis. Treatment of medial
epicondylitis in the presence of these other diagnoses typically yields
unpredictable results unless they are addressed appropriately.
consisted of weight lifters and body builders with high functional
demands. All patients in this group were satisfied with their
functional and cosmetic result and returned to full, unrestricted
activities. Isokinetic testing demonstrated that supination strength
and endurance was normal; in flexion, the patients had normal strength
but averaged 20% less endurance. Morrey et al. (50)
reported on seven patients with at least 15 months follow-up after a
two-incision technique. Those treated early had at least 95%
restoration of strength in all planes. Those who had a late
reattachment or a late insertion into the brachialis had deficits in
flexion and supination.
active population such as weight lifters and heavy laborers, it may
occur in anyone, especially with a traumatic injury. Unlike a proximal
biceps tendon rupture, a distal biceps tendon rupture left untreated
will yield significant functional deficits. Except for those
individuals who are otherwise inactive, an acute repair of the distal
biceps tendon will yield predictable results with restoration of ROM
and function. The two-incision technique is preferred because of the
low incidence of radial nerve problems, but a muscle-splitting approach
must be utilized to lower the incidence of postoperative radioulnar
synostosis.
the results of immediate or delayed repair have been uniformly good,
with restoration of normal strength and full ROM. Approximately 20% of
patients will develop a loss of 5° of terminal extension.
rare, the physician treating an active athlete or heavy laborer
population will undoubtedly encounter the injury. In cases of complete
disruption, prompt diagnosis and surgical repair result in a
predictable optimal outcome with excellent functional recovery.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
JA, Jokl P, Shaw R, et al. Clinical Study of Baseball Pitchers:
Correlation of Injury of the Throwing Arm with Method of Delivery. Am J Sports Med 1978;6:15.
RE, Barron J, Jobe FW, et al. An Electromyographic Analysis of the
Elbow in Normal and Injured Pitchers with Medial Collateral Ligament
Insufficiency. Am J Sports Med 1992;20:311.
JL, Nirschl RP. A Mechanical and Electromyographical Analysis of the
Effects of Various Joint Count Forcebraces on the Tennis Player. Am J Sports Med 1986;14:195.
SE, Jobe FW. Anatomy and Histology of the Collateral Ligaments of the
Elbow. Presented at the Seventh Open Meeting of the American Shoulder
and Elbow Surgeons, San Francisco, 1993.
DS, Hankin FM, Eckenrode JR, Jobe FW, Perry J, et al. Distal Biceps
Brachii Tendon Avulsion: A Simplified Method of Operative Repair. Am J Sports Med 1986;14:234.
SW, Morrey BF, Korinek S, et al. Posterolateral Rotatory Instability of
the Elbow: A Kinematic and Biomechanical analysis. Orthop Trans Res Soc 1990;15:6.