COMPARTMENT SYNDROMES
II – FRACTURES, DISLOCATIONS, NONUNIONS, AND MALUNIONS > General
> CHAPTER 13 – COMPARTMENT SYNDROMES
Orthopaedics, University of California, San Diego, and Pediatric
Orthopaedic and Scoliosis Center, Children’s Hospital, San Diego,
California 92123.
accumulating fluid and/or external compression creates high pressure
within a closed fascial space, reducing perfusion of the tissues within
that compartment below a level necessary for viability. Compartment
syndromes develop in skeletal muscles enclosed by relatively
noncompliant osseofascial boundaries. A buildup of pressure within
these compartments is not easily dissipated because of the inelasticity
of the muscle-investing fascia and surrounding bone. If pressure
remains sufficiently high for several hours, normal function of the
muscles and nerves is jeopardized, and necrosis eventually results.
Permanent loss of function and a Volkmann’s limb contracture may occur.
Prompt diagnosis is therefore essential, with immediate treatment to
reinstate capillary perfusion and prevent irreversible sequelae.
Surgical decompression is accomplished by fasciotomy, which allows the
muscles to increase in volume and reduces pressure within the fascial
enclosure.
An acute compartment syndrome (ACS) is a severe form, usually following
trauma, in which intracompartmental pressure is elevated to a high
level for long enough that capillary flow is impeded and decompression
is necessary to prevent necrosis
and
preserve limb viability. Commonly used terms for ACS are anterior
tibial syndrome, calf hypertension, compartmental syndrome, Volkmann’s
ischemia, and impending ischemic contracture (27).
The terms Volkmann’s ischemia and impending ischemic contracture should
not be used, however, because they do not define the cause of the
ischemic problem (e.g., compartment syndrome or arterial injury).
Volkmann’s contracture is the residual limb deformity that is the last
stage of muscle and nerve necrosis after an ACS.
recurrent. It is most commonly associated with exercise and occurs when
intracompartmental pressure is raised sufficiently to produce ischemia,
pain, and/or neurologic deficit. The symptoms spontaneously resolve
with rest; in the rare instance when exercise is continued despite pain
and neuromuscular deficit, however, a CCS may evolve into a full-blown
acute form that requires immediate decompression. Synonyms for CCS
include chronic exertional compartment syndrome, exercise ischemia,
exercise myopathy, and recurrent compartment syndrome.
intracompartmental pressure with subsequent ischemia of muscle, nerve,
and other compartment contents. In ACS the cause of the elevated
pressure is fluid accumulation from hemorrhage, extracellular edema,
and intracellular edema within the confines of a closed, noncompliant
osseofascial compartment (41). Intracellular
fluid accumulation is associated with membrane pump changes that lead
to an increase in intracellular calcium concentration and subsequent
shifts of water into the cell. Interstitial fluid accumulation results,
at least in part, from elevated capillary permeability, which is caused
by ischemia (41). The elevated
intracompartmental pressure causes further muscle ischemia, which leads
to further edema production. An edema–ischemia cycle is thus
established; without decompression, muscle infarction and other
irreversible damage will ensue (Fig. 13.1).
 |
|
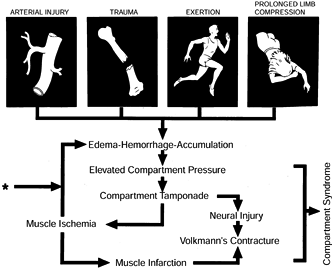
Figure 13.1.
Pathophysiology of a compartment syndrome, which can be initiated by arterial injury, trauma, exercise, or prolonged limb compression associated with alcohol or drug overdose. Common to all compartment syndromes are elevated intracompartmental pressure and subsequent ischemia. The asterisk marks the point of entry of decompression (fasciotomy) into the cycle. Without decompression, a self-perpetuating ischemia–edema process occurs, and irreversible damage, including Volkmann’s contracture, may ensue. (From Mubarak SJ, Hargens AR, Owen CA, and Akeson WH. Muscle Pressure Measurement with the Wick Catheter. In: Goldsmith HS, ed. Practice of Surgery. New York: Harper & Row, 1978.) |
capillary wall. Variables included in this equation include hemodynamic
factors (capillary pressures), colloid osmotic factors, and
permeability factors (41). In tissues at heart
level, capillary blood pressure normally ranges from 20 to 30 mm Hg;
when compartment pressure rises above this level, muscle perfusion is
jeopardized (3,8).
Hargens et al. confirmed that the capillary pressure in the
anterolateral compartment, at heart level, of normotensive dogs was 20
to 30 mm Hg and believed that intracompartmental pressures above this
level led to collapse of the thin-walled capillaries (18).
capillary perfusion from elevated intracompartmental pressure, arterial
pressure is almost always adequate to maintain distal flow in the
larger vessels. Therefore, distal pulses are usually present and should
not be used to rule out the presence of a compartment syndrome.
al. have shown that normal muscle contractions during exercise can
generate very high pressures (100 to 200 mm Hg) and that the muscle is
perfused during the periods of muscle relaxation (60).
In cases of CCS, the compartment pressure during relaxation is
significantly higher than normal (above 35 mm Hg), and muscle blood
flow decreases. This presumably leads to ischemic pain, although this
has not been proved. The primary cause of elevated relaxation pressures
is also not known. Hypotheses include muscle hypertrophy, fascial
thickening, aberrant anatomy, and vessel occlusion (10,32,57).
in detail in this chapter. Compartment syndromes of the hand and foot are discussed in Chapter 33, Chapter 45, Chapter 65, and Chapter 11.
Compartment syndromes can also arise in the shoulder, arm, thigh,
buttocks, and spine. It is essential to understand the compartmental
anatomy of the extremities in order to diagnose and treat acute and
chronic compartment syndromes effectively.
The volar compartment contains the flexors and pronators of the forearm
and wrist. These muscles may be further subdivided into (a) a
superficial group consisting of the pronator teres, flexor carpi
radialis, palmaris longus, flexor carpi ulnaris, and flexor digitorum
and (b) a deep group consisting of the flexor digitorum profundus,
flexor pollicis longus, and pronator quadratus. It is essential to
decompress adequately the entire course of the median nerve as it
courses through this compartment. Proximally it enters the forearm
between the two heads of the pronator teres muscle and then runs the
length of the forearm between the flexor digitorum superficialis and
profundus muscles before it enters the carpal tunnel.
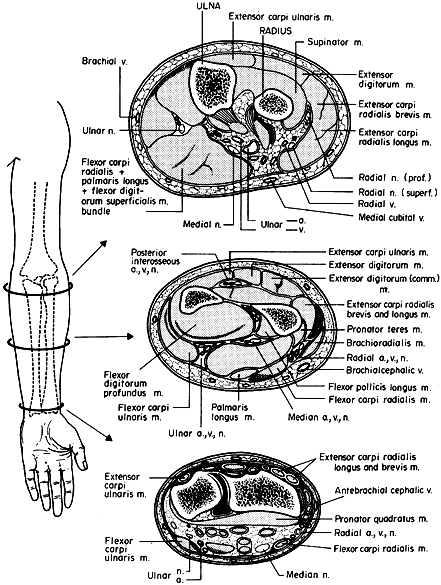 |
|
Figure 13.2. Forearm compartments: transverse sections through the left forearm at various levels. (From Mubarak SJ, Hargens AR. Compartment Syndromes and Volkmann’s Contracture. Philadelphia: WB Saunders, 1981.)
|
contains two groups of muscles. The superficial layer consists of the
extensor digitorum communis, extensor digitorum minimi, and extensor
carpi ulnaris. The deep layer consists of the supinator, abductor
pollicis longus, extensor pollicis brevis, and extensor pollicis longus.
consists of three muscles: the extensor carpi radialis longus, extensor
carpi radialis brevis, and brachioradialis.
involved in compartment syndrome. There are four compartments in the
leg, with the anterior compartment the one most commonly involved in
both ACS and CCS (Fig. 13.3). The anterior
compartment contains the tibialis anterior, extensor digitorum longus,
extensor hallucis longus, and peroneus tertius muscles as well as the
deep peroneal nerve, which supplies all of them. The deep peroneal
nerve also supplies the extensor digitorum brevis and supplies
sensation to the first dorsal web space in the foot.
 |
|
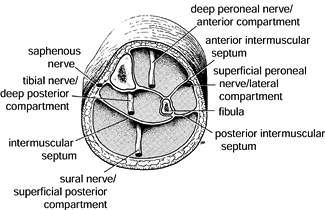
Figure 13.3.
Cross section of the middle lower third of the left leg, illustrating the four compartments with their associated peripheral nerves. (From Mubarak SJ, Owen CA. Double-Incision Fasciotomy of the Leg for Decompression in Compartment Syndromes. J Bone Joint Surg 1977;59-A:184.) |
longus and brevis muscles. The common peroneal nerve runs in this
compartment as it winds around the neck of the fibula, and after the
deep peroneal branch enters the anterior compartment, the superficial
peroneal nerve continues down the leg in the lateral compartment. In
the lower third of the leg it pierces the fascia and runs in the
subcutaneous tissues to the foot, where its two terminal branches, the
medial and intermediate dorsal cutaneous nerves, supply sensation to
the dorsum of the foot.
troublesome because it is not as accessible to palpation as are the
other compartments. This compartment contains the popliteus, flexor
hallucis longus, flexor digitorum longus, and tibialis posterior
muscles as well as the tibial nerve, which supplies them. The tibial
nerve runs between the soleus and tibialis posterior muscles proximally
and between the flexor digitorum longus and flexor hallucis longus
muscles distally before it enters the foot to supply the plantar
muscles and provide sensation to the sole of the foot. Clawing of the
toes after a tibial fracture suggests previous compartment syndrome of
the deep posterior compartment of the leg or the deep calcaneal
compartment of the foot.
superficial posterior compartment, whose surrounding fascia is less
constrained. Contained in this compartment are the plantaris,
gastrocnemius, and soleus muscles, as well as the sural nerve, which
pierces the fascia in the lower third of the leg and supplies sensation
to the lateral aspect of the foot.
increase in compartment volume (e.g., postfracture swelling) or a
decrease in compartment size (e.g., tight circumferential cast). In
some cases, both factors may play a role (e.g., casted tibia fracture).
Other causes include soft-tissue injuries, osteotomies, bleeding
disorders, burns, crush injuries, limb compression (e.g., drug
overdose), and postischemic swelling following arterial occlusion.
Compartment syndrome has also been reported following snake bites (13), massive fluid resuscitation in critically injured patients (5), inadvertent use of hypertonic saline during intravenous regional anesthesia (22,43), inadvertent extravasation of toxic substances during intravenous or intraarterial administration (4), intraosseous fluid administration in children (52), and the use of fluid pumps during arthroscopy (6,28,47).
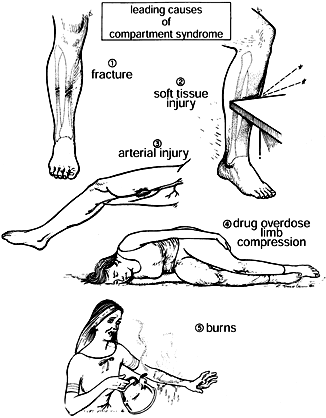 |
|
Figure 13.4. Leading causes of compartment syndrome. (From Mubarak SJ, Hargens AR. Compartment Syndromes and Volkmann’s Contracture. Philadelphia: WB Saunders, 1981.)
|
constriction may be extrinsic or intrinsic. Extrinsic compartment
constriction may be caused by a tight bandage or cast or by the
noncompliant eschar that forms in severe burns. Intrinsic constriction
may be iatrogenic in nature by the surgical closure of fascial defects.
These fascial defects may represent autofasciotomy from increased
intracompartmental pressure or may be the result of fascial incisions
used in the operative treatment of fractures (e.g., tibial plateau).
compartment size is stretching of a relaxed compartment. Gershuni et
al. showed that passive ankle and knee position significantly affected
intracompartmental pressures of the leg (16).
Pressures as high as 40 mm Hg were noted in the deep posterior
compartment of normal volunteers with full passive ankle dorsiflexion.
treatment of fractures. For example, late treatment of a shortened long
bone fracture by open reduction and internal fixation, such as in a
nonunion and/or malunion, can stretch the compartments back to their
original length. This effectively reduces the compartment size and can
significantly increase intracompartmental pressure. Similarly, in the
operative treatment of acute femur or tibia fractures, compartment
syndrome is occasionally seen following intramedullary nailing. In such
cases, there is considerable swelling of the thigh or leg with
resultant shortening of the fracture. When the limb is placed in
traction and the fracture is reduced, the size of the compartments is
decreased; this can lead to a sudden increase in intracompartmental
pressure.
cause of an ACS, less is known about the etiology of CCS. As mentioned
above, there are several hypotheses, all related to anatomy. Styf
suggested that CCS of the anterior compartment of the leg may be
related to vessel occlusion by local muscle herniations (57).
Chronic exercise may lead to significant muscular hypertrophy, which
essentially “outgrows” its noncompliant fascial covering. The fascia
itself may be abnormally thick or tight. Martens and Moeyersoons have
hypothesized that the fascia in these patients is abnormally
noncompliant and does not accommodate the increased muscle volume seen
during exercise (32). Detmer et al. reported increased fascial thickening in 25 of 36 samples from patients with CCS (10). Fascial scarring may also be present in cases where a specific traumatic event is associated with CCS (30,45,61).
acute compartment syndrome is pain that is greater than expected from
the primary clinical problem, such as a fracture or contusion.
Frequently, the patient has been observed for a period of time with
minimal or stable pain and then rapidly develops pain out of proportion
to what is expected. This may be associated with a need for larger and
larger doses of narcotic analgesia. The pain is usually described as a
deep, throbbing feeling of unrelenting pressure and is not responsive
to change in position of the extremity. The absence of pain in ACS is
almost always related to a superimposed central or peripheral sensory
deficit.
syndrome is a swollen, palpably tense compartment that is a direct
manifestation of increased intracompartmental pressure (Fig. 13.5).
Subcutaneous edema can mask the underlying swelling and increased
pressure of the compartment. Despite its relative specificity for
compartment
syndrome,
the tenseness of a compartment is difficult to quantify and remains a
crude indicator of increased intracompartmental pressure. In addition,
the deep posterior compartment is difficult to palpate, and an isolated
syndrome of this compartment can be missed if the examiner feels a
sense of security in the absence of palpably tense compartments.
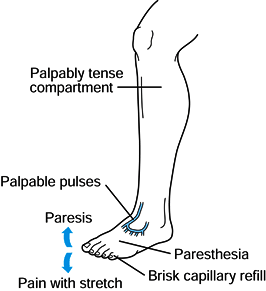 |
|
Figure 13.5.
Early findings of a compartment syndrome. Increased pressure leading to a palpably tense compartment is the earliest sign. (Redrawn from Mubarak SJ, Hargens AR, Owen CA, and Akeson WH. Muscle Pressure Measurement with the Wick Catheter. In: Goldsmith HS, ed. Practice of Surgery. New York: Harper & Row, 1978.) |
compartment(s) is a common finding. Unfortunately, pain is subjective
and depends on the reliability of the patient and the patient’s
threshold of pain. After any injury associated with a compartment
syndrome, all patients have pain, and differentiating this from
ischemic muscle pain can be quite difficult. The examiner must be wary
of the fact that pain on stretch may be absent later in the course of
the compartment syndrome because of anesthesia secondary to nerve
ischemia, which complicates the evaluation of pain.
compartment is likewise difficult to interpret and may arise secondary
to nerve involvement, primary muscle ischemia, guarding because of
pain, or a combination of all three.
the involved compartments is present. Provided that the patient is
conscious and can cooperate, a careful sensory examination is extremely
helpful in evaluating a patient with ACS. Each compartment of the
forearm and leg has at least one peripheral nerve coursing through it,
and careful sensory examination of the hand or foot can help confirm
the compartment(s) involved. Initially, the sensory deficit may
manifest itself as paresthesia only. With delay in treatment,
hypoesthesia progressing to anesthesia is inevitable. In cooperative
patients, any sensory deficit after an injury must be explained.
arterial injury or disease, peripheral pulses are palpable and brisk
capillary refill is found routinely in the patient with ACS. Although
the compartment pressure may be high enough to cause muscle and nerve
ischemia, only on rare occasions is it elevated sufficiently to occlude
a major artery (Fig. 13.6). If palpation of
distal pulses is difficult because of soft-tissue swelling, Doppler
studies are used to confirm their presence. It is crucial that the
treating physician be aware that the peripheral pulses are generally
intact with compartment syndromes and avoid a false sense of security
by palpating a pulse and deciding all is well. Moreover, the skin
circulation is satisfactory and the hand and foot are pink and viable,
unlike the ischemic appearance seen with an arterial injury.
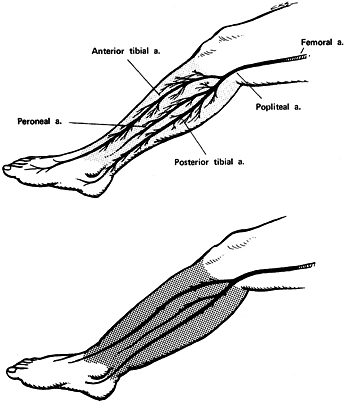 |
|
Figure 13.6. Ischemia associated with compartment syndromes. A: At tissue fluid pressures below 30 mm Hg, blood flows normally from arteries to arterioles and into capillaries. B:
When intracompartmental pressure rises above 30 mm Hg, blood flow is confined to large arteries, veins, and nonnutritional arteriovenous anastomoses. Typically, pulses are present distal to the region of the elevated tissue pressure. This may give the physician a false sense of security because he may assume that normal circulation exists in the muscle compartment. (From Mubarak SJ, Hargens AR. Compartment Syndromes and Volkmann’s Contracture. Philadelphia: WB Saunders, 1981.) |
injuries and neurovascular deficits is primarily limited to compartment
syndrome, arterial injury, and peripheral nerve injury (Table 13.1).
Identification of these problems is important because the treatments
are vastly different. A compartment syndrome must be treated by
immediate fasciotomy. A major arterial injury requires immediate
surgical restoration (e.g., repair of the artery or thrombectomy). A
peripheral nerve injury associated with a fracture or severe
soft-tissue injury is most commonly a neuropraxia, and the initial
treatment of choice is usually observation.
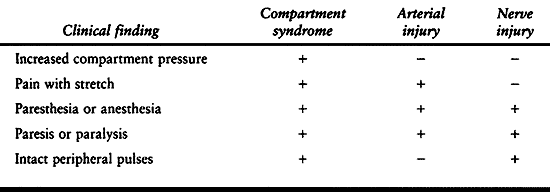 |
|
Table 13.1. Clinical Findings of Compartment Syndrome, Arterial Occlusion, and Neuropraxia
|
All three may have an associated motor or sensory deficit and pain. An
arterial injury usually results in absent pulses, poor skin color, and
decreased skin temperature. Unfortunately, however, there can be a
delay in diagnosis when poor blood flow is attributed to other factors
such as hypovolemia, local compression, or “kinking” of the vessel from
significant limb deformity. As mentioned, the patient with a
compartment syndrome routinely presents with intact peripheral
circulation. Isolated nerve injuries usually give
little
pain, and the diagnosis is often by exclusion of the other two
entities. Doppler studies, arteriography, and intracompartmental
pressure measurements are frequently required to aid in the
differential diagnosis of these three conditions.
exercise or other exertion. The treating physician must have a high
index of suspicion to correctly identify patients with CCS.
jogger to an enthusiastic marathon runner. Nonrunners may also be at
risk (e.g., weightlifters). The majority are young, active men, and the
symptoms are frequently bilateral. In most cases, the patient reports
recurrent pain that is initiated by exercise over the anterior or
lateral compartment of the leg. Symptoms have usually been present for
months by the time medical attention is sought. For runners, the onset
of pain is reproducible for a specific speed and distance. It is
usually necessary for the patient to discontinue his run and rest for a
few minutes, similar to the situation in elderly patients with vascular
claudication. This is variable, however, and some individuals may
continue to run at a reduced speed. The pain may persist for hours.
cramping in the compartment and may be achy, sharp, or dull.
Occasionally, there may also be associated numbness or weakness. The
most common compartments involved in CCS are the anterior and/or
lateral compartments of the leg; however, it has also been reported in
the posterior compartments of the leg (46), thigh (48), foot (31), back (62), forearm (30,45), and hand (59).
before the patient exercises. Muscle hernias, occurring in 60% of
Reneman’s patients, may be clinically more obvious after exercise (50,51). Pedowitz et al. summarized the physical examination findings in 45 patients with CCS (48).
Muscle herniation through a fascial defect was present in 46% of these
patients. Most of these defects are located in the lower third of the
leg overlying the anterior intermuscular septum between the anterior
and lateral compartments (Fig. 13.7). In this
location, the fascial defect may represent an enlargement of the
orifice through which a branch of the superficial peroneal nerve (e.g.,
medial dorsal cutaneous nerve) exits the lateral compartment. Muscle
herniation may cause superficial peroneal nerve irritation and even
neuroma formation (12). After exercise, a
sensation of increased fullness over the anterior compartment may be
experienced, and occasionally hypoesthesia on the dorsum of the foot is
documented. There should be no changes in the peripheral pulses after
exercise.
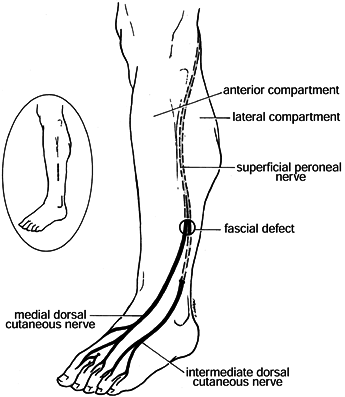 |
|
Figure 13.7.
Relationship of superficial peroneal nerve branches to fascial defect, commonly seen with chronic exertional compartment syndromes. Inset: Incision used for fasciotomy and exploration of the defect and nerves. (From Garfin SR, Mubarak SJ, Owen CA. Exertional Anterolateral Compartment Syndrome: Case Report with Fascial Defect, Muscle Herniation and Superficial Peroneal Nerve Entrapment. J Bone Joint Surg 1977;59-A:404.) |
are usually inadequate for the definitive diagnosis of CCS, objective
intracompartmental pressure measurements are needed. Unfortunately,
there still exists significant controversy over the critical pressure
criteria used for the diagnosis of CCS. In addition, different
investigators have used different catheter systems to obtain their
data. It is important to define abnormally high pressures in relation
to normative data from a control population using the same pressure
measurement system.
obtain intracompartmental pressures during the period of muscle
relaxation when microcirculatory perfusion occurs (60). In CCS, resting intracompartmental pressure is elevated (Fig. 13.8).
In our lab, objective criteria for the diagnosis of CCS were developed
by Pedowitz et al. using slit catheter determinations of static
pressures before and after exercise (46).
Pressures are measured before exercise and 1 and 5 minutes after
exercise. One or more of the following criteria are diagnostic of CCS:
rest pressure ≥15 mm Hg, 1-minute postexercise pressure ≥30 mm Hg; and
5-minute postexercise pressure ≥20 mm Hg.
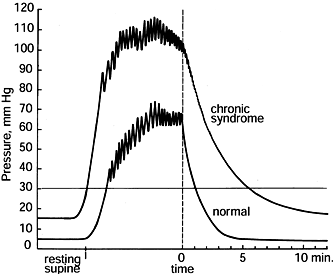 |
|
Figure 13.8.
Illustrative anterior compartmental pressures recorded with the wick catheter during exercise of a normal person and a patient afflicted with a chronic anterior compartment syndrome. The resting pressure of the chronic syndrome is elevated over that of the normal control. During exercise, the pressure rises to more than 75 mm Hg and remains greater than 30 mm Hg for more than 5 minutes in this patient with chronic compartment syndrome. (From Mubarak SJ, Hargens AR. Compartment Syndromes and Volkmann’s Contracture. Philadelphia: WB Saunders, 1981.) |
invasive, and recently there have been studies using a noninvasive
technique of near-infrared spectroscopy to diagnose CCS (7,37).
This technique holds promise for future research and clinical diagnosis
in patients with CCS and other skeletal muscle disorders.
of exercise-induced extremity pain. These include stress fracture,
periostitis, tendinitis, peripheral nerve entrapment, vascular
claudication, venous stasis, and neurogenic claudication. Plain
radiography, bone scintigraphy, ultrasound, magnetic resonance imaging,
electromyography, and nerve conduction studies may be useful in
determining the correct diagnosis other than CCS.
We have employed the wick and slit catheter technique for measurement
of tissue pressure in compartment syndromes. The wick technique was
first described in 1968 by Scholander et al. (56).
The wick catheter used in our patients was based on the original
design, but instead of cotton a piece of braided polyglycolic acid
suture was employed as the wick in the end of the catheter. The
development of the clinical wick catheter began in 1973, and the first
human use studies began in 1974 (Fig. 13.9). (40).
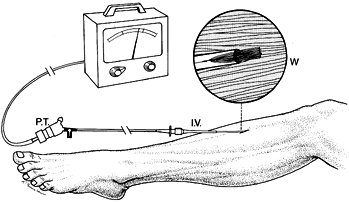 |
|
Figure 13.9.
Wick catheter technique for measuring equilibrium tissue fluid pressure continuously in a muscle compartment. Intravenous placement unit (I.V.) aids insertion of the sterilized saline-filled catheter. Before insertion, connect the wick catheter to a pressure transducer (P.T.) and recorder and calibrate it. A close-up view of the catheter tip (W) illustrates how catheter patency and continuous fluid transmission are maintained by the numerous wick fibers. (From Owen CA, Mubarek SJ, Hargens AR, et al. Intramuscular Pressure with Limb Compression. Clarification of the Pathogenesis of the Drug-Induced Compartment Syndrome/Crush Syndrome. N Engl J Med 1979;300:1169.) |
Rutherford, NJ), first developed by Rorabeck et al., is a modification
of the wick catheter principle and incorporates its features: accuracy,
reproducibility, large area of tissue fluid pickup, immediate
measurement of equilibrium tissue pressure, and continuous monitoring
of intramuscular pressure during contraction and exercise (Fig. 13.10) (55).
The primary advantages of the slit catheter are that it is less prone
to coagulation in long-term measurements, it has a faster response time
during exercise studies, and its manufacture is readily accomplished
and is more uniform than that of the wick catheter. Styf and Koörner
have described a microcapillary infusion technique using a catheter
with side holes near the tip that has excellent dynamic
properties when compared with the wick, and is very useful in studying chronic compartment syndromes (58).
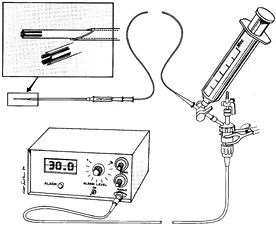 |
|
Figure 13.10.
Slit catheter technique for continuous measurement of intracompartmental pressure. Before inserting it into a muscle compartment, connect the sterile slit catheter to a pressure transducer and digital recorder and then fill it with saline using a 30-ml syringe. The catheter tip protrudes from the insertion needle during filling so that the tip can be checked for air bubbles (upper left). Before insertion into muscle, pull the catheter entirely within the needle. (From Mubarak SJ, Hargens AR. Compartment Syndromes and Volkmann’s Contracture. Philadelphia: WB Saunders, 1981.) |
pressure-monitoring catheter system (Camino Laboratories, San Diego,
CA) that uses a unique method of obtaining pressure measurements
without the disadvantages of the long fluid-filled catheters with
external transducers (9). The Camino catheter
operates by using a light-sending and receiving system that responds to
movements of a mirror diaphragm when pressure is applied to the
catheter tip. The movement is sensed by light signals emitted from a
“sending” light fiber; the reflected light is sent to an amplifier
through a “receiving” light fiber. The light sent and received is
analyzed by a microcomputer and converted into digital and analog
signals directly related to the applied pressure. This
pressure-measuring instrument has its sensor near the catheter tip,
which eliminates the need to make adjustments for hydrostatic pressure.
The fiber optic cable has a relatively large diameter, however, and can
be uncomfortable during long-term monitoring.
been developed. These work best, and are most accurate, when used with
high-pressure tubing and a slit catheter at the end. Simple needles
often plug with tissue and are much less accurate. An example of a
portable unit commonly used is the Stryker Pressure Monitor System
(Stryker, Kalamazoo, MI). The Stryker system is hand-held, with a
prefilled, sterile, disposable syringe (Fig. 13.11). It can be used for intermittent measurement or
continuous monitoring. When possible, it should be used with a slit-tip catheter rather than the fenestrated needle.
 |
|
Figure 13.11.
The Stryker system is a small portable monitoring system (Stryker, Kalamazoo, MI). It is most accurate if used with high-pressure tubing and a slit catheter. |
immediate access to a wick catheter, slit catheter, or even a portable
unit with a side-ported needle. In these situations, intracompartmental
pressure measurements can be readily obtained with an existing arterial
line setup and a catheter that has been cut at the tip. A simple
18-gauge needle and a mercury manometer (Whitesides technique) (63)
can also be used, but it is important to recognize that this method is
considered the least accurate. Moed et al. compared measurement of
intracompartmental pressures using a slit catheter, a Stryker portable
unit with a side-ported needle, and a simple 18-gauge needle with a
mercury manometer (36). They found that the
values obtained with the simple needle were consistently higher, by an
average of 18 to 19 mm Hg, than those obtained with the other two
methods. They found no statistical difference between the values
obtained with the slit catheter compared with the side-ported needle
unit.
compartment syndrome, obtain multiple pressure measurements throughout
each compartment. This is particularly important in patients with a
long bone fracture and suspected compartment syndrome. Heckman et al.
showed that, in cases of closed tibia fractures, there exists a
relationship between intracompartmental pressure and distance from the
actual fracture site (24). The highest
pressures were routinely found at or within 5 cm of the fracture site.
On the basis of these data, the authors recommend using the highest
recorded intracompartmental pressure as an indicator for fasciotomy.
without the need for intracompartmental pressure measurements. In these
cases, documentation of the elevated compartment pressures is
confirmatory. However, patients are frequently encountered in whom
there is difficulty in eliciting or interpreting the physical
examination findings. In these patients, the measurement of
intracompartmental pressure is particularly valuable in the diagnosis
and as a criterion for decompression.
physical examination may be difficult, and measurement of
intracompartmental pressures may be extremely valuable. Children with
fractures and other severe soft-tissue injuries may be so frightened
that examination is impossible. Polytrauma patients frequently have
closed head injuries and may be intoxicated from drugs and/or alcohol,
making examination difficult.
unresponsive individuals. Obtunded patients, intubated and paralyzed
patients, and comatose patients are unable to cooperate with a physical
examination, and palpably tense compartments are the only finding they
can demonstrate. It is essential in these patients to have a high index
of suspicion.
in whom the diagnosis is inconclusive. A patient may have an evolving
or early compartment syndrome in which their pain is clearly out of
proportion to what is expected from their injury. On examination they
may have palpably tense compartments but may not yet have developed
other classical findings such as pain on passive muscle stretch and
sensory deficits. A patient may also have a nerve deficit attributable
to other causes. It is often difficult to differentiate neuropraxia
secondary to stretch or contusion of a peripheral nerve from neurologic
deficits resulting from increased intracompartmental pressure.
clinically for more than 100 years. Despite significant advances in the
understanding of the etiology and pathophysiology of this syndrome,
disagreement still remains on the pressure threshold for fasciotomy.
One possible reason for the different published thresholds may be the
technique of measurement. As discussed above, the needle techniques
usually give higher pressure readings when compared with a slit
catheter or side-ported needle. In addition, there remains disagreement
on whether there exists an absolute critical closing pressure, that is,
a tissue pressure beyond which capillary circulation ceases and
ischemia ensues, or whether this ischemic threshold is relative to the
patient’s systemic blood pressure.
intracompartmental tissue pressure rose to within 10 to 30 mm Hg of the
patient’s diastolic blood pressure (64). This
recommendation was then modified slightly by Heckman et al. to perform
fasciotomy when the measured intracompartmental pressure rises to
within 10 to 20 mm Hg of the patient’s diastolic blood pressure (23).
For example, this would represent an absolute pressure of 50 to 60 mm
Hg in a patient with a diastolic blood pressure of 70 mm Hg. This
recommendation is based on their work in a canine model where
histologic evidence of muscle regeneration did not occur until the
8-hour pressurization level was within 20 mm Hg of the dog’s diastolic
blood pressure. Necrosis and fibrosis of muscle did not occur until the
8-hour pressurization level was within 10 mm Hg of the dog’s diastolic
blood pressure.
magnetic resonance spectroscopy to measure the metabolic activity of
muscle, also concluded that the perfusion pressure gradient between
systemic blood pressure and intracompartmental tissue pressure was
important in determining the ischemic threshold for muscle (25,26). In their canine model, using a slit catheter to determine intracompartmental pressures, they determined that normal
cellular metabolism can occur as long as the intracompartmental tissue
pressure was no closer than 30 mm Hg to the mean arterial blood
pressure (MAP). Hypertension would therefore have a “protective” effect
on muscle perfusion and would raise the absolute pressure threshold for
fasciotomy, whereas hypotension, as is frequently seen in the
polytraumatized patient, would effectively lower the threshold. In
addition, these authors noted that in traumatized muscle, cellular
damage was noted when the tissue pressure was within 40 mm Hg of the
animal’s MAP.
needle-infusion technique, reported that no patient with an absolute
pressure less than 45 mm Hg developed a compartment syndrome (35).
Almost all patients with intracompartmental pressures greater than 50
mm Hg, however, were noted to have true compartment syndromes. Matsen
et al., using an external compression device to elevate
intracompartmental pressure, and using themselves as volunteers, also
demonstrated that elevating the limb to approximately 50 cm above the
level of the heart lowered the tolerance of the leg to increased
intracompartmental pressures by as much as 35 mm Hg (34). They attributed this to the relative hypotension of the limb and to decreased perfusion pressure.
We have used similar pressure thresholds in our practice. Our clinical
and animal studies support the conclusion that the threshold
intracompartmental pressure at which fasciotomy is recommended is 30 mm
Hg for 8-hour pressurization in an acute compartment syndrome (18,19 and 20,40). Using a similar dog model, our lab has also demonstrated that the pressure threshold is lowered during systemic hypotension (65).
Because the total time of elevated tissue pressure and the absolute
pressure levels before measurement are usually unknown in most cases of
ACS, we recommend that intracompartmental pressure greater than 30 to
35 mm Hg, combined with other positive clinical findings, warrants
fasciotomy (Fig. 13.12).
 |
|
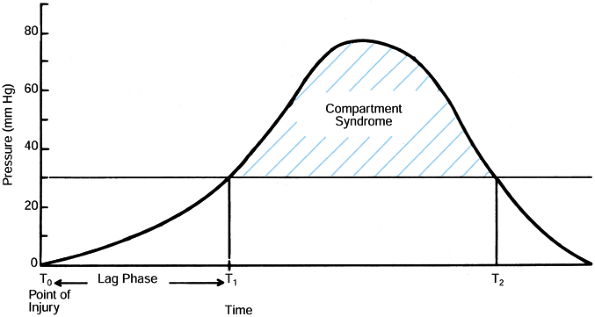
Figure 13.12. The time between injury and the onset of a compartment syndrome may vary from hours to days (lag phase, T0 to T1). The ischemia of the muscle and nerves of the compartment does not take place until the pressure rises to more than 30 mm Hg (horizontal line).
Thus, the time from injury to diagnosis and treatment is only a relative indicator of the ischemic period unless the tissue pressure has been monitored from the point of injury. (From Gelberman RH, Garfin SR, Hergenroeder PT, et al. Compartment Syndromes of the Forearm: Diagnosis and Treatment. Clin Orthop 1981;161:252.) |
for irreversible tissue damage, all authors agree that
intracompartmental pressure measurement is only one of many factors
that play a role in determining the need for fasciotomy. Any absolute
pressure threshold is a relative indication for decompression that
should be tempered by several patient factors, including overall
condition, systemic blood pressure, peripheral perfusion, trend of
symptoms and signs, trend of intracompartmental pressures, and the
cooperation and reliability of the patient.
carefully document the time of injury as well as the initial and
subsequent examinations. Perform and record a sensory examination to
include light touch, pin prick, and two-point discrimination. The
nurses and treating physician must monitor these values frequently.
Perform intracompartmental pressure measurements when indicated and
carefully document them as well.
possible compartment syndrome. The consequences for a patient from a
missed compartment syndrome can be devastating. The best way to ensure
early diagnosis and treatment is to understand the pathophysiology of
the syndrome, take all possible cases seriously, and deal with them on
an emergency basis.
considered, but the exam is equivocal and/or the intracompartmental
pressure is not high enough to warrant a fasciotomy, take all measures
possible to attempt to prevent a full-blown compartment syndrome from
occurring. Initially, remove all constrictive dressings and split or
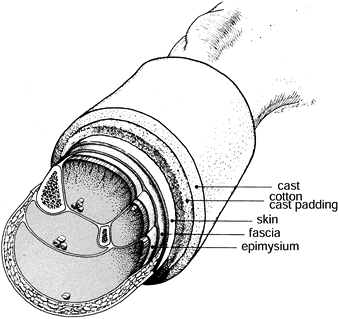
remove any casts (Fig. 13.13). Garfin et al. used a canine model to show that a cast can restrict volume expansion of a compartment by 40% (14). In addition, univalving and spreading the cast decreased compartment pressure by as much as 65%.
 |
|
Figure 13.13.
The circumferential cast is the first envelope that must be opened to decrease intracompartmental pressures. In laboratory studies, we have demonstrated that cast univalving and spreading decreased the intracompartmental pressure by as much as 65%. (From Garfin SR, Mubarak SJ, Evans KL, et al. Quantification of Intracompartmental Pressure and Volume under Plaster Casts. J Bone Joint Surg 1981;63-A:449.) |
developing a compartment syndrome is important. Frequently the limb is
elevated to decrease swelling. Matsen and colleagues have shown,
however, that elevation of the limb reduces mean arterial pressure as
well as the arteriovenous gradient and thereby reduces capillary flow (34).
Leaving the limb dependent may increase the mean arterial pressure, but
significant swelling may occur and also increase the risk of developing
a compartment syndrome. Therefore, place any extremity with an
incipient compartment syndrome, or at risk of developing a compartment
syndrome, at the level of the heart.
-
Place a tourniquet on the limb but do not
elevate it unless absolutely necessary. Administer prophylactic
antibiotics intravenously. -
We recommend long extensile skin
incisions, both to supplement the fascial decompression and to prevent
iatrogenic injury to nearby vessels, tendons, and nerves. Carefully
debride obvious areas of muscle necrosis at the initial operation, but
areas of muscle with uncertain vascularity can be left until the
patient is returned to the operating room in 24 to 72 hours, when
muscle viability can be determined more accurately. -
At the completion of all fasciotomies,
measure the pressure in several areas of each compartment to ensure
adequate decompression.
structures of the forearm, particularly the median nerve, when
decompressing a forearm compartment syndrome. For this reason, use an
extensive volar skin incision, avoiding a more limited “percutaneous”
approach. Two common approaches to the volar forearm and a single
dorsal approach are used to release the three forearm compartments.
-
Begin the skin incision proximal to the antecubital fossa on the ulnar aspect of the arm and cross the antecubital
P.405
fossa horizontally in the flexion crease (Fig. 13.14).
Extend the incision down the forearm in an S shape to the wrist flexion
crease and then cross the crease into the palm to allow release of the
carpal tunnel.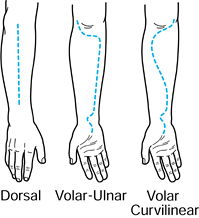 Figure 13.14.
Figure 13.14.
The dorsal and volar incisions used for forearm decompression. Both
volar incisions provide excellent exposure to the major neurovascular
structures. The volar curvilinear incision also provides access to the
mobile wad compartment. (Redrawn from Gelberman RH, Zakaib GS, Mubarak
SJ, et al. Decompression of Forearm Compartment Syndromes. Clin Orthop 1978;134:225.) -
Incise the superficial fascia throughout
the length of the forearm. Identify the median nerve proximally and
release the lacertus fibrosus. -
Release the proximal edge of the pronator
teres and the proximal edge of the flexor digitorum superficialis.
Follow the median nerve into the forearm between the flexor digitorum
superficialis and profundus and decompress it all the way to the carpal
tunnel. Preserve the palmar cutaneous branch of the median nerve. -
Incise the transverse carpal ligament to release the carpal tunnel.
-
Inspect the deep volar muscles (flexor
digitorum profundus, flexor pollicis longus, and pronator quadratus)
and release their respective fascial coverings as needed. With
undermining of the skin, the mobile wad can also be reached through
this approach.
-
Begin the incision on the lateral aspect
of the arm above the antecubital fossa. Extend it horizontally across
the elbow flexion crease, down the ulnar border of the forearm, and
then continue into the palm (Fig. 13.14). -
Carry out the remainder of the decompression as described above for the Henry approach.
the forearm and found no significant differences between the two in
regard to adequate decompression (15). The
mobile wad, however, cannot be easily reached through the volar ulnar
approach, and a second dorsal incision is needed to release this
compartment.
dorsal and mobile wad compartments can be measured to determine whether
decompression is needed. If there is any question as to the need for
decompression, proceed with fasciotomy. The dorsal incision is similar
to that used in the Thompson approach and can extend from the lateral
epicondyle to the midportion of the wrist (Fig. 13.14).
Usually the entire skin incision is not needed, and with adequate
undermining of the skin, the fascia of the dorsal and mobile wad
compartments is readily released. As previously mentioned, the mobile
wad can also be released from the volar curvilinear approach of Henry.
compartment syndrome. As described above, there are four compartments
in the leg. In general, all compartments must be released in acute
compartment syndrome of the leg.
used routinely in our practice for fasciotomy of the four leg
compartments (38).
-
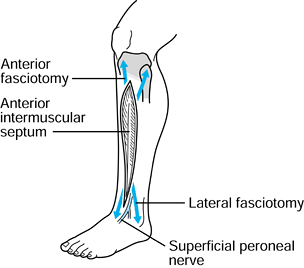
To approach the anterior and lateral
compartments, make a longitudinal skin incision 20 to 25 cm long,
halfway between the fibular shaft and tibial crest (Fig. 13.15). This lies approximately over the anterior intermuscular
P.406
septum dividing the anterior and lateral compartments and allows easy
access to both. Undermine the skin edges proximally and distally to
provide wide exposure of the fascia.![]() Figure 13.15.
Figure 13.15.
The two-incision technique for four-compartment release of the leg.
Anterolateral incision, step 1. (Redrawn from Mubarak SJ, Hargens AR.
Diagnosis and Management of Compartment Syndromes. In: AAOS: Symposium on Trauma to the Leg and Its Sequelae. St. Louis: CV Mosby, 1981.) -
Make a small transverse incision in the
midportion of the leg, just through the fascia, to identify the
anterior intermuscular septum that separates the anterior compartment
from the lateral compartment (Fig. 13.16).
Identification of this septum is necessary to identify the superficial
peroneal nerve, which lies in the lateral compartment next to the
septum.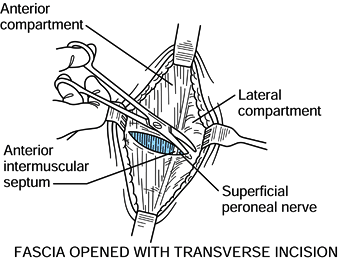 Figure 13.16. Anterolateral incision, step 2. (Redrawn from Mubarak SJ, Hargens AR. Diagnosis and Management of Compartment Syndromes. In: AAOS: Symposium on Trauma to the Leg and Its Sequelae. St. Louis: CV Mosby, 1981.)
Figure 13.16. Anterolateral incision, step 2. (Redrawn from Mubarak SJ, Hargens AR. Diagnosis and Management of Compartment Syndromes. In: AAOS: Symposium on Trauma to the Leg and Its Sequelae. St. Louis: CV Mosby, 1981.) -
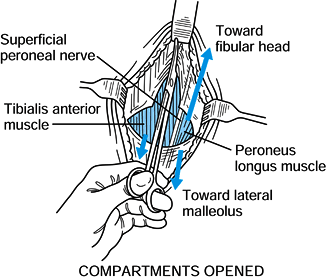
Using a 12-in. (31-cm) Metzenbaum
scissors, open the anterior compartment fascia. Visualization is aided
by retraction with right-angle retractors. Push the scissors with the
tips open slightly in the direction of the great toe distally and
proximally toward the patella (Fig. 13.17). If
there is any question whether the tip of the scissors has strayed from
the fascia, lengthen the skin incision to ensure complete fascial
release. Blind repeat attempts to complete the fasciotomy are dangerous
and can injure vascular, nervous, and tendinous structures.![]() Figure 13.17. Anterolateral incision, step 3. (Redrawn from Mubarak SJ, Hargens AR. Diagnosis and Management of Compartment Syndromes. In: AAOS: Symposium on Trauma to the Leg and Its Sequelae. St. Louis: CV Mosby, 1981.)
Figure 13.17. Anterolateral incision, step 3. (Redrawn from Mubarak SJ, Hargens AR. Diagnosis and Management of Compartment Syndromes. In: AAOS: Symposium on Trauma to the Leg and Its Sequelae. St. Louis: CV Mosby, 1981.) -
Make the lateral compartment fasciotomy
in line with the fibular shaft. Direct the scissors proximally toward
the fibular head and distally toward the lateral malleolus. In this way
the fascial incision is posterior to the superficial peroneal nerve.
Adequate visualization is essential, so do not hesitate to extend the
skin incision if necessary. -
Make a second longitudinal incision, 20
to 25 cm long, on the posteromedial side of the leg to approach the
superficial and deep posterior compartments (Fig. 13.18).
Place this incision 2 to 3 cm posterior to the posterior tibial margin.
If the incision is too anterior, the tibia may be left exposed after
the skin edges retract. An anterior incision also risks injury to the
saphenous vein and nerve, which course along the posterior margin of
the tibia.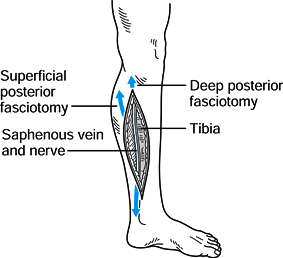 Figure 13.18. Posteromedial incision, step 1. (Redrawn from Mubarak SJ, Hargens AR. Diagnosis and Management of Compartment Syndromes. In: AAOS: Symposium on Trauma to the Leg and Its Sequelae. St. Louis: CV Mosby, 1981.)
Figure 13.18. Posteromedial incision, step 1. (Redrawn from Mubarak SJ, Hargens AR. Diagnosis and Management of Compartment Syndromes. In: AAOS: Symposium on Trauma to the Leg and Its Sequelae. St. Louis: CV Mosby, 1981.) -
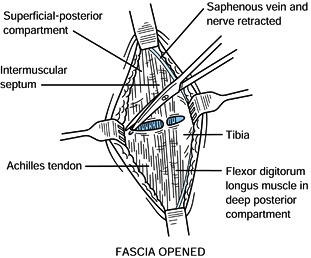
Undermine the skin edges and retract the
saphenous vein and nerve anteriorly. Make a transverse fascial incision
to allow identification of the septum between the deep and superficial
posterior compartments (Fig. 13.19). Identify
the tendon of the flexor digitorum longus in the deep posterior
compartment and the Achilles tendon in the superficial posterior
compartment.![]() Figure 13.19. Posteromedial incision, step 2. (Redrawn from Mubarak SJ, Hargens AR. Diagnosis and Management of Compartment Syndromes. In: AAOS: Symposium on Trauma to the Leg and Its Sequelae. St. Louis: CV Mosby, 1981.)
Figure 13.19. Posteromedial incision, step 2. (Redrawn from Mubarak SJ, Hargens AR. Diagnosis and Management of Compartment Syndromes. In: AAOS: Symposium on Trauma to the Leg and Its Sequelae. St. Louis: CV Mosby, 1981.) -
It is usually easiest to decompress the superficial posterior compartment first by extending the fasciotomy
P.407
proximally as far as possible and then distally behind the medial
malleolus. Release the deep posterior compartment distally and then
proximally under the soleus bridge (Fig. 13.20).
Release the soleus if it attaches to the tibia distally more than
halfway. In cases where both the deep and superficial compartments are
anchored to the posteromedial edge of the tibia, there is no
identifiable intermuscular septum and the deep posterior compartment
should be released from within the superficial posterior compartment by
retraction of the soleus off the tibia.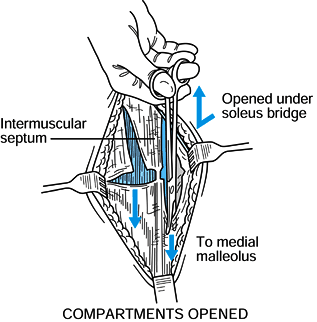 Figure 13.20. Posteromedial incision, step 3. (Redrawn from Mubarak SJ, Hargens AR. Diagnosis and Management of Compartment Syndromes. In: AAOS: Symposium on Trauma to the Leg and Its Sequelae. St. Louis: CV Mosby, 1981.)
Figure 13.20. Posteromedial incision, step 3. (Redrawn from Mubarak SJ, Hargens AR. Diagnosis and Management of Compartment Syndromes. In: AAOS: Symposium on Trauma to the Leg and Its Sequelae. St. Louis: CV Mosby, 1981.) -
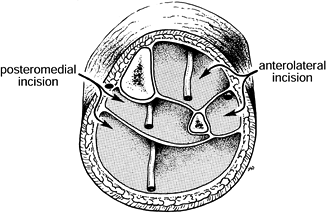
This completes a four-compartment decompression (Fig. 13.21).
If intraoperative pressure monitoring was used during the procedure, or
if there is any doubt as to the adequacy of the decompression, check
final intracompartmental pressures.![]() Figure 13.21.
Figure 13.21.
Decompression of the four compartments. (From Mubarak SJ, Owen CA.
Double-Incision Fasciotomy of the Leg for Decompression in Compartment
Syndromes. J Bone Joint Surg 1977;59-A:184.)
Centered over the lateral compartment, the incision must be extensile
in order to allow easy decompression of all four compartments.
-
Make an incision from the head of the
fibula to the lateral malleolus. Undermine the skin at the level of the
deep fascia both anteriorly and posteriorly in the middle third of the
wound sufficiently to visualize the posterior edge of the anterior
compartment, the lateral compartment, and the anterior edge of the
superficial posterior compartment. -
Make a transverse incision with a knife
through the deep fascia, beginning in the anterior compartment,
crossing the lateral compartment, and extending into the posterior
compartment. With careful inspection you can now identify the anterior
intermuscular septum running longitudinally between the anterior and
lateral compartments and the posterior intermuscular septum running
between the lateral and posterior compartments. It is very important to
take this step, because identification of the three compartments can be
difficult in the traumatized, distorted, and swollen limb. This
transverse incision is similar to that illustrated above for the
two-incision technique (Fig. 13.19 and Fig. 13.20). -
Using a knife or Metzenbaum scissors, as
described above for the two-incision technique, incise the deep fascia
of the anterior, lateral, and superficial posterior compartments for
the full length of each compartment. Be certain to decompress all
muscle proximally. Distally, at the level of the musculotendinous
junctions, further decompression is usually not necessary. -
Identify the deep posterior compartment.
Dissect the soleus free from its origin on the posterior intermuscular
septum in the middle or proximal third of the wound. In the nonswollen
limb the deep compartment lies about 1 cm deep to the superficial
posterior compartment, but in a limb with severe compartment syndrome
this compartment may be as deep as 2.5 cm. Release the deep fascia of
this compartment throughout its full length in a similar manner. As
with any technique, take care to avoid injury to the neurovascular
structures, particularly the common peroneal nerve proximally and its
branches distally.
We do not advise fibulectomy, as it destabilizes tibia fractures and
removes an important structure for reconstruction of the leg. It is
mentioned for historic interest only.
any of the long bones, perform external or internal fixation of the
fracture at the time of compartment decompression (17,33,53).
The obvious advantage of immobilizing the fracture is that care of the
fasciotomy wound is facilitated. The technique chosen for stabilization
should minimize further soft tissue damage if possible, and in the
lower extremity intramedullary nailing is an excellent choice. In some
lower extremity fractures, particularly high-grade tibia fractures,
intramedullary nailing may not be the optimal technique, and external
fixation may be a more prudent choice. The major disadvantage of an
external fixator is that mobilization of the skin for delayed primary
closure is more difficult, and skin grafting is usually required. For
forearm fractures, compression plates are the recommended choice for
fixation.
the high risk that exists for the development of an acute compartment
syndrome. Gershuni et al. have shown that, in the case of tibia
fractures and acute compartment syndrome, a good functional result can
be obtained if the compartment syndrome is readily identified and
adequately treated before irreversible muscle and nerve damage occur (17).
In cases where the compartment syndrome is missed, or treatment is
delayed, the patient may have a poor functional outcome despite
adequate treatment and healing of the tibia fracture.
particularly in the treatment of femur and tibia fractures with
intramedullary nailing, you should be aware of the possibility of
inducing a compartment syndrome. This was discussed above in the
etiology of acute compartment syndromes. In all cases of intramedullary
nailing of the femur and tibia, carefully inspect the thigh and leg for
tight compartments. If there is any evidence of increased
intracompartmental pressure, measure the pressure in all compartments
before the end of the procedure. If indicated, perform fasciotomies
immediately.
Anterior and lateral compartment fasciotomies should be considered in
patients undergoing tibia osteotomies or leg-lengthening procedures, or
when the tibia is used as a donor bone graft site. When debriding an
open tibial fracture, release those compartments accessible through the
exposed wound.
developed a thrombus or who have had a femoral artery bypass are
especially prone to developing compartment syndromes. In our
experience, patients with postischemic compartment syndrome have a poor
prognosis because the period of ischemia caused by the arterial injury
is added to the compartment syndrome that results after reinstitution
of arterial flow. If the arterial ischemia has been present for more
than 4 to 6 hours, prophylactic fasciotomy of the leg or forearm is
warranted at the time of arterial repair.
been established by history, physical examination, and
intracompartmental pressure measurement, fasciotomy is usually
required. After the diagnosis and surgical treatment are outlined to
patients, however, many prefer to limit their running or alter their
exercise program. The patient choosing nonoperative treatment must also
be counseled about the possibility of developing an acute compartment
syndrome if the symptoms are ignored and the activity is not modified (39). In our experience, most patients who desire to maintain a given level of jogging or running require fasciotomy.
same as that used for ACS; however, the skin incisions can be much
smaller, in the range of 5 to 6 cm (Fig. 13.22).
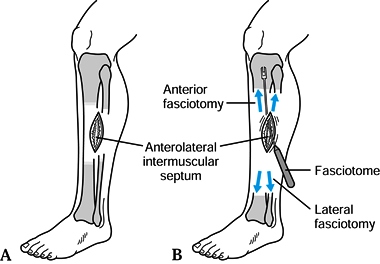 |
|
Figure 13.22.
Chronic anterior compartment decompression: anterolateral fasciotomies in the absence of a fascial hernia. (Redrawn from Fronek J, Mubarak SJ, Hargens AR, et al. Management of Chronic Exertional Anterior Compartment Syndrome of the Lower Extremity. Clin Orthop 1987;220:217.). |
-
Place a small 5-cm incision between the
tibial crest and fibular shaft, overlying the anterolateral
intermuscular septum. In the absence of a fascial hernia, place the
incision in the midportion of the leg. -
Extensive undermining and a fasciotome
aid limiting the skin incision while still performing a safe and
satisfactory fasciotomy (Fig. 13.23).![]() Figure 13.23. A 12-in. Metzenbaum scissors (bottom) or a fasciotome (top) is useful for decompression of the leg. (From Mubarak SJ, Hargens AR. Diagnosis and Management of Compartment Syndromes. In: AAOS: Symposium on Trauma to the Leg and Its Sequelae. St. Louis: CV Mosby, 1981.)
Figure 13.23. A 12-in. Metzenbaum scissors (bottom) or a fasciotome (top) is useful for decompression of the leg. (From Mubarak SJ, Hargens AR. Diagnosis and Management of Compartment Syndromes. In: AAOS: Symposium on Trauma to the Leg and Its Sequelae. St. Louis: CV Mosby, 1981.) -
Muscle hernias frequently occur in the
lower third of the leg in the area overlying the anterior intermuscular
septum. This is the site of emergence through the fascia of one or both
sensory branches of the superficial peroneal nerve (Fig. 13.24).
For this reason, make the skin incision over the hernia and perform the
fasciotomy at the fascial defect. This allows easy decompression of
both the anterior and lateral compartments and allows simultaneous
decompression of the nerve.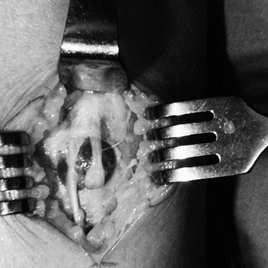 Figure 13.24.
Figure 13.24.
Intraoperative photograph of a fascial defect, bulging peroneus brevis
muscle, and a medial dorsal cutaneous nerve exiting from the defect.
The intermediate dorsal cutaneous nerve has developed a neuroma. (From
Garfin SR, Mubarak SJ, Owen CA. Exertional Anterolateral Compartment
Syndrome: Case Report with Fascial Defect, Muscle Herniation, and
Superficial Peroneal Nerve Entrapment. J Bone Joint Surg 1977;59-A:404.) -
Do not close the fascial defect
associated with a muscle hernia because of the risk of precipitating an
acute compartment syndrome. -
After making the skin incision, carry out decompression of the anterior and lateral compartments as described earlier (Fig. 13.25).
![]() Figure 13.25. Chronic anterior compartment decompression: anterolateral fasciotomies in the presence of a fascial hernia. A: Place the incision over the fascial defect, with attention to the superficial peroneal nerve and its branches. B: Enlarge the defect across the intermuscular septum (1) to gain entry into both compartments. C:
Figure 13.25. Chronic anterior compartment decompression: anterolateral fasciotomies in the presence of a fascial hernia. A: Place the incision over the fascial defect, with attention to the superficial peroneal nerve and its branches. B: Enlarge the defect across the intermuscular septum (1) to gain entry into both compartments. C:
Achieve complete longitudinal release of the anterior compartment by
passing the fasciotome in the direction of the patella proximally (2) and distally in the direction of the great toe (3). D: In the lateral compartment, direct the scissors or fasciotome posterior to the fibular head (4) and lateral malleolus (5).
(Redrawn from Fronek J, Mubarak SJ, Hargens AR, et al. Management of
Chronic Exertional Anterior Compartment Syndrome of the Lower
Extremity. Clin Orthop 1987;220:217.) -
With CCS of the anterior compartment, we
usually recommend release of both the anterior and lateral
compartments. In cases of posterior compartment decompression, perform
fasciotomy of the posterior compartments as described above for ACS,
but through a smaller incision.
-
Following adequate compartment
decompression, pack the wounds loosely open with saline-dampened gauze
and apply a bulky dressing. -
Never close the skin incisions
immediately after fasciotomy. At 24 to 72 hours after fasciotomy,
return the patient to the operating room for repeat debridement if
necessary and partial or even complete skin closure. Frequently the
compartment syndrome is associated with an open fracture, and the
patient is returned to the operating room several times for repeat
debridement related to the fracture and soft tissue injury. -
Most commonly, fasciotomy wounds are not
amenable to delayed primary closure, and split-thickness skin grafting
is necessary for wound closure. With the two-incision technique in the
leg, one wound can usually be closed primarily on a delayed basis, and
the other wound covered with a split-thickness skin graft. In the
P.411
case
of compartment syndrome associated with open fractures, quantitative
cultures can be used to determine the appropriate time for skin
grafting. -
Begin active and active-assisted range of
motion of the adjacent joints on the second day after fasciotomy. After
split-thickness skin grafting, immobilize the limb for 3 to 5 days to
decrease sheer forces across the graft and allow full incorporation.
When the skin graft has incorporated, reinstitute range-of-motion
exercises. -
In contrast to fasciotomy after ACS,
close the wound with an interdermal running stitch after fasciotomy for
CCS. Apply a light dressing and allow the patient weight bearing as
tolerated with the use of crutches as needed. After suture removal at 2
to 3 weeks, initiate light running and progress exercises as tolerated
over the next 3 to 6 weeks according to the patient’s abilities and
pain tolerance.
are related to a delay in diagnosis and treatment and/or to inadequate
decompression. The sequelae in such cases can be devastating to the
patient. Despite an abundance of clinical and basic science work on the
subject, compartment syndromes remain poorly understood. Because of
their relatively infrequent occurrence, they may be overlooked by a
treating physician who does not deal often with patients at risk,
namely the polytraumatized patient and the intravenous drug user. In
addition, the clinical findings of a compartment syndrome are rather
subjective and rely heavily on patient cooperation.
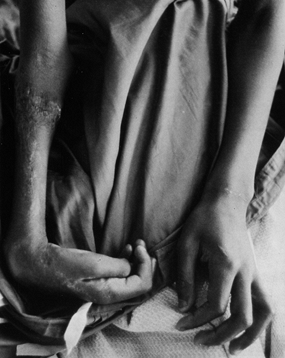
treated compartment syndrome is Volkmann’s ischemic contracture of the
extremity (Fig. 13.26). This results in a limb
with a varying amount of deformity and functional loss, depending on
the severity of the muscle and nerve injury. Muscles, nerves, tendons,
bone, vessels, and skin can all be affected by the ischemia from a
compartment syndrome. Irreversible muscle damage has been shown to
occur after 4 to 6 hours of complete ischemia (21). Hargens et al. showed in a canine model that, at a pressure of 30 mm Hg, irreversible muscle necrosis occurred at 8 hours (20).
In addition, they noted that this 8-hour threshold can be shorter in
cases of hypotension. Irreversible nerve damage occurs after 6 to 12
hours of ischemia at intracompartmental pressures between 30 and 40 mm
Hg (19,54).
 |
|
Figure 13.26.
Volkmann’s ischemic contracture in a child who suffered compartment syndrome of the forearm. Note the deformities of the wrist and hand. (From Mubarak SJ, Hargens AR. Compartment Syndromes and Volkmann’s Contracture. Philadelphia: WB Saunders, 1981.) |
immediate institution of treatment will help prevent Volkmann’s
ischemic contracture and restore normal function to a limb that has
suffered a compartment syndrome. In addition, if there is any question
as to the adequacy of decompression, intracompartmental pressure
measurements should be obtained in the operating room after fasciotomy.
iatrogenic injury during the treatment of compartment syndrome. Nerves,
blood vessels, and even tendons can be injured during compartment
release if careful attention is not paid to compartmental anatomy and
if there is inadequate visualization. In such cases, immediately repair
the injured structure(s).
its possible sequelae is to understand its pathophysiology and focus on
prevention of the disorder. We routinely
univalve
and spread all casts used in the treatment of closed fractures of the
long bones or use splints in the acute postinjury period before
placement of a potentially constricting cast. In patients undergoing
surgical procedures that have an associated risk of compartment
syndrome, such as tibial osteotomies and open treatment of tibial
plateau fractures, we usually perform a prophylactic fasciotomy.
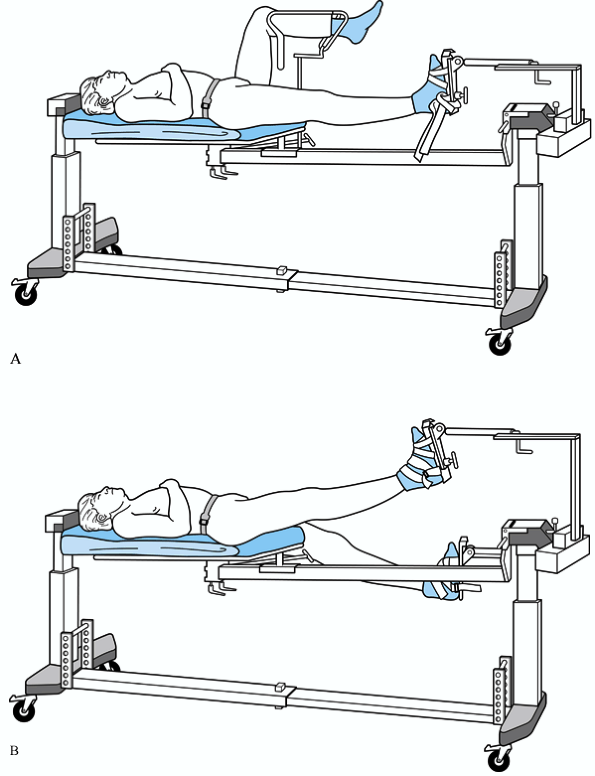
development of compartment syndrome in the well leg from positioning on
the fracture table (2,11).
Similarly, there have been many reports in the urology and gynecology
literature on the development of compartment syndrome in legs placed in
the lithotomy position (1,42,49).
Most commonly, these patients are in the lithotomy or hemilithotomy
position for prolonged periods of time (>4 hours). Heavy patients
and periods of intraoperative hypotension may also be risk factors,
although this has not been studied.
syndrome in the well leg from positioning on the fracture table. All
cases involved heavy patients and prolonged time in the well-leg
holder. These cases, along with those reported in the literature,
prompted an investigation in our lab into the pathophysiology of this
complication.
hemilithotomy position on a fracture table and found that the well-leg
holder caused a significant increase in calf intramuscular pressure and
a significant decrease in ankle blood pressure. More importantly, the
well-leg holder caused a significant decrease in P, the difference between ankle blood pressure and calf intramuscular pressure. In the lateral compartment P averaged only 31 mm Hg, a level very close to the ischemic threshold of muscle as reported by Heppenstall et al. (25).
complicated fracture cases which may take several hours to complete. We
frequently place both legs in traction boots and “scissor” the limbs (Fig. 13.27),
or use the lateral position on the fracture table. Supine or lateral
positioning on an image table without the use of traction are also good
alternatives. If the hemilithotomy position is used, we recommend
palpating the well-leg compartments and temporarily lowering the limb
every 2 to 3 hours. After all fluoroscopic images have been obtained,
the well leg should be immediately placed into a more relaxed, lowered
position.
 |
|
Figure 13.27.
Several cases have been reported in the literature of compartment syndrome of the leg from the use of a well-leg holder. This places the limb at 90° of flexion at both the hip and knee, and the elevated leg rests on a support (A). In complicated fracture cases, which may take several hours to complete, we recommend alternate limb positioning, such as the “scissors” position (B). |
nurses to examine the peripheral neurovascular status are extremely
important. We educate our nurses, medical students, and residents on
the evaluation and early recognition of patients with compartment
syndrome.
recognize an early compartment syndrome. Often, the patient is unable
or unwilling to cooperate with a physical exam, and the treating
physician must rely on his or her knowledge of the disorder and use
early intracompartmental pressure monitoring. The risk of measuring
compartment pressures is nominal compared with the risk of missing a
compartment syndrome. We generally teach residents and students that
the time to measure intracompartmental pressures is when they first
think about it.
arterial line pressure transducer and recorder to measure compartment
pressures. These are readily available in intensive care units,
recovery rooms, emergency rooms, and, of course, the operating room. We
also use the hand-held Stryker unit with the slit catheter or the
side-ported needle. We avoid the use of an 18-gauge needle alone. When
measuring pressures, we take multiple readings throughout each
compartment and immediately document them. In patients who are obtunded
or comatose and who are in a monitored setting, we frequently employ
continuous intracompartmental pressure monitoring when initial
pressures are elevated but not above the threshold for fasciotomy.
emergency. Although it may take 4 to 6 hours for irreversible muscle
damage to occur, it is usually impossible to determine the length of
time the compartment pressure has been elevated and at what pressure.
Furthermore, delaying treatment usually leads to poor outcome.
curvilinear volar incision, as this allows access to the entire
forearm, neurovascular structures, mobile wad, and radius if fixation
is required. After volar release, the dorsal compartment is measured,
and, if necessary, this compartment is released through a single dorsal
incision.
the two-incision technique. This allows excellent visualization and
easy release of all compartments. The deep posterior compartment is
readily accessible through the medial incision, even in patients with
severe swelling. Usually one of the incisions can be closed in a
delayed fashion, and skin grafting is then required only for the second
wound. A potential disadvantage to the two-incision technique is that
it tempts the inexperienced surgeon to use short incisions in the
midcalf. We use fairly extensive incisions,
as
the risks of “blind” fasciotomy are significant. Another potential
disadvantage to this technique is uncovering of the posteromedial edge
of the tibia if the skin incision is not properly placed. It is very
important to make the posteromedial incision several centimeters below
the posterior margin of the tibia in order to prevent this complication.
One advantage to this technique is that it is cosmetically more
acceptable, as it is less visible from the front. It must be an
extensile incision, and this minimizes the risk of injury to
neurovascular structures. Although the wound could potentially be
closed secondarily in its entirety, this is usually not the case;
similar to the two-incision technique, a portion of the wound usually
requires coverage with a
split-thickness
skin graft. A potential disadvantage to the single-incision technique
is that the deep posterior compartment is more difficult to reach,
particularly in a very swollen limb. The approach, however, is similar
to the Harmon posterolateral approach to the tibia for bone grafting,
an approach used commonly by many orthopaedic surgeons.
elevate it unless absolutely necessary. Inflation of the tourniquet
during compartment release simply adds to the ischemia time. With
careful dissection, blood loss can be kept to a minimum. Debridement of
obviously necrotic and other nonviable muscle is done primarily at the
time of compartment decompression. If, however, the viability of an
area of muscle is in question, we do not debride this area but rather
reevaluate the wound in 24 to 48 hours. In acute compartment syndrome,
we never close the fasciotomy wounds primarily and always return the
patient to the operating room in 24 to 48 hours for repeat debridement.
Frequently, a patient may require multiple debridements before the
wound is clean enough for delayed primary closure or split-thickness
skin grafting.
through staples placed near the wound edges. This prevents the skin
edges from retracting significantly, and they can also be
intermittently tightened to help decrease the wound size, particularly
in the sick patient who is too unstable to return to the operating room
within the first few days after fasciotomy. It is very important not to
place the vessel loops too tight initially. The dermotomy is an
essential component to compartmental release, and tightening the skin
could significantly increase the intracompartmental pressure.
system, such as the Sure-Closure (Life Medical Sciences, Inc.,
Princeton, NJ), to help close fasciotomy wounds in a delayed fashion.
There are several such devices on the market; when properly used, they
can obviate the need for a skin graft or at least significantly
decrease the size of the graft.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
LM, Loughlin JS, Morin CJ, Haning RV Jr. Bilateral Compartment Syndrome
after a Long Gynecologic Operation in the Lithotomy Position. Am J Obstet Gynecol 1990;162:1271.
BC, Hurley PE, Clark CA, McLaughlin CS. Complications Associated with
the Use of an Infusion Pump During Knee Arthroscopy. Arthroscopy 1992;8:224.
GA, Gross JH, Watenpaugh DE, et al. Near-Infrared Spectroscopy for
Monitoring of Tissue Oxygenation of Exercising Skeletal Muscle in a
Chronic Compartment Syndrome Model. J Bone Joint Surg 1997;79-A:838.
AG, Styf JR, Mubarak SJ, Hargens AR. A New Transducer-Tipped Fiber
Optic Catheter for Measuring Intramuscular Pressures. J Orthop Res 1990;8:464.
TW, Schutzer SF, Deafenbaugh MK, Bartosh RA. Compartment Syndrome
Complicating Use of the Hemilithotomy Position During Femoral Nailing:
A Report of Two Cases. J Bone Joint Surg. 1989;71-A:1556.
SR, Mubarak SJ, Owen CA. Exertional Anterolateral Compartment Syndrome:
Case Report with Fascial Defect, Muscle Herniation, and Superficial
Peroneal Nerve Entrapment. J Bone Joint Surg. 1977;59-A:404.
DH, Yaru NC, Hargens AR, et al. Ankle and Knee Position as a Factor
Modifying Intracompartmental Pressure in the Human Leg. J Bone Joint Surg 1984;66-A:1415.
AR, Akeson WH, Mubarak SJ, et al. Fluid Balance within the Canine
Anterolateral Compartment and Its Relationship to Compartment
Syndromes. J Bone Joint Surg 1978;60-A:499.
MM, Whitesides TE Jr, Grewe SR, et al. Histologic Determination of the
Ischemic Threshold of Muscle in the Canine Compartment Syndrome Model. J Orthop Trauma 1993;7:199.
MM, Whitesides TE Jr, Grewe SR, Rooks MD. Compartment Pressure in
Association with Closed Tibial Fractures. The Relationship between
Tissue Pressure, Compartment, and the Distance from the Site of the
Fracture. J Bone Joint Surg 1994;76-A:1285.
RB, Sapega AA, Scott R, et al. The Compartment Syndrome. An
Experimental and Clinical Study of Muscular Energy Metabolism Using
Phosphorus Nuclear Magnetic Resonance Spectroscopy. Clin Orthop 1988;226:138.
BP, Carr CF, Shirreffs TG. Compartment Syndrome after Arthroscopic
Surgery of the Knee. A Report of Two Cases Managed Nonperatively. Am J Sports Med 1997;25:123.
FA III, Mayo KA, Krugmire RB, et al. A Model Compartmental Syndrome in
Man with Particular Reference to the Quantification of Nerve Function. J Bone Joint Surg 1977;59-A:648.
BR, Thorderson PK. Measurement of Intracompartmental Pressure: A
Comparison of the Slit Catheter, Side-Ported Needle, and Simple Needle.
J Bone Joint Surg 1993;75-A:231.
LR, Styf JR, Pedowitz RA, et al. Intramuscular Deoxygenation During
Exercise in Patients Who Have Chronic Anterior Compartment Syndrome of
the Leg. J Bone Joint Surg 1997;79-A:844.
RA, Hargens AR, Mubarak SJ, Gershuni DH. Modified Criteria for
Objective Diagnosis of Chronic Compartment Syndrome of the Leg. Am J Sports Med 1990;18:35.
JA, Price CT, Knapp RD Jr. Compartment Syndrome of the Lower Extremity
after Intraosseous Infusion of Fluid: A Report of Two Cases. J Bone Joint Surg 1993;75-A:430.
CH, Castle GSP, Hardie R, Logan J. Compartmental Pressure Measurements:
An Experimental Investigation Using the Slit Catheter. J Trauma 1981;21:446.
SS, Hargens AR, Evans KL, et al. Skeletal Muscle Necrosis in
Pressurized Compartments Associated with Hemorrhagic Hypotension. J Trauma 1980;20:941.