Brachial Plexus Blocks
II – Single-Injection Peripheral Blocks > A – Upper Extremity > 7
– Brachial Plexus Blocks
The cricoid cartilage (indicative of the transverse process of C6), the
clavicular head of the sternocleidomastoid muscle, and the anterior and
middle scalene muscles with the interscalene groove in between (Fig. 7-1).
The cricoid cartilage is identified with the head in the neutral
position. The patient is then instructed to turn his or her head 30° to
the opposite side and to raise the head slightly off the bed. The two
heads of the sternocleidomastoid muscle are identified with particular
emphasis being placed on the lateral border of the clavicular head (Fig. 7-2A).
Next, the patient is asked to place the head back on the bed and relax.
The anterior scalene muscle is then identified immediately lateral and
deep to the lateral border of the clavicular head, and the fingers are
rolled or “walked” laterally into the interscalene groove between the
anterior and middle scalene muscles. The interscalene groove is marked.
A horizontal line is drawn at the level of the cricoid cartilage
laterally to intersect the interscalene groove. The insulated needle
connected to a nerve stimulator (1.0 mA, 2 Hz, 0.1 ms) is inserted at
the point of intersection between these two lines and is directed
perpendicularly to the skin in a medial, caudal, and posterior
direction (Fig. 7-2B).
The position of the needle is adjusted to maintain the same motor
response with a current less than 0.5 mA. After negative aspiration for
blood, the local anesthetic is slowly
injected with repeated aspiration for blood every 5 mL to be distributed around the brachial plexus (Fig. 7-3).
 |
|
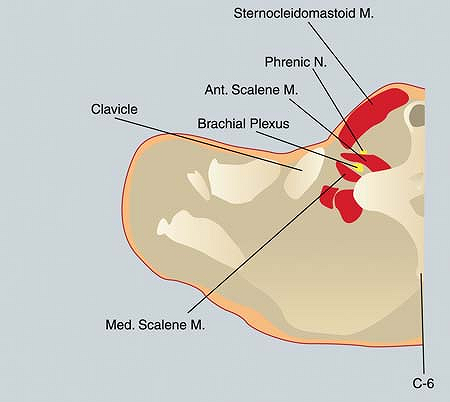
Figure 7-1.
The cricoid cartilage, the clavicular head of the sternocleidomastoid muscle, and the anterior and middle scalene muscles with the interscalene groove in between. |
Inability to elevate the arm (the “deltoid sign”). (b) The “money
sign,” in which the patient rubs the thumb against the index and middle
fingers indicating the onset of paresthesia or numbness in the
distribution of C6 and C7.
-
Sedation should be minimal, its purpose to allay anxiety without inhibiting the ability of the patient to communicate.
-
In a limited number of patients, the
clavicular head of the sternocleidomastoid muscle is either not present
or exists as an indistinct band. In these situations, the interscalene
groove is situated 3 cm lateral to the lateral border of the main belly
of the sternocleidomastoid muscle at the level of the cricoid
cartilage. This “3-cm mark” is a useful aid in obese individuals as
well. Additional landmarks include the external jugular vein and the
pulsation of the subclavian artery as it crosses over the first rib
between the anterior and middle scalene muscles at the lower end of the
interscalene groove. -
Patient discomfort is reduced by superficially infiltrating the skin with lidocaine using a 26-gauge needle.
-
To facilitate the insertion of the insulated needle, the skin can first be punctured with an 18-gauge needle.P.60
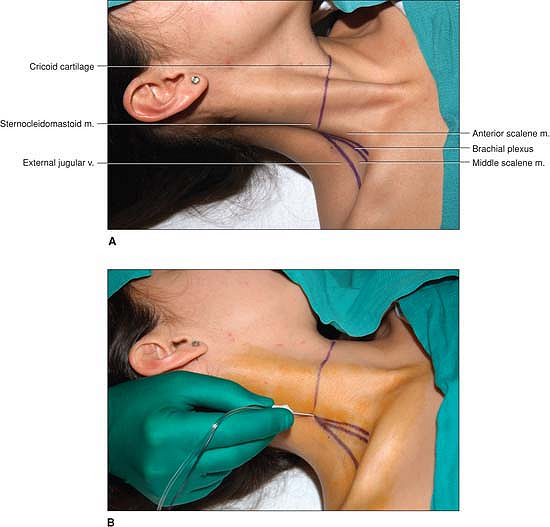 Figure 7-2. A:
Figure 7-2. A:
The two heads of the sternocleidomastoid muscle are identified with
particular emphasis being placed on the lateral border of the
clavicular head. B: The insulated needle
connected to a nerve stimulator is inserted at the point of
intersection between these two lines and directed perpendicularly to
the skin in a medial, caudal, and posterior direction. -
Insertion of the insulated needle with
the bevel facing the sheath increases the likelihood of feeling the
tactile sensation of “popping through the sheath.” -
Diaphragmatic movement is indicative of
stimulation of the phrenic nerve and of needle placement
medial/anterior to the interscalene groove. The needle should be
withdrawn and redirected in a more lateral/posterior direction. In
contrast, a suprascapular or trapezius muscle contraction is indicative
of needle placement lateral to the interscalene groove. The needle
should be withdrawn and redirected in a more medial/anterior direction. -
Positioning of the needle to maintain a motor response at 0.3 to 0.5 mA is associated with a high success rate.
-
It is essential that the caudal direction of the needle be maintained (Fig. 7-4). Insertion of the needle in a neutral/horizontal direction (i.e., midway between caudal
P.61
and cephalad) or in a cephalad direction facilitates neuraxial
positioning of the needle as well as the vertebral artery injection of
local anesthetic.![]() Figure 7-3.
Figure 7-3.
The local anesthetic is slowly injected with repeated aspiration for
blood every 5 mL to be distributed around the brachial plexus. -
A triangular-shaped swelling of the apex
at the point of needle insertion and the base at the lower end of the
interscalene groove may be noted, especially in thin individuals, as
the injection progresses and is indicative of correct needle placement.
In contrast, a circular swelling around the point of needle insertion
is more likely indicative of injection into the subcutaneous tissues
(i.e., superficial to the brachial plexus sheath). -
“Pressure paresthesia,” which is
described as a dull ache following the rapid injection of 2 to 3 mL of
local anesthetic, should be differentiated from the severe pain
produced by an intraneural injection. This “pressure paresthesia”
appears to be more commonly encountered with an interscalene block than
with other peripheral nerve blocks and may be referred to the shoulder
or more distally. -
This block is not indicated alone for a
surgery involving an area around the axilla (e.g., inferior capsular
shift). In addition, variations in the extent of T2 innervation may
result in inadequate anesthesia of the anterior and posterior
arthroscopic portal sites. These limitations can be overcome by local
infiltration to the appropriate sites. -
An interscalene block is not indicated
alone for surgery to the medial aspect of the upper extremity, as nerve
roots C8 and T1 are not consistently blocked with this approach to the
brachial plexus.P.62 Figure 7-4. The caudal direction of the needle needs to be maintained.
Figure 7-4. The caudal direction of the needle needs to be maintained. -
Side effects are commonly associated with
an interscalene block. A 100% incidence of phrenic nerve blockade (due
to the phrenic nerve’s C3-5 derivation) occurs, resulting in paresis of
the ipsilateral diaphragm. This results in a 25% to 30% reduction in
pulmonary function volumes. Caution is therefore advised in patients
with significantly reduced lung function. The patient should be
reassured that hoarseness (vasodilation of the larynx and arytenoids or
blockade of the recurrent laryngeal nerve) and Horner syndrome
(cervical sympathetic nerve block) are benign and transient. -
Serious complications associated with the
performance of this block include epidural, subdural, and spinal
injections and even injections directly into the spinal cord; arterial
(vertebral artery) and venous intravascular injections; as well as
pneumothorax.
JL. Permanent loss of cervical spinal cord function associated with
interscalene block performed under general anesthesia. Anesthesiology 2000;93:1541–1544.
A, Ekatodramis G, Kalberer F, et al. Acute and nonacute complications
associated with interscalene block and shoulder surgery. Anesthesiology 2001;95:875–880.
AR. The use of a “reverse” axis (axillary-interscalene) block in a
patient presenting with fractures of the left shoulder and elbow. Anesth Analg 2001;93:1618–1620.
A, Fanelli G, Aldegheri G, et al. Interscalene brachial plexus
anaesthesia with 0.5%, 0.75% or 1% ropivacaine: a double-blind
comparison with 2% mepivacaine. Br J Anaesth 1999;83: 872–875.
S, Greengrass R, Steele S, et al. A comparison of 0.5% bupivacaine,
0.5% ropivacaine, and 0.75% ropivacaine for interscalene brachial
plexus block. Anesth Analg 1998;87:1316–1319.
W, Saiyed M, Brown A. Interscalene block with a nerve stimulator: a
deltoid motor response is a satisfactory endpoint for successful block.
Reg Anesth Pain Manag 2000;25:356–359.
W, McDonald M. Hemidiaphragmatic paresis during interscalene brachial
plexus block: effects on pulmonary function and chest wall mechanics. Anesth Analg 1992;74:352–357.
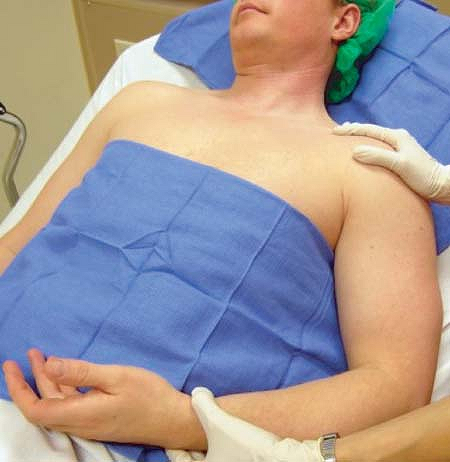
The patient is placed in a semi-sitting position, about 35° to 45° from
the horizontal plane, with the head turned to the opposite side. The
arm on the operative side is adducted, the shoulder is down and the
elbow is flexed, as shown in Figure 7-5.
Anesthesia and postoperative analgesia for any surgical procedure on
the upper extremity that does not involve the shoulder. It is an ideal
technique for surgery on the elbow, the forearm, the wrist, as well as
the hand.
 |
|
Figure 7-5. Patient positioning with shoulder down and elbow flexed.
|
 |
|
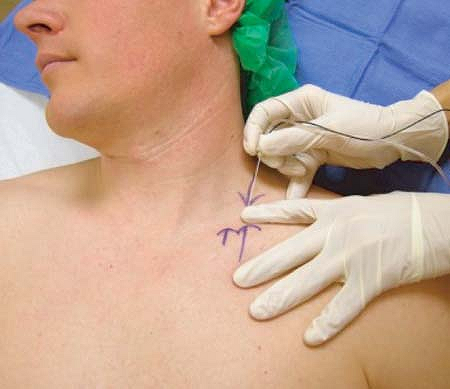
Figure 7-6.
Needle insertion: Two new arrows are drawn lateral to the original one. The upper arrow pointing down shows the needle insertion point. The lower arrow pointing up together with the upper arrow show the direction of needle insertion, which is caudad and parallel to the patient’s midline. |
The lateral (posterior) border of the sternocleidomastoid is identified
and traced caudally to the point where it meets the clavicle. This
point is marked with an arrow on the skin covering the clavicle. This
mark is used as a reference to find the needle insertion point, which
in adults, lies at a distance of approximately 1 in (2.5 cm) lateral to
it and one fingerbreadth above the clavicle.
cephalad and parallel to the clavicle at this level where the operator
usually is able to palpate the elements of the brachial plexus. The
needle insertion point is located immediately cephalad to the palpating
finger or one fingerbreadth above the clavicle as indicated by the
lateral upper arrow in Figure 7-6.
A small skin wheal of local anesthetic is raised at this level and the
insulated needle connected to a nerve stimulator (0.8–0.9 mA, 1 Hz, 0.1
ms) is inserted first perpendicular to the skin (easier penetration)
and then under the palpating finger in a caudal direction that is also
parallel to the patient’s midline as shown in Figure 7-6.
(upper trunk). The needle is then slowly advanced until a twitch of the
fingers, either in flexion or extension is visible. The local
anesthetic solution is slowly injected with frequent aspirations.
-
As it is the case with any regional
anesthesia technique, positioning of the patient is very important. No
attempts should be made at identifying any structures before
P.65
achieving
satisfactory posture. While the block can be performed with the patient
supine it is more comfortable for the patient—and no more complicated
for the operator—to have the patient sit up. Bringing the patient’s
shoulder down “opens” the supraclavicular area and facilitates the
technique. -
The point at which the lateral head of
the sternocleidomastoid meets the clavicle is where the “plumb bob”
technique is attempted. This point is located too close to the dome of
the pleura so we use it only as a reference point from which we locate
the needle insertion point in adults at about 1 in (2.5 cm) lateral to
it. We call this distance “margin of safety.” -
In patients with well-developed
sternocleidomastoid muscles, the width of its clavicular head can be
used to determine this margin of safety. -
We do not routinely rely on the palpation of either the subclavian artery pulse or the scalene muscles.
-
To easier penetrate the skin the needle
is first inserted perpendicular to it before changing its direction to
advance it caudally, parallel to the patient’s midline. -
A motor response from one of the trunks
of the plexus should be obtained in every patient at a depth of 2 cm or
less. If no motor response is obtained, the nerve stimulator status and
the indemnity of the electric circuit are checked before proceeding to
reassess the landmarks if necessary. -
With the supraclavicular block the type
of response elicited (i.e., fingers twitch) is more important than the
output at which such response is obtained provided that the output is
no greater than 0.9 mA, as we demonstrated in a prospective study. -
Flexion and extension of the wrist are
acceptable motor responses but supination or pronation of the wrist and
other more proximal responses are not. -
While the block may be followed by 50%
phrenic nerve paralysis it did not trigger symptoms in healthy
volunteers according to a study by Neal and collaborators. Our
experience confirms this. -
A block of the intercostobrachial
branches, also called “tourniquet block” or T2 block, is usually
unnecessary unless the surgical incision falls in the upper medial side
of the arm. Tourniquet pain usually is unavoidable after 2-h tourniquet
time whether this supplemental block is performed or not. This time
also marks the maximum allowable time for a tourniquet to remain
inflated.
CD, Domashevich V, Voronov G, Rafizad A, Jelev T. The supraclavicular
block with a nerve stimulator: to decrease or not to decrease, that is
the question. Anesth Analg 2004;98:1167–1171.
CD, Gloss FJ, Voronov G, Tyler SG, Stojiljkovic LS. Supraclavicular
block in the obese population: an analysis of 2020 blocks. Anesth Analg 2006;102:1252–1254.
JM, Moore JM, Kopacz DJ, Liu SS, Krammer DJ, Plorde JJ. Quantitative
Analysis of Respiratory, Motor, and Sensory Function After
Supraclavicular Block. Anesth Analg 1998;86:1239–44
Supine, with the hand of the side to be blocked positioned in a relaxed
manner on the abdomen, and the head slightly turned to the
contralateral side.
Anesthesia and immediate postoperative analgesia of any surgical
procedure in the region of the distal upper arm, the forearm, and the
hand.
The brachial plexus crosses beneath the clavicle in the vicinity of the
middle of the clavicular line drawn between the halfway point of the
ventral apophysis of the acromion and the jugular notch. In dissected
cadavers, the plexus lay at a maximum depth of 4 cm lateral to the
axillary artery and vein, where its three cords always converge at the
entrance to the trigonum of the clavipectoral fascia.
The ventral apophysis of the acromion and the jugular notch is
identified and the line joining these two points is drawn. The middle
of this line determines the site of introduction of the needle. The
insulated needle connected to a nerve stimulator (1.5 mA, 2 Hz, 0.1 ms)
is introduced directly beneath the clavicle and in a strictly vertical
direction (Fig. 7-7).
Usually, the lateral cord (contractions of the biceps muscle) is
stimulated. The position of the needle is adjusted to maintain the same
motor response with a current less than 0.3 mA. After negative
aspiration for blood, the appropriate volume of local anesthetic is
slowly injected, with repeat aspiration for blood every 5 mL to be
distributed around the brachial plexus (Fig. 7-8).
-
A complete blockade will develop within 5 to 15 minutes.
-
The most likely motor response predictive
of a complete block is the contraction of the finger muscles: extensors
or flexors (stimulation of either the radial or median nerve). -
Precise determination of the specific
anatomical landmarks is essential for the success and safety of this
block. Following the described landmarks, no injury of nerves, vessels,
or even the pleura have been induced in cadaver studies. -
In contrast to the jugular notch, the
exact determination of the ventral apophysis of the acromion lateral
point is occasionally more difficult. This point, however, is essential
for accurate determination of the puncture site. For that purpose, the
clavicle
P.67
may
be palpated from medial to lateral leads to the acromioclavicular
joint. This means the ventral apophysis of the acromion must be sought
ventrally and slightly laterally. Another approach is based on
following the crest of the scapula up to the acromion with the
assumption that the ventral apophysis is posterior. To rule out the
head of the humerus, the arm is passively moved and at the same time
the assumed landmark is identified. The latter should not move in
conjunction with this manipulation. The coracoid is considerably more
medial and may be clearly felt in most patients.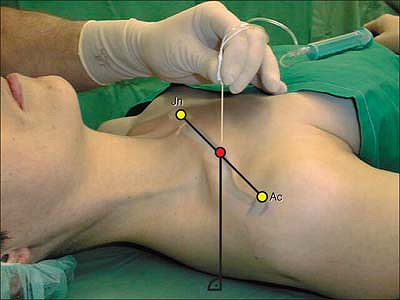 Figure 7-7.
Figure 7-7.
The insulated needle connected to a nerve stimulator is introduced
directly beneath the clavicle and in a strictly vertical direction. -
The measurement must be started from exactly in the middle of the jugular notch.
-
The distance between the puncture site
and the clavicle must be kept at a minimum (however, painful contact
with the periosteum should be avoided). -
If blood is aspirated, it means that the
puncture site is too medial. In contrast, if no motor responses are
elicited, the appropriateness of the puncture site should be confirmed.
If the second attempt is unsuccessful, the puncture site should be
shifted 0.5 to 1.0 cm laterally. If this correction of the puncture
site still does not produce the desired stimulation response, adjust
the position 0.5 to 1.0 cm in a medial direction from the original site. -
If primarily biceps muscle motor response
is elicited, the position of the needle needs to be slightly more
lateral and deeper (the perpendicular/vertical direction to meet the
posterior cord). Finally, in the exceptional case that the puncture
site cannot be defined with certainty, then another procedure should be
used. Under no circumstances should one simply “poke about” or ever
change from a vertical puncture direction. -
The appropriate positioning of the patient allows optimal observation of the peripheral muscle contractions.
-
Cardinal mistakes: (a) Puncture site too
lateral: false localization of the lateral landmark associated with a
high risk for axillary artery or vein injury. (b) Puncture depth
P.68
greater
than 6 cm: In cadavers, injury to the pleura can occur (pneumothorax);
however, the first rib provides relatively good protection, especially
in the event of faulty medial punctures. (c) Puncture site too medial
(subclavian artery, subclavian vein, or cephalic vein puncture).![]() Figure 7-8.
Figure 7-8.
After negative aspiration for blood, the appropriate volume of local
anesthetic is slowly injected, with repeat aspiration for blood every 5
mL to be distributed around the brachial plexus.
M, Kaiser H, Uhl M. Biometric data on risk of pneumothorax from
vertical infraclavicular brachial plexus block. A magnetic resonance
imaging study. Anästhesist 2001;50:511–516.
Supine, arm resting at patient’s side with the palm up (to make
hand/wrist motion with nerve stimulation easier to identify). Patient
may use a pillow behind the head, but the entire shoulder and back
should lie flat against the gurney. The operator stands on the side
ipsilateral to the extremity to be blocked, facing cephalad.
Surgery of the upper extremity at, or distal to, the elbow. The
intercostobrachial (ICB) nerve, which often helps to innervate the skin
over the medial epicondyle, is often spared surgical anesthesia with
this block.
The brachial plexus is intersected between 2 and 8 cm deep to the skin
(average: 4 cm). Therefore, for small and average-sized patients, a
5-cm, 22-gauge insulated stimulating needle is preferred. However, with
larger patients, a longer needle is often necessary.
40 to 50 mL for adults. The medial or lateral cord is most often
initially identified using the coracoid technique, and a relatively
large volume of local anesthetic is usually necessary to reach the
posterior cord, located posterior to the axillary artery.
The coracoid process of the scapula is the sole anatomic landmark. To
find the coracoid process, place two fingers in the groove between the
deltoid and pectoralis major muscles, and gently palpate laterally.
From the center of the coracoid process, mark a point that is exactly 2
cm medial and 2 cm cauda1 (Fig. 7-9). This is the needle entry point.
Raise a skin wheal at the needle entry point. The needle is then
inserted through the skin wheal with long axis of the needle
perpendicular to the gurney in all planes (Fig. 7-9).
With continuous aspiration and the nerve stimulator initially set at
1.2 mA and 2 Hz, the needle is advanced directly posterior. If the
brachial plexus is not identified after 5 to 8 cm of insertion,
depending on patient habitus, the needle is withdrawn to the skin and
redirected either cephalad or caudal in the paramedian sagittal plane
until discrete, stimulated motion occurs in any digit(s) with a current
<0.50 mA. Directing the needle tip out of the paramedian sagittal
plane must be avoided—neither medially
toward the lung, nor laterally, toward the individual terminal nerves
of the brachial plexus. Flexion or extension at the elbow or wrist that
results in motion of the fingers, without intrinsic hand/digit motion,
should be rejected.
-
Directing the needle tip out of the
paramedian sagittal plane medially toward the chest wall increases the
risk of a pneumothorax. -
Directing the needle tip out of the
paramedian sagittal plane laterally may place the needle tip lateral to
the cords and result in anesthesia of only one or two terminal nerves
of the arm. -
Frequently, the first motion elicited
results from direct stimulation of the pectoral major and minor
muscles. The needle must be advanced further since the brachial plexus
lies posterior to these muscles. -
Any limb motion other than intrinsic
finger flexion or extension must be rejected as these endpoints result
in a 60% failure rate.P.70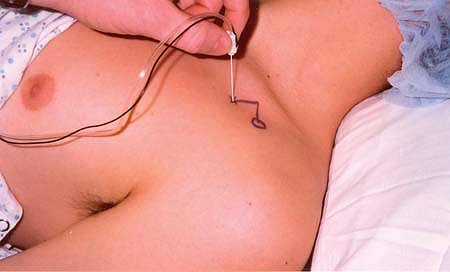 Figure 7-9. From the center of coracoid process, mark a point that is exactly 2 cm medial and 2 cm caudal. This is the needle entry point.
Figure 7-9. From the center of coracoid process, mark a point that is exactly 2 cm medial and 2 cm caudal. This is the needle entry point. -
If biceps or forearm motion occurs,
redirect the needle caudal in the paramedian sagittal plane. Changes to
the needle trajectory should be made in small increments. -
The ICB nerve courses adjacent to the
brachial plexus at the level of the cords. The infraclavicular block
will usually produce analgesia in this nerve to cover the majority of
tourniquet pain. However, while surgical anesthesia of the ICB
frequently occurs, this block should not be relied on to consistently
produce surgical anesthesia of the medial aspect of the arm above the
elbow. -
A “vertical” technique using a needle
entry point just caudal to the clavicle and medial to the entry point
of the coracoid technique has resulted in a number of reported
pneumothoraxes. The investigator who first described the coracoid
technique reported that even with deliberate attempts to penetrate the
thoracic cavity in cadavers, it proved impossible to enter the lung
using the coracoid approach.
PB, Nowitz M. A magnetic resonance imaging analysis of the
infraclavicular region: can brachial plexus depth be estimated before
needle insertion? Anesth Analg 2005;100:1184–1188.
J. The infraclavicular brachial plexus block by the coracoid approach
is clinically effective: an observational study of 150 patients. Can J Anaesth 2003;50:253–257.
O, Lilleas FG, Rotnes JS, et al. Magnetic resonance imaging
demonstrates lack of precision in needle placement by the
infraclavicular brachial plexus block described by Raj et al. Anesth Analg 1999;88:593–598.
H, Mayfield J, Rosow L, Chang Y, Carter C, Rosow C. Stimulation of the
posterior cord predicts successful infraclavicular block. Anesth Analg 2006;102:1564–1568.
V, Asehnoune K, Chassery C, et al. Resident versus staff
anesthesiologist performance: coracoid approach to infraclavicular
brachial plexus blocks using a double-stimulation technique. Reg Anesth Pain Med 2005;30:233–237.
V, N’guyen L, Chassery C, et al. A modified coracoid approach to
infraclavicular brachial plexus blocks using a double-stimulation
technique in 300 patients. Anesth Analg 2005;100:2263–2265.
J, Barcena M, Alvarez J. Restricted infraclavicular distribution of the
local anesthetic solution after infraclavicular brachial plexus block. Reg Anesth Pain Med 2003;28:33–36.
J, Barcena M, Taboada-Muniz M, et al. A comparison of single versus
multiple injections on the extent of anesthesia with coracoid
infraclavicular brachial plexus block. Anesth Analg 2004;99:1225–1230.
J, Taboada-Muniz M, Barcena M, Alvarez J. Median versus
musculocutaneous nerve response with single-injection infraclavicular
coracoid block. Reg Anesth Pain Med 2004;29:534–538.
JL, Brown DL, Wong GY, et al. Infraclavicular brachial plexus block:
parasagittal anatomy important to the coracoid technique. Anesth Analg 1998;87:870–873.