BONE GRAFTING, BONE GRAFT SUBSTITUTES, AND GROWTH FACTORS
– SURGICAL PRINCIPLES AND TECHNIQUES > CHAPTER 9 – BONE GRAFTING,
BONE GRAFT SUBSTITUTES, AND GROWTH FACTORS
first recorded bone implant was performed in 1668. Bone grafts are used
to treat various disorders, including delayed union and nonunion of
fractures, congenital pseudoarthrosis, and osseous defects from trauma,
infection, and tumors (157). Grafts are used in
reconstructive surgery to help secure prosthetic devices and perform
arthrodeses, and in dentistry to reconstruct the mandibular and
maxillary ridges and to treat periodontal disease. Bone grafts are also
used in plastic and facial surgery for reconstruction.
and growth factors induce bone formation by the host (7,8,9,22,30,31,48,50,51,53,62,63,65,73,74,103,123,127,147,148,149,150,151,152,153 and 154,159,162,166).
He believed that transplanted bone was invaded by vascular granulation
tissue, causing the old bone to be resorbed and subsequently replaced
by the host with new bone. Phemister’s concept remains valid; however,
Abbott and associates have shown that, in addition, surface cells in
the bone graft survive and participate in new bone formation (1,2). Ray and Sabet (124) and Arora and Laskin (5)
also confirmed the fact that superficial cells in the bone graft
probably survive transplantation and contribute to new bone formation.
The percentage of cells that survive transplantation is unknown, but
cell survival seems to be improved by minimizing the interval between
harvest and implantation and by keeping the graft moist and at
physiologic temperatures.
spaces and haversian canals is removed by macrophages. Granulation
tissue, preceded by the advance of capillaries, invades the areas of
resorption (122). Pluripotential mesenchymal
cells differentiate into osteoblasts, which begin to lay seams of
osteoid along the dead trabeculae of the bone graft. Osteoclasts resorb
the necrotic bone, and eventually most of the bone graft is replaced by
new host bone. Finally, the old marrow space is filled by new marrow
cells (25).
similar but much slower, because invasion of the graft must be through
the haversian canals of the transplant (38).
Osteoclasts resorb the surface of the canals, creating larger spaces
into which granulation tissue grows. As this granulation tissue
penetrates the center of the cortical graft, new bone is laid
throughout the graft along enlarged haversian canals. Depending on the
size of the graft, complete replacement may take many months to a year
or more (46).
corticocancellous. If structural strength is required, cortical bone
grafts must be used. However, the process of replacement produces
resorption as early as 6 weeks after implantation; in dogs, it may take
up to 1 year before the graft begins to regain its original mechanical
strength (45). Drilling holes in the graft does
not appear to accelerate the process of repair, but it may lead to the
early formation of biologic pegs that enhance graft union to host bone (17).
loaded in compression after being packed into an area of bone defect.
For example, in repairing fractures of the tibial plateaus in which
cancellous bone has been lost as a result of crushing, cancellous bone
graft can be packed to support the articular surface of the plateau,
and can bear significant loads during rehabilitation. When the strength
of cortical bone combined with the more rapid incorporation that is
characteristic of cancellous bone is desirable, combined
corticocancellous grafts may be used. When a bone graft has a cortical
and cancellous surface, incorporation is enhanced by exposing the
cancellous portion to the surrounding soft tissues to facilitate
vascular invasion.
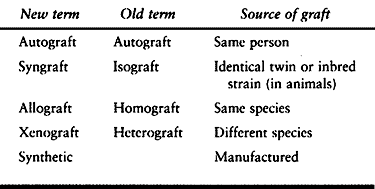
confusion. In this text, we use the new terminology. The correlation
between the two is shown in Table 9.1. For
most applications, autogenous bone graft is indicated. Other types of
bone grafts are indicated only if autogenous bone graft is unavailable
or if it is insufficient and must be augmented. Another exception is
when structural whole or partial bones, with or without joint articular
surfaces, are needed for reconstruction of massive whole or partial
bone defects (10,13,19,20,44,56,57 and 58,76,81,91,92,95,106,108,146,156,165). This occurs most commonly in tumor surgery, in which preserved or fresh allografts are used (49,66,72,78,88,89,100,101,111,112). This is discussed in more detail later in this chapter and in Chapter 20, Chapter 106, Chapter 126, and Chapter 128.
For practical reasons, isografts are almost never used in human
surgery. Although xenografts have been tried in various forms in the
past, they have never met with much success, because of the immunologic
response of the host.
 |
|
Table 9.1. Bone Graft Terminology
|
most osteogenic bone graft available and, therefore, is the most widely
used. Cancellous bone can be taken in strips or morcellized into fine
pieces that pack readily into small cracks or holes, or it can be used
to fill large, irregular voids. When packed firmly and contained,
cancellous bone provides good support against compression.The
best
source of cancellous bone is the ilium, although the metaphyseal region
of most long bones can be a source of bone graft. It is the principle
type of graft used for fractures, nonunions, and for arthrodesis of the
spine. If cancellous bone is in limited supply, it can be taken as
corticocancellous strips.
commonly before the development of good quality internal fixation and
was employed for both osteogenesis and fixation in the treatment of
nonunions (Fig. 9.1A). The tibia is the most
common source of this graft; the split fibula can also be used. The
most common indication for this graft today is bone grafting and
stabilizing the cervical spine, although with modern techniques it is
rarely used. In osteoporotic bone, screws commonly do not achieve
adequate fixation. Even metal washers and nuts are inadequate, because
the bone collapses when they are tightened. In this situation, a
nonunion or fracture site can be bridged with a cortical bone graft and
a plate applied to the opposite cortex. The screws can then be placed
through the bone into the bone graft, resulting in a reasonably solid
construct. For this purpose, it is preferable to use one half or three
quarters of the thickened portion of the superior border of the iliac
crest rather than the tibia. If a long, straight graft is needed, the
fibula is preferred. The tibia is used as a last resort, because a
donor site of this size creates a large stress riser in the tibia that,
unless protected for a prolonged period of time, can lead to pathologic
fracture.
 |
|
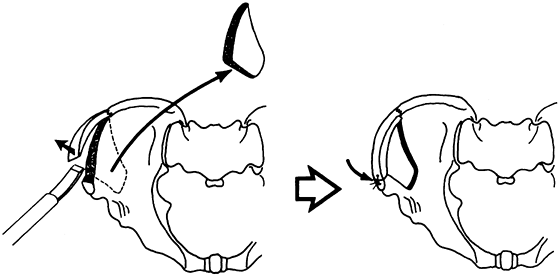
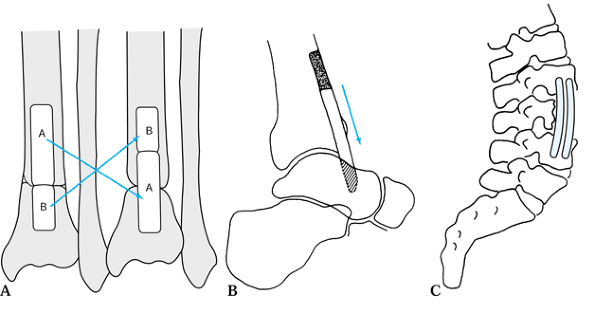
Figure 9.1. A:
A single-onlay cortical bone graft is shown. A single-onlay cortical graft may be used to treat nonunion of the humerus. A cancellous graft is placed at the nonunion site. We have not used this technique in the last 20 years; instead, we would internally fix the nonunion with a compression plate, performing an onlay cancellous bone graft. B: The use of the classic dual onlay cortical bone graft is almost exclusively limited to the treatment of congenital pseudoarthrosis of the tibia. The space between the two grafts and between the two ends of the bone at the nonunion site is packed with cancellous bone. In some nonunions of the humerus with osteoporotic bone, a compression plate is used, but this is supplemented on the opposite cortex by a cortical onlay graft to provide good screw fixation. Although only four screws are illustrated, this type of grafting requires a minimum of six screws with good fixation of both cortices on either side of the nonunion site. Dual onlay grafting is mostly of historic interest. See the text for further information. |
technique in 1941 for the treatment of congenital pseudarthrosis of the
tibia (12). This technique is interesting for its historical significance (Fig. 9.1B).We
have not found an indication for this graft since the early 1980s.
Ilizarov techniques are more useful for severe problems of this type. A
version of this technique using allograft is useful for revision total
joint arthroplasty to replace bone insufficiency.
Inlay grafts are created by a sliding technique, graft reversal
technique, or as a strut graft. Although originally designed for the
treatment of nonunion of the tibia, these techniques are also used for
arthrodesis and epiphyseal arrest (Fig. 9.2A, Fig. 9.2B and Fig. 9.2C).
 |
|
Figure 9.2. A: A sliding graft may be used to treat nonunion of the distal tibia. B:
In this case, a sliding graft is used as a component of ankle arthrodesis. This type of graft is more likely to be used for a previously failed ankle fusion or for fusion in the absence of the body of the talus. Internal fixation and additional graft in the ankle joint is not illustrated. C: Strut grafts for anterior spinal fusion. Strut grafts are very useful for bridging defects in the anterior spine and for providing support for anterior spinal fusion. Grafts from the ribs, fibula, and bicortical iliac crest are useful for strut grafting, depending on the size of the graft needed. Two rib grafts are shown bridging L-2–L-4. |
-
Drill four holes at each corner of the sliding graft. Cut the rectangular graft with a water-cooled saw blade.
-
After the graft is removed, if there is a
solid fibrous union between sections A and B, it is simply flipped end
for end and then impacted back into the slot. If it is in two pieces,
section A is slid distally, and section B is placed proximally. Section
A now bridges the fracture site (Fig. 9.2A).
fixation combined with onlay cancellous bone graft provides a better
result. This technique may be combined with internal fixation if there
is limited space to place a cancellous graft. The disadvantages of the
sliding or reversed bone graft are that, after the cuts are made, the
graft fits loosely in the bed, and it creates stress risers proximally
and distally to the nonunion site. It is most safely used in
metaphyseal rather than diaphyseal regions. Its use today
has
been supplanted by rigid internal fixation and cancellous onlay bone
grafting. Onlay bone grafting has the additional advantage of providing
a higher polar moment of inertia than does the inlay technique. A
common use of the strut graft is for anterior vertebral fusion at all
levels of the spine. Ribs, fibula, or sections of iliac crest are
useful for this application (Fig. 9.2C). The reverse inlay graft is useful for nonunions of the medial malleolus.
harvested from the ilium and is specifically designed to achieve
posterior fusion of the cervical spine.
nonunions in anatomic areas, such as the scaphoid and femoral neck,
where onlay bone grafting was impractical. In the carpal scaphoid, the
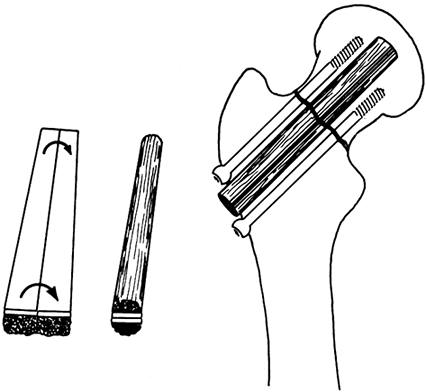
dowel is fashioned from dense cancellous bone (see Chapter 42). The use of the dowel graft for the management of nonunion of the femoral neck is illustrated in Figure 9.3.
Free microvascularized fibula grafts are more commonly used today. A
corticocancellous graft of appropriate length and approximately 25 mm
wide is harvested from the ilium or the tibia. The curvature of the
ilium often makes it difficult to obtain a straight graft of sufficient
length.
 |
|
Figure 9.3. Dowel graft for nonunion of the femoral neck.
|
-
Use a water-cooled power saw to cut this graft to avoid breakage.
-
Split the graft into two longitudinal sections. Make the cut in situ.
-
Place the two longitudinal grafts back to back, with the cortex in the interior of the graft; then shape it to form a dowel.
-
Temporarily fasten the two sections together with cerclage absorbable sutures.
-
Ream a hole from the lateral cortex of
the femur, across the femoral neck nonunion site, and into the femoral
head using a standard, hip-nail reamer. -
Drive the graft into this hole across the nonunion site, taking care not to break the graft.
-
Achieve additional fixation with multiple
compression screws. This graft can also be combined with a compression
hip screw if sufficient space is available. A fibula can be used for
this purpose as well and is mechanically stronger. Yoo (163), following the technique originated by Urbaniak (see Chapter 125),
uses a free microvascularized fibula with anastomosis to the lateral
circumflex femoral artery for avascular necrosis of the femoral head.
The usefulness of this technique in nonunions has not been established,
however. On occasion, a Meyers muscle-pedicle bone graft (96) or a valgus osteotomy is used (see Chapter 29).
microvascularized fibular grafts and, on occasion, the quadratus
muscle-pedicle graft popularized by Meyers (see Chapter 29) or valgus osteotomy (96,97,98 and 99,163). Peg grafts have also been used to bridge the tibia and fibula to produce proximal and distal tibiofibular synostosis (94). Occasionally, these grafts are used for spine fusions as well (84).
major long bones. Grafts in this location interfere with restoration of
endosteal blood supply; because they are in the central axis of the
bone, they resorb rather than incorporate. The only possible use for a
medullary graft is in the metacarpals and the metatarsals, where the
small size of the bone enhances incorporation. Even in this location,
however, internal fixation with onlay or intercalary cancellous bone
grafting may be a superior method.
with chips of cortical bone. These grafts have not been proven to be
superior to onlay cancellous bone grafting, are more difficult than
cancellous bone to harvest, and may involve greater morbidity; they are
rarely used today.
In local muscle-pedicle bone grafts, an attempt is made to preserve the
viability of the graft by maintaining muscle and ligament attachments
carrying blood supply to the bone or, in the case of diaphyseal bone,
by maintaining the nutrient artery. Two examples are the transfer of
the anterior iliac crest on the muscle attachments of the sartorius and
rectus femoris for use in the Davis type of hip fusion (see Chapter 106)
and the transfer of the posterior portion of the greater trochanter on
a quadratus muscle pedicle for nonunions of the femoral neck (see Chapter 29) (96,97,98 and 99).
Although technically more difficult, pedicle grafts have the advantages
of a high percentage of cell survival, rapid incorporation, and
increased active participation of the grafted cells in the healing
process. Free, microvascularized fibular grafts are used to replace
major deficiencies in long bones (see Chapter 36) and have been effectively used to treat avascular necrosis of the femoral head (see Chapter 125) (163).
it is relatively subcutaneous, has natural curvatures that are useful
in fashioning grafts, has ample cancellous bone, and has cortical bone
of varying thickness. Removal of the bone carries minimal risk and
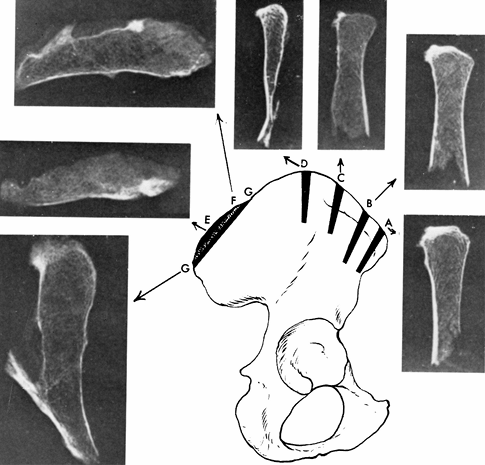
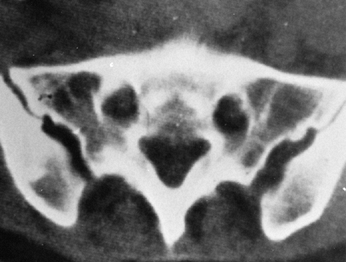
usually there is no significant residual disability. Abbott (1) demonstrated that the posterior third of the ilium is thickest (Fig. 9.4). This is confirmed by computer tomography (CT) scans (Fig. 9.5).
 |
|
Figure 9.4.
These cross sections of the iliac crest show the width of the bone and the cancellous bone available for grafting. The posterosuperior iliac spine area provides the most bone, and the central section of the ilium at point D is quite thin and is of no use in bone grafting. (From Abbott LC. The Use of Iliac Bone in the Treatment of Ununited Fractures. In American Academy of Orthopaedic Surgeons: Instructional Course Lectures, Vol. 2. Ann Arbor, MI: J. W. Edwards, 1944, with permission.) |
 |
|
Figure 9.5.
This CT scan of the pelvis at the level of the posterosuperior iliac spine illustrates the thickness of the ilium posteriorly and the amount of cancellous bone available. |
-
To procure bone, position the patient in
the prone position. It can also be harvested from the uppermost crest
in the lateral decubitus position. Prepare a wide area of skin and
eliminate possible contamination from the rectal area by placing an
adhesive plastic sheet transversely across the buttocks, fitting it
carefully into the gluteal crease cephalad to the rectum. -
Make a straight vertical incision
directly over the posterosuperior iliac spine or a curvilinear incision
that parallels the iliac crest (Fig. 9.6A). The
length of incision depends on the size of the graft needed and the
obesity of the patient, but an incision of at least 7.5 cm is needed.
To prevent injury to the cluneal nerves, avoid straight transverse
incisions and try not to carry incisions too far laterally. A
transverse incision is more likely to result in dehiscence and can be
painful if it lies along the belt line.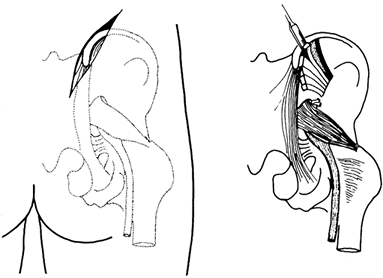 Figure 9.6. A posterior iliac graft is shown.
Figure 9.6. A posterior iliac graft is shown. -
Dissect sharply through the fat to the
prominence of the posterior iliac crest. Identify the origin and fascia
of the gluteus maximus insertion on the crest. With a cautery knife,
incise the origin of the gluteus maximus and dissect it free from the
crest subperiosteally. If the entire posterior iliac area is to be
harvested, take down
P.186P.187
the
gluteus from approximately 2.5 cm superior to the posterosuperior iliac
spine and inferior as far as the posteroinferior spine. With a large
key elevator, elevate the gluteus off the outer wall of the ilium down
to the level of the sciatic notch. Avoid injury to the superior gluteal
nerve and vessels. Obtain retraction with two Taylor retractors. -
The outer wall of the ilium is removed by
first outlining the area to be harvested by cutting through the outer
table of the ilium with a sharp osteotome, as illustrated in Figure 9.6B.
If an onlay cancellous bone graft is to be performed, harvest
corticocancellous strips with a curved gouge. Remove all underlying
cancellous bone down to the inner table of the ilium with a curved
gouge and curets of an appropriate size. There is considerable
cancellous bone superiorly and medially under the rim of the ilium that
requires a curet for removal. Try not to perforate the inner wall of
the ilium. -
Obtain hemostasis by applying a thin
layer of bone wax over bleeding points on the bone. Irrigate the donor
site thoroughly and remove excess wax. Perform routine closure over
suction drainage. Because bone graft sites tend to ooze considerably
after surgery, apply a large, bulky dressing.
 |
|
Figure 9.7. A, B: Harvesting of an anterior iliac graft is shown. C: This cross-sectional view shows the extent of the bone harvested. The outer table is not broached. (From Weber BG, Cech O. Pseudarthrosis. Bern, Switzerland: Hans Huber, 1976, with permission.)
|
-
Place the patient in a supine position.
Make an incision parallel to the crest and 1 cm proximal to it. Do not
incise anterior to the anterior superior iliac spine to avoid injury to
the lateral femoral cutaneous nerve to the thigh. Incise with a cautery
knife along the iliac crest, avoiding muscle. Posteriorly retract the
overhanging abdominal musculature proximally to remain on the
subcutaneous border of the ileum. This approach
P.188
will
significantly reduce hemorrhage and postoperative pain.
Subperiosteally, dissect the abdominal musculature and, subsequently,
the iliacus from the inner wall of the ilium. -
Outline the area to be harvested with straight and curved osteotomes (Fig. 9.7A).
Cut the strips, which will be removed. The middle ilium is paper thin,
but the anterior column just above the acetabulum is quite thick. -
Harvest the corticocancellous strips with a gouge.
-
Remove additional cancellous bone with gouges and curets (Fig. 9.7B). Do not broach the outer table (Fig. 9.7C).
-
Closure and postoperative management are
the same as for a posterior graft. We prefer to harvest from the inner
wall, because it is much easier for the surgeon and the patient has
less postoperative pain than with other sites. The outer wall can be
harvested as well. Watch for postoperative ileus and treat
appropriately.
-
Position the patient for harvest of the pelvis to optimize skin preparation, draping, and surgical access to the donor site.
-
Exclude the perineum from the operative field with an adherent plastic drape.
-
Thoroughly prepare the skin for surgery
and drape over a sufficiently wide area so that the skin incision can
be made entirely through an iodophor-impregnated adherent plastic drape. -
Place skin incisions slightly above or below the crest to avoid painful scars over bony prominences.
-
Avoid surgical injury to the lateral
femoral cutaneous nerve anteriorly and the cluneal as well as sciatic
nerves posteriorly through proper incision placement and careful
dissection. -
Dissect directly down to the bone, minimizing any undermining of subcutaneous fat off the fascia.
-
Expose bone by using an electrocautery knife on the lowest setting possible to detach the fascial origins and insertions.
-
Dissect muscle off bone subperiosteally with a large key-type elevator to avoid wandering into and injuring muscle.
-
Minimize soft-tissue dissection and stripping, exposing only those areas necessary to harvest the graft.
-
Take only as much bone graft as is needed.
-
Before beginning the bone graft harvest, be certain that there is complete control of bleeding from the soft tissues.
-
Immediately control bleeding from any significant bone bleeders.
-
After harvest is completed, use hemostatic agents to minimize postoperative bleeding.
-
Use postoperative wound suction in nearly all donor sites because postoperative hemorrhage is difficult to predict.
-
Monitor postoperative blood loss closely
to permit timely management of unexpected blood loss by temporary
cessation of suction, possible re-exploration, and blood replacement. -
Close the wound in layers using
meticulous technique with watertight closure of the fascia, closure of
the subcutaneous fat in two or more layers in obese patients, and
meticulous skin closure to minimize scar formation. -
Change postoperative dressings as frequently as necessary to maintain a dry dressing.
-
Protect donor sites postoperatively with
limited weight bearing and activities where the risk of pathologic
fracture through the donor site is high (older osteoporotic women or
long bone harvest sites).
-
Keep the skin incision small and avoid lateral extension, which risks injury to the cluneal nerves.
-
A curved linear incision parallel to the
posterior iliac crest placed directly over the posterior iliac spine
somewhat superior and medial to the crest approximately 5 to 6 cm in
length usually permits harvesting of the entire outer cortex and
underlying cancellous bone. Obese patients require larger incisions. -
Do not remove the origins and insertions
of the spinal fascia and musculature from the ileum unless the entire
nonarticular posterior superior iliac spine portion of the crest is to
be harvested. -
Identify the location of the superior and
inferior gluteal neurovascular bundles as well as the sciatic nerve and
avoid injury to these structures by avoiding excessive retraction and
appropriate placement of retractors. -
When harvesting large grafts, avoid penetration into the sacroiliac joint.
-
Perform soft-tissue closure as described above, under General Principles.
-
Restriction of activity and protected
weight bearing postoperatively is necessary only to relieve pain.
Pathologic fracture secondary to bone graft harvest is nearly unheard
of in this area.
-
Avoid injury to the lateral femoral cutaneous nerve by keeping all dissection posterior to the anterosuperior iliac spine.
-
Minimize soft-tissue dissection and use
small windows on the superior aspect of the crest when limited amounts
of only cancellous bone are to be harvested. -
Where corticocancellous graft is
required, remain in the anterior one quarter to one third of the crest
above the acetabulum, avoiding the thin, nearly purely cortical bone in
the middle third. -
Expose and harvest only the inner or
outer table (we prefer the inner table to avoid injuring the abductor
muscles) when corticocancellous graft is required. -
When full-thickness crest is harvested,
avoid fracture by remaining at least 3 cm posterior to the
anterosuperior iliac spine, harvesting the graft with a water-cooled
oscillating saw rather than an osteotome, and placing drill holes at
the end of the saw cuts, if necessary, to minimize the stress riser
effect. -
When large anterior grafts are harvested
that require more posterior exposure of the crest, avoid incising the
abdominal musculature by carefully identifying the interval between the
abductor hip musculature and the abdominal muscles, retracting the
abdominal muscles superiorward, and dissecting along the subcutaneous
border of the crest. -
Meticulously close the abdominal muscles to the abductor musculature to minimize the risk of hernia.
-
Protect patients postoperatively with limited weight bearing where the risk of fracture is high.
spinal fusion or for replacement of major bone defects in metaphyseal
regions, such as in nonunions of the distal humerus or in opening wedge
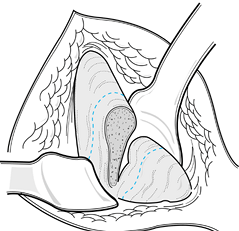
osteotomies. Coronal grafts are illustrated in Figure 9.8.
 |
|
Figure 9.8.
Full-thickness segments of the ilium are useful for many types of bone grafting, particularly for anterior arthrodesis of the spine and fusion of the ankle and subtalar joints. Segments shorter than 30 mm rarely cause cosmetic problems. (From Abbott LC. The Use of Iliac Bone in the Treatment of Ununited Fractures. In: American Academy of Orthopaedic Surgeons: Instructional Course Lectures, Vol. 2. Ann Arbor, MI: J. C. Edwards, 1944, with permission.) |
-
Expose both sides of the ilium from the anterosuperior iliac spine posteriorly as far as is needed for the graft desired (Fig. 9.9).
![]() Figure 9.9. A large bicortical graft is shown.
Figure 9.9. A large bicortical graft is shown. -
With a thin, curved osteotome that is 1
cm wide, enter the iliac crest approximately 15 to 20 mm posterior to
the anterosuperior iliac spine. -
Make a cut parallel to and beneath the
thickened rim of the crest for a distance of 7 to 10 cm, depending on
the size of the patient and the size of the graft needed. -
Gently pry the crest upward, producing a
greenstick fracture at its posterior aspect. Posteriorly, leave the
periosteum and muscle attachments intact to prevent the fragment from
becoming free. -
Remaining at least 2 cm posterior to the
anterior border of the ilium between the two iliac spines, drive an
osteotome downward to the depth of graft desired, or use an oscillating
saw. It is important to remain at least 2 cm or more superior to the
dome of the acetabulum. -
Make a second posterior cut to produce
the width of graft desired, and then connect these cuts in the depths
of the wound on both sides with a curved osteotome. Using a saw results
in a stronger graft. -
Carefully remove the graft and place it in a moist sponge in a basin on the back table until it is ready for use.
-
Obtain hemostasis, and return the iliac crest to its anatomic
P.190
position. Place 2 mm drill holes on either side of the initial
osteotomy on the top of the crest. Through these holes, place a #1
nonabsorbable suture and tie it securely to hold the crest in place.
This fixation is surprisingly stable. -
Securely suture the periosteum and
overlying muscles with interrupted sutures to help hold the crest in
place. Use suction drainage.
anterior spine fusion, are best harvested with a saw, because they are
stronger than those harvested with osteotomes. We use this graft about
two to three times per year, and we have yet to see the donor site fill
in. One fracture did occur through the anterior remnant of the ilium at
its base, but this fracture healed without problems by having the
patient limit activities and use crutches. No hernias have occurred,
and most patients have no symptoms within 6 months of graft harvest.
small tumors, it may be most convenient to harvest the graft from the
ipsilateral extremity undergoing operation. The graft can often be
taken through the same incision or through a small, separate incision.
Most of these sites can be harvested through a small, 2.5 to 5.0 cm
longitudinal incision placed over the subcutaneous surface of the end
of the bone.
-
Make a small cortical window in the
metaphyseal or epiphyseal region with a 6 mm osteotome or large drill.
Use curets to harvest the graft. Typical sites are illustrated in Figure 9.10 (64).
If the greater trochanter of the femur is used, avoid taking the graft
from the lateral aspect rather than the preferred anterior site. The
lateral location may result in trochanteric bursitis or a snapping
iliotibial band. A similar technique is used to harvest bone from
between the two tables of the ileum through a small window in the crest
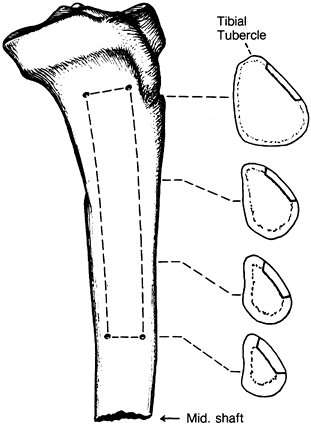
just posterior to the anterosuperior iliac spine.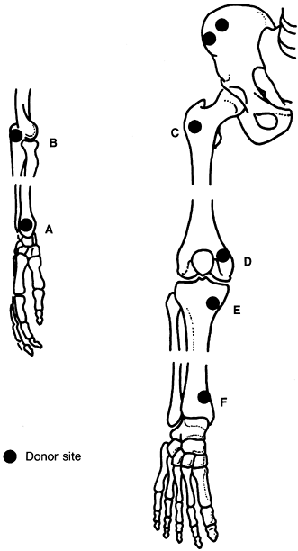 Figure 9.10.
Figure 9.10.
Peripheral sources of cancellous bone graft are illustrated. If only a
small amount of cancellous bone is needed or if it is contraindicated
or inconvenient to use the iliac crest, other sites of cancellous bone
are the styloid of the radius (A), the olecranon (B), the anterior aspect of the greater trochanter (C), the distal femoral condyle (D), the proximal tibia (E), and the distal tibia (F). (From Heim U, Pfeiffer KM. Small Fragment Set Manual. New York: Springer-Verlag, 1974, with permission.)
-
Use a tourniquet, and prepare and drape the extremity, as described in Chapter 5.
Make a straight, longitudinal incision over the middle of the
anteromedial surface of the tibia. Expose the anteromedial surface of
the tibia by subperiosteal dissection. Transverse incisions through the
periosteum at the proximal and distal ends of the longitudinal incision
improve exposure. -
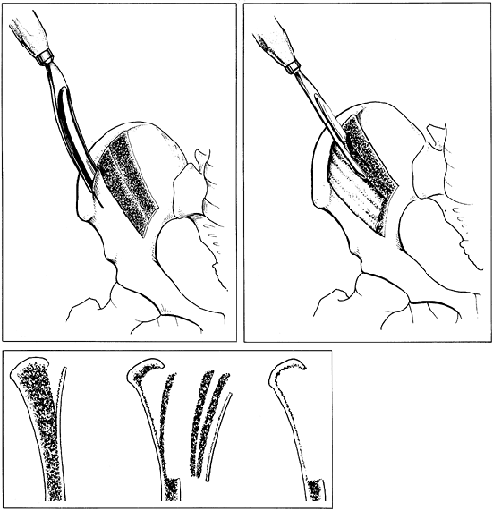
Outline the graft to be taken and drill a 3 to 4 mm hole in the cortex at each of the four corners of the graft (Fig. 9.11).
With a water-cooled oscillating saw, cut the graft. Avoid cutting
beyond the drill holes, because fracture of the tibia is then much more
likely to occur. Cut through the cortex at an oblique angle to assist
in removal of the graft.![]() Figure 9.11.
Figure 9.11.
A tibial graft is shown. A large, corticocancellous graft can be
removed from the proximal tibia on its anteromedial surface. (From Boyd
HB, Lapinski SP. Causes and Treatment of Nonunion of the Shafts of the
Long Bones. In: American Academy of Orthopaedic Surgeons: Instructional Course Lectures, Vol. 17. St. Louis: C. V. Mosby, 1960.) -
Carefully pry the graft out of the tibia
with a curved osteotome. To avoid dropping the graft, it is prudent to
insert a threaded Steinmann pin into one end of the graft; the
assistant can then use this pin to help pull the graft out of the donor
site and to hold onto it. Remove cancellous bone from the proximal
tibia as necessary. Avoid the subchondral bone of the proximal tibia
and, in children, avoid the physeal plate. -
After removal of the graft, release the
tourniquet and secure hemostasis. Because bone wax interferes somewhat
with bone regeneration in this location, it is best to control bleeding
with a collagen product. In adults, the periosteum and subcutaneous
tissue are closed in one layer. Drainage is important, but avoid
excessive, early suction because this can promote bleeding from the
bone graft site.
-
The entire proximal three quarters can be used, if necessary,
P.192
but rarely is the proximal end needed. Avoid injury to the peroneal
nerve. Never remove the distal fourth of the fibula, because it is
essential to the stability of the ankle. The syndesmosis ligaments must
be left intact, and a short portion with the interosseous membrane
attached must also be retained.
-
Harvest is usually best from the ipsilateral extremity being operated on to avoid morbidity in another extremity.
-
Long bone metaphyseal bone is much less
dense than the iliac crest in most adults, and in older patients it
contains less red marrow. For that reason, use metaphyseal cancellous
bone only when small amounts of less dense cancellous bone are required. -
Keep skin incisions small; expose only
the area to be harvested; avoid injury to local neurovascular
structures; and avoid harvesting through sites where chronic bursitis
or tendonitis might be a problem, such as the tip of the olecranon or
the lateral prominence of the greater trochanter. -
Avoid harvesting excessive bone immediately adjacent to the subchondral bone of the joint.
-
Avoid penetration of joints.
-
The entire fibula can be removed through
the interval between the peroneal muscles and the posterior
compartment, avoiding transection of any muscles. Remove the fibula
subperiosteally from distal to proximal to avoid injury to muscles. A
number of perforating blood vessels may be encountered; these vessels
must be coagulated or ligated. -
Facilitate removal by making the distal
transverse cut first. Carefully dissect circumferentially around the
fibula, avoiding injury to the peroneal vessels. Place two small, blunt
retractors anteriorly and posteriorly to protect the muscles.
-
For specific techniques related to free microvascular transfer of the fibula, see Chapter 36.
-
As opposed to the advice given earlier
regarding minimizing the size of skin incision, in this case a generous
longitudinal incision over the subcutaneous border of the fibula is
usually necessary to minimize the risk of complications to
neurovascular structures. -
Depending on the level of harvest,
carefully identify the portions of the common peroneal nerve at risk in
the exposure used. Protect these nerves, avoiding excessive retraction. -
Use intermuscular intervals to dissect to
the fibula and expose it by careful subperiosteal dissection. Avoid
diving into the deep soft tissues, which risks injury to the deep
neurovascular structures. -
Identify and carefully ligate arterial perforators to the fibula.
-
Never harvest the distal quarter of the
fibula. Leave the syndesmosis and a portion of the intraosseous
ligaments intact and attached to the distal portion. -
Use Henry’s extensile exposure for the
common peroneal nerve and proximal fibula in harvesting the proximal
third to avoid injury to neurovascular structures.
-
In general, the harvest of diaphyseal
cortical bone from the upper extremity and the femur is contraindicated
because of the risk of fracture. This should be done only if this is
the only reasonable alternative. In our careers, we have never
harvested from these sites. -
In our experience, harvest of tibial
diaphyseal bone is virtually never necessary today because of the
availability of alternative techniques. Neither of us have harvested a
diaphyseal tibial cortical graft in the last 20 years. -
If a tibial cortical graft is the only graft possible, then take the following steps to minimize the risk of complications:
-
Keep the bone graft as small as possible.
-
Use adequate-sized drill holes at the
corners of the harvest site and use a sharp water-cooled oscillating
saw that is kept constantly cool to harvest the graft, avoiding any
overcutting beyond the drill holes. -
If possible, harvest from either the
lateral or posterior surfaces of the tibia because the muscle coverage
lessens the risk of soft-tissue complications and makes it more likely
that the defect will heal to the greatest extent possible. -
Harvest from the subcutaneous border is
most convenient and provides the largest surface for graft but is
problematic because of the thin soft-tissue coverage. -
Tibial diaphyseal harvest sites must be
protected for a prolonged period of time, probably for at least 6
months in a well-fitted cast brace, with the patient’s activities
limited to normal sedentary walking using crutches initially and then a
cane. Sports activities or more vigorous activities must be avoided for
at least 1 year and, in some individuals with larger graft sites,
perhaps for several years. -
Decisions regarding when to cease
protection and allow the patient more vigorous activities are probably
best judged through the use of CT scans of the donor site. -
Because these grafts are exceedingly rare
today, no definite guidelines can be given. One of us has seen only two
diaphyseal tibial graft harvest sites in the past 20 years, both of
which were referred to him for secondary fracture of the tibia. One
patient responded to plate fixation and autologous bone grafting. The
other patient, who was infected, required multiple surgical procedures
but eventually healed, after control of the infection and double plate
fixation and autologous bone graft.
-
Use a water-cooled saw to transect the
fibula. Grasp the segment to be removed with bone-holding forceps,
maintaining gentle traction on the bone, and dissect subperiosteally
from distal to proximal until the segment of the fibula to be removed
is exposed. Transect the upper end in a similar fashion. If the entire
proximal fibula is to be removed, avoid injury to the peroneal nerve
and the trifurcation of the popliteal artery. -
After removal, repair the insertion of
the biceps tendon with strong, nonresorbable suture to the remnants of
the proximal tibiofibular ligaments.
prevalent in the first two decades of the 20th century, after the
experimental work of Ollier and Axhausen. In 1907, Lexer (82)
was the first to perform allogeneic whole joint transplantation, and he
had performed 25 by 1925. In 1942, Inclan reported the storage of
autogeneic and allogeneic bone, and this report stimulated many similar
clinical efforts at preservation, sterilization, and delayed
reimplantation. Although the superiority of fresh autogeneic grafts
repeatedly has been confirmed in experimental studies and clinical
experience, allogeneic implants preserved by freezing, freeze-drying
after sterilization with ethylene oxide, chemical sterilization, or
gamma irradiation are nonetheless widely used. Bone is more commonly
transplanted in the body than any other tissue or organ except blood.
Only recently has attention been paid to other musculoskeletal tissues
for allografting, such as cartilage, tendons, ligaments, and menisci.
for nonviable bone; an example is frozen, freeze-dried, sterilized
bone, a derivative of whole bone that lacks viable cellular components
but potentially contains inductive protein that can stimulate
osteogenesis.
in the recipient that it occupied in the donor, e.g., distal femur to
distal femur); heterotopic (transplanted to a different site but one
occupied by the same tissue as in the donor, e.g., fibula to spine); or
ectopic (transplanted to a site normally occupied by a different type
of tissue, e.g., fascia lata as a tendon graft). Ectopic sites for bone
grafting have been used mainly in investigating osteogenesis and,
rarely, for temporary clinical storage of bone.
replace missing bone segments and to promote the healing of nonunited
fractures. Cancellous bone or morcellized cortical bone is most often
used for filling cysts or cavities (Fig. 9.12); cortical bone is optimal for reconstructing defects that require a certain form and strength (Fig. 9.13).
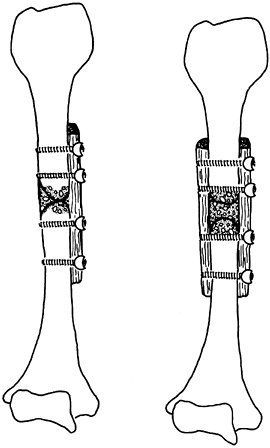
Although a cortical bone graft is strong when first implanted, the
incorporation process frequently weakens it, so a fatigue fracture may
occur many months to years after implantation (14). Therefore, plates or intramedullary devices are frequently used to augment the strength of the graft during incorporation.
 |
|
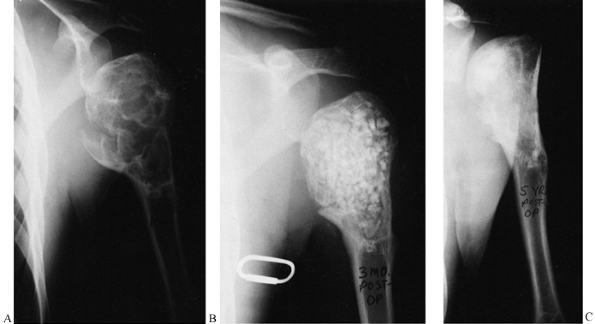
Figure 9.12.
This is a unicameral bone cyst in a young adult with a pathologic fracture. It was curetted and packed with freeze-dried and partially decalcified allograft (AAA) bone (B). Five-year followup shows healing with excellent graft incorporation and remodeling (C). |
 |
|
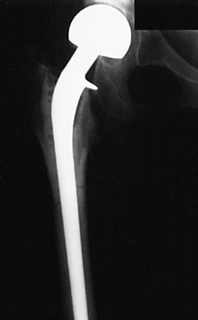
Figure 9.13.
A proximal femoral allograft was used to replace the proximal femur in a patient with a giant cell tumor. A custom metallic prosthesis was used to thread through the allograft. |
use of bone have been established by the American Association of Tissue
Banks. The goals of bone banking are to preserve the physical integrity
of the implant and its inductive proteins, reduce immunogenicity, and
ensure sterility. In general, a minimal interval (less than 24 hours)
between the death of the donor and the time of procurement is
desirable. Following the proper procedure for consent is essential.
shapes, and soft tissues and cells are removed to reduce
immunogenicity. Freezing to -70°C in a sterile state effectively
decreases immunogenicity and maintains sterility; this is generally
recommended for osteoarticular allografts (86).
Because the osteoarticular allografts frequently need to be matched for
size, the height and sex of the donor should be recorded by the bone
bank to permit as close a match as possible with the recipient (68). This method of storage decreases the strength of the allograft by about 10% (7).
Ethylene oxide sterilization also is effective, although it may destroy
bone-inductive proteins. The bone is preserved by freeze drying after
removal of ethylene oxide (79,118).
Freezing to -70°C and freeze drying reduce the immunogenicity of the
implant but with some compromise to its mechanical strength. Freezing
bone decreases its tensile and compressive strength by about 10% each,
whereas freeze drying decreases torsional strength by about 50% and
compressive strength by about 10% (114). The
process of replacement by host bone entails a transient reduction of
approximately 50% of strength. Sterilization of bone by heating to more
than 62°C, by autoclaving, or by gamma irradiation disrupts the
physical and chemical nature of bone and alters its mechanical
properties (18,35).
Bone implants subjected to these physically damaging processing methods
perform poorly. Bone subjected to freeze drying, partial
demineralization, or freezing incorporates more slowly than fresh
autografts or syngeneic grafts (11,107,161).
fresh state for 24 to 72 hours until the donor has been adequately
screened. The joint surfaces usually are stored in situ in the donor body at 4°C in a morgue environment. This ensures adequate viability of the cartilage for transplantation (133,146).
transfer of bacterial, fungal, or viral pathogens to the recipient.
Such measures should include patients’ histories and screening tests
for hepatitis, acquired immunodeficiency syndrome (AIDS), and syphilis.
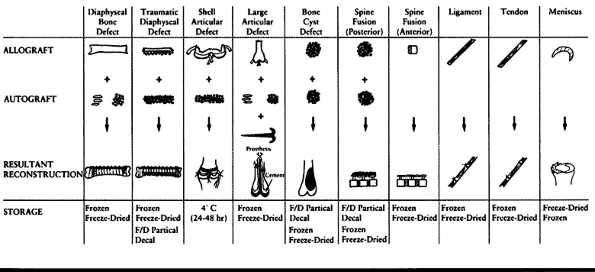
techniques commonly used for various types of musculoskeletal
allografts. Their applications have been well summarized by Czitrom (37).
 |
|
Table 9.2. Various Methods of Storing and Using Musculoskeletal Allografts for Different Orthopaedic Needs
|
into the skeletal system of the host. In the process of incorporation,
new bone deposited by the recipient envelopes and replaces the donor
bone tissue. How rapidly the graft is incorporated depends on its size,
structure, position, fixation, and genetic composition. The role of the
graft in stimulating incorporation may encompass osteoconduction,
osteoinduction, and osteogenesis.
autoclaved deproteinized bone all provide a scaffold into and around
which bone formation can occur (150). This function of the graft as a scaffold for ingrowth is referred to as osteoconduction.
natures or properties become intimately associated, and alterations of the developmental course of the interactants results” (150).
Bone matrix contains protein inductive factors, such as bone
morphogenetic proteins (BMP), which, if released from the matrix by
fracture, osteoclastic resorption, or chemical treatment, can induce
nearby mesenchymal tissues to differentiate into osteoblasts. This
factor is discussed in more detail later in this chapter.
destroyed the BMP are capable of osteoinduction in addition to
osteoconduction. An osteoinductive graft may be incorporated more
rapidly than one that is merely osteoconductive.
of new bone by living cells of the graft. Osteogenesis, together with
the absence of an immune response, is the basis for the superior
clinical performance of autografts. Although most of the autogenically
transplanted osteocytes die and leave empty lacunae, preosteoclasts,
preosteoblasts, and osteoclasts can survive (149).
In contrast, cells from a fresh allograft quickly elicit antibody
production and cell-mediated immunity, and are destroyed by the
recipient.
microanastomosis with existing microvessels. This process is more
common in cancellous bone and is aided in cortical bone by removal of
the periosteum (18). Most autografts and
alloimplants revascularize only by invasion of capillary sprouts from
the host bed during the resorption of the old matrix (creeping
substitution). Creeping substitution involves invasion of the allograft
by osteoclasts, and these, in turn, are followed by a blood vessel bud.
New osteons are laid down around the many blood vessels that invade the
graft (115).
also may be incorporated by a process of serial stress fractures that
result in graft remodeling. Periodically a region of stress
concentration may microfracture, followed by local remodeling. Later,
this process may occur in another region. The clinical manifestation of
this may be a complaint of rapid onset of pain, often after very minor
trauma, frequently with negative radiographic findings. The patient
should be put on crutches or other support until the pain is gone, in
order to prevent a catastrophic failure of the graft.
from the goal of restoring structural stability to bones or joints with
mechanical properties equal to adjacent bone and with an acceptable
cosmetic result. Therefore, the graft should be strong, potentially
viable, nonreactive (nontoxic, noncarcinogenic), sterile, storable,
capable of being shaped during surgery, and affordable.
structural and osteogenic functions desired, the size and shape of
various donor bones, and whether viable articular
cartilage
needs to be transplanted. The morbidity attendant on procuring an
autogeneic graft is often a limiting factor in bone graft surgery;
however, the use of some autogeneic corticocancellous bone is almost
essential in certain applications (such as posterolateral lumbar and
cervical spine fusions and to supplement allografts at the
osteosynthesis site). Alloimplants seldom result in fusion across
transverse processes or posterior arch structures; autolyzed,
antigen-extracted allogeneic (AAA) bone may be an exception (151).
Some autograft bone should almost always be used with allografts
because it helps to avoid nonunion and stimulates incorporation.
transplantation because of the major immune response to the
transplanted tissues. As discussed above, in the section on Bone and Tissue Banking,
freezing and freeze drying are useful storage measures that reduce the
immunogenicity of the implant. Alloimplants may be successfully used in
many applications, from craniofacial restoration to spinal operations
and whole joint transplantation. Donor bone is either removed
aseptically or secondarily sterilized with gaseous ethylene oxide or
gamma irradiation (119). The bone is then frozen or freeze dried for preservation and reduction of immunogenicity.
Furthermore, while in the frozen or freeze-dried state, bone loses its
inductive factors due to enzymatic autodigestion by intracellular and
extracellular enzymes. Accordingly, Urist developed a protocol for the
preparation of chemosterilized, AAA bone that would preserve the
inductive factors (149,150 and 151,153).
In this method, chloroform-methanol is used to extract lipids and cell
membrane lipoproteins (4 hours); 0.6 N hydrochloric acid extracts
acid-soluble proteins and demineralizes the surface (24 hours); and
neural phosphate buffer in the presence of sulfhydryl-group enzyme
inhibitors removes endogenous intracellular and extracellular
transplantation antigens (72 hours). The bone is then frozen and freeze
dried.
within 8 to 12 hours of death (minimal biodegradation time) if the
donor has not been stored in a morgue environment (4°C). If morgue
storage has been used, a 12- to 24-hour wait is acceptable. Prolonged
storage at -18° to 0°C and immediate freeze drying must be avoided,
because both reduce the level of inductive factors through
autodigestion. Sterilization by irradiation with more than 2.0 mrad
further denatures the BMP. The principal disadvantage of AAA bone is
decreased strength; therefore, it is rarely used when strength is an
important consideration. Urist and Dawson have used it for spine
fusions with a clinical success rate of 80% and a pseudarthrosis rate
of 12% (151). Urist also reported its successful application in 8 of 10 patients who underwent arthrodesis of the knee, ankle, and wrist (150).
The addition of fresh autogenic cancellous bone (including marrow) to
AAA bone creates a composite graft with enhanced osteogenic capacity.
AAA bone is not generally available for clinical use at this time;
however, other methods of partial demineralization are being used for
some preparations.
immune response after transplantation because the potentially
immunogenic chondrocytes are buried in the cartilage matrix and,
therefore, are inaccessible to the host’s immune system. Late immune
responses, such as lymph node hyperplasia, have been noted, however,
and the role of cartilage in eliciting an immune response remains
controversial (33,34,39,40,41,42 and 43,67,80,160).
If this immune response is strong, it can lead to destruction of
cartilage by a pannuslike reaction and a joint fluid inflammatory
response (128,130,131,132,133,134,135,136 and 137).
clearly understood, but again this could be an immune response,
cellular or humoral, to histocompatibility antigens (20,60,66,113,121).
Deterioration of the joint surfaces also may be the result of misfit
and eventual mechanical degeneration. Avoidance of these problems
requires exacting surgical technique (109) and excellent matching of donor and recipient sizes (68,129).
Mankin and colleagues reported preliminary results of massive allograft
transplantations performed for malignant or aggressive bone tumors (76,89).
Their improved allograft procurement technique involved freezing the
segments to decrease the immunogenicity of the bony portion and
glycerinization of the cartilage to maintain chondrocyte viability
during freezing and thawing. The complication rate was high, but the
tumor recurrence rate was low and the outcome generally successful.
Poitout has also reported high success rates using a similar technique
of liquid nitrogen storage and cartilage cryopreservation (116). Koskinen proposed the addition of autograft bone at the osteosynthesis site and noted an improvement in the union rate (78).
He also advocated strong internal fixation throughout the entire length
of the allograft to protect it from late fatigue fracture.
recipient; consequently, xenograft tissues must be treated to remove
antigenic proteins. Xenograft cancellous bone usually is obtained from
cattle. The freshly harvested bone is washed in water, extracted with
hydrogen peroxide to oxidize and partially remove proteins, delipidized
with fat solvents, and dried in acetone. It is then sterilized by gamma
irradiation. The resulting product (Kiel bone or Surgibone) is
structurally strong and elicits only a very weak antigenic response in
humans. The deproteinizing process also deactivates or removes
inductive factors, so the Kiel bone serves as an osteoconductive
scaffold; it is not inductive, however, as is demineralized human bone
matrix. Salama and Weismann report excellent results using Kiel bone
soaked in autogeneic marrow as a composite graft material (138). Xenogenic bovine bone is more widely used in Europe than in North America.
The collagen is dissolved and then reconstituted, leading to fibrillar
type I collagen with other antigenic proteins removed. Collagen by
itself is low in antigenicity. Collagen xenografts are most widely used
in dermatology and plastic surgery; collagen sponges also are used for
hemostasis in general surgery. For orthopaedic applications, collagen
may be combined with autogenous marrow and a ceramic composed of
sintered hydroxyapatite and tricalcium phosphate to form a composite
implant; such implants have been shown of be equal in effectiveness to
autologous bone in fresh bone defects in humans (24,32).
have noted that composite implants formed from various combinations of
ceramic, demineralized bone matrix, marrow, and type I collagen exhibit
a synergism among the components, resulting in faster and more abundant
bone formation. Although the biochemical basis of this process is not
well understood, it seems likely that such composite implants may prove
to be clinically useful.
have demonstrated little evidence for antihistocompatibility (anti-HLA)
antibodies in the serum of the hosts, whereas patients receiving
massive frozen allografts in the series reported by Rodrigo et al. (130) showed high titers of anti-HLA antibodies that remained positive as long as 5 years after transplantation.
immunogenicity and fresh subchondral bone that is highly immunogenic
are transplanted. With the use of a free avascular osteochondral
allograft for replacement of the end of a long bone, one desires the
devitalized bone to be remodeled through “creeping substitution” at an
appropriate rate (52,128)
and the cartilaginous surface to remain viable. Cartilage is
transplanted primarily for destroyed joints in the form of shell
allografts (joint cartilage plus 5 to 10 mm of subchondral bone). A
strong immune response to the bone can destroy the adjacent cartilage,
even though cartilage resists destruction by antibody and cellular
resorptive mechanisms (33,34).
In addition, the cartilage may become more immunogenic with time after
transplantation. One study indicated that the cartilage cells did not
express class I and class II antigens before transplantation, but they
did after transplantation; the expression of antigen became stronger
with time. It was suggested that an immune or inflammatory response
must occur first to stimulate the chondrocytes’ expression of HLA
antigens by the release of lymphocytes. If the recipient develops an
immune response against donor histocompatibility antigens, the
protection of cartilage from destruction is only relative; a low grade,
slow, immunologically mediated inflammatory response ensues,
characterized by increased synovial fluid and white blood cell counts,
antibody response, and pannus reactions that can destroy the cartilage (132,133).
bone elicits an immune response that delays healing at the
osteosynthesis site and blocks revascularization, resorption, and
appositional new bone formation. Long-term studies show no difference
in the morphology of eventual repair of autografts and allografts (77,126,158). Decalcified allografts repair at a faster rate than those that are only frozen, although they are mechanically weaker (106).
and spatial constraints of the area to be stabilized and the osteogenic
capacity of the host bed in orthotopic bone grafting. In general,
vertebral bodies, which are rich in cancellous bone and marrow, provide
far greater numbers of osteoprogenitor cells for remodeling a graft
than spinous processes, facets, and transverse processes, which are
composed mostly of cortical bone. Hence, demands placed on a graft
destined for posterolateral areas of the spine are greater than those
imposed on bone grafted to an intervertebral body location. For
posterolateral grafting of the spine, fresh autogeneic bone, either
alone or in a composite graft, is considered essential because the
graft provides surviving osteogenic precursor cells and inductive
factors. Frozen or freeze-dried devitalized allogeneic cortical and
cancellous bone may be used for cervical and lumbar interbody fusions
when less is demanded of the graft and when the recipient bed is rich
in marrow. At these locations, the incidence of fusion is the same with
allogeneic
bone and fresh autografts, although the rate at which the allograft fuses is slower (6,26,91,142).
and lumbar spine are lost because of degenerative processes, infection,
or neoplasia. Struts of allogeneic fibular bone can be used effectively
to restore stability; these struts are eventually incorporated and
remodeled. Even in the presence of recent osteomyelitis, devitalized
allogeneic bone can fuse and provide structural support for the
weakened spine (16). For large, multilevel
defects of several vertebral bodies, a femoral allograft frequently
will fill the defect and provide the correct curvature. In these cases,
additional internal fixation to protect the graft throughout its length
is advocated.
scoliosis frequently are performed in young children who do not have
enough bone present in the iliac crests to provide adequate amounts of
autograft. In these cases, allograft bone (frozen, freeze-dried, or AAA
bone) may be morcellized and mixed with the child’s own bone to provide
adequate amounts of graft material. More recently, titanium cages
filled with various grafting materials have been used for interbody
fusions. See Chapter 146 for more details.
-
During an operation, protect the
sterility of the graft at all stages. Avoid prolonged exposure to air
and saline and prolonged heating, which can destroy cells and inductive
factors (7,120,153).
Create an optimal host bed, preferably with marrow-rich cancellous bone
margins. Heating the recipient bed with power burrs or coating bleeding
interface with bone wax also destroys viable host cells and impairs
subsequent incorporation. -
Fashion the graft during the operative
procedure to mortise implants tightly into host bone. Spread the
allograft contact sites with autograft, and apply supplemental barrel
stave–like strips of bone graft to the osteosynthesis site. Orient the
graft in situ to provide axial alignment
of donor cortical bone with erect posture of the patient and maximum
exposure of the cancellous areas of donor bone to the host’s
marrow-containing cancellous bone. Forces across the host–graft
interface should maximize contact compression with ambulation of the
patient. -
Use suction for the first 48 hours after
the operation to reduce hematoma formation within perosseous soft
tissues, thereby reducing pain and preventing donor bone from floating
in a large blood clot. Early postoperative ambulation and mild exercise
stimulate blood flow and osteogenesis within the graft, provided the
graft is secure within the recipient bed. External splinting
appliances, as well as the patient’s muscle spasm and pain, tend to
stabilize the graft. Educating the patient with respect to proper
posture, weight bearing, turning, and exercising, especially when the
graft is most vulnerable, is critical for avoiding fatigue fractures
and pseudarthrosis.
the past 50 years, and recent results have approached 85% good or
excellent (72,76,116,124).
The needs for grafting in tumor surgery vary considerably, depending on
whether the defect is diaphyseal, ligamentous, tendinous, or cavitary
(cysts).
Other alternatives for replacement include the following: sliding
cortical autografts from the remaining bone above or below the defect,
large corticocancellous bone grafts from the iliac crest, vascularized
fibular autografts, morcellized autograft placed around an
intramedullary rod (23), and allograft bone with or without autograft (78,88).
Autogeneic bone alone is always preferable to an unsupplemented
allograft bone. When autograft sources are insufficient, allogeneic
bone can be used in combination with the autograft. When allograft bone
is used alone, it should be protected by a plate or rod throughout its
entire length to prevent a fatigue fracture when the allograft bone
weakens because of remodeling. Many years later, when the plate or rod
begins to weaken, the allograft bone will have been replaced
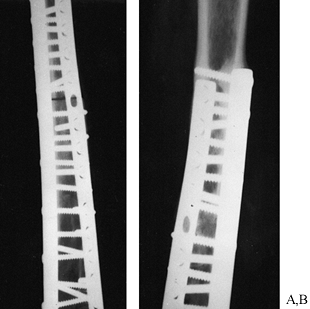
sufficiently to provide the needed strength (Fig. 9.14). In many
cases, gradual stepwise removal of the plates and screws is advisable to dynamize the allograft.
 |
|
Figure 9.14.
A midshaft femur freeze-dried allograft for a central chondrosarcoma is shown. The allograft showed good revascularization 8 years postoperatively, when one plate was removed for bursitis. |
for replacement include the following: a large osteoarticular
allograft, a large diaphyseal allograft with a custom metallic joint
replacement threaded through the allograft, and a joint fusion using
sliding autografts with or without allograft bone. Preliminary evidence
suggests that diaphyseal allografts used in combination with joint
replacements are superior to large osteoarticular allografts for
replacement of large osteoarticular defects (10).
Results were varied, and in all cases complications led to a failure of
normal joint function; however, the operations worked well as salvage
procedures. The more recent technique of threading a long-stem
customized joint replacement through a large diaphyseal allograft (10) appears to give better results, at least in the short term.
All of these grafts are best protected by screws, a porous fixed
acetabular component, a plate, or some combination of these items.
 |
|
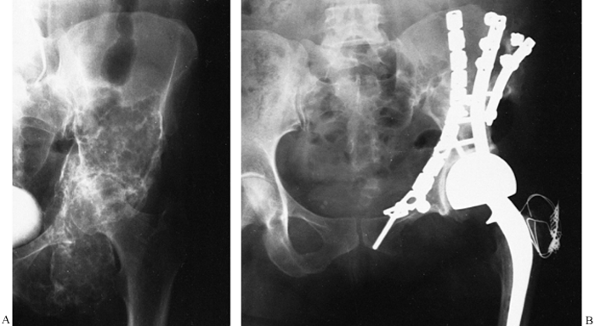
Figure 9.15. A large acetabular allograft (B) for a low-grade fibrous histiocytoma (A) of the acetabulum is shown.
|
can be filled with autografts in most cases. However, when the cysts
are so large that there is inadequate autogeneic bone available from
the iliac crests, allogeneic bone (preferably AAA bone) may be
morcellized and supplemented with autograft to fill the cyst cavity (Fig. 9.12).
biologic resurfacing of joint defects is being tried. The concept of an
autogenous free periosteal graft coupled with continuous passive motion
(105) or autogenous cartilage cell implant
combined with perichondrium is in early clinical trials and has
demonstrated mixed success (see Chapter 86). The use of autogenous osteoarticular autografts is an acceptable means of reconstruction (134,140,141).
However, this technique has the disadvantage that the available graft
materials seldom have a suitable shape for reconstructing a given
defect.
involving the transplantation of a devascularized, osteoarticular
allograft with a small bony component. Studies of osteoarticular shell
allografts in humans have not been followed long enough or often enough
to determine their outcome, but several authors have reported promising
early results (56,57 and 58,97). Of the shell allografts that have failed, some appear to have had a significant pannus reaction (75), suggesting that an immune response was induced by the cells of the subchondral bone (36,136).
-
If a small joint surface defect exists,
then make an attempt to resect only that part of the joint surface.
Press fit a clear plastic or wax material over the joint surface defect
to use as a template, and make a cut in the cartilage approximately 3
to 4 mm around the defect. In this way, a circumferential or geometric
cut is made in good viable cartilage just to the edge of the defective
cartilage. -
Cut approximately 5 to 10 mm into
subchondral bone with an osteotome, and remove the entire joint surface
defect. Inspect the subchondral bone, and curet any remaining defective
subchondral bone. If there is a large crater, as in avascular necrosis
of the hip, pack it with a fresh autogeneic iliac crest bone graft. -
Prepare the small allograft piece from a
donor joint that has been recently procured. Procurement requires
taking the specimen in a sterile fashion and keeping it sterilely
stored at 4°C with capsule intact. The end of a long bone is sufficient
for small defects. -
Place the plastic template that was used
to mark the size of the defect over the donor cartilage surface; mark
the donor surface cartilage with a knife, and cut a corresponding piece
from the donor surface, being sure to make the piece slightly larger
than what was removed from the recipient. Remove approximately 1 cm of
subchondral bone with the donor cartilage. Press fit the perfectly
sized donor piece into the defect. -
If there is good fixation at this point,
no further fixation is needed. However, it is better to err on the side
of obtaining good fixation, if possible. Small threaded Steinmann pins
may be drilled from the side into the subchondral bone, and small
screws also may be used for this purpose. Either procedure would be
better than passing screws across the cartilaginous surface through the
tidemark. If the anatomy of the joint does not allow this technique,
then drill four small (2.7 mm) holes in the four quadrants of the
graft, bringing the drill holes out through cortical bone at some place
distant from the graft along the metaphyseal region of the bone. Pass
another suture in a crisscross fashion through the other two holes, and
tie it over bone. This approach provides fixation of the graft until
the subchondral bone heals, and by that time the suture will dissolve.
The latter method is not as good as using screws or small pins, because
it involves drilling through the tidemark and the pressure of suture
across the donor cartilaginous surface. Another reasonable alternative
is the use of bioresorbable pins placed in a divergent pattern through
the allograft into recipient bone. -
Place the patient’s joint in a temporary
splint after adequate closure is obtained, and allow the joint to rest
for 2 to 3 days. Once the bleeding has stopped and the wound has begun
sufficient healing, start continuous passive motion if adequate
fixation of the graft has been obtained at surgery. Continue the
continuous passive motion for 3 months, if possible. Do not allow full
weight bearing for 6 to 9 months, at which time radiographs should show
adequate healing of the subchondral bone interfaces. If possible,
evaluate the graft approximately 1 year after surgery using diagnostic
arthroscopy to assess for an immune rejection phenomenon, which is
characterized by pannus covering the graft. If this reaction occurs,
the prognosis for the graft is poor. -
Preoperative and postoperative serum
studies can help predict an immune response. Obtain serum samples
before surgery and at 6 weeks, 12 weeks, and 1 year after the surgery.
Test these serum samples against donor lymphocytes, if they are
obtained at the time of the graft procurement, or a panel of typed
lymphocytes in a standard lymphocyte toxicity assay. A strong antibody
response does not necessarily mean that the graft will be rapidly
destroyed. Diagnostic arthroscopy is the only sure way to determine the
health of the graft. -
If a large shell allograft is needed,
such as in replacement of the entire hemijoint surface of a joint or
replacement of a medial or lateral side of both the proximal and distal
joint surfaces, then different techniques are needed. In these cases
there is usually a large defect due to a severe traumatic episode, such
as a gunshot wound. -
Procure the allograft specimen as an
entire joint specimen, and transport it to the operating room in
sterile saline at 4°C with the capsule intact. Transplantation of the
joint surfaces should be done approximately 48 hours from the time of
procurement. There is evidence to suggest that with this type of
storage, the subchondral bone cells will die before the cartilage
cells; this may be advantageous when trying to prevent an immune
response against the subchondral bone that could eventually damage the
donor cartilage (133). For this reason the specimens are not transplanted when they are fresh and warm. -
Debride the defect in the joint of all
scar tissue down to healthy bleeding bone. Fashion the shell allograft
pieces to fit the defect, leaving intact on the graft any ligaments
needed to replace ligaments missing in the
P.201
recipient.
Fix the graft through subchondral bone with threaded Steinmann pins,
screws, or staples placed from the side and not passing through the
donor cartilage surface. -
If good fixation is obtained at the time
of surgery, then begin continuous passive motion within a short time
after surgery. If only marginal fixation is obtained, however, then
immobilize the entire joint for 6 weeks or, at most, allow a few
degrees of motion in a protective brace. Allow partial weight bearing
for 3 to 6 months until adequate healing of the subchondral bone
interfaces is evident on radiography; this may require tomographic
evaluation. A second assessment with an arthroscope 1 year after
surgery may be desirable to determine whether a severe immune reaction
is occurring. Serial preoperative and postoperative serum
histocompatibility testing also can be done to monitor the immune
response.
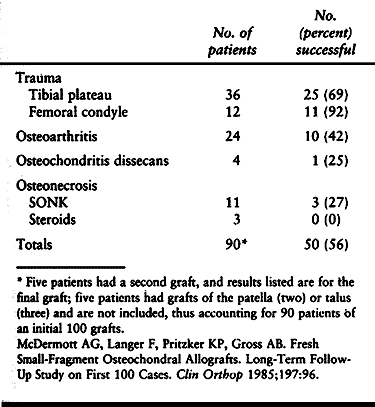
These patients have had success rates of 69% to 92%. Patients with
osteoarthritis also have satisfactory results with a success rate of
42%. Patients with osteochondritis dissecans and avascular necrosis
have had the poorest results, with a success rate of only about 25%.
The reason for this poor showing may be that there is inadequate
healing of the osteosynthesis site because of a poor blood supply to
the subchondral bone.
 |
|
Table 9.3. Effects of Etiology on the Results of Shell Allografts
|
primarily in the hip. Major segmental defects in the acetabulum as well
as the proximal femur may occur after multiple revision arthroplasties.
These defects can be reconstructed with allografts (see Chapter 106). Acetabular defects may be divided into rim defects, medial wall defects, and global defects.
posterior rim, or combinations of superior and posterior rim defects.
Most of the superior and posterior rim defects can be reconstructed
using a bicortical piece of autogenous iliac crest. If the defect is
large, as in the superior and posterior rim defect, a proximal femoral
head or distal femur allograft is usually better to reconstruct the
defect.
-
Cut and shape the proximal graft to provide the bone stock that is necessary, and fix it to the pelvis with two to three screws.
-
Use a screw-fixed porous ingrowth type of
acetabular component that has screw holes near the rim, so that the
screws securing the component can be used as additional fixation of the
allograft. -
Butter the allograft with autogeneic marrow and morcellized iliac crest bone to stimulate healing at the osteosynthesis site.
-
The screws going through the metal
backing of the acetabulum component should pass into good recipient
bone to protect the allograft during its remodeling. These grafts may
take up to 10 years to be completely resorbed and replaced by recipient
bone; therefore, they should be protected with partial weight bearing
with a cane during this time.
use morcellized autograft packed under a fixed porous ingrowth
acetabular component. If the medial wall defect is segmental and large,
then it may be adequate to use a large outer table of autogeneic iliac
crest, plus morcellized graft with a fixed porous component. It may be
necessary also to use a femoral head allograft fashioned to fill the
defect. Again, a porous acetabular component is desirable because it
will fix the allograft to the recipient autogeneic surfaces and protect
it during remodeling.
results in a very large defect. In these cases, use a large femoral
head allograft or distal femur allograft.
-
Debride the allograft of all cartilage
and soft tissue, and fix it with a few screws to the recipient pelvic
wall. Coat the surfaces with autogeneic bone and marrow before fixation. -
Once it is in position, ream the
allograft using standard reaming techniques, and place a screw-fixed
acetabular component, fastening additional screws through the porous
component into the allograft and into the remaining recipient pelvic
wall. -
Occasionally, an acetabular reinforcement
cage with an acetabular component cemented into it is necessary.
Prolonged protection with partial weight bearing using a cane for up to
5 years is recommended to allow complete incorporation of the allograft
material.
allograft is necessary. When harvesting the allograft for these cases,
preserve the ischial ramus, pubic ramus, and superior acetabular bone.
-
Fix the graft with screws and plates to
the recipient pelvic bone. If a bipolar proximal femoral component is
to be used, then use an anterior as well as posterior column plate to
fix the allograft. -
Coat the surfaces of the allograft with autograft to stimulate healing and union.
-
If a total hip replacement is to be used,
install an acetabular cage after reaming the allograft acetabulum and
coating it with autogeneic bone. Place the screws fixing the acetabular
component so that they pass through the allograft and into recipient
autogenous bone. Take care to keep the ischial part of the cage on the
anterior ischial tuberosity so it does not irritate the sciatic nerve.
three types. The first is an intramedullary defect due to diffuse focal
resorption of intramedullary bone around the bone cement interface; the
second is a medial calcar defect; and the third is a global proximal
femoral defect.
-
The intramedullary defect is best treated
with morcellized autogenous bone plus a porous ingrowth type of
prosthesis. If the amount of bone lost is too great to be replaced with
autogenous bone, however, mix autogenous iliac crest bone with
morcellized allograft bone as a slurry, and pack the defect with this
combination autograft and allograft before seating the proximal femoral
component. -
For medial calcar defects, cut and
fashion a medial calcar allograft to fit the defect. Fix this graft
with wires, screws, or a combination of both before seating the
proximal femoral component. In these cases, the porous coating should
be at least two thirds the length of the stem. -
A global defect of the proximal femur may
encompass bone loss of several centimeters in length. In these cases,
cut an entire proximal femur allograft and fashion it to fit the
defect, making a step cut at the osteosynthesis site. Prepare allograft
on the back table including reaming, then cement the proximal femoral
component into place in the allograft. -
Remove all excess cement from the
osteosynthesis step cut site on the allograft, and then cement the
distal stem into place. Additional fixation is usually necessary at the
osteosynthesis site in the form of a plate, screws, or cerclage wires. -
In addition, place strips of autogeneic
iliac crest around the circumference of the osteosynthesis site, and
hold them in place with cerclage wires. This method stimulates healing
at the osteosynthesis site. -
Postoperative management of these
patients includes suction drainage of any postoperative hematomas and
bed rest for 3 to 4 days until bleeding has stopped. At this time,
mobilize the patient and allow partial weight bearing using a walker or
crutches for several months until the osteosynthesis site has
adequately healed. Follow graft healing on serial radiographs. Healing
of the osteosynthesis site can be expected to take twice as long as
normal bone healing, that is, 3 to 6 months rather than 6 weeks to 3
months. Replacement of the large grafts may take several years, and for
this reason patients should use a cane for several years to protect the
prosthesis and the graft until it has been solidly incorporated and
replaced by recipient bone. Serial bone scans are helpful in
interpreting this replacement process. The allograft will become “hot”
on bone scan within a few weeks to a few months after the operative
procedure, and it will remain hot for several years. If quantitative
bone scans can be done when the scan turns “colder,” this is probably
an indication that significant strength has been regained to allow full
weight bearing.
traumatic destruction of skeletal parts presents many more difficult
problems than replacement of tumor or arthritis defects. Severe
soft-tissue injuries usually accompany the bony trauma, and many of the
wounds have been previously infected (Fig. 9.16).
Therefore, the surgeon is frequently operating in an area of decreased
blood supply, poor skin coverage, and persisting organisms from
previous infections. Most of the experience with allograft use in the
treatment of traumatic defects has come from the wartime experience,
and the following important prerequisites should be followed (15):
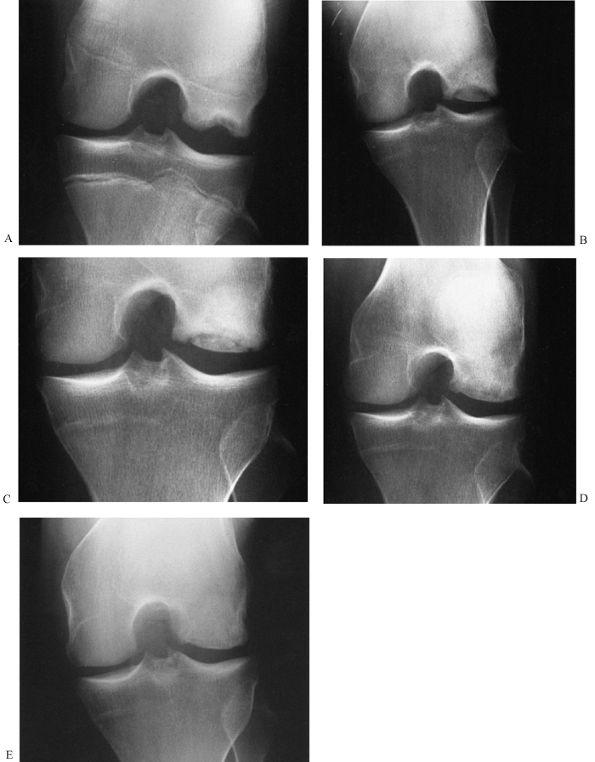 |
|
Figure 9.16. This is a femoral condyle shell allograft. Shown are a preoperative traumatic defect (A); immediate postoperative radiograph (B); replacement of subchondral bone in progress (C); full replacement of subchondral bone at 2 years after surgery (D); and appearance of the graft 11 years after surgery (E). (Courtesy of Dr. Frank Palumbo, Carmichael, CA.)
|
-
The distal anatomic parts are functionally intact.
-
Good skin coverage must be present or
obtainable at the time of surgery. (Remember that a collapsed
soft-tissue space will be filled with bone, which creates a stress on
the previous skin coverage.) -
Evidence of infection is absent.
-
Circulation is adequate.
-
Abundant cancellous autograft is available for supplementation of the bony surfaces of the allograft.
-
The graft is protected until healing is complete.
the treatment of diaphyseal defects. First, morcellized bone appears to
incorporate faster than large segmental allografts. Jeshrani and
Bencivenga found that 5 of 5 cortical grafts without morcellized grafts
failed, 3 of 3 cortical grafts with morcellized graft succeeded, and 17
of 17 grafts with only morcellized bone healed well (71).
Osteogenesis and revascularization of the morcellized bone begins to
occur within 10 days, and bacteria are much less likely to survive in
these small, rapidly revascularized pieces than in a large, nonviable
piece of allograft bone (18).
a gap in the femur, humerus, or other isolated long bone, it is
recommended that half of an allograft shaft be used with morcellized
autogeneic bone packed around it (Fig. 9.17) (9).
This approach has two advantages: It maximizes the morcellized graft
material, and it decreases the volume of cortical bone that might
distend the already shrunken surrounding soft tissue. Extreme care must
be taken to protect the large cortical allograft until it has been
incorporated; this may take as long as 2 to 3 years. The protection can
be provided by a cast, brace, internal fixation with rods or plates, or
a combination of these techniques.
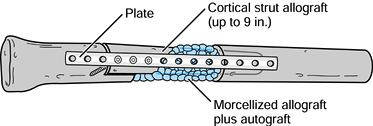 |
|
Figure 9.17. Diaphyseal strut allograft after trauma should be reduced in volume and surrounded by abundant morcellized graft.
|
cortical allograft for tibial bone loss. In this case, a proximal and
distal tibiofibular synostosis is obtained first; then progressive
grafts made of morcellized, mixed autograft, and allograft bone are
used to bridge the defect (94). This may
require a number of successive grafts. It is possible that synthetic
bone materials mixed with autogenous graft may prove to be at least as
good as allograft material for filling such defects. Alternatively,
bone transport by Ilizarov techniques might be the best way to bridge
the defect (see Chapter 32).
the maximal use of morcellized autografts with allografts can give the
results shown in Table 9.4 (15).
A good result is defined as restored bone continuity and no need for a
brace. Satisfactory results are those with restored bone continuity but
with the need for bracing. A failure is defined as an amputation.
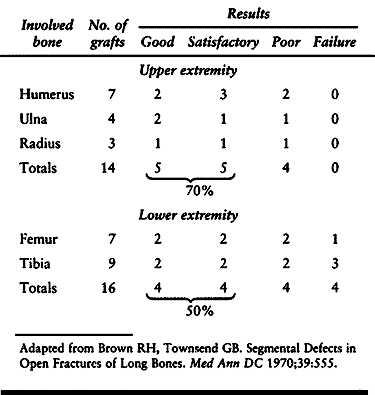 |
|
Table 9.4. Results of Allograft-Autografts for Traumatic Bone Loss
|
replacement is the same as previously described in this chapter. The
success rate is considerably better when shell allografts are used for
traumatic defects than when used for primary arthritic conditions such
as osteoarthritis and avascular necrosis (Table 9.2).
allografts has not yet been determined for posttraumatic conditions.
The success rates could be considerably worse because of problems with
avascular necrosis and collapse of subchondral bone. Using artificial
joints threaded through the allograft may prove to be a better method
to treat posttraumatic
defects of the ends of long bone, as has been the case with large tumors or failed total joint replacements.
pluripotential, primitive mesenchymal stem cells to differentiate into
osteoblasts. The process of induction has been intensely investigated
by Urist et al., and others (7,8,143,147,148,149,150,151,152,153 and 154).
Induction of bone formation occurs, in part, as a result of nonsoluble,
noncollagenous, bone morphogenetic proteins, as well as other inductive
proteins (21,22,48,50,51,53,103,127,147,148,149,150,151,152,153 and 154,159). The most intensely studied of these proteins is from those composing the BMP super family. These proteins are summarized in Table 9.5.
 |
|
Table 9.5. The BMP Superfamily in Mammals*
|
been recombinant BMP2 (rhBMP-2) and rhBMP-7, also known as osteogenic
protein-1 (OP-1).
found that when species-specific BMP was placed in a canine radius
nonunion model using a polylactic acid carrier, that a higher incidence
of union occurred as compared with that of controls. Yasko et al. (162),
using human rhBMP2 in a critical defect model in the femora of rats,
were able to demonstrate successful union compared with controls. This
was dose related, requiring a large dose of 11.0 µg to achieve union.
Gerhart et al. (48), using the same protein but
in a segmental defect in the femur of sheep stabilized by a plate,
showed no bony union in their negative control groups but complete
union in their experimental group and autograph controls. Similar
results were demonstrated in a rabbit critical defect model (166).
A significant number of human clinical trials are now under way with
these very interesting proteins. Recently published studies have
demonstrated successful use of a human BMP to augment one-stage
lengthening of femoral nonunions (73). When the
appropriate carrier vehicles are combined with the appropriate protein
or proteins, there is a high likelihood that they may replace the use
of autologous bone graft and nonstructural allografts to a great
extent. Future research in tissue engineering may result in synthetic
structural grafts as well.
aspiration in adults from the axial skeleton, contains a small but
significant number of pluripotential mesenchymal stem cells and
inductive factors that have been used to treat nonunions (27,28). Connolly et al. (27,28) have demonstrated the efficacy of repeated injection of tibial nonunions as a substitute for open operative grafting.
consists of the growth of host bone into a bone graft or matrix by the
growth into available channels in the graft of vascular buds, followed
by the laying down of osteoblasts along these channels with subsequent
bone formation. In some cases, osteoclastic resorption along the
channels may be a part of this process, but this is not necessary with
many materials. From the standpoint of a material introduced into a
bone defect site, this represents a passive rather than an active
phenomenon as in bone induction. Bone conduction can occur through an
open matrix, such as one would find in granules packed loosely
together, or through structural channels, such as found in some types
of coral, in which the ideal size appears to be somewhere between 250
and 600 µ in diameter. A number of synthetic or naturally derived
bone-filling materials, some of which promote bone formation through
conduction, are now on the market or under investigation. If these
materials are used as a carrier vehicle for inductive proteins, then
induction would of course also play a role. In their review of the role
of bone substitutes, Hollenger et al. (69) provide a list of those available in the United States as of 1996. This list is summarized in Table 9.6.
 |
|
Table 9.6. Bone Substitutes Available in the United States
|
materials at this time are Collagraft, Grafton, and ProOsteon 500. The
Norian SRS material has received considerable attention recently, but
it is still in clinical trials and is not available for general use.
nonstructural and supplied as a particulate. It may retain some bone
induction capability. Most surgeons use it to graft fracture repairs
where some assistance is desired but the surgeon either does not wish
to incur the morbidity of harvesting a bone graft or is not prepared to
harvest a bone graft. Otherwise, most surgeons seem to be using it as
an adjunct to autologous bone grafting to expand the bone graft,
particularly in spine fusions.
that, after rehydration, becomes soft and can be packed into bone
defects or laid on the surface of bone. In laboratory and clinical
studies, it has proven to be equally efficacious as autologous bone
graft in the experimental models and clinical applications chosen (24,32,59,90,93,102).
Collagraft has not experienced much popularity, however, because the
most common application of this type of material is to fill metaphyseal
defects where some structural integrity is necessary that the
Collagraft material does not offer.
porosities with cross-connected channels and is manufactured by the
chemical transformation of the calcium carbonate skeleton of a marine
coral. It has been shown in canine critical defect models to
incorporate well with excellent bone growth into the open channels of
the graft (90,93).
Currently this material is approved by the Food and Drug Administration
(FDA) for the filling of metaphyseal defects. It is used at our
institution for filling the defects in juxtaarticular fractures such as
those in the distal radius, in tibial plateau fractures, and in pilon
ankle fractures. We also use it to fill defects created by the removal
of small benign bone tumors (104). This
material is available both in block and granular form. Because it is a
very brittle material it is difficult to work with. It is quite weak in
shear and tension. When contained in a bone cavity totally surrounded
by bone, where the cavity is firmly packed with the material, it offers
reasonable resistance to compression, sufficient enough that it is
practical for reconstruction of tibial plateau fractures. Additional
support with internal fixation is required. Excessive packing can crush
the blocks and granules and reduce their porosity, potentially
interfering with bone ingrowth, but this problem has not been
documented in practice.
Because it is essentially pure hydroxyapatite, it illicits no immune
response. Occasionally, joint surfaces will settle because of
structural weakness in the material, but this can usually be avoided by
appropriate internal fixation. Because it is a foreign body, it could
potentially be a problem if infection were to occur. However, the
studies noted earlier actually show a slightly lower infection rate
with ProOsteon when compared with autologous bone. If significant
infection were to occur, then resolution of the infection would most
likely require removal of the ProOsteon. Lastly, the granules can
occasionally get into anatomic sites that are not desirable. In packing
the granules into bony defects, they often become adherent to the
surrounding soft tissues. Although in our experience this results in no
untoward effects, it does produce a less pleasing radiograph. It is a
somewhat laborious task to pick each of the particles off the soft
tissue. In addition, we have seen one case of a ProOsteon 500
arthrogram of a knee in which a surgeon packed the granules into the
defect of
a
severe tibial plateau fracture, apparently unaware that there was an
opening in the joint surface that allowed passage of the granules into
the joint space. One year after this occurrence, we arthroscoped the
knee and found the knee joint cartilage and menisci to be in perfect
condition, with no evidence of untoward effects from the granules. The
granules were all adherent to the synovium and were visible through the
arthroscope but appeared to be covered by a thin membrane as they were
apparently incorporated into the synovium. Therefore, removal of the
granules was impractical and seemingly unnecessary. Whether there will
be any long-term untoward effects of this complication remains to be
seen.
injectable calcium phosphates. To a mixture of three different calcium
phosphates and calcium carbonate, a sodium phosphate solution is added,
resulting in a paste that is formable and injectable for approximately
5 minutes. It is biocompatible as it sets at physiologic temperatures
and Ph. The dahllite in the paste hardens after about 10 minutes,
obtaining an initial compressive strength of approximately 10 mPa.
Within 12 hours, full strength is achieved, with a maximum compressive
strength of approximately 55 mPa and tensile strength of 2.1 mPa (29). Orthopaedic surgeons have been seeking some type of bone glue and
injectable synthetic bone replacement for years, and this is the first
commercially available implant of its type to be shown to be clinically
useful. It is not truly a bone glue but can immobilize and fix
cancellous bone fragments into place by mechanical interlocking.
Cortical bone fragments pressed into the material can likewise be
immobilized by mechanical interlocking. When the material has set, it
is a relatively solid material that does not offer any porosity for
bone conduction. Animal studies show that the material is replaced by
host bone in a manner similar to bone remodeling on the surface of the
implant. The rate of remodeling and whether or not the Norian material
will eventually be entirely replaced by host bone in humans has not yet
been established. Preliminary clinical studies suggest that the Norian
SRS material may be useful in weak osteoporotic bone, Colles’ fractures
of the distal radius, tibial plateau fractures, other juxtaarticular
fractures involving cancellous bone, and possibly in spinal
reconstruction and revision acetabular arthroplasty (29,54).
At the time of this writing, this material was not yet approved by the
FDA for general use. Within the lifetime of this edition of Chapman’s Orthopaedic Surgery,
no doubt this material will be widely employed. Two potential
complications exist: Material may penetrate into the intraarticular
space when it is injected percutaneously; and when a large amount of
material is used relative to the size of the bone, particularly if it
covers the vast majority of the fracture interfaces, delayed or
nonunion could occur. To the best of our knowledge, neither of these
problems have been reported in the literature.
exclusively to the usual surgical complications that can occur at the
bone graft harvest site. Complications in the use of allografts result
primarily from the problem of graft rejection due to immunologic
intolerance, and late graft failure due to weakening of the allograft
through bone remodeling. The other major concern is disease
transmission, in particular the viruses of hepatitis B, and C, and
human immunodeficiency virus (HIV). Through donor screening, testing,
and implant processing, these risks have been minimized. The major risk
remains with fresh composite allografts in which bone and cartilage are
transplanted. Donor screening programs have minimized this risk, but
they remain real. The complications and risks associated with the use
of allografts are discussed in some detail earlier and will not be
considered further here.
graft materials and other types of processed bone are quite small and
related primarily to either premature mechanical failure of the graft,
or nonunion due to lack of incorporation of the graft, lack of
ingrowth, or inappropriate use of the material for the clinical
situation. The most common problem we have seen has been the packing of
a fresh fracture defect site or site of a nonunion with an
osteoconductive material that offers no bone induction where the host
bed is incapable of thoroughly healing the gap site, resulting in
nonunion of the fracture or persistence of the nonunion. This problem
can be avoided by using autologous bone and adding purely conductive
materials only when necessary.
available in the United States for general use and because the clinical
trials reported are quite limited, potential complications of these
proteins, other than lack of efficacy, or perhaps immunologic adverse
response, are not yet known.
reported a major complication rate of 8.5% and minor complication rate
of 20.6% at 243 bone graft donor sites harvested by all types of
surgeons at their institution. Potential early complications include
wound dehiscence, infection, seromas and hematomas, pain, inadvertent
injury of adjacent joints, muscle or bowel herniation, and injury to
important structures such as nerves, vessels, and the ureter.
Intermediate and long-term complications include an ugly or painful
scar; bony deformity; fracture, with or without nonunion; chronic pain;
persistence of neurologic injury; and reflex sympathetic dystropy, as
well as others. Younger and Chapman reported a higher incidence of
complications in patients undergoing posterior spine fusion in which
the bone graft was harvested through the same incision as used for the
spine surgery. Hugh and Bohlman (70), however,
focused on the incidence of fractures at the iliac bone graft harvest
site, finding 14 patients with fractures out of approximately 400
fusions divided equally between the cervical and lumbar spine. These
were either avulsion fractures of the anterosuperior iliac spine or
fractures of the iliac wing. All patients were treated nonoperatively
with excellent long-term outcomes. They identified older women with
osteopenic bone as being particularly prone to this complication. In
1997, Goulet et al. (55) reviewed 170 patients
from whom bone grafts were harvested. Major complications occurred in
four (2.4%) in whom deep infection developed. Thirty-seven patients
(21.8%) had minor complications. Several of their patients (18.7%)
continued to report pain more than 2 years postoperatively. In spite of
this incidence, the vast majority of patients were functioning well at
2 years postoperatively. Simple rules to minimize the risks of
complications in bone graft donor sites are detailed in the Hints and
Tricks sidebars throughout this chapter.
site with clear or serosanguineous drainage. These lesioms are usually
not a problem if they are contained by a tightly closed fascia.
However, a large seroma or hematoma presenting in the subcutaneous area
may require aspiration and compression dressing or exploration of the
wound for drainage, control of bleeding points, and repeat closure over
suction drainage. This is important because these lesions can be very
slow to resolve, and persistent drainage can lead to retrograde
infection of the donor site.
early, within the first 1 to 3 weeks. Early surgical intervention to
drain and debride the operative site is nearly always indicated. Gram’s
stain and deep cultures are important, along with early institution of
appropriate intravenous bacteriocidal antibiotics. If surgical and
medical treatment is performed early, most patients respond quickly to
treatment, and chronic osteomyelitis is rare. Chronic osteomyelitis in
a donor site requires thorough debridement of all nonviable bone and
treatment, as outlined in Chapter 133.
sensation, some develop painful neuromas that can be disabling and even
lead to reflex sympathetic dystropy. Simple loss of sensation due to a
neuropraxia of the nerve secondary to retraction is usually managed
well by appropriate counseling of the patient and watchful waiting.
Persistent complaints with a definite trigger point, Tennel’s sign, or
palpable neuroma usually merit surgical exploration of the nerve.
Magnification should be used for careful inspection of the nerve. If an
intact nerve is found with minimal intraneural scarring, then simple
decompression with neurolysis may suffice. If a neuroma or dense
intraneural scarring is found, then resection of the injured segment
with repair of the nerve, or in some cases resection with burying of
the proximal stump, may be indicated depending on the nerve involved
and the patient.
viability of important structures or the extremity are usually dealt
with at the time of initial injury with ligation of the vessel. Injury
of larger vessels that are important to function or limb survival
require acute repair and intervention of a vascular surgeon when the
orthopaedic surgeon is not qualified to perform vascular repairs. The
most vexing late complication of a vascular injury is a pseudoaneurysm
in which the initial vascular injury was not appreciated by the
surgeon. Most typically, these lesions present as a mass or swelling
that may or may not be painful. On physical examination, they may be
pulsatile or have a bruit on oscultation or palpation. Ultrasound or
arteriogram, or both, is usually necessary to make a diagnosis, and
vascular surgery consultation for surgical correction is nearly always
indicated.
anterosuperior iliac spine or a split in the anterior column resulting
in inferior and anterior displacement of the anterior fragment to which
the sartorius and occasionally the rectus femuris are attached. The
vast majority of these fractures can be treated nonoperatively with
limited weight bearing with crutches. Union usually occurs within 6 to
10 weeks. The patient may be left with some deformity of the pelvis,
but usually this is asymptomatic and not of cosmetic concern. For the
most part, these fractures occur in elderly osteoporotic women.
Occasionally, they occur in young active athletes. In these cases, open
reduction and internal fixation may be indicated.
metaphyseal areas with minimal displacement can usually be treated
nonoperatively. Displaced unstable mid-diaphyseal fractures,
particularly in the tibia and femur, are usually best treated with
closed reamed intramedullary nailing. Reaming of the medullary canal
grafts the defect site, and if necessary, additional cancellous bone
can be added by the intramedullary or subperiosteal route (23).
Intramedullary nailing usually allows early weight bearing and return
to function. Because these fractures can be very slow to heal, the
intramedullary nail provides prolonged protection of the fracture site.
bone graft just harvested from the patient is accidentally dropped to
the floor of the operating room. Little has been written about what to
do in this circumstance other than to abandon the graft and harvest
another one, if available. Presnal and Kimbrough (117)
found that 50 surplus bone grafts dropped in the operating room in
their institution and submitted for culture were sterile. Therefore,
they recommended that, if a bone graft that had been dropped on the
operating room floor was to be implanted in the patient, extensive
sterilization was not essential. Our approach to the dropped graft is
as follows:
-
If a second graft can be harvested with
no or minimal additional morbidity for the patient, then discard the
dropped graft and harvest a second graft. -
If the dropped graft is essential and an
additional harvest would subject the patient to additional morbidity,
then treat the graft as follows and use it: -
Irrigate the graft using a pulsatile irrigator with two or more liters of saline depending on the size of the graft.
-
Then soak the graft in a basin on the
back table for at least 10 minutes in either an antibiotic solution or
in a very dilute solution of povidone-iodine. Povidone has been shown
to be cytotoxic to osteoblasts, so the solution should be weak and the
surgeon must realize that this may result in death of any viable
osteoblasts in the graft. -
The graft can then be inserted directly
from the antibiotic solution, or, if povidone solution was used, it
should then again be irrigated to remove any residual iodophor. -
If the graft happens to be cancellous
bone, then adding an antibiotic such as tobramycin powder to the graft
may be useful because cancellous bone graft has been recommended as a
vehicle for local antibiotic delivery. Lindsey et al. (85)
have shown that local Tobramycin does not appear to affect the normal
healing characteristics of bone graft in a canine femoral defect.
appropriate postoperative intravenous bacteriocidal antibiotics are
appropriate. Counseling of the patient is wise.
fractures for which Ilizarov techniques are not being used, we prefer
autologous bone graft harvested from the iliac crest. If any
significant amount of cancellous bone is needed, then we nearly always
use the posterior ilium, in spite of the inconvenience of this
operative site because of the large amount of bone available from this
location. In addition, the morbidity of this harvest site is much less
than one located anteriorly. Neither one of us does spine surgery, so
the issues of bone grafting and spine arthrodesis will be addressed in
the spine section.
from the metaphyseal section of the same limb segment or ipsilateral
limb. For contained metaphyseal defects secondary to fractures such as
in the tibial plateau, or for small benign tumors requiring bone graft,
we use Pro-Osteon 500 granules and blocks usually in combination. One
of the authors of this chapter (JRR), whose practice is predominantly
revision total joint arthroplasty, uses a considerable amount of
allograft, both in a morcellized form for impaction bone grafting in
cavitary defects of the femur and as structural grafts in major
proximal femoral, acetabular, or other areas. In addition, he uses
whole bone allografts with joint surfaces in an occasional resection
for malignant tumors and an occasional shell allograft for joint
resurfacing. Although his experience is small, it is consistent with
the literature discussed in this chapter. The techniques used by both
of us are reflected in the sections of this chapter discussing
indications and surgical technique.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.,
LC, Schottstaedt ER, Saunders JB, et al. The Evaluation of Cortical and
Cancellous Bone as Grafting Material. A Clinical and Experimental
Study. J Bone Joint Surg 1947;29:381.
SD, Schimandle JH, Hutton WC, et al. Lumbar Intertransverse-Process
Spinal Arthrodesis with Use of a Bovine Bone-Derived Osteoinductive
Protein. J Bone Joint Surg 1995;77-A:1404.
GD, Goldberg VM, Gordon NH, et al. The Long-Term Fate of Fresh and
Frozen Orthotopic Bone Allografts in Genetically Defined Rats. Clin Orthop 1985;197:245.
TD, Rodin EL, Martin RB, Burn OB. Finite Element Studies of Some
Juxta-articular Stress Changes due to Localized Subchondral Stiffening.
J Biomed 1984;17:11.
MW, Bucholz R, Cornell C. Treatment of Acute Fractures with a
Collagen-Calcium Phosphate Graft Material: A Randomized Clinical Trial.
J Bone Joint Surg 1997;79A:495.
JF, Guse R, Tiedeman J, Dehne R. Autologous Marrow Injection as a
Substitute for Operative Grafting of Tibial Nonunions. Clin Orthop 1991;266:259.
SD, Baffes GC, Wolfe MW, et al. The Effect of Recombinant Human
Osteogenic Protein-1 on Healing of Large Segmental Bone Defects. J Bone Joint Surg 1994;76-A:827.
PL, Urist MR. Osteogenesis by Radio-isotope Labelled Cell Populations
in Implants of Bone Matrix under the Influence of Ionizing Radiation. Clin Orthop 1971;76:231.
C, Verhelpen M, d’ Hemricourt J, et al. Morphometric and Physical
Investigations of Segmental Cortical Bone Autografts in Canine Ulnar
Defects. Clin Orthop 1992;282:273.
MW, Ford CH. The Development of Humoral Cytotoxic Antibodies after the
Allografting of Articular Surfaces of the Knee Joint in Sheep. J Bone Joint Surg 1971;53-B:554.
RH, Malinin TI, Cuellar AD, et al. Cortical Strut Allografts on the
Reconstruction of the Femur in Revision Total Hip Arthroplasty. Clinical Orthop 1992;285:35.
TN, Kirker-Head VS, Kriz MJ, et al. Healing Segmental Femoral Defects
in Sheep Using Recombinant Human Bone Morphogenetic Protein. Clin Orthop 1993;293:317.
S, Heligman D, Quill G, Piasecki P. The Use of Large Allografts for
Tumor Reconstruction and Salvage of the Failed Total Hip Arthroplasty. Clin Orthop 1988;231:62.
J, Kaban CB, Murray JE, et al. Application of the Biological Principle
of Induced Osteogenesis for Craniofacial Defects. Lancet 1981;1:959.
AE, McKee NH, Langer F, Pritzker K. Surgical Techniques and Clinical
Experience with Articular Allografts at the Knee. In: Friedlaender GE,
Mankin HJ, Sell KW, eds. Osteochondral Allografts: Biology, Banking, and Clinical Applications. Boston: Little Brown & Co, 1984.
AE, Silverstein EA, Fal J, et al. The Allotransplantation of Partial
Joints in the Treatment of Osteoarthritis of the Knee. Clin Orthop 1975;108:7.
RE, Chapman MW, Yee T, Moore DC. Autogenous Bone Marrow and Porous
Biphasic Calcium-Phosphate Ceramic for Segmental Bone Defects in the
Canine Ulna. Clin Orthop 1991;266:244.
KD, Johnston JO, Kaufer HN, Luck JV Jr. Limb Salvage and Prosthetic
Joint Reconstruction for Low-grade and Selected High-grade Sarcomas of
Bone after Wide Resection and Replacement by Autoclaved Autogeneic
Grafts. Clin Orthop 1986;211:180.
JD, Boyan BD, Aufdemorte TB, Abbott JT. The Use of Bone Morphogenetic
Protein in the Treatment of Non-Union in a Canine Model. J Bone Joint Surg 1991;73A:750.
CL, Jackson A, Aschliman M, Meske NB. The Estimation of Femoral Condyle
Size. An Important Component in Osteochondral Allografts. Clin Orthop 1989;246:225.
MK, Bencivenga A. Comparative Value of Cortical and Cancellous Bone as
Grafting Material in Bone Defect of Different Origins. East Afr Med J 1978;55:228.
MH, Gebhardt MC, Tomford WW, Mankin HJ. Reconstruction for Defects of
the Proximal Part of the Femur Using Allograft Arthroplasty. J Bone Joint Surg 1988;70-A:507.
EE, Urist MR, Finerman GA. Repair of Segmental Defects of the Tibia
With Cancellous Bone Grafts Augmented with Human Bone Morphogenetic
Protein. A Preliminary Report. Clin Orthop 1988;236:249.
EVS. Wide Resection of Primary Tumors of Bone and Replacement with
Massive Bone Grafts. An Improved Technique for Transplanting Allogeneic
Bone Grafts. Clin Orthop 1978;134:302.
HJ, Fogelson FS, Thrasher AZ, Jaffer F. Resection and Allograft
Transplantation in the Treatment of Malignant Bone Tumors. N Engl J Med 1976;294:1247.
RB, Chapman MW, Holmes RE, et al. The Effects of Bone Ingrowth on the
Strength and Noninvasive Assessment of a Coralline Hydroxyapatite
Material. Biomaterials 1989;10:481.
AG, Langer F, Pritzker KP, Gross AE. Fresh Small-fragment Osteochondral
Allografts. Long-Term Follow Up Study on First 100 Cases. Clin Orthop 1985;197:96.
JT, Bowers GM, Bailey RC. Comparison of Bone Graft Materials: II. New
Bone Formation with Autografts and Allografts: A Histological
Evaulation. J Periodontol 1981;52:297.
MH, Bucholz RW, Jones RE. Surgical Techniques and Clinical Experience
with Fresh Osteochondral Allografts of the Hip. In: Friedlaender GE,
Mankin JH, Sell KW, eds. Osteochondral Allografts: Biology, Banking,
and Clinical Applications. Boston: Little, Brown & Co, 1984.
MH, Harvey JP, Moore TM. Treatment of Displaced Subcapital and
Transcervical Fractures of the Femoral Neck by Muscle-Pedicle Bone
Graft and Internal Fixation. J Bone Joint Surg 1973;55-A:257.
MH, Harvey JP, Moore TM. The Muscle-Pedicle Bone Graft in the Treatment
of Displaced Fractures of the Femoral Neck: Indications, Operative
Technique, and Results. Orthop Clin North Am 1974;5:779.
W, Malinen TI, Makley JT, Dick HM. Massive Osteoarticular Allografts in
the Reconstruction of Extremities Following Resection of Tumors not
Requiring Chemotherapy and Radiation. Clin Orthop 1985;197:76.
DC, Chapman MW, Manske D. The Evaluation of a Biphasic Calcium
Phosphate Ceramic for Use in Grafting Long-bone Diaphyseal Defects. J Orthop Res 1987;5:356.
SW, Salter RB. The Induction Neochondrogenesis in Free Intra-articular
Autografts under the Influence of Continous Passive Motion. J Bone Joint Surg 1984;66-A:1248.
SA, Prolo DJ, Gutierrez RV, King SE. Quantitative Comparisons of
Healing in Cranial Fresh Autografts, Frozen Autografts, and Processed
Autografts and Allografts in Canine Skull Defects. Clin Orthop 1986;205:269.
RR, Friedlaender GE, Markham TC, et al. Effects of Freezing and
Freeze-Drying on the Biomechanical Properties of Rat Bone. J Orthop Res 1984;1:405.
DJ, Oklund SA. Composite Autogeneic Human Cranioplasty: Frozen Skull
Supplemented with Fresh Iliac Corticocancellous Bone. Neurosurgery 1984;15:846.
J. Reorganization of Fresh and Preserved Bone Transplants: An
Experimental Study in Rabbits Using Tetracycline Labelling. Acta Orthop Scand 1966;(Suppl)92:9.
JJ, Gray JM, Thompson EC. Temporary Suppression of Humoral Cytotoxic
Antibodies with Double Dose Cyclosphosamide Treatment in Rats Receiving
Distal Femur Allografts. Trans Orthop Res Soc 1984;9:265.
JJ, Sakovich L. Osteocartilaginous Allografts as Compared with
Autografts in the Treatment of Knee Joint Osteocartilaginous Defects in
Dogs. Trans Orthop Res Soc 1977;2:92.
JJ, Sakovich L, Travis C, Smith G. Osteocartilginous Allografts as
Compared with Autografts in the Treatment of Knee Joint
Osteocartilaginous Defects in Dogs. Clin Orthop 1978;134:342.
JJ, Schnaser AM, Reynolds HM Jr, et al. Inhibition of the Immune
Response to Experimental Fresh Osteoarticular Allografts. Clin Orthop 1989;243:235.
MS, Endrizzi DP, Cannon RM, Dick HM. Treatment of Tibial Defects and
Nonunions Using ipsilateral Vascularized Fibular Transposition. Clin Orthop 1993;296:207.
MR. Chemosterilized Antigen-Extracted Surface-Demineralized Allogeneic
(AAA) Bone for Arthrodesis. In: Friedlaender GE, Mankin HJ, Sell KW,
eds. Osteochondral Allografts. Boston: Little, Brown & Co, 1983.
MR, Dawson E. Intertransverse Process Fusion with the Aid of
Chemosterilized Autolyzed Antigen-Extracted Allogeneic (AAA) Bone. Clin Orthop 1981;154:97.
EK, Lane JM, Fellinger ER, et al. The Healing of Segmental Bone
Defects, Induced by Recombinant Human Bone Morphogenetic (rhBMP-2). J Bone Joint Surg 1992;74-A:659.